User login
Cardiovascular cost of smoking may last up to 25 years
Quitting smoking significantly reduces the risk of cardiovascular disease, but past smokers are still at elevated cardiovascular risk, compared with nonsmokers, for up to 25 years after smoking cessation, research in JAMA suggests.
A retrospective analysis of data from 8,770 individuals in the Framingham Heart Study compared the incidence of myocardial infarction, stroke, heart failure, or cardiovascular death in ever-smokers with that of never smokers.
Only 40% of the total cohort had never smoked. Of the 4,115 current smokers at baseline, 38.6% quit during the course of the study and did not relapse but 51.4% continued to smoke until they developed cardiovascular disease or dropped out of the study.
Current smokers had a significant 4.68-fold higher incidence of cardiovascular disease, compared with those who had never smoked, but those who stopped smoking showed a 39% decline in their risk of cardiovascular disease within 5 years of cessation.
However, individuals who were formerly heavy smokers – defined as at least 20 pack-years of smoking – retained a risk of cardiovascular disease 25% higher than that of never smokers until 10-15 years after quitting smoking. At 16 years, the 95% confidence interval for cardiovascular disease risk among former smokers versus that of never smokers finally and consistently included the null value of 1.
The study pooled two cohorts; the original cohort, who attended their fourth examination during 1954-1958 and an offspring cohort who attended their first examination during 1971-1975. The authors saw a difference between the two cohorts in the time course of cardiovascular disease risk in heavy smokers.
In the original cohort, former heavy smoking ceased to be significantly associated with increased cardiovascular disease risk within 5-10 years of cessation, but in the offspring cohort, it took 25 years after cessation for the incidence to decline to the same level of risk seen in never smokers.
“The upper estimate of this time course is a decade longer than that of the Nurses’ Health Study results for coronary heart disease and cardiovascular death and more than 20 years longer than in some prior reports for coronary heart disease and stroke,” wrote Meredith S. Duncan from the division of cardiovascular medicine at the Vanderbilt University Medical Center, Nashville, Tenn., and coauthors. “Although the exact amount of time after quitting at which former smokers’ CVD risk ceases to differ significantly from that of never smokers is unknown (and may further depend on cumulative exposure), these findings support a longer time course of risk reduction than was previously thought, yielding implications for CVD risk stratification of former smokers.”
However, they did note that the study could not account for environmental tobacco smoke exposure and that the participants were mostly of white European ancestry, which limited the generalizability of the findings to other populations.
The Framingham Health Study was supported by the National Heart, Lung, and Blood Institute. One author declared a consultancy with a pharmaceutical company on a proposed clinical trial. No other conflicts of interest were declared.
SOURCE: Duncan M et al. JAMA 2019. doi: 10.1001/jama.2019.10298.
Quitting smoking significantly reduces the risk of cardiovascular disease, but past smokers are still at elevated cardiovascular risk, compared with nonsmokers, for up to 25 years after smoking cessation, research in JAMA suggests.
A retrospective analysis of data from 8,770 individuals in the Framingham Heart Study compared the incidence of myocardial infarction, stroke, heart failure, or cardiovascular death in ever-smokers with that of never smokers.
Only 40% of the total cohort had never smoked. Of the 4,115 current smokers at baseline, 38.6% quit during the course of the study and did not relapse but 51.4% continued to smoke until they developed cardiovascular disease or dropped out of the study.
Current smokers had a significant 4.68-fold higher incidence of cardiovascular disease, compared with those who had never smoked, but those who stopped smoking showed a 39% decline in their risk of cardiovascular disease within 5 years of cessation.
However, individuals who were formerly heavy smokers – defined as at least 20 pack-years of smoking – retained a risk of cardiovascular disease 25% higher than that of never smokers until 10-15 years after quitting smoking. At 16 years, the 95% confidence interval for cardiovascular disease risk among former smokers versus that of never smokers finally and consistently included the null value of 1.
The study pooled two cohorts; the original cohort, who attended their fourth examination during 1954-1958 and an offspring cohort who attended their first examination during 1971-1975. The authors saw a difference between the two cohorts in the time course of cardiovascular disease risk in heavy smokers.
In the original cohort, former heavy smoking ceased to be significantly associated with increased cardiovascular disease risk within 5-10 years of cessation, but in the offspring cohort, it took 25 years after cessation for the incidence to decline to the same level of risk seen in never smokers.
“The upper estimate of this time course is a decade longer than that of the Nurses’ Health Study results for coronary heart disease and cardiovascular death and more than 20 years longer than in some prior reports for coronary heart disease and stroke,” wrote Meredith S. Duncan from the division of cardiovascular medicine at the Vanderbilt University Medical Center, Nashville, Tenn., and coauthors. “Although the exact amount of time after quitting at which former smokers’ CVD risk ceases to differ significantly from that of never smokers is unknown (and may further depend on cumulative exposure), these findings support a longer time course of risk reduction than was previously thought, yielding implications for CVD risk stratification of former smokers.”
However, they did note that the study could not account for environmental tobacco smoke exposure and that the participants were mostly of white European ancestry, which limited the generalizability of the findings to other populations.
The Framingham Health Study was supported by the National Heart, Lung, and Blood Institute. One author declared a consultancy with a pharmaceutical company on a proposed clinical trial. No other conflicts of interest were declared.
SOURCE: Duncan M et al. JAMA 2019. doi: 10.1001/jama.2019.10298.
Quitting smoking significantly reduces the risk of cardiovascular disease, but past smokers are still at elevated cardiovascular risk, compared with nonsmokers, for up to 25 years after smoking cessation, research in JAMA suggests.
A retrospective analysis of data from 8,770 individuals in the Framingham Heart Study compared the incidence of myocardial infarction, stroke, heart failure, or cardiovascular death in ever-smokers with that of never smokers.
Only 40% of the total cohort had never smoked. Of the 4,115 current smokers at baseline, 38.6% quit during the course of the study and did not relapse but 51.4% continued to smoke until they developed cardiovascular disease or dropped out of the study.
Current smokers had a significant 4.68-fold higher incidence of cardiovascular disease, compared with those who had never smoked, but those who stopped smoking showed a 39% decline in their risk of cardiovascular disease within 5 years of cessation.
However, individuals who were formerly heavy smokers – defined as at least 20 pack-years of smoking – retained a risk of cardiovascular disease 25% higher than that of never smokers until 10-15 years after quitting smoking. At 16 years, the 95% confidence interval for cardiovascular disease risk among former smokers versus that of never smokers finally and consistently included the null value of 1.
The study pooled two cohorts; the original cohort, who attended their fourth examination during 1954-1958 and an offspring cohort who attended their first examination during 1971-1975. The authors saw a difference between the two cohorts in the time course of cardiovascular disease risk in heavy smokers.
In the original cohort, former heavy smoking ceased to be significantly associated with increased cardiovascular disease risk within 5-10 years of cessation, but in the offspring cohort, it took 25 years after cessation for the incidence to decline to the same level of risk seen in never smokers.
“The upper estimate of this time course is a decade longer than that of the Nurses’ Health Study results for coronary heart disease and cardiovascular death and more than 20 years longer than in some prior reports for coronary heart disease and stroke,” wrote Meredith S. Duncan from the division of cardiovascular medicine at the Vanderbilt University Medical Center, Nashville, Tenn., and coauthors. “Although the exact amount of time after quitting at which former smokers’ CVD risk ceases to differ significantly from that of never smokers is unknown (and may further depend on cumulative exposure), these findings support a longer time course of risk reduction than was previously thought, yielding implications for CVD risk stratification of former smokers.”
However, they did note that the study could not account for environmental tobacco smoke exposure and that the participants were mostly of white European ancestry, which limited the generalizability of the findings to other populations.
The Framingham Health Study was supported by the National Heart, Lung, and Blood Institute. One author declared a consultancy with a pharmaceutical company on a proposed clinical trial. No other conflicts of interest were declared.
SOURCE: Duncan M et al. JAMA 2019. doi: 10.1001/jama.2019.10298.
FROM JAMA
Key clinical point:
Major finding: In the offspring cohort, heavy smokers showed elevated incidence of CVD for up to 25 years after quitting smoking.
Study details: A retrospective analysis of data from 8,770 individuals in the Framingham Heart Study.
Disclosures: The Framingham Health Study was supported by the National Heart, Lung, and Blood Institute. One author declared a consultancy with a pharmaceutical company on a proposed clinical trial. No other conflicts of interest were declared.
Source: Duncan M et al. JAMA. 2019. doi: 10.1001/jama.2019.10298.
Quality of Care for Veterans With In-Hospital Stroke
Stroke is a leading cause of death and long-term disability in the US.1 Quality improvement efforts for acute stroke care delivery have successfully led to increased rates of thrombolytic utilization.2 Increasing attention is now being paid to additional quality metrics for stroke care, including hospital management and initiation of appropriate secondary stroke prevention measures at discharge. Many organizations, including the Veterans Health Administration (VHA), use these measures to monitor health care quality and certify centers that are committed to excellence in stroke care.3-6 It is anticipated that collection, evaluation, and feedback from these data may lead to improvements in outcomes after stroke.7
Patients who experience onset of stroke symptoms while already admitted to a hospital may be uniquely suited for quality improvement strategies. In-hospital strokes (IHS) are not uncommon and have been associated with higher stroke severity and increased mortality compared with patients with stroke symptoms prior to arriving at the emergency department (ED).8-10 A potential reason for the higher observed mortality is that patients with IHS may have poorer access to acute stroke resources, such as stroke teams and neuroimaging, as well as increased rates of medical comorbidities.9,11,12 Furthermore, stroke management protocols are typically created based on ED resources, which may not be equivalent to resources available on inpatient settings.
Although many studies have examined clinical characteristics of patients with IHS, few studies have looked at the quality of stroke care for IHS. Information on stroke quality data is even more limited in VHA hospitals due to the small number of admitted patients with stroke.13 VHA released a directive on Acute Stroke Treatment (Directive 2011-03) in 2011 with a recent update in 2018, which aimed to implement quality improvement strategies for stroke care in VHA hospitals.14 Although focusing primarily on acute stroke care in the ED, this directive has led to increased awareness of areas for improvement, particularly among larger VHA hospitals. Prior to this directive, although national stroke guidelines were well-defined, more variability likely existed in stroke protocols and the manner in which stroke care was delivered across care settings. As efforts to measure and improve stroke care evolve, it is important to ensure that strategies used in ED settings also are implemented for patients already admitted to the hospital. This study seeks to define the quality of care in VHA hospitals between patients having an in-hospital ischemic stroke compared with those presenting to the ED.
Methods
As a secondary analysis, we examined stroke care quality data from an 11-site VHA stroke quality improvement study.15 Sites participating in this study were high stroke volume VHA hospitals from various geographic regions of the US. This study collected data on ICD-9 discharge diagnosis-defined ischemic stroke admissions between January 2009 and June 2012. Patient charts were reviewed by a group of central, trained abstractors who collected information on patient demographics, clinical history, and stroke characteristics. Stroke severity was defined using the National Institutes of Health Stroke Scale (NIHSS), assessed by standardized retrospective review of admission physical examination documentation.16 A multidisciplinary team defined 11 stroke quality indicators (QIs; the 8 Joint Commission indictors and 3 additional indicators: smoking cessation and dysphagia screening, and NIHSS assessment), and the chart abstractors’ data were used to evaluate eligibility and passing rates for each QI.
For our analysis, patients were stratified into 2 categories: patients admitted to the hospital for another diagnosis who developed an IHS, and patients presenting with stroke to the ED. We excluded patients transferred from other facilities. We then compared the demographic and clinical features of the 2 groups as well as eligibility and passing rates for each of the 11 QIs. Patients were recorded as eligible if they did not have any clinical contraindication to receiving the assessment or intervention measured by the quality metric. Passing rates were defined by the presence of clear documentation in the patient record that the quality metric was met or fulfilled. Comparisons were made using nonparametric Mann-Whitney U tests and chi-square tests. All tests were performed at α .05 level.
Results
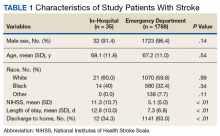
A total of 1823 patients were included in this analysis: 35 IHS and 1788 ED strokes. The 2 groups did not differ with respect to age, race, or sex (Table 1). Patients with IHS had higher stroke severity (mean NIHSS 11.3 vs 5.1, P <.01) and longer length of stay than did ED patients with stroke (mean 12.8 vs 7.3 days, P < .01). Patients with IHS also were less likely to be discharged home when compared with ED patients with stroke (34.3% vs 63.8%, P < .01).
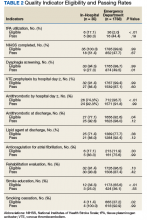
Table 2 summarizes our findings on eligibility and passing rates for the 11 QIs. For acute care metrics, we found that stroke severity documentation rates did not differ but were low for each patient group (51% vs 48%, P = .07). Patients with IHS were more likely to be eligible for IV tissue plasminogen activator (tPA; P < .01) although utilization rates did not differ. Only 2% of ED patients met eligibility criteria to receive tPA (36 of 1788), and among these patients only 16 actually received the drug. By comparison, 5 of 6 of eligible patients with IHS received tPA. Rates of dysphagia screening also were low for both groups, and patients with IHS were less likely to receive this screen prior to initiation of oral intake than were ED patients with stroke (27% vs 50%, P = .01).
Beyond the acute period, we found that patients with IHS were less likely than were ED patients with stroke to be eligible to receive antithrombotic therapy by 2 days after their initial stroke evaluation (74% vs 96%, P < .01), although treatment rates were similar between the 2 groups (P = .99). In patients with documented atrial fibrillation, initiation of anticoagulation therapy also did not differ (P = .99). The 2 groups were similar with respect to initiation of venous thromboembolism (VTE) prophylaxis (P = .596) and evaluation for rehabilitation needs (P = .42). Although rates of smoking cessation counseling and stroke education prior to discharge did not differ, overall rates of stroke education were very low for both groups (25% vs 36%, P = .55).
Similar to initiation of antithrombotic therapy in the hospital, we found lower rates of eligibility to receive antithrombotic therapy on discharge in the IHS group when compared with the ED group (77% vs 93%, P = .04). However, actual treatment initiation rates did not differ (P = .12). Use of lipid-lowering agents was similar for the 2 groups (P = .12).
Discussion
Our study found that veterans who develop an IHS received similar quality of care as did those presenting to the ED with stroke symptoms for many QIs, although there were some notable differences. We were pleased to find that overall rates of secondary stroke prevention initiation (antithrombotic and statin therapy), VTE prophylaxis, rehabilitation evaluations, and smoking cessation counseling were high for both groups, in keeping with evidence-based guidelines.17 This likely reflected the fact that these metrics typically involve care outside of the acute period and are less likely to be influenced by the location of initial stroke evaluation. Furthermore, efforts to improve smoking cessation and VTE prophylaxis are not exclusive to stroke care and have been the target of several nonstroke quality projects in the VHA. Many aspects of acute stroke care did differ, and present opportunities for quality improvement in the future.
In our sample, patients with IHS had higher IV thrombolytic eligibility, which has not typically been reported in other samples.10,11,18 In these studies, hospitalized patients have been reported to more often have contraindications to tPA, such as recent surgery or lack of stroke symptom recognition due to delirium or medication effects. Interestingly, patients presenting to VHA EDs had extremely low rates of tPA eligibility (2%), which is lower than many reported estimates of tPA eligibility outside of the VHA.19,20 This may be due to multiple influences, such as geographic barriers, patient perceptions about stroke symptoms, access to emergency medical services (EMS), EMS routing patterns, and social/cultural factors. Although not statistically significant due to small sample size, tPA use also was twice as high in the IHS group.
Given that a significant proportion of patients with IHS in the VHA system may be eligible for acute thrombolysis, our findings highlight the need for acute stroke protocols to ensure that patients with IHS receive the same rapid stroke assessment and access to thrombolytics as do patients evaluated in the ED. Further investigation is needed to determine whether there are unique features of patients with IHS in VHA hospitals, which may make them more eligible for IV thrombolysis.
Dysphagia is associated with increased risks for aspiration pneumonia in stroke patients.21 We found that patients with IHS were less likely to receive dysphagia screening compared with that of stroke patients admitted through the ED. This finding is consistent with the fact that care for patients with IHS is less frequently guided by specific stroke care protocols and algorithms that are more often used in EDs.8,11 Although attention to swallowing function may lead to improved outcomes in stroke, this can be easily overlooked in patients with IHS.22 However, low dysphagia screening also was found in patients admitted through the ED, suggesting that low screening rates cannot be solely explained by differences in where the initial stroke evaluation is occurring. These findings suggest a need for novel approaches to dysphagia screening in VHA stroke patients that can be universally implemented throughout the hospital.
Finally, we also found very low rates of stroke education prior to discharge for both groups. Given the risk of stroke recurrence and the overall poor level of public knowledge about stroke, providing patients with stroke with formal oral and written information on stroke is a critical component of secondary prevention.23,24 Educational tools, including those that are veteran specific, are now available for use in VHA hospitals and should be incorporated into quality improvement strategies for stroke care in VHA hospitals.
In 2012, the VHA Acute Stroke Treatment Directive was published in an effort to improve stroke care systemwide. Several of the metrics examined in this study are addressed in this directive. The data presented in this study is one of the only samples of stroke quality metrics within the VHA that largely predates the directive and can serve as a baseline comparator for future work examining stroke care after release of the directive. At present, although continuous internal reviews of quality data are ongoing, longitudinal description of stroke care quality since publication of the directive will help to inform future efforts to improve stroke care for veterans.
Limitations
Despite the strength of being a multicenter sampling of stroke care in high volume VHA hospitals, our study had several limitations. The IHS sample size was small, which limited our ability to evaluate differences between the groups, to evaluate generalizability, and account for estimation error.13 It is possible that differences existed between the groups that could not be observed in this sample due to small size (type II error) or that patient-specific characteristics not captured by these data could influence these metrics. Assessments of eligibility and passing were based on retrospective chart review and post hoc coding. Our sample assessed only patients who presented to larger VHA hospitals with higher stroke volumes, thus these findings may not be generalizable to smaller VHA hospitals with less systematized stroke care. This sample did not describe the specialty care services that were received by each patient, which may have influenced their stroke care. Finally, this study is an analysis of use of QIs in stroke care and did not examine how these indicators affect outcomes.
Conclusion
Despite reassuring findings for several inpatient ischemic stroke quality metrics, we found several differences in stroke care between patients with IHS compared with those presenting to the ED, emphasizing the need for standardized approaches to stroke care regardless of care setting. Although patients with IHS may be more likely to be eligible for tPA, these patients received dysphagia screening and less often than did ED patients with stroke. Ongoing quality initiatives should continue to place emphasis on improving all quality metrics (particularly dysphagia screening, stroke severity documentation, and stroke education) for patients with stroke at VHA hospitals across all care settings. Future work will be needed to examine how specific patient characteristics and revisions to stroke protocols may affect stroke quality metrics and outcomes between patients with IHS and those presenting to the ED.
Acknowledgments
The authors would like to thank Danielle Sager for her contributions to this project.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292.
2. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines—Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
3. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
4. The Joint Commission. Certificate of distinction for primary stroke centers. https://www.jointcommission.org/certificate_of_distinction_for_primary_stroke_centers_/.Published April 30, 2012. Accessed July 9, 2019.
5. US Department of Veterans Affairs. Center highlight: acute ischemic stroke care for veterans. https://www.queri.research.va.gov/center_highlights/stroke.cfm. Updated February 20, 2014. Accessed July 16, 2019.
6. Chumbler NR, Jia H, Phipps MS, et al. Does inpatient quality of care differ by age among US veterans with ischemic stroke? J Stroke Cerebrovasc Dis. 2012;21(8):844-851.
7. Katzan IL, Spertus J, Bettger JP, et al; American Heart Association Stroke Council; Council on Quality of Care and Outcomes Research; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Risk adjustment of ischemic stroke outcomes for comparing hospital performance: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(3):918-944.
8. Cumbler E, Wald H, Bhatt DL, et al. Quality of care and outcomes for in-hospital ischemic stroke: findings from the National Get With the Guidelines—Stroke. Stroke. 2014;45(1):231-238.
9. Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
10. Farooq MU, Reeves MJ, Gargano J, Wehner S, Hickenbottom S, Majid A; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. In-hospital stroke in a statewide stroke registry. Cerebrovascular Dis. 2008;25(1-2):12-20.
11. Bhalla A, Smeeton N, Rudd AG, Heuschmann P, Wolfe CD. A comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis. 2010;19:(5)357-363.
12. Garcia-Santibanez R, Liang J, Walker A, Matos-Diaz I, Kahkeshani K, Boniece I. Comparison of stroke codes in the emergency room and inpatient setting. J Stroke Cerebrovasc Dis. 2015;24(8):1948-1950.
13. Arling G, Reeves M, Ross J, et al. Estimating and reporting on the quality of inpatient stroke care by Veterans Health Administration medical centers. Circ Cardiovasc Qual Outcomes. 2012;5(1):44-51.
14. US Department of Veterans Affairs. Treatment of Acute Ischemic Stroke (AIS). VHA Directive 2011-038. https://www.hsrd.research.va.gov/news/feature/stroke.cfm. Updated January 20, 2014. Accessed July 17, 2019.
15. Williams LS, Daggett V, Slaven J, et al. Abstract 18: Does quality improvement training add to audit and feedback for inpatient stroke care processes? [International Stroke Conference abstract 18] Stroke. 2014;45(suppl 1):A18.
16. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858-862.
17. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
18. Park HJ, Cho HJ, Kim YD, et al. Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol. 2009;16(5):582-588.
19. Messé SR, Fonarow GC, Smith EE, et al. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2012;5(3):321-326.
20. Allen NB, Kaltenbach L, Goldstein LB, et al. Regional variation in recommended treatments for ischemic stroke and TIA: Get With the Guidelines—Stroke 2003-2010. Stroke. 2012;43(7):1858-1864.
21. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756-2763.
22. Bravata DM, Wells CK, Lo AC, et al. Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170(9):804-810.
23. Mosley I, Nicol M, Donnan G, Patrick I, Dewey H. Stroke symptoms and the decision to call for an ambulance. Stroke; a journal of cerebral circulation. 2007;38(2):361-366.
24. Jurkowski JM, Maniccia DM, Dennison BA, Samuels SJ, Spicer DA. Awareness of necessity to call 9-1-1 for stroke symptoms, upstate New York. Prev Chronic Dis. 2008;5(2):A41.
Stroke is a leading cause of death and long-term disability in the US.1 Quality improvement efforts for acute stroke care delivery have successfully led to increased rates of thrombolytic utilization.2 Increasing attention is now being paid to additional quality metrics for stroke care, including hospital management and initiation of appropriate secondary stroke prevention measures at discharge. Many organizations, including the Veterans Health Administration (VHA), use these measures to monitor health care quality and certify centers that are committed to excellence in stroke care.3-6 It is anticipated that collection, evaluation, and feedback from these data may lead to improvements in outcomes after stroke.7
Patients who experience onset of stroke symptoms while already admitted to a hospital may be uniquely suited for quality improvement strategies. In-hospital strokes (IHS) are not uncommon and have been associated with higher stroke severity and increased mortality compared with patients with stroke symptoms prior to arriving at the emergency department (ED).8-10 A potential reason for the higher observed mortality is that patients with IHS may have poorer access to acute stroke resources, such as stroke teams and neuroimaging, as well as increased rates of medical comorbidities.9,11,12 Furthermore, stroke management protocols are typically created based on ED resources, which may not be equivalent to resources available on inpatient settings.
Although many studies have examined clinical characteristics of patients with IHS, few studies have looked at the quality of stroke care for IHS. Information on stroke quality data is even more limited in VHA hospitals due to the small number of admitted patients with stroke.13 VHA released a directive on Acute Stroke Treatment (Directive 2011-03) in 2011 with a recent update in 2018, which aimed to implement quality improvement strategies for stroke care in VHA hospitals.14 Although focusing primarily on acute stroke care in the ED, this directive has led to increased awareness of areas for improvement, particularly among larger VHA hospitals. Prior to this directive, although national stroke guidelines were well-defined, more variability likely existed in stroke protocols and the manner in which stroke care was delivered across care settings. As efforts to measure and improve stroke care evolve, it is important to ensure that strategies used in ED settings also are implemented for patients already admitted to the hospital. This study seeks to define the quality of care in VHA hospitals between patients having an in-hospital ischemic stroke compared with those presenting to the ED.
Methods
As a secondary analysis, we examined stroke care quality data from an 11-site VHA stroke quality improvement study.15 Sites participating in this study were high stroke volume VHA hospitals from various geographic regions of the US. This study collected data on ICD-9 discharge diagnosis-defined ischemic stroke admissions between January 2009 and June 2012. Patient charts were reviewed by a group of central, trained abstractors who collected information on patient demographics, clinical history, and stroke characteristics. Stroke severity was defined using the National Institutes of Health Stroke Scale (NIHSS), assessed by standardized retrospective review of admission physical examination documentation.16 A multidisciplinary team defined 11 stroke quality indicators (QIs; the 8 Joint Commission indictors and 3 additional indicators: smoking cessation and dysphagia screening, and NIHSS assessment), and the chart abstractors’ data were used to evaluate eligibility and passing rates for each QI.
For our analysis, patients were stratified into 2 categories: patients admitted to the hospital for another diagnosis who developed an IHS, and patients presenting with stroke to the ED. We excluded patients transferred from other facilities. We then compared the demographic and clinical features of the 2 groups as well as eligibility and passing rates for each of the 11 QIs. Patients were recorded as eligible if they did not have any clinical contraindication to receiving the assessment or intervention measured by the quality metric. Passing rates were defined by the presence of clear documentation in the patient record that the quality metric was met or fulfilled. Comparisons were made using nonparametric Mann-Whitney U tests and chi-square tests. All tests were performed at α .05 level.
Results
A total of 1823 patients were included in this analysis: 35 IHS and 1788 ED strokes. The 2 groups did not differ with respect to age, race, or sex (Table 1). Patients with IHS had higher stroke severity (mean NIHSS 11.3 vs 5.1, P <.01) and longer length of stay than did ED patients with stroke (mean 12.8 vs 7.3 days, P < .01). Patients with IHS also were less likely to be discharged home when compared with ED patients with stroke (34.3% vs 63.8%, P < .01).
Table 2 summarizes our findings on eligibility and passing rates for the 11 QIs. For acute care metrics, we found that stroke severity documentation rates did not differ but were low for each patient group (51% vs 48%, P = .07). Patients with IHS were more likely to be eligible for IV tissue plasminogen activator (tPA; P < .01) although utilization rates did not differ. Only 2% of ED patients met eligibility criteria to receive tPA (36 of 1788), and among these patients only 16 actually received the drug. By comparison, 5 of 6 of eligible patients with IHS received tPA. Rates of dysphagia screening also were low for both groups, and patients with IHS were less likely to receive this screen prior to initiation of oral intake than were ED patients with stroke (27% vs 50%, P = .01).
Beyond the acute period, we found that patients with IHS were less likely than were ED patients with stroke to be eligible to receive antithrombotic therapy by 2 days after their initial stroke evaluation (74% vs 96%, P < .01), although treatment rates were similar between the 2 groups (P = .99). In patients with documented atrial fibrillation, initiation of anticoagulation therapy also did not differ (P = .99). The 2 groups were similar with respect to initiation of venous thromboembolism (VTE) prophylaxis (P = .596) and evaluation for rehabilitation needs (P = .42). Although rates of smoking cessation counseling and stroke education prior to discharge did not differ, overall rates of stroke education were very low for both groups (25% vs 36%, P = .55).
Similar to initiation of antithrombotic therapy in the hospital, we found lower rates of eligibility to receive antithrombotic therapy on discharge in the IHS group when compared with the ED group (77% vs 93%, P = .04). However, actual treatment initiation rates did not differ (P = .12). Use of lipid-lowering agents was similar for the 2 groups (P = .12).
Discussion
Our study found that veterans who develop an IHS received similar quality of care as did those presenting to the ED with stroke symptoms for many QIs, although there were some notable differences. We were pleased to find that overall rates of secondary stroke prevention initiation (antithrombotic and statin therapy), VTE prophylaxis, rehabilitation evaluations, and smoking cessation counseling were high for both groups, in keeping with evidence-based guidelines.17 This likely reflected the fact that these metrics typically involve care outside of the acute period and are less likely to be influenced by the location of initial stroke evaluation. Furthermore, efforts to improve smoking cessation and VTE prophylaxis are not exclusive to stroke care and have been the target of several nonstroke quality projects in the VHA. Many aspects of acute stroke care did differ, and present opportunities for quality improvement in the future.
In our sample, patients with IHS had higher IV thrombolytic eligibility, which has not typically been reported in other samples.10,11,18 In these studies, hospitalized patients have been reported to more often have contraindications to tPA, such as recent surgery or lack of stroke symptom recognition due to delirium or medication effects. Interestingly, patients presenting to VHA EDs had extremely low rates of tPA eligibility (2%), which is lower than many reported estimates of tPA eligibility outside of the VHA.19,20 This may be due to multiple influences, such as geographic barriers, patient perceptions about stroke symptoms, access to emergency medical services (EMS), EMS routing patterns, and social/cultural factors. Although not statistically significant due to small sample size, tPA use also was twice as high in the IHS group.
Given that a significant proportion of patients with IHS in the VHA system may be eligible for acute thrombolysis, our findings highlight the need for acute stroke protocols to ensure that patients with IHS receive the same rapid stroke assessment and access to thrombolytics as do patients evaluated in the ED. Further investigation is needed to determine whether there are unique features of patients with IHS in VHA hospitals, which may make them more eligible for IV thrombolysis.
Dysphagia is associated with increased risks for aspiration pneumonia in stroke patients.21 We found that patients with IHS were less likely to receive dysphagia screening compared with that of stroke patients admitted through the ED. This finding is consistent with the fact that care for patients with IHS is less frequently guided by specific stroke care protocols and algorithms that are more often used in EDs.8,11 Although attention to swallowing function may lead to improved outcomes in stroke, this can be easily overlooked in patients with IHS.22 However, low dysphagia screening also was found in patients admitted through the ED, suggesting that low screening rates cannot be solely explained by differences in where the initial stroke evaluation is occurring. These findings suggest a need for novel approaches to dysphagia screening in VHA stroke patients that can be universally implemented throughout the hospital.
Finally, we also found very low rates of stroke education prior to discharge for both groups. Given the risk of stroke recurrence and the overall poor level of public knowledge about stroke, providing patients with stroke with formal oral and written information on stroke is a critical component of secondary prevention.23,24 Educational tools, including those that are veteran specific, are now available for use in VHA hospitals and should be incorporated into quality improvement strategies for stroke care in VHA hospitals.
In 2012, the VHA Acute Stroke Treatment Directive was published in an effort to improve stroke care systemwide. Several of the metrics examined in this study are addressed in this directive. The data presented in this study is one of the only samples of stroke quality metrics within the VHA that largely predates the directive and can serve as a baseline comparator for future work examining stroke care after release of the directive. At present, although continuous internal reviews of quality data are ongoing, longitudinal description of stroke care quality since publication of the directive will help to inform future efforts to improve stroke care for veterans.
Limitations
Despite the strength of being a multicenter sampling of stroke care in high volume VHA hospitals, our study had several limitations. The IHS sample size was small, which limited our ability to evaluate differences between the groups, to evaluate generalizability, and account for estimation error.13 It is possible that differences existed between the groups that could not be observed in this sample due to small size (type II error) or that patient-specific characteristics not captured by these data could influence these metrics. Assessments of eligibility and passing were based on retrospective chart review and post hoc coding. Our sample assessed only patients who presented to larger VHA hospitals with higher stroke volumes, thus these findings may not be generalizable to smaller VHA hospitals with less systematized stroke care. This sample did not describe the specialty care services that were received by each patient, which may have influenced their stroke care. Finally, this study is an analysis of use of QIs in stroke care and did not examine how these indicators affect outcomes.
Conclusion
Despite reassuring findings for several inpatient ischemic stroke quality metrics, we found several differences in stroke care between patients with IHS compared with those presenting to the ED, emphasizing the need for standardized approaches to stroke care regardless of care setting. Although patients with IHS may be more likely to be eligible for tPA, these patients received dysphagia screening and less often than did ED patients with stroke. Ongoing quality initiatives should continue to place emphasis on improving all quality metrics (particularly dysphagia screening, stroke severity documentation, and stroke education) for patients with stroke at VHA hospitals across all care settings. Future work will be needed to examine how specific patient characteristics and revisions to stroke protocols may affect stroke quality metrics and outcomes between patients with IHS and those presenting to the ED.
Acknowledgments
The authors would like to thank Danielle Sager for her contributions to this project.
Stroke is a leading cause of death and long-term disability in the US.1 Quality improvement efforts for acute stroke care delivery have successfully led to increased rates of thrombolytic utilization.2 Increasing attention is now being paid to additional quality metrics for stroke care, including hospital management and initiation of appropriate secondary stroke prevention measures at discharge. Many organizations, including the Veterans Health Administration (VHA), use these measures to monitor health care quality and certify centers that are committed to excellence in stroke care.3-6 It is anticipated that collection, evaluation, and feedback from these data may lead to improvements in outcomes after stroke.7
Patients who experience onset of stroke symptoms while already admitted to a hospital may be uniquely suited for quality improvement strategies. In-hospital strokes (IHS) are not uncommon and have been associated with higher stroke severity and increased mortality compared with patients with stroke symptoms prior to arriving at the emergency department (ED).8-10 A potential reason for the higher observed mortality is that patients with IHS may have poorer access to acute stroke resources, such as stroke teams and neuroimaging, as well as increased rates of medical comorbidities.9,11,12 Furthermore, stroke management protocols are typically created based on ED resources, which may not be equivalent to resources available on inpatient settings.
Although many studies have examined clinical characteristics of patients with IHS, few studies have looked at the quality of stroke care for IHS. Information on stroke quality data is even more limited in VHA hospitals due to the small number of admitted patients with stroke.13 VHA released a directive on Acute Stroke Treatment (Directive 2011-03) in 2011 with a recent update in 2018, which aimed to implement quality improvement strategies for stroke care in VHA hospitals.14 Although focusing primarily on acute stroke care in the ED, this directive has led to increased awareness of areas for improvement, particularly among larger VHA hospitals. Prior to this directive, although national stroke guidelines were well-defined, more variability likely existed in stroke protocols and the manner in which stroke care was delivered across care settings. As efforts to measure and improve stroke care evolve, it is important to ensure that strategies used in ED settings also are implemented for patients already admitted to the hospital. This study seeks to define the quality of care in VHA hospitals between patients having an in-hospital ischemic stroke compared with those presenting to the ED.
Methods
As a secondary analysis, we examined stroke care quality data from an 11-site VHA stroke quality improvement study.15 Sites participating in this study were high stroke volume VHA hospitals from various geographic regions of the US. This study collected data on ICD-9 discharge diagnosis-defined ischemic stroke admissions between January 2009 and June 2012. Patient charts were reviewed by a group of central, trained abstractors who collected information on patient demographics, clinical history, and stroke characteristics. Stroke severity was defined using the National Institutes of Health Stroke Scale (NIHSS), assessed by standardized retrospective review of admission physical examination documentation.16 A multidisciplinary team defined 11 stroke quality indicators (QIs; the 8 Joint Commission indictors and 3 additional indicators: smoking cessation and dysphagia screening, and NIHSS assessment), and the chart abstractors’ data were used to evaluate eligibility and passing rates for each QI.
For our analysis, patients were stratified into 2 categories: patients admitted to the hospital for another diagnosis who developed an IHS, and patients presenting with stroke to the ED. We excluded patients transferred from other facilities. We then compared the demographic and clinical features of the 2 groups as well as eligibility and passing rates for each of the 11 QIs. Patients were recorded as eligible if they did not have any clinical contraindication to receiving the assessment or intervention measured by the quality metric. Passing rates were defined by the presence of clear documentation in the patient record that the quality metric was met or fulfilled. Comparisons were made using nonparametric Mann-Whitney U tests and chi-square tests. All tests were performed at α .05 level.
Results
A total of 1823 patients were included in this analysis: 35 IHS and 1788 ED strokes. The 2 groups did not differ with respect to age, race, or sex (Table 1). Patients with IHS had higher stroke severity (mean NIHSS 11.3 vs 5.1, P <.01) and longer length of stay than did ED patients with stroke (mean 12.8 vs 7.3 days, P < .01). Patients with IHS also were less likely to be discharged home when compared with ED patients with stroke (34.3% vs 63.8%, P < .01).
Table 2 summarizes our findings on eligibility and passing rates for the 11 QIs. For acute care metrics, we found that stroke severity documentation rates did not differ but were low for each patient group (51% vs 48%, P = .07). Patients with IHS were more likely to be eligible for IV tissue plasminogen activator (tPA; P < .01) although utilization rates did not differ. Only 2% of ED patients met eligibility criteria to receive tPA (36 of 1788), and among these patients only 16 actually received the drug. By comparison, 5 of 6 of eligible patients with IHS received tPA. Rates of dysphagia screening also were low for both groups, and patients with IHS were less likely to receive this screen prior to initiation of oral intake than were ED patients with stroke (27% vs 50%, P = .01).
Beyond the acute period, we found that patients with IHS were less likely than were ED patients with stroke to be eligible to receive antithrombotic therapy by 2 days after their initial stroke evaluation (74% vs 96%, P < .01), although treatment rates were similar between the 2 groups (P = .99). In patients with documented atrial fibrillation, initiation of anticoagulation therapy also did not differ (P = .99). The 2 groups were similar with respect to initiation of venous thromboembolism (VTE) prophylaxis (P = .596) and evaluation for rehabilitation needs (P = .42). Although rates of smoking cessation counseling and stroke education prior to discharge did not differ, overall rates of stroke education were very low for both groups (25% vs 36%, P = .55).
Similar to initiation of antithrombotic therapy in the hospital, we found lower rates of eligibility to receive antithrombotic therapy on discharge in the IHS group when compared with the ED group (77% vs 93%, P = .04). However, actual treatment initiation rates did not differ (P = .12). Use of lipid-lowering agents was similar for the 2 groups (P = .12).
Discussion
Our study found that veterans who develop an IHS received similar quality of care as did those presenting to the ED with stroke symptoms for many QIs, although there were some notable differences. We were pleased to find that overall rates of secondary stroke prevention initiation (antithrombotic and statin therapy), VTE prophylaxis, rehabilitation evaluations, and smoking cessation counseling were high for both groups, in keeping with evidence-based guidelines.17 This likely reflected the fact that these metrics typically involve care outside of the acute period and are less likely to be influenced by the location of initial stroke evaluation. Furthermore, efforts to improve smoking cessation and VTE prophylaxis are not exclusive to stroke care and have been the target of several nonstroke quality projects in the VHA. Many aspects of acute stroke care did differ, and present opportunities for quality improvement in the future.
In our sample, patients with IHS had higher IV thrombolytic eligibility, which has not typically been reported in other samples.10,11,18 In these studies, hospitalized patients have been reported to more often have contraindications to tPA, such as recent surgery or lack of stroke symptom recognition due to delirium or medication effects. Interestingly, patients presenting to VHA EDs had extremely low rates of tPA eligibility (2%), which is lower than many reported estimates of tPA eligibility outside of the VHA.19,20 This may be due to multiple influences, such as geographic barriers, patient perceptions about stroke symptoms, access to emergency medical services (EMS), EMS routing patterns, and social/cultural factors. Although not statistically significant due to small sample size, tPA use also was twice as high in the IHS group.
Given that a significant proportion of patients with IHS in the VHA system may be eligible for acute thrombolysis, our findings highlight the need for acute stroke protocols to ensure that patients with IHS receive the same rapid stroke assessment and access to thrombolytics as do patients evaluated in the ED. Further investigation is needed to determine whether there are unique features of patients with IHS in VHA hospitals, which may make them more eligible for IV thrombolysis.
Dysphagia is associated with increased risks for aspiration pneumonia in stroke patients.21 We found that patients with IHS were less likely to receive dysphagia screening compared with that of stroke patients admitted through the ED. This finding is consistent with the fact that care for patients with IHS is less frequently guided by specific stroke care protocols and algorithms that are more often used in EDs.8,11 Although attention to swallowing function may lead to improved outcomes in stroke, this can be easily overlooked in patients with IHS.22 However, low dysphagia screening also was found in patients admitted through the ED, suggesting that low screening rates cannot be solely explained by differences in where the initial stroke evaluation is occurring. These findings suggest a need for novel approaches to dysphagia screening in VHA stroke patients that can be universally implemented throughout the hospital.
Finally, we also found very low rates of stroke education prior to discharge for both groups. Given the risk of stroke recurrence and the overall poor level of public knowledge about stroke, providing patients with stroke with formal oral and written information on stroke is a critical component of secondary prevention.23,24 Educational tools, including those that are veteran specific, are now available for use in VHA hospitals and should be incorporated into quality improvement strategies for stroke care in VHA hospitals.
In 2012, the VHA Acute Stroke Treatment Directive was published in an effort to improve stroke care systemwide. Several of the metrics examined in this study are addressed in this directive. The data presented in this study is one of the only samples of stroke quality metrics within the VHA that largely predates the directive and can serve as a baseline comparator for future work examining stroke care after release of the directive. At present, although continuous internal reviews of quality data are ongoing, longitudinal description of stroke care quality since publication of the directive will help to inform future efforts to improve stroke care for veterans.
Limitations
Despite the strength of being a multicenter sampling of stroke care in high volume VHA hospitals, our study had several limitations. The IHS sample size was small, which limited our ability to evaluate differences between the groups, to evaluate generalizability, and account for estimation error.13 It is possible that differences existed between the groups that could not be observed in this sample due to small size (type II error) or that patient-specific characteristics not captured by these data could influence these metrics. Assessments of eligibility and passing were based on retrospective chart review and post hoc coding. Our sample assessed only patients who presented to larger VHA hospitals with higher stroke volumes, thus these findings may not be generalizable to smaller VHA hospitals with less systematized stroke care. This sample did not describe the specialty care services that were received by each patient, which may have influenced their stroke care. Finally, this study is an analysis of use of QIs in stroke care and did not examine how these indicators affect outcomes.
Conclusion
Despite reassuring findings for several inpatient ischemic stroke quality metrics, we found several differences in stroke care between patients with IHS compared with those presenting to the ED, emphasizing the need for standardized approaches to stroke care regardless of care setting. Although patients with IHS may be more likely to be eligible for tPA, these patients received dysphagia screening and less often than did ED patients with stroke. Ongoing quality initiatives should continue to place emphasis on improving all quality metrics (particularly dysphagia screening, stroke severity documentation, and stroke education) for patients with stroke at VHA hospitals across all care settings. Future work will be needed to examine how specific patient characteristics and revisions to stroke protocols may affect stroke quality metrics and outcomes between patients with IHS and those presenting to the ED.
Acknowledgments
The authors would like to thank Danielle Sager for her contributions to this project.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292.
2. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines—Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
3. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
4. The Joint Commission. Certificate of distinction for primary stroke centers. https://www.jointcommission.org/certificate_of_distinction_for_primary_stroke_centers_/.Published April 30, 2012. Accessed July 9, 2019.
5. US Department of Veterans Affairs. Center highlight: acute ischemic stroke care for veterans. https://www.queri.research.va.gov/center_highlights/stroke.cfm. Updated February 20, 2014. Accessed July 16, 2019.
6. Chumbler NR, Jia H, Phipps MS, et al. Does inpatient quality of care differ by age among US veterans with ischemic stroke? J Stroke Cerebrovasc Dis. 2012;21(8):844-851.
7. Katzan IL, Spertus J, Bettger JP, et al; American Heart Association Stroke Council; Council on Quality of Care and Outcomes Research; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Risk adjustment of ischemic stroke outcomes for comparing hospital performance: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(3):918-944.
8. Cumbler E, Wald H, Bhatt DL, et al. Quality of care and outcomes for in-hospital ischemic stroke: findings from the National Get With the Guidelines—Stroke. Stroke. 2014;45(1):231-238.
9. Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
10. Farooq MU, Reeves MJ, Gargano J, Wehner S, Hickenbottom S, Majid A; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. In-hospital stroke in a statewide stroke registry. Cerebrovascular Dis. 2008;25(1-2):12-20.
11. Bhalla A, Smeeton N, Rudd AG, Heuschmann P, Wolfe CD. A comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis. 2010;19:(5)357-363.
12. Garcia-Santibanez R, Liang J, Walker A, Matos-Diaz I, Kahkeshani K, Boniece I. Comparison of stroke codes in the emergency room and inpatient setting. J Stroke Cerebrovasc Dis. 2015;24(8):1948-1950.
13. Arling G, Reeves M, Ross J, et al. Estimating and reporting on the quality of inpatient stroke care by Veterans Health Administration medical centers. Circ Cardiovasc Qual Outcomes. 2012;5(1):44-51.
14. US Department of Veterans Affairs. Treatment of Acute Ischemic Stroke (AIS). VHA Directive 2011-038. https://www.hsrd.research.va.gov/news/feature/stroke.cfm. Updated January 20, 2014. Accessed July 17, 2019.
15. Williams LS, Daggett V, Slaven J, et al. Abstract 18: Does quality improvement training add to audit and feedback for inpatient stroke care processes? [International Stroke Conference abstract 18] Stroke. 2014;45(suppl 1):A18.
16. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858-862.
17. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
18. Park HJ, Cho HJ, Kim YD, et al. Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol. 2009;16(5):582-588.
19. Messé SR, Fonarow GC, Smith EE, et al. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2012;5(3):321-326.
20. Allen NB, Kaltenbach L, Goldstein LB, et al. Regional variation in recommended treatments for ischemic stroke and TIA: Get With the Guidelines—Stroke 2003-2010. Stroke. 2012;43(7):1858-1864.
21. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756-2763.
22. Bravata DM, Wells CK, Lo AC, et al. Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170(9):804-810.
23. Mosley I, Nicol M, Donnan G, Patrick I, Dewey H. Stroke symptoms and the decision to call for an ambulance. Stroke; a journal of cerebral circulation. 2007;38(2):361-366.
24. Jurkowski JM, Maniccia DM, Dennison BA, Samuels SJ, Spicer DA. Awareness of necessity to call 9-1-1 for stroke symptoms, upstate New York. Prev Chronic Dis. 2008;5(2):A41.
1. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28-e292.
2. Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With the Guidelines—Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
3. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
4. The Joint Commission. Certificate of distinction for primary stroke centers. https://www.jointcommission.org/certificate_of_distinction_for_primary_stroke_centers_/.Published April 30, 2012. Accessed July 9, 2019.
5. US Department of Veterans Affairs. Center highlight: acute ischemic stroke care for veterans. https://www.queri.research.va.gov/center_highlights/stroke.cfm. Updated February 20, 2014. Accessed July 16, 2019.
6. Chumbler NR, Jia H, Phipps MS, et al. Does inpatient quality of care differ by age among US veterans with ischemic stroke? J Stroke Cerebrovasc Dis. 2012;21(8):844-851.
7. Katzan IL, Spertus J, Bettger JP, et al; American Heart Association Stroke Council; Council on Quality of Care and Outcomes Research; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Risk adjustment of ischemic stroke outcomes for comparing hospital performance: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(3):918-944.
8. Cumbler E, Wald H, Bhatt DL, et al. Quality of care and outcomes for in-hospital ischemic stroke: findings from the National Get With the Guidelines—Stroke. Stroke. 2014;45(1):231-238.
9. Blacker DJ. In-hospital stroke. Lancet Neurol. 2003;2(12):741-746.
10. Farooq MU, Reeves MJ, Gargano J, Wehner S, Hickenbottom S, Majid A; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. In-hospital stroke in a statewide stroke registry. Cerebrovascular Dis. 2008;25(1-2):12-20.
11. Bhalla A, Smeeton N, Rudd AG, Heuschmann P, Wolfe CD. A comparison of characteristics and resource use between in-hospital and admitted patients with stroke. J Stroke Cerebrovasc Dis. 2010;19:(5)357-363.
12. Garcia-Santibanez R, Liang J, Walker A, Matos-Diaz I, Kahkeshani K, Boniece I. Comparison of stroke codes in the emergency room and inpatient setting. J Stroke Cerebrovasc Dis. 2015;24(8):1948-1950.
13. Arling G, Reeves M, Ross J, et al. Estimating and reporting on the quality of inpatient stroke care by Veterans Health Administration medical centers. Circ Cardiovasc Qual Outcomes. 2012;5(1):44-51.
14. US Department of Veterans Affairs. Treatment of Acute Ischemic Stroke (AIS). VHA Directive 2011-038. https://www.hsrd.research.va.gov/news/feature/stroke.cfm. Updated January 20, 2014. Accessed July 17, 2019.
15. Williams LS, Daggett V, Slaven J, et al. Abstract 18: Does quality improvement training add to audit and feedback for inpatient stroke care processes? [International Stroke Conference abstract 18] Stroke. 2014;45(suppl 1):A18.
16. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858-862.
17. Jauch EC, Saver JL, Adams HP Jr, et al; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947.
18. Park HJ, Cho HJ, Kim YD, et al. Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol. 2009;16(5):582-588.
19. Messé SR, Fonarow GC, Smith EE, et al. Use of tissue-type plasminogen activator before and after publication of the European Cooperative Acute Stroke Study III in Get With the Guidelines-Stroke. Circ Cardiovasc Qual Outcomes. 2012;5(3):321-326.
20. Allen NB, Kaltenbach L, Goldstein LB, et al. Regional variation in recommended treatments for ischemic stroke and TIA: Get With the Guidelines—Stroke 2003-2010. Stroke. 2012;43(7):1858-1864.
21. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756-2763.
22. Bravata DM, Wells CK, Lo AC, et al. Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170(9):804-810.
23. Mosley I, Nicol M, Donnan G, Patrick I, Dewey H. Stroke symptoms and the decision to call for an ambulance. Stroke; a journal of cerebral circulation. 2007;38(2):361-366.
24. Jurkowski JM, Maniccia DM, Dennison BA, Samuels SJ, Spicer DA. Awareness of necessity to call 9-1-1 for stroke symptoms, upstate New York. Prev Chronic Dis. 2008;5(2):A41.
Does endovascular thrombectomy benefit stroke patients with large infarcts?
Endovascular thrombectomy may benefit patients with stroke with large infarcts, an analysis suggests. The intervention may be more likely to benefit patients who “are treated early and have a core volume less than 100 cm3,” researchers reported in JAMA Neurology.
Clinical trials evaluating thrombectomy have largely excluded patients with large ischemic cores. To examine whether thrombectomy produces reasonable functional and safety outcomes in patients with stroke with large infarcts, compared with medical management alone, the investigators conducted a prespecified secondary analysis of data from the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
A nonrandomized study
Amrou Sarraj, MD, of the University of Texas, Houston, and his coauthors analyzed data from 105 patients in the prospective, multicenter cohort study, which enrolled patients between January 2016 and February 2018. Their analysis included data from patients who had large ischemic cores on CT (Alberta Stroke Program Early CT Score, 0-5) or on CT perfusion images (an ischemic core volume of at least 50 cm3). The SELECT study included patients with moderate to severe stroke and anterior circulation large-vessel occlusion who presented up to 24 hours from the time they last were known to be well. In the SELECT study, local investigators decided whether patients received endovascular thrombectomy or medical management alone in a nonrandomized fashion.
The 105 patients had a median age of 66 years, and 43% were female. Of the patients with large infarcts, 62 (59%) received endovascular thrombectomy plus medical management, and the rest received medical management alone.
At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27). In addition, endovascular thrombectomy was associated with better functional outcome, less infarct growth (44 vs. 98 mL), and smaller final infarct volume (97 vs. 190 mL).
The rates of neurologic worsening and symptomatic intracerebral hemorrhage were similar in both treatment groups, while mortality was lower among patients who received thrombectomy (29% vs. 42%). The likelihood of functional independence with endovascular thrombectomy decreased by 40% with each 1-hour delay in treatment and by 42% with each 10-cm3 increase in stroke volume.
Of 10 patients with core volumes greater than 100 cm3 who received endovascular thrombectomy, none had a favorable outcome.
“Although the odds of good outcomes for patients with large cores who received [endovascular thrombectomy] markedly decline with increasing core size and time to treatment, these data suggest potential benefits,” Dr. Sarraj and colleagues concluded. “Randomized clinical trials are needed.”
The authors noted that the results “did not reach significance after adjusting for baseline imbalances” and that “the small sample size limits the power of this analysis.”
The study was funded by an unrestricted grant from Stryker Neurovascular to the University of Texas. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. In addition, he is a site principal investigator for the TREVO Registry and DEFUSE 3 trials. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
SOURCE: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.
Patients who had thrombectomies had improved outcomes in an unadjusted statistical analysis, but these differences did not remain significant after adjustment for baseline age, clinical severity, and other key prognostic variables. However, the analysis was underpowered.
A key finding was that favorable outcomes in patients with large core volumes was strongly time dependent, which was consistent with previous data from the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration.
Faster treatment is the key to maximizing benefit for patients with poor collateral blood flow and a large ischemic core at baseline. As treatment work flow improves and more patients are transported directly to a thrombectomy-capable center, the number who benefit from reperfusion, despite a large ischemic core, is likely to further increase.
Ongoing randomized clinical trials are assessing the practical question of who to treat with thrombectomy when the estimated ischemic core volume is large.
Bruce C. V. Campbell, MBBS, PhD , of the University of Melbourne made these comments in an accompanying editorial. He reported research support from the several Australian research foundations. He also reported unrestricted grant funding for the Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial (EXTEND-IA) trial to the Florey Institute of Neuroscience and Mental Health in Parkville, Australia, from Covidien (Medtronic).
Patients who had thrombectomies had improved outcomes in an unadjusted statistical analysis, but these differences did not remain significant after adjustment for baseline age, clinical severity, and other key prognostic variables. However, the analysis was underpowered.
A key finding was that favorable outcomes in patients with large core volumes was strongly time dependent, which was consistent with previous data from the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration.
Faster treatment is the key to maximizing benefit for patients with poor collateral blood flow and a large ischemic core at baseline. As treatment work flow improves and more patients are transported directly to a thrombectomy-capable center, the number who benefit from reperfusion, despite a large ischemic core, is likely to further increase.
Ongoing randomized clinical trials are assessing the practical question of who to treat with thrombectomy when the estimated ischemic core volume is large.
Bruce C. V. Campbell, MBBS, PhD , of the University of Melbourne made these comments in an accompanying editorial. He reported research support from the several Australian research foundations. He also reported unrestricted grant funding for the Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial (EXTEND-IA) trial to the Florey Institute of Neuroscience and Mental Health in Parkville, Australia, from Covidien (Medtronic).
Patients who had thrombectomies had improved outcomes in an unadjusted statistical analysis, but these differences did not remain significant after adjustment for baseline age, clinical severity, and other key prognostic variables. However, the analysis was underpowered.
A key finding was that favorable outcomes in patients with large core volumes was strongly time dependent, which was consistent with previous data from the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration.
Faster treatment is the key to maximizing benefit for patients with poor collateral blood flow and a large ischemic core at baseline. As treatment work flow improves and more patients are transported directly to a thrombectomy-capable center, the number who benefit from reperfusion, despite a large ischemic core, is likely to further increase.
Ongoing randomized clinical trials are assessing the practical question of who to treat with thrombectomy when the estimated ischemic core volume is large.
Bruce C. V. Campbell, MBBS, PhD , of the University of Melbourne made these comments in an accompanying editorial. He reported research support from the several Australian research foundations. He also reported unrestricted grant funding for the Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial (EXTEND-IA) trial to the Florey Institute of Neuroscience and Mental Health in Parkville, Australia, from Covidien (Medtronic).
Endovascular thrombectomy may benefit patients with stroke with large infarcts, an analysis suggests. The intervention may be more likely to benefit patients who “are treated early and have a core volume less than 100 cm3,” researchers reported in JAMA Neurology.
Clinical trials evaluating thrombectomy have largely excluded patients with large ischemic cores. To examine whether thrombectomy produces reasonable functional and safety outcomes in patients with stroke with large infarcts, compared with medical management alone, the investigators conducted a prespecified secondary analysis of data from the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
A nonrandomized study
Amrou Sarraj, MD, of the University of Texas, Houston, and his coauthors analyzed data from 105 patients in the prospective, multicenter cohort study, which enrolled patients between January 2016 and February 2018. Their analysis included data from patients who had large ischemic cores on CT (Alberta Stroke Program Early CT Score, 0-5) or on CT perfusion images (an ischemic core volume of at least 50 cm3). The SELECT study included patients with moderate to severe stroke and anterior circulation large-vessel occlusion who presented up to 24 hours from the time they last were known to be well. In the SELECT study, local investigators decided whether patients received endovascular thrombectomy or medical management alone in a nonrandomized fashion.
The 105 patients had a median age of 66 years, and 43% were female. Of the patients with large infarcts, 62 (59%) received endovascular thrombectomy plus medical management, and the rest received medical management alone.
At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27). In addition, endovascular thrombectomy was associated with better functional outcome, less infarct growth (44 vs. 98 mL), and smaller final infarct volume (97 vs. 190 mL).
The rates of neurologic worsening and symptomatic intracerebral hemorrhage were similar in both treatment groups, while mortality was lower among patients who received thrombectomy (29% vs. 42%). The likelihood of functional independence with endovascular thrombectomy decreased by 40% with each 1-hour delay in treatment and by 42% with each 10-cm3 increase in stroke volume.
Of 10 patients with core volumes greater than 100 cm3 who received endovascular thrombectomy, none had a favorable outcome.
“Although the odds of good outcomes for patients with large cores who received [endovascular thrombectomy] markedly decline with increasing core size and time to treatment, these data suggest potential benefits,” Dr. Sarraj and colleagues concluded. “Randomized clinical trials are needed.”
The authors noted that the results “did not reach significance after adjusting for baseline imbalances” and that “the small sample size limits the power of this analysis.”
The study was funded by an unrestricted grant from Stryker Neurovascular to the University of Texas. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. In addition, he is a site principal investigator for the TREVO Registry and DEFUSE 3 trials. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
SOURCE: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.
Endovascular thrombectomy may benefit patients with stroke with large infarcts, an analysis suggests. The intervention may be more likely to benefit patients who “are treated early and have a core volume less than 100 cm3,” researchers reported in JAMA Neurology.
Clinical trials evaluating thrombectomy have largely excluded patients with large ischemic cores. To examine whether thrombectomy produces reasonable functional and safety outcomes in patients with stroke with large infarcts, compared with medical management alone, the investigators conducted a prespecified secondary analysis of data from the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
A nonrandomized study
Amrou Sarraj, MD, of the University of Texas, Houston, and his coauthors analyzed data from 105 patients in the prospective, multicenter cohort study, which enrolled patients between January 2016 and February 2018. Their analysis included data from patients who had large ischemic cores on CT (Alberta Stroke Program Early CT Score, 0-5) or on CT perfusion images (an ischemic core volume of at least 50 cm3). The SELECT study included patients with moderate to severe stroke and anterior circulation large-vessel occlusion who presented up to 24 hours from the time they last were known to be well. In the SELECT study, local investigators decided whether patients received endovascular thrombectomy or medical management alone in a nonrandomized fashion.
The 105 patients had a median age of 66 years, and 43% were female. Of the patients with large infarcts, 62 (59%) received endovascular thrombectomy plus medical management, and the rest received medical management alone.
At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27). In addition, endovascular thrombectomy was associated with better functional outcome, less infarct growth (44 vs. 98 mL), and smaller final infarct volume (97 vs. 190 mL).
The rates of neurologic worsening and symptomatic intracerebral hemorrhage were similar in both treatment groups, while mortality was lower among patients who received thrombectomy (29% vs. 42%). The likelihood of functional independence with endovascular thrombectomy decreased by 40% with each 1-hour delay in treatment and by 42% with each 10-cm3 increase in stroke volume.
Of 10 patients with core volumes greater than 100 cm3 who received endovascular thrombectomy, none had a favorable outcome.
“Although the odds of good outcomes for patients with large cores who received [endovascular thrombectomy] markedly decline with increasing core size and time to treatment, these data suggest potential benefits,” Dr. Sarraj and colleagues concluded. “Randomized clinical trials are needed.”
The authors noted that the results “did not reach significance after adjusting for baseline imbalances” and that “the small sample size limits the power of this analysis.”
The study was funded by an unrestricted grant from Stryker Neurovascular to the University of Texas. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. In addition, he is a site principal investigator for the TREVO Registry and DEFUSE 3 trials. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
SOURCE: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.
FROM JAMA NEUROLOGY
Key clinical point: and who have a core volume less than 100 mL.
Major finding: At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27).
Study details: A prespecified secondary analysis of nonrandomized data from 105 patients in the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
Disclosures: The study was funded by an unrestricted grant from Stryker Neurovascular. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
Source: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.
Statins crush early seizure risk poststroke
BANGKOK – Statin therapy, even when initiated only upon hospitalization for acute ischemic stroke, was associated with a striking reduction in the risk of early poststroke symptomatic seizure in a large observational study.
Using propensity-score matching to control for potential confounders, use of a statin during acute stroke management was associated with a “robust” 77% reduction in the risk of developing a symptomatic seizure within 7 days after hospital admission, Soichiro Matsubara, MD, reported at the International Epilepsy Congress.
This is an important finding because early symptomatic seizure (ESS) occurs in 2%-7% of patients following an acute ischemic stroke. Moreover, an Italian meta-analysis concluded that ESS was associated with a 4.4-fold increased risk of developing poststroke epilepsy (Epilepsia. 2016 Aug;57[8]:1205-14), noted Dr. Matsubara, a neurologist at the National Cerebral and Cardiovascular Center in Suita, Japan, as well as at Kumamoto (Japan) University.
He presented a study of 2,969 consecutive acute ischemic stroke patients with no history of epilepsy who were admitted to the Japanese comprehensive stroke center, of whom 2.2% experienced ESS. At physician discretion, 19% of the ESS cohort were on a statin during their acute stroke management, as were 55% of the no-ESS group. Four-fifths of patients on a statin initiated the drug only upon hospital admission.
Strokes tended to be more severe in the ESS group, with a median initial National Institutes of Health Stroke Scale score of 12.5, compared with 4 in the seizure-free patients. A cortical stroke lesion was evident upon imaging in 89% of the ESS group and 55% of no-ESS patients. Among ESS patients, 46% had a cardiometabolic stroke, compared with 34% of the no-ESS cohort. Mean C-reactive protein levels and white blood cell counts were significantly higher in the ESS cohort as well. Their median hospital length of stay was 25.5 days, versus 18 days in the no-ESS group, Dr. Matsubara said at the congress sponsored by the International League Against Epilepsy.
Of the 76 ESSs that occurred in 66 patients, 37% were focal awareness seizures, 35% were focal to bilateral tonic-clonic seizures, and 28% were focal impaired awareness seizures.
In a multivariate analysis adjusted for age, sex, body mass index, stroke subtype, and other potential confounders, statin therapy during acute management of stroke was independently associated with a 56% reduction in the relative risk of ESS. In contrast, a cortical stroke lesion was associated with a 2.83-fold increased risk.
Since this wasn’t a randomized trial of statin therapy, Dr. Matsubara and his coinvestigators felt the need to go further in analyzing the data. After extensive propensity score matching for atrial fibrillation, current smoking, systolic blood pressure, the presence or absence of a cortical stroke lesion, large vessel stenosis, and other possible confounders, they were left with two closely comparable groups: 886 statin-treated stroke patients and an equal number who were not on statin therapy during their acute stroke management. The key finding: The risk of ESS was reduced by a whopping 77% in the patients on statin therapy.
The neurologist observed that these new findings in acute ischemic stroke patients are consistent with an earlier study in a U.S. Veterans Affairs population, which demonstrated that statin therapy was associated with a significantly lower risk of new-onset geriatric epilepsy (J Am Geriatr Soc. 2009 Feb;57[2]:237-42).
As to the possible mechanism by which statins may protect against ESS, Dr. Matsubara noted that acute ischemic stroke causes toxic neuronal excitation because of blood-brain barrier disruption, ion channel dysfunction, altered gene expression, and increased release of neurotransmitters. In animal models, statins provide a neuroprotective effect by reducing glutamate levels, activating endothelial nitric oxide synthase, and inhibiting production of interleukin-6, tumor necrosis factor-alpha, and other inflammatory cytokines.
Asked about the intensity of the statin therapy, Dr. Matsubara replied that the target was typically an LDL cholesterol below 100 mg/dL.
He reported having no financial conflicts regarding the study, conducted free of commercial support.
SOURCE: Matsubara S et al. IEC 219, Abstract P002.
BANGKOK – Statin therapy, even when initiated only upon hospitalization for acute ischemic stroke, was associated with a striking reduction in the risk of early poststroke symptomatic seizure in a large observational study.
Using propensity-score matching to control for potential confounders, use of a statin during acute stroke management was associated with a “robust” 77% reduction in the risk of developing a symptomatic seizure within 7 days after hospital admission, Soichiro Matsubara, MD, reported at the International Epilepsy Congress.
This is an important finding because early symptomatic seizure (ESS) occurs in 2%-7% of patients following an acute ischemic stroke. Moreover, an Italian meta-analysis concluded that ESS was associated with a 4.4-fold increased risk of developing poststroke epilepsy (Epilepsia. 2016 Aug;57[8]:1205-14), noted Dr. Matsubara, a neurologist at the National Cerebral and Cardiovascular Center in Suita, Japan, as well as at Kumamoto (Japan) University.
He presented a study of 2,969 consecutive acute ischemic stroke patients with no history of epilepsy who were admitted to the Japanese comprehensive stroke center, of whom 2.2% experienced ESS. At physician discretion, 19% of the ESS cohort were on a statin during their acute stroke management, as were 55% of the no-ESS group. Four-fifths of patients on a statin initiated the drug only upon hospital admission.
Strokes tended to be more severe in the ESS group, with a median initial National Institutes of Health Stroke Scale score of 12.5, compared with 4 in the seizure-free patients. A cortical stroke lesion was evident upon imaging in 89% of the ESS group and 55% of no-ESS patients. Among ESS patients, 46% had a cardiometabolic stroke, compared with 34% of the no-ESS cohort. Mean C-reactive protein levels and white blood cell counts were significantly higher in the ESS cohort as well. Their median hospital length of stay was 25.5 days, versus 18 days in the no-ESS group, Dr. Matsubara said at the congress sponsored by the International League Against Epilepsy.
Of the 76 ESSs that occurred in 66 patients, 37% were focal awareness seizures, 35% were focal to bilateral tonic-clonic seizures, and 28% were focal impaired awareness seizures.
In a multivariate analysis adjusted for age, sex, body mass index, stroke subtype, and other potential confounders, statin therapy during acute management of stroke was independently associated with a 56% reduction in the relative risk of ESS. In contrast, a cortical stroke lesion was associated with a 2.83-fold increased risk.
Since this wasn’t a randomized trial of statin therapy, Dr. Matsubara and his coinvestigators felt the need to go further in analyzing the data. After extensive propensity score matching for atrial fibrillation, current smoking, systolic blood pressure, the presence or absence of a cortical stroke lesion, large vessel stenosis, and other possible confounders, they were left with two closely comparable groups: 886 statin-treated stroke patients and an equal number who were not on statin therapy during their acute stroke management. The key finding: The risk of ESS was reduced by a whopping 77% in the patients on statin therapy.
The neurologist observed that these new findings in acute ischemic stroke patients are consistent with an earlier study in a U.S. Veterans Affairs population, which demonstrated that statin therapy was associated with a significantly lower risk of new-onset geriatric epilepsy (J Am Geriatr Soc. 2009 Feb;57[2]:237-42).
As to the possible mechanism by which statins may protect against ESS, Dr. Matsubara noted that acute ischemic stroke causes toxic neuronal excitation because of blood-brain barrier disruption, ion channel dysfunction, altered gene expression, and increased release of neurotransmitters. In animal models, statins provide a neuroprotective effect by reducing glutamate levels, activating endothelial nitric oxide synthase, and inhibiting production of interleukin-6, tumor necrosis factor-alpha, and other inflammatory cytokines.
Asked about the intensity of the statin therapy, Dr. Matsubara replied that the target was typically an LDL cholesterol below 100 mg/dL.
He reported having no financial conflicts regarding the study, conducted free of commercial support.
SOURCE: Matsubara S et al. IEC 219, Abstract P002.
BANGKOK – Statin therapy, even when initiated only upon hospitalization for acute ischemic stroke, was associated with a striking reduction in the risk of early poststroke symptomatic seizure in a large observational study.
Using propensity-score matching to control for potential confounders, use of a statin during acute stroke management was associated with a “robust” 77% reduction in the risk of developing a symptomatic seizure within 7 days after hospital admission, Soichiro Matsubara, MD, reported at the International Epilepsy Congress.
This is an important finding because early symptomatic seizure (ESS) occurs in 2%-7% of patients following an acute ischemic stroke. Moreover, an Italian meta-analysis concluded that ESS was associated with a 4.4-fold increased risk of developing poststroke epilepsy (Epilepsia. 2016 Aug;57[8]:1205-14), noted Dr. Matsubara, a neurologist at the National Cerebral and Cardiovascular Center in Suita, Japan, as well as at Kumamoto (Japan) University.
He presented a study of 2,969 consecutive acute ischemic stroke patients with no history of epilepsy who were admitted to the Japanese comprehensive stroke center, of whom 2.2% experienced ESS. At physician discretion, 19% of the ESS cohort were on a statin during their acute stroke management, as were 55% of the no-ESS group. Four-fifths of patients on a statin initiated the drug only upon hospital admission.
Strokes tended to be more severe in the ESS group, with a median initial National Institutes of Health Stroke Scale score of 12.5, compared with 4 in the seizure-free patients. A cortical stroke lesion was evident upon imaging in 89% of the ESS group and 55% of no-ESS patients. Among ESS patients, 46% had a cardiometabolic stroke, compared with 34% of the no-ESS cohort. Mean C-reactive protein levels and white blood cell counts were significantly higher in the ESS cohort as well. Their median hospital length of stay was 25.5 days, versus 18 days in the no-ESS group, Dr. Matsubara said at the congress sponsored by the International League Against Epilepsy.
Of the 76 ESSs that occurred in 66 patients, 37% were focal awareness seizures, 35% were focal to bilateral tonic-clonic seizures, and 28% were focal impaired awareness seizures.
In a multivariate analysis adjusted for age, sex, body mass index, stroke subtype, and other potential confounders, statin therapy during acute management of stroke was independently associated with a 56% reduction in the relative risk of ESS. In contrast, a cortical stroke lesion was associated with a 2.83-fold increased risk.
Since this wasn’t a randomized trial of statin therapy, Dr. Matsubara and his coinvestigators felt the need to go further in analyzing the data. After extensive propensity score matching for atrial fibrillation, current smoking, systolic blood pressure, the presence or absence of a cortical stroke lesion, large vessel stenosis, and other possible confounders, they were left with two closely comparable groups: 886 statin-treated stroke patients and an equal number who were not on statin therapy during their acute stroke management. The key finding: The risk of ESS was reduced by a whopping 77% in the patients on statin therapy.
The neurologist observed that these new findings in acute ischemic stroke patients are consistent with an earlier study in a U.S. Veterans Affairs population, which demonstrated that statin therapy was associated with a significantly lower risk of new-onset geriatric epilepsy (J Am Geriatr Soc. 2009 Feb;57[2]:237-42).
As to the possible mechanism by which statins may protect against ESS, Dr. Matsubara noted that acute ischemic stroke causes toxic neuronal excitation because of blood-brain barrier disruption, ion channel dysfunction, altered gene expression, and increased release of neurotransmitters. In animal models, statins provide a neuroprotective effect by reducing glutamate levels, activating endothelial nitric oxide synthase, and inhibiting production of interleukin-6, tumor necrosis factor-alpha, and other inflammatory cytokines.
Asked about the intensity of the statin therapy, Dr. Matsubara replied that the target was typically an LDL cholesterol below 100 mg/dL.
He reported having no financial conflicts regarding the study, conducted free of commercial support.
SOURCE: Matsubara S et al. IEC 219, Abstract P002.
REPORTING FROM IEC 2019
Systolic, diastolic BP each tied to adverse CV outcomes
Both systolic and diastolic hypertension independently predict myocardial infarction and strokes, but systolic blood pressure is more strongly linked to adverse outcomes.
That’s according to a study of more than 1 million patients and 36 million outpatient blood pressure measurements published in the New England Journal of Medicine.
Systolic and diastolic hypertension predicted adverse outcomes at cutpoints of 140/90 and 130/80 mm Hg in the large retrospective cohort study, supporting the recent guideline changes that made blood pressure targets more stringent for higher-risk patients, said lead investigator Alexander C. Flint, MD, of Kaiser Permanente Northern California (KPNC) in Oakland.
“While systolic does count for more, in the fact that it is a stronger driver of the risk of heart attack and stroke, diastolic absolutely does as well, and it does so independently. So we ignore our diastolic hypertension at our own peril,” Dr. Flint said in an interview.
Systolic hypertension began to overshadow diastolic after the Framingham Heart Study and others that suggested it is a more important predictor of adverse cardiovascular outcomes, Dr. Flint and coauthors said in a report on their study.
Those findings caused some to say diastole should be abandoned, and led to a “near-exclusive focus” on systolic hypertension in a 2000 advisory statement from the National High Blood Pressure Education Program, they say in their report.
While current guidelines emphasize the importance of both systolic and diastolic targets, many clinicians today often assign little importance to diastolic blood pressure values, the report adds.
“The pendulum needs to swing back, right down in the middle,” Dr. Flint said in the interview.
The study by Dr. Flint and colleagues comprised a cohort of approximately 1.3 million outpatients from KPNC who had at least one baseline blood pressure reading in during 2007-2008, and two or more follow-up measurements between 2009 and 2016, for a total of about 36.8 million data points.
Systolic hypertension burden was linked to the composite of MI or stroke, with a hazard ratio of 1.18 (95% confidence interval, 1.17-1.18; P less than .001) per unit increase in z score, according to results of a multivariable regression analysis. Likewise, diastolic hypertension burden was linked to those adverse outcomes, with a hazard ratio of 1.06 (95% CI, 1.06-1.07; P less than .001).
Put in terms of estimated risk of MI or stroke, patients with a systolic blood pressure around 160 mm Hg – 3 standard deviations from the mean – was 4.8%, compared to a predicted risk of just 1.9% for a systolic blood pressure near 136 mm Hg, the investigators said in their report.
Similarly, predicted risk was 3.6% for a diastolic pressure of about 96 mm Hg, also 3 standard deviations from the mean, and 1.9% for a diastolic BP near 81 mm Hg.
“The two are not that separate,” Dr. Flint said of the risks associated with systolic and diastolic hypertension at that 3-standard-deviation point. Beyond that, increased systolic blood pressure is associated with more risk relative to increased diastolic blood pressure, the logistic regression modeling shows.
Taken together, findings from this large cohort study emphasize the importance of making lifestyle modifications and adjusting medication to ensure that both systolic and diastolic targets are met, according to Dr. Flint.
“Rises in systolic blood pressure count for more in influencing the risk of heart attack and stroke,” he said, “but diastolic independently counts for quite a lot. It’s a close second.”
Dr. Flint reported no disclosures. Senior author Deepak L. Bhatt, MD, MPH, reported disclosures with Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, Takeda, The Medicines Company, and others. The remaining authors had no disclosures.
SOURCE: Flint AC et al. N Engl J Med. 2019 Jul 18. doi: 10.1056/NEJMoa1803180.
Both systolic and diastolic hypertension independently predict myocardial infarction and strokes, but systolic blood pressure is more strongly linked to adverse outcomes.
That’s according to a study of more than 1 million patients and 36 million outpatient blood pressure measurements published in the New England Journal of Medicine.
Systolic and diastolic hypertension predicted adverse outcomes at cutpoints of 140/90 and 130/80 mm Hg in the large retrospective cohort study, supporting the recent guideline changes that made blood pressure targets more stringent for higher-risk patients, said lead investigator Alexander C. Flint, MD, of Kaiser Permanente Northern California (KPNC) in Oakland.
“While systolic does count for more, in the fact that it is a stronger driver of the risk of heart attack and stroke, diastolic absolutely does as well, and it does so independently. So we ignore our diastolic hypertension at our own peril,” Dr. Flint said in an interview.
Systolic hypertension began to overshadow diastolic after the Framingham Heart Study and others that suggested it is a more important predictor of adverse cardiovascular outcomes, Dr. Flint and coauthors said in a report on their study.
Those findings caused some to say diastole should be abandoned, and led to a “near-exclusive focus” on systolic hypertension in a 2000 advisory statement from the National High Blood Pressure Education Program, they say in their report.
While current guidelines emphasize the importance of both systolic and diastolic targets, many clinicians today often assign little importance to diastolic blood pressure values, the report adds.
“The pendulum needs to swing back, right down in the middle,” Dr. Flint said in the interview.
The study by Dr. Flint and colleagues comprised a cohort of approximately 1.3 million outpatients from KPNC who had at least one baseline blood pressure reading in during 2007-2008, and two or more follow-up measurements between 2009 and 2016, for a total of about 36.8 million data points.
Systolic hypertension burden was linked to the composite of MI or stroke, with a hazard ratio of 1.18 (95% confidence interval, 1.17-1.18; P less than .001) per unit increase in z score, according to results of a multivariable regression analysis. Likewise, diastolic hypertension burden was linked to those adverse outcomes, with a hazard ratio of 1.06 (95% CI, 1.06-1.07; P less than .001).
Put in terms of estimated risk of MI or stroke, patients with a systolic blood pressure around 160 mm Hg – 3 standard deviations from the mean – was 4.8%, compared to a predicted risk of just 1.9% for a systolic blood pressure near 136 mm Hg, the investigators said in their report.
Similarly, predicted risk was 3.6% for a diastolic pressure of about 96 mm Hg, also 3 standard deviations from the mean, and 1.9% for a diastolic BP near 81 mm Hg.
“The two are not that separate,” Dr. Flint said of the risks associated with systolic and diastolic hypertension at that 3-standard-deviation point. Beyond that, increased systolic blood pressure is associated with more risk relative to increased diastolic blood pressure, the logistic regression modeling shows.
Taken together, findings from this large cohort study emphasize the importance of making lifestyle modifications and adjusting medication to ensure that both systolic and diastolic targets are met, according to Dr. Flint.
“Rises in systolic blood pressure count for more in influencing the risk of heart attack and stroke,” he said, “but diastolic independently counts for quite a lot. It’s a close second.”
Dr. Flint reported no disclosures. Senior author Deepak L. Bhatt, MD, MPH, reported disclosures with Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, Takeda, The Medicines Company, and others. The remaining authors had no disclosures.
SOURCE: Flint AC et al. N Engl J Med. 2019 Jul 18. doi: 10.1056/NEJMoa1803180.
Both systolic and diastolic hypertension independently predict myocardial infarction and strokes, but systolic blood pressure is more strongly linked to adverse outcomes.
That’s according to a study of more than 1 million patients and 36 million outpatient blood pressure measurements published in the New England Journal of Medicine.
Systolic and diastolic hypertension predicted adverse outcomes at cutpoints of 140/90 and 130/80 mm Hg in the large retrospective cohort study, supporting the recent guideline changes that made blood pressure targets more stringent for higher-risk patients, said lead investigator Alexander C. Flint, MD, of Kaiser Permanente Northern California (KPNC) in Oakland.
“While systolic does count for more, in the fact that it is a stronger driver of the risk of heart attack and stroke, diastolic absolutely does as well, and it does so independently. So we ignore our diastolic hypertension at our own peril,” Dr. Flint said in an interview.
Systolic hypertension began to overshadow diastolic after the Framingham Heart Study and others that suggested it is a more important predictor of adverse cardiovascular outcomes, Dr. Flint and coauthors said in a report on their study.
Those findings caused some to say diastole should be abandoned, and led to a “near-exclusive focus” on systolic hypertension in a 2000 advisory statement from the National High Blood Pressure Education Program, they say in their report.
While current guidelines emphasize the importance of both systolic and diastolic targets, many clinicians today often assign little importance to diastolic blood pressure values, the report adds.
“The pendulum needs to swing back, right down in the middle,” Dr. Flint said in the interview.
The study by Dr. Flint and colleagues comprised a cohort of approximately 1.3 million outpatients from KPNC who had at least one baseline blood pressure reading in during 2007-2008, and two or more follow-up measurements between 2009 and 2016, for a total of about 36.8 million data points.
Systolic hypertension burden was linked to the composite of MI or stroke, with a hazard ratio of 1.18 (95% confidence interval, 1.17-1.18; P less than .001) per unit increase in z score, according to results of a multivariable regression analysis. Likewise, diastolic hypertension burden was linked to those adverse outcomes, with a hazard ratio of 1.06 (95% CI, 1.06-1.07; P less than .001).
Put in terms of estimated risk of MI or stroke, patients with a systolic blood pressure around 160 mm Hg – 3 standard deviations from the mean – was 4.8%, compared to a predicted risk of just 1.9% for a systolic blood pressure near 136 mm Hg, the investigators said in their report.
Similarly, predicted risk was 3.6% for a diastolic pressure of about 96 mm Hg, also 3 standard deviations from the mean, and 1.9% for a diastolic BP near 81 mm Hg.
“The two are not that separate,” Dr. Flint said of the risks associated with systolic and diastolic hypertension at that 3-standard-deviation point. Beyond that, increased systolic blood pressure is associated with more risk relative to increased diastolic blood pressure, the logistic regression modeling shows.
Taken together, findings from this large cohort study emphasize the importance of making lifestyle modifications and adjusting medication to ensure that both systolic and diastolic targets are met, according to Dr. Flint.
“Rises in systolic blood pressure count for more in influencing the risk of heart attack and stroke,” he said, “but diastolic independently counts for quite a lot. It’s a close second.”
Dr. Flint reported no disclosures. Senior author Deepak L. Bhatt, MD, MPH, reported disclosures with Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, Takeda, The Medicines Company, and others. The remaining authors had no disclosures.
SOURCE: Flint AC et al. N Engl J Med. 2019 Jul 18. doi: 10.1056/NEJMoa1803180.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: .
Major finding: Systolic and diastolic hypertension burden were linked to the composite endpoint with hazard ratios of 1.18 and 1.06 per unit increase in z score, respectively.
Study details: A retrospective cohort study of roughly 1.3 million outpatients with 36.8 million BP measurements.
Disclosures: The senior author of the study reported disclosures with Amarin, AstraZeneca, Bristol Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, Takeda, The Medicines Company, and others. The remaining authors had no disclosures.
Source: Flint AC et al. N Engl J Med. 2019 Jul 18. doi: 10.1056/NEJMoa1803180.
Lipoprotein(a) levels tied to higher ischemic stroke risk
High levels of lipoprotein(a) [Lp(a)] and LPA genotypes were linked to increased ischemic stroke risk in a recent large, contemporary general population study, investigators are reporting in the Journal of the American College of Cardiology.
Anne Langsted, MD, with Copenhagen University Hospital and the University of Copenhagen in Denmark, and her co-researchers evaluated the impact of high Lp(a) levels in a large contemporary cohort of 49,699 individuals in the Copenhagen General Population Study, and another 10,813 individuals in the Copenhagen City Heart Study.
Measurements assessed included plasma lipoprotein(a) levels and carrier or noncarrier status for LPA rs10455872. The endpoint of ischemic stroke was ascertained from Danish national health registries and confirmed by physicians.
Although risk estimates were less pronounced than what was reported before regarding the link between Lp(a) for ischemic heart disease and aortic valve stenosis, the risk of stroke was increased by a factor of 1.6 among individuals with high Lp(a) levels as compared to those with lower levels, the investigators said.
Compared with noncarriers of LPA rs1045572, the hazard ratio for ischemic stroke was 1.23 for carriers of LPA rs1045572, which was associated with high levels plasma lipoprotein(a) levels, according to the researchers.
“Our results indicate a causal association of Lp(a) with risk of ischemic stroke, and emphasize the need for randomized, controlled clinical trials on the effect of Lp(a)-lowering to prevent cardiovascular disease including ischemic stroke,” About 20% of the general population have high Lp(a) levels, and some individuals have extremely high levels, Dr. Langsted and co-authors said in their report.
Interest in Lp(a) as a risk factor for cardiovascular disease has been reignited following large studies showing that high Lp(a) levels were linked to increased risk of myocardial infarction and aortic valve stenosis, according to the investigators.
However, results of various studies are conflicting as to whether high Lp(a) levels increase risk of hemorrhagic or ischemic stroke, they said.
Both cohort studies used in the analysis were supported by sources in Denmark including the Danish Medical Research Council and Copenhagen University Hospital. Dr. Langsted had no disclosures. One co-author reported disclosures related to Akcea, Amgen, Sanofi, Regeneron, and AstraZeneca.
SOURCE: Langsted A, et al. JACC 2019;74[1]: 54-66. doi: 10.1016/j.jacc.2019.03.524
This study linking high lipoprotein(a) [Lp(a)] levels to stroke risk, taken together with previous research, provide a sound basis to routinely perform one-time screening so that individuals with inherited high levels can try to avoid adverse cardiovascular outcomes, according to Christie M. Ballantyne, MD.
“As someone in the dual role of preventive cardiologist and patient with a strong family history of cardiovascular disease, I think that we have sufficient evidence that high Lp(a) is strongly associated with an increased risk of myocardial infarction, stroke, and aortic valve stenosis,” Dr. Ballantyne wrote in an editorial comment on the study.
Evidence is now “overwhelming” that high Lp(a) is linked to myocardial infarction and stroke, and it’s known that statins and aspirin reduce risk of these outcomes, he said in the commentary.
Despite that, scientific statements do not recommend routine Lp(a) testing due to a lack of clinical trials evidence; as a result, clinical trials are not including Lp(a) as a routine measurement: “We thus have a loop of futility—lack of routine measurement leads to lack of data,” he said.
This most recent study from Langsted and colleagues demonstrates that high Lp(a) levels, and genetic variants associated with Lp(a), are associated with increased ischemic stroke risk. “The genetics strongly supported that high Lp(a) levels were in the causal pathway for ischemic stroke and coronary heart disease,” Dr. Ballantyne said.
One major strength and weakness of the study is its large and relatively homogeneous European population—that bolstered the genetic analyses, but also means the data can’t be extrapolated to other populations, such as Africans and East Asians, who have higher stroke rates compared with Europeans, Dr. Ballantyne said.
Dr. Ballantyne is with the Department of Medicine and Center for Cardiometabolic Disease Prevention, Baylor College of Medicine, Houston, Tex. His editorial comment appears in the Journal of the American College of Cardiology (2019;74[1]:67-9. doi:10.1016/j.jacc.2019.05.029 . Dr. Ballantyne reported disclosures related to Akcea, Amgen, and Novartis.
This study linking high lipoprotein(a) [Lp(a)] levels to stroke risk, taken together with previous research, provide a sound basis to routinely perform one-time screening so that individuals with inherited high levels can try to avoid adverse cardiovascular outcomes, according to Christie M. Ballantyne, MD.
“As someone in the dual role of preventive cardiologist and patient with a strong family history of cardiovascular disease, I think that we have sufficient evidence that high Lp(a) is strongly associated with an increased risk of myocardial infarction, stroke, and aortic valve stenosis,” Dr. Ballantyne wrote in an editorial comment on the study.
Evidence is now “overwhelming” that high Lp(a) is linked to myocardial infarction and stroke, and it’s known that statins and aspirin reduce risk of these outcomes, he said in the commentary.
Despite that, scientific statements do not recommend routine Lp(a) testing due to a lack of clinical trials evidence; as a result, clinical trials are not including Lp(a) as a routine measurement: “We thus have a loop of futility—lack of routine measurement leads to lack of data,” he said.
This most recent study from Langsted and colleagues demonstrates that high Lp(a) levels, and genetic variants associated with Lp(a), are associated with increased ischemic stroke risk. “The genetics strongly supported that high Lp(a) levels were in the causal pathway for ischemic stroke and coronary heart disease,” Dr. Ballantyne said.
One major strength and weakness of the study is its large and relatively homogeneous European population—that bolstered the genetic analyses, but also means the data can’t be extrapolated to other populations, such as Africans and East Asians, who have higher stroke rates compared with Europeans, Dr. Ballantyne said.
Dr. Ballantyne is with the Department of Medicine and Center for Cardiometabolic Disease Prevention, Baylor College of Medicine, Houston, Tex. His editorial comment appears in the Journal of the American College of Cardiology (2019;74[1]:67-9. doi:10.1016/j.jacc.2019.05.029 . Dr. Ballantyne reported disclosures related to Akcea, Amgen, and Novartis.
This study linking high lipoprotein(a) [Lp(a)] levels to stroke risk, taken together with previous research, provide a sound basis to routinely perform one-time screening so that individuals with inherited high levels can try to avoid adverse cardiovascular outcomes, according to Christie M. Ballantyne, MD.
“As someone in the dual role of preventive cardiologist and patient with a strong family history of cardiovascular disease, I think that we have sufficient evidence that high Lp(a) is strongly associated with an increased risk of myocardial infarction, stroke, and aortic valve stenosis,” Dr. Ballantyne wrote in an editorial comment on the study.
Evidence is now “overwhelming” that high Lp(a) is linked to myocardial infarction and stroke, and it’s known that statins and aspirin reduce risk of these outcomes, he said in the commentary.
Despite that, scientific statements do not recommend routine Lp(a) testing due to a lack of clinical trials evidence; as a result, clinical trials are not including Lp(a) as a routine measurement: “We thus have a loop of futility—lack of routine measurement leads to lack of data,” he said.
This most recent study from Langsted and colleagues demonstrates that high Lp(a) levels, and genetic variants associated with Lp(a), are associated with increased ischemic stroke risk. “The genetics strongly supported that high Lp(a) levels were in the causal pathway for ischemic stroke and coronary heart disease,” Dr. Ballantyne said.
One major strength and weakness of the study is its large and relatively homogeneous European population—that bolstered the genetic analyses, but also means the data can’t be extrapolated to other populations, such as Africans and East Asians, who have higher stroke rates compared with Europeans, Dr. Ballantyne said.
Dr. Ballantyne is with the Department of Medicine and Center for Cardiometabolic Disease Prevention, Baylor College of Medicine, Houston, Tex. His editorial comment appears in the Journal of the American College of Cardiology (2019;74[1]:67-9. doi:10.1016/j.jacc.2019.05.029 . Dr. Ballantyne reported disclosures related to Akcea, Amgen, and Novartis.
High levels of lipoprotein(a) [Lp(a)] and LPA genotypes were linked to increased ischemic stroke risk in a recent large, contemporary general population study, investigators are reporting in the Journal of the American College of Cardiology.
Anne Langsted, MD, with Copenhagen University Hospital and the University of Copenhagen in Denmark, and her co-researchers evaluated the impact of high Lp(a) levels in a large contemporary cohort of 49,699 individuals in the Copenhagen General Population Study, and another 10,813 individuals in the Copenhagen City Heart Study.
Measurements assessed included plasma lipoprotein(a) levels and carrier or noncarrier status for LPA rs10455872. The endpoint of ischemic stroke was ascertained from Danish national health registries and confirmed by physicians.
Although risk estimates were less pronounced than what was reported before regarding the link between Lp(a) for ischemic heart disease and aortic valve stenosis, the risk of stroke was increased by a factor of 1.6 among individuals with high Lp(a) levels as compared to those with lower levels, the investigators said.
Compared with noncarriers of LPA rs1045572, the hazard ratio for ischemic stroke was 1.23 for carriers of LPA rs1045572, which was associated with high levels plasma lipoprotein(a) levels, according to the researchers.
“Our results indicate a causal association of Lp(a) with risk of ischemic stroke, and emphasize the need for randomized, controlled clinical trials on the effect of Lp(a)-lowering to prevent cardiovascular disease including ischemic stroke,” About 20% of the general population have high Lp(a) levels, and some individuals have extremely high levels, Dr. Langsted and co-authors said in their report.
Interest in Lp(a) as a risk factor for cardiovascular disease has been reignited following large studies showing that high Lp(a) levels were linked to increased risk of myocardial infarction and aortic valve stenosis, according to the investigators.
However, results of various studies are conflicting as to whether high Lp(a) levels increase risk of hemorrhagic or ischemic stroke, they said.
Both cohort studies used in the analysis were supported by sources in Denmark including the Danish Medical Research Council and Copenhagen University Hospital. Dr. Langsted had no disclosures. One co-author reported disclosures related to Akcea, Amgen, Sanofi, Regeneron, and AstraZeneca.
SOURCE: Langsted A, et al. JACC 2019;74[1]: 54-66. doi: 10.1016/j.jacc.2019.03.524
High levels of lipoprotein(a) [Lp(a)] and LPA genotypes were linked to increased ischemic stroke risk in a recent large, contemporary general population study, investigators are reporting in the Journal of the American College of Cardiology.
Anne Langsted, MD, with Copenhagen University Hospital and the University of Copenhagen in Denmark, and her co-researchers evaluated the impact of high Lp(a) levels in a large contemporary cohort of 49,699 individuals in the Copenhagen General Population Study, and another 10,813 individuals in the Copenhagen City Heart Study.
Measurements assessed included plasma lipoprotein(a) levels and carrier or noncarrier status for LPA rs10455872. The endpoint of ischemic stroke was ascertained from Danish national health registries and confirmed by physicians.
Although risk estimates were less pronounced than what was reported before regarding the link between Lp(a) for ischemic heart disease and aortic valve stenosis, the risk of stroke was increased by a factor of 1.6 among individuals with high Lp(a) levels as compared to those with lower levels, the investigators said.
Compared with noncarriers of LPA rs1045572, the hazard ratio for ischemic stroke was 1.23 for carriers of LPA rs1045572, which was associated with high levels plasma lipoprotein(a) levels, according to the researchers.
“Our results indicate a causal association of Lp(a) with risk of ischemic stroke, and emphasize the need for randomized, controlled clinical trials on the effect of Lp(a)-lowering to prevent cardiovascular disease including ischemic stroke,” About 20% of the general population have high Lp(a) levels, and some individuals have extremely high levels, Dr. Langsted and co-authors said in their report.
Interest in Lp(a) as a risk factor for cardiovascular disease has been reignited following large studies showing that high Lp(a) levels were linked to increased risk of myocardial infarction and aortic valve stenosis, according to the investigators.
However, results of various studies are conflicting as to whether high Lp(a) levels increase risk of hemorrhagic or ischemic stroke, they said.
Both cohort studies used in the analysis were supported by sources in Denmark including the Danish Medical Research Council and Copenhagen University Hospital. Dr. Langsted had no disclosures. One co-author reported disclosures related to Akcea, Amgen, Sanofi, Regeneron, and AstraZeneca.
SOURCE: Langsted A, et al. JACC 2019;74[1]: 54-66. doi: 10.1016/j.jacc.2019.03.524
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point:
Major finding: Stroke risk was 1.6X higher with high Lp(a) levels.
Study details: Analysis of 49,699 individuals in the Copenhagen General Population Study, and 10,813 individuals in the Copenhagen City Heart Study.
Disclosures: Both studies were supported by the sources in Denmark including the Danish Medical Research Council and Copenhagen University Hospital. Dr. Langsted had no disclosures.
Source: Langsted A, et al. JACC 2019;74[1]: 54-66. doi: 10.1016/j.jacc.2019.03.524
DOACs surpass warfarin in low-weight AFib patients
SAN FRANCISCO – The direct-acting anticoagulants, as a class, were more effective and at least as safe as warfarin in low-weight and very-low-weight patients with atrial fibrillation in an adjusted analysis of real-world outcomes data from more than 21,000 Korean patients.
The analysis also showed that the direct-acting oral anticoagulants (DOACs) had the best safety and efficacy on low-weight patients when used at the labeled dosages, with blunted efficacy and safety at dosages that either exceeded or fell short of labeled levels, So-Ryoung Lee, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The overall superiority of DOACs by both efficacy and safety also generally extended to the subgroup of very-low-weight patients, those with weights of less than 50 kg. In this subgroup, which was 28% of the total population studied, the composite adverse event outcome occurred 33% less often among patients treated with a DOAC relative to patients treated with warfarin, a statistically significant difference, said Dr. Lee, a cardiologist at Seoul (South Korea) National University Hospital. Among all patients with weights of 60 kg (132 pounds) or less, the composite outcome occurred 34% less often in the DOAC-treated patients relative to the warfarin-treated patients, also a statistically significant difference.
Dr. Lee and colleagues used a Korean National Health Insurance database that included information on more than 600,000 adults with atrial fibrillation (AFib) as of January 2013. The researchers whittled this down to 21,678 patients who began for the first time treatment with an oral anticoagulant starting during or after January 2014; had no history of a stroke, intracranial hemorrhage, or gastrointestinal bleed; and weighed no more than 60 kg. This cohort included 7,575 (35%) who received warfarin treatment, and 14,103 (65%) who received a DOAC. Within the DOAC-treated group, 42% received rivaroxaban (Xarelto), 26% dabigatran (Pradaxa), 24% apixaban (Eliquis), and 8% edoxaban (Savaysa).
To account for baseline differences in demographics and comorbidities between the patients treated with a DOAC and those who received warfarin, Dr. Lee and her associates did propensity score adjustment, which resulted in similar cohorts of 6,692 patients treated with warfarin and 12,810 patients treated with a DOAC. The average age of these patients was 73 years, a third were men, and the average body mass index was just over 22 kg/m2.
The events that the researchers tallied during follow-up through December 2016 included rates of all-cause death, ischemic stroke, intracranial hemorrhage, hospitalization for GI bleeding, hospitalization for major bleeding, and the composite of these five outcomes.
In the propensity-score adjusted full cohort of all patients who weighed 60 kg or less, the rate of each of these five outcomes, as well as the composite outcome, occurred statistically significantly less often among the DOAC-treated patients than in those on warfarin. The reductions ranged from a 41% lower incidence of ischemic stroke on DOAC treatment compared with warfarin treatment, to an 18% reduced rate of hospitalization for a GI bleed, compared with warfarin-treated patients. In the subgroup of patients who weighed less than 50 kg (110 pounds), the reductions ranged from a 41% cut in ischemic stroke on a DOAC compared with warfarin to a 24% relative reduction in the rate of hospitalization for a major bleed, a difference that just reached statistical significance. The outcome of hospitalization for a GI bleed showed no significant between-group difference among very-low-weight patients, but the rates of intracranial hemorrhage and all-cause death also showed statistically significant lower rates among DOAC-treated patients.
Nearly two-thirds of the patients on a DOAC received the label-appropriate dose of the drug, but 31% received a dosage that was below the labeled level while 4% received a dosage above the labeled level. An analysis that divided the NOAC patients by the appropriateness of their treatment dosages showed that patients on the correct dosages fared best. For example, in the total cohort of patients who weighed 60 kg or less, those on the correct DOAC dosage had a 9.1% rate of the combined endpoint. Patients on a low DOAC dosage did about as well as did the patients on warfarin, with a combined event rate of 11.6% in each of these subgroups. The worst outcomes occurred among the small number of patients on an inappropriately-high DOAC dosage, with a combined event rate of 15.4%. The researchers found a similar pattern among patients who weighed less than 50 kg.
Dr. Lee had no disclosures.
SOURCE: Lee SR et al. HRS 2019, Abstract S-AB30-05.
SAN FRANCISCO – The direct-acting anticoagulants, as a class, were more effective and at least as safe as warfarin in low-weight and very-low-weight patients with atrial fibrillation in an adjusted analysis of real-world outcomes data from more than 21,000 Korean patients.
The analysis also showed that the direct-acting oral anticoagulants (DOACs) had the best safety and efficacy on low-weight patients when used at the labeled dosages, with blunted efficacy and safety at dosages that either exceeded or fell short of labeled levels, So-Ryoung Lee, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The overall superiority of DOACs by both efficacy and safety also generally extended to the subgroup of very-low-weight patients, those with weights of less than 50 kg. In this subgroup, which was 28% of the total population studied, the composite adverse event outcome occurred 33% less often among patients treated with a DOAC relative to patients treated with warfarin, a statistically significant difference, said Dr. Lee, a cardiologist at Seoul (South Korea) National University Hospital. Among all patients with weights of 60 kg (132 pounds) or less, the composite outcome occurred 34% less often in the DOAC-treated patients relative to the warfarin-treated patients, also a statistically significant difference.
Dr. Lee and colleagues used a Korean National Health Insurance database that included information on more than 600,000 adults with atrial fibrillation (AFib) as of January 2013. The researchers whittled this down to 21,678 patients who began for the first time treatment with an oral anticoagulant starting during or after January 2014; had no history of a stroke, intracranial hemorrhage, or gastrointestinal bleed; and weighed no more than 60 kg. This cohort included 7,575 (35%) who received warfarin treatment, and 14,103 (65%) who received a DOAC. Within the DOAC-treated group, 42% received rivaroxaban (Xarelto), 26% dabigatran (Pradaxa), 24% apixaban (Eliquis), and 8% edoxaban (Savaysa).
To account for baseline differences in demographics and comorbidities between the patients treated with a DOAC and those who received warfarin, Dr. Lee and her associates did propensity score adjustment, which resulted in similar cohorts of 6,692 patients treated with warfarin and 12,810 patients treated with a DOAC. The average age of these patients was 73 years, a third were men, and the average body mass index was just over 22 kg/m2.
The events that the researchers tallied during follow-up through December 2016 included rates of all-cause death, ischemic stroke, intracranial hemorrhage, hospitalization for GI bleeding, hospitalization for major bleeding, and the composite of these five outcomes.
In the propensity-score adjusted full cohort of all patients who weighed 60 kg or less, the rate of each of these five outcomes, as well as the composite outcome, occurred statistically significantly less often among the DOAC-treated patients than in those on warfarin. The reductions ranged from a 41% lower incidence of ischemic stroke on DOAC treatment compared with warfarin treatment, to an 18% reduced rate of hospitalization for a GI bleed, compared with warfarin-treated patients. In the subgroup of patients who weighed less than 50 kg (110 pounds), the reductions ranged from a 41% cut in ischemic stroke on a DOAC compared with warfarin to a 24% relative reduction in the rate of hospitalization for a major bleed, a difference that just reached statistical significance. The outcome of hospitalization for a GI bleed showed no significant between-group difference among very-low-weight patients, but the rates of intracranial hemorrhage and all-cause death also showed statistically significant lower rates among DOAC-treated patients.
Nearly two-thirds of the patients on a DOAC received the label-appropriate dose of the drug, but 31% received a dosage that was below the labeled level while 4% received a dosage above the labeled level. An analysis that divided the NOAC patients by the appropriateness of their treatment dosages showed that patients on the correct dosages fared best. For example, in the total cohort of patients who weighed 60 kg or less, those on the correct DOAC dosage had a 9.1% rate of the combined endpoint. Patients on a low DOAC dosage did about as well as did the patients on warfarin, with a combined event rate of 11.6% in each of these subgroups. The worst outcomes occurred among the small number of patients on an inappropriately-high DOAC dosage, with a combined event rate of 15.4%. The researchers found a similar pattern among patients who weighed less than 50 kg.
Dr. Lee had no disclosures.
SOURCE: Lee SR et al. HRS 2019, Abstract S-AB30-05.
SAN FRANCISCO – The direct-acting anticoagulants, as a class, were more effective and at least as safe as warfarin in low-weight and very-low-weight patients with atrial fibrillation in an adjusted analysis of real-world outcomes data from more than 21,000 Korean patients.
The analysis also showed that the direct-acting oral anticoagulants (DOACs) had the best safety and efficacy on low-weight patients when used at the labeled dosages, with blunted efficacy and safety at dosages that either exceeded or fell short of labeled levels, So-Ryoung Lee, MD, said at the annual scientific sessions of the Heart Rhythm Society.
The overall superiority of DOACs by both efficacy and safety also generally extended to the subgroup of very-low-weight patients, those with weights of less than 50 kg. In this subgroup, which was 28% of the total population studied, the composite adverse event outcome occurred 33% less often among patients treated with a DOAC relative to patients treated with warfarin, a statistically significant difference, said Dr. Lee, a cardiologist at Seoul (South Korea) National University Hospital. Among all patients with weights of 60 kg (132 pounds) or less, the composite outcome occurred 34% less often in the DOAC-treated patients relative to the warfarin-treated patients, also a statistically significant difference.
Dr. Lee and colleagues used a Korean National Health Insurance database that included information on more than 600,000 adults with atrial fibrillation (AFib) as of January 2013. The researchers whittled this down to 21,678 patients who began for the first time treatment with an oral anticoagulant starting during or after January 2014; had no history of a stroke, intracranial hemorrhage, or gastrointestinal bleed; and weighed no more than 60 kg. This cohort included 7,575 (35%) who received warfarin treatment, and 14,103 (65%) who received a DOAC. Within the DOAC-treated group, 42% received rivaroxaban (Xarelto), 26% dabigatran (Pradaxa), 24% apixaban (Eliquis), and 8% edoxaban (Savaysa).
To account for baseline differences in demographics and comorbidities between the patients treated with a DOAC and those who received warfarin, Dr. Lee and her associates did propensity score adjustment, which resulted in similar cohorts of 6,692 patients treated with warfarin and 12,810 patients treated with a DOAC. The average age of these patients was 73 years, a third were men, and the average body mass index was just over 22 kg/m2.
The events that the researchers tallied during follow-up through December 2016 included rates of all-cause death, ischemic stroke, intracranial hemorrhage, hospitalization for GI bleeding, hospitalization for major bleeding, and the composite of these five outcomes.
In the propensity-score adjusted full cohort of all patients who weighed 60 kg or less, the rate of each of these five outcomes, as well as the composite outcome, occurred statistically significantly less often among the DOAC-treated patients than in those on warfarin. The reductions ranged from a 41% lower incidence of ischemic stroke on DOAC treatment compared with warfarin treatment, to an 18% reduced rate of hospitalization for a GI bleed, compared with warfarin-treated patients. In the subgroup of patients who weighed less than 50 kg (110 pounds), the reductions ranged from a 41% cut in ischemic stroke on a DOAC compared with warfarin to a 24% relative reduction in the rate of hospitalization for a major bleed, a difference that just reached statistical significance. The outcome of hospitalization for a GI bleed showed no significant between-group difference among very-low-weight patients, but the rates of intracranial hemorrhage and all-cause death also showed statistically significant lower rates among DOAC-treated patients.
Nearly two-thirds of the patients on a DOAC received the label-appropriate dose of the drug, but 31% received a dosage that was below the labeled level while 4% received a dosage above the labeled level. An analysis that divided the NOAC patients by the appropriateness of their treatment dosages showed that patients on the correct dosages fared best. For example, in the total cohort of patients who weighed 60 kg or less, those on the correct DOAC dosage had a 9.1% rate of the combined endpoint. Patients on a low DOAC dosage did about as well as did the patients on warfarin, with a combined event rate of 11.6% in each of these subgroups. The worst outcomes occurred among the small number of patients on an inappropriately-high DOAC dosage, with a combined event rate of 15.4%. The researchers found a similar pattern among patients who weighed less than 50 kg.
Dr. Lee had no disclosures.
SOURCE: Lee SR et al. HRS 2019, Abstract S-AB30-05.
REPORTING FROM HEART RHYTHM 2019
Stroke policy recommendations incorporate advances in endovascular therapy
Stroke centers need to collaborate within their regions to assure best practices and optimal access to comprehensive stroke centers as well as newly-designated thrombectomy-capable stroke centers, according to an updated policy statement from the American Stroke Association published in Stroke.
Opeolu Adeoye, MD, associate professor of emergency medicine and neurosurgery at the University of Cincinnati – and chair of the policy statement writing group – and coauthors updated the ASA’s 2005 recommendations for policy makers and public health care agencies to reflect current evidence, the increased availability of endovascular therapy, and new stroke center certifications.
“We have seen monumental advancements in acute stroke care over the past 14 years, and our concept of a comprehensive stroke system of care has evolved as a result,” Dr. Adeoye said in a news release.
While a recommendation to support the initiation of stroke prevention regimens remains unchanged from the 2005 recommendations, the 2019 update emphasizes a need to support long-term adherence to such regimens. To that end, researchers should examine the potential benefits of stroke prevention efforts that incorporate social media, gamification, and other technologies and principles to promote healthy behavior, the authors suggested. Furthermore, technology may allow for the passive surveillance of baseline behaviors and enable researchers to track changes in behavior over time.
Thrombectomy-capable centers
Thrombectomy-capable stroke centers, which have capabilities between those of primary stroke centers and comprehensive stroke centers, provide a relatively new level of acute stroke care. In communities that do not otherwise have access to thrombectomy, these centers play a clear role. In communities with comprehensive stroke centers, their role “is more controversial, and routing plans for patients with a suspected LVO [large vessel occlusion] should always seek the center of highest capability when travel time differences are short,” the statement says.
Timely parenchymal and arterial imaging via CT or MRI are needed to identify the subset of patients who may benefit from thrombectomy. All centers managing stroke patients should develop a plan for the definitive identification and treatment of these patients. Imaging techniques that assess penumbral patterns to identify candidates for endovascular therapy between 6 and 24 hours after patients were last known to be normal “merit broader adoption,” the statement says.
Hospitals without thrombectomy capability should have transfer protocols to allow the rapid treatment of these patients to hospitals with the appropriate level of care. In rural facilities that lack 24/7 imaging and radiology capabilities, this may mean rapid transfer of patients with clinically suspected LVO to hospitals where their work-up may be expedited.
To improve process, centers providing thrombectomy should rigorously track patient flow at all time points from presentation to imaging to intervention. Reperfusion rates, procedural complications, and patient clinical outcomes must be tracked and reported.
Travel times
Triage paradigms and protocols should be developed to ensure that emergency medical service (EMS) providers are able to rapidly identify all patients with a known or suspected stroke and to assess them with a validated and standardized instrument for stroke screening such as FAST (Face, Arm, Speech, Time), Los Angeles Prehospital Stroke Screen, or Cincinnati Prehospital Stroke Scale.
In prehospital patients who screen positive for suspected stroke, a standard prehospital stroke severity assessment tool such as the Cincinnati Stroke Triage Assessment Tool, Rapid Arterial Occlusion Evaluation, Los Angeles Motor Scale, or Field Assessment Stroke Triage for Emergency Destination should be used. “Further research is needed to establish the most effective prehospital stroke severity triage scale,” the authors noted. In all cases, EMS should notify hospitals that a stroke patient is en route.
“When there are several intravenous alteplase–capable hospitals in a well-defined geographic region, extra transportation times to reach a facility capable of endovascular thrombectomy should be limited to no more than 15 minutes in patients with a prehospital stroke severity score suggestive of LVO,” according to the recommendations. “When several hospital options exist within similar travel times, EMS should seek care at the facility capable of offering the highest level of stroke care. Further research is needed to establish travel time parameters for hospital bypass in cases of prehospital suspicion of LVO.”
Outcomes and discharge
Centers should track various treatment and patient outcomes, and all patients discharged to their homes should have appropriate follow-up with specialized stroke services and primary care and be screened for postacute complications.
Government institutions should standardize the organization of stroke care, ensure that stroke patients receive timely care at appropriate hospitals, and facilitate access to secondary prevention and rehabilitation resources after stroke, the authors wrote.
“Programs geared at further improving the knowledge of the public, encouraging primordial and primary prevention, advancing and facilitating acute therapy, improving secondary prevention and recovery from stroke, and reducing disparities in stroke care should be actively developed in a coordinated and collaborative fashion by providers and policymakers at the local, state, and national levels,” the authors concluded. “Such efforts will continue to mitigate the effects of stroke on society.”
Dr. Adeoye had no disclosures. Some coauthors reported research grants and consultant or advisory board positions.
SOURCE: Adeoye O et al. Stroke. 2019 May 20. doi: 10.1161/STR.0000000000000173.
When determining where to transport a patient with stroke, uncertainty about the patient’s diagnosis and eligibility for thrombectomy is a necessary consideration, said Robert A. Harrington, MD, of Stanford University (Calif.), in an accompanying editorial.
In lieu of better data, stroke systems should follow the recommendation of the Mission: Lifeline Severity-based Stroke Triage Algorithm for emergency medical services to avoid more than 15 minutes of additional travel time to transport a patient to a center that can perform endovascular therapy when the patient may be eligible for intravenous tissue plasminogen activator (tPA), said Dr. Harrington.
Delays in initiating tPA could lead to some patients not receiving treatment. “Some patients with suspected LVO [large vessel occlusion] either will not have thrombectomy or will not be eligible for it, and they also run the risk of not receiving any acute reperfusion therapy. Consequently, transport algorithms and models must take into account the uncertainty in prehospital diagnosis when considering the most appropriate facility,” he said.
Forthcoming acute stroke guidelines “will recommend intravenous tPA for all eligible subjects” because administration of tPA before endovascular thrombectomy does not appear to be harmful, Dr. Harrington noted.
Ultimately, approaches to routing patients may vary by region. “It is up to local and regional communities ... to define how best to implement these elements into a stroke system of care that meets their needs and resources and to define the types of hospitals that should qualify as points of entry for patients with suspected LVO strokes,” Dr. Harrington said.
A group convened by the American Heart Association and American Stroke Association is drafting further guiding principles for stroke systems of care in various regional settings.
Dr. Harrington is president-elect of the American Heart Association. He reported receiving research grants from AstraZeneca and Bristol-Myers Squibb.
When determining where to transport a patient with stroke, uncertainty about the patient’s diagnosis and eligibility for thrombectomy is a necessary consideration, said Robert A. Harrington, MD, of Stanford University (Calif.), in an accompanying editorial.
In lieu of better data, stroke systems should follow the recommendation of the Mission: Lifeline Severity-based Stroke Triage Algorithm for emergency medical services to avoid more than 15 minutes of additional travel time to transport a patient to a center that can perform endovascular therapy when the patient may be eligible for intravenous tissue plasminogen activator (tPA), said Dr. Harrington.
Delays in initiating tPA could lead to some patients not receiving treatment. “Some patients with suspected LVO [large vessel occlusion] either will not have thrombectomy or will not be eligible for it, and they also run the risk of not receiving any acute reperfusion therapy. Consequently, transport algorithms and models must take into account the uncertainty in prehospital diagnosis when considering the most appropriate facility,” he said.
Forthcoming acute stroke guidelines “will recommend intravenous tPA for all eligible subjects” because administration of tPA before endovascular thrombectomy does not appear to be harmful, Dr. Harrington noted.
Ultimately, approaches to routing patients may vary by region. “It is up to local and regional communities ... to define how best to implement these elements into a stroke system of care that meets their needs and resources and to define the types of hospitals that should qualify as points of entry for patients with suspected LVO strokes,” Dr. Harrington said.
A group convened by the American Heart Association and American Stroke Association is drafting further guiding principles for stroke systems of care in various regional settings.
Dr. Harrington is president-elect of the American Heart Association. He reported receiving research grants from AstraZeneca and Bristol-Myers Squibb.
When determining where to transport a patient with stroke, uncertainty about the patient’s diagnosis and eligibility for thrombectomy is a necessary consideration, said Robert A. Harrington, MD, of Stanford University (Calif.), in an accompanying editorial.
In lieu of better data, stroke systems should follow the recommendation of the Mission: Lifeline Severity-based Stroke Triage Algorithm for emergency medical services to avoid more than 15 minutes of additional travel time to transport a patient to a center that can perform endovascular therapy when the patient may be eligible for intravenous tissue plasminogen activator (tPA), said Dr. Harrington.
Delays in initiating tPA could lead to some patients not receiving treatment. “Some patients with suspected LVO [large vessel occlusion] either will not have thrombectomy or will not be eligible for it, and they also run the risk of not receiving any acute reperfusion therapy. Consequently, transport algorithms and models must take into account the uncertainty in prehospital diagnosis when considering the most appropriate facility,” he said.
Forthcoming acute stroke guidelines “will recommend intravenous tPA for all eligible subjects” because administration of tPA before endovascular thrombectomy does not appear to be harmful, Dr. Harrington noted.
Ultimately, approaches to routing patients may vary by region. “It is up to local and regional communities ... to define how best to implement these elements into a stroke system of care that meets their needs and resources and to define the types of hospitals that should qualify as points of entry for patients with suspected LVO strokes,” Dr. Harrington said.
A group convened by the American Heart Association and American Stroke Association is drafting further guiding principles for stroke systems of care in various regional settings.
Dr. Harrington is president-elect of the American Heart Association. He reported receiving research grants from AstraZeneca and Bristol-Myers Squibb.
Stroke centers need to collaborate within their regions to assure best practices and optimal access to comprehensive stroke centers as well as newly-designated thrombectomy-capable stroke centers, according to an updated policy statement from the American Stroke Association published in Stroke.
Opeolu Adeoye, MD, associate professor of emergency medicine and neurosurgery at the University of Cincinnati – and chair of the policy statement writing group – and coauthors updated the ASA’s 2005 recommendations for policy makers and public health care agencies to reflect current evidence, the increased availability of endovascular therapy, and new stroke center certifications.
“We have seen monumental advancements in acute stroke care over the past 14 years, and our concept of a comprehensive stroke system of care has evolved as a result,” Dr. Adeoye said in a news release.
While a recommendation to support the initiation of stroke prevention regimens remains unchanged from the 2005 recommendations, the 2019 update emphasizes a need to support long-term adherence to such regimens. To that end, researchers should examine the potential benefits of stroke prevention efforts that incorporate social media, gamification, and other technologies and principles to promote healthy behavior, the authors suggested. Furthermore, technology may allow for the passive surveillance of baseline behaviors and enable researchers to track changes in behavior over time.
Thrombectomy-capable centers
Thrombectomy-capable stroke centers, which have capabilities between those of primary stroke centers and comprehensive stroke centers, provide a relatively new level of acute stroke care. In communities that do not otherwise have access to thrombectomy, these centers play a clear role. In communities with comprehensive stroke centers, their role “is more controversial, and routing plans for patients with a suspected LVO [large vessel occlusion] should always seek the center of highest capability when travel time differences are short,” the statement says.
Timely parenchymal and arterial imaging via CT or MRI are needed to identify the subset of patients who may benefit from thrombectomy. All centers managing stroke patients should develop a plan for the definitive identification and treatment of these patients. Imaging techniques that assess penumbral patterns to identify candidates for endovascular therapy between 6 and 24 hours after patients were last known to be normal “merit broader adoption,” the statement says.
Hospitals without thrombectomy capability should have transfer protocols to allow the rapid treatment of these patients to hospitals with the appropriate level of care. In rural facilities that lack 24/7 imaging and radiology capabilities, this may mean rapid transfer of patients with clinically suspected LVO to hospitals where their work-up may be expedited.
To improve process, centers providing thrombectomy should rigorously track patient flow at all time points from presentation to imaging to intervention. Reperfusion rates, procedural complications, and patient clinical outcomes must be tracked and reported.
Travel times
Triage paradigms and protocols should be developed to ensure that emergency medical service (EMS) providers are able to rapidly identify all patients with a known or suspected stroke and to assess them with a validated and standardized instrument for stroke screening such as FAST (Face, Arm, Speech, Time), Los Angeles Prehospital Stroke Screen, or Cincinnati Prehospital Stroke Scale.
In prehospital patients who screen positive for suspected stroke, a standard prehospital stroke severity assessment tool such as the Cincinnati Stroke Triage Assessment Tool, Rapid Arterial Occlusion Evaluation, Los Angeles Motor Scale, or Field Assessment Stroke Triage for Emergency Destination should be used. “Further research is needed to establish the most effective prehospital stroke severity triage scale,” the authors noted. In all cases, EMS should notify hospitals that a stroke patient is en route.
“When there are several intravenous alteplase–capable hospitals in a well-defined geographic region, extra transportation times to reach a facility capable of endovascular thrombectomy should be limited to no more than 15 minutes in patients with a prehospital stroke severity score suggestive of LVO,” according to the recommendations. “When several hospital options exist within similar travel times, EMS should seek care at the facility capable of offering the highest level of stroke care. Further research is needed to establish travel time parameters for hospital bypass in cases of prehospital suspicion of LVO.”
Outcomes and discharge
Centers should track various treatment and patient outcomes, and all patients discharged to their homes should have appropriate follow-up with specialized stroke services and primary care and be screened for postacute complications.
Government institutions should standardize the organization of stroke care, ensure that stroke patients receive timely care at appropriate hospitals, and facilitate access to secondary prevention and rehabilitation resources after stroke, the authors wrote.
“Programs geared at further improving the knowledge of the public, encouraging primordial and primary prevention, advancing and facilitating acute therapy, improving secondary prevention and recovery from stroke, and reducing disparities in stroke care should be actively developed in a coordinated and collaborative fashion by providers and policymakers at the local, state, and national levels,” the authors concluded. “Such efforts will continue to mitigate the effects of stroke on society.”
Dr. Adeoye had no disclosures. Some coauthors reported research grants and consultant or advisory board positions.
SOURCE: Adeoye O et al. Stroke. 2019 May 20. doi: 10.1161/STR.0000000000000173.
Stroke centers need to collaborate within their regions to assure best practices and optimal access to comprehensive stroke centers as well as newly-designated thrombectomy-capable stroke centers, according to an updated policy statement from the American Stroke Association published in Stroke.
Opeolu Adeoye, MD, associate professor of emergency medicine and neurosurgery at the University of Cincinnati – and chair of the policy statement writing group – and coauthors updated the ASA’s 2005 recommendations for policy makers and public health care agencies to reflect current evidence, the increased availability of endovascular therapy, and new stroke center certifications.
“We have seen monumental advancements in acute stroke care over the past 14 years, and our concept of a comprehensive stroke system of care has evolved as a result,” Dr. Adeoye said in a news release.
While a recommendation to support the initiation of stroke prevention regimens remains unchanged from the 2005 recommendations, the 2019 update emphasizes a need to support long-term adherence to such regimens. To that end, researchers should examine the potential benefits of stroke prevention efforts that incorporate social media, gamification, and other technologies and principles to promote healthy behavior, the authors suggested. Furthermore, technology may allow for the passive surveillance of baseline behaviors and enable researchers to track changes in behavior over time.
Thrombectomy-capable centers
Thrombectomy-capable stroke centers, which have capabilities between those of primary stroke centers and comprehensive stroke centers, provide a relatively new level of acute stroke care. In communities that do not otherwise have access to thrombectomy, these centers play a clear role. In communities with comprehensive stroke centers, their role “is more controversial, and routing plans for patients with a suspected LVO [large vessel occlusion] should always seek the center of highest capability when travel time differences are short,” the statement says.
Timely parenchymal and arterial imaging via CT or MRI are needed to identify the subset of patients who may benefit from thrombectomy. All centers managing stroke patients should develop a plan for the definitive identification and treatment of these patients. Imaging techniques that assess penumbral patterns to identify candidates for endovascular therapy between 6 and 24 hours after patients were last known to be normal “merit broader adoption,” the statement says.
Hospitals without thrombectomy capability should have transfer protocols to allow the rapid treatment of these patients to hospitals with the appropriate level of care. In rural facilities that lack 24/7 imaging and radiology capabilities, this may mean rapid transfer of patients with clinically suspected LVO to hospitals where their work-up may be expedited.
To improve process, centers providing thrombectomy should rigorously track patient flow at all time points from presentation to imaging to intervention. Reperfusion rates, procedural complications, and patient clinical outcomes must be tracked and reported.
Travel times
Triage paradigms and protocols should be developed to ensure that emergency medical service (EMS) providers are able to rapidly identify all patients with a known or suspected stroke and to assess them with a validated and standardized instrument for stroke screening such as FAST (Face, Arm, Speech, Time), Los Angeles Prehospital Stroke Screen, or Cincinnati Prehospital Stroke Scale.
In prehospital patients who screen positive for suspected stroke, a standard prehospital stroke severity assessment tool such as the Cincinnati Stroke Triage Assessment Tool, Rapid Arterial Occlusion Evaluation, Los Angeles Motor Scale, or Field Assessment Stroke Triage for Emergency Destination should be used. “Further research is needed to establish the most effective prehospital stroke severity triage scale,” the authors noted. In all cases, EMS should notify hospitals that a stroke patient is en route.
“When there are several intravenous alteplase–capable hospitals in a well-defined geographic region, extra transportation times to reach a facility capable of endovascular thrombectomy should be limited to no more than 15 minutes in patients with a prehospital stroke severity score suggestive of LVO,” according to the recommendations. “When several hospital options exist within similar travel times, EMS should seek care at the facility capable of offering the highest level of stroke care. Further research is needed to establish travel time parameters for hospital bypass in cases of prehospital suspicion of LVO.”
Outcomes and discharge
Centers should track various treatment and patient outcomes, and all patients discharged to their homes should have appropriate follow-up with specialized stroke services and primary care and be screened for postacute complications.
Government institutions should standardize the organization of stroke care, ensure that stroke patients receive timely care at appropriate hospitals, and facilitate access to secondary prevention and rehabilitation resources after stroke, the authors wrote.
“Programs geared at further improving the knowledge of the public, encouraging primordial and primary prevention, advancing and facilitating acute therapy, improving secondary prevention and recovery from stroke, and reducing disparities in stroke care should be actively developed in a coordinated and collaborative fashion by providers and policymakers at the local, state, and national levels,” the authors concluded. “Such efforts will continue to mitigate the effects of stroke on society.”
Dr. Adeoye had no disclosures. Some coauthors reported research grants and consultant or advisory board positions.
SOURCE: Adeoye O et al. Stroke. 2019 May 20. doi: 10.1161/STR.0000000000000173.
FROM STROKE
ICYMI: Dabigatran no better than aspirin for recurrent stroke prevention
Dabigatran was slightly but not significantly superior to aspirin at the prevention of recurring stroke in patients with a recent history of embolic stroke of undetermined source over a median follow-up of 19 months (6.6% rate of recurrent stroke for dabigatran vs. 7.7% for aspirin; hazard ratio, 0.85; 95% confidence interval, 0.69-1.03; P = 0.10), according to RE-SPECT ESUS, a multicenter, randomized, double-blind trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813959).
We first reported on the results of this trial when they were presented at the World Stroke Congress by lead investigator Hans-Christoph Diener, MD. Find our coverage at the link below.
Dabigatran was slightly but not significantly superior to aspirin at the prevention of recurring stroke in patients with a recent history of embolic stroke of undetermined source over a median follow-up of 19 months (6.6% rate of recurrent stroke for dabigatran vs. 7.7% for aspirin; hazard ratio, 0.85; 95% confidence interval, 0.69-1.03; P = 0.10), according to RE-SPECT ESUS, a multicenter, randomized, double-blind trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813959).
We first reported on the results of this trial when they were presented at the World Stroke Congress by lead investigator Hans-Christoph Diener, MD. Find our coverage at the link below.
Dabigatran was slightly but not significantly superior to aspirin at the prevention of recurring stroke in patients with a recent history of embolic stroke of undetermined source over a median follow-up of 19 months (6.6% rate of recurrent stroke for dabigatran vs. 7.7% for aspirin; hazard ratio, 0.85; 95% confidence interval, 0.69-1.03; P = 0.10), according to RE-SPECT ESUS, a multicenter, randomized, double-blind trial published in the New England Journal of Medicine (2019 May 15. doi: 10.1056/NEJMoa1813959).
We first reported on the results of this trial when they were presented at the World Stroke Congress by lead investigator Hans-Christoph Diener, MD. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Severe OSA increases cardiovascular risk after surgery
Unrecognized severe obstructive sleep apnea is a risk factor for cardiovascular complications after major noncardiac surgery, according to a study published in JAMA.
The researchers state that perioperative mismanagement of obstructive sleep apnea can lead to serious medical consequences. “General anesthetics, sedatives, and postoperative analgesics are potent respiratory depressants that relax the upper airway dilator muscles and impair ventilatory response to hypoxemia and hypercapnia. Each of these events exacerbates [obstructive sleep apnea] and may predispose patients to postoperative cardiovascular complications,” said researchers who conducted the The Postoperative vascular complications in unrecognised Obstructive Sleep apnoea (POSA) study (NCT01494181).
They undertook a prospective observational cohort study involving 1,218 patients undergoing major noncardiac surgery, who were already considered at high risk of postoperative cardiovascular events – having, for example, a history of coronary artery disease, stroke, diabetes, or renal impairment. However, none had a prior diagnosis of obstructive sleep apnea.
Preoperative sleep monitoring revealed that two-thirds of the cohort had unrecognized and untreated obstructive sleep apnea, including 11.2% with severe obstructive sleep apnea.
At 30 days after surgery, patients with obstructive sleep apnea had a 49% higher risk of the primary outcome of myocardial injury, cardiac death, heart failure, thromboembolism, atrial fibrillation, or stroke, compared with those without obstructive sleep apnea.
However, this association was largely due to a significant 2.23-fold higher risk among patients with severe obstructive sleep apnea, while those with only moderate or mild sleep apnea did not show a significant increased risk of cardiovascular complications.
Patients in this study with severe obstructive sleep apnea had a 13-fold higher risk of cardiac death, 80% higher risk of myocardial injury, more than sixfold higher risk of heart failure, and nearly fourfold higher risk of atrial fibrillation.
Researchers also saw an association between obstructive sleep apnea and increased risk of infective outcomes, unplanned tracheal intubation, postoperative lung ventilation, and readmission to the ICU.
The majority of patients received nocturnal oximetry monitoring during their first 3 nights after surgery. This revealed that patients without obstructive sleep apnea had significant increases in oxygen desaturation index during their first night after surgery, while those with sleep apnea did not return to their baseline oxygen desaturation index until the third night after surgery.
“Despite a substantial decrease in ODI [oxygen desaturation index] with oxygen therapy in patients with OSA during the first 3 postoperative nights, supplemental oxygen did not modify the association between OSA and postoperative cardiovascular event,” wrote Matthew T.V. Chan, MD, of Chinese University of Hong Kong, Prince of Wales Hospital, and coauthors.
Given that the events were associated with longer durations of severe oxyhemoglobin desaturation, more aggressive interventions such as positive airway pressure or oral appliances may be required, they noted.
“However, high-level evidence demonstrating the effect of these measures on perioperative outcomes is lacking [and] further clinical trials are now required to test if additional monitoring or alternative interventions would reduce the risk,” they wrote.
The study was supported by the Health and Medical Research Fund (Hong Kong), National Healthcare Group–Khoo Teck Puat Hospital, University Health Network Foundation, University of Malaya, Malaysian Society of Anaesthesiologists, Auckland Medical Research Foundation, and ResMed. One author declared grants from private industry and a patent pending on an obstructive sleep apnea risk questionnaire used in the study.
SOURCE: Chan M et al. JAMA 2019;321[18]:1788-98. doi: 10.1001/jama.2019.4783.
This study is large, prospective, and rigorous and adds important new information to the puzzle of the impact of sleep apnea on postoperative risk, Dennis Auckley, MD, and Stavros Memtsoudis, MD, wrote in an editorial accompanying this study. The study focused on predetermined clinically significant and measurable events, used standardized and objective sleep apnea testing, and attempted to control for many of the confounders that might have influenced outcomes.
The results suggest that obstructive sleep apnea should be recognized as a major perioperative risk factor, and it should receive the same attention and optimization efforts as comorbidities such as diabetes.
Dr. Auckley is from the division of pulmonary, critical care and sleep medicine at MetroHealth Medical Center, Case Western Reserve University, Cleveland, and Dr. Memtsoudis is clinical professor of anesthesiology at Cornell University, New York. These comments are adapted from an editorial (JAMA 2019;231[18]:1775-6). Both declared board and executive positions with the Society of Anesthesia and Sleep Medicine. Dr. Auckley declared research funding from Medtronic, and Dr. Memtsoudis declared personal fees from Teikoku and Sandoz.
This study is large, prospective, and rigorous and adds important new information to the puzzle of the impact of sleep apnea on postoperative risk, Dennis Auckley, MD, and Stavros Memtsoudis, MD, wrote in an editorial accompanying this study. The study focused on predetermined clinically significant and measurable events, used standardized and objective sleep apnea testing, and attempted to control for many of the confounders that might have influenced outcomes.
The results suggest that obstructive sleep apnea should be recognized as a major perioperative risk factor, and it should receive the same attention and optimization efforts as comorbidities such as diabetes.
Dr. Auckley is from the division of pulmonary, critical care and sleep medicine at MetroHealth Medical Center, Case Western Reserve University, Cleveland, and Dr. Memtsoudis is clinical professor of anesthesiology at Cornell University, New York. These comments are adapted from an editorial (JAMA 2019;231[18]:1775-6). Both declared board and executive positions with the Society of Anesthesia and Sleep Medicine. Dr. Auckley declared research funding from Medtronic, and Dr. Memtsoudis declared personal fees from Teikoku and Sandoz.
This study is large, prospective, and rigorous and adds important new information to the puzzle of the impact of sleep apnea on postoperative risk, Dennis Auckley, MD, and Stavros Memtsoudis, MD, wrote in an editorial accompanying this study. The study focused on predetermined clinically significant and measurable events, used standardized and objective sleep apnea testing, and attempted to control for many of the confounders that might have influenced outcomes.
The results suggest that obstructive sleep apnea should be recognized as a major perioperative risk factor, and it should receive the same attention and optimization efforts as comorbidities such as diabetes.
Dr. Auckley is from the division of pulmonary, critical care and sleep medicine at MetroHealth Medical Center, Case Western Reserve University, Cleveland, and Dr. Memtsoudis is clinical professor of anesthesiology at Cornell University, New York. These comments are adapted from an editorial (JAMA 2019;231[18]:1775-6). Both declared board and executive positions with the Society of Anesthesia and Sleep Medicine. Dr. Auckley declared research funding from Medtronic, and Dr. Memtsoudis declared personal fees from Teikoku and Sandoz.
Unrecognized severe obstructive sleep apnea is a risk factor for cardiovascular complications after major noncardiac surgery, according to a study published in JAMA.
The researchers state that perioperative mismanagement of obstructive sleep apnea can lead to serious medical consequences. “General anesthetics, sedatives, and postoperative analgesics are potent respiratory depressants that relax the upper airway dilator muscles and impair ventilatory response to hypoxemia and hypercapnia. Each of these events exacerbates [obstructive sleep apnea] and may predispose patients to postoperative cardiovascular complications,” said researchers who conducted the The Postoperative vascular complications in unrecognised Obstructive Sleep apnoea (POSA) study (NCT01494181).
They undertook a prospective observational cohort study involving 1,218 patients undergoing major noncardiac surgery, who were already considered at high risk of postoperative cardiovascular events – having, for example, a history of coronary artery disease, stroke, diabetes, or renal impairment. However, none had a prior diagnosis of obstructive sleep apnea.
Preoperative sleep monitoring revealed that two-thirds of the cohort had unrecognized and untreated obstructive sleep apnea, including 11.2% with severe obstructive sleep apnea.
At 30 days after surgery, patients with obstructive sleep apnea had a 49% higher risk of the primary outcome of myocardial injury, cardiac death, heart failure, thromboembolism, atrial fibrillation, or stroke, compared with those without obstructive sleep apnea.
However, this association was largely due to a significant 2.23-fold higher risk among patients with severe obstructive sleep apnea, while those with only moderate or mild sleep apnea did not show a significant increased risk of cardiovascular complications.
Patients in this study with severe obstructive sleep apnea had a 13-fold higher risk of cardiac death, 80% higher risk of myocardial injury, more than sixfold higher risk of heart failure, and nearly fourfold higher risk of atrial fibrillation.
Researchers also saw an association between obstructive sleep apnea and increased risk of infective outcomes, unplanned tracheal intubation, postoperative lung ventilation, and readmission to the ICU.
The majority of patients received nocturnal oximetry monitoring during their first 3 nights after surgery. This revealed that patients without obstructive sleep apnea had significant increases in oxygen desaturation index during their first night after surgery, while those with sleep apnea did not return to their baseline oxygen desaturation index until the third night after surgery.
“Despite a substantial decrease in ODI [oxygen desaturation index] with oxygen therapy in patients with OSA during the first 3 postoperative nights, supplemental oxygen did not modify the association between OSA and postoperative cardiovascular event,” wrote Matthew T.V. Chan, MD, of Chinese University of Hong Kong, Prince of Wales Hospital, and coauthors.
Given that the events were associated with longer durations of severe oxyhemoglobin desaturation, more aggressive interventions such as positive airway pressure or oral appliances may be required, they noted.
“However, high-level evidence demonstrating the effect of these measures on perioperative outcomes is lacking [and] further clinical trials are now required to test if additional monitoring or alternative interventions would reduce the risk,” they wrote.
The study was supported by the Health and Medical Research Fund (Hong Kong), National Healthcare Group–Khoo Teck Puat Hospital, University Health Network Foundation, University of Malaya, Malaysian Society of Anaesthesiologists, Auckland Medical Research Foundation, and ResMed. One author declared grants from private industry and a patent pending on an obstructive sleep apnea risk questionnaire used in the study.
SOURCE: Chan M et al. JAMA 2019;321[18]:1788-98. doi: 10.1001/jama.2019.4783.
Unrecognized severe obstructive sleep apnea is a risk factor for cardiovascular complications after major noncardiac surgery, according to a study published in JAMA.
The researchers state that perioperative mismanagement of obstructive sleep apnea can lead to serious medical consequences. “General anesthetics, sedatives, and postoperative analgesics are potent respiratory depressants that relax the upper airway dilator muscles and impair ventilatory response to hypoxemia and hypercapnia. Each of these events exacerbates [obstructive sleep apnea] and may predispose patients to postoperative cardiovascular complications,” said researchers who conducted the The Postoperative vascular complications in unrecognised Obstructive Sleep apnoea (POSA) study (NCT01494181).
They undertook a prospective observational cohort study involving 1,218 patients undergoing major noncardiac surgery, who were already considered at high risk of postoperative cardiovascular events – having, for example, a history of coronary artery disease, stroke, diabetes, or renal impairment. However, none had a prior diagnosis of obstructive sleep apnea.
Preoperative sleep monitoring revealed that two-thirds of the cohort had unrecognized and untreated obstructive sleep apnea, including 11.2% with severe obstructive sleep apnea.
At 30 days after surgery, patients with obstructive sleep apnea had a 49% higher risk of the primary outcome of myocardial injury, cardiac death, heart failure, thromboembolism, atrial fibrillation, or stroke, compared with those without obstructive sleep apnea.
However, this association was largely due to a significant 2.23-fold higher risk among patients with severe obstructive sleep apnea, while those with only moderate or mild sleep apnea did not show a significant increased risk of cardiovascular complications.
Patients in this study with severe obstructive sleep apnea had a 13-fold higher risk of cardiac death, 80% higher risk of myocardial injury, more than sixfold higher risk of heart failure, and nearly fourfold higher risk of atrial fibrillation.
Researchers also saw an association between obstructive sleep apnea and increased risk of infective outcomes, unplanned tracheal intubation, postoperative lung ventilation, and readmission to the ICU.
The majority of patients received nocturnal oximetry monitoring during their first 3 nights after surgery. This revealed that patients without obstructive sleep apnea had significant increases in oxygen desaturation index during their first night after surgery, while those with sleep apnea did not return to their baseline oxygen desaturation index until the third night after surgery.
“Despite a substantial decrease in ODI [oxygen desaturation index] with oxygen therapy in patients with OSA during the first 3 postoperative nights, supplemental oxygen did not modify the association between OSA and postoperative cardiovascular event,” wrote Matthew T.V. Chan, MD, of Chinese University of Hong Kong, Prince of Wales Hospital, and coauthors.
Given that the events were associated with longer durations of severe oxyhemoglobin desaturation, more aggressive interventions such as positive airway pressure or oral appliances may be required, they noted.
“However, high-level evidence demonstrating the effect of these measures on perioperative outcomes is lacking [and] further clinical trials are now required to test if additional monitoring or alternative interventions would reduce the risk,” they wrote.
The study was supported by the Health and Medical Research Fund (Hong Kong), National Healthcare Group–Khoo Teck Puat Hospital, University Health Network Foundation, University of Malaya, Malaysian Society of Anaesthesiologists, Auckland Medical Research Foundation, and ResMed. One author declared grants from private industry and a patent pending on an obstructive sleep apnea risk questionnaire used in the study.
SOURCE: Chan M et al. JAMA 2019;321[18]:1788-98. doi: 10.1001/jama.2019.4783.
FROM JAMA