User login
Diabetic dyslipidemia with eruptive xanthoma
A workup for secondary causes of hypertriglyceridemia was negative for hypothyroidism and nephrotic syndrome. She was currently taking no medications. She had no family history of dyslipidemia, and she denied alcohol consumption.
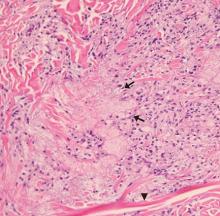
Based on the patient’s presentation, history, and the results of laboratory testing and skin biopsy, the diagnosis was eruptive xanthoma.
A RESULT OF ELEVATED TRIGLYCERIDES
Eruptive xanthoma is associated with elevation of chylomicrons and triglycerides.1 Hyperlipidemia that causes eruptive xanthoma may be familial (ie, due to a primary genetic defect) or secondary to another disease, or both.
Types of primary hypertriglyceridemia include elevated chylomicrons (Frederickson classification type I), elevated very-low-density lipoprotein (VLDL) (Frederickson type IV), and elevation of both chylomicrons and VLDL (Frederickson type V).2,3 Hypertriglyceridemia may also be secondary to obesity, diabetes mellitus, hypothyroidism, nephrotic syndrome, liver cirrhosis, excess ethanol ingestion, and medicines such as retinoids and estrogens.2,3
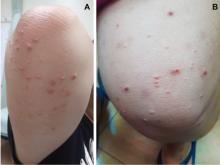
Lesions of eruptive xanthoma are yellowish papules 2 to 5 mm in diameter surrounded by an erythematous border. They are formed by clusters of foamy cells caused by phagocytosis of macrophages as a consequence of increased accumulations of intracellular lipids. The most common sites are the buttocks, extensor surfaces of the arms, and the back.4
Eruptive xanthoma occurs with markedly elevated triglyceride levels (ie, > 1,000 mg/dL),5 with an estimated prevalence of 18 cases per 100,000 people (< 0.02%).6 Diagnosis is usually established through the clinical history, physical examination, and prompt laboratory confirmation of hypertriglyceridemia. Skin biopsy is rarely if ever needed.
RECOGNIZE AND TREAT PROMPTLY TO AVOID FURTHER COMPLICATIONS
Severe hypertriglyceridemia poses an increased risk of acute pancreatitis. Early recognition and medical treatment in our patient prevented serious complications.
Treatment of eruptive xanthoma includes identifying the underlying cause of hypertriglyceridemia and commencing lifestyle modifications that include weight reduction, aerobic exercise, a strict low-fat diet with avoidance of simple carbohydrates and alcohol,7 and drug therapy.
The patient’s treatment plan
Although HMG-CoA reductase inhibitors (statins) have a modest triglyceride-lowering effect and are useful to modify cardiovascular risk, fibric acid derivatives (eg, gemfibrozil, fenofibrate) are the first-line therapy.8 Omega-3 fatty acids, statins, or niacin may be added if necessary.8
Our patient’s uncontrolled glycemia caused marked hypertriglyceridemia, perhaps from a decrease in lipoprotein lipase activity in adipose tissue and muscle. Lifestyle modifications, glucose-lowering agents (metformin, glimepiride), and fenofibrate were prescribed. She was also advised to seek medical attention if she developed upper-abdominal pain, which could be a symptom of pancreatitis.
- Flynn PD, Burns T, Breathnach S, Cox N, Griffiths C. Xanthomas and abnormalities of lipid metabolism and storage. In: Rook’s Textbook of Dermatology. 8th ed. Oxford: Blackwell Science; 2010.
- Breckenridge WC, Alaupovic P, Cox DW, Little JA. Apolipoprotein and lipoprotein concentrations in familial apolipoprotein C-II deficiency. Atherosclerosis 1982; 44(2):223–235. pmid:7138621
- Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 1998; 27(3):551–567. pmid:9785052
- Melmed S, Polonsky KS, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Philadelphia: Elsevier; 2016.
- Zak A, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014; 158(2):181–188. doi:10.5507/bp.2014.016
- Leaf DA. Chylomicronemia and the chylomicronemia syndrome: a practical approach to management. Am J Med 2008; 121(1):10–12. doi:10.1016/j.amjmed.2007.10.004
- Hegele RA, Ginsberg HN, Chapman MJ, et al; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014; 2(8):655–666. doi:10.1016/S2213-8587(13)70191-8
- Berglund L, Brunzell JD, Goldberg AC, et al; Endocrine Society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(9):2969–2989. doi:10.1210/jc.2011-3213
A workup for secondary causes of hypertriglyceridemia was negative for hypothyroidism and nephrotic syndrome. She was currently taking no medications. She had no family history of dyslipidemia, and she denied alcohol consumption.
Based on the patient’s presentation, history, and the results of laboratory testing and skin biopsy, the diagnosis was eruptive xanthoma.
A RESULT OF ELEVATED TRIGLYCERIDES
Eruptive xanthoma is associated with elevation of chylomicrons and triglycerides.1 Hyperlipidemia that causes eruptive xanthoma may be familial (ie, due to a primary genetic defect) or secondary to another disease, or both.
Types of primary hypertriglyceridemia include elevated chylomicrons (Frederickson classification type I), elevated very-low-density lipoprotein (VLDL) (Frederickson type IV), and elevation of both chylomicrons and VLDL (Frederickson type V).2,3 Hypertriglyceridemia may also be secondary to obesity, diabetes mellitus, hypothyroidism, nephrotic syndrome, liver cirrhosis, excess ethanol ingestion, and medicines such as retinoids and estrogens.2,3
Lesions of eruptive xanthoma are yellowish papules 2 to 5 mm in diameter surrounded by an erythematous border. They are formed by clusters of foamy cells caused by phagocytosis of macrophages as a consequence of increased accumulations of intracellular lipids. The most common sites are the buttocks, extensor surfaces of the arms, and the back.4
Eruptive xanthoma occurs with markedly elevated triglyceride levels (ie, > 1,000 mg/dL),5 with an estimated prevalence of 18 cases per 100,000 people (< 0.02%).6 Diagnosis is usually established through the clinical history, physical examination, and prompt laboratory confirmation of hypertriglyceridemia. Skin biopsy is rarely if ever needed.
RECOGNIZE AND TREAT PROMPTLY TO AVOID FURTHER COMPLICATIONS
Severe hypertriglyceridemia poses an increased risk of acute pancreatitis. Early recognition and medical treatment in our patient prevented serious complications.
Treatment of eruptive xanthoma includes identifying the underlying cause of hypertriglyceridemia and commencing lifestyle modifications that include weight reduction, aerobic exercise, a strict low-fat diet with avoidance of simple carbohydrates and alcohol,7 and drug therapy.
The patient’s treatment plan
Although HMG-CoA reductase inhibitors (statins) have a modest triglyceride-lowering effect and are useful to modify cardiovascular risk, fibric acid derivatives (eg, gemfibrozil, fenofibrate) are the first-line therapy.8 Omega-3 fatty acids, statins, or niacin may be added if necessary.8
Our patient’s uncontrolled glycemia caused marked hypertriglyceridemia, perhaps from a decrease in lipoprotein lipase activity in adipose tissue and muscle. Lifestyle modifications, glucose-lowering agents (metformin, glimepiride), and fenofibrate were prescribed. She was also advised to seek medical attention if she developed upper-abdominal pain, which could be a symptom of pancreatitis.
A workup for secondary causes of hypertriglyceridemia was negative for hypothyroidism and nephrotic syndrome. She was currently taking no medications. She had no family history of dyslipidemia, and she denied alcohol consumption.
Based on the patient’s presentation, history, and the results of laboratory testing and skin biopsy, the diagnosis was eruptive xanthoma.
A RESULT OF ELEVATED TRIGLYCERIDES
Eruptive xanthoma is associated with elevation of chylomicrons and triglycerides.1 Hyperlipidemia that causes eruptive xanthoma may be familial (ie, due to a primary genetic defect) or secondary to another disease, or both.
Types of primary hypertriglyceridemia include elevated chylomicrons (Frederickson classification type I), elevated very-low-density lipoprotein (VLDL) (Frederickson type IV), and elevation of both chylomicrons and VLDL (Frederickson type V).2,3 Hypertriglyceridemia may also be secondary to obesity, diabetes mellitus, hypothyroidism, nephrotic syndrome, liver cirrhosis, excess ethanol ingestion, and medicines such as retinoids and estrogens.2,3
Lesions of eruptive xanthoma are yellowish papules 2 to 5 mm in diameter surrounded by an erythematous border. They are formed by clusters of foamy cells caused by phagocytosis of macrophages as a consequence of increased accumulations of intracellular lipids. The most common sites are the buttocks, extensor surfaces of the arms, and the back.4
Eruptive xanthoma occurs with markedly elevated triglyceride levels (ie, > 1,000 mg/dL),5 with an estimated prevalence of 18 cases per 100,000 people (< 0.02%).6 Diagnosis is usually established through the clinical history, physical examination, and prompt laboratory confirmation of hypertriglyceridemia. Skin biopsy is rarely if ever needed.
RECOGNIZE AND TREAT PROMPTLY TO AVOID FURTHER COMPLICATIONS
Severe hypertriglyceridemia poses an increased risk of acute pancreatitis. Early recognition and medical treatment in our patient prevented serious complications.
Treatment of eruptive xanthoma includes identifying the underlying cause of hypertriglyceridemia and commencing lifestyle modifications that include weight reduction, aerobic exercise, a strict low-fat diet with avoidance of simple carbohydrates and alcohol,7 and drug therapy.
The patient’s treatment plan
Although HMG-CoA reductase inhibitors (statins) have a modest triglyceride-lowering effect and are useful to modify cardiovascular risk, fibric acid derivatives (eg, gemfibrozil, fenofibrate) are the first-line therapy.8 Omega-3 fatty acids, statins, or niacin may be added if necessary.8
Our patient’s uncontrolled glycemia caused marked hypertriglyceridemia, perhaps from a decrease in lipoprotein lipase activity in adipose tissue and muscle. Lifestyle modifications, glucose-lowering agents (metformin, glimepiride), and fenofibrate were prescribed. She was also advised to seek medical attention if she developed upper-abdominal pain, which could be a symptom of pancreatitis.
- Flynn PD, Burns T, Breathnach S, Cox N, Griffiths C. Xanthomas and abnormalities of lipid metabolism and storage. In: Rook’s Textbook of Dermatology. 8th ed. Oxford: Blackwell Science; 2010.
- Breckenridge WC, Alaupovic P, Cox DW, Little JA. Apolipoprotein and lipoprotein concentrations in familial apolipoprotein C-II deficiency. Atherosclerosis 1982; 44(2):223–235. pmid:7138621
- Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 1998; 27(3):551–567. pmid:9785052
- Melmed S, Polonsky KS, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Philadelphia: Elsevier; 2016.
- Zak A, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014; 158(2):181–188. doi:10.5507/bp.2014.016
- Leaf DA. Chylomicronemia and the chylomicronemia syndrome: a practical approach to management. Am J Med 2008; 121(1):10–12. doi:10.1016/j.amjmed.2007.10.004
- Hegele RA, Ginsberg HN, Chapman MJ, et al; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014; 2(8):655–666. doi:10.1016/S2213-8587(13)70191-8
- Berglund L, Brunzell JD, Goldberg AC, et al; Endocrine Society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(9):2969–2989. doi:10.1210/jc.2011-3213
- Flynn PD, Burns T, Breathnach S, Cox N, Griffiths C. Xanthomas and abnormalities of lipid metabolism and storage. In: Rook’s Textbook of Dermatology. 8th ed. Oxford: Blackwell Science; 2010.
- Breckenridge WC, Alaupovic P, Cox DW, Little JA. Apolipoprotein and lipoprotein concentrations in familial apolipoprotein C-II deficiency. Atherosclerosis 1982; 44(2):223–235. pmid:7138621
- Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 1998; 27(3):551–567. pmid:9785052
- Melmed S, Polonsky KS, Larsen PR, Kronenberg H. Williams Textbook of Endocrinology. 13th ed. Philadelphia: Elsevier; 2016.
- Zak A, Zeman M, Slaby A, Vecka M. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014; 158(2):181–188. doi:10.5507/bp.2014.016
- Leaf DA. Chylomicronemia and the chylomicronemia syndrome: a practical approach to management. Am J Med 2008; 121(1):10–12. doi:10.1016/j.amjmed.2007.10.004
- Hegele RA, Ginsberg HN, Chapman MJ, et al; European Atherosclerosis Society Consensus Panel. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014; 2(8):655–666. doi:10.1016/S2213-8587(13)70191-8
- Berglund L, Brunzell JD, Goldberg AC, et al; Endocrine Society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97(9):2969–2989. doi:10.1210/jc.2011-3213
Click for Credit: Fasting rules for surgery; Biomarkers for PSA vs OA; more
Here are 5 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. No birth rate gains from levothyroxine in pregnancy
To take the posttest, go to: https://bit.ly/2ZoXzK8
Expires March 23, 2020
2. Simple screening for risk of falling in elderly can guide prevention
To take the posttest, go to: https://bit.ly/2NKXxu3
Expires March 24, 2020
3. Time to revisit fasting rules for surgery patients
To take the posttest, go to: https://bit.ly/2HHwHiD
Expires March 26, 2020
4. Four biomarkers could distinguish psoriatic arthritis from osteoarthritis
To take the posttest, go to: https://bit.ly/344WPNS
Expires March 28, 2020
5. More chest compression–only CPR leads to increased survival rates
To take the posttest, go to: https://bit.ly/30CahGF
Expires April 1, 2020
Here are 5 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. No birth rate gains from levothyroxine in pregnancy
To take the posttest, go to: https://bit.ly/2ZoXzK8
Expires March 23, 2020
2. Simple screening for risk of falling in elderly can guide prevention
To take the posttest, go to: https://bit.ly/2NKXxu3
Expires March 24, 2020
3. Time to revisit fasting rules for surgery patients
To take the posttest, go to: https://bit.ly/2HHwHiD
Expires March 26, 2020
4. Four biomarkers could distinguish psoriatic arthritis from osteoarthritis
To take the posttest, go to: https://bit.ly/344WPNS
Expires March 28, 2020
5. More chest compression–only CPR leads to increased survival rates
To take the posttest, go to: https://bit.ly/30CahGF
Expires April 1, 2020
Here are 5 articles from the September issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. No birth rate gains from levothyroxine in pregnancy
To take the posttest, go to: https://bit.ly/2ZoXzK8
Expires March 23, 2020
2. Simple screening for risk of falling in elderly can guide prevention
To take the posttest, go to: https://bit.ly/2NKXxu3
Expires March 24, 2020
3. Time to revisit fasting rules for surgery patients
To take the posttest, go to: https://bit.ly/2HHwHiD
Expires March 26, 2020
4. Four biomarkers could distinguish psoriatic arthritis from osteoarthritis
To take the posttest, go to: https://bit.ly/344WPNS
Expires March 28, 2020
5. More chest compression–only CPR leads to increased survival rates
To take the posttest, go to: https://bit.ly/30CahGF
Expires April 1, 2020
ACIP issues 2 new recs on HPV vaccination
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
References
1. Markowitz L. Overview and background (HPV). CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-2-Markowitz-508.pdf. Presented February 27, 2019. Accessed August 1, 2019.
2. Brisson M, Laprise J-F. Cost-effectiveness of extending HPV vaccination above age 26 years in the U.S. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-3-Brisson-508.pdf. Presented February 2019. Accessed August 1, 2019.
3. Markowitz L. Recommendations for mid-adult HPV vaccination work group considerations. CDC Web site. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/HPV-7-Markowitz-508.pdf, Presented February 27, 2019. Accessed August 1, 2019.
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Zoledronate maintains bone loss after denosumab discontinuation
Women with postmenopausal osteoporosis who discontinued denosumab treatment after achieving osteopenia maintained bone mineral density at the spine and hip with a single infusion of zoledronate given 6 months after the last infusion of denosumab, according to results from a small, multicenter, randomized trial published in the Journal of Bone and Mineral Research.
The cessation of the monoclonal antibody denosumab is typically followed by a “rebound phenomenon” often attributed to an increase in bone turnover above pretreatment values caused by the up-regulation of osteoclastogenesis, according to Athanasios D. Anastasilakis, MD, of 424 General Military Hospital, Thessaloníki, Greece, and colleagues. Guidelines recommend that patients take a bisphosphonate to prevent this effect, but the optimal bisphosphonate regimen is unknown and evidence is inconsistent.
To address this question, the investigators randomized 57 postmenopausal women with osteoporosis who had received six monthly injections of denosumab (for an average of 2.2 years) and had achieved nonosteoporotic bone mineral density (BMD) T scores greater than –2.5 but no greater than –1 at the hip or the spine. A total of 27 received a single IV infusion of zoledronate 5 mg given 6 months after the last denosumab injection with a 3-week window, and 30 continued denosumab and received two additional monthly 60-mg injections. Following either the zoledronate infusion or the last denosumab injection, all women received no treatment and were followed until 2 years from randomization. All women were given vitamin D supplements and were seen in clinic appointments at baseline, 6, 12, 15, 18, and 24 months.
Areal BMD of the lumbar spine and femoral neck of the nondominant hip were measured at baseline, 12, and 24 months by dual-energy x-ray absorptiometry, and least significant changes were 5% or less at the spine and 4% or less at the femoral neck, based on proposals from the International Foundation for Osteoporosis and the National Osteoporosis Foundation USA.
At 24 months, lumbar spine BMD (LS‐BMD) returned to baseline in the zoledronate group, but decreased in the denosumab group by 4.82% from the 12‐month value (P less than .001).
The difference in LS-BMD changes between the two groups from month 12 to 24, the primary endpoint of the study, was statistically significant (–0.018 with zoledronate vs. –0.045 with denosumab; P = .025). Differences in changes of femoral neck BMD were also statistically significant (–0.004 with zoledronate vs. –0.038 with denosumab; P = .005), the researchers reported.
The differences in BMD changes between the two groups 24 and 12 months after discontinuation of denosumab (6 months after the last injection) for the zoledronate and denosumab group respectively were also statistically significant both at the lumbar spine (–0.002 with zoledronate vs. –0.045 with denosumab; P = .03) and at the femoral neck (–0.004 with zoledronate vs. –0.038 with denosumab; P = .007).
The authors observed no relationship between the number of denosumab injections and LS-BMD changes in either group of women; however, they noted that responses of individual patients to zoledronate were variable. For example, three women who took zoledronate experienced decreases of LS-BMD greater than the least significant change observed at 24 months, a finding which could not be explained by the timing of the infusion, baseline rate of bone turnover, or baseline BMD.
“It appears that intrinsic factors that still need to be defined may affect the response of a few individuals,” they wrote.
This was further illustrated by one patient in the zoledronate group who sustained clinical vertebral fractures associated with significant, unexplained decreases of BMD that could not be prevented with the zoledronate infusion.
“In clinical practice, it is, therefore, advisable to measure BMD at 12 months after the zoledronate infusion and decide whether additional treatment may be required,” the authors wrote.
Another significant finding reported by the authors was that neither baseline nor 12‐month bone turnover marker (BTM) values were associated with BMD changes in either group of women during the entire study period.
“Particularly important for clinical practice was the lack of a relationship in zoledronate-treated women; even when women were divided according to baseline median BTM values (below or above) there were no significant difference in BMD changes at 12 or 24 months,” they wrote.
“In a substantial number of women in the denosumab group BTMs were still above the upper limit of normal of the postmenopausal age 18 months after the last Dmab [denosumab] injection but also in 7.4% of patients treated with zoledronate at 2 years,” they added.
“Whether in the latter patients BTMs were also increased before the start of Dmab treatment, as it is known to occur in some patients with osteoporosis, or are due to a prolonged effect of Dmab withdrawal on bone metabolism could not be prevented by zoledronate, is not known because pretreatment data were not available,” the study authors noted.
For adverse events, in addition to the one patient in the zoledronate group with clinical vertebral fractures, three patients in the denosumab group sustained vertebral fractures.
“Prevalent vertebral fractures have been previously reported as the most important risk factor for clinical vertebral fractures following cessation of Dmab therapy [which] strongly suggest that spine x-rays should be performed in all patients in whom discontinuation of Dmab treatment is considered,” the authors wrote.
“In most women with postmenopausal osteoporosis treated with [denosumab] in whom discontinuation of treatment is considered when a nonosteoporotic BMD is achieved, a single intravenous infusion of zoledronate 5 mg given 6 months after the last Dmab injection prevents bone loss for at least 2 years independently of the rate of bone turnover. Follow-up is recommended, as in a few patients treatment might not have the expected effect at 2 years for currently unknown reasons,” they concluded.
The study was funded by institutional funds and the Hellenic Endocrine Society. Several authors reported receiving consulting or lecture fees from Amgen, which markets denosumab, as well as other pharmaceutical companies.
SOURCE: Anastasilakis A et al. J Bone Miner Res. 2019 Aug 21. doi: 10.1002/jbmr.3853.
Women with postmenopausal osteoporosis who discontinued denosumab treatment after achieving osteopenia maintained bone mineral density at the spine and hip with a single infusion of zoledronate given 6 months after the last infusion of denosumab, according to results from a small, multicenter, randomized trial published in the Journal of Bone and Mineral Research.
The cessation of the monoclonal antibody denosumab is typically followed by a “rebound phenomenon” often attributed to an increase in bone turnover above pretreatment values caused by the up-regulation of osteoclastogenesis, according to Athanasios D. Anastasilakis, MD, of 424 General Military Hospital, Thessaloníki, Greece, and colleagues. Guidelines recommend that patients take a bisphosphonate to prevent this effect, but the optimal bisphosphonate regimen is unknown and evidence is inconsistent.
To address this question, the investigators randomized 57 postmenopausal women with osteoporosis who had received six monthly injections of denosumab (for an average of 2.2 years) and had achieved nonosteoporotic bone mineral density (BMD) T scores greater than –2.5 but no greater than –1 at the hip or the spine. A total of 27 received a single IV infusion of zoledronate 5 mg given 6 months after the last denosumab injection with a 3-week window, and 30 continued denosumab and received two additional monthly 60-mg injections. Following either the zoledronate infusion or the last denosumab injection, all women received no treatment and were followed until 2 years from randomization. All women were given vitamin D supplements and were seen in clinic appointments at baseline, 6, 12, 15, 18, and 24 months.
Areal BMD of the lumbar spine and femoral neck of the nondominant hip were measured at baseline, 12, and 24 months by dual-energy x-ray absorptiometry, and least significant changes were 5% or less at the spine and 4% or less at the femoral neck, based on proposals from the International Foundation for Osteoporosis and the National Osteoporosis Foundation USA.
At 24 months, lumbar spine BMD (LS‐BMD) returned to baseline in the zoledronate group, but decreased in the denosumab group by 4.82% from the 12‐month value (P less than .001).
The difference in LS-BMD changes between the two groups from month 12 to 24, the primary endpoint of the study, was statistically significant (–0.018 with zoledronate vs. –0.045 with denosumab; P = .025). Differences in changes of femoral neck BMD were also statistically significant (–0.004 with zoledronate vs. –0.038 with denosumab; P = .005), the researchers reported.
The differences in BMD changes between the two groups 24 and 12 months after discontinuation of denosumab (6 months after the last injection) for the zoledronate and denosumab group respectively were also statistically significant both at the lumbar spine (–0.002 with zoledronate vs. –0.045 with denosumab; P = .03) and at the femoral neck (–0.004 with zoledronate vs. –0.038 with denosumab; P = .007).
The authors observed no relationship between the number of denosumab injections and LS-BMD changes in either group of women; however, they noted that responses of individual patients to zoledronate were variable. For example, three women who took zoledronate experienced decreases of LS-BMD greater than the least significant change observed at 24 months, a finding which could not be explained by the timing of the infusion, baseline rate of bone turnover, or baseline BMD.
“It appears that intrinsic factors that still need to be defined may affect the response of a few individuals,” they wrote.
This was further illustrated by one patient in the zoledronate group who sustained clinical vertebral fractures associated with significant, unexplained decreases of BMD that could not be prevented with the zoledronate infusion.
“In clinical practice, it is, therefore, advisable to measure BMD at 12 months after the zoledronate infusion and decide whether additional treatment may be required,” the authors wrote.
Another significant finding reported by the authors was that neither baseline nor 12‐month bone turnover marker (BTM) values were associated with BMD changes in either group of women during the entire study period.
“Particularly important for clinical practice was the lack of a relationship in zoledronate-treated women; even when women were divided according to baseline median BTM values (below or above) there were no significant difference in BMD changes at 12 or 24 months,” they wrote.
“In a substantial number of women in the denosumab group BTMs were still above the upper limit of normal of the postmenopausal age 18 months after the last Dmab [denosumab] injection but also in 7.4% of patients treated with zoledronate at 2 years,” they added.
“Whether in the latter patients BTMs were also increased before the start of Dmab treatment, as it is known to occur in some patients with osteoporosis, or are due to a prolonged effect of Dmab withdrawal on bone metabolism could not be prevented by zoledronate, is not known because pretreatment data were not available,” the study authors noted.
For adverse events, in addition to the one patient in the zoledronate group with clinical vertebral fractures, three patients in the denosumab group sustained vertebral fractures.
“Prevalent vertebral fractures have been previously reported as the most important risk factor for clinical vertebral fractures following cessation of Dmab therapy [which] strongly suggest that spine x-rays should be performed in all patients in whom discontinuation of Dmab treatment is considered,” the authors wrote.
“In most women with postmenopausal osteoporosis treated with [denosumab] in whom discontinuation of treatment is considered when a nonosteoporotic BMD is achieved, a single intravenous infusion of zoledronate 5 mg given 6 months after the last Dmab injection prevents bone loss for at least 2 years independently of the rate of bone turnover. Follow-up is recommended, as in a few patients treatment might not have the expected effect at 2 years for currently unknown reasons,” they concluded.
The study was funded by institutional funds and the Hellenic Endocrine Society. Several authors reported receiving consulting or lecture fees from Amgen, which markets denosumab, as well as other pharmaceutical companies.
SOURCE: Anastasilakis A et al. J Bone Miner Res. 2019 Aug 21. doi: 10.1002/jbmr.3853.
Women with postmenopausal osteoporosis who discontinued denosumab treatment after achieving osteopenia maintained bone mineral density at the spine and hip with a single infusion of zoledronate given 6 months after the last infusion of denosumab, according to results from a small, multicenter, randomized trial published in the Journal of Bone and Mineral Research.
The cessation of the monoclonal antibody denosumab is typically followed by a “rebound phenomenon” often attributed to an increase in bone turnover above pretreatment values caused by the up-regulation of osteoclastogenesis, according to Athanasios D. Anastasilakis, MD, of 424 General Military Hospital, Thessaloníki, Greece, and colleagues. Guidelines recommend that patients take a bisphosphonate to prevent this effect, but the optimal bisphosphonate regimen is unknown and evidence is inconsistent.
To address this question, the investigators randomized 57 postmenopausal women with osteoporosis who had received six monthly injections of denosumab (for an average of 2.2 years) and had achieved nonosteoporotic bone mineral density (BMD) T scores greater than –2.5 but no greater than –1 at the hip or the spine. A total of 27 received a single IV infusion of zoledronate 5 mg given 6 months after the last denosumab injection with a 3-week window, and 30 continued denosumab and received two additional monthly 60-mg injections. Following either the zoledronate infusion or the last denosumab injection, all women received no treatment and were followed until 2 years from randomization. All women were given vitamin D supplements and were seen in clinic appointments at baseline, 6, 12, 15, 18, and 24 months.
Areal BMD of the lumbar spine and femoral neck of the nondominant hip were measured at baseline, 12, and 24 months by dual-energy x-ray absorptiometry, and least significant changes were 5% or less at the spine and 4% or less at the femoral neck, based on proposals from the International Foundation for Osteoporosis and the National Osteoporosis Foundation USA.
At 24 months, lumbar spine BMD (LS‐BMD) returned to baseline in the zoledronate group, but decreased in the denosumab group by 4.82% from the 12‐month value (P less than .001).
The difference in LS-BMD changes between the two groups from month 12 to 24, the primary endpoint of the study, was statistically significant (–0.018 with zoledronate vs. –0.045 with denosumab; P = .025). Differences in changes of femoral neck BMD were also statistically significant (–0.004 with zoledronate vs. –0.038 with denosumab; P = .005), the researchers reported.
The differences in BMD changes between the two groups 24 and 12 months after discontinuation of denosumab (6 months after the last injection) for the zoledronate and denosumab group respectively were also statistically significant both at the lumbar spine (–0.002 with zoledronate vs. –0.045 with denosumab; P = .03) and at the femoral neck (–0.004 with zoledronate vs. –0.038 with denosumab; P = .007).
The authors observed no relationship between the number of denosumab injections and LS-BMD changes in either group of women; however, they noted that responses of individual patients to zoledronate were variable. For example, three women who took zoledronate experienced decreases of LS-BMD greater than the least significant change observed at 24 months, a finding which could not be explained by the timing of the infusion, baseline rate of bone turnover, or baseline BMD.
“It appears that intrinsic factors that still need to be defined may affect the response of a few individuals,” they wrote.
This was further illustrated by one patient in the zoledronate group who sustained clinical vertebral fractures associated with significant, unexplained decreases of BMD that could not be prevented with the zoledronate infusion.
“In clinical practice, it is, therefore, advisable to measure BMD at 12 months after the zoledronate infusion and decide whether additional treatment may be required,” the authors wrote.
Another significant finding reported by the authors was that neither baseline nor 12‐month bone turnover marker (BTM) values were associated with BMD changes in either group of women during the entire study period.
“Particularly important for clinical practice was the lack of a relationship in zoledronate-treated women; even when women were divided according to baseline median BTM values (below or above) there were no significant difference in BMD changes at 12 or 24 months,” they wrote.
“In a substantial number of women in the denosumab group BTMs were still above the upper limit of normal of the postmenopausal age 18 months after the last Dmab [denosumab] injection but also in 7.4% of patients treated with zoledronate at 2 years,” they added.
“Whether in the latter patients BTMs were also increased before the start of Dmab treatment, as it is known to occur in some patients with osteoporosis, or are due to a prolonged effect of Dmab withdrawal on bone metabolism could not be prevented by zoledronate, is not known because pretreatment data were not available,” the study authors noted.
For adverse events, in addition to the one patient in the zoledronate group with clinical vertebral fractures, three patients in the denosumab group sustained vertebral fractures.
“Prevalent vertebral fractures have been previously reported as the most important risk factor for clinical vertebral fractures following cessation of Dmab therapy [which] strongly suggest that spine x-rays should be performed in all patients in whom discontinuation of Dmab treatment is considered,” the authors wrote.
“In most women with postmenopausal osteoporosis treated with [denosumab] in whom discontinuation of treatment is considered when a nonosteoporotic BMD is achieved, a single intravenous infusion of zoledronate 5 mg given 6 months after the last Dmab injection prevents bone loss for at least 2 years independently of the rate of bone turnover. Follow-up is recommended, as in a few patients treatment might not have the expected effect at 2 years for currently unknown reasons,” they concluded.
The study was funded by institutional funds and the Hellenic Endocrine Society. Several authors reported receiving consulting or lecture fees from Amgen, which markets denosumab, as well as other pharmaceutical companies.
SOURCE: Anastasilakis A et al. J Bone Miner Res. 2019 Aug 21. doi: 10.1002/jbmr.3853.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
ACOG advises bleeding disorder screening for teens with heavy menstruation
Adolescent girls with heavy menstrual bleeding should be assessed for bleeding disorders, according to a Committee Opinion issued by the American College of Obstetricians and Gynecologists.
A bleeding disorder is secondary only to anovulation as a cause of heavy menstrual bleeding in adolescents.
Bleeding disorders affect 1%-2% of the general population, but are “found in approximately 20% of adolescent girls who present for evaluation of heavy menstrual bleeding and in 33% of adolescent girls hospitalized for heavy menstrual bleeding,” wrote Oluyemisi Adeyemi-Fowode, MD, and Judith Simms-Cendan, MD, and members of the ACOG Committee on Adolescent Health Care in the opinion, published in Obstetrics & Gynecology.
The committee advised that physical examination of teens with acute heavy menstrual bleeding should include assessment of hemodynamic stability with orthostatic blood pressure and pulse measurements. A speculum exam is not usually needed in teen girls with heavy menstrual bleeding. Evaluation should include screening for anemia attributable to blood loss with serum ferritin, endocrine disorders, and bleeding disorders. In suspected cases of bleeding disorders, laboratory evaluation and medical management should be done in consultation with a hematologist.
Those who are actively bleeding or hemodynamically unstable should be hospitalized for medical management, they said.
Ultrasonography is not necessary for an initial work-up of teens with heavy menstrual bleeding, but could be useful in patients who fail to respond to medical management.
Adolescent girls without contraindications to estrogen can be treated with hormone therapy in various forms including intravenous conjugated estrogen every 4-6 hours or oral 30-50 mg ethinyl estradiol every 6-8 hours until cessation of bleeding. Antifibrinolytics also can be used to stop bleeding.
Maintenance therapy after correction of acute heavy bleeding can include a combination of treatments such as hormonal contraceptives, oral and injectable progestins, and levonorgestrel-releasing intrauterine devices, the committee wrote. They also recommended oral iron replacement therapy for all women of reproductive age with anemia caused by menstrual bleeding.
If a patient fails to respond to medical therapy, nonmedical options or surgery may be considered, according to the committee. In addition, all teen girls with bleeding disorders should be advised about safe medication use, including the use of aspirin or NSAIDs only on the recommendation of a hematologist.
Patients and their families need education on menstrual issues including possible options for surgery in the future if heavy menstruation does not resolve. If a patient has a known bleeding disorder and is considering surgery, preoperative evaluation should include a consultation with a hematologist and an anesthesiologist, the committee noted.
Melissa Kottke, MD, MPH, said in an interview, “Every ob.gyn. will see a young patient with ‘heavy menstrual bleeding.’ And it becomes part of the art and challenge to work with the patient and family to collectively explore if this is, indeed, ‘heavy’ and of concern … or is it is a ‘normal’ menstrual period and simply reflects a newer life experience that would benefit from some education? And the stakes are high. Young people who have heavy menstrual cycles are much more likely to have an underlying bleeding disorder than the general population (20% vs. 1%-2%), and 75%-80% of adolescents with bleeding disorders report heavy menses as the most common clinical manifestation of their disorder.
“Fortunately, Committee Opinion 785, ‘Screening and Management of Bleeding Disorders in Adolescents with Heavy Menstrual Bleeding’ from the ACOG Committee on Adolescent Health Care is detailed and pragmatic. It outlines how to translate everyday conversations with young people about their menses into a quantifiable estimate of bleeding, including a very teen-friendly Pictorial Blood Loss Assessment Chart. It also gives ob.gyns. ever-important guidance about what to do next for evaluation and diagnosis. This committee opinion nicely outlines how to help manage heavy bleeding in an adolescent with a detailed algorithm. And very importantly, it gives clear management guidance and encourages ob.gyns. to avoid frequently unnecessary (speculum exams and ultrasounds) and excessive (early transfusion or surgical interventions) approaches to management for the young patient. I think it will be a great resource for any provider who is taking care of heavy menstrual bleeding for a young person,” said Dr. Kottke, who is director of the Jane Fonda Center for Adolescent Reproductive Health and associate professor of gynecology and obstetrics, both at Emory University, Atlanta. Dr. Kottke is not a member of the ACOG Committee on Adolescent Health and was asked to comment on the opinion.*
The complete opinion, ACOG Committee Opinion number 785, includes recommended laboratory tests, an eight-question screening tool, and a management algorithm.
The committee members had no financial conflicts to disclose. Dr. Kottke said she had no relevant financial disclosures.
SOURCE: Adeyemi-Fowode O and Simms-Cendan J. Obstet Gynecol. 2019 Sep. 134:e71-83.
*This article was updated on 9/9/2019.
Adolescent girls with heavy menstrual bleeding should be assessed for bleeding disorders, according to a Committee Opinion issued by the American College of Obstetricians and Gynecologists.
A bleeding disorder is secondary only to anovulation as a cause of heavy menstrual bleeding in adolescents.
Bleeding disorders affect 1%-2% of the general population, but are “found in approximately 20% of adolescent girls who present for evaluation of heavy menstrual bleeding and in 33% of adolescent girls hospitalized for heavy menstrual bleeding,” wrote Oluyemisi Adeyemi-Fowode, MD, and Judith Simms-Cendan, MD, and members of the ACOG Committee on Adolescent Health Care in the opinion, published in Obstetrics & Gynecology.
The committee advised that physical examination of teens with acute heavy menstrual bleeding should include assessment of hemodynamic stability with orthostatic blood pressure and pulse measurements. A speculum exam is not usually needed in teen girls with heavy menstrual bleeding. Evaluation should include screening for anemia attributable to blood loss with serum ferritin, endocrine disorders, and bleeding disorders. In suspected cases of bleeding disorders, laboratory evaluation and medical management should be done in consultation with a hematologist.
Those who are actively bleeding or hemodynamically unstable should be hospitalized for medical management, they said.
Ultrasonography is not necessary for an initial work-up of teens with heavy menstrual bleeding, but could be useful in patients who fail to respond to medical management.
Adolescent girls without contraindications to estrogen can be treated with hormone therapy in various forms including intravenous conjugated estrogen every 4-6 hours or oral 30-50 mg ethinyl estradiol every 6-8 hours until cessation of bleeding. Antifibrinolytics also can be used to stop bleeding.
Maintenance therapy after correction of acute heavy bleeding can include a combination of treatments such as hormonal contraceptives, oral and injectable progestins, and levonorgestrel-releasing intrauterine devices, the committee wrote. They also recommended oral iron replacement therapy for all women of reproductive age with anemia caused by menstrual bleeding.
If a patient fails to respond to medical therapy, nonmedical options or surgery may be considered, according to the committee. In addition, all teen girls with bleeding disorders should be advised about safe medication use, including the use of aspirin or NSAIDs only on the recommendation of a hematologist.
Patients and their families need education on menstrual issues including possible options for surgery in the future if heavy menstruation does not resolve. If a patient has a known bleeding disorder and is considering surgery, preoperative evaluation should include a consultation with a hematologist and an anesthesiologist, the committee noted.
Melissa Kottke, MD, MPH, said in an interview, “Every ob.gyn. will see a young patient with ‘heavy menstrual bleeding.’ And it becomes part of the art and challenge to work with the patient and family to collectively explore if this is, indeed, ‘heavy’ and of concern … or is it is a ‘normal’ menstrual period and simply reflects a newer life experience that would benefit from some education? And the stakes are high. Young people who have heavy menstrual cycles are much more likely to have an underlying bleeding disorder than the general population (20% vs. 1%-2%), and 75%-80% of adolescents with bleeding disorders report heavy menses as the most common clinical manifestation of their disorder.
“Fortunately, Committee Opinion 785, ‘Screening and Management of Bleeding Disorders in Adolescents with Heavy Menstrual Bleeding’ from the ACOG Committee on Adolescent Health Care is detailed and pragmatic. It outlines how to translate everyday conversations with young people about their menses into a quantifiable estimate of bleeding, including a very teen-friendly Pictorial Blood Loss Assessment Chart. It also gives ob.gyns. ever-important guidance about what to do next for evaluation and diagnosis. This committee opinion nicely outlines how to help manage heavy bleeding in an adolescent with a detailed algorithm. And very importantly, it gives clear management guidance and encourages ob.gyns. to avoid frequently unnecessary (speculum exams and ultrasounds) and excessive (early transfusion or surgical interventions) approaches to management for the young patient. I think it will be a great resource for any provider who is taking care of heavy menstrual bleeding for a young person,” said Dr. Kottke, who is director of the Jane Fonda Center for Adolescent Reproductive Health and associate professor of gynecology and obstetrics, both at Emory University, Atlanta. Dr. Kottke is not a member of the ACOG Committee on Adolescent Health and was asked to comment on the opinion.*
The complete opinion, ACOG Committee Opinion number 785, includes recommended laboratory tests, an eight-question screening tool, and a management algorithm.
The committee members had no financial conflicts to disclose. Dr. Kottke said she had no relevant financial disclosures.
SOURCE: Adeyemi-Fowode O and Simms-Cendan J. Obstet Gynecol. 2019 Sep. 134:e71-83.
*This article was updated on 9/9/2019.
Adolescent girls with heavy menstrual bleeding should be assessed for bleeding disorders, according to a Committee Opinion issued by the American College of Obstetricians and Gynecologists.
A bleeding disorder is secondary only to anovulation as a cause of heavy menstrual bleeding in adolescents.
Bleeding disorders affect 1%-2% of the general population, but are “found in approximately 20% of adolescent girls who present for evaluation of heavy menstrual bleeding and in 33% of adolescent girls hospitalized for heavy menstrual bleeding,” wrote Oluyemisi Adeyemi-Fowode, MD, and Judith Simms-Cendan, MD, and members of the ACOG Committee on Adolescent Health Care in the opinion, published in Obstetrics & Gynecology.
The committee advised that physical examination of teens with acute heavy menstrual bleeding should include assessment of hemodynamic stability with orthostatic blood pressure and pulse measurements. A speculum exam is not usually needed in teen girls with heavy menstrual bleeding. Evaluation should include screening for anemia attributable to blood loss with serum ferritin, endocrine disorders, and bleeding disorders. In suspected cases of bleeding disorders, laboratory evaluation and medical management should be done in consultation with a hematologist.
Those who are actively bleeding or hemodynamically unstable should be hospitalized for medical management, they said.
Ultrasonography is not necessary for an initial work-up of teens with heavy menstrual bleeding, but could be useful in patients who fail to respond to medical management.
Adolescent girls without contraindications to estrogen can be treated with hormone therapy in various forms including intravenous conjugated estrogen every 4-6 hours or oral 30-50 mg ethinyl estradiol every 6-8 hours until cessation of bleeding. Antifibrinolytics also can be used to stop bleeding.
Maintenance therapy after correction of acute heavy bleeding can include a combination of treatments such as hormonal contraceptives, oral and injectable progestins, and levonorgestrel-releasing intrauterine devices, the committee wrote. They also recommended oral iron replacement therapy for all women of reproductive age with anemia caused by menstrual bleeding.
If a patient fails to respond to medical therapy, nonmedical options or surgery may be considered, according to the committee. In addition, all teen girls with bleeding disorders should be advised about safe medication use, including the use of aspirin or NSAIDs only on the recommendation of a hematologist.
Patients and their families need education on menstrual issues including possible options for surgery in the future if heavy menstruation does not resolve. If a patient has a known bleeding disorder and is considering surgery, preoperative evaluation should include a consultation with a hematologist and an anesthesiologist, the committee noted.
Melissa Kottke, MD, MPH, said in an interview, “Every ob.gyn. will see a young patient with ‘heavy menstrual bleeding.’ And it becomes part of the art and challenge to work with the patient and family to collectively explore if this is, indeed, ‘heavy’ and of concern … or is it is a ‘normal’ menstrual period and simply reflects a newer life experience that would benefit from some education? And the stakes are high. Young people who have heavy menstrual cycles are much more likely to have an underlying bleeding disorder than the general population (20% vs. 1%-2%), and 75%-80% of adolescents with bleeding disorders report heavy menses as the most common clinical manifestation of their disorder.
“Fortunately, Committee Opinion 785, ‘Screening and Management of Bleeding Disorders in Adolescents with Heavy Menstrual Bleeding’ from the ACOG Committee on Adolescent Health Care is detailed and pragmatic. It outlines how to translate everyday conversations with young people about their menses into a quantifiable estimate of bleeding, including a very teen-friendly Pictorial Blood Loss Assessment Chart. It also gives ob.gyns. ever-important guidance about what to do next for evaluation and diagnosis. This committee opinion nicely outlines how to help manage heavy bleeding in an adolescent with a detailed algorithm. And very importantly, it gives clear management guidance and encourages ob.gyns. to avoid frequently unnecessary (speculum exams and ultrasounds) and excessive (early transfusion or surgical interventions) approaches to management for the young patient. I think it will be a great resource for any provider who is taking care of heavy menstrual bleeding for a young person,” said Dr. Kottke, who is director of the Jane Fonda Center for Adolescent Reproductive Health and associate professor of gynecology and obstetrics, both at Emory University, Atlanta. Dr. Kottke is not a member of the ACOG Committee on Adolescent Health and was asked to comment on the opinion.*
The complete opinion, ACOG Committee Opinion number 785, includes recommended laboratory tests, an eight-question screening tool, and a management algorithm.
The committee members had no financial conflicts to disclose. Dr. Kottke said she had no relevant financial disclosures.
SOURCE: Adeyemi-Fowode O and Simms-Cendan J. Obstet Gynecol. 2019 Sep. 134:e71-83.
*This article was updated on 9/9/2019.
FROM OBSTETRICS AND GYNECOLOGY
High-dose vitamin D for bone health may do more harm than good
In fact, rather than a hypothesized increase in volumetric bone mineral density (BMD) with doses well above the recommended dietary allowance, a negative dose-response relationship was observed, Lauren A. Burt, PhD, of the McCaig Institute for Bone and Joint Health at the University of Calgary (Alta.) and colleagues found.
The total volumetric radial BMD was significantly lower in 101 and 97 study participants randomized to receive daily vitamin D3 doses of 10,000 IU or 4,000 IU for 3 years, respectively (–7.5 and –3.9 mg of calcium hydroxyapatite [HA] per cm3), compared with 105 participants randomized to a reference group that received 400 IU (mean percent changes, –3.5%, –2.4%, and –1.2%, respectively). Total volumetric tibial BMD was also significantly lower in the 10,000 IU arm, compared with the reference arm (–4.1 mg HA per cm3; mean percent change –1.7% vs. –0.4%), the investigators reported Aug. 27 in JAMA.
There also were no significant differences seen between the three groups for the coprimary endpoint of bone strength at either the radius or tibia.
Participants in the double-blind trial were community-dwelling healthy men and women aged 55-70 years (mean age, 62.2 years) without osteoporosis and with baseline levels of 25-hydroxyvitamin D (25[OH]D) of 30-125 nmol/L. They were enrolled from a single center between August 2013 and December 2017 and treated with daily oral vitamin D3 drops at the assigned dosage for 3 years and with calcium supplementation if dietary calcium intake was less than 1,200 mg daily.
Mean supplementation adherence was 99% among the 303 participants who completed the trial (out of 311 enrolled), and adherence was similar across the groups.
Baseline 25(OH)D levels in the 400 IU group were 76.3 nmol/L at baseline, 76.7 nmol/L at 3 months, and 77.4 nmol/L at 3 years. The corresponding measures for the 4,000 IU group were 81.3, 115.3, and 132.2 nmol/L, and for the 10,000 IU group, they were 78.4, 188.0, and 144.4, the investigators said, noting that significant group-by-time interactions were noted for volumetric BMD.
Bone strength decreased over time, but group-by-time interactions for that measure were not statistically significant, they said.
A total of 44 serious adverse events occurred in 38 participants (12.2%), and one death from presumed myocardial infarction occurred in the 400 IU group. Of eight prespecified adverse events, only hypercalcemia and hypercalciuria had significant dose-response effects; all episodes of hypercalcemia were mild and had resolved at follow-up, and the two hypercalcemia events, which occurred in one participant in the 10,000 IU group, were also transient. No significant difference in fall rates was seen in the three groups, they noted.
Vitamin D is considered beneficial for preventing and treating osteoporosis, and data support supplementation in individuals with 25(OH)D levels less than 30 nmol/L, but recent meta-analyses did not find a major treatment benefit for osteoporosis or for preventing falls and fractures, the investigators said.
Further, while most supplementation recommendations call for 400-2,000 IU daily, with a tolerable upper intake level of 4,000-10,000 IU, 3% of U.S. adults in 2013-2014 reported intake of at least 4,000 IU per day, but few studies have assessed the effects of doses at or above the upper intake level for 12 months or longer, they noted, adding that this study was “motivated by the prevalence of high-dose vitamin D supplementation among healthy adults.”
“It was hypothesized that a higher dose of vitamin D has a positive effect on high-resolution peripheral quantitative CT measures of volumetric density and strength, perhaps via suppression of parathyroid hormone (PTH)–mediated bone turnover,” they wrote.
However, based on the significantly lower radial BMD seen with both 4,000 and 10,000 IU, compared with 400 IU; the lower tibial BMD with 10,000 IU, compared with 400 IU; and the lack of a difference in bone strength at the radius and tibia, the findings do not support a benefit of high-dose vitamin D supplementation for bone health, they said, noting that additional study is needed to determine whether such doses are harmful.
“Because these results are in the opposite direction of the research hypothesis, this evidence of high-dose vitamin D having a negative effect on bone should be regarded as hypothesis generating, requiring confirmation with further research,” they concluded.
SOURCE: Burt L et al. JAMA. 2019 Aug 27;322(8):736-45.
In fact, rather than a hypothesized increase in volumetric bone mineral density (BMD) with doses well above the recommended dietary allowance, a negative dose-response relationship was observed, Lauren A. Burt, PhD, of the McCaig Institute for Bone and Joint Health at the University of Calgary (Alta.) and colleagues found.
The total volumetric radial BMD was significantly lower in 101 and 97 study participants randomized to receive daily vitamin D3 doses of 10,000 IU or 4,000 IU for 3 years, respectively (–7.5 and –3.9 mg of calcium hydroxyapatite [HA] per cm3), compared with 105 participants randomized to a reference group that received 400 IU (mean percent changes, –3.5%, –2.4%, and –1.2%, respectively). Total volumetric tibial BMD was also significantly lower in the 10,000 IU arm, compared with the reference arm (–4.1 mg HA per cm3; mean percent change –1.7% vs. –0.4%), the investigators reported Aug. 27 in JAMA.
There also were no significant differences seen between the three groups for the coprimary endpoint of bone strength at either the radius or tibia.
Participants in the double-blind trial were community-dwelling healthy men and women aged 55-70 years (mean age, 62.2 years) without osteoporosis and with baseline levels of 25-hydroxyvitamin D (25[OH]D) of 30-125 nmol/L. They were enrolled from a single center between August 2013 and December 2017 and treated with daily oral vitamin D3 drops at the assigned dosage for 3 years and with calcium supplementation if dietary calcium intake was less than 1,200 mg daily.
Mean supplementation adherence was 99% among the 303 participants who completed the trial (out of 311 enrolled), and adherence was similar across the groups.
Baseline 25(OH)D levels in the 400 IU group were 76.3 nmol/L at baseline, 76.7 nmol/L at 3 months, and 77.4 nmol/L at 3 years. The corresponding measures for the 4,000 IU group were 81.3, 115.3, and 132.2 nmol/L, and for the 10,000 IU group, they were 78.4, 188.0, and 144.4, the investigators said, noting that significant group-by-time interactions were noted for volumetric BMD.
Bone strength decreased over time, but group-by-time interactions for that measure were not statistically significant, they said.
A total of 44 serious adverse events occurred in 38 participants (12.2%), and one death from presumed myocardial infarction occurred in the 400 IU group. Of eight prespecified adverse events, only hypercalcemia and hypercalciuria had significant dose-response effects; all episodes of hypercalcemia were mild and had resolved at follow-up, and the two hypercalcemia events, which occurred in one participant in the 10,000 IU group, were also transient. No significant difference in fall rates was seen in the three groups, they noted.
Vitamin D is considered beneficial for preventing and treating osteoporosis, and data support supplementation in individuals with 25(OH)D levels less than 30 nmol/L, but recent meta-analyses did not find a major treatment benefit for osteoporosis or for preventing falls and fractures, the investigators said.
Further, while most supplementation recommendations call for 400-2,000 IU daily, with a tolerable upper intake level of 4,000-10,000 IU, 3% of U.S. adults in 2013-2014 reported intake of at least 4,000 IU per day, but few studies have assessed the effects of doses at or above the upper intake level for 12 months or longer, they noted, adding that this study was “motivated by the prevalence of high-dose vitamin D supplementation among healthy adults.”
“It was hypothesized that a higher dose of vitamin D has a positive effect on high-resolution peripheral quantitative CT measures of volumetric density and strength, perhaps via suppression of parathyroid hormone (PTH)–mediated bone turnover,” they wrote.
However, based on the significantly lower radial BMD seen with both 4,000 and 10,000 IU, compared with 400 IU; the lower tibial BMD with 10,000 IU, compared with 400 IU; and the lack of a difference in bone strength at the radius and tibia, the findings do not support a benefit of high-dose vitamin D supplementation for bone health, they said, noting that additional study is needed to determine whether such doses are harmful.
“Because these results are in the opposite direction of the research hypothesis, this evidence of high-dose vitamin D having a negative effect on bone should be regarded as hypothesis generating, requiring confirmation with further research,” they concluded.
SOURCE: Burt L et al. JAMA. 2019 Aug 27;322(8):736-45.
In fact, rather than a hypothesized increase in volumetric bone mineral density (BMD) with doses well above the recommended dietary allowance, a negative dose-response relationship was observed, Lauren A. Burt, PhD, of the McCaig Institute for Bone and Joint Health at the University of Calgary (Alta.) and colleagues found.
The total volumetric radial BMD was significantly lower in 101 and 97 study participants randomized to receive daily vitamin D3 doses of 10,000 IU or 4,000 IU for 3 years, respectively (–7.5 and –3.9 mg of calcium hydroxyapatite [HA] per cm3), compared with 105 participants randomized to a reference group that received 400 IU (mean percent changes, –3.5%, –2.4%, and –1.2%, respectively). Total volumetric tibial BMD was also significantly lower in the 10,000 IU arm, compared with the reference arm (–4.1 mg HA per cm3; mean percent change –1.7% vs. –0.4%), the investigators reported Aug. 27 in JAMA.
There also were no significant differences seen between the three groups for the coprimary endpoint of bone strength at either the radius or tibia.
Participants in the double-blind trial were community-dwelling healthy men and women aged 55-70 years (mean age, 62.2 years) without osteoporosis and with baseline levels of 25-hydroxyvitamin D (25[OH]D) of 30-125 nmol/L. They were enrolled from a single center between August 2013 and December 2017 and treated with daily oral vitamin D3 drops at the assigned dosage for 3 years and with calcium supplementation if dietary calcium intake was less than 1,200 mg daily.
Mean supplementation adherence was 99% among the 303 participants who completed the trial (out of 311 enrolled), and adherence was similar across the groups.
Baseline 25(OH)D levels in the 400 IU group were 76.3 nmol/L at baseline, 76.7 nmol/L at 3 months, and 77.4 nmol/L at 3 years. The corresponding measures for the 4,000 IU group were 81.3, 115.3, and 132.2 nmol/L, and for the 10,000 IU group, they were 78.4, 188.0, and 144.4, the investigators said, noting that significant group-by-time interactions were noted for volumetric BMD.
Bone strength decreased over time, but group-by-time interactions for that measure were not statistically significant, they said.
A total of 44 serious adverse events occurred in 38 participants (12.2%), and one death from presumed myocardial infarction occurred in the 400 IU group. Of eight prespecified adverse events, only hypercalcemia and hypercalciuria had significant dose-response effects; all episodes of hypercalcemia were mild and had resolved at follow-up, and the two hypercalcemia events, which occurred in one participant in the 10,000 IU group, were also transient. No significant difference in fall rates was seen in the three groups, they noted.
Vitamin D is considered beneficial for preventing and treating osteoporosis, and data support supplementation in individuals with 25(OH)D levels less than 30 nmol/L, but recent meta-analyses did not find a major treatment benefit for osteoporosis or for preventing falls and fractures, the investigators said.
Further, while most supplementation recommendations call for 400-2,000 IU daily, with a tolerable upper intake level of 4,000-10,000 IU, 3% of U.S. adults in 2013-2014 reported intake of at least 4,000 IU per day, but few studies have assessed the effects of doses at or above the upper intake level for 12 months or longer, they noted, adding that this study was “motivated by the prevalence of high-dose vitamin D supplementation among healthy adults.”
“It was hypothesized that a higher dose of vitamin D has a positive effect on high-resolution peripheral quantitative CT measures of volumetric density and strength, perhaps via suppression of parathyroid hormone (PTH)–mediated bone turnover,” they wrote.
However, based on the significantly lower radial BMD seen with both 4,000 and 10,000 IU, compared with 400 IU; the lower tibial BMD with 10,000 IU, compared with 400 IU; and the lack of a difference in bone strength at the radius and tibia, the findings do not support a benefit of high-dose vitamin D supplementation for bone health, they said, noting that additional study is needed to determine whether such doses are harmful.
“Because these results are in the opposite direction of the research hypothesis, this evidence of high-dose vitamin D having a negative effect on bone should be regarded as hypothesis generating, requiring confirmation with further research,” they concluded.
SOURCE: Burt L et al. JAMA. 2019 Aug 27;322(8):736-45.
FROM JAMA
Predictive model estimates likelihood of failing induction of labor in obese patients
reported researchers from the University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
The ten variables included in the model were prior vaginal delivery; prior cesarean delivery; maternal height, age, and weight at delivery; parity; gestational weight gain; Medicaid insurance; pregestational diabetes; and chronic hypertension, said Robert M. Rossi, MD, of the university and associates, who developed the model.
“Our hope is that this model may be useful as a tool to estimate an individualized risk based on commonly available prenatal factors that may assist in delivery planning and allocation of appropriate resources,” the investigators said in a study summarizing their findings, published in Obstetrics & Gynecology.
The researchers conducted a population-based, retrospective cohort study of delivery records from 1,098,981 obese women in a National Center for Health Statistics birth-death cohort database who underwent induction of labor between 2012 and 2016. Of these women, 825,797 (75%) women succeeded in delivering after induction, while 273,184 (25%) women failed to deliver after induction of labor and instead underwent cesarean section. The women included in the study had a body mass index of 30 or higher and underwent induction between 37 weeks and 44 weeks of gestation.
The class of obesity prior to pregnancy impacted the rate of induction failure, as patients with class I obesity had a rate of cesarean section of 21.6% (95% confidence interval, 21.4%-21.7%), while women with class II obesity had a rate of 25% (95% CI, 24.8%-25.2%) and women with class III obesity had a rate of 31% (95% CI, 30.8%-31.3%). Women also were more likely to fail induction if they had received fertility treatment, if they were older than 35 years, if they were of non-Hispanic black race, if they had gestational weight gain or maternal weight gain, if they had pregestational diabetes or gestational diabetes, or if they had gestational hypertension or preeclampsia (all P less than .001). Factors that made a woman less likely to undergo cesarean delivery were Medicaid insurance status or receiving Special Supplemental Nutrition Program for Women, Infant and Children (SNAP WIC) support.
Under the predictive model, the receiver operator characteristic curve (ROC) had an area under the curve (AUC) of 0.79 (95% CI, 0.78-0.79), and subsequent validation of the model using a different external U.S. birth cohort dataset showed an AUC of 0.77 (95% CI, 0.76-0.77). In both datasets, the model was calibrated to predict failure of induction of labor up to 75%, at which point the model overestimated the risk in patients, Dr. Rossi and associates said.
“Although we do not stipulate that an elective cesarean delivery should be offered for ‘high risk’ obese women, this tool may allow the provider to have a heightened awareness and prepare accordingly with timing of delivery, increased staffing, and anesthesia presence, particularly given the higher rates of maternal and neonatal adverse outcomes after a failed induction of labor,” said Dr. Rossi and colleagues.
Martina Louise Badell, MD, commented in an interview, “This is well-designed, large, population-based cohort study of more than 1 million obese women with a singleton pregnancy who underwent induction of labor. To determine the chance of successful induction of labor, a 10-variable model was created. This model achieved an AUC of 0.79, which is fairly good accuracy.
“They created an easy-to-use risk calculator as a tool to help identify chance of successful induction of labor in obese women. Similar to the VBAC [vaginal birth after cesarean] calculator, this calculator may help clinicians with patient-specific counseling, risk stratifying, and delivery planning,” said Dr. Badell, a maternal-fetal medicine specialist who is director of the Emory Perinatal Center at Emory University, Atlanta. Dr. Badell, who was not a coauthor of this study, was asked to comment on the study’s merit.
The authors reported no relevant financial disclosures. Dr. Badell had no relevant financial disclosures. There was no external funding.
SOURCE: Rossi R et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003377.
reported researchers from the University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
The ten variables included in the model were prior vaginal delivery; prior cesarean delivery; maternal height, age, and weight at delivery; parity; gestational weight gain; Medicaid insurance; pregestational diabetes; and chronic hypertension, said Robert M. Rossi, MD, of the university and associates, who developed the model.
“Our hope is that this model may be useful as a tool to estimate an individualized risk based on commonly available prenatal factors that may assist in delivery planning and allocation of appropriate resources,” the investigators said in a study summarizing their findings, published in Obstetrics & Gynecology.
The researchers conducted a population-based, retrospective cohort study of delivery records from 1,098,981 obese women in a National Center for Health Statistics birth-death cohort database who underwent induction of labor between 2012 and 2016. Of these women, 825,797 (75%) women succeeded in delivering after induction, while 273,184 (25%) women failed to deliver after induction of labor and instead underwent cesarean section. The women included in the study had a body mass index of 30 or higher and underwent induction between 37 weeks and 44 weeks of gestation.
The class of obesity prior to pregnancy impacted the rate of induction failure, as patients with class I obesity had a rate of cesarean section of 21.6% (95% confidence interval, 21.4%-21.7%), while women with class II obesity had a rate of 25% (95% CI, 24.8%-25.2%) and women with class III obesity had a rate of 31% (95% CI, 30.8%-31.3%). Women also were more likely to fail induction if they had received fertility treatment, if they were older than 35 years, if they were of non-Hispanic black race, if they had gestational weight gain or maternal weight gain, if they had pregestational diabetes or gestational diabetes, or if they had gestational hypertension or preeclampsia (all P less than .001). Factors that made a woman less likely to undergo cesarean delivery were Medicaid insurance status or receiving Special Supplemental Nutrition Program for Women, Infant and Children (SNAP WIC) support.
Under the predictive model, the receiver operator characteristic curve (ROC) had an area under the curve (AUC) of 0.79 (95% CI, 0.78-0.79), and subsequent validation of the model using a different external U.S. birth cohort dataset showed an AUC of 0.77 (95% CI, 0.76-0.77). In both datasets, the model was calibrated to predict failure of induction of labor up to 75%, at which point the model overestimated the risk in patients, Dr. Rossi and associates said.
“Although we do not stipulate that an elective cesarean delivery should be offered for ‘high risk’ obese women, this tool may allow the provider to have a heightened awareness and prepare accordingly with timing of delivery, increased staffing, and anesthesia presence, particularly given the higher rates of maternal and neonatal adverse outcomes after a failed induction of labor,” said Dr. Rossi and colleagues.
Martina Louise Badell, MD, commented in an interview, “This is well-designed, large, population-based cohort study of more than 1 million obese women with a singleton pregnancy who underwent induction of labor. To determine the chance of successful induction of labor, a 10-variable model was created. This model achieved an AUC of 0.79, which is fairly good accuracy.
“They created an easy-to-use risk calculator as a tool to help identify chance of successful induction of labor in obese women. Similar to the VBAC [vaginal birth after cesarean] calculator, this calculator may help clinicians with patient-specific counseling, risk stratifying, and delivery planning,” said Dr. Badell, a maternal-fetal medicine specialist who is director of the Emory Perinatal Center at Emory University, Atlanta. Dr. Badell, who was not a coauthor of this study, was asked to comment on the study’s merit.
The authors reported no relevant financial disclosures. Dr. Badell had no relevant financial disclosures. There was no external funding.
SOURCE: Rossi R et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003377.
reported researchers from the University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
The ten variables included in the model were prior vaginal delivery; prior cesarean delivery; maternal height, age, and weight at delivery; parity; gestational weight gain; Medicaid insurance; pregestational diabetes; and chronic hypertension, said Robert M. Rossi, MD, of the university and associates, who developed the model.
“Our hope is that this model may be useful as a tool to estimate an individualized risk based on commonly available prenatal factors that may assist in delivery planning and allocation of appropriate resources,” the investigators said in a study summarizing their findings, published in Obstetrics & Gynecology.
The researchers conducted a population-based, retrospective cohort study of delivery records from 1,098,981 obese women in a National Center for Health Statistics birth-death cohort database who underwent induction of labor between 2012 and 2016. Of these women, 825,797 (75%) women succeeded in delivering after induction, while 273,184 (25%) women failed to deliver after induction of labor and instead underwent cesarean section. The women included in the study had a body mass index of 30 or higher and underwent induction between 37 weeks and 44 weeks of gestation.
The class of obesity prior to pregnancy impacted the rate of induction failure, as patients with class I obesity had a rate of cesarean section of 21.6% (95% confidence interval, 21.4%-21.7%), while women with class II obesity had a rate of 25% (95% CI, 24.8%-25.2%) and women with class III obesity had a rate of 31% (95% CI, 30.8%-31.3%). Women also were more likely to fail induction if they had received fertility treatment, if they were older than 35 years, if they were of non-Hispanic black race, if they had gestational weight gain or maternal weight gain, if they had pregestational diabetes or gestational diabetes, or if they had gestational hypertension or preeclampsia (all P less than .001). Factors that made a woman less likely to undergo cesarean delivery were Medicaid insurance status or receiving Special Supplemental Nutrition Program for Women, Infant and Children (SNAP WIC) support.
Under the predictive model, the receiver operator characteristic curve (ROC) had an area under the curve (AUC) of 0.79 (95% CI, 0.78-0.79), and subsequent validation of the model using a different external U.S. birth cohort dataset showed an AUC of 0.77 (95% CI, 0.76-0.77). In both datasets, the model was calibrated to predict failure of induction of labor up to 75%, at which point the model overestimated the risk in patients, Dr. Rossi and associates said.
“Although we do not stipulate that an elective cesarean delivery should be offered for ‘high risk’ obese women, this tool may allow the provider to have a heightened awareness and prepare accordingly with timing of delivery, increased staffing, and anesthesia presence, particularly given the higher rates of maternal and neonatal adverse outcomes after a failed induction of labor,” said Dr. Rossi and colleagues.
Martina Louise Badell, MD, commented in an interview, “This is well-designed, large, population-based cohort study of more than 1 million obese women with a singleton pregnancy who underwent induction of labor. To determine the chance of successful induction of labor, a 10-variable model was created. This model achieved an AUC of 0.79, which is fairly good accuracy.
“They created an easy-to-use risk calculator as a tool to help identify chance of successful induction of labor in obese women. Similar to the VBAC [vaginal birth after cesarean] calculator, this calculator may help clinicians with patient-specific counseling, risk stratifying, and delivery planning,” said Dr. Badell, a maternal-fetal medicine specialist who is director of the Emory Perinatal Center at Emory University, Atlanta. Dr. Badell, who was not a coauthor of this study, was asked to comment on the study’s merit.
The authors reported no relevant financial disclosures. Dr. Badell had no relevant financial disclosures. There was no external funding.
SOURCE: Rossi R et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003377.
FROM OBSTETRICS & GYNECOLOGY
Endometriosis is linked to adverse pregnancy outcomes
a large study has found.
Leslie V. Farland, ScD, of the University of Arizona, Tucson, and coauthors reported their analysis of data from 196,722 pregnancies in 116,429 women aged 25-42 years enrolled in the Nurses Health Study II cohort in Obstetrics & Gynecology.
Among the women with eligible pregnancies, 4.5% had laparoscopically confirmed endometriosis. These women were found to have a 40% higher risk of spontaneous abortion than were women without endometriosis (19.3% vs. 12.3%) and a 46% higher risk of ectopic pregnancy (1.8% vs. 0.8%). The risk of ectopic pregnancy was even more pronounced in women without a history of infertility.
Researchers also saw a 16% higher risk of preterm birth in women with endometriosis (12% in women with endometriosis vs. 8.1% in women without endometriosis), and a 16% greater risk of low-birth-weight babies (5.6% in women with endometriosis vs. 3.6% in women without endometriosis).
There also was the suggestion of an increased risk of stillbirth, although the researchers said this finding should be interpreted with caution because of the small sample size.
Women with endometriosis also had a 35% greater risk of gestational diabetes than did women without endometriosis. This association was stronger in women younger than age 35 years, in women without a history of infertility, and in women undergoing their second or later pregnancy. Endometriosis also was associated with a 30% greater risk of hypertensive disorders of pregnancy, particularly in second or later pregnancies.
Dr. Farland and associates wrote that recent research on the relationship between endometriosis and pregnancy outcomes had yielded “mixed results.”
“For example, much of the research to date has been conducted among women attending infertility clinics, which may conflate the influence of advanced maternal age, fertility treatment, and infertility itself with endometriosis, given the known elevated risk of adverse pregnancy outcomes in this population,” they wrote.
They suggested that one possible mechanism for the association between endometriosis and adverse pregnancy outcomes was progesterone resistance, which was hypothesized to affect genes important for embryo implantation and therefore contribute to pregnancy loss. Another mechanism could be increased inflammation, which may increase the risk of preterm birth and abnormal placentation.
“Elucidating mechanisms of association and possible pathways for intervention or screening procedures will be critical to improve the health of women with endometriosis and their children,” they wrote.
Katrina Mark, MD, commented in an interview, “This study, which identifies an increased risk of adverse pregnancy outcomes in women with endometriosis, is an important step in improving reproductive success.
“Although some explanations for these findings were postulated by the researchers, the next step will be to study the underlying physiology that leads to these complications so that interventions can be offered to improve outcomes,” said Dr. Mark, who is an associate professor of obstetrics, gynecology & reproductive sciences at the University of Maryland School of Medicine. Dr. Mark, who is not a coauthor of the study, was asked to comment on the study’s merit.
The study was supported by grants from the National Institutes of Health. Daniela A. Carusi, MD, received funding from UpToDate; Andrew W. Horne, MB, ChB, PhD, declared European government grants funding and consultancies with the pharmaceutical sector unrelated to the present study; Jorge E. Chavarro, MD, and Stacey A. Missmer, ScD, declared institutional funding from the NIH, and Dr. Missmer also received institutional funding from other funding bodies, as well as consulting fees. Dr. Farland and the remaining coauthors had no relevant financial disclosures. Dr. Mark has no relevant financial disclosures.
SOURCE: Farland LV et al. Obstetr Gynecol. 2019. doi: 10.1097/AOG.0000000000003410.
a large study has found.
Leslie V. Farland, ScD, of the University of Arizona, Tucson, and coauthors reported their analysis of data from 196,722 pregnancies in 116,429 women aged 25-42 years enrolled in the Nurses Health Study II cohort in Obstetrics & Gynecology.
Among the women with eligible pregnancies, 4.5% had laparoscopically confirmed endometriosis. These women were found to have a 40% higher risk of spontaneous abortion than were women without endometriosis (19.3% vs. 12.3%) and a 46% higher risk of ectopic pregnancy (1.8% vs. 0.8%). The risk of ectopic pregnancy was even more pronounced in women without a history of infertility.
Researchers also saw a 16% higher risk of preterm birth in women with endometriosis (12% in women with endometriosis vs. 8.1% in women without endometriosis), and a 16% greater risk of low-birth-weight babies (5.6% in women with endometriosis vs. 3.6% in women without endometriosis).
There also was the suggestion of an increased risk of stillbirth, although the researchers said this finding should be interpreted with caution because of the small sample size.
Women with endometriosis also had a 35% greater risk of gestational diabetes than did women without endometriosis. This association was stronger in women younger than age 35 years, in women without a history of infertility, and in women undergoing their second or later pregnancy. Endometriosis also was associated with a 30% greater risk of hypertensive disorders of pregnancy, particularly in second or later pregnancies.
Dr. Farland and associates wrote that recent research on the relationship between endometriosis and pregnancy outcomes had yielded “mixed results.”
“For example, much of the research to date has been conducted among women attending infertility clinics, which may conflate the influence of advanced maternal age, fertility treatment, and infertility itself with endometriosis, given the known elevated risk of adverse pregnancy outcomes in this population,” they wrote.
They suggested that one possible mechanism for the association between endometriosis and adverse pregnancy outcomes was progesterone resistance, which was hypothesized to affect genes important for embryo implantation and therefore contribute to pregnancy loss. Another mechanism could be increased inflammation, which may increase the risk of preterm birth and abnormal placentation.
“Elucidating mechanisms of association and possible pathways for intervention or screening procedures will be critical to improve the health of women with endometriosis and their children,” they wrote.
Katrina Mark, MD, commented in an interview, “This study, which identifies an increased risk of adverse pregnancy outcomes in women with endometriosis, is an important step in improving reproductive success.
“Although some explanations for these findings were postulated by the researchers, the next step will be to study the underlying physiology that leads to these complications so that interventions can be offered to improve outcomes,” said Dr. Mark, who is an associate professor of obstetrics, gynecology & reproductive sciences at the University of Maryland School of Medicine. Dr. Mark, who is not a coauthor of the study, was asked to comment on the study’s merit.
The study was supported by grants from the National Institutes of Health. Daniela A. Carusi, MD, received funding from UpToDate; Andrew W. Horne, MB, ChB, PhD, declared European government grants funding and consultancies with the pharmaceutical sector unrelated to the present study; Jorge E. Chavarro, MD, and Stacey A. Missmer, ScD, declared institutional funding from the NIH, and Dr. Missmer also received institutional funding from other funding bodies, as well as consulting fees. Dr. Farland and the remaining coauthors had no relevant financial disclosures. Dr. Mark has no relevant financial disclosures.
SOURCE: Farland LV et al. Obstetr Gynecol. 2019. doi: 10.1097/AOG.0000000000003410.
a large study has found.
Leslie V. Farland, ScD, of the University of Arizona, Tucson, and coauthors reported their analysis of data from 196,722 pregnancies in 116,429 women aged 25-42 years enrolled in the Nurses Health Study II cohort in Obstetrics & Gynecology.
Among the women with eligible pregnancies, 4.5% had laparoscopically confirmed endometriosis. These women were found to have a 40% higher risk of spontaneous abortion than were women without endometriosis (19.3% vs. 12.3%) and a 46% higher risk of ectopic pregnancy (1.8% vs. 0.8%). The risk of ectopic pregnancy was even more pronounced in women without a history of infertility.
Researchers also saw a 16% higher risk of preterm birth in women with endometriosis (12% in women with endometriosis vs. 8.1% in women without endometriosis), and a 16% greater risk of low-birth-weight babies (5.6% in women with endometriosis vs. 3.6% in women without endometriosis).
There also was the suggestion of an increased risk of stillbirth, although the researchers said this finding should be interpreted with caution because of the small sample size.
Women with endometriosis also had a 35% greater risk of gestational diabetes than did women without endometriosis. This association was stronger in women younger than age 35 years, in women without a history of infertility, and in women undergoing their second or later pregnancy. Endometriosis also was associated with a 30% greater risk of hypertensive disorders of pregnancy, particularly in second or later pregnancies.
Dr. Farland and associates wrote that recent research on the relationship between endometriosis and pregnancy outcomes had yielded “mixed results.”
“For example, much of the research to date has been conducted among women attending infertility clinics, which may conflate the influence of advanced maternal age, fertility treatment, and infertility itself with endometriosis, given the known elevated risk of adverse pregnancy outcomes in this population,” they wrote.
They suggested that one possible mechanism for the association between endometriosis and adverse pregnancy outcomes was progesterone resistance, which was hypothesized to affect genes important for embryo implantation and therefore contribute to pregnancy loss. Another mechanism could be increased inflammation, which may increase the risk of preterm birth and abnormal placentation.
“Elucidating mechanisms of association and possible pathways for intervention or screening procedures will be critical to improve the health of women with endometriosis and their children,” they wrote.
Katrina Mark, MD, commented in an interview, “This study, which identifies an increased risk of adverse pregnancy outcomes in women with endometriosis, is an important step in improving reproductive success.
“Although some explanations for these findings were postulated by the researchers, the next step will be to study the underlying physiology that leads to these complications so that interventions can be offered to improve outcomes,” said Dr. Mark, who is an associate professor of obstetrics, gynecology & reproductive sciences at the University of Maryland School of Medicine. Dr. Mark, who is not a coauthor of the study, was asked to comment on the study’s merit.
The study was supported by grants from the National Institutes of Health. Daniela A. Carusi, MD, received funding from UpToDate; Andrew W. Horne, MB, ChB, PhD, declared European government grants funding and consultancies with the pharmaceutical sector unrelated to the present study; Jorge E. Chavarro, MD, and Stacey A. Missmer, ScD, declared institutional funding from the NIH, and Dr. Missmer also received institutional funding from other funding bodies, as well as consulting fees. Dr. Farland and the remaining coauthors had no relevant financial disclosures. Dr. Mark has no relevant financial disclosures.
SOURCE: Farland LV et al. Obstetr Gynecol. 2019. doi: 10.1097/AOG.0000000000003410.
FROM OBSTETRICS & GYNECOLOGY
Ovarian cancer and perineal talc exposure: An epidemiologic dilemma
Many readers may be aware of large payments made by such companies as Johnson & Johnson to compensate women with a history of ovarian cancer who have claimed that perineal application of talc played a causative role in their cancer development. This column serves to review the purported role of perineal talc use in the development of ovarian cancer, and explore some of the pitfalls of observational science.
Talc, a hydrated magnesium silicate, is the softest mineral on earth, and has been sold as a personal hygiene product for many decades. Perineal application of talc to sanitary pads, perineal skin, undergarments, and diapers has been a common practice to decrease friction, moisture build-up, and as a deodorant. Talc is chemically similar, although not identical, to asbestos and is geologically located in close proximity to the known carcinogen. In the 1970s, there were concerns raised regarding the possible contamination of cosmetic-grade talc with asbestos, which led to the development of asbestos-free forms of the substance. Given that a strong causal relationship had been established between asbestos exposure and lung and pleural cancers, there was concern that exposure to perineal talc might increase cancer risk.
In the 1980s, an association between perineal talc exposure and ovarian cancer was observed in a case-control study.1 Since that time, multiple other observational studies, predominately case-control studies, have observed an increased ovarian cancer risk among users of perineal talc including the findings of a meta-analysis which estimated a 24%-39% increased risk for ovarian cancer among users.2 Does this establish a causal relationship? For the purposes of legal cases, these associations are adequate. However, science demands a different standard when determining cause and effect.
It is not unusual to rely on observational studies to establish a causal relationship between exposure and disease when it is unethical to randomize subjects in a clinical trial to exposure of the potential harmful agent. This was the necessary methodology behind establishing that smoking causes lung cancer. Several factors must be present when relying on observational studies to establish plausible causation including an observable biologic mechanism, dose-effect response, temporal relationship, consistent effect observed in multiple study populations, and statistical strength of response. These elements should be present in a consistent and powerful enough way to balance the pitfalls of observational studies, namely biases.
A particularly problematic bias is one of recall bias, which plagues case-control studies. Case-control studies are a popular tool to measure a relationship between an exposure and a rare disease, because they are more feasible than the prospective, observational cohort studies that require very large study populations observed over very long periods of time to capture enough events of interest (in this case, cases of ovarian cancer). In case-control studies, researchers identify a cohort of patients with the outcome of interest (ovarian cancer) and compare this population to a control group of similar demographic features. They then survey directly or indirectly (through medical records) for the exposure of interest (perineal talc use).
Recall bias occurs when subjects who have the disease are more likely to have memory of exposure than do control subjects because of the natural instincts individuals have toward attribution. This is emphasized when there is public commentary, justified or not, about the potential risks of that exposure. Given the significant publicity that these lawsuits have had with companies that produced cosmetic talc, it is plausible that ovarian cancer survivors are more likely to remember and negatively attribute their talc exposure to their cancer than are subjects without cancer. Additionally, their memory of volume and duration of exposure generally is enhanced by the same pressures. The potential for this bias is eliminated in prospective, cohort observational studies such as the Women’s Health Initiative Observational Study which, among 61,576 women, half of whom reported perineal talc exposure, did not measure a difference in the development of ovarian cancers during their 12 years of mean follow-up.3
Given these inherent biases, The biologic mechanism of talc carcinogenesis is largely theoretical. As mentioned earlier, prior to the 1970s, there was some observed contamination of talc with asbestos likely caused by the geologic proximity of these minerals. Asbestos is a known carcinogen, and therefore possibly could be harmful if a contaminant of talc. However, it is not known if this level of contamination was enough to be achieve ovarian carcinogenesis. Most theories of talc carcinogenesis are based on foreign body inflammatory reaction via talc particle ascent through the genital tract. This is proposed to induce an inflammatory release of prostaglandins and cytokines, which could cause a mutagenic effect promoting carcinogenesis. The foreign body inflammatory mechanism is further supported by the observation of a decreased incidence of ovarian cancer after hysterectomy or tubal ligation.4 However, inconsistently, a protective effect of NSAIDs has not been observed in ovarian cancer.5
A recent meta-analysis, which reviewed 27 of the largest, best-quality observational studies, identified a dose-effect response with an increased risk for ovarian cancer with greater than 3,600 lifetime applications, compared with less than 3,600 applications.2 The observed association between perineal talc exposure and increased risk of ovarian cancer appears to be consistent across a number of observational studies, including both case-control studies and prospective cohort studies (although somewhat mitigated in the latter). Additionally, there appears to be consistency in the finding that the risk is present for the epithelial subtypes of serous and endometrioid, but not mucinous or clear cell cancer. However, when considering the magnitude of effect, this remains somewhat small (odds ratio, 1.31; 95% confidence interval, 1.24-1.39) when compared with other better established carcinogenic relationships such as smoking and lung cancer where the hazard ratio is 12.12 (95% CI, 6.94-21.17).2,6
If talc does not cause ovarian cancer, why would this association be observed at all? One explanation could be that talc use is a confounder for the true causative mechanism. A theoretical example of this would be if the genital microbiome (a subject we have reviewed previously in this column) was the true culprit. If a particular microbiome profile promotes both oncogenic change in the ovary while also causing vaginal discharge and odor, it might increase the likelihood that perineal talc use is reported in the history of these cancer patients. This is purely speculative, but it always is important to consider the potential for confounding variables when utilizing observational studies to attribute cause and effect.
Therefore, there is a consistently observed association between perineal talc application and ovarian cancer, however, the relationship does not appear to be strong enough, associated with a proven carcinogenic mechanism, or free from interfering recall bias such to definitively state that perineal talc exposure causes ovarian cancer. Given these findings, it is reasonable to recommend patients avoid the use of perineal talc application until further definitive safety evidence is provided. In the meantime, it should be noted that even though talc-containing products are not commercially labeled as carcinogens, many pharmaceutical and cosmetic companies have replaced the mineral talc with corn starch in their powders.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at [email protected].
References
1. Cancer. 1982 Jul 15;50(2):372-6.
2. Epidemiology. 2018 Jan;29(1):41-9.
3. J Natl Cancer Inst. 2014 Sep 10;106(9). pii: dju208.
4. Am J Epidemiol. 1991 Aug 15;134(4):362-9.
5. Int J Cancer. 2008 Jan 1;122(1):170-6.
6. J Natl Cancer Inst. 2018 Nov 1;110(11):1201-7.
Many readers may be aware of large payments made by such companies as Johnson & Johnson to compensate women with a history of ovarian cancer who have claimed that perineal application of talc played a causative role in their cancer development. This column serves to review the purported role of perineal talc use in the development of ovarian cancer, and explore some of the pitfalls of observational science.
Talc, a hydrated magnesium silicate, is the softest mineral on earth, and has been sold as a personal hygiene product for many decades. Perineal application of talc to sanitary pads, perineal skin, undergarments, and diapers has been a common practice to decrease friction, moisture build-up, and as a deodorant. Talc is chemically similar, although not identical, to asbestos and is geologically located in close proximity to the known carcinogen. In the 1970s, there were concerns raised regarding the possible contamination of cosmetic-grade talc with asbestos, which led to the development of asbestos-free forms of the substance. Given that a strong causal relationship had been established between asbestos exposure and lung and pleural cancers, there was concern that exposure to perineal talc might increase cancer risk.
In the 1980s, an association between perineal talc exposure and ovarian cancer was observed in a case-control study.1 Since that time, multiple other observational studies, predominately case-control studies, have observed an increased ovarian cancer risk among users of perineal talc including the findings of a meta-analysis which estimated a 24%-39% increased risk for ovarian cancer among users.2 Does this establish a causal relationship? For the purposes of legal cases, these associations are adequate. However, science demands a different standard when determining cause and effect.
It is not unusual to rely on observational studies to establish a causal relationship between exposure and disease when it is unethical to randomize subjects in a clinical trial to exposure of the potential harmful agent. This was the necessary methodology behind establishing that smoking causes lung cancer. Several factors must be present when relying on observational studies to establish plausible causation including an observable biologic mechanism, dose-effect response, temporal relationship, consistent effect observed in multiple study populations, and statistical strength of response. These elements should be present in a consistent and powerful enough way to balance the pitfalls of observational studies, namely biases.
A particularly problematic bias is one of recall bias, which plagues case-control studies. Case-control studies are a popular tool to measure a relationship between an exposure and a rare disease, because they are more feasible than the prospective, observational cohort studies that require very large study populations observed over very long periods of time to capture enough events of interest (in this case, cases of ovarian cancer). In case-control studies, researchers identify a cohort of patients with the outcome of interest (ovarian cancer) and compare this population to a control group of similar demographic features. They then survey directly or indirectly (through medical records) for the exposure of interest (perineal talc use).
Recall bias occurs when subjects who have the disease are more likely to have memory of exposure than do control subjects because of the natural instincts individuals have toward attribution. This is emphasized when there is public commentary, justified or not, about the potential risks of that exposure. Given the significant publicity that these lawsuits have had with companies that produced cosmetic talc, it is plausible that ovarian cancer survivors are more likely to remember and negatively attribute their talc exposure to their cancer than are subjects without cancer. Additionally, their memory of volume and duration of exposure generally is enhanced by the same pressures. The potential for this bias is eliminated in prospective, cohort observational studies such as the Women’s Health Initiative Observational Study which, among 61,576 women, half of whom reported perineal talc exposure, did not measure a difference in the development of ovarian cancers during their 12 years of mean follow-up.3
Given these inherent biases, The biologic mechanism of talc carcinogenesis is largely theoretical. As mentioned earlier, prior to the 1970s, there was some observed contamination of talc with asbestos likely caused by the geologic proximity of these minerals. Asbestos is a known carcinogen, and therefore possibly could be harmful if a contaminant of talc. However, it is not known if this level of contamination was enough to be achieve ovarian carcinogenesis. Most theories of talc carcinogenesis are based on foreign body inflammatory reaction via talc particle ascent through the genital tract. This is proposed to induce an inflammatory release of prostaglandins and cytokines, which could cause a mutagenic effect promoting carcinogenesis. The foreign body inflammatory mechanism is further supported by the observation of a decreased incidence of ovarian cancer after hysterectomy or tubal ligation.4 However, inconsistently, a protective effect of NSAIDs has not been observed in ovarian cancer.5
A recent meta-analysis, which reviewed 27 of the largest, best-quality observational studies, identified a dose-effect response with an increased risk for ovarian cancer with greater than 3,600 lifetime applications, compared with less than 3,600 applications.2 The observed association between perineal talc exposure and increased risk of ovarian cancer appears to be consistent across a number of observational studies, including both case-control studies and prospective cohort studies (although somewhat mitigated in the latter). Additionally, there appears to be consistency in the finding that the risk is present for the epithelial subtypes of serous and endometrioid, but not mucinous or clear cell cancer. However, when considering the magnitude of effect, this remains somewhat small (odds ratio, 1.31; 95% confidence interval, 1.24-1.39) when compared with other better established carcinogenic relationships such as smoking and lung cancer where the hazard ratio is 12.12 (95% CI, 6.94-21.17).2,6
If talc does not cause ovarian cancer, why would this association be observed at all? One explanation could be that talc use is a confounder for the true causative mechanism. A theoretical example of this would be if the genital microbiome (a subject we have reviewed previously in this column) was the true culprit. If a particular microbiome profile promotes both oncogenic change in the ovary while also causing vaginal discharge and odor, it might increase the likelihood that perineal talc use is reported in the history of these cancer patients. This is purely speculative, but it always is important to consider the potential for confounding variables when utilizing observational studies to attribute cause and effect.
Therefore, there is a consistently observed association between perineal talc application and ovarian cancer, however, the relationship does not appear to be strong enough, associated with a proven carcinogenic mechanism, or free from interfering recall bias such to definitively state that perineal talc exposure causes ovarian cancer. Given these findings, it is reasonable to recommend patients avoid the use of perineal talc application until further definitive safety evidence is provided. In the meantime, it should be noted that even though talc-containing products are not commercially labeled as carcinogens, many pharmaceutical and cosmetic companies have replaced the mineral talc with corn starch in their powders.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at [email protected].
References
1. Cancer. 1982 Jul 15;50(2):372-6.
2. Epidemiology. 2018 Jan;29(1):41-9.
3. J Natl Cancer Inst. 2014 Sep 10;106(9). pii: dju208.
4. Am J Epidemiol. 1991 Aug 15;134(4):362-9.
5. Int J Cancer. 2008 Jan 1;122(1):170-6.
6. J Natl Cancer Inst. 2018 Nov 1;110(11):1201-7.
Many readers may be aware of large payments made by such companies as Johnson & Johnson to compensate women with a history of ovarian cancer who have claimed that perineal application of talc played a causative role in their cancer development. This column serves to review the purported role of perineal talc use in the development of ovarian cancer, and explore some of the pitfalls of observational science.
Talc, a hydrated magnesium silicate, is the softest mineral on earth, and has been sold as a personal hygiene product for many decades. Perineal application of talc to sanitary pads, perineal skin, undergarments, and diapers has been a common practice to decrease friction, moisture build-up, and as a deodorant. Talc is chemically similar, although not identical, to asbestos and is geologically located in close proximity to the known carcinogen. In the 1970s, there were concerns raised regarding the possible contamination of cosmetic-grade talc with asbestos, which led to the development of asbestos-free forms of the substance. Given that a strong causal relationship had been established between asbestos exposure and lung and pleural cancers, there was concern that exposure to perineal talc might increase cancer risk.
In the 1980s, an association between perineal talc exposure and ovarian cancer was observed in a case-control study.1 Since that time, multiple other observational studies, predominately case-control studies, have observed an increased ovarian cancer risk among users of perineal talc including the findings of a meta-analysis which estimated a 24%-39% increased risk for ovarian cancer among users.2 Does this establish a causal relationship? For the purposes of legal cases, these associations are adequate. However, science demands a different standard when determining cause and effect.
It is not unusual to rely on observational studies to establish a causal relationship between exposure and disease when it is unethical to randomize subjects in a clinical trial to exposure of the potential harmful agent. This was the necessary methodology behind establishing that smoking causes lung cancer. Several factors must be present when relying on observational studies to establish plausible causation including an observable biologic mechanism, dose-effect response, temporal relationship, consistent effect observed in multiple study populations, and statistical strength of response. These elements should be present in a consistent and powerful enough way to balance the pitfalls of observational studies, namely biases.
A particularly problematic bias is one of recall bias, which plagues case-control studies. Case-control studies are a popular tool to measure a relationship between an exposure and a rare disease, because they are more feasible than the prospective, observational cohort studies that require very large study populations observed over very long periods of time to capture enough events of interest (in this case, cases of ovarian cancer). In case-control studies, researchers identify a cohort of patients with the outcome of interest (ovarian cancer) and compare this population to a control group of similar demographic features. They then survey directly or indirectly (through medical records) for the exposure of interest (perineal talc use).
Recall bias occurs when subjects who have the disease are more likely to have memory of exposure than do control subjects because of the natural instincts individuals have toward attribution. This is emphasized when there is public commentary, justified or not, about the potential risks of that exposure. Given the significant publicity that these lawsuits have had with companies that produced cosmetic talc, it is plausible that ovarian cancer survivors are more likely to remember and negatively attribute their talc exposure to their cancer than are subjects without cancer. Additionally, their memory of volume and duration of exposure generally is enhanced by the same pressures. The potential for this bias is eliminated in prospective, cohort observational studies such as the Women’s Health Initiative Observational Study which, among 61,576 women, half of whom reported perineal talc exposure, did not measure a difference in the development of ovarian cancers during their 12 years of mean follow-up.3
Given these inherent biases, The biologic mechanism of talc carcinogenesis is largely theoretical. As mentioned earlier, prior to the 1970s, there was some observed contamination of talc with asbestos likely caused by the geologic proximity of these minerals. Asbestos is a known carcinogen, and therefore possibly could be harmful if a contaminant of talc. However, it is not known if this level of contamination was enough to be achieve ovarian carcinogenesis. Most theories of talc carcinogenesis are based on foreign body inflammatory reaction via talc particle ascent through the genital tract. This is proposed to induce an inflammatory release of prostaglandins and cytokines, which could cause a mutagenic effect promoting carcinogenesis. The foreign body inflammatory mechanism is further supported by the observation of a decreased incidence of ovarian cancer after hysterectomy or tubal ligation.4 However, inconsistently, a protective effect of NSAIDs has not been observed in ovarian cancer.5
A recent meta-analysis, which reviewed 27 of the largest, best-quality observational studies, identified a dose-effect response with an increased risk for ovarian cancer with greater than 3,600 lifetime applications, compared with less than 3,600 applications.2 The observed association between perineal talc exposure and increased risk of ovarian cancer appears to be consistent across a number of observational studies, including both case-control studies and prospective cohort studies (although somewhat mitigated in the latter). Additionally, there appears to be consistency in the finding that the risk is present for the epithelial subtypes of serous and endometrioid, but not mucinous or clear cell cancer. However, when considering the magnitude of effect, this remains somewhat small (odds ratio, 1.31; 95% confidence interval, 1.24-1.39) when compared with other better established carcinogenic relationships such as smoking and lung cancer where the hazard ratio is 12.12 (95% CI, 6.94-21.17).2,6
If talc does not cause ovarian cancer, why would this association be observed at all? One explanation could be that talc use is a confounder for the true causative mechanism. A theoretical example of this would be if the genital microbiome (a subject we have reviewed previously in this column) was the true culprit. If a particular microbiome profile promotes both oncogenic change in the ovary while also causing vaginal discharge and odor, it might increase the likelihood that perineal talc use is reported in the history of these cancer patients. This is purely speculative, but it always is important to consider the potential for confounding variables when utilizing observational studies to attribute cause and effect.
Therefore, there is a consistently observed association between perineal talc application and ovarian cancer, however, the relationship does not appear to be strong enough, associated with a proven carcinogenic mechanism, or free from interfering recall bias such to definitively state that perineal talc exposure causes ovarian cancer. Given these findings, it is reasonable to recommend patients avoid the use of perineal talc application until further definitive safety evidence is provided. In the meantime, it should be noted that even though talc-containing products are not commercially labeled as carcinogens, many pharmaceutical and cosmetic companies have replaced the mineral talc with corn starch in their powders.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at [email protected].
References
1. Cancer. 1982 Jul 15;50(2):372-6.
2. Epidemiology. 2018 Jan;29(1):41-9.
3. J Natl Cancer Inst. 2014 Sep 10;106(9). pii: dju208.
4. Am J Epidemiol. 1991 Aug 15;134(4):362-9.
5. Int J Cancer. 2008 Jan 1;122(1):170-6.
6. J Natl Cancer Inst. 2018 Nov 1;110(11):1201-7.
Pretreatment CT data may help predict immunotherapy benefit in ovarian cancer
Pretreatment CT data may help identify responders to immunotherapy in ovarian cancer, according to a new study.
Specifically, fewer sites of disease and lower intratumor heterogeneity on contrast-enhanced CT may indicate a higher likelihood of durable response to immune checkpoint inhibitors, according to results of the retrospective study, recently published in JCO Precision Oncology.
“Our results suggest that quantitative analysis of baseline contrast-enhanced CT may facilitate the delivery of precision medicine to patients with ovarian cancer by identifying patients who may benefit from immunotherapy,” wrote Yuki Himoto, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues.
The study leverages findings from the emerging field of radiomics, which the investigators note allows for “virtual sampling” of tumor heterogeneity within a single lesion and between lesions.
“This information may complement molecular profiling in personalizing medical decisions,” Dr. Himoto and coauthors explained.
The study cohort included 75 patients with recurrent ovarian cancer who were enrolled in ongoing, prospective trials of immunotherapy, according to the researchers. Of that group, just under one in five derived a durable clinical benefit, defined as progression-free survival lasting at least 24 weeks.
In univariable analysis, they found a number of contrast-enhanced CT variables were linked to durable clinical benefit, including fewer disease sites, lower cluster-site entropy and dissimilarity, which they wrote were an indicator of lower intertumor heterogeneity, and higher energy in the largest-volume lesion, which they described as an indicator of lower intratumor heterogeneity.
However, in multivariable analysis, the only variables that were still associated with durable clinical benefit were fewer disease sites (odds ratio, 1.64; 95% confidence interval, 1.19-2.27; P = .012) and higher energy in the largest lesion (odds ratio, 1.41; 95% CI, 1.11-1.81; P = .006), according to the report.
Those two factors combined were a composite indicator of durable clinical benefit (C-index, 0.821).
These findings could represent a step forward in the provision of immunotherapy in ovarian cancer, which exhibits poor response to immune checkpoint inhibitors, compared with some other cancer types, the investigators wrote.
More insights are needed, however, to help personalize the selection of immunotherapy in ovarian cancer, including a better understanding of cancer immune reactions and retooling of immune response criteria, they added.
“Composite multimodal multifaceted biomarkers that noninvasively capture spatiotemporal tumor heterogeneity will likely be necessary to comprehensively assess immune the tumor microenvironment and serve as clinical decision support for prognosis inference and prediction of response,” Dr. Himoto and associates wrote.
The study was supported by the National Cancer Institute, among other sources. Study authors reported disclosures related to Merck, Bristol-Myers Squibb, Genentech, Celgene, AstraZeneca, Y-mAbs Therapeutics, and others.
SOURCE: Himoto Y et al. JCO Precis Oncol. 2019 Aug 13. doi: 10.1200/PO.19.00038.
Pretreatment CT data may help identify responders to immunotherapy in ovarian cancer, according to a new study.
Specifically, fewer sites of disease and lower intratumor heterogeneity on contrast-enhanced CT may indicate a higher likelihood of durable response to immune checkpoint inhibitors, according to results of the retrospective study, recently published in JCO Precision Oncology.
“Our results suggest that quantitative analysis of baseline contrast-enhanced CT may facilitate the delivery of precision medicine to patients with ovarian cancer by identifying patients who may benefit from immunotherapy,” wrote Yuki Himoto, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues.
The study leverages findings from the emerging field of radiomics, which the investigators note allows for “virtual sampling” of tumor heterogeneity within a single lesion and between lesions.
“This information may complement molecular profiling in personalizing medical decisions,” Dr. Himoto and coauthors explained.
The study cohort included 75 patients with recurrent ovarian cancer who were enrolled in ongoing, prospective trials of immunotherapy, according to the researchers. Of that group, just under one in five derived a durable clinical benefit, defined as progression-free survival lasting at least 24 weeks.
In univariable analysis, they found a number of contrast-enhanced CT variables were linked to durable clinical benefit, including fewer disease sites, lower cluster-site entropy and dissimilarity, which they wrote were an indicator of lower intertumor heterogeneity, and higher energy in the largest-volume lesion, which they described as an indicator of lower intratumor heterogeneity.
However, in multivariable analysis, the only variables that were still associated with durable clinical benefit were fewer disease sites (odds ratio, 1.64; 95% confidence interval, 1.19-2.27; P = .012) and higher energy in the largest lesion (odds ratio, 1.41; 95% CI, 1.11-1.81; P = .006), according to the report.
Those two factors combined were a composite indicator of durable clinical benefit (C-index, 0.821).
These findings could represent a step forward in the provision of immunotherapy in ovarian cancer, which exhibits poor response to immune checkpoint inhibitors, compared with some other cancer types, the investigators wrote.
More insights are needed, however, to help personalize the selection of immunotherapy in ovarian cancer, including a better understanding of cancer immune reactions and retooling of immune response criteria, they added.
“Composite multimodal multifaceted biomarkers that noninvasively capture spatiotemporal tumor heterogeneity will likely be necessary to comprehensively assess immune the tumor microenvironment and serve as clinical decision support for prognosis inference and prediction of response,” Dr. Himoto and associates wrote.
The study was supported by the National Cancer Institute, among other sources. Study authors reported disclosures related to Merck, Bristol-Myers Squibb, Genentech, Celgene, AstraZeneca, Y-mAbs Therapeutics, and others.
SOURCE: Himoto Y et al. JCO Precis Oncol. 2019 Aug 13. doi: 10.1200/PO.19.00038.
Pretreatment CT data may help identify responders to immunotherapy in ovarian cancer, according to a new study.
Specifically, fewer sites of disease and lower intratumor heterogeneity on contrast-enhanced CT may indicate a higher likelihood of durable response to immune checkpoint inhibitors, according to results of the retrospective study, recently published in JCO Precision Oncology.
“Our results suggest that quantitative analysis of baseline contrast-enhanced CT may facilitate the delivery of precision medicine to patients with ovarian cancer by identifying patients who may benefit from immunotherapy,” wrote Yuki Himoto, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, and colleagues.
The study leverages findings from the emerging field of radiomics, which the investigators note allows for “virtual sampling” of tumor heterogeneity within a single lesion and between lesions.
“This information may complement molecular profiling in personalizing medical decisions,” Dr. Himoto and coauthors explained.
The study cohort included 75 patients with recurrent ovarian cancer who were enrolled in ongoing, prospective trials of immunotherapy, according to the researchers. Of that group, just under one in five derived a durable clinical benefit, defined as progression-free survival lasting at least 24 weeks.
In univariable analysis, they found a number of contrast-enhanced CT variables were linked to durable clinical benefit, including fewer disease sites, lower cluster-site entropy and dissimilarity, which they wrote were an indicator of lower intertumor heterogeneity, and higher energy in the largest-volume lesion, which they described as an indicator of lower intratumor heterogeneity.
However, in multivariable analysis, the only variables that were still associated with durable clinical benefit were fewer disease sites (odds ratio, 1.64; 95% confidence interval, 1.19-2.27; P = .012) and higher energy in the largest lesion (odds ratio, 1.41; 95% CI, 1.11-1.81; P = .006), according to the report.
Those two factors combined were a composite indicator of durable clinical benefit (C-index, 0.821).
These findings could represent a step forward in the provision of immunotherapy in ovarian cancer, which exhibits poor response to immune checkpoint inhibitors, compared with some other cancer types, the investigators wrote.
More insights are needed, however, to help personalize the selection of immunotherapy in ovarian cancer, including a better understanding of cancer immune reactions and retooling of immune response criteria, they added.
“Composite multimodal multifaceted biomarkers that noninvasively capture spatiotemporal tumor heterogeneity will likely be necessary to comprehensively assess immune the tumor microenvironment and serve as clinical decision support for prognosis inference and prediction of response,” Dr. Himoto and associates wrote.
The study was supported by the National Cancer Institute, among other sources. Study authors reported disclosures related to Merck, Bristol-Myers Squibb, Genentech, Celgene, AstraZeneca, Y-mAbs Therapeutics, and others.
SOURCE: Himoto Y et al. JCO Precis Oncol. 2019 Aug 13. doi: 10.1200/PO.19.00038.
FROM JCO PRECISION ONCOLOGY