User login
Use of genetic testing for congenital heart defect management
The average student in America learns that genes form the building blocks of what makes us human by the time they receive their high school diploma. Indeed, the completion of the Human Genome Project in 2003 paved the way for our genetic makeup, much like our medical history, to become a routine part of our health care. For example, our faculty at the University of Maryland School of Medicine discovered an important gene – CYP2C19 – which is involved in the metabolism of the antiplatelet medicine clopidogrel (Plavix). Although most people have this gene, some don’t. Therefore, when we manage a patient with coronary disease, we use a genetic screen to determine whether that patient has CYP2C19 and then modify therapy based on these results.
Our genes also have become commodities – from companies willing to analyze our genes to determine our racial and ethnic ancestry or propensity for certain diseases to those that can sequence the family dog’s genes.
Advances in genomics similarly have impacted ob.gyn. practice. Because of rapidly evolving gene analysis tools, we can now, for example, noninvasively test a developing fetus’s risk for chromosomal abnormalities and determine a baby’s sex by merely examining fetal DNA in a pregnant woman’s bloodstream. Although not diagnostic, these gene-based prenatal screening tests have reduced the need for unnecessary, costly, and highly invasive procedures for many of our patients.
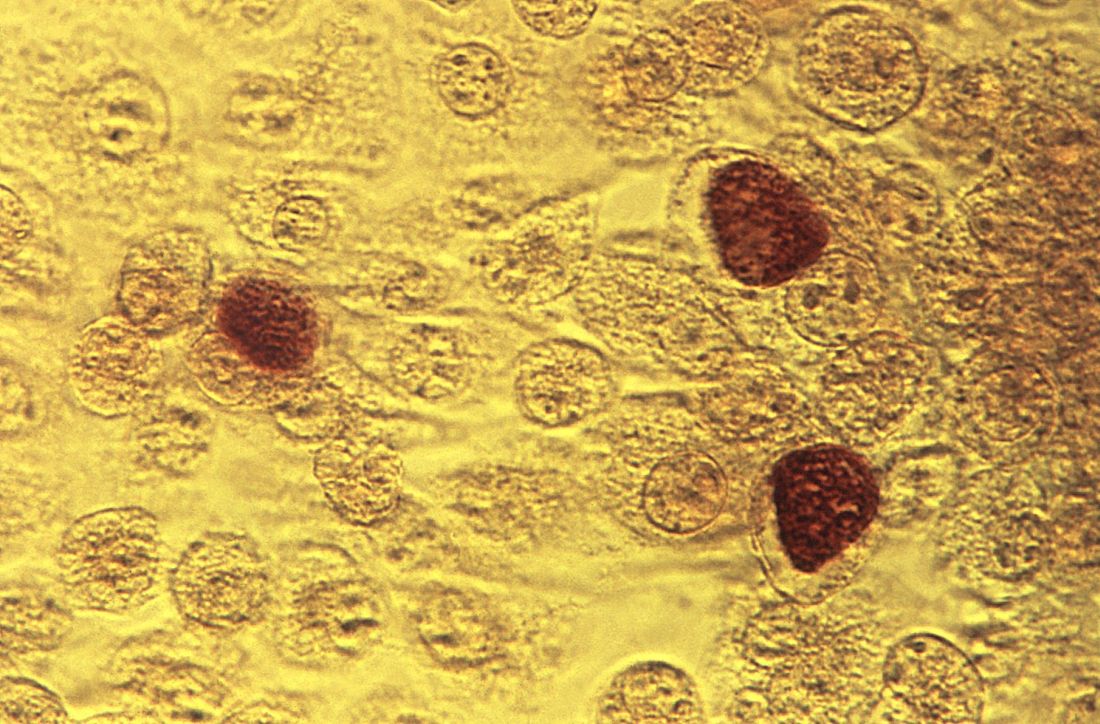
Importantly, our recognition that certain genes can confer a higher risk of disease has meant that performing a prenatal genetic evaluation can greatly inform the mother and her care team about potential problems her baby may have that may require additional management. For babies who have congenital heart defects, a genetic evaluation performed in addition to sonographic examination can provide ob.gyns. with crucial details to enhance pregnancy management and postnatal care decisions.
The importance of genetic testing and analysis in the detection, treatment, and prevention of congenital heart defects is the topic of part two of this two-part Master Class series authored by Shifa Turan, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. By using a combination of three- and four-dimensional ultrasound with gene assays, Dr. Turan and her colleagues can greatly enhance and personalize the care they deliver to their patients.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The average student in America learns that genes form the building blocks of what makes us human by the time they receive their high school diploma. Indeed, the completion of the Human Genome Project in 2003 paved the way for our genetic makeup, much like our medical history, to become a routine part of our health care. For example, our faculty at the University of Maryland School of Medicine discovered an important gene – CYP2C19 – which is involved in the metabolism of the antiplatelet medicine clopidogrel (Plavix). Although most people have this gene, some don’t. Therefore, when we manage a patient with coronary disease, we use a genetic screen to determine whether that patient has CYP2C19 and then modify therapy based on these results.
Our genes also have become commodities – from companies willing to analyze our genes to determine our racial and ethnic ancestry or propensity for certain diseases to those that can sequence the family dog’s genes.
Advances in genomics similarly have impacted ob.gyn. practice. Because of rapidly evolving gene analysis tools, we can now, for example, noninvasively test a developing fetus’s risk for chromosomal abnormalities and determine a baby’s sex by merely examining fetal DNA in a pregnant woman’s bloodstream. Although not diagnostic, these gene-based prenatal screening tests have reduced the need for unnecessary, costly, and highly invasive procedures for many of our patients.
Importantly, our recognition that certain genes can confer a higher risk of disease has meant that performing a prenatal genetic evaluation can greatly inform the mother and her care team about potential problems her baby may have that may require additional management. For babies who have congenital heart defects, a genetic evaluation performed in addition to sonographic examination can provide ob.gyns. with crucial details to enhance pregnancy management and postnatal care decisions.
The importance of genetic testing and analysis in the detection, treatment, and prevention of congenital heart defects is the topic of part two of this two-part Master Class series authored by Shifa Turan, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. By using a combination of three- and four-dimensional ultrasound with gene assays, Dr. Turan and her colleagues can greatly enhance and personalize the care they deliver to their patients.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
The average student in America learns that genes form the building blocks of what makes us human by the time they receive their high school diploma. Indeed, the completion of the Human Genome Project in 2003 paved the way for our genetic makeup, much like our medical history, to become a routine part of our health care. For example, our faculty at the University of Maryland School of Medicine discovered an important gene – CYP2C19 – which is involved in the metabolism of the antiplatelet medicine clopidogrel (Plavix). Although most people have this gene, some don’t. Therefore, when we manage a patient with coronary disease, we use a genetic screen to determine whether that patient has CYP2C19 and then modify therapy based on these results.
Our genes also have become commodities – from companies willing to analyze our genes to determine our racial and ethnic ancestry or propensity for certain diseases to those that can sequence the family dog’s genes.
Advances in genomics similarly have impacted ob.gyn. practice. Because of rapidly evolving gene analysis tools, we can now, for example, noninvasively test a developing fetus’s risk for chromosomal abnormalities and determine a baby’s sex by merely examining fetal DNA in a pregnant woman’s bloodstream. Although not diagnostic, these gene-based prenatal screening tests have reduced the need for unnecessary, costly, and highly invasive procedures for many of our patients.
Importantly, our recognition that certain genes can confer a higher risk of disease has meant that performing a prenatal genetic evaluation can greatly inform the mother and her care team about potential problems her baby may have that may require additional management. For babies who have congenital heart defects, a genetic evaluation performed in addition to sonographic examination can provide ob.gyns. with crucial details to enhance pregnancy management and postnatal care decisions.
The importance of genetic testing and analysis in the detection, treatment, and prevention of congenital heart defects is the topic of part two of this two-part Master Class series authored by Shifa Turan, MD, associate professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. By using a combination of three- and four-dimensional ultrasound with gene assays, Dr. Turan and her colleagues can greatly enhance and personalize the care they deliver to their patients.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland School of Medicine, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Genetic assessment for CHD: Case-specific, stepwise
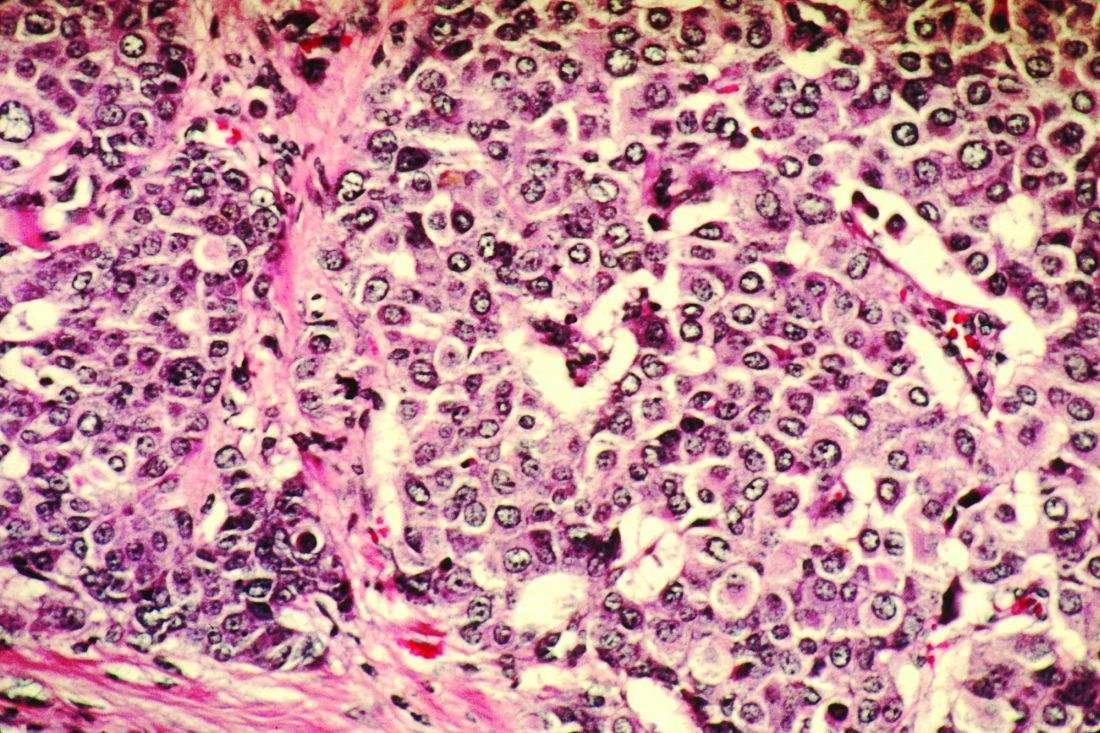
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.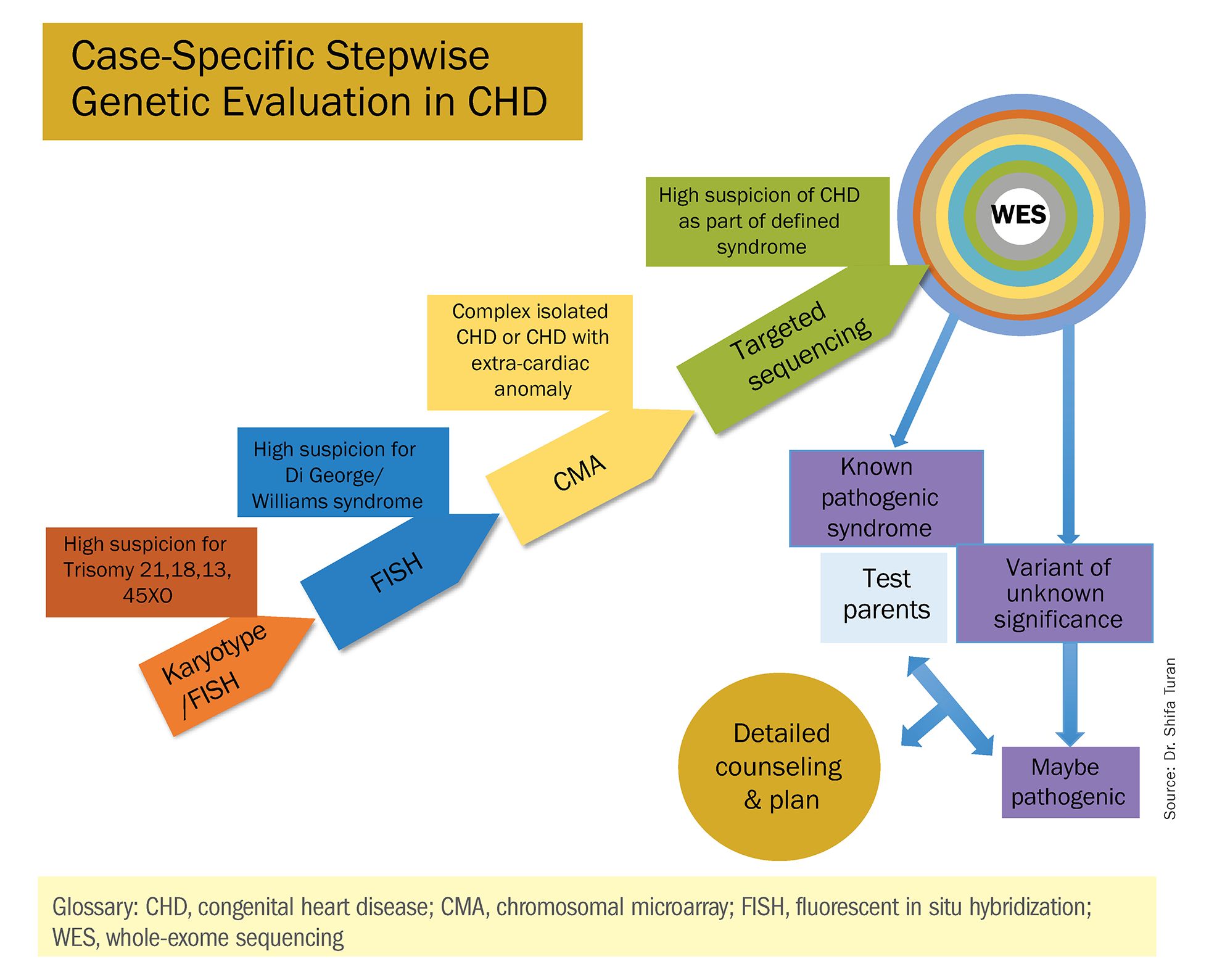
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.
At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
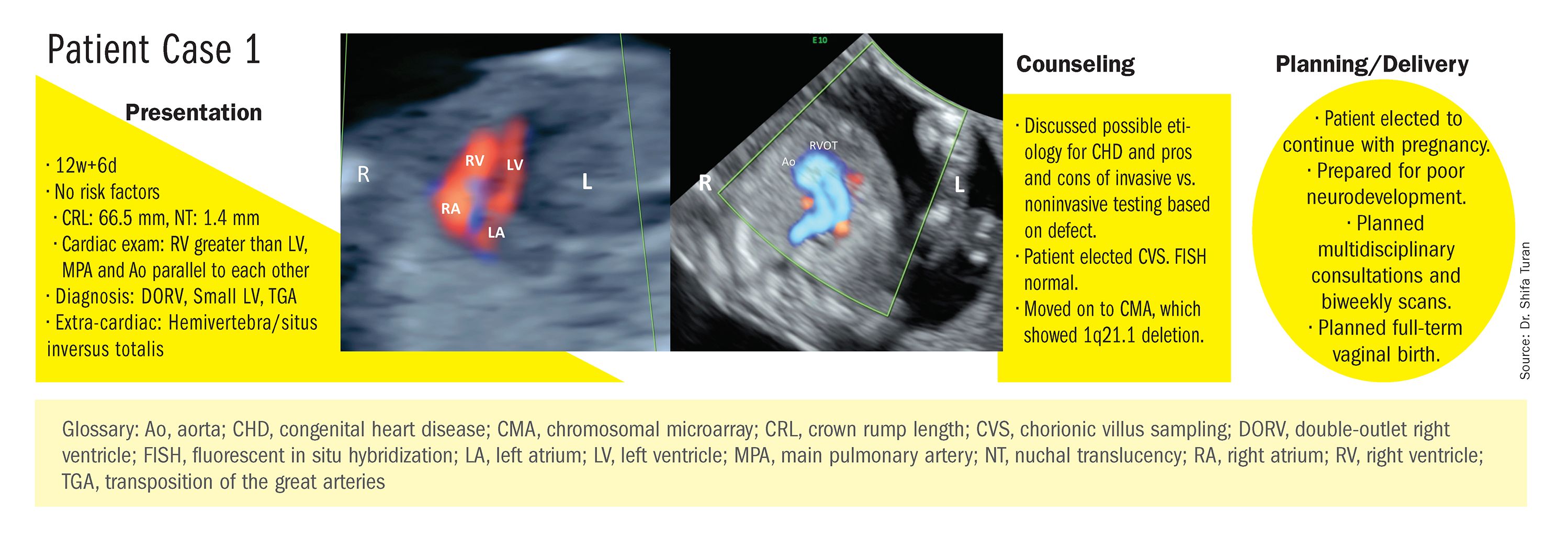
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
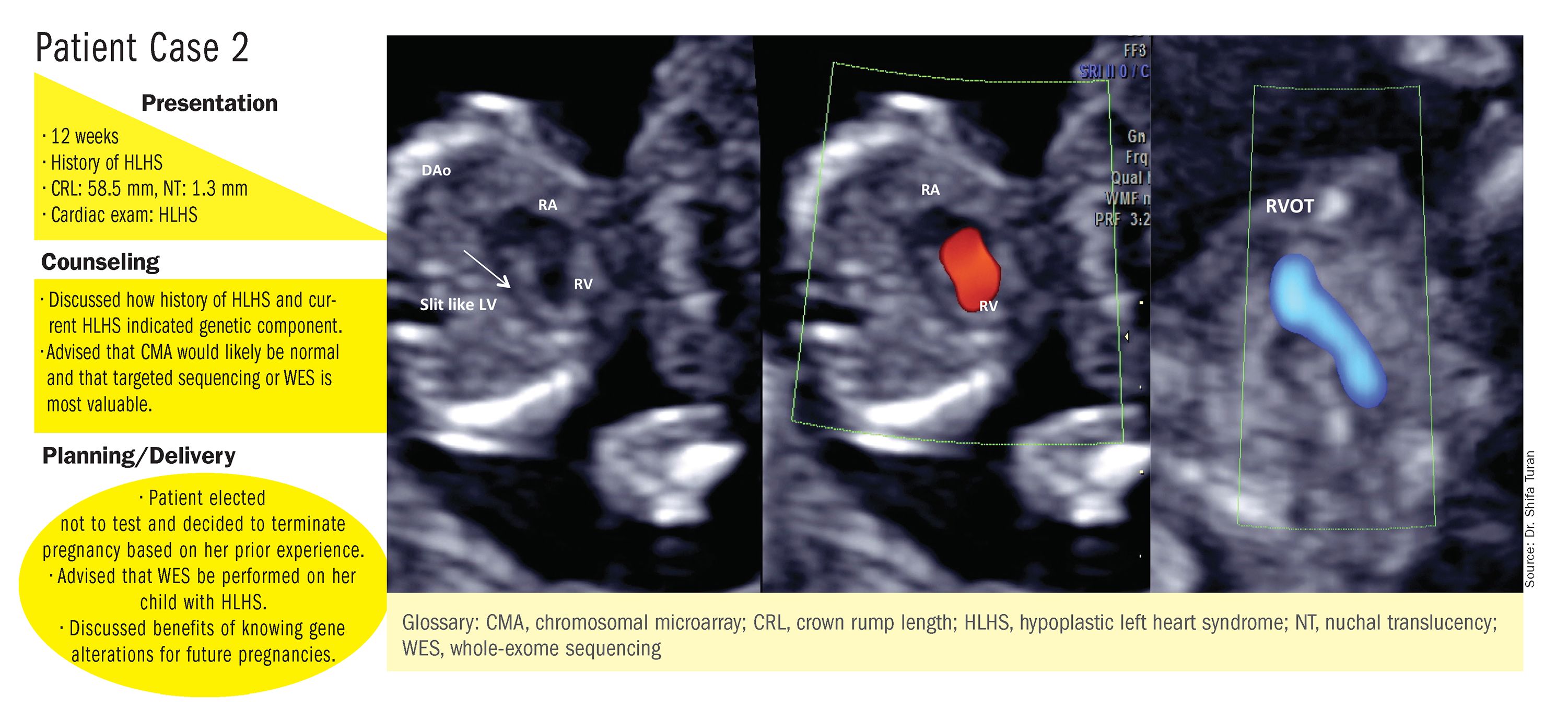
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.
These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at [email protected].
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.

At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.

These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at [email protected].
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
Congenital heart defects (CHDs) are etiologically heterogeneous, but in recent years it has become clear that genetics plays a larger role in the development of CHDs than was previously thought. Research has been shifting from a focus on risk – estimating the magnitude of increased risk, for instance, based on maternal or familial risk factors – to a focus on the etiology of cardiac defects.
In practice, advances in genetic testing technologies have made the underlying causes of CHDs increasingly detectable. Chromosomal microarray analysis (CMA) – technology that detects significantly more and smaller changes in the amount of chromosomal material than traditional karyotype – has been proven to increase the diagnostic yield in cases of isolated CHDs and CHDs with extracardiac anomalies. Targeted next-generation sequencing also is now available as an additional approach in selective cases, and a clinically viable option for whole-exome sequencing is fast approaching.
For researchers, genetic evaluation carries the potential to unravel remaining mysteries about underlying causes of CHDs – to provide pathological insights and identify potential therapeutic targets. Currently, about 6 % of the total pie of presumed genetic determinants of CHDs is attributed to chromosomal anomalies, 10% to copy number variants, and 12% to single-gene defects. The remaining 72% of etiology, approximately, is undetermined.
As Helen Taussig, MD, (known as the founder of pediatric cardiology) once said, common cardiac malformations occurring in otherwise “normal” individuals “must be genetic in origin.”1 Greater use of genetic testing – and in particular, of whole-exome sequencing – will drive down this “undetermined” piece of the genetics pie.
For clinicians and patients, prenatal genetic evaluation can inform clinical management, guiding decisions on the mode, timing, and location of delivery. Genetic assessments help guide the neonatal health care team in taking optimal care of the infant, and the surgeon in preparing for neonatal surgeries and postsurgical complications.
In a recent analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database, prenatal diagnosis was associated with a lower overall prevalence of major preoperative risk factors for cardiac surgery.2 Surgical outcomes themselves also have been shown to be better after the prenatal diagnosis of complex CHDs, mainly because of improvements in perioperative care.3
When genetic etiology is elucidated, the cardiologist also is better able to counsel patients about anticipated challenges – such as the propensity, with certain genetic variants of CHD, to develop neurodevelopmental delays or other cardiac complications – and to target patient follow-up. Patients also can make informed decisions about termination or continuation of a current pregnancy and about family planning in the future.
Fortunately, advances in genetics technology have paralleled technological advancements in ultrasound. As I discussed in part one of this two-part Master Class series, it is now possible to detect many major CHDs well before 16 weeks’ gestation. Checking the structure of the fetal heart at the first-trimester screening and sonography (11-14 weeks of gestation) offers the opportunity for early genetic assessment, counseling, and planning when anomalies are detected.
A personalized approach
There has been growing interest in recent years in CMA for the prenatal genetic workup of CHDs. Microarray targets chromosomal regions at a much higher resolution than traditional karyotype. Traditional karyotype assesses both changes in chromosome number as well as more subtle structural changes such as chromosomal deletions and duplications. CMA finds what traditional karyotype identifies, but in addition, it identifies much smaller, clinically relevant chromosomal deletions and duplications that are not detected by karyotype performed with or without fluorescence in-situ hybridization (FISH). FISH uses DNA probes that carry fluorescent tags to detect chromosomal DNA.

At our center, we studied the prenatal genetic test results of 145 fetuses diagnosed with CHDs. Each case involved FISH for aneuploidy/karyotype, followed by CMA in cases of a negative karyotype result. CMA increased the diagnostic yield in cases of CHD by 19.8% overall – 17.4% in cases of isolated CHD and 24.5% in cases of CHD plus extracardiac anomalies.4
Indeed, although a microarray costs more and takes an additional 2 weeks to run, CMA should be strongly considered as first-line testing for the prenatal genetic evaluation of fetuses with major structural cardiac abnormalities detected by ultrasound. However, there still are cases in which a karyotype might be sufficient. For instance, if I see that a fetus has an atrial-ventricular septal defect on a prenatal ultrasound, and there are markers for trisomy 21, 13, or 18, or Turner’s syndrome (45 XO), I usually recommend a karyotype or FISH rather than an initial CMA. If the karyotype is abnormal – which is likely in such a scenario – there isn’t a need for more extensive testing.
Similarly, when there is high suspicion for DiGeorge syndrome (the 22q11.2 deletion, which often includes cleft palate and aortic arch abnormalities), usually it is most appropriate to perform a FISH test.
CMA is the preferred first modality, however, when prenatal imaging suggests severe CHD – for instance, when there are signs of hypoplastic left heart syndrome or tetralogy of Fallot (a conotruncal defect) – or complex CHD with extracardiac anomalies. In these cases, there is a high likelihood of detecting a small deletion or duplication that would be missed with karyotype.
In the past decade, karyotype and CMA have become the major methods used in our practice. However, targeted next‐generation sequencing and whole‐exome sequencing may become more widely used because these technologies enable rapid analysis of a large number of gene sequences and facilitate discovery of novel causative genes in many genetic diseases that cause CHDs.
Currently, targeted next-generation sequencing has mainly been used in the postnatal setting, and there are limited data available on its prenatal use. Compared with whole-exome sequencing, which sequences all of the protein-coding regions of the genome, targeted next-generation sequencing panels select regions of genes that are known to be associated with diseases of interest.
For CHDs, some perinatal centers have begun using a customized gene panel that targets 77 CHD-associated genes. This particular panel has been shown to be useful in addition to current methods and is an effective tool for prenatal genetic diagnosis.5
Whole-exome sequencing is currently expensive and time consuming. While sometimes it is used in the postnatal context, it is not yet part of routine practice as a prenatal diagnostic tool. As technology advances this will change – early in the next decade, I believe. For now, whole-exome sequencing may be an option for some patients who want to know more when severe CHD is evident on ultrasound and there are negative results from CMA or targeted sequencing. We have diagnosed some rare genetic syndromes using whole-exome sequencing; these diagnoses helped us to better manage the pregnancies.

These choices are part of the case-specific, stepwise approach to genetic evaluation that we take in our fetal heart program. Our goal is to pursue information that will be accurate and valuable for the patient and clinicians, in the most cost-effective and timely manner.
Limitations of noninvasive screening
In our fetal heart program we see increasing numbers of referred patients who have chosen noninvasive cell-free fetal DNA screening (cfDNA) after a cardiac anomaly is detected on ultrasound examination, and who believe that their “low risk” results demonstrate very little or no risk of CHD. Many of these patients express a belief that noninvasive testing is highly sensitive and accurate for fetal anomalies, including CHDs, and are not easily convinced of the value of other genetic tests.
We recently conducted a retrospective chart analysis (unpublished) in which we found that 41% of cases of CHD with abnormal genetics results were not detectable by cfDNA screening.
In the case of atrial-ventricular septal defects and conotruncal abnormalities that often are more associated with common aneuploidies (trisomy 21, 18, 13, and 45 XO), a “high-risk” result from cfDNA screening may offer the family and cardiology/neonatal team some guidance, but a “low-risk” result does not eliminate the risk of a microarray abnormality and thus may provide false reassurance.
Other research has shown that noninvasive screening will miss up to 7.3% of karyotype abnormalities in pregnancies at high risk for common aneuploidies.6
While invasive testing poses a very small risk of miscarriage, it is hard without such testing to elucidate the potential genetic etiologies of CHDs and truly understand the problems. We must take time to thoughtfully counsel patients who decline invasive testing about the limitations of cfDNA screening for CHDs and other anomalies.
Dr. Turan is an associate professor of obstetrics, gynecology, and reproductive sciences, and director of the fetal heart program at the University of Maryland School of Medicine and director of the Fetal Heart Program at the University of Maryland Medical Center. Dr. Turan reported that she has no disclosures relevant to this Master Class. Email her at [email protected].
References
1. J Am Coll Cardiol. 1988 Oct;12(4):1079-86.
2. Pediatr Cardiol. 2019 Mar;40(3):489-96.
3. Ann Pediatr Cardiol. 2017 May-Aug;10(2):126-30.
4. Eur J Obstet Gynecol Reprod Biol 2018;221:172-76.
5. Ultrasound Obstet Gynecol. 2018 Aug;52(2):205-11.
6. PLoS One. 2016 Jan 15;11(1):e0146794.
Try testosterone for some women with sexual dysfunction, but not others
A new international position statement on testosterone therapy for women concludes that a trial of testosterone is appropriate for postmenopausal women with hypoactive sexual desire dysfunction (HSDD) and that its use for any other condition, symptom, or reason is not supported by available evidence.
The seven-page position statement, developed by an international task force of experts from the Endocrine Society, the American College of Gynecologists and Obstetricians, and multiple other medical societies, also emphasized that blood concentrations of testosterone should approximate premenopausal physiological conditions.
“When testosterone therapy is given, the resultant blood levels should not be above those seen in healthy young women,” said lead author Susan Ruth Davis, PhD, MBBS, of Monash University in Melbourne, Australia, in a press release issued by the Endocrine Society. Dr. Davis is president of the International Menopause Society, which coordinated the panel.
The statement was published in the Journal of Clinical Endocrinology & Metabolism and three other medical journals.
Margaret E. Wierman, MD, who represented the Endocrine Society on the task force, said in an interview that there has been “growing concern about testosterone being prescribed for a variety of signs and symptoms without data to support” such use. At the same time, there is significant concern about the ongoing lack of approved formulations licensed specifically for women, she said.
In part, the statement is about a renewed “call to industry to make some [female-specific] formulations so that we can examine other potential roles of testosterone in women,” said Dr. Wierman, professor of medicine and physiology at the University of Colorado at Denver, Aurora, and chief of endocrinology at the Rocky Mountain Regional Veterans Affairs Medical Center in Aurora.
“Testosterone may be useful [for indications other than HSDD], but we don’t know. There may be no [breast or cardiovascular disease risk], but we don’t know,” she said. “And without a formulation to study potential benefits and risks, it’s good to be cautious. It’s good to really outline where we have data and where we don’t.”
The Endocrine Society’s 2014 clinical practice guideline on androgen therapy in women, for which Dr. Wierman was the lead author, also recommended against the off-label use of testosterone for sexual dysfunction other than HSDD or for any other reason, such as cognitive, cardiovascular, metabolic, or bone health. As with the new statement, the society’s position statement was guided by an international, multisociety task force, albeit a smaller one.
For the new global position statement, the task force’s review of evidence includes a recently published systematic review and meta-analysis of randomized controlled trial data – of at least 12 weeks’ duration – on the use of testosterone for sexual function, cardiometabolic variables, cognitive measures, and musculoskeletal health. Some of the data from the randomized controlled trials were unpublished.
The meta-analysis, led by Dr. Davis and published in July in the Lancet Diabetes & Endocrinology, found that, compared with placebo or a comparator (such as estrogen, with or without progesterone), testosterone in either oral or transdermal form significantly improved sexual function in postmenopausal women. However, data about the effects of testosterone for other indications, its long-term safety, and its use in premenopausal women, were insufficient for drawing any conclusions (Lancet Diabetes Endocrinol. 2019 Jul 25. doi: 10.1016/S2213-8587[19]30189-5).
In addition, testosterone administered orally – but not nonorally (patch or cream) – was associated with adverse lipid profiles, Dr. Davis and her colleagues reported.
Another systematic review and meta-analysis, published in Fertility and Sterility in 2017 and included in the task force’s evidence review, focused specifically on transdermal testosterone for menopausal women with HSDD, with or without estrogen and progestin therapy. It also showed short-term efficacy in terms of improvement in sexual function, as well as short-term safety (Fertil Steril. 2017;107(2):475-82).
The new position statement warns about the lack of long-term safety data, stating that “safety data for testosterone in physiologic doses are not available beyond 24 months of treatment.”
In the short term, testosterone therapy for postmenopausal women (in doses approximating testosterone concentrations for premenopausal women), is associated with mild increases in acne and body/facial hair growth in some women, but not with alopecia, clitoromegaly, or voice change. Short-term transdermal therapy also does not seem to affect breast cancer risk or have any significant effects on lipid profiles, the statement says.
The panel points out, however, that randomized controlled trials with testosterone therapy have excluded women who are at high risk of cardiometabolic disease, and that women with a previous diagnosis of breast cancer have also been excluded from randomized trials of testosterone in women with HSDD. This is a “big issue,” said Dr. Wierman, and means that recommendations regarding the effect of testosterone in postmenopausal women with HSDD may not be generalizable to possible at-risk subpopulations.
The panel endorsed testosterone therapy specifically for women with HSDD because most of the studies reporting on sexual function have recruited women with diagnosed HSDD. Demonstrated benefits of testosterone in these cases include improved sexual desire, arousal, orgasm, and pleasure, and reduced concerns and distress about sex. HSDD should be diagnosed after formal biopsychosocial assessment, the statement notes.
“We don’t completely understand the control of sexual function in women, but it’s very dependent on estrogen status. And it’s also dependent on psychosocial factors, emotional health, relationship issues, and physical issues,” Dr. Wierman said in the interview.
“In practice, we look at all these issues, and we first optimize estrogen status. Once that’s done, and we’ve looked at all the other components of sexual function, then we can consider off-label use of testosterone,” she said. “If there’s no response in 3-6 months, we stop it.”
Testosterone levels do not correlate with sexual dysfunction, Dr. Wierman emphasized, and direct assays for the measurement of total and free testosterone are unreliable. The statement acknowledges that but still recommends measurement of testosterone using direct assays, in cases in which liquid/gas chromatography and tandem mass spectrometry assay (which has “high accuracy and reproducibility”) are not available. This is “to exclude high baseline concentrations and also to exclude supraphysiological concentrations during treatment,” the panel said.
Most endocrinologists and other experts who prescribe testosterone therapy for women use an approved male formulation off label and adjust it – an approach that the panel says is reasonable as long as hormone concentrations are “maintained in the physiologic female range.”
Compounded “bioidentical” testosterone therapy “cannot be recommended for the treatment of HSDD because of the lack of evidence for safety and efficacy,” the statement says.
“A big concern of many endocrinologists,” Dr. Wierman added, “is the recent explosion of using pharmacological levels of both estrogen and testosterone in either [injections] or pellets.” The Endocrine Society and other societies have alerted the Food and Drug Administration to “this new cottage industry, which may have significant side effects and risks for our patients,” she said.
Dr. Wierman reported received funding from Corcept Therapeutics, Novartis, and the Cancer League of Colorado, and honoraria or consultation fees from Pfizer to review ASPIRE grant applications for studies of acromegaly as well as Endocrine Society honorarium for teaching in the Endocrine Board Review and Clinical Endocrine Update. Dr. Davis reported receiving funding from a National Health and Medical Research Council Project Grant, a National Breast Foundation accelerator grant, and the Grollo-Ruzenne Foundation, as well as honoraria from Besins and Pfizer Australia. She has been a consultant to Besins Healthcare, Mayne Pharmaceuticals, Lawley Pharmaceuticals, and Que Oncology. Disclosures for other authors of the position statement are listed with the statement.
SOURCE: Davis SR et al. J Clin Endocrinol Metab. 2019 Sep 2. doi: 10.1210/jc.2019-01603.
A new international position statement on testosterone therapy for women concludes that a trial of testosterone is appropriate for postmenopausal women with hypoactive sexual desire dysfunction (HSDD) and that its use for any other condition, symptom, or reason is not supported by available evidence.
The seven-page position statement, developed by an international task force of experts from the Endocrine Society, the American College of Gynecologists and Obstetricians, and multiple other medical societies, also emphasized that blood concentrations of testosterone should approximate premenopausal physiological conditions.
“When testosterone therapy is given, the resultant blood levels should not be above those seen in healthy young women,” said lead author Susan Ruth Davis, PhD, MBBS, of Monash University in Melbourne, Australia, in a press release issued by the Endocrine Society. Dr. Davis is president of the International Menopause Society, which coordinated the panel.
The statement was published in the Journal of Clinical Endocrinology & Metabolism and three other medical journals.
Margaret E. Wierman, MD, who represented the Endocrine Society on the task force, said in an interview that there has been “growing concern about testosterone being prescribed for a variety of signs and symptoms without data to support” such use. At the same time, there is significant concern about the ongoing lack of approved formulations licensed specifically for women, she said.
In part, the statement is about a renewed “call to industry to make some [female-specific] formulations so that we can examine other potential roles of testosterone in women,” said Dr. Wierman, professor of medicine and physiology at the University of Colorado at Denver, Aurora, and chief of endocrinology at the Rocky Mountain Regional Veterans Affairs Medical Center in Aurora.
“Testosterone may be useful [for indications other than HSDD], but we don’t know. There may be no [breast or cardiovascular disease risk], but we don’t know,” she said. “And without a formulation to study potential benefits and risks, it’s good to be cautious. It’s good to really outline where we have data and where we don’t.”
The Endocrine Society’s 2014 clinical practice guideline on androgen therapy in women, for which Dr. Wierman was the lead author, also recommended against the off-label use of testosterone for sexual dysfunction other than HSDD or for any other reason, such as cognitive, cardiovascular, metabolic, or bone health. As with the new statement, the society’s position statement was guided by an international, multisociety task force, albeit a smaller one.
For the new global position statement, the task force’s review of evidence includes a recently published systematic review and meta-analysis of randomized controlled trial data – of at least 12 weeks’ duration – on the use of testosterone for sexual function, cardiometabolic variables, cognitive measures, and musculoskeletal health. Some of the data from the randomized controlled trials were unpublished.
The meta-analysis, led by Dr. Davis and published in July in the Lancet Diabetes & Endocrinology, found that, compared with placebo or a comparator (such as estrogen, with or without progesterone), testosterone in either oral or transdermal form significantly improved sexual function in postmenopausal women. However, data about the effects of testosterone for other indications, its long-term safety, and its use in premenopausal women, were insufficient for drawing any conclusions (Lancet Diabetes Endocrinol. 2019 Jul 25. doi: 10.1016/S2213-8587[19]30189-5).
In addition, testosterone administered orally – but not nonorally (patch or cream) – was associated with adverse lipid profiles, Dr. Davis and her colleagues reported.
Another systematic review and meta-analysis, published in Fertility and Sterility in 2017 and included in the task force’s evidence review, focused specifically on transdermal testosterone for menopausal women with HSDD, with or without estrogen and progestin therapy. It also showed short-term efficacy in terms of improvement in sexual function, as well as short-term safety (Fertil Steril. 2017;107(2):475-82).
The new position statement warns about the lack of long-term safety data, stating that “safety data for testosterone in physiologic doses are not available beyond 24 months of treatment.”
In the short term, testosterone therapy for postmenopausal women (in doses approximating testosterone concentrations for premenopausal women), is associated with mild increases in acne and body/facial hair growth in some women, but not with alopecia, clitoromegaly, or voice change. Short-term transdermal therapy also does not seem to affect breast cancer risk or have any significant effects on lipid profiles, the statement says.
The panel points out, however, that randomized controlled trials with testosterone therapy have excluded women who are at high risk of cardiometabolic disease, and that women with a previous diagnosis of breast cancer have also been excluded from randomized trials of testosterone in women with HSDD. This is a “big issue,” said Dr. Wierman, and means that recommendations regarding the effect of testosterone in postmenopausal women with HSDD may not be generalizable to possible at-risk subpopulations.
The panel endorsed testosterone therapy specifically for women with HSDD because most of the studies reporting on sexual function have recruited women with diagnosed HSDD. Demonstrated benefits of testosterone in these cases include improved sexual desire, arousal, orgasm, and pleasure, and reduced concerns and distress about sex. HSDD should be diagnosed after formal biopsychosocial assessment, the statement notes.
“We don’t completely understand the control of sexual function in women, but it’s very dependent on estrogen status. And it’s also dependent on psychosocial factors, emotional health, relationship issues, and physical issues,” Dr. Wierman said in the interview.
“In practice, we look at all these issues, and we first optimize estrogen status. Once that’s done, and we’ve looked at all the other components of sexual function, then we can consider off-label use of testosterone,” she said. “If there’s no response in 3-6 months, we stop it.”
Testosterone levels do not correlate with sexual dysfunction, Dr. Wierman emphasized, and direct assays for the measurement of total and free testosterone are unreliable. The statement acknowledges that but still recommends measurement of testosterone using direct assays, in cases in which liquid/gas chromatography and tandem mass spectrometry assay (which has “high accuracy and reproducibility”) are not available. This is “to exclude high baseline concentrations and also to exclude supraphysiological concentrations during treatment,” the panel said.
Most endocrinologists and other experts who prescribe testosterone therapy for women use an approved male formulation off label and adjust it – an approach that the panel says is reasonable as long as hormone concentrations are “maintained in the physiologic female range.”
Compounded “bioidentical” testosterone therapy “cannot be recommended for the treatment of HSDD because of the lack of evidence for safety and efficacy,” the statement says.
“A big concern of many endocrinologists,” Dr. Wierman added, “is the recent explosion of using pharmacological levels of both estrogen and testosterone in either [injections] or pellets.” The Endocrine Society and other societies have alerted the Food and Drug Administration to “this new cottage industry, which may have significant side effects and risks for our patients,” she said.
Dr. Wierman reported received funding from Corcept Therapeutics, Novartis, and the Cancer League of Colorado, and honoraria or consultation fees from Pfizer to review ASPIRE grant applications for studies of acromegaly as well as Endocrine Society honorarium for teaching in the Endocrine Board Review and Clinical Endocrine Update. Dr. Davis reported receiving funding from a National Health and Medical Research Council Project Grant, a National Breast Foundation accelerator grant, and the Grollo-Ruzenne Foundation, as well as honoraria from Besins and Pfizer Australia. She has been a consultant to Besins Healthcare, Mayne Pharmaceuticals, Lawley Pharmaceuticals, and Que Oncology. Disclosures for other authors of the position statement are listed with the statement.
SOURCE: Davis SR et al. J Clin Endocrinol Metab. 2019 Sep 2. doi: 10.1210/jc.2019-01603.
A new international position statement on testosterone therapy for women concludes that a trial of testosterone is appropriate for postmenopausal women with hypoactive sexual desire dysfunction (HSDD) and that its use for any other condition, symptom, or reason is not supported by available evidence.
The seven-page position statement, developed by an international task force of experts from the Endocrine Society, the American College of Gynecologists and Obstetricians, and multiple other medical societies, also emphasized that blood concentrations of testosterone should approximate premenopausal physiological conditions.
“When testosterone therapy is given, the resultant blood levels should not be above those seen in healthy young women,” said lead author Susan Ruth Davis, PhD, MBBS, of Monash University in Melbourne, Australia, in a press release issued by the Endocrine Society. Dr. Davis is president of the International Menopause Society, which coordinated the panel.
The statement was published in the Journal of Clinical Endocrinology & Metabolism and three other medical journals.
Margaret E. Wierman, MD, who represented the Endocrine Society on the task force, said in an interview that there has been “growing concern about testosterone being prescribed for a variety of signs and symptoms without data to support” such use. At the same time, there is significant concern about the ongoing lack of approved formulations licensed specifically for women, she said.
In part, the statement is about a renewed “call to industry to make some [female-specific] formulations so that we can examine other potential roles of testosterone in women,” said Dr. Wierman, professor of medicine and physiology at the University of Colorado at Denver, Aurora, and chief of endocrinology at the Rocky Mountain Regional Veterans Affairs Medical Center in Aurora.
“Testosterone may be useful [for indications other than HSDD], but we don’t know. There may be no [breast or cardiovascular disease risk], but we don’t know,” she said. “And without a formulation to study potential benefits and risks, it’s good to be cautious. It’s good to really outline where we have data and where we don’t.”
The Endocrine Society’s 2014 clinical practice guideline on androgen therapy in women, for which Dr. Wierman was the lead author, also recommended against the off-label use of testosterone for sexual dysfunction other than HSDD or for any other reason, such as cognitive, cardiovascular, metabolic, or bone health. As with the new statement, the society’s position statement was guided by an international, multisociety task force, albeit a smaller one.
For the new global position statement, the task force’s review of evidence includes a recently published systematic review and meta-analysis of randomized controlled trial data – of at least 12 weeks’ duration – on the use of testosterone for sexual function, cardiometabolic variables, cognitive measures, and musculoskeletal health. Some of the data from the randomized controlled trials were unpublished.
The meta-analysis, led by Dr. Davis and published in July in the Lancet Diabetes & Endocrinology, found that, compared with placebo or a comparator (such as estrogen, with or without progesterone), testosterone in either oral or transdermal form significantly improved sexual function in postmenopausal women. However, data about the effects of testosterone for other indications, its long-term safety, and its use in premenopausal women, were insufficient for drawing any conclusions (Lancet Diabetes Endocrinol. 2019 Jul 25. doi: 10.1016/S2213-8587[19]30189-5).
In addition, testosterone administered orally – but not nonorally (patch or cream) – was associated with adverse lipid profiles, Dr. Davis and her colleagues reported.
Another systematic review and meta-analysis, published in Fertility and Sterility in 2017 and included in the task force’s evidence review, focused specifically on transdermal testosterone for menopausal women with HSDD, with or without estrogen and progestin therapy. It also showed short-term efficacy in terms of improvement in sexual function, as well as short-term safety (Fertil Steril. 2017;107(2):475-82).
The new position statement warns about the lack of long-term safety data, stating that “safety data for testosterone in physiologic doses are not available beyond 24 months of treatment.”
In the short term, testosterone therapy for postmenopausal women (in doses approximating testosterone concentrations for premenopausal women), is associated with mild increases in acne and body/facial hair growth in some women, but not with alopecia, clitoromegaly, or voice change. Short-term transdermal therapy also does not seem to affect breast cancer risk or have any significant effects on lipid profiles, the statement says.
The panel points out, however, that randomized controlled trials with testosterone therapy have excluded women who are at high risk of cardiometabolic disease, and that women with a previous diagnosis of breast cancer have also been excluded from randomized trials of testosterone in women with HSDD. This is a “big issue,” said Dr. Wierman, and means that recommendations regarding the effect of testosterone in postmenopausal women with HSDD may not be generalizable to possible at-risk subpopulations.
The panel endorsed testosterone therapy specifically for women with HSDD because most of the studies reporting on sexual function have recruited women with diagnosed HSDD. Demonstrated benefits of testosterone in these cases include improved sexual desire, arousal, orgasm, and pleasure, and reduced concerns and distress about sex. HSDD should be diagnosed after formal biopsychosocial assessment, the statement notes.
“We don’t completely understand the control of sexual function in women, but it’s very dependent on estrogen status. And it’s also dependent on psychosocial factors, emotional health, relationship issues, and physical issues,” Dr. Wierman said in the interview.
“In practice, we look at all these issues, and we first optimize estrogen status. Once that’s done, and we’ve looked at all the other components of sexual function, then we can consider off-label use of testosterone,” she said. “If there’s no response in 3-6 months, we stop it.”
Testosterone levels do not correlate with sexual dysfunction, Dr. Wierman emphasized, and direct assays for the measurement of total and free testosterone are unreliable. The statement acknowledges that but still recommends measurement of testosterone using direct assays, in cases in which liquid/gas chromatography and tandem mass spectrometry assay (which has “high accuracy and reproducibility”) are not available. This is “to exclude high baseline concentrations and also to exclude supraphysiological concentrations during treatment,” the panel said.
Most endocrinologists and other experts who prescribe testosterone therapy for women use an approved male formulation off label and adjust it – an approach that the panel says is reasonable as long as hormone concentrations are “maintained in the physiologic female range.”
Compounded “bioidentical” testosterone therapy “cannot be recommended for the treatment of HSDD because of the lack of evidence for safety and efficacy,” the statement says.
“A big concern of many endocrinologists,” Dr. Wierman added, “is the recent explosion of using pharmacological levels of both estrogen and testosterone in either [injections] or pellets.” The Endocrine Society and other societies have alerted the Food and Drug Administration to “this new cottage industry, which may have significant side effects and risks for our patients,” she said.
Dr. Wierman reported received funding from Corcept Therapeutics, Novartis, and the Cancer League of Colorado, and honoraria or consultation fees from Pfizer to review ASPIRE grant applications for studies of acromegaly as well as Endocrine Society honorarium for teaching in the Endocrine Board Review and Clinical Endocrine Update. Dr. Davis reported receiving funding from a National Health and Medical Research Council Project Grant, a National Breast Foundation accelerator grant, and the Grollo-Ruzenne Foundation, as well as honoraria from Besins and Pfizer Australia. She has been a consultant to Besins Healthcare, Mayne Pharmaceuticals, Lawley Pharmaceuticals, and Que Oncology. Disclosures for other authors of the position statement are listed with the statement.
SOURCE: Davis SR et al. J Clin Endocrinol Metab. 2019 Sep 2. doi: 10.1210/jc.2019-01603.
FROM JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Zulresso: Hope and lingering questions
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at [email protected].
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at [email protected].
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The last decade has brought increasing awareness of the need to effectively screen for postpartum depression, with a majority of states across the country now having some sort of formal program by which women are screened for mood disorder during the postnatal period, typically with scales such as the Edinburgh Postnatal Depression Scale (EPDS).
In addition to effective screening is a pressing need for effective referral networks of clinicians who have both the expertise and time to manage the 10%-15% of women who have been identified and who suffer from postpartum psychiatric disorders – both postpartum mood and anxiety disorders. Several studies have suggested that only a small percentage of postpartum women who score with clinically significant level of depressive symptoms actually get to a clinician or, if they do get to a clinician, receive adequate treatment restoring their emotional well-being (J Clin Psychiatry. 2016 Sep;77[9]:1189-200).
Zulresso (brexanolone), a novel new antidepressant medication which recently received Food and Drug Administration approval for the treatment of postpartum depression, is a first-in-class molecule to get such approval. Zulresso is a neurosteroid, an analogue of allopregnanolone and a GABAA receptor–positive allosteric modulator, a primary inhibitory neurotransmitter in the brain.
There is every reason to believe that, as a class, this group of neurosteroid molecules are effective in treating depression in other populations aside from women with postpartum depression and hence may not be specific to the postpartum period. For example, recent presentations of preliminary data suggest other neurosteroids such as zuranolone (an oral medication also developed by Sage Therapeutics) is effective for both men and women who have major depression in addition to women suffering from postpartum depression.
Zulresso is approved through a Risk Evaluation and Mitigation Strategy–restricted program and, per that protocol, needs to be administered by a health care provider in a recognized health care setting intravenously over 2.5 days (60 hours). Because of concerns regarding increased sedation, continuous pulse oximetry is required, and this is outlined in a boxed warning in the prescribing information. Zulresso has been classified by the Drug Enforcement Administration (DEA) as a Schedule IV injection and is subject to the prescribing regulations for a controlled substance.
Since Zulresso’s approval, my colleagues and I at the Center for Women’s Mental Health have received numerous queries from patients and colleagues about our clinical impression of this new molecule with a different mechanism of action – a welcome addition to the antidepressant pharmacopeia. The question posed to us essentially is: Where does brexanolone fit into our algorithm for treating women who suffer from postpartum depression? And frequently, the follow-up query is: Because subjects in the clinical trials for this medication included women who had onset of depression either late in pregnancy or during the postpartum period, how specific is brexanolone with respect to being a targeted therapy for postpartum depression, compared with depression encountered in other clinical settings.
What clearly can be stated is that Zulresso has a rapid onset of action and was demonstrated across clinical trials to have sustained benefit up to 30 days after IV administration. The question is whether patients have sustained benefit after 30 days or if this is a medicine to be considered as a “bridge” to other treatment. Data answering that critical clinical question are unavailable at this time. From a clinical standpoint, do patients receive this treatment and get sent home on antidepressants, as we would for patients who receive ECT, often discharging them with prophylactic antidepressants to sustain the benefit of the treatment? Or do patients receive this new medicine with the clinician providing close follow-up, assuming a wait-and-see approach? Because data informing the answer to that question are not available, this decision will be made empirically, frequently factoring in the patient’s past clinical history where presumably more liberal use of antidepressant immediately after the administration of Zulresso will be pursued in those with histories of highly recurrent major depression.
So where might this new medicine fit into the treatment of postpartum depression of moderate severity, or modest to moderate severity? It should be kept in mind that for patients with mild to moderate postpartum depression, there are data supporting the efficacy of cognitive-behavioral therapy (CBT). CBT frequently is pursued with concurrent mobilization of substantial social support with good outcomes. In patients with more severe postpartum depression, there are data supporting the use of antidepressants, and in these patients as well, use of established support from the ever-growing network of community-based support groups and services can be particularly helpful. It is unlikely that Zulresso will be a first-line medication for the treatment of postpartum depression, but it may be particularly appropriate for patients with severe illness who have not responded to other interventions.
Other practical considerations regarding use of Zulresso include the requirement that the medicine be administered in hospitals that have established clinical infrastructure to accommodate this particular population of patients and where pharmacists and other relevant parties in hospitals have accepted the medicine into its drug formulary. While coverage by various insurance policies may vary, the cost of this new medication is substantial, between $24,000 and $34,000 per treatment, according to reports.
Where Zulresso fits into the pharmacopeia for treating postpartum depression may fall well beyond the issues of efficacy. Given all of the attention to this first-in-class medicine, Zulresso has reinforced the growing interest in the substantial prevalence and the morbidity associated with postpartum depression. It is hard to imagine Zulresso being used in cases of more mild to moderate depression, in which there is nonemergent opportunity to pursue options that do not require a new mom to absent herself from homelife with a newborn. However, in picking cases of severe new onset or recurrence of depression in postpartum women, the rapid onset of benefit that was noted within days could be an extraordinary relief and be the beginning of a road to wellness for some women.
Ultimately, the collaboration of patients with their doctors, the realities of cost, and the acceptability of use in various clinical settings will determine how Zulresso is incorporated into seeking treatment to mitigate the suffering associated with postpartum depression. We at the Center for Women’s Mental Health are interested in user experience with respect to this medicine and welcome comments from both patients and their doctors at [email protected].
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. This center was an investigator site for one of the studies supported by Sage Therapeutics, the manufacturer of Zulresso. Dr. Cohen is also the Edmund and Carroll Carpenter professor of psychiatry at Harvard Medical School, also in Boston. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Oral drug for postpartum depression aces phase 3 trial
COPENHAGEN – A first-in-class, once-daily, orally administered neuroactive steroid known for now as SAGE-217 aced all of its primary and secondary outcomes for the treatment of postpartum depression in the phase 3, randomized, double-blind, placebo-controlled ROBIN study, Eduard Vieta, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“I think this changes the paradigm in the treatment of postpartum depression,” declared Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Like brexanolone (Zulresso), an intravenous formulation of allopregnanolone approved by the Food and Drug Administration in March 2019 as the first-ever drug specifically targeting postpartum depression, SAGE-217 is a positive allosteric modifier of synaptic and extrasynaptic GABA-A receptors. That differentiates the two drugs from benzodiazepines, which target only synaptic receptors. Both brexanolone and SAGE-217 are drugs developed by Sage Therapeutics. But SAGE-217, an investigational agent, is vastly more convenient to use than brexanolone since, as an oral drug, it doesn’t require hospitalization for intravenous administration.
Dr. Vieta ticked off five reasons why he considers SAGE-217 a game changer in the treatment of postpartum depression: “It’s an amazingly effective compound, with an effect size that’s bigger than we usually see with antidepressants. It has an early onset of action, similar to what we see with glutaminergic agents, although with an opposite mechanism: enhancing GABA rather than opposing glutamate. It has excellent tolerability, similar to placebo. It’s made to be used orally, a major advantage over other drugs that are available or close to becoming available, which have to be given IV. And last but not least, a patient will get it for only 2 weeks. The treatment can be stopped after 2 weeks, and there is long-term improvement.”
The ROBIN trial included 151 patients with postpartum depression as defined by a baseline Hamilton Rating Scale for Depression (HAM-D) score of at least 26 who were randomized double-blind to 14 days of SAGE-217 at 30 mg once daily or to placebo. The primary endpoint was the change in HAM-D scores between baseline and day 15. The key finding was that the SAGE-217 group averaged a 17.8-point reduction, significantly greater than the 13.6-point improvement with placebo. This advantage was maintained at assessment on day 45 – a full month after treatment stopped – with a 24.8-point improvement over baseline in the SAGE-217 recipients, compared with a 19-point reduction in controls. The advantage favoring SAGE-217 was significant as early as day 3, the first assessment, at which point the average improvement in HAM-D was 12.5 points, compared with 9.8 points in controls.
Other secondary endpoints included change from baseline to day 15 on the Montgomery-Åsberg Depression Rating Scale (MADRS): a 22.8-point improvement in the SAGE-217 group, significantly greater than the 17.6-point improvement in the placebo arm. The same pattern was evident at day 45, with reductions in MADRS of 24.8 and 19 points, respectively, in the SAGE-217 and placebo groups.
Another key prespecified secondary endpoint was change in scores on the Hamilton Rating Scale for Anxiety through day 15. There was a mean 16.6-point drop in the active treatment arm, compared with a 12.7-point improvement with placebo, again a statistically significant and clinically meaningful between-group difference. This is an important endpoint because comorbid anxiety is common in the setting of postpartum depression, the psychiatrist continued.
The SAGE-217 group also demonstrated significantly higher rates of HAM-D response as defined by a 50% or greater reduction in total score at day 15, as well as in HAM-D remission, which entails having a score of 7 or less.
Treatment-emergent adverse events in the SAGE-217 and placebo arms were similar in frequency and type. The most common adverse events associated with SAGE-217 – all occurring in single-digit frequencies – were sleepiness, headache, dizziness, upper respiratory infections, and diarrhea. There was no signal of increased suicidal thoughts or behavior as assessed using the Columbia Suicide Severity Rating Scale.
SAGE-217 also is the focus of an ongoing pivotal phase 3 trial in patients with major depression. In addition, the drug is under study for bipolar depression, major depressive disorder with comorbid insomnia, and generalized anxiety disorder.
Dr. Vieta reported serving on advisory boards for Sage Therapeutics, the study sponsor, as well as for two dozen other pharmaceutical companies. He receives research funding from the Spanish Ministry of Science and Education, the Stanley Medical Research Institute, and more than a dozen pharmaceutical companies.
COPENHAGEN – A first-in-class, once-daily, orally administered neuroactive steroid known for now as SAGE-217 aced all of its primary and secondary outcomes for the treatment of postpartum depression in the phase 3, randomized, double-blind, placebo-controlled ROBIN study, Eduard Vieta, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“I think this changes the paradigm in the treatment of postpartum depression,” declared Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Like brexanolone (Zulresso), an intravenous formulation of allopregnanolone approved by the Food and Drug Administration in March 2019 as the first-ever drug specifically targeting postpartum depression, SAGE-217 is a positive allosteric modifier of synaptic and extrasynaptic GABA-A receptors. That differentiates the two drugs from benzodiazepines, which target only synaptic receptors. Both brexanolone and SAGE-217 are drugs developed by Sage Therapeutics. But SAGE-217, an investigational agent, is vastly more convenient to use than brexanolone since, as an oral drug, it doesn’t require hospitalization for intravenous administration.
Dr. Vieta ticked off five reasons why he considers SAGE-217 a game changer in the treatment of postpartum depression: “It’s an amazingly effective compound, with an effect size that’s bigger than we usually see with antidepressants. It has an early onset of action, similar to what we see with glutaminergic agents, although with an opposite mechanism: enhancing GABA rather than opposing glutamate. It has excellent tolerability, similar to placebo. It’s made to be used orally, a major advantage over other drugs that are available or close to becoming available, which have to be given IV. And last but not least, a patient will get it for only 2 weeks. The treatment can be stopped after 2 weeks, and there is long-term improvement.”
The ROBIN trial included 151 patients with postpartum depression as defined by a baseline Hamilton Rating Scale for Depression (HAM-D) score of at least 26 who were randomized double-blind to 14 days of SAGE-217 at 30 mg once daily or to placebo. The primary endpoint was the change in HAM-D scores between baseline and day 15. The key finding was that the SAGE-217 group averaged a 17.8-point reduction, significantly greater than the 13.6-point improvement with placebo. This advantage was maintained at assessment on day 45 – a full month after treatment stopped – with a 24.8-point improvement over baseline in the SAGE-217 recipients, compared with a 19-point reduction in controls. The advantage favoring SAGE-217 was significant as early as day 3, the first assessment, at which point the average improvement in HAM-D was 12.5 points, compared with 9.8 points in controls.
Other secondary endpoints included change from baseline to day 15 on the Montgomery-Åsberg Depression Rating Scale (MADRS): a 22.8-point improvement in the SAGE-217 group, significantly greater than the 17.6-point improvement in the placebo arm. The same pattern was evident at day 45, with reductions in MADRS of 24.8 and 19 points, respectively, in the SAGE-217 and placebo groups.
Another key prespecified secondary endpoint was change in scores on the Hamilton Rating Scale for Anxiety through day 15. There was a mean 16.6-point drop in the active treatment arm, compared with a 12.7-point improvement with placebo, again a statistically significant and clinically meaningful between-group difference. This is an important endpoint because comorbid anxiety is common in the setting of postpartum depression, the psychiatrist continued.
The SAGE-217 group also demonstrated significantly higher rates of HAM-D response as defined by a 50% or greater reduction in total score at day 15, as well as in HAM-D remission, which entails having a score of 7 or less.
Treatment-emergent adverse events in the SAGE-217 and placebo arms were similar in frequency and type. The most common adverse events associated with SAGE-217 – all occurring in single-digit frequencies – were sleepiness, headache, dizziness, upper respiratory infections, and diarrhea. There was no signal of increased suicidal thoughts or behavior as assessed using the Columbia Suicide Severity Rating Scale.
SAGE-217 also is the focus of an ongoing pivotal phase 3 trial in patients with major depression. In addition, the drug is under study for bipolar depression, major depressive disorder with comorbid insomnia, and generalized anxiety disorder.
Dr. Vieta reported serving on advisory boards for Sage Therapeutics, the study sponsor, as well as for two dozen other pharmaceutical companies. He receives research funding from the Spanish Ministry of Science and Education, the Stanley Medical Research Institute, and more than a dozen pharmaceutical companies.
COPENHAGEN – A first-in-class, once-daily, orally administered neuroactive steroid known for now as SAGE-217 aced all of its primary and secondary outcomes for the treatment of postpartum depression in the phase 3, randomized, double-blind, placebo-controlled ROBIN study, Eduard Vieta, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
“I think this changes the paradigm in the treatment of postpartum depression,” declared Dr. Vieta, professor of psychiatry and head of the bipolar disorders program at the University of Barcelona.
Like brexanolone (Zulresso), an intravenous formulation of allopregnanolone approved by the Food and Drug Administration in March 2019 as the first-ever drug specifically targeting postpartum depression, SAGE-217 is a positive allosteric modifier of synaptic and extrasynaptic GABA-A receptors. That differentiates the two drugs from benzodiazepines, which target only synaptic receptors. Both brexanolone and SAGE-217 are drugs developed by Sage Therapeutics. But SAGE-217, an investigational agent, is vastly more convenient to use than brexanolone since, as an oral drug, it doesn’t require hospitalization for intravenous administration.
Dr. Vieta ticked off five reasons why he considers SAGE-217 a game changer in the treatment of postpartum depression: “It’s an amazingly effective compound, with an effect size that’s bigger than we usually see with antidepressants. It has an early onset of action, similar to what we see with glutaminergic agents, although with an opposite mechanism: enhancing GABA rather than opposing glutamate. It has excellent tolerability, similar to placebo. It’s made to be used orally, a major advantage over other drugs that are available or close to becoming available, which have to be given IV. And last but not least, a patient will get it for only 2 weeks. The treatment can be stopped after 2 weeks, and there is long-term improvement.”
The ROBIN trial included 151 patients with postpartum depression as defined by a baseline Hamilton Rating Scale for Depression (HAM-D) score of at least 26 who were randomized double-blind to 14 days of SAGE-217 at 30 mg once daily or to placebo. The primary endpoint was the change in HAM-D scores between baseline and day 15. The key finding was that the SAGE-217 group averaged a 17.8-point reduction, significantly greater than the 13.6-point improvement with placebo. This advantage was maintained at assessment on day 45 – a full month after treatment stopped – with a 24.8-point improvement over baseline in the SAGE-217 recipients, compared with a 19-point reduction in controls. The advantage favoring SAGE-217 was significant as early as day 3, the first assessment, at which point the average improvement in HAM-D was 12.5 points, compared with 9.8 points in controls.
Other secondary endpoints included change from baseline to day 15 on the Montgomery-Åsberg Depression Rating Scale (MADRS): a 22.8-point improvement in the SAGE-217 group, significantly greater than the 17.6-point improvement in the placebo arm. The same pattern was evident at day 45, with reductions in MADRS of 24.8 and 19 points, respectively, in the SAGE-217 and placebo groups.
Another key prespecified secondary endpoint was change in scores on the Hamilton Rating Scale for Anxiety through day 15. There was a mean 16.6-point drop in the active treatment arm, compared with a 12.7-point improvement with placebo, again a statistically significant and clinically meaningful between-group difference. This is an important endpoint because comorbid anxiety is common in the setting of postpartum depression, the psychiatrist continued.
The SAGE-217 group also demonstrated significantly higher rates of HAM-D response as defined by a 50% or greater reduction in total score at day 15, as well as in HAM-D remission, which entails having a score of 7 or less.
Treatment-emergent adverse events in the SAGE-217 and placebo arms were similar in frequency and type. The most common adverse events associated with SAGE-217 – all occurring in single-digit frequencies – were sleepiness, headache, dizziness, upper respiratory infections, and diarrhea. There was no signal of increased suicidal thoughts or behavior as assessed using the Columbia Suicide Severity Rating Scale.
SAGE-217 also is the focus of an ongoing pivotal phase 3 trial in patients with major depression. In addition, the drug is under study for bipolar depression, major depressive disorder with comorbid insomnia, and generalized anxiety disorder.
Dr. Vieta reported serving on advisory boards for Sage Therapeutics, the study sponsor, as well as for two dozen other pharmaceutical companies. He receives research funding from the Spanish Ministry of Science and Education, the Stanley Medical Research Institute, and more than a dozen pharmaceutical companies.
REPORTING FROM THE ECNP 2019
Chronic hypertension in pregnancy increased 13-fold since 1970
The rate of chronic hypertension during pregnancy has increased significantly in the United States since 1970 and is more common in older women and in black women, according to a population-based, cross-sectional analysis.
Researchers analyzed data from more than 151 million women with delivery-related hospitalizations in the United States between 1970 and 2010 and found that the rate of chronic hypertension in pregnancy increased steadily over time from 1970 to 1990, plateaued from 1990 to 2000, then increased again to 2010.
The analysis revealed an average annual increase of 6% – which was higher among white women than among black women – and an overall 13-fold increase from 1970 to 2010. These increases appeared to be independent of rates of obesity and smoking. The findings were published in Hypertension.
The rates of chronic hypertension also increased with maternal age, among both black and white women.
“The strong association between age and rates of chronic hypertension underscores the potential for both biological and social determinants of health to influence risk,” wrote Cande V. Ananth, PhD, from the Rutgers University, New Brunswick, N.J., and coauthors. “The period effect in chronic hypertension in pregnancy is thus largely a product of the age effect and the increasing mean age at first birth in the U.S.”
The overall prevalence of chronic hypertension in pregnancy was 0.63%, but was twofold higher in black women, compared with white women (1.24% vs. 0.53%). The authors noted that black women experienced disproportionally higher rates of ischemic placental disease, pregestational and gestational diabetes, preterm delivery and perinatal mortality, which may be a consequences of higher rates of obesity, social disadvantage, smoking, and less access to care.
“This disparity may also be related to the higher tendency of black women to develop vascular disease at an earlier age than white women, which may also explain why the age-associated increase in chronic hypertension among black women is relatively smaller than white women,” they wrote. “The persistent race disparity in chronic hypertension is also a cause for continued concern and underscores the role of complex population dynamics that shape risks.”
This was the largest study to evaluate changes in the prevalence of chronic hypertension in pregnancy over time and particularly how the prevalence is influenced by age, period, and birth cohort.
In regard to the 13-fold increase from 1970 to 2010, the researchers suggested that changing diagnostic criteria for hypertension, as well as earlier access to prenatal care, may have played a part. For example, the American College of Cardiology recently modified their guidelines to include patients with systolic and diastolic blood pressures of 130-139 mm Hg and 80-89 mm Hg as stage 1 hypertension, which they noted would increase the prevalence rates of chronic hypertension during pregnancy.
The researchers reported having no outside funding and no conflicts of interest.
SOURCE: Ananth CV et al. Hypertension. 2019 Sept 9. doi: 10.1161/HYPERTENSIONAHA.119.12968.
The rate of chronic hypertension during pregnancy has increased significantly in the United States since 1970 and is more common in older women and in black women, according to a population-based, cross-sectional analysis.
Researchers analyzed data from more than 151 million women with delivery-related hospitalizations in the United States between 1970 and 2010 and found that the rate of chronic hypertension in pregnancy increased steadily over time from 1970 to 1990, plateaued from 1990 to 2000, then increased again to 2010.
The analysis revealed an average annual increase of 6% – which was higher among white women than among black women – and an overall 13-fold increase from 1970 to 2010. These increases appeared to be independent of rates of obesity and smoking. The findings were published in Hypertension.
The rates of chronic hypertension also increased with maternal age, among both black and white women.
“The strong association between age and rates of chronic hypertension underscores the potential for both biological and social determinants of health to influence risk,” wrote Cande V. Ananth, PhD, from the Rutgers University, New Brunswick, N.J., and coauthors. “The period effect in chronic hypertension in pregnancy is thus largely a product of the age effect and the increasing mean age at first birth in the U.S.”
The overall prevalence of chronic hypertension in pregnancy was 0.63%, but was twofold higher in black women, compared with white women (1.24% vs. 0.53%). The authors noted that black women experienced disproportionally higher rates of ischemic placental disease, pregestational and gestational diabetes, preterm delivery and perinatal mortality, which may be a consequences of higher rates of obesity, social disadvantage, smoking, and less access to care.
“This disparity may also be related to the higher tendency of black women to develop vascular disease at an earlier age than white women, which may also explain why the age-associated increase in chronic hypertension among black women is relatively smaller than white women,” they wrote. “The persistent race disparity in chronic hypertension is also a cause for continued concern and underscores the role of complex population dynamics that shape risks.”
This was the largest study to evaluate changes in the prevalence of chronic hypertension in pregnancy over time and particularly how the prevalence is influenced by age, period, and birth cohort.
In regard to the 13-fold increase from 1970 to 2010, the researchers suggested that changing diagnostic criteria for hypertension, as well as earlier access to prenatal care, may have played a part. For example, the American College of Cardiology recently modified their guidelines to include patients with systolic and diastolic blood pressures of 130-139 mm Hg and 80-89 mm Hg as stage 1 hypertension, which they noted would increase the prevalence rates of chronic hypertension during pregnancy.
The researchers reported having no outside funding and no conflicts of interest.
SOURCE: Ananth CV et al. Hypertension. 2019 Sept 9. doi: 10.1161/HYPERTENSIONAHA.119.12968.
The rate of chronic hypertension during pregnancy has increased significantly in the United States since 1970 and is more common in older women and in black women, according to a population-based, cross-sectional analysis.
Researchers analyzed data from more than 151 million women with delivery-related hospitalizations in the United States between 1970 and 2010 and found that the rate of chronic hypertension in pregnancy increased steadily over time from 1970 to 1990, plateaued from 1990 to 2000, then increased again to 2010.
The analysis revealed an average annual increase of 6% – which was higher among white women than among black women – and an overall 13-fold increase from 1970 to 2010. These increases appeared to be independent of rates of obesity and smoking. The findings were published in Hypertension.
The rates of chronic hypertension also increased with maternal age, among both black and white women.
“The strong association between age and rates of chronic hypertension underscores the potential for both biological and social determinants of health to influence risk,” wrote Cande V. Ananth, PhD, from the Rutgers University, New Brunswick, N.J., and coauthors. “The period effect in chronic hypertension in pregnancy is thus largely a product of the age effect and the increasing mean age at first birth in the U.S.”
The overall prevalence of chronic hypertension in pregnancy was 0.63%, but was twofold higher in black women, compared with white women (1.24% vs. 0.53%). The authors noted that black women experienced disproportionally higher rates of ischemic placental disease, pregestational and gestational diabetes, preterm delivery and perinatal mortality, which may be a consequences of higher rates of obesity, social disadvantage, smoking, and less access to care.
“This disparity may also be related to the higher tendency of black women to develop vascular disease at an earlier age than white women, which may also explain why the age-associated increase in chronic hypertension among black women is relatively smaller than white women,” they wrote. “The persistent race disparity in chronic hypertension is also a cause for continued concern and underscores the role of complex population dynamics that shape risks.”
This was the largest study to evaluate changes in the prevalence of chronic hypertension in pregnancy over time and particularly how the prevalence is influenced by age, period, and birth cohort.
In regard to the 13-fold increase from 1970 to 2010, the researchers suggested that changing diagnostic criteria for hypertension, as well as earlier access to prenatal care, may have played a part. For example, the American College of Cardiology recently modified their guidelines to include patients with systolic and diastolic blood pressures of 130-139 mm Hg and 80-89 mm Hg as stage 1 hypertension, which they noted would increase the prevalence rates of chronic hypertension during pregnancy.
The researchers reported having no outside funding and no conflicts of interest.
SOURCE: Ananth CV et al. Hypertension. 2019 Sept 9. doi: 10.1161/HYPERTENSIONAHA.119.12968.
FROM HYPERTENSION
Chlamydia trachomatis is associated with adverse reproductive health outcomes
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
compared with women who have tested negative for C. trachomatis or who have not been tested for the bacterium, according to a retrospective cohort study.
The risk of PID increases with repeat chlamydial infections, and the use of antibiotics that are effective against C. trachomatis does not decrease the risk of subsequent PID, the researchers reported in Clinical Infectious Diseases.
Prior studies have yielded different estimates of the risk of reproductive complications after chlamydia infection, said Casper den Heijer, MD, PhD, a researcher at Utrecht Institute of Pharmaceutical Sciences in Heerlen, the Netherlands, and colleagues. To assess the risk of PID, ectopic pregnancy, and infertility in women with a previous C. trachomatis diagnosis, Dr. den Heijer and coauthors conducted a retrospective study of women aged 12-25 years at baseline in the Clinical Practice Research Datalink GOLD database. Their analysis included data from women living in England between 2000 and 2013. The investigators used Cox proportional hazard models to evaluate the risk of adverse outcomes.
The researchers analyzed data from 857,324 women with a mean follow-up of 7.5 years. Patients’ mean age at baseline was 15 years. In all, the participants had 8,346 occurrences of PID, 2,484 occurrences of ectopic pregnancy, and 2,066 occurrences of female infertility.
For PID, incidence rates per 1,000 person-years were 1.1 among women untested for C. trachomatis, 1.4 among women who tested negative, and 5.4 among women who tested positive. For ectopic pregnancy, the incidence rates were 0.3 for untested women, 0.4 for negatively tested women, and 1.2 for positively tested women. Infertility incidence rates were 0.3 for untested women, 0.3 for negatively tested women, and 0.9 for positively tested women.
Compared with women who tested negative for C. trachomatis, women who tested positive had an increased risk of PID (adjusted hazard ratio, 2.36), ectopic pregnancy (aHR, 1.87), and female infertility (aHR, 1.85). Untested women had a lower risk for PID, compared with women who tested negative (aHR, 0.57).
C. trachomatis–effective antibiotic use was associated with higher PID risk, and that risk increased as the women used more of the antibiotic prescriptions, Dr. den Heijer and associates said. This occurred in all three groups of women. A possible explanation for this association between the antibiotics and higher PID risk could be that PID can be caused by other infectious diseases that could be treated with C. trachomatis–effective antibiotics.
While the study relied on primary care data, genitourinary medicine clinics diagnose and treat “a sizable proportion” of sexually transmitted infections in the United Kingdom, the authors noted. This limitation means that the study underestimates the number of C. trachomatis diagnoses in the cohort, they said.
Nonetheless, “Our results confirm the reproductive health burden of [C. trachomatis] and show the need for adequate public health interventions,” Dr. den Heijer and associates concluded.
Iris Krishna, MD, said in an interview, “This is a well-designed population-based retrospective cohort study evaluating the incidence of PID, ectopic pregnancy, and female infertility amongst more than 850,000 women in a primary care setting with a previous diagnosis of C. trachomatis, compared with women who have tested negative for C. trachomatis and women who have not been tested for C. trachomatis. This study also evaluated the impact of antibiotic use on PID.”
Dr. Krishna, assistant professor of gynecology and obstetrics in the division of maternal-fetal medicine at Emory University in Atlanta, continued, “This study demonstrates an association between C. trachomatis infection and adverse reproductive health outcomes. It highlights the importance of prompt diagnosis and treatment of C. trachomatis to reduce the risk of both short- and long-term reproductive health complications, as well as highlighting the importance of preventing recurrent C. trachomatis infections. It also emphasizes the importance of targeted screening for high-risk groups and appropriate follow-up to ensure that optimal antibiotic treatment is provided, especially amongst women who have recently used C. trachomatis–effective antibiotics.
“The finding of progression to PID despite C. trachomatis-effective antibiotic use indicates a more complex relationship where perhaps host immunological factors or effects of antibiotics on the vaginal microbiome may play a role and requires further study,” concluded Dr. Krishna. She was not involved in the current study, and was asked to comment on the findings.
The study was supported by the Netherlands Organization for Health Research and Development. Dr. den Heijer had no relevant disclosures. Dr. Krishna said she had no relevant financial disclosures.
SOURCE: den Heijer CDJ et al. Clin Infect Dis. 2019 Aug 24. doi: 10.1093/cid/ciz429.
FROM CLINICAL INFECTIOUS DISEASES
How safe and effective is ondansetron for nausea and vomiting in pregnancy?
EVIDENCE SUMMARY
Efficacy. A 2014 double-blind RCT compared ondansetron with pyridoxine plus doxylamine (standard care) for outpatient treatment of nausea and vomiting in pregnancy.1 The 36 patients had an average gestational age of 8 weeks and received either 4 mg oral ondansetron plus placebo or 25 mg pyridoxine plus 12.5 mg doxylamine 3 times daily for 5 days. Nausea and vomiting severity was measured using 2 separate 10-cm visual analog scales (VAS) with scores ranging from 0 to 10 (worst nausea or vomiting imaginable). Researchers determined that a VAS score reduction of 2.5 cm was clinically significant.
Patients treated with ondansetron described greater improvements in nausea (mean VAS change −5.1 cm vs −2 cm; P = .019) and vomiting (mean VAS change −4.1 cm vs −1.7 cm; P = .049). No patient required hospitalization. The researchers didn’t report on adverse effects or birth outcomes. The study was limited by the small sample size and a high rate (17%) of patients with missing data or who were lost to follow-up.
IV ondansetron vs metoclopramide: Similar efficacy, fewer adverse effects
A 2014 double-blind RCT compared IV ondansetron with IV metoclopramide (standard care) for treating hyperemesis gravidarum.2 The 160 patients had an average gestational age of 9.5 weeks and intractable nausea and vomiting severe enough to cause dehydration, metabolic disturbance, and hospitalization. Patients received either 4 mg ondansetron or 10 mg metoclopramide IV every 8 hours for 24 hours. The primary outcomes were number of episodes of vomiting over 24 hours and self-reported sense of well-being rated on a 10-point scale.
No differences were found between the ondansetron- and metoclopramide-treated groups in terms of vomiting over 24 hours (median episodes 1 and 1; P = .38) or sense of well-being (mean scores 8.7 vs 8.3; P = .13). Patients treated with ondansetron were less likely to have persistent ketonuria at 24 hours (relative risk [RR] = 0.3; 95% confidence interval [CI], 0.1-0.8; number needed to treat [NNT] = 6). They also were less likely to feel drowsy (RR = 0.3; 95% CI, 0.1–0.8; NNT = 6) or complain of dry mouth (RR = 0.4; 95% CI, 0.1-0.9; NNT = 8). The study didn’t report birth outcomes or adverse fetal effects.
Oral ondansetron outperforms oral metoclopramide in small study
A 2013 double-blind RCT compared ondansetron with metoclopramide (standard care) for controlling severe nausea and vomiting.3 The 83 patients, with an average gestational age of 8.7 weeks, had more than 3 vomiting episodes daily, weight loss, and ketonuria. They received either 4 mg oral ondansetron or 10 mg oral metoclopramide for 2 weeks as follows: 3 times daily for 1 week, then twice daily for 3 days, then once daily for 4 days. Patients rated nausea severity using a 10-cm VAS from 0 to 10 (severe nausea) and recorded the number of vomiting episodes.
Women treated with ondansetron had significantly lower VAS scores on Days 3 and 4 of treatment (5.4 vs 6, P = .024 on Day 3; 4.1 vs 5.7, P = .023 on Day 4). They also had fewer episodes of vomiting on Days 2, 3, and 4 (3.7 vs 6, P = .006 on Day 2; 3.2 vs 5.3, P = .006 on Day 3; and 3.3 vs 5, P = .013 on Day 4). The study was limited by the small sample size.
Safety. A 2016 systematic review examining the risk of birth defects associated with ondansetron exposure in pregnancy found 8 reports: 5 birth registries, 2 case-control studies, and 1 prospective cohort study.4 Investigators compared rates of major malformations—cleft lips, cleft palates, neural tube defects, cardiac defects, and hypospadias—in 5101 women exposed to ondansetron in the first trimester with birth defect rates in more than 3.1 million nonexposed women.
Continue to: No study demonstrated...
No study demonstrated an increased rate of major malformations associated with ondansetron exposure except for 2 disease registry studies with nearly 2.4 million patients that reported a slight increase in the risk of cardiac defects (odds ratio [OR] = 2; 95% CI, 1.3-3.1; OR = 1.6, 95% CI, 1-2.1). Comparisons of other birth defect rates associated with ondansetron exposure were inconsistent, with studies showing small increases, decreases, or no difference in rates between exposed and nonexposed women.
Exposure vs nonexposure: No difference in adverse outcomes
A 2013 retrospective cohort study looked at 608,385 pregnancies among women in Denmark, of whom 1970 (0.3%) had been exposed to ondansetron.5 The study found that exposure to ondansetron compared with nonexposure was associated with a lower risk for spontaneous abortion between 7 and 12 weeks’ gestation (1.1% vs 3.7%; hazard ratio [HR] = 0.5; 95% CI, 0.3-0.9).
No significant differences between ondansetron exposure and nonexposure were found for the following adverse outcomes: spontaneous abortion between 13 and 22 weeks’ gestation (1% vs 2.1%; HR = 0.6; 95% CI, 0.3-1.2); stillbirth (0.3% vs 0.4%; HR = 0.4; 95% CI, 0.1-1.7); any major birth defect (2.9% in both exposed and nonexposed women; OR = 1.12; 95% CI, 0.69-1.82); preterm delivery (6.2% vs 5.2%; OR = 0.9; 95% CI, 0.7-1.3), low birth weight infant (4.1% vs 3.7%; OR = 0.8; 95% CI, 0.5-1.1); and small-for-gestational-age infant (10.4% vs 9.2%; OR = 1.1; 95% CI, 0.9-1.4).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) states that insufficient data exist regarding the safety of ondansetron for the fetus.6 ACOG recommends individualizing the use of ondansetron before 10 weeks of pregnancy after weighing the risks and benefits. ACOG also recommends adding ondansetron as third-line treatment for nausea and vomiting unresponsive to first- and second-line treatments.
EDITOR'S TAKEAWAY
Higher-quality studies showed ondansetron to be an effective treatment for hyperemesis gravidarum. Lower-quality studies raised some concerns about adverse fetal effects. Although the adverse effects were rare and the quality of the evidence was lower, the cautionary principle suggests that ondansetron should be a second-line option.
1. Oliveira LG, Capp SM, You WB, et al. Ondansetron compared with doxylamine and pyridoxine for treatment of nausea in pregnancy: a randomized controlled trial. Obstet Gynecol. 2014;124:735-742.
2. Abas MN, Tan PC, Azmi N, et al. Ondansetron compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2014;123:1272-1279.
3. Kashifard M, Basirat Z, Kashifard M, et al. Ondansetrone or metoclopromide? Which is more effective in severe nausea and vomiting of pregnancy? A randomized trial double-blind study. Clin Exp Obstet Gynecol. 2013;40:127-130.
4. Carstairs SD. Ondansetron use in pregnancy and birth defects: a systematic review. Obstet Gynecol. 2016;127:878-883.
5. Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013;368:814-823.
6. American College of Obstetricians and Gynecologists, Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131:e15-e30.
EVIDENCE SUMMARY
Efficacy. A 2014 double-blind RCT compared ondansetron with pyridoxine plus doxylamine (standard care) for outpatient treatment of nausea and vomiting in pregnancy.1 The 36 patients had an average gestational age of 8 weeks and received either 4 mg oral ondansetron plus placebo or 25 mg pyridoxine plus 12.5 mg doxylamine 3 times daily for 5 days. Nausea and vomiting severity was measured using 2 separate 10-cm visual analog scales (VAS) with scores ranging from 0 to 10 (worst nausea or vomiting imaginable). Researchers determined that a VAS score reduction of 2.5 cm was clinically significant.
Patients treated with ondansetron described greater improvements in nausea (mean VAS change −5.1 cm vs −2 cm; P = .019) and vomiting (mean VAS change −4.1 cm vs −1.7 cm; P = .049). No patient required hospitalization. The researchers didn’t report on adverse effects or birth outcomes. The study was limited by the small sample size and a high rate (17%) of patients with missing data or who were lost to follow-up.
IV ondansetron vs metoclopramide: Similar efficacy, fewer adverse effects
A 2014 double-blind RCT compared IV ondansetron with IV metoclopramide (standard care) for treating hyperemesis gravidarum.2 The 160 patients had an average gestational age of 9.5 weeks and intractable nausea and vomiting severe enough to cause dehydration, metabolic disturbance, and hospitalization. Patients received either 4 mg ondansetron or 10 mg metoclopramide IV every 8 hours for 24 hours. The primary outcomes were number of episodes of vomiting over 24 hours and self-reported sense of well-being rated on a 10-point scale.
No differences were found between the ondansetron- and metoclopramide-treated groups in terms of vomiting over 24 hours (median episodes 1 and 1; P = .38) or sense of well-being (mean scores 8.7 vs 8.3; P = .13). Patients treated with ondansetron were less likely to have persistent ketonuria at 24 hours (relative risk [RR] = 0.3; 95% confidence interval [CI], 0.1-0.8; number needed to treat [NNT] = 6). They also were less likely to feel drowsy (RR = 0.3; 95% CI, 0.1–0.8; NNT = 6) or complain of dry mouth (RR = 0.4; 95% CI, 0.1-0.9; NNT = 8). The study didn’t report birth outcomes or adverse fetal effects.
Oral ondansetron outperforms oral metoclopramide in small study
A 2013 double-blind RCT compared ondansetron with metoclopramide (standard care) for controlling severe nausea and vomiting.3 The 83 patients, with an average gestational age of 8.7 weeks, had more than 3 vomiting episodes daily, weight loss, and ketonuria. They received either 4 mg oral ondansetron or 10 mg oral metoclopramide for 2 weeks as follows: 3 times daily for 1 week, then twice daily for 3 days, then once daily for 4 days. Patients rated nausea severity using a 10-cm VAS from 0 to 10 (severe nausea) and recorded the number of vomiting episodes.
Women treated with ondansetron had significantly lower VAS scores on Days 3 and 4 of treatment (5.4 vs 6, P = .024 on Day 3; 4.1 vs 5.7, P = .023 on Day 4). They also had fewer episodes of vomiting on Days 2, 3, and 4 (3.7 vs 6, P = .006 on Day 2; 3.2 vs 5.3, P = .006 on Day 3; and 3.3 vs 5, P = .013 on Day 4). The study was limited by the small sample size.
Safety. A 2016 systematic review examining the risk of birth defects associated with ondansetron exposure in pregnancy found 8 reports: 5 birth registries, 2 case-control studies, and 1 prospective cohort study.4 Investigators compared rates of major malformations—cleft lips, cleft palates, neural tube defects, cardiac defects, and hypospadias—in 5101 women exposed to ondansetron in the first trimester with birth defect rates in more than 3.1 million nonexposed women.
Continue to: No study demonstrated...
No study demonstrated an increased rate of major malformations associated with ondansetron exposure except for 2 disease registry studies with nearly 2.4 million patients that reported a slight increase in the risk of cardiac defects (odds ratio [OR] = 2; 95% CI, 1.3-3.1; OR = 1.6, 95% CI, 1-2.1). Comparisons of other birth defect rates associated with ondansetron exposure were inconsistent, with studies showing small increases, decreases, or no difference in rates between exposed and nonexposed women.
Exposure vs nonexposure: No difference in adverse outcomes
A 2013 retrospective cohort study looked at 608,385 pregnancies among women in Denmark, of whom 1970 (0.3%) had been exposed to ondansetron.5 The study found that exposure to ondansetron compared with nonexposure was associated with a lower risk for spontaneous abortion between 7 and 12 weeks’ gestation (1.1% vs 3.7%; hazard ratio [HR] = 0.5; 95% CI, 0.3-0.9).
No significant differences between ondansetron exposure and nonexposure were found for the following adverse outcomes: spontaneous abortion between 13 and 22 weeks’ gestation (1% vs 2.1%; HR = 0.6; 95% CI, 0.3-1.2); stillbirth (0.3% vs 0.4%; HR = 0.4; 95% CI, 0.1-1.7); any major birth defect (2.9% in both exposed and nonexposed women; OR = 1.12; 95% CI, 0.69-1.82); preterm delivery (6.2% vs 5.2%; OR = 0.9; 95% CI, 0.7-1.3), low birth weight infant (4.1% vs 3.7%; OR = 0.8; 95% CI, 0.5-1.1); and small-for-gestational-age infant (10.4% vs 9.2%; OR = 1.1; 95% CI, 0.9-1.4).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) states that insufficient data exist regarding the safety of ondansetron for the fetus.6 ACOG recommends individualizing the use of ondansetron before 10 weeks of pregnancy after weighing the risks and benefits. ACOG also recommends adding ondansetron as third-line treatment for nausea and vomiting unresponsive to first- and second-line treatments.
EDITOR'S TAKEAWAY
Higher-quality studies showed ondansetron to be an effective treatment for hyperemesis gravidarum. Lower-quality studies raised some concerns about adverse fetal effects. Although the adverse effects were rare and the quality of the evidence was lower, the cautionary principle suggests that ondansetron should be a second-line option.
EVIDENCE SUMMARY
Efficacy. A 2014 double-blind RCT compared ondansetron with pyridoxine plus doxylamine (standard care) for outpatient treatment of nausea and vomiting in pregnancy.1 The 36 patients had an average gestational age of 8 weeks and received either 4 mg oral ondansetron plus placebo or 25 mg pyridoxine plus 12.5 mg doxylamine 3 times daily for 5 days. Nausea and vomiting severity was measured using 2 separate 10-cm visual analog scales (VAS) with scores ranging from 0 to 10 (worst nausea or vomiting imaginable). Researchers determined that a VAS score reduction of 2.5 cm was clinically significant.
Patients treated with ondansetron described greater improvements in nausea (mean VAS change −5.1 cm vs −2 cm; P = .019) and vomiting (mean VAS change −4.1 cm vs −1.7 cm; P = .049). No patient required hospitalization. The researchers didn’t report on adverse effects or birth outcomes. The study was limited by the small sample size and a high rate (17%) of patients with missing data or who were lost to follow-up.
IV ondansetron vs metoclopramide: Similar efficacy, fewer adverse effects
A 2014 double-blind RCT compared IV ondansetron with IV metoclopramide (standard care) for treating hyperemesis gravidarum.2 The 160 patients had an average gestational age of 9.5 weeks and intractable nausea and vomiting severe enough to cause dehydration, metabolic disturbance, and hospitalization. Patients received either 4 mg ondansetron or 10 mg metoclopramide IV every 8 hours for 24 hours. The primary outcomes were number of episodes of vomiting over 24 hours and self-reported sense of well-being rated on a 10-point scale.
No differences were found between the ondansetron- and metoclopramide-treated groups in terms of vomiting over 24 hours (median episodes 1 and 1; P = .38) or sense of well-being (mean scores 8.7 vs 8.3; P = .13). Patients treated with ondansetron were less likely to have persistent ketonuria at 24 hours (relative risk [RR] = 0.3; 95% confidence interval [CI], 0.1-0.8; number needed to treat [NNT] = 6). They also were less likely to feel drowsy (RR = 0.3; 95% CI, 0.1–0.8; NNT = 6) or complain of dry mouth (RR = 0.4; 95% CI, 0.1-0.9; NNT = 8). The study didn’t report birth outcomes or adverse fetal effects.
Oral ondansetron outperforms oral metoclopramide in small study
A 2013 double-blind RCT compared ondansetron with metoclopramide (standard care) for controlling severe nausea and vomiting.3 The 83 patients, with an average gestational age of 8.7 weeks, had more than 3 vomiting episodes daily, weight loss, and ketonuria. They received either 4 mg oral ondansetron or 10 mg oral metoclopramide for 2 weeks as follows: 3 times daily for 1 week, then twice daily for 3 days, then once daily for 4 days. Patients rated nausea severity using a 10-cm VAS from 0 to 10 (severe nausea) and recorded the number of vomiting episodes.
Women treated with ondansetron had significantly lower VAS scores on Days 3 and 4 of treatment (5.4 vs 6, P = .024 on Day 3; 4.1 vs 5.7, P = .023 on Day 4). They also had fewer episodes of vomiting on Days 2, 3, and 4 (3.7 vs 6, P = .006 on Day 2; 3.2 vs 5.3, P = .006 on Day 3; and 3.3 vs 5, P = .013 on Day 4). The study was limited by the small sample size.
Safety. A 2016 systematic review examining the risk of birth defects associated with ondansetron exposure in pregnancy found 8 reports: 5 birth registries, 2 case-control studies, and 1 prospective cohort study.4 Investigators compared rates of major malformations—cleft lips, cleft palates, neural tube defects, cardiac defects, and hypospadias—in 5101 women exposed to ondansetron in the first trimester with birth defect rates in more than 3.1 million nonexposed women.
Continue to: No study demonstrated...
No study demonstrated an increased rate of major malformations associated with ondansetron exposure except for 2 disease registry studies with nearly 2.4 million patients that reported a slight increase in the risk of cardiac defects (odds ratio [OR] = 2; 95% CI, 1.3-3.1; OR = 1.6, 95% CI, 1-2.1). Comparisons of other birth defect rates associated with ondansetron exposure were inconsistent, with studies showing small increases, decreases, or no difference in rates between exposed and nonexposed women.
Exposure vs nonexposure: No difference in adverse outcomes
A 2013 retrospective cohort study looked at 608,385 pregnancies among women in Denmark, of whom 1970 (0.3%) had been exposed to ondansetron.5 The study found that exposure to ondansetron compared with nonexposure was associated with a lower risk for spontaneous abortion between 7 and 12 weeks’ gestation (1.1% vs 3.7%; hazard ratio [HR] = 0.5; 95% CI, 0.3-0.9).
No significant differences between ondansetron exposure and nonexposure were found for the following adverse outcomes: spontaneous abortion between 13 and 22 weeks’ gestation (1% vs 2.1%; HR = 0.6; 95% CI, 0.3-1.2); stillbirth (0.3% vs 0.4%; HR = 0.4; 95% CI, 0.1-1.7); any major birth defect (2.9% in both exposed and nonexposed women; OR = 1.12; 95% CI, 0.69-1.82); preterm delivery (6.2% vs 5.2%; OR = 0.9; 95% CI, 0.7-1.3), low birth weight infant (4.1% vs 3.7%; OR = 0.8; 95% CI, 0.5-1.1); and small-for-gestational-age infant (10.4% vs 9.2%; OR = 1.1; 95% CI, 0.9-1.4).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) states that insufficient data exist regarding the safety of ondansetron for the fetus.6 ACOG recommends individualizing the use of ondansetron before 10 weeks of pregnancy after weighing the risks and benefits. ACOG also recommends adding ondansetron as third-line treatment for nausea and vomiting unresponsive to first- and second-line treatments.
EDITOR'S TAKEAWAY
Higher-quality studies showed ondansetron to be an effective treatment for hyperemesis gravidarum. Lower-quality studies raised some concerns about adverse fetal effects. Although the adverse effects were rare and the quality of the evidence was lower, the cautionary principle suggests that ondansetron should be a second-line option.
1. Oliveira LG, Capp SM, You WB, et al. Ondansetron compared with doxylamine and pyridoxine for treatment of nausea in pregnancy: a randomized controlled trial. Obstet Gynecol. 2014;124:735-742.
2. Abas MN, Tan PC, Azmi N, et al. Ondansetron compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2014;123:1272-1279.
3. Kashifard M, Basirat Z, Kashifard M, et al. Ondansetrone or metoclopromide? Which is more effective in severe nausea and vomiting of pregnancy? A randomized trial double-blind study. Clin Exp Obstet Gynecol. 2013;40:127-130.
4. Carstairs SD. Ondansetron use in pregnancy and birth defects: a systematic review. Obstet Gynecol. 2016;127:878-883.
5. Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013;368:814-823.
6. American College of Obstetricians and Gynecologists, Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131:e15-e30.
1. Oliveira LG, Capp SM, You WB, et al. Ondansetron compared with doxylamine and pyridoxine for treatment of nausea in pregnancy: a randomized controlled trial. Obstet Gynecol. 2014;124:735-742.
2. Abas MN, Tan PC, Azmi N, et al. Ondansetron compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2014;123:1272-1279.
3. Kashifard M, Basirat Z, Kashifard M, et al. Ondansetrone or metoclopromide? Which is more effective in severe nausea and vomiting of pregnancy? A randomized trial double-blind study. Clin Exp Obstet Gynecol. 2013;40:127-130.
4. Carstairs SD. Ondansetron use in pregnancy and birth defects: a systematic review. Obstet Gynecol. 2016;127:878-883.
5. Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013;368:814-823.
6. American College of Obstetricians and Gynecologists, Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131:e15-e30.
EVIDENCE-BASED ANSWER:
Oral ondansetron is more effective than a combination of pyridoxine and doxylamine for outpatient treatment of nausea and vomiting in pregnancy (strength of recommendation [SOR]: B, randomized controlled trial [RCT]).
For moderate to severe nausea and vomiting, intravenous (IV) ondansetron is at least as effective as IV metoclopramide and may cause fewer adverse reactions (SOR: B, RCTs).
Disease registry, case-control, and cohort studies report a slight increase in the risk of cardiac defects with ondansetron use in first-trimester pregnancies, but no major or other birth defects are associated with ondansetron exposure (SOR: B, a systematic review of observational trials and a single retrospective cohort study).
A specialty society guideline recommends weighing the risks and benefits of ondansetron use before 10 weeks’ gestational age and suggests reserving ondansetron for patients who have persistent nausea and vomiting unresponsive to first- and second-line treatments (SOR: C, expert opinion).
How best to address breast pain in nonbreastfeeding women
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).
Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15
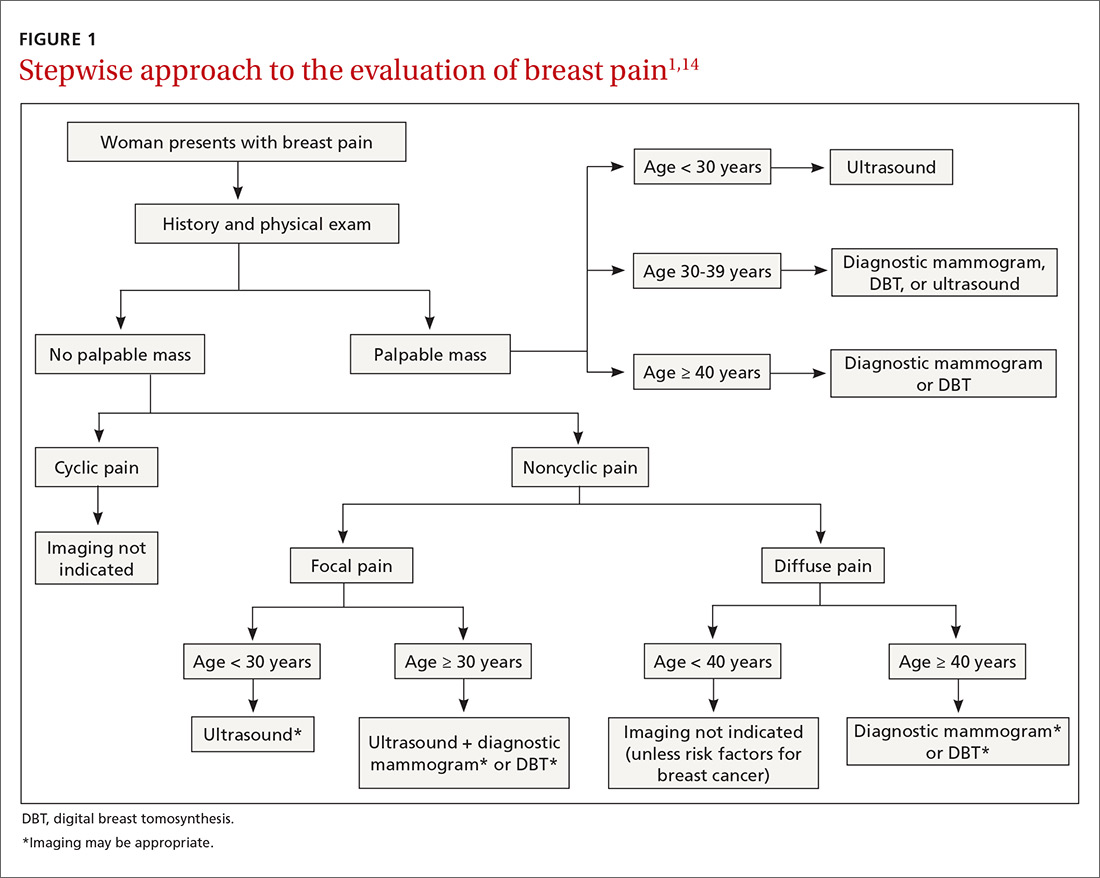
Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15
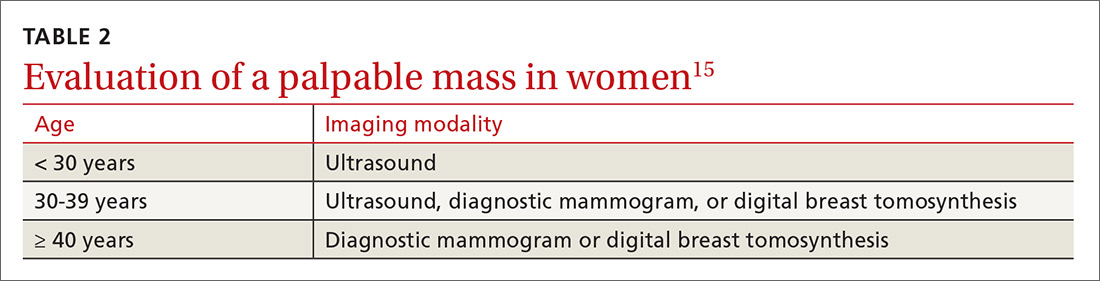
Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).
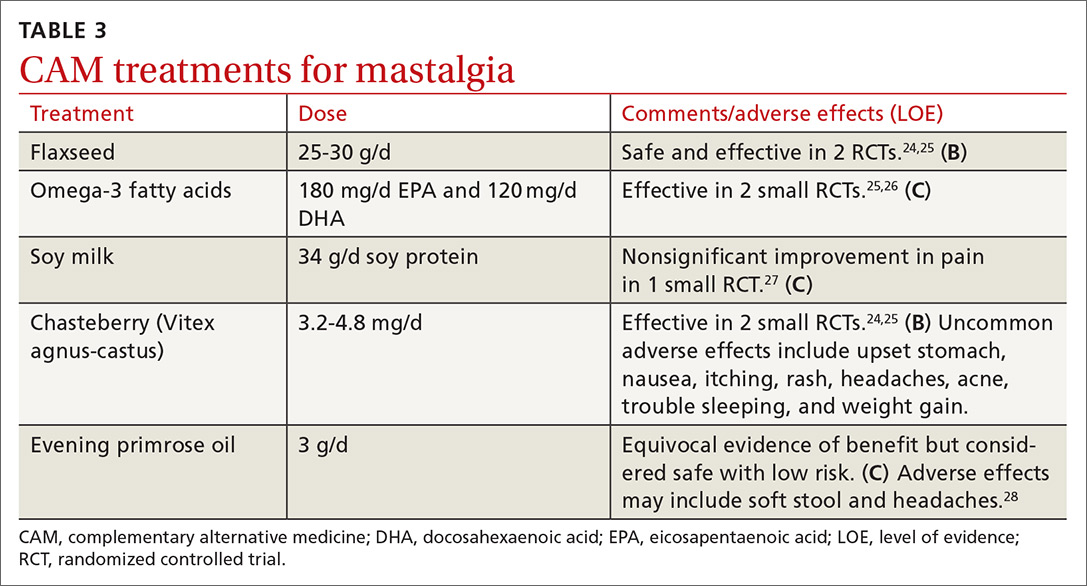
Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38
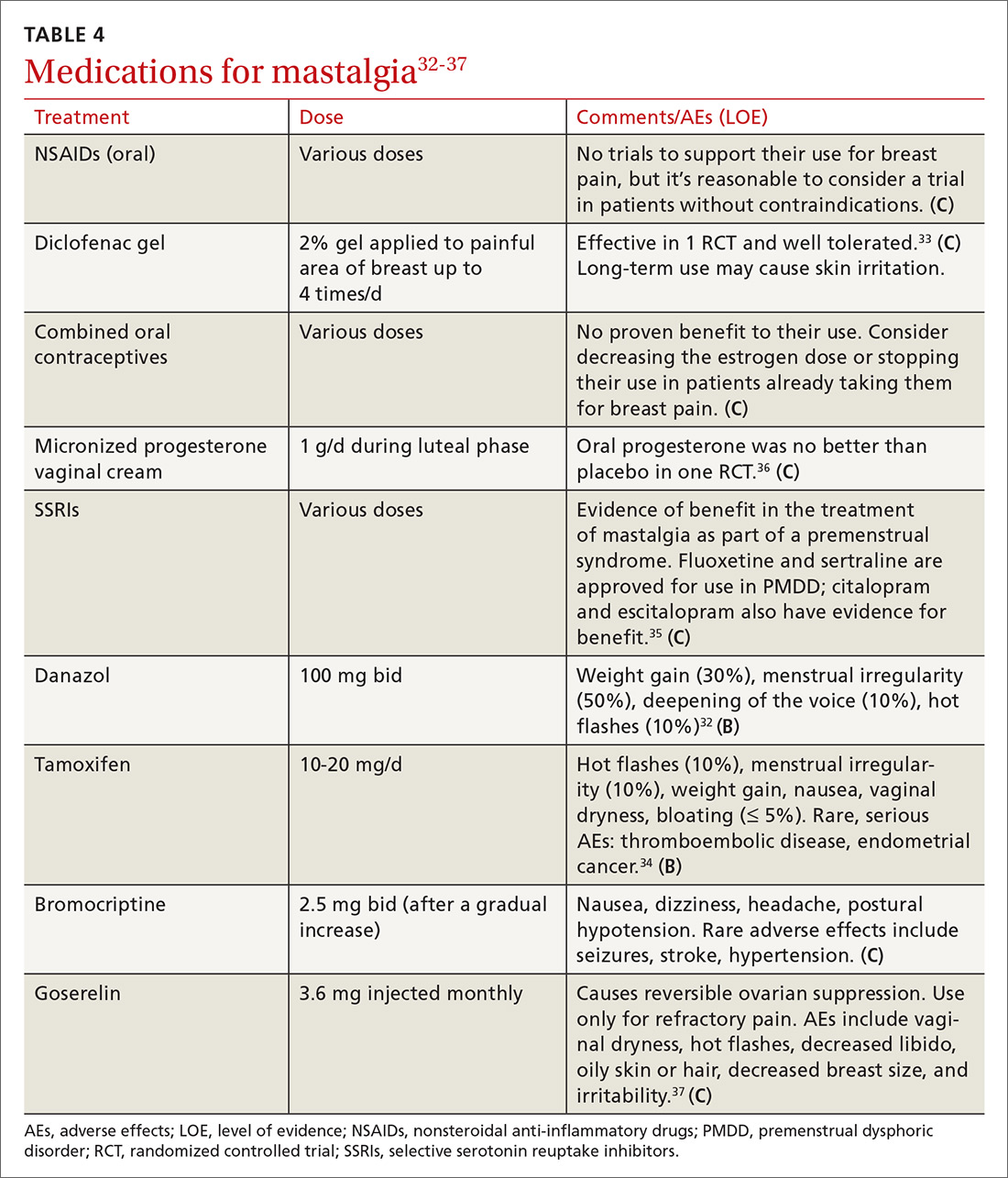
SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35
Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; [email protected].
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).

Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15

Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15

Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).

Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38

SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35

Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; [email protected].
CASE 1
Robin S is a 40-year-old woman who has never had children or been pregnant. She is in a relationship with a woman so does not use contraception. She has no family history of cancer. She presents with worsening bilateral breast pain that starts 10 days before the onset of her period. The pain has been present for about 4 years, but it has worsened over the last 6 months such that she is unable to wear a bra during these 10 days, finds lying in bed on her side too painful for sleep, and is unable to exercise. She has tried to eliminate caffeine from her diet and takes ibuprofen, but neither of these interventions has controlled her pain. Her breast exam is normal except for diffuse tenderness over both breasts.
CASE 2
Meg R is a 50-year-old healthy woman. She is a G2P2 who breastfed each of her children for 1 year. She does not smoke. She has no family history of breast cancer or other malignancies. She presents with 2 months of deep, left-sided breast pain. She describes the pain as constant, progressive, dull, and achy. She points to a spot in the upper outer quadrant of her left breast and describes the pain as being close to her ribs. She had a screening mammogram 3 weeks earlier that was normal, with findings of dense breasts. She did not tell the technician that she was having pain. Clinical breast examination of both breasts reveals tenderness to deep palpation of the left breast. She has dense breasts but a focal mass is not palpated.
Mastalgia, or breast pain, is one of the most common breast symptoms seen in primary care and a common reason for referrals to breast surgeons. Up to 70% of women will experience breast pain during their lifetime—most in their premenopausal years.1,2
The most common type of breast pain is cyclic (ie, relating to the menstrual cycle); it accounts for up to 70% of all cases of breast pain in women.1,3 The other 2 types of breast pain are noncyclic and extramammary. The cause of cyclic breast pain is unclear, but it is likely hormonally mediated and multifactorial. In the vast majority of women with breast pain, no distinct etiology is found, and there is a very low incidence of breast cancer.2,4
In this review, we describe how to proceed when a woman who is not breastfeeding presents with cyclic or noncyclic breast pain.
Evaluation: Focus on the pain, medications, and history
Evaluation of breast pain should begin with the patient describing the pain, including its quality, location, radiation, and relationship to the menstrual cycle. It’s important to inquire about recent trauma or aggravating activities and to order a pregnancy test for women of childbearing age.1
Cyclic mastalgia is typically described as diffuse, either unilateral or bilateral, with an aching or heavy quality. The pain is often felt in the upper outer quadrant of the breast with radiation to the axilla. It most commonly occurs during the luteal phase of the menstrual cycle, improves with the onset of menses, and is thought to be related to the increased water content in breast stroma caused by increasing hormone levels during the luteal phase.5-7
Continue to: Noncyclic mastalgia
Noncyclic mastalgia is typically unilateral and localized within 1 quadrant of the breast; however, women may report diffuse pain with radiation to the axilla. The pain is often described as burning, achy, or as soreness.5,6 There can be considerable overlap in the presentations of cyclic and noncyclic pain and differentiating between the 2 is often not necessary as management is similar.8
A thorough review of medications is important as several drugs have been associated with breast pain. These include oral contraceptives, hormone therapy, antidepressants (selective serotonin reuptake inhibitors [SSRIs], venlafaxine, mirtazapine), antipsychotics (haloperidol), and some cardiovascular agents (spironolactone, digoxin).5
Inquiring about stress, caffeine intake, smoking status, and bra usage may also yield useful information. Increased stress and caffeine intake have been associated with mastalgia,7 and women who are heavy smokers are more likely to have noncyclic hypersensitive breast pain.9 In addition, women with large breasts often have noncyclic breast pain, particularly if they don’t wear a sufficiently supportive bra.3
Medical, surgical, family history. Relevant aspects of a woman’s past medical, surgical, and family history include prior breast mass or biopsy, breast surgery, and risk factors associated with breast cancer (menarche age < 12 years, menopause age > 55 years, nulliparity, exposure to ionizing radiation, and family history of breast or ovarian cancer).1 A thorough history should include questions to evaluate for extra-mammary etiologies of breast pain such as those that are musculoskeletal or dermatologic in nature (TABLE 11,5,8,10).

Using an objective measure of pain is not only helpful for evaluating the pain itself, but also for determining the effectiveness of treatment strategies. When using the Cardiff Breast Pain Chart, for example, menstrual cycle and level of pain are recorded on a calendar (see www.breastcancercare.org.uk/sites/default/files/files/breast_pain_chart.pdf).11 If the pain is determined to be cyclic, the concern for malignancy is significantly lower.2
Continue to: Ensure that the physical exam is thorough
Ensure that the physical exam is thorough
Women presenting with breast pain should undergo a clinical breast exam in both the upright and supine positions. Inspect for asymmetry, erythema, rashes, skin dimpling, nipple discharge, and retraction/inversion. Palpate the breasts for any suspicious masses, asymmetry, or tenderness, as well as for axillary and/or supraclavicular lymphadenopathy and chest wall tenderness. This is facilitated by having the patient lie in the lateral decubitus position, allowing the breast to fall away from the chest wall.5,12,13
Imaging: Preferred method depends on the age of the patient
Women with a palpable mass should be referred for diagnostic imaging (FIGURE 11,14). Ultrasonography is the recommended modality for women < 30 years of age (TABLE 215). For women between the ages of 30 and 39 years, appropriate initial imaging includes ultrasound, diagnostic mammography, or digital breast tomosynthesis (DBT). For women ≥ 40 years of age, diagnostic mammography or DBT is recommended.15

Cyclic breast pain. Women with cyclic breast pain do not require further evaluation with imaging. Reassurance and symptomatic treatment is appropriate in most cases, as the risk of malignancy is very low in the absence of other concerning signs or symptoms. A screening mammogram may be appropriate for women > 40 years of age who have not had one in the preceding 12 months.1-3,10,12,15

Noncyclic breast pain. In contrast, imaging may be appropriate in women who present with noncyclic breast pain depending on the woman’s age and whether the pain is focal (≤ 25% of the breast and axillary tissue) or diffuse (> 25% of the breast and axillary tissue). Although evidence suggests that the risk of malignancy in women with noncyclic breast pain is low, the American College of Radiology advises that imaging may be useful in some patients to provide reassurance and to exclude a treatable cause of breast pain.3,14 In women with focal pain, ultrasound alone is the preferred modality for women < 30 years of age and ultrasound plus diagnostic mammography is recommended for women ≥ 30 years of age.3,14
In one small study, the use of ultrasonography in women ages < 30 years with focal breast pain had a sensitivity of 100% and a negative predictive value of 100%.16 Similarly, another small retrospective study in older women (average age 56 years) with focal breast pain and no palpable mass showed that ultrasound plus diagnostic mammography had a negative predictive value of 100%.4 DBT may be used in place of mammography to rule out malignancy in this setting.
Continue to: In general...
In general, routine imaging is not indicated for women with noncyclic diffuse breast pain, although diagnostic mammography or DBT may be considered in women ≥ 40 years of age 14 (see “Less common diagnoses with breast pain”4,5,17-21).
SIDEBAR
Less common diagnoses with breast pain
Many women presenting with breast pain are concerned about malignancy. Breast cancer is an uncommon cause of breast pain; only 0.5% of patients presenting with mastalgia without other clinical findings have a malignancy.4 Mastalgia is not a risk factor for breast cancer.
When mastalgia is associated with breast cancer, it is more likely to be unilateral, intense, noncyclic, and progressive.5 Concerning features that warrant further evaluation include new onset focal pain with or without an abnormal exam. If symptoms cannot be explained by an obvious cause (such as trauma, costochondritis, radicular back or intercostal pain, herpes zoster, or superficial thrombophlebitis that does not resolve), diagnostic breast imaging is indicated.
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that initially presents with breast pain and rapidly enlarging diffuse erythema of the breast in the absence of a discrete breast lump. The initial presentation is similar to that seen with benign inflammatory etiologies of the breast tissue like cellulitis or abscess, duct ectasia, mastitis, phlebitis of the thoracoepigastric vein (Mondor’s disease), or fat necrosis.17 Benign breast conditions due to these causes will generally resolve with appropriate treatment for those conditions within 7 days and will generally not present with the warning signs of IBC, which include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Although uncommon (accounting for 1%-6% of all breast cancer diagnoses), IBC spreads rapidly over a few weeks; thus, urgent imaging is warranted.17
Mastitis is inflammation of the breast tissue that may or may not be associated with a bacterial infection and uncommonly occurs in nonbreastfeeding women. Periductal mastitis is characterized by inflammation of the subareolar ducts and can present with pain, periareolar inflammation, and purulent nipple discharge.18 The condition is typically chronic, and the inflamed ducts may become secondarily infected leading to duct damage and abscess formation. Treatment generally includes antibiotics along with incision and drainage of any associated abscesses or duct excision.18,19
Idiopathic granulomatous mastitis (IGM) is a rare inflammatory breast disease that typically affects young parous women. The presentation can vary from a single peripheral breast mass to multiple areas of infection with abscesses and skin ulceration. The etiology is unknown. Diagnosis requires a core needle biopsy to rule out malignancy or other causes of granulomatous disease. IGM is a benign condition and typically resolves without treatment over the course of several months, although antibiotics and/or drainage may be required for secondary infections.20,21
Continue to: Treatment...
Treatment: When reassurance isn’t enough
Nonrandomized studies suggest that reassurance that mastalgia is benign is enough to treat up to 70% of women.8,22,23 Cyclic breast pain is usually treated symptomatically since the likelihood of breast cancer is extremely low in absence of clinical breast examination abnormalities.2 Because treatment for cyclic and noncyclic mastalgia overlaps, available treatments are discussed together on the following pages.
Lifestyle factors associated with breast pain include stress, caffeine consumption, smoking, and having breastfed 3 or more children (P < .05).9 Although restriction of caffeine, fat, and salt intake may be attempted to address breast pain, no randomized control trials (RCTs) of these interventions have demonstrated effectiveness in reducing mastalgia.8,10
Although not supported by RCTs, first-line treatment of mastalgia includes a recommendation that women, particularly those with large, heavy breasts, wear a well-fitted and supportive bra.8,10
Complementary and alternative medicine treatments for mastalgia
A number of complementary and alternative medicine treatments have demonstrated benefit in treating mastalgia and are often tried before pharmacologic agents (TABLE 324-28). Keep in mind, though, that these therapies are not regulated by the US Food and Drug Administration (FDA). So it’s wise to review particular products with your patient before she buys them (or ask her to bring in any bottles of product for you to review).

Flaxseed, omega-3 fatty acids, and soy milk. Flaxseed, a source of phytoestrogens and omega-3 fatty acids, has been shown to reduce cyclic breast pain in 2 small RCTs.24,25 Breast pain scores were significantly lower for patients ingesting 25 g/d of flaxseed powder compared with placebo.24,25 Omega-3 fatty acids were also more effective than placebo for relief of cyclic breast pain in 2 small RCTs.25,26 Another small RCT demonstrated that women who drank soy milk had a nonsignificant improvement in breast pain compared with those who drank cow’s milk.27
Continue to: Chasteberry
Chasteberry. One RCT demonstrated that Vitex agnus-castus, a chasteberry fruit extract, produced significant and clinically meaningful improvement in visual analogue pain scores for mastalgia, with few adverse effects.29 Another RCT assessing breast fullness as part of the premenstrual syndrome showed significant improvement in breast discomfort for women treated with Vitex agnus-castus.30
Evening primrose oil (EPO). In at least one small study, EPO was effective in controlling breast pain.28 A more recent meta-analysis of all of the EPO trials including gamolenic acid (the active ingredient of EPO) showed no significant difference in mastalgia compared with placebo.31
Pharmacologic Tx options: Start with NSAIDs
Oral nonsteroidal anti-inflammatory drugs (NSAIDs) are often recommended as a first-line treatment for mastalgia and are likely effective for some women; however, there is currently insufficient evidence that oral NSAIDs (or acetaminophen) improve pain (TABLE 432-37; FIGURE 25,13,17). Nevertheless, the potential benefits are thought to outweigh the risk of adverse effects in most patients. A small RCT did demonstrate that topical diclofenac was effective in patients with cyclic and noncyclic mastalgia.38

SSRIs. A meta-analysis of 10 double-blind RCTs of SSRIs used in women with premenstrual symptoms, including 4 studies that specifically included physical symptoms such as breast pain, showed SSRIs to be more effective than placebo at relieving breast pain.35

Progesterones. Several studies have found topical, oral, and injected progesterone ineffective at reducing breast pain.8,36,39 However, one RCT did show topical vaginal micronized progesterone used in the luteal phase to be effective in reducing breast pain by at least 50%.36
Continue to: Oral contraceptives
Oral contraceptives. For women who use oral contraceptive pills and experience cyclic breast pain, continuous dosing (skipping the pill-free week) or using a lower dose of estrogen may improve symptoms. Postmenopausal women with mastalgia that developed with initiation of hormone therapy may benefit from discontinuing hormone therapy or decreasing the estrogen dose; however, there are no RCTs to offer conclusive evidence of the effectiveness of these interventions.10
Danazol. Women with severe mastalgia that does not respond to more benign therapies may require hormone therapy. As with all symptom management, it is imperative to engage the patient in a shared decision-making conversation about the risks and benefits of this treatment strategy. Women must be able to balance the potential adverse effects of agents such as danazol and tamoxifen with the need to alleviate pain and improve quality of life.
Danazol is the only medication FDA-approved for the treatment of mastalgia. Danazol is an androgen that blocks the release of other gonadotropins to limit hormonal stimulation of breast tissue. One RCT demonstrated that danazol (100 mg bid) reduces breast pain in 60% to 90% of women, although adverse effects often limit utility.40 Adverse effects of danazol include weight gain, hot flashes, deepening of the voice, hirsutism, menorrhagia or amenorrhea, muscle cramps, and androgenic effects on a fetus.8,31,40 Danazol may be best used cyclically during the luteal phase of the menstrual cycle to limit these adverse effects with reduction of the dose to 100 mg/d after relief of symptoms.31,40
Tamoxifen, a selective estrogen receptor modulator, has been shown to reduce breast pain in 80% to 90% of women, although it is not indicated for mastalgia.40 Tamoxifen may cause endometrial thickening, hot flashes, menstrual irregularity, venous thromboembolism, and teratogenicity. The 10 mg/d dose appears to be as effective at improving symptoms as the 20 mg/d dose with fewer adverse effects.8,31,40
In a head-to-head randomized trial, tamoxifen was superior to danazol for relief of breast pain with fewer adverse effects.34 Experts recommend limiting use of tamoxifen and danazol to 3 to 6 months. Neither of these drugs is considered safe in pregnancy.
Continue to: Bromocriptine
Bromocriptine, a prolactin inhibitor, has been shown to be more effective than placebo in reducing breast pain, although nausea and dizziness contribute to high discontinuation rates. Bromocriptine is less effective than danazol.40
Goserelin, which is not available in the United States, is a gonadorelin analog (luteinizing hormone-releasing hormone analog) that produces reversible ovarian suppression. One RCT showed that goserelin injection may be more effective than placebo in reducing breast pain.37 Adverse effects include vaginal dryness, hot flashes, decreased libido, oily skin or hair, decreased breast size, and irritability. It is recommended as treatment only for severe refractory mastalgia and that it be used no longer than 6 months.31,37
CASE 1
You reassure Ms. S that her history and physical exam are consistent with cyclic breast pain and not malignancy. You review the current US Preventive Services Task Force recommendations for breast cancer screening in women ages 40 to 49 years (Grade C; women who place a higher value on the potential benefit than the potential harms may choose screening).41 Based on shared decision-making,you offer her a screening mammogram, which returns normal. After confirming that she is using an appropriately-sized supportive bra, you recommend adding 25 g/d of ground flaxseed to her diet.
After 2 months she reports a 30% improvement in her pain. You then recommend chasteberry extract 4.2 mg/d, which provides additional relief to the point where she can now sleep better and walk for exercise.
CASE 2
You order a diagnostic mammogram of the left breast, which is normal, and an ultrasound that demonstrates a 6-cm deep mass. A biopsy determines that Ms. R has invasive lobular breast cancer—an extremely unlikely outcome of breast pain. She elects to have a double mastectomy and reconstruction and is doing well 4 years later.
CORRESPONDENCE
Sarina Schrager, MD, MS, University of Wisconsin Department of Family Medicine and Community Health, 1100 Delaplaine Ct., Madison, WI, 53715; [email protected].
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
1. Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician. 2012;86:343-349.
2. Chetlen AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
3. Expert Panel on Breast Imaging: Jokich PM, Bailey L, D’Orsi C, et al. ACR Appropriateness Criteria Breast Pain. J Am Coll Radiol. 2017;14:S25-S33.
4. Arslan M, Küçükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
5. Smith RL, Pruthi S, Fitzpatrick LA. Evaluation and management of breast pain. Mayo Clin Proc. 2004;79:353-372.
6. Mansel RE. ABC of breast diseases. Breast pain. BMJ. 1994;309:866-868.
7. Ader DN, South-Paul J, Adera T, et al. Cyclical mastalgia: prevalence and associated health and behavioral factors. J Psychosom Obstet Gynaecol. 2001;22:71-76.
8. Iddon J, Dixon JM. Mastalgia. BMJ. 2013;347:f3288.
9. Eren T, Aslan A, Ozemir IA, et al. Factors effecting mastalgia. Breast Care (Basel). 2016;11:188-193.
10. Pearlman MD, Griffin JL. Benign breast disease. Obstet Gynecol. 2010;116:747-758.
11. Gateley CA, Mansel RE. The Cardiff Breast Score. Br J Hosp Med. 1991;45:16.
12. Michigan Medicine. University of Michigan. Common breast problems: guidelines for clinical care. https://www.med.umich.edu/1info/FHP/practiceguides/breast/breast.pdf. Updated June 2013. Accessed September 3, 2019.
13. Millet AV, Dirbas FM. Clinical management of breast pain: a review. Obstet Gynecol Surv. 2002;57:451-461.
14. American College of Radiology. ACR Appropriateness Criteria: Breast Pain. https://acsearch.acr.org/docs/3091546/Narrative/. Revised 2018. Accessed July 2, 2019.
15. American College of Radiology. ACR Appropriateness Criteria: Palpable Breast Masses. https://acsearch.acr.org/docs/69495/Narrative/. Revised 2016. Accessed September 3, 2019.
16. Loving VA, DeMartini WB, Eby PR, et al. Targeted ultrasound in women younger than 30 years with focal breast signs or symptoms: outcomes analyses and management implications. AJR Am J Roentgenol. 2010;195:1472-1477.
17. Molckovsky A, Fitzgerald B, Freedman O, et al. Approach to inflammatory breast cancer. Can Fam Physician. 2009;55:25-31.
18. Ammari FF, Yaghan RJ, Omari AK. Periductal mastitis: clinical characteristics and outcome. Saudi Med J. 2002;23:819-822.
19. Lannin DR. Twenty-two year experience with recurring subareolar abscess and lactiferous duct fistula treated by a single breast surgeon. Am J Surg. 2004;188:407-410.
20. Wilson JP, Massoll N, Marshall J, et al. Idiopathic granulomatous mastitis: in search of a therapeutic paradigm. Am Surg. 2007;73:798-802.
21. Bouton ME, Jayaram L, O’Neill PJ, et al. Management of idiopathic granulomatous mastitis with observation. Am J Surg. 2015;210:258-262.
22. Olawaiye A, Withiam-Leitch M, Danakas G, et al. Mastalgia: a review of management. J Reprod Med. 2005;50:933-939.
23. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Gynecology. Practice Bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol. 2016;127:e141-e156.
24. Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, et al. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med. 2016;24:90-95.
25. Vaziri F, Zamani Lari M, Sansami Dehaghani A, et al. Comparing the effects of dietary flaxseed and omega-3 fatty acids supplement on cyclical mastalgia in Iranian women: a randomized clinical trial. Int J Fam Med. 2014;2014:174532.
26. Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21:141-146.
27. McFayden IJ, Chetty U, Setchell KD, et al. A randomized double blind-cross over trial of soya protein for the treatment of cyclical breast pain. Breast. 2000;9:271-276.
28. Pruthi S, Wahner-Roedler DL, Torkelson CJ, et al. Vitamin E and evening primrose oil for management of cyclical mastalgia: a randomized pilot study. Altern Med Rev. 2010;15:59-67.
29. Halaska M, Raus K, Beles P, et al. Treatment of cyclical mastodynia using an extract of Vitex agnus castus: results of a double-blind comparison with a placebo. Ceska Gynekol. 1998;63:388-392.
30. Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective randomised placebo controlled study. BMJ. 2001;322:134-137.
31. Goyal A. Breast pain. BMJ Clin Evid. 2011;2011:0812.
32. Maddox PR, Harrison BJ, Mansel RE. Low-dose danazol for mastalgia. Br J Clin Pract Suppl. 1989;68:43-47.
33. Ahmadinejad M, Delfan B, Haghdani S, et al. Comparing the effect of diclofenac gel and piroxicam gel on mastalgia. Breast J. 2010;16:213-214.
34. Kontostolis E, Stefanidis K, Navrozoglou I, et al. Comparison of tamoxifen with danazol for treatment of cyclical mastalgia. Gynecol Endocrinol. 1997;11:393-397.
35. Marjoribanks J, Brown J, O’Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6):CD001396. doi: 10.1002/14651858.CD001396.pub3.
36. Nappi C, Affinito P, Di Carlo C, et al. Double-blind controlled trial of progesterone vaginal cream treatment for cyclical mastodynia in women with benign breast disease. J Endocrinol Invest. 1992;15:801-806.
37. Mansel RE, Goyal A, Preece P, et al. European randomized, multicenter study of goserelin (Zoladex) in the management of mastalgia. Am J Obstet Gynecol. 2004;191:1942-1949.
38. Colak T, Ipek T, Kanik A, et al. Efficacy of topical nonsteroidal antiinflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196:525-530.
39. Goyal A. Breast pain. Am Fam Physician. 2016;93:872-873.
40. Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
41. US Preventive Services Task Force. Breast cancer: Screening. Release date: January 2016. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1. Accessed August 13, 2019.
PRACTICE RECOMMENDATIONS
› Instruct patients to maintain a pain diary, which, along with a careful history and physical examination, helps to determine the cause of breast pain and the type of evaluation needed. C
› Treat cyclic, bilateral breast pain with chasteberry and flaxseed. B
› Consider short-term treatment with danazol or tamoxifen for women with severe pain. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
USPSTF recommends preventive breast cancer medications only for women at risk
Medication to help prevent breast cancer is not recommended for women without increased risk, but could benefit women at increased risk for the disease, according to an update from the U.S. Preventive Services Task Force.
“Although evidence on the best interval at which to reassess risk and indications for risk-reducing medications is not available, a pragmatic approach would be to repeat risk assessment when there is a significant change in breast cancer risk factors; for instance, when a family member is diagnosed with breast cancer or when there is a new diagnosis of atypical hyperplasia or lobular carcinoma in situ on breast biopsy,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University and members of the task force.
The recommendation applies to asymptomatic women aged 35 years and older, including women with a history of benign breast lesions, but does not apply to women with current or previous breast cancer or ductal carcinoma in situ. The recommendation remains essentially unchanged from the 2013 version, with the addition of aromatase inhibitors (AIs) in the list of options for risk-reducing medications.
In an evidence report accompanying the recommendation, researchers reviewed data from 46 studies including 82 articles and more than 5 million individuals. Overall, among 10 placebo-controlled trials, tamoxifen, raloxifene, and AIs were associated with lower incidence of invasive breast cancer, with risk ratios of 0.69, 0.44, and 0.45, respectively.
However, based on the risk of adverse effects including thromboembolic events, endometrial cancer, and cataracts, the task force determined that the benefits of these medications were no greater than small in women with no risk factors. In addition, 18 risk assessments in 25 studies showed low levels of accuracy in predicting breast cancer risk.
Data from the studies reviewed by the USPSTF showed that the harms of AIs included vasomotor symptoms, GI symptoms, musculoskeletal pain, and potential increased risk of cardiovascular events and fractures. Potential harms of other medications to help prevent breast cancer (tamoxifen and raloxifene) included increased risk for venous thromboembolic events, endometrial cancer, cataracts, and hot flashes.
The findings were limited by several factors including possible publication bias, variation in risk assessment studies, and inability to conduct subgroup analysis, wrote Heidi D. Nelson, MD, of Oregon Health & Sciences University, Portland, and colleagues in the evidence report.
“Although most results are consistent with the 2013 USPSTF review, this update provides additional evidence of the inaccuracy of risk assessment methods,” they noted.
“The USPSTF recommendations, and the accompanying systematic evidence review by Nelson and colleagues rightfully focus on the need to identify women for whom the benefits are likely to outweigh harms, but they also underscore persistent uncertainties about how to accomplish that goal,” wrote Lydia E. Pace, MD, and Nancy L. Keating, MD, both of Brigham and Women’s Hospital in Boston, in an accompanying editorial (JAMA. 2019 Sept 3;322:821-23).
“Identifying safer and more effective preventive medications would help mitigate the low discriminatory accuracy of existing breast cancer risk models,” the editorialists wrote. “Meanwhile, considering risk-reducing medications for women with 5-year risk greater than 3% seems reasonable, as well as for women with atypical hyperplasia and [lobular carcinoma in situ].”
The research was funded by the Agency for Healthcare Research and Quality. Neither the task force researchers nor the editorialists reported relevant financial conflicts.
SOURCEs: Owens DK et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.11885; Nelson HD et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.5780.
Medication to help prevent breast cancer is not recommended for women without increased risk, but could benefit women at increased risk for the disease, according to an update from the U.S. Preventive Services Task Force.
“Although evidence on the best interval at which to reassess risk and indications for risk-reducing medications is not available, a pragmatic approach would be to repeat risk assessment when there is a significant change in breast cancer risk factors; for instance, when a family member is diagnosed with breast cancer or when there is a new diagnosis of atypical hyperplasia or lobular carcinoma in situ on breast biopsy,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University and members of the task force.
The recommendation applies to asymptomatic women aged 35 years and older, including women with a history of benign breast lesions, but does not apply to women with current or previous breast cancer or ductal carcinoma in situ. The recommendation remains essentially unchanged from the 2013 version, with the addition of aromatase inhibitors (AIs) in the list of options for risk-reducing medications.
In an evidence report accompanying the recommendation, researchers reviewed data from 46 studies including 82 articles and more than 5 million individuals. Overall, among 10 placebo-controlled trials, tamoxifen, raloxifene, and AIs were associated with lower incidence of invasive breast cancer, with risk ratios of 0.69, 0.44, and 0.45, respectively.
However, based on the risk of adverse effects including thromboembolic events, endometrial cancer, and cataracts, the task force determined that the benefits of these medications were no greater than small in women with no risk factors. In addition, 18 risk assessments in 25 studies showed low levels of accuracy in predicting breast cancer risk.
Data from the studies reviewed by the USPSTF showed that the harms of AIs included vasomotor symptoms, GI symptoms, musculoskeletal pain, and potential increased risk of cardiovascular events and fractures. Potential harms of other medications to help prevent breast cancer (tamoxifen and raloxifene) included increased risk for venous thromboembolic events, endometrial cancer, cataracts, and hot flashes.
The findings were limited by several factors including possible publication bias, variation in risk assessment studies, and inability to conduct subgroup analysis, wrote Heidi D. Nelson, MD, of Oregon Health & Sciences University, Portland, and colleagues in the evidence report.
“Although most results are consistent with the 2013 USPSTF review, this update provides additional evidence of the inaccuracy of risk assessment methods,” they noted.
“The USPSTF recommendations, and the accompanying systematic evidence review by Nelson and colleagues rightfully focus on the need to identify women for whom the benefits are likely to outweigh harms, but they also underscore persistent uncertainties about how to accomplish that goal,” wrote Lydia E. Pace, MD, and Nancy L. Keating, MD, both of Brigham and Women’s Hospital in Boston, in an accompanying editorial (JAMA. 2019 Sept 3;322:821-23).
“Identifying safer and more effective preventive medications would help mitigate the low discriminatory accuracy of existing breast cancer risk models,” the editorialists wrote. “Meanwhile, considering risk-reducing medications for women with 5-year risk greater than 3% seems reasonable, as well as for women with atypical hyperplasia and [lobular carcinoma in situ].”
The research was funded by the Agency for Healthcare Research and Quality. Neither the task force researchers nor the editorialists reported relevant financial conflicts.
SOURCEs: Owens DK et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.11885; Nelson HD et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.5780.
Medication to help prevent breast cancer is not recommended for women without increased risk, but could benefit women at increased risk for the disease, according to an update from the U.S. Preventive Services Task Force.
“Although evidence on the best interval at which to reassess risk and indications for risk-reducing medications is not available, a pragmatic approach would be to repeat risk assessment when there is a significant change in breast cancer risk factors; for instance, when a family member is diagnosed with breast cancer or when there is a new diagnosis of atypical hyperplasia or lobular carcinoma in situ on breast biopsy,” wrote Douglas K. Owens, MD, of Stanford (Calif.) University and members of the task force.
The recommendation applies to asymptomatic women aged 35 years and older, including women with a history of benign breast lesions, but does not apply to women with current or previous breast cancer or ductal carcinoma in situ. The recommendation remains essentially unchanged from the 2013 version, with the addition of aromatase inhibitors (AIs) in the list of options for risk-reducing medications.
In an evidence report accompanying the recommendation, researchers reviewed data from 46 studies including 82 articles and more than 5 million individuals. Overall, among 10 placebo-controlled trials, tamoxifen, raloxifene, and AIs were associated with lower incidence of invasive breast cancer, with risk ratios of 0.69, 0.44, and 0.45, respectively.
However, based on the risk of adverse effects including thromboembolic events, endometrial cancer, and cataracts, the task force determined that the benefits of these medications were no greater than small in women with no risk factors. In addition, 18 risk assessments in 25 studies showed low levels of accuracy in predicting breast cancer risk.
Data from the studies reviewed by the USPSTF showed that the harms of AIs included vasomotor symptoms, GI symptoms, musculoskeletal pain, and potential increased risk of cardiovascular events and fractures. Potential harms of other medications to help prevent breast cancer (tamoxifen and raloxifene) included increased risk for venous thromboembolic events, endometrial cancer, cataracts, and hot flashes.
The findings were limited by several factors including possible publication bias, variation in risk assessment studies, and inability to conduct subgroup analysis, wrote Heidi D. Nelson, MD, of Oregon Health & Sciences University, Portland, and colleagues in the evidence report.
“Although most results are consistent with the 2013 USPSTF review, this update provides additional evidence of the inaccuracy of risk assessment methods,” they noted.
“The USPSTF recommendations, and the accompanying systematic evidence review by Nelson and colleagues rightfully focus on the need to identify women for whom the benefits are likely to outweigh harms, but they also underscore persistent uncertainties about how to accomplish that goal,” wrote Lydia E. Pace, MD, and Nancy L. Keating, MD, both of Brigham and Women’s Hospital in Boston, in an accompanying editorial (JAMA. 2019 Sept 3;322:821-23).
“Identifying safer and more effective preventive medications would help mitigate the low discriminatory accuracy of existing breast cancer risk models,” the editorialists wrote. “Meanwhile, considering risk-reducing medications for women with 5-year risk greater than 3% seems reasonable, as well as for women with atypical hyperplasia and [lobular carcinoma in situ].”
The research was funded by the Agency for Healthcare Research and Quality. Neither the task force researchers nor the editorialists reported relevant financial conflicts.
SOURCEs: Owens DK et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.11885; Nelson HD et al. JAMA. 2019 Sept 3. doi: 10.1001/jama.2019.5780.
FROM JAMA