User login
Polio in 2022: Some concerns but vaccine still works
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Who would have thought we would need to refresh our knowledge on polio virus in 2022? Fate seems cruel to add this concern on the heels of SARS-CoV-2, monkeypox, abnormal seasons for RSV, acute flaccid myelitis (AFM) linked to enteroviruses, and a summer of parechovirus causing infant meningitis. But confirmation that indeed an adult had polio with paralytic disease raises concerns among public health groups and ordinary citizens alike, particularly those who remember polio in its heyday.
History: In the summer of 1952, polio was among the most feared diseases on the planet. Families were advised to not allow children to congregate in groups or use public swimming pools; little league baseball games were being canceled and there was talk of not opening schools for the fall. Every parent’s nightmare seemed to be the nonspecific febrile summer illness that led to paralytic sequelae. TV news included videos of the iron lung wards in hospitals across the country. Medical providers felt powerless, only able to give nonspecific preventive advice. There was no specific antiviral (there still isn’t) and vaccines seemed a long way off.
Then came the news that Dr. Jonas Salk’s group had gotten an inactivated polio vaccine (IPV) approved for general use in 1955. Families were excited to have their children vaccinated. Paralytic polio cases dropped like a rock from approximately 22,000/year in 1952 to approximately 2,200 in 1956. A surge to near 6,000 cases in 1959 led to Dr. Albert Sabin’s oral polio vaccine (OPV), which supplanted IPV in 1961. OPV had the advantages of: 1) Inducing mucosal as well as serum antibodies, 2) more durable responses, and 3) immunity in unvaccinated persons exposed to vaccine virus that had been shed in stools into wastewater and rivers.
By 1964, polio had nearly disappeared. The last wild-type indigenous U.S. case was in 1979. By 1994, all the Americas were declared polio free. Because the only U.S. paralytic polio cases thereafter were foreign imports or were associated with oral vaccine strains (so-called vaccine-associated paralytic polio [VAPP]), OPV was replaced by an enhanced IPV in 2000 to prevent further VAPP.
Polio facts: Polio is asymptomatic in about 70% of infections. Among the 30% with symptoms, paralysis occurs infrequently, with the overall rate of paralytic infections being 0.5% (rate varies by virus type with type 3 having the highest rate).1 Why then was the world so afraid of polio? If every person in a U.S. birth cohort (about 3.7 million) was unvaccinated and became infected with poliovirus, more than 18,000 would get paralytic polio and almost 1,300 would die. Of note, adults have a higher chance of paralytic polio after infection than children.
Concerns in 2022: Persons vaccinated with at least three doses of either IPV or OPV have historically been protected from paralytic polio (99% protection). But are we sure that the United States remains protected against polio after 2 decades of IPV being the only vaccine? Polio could be reintroduced at any time to the United States from countries with reported cases that likely arose because of low vaccination rates related to war, famine, or political upheavals (Malawi, Mozambique, Nigeria, Pakistan, and Afghanistan).2 The proof? The recent confirmed New York case.
International efforts resulted in global eradication of two polio wild-types viruses (type 2 in 2015 and type 3 in 2019). Nevertheless, vaccine-derived, virulent polio virus (VDPV) type 2 and VDPV-3 still circulate in some areas, particularly Africa (VDPV-2) and Israel (VDPV-3). The above-mentioned U.S. case is an unvaccinated adult traveler who went to an area where VDPV-2 circulates and developed disease after returning home.3 So, it was not an indigenous reappearance in the United States and it was not a breakthrough case in a vaccinated person. But it is sobering to realize that all who are unvaccinated remain at risk for paralytic polio in 2022, particularly because vaccination rates declined nearly everywhere during the initial COVID-19 pandemic. We are still catching up, with vaccination rates under 50% in some ZIP codes.4
Are VDPVs circulating in some parts of the United States? Interestingly, wastewater surveillance programs may be the most economical and practical way to perform polio surveillance. Such a program detected polio virus in London wastewater in June 2022.5 New York has recently detected polio in wastewater during testing begun because of the recent case.6
Good news: For paralytic polio, seropositivity at any titer indicates protection, so U.S. serosurveillance data would also be informative. How durable is polio protection in the IPV era? Available data suggest that even though we have used only IPV these past 20 years, seropositivity rates among vaccinees with at least three doses of either IPV or OPV should persist for decades and likely for life. Even before polio became a concern this year, the Centers for Disease Control and Prevention, being proactive, wanted to ensure that the enhanced IPV was producing durable immunity and that persons of all ages remained seropositive to the three polio virus types over 10 years after discontinuing OPV use in 2012.
The CDC collaborated with investigators in Kansas City, Mo., to evaluate titers and seropositivity to all three types in a 2- to 85-year-old otherwise healthy cohort with demographics that mirrored the 2010 census for the Kansas City region, which in turn mirrored the national 2021 census data.7 There were approximately 100 persons in each age cohort, with 200 below age 11 years (the cohort that had received only IPV). Serology was performed at the CDC.
Overall seropositivity rates were high, but lower for type 3 (83.3%) and type 2 (90.7%) than type 1 (94.4%). Of note, most of those seronegative for one or more types were among 2- to 3-year-olds who had not completed their full IPV series, with most seronegative results being against polio types 1 and 3. Further, five, who were confirmed as having received no polio vaccine, were seronegative for all three types. Two with no available vaccine records (over 18 years old) were also seronegative for all three types.
So, regardless of the era in which one got polio vaccine, vaccine protection appears to persist indefinitely after three doses. Even 80-year-olds were still seropositive if they had three doses. We can confidently reassure our patients that the vaccine still works; the persons who need to fear polio in 2022 are those who are not vaccinated or have had fewer than three doses, particularly if they travel to areas of persistent polio. Wild type 1 virus persists in a few countries as does VDPV type 2 and VDPV type 3. Importantly, wild type 2 and wild type 3 (with the lowest seropositivity in 2012 study) have been eliminated globally so the only circulating type 2 and type 3 polio virus is VDPV in a few countries. Travel to these countries warrants review of polio vaccine records and CDC or WHO current recommendations for travelers to those countries.
Dr. Harrison is a professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at [email protected].
References
1. Poliomyelitis. World Health Organization fact sheet, 2022 Jul 4..
2. Franco-Paredes C et al. Lancet Infect Dis. 2022 Aug 16. doi: 10.1016/S1473-3099(22)00548-5.
3. Link-Gelles R et al. MMWR Morb Mortal Wkly Rep. 2022 Aug 19;71(33):1065-8.
4. “Polio vaccination rate for 2-year-olds is as low as 37% in parts of N.Y. county where paralysis case was found,” NBC News, Erika Edwards, 2022 Aug 16. 5. Vaccine-derived poliovirus type 2 (VDPV2) detected in environmental samples in London. Polioeradication.org. 2022 Jun 22.
6. “NYSDOH and NYCDOHMH wastewater monitoring identifies polio in New York City and urges unvaccinated New Yorkers to get vaccinated now,” nyc.gov. 2022 Aug 12.
7. Wallace GS et al. Hum Vaccin Immunother. 2017;13(4):776-83.
Improving Inpatient COVID-19 Vaccination Rates Among Adult Patients at a Tertiary Academic Medical Center
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.
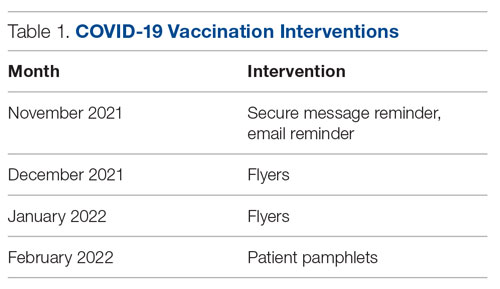
In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.
Results
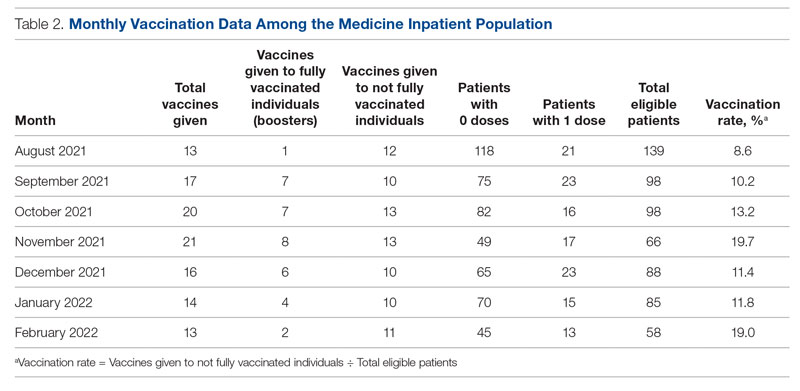
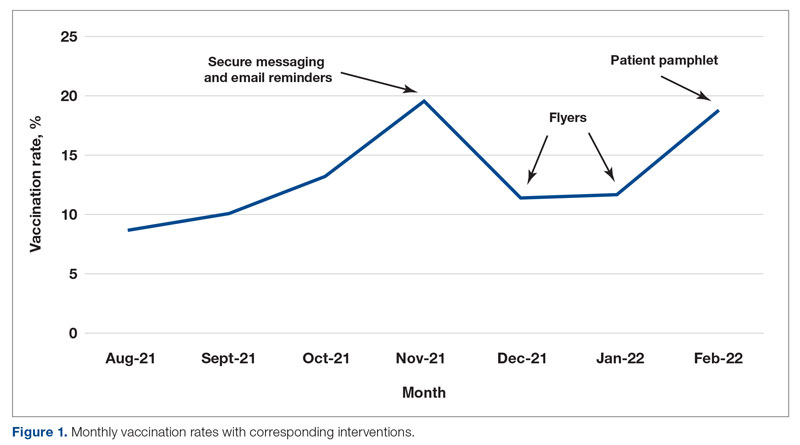
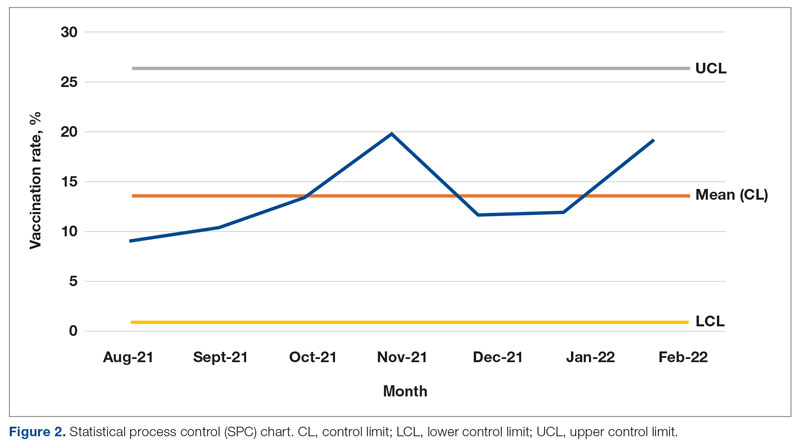
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).
Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
From the Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC.
Abstract
Objective: Inpatient vaccination initiatives are well described in the literature. During the COVID-19 pandemic, hospitals began administering COVID-19 vaccines to hospitalized patients. Although vaccination rates increased, there remained many unvaccinated patients despite community efforts. This quality improvement project aimed to increase the COVID-19 vaccination rates of hospitalized patients on the medicine service at the George Washington University Hospital (GWUH).
Methods: From November 2021 through February 2022, we conducted a Plan-Do-Study-Act (PDSA) cycle with 3 phases. Initial steps included gathering baseline data from the electronic health record and consulting stakeholders. The first 2 phases focused on educating housestaff on the availability, ordering process, and administration of the Pfizer vaccine. The third phase consisted of developing educational pamphlets for patients to be included in their admission packets.
Results: The baseline mean COVID-19 vaccination rate (August to October 2021) of eligible patients on the medicine service was 10.7%. In the months after we implemented the PDSA cycle (November 2021 to February 2022), the mean vaccination rate increased to 15.4%.
Conclusion: This quality improvement project implemented measures to increase administration of the Pfizer vaccine to eligible patients admitted to the medicine service at GWUH. The mean vaccination rate increased from 10.7% in the 3 months prior to implementation to 15.4% during the 4 months post implementation. Other measures to consider in the future include increasing the availability of other COVID-19 vaccines at our hospital and incorporating the vaccine into the admission order set to help facilitate vaccination early in the hospital course.
Keywords: housestaff, quality improvement, PDSA, COVID-19, BNT162b2 vaccine, patient education
Throughout the COVID-19 pandemic, case rates in the United States have fluctuated considerably, corresponding to epidemic waves. In 2021, US daily cases of COVID-19 peaked at nearly 300,000 in early January and reached a nadir of 8000 cases in mid-June.1 In September 2021, new cases had increased to 200,000 per day due to the prevalence of the Delta variant.1 Particularly with the emergence of new variants of SARS-CoV-2, vaccination efforts to limit the spread of infection and severity of illness are critical. Data have shown that 2 doses of the BNT162b2 vaccine (Pfizer-BioNTech) were largely protective against severe infection for approximately 6 months.2,3 When we began this quality improvement (QI) project in September 2021, only 179 million Americans had been fully vaccinated, according to data from the Centers for Disease Control and Prevention, which is just over half of the US population.4 An electronic survey conducted in the United States with more than 5 million responses found that, of those who were hesitant about receiving the vaccine, 49% reported a fear of adverse effects and 48% reported a lack of trust in the vaccine.5
This QI project sought to target unvaccinated individuals admitted to the internal medicine inpatient service. Vaccinating hospitalized patients is especially important since they are sicker than the general population and at higher risk of having poor outcomes from COVID-19. Inpatient vaccine initiatives, such as administering influenza vaccine prior to discharge, have been successfully implemented in the past.6 One large COVID-19 vaccination program featured an admission order set to increase the rates of vaccination among hospitalized patients.7 Our QI project piloted a multidisciplinary approach involving the nursing staff, pharmacy, information technology (IT) department, and internal medicine housestaff to increase COVID-19 vaccination rates among hospitalized patients on the medical service. This project aimed to increase inpatient vaccination rates through interventions targeting both primary providers as well as the patients themselves.
Methods
Setting and Interventions
This project was conducted at the George Washington University Hospital (GWUH) in Washington, DC. The clinicians involved in the study were the internal medicine housestaff, and the patients included were adults admitted to the resident medicine ward teams. The project was exempt by the institutional review board and did not require informed consent.
The quality improvement initiative had 3 phases, each featuring a different intervention (Table 1). The first phase involved sending a weekly announcement (via email and a secure health care messaging app) to current residents rotating on the inpatient medicine service. The announcement contained information regarding COVID-19 vaccine availability at the hospital, instructions on ordering the vaccine, and the process of coordinating with pharmacy to facilitate vaccine administration. Thereafter, residents were educated on the process of giving a COVID-19 vaccine to a patient from start to finish. Due to the nature of the residency schedule, different housestaff members rotated in and out of the medicine wards during the intervention periods. The weekly email was sent to the entire internal medicine housestaff, informing all residents about the QI project, while the weekly secure messages served as reminders and were only sent to residents currently on the medicine wards.

In the second phase, we posted paper flyers throughout the hospital to remind housestaff to give the vaccine and again educate them on the process of ordering the vaccine. For the third intervention, a COVID-19 vaccine educational pamphlet was developed for distribution to inpatients at GWUH. The pamphlet included information on vaccine efficacy, safety, side effects, and eligibility. The pamphlet was incorporated in the admission packet that every patient receives upon admission to the hospital. The patients reviewed the pamphlets with nursing staff, who would answer any questions, with residents available to discuss any outstanding concerns.
Measures and Data Gathering
The primary endpoint of the study was inpatient vaccination rate, defined as the number of COVID-19 vaccines administered divided by the number of patients eligible to receive a vaccine (not fully vaccinated). During initial triage, nursing staff documented vaccination status in the electronic health record (EHR), checking a box in a data entry form if a patient had received 0, 1, or 2 doses of the COVID-19 vaccine. The GWUH IT department generated data from this form to determine the number of patients eligible to receive a COVID-19 vaccine. Data were extracted from the medication administration record in the EHR to determine the number of vaccines that were administered to patients during their hospitalization on the inpatient medical service. Each month, the IT department extracted data for the number of eligible patients and the number of vaccines administered. This yielded the monthly vaccination rates. The monthly vaccination rates in the period prior to starting the QI initiative were compared to the rates in the period after the interventions were implemented.
Of note, during the course of this project, patients became eligible for a third COVID-19 vaccine (booster). We decided to continue with the original aim of vaccinating adults who had only received 0 or 1 dose of the vaccine. Therefore, the eligibility criteria remained the same throughout the study. We obtained retrospective data to ensure that the vaccines being counted toward the vaccination rate were vaccines given to patients not yet fully vaccinated and not vaccines given as boosters.

Results
From August to October 2021, the baseline average monthly vaccination rate of patients on the medicine service who were eligible to receive a COVID-19 vaccine was 10.7%. After the first intervention, the vaccination rate increased to 19.7% in November 2021 (Table 2). The second intervention yielded vaccination rates of 11.4% and 11.8% in December 2021 and January 2022, respectively. During the final phase in February 2022, the vaccination rate was 19.0%. At the conclusion of the study, the mean vaccination rate for the intervention months was 15.4% (Figure 1). Process stability and variation are demonstrated with a statistical process control chart (Figure 2).


Discussion
For this housestaff-driven QI project, we implemented an inpatient COVID-19 vaccination campaign consisting of 3 phases that targeted both providers and patients. During the intervention period, we observed an increased vaccination rate compared to the period just prior to implementation of the QI project. While our interventions may certainly have boosted vaccination rates, we understand other variables could have contributed to increased rates as well. The emergence of variants in the United States, such as omicron in December 2021,8 could have precipitated a demand for vaccinations among patients. Holidays in November and December may also have increased patients’ desire to get vaccinated before travel.
We encountered a number of roadblocks that challenged our project, including difficulty identifying patients who were eligible for the vaccine, logistical vaccine administration challenges, and hesitancy among the inpatient population. Accurately identifying patients who were eligible for a vaccine in the EHR was especially challenging in the setting of rapidly changing guidelines regarding COVID-19 vaccination. In September 2021, the US Food and Drug Administration authorized the Pfizer booster for certain populations and later, in November 2021, for all adults. This meant that some fully vaccinated hospitalized patients (those with 2 doses) then qualified for an additional dose of the vaccine and received a dose during hospitalization. To determine the true vaccination rate, we obtained retrospective data that allowed us to track each vaccine administered. If a patient had already received 2 doses of the COVID-19 vaccine, the vaccine administered was counted as a booster and excluded from the calculation of the vaccination rate. Future PDSA cycles could include updating the EHR to capture the whole range of COVID-19 vaccination status (unvaccinated, partially vaccinated, fully vaccinated, fully vaccinated with 1 booster, fully vaccinated with 2 boosters).
We also encountered logistical challenges with the administration of the COVID-19 vaccine to hospitalized patients. During the intervention period, our pharmacy department required 5 COVID-19 vaccination orders before opening a vial and administering the vaccine doses in order to reduce waste. This policy may have limited our ability to vaccinate eligible inpatients because we were not always able to identify 5 patients simultaneously on the service who were eligible and consented to the vaccine.
The majority of patients who were interested in receiving COVID-19 vaccination had already been vaccinated in the outpatient setting. This fact made the inpatient internal medicine subset of patients a particularly challenging population to target, given their possible hesitancy regarding vaccination. By utilizing a multidisciplinary team and increasing communication of providers and nursing staff, we helped to increase the COVID-19 vaccination rates at our hospital from 10.7% to 15.4%.
Future Directions
Future interventions to consider include increasing the availability of other approved COVID-19 vaccines at our hospital besides the Pfizer-BioNTech vaccine. Furthermore, incorporating the vaccine into the admission order set would help initiate the vaccination process early in the hospital course. We encourage other institutions to utilize similar approaches to not only remind providers about inpatient vaccination, but also educate and encourage patients to receive the vaccine. These measures will help institutions increase inpatient COVID-19 vaccination rates in a high-risk population.
Corresponding author: Anna Rubin, MD, Department of Medicine, The George Washington University School of Medicine and Health Sciences, Washington, DC; [email protected]
Disclosures: None reported.
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
1. Trends in number of COVID-19 cases and deaths in the US reported to CDC, by state/territory. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#trends_dailycases
2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162B2 MRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi:10.1056/nejmoa2034577
3. Hall V, Foulkes S, Insalata F, et al. Protection against SARS-COV-2 after covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207-1220. doi:10.1056/nejmoa2118691
4. Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed February 25, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum
5. King WC, Rubinstein M, Reinhart A, Mejia R. Time trends, factors associated with, and reasons for covid-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLOS ONE. 2021;16(12). doi:10.1371/journal.pone.0260731
6. Cohen ES, Ogrinc G, Taylor T, et al. Influenza vaccination rates for hospitalised patients: A multiyear quality improvement effort. BMJ Qual Saf. 2015;24(3):221-227. doi:10.1136/bmjqs-2014-003556
7. Berger RE, Diaz DC, Chacko S, et al. Implementation of an inpatient covid-19 vaccination program. NEJM Catalyst. 2021;2(10). doi:10.1056/cat.21.0235
8. CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(50):1731-1734. doi:10.15585/mmwr.mm7050e1
N.Y. governor declares state disaster emergency to boost polio vaccination
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
New York Governor Kathy Hochul declared a state disaster emergency on Sept. 9 after the polio virus has been detected in another county. The order allows EMS workers, midwives, and pharmacists to administer the vaccine and permits physicians and nurse practitioners to issue standing orders for polio vaccines.
“On polio, we simply cannot roll the dice,” New York State Health Commissioner Dr. Mary T. Bassett said in a news release. “If you or your child are unvaccinated or not up to date with vaccinations, the risk of paralytic disease is real. I urge New Yorkers to not accept any risk at all.”
In July, an unvaccinated adult man in Rockland County, which is north of New York City, was diagnosed with polio virus. It was the first confirmed case of the virus in the United States since 2013.
New York state health officials have not announced any additional polio cases. Since as early as April, polio has also been detected in wastewater samples in New York City and in Rockland, Orange, and Sullivan counties. In August, the virus was detected in wastewater from Nassau County on Long Island.
New York’s statewide polio vaccination rate is 79%, and the New York State Department of Health is aiming for a rate over 90%, the announcement said. In some counties, vaccination rates are far below the state average, including Rockland County (60%), Orange County (59%), and Sullivan County (62%). Nassau County’s polio vaccination rate is similar to the state average.
“Polio immunization is safe and effective – protecting nearly all people against disease who receive the recommended doses,” Dr. Basset said; “Do not wait to vaccinate.”
A version of this article first appeared on Medscape.com.
Congenital cytomegalovirus declined in wake of COVID-19
Congenital cytomegalovirus cases declined significantly during the COVID-19 pandemic, compared with a period before the pandemic, based on data from nearly 20,000 newborns.
A study originated to explore racial and ethnic differences in congenital cytomegalovirus (cCMV) began in 2016, but was halted in April 2020 because of the COVID-19 pandemic, wrote Mark R. Schleiss, MD, of the University of Minnesota, Minneapolis, and colleagues. The study resumed for a period from August 2020 to December 2021, and the researchers compared data on cCMV before and during the pandemic. The prepandemic period included data from April 2016 to March 2020.
“We have been screening for congenital CMV infection in Minnesota for 6 years as a part of a multicenter collaborative study that I lead as the primary investigator,” Dr. Schleiss said in an interview. “Our efforts have contributed to the decision, vetted through the Minnesota Legislature and signed into law in 2021 (the “Vivian Act”), to begin universal screening for all newborns in Minnesota in 2023. In the context of this ongoing screening/surveillance study, it was important and scientifically very interesting to examine the impact of the COVID-19 pandemic on the risk of congenital CMV infection,” he explained.
The findings were published in a research letter in JAMA Network Open. A total of 15,697 newborns were screened before the pandemic and 4,222 were screened during the pandemic period at six hospitals. The majority of the mothers participating during the prepandemic and pandemic periods were non-Hispanic White (71% and 60%, respectively).
Overall, the percentage screened prevalence for cCMV was 79% in the prepandemic period and 21% during the pandemic, with rates of 4.5 per 1,000 and 1.4 per 1,000, respectively.
Although the highest percentage of cCMV cases occurred in newborns of mothers aged 25 years and older (86%), the prevalence was highest among newborns of mothers aged 24 years and younger (6.0 per 1,000). The prevalence of cCMV overall was higher in infants of non-Hispanic Black mothers vs. non-Hispanic White mothers, but not significantly different (5.1 per 1,000 vs. 4.6 per 1,000) and among second newborns vs. first newborns (6.0 vs. 3.2 per 1,000, respectively).
Factors related to COVID-19, including reduced day care attendance, behavioral changes, and mitigation measures at childcare facilities such as smaller classes and increased hand hygiene and disinfection may have contributed to this decrease in cCMV in the pandemic period, the researchers wrote in their discussion.
The comparable prevalence in newborns of non-Hispanic Black and White mothers contrasts with previous studies showing a higher prevalence in children of non-Hispanic Black mothers, the researchers noted in their discussion.
The study was limited by several factors, including the variation in time points for enrollment at different sites and the exclusion of families in the newborn nursery with positive COVID-19 results during the pandemic, they wrote. More research is needed on the potential effects of behavioral interventions to reduce CMV risk during pregnancy, as well as future CMV vaccination for childbearing-aged women and young children, they concluded.
However, the researchers were surprised by the impact of COVID-19 on the prevalence of cCMV, Dr. Schleiss said in an interview. “We have had the knowledge for many years that CMV infections in young women are commonly acquired through interactions with their toddlers. These interactions – sharing food, wiping drool and nasal discharge from the toddler’s nose, changing diapers, kissing the child on the mouth – can transmit CMV,” he said. In addition, toddlers may acquire CMV from group day care; the child then sheds CMV and transmits the virus to their pregnant mother, who then transmits the virus across the placenta, leading to cCMV infection in the newborn, Dr. Schleiss explained.
Although the researchers expected a decrease in CMV in the wake of closures of group day care, increased home schooling, decreased interactions among children, hygienic precautions, and social isolation, the decrease exceeded their expectations, said Dr. Schleiss. “Our previous work showed that in the 5-year period leading up to the pandemic, about one baby in every 200 births was born with CMV. Between August 2020 and December 2021, the number decreased to one baby in every 1,000 births,” a difference he and his team found striking.
The message from the study is that CMV can be prevented, said Dr. Schleiss. “Hygienic precautions during pregnancy had a big impact. Since congenital CMV infection is the most common congenital infection in the United States, and probably globally, that causes disabilities in children, the implications are highly significant,” he said. “The hygienic precautions we all have engaged in during the pandemic, such as masking, handwashing, and infection prevention behaviors, were almost certainly responsible for the reduction in CMV transmission, which in turn protected mothers and newborns from the potentially devastating effects of the CMV virus,” he noted.
Looking ahead, “Vaccines are moving forward in clinical trials that aim to confer immunity on young women of childbearing age to protect future pregnancies against transmission of CMV to the newborn infant; it would be very important to examine in future studies whether hygienic precautions would have the same impact as a potential vaccine,” Dr. Schleiss said. More research is needed to examine the effect of education of women about CMV transmission, he added. “We think it is very important to share this knowledge from our study with the pediatric community, since pediatricians can be important in counseling women about future pregnancies and the risks of CMV acquisition and transmission,” he noted.
Implications for other viruses
Although CMV poses minimal risk for healthy populations, irreversible complications for infants born with congenital CMV, especially hearing loss, are very concerning, said Catherine Haut, DNP, CPNP-AC/PC, a pediatric nurse practitioner in Rehoboth Beach, Del., in an interview.
“The study of viral transmission during a time of isolation, masking, and other mitigation procedures for COVID-19 assists in awareness that other viruses may also be limited with the use of these measures,” she said.
Dr. Haut was not surprised by the findings, given that CMV is transmitted primarily through direct contact with body fluids and that more than 50% of American adults have been infected by age 40, according to the Centers for Disease Control and Prevention, she said.
The take-home message for pediatricians, Dr. Haut said, is measures to prevent transmission of viral infection can yield significant positive health outcomes for the pediatric population; however, the effect of isolation, which has been associated with a higher rate of mental health problems, should not be ignored.
“Despite appropriate statistical analyses and presentation of findings in this study, the population sampled during the pandemic was less than 30% of the pre-COVID sampling, representing a study limitation,” and conducting research in a single state limits generalizability, Dr. Haut noted. “I agree with the authors that additional study is necessary to better understand prevention measures and apply these methods to reduce CMV transmission. Pursuit of CMV immunization opportunities is also needed,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Vaccine Program Office, the Minnesota Department of Health Newborn Screening Program, and the University of South Carolina Disability Research and Dissemination Center. Lead author Dr. Schleiss disclosed grants from the CDC, the National Institutes of Health, and the DRDC during the conduct of the study; he also disclosed receiving personal fees from Moderna, Sanofi, GlaxoSmithKline, and Merck unrelated to the study. Dr. Haut had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Congenital cytomegalovirus cases declined significantly during the COVID-19 pandemic, compared with a period before the pandemic, based on data from nearly 20,000 newborns.
A study originated to explore racial and ethnic differences in congenital cytomegalovirus (cCMV) began in 2016, but was halted in April 2020 because of the COVID-19 pandemic, wrote Mark R. Schleiss, MD, of the University of Minnesota, Minneapolis, and colleagues. The study resumed for a period from August 2020 to December 2021, and the researchers compared data on cCMV before and during the pandemic. The prepandemic period included data from April 2016 to March 2020.
“We have been screening for congenital CMV infection in Minnesota for 6 years as a part of a multicenter collaborative study that I lead as the primary investigator,” Dr. Schleiss said in an interview. “Our efforts have contributed to the decision, vetted through the Minnesota Legislature and signed into law in 2021 (the “Vivian Act”), to begin universal screening for all newborns in Minnesota in 2023. In the context of this ongoing screening/surveillance study, it was important and scientifically very interesting to examine the impact of the COVID-19 pandemic on the risk of congenital CMV infection,” he explained.
The findings were published in a research letter in JAMA Network Open. A total of 15,697 newborns were screened before the pandemic and 4,222 were screened during the pandemic period at six hospitals. The majority of the mothers participating during the prepandemic and pandemic periods were non-Hispanic White (71% and 60%, respectively).
Overall, the percentage screened prevalence for cCMV was 79% in the prepandemic period and 21% during the pandemic, with rates of 4.5 per 1,000 and 1.4 per 1,000, respectively.
Although the highest percentage of cCMV cases occurred in newborns of mothers aged 25 years and older (86%), the prevalence was highest among newborns of mothers aged 24 years and younger (6.0 per 1,000). The prevalence of cCMV overall was higher in infants of non-Hispanic Black mothers vs. non-Hispanic White mothers, but not significantly different (5.1 per 1,000 vs. 4.6 per 1,000) and among second newborns vs. first newborns (6.0 vs. 3.2 per 1,000, respectively).
Factors related to COVID-19, including reduced day care attendance, behavioral changes, and mitigation measures at childcare facilities such as smaller classes and increased hand hygiene and disinfection may have contributed to this decrease in cCMV in the pandemic period, the researchers wrote in their discussion.
The comparable prevalence in newborns of non-Hispanic Black and White mothers contrasts with previous studies showing a higher prevalence in children of non-Hispanic Black mothers, the researchers noted in their discussion.
The study was limited by several factors, including the variation in time points for enrollment at different sites and the exclusion of families in the newborn nursery with positive COVID-19 results during the pandemic, they wrote. More research is needed on the potential effects of behavioral interventions to reduce CMV risk during pregnancy, as well as future CMV vaccination for childbearing-aged women and young children, they concluded.
However, the researchers were surprised by the impact of COVID-19 on the prevalence of cCMV, Dr. Schleiss said in an interview. “We have had the knowledge for many years that CMV infections in young women are commonly acquired through interactions with their toddlers. These interactions – sharing food, wiping drool and nasal discharge from the toddler’s nose, changing diapers, kissing the child on the mouth – can transmit CMV,” he said. In addition, toddlers may acquire CMV from group day care; the child then sheds CMV and transmits the virus to their pregnant mother, who then transmits the virus across the placenta, leading to cCMV infection in the newborn, Dr. Schleiss explained.
Although the researchers expected a decrease in CMV in the wake of closures of group day care, increased home schooling, decreased interactions among children, hygienic precautions, and social isolation, the decrease exceeded their expectations, said Dr. Schleiss. “Our previous work showed that in the 5-year period leading up to the pandemic, about one baby in every 200 births was born with CMV. Between August 2020 and December 2021, the number decreased to one baby in every 1,000 births,” a difference he and his team found striking.
The message from the study is that CMV can be prevented, said Dr. Schleiss. “Hygienic precautions during pregnancy had a big impact. Since congenital CMV infection is the most common congenital infection in the United States, and probably globally, that causes disabilities in children, the implications are highly significant,” he said. “The hygienic precautions we all have engaged in during the pandemic, such as masking, handwashing, and infection prevention behaviors, were almost certainly responsible for the reduction in CMV transmission, which in turn protected mothers and newborns from the potentially devastating effects of the CMV virus,” he noted.
Looking ahead, “Vaccines are moving forward in clinical trials that aim to confer immunity on young women of childbearing age to protect future pregnancies against transmission of CMV to the newborn infant; it would be very important to examine in future studies whether hygienic precautions would have the same impact as a potential vaccine,” Dr. Schleiss said. More research is needed to examine the effect of education of women about CMV transmission, he added. “We think it is very important to share this knowledge from our study with the pediatric community, since pediatricians can be important in counseling women about future pregnancies and the risks of CMV acquisition and transmission,” he noted.
Implications for other viruses
Although CMV poses minimal risk for healthy populations, irreversible complications for infants born with congenital CMV, especially hearing loss, are very concerning, said Catherine Haut, DNP, CPNP-AC/PC, a pediatric nurse practitioner in Rehoboth Beach, Del., in an interview.
“The study of viral transmission during a time of isolation, masking, and other mitigation procedures for COVID-19 assists in awareness that other viruses may also be limited with the use of these measures,” she said.
Dr. Haut was not surprised by the findings, given that CMV is transmitted primarily through direct contact with body fluids and that more than 50% of American adults have been infected by age 40, according to the Centers for Disease Control and Prevention, she said.
The take-home message for pediatricians, Dr. Haut said, is measures to prevent transmission of viral infection can yield significant positive health outcomes for the pediatric population; however, the effect of isolation, which has been associated with a higher rate of mental health problems, should not be ignored.
“Despite appropriate statistical analyses and presentation of findings in this study, the population sampled during the pandemic was less than 30% of the pre-COVID sampling, representing a study limitation,” and conducting research in a single state limits generalizability, Dr. Haut noted. “I agree with the authors that additional study is necessary to better understand prevention measures and apply these methods to reduce CMV transmission. Pursuit of CMV immunization opportunities is also needed,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Vaccine Program Office, the Minnesota Department of Health Newborn Screening Program, and the University of South Carolina Disability Research and Dissemination Center. Lead author Dr. Schleiss disclosed grants from the CDC, the National Institutes of Health, and the DRDC during the conduct of the study; he also disclosed receiving personal fees from Moderna, Sanofi, GlaxoSmithKline, and Merck unrelated to the study. Dr. Haut had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Congenital cytomegalovirus cases declined significantly during the COVID-19 pandemic, compared with a period before the pandemic, based on data from nearly 20,000 newborns.
A study originated to explore racial and ethnic differences in congenital cytomegalovirus (cCMV) began in 2016, but was halted in April 2020 because of the COVID-19 pandemic, wrote Mark R. Schleiss, MD, of the University of Minnesota, Minneapolis, and colleagues. The study resumed for a period from August 2020 to December 2021, and the researchers compared data on cCMV before and during the pandemic. The prepandemic period included data from April 2016 to March 2020.
“We have been screening for congenital CMV infection in Minnesota for 6 years as a part of a multicenter collaborative study that I lead as the primary investigator,” Dr. Schleiss said in an interview. “Our efforts have contributed to the decision, vetted through the Minnesota Legislature and signed into law in 2021 (the “Vivian Act”), to begin universal screening for all newborns in Minnesota in 2023. In the context of this ongoing screening/surveillance study, it was important and scientifically very interesting to examine the impact of the COVID-19 pandemic on the risk of congenital CMV infection,” he explained.
The findings were published in a research letter in JAMA Network Open. A total of 15,697 newborns were screened before the pandemic and 4,222 were screened during the pandemic period at six hospitals. The majority of the mothers participating during the prepandemic and pandemic periods were non-Hispanic White (71% and 60%, respectively).
Overall, the percentage screened prevalence for cCMV was 79% in the prepandemic period and 21% during the pandemic, with rates of 4.5 per 1,000 and 1.4 per 1,000, respectively.
Although the highest percentage of cCMV cases occurred in newborns of mothers aged 25 years and older (86%), the prevalence was highest among newborns of mothers aged 24 years and younger (6.0 per 1,000). The prevalence of cCMV overall was higher in infants of non-Hispanic Black mothers vs. non-Hispanic White mothers, but not significantly different (5.1 per 1,000 vs. 4.6 per 1,000) and among second newborns vs. first newborns (6.0 vs. 3.2 per 1,000, respectively).
Factors related to COVID-19, including reduced day care attendance, behavioral changes, and mitigation measures at childcare facilities such as smaller classes and increased hand hygiene and disinfection may have contributed to this decrease in cCMV in the pandemic period, the researchers wrote in their discussion.
The comparable prevalence in newborns of non-Hispanic Black and White mothers contrasts with previous studies showing a higher prevalence in children of non-Hispanic Black mothers, the researchers noted in their discussion.
The study was limited by several factors, including the variation in time points for enrollment at different sites and the exclusion of families in the newborn nursery with positive COVID-19 results during the pandemic, they wrote. More research is needed on the potential effects of behavioral interventions to reduce CMV risk during pregnancy, as well as future CMV vaccination for childbearing-aged women and young children, they concluded.
However, the researchers were surprised by the impact of COVID-19 on the prevalence of cCMV, Dr. Schleiss said in an interview. “We have had the knowledge for many years that CMV infections in young women are commonly acquired through interactions with their toddlers. These interactions – sharing food, wiping drool and nasal discharge from the toddler’s nose, changing diapers, kissing the child on the mouth – can transmit CMV,” he said. In addition, toddlers may acquire CMV from group day care; the child then sheds CMV and transmits the virus to their pregnant mother, who then transmits the virus across the placenta, leading to cCMV infection in the newborn, Dr. Schleiss explained.
Although the researchers expected a decrease in CMV in the wake of closures of group day care, increased home schooling, decreased interactions among children, hygienic precautions, and social isolation, the decrease exceeded their expectations, said Dr. Schleiss. “Our previous work showed that in the 5-year period leading up to the pandemic, about one baby in every 200 births was born with CMV. Between August 2020 and December 2021, the number decreased to one baby in every 1,000 births,” a difference he and his team found striking.
The message from the study is that CMV can be prevented, said Dr. Schleiss. “Hygienic precautions during pregnancy had a big impact. Since congenital CMV infection is the most common congenital infection in the United States, and probably globally, that causes disabilities in children, the implications are highly significant,” he said. “The hygienic precautions we all have engaged in during the pandemic, such as masking, handwashing, and infection prevention behaviors, were almost certainly responsible for the reduction in CMV transmission, which in turn protected mothers and newborns from the potentially devastating effects of the CMV virus,” he noted.
Looking ahead, “Vaccines are moving forward in clinical trials that aim to confer immunity on young women of childbearing age to protect future pregnancies against transmission of CMV to the newborn infant; it would be very important to examine in future studies whether hygienic precautions would have the same impact as a potential vaccine,” Dr. Schleiss said. More research is needed to examine the effect of education of women about CMV transmission, he added. “We think it is very important to share this knowledge from our study with the pediatric community, since pediatricians can be important in counseling women about future pregnancies and the risks of CMV acquisition and transmission,” he noted.
Implications for other viruses
Although CMV poses minimal risk for healthy populations, irreversible complications for infants born with congenital CMV, especially hearing loss, are very concerning, said Catherine Haut, DNP, CPNP-AC/PC, a pediatric nurse practitioner in Rehoboth Beach, Del., in an interview.
“The study of viral transmission during a time of isolation, masking, and other mitigation procedures for COVID-19 assists in awareness that other viruses may also be limited with the use of these measures,” she said.
Dr. Haut was not surprised by the findings, given that CMV is transmitted primarily through direct contact with body fluids and that more than 50% of American adults have been infected by age 40, according to the Centers for Disease Control and Prevention, she said.
The take-home message for pediatricians, Dr. Haut said, is measures to prevent transmission of viral infection can yield significant positive health outcomes for the pediatric population; however, the effect of isolation, which has been associated with a higher rate of mental health problems, should not be ignored.
“Despite appropriate statistical analyses and presentation of findings in this study, the population sampled during the pandemic was less than 30% of the pre-COVID sampling, representing a study limitation,” and conducting research in a single state limits generalizability, Dr. Haut noted. “I agree with the authors that additional study is necessary to better understand prevention measures and apply these methods to reduce CMV transmission. Pursuit of CMV immunization opportunities is also needed,” she said.
The study was supported by the Centers for Disease Control and Prevention, the National Vaccine Program Office, the Minnesota Department of Health Newborn Screening Program, and the University of South Carolina Disability Research and Dissemination Center. Lead author Dr. Schleiss disclosed grants from the CDC, the National Institutes of Health, and the DRDC during the conduct of the study; he also disclosed receiving personal fees from Moderna, Sanofi, GlaxoSmithKline, and Merck unrelated to the study. Dr. Haut had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
FROM JAMA NETWORK OPEN
Why some infectious disease docs are ‘encouraged’ by new bivalent COVID vaccines
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
A panel of infectious disease experts shared their take recently on the importance of the newly approved bivalent COVID-19 vaccines, why authorization without human data is not for them a cause for alarm, and what they are most optimistic about at this stage of the pandemic.
“I’m very encouraged by this new development,” Kathryn M. Edwards, MD, said during a media briefing sponsored by the Infectious Diseases Society of America (IDSA).
, she said. “It does seem that if you have a circulating strain BA.4 and BA.5, hitting it with the appropriate vaccine targeted for that is most immunogenic, certainly. We will hopefully see that in terms of effectiveness.”
Changing the vaccines at this point is appropriate, Walter A. Orenstein, MD, said. “One of our challenges is that this virus mutates. Our immune response is focused on an area of the virus that can change and be evaded,” said Dr. Orenstein, professor and associate director of the Emory Vaccine Center at Emory University, Atlanta.
“This is different than measles or polio,” he said. “But for influenza and now with SARS-CoV-2 ... we have to update our vaccines, because the virus changes.”
Man versus mouse
Dr. Edwards addressed the controversy over a lack of human data specific to these next-generation Pfizer/BioNTech and Moderna vaccines. “I do not want people to be unhappy or worried that the bivalent vaccine will act in a different way than the ones that we have been administering for the past 2 years.”
The Food and Drug Administration emergency use authorization may have relied primarily on animal studies, she said, but mice given a vaccine specific to BA.4 and BA.5 “have a much more robust immune response,” compared with those given a BA.1 vaccine.
Also, “over and over and over again we have seen with these SARS-CoV-2 vaccines that the mouse responses mirror the human responses,” said Dr. Edwards, scientific director of the Vanderbilt Vaccine Research Program at Vanderbilt University, Nashville, Tenn., and an IDSA fellow.
“Human data will be coming very soon to look at the immunogenicity,” she said.
A ‘glass half full’ perspective
When asked what they are most optimistic about at this point in the COVID-19 pandemic, Dr. Orenstein said, “I’m really positive in the sense that the vaccines we have are already very effective against severe disease, death, and hospitalization. I feel really good about that. And we have great tools.
“The bottom line for me is, I want to get it myself,” he said regarding the bivalent vaccine.
“There are a lot of things to be happy with,” Dr. Edwards said. “I’m kind of a glass-half-full kind of person.”
Dr. Edwards is confident that the surveillance systems now in place can accurately detect major changes in the virus, including new variants. She is also optimistic about the mRNA technology that allows rapid updates to COVID-19 vaccines.
Furthermore, “I’m happy that we’re beginning to open up – that we can go do different things that we have done in the past and feel much more comfortable,” she said.
More motivational messaging needed
Now is also a good time to renew efforts to get people vaccinated.
“We invested a lot into developing these vaccines, but I think we also need to invest in what I call ‘implementation science research,’ ” Dr. Orenstein said, the goal being to convince people to get vaccinated.
He pointed out that it’s vaccinations, not vaccines, that saves lives. “Vaccine doses that remain in the vial are 0% effective.
“When I was director of the United States’ immunization program at the CDC,” Dr. Orenstein said, “my director of communications used to say that you need the right message delivered by the right messenger through the right communications channel.”
Dr. Edwards agreed that listening to people’s concerns and respecting their questions are important. “We also need to make sure that we use the proper messenger, just as Walt said. Maybe the proper messenger isn’t an old gray-haired lady,” she said, referring to herself, “but it’s someone that lives in your community or is your primary care doctor who has taken care of you or your children for many years.”
Research on how to better motivate people to get vaccinated is warranted, Dr. Edwards said, as well as on “how to make sure that this is really a medical issue and not a political issue. That’s been a really big problem.”
A version of this article first appeared on Medscape.com.
Influenza vaccine may offer much more than flu prevention
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
New study supports safety of COVID-19 boosters during pregnancy
Doctors and health professionals continue to recommend COVID-19 vaccine boosters or third doses for adolescents and adults more than 5 months after their initial vaccinations with the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 primary vaccine series or more than 2 months after receiving the Janssen JNJ-78436735 vaccine, Alisa Kachikis, MD, of the University of Washington, Seattle, and colleagues wrote in JAMA Network Open.
Although multiple studies have shown that the COVID-19 primary series is safe and well tolerated in pregnant and lactating women, information on the safety and tolerability of boosters are lacking, the researchers noted.
“COVID-19 will be with us for a while, and it is important to continue to provide data on COVID-19 vaccines in these groups, particularly because there still are many questions about the vaccine, and because pregnant individuals have been, understandably, more hesitant to receive COVID-19 vaccines,” Dr. Kachikis said in an interview. “The findings of this study that COVID-19 booster doses are well tolerated among pregnant and lactating individuals are especially pertinent with the new COVID-19 boosters available this fall.”
In the new study, the researchers reviewed data from 17,014 participants who were part of an ongoing online prospective study of COVID-19 vaccines in pregnant and lactating individuals. Data were collected between October 2021 and April 2022 through an online survey.
The study population included 2,009 participants (11.8%) who were pregnant at the time of their booster or third dose, 10,279 (60.4%) who were lactating, and 4,726 (27.8%) who were neither pregnant nor lactating. The mean age of the participants was 33.3 years; 92.1% self-identified as White, 94.5% self-identified as non-Hispanic, and 99.7% self-identified as female.
The receipt of a booster was similar across trimesters; 26.4%, 36.5%, and 37.1% of participants received boosters or third doses in the first, second, and third trimester, respectively. The primary outcome was self-reported vaccine reactions within 24 hours of the dose.
Overall, 82.8% of the respondents reported a reaction at the site of the injection, such as redness, pain, or swelling, and 67.9% reported at least one systemic symptom, such as aches and pains, headache, chills, or fever. The most frequently reported symptoms across all groups were injection-site pain (82.2%) and fatigue (54.4%).
The pregnant women were significantly more likely than nonpregnant or nonlactating individuals to report any local reaction at the injection site (adjusted odds ratio, 1.2; P = .01), but less likely to report any systemic reaction (aOR, 0.7; P < .001).
The majority (97.6%) of the pregnant respondents and 96.0% of those lactating reported no obstetric or lactation concerns after vaccination.
Overall, a majority of the respondents reported that recommendations from public health authorities were helpful in their decision to receive a COVID-19 booster or third dose (90.0% of pregnant respondents, 89.9% of lactating respondents, and 88.1% of those neither pregnant nor lactating).
Although vaccine uptake in the current study population was high (91.1% overall and 95.0% of those pregnant), “the importance of the health care professional’s recommendation is pertinent given the ongoing increased vaccine hesitancy among pregnant individuals in the context of the COVID-19 vaccine,” the researchers emphasized.
The study findings were limited by several factors including the reliance on self-reports and a convenience sample composed mainly of health care workers because of their vaccine eligibility at the time the study started, which limits generalizability, the researchers noted. Analyses on the pregnancy outcomes of those who were pregnant when vaccinated are in progress.
The results were strengthened by the large study population that included participants from all 50 states and several territories, and ability to compare results between pregnant and lactating individuals with those who were neither pregnant nor lactating, but were of childbearing age, they said.
The results support the safety of COVID-19 boosters for pregnant and breastfeeding individuals, and these data are important to inform discussions between patients and clinicians to boost vaccine uptake and acceptance in this population, they concluded.
“Our earlier data analysis showed that pregnant and lactating individuals did very well with the initial COVID-19 vaccine series, so it was not very surprising that they also did well with COVID-19 booster or third doses,” Dr. Kachikis said in an interview.
There are two takeaway messages for clinicians, she said: “First, pregnant and lactating individuals tolerated the COVID-19 booster well. The second is that clinicians are very important when it comes to vaccine acceptance.”
“In our study, we found that, while pregnant participants were more likely to report that they were hesitant to receive the booster, they also were more likely to have discussed the COVID-19 booster with their health care provider, and to have received a recommendation to receive the booster. So, spending a little bit of extra time with patients discussing COVID-19 boosters and recommending them can make a significant difference,” she said.
The message of the study is highly reassuring for pregnant and lactating individuals, Dr. Kachikis added. “Most of the participants reported that they had fewer symptoms with the COVID-19 booster compared to the primary vaccine series, which is good news, especially since a new COVID-19 booster is being recommended for the fall.”
Reassuring findings for doctors and patients
The current study is especially timely, as updated COVID-19 boosters have now been recommended for most individuals by the Centers for Disease Control and Prevention, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
The findings support previous studies on the tolerability of COVID-19 vaccinations in pregnant and lactating persons, said Dr. Badell, who was not involved in the study.
The reassuring message for clinicians is that COVID-19 booster vaccinations are similarly well tolerated in pregnancy and lactation as they are in nonpregnant individuals, said Dr. Badell. “Given the risks of COVID infections in pregnancy and neonates, reassuring data on the tolerability and safety of vaccination in this population is very important.” Also, the researchers found that all three cohorts reported that recommendations from public or medical health authorities helped them make a decision about vaccination; “thus the more data to support these recommendations, the better,” she emphasized.
If you are pregnant or breastfeeding, the message from the study is that COVID-19 booster vaccinations are similarly well tolerated by those who are pregnant or breastfeeding and those who are not, said Dr. Badell.
“This study provides additional support for the strong recommendation to encourage not only COVID-19 vaccination in pregnancy and lactation, but booster vaccinations specifically,” and pregnant and breastfeeding individuals should not be excluded from the new CDC recommendations for COVID-19 boosters, she said.
Future research suggestions
Next steps for research include evaluating the obstetrical and neonatal outcomes in pregnancy and lactation following COVID- 19 boosters, Dr. Badell added.
Dr. Kachikis suggested studies try to answer the remaining questions about COVID-19 vaccines and the immunity of pregnant and lactating persons, particularly since they were excluded from the early clinical trials in 2020.
The study was supported by the National Institute of Allergy and Infectious Diseases, a Women’s Reproductive Health Research Award, and the National Center for Advancing Translational Sciences of the National Institutes of Health. \Dr. Kachikis disclosed serving as a research consultant for Pfizer and GlaxoSmithKline and as an unpaid consultant for GlaxoSmithKline unrelated to the current study, as well as grant support from Merck and Pfizer unrelated to the current study. Dr. Badell had no financial conflicts to disclose.
Doctors and health professionals continue to recommend COVID-19 vaccine boosters or third doses for adolescents and adults more than 5 months after their initial vaccinations with the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 primary vaccine series or more than 2 months after receiving the Janssen JNJ-78436735 vaccine, Alisa Kachikis, MD, of the University of Washington, Seattle, and colleagues wrote in JAMA Network Open.
Although multiple studies have shown that the COVID-19 primary series is safe and well tolerated in pregnant and lactating women, information on the safety and tolerability of boosters are lacking, the researchers noted.
“COVID-19 will be with us for a while, and it is important to continue to provide data on COVID-19 vaccines in these groups, particularly because there still are many questions about the vaccine, and because pregnant individuals have been, understandably, more hesitant to receive COVID-19 vaccines,” Dr. Kachikis said in an interview. “The findings of this study that COVID-19 booster doses are well tolerated among pregnant and lactating individuals are especially pertinent with the new COVID-19 boosters available this fall.”
In the new study, the researchers reviewed data from 17,014 participants who were part of an ongoing online prospective study of COVID-19 vaccines in pregnant and lactating individuals. Data were collected between October 2021 and April 2022 through an online survey.
The study population included 2,009 participants (11.8%) who were pregnant at the time of their booster or third dose, 10,279 (60.4%) who were lactating, and 4,726 (27.8%) who were neither pregnant nor lactating. The mean age of the participants was 33.3 years; 92.1% self-identified as White, 94.5% self-identified as non-Hispanic, and 99.7% self-identified as female.
The receipt of a booster was similar across trimesters; 26.4%, 36.5%, and 37.1% of participants received boosters or third doses in the first, second, and third trimester, respectively. The primary outcome was self-reported vaccine reactions within 24 hours of the dose.
Overall, 82.8% of the respondents reported a reaction at the site of the injection, such as redness, pain, or swelling, and 67.9% reported at least one systemic symptom, such as aches and pains, headache, chills, or fever. The most frequently reported symptoms across all groups were injection-site pain (82.2%) and fatigue (54.4%).
The pregnant women were significantly more likely than nonpregnant or nonlactating individuals to report any local reaction at the injection site (adjusted odds ratio, 1.2; P = .01), but less likely to report any systemic reaction (aOR, 0.7; P < .001).
The majority (97.6%) of the pregnant respondents and 96.0% of those lactating reported no obstetric or lactation concerns after vaccination.
Overall, a majority of the respondents reported that recommendations from public health authorities were helpful in their decision to receive a COVID-19 booster or third dose (90.0% of pregnant respondents, 89.9% of lactating respondents, and 88.1% of those neither pregnant nor lactating).
Although vaccine uptake in the current study population was high (91.1% overall and 95.0% of those pregnant), “the importance of the health care professional’s recommendation is pertinent given the ongoing increased vaccine hesitancy among pregnant individuals in the context of the COVID-19 vaccine,” the researchers emphasized.
The study findings were limited by several factors including the reliance on self-reports and a convenience sample composed mainly of health care workers because of their vaccine eligibility at the time the study started, which limits generalizability, the researchers noted. Analyses on the pregnancy outcomes of those who were pregnant when vaccinated are in progress.
The results were strengthened by the large study population that included participants from all 50 states and several territories, and ability to compare results between pregnant and lactating individuals with those who were neither pregnant nor lactating, but were of childbearing age, they said.
The results support the safety of COVID-19 boosters for pregnant and breastfeeding individuals, and these data are important to inform discussions between patients and clinicians to boost vaccine uptake and acceptance in this population, they concluded.
“Our earlier data analysis showed that pregnant and lactating individuals did very well with the initial COVID-19 vaccine series, so it was not very surprising that they also did well with COVID-19 booster or third doses,” Dr. Kachikis said in an interview.
There are two takeaway messages for clinicians, she said: “First, pregnant and lactating individuals tolerated the COVID-19 booster well. The second is that clinicians are very important when it comes to vaccine acceptance.”
“In our study, we found that, while pregnant participants were more likely to report that they were hesitant to receive the booster, they also were more likely to have discussed the COVID-19 booster with their health care provider, and to have received a recommendation to receive the booster. So, spending a little bit of extra time with patients discussing COVID-19 boosters and recommending them can make a significant difference,” she said.
The message of the study is highly reassuring for pregnant and lactating individuals, Dr. Kachikis added. “Most of the participants reported that they had fewer symptoms with the COVID-19 booster compared to the primary vaccine series, which is good news, especially since a new COVID-19 booster is being recommended for the fall.”
Reassuring findings for doctors and patients
The current study is especially timely, as updated COVID-19 boosters have now been recommended for most individuals by the Centers for Disease Control and Prevention, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
The findings support previous studies on the tolerability of COVID-19 vaccinations in pregnant and lactating persons, said Dr. Badell, who was not involved in the study.
The reassuring message for clinicians is that COVID-19 booster vaccinations are similarly well tolerated in pregnancy and lactation as they are in nonpregnant individuals, said Dr. Badell. “Given the risks of COVID infections in pregnancy and neonates, reassuring data on the tolerability and safety of vaccination in this population is very important.” Also, the researchers found that all three cohorts reported that recommendations from public or medical health authorities helped them make a decision about vaccination; “thus the more data to support these recommendations, the better,” she emphasized.
If you are pregnant or breastfeeding, the message from the study is that COVID-19 booster vaccinations are similarly well tolerated by those who are pregnant or breastfeeding and those who are not, said Dr. Badell.
“This study provides additional support for the strong recommendation to encourage not only COVID-19 vaccination in pregnancy and lactation, but booster vaccinations specifically,” and pregnant and breastfeeding individuals should not be excluded from the new CDC recommendations for COVID-19 boosters, she said.
Future research suggestions
Next steps for research include evaluating the obstetrical and neonatal outcomes in pregnancy and lactation following COVID- 19 boosters, Dr. Badell added.
Dr. Kachikis suggested studies try to answer the remaining questions about COVID-19 vaccines and the immunity of pregnant and lactating persons, particularly since they were excluded from the early clinical trials in 2020.
The study was supported by the National Institute of Allergy and Infectious Diseases, a Women’s Reproductive Health Research Award, and the National Center for Advancing Translational Sciences of the National Institutes of Health. \Dr. Kachikis disclosed serving as a research consultant for Pfizer and GlaxoSmithKline and as an unpaid consultant for GlaxoSmithKline unrelated to the current study, as well as grant support from Merck and Pfizer unrelated to the current study. Dr. Badell had no financial conflicts to disclose.
Doctors and health professionals continue to recommend COVID-19 vaccine boosters or third doses for adolescents and adults more than 5 months after their initial vaccinations with the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 primary vaccine series or more than 2 months after receiving the Janssen JNJ-78436735 vaccine, Alisa Kachikis, MD, of the University of Washington, Seattle, and colleagues wrote in JAMA Network Open.
Although multiple studies have shown that the COVID-19 primary series is safe and well tolerated in pregnant and lactating women, information on the safety and tolerability of boosters are lacking, the researchers noted.
“COVID-19 will be with us for a while, and it is important to continue to provide data on COVID-19 vaccines in these groups, particularly because there still are many questions about the vaccine, and because pregnant individuals have been, understandably, more hesitant to receive COVID-19 vaccines,” Dr. Kachikis said in an interview. “The findings of this study that COVID-19 booster doses are well tolerated among pregnant and lactating individuals are especially pertinent with the new COVID-19 boosters available this fall.”
In the new study, the researchers reviewed data from 17,014 participants who were part of an ongoing online prospective study of COVID-19 vaccines in pregnant and lactating individuals. Data were collected between October 2021 and April 2022 through an online survey.
The study population included 2,009 participants (11.8%) who were pregnant at the time of their booster or third dose, 10,279 (60.4%) who were lactating, and 4,726 (27.8%) who were neither pregnant nor lactating. The mean age of the participants was 33.3 years; 92.1% self-identified as White, 94.5% self-identified as non-Hispanic, and 99.7% self-identified as female.
The receipt of a booster was similar across trimesters; 26.4%, 36.5%, and 37.1% of participants received boosters or third doses in the first, second, and third trimester, respectively. The primary outcome was self-reported vaccine reactions within 24 hours of the dose.
Overall, 82.8% of the respondents reported a reaction at the site of the injection, such as redness, pain, or swelling, and 67.9% reported at least one systemic symptom, such as aches and pains, headache, chills, or fever. The most frequently reported symptoms across all groups were injection-site pain (82.2%) and fatigue (54.4%).
The pregnant women were significantly more likely than nonpregnant or nonlactating individuals to report any local reaction at the injection site (adjusted odds ratio, 1.2; P = .01), but less likely to report any systemic reaction (aOR, 0.7; P < .001).
The majority (97.6%) of the pregnant respondents and 96.0% of those lactating reported no obstetric or lactation concerns after vaccination.
Overall, a majority of the respondents reported that recommendations from public health authorities were helpful in their decision to receive a COVID-19 booster or third dose (90.0% of pregnant respondents, 89.9% of lactating respondents, and 88.1% of those neither pregnant nor lactating).
Although vaccine uptake in the current study population was high (91.1% overall and 95.0% of those pregnant), “the importance of the health care professional’s recommendation is pertinent given the ongoing increased vaccine hesitancy among pregnant individuals in the context of the COVID-19 vaccine,” the researchers emphasized.
The study findings were limited by several factors including the reliance on self-reports and a convenience sample composed mainly of health care workers because of their vaccine eligibility at the time the study started, which limits generalizability, the researchers noted. Analyses on the pregnancy outcomes of those who were pregnant when vaccinated are in progress.
The results were strengthened by the large study population that included participants from all 50 states and several territories, and ability to compare results between pregnant and lactating individuals with those who were neither pregnant nor lactating, but were of childbearing age, they said.
The results support the safety of COVID-19 boosters for pregnant and breastfeeding individuals, and these data are important to inform discussions between patients and clinicians to boost vaccine uptake and acceptance in this population, they concluded.
“Our earlier data analysis showed that pregnant and lactating individuals did very well with the initial COVID-19 vaccine series, so it was not very surprising that they also did well with COVID-19 booster or third doses,” Dr. Kachikis said in an interview.
There are two takeaway messages for clinicians, she said: “First, pregnant and lactating individuals tolerated the COVID-19 booster well. The second is that clinicians are very important when it comes to vaccine acceptance.”
“In our study, we found that, while pregnant participants were more likely to report that they were hesitant to receive the booster, they also were more likely to have discussed the COVID-19 booster with their health care provider, and to have received a recommendation to receive the booster. So, spending a little bit of extra time with patients discussing COVID-19 boosters and recommending them can make a significant difference,” she said.
The message of the study is highly reassuring for pregnant and lactating individuals, Dr. Kachikis added. “Most of the participants reported that they had fewer symptoms with the COVID-19 booster compared to the primary vaccine series, which is good news, especially since a new COVID-19 booster is being recommended for the fall.”
Reassuring findings for doctors and patients
The current study is especially timely, as updated COVID-19 boosters have now been recommended for most individuals by the Centers for Disease Control and Prevention, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
The findings support previous studies on the tolerability of COVID-19 vaccinations in pregnant and lactating persons, said Dr. Badell, who was not involved in the study.
The reassuring message for clinicians is that COVID-19 booster vaccinations are similarly well tolerated in pregnancy and lactation as they are in nonpregnant individuals, said Dr. Badell. “Given the risks of COVID infections in pregnancy and neonates, reassuring data on the tolerability and safety of vaccination in this population is very important.” Also, the researchers found that all three cohorts reported that recommendations from public or medical health authorities helped them make a decision about vaccination; “thus the more data to support these recommendations, the better,” she emphasized.
If you are pregnant or breastfeeding, the message from the study is that COVID-19 booster vaccinations are similarly well tolerated by those who are pregnant or breastfeeding and those who are not, said Dr. Badell.
“This study provides additional support for the strong recommendation to encourage not only COVID-19 vaccination in pregnancy and lactation, but booster vaccinations specifically,” and pregnant and breastfeeding individuals should not be excluded from the new CDC recommendations for COVID-19 boosters, she said.
Future research suggestions
Next steps for research include evaluating the obstetrical and neonatal outcomes in pregnancy and lactation following COVID- 19 boosters, Dr. Badell added.
Dr. Kachikis suggested studies try to answer the remaining questions about COVID-19 vaccines and the immunity of pregnant and lactating persons, particularly since they were excluded from the early clinical trials in 2020.
The study was supported by the National Institute of Allergy and Infectious Diseases, a Women’s Reproductive Health Research Award, and the National Center for Advancing Translational Sciences of the National Institutes of Health. \Dr. Kachikis disclosed serving as a research consultant for Pfizer and GlaxoSmithKline and as an unpaid consultant for GlaxoSmithKline unrelated to the current study, as well as grant support from Merck and Pfizer unrelated to the current study. Dr. Badell had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Unvaccinated 10 times more likely to be hospitalized for Omicron
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
FROM JAMA INTERNAL MEDICINE
Pediatricians urge flu vaccine for children
Attention parents: The nation’s leading pediatric medical society is urging you to make sure your children get a flu shot this fall to prevent and control the spread of the illness.
The American Academy of Pediatrics recently called on parents and caregivers to seek flu vaccines for their children as soon as they are available in the fall. The group is encouraging parents to catch up on all other vaccines for their children, too.
“As a pediatrician and a parent, I consider the flu vaccine as critical for all family members,” Kristina A. Bryant, MD, said in a statement about the academy’s recommendations. “We should not underestimate the flu, especially when other respiratory viruses like COVID-19 are circulating within our communities. Besides making your child miserable and wreaking havoc on your family’s routine, influenza can also be serious and even deadly in children.”
Only 55% of children aged 6 months to 17 years had been vaccinated against influenza as of early April – down 2% from the previous April – and coverage levels were 8.1% lower for Black children compared with non-Hispanic White children, according to the CDC. In the 2019-2020 flu season, 188 children in the United States died of the infection, equaling the high mark for deaths set in the 2017-2018 season, the agency reported.
American Academy of Pediatrics guidelines recommend children aged 6 months and older be vaccinated with the flu vaccine every year. Depending on the child’s age and health, they may receive either a shot, which has an inactive version of the flu virus, or the nasal spray, which has a weakened form of the virus. The academy has more information about the different vaccines.
Children aged 6-8 months who are getting flu vaccines for the first time should receive two doses at least 4 weeks apart. Pregnant women can get the flu vaccine any time in their pregnancy. Influenza vaccines are safe for developing fetuses, according to the academy.
The group stressed the importance of flu vaccines for high-risk and medically vulnerable children and acknowledged the need to end barriers to immunizations for all people, regardless of income or insurance coverage. In 2020, an estimated 16.1% of children in the United States were living in poverty, up from 14.4% in 2019, according to the U.S. Census Bureau.
A version of this article first appeared on WebMD.com.
Attention parents: The nation’s leading pediatric medical society is urging you to make sure your children get a flu shot this fall to prevent and control the spread of the illness.
The American Academy of Pediatrics recently called on parents and caregivers to seek flu vaccines for their children as soon as they are available in the fall. The group is encouraging parents to catch up on all other vaccines for their children, too.
“As a pediatrician and a parent, I consider the flu vaccine as critical for all family members,” Kristina A. Bryant, MD, said in a statement about the academy’s recommendations. “We should not underestimate the flu, especially when other respiratory viruses like COVID-19 are circulating within our communities. Besides making your child miserable and wreaking havoc on your family’s routine, influenza can also be serious and even deadly in children.”
Only 55% of children aged 6 months to 17 years had been vaccinated against influenza as of early April – down 2% from the previous April – and coverage levels were 8.1% lower for Black children compared with non-Hispanic White children, according to the CDC. In the 2019-2020 flu season, 188 children in the United States died of the infection, equaling the high mark for deaths set in the 2017-2018 season, the agency reported.
American Academy of Pediatrics guidelines recommend children aged 6 months and older be vaccinated with the flu vaccine every year. Depending on the child’s age and health, they may receive either a shot, which has an inactive version of the flu virus, or the nasal spray, which has a weakened form of the virus. The academy has more information about the different vaccines.
Children aged 6-8 months who are getting flu vaccines for the first time should receive two doses at least 4 weeks apart. Pregnant women can get the flu vaccine any time in their pregnancy. Influenza vaccines are safe for developing fetuses, according to the academy.
The group stressed the importance of flu vaccines for high-risk and medically vulnerable children and acknowledged the need to end barriers to immunizations for all people, regardless of income or insurance coverage. In 2020, an estimated 16.1% of children in the United States were living in poverty, up from 14.4% in 2019, according to the U.S. Census Bureau.
A version of this article first appeared on WebMD.com.
Attention parents: The nation’s leading pediatric medical society is urging you to make sure your children get a flu shot this fall to prevent and control the spread of the illness.
The American Academy of Pediatrics recently called on parents and caregivers to seek flu vaccines for their children as soon as they are available in the fall. The group is encouraging parents to catch up on all other vaccines for their children, too.
“As a pediatrician and a parent, I consider the flu vaccine as critical for all family members,” Kristina A. Bryant, MD, said in a statement about the academy’s recommendations. “We should not underestimate the flu, especially when other respiratory viruses like COVID-19 are circulating within our communities. Besides making your child miserable and wreaking havoc on your family’s routine, influenza can also be serious and even deadly in children.”
Only 55% of children aged 6 months to 17 years had been vaccinated against influenza as of early April – down 2% from the previous April – and coverage levels were 8.1% lower for Black children compared with non-Hispanic White children, according to the CDC. In the 2019-2020 flu season, 188 children in the United States died of the infection, equaling the high mark for deaths set in the 2017-2018 season, the agency reported.
American Academy of Pediatrics guidelines recommend children aged 6 months and older be vaccinated with the flu vaccine every year. Depending on the child’s age and health, they may receive either a shot, which has an inactive version of the flu virus, or the nasal spray, which has a weakened form of the virus. The academy has more information about the different vaccines.
Children aged 6-8 months who are getting flu vaccines for the first time should receive two doses at least 4 weeks apart. Pregnant women can get the flu vaccine any time in their pregnancy. Influenza vaccines are safe for developing fetuses, according to the academy.
The group stressed the importance of flu vaccines for high-risk and medically vulnerable children and acknowledged the need to end barriers to immunizations for all people, regardless of income or insurance coverage. In 2020, an estimated 16.1% of children in the United States were living in poverty, up from 14.4% in 2019, according to the U.S. Census Bureau.
A version of this article first appeared on WebMD.com.
COVID-19 vaccination recap: The latest developments
In recent weeks, the COVID-19 vaccine arsenal has grown more robust. Here’s what you need to know:
Variant-specific boosters. On September 1, the Advisory Committee on Immunization Practices (ACIP) adopted a recommendation for a booster of either a new bivalent Pfizer-BioNTech COVID-19 vaccine (for individuals ages 12 years and older) or bivalent Moderna COVID-19 vaccine (for individuals ages 18 years and older) at least 2 months after receipt of a primary series or prior monovalent booster dose. Both bivalent vaccines were recently approved by the Food and Drug Administration (FDA) under an Emergency Use Authorization (EUA) and offer protection against one of the more common circulating strains of SARS-COV-2 (BA.1) while boosting immunity to the original strain. Both options are approved only as booster shots, not as an original COVID vaccine series.1
Novavax vaccine. This summer, the FDA issued an EUA for the Novavax COVID-19 vaccine in adults and a later EUA for adolescents (ages 12 to 17 years).2 Novavax is the fourth vaccine available to combat COVID-19 infection. This newest addition to the COVID armamentarium consists of coronavirus protein subunits, produced using recombinant technology, and a matrix adjuvant. The primary series consists of 2 doses administered at least 3 weeks apart.3,4
A few caveats: The Novavax vaccine comes in 10-dose vials, which should be kept refrigerated until use. Once the first dose is used, the vial should be discarded after 6 hours. This may present some scheduling and logistical issues. Also, the Novavax vaccine is not currently approved for use in children younger than 12 years, or as a booster to other vaccines.3,4
The effectiveness and safety of the Novavax vaccine appears to be comparable to that of the other vaccines approved to date, although measuring vaccine effectiveness is a tricky business given the rapid mutation of the virus and changing dominant strains.3,4 The Novavax vaccine’s efficacy against currently circulating Omicron variants of the virus (eg, BA.2.12.1, BA.4, BA.5) remains to be determined.
As far as safety, preliminary studies indicate that Novavax may be associated with rare cases of myocarditis.3,4 Myocarditis can result from the COVID infection itself at an overall rate of 1 to 2 per 1000, which is 16 times the rate in adults without COVID.5
Could it provide reassurance to the hesitant? The Novavax COVID vaccine was developed using a vaccine platform and production process similar to that of other commonly administered vaccines, such as hepatitis B vaccine and human papillomavirus vaccine. This may make it an appealing option for patients who have shown hesitancy toward new vaccine technologies.
And, of course, there are the Pfizer and Moderna vaccines. Currently, there are 2 vaccines approved under the normal licensing process for adults, both of which are mRNA-based vaccines: Pfizer/BioNTech (Comirnaty) for those ages 12 years and older and Moderna (Spikevax) for those ages 18 and older. A third COVID vaccine option is manufactured by Johnson & Johnson (Janssen) and uses an adenovirus platform. The FDA revised its EUA in May to limit its use.6 The Johnson & Johnson vaccine has been associated with rare but serious reactions called thrombosis with thrombocytopenia. ACIP recommends all other vaccines in preference to the Johnson & Johnson vaccine.
For more on COVID vaccination for patients of all ages, see: www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
1. Oliver S. Evidence to recommendations framework: Bivalent COVID-19 vaccine booster doses. Presented to the Advisory Committee on Immunization Practices, September 1, 2002. Accessed September 6, 2002. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/08-COVID-Oliver-508.pdf
2. FDA. Novavax COVID-19 vaccine, adjuvanted. Updated August 19, 2022. Accessed August 23, 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/novavax-covid-19-vaccine-adjuvanted
3. Dubovsky F. NVX-CoV2373 (Novavax COVID-19 vaccine) in adults (≥ 18 years of age). Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/04-covid-dubovsky-508.pdf
4. Twentyman E. Evidence to recommendation framework: Novavax COVID-19 vaccine, adjuvanted in adults ages 18 years and older. Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/05-covid-twentyman-508.pdf
5. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. Morbid Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
6. American Hospital Association. FDA limits J&J COVID-19 vaccine use to certain adults. Published May 6, 2022. Accessed September 6, 2022. www.aha.org/news/headline/2022-05-06-fda-limits-jj-covid-19-vaccine-use-certain-adults
In recent weeks, the COVID-19 vaccine arsenal has grown more robust. Here’s what you need to know:
Variant-specific boosters. On September 1, the Advisory Committee on Immunization Practices (ACIP) adopted a recommendation for a booster of either a new bivalent Pfizer-BioNTech COVID-19 vaccine (for individuals ages 12 years and older) or bivalent Moderna COVID-19 vaccine (for individuals ages 18 years and older) at least 2 months after receipt of a primary series or prior monovalent booster dose. Both bivalent vaccines were recently approved by the Food and Drug Administration (FDA) under an Emergency Use Authorization (EUA) and offer protection against one of the more common circulating strains of SARS-COV-2 (BA.1) while boosting immunity to the original strain. Both options are approved only as booster shots, not as an original COVID vaccine series.1
Novavax vaccine. This summer, the FDA issued an EUA for the Novavax COVID-19 vaccine in adults and a later EUA for adolescents (ages 12 to 17 years).2 Novavax is the fourth vaccine available to combat COVID-19 infection. This newest addition to the COVID armamentarium consists of coronavirus protein subunits, produced using recombinant technology, and a matrix adjuvant. The primary series consists of 2 doses administered at least 3 weeks apart.3,4
A few caveats: The Novavax vaccine comes in 10-dose vials, which should be kept refrigerated until use. Once the first dose is used, the vial should be discarded after 6 hours. This may present some scheduling and logistical issues. Also, the Novavax vaccine is not currently approved for use in children younger than 12 years, or as a booster to other vaccines.3,4
The effectiveness and safety of the Novavax vaccine appears to be comparable to that of the other vaccines approved to date, although measuring vaccine effectiveness is a tricky business given the rapid mutation of the virus and changing dominant strains.3,4 The Novavax vaccine’s efficacy against currently circulating Omicron variants of the virus (eg, BA.2.12.1, BA.4, BA.5) remains to be determined.
As far as safety, preliminary studies indicate that Novavax may be associated with rare cases of myocarditis.3,4 Myocarditis can result from the COVID infection itself at an overall rate of 1 to 2 per 1000, which is 16 times the rate in adults without COVID.5
Could it provide reassurance to the hesitant? The Novavax COVID vaccine was developed using a vaccine platform and production process similar to that of other commonly administered vaccines, such as hepatitis B vaccine and human papillomavirus vaccine. This may make it an appealing option for patients who have shown hesitancy toward new vaccine technologies.
And, of course, there are the Pfizer and Moderna vaccines. Currently, there are 2 vaccines approved under the normal licensing process for adults, both of which are mRNA-based vaccines: Pfizer/BioNTech (Comirnaty) for those ages 12 years and older and Moderna (Spikevax) for those ages 18 and older. A third COVID vaccine option is manufactured by Johnson & Johnson (Janssen) and uses an adenovirus platform. The FDA revised its EUA in May to limit its use.6 The Johnson & Johnson vaccine has been associated with rare but serious reactions called thrombosis with thrombocytopenia. ACIP recommends all other vaccines in preference to the Johnson & Johnson vaccine.
For more on COVID vaccination for patients of all ages, see: www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
In recent weeks, the COVID-19 vaccine arsenal has grown more robust. Here’s what you need to know:
Variant-specific boosters. On September 1, the Advisory Committee on Immunization Practices (ACIP) adopted a recommendation for a booster of either a new bivalent Pfizer-BioNTech COVID-19 vaccine (for individuals ages 12 years and older) or bivalent Moderna COVID-19 vaccine (for individuals ages 18 years and older) at least 2 months after receipt of a primary series or prior monovalent booster dose. Both bivalent vaccines were recently approved by the Food and Drug Administration (FDA) under an Emergency Use Authorization (EUA) and offer protection against one of the more common circulating strains of SARS-COV-2 (BA.1) while boosting immunity to the original strain. Both options are approved only as booster shots, not as an original COVID vaccine series.1
Novavax vaccine. This summer, the FDA issued an EUA for the Novavax COVID-19 vaccine in adults and a later EUA for adolescents (ages 12 to 17 years).2 Novavax is the fourth vaccine available to combat COVID-19 infection. This newest addition to the COVID armamentarium consists of coronavirus protein subunits, produced using recombinant technology, and a matrix adjuvant. The primary series consists of 2 doses administered at least 3 weeks apart.3,4
A few caveats: The Novavax vaccine comes in 10-dose vials, which should be kept refrigerated until use. Once the first dose is used, the vial should be discarded after 6 hours. This may present some scheduling and logistical issues. Also, the Novavax vaccine is not currently approved for use in children younger than 12 years, or as a booster to other vaccines.3,4
The effectiveness and safety of the Novavax vaccine appears to be comparable to that of the other vaccines approved to date, although measuring vaccine effectiveness is a tricky business given the rapid mutation of the virus and changing dominant strains.3,4 The Novavax vaccine’s efficacy against currently circulating Omicron variants of the virus (eg, BA.2.12.1, BA.4, BA.5) remains to be determined.
As far as safety, preliminary studies indicate that Novavax may be associated with rare cases of myocarditis.3,4 Myocarditis can result from the COVID infection itself at an overall rate of 1 to 2 per 1000, which is 16 times the rate in adults without COVID.5
Could it provide reassurance to the hesitant? The Novavax COVID vaccine was developed using a vaccine platform and production process similar to that of other commonly administered vaccines, such as hepatitis B vaccine and human papillomavirus vaccine. This may make it an appealing option for patients who have shown hesitancy toward new vaccine technologies.
And, of course, there are the Pfizer and Moderna vaccines. Currently, there are 2 vaccines approved under the normal licensing process for adults, both of which are mRNA-based vaccines: Pfizer/BioNTech (Comirnaty) for those ages 12 years and older and Moderna (Spikevax) for those ages 18 and older. A third COVID vaccine option is manufactured by Johnson & Johnson (Janssen) and uses an adenovirus platform. The FDA revised its EUA in May to limit its use.6 The Johnson & Johnson vaccine has been associated with rare but serious reactions called thrombosis with thrombocytopenia. ACIP recommends all other vaccines in preference to the Johnson & Johnson vaccine.
For more on COVID vaccination for patients of all ages, see: www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf
1. Oliver S. Evidence to recommendations framework: Bivalent COVID-19 vaccine booster doses. Presented to the Advisory Committee on Immunization Practices, September 1, 2002. Accessed September 6, 2002. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/08-COVID-Oliver-508.pdf
2. FDA. Novavax COVID-19 vaccine, adjuvanted. Updated August 19, 2022. Accessed August 23, 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/novavax-covid-19-vaccine-adjuvanted
3. Dubovsky F. NVX-CoV2373 (Novavax COVID-19 vaccine) in adults (≥ 18 years of age). Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/04-covid-dubovsky-508.pdf
4. Twentyman E. Evidence to recommendation framework: Novavax COVID-19 vaccine, adjuvanted in adults ages 18 years and older. Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/05-covid-twentyman-508.pdf
5. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. Morbid Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
6. American Hospital Association. FDA limits J&J COVID-19 vaccine use to certain adults. Published May 6, 2022. Accessed September 6, 2022. www.aha.org/news/headline/2022-05-06-fda-limits-jj-covid-19-vaccine-use-certain-adults
1. Oliver S. Evidence to recommendations framework: Bivalent COVID-19 vaccine booster doses. Presented to the Advisory Committee on Immunization Practices, September 1, 2002. Accessed September 6, 2002. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/08-COVID-Oliver-508.pdf
2. FDA. Novavax COVID-19 vaccine, adjuvanted. Updated August 19, 2022. Accessed August 23, 2022. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/novavax-covid-19-vaccine-adjuvanted
3. Dubovsky F. NVX-CoV2373 (Novavax COVID-19 vaccine) in adults (≥ 18 years of age). Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/04-covid-dubovsky-508.pdf
4. Twentyman E. Evidence to recommendation framework: Novavax COVID-19 vaccine, adjuvanted in adults ages 18 years and older. Presented to the Advisory Committee on Immunization Practices, July 19, 2022. Accessed August 17, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/05-covid-twentyman-508.pdf
5. Boehmer TK, Kompaniyets L, Lavery AM, et al. Association between COVID-19 and myocarditis using hospital-based administrative data—United States, March 2020–January 2021. Morbid Mortal Wkly Rep. 2021;70:1228-1232. doi: 10.15585/mmwr.mm7035e5
6. American Hospital Association. FDA limits J&J COVID-19 vaccine use to certain adults. Published May 6, 2022. Accessed September 6, 2022. www.aha.org/news/headline/2022-05-06-fda-limits-jj-covid-19-vaccine-use-certain-adults