User login
Influenza Vaccination Recommendations During Use of Select Immunosuppressants for Psoriasis
A 42-year-old woman with psoriasis presents for a checkup at the dermatology clinic. Her psoriasis has been fairly stable on methotrexate with no recent flares. She presents her concern of the coronavirus pandemic continuing into the flu season and mentions she would like to minimize her chances of having a respiratory illness. The influenza vaccine has just become available, and she inquires when she can get the vaccine and whether it will interfere with her treatment. What are your recommendations for the patient?
Psoriasis is an immune-mediated, inflammatory skin condition stemming from hyperproliferation of keratinocytes that classically involves erythematous skin plaques with overlying scale. Treatment options vary widely and include topical modalities, phototherapy, immunosuppressants, and biologic agents. Selection of treatment largely depends on the severity and extent of body surface area involvement; systemic therapy generally is indicated when the affected body surface area is greater than 5% to 10%. In patients on systemic therapy, increased susceptibility to infection is a priority concern for prescribing physicians. In the context of continuing immunosuppressive medications, vaccines that reduce susceptibility to infectious diseases can play an important role in reducing morbidity and mortality for these patients; however, an important consideration is that in patients with chronic conditions and frequent hospital visits, vaccines may be administered by various clinicians who may not be familiar with the management of immunosuppressive treatments. It is pivotal for prescribing dermatologists to provide appropriate vaccination instructions for the patient and any future clinicians to ensure vaccine efficacy in these patients.
The intramuscular influenza vaccine is a killed vaccine that is administered annually and has been shown to be safe for use in both immunocompetent and immunocompromised patients.1,2 Despite its safety, questions remain regarding the efficacy of vaccines while a patient is unable to mount a normal immune response and whether the treatment must be altered to maximize immunogenicity. The common systemic treatment options for psoriasis and any recommendations that can be made regarding administration of the influenza vaccine in that context are outlined in the Table. Given the sparsity of clinical data measuring vaccine immunogenicity in patients with psoriasis, vaccine guidelines are drawn from patients with various conditions who are receiving the same dose of medication as indicated for psoriasis.

Immunosuppressants and biologics commonly are used in dermatology for the management of many conditions, including psoriasis. As flu season approaches in the setting of a global pandemic, it is critical to understand the effects of commonly used psoriasis medications on the influenza vaccine. Through a brief review of the latest data concerning their interactions, dermatologists will be able to provide appropriate recommendations that maximize a patient’s immune response to the vaccine while minimizing adverse effects from holding medication.
- Zbinden D, Manuel O. Influenza vaccination in immunocompromised patients: efficacy and safety. Immunotherapy. 2014;6:131-139.
- Milanovic M, Stojanovich L, Djokovic A, et al. Influenza vaccination in autoimmune rheumatic disease patients. Tohoku J Exp Med. 2013;229:29-34.
- Dengler TJ, Strnad N, Bühring I, et al. Differential immune response to influenza and pneumococcal vaccination in immunosuppressed patients after heart transplantation. Transplantation. 1998;66:1340-1347.
- Willcocks LC, Chaudhry AN, Smith JC, et al. The effect of sirolimus therapy on vaccine responses in transplant recipients. Am J Transplant. 2007;7:2006-2011.
- Chioato A, Noseda E, Stevens M, et al. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: results of an open-label, parallel-group, randomized single-center study. Clin Vaccine Immunol. 2012;19:1597-1602.
- Richi P, Martín MD, de Ory F, et al. Secukinumab does not impair the immunogenic response to the influenza vaccine in patients. RMD Open. 2019;5:e001018.
- Furer V, Zisman D, Kaufman I, et al. Immunogenicity and safety of vaccination against seasonal influenza vaccine in patients with psoriatic arthritis treated with secukinumab. Vaccine. 2020;38:847-851.
- Hua C, Barnetche T, Combe B, et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. 2014;66:1016-1026.
- Park JK, Choi Y, Winthrop KL, et al. Optimal time between the last methotrexate administration and seasonal influenza vaccination in rheumatoid arthritis: post hoc analysis of a randomised clinical trial. Ann Rheum Dis. 2019;78:1283-1284.
- Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76:1559-1565.
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898-904.
- Shirai S, Hara M, Sakata Y, et al. Immunogenicity of quadrivalent influenza vaccine for patients with inflammatory bowel disease undergoing immunosuppressive therapy. Inflamm Bowel Dis. 2018;24:1082-1091.
- Fomin I. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF blockers. Ann Rheum Dis. 2006;65:191-194.
- Bosaeed M, Kumar D. Seasonal influenza vaccine in immunocompromised persons. Hum Vaccin Immunother. 2018;14:1311-1322.
- Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34:272-279.
A 42-year-old woman with psoriasis presents for a checkup at the dermatology clinic. Her psoriasis has been fairly stable on methotrexate with no recent flares. She presents her concern of the coronavirus pandemic continuing into the flu season and mentions she would like to minimize her chances of having a respiratory illness. The influenza vaccine has just become available, and she inquires when she can get the vaccine and whether it will interfere with her treatment. What are your recommendations for the patient?
Psoriasis is an immune-mediated, inflammatory skin condition stemming from hyperproliferation of keratinocytes that classically involves erythematous skin plaques with overlying scale. Treatment options vary widely and include topical modalities, phototherapy, immunosuppressants, and biologic agents. Selection of treatment largely depends on the severity and extent of body surface area involvement; systemic therapy generally is indicated when the affected body surface area is greater than 5% to 10%. In patients on systemic therapy, increased susceptibility to infection is a priority concern for prescribing physicians. In the context of continuing immunosuppressive medications, vaccines that reduce susceptibility to infectious diseases can play an important role in reducing morbidity and mortality for these patients; however, an important consideration is that in patients with chronic conditions and frequent hospital visits, vaccines may be administered by various clinicians who may not be familiar with the management of immunosuppressive treatments. It is pivotal for prescribing dermatologists to provide appropriate vaccination instructions for the patient and any future clinicians to ensure vaccine efficacy in these patients.
The intramuscular influenza vaccine is a killed vaccine that is administered annually and has been shown to be safe for use in both immunocompetent and immunocompromised patients.1,2 Despite its safety, questions remain regarding the efficacy of vaccines while a patient is unable to mount a normal immune response and whether the treatment must be altered to maximize immunogenicity. The common systemic treatment options for psoriasis and any recommendations that can be made regarding administration of the influenza vaccine in that context are outlined in the Table. Given the sparsity of clinical data measuring vaccine immunogenicity in patients with psoriasis, vaccine guidelines are drawn from patients with various conditions who are receiving the same dose of medication as indicated for psoriasis.

Immunosuppressants and biologics commonly are used in dermatology for the management of many conditions, including psoriasis. As flu season approaches in the setting of a global pandemic, it is critical to understand the effects of commonly used psoriasis medications on the influenza vaccine. Through a brief review of the latest data concerning their interactions, dermatologists will be able to provide appropriate recommendations that maximize a patient’s immune response to the vaccine while minimizing adverse effects from holding medication.
A 42-year-old woman with psoriasis presents for a checkup at the dermatology clinic. Her psoriasis has been fairly stable on methotrexate with no recent flares. She presents her concern of the coronavirus pandemic continuing into the flu season and mentions she would like to minimize her chances of having a respiratory illness. The influenza vaccine has just become available, and she inquires when she can get the vaccine and whether it will interfere with her treatment. What are your recommendations for the patient?
Psoriasis is an immune-mediated, inflammatory skin condition stemming from hyperproliferation of keratinocytes that classically involves erythematous skin plaques with overlying scale. Treatment options vary widely and include topical modalities, phototherapy, immunosuppressants, and biologic agents. Selection of treatment largely depends on the severity and extent of body surface area involvement; systemic therapy generally is indicated when the affected body surface area is greater than 5% to 10%. In patients on systemic therapy, increased susceptibility to infection is a priority concern for prescribing physicians. In the context of continuing immunosuppressive medications, vaccines that reduce susceptibility to infectious diseases can play an important role in reducing morbidity and mortality for these patients; however, an important consideration is that in patients with chronic conditions and frequent hospital visits, vaccines may be administered by various clinicians who may not be familiar with the management of immunosuppressive treatments. It is pivotal for prescribing dermatologists to provide appropriate vaccination instructions for the patient and any future clinicians to ensure vaccine efficacy in these patients.
The intramuscular influenza vaccine is a killed vaccine that is administered annually and has been shown to be safe for use in both immunocompetent and immunocompromised patients.1,2 Despite its safety, questions remain regarding the efficacy of vaccines while a patient is unable to mount a normal immune response and whether the treatment must be altered to maximize immunogenicity. The common systemic treatment options for psoriasis and any recommendations that can be made regarding administration of the influenza vaccine in that context are outlined in the Table. Given the sparsity of clinical data measuring vaccine immunogenicity in patients with psoriasis, vaccine guidelines are drawn from patients with various conditions who are receiving the same dose of medication as indicated for psoriasis.

Immunosuppressants and biologics commonly are used in dermatology for the management of many conditions, including psoriasis. As flu season approaches in the setting of a global pandemic, it is critical to understand the effects of commonly used psoriasis medications on the influenza vaccine. Through a brief review of the latest data concerning their interactions, dermatologists will be able to provide appropriate recommendations that maximize a patient’s immune response to the vaccine while minimizing adverse effects from holding medication.
- Zbinden D, Manuel O. Influenza vaccination in immunocompromised patients: efficacy and safety. Immunotherapy. 2014;6:131-139.
- Milanovic M, Stojanovich L, Djokovic A, et al. Influenza vaccination in autoimmune rheumatic disease patients. Tohoku J Exp Med. 2013;229:29-34.
- Dengler TJ, Strnad N, Bühring I, et al. Differential immune response to influenza and pneumococcal vaccination in immunosuppressed patients after heart transplantation. Transplantation. 1998;66:1340-1347.
- Willcocks LC, Chaudhry AN, Smith JC, et al. The effect of sirolimus therapy on vaccine responses in transplant recipients. Am J Transplant. 2007;7:2006-2011.
- Chioato A, Noseda E, Stevens M, et al. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: results of an open-label, parallel-group, randomized single-center study. Clin Vaccine Immunol. 2012;19:1597-1602.
- Richi P, Martín MD, de Ory F, et al. Secukinumab does not impair the immunogenic response to the influenza vaccine in patients. RMD Open. 2019;5:e001018.
- Furer V, Zisman D, Kaufman I, et al. Immunogenicity and safety of vaccination against seasonal influenza vaccine in patients with psoriatic arthritis treated with secukinumab. Vaccine. 2020;38:847-851.
- Hua C, Barnetche T, Combe B, et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. 2014;66:1016-1026.
- Park JK, Choi Y, Winthrop KL, et al. Optimal time between the last methotrexate administration and seasonal influenza vaccination in rheumatoid arthritis: post hoc analysis of a randomised clinical trial. Ann Rheum Dis. 2019;78:1283-1284.
- Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76:1559-1565.
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898-904.
- Shirai S, Hara M, Sakata Y, et al. Immunogenicity of quadrivalent influenza vaccine for patients with inflammatory bowel disease undergoing immunosuppressive therapy. Inflamm Bowel Dis. 2018;24:1082-1091.
- Fomin I. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF blockers. Ann Rheum Dis. 2006;65:191-194.
- Bosaeed M, Kumar D. Seasonal influenza vaccine in immunocompromised persons. Hum Vaccin Immunother. 2018;14:1311-1322.
- Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34:272-279.
- Zbinden D, Manuel O. Influenza vaccination in immunocompromised patients: efficacy and safety. Immunotherapy. 2014;6:131-139.
- Milanovic M, Stojanovich L, Djokovic A, et al. Influenza vaccination in autoimmune rheumatic disease patients. Tohoku J Exp Med. 2013;229:29-34.
- Dengler TJ, Strnad N, Bühring I, et al. Differential immune response to influenza and pneumococcal vaccination in immunosuppressed patients after heart transplantation. Transplantation. 1998;66:1340-1347.
- Willcocks LC, Chaudhry AN, Smith JC, et al. The effect of sirolimus therapy on vaccine responses in transplant recipients. Am J Transplant. 2007;7:2006-2011.
- Chioato A, Noseda E, Stevens M, et al. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: results of an open-label, parallel-group, randomized single-center study. Clin Vaccine Immunol. 2012;19:1597-1602.
- Richi P, Martín MD, de Ory F, et al. Secukinumab does not impair the immunogenic response to the influenza vaccine in patients. RMD Open. 2019;5:e001018.
- Furer V, Zisman D, Kaufman I, et al. Immunogenicity and safety of vaccination against seasonal influenza vaccine in patients with psoriatic arthritis treated with secukinumab. Vaccine. 2020;38:847-851.
- Hua C, Barnetche T, Combe B, et al. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. 2014;66:1016-1026.
- Park JK, Choi Y, Winthrop KL, et al. Optimal time between the last methotrexate administration and seasonal influenza vaccination in rheumatoid arthritis: post hoc analysis of a randomised clinical trial. Ann Rheum Dis. 2019;78:1283-1284.
- Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76:1559-1565.
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898-904.
- Shirai S, Hara M, Sakata Y, et al. Immunogenicity of quadrivalent influenza vaccine for patients with inflammatory bowel disease undergoing immunosuppressive therapy. Inflamm Bowel Dis. 2018;24:1082-1091.
- Fomin I. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF blockers. Ann Rheum Dis. 2006;65:191-194.
- Bosaeed M, Kumar D. Seasonal influenza vaccine in immunocompromised persons. Hum Vaccin Immunother. 2018;14:1311-1322.
- Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34:272-279.
Practice Points
- Patients receiving methotrexate appear to benefit from suspending treatment for 2 weeks following influenza vaccination, as it maximizes the seroprotective response.
- Patients receiving tumor necrosis factor α inhibitors and low-dose IL-17 inhibitors have an unaltered humoral response to vaccination and attain protection equal to that of the general population.
- Patients treated with cyclosporine should be closely monitored for influenza symptoms even after vaccination, as approximately half of patients do not achieve a seroprotective response.
- Consider the increased risk for psoriatic flare during treatment suspension and the possibility of failed seroprotection, warranting close monitoring and clinical judgement tailored to each individual.
Risk for Deep Fungal Infections During IL-17 and IL-23 Inhibitor Therapy for Psoriasis
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
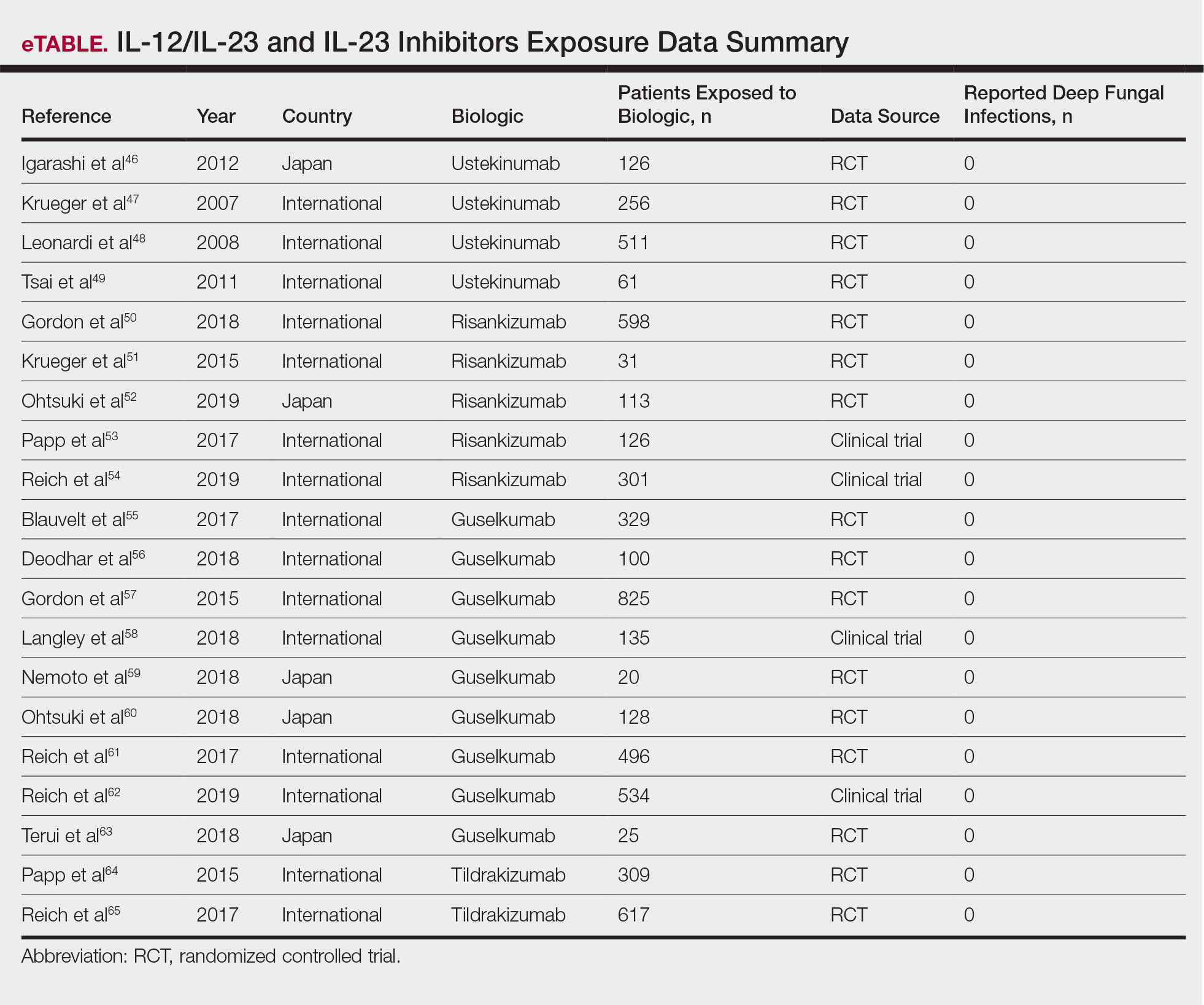
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.
IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.

IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.

IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
Practice Points
- The use of IL-17, IL-12/IL-23, and IL-23 inhibitors for psoriasis and other inflammatory conditions does not appear to increase the risk for deep fungal infections.
- Physicians should still be cautiously optimistic in prescribing these medications, as IL-17 and IL-23 play a central role in immunologic defenses, particularly against fungi.
- A high index of suspicion should be maintained for patients from endemic areas who are being treated with biologics.
Study highlights differences between White and Latino patients with psoriasis
in the same studies, according to new data presented at the virtual Skin of Color Update 2020.
“Our findings demonstrate that, though White psoriasis patients may have higher severity in certain body regions such as the trunk, axilla, and groin areas, Latino psoriasis patients have a greater distribution of involvement, particularly in their upper limbs,” reported Alyssa G. Ashbaugh, a third-year medical student at the University of California, Irvine.
The study also found that psoriasis had a greater adverse impact on well-being, as measured with the Dermatology Life Quality Index (DLQI). At entry into the trials from which these patients were drawn, the higher DLQI score, significantly lower quality of life, was nearly two times higher (13.78 vs. 7.31; P = .01) among the Latino patients, compared with White patients.
This is not the first study to show a greater negative impact from psoriasis on Latinos than Whites, according to Ms. Ashbaugh. For example, Latinos had the worse quality of life at baseline by DLQI score than White, Asians, or Black participants in a trial of etanercept that enrolled more than 2000 patients.
In this retrospective chart review, patient characteristics were evaluated in all 21 Latino patients enrolled in psoriasis clinical trials at the University of California, Irvine, in a recent period. They were matched by age and gender to an equal number of White patients participating in the same trials.
The mean age at diagnosis of psoriasis was older in the Latino group than in the White population (42.4 vs. 35.6 years; P = .20), but the difference did not reach statistical significance. The proportion of patients with severe disease on investigator global assessment was also greater but not significantly different in the Latino group, compared with the White group, respectively (42.9% vs. 28.6%; P = .10).
However, differences in the patterns of disease did reach significance. This included a lower mean Psoriasis Assessment Severity Index score of the trunk, axilla, and groin in Latinos (4.74 vs. 9.73; P = .02). But compared with White participants, Latinos had a higher mean percentage of body surface area involvement in the upper limbs (4.78 vs. 1.85; P = .004) and a higher percentage of total body surface area involvement (20.50 vs. 10.03; P = .02).
“While White patients were found to have lived many more years with psoriasis, it is important for future studies to examine whether this is due to earlier onset or delayed diagnosis, given the fact that minorities are less likely to have access to a dermatologist,” reported Ms. Ashbaugh, who performed this work under the guidance of the senior author, Natasha Mesinkovska, MD, PhD, with the department of dermatology, University of California, Irvine.
Overall, the study suggested that body surface coverage and severity is not similarly distributed in Latinos relative to Whites. Although Ms. Ashbaugh conceded that the small sample size and retrospective design of this study are important limitations, she believes that her study, along with previously published studies that suggest psoriasis characteristics may differ meaningfully by race or ethnicity, raises issues that should be explored in future studies designed to confirm differences and whether those differences should affect management.
Other studies have suggested “there are notable differences in the presentation of psoriasis between racial and ethnic groups with the Latino population often presenting to physicians with more severe psoriasis and increased body surface area involvement,” Ms. Ashbaugh noted. Although this appears to be one of the first studies to examine psoriasis characteristics in Latinos relative to Whites, she believes this is an area ripe for further analysis.
Psoriasis “is not a rare occurrence” in non-White populations even if U.S. data suggest that the prevalence in “people of color is lower than that of psoriasis in the U.S. white population,” Amy McMichael, MD, chair of the department of dermatology, Wake Forest Baptist Medical Center, Winston-Salem, N.C., commented in an interview after the meeting. She agreed that it cannot be assumed that psoriasis in skin of color has the same manifestations or responds to treatment in the same way as in White patients.
“Studies have suggested that lesion thickness and, often, extent of disease can be worse in patients of color. Few studies to date have examined the efficacy of treatments and impact of disease in these populations,” she said.
One exception was a study Dr. McMichael and colleagues published last year on the efficacy and safety of the interleukin-17 receptor A antagonist brodalumab for psoriasis in patients of color. The study showed that Black, Latino, and Asian patients participating in the AMAGINE-2 and AMAGINE-3 trials achieved similar outcomes as White participants.
“We published this study because this is one of the first, if not the first, to have enough patients of color to actually draw conclusions about the efficacy of the biologic as well as the patient-reported outcomes,” she explained.
Like the author of the evaluation of Latino patients undertaken at the University of California, Irvine, Dr. McMichael said studies of psoriasis specific to patients of color are needed.
“We cannot assume all patients of color will have the same outcomes as their Caucasian counterparts. It is imperative to include those of color in future psoriasis treatment trials in order to determine the efficacy of new medications,” she added, specifically calling for collection of data on patient-reported outcomes.
Ms. Ashbaugh has no relevant financial relationships to disclose. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Johnson & Johnson, and Aclaris.
in the same studies, according to new data presented at the virtual Skin of Color Update 2020.
“Our findings demonstrate that, though White psoriasis patients may have higher severity in certain body regions such as the trunk, axilla, and groin areas, Latino psoriasis patients have a greater distribution of involvement, particularly in their upper limbs,” reported Alyssa G. Ashbaugh, a third-year medical student at the University of California, Irvine.
The study also found that psoriasis had a greater adverse impact on well-being, as measured with the Dermatology Life Quality Index (DLQI). At entry into the trials from which these patients were drawn, the higher DLQI score, significantly lower quality of life, was nearly two times higher (13.78 vs. 7.31; P = .01) among the Latino patients, compared with White patients.
This is not the first study to show a greater negative impact from psoriasis on Latinos than Whites, according to Ms. Ashbaugh. For example, Latinos had the worse quality of life at baseline by DLQI score than White, Asians, or Black participants in a trial of etanercept that enrolled more than 2000 patients.
In this retrospective chart review, patient characteristics were evaluated in all 21 Latino patients enrolled in psoriasis clinical trials at the University of California, Irvine, in a recent period. They were matched by age and gender to an equal number of White patients participating in the same trials.
The mean age at diagnosis of psoriasis was older in the Latino group than in the White population (42.4 vs. 35.6 years; P = .20), but the difference did not reach statistical significance. The proportion of patients with severe disease on investigator global assessment was also greater but not significantly different in the Latino group, compared with the White group, respectively (42.9% vs. 28.6%; P = .10).
However, differences in the patterns of disease did reach significance. This included a lower mean Psoriasis Assessment Severity Index score of the trunk, axilla, and groin in Latinos (4.74 vs. 9.73; P = .02). But compared with White participants, Latinos had a higher mean percentage of body surface area involvement in the upper limbs (4.78 vs. 1.85; P = .004) and a higher percentage of total body surface area involvement (20.50 vs. 10.03; P = .02).
“While White patients were found to have lived many more years with psoriasis, it is important for future studies to examine whether this is due to earlier onset or delayed diagnosis, given the fact that minorities are less likely to have access to a dermatologist,” reported Ms. Ashbaugh, who performed this work under the guidance of the senior author, Natasha Mesinkovska, MD, PhD, with the department of dermatology, University of California, Irvine.
Overall, the study suggested that body surface coverage and severity is not similarly distributed in Latinos relative to Whites. Although Ms. Ashbaugh conceded that the small sample size and retrospective design of this study are important limitations, she believes that her study, along with previously published studies that suggest psoriasis characteristics may differ meaningfully by race or ethnicity, raises issues that should be explored in future studies designed to confirm differences and whether those differences should affect management.
Other studies have suggested “there are notable differences in the presentation of psoriasis between racial and ethnic groups with the Latino population often presenting to physicians with more severe psoriasis and increased body surface area involvement,” Ms. Ashbaugh noted. Although this appears to be one of the first studies to examine psoriasis characteristics in Latinos relative to Whites, she believes this is an area ripe for further analysis.
Psoriasis “is not a rare occurrence” in non-White populations even if U.S. data suggest that the prevalence in “people of color is lower than that of psoriasis in the U.S. white population,” Amy McMichael, MD, chair of the department of dermatology, Wake Forest Baptist Medical Center, Winston-Salem, N.C., commented in an interview after the meeting. She agreed that it cannot be assumed that psoriasis in skin of color has the same manifestations or responds to treatment in the same way as in White patients.
“Studies have suggested that lesion thickness and, often, extent of disease can be worse in patients of color. Few studies to date have examined the efficacy of treatments and impact of disease in these populations,” she said.
One exception was a study Dr. McMichael and colleagues published last year on the efficacy and safety of the interleukin-17 receptor A antagonist brodalumab for psoriasis in patients of color. The study showed that Black, Latino, and Asian patients participating in the AMAGINE-2 and AMAGINE-3 trials achieved similar outcomes as White participants.
“We published this study because this is one of the first, if not the first, to have enough patients of color to actually draw conclusions about the efficacy of the biologic as well as the patient-reported outcomes,” she explained.
Like the author of the evaluation of Latino patients undertaken at the University of California, Irvine, Dr. McMichael said studies of psoriasis specific to patients of color are needed.
“We cannot assume all patients of color will have the same outcomes as their Caucasian counterparts. It is imperative to include those of color in future psoriasis treatment trials in order to determine the efficacy of new medications,” she added, specifically calling for collection of data on patient-reported outcomes.
Ms. Ashbaugh has no relevant financial relationships to disclose. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Johnson & Johnson, and Aclaris.
in the same studies, according to new data presented at the virtual Skin of Color Update 2020.
“Our findings demonstrate that, though White psoriasis patients may have higher severity in certain body regions such as the trunk, axilla, and groin areas, Latino psoriasis patients have a greater distribution of involvement, particularly in their upper limbs,” reported Alyssa G. Ashbaugh, a third-year medical student at the University of California, Irvine.
The study also found that psoriasis had a greater adverse impact on well-being, as measured with the Dermatology Life Quality Index (DLQI). At entry into the trials from which these patients were drawn, the higher DLQI score, significantly lower quality of life, was nearly two times higher (13.78 vs. 7.31; P = .01) among the Latino patients, compared with White patients.
This is not the first study to show a greater negative impact from psoriasis on Latinos than Whites, according to Ms. Ashbaugh. For example, Latinos had the worse quality of life at baseline by DLQI score than White, Asians, or Black participants in a trial of etanercept that enrolled more than 2000 patients.
In this retrospective chart review, patient characteristics were evaluated in all 21 Latino patients enrolled in psoriasis clinical trials at the University of California, Irvine, in a recent period. They were matched by age and gender to an equal number of White patients participating in the same trials.
The mean age at diagnosis of psoriasis was older in the Latino group than in the White population (42.4 vs. 35.6 years; P = .20), but the difference did not reach statistical significance. The proportion of patients with severe disease on investigator global assessment was also greater but not significantly different in the Latino group, compared with the White group, respectively (42.9% vs. 28.6%; P = .10).
However, differences in the patterns of disease did reach significance. This included a lower mean Psoriasis Assessment Severity Index score of the trunk, axilla, and groin in Latinos (4.74 vs. 9.73; P = .02). But compared with White participants, Latinos had a higher mean percentage of body surface area involvement in the upper limbs (4.78 vs. 1.85; P = .004) and a higher percentage of total body surface area involvement (20.50 vs. 10.03; P = .02).
“While White patients were found to have lived many more years with psoriasis, it is important for future studies to examine whether this is due to earlier onset or delayed diagnosis, given the fact that minorities are less likely to have access to a dermatologist,” reported Ms. Ashbaugh, who performed this work under the guidance of the senior author, Natasha Mesinkovska, MD, PhD, with the department of dermatology, University of California, Irvine.
Overall, the study suggested that body surface coverage and severity is not similarly distributed in Latinos relative to Whites. Although Ms. Ashbaugh conceded that the small sample size and retrospective design of this study are important limitations, she believes that her study, along with previously published studies that suggest psoriasis characteristics may differ meaningfully by race or ethnicity, raises issues that should be explored in future studies designed to confirm differences and whether those differences should affect management.
Other studies have suggested “there are notable differences in the presentation of psoriasis between racial and ethnic groups with the Latino population often presenting to physicians with more severe psoriasis and increased body surface area involvement,” Ms. Ashbaugh noted. Although this appears to be one of the first studies to examine psoriasis characteristics in Latinos relative to Whites, she believes this is an area ripe for further analysis.
Psoriasis “is not a rare occurrence” in non-White populations even if U.S. data suggest that the prevalence in “people of color is lower than that of psoriasis in the U.S. white population,” Amy McMichael, MD, chair of the department of dermatology, Wake Forest Baptist Medical Center, Winston-Salem, N.C., commented in an interview after the meeting. She agreed that it cannot be assumed that psoriasis in skin of color has the same manifestations or responds to treatment in the same way as in White patients.
“Studies have suggested that lesion thickness and, often, extent of disease can be worse in patients of color. Few studies to date have examined the efficacy of treatments and impact of disease in these populations,” she said.
One exception was a study Dr. McMichael and colleagues published last year on the efficacy and safety of the interleukin-17 receptor A antagonist brodalumab for psoriasis in patients of color. The study showed that Black, Latino, and Asian patients participating in the AMAGINE-2 and AMAGINE-3 trials achieved similar outcomes as White participants.
“We published this study because this is one of the first, if not the first, to have enough patients of color to actually draw conclusions about the efficacy of the biologic as well as the patient-reported outcomes,” she explained.
Like the author of the evaluation of Latino patients undertaken at the University of California, Irvine, Dr. McMichael said studies of psoriasis specific to patients of color are needed.
“We cannot assume all patients of color will have the same outcomes as their Caucasian counterparts. It is imperative to include those of color in future psoriasis treatment trials in order to determine the efficacy of new medications,” she added, specifically calling for collection of data on patient-reported outcomes.
Ms. Ashbaugh has no relevant financial relationships to disclose. Dr. McMichael’s disclosures included serving as an investigator and/or consultant for companies that included Allergan, Procter & Gamble, Johnson & Johnson, and Aclaris.
FROM SOC 2020
Shingrix effective in older adults with preexisting immune-mediated disorders
The adjuvanted recombinant zoster vaccine Shingrix appears to be effective in older adults with autoimmune diseases who are not receiving treatment regimens that suppress the immune system, according to a post hoc analysis of patients in two clinical trials.
A two-dose regimen of Shingrix was effective in 90.5% of a subset of patients in two phase 3 clinical trials of adults who were aged at least 50 years, according to Alemnew F. Dagnew, MD, of GlaxoSmithKline and colleagues. The lowest rates of effectiveness with Shingrix, for patients aged between 70-79 years, was 84.4%, the researchers reported in Rheumatology.
The CDC recommends adults aged at least 50 years receive two doses of Shingrix to help prevent reoccurrence of herpes zoster, or Zostavax (zoster vaccine live) if adults are allergic to components of the Shingrix vaccine or have tested negative for varicella zoster virus immunity.
Dr. Dagnew and colleagues evaluated Shingrix in 983 patients who received two doses of Shingrix and 960 patients who received placebo from the ZOE-50 and ZOE-70 trials, where each dose was administered at least 2 months apart. The mean age of patients in both groups was 68.8 years in the Shingrix group and 69.4 years in the placebo group, and more than half of patients in both Shingrix (59.9%) and placebo groups (60.8%) were women. About 7% of the patients in two clinical trial had a pIMD.
At enrollment, the most common preexisting immune-mediated disorders (pIMDs) were psoriasis (215 patients taking Shingrix vs. 239 patients on placebo), spondyloarthropathy (109 patients taking Shingrix vs. 89 patients on placebo), rheumatoid arthritis (96 patients taking Shingrix vs. 94 patients on placebo), and celiac disease (41 patients taking Shingrix vs. 34 patients on placebo). Dr. Dagnew and colleagues examined the subgroup of patients with pIMDs for safety and vaccine efficacy, which was defined as not developing herpes zoster before the second dose.
Overall, the efficacy of Shingrix was 90.5% across all age groups (95% confidence interval, 73.5%-97.5%), with the group aged between 70-79 years having the lowest rate of effectiveness (95% CI, 30.8%-98.3%). The rate of severe adverse events was 14.6% in the Shingrix group and 11.7% in the placebo group between the first Shingrix dose and for up to 1 year after the second dose. The most common adverse events were infections and infestations as well as cardiac disorders. “Our data show a balance between study groups in the frequency and nature of SAEs, confirming the favorable safety profile of [Shingrix] in populations with pIMDs,” Dr. Dagnew and colleagues wrote.
The researchers acknowledged that the ZOE-50/70 studies were underpowered to detect the efficacy and safety of Shingrix in individuals with pIMDs but said that the large number of participants in the studies let them estimate efficacy and adverse events for this subgroup. They also noted there was no randomization of pIMDs at enrollment, even though pIMDs occurred at similar rates between Shingrix and placebo groups.
This study was funded by GlaxoSmithKline; the company helped with conducting and analyzing the study and also provided the costs associated with publishing it. Five authors reported being an employee of GlaxoSmithKline during the time the work was conducted, and four of the five own stock in the company. One author is now an employee of UCB. One author reported having served on the advisory boards for Merck Sharp & Dohme, GlaxoSmithKline, and Curevo.
SOURCE: Dagnew AF et al. Rheumatology. 2020 Sep 10. doi: 10.1093/rheumatology/keaa424.
The adjuvanted recombinant zoster vaccine Shingrix appears to be effective in older adults with autoimmune diseases who are not receiving treatment regimens that suppress the immune system, according to a post hoc analysis of patients in two clinical trials.
A two-dose regimen of Shingrix was effective in 90.5% of a subset of patients in two phase 3 clinical trials of adults who were aged at least 50 years, according to Alemnew F. Dagnew, MD, of GlaxoSmithKline and colleagues. The lowest rates of effectiveness with Shingrix, for patients aged between 70-79 years, was 84.4%, the researchers reported in Rheumatology.
The CDC recommends adults aged at least 50 years receive two doses of Shingrix to help prevent reoccurrence of herpes zoster, or Zostavax (zoster vaccine live) if adults are allergic to components of the Shingrix vaccine or have tested negative for varicella zoster virus immunity.
Dr. Dagnew and colleagues evaluated Shingrix in 983 patients who received two doses of Shingrix and 960 patients who received placebo from the ZOE-50 and ZOE-70 trials, where each dose was administered at least 2 months apart. The mean age of patients in both groups was 68.8 years in the Shingrix group and 69.4 years in the placebo group, and more than half of patients in both Shingrix (59.9%) and placebo groups (60.8%) were women. About 7% of the patients in two clinical trial had a pIMD.
At enrollment, the most common preexisting immune-mediated disorders (pIMDs) were psoriasis (215 patients taking Shingrix vs. 239 patients on placebo), spondyloarthropathy (109 patients taking Shingrix vs. 89 patients on placebo), rheumatoid arthritis (96 patients taking Shingrix vs. 94 patients on placebo), and celiac disease (41 patients taking Shingrix vs. 34 patients on placebo). Dr. Dagnew and colleagues examined the subgroup of patients with pIMDs for safety and vaccine efficacy, which was defined as not developing herpes zoster before the second dose.
Overall, the efficacy of Shingrix was 90.5% across all age groups (95% confidence interval, 73.5%-97.5%), with the group aged between 70-79 years having the lowest rate of effectiveness (95% CI, 30.8%-98.3%). The rate of severe adverse events was 14.6% in the Shingrix group and 11.7% in the placebo group between the first Shingrix dose and for up to 1 year after the second dose. The most common adverse events were infections and infestations as well as cardiac disorders. “Our data show a balance between study groups in the frequency and nature of SAEs, confirming the favorable safety profile of [Shingrix] in populations with pIMDs,” Dr. Dagnew and colleagues wrote.
The researchers acknowledged that the ZOE-50/70 studies were underpowered to detect the efficacy and safety of Shingrix in individuals with pIMDs but said that the large number of participants in the studies let them estimate efficacy and adverse events for this subgroup. They also noted there was no randomization of pIMDs at enrollment, even though pIMDs occurred at similar rates between Shingrix and placebo groups.
This study was funded by GlaxoSmithKline; the company helped with conducting and analyzing the study and also provided the costs associated with publishing it. Five authors reported being an employee of GlaxoSmithKline during the time the work was conducted, and four of the five own stock in the company. One author is now an employee of UCB. One author reported having served on the advisory boards for Merck Sharp & Dohme, GlaxoSmithKline, and Curevo.
SOURCE: Dagnew AF et al. Rheumatology. 2020 Sep 10. doi: 10.1093/rheumatology/keaa424.
The adjuvanted recombinant zoster vaccine Shingrix appears to be effective in older adults with autoimmune diseases who are not receiving treatment regimens that suppress the immune system, according to a post hoc analysis of patients in two clinical trials.
A two-dose regimen of Shingrix was effective in 90.5% of a subset of patients in two phase 3 clinical trials of adults who were aged at least 50 years, according to Alemnew F. Dagnew, MD, of GlaxoSmithKline and colleagues. The lowest rates of effectiveness with Shingrix, for patients aged between 70-79 years, was 84.4%, the researchers reported in Rheumatology.
The CDC recommends adults aged at least 50 years receive two doses of Shingrix to help prevent reoccurrence of herpes zoster, or Zostavax (zoster vaccine live) if adults are allergic to components of the Shingrix vaccine or have tested negative for varicella zoster virus immunity.
Dr. Dagnew and colleagues evaluated Shingrix in 983 patients who received two doses of Shingrix and 960 patients who received placebo from the ZOE-50 and ZOE-70 trials, where each dose was administered at least 2 months apart. The mean age of patients in both groups was 68.8 years in the Shingrix group and 69.4 years in the placebo group, and more than half of patients in both Shingrix (59.9%) and placebo groups (60.8%) were women. About 7% of the patients in two clinical trial had a pIMD.
At enrollment, the most common preexisting immune-mediated disorders (pIMDs) were psoriasis (215 patients taking Shingrix vs. 239 patients on placebo), spondyloarthropathy (109 patients taking Shingrix vs. 89 patients on placebo), rheumatoid arthritis (96 patients taking Shingrix vs. 94 patients on placebo), and celiac disease (41 patients taking Shingrix vs. 34 patients on placebo). Dr. Dagnew and colleagues examined the subgroup of patients with pIMDs for safety and vaccine efficacy, which was defined as not developing herpes zoster before the second dose.
Overall, the efficacy of Shingrix was 90.5% across all age groups (95% confidence interval, 73.5%-97.5%), with the group aged between 70-79 years having the lowest rate of effectiveness (95% CI, 30.8%-98.3%). The rate of severe adverse events was 14.6% in the Shingrix group and 11.7% in the placebo group between the first Shingrix dose and for up to 1 year after the second dose. The most common adverse events were infections and infestations as well as cardiac disorders. “Our data show a balance between study groups in the frequency and nature of SAEs, confirming the favorable safety profile of [Shingrix] in populations with pIMDs,” Dr. Dagnew and colleagues wrote.
The researchers acknowledged that the ZOE-50/70 studies were underpowered to detect the efficacy and safety of Shingrix in individuals with pIMDs but said that the large number of participants in the studies let them estimate efficacy and adverse events for this subgroup. They also noted there was no randomization of pIMDs at enrollment, even though pIMDs occurred at similar rates between Shingrix and placebo groups.
This study was funded by GlaxoSmithKline; the company helped with conducting and analyzing the study and also provided the costs associated with publishing it. Five authors reported being an employee of GlaxoSmithKline during the time the work was conducted, and four of the five own stock in the company. One author is now an employee of UCB. One author reported having served on the advisory boards for Merck Sharp & Dohme, GlaxoSmithKline, and Curevo.
SOURCE: Dagnew AF et al. Rheumatology. 2020 Sep 10. doi: 10.1093/rheumatology/keaa424.
FROM RHEUMATOLOGY
Psoriasis and Psoriatic Arthritis
Psoriasis and Psoriatic Arthritis is a supplement to Dermatology News with commentary from Joel M. Gelfand, MD, MSCE and Alan Menter, MD.
- Exploring new guidelines and scientific advances
- ‘Loss-framed’ approach makes psoriasis patients more agreeable to treatment
- Topical PDE-4 inhibitor for psoriasis effective in phase 2b trial
- Biologics may delay psoriatic arthritis
- AAD-NPF releases guidelines for systemic nonbiologic treatments of psoriasis
- Specific markers detect psoriatic disease inflammation without elevated CRP
- Beyond PASI 100: Striving for molecular clearance
- Ultrasound improves specificity of psoriatic arthritis referrals
- Registry data reveal temporal relationship between psoriasis symptoms and PsA onset
- New psoriasis guidelines focus on topical and alternative treatments, and severity measures
- Psoriasis topical combination maintenance strategy hits mark at 1 year
Read the supplement.
Psoriasis and Psoriatic Arthritis is a supplement to Dermatology News with commentary from Joel M. Gelfand, MD, MSCE and Alan Menter, MD.
- Exploring new guidelines and scientific advances
- ‘Loss-framed’ approach makes psoriasis patients more agreeable to treatment
- Topical PDE-4 inhibitor for psoriasis effective in phase 2b trial
- Biologics may delay psoriatic arthritis
- AAD-NPF releases guidelines for systemic nonbiologic treatments of psoriasis
- Specific markers detect psoriatic disease inflammation without elevated CRP
- Beyond PASI 100: Striving for molecular clearance
- Ultrasound improves specificity of psoriatic arthritis referrals
- Registry data reveal temporal relationship between psoriasis symptoms and PsA onset
- New psoriasis guidelines focus on topical and alternative treatments, and severity measures
- Psoriasis topical combination maintenance strategy hits mark at 1 year
Read the supplement.
Psoriasis and Psoriatic Arthritis is a supplement to Dermatology News with commentary from Joel M. Gelfand, MD, MSCE and Alan Menter, MD.
- Exploring new guidelines and scientific advances
- ‘Loss-framed’ approach makes psoriasis patients more agreeable to treatment
- Topical PDE-4 inhibitor for psoriasis effective in phase 2b trial
- Biologics may delay psoriatic arthritis
- AAD-NPF releases guidelines for systemic nonbiologic treatments of psoriasis
- Specific markers detect psoriatic disease inflammation without elevated CRP
- Beyond PASI 100: Striving for molecular clearance
- Ultrasound improves specificity of psoriatic arthritis referrals
- Registry data reveal temporal relationship between psoriasis symptoms and PsA onset
- New psoriasis guidelines focus on topical and alternative treatments, and severity measures
- Psoriasis topical combination maintenance strategy hits mark at 1 year
Read the supplement.
Pregnancy studies on psoriasis, PsA medications pick up
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
Christina Chambers, PhD, MPH, who runs the MotherToBaby Pregnancy Studies research center at the University of California, San Diego, has found most pregnant women to be “entirely altruistic” about sharing their experiences with drug treatment during pregnancy.
– but dermatologists, rheumatologists, and their female patients with psoriasis and psoriatic arthritis (PsA) want much more.
And women’s participation in the MotherToBaby studies conducted by the nonprofit Organization of Teratology Information Specialists (OTIS) is key, say physicians who are treating women of reproductive age. OTIS is now listed in drug labeling as the “pregnancy registry” contact for many of the medications they may be discussing with patients.
Dr. Chambers said that most women appreciate “that participating in a study may not help her with her pregnancy, but it can help her sister or her friend or someone else who has these same questions in planning a pregnancy of ‘Can I stay on my treatment?’ or, in the case of an unplanned pregnancy, ‘Should I be concerned?’ ”
OTIS has enrolled women with psoriasis and/or PsA in studies of nine medications, most of them biologics (both TNF-alpha blockers and newer anti-interleukin agents).
Four of the studies – those evaluating etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), and ustekinumab (Stelara) – are now closed to enrollment with analyses either underway or completed. The other five are currently enrolling patients and involve treatment with certolizumab pegol (Cimzia), tildrakizumab (Ilumya), apremilast (Otezla), guselkumab (Tremfya), and tofacitinib (Xeljanz).
Lisa R. Sammaritano, MD, a rheumatologist at the Hospital for Special Surgery, New York, who led the development of the American College of Rheumatology’s first guideline for the management of reproductive health in rheumatic and musculoskeletal diseases, recommends to some of her patients that they contact OTIS. “Their pregnancy registry studies have added important information to the field over the years,” she said.
Most recently, a study of the anti–TNF-alpha medication adalimumab that began in 2004 in pregnant patients with RA and Crohn’s disease culminated in a 2019 PLOS ONE paper reporting no associations between exposure to the medication and an increased risk of adverse outcomes. The outcomes studied were major structural birth defects, minor defects, spontaneous abortion, preterm delivery, prenatal and postnatal growth deficiency, serious or opportunistic infections, and malignancies.
An analysis is underway of adalimumab exposure in women with PsA – a patient subset that was added after the study started. But in the meantime, Dr. Chambers said, the 2019 research article is relevant to questions of drug safety across indications.
OTIS’s MothertoBaby studies are structured as prospective cohort studies. Dr. Chambers, a perinatal epidemiologist, is president of OTIS, which recruits women who have an exposure to the medication under study – at least one dose, for any length of time. And in most cases, it also recruits women with the underlying condition but no exposure and healthy women without the condition to represent the general population.
It’s the disease-matched comparison group that makes OTIS’s studies different from traditional pregnancy registries involving “a simple exposure series and outcomes that are described in the context of what you’d expect in the general population,” said Dr. Chambers, professor in the department of pediatrics, as well as family and preventative medicine, at UCSD and codirector of the Center for Better Beginnings at that university. “Many maternal conditions themselves [or their comorbidities] carry some risk of adverse outcomes in pregnancy.”
The OTIS studies typically involve at least 100 exposed pregnancies and a similar number of unexposed pregnancies; some have cohorts of 200-300.
The recently published study of adalimumab, for instance, included 257 women with exposure to the drug and 120 women in a disease comparison group with no exposure. In addition to finding no associations between drug exposure and adverse outcomes, the study found that women with RA or Crohn’s were at increased risk of preterm delivery, irrespective of adalimumab exposure.
“There’s insufficient [power with any of these numbers] to come to the conclusion that a drug is safe,” she said. “But what we have been able to say [through our studies] is that we’ve looked carefully at the whole array of outcomes ... and we don’t see anything unusual. That early view can be reassuring” until large population-based studies or claims analyses become possible.
Dr. Sammaritano, also with Weill Cornell Medicine, New York, said that she does not recommend registry participation for patients who stop biologics at the diagnosis of pregnancy. Since “the start of IgG antibody transfer during pregnancy is about 16 weeks,” she worries that including these patients might lead to falsely reassuring findings. “We are most interested in [knowing the outcomes of] patients who must continue the drugs through pregnancy,” she said.
Dr. Chambers, however, said that in her view, placental transfer is not a requirement for a medication to have some effect on the outcome of pregnancy. “The outcome could be influenced by an effect of the medication that doesn’t require placental transfer or require placental transfer in large amounts,” she said. “So it’s relevant to examine exposures that have occurred only in the first trimester, and this is especially true for the outcome of major birth defects, most of which are initiated in the first trimester.”
The MotherToBaby studies typically include both early, short exposures and longer exposures, she said. “And certainly, duration of use is a factor that we do consider in looking at specific outcomes such as growth, preterm delivery, and risk of serious or opportunistic infections.”
(In the published study of adalimumab, 65.3% of women in the medication-exposed cohort used the medication in all three trimesters, 10.5% in the first and second trimesters, and 22.4% in the first trimester only.)
Women participating in the MotherToBaby studies complete two to four interviews during pregnancy and may be interviewed again after delivery. They are asked for their permission to share a copy of their medical records – and their baby’s medical records – and their babies receive a follow-up pediatric exam by a pediatrician with expertise in dysmorphology/genetics (who is blinded to exposure status), most commonly in the participant’s home. Providers are not asked to enter any data.
Eliza Chakravarty, MD, a rheumatologist with the Oklahoma Medical Research Foundation in Oklahoma City who treats patients with PsA who are pregnant or considering pregnancy, said that her referrals for research participation “have been mostly to MothertoBaby.”
“Most drug companies [in the autoimmune space] are now contracting with them [for their pregnancy exposure research],” she said. “I really like that it’s become so centralized.”
She tells patients that many questions can be answered through research, that their experience matters, and that “there are benefits” to the extra pediatric examination. “I give them the information and let them decide whether or not they want to call [MotherToBaby],” she said. “I don’t want to impose. I want to make them aware.”
Dr. Chambers emphasizes to patients and physicians that the studies are strictly observational and do not require any changes in personal or medical regimens. “When people hear the word ‘research’ they think of clinical trials. We’re saying, you and your provider do everything you normally would do, just let us observe what happens during your pregnancy.”
Physicians should assure patients, moreover, that “just because the drug is being studied doesn’t mean there’s a known risk or even a suspected risk,” she said.
The MotherToBaby studies receive funding from the pharmaceutical companies, which are required by the Food and Drug Administration to conduct pregnancy exposure registries for medications used during pregnancy or in women of reproductive age. OTIS has an independent advisory board, however, and independently analyzes and publishes its findings. Progress reports are shared with the pharmaceutical companies, and in turn, the FDA, Dr. Chambers said.
To refer patients for MotherToBaby studies, physicians can use an online referral form found on the MothertoBaby web site, a service of OTIS, or call the pregnancy studies team at 877-311-8972 to provide them with the patient’s name or number. Patients may also be given the number and advised to consider calling. MotherToBaby offers medication fact sheets that answer questions about exposures during pregnancy and breastfeeding, and runs a free and confidential teratogen counseling service: 866-626-6847.
Active Comparator Trial Designs Used to Promote Development of Innovative New Medications
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Practice Points
- When evaluating a new treatment, it is important to consider not only whether it is effective but also whether it provides additional value compared to existing treatment options.
- Encouraging active comparator trials will provide clinicians and patients with important data to guide decision-making regarding the most appropriate treatment options.
Study results suggest ustekinumab may trigger acute CV events early in treatment
in susceptible patients, according to a large French case-time-control analysis.
Investigators led by Florence Poizeau, MD, of the department of dermatology at Rennes (France) University Hospital, found high-risk patients had more than four times the risk of an acute SCE in the 6 months after starting treatment. Although ustekinumab (Stelara) effectively treats moderate to severe psoriasis, psoriatic arthritis (PsA), and Crohn’s disease (indications approved by the Food and Drug Administration), the early months after ustekinumab initiation may be associated with atherosclerotic plaque destabilization via the inhibition of helper T cell subtype 17, the group reported in JAMA Dermatology.
The observational study drew on France’s 66 million–registrant health insurance database to identify all patients exposed to ustekinumab between April 1, 2010, and Dec. 31, 2016. Classified by high or low cardiovascular risk level, ustekinumab recipients served as their own controls, being compared during two time windows: the risk period covered the 6 months after initiating treatment and leading up to the SCE, defined as acute coronary syndrome (ACS) or stroke, while a reference period spanned the 6-12 months leading up to the risk period.
In the statistical analysis of 9,290 ustekinumab-exposed patients (mean age 43 years, 52% male), conducted from September 2017 to July 2018, 7,588 (82%) received ustekinumab for psoriasis or PsA, and 724 (8%) for Crohn’s disease. (The remaining indications were for psoriasis or PsA and Crohn’s disease, or were undetermined.)
Of these patients, 98 experienced SCEs (52 with ACS admitted to the ICU and 46 with strokes). In patients deemed at high cardiovascular risk – those with two risk factors or a personal history of atherosclerotic disease – there was a statistically significant association between starting ustekinumab and SCE occurrence, for an odds ratio of 4.17 (95% confidence interval, 1.19-14.59). In contrast, no such association emerged in ustekinumab users at low cardiovascular risk, for an OR of 0.30 (95% CI, 0.03-3.13). The OR for all was 2.41 (95% CI, 0.83-7.01).
Of the 98 patients included in the final case-time-control analysis, 62 were men (63%), the median age was 57 years, and 76 (78%) were at high cardiovascular risk. A total of 89 patients (91%) had psoriasis, four (4%) had Crohn’s disease, and two (2%) had both.
The investigators also did an analysis including these 98 patients plus 13 patients with ACS who were not hospitalized in an ICU, and 68 with unstable angina, for a total of 179. In this group, the ORs for SCE were 1.75 (95% CI, 0.86-3.56) overall, compared with 3.20 (95% CI, 1.29-7.92) among those at high cardiovascular risk and 0.21 (95% CI, 0.02-1.69) among those at low cardiovascular risk.
The Rennes investigators’ decision to focus on early SCEs stemmed in part from a meta-analysis of randomized clinical trials that reported a possible excess of early SCEs in adults exposed to anti–IL-12/23p40 antibodies, which at that time included the now-discontinued experimental antibody briakinumab. Briakinumab trials were aborted and the drug was never brought to market, leaving ustekinumab as the only antibody of this type.
The finding of “an association between ustekinumab initiation and SCE among patients with cardiovascular risk factors suggests the need for caution regarding the prescription of ustekinumab in this population,” Dr. Poizeau and colleagues wrote. The risk “seems to concern patients with psoriasis” rather than Crohn’s disease, which may be related to the older age and greater cardiovascular risk of the former. “A close collaboration between cardiologists and biologic prescribers could be beneficial to evaluate the risk of SCEs for patients who are receiving ustekinumab,” they added, recommending further research into the physiopathological mechanisms of action.
Offering a U.S. clinician’s perspective on the French study, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, called the findings “unique and interesting with very robust odds ratios. These posttreatment associations have actually been a big area of research over the past decade but not with such defined time periods.”
No significant increases in risk have been seen with other biologics, Dr. Fernandez added, with the exception of briakinumab. “But still, the current study does not definitively answer the question whether ustekinumab can trigger acute events within 6 months of treatment. There’s smoke, but we haven’t clearly seen a fire.”
As to ustekinumab’s possible pathogenic mechanism of action, Dr. Fernandez pointed to data suggesting that IL-17A can be stabilizing to atherosclerotic plaques. “So there’s a hypothesis that blocking the 17/23 pathway may destabilize plaques and make patients more prone to acute cardiovascular events.”
In other comments from clinicians not involved in the study, Seoyoung Kim, MD, ScD, MSCE, director of the program in rheumatologic, immunologic, and musculoskeletal pharmacoepidemiology (PRIME) at Brigham and Women’s Hospital, Boston, noted that, while the investigators controlled for the trend over time and their design choice included time-fixed covariates such as age, sex, and family history within individuals, the case-crossover study could not control for time-varying confounders within individuals.
“In other words, it’s possible that some of the patients had a lot more disease activity and systemic inflammation and used more NSAIDs, steroids, and other medications potentially related to cardiovascular risk a few months before they started ustekinumab, compared with 6-12 months prior,“ Dr. Kim said in an interview. “I would be curious to know if they would find the same thing or not if they studied a different type of biologic drug.”
She also pointed out that the number of outcomes overall was small, leading to imprecise estimates and wide confidence intervals.
Last year Dr. Kim and associates published a study comparing ustekinumab with tumor necrosis factor inhibitor therapy in younger psoriasis and psoriatic arthritis patients and found no difference between the two groups in major cardiovascular events or atrial fibrillation.
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, has more reservations about the findings. “The Poizeau study was methodologically flawed, making the results unreliable,” he said in an interview. “There is a breadth of data from clinical trials and observational studies that do not demonstrate an increased risk of major acute cardiovascular events with ustekinumab and the results of the Poizeau study should not impact clinical practice.”
In an interview, Mark G. Lebwohl, MD, professor and chairman of the department of dermatology and chief for clinical therapeutics at the Icahn School of Medicine at Mount Sinai, New York, said that, in his view, the investigators used early reports of a small number cardiovascular events to look at the issue from a faulty perspective, and hence their findings would have no impact on his clinical practice.
“This study looked at the issue incorrectly. It looked at people put on drug who already had two risk factors for heart attack. And psoriasis itself is a third risk factor,” he said. “So lo and behold, big surprise, some of them had cardiovascular events.”
Dr. Lebwohl noted that a wealth of carefully compiled data has found no increase over time in cardiovascular events with this drug in psoriasis patients. The risk of cardiovascular events actually goes down with time because of the drug’s anti-inflammatory effects.
Dr. Fernandez takes a more positive view of the French findings. “The data certainly support the need for further research in this area,” he said in an interview, “and in the meantime this paper will probably make me extra cautious in using ustekinumab in those at significant risk.”
The French study was supported by a grant from the French National Agency for Medicines and Health Products Safety. Dr. Poizeau and seven coauthors had no disclosures. The remaining five reported disclosures that included receiving fees from AbbVie, Admiral, Amgen, Baxalta, Cologne, Dermavant, Eli Lilly, Janssen, Kyowa Kirin, Novartis, Mylan, Sun Pharmaceuticals, and UCB, as well as grants and personal fees from Boehringer Ingelheim, Leo Pharma, and Pfizer outside the submitted work, and personal fees from Pfizer, AbbVie, UCB Pharma, and Lilly during the conduct of the study. Dr. Fernandez reported consulting work for AbbVie and research grants from Novartis. Dr. Kim disclosed research grants from Brigham and Women’s Hospital and from Pfizer, Abbvie, Roche, and Bristol-Myers Squibb for unrelated studies. Dr. Gelfand reported varying financial ties to Bristol-Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Janssen Biologics, Regeneron, UCB, Sanofi, Pfizer, Celgene, OrthoDermatolgics, AbbVie, Novartis, and Eli Lilly. He is copatent holder of a treatment for cutaneous T-cell lymphoma. Dr. Lebwohl reported unpaid consulting for most manufacturers of psoriasis drugs, with all fees going directly to Mount Sinai.
Source: Poizeau F et al. JAMA Dermatol. 2020 Sep 9. doi: 10.1001/jamadermatol.2020.2977.
in susceptible patients, according to a large French case-time-control analysis.
Investigators led by Florence Poizeau, MD, of the department of dermatology at Rennes (France) University Hospital, found high-risk patients had more than four times the risk of an acute SCE in the 6 months after starting treatment. Although ustekinumab (Stelara) effectively treats moderate to severe psoriasis, psoriatic arthritis (PsA), and Crohn’s disease (indications approved by the Food and Drug Administration), the early months after ustekinumab initiation may be associated with atherosclerotic plaque destabilization via the inhibition of helper T cell subtype 17, the group reported in JAMA Dermatology.
The observational study drew on France’s 66 million–registrant health insurance database to identify all patients exposed to ustekinumab between April 1, 2010, and Dec. 31, 2016. Classified by high or low cardiovascular risk level, ustekinumab recipients served as their own controls, being compared during two time windows: the risk period covered the 6 months after initiating treatment and leading up to the SCE, defined as acute coronary syndrome (ACS) or stroke, while a reference period spanned the 6-12 months leading up to the risk period.
In the statistical analysis of 9,290 ustekinumab-exposed patients (mean age 43 years, 52% male), conducted from September 2017 to July 2018, 7,588 (82%) received ustekinumab for psoriasis or PsA, and 724 (8%) for Crohn’s disease. (The remaining indications were for psoriasis or PsA and Crohn’s disease, or were undetermined.)
Of these patients, 98 experienced SCEs (52 with ACS admitted to the ICU and 46 with strokes). In patients deemed at high cardiovascular risk – those with two risk factors or a personal history of atherosclerotic disease – there was a statistically significant association between starting ustekinumab and SCE occurrence, for an odds ratio of 4.17 (95% confidence interval, 1.19-14.59). In contrast, no such association emerged in ustekinumab users at low cardiovascular risk, for an OR of 0.30 (95% CI, 0.03-3.13). The OR for all was 2.41 (95% CI, 0.83-7.01).
Of the 98 patients included in the final case-time-control analysis, 62 were men (63%), the median age was 57 years, and 76 (78%) were at high cardiovascular risk. A total of 89 patients (91%) had psoriasis, four (4%) had Crohn’s disease, and two (2%) had both.
The investigators also did an analysis including these 98 patients plus 13 patients with ACS who were not hospitalized in an ICU, and 68 with unstable angina, for a total of 179. In this group, the ORs for SCE were 1.75 (95% CI, 0.86-3.56) overall, compared with 3.20 (95% CI, 1.29-7.92) among those at high cardiovascular risk and 0.21 (95% CI, 0.02-1.69) among those at low cardiovascular risk.
The Rennes investigators’ decision to focus on early SCEs stemmed in part from a meta-analysis of randomized clinical trials that reported a possible excess of early SCEs in adults exposed to anti–IL-12/23p40 antibodies, which at that time included the now-discontinued experimental antibody briakinumab. Briakinumab trials were aborted and the drug was never brought to market, leaving ustekinumab as the only antibody of this type.
The finding of “an association between ustekinumab initiation and SCE among patients with cardiovascular risk factors suggests the need for caution regarding the prescription of ustekinumab in this population,” Dr. Poizeau and colleagues wrote. The risk “seems to concern patients with psoriasis” rather than Crohn’s disease, which may be related to the older age and greater cardiovascular risk of the former. “A close collaboration between cardiologists and biologic prescribers could be beneficial to evaluate the risk of SCEs for patients who are receiving ustekinumab,” they added, recommending further research into the physiopathological mechanisms of action.
Offering a U.S. clinician’s perspective on the French study, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, called the findings “unique and interesting with very robust odds ratios. These posttreatment associations have actually been a big area of research over the past decade but not with such defined time periods.”
No significant increases in risk have been seen with other biologics, Dr. Fernandez added, with the exception of briakinumab. “But still, the current study does not definitively answer the question whether ustekinumab can trigger acute events within 6 months of treatment. There’s smoke, but we haven’t clearly seen a fire.”
As to ustekinumab’s possible pathogenic mechanism of action, Dr. Fernandez pointed to data suggesting that IL-17A can be stabilizing to atherosclerotic plaques. “So there’s a hypothesis that blocking the 17/23 pathway may destabilize plaques and make patients more prone to acute cardiovascular events.”
In other comments from clinicians not involved in the study, Seoyoung Kim, MD, ScD, MSCE, director of the program in rheumatologic, immunologic, and musculoskeletal pharmacoepidemiology (PRIME) at Brigham and Women’s Hospital, Boston, noted that, while the investigators controlled for the trend over time and their design choice included time-fixed covariates such as age, sex, and family history within individuals, the case-crossover study could not control for time-varying confounders within individuals.
“In other words, it’s possible that some of the patients had a lot more disease activity and systemic inflammation and used more NSAIDs, steroids, and other medications potentially related to cardiovascular risk a few months before they started ustekinumab, compared with 6-12 months prior,“ Dr. Kim said in an interview. “I would be curious to know if they would find the same thing or not if they studied a different type of biologic drug.”
She also pointed out that the number of outcomes overall was small, leading to imprecise estimates and wide confidence intervals.
Last year Dr. Kim and associates published a study comparing ustekinumab with tumor necrosis factor inhibitor therapy in younger psoriasis and psoriatic arthritis patients and found no difference between the two groups in major cardiovascular events or atrial fibrillation.
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, has more reservations about the findings. “The Poizeau study was methodologically flawed, making the results unreliable,” he said in an interview. “There is a breadth of data from clinical trials and observational studies that do not demonstrate an increased risk of major acute cardiovascular events with ustekinumab and the results of the Poizeau study should not impact clinical practice.”
In an interview, Mark G. Lebwohl, MD, professor and chairman of the department of dermatology and chief for clinical therapeutics at the Icahn School of Medicine at Mount Sinai, New York, said that, in his view, the investigators used early reports of a small number cardiovascular events to look at the issue from a faulty perspective, and hence their findings would have no impact on his clinical practice.
“This study looked at the issue incorrectly. It looked at people put on drug who already had two risk factors for heart attack. And psoriasis itself is a third risk factor,” he said. “So lo and behold, big surprise, some of them had cardiovascular events.”
Dr. Lebwohl noted that a wealth of carefully compiled data has found no increase over time in cardiovascular events with this drug in psoriasis patients. The risk of cardiovascular events actually goes down with time because of the drug’s anti-inflammatory effects.
Dr. Fernandez takes a more positive view of the French findings. “The data certainly support the need for further research in this area,” he said in an interview, “and in the meantime this paper will probably make me extra cautious in using ustekinumab in those at significant risk.”
The French study was supported by a grant from the French National Agency for Medicines and Health Products Safety. Dr. Poizeau and seven coauthors had no disclosures. The remaining five reported disclosures that included receiving fees from AbbVie, Admiral, Amgen, Baxalta, Cologne, Dermavant, Eli Lilly, Janssen, Kyowa Kirin, Novartis, Mylan, Sun Pharmaceuticals, and UCB, as well as grants and personal fees from Boehringer Ingelheim, Leo Pharma, and Pfizer outside the submitted work, and personal fees from Pfizer, AbbVie, UCB Pharma, and Lilly during the conduct of the study. Dr. Fernandez reported consulting work for AbbVie and research grants from Novartis. Dr. Kim disclosed research grants from Brigham and Women’s Hospital and from Pfizer, Abbvie, Roche, and Bristol-Myers Squibb for unrelated studies. Dr. Gelfand reported varying financial ties to Bristol-Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Janssen Biologics, Regeneron, UCB, Sanofi, Pfizer, Celgene, OrthoDermatolgics, AbbVie, Novartis, and Eli Lilly. He is copatent holder of a treatment for cutaneous T-cell lymphoma. Dr. Lebwohl reported unpaid consulting for most manufacturers of psoriasis drugs, with all fees going directly to Mount Sinai.
Source: Poizeau F et al. JAMA Dermatol. 2020 Sep 9. doi: 10.1001/jamadermatol.2020.2977.
in susceptible patients, according to a large French case-time-control analysis.
Investigators led by Florence Poizeau, MD, of the department of dermatology at Rennes (France) University Hospital, found high-risk patients had more than four times the risk of an acute SCE in the 6 months after starting treatment. Although ustekinumab (Stelara) effectively treats moderate to severe psoriasis, psoriatic arthritis (PsA), and Crohn’s disease (indications approved by the Food and Drug Administration), the early months after ustekinumab initiation may be associated with atherosclerotic plaque destabilization via the inhibition of helper T cell subtype 17, the group reported in JAMA Dermatology.
The observational study drew on France’s 66 million–registrant health insurance database to identify all patients exposed to ustekinumab between April 1, 2010, and Dec. 31, 2016. Classified by high or low cardiovascular risk level, ustekinumab recipients served as their own controls, being compared during two time windows: the risk period covered the 6 months after initiating treatment and leading up to the SCE, defined as acute coronary syndrome (ACS) or stroke, while a reference period spanned the 6-12 months leading up to the risk period.
In the statistical analysis of 9,290 ustekinumab-exposed patients (mean age 43 years, 52% male), conducted from September 2017 to July 2018, 7,588 (82%) received ustekinumab for psoriasis or PsA, and 724 (8%) for Crohn’s disease. (The remaining indications were for psoriasis or PsA and Crohn’s disease, or were undetermined.)
Of these patients, 98 experienced SCEs (52 with ACS admitted to the ICU and 46 with strokes). In patients deemed at high cardiovascular risk – those with two risk factors or a personal history of atherosclerotic disease – there was a statistically significant association between starting ustekinumab and SCE occurrence, for an odds ratio of 4.17 (95% confidence interval, 1.19-14.59). In contrast, no such association emerged in ustekinumab users at low cardiovascular risk, for an OR of 0.30 (95% CI, 0.03-3.13). The OR for all was 2.41 (95% CI, 0.83-7.01).
Of the 98 patients included in the final case-time-control analysis, 62 were men (63%), the median age was 57 years, and 76 (78%) were at high cardiovascular risk. A total of 89 patients (91%) had psoriasis, four (4%) had Crohn’s disease, and two (2%) had both.
The investigators also did an analysis including these 98 patients plus 13 patients with ACS who were not hospitalized in an ICU, and 68 with unstable angina, for a total of 179. In this group, the ORs for SCE were 1.75 (95% CI, 0.86-3.56) overall, compared with 3.20 (95% CI, 1.29-7.92) among those at high cardiovascular risk and 0.21 (95% CI, 0.02-1.69) among those at low cardiovascular risk.
The Rennes investigators’ decision to focus on early SCEs stemmed in part from a meta-analysis of randomized clinical trials that reported a possible excess of early SCEs in adults exposed to anti–IL-12/23p40 antibodies, which at that time included the now-discontinued experimental antibody briakinumab. Briakinumab trials were aborted and the drug was never brought to market, leaving ustekinumab as the only antibody of this type.
The finding of “an association between ustekinumab initiation and SCE among patients with cardiovascular risk factors suggests the need for caution regarding the prescription of ustekinumab in this population,” Dr. Poizeau and colleagues wrote. The risk “seems to concern patients with psoriasis” rather than Crohn’s disease, which may be related to the older age and greater cardiovascular risk of the former. “A close collaboration between cardiologists and biologic prescribers could be beneficial to evaluate the risk of SCEs for patients who are receiving ustekinumab,” they added, recommending further research into the physiopathological mechanisms of action.
Offering a U.S. clinician’s perspective on the French study, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, called the findings “unique and interesting with very robust odds ratios. These posttreatment associations have actually been a big area of research over the past decade but not with such defined time periods.”
No significant increases in risk have been seen with other biologics, Dr. Fernandez added, with the exception of briakinumab. “But still, the current study does not definitively answer the question whether ustekinumab can trigger acute events within 6 months of treatment. There’s smoke, but we haven’t clearly seen a fire.”
As to ustekinumab’s possible pathogenic mechanism of action, Dr. Fernandez pointed to data suggesting that IL-17A can be stabilizing to atherosclerotic plaques. “So there’s a hypothesis that blocking the 17/23 pathway may destabilize plaques and make patients more prone to acute cardiovascular events.”
In other comments from clinicians not involved in the study, Seoyoung Kim, MD, ScD, MSCE, director of the program in rheumatologic, immunologic, and musculoskeletal pharmacoepidemiology (PRIME) at Brigham and Women’s Hospital, Boston, noted that, while the investigators controlled for the trend over time and their design choice included time-fixed covariates such as age, sex, and family history within individuals, the case-crossover study could not control for time-varying confounders within individuals.
“In other words, it’s possible that some of the patients had a lot more disease activity and systemic inflammation and used more NSAIDs, steroids, and other medications potentially related to cardiovascular risk a few months before they started ustekinumab, compared with 6-12 months prior,“ Dr. Kim said in an interview. “I would be curious to know if they would find the same thing or not if they studied a different type of biologic drug.”
She also pointed out that the number of outcomes overall was small, leading to imprecise estimates and wide confidence intervals.
Last year Dr. Kim and associates published a study comparing ustekinumab with tumor necrosis factor inhibitor therapy in younger psoriasis and psoriatic arthritis patients and found no difference between the two groups in major cardiovascular events or atrial fibrillation.
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, has more reservations about the findings. “The Poizeau study was methodologically flawed, making the results unreliable,” he said in an interview. “There is a breadth of data from clinical trials and observational studies that do not demonstrate an increased risk of major acute cardiovascular events with ustekinumab and the results of the Poizeau study should not impact clinical practice.”
In an interview, Mark G. Lebwohl, MD, professor and chairman of the department of dermatology and chief for clinical therapeutics at the Icahn School of Medicine at Mount Sinai, New York, said that, in his view, the investigators used early reports of a small number cardiovascular events to look at the issue from a faulty perspective, and hence their findings would have no impact on his clinical practice.
“This study looked at the issue incorrectly. It looked at people put on drug who already had two risk factors for heart attack. And psoriasis itself is a third risk factor,” he said. “So lo and behold, big surprise, some of them had cardiovascular events.”
Dr. Lebwohl noted that a wealth of carefully compiled data has found no increase over time in cardiovascular events with this drug in psoriasis patients. The risk of cardiovascular events actually goes down with time because of the drug’s anti-inflammatory effects.
Dr. Fernandez takes a more positive view of the French findings. “The data certainly support the need for further research in this area,” he said in an interview, “and in the meantime this paper will probably make me extra cautious in using ustekinumab in those at significant risk.”
The French study was supported by a grant from the French National Agency for Medicines and Health Products Safety. Dr. Poizeau and seven coauthors had no disclosures. The remaining five reported disclosures that included receiving fees from AbbVie, Admiral, Amgen, Baxalta, Cologne, Dermavant, Eli Lilly, Janssen, Kyowa Kirin, Novartis, Mylan, Sun Pharmaceuticals, and UCB, as well as grants and personal fees from Boehringer Ingelheim, Leo Pharma, and Pfizer outside the submitted work, and personal fees from Pfizer, AbbVie, UCB Pharma, and Lilly during the conduct of the study. Dr. Fernandez reported consulting work for AbbVie and research grants from Novartis. Dr. Kim disclosed research grants from Brigham and Women’s Hospital and from Pfizer, Abbvie, Roche, and Bristol-Myers Squibb for unrelated studies. Dr. Gelfand reported varying financial ties to Bristol-Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Janssen Biologics, Regeneron, UCB, Sanofi, Pfizer, Celgene, OrthoDermatolgics, AbbVie, Novartis, and Eli Lilly. He is copatent holder of a treatment for cutaneous T-cell lymphoma. Dr. Lebwohl reported unpaid consulting for most manufacturers of psoriasis drugs, with all fees going directly to Mount Sinai.
Source: Poizeau F et al. JAMA Dermatol. 2020 Sep 9. doi: 10.1001/jamadermatol.2020.2977.
Biologics for psoriasis may also reduce coronary plaque
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
Biologics used as treatment for psoriasis may also help reduce lipid-rich necrotic core (LRNC), a high-risk plaque associated with cardiovascular events, recent research from a prospective, observational study suggests.
Cardiac CT scans performed on patients with psoriasis 1 year after starting biologic therapy revealed a reduction in LRNC, compared with patients who were not receiving biologics, according to Harry Choi, MD, of the National Heart, Lung, and Blood Institute at the National Institutes of Health and colleagues. The association with reduction in LRNC and biologic therapy remained significant when adjusted for type of biologic. “These findings demonstrate that LRNC may be modulated by the control of systemic inflammation,” the researchers wrote in their study, published Sept. 15 in Circulation: Cardiovascular Imaging.
Dr. Choi and colleagues evaluated 289 patients with psoriasis within the Psoriasis Atherosclerosis and Cardiometabolic Disease Initiative cohort. The patients had a mean age of 50 years and a mean body mass index of 29.4 kg/m2, as well as a mean Psoriasis Area and Severity Index (PASI) score of 6.0. At baseline, 29% of patients had hypertension, 41% had hyperlipidemia, their mean Framingham risk score was 1.9, and a three-quarters (212 of 289) had mild to moderate psoriasis.
Changes in LRNC were observed at 1 year, compared with baseline prior to and after receiving biologic therapy (124 patients) in comparison with patients who did not undergo biologic therapy (85 patients). Biologic therapies were grouped by type, which included anti–tumor necrosis factor (anti-TNF), anti–interleukin (IL)–12/23, and anti–IL-17 biologics.
There were a significant associations between LRNC and Framingham risk score (standardized beta coefficient, 0.12; 95% confidence interval, 0.00-0.15; P = .045) and severity of psoriasis (beta, 0.13; 95% CI, 0.01-0.26; P = .029) at baseline.
Key findings
The researchers found a significant reduction in LRNC 1 year after patients began biologic therapy (median, 2.97 mm2; interquartile range, 1.99-4.66), compared with baseline (median, 3.12 mm2; IQR, 1.84-4.35) (P = .028), while patients who did not receive biologic therapy had nonsignificantly higher LRNC after 1 year (median, 3.12 mm2; IQR, 1.82-4.60), compared with baseline measurements (median, 3.34 mm2; IQR, 2.04–4.74) (P = .06).
The results remained significant after the researchers adjusted for psoriasis severity, Framingham risk score, BMI, use of statins (beta, −0.09; 95% CI, −0.01 to −0.18; P = .033). Significant reductions in LRNC also remained when analyzing patients receiving anti-TNF, anti–IL-12/23, and anti–IL-17 biologics independently, and there were no significant between-group differences in reduction of LRNC.
The potential of biologics for improving vascular health
Discussing the study results in a press release from the American Heart Association, senior author Nehal N. Mehta, MD, MSCE, FAHA, chief of the Lab of Inflammation and Cardiometabolic Diseases at the NHLBI at NIH, compared the effect biologic therapy had on coronary plaque reduction with that of statins.
“There is approximately 6%-8% reduction in coronary plaque following therapy with statins. Similarly, our treatment with biologic therapy reduced coronary plaque by the same amount after one year. These findings suggest that biologic therapy to treat psoriasis may be just as beneficial as statin therapy on heart arteries,” Dr. Mehta said in the release.
In an interview, Nieca Goldberg, MD, medical director of NYU Women’s Heart Program at NYU Langone Health, echoed Dr. Mehta’s commments and said psoriasis carries the “potential to treat two conditions with the same drug.”
“We know conditions such as psoriatic arthritis and rheumatoid arthritis cause chronic inflammation. Chronic inflammation causes injury to blood vessels and high-risk coronary plaque. Individuals with these inflammatory conditions are at high risk for heart attack,” she said. “This study shows that biologic treatment for psoriatic arthritis can reduce the presence of high-risk plaque. It shows the potential to treat chronic inflammation and high-risk coronary plaque.”
While the results show an association between use of biologics and LRNC reduction, the study design was observational and patients had a short follow-up period. Dr. Goldberg noted more studies are needed to evaluate the effect of biologics on reducing cardiovascular events such as a myocardial infarction.
“We have never before been able to show healing of an inflamed plaque like this in humans. Biologic therapy reduces systemic inflammation and immune activation, and it has a favorable impact on improving overall vascular health,” Dr. Mehta said in the press release. “Imagine if we can treat both psoriasis and coronary heart disease with one therapy – that is the question to be asked in future studies.”
This study was funded with support from the NHLBI Intramural Research Program and the NIH Medical Research Scholars Program at the National Institutes of Health. One investigator reports financial relationships with numerous pharmaceutical companies. The other authors report no relevant conflicts of interest. Dr. Mehta also reports numerous such relationships. Dr. Goldberg reports no relevant conflicts of interest.
SOURCE: Choi H et al. Circ Cardiovasc Imaging. 2020 Sep;13(9):e011199.
FROM CIRCULATION: CARDIOVASCULAR IMAGING
COVID-19 outcomes no worse in patients on TNF inhibitors or methotrexate
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
Continued use of tumor necrosis factor inhibitors or methotrexate is acceptable in most patients who acquire COVID-19, results of a recent cohort study suggest.
Among patients on tumor necrosis factor inhibitors (TNFi) or methotrexate who developed COVID-19, death and hospitalization rates were similar to matched COVID-19 patients not on those medications, according to authors of the multicenter research network study.
Reassuringly, likelihood of hospitalization and mortality were not significantly different between 214 patients with COVID-19 taking TNFi or methotrexate and 31,862 matched COVID-19 patients not on those medications, according to the investigators, whose findings were published recently in the Journal of the American Academy of Dermatology.
Zachary Zinn, MD, corresponding author on the study, said in an interview that the findings suggest these medicines can be safely continued in the majority of patients taking them during the COVID-19 pandemic.
“If you’re a prescribing physician who’s giving patients TNF inhibitors or methotrexate or both, I think you can comfortably tell your patients there is good data that these do not lead to worse outcomes if you get COVID-19,” said Dr. Zinn, associate professor in the department of dermatology at West Virginia University, Morgantown.
The findings from these researchers corroborate a growing body of evidence suggesting that immunosuppressive treatments can be continued in patients with dermatologic and rheumatic conditions.
In recent guidance from the National Psoriasis Foundation, released Sept. 4, an expert consensus panel cited 15 studies that they said suggested that treatments for psoriasis or psoriatic arthritis “do not meaningfully alter the risk of acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.”
That said, the data to date are mainly from small case series and registry studies based on spontaneously reported COVID-19 cases, which suggests a continued need for shared decision making. In addition, chronic systemic corticosteroids should be avoided for management of psoriatic arthritis, the guidance states, based on rheumatology and gastroenterology literature suggesting this treatment is linked to worse COVID-19 outcomes.
In the interview, Dr. Zinn noted that some previous studies of immunosuppressive treatments in patients who acquire COVID-19 have aggregated data on numerous classes of biologic medications, lessening the strength of data for each specific medication.
“By focusing specifically on TNF inhibitors and methotrexate, this study gives better guidance to prescribers of these medications,” he said.
To see whether TNFi or methotrexate increased risk of worsened COVID-19 outcomes, Dr. Zinn and coinvestigators evaluated data from TriNetX, a research network that includes approximately 53 million unique patient records, predominantly in the United States.
They identified 32,076 adult patients with COVID-19, of whom 214 had recent exposure to TNFi or methotrexate. The patients in the TNFi/methotrexate group were similar in age to those without exposure to those drugs, at 55.1 versus 53.2 years, respectively. However, patients in the drug exposure group were more frequently White, female, and had substantially more comorbidities, including diabetes and obesity, according to the investigators.
Nevertheless, the likelihood of hospitalization was not statistically different in the TNFi/methotrexate group versus the non-TNFi/methotrexate group, with a risk ratio of 0.91 (95% confidence interval, 0.68-1.22; P = .5260).
Likewise, the likelihood of death was not different between groups, with a RR of 0.87 (95% CI, 0.42-1.78; P = .6958). Looking at subgroups of patients exposed to TNFi or methotrexate only didn’t change the results, the investigators added.
Taken together, the findings argue against interruption of these treatments because of the fear of the possibly worse COVID-19 outcomes, the investigators concluded, although they emphasized the need for more research.
“Because the COVID-19 pandemic is ongoing, there is a desperate need for evidence-based data on biologic and immunomodulator exposure in the setting of COVID-19 infection,” they wrote.
Dr. Zinn and coauthors reported no conflicts of interest and no funding sources related to the study.
SOURCE: Zinn Z et al. J Am Acad Dermatol. 2020 Sep 11. doi: 10.1016/j.jaad.2020.09.009.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY