User login
Chronic inflammatory diseases vary widely in CHD risk
Not all chronic systemic inflammatory diseases are equal enhancers of atherosclerotic cardiovascular disease risk, according to a large case-control study.
Current AHA/American College of Cardiology guidelines cite three chronic inflammatory diseases as atherosclerotic cardiovascular disease risk enhancers: rheumatoid arthritis, psoriasis, and HIV infection. But this study of those three diseases, along with three others marked by elevated high sensitivity C-reactive protein (systemic sclerosis, inflammatory bowel disease, and systemic lupus erythematosus [SLE]), showed that chronic inflammatory diseases are not monolithic in terms of their associated risk of incident coronary heart disease (CHD).
Indeed, two of the six inflammatory diseases – psoriasis and inflammatory bowel disease – turned out to be not at all associated with increased cardiovascular risk in the 37,117-patient study. The highest-risk disease was SLE, not specifically mentioned in the guidelines, Arjun Sinha, MD, a cardiology fellow at Northwestern University, Chicago, noted in his presentation at the virtual American Heart Association scientific sessions.
The study included 18,129 patients with one of the six chronic inflammatory diseases and 18,988 matched controls, none with CHD at baseline. All regularly received outpatient care at Northwestern during 2000-2019. There were 1,011 incident CHD events during a median of 3.5 years of follow-up.
In a Cox proportional hazards analysis adjusted for demographics, insurance status, hypertension, diabetes, current smoking, total cholesterol, and estimated glomerular filtration rate, here’s how the chronic inflammatory diseases stacked up in terms of incident CHD and MI risks:
- SLE: hazard ratio for CHD, 2.85; for MI, 4.76.
- Systemic sclerosis: HR for CHD, 2.14; for MI, 3.19.
- HIV: HR for CHD, 1.38; for MI, 1.69.
- Rheumatoid arthritis: HR for CHD, 1.22; for MI, 1.45.
- Psoriasis: no significant increase.
- Inflammatory bowel disease: no significant increase.
In an exploratory analysis, Dr. Sinha and coinvestigators evaluated the risk of incident CHD stratified by disease severity. For lack of standardized disease severity scales, the investigators relied upon tertiles of CD4 T cell count in the HIV group and CRP in the others. The HR for new-onset CHD in the more than 5,000 patients with psoriasis didn’t vary by CRP tertile. However, there was a nonsignificant trend for greater disease severity, as reflected by CRP tertile, to be associated with increased incident CHD risk in the HIV and inflammatory bowel disease groups.
In contrast, patients with rheumatoid arthritis or systemic sclerosis who were in the top CRP tertile had a significantly greater risk of developing CHD than that of controls, with HRs of 2.11 in the rheumatoid arthritis group and 4.59 with systemic sclerosis, although patients in the other two tertiles weren’t at significantly increased risk. But all three tertiles of CRP in patients with SLE were associated with significantly increased CHD risk: 3.17-fold in the lowest tertile of lupus severity, 5.38-fold in the middle tertile, and 4.04-fold in the top tertile for inflammation.
These findings could be used in clinical practice to fine-tune atherosclerotic cardiovascular disease risk assessment based upon chronic inflammatory disease type and severity. That’s information which in turn can help guide the timing and intensity of preventive therapy for patients with each disease type.
But studying the association between chronic systemic inflammatory diseases and CHD risk can be useful in additional ways, according to Dr. Sinha. These inflammatory diseases can serve as models of atherosclerosis that shed light on the non–lipid-related mechanisms involved in cardiovascular disease.
“The gradient in risk may be hypothesis-generating with respect to which specific inflammatory pathways may contribute to CHD,” he explained.
Each of these six chronic inflammatory diseases is characterized by a different form of major immune dysfunction, Dr. Sinha continued. A case in point is SLE, the inflammatory disease associated with the highest risk of CHD and MI. Lupus is characterized by a form of neutrophil dysfunction marked by increased formation and reduced degradation of neutrophil extracellular traps, or NETs, as well as by an increase in autoreactive B cells and dysfunctional CD4+ T helper cells. The increase in NETs of of particular interest because NETs have also been shown to contribute to the development of atherosclerosis, endothelial dysfunction, plaque erosion, and thrombosis.
In another exploratory analysis, Dr. Sinha and coworkers found that SLE patients with a neutrophil count above the median level were twice as likely to develop CHD than were those with a neutrophil count below the median.
A better understanding of the upstream pathways linking NET formation in SLE and atherosclerosis could lead to development of new or repurposed medications that target immune dysfunction in order to curb atherosclerosis, said Dr. Sinha, whose study won the AHA’s Samuel A. Levine Early Career Clinical Investigator Award.
He reported having no financial conflicts regarding his study.
Not all chronic systemic inflammatory diseases are equal enhancers of atherosclerotic cardiovascular disease risk, according to a large case-control study.
Current AHA/American College of Cardiology guidelines cite three chronic inflammatory diseases as atherosclerotic cardiovascular disease risk enhancers: rheumatoid arthritis, psoriasis, and HIV infection. But this study of those three diseases, along with three others marked by elevated high sensitivity C-reactive protein (systemic sclerosis, inflammatory bowel disease, and systemic lupus erythematosus [SLE]), showed that chronic inflammatory diseases are not monolithic in terms of their associated risk of incident coronary heart disease (CHD).
Indeed, two of the six inflammatory diseases – psoriasis and inflammatory bowel disease – turned out to be not at all associated with increased cardiovascular risk in the 37,117-patient study. The highest-risk disease was SLE, not specifically mentioned in the guidelines, Arjun Sinha, MD, a cardiology fellow at Northwestern University, Chicago, noted in his presentation at the virtual American Heart Association scientific sessions.
The study included 18,129 patients with one of the six chronic inflammatory diseases and 18,988 matched controls, none with CHD at baseline. All regularly received outpatient care at Northwestern during 2000-2019. There were 1,011 incident CHD events during a median of 3.5 years of follow-up.
In a Cox proportional hazards analysis adjusted for demographics, insurance status, hypertension, diabetes, current smoking, total cholesterol, and estimated glomerular filtration rate, here’s how the chronic inflammatory diseases stacked up in terms of incident CHD and MI risks:
- SLE: hazard ratio for CHD, 2.85; for MI, 4.76.
- Systemic sclerosis: HR for CHD, 2.14; for MI, 3.19.
- HIV: HR for CHD, 1.38; for MI, 1.69.
- Rheumatoid arthritis: HR for CHD, 1.22; for MI, 1.45.
- Psoriasis: no significant increase.
- Inflammatory bowel disease: no significant increase.
In an exploratory analysis, Dr. Sinha and coinvestigators evaluated the risk of incident CHD stratified by disease severity. For lack of standardized disease severity scales, the investigators relied upon tertiles of CD4 T cell count in the HIV group and CRP in the others. The HR for new-onset CHD in the more than 5,000 patients with psoriasis didn’t vary by CRP tertile. However, there was a nonsignificant trend for greater disease severity, as reflected by CRP tertile, to be associated with increased incident CHD risk in the HIV and inflammatory bowel disease groups.
In contrast, patients with rheumatoid arthritis or systemic sclerosis who were in the top CRP tertile had a significantly greater risk of developing CHD than that of controls, with HRs of 2.11 in the rheumatoid arthritis group and 4.59 with systemic sclerosis, although patients in the other two tertiles weren’t at significantly increased risk. But all three tertiles of CRP in patients with SLE were associated with significantly increased CHD risk: 3.17-fold in the lowest tertile of lupus severity, 5.38-fold in the middle tertile, and 4.04-fold in the top tertile for inflammation.
These findings could be used in clinical practice to fine-tune atherosclerotic cardiovascular disease risk assessment based upon chronic inflammatory disease type and severity. That’s information which in turn can help guide the timing and intensity of preventive therapy for patients with each disease type.
But studying the association between chronic systemic inflammatory diseases and CHD risk can be useful in additional ways, according to Dr. Sinha. These inflammatory diseases can serve as models of atherosclerosis that shed light on the non–lipid-related mechanisms involved in cardiovascular disease.
“The gradient in risk may be hypothesis-generating with respect to which specific inflammatory pathways may contribute to CHD,” he explained.
Each of these six chronic inflammatory diseases is characterized by a different form of major immune dysfunction, Dr. Sinha continued. A case in point is SLE, the inflammatory disease associated with the highest risk of CHD and MI. Lupus is characterized by a form of neutrophil dysfunction marked by increased formation and reduced degradation of neutrophil extracellular traps, or NETs, as well as by an increase in autoreactive B cells and dysfunctional CD4+ T helper cells. The increase in NETs of of particular interest because NETs have also been shown to contribute to the development of atherosclerosis, endothelial dysfunction, plaque erosion, and thrombosis.
In another exploratory analysis, Dr. Sinha and coworkers found that SLE patients with a neutrophil count above the median level were twice as likely to develop CHD than were those with a neutrophil count below the median.
A better understanding of the upstream pathways linking NET formation in SLE and atherosclerosis could lead to development of new or repurposed medications that target immune dysfunction in order to curb atherosclerosis, said Dr. Sinha, whose study won the AHA’s Samuel A. Levine Early Career Clinical Investigator Award.
He reported having no financial conflicts regarding his study.
Not all chronic systemic inflammatory diseases are equal enhancers of atherosclerotic cardiovascular disease risk, according to a large case-control study.
Current AHA/American College of Cardiology guidelines cite three chronic inflammatory diseases as atherosclerotic cardiovascular disease risk enhancers: rheumatoid arthritis, psoriasis, and HIV infection. But this study of those three diseases, along with three others marked by elevated high sensitivity C-reactive protein (systemic sclerosis, inflammatory bowel disease, and systemic lupus erythematosus [SLE]), showed that chronic inflammatory diseases are not monolithic in terms of their associated risk of incident coronary heart disease (CHD).
Indeed, two of the six inflammatory diseases – psoriasis and inflammatory bowel disease – turned out to be not at all associated with increased cardiovascular risk in the 37,117-patient study. The highest-risk disease was SLE, not specifically mentioned in the guidelines, Arjun Sinha, MD, a cardiology fellow at Northwestern University, Chicago, noted in his presentation at the virtual American Heart Association scientific sessions.
The study included 18,129 patients with one of the six chronic inflammatory diseases and 18,988 matched controls, none with CHD at baseline. All regularly received outpatient care at Northwestern during 2000-2019. There were 1,011 incident CHD events during a median of 3.5 years of follow-up.
In a Cox proportional hazards analysis adjusted for demographics, insurance status, hypertension, diabetes, current smoking, total cholesterol, and estimated glomerular filtration rate, here’s how the chronic inflammatory diseases stacked up in terms of incident CHD and MI risks:
- SLE: hazard ratio for CHD, 2.85; for MI, 4.76.
- Systemic sclerosis: HR for CHD, 2.14; for MI, 3.19.
- HIV: HR for CHD, 1.38; for MI, 1.69.
- Rheumatoid arthritis: HR for CHD, 1.22; for MI, 1.45.
- Psoriasis: no significant increase.
- Inflammatory bowel disease: no significant increase.
In an exploratory analysis, Dr. Sinha and coinvestigators evaluated the risk of incident CHD stratified by disease severity. For lack of standardized disease severity scales, the investigators relied upon tertiles of CD4 T cell count in the HIV group and CRP in the others. The HR for new-onset CHD in the more than 5,000 patients with psoriasis didn’t vary by CRP tertile. However, there was a nonsignificant trend for greater disease severity, as reflected by CRP tertile, to be associated with increased incident CHD risk in the HIV and inflammatory bowel disease groups.
In contrast, patients with rheumatoid arthritis or systemic sclerosis who were in the top CRP tertile had a significantly greater risk of developing CHD than that of controls, with HRs of 2.11 in the rheumatoid arthritis group and 4.59 with systemic sclerosis, although patients in the other two tertiles weren’t at significantly increased risk. But all three tertiles of CRP in patients with SLE were associated with significantly increased CHD risk: 3.17-fold in the lowest tertile of lupus severity, 5.38-fold in the middle tertile, and 4.04-fold in the top tertile for inflammation.
These findings could be used in clinical practice to fine-tune atherosclerotic cardiovascular disease risk assessment based upon chronic inflammatory disease type and severity. That’s information which in turn can help guide the timing and intensity of preventive therapy for patients with each disease type.
But studying the association between chronic systemic inflammatory diseases and CHD risk can be useful in additional ways, according to Dr. Sinha. These inflammatory diseases can serve as models of atherosclerosis that shed light on the non–lipid-related mechanisms involved in cardiovascular disease.
“The gradient in risk may be hypothesis-generating with respect to which specific inflammatory pathways may contribute to CHD,” he explained.
Each of these six chronic inflammatory diseases is characterized by a different form of major immune dysfunction, Dr. Sinha continued. A case in point is SLE, the inflammatory disease associated with the highest risk of CHD and MI. Lupus is characterized by a form of neutrophil dysfunction marked by increased formation and reduced degradation of neutrophil extracellular traps, or NETs, as well as by an increase in autoreactive B cells and dysfunctional CD4+ T helper cells. The increase in NETs of of particular interest because NETs have also been shown to contribute to the development of atherosclerosis, endothelial dysfunction, plaque erosion, and thrombosis.
In another exploratory analysis, Dr. Sinha and coworkers found that SLE patients with a neutrophil count above the median level were twice as likely to develop CHD than were those with a neutrophil count below the median.
A better understanding of the upstream pathways linking NET formation in SLE and atherosclerosis could lead to development of new or repurposed medications that target immune dysfunction in order to curb atherosclerosis, said Dr. Sinha, whose study won the AHA’s Samuel A. Levine Early Career Clinical Investigator Award.
He reported having no financial conflicts regarding his study.
FROM AHA 2020
Methotrexate users need tuberculosis tests in high-TB areas
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
People taking even low-dose methotrexate need tuberculosis screening and ongoing clinical care if they live in areas where TB is common, results of a study presented at the virtual annual meeting of the American College of Rheumatology suggest.
Coauthor Carol Hitchon, MD, MSc, a rheumatologist with the University of Manitoba in Winnipeg, who presented the findings, warned that methotrexate (MTX) users who also take corticosteroids or other immunosuppressants are at particular risk and need TB screening.
Current management guidelines for rheumatic disease address TB in relation to biologics, but not in relation to methotrexate, Dr. Hitchon said.
“We know that methotrexate is the foundational DMARD [disease-modifying antirheumatic drug] for many rheumatic diseases, especially rheumatoid arthritis,” Dr. Hitchon noted at a press conference. “It’s safe and effective when dosed properly. However, methotrexate does have the potential for significant liver toxicity as well as infection, particularly for infectious organisms that are targeted by cell-mediated immunity, and TB is one of those agents.”
Using multiple databases, researchers conducted a systematic review of the literature published from 1990 to 2018 on TB rates among people who take less than 30 mg of methotrexate a week. Of the 4,700 studies they examined, 31 fit the criteria for this analysis.
They collected data on tuberculosis incidence or new TB diagnoses vs. reactivation of latent TB infection as well as TB outcomes, such as pulmonary symptoms, dissemination, and mortality.
They found a modest increase in the risk of TB infections in the setting of low-dose methotrexate. In addition, rates of TB in people with rheumatic disease who are treated with either methotrexate or biologics are generally higher than in the general population.
They also found that methotrexate users had higher rates of the type of TB that spreads beyond a patient’s lungs, compared with the general population.
Safety of INH with methotrexate
Researchers also looked at the safety of isoniazid (INH), the antibiotic used to treat TB, and found that isoniazid-related liver toxicity and neutropenia were more common when people took the antibiotic along with methotrexate, but those effects were usually reversible.
TB is endemic in various regions around the world. Historically there hasn’t been much rheumatology capacity in many of these areas, but as that capacity increases more people who are at high risk for developing or reactivating TB will be receiving methotrexate for rheumatic diseases, Dr. Hitchon said.
“It’s prudent for people managing patients who may be at higher risk for TB either from where they live or from where they travel that we should have a high suspicion for TB and consider screening as part of our workup in the course of initiating treatment like methotrexate,” she said.
Narender Annapureddy, MD, a rheumatologist at Vanderbilt University, Nashville, Tenn., who was not involved in the research, pointed out that a limitation of the work is that only 27% of the studies are from developing countries, which are more likely to have endemic TB, and those studies had very few cases.
“This finding needs to be studied in larger populations in TB-endemic areas and in high-risk populations,” he said in an interview.
As for practice implications in the United States, Dr. Annapureddy noted that TB is rare in the United States and most of the cases occur in people born in other countries.
“This population may be at risk for TB and should probably be screened for TB before initiating methotrexate,” he said. “Since biologics are usually the next step, especially in RA after patients fail methotrexate, having information on TB status may also help guide management options after MTX failure.
“Since high-dose steroids are another important risk factor for TB activation,” Dr. Annapureddy continued, “rheumatologists should likely consider screening patients who are going to be on moderate to high doses of steroids with MTX.”
A version of this article originally appeared on Medscape.com.
Topical tapinarof effective in pivotal psoriasis trials
in two identical pivotal phase 3, randomized trials, Mark G. Lebwohl, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Tapinarof cream has the potential to be a first-in-class topical therapeutic aryl hydrocarbon receptor modulating agent and will provide physicians and patients with a novel nonsteroidal topical treatment option that’s effective and well tolerated,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dermavant Sciences, the company developing topical tapinarof for treatment of atopic dermatitis as well as psoriasis, announced that upon completion of an ongoing long-term extension study the company plans to file for approval of the drug for psoriasis in 2021.
The two pivotal phase 3 trials, PSOARING 1 and PSOARING 2, randomized a total of 1,025 patients with plaque psoriasis to once-daily tapinarof cream 1% or its vehicle. “This was a fairly difficult group of patients,” Dr. Lebwohl said. Roughly 80% had moderate psoriasis as defined by a baseline Physician Global Assessment (PGA) score of 3, with the remainder split evenly between mild and severe disease. Participants averaged 8% body surface area involvement. Body mass index was on average greater than 31 kg/m2.
The primary efficacy endpoint was a PGA score of 0 or 1 – that is, clear or almost clear – plus at least a 2-grade improvement in PGA from baseline at week 12. This was achieved in 35.4% of patients on tapinarof cream once daily in PSOARING 1 and 40.2% in PSOARING 2, compared with 6.0% and 6.3% of vehicle-treated controls, a highly significant difference (both P < .0001).
The prespecified secondary endpoint was a 75% improvement in Psoriasis Area and Severity Index (PASI) score from baseline to week 12. The PASI 75 rates were 36.1% and 47.6% with tapinarof, significantly better than the 10.2% and 6.9% rates in controls.
The most common adverse event associated with tapinarof was folliculitis, which occurred in 20.6% of treated patients in PSOARING 1 and in 15.7% in PSOARING 2. More than 98% of cases were mild or moderate. The folliculitis led to study discontinuation in only 1.8% and 0.9% of subjects in the two trials.
The other noteworthy adverse event was contact dermatitis. It occurred in 3.8% and 4.7% of tapinarof-treated patients, again with low study discontinuation rates of 1.5% and 2.2%.
During the audience discussion, Linda Stein Gold, MD, lead investigator for PSOARING 2, was asked about this folliculitis. She said the mechanism is unclear, as is the best management. She encountered it in patients, didn’t treat it, and it went away on its own. It’s not a bacterial folliculitis; when cultured it invariably proved culture negative, she noted.
The comparative efficacy of tapinarof cream versus the potent and superpotent topical corticosteroids commonly used in the treatment of psoriasis hasn’t been evaluated in head-to-head studies. Her experience and that of the other investigators has been that tapinarof’s efficacy is comparably strong, “but we don’t have the steroid side effects,” said Dr. Stein Gold, director of dermatology clinical research at Henry Ford Health System in Detroit.
In an interview, Dr. Lebwohl said tapinarof, if approved, could help meet a major unmet need for new and better topical therapies for psoriasis.
“You keep hearing about all these biologic agents and small-molecule pills coming out, but the majority of patients still only need topical therapy,” he observed.
Moreover, even when patients with more severe disease achieve a PASI 75 or PASI 90 response with systemic therapy, they usually still need supplemental topical therapy to get them closer to the goal of clear skin.
The superpotent steroids that are the current mainstay of topical therapy come with predictable side effects that dictate a 2- to 4-week limit on their approved use. Also, they’re not supposed to be applied to the face or to intertriginous sites, including the groin, axillae, and under the breasts. In contrast, tapinarof has proved safe and effective in these sensitive areas.
Asked to predict how tapinarof is likely to be used in clinical practice, Dr. Lebwohl replied: “The efficacy was equivalent to strong topical steroids, so I think it could be used first line in place of topical steroids. And in particular, in patients with psoriasis at facial and intertriginous sites, I think an argument can be made for insisting that it be first line.”
He also expects that physicians will end up utilizing tapinarof for a varied group of steroid-responsive dermatoses beyond psoriasis and atopic dermatitis.
“It clearly reduces inflammation, which is why I would expect it would work well for those,” the dermatologist said.
The mechanism of action of tapinarof has been worked out. The drug enters the cell and binds to the aryl hydrocarbon receptor, forming a complex that enters the nucleus. There it joins with the aryl hydrocarbon receptor nuclear translocator, which regulates gene expression so as to reduce production of inflammatory cytokines while promoting an increase in skin barrier proteins, which is why tapinarof is also being developed as an atopic dermatitis therapy.
Dr. Lebwohl and Dr. Stein Gold reported receiving research funds from and serving as consultants to Dermavant Sciences as well as numerous other pharmaceutical companies.
in two identical pivotal phase 3, randomized trials, Mark G. Lebwohl, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Tapinarof cream has the potential to be a first-in-class topical therapeutic aryl hydrocarbon receptor modulating agent and will provide physicians and patients with a novel nonsteroidal topical treatment option that’s effective and well tolerated,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dermavant Sciences, the company developing topical tapinarof for treatment of atopic dermatitis as well as psoriasis, announced that upon completion of an ongoing long-term extension study the company plans to file for approval of the drug for psoriasis in 2021.
The two pivotal phase 3 trials, PSOARING 1 and PSOARING 2, randomized a total of 1,025 patients with plaque psoriasis to once-daily tapinarof cream 1% or its vehicle. “This was a fairly difficult group of patients,” Dr. Lebwohl said. Roughly 80% had moderate psoriasis as defined by a baseline Physician Global Assessment (PGA) score of 3, with the remainder split evenly between mild and severe disease. Participants averaged 8% body surface area involvement. Body mass index was on average greater than 31 kg/m2.
The primary efficacy endpoint was a PGA score of 0 or 1 – that is, clear or almost clear – plus at least a 2-grade improvement in PGA from baseline at week 12. This was achieved in 35.4% of patients on tapinarof cream once daily in PSOARING 1 and 40.2% in PSOARING 2, compared with 6.0% and 6.3% of vehicle-treated controls, a highly significant difference (both P < .0001).
The prespecified secondary endpoint was a 75% improvement in Psoriasis Area and Severity Index (PASI) score from baseline to week 12. The PASI 75 rates were 36.1% and 47.6% with tapinarof, significantly better than the 10.2% and 6.9% rates in controls.
The most common adverse event associated with tapinarof was folliculitis, which occurred in 20.6% of treated patients in PSOARING 1 and in 15.7% in PSOARING 2. More than 98% of cases were mild or moderate. The folliculitis led to study discontinuation in only 1.8% and 0.9% of subjects in the two trials.
The other noteworthy adverse event was contact dermatitis. It occurred in 3.8% and 4.7% of tapinarof-treated patients, again with low study discontinuation rates of 1.5% and 2.2%.
During the audience discussion, Linda Stein Gold, MD, lead investigator for PSOARING 2, was asked about this folliculitis. She said the mechanism is unclear, as is the best management. She encountered it in patients, didn’t treat it, and it went away on its own. It’s not a bacterial folliculitis; when cultured it invariably proved culture negative, she noted.
The comparative efficacy of tapinarof cream versus the potent and superpotent topical corticosteroids commonly used in the treatment of psoriasis hasn’t been evaluated in head-to-head studies. Her experience and that of the other investigators has been that tapinarof’s efficacy is comparably strong, “but we don’t have the steroid side effects,” said Dr. Stein Gold, director of dermatology clinical research at Henry Ford Health System in Detroit.
In an interview, Dr. Lebwohl said tapinarof, if approved, could help meet a major unmet need for new and better topical therapies for psoriasis.
“You keep hearing about all these biologic agents and small-molecule pills coming out, but the majority of patients still only need topical therapy,” he observed.
Moreover, even when patients with more severe disease achieve a PASI 75 or PASI 90 response with systemic therapy, they usually still need supplemental topical therapy to get them closer to the goal of clear skin.
The superpotent steroids that are the current mainstay of topical therapy come with predictable side effects that dictate a 2- to 4-week limit on their approved use. Also, they’re not supposed to be applied to the face or to intertriginous sites, including the groin, axillae, and under the breasts. In contrast, tapinarof has proved safe and effective in these sensitive areas.
Asked to predict how tapinarof is likely to be used in clinical practice, Dr. Lebwohl replied: “The efficacy was equivalent to strong topical steroids, so I think it could be used first line in place of topical steroids. And in particular, in patients with psoriasis at facial and intertriginous sites, I think an argument can be made for insisting that it be first line.”
He also expects that physicians will end up utilizing tapinarof for a varied group of steroid-responsive dermatoses beyond psoriasis and atopic dermatitis.
“It clearly reduces inflammation, which is why I would expect it would work well for those,” the dermatologist said.
The mechanism of action of tapinarof has been worked out. The drug enters the cell and binds to the aryl hydrocarbon receptor, forming a complex that enters the nucleus. There it joins with the aryl hydrocarbon receptor nuclear translocator, which regulates gene expression so as to reduce production of inflammatory cytokines while promoting an increase in skin barrier proteins, which is why tapinarof is also being developed as an atopic dermatitis therapy.
Dr. Lebwohl and Dr. Stein Gold reported receiving research funds from and serving as consultants to Dermavant Sciences as well as numerous other pharmaceutical companies.
in two identical pivotal phase 3, randomized trials, Mark G. Lebwohl, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Tapinarof cream has the potential to be a first-in-class topical therapeutic aryl hydrocarbon receptor modulating agent and will provide physicians and patients with a novel nonsteroidal topical treatment option that’s effective and well tolerated,” predicted Dr. Lebwohl, professor and chair of the department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dermavant Sciences, the company developing topical tapinarof for treatment of atopic dermatitis as well as psoriasis, announced that upon completion of an ongoing long-term extension study the company plans to file for approval of the drug for psoriasis in 2021.
The two pivotal phase 3 trials, PSOARING 1 and PSOARING 2, randomized a total of 1,025 patients with plaque psoriasis to once-daily tapinarof cream 1% or its vehicle. “This was a fairly difficult group of patients,” Dr. Lebwohl said. Roughly 80% had moderate psoriasis as defined by a baseline Physician Global Assessment (PGA) score of 3, with the remainder split evenly between mild and severe disease. Participants averaged 8% body surface area involvement. Body mass index was on average greater than 31 kg/m2.
The primary efficacy endpoint was a PGA score of 0 or 1 – that is, clear or almost clear – plus at least a 2-grade improvement in PGA from baseline at week 12. This was achieved in 35.4% of patients on tapinarof cream once daily in PSOARING 1 and 40.2% in PSOARING 2, compared with 6.0% and 6.3% of vehicle-treated controls, a highly significant difference (both P < .0001).
The prespecified secondary endpoint was a 75% improvement in Psoriasis Area and Severity Index (PASI) score from baseline to week 12. The PASI 75 rates were 36.1% and 47.6% with tapinarof, significantly better than the 10.2% and 6.9% rates in controls.
The most common adverse event associated with tapinarof was folliculitis, which occurred in 20.6% of treated patients in PSOARING 1 and in 15.7% in PSOARING 2. More than 98% of cases were mild or moderate. The folliculitis led to study discontinuation in only 1.8% and 0.9% of subjects in the two trials.
The other noteworthy adverse event was contact dermatitis. It occurred in 3.8% and 4.7% of tapinarof-treated patients, again with low study discontinuation rates of 1.5% and 2.2%.
During the audience discussion, Linda Stein Gold, MD, lead investigator for PSOARING 2, was asked about this folliculitis. She said the mechanism is unclear, as is the best management. She encountered it in patients, didn’t treat it, and it went away on its own. It’s not a bacterial folliculitis; when cultured it invariably proved culture negative, she noted.
The comparative efficacy of tapinarof cream versus the potent and superpotent topical corticosteroids commonly used in the treatment of psoriasis hasn’t been evaluated in head-to-head studies. Her experience and that of the other investigators has been that tapinarof’s efficacy is comparably strong, “but we don’t have the steroid side effects,” said Dr. Stein Gold, director of dermatology clinical research at Henry Ford Health System in Detroit.
In an interview, Dr. Lebwohl said tapinarof, if approved, could help meet a major unmet need for new and better topical therapies for psoriasis.
“You keep hearing about all these biologic agents and small-molecule pills coming out, but the majority of patients still only need topical therapy,” he observed.
Moreover, even when patients with more severe disease achieve a PASI 75 or PASI 90 response with systemic therapy, they usually still need supplemental topical therapy to get them closer to the goal of clear skin.
The superpotent steroids that are the current mainstay of topical therapy come with predictable side effects that dictate a 2- to 4-week limit on their approved use. Also, they’re not supposed to be applied to the face or to intertriginous sites, including the groin, axillae, and under the breasts. In contrast, tapinarof has proved safe and effective in these sensitive areas.
Asked to predict how tapinarof is likely to be used in clinical practice, Dr. Lebwohl replied: “The efficacy was equivalent to strong topical steroids, so I think it could be used first line in place of topical steroids. And in particular, in patients with psoriasis at facial and intertriginous sites, I think an argument can be made for insisting that it be first line.”
He also expects that physicians will end up utilizing tapinarof for a varied group of steroid-responsive dermatoses beyond psoriasis and atopic dermatitis.
“It clearly reduces inflammation, which is why I would expect it would work well for those,” the dermatologist said.
The mechanism of action of tapinarof has been worked out. The drug enters the cell and binds to the aryl hydrocarbon receptor, forming a complex that enters the nucleus. There it joins with the aryl hydrocarbon receptor nuclear translocator, which regulates gene expression so as to reduce production of inflammatory cytokines while promoting an increase in skin barrier proteins, which is why tapinarof is also being developed as an atopic dermatitis therapy.
Dr. Lebwohl and Dr. Stein Gold reported receiving research funds from and serving as consultants to Dermavant Sciences as well as numerous other pharmaceutical companies.
FROM THE EADV CONGRESS
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis in Pediatric Patients
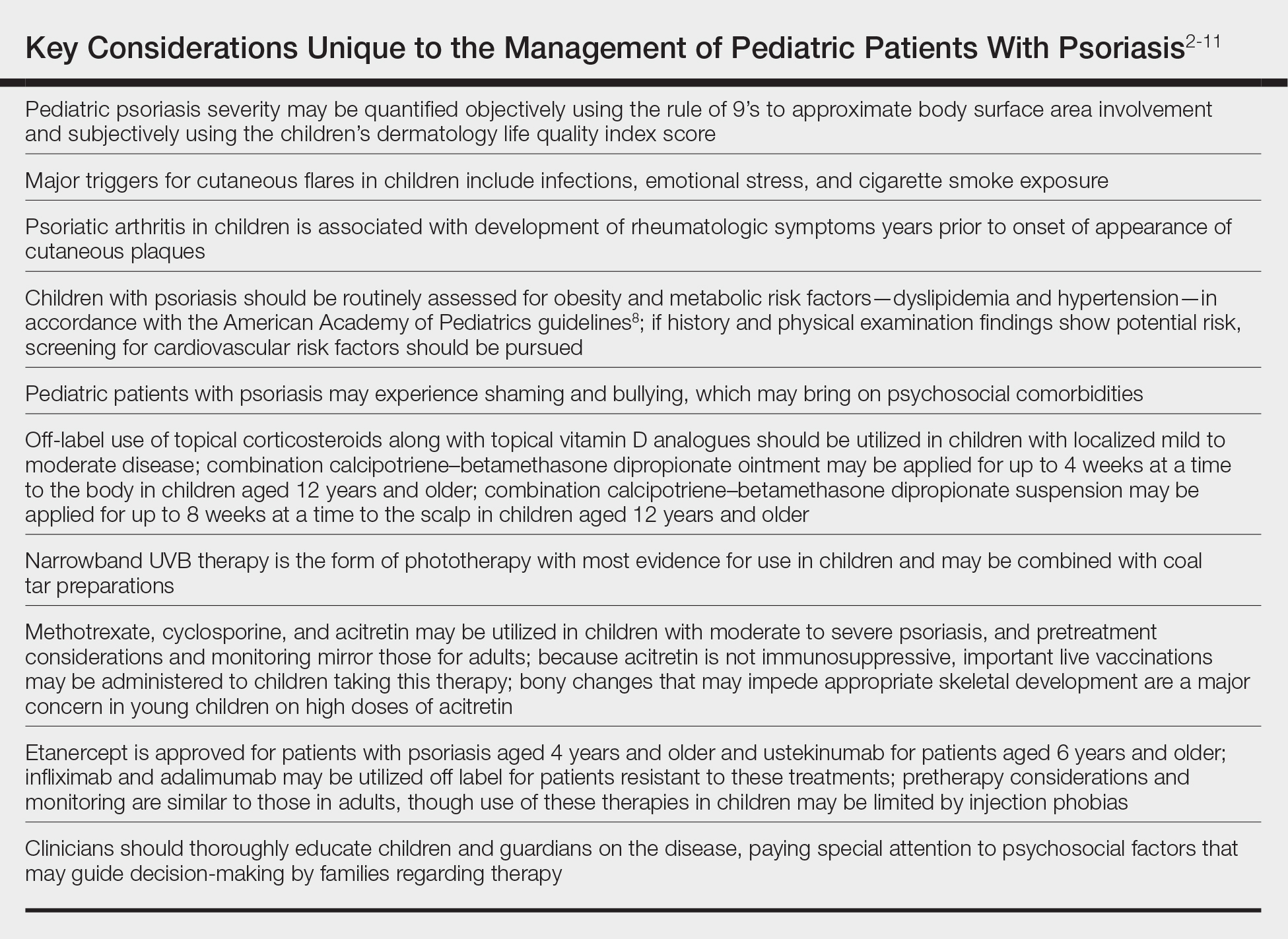
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).
Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.
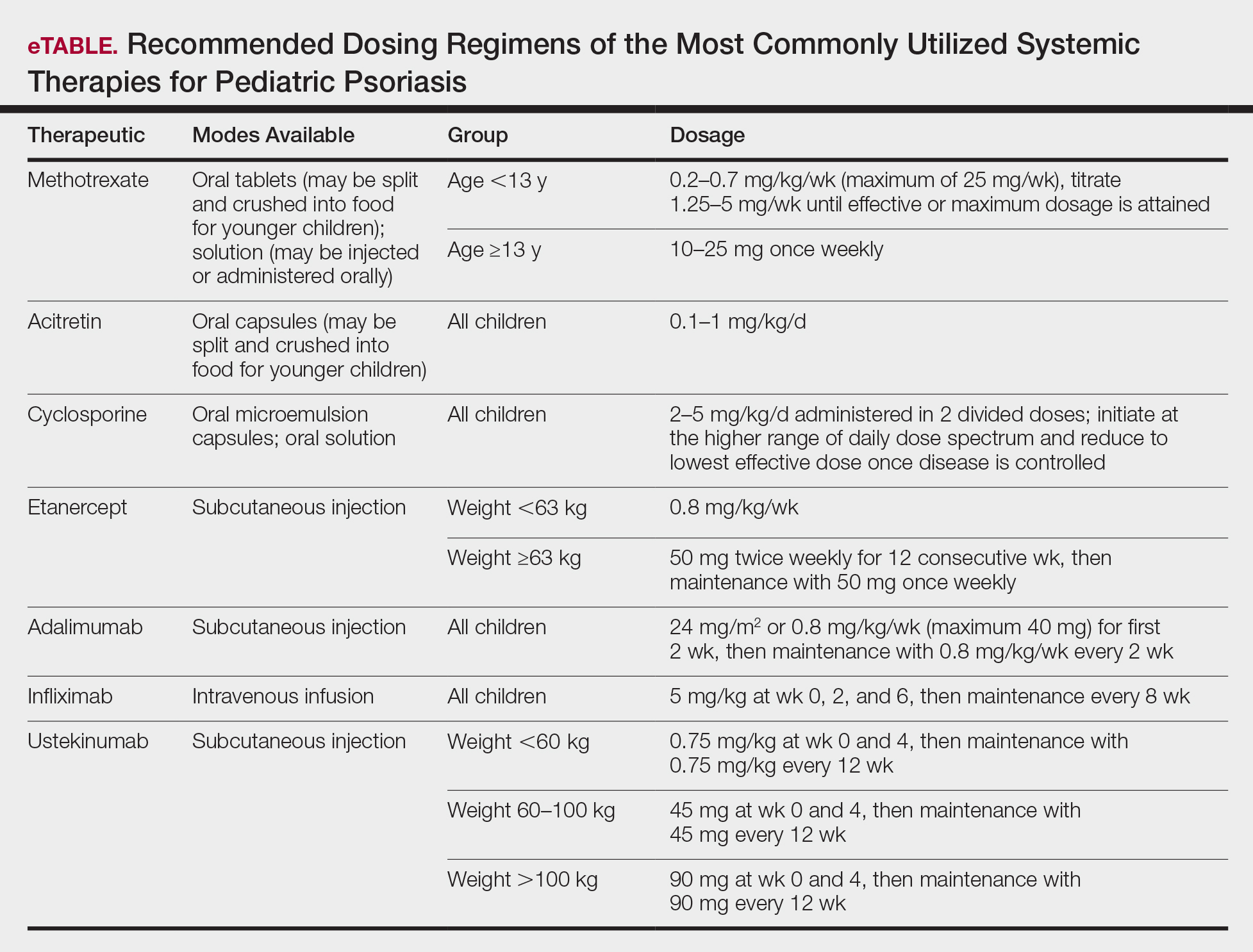
Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).

Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.

Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).

Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.

Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
Practice Points
- For children, several environmental factors may prompt psoriasis flares, and it is critical to identify and eliminate these triggers.
- Although the use of biologics may be limited by cost and injection phobias in children, they may be an appropriate option for children with moderate to severe psoriasis when other therapies have failed. A growing body of literature is establishing the safety and effectiveness of biologics in children.
- Clinicians should thoroughly educate parents/ guardians on the course of psoriasis and treatment options as well as pay special attention to treatment goals and psychosocial factors that may guide decision-making regarding therapy.
Biologics in Pediatric Psoriasis and Atopic Dermatitis: Revolutionizing the Treatment Landscape
Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.
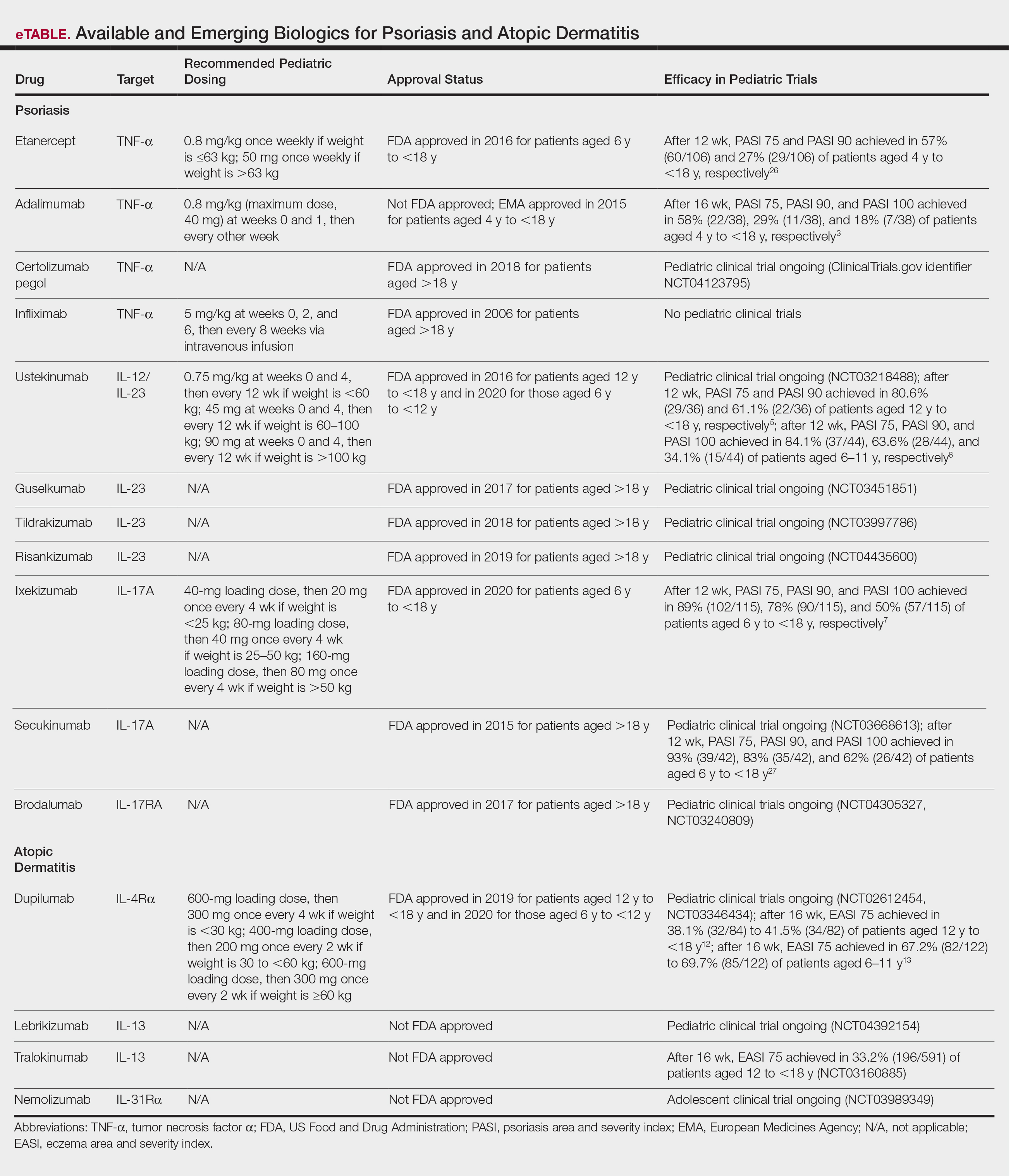
- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.

Psoriasis and atopic dermatitis (AD) can impact quality of life (QOL) in pediatric patients, warranting early recognition and treatment.1 Topical agents often are inadequate to treat moderate to severe disease, but the potential toxicity of systemic agents, which largely include immunosuppressives, limit their use in this population despite their effectiveness. Our expanding knowledge of the pathogenesis of psoriasis (tumor necrosis factor [TNF] α and IL-23/TH17 pathways) and AD has led to targeted interventions, particularly monoclonal antibody biologics, which have revolutionized treatment for affected adults and more recently children. Several agents are approved by the US Food and Drug Administration (FDA) for pediatric psoriasis, and dupilumab is approved for pediatric AD. Herein, we discuss the latest developments in the treatment landscape for pediatric psoriasis and AD.
Pediatric Psoriasis
Methotrexate (MTX) and cyclosporine have been FDA approved for psoriasis in adults since 1972 and 1997, respectively.2 Before biologics, MTX was the primary systemic agent used to treat pediatric psoriasis, given its lower toxicity vs cyclosporine. The TNF-α inhibitor etanercept became the first FDA-approved biologic for pediatric psoriasis in 2016. Adalimumab has been available in Europe for children since 2015 but is not FDA approved. Certolizumab, a pegylated TNF-α inhibitor that distinctly fails to cross the placental barrier currently is in clinical trials (ClinicalTrials.gov identifier NCT04123795). Tumor necrosis factor α inhibitors have shown more rapid onset and greater efficacy during the first 16 weeks of use than MTX, including a head-to-head trial comparing MTX to adalimumab.3 A recent real-world study showed that pediatric patients receiving biologics, primarily TNF-α inhibitors, were more likely to achieve psoriasis area and severity index (PASI) 75 or clear/almost clear status (similar to PASI 90) than MTX and had higher drug survival rates.4
Ustekinumab targets both IL-12 and IL-23, which share the IL-23 receptor p40 subunit. It was the first biologic to target IL-23, which promotes the proliferation and survival of helper T cells (TH17). Ustekinumab has led to greater reductions in PASI scores than TNF-α inhibitors.5,6 Pediatric trials of guselkumab, risankizumab, and tildrakizumab, all targeting the IL-23 receptor–specific p19 subunit, are completed or currently recruiting (NCT03451851, NCT03997786, NCT04435600). Ixekizumab is the first IL-17A–targeting biologic approved for children.7 Secukinumab and the IL-17 receptor inhibitor brodalumab are in pediatric trials (NCT03668613, NCT04305327, NCT03240809). One potential issue with
Skin disease can profoundly affect QOL during childhood and adolescence, a critical time for psychosocial development. In psoriasis, improvement in QOL is proportional to clearance and is greater when PASI 90 is achieved vs PASI 75.8 The high efficacy of IL-23 and IL-17A pathway inhibitors now makes achieving at least PASI 90 the new standard, which can be reached in most patients.
Pediatric AD
For AD in the pediatric population, systemic treatments primarily include corticosteroids, mycophenolate mofetil, azathioprine, cyclosporine, and MTX. Although cyclosporine was the favored systemic agent among pediatric dermatologists in one study,9 claims data analyses show that systemic corticosteroids are used much more often overall, prescribed in 24.4% (116,635 total cases) of children with AD vs nonsteroidal immunosuppressants in less than 0.5%.10 Systemic steroids are impractical given their side effects and risk for disease rebound; however, no immunosuppressants are safe for long-term use, and all require frequent laboratory monitoring. The development of biologics for AD largely involves targeting TH2-driven inflammation.11 Dupilumab is the only FDA-approved biologic for moderate to severe pediatric AD, including in patients as young as 6 years of age. Dupilumab inhibits activation of the IL-4Rα subunit, thereby blocking responses to its ligands, IL-4 and IL-13. Phase 3 trials are now underway in children aged 6 months to 5 years (NCT02612454, NCT03346434). The concomitant ameliorative effects of dupilumab on asthma and other allergic disorders, occurring in approximately 90% of children with moderate to severe AD, is an added benefit.12 Although dupilumab does not appear to modify the disease course in children with AD, the possibility that early introduction could reduce the risk for later developing allergic disease is intriguing.
Adolescent trials have been started for lebrikizumab (NCT04392154) and have been completed for tralokinumab (NCT03160885). Both agents selectively target IL-13 to block TH2 pathway inflammation. The only reported adverse effects of IL-4Rα and IL-13 inhibitors have been injection-site pain/reactions and increased conjunctivitis.13
The only other biologic for AD currently in clinical trials for adolescents is nemolizumab, targeting the receptor for IL-31, a predominantly TH2 cytokine that causes pruritus (NCT03989349). In adults, nemolizumab has shown rapid and potent suppression of itch (but not inflammation) without adding topical corticosteroids.14
Advantages of Biologics and Laboratory Monitoring
By targeting specific cytokines, biologics have greater and more rapid efficacy, fewer side effects, fewer drug interactions, less frequent dosing, and less immunosuppression compared to other systemic agents.3,4,15,16
Recent pediatric-specific guidelines for psoriasis recommend baseline monitoring for tuberculosis for all biologics but yearly tuberculosis testing only for TNF-α inhibitors unless the individual patient is at increased risk.2 No tuberculosis testing is needed for dupilumab, and no other laboratory monitoring is recommended for any biologic in children unless warranted by risk. This difference in recommended monitoring suggests the safety of biologics and is advantageous in managing pediatric therapy.
Unanswered Questions: Vaccines and Antidrug Antibodies
Although administration of killed vaccines is considered safe with all approved biologics, questions remain about the safety of administering live vaccines while on biologics, a particularly pertinent issue in younger children treated with dupilumab and other biologics for AD. Another unanswered question is the potential reduction in clinical response and drug durability with intermittent use of biologics due to the potential development of neutralizing antidrug antibodies (ADAs). The ability to discontinue medication intermittently is desirable, both to determine the natural course of the underlying disease and give a holiday as tolerated. Newer biologics are thought to have lower immunogenicity and less frequent ADA development.17-19 Even with TNF-α inhibitors, the presence of anti-ADAs is not temporally related to response in children with psoriasis.20 Long-term outcomes of the use of biologics in adults have been reassuring, and safety profiles of biologics studied thus far appear to be similar in children.21,22 However, understanding the potential long-term effects from the use of newly approved and emerging biologics in the pediatric population will require decades of study to ensure safety, including nonrandomized studies and postmarketing reports from regulatory agencies.
Cost Considerations
Biologics are disease and QOL altering for children with moderate to severe psoriasis or AD; however, access to biologics often is an obstacle for patients and practitioners. Biologics cost $30,000 to $60,000 annually, while conventional systemic treatments such as MTX, cyclosporine, and acitretin cost $100 to $3000 annually, raising the question of cost effectiveness. In 2016, the Institute for Clinical and Economic Review concluded that biologics for psoriasis had reasonably good value based on improved QOL and concluded in 2017 that dupilumab had a benefit that outweighed its cost.23,24 Prior authorizations and multiple appeals have been necessary to obtain approval, especially in the pediatric population.25 This difficulty highlights the need for programs to cover the cost of biologics for all children, as well as registries to further assess effectiveness and long-term safety, especially compared to traditional systemic agents.
On the Horizon
Clinical trials for other therapies for children and adolescents are ongoing. Details on recommended dosing, approval status, and efficacy in trials are provided in the eTable. Given their high efficacy in adults with psoriasis, IL-23–specific and TH17 pathway biologics likely are similarly efficacious and raise the bar for the expectation of achieving PASI 90 and PASI 100 responses. The long-term safety, durability of responses, and ability to modify disease, particularly when started early in life (eg, preadolescence) and early in the disease course, remains to be determined.

- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
- Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel). 2019;6:133.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82:161-201.
- Papp K, Thaci D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet. 2017;390:40-49.
- Bronckers I, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156:384-392.
- Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73:594-603.
- Philipp S, Menter A, Nikkels AF, et al. Ustekinumab for the treatmentof moderate-to-severe plaque psoriasis in paediatric patients (>/= 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183:664-672.
- Paller AS, Seyger MMB, Alejandro Magarinos G, et al. Efficacy and safety of ixekizumab in a phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183:231-241.
- Bruins FM, Bronckers I, Groenewoud HMM, et al. Association between quality of life and improvement in psoriasis severity and extent in pediatric patients. JAMA Dermatol. 2020;156:72-78.
- Totri CR, Eichenfield LF, Logan K, et al. Prescribing practices for systemic agents in the treatment of severe pediatric atopic dermatitis in the US and Canada: the PeDRA TREAT survey. J Am Acad Dermatol. 2017;76:281-285.
- Paller AS, Siegfried EC, Vekeman F, et al. Treatment patterns of pediatric patients with atopic dermatitis: a claims data analysis. J Am Acad Dermatol. 2020;82:651-660.
- Tsianakas A, Ständer S. Dupilumab: a milestone in the treatment of atopic dermatitis. The Lancet. 2016;10013:4-5.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56.
- Paller AS, Siegfried EC, Thaci D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293.
- Bagci IS, Ruzicka T. IL-31: a new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141:858-866.
- Schwartz G, Paller AS. Targeted therapies for pediatric psoriasis. Semin Cutan Med Surg. 2018;37:167-172.
- Dommasch ED, Kim SC, Lee MP, et al. Risk of serious infection in patients receiving systemic medications for the treatment of psoriasis. JAMA Dermatol. 2019;155:1142-1152.
- Reich K, Blauvelt A, Armstrong A, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits minimal immunogenicity in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2017;176:752-758.
- Bagel J, Lebwohl M, Israel RJ, et al. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. 2020;82:344-351.
- Zhu Y, Marini JC, Song M, et al. Immunogenicity of guselkumab is not clinically relevant in patients with moderate-to-severe plaque psoriasis. J Invest Dermatol. 2019;139:1830.e6-1834.e6.
- Langley RG, Kasichayanula S, Trivedi M, et al. Pharmacokinetics, immunogenicity, and efficacy of etanercept in pediatric patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2018;58:340-346.
- Paller AS, Siegfried EC, Pariser DM, et al. Long-term safety and efficacy of etanercept in children and adolescents with plaque psoriasis. J Am Acad Dermatol. 2016;74:280-287.e1-3.
- Papp K, Gottlieb AB, Naldi L, et al. Safety surveillance for ustekinumab and other psoriasis treatments from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714.
- Targeted immunomodulators for the treatment of moderate-to-severe plaque psoriasis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2017/11/ICER_Psoriasis_Update_Draft_Report_04272018.pdf. Published December 2, 2016. Accessed October 26, 2020.
- Dupilumab and crisaborole for atopic dermatitis: effectiveness and value. Institute for Clinical and Economic Review website. https://icer-review.org/wp-content/uploads/2016/10/MWCEPAC_ATOPIC_EVIDENCE_REPORT_051217.pdf. Published May 12, 2017. Accessed October 26, 2020.
- Siegfried EC, Igelman S, Jaworski JC, et al. Use of dupilumab in pediatric atopic dermatitis: access, dosing, and implications for managing severe atopic dermatitis. Pediatr Dermatol. 2019;36:172-176.
- Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241-251.
- Reich A. Secukinumab is highly efficacious and has a favorable safety profile in pediatric patients with moderate-to-severe plaque psoriasis. Presented at: AAD Virtual Meeting Experience; June 12–14, 2020.
Biologics may protect psoriasis patients against severe COVID-19
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
FROM THE EADV CONGRESS
Mirikizumab beats placebo, secukinumab for psoriasis
The investigational monoclonal antibody according to new long-term OASIS-2 trial data.
Both doses of mirikizumab in the international, double-blind trial achieved improvements in Psoriasis Area and Severity Index (PASI) scores in larger numbers of participants at week 52 than secukinumab (Cosentyx), with low adverse event rates.
If approved, mirikizumab, which binds the p19 subunit of IL-23, would join three other IL-23 drugs already marketed in the United States for moderate to severe psoriasis, said OASIS-2 lead investigator Kim A. Papp, MD, PhD, founder and president of Probity Medical Research in Waterloo, Ont.
But Dr. Papp feels larger studies “will be necessary to put these data into perspective,” he said during a presentation at the virtual annual European Academy of Dermatology and Venereology Congress.
“Probably the most important takeaway here is that we may have another option to choose from,” Dr. Papp said in an interview. “People tend to think we have an adequate stable of treatment options, and I would argue we do not.”
“There are variations over time that occur in terms of an individual’s biological response, and the consequence is that nothing we have works for everyone, and nothing we have works forever,” he added. Psoriasis biologics “are increasingly competent, compared to medications we had even 5 or 10 years ago ... but they still don’t satisfy all our needs, so we do need to keep replenishing our stock.”
The multicenter trial included 1,465 patients who were randomly split into four groups. Subcutaneously, one group received 250 mg of mirikizumab every 4 weeks, and then 250 mg of the drug every 8 weeks starting at week 16. Another group received 250 mg of mirikizumab every 4 weeks and then 125 mg every 8 weeks starting at week 16.
The third group received 300 mg of secukinumab weekly for 4 weeks and then every 4 weeks starting at week 4. The last group received placebo every 4 weeks, and then 250 mg of mirikizumab every 4 weeks from week 16 to 32 and every 8 weeks thereafter.
Primary endpoints measured the percentage of patients achieving a static Physician’s Global Assessment (sPGA) of 0 or 1, with an improvement of at least 2 points from baseline; and the proportion of patients with PASI 90 at week 16, compared with placebo.
Major secondary endpoints were PASI 75 and PASI 100, compared with placebo at week 16; an sPGA of 0 or 1 and PASI 90 noninferiority, compared with secukinumab at week 16; and sPGA of 0 or 1, PASI 90, and PASI 100 superiority, compared with secukinumab at week 52.
More than 91% of participants completed all 52 weeks in the trial. Mirikizumab met primary endpoints compared with placebo and major secondary endpoints vs secukinumab at week 16 (P < .001). PASI 90 and sPGA (0,1) response rates far exceeded placebo for both 250 mg mirikizumab (74.4% and 79.7%, respectively) and secukinumab (72.8% and 76.3%, respectively).
At week 52, major secondary endpoints for both mirikizumab doses were superior to secukinumab (all P < .001). PASI 90 was achieved by 81.4% of 125 mg and 82.4% of 250 mg mirikizumab patients versus 69.4% of secukinumab patients; sPGA (0,1) by 83.1% of 125 mg and 83.3% of 250 mg mirikizumab patients versus 68.5% of secukinumab patients; and PASI 100 by 53.9% of 125 mg and 58.8% of 250 mg mirikizumab patients versus 42.9% of secukinumab patients.
Treatment-associated adverse effects were similar across all treatment groups and study periods. The most common were nasopharyngitis, upper respiratory tract infection, headache, back pain, and arthralgia. But serious adverse effects were minimal, Dr. Papp said. One death occurred in a mirikizumab patient from acute MI, which was deemed unrelated to the study drug.
Myrto Georgia Trakatelli, MD, PhD, from Aristotle University of Thessaloniki (Greece), said the results indicate that dermatologists “should not be afraid to use” mirikizumab long term if it is approved by the Food and Drug Administration.
“Sometimes patients use many treatments for a long time and all of a sudden, they stop working,” Dr. Trakatelli said in an interview. “A new biologic is always welcome because we do see patients not responding to other treatment.”
But Dr. Trakatelli said “a point that troubled me in the study” was that mirikizumab was compared with an IL-17 inhibitor “instead of a molecule targeting IL-23, such as guselkumab [Tremfya], for example.”
“I would have liked to see a head-to-head comparison with a molecule that blocks the same target,” said Dr. Trakatelli, chair of the EADV education committee.
Dr. Papp countered that “there are various reasons for running comparator studies.” Secukinumab, he said, “was the market leader and was widely used, so it makes sense that one is going to compare against a product as the market lead.”
“Not to say there won’t be future studies” in which mirikizumab is compared “head to head with IL-23s,” Dr. Papp added.
But larger patient numbers and longer treatment times are still needed with mirikizumab “to characterize the level of response, duration of response, and any adverse event profiles,” Dr. Papp stressed.
“One study does not a drug make,” he said. “It’s just exciting that we still have things to offer. This is an important example, and of course opportunity, for patients.”
The trial was funded by Lilly. Dr. Papp disclosed financial relationships with AbbVie, Amgen, Astellas, Valeant, Baxalta, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coherus, Dermira, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda, and UCB. Dr. Trakatelli is a speaker for Novartis.
A version of this article originally appeared on Medscape.com.
The investigational monoclonal antibody according to new long-term OASIS-2 trial data.
Both doses of mirikizumab in the international, double-blind trial achieved improvements in Psoriasis Area and Severity Index (PASI) scores in larger numbers of participants at week 52 than secukinumab (Cosentyx), with low adverse event rates.
If approved, mirikizumab, which binds the p19 subunit of IL-23, would join three other IL-23 drugs already marketed in the United States for moderate to severe psoriasis, said OASIS-2 lead investigator Kim A. Papp, MD, PhD, founder and president of Probity Medical Research in Waterloo, Ont.
But Dr. Papp feels larger studies “will be necessary to put these data into perspective,” he said during a presentation at the virtual annual European Academy of Dermatology and Venereology Congress.
“Probably the most important takeaway here is that we may have another option to choose from,” Dr. Papp said in an interview. “People tend to think we have an adequate stable of treatment options, and I would argue we do not.”
“There are variations over time that occur in terms of an individual’s biological response, and the consequence is that nothing we have works for everyone, and nothing we have works forever,” he added. Psoriasis biologics “are increasingly competent, compared to medications we had even 5 or 10 years ago ... but they still don’t satisfy all our needs, so we do need to keep replenishing our stock.”
The multicenter trial included 1,465 patients who were randomly split into four groups. Subcutaneously, one group received 250 mg of mirikizumab every 4 weeks, and then 250 mg of the drug every 8 weeks starting at week 16. Another group received 250 mg of mirikizumab every 4 weeks and then 125 mg every 8 weeks starting at week 16.
The third group received 300 mg of secukinumab weekly for 4 weeks and then every 4 weeks starting at week 4. The last group received placebo every 4 weeks, and then 250 mg of mirikizumab every 4 weeks from week 16 to 32 and every 8 weeks thereafter.
Primary endpoints measured the percentage of patients achieving a static Physician’s Global Assessment (sPGA) of 0 or 1, with an improvement of at least 2 points from baseline; and the proportion of patients with PASI 90 at week 16, compared with placebo.
Major secondary endpoints were PASI 75 and PASI 100, compared with placebo at week 16; an sPGA of 0 or 1 and PASI 90 noninferiority, compared with secukinumab at week 16; and sPGA of 0 or 1, PASI 90, and PASI 100 superiority, compared with secukinumab at week 52.
More than 91% of participants completed all 52 weeks in the trial. Mirikizumab met primary endpoints compared with placebo and major secondary endpoints vs secukinumab at week 16 (P < .001). PASI 90 and sPGA (0,1) response rates far exceeded placebo for both 250 mg mirikizumab (74.4% and 79.7%, respectively) and secukinumab (72.8% and 76.3%, respectively).
At week 52, major secondary endpoints for both mirikizumab doses were superior to secukinumab (all P < .001). PASI 90 was achieved by 81.4% of 125 mg and 82.4% of 250 mg mirikizumab patients versus 69.4% of secukinumab patients; sPGA (0,1) by 83.1% of 125 mg and 83.3% of 250 mg mirikizumab patients versus 68.5% of secukinumab patients; and PASI 100 by 53.9% of 125 mg and 58.8% of 250 mg mirikizumab patients versus 42.9% of secukinumab patients.
Treatment-associated adverse effects were similar across all treatment groups and study periods. The most common were nasopharyngitis, upper respiratory tract infection, headache, back pain, and arthralgia. But serious adverse effects were minimal, Dr. Papp said. One death occurred in a mirikizumab patient from acute MI, which was deemed unrelated to the study drug.
Myrto Georgia Trakatelli, MD, PhD, from Aristotle University of Thessaloniki (Greece), said the results indicate that dermatologists “should not be afraid to use” mirikizumab long term if it is approved by the Food and Drug Administration.
“Sometimes patients use many treatments for a long time and all of a sudden, they stop working,” Dr. Trakatelli said in an interview. “A new biologic is always welcome because we do see patients not responding to other treatment.”
But Dr. Trakatelli said “a point that troubled me in the study” was that mirikizumab was compared with an IL-17 inhibitor “instead of a molecule targeting IL-23, such as guselkumab [Tremfya], for example.”
“I would have liked to see a head-to-head comparison with a molecule that blocks the same target,” said Dr. Trakatelli, chair of the EADV education committee.
Dr. Papp countered that “there are various reasons for running comparator studies.” Secukinumab, he said, “was the market leader and was widely used, so it makes sense that one is going to compare against a product as the market lead.”
“Not to say there won’t be future studies” in which mirikizumab is compared “head to head with IL-23s,” Dr. Papp added.
But larger patient numbers and longer treatment times are still needed with mirikizumab “to characterize the level of response, duration of response, and any adverse event profiles,” Dr. Papp stressed.
“One study does not a drug make,” he said. “It’s just exciting that we still have things to offer. This is an important example, and of course opportunity, for patients.”
The trial was funded by Lilly. Dr. Papp disclosed financial relationships with AbbVie, Amgen, Astellas, Valeant, Baxalta, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coherus, Dermira, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda, and UCB. Dr. Trakatelli is a speaker for Novartis.
A version of this article originally appeared on Medscape.com.
The investigational monoclonal antibody according to new long-term OASIS-2 trial data.
Both doses of mirikizumab in the international, double-blind trial achieved improvements in Psoriasis Area and Severity Index (PASI) scores in larger numbers of participants at week 52 than secukinumab (Cosentyx), with low adverse event rates.
If approved, mirikizumab, which binds the p19 subunit of IL-23, would join three other IL-23 drugs already marketed in the United States for moderate to severe psoriasis, said OASIS-2 lead investigator Kim A. Papp, MD, PhD, founder and president of Probity Medical Research in Waterloo, Ont.
But Dr. Papp feels larger studies “will be necessary to put these data into perspective,” he said during a presentation at the virtual annual European Academy of Dermatology and Venereology Congress.
“Probably the most important takeaway here is that we may have another option to choose from,” Dr. Papp said in an interview. “People tend to think we have an adequate stable of treatment options, and I would argue we do not.”
“There are variations over time that occur in terms of an individual’s biological response, and the consequence is that nothing we have works for everyone, and nothing we have works forever,” he added. Psoriasis biologics “are increasingly competent, compared to medications we had even 5 or 10 years ago ... but they still don’t satisfy all our needs, so we do need to keep replenishing our stock.”
The multicenter trial included 1,465 patients who were randomly split into four groups. Subcutaneously, one group received 250 mg of mirikizumab every 4 weeks, and then 250 mg of the drug every 8 weeks starting at week 16. Another group received 250 mg of mirikizumab every 4 weeks and then 125 mg every 8 weeks starting at week 16.
The third group received 300 mg of secukinumab weekly for 4 weeks and then every 4 weeks starting at week 4. The last group received placebo every 4 weeks, and then 250 mg of mirikizumab every 4 weeks from week 16 to 32 and every 8 weeks thereafter.
Primary endpoints measured the percentage of patients achieving a static Physician’s Global Assessment (sPGA) of 0 or 1, with an improvement of at least 2 points from baseline; and the proportion of patients with PASI 90 at week 16, compared with placebo.
Major secondary endpoints were PASI 75 and PASI 100, compared with placebo at week 16; an sPGA of 0 or 1 and PASI 90 noninferiority, compared with secukinumab at week 16; and sPGA of 0 or 1, PASI 90, and PASI 100 superiority, compared with secukinumab at week 52.
More than 91% of participants completed all 52 weeks in the trial. Mirikizumab met primary endpoints compared with placebo and major secondary endpoints vs secukinumab at week 16 (P < .001). PASI 90 and sPGA (0,1) response rates far exceeded placebo for both 250 mg mirikizumab (74.4% and 79.7%, respectively) and secukinumab (72.8% and 76.3%, respectively).
At week 52, major secondary endpoints for both mirikizumab doses were superior to secukinumab (all P < .001). PASI 90 was achieved by 81.4% of 125 mg and 82.4% of 250 mg mirikizumab patients versus 69.4% of secukinumab patients; sPGA (0,1) by 83.1% of 125 mg and 83.3% of 250 mg mirikizumab patients versus 68.5% of secukinumab patients; and PASI 100 by 53.9% of 125 mg and 58.8% of 250 mg mirikizumab patients versus 42.9% of secukinumab patients.
Treatment-associated adverse effects were similar across all treatment groups and study periods. The most common were nasopharyngitis, upper respiratory tract infection, headache, back pain, and arthralgia. But serious adverse effects were minimal, Dr. Papp said. One death occurred in a mirikizumab patient from acute MI, which was deemed unrelated to the study drug.
Myrto Georgia Trakatelli, MD, PhD, from Aristotle University of Thessaloniki (Greece), said the results indicate that dermatologists “should not be afraid to use” mirikizumab long term if it is approved by the Food and Drug Administration.
“Sometimes patients use many treatments for a long time and all of a sudden, they stop working,” Dr. Trakatelli said in an interview. “A new biologic is always welcome because we do see patients not responding to other treatment.”
But Dr. Trakatelli said “a point that troubled me in the study” was that mirikizumab was compared with an IL-17 inhibitor “instead of a molecule targeting IL-23, such as guselkumab [Tremfya], for example.”
“I would have liked to see a head-to-head comparison with a molecule that blocks the same target,” said Dr. Trakatelli, chair of the EADV education committee.
Dr. Papp countered that “there are various reasons for running comparator studies.” Secukinumab, he said, “was the market leader and was widely used, so it makes sense that one is going to compare against a product as the market lead.”
“Not to say there won’t be future studies” in which mirikizumab is compared “head to head with IL-23s,” Dr. Papp added.
But larger patient numbers and longer treatment times are still needed with mirikizumab “to characterize the level of response, duration of response, and any adverse event profiles,” Dr. Papp stressed.
“One study does not a drug make,” he said. “It’s just exciting that we still have things to offer. This is an important example, and of course opportunity, for patients.”
The trial was funded by Lilly. Dr. Papp disclosed financial relationships with AbbVie, Amgen, Astellas, Valeant, Baxalta, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Coherus, Dermira, Forward Pharma, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Kirin, LEO Pharma, Lilly, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Stiefel, Sun Pharma, Takeda, and UCB. Dr. Trakatelli is a speaker for Novartis.
A version of this article originally appeared on Medscape.com.
FROM THE EADV CONGRESS
Hand eczema: Pan-JAK inhibitor delgocitinib shows dose-dependent response in phase 2b trial
a new international phase 2b research suggests.
An investigational pan–Janus kinase inhibitor that blocks all four members of the JAK family, twice-daily delgocitinib doses of 8 mg/g and 20 mg/g demonstrated the highest efficacy in adults with mild to severe chronic hand eczema. By week 16, nearly 40% of patients receiving either dose were clear or almost clear of symptoms.
“By mode of action, we think delgocitinib is more selective in the way of acting,” said lead investigator Margitta Worm, MD, PhD, of the department of dermatology, venereology, and allergology at Charité University Hospital in Berlin, during a presentation of the results at the virtual annual congress of the European Academy of Dermatology and Venereology.
“We do know that JAKs play an important role in chronic inflammation and interfering with the JAK pathway can have anti-inflammatory effects,” Dr. Worm said in an interview. “Whenever it’s possible to use a molecule topically or locally, it’s advantageous for patients because it’s only acting where you apply it and there are no systemic side effects.”
Defined as lasting more than 3 months or relapsing twice or more within a year, chronic hand eczema is a particularly problematic form of atopic dermatitis because “we need our hands every day for almost every activity, so having eczema on your hands has a huge impact on quality of life,” Dr. Worm said.
Many people whose hands are integral to their occupations also have trouble working because of the disorder, she explained. But current topical treatments are limited to emollients, corticosteroids, and calcineurin inhibitors.
“Topical corticosteroids are efficacious, but can cause skin atrophy,” she said. “Their long-term side-effect profile limits their use.”
The number of patients in each treatment group was too small to focus on different subtypes of chronic hand eczema, “but this is something that will probably be looked at in the future,” Dr. Worm said. “At the moment it’s nice to see a dose-dependent clinical efficacy and good tolerability, and now we have to wait for phase 3 data in the future.”
Dr. Worm and colleagues aimed to establish the dose-response relationship of twice-daily applications of delgocitinib cream in doses of 1, 3, 8, and 20 mg/g and a delgocitinib cream vehicle for 16 weeks. The 258 participants (61% women; average age, 46 years) were randomly assigned in equal groups to each dose of delgocitinib cream or the vehicle cream twice daily at centers in Denmark, Germany, and the United States.
The primary endpoint for the double-blind, 26-center trial was the proportion of patients who achieved an Investigator’s Global Assessment score of 0 (“clear”) or 1 (“almost clear”), with a 2-point or higher improvement from baseline over the study period. A key secondary endpoint was a change in the Hand Eczema Severity Index (HECSI) from baseline to week 16.
At week 16, a statistically significant dose response was established for both primary and secondary endpoints (P < .025). More patients in the delgocitinib 8-mg/g and 20-mg/g groups met the primary endpoint (36.5% and 37.7%, respectively) than patients in the 1-mg/g and 3-mg/g groups (21.2% and 7.8%, respectively) and vehicle group (8%, P = .0004).
This primary skin clearance effect at week 16 was demonstrated from week 4 in the 8-mg/g group and week 6 in the 20-mg/g group. But all active doses achieved a statistically significant greater jump in HECSI from baseline to week 16 than the vehicle cream (P < .05).
“The strength of the trial is that there were different concentrations of the substance used,” Dr. Worm said. “When you look to the results, you can demonstrate a dose-dependent clinical efficacy. This is of great value to really compare the efficacy of single doses.”
Most adverse events reported were not considered treatment related and were mild or moderate. The most frequently reported side effects were nasopharyngitis, eczema, and headache.
Commenting on the results, Asli Bilgic, MD, from Akdeniz University in Antalya, Turkey, who was not involved with the study, said that phase 3 studies of delgocitinib should probe further into the effects of the 8-mg/g dosage in this patient group since it appears to show similar efficacy and safety to 20 mg/g.
It’s important for research to focus on hand eczema “because it’s a very common disease, and treatment options are really sparse,” Dr. Bilgic said in an interview.
“Especially in the COVID era, many health care professionals, along with cleaning, catering, and mechanical jobs” are essential workers affected by the condition, she said. “It affects people’s self-esteem and their ability to do their job.”
The study was funded by LEO Pharma. Dr. Worm received lecture honoraria from LEO Pharma. Dr. Bilgic disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new international phase 2b research suggests.
An investigational pan–Janus kinase inhibitor that blocks all four members of the JAK family, twice-daily delgocitinib doses of 8 mg/g and 20 mg/g demonstrated the highest efficacy in adults with mild to severe chronic hand eczema. By week 16, nearly 40% of patients receiving either dose were clear or almost clear of symptoms.
“By mode of action, we think delgocitinib is more selective in the way of acting,” said lead investigator Margitta Worm, MD, PhD, of the department of dermatology, venereology, and allergology at Charité University Hospital in Berlin, during a presentation of the results at the virtual annual congress of the European Academy of Dermatology and Venereology.
“We do know that JAKs play an important role in chronic inflammation and interfering with the JAK pathway can have anti-inflammatory effects,” Dr. Worm said in an interview. “Whenever it’s possible to use a molecule topically or locally, it’s advantageous for patients because it’s only acting where you apply it and there are no systemic side effects.”
Defined as lasting more than 3 months or relapsing twice or more within a year, chronic hand eczema is a particularly problematic form of atopic dermatitis because “we need our hands every day for almost every activity, so having eczema on your hands has a huge impact on quality of life,” Dr. Worm said.
Many people whose hands are integral to their occupations also have trouble working because of the disorder, she explained. But current topical treatments are limited to emollients, corticosteroids, and calcineurin inhibitors.
“Topical corticosteroids are efficacious, but can cause skin atrophy,” she said. “Their long-term side-effect profile limits their use.”
The number of patients in each treatment group was too small to focus on different subtypes of chronic hand eczema, “but this is something that will probably be looked at in the future,” Dr. Worm said. “At the moment it’s nice to see a dose-dependent clinical efficacy and good tolerability, and now we have to wait for phase 3 data in the future.”
Dr. Worm and colleagues aimed to establish the dose-response relationship of twice-daily applications of delgocitinib cream in doses of 1, 3, 8, and 20 mg/g and a delgocitinib cream vehicle for 16 weeks. The 258 participants (61% women; average age, 46 years) were randomly assigned in equal groups to each dose of delgocitinib cream or the vehicle cream twice daily at centers in Denmark, Germany, and the United States.
The primary endpoint for the double-blind, 26-center trial was the proportion of patients who achieved an Investigator’s Global Assessment score of 0 (“clear”) or 1 (“almost clear”), with a 2-point or higher improvement from baseline over the study period. A key secondary endpoint was a change in the Hand Eczema Severity Index (HECSI) from baseline to week 16.
At week 16, a statistically significant dose response was established for both primary and secondary endpoints (P < .025). More patients in the delgocitinib 8-mg/g and 20-mg/g groups met the primary endpoint (36.5% and 37.7%, respectively) than patients in the 1-mg/g and 3-mg/g groups (21.2% and 7.8%, respectively) and vehicle group (8%, P = .0004).
This primary skin clearance effect at week 16 was demonstrated from week 4 in the 8-mg/g group and week 6 in the 20-mg/g group. But all active doses achieved a statistically significant greater jump in HECSI from baseline to week 16 than the vehicle cream (P < .05).
“The strength of the trial is that there were different concentrations of the substance used,” Dr. Worm said. “When you look to the results, you can demonstrate a dose-dependent clinical efficacy. This is of great value to really compare the efficacy of single doses.”
Most adverse events reported were not considered treatment related and were mild or moderate. The most frequently reported side effects were nasopharyngitis, eczema, and headache.
Commenting on the results, Asli Bilgic, MD, from Akdeniz University in Antalya, Turkey, who was not involved with the study, said that phase 3 studies of delgocitinib should probe further into the effects of the 8-mg/g dosage in this patient group since it appears to show similar efficacy and safety to 20 mg/g.
It’s important for research to focus on hand eczema “because it’s a very common disease, and treatment options are really sparse,” Dr. Bilgic said in an interview.
“Especially in the COVID era, many health care professionals, along with cleaning, catering, and mechanical jobs” are essential workers affected by the condition, she said. “It affects people’s self-esteem and their ability to do their job.”
The study was funded by LEO Pharma. Dr. Worm received lecture honoraria from LEO Pharma. Dr. Bilgic disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
a new international phase 2b research suggests.
An investigational pan–Janus kinase inhibitor that blocks all four members of the JAK family, twice-daily delgocitinib doses of 8 mg/g and 20 mg/g demonstrated the highest efficacy in adults with mild to severe chronic hand eczema. By week 16, nearly 40% of patients receiving either dose were clear or almost clear of symptoms.
“By mode of action, we think delgocitinib is more selective in the way of acting,” said lead investigator Margitta Worm, MD, PhD, of the department of dermatology, venereology, and allergology at Charité University Hospital in Berlin, during a presentation of the results at the virtual annual congress of the European Academy of Dermatology and Venereology.
“We do know that JAKs play an important role in chronic inflammation and interfering with the JAK pathway can have anti-inflammatory effects,” Dr. Worm said in an interview. “Whenever it’s possible to use a molecule topically or locally, it’s advantageous for patients because it’s only acting where you apply it and there are no systemic side effects.”
Defined as lasting more than 3 months or relapsing twice or more within a year, chronic hand eczema is a particularly problematic form of atopic dermatitis because “we need our hands every day for almost every activity, so having eczema on your hands has a huge impact on quality of life,” Dr. Worm said.
Many people whose hands are integral to their occupations also have trouble working because of the disorder, she explained. But current topical treatments are limited to emollients, corticosteroids, and calcineurin inhibitors.
“Topical corticosteroids are efficacious, but can cause skin atrophy,” she said. “Their long-term side-effect profile limits their use.”
The number of patients in each treatment group was too small to focus on different subtypes of chronic hand eczema, “but this is something that will probably be looked at in the future,” Dr. Worm said. “At the moment it’s nice to see a dose-dependent clinical efficacy and good tolerability, and now we have to wait for phase 3 data in the future.”
Dr. Worm and colleagues aimed to establish the dose-response relationship of twice-daily applications of delgocitinib cream in doses of 1, 3, 8, and 20 mg/g and a delgocitinib cream vehicle for 16 weeks. The 258 participants (61% women; average age, 46 years) were randomly assigned in equal groups to each dose of delgocitinib cream or the vehicle cream twice daily at centers in Denmark, Germany, and the United States.
The primary endpoint for the double-blind, 26-center trial was the proportion of patients who achieved an Investigator’s Global Assessment score of 0 (“clear”) or 1 (“almost clear”), with a 2-point or higher improvement from baseline over the study period. A key secondary endpoint was a change in the Hand Eczema Severity Index (HECSI) from baseline to week 16.
At week 16, a statistically significant dose response was established for both primary and secondary endpoints (P < .025). More patients in the delgocitinib 8-mg/g and 20-mg/g groups met the primary endpoint (36.5% and 37.7%, respectively) than patients in the 1-mg/g and 3-mg/g groups (21.2% and 7.8%, respectively) and vehicle group (8%, P = .0004).
This primary skin clearance effect at week 16 was demonstrated from week 4 in the 8-mg/g group and week 6 in the 20-mg/g group. But all active doses achieved a statistically significant greater jump in HECSI from baseline to week 16 than the vehicle cream (P < .05).
“The strength of the trial is that there were different concentrations of the substance used,” Dr. Worm said. “When you look to the results, you can demonstrate a dose-dependent clinical efficacy. This is of great value to really compare the efficacy of single doses.”
Most adverse events reported were not considered treatment related and were mild or moderate. The most frequently reported side effects were nasopharyngitis, eczema, and headache.
Commenting on the results, Asli Bilgic, MD, from Akdeniz University in Antalya, Turkey, who was not involved with the study, said that phase 3 studies of delgocitinib should probe further into the effects of the 8-mg/g dosage in this patient group since it appears to show similar efficacy and safety to 20 mg/g.
It’s important for research to focus on hand eczema “because it’s a very common disease, and treatment options are really sparse,” Dr. Bilgic said in an interview.
“Especially in the COVID era, many health care professionals, along with cleaning, catering, and mechanical jobs” are essential workers affected by the condition, she said. “It affects people’s self-esteem and their ability to do their job.”
The study was funded by LEO Pharma. Dr. Worm received lecture honoraria from LEO Pharma. Dr. Bilgic disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE EADV CONGRESS
Data on potential risks of COVID-19 in psoriasis patients limited, but reassuring
The available according to a summary of published studies and expert opinions summarized at the annual Coastal Dermatology Symposium, held virtually.
For patients with psoriasis concerned about their outcome if infected with COVID-19, “there is no evidence to support stopping biologics or systemic agents, so I am asking my patients to continue,” Kristina C. Duffin, MD, professor and chair of dermatology at the University of Utah, Salt Lake City, said at the meeting.
The National Psoriasis Foundation, which created a COVID-19 task force and maintains a COVID-19 Resource Center on its website, has provided similar advice. Many statements are phrased cautiously and clinicians are encouraged to practice shared decision-making, but the NPF guidance supports continuing effective therapy – or, in newly diagnosed patients, starting effective therapy – among those who are not infected with SARS-CoV2.
Patients with a new diagnosis of psoriasis “should be aware that untreated psoriatic disease is associated with serious impact on physical and emotional health, and in the case of psoriatic arthritis, can lead to permanent joint damage and disability,” according to the NPF guidance.
Overall, the “existing data generally suggest” that most treatments for psoriasis and psoriatic arthritis “do not meaningfully alter the risks of contracting SARS-CoV2 or having a worse course of COVID-19 illness,” the current guidance states. Yet, because of limited data this “is not known with certainty.”
Chronic systemic steroids are an exception. In a review of recently published studies evaluating whether psoriasis or its therapies increase risk of adverse outcomes in patients with COVID-19 infection, Dr. Duffin pointed to several that associated systemic steroids with hospitalization or other markers of severe disease.
The NPF guidance also recommends avoiding chronic systemic steroids in patients with psoriasis during the current COVID-19 era “if possible.” In patients with psoriatic arthritis who require systemic steroids, the guidance recommends “the lowest dose necessary to achieve the desired therapeutic effect.”
This is not necessarily true in patients with psoriasis and COVID-19 infection. Based on the potential for systemic steroids to improve outcomes in hospitalized COVID-19 patients requiring oxygen, steroids “should not be withheld” even when the justification is concern about the potential risk of flares with withdrawal, according to the NPF guidance statement.
The NPF guidance specifically cautions against use of hydroxychloroquine or chloroquine for prevention or treatment of COVID-19. In addition to an uncertain benefit, these antimalarial drugs have been associated previously with flares of psoriasis.
Dr. Duffin agreed and went on to warn that COVID-19 infection itself is a potential trigger for flares. She cited two published case reports of flares associated with psoriasis. Although one patient had also been exposed to hydroxychloroquine, she said the risk of psoriasis-induced flare “makes sense” based on previous associations made between flares and other viral infections and stress.
In patients with psoriasis who contract COVID-19 infection, Dr. Duffin concurred with the NPF guidance that management decisions should be made on a “case-by-case basis.” Although the NPF guidance states that “most patients can restart psoriasis and/or psoriatic arthritis treatments after complete resolution of COVID-19 symptoms,” no specific advice was offered on the decision to stop treatments.
For protecting psoriasis patients from infection and managing COVID-19 in those who become infected, much of the NPF advice is consistent with that offered to patients without psoriasis. This involves practicing infection control that reduces risk of transmission. Both the NPF guidance and Dr. Duffin suggested telemedicine is appropriate for limiting in-patient visits under pandemic conditions.
Although patients with psoriasis are more likely than the general population to have the comorbidities associated with bad COVID-19 infection outcomes, according to the NPF guidance, Dr. Duffin called the overall data evaluating susceptibility among psoriasis patients “reassuring.” She cautioned that the data are still limited, but the evidence so far suggests that neither psoriasis nor biologics are independent risk factors for acquiring COVID-19 or having a worse outcome if infected.
Yet, more definitive data are needed, and Dr. Duffin advised clinicians and patients to consult the NPF website for updates. “More up-to-date information will certainly be added as we go forward,” she said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
This NPF task force on COVID-19 is meeting every 2 weeks, according to Joel M. Gelfand, MD, professor of dermatology, University of Pennsylvania, Philadelphia, and cochair of the task force. Dr. Gelfand reported that updates are based on a discussion of the available data.
“We will be releasing additional recommendations as necessary based on the developments,” he said in an interview. Updates are not necessarily required at this frequency but can be if appropriate. The goal is to keep recommendations current and evidence-based.
Dr. Duffin reported financial relationships with Amgen, AbbVie, Bristol-Myers Squibb, Boehringer-Ingelheim, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Siena, and UCB. Dr. Gelfand reported financial relationships with AbbVie, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Pfizer, Roche, and UCB.
This publication and Global Academy for Medical Education are owned by the same parent company.
The available according to a summary of published studies and expert opinions summarized at the annual Coastal Dermatology Symposium, held virtually.
For patients with psoriasis concerned about their outcome if infected with COVID-19, “there is no evidence to support stopping biologics or systemic agents, so I am asking my patients to continue,” Kristina C. Duffin, MD, professor and chair of dermatology at the University of Utah, Salt Lake City, said at the meeting.
The National Psoriasis Foundation, which created a COVID-19 task force and maintains a COVID-19 Resource Center on its website, has provided similar advice. Many statements are phrased cautiously and clinicians are encouraged to practice shared decision-making, but the NPF guidance supports continuing effective therapy – or, in newly diagnosed patients, starting effective therapy – among those who are not infected with SARS-CoV2.
Patients with a new diagnosis of psoriasis “should be aware that untreated psoriatic disease is associated with serious impact on physical and emotional health, and in the case of psoriatic arthritis, can lead to permanent joint damage and disability,” according to the NPF guidance.
Overall, the “existing data generally suggest” that most treatments for psoriasis and psoriatic arthritis “do not meaningfully alter the risks of contracting SARS-CoV2 or having a worse course of COVID-19 illness,” the current guidance states. Yet, because of limited data this “is not known with certainty.”
Chronic systemic steroids are an exception. In a review of recently published studies evaluating whether psoriasis or its therapies increase risk of adverse outcomes in patients with COVID-19 infection, Dr. Duffin pointed to several that associated systemic steroids with hospitalization or other markers of severe disease.
The NPF guidance also recommends avoiding chronic systemic steroids in patients with psoriasis during the current COVID-19 era “if possible.” In patients with psoriatic arthritis who require systemic steroids, the guidance recommends “the lowest dose necessary to achieve the desired therapeutic effect.”
This is not necessarily true in patients with psoriasis and COVID-19 infection. Based on the potential for systemic steroids to improve outcomes in hospitalized COVID-19 patients requiring oxygen, steroids “should not be withheld” even when the justification is concern about the potential risk of flares with withdrawal, according to the NPF guidance statement.
The NPF guidance specifically cautions against use of hydroxychloroquine or chloroquine for prevention or treatment of COVID-19. In addition to an uncertain benefit, these antimalarial drugs have been associated previously with flares of psoriasis.
Dr. Duffin agreed and went on to warn that COVID-19 infection itself is a potential trigger for flares. She cited two published case reports of flares associated with psoriasis. Although one patient had also been exposed to hydroxychloroquine, she said the risk of psoriasis-induced flare “makes sense” based on previous associations made between flares and other viral infections and stress.
In patients with psoriasis who contract COVID-19 infection, Dr. Duffin concurred with the NPF guidance that management decisions should be made on a “case-by-case basis.” Although the NPF guidance states that “most patients can restart psoriasis and/or psoriatic arthritis treatments after complete resolution of COVID-19 symptoms,” no specific advice was offered on the decision to stop treatments.
For protecting psoriasis patients from infection and managing COVID-19 in those who become infected, much of the NPF advice is consistent with that offered to patients without psoriasis. This involves practicing infection control that reduces risk of transmission. Both the NPF guidance and Dr. Duffin suggested telemedicine is appropriate for limiting in-patient visits under pandemic conditions.
Although patients with psoriasis are more likely than the general population to have the comorbidities associated with bad COVID-19 infection outcomes, according to the NPF guidance, Dr. Duffin called the overall data evaluating susceptibility among psoriasis patients “reassuring.” She cautioned that the data are still limited, but the evidence so far suggests that neither psoriasis nor biologics are independent risk factors for acquiring COVID-19 or having a worse outcome if infected.
Yet, more definitive data are needed, and Dr. Duffin advised clinicians and patients to consult the NPF website for updates. “More up-to-date information will certainly be added as we go forward,” she said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
This NPF task force on COVID-19 is meeting every 2 weeks, according to Joel M. Gelfand, MD, professor of dermatology, University of Pennsylvania, Philadelphia, and cochair of the task force. Dr. Gelfand reported that updates are based on a discussion of the available data.
“We will be releasing additional recommendations as necessary based on the developments,” he said in an interview. Updates are not necessarily required at this frequency but can be if appropriate. The goal is to keep recommendations current and evidence-based.
Dr. Duffin reported financial relationships with Amgen, AbbVie, Bristol-Myers Squibb, Boehringer-Ingelheim, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Siena, and UCB. Dr. Gelfand reported financial relationships with AbbVie, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Pfizer, Roche, and UCB.
This publication and Global Academy for Medical Education are owned by the same parent company.
The available according to a summary of published studies and expert opinions summarized at the annual Coastal Dermatology Symposium, held virtually.
For patients with psoriasis concerned about their outcome if infected with COVID-19, “there is no evidence to support stopping biologics or systemic agents, so I am asking my patients to continue,” Kristina C. Duffin, MD, professor and chair of dermatology at the University of Utah, Salt Lake City, said at the meeting.
The National Psoriasis Foundation, which created a COVID-19 task force and maintains a COVID-19 Resource Center on its website, has provided similar advice. Many statements are phrased cautiously and clinicians are encouraged to practice shared decision-making, but the NPF guidance supports continuing effective therapy – or, in newly diagnosed patients, starting effective therapy – among those who are not infected with SARS-CoV2.
Patients with a new diagnosis of psoriasis “should be aware that untreated psoriatic disease is associated with serious impact on physical and emotional health, and in the case of psoriatic arthritis, can lead to permanent joint damage and disability,” according to the NPF guidance.
Overall, the “existing data generally suggest” that most treatments for psoriasis and psoriatic arthritis “do not meaningfully alter the risks of contracting SARS-CoV2 or having a worse course of COVID-19 illness,” the current guidance states. Yet, because of limited data this “is not known with certainty.”
Chronic systemic steroids are an exception. In a review of recently published studies evaluating whether psoriasis or its therapies increase risk of adverse outcomes in patients with COVID-19 infection, Dr. Duffin pointed to several that associated systemic steroids with hospitalization or other markers of severe disease.
The NPF guidance also recommends avoiding chronic systemic steroids in patients with psoriasis during the current COVID-19 era “if possible.” In patients with psoriatic arthritis who require systemic steroids, the guidance recommends “the lowest dose necessary to achieve the desired therapeutic effect.”
This is not necessarily true in patients with psoriasis and COVID-19 infection. Based on the potential for systemic steroids to improve outcomes in hospitalized COVID-19 patients requiring oxygen, steroids “should not be withheld” even when the justification is concern about the potential risk of flares with withdrawal, according to the NPF guidance statement.
The NPF guidance specifically cautions against use of hydroxychloroquine or chloroquine for prevention or treatment of COVID-19. In addition to an uncertain benefit, these antimalarial drugs have been associated previously with flares of psoriasis.
Dr. Duffin agreed and went on to warn that COVID-19 infection itself is a potential trigger for flares. She cited two published case reports of flares associated with psoriasis. Although one patient had also been exposed to hydroxychloroquine, she said the risk of psoriasis-induced flare “makes sense” based on previous associations made between flares and other viral infections and stress.
In patients with psoriasis who contract COVID-19 infection, Dr. Duffin concurred with the NPF guidance that management decisions should be made on a “case-by-case basis.” Although the NPF guidance states that “most patients can restart psoriasis and/or psoriatic arthritis treatments after complete resolution of COVID-19 symptoms,” no specific advice was offered on the decision to stop treatments.
For protecting psoriasis patients from infection and managing COVID-19 in those who become infected, much of the NPF advice is consistent with that offered to patients without psoriasis. This involves practicing infection control that reduces risk of transmission. Both the NPF guidance and Dr. Duffin suggested telemedicine is appropriate for limiting in-patient visits under pandemic conditions.
Although patients with psoriasis are more likely than the general population to have the comorbidities associated with bad COVID-19 infection outcomes, according to the NPF guidance, Dr. Duffin called the overall data evaluating susceptibility among psoriasis patients “reassuring.” She cautioned that the data are still limited, but the evidence so far suggests that neither psoriasis nor biologics are independent risk factors for acquiring COVID-19 or having a worse outcome if infected.
Yet, more definitive data are needed, and Dr. Duffin advised clinicians and patients to consult the NPF website for updates. “More up-to-date information will certainly be added as we go forward,” she said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
This NPF task force on COVID-19 is meeting every 2 weeks, according to Joel M. Gelfand, MD, professor of dermatology, University of Pennsylvania, Philadelphia, and cochair of the task force. Dr. Gelfand reported that updates are based on a discussion of the available data.
“We will be releasing additional recommendations as necessary based on the developments,” he said in an interview. Updates are not necessarily required at this frequency but can be if appropriate. The goal is to keep recommendations current and evidence-based.
Dr. Duffin reported financial relationships with Amgen, AbbVie, Bristol-Myers Squibb, Boehringer-Ingelheim, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Siena, and UCB. Dr. Gelfand reported financial relationships with AbbVie, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Pfizer, Roche, and UCB.
This publication and Global Academy for Medical Education are owned by the same parent company.
FROM COASTAL DERM
Non-Whites remain sorely underrepresented in phase 3 psoriasis trials
Non-White patient participation in phase 3 therapeutic trials for plaque psoriasis is less than 15%, according to a recently published analysis of data from the ClinicalTrials.gov database.
The exact figure drawn from the survey of 82 trials was 14.2%, but 20 (24%) of the trials did not include ethnoracial data at all, and only 65% of those with data had complete data, according to a report in the British Journal of Dermatology by a team of investigators from the department of dermatology at the University of California, San Francisco.
“The remaining studies reported the percentage of white participants only or white participants and one additional ethnoracial group,” reported the investigators, led by Vidhatha D. Reddy, a medical student at UCSF.
The investigators broke down participation by race in all phase 3 plaque psoriasis trials that enrolled adults and had posted results by May 2020. Data from trials of medications yet to be approved were excluded.
Most trials were multinational. The medications evaluated included 11 biologics, 10 topicals, 2 oral systemic agents, and a phosphodiesterase type-4 inhibitor. The 82 trials included in this analysis enrolled 48,846 collectively.
From trials that identified race, 85.8% of 39,161 participants were White, 3.09% of 25,565 patients were Black, 19.55% of 11,364 patients were Hispanic or Latino, and 9.21% of 30,009 patients were Asian. Of trials that included Native Americans or Pacific Islanders, fewer than 2% of participants represented this category.
Non-White patients remain underrepresented even when recognizing differences in the prevalence of psoriasis. For example, one recent survey found the U.S, prevalence of psoriasis to be about half as great in Blacks as it is in Whites (1.9% vs. 3.9%), but the representation of Blacks in the phase 3 trials evaluated by Mr. Reddy and colleagues was more than 20 times lower.
There are many reasons to suspect that lack of diversification in psoriasis trials is impeding optimal care in those underrepresented. Of several examples offered by the authors, one involved differential responses to adalimumab among patients with hidradenitis suppurativa with genetic variants in the BCL2 gene, but the authors reported racially associated genetic differences are not uncommon.
“Estimates have shown that approximately one-fifth of newly developed medications demonstrate interracial/ethnic variability in regard to various factors, such as pharmacokinetics, safety and efficacy profiles, dosing, and pharmacogenetics,” Mr. Reddy and his coinvestigators stated.
Although racial diversity in the design and recruitment for clinical trials has not been a priority in trials involving psoriasis, other skin diseases, or most diseases in general, the authors cited some evidence that this is changing.
“Since 2017, research funded by the National Institutes of Health has been required to report race and ethnicity of participants following an amendment to the Health Revitalization Act,” according to the authors, who suggested that other such initiatives are needed. They advocated “explicit goals to increase recruitment of people of color” as a standard step in clinical trial conduct.
Hypertension trials were cited as an example in which diversity has made a difference.
“Although Black patients are at an elevated risk of developing hypertension, it was not until the enrollment of a substantial proportion of black participants in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) that enough data on Black patients were available to make specific treatment recommendations in this population,” they noted.
Impossible to know treatment benefits without ethnoracial data
Another investigator who has considered this issue, Junko Takeshita, MD, PhD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, agreed.
“Lack of diversity among participants in phase 3 clinical trials for psoriasis is a problem,” said Dr. Takeshita, who led a study of racial differences in perceptions of psoriasis therapies that was published last year.
In that study, “my research group not only found differences in perceptions about biologics between Black and White patients with psoriasis, but we have also shown that Black patients with psoriasis are less likely to receive biologic treatment,” she reported. There are many explanations. For example, she found in another study that Black patients are underrepresented in direct-to-consumer advertisements for biologics.
This problem is not unique to psoriasis. Underrepresentation of Blacks and other ethnoracial groups is true of other skin diseases and many diseases in general, according to Dr. Takeshita. However, she cautioned that the 3% figure for Black participation in psoriasis trials reported by Mr. Reddy and colleagues is not necessarily reflective of trials in the United States.
“This study included international study sites that are recruiting patients from populations with different demographics than the U.S.,” she noted. By including sites with only Asian patients or countries with few Blacks in the population, it dilutes Black representation. She would expect the exact proportion of Black participants to be somewhat higher even if they are “still likely to be underrepresented” if the analysis has been limited to U.S. data.
The research had no funding source. Three of the nine authors reported financial relationships with pharmaceutical companies.
SOURCE: Reddy VD et al. Br J Dermatol. 2020 Sep 17. doi: 10.1111/bjd.19468.
Non-White patient participation in phase 3 therapeutic trials for plaque psoriasis is less than 15%, according to a recently published analysis of data from the ClinicalTrials.gov database.
The exact figure drawn from the survey of 82 trials was 14.2%, but 20 (24%) of the trials did not include ethnoracial data at all, and only 65% of those with data had complete data, according to a report in the British Journal of Dermatology by a team of investigators from the department of dermatology at the University of California, San Francisco.
“The remaining studies reported the percentage of white participants only or white participants and one additional ethnoracial group,” reported the investigators, led by Vidhatha D. Reddy, a medical student at UCSF.
The investigators broke down participation by race in all phase 3 plaque psoriasis trials that enrolled adults and had posted results by May 2020. Data from trials of medications yet to be approved were excluded.
Most trials were multinational. The medications evaluated included 11 biologics, 10 topicals, 2 oral systemic agents, and a phosphodiesterase type-4 inhibitor. The 82 trials included in this analysis enrolled 48,846 collectively.
From trials that identified race, 85.8% of 39,161 participants were White, 3.09% of 25,565 patients were Black, 19.55% of 11,364 patients were Hispanic or Latino, and 9.21% of 30,009 patients were Asian. Of trials that included Native Americans or Pacific Islanders, fewer than 2% of participants represented this category.
Non-White patients remain underrepresented even when recognizing differences in the prevalence of psoriasis. For example, one recent survey found the U.S, prevalence of psoriasis to be about half as great in Blacks as it is in Whites (1.9% vs. 3.9%), but the representation of Blacks in the phase 3 trials evaluated by Mr. Reddy and colleagues was more than 20 times lower.
There are many reasons to suspect that lack of diversification in psoriasis trials is impeding optimal care in those underrepresented. Of several examples offered by the authors, one involved differential responses to adalimumab among patients with hidradenitis suppurativa with genetic variants in the BCL2 gene, but the authors reported racially associated genetic differences are not uncommon.
“Estimates have shown that approximately one-fifth of newly developed medications demonstrate interracial/ethnic variability in regard to various factors, such as pharmacokinetics, safety and efficacy profiles, dosing, and pharmacogenetics,” Mr. Reddy and his coinvestigators stated.
Although racial diversity in the design and recruitment for clinical trials has not been a priority in trials involving psoriasis, other skin diseases, or most diseases in general, the authors cited some evidence that this is changing.
“Since 2017, research funded by the National Institutes of Health has been required to report race and ethnicity of participants following an amendment to the Health Revitalization Act,” according to the authors, who suggested that other such initiatives are needed. They advocated “explicit goals to increase recruitment of people of color” as a standard step in clinical trial conduct.
Hypertension trials were cited as an example in which diversity has made a difference.
“Although Black patients are at an elevated risk of developing hypertension, it was not until the enrollment of a substantial proportion of black participants in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) that enough data on Black patients were available to make specific treatment recommendations in this population,” they noted.
Impossible to know treatment benefits without ethnoracial data
Another investigator who has considered this issue, Junko Takeshita, MD, PhD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, agreed.
“Lack of diversity among participants in phase 3 clinical trials for psoriasis is a problem,” said Dr. Takeshita, who led a study of racial differences in perceptions of psoriasis therapies that was published last year.
In that study, “my research group not only found differences in perceptions about biologics between Black and White patients with psoriasis, but we have also shown that Black patients with psoriasis are less likely to receive biologic treatment,” she reported. There are many explanations. For example, she found in another study that Black patients are underrepresented in direct-to-consumer advertisements for biologics.
This problem is not unique to psoriasis. Underrepresentation of Blacks and other ethnoracial groups is true of other skin diseases and many diseases in general, according to Dr. Takeshita. However, she cautioned that the 3% figure for Black participation in psoriasis trials reported by Mr. Reddy and colleagues is not necessarily reflective of trials in the United States.
“This study included international study sites that are recruiting patients from populations with different demographics than the U.S.,” she noted. By including sites with only Asian patients or countries with few Blacks in the population, it dilutes Black representation. She would expect the exact proportion of Black participants to be somewhat higher even if they are “still likely to be underrepresented” if the analysis has been limited to U.S. data.
The research had no funding source. Three of the nine authors reported financial relationships with pharmaceutical companies.
SOURCE: Reddy VD et al. Br J Dermatol. 2020 Sep 17. doi: 10.1111/bjd.19468.
Non-White patient participation in phase 3 therapeutic trials for plaque psoriasis is less than 15%, according to a recently published analysis of data from the ClinicalTrials.gov database.
The exact figure drawn from the survey of 82 trials was 14.2%, but 20 (24%) of the trials did not include ethnoracial data at all, and only 65% of those with data had complete data, according to a report in the British Journal of Dermatology by a team of investigators from the department of dermatology at the University of California, San Francisco.
“The remaining studies reported the percentage of white participants only or white participants and one additional ethnoracial group,” reported the investigators, led by Vidhatha D. Reddy, a medical student at UCSF.
The investigators broke down participation by race in all phase 3 plaque psoriasis trials that enrolled adults and had posted results by May 2020. Data from trials of medications yet to be approved were excluded.
Most trials were multinational. The medications evaluated included 11 biologics, 10 topicals, 2 oral systemic agents, and a phosphodiesterase type-4 inhibitor. The 82 trials included in this analysis enrolled 48,846 collectively.
From trials that identified race, 85.8% of 39,161 participants were White, 3.09% of 25,565 patients were Black, 19.55% of 11,364 patients were Hispanic or Latino, and 9.21% of 30,009 patients were Asian. Of trials that included Native Americans or Pacific Islanders, fewer than 2% of participants represented this category.
Non-White patients remain underrepresented even when recognizing differences in the prevalence of psoriasis. For example, one recent survey found the U.S, prevalence of psoriasis to be about half as great in Blacks as it is in Whites (1.9% vs. 3.9%), but the representation of Blacks in the phase 3 trials evaluated by Mr. Reddy and colleagues was more than 20 times lower.
There are many reasons to suspect that lack of diversification in psoriasis trials is impeding optimal care in those underrepresented. Of several examples offered by the authors, one involved differential responses to adalimumab among patients with hidradenitis suppurativa with genetic variants in the BCL2 gene, but the authors reported racially associated genetic differences are not uncommon.
“Estimates have shown that approximately one-fifth of newly developed medications demonstrate interracial/ethnic variability in regard to various factors, such as pharmacokinetics, safety and efficacy profiles, dosing, and pharmacogenetics,” Mr. Reddy and his coinvestigators stated.
Although racial diversity in the design and recruitment for clinical trials has not been a priority in trials involving psoriasis, other skin diseases, or most diseases in general, the authors cited some evidence that this is changing.
“Since 2017, research funded by the National Institutes of Health has been required to report race and ethnicity of participants following an amendment to the Health Revitalization Act,” according to the authors, who suggested that other such initiatives are needed. They advocated “explicit goals to increase recruitment of people of color” as a standard step in clinical trial conduct.
Hypertension trials were cited as an example in which diversity has made a difference.
“Although Black patients are at an elevated risk of developing hypertension, it was not until the enrollment of a substantial proportion of black participants in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) that enough data on Black patients were available to make specific treatment recommendations in this population,” they noted.
Impossible to know treatment benefits without ethnoracial data
Another investigator who has considered this issue, Junko Takeshita, MD, PhD, an assistant professor of dermatology at the University of Pennsylvania, Philadelphia, agreed.
“Lack of diversity among participants in phase 3 clinical trials for psoriasis is a problem,” said Dr. Takeshita, who led a study of racial differences in perceptions of psoriasis therapies that was published last year.
In that study, “my research group not only found differences in perceptions about biologics between Black and White patients with psoriasis, but we have also shown that Black patients with psoriasis are less likely to receive biologic treatment,” she reported. There are many explanations. For example, she found in another study that Black patients are underrepresented in direct-to-consumer advertisements for biologics.
This problem is not unique to psoriasis. Underrepresentation of Blacks and other ethnoracial groups is true of other skin diseases and many diseases in general, according to Dr. Takeshita. However, she cautioned that the 3% figure for Black participation in psoriasis trials reported by Mr. Reddy and colleagues is not necessarily reflective of trials in the United States.
“This study included international study sites that are recruiting patients from populations with different demographics than the U.S.,” she noted. By including sites with only Asian patients or countries with few Blacks in the population, it dilutes Black representation. She would expect the exact proportion of Black participants to be somewhat higher even if they are “still likely to be underrepresented” if the analysis has been limited to U.S. data.
The research had no funding source. Three of the nine authors reported financial relationships with pharmaceutical companies.
SOURCE: Reddy VD et al. Br J Dermatol. 2020 Sep 17. doi: 10.1111/bjd.19468.
FROM THE BRITISH JOURNAL OF DERMATOLOGY