User login
For many, long COVID’s impacts go on and on, major study says
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
in the same time frame, a large study out of Scotland found.
Multiple studies are evaluating people with long COVID in the hopes of figuring out why some people experience debilitating symptoms long after their primary infection ends and others either do not or recover more quickly.
This current study is notable for its large size – 96,238 people. Researchers checked in with participants at 6, 12, and 18 months, and included a group of people never infected with the coronavirus to help investigators make a stronger case.
“A lot of the symptoms of long COVID are nonspecific and therefore can occur in people never infected,” says senior study author Jill P. Pell, MD, head of the School of Health and Wellbeing at the University of Glasgow in Scotland.
Ruling out coincidence
This study shows that people experienced a wide range of symptoms after becoming infected with COVID-19 at a significantly higher rate than those who were never infected, “thereby confirming that they were genuinely associated with COVID and not merely a coincidence,” she said.
Among 21,525 people who had COVID-19 and had symptoms, tiredness, headache and muscle aches or muscle weakness were the most common ongoing symptoms.
Loss of smell was almost nine times more likely in this group compared to the never-infected group in one analysis where researchers controlled for other possible factors. The risk for loss of taste was almost six times greater, followed by risk of breathlessness at three times higher.
Long COVID risk was highest after a severe original infection and among older people, women, Black, and South Asian populations, people with socioeconomic disadvantages, and those with more than one underlying health condition.
Adding up the 6% with no recovery after 18 months and 42% with partial recovery means that between 6 and 18 months following symptomatic coronavirus infection, almost half of those infected still experience persistent symptoms.
Vaccination validated
On the plus side, people vaccinated against COVID-19 before getting infected had a lower risk for some persistent symptoms. In addition, Dr. Pell and colleagues found no evidence that people who experienced asymptomatic infection were likely to experience long COVID symptoms or challenges with activities of daily living.
The findings of the Long-COVID in Scotland Study (Long-CISS) were published in the journal Nature Communications.
‘More long COVID than ever before’
“Unfortunately, these long COVID symptoms are not getting better as the cases of COVID get milder,” said Thomas Gut, DO, medical director for the post-COVID recovery program at Staten Island (N.Y.) University Hospital. “Quite the opposite – this infection has become so common in a community because it’s so mild and spreading so rapidly that we’re seeing more long COVID symptoms than ever before.”
Although most patients he sees with long COVID resolve their symptoms within 3-6 months, “We do see some patients who require short-term disability because their symptoms continue past 6 months and out to 2 years,” said Dr. Gut, a hospitalist at Staten Island University Hospital, a member hospital of Northwell Health.
Patients with fatigue and neurocognitive symptoms “have a very tough time going back to work. Short-term disability gives them the time and finances to pursue specialty care with cardiology, pulmonary, and neurocognitive testing,” he said.
Support the whole person
The burden of living with long COVID goes beyond the persistent symptoms. “Long COVID can have wide-ranging impacts – not only on health but also quality of life and activities of daily living [including] work, mobility, self-care and more,” Dr. Pell said. “So, people with long COVID need support relevant to their individual needs and this may extend beyond the health care sector, for example including social services, school or workplace.”
Still, Lisa Penziner, RN, founder of the COVID Long Haulers Support Group in Westchester and Long Island, N.Y., said while people with the most severe cases of COVID-19 tended to have the worst long COVID symptoms, they’re not the only ones.
“We saw many post-COVID members who had mild cases and their long-haul symptoms were worse weeks later than the virus itself,” said Md. Penziner.
She estimates that 80%-90% of her support group members recover within 6 months. “However, there are others who were experiencing symptoms for much longer.”
Respiratory treatment, physical therapy, and other follow-up doctor visits are common after 6 months, for example.
“Additionally, there is a mental health component to recovery as well, meaning that the patient must learn to live while experiencing lingering, long-haul COVID symptoms in work and daily life,” said Ms. Penziner, director of special projects at North Westchester Restorative Therapy & Nursing.
In addition to ongoing medical care, people with long COVID need understanding, she said.
“While long-haul symptoms do not happen to everyone, it is proven that many do experience long-haul symptoms, and the support of the community in understanding is important.”
Limitations of the study
Dr. Pell and colleagues noted some strengths and weaknesses to their study. For example, “as a general population study, our findings provide a better indication of the overall risk and burden of long COVID than hospitalized cohorts,” they noted.
Also, the Scottish population is 96% White, so other long COVID studies with more diverse participants are warranted.
Another potential weakness is the response rate of 16% among those invited to participate in the study, which Dr. Pell and colleagues addressed: “Our cohort included a large sample (33,281) of people previously infected and the response rate of 16% overall and 20% among people who had symptomatic infection was consistent with previous studies that have used SMS text invitations as the sole method of recruitment.”
“We tell patients this should last 3-6 months, but some patients have longer recovery periods,” Dr. Gut said. “We’re here for them. We have a lot of services available to help get them through the recovery process, and we have a lot of options to help support them.”
“What we found most helpful is when there is peer-to-peer support, reaffirming to the member that they are not alone in the long-haul battle, which has been a major benefit of the support group,” Ms. Penziner said.
A version of this article first appeared on WebMD.com.
FROM NATURE COMMUNICATIONS
FDA: Newborns protected by whooping cough vaccine
The Food and Drug Administration has approved a whooping cough vaccine that protects newborns under 2 months of age.
The vaccine, manufactured by GlaxoSmithKline, was previously approved among pregnant people for their own protection.
“Infants younger than 2 months of age are too young to be protected by the childhood pertussis vaccine series,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a press release. “This is the first vaccine approved specifically for use during pregnancy to prevent a disease in young infants whose mothers are vaccinated during pregnancy.”
Pertussis is a highly contagious respiratory tract infection caused by the bacterium Bordetella pertussis. Most cases that result in hospitalizations and death are among infants within 2 months of birth.
The FDA said its decision was based on data from observational studies, which included 108 cases of pertussis in infants younger than 2 months old. According to data evaluated by the agency, the vaccine was 78% effective in preventing whooping cough.
Boostrix is administered as a single 0.5-mL dose.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a whooping cough vaccine that protects newborns under 2 months of age.
The vaccine, manufactured by GlaxoSmithKline, was previously approved among pregnant people for their own protection.
“Infants younger than 2 months of age are too young to be protected by the childhood pertussis vaccine series,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a press release. “This is the first vaccine approved specifically for use during pregnancy to prevent a disease in young infants whose mothers are vaccinated during pregnancy.”
Pertussis is a highly contagious respiratory tract infection caused by the bacterium Bordetella pertussis. Most cases that result in hospitalizations and death are among infants within 2 months of birth.
The FDA said its decision was based on data from observational studies, which included 108 cases of pertussis in infants younger than 2 months old. According to data evaluated by the agency, the vaccine was 78% effective in preventing whooping cough.
Boostrix is administered as a single 0.5-mL dose.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a whooping cough vaccine that protects newborns under 2 months of age.
The vaccine, manufactured by GlaxoSmithKline, was previously approved among pregnant people for their own protection.
“Infants younger than 2 months of age are too young to be protected by the childhood pertussis vaccine series,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a press release. “This is the first vaccine approved specifically for use during pregnancy to prevent a disease in young infants whose mothers are vaccinated during pregnancy.”
Pertussis is a highly contagious respiratory tract infection caused by the bacterium Bordetella pertussis. Most cases that result in hospitalizations and death are among infants within 2 months of birth.
The FDA said its decision was based on data from observational studies, which included 108 cases of pertussis in infants younger than 2 months old. According to data evaluated by the agency, the vaccine was 78% effective in preventing whooping cough.
Boostrix is administered as a single 0.5-mL dose.
A version of this article first appeared on Medscape.com.
HPV infection in pregnancy higher among women living with HIV
Pregnant women living with HIV were more likely to be infected with human papillomavirus (HPV) than were pregnant women without HIV, a recent systematic review and meta-analysis reports.
“High prevalence of HPV was documented in pregnant WLWH [women living with HIV], exceeding the prevalence among pregnant women without HIV,” Elisabeth McClymont, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in the Journal of Acquired Immune Deficiency Syndrome.
Their results contribute to two major global public health goals: eliminating cervical cancer and improving the health outcomes of newborn babies.
“Our findings of a high prevalence of HPV infection during pregnancy in WLWH, particularly of highly oncogenic HPV types, emphasize the need for HPV screening and vaccination in WLWH,” they added. “WLWH are a key population for both HPV and adverse pregnancy outcome prevention.”
Emerging evidence suggests that being infected with HPV during pregnancy may be linked with adverse pregnancy outcomes. Although women living with HIV have higher rates of HPV infection and adverse pregnancy outcomes, no prior reviews have reported on HPV infection during pregnancy in women living with HIV, the authors explained.
A study of studies
Dr. McClymont and colleagues searched the standard medical research databases through Jan. 18, 2022, for pooled and type-specific HPV prevalence and associated pregnancy outcomes among pregnant women living with HIV, including available within-study comparators of women without HIV.
They performed subgroup analyses according to polymerase chain reaction primers used to detect HPV type and according to region (Africa, Asia and Europe, the Americas).
Their analysis of 10 studies describing HPV prevalence in 1,594 pregnant women living with HIV found:
- The pooled HPV prevalence in pregnant women living with HIV was 75.5% (95% confidence interval, 50.2%-90.4%) but ranged from 23% to 98% between individual studies.
- Among the five studies that also analyzed HPV prevalence in pregnant women without HIV, the pooled prevalence was 48.1% (95% CI, 27.1%-69.8%).
- Pregnant women living with HIV had 54% higher odds of being HPV positive than did pregnant women without HIV.
- HPV-16 was the most common HPV type detected in pregnant women living with HIV, followed by HPV-52; other common types included HPV-18 and HPV-58.
- One study provided data on pregnancy outcomes in women living with HIV but did not correlate pregnancy outcomes with HPV status.
Experts urge HPV, cervical cancer screening for women living with HIV
“HPV is a common virus that can lead to cervical dysplasia and cervical cancer,” cautioned Clara Paik, MD, professor and clinic medical director of obstetrics and gynecology at UC Davis Health, Sacramento.
“HPV can also be associated with adverse pregnancy outcomes, including preterm birth and premature membrane rupture,” she said in an interview. “It is important to know the prevalence of HPV infection in pregnant women living with HIV in order to assess if this specific population is at higher risk for adverse pregnancy outcomes.”
Dr. Paik, who was not involved in the study, would like these results to lead to better HPV screening in pregnant women living with HIV.
“The study’s strengths include the large number of women studied when all the research studies were pooled,” she said. “A weakness is that, if individual studies had limitations, a systematic review based on weaker studies may not necessarily yield results that are conclusive.”
Linda Eckert, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, said that the study highlights the importance of including cervical cancer screening in antepartum care, especially in areas of high HIV prevalence.
“Women living with HIV have a sixfold increased rate of developing cervical cancer compared to women without HIV,” she added, citing a 2020 analysis in The Lancet Global Health that estimated global cervical cancer risk among women living with HIV.
“This [new] study allows us to definitively say that pregnant women living with HIV have higher rates of HPV than do pregnant women without HIV,” noted Dr. Eckert, who was not involved in either study. “And HPV type 16 – the HPV type most associated with developing cervical cancer – was the most common high-risk HPV type found in these patients.”
HPV vaccination recommended
The World Health Organization’s call to eliminate cervical cancer has generated interest and funding for cervical cancer screening of women with HIV, Dr. Eckert said. “WHO recommends that women living with HIV who are 25 years of age and above be screened for cervical cancer annually.”
The authors urged that women living with HIV not only be screened for HPV but that they also be vaccinated against HPV.
“We know that HPV vaccination is unprecedented in its ability to prevent HPV infections when it is received prior to acquiring HPV infection,” Dr. Eckert said, “but currently data showing that HPV vaccination would treat HPV16 in pregnant women already infected with HPV16 are lacking.
“This study points to the need for a trial to investigate HPV vaccination in pregnant women living with HIV who have the high-risk HPV types,” she suggested.
Dr. Eckert contributed to the American College of Obstetricians and Gynecologists’ 2020 Human Papillomavirus Vaccination Committee Opinion. One study coauthor reported financial relationships with Merck. Dr. McClymont, the other coauthors, as well as Dr. Paik and Dr. Eckert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women living with HIV were more likely to be infected with human papillomavirus (HPV) than were pregnant women without HIV, a recent systematic review and meta-analysis reports.
“High prevalence of HPV was documented in pregnant WLWH [women living with HIV], exceeding the prevalence among pregnant women without HIV,” Elisabeth McClymont, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in the Journal of Acquired Immune Deficiency Syndrome.
Their results contribute to two major global public health goals: eliminating cervical cancer and improving the health outcomes of newborn babies.
“Our findings of a high prevalence of HPV infection during pregnancy in WLWH, particularly of highly oncogenic HPV types, emphasize the need for HPV screening and vaccination in WLWH,” they added. “WLWH are a key population for both HPV and adverse pregnancy outcome prevention.”
Emerging evidence suggests that being infected with HPV during pregnancy may be linked with adverse pregnancy outcomes. Although women living with HIV have higher rates of HPV infection and adverse pregnancy outcomes, no prior reviews have reported on HPV infection during pregnancy in women living with HIV, the authors explained.
A study of studies
Dr. McClymont and colleagues searched the standard medical research databases through Jan. 18, 2022, for pooled and type-specific HPV prevalence and associated pregnancy outcomes among pregnant women living with HIV, including available within-study comparators of women without HIV.
They performed subgroup analyses according to polymerase chain reaction primers used to detect HPV type and according to region (Africa, Asia and Europe, the Americas).
Their analysis of 10 studies describing HPV prevalence in 1,594 pregnant women living with HIV found:
- The pooled HPV prevalence in pregnant women living with HIV was 75.5% (95% confidence interval, 50.2%-90.4%) but ranged from 23% to 98% between individual studies.
- Among the five studies that also analyzed HPV prevalence in pregnant women without HIV, the pooled prevalence was 48.1% (95% CI, 27.1%-69.8%).
- Pregnant women living with HIV had 54% higher odds of being HPV positive than did pregnant women without HIV.
- HPV-16 was the most common HPV type detected in pregnant women living with HIV, followed by HPV-52; other common types included HPV-18 and HPV-58.
- One study provided data on pregnancy outcomes in women living with HIV but did not correlate pregnancy outcomes with HPV status.
Experts urge HPV, cervical cancer screening for women living with HIV
“HPV is a common virus that can lead to cervical dysplasia and cervical cancer,” cautioned Clara Paik, MD, professor and clinic medical director of obstetrics and gynecology at UC Davis Health, Sacramento.
“HPV can also be associated with adverse pregnancy outcomes, including preterm birth and premature membrane rupture,” she said in an interview. “It is important to know the prevalence of HPV infection in pregnant women living with HIV in order to assess if this specific population is at higher risk for adverse pregnancy outcomes.”
Dr. Paik, who was not involved in the study, would like these results to lead to better HPV screening in pregnant women living with HIV.
“The study’s strengths include the large number of women studied when all the research studies were pooled,” she said. “A weakness is that, if individual studies had limitations, a systematic review based on weaker studies may not necessarily yield results that are conclusive.”
Linda Eckert, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, said that the study highlights the importance of including cervical cancer screening in antepartum care, especially in areas of high HIV prevalence.
“Women living with HIV have a sixfold increased rate of developing cervical cancer compared to women without HIV,” she added, citing a 2020 analysis in The Lancet Global Health that estimated global cervical cancer risk among women living with HIV.
“This [new] study allows us to definitively say that pregnant women living with HIV have higher rates of HPV than do pregnant women without HIV,” noted Dr. Eckert, who was not involved in either study. “And HPV type 16 – the HPV type most associated with developing cervical cancer – was the most common high-risk HPV type found in these patients.”
HPV vaccination recommended
The World Health Organization’s call to eliminate cervical cancer has generated interest and funding for cervical cancer screening of women with HIV, Dr. Eckert said. “WHO recommends that women living with HIV who are 25 years of age and above be screened for cervical cancer annually.”
The authors urged that women living with HIV not only be screened for HPV but that they also be vaccinated against HPV.
“We know that HPV vaccination is unprecedented in its ability to prevent HPV infections when it is received prior to acquiring HPV infection,” Dr. Eckert said, “but currently data showing that HPV vaccination would treat HPV16 in pregnant women already infected with HPV16 are lacking.
“This study points to the need for a trial to investigate HPV vaccination in pregnant women living with HIV who have the high-risk HPV types,” she suggested.
Dr. Eckert contributed to the American College of Obstetricians and Gynecologists’ 2020 Human Papillomavirus Vaccination Committee Opinion. One study coauthor reported financial relationships with Merck. Dr. McClymont, the other coauthors, as well as Dr. Paik and Dr. Eckert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women living with HIV were more likely to be infected with human papillomavirus (HPV) than were pregnant women without HIV, a recent systematic review and meta-analysis reports.
“High prevalence of HPV was documented in pregnant WLWH [women living with HIV], exceeding the prevalence among pregnant women without HIV,” Elisabeth McClymont, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in the Journal of Acquired Immune Deficiency Syndrome.
Their results contribute to two major global public health goals: eliminating cervical cancer and improving the health outcomes of newborn babies.
“Our findings of a high prevalence of HPV infection during pregnancy in WLWH, particularly of highly oncogenic HPV types, emphasize the need for HPV screening and vaccination in WLWH,” they added. “WLWH are a key population for both HPV and adverse pregnancy outcome prevention.”
Emerging evidence suggests that being infected with HPV during pregnancy may be linked with adverse pregnancy outcomes. Although women living with HIV have higher rates of HPV infection and adverse pregnancy outcomes, no prior reviews have reported on HPV infection during pregnancy in women living with HIV, the authors explained.
A study of studies
Dr. McClymont and colleagues searched the standard medical research databases through Jan. 18, 2022, for pooled and type-specific HPV prevalence and associated pregnancy outcomes among pregnant women living with HIV, including available within-study comparators of women without HIV.
They performed subgroup analyses according to polymerase chain reaction primers used to detect HPV type and according to region (Africa, Asia and Europe, the Americas).
Their analysis of 10 studies describing HPV prevalence in 1,594 pregnant women living with HIV found:
- The pooled HPV prevalence in pregnant women living with HIV was 75.5% (95% confidence interval, 50.2%-90.4%) but ranged from 23% to 98% between individual studies.
- Among the five studies that also analyzed HPV prevalence in pregnant women without HIV, the pooled prevalence was 48.1% (95% CI, 27.1%-69.8%).
- Pregnant women living with HIV had 54% higher odds of being HPV positive than did pregnant women without HIV.
- HPV-16 was the most common HPV type detected in pregnant women living with HIV, followed by HPV-52; other common types included HPV-18 and HPV-58.
- One study provided data on pregnancy outcomes in women living with HIV but did not correlate pregnancy outcomes with HPV status.
Experts urge HPV, cervical cancer screening for women living with HIV
“HPV is a common virus that can lead to cervical dysplasia and cervical cancer,” cautioned Clara Paik, MD, professor and clinic medical director of obstetrics and gynecology at UC Davis Health, Sacramento.
“HPV can also be associated with adverse pregnancy outcomes, including preterm birth and premature membrane rupture,” she said in an interview. “It is important to know the prevalence of HPV infection in pregnant women living with HIV in order to assess if this specific population is at higher risk for adverse pregnancy outcomes.”
Dr. Paik, who was not involved in the study, would like these results to lead to better HPV screening in pregnant women living with HIV.
“The study’s strengths include the large number of women studied when all the research studies were pooled,” she said. “A weakness is that, if individual studies had limitations, a systematic review based on weaker studies may not necessarily yield results that are conclusive.”
Linda Eckert, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, said that the study highlights the importance of including cervical cancer screening in antepartum care, especially in areas of high HIV prevalence.
“Women living with HIV have a sixfold increased rate of developing cervical cancer compared to women without HIV,” she added, citing a 2020 analysis in The Lancet Global Health that estimated global cervical cancer risk among women living with HIV.
“This [new] study allows us to definitively say that pregnant women living with HIV have higher rates of HPV than do pregnant women without HIV,” noted Dr. Eckert, who was not involved in either study. “And HPV type 16 – the HPV type most associated with developing cervical cancer – was the most common high-risk HPV type found in these patients.”
HPV vaccination recommended
The World Health Organization’s call to eliminate cervical cancer has generated interest and funding for cervical cancer screening of women with HIV, Dr. Eckert said. “WHO recommends that women living with HIV who are 25 years of age and above be screened for cervical cancer annually.”
The authors urged that women living with HIV not only be screened for HPV but that they also be vaccinated against HPV.
“We know that HPV vaccination is unprecedented in its ability to prevent HPV infections when it is received prior to acquiring HPV infection,” Dr. Eckert said, “but currently data showing that HPV vaccination would treat HPV16 in pregnant women already infected with HPV16 are lacking.
“This study points to the need for a trial to investigate HPV vaccination in pregnant women living with HIV who have the high-risk HPV types,” she suggested.
Dr. Eckert contributed to the American College of Obstetricians and Gynecologists’ 2020 Human Papillomavirus Vaccination Committee Opinion. One study coauthor reported financial relationships with Merck. Dr. McClymont, the other coauthors, as well as Dr. Paik and Dr. Eckert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ACQUIRED IMMUNE DEFICIENCY SYNDROME.
Is another COVID-19 booster really needed?
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Death of son reinforces flu vaccination message
“It was what the CDC [Centers for Disease Control and Prevention] would call classic influenza-like illness,” Dr. Teichman said. “It was too late to start antivirals, so I gave him advice on symptomatic treatment. We texted the next day, and I was glad to hear that his fever was trending down and that he was feeling a little bit better.”
Two days later, his son called again.
“He said he was having trouble breathing, and over the phone I could hear him hyperventilating.” The retired pediatrician and health care executive told his son to seek medical care.
“Then I got the call that no parent wants to get.”
Brent’s cousin Jake called saying he couldn’t wake Brent up.
“I called Jake back a few minutes later and asked him to hold up the phone,” Dr. Teichman said. “I listened to EMS working on my son, calling for round after round of many medications. He was in arrest and they couldn’t revive him.”
“To this day when I close my eyes at night, I still hear the beeping of those monitors.”
Brent had no health conditions to put him at higher risk for complications of the flu. “Brent was a wonderful son, brother, uncle, and friend. He had a passion for everything he did, and that included his chosen calling of the culinary arts but also included University of Kentucky sports,” Dr. Teichman said.
Brent planned to get a flu vaccine but had not done it yet. “In his obituary, we requested that, in lieu of flowers or donations, people go get their flu shot,” Dr. Teichman said.
“I’m here today to put a face on influenza,” Dr. Teichman said at a news briefing Oct. 4 on preventing the flu and pneumococcal disease, sponsored by the National Foundation for Infectious Diseases.
New survey numbers ‘alarming’
The NFID commissioned a national survey of more than 1,000 U.S. adults to better understand their knowledge and attitudes about the flu, pneumococcal disease, vaccines, and the impact of COVID-19.
“We were alarmed to learn that only 49% of U.S. adults plan to get their flu vaccine this season,” said Patricia A. “Patsy” Stinchfield, a registered nurse, NFID president, and moderator of the news briefing. “That is not good enough.”
In addition, 22% of people at higher risk for flu-related complications do not plan to get vaccinated this season. “That’s a dangerous risk to take,” Ms. Stinchfield said.
An encouraging finding, she said, is that 69% of adults surveyed recognize that an annual flu vaccination is the best way to prevent flu-related hospitalizations and death.
“So, most people know what to do. We just need to do it,” she said.
The top reason for not getting a flu shot in 2022 mentioned by 41% of people surveyed, is they do not think vaccines work very well. Another 39% are concerned about vaccine side effects, and 28% skip the vaccine because they “never get the flu.”
The experts on the panel emphasized the recommendation that all Americans 6 months or older get the flu vaccine, preferably by the end of October. Vaccination is especially important for those at higher risk of complications from the flu, including children under 5, pregnant women, people with one or more health conditions, the immunocompromised, and Americans 65 years and older.
Ms. Stinchfield acknowledged that the effectiveness of the flu vaccine varies season to season, but even if the vaccine does not completely match the circulating viruses, it can help prevent serious outcomes like hospitalization and death. One of the serious potential complications is pneumonia or “pneumococcal disease.”
“Our survey shows that only 29% of those at risk have been advised to receive a pneumococcal vaccine,” Ms. Stinchfield said. “The good news is that, among those who were advised to get the vaccine, 74% did receive their pneumococcal vaccine,” she said. “This underscores a key point to you, my fellow clinicians: As health professionals, our recommendations matter.”
Higher doses for 65+ Americans
The CDC updated recommendations this flu season for adults 65 and older to receive one of three preferentially recommended flu vaccines, said CDC Director Rochelle Walensky, MD. The CDC is recommending higher-dose, stronger vaccines for older Americans “based on a review of the available studies, which suggested that in this age group, these vaccines are potentially more effective than standard-dose ... vaccines.”
During most seasons, people 65 and older bear the greatest burden of severe flu disease, accounting for most flu-related hospitalizations and deaths.
“They are the largest vulnerable segment of our society,” Dr. Walensky said.
What will this flu season be like?
Health officials in the flu vaccine business also tend to be in the flu season prediction business. That includes Dr. Walensky.
“While we will never exactly know what each flu season will hold, we do know that every year, the best way you can protect yourself and those around you is to get your annual flu vaccine,” she said while taking part remotely in the briefing.
How severe will the flu season be in 2022-23? William Schaffner, MD, said he gets that question a lot. “Don’t think about that. Just focus on the fact that flu will be with us each year.
“We were a little bit spoiled. We’ve had two mild influenza seasons,” said Dr. Schaffner, medical director of NFID and a professor of infectious diseases and preventive medicine at Vanderbilt University, Nashville, Tenn. “I think with all the interest in COVID, people have rather forgotten about influenza. I’ve had to remind them that this is yet another serious winter respiratory virus.
“As I like to say, flu is fickle. It’s difficult to predict how serious this next outbreak of influenza this season is going to be. We could look at what happened in the Southern Hemisphere,” he said.
For example, Australia had the worst influenza season in the past 5 years, Schaffner said. “If you want a hint of what might happen here and you want yet another reason to be vaccinated, there it is.”
What we do know, Dr. Walensky said, is that the timing and severity of the past two flu seasons in the U.S. have been different than typical flu seasons. “And this is likely due to the COVID mitigation measures and other changes in circulating respiratory viruses.” Also, although last flu season was “relatively mild,” there was more flu activity than in the prior, 2020-21 season.
Also, Dr. Walensky said, last season’s flu cases began to increase in November and remained elevated until mid-June, “making it the latest season on record.”
The official cause of Brent Teichman’s death was multilobar pneumonia, cause undetermined. “But after 30-plus years as a pediatrician ... I know influenza when I see it,” Dr. Teichman said.
“There’s a hole in our hearts that will never heal. Loss of a child is devastating,” he said. The flu “can take the life of a healthy young person, as it did to my son.
“And for all those listening to my story who are vaccine hesitant, do it for those who love you. So that they won’t walk the path that we and many other families in this country have walked.”
To prove their point, Dr. Teichman and Ms. Stinchfield raised their sleeves and received flu shots during the news briefing.
“This one is for Brent,” Dr. Teichman said.
A version of this article first appeared on WebMD.com.
“It was what the CDC [Centers for Disease Control and Prevention] would call classic influenza-like illness,” Dr. Teichman said. “It was too late to start antivirals, so I gave him advice on symptomatic treatment. We texted the next day, and I was glad to hear that his fever was trending down and that he was feeling a little bit better.”
Two days later, his son called again.
“He said he was having trouble breathing, and over the phone I could hear him hyperventilating.” The retired pediatrician and health care executive told his son to seek medical care.
“Then I got the call that no parent wants to get.”
Brent’s cousin Jake called saying he couldn’t wake Brent up.
“I called Jake back a few minutes later and asked him to hold up the phone,” Dr. Teichman said. “I listened to EMS working on my son, calling for round after round of many medications. He was in arrest and they couldn’t revive him.”
“To this day when I close my eyes at night, I still hear the beeping of those monitors.”
Brent had no health conditions to put him at higher risk for complications of the flu. “Brent was a wonderful son, brother, uncle, and friend. He had a passion for everything he did, and that included his chosen calling of the culinary arts but also included University of Kentucky sports,” Dr. Teichman said.
Brent planned to get a flu vaccine but had not done it yet. “In his obituary, we requested that, in lieu of flowers or donations, people go get their flu shot,” Dr. Teichman said.
“I’m here today to put a face on influenza,” Dr. Teichman said at a news briefing Oct. 4 on preventing the flu and pneumococcal disease, sponsored by the National Foundation for Infectious Diseases.
New survey numbers ‘alarming’
The NFID commissioned a national survey of more than 1,000 U.S. adults to better understand their knowledge and attitudes about the flu, pneumococcal disease, vaccines, and the impact of COVID-19.
“We were alarmed to learn that only 49% of U.S. adults plan to get their flu vaccine this season,” said Patricia A. “Patsy” Stinchfield, a registered nurse, NFID president, and moderator of the news briefing. “That is not good enough.”
In addition, 22% of people at higher risk for flu-related complications do not plan to get vaccinated this season. “That’s a dangerous risk to take,” Ms. Stinchfield said.
An encouraging finding, she said, is that 69% of adults surveyed recognize that an annual flu vaccination is the best way to prevent flu-related hospitalizations and death.
“So, most people know what to do. We just need to do it,” she said.
The top reason for not getting a flu shot in 2022 mentioned by 41% of people surveyed, is they do not think vaccines work very well. Another 39% are concerned about vaccine side effects, and 28% skip the vaccine because they “never get the flu.”
The experts on the panel emphasized the recommendation that all Americans 6 months or older get the flu vaccine, preferably by the end of October. Vaccination is especially important for those at higher risk of complications from the flu, including children under 5, pregnant women, people with one or more health conditions, the immunocompromised, and Americans 65 years and older.
Ms. Stinchfield acknowledged that the effectiveness of the flu vaccine varies season to season, but even if the vaccine does not completely match the circulating viruses, it can help prevent serious outcomes like hospitalization and death. One of the serious potential complications is pneumonia or “pneumococcal disease.”
“Our survey shows that only 29% of those at risk have been advised to receive a pneumococcal vaccine,” Ms. Stinchfield said. “The good news is that, among those who were advised to get the vaccine, 74% did receive their pneumococcal vaccine,” she said. “This underscores a key point to you, my fellow clinicians: As health professionals, our recommendations matter.”
Higher doses for 65+ Americans
The CDC updated recommendations this flu season for adults 65 and older to receive one of three preferentially recommended flu vaccines, said CDC Director Rochelle Walensky, MD. The CDC is recommending higher-dose, stronger vaccines for older Americans “based on a review of the available studies, which suggested that in this age group, these vaccines are potentially more effective than standard-dose ... vaccines.”
During most seasons, people 65 and older bear the greatest burden of severe flu disease, accounting for most flu-related hospitalizations and deaths.
“They are the largest vulnerable segment of our society,” Dr. Walensky said.
What will this flu season be like?
Health officials in the flu vaccine business also tend to be in the flu season prediction business. That includes Dr. Walensky.
“While we will never exactly know what each flu season will hold, we do know that every year, the best way you can protect yourself and those around you is to get your annual flu vaccine,” she said while taking part remotely in the briefing.
How severe will the flu season be in 2022-23? William Schaffner, MD, said he gets that question a lot. “Don’t think about that. Just focus on the fact that flu will be with us each year.
“We were a little bit spoiled. We’ve had two mild influenza seasons,” said Dr. Schaffner, medical director of NFID and a professor of infectious diseases and preventive medicine at Vanderbilt University, Nashville, Tenn. “I think with all the interest in COVID, people have rather forgotten about influenza. I’ve had to remind them that this is yet another serious winter respiratory virus.
“As I like to say, flu is fickle. It’s difficult to predict how serious this next outbreak of influenza this season is going to be. We could look at what happened in the Southern Hemisphere,” he said.
For example, Australia had the worst influenza season in the past 5 years, Schaffner said. “If you want a hint of what might happen here and you want yet another reason to be vaccinated, there it is.”
What we do know, Dr. Walensky said, is that the timing and severity of the past two flu seasons in the U.S. have been different than typical flu seasons. “And this is likely due to the COVID mitigation measures and other changes in circulating respiratory viruses.” Also, although last flu season was “relatively mild,” there was more flu activity than in the prior, 2020-21 season.
Also, Dr. Walensky said, last season’s flu cases began to increase in November and remained elevated until mid-June, “making it the latest season on record.”
The official cause of Brent Teichman’s death was multilobar pneumonia, cause undetermined. “But after 30-plus years as a pediatrician ... I know influenza when I see it,” Dr. Teichman said.
“There’s a hole in our hearts that will never heal. Loss of a child is devastating,” he said. The flu “can take the life of a healthy young person, as it did to my son.
“And for all those listening to my story who are vaccine hesitant, do it for those who love you. So that they won’t walk the path that we and many other families in this country have walked.”
To prove their point, Dr. Teichman and Ms. Stinchfield raised their sleeves and received flu shots during the news briefing.
“This one is for Brent,” Dr. Teichman said.
A version of this article first appeared on WebMD.com.
“It was what the CDC [Centers for Disease Control and Prevention] would call classic influenza-like illness,” Dr. Teichman said. “It was too late to start antivirals, so I gave him advice on symptomatic treatment. We texted the next day, and I was glad to hear that his fever was trending down and that he was feeling a little bit better.”
Two days later, his son called again.
“He said he was having trouble breathing, and over the phone I could hear him hyperventilating.” The retired pediatrician and health care executive told his son to seek medical care.
“Then I got the call that no parent wants to get.”
Brent’s cousin Jake called saying he couldn’t wake Brent up.
“I called Jake back a few minutes later and asked him to hold up the phone,” Dr. Teichman said. “I listened to EMS working on my son, calling for round after round of many medications. He was in arrest and they couldn’t revive him.”
“To this day when I close my eyes at night, I still hear the beeping of those monitors.”
Brent had no health conditions to put him at higher risk for complications of the flu. “Brent was a wonderful son, brother, uncle, and friend. He had a passion for everything he did, and that included his chosen calling of the culinary arts but also included University of Kentucky sports,” Dr. Teichman said.
Brent planned to get a flu vaccine but had not done it yet. “In his obituary, we requested that, in lieu of flowers or donations, people go get their flu shot,” Dr. Teichman said.
“I’m here today to put a face on influenza,” Dr. Teichman said at a news briefing Oct. 4 on preventing the flu and pneumococcal disease, sponsored by the National Foundation for Infectious Diseases.
New survey numbers ‘alarming’
The NFID commissioned a national survey of more than 1,000 U.S. adults to better understand their knowledge and attitudes about the flu, pneumococcal disease, vaccines, and the impact of COVID-19.
“We were alarmed to learn that only 49% of U.S. adults plan to get their flu vaccine this season,” said Patricia A. “Patsy” Stinchfield, a registered nurse, NFID president, and moderator of the news briefing. “That is not good enough.”
In addition, 22% of people at higher risk for flu-related complications do not plan to get vaccinated this season. “That’s a dangerous risk to take,” Ms. Stinchfield said.
An encouraging finding, she said, is that 69% of adults surveyed recognize that an annual flu vaccination is the best way to prevent flu-related hospitalizations and death.
“So, most people know what to do. We just need to do it,” she said.
The top reason for not getting a flu shot in 2022 mentioned by 41% of people surveyed, is they do not think vaccines work very well. Another 39% are concerned about vaccine side effects, and 28% skip the vaccine because they “never get the flu.”
The experts on the panel emphasized the recommendation that all Americans 6 months or older get the flu vaccine, preferably by the end of October. Vaccination is especially important for those at higher risk of complications from the flu, including children under 5, pregnant women, people with one or more health conditions, the immunocompromised, and Americans 65 years and older.
Ms. Stinchfield acknowledged that the effectiveness of the flu vaccine varies season to season, but even if the vaccine does not completely match the circulating viruses, it can help prevent serious outcomes like hospitalization and death. One of the serious potential complications is pneumonia or “pneumococcal disease.”
“Our survey shows that only 29% of those at risk have been advised to receive a pneumococcal vaccine,” Ms. Stinchfield said. “The good news is that, among those who were advised to get the vaccine, 74% did receive their pneumococcal vaccine,” she said. “This underscores a key point to you, my fellow clinicians: As health professionals, our recommendations matter.”
Higher doses for 65+ Americans
The CDC updated recommendations this flu season for adults 65 and older to receive one of three preferentially recommended flu vaccines, said CDC Director Rochelle Walensky, MD. The CDC is recommending higher-dose, stronger vaccines for older Americans “based on a review of the available studies, which suggested that in this age group, these vaccines are potentially more effective than standard-dose ... vaccines.”
During most seasons, people 65 and older bear the greatest burden of severe flu disease, accounting for most flu-related hospitalizations and deaths.
“They are the largest vulnerable segment of our society,” Dr. Walensky said.
What will this flu season be like?
Health officials in the flu vaccine business also tend to be in the flu season prediction business. That includes Dr. Walensky.
“While we will never exactly know what each flu season will hold, we do know that every year, the best way you can protect yourself and those around you is to get your annual flu vaccine,” she said while taking part remotely in the briefing.
How severe will the flu season be in 2022-23? William Schaffner, MD, said he gets that question a lot. “Don’t think about that. Just focus on the fact that flu will be with us each year.
“We were a little bit spoiled. We’ve had two mild influenza seasons,” said Dr. Schaffner, medical director of NFID and a professor of infectious diseases and preventive medicine at Vanderbilt University, Nashville, Tenn. “I think with all the interest in COVID, people have rather forgotten about influenza. I’ve had to remind them that this is yet another serious winter respiratory virus.
“As I like to say, flu is fickle. It’s difficult to predict how serious this next outbreak of influenza this season is going to be. We could look at what happened in the Southern Hemisphere,” he said.
For example, Australia had the worst influenza season in the past 5 years, Schaffner said. “If you want a hint of what might happen here and you want yet another reason to be vaccinated, there it is.”
What we do know, Dr. Walensky said, is that the timing and severity of the past two flu seasons in the U.S. have been different than typical flu seasons. “And this is likely due to the COVID mitigation measures and other changes in circulating respiratory viruses.” Also, although last flu season was “relatively mild,” there was more flu activity than in the prior, 2020-21 season.
Also, Dr. Walensky said, last season’s flu cases began to increase in November and remained elevated until mid-June, “making it the latest season on record.”
The official cause of Brent Teichman’s death was multilobar pneumonia, cause undetermined. “But after 30-plus years as a pediatrician ... I know influenza when I see it,” Dr. Teichman said.
“There’s a hole in our hearts that will never heal. Loss of a child is devastating,” he said. The flu “can take the life of a healthy young person, as it did to my son.
“And for all those listening to my story who are vaccine hesitant, do it for those who love you. So that they won’t walk the path that we and many other families in this country have walked.”
To prove their point, Dr. Teichman and Ms. Stinchfield raised their sleeves and received flu shots during the news briefing.
“This one is for Brent,” Dr. Teichman said.
A version of this article first appeared on WebMD.com.
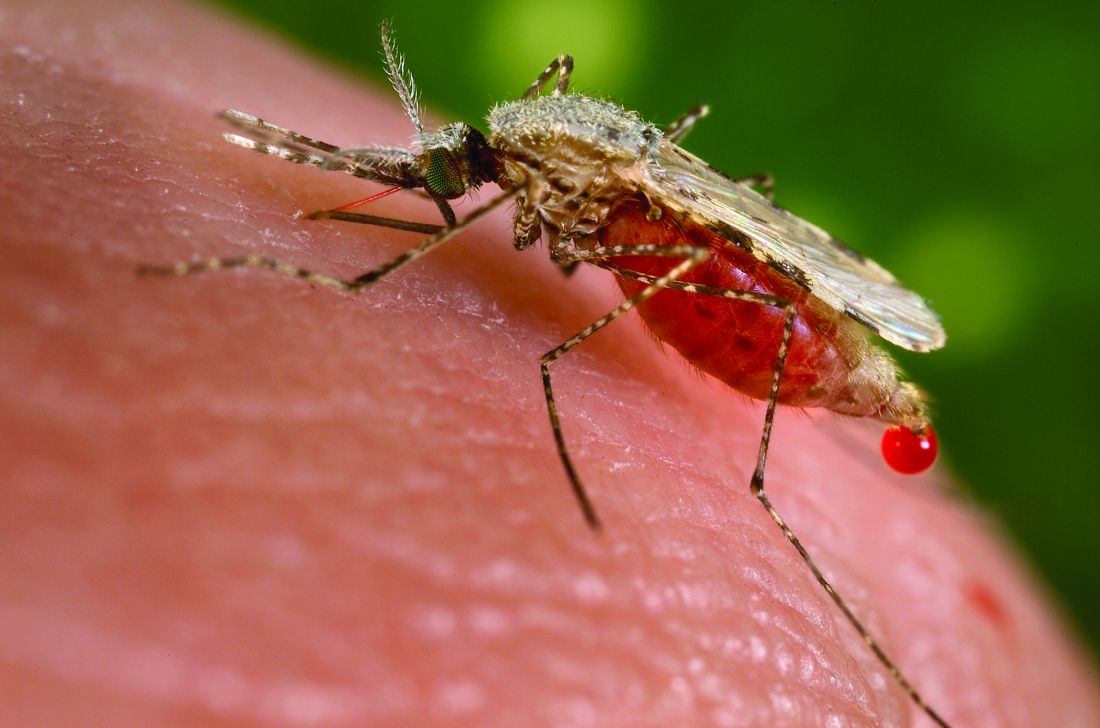
Malaria vaccine gets special delivery by tiny health personnel
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Don’t like needles? Have we got a vaccine for you
Here’s a quick question: How do you turn the most annoying thing ever into something positive?
No, we’re not talking about politicians this time. No, not Elon Musk, either. Infomercials? Guess again. Humidity? Nope, even more annoying than that.
Give up? The most annoying thing ever is mosquitoes. This time, however, NPR reports that mosquitoes have been used to deliver a vaccine for the very disease they’ve been transmitting to their human food sources all these years.
In a recent proof-of-concept trial, investigators used CRISPR technology to genetically modify malaria-causing Plasmodium falciparum sporozoites, which just happen to live in the salivary glands of Anopheles mosquitoes. And since the Plasmodium parasites are already in the mosquitoes, it made sense to use the buzzy little critters as the delivery device for the vaccine.
More sense than a syringe, you ask? Have you ever tried to poke a syringe into the salivary gland of a mosquito? No, we thought not. Well, we can tell you from experience that it’s really, really hard. Never mind how we know. We just do.
The 14 study volunteers – who were paid $4,100 for their participation – were first exposed to hundreds of mosquitoes carrying the altered Plasmodium parasites. Then, to test the vaccine, they were exposed to mosquitoes that had actual, malaria-carrying Plasmodium. Half of the subjects got malaria, so the vaccine was only 50% effective, meaning there’s still work to do.
Meanwhile, the scientists here at LOTMEco are all over this mosquito-delivery business, working on a vaccine to prevent Elon Musk. Plan B involves some sort of really big swatter.
Climate change: Sleeping your life away
It’s no secret that climate change is raising the temperature on everything. You may think you’re getting relief when the sun goes down, but in some places it’s still hot. A new survey conducted in central Japan shows how bad it can be and how higher nighttime temperatures can have a serious impact on people’s health.
That online survey, the Sleep Quality Index for Daily Sleep, enabled the investigators to correlate sleep quality with daily temperature for 1,284 adults in 2011 and 2012 who completed the survey over 10 days.
Not only was there a significant difference in sleep disturbance among younger men (higher) versus older men, but the prevalence of sleep disturbance went up when the daytime temperature was above 24.8° C. They also found that disability-adjusted life-years (DALYs), which measure time lost through premature death and time lived in certain conditions that put one’s health at risk, were 81.8 years for the city of Nagoya (population, 2.2 million) in 2012.
The damage to health from sleep disorders caused by daily temperatures higher than 25° C “is comparable to that of heatstroke and must be addressed,” lead author Tomohiko Ihara of the University of Tokyo said in a written statement.
The researchers hope that this information will help sway legislators to consider the impact of higher nighttime temperatures and that it can be used to provide guidance for better sleep. The solution for now? Sleep with the air conditioner on. Your energy bill might increase, but just think about those DALYs. If using the AC lowers DALYs and increases time lived, then we say it’s worth it.
Maybe it would have been a dragon WITH cancer
If you ask a random person on the street to tell you all they know about the country of Wales, they’ll probably mention two things: One, the contorted collection of jumbled-up letters that is the Welsh language (looking at you, Llanfairpwllgwyngyllgogerychwyrndrobwllllantysiliogogogoch) and, two, the association with dragons. The Welsh flag even has a dragon on it.
With that in mind, take a guess as to what sort of statue art dealer Simon Wingett wanted to build in the Welsh town of Wrexham. No, not a monument to the second-longest place name in the world. Try again. His dragon would not be some piddly little thing either; he wanted a virtual kaiju overlooking the town, with the whole statue to stand about 60 meters high. That’s taller than the original 1954 Godzilla.
Artistic masterpieces may sell for frankly insane prices, but art dealers themselves are not the wealthiest of individuals, so Mr. Wingett needed money to fund his dragon-based dream. Lucky for him, he also happened to be the manager of a cancer charity – initially set up by Mr. Wingett’s father, who had throat cancer – which nominally aimed to provide equipment and resources to cancer patients in the Wrexham area.
Yes, this is going precisely where you think it’s going. From 2011 to 2018, when the charity closed, Mr. Wingett used the charity’s donations to fund his dragon statue – which never actually got built, by the way – to the tune of over 400,000 pounds. Of course, Mr. Wingett came under scrutiny when people started to notice that his cancer charity hadn’t actually done anything charitable since 2011, and he was recently banned by the Welsh High Court from serving as trustee of any charity for 10 years. Oh no, tragedy and horror! Truly a punishment worse than death itself.
Okay fine, he also has to pay back 117,000 pounds to actual legitimate cancer charities. The astute mathematicians out there may notice that 117,000 is a lot less than 400,000. But it’s just as the old saying goes: One-quarter of crime doesn’t pay. You can keep three-quarters of it, though, that’s completely fine.
Breakthrough COVID studies lend support to use of new boosters in immunosuppressed patients
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
People with immune-mediated inflammatory diseases who are taking immunosuppressants don’t mount as strong of an immune defense against the Omicron variant as they did against the original SARS-CoV-2 wild-type virus, according to two studies published in Annals of the Rheumatic Diseases. One of the studies further showed that vaccinated individuals taking immunosuppressants have poorer cross-neutralizing responses to Omicron than do healthy vaccinated individuals, even after three doses of the COVID-19 mRNA vaccines.
“We carefully suggest that if Omicron-specific vaccination can be administered, it may be an effective way to reduce the risk of breakthrough infections in patients with autoimmune rheumatic disease,” Sang Tae Choi, MD, PhD, of the University College of Medicine, Seoul, Korea, and one of the authors of the study on cross-neutralizing protection, told this news organization. “However, further research is needed on Omicron-specific vaccine effectiveness in patients with immune dysfunctions. We believe that these study results can be of great benefit in determining the strategy of vaccination in the future.”
The earlier study, published in July, examined the ability of COVID-19 vaccines to induce cross-reactive antibody responses against Omicron infections in patients with autoimmune rheumatic diseases (ARDs). The observational study involved 149 patients with ARDs and 94 health care workers as controls, all of whom provided blood samples a median 15 weeks after their second COVID vaccine dose or a median 8 weeks after their third dose. A little more than two-thirds of the patients (68.5%) had received a third mRNA vaccine dose. None of the participants previously had COVID-19.
The researchers compared the rate of breakthrough infections with the Omicron variant to the neutralizing responses in patients’ blood, specifically the cross-neutralizing antibody responses because the original mRNA vaccines targeted a different variant than Omicron. Breakthrough infections were assessed by survey questions.
“Our findings suggested that neither primary series vaccinations nor booster doses are sufficient to induce Omicron-neutralizing responses above the threshold in patients with ARDs, although responses were noticeably increased following the third dose of an mRNA vaccine,” write Woo-Joong Kim, of the Chung-Ang University College of Medicine, Seoul, Korea, and his colleagues. “This impairment of cross-neutralization responses across most of our patients contrasts starkly with a potent elicitation of the Omicron-neutralizing responses after the third vaccination in healthy recipients.”
The average neutralizing responses against the original SARS-CoV-2 strain were similar in both groups: 76% in patients with ARDs and 72% in health care workers after the second dose. The mean response after a third dose was 97% in health care workers and 88% in patients.
The average cross-neutralizing response against the Omicron variant was far lower, particularly in those with rheumatic disease: only 11.5%, which rose to 27% after the third dose. Only 39% of the patient sera showed neutralization of Omicron, even after the third dose. Meanwhile, the mean cross-neutralizing response in health care workers was 18% after the second dose and 50% after the third.
When the researchers compared seropositivity rates against the original virus to neutralizing responses against Omicron, the association between these was stronger in health care workers than in those with ARDs. In fact, among patients with ARDs who seroconverted, only 41% showed any response against Omicron. Among all the patients, most of those who didn’t respond to Omicron (93.5%) had initially seroconverted.
The researchers also looked at the ability to neutralize Omicron on the basis of disease in those who received three doses of the vaccine. About half of those with lupus (52%) showed any neutralization against Omicron, compared with 25% of those with rheumatoid arthritis, 37.5% of those with ankylosing spondylitis, 33% of those with Behçet snydrome, and all of those with adult-onset Still’s disease.
The rate of breakthrough infections was lower in patients (19%) than in health care workers (33%). A similar pattern was seen in the more recent study published Sept. 5. Researchers used data from a prospective cohort study in the Netherlands to examine incidence and severity of Omicron breakthrough infections in patients with immune-mediated inflammatory diseases. The researchers compared infection rates and severity among 1,593 vaccinated patients with inflammatory disease who were taking immunosuppressants and 579 vaccinated controls (418 patients with inflammatory disease not on immunosuppressants and 161 healthy controls).
One in five patients with inflammatory disease (21%) were taking immunosuppressants that substantially impair antibodies, such as anti-CD20 therapy, S1P modulators, or mycophenolate mofetil combination therapy, and 48% of these patients seroconverted after primary vaccination, compared with 96% of patients taking other immunosuppressants and 98% of controls.
Breakthrough infection rates were similar between the control group (31%) and those taking immunosuppressants (30%). Only three participants had severe disease requiring hospitalization: one control and two patients taking immunosuppressants.
“In both studies, the controls had similar or higher rates of breakthrough infections, compared with the immunosuppressed,” noted Alfred Kim, MD, an assistant professor of medicine at Washington University, St Louis, but he added, “one has to consider differences in mitigation strategies, such as masking, that may explain these findings.” That is, patients taking immunosuppressants may be taking fewer risks in the community or have fewer potential exposures, especially in the Korean study, wherein the controls were health care workers.
A greater disparity in infections occurred when considering seroconversion rates. Breakthrough incidence was 38% among those taking immunosuppressants who did not seroconvert, compared with 29% among those who did. A similar trend was seen in breakthrough incidence between those taking strongly antibody-impairing immunosuppressants (36% breakthrough rate) and those taking other immunosuppressants (28%).
Among those taking immunosuppressants who seroconverted, a primary series of vaccination reduced the risk of a breakthrough infection by 29%. Protection became more robust with a booster or prior infection, both of which reduced breakthrough infection risk by 39% in those taking immunosuppressants who seroconverted.
“We demonstrate in patients with immune-mediated inflammatory diseases on immunosuppressants that additional vaccinations are associated with decreased risk of SARS-CoV-2 Omicron breakthrough infections,” wrote Eileen W. Stalman, MD, PhD, of Amsterdam UMC in the Netherlands, and her colleagues.
Though neither study broke down immune response or breakthrough infection based on individual medications, Kim said that previous research allows one to extrapolate “that prior culprits of poor vaccine responses [such as B-cell depleting drugs, mycophenolate, and TNF [tumor necrosis factor] inhibitors will continue to bear the greatest burden in breakthrough infection, including Omicron.”
Overall, he found the data from both studies relatively consistent with one another.
“Those on immunosuppression, particularly mechanisms that have been established as risk factors for poor vaccine responses, are at risk of breakthrough infection during the era of Omicron,” Dr. Kim said.
The earlier study from Korea also found that “the median time between the third-dose vaccination and the date of confirmed breakthrough infection in patients with ARDs was significantly shorter, compared with that in health care workers” at just 93 days in patients versus 122 days in health care workers. They postulated that this population’s limited neutralization of Omicron explained this short-lived protection.
Most of the patients with breakthrough infections (74%) in that study showed no neutralization against Omicron, including the only two hospitalized patients, both of whom had strong responses against the original SARS-CoV-2 strain. The significant decline over time of neutralization against Omicron suggested “the potential for a substantial loss of the protection from breakthrough infection,” the authors write.
“The third dose of an mRNA vaccine could improve the cross-neutralization of the SARS-CoV-2 Omicron variant in patients with autoimmune rheumatic disease [although] more than half of the patients failed to generate Omicron-neutralizing antibodies,” Tae Choi said in an interview. “Our study sheds light on the relative deficiency of the Omicron-specific neutralizing responses in patients with autoimmune rheumatic disease and their anticipated vulnerability to breakthrough infection.”
The message for clinicians, Dr. Kim said, is to “continue to urge our patients to maintain additional and boosting doses per guidance, use pre-exposure prophylaxis such as Evusheld, and continue other mitigation strategies as they have done.”
The Dutch study was funded by The Netherlands Organization for Health Research and Development; the Korean study used no external funding.
The authors of the Korean study had no disclosures. The Dutch study’s authors reported a wide range of disclosures involving more than a dozen pharmaceutical companies but not including Pfizer or Moderna. Dr. Kim’s industry disclosures include Alexion, ANI, AstraZeneca, Aurinia, Exagen, Foghorn Therapeutics, GlaxoSmithKline, Kypha, and Pfizer.
A version of this article first appeared on Medscape.com.
Meet the JCOM Author with Dr. Barkoudah: Improving Inpatient COVID-19 Vaccination Rates
How well are we doing with adolescent vaccination?
Every year, the Centers for Disease Control and Prevention (CDC) conducts a national survey to provide an estimate of vaccination rates among adolescents ages 13 to 17 years. The results for 2021, published recently, illustrate the progress that we’ve made and the areas in which improvement is still needed; notably, human papillomavirus (HPV) vaccine is an example of both.1
First, what’s recommended? The CDC recommends the following vaccines at age 11 to 12 years: tetanus, diphtheria, and acellular pertussis vaccine (Tdap); HPV vaccine series (2 doses if the first dose is received prior to age 15 years; 3 doses if the first dose is received at age 15 years or older); and quadrivalent meningococcal conjugate vaccine (MenACWY). A second (booster) dose of MenACWY is recommended at age 16 years. Adolescents should also receive an annual influenza vaccine and a COVID-19 vaccine series.2
For adolescents not fully vaccinated in childhood, catch-up vaccination is recommended for hepatitis A (HepA); hepatitis B (HepB); measles, mumps, and rubella (MMR); and varicella (VAR).2
How are we doing? In 2021, 89.6% of adolescents had received ≥ 1 Tdap dose and 89.0% had received ≥ 1 MenACWY dose; both these rates remained stable from the year before. For HPV vaccine, 76.9% had received ≥ 1 dose (an increase of 1.8 percentage points from 2020); 61.7% were HPV vaccine “up to date” (an increase of 3.1 percentage points). The teen HPV vaccination rate has increased slowly but progressively since the first recommendation for routine HPV vaccination was made for females in 2006 and for males in 2011.1
Among those age 17 years, coverage with ≥ 2 MenACWY doses was 60.0% (an increase of 5.6 percentage points from 2020). Coverage was 85% for ≥ 2 HepA doses (an increase of 2.9 percentage points from 2020) and remained stable at > 90% for each of the following: ≥ 2 doses of MMR, ≥ 3 doses of HepB, and both VAR doses.1
Keeping the momentum. As a country, we continue to make progress at increasing vaccination rates among US adolescents—but there is still plenty of room for improvement. Family physicians should check vaccine status at each clinical encounter and encourage parents and caregivers to schedule future wellness and vaccine visits for these young patients. This may be especially important among adolescents who were due for and missed a vaccination during the COVID-19 pandemic.
1. Pingali C, Yankey D, Elam-Evans LD, et al. National vaccination coverage among adolescents aged 13-17 years—National Immunization Survey-Teen, United States, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:1101-1108.
2. Wodi AP, Murthy N, Bernstein H, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:234-237.
Every year, the Centers for Disease Control and Prevention (CDC) conducts a national survey to provide an estimate of vaccination rates among adolescents ages 13 to 17 years. The results for 2021, published recently, illustrate the progress that we’ve made and the areas in which improvement is still needed; notably, human papillomavirus (HPV) vaccine is an example of both.1
First, what’s recommended? The CDC recommends the following vaccines at age 11 to 12 years: tetanus, diphtheria, and acellular pertussis vaccine (Tdap); HPV vaccine series (2 doses if the first dose is received prior to age 15 years; 3 doses if the first dose is received at age 15 years or older); and quadrivalent meningococcal conjugate vaccine (MenACWY). A second (booster) dose of MenACWY is recommended at age 16 years. Adolescents should also receive an annual influenza vaccine and a COVID-19 vaccine series.2
For adolescents not fully vaccinated in childhood, catch-up vaccination is recommended for hepatitis A (HepA); hepatitis B (HepB); measles, mumps, and rubella (MMR); and varicella (VAR).2
How are we doing? In 2021, 89.6% of adolescents had received ≥ 1 Tdap dose and 89.0% had received ≥ 1 MenACWY dose; both these rates remained stable from the year before. For HPV vaccine, 76.9% had received ≥ 1 dose (an increase of 1.8 percentage points from 2020); 61.7% were HPV vaccine “up to date” (an increase of 3.1 percentage points). The teen HPV vaccination rate has increased slowly but progressively since the first recommendation for routine HPV vaccination was made for females in 2006 and for males in 2011.1
Among those age 17 years, coverage with ≥ 2 MenACWY doses was 60.0% (an increase of 5.6 percentage points from 2020). Coverage was 85% for ≥ 2 HepA doses (an increase of 2.9 percentage points from 2020) and remained stable at > 90% for each of the following: ≥ 2 doses of MMR, ≥ 3 doses of HepB, and both VAR doses.1
Keeping the momentum. As a country, we continue to make progress at increasing vaccination rates among US adolescents—but there is still plenty of room for improvement. Family physicians should check vaccine status at each clinical encounter and encourage parents and caregivers to schedule future wellness and vaccine visits for these young patients. This may be especially important among adolescents who were due for and missed a vaccination during the COVID-19 pandemic.
Every year, the Centers for Disease Control and Prevention (CDC) conducts a national survey to provide an estimate of vaccination rates among adolescents ages 13 to 17 years. The results for 2021, published recently, illustrate the progress that we’ve made and the areas in which improvement is still needed; notably, human papillomavirus (HPV) vaccine is an example of both.1
First, what’s recommended? The CDC recommends the following vaccines at age 11 to 12 years: tetanus, diphtheria, and acellular pertussis vaccine (Tdap); HPV vaccine series (2 doses if the first dose is received prior to age 15 years; 3 doses if the first dose is received at age 15 years or older); and quadrivalent meningococcal conjugate vaccine (MenACWY). A second (booster) dose of MenACWY is recommended at age 16 years. Adolescents should also receive an annual influenza vaccine and a COVID-19 vaccine series.2
For adolescents not fully vaccinated in childhood, catch-up vaccination is recommended for hepatitis A (HepA); hepatitis B (HepB); measles, mumps, and rubella (MMR); and varicella (VAR).2
How are we doing? In 2021, 89.6% of adolescents had received ≥ 1 Tdap dose and 89.0% had received ≥ 1 MenACWY dose; both these rates remained stable from the year before. For HPV vaccine, 76.9% had received ≥ 1 dose (an increase of 1.8 percentage points from 2020); 61.7% were HPV vaccine “up to date” (an increase of 3.1 percentage points). The teen HPV vaccination rate has increased slowly but progressively since the first recommendation for routine HPV vaccination was made for females in 2006 and for males in 2011.1
Among those age 17 years, coverage with ≥ 2 MenACWY doses was 60.0% (an increase of 5.6 percentage points from 2020). Coverage was 85% for ≥ 2 HepA doses (an increase of 2.9 percentage points from 2020) and remained stable at > 90% for each of the following: ≥ 2 doses of MMR, ≥ 3 doses of HepB, and both VAR doses.1
Keeping the momentum. As a country, we continue to make progress at increasing vaccination rates among US adolescents—but there is still plenty of room for improvement. Family physicians should check vaccine status at each clinical encounter and encourage parents and caregivers to schedule future wellness and vaccine visits for these young patients. This may be especially important among adolescents who were due for and missed a vaccination during the COVID-19 pandemic.
1. Pingali C, Yankey D, Elam-Evans LD, et al. National vaccination coverage among adolescents aged 13-17 years—National Immunization Survey-Teen, United States, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:1101-1108.
2. Wodi AP, Murthy N, Bernstein H, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:234-237.
1. Pingali C, Yankey D, Elam-Evans LD, et al. National vaccination coverage among adolescents aged 13-17 years—National Immunization Survey-Teen, United States, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:1101-1108.
2. Wodi AP, Murthy N, Bernstein H, et al. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:234-237.
Can we eliminate measles and rubella worldwide?
A study in The Lancet Global Health takes a pessimistic view of our ability to eradicate measles by 2100, although rubella forecasts look a bit more promising.
So far, measles has been eliminated in 81 countries and rubella in 93. But factors such as antivaccination sentiment and misinformation linking vaccination to autism have led to occasional outbreaks. In addition, because the COVID-19 pandemic fueled lower routine vaccination coverage and postponed public health campaigns, some countries have also lost previously gained ground.
The study, which is slated for publication in the Oct. 1 issue of the Lancet Global Health, explored the likelihood of eliminating measles and rubella, based on vaccination strategies in 93 countries with the highest measles and rubella burden, under two vaccination scenarios: 1) a “business as usual” approach, that is, continuing current vaccination coverage via routine childhood immunization schedules and intermittent vaccination campaigns that target age groups to vaccinate quickly (known as SIAs); and 2) an “intensified investment approach” that scales up SIA vaccination coverage into the future.
Both vaccination scenarios were evaluated within the context of two national models (Johns Hopkins University and Public Health England), and one subnational model (Nigeria) for rubella transmission.
Lead author Amy Winter, PhD, assistant professor of epidemiology and biostatistics, University of Georgia College of Public Health, Athens, told this news organization that “under the intensified investment scenario, rubella elimination is likely to be achieved in all 93 countries that were modeled [but] measles elimination is likely in some but not all countries.”
This is especially the case if the goal is cessation of vaccination campaigns, study authors noted when placing the research in context.
But Dr. Winter also emphasized that Nigeria offered specific lessons not seen in the national models.
For one,
In addition, she stressed a need to improve vaccine equity by focusing on areas with really low coverage and then moving into areas with higher coverage.
“The Nigerian subnational analysis definitely illustrates the importance of achieving equitable vaccination and the need for potentially targeted strategies to improve vaccination,” she said. “The initial focus should be on getting areas with low coverage up to par.”
Still, “even with the intensified investment approach, we won’t be able to eradicate measles,” William Moss, MD, professor of epidemiology and executive director, International Vaccine Access Center, Johns Hopkins University, Baltimore, who was not directly involved in the study, told this news organization.
Pandemic interruptions, future strategies
In a related editorial (The Lancet Global Health. 2022 Oct 1. doi: 10.1016/S2214-109X[22]00388-6), the authors noted that COVID-19 has markedly disrupted vaccination campaigns globally.
In 2017, 118 (61%) countries achieved the Global Vaccine Action Plan 2020 target of 90% or more national MCV1 (first dose of measles vaccine) coverage. Since that time, measles coverage has declined from 84%-85% in 2017 to 81% in 2021, leaving 24.7 million completely unprotected (also known as zero-dose children) and 14.7 million children underimmunized (that is, recipients of only 1 dose).
Notably, this is the lowest immunization level since 2008, with more than 5 million more children missing their first measles dose.
Dr. Moss has previously written on the biological feasibility of measles eradication and said that it’s not tenable to rely on increased vaccination coverage alone.
We need “new tools and the new strategies. One of the ones that we’re most excited about [is] microarray patches,” he said, noting that they are thermostable and can be administered by anyone.
Dr. Moss also said that, while he is hoping for point-of-care rapid diagnostics, the focus of the efforts needs to change.
“Where’s [the] measles virus coming from? Where’s it being exported from and where is it being imported to?” he posited, adding that the focus should be on these areas “to try to shut down transmission … a radical kind of second phase of a measles eradication puts aside equity and focuses on sources and sinks.”
In the interim, rubella elimination looks promising.
“It’s not as contagious [as measles] and has a lower sort of herd immunity threshold because of it,” Dr. Winter said.
Dr. Winter and Dr. Moss report no relevant financial relationships. The study was funded by the World Health Organization, Gavi, the Vaccine Alliance, the Centers for Disease Control and Prevention, and the Bill & Melinda Gates Foundation.
A version of this article first appeared on Medscape.com.
A study in The Lancet Global Health takes a pessimistic view of our ability to eradicate measles by 2100, although rubella forecasts look a bit more promising.
So far, measles has been eliminated in 81 countries and rubella in 93. But factors such as antivaccination sentiment and misinformation linking vaccination to autism have led to occasional outbreaks. In addition, because the COVID-19 pandemic fueled lower routine vaccination coverage and postponed public health campaigns, some countries have also lost previously gained ground.
The study, which is slated for publication in the Oct. 1 issue of the Lancet Global Health, explored the likelihood of eliminating measles and rubella, based on vaccination strategies in 93 countries with the highest measles and rubella burden, under two vaccination scenarios: 1) a “business as usual” approach, that is, continuing current vaccination coverage via routine childhood immunization schedules and intermittent vaccination campaigns that target age groups to vaccinate quickly (known as SIAs); and 2) an “intensified investment approach” that scales up SIA vaccination coverage into the future.
Both vaccination scenarios were evaluated within the context of two national models (Johns Hopkins University and Public Health England), and one subnational model (Nigeria) for rubella transmission.
Lead author Amy Winter, PhD, assistant professor of epidemiology and biostatistics, University of Georgia College of Public Health, Athens, told this news organization that “under the intensified investment scenario, rubella elimination is likely to be achieved in all 93 countries that were modeled [but] measles elimination is likely in some but not all countries.”
This is especially the case if the goal is cessation of vaccination campaigns, study authors noted when placing the research in context.
But Dr. Winter also emphasized that Nigeria offered specific lessons not seen in the national models.
For one,
In addition, she stressed a need to improve vaccine equity by focusing on areas with really low coverage and then moving into areas with higher coverage.
“The Nigerian subnational analysis definitely illustrates the importance of achieving equitable vaccination and the need for potentially targeted strategies to improve vaccination,” she said. “The initial focus should be on getting areas with low coverage up to par.”
Still, “even with the intensified investment approach, we won’t be able to eradicate measles,” William Moss, MD, professor of epidemiology and executive director, International Vaccine Access Center, Johns Hopkins University, Baltimore, who was not directly involved in the study, told this news organization.
Pandemic interruptions, future strategies
In a related editorial (The Lancet Global Health. 2022 Oct 1. doi: 10.1016/S2214-109X[22]00388-6), the authors noted that COVID-19 has markedly disrupted vaccination campaigns globally.
In 2017, 118 (61%) countries achieved the Global Vaccine Action Plan 2020 target of 90% or more national MCV1 (first dose of measles vaccine) coverage. Since that time, measles coverage has declined from 84%-85% in 2017 to 81% in 2021, leaving 24.7 million completely unprotected (also known as zero-dose children) and 14.7 million children underimmunized (that is, recipients of only 1 dose).
Notably, this is the lowest immunization level since 2008, with more than 5 million more children missing their first measles dose.
Dr. Moss has previously written on the biological feasibility of measles eradication and said that it’s not tenable to rely on increased vaccination coverage alone.
We need “new tools and the new strategies. One of the ones that we’re most excited about [is] microarray patches,” he said, noting that they are thermostable and can be administered by anyone.
Dr. Moss also said that, while he is hoping for point-of-care rapid diagnostics, the focus of the efforts needs to change.
“Where’s [the] measles virus coming from? Where’s it being exported from and where is it being imported to?” he posited, adding that the focus should be on these areas “to try to shut down transmission … a radical kind of second phase of a measles eradication puts aside equity and focuses on sources and sinks.”
In the interim, rubella elimination looks promising.
“It’s not as contagious [as measles] and has a lower sort of herd immunity threshold because of it,” Dr. Winter said.
Dr. Winter and Dr. Moss report no relevant financial relationships. The study was funded by the World Health Organization, Gavi, the Vaccine Alliance, the Centers for Disease Control and Prevention, and the Bill & Melinda Gates Foundation.
A version of this article first appeared on Medscape.com.
A study in The Lancet Global Health takes a pessimistic view of our ability to eradicate measles by 2100, although rubella forecasts look a bit more promising.
So far, measles has been eliminated in 81 countries and rubella in 93. But factors such as antivaccination sentiment and misinformation linking vaccination to autism have led to occasional outbreaks. In addition, because the COVID-19 pandemic fueled lower routine vaccination coverage and postponed public health campaigns, some countries have also lost previously gained ground.
The study, which is slated for publication in the Oct. 1 issue of the Lancet Global Health, explored the likelihood of eliminating measles and rubella, based on vaccination strategies in 93 countries with the highest measles and rubella burden, under two vaccination scenarios: 1) a “business as usual” approach, that is, continuing current vaccination coverage via routine childhood immunization schedules and intermittent vaccination campaigns that target age groups to vaccinate quickly (known as SIAs); and 2) an “intensified investment approach” that scales up SIA vaccination coverage into the future.
Both vaccination scenarios were evaluated within the context of two national models (Johns Hopkins University and Public Health England), and one subnational model (Nigeria) for rubella transmission.
Lead author Amy Winter, PhD, assistant professor of epidemiology and biostatistics, University of Georgia College of Public Health, Athens, told this news organization that “under the intensified investment scenario, rubella elimination is likely to be achieved in all 93 countries that were modeled [but] measles elimination is likely in some but not all countries.”
This is especially the case if the goal is cessation of vaccination campaigns, study authors noted when placing the research in context.
But Dr. Winter also emphasized that Nigeria offered specific lessons not seen in the national models.
For one,
In addition, she stressed a need to improve vaccine equity by focusing on areas with really low coverage and then moving into areas with higher coverage.
“The Nigerian subnational analysis definitely illustrates the importance of achieving equitable vaccination and the need for potentially targeted strategies to improve vaccination,” she said. “The initial focus should be on getting areas with low coverage up to par.”
Still, “even with the intensified investment approach, we won’t be able to eradicate measles,” William Moss, MD, professor of epidemiology and executive director, International Vaccine Access Center, Johns Hopkins University, Baltimore, who was not directly involved in the study, told this news organization.
Pandemic interruptions, future strategies
In a related editorial (The Lancet Global Health. 2022 Oct 1. doi: 10.1016/S2214-109X[22]00388-6), the authors noted that COVID-19 has markedly disrupted vaccination campaigns globally.
In 2017, 118 (61%) countries achieved the Global Vaccine Action Plan 2020 target of 90% or more national MCV1 (first dose of measles vaccine) coverage. Since that time, measles coverage has declined from 84%-85% in 2017 to 81% in 2021, leaving 24.7 million completely unprotected (also known as zero-dose children) and 14.7 million children underimmunized (that is, recipients of only 1 dose).
Notably, this is the lowest immunization level since 2008, with more than 5 million more children missing their first measles dose.
Dr. Moss has previously written on the biological feasibility of measles eradication and said that it’s not tenable to rely on increased vaccination coverage alone.
We need “new tools and the new strategies. One of the ones that we’re most excited about [is] microarray patches,” he said, noting that they are thermostable and can be administered by anyone.
Dr. Moss also said that, while he is hoping for point-of-care rapid diagnostics, the focus of the efforts needs to change.
“Where’s [the] measles virus coming from? Where’s it being exported from and where is it being imported to?” he posited, adding that the focus should be on these areas “to try to shut down transmission … a radical kind of second phase of a measles eradication puts aside equity and focuses on sources and sinks.”
In the interim, rubella elimination looks promising.
“It’s not as contagious [as measles] and has a lower sort of herd immunity threshold because of it,” Dr. Winter said.
Dr. Winter and Dr. Moss report no relevant financial relationships. The study was funded by the World Health Organization, Gavi, the Vaccine Alliance, the Centers for Disease Control and Prevention, and the Bill & Melinda Gates Foundation.
A version of this article first appeared on Medscape.com.
FROM THE LANCET GLOBAL HEALTH