User login
Flu activity dropped in early December
according to the Centers for Disease Control and Prevention.
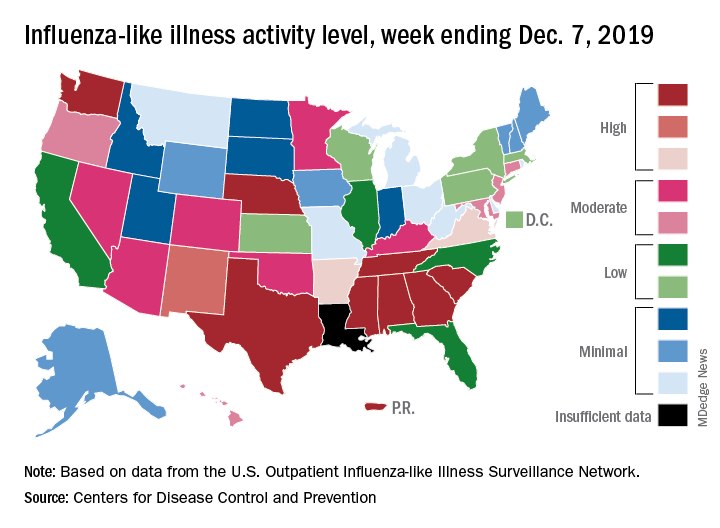
Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
according to the Centers for Disease Control and Prevention.

Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
according to the Centers for Disease Control and Prevention.

Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
Asthma exacerbation in pregnancy impacts mothers, infants
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
Women with asthma who suffer asthma exacerbation while pregnant are at increased risk for complications during pregnancy and delivery, and their infants are at increased risk for respiratory problems, according to data from a longitudinal study of 58,524 women with asthma.
“Asthma exacerbation during pregnancy has been found to be associated with adverse perinatal and pregnancy outcomes such as low birth weight, small for gestational age, preterm delivery, congenital malformation, preeclampsia, and perinatal mortality,” but previous studies have been small and limited to comparisons of asthmatic and nonasthmatic women, wrote Kawsari Abdullah, PhD, of Children’s Hospital of Eastern Ontario, Ottawa, and colleagues.
To determine the impact of asthma exacerbation on maternal and fetal outcomes, the researchers analyzed data from the Ontario Asthma Surveillance Information System to identify women with asthma who had at least one pregnancy resulting in a live or still birth between 2006 and 2012.
Overall, significantly more women with exacerbated asthma had preeclampsia or pregnancy-induced hypertension, compared with asthmatic women who had no exacerbations, at 5% vs. 4% and 7% vs. 5%, respectively (P less than .001), according to the study published in the European Respiratory Journal.
Adverse perinatal outcomes were significantly more likely among babies of mothers with exacerbated asthma, compared with those who had no exacerbations, including low birth weight (7% vs. 5%), small for gestational age (3% vs. 2%), preterm birth (8% vs. 7%), and congenital malformation (6% vs. 5%). All P values were less than .001, except for small for gestational age, which was P = .008.
In addition, significantly more babies of asthmatic women with exacerbated asthma during pregnancy had respiratory problems including asthma and pneumonia, compared with those of asthmatic women who had no exacerbations during pregnancy, at 38% vs. 31% and 24% vs. 22% (P less than .001 for both). The researchers found no significant interactions between maternal age and smoking and asthma exacerbations.
The findings were limited by several factors, including the lack of a validated algorithm for asthma exacerbation, which the researchers defined as five or more visits to a general practice clinician for asthma during pregnancy. Other limitations included the lack of categorizing asthma exacerbation by severity, and the inability to include the potential effects of asthma medication on maternal and fetal outcomes, Dr. Abdullah and colleagues noted.
However, the results were strengthened by the large sample size and ability to follow babies from birth until 5 years of age, they said.
“Targeting women with asthma during pregnancy and ensuring appropriate asthma management and postpartum follow-up may help to reduce the risk of pregnancy complications, adverse perinatal outcomes, and early childhood respiratory disorders,” they concluded.
This study is important because asthma is a common, potentially serious medical condition that complicates approximately 4%-8% of pregnancies, and one in three women with asthma experience an exacerbation during pregnancy, Iris Krishna, MD, a specialist in maternal/fetal medicine at Emory University, Atlanta, said in an interview.
“This study is unique in that it uses population-level data to assess the association between an asthma exacerbation during pregnancy and adverse perinatal outcomes,” Dr. Krishna said. “After adjusting for confounders, and consistent with previous studies, study findings suggest an increased risk for women with asthma who have an asthma exacerbation during pregnancy for preeclampsia [odds ratio, 1.3; P less than .001], pregnancy-induced hypertension [OR, 1.17; P less than .05], low-birth-weight infant [OR, 1.14; P less than .05], preterm birth [OR, 1.14; P less than .05], and congenital malformations [OR, 1.21; P less than .001].”
Dr. Krishna also noted the impact on early childhood outcomes. “In this study, children born to women who had an asthma exacerbation during pregnancy had a 23% higher risk of developing asthma before 5 years of age, which is consistent with previous studies. [The] investigators also reported a 12% higher risk of having pneumonia during the first 5 years of life for children born to women who had an asthma exacerbation during pregnancy.”
“Previous studies have suggested children born to mothers with uncontrolled asthma have an increased risk for respiratory infections, but this study is the first to report an association with pneumonia,” she said. This increased risk for childhood respiratory disorders warrants further study.
Consequently, “Women with asthma during pregnancy should have appropriate management to ensure good control to optimize pregnancy outcome,” Dr. Krishna emphasized. “Women who experience asthma exacerbations in pregnancy are at increased risk for preeclampsia, [pregnancy-induced hypertension], low birth weight, and preterm delivery and may require closer monitoring.”
The study was supported by the Institute for Clinical Evaluative Sciences. The researchers and Dr. Krishna had no financial conflicts to disclose.
SOURCE: Abdullah K et al. Eur Respir J. 2019 Nov 26. doi: 10.1183/13993003.01335-2019.
FROM THE EUROPEAN RESPIRATORY JOURNAL
EVALI outbreak ongoing, but new cases decline
The vaping lung disease outbreak continues, but according to the Centers for Disease Control and Prevention, it may have peaked and the number of new hospitalized cases reported to the CDC may be decreasing.
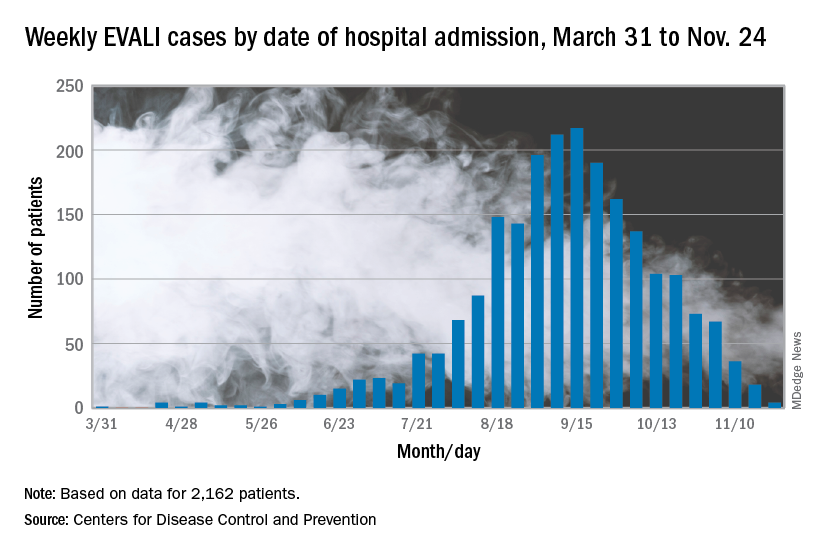
In the Dec. 6, 2019, Morbidity and Mortality Weekly Report, the CDC has updated information about cases of e-cigarette, or vaping, product use–associated lung injury (EVALI): As of Dec. 3, there have been 2,291 cases reported from all 50 states, Washington, D.C., and two U.S. territories (Puerto Rico and U.S. Virgin Islands). A total of 48 deaths have been confirmed in 25 states and Washington, D.C., the CDC reported.
The largest number of weekly hospitalized cases occurred during the week of Sept. 15, 2019; since then, hospitalized cases have steadily declined. “Among all hospitalized EVALI patients reported to CDC weekly, the percentage of recent cases (patients hospitalized within the preceding 3 weeks) declined from 58% reported November 12 to 30% reported December 3,” the report stated.
About 80%of hospitalized EVALI patients reported using tetrahydrocannabinol (THC)–containing e-cigarette, or vaping, products. “Dank Vapes,” counterfeit THC-containing products of unknown origin, were the most commonly reported THC-containing branded products used. Dank Vapes were used by 56% of hospitalized EVALI patients nationwide, followed by TKO brand (15%), Smart Cart (13%), and Rove (12%).
Of EVALI patients for whom data were available, 67% were male, and the median age was 24 years (range, 13-77 years); 78% were aged under 35 years and 16% were under 18 years. About 75% of EVALI patients were non-Hispanic white and 16% were Hispanic. Among the 48 deaths, 54% of patients were male, and the median age was 52 years (range, 17-75 years).
CDC research on EVALI continues to be limited by the self-reported data, lack of data on substances used, missing data, loss to follow-up, and reporting lags, but the intensive investigation and data collection is ongoing.
The report concludes: “While the investigation continues, persons should consider refraining from the use of all e-cigarette, or vaping, products. Adults using e-cigarette, or vaping, products to quit smoking should not return to smoking cigarettes; they should weigh all risks and benefits and consider using [Food and Drug Administration]–approved cessation medications. Adults who continue to use e-cigarette, or vaping, products should carefully monitor themselves for symptoms and see a health care provider immediately if they develop symptoms similar to those reported in this outbreak. Irrespective of the ongoing investigation, e-cigarette, or vaping, products should never be used by youths, young adults or pregnant women.”
Information on the current investigation, reporting of cases, and other resources can be found on the CDC website.
SOURCE: Lozier MJ et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 6. doi: 10.15585/mmwr.mm6849e1.
The vaping lung disease outbreak continues, but according to the Centers for Disease Control and Prevention, it may have peaked and the number of new hospitalized cases reported to the CDC may be decreasing.
In the Dec. 6, 2019, Morbidity and Mortality Weekly Report, the CDC has updated information about cases of e-cigarette, or vaping, product use–associated lung injury (EVALI): As of Dec. 3, there have been 2,291 cases reported from all 50 states, Washington, D.C., and two U.S. territories (Puerto Rico and U.S. Virgin Islands). A total of 48 deaths have been confirmed in 25 states and Washington, D.C., the CDC reported.
The largest number of weekly hospitalized cases occurred during the week of Sept. 15, 2019; since then, hospitalized cases have steadily declined. “Among all hospitalized EVALI patients reported to CDC weekly, the percentage of recent cases (patients hospitalized within the preceding 3 weeks) declined from 58% reported November 12 to 30% reported December 3,” the report stated.
About 80%of hospitalized EVALI patients reported using tetrahydrocannabinol (THC)–containing e-cigarette, or vaping, products. “Dank Vapes,” counterfeit THC-containing products of unknown origin, were the most commonly reported THC-containing branded products used. Dank Vapes were used by 56% of hospitalized EVALI patients nationwide, followed by TKO brand (15%), Smart Cart (13%), and Rove (12%).
Of EVALI patients for whom data were available, 67% were male, and the median age was 24 years (range, 13-77 years); 78% were aged under 35 years and 16% were under 18 years. About 75% of EVALI patients were non-Hispanic white and 16% were Hispanic. Among the 48 deaths, 54% of patients were male, and the median age was 52 years (range, 17-75 years).

CDC research on EVALI continues to be limited by the self-reported data, lack of data on substances used, missing data, loss to follow-up, and reporting lags, but the intensive investigation and data collection is ongoing.
The report concludes: “While the investigation continues, persons should consider refraining from the use of all e-cigarette, or vaping, products. Adults using e-cigarette, or vaping, products to quit smoking should not return to smoking cigarettes; they should weigh all risks and benefits and consider using [Food and Drug Administration]–approved cessation medications. Adults who continue to use e-cigarette, or vaping, products should carefully monitor themselves for symptoms and see a health care provider immediately if they develop symptoms similar to those reported in this outbreak. Irrespective of the ongoing investigation, e-cigarette, or vaping, products should never be used by youths, young adults or pregnant women.”
Information on the current investigation, reporting of cases, and other resources can be found on the CDC website.
SOURCE: Lozier MJ et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 6. doi: 10.15585/mmwr.mm6849e1.
The vaping lung disease outbreak continues, but according to the Centers for Disease Control and Prevention, it may have peaked and the number of new hospitalized cases reported to the CDC may be decreasing.
In the Dec. 6, 2019, Morbidity and Mortality Weekly Report, the CDC has updated information about cases of e-cigarette, or vaping, product use–associated lung injury (EVALI): As of Dec. 3, there have been 2,291 cases reported from all 50 states, Washington, D.C., and two U.S. territories (Puerto Rico and U.S. Virgin Islands). A total of 48 deaths have been confirmed in 25 states and Washington, D.C., the CDC reported.
The largest number of weekly hospitalized cases occurred during the week of Sept. 15, 2019; since then, hospitalized cases have steadily declined. “Among all hospitalized EVALI patients reported to CDC weekly, the percentage of recent cases (patients hospitalized within the preceding 3 weeks) declined from 58% reported November 12 to 30% reported December 3,” the report stated.
About 80%of hospitalized EVALI patients reported using tetrahydrocannabinol (THC)–containing e-cigarette, or vaping, products. “Dank Vapes,” counterfeit THC-containing products of unknown origin, were the most commonly reported THC-containing branded products used. Dank Vapes were used by 56% of hospitalized EVALI patients nationwide, followed by TKO brand (15%), Smart Cart (13%), and Rove (12%).
Of EVALI patients for whom data were available, 67% were male, and the median age was 24 years (range, 13-77 years); 78% were aged under 35 years and 16% were under 18 years. About 75% of EVALI patients were non-Hispanic white and 16% were Hispanic. Among the 48 deaths, 54% of patients were male, and the median age was 52 years (range, 17-75 years).

CDC research on EVALI continues to be limited by the self-reported data, lack of data on substances used, missing data, loss to follow-up, and reporting lags, but the intensive investigation and data collection is ongoing.
The report concludes: “While the investigation continues, persons should consider refraining from the use of all e-cigarette, or vaping, products. Adults using e-cigarette, or vaping, products to quit smoking should not return to smoking cigarettes; they should weigh all risks and benefits and consider using [Food and Drug Administration]–approved cessation medications. Adults who continue to use e-cigarette, or vaping, products should carefully monitor themselves for symptoms and see a health care provider immediately if they develop symptoms similar to those reported in this outbreak. Irrespective of the ongoing investigation, e-cigarette, or vaping, products should never be used by youths, young adults or pregnant women.”
Information on the current investigation, reporting of cases, and other resources can be found on the CDC website.
SOURCE: Lozier MJ et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 6. doi: 10.15585/mmwr.mm6849e1.
FROM THE MMWR
E-cigarette use, interest in flavors remains high among youth
, according to new findings from the Centers for Disease Control and Prevention.
Just over half of high school students and about a quarter of middle school students have ever tried a tobacco product, and more than a third of students have ever tried an e-cigarette, according to results from the 2019 National Youth Tobacco Survey. These results were published in the Morbidity and Mortality Weekly Report on Dec. 6.
Adolescent cigarette smoking rates have continued their decline, hitting their lowest rate ever in 2019, but e-cigarette use, or “vaping,” has continued to increase. E-cigarette use surpassed that of all other tobacco products in 2014 and has remained the most common—as well as the least likely to be perceived as harmful, researchers reported.
“Although most current youth tobacco product users are not daily users, estimates of frequent e-cigarette use among high school students were comparable to those observed for cigarette and smokeless tobacco product users in 2019,” wrote Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, and associates at the CDC and Food and Drug Administration. “Youth use of tobacco products in any form is unsafe, regardless of whether the products are smoked, smokeless, or electronic.”
The high prevalence of e-cigarette use was no surprise to Karen Wilson, MD, chief of the division of general pediatrics at the Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital, New York, and chair of the American Academy of Pediatrics’ Tobacco Consortium.
“It also fits with what we’re seeing anecdotally,” Dr. Wilson said in an interview. “We hear the statistic that 30% of high school students are using them, but high school students will say it’s much more than that.”
It’s therefore important for physicians to be proactive in talking to youth about these products. “They should absolutely be screening for vaping and know all about the different products,” including JUUL, Suorin, nicotine toothpicks, and candies and other products, Dr. Wilson said. “Pediatricians need to be asking their teenagers open-ended questions about what are kids using now.”
The American Academy of Pediatrics has resources available to help pediatricians and families of youth using e-cigarettes and vaping devices, she added.
Main findings
The researchers reported data from the annual, cross-sectional National Youth Tobacco Survey, administered to U.S. students in public and private schools in all 50 states and the District of Columbia. The results were divided into middle school (grades 6-8) and high school (grades 9-12) from 251 participating schools between February 2019 and May 2019.
The survey has been done using pencil and paper questionnaires since it began in 1999, but this year’s surveys were digital for the first time. Among the 19,018 questionnaires completed (student response rate 85.3%), 8,837 were middle school and 10,097 were high school. The weighted analysis of results represents 27 million students: 11.9 million in middle school and 15 million in high school.
More than half (53.3%) of high school students reported ever having tried a tobacco product, and 31.2% reported having used one in the past 30 days. In middle school, 24.3% of students reported ever using a tobacco product, and 12.5% have used one in the past month.
Tobacco products include cigarettes (traditional/combusted), electronic cigarettes, cigars, smokeless tobacco, hookahs, pipe tobacco, and bidis, which are small brown cigarettes wrapped in leaves. Among the electronic tobacco products mentioned in the survey were NJOY, Blu, Vuse, MarkTen, Logic, Vapin Plus, eGo and Halo.
The most common product for youth to try was e-cigarettes, which 35% of middle and high school students had ever tried. Just under a quarter of students (23%) had used a tobacco product in the past month, and e-cigarettes were again the most commonly used overall by that group, cited by 20% of recent users. Cigars (5.3%), cigarettes (4.3%), smokeless tobacco (3.5%), hookahs (2.6%) and pipes (under 1%) were used much less frequently.
Frequent use, defined as at least 20 of the previous 30 days, was most common among youth using smokeless tobacco (34.1% of current users) and e-cigarettes (30.4%) and least common among cigar smokers (16.8%). Among those currently using any tobacco product, 24.7% said they had cravings for a product within the past month, and 13.7% wanted to use it within a half hour of waking up.
More than half of those who currently used any tobacco products (57.8%) were seriously considering quitting, and a similar proportion (57.5%) had stopped using all tobacco products for at least 1 day in an attempt to quit.
“Many [adolescents] will tell you they will use it until they don’t have the availability of getting it,” Dr. Wilson said. “The problem is that they’re becoming so addicted to the high-nicotine products that they’re going farther and farther out of their way to try to get these products so that they can satisfy their addictions.”
Policies restricting access, such as increasing the age for sales to 21 and increasing taxes on products, can reduce tobacco use among youth, Dr. Wilson said.
“It will encourage teenagers to get help for their addiction by using FDA-approved devices or nicotine replacement therapy and behavioral interventions rather than relying on an unproven and potentially dangerous product,” she said.
Reasons for use, flavor, and harm perception
The most common flavored tobacco product used among youth was e-cigarettes, reported by 68.8% of current e-cigarette users, followed by smokeless tobacco (48%), cigarettes (46.7%, only menthol), cigars, pipe tobacco, and hookahs.
The top reasons youth cited for trying e-cigarettes were curiosity (55.3%), a friend or family member’s use (30.8%), and their availability in a wide range of flavors (22.4%). Almost as popular as flavor availability was e-cigarette users’ interest in doing “tricks” with the product (21.2%).
The cross-sectional questionnaire method of the study precluded the ability to draw conclusions about why students might perceive a particular tobacco product as more or less harmful. However, public health officials have expressed concern that flavors reduce the perceived harm that can come from the products. Dr. Wilson said the attraction to e-cigarette flavors is “huge.”
“If electronic cigarettes were only available in tobacco flavor, I do not believe that many teenagers at all would try them,” Dr. Wilson said. “They think because they’re sweet and flavored that they actually aren’t harmful. It makes the kids think these are safe products.”
More than one in four students (28.2%) perceived intermittent e-cigarette use as causing little to no harm, and only 16.4% similarly saw little or no harm from intermittent hookah use, compared with 11.5% for smokeless tobacco and 9.5% for cigarettes. Less than a third of respondents (32.3%) saw intermittent e-cigarette use as causing a lot of harm, compared with much higher percentages for cigarettes (54.9%) and smokeless tobacco (52.5%).
Part of the problem with harm perception is the narrative promoted by e-cigarette companies, Dr. Wilson said.
“From the very beginning, they started with a campaign that called this harmless water vapor, which it is absolutely not,” she said. “It’s an aerosol of toxic chemicals and nicotine, which is addictive. We know that nicotine that can impact scores of cognitive tests and impulsivity. We have no idea what these really high levels [of nicotine] will do.”
Further, potential long-term harm is still an open question, she pointed out.
“We also know that these are particulates and toxins that are being inhaled into the lungs,” Dr. Wilson said. “We know they have some impact on asthma, and we don’t know what the impact is for using for 10 or 20 years.”
Curiosity about e-cigarettes and about traditional cigarettes were prevalent in similar proportions among youth who had never tried a tobacco product: 39.1% of never-users were curious about e-cigarettes, and 37% about traditional cigarettes. In addition to curiosity, researchers assess susceptibility among those who have never tried a tobacco product and found nearly identical susceptibility to e-cigarettes (45%) and traditional cigarettes (45.9%).
The survey also asked students about their exposure to tobacco advertising or promotions from a wide range of sources: convenience stores, supermarkets, gas stations, the Internet, television, video streaming, cinemas, and newspapers or magazines. Among the students who reported going to these sources, 69.3% had seen e-cigarette marketing, and 81.7% had seen marketing for other tobacco products, including cigarettes.
SOURCE: Wang TW et al. MMWR Surveill Summ. 2019 Nov 6;68(12):1-22. doi: 10.15585/mmwr.ss6812a1.
, according to new findings from the Centers for Disease Control and Prevention.
Just over half of high school students and about a quarter of middle school students have ever tried a tobacco product, and more than a third of students have ever tried an e-cigarette, according to results from the 2019 National Youth Tobacco Survey. These results were published in the Morbidity and Mortality Weekly Report on Dec. 6.
Adolescent cigarette smoking rates have continued their decline, hitting their lowest rate ever in 2019, but e-cigarette use, or “vaping,” has continued to increase. E-cigarette use surpassed that of all other tobacco products in 2014 and has remained the most common—as well as the least likely to be perceived as harmful, researchers reported.
“Although most current youth tobacco product users are not daily users, estimates of frequent e-cigarette use among high school students were comparable to those observed for cigarette and smokeless tobacco product users in 2019,” wrote Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, and associates at the CDC and Food and Drug Administration. “Youth use of tobacco products in any form is unsafe, regardless of whether the products are smoked, smokeless, or electronic.”
The high prevalence of e-cigarette use was no surprise to Karen Wilson, MD, chief of the division of general pediatrics at the Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital, New York, and chair of the American Academy of Pediatrics’ Tobacco Consortium.
“It also fits with what we’re seeing anecdotally,” Dr. Wilson said in an interview. “We hear the statistic that 30% of high school students are using them, but high school students will say it’s much more than that.”
It’s therefore important for physicians to be proactive in talking to youth about these products. “They should absolutely be screening for vaping and know all about the different products,” including JUUL, Suorin, nicotine toothpicks, and candies and other products, Dr. Wilson said. “Pediatricians need to be asking their teenagers open-ended questions about what are kids using now.”
The American Academy of Pediatrics has resources available to help pediatricians and families of youth using e-cigarettes and vaping devices, she added.
Main findings
The researchers reported data from the annual, cross-sectional National Youth Tobacco Survey, administered to U.S. students in public and private schools in all 50 states and the District of Columbia. The results were divided into middle school (grades 6-8) and high school (grades 9-12) from 251 participating schools between February 2019 and May 2019.
The survey has been done using pencil and paper questionnaires since it began in 1999, but this year’s surveys were digital for the first time. Among the 19,018 questionnaires completed (student response rate 85.3%), 8,837 were middle school and 10,097 were high school. The weighted analysis of results represents 27 million students: 11.9 million in middle school and 15 million in high school.
More than half (53.3%) of high school students reported ever having tried a tobacco product, and 31.2% reported having used one in the past 30 days. In middle school, 24.3% of students reported ever using a tobacco product, and 12.5% have used one in the past month.
Tobacco products include cigarettes (traditional/combusted), electronic cigarettes, cigars, smokeless tobacco, hookahs, pipe tobacco, and bidis, which are small brown cigarettes wrapped in leaves. Among the electronic tobacco products mentioned in the survey were NJOY, Blu, Vuse, MarkTen, Logic, Vapin Plus, eGo and Halo.
The most common product for youth to try was e-cigarettes, which 35% of middle and high school students had ever tried. Just under a quarter of students (23%) had used a tobacco product in the past month, and e-cigarettes were again the most commonly used overall by that group, cited by 20% of recent users. Cigars (5.3%), cigarettes (4.3%), smokeless tobacco (3.5%), hookahs (2.6%) and pipes (under 1%) were used much less frequently.
Frequent use, defined as at least 20 of the previous 30 days, was most common among youth using smokeless tobacco (34.1% of current users) and e-cigarettes (30.4%) and least common among cigar smokers (16.8%). Among those currently using any tobacco product, 24.7% said they had cravings for a product within the past month, and 13.7% wanted to use it within a half hour of waking up.
More than half of those who currently used any tobacco products (57.8%) were seriously considering quitting, and a similar proportion (57.5%) had stopped using all tobacco products for at least 1 day in an attempt to quit.
“Many [adolescents] will tell you they will use it until they don’t have the availability of getting it,” Dr. Wilson said. “The problem is that they’re becoming so addicted to the high-nicotine products that they’re going farther and farther out of their way to try to get these products so that they can satisfy their addictions.”
Policies restricting access, such as increasing the age for sales to 21 and increasing taxes on products, can reduce tobacco use among youth, Dr. Wilson said.
“It will encourage teenagers to get help for their addiction by using FDA-approved devices or nicotine replacement therapy and behavioral interventions rather than relying on an unproven and potentially dangerous product,” she said.
Reasons for use, flavor, and harm perception
The most common flavored tobacco product used among youth was e-cigarettes, reported by 68.8% of current e-cigarette users, followed by smokeless tobacco (48%), cigarettes (46.7%, only menthol), cigars, pipe tobacco, and hookahs.
The top reasons youth cited for trying e-cigarettes were curiosity (55.3%), a friend or family member’s use (30.8%), and their availability in a wide range of flavors (22.4%). Almost as popular as flavor availability was e-cigarette users’ interest in doing “tricks” with the product (21.2%).
The cross-sectional questionnaire method of the study precluded the ability to draw conclusions about why students might perceive a particular tobacco product as more or less harmful. However, public health officials have expressed concern that flavors reduce the perceived harm that can come from the products. Dr. Wilson said the attraction to e-cigarette flavors is “huge.”
“If electronic cigarettes were only available in tobacco flavor, I do not believe that many teenagers at all would try them,” Dr. Wilson said. “They think because they’re sweet and flavored that they actually aren’t harmful. It makes the kids think these are safe products.”
More than one in four students (28.2%) perceived intermittent e-cigarette use as causing little to no harm, and only 16.4% similarly saw little or no harm from intermittent hookah use, compared with 11.5% for smokeless tobacco and 9.5% for cigarettes. Less than a third of respondents (32.3%) saw intermittent e-cigarette use as causing a lot of harm, compared with much higher percentages for cigarettes (54.9%) and smokeless tobacco (52.5%).
Part of the problem with harm perception is the narrative promoted by e-cigarette companies, Dr. Wilson said.
“From the very beginning, they started with a campaign that called this harmless water vapor, which it is absolutely not,” she said. “It’s an aerosol of toxic chemicals and nicotine, which is addictive. We know that nicotine that can impact scores of cognitive tests and impulsivity. We have no idea what these really high levels [of nicotine] will do.”
Further, potential long-term harm is still an open question, she pointed out.
“We also know that these are particulates and toxins that are being inhaled into the lungs,” Dr. Wilson said. “We know they have some impact on asthma, and we don’t know what the impact is for using for 10 or 20 years.”
Curiosity about e-cigarettes and about traditional cigarettes were prevalent in similar proportions among youth who had never tried a tobacco product: 39.1% of never-users were curious about e-cigarettes, and 37% about traditional cigarettes. In addition to curiosity, researchers assess susceptibility among those who have never tried a tobacco product and found nearly identical susceptibility to e-cigarettes (45%) and traditional cigarettes (45.9%).
The survey also asked students about their exposure to tobacco advertising or promotions from a wide range of sources: convenience stores, supermarkets, gas stations, the Internet, television, video streaming, cinemas, and newspapers or magazines. Among the students who reported going to these sources, 69.3% had seen e-cigarette marketing, and 81.7% had seen marketing for other tobacco products, including cigarettes.
SOURCE: Wang TW et al. MMWR Surveill Summ. 2019 Nov 6;68(12):1-22. doi: 10.15585/mmwr.ss6812a1.
, according to new findings from the Centers for Disease Control and Prevention.
Just over half of high school students and about a quarter of middle school students have ever tried a tobacco product, and more than a third of students have ever tried an e-cigarette, according to results from the 2019 National Youth Tobacco Survey. These results were published in the Morbidity and Mortality Weekly Report on Dec. 6.
Adolescent cigarette smoking rates have continued their decline, hitting their lowest rate ever in 2019, but e-cigarette use, or “vaping,” has continued to increase. E-cigarette use surpassed that of all other tobacco products in 2014 and has remained the most common—as well as the least likely to be perceived as harmful, researchers reported.
“Although most current youth tobacco product users are not daily users, estimates of frequent e-cigarette use among high school students were comparable to those observed for cigarette and smokeless tobacco product users in 2019,” wrote Teresa W. Wang, PhD, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, and associates at the CDC and Food and Drug Administration. “Youth use of tobacco products in any form is unsafe, regardless of whether the products are smoked, smokeless, or electronic.”
The high prevalence of e-cigarette use was no surprise to Karen Wilson, MD, chief of the division of general pediatrics at the Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital, New York, and chair of the American Academy of Pediatrics’ Tobacco Consortium.
“It also fits with what we’re seeing anecdotally,” Dr. Wilson said in an interview. “We hear the statistic that 30% of high school students are using them, but high school students will say it’s much more than that.”
It’s therefore important for physicians to be proactive in talking to youth about these products. “They should absolutely be screening for vaping and know all about the different products,” including JUUL, Suorin, nicotine toothpicks, and candies and other products, Dr. Wilson said. “Pediatricians need to be asking their teenagers open-ended questions about what are kids using now.”
The American Academy of Pediatrics has resources available to help pediatricians and families of youth using e-cigarettes and vaping devices, she added.
Main findings
The researchers reported data from the annual, cross-sectional National Youth Tobacco Survey, administered to U.S. students in public and private schools in all 50 states and the District of Columbia. The results were divided into middle school (grades 6-8) and high school (grades 9-12) from 251 participating schools between February 2019 and May 2019.
The survey has been done using pencil and paper questionnaires since it began in 1999, but this year’s surveys were digital for the first time. Among the 19,018 questionnaires completed (student response rate 85.3%), 8,837 were middle school and 10,097 were high school. The weighted analysis of results represents 27 million students: 11.9 million in middle school and 15 million in high school.
More than half (53.3%) of high school students reported ever having tried a tobacco product, and 31.2% reported having used one in the past 30 days. In middle school, 24.3% of students reported ever using a tobacco product, and 12.5% have used one in the past month.
Tobacco products include cigarettes (traditional/combusted), electronic cigarettes, cigars, smokeless tobacco, hookahs, pipe tobacco, and bidis, which are small brown cigarettes wrapped in leaves. Among the electronic tobacco products mentioned in the survey were NJOY, Blu, Vuse, MarkTen, Logic, Vapin Plus, eGo and Halo.
The most common product for youth to try was e-cigarettes, which 35% of middle and high school students had ever tried. Just under a quarter of students (23%) had used a tobacco product in the past month, and e-cigarettes were again the most commonly used overall by that group, cited by 20% of recent users. Cigars (5.3%), cigarettes (4.3%), smokeless tobacco (3.5%), hookahs (2.6%) and pipes (under 1%) were used much less frequently.
Frequent use, defined as at least 20 of the previous 30 days, was most common among youth using smokeless tobacco (34.1% of current users) and e-cigarettes (30.4%) and least common among cigar smokers (16.8%). Among those currently using any tobacco product, 24.7% said they had cravings for a product within the past month, and 13.7% wanted to use it within a half hour of waking up.
More than half of those who currently used any tobacco products (57.8%) were seriously considering quitting, and a similar proportion (57.5%) had stopped using all tobacco products for at least 1 day in an attempt to quit.
“Many [adolescents] will tell you they will use it until they don’t have the availability of getting it,” Dr. Wilson said. “The problem is that they’re becoming so addicted to the high-nicotine products that they’re going farther and farther out of their way to try to get these products so that they can satisfy their addictions.”
Policies restricting access, such as increasing the age for sales to 21 and increasing taxes on products, can reduce tobacco use among youth, Dr. Wilson said.
“It will encourage teenagers to get help for their addiction by using FDA-approved devices or nicotine replacement therapy and behavioral interventions rather than relying on an unproven and potentially dangerous product,” she said.
Reasons for use, flavor, and harm perception
The most common flavored tobacco product used among youth was e-cigarettes, reported by 68.8% of current e-cigarette users, followed by smokeless tobacco (48%), cigarettes (46.7%, only menthol), cigars, pipe tobacco, and hookahs.
The top reasons youth cited for trying e-cigarettes were curiosity (55.3%), a friend or family member’s use (30.8%), and their availability in a wide range of flavors (22.4%). Almost as popular as flavor availability was e-cigarette users’ interest in doing “tricks” with the product (21.2%).
The cross-sectional questionnaire method of the study precluded the ability to draw conclusions about why students might perceive a particular tobacco product as more or less harmful. However, public health officials have expressed concern that flavors reduce the perceived harm that can come from the products. Dr. Wilson said the attraction to e-cigarette flavors is “huge.”
“If electronic cigarettes were only available in tobacco flavor, I do not believe that many teenagers at all would try them,” Dr. Wilson said. “They think because they’re sweet and flavored that they actually aren’t harmful. It makes the kids think these are safe products.”
More than one in four students (28.2%) perceived intermittent e-cigarette use as causing little to no harm, and only 16.4% similarly saw little or no harm from intermittent hookah use, compared with 11.5% for smokeless tobacco and 9.5% for cigarettes. Less than a third of respondents (32.3%) saw intermittent e-cigarette use as causing a lot of harm, compared with much higher percentages for cigarettes (54.9%) and smokeless tobacco (52.5%).
Part of the problem with harm perception is the narrative promoted by e-cigarette companies, Dr. Wilson said.
“From the very beginning, they started with a campaign that called this harmless water vapor, which it is absolutely not,” she said. “It’s an aerosol of toxic chemicals and nicotine, which is addictive. We know that nicotine that can impact scores of cognitive tests and impulsivity. We have no idea what these really high levels [of nicotine] will do.”
Further, potential long-term harm is still an open question, she pointed out.
“We also know that these are particulates and toxins that are being inhaled into the lungs,” Dr. Wilson said. “We know they have some impact on asthma, and we don’t know what the impact is for using for 10 or 20 years.”
Curiosity about e-cigarettes and about traditional cigarettes were prevalent in similar proportions among youth who had never tried a tobacco product: 39.1% of never-users were curious about e-cigarettes, and 37% about traditional cigarettes. In addition to curiosity, researchers assess susceptibility among those who have never tried a tobacco product and found nearly identical susceptibility to e-cigarettes (45%) and traditional cigarettes (45.9%).
The survey also asked students about their exposure to tobacco advertising or promotions from a wide range of sources: convenience stores, supermarkets, gas stations, the Internet, television, video streaming, cinemas, and newspapers or magazines. Among the students who reported going to these sources, 69.3% had seen e-cigarette marketing, and 81.7% had seen marketing for other tobacco products, including cigarettes.
SOURCE: Wang TW et al. MMWR Surveill Summ. 2019 Nov 6;68(12):1-22. doi: 10.15585/mmwr.ss6812a1.
FROM THE MMWR
When guideline treatment of asthma fails, consider a macrolide antibiotic
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3
This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29
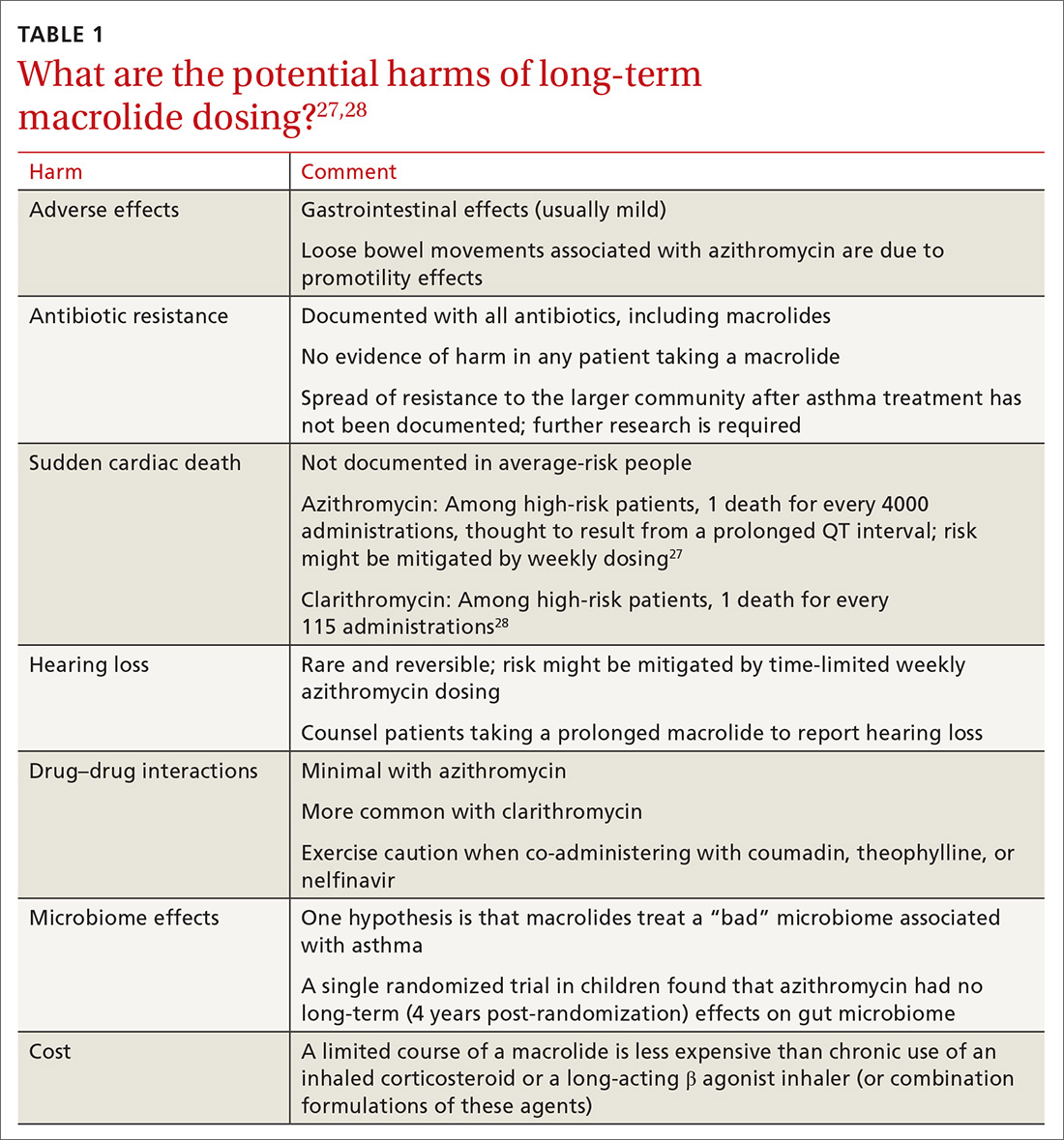
Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.
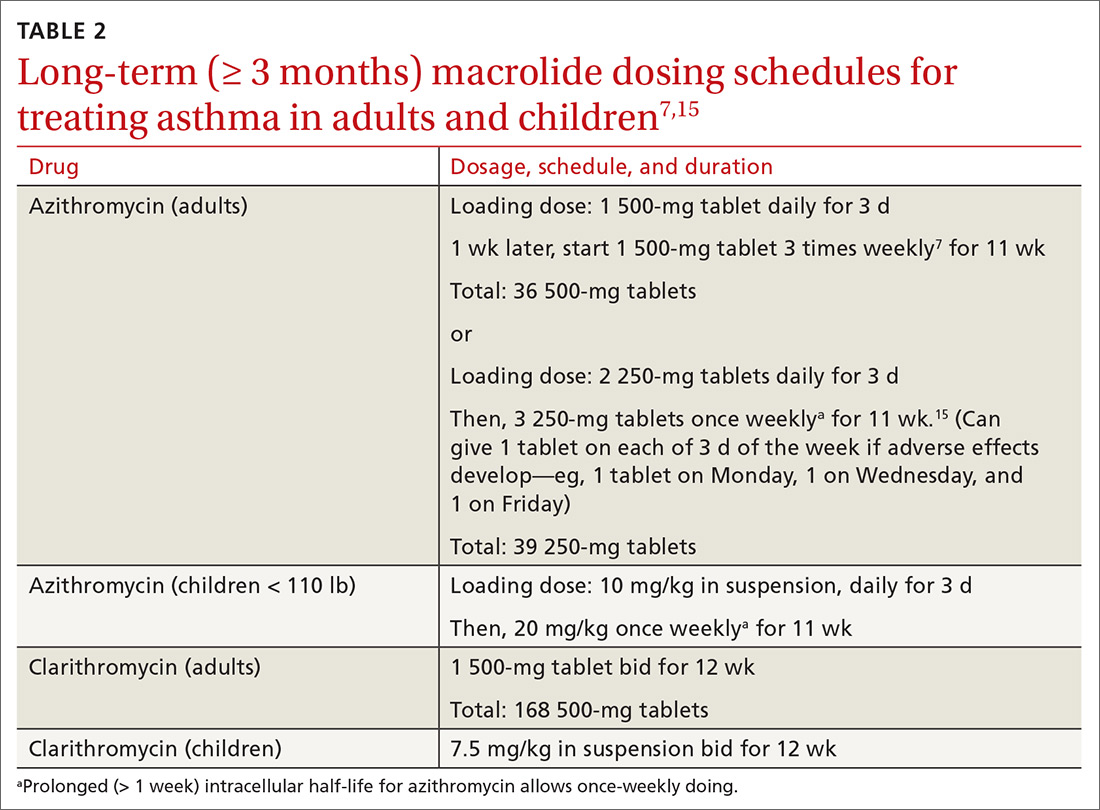
Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3

This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29

Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.

Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3

This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29

Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.

Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
PRACTICE RECOMMENDATIONS
› Consider a trial of azithromycin for patients who have poorly controlled persistent asthma and are not responding to guideline treatment with the combination of an inhaled corticosteroid and either a long-acting bronchodilator or long-acting muscarinic antagonist. B
› Consider a trial of azithromycin in addition to first-line guideline therapy for patients who have new-onset asthma. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
An update on the current standard for ultrasound education in fellowship
Point-of-care ultrasound (POCUS) is an essential part of ICU care. It has been demonstrated to improve patient safety and outcomes through procedural guidance (Brass P, et al. Cochrane Database Syst Rev. 2015 Jan 9;1:CD006962) and aid in accurate and timely diagnosis of cardiopulmonary failure (Lichtenstein DA, Mezière GA. Chest. 2008 Jul;134[1]:117-25). Due in part to increasing affordability and portability of ultrasound technologies, the use of POCUS has become seemingly ubiquitous and will continue to increase in coming years. According to expert groups representing 12 critical care societies worldwide, general critical care ultrasound and basic critical care echocardiography should be mandatory training for ICU physicians (Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37[7]:1077-83).
Currently, POCUS is not universally taught to pulmonary and critical care fellows (PCCM); and when training does exist, curriculums are not standardized. This is in part due to the broadly worded requirements set forth from the ACGME for pulmonary disease and critical care medicine. The totality of ACGME common program requirements as it regards to ultrasound training are as follows: 1. “Fellows must demonstrate competence in procedural and technical skills, including ... use of ultrasound techniques to perform thoracentesis and place intravascular and intracavitary tubes and catheters”; and 2. “Fellows must demonstrate knowledge of imaging techniques commonly employed in the evaluation of patients with pulmonary disease or critical illness, including the use of ultrasound” (ACGME Program Requirements for Graduate Medical Education in Pulmonary Disease and Critical Care Medicine).
In comparison, recently updated ACGME common program requirements for ultrasound in emergency medicine and anesthesiology residencies are robust and detailed. Requirements for anesthesia residency training include: ” ... competency in using surface ultrasound ... and transthoracic echocardiography to guide the performance of invasive procedures and to evaluate organ function and pathology ... understanding the principles of ultrasound, including the physics of ultrasound transmission, ultrasound transducer construction, and transducer selection for specific applications, to include being able to obtain images with an understanding of limitations and artifacts ... obtaining standard views of the heart and inferior vena cava with transthoracic echocardiography allowing the evaluation of myocardial function, estimation of central venous pressure, and gross pericardial/cardiac pathology (eg, large pericardial effusion) ... using transthoracic ultrasound for the detection of pneumothorax and pleural effusion ... using surface ultrasound to guide vascular access (both central and peripheral) ... describing techniques, views, and findings in standard language” (ACGME Program Requirements for Graduate Medical Education In Anesthesiology).
Herein lies a stark contrast in what is required of programs that train physicians to care for unstable patients and the critically ill. Current requirements leave graduates of PCCM training programs vulnerable to completing ACGME milestones without being adequately prepared to evaluate patients in a modern ICU setting. Increasingly, hospitals credentialing committees expect PCCM graduates to be suitably trained in ultrasound. Regrettably, there is no assurance that is true, or standardized, with current PCCM fellowship training requirements.
There is not a national standard for competency assessment or requirements for credentialing in POCUS for critical care physicians at this time. However, multiple national and international critical care societies, including CHEST, have consensus statements and recommendations outlining the areas of competence expected in critical care ultrasound (Mayo PH, et al. Chest. 2009 Apr;135[4];1050-60, Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37(7):1077-83). The PCCM ACGME requirements should be updated to reflect such recommendations, thereby placing greater emphasis on ultrasound teaching requirements and standardized curriculums. Despite the current ACGME program requirements, it is incumbent upon critical care training programs to provide competency-based education of this now “standard of care” technology.
Barriers to universal POCUS training exist. Fellowship programs may lack trained, ultrasound confident faculty, time, and funding to successfully develop and sustain an ultrasound curriculum. (Eisen LA, et al. Crit Care Med. 2010;38[10]:1978-83; Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print].)
Although access to adequate quality and quantity of ultrasound machines is less often a problem than in the past, many institutions lack archival and image review software that allows for quality assurance of image acquisition, and some still may not have a faculty member with expertise and ability to champion the cause.
In attempts to mitigate the local faculty gaps, national and regional solutions have been developed for ultrasonography education. CHEST has educated more than 1,400 learners in the Ultrasound Essentials course since 2013. Also, grassroots efforts have led to the development of courses specifically designed to teach incoming PCCM fellows. Using a collaborative and cost-effective model, these regional programs pool faculty and experts in the field to train multiple fellowship programs simultaneously. The first of these was created over a decade ago in New York City (Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12:[Epub ahead of print].)
Currently, there are at least four regional annual ultrasound courses directed at teaching PCCM fellows. These courses are typically held over multiple days and encompass the basics of critical care ultrasound, including vascular, thoracic, abdominal, cardiac, and procedural imaging. By estimation, these four courses provide a basic ultrasonography education to approximately two-thirds of first year pulmonary and critical care fellows in the United States. In addition to training fellows, these programs also serve as a platform for the development of local faculty experts, so that training can continue at their institutions.
Introductory courses are highly effective (Dinh VA, et al. Crit Care Res Pract. 2015 Aug 5:675041 Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print]), but ongoing education, assessment, and quality assurance is required to achieve sustained competence. Ideally, training in POCUS should entail a dedicated, intensive introduction to the competencies of critical care ultrasound (such as the above regional courses or CHEST ultrasound courses), followed by a formal curriculum within the PCCM fellowship programs. This curriculum should afford the trainee exposure to critically ill patients in an environment with adequate ultrasound equipment and a method to record studies. The trainee then interprets the acquired studies in clinical context. Preferably, the program will afford the trainee real-time quality assurance for image acquisition and interpretation by a program champion. Quality assurance can be provided on site or remotely using fixed interval review sessions. Lastly, the program should have internal milestones to evaluate when a trainee has reached competency to perform these tasks independently. The completion of training should include a letter to any future employee attesting to the trainee’s acquisition of these skills and ability to apply them safely while caring for the critically ill. This robust education is occurring in many centers across the country. PCCM fellowship programs owe it to their trainees, and patients, that competency-based critical care ultrasound training is robust, standardized, and supported.
Point-of-care ultrasound (POCUS) is an essential part of ICU care. It has been demonstrated to improve patient safety and outcomes through procedural guidance (Brass P, et al. Cochrane Database Syst Rev. 2015 Jan 9;1:CD006962) and aid in accurate and timely diagnosis of cardiopulmonary failure (Lichtenstein DA, Mezière GA. Chest. 2008 Jul;134[1]:117-25). Due in part to increasing affordability and portability of ultrasound technologies, the use of POCUS has become seemingly ubiquitous and will continue to increase in coming years. According to expert groups representing 12 critical care societies worldwide, general critical care ultrasound and basic critical care echocardiography should be mandatory training for ICU physicians (Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37[7]:1077-83).
Currently, POCUS is not universally taught to pulmonary and critical care fellows (PCCM); and when training does exist, curriculums are not standardized. This is in part due to the broadly worded requirements set forth from the ACGME for pulmonary disease and critical care medicine. The totality of ACGME common program requirements as it regards to ultrasound training are as follows: 1. “Fellows must demonstrate competence in procedural and technical skills, including ... use of ultrasound techniques to perform thoracentesis and place intravascular and intracavitary tubes and catheters”; and 2. “Fellows must demonstrate knowledge of imaging techniques commonly employed in the evaluation of patients with pulmonary disease or critical illness, including the use of ultrasound” (ACGME Program Requirements for Graduate Medical Education in Pulmonary Disease and Critical Care Medicine).
In comparison, recently updated ACGME common program requirements for ultrasound in emergency medicine and anesthesiology residencies are robust and detailed. Requirements for anesthesia residency training include: ” ... competency in using surface ultrasound ... and transthoracic echocardiography to guide the performance of invasive procedures and to evaluate organ function and pathology ... understanding the principles of ultrasound, including the physics of ultrasound transmission, ultrasound transducer construction, and transducer selection for specific applications, to include being able to obtain images with an understanding of limitations and artifacts ... obtaining standard views of the heart and inferior vena cava with transthoracic echocardiography allowing the evaluation of myocardial function, estimation of central venous pressure, and gross pericardial/cardiac pathology (eg, large pericardial effusion) ... using transthoracic ultrasound for the detection of pneumothorax and pleural effusion ... using surface ultrasound to guide vascular access (both central and peripheral) ... describing techniques, views, and findings in standard language” (ACGME Program Requirements for Graduate Medical Education In Anesthesiology).
Herein lies a stark contrast in what is required of programs that train physicians to care for unstable patients and the critically ill. Current requirements leave graduates of PCCM training programs vulnerable to completing ACGME milestones without being adequately prepared to evaluate patients in a modern ICU setting. Increasingly, hospitals credentialing committees expect PCCM graduates to be suitably trained in ultrasound. Regrettably, there is no assurance that is true, or standardized, with current PCCM fellowship training requirements.
There is not a national standard for competency assessment or requirements for credentialing in POCUS for critical care physicians at this time. However, multiple national and international critical care societies, including CHEST, have consensus statements and recommendations outlining the areas of competence expected in critical care ultrasound (Mayo PH, et al. Chest. 2009 Apr;135[4];1050-60, Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37(7):1077-83). The PCCM ACGME requirements should be updated to reflect such recommendations, thereby placing greater emphasis on ultrasound teaching requirements and standardized curriculums. Despite the current ACGME program requirements, it is incumbent upon critical care training programs to provide competency-based education of this now “standard of care” technology.
Barriers to universal POCUS training exist. Fellowship programs may lack trained, ultrasound confident faculty, time, and funding to successfully develop and sustain an ultrasound curriculum. (Eisen LA, et al. Crit Care Med. 2010;38[10]:1978-83; Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print].)
Although access to adequate quality and quantity of ultrasound machines is less often a problem than in the past, many institutions lack archival and image review software that allows for quality assurance of image acquisition, and some still may not have a faculty member with expertise and ability to champion the cause.
In attempts to mitigate the local faculty gaps, national and regional solutions have been developed for ultrasonography education. CHEST has educated more than 1,400 learners in the Ultrasound Essentials course since 2013. Also, grassroots efforts have led to the development of courses specifically designed to teach incoming PCCM fellows. Using a collaborative and cost-effective model, these regional programs pool faculty and experts in the field to train multiple fellowship programs simultaneously. The first of these was created over a decade ago in New York City (Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12:[Epub ahead of print].)
Currently, there are at least four regional annual ultrasound courses directed at teaching PCCM fellows. These courses are typically held over multiple days and encompass the basics of critical care ultrasound, including vascular, thoracic, abdominal, cardiac, and procedural imaging. By estimation, these four courses provide a basic ultrasonography education to approximately two-thirds of first year pulmonary and critical care fellows in the United States. In addition to training fellows, these programs also serve as a platform for the development of local faculty experts, so that training can continue at their institutions.
Introductory courses are highly effective (Dinh VA, et al. Crit Care Res Pract. 2015 Aug 5:675041 Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print]), but ongoing education, assessment, and quality assurance is required to achieve sustained competence. Ideally, training in POCUS should entail a dedicated, intensive introduction to the competencies of critical care ultrasound (such as the above regional courses or CHEST ultrasound courses), followed by a formal curriculum within the PCCM fellowship programs. This curriculum should afford the trainee exposure to critically ill patients in an environment with adequate ultrasound equipment and a method to record studies. The trainee then interprets the acquired studies in clinical context. Preferably, the program will afford the trainee real-time quality assurance for image acquisition and interpretation by a program champion. Quality assurance can be provided on site or remotely using fixed interval review sessions. Lastly, the program should have internal milestones to evaluate when a trainee has reached competency to perform these tasks independently. The completion of training should include a letter to any future employee attesting to the trainee’s acquisition of these skills and ability to apply them safely while caring for the critically ill. This robust education is occurring in many centers across the country. PCCM fellowship programs owe it to their trainees, and patients, that competency-based critical care ultrasound training is robust, standardized, and supported.
Point-of-care ultrasound (POCUS) is an essential part of ICU care. It has been demonstrated to improve patient safety and outcomes through procedural guidance (Brass P, et al. Cochrane Database Syst Rev. 2015 Jan 9;1:CD006962) and aid in accurate and timely diagnosis of cardiopulmonary failure (Lichtenstein DA, Mezière GA. Chest. 2008 Jul;134[1]:117-25). Due in part to increasing affordability and portability of ultrasound technologies, the use of POCUS has become seemingly ubiquitous and will continue to increase in coming years. According to expert groups representing 12 critical care societies worldwide, general critical care ultrasound and basic critical care echocardiography should be mandatory training for ICU physicians (Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37[7]:1077-83).
Currently, POCUS is not universally taught to pulmonary and critical care fellows (PCCM); and when training does exist, curriculums are not standardized. This is in part due to the broadly worded requirements set forth from the ACGME for pulmonary disease and critical care medicine. The totality of ACGME common program requirements as it regards to ultrasound training are as follows: 1. “Fellows must demonstrate competence in procedural and technical skills, including ... use of ultrasound techniques to perform thoracentesis and place intravascular and intracavitary tubes and catheters”; and 2. “Fellows must demonstrate knowledge of imaging techniques commonly employed in the evaluation of patients with pulmonary disease or critical illness, including the use of ultrasound” (ACGME Program Requirements for Graduate Medical Education in Pulmonary Disease and Critical Care Medicine).
In comparison, recently updated ACGME common program requirements for ultrasound in emergency medicine and anesthesiology residencies are robust and detailed. Requirements for anesthesia residency training include: ” ... competency in using surface ultrasound ... and transthoracic echocardiography to guide the performance of invasive procedures and to evaluate organ function and pathology ... understanding the principles of ultrasound, including the physics of ultrasound transmission, ultrasound transducer construction, and transducer selection for specific applications, to include being able to obtain images with an understanding of limitations and artifacts ... obtaining standard views of the heart and inferior vena cava with transthoracic echocardiography allowing the evaluation of myocardial function, estimation of central venous pressure, and gross pericardial/cardiac pathology (eg, large pericardial effusion) ... using transthoracic ultrasound for the detection of pneumothorax and pleural effusion ... using surface ultrasound to guide vascular access (both central and peripheral) ... describing techniques, views, and findings in standard language” (ACGME Program Requirements for Graduate Medical Education In Anesthesiology).
Herein lies a stark contrast in what is required of programs that train physicians to care for unstable patients and the critically ill. Current requirements leave graduates of PCCM training programs vulnerable to completing ACGME milestones without being adequately prepared to evaluate patients in a modern ICU setting. Increasingly, hospitals credentialing committees expect PCCM graduates to be suitably trained in ultrasound. Regrettably, there is no assurance that is true, or standardized, with current PCCM fellowship training requirements.
There is not a national standard for competency assessment or requirements for credentialing in POCUS for critical care physicians at this time. However, multiple national and international critical care societies, including CHEST, have consensus statements and recommendations outlining the areas of competence expected in critical care ultrasound (Mayo PH, et al. Chest. 2009 Apr;135[4];1050-60, Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37(7):1077-83). The PCCM ACGME requirements should be updated to reflect such recommendations, thereby placing greater emphasis on ultrasound teaching requirements and standardized curriculums. Despite the current ACGME program requirements, it is incumbent upon critical care training programs to provide competency-based education of this now “standard of care” technology.
Barriers to universal POCUS training exist. Fellowship programs may lack trained, ultrasound confident faculty, time, and funding to successfully develop and sustain an ultrasound curriculum. (Eisen LA, et al. Crit Care Med. 2010;38[10]:1978-83; Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print].)
Although access to adequate quality and quantity of ultrasound machines is less often a problem than in the past, many institutions lack archival and image review software that allows for quality assurance of image acquisition, and some still may not have a faculty member with expertise and ability to champion the cause.
In attempts to mitigate the local faculty gaps, national and regional solutions have been developed for ultrasonography education. CHEST has educated more than 1,400 learners in the Ultrasound Essentials course since 2013. Also, grassroots efforts have led to the development of courses specifically designed to teach incoming PCCM fellows. Using a collaborative and cost-effective model, these regional programs pool faculty and experts in the field to train multiple fellowship programs simultaneously. The first of these was created over a decade ago in New York City (Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12:[Epub ahead of print].)
Currently, there are at least four regional annual ultrasound courses directed at teaching PCCM fellows. These courses are typically held over multiple days and encompass the basics of critical care ultrasound, including vascular, thoracic, abdominal, cardiac, and procedural imaging. By estimation, these four courses provide a basic ultrasonography education to approximately two-thirds of first year pulmonary and critical care fellows in the United States. In addition to training fellows, these programs also serve as a platform for the development of local faculty experts, so that training can continue at their institutions.
Introductory courses are highly effective (Dinh VA, et al. Crit Care Res Pract. 2015 Aug 5:675041 Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print]), but ongoing education, assessment, and quality assurance is required to achieve sustained competence. Ideally, training in POCUS should entail a dedicated, intensive introduction to the competencies of critical care ultrasound (such as the above regional courses or CHEST ultrasound courses), followed by a formal curriculum within the PCCM fellowship programs. This curriculum should afford the trainee exposure to critically ill patients in an environment with adequate ultrasound equipment and a method to record studies. The trainee then interprets the acquired studies in clinical context. Preferably, the program will afford the trainee real-time quality assurance for image acquisition and interpretation by a program champion. Quality assurance can be provided on site or remotely using fixed interval review sessions. Lastly, the program should have internal milestones to evaluate when a trainee has reached competency to perform these tasks independently. The completion of training should include a letter to any future employee attesting to the trainee’s acquisition of these skills and ability to apply them safely while caring for the critically ill. This robust education is occurring in many centers across the country. PCCM fellowship programs owe it to their trainees, and patients, that competency-based critical care ultrasound training is robust, standardized, and supported.
Influenza already in midseason form
It’s been a decade since flu activity levels were this high this early in the season.
For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
It’s been a decade since flu activity levels were this high this early in the season.

For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
It’s been a decade since flu activity levels were this high this early in the season.

For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
Have lower readmission rates led to higher mortality for patients with COPD?
Be careful what you wish for
There is at least one aspect of “Obamacare” that my mother-in-law and I can firmly agree on: Hospitals should not get paid for frequent readmissions.
The Hospital Readmission Reduction Program (HRRP), enacted by the Centers for Medicare & Medicaid Services in 2012 with the goal of penalizing hospitals for excessive readmissions, has great face validity – and noble intentions. Does it also have a potentially disastrous downside?
On one side of the coin, the HRRP has been a remarkable success. It moved the national needle significantly on readmission rates. Yes, there are some caveats about increases in observation status patients and other shifts that could account for some of the difference, but it is fairly uncontroversial that overall, there are fewer 30-day readmissions across the country following initiation of HRRP. That is perhaps encouraging evidence of the potential positive impact that policy can make to drive changes for specific targets.
However, there is also a murkier – and more controversial – side. There have been a number of studies that have suggested reductions in readmission rates may have been associated with an increase in mortality in some patient groups. You discharge a patient and hope they won’t return to the hospital, but perhaps you should be more careful what you actually wish for.
Overall, the evidence of an association between readmissions and mortality has been complicated and conflicting. Headlines have alternately raised alarm about increased deaths and then reassured that there has been no change or perhaps even some concordant improvements in mortality. Not necessarily surprising, considering that these studies are all unavoidably of observational design and use different criteria, datasets and analytic models, which then drive their seemingly conflicting results.
An article published recently in the Journal of Hospital Medicine enters into this fray. The researchers examined the potential association between changes in rates of chronic obstructive pulmonary disease (COPD) readmissions and 30-day mortality following HRRP introduction. While the initial HRRP program and subsequent analyses included patients with heart failure, acute MI, and pneumonia, the program was extended in 2014 to include patients with COPD. So, what happened in this patient group?
Through a number of statistical gymnastics, which as a nonstatistician I am having difficulty truly wrapping my head around, the researchers seem to have found a number of important insights:
- The all-cause 30-day risk-standardized readmission rate declined from 2010 to 2017.
- The all-cause 30-day risk-standardized mortality rate increased from 2010 to 2017, and the rate of increase in mortality appears to be accelerating.
- Hospitals with higher readmission rates prior to COPD readmission penalties had a lower rate of increase in mortalities.
- Hospitals that had a larger decrease in readmission rates had a larger rate of increase in mortality.
These researchers could not evaluate data at the patient level and could not adjust for changes in disease severity. However, taken together, these findings suggest that something bad may be truly happening here.
The authors of this study also point out that the associations with increased mortality have largely been seen in patients with heart failure – and now in patients with COPD – which are both chronic diseases characterized by exacerbations, as opposed to acute MI and pneumonia, which are episodic and treatable. Perhaps in those types of disease, efforts to avoid readmissions may be more universally helpful. Maybe.
Even if it is challenging for me to adjudicate the complicated methods and results of this study, I find it concerning that there is “biological plausibility” for this association. Hospitalists know exactly how this might have happened. Have you heard of the pop-up alerts that fire in the emergency department to let the physicians know that this patient was discharged within the past 30 days? You know that alert is not meant to tell you what to do, but you just might want to consider trying to discharge them or at least place them in observation – use your clinical judgment, if you know what I mean.
Within the past decade, observation units quickly cropped up all over the country, often not staffed by hospitalists nor cardiologists, where patients with decompensated heart failure, chest pain, and/or COPD, can be given Lasix and/or nebulizer treatments – at least just enough to let them walk on back out that door without a hospital admission.
At the end of the day, whether mortality rates have truly increased in the real world, this well-intentioned program seems to have serious issues. As Ashish Jha, MD, wrote in 2018, “Right now, a high-readmission, low-mortality hospital will be penalized at 6-10 times the rate of a low-readmission, high-mortality hospital. The signal from policy makers is clear – readmissions matter a lot more than mortality – and this signal needs to stop.”
Dr. Moriates is a hospitalist, the assistant dean for health care value, and an associate professor of internal medicine at Dell Medical School at University of Texas, Austin. He is also director of implementation initiatives at Costs of Care. This article first appeared on the Hospital Leader, SHM’s official blog, at hospitalleader.org.
Be careful what you wish for
Be careful what you wish for
There is at least one aspect of “Obamacare” that my mother-in-law and I can firmly agree on: Hospitals should not get paid for frequent readmissions.
The Hospital Readmission Reduction Program (HRRP), enacted by the Centers for Medicare & Medicaid Services in 2012 with the goal of penalizing hospitals for excessive readmissions, has great face validity – and noble intentions. Does it also have a potentially disastrous downside?
On one side of the coin, the HRRP has been a remarkable success. It moved the national needle significantly on readmission rates. Yes, there are some caveats about increases in observation status patients and other shifts that could account for some of the difference, but it is fairly uncontroversial that overall, there are fewer 30-day readmissions across the country following initiation of HRRP. That is perhaps encouraging evidence of the potential positive impact that policy can make to drive changes for specific targets.
However, there is also a murkier – and more controversial – side. There have been a number of studies that have suggested reductions in readmission rates may have been associated with an increase in mortality in some patient groups. You discharge a patient and hope they won’t return to the hospital, but perhaps you should be more careful what you actually wish for.
Overall, the evidence of an association between readmissions and mortality has been complicated and conflicting. Headlines have alternately raised alarm about increased deaths and then reassured that there has been no change or perhaps even some concordant improvements in mortality. Not necessarily surprising, considering that these studies are all unavoidably of observational design and use different criteria, datasets and analytic models, which then drive their seemingly conflicting results.
An article published recently in the Journal of Hospital Medicine enters into this fray. The researchers examined the potential association between changes in rates of chronic obstructive pulmonary disease (COPD) readmissions and 30-day mortality following HRRP introduction. While the initial HRRP program and subsequent analyses included patients with heart failure, acute MI, and pneumonia, the program was extended in 2014 to include patients with COPD. So, what happened in this patient group?
Through a number of statistical gymnastics, which as a nonstatistician I am having difficulty truly wrapping my head around, the researchers seem to have found a number of important insights:
- The all-cause 30-day risk-standardized readmission rate declined from 2010 to 2017.
- The all-cause 30-day risk-standardized mortality rate increased from 2010 to 2017, and the rate of increase in mortality appears to be accelerating.
- Hospitals with higher readmission rates prior to COPD readmission penalties had a lower rate of increase in mortalities.
- Hospitals that had a larger decrease in readmission rates had a larger rate of increase in mortality.
These researchers could not evaluate data at the patient level and could not adjust for changes in disease severity. However, taken together, these findings suggest that something bad may be truly happening here.
The authors of this study also point out that the associations with increased mortality have largely been seen in patients with heart failure – and now in patients with COPD – which are both chronic diseases characterized by exacerbations, as opposed to acute MI and pneumonia, which are episodic and treatable. Perhaps in those types of disease, efforts to avoid readmissions may be more universally helpful. Maybe.
Even if it is challenging for me to adjudicate the complicated methods and results of this study, I find it concerning that there is “biological plausibility” for this association. Hospitalists know exactly how this might have happened. Have you heard of the pop-up alerts that fire in the emergency department to let the physicians know that this patient was discharged within the past 30 days? You know that alert is not meant to tell you what to do, but you just might want to consider trying to discharge them or at least place them in observation – use your clinical judgment, if you know what I mean.
Within the past decade, observation units quickly cropped up all over the country, often not staffed by hospitalists nor cardiologists, where patients with decompensated heart failure, chest pain, and/or COPD, can be given Lasix and/or nebulizer treatments – at least just enough to let them walk on back out that door without a hospital admission.
At the end of the day, whether mortality rates have truly increased in the real world, this well-intentioned program seems to have serious issues. As Ashish Jha, MD, wrote in 2018, “Right now, a high-readmission, low-mortality hospital will be penalized at 6-10 times the rate of a low-readmission, high-mortality hospital. The signal from policy makers is clear – readmissions matter a lot more than mortality – and this signal needs to stop.”
Dr. Moriates is a hospitalist, the assistant dean for health care value, and an associate professor of internal medicine at Dell Medical School at University of Texas, Austin. He is also director of implementation initiatives at Costs of Care. This article first appeared on the Hospital Leader, SHM’s official blog, at hospitalleader.org.
There is at least one aspect of “Obamacare” that my mother-in-law and I can firmly agree on: Hospitals should not get paid for frequent readmissions.
The Hospital Readmission Reduction Program (HRRP), enacted by the Centers for Medicare & Medicaid Services in 2012 with the goal of penalizing hospitals for excessive readmissions, has great face validity – and noble intentions. Does it also have a potentially disastrous downside?
On one side of the coin, the HRRP has been a remarkable success. It moved the national needle significantly on readmission rates. Yes, there are some caveats about increases in observation status patients and other shifts that could account for some of the difference, but it is fairly uncontroversial that overall, there are fewer 30-day readmissions across the country following initiation of HRRP. That is perhaps encouraging evidence of the potential positive impact that policy can make to drive changes for specific targets.
However, there is also a murkier – and more controversial – side. There have been a number of studies that have suggested reductions in readmission rates may have been associated with an increase in mortality in some patient groups. You discharge a patient and hope they won’t return to the hospital, but perhaps you should be more careful what you actually wish for.
Overall, the evidence of an association between readmissions and mortality has been complicated and conflicting. Headlines have alternately raised alarm about increased deaths and then reassured that there has been no change or perhaps even some concordant improvements in mortality. Not necessarily surprising, considering that these studies are all unavoidably of observational design and use different criteria, datasets and analytic models, which then drive their seemingly conflicting results.
An article published recently in the Journal of Hospital Medicine enters into this fray. The researchers examined the potential association between changes in rates of chronic obstructive pulmonary disease (COPD) readmissions and 30-day mortality following HRRP introduction. While the initial HRRP program and subsequent analyses included patients with heart failure, acute MI, and pneumonia, the program was extended in 2014 to include patients with COPD. So, what happened in this patient group?
Through a number of statistical gymnastics, which as a nonstatistician I am having difficulty truly wrapping my head around, the researchers seem to have found a number of important insights:
- The all-cause 30-day risk-standardized readmission rate declined from 2010 to 2017.
- The all-cause 30-day risk-standardized mortality rate increased from 2010 to 2017, and the rate of increase in mortality appears to be accelerating.
- Hospitals with higher readmission rates prior to COPD readmission penalties had a lower rate of increase in mortalities.
- Hospitals that had a larger decrease in readmission rates had a larger rate of increase in mortality.
These researchers could not evaluate data at the patient level and could not adjust for changes in disease severity. However, taken together, these findings suggest that something bad may be truly happening here.
The authors of this study also point out that the associations with increased mortality have largely been seen in patients with heart failure – and now in patients with COPD – which are both chronic diseases characterized by exacerbations, as opposed to acute MI and pneumonia, which are episodic and treatable. Perhaps in those types of disease, efforts to avoid readmissions may be more universally helpful. Maybe.
Even if it is challenging for me to adjudicate the complicated methods and results of this study, I find it concerning that there is “biological plausibility” for this association. Hospitalists know exactly how this might have happened. Have you heard of the pop-up alerts that fire in the emergency department to let the physicians know that this patient was discharged within the past 30 days? You know that alert is not meant to tell you what to do, but you just might want to consider trying to discharge them or at least place them in observation – use your clinical judgment, if you know what I mean.
Within the past decade, observation units quickly cropped up all over the country, often not staffed by hospitalists nor cardiologists, where patients with decompensated heart failure, chest pain, and/or COPD, can be given Lasix and/or nebulizer treatments – at least just enough to let them walk on back out that door without a hospital admission.
At the end of the day, whether mortality rates have truly increased in the real world, this well-intentioned program seems to have serious issues. As Ashish Jha, MD, wrote in 2018, “Right now, a high-readmission, low-mortality hospital will be penalized at 6-10 times the rate of a low-readmission, high-mortality hospital. The signal from policy makers is clear – readmissions matter a lot more than mortality – and this signal needs to stop.”
Dr. Moriates is a hospitalist, the assistant dean for health care value, and an associate professor of internal medicine at Dell Medical School at University of Texas, Austin. He is also director of implementation initiatives at Costs of Care. This article first appeared on the Hospital Leader, SHM’s official blog, at hospitalleader.org.