User login
Psychiatric illnesses share common brain network
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.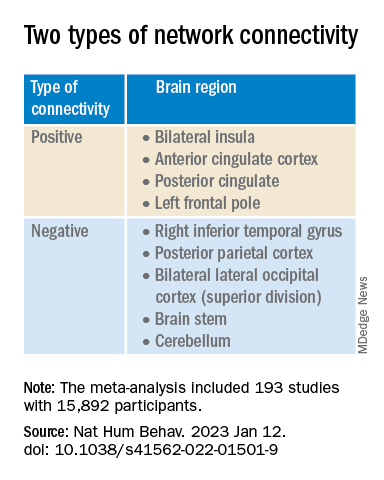
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators used coordinate and lesion network mapping to assess whether there was a shared brain network common to multiple psychiatric disorders. In a meta-analysis of almost 200 studies encompassing more than 15,000 individuals, they found that atrophy coordinates across these six psychiatric conditions all mapped to a common brain network.
Moreover, lesion damage to this network in patients with penetrating head trauma correlated with the number of psychiatric illnesses that the patients were diagnosed with post trauma.
The findings have “bigger-picture potential implications,” lead author Joseph Taylor, MD, PhD, medical director of transcranial magnetic stimulation at Brigham and Women’s Hospital’s Center for Brain Circuit Therapeutics, Boston, told this news organization.
“In psychiatry, we talk about symptoms and define our disorders based on symptom checklists, which are fairly reliable but don’t have neurobiological underpinnings,” said Dr. Taylor, who is also an associate psychiatrist in Brigham’s department of psychiatry.
By contrast, “in neurology, we ask: ‘Where is the lesion?’ Studying brain networks could potentially help us diagnose and treat people with psychiatric illness more effectively, just as we treat neurological disorders,” he added.
The findings were published online in Nature Human Behavior.
Beyond symptom checklists
Dr. Taylor noted that, in the field of psychiatry, “we often study disorders in isolation,” such as generalized anxiety disorder and major depressive disorder.
“But what see clinically is that half of patients meet the criteria for more than one psychiatric disorder,” he said. “It can be difficult to diagnose and treat these patients, and there are worse treatment outcomes.”
There is also a “discrepancy” between how these disorders are studied (one at a time) and how patients are treated in clinic, Dr. Taylor noted. And there is increasing evidence that psychiatric disorders may share a common neurobiology.
This “highlights the possibility of potentially developing transdiagnostic treatments based on common neurobiology, not just symptom checklists,” Dr. Taylor said.
Prior work “has attempted to map abnormalities to common brain regions rather than to a common brain network,” the investigators wrote. Moreover, “prior studies have rarely tested specificity by comparing psychiatric disorders to other brain disorders.”
In the current study, the researchers used “morphometric brain lesion datasets coupled with a wiring diagram of the human brain to derive a convergent brain network for psychiatric illness.”
They analyzed four large published datasets. Dataset 1 was sourced from an activation likelihood estimation meta-analysis (ALE) of whole-brain voxel-based studies that compared patients with psychiatric disorders such as schizophrenia, BD, depression, addiction, OCD, and anxiety to healthy controls (n = 193 studies; 15,892 individuals in total).
Dataset 2 was drawn from published neuroimaging studies involving patients with Alzheimer’s disease (AD) and other neurodegenerative conditions (n = 72 studies). They reported coordinates regarding which patients with these disorders had more atrophy compared with control persons.
Dataset 3 was sourced from the Vietnam Head Injury study, which followed veterans with and those without penetrating head injuries (n = 194 veterans with injuries). Dataset 4 was sourced from published neurosurgical ablation coordinates for depression.
Shared neurobiology
Upon analyzing dataset 1, the researchers found decreased gray matter in the bilateral anterior insula, dorsal anterior cingulate cortex, dorsomedial prefrontal cortex, thalamus, amygdala, hippocampus, and parietal operculum – findings that are “consistent with prior work.”
However, fewer than 35% of the studies contributed to any single cluster; and no cluster was specific to psychiatric versus neurodegenerative coordinates (drawn from dataset 2).
On the other hand, coordinate network mapping yielded “more statistically robust” (P < .001) results, which were found in 85% of the studies. “Psychiatric atrophy coordinates were functionally connected to the same network of brain regions,” the researchers reported.
This network was defined by two types of connectivity, positive and negative.
“The topography of this transdiagnostic network was independent of the statistical threshold and specific to psychiatric (vs. neurodegenerative) disorders, with the strongest peak occurring in the posterior parietal cortex (Brodmann Area 7) near the intraparietal sulcus,” the investigators wrote.
When lesions from dataset 3 were overlaid onto the ALE map and the transdiagnostic network in order to evaluate whether damage to either map correlated with number of post-lesion psychiatric diagnosis, results showed no evidence of a correlation between psychiatric comorbidity and damage on the ALE map (Pearson r, 0.02; P = .766).
However, when the same approach was applied to the transdiagnostic network, a statistically significant correlation was found between psychiatric comorbidity and lesion damage (Pearson r, –0.21; P = .01). A multiple regression model showed that the transdiagnostic, but not the ALE, network “independently predicted the number of post-lesion psychiatric diagnoses” (P = .003 vs. P = .1), the investigators reported.
All four neurosurgical ablative targets for psychiatric disorders found on analysis of dataset 4 “intersected” and aligned with the transdiagnostic network.
“The study does not immediately impact clinical practice, but it would be helpful for practicing clinicians to know that psychiatric disorders commonly co-occur and might share common neurobiology and a convergent brain network,” Dr. Taylor said.
“Future work based on our findings could potentially influence clinical trials and clinical practice, especially in the area of brain stimulation,” he added.
‘Exciting new targets’
In a comment, Desmond Oathes, PhD, associate director, Center for Neuromodulation and Stress, University of Pennsylvania, Philadelphia, said the “next step in the science is to combine individual brain imaging, aka, ‘individualized connectomes,’ with these promising group maps to determine something meaningful at the individual patient level.”
Dr. Oathes, who is also a faculty clinician at the Center for the Treatment and Study of Anxiety and was not involved with the study, noted that an open question is whether the brain volume abnormalities/atrophy “can be changed with treatment and in what direction.”
A “strong take-home message from this paper is that brain volume measures from single coordinates are noisy as measures of psychiatric abnormality, whereas network effects seem to be especially sensitive for capturing these effects,” Dr. Oathes said.
The “abnormal networks across these disorders do not fit easily into well-known networks from healthy participants. However, they map well onto other databases relevant to psychiatric disorders and offer exciting new potential targets for prospective treatment studies,” he added.
The investigators received no specific funding for this work. Dr. Taylor reported no relevant financial relationships. Dr. Oathes reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE HUMAN BEHAVIOR
Evaluation after a suicide attempt: What to ask
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
Gut microbiota and symptoms of psychosis: Is there a link?
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gut microbiome and the brain interact are collectively known as the gut-microbiota-brain axis.7
How do we acquire our gut microbiome, and how does it come to influence our brain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34 Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.
Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49 Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gut microbiome and the brain interact are collectively known as the gut-microbiota-brain axis.7
How do we acquire our gut microbiome, and how does it come to influence our brain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34 Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.

Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49 Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gut microbiome and the brain interact are collectively known as the gut-microbiota-brain axis.7
How do we acquire our gut microbiome, and how does it come to influence our brain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34 Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.

Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49 Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
Depression and schizophrenia: Many biological and clinical similarities
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
Discontinuing a long-acting injectable antipsychotic: What to consider
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.
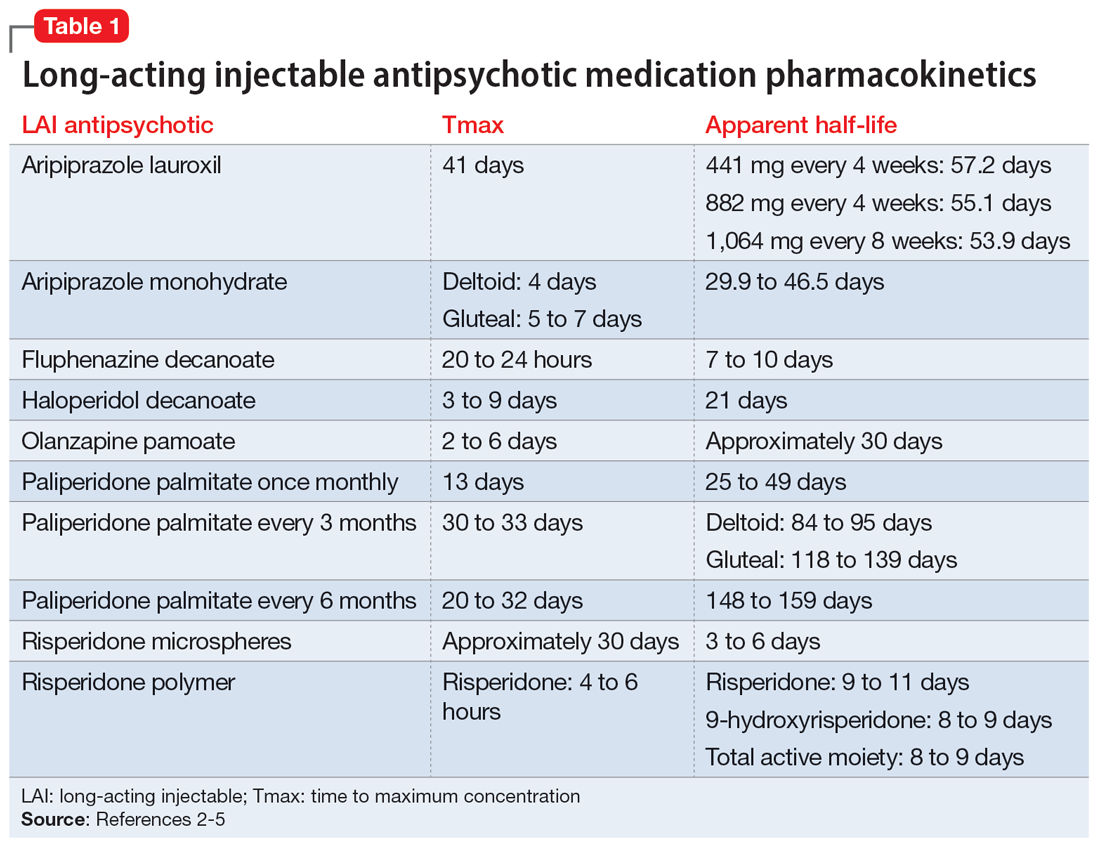
Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.
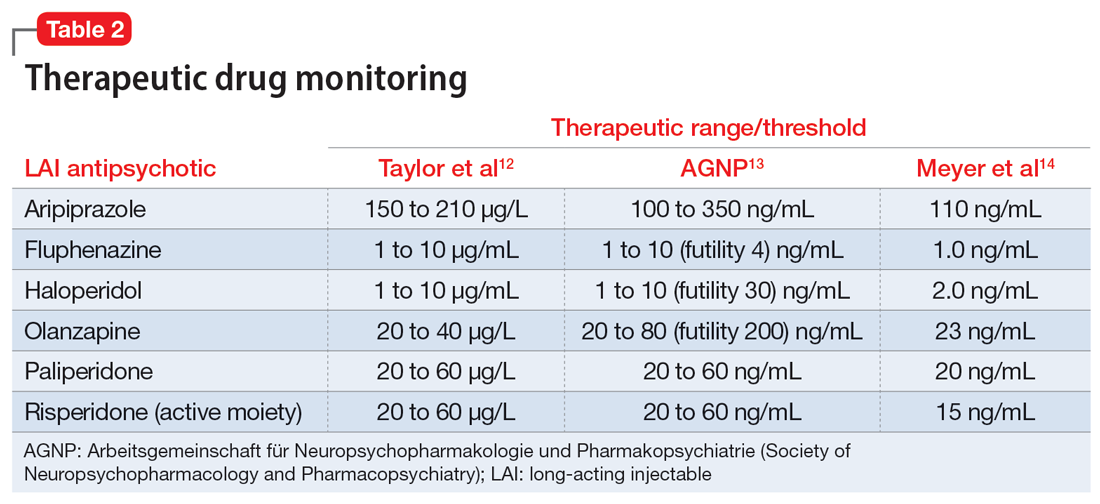
No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.

Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.

No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.

Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.

No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
‘Concerning’ uptick in pediatric antipsychotic prescribing
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
“This study demonstrates a concerning trend in antipsychotic prescribing in children and adolescents,” study investigator Matthias Pierce, PhD, senior research fellow at the University of Manchester (England) Center for Women’s Mental Health, who jointly led the study, said in a news release.
“We do not think the changes in prescribing necessarily relate to changes in clinical need; rather, it may be more likely to reflect changes in prescribing practice by clinicians,” Dr. Pierce said.
The study was published online in The Lancet Psychiatry.
Increase in long-term use
Between 2000 and 2019, prescriptions for antipsychotics nearly doubled from 0.06% to 0.11%.
The investigators note that the U.K.’s National Institute for Health and Care Excellence has approved the use of some antipsychotics in patients younger than age 18 with schizophrenia, bipolar disorder, and severely aggressive behavior attributable to conduct disorder.
However, these data suggest antipsychotics are being prescribed for an increasingly broad range of conditions, most commonly autism, but also for attention-deficit/ hyperactivity disorder, tic disorders like Tourrette syndrome, and learning difficulties.
“Broadening use of antipsychotics in developing young people begs questions about their safety over time and demands more research on this topic,” senior author Kathryn Abel, MBBS, PhD, from the University of Manchester said in the news release.
During the study period, antipsychotic prescribing in primary care increased by an average of 3.3% per year and the rate of first prescriptions increased by 2.2% per year.
The data also suggest that more children and adolescents are taking these powerful drugs for longer periods of time. The proportion receiving antipsychotics for at least 6 months after an initial prescription rose from 41.9% in 2000 to 62.8% in 2018.
Prescribing inequities
From 2009 onwards, more than 90% of prescriptions were for atypical antipsychotics.
Over time, risperidone dominated, with more than 60% of all prescriptions, followed by aripiprazole, quetiapine, olanzapine, and haloperidol as the most prescribed antipsychotics.
Boys and older children aged 15-18 years were most likely to receive an antipsychotic. However, the increasing trends were evident in all groups.
The data also point to inequities in prescribing as a result of deprivation levels, with typical antipsychotics prescribed more frequently in more deprived areas over time.
Dr. Pierce said he hopes this study will “help clinicians to evaluate the prescribing of antipsychotics to children more fully and will encourage them to consider better access to alternatives.”
Dr. Abel noted that antipsychotic medications “continue to have a valuable role in the treatment of serious mental illness. These findings represent a descriptive account of antipsychotic prescribing to children and adolescents in the U.K. today and provide a window onto current practice.”
Findings are no surprise
Emily Simonoff, MD, professor of child and adolescent psychiatry, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, offered perspective on the study in a statement from the U.K. nonprofit Science Media Centre.
“To clinicians, it will not be surprising that the authors demonstrate an increase in rates of prescriptions over that time period, as there has been a steadily emerging evidence base for the benefits of this group of medication for a range of different indications, which has been further supported by new licensing indications and recommendations from NICE,” Dr. Simonoff said.
For example, “there is good evidence for their benefits for other conditions such as irritability in autism spectrum disorder.
“However, it should also be noted that NICE recommendations for their use in many conditions is as part of a multimodal treatment plan, for example including psychological or behavioral interventions. It’s unclear from the study whether such recommendations were being followed or medication was being used on its own,” she added.
Dr. Simonoff also said it’s “reassuring” that prescribing rates remain very low in the youngest children and notes that the authors “rightly highlight the need for high-quality, longer-term studies on efficacy and, most importantly, adverse effects. This should be a research priority.”
The study had no funding. The authors report no relevant financial relationships. Dr. Simonoff is a member of the NICE guideline development group for the management of autism and has published on the efficacy of antipsychotic medication for irritability in autism.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY
Novel antipsychotic ‘encouraging’ for resistant schizophrenia
The topline results from an exploratory study, which were released by the developer Newron Pharmaceuticals, are “very encouraging,” Stephen R. Marder, MD, professor of psychiatry and biobehavioral sciences at the University of California, Los Angeles, said in a company news release.
“The magnitude of the improvements experienced by these TRS patients, not responding to their current antipsychotic, on evenamide was substantial, improved over time, and was likely to be clinically meaningful,” Dr. Marder said.
First 100 patients
The topline results are based on the first 100 patients enrolled in study 014 and randomly assigned to receive evenamide at 7.5 mg, 15 mg, or 30 mg twice daily, as well as patients in the extension arm (study 015) that have completed 30 weeks.
Key findings released by the company included statistically significant improvement over baseline at 30 weeks (P < .001) in Positive and Negative Syndrome Scale (PANSS) scores, with continued improvement over that seen at 6 weeks.
The proportion of patients with clinically meaningful PANSS improvement at 30 weeks more than doubled from 16.5% at 6 weeks.
In addition, results showed statistically significant improvement (P < .001) at week 30 compared with baseline in illness severity as measured by the Clinical Global Impression of Severity (CGI-S), with continued improvement over that seen at 6 weeks.
The proportion of patients whose illness improved by at least one level of severity was 60% at week 6 and increased approximately by an additional 20% at week 30.
The proportion of patients judged to have clinically meaningful improvement, defined as at least “much improved,” on the Clinical Global Impression of Change (CGI-C) was 27% at week 6 – and increased a further 10% at week 30.
Evenamide was also well tolerated, with few adverse effects reported, and 85 of 100 patients remained on treatment at 30 weeks.
New options ‘desperately needed’
Newron plans to present the full results from study 014 at the European Congress of Psychiatry, scheduled for March 25-28 in Paris.
The extension study 015 is ongoing and will provide results on evenamide treatment for up to 1 year by the second quarter of 2023.
The company reported it expects to launch a randomized, placebo-controlled study (study 003) of the drug in TRS this year.
If the current results are confirmed in the randomized controlled trial, “evenamide would be the first medication that could be added to an antipsychotic to improve symptoms in treatment-refractory schizophrenia,” Dr. Marder said.
New therapeutic options for TRS, which occurs in about one-third of patients, are “desperately needed,” Ravi Anand, MD, chief medical officer at Newron, said in the release.
The reported data, comparing the effect of evenamide at 6 weeks vs. 6 months, “suggest that not only was there sustained improvement in the key measures, but the proportion of patients achieving clinically meaningful improvement increased over time,” Dr. Anand added.
A version of this article first appeared on Medscape.com.
The topline results from an exploratory study, which were released by the developer Newron Pharmaceuticals, are “very encouraging,” Stephen R. Marder, MD, professor of psychiatry and biobehavioral sciences at the University of California, Los Angeles, said in a company news release.
“The magnitude of the improvements experienced by these TRS patients, not responding to their current antipsychotic, on evenamide was substantial, improved over time, and was likely to be clinically meaningful,” Dr. Marder said.
First 100 patients
The topline results are based on the first 100 patients enrolled in study 014 and randomly assigned to receive evenamide at 7.5 mg, 15 mg, or 30 mg twice daily, as well as patients in the extension arm (study 015) that have completed 30 weeks.
Key findings released by the company included statistically significant improvement over baseline at 30 weeks (P < .001) in Positive and Negative Syndrome Scale (PANSS) scores, with continued improvement over that seen at 6 weeks.
The proportion of patients with clinically meaningful PANSS improvement at 30 weeks more than doubled from 16.5% at 6 weeks.
In addition, results showed statistically significant improvement (P < .001) at week 30 compared with baseline in illness severity as measured by the Clinical Global Impression of Severity (CGI-S), with continued improvement over that seen at 6 weeks.
The proportion of patients whose illness improved by at least one level of severity was 60% at week 6 and increased approximately by an additional 20% at week 30.
The proportion of patients judged to have clinically meaningful improvement, defined as at least “much improved,” on the Clinical Global Impression of Change (CGI-C) was 27% at week 6 – and increased a further 10% at week 30.
Evenamide was also well tolerated, with few adverse effects reported, and 85 of 100 patients remained on treatment at 30 weeks.
New options ‘desperately needed’
Newron plans to present the full results from study 014 at the European Congress of Psychiatry, scheduled for March 25-28 in Paris.
The extension study 015 is ongoing and will provide results on evenamide treatment for up to 1 year by the second quarter of 2023.
The company reported it expects to launch a randomized, placebo-controlled study (study 003) of the drug in TRS this year.
If the current results are confirmed in the randomized controlled trial, “evenamide would be the first medication that could be added to an antipsychotic to improve symptoms in treatment-refractory schizophrenia,” Dr. Marder said.
New therapeutic options for TRS, which occurs in about one-third of patients, are “desperately needed,” Ravi Anand, MD, chief medical officer at Newron, said in the release.
The reported data, comparing the effect of evenamide at 6 weeks vs. 6 months, “suggest that not only was there sustained improvement in the key measures, but the proportion of patients achieving clinically meaningful improvement increased over time,” Dr. Anand added.
A version of this article first appeared on Medscape.com.
The topline results from an exploratory study, which were released by the developer Newron Pharmaceuticals, are “very encouraging,” Stephen R. Marder, MD, professor of psychiatry and biobehavioral sciences at the University of California, Los Angeles, said in a company news release.
“The magnitude of the improvements experienced by these TRS patients, not responding to their current antipsychotic, on evenamide was substantial, improved over time, and was likely to be clinically meaningful,” Dr. Marder said.
First 100 patients
The topline results are based on the first 100 patients enrolled in study 014 and randomly assigned to receive evenamide at 7.5 mg, 15 mg, or 30 mg twice daily, as well as patients in the extension arm (study 015) that have completed 30 weeks.
Key findings released by the company included statistically significant improvement over baseline at 30 weeks (P < .001) in Positive and Negative Syndrome Scale (PANSS) scores, with continued improvement over that seen at 6 weeks.
The proportion of patients with clinically meaningful PANSS improvement at 30 weeks more than doubled from 16.5% at 6 weeks.
In addition, results showed statistically significant improvement (P < .001) at week 30 compared with baseline in illness severity as measured by the Clinical Global Impression of Severity (CGI-S), with continued improvement over that seen at 6 weeks.
The proportion of patients whose illness improved by at least one level of severity was 60% at week 6 and increased approximately by an additional 20% at week 30.
The proportion of patients judged to have clinically meaningful improvement, defined as at least “much improved,” on the Clinical Global Impression of Change (CGI-C) was 27% at week 6 – and increased a further 10% at week 30.
Evenamide was also well tolerated, with few adverse effects reported, and 85 of 100 patients remained on treatment at 30 weeks.
New options ‘desperately needed’
Newron plans to present the full results from study 014 at the European Congress of Psychiatry, scheduled for March 25-28 in Paris.
The extension study 015 is ongoing and will provide results on evenamide treatment for up to 1 year by the second quarter of 2023.
The company reported it expects to launch a randomized, placebo-controlled study (study 003) of the drug in TRS this year.
If the current results are confirmed in the randomized controlled trial, “evenamide would be the first medication that could be added to an antipsychotic to improve symptoms in treatment-refractory schizophrenia,” Dr. Marder said.
New therapeutic options for TRS, which occurs in about one-third of patients, are “desperately needed,” Ravi Anand, MD, chief medical officer at Newron, said in the release.
The reported data, comparing the effect of evenamide at 6 weeks vs. 6 months, “suggest that not only was there sustained improvement in the key measures, but the proportion of patients achieving clinically meaningful improvement increased over time,” Dr. Anand added.
A version of this article first appeared on Medscape.com.
‘Affect discrepancies’ may underlie negative symptoms in schizophrenia
Anhedonia is common in schizophrenia patients, but treatments have not been especially successful, possibly because of a lack of understanding the mechanisms behind anhedonia in these patients, Sydney H. James, a PhD candidate at the University of Georgia, Athens, and colleagues wrote.
Although many schizophrenia (SZ) patients exhibit anhedonia on diagnosis in a clinical interview setting, other recent research shows comparable response to pleasant stimuli between schizophrenic patients and healthy controls. The researchers proposed that anhedonia “reflects abnormalities in the valuation of desired affective states in individuals with SZ,” with differences between actual and ideal affect.
In a study published in the Journal of Psychiatric Research, the researchers identified 32 outpatients with schizophrenia and 29 healthy controls. The SZ participants were recruited from community outpatient mental health services in Georgia. All participants completed Structured Clinical Interview for DSM-5 Disorders and the SCID-5 Personality Disorders. Participants then completed the Affect Valuation Index and measures of negative symptom severity. Negative symptom severity was measured using the Negative Symptom Inventory-Self-Report, an 11-item questionnaire assessing three specific experiential and behavioral components (anhedonia, avolition, and asociality) over the past week.
The average age of the SZ patients and controls was approximately 40 years, and 10 SZ patients and 5 controls were male.
Overall, the researchers found a significant main effect of group, a significant main effect of arousal, and a significant group X arousal interaction for positive affect discrepancy scores. For negative affect discrepancy scores, they found a significant main effect on group, nonsignificant main effect of arousal, and significant group X arousal interaction.
Individuals with SZ showed greater positive and negative emotion discrepancy scores, compared with controls, in contrast to the researchers’ hypothesis. “Those diagnosed with SZ were more likely to want to feel less negative than they actually did,” they wrote. The negative affect discrepancy scores were positively associated with negative symptoms. The discrepancies between actual and ideal affect may be impacted by social interactions and the perceived expectations of others for levels of negative affect.
The study findings were limited by the small sample size and inability to test the relationship between ideal and actual affect as related to low-pleasure beliefs, which merits further study, the researchers noted. Other limitations include the focus on an outpatient population with mild to moderate SZ, and the use of a trait format to measure affect rather than experiential emotion knowledge.
However, the results have practical implications for treatment and suggest that, “given the positive associations between negative symptom and affect discrepancy scores, psychosocial treatments could target expectations for future positive and negative emotional experience,” and ecological momentary assessment could be used to track affect through a period of treatment and prompt conversations between SZ patients and therapists about discrepancies, they concluded.
The study participants were compensated by the National Institute of Mental Health through a grant to a corresponding author. Ms. James had no financial conflicts to disclose.
Anhedonia is common in schizophrenia patients, but treatments have not been especially successful, possibly because of a lack of understanding the mechanisms behind anhedonia in these patients, Sydney H. James, a PhD candidate at the University of Georgia, Athens, and colleagues wrote.
Although many schizophrenia (SZ) patients exhibit anhedonia on diagnosis in a clinical interview setting, other recent research shows comparable response to pleasant stimuli between schizophrenic patients and healthy controls. The researchers proposed that anhedonia “reflects abnormalities in the valuation of desired affective states in individuals with SZ,” with differences between actual and ideal affect.
In a study published in the Journal of Psychiatric Research, the researchers identified 32 outpatients with schizophrenia and 29 healthy controls. The SZ participants were recruited from community outpatient mental health services in Georgia. All participants completed Structured Clinical Interview for DSM-5 Disorders and the SCID-5 Personality Disorders. Participants then completed the Affect Valuation Index and measures of negative symptom severity. Negative symptom severity was measured using the Negative Symptom Inventory-Self-Report, an 11-item questionnaire assessing three specific experiential and behavioral components (anhedonia, avolition, and asociality) over the past week.
The average age of the SZ patients and controls was approximately 40 years, and 10 SZ patients and 5 controls were male.
Overall, the researchers found a significant main effect of group, a significant main effect of arousal, and a significant group X arousal interaction for positive affect discrepancy scores. For negative affect discrepancy scores, they found a significant main effect on group, nonsignificant main effect of arousal, and significant group X arousal interaction.
Individuals with SZ showed greater positive and negative emotion discrepancy scores, compared with controls, in contrast to the researchers’ hypothesis. “Those diagnosed with SZ were more likely to want to feel less negative than they actually did,” they wrote. The negative affect discrepancy scores were positively associated with negative symptoms. The discrepancies between actual and ideal affect may be impacted by social interactions and the perceived expectations of others for levels of negative affect.
The study findings were limited by the small sample size and inability to test the relationship between ideal and actual affect as related to low-pleasure beliefs, which merits further study, the researchers noted. Other limitations include the focus on an outpatient population with mild to moderate SZ, and the use of a trait format to measure affect rather than experiential emotion knowledge.
However, the results have practical implications for treatment and suggest that, “given the positive associations between negative symptom and affect discrepancy scores, psychosocial treatments could target expectations for future positive and negative emotional experience,” and ecological momentary assessment could be used to track affect through a period of treatment and prompt conversations between SZ patients and therapists about discrepancies, they concluded.
The study participants were compensated by the National Institute of Mental Health through a grant to a corresponding author. Ms. James had no financial conflicts to disclose.
Anhedonia is common in schizophrenia patients, but treatments have not been especially successful, possibly because of a lack of understanding the mechanisms behind anhedonia in these patients, Sydney H. James, a PhD candidate at the University of Georgia, Athens, and colleagues wrote.
Although many schizophrenia (SZ) patients exhibit anhedonia on diagnosis in a clinical interview setting, other recent research shows comparable response to pleasant stimuli between schizophrenic patients and healthy controls. The researchers proposed that anhedonia “reflects abnormalities in the valuation of desired affective states in individuals with SZ,” with differences between actual and ideal affect.
In a study published in the Journal of Psychiatric Research, the researchers identified 32 outpatients with schizophrenia and 29 healthy controls. The SZ participants were recruited from community outpatient mental health services in Georgia. All participants completed Structured Clinical Interview for DSM-5 Disorders and the SCID-5 Personality Disorders. Participants then completed the Affect Valuation Index and measures of negative symptom severity. Negative symptom severity was measured using the Negative Symptom Inventory-Self-Report, an 11-item questionnaire assessing three specific experiential and behavioral components (anhedonia, avolition, and asociality) over the past week.
The average age of the SZ patients and controls was approximately 40 years, and 10 SZ patients and 5 controls were male.
Overall, the researchers found a significant main effect of group, a significant main effect of arousal, and a significant group X arousal interaction for positive affect discrepancy scores. For negative affect discrepancy scores, they found a significant main effect on group, nonsignificant main effect of arousal, and significant group X arousal interaction.
Individuals with SZ showed greater positive and negative emotion discrepancy scores, compared with controls, in contrast to the researchers’ hypothesis. “Those diagnosed with SZ were more likely to want to feel less negative than they actually did,” they wrote. The negative affect discrepancy scores were positively associated with negative symptoms. The discrepancies between actual and ideal affect may be impacted by social interactions and the perceived expectations of others for levels of negative affect.
The study findings were limited by the small sample size and inability to test the relationship between ideal and actual affect as related to low-pleasure beliefs, which merits further study, the researchers noted. Other limitations include the focus on an outpatient population with mild to moderate SZ, and the use of a trait format to measure affect rather than experiential emotion knowledge.
However, the results have practical implications for treatment and suggest that, “given the positive associations between negative symptom and affect discrepancy scores, psychosocial treatments could target expectations for future positive and negative emotional experience,” and ecological momentary assessment could be used to track affect through a period of treatment and prompt conversations between SZ patients and therapists about discrepancies, they concluded.
The study participants were compensated by the National Institute of Mental Health through a grant to a corresponding author. Ms. James had no financial conflicts to disclose.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
One in four cardiologists worldwide report mental health issues
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
ranging from anxiety or anger issues to major depression or other psychiatric disorders.
Such conditions varied in prevalence by cardiology subspecialty and years in the field, were more common in women than in men, and were closely linked to enduring hostile work environments and other strains of professional life.
The survey, conducted only months before the COVID-19 pandemic and with its share of limitations, still paints a picture that’s not pretty.
For example, mental health concerns were reported by about 42% of respondents who cited a hostile work environment, defined as workplace experience of discrimination based on age, sex, religion, race or ethnicity, or emotional or sexual harassment. Conversely, the prevalence of these concerns reached only 17% among those without such workplace conditions.
The study shows substantial overlap between cardiologists reporting hostility at work and those with mental health concerns, “and that was a significant finding,” Garima Sharma, MD, Johns Hopkins University, Baltimore, said in an interview.
Still, only 31% of male and 42% of female cardiologists (P < .001) reporting mental health concerns also said they had sought professional help either within or outside their own institutions.
That means “there is a lot of silent suffering” in the field, said Dr. Sharma, who is lead author on the study, published in the Journal of the American College of Cardiology.
Bringing back the conversation
The survey findings, she added, point to at least two potential ways the cardiology community can strive to diminish what may be a major underlying cause of the mental health concerns and their consequences.
“If you work towards reducing hostility at work and making mental health a priority for your workforce, then those experiencing these types of egregious conditions based on age, gender, race, ethnicity, or sexual orientation are less likely to be harmed.”
Mental health concerns among cardiologists are seldom openly discussed, so the current study can be “a way to bring them back into the conversation,” Dr. Sharma said. Clinician mental health “is extremely important because it directly impacts patient care and productivity.”
The survey’s reported mental health conditions “are an issue across the board in medicine, and amongst our medical students as well,” senior author Laxmi S. Mehta, MD, professor of internal medicine at Ohio State University, Columbus, said in an interview. The current study provides new details about their prevalence and predictors in cardiology and, she hopes, may improve the field’s awareness of and efforts to address the problem.
“We need to support those who have underlying mental health conditions, as well as improve the work environment to reduce contributory factors to mental illnesses. And we also need to work on reducing the stigma associated with seeking treatment and on reducing the barriers to receiving treatment,” said Dr. Mehta, who chairs the Workgroup on Clinician Well-Being of the ACC, which conducted the survey in 2019.
A global perspective
Cardiologists in Africa, the Americas, Asia, Europe, the Middle East, and Oceania – 5,890 in all – responded to mental health questions on the survey, which was novel for its global reach and insights across continents and cultures.
Respondents in South America and Central America reported the highest prevalences of mental health concerns, outliers at about 39% and 33%, respectively. Rates for most other geographic regions ranged narrowly from about 20% to 26%, the lowest reported in Asia and the Middle East.
Dr. Sharma acknowledged that the countries probably varied widely in social and cultural factors likely to influence survey responses, such as interpretation of the questionnaire’s mental health terminology or the degree to which the disorders are stigmatized.
“I think it’s hard to say how people may or may not respond culturally to a certain word or metric,” she said. But on the survey results, “whether you’re practicing in rural America, in rural India, or in the United Arab Emirates, Oceania, or Eastern Europe, there is a level of consistency, across the board, in what people are recognizing as mental health conditions.”
Junior vs. senior physicians
The global perspective “is a nice positive of the study, and the high rates in Central America and South America I think were something the field was not aware of and are an important contribution,” Srijan Sen, MD, PhD, said in an interview.
The psychological toll of hostile work environments is an issue throughout medicine, “but it seems greater in certain specialties, and cardiology may be one where it’s more of a problem,” observed Dr. Sen, who studies physician mental health at the University of Michigan, Ann Arbor, and wasn’t associated with the survey.
Mental health concerns in the survey were significantly more common among women than men (33.7% vs 26.3%), and for younger cardiologists, compared with older cardiologists (32.2% for those < 40 vs. 22.1% and 16.8% for those 55-69 and 70 or older, respectively).
Those findings seem to make sense, Dr. Sen observed. “Generally, cardiology and medicine broadly are hierarchical, so being more junior can be stressful.” And if there’s more hostility in the workplace, “it might fall on junior people.”
In other studies, moreover, “a high level of work-family conflict has been a real driver of depression and burnout, and that likely is affecting younger physicians, particularly young women physicians,” who may have smaller children and a greater burden of childcare than their seniors.
He pointed to the survey’s low response rate as an important limitation of the study. Of the 71,022 cardiologists invited to participate, only 5,890 (8.3%) responded and answered the queries on mental health.
With a response rate that low, a survey “can be biased in ways that we can’t predict,” Dr. Sen noted. Also, anyone concerned about the toxicity of their own workplace might be “more likely to respond to the survey than if they worked in a more pleasant place. That would provide a skewed sense of the overall experience of cardiologists.”
Those issues might not be a concern with the current survey, however, “because the results are consistent with other studies with higher response rates.”
‘Sobering report’
An accompanying editorial said Dr. Sharm and colleagues have provided “a sobering report on the global prevalence and potential contributors to mental health concerns” in the surveyed population.
Based on its lessons, Andrew J. Sauer, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., proposed several potential “interventions” the field could enact.
It could “selectively promote leaders who strive to mitigate implicit bias, discrimination, and harassment while advancing diversity, equity, and inclusion within the broad ranks of cardiologists.”
Also, he continued, “we must eliminate the stigmatization of mental illness among physicians. We need to handle mental health concerns with compassion and without blaming, like how we strive to treat our veterans who suffer from posttraumatic stress disorder.”
Lastly, Dr. Sauer wrote, “mentorship programs should be formalized to assist the cardiologist in transition zones from early to mid-career, with particular attention to women and those experiencing a simultaneously increased load of family burdens that compound existing workplace contributors to burnout and psychological distress.”
Years in practice
Of the cardiologists who responded to the survey’s mental health questions, 28% reported they have experienced mental health issues that could include alcohol/drug use disorder, suicidal tendencies, psychological distress (including anxiety, irritability, or anger), “other psychiatric disorders” (such as panic disorder, posttraumatic stress, or eating disorders) or major psychiatric disorders such as major depression, bipolar disorder, or schizophrenia.
Cardiologists with 5-10 years of practice post-training were more likely than cardiologists practicing for at least 20 years to have mental health concerns (31.9% vs. 22.6%, P < .001).
Mental health concerns were cited by 42% of respondents who cited “any type of discrimination” based on age, sex, race or ethnicity, or sexual orientation, the report noted.
Among those reporting any mental health concern, 2.7% considered suicide within the past year and 2.9% considered suicide more than 12 months previously. Women were more likely than men to consider suicide within the past year (3.8% vs. 2.3%) but were also more likely to seek help (42.3% vs. 31.1%; P < .001 for both differences), the authors wrote.
In multivariate analysis, predictors of mental health concerns included emotional harassment, 2.81 (odds ratio, 2.81; 95% confidence interval, 2.46-3.20), any discrimination (OR, 1.85; 95% CI, 1.61-2.12), being divorced (OR, 1.73; 95% CI, 1.26-2.36, age less than 55 years (OR, 1.43; 95% CI, 1.24-1.66), and being mid-career versus late (OR, 1.36; 95% CI, 1.14-1.62).
Because the survey was conducted from September to October 2019, before the pandemic’s traumatic effects unfolded on health care nearly everywhere, “I think there needs to be a follow-up at some point when everything has leveled out,” Dr. Sharma said. The current study is “a baseline, and not a healthy baseline,” for the field’s state of mental health that has likely grown worse during the pandemic.
But even without such a follow-up, the current study “is actionable enough that it forces us to do something about it right now.”
Dr. Sharma, Dr. Mehta, their coauthors, Dr. Sen, and Dr. Sauer reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Contemporary psychiatry: A SWOT analysis
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at [email protected].
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at [email protected].
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at [email protected].
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.