User login
Halogenated anesthetic linked to less chronic postop mastectomy pain
The use of halogenated agents for anesthetic during a mastectomy operation may be associated with a lower incidence of long-term chronic postmastectomy pain (CPMP), according to a paper published in the the Journal of Clinical Anesthesia.
The retrospective cross-sectional survey of 128 women who underwent mastectomy with axillary lymph node dissection set out to determine whether the anesthetic or analgesic regimen used perioperatively had any impact on the risk of long-term chronic postoperative pain.
Overall, 43.8% of the women reported chronic pain, and nearly half of these showed neuropathic characteristics with an ID Pain score greater than or equal to 2 (J Clin Anesthesia. 2016;33:20-25. doi: 10.1016/j.jclinane.2015.07.010).
Those who were given a halogenated agent for anesthesia during the operation – 64% of patients in the survey - had a significant 19% lower incidence of chronic long-term postoperative mastectomy pain (95% CI, 0.70-0.95; P = .012).
Arnaud Steyaert, MD, and colleagues at the Catholic University of Louvain (Belgium) described this result as surprising, noting that sevoflurane use was recently found to be a risk factor for chronic pain after breast cancer surgery.
“An explanation for this discrepancy could be that the influence of sevoflurane on the development of CPMP depends on the other components of the anesthetic regimen,” the authors wrote, pointing out that the aforementioned study included the use of remifentanil in all patients, which can trigger acute opioid-induced hyperalgesia and chronic pain after surgery.
Apart from this effect, the authors said they did not see any impact from other analgesics – which included sufentanil, ketamine, clonidine, NSAIDs, and/or magnesium sulfate – on the risk of long-term chronic pain. However, patients treated with piritramide in the recovery room did have a significant 30% greater risk of chronic postoperative pain.
The study also found that patients who needed strong opioids in the postanesthesia care unit had a 30% higher risk of chronic long-term pain (95% CI, 1.11-1.53). “This was expected, as more intense acute postoperative pain is a known risk factor for developing chronic postsurgical pain, including CPMP,” the authors wrote.
Patients who had received adjuvant chemotherapy had a 32% higher incidence of chronic long-term pain, but there was no increase in risk associated with adjuvant radiotherapy. Both are known to cause neurotoxicity and therefore neuropathic pain, the authors commented.
No conflicts of interest were declared.
The use of halogenated agents for anesthetic during a mastectomy operation may be associated with a lower incidence of long-term chronic postmastectomy pain (CPMP), according to a paper published in the the Journal of Clinical Anesthesia.
The retrospective cross-sectional survey of 128 women who underwent mastectomy with axillary lymph node dissection set out to determine whether the anesthetic or analgesic regimen used perioperatively had any impact on the risk of long-term chronic postoperative pain.
Overall, 43.8% of the women reported chronic pain, and nearly half of these showed neuropathic characteristics with an ID Pain score greater than or equal to 2 (J Clin Anesthesia. 2016;33:20-25. doi: 10.1016/j.jclinane.2015.07.010).
Those who were given a halogenated agent for anesthesia during the operation – 64% of patients in the survey - had a significant 19% lower incidence of chronic long-term postoperative mastectomy pain (95% CI, 0.70-0.95; P = .012).
Arnaud Steyaert, MD, and colleagues at the Catholic University of Louvain (Belgium) described this result as surprising, noting that sevoflurane use was recently found to be a risk factor for chronic pain after breast cancer surgery.
“An explanation for this discrepancy could be that the influence of sevoflurane on the development of CPMP depends on the other components of the anesthetic regimen,” the authors wrote, pointing out that the aforementioned study included the use of remifentanil in all patients, which can trigger acute opioid-induced hyperalgesia and chronic pain after surgery.
Apart from this effect, the authors said they did not see any impact from other analgesics – which included sufentanil, ketamine, clonidine, NSAIDs, and/or magnesium sulfate – on the risk of long-term chronic pain. However, patients treated with piritramide in the recovery room did have a significant 30% greater risk of chronic postoperative pain.
The study also found that patients who needed strong opioids in the postanesthesia care unit had a 30% higher risk of chronic long-term pain (95% CI, 1.11-1.53). “This was expected, as more intense acute postoperative pain is a known risk factor for developing chronic postsurgical pain, including CPMP,” the authors wrote.
Patients who had received adjuvant chemotherapy had a 32% higher incidence of chronic long-term pain, but there was no increase in risk associated with adjuvant radiotherapy. Both are known to cause neurotoxicity and therefore neuropathic pain, the authors commented.
No conflicts of interest were declared.
The use of halogenated agents for anesthetic during a mastectomy operation may be associated with a lower incidence of long-term chronic postmastectomy pain (CPMP), according to a paper published in the the Journal of Clinical Anesthesia.
The retrospective cross-sectional survey of 128 women who underwent mastectomy with axillary lymph node dissection set out to determine whether the anesthetic or analgesic regimen used perioperatively had any impact on the risk of long-term chronic postoperative pain.
Overall, 43.8% of the women reported chronic pain, and nearly half of these showed neuropathic characteristics with an ID Pain score greater than or equal to 2 (J Clin Anesthesia. 2016;33:20-25. doi: 10.1016/j.jclinane.2015.07.010).
Those who were given a halogenated agent for anesthesia during the operation – 64% of patients in the survey - had a significant 19% lower incidence of chronic long-term postoperative mastectomy pain (95% CI, 0.70-0.95; P = .012).
Arnaud Steyaert, MD, and colleagues at the Catholic University of Louvain (Belgium) described this result as surprising, noting that sevoflurane use was recently found to be a risk factor for chronic pain after breast cancer surgery.
“An explanation for this discrepancy could be that the influence of sevoflurane on the development of CPMP depends on the other components of the anesthetic regimen,” the authors wrote, pointing out that the aforementioned study included the use of remifentanil in all patients, which can trigger acute opioid-induced hyperalgesia and chronic pain after surgery.
Apart from this effect, the authors said they did not see any impact from other analgesics – which included sufentanil, ketamine, clonidine, NSAIDs, and/or magnesium sulfate – on the risk of long-term chronic pain. However, patients treated with piritramide in the recovery room did have a significant 30% greater risk of chronic postoperative pain.
The study also found that patients who needed strong opioids in the postanesthesia care unit had a 30% higher risk of chronic long-term pain (95% CI, 1.11-1.53). “This was expected, as more intense acute postoperative pain is a known risk factor for developing chronic postsurgical pain, including CPMP,” the authors wrote.
Patients who had received adjuvant chemotherapy had a 32% higher incidence of chronic long-term pain, but there was no increase in risk associated with adjuvant radiotherapy. Both are known to cause neurotoxicity and therefore neuropathic pain, the authors commented.
No conflicts of interest were declared.
FROM THE JOURNAL OF CLINICAL ANESTHESIA
Key clinical point: The use of halogenated agents for anesthetic during a mastectomy operation may be associated with a lower incidence of long-term chronic postmastectomy pain.
Major finding: Patients given a halogenated agent for anesthesia during a mastectomy had a significant 19% lower incidence of chronic long-term postoperative mastectomy pain.
Data source: A retrospective cross-sectional survey.
Disclosures: No conflicts of interest were declared.
Surgery for bowel obstruction in cancer patients didn’t increase 90-day mortality
CORONADO, CALIF. – Among advanced cancer patients with bowel obstruction, surgery was not an independent predictor of the ability to eat at discharge or survival within 90 days of consultation, results from a long-term retrospective study showed.
“I think this represents the complexity in treating these patients,” lead study author Brian D. Badgwell, MD, said at the annual meeting of the Western Surgical Association. “We need future studies to identify the optimal outcome measures.”
For the current study, the researchers retrospectively reviewed the medical records of 490 patients who required surgical consultation for bowel obstruction at MD Anderson Cancer Center between January 2000 and May 2014. They set out to determine the incidence of obstruction due to intra-abdominal tumor and to identify variables associated with the ability to eat at hospital discharge and 90-day survival. They excluded patients without clinical or radiologic features of mechanical bowel obstruction. Clinical variables of interest included obstruction site, tumor vs. non-tumor cause, laboratory parameters, radiologic extent of malignancy, and the type of treatment performed (surgical, medical, or interventional, defined as interventional radiology or endoscopy). Overall survival was calculated from the date of first surgical evaluation for bowel obstruction to any cause mortality or last follow-up. Univariate and multivariate analyses were performed for ability to eat and a Cox proportional hazards model for 90-day survival.
Dr. Badgwell reported that the most common obstruction site in the 490 patients was the small bowel (64%), followed by large bowel (20%) and gastric outlet (16%). Obstruction etiology was identified as tumor-related in 68% of cases, followed by adhesion-related (20%) and unclear (12%). Nearly half of patients (46%) received chemotherapy within 6 weeks of their surgical consultation, but only 4% were neutropenic. More than half of patients (52%) had an albumin level of less than 3.5 g/dL, 52% had a hemoglobin of 10 g/dL or greater, 36% had lymphadenopathy, 35% had ascites, 34% had peritoneal disease, and 31% had a primary or recurrent tumor in place. In addition, 53% had an abdominal visceral malignancy, 9% had bone metastases, and 14% had lung metastases.
About half of patients (49%) received medical management as their treatment, followed by surgical and procedural treatment (32% and 17%, respectively). Fifteen percent were discharged to in-home hospice or to an inpatient hospice facility. More than two-thirds (68%) were able to eat at the time of discharge, and 43% died within 90 days of surgical consultation.
Multivariate analysis revealed that the following factors were negatively associated with eating at discharge: an intact/primary local recurrence (odds ratio, 0.46), carcinomatosis (OR, 0.34), and albumin level of less than 3.5 g/dL (OR, 0.55). At the same time, variables associated with death within 90 days of consultation included having an intact primary/local recurrence (hazard ratio, 1.75), carcinomatosis (HR, 1.98), and abdominal visceral metastasis (HR, 1.75). Finally, compared with procedural treatment, both medical management and surgical management were negatively associated with death within 90 days (HR of 0.51 and 0.44, respectively).
“There is a high rate of non-mechanical bowel dysfunction in patients undergoing surgical consultation for bowel obstruction,” Dr. Badgwell concluded. “It’s very difficult to categorize these cases preoperatively. They do require a selective approach. Variables associated with outcome measures support caution in patients with carcinomatosis, hypoalbuminemia, and multiple sites of disease on imaging.”
Dr. Badgwell reported having no financial disclosures.
CORONADO, CALIF. – Among advanced cancer patients with bowel obstruction, surgery was not an independent predictor of the ability to eat at discharge or survival within 90 days of consultation, results from a long-term retrospective study showed.
“I think this represents the complexity in treating these patients,” lead study author Brian D. Badgwell, MD, said at the annual meeting of the Western Surgical Association. “We need future studies to identify the optimal outcome measures.”
For the current study, the researchers retrospectively reviewed the medical records of 490 patients who required surgical consultation for bowel obstruction at MD Anderson Cancer Center between January 2000 and May 2014. They set out to determine the incidence of obstruction due to intra-abdominal tumor and to identify variables associated with the ability to eat at hospital discharge and 90-day survival. They excluded patients without clinical or radiologic features of mechanical bowel obstruction. Clinical variables of interest included obstruction site, tumor vs. non-tumor cause, laboratory parameters, radiologic extent of malignancy, and the type of treatment performed (surgical, medical, or interventional, defined as interventional radiology or endoscopy). Overall survival was calculated from the date of first surgical evaluation for bowel obstruction to any cause mortality or last follow-up. Univariate and multivariate analyses were performed for ability to eat and a Cox proportional hazards model for 90-day survival.
Dr. Badgwell reported that the most common obstruction site in the 490 patients was the small bowel (64%), followed by large bowel (20%) and gastric outlet (16%). Obstruction etiology was identified as tumor-related in 68% of cases, followed by adhesion-related (20%) and unclear (12%). Nearly half of patients (46%) received chemotherapy within 6 weeks of their surgical consultation, but only 4% were neutropenic. More than half of patients (52%) had an albumin level of less than 3.5 g/dL, 52% had a hemoglobin of 10 g/dL or greater, 36% had lymphadenopathy, 35% had ascites, 34% had peritoneal disease, and 31% had a primary or recurrent tumor in place. In addition, 53% had an abdominal visceral malignancy, 9% had bone metastases, and 14% had lung metastases.
About half of patients (49%) received medical management as their treatment, followed by surgical and procedural treatment (32% and 17%, respectively). Fifteen percent were discharged to in-home hospice or to an inpatient hospice facility. More than two-thirds (68%) were able to eat at the time of discharge, and 43% died within 90 days of surgical consultation.
Multivariate analysis revealed that the following factors were negatively associated with eating at discharge: an intact/primary local recurrence (odds ratio, 0.46), carcinomatosis (OR, 0.34), and albumin level of less than 3.5 g/dL (OR, 0.55). At the same time, variables associated with death within 90 days of consultation included having an intact primary/local recurrence (hazard ratio, 1.75), carcinomatosis (HR, 1.98), and abdominal visceral metastasis (HR, 1.75). Finally, compared with procedural treatment, both medical management and surgical management were negatively associated with death within 90 days (HR of 0.51 and 0.44, respectively).
“There is a high rate of non-mechanical bowel dysfunction in patients undergoing surgical consultation for bowel obstruction,” Dr. Badgwell concluded. “It’s very difficult to categorize these cases preoperatively. They do require a selective approach. Variables associated with outcome measures support caution in patients with carcinomatosis, hypoalbuminemia, and multiple sites of disease on imaging.”
Dr. Badgwell reported having no financial disclosures.
CORONADO, CALIF. – Among advanced cancer patients with bowel obstruction, surgery was not an independent predictor of the ability to eat at discharge or survival within 90 days of consultation, results from a long-term retrospective study showed.
“I think this represents the complexity in treating these patients,” lead study author Brian D. Badgwell, MD, said at the annual meeting of the Western Surgical Association. “We need future studies to identify the optimal outcome measures.”
For the current study, the researchers retrospectively reviewed the medical records of 490 patients who required surgical consultation for bowel obstruction at MD Anderson Cancer Center between January 2000 and May 2014. They set out to determine the incidence of obstruction due to intra-abdominal tumor and to identify variables associated with the ability to eat at hospital discharge and 90-day survival. They excluded patients without clinical or radiologic features of mechanical bowel obstruction. Clinical variables of interest included obstruction site, tumor vs. non-tumor cause, laboratory parameters, radiologic extent of malignancy, and the type of treatment performed (surgical, medical, or interventional, defined as interventional radiology or endoscopy). Overall survival was calculated from the date of first surgical evaluation for bowel obstruction to any cause mortality or last follow-up. Univariate and multivariate analyses were performed for ability to eat and a Cox proportional hazards model for 90-day survival.
Dr. Badgwell reported that the most common obstruction site in the 490 patients was the small bowel (64%), followed by large bowel (20%) and gastric outlet (16%). Obstruction etiology was identified as tumor-related in 68% of cases, followed by adhesion-related (20%) and unclear (12%). Nearly half of patients (46%) received chemotherapy within 6 weeks of their surgical consultation, but only 4% were neutropenic. More than half of patients (52%) had an albumin level of less than 3.5 g/dL, 52% had a hemoglobin of 10 g/dL or greater, 36% had lymphadenopathy, 35% had ascites, 34% had peritoneal disease, and 31% had a primary or recurrent tumor in place. In addition, 53% had an abdominal visceral malignancy, 9% had bone metastases, and 14% had lung metastases.
About half of patients (49%) received medical management as their treatment, followed by surgical and procedural treatment (32% and 17%, respectively). Fifteen percent were discharged to in-home hospice or to an inpatient hospice facility. More than two-thirds (68%) were able to eat at the time of discharge, and 43% died within 90 days of surgical consultation.
Multivariate analysis revealed that the following factors were negatively associated with eating at discharge: an intact/primary local recurrence (odds ratio, 0.46), carcinomatosis (OR, 0.34), and albumin level of less than 3.5 g/dL (OR, 0.55). At the same time, variables associated with death within 90 days of consultation included having an intact primary/local recurrence (hazard ratio, 1.75), carcinomatosis (HR, 1.98), and abdominal visceral metastasis (HR, 1.75). Finally, compared with procedural treatment, both medical management and surgical management were negatively associated with death within 90 days (HR of 0.51 and 0.44, respectively).
“There is a high rate of non-mechanical bowel dysfunction in patients undergoing surgical consultation for bowel obstruction,” Dr. Badgwell concluded. “It’s very difficult to categorize these cases preoperatively. They do require a selective approach. Variables associated with outcome measures support caution in patients with carcinomatosis, hypoalbuminemia, and multiple sites of disease on imaging.”
Dr. Badgwell reported having no financial disclosures.
AT WSA 2016
Key clinical point:
Major finding: Compared with procedural treatment of bowel obstruction, both medical management and surgical management were negatively associated with death within 90 days (HR of 0.51 and 0.44, respectively).
Data source: A retrospective review of 490 patients with advanced cancer who required surgical consultation for bowel obstruction at MD Anderson Cancer Center, Houston, between January 2000 and May 2014.
Disclosures: Dr. Badgwell reported having no financial disclosures.
Tumor boards linked to improved survival in hepatocellular carcinoma
BOSTON – Veterans were about 13% less likely to die within 5 years of hepatocellular carcinoma diagnosis when multidisciplinary tumor boards managed their care than if they did not, according to a large, multicenter observational study.
Seeing a hepatologist or surgeon within 30 days of diagnosis also significantly improved 5-year overall survival, even after controlling for age, race, Charlson-Deyo comorbidity index, Barcelona Clinic Liver Cancer (BCLC) stage, academic center and geographic region of care, and the distance patients lived from the nearest Veterans Affairs transplant center, Marina Serper, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases. “More studies are needed to understand how to best use multidisciplinary tumor boards to improve the care of patients with hepatocellular carcinoma,” she said.
Outcomes data for hepatocellular carcinoma mostly come from clinical trials; transplant centers; and Surveillance, Epidemiology, and End Results-Medicare analyses, noted Dr. Serper of the University of Pennsylvania in Philadelphia.
For a better look at veterans, she and her associates combined administrative, laboratory, and death data with medical chart reviews and information from the Organ Procurement and Transplantation Network’s Standard Transplant Analysis and Research file. The initial cohort included more than 6,800 veterans whose ICD-9CM diagnosis code indicated a malignant hepatic neoplasm. Excluding patients with neoplasms such as cholangiocarcinoma and those managed outside the VA left 3,989 VA patients with hepatocellular carcinoma.
In the multivariable analysis, use of multidisciplinary tumor boards was associated with a statistically significant 13% improvement in 5-year overall survival (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001). Improved survival also was linked with seeing certain specialists within 30 days of diagnosis, including hepatologists (HR, 0.77; P less than .001) and surgeons (HR, 0.72; P less than .001). Consulting with a hepatologist within 30 days of diagnosis, however, did not improve the chances of receiving curative therapy, such as liver transplantation, resection, local ablation, transarterial chemoembolization, or Y-90 radioembolization.
Care also varied substantially geographically and by academic affiliation, Dr. Serper noted. “Treatment of hepatocellular carcinoma is complex, as it depends as much on liver function as it does on tumor staging,” she emphasized. “Studies to improve multidisciplinary approaches for hepatocellular carcinoma in the community are needed to increase rates of curative therapy and improve clinical outcomes.”
Patients in this study averaged 62 years of age at diagnosis, 54% were white, 36% were within Milan criteria, and 45% had a Child-Turcotte-Pugh score of B or higher. Nearly 18% had macrovascular invasion at diagnosis, and 7% had metastatic disease. Nearly two-thirds of patients were BCLC stage A or B at diagnosis, and more than a third had underlying alcohol misuse and chronic hepatitis C virus infection.
The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
BOSTON – Veterans were about 13% less likely to die within 5 years of hepatocellular carcinoma diagnosis when multidisciplinary tumor boards managed their care than if they did not, according to a large, multicenter observational study.
Seeing a hepatologist or surgeon within 30 days of diagnosis also significantly improved 5-year overall survival, even after controlling for age, race, Charlson-Deyo comorbidity index, Barcelona Clinic Liver Cancer (BCLC) stage, academic center and geographic region of care, and the distance patients lived from the nearest Veterans Affairs transplant center, Marina Serper, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases. “More studies are needed to understand how to best use multidisciplinary tumor boards to improve the care of patients with hepatocellular carcinoma,” she said.
Outcomes data for hepatocellular carcinoma mostly come from clinical trials; transplant centers; and Surveillance, Epidemiology, and End Results-Medicare analyses, noted Dr. Serper of the University of Pennsylvania in Philadelphia.
For a better look at veterans, she and her associates combined administrative, laboratory, and death data with medical chart reviews and information from the Organ Procurement and Transplantation Network’s Standard Transplant Analysis and Research file. The initial cohort included more than 6,800 veterans whose ICD-9CM diagnosis code indicated a malignant hepatic neoplasm. Excluding patients with neoplasms such as cholangiocarcinoma and those managed outside the VA left 3,989 VA patients with hepatocellular carcinoma.
In the multivariable analysis, use of multidisciplinary tumor boards was associated with a statistically significant 13% improvement in 5-year overall survival (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001). Improved survival also was linked with seeing certain specialists within 30 days of diagnosis, including hepatologists (HR, 0.77; P less than .001) and surgeons (HR, 0.72; P less than .001). Consulting with a hepatologist within 30 days of diagnosis, however, did not improve the chances of receiving curative therapy, such as liver transplantation, resection, local ablation, transarterial chemoembolization, or Y-90 radioembolization.
Care also varied substantially geographically and by academic affiliation, Dr. Serper noted. “Treatment of hepatocellular carcinoma is complex, as it depends as much on liver function as it does on tumor staging,” she emphasized. “Studies to improve multidisciplinary approaches for hepatocellular carcinoma in the community are needed to increase rates of curative therapy and improve clinical outcomes.”
Patients in this study averaged 62 years of age at diagnosis, 54% were white, 36% were within Milan criteria, and 45% had a Child-Turcotte-Pugh score of B or higher. Nearly 18% had macrovascular invasion at diagnosis, and 7% had metastatic disease. Nearly two-thirds of patients were BCLC stage A or B at diagnosis, and more than a third had underlying alcohol misuse and chronic hepatitis C virus infection.
The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
BOSTON – Veterans were about 13% less likely to die within 5 years of hepatocellular carcinoma diagnosis when multidisciplinary tumor boards managed their care than if they did not, according to a large, multicenter observational study.
Seeing a hepatologist or surgeon within 30 days of diagnosis also significantly improved 5-year overall survival, even after controlling for age, race, Charlson-Deyo comorbidity index, Barcelona Clinic Liver Cancer (BCLC) stage, academic center and geographic region of care, and the distance patients lived from the nearest Veterans Affairs transplant center, Marina Serper, MD, reported at the annual meeting of the American Association for the Study of Liver Diseases. “More studies are needed to understand how to best use multidisciplinary tumor boards to improve the care of patients with hepatocellular carcinoma,” she said.
Outcomes data for hepatocellular carcinoma mostly come from clinical trials; transplant centers; and Surveillance, Epidemiology, and End Results-Medicare analyses, noted Dr. Serper of the University of Pennsylvania in Philadelphia.
For a better look at veterans, she and her associates combined administrative, laboratory, and death data with medical chart reviews and information from the Organ Procurement and Transplantation Network’s Standard Transplant Analysis and Research file. The initial cohort included more than 6,800 veterans whose ICD-9CM diagnosis code indicated a malignant hepatic neoplasm. Excluding patients with neoplasms such as cholangiocarcinoma and those managed outside the VA left 3,989 VA patients with hepatocellular carcinoma.
In the multivariable analysis, use of multidisciplinary tumor boards was associated with a statistically significant 13% improvement in 5-year overall survival (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001). Improved survival also was linked with seeing certain specialists within 30 days of diagnosis, including hepatologists (HR, 0.77; P less than .001) and surgeons (HR, 0.72; P less than .001). Consulting with a hepatologist within 30 days of diagnosis, however, did not improve the chances of receiving curative therapy, such as liver transplantation, resection, local ablation, transarterial chemoembolization, or Y-90 radioembolization.
Care also varied substantially geographically and by academic affiliation, Dr. Serper noted. “Treatment of hepatocellular carcinoma is complex, as it depends as much on liver function as it does on tumor staging,” she emphasized. “Studies to improve multidisciplinary approaches for hepatocellular carcinoma in the community are needed to increase rates of curative therapy and improve clinical outcomes.”
Patients in this study averaged 62 years of age at diagnosis, 54% were white, 36% were within Milan criteria, and 45% had a Child-Turcotte-Pugh score of B or higher. Nearly 18% had macrovascular invasion at diagnosis, and 7% had metastatic disease. Nearly two-thirds of patients were BCLC stage A or B at diagnosis, and more than a third had underlying alcohol misuse and chronic hepatitis C virus infection.
The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
AT THE LIVER MEETING 2016
Key clinical point: The use of multidisciplinary tumor boards was associated with significantly improved overall survival in patients with hepatocellular carcinoma.
Major finding: The risk of death within 5 years dropped by about 13% (hazard ratio, 0.87; 95% confidence interval, 0.81-0.94; P less than .001).
Data source: A retrospective study of 3,989 Veterans Affairs patients with hepatocellular carcinoma.
Disclosures: The work was funded by unrestricted grants from Bayer Healthcare Pharmaceuticals and the VA’s HIV, Hepatitis and Public Health Pathogens Programs. The investigators had no relevant financial disclosures.
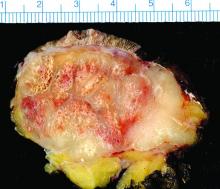
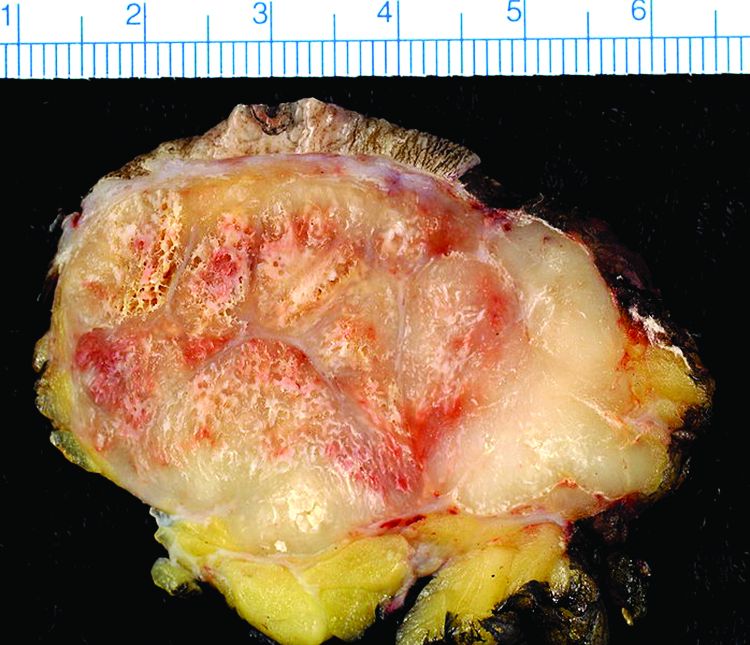
Study finds 19% of Merkel cell carcinomas are virus negative
Nineteen percent of Merkel cell carcinomas are not driven by the Merkel cell polyomavirus and are substantially more aggressive than those that are virus positive, according to a report published online in the Journal of Investigative Dermatology.
This and other findings from a retrospective analysis of samples from 282 Merkel cell carcinomas in a Seattle repository “suggest that it may be clinically indicated to determine tumor viral status at the time of diagnosis, as the results may affect prognosis as well as optimal clinical management,” wrote Ata Moshiri, MD, who was with the University of Washington, Seattle, at the time of the study, and his associates.
Given that virus-negative Merkel cell carcinomas carry a markedly higher risk of recurrence, progression, and patient mortality, “clinicians may consider larger initial surgical margins, larger radiotherapy fields, and the use of regional nodal therapy even in the absence of documented nodal metastasis. Closer clinical follow-up and more frequent radiologic surveillance may be justified for patients with virus-negative tumors because ... serologic monitoring is not feasible for this patient population,” the investigators noted.
The incidence of Merkel cell carcinoma, a rare and aggressive neuroendocrine skin cancer with an overall disease-related mortality of 40%, has quadrupled during the last 20 years. This is likely because of the increasing prevalence of risk factors for the cancer, including advanced age, increased cumulative exposure to ultraviolet light, and systemic immune suppression.
Data concerning the presence of Merkel cell polyomavirus in these cancers are conflicting, with estimates of virus positivity ranging from 20% all the way to 100% in some studies. Part of the reason for this wide range of estimates is that there is no accepted preferred method for measuring the viral status of these tumors. Moreover, the prognostic significance of that viral status is also debated. Thus, most Merkel cell cancers are not routinely analyzed for the presence of Merkel cell polyomavirus.
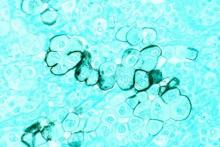
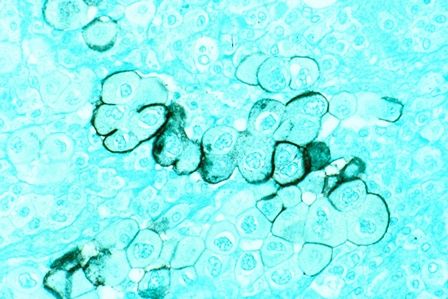
To pin down the prevalence of virus positivity and establish whether it impacts clinical outcomes, Dr. Moshiri and his associates analyzed 282 Merkel cell specimens collected since 1980 and stored in a Seattle repository, along with clinical data. They tested each specimen using an immunohistochemical assay to detect one antibody (CM2B4), a different immunohistochemical assay to detect another antibody (Ab3), and a quantitative PCR assay for polyomavirus DNA. To be considered virus positive, each specimen had to show the presence of the virus on at least two of these tests.
By these criteria, 53 tumors (18.8%) were found to be virus negative and 229 (81.2%) to be virus positive.
Virus-negative tumors tended to be smaller than virus-positive tumors at presentation. Despite their smaller size, virus-negative tumors tended to be more advanced at presentation: 66.7% had nodal or distant metastases, compared with 48.3% of virus-positive tumors.
A total of 66.7% of virus-negative carcinomas progressed, compared with only 43.6% of virus-positive carcinomas. The median time to progression was 1.2 years for virus-negative cancers, but was not reached for virus-positive cancers. In a univariate analysis, virus-negative tumors had a nearly twofold higher risk of progression. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell slightly to 1.55.
Cancer-specific mortality was 45.3% for virus-negative tumors, compared with 26.3% for virus-positive tumors. Median time to death from Merkel cell carcinoma was 3.7 years for virus-negative tumors but was not reached for virus-positive tumors. In a univariate analysis, virus-negative tumors carried a nearly twofold higher risk of death from Merkel cell carcinoma. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell somewhat to 1.50.
Median overall survival was 3.3 years for patients with virus-negative tumors, compared with 4.6 years for patients with virus-positive tumors.
These findings indicate that a more advanced cancer stage at diagnosis accounts for some but not all of the poorer clinical outcomes seen with virus-negative tumors, the investigators said.
This study could not assess why virus-negative Merkel cell carcinomas are more aggressive and lethal than virus-positive ones, but previous studies have proposed some plausible biological mechanisms. Virus-negative tumors carry a greater number of chromosomal aberrations, a greater burden of nucleotide mutations, and a greater number of mutations in known oncogenic pathways. They also may be more immunogenic “due to their constitutive expression of oncoproteins that may serve as targets for cytotoxic tumor-infiltrating lymphocytes,” Dr. Moshiri and his associates said.
They added that in this study, the immunohistochemical assay for CM2B4 antibodies was the test that most accurately identified tumors that had worse outcomes. “We believe that the CM2B4 antibody test may be well-suited for routine clinical use” because of its sensitivity and specificity in this application, its commercial availability, “and the ease with which it could be included in the work flow of clinical laboratories accustomed to immunohistochemistry.”
Dr. Moshiri is currently at the University of Pennsylvania, Philadelphia.
The National Institutes of Health, the Colin Johnston Fund, and the Janet Canning Fund supported the study. Dr. Moshiri reported having no relevant financial disclosures; one of his associates reported that her institute received research funding from Valeant and Pfizer unrelated to this work.
Nineteen percent of Merkel cell carcinomas are not driven by the Merkel cell polyomavirus and are substantially more aggressive than those that are virus positive, according to a report published online in the Journal of Investigative Dermatology.
This and other findings from a retrospective analysis of samples from 282 Merkel cell carcinomas in a Seattle repository “suggest that it may be clinically indicated to determine tumor viral status at the time of diagnosis, as the results may affect prognosis as well as optimal clinical management,” wrote Ata Moshiri, MD, who was with the University of Washington, Seattle, at the time of the study, and his associates.
Given that virus-negative Merkel cell carcinomas carry a markedly higher risk of recurrence, progression, and patient mortality, “clinicians may consider larger initial surgical margins, larger radiotherapy fields, and the use of regional nodal therapy even in the absence of documented nodal metastasis. Closer clinical follow-up and more frequent radiologic surveillance may be justified for patients with virus-negative tumors because ... serologic monitoring is not feasible for this patient population,” the investigators noted.
The incidence of Merkel cell carcinoma, a rare and aggressive neuroendocrine skin cancer with an overall disease-related mortality of 40%, has quadrupled during the last 20 years. This is likely because of the increasing prevalence of risk factors for the cancer, including advanced age, increased cumulative exposure to ultraviolet light, and systemic immune suppression.
Data concerning the presence of Merkel cell polyomavirus in these cancers are conflicting, with estimates of virus positivity ranging from 20% all the way to 100% in some studies. Part of the reason for this wide range of estimates is that there is no accepted preferred method for measuring the viral status of these tumors. Moreover, the prognostic significance of that viral status is also debated. Thus, most Merkel cell cancers are not routinely analyzed for the presence of Merkel cell polyomavirus.
To pin down the prevalence of virus positivity and establish whether it impacts clinical outcomes, Dr. Moshiri and his associates analyzed 282 Merkel cell specimens collected since 1980 and stored in a Seattle repository, along with clinical data. They tested each specimen using an immunohistochemical assay to detect one antibody (CM2B4), a different immunohistochemical assay to detect another antibody (Ab3), and a quantitative PCR assay for polyomavirus DNA. To be considered virus positive, each specimen had to show the presence of the virus on at least two of these tests.
By these criteria, 53 tumors (18.8%) were found to be virus negative and 229 (81.2%) to be virus positive.
Virus-negative tumors tended to be smaller than virus-positive tumors at presentation. Despite their smaller size, virus-negative tumors tended to be more advanced at presentation: 66.7% had nodal or distant metastases, compared with 48.3% of virus-positive tumors.
A total of 66.7% of virus-negative carcinomas progressed, compared with only 43.6% of virus-positive carcinomas. The median time to progression was 1.2 years for virus-negative cancers, but was not reached for virus-positive cancers. In a univariate analysis, virus-negative tumors had a nearly twofold higher risk of progression. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell slightly to 1.55.
Cancer-specific mortality was 45.3% for virus-negative tumors, compared with 26.3% for virus-positive tumors. Median time to death from Merkel cell carcinoma was 3.7 years for virus-negative tumors but was not reached for virus-positive tumors. In a univariate analysis, virus-negative tumors carried a nearly twofold higher risk of death from Merkel cell carcinoma. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell somewhat to 1.50.
Median overall survival was 3.3 years for patients with virus-negative tumors, compared with 4.6 years for patients with virus-positive tumors.
These findings indicate that a more advanced cancer stage at diagnosis accounts for some but not all of the poorer clinical outcomes seen with virus-negative tumors, the investigators said.
This study could not assess why virus-negative Merkel cell carcinomas are more aggressive and lethal than virus-positive ones, but previous studies have proposed some plausible biological mechanisms. Virus-negative tumors carry a greater number of chromosomal aberrations, a greater burden of nucleotide mutations, and a greater number of mutations in known oncogenic pathways. They also may be more immunogenic “due to their constitutive expression of oncoproteins that may serve as targets for cytotoxic tumor-infiltrating lymphocytes,” Dr. Moshiri and his associates said.
They added that in this study, the immunohistochemical assay for CM2B4 antibodies was the test that most accurately identified tumors that had worse outcomes. “We believe that the CM2B4 antibody test may be well-suited for routine clinical use” because of its sensitivity and specificity in this application, its commercial availability, “and the ease with which it could be included in the work flow of clinical laboratories accustomed to immunohistochemistry.”
Dr. Moshiri is currently at the University of Pennsylvania, Philadelphia.
The National Institutes of Health, the Colin Johnston Fund, and the Janet Canning Fund supported the study. Dr. Moshiri reported having no relevant financial disclosures; one of his associates reported that her institute received research funding from Valeant and Pfizer unrelated to this work.
Nineteen percent of Merkel cell carcinomas are not driven by the Merkel cell polyomavirus and are substantially more aggressive than those that are virus positive, according to a report published online in the Journal of Investigative Dermatology.
This and other findings from a retrospective analysis of samples from 282 Merkel cell carcinomas in a Seattle repository “suggest that it may be clinically indicated to determine tumor viral status at the time of diagnosis, as the results may affect prognosis as well as optimal clinical management,” wrote Ata Moshiri, MD, who was with the University of Washington, Seattle, at the time of the study, and his associates.
Given that virus-negative Merkel cell carcinomas carry a markedly higher risk of recurrence, progression, and patient mortality, “clinicians may consider larger initial surgical margins, larger radiotherapy fields, and the use of regional nodal therapy even in the absence of documented nodal metastasis. Closer clinical follow-up and more frequent radiologic surveillance may be justified for patients with virus-negative tumors because ... serologic monitoring is not feasible for this patient population,” the investigators noted.
The incidence of Merkel cell carcinoma, a rare and aggressive neuroendocrine skin cancer with an overall disease-related mortality of 40%, has quadrupled during the last 20 years. This is likely because of the increasing prevalence of risk factors for the cancer, including advanced age, increased cumulative exposure to ultraviolet light, and systemic immune suppression.
Data concerning the presence of Merkel cell polyomavirus in these cancers are conflicting, with estimates of virus positivity ranging from 20% all the way to 100% in some studies. Part of the reason for this wide range of estimates is that there is no accepted preferred method for measuring the viral status of these tumors. Moreover, the prognostic significance of that viral status is also debated. Thus, most Merkel cell cancers are not routinely analyzed for the presence of Merkel cell polyomavirus.
To pin down the prevalence of virus positivity and establish whether it impacts clinical outcomes, Dr. Moshiri and his associates analyzed 282 Merkel cell specimens collected since 1980 and stored in a Seattle repository, along with clinical data. They tested each specimen using an immunohistochemical assay to detect one antibody (CM2B4), a different immunohistochemical assay to detect another antibody (Ab3), and a quantitative PCR assay for polyomavirus DNA. To be considered virus positive, each specimen had to show the presence of the virus on at least two of these tests.
By these criteria, 53 tumors (18.8%) were found to be virus negative and 229 (81.2%) to be virus positive.
Virus-negative tumors tended to be smaller than virus-positive tumors at presentation. Despite their smaller size, virus-negative tumors tended to be more advanced at presentation: 66.7% had nodal or distant metastases, compared with 48.3% of virus-positive tumors.
A total of 66.7% of virus-negative carcinomas progressed, compared with only 43.6% of virus-positive carcinomas. The median time to progression was 1.2 years for virus-negative cancers, but was not reached for virus-positive cancers. In a univariate analysis, virus-negative tumors had a nearly twofold higher risk of progression. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell slightly to 1.55.
Cancer-specific mortality was 45.3% for virus-negative tumors, compared with 26.3% for virus-positive tumors. Median time to death from Merkel cell carcinoma was 3.7 years for virus-negative tumors but was not reached for virus-positive tumors. In a univariate analysis, virus-negative tumors carried a nearly twofold higher risk of death from Merkel cell carcinoma. In a multivariate analysis that adjusted for differences in disease stage at presentation, the HR fell somewhat to 1.50.
Median overall survival was 3.3 years for patients with virus-negative tumors, compared with 4.6 years for patients with virus-positive tumors.
These findings indicate that a more advanced cancer stage at diagnosis accounts for some but not all of the poorer clinical outcomes seen with virus-negative tumors, the investigators said.
This study could not assess why virus-negative Merkel cell carcinomas are more aggressive and lethal than virus-positive ones, but previous studies have proposed some plausible biological mechanisms. Virus-negative tumors carry a greater number of chromosomal aberrations, a greater burden of nucleotide mutations, and a greater number of mutations in known oncogenic pathways. They also may be more immunogenic “due to their constitutive expression of oncoproteins that may serve as targets for cytotoxic tumor-infiltrating lymphocytes,” Dr. Moshiri and his associates said.
They added that in this study, the immunohistochemical assay for CM2B4 antibodies was the test that most accurately identified tumors that had worse outcomes. “We believe that the CM2B4 antibody test may be well-suited for routine clinical use” because of its sensitivity and specificity in this application, its commercial availability, “and the ease with which it could be included in the work flow of clinical laboratories accustomed to immunohistochemistry.”
Dr. Moshiri is currently at the University of Pennsylvania, Philadelphia.
The National Institutes of Health, the Colin Johnston Fund, and the Janet Canning Fund supported the study. Dr. Moshiri reported having no relevant financial disclosures; one of his associates reported that her institute received research funding from Valeant and Pfizer unrelated to this work.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Nineteen percent of Merkel cell carcinomas are not driven by the Merkel cell polyomavirus and are substantially more aggressive than those that are virus positive.
Major finding: The 53 virus-negative tumors carried a cancer-specific mortality of 45.3%, while the 229 virus-positive tumors carried a cancer-specific mortality of 26.2%.
Data source: A retrospective molecular analysis of samples from 282 Merkel cell carcinomas in a Seattle repository for the presence of Merkel cell polyomavirus.
Disclosures: The National Institutes of Health, the Colin Johnston Fund, and the Janet Canning Fund supported the study. Dr. Moshiri reported having no relevant financial disclosures; one of his associates reported that her institute received research funding from Valeant and Pfizer unrelated to this work.
Delayed bleeding possible with EBUS-TBNA on antiplatelets
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Ten percent of patients on dual antiplatelet therapy bled within 48 hours, versus 1.1% of those not on antiplatelet therapy.
Data source: Single-center review of 404 patients.
Disclosures: There was no industry funding for the work, and the investigators had no disclosures.
Surgical infections, early discharge hike readmissions in extrahepatic cholangiocarcinoma
WASHINGTON – Hospital readmissions are common after resection of extrahepatic cholangiocarcinoma, with about 20% of patients returning in the first 90 days after surgery.
Two factors – surgical site infections and an abbreviated length of stay – both quadrupled the risk of readmission, Michail Mavros, MD, said at the American College of Surgeons Clinical Congress.
“Surgeons are scrutinized over length of stay and, as a result, these fast-track recovery pathways are increasingly important. Readmission rates are being used as a quality metric and performance indicator, and tied to reimbursement. But our data suggest that we should be somewhat cautious in implementing those with this surgery. The patient may look great with good pain control, and be eating and ambulating by day 4 or 5, but it may be premature to discharge at that point, and safer to wait a little longer. The financial penalty for readmission is probably not worth that small bonus we get for early discharge.”
The study comprised 422 patients who underwent resection with curative intent for extrahepatic cholangiocarcinoma. This is a rare tumor with about 5,000 cases presenting each year. Dr. Mavros and his colleagues extracted their data from the U.S. Extrahepatic Cholangiocarcinoma Collaborative. The primary outcomes were 30- and 90-day readmission rates.
The patients’ median age was 67 years. About a third had mild comorbidities with an American Society of Anesthesiologist (ASA) comorbidity class of 1-2. The rest had moderate to severe comorbidities (ASA class 3-4). Hypertension was common (48%); 18% had diabetes.
Tumor location was split almost equally between distal and hilar; the median tumor size was 2.3 cm.
Final margins were positive in 28% and half of the cohort had positive regional lymph nodes.
The procedures were quite varied, and included common bile duct resection (18%); hepatectomy plus common bile duct resection (40%); and Whipple procedure (42%). The median estimated blood loss was 500 cc; 28% of the cohort required transfusion with packed red blood cells and 8% with fresh frozen plasma.
Postoperative complications were common (63%), with half of those being classed as serious. Infectious complications were most common, including superficial (11%), deep (7%), and organ space infections (16%).
Bile leaks occurred in 4% of cases. Reoperations were necessary in 7%. The 30-day mortality was 4.5% and 90-day mortality, 8%.The median length of stay was 8 days but this ranged from 7 to 18 days.
The 30-day readmission rate was 19% and the 90-day readmission rate was 23%. Most readmissions occurred fairly quickly – the median time to readmission was 12 days, with a range of 6-24 days.
The investigators conducted a multivariate analysis to determine independent predictors of readmission. The strongest predictors were any surgical complications (odds ratio, 8.4); organ-space infection (OR, 4.5); and length of stay of 8 days or less (OR, 4.3). Other predictors were advancing age (OR, 1.5 for each 10 years) and having had a liver resection (OR, 2.0).
“It’s clear from these results that avoidance of complications, especially infectious complications, may improve readmission rates dramatically,” Dr. Mavros said. “We would advise caution in implementing any fast-track protocols with these patients, given the finding that early discharge was associated with a higher rate of readmission.”
Dr. Mavros had no financial disclosures.
[email protected]
On Twitter @alz_gal
WASHINGTON – Hospital readmissions are common after resection of extrahepatic cholangiocarcinoma, with about 20% of patients returning in the first 90 days after surgery.
Two factors – surgical site infections and an abbreviated length of stay – both quadrupled the risk of readmission, Michail Mavros, MD, said at the American College of Surgeons Clinical Congress.
“Surgeons are scrutinized over length of stay and, as a result, these fast-track recovery pathways are increasingly important. Readmission rates are being used as a quality metric and performance indicator, and tied to reimbursement. But our data suggest that we should be somewhat cautious in implementing those with this surgery. The patient may look great with good pain control, and be eating and ambulating by day 4 or 5, but it may be premature to discharge at that point, and safer to wait a little longer. The financial penalty for readmission is probably not worth that small bonus we get for early discharge.”
The study comprised 422 patients who underwent resection with curative intent for extrahepatic cholangiocarcinoma. This is a rare tumor with about 5,000 cases presenting each year. Dr. Mavros and his colleagues extracted their data from the U.S. Extrahepatic Cholangiocarcinoma Collaborative. The primary outcomes were 30- and 90-day readmission rates.
The patients’ median age was 67 years. About a third had mild comorbidities with an American Society of Anesthesiologist (ASA) comorbidity class of 1-2. The rest had moderate to severe comorbidities (ASA class 3-4). Hypertension was common (48%); 18% had diabetes.
Tumor location was split almost equally between distal and hilar; the median tumor size was 2.3 cm.
Final margins were positive in 28% and half of the cohort had positive regional lymph nodes.
The procedures were quite varied, and included common bile duct resection (18%); hepatectomy plus common bile duct resection (40%); and Whipple procedure (42%). The median estimated blood loss was 500 cc; 28% of the cohort required transfusion with packed red blood cells and 8% with fresh frozen plasma.
Postoperative complications were common (63%), with half of those being classed as serious. Infectious complications were most common, including superficial (11%), deep (7%), and organ space infections (16%).
Bile leaks occurred in 4% of cases. Reoperations were necessary in 7%. The 30-day mortality was 4.5% and 90-day mortality, 8%.The median length of stay was 8 days but this ranged from 7 to 18 days.
The 30-day readmission rate was 19% and the 90-day readmission rate was 23%. Most readmissions occurred fairly quickly – the median time to readmission was 12 days, with a range of 6-24 days.
The investigators conducted a multivariate analysis to determine independent predictors of readmission. The strongest predictors were any surgical complications (odds ratio, 8.4); organ-space infection (OR, 4.5); and length of stay of 8 days or less (OR, 4.3). Other predictors were advancing age (OR, 1.5 for each 10 years) and having had a liver resection (OR, 2.0).
“It’s clear from these results that avoidance of complications, especially infectious complications, may improve readmission rates dramatically,” Dr. Mavros said. “We would advise caution in implementing any fast-track protocols with these patients, given the finding that early discharge was associated with a higher rate of readmission.”
Dr. Mavros had no financial disclosures.
[email protected]
On Twitter @alz_gal
WASHINGTON – Hospital readmissions are common after resection of extrahepatic cholangiocarcinoma, with about 20% of patients returning in the first 90 days after surgery.
Two factors – surgical site infections and an abbreviated length of stay – both quadrupled the risk of readmission, Michail Mavros, MD, said at the American College of Surgeons Clinical Congress.
“Surgeons are scrutinized over length of stay and, as a result, these fast-track recovery pathways are increasingly important. Readmission rates are being used as a quality metric and performance indicator, and tied to reimbursement. But our data suggest that we should be somewhat cautious in implementing those with this surgery. The patient may look great with good pain control, and be eating and ambulating by day 4 or 5, but it may be premature to discharge at that point, and safer to wait a little longer. The financial penalty for readmission is probably not worth that small bonus we get for early discharge.”
The study comprised 422 patients who underwent resection with curative intent for extrahepatic cholangiocarcinoma. This is a rare tumor with about 5,000 cases presenting each year. Dr. Mavros and his colleagues extracted their data from the U.S. Extrahepatic Cholangiocarcinoma Collaborative. The primary outcomes were 30- and 90-day readmission rates.
The patients’ median age was 67 years. About a third had mild comorbidities with an American Society of Anesthesiologist (ASA) comorbidity class of 1-2. The rest had moderate to severe comorbidities (ASA class 3-4). Hypertension was common (48%); 18% had diabetes.
Tumor location was split almost equally between distal and hilar; the median tumor size was 2.3 cm.
Final margins were positive in 28% and half of the cohort had positive regional lymph nodes.
The procedures were quite varied, and included common bile duct resection (18%); hepatectomy plus common bile duct resection (40%); and Whipple procedure (42%). The median estimated blood loss was 500 cc; 28% of the cohort required transfusion with packed red blood cells and 8% with fresh frozen plasma.
Postoperative complications were common (63%), with half of those being classed as serious. Infectious complications were most common, including superficial (11%), deep (7%), and organ space infections (16%).
Bile leaks occurred in 4% of cases. Reoperations were necessary in 7%. The 30-day mortality was 4.5% and 90-day mortality, 8%.The median length of stay was 8 days but this ranged from 7 to 18 days.
The 30-day readmission rate was 19% and the 90-day readmission rate was 23%. Most readmissions occurred fairly quickly – the median time to readmission was 12 days, with a range of 6-24 days.
The investigators conducted a multivariate analysis to determine independent predictors of readmission. The strongest predictors were any surgical complications (odds ratio, 8.4); organ-space infection (OR, 4.5); and length of stay of 8 days or less (OR, 4.3). Other predictors were advancing age (OR, 1.5 for each 10 years) and having had a liver resection (OR, 2.0).
“It’s clear from these results that avoidance of complications, especially infectious complications, may improve readmission rates dramatically,” Dr. Mavros said. “We would advise caution in implementing any fast-track protocols with these patients, given the finding that early discharge was associated with a higher rate of readmission.”
Dr. Mavros had no financial disclosures.
[email protected]
On Twitter @alz_gal
Key clinical point:
Major finding: Organ space infections and a shorter length of stay both quadrupled the risk of a readmission.
Data source: The database review comprised 422 patients.
Disclosures: Dr. Mavros had no financial disclosures.
Biomarker identifies precancerous pancreatic cysts
LAS VEGAS – In fluid derived from pancreatic cysts, methylated DNA markers predict the presence of high-grade dysplasia (HGD) or cancer, and could help physicians decide whether to surgically remove cysts – a procedure that often has serious complications.
If validated in larger studies, the biomarkers have the potential to supplant the Fukuoka criteria that is currently used. “The markers could cause a paradigm shift in how we approach these lesions in our clinical practice,” Shounak Majumder, MD, a fellow at the Mayo Clinic in Rochester, Minn., said in an interview.
Less than 50% of cysts that are surgically resected turn out to be HGD or cancerous. “Having a cyst fluid marker could identify the patients that would benefit the most from surgery. If you’re going to go through a pancreatic resection, we’d rather give you the best chance of saying that we removed something that either has early cancer in it or will turn into cancer in the near future,” said Dr. Majumder.
The study looked at pancreatic cyst fluid from 83 cysts that had been surgically resected. The DNA samples were taken from the cyst fluid. Dr. Majumder believes that the cells shed from the cyst wall into the fluid. As a result, DNA from the fluid captures heterogeneity in the cyst more effectively than a biopsied sample.
The researchers found five methylated DNA markers that distinguished cancer or HGD from controls with areas under the ROC curve of 0.90 or higher. The top two (BMP3, EMX1) detected 93% of cases (95% CI, 66%-100%) at a specificity of 90% (95% CI, 80%-96%). Applied to eight cysts with intermediate-grade dysplasia, the biomarkers would have identified three at 95% specificity.
By comparison, the Fukuoka guidelines have 56% sensitivity and 73% specificity.
A limitation to the technique is that DNA cannot be extracted from all samples. About 5%-10% of pancreatic fluid samples are unusable, according to Somashekar Krishna, MD, MPH, assistant professor of medicine at the Ohio State University Medical Center, who attended the session. Dr. Krishna is conducting research combining endomicroscopy with molecular markers.
“We should have a foolproof system where if one fails, the other kicks in, and we have an answer for every patient. My opinion is that endomicroscopy has to be combined with molecular studies. I think combined we’ll have an excellent diagnostic yield,” Dr. Krishna said in an interview.
Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
LAS VEGAS – In fluid derived from pancreatic cysts, methylated DNA markers predict the presence of high-grade dysplasia (HGD) or cancer, and could help physicians decide whether to surgically remove cysts – a procedure that often has serious complications.
If validated in larger studies, the biomarkers have the potential to supplant the Fukuoka criteria that is currently used. “The markers could cause a paradigm shift in how we approach these lesions in our clinical practice,” Shounak Majumder, MD, a fellow at the Mayo Clinic in Rochester, Minn., said in an interview.
Less than 50% of cysts that are surgically resected turn out to be HGD or cancerous. “Having a cyst fluid marker could identify the patients that would benefit the most from surgery. If you’re going to go through a pancreatic resection, we’d rather give you the best chance of saying that we removed something that either has early cancer in it or will turn into cancer in the near future,” said Dr. Majumder.
The study looked at pancreatic cyst fluid from 83 cysts that had been surgically resected. The DNA samples were taken from the cyst fluid. Dr. Majumder believes that the cells shed from the cyst wall into the fluid. As a result, DNA from the fluid captures heterogeneity in the cyst more effectively than a biopsied sample.
The researchers found five methylated DNA markers that distinguished cancer or HGD from controls with areas under the ROC curve of 0.90 or higher. The top two (BMP3, EMX1) detected 93% of cases (95% CI, 66%-100%) at a specificity of 90% (95% CI, 80%-96%). Applied to eight cysts with intermediate-grade dysplasia, the biomarkers would have identified three at 95% specificity.
By comparison, the Fukuoka guidelines have 56% sensitivity and 73% specificity.
A limitation to the technique is that DNA cannot be extracted from all samples. About 5%-10% of pancreatic fluid samples are unusable, according to Somashekar Krishna, MD, MPH, assistant professor of medicine at the Ohio State University Medical Center, who attended the session. Dr. Krishna is conducting research combining endomicroscopy with molecular markers.
“We should have a foolproof system where if one fails, the other kicks in, and we have an answer for every patient. My opinion is that endomicroscopy has to be combined with molecular studies. I think combined we’ll have an excellent diagnostic yield,” Dr. Krishna said in an interview.
Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
LAS VEGAS – In fluid derived from pancreatic cysts, methylated DNA markers predict the presence of high-grade dysplasia (HGD) or cancer, and could help physicians decide whether to surgically remove cysts – a procedure that often has serious complications.
If validated in larger studies, the biomarkers have the potential to supplant the Fukuoka criteria that is currently used. “The markers could cause a paradigm shift in how we approach these lesions in our clinical practice,” Shounak Majumder, MD, a fellow at the Mayo Clinic in Rochester, Minn., said in an interview.
Less than 50% of cysts that are surgically resected turn out to be HGD or cancerous. “Having a cyst fluid marker could identify the patients that would benefit the most from surgery. If you’re going to go through a pancreatic resection, we’d rather give you the best chance of saying that we removed something that either has early cancer in it or will turn into cancer in the near future,” said Dr. Majumder.
The study looked at pancreatic cyst fluid from 83 cysts that had been surgically resected. The DNA samples were taken from the cyst fluid. Dr. Majumder believes that the cells shed from the cyst wall into the fluid. As a result, DNA from the fluid captures heterogeneity in the cyst more effectively than a biopsied sample.
The researchers found five methylated DNA markers that distinguished cancer or HGD from controls with areas under the ROC curve of 0.90 or higher. The top two (BMP3, EMX1) detected 93% of cases (95% CI, 66%-100%) at a specificity of 90% (95% CI, 80%-96%). Applied to eight cysts with intermediate-grade dysplasia, the biomarkers would have identified three at 95% specificity.
By comparison, the Fukuoka guidelines have 56% sensitivity and 73% specificity.
A limitation to the technique is that DNA cannot be extracted from all samples. About 5%-10% of pancreatic fluid samples are unusable, according to Somashekar Krishna, MD, MPH, assistant professor of medicine at the Ohio State University Medical Center, who attended the session. Dr. Krishna is conducting research combining endomicroscopy with molecular markers.
“We should have a foolproof system where if one fails, the other kicks in, and we have an answer for every patient. My opinion is that endomicroscopy has to be combined with molecular studies. I think combined we’ll have an excellent diagnostic yield,” Dr. Krishna said in an interview.
Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
AT ACG 2016
Key clinical point:
Major finding: DNA markers isolated from pancreatic fluid predicted cancer or high-grade dysplasia with 90% specificity and 93% sensitivity.
Data source: Pilot study, retrospective analysis.
Disclosures: Dr. Majumder and Dr. Krishna have declared no conflicts of interest.
Enhanced recovery pathways in gynecology
Enhanced recovery surgical principles were first described in the 1990s.1 These principles postulate that the body’s stress response to surgical injury and deviation from normal physiology is the source of postoperative morbidity. Thus, enhanced recovery programs are designed around perioperative interventions that mitigate and help the body cope with the surgical stress response.
Many of these interventions run counter to traditional perioperative care paradigms. Enhanced recovery protocols are diverse but have common themes of avoiding preoperative fasting and bowel preparation, early oral intake, limiting use of drains and catheters, multimodal analgesia, early ambulation, and prioritizing euvolemia and normothermia. Individual interventions in these areas are combined to create a master protocol, which is implemented as a bundle to improve surgical outcomes.
Current components
Minimizing preoperative fasting, early postoperative refeeding, and preoperative carbohydrate-loading drinks are all key aspects of enhanced recovery protocols. “NPO after midnight” has been a longstanding rule due to the risk of aspiration with intubation. However, a Cochrane review found no evidence that a shortened period of fasting was associated with an increased risk of aspiration or related morbidity. Currently, the American Society of Anesthesiologists recommends only a 6-hour fast for solid foods and 2 hours for clear liquids.2,3
Preoperative fasting causes depletion of glycogen stores leading to insulin resistance and hyperglycemia, which are both associated with postoperative complications and morbidity.4 Preoperative carbohydrate-loading drinks can reverse some of the effects of limited preoperative fasting including preventing insulin resistance and hyperglycemia.5
Postoperative fasting should also be avoided. Early enteral intake is very important to decrease time spent in a catabolic state and decrease insulin resistance. In gynecology patients, early refeeding is associated with a faster return of bowel function and a decreased length of stay without an increase in postoperative complications.6 Notably, patients undergoing early feeding consistently experience more nausea and vomiting, but this is not associated with complications.7
The fluid management goal in enhanced recovery is to maintain perioperative euvolemia, as both hypovolemia and hypervolemia have negative physiologic consequences. When studied, fluid protocols designed to minimize the use of postoperative fluids have resulted in decreased cardiopulmonary complications, decreased postoperative morbidity, faster return of bowel function, and shorter hospital stays.8 Given the morbidity associated with fluid overload, enhanced recovery protocols recommend that minimal fluids be given in the operating room and intravenous fluids be removed as quickly as possible, often with first oral intake or postoperative day 1 at the latest.
Engagement of the patient in their perioperative recovery with patient education materials and expectations for postoperative tasks, such as early refeeding, spirometry, and ambulation are all important components of enhanced recovery. Patients become partners in achieving postoperative milestones, and this results in improved outcomes such as decreased pain scores and shorter recoveries.
Evidence in gynecology
Enhanced recovery has been studied in many surgical disciplines including urology, colorectal surgery, hepatobiliary surgery, and gynecology. High-quality studies of abdominal and vaginal hysterectomy patients have consistently found a decrease in length of stay with no difference in readmission or postoperative complication rates.9 An interesting study also found that an enhanced recovery program was associated with decreased nursing time required for patient care.10
For ovarian cancer patients, enhanced recovery is associated with decreased length of stay, decreased time to return of bowel function, and improved quality of life. Enhanced recovery is also cost saving, saving $257-$697 per vaginal hysterectomy patient and $5,410-$7,600 per ovarian cancer patient.11
Enhanced recovery protocols are safe, evidenced based, cost saving, and are increasingly being adopted as clinicians and health systems become aware of their benefits.
References
1. Br J Anaesth. 1997 May;78(5):606-17.
2. Cochrane Database Syst Rev. 2003 Oct 20;(4):CD004423.
3. Anesthesiology. 1999 Mar;90(3):896-905.
4. J Am Coll Surg. 2012 Jan;214(1):68-80.
5. Clin Nutr. 1998 Apr;17(2):65-71.
6. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD004508.
7. Obstet Gynecol. 1998 Jul;92(1):94-7.
8. Br J Surg. 2009 Apr;96(4):331-41.
9. Obstet Gynecol. 2013 Aug;122(2 Pt 1):319-28.
10. Qual Saf Health Care. 2009 Jun;18(3):236-40.
11. Gynecol Oncol. 2008 Feb;108(2):282-6.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Barber is a third-year fellow in gynecologic oncology at the university. They reported having no financial disclosures relevant to this column. Email them at [email protected].
Enhanced recovery surgical principles were first described in the 1990s.1 These principles postulate that the body’s stress response to surgical injury and deviation from normal physiology is the source of postoperative morbidity. Thus, enhanced recovery programs are designed around perioperative interventions that mitigate and help the body cope with the surgical stress response.
Many of these interventions run counter to traditional perioperative care paradigms. Enhanced recovery protocols are diverse but have common themes of avoiding preoperative fasting and bowel preparation, early oral intake, limiting use of drains and catheters, multimodal analgesia, early ambulation, and prioritizing euvolemia and normothermia. Individual interventions in these areas are combined to create a master protocol, which is implemented as a bundle to improve surgical outcomes.
Current components
Minimizing preoperative fasting, early postoperative refeeding, and preoperative carbohydrate-loading drinks are all key aspects of enhanced recovery protocols. “NPO after midnight” has been a longstanding rule due to the risk of aspiration with intubation. However, a Cochrane review found no evidence that a shortened period of fasting was associated with an increased risk of aspiration or related morbidity. Currently, the American Society of Anesthesiologists recommends only a 6-hour fast for solid foods and 2 hours for clear liquids.2,3
Preoperative fasting causes depletion of glycogen stores leading to insulin resistance and hyperglycemia, which are both associated with postoperative complications and morbidity.4 Preoperative carbohydrate-loading drinks can reverse some of the effects of limited preoperative fasting including preventing insulin resistance and hyperglycemia.5
Postoperative fasting should also be avoided. Early enteral intake is very important to decrease time spent in a catabolic state and decrease insulin resistance. In gynecology patients, early refeeding is associated with a faster return of bowel function and a decreased length of stay without an increase in postoperative complications.6 Notably, patients undergoing early feeding consistently experience more nausea and vomiting, but this is not associated with complications.7
The fluid management goal in enhanced recovery is to maintain perioperative euvolemia, as both hypovolemia and hypervolemia have negative physiologic consequences. When studied, fluid protocols designed to minimize the use of postoperative fluids have resulted in decreased cardiopulmonary complications, decreased postoperative morbidity, faster return of bowel function, and shorter hospital stays.8 Given the morbidity associated with fluid overload, enhanced recovery protocols recommend that minimal fluids be given in the operating room and intravenous fluids be removed as quickly as possible, often with first oral intake or postoperative day 1 at the latest.
Engagement of the patient in their perioperative recovery with patient education materials and expectations for postoperative tasks, such as early refeeding, spirometry, and ambulation are all important components of enhanced recovery. Patients become partners in achieving postoperative milestones, and this results in improved outcomes such as decreased pain scores and shorter recoveries.
Evidence in gynecology
Enhanced recovery has been studied in many surgical disciplines including urology, colorectal surgery, hepatobiliary surgery, and gynecology. High-quality studies of abdominal and vaginal hysterectomy patients have consistently found a decrease in length of stay with no difference in readmission or postoperative complication rates.9 An interesting study also found that an enhanced recovery program was associated with decreased nursing time required for patient care.10
For ovarian cancer patients, enhanced recovery is associated with decreased length of stay, decreased time to return of bowel function, and improved quality of life. Enhanced recovery is also cost saving, saving $257-$697 per vaginal hysterectomy patient and $5,410-$7,600 per ovarian cancer patient.11
Enhanced recovery protocols are safe, evidenced based, cost saving, and are increasingly being adopted as clinicians and health systems become aware of their benefits.
References
1. Br J Anaesth. 1997 May;78(5):606-17.
2. Cochrane Database Syst Rev. 2003 Oct 20;(4):CD004423.
3. Anesthesiology. 1999 Mar;90(3):896-905.
4. J Am Coll Surg. 2012 Jan;214(1):68-80.
5. Clin Nutr. 1998 Apr;17(2):65-71.
6. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD004508.
7. Obstet Gynecol. 1998 Jul;92(1):94-7.
8. Br J Surg. 2009 Apr;96(4):331-41.
9. Obstet Gynecol. 2013 Aug;122(2 Pt 1):319-28.
10. Qual Saf Health Care. 2009 Jun;18(3):236-40.
11. Gynecol Oncol. 2008 Feb;108(2):282-6.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Barber is a third-year fellow in gynecologic oncology at the university. They reported having no financial disclosures relevant to this column. Email them at [email protected].
Enhanced recovery surgical principles were first described in the 1990s.1 These principles postulate that the body’s stress response to surgical injury and deviation from normal physiology is the source of postoperative morbidity. Thus, enhanced recovery programs are designed around perioperative interventions that mitigate and help the body cope with the surgical stress response.
Many of these interventions run counter to traditional perioperative care paradigms. Enhanced recovery protocols are diverse but have common themes of avoiding preoperative fasting and bowel preparation, early oral intake, limiting use of drains and catheters, multimodal analgesia, early ambulation, and prioritizing euvolemia and normothermia. Individual interventions in these areas are combined to create a master protocol, which is implemented as a bundle to improve surgical outcomes.
Current components
Minimizing preoperative fasting, early postoperative refeeding, and preoperative carbohydrate-loading drinks are all key aspects of enhanced recovery protocols. “NPO after midnight” has been a longstanding rule due to the risk of aspiration with intubation. However, a Cochrane review found no evidence that a shortened period of fasting was associated with an increased risk of aspiration or related morbidity. Currently, the American Society of Anesthesiologists recommends only a 6-hour fast for solid foods and 2 hours for clear liquids.2,3
Preoperative fasting causes depletion of glycogen stores leading to insulin resistance and hyperglycemia, which are both associated with postoperative complications and morbidity.4 Preoperative carbohydrate-loading drinks can reverse some of the effects of limited preoperative fasting including preventing insulin resistance and hyperglycemia.5
Postoperative fasting should also be avoided. Early enteral intake is very important to decrease time spent in a catabolic state and decrease insulin resistance. In gynecology patients, early refeeding is associated with a faster return of bowel function and a decreased length of stay without an increase in postoperative complications.6 Notably, patients undergoing early feeding consistently experience more nausea and vomiting, but this is not associated with complications.7
The fluid management goal in enhanced recovery is to maintain perioperative euvolemia, as both hypovolemia and hypervolemia have negative physiologic consequences. When studied, fluid protocols designed to minimize the use of postoperative fluids have resulted in decreased cardiopulmonary complications, decreased postoperative morbidity, faster return of bowel function, and shorter hospital stays.8 Given the morbidity associated with fluid overload, enhanced recovery protocols recommend that minimal fluids be given in the operating room and intravenous fluids be removed as quickly as possible, often with first oral intake or postoperative day 1 at the latest.
Engagement of the patient in their perioperative recovery with patient education materials and expectations for postoperative tasks, such as early refeeding, spirometry, and ambulation are all important components of enhanced recovery. Patients become partners in achieving postoperative milestones, and this results in improved outcomes such as decreased pain scores and shorter recoveries.
Evidence in gynecology
Enhanced recovery has been studied in many surgical disciplines including urology, colorectal surgery, hepatobiliary surgery, and gynecology. High-quality studies of abdominal and vaginal hysterectomy patients have consistently found a decrease in length of stay with no difference in readmission or postoperative complication rates.9 An interesting study also found that an enhanced recovery program was associated with decreased nursing time required for patient care.10
For ovarian cancer patients, enhanced recovery is associated with decreased length of stay, decreased time to return of bowel function, and improved quality of life. Enhanced recovery is also cost saving, saving $257-$697 per vaginal hysterectomy patient and $5,410-$7,600 per ovarian cancer patient.11
Enhanced recovery protocols are safe, evidenced based, cost saving, and are increasingly being adopted as clinicians and health systems become aware of their benefits.
References
1. Br J Anaesth. 1997 May;78(5):606-17.
2. Cochrane Database Syst Rev. 2003 Oct 20;(4):CD004423.
3. Anesthesiology. 1999 Mar;90(3):896-905.
4. J Am Coll Surg. 2012 Jan;214(1):68-80.
5. Clin Nutr. 1998 Apr;17(2):65-71.
6. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD004508.
7. Obstet Gynecol. 1998 Jul;92(1):94-7.
8. Br J Surg. 2009 Apr;96(4):331-41.
9. Obstet Gynecol. 2013 Aug;122(2 Pt 1):319-28.
10. Qual Saf Health Care. 2009 Jun;18(3):236-40.
11. Gynecol Oncol. 2008 Feb;108(2):282-6.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Barber is a third-year fellow in gynecologic oncology at the university. They reported having no financial disclosures relevant to this column. Email them at [email protected].
VIDEO: Open, robotic, laparoscopic approaches equally effective in pancreatectomy
WASHINGTON – Minimally invasive surgery – whether robotic or laparoscopic – is just as effective as open surgery in pancreatectomy.
Both minimally invasive approaches had perioperative and oncologic outcomes that were similar to open approaches, as well as to each other, Katelin Mirkin, MD, reported at the annual clinical congress of the American College of Surgeons. And while minimally invasive surgery (MIS) techniques were associated with a slightly faster move to neoadjuvant chemotherapy, survival outcomes in all three surgical approaches were similar.
Dr. Mirkin, a surgery resident at Penn State Milton S. Hershey Medical Center, Hershey, Pa., plumbed the National Cancer Database for patients with stage I-III pancreatic cancer who were treated by surgical resection from 2010 to 2012. Her cohort comprised 9,047 patients; of these, 7,924 were treated with open surgery, 992 with laparoscopic surgery, and 131 with robotic surgery. She examined a number of factors including lymph node harvest and surgical margins, length of stay and time to adjuvant chemotherapy, and survival.
Patients who had MIS were older (67 vs. 66 years) and more often treated at an academic center, but otherwise there were no significant baseline differences.
Dr. Mirkin first compared the open surgeries with MIS. There were no significant associations with surgical approach and cancer stage. However, distal resections were significantly more likely to be dealt with by MIS, and Whipple procedures by open approaches. There were also more open than MIS total resections.
MIS was more likely to conclude with negative surgical margins (79% vs. 75%), and open surgery more likely to end with positive margins (22% vs. 19%).
Perioperative outcomes favored MIS approaches for all types of surgery, with a mean overall stay of 9.5 days vs. 11.3 days for open surgery. The mean length of stay for a distal resection was 7 days for MIS vs. 8 for open. For a Whipple procedure, the mean stay was 10.7 vs. 11.9 days. For a total resection, it was 10 vs. 11.8 days.
MIS was also associated with a significantly shorter time to the initiation of adjuvant chemotherapy overall (56 vs. 59 days). For a Whipple, time to chemotherapy was 58 vs. 60 days, respectively. For a distal resection, it was 52 vs. 56 days, and for a total resection, 52 vs. 58 days.
Neither approach offered a survival benefit over the other, Dr. Mirkin noted. For stage I cancers, less than 50% of MIS patients and less than 25% of open patients were alive by 50 months. For those with stage II tumors, less than 25% of each group was alive by 40 months. For stage III tumors, the 40-month survival rates were about 10% for MIS patients and 15% for open patients.
Dr. Mirkin then examined perioperative, oncologic, and survival outcomes among those who underwent laparoscopic and robotic surgeries. There were no demographic differences between these groups.
Oncologic outcomes were almost identical with regard to the number of positive regional nodes harvested (six), and surgical margins. Nodes were negative in 82% of robotic cases vs. 78% of laparoscopic cases and positive in 17.6% of robotic cases and 19.4% of laparoscopic cases.
Length of stay was significantly shorter for a laparoscopic approach overall (10 vs. 9.4 days) and particularly in distal resection (7 vs. 10 days). However, there were no differences in length of stay in any other surgery type. Nor was there any difference in the time to neoadjuvant chemotherapy.
Survival outcomes were similar as well. For stage I cancers, 40-month survival was about 40% in the laparoscopic group and 25% in the robotic group. For stage II cancers, 40-month survival was about 15% and 25%, respectively. For stage III tumors, 20-month survival in the robotic group was near 0 and 25% in the laparoscopic group. By 40 months almost all patients were deceased.
A multivariate survival analysis controlled for age, sex, race, comorbidities, facility type and location, surgery type, surgical margins, pathologic stage, and systemic therapy. It found only one significant association: Patients with 12 or more lymph nodes harvested were 19% more likely to die than those with fewer than 12 nodes harvested.
Time to chemotherapy (longer or shorter than 57 days) did not significantly impact survival, Dr. Mirkin said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
WASHINGTON – Minimally invasive surgery – whether robotic or laparoscopic – is just as effective as open surgery in pancreatectomy.
Both minimally invasive approaches had perioperative and oncologic outcomes that were similar to open approaches, as well as to each other, Katelin Mirkin, MD, reported at the annual clinical congress of the American College of Surgeons. And while minimally invasive surgery (MIS) techniques were associated with a slightly faster move to neoadjuvant chemotherapy, survival outcomes in all three surgical approaches were similar.
Dr. Mirkin, a surgery resident at Penn State Milton S. Hershey Medical Center, Hershey, Pa., plumbed the National Cancer Database for patients with stage I-III pancreatic cancer who were treated by surgical resection from 2010 to 2012. Her cohort comprised 9,047 patients; of these, 7,924 were treated with open surgery, 992 with laparoscopic surgery, and 131 with robotic surgery. She examined a number of factors including lymph node harvest and surgical margins, length of stay and time to adjuvant chemotherapy, and survival.
Patients who had MIS were older (67 vs. 66 years) and more often treated at an academic center, but otherwise there were no significant baseline differences.
Dr. Mirkin first compared the open surgeries with MIS. There were no significant associations with surgical approach and cancer stage. However, distal resections were significantly more likely to be dealt with by MIS, and Whipple procedures by open approaches. There were also more open than MIS total resections.
MIS was more likely to conclude with negative surgical margins (79% vs. 75%), and open surgery more likely to end with positive margins (22% vs. 19%).
Perioperative outcomes favored MIS approaches for all types of surgery, with a mean overall stay of 9.5 days vs. 11.3 days for open surgery. The mean length of stay for a distal resection was 7 days for MIS vs. 8 for open. For a Whipple procedure, the mean stay was 10.7 vs. 11.9 days. For a total resection, it was 10 vs. 11.8 days.
MIS was also associated with a significantly shorter time to the initiation of adjuvant chemotherapy overall (56 vs. 59 days). For a Whipple, time to chemotherapy was 58 vs. 60 days, respectively. For a distal resection, it was 52 vs. 56 days, and for a total resection, 52 vs. 58 days.
Neither approach offered a survival benefit over the other, Dr. Mirkin noted. For stage I cancers, less than 50% of MIS patients and less than 25% of open patients were alive by 50 months. For those with stage II tumors, less than 25% of each group was alive by 40 months. For stage III tumors, the 40-month survival rates were about 10% for MIS patients and 15% for open patients.
Dr. Mirkin then examined perioperative, oncologic, and survival outcomes among those who underwent laparoscopic and robotic surgeries. There were no demographic differences between these groups.
Oncologic outcomes were almost identical with regard to the number of positive regional nodes harvested (six), and surgical margins. Nodes were negative in 82% of robotic cases vs. 78% of laparoscopic cases and positive in 17.6% of robotic cases and 19.4% of laparoscopic cases.
Length of stay was significantly shorter for a laparoscopic approach overall (10 vs. 9.4 days) and particularly in distal resection (7 vs. 10 days). However, there were no differences in length of stay in any other surgery type. Nor was there any difference in the time to neoadjuvant chemotherapy.
Survival outcomes were similar as well. For stage I cancers, 40-month survival was about 40% in the laparoscopic group and 25% in the robotic group. For stage II cancers, 40-month survival was about 15% and 25%, respectively. For stage III tumors, 20-month survival in the robotic group was near 0 and 25% in the laparoscopic group. By 40 months almost all patients were deceased.
A multivariate survival analysis controlled for age, sex, race, comorbidities, facility type and location, surgery type, surgical margins, pathologic stage, and systemic therapy. It found only one significant association: Patients with 12 or more lymph nodes harvested were 19% more likely to die than those with fewer than 12 nodes harvested.
Time to chemotherapy (longer or shorter than 57 days) did not significantly impact survival, Dr. Mirkin said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
WASHINGTON – Minimally invasive surgery – whether robotic or laparoscopic – is just as effective as open surgery in pancreatectomy.
Both minimally invasive approaches had perioperative and oncologic outcomes that were similar to open approaches, as well as to each other, Katelin Mirkin, MD, reported at the annual clinical congress of the American College of Surgeons. And while minimally invasive surgery (MIS) techniques were associated with a slightly faster move to neoadjuvant chemotherapy, survival outcomes in all three surgical approaches were similar.
Dr. Mirkin, a surgery resident at Penn State Milton S. Hershey Medical Center, Hershey, Pa., plumbed the National Cancer Database for patients with stage I-III pancreatic cancer who were treated by surgical resection from 2010 to 2012. Her cohort comprised 9,047 patients; of these, 7,924 were treated with open surgery, 992 with laparoscopic surgery, and 131 with robotic surgery. She examined a number of factors including lymph node harvest and surgical margins, length of stay and time to adjuvant chemotherapy, and survival.
Patients who had MIS were older (67 vs. 66 years) and more often treated at an academic center, but otherwise there were no significant baseline differences.
Dr. Mirkin first compared the open surgeries with MIS. There were no significant associations with surgical approach and cancer stage. However, distal resections were significantly more likely to be dealt with by MIS, and Whipple procedures by open approaches. There were also more open than MIS total resections.
MIS was more likely to conclude with negative surgical margins (79% vs. 75%), and open surgery more likely to end with positive margins (22% vs. 19%).
Perioperative outcomes favored MIS approaches for all types of surgery, with a mean overall stay of 9.5 days vs. 11.3 days for open surgery. The mean length of stay for a distal resection was 7 days for MIS vs. 8 for open. For a Whipple procedure, the mean stay was 10.7 vs. 11.9 days. For a total resection, it was 10 vs. 11.8 days.
MIS was also associated with a significantly shorter time to the initiation of adjuvant chemotherapy overall (56 vs. 59 days). For a Whipple, time to chemotherapy was 58 vs. 60 days, respectively. For a distal resection, it was 52 vs. 56 days, and for a total resection, 52 vs. 58 days.
Neither approach offered a survival benefit over the other, Dr. Mirkin noted. For stage I cancers, less than 50% of MIS patients and less than 25% of open patients were alive by 50 months. For those with stage II tumors, less than 25% of each group was alive by 40 months. For stage III tumors, the 40-month survival rates were about 10% for MIS patients and 15% for open patients.
Dr. Mirkin then examined perioperative, oncologic, and survival outcomes among those who underwent laparoscopic and robotic surgeries. There were no demographic differences between these groups.
Oncologic outcomes were almost identical with regard to the number of positive regional nodes harvested (six), and surgical margins. Nodes were negative in 82% of robotic cases vs. 78% of laparoscopic cases and positive in 17.6% of robotic cases and 19.4% of laparoscopic cases.
Length of stay was significantly shorter for a laparoscopic approach overall (10 vs. 9.4 days) and particularly in distal resection (7 vs. 10 days). However, there were no differences in length of stay in any other surgery type. Nor was there any difference in the time to neoadjuvant chemotherapy.
Survival outcomes were similar as well. For stage I cancers, 40-month survival was about 40% in the laparoscopic group and 25% in the robotic group. For stage II cancers, 40-month survival was about 15% and 25%, respectively. For stage III tumors, 20-month survival in the robotic group was near 0 and 25% in the laparoscopic group. By 40 months almost all patients were deceased.
A multivariate survival analysis controlled for age, sex, race, comorbidities, facility type and location, surgery type, surgical margins, pathologic stage, and systemic therapy. It found only one significant association: Patients with 12 or more lymph nodes harvested were 19% more likely to die than those with fewer than 12 nodes harvested.
Time to chemotherapy (longer or shorter than 57 days) did not significantly impact survival, Dr. Mirkin said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @alz_gal
AT THE ACS CLINICAL CONGRESS
Key clinical point:
Major finding: For stage I cancers, less than 50% of minimally invasive surgery patients and less than 25% of open surgery patients were alive by 50 months. For those with stage II tumors, less than 25% of each group was alive by 40 months.
Data source: The database review comprised 9,047 cases.
Disclosures: Dr. Mirkin had no financial disclosures.
Laparoscopy comparable to open staging for uterine papillary serous cancer
BOSTON – Laparoscopic staging of patients with uterine papillary serous carcinoma is a safe alternative to open staging and may offer some advantages, according to findings presented at the annual Minimally Invasive Surgery Week.
“Traditionally, serous papillary cancer has been treated different than the other endometrial cancers, the reason being is that it tends to behave more like ovarian cancer,” Jeanette Voice, MD, of Richmond University Medical Center in Staten Island, N.Y., said at the meeting, which was held by the Society for Laparoendoscopic Surgeons. “Patients with serous papillary cancer tend to be older [so] these patients may benefit from a less invasive surgical approach.”
Dr. Voice and her coinvestigators conducted an 8-year retrospective study of laparoscopic and open-staged cases treated from March 2007 through May 2015. Initially, 59 patients with pathology-confirmed uterine papillary serous carcinoma were identified over that time period, and were divided into two cohorts: one receiving open surgery (37 patients) and one receiving laparoscopic surgery (22 patients).
Median age, body mass index, and prior abdominal surgery rate were not significantly different between the two cohorts.
In terms of intraoperative factors, median operative times for the open and laparoscopic cohorts were similar: 196 minutes versus 216 minutes, respectively (P = .561). Similarly, the number of pelvic lymph node dissections and rate of omentectomy were also not significantly different: 18 nodes (open) versus 16 nodes (laparoscopic) (P = .96), and 100% (open) versus 91% (laparoscopic) (P = .08).
However, laparoscopic patients had more favorable median estimated blood loss (310 mL versus 175 mL, P = .048) and shorter hospital stays (4 days versus 1 day, P less than .042).
Laparoscopic patients also achieved more robust debulking to zero centimeter residual disease, with 90.5% of patients achieving it versus 65.7% of those in the open surgery cohort, but the difference was not statistically significant (P = .1).
In terms of postoperative adjuvant therapy – brachytherapy, external beam radiation, and chemotherapy – there were no significant differences in outcomes between the two cohorts. Recurrence rates were also similar, with nine recurrences in the open cohort and eight recurrences in the laparoscopic cohort. The estimated 36-month progression-free survival rates were “almost identical,” with 55.3% in the open cohort versus 53.3% in the laparoscopic (P = .727), according to Dr. Voice.
Postoperative complications were more common in the open surgery cohort (29.7%), compared with 13.6% in the laparoscopic cohort, but no statistically significant difference was found between them (P = .16).
Dr. Voice did not report information on financial disclosures.
BOSTON – Laparoscopic staging of patients with uterine papillary serous carcinoma is a safe alternative to open staging and may offer some advantages, according to findings presented at the annual Minimally Invasive Surgery Week.
“Traditionally, serous papillary cancer has been treated different than the other endometrial cancers, the reason being is that it tends to behave more like ovarian cancer,” Jeanette Voice, MD, of Richmond University Medical Center in Staten Island, N.Y., said at the meeting, which was held by the Society for Laparoendoscopic Surgeons. “Patients with serous papillary cancer tend to be older [so] these patients may benefit from a less invasive surgical approach.”
Dr. Voice and her coinvestigators conducted an 8-year retrospective study of laparoscopic and open-staged cases treated from March 2007 through May 2015. Initially, 59 patients with pathology-confirmed uterine papillary serous carcinoma were identified over that time period, and were divided into two cohorts: one receiving open surgery (37 patients) and one receiving laparoscopic surgery (22 patients).
Median age, body mass index, and prior abdominal surgery rate were not significantly different between the two cohorts.
In terms of intraoperative factors, median operative times for the open and laparoscopic cohorts were similar: 196 minutes versus 216 minutes, respectively (P = .561). Similarly, the number of pelvic lymph node dissections and rate of omentectomy were also not significantly different: 18 nodes (open) versus 16 nodes (laparoscopic) (P = .96), and 100% (open) versus 91% (laparoscopic) (P = .08).
However, laparoscopic patients had more favorable median estimated blood loss (310 mL versus 175 mL, P = .048) and shorter hospital stays (4 days versus 1 day, P less than .042).
Laparoscopic patients also achieved more robust debulking to zero centimeter residual disease, with 90.5% of patients achieving it versus 65.7% of those in the open surgery cohort, but the difference was not statistically significant (P = .1).
In terms of postoperative adjuvant therapy – brachytherapy, external beam radiation, and chemotherapy – there were no significant differences in outcomes between the two cohorts. Recurrence rates were also similar, with nine recurrences in the open cohort and eight recurrences in the laparoscopic cohort. The estimated 36-month progression-free survival rates were “almost identical,” with 55.3% in the open cohort versus 53.3% in the laparoscopic (P = .727), according to Dr. Voice.
Postoperative complications were more common in the open surgery cohort (29.7%), compared with 13.6% in the laparoscopic cohort, but no statistically significant difference was found between them (P = .16).
Dr. Voice did not report information on financial disclosures.
BOSTON – Laparoscopic staging of patients with uterine papillary serous carcinoma is a safe alternative to open staging and may offer some advantages, according to findings presented at the annual Minimally Invasive Surgery Week.
“Traditionally, serous papillary cancer has been treated different than the other endometrial cancers, the reason being is that it tends to behave more like ovarian cancer,” Jeanette Voice, MD, of Richmond University Medical Center in Staten Island, N.Y., said at the meeting, which was held by the Society for Laparoendoscopic Surgeons. “Patients with serous papillary cancer tend to be older [so] these patients may benefit from a less invasive surgical approach.”
Dr. Voice and her coinvestigators conducted an 8-year retrospective study of laparoscopic and open-staged cases treated from March 2007 through May 2015. Initially, 59 patients with pathology-confirmed uterine papillary serous carcinoma were identified over that time period, and were divided into two cohorts: one receiving open surgery (37 patients) and one receiving laparoscopic surgery (22 patients).
Median age, body mass index, and prior abdominal surgery rate were not significantly different between the two cohorts.
In terms of intraoperative factors, median operative times for the open and laparoscopic cohorts were similar: 196 minutes versus 216 minutes, respectively (P = .561). Similarly, the number of pelvic lymph node dissections and rate of omentectomy were also not significantly different: 18 nodes (open) versus 16 nodes (laparoscopic) (P = .96), and 100% (open) versus 91% (laparoscopic) (P = .08).
However, laparoscopic patients had more favorable median estimated blood loss (310 mL versus 175 mL, P = .048) and shorter hospital stays (4 days versus 1 day, P less than .042).
Laparoscopic patients also achieved more robust debulking to zero centimeter residual disease, with 90.5% of patients achieving it versus 65.7% of those in the open surgery cohort, but the difference was not statistically significant (P = .1).
In terms of postoperative adjuvant therapy – brachytherapy, external beam radiation, and chemotherapy – there were no significant differences in outcomes between the two cohorts. Recurrence rates were also similar, with nine recurrences in the open cohort and eight recurrences in the laparoscopic cohort. The estimated 36-month progression-free survival rates were “almost identical,” with 55.3% in the open cohort versus 53.3% in the laparoscopic (P = .727), according to Dr. Voice.
Postoperative complications were more common in the open surgery cohort (29.7%), compared with 13.6% in the laparoscopic cohort, but no statistically significant difference was found between them (P = .16).
Dr. Voice did not report information on financial disclosures.
AT MINIMALLY INVASIVE SURGERY WEEK
Key clinical point:
Major finding: Laparoscopic patients had lower median estimated blood loss (310 mL v. 175 mL, P = .048) and shorter hospital stays (4 days v. 1 day, P less than .042).
Data source: Retrospective review of data on 59 open and laparoscopic patients over 8 years.
Disclosures: Dr. Voice did not report information on financial disclosures.