User login
How to Reduce Cardiovascular Morbidity and Mortality in Psoriasis and PsA
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.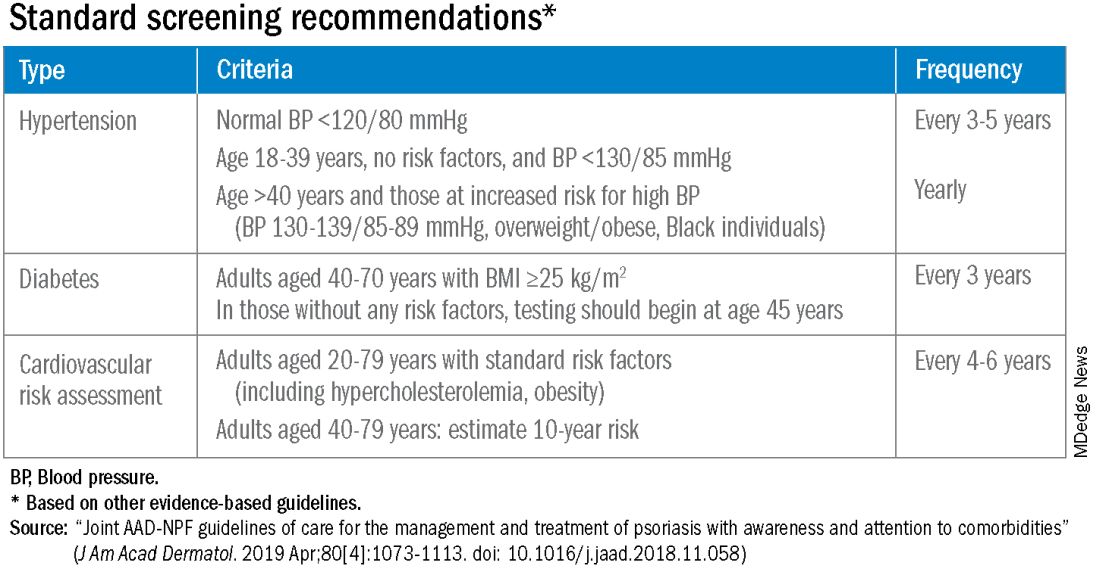
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Progressive joint pain and swelling
Psoriatic arthritis (PsA) is consistent with the patient's joint pain, dactylitis, enthesitis, skin plaques, and radiographic findings, making it the most likely diagnosis.
Rheumatoid arthritis (RA) is possible because of the patient's joint symptoms; however, it is not the correct answer because of negative RF and ACPA tests and skin plaques.
Osteoarthritis might cause joint pain but does not typically present with prolonged morning stiffness, skin plaques, or the "pencil-in-cup" radiographic finding.
Gout, an inflammatory arthritis, primarily affects the big toe and does not align with the patient's skin and radiographic manifestations.
PsA is a chronic inflammatory arthritis that often develops in people with psoriasis. It affects roughly 0.05%- 0.25% of the general population and up to 41% of people with psoriasis. PsA is most seen in White patients between 35 and 55 years and affects both men and women equally. PsA is linked to a higher risk for obesity, hypertension, hyperlipidemia, type 2 diabetes, metabolic syndrome, and other conditions, including uveitis and inflammatory bowel disease.
Clinically, PsA presents with a diverse range of manifestations, encompassing peripheral joint inflammation, often with an asymmetric distribution; axial skeletal involvement reminiscent of spondylitis; dactylitis characterized by sausage-like swelling of fingers or toes; and enthesitis. Common symptoms or findings include early morning stiffness for > 30 minutes; joint pain, tenderness, and swelling; back pain aggravated by rest and relieved by exercise; limited joint motion; and deformity. Although most patients have a preceding condition in skin psoriasis, diagnosis of PsA is often delayed. Furthermore, nearly 80% of patients may exhibit nail changes, such as pitting or onycholysis, compared with about 40% of patients with psoriasis without arthritis. The heterogeneity of its clinical features often necessitates a comprehensive differential diagnosis to distinguish PsA from other spondyloarthropathies and rheumatic diseases. The most accepted classification criteria for PsA are the Classification of Psoriatic Arthritis (CASPAR) criteria, which have been used since 2006.
No laboratory tests are specific for PsA; however, a normal ESR and CRP level should not be used to rule out a diagnosis of PsA because these values are increased in only about 40% of patients. RF and ACPA are classically considered absent in PsA, and a negative RF is regarded as a criterion for diagnosing PsA per the CASPAR classification criteria. Radiographic changes show some characteristic patterns in PsA, including erosive damage, gross joint destruction, joint space narrowing, and "pencil-in-cup" deformity.
PsA treatment options have evolved over the years. Whereas in the past, nonsteroidal anti-inflammatory drugs, glucocorticoids, methotrexate, sulfasalazine, and cyclosporine were commonly prescribed, the development of immunologically targeted biological disease-modifying antirheumatic drugs (DMARDs) and targeted synthetic DMARDs since 2000 has revolutionized the treatment of PsA. Tumor necrosis factor inhibitors (ie, etanercept, infliximab, and adalimumab) have been shown to improve all domains (psoriatic and articular disease) of PsA and are considered a milestone in managing the condition. Other emerging therapeutic strategies in recent years have demonstrated efficacy in treating PsA, including monoclonal antibodies targeting interleukin (IL)-12, IL-23, and IL-17, as well as small-molecule phosphodiesterase 4 and Janus kinase inhibitors.
Although most of these options have the potential to be effective in all clinical domains of the disease, their cross-domain efficacy can vary from patient to patient. In some cases, treatment may not be practical or can lose effectiveness over time, and true disease remission is rare. As a result, clinicians must regularly assess each domain and aim to achieve remission or low disease activity across the different active domains while also being aware of potential adverse events.
Alan Irvine, MD, DSc, Consultant Dermatologist, ADI Dermatology LTD, Dublin, Ireland
Alan Irvine, MD, DSc, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Serve(d) as a speaker or member of a speakers bureau for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Received income in an amount equal to or greater than $250 from: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is consistent with the patient's joint pain, dactylitis, enthesitis, skin plaques, and radiographic findings, making it the most likely diagnosis.
Rheumatoid arthritis (RA) is possible because of the patient's joint symptoms; however, it is not the correct answer because of negative RF and ACPA tests and skin plaques.
Osteoarthritis might cause joint pain but does not typically present with prolonged morning stiffness, skin plaques, or the "pencil-in-cup" radiographic finding.
Gout, an inflammatory arthritis, primarily affects the big toe and does not align with the patient's skin and radiographic manifestations.
PsA is a chronic inflammatory arthritis that often develops in people with psoriasis. It affects roughly 0.05%- 0.25% of the general population and up to 41% of people with psoriasis. PsA is most seen in White patients between 35 and 55 years and affects both men and women equally. PsA is linked to a higher risk for obesity, hypertension, hyperlipidemia, type 2 diabetes, metabolic syndrome, and other conditions, including uveitis and inflammatory bowel disease.
Clinically, PsA presents with a diverse range of manifestations, encompassing peripheral joint inflammation, often with an asymmetric distribution; axial skeletal involvement reminiscent of spondylitis; dactylitis characterized by sausage-like swelling of fingers or toes; and enthesitis. Common symptoms or findings include early morning stiffness for > 30 minutes; joint pain, tenderness, and swelling; back pain aggravated by rest and relieved by exercise; limited joint motion; and deformity. Although most patients have a preceding condition in skin psoriasis, diagnosis of PsA is often delayed. Furthermore, nearly 80% of patients may exhibit nail changes, such as pitting or onycholysis, compared with about 40% of patients with psoriasis without arthritis. The heterogeneity of its clinical features often necessitates a comprehensive differential diagnosis to distinguish PsA from other spondyloarthropathies and rheumatic diseases. The most accepted classification criteria for PsA are the Classification of Psoriatic Arthritis (CASPAR) criteria, which have been used since 2006.
No laboratory tests are specific for PsA; however, a normal ESR and CRP level should not be used to rule out a diagnosis of PsA because these values are increased in only about 40% of patients. RF and ACPA are classically considered absent in PsA, and a negative RF is regarded as a criterion for diagnosing PsA per the CASPAR classification criteria. Radiographic changes show some characteristic patterns in PsA, including erosive damage, gross joint destruction, joint space narrowing, and "pencil-in-cup" deformity.
PsA treatment options have evolved over the years. Whereas in the past, nonsteroidal anti-inflammatory drugs, glucocorticoids, methotrexate, sulfasalazine, and cyclosporine were commonly prescribed, the development of immunologically targeted biological disease-modifying antirheumatic drugs (DMARDs) and targeted synthetic DMARDs since 2000 has revolutionized the treatment of PsA. Tumor necrosis factor inhibitors (ie, etanercept, infliximab, and adalimumab) have been shown to improve all domains (psoriatic and articular disease) of PsA and are considered a milestone in managing the condition. Other emerging therapeutic strategies in recent years have demonstrated efficacy in treating PsA, including monoclonal antibodies targeting interleukin (IL)-12, IL-23, and IL-17, as well as small-molecule phosphodiesterase 4 and Janus kinase inhibitors.
Although most of these options have the potential to be effective in all clinical domains of the disease, their cross-domain efficacy can vary from patient to patient. In some cases, treatment may not be practical or can lose effectiveness over time, and true disease remission is rare. As a result, clinicians must regularly assess each domain and aim to achieve remission or low disease activity across the different active domains while also being aware of potential adverse events.
Alan Irvine, MD, DSc, Consultant Dermatologist, ADI Dermatology LTD, Dublin, Ireland
Alan Irvine, MD, DSc, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Serve(d) as a speaker or member of a speakers bureau for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Received income in an amount equal to or greater than $250 from: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is consistent with the patient's joint pain, dactylitis, enthesitis, skin plaques, and radiographic findings, making it the most likely diagnosis.
Rheumatoid arthritis (RA) is possible because of the patient's joint symptoms; however, it is not the correct answer because of negative RF and ACPA tests and skin plaques.
Osteoarthritis might cause joint pain but does not typically present with prolonged morning stiffness, skin plaques, or the "pencil-in-cup" radiographic finding.
Gout, an inflammatory arthritis, primarily affects the big toe and does not align with the patient's skin and radiographic manifestations.
PsA is a chronic inflammatory arthritis that often develops in people with psoriasis. It affects roughly 0.05%- 0.25% of the general population and up to 41% of people with psoriasis. PsA is most seen in White patients between 35 and 55 years and affects both men and women equally. PsA is linked to a higher risk for obesity, hypertension, hyperlipidemia, type 2 diabetes, metabolic syndrome, and other conditions, including uveitis and inflammatory bowel disease.
Clinically, PsA presents with a diverse range of manifestations, encompassing peripheral joint inflammation, often with an asymmetric distribution; axial skeletal involvement reminiscent of spondylitis; dactylitis characterized by sausage-like swelling of fingers or toes; and enthesitis. Common symptoms or findings include early morning stiffness for > 30 minutes; joint pain, tenderness, and swelling; back pain aggravated by rest and relieved by exercise; limited joint motion; and deformity. Although most patients have a preceding condition in skin psoriasis, diagnosis of PsA is often delayed. Furthermore, nearly 80% of patients may exhibit nail changes, such as pitting or onycholysis, compared with about 40% of patients with psoriasis without arthritis. The heterogeneity of its clinical features often necessitates a comprehensive differential diagnosis to distinguish PsA from other spondyloarthropathies and rheumatic diseases. The most accepted classification criteria for PsA are the Classification of Psoriatic Arthritis (CASPAR) criteria, which have been used since 2006.
No laboratory tests are specific for PsA; however, a normal ESR and CRP level should not be used to rule out a diagnosis of PsA because these values are increased in only about 40% of patients. RF and ACPA are classically considered absent in PsA, and a negative RF is regarded as a criterion for diagnosing PsA per the CASPAR classification criteria. Radiographic changes show some characteristic patterns in PsA, including erosive damage, gross joint destruction, joint space narrowing, and "pencil-in-cup" deformity.
PsA treatment options have evolved over the years. Whereas in the past, nonsteroidal anti-inflammatory drugs, glucocorticoids, methotrexate, sulfasalazine, and cyclosporine were commonly prescribed, the development of immunologically targeted biological disease-modifying antirheumatic drugs (DMARDs) and targeted synthetic DMARDs since 2000 has revolutionized the treatment of PsA. Tumor necrosis factor inhibitors (ie, etanercept, infliximab, and adalimumab) have been shown to improve all domains (psoriatic and articular disease) of PsA and are considered a milestone in managing the condition. Other emerging therapeutic strategies in recent years have demonstrated efficacy in treating PsA, including monoclonal antibodies targeting interleukin (IL)-12, IL-23, and IL-17, as well as small-molecule phosphodiesterase 4 and Janus kinase inhibitors.
Although most of these options have the potential to be effective in all clinical domains of the disease, their cross-domain efficacy can vary from patient to patient. In some cases, treatment may not be practical or can lose effectiveness over time, and true disease remission is rare. As a result, clinicians must regularly assess each domain and aim to achieve remission or low disease activity across the different active domains while also being aware of potential adverse events.
Alan Irvine, MD, DSc, Consultant Dermatologist, ADI Dermatology LTD, Dublin, Ireland
Alan Irvine, MD, DSc, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Serve(d) as a speaker or member of a speakers bureau for: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Received income in an amount equal to or greater than $250 from: Sanofi; Abbvie; Regeneron; Leo; Pfizer; Janssen.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 45-year-old man visited the rheumatology clinic with a 6-month history of progressive joint pain and swelling. He described experiencing morning stiffness that lasted about an hour, with the pain showing improvement with activity. Interestingly, he also mentioned having rashes for the past 10 years, which he initially attributed to eczema and managed with over-the-counter creams.
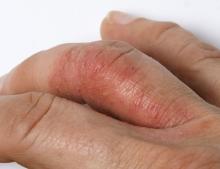
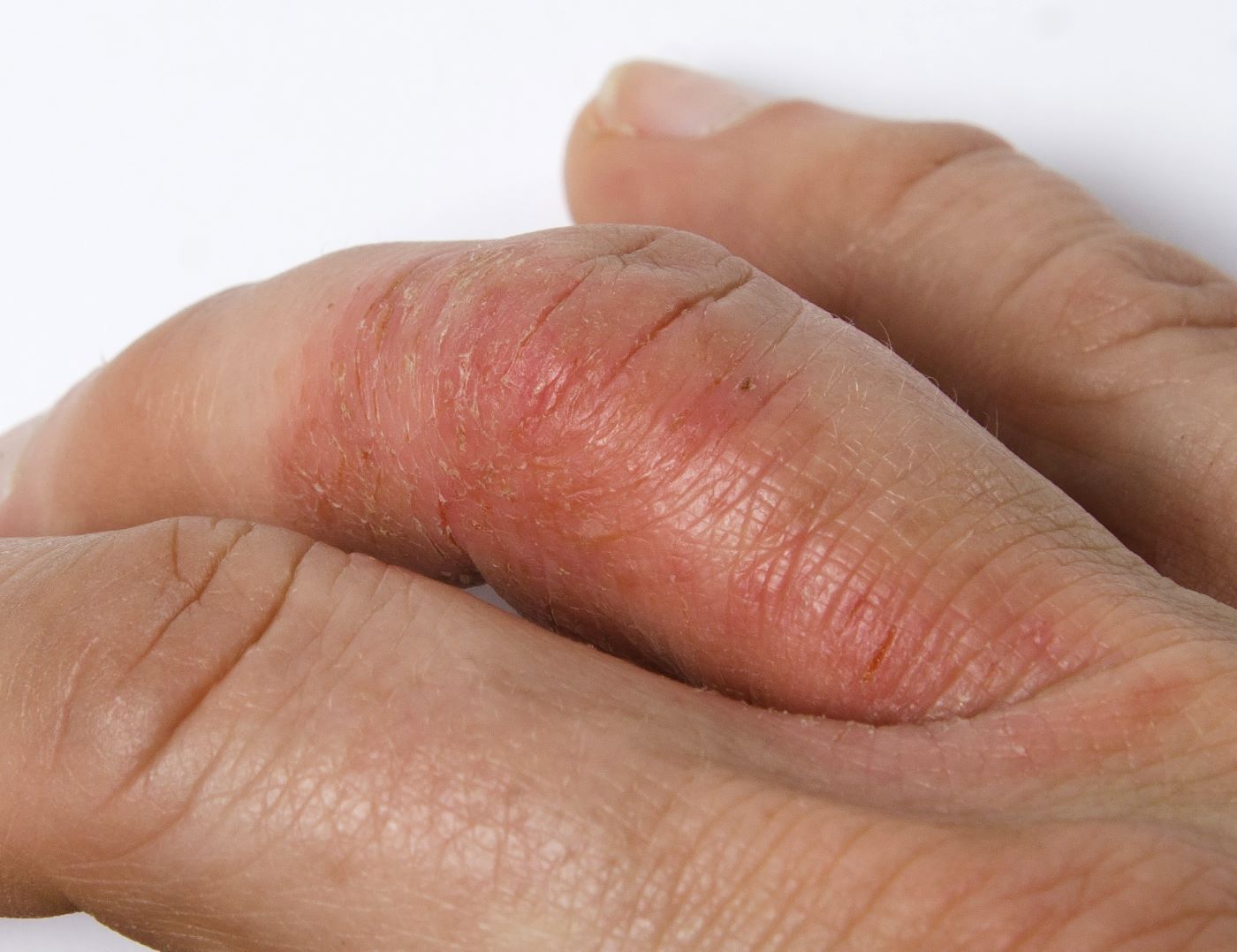
On physical examination, there was noticeable swelling and tenderness in the distal interphalangeal (DIP) joints of both hands. The fourth finger on the right hand exhibited dactylitis with a well-circumscribed, erythematous, scaly lesion (see image). Physical exam suggested enthesitis at the insertion of the Achilles tendon. Skin examination revealed plaques with a characteristic silver scaling on the elbows and knees. Laboratory tests indicated elevated C-reactive protein (CRP) levels and erythrocyte sedimentation rate (ESR). Notably, both the rheumatoid factor (RF) and anti-citrullinated protein antibody (ACPA) tests returned negative results. Radiography of the hands showed periarticular erosions and a "pencil-in-cup" deformity at the DIP joints.
Bimekizumab shows promise for palmoplantar pustular psoriasis
.
PPP is a type of pustular psoriasis that remains a treatment challenge, and available treatments for palmoplantar plaque psoriasis with pustules also “remain unsatisfactory,” according to Thierry Passeron, MD, PhD, of the dermatology service at Centre Hospitalier Universitaire de Nice (France), and colleagues. Bimekizumab, an anti-interleukin (IL)-17A and anti-IL-17F antibody therapy, has been used for psoriasis and psoriatic arthritis (PsA), but its effectiveness for PPP has not been studied, they said. In the United States, bimekizumab (Bimzelx), administered subcutaneously, was recently approved for treating moderate to severe plaque psoriasis in adults; in the European Union, it is approved for treating psoriasis, in addition to psoriatic arthritis, axial spondyloarthritis and ankylosing spondylitis.
In the case series published in JAMA Dermatology, Dr. Passeron and coinvestigators identified 11 adults with PPP and 10 with palmoplantar plaque psoriasis with pustules who were treated at one of seven tertiary dermatology centers in France from September 2022 through June 2023. PPP also has been associated with bone and joint inflammation in SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome.
All patients received bimekizumab for at least 3 months. The patients — 19 women and 2 men — ranged in age from 24 to 68 years (mean age, 46 years). The primary outcome was complete clearance, defined as an Investigator Global Assessment (IGA) score of 0.
A total of 17 patients achieved an IGA score of zero in 1-4 months. Over 3-6 months, three patients achieved an IGA score of 1 (almost clear), and one patient achieved an IGA score of 2 (mild).
Three patients with PPP also had acrodermatitis continua of Hallopeau; in these patients, nail involvement improved by 50%-70% after 4-6 months of bimekizumab use. Two patients with SAPHO experienced complete clearance of skin lesions associated with improvement in joint pain.
Four patients developed oral and genital candidiasis during treatment, but all were treated successfully with antifungals. None of the patients discontinued bimekizumab because of adverse events. “All patients are still receiving treatment, and their psoriatic lesions remain controlled,” the authors wrote.
“The rapid and consistent improvement observed in the present case series supports the effectiveness of bimekizumab therapy in managing PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome,” they said in their discussion.
The study findings were limited by several factors including the small sample size and short follow-up period, and by the inclusion of only patients with severe disease; and prospective, placebo-controlled studies are needed to confirm the results, the researchers noted.
However, the results suggest that bimekizumab could be a treatment approach for PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome, and warrant a prospective, randomized, placebo-controlled, randomized clinical trial to confirm the findings, they concluded.
Dr. Passeron disclosed fees from AbbVie, ACM Pharma, Almirall, Boehringer Ingelheim, Bristol Myers Squibb, Calypso, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte, Janssen, LEO Pharma, Eli Lilly, Novartis, Roivant Sciences, Sun Pharmaceuticals, and VYNE Therapeutics outside the current study; he is a cofounder of Yukin Therapeutics. Three authors disclosed receiving personal fees from UCB, manufacturer of bimekizumab, outside of the submitted work, another author disclosed receiving personal fees from UCB during the conduct of the study, and another reported receiving grants from UCB and several other companies, outside the submitted work.
The study findings were also presented at a meeting, Les Journées Dermatologiques de Paris 2023, on December 6, in Paris.
.
PPP is a type of pustular psoriasis that remains a treatment challenge, and available treatments for palmoplantar plaque psoriasis with pustules also “remain unsatisfactory,” according to Thierry Passeron, MD, PhD, of the dermatology service at Centre Hospitalier Universitaire de Nice (France), and colleagues. Bimekizumab, an anti-interleukin (IL)-17A and anti-IL-17F antibody therapy, has been used for psoriasis and psoriatic arthritis (PsA), but its effectiveness for PPP has not been studied, they said. In the United States, bimekizumab (Bimzelx), administered subcutaneously, was recently approved for treating moderate to severe plaque psoriasis in adults; in the European Union, it is approved for treating psoriasis, in addition to psoriatic arthritis, axial spondyloarthritis and ankylosing spondylitis.
In the case series published in JAMA Dermatology, Dr. Passeron and coinvestigators identified 11 adults with PPP and 10 with palmoplantar plaque psoriasis with pustules who were treated at one of seven tertiary dermatology centers in France from September 2022 through June 2023. PPP also has been associated with bone and joint inflammation in SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome.
All patients received bimekizumab for at least 3 months. The patients — 19 women and 2 men — ranged in age from 24 to 68 years (mean age, 46 years). The primary outcome was complete clearance, defined as an Investigator Global Assessment (IGA) score of 0.
A total of 17 patients achieved an IGA score of zero in 1-4 months. Over 3-6 months, three patients achieved an IGA score of 1 (almost clear), and one patient achieved an IGA score of 2 (mild).
Three patients with PPP also had acrodermatitis continua of Hallopeau; in these patients, nail involvement improved by 50%-70% after 4-6 months of bimekizumab use. Two patients with SAPHO experienced complete clearance of skin lesions associated with improvement in joint pain.
Four patients developed oral and genital candidiasis during treatment, but all were treated successfully with antifungals. None of the patients discontinued bimekizumab because of adverse events. “All patients are still receiving treatment, and their psoriatic lesions remain controlled,” the authors wrote.
“The rapid and consistent improvement observed in the present case series supports the effectiveness of bimekizumab therapy in managing PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome,” they said in their discussion.
The study findings were limited by several factors including the small sample size and short follow-up period, and by the inclusion of only patients with severe disease; and prospective, placebo-controlled studies are needed to confirm the results, the researchers noted.
However, the results suggest that bimekizumab could be a treatment approach for PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome, and warrant a prospective, randomized, placebo-controlled, randomized clinical trial to confirm the findings, they concluded.
Dr. Passeron disclosed fees from AbbVie, ACM Pharma, Almirall, Boehringer Ingelheim, Bristol Myers Squibb, Calypso, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte, Janssen, LEO Pharma, Eli Lilly, Novartis, Roivant Sciences, Sun Pharmaceuticals, and VYNE Therapeutics outside the current study; he is a cofounder of Yukin Therapeutics. Three authors disclosed receiving personal fees from UCB, manufacturer of bimekizumab, outside of the submitted work, another author disclosed receiving personal fees from UCB during the conduct of the study, and another reported receiving grants from UCB and several other companies, outside the submitted work.
The study findings were also presented at a meeting, Les Journées Dermatologiques de Paris 2023, on December 6, in Paris.
.
PPP is a type of pustular psoriasis that remains a treatment challenge, and available treatments for palmoplantar plaque psoriasis with pustules also “remain unsatisfactory,” according to Thierry Passeron, MD, PhD, of the dermatology service at Centre Hospitalier Universitaire de Nice (France), and colleagues. Bimekizumab, an anti-interleukin (IL)-17A and anti-IL-17F antibody therapy, has been used for psoriasis and psoriatic arthritis (PsA), but its effectiveness for PPP has not been studied, they said. In the United States, bimekizumab (Bimzelx), administered subcutaneously, was recently approved for treating moderate to severe plaque psoriasis in adults; in the European Union, it is approved for treating psoriasis, in addition to psoriatic arthritis, axial spondyloarthritis and ankylosing spondylitis.
In the case series published in JAMA Dermatology, Dr. Passeron and coinvestigators identified 11 adults with PPP and 10 with palmoplantar plaque psoriasis with pustules who were treated at one of seven tertiary dermatology centers in France from September 2022 through June 2023. PPP also has been associated with bone and joint inflammation in SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome.
All patients received bimekizumab for at least 3 months. The patients — 19 women and 2 men — ranged in age from 24 to 68 years (mean age, 46 years). The primary outcome was complete clearance, defined as an Investigator Global Assessment (IGA) score of 0.
A total of 17 patients achieved an IGA score of zero in 1-4 months. Over 3-6 months, three patients achieved an IGA score of 1 (almost clear), and one patient achieved an IGA score of 2 (mild).
Three patients with PPP also had acrodermatitis continua of Hallopeau; in these patients, nail involvement improved by 50%-70% after 4-6 months of bimekizumab use. Two patients with SAPHO experienced complete clearance of skin lesions associated with improvement in joint pain.
Four patients developed oral and genital candidiasis during treatment, but all were treated successfully with antifungals. None of the patients discontinued bimekizumab because of adverse events. “All patients are still receiving treatment, and their psoriatic lesions remain controlled,” the authors wrote.
“The rapid and consistent improvement observed in the present case series supports the effectiveness of bimekizumab therapy in managing PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome,” they said in their discussion.
The study findings were limited by several factors including the small sample size and short follow-up period, and by the inclusion of only patients with severe disease; and prospective, placebo-controlled studies are needed to confirm the results, the researchers noted.
However, the results suggest that bimekizumab could be a treatment approach for PPP, palmoplantar plaque psoriasis with pustules, and SAPHO syndrome, and warrant a prospective, randomized, placebo-controlled, randomized clinical trial to confirm the findings, they concluded.
Dr. Passeron disclosed fees from AbbVie, ACM Pharma, Almirall, Boehringer Ingelheim, Bristol Myers Squibb, Calypso, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte, Janssen, LEO Pharma, Eli Lilly, Novartis, Roivant Sciences, Sun Pharmaceuticals, and VYNE Therapeutics outside the current study; he is a cofounder of Yukin Therapeutics. Three authors disclosed receiving personal fees from UCB, manufacturer of bimekizumab, outside of the submitted work, another author disclosed receiving personal fees from UCB during the conduct of the study, and another reported receiving grants from UCB and several other companies, outside the submitted work.
The study findings were also presented at a meeting, Les Journées Dermatologiques de Paris 2023, on December 6, in Paris.
FROM JAMA DERMATOLOGY
Uveitis Associated with Psoriatic Arthritis: Characteristics, Approaches, and Treatment
With the growing number of treatment options for psoriatic arthritis (PsA), therapeutic decision-making has shifted to an increasingly tailored and patient-centered approach. A number of factors contribute to the treatment decision-making process, including age, insurance restrictions, route of administration, side effect profile, comorbidities, and extra-articular manifestations of the disease. In this article, we discuss an extra-articular comorbidity, uveitis, which is frequently seen in patients with PsA. We discuss clinical characteristics of uveitis associated with PsA and describe how the presence of uveitis influences our treatment approach to PsA, based on existing data.
Uveitis refers broadly to inflammation of the uvea, the vascularized and pigmented layer of the eye composed of the iris, the ciliary body, and the choroid. While infection is a common cause of uveitis, many cases are noninfectious and are often associated with an underlying autoimmune or systemic inflammatory disorder. Uveitis is frequently reported in diseases in the spondyloarthritis (SpA) family, including axial spondyloarthritis (AxSpA) and reactive arthritis, as well as PsA. Exact estimates of the prevalence of uveitis in PsA vary widely from 7%-25%, depending on the particular cohort studied.1,2 In all forms of SpA, the anterior chamber of the uvea is the most likely to be affected.3 However, compared to patients with AxSpA, patients with PsA appear to have a higher rate of posterior involvement. In addition, patients with PsA appear to have higher frequencies of insidious, bilateral uveitis, as compared to the acute, unilateral, anterior uveitis that is most characteristic of AxSpA.4 Women with PsA may be more likely than men to experience uveitis, although this has not been a consistent finding.5
Patients with PsA who are human leukocyte antigen B27 (HLA-B27) positive may be at risk for more severe and refractory anterior uveitis compared to those who do not express the allele.5 Those who are HLA-B27 positive are also known to have higher rates of axial involvement. It has therefore been postulated that 2 phenotypes of uveitis may exist in PsA: patients who are HLA-B27 positive who have axial disease and severe, unilateral anterior uveitis reminiscent of other forms of SpA, and patients who are HLA-B27 negative, often women, with peripheral-predominant arthritis who are prone to the classic anterior uveitis but may also develop atypical bilateral, insidious, and/or posterior involvement.4 Specific characteristics of PsA may also provide information about the risk for developing uveitis. For example, dactylitis has been linked to a higher risk of developing uveitis in some, but not all, cohorts of patients with PsA, and the risk of uveitis in PsA has been found in many studies to correlate with longer duration of disease.6-8
The presence of uveitis signals a disruption in the blood-retina barrier and the subsequent entrance of inflammatory cells into the eye. An entire explanation of pathogenesis is beyond the scope of this article; however, it is worth noting that many of the inflammatory mediators of active uveitis mirror those of PsA. For instance, both the mesenchymal cells in enthesitis and the cells of the ciliary body express receptors for interleukin (IL)-23, suggesting a potential role of the signaling pathways involving this cytokine in both diseases.9 Another study found increased serum levels of IL-17, a known mediator of PsA disease, in patients with active uveitis.10 Despite these common pathogenesis links, there are limited data on the utility of certain existing PsA treatments on uveitis manifestations.
Our approach is always to manage uveitis associated with PsA in collaboration with a specialized and experienced ophthalmologist. Uncontrolled uveitis can be vision threatening and contribute to long-term morbidity associated with PsA, so timely recognition, evaluation, and appropriate treatment are important. Ocular glucocorticoid (GC) drops may be used as first-line therapy, particularly for anterior uveitis, to quickly quell inflammation. Escalation to systemic GCs for more severe or posteriorly localized disease may be considered carefully, given the known risk of worsening skin psoriasis (PsO) with GC withdrawal after a course of therapy. Use of GC-sparing therapy should be determined on a case-by-case basis. While generalized, noninfectious uveitis often resolves with GC treatment, the risk of uveitis recurrence in patients with PsA and the challenges of systemic GCs with PsO lead us to frequently consider GC-sparing therapy that addresses ocular, musculoskeletal, and cutaneous manifestations. Tumor necrosis factor inhibitors (TNF-I) are our typical first-line considerations for GC-sparing therapy in patients with PsA with inflammatory joint symptoms and uveitis, although nonbiologic therapy can be considered first-line therapy in select populations.
Data establishing the efficacy of TNF-I come largely from randomized controlled trials (RCTs) of adalimumab (ADA) compared to placebo in noninfectious uveitis.11 While these trials focused on idiopathic posterior or pan-uveitis, these data have been extrapolated to SpA-associated anterior uveitis, and large registry analyses have supported use of TNF-I in this population.12 When selecting a particular TNF-I in a patient with current or past uveitis, we frequently start with ADA, based on supportive, albeit uncontrolled, data suggesting a reduction in the risk of recurrence with this agent in patients with SpA and uveitis.12 For patients who are unable to tolerate subcutaneous injections, who fail ADA, or who we suspect will require higher, titratable dosing, we favor infliximab infusions. Other data suggest that golimumab and certolizumab are also reasonable alternatives.13,14 We do not generally use etanercept, as the limited data that are available suggest that it is less effective at reducing risk of uveitis recurrence.12 Methotrexate or leflunomide may be an appropriate first line choice for patients with peripheral-predominant PsA and uveitis, but it is important to note that these agents are not effective for axial disease.
Despite the mechanistic data implicating the role of IL-17 in uveitis associated with PsA, the IL-17A inhibitor, secukinumab, failed to show a reduction in uveitis recurrence, compared to placebo, in pooled analysis of RCTs of noninfectious uveitis.15 However, a phase 2 trial of intravenous secukinumab in noninfectious uveitis showed promise, possibly because this dosing regimen can achieve higher effective concentrations.16 It is not our current practice to use secukinumab or ixekizumab as a first-line therapy in patients with PsA and concurrent uveitis, owing to a lack of data supporting efficacy. A novel IL-17A/F inhibitor, bimekizumab (BKZ), has recently been used in several successful phase 3 trials in patients with both TNF-naïve and TNF-nonresponder SpA, including AxSpA and PsA.17 Interestingly, data from the phase 2 and 3 trials of BKZ found low incidence rates of uveitis in patients with SpA treated with BKZ compared to placebo, suggesting that BKZ might be more effective in uveitis than other IL-17 inhibitors, but these data need to be confirmed.
Successful use of Janus kinase (JAK) inhibitors in noninfectious uveitis, including cases associated with inflammatory arthritis, has been described in case reports as well as in current phase 2 trials.18 The dual IL-12/IL-23 inhibitor, ustekinumab, also showed initial promise in a small, nonrandomized, uncontrolled phase 1/2 study of the treatment of posterior uveitis, as well as success in few case reports of PsA-associated uveitis.19 However, a post-hoc analysis of extra-intestinal manifestations, including uveitis and iritis, in patients with inflammatory bowel disease treated with ustekinumab found no benefit in preventing or treating ocular disease compared to placebo.20 Given the paucity of available data, JAK inhibitors and the IL-12/IL-23 inhibitor, ustekinumab, are not part of our typical treatment algorithm for patients with PsA-associated uveitis.
In conclusion, uveitis is a frequent extra-articular comorbidity of PsA, and it may present differently than the typical acute onset, unilateral anterior uveitis seen in SpA. While uveitis may share many different immunologic threads with PsA, the most convincing data support the use of TNF-I as a GC-sparing agent in this setting, particularly ADA, infliximab, golimumab, or certolizumab. Our approach is generally to start with these agents or methotrexate when directed therapy is needed for uveitis in PsA. Further investigation into the use of the IL-17A/F inhibitor BKZ and JAK inhibitors, as well as tyrosine kinase 2 inhibitors, in PsA associated uveitis may yield additional options for our patients.21
1. De Vicente Delmas A, Sanchez-Bilbao L, Calvo-Rio V, et al. Uveitis in psoriatic arthritis: study of 406 patients in a single university center and literature review. RMD Open. 2023;9(1):e002781.
2. Rademacher J, Poddubnyy D, Pleyer U. Uveitis in spondyloarthritis. Ther Adv Musculoskelet Dis. 2020;12:1759720X20951733.
3. Zeboulon N, Dougados M, Gossec L. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis. 2008;67(7):955-959.
4. Paiva ES, Macaluso DC, Edwards A, Rosenbaum JT. Characterisation of uveitis in patients with psoriatic arthritis. Ann Rheum Dis. 2000;59(1):67-70.
5. Fraga NA, Oliveira Mde F, Follador I, Rocha Bde O, Rego VR. Psoriasis and uveitis: a literature review. An Bras Dermatol. 2012;87(6):877-883.
6. Niccoli L, Nannini C, Cassara E, et al. Frequency of iridocyclitis in patients with early psoriatic arthritis: a prospective, follow up study. Int J Rheum Dis. 2012;15(4):414-418.
7. Yasar Bilge NS, Kalyoncu U, Atagunduz P, et al. Uveitis-related factors in patients with spondyloarthritis: TReasure Real-Life Results. Am J Ophthalmol. 2021;228:58-64.
8. Chia AYT, Ang GWX, Chan ASY, Chan W, Chong TKY, Leung YY. Managing psoriatic arthritis with inflammatory bowel disease and/or uveitis. Front Med (Lausanne). 2021;8:737256.
9. Reinhardt A, Yevsa T, Worbs T, et al. Interleukin-23-dependent gamma/delta T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol. 2016;68(10):2476-2486.
10. Jawad S, Liu B, Agron E, Nussenblatt RB, Sen HN. Elevated serum levels of interleukin-17A in uveitis patients. Ocul Immunol Inflamm. 2013;21(6):434-439.
11. Merrill PT, Vitale A, Zierhut M, et al. Efficacy of adalimumab in non-infectious uveitis across different etiologies: a post hoc analysis of the VISUAL I and VISUAL II Trials. Ocul Immunol Inflamm. 2021;29(7-8):1569-1575.
12. Lie E, Lindstrom U, Zverkova-Sandstrom T, et al. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis: results from the Swedish biologics register. Ann Rheum Dis. 2017;76(9):1515-1521.
13. van der Horst-Bruinsma I, van Bentum R, Verbraak FD, et al. The impact of certolizumab pegol treatment on the incidence of anterior uveitis flares in patients with axial spondyloarthritis: 48-week interim results from C-VIEW. RMD Open. 2020;6(1):e001161.
14. Calvo-Rio V, Blanco R, Santos-Gomez M, et al. Golimumab in refractory uveitis related to spondyloarthritis. Multicenter study of 15 patients. Semin Arthritis Rheum. 2016;46(1):95-101.
15. Dick AD, Tugal-Tutkun I, Foster S, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120(4):777-787.
16. Letko E, Yeh S, Foster CS, et al. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology. 2015;122(5):939-948.
17. van der Heijde D, Deodhar A, Baraliakos X, et al. Efficacy and safety of bimekizumab in axial spondyloarthritis: results of two parallel phase 3 randomised controlled trials. Ann Rheum Dis. 2023;82(4):515-526.
18. Dhillon S, Keam SJ. Filgotinib: first approval. Drugs. 2020;80(18):1987-1997.
19. Pepple KL, Lin P. Targeting interleukin-23 in the treatment of noninfectious uveitis. Ophthalmology. 2018;125(12):1977-1983.
20. Narula N, Aruljothy A, Wong ECL, et al. The impact of ustekinumab on extraintestinal manifestations of Crohn’s disease: a post hoc analysis of the UNITI studies. United European Gastroenterol J. 2021;9(5):581-589.
21. Rusinol L, Puig L. Tyk2 targeting in immune-mediated inflammatory diseases. Int J Mol Sci. 2023;24(4):3391.
With the growing number of treatment options for psoriatic arthritis (PsA), therapeutic decision-making has shifted to an increasingly tailored and patient-centered approach. A number of factors contribute to the treatment decision-making process, including age, insurance restrictions, route of administration, side effect profile, comorbidities, and extra-articular manifestations of the disease. In this article, we discuss an extra-articular comorbidity, uveitis, which is frequently seen in patients with PsA. We discuss clinical characteristics of uveitis associated with PsA and describe how the presence of uveitis influences our treatment approach to PsA, based on existing data.
Uveitis refers broadly to inflammation of the uvea, the vascularized and pigmented layer of the eye composed of the iris, the ciliary body, and the choroid. While infection is a common cause of uveitis, many cases are noninfectious and are often associated with an underlying autoimmune or systemic inflammatory disorder. Uveitis is frequently reported in diseases in the spondyloarthritis (SpA) family, including axial spondyloarthritis (AxSpA) and reactive arthritis, as well as PsA. Exact estimates of the prevalence of uveitis in PsA vary widely from 7%-25%, depending on the particular cohort studied.1,2 In all forms of SpA, the anterior chamber of the uvea is the most likely to be affected.3 However, compared to patients with AxSpA, patients with PsA appear to have a higher rate of posterior involvement. In addition, patients with PsA appear to have higher frequencies of insidious, bilateral uveitis, as compared to the acute, unilateral, anterior uveitis that is most characteristic of AxSpA.4 Women with PsA may be more likely than men to experience uveitis, although this has not been a consistent finding.5
Patients with PsA who are human leukocyte antigen B27 (HLA-B27) positive may be at risk for more severe and refractory anterior uveitis compared to those who do not express the allele.5 Those who are HLA-B27 positive are also known to have higher rates of axial involvement. It has therefore been postulated that 2 phenotypes of uveitis may exist in PsA: patients who are HLA-B27 positive who have axial disease and severe, unilateral anterior uveitis reminiscent of other forms of SpA, and patients who are HLA-B27 negative, often women, with peripheral-predominant arthritis who are prone to the classic anterior uveitis but may also develop atypical bilateral, insidious, and/or posterior involvement.4 Specific characteristics of PsA may also provide information about the risk for developing uveitis. For example, dactylitis has been linked to a higher risk of developing uveitis in some, but not all, cohorts of patients with PsA, and the risk of uveitis in PsA has been found in many studies to correlate with longer duration of disease.6-8
The presence of uveitis signals a disruption in the blood-retina barrier and the subsequent entrance of inflammatory cells into the eye. An entire explanation of pathogenesis is beyond the scope of this article; however, it is worth noting that many of the inflammatory mediators of active uveitis mirror those of PsA. For instance, both the mesenchymal cells in enthesitis and the cells of the ciliary body express receptors for interleukin (IL)-23, suggesting a potential role of the signaling pathways involving this cytokine in both diseases.9 Another study found increased serum levels of IL-17, a known mediator of PsA disease, in patients with active uveitis.10 Despite these common pathogenesis links, there are limited data on the utility of certain existing PsA treatments on uveitis manifestations.
Our approach is always to manage uveitis associated with PsA in collaboration with a specialized and experienced ophthalmologist. Uncontrolled uveitis can be vision threatening and contribute to long-term morbidity associated with PsA, so timely recognition, evaluation, and appropriate treatment are important. Ocular glucocorticoid (GC) drops may be used as first-line therapy, particularly for anterior uveitis, to quickly quell inflammation. Escalation to systemic GCs for more severe or posteriorly localized disease may be considered carefully, given the known risk of worsening skin psoriasis (PsO) with GC withdrawal after a course of therapy. Use of GC-sparing therapy should be determined on a case-by-case basis. While generalized, noninfectious uveitis often resolves with GC treatment, the risk of uveitis recurrence in patients with PsA and the challenges of systemic GCs with PsO lead us to frequently consider GC-sparing therapy that addresses ocular, musculoskeletal, and cutaneous manifestations. Tumor necrosis factor inhibitors (TNF-I) are our typical first-line considerations for GC-sparing therapy in patients with PsA with inflammatory joint symptoms and uveitis, although nonbiologic therapy can be considered first-line therapy in select populations.
Data establishing the efficacy of TNF-I come largely from randomized controlled trials (RCTs) of adalimumab (ADA) compared to placebo in noninfectious uveitis.11 While these trials focused on idiopathic posterior or pan-uveitis, these data have been extrapolated to SpA-associated anterior uveitis, and large registry analyses have supported use of TNF-I in this population.12 When selecting a particular TNF-I in a patient with current or past uveitis, we frequently start with ADA, based on supportive, albeit uncontrolled, data suggesting a reduction in the risk of recurrence with this agent in patients with SpA and uveitis.12 For patients who are unable to tolerate subcutaneous injections, who fail ADA, or who we suspect will require higher, titratable dosing, we favor infliximab infusions. Other data suggest that golimumab and certolizumab are also reasonable alternatives.13,14 We do not generally use etanercept, as the limited data that are available suggest that it is less effective at reducing risk of uveitis recurrence.12 Methotrexate or leflunomide may be an appropriate first line choice for patients with peripheral-predominant PsA and uveitis, but it is important to note that these agents are not effective for axial disease.
Despite the mechanistic data implicating the role of IL-17 in uveitis associated with PsA, the IL-17A inhibitor, secukinumab, failed to show a reduction in uveitis recurrence, compared to placebo, in pooled analysis of RCTs of noninfectious uveitis.15 However, a phase 2 trial of intravenous secukinumab in noninfectious uveitis showed promise, possibly because this dosing regimen can achieve higher effective concentrations.16 It is not our current practice to use secukinumab or ixekizumab as a first-line therapy in patients with PsA and concurrent uveitis, owing to a lack of data supporting efficacy. A novel IL-17A/F inhibitor, bimekizumab (BKZ), has recently been used in several successful phase 3 trials in patients with both TNF-naïve and TNF-nonresponder SpA, including AxSpA and PsA.17 Interestingly, data from the phase 2 and 3 trials of BKZ found low incidence rates of uveitis in patients with SpA treated with BKZ compared to placebo, suggesting that BKZ might be more effective in uveitis than other IL-17 inhibitors, but these data need to be confirmed.
Successful use of Janus kinase (JAK) inhibitors in noninfectious uveitis, including cases associated with inflammatory arthritis, has been described in case reports as well as in current phase 2 trials.18 The dual IL-12/IL-23 inhibitor, ustekinumab, also showed initial promise in a small, nonrandomized, uncontrolled phase 1/2 study of the treatment of posterior uveitis, as well as success in few case reports of PsA-associated uveitis.19 However, a post-hoc analysis of extra-intestinal manifestations, including uveitis and iritis, in patients with inflammatory bowel disease treated with ustekinumab found no benefit in preventing or treating ocular disease compared to placebo.20 Given the paucity of available data, JAK inhibitors and the IL-12/IL-23 inhibitor, ustekinumab, are not part of our typical treatment algorithm for patients with PsA-associated uveitis.
In conclusion, uveitis is a frequent extra-articular comorbidity of PsA, and it may present differently than the typical acute onset, unilateral anterior uveitis seen in SpA. While uveitis may share many different immunologic threads with PsA, the most convincing data support the use of TNF-I as a GC-sparing agent in this setting, particularly ADA, infliximab, golimumab, or certolizumab. Our approach is generally to start with these agents or methotrexate when directed therapy is needed for uveitis in PsA. Further investigation into the use of the IL-17A/F inhibitor BKZ and JAK inhibitors, as well as tyrosine kinase 2 inhibitors, in PsA associated uveitis may yield additional options for our patients.21
With the growing number of treatment options for psoriatic arthritis (PsA), therapeutic decision-making has shifted to an increasingly tailored and patient-centered approach. A number of factors contribute to the treatment decision-making process, including age, insurance restrictions, route of administration, side effect profile, comorbidities, and extra-articular manifestations of the disease. In this article, we discuss an extra-articular comorbidity, uveitis, which is frequently seen in patients with PsA. We discuss clinical characteristics of uveitis associated with PsA and describe how the presence of uveitis influences our treatment approach to PsA, based on existing data.
Uveitis refers broadly to inflammation of the uvea, the vascularized and pigmented layer of the eye composed of the iris, the ciliary body, and the choroid. While infection is a common cause of uveitis, many cases are noninfectious and are often associated with an underlying autoimmune or systemic inflammatory disorder. Uveitis is frequently reported in diseases in the spondyloarthritis (SpA) family, including axial spondyloarthritis (AxSpA) and reactive arthritis, as well as PsA. Exact estimates of the prevalence of uveitis in PsA vary widely from 7%-25%, depending on the particular cohort studied.1,2 In all forms of SpA, the anterior chamber of the uvea is the most likely to be affected.3 However, compared to patients with AxSpA, patients with PsA appear to have a higher rate of posterior involvement. In addition, patients with PsA appear to have higher frequencies of insidious, bilateral uveitis, as compared to the acute, unilateral, anterior uveitis that is most characteristic of AxSpA.4 Women with PsA may be more likely than men to experience uveitis, although this has not been a consistent finding.5
Patients with PsA who are human leukocyte antigen B27 (HLA-B27) positive may be at risk for more severe and refractory anterior uveitis compared to those who do not express the allele.5 Those who are HLA-B27 positive are also known to have higher rates of axial involvement. It has therefore been postulated that 2 phenotypes of uveitis may exist in PsA: patients who are HLA-B27 positive who have axial disease and severe, unilateral anterior uveitis reminiscent of other forms of SpA, and patients who are HLA-B27 negative, often women, with peripheral-predominant arthritis who are prone to the classic anterior uveitis but may also develop atypical bilateral, insidious, and/or posterior involvement.4 Specific characteristics of PsA may also provide information about the risk for developing uveitis. For example, dactylitis has been linked to a higher risk of developing uveitis in some, but not all, cohorts of patients with PsA, and the risk of uveitis in PsA has been found in many studies to correlate with longer duration of disease.6-8
The presence of uveitis signals a disruption in the blood-retina barrier and the subsequent entrance of inflammatory cells into the eye. An entire explanation of pathogenesis is beyond the scope of this article; however, it is worth noting that many of the inflammatory mediators of active uveitis mirror those of PsA. For instance, both the mesenchymal cells in enthesitis and the cells of the ciliary body express receptors for interleukin (IL)-23, suggesting a potential role of the signaling pathways involving this cytokine in both diseases.9 Another study found increased serum levels of IL-17, a known mediator of PsA disease, in patients with active uveitis.10 Despite these common pathogenesis links, there are limited data on the utility of certain existing PsA treatments on uveitis manifestations.
Our approach is always to manage uveitis associated with PsA in collaboration with a specialized and experienced ophthalmologist. Uncontrolled uveitis can be vision threatening and contribute to long-term morbidity associated with PsA, so timely recognition, evaluation, and appropriate treatment are important. Ocular glucocorticoid (GC) drops may be used as first-line therapy, particularly for anterior uveitis, to quickly quell inflammation. Escalation to systemic GCs for more severe or posteriorly localized disease may be considered carefully, given the known risk of worsening skin psoriasis (PsO) with GC withdrawal after a course of therapy. Use of GC-sparing therapy should be determined on a case-by-case basis. While generalized, noninfectious uveitis often resolves with GC treatment, the risk of uveitis recurrence in patients with PsA and the challenges of systemic GCs with PsO lead us to frequently consider GC-sparing therapy that addresses ocular, musculoskeletal, and cutaneous manifestations. Tumor necrosis factor inhibitors (TNF-I) are our typical first-line considerations for GC-sparing therapy in patients with PsA with inflammatory joint symptoms and uveitis, although nonbiologic therapy can be considered first-line therapy in select populations.
Data establishing the efficacy of TNF-I come largely from randomized controlled trials (RCTs) of adalimumab (ADA) compared to placebo in noninfectious uveitis.11 While these trials focused on idiopathic posterior or pan-uveitis, these data have been extrapolated to SpA-associated anterior uveitis, and large registry analyses have supported use of TNF-I in this population.12 When selecting a particular TNF-I in a patient with current or past uveitis, we frequently start with ADA, based on supportive, albeit uncontrolled, data suggesting a reduction in the risk of recurrence with this agent in patients with SpA and uveitis.12 For patients who are unable to tolerate subcutaneous injections, who fail ADA, or who we suspect will require higher, titratable dosing, we favor infliximab infusions. Other data suggest that golimumab and certolizumab are also reasonable alternatives.13,14 We do not generally use etanercept, as the limited data that are available suggest that it is less effective at reducing risk of uveitis recurrence.12 Methotrexate or leflunomide may be an appropriate first line choice for patients with peripheral-predominant PsA and uveitis, but it is important to note that these agents are not effective for axial disease.
Despite the mechanistic data implicating the role of IL-17 in uveitis associated with PsA, the IL-17A inhibitor, secukinumab, failed to show a reduction in uveitis recurrence, compared to placebo, in pooled analysis of RCTs of noninfectious uveitis.15 However, a phase 2 trial of intravenous secukinumab in noninfectious uveitis showed promise, possibly because this dosing regimen can achieve higher effective concentrations.16 It is not our current practice to use secukinumab or ixekizumab as a first-line therapy in patients with PsA and concurrent uveitis, owing to a lack of data supporting efficacy. A novel IL-17A/F inhibitor, bimekizumab (BKZ), has recently been used in several successful phase 3 trials in patients with both TNF-naïve and TNF-nonresponder SpA, including AxSpA and PsA.17 Interestingly, data from the phase 2 and 3 trials of BKZ found low incidence rates of uveitis in patients with SpA treated with BKZ compared to placebo, suggesting that BKZ might be more effective in uveitis than other IL-17 inhibitors, but these data need to be confirmed.
Successful use of Janus kinase (JAK) inhibitors in noninfectious uveitis, including cases associated with inflammatory arthritis, has been described in case reports as well as in current phase 2 trials.18 The dual IL-12/IL-23 inhibitor, ustekinumab, also showed initial promise in a small, nonrandomized, uncontrolled phase 1/2 study of the treatment of posterior uveitis, as well as success in few case reports of PsA-associated uveitis.19 However, a post-hoc analysis of extra-intestinal manifestations, including uveitis and iritis, in patients with inflammatory bowel disease treated with ustekinumab found no benefit in preventing or treating ocular disease compared to placebo.20 Given the paucity of available data, JAK inhibitors and the IL-12/IL-23 inhibitor, ustekinumab, are not part of our typical treatment algorithm for patients with PsA-associated uveitis.
In conclusion, uveitis is a frequent extra-articular comorbidity of PsA, and it may present differently than the typical acute onset, unilateral anterior uveitis seen in SpA. While uveitis may share many different immunologic threads with PsA, the most convincing data support the use of TNF-I as a GC-sparing agent in this setting, particularly ADA, infliximab, golimumab, or certolizumab. Our approach is generally to start with these agents or methotrexate when directed therapy is needed for uveitis in PsA. Further investigation into the use of the IL-17A/F inhibitor BKZ and JAK inhibitors, as well as tyrosine kinase 2 inhibitors, in PsA associated uveitis may yield additional options for our patients.21
1. De Vicente Delmas A, Sanchez-Bilbao L, Calvo-Rio V, et al. Uveitis in psoriatic arthritis: study of 406 patients in a single university center and literature review. RMD Open. 2023;9(1):e002781.
2. Rademacher J, Poddubnyy D, Pleyer U. Uveitis in spondyloarthritis. Ther Adv Musculoskelet Dis. 2020;12:1759720X20951733.
3. Zeboulon N, Dougados M, Gossec L. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis. 2008;67(7):955-959.
4. Paiva ES, Macaluso DC, Edwards A, Rosenbaum JT. Characterisation of uveitis in patients with psoriatic arthritis. Ann Rheum Dis. 2000;59(1):67-70.
5. Fraga NA, Oliveira Mde F, Follador I, Rocha Bde O, Rego VR. Psoriasis and uveitis: a literature review. An Bras Dermatol. 2012;87(6):877-883.
6. Niccoli L, Nannini C, Cassara E, et al. Frequency of iridocyclitis in patients with early psoriatic arthritis: a prospective, follow up study. Int J Rheum Dis. 2012;15(4):414-418.
7. Yasar Bilge NS, Kalyoncu U, Atagunduz P, et al. Uveitis-related factors in patients with spondyloarthritis: TReasure Real-Life Results. Am J Ophthalmol. 2021;228:58-64.
8. Chia AYT, Ang GWX, Chan ASY, Chan W, Chong TKY, Leung YY. Managing psoriatic arthritis with inflammatory bowel disease and/or uveitis. Front Med (Lausanne). 2021;8:737256.
9. Reinhardt A, Yevsa T, Worbs T, et al. Interleukin-23-dependent gamma/delta T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol. 2016;68(10):2476-2486.
10. Jawad S, Liu B, Agron E, Nussenblatt RB, Sen HN. Elevated serum levels of interleukin-17A in uveitis patients. Ocul Immunol Inflamm. 2013;21(6):434-439.
11. Merrill PT, Vitale A, Zierhut M, et al. Efficacy of adalimumab in non-infectious uveitis across different etiologies: a post hoc analysis of the VISUAL I and VISUAL II Trials. Ocul Immunol Inflamm. 2021;29(7-8):1569-1575.
12. Lie E, Lindstrom U, Zverkova-Sandstrom T, et al. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis: results from the Swedish biologics register. Ann Rheum Dis. 2017;76(9):1515-1521.
13. van der Horst-Bruinsma I, van Bentum R, Verbraak FD, et al. The impact of certolizumab pegol treatment on the incidence of anterior uveitis flares in patients with axial spondyloarthritis: 48-week interim results from C-VIEW. RMD Open. 2020;6(1):e001161.
14. Calvo-Rio V, Blanco R, Santos-Gomez M, et al. Golimumab in refractory uveitis related to spondyloarthritis. Multicenter study of 15 patients. Semin Arthritis Rheum. 2016;46(1):95-101.
15. Dick AD, Tugal-Tutkun I, Foster S, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120(4):777-787.
16. Letko E, Yeh S, Foster CS, et al. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology. 2015;122(5):939-948.
17. van der Heijde D, Deodhar A, Baraliakos X, et al. Efficacy and safety of bimekizumab in axial spondyloarthritis: results of two parallel phase 3 randomised controlled trials. Ann Rheum Dis. 2023;82(4):515-526.
18. Dhillon S, Keam SJ. Filgotinib: first approval. Drugs. 2020;80(18):1987-1997.
19. Pepple KL, Lin P. Targeting interleukin-23 in the treatment of noninfectious uveitis. Ophthalmology. 2018;125(12):1977-1983.
20. Narula N, Aruljothy A, Wong ECL, et al. The impact of ustekinumab on extraintestinal manifestations of Crohn’s disease: a post hoc analysis of the UNITI studies. United European Gastroenterol J. 2021;9(5):581-589.
21. Rusinol L, Puig L. Tyk2 targeting in immune-mediated inflammatory diseases. Int J Mol Sci. 2023;24(4):3391.
1. De Vicente Delmas A, Sanchez-Bilbao L, Calvo-Rio V, et al. Uveitis in psoriatic arthritis: study of 406 patients in a single university center and literature review. RMD Open. 2023;9(1):e002781.
2. Rademacher J, Poddubnyy D, Pleyer U. Uveitis in spondyloarthritis. Ther Adv Musculoskelet Dis. 2020;12:1759720X20951733.
3. Zeboulon N, Dougados M, Gossec L. Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis. 2008;67(7):955-959.
4. Paiva ES, Macaluso DC, Edwards A, Rosenbaum JT. Characterisation of uveitis in patients with psoriatic arthritis. Ann Rheum Dis. 2000;59(1):67-70.
5. Fraga NA, Oliveira Mde F, Follador I, Rocha Bde O, Rego VR. Psoriasis and uveitis: a literature review. An Bras Dermatol. 2012;87(6):877-883.
6. Niccoli L, Nannini C, Cassara E, et al. Frequency of iridocyclitis in patients with early psoriatic arthritis: a prospective, follow up study. Int J Rheum Dis. 2012;15(4):414-418.
7. Yasar Bilge NS, Kalyoncu U, Atagunduz P, et al. Uveitis-related factors in patients with spondyloarthritis: TReasure Real-Life Results. Am J Ophthalmol. 2021;228:58-64.
8. Chia AYT, Ang GWX, Chan ASY, Chan W, Chong TKY, Leung YY. Managing psoriatic arthritis with inflammatory bowel disease and/or uveitis. Front Med (Lausanne). 2021;8:737256.
9. Reinhardt A, Yevsa T, Worbs T, et al. Interleukin-23-dependent gamma/delta T cells produce interleukin-17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol. 2016;68(10):2476-2486.
10. Jawad S, Liu B, Agron E, Nussenblatt RB, Sen HN. Elevated serum levels of interleukin-17A in uveitis patients. Ocul Immunol Inflamm. 2013;21(6):434-439.
11. Merrill PT, Vitale A, Zierhut M, et al. Efficacy of adalimumab in non-infectious uveitis across different etiologies: a post hoc analysis of the VISUAL I and VISUAL II Trials. Ocul Immunol Inflamm. 2021;29(7-8):1569-1575.
12. Lie E, Lindstrom U, Zverkova-Sandstrom T, et al. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis: results from the Swedish biologics register. Ann Rheum Dis. 2017;76(9):1515-1521.
13. van der Horst-Bruinsma I, van Bentum R, Verbraak FD, et al. The impact of certolizumab pegol treatment on the incidence of anterior uveitis flares in patients with axial spondyloarthritis: 48-week interim results from C-VIEW. RMD Open. 2020;6(1):e001161.
14. Calvo-Rio V, Blanco R, Santos-Gomez M, et al. Golimumab in refractory uveitis related to spondyloarthritis. Multicenter study of 15 patients. Semin Arthritis Rheum. 2016;46(1):95-101.
15. Dick AD, Tugal-Tutkun I, Foster S, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120(4):777-787.
16. Letko E, Yeh S, Foster CS, et al. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology. 2015;122(5):939-948.
17. van der Heijde D, Deodhar A, Baraliakos X, et al. Efficacy and safety of bimekizumab in axial spondyloarthritis: results of two parallel phase 3 randomised controlled trials. Ann Rheum Dis. 2023;82(4):515-526.
18. Dhillon S, Keam SJ. Filgotinib: first approval. Drugs. 2020;80(18):1987-1997.
19. Pepple KL, Lin P. Targeting interleukin-23 in the treatment of noninfectious uveitis. Ophthalmology. 2018;125(12):1977-1983.
20. Narula N, Aruljothy A, Wong ECL, et al. The impact of ustekinumab on extraintestinal manifestations of Crohn’s disease: a post hoc analysis of the UNITI studies. United European Gastroenterol J. 2021;9(5):581-589.
21. Rusinol L, Puig L. Tyk2 targeting in immune-mediated inflammatory diseases. Int J Mol Sci. 2023;24(4):3391.
Nail psoriasis in Black patients often overlooked
NEW YORK – From clinical trials to textbooks, , even when the skin disease has already been diagnosed, according to Shari R. Lipner, MD.
In a recently published review of 45 randomized controlled trials of therapies for nail psoriasis, almost all included information about the gender of the patients enrolled, but only about 35% reported race and/or ethnicity, Dr. Lipner, associate professor of dermatology, Weill Cornell Medical College, New York, said at the Skin of Color Update 2023. The proportion climbed to 59% in trials that included at least one study site in the United States, although representation of non-White patients in studies conducted in the United States was not proportional to the population (13.4% vs. 39.9%), said Dr. Lipner, senior author of the review .
Black patients largely unrepresented in photos
When an Internet search was conducted for images of nail psoriasis, the proportion of images fell as the number of the Fitzpatrick scale increased. Fitzpatrick skin types 1 or 2 represented 70% of the images, skin types 3 to 4 represented about 27%, leaving just 3% represented by darker skin types, Dr. Lipner said.
“Unfortunately, things are not much better if you look at the dermatology and nail-specific textbooks. In fact, the percentages we see are almost identical,” said Dr. Lipner, noting that her review of images suggested that only about 3% of images in textbooks are of Fitzpatrick skin types 5 or 6, an obstacle for clinicians learning to recognize nail involvement in skin of color patients with psoriasis.
“We have written a couple of papers on this topic, including a call to action” in a letter to the editor in the Journal of the American Academy of Dermatology, Dr. Lipner noted. “To ensure access to safe and effective treatments for all patient populations,” she and her coauthor wrote, “we advocate the prioritized enrollment of racial and ethnic minority groups in psoriasis, PsA [psoriatic arthritis], and NP [nail psoriasis] clinical trials.”
Data from the 2009-2010 U.S. National Health and Nutrition Examination Survey (NHANES) confirms that psoriasis is less common in Blacks (1.9%) and Hispanics (1.6%) than Whites (3.6%). But these lower numbers still translate into substantial numbers nationally. Of those with psoriasis, the lifetime incidence of nail involvement has been variously estimated between 80% and 90%, Dr. Lipner said.
In about 10% of patients with psoriasis, nail involvement is isolated, occurring in the absence of skin lesions, a proportion that appears to be similar in Blacks and Whites according to Dr. Lipner.
Patient characteristics similar by race
In a study conducted at her own center, many of the characteristics of psoriasis were similar when those with a Fitzpatrick skin type 4 or higher were compared to those of 3 or lower. This included male-female distribution, smoking history, and presence of accompanying psoriatic arthritis. There was one discrepancy between lighter and darker skin.
“The big difference was that it took almost 3 years longer [on average] for darker skin to be diagnosed, and there was worse severity of disease,” Dr. Lipner said.
Like cutaneous manifestations of psoriasis, there are differences in appearance in the nail, many of which are simply produced by how skin color alters the appearance, such as the brownish hue of erythema in darker versus lighter skin. Dr. Lipner also noted that many of the features, such as keratosis, can be more severe in patients with darker skin types, but this is likely because of the delay in diagnosis.
The problem with overlooking nail psoriasis in patients of any skin color is the significant and independent adverse impact imposed by nail disease on quality of life, she added. She recounted the case of a 22-year-old Black patient whose nail psoriasis was overlooked even as she was being treated for her skin lesions.
“The diagnosis of nail psoriasis was missed for 3 years,” said Dr. Lipner, noting that the nail involvement was not trivial. “She had trouble doing her daily activities of life, but also, she was very embarrassed by her nails, not surprisingly.”
The problem of underrepresentation of Blacks in photos depicting nail diseases is not going unnoticed.
“Recently, there has been a concerted effort on the part of authors and editors to include more images of skin of color patients in published articles and textbooks,” said Jane S. Bellet, MD, professor of dermatology, Duke University, Durham, N.C.
An expert in nail disorders, particularly in children, Dr. Bellet said in an interview that this trend “must continue and increase in volume.” She said that the need for more images of nail disease in skin of color is not restricted to textbooks but includes “other learning materials, such as online atlases.”
Dr. Lipner and Dr. Bellet reported no potential conflicts of interest relative to this topic.
NEW YORK – From clinical trials to textbooks, , even when the skin disease has already been diagnosed, according to Shari R. Lipner, MD.
In a recently published review of 45 randomized controlled trials of therapies for nail psoriasis, almost all included information about the gender of the patients enrolled, but only about 35% reported race and/or ethnicity, Dr. Lipner, associate professor of dermatology, Weill Cornell Medical College, New York, said at the Skin of Color Update 2023. The proportion climbed to 59% in trials that included at least one study site in the United States, although representation of non-White patients in studies conducted in the United States was not proportional to the population (13.4% vs. 39.9%), said Dr. Lipner, senior author of the review .
Black patients largely unrepresented in photos
When an Internet search was conducted for images of nail psoriasis, the proportion of images fell as the number of the Fitzpatrick scale increased. Fitzpatrick skin types 1 or 2 represented 70% of the images, skin types 3 to 4 represented about 27%, leaving just 3% represented by darker skin types, Dr. Lipner said.
“Unfortunately, things are not much better if you look at the dermatology and nail-specific textbooks. In fact, the percentages we see are almost identical,” said Dr. Lipner, noting that her review of images suggested that only about 3% of images in textbooks are of Fitzpatrick skin types 5 or 6, an obstacle for clinicians learning to recognize nail involvement in skin of color patients with psoriasis.
“We have written a couple of papers on this topic, including a call to action” in a letter to the editor in the Journal of the American Academy of Dermatology, Dr. Lipner noted. “To ensure access to safe and effective treatments for all patient populations,” she and her coauthor wrote, “we advocate the prioritized enrollment of racial and ethnic minority groups in psoriasis, PsA [psoriatic arthritis], and NP [nail psoriasis] clinical trials.”
Data from the 2009-2010 U.S. National Health and Nutrition Examination Survey (NHANES) confirms that psoriasis is less common in Blacks (1.9%) and Hispanics (1.6%) than Whites (3.6%). But these lower numbers still translate into substantial numbers nationally. Of those with psoriasis, the lifetime incidence of nail involvement has been variously estimated between 80% and 90%, Dr. Lipner said.
In about 10% of patients with psoriasis, nail involvement is isolated, occurring in the absence of skin lesions, a proportion that appears to be similar in Blacks and Whites according to Dr. Lipner.
Patient characteristics similar by race
In a study conducted at her own center, many of the characteristics of psoriasis were similar when those with a Fitzpatrick skin type 4 or higher were compared to those of 3 or lower. This included male-female distribution, smoking history, and presence of accompanying psoriatic arthritis. There was one discrepancy between lighter and darker skin.
“The big difference was that it took almost 3 years longer [on average] for darker skin to be diagnosed, and there was worse severity of disease,” Dr. Lipner said.
Like cutaneous manifestations of psoriasis, there are differences in appearance in the nail, many of which are simply produced by how skin color alters the appearance, such as the brownish hue of erythema in darker versus lighter skin. Dr. Lipner also noted that many of the features, such as keratosis, can be more severe in patients with darker skin types, but this is likely because of the delay in diagnosis.
The problem with overlooking nail psoriasis in patients of any skin color is the significant and independent adverse impact imposed by nail disease on quality of life, she added. She recounted the case of a 22-year-old Black patient whose nail psoriasis was overlooked even as she was being treated for her skin lesions.
“The diagnosis of nail psoriasis was missed for 3 years,” said Dr. Lipner, noting that the nail involvement was not trivial. “She had trouble doing her daily activities of life, but also, she was very embarrassed by her nails, not surprisingly.”
The problem of underrepresentation of Blacks in photos depicting nail diseases is not going unnoticed.
“Recently, there has been a concerted effort on the part of authors and editors to include more images of skin of color patients in published articles and textbooks,” said Jane S. Bellet, MD, professor of dermatology, Duke University, Durham, N.C.
An expert in nail disorders, particularly in children, Dr. Bellet said in an interview that this trend “must continue and increase in volume.” She said that the need for more images of nail disease in skin of color is not restricted to textbooks but includes “other learning materials, such as online atlases.”
Dr. Lipner and Dr. Bellet reported no potential conflicts of interest relative to this topic.
NEW YORK – From clinical trials to textbooks, , even when the skin disease has already been diagnosed, according to Shari R. Lipner, MD.
In a recently published review of 45 randomized controlled trials of therapies for nail psoriasis, almost all included information about the gender of the patients enrolled, but only about 35% reported race and/or ethnicity, Dr. Lipner, associate professor of dermatology, Weill Cornell Medical College, New York, said at the Skin of Color Update 2023. The proportion climbed to 59% in trials that included at least one study site in the United States, although representation of non-White patients in studies conducted in the United States was not proportional to the population (13.4% vs. 39.9%), said Dr. Lipner, senior author of the review .
Black patients largely unrepresented in photos
When an Internet search was conducted for images of nail psoriasis, the proportion of images fell as the number of the Fitzpatrick scale increased. Fitzpatrick skin types 1 or 2 represented 70% of the images, skin types 3 to 4 represented about 27%, leaving just 3% represented by darker skin types, Dr. Lipner said.
“Unfortunately, things are not much better if you look at the dermatology and nail-specific textbooks. In fact, the percentages we see are almost identical,” said Dr. Lipner, noting that her review of images suggested that only about 3% of images in textbooks are of Fitzpatrick skin types 5 or 6, an obstacle for clinicians learning to recognize nail involvement in skin of color patients with psoriasis.
“We have written a couple of papers on this topic, including a call to action” in a letter to the editor in the Journal of the American Academy of Dermatology, Dr. Lipner noted. “To ensure access to safe and effective treatments for all patient populations,” she and her coauthor wrote, “we advocate the prioritized enrollment of racial and ethnic minority groups in psoriasis, PsA [psoriatic arthritis], and NP [nail psoriasis] clinical trials.”
Data from the 2009-2010 U.S. National Health and Nutrition Examination Survey (NHANES) confirms that psoriasis is less common in Blacks (1.9%) and Hispanics (1.6%) than Whites (3.6%). But these lower numbers still translate into substantial numbers nationally. Of those with psoriasis, the lifetime incidence of nail involvement has been variously estimated between 80% and 90%, Dr. Lipner said.
In about 10% of patients with psoriasis, nail involvement is isolated, occurring in the absence of skin lesions, a proportion that appears to be similar in Blacks and Whites according to Dr. Lipner.
Patient characteristics similar by race
In a study conducted at her own center, many of the characteristics of psoriasis were similar when those with a Fitzpatrick skin type 4 or higher were compared to those of 3 or lower. This included male-female distribution, smoking history, and presence of accompanying psoriatic arthritis. There was one discrepancy between lighter and darker skin.
“The big difference was that it took almost 3 years longer [on average] for darker skin to be diagnosed, and there was worse severity of disease,” Dr. Lipner said.
Like cutaneous manifestations of psoriasis, there are differences in appearance in the nail, many of which are simply produced by how skin color alters the appearance, such as the brownish hue of erythema in darker versus lighter skin. Dr. Lipner also noted that many of the features, such as keratosis, can be more severe in patients with darker skin types, but this is likely because of the delay in diagnosis.
The problem with overlooking nail psoriasis in patients of any skin color is the significant and independent adverse impact imposed by nail disease on quality of life, she added. She recounted the case of a 22-year-old Black patient whose nail psoriasis was overlooked even as she was being treated for her skin lesions.
“The diagnosis of nail psoriasis was missed for 3 years,” said Dr. Lipner, noting that the nail involvement was not trivial. “She had trouble doing her daily activities of life, but also, she was very embarrassed by her nails, not surprisingly.”
The problem of underrepresentation of Blacks in photos depicting nail diseases is not going unnoticed.
“Recently, there has been a concerted effort on the part of authors and editors to include more images of skin of color patients in published articles and textbooks,” said Jane S. Bellet, MD, professor of dermatology, Duke University, Durham, N.C.
An expert in nail disorders, particularly in children, Dr. Bellet said in an interview that this trend “must continue and increase in volume.” She said that the need for more images of nail disease in skin of color is not restricted to textbooks but includes “other learning materials, such as online atlases.”
Dr. Lipner and Dr. Bellet reported no potential conflicts of interest relative to this topic.
AT SOC 2023
Commentary: Examining DMARD Use in PsA, December 2023
Limiting radiographic progression is an important long-term goal of treatment of PsA. In a post hoc analysis that included 449 biologic-naive patients with PsA from the DISCOVER-2 trial who received 100 mg guselkumab every 4 or 8 weeks, Mease and colleagues demonstrated that a greater improvement in the Disease Activity Index for PsA (DAPSA) scores as early as week 8 and the achievement of DAPSA low disease activity at week 8 were associated with a significantly lower progression of radiographic joint damage (total PsA-modified van der Heijde-Sharp score) through week 100. Thus, patients who respond well early have better long-term outcomes.
The safety of targeted therapies is always of concern and is inadequately addressed by individual clinical trials. Meta-analyses may provide further insights. In a network meta-analysis of 94 randomized controlled trials that included a total of 54,369 patients with PsA or psoriasis who were treated with 14 biologics, five small molecules, or placebo, Chiu and colleagues found that for patients with psoriasis, infliximab, deucravacitinib, and bimekizumab had the highest risks for infection. In patients with PsA, bimekizumab, apremilast, and 30 mg upadacitinib led to a significantly higher risk for infection compared with placebo, and 30 mg upadacitinib also increasing the risk for serious infection compared with placebo. The risk for infection in patients with PsA did not increase with most bDMARD and targeted synthetic DMARD (tsDMARD), except bimekizumab, apremilast, and 30 mg upadacitinib.
There is increasing recognition of the difficulty in managing patients with refractory PsA. One approach to such difficult-to-treat disease is dual targeted therapy (DTT). However, the safety of these combinations is of major concern. There is currently an ongoing clinical trial comparing a combination of guselkumab and golimumab vs guselkumab alone for treatment-resistant PsA. In the meantime, Valero-Martinez and colleagues have reported results from an observational, retrospective, cross-sectional study that included patients with refractory PsA (n = 14) or spondyloarthritis (n = 22) who simultaneously received two bDMARD or tsDMARD with different therapeutic targets. The most commonly used combinations were a tumor necrosis factor (TNF) inhibitor plus an interleukin (IL)-12/23 pathway inhibitor, followed by a TNF inhibitor plus an IL-17 inhibitor. They found that at a median exposure of 14.86 months, the DTT retention rate in patients with PsA was 42.8%, with 40.0% and 53.3% of patients achieving remission or low activity and major clinical improvements, respectively. Treatment discontinuation due to adverse events was reported in one patient with PsA and multiple comorbidities. Thus, DTT led to satisfactory clinical improvements and no serious adverse events in patients with refractory PsA. The results of larger observational and randomized trials are awaited.
Limiting radiographic progression is an important long-term goal of treatment of PsA. In a post hoc analysis that included 449 biologic-naive patients with PsA from the DISCOVER-2 trial who received 100 mg guselkumab every 4 or 8 weeks, Mease and colleagues demonstrated that a greater improvement in the Disease Activity Index for PsA (DAPSA) scores as early as week 8 and the achievement of DAPSA low disease activity at week 8 were associated with a significantly lower progression of radiographic joint damage (total PsA-modified van der Heijde-Sharp score) through week 100. Thus, patients who respond well early have better long-term outcomes.
The safety of targeted therapies is always of concern and is inadequately addressed by individual clinical trials. Meta-analyses may provide further insights. In a network meta-analysis of 94 randomized controlled trials that included a total of 54,369 patients with PsA or psoriasis who were treated with 14 biologics, five small molecules, or placebo, Chiu and colleagues found that for patients with psoriasis, infliximab, deucravacitinib, and bimekizumab had the highest risks for infection. In patients with PsA, bimekizumab, apremilast, and 30 mg upadacitinib led to a significantly higher risk for infection compared with placebo, and 30 mg upadacitinib also increasing the risk for serious infection compared with placebo. The risk for infection in patients with PsA did not increase with most bDMARD and targeted synthetic DMARD (tsDMARD), except bimekizumab, apremilast, and 30 mg upadacitinib.
There is increasing recognition of the difficulty in managing patients with refractory PsA. One approach to such difficult-to-treat disease is dual targeted therapy (DTT). However, the safety of these combinations is of major concern. There is currently an ongoing clinical trial comparing a combination of guselkumab and golimumab vs guselkumab alone for treatment-resistant PsA. In the meantime, Valero-Martinez and colleagues have reported results from an observational, retrospective, cross-sectional study that included patients with refractory PsA (n = 14) or spondyloarthritis (n = 22) who simultaneously received two bDMARD or tsDMARD with different therapeutic targets. The most commonly used combinations were a tumor necrosis factor (TNF) inhibitor plus an interleukin (IL)-12/23 pathway inhibitor, followed by a TNF inhibitor plus an IL-17 inhibitor. They found that at a median exposure of 14.86 months, the DTT retention rate in patients with PsA was 42.8%, with 40.0% and 53.3% of patients achieving remission or low activity and major clinical improvements, respectively. Treatment discontinuation due to adverse events was reported in one patient with PsA and multiple comorbidities. Thus, DTT led to satisfactory clinical improvements and no serious adverse events in patients with refractory PsA. The results of larger observational and randomized trials are awaited.
Limiting radiographic progression is an important long-term goal of treatment of PsA. In a post hoc analysis that included 449 biologic-naive patients with PsA from the DISCOVER-2 trial who received 100 mg guselkumab every 4 or 8 weeks, Mease and colleagues demonstrated that a greater improvement in the Disease Activity Index for PsA (DAPSA) scores as early as week 8 and the achievement of DAPSA low disease activity at week 8 were associated with a significantly lower progression of radiographic joint damage (total PsA-modified van der Heijde-Sharp score) through week 100. Thus, patients who respond well early have better long-term outcomes.
The safety of targeted therapies is always of concern and is inadequately addressed by individual clinical trials. Meta-analyses may provide further insights. In a network meta-analysis of 94 randomized controlled trials that included a total of 54,369 patients with PsA or psoriasis who were treated with 14 biologics, five small molecules, or placebo, Chiu and colleagues found that for patients with psoriasis, infliximab, deucravacitinib, and bimekizumab had the highest risks for infection. In patients with PsA, bimekizumab, apremilast, and 30 mg upadacitinib led to a significantly higher risk for infection compared with placebo, and 30 mg upadacitinib also increasing the risk for serious infection compared with placebo. The risk for infection in patients with PsA did not increase with most bDMARD and targeted synthetic DMARD (tsDMARD), except bimekizumab, apremilast, and 30 mg upadacitinib.
There is increasing recognition of the difficulty in managing patients with refractory PsA. One approach to such difficult-to-treat disease is dual targeted therapy (DTT). However, the safety of these combinations is of major concern. There is currently an ongoing clinical trial comparing a combination of guselkumab and golimumab vs guselkumab alone for treatment-resistant PsA. In the meantime, Valero-Martinez and colleagues have reported results from an observational, retrospective, cross-sectional study that included patients with refractory PsA (n = 14) or spondyloarthritis (n = 22) who simultaneously received two bDMARD or tsDMARD with different therapeutic targets. The most commonly used combinations were a tumor necrosis factor (TNF) inhibitor plus an interleukin (IL)-12/23 pathway inhibitor, followed by a TNF inhibitor plus an IL-17 inhibitor. They found that at a median exposure of 14.86 months, the DTT retention rate in patients with PsA was 42.8%, with 40.0% and 53.3% of patients achieving remission or low activity and major clinical improvements, respectively. Treatment discontinuation due to adverse events was reported in one patient with PsA and multiple comorbidities. Thus, DTT led to satisfactory clinical improvements and no serious adverse events in patients with refractory PsA. The results of larger observational and randomized trials are awaited.
IV secukinumab, alternative to self-injections, reaches primary endpoints in PsA, axSpA
SAN DIEGO – Monthly use of intravenously administered secukinumab (Cosentyx) proved its efficacy over placebo in treating psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA) in two industry-sponsored, randomized, double-blinded, phase 3 trials of the drug’s second and newly approved route of administration.
The studies of the human monoclonal antibody secukinumab, an interleukin-17 inhibitor, were presented at the annual meeting of the American College of Rheumatology. A subcutaneously injectable formulation of the drug is available, and the Food and Drug Administration approved the IV form for the conditions in October, although at a recommended lower monthly dose than the new trials examined.
In the PsA trial, 191 patients took IV secukinumab, and 190 took placebo. For the primary endpoint, the percentages who reached at least a 50% improvement in American College of Rheumatology response criteria (ACR 50) at 16 weeks were 31.4% and 6.3%, respectively (P < .0001).
In the axSpA trial, 264 patients took IV secukinumab, and 262 took placebo. The primary endpoint, at least a 40% improvement in Assessment of the Spondyloarthritis International Society response criteria (ASAS 40), was met at 16 weeks by 40.9% and 22.9%, respectively (P < .0001).
“Both studies appear to present clear efficacy of IV route administration of secukinumab with no clear increase in safety signals,” consultant rheumatologist Nicola Goodson, MBChB, PhD, of Aintree University Hospital in Liverpool, England, said in an interview.
“Offering IV administration as an option to patients is helpful,” added Dr. Goodson, who was not involved with the study but is familiar with its findings.
As Dr. Goodson explained, secukinumab “was the first IL [interleukin]-17 inhibitor used to treat spondyloarthropathies, and we have been using subcutaneous secukinumab to treat psoriasis, psoriatic arthritis, and axial spondyloarthritis/ankylosing spondylitis since 2016 in the U.K. Our experience with this medication has been good with similar efficacy to anti-TNF [tumor necrosis factor] therapy in axial spondyloarthritis. The medication is generally well-tolerated, and the subcutaneous pen injection device is easy for patients to use.”
However, IV treatment may speed up onset of action, she said, and it may be useful in situations when compliance is a challenge.
PsA trial details
In the PsA trial, known as INVIGORATE-2, researchers recruited patients who met the CASPAR criteria for active PsA with symptoms for ≥ 6 months, and had ≥ 3 tender joints out of 78 joints and ≥ 3 swollen joints out of 76.
Participants with a mean age of 48, including 55% females, were randomized 1:1 to receive placebo or secukinumab (6 mg/kg at baseline followed by 3 mg/kg every 4 weeks). Those in the placebo group were switched to the same monthly doses of secukinumab at 16 weeks.
“Patients who switched from the placebo had a similar increase of efficacy as the original treated group,” rheumatologist Alan J. Kivitz, MD, of the Altoona Center for Clinical Research, in Duncansville, Penn., said in his presentation at the meeting. Specifically, at 52 weeks, the groups had similar ACR 50 response rates: 58% with secukinumab and 64% with placebo-to-secukinumab.
The fact that patients in the original placebo group who received 3 mg IV doses without 6-mg loading doses achieved ACR response rates similar to those who took secukinumab during the whole trial “could suggest that the IV loading dose may not be required. This would need to be explored in a randomized head-to-head study, but it’s an interesting observation that may reduce costs and exposure to higher doses of medication at the start of treatment,” Dr. Goodson said.
Among the patients who received secukinumab at any point in the study, 63% had a treatment-emergent adverse event, including 5.9% with serious events. One death was reported in the placebo group before week 16. No other deaths were reported.
AxSpA trial details
In the axSpA trial, called INVIGORATE-1, researchers recruited people aged ≥18 years with a diagnosis of active radiographic axSpA according to modified New York criteria or nonradiographic axSpA according to ASAS criteria, and all had inflammatory back pain for ≥6 months with an onset before age 45. They were randomized at a 1:1 ratio to receive IV secukinumab (6 mg/kg loading dose, followed by 3 mg/kg every 4 weeks) or placebo for 16 weeks. At that point, the placebo group switched to the same monthly doses of IV secukinumab.
Participants had a mean age of about 39, and about one-third were female.
Following the statistical superiority in ASAS 40 response rates seen with IV secukinumab at week 16, patients who from there switched from placebo to IV secukinumab achieved comparable ASAS 40 response rates to those of patients originally randomized to secukinumab by week 24, reaching 66.8% for those on secukinumab the whole time and 74.9% for those who switched.
Secondary outcome measures were similar in both groups at week 52.
Among all patients who took secukinumab – the percentage with any adverse event was 63.2%, and 6% had a nonfatal adverse event deemed serious. There was one death during secukinumab treatment not suspected to be related to treatment.
In a presentation about the axSpA study findings, Atul Deodhar, MD, of Oregon Health & Science University, noted that “having an IV biologic available in the U.S. has some advantages. There are certain insurance providers such as Medicare where it is more economical for the patient to have an IV drug available.”
Dr. Deodhar also noted that in October the FDA approved a recommended lower dose for the IV treatment than in the study: 1.75 mg/kg instead of 3 mg/kg following the loading dose. That’s because the 3 mg/kg dose caused blood levels to be higher than those in the subcutaneous form, he said.
The FDA made the same dose recommendation for PsA.
Study limitations
Dr. Goodson, the U.K. consultant rheumatologist, noted a limitation of the trials: “It would have been interesting to compare IV to subcutaneous route secukinumab.” Still, the findings suggest that “the safety and efficacy of IV administration appears comparable,” she said.
“IV administration will have associated costs of attending hospital or infusion clinics,” she added, “and the cost of additional staff and administration need to be considered.”
Novartis, the maker of secukinumab, funded both studies. The PsA study authors report multiple relationships with industry, and some, such as Dr. Kivitz, have connections to Novartis. The axSpA study authors also report multiple relationships with industry, and some, such as Dr. Deodhar, have connections to Novartis. Some authors of both studies are Novartis employees. Dr. Goodson disclosed financial relationships with UCB and AbbVie.
SAN DIEGO – Monthly use of intravenously administered secukinumab (Cosentyx) proved its efficacy over placebo in treating psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA) in two industry-sponsored, randomized, double-blinded, phase 3 trials of the drug’s second and newly approved route of administration.
The studies of the human monoclonal antibody secukinumab, an interleukin-17 inhibitor, were presented at the annual meeting of the American College of Rheumatology. A subcutaneously injectable formulation of the drug is available, and the Food and Drug Administration approved the IV form for the conditions in October, although at a recommended lower monthly dose than the new trials examined.
In the PsA trial, 191 patients took IV secukinumab, and 190 took placebo. For the primary endpoint, the percentages who reached at least a 50% improvement in American College of Rheumatology response criteria (ACR 50) at 16 weeks were 31.4% and 6.3%, respectively (P < .0001).
In the axSpA trial, 264 patients took IV secukinumab, and 262 took placebo. The primary endpoint, at least a 40% improvement in Assessment of the Spondyloarthritis International Society response criteria (ASAS 40), was met at 16 weeks by 40.9% and 22.9%, respectively (P < .0001).
“Both studies appear to present clear efficacy of IV route administration of secukinumab with no clear increase in safety signals,” consultant rheumatologist Nicola Goodson, MBChB, PhD, of Aintree University Hospital in Liverpool, England, said in an interview.
“Offering IV administration as an option to patients is helpful,” added Dr. Goodson, who was not involved with the study but is familiar with its findings.
As Dr. Goodson explained, secukinumab “was the first IL [interleukin]-17 inhibitor used to treat spondyloarthropathies, and we have been using subcutaneous secukinumab to treat psoriasis, psoriatic arthritis, and axial spondyloarthritis/ankylosing spondylitis since 2016 in the U.K. Our experience with this medication has been good with similar efficacy to anti-TNF [tumor necrosis factor] therapy in axial spondyloarthritis. The medication is generally well-tolerated, and the subcutaneous pen injection device is easy for patients to use.”
However, IV treatment may speed up onset of action, she said, and it may be useful in situations when compliance is a challenge.
PsA trial details
In the PsA trial, known as INVIGORATE-2, researchers recruited patients who met the CASPAR criteria for active PsA with symptoms for ≥ 6 months, and had ≥ 3 tender joints out of 78 joints and ≥ 3 swollen joints out of 76.
Participants with a mean age of 48, including 55% females, were randomized 1:1 to receive placebo or secukinumab (6 mg/kg at baseline followed by 3 mg/kg every 4 weeks). Those in the placebo group were switched to the same monthly doses of secukinumab at 16 weeks.
“Patients who switched from the placebo had a similar increase of efficacy as the original treated group,” rheumatologist Alan J. Kivitz, MD, of the Altoona Center for Clinical Research, in Duncansville, Penn., said in his presentation at the meeting. Specifically, at 52 weeks, the groups had similar ACR 50 response rates: 58% with secukinumab and 64% with placebo-to-secukinumab.
The fact that patients in the original placebo group who received 3 mg IV doses without 6-mg loading doses achieved ACR response rates similar to those who took secukinumab during the whole trial “could suggest that the IV loading dose may not be required. This would need to be explored in a randomized head-to-head study, but it’s an interesting observation that may reduce costs and exposure to higher doses of medication at the start of treatment,” Dr. Goodson said.
Among the patients who received secukinumab at any point in the study, 63% had a treatment-emergent adverse event, including 5.9% with serious events. One death was reported in the placebo group before week 16. No other deaths were reported.
AxSpA trial details
In the axSpA trial, called INVIGORATE-1, researchers recruited people aged ≥18 years with a diagnosis of active radiographic axSpA according to modified New York criteria or nonradiographic axSpA according to ASAS criteria, and all had inflammatory back pain for ≥6 months with an onset before age 45. They were randomized at a 1:1 ratio to receive IV secukinumab (6 mg/kg loading dose, followed by 3 mg/kg every 4 weeks) or placebo for 16 weeks. At that point, the placebo group switched to the same monthly doses of IV secukinumab.
Participants had a mean age of about 39, and about one-third were female.
Following the statistical superiority in ASAS 40 response rates seen with IV secukinumab at week 16, patients who from there switched from placebo to IV secukinumab achieved comparable ASAS 40 response rates to those of patients originally randomized to secukinumab by week 24, reaching 66.8% for those on secukinumab the whole time and 74.9% for those who switched.
Secondary outcome measures were similar in both groups at week 52.
Among all patients who took secukinumab – the percentage with any adverse event was 63.2%, and 6% had a nonfatal adverse event deemed serious. There was one death during secukinumab treatment not suspected to be related to treatment.
In a presentation about the axSpA study findings, Atul Deodhar, MD, of Oregon Health & Science University, noted that “having an IV biologic available in the U.S. has some advantages. There are certain insurance providers such as Medicare where it is more economical for the patient to have an IV drug available.”
Dr. Deodhar also noted that in October the FDA approved a recommended lower dose for the IV treatment than in the study: 1.75 mg/kg instead of 3 mg/kg following the loading dose. That’s because the 3 mg/kg dose caused blood levels to be higher than those in the subcutaneous form, he said.
The FDA made the same dose recommendation for PsA.
Study limitations
Dr. Goodson, the U.K. consultant rheumatologist, noted a limitation of the trials: “It would have been interesting to compare IV to subcutaneous route secukinumab.” Still, the findings suggest that “the safety and efficacy of IV administration appears comparable,” she said.
“IV administration will have associated costs of attending hospital or infusion clinics,” she added, “and the cost of additional staff and administration need to be considered.”
Novartis, the maker of secukinumab, funded both studies. The PsA study authors report multiple relationships with industry, and some, such as Dr. Kivitz, have connections to Novartis. The axSpA study authors also report multiple relationships with industry, and some, such as Dr. Deodhar, have connections to Novartis. Some authors of both studies are Novartis employees. Dr. Goodson disclosed financial relationships with UCB and AbbVie.
SAN DIEGO – Monthly use of intravenously administered secukinumab (Cosentyx) proved its efficacy over placebo in treating psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA) in two industry-sponsored, randomized, double-blinded, phase 3 trials of the drug’s second and newly approved route of administration.
The studies of the human monoclonal antibody secukinumab, an interleukin-17 inhibitor, were presented at the annual meeting of the American College of Rheumatology. A subcutaneously injectable formulation of the drug is available, and the Food and Drug Administration approved the IV form for the conditions in October, although at a recommended lower monthly dose than the new trials examined.
In the PsA trial, 191 patients took IV secukinumab, and 190 took placebo. For the primary endpoint, the percentages who reached at least a 50% improvement in American College of Rheumatology response criteria (ACR 50) at 16 weeks were 31.4% and 6.3%, respectively (P < .0001).
In the axSpA trial, 264 patients took IV secukinumab, and 262 took placebo. The primary endpoint, at least a 40% improvement in Assessment of the Spondyloarthritis International Society response criteria (ASAS 40), was met at 16 weeks by 40.9% and 22.9%, respectively (P < .0001).
“Both studies appear to present clear efficacy of IV route administration of secukinumab with no clear increase in safety signals,” consultant rheumatologist Nicola Goodson, MBChB, PhD, of Aintree University Hospital in Liverpool, England, said in an interview.
“Offering IV administration as an option to patients is helpful,” added Dr. Goodson, who was not involved with the study but is familiar with its findings.
As Dr. Goodson explained, secukinumab “was the first IL [interleukin]-17 inhibitor used to treat spondyloarthropathies, and we have been using subcutaneous secukinumab to treat psoriasis, psoriatic arthritis, and axial spondyloarthritis/ankylosing spondylitis since 2016 in the U.K. Our experience with this medication has been good with similar efficacy to anti-TNF [tumor necrosis factor] therapy in axial spondyloarthritis. The medication is generally well-tolerated, and the subcutaneous pen injection device is easy for patients to use.”
However, IV treatment may speed up onset of action, she said, and it may be useful in situations when compliance is a challenge.
PsA trial details
In the PsA trial, known as INVIGORATE-2, researchers recruited patients who met the CASPAR criteria for active PsA with symptoms for ≥ 6 months, and had ≥ 3 tender joints out of 78 joints and ≥ 3 swollen joints out of 76.
Participants with a mean age of 48, including 55% females, were randomized 1:1 to receive placebo or secukinumab (6 mg/kg at baseline followed by 3 mg/kg every 4 weeks). Those in the placebo group were switched to the same monthly doses of secukinumab at 16 weeks.
“Patients who switched from the placebo had a similar increase of efficacy as the original treated group,” rheumatologist Alan J. Kivitz, MD, of the Altoona Center for Clinical Research, in Duncansville, Penn., said in his presentation at the meeting. Specifically, at 52 weeks, the groups had similar ACR 50 response rates: 58% with secukinumab and 64% with placebo-to-secukinumab.
The fact that patients in the original placebo group who received 3 mg IV doses without 6-mg loading doses achieved ACR response rates similar to those who took secukinumab during the whole trial “could suggest that the IV loading dose may not be required. This would need to be explored in a randomized head-to-head study, but it’s an interesting observation that may reduce costs and exposure to higher doses of medication at the start of treatment,” Dr. Goodson said.
Among the patients who received secukinumab at any point in the study, 63% had a treatment-emergent adverse event, including 5.9% with serious events. One death was reported in the placebo group before week 16. No other deaths were reported.
AxSpA trial details
In the axSpA trial, called INVIGORATE-1, researchers recruited people aged ≥18 years with a diagnosis of active radiographic axSpA according to modified New York criteria or nonradiographic axSpA according to ASAS criteria, and all had inflammatory back pain for ≥6 months with an onset before age 45. They were randomized at a 1:1 ratio to receive IV secukinumab (6 mg/kg loading dose, followed by 3 mg/kg every 4 weeks) or placebo for 16 weeks. At that point, the placebo group switched to the same monthly doses of IV secukinumab.
Participants had a mean age of about 39, and about one-third were female.
Following the statistical superiority in ASAS 40 response rates seen with IV secukinumab at week 16, patients who from there switched from placebo to IV secukinumab achieved comparable ASAS 40 response rates to those of patients originally randomized to secukinumab by week 24, reaching 66.8% for those on secukinumab the whole time and 74.9% for those who switched.
Secondary outcome measures were similar in both groups at week 52.
Among all patients who took secukinumab – the percentage with any adverse event was 63.2%, and 6% had a nonfatal adverse event deemed serious. There was one death during secukinumab treatment not suspected to be related to treatment.
In a presentation about the axSpA study findings, Atul Deodhar, MD, of Oregon Health & Science University, noted that “having an IV biologic available in the U.S. has some advantages. There are certain insurance providers such as Medicare where it is more economical for the patient to have an IV drug available.”
Dr. Deodhar also noted that in October the FDA approved a recommended lower dose for the IV treatment than in the study: 1.75 mg/kg instead of 3 mg/kg following the loading dose. That’s because the 3 mg/kg dose caused blood levels to be higher than those in the subcutaneous form, he said.
The FDA made the same dose recommendation for PsA.
Study limitations
Dr. Goodson, the U.K. consultant rheumatologist, noted a limitation of the trials: “It would have been interesting to compare IV to subcutaneous route secukinumab.” Still, the findings suggest that “the safety and efficacy of IV administration appears comparable,” she said.
“IV administration will have associated costs of attending hospital or infusion clinics,” she added, “and the cost of additional staff and administration need to be considered.”
Novartis, the maker of secukinumab, funded both studies. The PsA study authors report multiple relationships with industry, and some, such as Dr. Kivitz, have connections to Novartis. The axSpA study authors also report multiple relationships with industry, and some, such as Dr. Deodhar, have connections to Novartis. Some authors of both studies are Novartis employees. Dr. Goodson disclosed financial relationships with UCB and AbbVie.
AT ACR 2023
Entheseal ultrasound by dermatologists may not be effective for screening PsA
Key clinical point: In patients with psoriasis, the German Psoriasis Arthritis Diagnostic (GEPARD) questionnaire followed by a dermatological assessment can detect psoriatic arthritis (PsA) risk almost accurately, and entheseal ultrasound by dermatologists shows no additional benefits in this regard.
Major finding: PsA was diagnosed in 6 out of 40 patients, with a sensitivity of 100%; however, dermatologic ultrasound evaluation of the entheses alone was unable to confirm these findings. Of the remaining 34 patients with psoriasis, 8 were deemed by dermatologists as possibly having PsA based on the ultrasound examination although rheumatologists detected ultrasound abnormalities in only 3 patients.
Study details: Findings are from a cross-sectional study including 40 patients with psoriasis who completed the GEPARD questionnaire and subsequently underwent an assessment for PsA by a dermatologist and an ultrasound examination.
Disclosures: This study was supported by a conditional research grant from Rheumazentrum Erlangen-Nuremberg e.V. and AGJR Verbundprojekt. The authors declared no conflicts of interest.
Source: Bartsch V et al. Screening for psoriatic arthritis in dermatological settings—Are handheld ultrasound devices the gamechangers we hoped for? Rheumatology (Oxford). 2023 (Nov 3). doi: 10.1093/rheumatology/kead592
Key clinical point: In patients with psoriasis, the German Psoriasis Arthritis Diagnostic (GEPARD) questionnaire followed by a dermatological assessment can detect psoriatic arthritis (PsA) risk almost accurately, and entheseal ultrasound by dermatologists shows no additional benefits in this regard.
Major finding: PsA was diagnosed in 6 out of 40 patients, with a sensitivity of 100%; however, dermatologic ultrasound evaluation of the entheses alone was unable to confirm these findings. Of the remaining 34 patients with psoriasis, 8 were deemed by dermatologists as possibly having PsA based on the ultrasound examination although rheumatologists detected ultrasound abnormalities in only 3 patients.
Study details: Findings are from a cross-sectional study including 40 patients with psoriasis who completed the GEPARD questionnaire and subsequently underwent an assessment for PsA by a dermatologist and an ultrasound examination.
Disclosures: This study was supported by a conditional research grant from Rheumazentrum Erlangen-Nuremberg e.V. and AGJR Verbundprojekt. The authors declared no conflicts of interest.
Source: Bartsch V et al. Screening for psoriatic arthritis in dermatological settings—Are handheld ultrasound devices the gamechangers we hoped for? Rheumatology (Oxford). 2023 (Nov 3). doi: 10.1093/rheumatology/kead592
Key clinical point: In patients with psoriasis, the German Psoriasis Arthritis Diagnostic (GEPARD) questionnaire followed by a dermatological assessment can detect psoriatic arthritis (PsA) risk almost accurately, and entheseal ultrasound by dermatologists shows no additional benefits in this regard.
Major finding: PsA was diagnosed in 6 out of 40 patients, with a sensitivity of 100%; however, dermatologic ultrasound evaluation of the entheses alone was unable to confirm these findings. Of the remaining 34 patients with psoriasis, 8 were deemed by dermatologists as possibly having PsA based on the ultrasound examination although rheumatologists detected ultrasound abnormalities in only 3 patients.
Study details: Findings are from a cross-sectional study including 40 patients with psoriasis who completed the GEPARD questionnaire and subsequently underwent an assessment for PsA by a dermatologist and an ultrasound examination.
Disclosures: This study was supported by a conditional research grant from Rheumazentrum Erlangen-Nuremberg e.V. and AGJR Verbundprojekt. The authors declared no conflicts of interest.
Source: Bartsch V et al. Screening for psoriatic arthritis in dermatological settings—Are handheld ultrasound devices the gamechangers we hoped for? Rheumatology (Oxford). 2023 (Nov 3). doi: 10.1093/rheumatology/kead592
Syndesmophyte formation is a rare event in PsA
Key clinical point: Patients with psoriatic arthritis (PsA), except those with elevated C-reactive protein (CRP) levels (≥10 mg/L) and radiographic sacroiliac involvement, generally had a very low likelihood of developing syndesmophytes.
Major finding: At 2 years of follow-up, the majority (~90%) of patients showed no new syndesmophyte development, with syndesmophyte development being reported in only 11 patients. The probability of developing vs not developing syndesmophytes increased numerically by 3 and 14 times in patients with radiographic sacroiliitis and in those with radiographic sacroiliitis plus elevated CRP levels, respectively.
Study details: Findings are from an analysis of the data of 150 patients with PsA from the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) trial who had baseline and 2-year follow-up radiographs available.
Disclosures: The BEPAS trial was funded by MSD Belgium. Some authors declared receiving consultancy fees, speaker fees, or research grants from or having other ties with various sources, including MSD.
Source: de Hooge M et al. Specific descriptions of axial involvement are associated with radiographic damage development after 2 years in psoriatic arthritis patients. Ann Rheum Dis. 2023 (Nov 2). doi: 10.1136/ard-2023-224501
Key clinical point: Patients with psoriatic arthritis (PsA), except those with elevated C-reactive protein (CRP) levels (≥10 mg/L) and radiographic sacroiliac involvement, generally had a very low likelihood of developing syndesmophytes.
Major finding: At 2 years of follow-up, the majority (~90%) of patients showed no new syndesmophyte development, with syndesmophyte development being reported in only 11 patients. The probability of developing vs not developing syndesmophytes increased numerically by 3 and 14 times in patients with radiographic sacroiliitis and in those with radiographic sacroiliitis plus elevated CRP levels, respectively.
Study details: Findings are from an analysis of the data of 150 patients with PsA from the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) trial who had baseline and 2-year follow-up radiographs available.
Disclosures: The BEPAS trial was funded by MSD Belgium. Some authors declared receiving consultancy fees, speaker fees, or research grants from or having other ties with various sources, including MSD.
Source: de Hooge M et al. Specific descriptions of axial involvement are associated with radiographic damage development after 2 years in psoriatic arthritis patients. Ann Rheum Dis. 2023 (Nov 2). doi: 10.1136/ard-2023-224501
Key clinical point: Patients with psoriatic arthritis (PsA), except those with elevated C-reactive protein (CRP) levels (≥10 mg/L) and radiographic sacroiliac involvement, generally had a very low likelihood of developing syndesmophytes.
Major finding: At 2 years of follow-up, the majority (~90%) of patients showed no new syndesmophyte development, with syndesmophyte development being reported in only 11 patients. The probability of developing vs not developing syndesmophytes increased numerically by 3 and 14 times in patients with radiographic sacroiliitis and in those with radiographic sacroiliitis plus elevated CRP levels, respectively.
Study details: Findings are from an analysis of the data of 150 patients with PsA from the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) trial who had baseline and 2-year follow-up radiographs available.
Disclosures: The BEPAS trial was funded by MSD Belgium. Some authors declared receiving consultancy fees, speaker fees, or research grants from or having other ties with various sources, including MSD.
Source: de Hooge M et al. Specific descriptions of axial involvement are associated with radiographic damage development after 2 years in psoriatic arthritis patients. Ann Rheum Dis. 2023 (Nov 2). doi: 10.1136/ard-2023-224501
Early efficacy with guselkumab predicts lower radiographic progression in PsA
Key clinical point: Improvement in the Disease Activity Index in PsA (DAPSA) scores as early as week 8 was associated with a lower progression of structural joint damage at 2 years in biologic-naive patients with psoriatic arthritis (PsA) who were treated with guselkumab.
Major finding: A greater improvement in DAPSA scores as early as week 8 (parameter estimate [β] −0.03, P = .0096) and the achievement of DAPSA low disease activity at week 8 (least squares mean difference −1.44; P = .0151) were associated with a significantly lower radiographic progression of joint damage through week 100.
Study details: This post hoc analysis included 449 biologic-naive patients with PsA from the DISCOVER-2 trial who received 100 mg guselkumab every 4 or 8 weeks.
Disclosures: The DISCOVER-2 trial was funded by Janssen Research & Development, LLC. Five authors declared being employees of Janssen or owning stock or stock options in Johnson & Johnson. The other authors declared ties with various sources, including Janssen.
Source: Mease PJ et al. Earlier clinical response predicts low rates of radiographic progression in biologic-naïve patients with active psoriatic arthritis receiving guselkumab treatment. Clin Rheumatol. 2023 (Oct 3). doi: 10.1007/s10067-023-06745-y
Key clinical point: Improvement in the Disease Activity Index in PsA (DAPSA) scores as early as week 8 was associated with a lower progression of structural joint damage at 2 years in biologic-naive patients with psoriatic arthritis (PsA) who were treated with guselkumab.
Major finding: A greater improvement in DAPSA scores as early as week 8 (parameter estimate [β] −0.03, P = .0096) and the achievement of DAPSA low disease activity at week 8 (least squares mean difference −1.44; P = .0151) were associated with a significantly lower radiographic progression of joint damage through week 100.
Study details: This post hoc analysis included 449 biologic-naive patients with PsA from the DISCOVER-2 trial who received 100 mg guselkumab every 4 or 8 weeks.
Disclosures: The DISCOVER-2 trial was funded by Janssen Research & Development, LLC. Five authors declared being employees of Janssen or owning stock or stock options in Johnson & Johnson. The other authors declared ties with various sources, including Janssen.
Source: Mease PJ et al. Earlier clinical response predicts low rates of radiographic progression in biologic-naïve patients with active psoriatic arthritis receiving guselkumab treatment. Clin Rheumatol. 2023 (Oct 3). doi: 10.1007/s10067-023-06745-y
Key clinical point: Improvement in the Disease Activity Index in PsA (DAPSA) scores as early as week 8 was associated with a lower progression of structural joint damage at 2 years in biologic-naive patients with psoriatic arthritis (PsA) who were treated with guselkumab.
Major finding: A greater improvement in DAPSA scores as early as week 8 (parameter estimate [β] −0.03, P = .0096) and the achievement of DAPSA low disease activity at week 8 (least squares mean difference −1.44; P = .0151) were associated with a significantly lower radiographic progression of joint damage through week 100.
Study details: This post hoc analysis included 449 biologic-naive patients with PsA from the DISCOVER-2 trial who received 100 mg guselkumab every 4 or 8 weeks.
Disclosures: The DISCOVER-2 trial was funded by Janssen Research & Development, LLC. Five authors declared being employees of Janssen or owning stock or stock options in Johnson & Johnson. The other authors declared ties with various sources, including Janssen.
Source: Mease PJ et al. Earlier clinical response predicts low rates of radiographic progression in biologic-naïve patients with active psoriatic arthritis receiving guselkumab treatment. Clin Rheumatol. 2023 (Oct 3). doi: 10.1007/s10067-023-06745-y