User login
E-cigarette users topped 8 million in 2018
according to a report from the National Center for Health Statistics.
Those 8.1 million individuals who were using e-cigarettes either every day or some days represented 3.2% of the total adult population, based on data from the 2018 National Health Interview Survey. An even larger proportion, 14.9%, said that they had at least tried an e-cigarette, Maria A. Villarroel, PhD, and associates at the NCHS said in a recent data brief.
Most cigarette smokers, both current and former, were even more likely to use e-cigarettes, they noted.
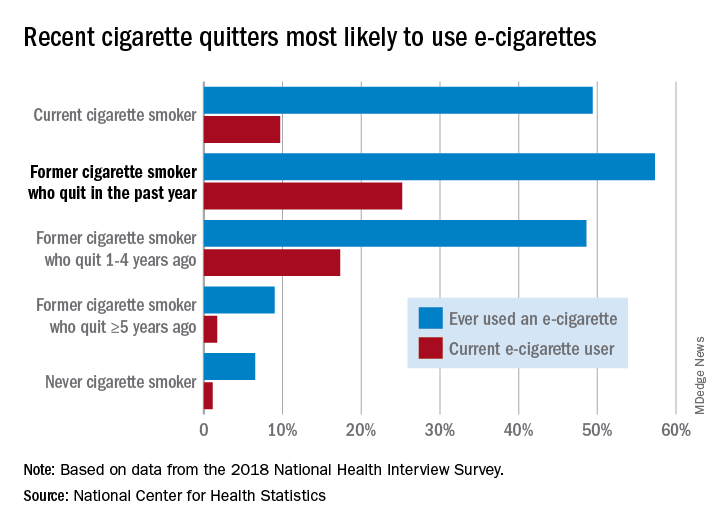
Former cigarette smokers who had quit within the last year were the most likely to use e-cigarettes – 57.3% had ever used one and 25.2% were current users – while current cigarette users (49.4% ever use and 9.7% current use) and former smokers who had quit 1-5 years before (48.6% ever use, 17.3% current) also were above-average e-cigarette consumers, they reported.
Use was significantly lower, however, among former cigarette smokers who had quit 5 or more years earlier (9.0% and 1.7%, respectively) and those who had never smoked (6.5% and 1.1%), the NCHS investigators said.
The survey data also showed much variation among the sociodemographic subgroups:
- E-cigarette ever/current use was significantly higher in men (17.9% and 4.2%) than women (12.3% and 2.3%).
- Whites were significantly more likely to use e-cigarettes (16.9% and 3.7%), compared with Hispanic (11.5% and 2.5%), black (10.0% and 1.6%), and Asian (10.2% and 2.2%) adults.
- There was significant trend of decreasing use from age 18-24 years (25.8% and 7.6%) to 65 years and older (4.7% and 0.8%).
SOURCE: Villarroel MA et al. NCHS Data Brief No. 365, April 2020.
according to a report from the National Center for Health Statistics.

Those 8.1 million individuals who were using e-cigarettes either every day or some days represented 3.2% of the total adult population, based on data from the 2018 National Health Interview Survey. An even larger proportion, 14.9%, said that they had at least tried an e-cigarette, Maria A. Villarroel, PhD, and associates at the NCHS said in a recent data brief.
Most cigarette smokers, both current and former, were even more likely to use e-cigarettes, they noted.
Former cigarette smokers who had quit within the last year were the most likely to use e-cigarettes – 57.3% had ever used one and 25.2% were current users – while current cigarette users (49.4% ever use and 9.7% current use) and former smokers who had quit 1-5 years before (48.6% ever use, 17.3% current) also were above-average e-cigarette consumers, they reported.
Use was significantly lower, however, among former cigarette smokers who had quit 5 or more years earlier (9.0% and 1.7%, respectively) and those who had never smoked (6.5% and 1.1%), the NCHS investigators said.
The survey data also showed much variation among the sociodemographic subgroups:
- E-cigarette ever/current use was significantly higher in men (17.9% and 4.2%) than women (12.3% and 2.3%).
- Whites were significantly more likely to use e-cigarettes (16.9% and 3.7%), compared with Hispanic (11.5% and 2.5%), black (10.0% and 1.6%), and Asian (10.2% and 2.2%) adults.
- There was significant trend of decreasing use from age 18-24 years (25.8% and 7.6%) to 65 years and older (4.7% and 0.8%).
SOURCE: Villarroel MA et al. NCHS Data Brief No. 365, April 2020.
according to a report from the National Center for Health Statistics.

Those 8.1 million individuals who were using e-cigarettes either every day or some days represented 3.2% of the total adult population, based on data from the 2018 National Health Interview Survey. An even larger proportion, 14.9%, said that they had at least tried an e-cigarette, Maria A. Villarroel, PhD, and associates at the NCHS said in a recent data brief.
Most cigarette smokers, both current and former, were even more likely to use e-cigarettes, they noted.
Former cigarette smokers who had quit within the last year were the most likely to use e-cigarettes – 57.3% had ever used one and 25.2% were current users – while current cigarette users (49.4% ever use and 9.7% current use) and former smokers who had quit 1-5 years before (48.6% ever use, 17.3% current) also were above-average e-cigarette consumers, they reported.
Use was significantly lower, however, among former cigarette smokers who had quit 5 or more years earlier (9.0% and 1.7%, respectively) and those who had never smoked (6.5% and 1.1%), the NCHS investigators said.
The survey data also showed much variation among the sociodemographic subgroups:
- E-cigarette ever/current use was significantly higher in men (17.9% and 4.2%) than women (12.3% and 2.3%).
- Whites were significantly more likely to use e-cigarettes (16.9% and 3.7%), compared with Hispanic (11.5% and 2.5%), black (10.0% and 1.6%), and Asian (10.2% and 2.2%) adults.
- There was significant trend of decreasing use from age 18-24 years (25.8% and 7.6%) to 65 years and older (4.7% and 0.8%).
SOURCE: Villarroel MA et al. NCHS Data Brief No. 365, April 2020.
With life in the balance, a pediatric palliative care program expands its work to adults
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.
In late March of 2020, when it became clear that hospitals in the greater New York City area would face a capacity crisis in caring for seriously ill patients with COVID-19, members of the leadership team at the Children’s Hospital at Montefiore (CHAM) in the Bronx, N.Y., convened to draft a response plan.
The recommendations put into action that day included moving the hospital’s emergency department from the lower level to the fourth floor, increasing the age limit for patients seen in the ED from 21 years of age to 30 and freeing up an entire hospital floor and a half to accommodate the anticipated surge of patients with COVID-19 admitted to Montefiore’s interconnected adult hospital, according to Sarah E. Norris, MD.
“We made multiple moves all at once,” said Dr. Norris, director of pediatric palliative care at CHAM. “It struck everyone as logical that palliative care had to be expanded, because all of the news we had received as the surge came to New York from around the world was full of death and uncertainty, and would require thoughtful conversations about end-of-life wishes at critical times and how to really respect the person and understand their values.”
When Dr. Norris left the leadership team meeting, she returned to her office, put her face in her hands, and sobbed as she began to process the gravity of what was ahead. “I cried because I knew that so many families were going to suffer a heartbreak, no matter how much we could do,” she said.
Stitching the QUILT
Over the next few days, Dr. Norris began recruiting colleagues from the large Montefiore Health System – most of whom she did not know – who met criteria for work deployment to expand CHAM’s palliative care program of clinician to 27 clinicians consisting of pediatricians, nurse practitioners, and psychologists, to meet the projected needs of COVID-19 patients and their families.
Some candidates for the effort, known as the Quality in Life Team (QUILT), were 65 years of age or older, considered at high risk for developing COVID-19-related complications themselves. Others were immunocompromised or had medical conditions that would not allow them to have direct contact with COVID-19 patients. “There were also clinicians in other parts of our health system whose practice hours were going to be severely reduced,” said Dr. Norris, who is board-certified in general pediatrics and in hospice and palliative care medicine.
Once she assembled QUILT, members participated in a 1-day rapid training webinar covering the basics of palliative care and grief, and readied themselves for one of three roles: physicians to provide face-to-face palliative care in CHAM; supportive callers to provide support to patients with COVID-19 and their families between 12:00-8:00 p.m. each day; and bereavement callers to reach out to families who lost loved ones to COVID-19 and provide grief counseling for 3 weeks.
“This allows families to have at least two contacts a day from the hospital: one from the medical team that’s giving them technical, medical information, and another from members of the QUILT team,” Dr. Norris said. “We provide support for the worry, anxiety, and fear that we know creeps in when you’re separated from your family member, especially during a pandemic when you watch TV and there’s a death count rising.”
During her early meetings with QUILT members via Zoom or on the phone, Dr. Norris encouraged them to stretch their skill sets and mindsets as they shifted from caring for children and adolescents to mostly adults. “Pediatricians are all about family; that’s why we get into this,” she said. “We’re used to treating your kids, but then, suddenly, the parent becomes our patient, like in COVID-19, or the grandparent becomes our patient. We treat you all the same; you’re part of our family. There has been no adult who has died ‘within our house’ that has died alone. There has either been a staff member at their bedside, or when possible, a family member. We are witnessing life until the last breath here.”
‘They have no loved ones with them’
One day, members of CHAM’s medical team contacted Dr. Norris about a patient with COVID-19 who’d been cared for by Montefiore clinicians all of his young life. The boy’s mother, who did not speak English, was at his bedside in the ICU, and the clinicians asked Dr. Norris to speak with her by cell phone while they prepared him for intubation.
“We were looking at each other through a glass window wall in our ICU,” Dr. Norris recalled. “I talked to her the entire time the team worked to put him on the breathing machine, through an interpreter. I asked her to tell me about her son and about her family, and she did. We developed a warm relationship. After that, every day I would see her son through the glass window wall. Every couple of days, I would have the privilege of talking to his mother by phone. At one point, she asked me, ‘Dr. Norris, do you think his lungs will heal?’ I had to tell her no. Almost selfishly, I was relieved we were on the phone, because she cried, and so did I. When he died, she was able to be by his side.”
Frederick J. Kaskel, MD, PhD, joined QUILT as a supportive caller after being asked to go home during his on-call shift on St. Patrick’s Day at CHAM, where he serves as chief emeritus of nephrology. “I was told that I was deemed to be at high risk because of my age,” the 75-year-old said. “The next day, a junior person took over for me, and 2 days later she got sick with COVID-19. She’s fine but she was home for 3 weeks sick as a dog. It was scary.”
In his role as a supportive caller, Dr. Kaskel found himself engaged in his share of detective work, trying to find phone numbers of next of kin for patients hospitalized with COVID-19. “When they come into the ER, they may not have been with a loved one or a family member; they may have been brought in by an EMT,” he said. “Some of them speak little English and others have little documentation with them. It takes a lot of work to get phone numbers.”
Once Dr. Kaskel reaches a loved one by phone, he introduces himself as a member of the QUILT team. “I tell them I’m not calling to update the medical status but just to talk to them about their loved one,” he said. “Then I usually ask, ‘So, how are you doing with this? The stress is enormous, the uncertainties.’ Then they open up and express their fears. I’ve had a lot of people say, ‘we have no money, and I don’t know how we’re going to pay rent for the apartment. We have to line up for food.’ I also ask what they do to alleviate stress. One guy said, ‘I drink a lot, but I’m careful.’ ”
Dr. Kaskel, who is also a past president of the American Society of Pediatric Nephrology, applies that same personable approach in daily conversations with adult patients hospitalized at CHAM with COVID-19, the majority of whom are African Americans in their 30s, 40s, and 50s. “Invariably, they ask, ‘Has my loved one been updated as to my status?’ ” he said. “The second thing they often say is, ‘I’m worried about infecting other people, but I also worry if I’m going to get through this. I’m really afraid I’m going to die.’ I say, ‘You have a wonderful team keeping track of you. They’re seeing you all the time and making changes to your medicines.’ ”
When patients express their fear of dying from the virus, Dr. Kaskel asks them how they’re coping with that fear. Most tell him that they pray.
“If they don’t answer, I ask if they have any hobbies, like ‘Are you watching TV? Are you reading? Do you have your cell phone?’ ” he said. “Then they open up and say things like, ‘I’m listening to music on the cell phone,’ or ‘I’m FaceTiming with my loved ones.’ The use of FaceTime is crucial, because they are in a hospital, critically ill, potentially dying alone with strangers. This really hit me on the first day [of this work]. They have no loved ones with them. They have strangers: the CHAM nurses, the medical residents, the social workers, and the doctors.”
No hospital cheeseburgers
QUILT began its work on April 6, and at one time provided palliative care services for a peak of 92 mostly adult patients with COVID-19. The supportive callers made 249 individual connections with patients and family members by phone from April 6-13, 162 connections from April 13-19, and 130 connections from April 20-26, according to Dr. Norris. As of April 28, the CHAM inpatient census of patients aged 18 years and over with COVID-19 was 42, “and we’re making 130 connections by phone to patients and family members each day,” she said.
QUILT bereavement callers are following 30 families, providing 3 weeks of acute grief counseling from the date of death. “A sad truth is that, here in New York, our entire funeral, burial, cremation system is overwhelmed in volume,” Dr. Norris said. “Only half of the patients we’re following 3 weeks out have been able to have their family member buried or cremated; many are still waiting. What strikes me here is that pediatricians are often partners in care. With time, we’re partners in care in heartbreak, and in the occasional victory. We mourn patients who have died. We’ve had colleagues who died from COVID-19 right here at our hospital. But we stand together like a family.”
Dr. Norris recalled an older woman who came into CHAM’s ICU on a ventilator, critically ill from COVID-19. She called her husband at home every day with updates. “I got to know her husband, and I got to know her through him,” Dr. Norris said. “We talked every single day and she was able to graduate off of the breathing tube and out of the ICU, which was amazing.” The woman was moved to a floor in the adult hospital, but Dr. Norris continues to visit her and to provide her husband with updates, “because I’m devoted to them,” she said.
Recently, physicians in the adult hospital consulted with Dr. Norris about the woman. “They were trying to figure out what to do with her next,” she said. “Could she go home, or did she need rehab? They said, ‘We called you, Dr. Norris, because her husband thinks he can take her home.’ We know that COVID-19 really weakens people, so I went over to see her myself. I thought, ‘No single person could take care of an adult so weak at home.’ So, I called her husband and said, ‘I’m here with your wife, and I have to tell you; if she were my mother, I couldn’t take her home today. I need you to trust me.’ He said, ‘OK. We trust you and know that you have her best interest at heart.’ ”
Dr. Kaskel relayed the story of an older patient who was slowly recovering from COVID-19. During a phone call, he asked the man if there was anything he wanted at that moment.
“He said, ‘I’d love to see my wife and my children and my grandkids. I know I’m going to see them again, but right now, doc, if you could get me a cheeseburger with lettuce and tomato and ketchup and French fries from outside of the hospital, I’d be the happiest man in the world.’
I said, ‘What’s the matter with the cheeseburger made at the hospital?’
He said, ‘No! They can’t make the cheeseburger I want.’
I promised him I’d relay that message to the social worker responsible for the patient. I told her please, if you buy this for him, I’ll pay you back.”
Self-care and the next chapter
Twice each week, QUILT members gather in front of their computer monitors for mandatory Zoom meetings facilitated by two psychologists to share challenges, best practices, and to discuss the difficult work they’re doing. “We meet, because you cannot help someone if you cannot help yourself,” Dr. Norris said. “We have been encouraged each and every meeting to practice self-compassion, and to recognize that things happen during a pandemic – some will be the best you can do.”
She described organizing and serving on QUILT as a grounding experience with important lessons for the delivery of health care after the pandemic subsides and the team members return to their respective practices. “I think we’ve all gained a greater sense of humility, and we understand that the badge I wear every day does not protect me from becoming a patient, or from having my own family fall ill,” she said. “Here, we think about it very simply: ‘I’m going to treat you like you’re part of my own family.’ ”
Dr. Kaskel said that serving on QUILT as a supportive caller is an experience he won’t soon forget.
“The human bond is so accessible if you accept it,” he said. “If someone is an introvert that might not be able to draw out a stranger on the phone, then [he or she] shouldn’t do this [work]. But the fact that you can make a bond with someone that you’re not even seeing in person and know that both sides of this phone call are getting good vibes, that’s a remarkable feeling that I never really knew before, because I’ve never really had to do that before. It brings up feelings like I had after 9/11 – a unified approach to surviving this as people, as a community, the idea that ‘we will get through this,’ even though it’s totally different than anything before. The idea that there’s still hope. Those are things you can’t put a price on.”
An article about how CHAM transformed to provide care to adult COVID-19 patients was published online May 4, 2020, in the Journal of Pediatrics: doi: 10.1016/j.jpeds.2020.04.060.
Evidence builds linking anticoagulation to COVID-19 survival
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
, a large study from the epicenter of the U.S. outbreak suggests.
Among nearly 3,000 patients with COVID-19 admitted to New York City’s Mount Sinai Health System beginning in mid-March, median survival increased from 14 days to 21 days with the addition of anticoagulation.
The results were particularly striking among sicker patients who required mechanical ventilation, in whom in-hospital mortality fell from 62.7% to 29.1% and median survival jumped from 9 days to 21 days.
Interestingly, the association with anticoagulation and improved survival remained even after adjusting for mechanical ventilation, the authors reported May 6 in the Journal of the American College of Cardiology.
“It’s important for the community to know, first of all, how this should be approached and, second, it’s really opening a door to a new reality,” senior corresponding author Valentin Fuster, MD, PhD, director of Mount Sinai’s Zena and Michael A. Wiener Cardiovascular Institute and JACC editor-in-chief.
“I can tell you any family of mine who will have this disease absolutely will be on antithrombotic therapy and, actually, so are all of the patients at Mount Sinai now,” he said in an interview. COVID-19 is thought to promote thrombosis but the exact role of anticoagulation in the management of COVID-19 and optimal regimen are unknown.
In late March, the International Society on Thrombosis and Haemostasis recommended that all hospitalized COVID-19 patients, even those not in the ICU, should receive prophylactic-dose low-molecular-weight heparin (LMWH), unless they have contraindications.
Last month, international consensus-based recommendations were published for the diagnosis and management of thrombotic disease in patients with COVID-19.
In early March, however, data were scare and only a minimal number of patients were receiving anticoagulants at Mount Sinai.
“But after a few weeks, we reached an intuitive feeling that anticoagulation was of benefit and, at the same time, the literature was beginning to say clots were important in this disease,” Dr. Fuster said. “So we took a very straightforward approach and set up a policy in our institution that all COVID-19 patients should be on antithrombotic therapy. It was a decision made without data, but it was a feeling.”
For the present study, the researchers examined mortality and bleeding among 2,773 patients hospitalized at Mount Sinai with confirmed COVID-19 between March 14 and April 11.
Of these, 786 (28%) received systemic anticoagulation including subcutaneous heparin, LMWH, fractionated heparin, and the novel oral anticoagulants apixaban and dabigatran, for a median of 3 days (range, 2-7 days). Tissue plasminogen activator was also used in some ICU cases.
Major bleeding was defined as hemoglobin less than 7 g/dL and any red blood cell transfusion; at least two units of red blood cell transfusion within 48 hours; or a diagnosis code for major bleeding, notably including intracranial hemorrhage.
Patients treated with anticoagulation were more likely to require invasive mechanical ventilation (29.8% vs. 8.1%) and to have significantly increased prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C-reactive protein, and d-dimer values. In-hospital mortality was 22.5% with anticoagulation and 22.8% without anticoagulation (median survival, 14 days vs. 21 days).
In multivariate analysis, longer anticoagulation duration was associated with a 14% lower adjusted risk of in-hospital death (hazard ratio, 0.86 per day; 95% confidence interval, 0.82-0.89; P < .001).
The model adjusted for several potential confounders such as age, ethnicity, body mass index, and prehospital anticoagulation use. To adjust for differential length of stay and anticoagulation initiation, anticoagulation duration was used as a covariate and intubation was treated as a time-dependent variable.
Bleeding events were similar in patients treated with and without anticoagulation (3% vs. 1.9%; P = .2) but were more common among the 375 intubated patients than among nonintubated patients (7.5% vs. 1.35%; P value not given). “The most important thing was there was no increase in bleeding,” said Dr. Fuster.
Additional support for a possible survival benefit was published April 27 and included 449 patients with severe COVID-19 treated with heparin (mostly LMWH) for at least 7 days in Hunan, China. Overall, 28-day mortality was similar between heparin users and nonusers (30.3% vs. 29.7%) but was significantly lower among heparin users who had a Sepsis-Induced Coagulopathy score of at least 4 (40% vs. 64.2%; P = .02) or d-dimer greater than sixfold the upper limit of normal (32.8% vs. 52.4%; P = .01).
In multivariate analysis, d-dimer, prothrombin time, and age were positively correlated with 28-day mortality, and platelet count was negatively correlated with 28-day mortality.
Victor F. Tapson, MD, who directs the pulmonary embolism response team at Cedars-Sinai Medical Center in Los Angeles and was not involved with the study, said, “The Chinese data were not enough for me to anticoagulate patients therapeutically” but the Mount Sinai data strengthen the case.
“They’re wise to call this a ‘suggestion of improved outcomes,’ but it’s pretty compelling that those patients who were on anticoagulation had improved survival after adjusting for mechanical ventilation,” he said in an interview. “These are sicker patients and sicker patients may get anticoagulated more, but they may bleed more. The bleed risks were a little different but they didn’t seem too concerning.”
“I think this helps move us forward some that we should consider anticoagulating with therapeutic anticoagulation certain patients that meet certain criteria,” Dr. Tapson said. “An easy example is a patient who comes to the hospital, has active cancer and is on a DOAC [direct oral anticoagulant], and comes up with COVID.”
At the same time, some clinicians want to increase prophylactic anticoagulation “using enoxaparin 40 mg once a day and maybe go to twice a day – not quite therapeutic doses but increased prophylaxis,” he observed. Anticoagulation was given at “relatively low doses” in the Mount Sinai study but that is evolving in light of the reassuring bleeding data, Dr. Fuster said. They now have three enoxaparin regimens and, for example, give patients who don’t require intensive care enoxaparin 30 mg twice a day, up from 40 mg a day initially.
Patients are also stratified by factors such as renal failure and obesity, creating an intermediate group between those not initially needing intensive care and ICU cases.
In the coming weeks, the researchers will evaluate anticoagulation regimens and a broader array of outcomes among 5,000 patients, two-thirds of whom received anticoagulation after Mount Sinai enacted its anticoagulation policy. “We’re now going to look at the difference between all these [regimens],” Dr. Fuster said. “My personal feeling and, for feasibility issues, I hope the winner is subcutaneous heparin.”
Three randomized trials are also planned. “Three questions we really want to ask are: what to give in the hospital, what to give those who go home after the hospital, and what to give those who are not hospitalized,” he said.
The work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fuster has disclosed no relevant financial relationships. Dr. Tapson reported consulting and clinical trial work for BMS, Janssen, Daiichi Medical, ECOS/BTG, Inari, and Penumbra.
A version of this article originally appeared on Medscape.com.
Volunteering during the pandemic: What doctors need to know
A couple of weeks ago, I posted a silly picture of myself with one N95 mask and asked the folks on Twitter what else I might need. In a matter of a few days, I had filled out a form online for volunteering through the Society of Critical Care Medicine, been assigned to work at a hospital in New York City, and booked a hotel and flight.
I was going to volunteer, although I wasn’t sure of exactly what I would be doing. I’m trained as a bariatric surgeon – not obviously suited for critical care, but arguably even less suited for medicine wards.
I undoubtedly would have been less prepared if I hadn’t sought guidance on what to bring with me and generally what to expect. Less than a day after seeking advice, two local women physicians donated N95s, face shields, gowns, bouffants, and coveralls to me. I also received a laminated photo of myself to attach to my gown in the mail from a stranger I met online.
Others suggested I bring goggles, chocolate, protein bars, hand sanitizer, powdered laundry detergent, and alcohol wipes. After running around all over town, I was able find everything but the wipes.
Just as others helped me achieve my goal of volunteering, I hope I can guide those who would like to do similar work by sharing details about my experience and other information I have collected about volunteering.
Below I answer some questions that those considering volunteering might have, including why I went, who I contacted to set this up, who paid for my flight, and what I observed in the hospital.
Motivation and logistics
I am currently serving in a nonclinical role at my institution. So when the pandemic hit the United States, I felt an immense amount of guilt for not being on the front lines caring for patients. I offered my services to local hospitals and registered for the California Health Corps. I live in northern California, which was the first part of the country to shelter in place. Since my home was actually relatively spared, my services weren’t needed.
As the weeks passed, I was slowly getting more and more fit, exercising in my house since there was little else I could do, and the guilt became a cloud gathering over my head.
I decided to volunteer in a place where demands for help were higher – New York. I tried very hard to sign up to volunteer through the state’s registry for health care volunteers, but was unable to do so. Coincidentally, around that same time, I saw on Twitter that Josh Mugele, MD, emergency medicine physician and program director of the emergency medicine residency at Northeast Georgia Medical Center in Gainesville, was on his way to New York. He shared the Society of Critical Care Medicine’s form for volunteering with me, and in less than 48 hours, I was assigned to a hospital in New York City. Five days later I was on a plane from San Francisco to my destination on the opposite side of the country. The airline paid for my flight.
This is not the only path to volunteering. Another volunteer, Sara Pauk, MD, ob.gyn. at the University of Washington, Seattle, found her volunteer role through contacting the New York City Health and Hospitals system directly. Other who have volunteered told me they had contacted specific hospitals or worked with agencies that were placing physicians.
PPE
The Brooklyn hospital where I volunteered provided me with two sets of scrubs and two N95s. Gowns were variably available on our unit, and there was no eye protection. As a colleague of mine, Ben Daxon, MD, anesthesia and critical care physician at the Mayo Clinic in Rochester, Minn., had suggested, anyone volunteering in this context should bring personal protective equipment (PPE) – That includes gowns, bouffants/scrub caps, eye protection, masks, and scrubs.
The “COVID corner”
Once I arrived in New York, I did not feel particularly safe in my hotel, so I moved to another the next day. Then I had to sort out how to keep the whole room from being contaminated. I created a “COVID corner” right by the door where I kept almost everything that had been outside the door.
Every time I walked in the door, I immediately took off my shoes and left them in that corner. I could not find alcohol wipes, even after looking around in the city, so I relied on time to kill the virus, which I presumed was on everything that came from outside.
Groceries stayed by the door for 48-72 hours if possible. After that, I would move them to the “clean” parts of the room. I wore the same outfit to and from the hospital everyday, putting it on right before I left and taking it off immediately after walking into the room (and then proceeding directly to the shower). Those clothes – “my COVID outfit” – lived in the COVID corner. Anything else I wore, including exercise clothes and underwear, got washed right after I wore it.
At the hospital, I would change into scrubs and leave my COVID outfit in a plastic bag inside my handbag. Note: I fully accepted that my handbag was now a COVID handbag. I kept a pair of clogs in the hospital for daily wear. Without alcohol wipes, my room did not feel clean. But I did start to become at peace with my system, even though it was inferior to the system I use in my own home.
Meal time
In addition to bringing snacks from home, I gathered some meal items at a grocery store during my first day in New York. These included water, yogurt, a few protein drinks, fruit, and some mini chocolate croissants. It’s a pandemic – chocolate is encouraged, right?
Neither any of the volunteers I knew nor I had access to a kitchen, so this was about the best I could do.
My first week I worked nights and ate sporadically. A couple of days I bought bagel sandwiches on the way back to the hotel in the morning. Other times, I would eat yogurt or a protein bar.
I had trouble sleeping, so I would wake up early and either do yoga in my room or go for a run in a nearby park. Usually I didn’t plan well enough to eat before I went into the hospital, so I would take yogurt, some fruit, and a croissant with me as I headed out. It was hard eating on the run with a mask on my face.
When I switched to working days, I actually ordered proper dinners from local Thai, Mexican, and Indian restaurants. I paid around $20 a meal.
One night I even had dinner with a coworker who was staying at a hotel close to mine – what a luxury! Prior to all this I had been sheltering in place alone for weeks, so in that sense, this experience was a delight. I interacted with other people, in person, every day!
My commute
My hotel was about 20 minutes from the hospital. Well-meaning folks informed me that Hertz had free car rentals and Uber had discounts for health care workers. When I investigated these options, I found that only employees of certain hospitals were eligible. As a volunteer, I was not eligible.
I ultimately took Uber back and forth, and I was lucky that a few friends had sent me Uber gift cards to defray the costs. Most days, I paid about $20 each way, although 1 day there actually was “surge pricing.” The grand total for the trip was close to $800.
Many of the Uber drivers had put up plastic partitions – reminiscent of the plastic Dexter would use to contain his crime scenes – to increase their separation from their passengers. It was a bit eerie, but also somewhat welcome.
New normal
The actual work at the hospital in Brooklyn where I volunteered was different from usual practice in numerous ways. One of the things I immediately noticed was how difficult it was to get chest x-rays. After placing an emergent chest tube for a tension pneumothorax, it took about 6 hours to get a chest x-ray to assess placement.
Because code medications were needed much more frequently than normal times, these medications were kept in an open supply closet for ease of access. Many of the ventilators looked like they were from the 1970s. (They had been borrowed from the Federal Emergency Management Agency.)
What was most distinct about this work was the sheer volume of deaths and dying patients -- at least one death on our unit occurred every day I was there -- and the way families communicated with their loved ones. Countless times I held my phone over the faces of my unconscious patients to let their family profess their love and beg them to fight. While I have had to deliver bad news over the phone many times in my career, I have never had to intrude on families’ last conversations with their dying loved ones or witness that conversation occurring via a tiny screen.
Reentry
In many ways, I am lucky that I do not do clinical work in my hometown. So while other volunteers were figuring out how many more vacation days they would have to use, or whether they would have to take unpaid leave, and when and how they would get tested, all I had to do was prepare to go back home and quarantine myself for a couple of weeks.
I used up 2 weeks of vacation to volunteer in New York, but luckily, I could resume my normal work the day after I returned home.
Obviously, living in the pandemic is unique to anything we have ever experienced. Recognizing that, I recorded video diaries the whole time I was in New York. I laughed (like when I tried to fit all of my PPE on my tiny head), and I cried – several times. I suppose 1 day I may actually watch them and be reminded of what it was like to have been able to serve in this historic moment. Until then, they will remain locked up on the same phone that served as the only communication vehicle between my patients and their loved ones.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University.
A couple of weeks ago, I posted a silly picture of myself with one N95 mask and asked the folks on Twitter what else I might need. In a matter of a few days, I had filled out a form online for volunteering through the Society of Critical Care Medicine, been assigned to work at a hospital in New York City, and booked a hotel and flight.
I was going to volunteer, although I wasn’t sure of exactly what I would be doing. I’m trained as a bariatric surgeon – not obviously suited for critical care, but arguably even less suited for medicine wards.
I undoubtedly would have been less prepared if I hadn’t sought guidance on what to bring with me and generally what to expect. Less than a day after seeking advice, two local women physicians donated N95s, face shields, gowns, bouffants, and coveralls to me. I also received a laminated photo of myself to attach to my gown in the mail from a stranger I met online.
Others suggested I bring goggles, chocolate, protein bars, hand sanitizer, powdered laundry detergent, and alcohol wipes. After running around all over town, I was able find everything but the wipes.
Just as others helped me achieve my goal of volunteering, I hope I can guide those who would like to do similar work by sharing details about my experience and other information I have collected about volunteering.
Below I answer some questions that those considering volunteering might have, including why I went, who I contacted to set this up, who paid for my flight, and what I observed in the hospital.
Motivation and logistics
I am currently serving in a nonclinical role at my institution. So when the pandemic hit the United States, I felt an immense amount of guilt for not being on the front lines caring for patients. I offered my services to local hospitals and registered for the California Health Corps. I live in northern California, which was the first part of the country to shelter in place. Since my home was actually relatively spared, my services weren’t needed.
As the weeks passed, I was slowly getting more and more fit, exercising in my house since there was little else I could do, and the guilt became a cloud gathering over my head.
I decided to volunteer in a place where demands for help were higher – New York. I tried very hard to sign up to volunteer through the state’s registry for health care volunteers, but was unable to do so. Coincidentally, around that same time, I saw on Twitter that Josh Mugele, MD, emergency medicine physician and program director of the emergency medicine residency at Northeast Georgia Medical Center in Gainesville, was on his way to New York. He shared the Society of Critical Care Medicine’s form for volunteering with me, and in less than 48 hours, I was assigned to a hospital in New York City. Five days later I was on a plane from San Francisco to my destination on the opposite side of the country. The airline paid for my flight.
This is not the only path to volunteering. Another volunteer, Sara Pauk, MD, ob.gyn. at the University of Washington, Seattle, found her volunteer role through contacting the New York City Health and Hospitals system directly. Other who have volunteered told me they had contacted specific hospitals or worked with agencies that were placing physicians.
PPE
The Brooklyn hospital where I volunteered provided me with two sets of scrubs and two N95s. Gowns were variably available on our unit, and there was no eye protection. As a colleague of mine, Ben Daxon, MD, anesthesia and critical care physician at the Mayo Clinic in Rochester, Minn., had suggested, anyone volunteering in this context should bring personal protective equipment (PPE) – That includes gowns, bouffants/scrub caps, eye protection, masks, and scrubs.
The “COVID corner”
Once I arrived in New York, I did not feel particularly safe in my hotel, so I moved to another the next day. Then I had to sort out how to keep the whole room from being contaminated. I created a “COVID corner” right by the door where I kept almost everything that had been outside the door.
Every time I walked in the door, I immediately took off my shoes and left them in that corner. I could not find alcohol wipes, even after looking around in the city, so I relied on time to kill the virus, which I presumed was on everything that came from outside.
Groceries stayed by the door for 48-72 hours if possible. After that, I would move them to the “clean” parts of the room. I wore the same outfit to and from the hospital everyday, putting it on right before I left and taking it off immediately after walking into the room (and then proceeding directly to the shower). Those clothes – “my COVID outfit” – lived in the COVID corner. Anything else I wore, including exercise clothes and underwear, got washed right after I wore it.
At the hospital, I would change into scrubs and leave my COVID outfit in a plastic bag inside my handbag. Note: I fully accepted that my handbag was now a COVID handbag. I kept a pair of clogs in the hospital for daily wear. Without alcohol wipes, my room did not feel clean. But I did start to become at peace with my system, even though it was inferior to the system I use in my own home.
Meal time
In addition to bringing snacks from home, I gathered some meal items at a grocery store during my first day in New York. These included water, yogurt, a few protein drinks, fruit, and some mini chocolate croissants. It’s a pandemic – chocolate is encouraged, right?
Neither any of the volunteers I knew nor I had access to a kitchen, so this was about the best I could do.
My first week I worked nights and ate sporadically. A couple of days I bought bagel sandwiches on the way back to the hotel in the morning. Other times, I would eat yogurt or a protein bar.
I had trouble sleeping, so I would wake up early and either do yoga in my room or go for a run in a nearby park. Usually I didn’t plan well enough to eat before I went into the hospital, so I would take yogurt, some fruit, and a croissant with me as I headed out. It was hard eating on the run with a mask on my face.
When I switched to working days, I actually ordered proper dinners from local Thai, Mexican, and Indian restaurants. I paid around $20 a meal.
One night I even had dinner with a coworker who was staying at a hotel close to mine – what a luxury! Prior to all this I had been sheltering in place alone for weeks, so in that sense, this experience was a delight. I interacted with other people, in person, every day!
My commute
My hotel was about 20 minutes from the hospital. Well-meaning folks informed me that Hertz had free car rentals and Uber had discounts for health care workers. When I investigated these options, I found that only employees of certain hospitals were eligible. As a volunteer, I was not eligible.
I ultimately took Uber back and forth, and I was lucky that a few friends had sent me Uber gift cards to defray the costs. Most days, I paid about $20 each way, although 1 day there actually was “surge pricing.” The grand total for the trip was close to $800.
Many of the Uber drivers had put up plastic partitions – reminiscent of the plastic Dexter would use to contain his crime scenes – to increase their separation from their passengers. It was a bit eerie, but also somewhat welcome.
New normal
The actual work at the hospital in Brooklyn where I volunteered was different from usual practice in numerous ways. One of the things I immediately noticed was how difficult it was to get chest x-rays. After placing an emergent chest tube for a tension pneumothorax, it took about 6 hours to get a chest x-ray to assess placement.
Because code medications were needed much more frequently than normal times, these medications were kept in an open supply closet for ease of access. Many of the ventilators looked like they were from the 1970s. (They had been borrowed from the Federal Emergency Management Agency.)
What was most distinct about this work was the sheer volume of deaths and dying patients -- at least one death on our unit occurred every day I was there -- and the way families communicated with their loved ones. Countless times I held my phone over the faces of my unconscious patients to let their family profess their love and beg them to fight. While I have had to deliver bad news over the phone many times in my career, I have never had to intrude on families’ last conversations with their dying loved ones or witness that conversation occurring via a tiny screen.
Reentry
In many ways, I am lucky that I do not do clinical work in my hometown. So while other volunteers were figuring out how many more vacation days they would have to use, or whether they would have to take unpaid leave, and when and how they would get tested, all I had to do was prepare to go back home and quarantine myself for a couple of weeks.
I used up 2 weeks of vacation to volunteer in New York, but luckily, I could resume my normal work the day after I returned home.
Obviously, living in the pandemic is unique to anything we have ever experienced. Recognizing that, I recorded video diaries the whole time I was in New York. I laughed (like when I tried to fit all of my PPE on my tiny head), and I cried – several times. I suppose 1 day I may actually watch them and be reminded of what it was like to have been able to serve in this historic moment. Until then, they will remain locked up on the same phone that served as the only communication vehicle between my patients and their loved ones.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University.
A couple of weeks ago, I posted a silly picture of myself with one N95 mask and asked the folks on Twitter what else I might need. In a matter of a few days, I had filled out a form online for volunteering through the Society of Critical Care Medicine, been assigned to work at a hospital in New York City, and booked a hotel and flight.
I was going to volunteer, although I wasn’t sure of exactly what I would be doing. I’m trained as a bariatric surgeon – not obviously suited for critical care, but arguably even less suited for medicine wards.
I undoubtedly would have been less prepared if I hadn’t sought guidance on what to bring with me and generally what to expect. Less than a day after seeking advice, two local women physicians donated N95s, face shields, gowns, bouffants, and coveralls to me. I also received a laminated photo of myself to attach to my gown in the mail from a stranger I met online.
Others suggested I bring goggles, chocolate, protein bars, hand sanitizer, powdered laundry detergent, and alcohol wipes. After running around all over town, I was able find everything but the wipes.
Just as others helped me achieve my goal of volunteering, I hope I can guide those who would like to do similar work by sharing details about my experience and other information I have collected about volunteering.
Below I answer some questions that those considering volunteering might have, including why I went, who I contacted to set this up, who paid for my flight, and what I observed in the hospital.
Motivation and logistics
I am currently serving in a nonclinical role at my institution. So when the pandemic hit the United States, I felt an immense amount of guilt for not being on the front lines caring for patients. I offered my services to local hospitals and registered for the California Health Corps. I live in northern California, which was the first part of the country to shelter in place. Since my home was actually relatively spared, my services weren’t needed.
As the weeks passed, I was slowly getting more and more fit, exercising in my house since there was little else I could do, and the guilt became a cloud gathering over my head.
I decided to volunteer in a place where demands for help were higher – New York. I tried very hard to sign up to volunteer through the state’s registry for health care volunteers, but was unable to do so. Coincidentally, around that same time, I saw on Twitter that Josh Mugele, MD, emergency medicine physician and program director of the emergency medicine residency at Northeast Georgia Medical Center in Gainesville, was on his way to New York. He shared the Society of Critical Care Medicine’s form for volunteering with me, and in less than 48 hours, I was assigned to a hospital in New York City. Five days later I was on a plane from San Francisco to my destination on the opposite side of the country. The airline paid for my flight.
This is not the only path to volunteering. Another volunteer, Sara Pauk, MD, ob.gyn. at the University of Washington, Seattle, found her volunteer role through contacting the New York City Health and Hospitals system directly. Other who have volunteered told me they had contacted specific hospitals or worked with agencies that were placing physicians.
PPE
The Brooklyn hospital where I volunteered provided me with two sets of scrubs and two N95s. Gowns were variably available on our unit, and there was no eye protection. As a colleague of mine, Ben Daxon, MD, anesthesia and critical care physician at the Mayo Clinic in Rochester, Minn., had suggested, anyone volunteering in this context should bring personal protective equipment (PPE) – That includes gowns, bouffants/scrub caps, eye protection, masks, and scrubs.
The “COVID corner”
Once I arrived in New York, I did not feel particularly safe in my hotel, so I moved to another the next day. Then I had to sort out how to keep the whole room from being contaminated. I created a “COVID corner” right by the door where I kept almost everything that had been outside the door.
Every time I walked in the door, I immediately took off my shoes and left them in that corner. I could not find alcohol wipes, even after looking around in the city, so I relied on time to kill the virus, which I presumed was on everything that came from outside.
Groceries stayed by the door for 48-72 hours if possible. After that, I would move them to the “clean” parts of the room. I wore the same outfit to and from the hospital everyday, putting it on right before I left and taking it off immediately after walking into the room (and then proceeding directly to the shower). Those clothes – “my COVID outfit” – lived in the COVID corner. Anything else I wore, including exercise clothes and underwear, got washed right after I wore it.
At the hospital, I would change into scrubs and leave my COVID outfit in a plastic bag inside my handbag. Note: I fully accepted that my handbag was now a COVID handbag. I kept a pair of clogs in the hospital for daily wear. Without alcohol wipes, my room did not feel clean. But I did start to become at peace with my system, even though it was inferior to the system I use in my own home.
Meal time
In addition to bringing snacks from home, I gathered some meal items at a grocery store during my first day in New York. These included water, yogurt, a few protein drinks, fruit, and some mini chocolate croissants. It’s a pandemic – chocolate is encouraged, right?
Neither any of the volunteers I knew nor I had access to a kitchen, so this was about the best I could do.
My first week I worked nights and ate sporadically. A couple of days I bought bagel sandwiches on the way back to the hotel in the morning. Other times, I would eat yogurt or a protein bar.
I had trouble sleeping, so I would wake up early and either do yoga in my room or go for a run in a nearby park. Usually I didn’t plan well enough to eat before I went into the hospital, so I would take yogurt, some fruit, and a croissant with me as I headed out. It was hard eating on the run with a mask on my face.
When I switched to working days, I actually ordered proper dinners from local Thai, Mexican, and Indian restaurants. I paid around $20 a meal.
One night I even had dinner with a coworker who was staying at a hotel close to mine – what a luxury! Prior to all this I had been sheltering in place alone for weeks, so in that sense, this experience was a delight. I interacted with other people, in person, every day!
My commute
My hotel was about 20 minutes from the hospital. Well-meaning folks informed me that Hertz had free car rentals and Uber had discounts for health care workers. When I investigated these options, I found that only employees of certain hospitals were eligible. As a volunteer, I was not eligible.
I ultimately took Uber back and forth, and I was lucky that a few friends had sent me Uber gift cards to defray the costs. Most days, I paid about $20 each way, although 1 day there actually was “surge pricing.” The grand total for the trip was close to $800.
Many of the Uber drivers had put up plastic partitions – reminiscent of the plastic Dexter would use to contain his crime scenes – to increase their separation from their passengers. It was a bit eerie, but also somewhat welcome.
New normal
The actual work at the hospital in Brooklyn where I volunteered was different from usual practice in numerous ways. One of the things I immediately noticed was how difficult it was to get chest x-rays. After placing an emergent chest tube for a tension pneumothorax, it took about 6 hours to get a chest x-ray to assess placement.
Because code medications were needed much more frequently than normal times, these medications were kept in an open supply closet for ease of access. Many of the ventilators looked like they were from the 1970s. (They had been borrowed from the Federal Emergency Management Agency.)
What was most distinct about this work was the sheer volume of deaths and dying patients -- at least one death on our unit occurred every day I was there -- and the way families communicated with their loved ones. Countless times I held my phone over the faces of my unconscious patients to let their family profess their love and beg them to fight. While I have had to deliver bad news over the phone many times in my career, I have never had to intrude on families’ last conversations with their dying loved ones or witness that conversation occurring via a tiny screen.
Reentry
In many ways, I am lucky that I do not do clinical work in my hometown. So while other volunteers were figuring out how many more vacation days they would have to use, or whether they would have to take unpaid leave, and when and how they would get tested, all I had to do was prepare to go back home and quarantine myself for a couple of weeks.
I used up 2 weeks of vacation to volunteer in New York, but luckily, I could resume my normal work the day after I returned home.
Obviously, living in the pandemic is unique to anything we have ever experienced. Recognizing that, I recorded video diaries the whole time I was in New York. I laughed (like when I tried to fit all of my PPE on my tiny head), and I cried – several times. I suppose 1 day I may actually watch them and be reminded of what it was like to have been able to serve in this historic moment. Until then, they will remain locked up on the same phone that served as the only communication vehicle between my patients and their loved ones.
Dr. Salles is a bariatric surgeon and is currently a Scholar in Residence at Stanford (Calif.) University.
Renal function data improve risk stratification in patients with PAH
The REVEAL-based risk-management strategy was significantly more effective than the current European Society of Cardiology guidelines at discriminating risk in adults with pulmonary arterial hypertension, and renal function significantly improved risk stratification, findings from a retrospective registry study suggest.
“Although the importance of identification of low or high risk is intuitive, the clinical utility of stratification into the intermediate-risk category is less certain” in patients with pulmonary arterial hypertension (PAH), wrote Jason G.E. Zelt, MSc, of the University of Ottawa and colleagues. “Despite the importance of renal function in the PAH population, it has not been formally incorporated into many of the contemporary PAH risk tools, including current guidelines,” they noted.
In a study published in the Journal of Heart and Lung Transplantation, the researchers compared several current research tools for risk assessment in PAH, including the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL) risk calculator, the French Pulmonary Hypertension Registry (FPHR), and guidelines from the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). They also reviewed REVEAL 2.0, an update that included the estimated glomerular filtration rate (eGFR) as a measure of renal function.
The study population included 211 adults with PAH seen at a single pulmonary hypertension clinic; the average age was 63 years and 65% were women. In addition, 42% had at least stage 3 chronic kidney disease. The primary endpoint was transplant-free survival, which was a median of 7 years. Creatinine was assessed at baseline in all patients. In addition, patients were grouped based on the percent change in renal function between diagnosis and 6 months.
Although the ESC and REVEAL algorithms significantly stratified transplant-free survival risk, the researchers found little agreement among the algorithms in stratifying transplant-free survival for patients in the intermediate-risk category.
However, using REVEAL 2.0, both renal function at diagnosis and renal function at 6 months were significant predictors (P < .0001 for both) from intermediate-risk to higher- or lower-risk groups, the researchers said.
“A decrease in renal function may be a harbinger of both [right ventricle] dysfunction and further PAH disease progression. However, further research is needed to confirm whether declining eGFR is a sentinel biomarker in prospective cohorts,” the researchers said.
The study findings were limited by several factors including the retrospective design and the use of mainly baseline data without information on long-term risk assessment, the researchers noted. However, “a key finding of our study was the ability of baseline eGFR to robustly restratify ESC/ERS-based risk strategies,” they said. “Our work highlights key limitations of the ESC/ERS-based risk assessment, and suggests that incorporating measures of kidney function are important strategies moving forward,” they concluded.
Mr. Zelt is an MD/PhD student and had no financial conflicts to disclose. Some coauthors disclosed relationships with Actelion Pharmaceuticals, Bayer Pharmaceuticals, and Northern Therapeutics.
SOURCE: Zelt JGE et al. J Heart Lung Transplant. 2020 Apr 5. doi: 10.1016/j.healun.2020.03.026.
The REVEAL-based risk-management strategy was significantly more effective than the current European Society of Cardiology guidelines at discriminating risk in adults with pulmonary arterial hypertension, and renal function significantly improved risk stratification, findings from a retrospective registry study suggest.
“Although the importance of identification of low or high risk is intuitive, the clinical utility of stratification into the intermediate-risk category is less certain” in patients with pulmonary arterial hypertension (PAH), wrote Jason G.E. Zelt, MSc, of the University of Ottawa and colleagues. “Despite the importance of renal function in the PAH population, it has not been formally incorporated into many of the contemporary PAH risk tools, including current guidelines,” they noted.
In a study published in the Journal of Heart and Lung Transplantation, the researchers compared several current research tools for risk assessment in PAH, including the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL) risk calculator, the French Pulmonary Hypertension Registry (FPHR), and guidelines from the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). They also reviewed REVEAL 2.0, an update that included the estimated glomerular filtration rate (eGFR) as a measure of renal function.
The study population included 211 adults with PAH seen at a single pulmonary hypertension clinic; the average age was 63 years and 65% were women. In addition, 42% had at least stage 3 chronic kidney disease. The primary endpoint was transplant-free survival, which was a median of 7 years. Creatinine was assessed at baseline in all patients. In addition, patients were grouped based on the percent change in renal function between diagnosis and 6 months.
Although the ESC and REVEAL algorithms significantly stratified transplant-free survival risk, the researchers found little agreement among the algorithms in stratifying transplant-free survival for patients in the intermediate-risk category.
However, using REVEAL 2.0, both renal function at diagnosis and renal function at 6 months were significant predictors (P < .0001 for both) from intermediate-risk to higher- or lower-risk groups, the researchers said.
“A decrease in renal function may be a harbinger of both [right ventricle] dysfunction and further PAH disease progression. However, further research is needed to confirm whether declining eGFR is a sentinel biomarker in prospective cohorts,” the researchers said.
The study findings were limited by several factors including the retrospective design and the use of mainly baseline data without information on long-term risk assessment, the researchers noted. However, “a key finding of our study was the ability of baseline eGFR to robustly restratify ESC/ERS-based risk strategies,” they said. “Our work highlights key limitations of the ESC/ERS-based risk assessment, and suggests that incorporating measures of kidney function are important strategies moving forward,” they concluded.
Mr. Zelt is an MD/PhD student and had no financial conflicts to disclose. Some coauthors disclosed relationships with Actelion Pharmaceuticals, Bayer Pharmaceuticals, and Northern Therapeutics.
SOURCE: Zelt JGE et al. J Heart Lung Transplant. 2020 Apr 5. doi: 10.1016/j.healun.2020.03.026.
The REVEAL-based risk-management strategy was significantly more effective than the current European Society of Cardiology guidelines at discriminating risk in adults with pulmonary arterial hypertension, and renal function significantly improved risk stratification, findings from a retrospective registry study suggest.
“Although the importance of identification of low or high risk is intuitive, the clinical utility of stratification into the intermediate-risk category is less certain” in patients with pulmonary arterial hypertension (PAH), wrote Jason G.E. Zelt, MSc, of the University of Ottawa and colleagues. “Despite the importance of renal function in the PAH population, it has not been formally incorporated into many of the contemporary PAH risk tools, including current guidelines,” they noted.
In a study published in the Journal of Heart and Lung Transplantation, the researchers compared several current research tools for risk assessment in PAH, including the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL) risk calculator, the French Pulmonary Hypertension Registry (FPHR), and guidelines from the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). They also reviewed REVEAL 2.0, an update that included the estimated glomerular filtration rate (eGFR) as a measure of renal function.
The study population included 211 adults with PAH seen at a single pulmonary hypertension clinic; the average age was 63 years and 65% were women. In addition, 42% had at least stage 3 chronic kidney disease. The primary endpoint was transplant-free survival, which was a median of 7 years. Creatinine was assessed at baseline in all patients. In addition, patients were grouped based on the percent change in renal function between diagnosis and 6 months.
Although the ESC and REVEAL algorithms significantly stratified transplant-free survival risk, the researchers found little agreement among the algorithms in stratifying transplant-free survival for patients in the intermediate-risk category.
However, using REVEAL 2.0, both renal function at diagnosis and renal function at 6 months were significant predictors (P < .0001 for both) from intermediate-risk to higher- or lower-risk groups, the researchers said.
“A decrease in renal function may be a harbinger of both [right ventricle] dysfunction and further PAH disease progression. However, further research is needed to confirm whether declining eGFR is a sentinel biomarker in prospective cohorts,” the researchers said.
The study findings were limited by several factors including the retrospective design and the use of mainly baseline data without information on long-term risk assessment, the researchers noted. However, “a key finding of our study was the ability of baseline eGFR to robustly restratify ESC/ERS-based risk strategies,” they said. “Our work highlights key limitations of the ESC/ERS-based risk assessment, and suggests that incorporating measures of kidney function are important strategies moving forward,” they concluded.
Mr. Zelt is an MD/PhD student and had no financial conflicts to disclose. Some coauthors disclosed relationships with Actelion Pharmaceuticals, Bayer Pharmaceuticals, and Northern Therapeutics.
SOURCE: Zelt JGE et al. J Heart Lung Transplant. 2020 Apr 5. doi: 10.1016/j.healun.2020.03.026.
FROM THE JOURNAL OF HEART AND LUNG TRANSPLANTATION
Expert discusses red flags for interstitial lung disease in pediatric rheumatology
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
MAUI, HAWAII – Anti-Ro52 autoantibodies are the latest and most potent of the autoantibody predictors of interstitial lung disease (ILD) discovered in patients with juvenile dermatomyositis, Anne M. Stevens, MD, PhD, said at the 2020 Rheumatology Winter Clinical Symposium.
In addition to detailing the autoantibody red flags for ILD in juvenile dermatomyositis (JDM), she called for “hypervigilance” in patients with systemic juvenile idiopathic arthritis (SJIA) who exhibit any of a series of risk factors for ILD.
“Most of the lung disease in kids with systemic JIA is asymptomatic until very late, but it can be reversible if we treat it. So it’s worth finding and monitoring and giving everyone PCP [pneumocystis pneumonia] prophylaxis, because they have a high incidence of PCP if they have any of those risk factors,” observed Dr. Stevens, a pediatric rheumatologist at the University of Washington, Seattle, and senior director for the adaptive immunity research program at Janssen Pharmaceuticals.
Autoantibodies predict ILD in JDM
Dr. Stevens highlighted recent work by Sara Sabbagh, DO, of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and coinvestigators in the Childhood Myositis Heterogeneity Collaborative Study Group. They reported the presence of anti-Ro52 autoantibodies in 14% of a cohort of 302 patients with JDM as well as in 12% of 25 patients with juvenile polymyositis and in 18% of 44 youths with an overlap of juvenile connective tissue disease and myositis. In addition, 13% of patients were positive for autoantibodies previously identified as being associated with ILD in these forms of juvenile myositis: Namely, 9% of the cohort were positive for antimelanoma differentiation–associated protein 5 (anti-MDA5) autoantibodies, and antiaminoacyl tRNA synthestase (anti-Jo-1) autoantibodies were present in 4%.
A total of 33 of the 371 juvenile myositis patients had ILD based upon CT imaging, chest X-ray, dyspnea on exertion, and/or biopsy. Most patients with anti-Ro52 also had other autoantibodies associated with ILD. Indeed, 31% of patients with anti-MDA5 autoantibodies also had anti-Ro52, as did 64% of those with anti-Jo-1. After controlling for the presence of these other myositis-specific autoantibodies, auto-Ro52 autoantibodies were independently associated with ILD, which was present in 36% of those with and just 4% of those without anti-Ro52 autoantibodies.
Importantly, if a patient with JDM or another form of juvenile myositis had both anti-Ro52 and another myositis-specific autoantibody, the risk for ILD rose dramatically, climbing to 70% in patients with anti-Ro52 and anti-MDA5 autoantibodies, and to 100% in those who were both anti-Ro52- and anti-Jo-1 positive.
Patients with anti-Ro52 autoantibodies had a worse prognosis, with more severe and chronic disease, Dr. Stevens noted.
Novel potential treatment for ILD in JDM: JAK inhibitors
Standard treatment of ILD in JDM in all cases includes high-dose pulsed corticosteroids, intravenous immunoglobulin (IVIG), and either methotrexate or mycophenolate mofetil. Consideration should be given to adding cyclosporine, particularly when a macrophage activation syndrome component is present. In addition, several exciting recent lines of evidence suggest a potential role for Janus kinase (JAK) inhibitors in the subset of JDM patients with anti-MDA5 autoantibody-positive disease, according to Dr. Stevens.
For one, Dr. Sabbagh and colleagues have reported impressive success with the use of the JAK 1/3 inhibitor tofacitinib (Xeljanz) in two patients with anti-MDA5 autoantibody-positive refractory JDM with ILD. Both patients experienced moderate clinical improvement in disease activity in their skin, muscles, and other target organs. But particularly striking was what the investigators termed the “remarkable” improvement in ILD, including near-resolution of abnormal findings on high-resolution CT imaging and a more robust performance on pulmonary function testing.
Both of these hitherto treatment-refractory patients were able to wean or discontinue their immunosuppressive medications. The patients’ elevated blood interferon-response gene signature improved significantly in response to tofacitinib, and their problematic upregulation of STAT1 phosphorylation of CD4+ T cells and monocytes stimulated with interferon-gamma was tamed, dropping to levels typically seen in healthy individuals.
Also, French pediatric rheumatologists have identified key phenotypic and cytokine differences between 13 patients with JDM or juvenile overlap myositis who were anti-MDA5 autoantibody positive at presentation and 51 others who were not. The anti-MDA5 autoantibody–positive group had a higher frequency of ILD, arthritis, skin ulcerations, and lupus features, but milder muscle involvement than did the anti-MDA5 autoantibody–negative group. The anti-MDA5 autoantibody–positive patients demonstrated enhanced interferon-alpha signaling based upon their significantly higher serum interferon-alpha levels, compared with the anti-MDA5-negative group, and those levels decreased following treatment with improvement in symptoms.
The French investigators proposed that interferon-alpha may constitute a novel therapeutic target in the subgroup of patients with severe, refractory juvenile myositis and anti-MDA5 autoantibodies – and, as it happens, it’s known that JAK inhibitors modulate the interferon pathway.
Risk factors for ILD in SJIA
In the past half-dozen years or so, pediatric rheumatologists have become increasingly aware of and concerned about a new development in SJIA: the occurrence of comorbid ILD. This is a poor-prognosis disease: In a cohort from the United Kingdom, 5-year mortality from the time of diagnosis was 41%, fully 40-fold higher than in patients with SJIA only.
Patient cohorts with SJIA and ILD have unusual clinical and laboratory features that aren’t part of the typical picture in SJIA. These include acute clubbing, lymphopenia, a fixed pruritic rash, unexplained abdominal pain, peripheral eosinophilia, facial swelling, and an increased ferritin level, a hallmark of acute macrophage activation syndrome. Onset of SJIA before 2 years of age is another red flag associated with increased risk for ILD. So is trisomy 21, which is up to 50 times more prevalent in patients with SJIA and ILD than in the general population or in patients with SJIA only. Another clue is an adverse reaction to tocilizumab (Actemra).
Any of these findings warrant hypervigilance: “Be on high alert and monitor these patients for ILD much more closely,” Dr. Stevens advised.
This means ordering a CT scan, prescribing PCP prophylaxis, and regularly measuring pulmonary function, admittedly a challenge in children under 7 years old. In these younger kids, practical solutions include measuring their oxygen saturation before and after running around the room to see if it drops. A 6-minute walk test and sleep oximetry are other options.
The explanation for the abrupt arrival of ILD as part of the picture in SJIA during the past decade remains unclear. The timing coincides with a major advance in the treatment of SJIA: the arrival of biologic agents blocking interleukin-1 and -6. Could this be a serious treatment side effect?
“It’s all association so far, and we’re not really sure why we’re seeing this association. Is it because we’re using a lot [fewer] corticosteroids now, and maybe those were preventing lung disease in the past?” Dr. Stevens speculated.
At this point, she and her fellow pediatric rheumatologists are awaiting further evidence before discussing a curb in their use of IL-1 or -6 inhibitors in patients with SJIA.
“These drugs have turned around the lives of kids with SJIA. They used to suffer through all our ineffective treatments for years, with terrible joint destruction and a pretty high mortality rate. These are great drugs for this disease, and we certainly don’t want to limit them,” she said.
Dr. Stevens reported research collaborations with Kineta and Seattle Genetics in addition to her employment at Janssen Pharmaceuticals.
REPORTING FROM RWCS 2020
Primary care physicians reshuffle their work, lives in a pandemic
During his shift at a COVID-19 drive-through triage screening area set up outside the University of Arkansas for Medical Sciences in Little Rock, Robert Hopkins Jr., MD, noticed a woman bowled over in the front seat of her car.
A nurse practitioner had just informed her that she had met the criteria for undergoing testing for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
“She was very upset and was crying nearly inconsolably,” said Dr. Hopkins, who directs the division of general internal medicine at the University of Arkansas Medical Sciences College of Medicine. “I went over and visited with her for a few minutes. She was scared to death that we [had] told her she was going to die. In her mind, if she had COVID-19 that meant a death sentence, and if we were testing her that meant she was likely to not survive.”
Dr. Hopkins tried his best to put testing in perspective for the woman. “At least she came to a level of comfort and realized that we were doing this for her, that this was not a death sentence, that this was not her fault,” he said. “She was worried about infecting her kids and her grandkids and ending up in the hospital and being a burden. Being able to spend that few minutes with her and help to bring down her level of anxiety – I think that’s where we need to put our efforts as physicians right now, helping people understand, ‘Yes, this is serious. Yes, we need to continue to social distance. Yes, we need to be cautious. But, we will get through this if we all work together to do so.’ ”
Prior to the COVID-19 pandemic, Dr. Hopkins spent part of his time seeing patients in the university’s main hospital, but most of it in an outpatient clinic where he and about 20 other primary care physicians care for patients and precept medical residents. Now, medical residents have been deployed to other services, primarily in the hospital, and he and his physician colleagues are conducting 80%-90% of patient visits by video conferencing or by telephone. It’s a whole new world.
“We’ve gone from a relatively traditional inpatient/outpatient practice where we’re seeing patients face to face to doing some face-to-face visits, but an awful lot of what we do now is in the technology domain,” said Dr. Hopkins, who also assisted with health care relief efforts during hurricanes Rita and Katrina.
“A group of six of us has been redeployed to assist with the surge unit for the inpatient facility, so our outpatient duties are being taken on by some of our partners.”
He also pitches in at the drive-through COVID-19 screening clinic, which was set up on March 27 and operates between 8 a.m. and 8 p.m., 7 days a week. “We’re able to measure people’s temperature, take a quick screening history, decide whether their risk is such that we need to do a COVID-19 PCR [polymerase chain reaction] test,” he said. “Then we make a determination of whether they need to go home on quarantine awaiting those results, or if they don’t have anything that needs to be evaluated, or whether they need to be triaged to an urgent care setting or to the emergency department.”
To minimize his risk of acquiring COVID-19, he follows personal hygiene practices recommended by the Centers for Disease Control and Prevention. He also places his work shoes in a shoebox, which he keeps in his car. “I put them on when I get to the parking deck at work, do my work, and then I put them in the shoebox, slip on another pair of shoes and drive home so I’m not tracking in things I potentially had on me,” said Dr. Hopkins, who is married and a father to two college-aged sons and a daughter in fourth grade. “When I get home I immediately shower, and then I exercise or have dinner with my family.”
Despite the longer-than-usual work hours and upheaval to the traditional medical practice model brought on by the pandemic, Dr. Hopkins, a self-described “glass half full person,” said that he does his best to keep watch over his patients and colleagues. “I’m trying to keep an eye out on my team members – physicians, nurses, medical assistants, and folks at the front desk – trying to make sure that people are getting rest, trying to make sure that people are not overcommitting,” he said. “Because if we’re not all working together and working for the long term, we’re going to be in trouble. This is not going to be a sprint; this is going to be a marathon for us to get through.”
To keep mentally centered, he engages in at least 40 minutes of exercise each day on his bicycle or on the elliptical machine at home. Dr. Hopkins hopes that the current efforts to redeploy resources, expand clinician skill sets, and forge relationships with colleagues in other disciplines will carry over into the delivery of health care when COVID-19 is a distant memory. “I hope that some of those relationships are going to continue and result in better care for all of our patients,” he said.
"We are in dire need of hugs"
Typically, Dr. Dakkak, a family physician at Boston University, practices a mix of clinic-based family medicine and obstetrics, and works in inpatient medicine 6 weeks a year. Currently, she is leading a COVID-19 team full time at Boston Medical Center, a 300-bed safety-net hospital located on the campus of Boston University Medical Center.
COVID-19 has also shaken up her life at home.
When Dr. Dakkak volunteered to take on her new role, the first thing that came to her mind was how making the switch would affect the well-being of her 8-year-old son and 10-year-old daughter.
“I thought, ‘How do I get my children somewhere where I don’t have to worry about them?’ ” Dr. Dakkak said.
She floated the idea with her husband of flying their children out to stay with her recently retired parents, who live outside of Sacramento, Calif., until the pandemic eases up. “I was thinking to myself, ‘Am I overreacting? Is the pandemic not going to be that bad?’ because the rest of the country seemed to be in some amount of denial,” she said. “So, I called my dad, who’s a retired pediatric anesthesiologist. He’s from Egypt so he’s done crisis medicine in his time. He encouraged me to send the kids.”
On the same day that Dr. Dakkak began her first 12-hour COVID-19 shift at the hospital, her husband and children boarded a plane to California, where the kids remain in the care of her parents. Her husband returned after staying there for 2 weeks. “Every day when I’m working, I validate my decision,” she said. “When I first started, I worked 5 nights in a row, had 2 days off, and then worked 6 nights in a row. I was busy so I didn’t think about [being away from my kids], but at the same time I was grateful that I didn’t have to come home and worry about homeschooling the kids or infecting them.”
She checks in with them as she can via cell phone or FaceTime. “My son has been very honest,” Dr. Dakkak said. “He says, ‘FaceTime makes me miss you more, and I don’t like it,’ which I understand. I’ll call my mom, and if they want to talk to me, they’ll talk to me. If they don’t want to talk to me, I’m okay. This is about them being healthy and safe. I sent them a care package a few days ago with cards and some workbooks. I’m optimistic that in June I can at least see them if not bring them home.”
Dr. Dakkak describes leading a COVID-19 team as a grueling experience that challenges her medical know-how nearly every day, with seemingly ever-changing algorithms. “Our knowledge of this disease is five steps behind, and changing at lightning speed,” said Dr. Dakkak, who completed a fellowship in surgical and high-risk obstetrics. “It’s hard to balance continuing to teach evidence-based medicine for everything else in medicine [with continuing] to practice minimal and ever-changing evidence-based COVID medicine. We just don’t know enough [about the virus] yet. This is nothing like we were taught in medical school. Everyone has elevated d-dimers with COVID-19, and we don’t get CT pulmonary angiograms [CTPAs] on all of them; we wouldn’t physically be able to. Some patients have d-dimers in the thousands, and only some are stable to get CTPAs. We are also finding pulmonary embolisms. Now we’re basing our algorithm on anticoagulation due to d-dimers because sometimes you can’t always do a CTPA even if you want to. On the other hand, we have people who are coming into the hospital too late. We’ve had a few who have come in after having days of stroke symptoms. I worry about our patients at home who hesitate to come in when they really should.”
Sometimes she feels sad for the medical residents on her team because their instinct is to go in and check on each patient, “but I don’t want them to get exposed,” she said. “So, we check in by phone, or if they need a physical check-in, we minimize the check-ins; only one of us goes in. I’m more willing to put myself in the room than to put them in the room. I also feel for them because they came into medicine for the humanity of medicine – not the charting or the ordering of medicine. I also worry about the acuity and sadness they’re seeing. This is a rough introduction to medicine for them.”
When interviewed for this story in late April, Dr. Dakkak had kept track of her intubated COVID-19 patients. “Most of my patients get to go home without having been intubated, but those aren’t the ones I worry about,” she said. “I have two patients I have been watching. One of them has just been extubated and I’m still worried about him, but I’m hoping he’s going to be fine. The other one is the first pregnant woman we intubated. She is now extubated, doing really well, and went home. Her fetus is doing well, never had any issues while she was intubated. Those cases make me happy. They were both under the age of 35. It is nice to follow those intubations and find that the majority are doing okay.”
The first patient she had cared for who died was a young man “who was always in good spirits,” she recalled. “We called his brother right before intubating him. After intubation, his oxygen saturation didn’t jump up, which made me worry a bit.” About a week later, the young man died. “I kept thinking, ‘We intubated him when he was still comfortable talking. Should I have put it off and had him call more people to say goodbye? Should I have known that he wasn’t going to wake up?’ ” said Dr. Dakkak, who is also women’s health director at Manet Community Health Centers. “A lot of us have worked on our end-of-life discussions in the past month, just being able to tell somebody, ‘This might be your last time to call family. Call family and talk to whoever you want.’ Guilt isn’t the right word, but it’s unsettling if I’m the last person a patient talks to. I feel that, if that’s the case, then I didn’t do a good enough job trying to get them to their family or friends. If I am worried about a patient’s clinical status declining, I tell families now, when I call them, ‘I hope I’m wrong; I hope they don’t need to be intubated, but I think this is the time to talk.’ ”
To keep herself grounded during off hours, Dr. Dakkak spends time resting, checking in with her family, journaling “to get a lot of feelings out,” gardening, hiking, and joining Zoom chats with friends. Once recentered, she draws from a sense of obligation to others as she prepares for her next shift caring for COVID-19 patients.
“I have a lot of love for the world that I get to expend by doing this hard work,” she said. “I love humanity and I love humanity in times of crisis. The interactions I have with patients and their families are still central to why I do this work. I love my medical teams, and I would never want to let them down. It is nice to feel the sense of teamwork across the hospital. The nurses that I sit with and experience this with are amazing. I keep saying that the only thing I want to do when this pandemic is over is hug everyone. I think we are in dire need of hugs.”
Finding light in the darkness
Internist Katie Jobbins, DO, also has worked in a professional role that was created because of COVID-19.
Shortly before Dr. Jobbins was deployed to Baystate Medical Center in Springfield, Ma., for 2 weeks in April of 2020 to help clinicians with an anticipated surge of COVID-19 cases, she encountered a patient who walked into Baystate’s High Street Health Center.
“I think I have COVID-19,” the patient proclaimed to her, at the outpatient clinic that serves mostly inner-city, Medicaid patients.
Prior to becoming an ambulatory internist, Dr. Jobbins was a surgical resident. “So I went into that mode of ‘I need to do this, this, and this,’ ” she said. “I went through a checklist in my head to make sure I was prepared to take care of the patient.”
She applied that same systems approach during her redeployment assignment in the tertiary care hospital, which typically involved 10-hour shifts overseeing internal medicine residents in a medical telemetry unit. “We would take care of people under investigation for COVID-19, but we were not assigned to the actual COVID unit,” said Dr. Jobbins, who is also associate program director for the internal medicine residency program at the University of Massachusetts Medical School–Baystate Springfield. “They tried to redeploy other people to those units who had special training, and we were trying to back fill into where those people that got moved to the COVID units or the ICU units were actually working. We were taking more of the medical side of the floors.”
Even so, one patient on the unit was suspected of having COVID-19, so Dr. Jobbins suited up with personal protective equipment and conducted a thorough exam with residents waiting outside the patient’s room, a safe distance away. “I explained everything I found on the exam to the residents, trying to give them some educational benefit, even though they couldn’t physically examine the patient because we’re trying to protect them since they’re in training,” she said. “It was anxiety provoking, on some level, knowing that there’s a potential risk of exposure [to the virus], but knowing that Baystate Health has gone to extraordinary measures to make sure we have the correct PPE and support us is reassuring. I knew I had the right equipment and the right tools to take care of the patient, which calmed my nerves and made me feel like I could do the job. That’s the most important thing as a physician during this time: knowing that you have people supporting you who have your back at all times.”
Like Dr. Dakkak, Dr. Jobbins had to make some adjustments to her interaction with her family.
Before she began the deployment, Dr. Jobbins engaged in a frank discussion with her husband and her two young boys about the risks she faced working in a hospital caring for patients with COVID-19. “My husband and I made sure our wills were up to date, and we talked about what we would do if either of us got the virus,” she said. To minimize the potential risk of transmitting the virus to her loved ones during the two-week deployment, she considered living away from her family in a nearby home owned by her father, but decided against that and to “take it day by day.” Following her hospital shifts, Dr. Jobbins changed into a fresh set of clothes before leaving the hospital. Once she arrived home, she showered to reduce the risk of possibly becoming a vector to her family.
She had to tell her kids: “You can’t kiss me right now.”
“As much as it’s hard for them to understand, we had a conversation [in which I explained] ‘This is a virus. It will go away eventually, but it’s a virus we’re fighting.’ It’s interesting to watch a 3-year-old try to process that and take his play samurai sword or Marvel toys and decide he’s going to run around the neighborhood and try to kill the virus.”
At the High Street Health Center, Dr. Jobbins and her colleagues have transitioned to conducting most patient encounters via telephone or video appointments. “We have tried to maintain as much continuity for our patients to address their chronic medical needs through these visits, such as hypertension management and diabetes care,” she said. “We have begun a rigorous screening process to triage and treat patients suspicious for COVID-19 through telehealth in hopes of keeping them safe and in their own homes. We also continue to see patients for nonrespiratory urgent care needs in person once they have screened negative for COVID-19.”
“In terms of the inpatient setting, we’ve noticed that a lot of people are choosing not to go to the hospital now, unless they’re extremely ill,” Dr. Jobbins noted. “We’re going to need to find a balance with when do people truly need to go to the hospital and when do they not? What can we manage as an outpatient versus having someone go to the emergency department? That’s really the role of the primary care physician. We need to help people understand, ‘You don’t need to go to the ED for everything, but here are the things you really need to go for.’ ”
“It will be interesting to see what health care looks like in 6 months or a year. I’m excited to see where we land,” Dr. Jobbins added.
Hopes for the Future of Telemedicine
When the practice of medicine enters a post–COVID-19 era, Dr. Jobbins hopes that telemedicine will be incorporated more into the delivery of patient care. “I’ve found that many of my patients who often are no-shows to the inpatient version of their visits have had a higher success rate of follow-through when we do the telephone visits,” she said. “It’s been very successful. I hope that the insurance companies and [Centers for Medicare & Medicaid] will continue to reimburse this as they see this is a benefit to our patients.
Dr. Hopkins is also hopeful that physicians will be able to successfully see patients via telemedicine in the postpandemic world.
“For the ups and downs we’ve had with telemedicine, I’d love for us to be able to enhance the positives and incorporate that into our practice going forward. If we can reach our patients and help treat them where they are, rather than them having to come to us, that may be a plus,” he said.
In the meantime, Dr. Jobbins presses on as the curve of COVID-19 cases flattens in Western Massachusetts and remains grateful that she chose to practice medicine.
“The commitment I have to being an educator in addition to being a physician is part of why I keep doing this,” Dr. Jobbins said. “I find this to be one of the most fulfilling jobs and careers you could ever have: being there for people when they need you the most. That’s really what a physician’s job is: being there for people when a family member has passed away or when they just need to talk because they’re having anxiety. At the end of the day, if we can impart that to those we work with and bring in a positive attitude, it’s infectious and it makes people see this is a reason we keep doing what we’re doing.”
She’s also been heartened by the kindness of strangers during this pandemic, from those who made and donated face shields when they were in short supply, to those who delivered food to the hospital as a gesture of thanks.
“I had a patient who made homemade masks and sent them to my office,” she said. “There’s obviously good and bad during this time, but I get hope from seeing all of the good things that are coming out of this, the whole idea of finding the light in the darkness.”
During his shift at a COVID-19 drive-through triage screening area set up outside the University of Arkansas for Medical Sciences in Little Rock, Robert Hopkins Jr., MD, noticed a woman bowled over in the front seat of her car.
A nurse practitioner had just informed her that she had met the criteria for undergoing testing for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
“She was very upset and was crying nearly inconsolably,” said Dr. Hopkins, who directs the division of general internal medicine at the University of Arkansas Medical Sciences College of Medicine. “I went over and visited with her for a few minutes. She was scared to death that we [had] told her she was going to die. In her mind, if she had COVID-19 that meant a death sentence, and if we were testing her that meant she was likely to not survive.”
Dr. Hopkins tried his best to put testing in perspective for the woman. “At least she came to a level of comfort and realized that we were doing this for her, that this was not a death sentence, that this was not her fault,” he said. “She was worried about infecting her kids and her grandkids and ending up in the hospital and being a burden. Being able to spend that few minutes with her and help to bring down her level of anxiety – I think that’s where we need to put our efforts as physicians right now, helping people understand, ‘Yes, this is serious. Yes, we need to continue to social distance. Yes, we need to be cautious. But, we will get through this if we all work together to do so.’ ”
Prior to the COVID-19 pandemic, Dr. Hopkins spent part of his time seeing patients in the university’s main hospital, but most of it in an outpatient clinic where he and about 20 other primary care physicians care for patients and precept medical residents. Now, medical residents have been deployed to other services, primarily in the hospital, and he and his physician colleagues are conducting 80%-90% of patient visits by video conferencing or by telephone. It’s a whole new world.
“We’ve gone from a relatively traditional inpatient/outpatient practice where we’re seeing patients face to face to doing some face-to-face visits, but an awful lot of what we do now is in the technology domain,” said Dr. Hopkins, who also assisted with health care relief efforts during hurricanes Rita and Katrina.
“A group of six of us has been redeployed to assist with the surge unit for the inpatient facility, so our outpatient duties are being taken on by some of our partners.”
He also pitches in at the drive-through COVID-19 screening clinic, which was set up on March 27 and operates between 8 a.m. and 8 p.m., 7 days a week. “We’re able to measure people’s temperature, take a quick screening history, decide whether their risk is such that we need to do a COVID-19 PCR [polymerase chain reaction] test,” he said. “Then we make a determination of whether they need to go home on quarantine awaiting those results, or if they don’t have anything that needs to be evaluated, or whether they need to be triaged to an urgent care setting or to the emergency department.”
To minimize his risk of acquiring COVID-19, he follows personal hygiene practices recommended by the Centers for Disease Control and Prevention. He also places his work shoes in a shoebox, which he keeps in his car. “I put them on when I get to the parking deck at work, do my work, and then I put them in the shoebox, slip on another pair of shoes and drive home so I’m not tracking in things I potentially had on me,” said Dr. Hopkins, who is married and a father to two college-aged sons and a daughter in fourth grade. “When I get home I immediately shower, and then I exercise or have dinner with my family.”
Despite the longer-than-usual work hours and upheaval to the traditional medical practice model brought on by the pandemic, Dr. Hopkins, a self-described “glass half full person,” said that he does his best to keep watch over his patients and colleagues. “I’m trying to keep an eye out on my team members – physicians, nurses, medical assistants, and folks at the front desk – trying to make sure that people are getting rest, trying to make sure that people are not overcommitting,” he said. “Because if we’re not all working together and working for the long term, we’re going to be in trouble. This is not going to be a sprint; this is going to be a marathon for us to get through.”
To keep mentally centered, he engages in at least 40 minutes of exercise each day on his bicycle or on the elliptical machine at home. Dr. Hopkins hopes that the current efforts to redeploy resources, expand clinician skill sets, and forge relationships with colleagues in other disciplines will carry over into the delivery of health care when COVID-19 is a distant memory. “I hope that some of those relationships are going to continue and result in better care for all of our patients,” he said.
"We are in dire need of hugs"
Typically, Dr. Dakkak, a family physician at Boston University, practices a mix of clinic-based family medicine and obstetrics, and works in inpatient medicine 6 weeks a year. Currently, she is leading a COVID-19 team full time at Boston Medical Center, a 300-bed safety-net hospital located on the campus of Boston University Medical Center.
COVID-19 has also shaken up her life at home.
When Dr. Dakkak volunteered to take on her new role, the first thing that came to her mind was how making the switch would affect the well-being of her 8-year-old son and 10-year-old daughter.
“I thought, ‘How do I get my children somewhere where I don’t have to worry about them?’ ” Dr. Dakkak said.
She floated the idea with her husband of flying their children out to stay with her recently retired parents, who live outside of Sacramento, Calif., until the pandemic eases up. “I was thinking to myself, ‘Am I overreacting? Is the pandemic not going to be that bad?’ because the rest of the country seemed to be in some amount of denial,” she said. “So, I called my dad, who’s a retired pediatric anesthesiologist. He’s from Egypt so he’s done crisis medicine in his time. He encouraged me to send the kids.”
On the same day that Dr. Dakkak began her first 12-hour COVID-19 shift at the hospital, her husband and children boarded a plane to California, where the kids remain in the care of her parents. Her husband returned after staying there for 2 weeks. “Every day when I’m working, I validate my decision,” she said. “When I first started, I worked 5 nights in a row, had 2 days off, and then worked 6 nights in a row. I was busy so I didn’t think about [being away from my kids], but at the same time I was grateful that I didn’t have to come home and worry about homeschooling the kids or infecting them.”
She checks in with them as she can via cell phone or FaceTime. “My son has been very honest,” Dr. Dakkak said. “He says, ‘FaceTime makes me miss you more, and I don’t like it,’ which I understand. I’ll call my mom, and if they want to talk to me, they’ll talk to me. If they don’t want to talk to me, I’m okay. This is about them being healthy and safe. I sent them a care package a few days ago with cards and some workbooks. I’m optimistic that in June I can at least see them if not bring them home.”
Dr. Dakkak describes leading a COVID-19 team as a grueling experience that challenges her medical know-how nearly every day, with seemingly ever-changing algorithms. “Our knowledge of this disease is five steps behind, and changing at lightning speed,” said Dr. Dakkak, who completed a fellowship in surgical and high-risk obstetrics. “It’s hard to balance continuing to teach evidence-based medicine for everything else in medicine [with continuing] to practice minimal and ever-changing evidence-based COVID medicine. We just don’t know enough [about the virus] yet. This is nothing like we were taught in medical school. Everyone has elevated d-dimers with COVID-19, and we don’t get CT pulmonary angiograms [CTPAs] on all of them; we wouldn’t physically be able to. Some patients have d-dimers in the thousands, and only some are stable to get CTPAs. We are also finding pulmonary embolisms. Now we’re basing our algorithm on anticoagulation due to d-dimers because sometimes you can’t always do a CTPA even if you want to. On the other hand, we have people who are coming into the hospital too late. We’ve had a few who have come in after having days of stroke symptoms. I worry about our patients at home who hesitate to come in when they really should.”
Sometimes she feels sad for the medical residents on her team because their instinct is to go in and check on each patient, “but I don’t want them to get exposed,” she said. “So, we check in by phone, or if they need a physical check-in, we minimize the check-ins; only one of us goes in. I’m more willing to put myself in the room than to put them in the room. I also feel for them because they came into medicine for the humanity of medicine – not the charting or the ordering of medicine. I also worry about the acuity and sadness they’re seeing. This is a rough introduction to medicine for them.”
When interviewed for this story in late April, Dr. Dakkak had kept track of her intubated COVID-19 patients. “Most of my patients get to go home without having been intubated, but those aren’t the ones I worry about,” she said. “I have two patients I have been watching. One of them has just been extubated and I’m still worried about him, but I’m hoping he’s going to be fine. The other one is the first pregnant woman we intubated. She is now extubated, doing really well, and went home. Her fetus is doing well, never had any issues while she was intubated. Those cases make me happy. They were both under the age of 35. It is nice to follow those intubations and find that the majority are doing okay.”
The first patient she had cared for who died was a young man “who was always in good spirits,” she recalled. “We called his brother right before intubating him. After intubation, his oxygen saturation didn’t jump up, which made me worry a bit.” About a week later, the young man died. “I kept thinking, ‘We intubated him when he was still comfortable talking. Should I have put it off and had him call more people to say goodbye? Should I have known that he wasn’t going to wake up?’ ” said Dr. Dakkak, who is also women’s health director at Manet Community Health Centers. “A lot of us have worked on our end-of-life discussions in the past month, just being able to tell somebody, ‘This might be your last time to call family. Call family and talk to whoever you want.’ Guilt isn’t the right word, but it’s unsettling if I’m the last person a patient talks to. I feel that, if that’s the case, then I didn’t do a good enough job trying to get them to their family or friends. If I am worried about a patient’s clinical status declining, I tell families now, when I call them, ‘I hope I’m wrong; I hope they don’t need to be intubated, but I think this is the time to talk.’ ”
To keep herself grounded during off hours, Dr. Dakkak spends time resting, checking in with her family, journaling “to get a lot of feelings out,” gardening, hiking, and joining Zoom chats with friends. Once recentered, she draws from a sense of obligation to others as she prepares for her next shift caring for COVID-19 patients.
“I have a lot of love for the world that I get to expend by doing this hard work,” she said. “I love humanity and I love humanity in times of crisis. The interactions I have with patients and their families are still central to why I do this work. I love my medical teams, and I would never want to let them down. It is nice to feel the sense of teamwork across the hospital. The nurses that I sit with and experience this with are amazing. I keep saying that the only thing I want to do when this pandemic is over is hug everyone. I think we are in dire need of hugs.”
Finding light in the darkness
Internist Katie Jobbins, DO, also has worked in a professional role that was created because of COVID-19.
Shortly before Dr. Jobbins was deployed to Baystate Medical Center in Springfield, Ma., for 2 weeks in April of 2020 to help clinicians with an anticipated surge of COVID-19 cases, she encountered a patient who walked into Baystate’s High Street Health Center.
“I think I have COVID-19,” the patient proclaimed to her, at the outpatient clinic that serves mostly inner-city, Medicaid patients.
Prior to becoming an ambulatory internist, Dr. Jobbins was a surgical resident. “So I went into that mode of ‘I need to do this, this, and this,’ ” she said. “I went through a checklist in my head to make sure I was prepared to take care of the patient.”
She applied that same systems approach during her redeployment assignment in the tertiary care hospital, which typically involved 10-hour shifts overseeing internal medicine residents in a medical telemetry unit. “We would take care of people under investigation for COVID-19, but we were not assigned to the actual COVID unit,” said Dr. Jobbins, who is also associate program director for the internal medicine residency program at the University of Massachusetts Medical School–Baystate Springfield. “They tried to redeploy other people to those units who had special training, and we were trying to back fill into where those people that got moved to the COVID units or the ICU units were actually working. We were taking more of the medical side of the floors.”
Even so, one patient on the unit was suspected of having COVID-19, so Dr. Jobbins suited up with personal protective equipment and conducted a thorough exam with residents waiting outside the patient’s room, a safe distance away. “I explained everything I found on the exam to the residents, trying to give them some educational benefit, even though they couldn’t physically examine the patient because we’re trying to protect them since they’re in training,” she said. “It was anxiety provoking, on some level, knowing that there’s a potential risk of exposure [to the virus], but knowing that Baystate Health has gone to extraordinary measures to make sure we have the correct PPE and support us is reassuring. I knew I had the right equipment and the right tools to take care of the patient, which calmed my nerves and made me feel like I could do the job. That’s the most important thing as a physician during this time: knowing that you have people supporting you who have your back at all times.”
Like Dr. Dakkak, Dr. Jobbins had to make some adjustments to her interaction with her family.
Before she began the deployment, Dr. Jobbins engaged in a frank discussion with her husband and her two young boys about the risks she faced working in a hospital caring for patients with COVID-19. “My husband and I made sure our wills were up to date, and we talked about what we would do if either of us got the virus,” she said. To minimize the potential risk of transmitting the virus to her loved ones during the two-week deployment, she considered living away from her family in a nearby home owned by her father, but decided against that and to “take it day by day.” Following her hospital shifts, Dr. Jobbins changed into a fresh set of clothes before leaving the hospital. Once she arrived home, she showered to reduce the risk of possibly becoming a vector to her family.
She had to tell her kids: “You can’t kiss me right now.”
“As much as it’s hard for them to understand, we had a conversation [in which I explained] ‘This is a virus. It will go away eventually, but it’s a virus we’re fighting.’ It’s interesting to watch a 3-year-old try to process that and take his play samurai sword or Marvel toys and decide he’s going to run around the neighborhood and try to kill the virus.”
At the High Street Health Center, Dr. Jobbins and her colleagues have transitioned to conducting most patient encounters via telephone or video appointments. “We have tried to maintain as much continuity for our patients to address their chronic medical needs through these visits, such as hypertension management and diabetes care,” she said. “We have begun a rigorous screening process to triage and treat patients suspicious for COVID-19 through telehealth in hopes of keeping them safe and in their own homes. We also continue to see patients for nonrespiratory urgent care needs in person once they have screened negative for COVID-19.”
“In terms of the inpatient setting, we’ve noticed that a lot of people are choosing not to go to the hospital now, unless they’re extremely ill,” Dr. Jobbins noted. “We’re going to need to find a balance with when do people truly need to go to the hospital and when do they not? What can we manage as an outpatient versus having someone go to the emergency department? That’s really the role of the primary care physician. We need to help people understand, ‘You don’t need to go to the ED for everything, but here are the things you really need to go for.’ ”
“It will be interesting to see what health care looks like in 6 months or a year. I’m excited to see where we land,” Dr. Jobbins added.
Hopes for the Future of Telemedicine
When the practice of medicine enters a post–COVID-19 era, Dr. Jobbins hopes that telemedicine will be incorporated more into the delivery of patient care. “I’ve found that many of my patients who often are no-shows to the inpatient version of their visits have had a higher success rate of follow-through when we do the telephone visits,” she said. “It’s been very successful. I hope that the insurance companies and [Centers for Medicare & Medicaid] will continue to reimburse this as they see this is a benefit to our patients.
Dr. Hopkins is also hopeful that physicians will be able to successfully see patients via telemedicine in the postpandemic world.
“For the ups and downs we’ve had with telemedicine, I’d love for us to be able to enhance the positives and incorporate that into our practice going forward. If we can reach our patients and help treat them where they are, rather than them having to come to us, that may be a plus,” he said.
In the meantime, Dr. Jobbins presses on as the curve of COVID-19 cases flattens in Western Massachusetts and remains grateful that she chose to practice medicine.
“The commitment I have to being an educator in addition to being a physician is part of why I keep doing this,” Dr. Jobbins said. “I find this to be one of the most fulfilling jobs and careers you could ever have: being there for people when they need you the most. That’s really what a physician’s job is: being there for people when a family member has passed away or when they just need to talk because they’re having anxiety. At the end of the day, if we can impart that to those we work with and bring in a positive attitude, it’s infectious and it makes people see this is a reason we keep doing what we’re doing.”
She’s also been heartened by the kindness of strangers during this pandemic, from those who made and donated face shields when they were in short supply, to those who delivered food to the hospital as a gesture of thanks.
“I had a patient who made homemade masks and sent them to my office,” she said. “There’s obviously good and bad during this time, but I get hope from seeing all of the good things that are coming out of this, the whole idea of finding the light in the darkness.”
During his shift at a COVID-19 drive-through triage screening area set up outside the University of Arkansas for Medical Sciences in Little Rock, Robert Hopkins Jr., MD, noticed a woman bowled over in the front seat of her car.
A nurse practitioner had just informed her that she had met the criteria for undergoing testing for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
“She was very upset and was crying nearly inconsolably,” said Dr. Hopkins, who directs the division of general internal medicine at the University of Arkansas Medical Sciences College of Medicine. “I went over and visited with her for a few minutes. She was scared to death that we [had] told her she was going to die. In her mind, if she had COVID-19 that meant a death sentence, and if we were testing her that meant she was likely to not survive.”
Dr. Hopkins tried his best to put testing in perspective for the woman. “At least she came to a level of comfort and realized that we were doing this for her, that this was not a death sentence, that this was not her fault,” he said. “She was worried about infecting her kids and her grandkids and ending up in the hospital and being a burden. Being able to spend that few minutes with her and help to bring down her level of anxiety – I think that’s where we need to put our efforts as physicians right now, helping people understand, ‘Yes, this is serious. Yes, we need to continue to social distance. Yes, we need to be cautious. But, we will get through this if we all work together to do so.’ ”
Prior to the COVID-19 pandemic, Dr. Hopkins spent part of his time seeing patients in the university’s main hospital, but most of it in an outpatient clinic where he and about 20 other primary care physicians care for patients and precept medical residents. Now, medical residents have been deployed to other services, primarily in the hospital, and he and his physician colleagues are conducting 80%-90% of patient visits by video conferencing or by telephone. It’s a whole new world.
“We’ve gone from a relatively traditional inpatient/outpatient practice where we’re seeing patients face to face to doing some face-to-face visits, but an awful lot of what we do now is in the technology domain,” said Dr. Hopkins, who also assisted with health care relief efforts during hurricanes Rita and Katrina.
“A group of six of us has been redeployed to assist with the surge unit for the inpatient facility, so our outpatient duties are being taken on by some of our partners.”
He also pitches in at the drive-through COVID-19 screening clinic, which was set up on March 27 and operates between 8 a.m. and 8 p.m., 7 days a week. “We’re able to measure people’s temperature, take a quick screening history, decide whether their risk is such that we need to do a COVID-19 PCR [polymerase chain reaction] test,” he said. “Then we make a determination of whether they need to go home on quarantine awaiting those results, or if they don’t have anything that needs to be evaluated, or whether they need to be triaged to an urgent care setting or to the emergency department.”
To minimize his risk of acquiring COVID-19, he follows personal hygiene practices recommended by the Centers for Disease Control and Prevention. He also places his work shoes in a shoebox, which he keeps in his car. “I put them on when I get to the parking deck at work, do my work, and then I put them in the shoebox, slip on another pair of shoes and drive home so I’m not tracking in things I potentially had on me,” said Dr. Hopkins, who is married and a father to two college-aged sons and a daughter in fourth grade. “When I get home I immediately shower, and then I exercise or have dinner with my family.”
Despite the longer-than-usual work hours and upheaval to the traditional medical practice model brought on by the pandemic, Dr. Hopkins, a self-described “glass half full person,” said that he does his best to keep watch over his patients and colleagues. “I’m trying to keep an eye out on my team members – physicians, nurses, medical assistants, and folks at the front desk – trying to make sure that people are getting rest, trying to make sure that people are not overcommitting,” he said. “Because if we’re not all working together and working for the long term, we’re going to be in trouble. This is not going to be a sprint; this is going to be a marathon for us to get through.”
To keep mentally centered, he engages in at least 40 minutes of exercise each day on his bicycle or on the elliptical machine at home. Dr. Hopkins hopes that the current efforts to redeploy resources, expand clinician skill sets, and forge relationships with colleagues in other disciplines will carry over into the delivery of health care when COVID-19 is a distant memory. “I hope that some of those relationships are going to continue and result in better care for all of our patients,” he said.
"We are in dire need of hugs"
Typically, Dr. Dakkak, a family physician at Boston University, practices a mix of clinic-based family medicine and obstetrics, and works in inpatient medicine 6 weeks a year. Currently, she is leading a COVID-19 team full time at Boston Medical Center, a 300-bed safety-net hospital located on the campus of Boston University Medical Center.
COVID-19 has also shaken up her life at home.
When Dr. Dakkak volunteered to take on her new role, the first thing that came to her mind was how making the switch would affect the well-being of her 8-year-old son and 10-year-old daughter.
“I thought, ‘How do I get my children somewhere where I don’t have to worry about them?’ ” Dr. Dakkak said.
She floated the idea with her husband of flying their children out to stay with her recently retired parents, who live outside of Sacramento, Calif., until the pandemic eases up. “I was thinking to myself, ‘Am I overreacting? Is the pandemic not going to be that bad?’ because the rest of the country seemed to be in some amount of denial,” she said. “So, I called my dad, who’s a retired pediatric anesthesiologist. He’s from Egypt so he’s done crisis medicine in his time. He encouraged me to send the kids.”
On the same day that Dr. Dakkak began her first 12-hour COVID-19 shift at the hospital, her husband and children boarded a plane to California, where the kids remain in the care of her parents. Her husband returned after staying there for 2 weeks. “Every day when I’m working, I validate my decision,” she said. “When I first started, I worked 5 nights in a row, had 2 days off, and then worked 6 nights in a row. I was busy so I didn’t think about [being away from my kids], but at the same time I was grateful that I didn’t have to come home and worry about homeschooling the kids or infecting them.”
She checks in with them as she can via cell phone or FaceTime. “My son has been very honest,” Dr. Dakkak said. “He says, ‘FaceTime makes me miss you more, and I don’t like it,’ which I understand. I’ll call my mom, and if they want to talk to me, they’ll talk to me. If they don’t want to talk to me, I’m okay. This is about them being healthy and safe. I sent them a care package a few days ago with cards and some workbooks. I’m optimistic that in June I can at least see them if not bring them home.”
Dr. Dakkak describes leading a COVID-19 team as a grueling experience that challenges her medical know-how nearly every day, with seemingly ever-changing algorithms. “Our knowledge of this disease is five steps behind, and changing at lightning speed,” said Dr. Dakkak, who completed a fellowship in surgical and high-risk obstetrics. “It’s hard to balance continuing to teach evidence-based medicine for everything else in medicine [with continuing] to practice minimal and ever-changing evidence-based COVID medicine. We just don’t know enough [about the virus] yet. This is nothing like we were taught in medical school. Everyone has elevated d-dimers with COVID-19, and we don’t get CT pulmonary angiograms [CTPAs] on all of them; we wouldn’t physically be able to. Some patients have d-dimers in the thousands, and only some are stable to get CTPAs. We are also finding pulmonary embolisms. Now we’re basing our algorithm on anticoagulation due to d-dimers because sometimes you can’t always do a CTPA even if you want to. On the other hand, we have people who are coming into the hospital too late. We’ve had a few who have come in after having days of stroke symptoms. I worry about our patients at home who hesitate to come in when they really should.”
Sometimes she feels sad for the medical residents on her team because their instinct is to go in and check on each patient, “but I don’t want them to get exposed,” she said. “So, we check in by phone, or if they need a physical check-in, we minimize the check-ins; only one of us goes in. I’m more willing to put myself in the room than to put them in the room. I also feel for them because they came into medicine for the humanity of medicine – not the charting or the ordering of medicine. I also worry about the acuity and sadness they’re seeing. This is a rough introduction to medicine for them.”
When interviewed for this story in late April, Dr. Dakkak had kept track of her intubated COVID-19 patients. “Most of my patients get to go home without having been intubated, but those aren’t the ones I worry about,” she said. “I have two patients I have been watching. One of them has just been extubated and I’m still worried about him, but I’m hoping he’s going to be fine. The other one is the first pregnant woman we intubated. She is now extubated, doing really well, and went home. Her fetus is doing well, never had any issues while she was intubated. Those cases make me happy. They were both under the age of 35. It is nice to follow those intubations and find that the majority are doing okay.”
The first patient she had cared for who died was a young man “who was always in good spirits,” she recalled. “We called his brother right before intubating him. After intubation, his oxygen saturation didn’t jump up, which made me worry a bit.” About a week later, the young man died. “I kept thinking, ‘We intubated him when he was still comfortable talking. Should I have put it off and had him call more people to say goodbye? Should I have known that he wasn’t going to wake up?’ ” said Dr. Dakkak, who is also women’s health director at Manet Community Health Centers. “A lot of us have worked on our end-of-life discussions in the past month, just being able to tell somebody, ‘This might be your last time to call family. Call family and talk to whoever you want.’ Guilt isn’t the right word, but it’s unsettling if I’m the last person a patient talks to. I feel that, if that’s the case, then I didn’t do a good enough job trying to get them to their family or friends. If I am worried about a patient’s clinical status declining, I tell families now, when I call them, ‘I hope I’m wrong; I hope they don’t need to be intubated, but I think this is the time to talk.’ ”
To keep herself grounded during off hours, Dr. Dakkak spends time resting, checking in with her family, journaling “to get a lot of feelings out,” gardening, hiking, and joining Zoom chats with friends. Once recentered, she draws from a sense of obligation to others as she prepares for her next shift caring for COVID-19 patients.
“I have a lot of love for the world that I get to expend by doing this hard work,” she said. “I love humanity and I love humanity in times of crisis. The interactions I have with patients and their families are still central to why I do this work. I love my medical teams, and I would never want to let them down. It is nice to feel the sense of teamwork across the hospital. The nurses that I sit with and experience this with are amazing. I keep saying that the only thing I want to do when this pandemic is over is hug everyone. I think we are in dire need of hugs.”
Finding light in the darkness
Internist Katie Jobbins, DO, also has worked in a professional role that was created because of COVID-19.
Shortly before Dr. Jobbins was deployed to Baystate Medical Center in Springfield, Ma., for 2 weeks in April of 2020 to help clinicians with an anticipated surge of COVID-19 cases, she encountered a patient who walked into Baystate’s High Street Health Center.
“I think I have COVID-19,” the patient proclaimed to her, at the outpatient clinic that serves mostly inner-city, Medicaid patients.
Prior to becoming an ambulatory internist, Dr. Jobbins was a surgical resident. “So I went into that mode of ‘I need to do this, this, and this,’ ” she said. “I went through a checklist in my head to make sure I was prepared to take care of the patient.”
She applied that same systems approach during her redeployment assignment in the tertiary care hospital, which typically involved 10-hour shifts overseeing internal medicine residents in a medical telemetry unit. “We would take care of people under investigation for COVID-19, but we were not assigned to the actual COVID unit,” said Dr. Jobbins, who is also associate program director for the internal medicine residency program at the University of Massachusetts Medical School–Baystate Springfield. “They tried to redeploy other people to those units who had special training, and we were trying to back fill into where those people that got moved to the COVID units or the ICU units were actually working. We were taking more of the medical side of the floors.”
Even so, one patient on the unit was suspected of having COVID-19, so Dr. Jobbins suited up with personal protective equipment and conducted a thorough exam with residents waiting outside the patient’s room, a safe distance away. “I explained everything I found on the exam to the residents, trying to give them some educational benefit, even though they couldn’t physically examine the patient because we’re trying to protect them since they’re in training,” she said. “It was anxiety provoking, on some level, knowing that there’s a potential risk of exposure [to the virus], but knowing that Baystate Health has gone to extraordinary measures to make sure we have the correct PPE and support us is reassuring. I knew I had the right equipment and the right tools to take care of the patient, which calmed my nerves and made me feel like I could do the job. That’s the most important thing as a physician during this time: knowing that you have people supporting you who have your back at all times.”
Like Dr. Dakkak, Dr. Jobbins had to make some adjustments to her interaction with her family.
Before she began the deployment, Dr. Jobbins engaged in a frank discussion with her husband and her two young boys about the risks she faced working in a hospital caring for patients with COVID-19. “My husband and I made sure our wills were up to date, and we talked about what we would do if either of us got the virus,” she said. To minimize the potential risk of transmitting the virus to her loved ones during the two-week deployment, she considered living away from her family in a nearby home owned by her father, but decided against that and to “take it day by day.” Following her hospital shifts, Dr. Jobbins changed into a fresh set of clothes before leaving the hospital. Once she arrived home, she showered to reduce the risk of possibly becoming a vector to her family.
She had to tell her kids: “You can’t kiss me right now.”
“As much as it’s hard for them to understand, we had a conversation [in which I explained] ‘This is a virus. It will go away eventually, but it’s a virus we’re fighting.’ It’s interesting to watch a 3-year-old try to process that and take his play samurai sword or Marvel toys and decide he’s going to run around the neighborhood and try to kill the virus.”
At the High Street Health Center, Dr. Jobbins and her colleagues have transitioned to conducting most patient encounters via telephone or video appointments. “We have tried to maintain as much continuity for our patients to address their chronic medical needs through these visits, such as hypertension management and diabetes care,” she said. “We have begun a rigorous screening process to triage and treat patients suspicious for COVID-19 through telehealth in hopes of keeping them safe and in their own homes. We also continue to see patients for nonrespiratory urgent care needs in person once they have screened negative for COVID-19.”
“In terms of the inpatient setting, we’ve noticed that a lot of people are choosing not to go to the hospital now, unless they’re extremely ill,” Dr. Jobbins noted. “We’re going to need to find a balance with when do people truly need to go to the hospital and when do they not? What can we manage as an outpatient versus having someone go to the emergency department? That’s really the role of the primary care physician. We need to help people understand, ‘You don’t need to go to the ED for everything, but here are the things you really need to go for.’ ”
“It will be interesting to see what health care looks like in 6 months or a year. I’m excited to see where we land,” Dr. Jobbins added.
Hopes for the Future of Telemedicine
When the practice of medicine enters a post–COVID-19 era, Dr. Jobbins hopes that telemedicine will be incorporated more into the delivery of patient care. “I’ve found that many of my patients who often are no-shows to the inpatient version of their visits have had a higher success rate of follow-through when we do the telephone visits,” she said. “It’s been very successful. I hope that the insurance companies and [Centers for Medicare & Medicaid] will continue to reimburse this as they see this is a benefit to our patients.
Dr. Hopkins is also hopeful that physicians will be able to successfully see patients via telemedicine in the postpandemic world.
“For the ups and downs we’ve had with telemedicine, I’d love for us to be able to enhance the positives and incorporate that into our practice going forward. If we can reach our patients and help treat them where they are, rather than them having to come to us, that may be a plus,” he said.
In the meantime, Dr. Jobbins presses on as the curve of COVID-19 cases flattens in Western Massachusetts and remains grateful that she chose to practice medicine.
“The commitment I have to being an educator in addition to being a physician is part of why I keep doing this,” Dr. Jobbins said. “I find this to be one of the most fulfilling jobs and careers you could ever have: being there for people when they need you the most. That’s really what a physician’s job is: being there for people when a family member has passed away or when they just need to talk because they’re having anxiety. At the end of the day, if we can impart that to those we work with and bring in a positive attitude, it’s infectious and it makes people see this is a reason we keep doing what we’re doing.”
She’s also been heartened by the kindness of strangers during this pandemic, from those who made and donated face shields when they were in short supply, to those who delivered food to the hospital as a gesture of thanks.
“I had a patient who made homemade masks and sent them to my office,” she said. “There’s obviously good and bad during this time, but I get hope from seeing all of the good things that are coming out of this, the whole idea of finding the light in the darkness.”
New study of diabetes drug for COVID-19 raises eyebrows
A just-launched study of the type 2 diabetes agent dapagliflozin (Farxiga, AstraZeneca) in patients with mild to moderate COVID-19 is raising eyebrows, given that several expert groups have advised that drugs in this class – the sodium-glucose cotransporter 2 (SGLT2) inhibitors – be stopped in all patients hospitalized with COVID-19 because of the increased risk for diabetic ketoacidosis (DKA).
The randomized, double-blind, placebo-controlled, phase 3 Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) study is sponsored by AstraZeneca and Saint Luke’s Mid America Heart Institute.
The trial will assess whether dapagliflozin reduces the risks of disease progression, clinical complications, and death because of COVID-19 in patients with type 2 diabetes, cardiovascular disease, and/or mild to moderate chronic kidney disease (CKD).
“Dapagliflozin has demonstrated cardio- and renal-protective benefits and improved outcomes in high-risk patients with type 2 diabetes, heart failure with reduced ejection fraction, and CKD,” said the principal investigator of DARE-19, Mikhail N. Kosiborod, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo.
And “patients with COVID-19 and underlying cardiometabolic disease appear to be at the highest risk of morbid complications,” he explained in an AstraZeneca statement.
“Through DARE-19, we hope to decrease the severity of illness, and prevent cardiovascular, respiratory, and kidney decompensation, which are common in patients with COVID-19,” Dr. Kosiborod continued.
However, advice to stop SGLT2 inhibitors in patients hospitalized with COVID-19 because of its associated DKA risk has come from several channels.
These include initial guidance from Diabetes UK; experts who spoke during an American Diabetes Association webinar; and most recently, an international panel of diabetes experts.
Some clinicians went so far as to say that they view the trial as potentially dangerous, while others said they could see some logic to it, as long as it is carefully managed.
“A dangerous proposition – a DARE I would not take”
Partha Kar, MD, of Portsmouth Hospitals NHS Trust and national clinical director of diabetes at NHS England, said in an interview: “It’s interesting to see [AstraZeneca] embark on a study with a particular class of drug whereby ... [in] the UK we have said that if you get sent to hospital with COVID-19 you should stop [SGLT2 inhibitors] immediately.”
It “sounds like a risky proposition to go ahead with, [and it] definitely made me raise an eyebrow,” he added.
Nephrologist Bruce R. Leslie, MD, of Seventh Doctor Consulting in Princeton, N.J., agreed with Dr. Kar.
“Giving SGLT2 inhibitors to patients in the DARE-19 study is a dangerous proposition because these drugs can induce ketoacidosis during the stress of acute illness such as COVID-19. ... Moreover, ketoacidosis is associated with hypercoagulability which could be especially dangerous in COVID-19, given that it has been causing thrombophilia with large-vessel occlusive strokes in young patients,” he said in an interview.
“One wonders how these risks were assessed by the authorities that approved the DARE-19 study,” said Dr. Leslie, who formerly worked for Bristol-Myers Squibb.
“How does the sponsor intend to secure informed consent given the risks? This is a DARE I would not take,” he said.
Asked to address these concerns, Dr. Kosiborod said in an interview that “the DARE-19 trial will assess both the efficacy and the safety of dapagliflozin in this patient population in a closely monitored environment of a rigorously designed randomized clinical trial. The trial protocol excludes patients with type 1 diabetes or at high risk for DKA.
“Furthermore, the protocol includes detailed specific instructions to ensure careful monitoring for DKA, including frequent assessments of acid-base status in the hospital setting. The safety data will be closely monitored by an independent data-monitoring committee,” he continued.
Dr. Kosiborod also pointed out that there is “no systematically collected information on the use of dapagliflozin or any other SGLT2 inhibitor in patients being treated for COVID-19, including the associated potential benefits, possible risks such as DKA, and the balance of these potential benefits and risks.”
DARE-19 design: Several outcomes will be examined
The DARE-19 trial is designed to enroll 900 adults with confirmed SARS-CoV-2 infection and oxygen saturation of 94% or greater.
Inclusion criteria include a medical history of hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure, and/or stage 3-4 CKD. Exclusion criteria include current SGLT2 inhibitor treatment, type 1 diabetes, severe CKD, and severe COVID-19.
Dapagliflozin is approved in the EU for use in some patients with type 1 diabetes; this is not the case in the United States, although SGLT2 inhibitors in general are sometimes used off label in these patients.
Patients in DARE-19 will be randomized to 10 mg/day dapagliflozin or placebo for 30 days, in addition to standard care, in participating hospital. Primary outcomes are time to first occurrence of either death or new or worsened organ dysfunction, including respiratory decompensation, new or worsening heart failure, requirement for vasopressor therapy, ventricular tachycardia, and renal failure.
Secondary outcomes include a composite of time to death from any cause, time to new/worsened organ dysfunction, clinical status at day 30, and time to hospital discharge.
Rationale for the study
Irl B. Hirsch, MD, professor and diabetes treatment and teaching chair at the University of Washington, Seattle, said in an interview that he does see some logic to the trial.
Admitting that he doesn’t know much about “COVID-19 cardiomyopathy” – which would be one of the targets of dapagliflozin – other than it is quite common, he said that this, along with the potential renal benefits of dapagliflozin in the setting of COVID-19, make the study “intriguing.”
“Perhaps there is some rationale to it,” he said. However, “my concern is these sick COVID-19 patients are often acidemic, and besides the very complex acid-base challenges we see with intubated patients, these patients likely have combination lactic and ketoacidemia, the latter at least some from starvation.
“Still, if enough dextrose and insulin are provided to prevent ketoacid accumulation, my guess is it would do at least as well as hydroxychloroquine,” he said.
And Simon Heller, MD, professor of clinical diabetes at the University of Sheffield (England), said in an interview: “I think it is quite a brave study, mainly because of the increased risk of DKA.
“However, on the basis that these patients will be carefully monitored, the risk of DKA shouldn’t be great. I think it is important that patients with type 2 diabetes can participate whenever possible in such trials,” he said.
The estimated completion date for DARE-19 is December 2020.
Dr. Kosiborod has reported receiving grant support, honoraria, and/or research support from AstraZeneca, Boehringer Ingelheim, Sanofi, Amgen, Novo Nordisk, Merck, Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia Therapeutics, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. Dr. Leslie has reported owning stock in Bristol-Myers Squibb, Pfizer, and Lilly. Dr. Hirsch has reported consulting for Abbott Diabetes Care, Roche, and Bigfoot Biomedical, conducting research for Medtronic, and is a diabetes editor for UpToDate. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, MSD, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic UK. He is on the advisory board for Medscape. Dr. Kar has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A just-launched study of the type 2 diabetes agent dapagliflozin (Farxiga, AstraZeneca) in patients with mild to moderate COVID-19 is raising eyebrows, given that several expert groups have advised that drugs in this class – the sodium-glucose cotransporter 2 (SGLT2) inhibitors – be stopped in all patients hospitalized with COVID-19 because of the increased risk for diabetic ketoacidosis (DKA).
The randomized, double-blind, placebo-controlled, phase 3 Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) study is sponsored by AstraZeneca and Saint Luke’s Mid America Heart Institute.
The trial will assess whether dapagliflozin reduces the risks of disease progression, clinical complications, and death because of COVID-19 in patients with type 2 diabetes, cardiovascular disease, and/or mild to moderate chronic kidney disease (CKD).
“Dapagliflozin has demonstrated cardio- and renal-protective benefits and improved outcomes in high-risk patients with type 2 diabetes, heart failure with reduced ejection fraction, and CKD,” said the principal investigator of DARE-19, Mikhail N. Kosiborod, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo.
And “patients with COVID-19 and underlying cardiometabolic disease appear to be at the highest risk of morbid complications,” he explained in an AstraZeneca statement.
“Through DARE-19, we hope to decrease the severity of illness, and prevent cardiovascular, respiratory, and kidney decompensation, which are common in patients with COVID-19,” Dr. Kosiborod continued.
However, advice to stop SGLT2 inhibitors in patients hospitalized with COVID-19 because of its associated DKA risk has come from several channels.
These include initial guidance from Diabetes UK; experts who spoke during an American Diabetes Association webinar; and most recently, an international panel of diabetes experts.
Some clinicians went so far as to say that they view the trial as potentially dangerous, while others said they could see some logic to it, as long as it is carefully managed.
“A dangerous proposition – a DARE I would not take”
Partha Kar, MD, of Portsmouth Hospitals NHS Trust and national clinical director of diabetes at NHS England, said in an interview: “It’s interesting to see [AstraZeneca] embark on a study with a particular class of drug whereby ... [in] the UK we have said that if you get sent to hospital with COVID-19 you should stop [SGLT2 inhibitors] immediately.”
It “sounds like a risky proposition to go ahead with, [and it] definitely made me raise an eyebrow,” he added.
Nephrologist Bruce R. Leslie, MD, of Seventh Doctor Consulting in Princeton, N.J., agreed with Dr. Kar.
“Giving SGLT2 inhibitors to patients in the DARE-19 study is a dangerous proposition because these drugs can induce ketoacidosis during the stress of acute illness such as COVID-19. ... Moreover, ketoacidosis is associated with hypercoagulability which could be especially dangerous in COVID-19, given that it has been causing thrombophilia with large-vessel occlusive strokes in young patients,” he said in an interview.
“One wonders how these risks were assessed by the authorities that approved the DARE-19 study,” said Dr. Leslie, who formerly worked for Bristol-Myers Squibb.
“How does the sponsor intend to secure informed consent given the risks? This is a DARE I would not take,” he said.
Asked to address these concerns, Dr. Kosiborod said in an interview that “the DARE-19 trial will assess both the efficacy and the safety of dapagliflozin in this patient population in a closely monitored environment of a rigorously designed randomized clinical trial. The trial protocol excludes patients with type 1 diabetes or at high risk for DKA.
“Furthermore, the protocol includes detailed specific instructions to ensure careful monitoring for DKA, including frequent assessments of acid-base status in the hospital setting. The safety data will be closely monitored by an independent data-monitoring committee,” he continued.
Dr. Kosiborod also pointed out that there is “no systematically collected information on the use of dapagliflozin or any other SGLT2 inhibitor in patients being treated for COVID-19, including the associated potential benefits, possible risks such as DKA, and the balance of these potential benefits and risks.”
DARE-19 design: Several outcomes will be examined
The DARE-19 trial is designed to enroll 900 adults with confirmed SARS-CoV-2 infection and oxygen saturation of 94% or greater.
Inclusion criteria include a medical history of hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure, and/or stage 3-4 CKD. Exclusion criteria include current SGLT2 inhibitor treatment, type 1 diabetes, severe CKD, and severe COVID-19.
Dapagliflozin is approved in the EU for use in some patients with type 1 diabetes; this is not the case in the United States, although SGLT2 inhibitors in general are sometimes used off label in these patients.
Patients in DARE-19 will be randomized to 10 mg/day dapagliflozin or placebo for 30 days, in addition to standard care, in participating hospital. Primary outcomes are time to first occurrence of either death or new or worsened organ dysfunction, including respiratory decompensation, new or worsening heart failure, requirement for vasopressor therapy, ventricular tachycardia, and renal failure.
Secondary outcomes include a composite of time to death from any cause, time to new/worsened organ dysfunction, clinical status at day 30, and time to hospital discharge.
Rationale for the study
Irl B. Hirsch, MD, professor and diabetes treatment and teaching chair at the University of Washington, Seattle, said in an interview that he does see some logic to the trial.
Admitting that he doesn’t know much about “COVID-19 cardiomyopathy” – which would be one of the targets of dapagliflozin – other than it is quite common, he said that this, along with the potential renal benefits of dapagliflozin in the setting of COVID-19, make the study “intriguing.”
“Perhaps there is some rationale to it,” he said. However, “my concern is these sick COVID-19 patients are often acidemic, and besides the very complex acid-base challenges we see with intubated patients, these patients likely have combination lactic and ketoacidemia, the latter at least some from starvation.
“Still, if enough dextrose and insulin are provided to prevent ketoacid accumulation, my guess is it would do at least as well as hydroxychloroquine,” he said.
And Simon Heller, MD, professor of clinical diabetes at the University of Sheffield (England), said in an interview: “I think it is quite a brave study, mainly because of the increased risk of DKA.
“However, on the basis that these patients will be carefully monitored, the risk of DKA shouldn’t be great. I think it is important that patients with type 2 diabetes can participate whenever possible in such trials,” he said.
The estimated completion date for DARE-19 is December 2020.
Dr. Kosiborod has reported receiving grant support, honoraria, and/or research support from AstraZeneca, Boehringer Ingelheim, Sanofi, Amgen, Novo Nordisk, Merck, Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia Therapeutics, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. Dr. Leslie has reported owning stock in Bristol-Myers Squibb, Pfizer, and Lilly. Dr. Hirsch has reported consulting for Abbott Diabetes Care, Roche, and Bigfoot Biomedical, conducting research for Medtronic, and is a diabetes editor for UpToDate. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, MSD, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic UK. He is on the advisory board for Medscape. Dr. Kar has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A just-launched study of the type 2 diabetes agent dapagliflozin (Farxiga, AstraZeneca) in patients with mild to moderate COVID-19 is raising eyebrows, given that several expert groups have advised that drugs in this class – the sodium-glucose cotransporter 2 (SGLT2) inhibitors – be stopped in all patients hospitalized with COVID-19 because of the increased risk for diabetic ketoacidosis (DKA).
The randomized, double-blind, placebo-controlled, phase 3 Dapagliflozin in Respiratory Failure in Patients With COVID-19 (DARE-19) study is sponsored by AstraZeneca and Saint Luke’s Mid America Heart Institute.
The trial will assess whether dapagliflozin reduces the risks of disease progression, clinical complications, and death because of COVID-19 in patients with type 2 diabetes, cardiovascular disease, and/or mild to moderate chronic kidney disease (CKD).
“Dapagliflozin has demonstrated cardio- and renal-protective benefits and improved outcomes in high-risk patients with type 2 diabetes, heart failure with reduced ejection fraction, and CKD,” said the principal investigator of DARE-19, Mikhail N. Kosiborod, MD, a cardiologist at Saint Luke’s Mid America Heart Institute, Kansas City, Mo.
And “patients with COVID-19 and underlying cardiometabolic disease appear to be at the highest risk of morbid complications,” he explained in an AstraZeneca statement.
“Through DARE-19, we hope to decrease the severity of illness, and prevent cardiovascular, respiratory, and kidney decompensation, which are common in patients with COVID-19,” Dr. Kosiborod continued.
However, advice to stop SGLT2 inhibitors in patients hospitalized with COVID-19 because of its associated DKA risk has come from several channels.
These include initial guidance from Diabetes UK; experts who spoke during an American Diabetes Association webinar; and most recently, an international panel of diabetes experts.
Some clinicians went so far as to say that they view the trial as potentially dangerous, while others said they could see some logic to it, as long as it is carefully managed.
“A dangerous proposition – a DARE I would not take”
Partha Kar, MD, of Portsmouth Hospitals NHS Trust and national clinical director of diabetes at NHS England, said in an interview: “It’s interesting to see [AstraZeneca] embark on a study with a particular class of drug whereby ... [in] the UK we have said that if you get sent to hospital with COVID-19 you should stop [SGLT2 inhibitors] immediately.”
It “sounds like a risky proposition to go ahead with, [and it] definitely made me raise an eyebrow,” he added.
Nephrologist Bruce R. Leslie, MD, of Seventh Doctor Consulting in Princeton, N.J., agreed with Dr. Kar.
“Giving SGLT2 inhibitors to patients in the DARE-19 study is a dangerous proposition because these drugs can induce ketoacidosis during the stress of acute illness such as COVID-19. ... Moreover, ketoacidosis is associated with hypercoagulability which could be especially dangerous in COVID-19, given that it has been causing thrombophilia with large-vessel occlusive strokes in young patients,” he said in an interview.
“One wonders how these risks were assessed by the authorities that approved the DARE-19 study,” said Dr. Leslie, who formerly worked for Bristol-Myers Squibb.
“How does the sponsor intend to secure informed consent given the risks? This is a DARE I would not take,” he said.
Asked to address these concerns, Dr. Kosiborod said in an interview that “the DARE-19 trial will assess both the efficacy and the safety of dapagliflozin in this patient population in a closely monitored environment of a rigorously designed randomized clinical trial. The trial protocol excludes patients with type 1 diabetes or at high risk for DKA.
“Furthermore, the protocol includes detailed specific instructions to ensure careful monitoring for DKA, including frequent assessments of acid-base status in the hospital setting. The safety data will be closely monitored by an independent data-monitoring committee,” he continued.
Dr. Kosiborod also pointed out that there is “no systematically collected information on the use of dapagliflozin or any other SGLT2 inhibitor in patients being treated for COVID-19, including the associated potential benefits, possible risks such as DKA, and the balance of these potential benefits and risks.”
DARE-19 design: Several outcomes will be examined
The DARE-19 trial is designed to enroll 900 adults with confirmed SARS-CoV-2 infection and oxygen saturation of 94% or greater.
Inclusion criteria include a medical history of hypertension, type 2 diabetes, atherosclerotic cardiovascular disease, heart failure, and/or stage 3-4 CKD. Exclusion criteria include current SGLT2 inhibitor treatment, type 1 diabetes, severe CKD, and severe COVID-19.
Dapagliflozin is approved in the EU for use in some patients with type 1 diabetes; this is not the case in the United States, although SGLT2 inhibitors in general are sometimes used off label in these patients.
Patients in DARE-19 will be randomized to 10 mg/day dapagliflozin or placebo for 30 days, in addition to standard care, in participating hospital. Primary outcomes are time to first occurrence of either death or new or worsened organ dysfunction, including respiratory decompensation, new or worsening heart failure, requirement for vasopressor therapy, ventricular tachycardia, and renal failure.
Secondary outcomes include a composite of time to death from any cause, time to new/worsened organ dysfunction, clinical status at day 30, and time to hospital discharge.
Rationale for the study
Irl B. Hirsch, MD, professor and diabetes treatment and teaching chair at the University of Washington, Seattle, said in an interview that he does see some logic to the trial.
Admitting that he doesn’t know much about “COVID-19 cardiomyopathy” – which would be one of the targets of dapagliflozin – other than it is quite common, he said that this, along with the potential renal benefits of dapagliflozin in the setting of COVID-19, make the study “intriguing.”
“Perhaps there is some rationale to it,” he said. However, “my concern is these sick COVID-19 patients are often acidemic, and besides the very complex acid-base challenges we see with intubated patients, these patients likely have combination lactic and ketoacidemia, the latter at least some from starvation.
“Still, if enough dextrose and insulin are provided to prevent ketoacid accumulation, my guess is it would do at least as well as hydroxychloroquine,” he said.
And Simon Heller, MD, professor of clinical diabetes at the University of Sheffield (England), said in an interview: “I think it is quite a brave study, mainly because of the increased risk of DKA.
“However, on the basis that these patients will be carefully monitored, the risk of DKA shouldn’t be great. I think it is important that patients with type 2 diabetes can participate whenever possible in such trials,” he said.
The estimated completion date for DARE-19 is December 2020.
Dr. Kosiborod has reported receiving grant support, honoraria, and/or research support from AstraZeneca, Boehringer Ingelheim, Sanofi, Amgen, Novo Nordisk, Merck, Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia Therapeutics, Novartis, Applied Therapeutics, Amarin, and Eli Lilly. Dr. Leslie has reported owning stock in Bristol-Myers Squibb, Pfizer, and Lilly. Dr. Hirsch has reported consulting for Abbott Diabetes Care, Roche, and Bigfoot Biomedical, conducting research for Medtronic, and is a diabetes editor for UpToDate. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, MSD, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic UK. He is on the advisory board for Medscape. Dr. Kar has reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Yale’s COVID-19 inpatient protocol: Hydroxychloroquine plus/minus tocilizumab
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.
Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
[email protected]
This article was updated with the latest treatment algorithm on 5/6/2020.
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.

Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
[email protected]
This article was updated with the latest treatment algorithm on 5/6/2020.
Hydroxychloroquine is currently first-line, and tocilizumab second-line, for people hospitalized with polymerase chain reaction–confirmed COVID-19 in the Yale New Haven (Conn.) Health System, which operates hospitals across Connecticut, many of them hard hit by the pandemic.
Patients enter the treatment algorithm if they have an oxygen saturation at or below 93% on room air or chronic supplementation, or by being acutely ill with fever, respiratory signs, or opacities on chest x-ray, plus risk factors for severe illness such as age over 60 years, chronic heart or lung disease, immunosuppression, diabetes, hypertension, or obesity, which makes it harder to ventilate.
Physicians at Yale have seen both presentations – oxygen desaturation and frank illness – and “wanted to make sure we weren’t missing anyone,” said Nihar Desai, MD, a Yale cardiologist who is helping to coordinate the health system’s response to COVID-19.
In either case, the initial treatment is the same at Yale hospitals: hydroxychloroquine for 5 days, with tocilizumab (Actemra) considered when not contraindicated and oxygen requirements reach or pass 3 L, or 2 L with C-reactive protein levels above 70 mg/L.
Patients are put on prophylactic enoxaparin to thin the blood unless contraindicated; inflammatory, cardiac, kidney, and other markers are checked every 12 or 24 hours; and ECGs are taken daily if telemetry isn’t used. Chest x-rays are repeated if clinical signs worsen, and transthoracic echocardiograms are ordered for suspected heart problems.
ICUs are notified early if the clinical situation worsens because patients “can deteriorate very quickly; at the first sign of trouble, people are really aggressive,” said Dr. Desai, also the associate chief of clinical operations in the Section of Cardiovascular Medicine at the Yale University, New Haven.
The haze of battle
Yale has updated its algorithm several times since the virus first hit Connecticut weeks ago. A team including pulmonologists, critical care physicians, pharmacologists, infectious disease experts, and cardiologists, including Dr. Desai, are constantly monitoring the situation and making changes as new information comes in.

Much of what’s being done at Yale and elsewhere is empiric because there are simply not much data to go on. “We are trying to do the best we can” in “the haze of battle. People really came together quickly to develop this. One hopes we never have to go through anything like this again,” he said.
Hydroxychloroquine is first-line at Yale because in-vitro data show potent inhibition of the virus and possible clinical benefit, which is about as good as evidence gets at the moment. Also, “it’s cheap, it’s been used for decades, and people are relatively comfortable with it,” Dr. Desai said.
Tocilizumab, an interleukin-6 (IL-6) receptor antagonist, is second-line because it might counter the cytokine storm thought to be at least partly responsible for severe complications, and retrospective data suggest possible benefit. The antiviral remdesivir and IL-6 blocker sarulimab (Kevzara) are also potential candidates, available through clinical trials.
Dr. Desai wanted to share the algorithm with other providers because, he noted, “there are a lot of places that may not have all the resources we have.”
His home institution, Yale New Haven Hospital, is almost half full with COVID-19 patients, at more than 400.
A moving target
Yale’s approach is similar in confirmed COVID-19 cases already in respiratory failure, including those on mechanical ventilation and extracorporeal membrane oxygenation: hydroxychloroquine and possibly tocilizumab, but also methylprednisolone if clinical status worsens or inflammatory markers go up. The steroid is for additional help battling the cytokine storm, Dr. Desai said.
The degree of anticoagulation in the ICU is based on d-dimer levels or suspicion or confirmation of venous thromboembolism. Telemetry is monitored closely for QTc prolongation, and point of care ultrasound is considered to check left ventricular function in the setting of markedly increased cardiac troponin levels, ECG abnormalities, or hemodynamic instability.
Previous versions of Yale’s algorithm included HIV protease inhibitors, but they were pulled after a recent trial found no benefit. Frequency of monitoring was also reduced from every 8 hours because it didn’t improve decision making and put staff collecting specimens at risk (N Engl J Med. 2020 Mar 18. doi: 10.1056/NEJMoa2001282).
Anticoagulation was added to newer versions after it became clear that COVID-19 is prothrombotic. “We are still seeing thrombotic events that might warrant further intensification,” Dr. Desai said.
Newer algorithms also have Yale watching QTc intervals more closely. It’s unclear if the prolongation risk is caused by the infection or hydroxychloroquine.
On April 24, the Food and Drug Administration reiterated it’s concern about the arrhythmia risk with hydroxychloroquine and emphasized that it should only be used for COVID-19 patients when they are hospitalized and it is not feasible for them to participate in a clinical trial.
To help keep patients safe, ECGs from confirmed or suspected COVID-19 cases are now first in line to be reviewed by cardiologists across Yale hospitals to pick up prolongations and notify providers as soon as possible. Hydroxychloroquine is held if there are no other explanations.
Cardiologists are on the fontline at Yale and elsewhere, Dr. Desai said, because heart complications like myocarditis and arrhythmias emerged early as common problems in hospitalized patients.
[email protected]
This article was updated with the latest treatment algorithm on 5/6/2020.
COVID-19: An opportunity, challenge for addiction treatment, NIDA boss says
The COVID-19 pandemic is posing significant challenges while also providing unique opportunities for patients with substance use disorders (SUD), a leading expert says.
Nora Volkow, MD, director of the National Institute on Drug Abuse, said that the pandemic has accelerated the use of telemedicine, making it easier for patients with SUD to access treatment. It has also led to the proliferation of more mental health hotlines, which is critical since the vast majority of these patients have comorbid mental illness.
In addition, COVID-19 has resulted in increased availability of “alternative” peer support mechanisms via cellphones or computers to aid individuals’ sobriety.
Dr. Volkow spoke at the virtual American Psychiatric Association Spring Highlights Meeting 2020, which is replacing the organization’s canceled annual meeting.
While methadone clinics have had to close during the pandemic, making it challenging for those on medically assisted treatment to receive methadone or buprenorphine, some of the rules and regulations have been relaxed in order to make these medications accessible without the need for in-person attendance at a clinic. In addition, the Substance Abuse and Mental Health Services Administration has relaxed some of its own regulations regarding telehealth and opioid treatment programs.
Social isolation, stigma intensified
A pandemic increases anxiety in the general population, but for patients with SUD who may be also be struggling with homelessness and comorbid mental illness, the situation can further exacerbate social stigma and isolation – leading to relapse, more overdoses, and overdose deaths, Dr. Volkow said. Social interaction is “extraordinarily important” for patients and “one of the most powerful tools we have” to build resilience.
Right now, said Dr. Volkow, “we are in the dark as to how COVID infections have affected the number of overdose deaths.”
However, she noted that
“So even through this devastation, we can actually extract something that may help others in future,” she said.
Dr. Volkow noted that during the pandemic it is critical to reinforce the importance of engaging in – and remaining in – treatment to SUD patients. It’s also crucial to make patients aware of social support systems and behavioral interventions to help them cope with stress and to mitigate relapse risk.
COVID-19 and relapse
Elie G. Aoun, MD, assistant professor of psychiatry at New York University and vice chair of the APA’s Council on Addiction Psychiatry, said in an interview that Dr. Volkow’s presentation provided “exactly the kind of accessible information” clinicians need.
Dr. Aoun said he sees the impact of the COVID-19 crisis in his practice every day. Patients with SUD “are getting the short end of the stick.”
Social distancing measures prompted by the pandemic can be “very triggering” for SUD patients, he said. One of his patients told him the current isolation, loneliness, movement restrictions, and boredom remind her of the way she felt when she used drugs.
Dr. Aoun said four of his patients have relapsed since the pandemic began. Two of them had just started treatment after years of using drugs, so this was a “major setback” for them.
He and his colleagues were “not really prepared” to provide care via video link, which he believes is not as effective as in-person sessions.
In addition to disrupting patient care, said Dr. Aoun, the pandemic is forcing the medical community to face social determinants of health, such as poverty and homelessness, as they relate to addiction disorders and whether or not someone receives care.
This article originally appeared on Medscape.com.
The COVID-19 pandemic is posing significant challenges while also providing unique opportunities for patients with substance use disorders (SUD), a leading expert says.
Nora Volkow, MD, director of the National Institute on Drug Abuse, said that the pandemic has accelerated the use of telemedicine, making it easier for patients with SUD to access treatment. It has also led to the proliferation of more mental health hotlines, which is critical since the vast majority of these patients have comorbid mental illness.
In addition, COVID-19 has resulted in increased availability of “alternative” peer support mechanisms via cellphones or computers to aid individuals’ sobriety.
Dr. Volkow spoke at the virtual American Psychiatric Association Spring Highlights Meeting 2020, which is replacing the organization’s canceled annual meeting.
While methadone clinics have had to close during the pandemic, making it challenging for those on medically assisted treatment to receive methadone or buprenorphine, some of the rules and regulations have been relaxed in order to make these medications accessible without the need for in-person attendance at a clinic. In addition, the Substance Abuse and Mental Health Services Administration has relaxed some of its own regulations regarding telehealth and opioid treatment programs.
Social isolation, stigma intensified
A pandemic increases anxiety in the general population, but for patients with SUD who may be also be struggling with homelessness and comorbid mental illness, the situation can further exacerbate social stigma and isolation – leading to relapse, more overdoses, and overdose deaths, Dr. Volkow said. Social interaction is “extraordinarily important” for patients and “one of the most powerful tools we have” to build resilience.
Right now, said Dr. Volkow, “we are in the dark as to how COVID infections have affected the number of overdose deaths.”
However, she noted that
“So even through this devastation, we can actually extract something that may help others in future,” she said.
Dr. Volkow noted that during the pandemic it is critical to reinforce the importance of engaging in – and remaining in – treatment to SUD patients. It’s also crucial to make patients aware of social support systems and behavioral interventions to help them cope with stress and to mitigate relapse risk.
COVID-19 and relapse
Elie G. Aoun, MD, assistant professor of psychiatry at New York University and vice chair of the APA’s Council on Addiction Psychiatry, said in an interview that Dr. Volkow’s presentation provided “exactly the kind of accessible information” clinicians need.
Dr. Aoun said he sees the impact of the COVID-19 crisis in his practice every day. Patients with SUD “are getting the short end of the stick.”
Social distancing measures prompted by the pandemic can be “very triggering” for SUD patients, he said. One of his patients told him the current isolation, loneliness, movement restrictions, and boredom remind her of the way she felt when she used drugs.
Dr. Aoun said four of his patients have relapsed since the pandemic began. Two of them had just started treatment after years of using drugs, so this was a “major setback” for them.
He and his colleagues were “not really prepared” to provide care via video link, which he believes is not as effective as in-person sessions.
In addition to disrupting patient care, said Dr. Aoun, the pandemic is forcing the medical community to face social determinants of health, such as poverty and homelessness, as they relate to addiction disorders and whether or not someone receives care.
This article originally appeared on Medscape.com.
The COVID-19 pandemic is posing significant challenges while also providing unique opportunities for patients with substance use disorders (SUD), a leading expert says.
Nora Volkow, MD, director of the National Institute on Drug Abuse, said that the pandemic has accelerated the use of telemedicine, making it easier for patients with SUD to access treatment. It has also led to the proliferation of more mental health hotlines, which is critical since the vast majority of these patients have comorbid mental illness.
In addition, COVID-19 has resulted in increased availability of “alternative” peer support mechanisms via cellphones or computers to aid individuals’ sobriety.
Dr. Volkow spoke at the virtual American Psychiatric Association Spring Highlights Meeting 2020, which is replacing the organization’s canceled annual meeting.
While methadone clinics have had to close during the pandemic, making it challenging for those on medically assisted treatment to receive methadone or buprenorphine, some of the rules and regulations have been relaxed in order to make these medications accessible without the need for in-person attendance at a clinic. In addition, the Substance Abuse and Mental Health Services Administration has relaxed some of its own regulations regarding telehealth and opioid treatment programs.
Social isolation, stigma intensified
A pandemic increases anxiety in the general population, but for patients with SUD who may be also be struggling with homelessness and comorbid mental illness, the situation can further exacerbate social stigma and isolation – leading to relapse, more overdoses, and overdose deaths, Dr. Volkow said. Social interaction is “extraordinarily important” for patients and “one of the most powerful tools we have” to build resilience.
Right now, said Dr. Volkow, “we are in the dark as to how COVID infections have affected the number of overdose deaths.”
However, she noted that
“So even through this devastation, we can actually extract something that may help others in future,” she said.
Dr. Volkow noted that during the pandemic it is critical to reinforce the importance of engaging in – and remaining in – treatment to SUD patients. It’s also crucial to make patients aware of social support systems and behavioral interventions to help them cope with stress and to mitigate relapse risk.
COVID-19 and relapse
Elie G. Aoun, MD, assistant professor of psychiatry at New York University and vice chair of the APA’s Council on Addiction Psychiatry, said in an interview that Dr. Volkow’s presentation provided “exactly the kind of accessible information” clinicians need.
Dr. Aoun said he sees the impact of the COVID-19 crisis in his practice every day. Patients with SUD “are getting the short end of the stick.”
Social distancing measures prompted by the pandemic can be “very triggering” for SUD patients, he said. One of his patients told him the current isolation, loneliness, movement restrictions, and boredom remind her of the way she felt when she used drugs.
Dr. Aoun said four of his patients have relapsed since the pandemic began. Two of them had just started treatment after years of using drugs, so this was a “major setback” for them.
He and his colleagues were “not really prepared” to provide care via video link, which he believes is not as effective as in-person sessions.
In addition to disrupting patient care, said Dr. Aoun, the pandemic is forcing the medical community to face social determinants of health, such as poverty and homelessness, as they relate to addiction disorders and whether or not someone receives care.
This article originally appeared on Medscape.com.