User login
Volunteer surgeon describes working at a New York hospital

In an April 18 Twitter post, Dr. Salles wrote that her unit had experienced three code blues and two deaths in a single night.
“I don’t know how many times I’ve called to tell someone their loved one has died,” she wrote in the post. “I had to do it again last night. ... Of the five patients I’ve personally been responsible for in the past two nights, two have come so close to dying that we called a code blue. That means 40% of my patients have coded. Never in my life has anything close to that happened,” she continued in the thread.
Dr. Salles, a minimally invasive and bariatric surgeon and scholar in residence at Stanford (Calif.) University, headed to New York in mid-April to assist with COVID-19 treatment efforts. Before the trip, she collected as many supplies and as much personal protective equipment as she could acquire, some of which were donated by Good Samaritans. On her first day as a volunteer, Dr. Salles recounted the stark differences between what she is used to seeing and her new environment and the novel challenges she has encountered in New York.
“Things that were not normal now seem normal,” she wrote in an April 15 Twitter post. “ICU patients in [a postanesthesia care unit] and Preop is the new normal. Patients satting in the 70s and 80s seems normal. ICU docs managing [continuous veno-venous hemodialysis] seems normal. Working with strangers seems normal. ... Obviously everyone walking around with barely any skin exposed is also the new normal.”
Similar to a “normal” ICU, new patients are admitted daily, Dr. Salles noted. However, the majority of those who leave the ICU do not go home, she wrote.
“Almost all of the ones who leave are doing so because they’ve died rather than getting better,” she wrote in the same April 19 Twitter thread. “There is a pervasive feeling of helplessness. ... The tools we are working with seem insufficient. For the sickest patients, there are no ventilator settings that seem to work, there are no medications that seem to help. I am not used to this.”
When patients are close to dying, health care workers do their best to connect the patient to loved ones through video calls, watching as family members say their last goodbyes through a screen, Dr. Salles detailed in a later post.
“Their voices cracked, and though they weren’t speaking English, I could hear their pain,” she wrote in an April 20 Twitter post. “For a moment, I imagined having to say goodbye to my mother this way. To not be able to be there, to not be able to hold her hand, to not be able to hug her. And I watched my colleague, who amazingly kept her composure until they had said everything they wanted to say. It was only after they hung up that I saw the tears well up in her eyes.”
But amid the dark days and bleak outcomes, Dr. Salles has found silver linings, humor, and gifts for which to be thankful.
“People are really generous,” she wrote in an April 15 post. “So many have offered to pay for transportation. Other docs in NY have offered to help me with supplies (and I am paying it forward). Grateful to you all!”
In another post, Dr. Salles joked that her “small head” makes it difficult to wear PPE.
“Wearing an N95 for hours really sucks,” she wrote. “It rides up, I pull it down. It digs into my cheeks, I pull it up. Repeat.”
The volunteer experience thus far has also made Dr. Salles question the future and worry about the mental health of her fellow health care professionals.
“The people who have been in NYC since the beginning of this, and those who work in Lombardy, Italy, and in Wuhan, China have faced loss for weeks to months,” she wrote in an April 18 Twitter post. “Not only do we not know when this will end, but it is likely that after it fades, it will come back in a second wave. I am lucky. I’m just a visitor here. I have the privilege to observe and learn and hopefully help, knowing I will be able to walk away. But what about those who can’t walk away? Social distancing is starting to work. But for healthcare workers, the ongoing devastation is very real. What is our long term plan?”
Dr. Salles expressed concern for health care workers who are witnessing “horrible things” with little time to process the experiences.
“It may be especially hard for those who are now working in specialties they are not used to, having to provide care they are not familiar with. They are all doing their best, but inevitably mistakes will be made, and they will likely blame themselves,” she wrote. “How do we best support them?”
Stay tuned for upcoming commentaries from Dr. Salles on her COVID-19 volunteer experience in New York City.

In an April 18 Twitter post, Dr. Salles wrote that her unit had experienced three code blues and two deaths in a single night.
“I don’t know how many times I’ve called to tell someone their loved one has died,” she wrote in the post. “I had to do it again last night. ... Of the five patients I’ve personally been responsible for in the past two nights, two have come so close to dying that we called a code blue. That means 40% of my patients have coded. Never in my life has anything close to that happened,” she continued in the thread.
Dr. Salles, a minimally invasive and bariatric surgeon and scholar in residence at Stanford (Calif.) University, headed to New York in mid-April to assist with COVID-19 treatment efforts. Before the trip, she collected as many supplies and as much personal protective equipment as she could acquire, some of which were donated by Good Samaritans. On her first day as a volunteer, Dr. Salles recounted the stark differences between what she is used to seeing and her new environment and the novel challenges she has encountered in New York.
“Things that were not normal now seem normal,” she wrote in an April 15 Twitter post. “ICU patients in [a postanesthesia care unit] and Preop is the new normal. Patients satting in the 70s and 80s seems normal. ICU docs managing [continuous veno-venous hemodialysis] seems normal. Working with strangers seems normal. ... Obviously everyone walking around with barely any skin exposed is also the new normal.”
Similar to a “normal” ICU, new patients are admitted daily, Dr. Salles noted. However, the majority of those who leave the ICU do not go home, she wrote.
“Almost all of the ones who leave are doing so because they’ve died rather than getting better,” she wrote in the same April 19 Twitter thread. “There is a pervasive feeling of helplessness. ... The tools we are working with seem insufficient. For the sickest patients, there are no ventilator settings that seem to work, there are no medications that seem to help. I am not used to this.”
When patients are close to dying, health care workers do their best to connect the patient to loved ones through video calls, watching as family members say their last goodbyes through a screen, Dr. Salles detailed in a later post.
“Their voices cracked, and though they weren’t speaking English, I could hear their pain,” she wrote in an April 20 Twitter post. “For a moment, I imagined having to say goodbye to my mother this way. To not be able to be there, to not be able to hold her hand, to not be able to hug her. And I watched my colleague, who amazingly kept her composure until they had said everything they wanted to say. It was only after they hung up that I saw the tears well up in her eyes.”
But amid the dark days and bleak outcomes, Dr. Salles has found silver linings, humor, and gifts for which to be thankful.
“People are really generous,” she wrote in an April 15 post. “So many have offered to pay for transportation. Other docs in NY have offered to help me with supplies (and I am paying it forward). Grateful to you all!”
In another post, Dr. Salles joked that her “small head” makes it difficult to wear PPE.
“Wearing an N95 for hours really sucks,” she wrote. “It rides up, I pull it down. It digs into my cheeks, I pull it up. Repeat.”
The volunteer experience thus far has also made Dr. Salles question the future and worry about the mental health of her fellow health care professionals.
“The people who have been in NYC since the beginning of this, and those who work in Lombardy, Italy, and in Wuhan, China have faced loss for weeks to months,” she wrote in an April 18 Twitter post. “Not only do we not know when this will end, but it is likely that after it fades, it will come back in a second wave. I am lucky. I’m just a visitor here. I have the privilege to observe and learn and hopefully help, knowing I will be able to walk away. But what about those who can’t walk away? Social distancing is starting to work. But for healthcare workers, the ongoing devastation is very real. What is our long term plan?”
Dr. Salles expressed concern for health care workers who are witnessing “horrible things” with little time to process the experiences.
“It may be especially hard for those who are now working in specialties they are not used to, having to provide care they are not familiar with. They are all doing their best, but inevitably mistakes will be made, and they will likely blame themselves,” she wrote. “How do we best support them?”
Stay tuned for upcoming commentaries from Dr. Salles on her COVID-19 volunteer experience in New York City.

In an April 18 Twitter post, Dr. Salles wrote that her unit had experienced three code blues and two deaths in a single night.
“I don’t know how many times I’ve called to tell someone their loved one has died,” she wrote in the post. “I had to do it again last night. ... Of the five patients I’ve personally been responsible for in the past two nights, two have come so close to dying that we called a code blue. That means 40% of my patients have coded. Never in my life has anything close to that happened,” she continued in the thread.
Dr. Salles, a minimally invasive and bariatric surgeon and scholar in residence at Stanford (Calif.) University, headed to New York in mid-April to assist with COVID-19 treatment efforts. Before the trip, she collected as many supplies and as much personal protective equipment as she could acquire, some of which were donated by Good Samaritans. On her first day as a volunteer, Dr. Salles recounted the stark differences between what she is used to seeing and her new environment and the novel challenges she has encountered in New York.
“Things that were not normal now seem normal,” she wrote in an April 15 Twitter post. “ICU patients in [a postanesthesia care unit] and Preop is the new normal. Patients satting in the 70s and 80s seems normal. ICU docs managing [continuous veno-venous hemodialysis] seems normal. Working with strangers seems normal. ... Obviously everyone walking around with barely any skin exposed is also the new normal.”
Similar to a “normal” ICU, new patients are admitted daily, Dr. Salles noted. However, the majority of those who leave the ICU do not go home, she wrote.
“Almost all of the ones who leave are doing so because they’ve died rather than getting better,” she wrote in the same April 19 Twitter thread. “There is a pervasive feeling of helplessness. ... The tools we are working with seem insufficient. For the sickest patients, there are no ventilator settings that seem to work, there are no medications that seem to help. I am not used to this.”
When patients are close to dying, health care workers do their best to connect the patient to loved ones through video calls, watching as family members say their last goodbyes through a screen, Dr. Salles detailed in a later post.
“Their voices cracked, and though they weren’t speaking English, I could hear their pain,” she wrote in an April 20 Twitter post. “For a moment, I imagined having to say goodbye to my mother this way. To not be able to be there, to not be able to hold her hand, to not be able to hug her. And I watched my colleague, who amazingly kept her composure until they had said everything they wanted to say. It was only after they hung up that I saw the tears well up in her eyes.”
But amid the dark days and bleak outcomes, Dr. Salles has found silver linings, humor, and gifts for which to be thankful.
“People are really generous,” she wrote in an April 15 post. “So many have offered to pay for transportation. Other docs in NY have offered to help me with supplies (and I am paying it forward). Grateful to you all!”
In another post, Dr. Salles joked that her “small head” makes it difficult to wear PPE.
“Wearing an N95 for hours really sucks,” she wrote. “It rides up, I pull it down. It digs into my cheeks, I pull it up. Repeat.”
The volunteer experience thus far has also made Dr. Salles question the future and worry about the mental health of her fellow health care professionals.
“The people who have been in NYC since the beginning of this, and those who work in Lombardy, Italy, and in Wuhan, China have faced loss for weeks to months,” she wrote in an April 18 Twitter post. “Not only do we not know when this will end, but it is likely that after it fades, it will come back in a second wave. I am lucky. I’m just a visitor here. I have the privilege to observe and learn and hopefully help, knowing I will be able to walk away. But what about those who can’t walk away? Social distancing is starting to work. But for healthcare workers, the ongoing devastation is very real. What is our long term plan?”
Dr. Salles expressed concern for health care workers who are witnessing “horrible things” with little time to process the experiences.
“It may be especially hard for those who are now working in specialties they are not used to, having to provide care they are not familiar with. They are all doing their best, but inevitably mistakes will be made, and they will likely blame themselves,” she wrote. “How do we best support them?”
Stay tuned for upcoming commentaries from Dr. Salles on her COVID-19 volunteer experience in New York City.
Sleep quality may affect COPD risk in African American smokers
African American smokers who logged more total sleep time and greater sleep efficacy performed better on a functional walk test than did those with poorer sleep, based on data from 209 adults.
African American smokers tend to develop COPD sooner and also report more sleep problems, compared with white smokers, wrote Andrew J. Gangemi, MD, of Temple University Hospital, Philadelphia, and colleagues.
In addition, African Americans tend to develop COPD at a younger age and with lower levels of smoking than do non-Hispanic whites, they said. “Sleep health may be a contributing factor to the lung and cardiovascular health disparity experienced by AA smokers,” in part because data suggest that insufficient sleep may be associated with increased risk of COPD exacerbation in smokers in general, they said.
In a study published in Chest, the researchers reviewed data from 209 African American adults aged 40-65 years who had smoked at least one cigarette in the past month. The average age of the participants was 55 years, 59% were women, and the average smoking habit was nine cigarettes per day.
The researchers measured functional exercise capacity of the participants using the 6-minute walk test (6MWT). Total sleep time (TST) and sleep efficacy (SE) were measured by way of a finger-based device.
Smokers of at least 10 cigarettes per day gained an additional 0.05-0.58 meters in distance covered on the 6MWT for every added minute of total sleep time in a multivariable regression analysis. Similarly, smokers of at least 10 cigarettes per day gained an additional 0.84-6.17–meter increase in distance covered on the 6MWT for every added percentage of sleep efficacy.
The reasons for the impact of SE and TST on functional exercise capacity in smokers remain unclear, the researchers said. “Heavier smokers have higher levels of autonomic imbalance, including higher resting heart rate and heart rate variability, impaired 24-hour cardiovascular sympathetic tone, and blunted cerebrovascular autonomic regulation and baroreflex response to hypercapnia,” they said.
Also unclear is the reason for the large magnitude of the association between SE and smoking vs. the lesser association between TST and smoking on 6MWT results, the researchers wrote. “Poor sleep efficiency, outside of traditional OSA scoring, is predictive of myocardial infarction, stroke, and cardiovascular-related mortality risk. Moreover, deficits in sleep efficiency have been consistently demonstrated in smokers versus nonsmokers,” they said.
The study findings were limited by several factors including inability to extrapolate data to other demographic groups and the cross-sectional design, the researchers noted. In addition, they did not address how TST and SE may relate to lung function.
However, the results “extend current knowledge about the potential role of improved sleep health to functional exercise capacity in AA smokers,” and set the stage for future studies of how changes in sleep health may affect lung and functional exercise capacity in smokers over time, as well as effects on inflammation and autonomic imbalance, the researchers concluded.
The study was supported by the National Institute on Minority Health and Health Disparities and by the National Institute of General Medical Sciences, both part of the National Institutes Health. The researchers had no financial conflicts to disclose.
SOURCE: Gangemi A et al. Chest 2020 Apr 23. doi: 10.1016/j.chest.2020.03.070.
African American smokers who logged more total sleep time and greater sleep efficacy performed better on a functional walk test than did those with poorer sleep, based on data from 209 adults.
African American smokers tend to develop COPD sooner and also report more sleep problems, compared with white smokers, wrote Andrew J. Gangemi, MD, of Temple University Hospital, Philadelphia, and colleagues.
In addition, African Americans tend to develop COPD at a younger age and with lower levels of smoking than do non-Hispanic whites, they said. “Sleep health may be a contributing factor to the lung and cardiovascular health disparity experienced by AA smokers,” in part because data suggest that insufficient sleep may be associated with increased risk of COPD exacerbation in smokers in general, they said.
In a study published in Chest, the researchers reviewed data from 209 African American adults aged 40-65 years who had smoked at least one cigarette in the past month. The average age of the participants was 55 years, 59% were women, and the average smoking habit was nine cigarettes per day.
The researchers measured functional exercise capacity of the participants using the 6-minute walk test (6MWT). Total sleep time (TST) and sleep efficacy (SE) were measured by way of a finger-based device.
Smokers of at least 10 cigarettes per day gained an additional 0.05-0.58 meters in distance covered on the 6MWT for every added minute of total sleep time in a multivariable regression analysis. Similarly, smokers of at least 10 cigarettes per day gained an additional 0.84-6.17–meter increase in distance covered on the 6MWT for every added percentage of sleep efficacy.
The reasons for the impact of SE and TST on functional exercise capacity in smokers remain unclear, the researchers said. “Heavier smokers have higher levels of autonomic imbalance, including higher resting heart rate and heart rate variability, impaired 24-hour cardiovascular sympathetic tone, and blunted cerebrovascular autonomic regulation and baroreflex response to hypercapnia,” they said.
Also unclear is the reason for the large magnitude of the association between SE and smoking vs. the lesser association between TST and smoking on 6MWT results, the researchers wrote. “Poor sleep efficiency, outside of traditional OSA scoring, is predictive of myocardial infarction, stroke, and cardiovascular-related mortality risk. Moreover, deficits in sleep efficiency have been consistently demonstrated in smokers versus nonsmokers,” they said.
The study findings were limited by several factors including inability to extrapolate data to other demographic groups and the cross-sectional design, the researchers noted. In addition, they did not address how TST and SE may relate to lung function.
However, the results “extend current knowledge about the potential role of improved sleep health to functional exercise capacity in AA smokers,” and set the stage for future studies of how changes in sleep health may affect lung and functional exercise capacity in smokers over time, as well as effects on inflammation and autonomic imbalance, the researchers concluded.
The study was supported by the National Institute on Minority Health and Health Disparities and by the National Institute of General Medical Sciences, both part of the National Institutes Health. The researchers had no financial conflicts to disclose.
SOURCE: Gangemi A et al. Chest 2020 Apr 23. doi: 10.1016/j.chest.2020.03.070.
African American smokers who logged more total sleep time and greater sleep efficacy performed better on a functional walk test than did those with poorer sleep, based on data from 209 adults.
African American smokers tend to develop COPD sooner and also report more sleep problems, compared with white smokers, wrote Andrew J. Gangemi, MD, of Temple University Hospital, Philadelphia, and colleagues.
In addition, African Americans tend to develop COPD at a younger age and with lower levels of smoking than do non-Hispanic whites, they said. “Sleep health may be a contributing factor to the lung and cardiovascular health disparity experienced by AA smokers,” in part because data suggest that insufficient sleep may be associated with increased risk of COPD exacerbation in smokers in general, they said.
In a study published in Chest, the researchers reviewed data from 209 African American adults aged 40-65 years who had smoked at least one cigarette in the past month. The average age of the participants was 55 years, 59% were women, and the average smoking habit was nine cigarettes per day.
The researchers measured functional exercise capacity of the participants using the 6-minute walk test (6MWT). Total sleep time (TST) and sleep efficacy (SE) were measured by way of a finger-based device.
Smokers of at least 10 cigarettes per day gained an additional 0.05-0.58 meters in distance covered on the 6MWT for every added minute of total sleep time in a multivariable regression analysis. Similarly, smokers of at least 10 cigarettes per day gained an additional 0.84-6.17–meter increase in distance covered on the 6MWT for every added percentage of sleep efficacy.
The reasons for the impact of SE and TST on functional exercise capacity in smokers remain unclear, the researchers said. “Heavier smokers have higher levels of autonomic imbalance, including higher resting heart rate and heart rate variability, impaired 24-hour cardiovascular sympathetic tone, and blunted cerebrovascular autonomic regulation and baroreflex response to hypercapnia,” they said.
Also unclear is the reason for the large magnitude of the association between SE and smoking vs. the lesser association between TST and smoking on 6MWT results, the researchers wrote. “Poor sleep efficiency, outside of traditional OSA scoring, is predictive of myocardial infarction, stroke, and cardiovascular-related mortality risk. Moreover, deficits in sleep efficiency have been consistently demonstrated in smokers versus nonsmokers,” they said.
The study findings were limited by several factors including inability to extrapolate data to other demographic groups and the cross-sectional design, the researchers noted. In addition, they did not address how TST and SE may relate to lung function.
However, the results “extend current knowledge about the potential role of improved sleep health to functional exercise capacity in AA smokers,” and set the stage for future studies of how changes in sleep health may affect lung and functional exercise capacity in smokers over time, as well as effects on inflammation and autonomic imbalance, the researchers concluded.
The study was supported by the National Institute on Minority Health and Health Disparities and by the National Institute of General Medical Sciences, both part of the National Institutes Health. The researchers had no financial conflicts to disclose.
SOURCE: Gangemi A et al. Chest 2020 Apr 23. doi: 10.1016/j.chest.2020.03.070.
FROM CHEST
Hospitals update hydroxychloroquine protocols after FDA warning
Across the country, hospitals are incorporating Friday’s warning from the US Food and Drug Administration (FDA) about the risks of prescribing hydroxychloroquine and chloroquine for COVID-19 into their treatment protocols.
For some hospitals, the message affirmed the cautious approach they were already taking with hydroxychloroquine. “From a New York state or Northwell perspective, there is no major change,” said Onisis Stefas, PharmD, vice president of pharmacy at Northwell Health in New York. “We were not prescribing it out in the community very early on because of the concerns associated with the heart arrhythmias.”
Brigham and Women’s Hospital in Boston, Massachusetts, is currently in the process of updating its publicly available COVID-19 protocols website “to incorporate the FDA’s updated safety assessment and ongoing clinical trials,” a hospital spokesperson told Medscape Medical News. Prior to the updates, the treatment protocols indicated that hydroxychloroquine should only be considered after weighing the risks and benefits for patients who are not candidates for other clinical trials and meet a specific set of health criteria.
The warning is a timely and important synthesis of what physicians know about the drugs so far and how cautiously clinicians across the country should be using them, said Rajesh T. Gandhi, MD, infectious diseases physician at Massachusetts General Hospital (MGH), Boston, professor of medicine at Harvard Medical School, member of the Infectious Diseases Society of America (IDSA), and chair-elect of the HIV Medicine Association.
“I think to be honest it’s a really important message to the public and clinicians across the country,” said Gandhi. “Because we all know there is just a ton of discussion around this drug ... and it came out fairly and said what we know right now.”
The two antimalarial drugs have been at the center of much political debate and scientific scrutiny in recent weeks, following President Trump’s endorsement and the FDA’s emergency use authorization for the two medications in March. Hospitals across the country had incorporated hydroxychloroquine and chloroquine into their constantly evolving treatment protocols for patients with COVID-19.
But the evidence that these drugs actually help treat COVID-19 remains scant. Some small studies suggest the therapies help patients with COVID-19, while others conclude the drugs have no effect or even harm patients. In the United States, medical societies including the American Heart Association have also warned about the serious cardiac issues that can accompany these drugs for some patients.
In the new warning, the FDA said it “cautions against use of hydroxychloroquine or chloroquine for COVID-19 patients outside of the hospital setting or a clinical trial” and urged “close supervision” of patients treated with these therapies, citing cardiac side effects.
The FDA also said it is aware that the outpatient prescription of these medications has increased since its March authorization, but the drugs still have not been shown to be safe or effective in treating or preventing COVID-19.
The FDA announcement is consistent with protocols established by the National Institutes of Health and IDSA earlier this month that recommend against using hydroxychloroquine or chloroquine, except when these drugs are administered as part of a clinical trial.
“We agree wholeheartedly with the FDA and have been hoping that the FDA would in fact issue that kind of clarification,” said Samuel Brown, MD, study committee cochair of the ORCHID clinical trial, a multicenter, blinded study investigating the safety and efficacy of hydroxychloroquine. These medications need to be tested in clinical trials that are able to focus closely on safety monitoring, he said. Experts at Vanderbilt University, one of the medical centers participating in the ORCHID clinical trial, decided before Tennessee had any cases of COVID-19 that unproven therapies like hydroxychloroquine would only be available through clinical trials, said Wesley Self, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tennessee, and chair of the ORCHID study committee.
Northwell Health, like other hospitals in New York, has been following a March executive order issued by Governor Andrew Cuomo limiting the use of these drugs for COVID-19 outside of clinical trials, said Stefas. At Northwell Health, patients with COVID-19 only receive hydroxychloroquine or chloroquine when treated in hospital, where they can be closely monitored, or as part of a clinical trial. The hospital system’s protocols currently do not recommend pairing hydroxychloroquine with azithromycin, said Stefas. The new FDA announcement is “very similar” to New York’s existing executive order, he said. “Reading through this reinforces a lot of what we originally thought.”
At MGH in Boston, the FDA safety warning is in line with and “solidifies” the hospital’s evolving protocols, said Gandhi. Clinicians at MGH have been steadily moving away from prescribing hydroxychloroquine outside of clinical trials as the efficacy has remained murky, the serious side effects have become more evident, and clinical trials to assess the drug have gotten underway in recent weeks, he said. Given the conflicting evidence, Gandhi feels the use of these drugs needs to be focused in clinical trials, where scientists can truly evaluate how much they help or harm.
“We know fundamentally that’s the way to do this,” Gandhi said. “We also don’t know that it doesn’t work, so it is ethical and incumbent upon us to do a study,” Gandhi said.
Other hospitals are already heeding the FDA’s warning. At UW Medicine in Washington state, for example, hydroxychloroquine was considered a possible treatment for COVID-19 prior to the FDA’s recent announcement. “Based on FDA guidance, hydroxychloroquine is no longer recommended as therapy for COVID-19 unless done through a clinical trial,” said Tim Dellit, MD, chief medical officer for UW Medicine.
Michigan Medicine stopped using hydroxychloroquine and azithromycin (both separately and in combination) about a month ago, said Daniel Kaul, MD, a professor of infectious disease at the University of Michigan. “When we reviewed the data that was available in more detail, we realized that it was essentially uninterpretable,” he said. As of Monday, the only patients receiving this drug at Michigan Medicine are those enrolled in the ORCHID clinical trial.
But that does not seem to be the case everywhere. Most patients transferred to Michigan Medicine from other hospitals have received these drugs, indicating they are still being widely used, said Kaul. “I think this FDA guidance is appropriate and may reduce usage of this drug and make people more aware of the potential side effects both in inpatient and outpatient settings.”
Hopefully, the FDA guidance will help slow the use of these drugs outside the appropriate clinical trial setting, said Kaul. “I think that the kind of politicization of this drug, which is pretty much unprecedented in my experience, created a really harmful environment where calm decision making and assessment of the relative risks and benefits became somewhat impossible,” said Kaul.
This article first appeared on Medscape.com.
Across the country, hospitals are incorporating Friday’s warning from the US Food and Drug Administration (FDA) about the risks of prescribing hydroxychloroquine and chloroquine for COVID-19 into their treatment protocols.
For some hospitals, the message affirmed the cautious approach they were already taking with hydroxychloroquine. “From a New York state or Northwell perspective, there is no major change,” said Onisis Stefas, PharmD, vice president of pharmacy at Northwell Health in New York. “We were not prescribing it out in the community very early on because of the concerns associated with the heart arrhythmias.”
Brigham and Women’s Hospital in Boston, Massachusetts, is currently in the process of updating its publicly available COVID-19 protocols website “to incorporate the FDA’s updated safety assessment and ongoing clinical trials,” a hospital spokesperson told Medscape Medical News. Prior to the updates, the treatment protocols indicated that hydroxychloroquine should only be considered after weighing the risks and benefits for patients who are not candidates for other clinical trials and meet a specific set of health criteria.
The warning is a timely and important synthesis of what physicians know about the drugs so far and how cautiously clinicians across the country should be using them, said Rajesh T. Gandhi, MD, infectious diseases physician at Massachusetts General Hospital (MGH), Boston, professor of medicine at Harvard Medical School, member of the Infectious Diseases Society of America (IDSA), and chair-elect of the HIV Medicine Association.
“I think to be honest it’s a really important message to the public and clinicians across the country,” said Gandhi. “Because we all know there is just a ton of discussion around this drug ... and it came out fairly and said what we know right now.”
The two antimalarial drugs have been at the center of much political debate and scientific scrutiny in recent weeks, following President Trump’s endorsement and the FDA’s emergency use authorization for the two medications in March. Hospitals across the country had incorporated hydroxychloroquine and chloroquine into their constantly evolving treatment protocols for patients with COVID-19.
But the evidence that these drugs actually help treat COVID-19 remains scant. Some small studies suggest the therapies help patients with COVID-19, while others conclude the drugs have no effect or even harm patients. In the United States, medical societies including the American Heart Association have also warned about the serious cardiac issues that can accompany these drugs for some patients.
In the new warning, the FDA said it “cautions against use of hydroxychloroquine or chloroquine for COVID-19 patients outside of the hospital setting or a clinical trial” and urged “close supervision” of patients treated with these therapies, citing cardiac side effects.
The FDA also said it is aware that the outpatient prescription of these medications has increased since its March authorization, but the drugs still have not been shown to be safe or effective in treating or preventing COVID-19.
The FDA announcement is consistent with protocols established by the National Institutes of Health and IDSA earlier this month that recommend against using hydroxychloroquine or chloroquine, except when these drugs are administered as part of a clinical trial.
“We agree wholeheartedly with the FDA and have been hoping that the FDA would in fact issue that kind of clarification,” said Samuel Brown, MD, study committee cochair of the ORCHID clinical trial, a multicenter, blinded study investigating the safety and efficacy of hydroxychloroquine. These medications need to be tested in clinical trials that are able to focus closely on safety monitoring, he said. Experts at Vanderbilt University, one of the medical centers participating in the ORCHID clinical trial, decided before Tennessee had any cases of COVID-19 that unproven therapies like hydroxychloroquine would only be available through clinical trials, said Wesley Self, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tennessee, and chair of the ORCHID study committee.
Northwell Health, like other hospitals in New York, has been following a March executive order issued by Governor Andrew Cuomo limiting the use of these drugs for COVID-19 outside of clinical trials, said Stefas. At Northwell Health, patients with COVID-19 only receive hydroxychloroquine or chloroquine when treated in hospital, where they can be closely monitored, or as part of a clinical trial. The hospital system’s protocols currently do not recommend pairing hydroxychloroquine with azithromycin, said Stefas. The new FDA announcement is “very similar” to New York’s existing executive order, he said. “Reading through this reinforces a lot of what we originally thought.”
At MGH in Boston, the FDA safety warning is in line with and “solidifies” the hospital’s evolving protocols, said Gandhi. Clinicians at MGH have been steadily moving away from prescribing hydroxychloroquine outside of clinical trials as the efficacy has remained murky, the serious side effects have become more evident, and clinical trials to assess the drug have gotten underway in recent weeks, he said. Given the conflicting evidence, Gandhi feels the use of these drugs needs to be focused in clinical trials, where scientists can truly evaluate how much they help or harm.
“We know fundamentally that’s the way to do this,” Gandhi said. “We also don’t know that it doesn’t work, so it is ethical and incumbent upon us to do a study,” Gandhi said.
Other hospitals are already heeding the FDA’s warning. At UW Medicine in Washington state, for example, hydroxychloroquine was considered a possible treatment for COVID-19 prior to the FDA’s recent announcement. “Based on FDA guidance, hydroxychloroquine is no longer recommended as therapy for COVID-19 unless done through a clinical trial,” said Tim Dellit, MD, chief medical officer for UW Medicine.
Michigan Medicine stopped using hydroxychloroquine and azithromycin (both separately and in combination) about a month ago, said Daniel Kaul, MD, a professor of infectious disease at the University of Michigan. “When we reviewed the data that was available in more detail, we realized that it was essentially uninterpretable,” he said. As of Monday, the only patients receiving this drug at Michigan Medicine are those enrolled in the ORCHID clinical trial.
But that does not seem to be the case everywhere. Most patients transferred to Michigan Medicine from other hospitals have received these drugs, indicating they are still being widely used, said Kaul. “I think this FDA guidance is appropriate and may reduce usage of this drug and make people more aware of the potential side effects both in inpatient and outpatient settings.”
Hopefully, the FDA guidance will help slow the use of these drugs outside the appropriate clinical trial setting, said Kaul. “I think that the kind of politicization of this drug, which is pretty much unprecedented in my experience, created a really harmful environment where calm decision making and assessment of the relative risks and benefits became somewhat impossible,” said Kaul.
This article first appeared on Medscape.com.
Across the country, hospitals are incorporating Friday’s warning from the US Food and Drug Administration (FDA) about the risks of prescribing hydroxychloroquine and chloroquine for COVID-19 into their treatment protocols.
For some hospitals, the message affirmed the cautious approach they were already taking with hydroxychloroquine. “From a New York state or Northwell perspective, there is no major change,” said Onisis Stefas, PharmD, vice president of pharmacy at Northwell Health in New York. “We were not prescribing it out in the community very early on because of the concerns associated with the heart arrhythmias.”
Brigham and Women’s Hospital in Boston, Massachusetts, is currently in the process of updating its publicly available COVID-19 protocols website “to incorporate the FDA’s updated safety assessment and ongoing clinical trials,” a hospital spokesperson told Medscape Medical News. Prior to the updates, the treatment protocols indicated that hydroxychloroquine should only be considered after weighing the risks and benefits for patients who are not candidates for other clinical trials and meet a specific set of health criteria.
The warning is a timely and important synthesis of what physicians know about the drugs so far and how cautiously clinicians across the country should be using them, said Rajesh T. Gandhi, MD, infectious diseases physician at Massachusetts General Hospital (MGH), Boston, professor of medicine at Harvard Medical School, member of the Infectious Diseases Society of America (IDSA), and chair-elect of the HIV Medicine Association.
“I think to be honest it’s a really important message to the public and clinicians across the country,” said Gandhi. “Because we all know there is just a ton of discussion around this drug ... and it came out fairly and said what we know right now.”
The two antimalarial drugs have been at the center of much political debate and scientific scrutiny in recent weeks, following President Trump’s endorsement and the FDA’s emergency use authorization for the two medications in March. Hospitals across the country had incorporated hydroxychloroquine and chloroquine into their constantly evolving treatment protocols for patients with COVID-19.
But the evidence that these drugs actually help treat COVID-19 remains scant. Some small studies suggest the therapies help patients with COVID-19, while others conclude the drugs have no effect or even harm patients. In the United States, medical societies including the American Heart Association have also warned about the serious cardiac issues that can accompany these drugs for some patients.
In the new warning, the FDA said it “cautions against use of hydroxychloroquine or chloroquine for COVID-19 patients outside of the hospital setting or a clinical trial” and urged “close supervision” of patients treated with these therapies, citing cardiac side effects.
The FDA also said it is aware that the outpatient prescription of these medications has increased since its March authorization, but the drugs still have not been shown to be safe or effective in treating or preventing COVID-19.
The FDA announcement is consistent with protocols established by the National Institutes of Health and IDSA earlier this month that recommend against using hydroxychloroquine or chloroquine, except when these drugs are administered as part of a clinical trial.
“We agree wholeheartedly with the FDA and have been hoping that the FDA would in fact issue that kind of clarification,” said Samuel Brown, MD, study committee cochair of the ORCHID clinical trial, a multicenter, blinded study investigating the safety and efficacy of hydroxychloroquine. These medications need to be tested in clinical trials that are able to focus closely on safety monitoring, he said. Experts at Vanderbilt University, one of the medical centers participating in the ORCHID clinical trial, decided before Tennessee had any cases of COVID-19 that unproven therapies like hydroxychloroquine would only be available through clinical trials, said Wesley Self, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tennessee, and chair of the ORCHID study committee.
Northwell Health, like other hospitals in New York, has been following a March executive order issued by Governor Andrew Cuomo limiting the use of these drugs for COVID-19 outside of clinical trials, said Stefas. At Northwell Health, patients with COVID-19 only receive hydroxychloroquine or chloroquine when treated in hospital, where they can be closely monitored, or as part of a clinical trial. The hospital system’s protocols currently do not recommend pairing hydroxychloroquine with azithromycin, said Stefas. The new FDA announcement is “very similar” to New York’s existing executive order, he said. “Reading through this reinforces a lot of what we originally thought.”
At MGH in Boston, the FDA safety warning is in line with and “solidifies” the hospital’s evolving protocols, said Gandhi. Clinicians at MGH have been steadily moving away from prescribing hydroxychloroquine outside of clinical trials as the efficacy has remained murky, the serious side effects have become more evident, and clinical trials to assess the drug have gotten underway in recent weeks, he said. Given the conflicting evidence, Gandhi feels the use of these drugs needs to be focused in clinical trials, where scientists can truly evaluate how much they help or harm.
“We know fundamentally that’s the way to do this,” Gandhi said. “We also don’t know that it doesn’t work, so it is ethical and incumbent upon us to do a study,” Gandhi said.
Other hospitals are already heeding the FDA’s warning. At UW Medicine in Washington state, for example, hydroxychloroquine was considered a possible treatment for COVID-19 prior to the FDA’s recent announcement. “Based on FDA guidance, hydroxychloroquine is no longer recommended as therapy for COVID-19 unless done through a clinical trial,” said Tim Dellit, MD, chief medical officer for UW Medicine.
Michigan Medicine stopped using hydroxychloroquine and azithromycin (both separately and in combination) about a month ago, said Daniel Kaul, MD, a professor of infectious disease at the University of Michigan. “When we reviewed the data that was available in more detail, we realized that it was essentially uninterpretable,” he said. As of Monday, the only patients receiving this drug at Michigan Medicine are those enrolled in the ORCHID clinical trial.
But that does not seem to be the case everywhere. Most patients transferred to Michigan Medicine from other hospitals have received these drugs, indicating they are still being widely used, said Kaul. “I think this FDA guidance is appropriate and may reduce usage of this drug and make people more aware of the potential side effects both in inpatient and outpatient settings.”
Hopefully, the FDA guidance will help slow the use of these drugs outside the appropriate clinical trial setting, said Kaul. “I think that the kind of politicization of this drug, which is pretty much unprecedented in my experience, created a really harmful environment where calm decision making and assessment of the relative risks and benefits became somewhat impossible,” said Kaul.
This article first appeared on Medscape.com.
Consensus recommendations on AMI management during COVID-19
A consensus statement from the American College of Cardiology (ACC), the American College of Emergency Physicians (ACEP), and the Society for Cardiovascular Angiography & Interventions (SCAI) outlines recommendations for a systematic approach for the care of patients with an acute myocardial infarction (AMI) during the COVID-19 pandemic.
The statement was published in the Journal of the American College of Cardiology.
During the COVID-19 pandemic, percutaneous coronary intervention (PCI) remains the standard of care for patients with ST-segment elevation MI (STEMI) at PCI-capable hospitals when it can be provided in a timely fashion in a dedicated cardiac catheterization laboratory with an expert care team wearing personal protection equipment (PPE), the writing group advised.
“A fibrinolysis-based strategy may be entertained at non-PCI capable referral hospitals or in specific situations where primary PCI cannot be executed or is not deemed the best option,” they said.
SCAI President Ehtisham Mahmud, MD, of the University of California, San Diego, and the writing group also said that clinicians should recognize that cardiovascular manifestations of COVID-19 are “complex” in patients presenting with AMI, myocarditis simulating a STEMI, stress cardiomyopathy, nonischemic cardiomyopathy, coronary spasm, or nonspecific myocardial injury.
A “broad differential diagnosis for ST elevations (including COVID-associated myocarditis) should be considered in the ED prior to choosing a reperfusion strategy,” they advised.
In the absence of hemodynamic instability or ongoing ischemic symptoms, non-STEMI patients with known or suspected COVID-19 are best managed with an initial medical stabilization strategy, the group said.
They also said it is “imperative that health care workers use appropriate PPE for all invasive procedures during this pandemic” and that new rapid COVID-19 testing be “expeditiously” disseminated to all hospitals that manage patients with AMI.
Major challenges are that the prevalence of the COVID-19 in the United States remains unknown and there is the risk for asymptomatic spread.
The writing group said it’s “critical” to “inform the public that we can minimize exposure to the coronavirus so they can continue to call the Emergency Medical System (EMS) for acute ischemic heart disease symptoms and therefore get the appropriate level of cardiac care that their presentation warrants.”
This research had no commercial funding. Dr. Mahmud reported receiving clinical trial research support from Corindus, Abbott Vascular, and CSI; consulting with Medtronic; and consulting and equity with Abiomed. A complete list of author disclosures is included with the original article.
A version of this article originally appeared on Medscape.com.
A consensus statement from the American College of Cardiology (ACC), the American College of Emergency Physicians (ACEP), and the Society for Cardiovascular Angiography & Interventions (SCAI) outlines recommendations for a systematic approach for the care of patients with an acute myocardial infarction (AMI) during the COVID-19 pandemic.
The statement was published in the Journal of the American College of Cardiology.
During the COVID-19 pandemic, percutaneous coronary intervention (PCI) remains the standard of care for patients with ST-segment elevation MI (STEMI) at PCI-capable hospitals when it can be provided in a timely fashion in a dedicated cardiac catheterization laboratory with an expert care team wearing personal protection equipment (PPE), the writing group advised.
“A fibrinolysis-based strategy may be entertained at non-PCI capable referral hospitals or in specific situations where primary PCI cannot be executed or is not deemed the best option,” they said.
SCAI President Ehtisham Mahmud, MD, of the University of California, San Diego, and the writing group also said that clinicians should recognize that cardiovascular manifestations of COVID-19 are “complex” in patients presenting with AMI, myocarditis simulating a STEMI, stress cardiomyopathy, nonischemic cardiomyopathy, coronary spasm, or nonspecific myocardial injury.
A “broad differential diagnosis for ST elevations (including COVID-associated myocarditis) should be considered in the ED prior to choosing a reperfusion strategy,” they advised.
In the absence of hemodynamic instability or ongoing ischemic symptoms, non-STEMI patients with known or suspected COVID-19 are best managed with an initial medical stabilization strategy, the group said.
They also said it is “imperative that health care workers use appropriate PPE for all invasive procedures during this pandemic” and that new rapid COVID-19 testing be “expeditiously” disseminated to all hospitals that manage patients with AMI.
Major challenges are that the prevalence of the COVID-19 in the United States remains unknown and there is the risk for asymptomatic spread.
The writing group said it’s “critical” to “inform the public that we can minimize exposure to the coronavirus so they can continue to call the Emergency Medical System (EMS) for acute ischemic heart disease symptoms and therefore get the appropriate level of cardiac care that their presentation warrants.”
This research had no commercial funding. Dr. Mahmud reported receiving clinical trial research support from Corindus, Abbott Vascular, and CSI; consulting with Medtronic; and consulting and equity with Abiomed. A complete list of author disclosures is included with the original article.
A version of this article originally appeared on Medscape.com.
A consensus statement from the American College of Cardiology (ACC), the American College of Emergency Physicians (ACEP), and the Society for Cardiovascular Angiography & Interventions (SCAI) outlines recommendations for a systematic approach for the care of patients with an acute myocardial infarction (AMI) during the COVID-19 pandemic.
The statement was published in the Journal of the American College of Cardiology.
During the COVID-19 pandemic, percutaneous coronary intervention (PCI) remains the standard of care for patients with ST-segment elevation MI (STEMI) at PCI-capable hospitals when it can be provided in a timely fashion in a dedicated cardiac catheterization laboratory with an expert care team wearing personal protection equipment (PPE), the writing group advised.
“A fibrinolysis-based strategy may be entertained at non-PCI capable referral hospitals or in specific situations where primary PCI cannot be executed or is not deemed the best option,” they said.
SCAI President Ehtisham Mahmud, MD, of the University of California, San Diego, and the writing group also said that clinicians should recognize that cardiovascular manifestations of COVID-19 are “complex” in patients presenting with AMI, myocarditis simulating a STEMI, stress cardiomyopathy, nonischemic cardiomyopathy, coronary spasm, or nonspecific myocardial injury.
A “broad differential diagnosis for ST elevations (including COVID-associated myocarditis) should be considered in the ED prior to choosing a reperfusion strategy,” they advised.
In the absence of hemodynamic instability or ongoing ischemic symptoms, non-STEMI patients with known or suspected COVID-19 are best managed with an initial medical stabilization strategy, the group said.
They also said it is “imperative that health care workers use appropriate PPE for all invasive procedures during this pandemic” and that new rapid COVID-19 testing be “expeditiously” disseminated to all hospitals that manage patients with AMI.
Major challenges are that the prevalence of the COVID-19 in the United States remains unknown and there is the risk for asymptomatic spread.
The writing group said it’s “critical” to “inform the public that we can minimize exposure to the coronavirus so they can continue to call the Emergency Medical System (EMS) for acute ischemic heart disease symptoms and therefore get the appropriate level of cardiac care that their presentation warrants.”
This research had no commercial funding. Dr. Mahmud reported receiving clinical trial research support from Corindus, Abbott Vascular, and CSI; consulting with Medtronic; and consulting and equity with Abiomed. A complete list of author disclosures is included with the original article.
A version of this article originally appeared on Medscape.com.
FDA grants Breakthrough Therapy status to sotatercept for PAH treatment
Approval for sotatercept, “a selective ligand trap for members of the TGF-beta [transforming growth factor-beta] superfamily which rebalances BMPR-II [bone morphogenetic protein receptor type II] signaling,” was based on two types of research. It was based on results of preclinical research indicating “reversed pulmonary vessel muscularization and improved indicators of right heart failure,” as well as results of the phase 2, placebo-controlled PULSAR study, in which sotatercept showed positive results, meeting primary and secondary endpoints.
Adverse events during PULSAR “were consistent with previously published data on sotatercept” in other diseases. The drug is also under investigation in the phase 2 SPECTRA trial, which includes patients with PAH.
“We believe that sotatercept has the potential to shift the current treatment paradigm and provide significant benefit to patients with PAH on top of currently available therapies. Thus, we’re thrilled that the FDA has granted this Breakthrough Therapy designation – a first for an Acceleron-discovered medicine and for a therapeutic candidate in PAH – as it supports and aligns with our mission to deliver novel therapeutic options to patients in need as quickly as possible,” Habib Dable, president and CEO of Acceleron Pharma, said in the press release.
Approval for sotatercept, “a selective ligand trap for members of the TGF-beta [transforming growth factor-beta] superfamily which rebalances BMPR-II [bone morphogenetic protein receptor type II] signaling,” was based on two types of research. It was based on results of preclinical research indicating “reversed pulmonary vessel muscularization and improved indicators of right heart failure,” as well as results of the phase 2, placebo-controlled PULSAR study, in which sotatercept showed positive results, meeting primary and secondary endpoints.
Adverse events during PULSAR “were consistent with previously published data on sotatercept” in other diseases. The drug is also under investigation in the phase 2 SPECTRA trial, which includes patients with PAH.
“We believe that sotatercept has the potential to shift the current treatment paradigm and provide significant benefit to patients with PAH on top of currently available therapies. Thus, we’re thrilled that the FDA has granted this Breakthrough Therapy designation – a first for an Acceleron-discovered medicine and for a therapeutic candidate in PAH – as it supports and aligns with our mission to deliver novel therapeutic options to patients in need as quickly as possible,” Habib Dable, president and CEO of Acceleron Pharma, said in the press release.
Approval for sotatercept, “a selective ligand trap for members of the TGF-beta [transforming growth factor-beta] superfamily which rebalances BMPR-II [bone morphogenetic protein receptor type II] signaling,” was based on two types of research. It was based on results of preclinical research indicating “reversed pulmonary vessel muscularization and improved indicators of right heart failure,” as well as results of the phase 2, placebo-controlled PULSAR study, in which sotatercept showed positive results, meeting primary and secondary endpoints.
Adverse events during PULSAR “were consistent with previously published data on sotatercept” in other diseases. The drug is also under investigation in the phase 2 SPECTRA trial, which includes patients with PAH.
“We believe that sotatercept has the potential to shift the current treatment paradigm and provide significant benefit to patients with PAH on top of currently available therapies. Thus, we’re thrilled that the FDA has granted this Breakthrough Therapy designation – a first for an Acceleron-discovered medicine and for a therapeutic candidate in PAH – as it supports and aligns with our mission to deliver novel therapeutic options to patients in need as quickly as possible,” Habib Dable, president and CEO of Acceleron Pharma, said in the press release.
Seniors with COVID-19 show unusual symptoms, doctors say
complicating efforts to ensure they get timely and appropriate treatment, according to physicians.
COVID-19 is typically signaled by three symptoms: a fever, an insistent cough, and shortness of breath. But older adults – the age group most at risk of severe complications or death from this condition – may have none of these characteristics.
Instead, seniors may seem “off” – not acting like themselves – early on after being infected by the coronavirus. They may sleep more than usual or stop eating. They may seem unusually apathetic or confused, losing orientation to their surroundings. They may become dizzy and fall. Sometimes, seniors stop speaking or simply collapse.
“With a lot of conditions, older adults don’t present in a typical way, and we’re seeing that with COVID-19 as well,” said Camille Vaughan, MD, section chief of geriatrics and gerontology at Emory University, Atlanta.
The reason has to do with how older bodies respond to illness and infection.
At advanced ages, “someone’s immune response may be blunted and their ability to regulate temperature may be altered,” said Dr. Joseph Ouslander, a professor of geriatric medicine at Florida Atlantic University in Boca Raton.
“Underlying chronic illnesses can mask or interfere with signs of infection,” he said. “Some older people, whether from age-related changes or previous neurologic issues such as a stroke, may have altered cough reflexes. Others with cognitive impairment may not be able to communicate their symptoms.”
Recognizing danger signs is important: If early signs of COVID-19 are missed, seniors may deteriorate before getting needed care. And people may go in and out of their homes without adequate protective measures, risking the spread of infection.
Quratulain Syed, MD, an Atlanta geriatrician, describes a man in his 80s whom she treated in mid-March. Over a period of days, this patient, who had heart disease, diabetes and moderate cognitive impairment, stopped walking and became incontinent and profoundly lethargic. But he didn’t have a fever or a cough. His only respiratory symptom: sneezing off and on.
The man’s elderly spouse called 911 twice. Both times, paramedics checked his vital signs and declared he was OK. After another worried call from the overwhelmed spouse, Dr. Syed insisted the patient be taken to the hospital, where he tested positive for COVID-19.
“I was quite concerned about the paramedics and health aides who’d been in the house and who hadn’t used PPE [personal protective equipment],” Dr. Syed said.
Dr. Sam Torbati, medical director of the emergency department at Cedars-Sinai Medical Center, Los Angeles, describes treating seniors who initially appear to be trauma patients but are found to have COVID-19.
“They get weak and dehydrated,” he said, “and when they stand to walk, they collapse and injure themselves badly.”
Dr. Torbati has seen older adults who are profoundly disoriented and unable to speak and who appear at first to have suffered strokes.
“When we test them, we discover that what’s producing these changes is a central nervous system effect of coronavirus,” he said.
Laura Perry, MD, of the University of California, San Francisco, saw a patient like this several weeks ago. The woman, in her 80s, had what seemed to be a cold before becoming very confused. In the hospital, she couldn’t identify where she was or stay awake during an examination. Dr. Perry diagnosed hypoactive delirium, an altered mental state in which people become inactive and drowsy. The patient tested positive for coronavirus and is still in the ICU.
Anthony Perry, MD, of the department of geriatric medicine at Rush University Medical Center in Chicago, tells of an 81-year-old woman with nausea, vomiting, and diarrhea who tested positive for COVID-19 in the emergency room. After receiving intravenous fluids, oxygen, and medication for her intestinal upset, she returned home after 2 days and is doing well.
Another 80-year-old Rush patient with similar symptoms – nausea and vomiting, but no cough, fever, or shortness of breath – is in intensive care after getting a positive COVID-19 test and due to be put on a ventilator. The difference? This patient is frail with “a lot of cardiovascular disease,” Dr. Perry said. Other than that, it’s not yet clear why some older patients do well while others do not.
So far, reports of cases like these have been anecdotal. But a few physicians are trying to gather more systematic information.
In Switzerland, Sylvain Nguyen, MD, a geriatrician at the University of Lausanne Hospital Center, put together a list of typical and atypical symptoms in older COVID-19 patients for a paper to be published in the Revue Médicale Suisse. Included on the atypical list are changes in a patient’s usual status, delirium, falls, fatigue, lethargy, low blood pressure, painful swallowing, fainting, diarrhea, nausea, vomiting, abdominal pain, and the loss of smell and taste.
Data come from hospitals and nursing homes in Switzerland, Italy, and France, Dr. Nguyen said in an email.
On the front lines, physicians need to make sure they carefully assess an older patient’s symptoms.
“While we have to have a high suspicion of COVID-19 because it’s so dangerous in the older population, there are many other things to consider,” said Kathleen Unroe, MD, a geriatrician at Indiana University, Indianapolis.
Seniors may also do poorly because their routines have changed. In nursing homes and most assisted living centers, activities have stopped and “residents are going to get weaker and more deconditioned because they’re not walking to and from the dining hall,” she said.
At home, isolated seniors may not be getting as much help with medication management or other essential needs from family members who are keeping their distance, other experts suggested. Or they may have become apathetic or depressed.
“I’d want to know ‘What’s the potential this person has had an exposure [to the coronavirus], especially in the last 2 weeks?’ ” said Dr. Vaughan of Emory. “Do they have home health personnel coming in? Have they gotten together with other family members? Are chronic conditions being controlled? Is there another diagnosis that seems more likely?”
“Someone may be just having a bad day. But if they’re not themselves for a couple of days, absolutely reach out to a primary care doctor or a local health system hotline to see if they meet the threshold for [coronavirus] testing,” Dr. Vaughan advised. “Be persistent. If you get a ‘no’ the first time and things aren’t improving, call back and ask again.”
Kaiser Health News (khn.org) is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
complicating efforts to ensure they get timely and appropriate treatment, according to physicians.
COVID-19 is typically signaled by three symptoms: a fever, an insistent cough, and shortness of breath. But older adults – the age group most at risk of severe complications or death from this condition – may have none of these characteristics.
Instead, seniors may seem “off” – not acting like themselves – early on after being infected by the coronavirus. They may sleep more than usual or stop eating. They may seem unusually apathetic or confused, losing orientation to their surroundings. They may become dizzy and fall. Sometimes, seniors stop speaking or simply collapse.
“With a lot of conditions, older adults don’t present in a typical way, and we’re seeing that with COVID-19 as well,” said Camille Vaughan, MD, section chief of geriatrics and gerontology at Emory University, Atlanta.
The reason has to do with how older bodies respond to illness and infection.
At advanced ages, “someone’s immune response may be blunted and their ability to regulate temperature may be altered,” said Dr. Joseph Ouslander, a professor of geriatric medicine at Florida Atlantic University in Boca Raton.
“Underlying chronic illnesses can mask or interfere with signs of infection,” he said. “Some older people, whether from age-related changes or previous neurologic issues such as a stroke, may have altered cough reflexes. Others with cognitive impairment may not be able to communicate their symptoms.”
Recognizing danger signs is important: If early signs of COVID-19 are missed, seniors may deteriorate before getting needed care. And people may go in and out of their homes without adequate protective measures, risking the spread of infection.
Quratulain Syed, MD, an Atlanta geriatrician, describes a man in his 80s whom she treated in mid-March. Over a period of days, this patient, who had heart disease, diabetes and moderate cognitive impairment, stopped walking and became incontinent and profoundly lethargic. But he didn’t have a fever or a cough. His only respiratory symptom: sneezing off and on.
The man’s elderly spouse called 911 twice. Both times, paramedics checked his vital signs and declared he was OK. After another worried call from the overwhelmed spouse, Dr. Syed insisted the patient be taken to the hospital, where he tested positive for COVID-19.
“I was quite concerned about the paramedics and health aides who’d been in the house and who hadn’t used PPE [personal protective equipment],” Dr. Syed said.
Dr. Sam Torbati, medical director of the emergency department at Cedars-Sinai Medical Center, Los Angeles, describes treating seniors who initially appear to be trauma patients but are found to have COVID-19.
“They get weak and dehydrated,” he said, “and when they stand to walk, they collapse and injure themselves badly.”
Dr. Torbati has seen older adults who are profoundly disoriented and unable to speak and who appear at first to have suffered strokes.
“When we test them, we discover that what’s producing these changes is a central nervous system effect of coronavirus,” he said.
Laura Perry, MD, of the University of California, San Francisco, saw a patient like this several weeks ago. The woman, in her 80s, had what seemed to be a cold before becoming very confused. In the hospital, she couldn’t identify where she was or stay awake during an examination. Dr. Perry diagnosed hypoactive delirium, an altered mental state in which people become inactive and drowsy. The patient tested positive for coronavirus and is still in the ICU.
Anthony Perry, MD, of the department of geriatric medicine at Rush University Medical Center in Chicago, tells of an 81-year-old woman with nausea, vomiting, and diarrhea who tested positive for COVID-19 in the emergency room. After receiving intravenous fluids, oxygen, and medication for her intestinal upset, she returned home after 2 days and is doing well.
Another 80-year-old Rush patient with similar symptoms – nausea and vomiting, but no cough, fever, or shortness of breath – is in intensive care after getting a positive COVID-19 test and due to be put on a ventilator. The difference? This patient is frail with “a lot of cardiovascular disease,” Dr. Perry said. Other than that, it’s not yet clear why some older patients do well while others do not.
So far, reports of cases like these have been anecdotal. But a few physicians are trying to gather more systematic information.
In Switzerland, Sylvain Nguyen, MD, a geriatrician at the University of Lausanne Hospital Center, put together a list of typical and atypical symptoms in older COVID-19 patients for a paper to be published in the Revue Médicale Suisse. Included on the atypical list are changes in a patient’s usual status, delirium, falls, fatigue, lethargy, low blood pressure, painful swallowing, fainting, diarrhea, nausea, vomiting, abdominal pain, and the loss of smell and taste.
Data come from hospitals and nursing homes in Switzerland, Italy, and France, Dr. Nguyen said in an email.
On the front lines, physicians need to make sure they carefully assess an older patient’s symptoms.
“While we have to have a high suspicion of COVID-19 because it’s so dangerous in the older population, there are many other things to consider,” said Kathleen Unroe, MD, a geriatrician at Indiana University, Indianapolis.
Seniors may also do poorly because their routines have changed. In nursing homes and most assisted living centers, activities have stopped and “residents are going to get weaker and more deconditioned because they’re not walking to and from the dining hall,” she said.
At home, isolated seniors may not be getting as much help with medication management or other essential needs from family members who are keeping their distance, other experts suggested. Or they may have become apathetic or depressed.
“I’d want to know ‘What’s the potential this person has had an exposure [to the coronavirus], especially in the last 2 weeks?’ ” said Dr. Vaughan of Emory. “Do they have home health personnel coming in? Have they gotten together with other family members? Are chronic conditions being controlled? Is there another diagnosis that seems more likely?”
“Someone may be just having a bad day. But if they’re not themselves for a couple of days, absolutely reach out to a primary care doctor or a local health system hotline to see if they meet the threshold for [coronavirus] testing,” Dr. Vaughan advised. “Be persistent. If you get a ‘no’ the first time and things aren’t improving, call back and ask again.”
Kaiser Health News (khn.org) is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
complicating efforts to ensure they get timely and appropriate treatment, according to physicians.
COVID-19 is typically signaled by three symptoms: a fever, an insistent cough, and shortness of breath. But older adults – the age group most at risk of severe complications or death from this condition – may have none of these characteristics.
Instead, seniors may seem “off” – not acting like themselves – early on after being infected by the coronavirus. They may sleep more than usual or stop eating. They may seem unusually apathetic or confused, losing orientation to their surroundings. They may become dizzy and fall. Sometimes, seniors stop speaking or simply collapse.
“With a lot of conditions, older adults don’t present in a typical way, and we’re seeing that with COVID-19 as well,” said Camille Vaughan, MD, section chief of geriatrics and gerontology at Emory University, Atlanta.
The reason has to do with how older bodies respond to illness and infection.
At advanced ages, “someone’s immune response may be blunted and their ability to regulate temperature may be altered,” said Dr. Joseph Ouslander, a professor of geriatric medicine at Florida Atlantic University in Boca Raton.
“Underlying chronic illnesses can mask or interfere with signs of infection,” he said. “Some older people, whether from age-related changes or previous neurologic issues such as a stroke, may have altered cough reflexes. Others with cognitive impairment may not be able to communicate their symptoms.”
Recognizing danger signs is important: If early signs of COVID-19 are missed, seniors may deteriorate before getting needed care. And people may go in and out of their homes without adequate protective measures, risking the spread of infection.
Quratulain Syed, MD, an Atlanta geriatrician, describes a man in his 80s whom she treated in mid-March. Over a period of days, this patient, who had heart disease, diabetes and moderate cognitive impairment, stopped walking and became incontinent and profoundly lethargic. But he didn’t have a fever or a cough. His only respiratory symptom: sneezing off and on.
The man’s elderly spouse called 911 twice. Both times, paramedics checked his vital signs and declared he was OK. After another worried call from the overwhelmed spouse, Dr. Syed insisted the patient be taken to the hospital, where he tested positive for COVID-19.
“I was quite concerned about the paramedics and health aides who’d been in the house and who hadn’t used PPE [personal protective equipment],” Dr. Syed said.
Dr. Sam Torbati, medical director of the emergency department at Cedars-Sinai Medical Center, Los Angeles, describes treating seniors who initially appear to be trauma patients but are found to have COVID-19.
“They get weak and dehydrated,” he said, “and when they stand to walk, they collapse and injure themselves badly.”
Dr. Torbati has seen older adults who are profoundly disoriented and unable to speak and who appear at first to have suffered strokes.
“When we test them, we discover that what’s producing these changes is a central nervous system effect of coronavirus,” he said.
Laura Perry, MD, of the University of California, San Francisco, saw a patient like this several weeks ago. The woman, in her 80s, had what seemed to be a cold before becoming very confused. In the hospital, she couldn’t identify where she was or stay awake during an examination. Dr. Perry diagnosed hypoactive delirium, an altered mental state in which people become inactive and drowsy. The patient tested positive for coronavirus and is still in the ICU.
Anthony Perry, MD, of the department of geriatric medicine at Rush University Medical Center in Chicago, tells of an 81-year-old woman with nausea, vomiting, and diarrhea who tested positive for COVID-19 in the emergency room. After receiving intravenous fluids, oxygen, and medication for her intestinal upset, she returned home after 2 days and is doing well.
Another 80-year-old Rush patient with similar symptoms – nausea and vomiting, but no cough, fever, or shortness of breath – is in intensive care after getting a positive COVID-19 test and due to be put on a ventilator. The difference? This patient is frail with “a lot of cardiovascular disease,” Dr. Perry said. Other than that, it’s not yet clear why some older patients do well while others do not.
So far, reports of cases like these have been anecdotal. But a few physicians are trying to gather more systematic information.
In Switzerland, Sylvain Nguyen, MD, a geriatrician at the University of Lausanne Hospital Center, put together a list of typical and atypical symptoms in older COVID-19 patients for a paper to be published in the Revue Médicale Suisse. Included on the atypical list are changes in a patient’s usual status, delirium, falls, fatigue, lethargy, low blood pressure, painful swallowing, fainting, diarrhea, nausea, vomiting, abdominal pain, and the loss of smell and taste.
Data come from hospitals and nursing homes in Switzerland, Italy, and France, Dr. Nguyen said in an email.
On the front lines, physicians need to make sure they carefully assess an older patient’s symptoms.
“While we have to have a high suspicion of COVID-19 because it’s so dangerous in the older population, there are many other things to consider,” said Kathleen Unroe, MD, a geriatrician at Indiana University, Indianapolis.
Seniors may also do poorly because their routines have changed. In nursing homes and most assisted living centers, activities have stopped and “residents are going to get weaker and more deconditioned because they’re not walking to and from the dining hall,” she said.
At home, isolated seniors may not be getting as much help with medication management or other essential needs from family members who are keeping their distance, other experts suggested. Or they may have become apathetic or depressed.
“I’d want to know ‘What’s the potential this person has had an exposure [to the coronavirus], especially in the last 2 weeks?’ ” said Dr. Vaughan of Emory. “Do they have home health personnel coming in? Have they gotten together with other family members? Are chronic conditions being controlled? Is there another diagnosis that seems more likely?”
“Someone may be just having a bad day. But if they’re not themselves for a couple of days, absolutely reach out to a primary care doctor or a local health system hotline to see if they meet the threshold for [coronavirus] testing,” Dr. Vaughan advised. “Be persistent. If you get a ‘no’ the first time and things aren’t improving, call back and ask again.”
Kaiser Health News (khn.org) is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Increased risk of lung cancer with COPD, even in never smokers
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.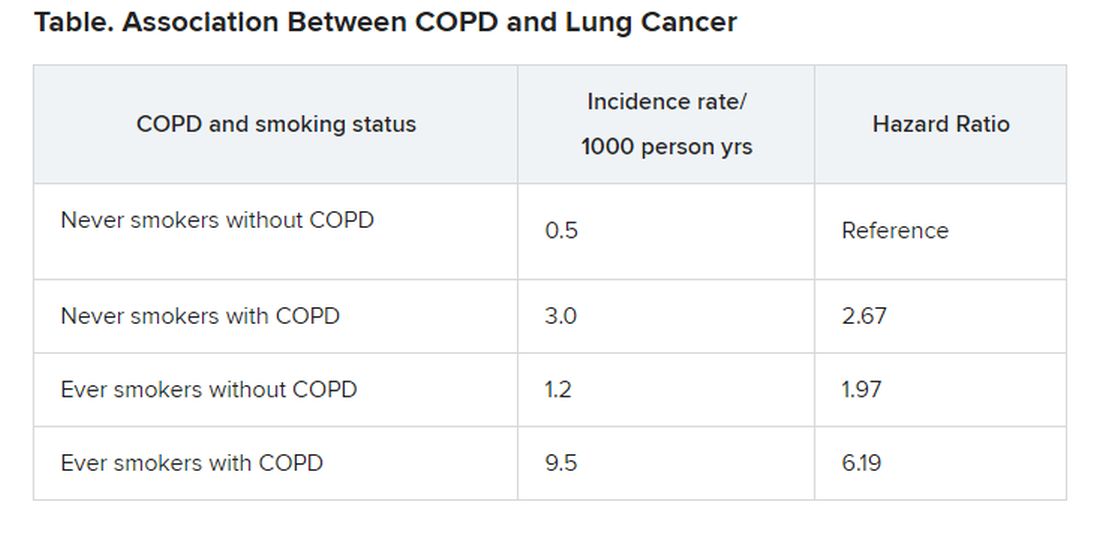
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
an observational cohort study has shown.
Patients with COPD who had never smoked had more than double the risk of developing lung cancer (with an adjusted hazard ratio [HR] of 2.67), compared to individuals without COPD who had never smoked.
This was slightly higher than the increased risk seen in individuals who had smoked but who did not have COPD. This group had an almost double the risk of developing lung cancer (adjusted HR, 1.97), again compared to never smokers, the investigators added.
The highest risk of lung cancer was in patients who had COPD and who had smoked; this group had a sixfold risk of developing lung cancer (adjusted HR, 6.19) compared with never smokers without COPD, they note.
“COPD was a strong independent risk factor for lung cancer incidence in never smokers,” conclude the authors, led by Hye Yun Park, MD, Samsung Medical Center, Seoul, South Korea.
“Future studies should evaluate whether COPD patients are candidates for lung cancer screening, irrespective of smoking status,” they suggest.
The study was published March 10 in the journal Thorax.
It was based on an analysis of data from the National Health Insurance (NHS) Service National Sample Cohort between January 2002 and December 2013.
“We included all men and women, 40 to 84 years of age, who underwent at least one health screening examination provided by the NHS during the study period,” Park and colleagues explain.
Overall, the cohort included 338,548 men and women. Participants were followed-up for a median of 7 years.
Over the study interval, 1834 participants developed lung cancer.
“The risk of disease [lung cancer] in never smokers with COPD was higher than that in ever smokers without COPD,” the investigators observe.
“Given that poor lung function in COPD is often a barrier to optimal lung cancer treatment due to increased risk of treatment-related morbidities, our study suggests that early detection of lung cancer in COPD patients may reduce the risk of treatment complications,” the authors write.
The study was supported by the National Research Foundation of Korea. The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
COVID-19 registry tracks pregnant women, newborns
A multidisciplinary team of researchers has created a national registry to study how COVID-19 affects pregnant women and their newborns.
“Pregnant women are generally considered healthy, but they are also a vulnerable group, and we currently have no data on COVID-19 in pregnancy,” coprincipal investigator Yalda Afshar, MD, PhD, an ob.gyn. at UCLA Health in Los Angeles, said in an interview.
“We expect this registry to provide data that will be critical in helping to improve care for pregnant women during this global pandemic,” Dr. Afshar, a fellow with UCLA Biodesign, stated in a news release.
The Pregnancy Coronavirus Outcomes Registry is enrolling pregnant women and those who have been pregnant or post partum within the past 6 weeks and who have either received a confirmed diagnosis of COVID-19 or are being evaluated for COVID-19.
Women are being recruited through their health care provider. A study coordinator contacts the participants by telephone. Women can also join the registry on their own without a referral by visiting the registry website.
The registry collects data on COVID-19 symptoms, clinical course, pregnancy, and neonatal outcomes and follows women from enrollment through the second and third trimesters and the postpartum period. The goal is to follow the mothers and babies for up to 1 year.
Hundreds of women already enrolled
Dr. Afshar noted that these kinds of registries often take months to design and to receive funding, but with COVID-19, “there was no time for that. We had to get it up and running ASAP.”
She said the team has been “blown away” by how quickly people have come forward to join the registry. Within 2 weeks of going live, the registry had enrolled more than 400 participants from across the United States. “At this rate, I think we will easily get 1,000 participants in a month or so,” Dr. Afshar said.
“With the global reach of this disease, the findings resulting from this work have the potential to impact millions of lives in an entire generation,” Johnese Spisso, CEO of UCLA Health, said in the news release.
Dr. Afshar noted that, although the impact of COVID-19 on pregnancy remains unknown, history suggests the disease will make some pregnancies and deliveries more challenging. “We know that in previous outbreaks of the regular flu, for example, there have been more deaths and poorer outcomes among pregnant women compared with nonpregnant women.”
Dr. Afshar is overseeing the study with colleagues at the University of California, Los Angeles, and the University of California, San Francisco, where the registry data will be coordinated.
“In addition to gaining a better understanding of the course of the disease, we will investigate disease transmission to determine if it can be passed from a mother to her baby in utero and during the postpartum period, such as in breast milk,” UCSF’s Stephanie Gaw, MD, PhD, who is leading the biospecimen core of the study, said in the release.
Health care providers interested in more information about the registry may send an email to [email protected]. A YouTube video on the registry is also available.
Dr. Afshar disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A multidisciplinary team of researchers has created a national registry to study how COVID-19 affects pregnant women and their newborns.
“Pregnant women are generally considered healthy, but they are also a vulnerable group, and we currently have no data on COVID-19 in pregnancy,” coprincipal investigator Yalda Afshar, MD, PhD, an ob.gyn. at UCLA Health in Los Angeles, said in an interview.
“We expect this registry to provide data that will be critical in helping to improve care for pregnant women during this global pandemic,” Dr. Afshar, a fellow with UCLA Biodesign, stated in a news release.
The Pregnancy Coronavirus Outcomes Registry is enrolling pregnant women and those who have been pregnant or post partum within the past 6 weeks and who have either received a confirmed diagnosis of COVID-19 or are being evaluated for COVID-19.
Women are being recruited through their health care provider. A study coordinator contacts the participants by telephone. Women can also join the registry on their own without a referral by visiting the registry website.
The registry collects data on COVID-19 symptoms, clinical course, pregnancy, and neonatal outcomes and follows women from enrollment through the second and third trimesters and the postpartum period. The goal is to follow the mothers and babies for up to 1 year.
Hundreds of women already enrolled
Dr. Afshar noted that these kinds of registries often take months to design and to receive funding, but with COVID-19, “there was no time for that. We had to get it up and running ASAP.”
She said the team has been “blown away” by how quickly people have come forward to join the registry. Within 2 weeks of going live, the registry had enrolled more than 400 participants from across the United States. “At this rate, I think we will easily get 1,000 participants in a month or so,” Dr. Afshar said.
“With the global reach of this disease, the findings resulting from this work have the potential to impact millions of lives in an entire generation,” Johnese Spisso, CEO of UCLA Health, said in the news release.
Dr. Afshar noted that, although the impact of COVID-19 on pregnancy remains unknown, history suggests the disease will make some pregnancies and deliveries more challenging. “We know that in previous outbreaks of the regular flu, for example, there have been more deaths and poorer outcomes among pregnant women compared with nonpregnant women.”
Dr. Afshar is overseeing the study with colleagues at the University of California, Los Angeles, and the University of California, San Francisco, where the registry data will be coordinated.
“In addition to gaining a better understanding of the course of the disease, we will investigate disease transmission to determine if it can be passed from a mother to her baby in utero and during the postpartum period, such as in breast milk,” UCSF’s Stephanie Gaw, MD, PhD, who is leading the biospecimen core of the study, said in the release.
Health care providers interested in more information about the registry may send an email to [email protected]. A YouTube video on the registry is also available.
Dr. Afshar disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A multidisciplinary team of researchers has created a national registry to study how COVID-19 affects pregnant women and their newborns.
“Pregnant women are generally considered healthy, but they are also a vulnerable group, and we currently have no data on COVID-19 in pregnancy,” coprincipal investigator Yalda Afshar, MD, PhD, an ob.gyn. at UCLA Health in Los Angeles, said in an interview.
“We expect this registry to provide data that will be critical in helping to improve care for pregnant women during this global pandemic,” Dr. Afshar, a fellow with UCLA Biodesign, stated in a news release.
The Pregnancy Coronavirus Outcomes Registry is enrolling pregnant women and those who have been pregnant or post partum within the past 6 weeks and who have either received a confirmed diagnosis of COVID-19 or are being evaluated for COVID-19.
Women are being recruited through their health care provider. A study coordinator contacts the participants by telephone. Women can also join the registry on their own without a referral by visiting the registry website.
The registry collects data on COVID-19 symptoms, clinical course, pregnancy, and neonatal outcomes and follows women from enrollment through the second and third trimesters and the postpartum period. The goal is to follow the mothers and babies for up to 1 year.
Hundreds of women already enrolled
Dr. Afshar noted that these kinds of registries often take months to design and to receive funding, but with COVID-19, “there was no time for that. We had to get it up and running ASAP.”
She said the team has been “blown away” by how quickly people have come forward to join the registry. Within 2 weeks of going live, the registry had enrolled more than 400 participants from across the United States. “At this rate, I think we will easily get 1,000 participants in a month or so,” Dr. Afshar said.
“With the global reach of this disease, the findings resulting from this work have the potential to impact millions of lives in an entire generation,” Johnese Spisso, CEO of UCLA Health, said in the news release.
Dr. Afshar noted that, although the impact of COVID-19 on pregnancy remains unknown, history suggests the disease will make some pregnancies and deliveries more challenging. “We know that in previous outbreaks of the regular flu, for example, there have been more deaths and poorer outcomes among pregnant women compared with nonpregnant women.”
Dr. Afshar is overseeing the study with colleagues at the University of California, Los Angeles, and the University of California, San Francisco, where the registry data will be coordinated.
“In addition to gaining a better understanding of the course of the disease, we will investigate disease transmission to determine if it can be passed from a mother to her baby in utero and during the postpartum period, such as in breast milk,” UCSF’s Stephanie Gaw, MD, PhD, who is leading the biospecimen core of the study, said in the release.
Health care providers interested in more information about the registry may send an email to [email protected]. A YouTube video on the registry is also available.
Dr. Afshar disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19: Frequently asked clinical questions
Question
How should patients on immunosuppressive therapy be advised during the COVID-19 pandemic?
Answer
In general, those patients who have not tested positive, have not been exposed, and are asymptomatic should continue their medications as prescribed.
The American College of Rheumatology issued a statement on April 14, recommending that stable patients continue their medications. Those with known exposure but without confirmed infection may continue hydroxychloroquine, sulfasalazine, and NSAIDs.
Immunosuppressants, non–IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily. Anti-malarial therapies (hydroxycholoroquine and chloroquine) may be continued and IL-6 inhibitors may be continued in select circumstances.1
The American Academy of Dermatology recommends that the discussion of continuation of biologics be based on a case-by-case basis, citing insufficient evidence to recommend against discontinuation at this time in those patients who have not tested positive. In patients who have tested positive for COVID-19 it is recommended that biologic therapy be suspended until symptoms have resolved.2
Question
Should I continue preventive services during peak COVID-19?
Answer
The Centers for Disease Control and Prevention recommends delaying all elective ambulatory provider visits. In general, preventative services, such as adult immunizations, lipid screening, and cancer screenings, should be delayed. Additionally, the CDC recommends reaching out to patients who are at high risk for complications from respiratory diseases to ensure medication adherence and provide resources if these patients become ill. Facilities can reduce transmission of COVID-19 by triaging and assessing patients through virtual visits through phone calls, video conferences, text-monitoring systems, and other telemedicine tools. Physicians should try to provide routine and chronic care through virtual visits when possible over in-person visits.3
Question
Should I continue to vaccinate my pediatric population during peak COVID-19?
Answer
Practices that schedule separate well visits and sick visits in different sessions or locations can continue to provide well child visits. A practice could, for example, schedule well visits in the morning and sick visits in the afternoon if a single facility is used. These practices should prioritize newborn care and vaccinations of children, especially for those under the age of 24 months.4
Question
Can physicians use telehealth (phone only or audiovisual) to conduct visits with Medicare patients even if they are new patients?
Answer
Effective March 1 through the duration of the pandemic, Medicare will pay physicians for telehealth services at the same rate as an in-office visit. On March 30th, the Centers for Medicare & Medcaid Services announced new policies for physicians and hospitals during the COVID-19 pandemic. These guidelines were updated on April 9.
Audio-only visits are now permitted and the limit on the number of these kinds of visits allowed per month has been waived. Controlled substances can be prescribed via telehealth; however, complying with each state’s individual laws is still required.
Use of any two-way, audiovisual device is permitted. The level of service billed for visits with both audio and visual components is the same as an in-office visit. Telemedicine can be used for both new and existing patients.5
A list of services that may be rendered via telehealth are available on the CMS website.6
It will be important to regularly check the references given, as information on some of these topics is updated frequently.
Dr. Chuong is a second-year resident in the family medicine residency, Dr. Flanagan is a third-year resident, and Dr. Matthews is an intern, all at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. ACR issues COVID-19 treatment guidance for rheumatic disease patients.
2. American Academy of Dermatology: Guidance on the use of biologic agents during COVID-19 outbreak.
3. Centers for Disease Control and Prevention. Actions to take in response to community transmission of COVID-19.
4. Centers for Disease Control and Prevention. Maintaining childhood immunizations during COVID19 pandemic.
5. Centers for Medicare & Medcaid Services. COVID-19 frequently asked questions (FAQs) on Medicare Fee-for-Service (FFS) billing.
6. Centers for Medicare & Medcaid Services. List of telehealth services.
Question
How should patients on immunosuppressive therapy be advised during the COVID-19 pandemic?
Answer
In general, those patients who have not tested positive, have not been exposed, and are asymptomatic should continue their medications as prescribed.
The American College of Rheumatology issued a statement on April 14, recommending that stable patients continue their medications. Those with known exposure but without confirmed infection may continue hydroxychloroquine, sulfasalazine, and NSAIDs.
Immunosuppressants, non–IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily. Anti-malarial therapies (hydroxycholoroquine and chloroquine) may be continued and IL-6 inhibitors may be continued in select circumstances.1
The American Academy of Dermatology recommends that the discussion of continuation of biologics be based on a case-by-case basis, citing insufficient evidence to recommend against discontinuation at this time in those patients who have not tested positive. In patients who have tested positive for COVID-19 it is recommended that biologic therapy be suspended until symptoms have resolved.2
Question
Should I continue preventive services during peak COVID-19?
Answer
The Centers for Disease Control and Prevention recommends delaying all elective ambulatory provider visits. In general, preventative services, such as adult immunizations, lipid screening, and cancer screenings, should be delayed. Additionally, the CDC recommends reaching out to patients who are at high risk for complications from respiratory diseases to ensure medication adherence and provide resources if these patients become ill. Facilities can reduce transmission of COVID-19 by triaging and assessing patients through virtual visits through phone calls, video conferences, text-monitoring systems, and other telemedicine tools. Physicians should try to provide routine and chronic care through virtual visits when possible over in-person visits.3
Question
Should I continue to vaccinate my pediatric population during peak COVID-19?
Answer
Practices that schedule separate well visits and sick visits in different sessions or locations can continue to provide well child visits. A practice could, for example, schedule well visits in the morning and sick visits in the afternoon if a single facility is used. These practices should prioritize newborn care and vaccinations of children, especially for those under the age of 24 months.4
Question
Can physicians use telehealth (phone only or audiovisual) to conduct visits with Medicare patients even if they are new patients?
Answer
Effective March 1 through the duration of the pandemic, Medicare will pay physicians for telehealth services at the same rate as an in-office visit. On March 30th, the Centers for Medicare & Medcaid Services announced new policies for physicians and hospitals during the COVID-19 pandemic. These guidelines were updated on April 9.
Audio-only visits are now permitted and the limit on the number of these kinds of visits allowed per month has been waived. Controlled substances can be prescribed via telehealth; however, complying with each state’s individual laws is still required.
Use of any two-way, audiovisual device is permitted. The level of service billed for visits with both audio and visual components is the same as an in-office visit. Telemedicine can be used for both new and existing patients.5
A list of services that may be rendered via telehealth are available on the CMS website.6
It will be important to regularly check the references given, as information on some of these topics is updated frequently.
Dr. Chuong is a second-year resident in the family medicine residency, Dr. Flanagan is a third-year resident, and Dr. Matthews is an intern, all at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. ACR issues COVID-19 treatment guidance for rheumatic disease patients.
2. American Academy of Dermatology: Guidance on the use of biologic agents during COVID-19 outbreak.
3. Centers for Disease Control and Prevention. Actions to take in response to community transmission of COVID-19.
4. Centers for Disease Control and Prevention. Maintaining childhood immunizations during COVID19 pandemic.
5. Centers for Medicare & Medcaid Services. COVID-19 frequently asked questions (FAQs) on Medicare Fee-for-Service (FFS) billing.
6. Centers for Medicare & Medcaid Services. List of telehealth services.
Question
How should patients on immunosuppressive therapy be advised during the COVID-19 pandemic?
Answer
In general, those patients who have not tested positive, have not been exposed, and are asymptomatic should continue their medications as prescribed.
The American College of Rheumatology issued a statement on April 14, recommending that stable patients continue their medications. Those with known exposure but without confirmed infection may continue hydroxychloroquine, sulfasalazine, and NSAIDs.
Immunosuppressants, non–IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily, pending a negative test or after two weeks without symptoms. In patients with confirmed positive COVID-19 infection, sulfasalazine, methotrexate, leflunomide, immunosuppressants, non-IL-6 biologics, and JAK inhibitors should be stopped temporarily. Anti-malarial therapies (hydroxycholoroquine and chloroquine) may be continued and IL-6 inhibitors may be continued in select circumstances.1
The American Academy of Dermatology recommends that the discussion of continuation of biologics be based on a case-by-case basis, citing insufficient evidence to recommend against discontinuation at this time in those patients who have not tested positive. In patients who have tested positive for COVID-19 it is recommended that biologic therapy be suspended until symptoms have resolved.2
Question
Should I continue preventive services during peak COVID-19?
Answer
The Centers for Disease Control and Prevention recommends delaying all elective ambulatory provider visits. In general, preventative services, such as adult immunizations, lipid screening, and cancer screenings, should be delayed. Additionally, the CDC recommends reaching out to patients who are at high risk for complications from respiratory diseases to ensure medication adherence and provide resources if these patients become ill. Facilities can reduce transmission of COVID-19 by triaging and assessing patients through virtual visits through phone calls, video conferences, text-monitoring systems, and other telemedicine tools. Physicians should try to provide routine and chronic care through virtual visits when possible over in-person visits.3
Question
Should I continue to vaccinate my pediatric population during peak COVID-19?
Answer
Practices that schedule separate well visits and sick visits in different sessions or locations can continue to provide well child visits. A practice could, for example, schedule well visits in the morning and sick visits in the afternoon if a single facility is used. These practices should prioritize newborn care and vaccinations of children, especially for those under the age of 24 months.4
Question
Can physicians use telehealth (phone only or audiovisual) to conduct visits with Medicare patients even if they are new patients?
Answer
Effective March 1 through the duration of the pandemic, Medicare will pay physicians for telehealth services at the same rate as an in-office visit. On March 30th, the Centers for Medicare & Medcaid Services announced new policies for physicians and hospitals during the COVID-19 pandemic. These guidelines were updated on April 9.
Audio-only visits are now permitted and the limit on the number of these kinds of visits allowed per month has been waived. Controlled substances can be prescribed via telehealth; however, complying with each state’s individual laws is still required.
Use of any two-way, audiovisual device is permitted. The level of service billed for visits with both audio and visual components is the same as an in-office visit. Telemedicine can be used for both new and existing patients.5
A list of services that may be rendered via telehealth are available on the CMS website.6
It will be important to regularly check the references given, as information on some of these topics is updated frequently.
Dr. Chuong is a second-year resident in the family medicine residency, Dr. Flanagan is a third-year resident, and Dr. Matthews is an intern, all at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
References
1. ACR issues COVID-19 treatment guidance for rheumatic disease patients.
2. American Academy of Dermatology: Guidance on the use of biologic agents during COVID-19 outbreak.
3. Centers for Disease Control and Prevention. Actions to take in response to community transmission of COVID-19.
4. Centers for Disease Control and Prevention. Maintaining childhood immunizations during COVID19 pandemic.
5. Centers for Medicare & Medcaid Services. COVID-19 frequently asked questions (FAQs) on Medicare Fee-for-Service (FFS) billing.
6. Centers for Medicare & Medcaid Services. List of telehealth services.
FDA reiterates hydroxychloroquine limitations for COVID-19
The U.S. Food and Drug Administration reinforced its March guidance on when it’s permissible to use hydroxychloroquine and chloroquine to treat COVID-19 patients and on the multiple risks these drugs pose in a Safety Communication on April 24.
The new communication reiterated the agency’s position from the Emergency Use Authorization (EUA) it granted on March 28 to allow hydroxychloroquine and chloroquine treatment of COVID-19 patients only when they are hospitalized and participation in a clinical trial is “not available,” or “not feasible.” The April 24 update to the EUA noted that “the FDA is aware of reports of serious heart rhythm problems in patients with COVID-19 treated with hydroxychloroquine or chloroquine, often in combination with azithromycin and other QT-prolonging medicines. We are also aware of increased use of these medicines through outpatient prescriptions.”
In addition to reiterating the prior limitations on permissible patients for these treatment the agency also said in the new communication that “close supervision is strongly recommended, “ specifying that “we recommend initial evaluation and monitoring when using hydroxychloroquine or chloroquine under the EUA or in clinical trials that investigate these medicines for the treatment or prevention of COVID-19. Monitoring may include baseline ECG, electrolytes, renal function, and hepatic tests.” The communication also highlighted several potential serious adverse effects from hydroxychloroquine or chloroquine that include QT prolongation with increased risk in patients with renal insufficiency or failure, increased insulin levels and insulin action causing increased risk of severe hypoglycemia, hemolysis in selected patients, and interaction with other medicines that cause QT prolongation.
“If a healthcare professional is considering use of hydroxychloroquine or chloroquine to treat or prevent COVID-19, FDA recommends checking www.clinicaltrials.gov for a suitable clinical trial and consider enrolling the patient,” the statement added.
The FDA’s Safety Communication came a day after the European Medicines Agency issued a similar reminder about the risk for serious adverse effects from treatment with hydroxychloroquine and chloroquine, the need for adverse effect monitoring, and the unproven status of purported benefits from these agents.
The statement came after ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite a lack of evidence.
The FDA’s communication cited recent case reports sent to the FDA, as well as published findings, and reports to the National Poison Data System that have described serious, heart-related adverse events and death in COVID-19 patients who received hydroxychloroquine and chloroquine, alone or in combination with azithromycin or another QT-prolonging drug. One recent, notable but not peer-reviewed report on 368 patients treated at any of several U.S. VA medical centers showed no apparent benefit to hospitalized COVID-19 patients treated with hydroxychloroquine and a signal for increased mortality among certain patients on this drug (medRxiv. 2020 Apr 23; doi: 10.1101/2020.04.16.20065920). Several cardiology societies have also highlighted the cardiac considerations for using these drugs in patients with COVID-19, including a summary coauthored by the presidents of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society (Circulation. 2020 Apr 8. doi: 10.1161/CIRCULATIONAHA.120.047521), and in guidance from the European Society of Cardiology.
The U.S. Food and Drug Administration reinforced its March guidance on when it’s permissible to use hydroxychloroquine and chloroquine to treat COVID-19 patients and on the multiple risks these drugs pose in a Safety Communication on April 24.
The new communication reiterated the agency’s position from the Emergency Use Authorization (EUA) it granted on March 28 to allow hydroxychloroquine and chloroquine treatment of COVID-19 patients only when they are hospitalized and participation in a clinical trial is “not available,” or “not feasible.” The April 24 update to the EUA noted that “the FDA is aware of reports of serious heart rhythm problems in patients with COVID-19 treated with hydroxychloroquine or chloroquine, often in combination with azithromycin and other QT-prolonging medicines. We are also aware of increased use of these medicines through outpatient prescriptions.”
In addition to reiterating the prior limitations on permissible patients for these treatment the agency also said in the new communication that “close supervision is strongly recommended, “ specifying that “we recommend initial evaluation and monitoring when using hydroxychloroquine or chloroquine under the EUA or in clinical trials that investigate these medicines for the treatment or prevention of COVID-19. Monitoring may include baseline ECG, electrolytes, renal function, and hepatic tests.” The communication also highlighted several potential serious adverse effects from hydroxychloroquine or chloroquine that include QT prolongation with increased risk in patients with renal insufficiency or failure, increased insulin levels and insulin action causing increased risk of severe hypoglycemia, hemolysis in selected patients, and interaction with other medicines that cause QT prolongation.
“If a healthcare professional is considering use of hydroxychloroquine or chloroquine to treat or prevent COVID-19, FDA recommends checking www.clinicaltrials.gov for a suitable clinical trial and consider enrolling the patient,” the statement added.
The FDA’s Safety Communication came a day after the European Medicines Agency issued a similar reminder about the risk for serious adverse effects from treatment with hydroxychloroquine and chloroquine, the need for adverse effect monitoring, and the unproven status of purported benefits from these agents.
The statement came after ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite a lack of evidence.
The FDA’s communication cited recent case reports sent to the FDA, as well as published findings, and reports to the National Poison Data System that have described serious, heart-related adverse events and death in COVID-19 patients who received hydroxychloroquine and chloroquine, alone or in combination with azithromycin or another QT-prolonging drug. One recent, notable but not peer-reviewed report on 368 patients treated at any of several U.S. VA medical centers showed no apparent benefit to hospitalized COVID-19 patients treated with hydroxychloroquine and a signal for increased mortality among certain patients on this drug (medRxiv. 2020 Apr 23; doi: 10.1101/2020.04.16.20065920). Several cardiology societies have also highlighted the cardiac considerations for using these drugs in patients with COVID-19, including a summary coauthored by the presidents of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society (Circulation. 2020 Apr 8. doi: 10.1161/CIRCULATIONAHA.120.047521), and in guidance from the European Society of Cardiology.
The U.S. Food and Drug Administration reinforced its March guidance on when it’s permissible to use hydroxychloroquine and chloroquine to treat COVID-19 patients and on the multiple risks these drugs pose in a Safety Communication on April 24.
The new communication reiterated the agency’s position from the Emergency Use Authorization (EUA) it granted on March 28 to allow hydroxychloroquine and chloroquine treatment of COVID-19 patients only when they are hospitalized and participation in a clinical trial is “not available,” or “not feasible.” The April 24 update to the EUA noted that “the FDA is aware of reports of serious heart rhythm problems in patients with COVID-19 treated with hydroxychloroquine or chloroquine, often in combination with azithromycin and other QT-prolonging medicines. We are also aware of increased use of these medicines through outpatient prescriptions.”
In addition to reiterating the prior limitations on permissible patients for these treatment the agency also said in the new communication that “close supervision is strongly recommended, “ specifying that “we recommend initial evaluation and monitoring when using hydroxychloroquine or chloroquine under the EUA or in clinical trials that investigate these medicines for the treatment or prevention of COVID-19. Monitoring may include baseline ECG, electrolytes, renal function, and hepatic tests.” The communication also highlighted several potential serious adverse effects from hydroxychloroquine or chloroquine that include QT prolongation with increased risk in patients with renal insufficiency or failure, increased insulin levels and insulin action causing increased risk of severe hypoglycemia, hemolysis in selected patients, and interaction with other medicines that cause QT prolongation.
“If a healthcare professional is considering use of hydroxychloroquine or chloroquine to treat or prevent COVID-19, FDA recommends checking www.clinicaltrials.gov for a suitable clinical trial and consider enrolling the patient,” the statement added.
The FDA’s Safety Communication came a day after the European Medicines Agency issued a similar reminder about the risk for serious adverse effects from treatment with hydroxychloroquine and chloroquine, the need for adverse effect monitoring, and the unproven status of purported benefits from these agents.
The statement came after ongoing promotion by the Trump administration of hydroxychloroquine, in particular, for COVID-19 despite a lack of evidence.
The FDA’s communication cited recent case reports sent to the FDA, as well as published findings, and reports to the National Poison Data System that have described serious, heart-related adverse events and death in COVID-19 patients who received hydroxychloroquine and chloroquine, alone or in combination with azithromycin or another QT-prolonging drug. One recent, notable but not peer-reviewed report on 368 patients treated at any of several U.S. VA medical centers showed no apparent benefit to hospitalized COVID-19 patients treated with hydroxychloroquine and a signal for increased mortality among certain patients on this drug (medRxiv. 2020 Apr 23; doi: 10.1101/2020.04.16.20065920). Several cardiology societies have also highlighted the cardiac considerations for using these drugs in patients with COVID-19, including a summary coauthored by the presidents of the American College of Cardiology, the American Heart Association, and the Heart Rhythm Society (Circulation. 2020 Apr 8. doi: 10.1161/CIRCULATIONAHA.120.047521), and in guidance from the European Society of Cardiology.
FROM THE FDA