User login
Gender, racial, socioeconomic differences found in obesity-depression link
Association holds for white women across income levels, black men with incomes of $100,000 or higher.
Among white women, obesity is positively associated with depressive symptoms across all income levels. However, among black women, no such associations are found – regardless of income. Meanwhile, among men, the link between obesity and depression appears strong for black men with high household incomes, a cross-sectional analysis of 12,220 adults suggests.
“This work underscores the importance of disentangling the association of race and [socioeconomic status] to gain a better understanding of how each operates to impact health outcomes,” wrote Caryn N. Bell, PhD, and her associates. The report is in Preventive Medicine.
The study comprised 3,755 black subjects, 55.5% of whom were women, and 8,465 white subjects, 51.8% of whom were women. They completed a detailed questionnaire as part of the 2007-2014 National Health and Nutrition Examination Survey and had a physical exam. Depressive symptoms were measured by the Patient Health Questionnaire-9 (PHQ-9), and obesity was defined as a body mass index of 30 kg/m2 or higher. About 1% of both black and white subjects had severe depressive symptoms, meaning a PHQ-9 score ranging from 20 to 27 points.
A greater percentage of black participants were obese (47.3% vs. 34.4%), and black participants were less likely to live in a household earning $100,000 per year or more (10.9% vs. 28.3%). Black participants were a bit younger (mean age 44.8 years vs. 49.2 years), and less likely to be currently married, college graduates, insured, and physically active. A higher percentage reported fair to poor health (23.9% vs. 14.6%). The differences were statistically significant.
For white women, the association between obesity and depression held across all income levels. For black women, this association was not found at any income level. For black men, the link between obesity and depression was limited to those with a household income of $100,000 or more (odds ratio, 4.65; 95% confidence interval, 1.48-14.59). And for white men, the association was limited to those with a household income of $35,000-$74,999 (OR, 1.44; 95% CI, 1.02-2.03).
The effect of race on obesity and depression has been well studied – it’s known, for instance, that the association between obesity and depression is strongest among white women – but the role of income as a modifier has not been well addressed, wrote Dr. Bell, an assistant professor in the department of African American studies at the University of Maryland, College Park, and her associates.
Dr. Bell and her associates wrote.
As for explanations, the authors suggested that strong, antiobesity stigma “may be present among white women at all income levels,” and may drive depression regardless of how much they make.
The prevalence of depressive symptoms at specific income levels among men suggests that something other than stigma is at work. Depression among obese, middle-income white men might be tied to “an unmeasured factor like subjective social status.” Meanwhile, obese black men with high household incomes “have less income and wealth than their white counterparts” because “of various forms of structural racism. ... This may be manifested with higher rates of depression through obesity-related factors like unhealthy coping behaviors and stress,” the investigators said.
Dr. Bell and her associates cited a few limitations. One is that the study looked only at those factors among black and white people. “Results could differ with other ethnic groups,” they wrote. In addition, income was self-reported, and three-way interactions – which are tough to interpret – were used. Nevertheless, they said, the study results have key public health implications.
The study had no financial disclosures, and the investigators reported having no conflicts of interest.
SOURCE: Bell CN et al. Prev Med. 2018 Dec 3. doi: 10.1016/j.ypmed.2018.11.024.
Association holds for white women across income levels, black men with incomes of $100,000 or higher.
Association holds for white women across income levels, black men with incomes of $100,000 or higher.
Among white women, obesity is positively associated with depressive symptoms across all income levels. However, among black women, no such associations are found – regardless of income. Meanwhile, among men, the link between obesity and depression appears strong for black men with high household incomes, a cross-sectional analysis of 12,220 adults suggests.
“This work underscores the importance of disentangling the association of race and [socioeconomic status] to gain a better understanding of how each operates to impact health outcomes,” wrote Caryn N. Bell, PhD, and her associates. The report is in Preventive Medicine.
The study comprised 3,755 black subjects, 55.5% of whom were women, and 8,465 white subjects, 51.8% of whom were women. They completed a detailed questionnaire as part of the 2007-2014 National Health and Nutrition Examination Survey and had a physical exam. Depressive symptoms were measured by the Patient Health Questionnaire-9 (PHQ-9), and obesity was defined as a body mass index of 30 kg/m2 or higher. About 1% of both black and white subjects had severe depressive symptoms, meaning a PHQ-9 score ranging from 20 to 27 points.
A greater percentage of black participants were obese (47.3% vs. 34.4%), and black participants were less likely to live in a household earning $100,000 per year or more (10.9% vs. 28.3%). Black participants were a bit younger (mean age 44.8 years vs. 49.2 years), and less likely to be currently married, college graduates, insured, and physically active. A higher percentage reported fair to poor health (23.9% vs. 14.6%). The differences were statistically significant.
For white women, the association between obesity and depression held across all income levels. For black women, this association was not found at any income level. For black men, the link between obesity and depression was limited to those with a household income of $100,000 or more (odds ratio, 4.65; 95% confidence interval, 1.48-14.59). And for white men, the association was limited to those with a household income of $35,000-$74,999 (OR, 1.44; 95% CI, 1.02-2.03).
The effect of race on obesity and depression has been well studied – it’s known, for instance, that the association between obesity and depression is strongest among white women – but the role of income as a modifier has not been well addressed, wrote Dr. Bell, an assistant professor in the department of African American studies at the University of Maryland, College Park, and her associates.
Dr. Bell and her associates wrote.
As for explanations, the authors suggested that strong, antiobesity stigma “may be present among white women at all income levels,” and may drive depression regardless of how much they make.
The prevalence of depressive symptoms at specific income levels among men suggests that something other than stigma is at work. Depression among obese, middle-income white men might be tied to “an unmeasured factor like subjective social status.” Meanwhile, obese black men with high household incomes “have less income and wealth than their white counterparts” because “of various forms of structural racism. ... This may be manifested with higher rates of depression through obesity-related factors like unhealthy coping behaviors and stress,” the investigators said.
Dr. Bell and her associates cited a few limitations. One is that the study looked only at those factors among black and white people. “Results could differ with other ethnic groups,” they wrote. In addition, income was self-reported, and three-way interactions – which are tough to interpret – were used. Nevertheless, they said, the study results have key public health implications.
The study had no financial disclosures, and the investigators reported having no conflicts of interest.
SOURCE: Bell CN et al. Prev Med. 2018 Dec 3. doi: 10.1016/j.ypmed.2018.11.024.
Among white women, obesity is positively associated with depressive symptoms across all income levels. However, among black women, no such associations are found – regardless of income. Meanwhile, among men, the link between obesity and depression appears strong for black men with high household incomes, a cross-sectional analysis of 12,220 adults suggests.
“This work underscores the importance of disentangling the association of race and [socioeconomic status] to gain a better understanding of how each operates to impact health outcomes,” wrote Caryn N. Bell, PhD, and her associates. The report is in Preventive Medicine.
The study comprised 3,755 black subjects, 55.5% of whom were women, and 8,465 white subjects, 51.8% of whom were women. They completed a detailed questionnaire as part of the 2007-2014 National Health and Nutrition Examination Survey and had a physical exam. Depressive symptoms were measured by the Patient Health Questionnaire-9 (PHQ-9), and obesity was defined as a body mass index of 30 kg/m2 or higher. About 1% of both black and white subjects had severe depressive symptoms, meaning a PHQ-9 score ranging from 20 to 27 points.
A greater percentage of black participants were obese (47.3% vs. 34.4%), and black participants were less likely to live in a household earning $100,000 per year or more (10.9% vs. 28.3%). Black participants were a bit younger (mean age 44.8 years vs. 49.2 years), and less likely to be currently married, college graduates, insured, and physically active. A higher percentage reported fair to poor health (23.9% vs. 14.6%). The differences were statistically significant.
For white women, the association between obesity and depression held across all income levels. For black women, this association was not found at any income level. For black men, the link between obesity and depression was limited to those with a household income of $100,000 or more (odds ratio, 4.65; 95% confidence interval, 1.48-14.59). And for white men, the association was limited to those with a household income of $35,000-$74,999 (OR, 1.44; 95% CI, 1.02-2.03).
The effect of race on obesity and depression has been well studied – it’s known, for instance, that the association between obesity and depression is strongest among white women – but the role of income as a modifier has not been well addressed, wrote Dr. Bell, an assistant professor in the department of African American studies at the University of Maryland, College Park, and her associates.
Dr. Bell and her associates wrote.
As for explanations, the authors suggested that strong, antiobesity stigma “may be present among white women at all income levels,” and may drive depression regardless of how much they make.
The prevalence of depressive symptoms at specific income levels among men suggests that something other than stigma is at work. Depression among obese, middle-income white men might be tied to “an unmeasured factor like subjective social status.” Meanwhile, obese black men with high household incomes “have less income and wealth than their white counterparts” because “of various forms of structural racism. ... This may be manifested with higher rates of depression through obesity-related factors like unhealthy coping behaviors and stress,” the investigators said.
Dr. Bell and her associates cited a few limitations. One is that the study looked only at those factors among black and white people. “Results could differ with other ethnic groups,” they wrote. In addition, income was self-reported, and three-way interactions – which are tough to interpret – were used. Nevertheless, they said, the study results have key public health implications.
The study had no financial disclosures, and the investigators reported having no conflicts of interest.
SOURCE: Bell CN et al. Prev Med. 2018 Dec 3. doi: 10.1016/j.ypmed.2018.11.024.
FROM PREVENTIVE MEDICINE
For pelvic pain, think outside the lower body
LAS VEGAS – An estimated 15%-25% of women aged 18-50 years suffer from chronic pelvic pain, a condition that commonly leads to sick days, reduced activity, and higher medication use. Treatments like surgery and opioids may seem feasible, but an obstetrician-gynecologist who studies pain urged colleagues to think twice.
In some cases, pelvic pain patients may suffer from centralized pain syndromes, conditions linked to the central nervous system that may not respond well to those common treatments, said Sawsan As-Sanie, MD, MPH, director of the University of Michigan Endometriosis Center, Ann Arbor.
“If we have laser vision on the pelvis, we may help some patients, but many of us will do harm,” said Dr. As-Sanie, who spoke at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Endometriosis is frequently linked to pelvic pain. But, she said, the link between the two is fuzzier than has been assumed.
“It would make sense that endometriosis or pelvic adhesions would activate nociceptive pain, and [there are] a lot of data to support that this is, in part, how endometriosis causes pain,” she said. “But I would argue it really isn’t that simple because the relationship between endometriosis and pelvic pain is very complex and not explained entirely by the lesion.” For example, “we know that pain recurs after medical and surgical therapy, often without evidence of recurrent endometriosis.” And, there’s little relationship between pain symptoms and the location or extent of endometriosis.
What’s going on? Dr. As-Sanie suggested central pain syndromes can play a significant role in pelvic pain. These syndromes are 1.5-2 times more common in women than men, and are triggered or exacerbated by stressors.
She also emphasized the wide-ranging effects of these syndromes. “We focus on pain, but it’s clearly not a just a pain disorder,” noting that patients can report fatigue, poor sleep, greater sensitivity to light and sound, and memory difficulties that produce “fibromyalgia fog.”
Research suggests that patients with central pain syndromes experience changes in both brain structure and function, she said. As for pelvic pain specifically, studies have linked it to increased pain sensitivity and altered central nervous system structure and function regardless of whether endometriosis is present.
How should patients with pelvic pain be treated in light of this information? Dr. As-Sanie suggests first trying “gold standard” approaches to treat contributing factors whether they’re gynecologic, urologic, gastrointestinal, musculoskeletal or nerve related.
If those strategies don’t work, she said, “consider treating centralized pain” with a blend of approaches: behavioral (such as diet and cognitive-behavior therapy), medical (such as hormone modulation), and interventional (such as physical therapy and surgery).
Also consider pharmacologic therapies, said Dr. As-Sanie, who identified dual reuptake inhibitors (venlafaxine [Effexor] and duloxetine [Cymbalta] are a class of antidepressants that block the reuptake of both serotonin and norepinephrine) and anticonvulsants as drugs with strong evidence as treatments for central pain syndromes.
“Start at low doses and titrate up,” she advised, and “if at any point a given medication doesn’t work, we should try another.”
The Pelvic Anatomy and Gynecologic Surgery Symposium was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Dr. As-Sanie discloses she is a consultant for AbbVie and Myovant.
LAS VEGAS – An estimated 15%-25% of women aged 18-50 years suffer from chronic pelvic pain, a condition that commonly leads to sick days, reduced activity, and higher medication use. Treatments like surgery and opioids may seem feasible, but an obstetrician-gynecologist who studies pain urged colleagues to think twice.
In some cases, pelvic pain patients may suffer from centralized pain syndromes, conditions linked to the central nervous system that may not respond well to those common treatments, said Sawsan As-Sanie, MD, MPH, director of the University of Michigan Endometriosis Center, Ann Arbor.
“If we have laser vision on the pelvis, we may help some patients, but many of us will do harm,” said Dr. As-Sanie, who spoke at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Endometriosis is frequently linked to pelvic pain. But, she said, the link between the two is fuzzier than has been assumed.
“It would make sense that endometriosis or pelvic adhesions would activate nociceptive pain, and [there are] a lot of data to support that this is, in part, how endometriosis causes pain,” she said. “But I would argue it really isn’t that simple because the relationship between endometriosis and pelvic pain is very complex and not explained entirely by the lesion.” For example, “we know that pain recurs after medical and surgical therapy, often without evidence of recurrent endometriosis.” And, there’s little relationship between pain symptoms and the location or extent of endometriosis.
What’s going on? Dr. As-Sanie suggested central pain syndromes can play a significant role in pelvic pain. These syndromes are 1.5-2 times more common in women than men, and are triggered or exacerbated by stressors.
She also emphasized the wide-ranging effects of these syndromes. “We focus on pain, but it’s clearly not a just a pain disorder,” noting that patients can report fatigue, poor sleep, greater sensitivity to light and sound, and memory difficulties that produce “fibromyalgia fog.”
Research suggests that patients with central pain syndromes experience changes in both brain structure and function, she said. As for pelvic pain specifically, studies have linked it to increased pain sensitivity and altered central nervous system structure and function regardless of whether endometriosis is present.
How should patients with pelvic pain be treated in light of this information? Dr. As-Sanie suggests first trying “gold standard” approaches to treat contributing factors whether they’re gynecologic, urologic, gastrointestinal, musculoskeletal or nerve related.
If those strategies don’t work, she said, “consider treating centralized pain” with a blend of approaches: behavioral (such as diet and cognitive-behavior therapy), medical (such as hormone modulation), and interventional (such as physical therapy and surgery).
Also consider pharmacologic therapies, said Dr. As-Sanie, who identified dual reuptake inhibitors (venlafaxine [Effexor] and duloxetine [Cymbalta] are a class of antidepressants that block the reuptake of both serotonin and norepinephrine) and anticonvulsants as drugs with strong evidence as treatments for central pain syndromes.
“Start at low doses and titrate up,” she advised, and “if at any point a given medication doesn’t work, we should try another.”
The Pelvic Anatomy and Gynecologic Surgery Symposium was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Dr. As-Sanie discloses she is a consultant for AbbVie and Myovant.
LAS VEGAS – An estimated 15%-25% of women aged 18-50 years suffer from chronic pelvic pain, a condition that commonly leads to sick days, reduced activity, and higher medication use. Treatments like surgery and opioids may seem feasible, but an obstetrician-gynecologist who studies pain urged colleagues to think twice.
In some cases, pelvic pain patients may suffer from centralized pain syndromes, conditions linked to the central nervous system that may not respond well to those common treatments, said Sawsan As-Sanie, MD, MPH, director of the University of Michigan Endometriosis Center, Ann Arbor.
“If we have laser vision on the pelvis, we may help some patients, but many of us will do harm,” said Dr. As-Sanie, who spoke at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Endometriosis is frequently linked to pelvic pain. But, she said, the link between the two is fuzzier than has been assumed.
“It would make sense that endometriosis or pelvic adhesions would activate nociceptive pain, and [there are] a lot of data to support that this is, in part, how endometriosis causes pain,” she said. “But I would argue it really isn’t that simple because the relationship between endometriosis and pelvic pain is very complex and not explained entirely by the lesion.” For example, “we know that pain recurs after medical and surgical therapy, often without evidence of recurrent endometriosis.” And, there’s little relationship between pain symptoms and the location or extent of endometriosis.
What’s going on? Dr. As-Sanie suggested central pain syndromes can play a significant role in pelvic pain. These syndromes are 1.5-2 times more common in women than men, and are triggered or exacerbated by stressors.
She also emphasized the wide-ranging effects of these syndromes. “We focus on pain, but it’s clearly not a just a pain disorder,” noting that patients can report fatigue, poor sleep, greater sensitivity to light and sound, and memory difficulties that produce “fibromyalgia fog.”
Research suggests that patients with central pain syndromes experience changes in both brain structure and function, she said. As for pelvic pain specifically, studies have linked it to increased pain sensitivity and altered central nervous system structure and function regardless of whether endometriosis is present.
How should patients with pelvic pain be treated in light of this information? Dr. As-Sanie suggests first trying “gold standard” approaches to treat contributing factors whether they’re gynecologic, urologic, gastrointestinal, musculoskeletal or nerve related.
If those strategies don’t work, she said, “consider treating centralized pain” with a blend of approaches: behavioral (such as diet and cognitive-behavior therapy), medical (such as hormone modulation), and interventional (such as physical therapy and surgery).
Also consider pharmacologic therapies, said Dr. As-Sanie, who identified dual reuptake inhibitors (venlafaxine [Effexor] and duloxetine [Cymbalta] are a class of antidepressants that block the reuptake of both serotonin and norepinephrine) and anticonvulsants as drugs with strong evidence as treatments for central pain syndromes.
“Start at low doses and titrate up,” she advised, and “if at any point a given medication doesn’t work, we should try another.”
The Pelvic Anatomy and Gynecologic Surgery Symposium was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
Dr. As-Sanie discloses she is a consultant for AbbVie and Myovant.
EXPERT ANALYSIS FROM PAGS
Poor-prognosis cancers linked to highest suicide risk in first year
Suicide risk significantly increases within the first year of a cancer diagnosis, with risk varying by type of cancer, according to investigators who conducted a retrospective analysis representing nearly 4.7 million patients.
Risk of suicide in that first year after diagnosis was especially high in pancreatic and lung cancers, while by contrast, breast and prostate cancer did not increase suicide risk, reported the researchers, led by Hesham Hamoda, MD, MPH, of Boston Children’s Hospital/Harvard Medical School, and Ahmad Alfaar, MBBCh, MSc, of Charité–Universitätsmedizin Berlin.
That variation in suicide risk by cancer type suggests that prognosis and 5-year relative survival play a role in increasing suicide rates, according to Dr. Hamoda, Dr. Alfaar, and their coauthors.
“After the diagnosis, it is important that health care providers be vigilant in screening for suicide and ensuring that patients have access to social and emotional support,” they wrote in a report published in Cancer. Their analysis was based on 4,671,989 patients with a diagnosis of cancer in the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2014. Out of 1,005,825 of those patients who died within the first year of diagnosis, the cause of death was suicide for 1,585, or 0.16%.
Overall, the risk of suicide increased significantly among cancer patients versus the general population, with an observed-to-expected (O/E) ratio of 2.51 per 10,000 person-years, the investigators found. The risk was highest in the first 6 months, with an O/E mortality of 3.13 versus 1.8 in the latter 6 months.
The highest ratios were seen for pancreatic cancer, with an O/E ratio of 8.01, and lung cancer, with a ratio of 6.05, the researchers found in further analysis.
Significant increases in suicide risk were also seen for colorectal cancer (2.08) and melanoma (1.45), though rates were not significantly different versus the general population for breast (1.23) and prostate (0.99), according to the reported data.
Suicide risk was relatively high for any cancer with distant metastases (5.63), though still significantly higher at 1.65 in persons with localized/regional disease, the data show.
The increased suicide risk persisted more than 1 year after the cancer diagnosis, though not to the degree observed within that first year, they added.
Most patients with suicide as a cause of death were white (90.2%) and male (87%). Nearly 60% were between the ages of 65 and 84 at the time of suicide.
Social support plays an integral role in suicide prevention among cancer patients, the researchers noted.
Previous studies suggest that support programs may decrease suicide risk by making patients better aware of their prognosis, receptive to decreased social stigma, or less likely to have stress related to cost of care, they said.
“Discussing the quality of life after diagnosis, the effectiveness of therapy, and the prognosis of the disease and maintaining a trusting relationship with health care professionals all decrease the likelihood of suicide immediately after a diagnosis of cancer,” they said.
Dr. Hamoda, Dr. Alfaar, and their coauthors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service (Dr. Alfaar).
SOURCE: Saad AM, et al. Cancer 2019 Jan 7. doi: 10.1002/cncr.31876.
Suicide risk significantly increases within the first year of a cancer diagnosis, with risk varying by type of cancer, according to investigators who conducted a retrospective analysis representing nearly 4.7 million patients.
Risk of suicide in that first year after diagnosis was especially high in pancreatic and lung cancers, while by contrast, breast and prostate cancer did not increase suicide risk, reported the researchers, led by Hesham Hamoda, MD, MPH, of Boston Children’s Hospital/Harvard Medical School, and Ahmad Alfaar, MBBCh, MSc, of Charité–Universitätsmedizin Berlin.
That variation in suicide risk by cancer type suggests that prognosis and 5-year relative survival play a role in increasing suicide rates, according to Dr. Hamoda, Dr. Alfaar, and their coauthors.
“After the diagnosis, it is important that health care providers be vigilant in screening for suicide and ensuring that patients have access to social and emotional support,” they wrote in a report published in Cancer. Their analysis was based on 4,671,989 patients with a diagnosis of cancer in the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2014. Out of 1,005,825 of those patients who died within the first year of diagnosis, the cause of death was suicide for 1,585, or 0.16%.
Overall, the risk of suicide increased significantly among cancer patients versus the general population, with an observed-to-expected (O/E) ratio of 2.51 per 10,000 person-years, the investigators found. The risk was highest in the first 6 months, with an O/E mortality of 3.13 versus 1.8 in the latter 6 months.
The highest ratios were seen for pancreatic cancer, with an O/E ratio of 8.01, and lung cancer, with a ratio of 6.05, the researchers found in further analysis.
Significant increases in suicide risk were also seen for colorectal cancer (2.08) and melanoma (1.45), though rates were not significantly different versus the general population for breast (1.23) and prostate (0.99), according to the reported data.
Suicide risk was relatively high for any cancer with distant metastases (5.63), though still significantly higher at 1.65 in persons with localized/regional disease, the data show.
The increased suicide risk persisted more than 1 year after the cancer diagnosis, though not to the degree observed within that first year, they added.
Most patients with suicide as a cause of death were white (90.2%) and male (87%). Nearly 60% were between the ages of 65 and 84 at the time of suicide.
Social support plays an integral role in suicide prevention among cancer patients, the researchers noted.
Previous studies suggest that support programs may decrease suicide risk by making patients better aware of their prognosis, receptive to decreased social stigma, or less likely to have stress related to cost of care, they said.
“Discussing the quality of life after diagnosis, the effectiveness of therapy, and the prognosis of the disease and maintaining a trusting relationship with health care professionals all decrease the likelihood of suicide immediately after a diagnosis of cancer,” they said.
Dr. Hamoda, Dr. Alfaar, and their coauthors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service (Dr. Alfaar).
SOURCE: Saad AM, et al. Cancer 2019 Jan 7. doi: 10.1002/cncr.31876.
Suicide risk significantly increases within the first year of a cancer diagnosis, with risk varying by type of cancer, according to investigators who conducted a retrospective analysis representing nearly 4.7 million patients.
Risk of suicide in that first year after diagnosis was especially high in pancreatic and lung cancers, while by contrast, breast and prostate cancer did not increase suicide risk, reported the researchers, led by Hesham Hamoda, MD, MPH, of Boston Children’s Hospital/Harvard Medical School, and Ahmad Alfaar, MBBCh, MSc, of Charité–Universitätsmedizin Berlin.
That variation in suicide risk by cancer type suggests that prognosis and 5-year relative survival play a role in increasing suicide rates, according to Dr. Hamoda, Dr. Alfaar, and their coauthors.
“After the diagnosis, it is important that health care providers be vigilant in screening for suicide and ensuring that patients have access to social and emotional support,” they wrote in a report published in Cancer. Their analysis was based on 4,671,989 patients with a diagnosis of cancer in the Surveillance, Epidemiology, and End Results (SEER) database between 2000 and 2014. Out of 1,005,825 of those patients who died within the first year of diagnosis, the cause of death was suicide for 1,585, or 0.16%.
Overall, the risk of suicide increased significantly among cancer patients versus the general population, with an observed-to-expected (O/E) ratio of 2.51 per 10,000 person-years, the investigators found. The risk was highest in the first 6 months, with an O/E mortality of 3.13 versus 1.8 in the latter 6 months.
The highest ratios were seen for pancreatic cancer, with an O/E ratio of 8.01, and lung cancer, with a ratio of 6.05, the researchers found in further analysis.
Significant increases in suicide risk were also seen for colorectal cancer (2.08) and melanoma (1.45), though rates were not significantly different versus the general population for breast (1.23) and prostate (0.99), according to the reported data.
Suicide risk was relatively high for any cancer with distant metastases (5.63), though still significantly higher at 1.65 in persons with localized/regional disease, the data show.
The increased suicide risk persisted more than 1 year after the cancer diagnosis, though not to the degree observed within that first year, they added.
Most patients with suicide as a cause of death were white (90.2%) and male (87%). Nearly 60% were between the ages of 65 and 84 at the time of suicide.
Social support plays an integral role in suicide prevention among cancer patients, the researchers noted.
Previous studies suggest that support programs may decrease suicide risk by making patients better aware of their prognosis, receptive to decreased social stigma, or less likely to have stress related to cost of care, they said.
“Discussing the quality of life after diagnosis, the effectiveness of therapy, and the prognosis of the disease and maintaining a trusting relationship with health care professionals all decrease the likelihood of suicide immediately after a diagnosis of cancer,” they said.
Dr. Hamoda, Dr. Alfaar, and their coauthors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service (Dr. Alfaar).
SOURCE: Saad AM, et al. Cancer 2019 Jan 7. doi: 10.1002/cncr.31876.
FROM CANCER
Key clinical point: A cancer diagnosis significantly increases risk of suicide in comparison to the general population, particularly for poorer-prognosis cancers.
Major finding: The observed-to-expected mortality ratio was substantially higher for pancreatic cancer (8.01), and lung cancer (6.05), but not significantly increased for breast (1.23) and prostate (0.99).
Study details: A retrospective population-based study of 4,671,989 cancer patients.
Disclosures: The authors reported no conflicts of interest. Funding for the study came in part from the German Academic Exchange Service.
Source: Saad AM et al. Cancer. 2019 Jan 7. doi: 10.1002/cncr.31876.
Cerebral small vessel and cognitive impairment
Also today, antidepressants are tied to greater hip fracture incidence, a hospital readmission reduction program may be doing more harm than good, and the flu season rages on with 19 states showing high activity in the final week of 2018.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, antidepressants are tied to greater hip fracture incidence, a hospital readmission reduction program may be doing more harm than good, and the flu season rages on with 19 states showing high activity in the final week of 2018.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, antidepressants are tied to greater hip fracture incidence, a hospital readmission reduction program may be doing more harm than good, and the flu season rages on with 19 states showing high activity in the final week of 2018.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Click for Credit: STIs on the rise; psoriasis & cardiac risk; more
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
Here are 5 articles from the January issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Can ultrasound screening improve survival in ovarian cancer?
To take the posttest, go to: https://bit.ly/2Vtuc8F
Expires October 17, 2019
2. Higher BMI associated with greater loss of gray matter volume in MS
To take the posttest, go to: https://bit.ly/2ArvFDp
Expires October 29, 2019
3. Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
To take the posttest, go to: https://bit.ly/2sbnkiS
Expires October 31, 2019
4. Fever, intestinal symptoms may delay diagnosis of Kawasaki disease in children
To take the posttest, go to: https://bit.ly/2RdPoBi
Expires October 31, 2019
5. Rate of STIs is rising, and many U.S. teens are sexually active
To take the posttest, go to: https://bit.ly/2CPuYFW
Expires November 8, 2019
Endometriosis surgery: Women can expect years-long benefits
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
LAS VEGAS – according to a survey study from the University of Pittsburgh.
The work was likely the first to assess long-term outcomes after laparoscopic endometriosis excision with a disease-specific questionnaire, the Endometriosis Health Profile-30 (EHP-30). The findings should reassure both surgeons and patients. “I really feel these results can help us as endometriosis providers” to counsel women, said lead investigator Nicole M. Donnellan, MD, a gynecologic surgeon at the University of Pittsburgh.
Surgery “offers lasting improvement in all quality of life domains ... measured by the EHP-30”: pain; control/powerlessness; emotional well-being; social support; and self-image, with supplemental questions about work, sexual function, and other matters. Because “definitive surgery was not associated with improved outcomes when compared with fertility-sparing surgery ... fertility preservation should continue to be offered as first-line surgery for treatment of symptomatic disease,” Dr. Donnellan and her team concluded at a meeting sponsored by AAGL.
Surgery is the gold standard for endometriosis, but there just hasn’t been much data on long-term outcomes until now, especially with a potent questionnaire like the EHP-30. The gap left surgeons in the lurch on what to tell women how they’ll do, especially because results from previous, shorter, and less-rigorous studies have been mixed. The Pittsburgh results mean that competent surgeons can breathe easier and be confident in telling women what to expect.
The team administered EHP-30 to 61 women before surgery and at 4 weeks postoperatively; 45 patients (74%) had fertility-sparing excisions, 7 (11%) had hysterectomy with adnexa preservation, and 9 (15%) had hysterectomy with bilateral salpingo-oophorectomy. The women were contacted again in 2017 to fill out the survey anywhere from 3 to 7 years after their operation; 45 women agreed, a response rate of 74%.
There was a definitive, statistically significant reduction in scores across all five domains of the survey, both at 4 weeks and out to 7 years, and the improvements did not vary by endometriosis stage or the type of surgery women had.
The overall score – a combination of the five domains – fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at long-term follow-up. Pain scores fell about the same amount; the greatest improvements were on questions that focused on sense of control and empowerment.
At long-term follow-up, overall scores improved a median of 43 points in women with American Society for Reproductive Medicine stage 1 endometriosis and 28 points among women with stage 4 disease (P = .705). Although the differences were not statistically significant, women with stage 1 disease generally reported the greatest improvements, except on the control and empowerment scale, where women reported the same improvement across all four stages, about 50 points out of 100.
Long-term score improvements were pretty much identical among women who had fertility-sparing surgery and those who had hysterectomies, with, for instance, both groups reporting about a 33-point improvement in pain scores. The two groups separated out only on emotional well-being scores, a 38-point improvement in the hysterectomy group versus 21 points, but the difference was not statistically significant (P = .525).
The long-term results remained the same when eight women who had subsequent gynecologic surgery were excluded.
In the end, the take home is that “all of these women improved,” Dr. Donnellan said.
The investigators didn’t report any disclosures.
SOURCE: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
REPORTING FROM THE AAGL GLOBAL CONGRESS
Key clinical point: Endometriosis excision improves quality of life for at least 7 years, even when women have conservative, fertility-sparing surgery.
Major finding: The overall score on the Endometriosis Health Profile-30 fell from a preoperative median of 50 points out of a possible 100, with 100 being the worst possible score, to a median of about 20 points 4 weeks after surgery, and a median of about 10 points at the 7-year follow-up.
Study details: A review of 61 cases
Disclosures: The investigators didn’t report any disclosures.
Source: Donnellan NM et al. 2018 AAGL Global Congress, Abstract 82.
Hypertension guidelines: Treat patients, not numbers
When treating high blood pressure, how low should we try to go? Debate continues about optimal blood pressure goals after publication of guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) in 2017 that set or permitted a treatment goal of less than 130 mm Hg, depending on the population.1
In this article, we summarize the evolution of hypertension guidelines and the evidence behind them.
HOW THE GOALS EVOLVED
JNC 7, 2003: 140/90 or 130/80
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),2 published in 2003, specified treatment goals of:
- < 140/90 mm Hg for most patients
- < 130/80 mm Hg for those with diabetes or chronic kidney disease.
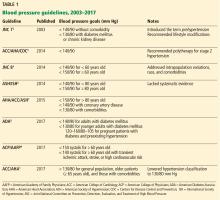
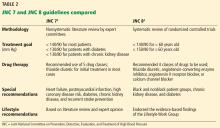
JNC 7 provided much-needed clarity and uniformity to managing hypertension. Since then, various scientific groups have published their own guidelines (Table 1).1–9
ACC/AHA/CDC 2014: 140/90
In 2014, the ACC, AHA, and US Centers for Disease Control and Prevention (CDC) published an evidence-based algorithm for hypertension management.3 As in JNC 7, they suggested a blood pressure goal of less than 140/90 mm Hg, lifestyle modification, and polytherapy, eg, a thiazide diuretic for stage 1 hypertension (< 160/100 mm Hg) and combination therapy with a thiazide diuretic and an angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blocker (ARB), or calcium channel blocker for stage 2 hypertension (≥ 160/100 mm Hg).
JNC 8 2014: 140/90 or 150/90
Soon after, the much-anticipated report of the panel members appointed to the eighth JNC (JNC 8) was published.4 Previous JNC reports were written and published under the auspices of the National Heart, Lung, and Blood Institute, but while the JNC 8 report was being prepared, this government body announced it would no longer publish guidelines.
In contrast to JNC 7, the JNC 8 panel based its recommendations on a systematic review of randomized clinical trials. However, the process and methodology were controversial, especially as the panel excluded some important clinical trials from the analysis.
JNC 8 relaxed the targets in several subgroups, such as patients over age 60 and those with diabetes and chronic kidney disease, due to a lack of definitive evidence on the impact of blood pressure targets lower than 140/90 mm Hg in these groups. Thus, their goals were:
- < 140/90 mm Hg for patients under age 60
- < 150/90 mm Hg for patients age 60 and older.
Of note, a minority of the JNC 8 panel disagreed with the new targets and provided evidence for keeping the systolic blood pressure target below 140 mm Hg for patients 60 and older.5 Further, the JNC 8 report was not endorsed by several important societies, ie, the AHA, ACC, National Heart, Lung, and Blood Institute, and American Society of Hypertension (ASH). These issues compromised the acceptance and applicability of the guidelines.
ASH/ISH 2014: 140/90 or 150/90
Also in 2014, the ASH and the International Society of Hypertension released their own report.6 Their goals:
- < 140/90 mm Hg for most patients
- < 150/90 mm Hg for patients age 80 and older.
AHA/ACC/ASH 2015: Goals in subgroups
In 2015, the AHA, ACC, and ASH released a joint scientific statement outlining hypertension goals for specific patient populations7:
- < 150/90 mm Hg for those age 80 and older
- < 140/90 mm Hg for those with coronary artery disease
- < 130/80 mm Hg for those with comorbidities such as diabetes and cardiovascular disease.
ADA 2016: Goals for patients with diabetes
In 2016, the American Diabetes Association (ADA) set the following blood pressure goals for patients with diabetes8:
- < 140/90 mm Hg for adults with diabetes
- < 130/80 mm Hg for younger adults with diabetes and adults with a high risk of cardiovascular disease
- 120–160/80–105 mm Hg for pregnant patients with diabetes and preexisting hypertension who are treated with antihypertensive therapy.
ACP/AAFP 2017: Systolic 150 or 130
In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 140 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9
ACC/AHA 2017: 130/80
The 2017 ACC/AHA guidelines recommended a more aggressive goal of below 130/80 for all, including patients age 65 and older.1
This is a class I (strong) recommendation for patients with known cardiovascular disease or a 10-year risk of a cardiovascular event of 10% or higher, with a B-R level of evidence for the systolic goal (ie, moderate-quality, based on systematic review of randomized controlled trials) and a C-EO level of evidence for the diastolic goal (ie, based on expert opinion).
For patients who do not have cardiovascular disease and who are at lower risk of it, this is a class IIb (weak) recommendation, ie, it “may be reasonable,” with a B-NR level of evidence (moderate-quality, based on nonrandomized studies) for the systolic goal and C-EO (expert opinion) for the diastolic goal.
For many patients, this involves drug treatment. For those with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, the ACC/AHA guidelines say that drug treatment “is recommended” if their average blood pressure is 130/80 mm Hg or higher (class I recommendation, based on strong evidence for the systolic threshold and expert option for the diastolic). For those without cardiovascular disease and at lower risk, drug treatment is recommended if their average blood pressure is 140/90 mm Hg or higher (also class I, but based on limited data).
EVERYONE AGREES ON LIFESTYLE
Although the guidelines differ in their blood pressure targets, they consistently recommend lifestyle modifications.
Lifestyle modifications, first described in JNC 7, included weight loss, sodium restriction, and the DASH diet, which is rich in fruits, vegetables, low-fat dairy products, whole grains, poultry, and fish, and low in red meat, sweets, cholesterol, and total and saturated fat.2
These recommendations were based on results from 3 large randomized controlled trials in patients with and without hypertension.10–12 In patients with no history of hypertension, interventions to promote weight loss and sodium restriction significantly reduced blood pressure and the incidence of hypertension (the latter by as much as 77%) compared with usual care.10,11
In patients with and without hypertension, lowering sodium intake in conjunction with the DASH diet was associated with substantially larger reductions in systolic blood pressure.12
The recommendation to lower sodium intake has not changed in the guideline revisions. Meanwhile, other modifications have been added, such as incorporating both aerobic and resistance exercise and moderating alcohol intake. These recommendations have a class I level of evidence (ie, strongest level) in the 2017 ACC/AHA guidelines.1
HYPERTENSION BEGINS AT 130/80
The definition of hypertension changed in the 2017 ACC/AHA guidelines1: previously set at 140/90 mm Hg or higher, it is now 130/80 mm Hg or higher for all age groups. Adults with systolic blood pressure of 130 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg are now classified as having stage 1 hypertension.
Under the new definition, the number of US adults who have hypertension expanded to 45.6% of the general population,13 up from 31.9% under the JNC 7 definition. Thus, overall, 103.3 million US adults now have hypertension, compared with 72.2 million under the JNC 7 criteria.
In addition, the new guidelines expanded the population of adults for whom antihypertensive drug treatment is recommended to 36.2% (81.9 million). However, this represents only a 1.9% absolute increase over the JNC 7 recommendations (34.3%) and a 5.1% absolute increase over the JNC 8 recommendations.14
SPRINT: INTENSIVE TREATMENT IS BENEFICIAL
The new ACC/AHA guidelines1 were based on evidence from several trials, including the Systolic Blood Pressure Intervention Trial (SPRINT).15
This multicenter trial investigated the effect of intensive blood pressure treatment on cardiovascular disease risk.16 The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, and heart failure.
The trial enrolled 9,361 participants at least 50 years of age with systolic blood pressure 130 mm Hg or higher and at least 1 additional risk factor for cardiovascular disease. It excluded anyone with a history of diabetes mellitus, stroke, symptomatic heart failure, or end-stage renal disease.
Two interventions were compared:
- Intensive treatment, with a systolic blood pressure goal of less than 120 mm Hg: the protocol called for polytherapy, even for participants who were 75 or older if their blood pressure was 140 mm Hg or higher
- Standard treatment, with a systolic blood pressure goal of less than 140 mm Hg: it used polytherapy for patients whose systolic blood pressure was 160 mm Hg or higher.
The trial was intended to last 5 years but was stopped early at a median of 3.26 years owing to a significantly lower rate of the primary composite outcome in the intensive-treatment group: 1.65% per year vs 2.19%, a 25% relative risk reduction (P < .001) or a 0.54% absolute risk reduction. We calculate the number needed to treat (NNT) for 1 year to prevent 1 event as 185, and over the 3.26 years of the trial, the investigators calculated the NNT as 61. Similarly, the rate of death from any cause was also lower with intensive treatment, 1.03% per year vs 1.40% per year, a 27% relative risk reduction (P = .003) or a 0.37% absolute risk reduction, NNT 270.
Using these findings, Bress et al16 estimated that implementing intensive blood pressure goals could prevent 107,500 deaths annually.
The downside is adverse effects. In SPRINT,15 the intensive-treatment group experienced significantly higher rates of serious adverse effects than the standard-treatment group, ie:
- Hypotension 2.4% vs 1.4%, P = .001
- Syncope 2.3% vs 1.7%, P = .05
- Electrolyte abnormalities 3.1% vs 2.3%, P = .02)
- Acute kidney injury or kidney failure 4.1% vs 2.5%, P < .001
- Any treatment-related adverse event 4.7% vs 2.5%, P = .001.
Thus, Bress et al16 estimated that fully implementing the intensive-treatment goals could cause an additional 56,100 episodes of hypotension per year, 34,400 cases of syncope, 43,400 serious electrolyte disorders, and 88,700 cases of acute kidney injury. All told, about 3 million Americans could suffer a serious adverse effect under the intensive-treatment goals.
SPRINT caveats and limitations
SPRINT15 was stopped early, after 3.26 years instead of the planned 5 years. The true risk-benefit ratio may have been different if the trial had been extended longer.
In addition, SPRINT used automated office blood pressure measurements in which patients were seated alone and a device (Model 907, Omron Healthcare) took 3 blood pressure measurements at 1-minute intervals after 5 minutes of quiet rest. This was designed to reduce elevated blood pressure readings in the presence of a healthcare professional in a medical setting (ie, “white coat” hypertension).
Many physicians are still taking blood pressure manually, which tends to give higher readings. Therefore, if they aim for a lower goal, they may risk overtreating the patient.
About 50% of patients did not achieve the target systolic blood pressure (< 120 mm Hg) despite receiving an average of 2.8 antihypertensive medications in the intensive-treatment group and 1.8 in the standard-treatment group. The use of antihypertensive medications, however, was not a controlled variable in the trial, and practitioners chose the appropriate drugs for their patients.
Diastolic pressure, which can be markedly lower in older hypertensive patients, was largely ignored, although lower diastolic pressure may have contributed to higher syncope rates in response to alpha blockers and calcium blockers.
Moreover, the trial excluded those with significant comorbidities and those younger than 50 (the mean age was 67.9), which limits the generalizability of the results.
JNC 8 VS SPRINT GOALS: WHAT'S THE EFFECT ON OUTCOMES?
JNC 84 recommended a relaxed target of less than 140/90 mm Hg for adults younger than 60, including those with chronic kidney disease or diabetes, and less than 150/90 mm Hg for adults 60 and older. The SPRINT findings upended those recommendations, showing that intensive treatment in adults age 75 or older significantly improved the composite cardiovascular disease outcome (2.59 vs 3.85 events per year; P < .001) and all-cause mortality (1.78 vs 2.63 events per year; P < .05) compared with standard treatment.17 Also, a subset review of SPRINT trial data found no difference in benefit based on chronic kidney disease status.18
A meta-analysis of 74 clinical trials (N = 306,273) offers a compromise between the SPRINT findings and the JNC 8 recommendations.19 It found that the beneficial effect of blood pressure treatment depended on the patient’s baseline systolic blood pressure. In those with a baseline systolic pressure of 160 mm Hg or higher, treatment reduced cardiovascular mortality by about 15% (relative risk [RR] 0.85; 95% confidence interval [CI] 0.77–0.95). In patients with systolic pressure below 140 mm Hg, treatment effects were neutral (RR 1.03, 95% CI 0.87–1.20) and not associated with any benefit as primary prevention, although data suggest it may reduce the risk of adverse outcomes in patients with coronary heart disease.
OTHER TRIALS THAT INFLUENCED THE GUIDELINES
SHEP and HYVET (the Systolic Hypertension in the Elderly Program20 and the Hypertension in the Very Elderly Trial)21 supported intensive blood pressure treatment for older patients by reporting a reduction in fatal and nonfatal stroke risks for those with a systolic blood pressure above 160 mm Hg.
FEVER (the Felodipine Event Reduction study)22 found that treatment with a calcium channel blocker in even a low dose can significantly decrease cardiovascular events, cardiovascular disease, and heart failure compared with no treatment.
JATOS and VALISH (the Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients23 and the Valsartan in Elderly Isolated Systolic Hypertension study)24 found that outcomes were similar with intensive vs standard treatment.
Ettehad et al25 performed a meta-analysis of 123 studies with more than 600,000 participants that provided strong evidence supporting blood pressure treatment goals below 130/90 mm Hg, in line with the SPRINT trial results.
BLOOD PRESSURE ISN’T EVERYTHING
Other trials remind us that although blood pressure is important, it is not the only factor affecting cardiovascular risk.
HOPE (the Heart Outcomes Prevention Evaluation)26 investigated the use of ramipril (an ACE inhibitor) in preventing myocardial infarction, stroke, or cardiovascular death in patients at high risk of cardiovascular events. The study included 9,297 participants over age 55 (mean age 66) with a baseline blood pressure 139/79 mm Hg. Follow-up was 4.5 years.
Ramipril was better than placebo, with significantly fewer patients experiencing adverse end points in the ramipril group compared with the placebo group:
- Myocardial infarction 9.9% vs 12.3%, RR 0.80, P < .001
- Cardiovascular death 6.1% vs 8.1%, RR 0.74, P < .001
- Stroke 3.4% vs 4.9%, RR = .68, P < .001
- The composite end point 14.0% vs 17.8%, RR 0.78, P < .001).
Results were even better in the subset of patients who had diabetes.27 However, the decrease in blood pressure attributable to antihypertensive therapy with ramipril was minimal (3–4 mm Hg systolic and 1–2 mm Hg diastolic). This slight change should not have been enough to produce significant differences in clinical outcomes, a major limitation of this trial. The investigators speculated that the positive results may be due to a class effect of ACE inhibitors.26
HOPE 328–30 explored the effect of blood pressure- and cholesterol-controlling drugs on the same primary end points but in patients at intermediate risk of major cardiovascular events. Investigators randomized the 12,705 patients to 4 treatment groups:
- Blood pressure control with candesartan (an ARB) plus hydrochlorothiazide (a thiazide diuretic)
- Cholesterol control with rosuvastatin (a statin)
- Blood pressure plus cholesterol control
- Placebo.
Therapy was started at a systolic blood pressure above 140 mm Hg.
Compared with placebo, the rate of composite events was significantly reduced in the rosuvastatin group (3.7% vs 4.8%, HR 0.76, P = .002)28 and the candesartan-hydrochlorothiazide-rosuvastatin group (3.6% vs 5.0%, HR 0.71; P = .005)29 but not in the candesartan-hydrochlorothiazide group (4.1% vs 4.4%; HR 0.93; P = .40).30
In addition, a subgroup analysis comparing active treatment vs placebo found a significant reduction in major cardiovascular events for treated patients whose baseline systolic blood pressure was in the upper third (> 143.5 mm Hg, mean 154.1 mm Hg), while treated patients in the lower middle and lower thirds had no significant reduction.30
These results suggest that intensive treatment to achieve a systolic blood pressure below 140 mm Hg in patients at intermediate risk may not be helpful. Nevertheless, there seems to be agreement that intensive treatment generally leads to a reduction in cardiovascular events. The results also show the benefit of lowering cholesterol.
Bundy et al31 performed a meta-analysis that provides support for intensive antihypertensive treatment. Reviewing 42 clinical trials in more than 144,000 patients, they found that treating to reach a target systolic blood pressure of 120 to 124 mm Hg can reduce cardiovascular events and all-cause mortality.
The trade-off is a minimal increase in the risk of adverse events. Also, the risk-benefit ratio of intensive treatment seems to vary in different patient subgroups.
WHAT ABOUT PATIENTS WITH COMORBIDITIES?
The debate over intensive vs standard treatment in blood pressure management extends beyond hypertension and includes important comorbidities such as diabetes, stroke, and renal disease. Patients with a history of stroke or end-stage renal disease have only a minimal mention in the AHA/ACC guidelines.
Diabetes
Emdin et al,32 in a meta-analysis of 40 trials that included more than 100,000 patients with diabetes, concluded that a 10-mm Hg lowering of systolic blood pressure significantly reduces the rates of all-cause mortality, cardiovascular disease, coronary heart disease, stroke, albuminuria, and retinopathy. Stratifying the results according to the systolic blood pressure achieved (≥ 130 or < 130 mm Hg), the relative risks of mortality, coronary heart disease, cardiovascular disease, heart failure, and albuminuria were actually lower in the higher stratum than in the lower.
ACCORD (the Action to Control Cardiovascular Risk in Diabetes)33 study provides contrary results. It examined intensive and standard blood pressure control targets in patients with type 2 diabetes at high risk of cardiovascular events, using primary outcome measures similar to those in SPRINT. It found no significant difference in fatal and nonfatal cardiovascular events between the intensive and standard blood pressure target arms.
Despite those results, the ACC/AHA guidelines still advocate for more intensive treatment (goal < 130/80 mm Hg) in all patients, including those with diabetes.1
The ADA position statement (September 2017) recommended a target below 140/90 mm Hg in patients with diabetes and hypertension.8 However, they also noted that lower systolic and diastolic blood pressure targets, such as below 130/80 mm Hg, may be appropriate for patients at high risk of cardiovascular disease “if they can be achieved without undue treatment burden.”8 Thus, it is not clear which blood pressure targets in patients with diabetes are the best.
Stroke
In patients with stroke, AHA/ACC guidelines1 recommend treatment if the blood pressure is 140/90 mm Hg or higher because antihypertensive therapy has been associated with a decrease in the recurrence of transient ischemic attack and stroke. The ideal target blood pressure is not known, but a goal of less than 130/80 mm Hg may be reasonable.
In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, a retrospective open-label trial, a target blood pressure below 130/80 mm Hg in patients with a history of lacunar stroke was associated with a lower risk of intracranial hemorrhage, but the difference was not statistically significant.34 For this reason, the ACC/AHA guidelines consider it reasonable to aim for a systolic blood pressure below 130 mm Hg in these patients.1
Renal disease
The ACC/AHA guidelines do not address how to manage hypertension in patients with end-stage renal disease, but for patients with chronic kidney disease they recommend a blood pressure target below 130/80 mm Hg.1 This recommendation is derived from the SPRINT trial,15 in which patients with stage 3 or 4 chronic kidney disease accounted for 28% of the study population. In that subgroup, intensive blood pressure control seemed to provide the same benefits for reduction in cardiovascular death and all-cause mortality.
TREAT PATIENTS, NOT NUMBERS
Blood pressure targets should be applied in the appropriate clinical context and on a patient-by-patient basis. In clinical practice, one size does not always fit all, as special cases exist.
For example, blood pressure can oscillate widely in patients with autonomic nerve disorders, making it difficult to strive for a specific target, especially an intensive one. Thus, it may be necessary to allow higher systolic blood pressure in these patients. Similarly, patients with diabetes or chronic kidney disease may be at higher risk of kidney injury with more intensive blood pressure management.
Treating numbers rather than patients may result in unbalanced patient care. The optimal approach to blood pressure management relies on a comprehensive risk factor assessment and shared decision-making with the patient before setting specific blood pressure targets.
OUR APPROACH
We aim for a blood pressure goal below 130/80 mm Hg for all patients with cardiovascular disease, according to the AHA/ACC guidelines. We aim for that same target in patients without cardiovascular disease but who have an elevated estimated cardiovascular risk (> 10%) over the next 10 years.
We recognize, however, that the benefits of aggressive blood pressure reduction may not be as clear in all patients, such as those with diabetes. We also recognize that some patient subgroups are at high risk of adverse events, including those with low diastolic pressure, chronic kidney disease, a history of falls, and older age. In those patients, we are extremely judicious when titrating antihypertensive medications. We often make smaller titrations, at longer intervals, and with more frequent laboratory testing and in-office follow-up.
Our process of managing hypertension through intensive blood pressure control to achieve lower systolic blood pressure targets requires a concerted effort among healthcare providers at all levels. It especially requires more involvement and investment from primary care providers to individualize treatment in their patients. This process has helped us to reach our treatment goals while limiting adverse effects of lower blood pressure targets.
MOVING FORWARD
Hypertension is a major risk factor for cardiovascular disease, and intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease. Thus, a general consensus on the definition of hypertension and treatment goals is essential to reduce the risk of cardiovascular events in this large patient population.
Intensive blood pressure treatment has shown efficacy, but it has a small accompanying risk of adverse events, which varies in patient subgroups and affects the benefit-risk ratio of this therapy. For example, the cardiovascular benefit of intensive treatment is less clear in diabetic patients, and the risk of adverse events may be higher in older patients with chronic kidney disease.
Moving forward, more research is needed into the effects of intensive and standard treatment on patients of all ages, those with common comorbid conditions, and those with other important factors such as diastolic hypertension.
Finally, the various medical societies should collaborate on hypertension guideline development. This would require considerable planning and coordination but would ultimately be useful in creating a generalizable approach to hypertension management.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Go AS, Bauman MA, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2014; 63(4):878–885. doi:10.1161/HYP.0000000000000003
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Weber MA, Schiffrin EL, White WB, et al. Notice of duplicate publication [duplicate publication of Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens 2014; 16(1):14–26. doi:10.1111/jch.12237] J Hypertens 2014; 32(1):3–15. doi:10.1097/HJH.0000000000000065
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Soc Hypertens 2015; 9(6):453–498. doi:10.1016/j.jash.2015.03.002
- de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6):430–437. doi:10.7326/M16-1785
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in over-weight people with high normal blood pressure: the Trials of Hypertension Prevention, phase II. Arch Intern Med 1997; 157(6):657–667. pmid:9080920
- He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 2000; 35(2):544–549. pmid:10679495
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 10; 2014(260):1–161. pmid:24819891
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018; 71(2):109–118. doi:10.1016/j.jacc.2017.10.073
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Bress AP, Kramer H, Khatib R, et al. Potential deaths averted and serious adverse events incurred from adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) intensive blood pressure regimen in the United States: Projections from NHANES (National Health and Nutrition Examination Survey). Circulation 2017; 135(17):1617–1628. doi:10.1161/CIRCULATIONAHA.116.025322
- Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315(24):2673–2682. doi:10.1001/jama.2016.7050
- Beddhu S, Rocco MV, Toto R, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med 2017; 167(6):375–383. doi:10.7326/M16-2966
- Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med 2018; 178(1):28–36. doi:10.1001/jamainternmed.2017.6015
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265(24):3255–3264. pmid:2046107
- Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens 2003; 21(12):2409–2417. doi:10.1097/01.hjh.0000084782.15238.a2
- Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23(12):2157–2172. pmid:16269957
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31(12):2115–2127. doi:10.1291/hypres.31.2115
- Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56(2):196–202. doi:10.1161/HYPERTENSIONAHA.109.146035
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
- Sleight P. The HOPE study (Heart Outcomes Prevention Evaluation). J Renin Angiotensin Aldosterone Syst 2000; 1(1):18–20. doi:10.3317/jraas.2000.002
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355(9200):253–259. pmid:10675071
- Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2021–2031. doi:10.1056/NEJMoa1600176
- Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016; 374(21):2032–2043. doi:10.1056/NEJMoa1600177
- Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2009–2020. doi:10.1056/NEJMoa1600175
- Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017; 2(7):775–781. doi:10.1001/jamacardio.2017.1421
- Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313(6):603–615. doi:10.1001/jama.2014.18574
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382(9891):507–515. doi:10.1016/S0140-6736(13)60852-1
When treating high blood pressure, how low should we try to go? Debate continues about optimal blood pressure goals after publication of guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) in 2017 that set or permitted a treatment goal of less than 130 mm Hg, depending on the population.1
In this article, we summarize the evolution of hypertension guidelines and the evidence behind them.
HOW THE GOALS EVOLVED
JNC 7, 2003: 140/90 or 130/80
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),2 published in 2003, specified treatment goals of:
- < 140/90 mm Hg for most patients
- < 130/80 mm Hg for those with diabetes or chronic kidney disease.
JNC 7 provided much-needed clarity and uniformity to managing hypertension. Since then, various scientific groups have published their own guidelines (Table 1).1–9
ACC/AHA/CDC 2014: 140/90
In 2014, the ACC, AHA, and US Centers for Disease Control and Prevention (CDC) published an evidence-based algorithm for hypertension management.3 As in JNC 7, they suggested a blood pressure goal of less than 140/90 mm Hg, lifestyle modification, and polytherapy, eg, a thiazide diuretic for stage 1 hypertension (< 160/100 mm Hg) and combination therapy with a thiazide diuretic and an angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blocker (ARB), or calcium channel blocker for stage 2 hypertension (≥ 160/100 mm Hg).
JNC 8 2014: 140/90 or 150/90
Soon after, the much-anticipated report of the panel members appointed to the eighth JNC (JNC 8) was published.4 Previous JNC reports were written and published under the auspices of the National Heart, Lung, and Blood Institute, but while the JNC 8 report was being prepared, this government body announced it would no longer publish guidelines.
In contrast to JNC 7, the JNC 8 panel based its recommendations on a systematic review of randomized clinical trials. However, the process and methodology were controversial, especially as the panel excluded some important clinical trials from the analysis.
JNC 8 relaxed the targets in several subgroups, such as patients over age 60 and those with diabetes and chronic kidney disease, due to a lack of definitive evidence on the impact of blood pressure targets lower than 140/90 mm Hg in these groups. Thus, their goals were:
- < 140/90 mm Hg for patients under age 60
- < 150/90 mm Hg for patients age 60 and older.
Of note, a minority of the JNC 8 panel disagreed with the new targets and provided evidence for keeping the systolic blood pressure target below 140 mm Hg for patients 60 and older.5 Further, the JNC 8 report was not endorsed by several important societies, ie, the AHA, ACC, National Heart, Lung, and Blood Institute, and American Society of Hypertension (ASH). These issues compromised the acceptance and applicability of the guidelines.
ASH/ISH 2014: 140/90 or 150/90
Also in 2014, the ASH and the International Society of Hypertension released their own report.6 Their goals:
- < 140/90 mm Hg for most patients
- < 150/90 mm Hg for patients age 80 and older.
AHA/ACC/ASH 2015: Goals in subgroups
In 2015, the AHA, ACC, and ASH released a joint scientific statement outlining hypertension goals for specific patient populations7:
- < 150/90 mm Hg for those age 80 and older
- < 140/90 mm Hg for those with coronary artery disease
- < 130/80 mm Hg for those with comorbidities such as diabetes and cardiovascular disease.
ADA 2016: Goals for patients with diabetes
In 2016, the American Diabetes Association (ADA) set the following blood pressure goals for patients with diabetes8:
- < 140/90 mm Hg for adults with diabetes
- < 130/80 mm Hg for younger adults with diabetes and adults with a high risk of cardiovascular disease
- 120–160/80–105 mm Hg for pregnant patients with diabetes and preexisting hypertension who are treated with antihypertensive therapy.
ACP/AAFP 2017: Systolic 150 or 130
In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 140 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9
ACC/AHA 2017: 130/80
The 2017 ACC/AHA guidelines recommended a more aggressive goal of below 130/80 for all, including patients age 65 and older.1
This is a class I (strong) recommendation for patients with known cardiovascular disease or a 10-year risk of a cardiovascular event of 10% or higher, with a B-R level of evidence for the systolic goal (ie, moderate-quality, based on systematic review of randomized controlled trials) and a C-EO level of evidence for the diastolic goal (ie, based on expert opinion).
For patients who do not have cardiovascular disease and who are at lower risk of it, this is a class IIb (weak) recommendation, ie, it “may be reasonable,” with a B-NR level of evidence (moderate-quality, based on nonrandomized studies) for the systolic goal and C-EO (expert opinion) for the diastolic goal.
For many patients, this involves drug treatment. For those with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, the ACC/AHA guidelines say that drug treatment “is recommended” if their average blood pressure is 130/80 mm Hg or higher (class I recommendation, based on strong evidence for the systolic threshold and expert option for the diastolic). For those without cardiovascular disease and at lower risk, drug treatment is recommended if their average blood pressure is 140/90 mm Hg or higher (also class I, but based on limited data).
EVERYONE AGREES ON LIFESTYLE
Although the guidelines differ in their blood pressure targets, they consistently recommend lifestyle modifications.
Lifestyle modifications, first described in JNC 7, included weight loss, sodium restriction, and the DASH diet, which is rich in fruits, vegetables, low-fat dairy products, whole grains, poultry, and fish, and low in red meat, sweets, cholesterol, and total and saturated fat.2
These recommendations were based on results from 3 large randomized controlled trials in patients with and without hypertension.10–12 In patients with no history of hypertension, interventions to promote weight loss and sodium restriction significantly reduced blood pressure and the incidence of hypertension (the latter by as much as 77%) compared with usual care.10,11
In patients with and without hypertension, lowering sodium intake in conjunction with the DASH diet was associated with substantially larger reductions in systolic blood pressure.12
The recommendation to lower sodium intake has not changed in the guideline revisions. Meanwhile, other modifications have been added, such as incorporating both aerobic and resistance exercise and moderating alcohol intake. These recommendations have a class I level of evidence (ie, strongest level) in the 2017 ACC/AHA guidelines.1
HYPERTENSION BEGINS AT 130/80
The definition of hypertension changed in the 2017 ACC/AHA guidelines1: previously set at 140/90 mm Hg or higher, it is now 130/80 mm Hg or higher for all age groups. Adults with systolic blood pressure of 130 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg are now classified as having stage 1 hypertension.
Under the new definition, the number of US adults who have hypertension expanded to 45.6% of the general population,13 up from 31.9% under the JNC 7 definition. Thus, overall, 103.3 million US adults now have hypertension, compared with 72.2 million under the JNC 7 criteria.
In addition, the new guidelines expanded the population of adults for whom antihypertensive drug treatment is recommended to 36.2% (81.9 million). However, this represents only a 1.9% absolute increase over the JNC 7 recommendations (34.3%) and a 5.1% absolute increase over the JNC 8 recommendations.14
SPRINT: INTENSIVE TREATMENT IS BENEFICIAL
The new ACC/AHA guidelines1 were based on evidence from several trials, including the Systolic Blood Pressure Intervention Trial (SPRINT).15
This multicenter trial investigated the effect of intensive blood pressure treatment on cardiovascular disease risk.16 The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, and heart failure.
The trial enrolled 9,361 participants at least 50 years of age with systolic blood pressure 130 mm Hg or higher and at least 1 additional risk factor for cardiovascular disease. It excluded anyone with a history of diabetes mellitus, stroke, symptomatic heart failure, or end-stage renal disease.
Two interventions were compared:
- Intensive treatment, with a systolic blood pressure goal of less than 120 mm Hg: the protocol called for polytherapy, even for participants who were 75 or older if their blood pressure was 140 mm Hg or higher
- Standard treatment, with a systolic blood pressure goal of less than 140 mm Hg: it used polytherapy for patients whose systolic blood pressure was 160 mm Hg or higher.
The trial was intended to last 5 years but was stopped early at a median of 3.26 years owing to a significantly lower rate of the primary composite outcome in the intensive-treatment group: 1.65% per year vs 2.19%, a 25% relative risk reduction (P < .001) or a 0.54% absolute risk reduction. We calculate the number needed to treat (NNT) for 1 year to prevent 1 event as 185, and over the 3.26 years of the trial, the investigators calculated the NNT as 61. Similarly, the rate of death from any cause was also lower with intensive treatment, 1.03% per year vs 1.40% per year, a 27% relative risk reduction (P = .003) or a 0.37% absolute risk reduction, NNT 270.
Using these findings, Bress et al16 estimated that implementing intensive blood pressure goals could prevent 107,500 deaths annually.
The downside is adverse effects. In SPRINT,15 the intensive-treatment group experienced significantly higher rates of serious adverse effects than the standard-treatment group, ie:
- Hypotension 2.4% vs 1.4%, P = .001
- Syncope 2.3% vs 1.7%, P = .05
- Electrolyte abnormalities 3.1% vs 2.3%, P = .02)
- Acute kidney injury or kidney failure 4.1% vs 2.5%, P < .001
- Any treatment-related adverse event 4.7% vs 2.5%, P = .001.
Thus, Bress et al16 estimated that fully implementing the intensive-treatment goals could cause an additional 56,100 episodes of hypotension per year, 34,400 cases of syncope, 43,400 serious electrolyte disorders, and 88,700 cases of acute kidney injury. All told, about 3 million Americans could suffer a serious adverse effect under the intensive-treatment goals.
SPRINT caveats and limitations
SPRINT15 was stopped early, after 3.26 years instead of the planned 5 years. The true risk-benefit ratio may have been different if the trial had been extended longer.
In addition, SPRINT used automated office blood pressure measurements in which patients were seated alone and a device (Model 907, Omron Healthcare) took 3 blood pressure measurements at 1-minute intervals after 5 minutes of quiet rest. This was designed to reduce elevated blood pressure readings in the presence of a healthcare professional in a medical setting (ie, “white coat” hypertension).
Many physicians are still taking blood pressure manually, which tends to give higher readings. Therefore, if they aim for a lower goal, they may risk overtreating the patient.
About 50% of patients did not achieve the target systolic blood pressure (< 120 mm Hg) despite receiving an average of 2.8 antihypertensive medications in the intensive-treatment group and 1.8 in the standard-treatment group. The use of antihypertensive medications, however, was not a controlled variable in the trial, and practitioners chose the appropriate drugs for their patients.
Diastolic pressure, which can be markedly lower in older hypertensive patients, was largely ignored, although lower diastolic pressure may have contributed to higher syncope rates in response to alpha blockers and calcium blockers.
Moreover, the trial excluded those with significant comorbidities and those younger than 50 (the mean age was 67.9), which limits the generalizability of the results.
JNC 8 VS SPRINT GOALS: WHAT'S THE EFFECT ON OUTCOMES?
JNC 84 recommended a relaxed target of less than 140/90 mm Hg for adults younger than 60, including those with chronic kidney disease or diabetes, and less than 150/90 mm Hg for adults 60 and older. The SPRINT findings upended those recommendations, showing that intensive treatment in adults age 75 or older significantly improved the composite cardiovascular disease outcome (2.59 vs 3.85 events per year; P < .001) and all-cause mortality (1.78 vs 2.63 events per year; P < .05) compared with standard treatment.17 Also, a subset review of SPRINT trial data found no difference in benefit based on chronic kidney disease status.18
A meta-analysis of 74 clinical trials (N = 306,273) offers a compromise between the SPRINT findings and the JNC 8 recommendations.19 It found that the beneficial effect of blood pressure treatment depended on the patient’s baseline systolic blood pressure. In those with a baseline systolic pressure of 160 mm Hg or higher, treatment reduced cardiovascular mortality by about 15% (relative risk [RR] 0.85; 95% confidence interval [CI] 0.77–0.95). In patients with systolic pressure below 140 mm Hg, treatment effects were neutral (RR 1.03, 95% CI 0.87–1.20) and not associated with any benefit as primary prevention, although data suggest it may reduce the risk of adverse outcomes in patients with coronary heart disease.
OTHER TRIALS THAT INFLUENCED THE GUIDELINES
SHEP and HYVET (the Systolic Hypertension in the Elderly Program20 and the Hypertension in the Very Elderly Trial)21 supported intensive blood pressure treatment for older patients by reporting a reduction in fatal and nonfatal stroke risks for those with a systolic blood pressure above 160 mm Hg.
FEVER (the Felodipine Event Reduction study)22 found that treatment with a calcium channel blocker in even a low dose can significantly decrease cardiovascular events, cardiovascular disease, and heart failure compared with no treatment.
JATOS and VALISH (the Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients23 and the Valsartan in Elderly Isolated Systolic Hypertension study)24 found that outcomes were similar with intensive vs standard treatment.
Ettehad et al25 performed a meta-analysis of 123 studies with more than 600,000 participants that provided strong evidence supporting blood pressure treatment goals below 130/90 mm Hg, in line with the SPRINT trial results.
BLOOD PRESSURE ISN’T EVERYTHING
Other trials remind us that although blood pressure is important, it is not the only factor affecting cardiovascular risk.
HOPE (the Heart Outcomes Prevention Evaluation)26 investigated the use of ramipril (an ACE inhibitor) in preventing myocardial infarction, stroke, or cardiovascular death in patients at high risk of cardiovascular events. The study included 9,297 participants over age 55 (mean age 66) with a baseline blood pressure 139/79 mm Hg. Follow-up was 4.5 years.
Ramipril was better than placebo, with significantly fewer patients experiencing adverse end points in the ramipril group compared with the placebo group:
- Myocardial infarction 9.9% vs 12.3%, RR 0.80, P < .001
- Cardiovascular death 6.1% vs 8.1%, RR 0.74, P < .001
- Stroke 3.4% vs 4.9%, RR = .68, P < .001
- The composite end point 14.0% vs 17.8%, RR 0.78, P < .001).
Results were even better in the subset of patients who had diabetes.27 However, the decrease in blood pressure attributable to antihypertensive therapy with ramipril was minimal (3–4 mm Hg systolic and 1–2 mm Hg diastolic). This slight change should not have been enough to produce significant differences in clinical outcomes, a major limitation of this trial. The investigators speculated that the positive results may be due to a class effect of ACE inhibitors.26
HOPE 328–30 explored the effect of blood pressure- and cholesterol-controlling drugs on the same primary end points but in patients at intermediate risk of major cardiovascular events. Investigators randomized the 12,705 patients to 4 treatment groups:
- Blood pressure control with candesartan (an ARB) plus hydrochlorothiazide (a thiazide diuretic)
- Cholesterol control with rosuvastatin (a statin)
- Blood pressure plus cholesterol control
- Placebo.
Therapy was started at a systolic blood pressure above 140 mm Hg.
Compared with placebo, the rate of composite events was significantly reduced in the rosuvastatin group (3.7% vs 4.8%, HR 0.76, P = .002)28 and the candesartan-hydrochlorothiazide-rosuvastatin group (3.6% vs 5.0%, HR 0.71; P = .005)29 but not in the candesartan-hydrochlorothiazide group (4.1% vs 4.4%; HR 0.93; P = .40).30
In addition, a subgroup analysis comparing active treatment vs placebo found a significant reduction in major cardiovascular events for treated patients whose baseline systolic blood pressure was in the upper third (> 143.5 mm Hg, mean 154.1 mm Hg), while treated patients in the lower middle and lower thirds had no significant reduction.30
These results suggest that intensive treatment to achieve a systolic blood pressure below 140 mm Hg in patients at intermediate risk may not be helpful. Nevertheless, there seems to be agreement that intensive treatment generally leads to a reduction in cardiovascular events. The results also show the benefit of lowering cholesterol.
Bundy et al31 performed a meta-analysis that provides support for intensive antihypertensive treatment. Reviewing 42 clinical trials in more than 144,000 patients, they found that treating to reach a target systolic blood pressure of 120 to 124 mm Hg can reduce cardiovascular events and all-cause mortality.
The trade-off is a minimal increase in the risk of adverse events. Also, the risk-benefit ratio of intensive treatment seems to vary in different patient subgroups.
WHAT ABOUT PATIENTS WITH COMORBIDITIES?
The debate over intensive vs standard treatment in blood pressure management extends beyond hypertension and includes important comorbidities such as diabetes, stroke, and renal disease. Patients with a history of stroke or end-stage renal disease have only a minimal mention in the AHA/ACC guidelines.
Diabetes
Emdin et al,32 in a meta-analysis of 40 trials that included more than 100,000 patients with diabetes, concluded that a 10-mm Hg lowering of systolic blood pressure significantly reduces the rates of all-cause mortality, cardiovascular disease, coronary heart disease, stroke, albuminuria, and retinopathy. Stratifying the results according to the systolic blood pressure achieved (≥ 130 or < 130 mm Hg), the relative risks of mortality, coronary heart disease, cardiovascular disease, heart failure, and albuminuria were actually lower in the higher stratum than in the lower.
ACCORD (the Action to Control Cardiovascular Risk in Diabetes)33 study provides contrary results. It examined intensive and standard blood pressure control targets in patients with type 2 diabetes at high risk of cardiovascular events, using primary outcome measures similar to those in SPRINT. It found no significant difference in fatal and nonfatal cardiovascular events between the intensive and standard blood pressure target arms.
Despite those results, the ACC/AHA guidelines still advocate for more intensive treatment (goal < 130/80 mm Hg) in all patients, including those with diabetes.1
The ADA position statement (September 2017) recommended a target below 140/90 mm Hg in patients with diabetes and hypertension.8 However, they also noted that lower systolic and diastolic blood pressure targets, such as below 130/80 mm Hg, may be appropriate for patients at high risk of cardiovascular disease “if they can be achieved without undue treatment burden.”8 Thus, it is not clear which blood pressure targets in patients with diabetes are the best.
Stroke
In patients with stroke, AHA/ACC guidelines1 recommend treatment if the blood pressure is 140/90 mm Hg or higher because antihypertensive therapy has been associated with a decrease in the recurrence of transient ischemic attack and stroke. The ideal target blood pressure is not known, but a goal of less than 130/80 mm Hg may be reasonable.
In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, a retrospective open-label trial, a target blood pressure below 130/80 mm Hg in patients with a history of lacunar stroke was associated with a lower risk of intracranial hemorrhage, but the difference was not statistically significant.34 For this reason, the ACC/AHA guidelines consider it reasonable to aim for a systolic blood pressure below 130 mm Hg in these patients.1
Renal disease
The ACC/AHA guidelines do not address how to manage hypertension in patients with end-stage renal disease, but for patients with chronic kidney disease they recommend a blood pressure target below 130/80 mm Hg.1 This recommendation is derived from the SPRINT trial,15 in which patients with stage 3 or 4 chronic kidney disease accounted for 28% of the study population. In that subgroup, intensive blood pressure control seemed to provide the same benefits for reduction in cardiovascular death and all-cause mortality.
TREAT PATIENTS, NOT NUMBERS
Blood pressure targets should be applied in the appropriate clinical context and on a patient-by-patient basis. In clinical practice, one size does not always fit all, as special cases exist.
For example, blood pressure can oscillate widely in patients with autonomic nerve disorders, making it difficult to strive for a specific target, especially an intensive one. Thus, it may be necessary to allow higher systolic blood pressure in these patients. Similarly, patients with diabetes or chronic kidney disease may be at higher risk of kidney injury with more intensive blood pressure management.
Treating numbers rather than patients may result in unbalanced patient care. The optimal approach to blood pressure management relies on a comprehensive risk factor assessment and shared decision-making with the patient before setting specific blood pressure targets.
OUR APPROACH
We aim for a blood pressure goal below 130/80 mm Hg for all patients with cardiovascular disease, according to the AHA/ACC guidelines. We aim for that same target in patients without cardiovascular disease but who have an elevated estimated cardiovascular risk (> 10%) over the next 10 years.
We recognize, however, that the benefits of aggressive blood pressure reduction may not be as clear in all patients, such as those with diabetes. We also recognize that some patient subgroups are at high risk of adverse events, including those with low diastolic pressure, chronic kidney disease, a history of falls, and older age. In those patients, we are extremely judicious when titrating antihypertensive medications. We often make smaller titrations, at longer intervals, and with more frequent laboratory testing and in-office follow-up.
Our process of managing hypertension through intensive blood pressure control to achieve lower systolic blood pressure targets requires a concerted effort among healthcare providers at all levels. It especially requires more involvement and investment from primary care providers to individualize treatment in their patients. This process has helped us to reach our treatment goals while limiting adverse effects of lower blood pressure targets.
MOVING FORWARD
Hypertension is a major risk factor for cardiovascular disease, and intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease. Thus, a general consensus on the definition of hypertension and treatment goals is essential to reduce the risk of cardiovascular events in this large patient population.
Intensive blood pressure treatment has shown efficacy, but it has a small accompanying risk of adverse events, which varies in patient subgroups and affects the benefit-risk ratio of this therapy. For example, the cardiovascular benefit of intensive treatment is less clear in diabetic patients, and the risk of adverse events may be higher in older patients with chronic kidney disease.
Moving forward, more research is needed into the effects of intensive and standard treatment on patients of all ages, those with common comorbid conditions, and those with other important factors such as diastolic hypertension.
Finally, the various medical societies should collaborate on hypertension guideline development. This would require considerable planning and coordination but would ultimately be useful in creating a generalizable approach to hypertension management.
When treating high blood pressure, how low should we try to go? Debate continues about optimal blood pressure goals after publication of guidelines from the American College of Cardiology and American Heart Association (ACC/AHA) in 2017 that set or permitted a treatment goal of less than 130 mm Hg, depending on the population.1
In this article, we summarize the evolution of hypertension guidelines and the evidence behind them.
HOW THE GOALS EVOLVED
JNC 7, 2003: 140/90 or 130/80
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7),2 published in 2003, specified treatment goals of:
- < 140/90 mm Hg for most patients
- < 130/80 mm Hg for those with diabetes or chronic kidney disease.
JNC 7 provided much-needed clarity and uniformity to managing hypertension. Since then, various scientific groups have published their own guidelines (Table 1).1–9
ACC/AHA/CDC 2014: 140/90
In 2014, the ACC, AHA, and US Centers for Disease Control and Prevention (CDC) published an evidence-based algorithm for hypertension management.3 As in JNC 7, they suggested a blood pressure goal of less than 140/90 mm Hg, lifestyle modification, and polytherapy, eg, a thiazide diuretic for stage 1 hypertension (< 160/100 mm Hg) and combination therapy with a thiazide diuretic and an angiotensin-converting enzyme (ACE) inhibitor, angiotensin II receptor blocker (ARB), or calcium channel blocker for stage 2 hypertension (≥ 160/100 mm Hg).
JNC 8 2014: 140/90 or 150/90
Soon after, the much-anticipated report of the panel members appointed to the eighth JNC (JNC 8) was published.4 Previous JNC reports were written and published under the auspices of the National Heart, Lung, and Blood Institute, but while the JNC 8 report was being prepared, this government body announced it would no longer publish guidelines.
In contrast to JNC 7, the JNC 8 panel based its recommendations on a systematic review of randomized clinical trials. However, the process and methodology were controversial, especially as the panel excluded some important clinical trials from the analysis.
JNC 8 relaxed the targets in several subgroups, such as patients over age 60 and those with diabetes and chronic kidney disease, due to a lack of definitive evidence on the impact of blood pressure targets lower than 140/90 mm Hg in these groups. Thus, their goals were:
- < 140/90 mm Hg for patients under age 60
- < 150/90 mm Hg for patients age 60 and older.
Of note, a minority of the JNC 8 panel disagreed with the new targets and provided evidence for keeping the systolic blood pressure target below 140 mm Hg for patients 60 and older.5 Further, the JNC 8 report was not endorsed by several important societies, ie, the AHA, ACC, National Heart, Lung, and Blood Institute, and American Society of Hypertension (ASH). These issues compromised the acceptance and applicability of the guidelines.
ASH/ISH 2014: 140/90 or 150/90
Also in 2014, the ASH and the International Society of Hypertension released their own report.6 Their goals:
- < 140/90 mm Hg for most patients
- < 150/90 mm Hg for patients age 80 and older.
AHA/ACC/ASH 2015: Goals in subgroups
In 2015, the AHA, ACC, and ASH released a joint scientific statement outlining hypertension goals for specific patient populations7:
- < 150/90 mm Hg for those age 80 and older
- < 140/90 mm Hg for those with coronary artery disease
- < 130/80 mm Hg for those with comorbidities such as diabetes and cardiovascular disease.
ADA 2016: Goals for patients with diabetes
In 2016, the American Diabetes Association (ADA) set the following blood pressure goals for patients with diabetes8:
- < 140/90 mm Hg for adults with diabetes
- < 130/80 mm Hg for younger adults with diabetes and adults with a high risk of cardiovascular disease
- 120–160/80–105 mm Hg for pregnant patients with diabetes and preexisting hypertension who are treated with antihypertensive therapy.
ACP/AAFP 2017: Systolic 150 or 130
In 2017, the American College of Physicians (ACP) and the American Academy of Family Physicians (AAFP) recommended a relaxed systolic blood pressure target, ie, below 150 mm Hg, for adults over age 60, but a tighter goal of less than 140 mm Hg for the same age group if they have transient ischemic attack, stroke, or high cardiovascular risk.9
ACC/AHA 2017: 130/80
The 2017 ACC/AHA guidelines recommended a more aggressive goal of below 130/80 for all, including patients age 65 and older.1
This is a class I (strong) recommendation for patients with known cardiovascular disease or a 10-year risk of a cardiovascular event of 10% or higher, with a B-R level of evidence for the systolic goal (ie, moderate-quality, based on systematic review of randomized controlled trials) and a C-EO level of evidence for the diastolic goal (ie, based on expert opinion).
For patients who do not have cardiovascular disease and who are at lower risk of it, this is a class IIb (weak) recommendation, ie, it “may be reasonable,” with a B-NR level of evidence (moderate-quality, based on nonrandomized studies) for the systolic goal and C-EO (expert opinion) for the diastolic goal.
For many patients, this involves drug treatment. For those with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, the ACC/AHA guidelines say that drug treatment “is recommended” if their average blood pressure is 130/80 mm Hg or higher (class I recommendation, based on strong evidence for the systolic threshold and expert option for the diastolic). For those without cardiovascular disease and at lower risk, drug treatment is recommended if their average blood pressure is 140/90 mm Hg or higher (also class I, but based on limited data).
EVERYONE AGREES ON LIFESTYLE
Although the guidelines differ in their blood pressure targets, they consistently recommend lifestyle modifications.
Lifestyle modifications, first described in JNC 7, included weight loss, sodium restriction, and the DASH diet, which is rich in fruits, vegetables, low-fat dairy products, whole grains, poultry, and fish, and low in red meat, sweets, cholesterol, and total and saturated fat.2
These recommendations were based on results from 3 large randomized controlled trials in patients with and without hypertension.10–12 In patients with no history of hypertension, interventions to promote weight loss and sodium restriction significantly reduced blood pressure and the incidence of hypertension (the latter by as much as 77%) compared with usual care.10,11
In patients with and without hypertension, lowering sodium intake in conjunction with the DASH diet was associated with substantially larger reductions in systolic blood pressure.12
The recommendation to lower sodium intake has not changed in the guideline revisions. Meanwhile, other modifications have been added, such as incorporating both aerobic and resistance exercise and moderating alcohol intake. These recommendations have a class I level of evidence (ie, strongest level) in the 2017 ACC/AHA guidelines.1
HYPERTENSION BEGINS AT 130/80
The definition of hypertension changed in the 2017 ACC/AHA guidelines1: previously set at 140/90 mm Hg or higher, it is now 130/80 mm Hg or higher for all age groups. Adults with systolic blood pressure of 130 to 139 mm Hg or diastolic blood pressure of 80 to 89 mm Hg are now classified as having stage 1 hypertension.
Under the new definition, the number of US adults who have hypertension expanded to 45.6% of the general population,13 up from 31.9% under the JNC 7 definition. Thus, overall, 103.3 million US adults now have hypertension, compared with 72.2 million under the JNC 7 criteria.
In addition, the new guidelines expanded the population of adults for whom antihypertensive drug treatment is recommended to 36.2% (81.9 million). However, this represents only a 1.9% absolute increase over the JNC 7 recommendations (34.3%) and a 5.1% absolute increase over the JNC 8 recommendations.14
SPRINT: INTENSIVE TREATMENT IS BENEFICIAL
The new ACC/AHA guidelines1 were based on evidence from several trials, including the Systolic Blood Pressure Intervention Trial (SPRINT).15
This multicenter trial investigated the effect of intensive blood pressure treatment on cardiovascular disease risk.16 The primary outcome was a composite of myocardial infarction, acute coronary syndrome, stroke, and heart failure.
The trial enrolled 9,361 participants at least 50 years of age with systolic blood pressure 130 mm Hg or higher and at least 1 additional risk factor for cardiovascular disease. It excluded anyone with a history of diabetes mellitus, stroke, symptomatic heart failure, or end-stage renal disease.
Two interventions were compared:
- Intensive treatment, with a systolic blood pressure goal of less than 120 mm Hg: the protocol called for polytherapy, even for participants who were 75 or older if their blood pressure was 140 mm Hg or higher
- Standard treatment, with a systolic blood pressure goal of less than 140 mm Hg: it used polytherapy for patients whose systolic blood pressure was 160 mm Hg or higher.
The trial was intended to last 5 years but was stopped early at a median of 3.26 years owing to a significantly lower rate of the primary composite outcome in the intensive-treatment group: 1.65% per year vs 2.19%, a 25% relative risk reduction (P < .001) or a 0.54% absolute risk reduction. We calculate the number needed to treat (NNT) for 1 year to prevent 1 event as 185, and over the 3.26 years of the trial, the investigators calculated the NNT as 61. Similarly, the rate of death from any cause was also lower with intensive treatment, 1.03% per year vs 1.40% per year, a 27% relative risk reduction (P = .003) or a 0.37% absolute risk reduction, NNT 270.
Using these findings, Bress et al16 estimated that implementing intensive blood pressure goals could prevent 107,500 deaths annually.
The downside is adverse effects. In SPRINT,15 the intensive-treatment group experienced significantly higher rates of serious adverse effects than the standard-treatment group, ie:
- Hypotension 2.4% vs 1.4%, P = .001
- Syncope 2.3% vs 1.7%, P = .05
- Electrolyte abnormalities 3.1% vs 2.3%, P = .02)
- Acute kidney injury or kidney failure 4.1% vs 2.5%, P < .001
- Any treatment-related adverse event 4.7% vs 2.5%, P = .001.
Thus, Bress et al16 estimated that fully implementing the intensive-treatment goals could cause an additional 56,100 episodes of hypotension per year, 34,400 cases of syncope, 43,400 serious electrolyte disorders, and 88,700 cases of acute kidney injury. All told, about 3 million Americans could suffer a serious adverse effect under the intensive-treatment goals.
SPRINT caveats and limitations
SPRINT15 was stopped early, after 3.26 years instead of the planned 5 years. The true risk-benefit ratio may have been different if the trial had been extended longer.
In addition, SPRINT used automated office blood pressure measurements in which patients were seated alone and a device (Model 907, Omron Healthcare) took 3 blood pressure measurements at 1-minute intervals after 5 minutes of quiet rest. This was designed to reduce elevated blood pressure readings in the presence of a healthcare professional in a medical setting (ie, “white coat” hypertension).
Many physicians are still taking blood pressure manually, which tends to give higher readings. Therefore, if they aim for a lower goal, they may risk overtreating the patient.
About 50% of patients did not achieve the target systolic blood pressure (< 120 mm Hg) despite receiving an average of 2.8 antihypertensive medications in the intensive-treatment group and 1.8 in the standard-treatment group. The use of antihypertensive medications, however, was not a controlled variable in the trial, and practitioners chose the appropriate drugs for their patients.
Diastolic pressure, which can be markedly lower in older hypertensive patients, was largely ignored, although lower diastolic pressure may have contributed to higher syncope rates in response to alpha blockers and calcium blockers.
Moreover, the trial excluded those with significant comorbidities and those younger than 50 (the mean age was 67.9), which limits the generalizability of the results.
JNC 8 VS SPRINT GOALS: WHAT'S THE EFFECT ON OUTCOMES?
JNC 84 recommended a relaxed target of less than 140/90 mm Hg for adults younger than 60, including those with chronic kidney disease or diabetes, and less than 150/90 mm Hg for adults 60 and older. The SPRINT findings upended those recommendations, showing that intensive treatment in adults age 75 or older significantly improved the composite cardiovascular disease outcome (2.59 vs 3.85 events per year; P < .001) and all-cause mortality (1.78 vs 2.63 events per year; P < .05) compared with standard treatment.17 Also, a subset review of SPRINT trial data found no difference in benefit based on chronic kidney disease status.18
A meta-analysis of 74 clinical trials (N = 306,273) offers a compromise between the SPRINT findings and the JNC 8 recommendations.19 It found that the beneficial effect of blood pressure treatment depended on the patient’s baseline systolic blood pressure. In those with a baseline systolic pressure of 160 mm Hg or higher, treatment reduced cardiovascular mortality by about 15% (relative risk [RR] 0.85; 95% confidence interval [CI] 0.77–0.95). In patients with systolic pressure below 140 mm Hg, treatment effects were neutral (RR 1.03, 95% CI 0.87–1.20) and not associated with any benefit as primary prevention, although data suggest it may reduce the risk of adverse outcomes in patients with coronary heart disease.
OTHER TRIALS THAT INFLUENCED THE GUIDELINES
SHEP and HYVET (the Systolic Hypertension in the Elderly Program20 and the Hypertension in the Very Elderly Trial)21 supported intensive blood pressure treatment for older patients by reporting a reduction in fatal and nonfatal stroke risks for those with a systolic blood pressure above 160 mm Hg.
FEVER (the Felodipine Event Reduction study)22 found that treatment with a calcium channel blocker in even a low dose can significantly decrease cardiovascular events, cardiovascular disease, and heart failure compared with no treatment.
JATOS and VALISH (the Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients23 and the Valsartan in Elderly Isolated Systolic Hypertension study)24 found that outcomes were similar with intensive vs standard treatment.
Ettehad et al25 performed a meta-analysis of 123 studies with more than 600,000 participants that provided strong evidence supporting blood pressure treatment goals below 130/90 mm Hg, in line with the SPRINT trial results.
BLOOD PRESSURE ISN’T EVERYTHING
Other trials remind us that although blood pressure is important, it is not the only factor affecting cardiovascular risk.
HOPE (the Heart Outcomes Prevention Evaluation)26 investigated the use of ramipril (an ACE inhibitor) in preventing myocardial infarction, stroke, or cardiovascular death in patients at high risk of cardiovascular events. The study included 9,297 participants over age 55 (mean age 66) with a baseline blood pressure 139/79 mm Hg. Follow-up was 4.5 years.
Ramipril was better than placebo, with significantly fewer patients experiencing adverse end points in the ramipril group compared with the placebo group:
- Myocardial infarction 9.9% vs 12.3%, RR 0.80, P < .001
- Cardiovascular death 6.1% vs 8.1%, RR 0.74, P < .001
- Stroke 3.4% vs 4.9%, RR = .68, P < .001
- The composite end point 14.0% vs 17.8%, RR 0.78, P < .001).
Results were even better in the subset of patients who had diabetes.27 However, the decrease in blood pressure attributable to antihypertensive therapy with ramipril was minimal (3–4 mm Hg systolic and 1–2 mm Hg diastolic). This slight change should not have been enough to produce significant differences in clinical outcomes, a major limitation of this trial. The investigators speculated that the positive results may be due to a class effect of ACE inhibitors.26
HOPE 328–30 explored the effect of blood pressure- and cholesterol-controlling drugs on the same primary end points but in patients at intermediate risk of major cardiovascular events. Investigators randomized the 12,705 patients to 4 treatment groups:
- Blood pressure control with candesartan (an ARB) plus hydrochlorothiazide (a thiazide diuretic)
- Cholesterol control with rosuvastatin (a statin)
- Blood pressure plus cholesterol control
- Placebo.
Therapy was started at a systolic blood pressure above 140 mm Hg.
Compared with placebo, the rate of composite events was significantly reduced in the rosuvastatin group (3.7% vs 4.8%, HR 0.76, P = .002)28 and the candesartan-hydrochlorothiazide-rosuvastatin group (3.6% vs 5.0%, HR 0.71; P = .005)29 but not in the candesartan-hydrochlorothiazide group (4.1% vs 4.4%; HR 0.93; P = .40).30
In addition, a subgroup analysis comparing active treatment vs placebo found a significant reduction in major cardiovascular events for treated patients whose baseline systolic blood pressure was in the upper third (> 143.5 mm Hg, mean 154.1 mm Hg), while treated patients in the lower middle and lower thirds had no significant reduction.30
These results suggest that intensive treatment to achieve a systolic blood pressure below 140 mm Hg in patients at intermediate risk may not be helpful. Nevertheless, there seems to be agreement that intensive treatment generally leads to a reduction in cardiovascular events. The results also show the benefit of lowering cholesterol.
Bundy et al31 performed a meta-analysis that provides support for intensive antihypertensive treatment. Reviewing 42 clinical trials in more than 144,000 patients, they found that treating to reach a target systolic blood pressure of 120 to 124 mm Hg can reduce cardiovascular events and all-cause mortality.
The trade-off is a minimal increase in the risk of adverse events. Also, the risk-benefit ratio of intensive treatment seems to vary in different patient subgroups.
WHAT ABOUT PATIENTS WITH COMORBIDITIES?
The debate over intensive vs standard treatment in blood pressure management extends beyond hypertension and includes important comorbidities such as diabetes, stroke, and renal disease. Patients with a history of stroke or end-stage renal disease have only a minimal mention in the AHA/ACC guidelines.
Diabetes
Emdin et al,32 in a meta-analysis of 40 trials that included more than 100,000 patients with diabetes, concluded that a 10-mm Hg lowering of systolic blood pressure significantly reduces the rates of all-cause mortality, cardiovascular disease, coronary heart disease, stroke, albuminuria, and retinopathy. Stratifying the results according to the systolic blood pressure achieved (≥ 130 or < 130 mm Hg), the relative risks of mortality, coronary heart disease, cardiovascular disease, heart failure, and albuminuria were actually lower in the higher stratum than in the lower.
ACCORD (the Action to Control Cardiovascular Risk in Diabetes)33 study provides contrary results. It examined intensive and standard blood pressure control targets in patients with type 2 diabetes at high risk of cardiovascular events, using primary outcome measures similar to those in SPRINT. It found no significant difference in fatal and nonfatal cardiovascular events between the intensive and standard blood pressure target arms.
Despite those results, the ACC/AHA guidelines still advocate for more intensive treatment (goal < 130/80 mm Hg) in all patients, including those with diabetes.1
The ADA position statement (September 2017) recommended a target below 140/90 mm Hg in patients with diabetes and hypertension.8 However, they also noted that lower systolic and diastolic blood pressure targets, such as below 130/80 mm Hg, may be appropriate for patients at high risk of cardiovascular disease “if they can be achieved without undue treatment burden.”8 Thus, it is not clear which blood pressure targets in patients with diabetes are the best.
Stroke
In patients with stroke, AHA/ACC guidelines1 recommend treatment if the blood pressure is 140/90 mm Hg or higher because antihypertensive therapy has been associated with a decrease in the recurrence of transient ischemic attack and stroke. The ideal target blood pressure is not known, but a goal of less than 130/80 mm Hg may be reasonable.
In the Secondary Prevention of Small Subcortical Strokes (SPS3) trial, a retrospective open-label trial, a target blood pressure below 130/80 mm Hg in patients with a history of lacunar stroke was associated with a lower risk of intracranial hemorrhage, but the difference was not statistically significant.34 For this reason, the ACC/AHA guidelines consider it reasonable to aim for a systolic blood pressure below 130 mm Hg in these patients.1
Renal disease
The ACC/AHA guidelines do not address how to manage hypertension in patients with end-stage renal disease, but for patients with chronic kidney disease they recommend a blood pressure target below 130/80 mm Hg.1 This recommendation is derived from the SPRINT trial,15 in which patients with stage 3 or 4 chronic kidney disease accounted for 28% of the study population. In that subgroup, intensive blood pressure control seemed to provide the same benefits for reduction in cardiovascular death and all-cause mortality.
TREAT PATIENTS, NOT NUMBERS
Blood pressure targets should be applied in the appropriate clinical context and on a patient-by-patient basis. In clinical practice, one size does not always fit all, as special cases exist.
For example, blood pressure can oscillate widely in patients with autonomic nerve disorders, making it difficult to strive for a specific target, especially an intensive one. Thus, it may be necessary to allow higher systolic blood pressure in these patients. Similarly, patients with diabetes or chronic kidney disease may be at higher risk of kidney injury with more intensive blood pressure management.
Treating numbers rather than patients may result in unbalanced patient care. The optimal approach to blood pressure management relies on a comprehensive risk factor assessment and shared decision-making with the patient before setting specific blood pressure targets.
OUR APPROACH
We aim for a blood pressure goal below 130/80 mm Hg for all patients with cardiovascular disease, according to the AHA/ACC guidelines. We aim for that same target in patients without cardiovascular disease but who have an elevated estimated cardiovascular risk (> 10%) over the next 10 years.
We recognize, however, that the benefits of aggressive blood pressure reduction may not be as clear in all patients, such as those with diabetes. We also recognize that some patient subgroups are at high risk of adverse events, including those with low diastolic pressure, chronic kidney disease, a history of falls, and older age. In those patients, we are extremely judicious when titrating antihypertensive medications. We often make smaller titrations, at longer intervals, and with more frequent laboratory testing and in-office follow-up.
Our process of managing hypertension through intensive blood pressure control to achieve lower systolic blood pressure targets requires a concerted effort among healthcare providers at all levels. It especially requires more involvement and investment from primary care providers to individualize treatment in their patients. This process has helped us to reach our treatment goals while limiting adverse effects of lower blood pressure targets.
MOVING FORWARD
Hypertension is a major risk factor for cardiovascular disease, and intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease. Thus, a general consensus on the definition of hypertension and treatment goals is essential to reduce the risk of cardiovascular events in this large patient population.
Intensive blood pressure treatment has shown efficacy, but it has a small accompanying risk of adverse events, which varies in patient subgroups and affects the benefit-risk ratio of this therapy. For example, the cardiovascular benefit of intensive treatment is less clear in diabetic patients, and the risk of adverse events may be higher in older patients with chronic kidney disease.
Moving forward, more research is needed into the effects of intensive and standard treatment on patients of all ages, those with common comorbid conditions, and those with other important factors such as diastolic hypertension.
Finally, the various medical societies should collaborate on hypertension guideline development. This would require considerable planning and coordination but would ultimately be useful in creating a generalizable approach to hypertension management.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Go AS, Bauman MA, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2014; 63(4):878–885. doi:10.1161/HYP.0000000000000003
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Weber MA, Schiffrin EL, White WB, et al. Notice of duplicate publication [duplicate publication of Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens 2014; 16(1):14–26. doi:10.1111/jch.12237] J Hypertens 2014; 32(1):3–15. doi:10.1097/HJH.0000000000000065
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Soc Hypertens 2015; 9(6):453–498. doi:10.1016/j.jash.2015.03.002
- de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6):430–437. doi:10.7326/M16-1785
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in over-weight people with high normal blood pressure: the Trials of Hypertension Prevention, phase II. Arch Intern Med 1997; 157(6):657–667. pmid:9080920
- He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 2000; 35(2):544–549. pmid:10679495
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 10; 2014(260):1–161. pmid:24819891
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018; 71(2):109–118. doi:10.1016/j.jacc.2017.10.073
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Bress AP, Kramer H, Khatib R, et al. Potential deaths averted and serious adverse events incurred from adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) intensive blood pressure regimen in the United States: Projections from NHANES (National Health and Nutrition Examination Survey). Circulation 2017; 135(17):1617–1628. doi:10.1161/CIRCULATIONAHA.116.025322
- Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315(24):2673–2682. doi:10.1001/jama.2016.7050
- Beddhu S, Rocco MV, Toto R, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med 2017; 167(6):375–383. doi:10.7326/M16-2966
- Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med 2018; 178(1):28–36. doi:10.1001/jamainternmed.2017.6015
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265(24):3255–3264. pmid:2046107
- Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens 2003; 21(12):2409–2417. doi:10.1097/01.hjh.0000084782.15238.a2
- Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23(12):2157–2172. pmid:16269957
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31(12):2115–2127. doi:10.1291/hypres.31.2115
- Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56(2):196–202. doi:10.1161/HYPERTENSIONAHA.109.146035
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
- Sleight P. The HOPE study (Heart Outcomes Prevention Evaluation). J Renin Angiotensin Aldosterone Syst 2000; 1(1):18–20. doi:10.3317/jraas.2000.002
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355(9200):253–259. pmid:10675071
- Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2021–2031. doi:10.1056/NEJMoa1600176
- Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016; 374(21):2032–2043. doi:10.1056/NEJMoa1600177
- Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2009–2020. doi:10.1056/NEJMoa1600175
- Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017; 2(7):775–781. doi:10.1001/jamacardio.2017.1421
- Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313(6):603–615. doi:10.1001/jama.2014.18574
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382(9891):507–515. doi:10.1016/S0140-6736(13)60852-1
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71(19):e127–e248. doi:10.1016/j.jacc.2017.11.006
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2572. doi:10.1001/jama.289.19.2560
- Go AS, Bauman MA, King SM, et al. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension 2014; 63(4):878–885. doi:10.1161/HYP.0000000000000003
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT Jr, Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Weber MA, Schiffrin EL, White WB, et al. Notice of duplicate publication [duplicate publication of Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens 2014; 16(1):14–26. doi:10.1111/jch.12237] J Hypertens 2014; 32(1):3–15. doi:10.1097/HJH.0000000000000065
- Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. J Am Soc Hypertens 2015; 9(6):453–498. doi:10.1016/j.jash.2015.03.002
- de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6):430–437. doi:10.7326/M16-1785
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in over-weight people with high normal blood pressure: the Trials of Hypertension Prevention, phase II. Arch Intern Med 1997; 157(6):657–667. pmid:9080920
- He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension 2000; 35(2):544–549. pmid:10679495
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for US adults: National Health Interview Survey, 2012. National Center for Health Statistics. Vital Health Stat 10; 2014(260):1–161. pmid:24819891
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018; 71(2):109–118. doi:10.1016/j.jacc.2017.10.073
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Bress AP, Kramer H, Khatib R, et al. Potential deaths averted and serious adverse events incurred from adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) intensive blood pressure regimen in the United States: Projections from NHANES (National Health and Nutrition Examination Survey). Circulation 2017; 135(17):1617–1628. doi:10.1161/CIRCULATIONAHA.116.025322
- Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥ 75 years: a randomized clinical trial. JAMA 2016; 315(24):2673–2682. doi:10.1001/jama.2016.7050
- Beddhu S, Rocco MV, Toto R, et al. Effects of intensive systolic blood pressure control on kidney and cardiovascular outcomes in persons without kidney disease: a secondary analysis of a randomized trial. Ann Intern Med 2017; 167(6):375–383. doi:10.7326/M16-2966
- Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med 2018; 178(1):28–36. doi:10.1001/jamainternmed.2017.6015
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265(24):3255–3264. pmid:2046107
- Bulpitt CJ, Beckett NS, Cooke J, et al. Results of the pilot study for the Hypertension in the Very Elderly Trial. J Hypertens 2003; 21(12):2409–2417. doi:10.1097/01.hjh.0000084782.15238.a2
- Liu L, Zhang Y, Liu G, et al. The Felodipine Event Reduction (FEVER) study: a randomized long-term placebo-controlled trial in Chinese hypertensive patients. J Hypertens 2005; 23(12):2157–2172. pmid:16269957
- JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res 2008; 31(12):2115–2127. doi:10.1291/hypres.31.2115
- Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension 2010; 56(2):196–202. doi:10.1161/HYPERTENSIONAHA.109.146035
- Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387(10022):957–967. doi:10.1016/S0140-6736(15)01225-8
- Sleight P. The HOPE study (Heart Outcomes Prevention Evaluation). J Renin Angiotensin Aldosterone Syst 2000; 1(1):18–20. doi:10.3317/jraas.2000.002
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355(9200):253–259. pmid:10675071
- Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2021–2031. doi:10.1056/NEJMoa1600176
- Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016; 374(21):2032–2043. doi:10.1056/NEJMoa1600177
- Lonn EM, Bosch J, López-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016; 374(21):2009–2020. doi:10.1056/NEJMoa1600175
- Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol 2017; 2(7):775–781. doi:10.1001/jamacardio.2017.1421
- Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA 2015; 313(6):603–615. doi:10.1001/jama.2014.18574
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382(9891):507–515. doi:10.1016/S0140-6736(13)60852-1
KEY POINTS
- The 2017 ACC/AHA guidelines lowered the definition of hypertension to 130/80 mm Hg or higher, thereby in-creasing the number of US adults with hypertension from 31.9% to 45.6%.
- For patients with known cardiovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, drug treatment “is recommended” if the average blood pressure is 130/80 mm Hg or higher. For those without cardiovascular disease and at lower risk, drug treatment is recommended if the aver-age blood pressure is 140/90 mm Hg or higher.
- A treatment goal of less than 130/80 mm Hg “is recommended” for patients with hypertension and known car-diovascular disease or a 10-year risk of an atherosclerotic cardiovascular disease event of 10% or higher, and “may be reasonable” for those without additional markers of increased cardiovascular risk.
- Intensive blood pressure control has the potential to significantly reduce rates of morbidity and death associated with cardiovascular disease, at the price of causing more adverse effects.
Common benign breast concerns for the primary care physician
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
Breast concerns account for approximately 3% of all female visits to a primary care practice.1 The most common symptoms are breast lumps and breast pain.
Because breast cancer is the most common malignancy in women in the United States, affecting nearly 1 in 8 women in their lifetime, women with breast problems often fear the worst. However, only about 3.5% of women reporting a concern have cancer; most problems are benign (Table 1).1
Here, we present an evidence-based review of common breast problems in primary care practice and discuss how to evaluate and manage them.
GENERAL APPROACH
The evaluation of a breast concern requires a systematic approach, beginning with a history that documents the onset, severity, and frequency of symptoms. If the concern is a lump or mass, ask whether it becomes more tender or increases in size at any point during the menstrual cycle.
Focus the physical examination on the cervical, supraclavicular, infraclavicular, and axillary lymph nodes and on the breast itself. Assess breast symmetry, note any skin changes such as dimpling, and check the nipples for discharge and inversion. Palpate the breasts for masses.
PALPABLE BREAST MASS: IMAGING NEEDED
If a mass is present, it is more likely to be malignant if any of the following is true:
- Firm to hard texture or indistinct margins
- Attached to the underlying deep fascia or skin
- Associated nipple inversion or skin dimpling.2
Breast masses are more likely benign if they have discrete, well-defined margins, are mobile with a soft to rubbery consistency, and change with the menstrual cycle. However, clinical features are unreliable indicators of cause, and thus additional investigation with breast imaging is warranted.
Mammography remains the diagnostic test of choice for all women age 30 or older who have a palpable breast mass. It is less effective in younger women because they are more likely to have extremely dense fibroglandular tissue that will limit its sensitivity to imaging.
Order diagnostic mammography, which includes additional views focused on the area of concern, rather than screening mammography, which includes only standard craniocaudal and mediolateral oblique views. A skin marker should be applied over the palpable lump to aid imaging. Because a breast that contains a mass may be denser than the opposite breast or may show asymmetry, both breasts should be imaged. The sensitivity of diagnostic mammography varies from 85% to 90%, so a negative mammogram does not rule out malignancy.2,3
Targeted ultrasonography of the palpable mass helps identify solid masses such as fibroadenomas or malignant tumors, classifies the margins (lobulated, smooth, or irregular), and assesses vascularity. Ultrasonography is particularly useful for characterizing cystic lesions (eg, simple, septated, or clustered cysts) and cysts with internal echoes. It can also identify lipomas or sebaceous cysts.
If the findings on both mammography and ultrasonography are benign, the likelihood of cancer is very low, with an estimated negative predictive value of 97% to 100%.2,3 Additionally, the likelihood of nonmalignant findings on biopsy after benign imaging is approximately 99%.3
Although radiologic imaging can define palpable masses, it is intended as a clinical aid. Suspicious findings on clinical examination should never be ignored even if findings on imaging are reassuring, as studies have documented that about 5% of breast cancers may be detected on clinical breast examination alone.4
Other imaging tests such as magnetic resonance imaging may be considered occasionally if clinical suspicion remains high after negative mammography and ultrasonography, but they cannot confirm a diagnosis of malignancy. In that case, refer the patient to a surgeon for consideration of excisional biopsy.
Patients with an indeterminate lesion can return in 3 to 12 weeks for a follow-up examination and repeat imaging, which helps assess interval clinical stability. The latter option is especially helpful for patients with masses that are of low suspicion or for patients who prefer to avoid invasive tissue biopsy.
Patients with clinical and radiologic findings that suggest a benign cause can return for short-term follow-up in 6 months or in 12 months for their regular mammogram.
BREAST PAIN: RARELY MALIGNANT
More than 50% of women experience breast pain at some point in their life.5 Of these, 35% report that the pain adversely affects their sleep, and 41% note that the pain detrimentally affects their sexual quality of life. Up to 66% of breast pain correlates directly with the patient’s menstrual cycle.5 Breast pain is rarely associated with malignancy.
Regardless of its severity and the low likelihood of malignancy, breast pain can be a significant source of distress for the patient, primarily because of concerns about underlying malignancy. If the patient has a focal area of pain on examination, order mammography in combination with targeted ultrasonography. The sensitivity and negative predictive value of benign findings on combination mammography and ultrasonography in this setting are as high as 100%. The incidence of underlying cancer in patients with focal breast pain and no palpable mass is approximately 1.2%.6
The long-term prognosis in women with diffuse, often bilateral breast pain (in the absence of additional clinical findings) is excellent. In one study, the incidence of a breast cancer diagnosis was 1.8% after a median of 51 months of follow-up.7 Therefore, patients presenting with diffuse pain, no palpable abnormalities, and benign imaging can be safely reassured. Magnetic resonance imaging is rarely indicated in patients with breast pain unless other clinical findings, such as a mass or skin changes, are noted and the results of mammography and ultrasonography are negative.
Treating breast pain
Treating breast pain remains a challenge. The first step is to reassure the patient about her prognosis and help her make appropriate lifestyle modifications.
A well-fitting bra. Suggest getting a professional bra fitting. Wearing a well-fitted bra that offers lift, support, and compression and reduces excess motion can help improve benign breast pain. A bra fitting is especially important for women with large breasts because it can be difficult for these women to get an accurate size. Wearing a lightly fitted bra at night may also provide comfort if there is nighttime pain with breast tissue movement.
Reducing daily caffeine intake is often advised for pain management, but strong evidence of its efficacy is lacking.
Anti-inflammatory drugs can be beneficial if used short-term, especially if costochondritis is suspected.
Danazol improves pain in more than 70% of patients with cyclical symptoms and in up to 48% of those with noncyclical symptoms.
Bromocriptine is effective in up to 54% of those with cyclical symptoms and in up to 33% of those with noncyclical symptoms.8 However, the US Food and Drug Administration (FDA) withdrew approval for this indication because of adverse effects.
Tamoxifen, in contrast, provides relief in 94% of those with cyclical symptoms and in 56% of those with noncyclical symptoms.9
Adverse effects, however, limit the use of danazol, bromocriptine, and tamoxifen, and they should be prescribed only for short-term use (3 to 6 months) and only in women with chronic debilitating pain.
A few small studies have evaluated alternative options.
Toremifene is a triphenylethylene derivative similar to tamoxifen that is also used in the adjuvant treatment of postmenopausal breast cancer (but with fewer adverse effects). It has been documented to have a significant effect on premenstrual breast pain, with a 64% reduction in breast pain scores compared with a 26% reduction with placebo.10 However, the FDA has not approved it for this indication, and it can be cost-prohibitive.
Over-the-counter medications that may provide relief for cyclic breast pain include vitamin E or B6, products containing oil of Vitex agnus castus (chaste tree or chasteberry), and flaxseed.11,12
Acupuncture has been evaluated in patients with noncyclic breast pain and was found to reduce pain by 56% to 67% in one study,13 although it did not affect quality of life.
NIPPLE DISCHARGE
From 5% to 7% of women seek medical attention for nipple discharge.14,15 Breast cancer is found in 5% to 15% of women who undergo surgery for nipple discharge.16,17
Review the patient’s current medications and inquire about health conditions such as thyroid dysfunction or visual field changes that suggest a pituitary mass (which can lead to nipple discharge by causing hormonal dysregulation or hyperprolactinemia).
Palpate the breasts for an underlying mass, look for lesions on the nipple, and assess the color of the fluid. Also note whether there is discharge from one or both breasts, whether it is spontaneous or expressive, and whether it occurs from a single or multiple ducts. Nipple lesions may require further testing with punch biopsy.
Nonlactational nipple discharge is classified as physiologic or pathologic. Physiologic nipple discharge is typically bilateral, involving multiple ducts, and is often clear or straw-colored but may also be green, gray, or brown.
White, opaque fluid is often related to galactorrhea as a result of hyperprolactinemia, hypothyroidism, or medications such as antipsychotic drugs (eg, haloperidol and fluphenazine) and gastrointestinal motility agents such as metoclopramide. Discharge also commonly results from benign underlying ductal abnormalities such as intraductal papilloma, periductal mastitis, and duct ectasia.
Pathologic nipple discharge is often unilateral and persistent, occurring spontaneously from a solitary duct, and may be bloody or serous.
For women with pathologic nipple discharge who are 30 or older, diagnostic imaging with mammography and subareolar ultrasonography is recommended. If the patient is younger than 30, ultrasonography of the subareolar region alone can be used. Targeted ultrasonography of any palpable area is also advised.
Cytologic assessment of the fluid is not recommended because it can often lead to a false-positive finding of atypical cells. Imaging studies such as ductography, duct lavage, ductoscopy, and magnetic resonance imaging are also generally unnecessary; instead, a persistent clinical concern should prompt a surgical referral for consideration of duct excision.
When a patient has pathologic nipple discharge with a negative physical examination and breast imaging, studies have shown that the risk of cancer is 3% or less.18
Patients with spontaneous bloody or serous single-duct discharge with negative results on mammography and ultrasonography should be reassured that they have a low risk of underlying cancer. If the patient prefers, one approachto management is follow-up mammography and ultrasonography at 6 months and clinical examination for up to 2 years or until the discharge resolves on its own.
On the other hand, if the discharge is distressing to the patient, subareolar duct excision can be performed with both a diagnostic and therapeutic purpose.
NIPPLE-AREOLAR RASH: CONSIDER PAGET DISEASE
A rash on the nipple or areolar region warrants careful evaluation because it may be the first sign of Paget disease of the breast.
In the clinical breast examination, assess the extent of the rash and the presence of any underlying breast mass or nipple discharge. Dermatitis often starts on the areola and resolves quickly with topical therapy. However, Paget disease tends to start directly on the nipple itself, is unresponsive or only partially responsive to topical therapy, and progresses gradually, leading to erosions and ultimately effacement of the nipple itself.
If the clinical examination suggests mild dermatitis and the results of breast imaging are negative, treat the patient with a topical medication because benign conditions such as dermatitis and eczema are common. However, continued follow-up is mandatory until the rash completely resolves: Paget disease sometimes initially improves with topical therapy due to its inflammatory nature.
If you suspect Paget disease or the rash does not fully resolve after 2 to 3 weeks of topical therapy, refer the patient to a dermatologist for full-thickness punch biopsy to establish the diagnosis.
Paget disease of the breast may or may not be associated with underlying ductal carcinoma in situ or invasive breast cancer.19 The absence of clinical or imaging abnormalities in a patient with Paget disease does not rule out underlying malignancy.20
DENSE BREASTS
Increased breast density has been shown to be a risk factor for breast cancer and may be prognostically useful when combined with the Tyrer-Cuzick model or the Gail model of breast cancer risk.24
Additionally, increased density can mask cancers on mammography, significantly reducing its sensitivity. In women with heterogeneously or extremely dense breasts, the sensitivity of mammography for detecting cancer is only 25% to 50%.21 Due to this low sensitivity, supplemental imaging is helpful, particularly in women already at risk of breast cancer based on family history.
Supplemental screening
Digital mammography with tomosynthesis was approved by the FDA in 2011 for use in combination with standard digital mammography for breast cancer screening. Compared with traditional 2-dimensional mammography alone, adding 3-D tomosynthesis decreases the recall rate and increases the cancer detection rate.25
Tomosynthesis tends to perform better in women with heterogeneously dense breasts (BI-RADS category C). There is no significant improvement in cancer detection in women with extremely dense breasts (BI-RADS category D).26
Depending on the methodology, radiation exposure can be either higher or lower than with traditional mammography. However, in all forms, the very small amount of radiation is considered safe.
Whole breast ultrasonography. When whole breast ultrasonography is used to supplement mammography, the recall rate is higher than when mammography is used alone (14% vs 7%–11%).22 It also increases the cancer detection rate by 4.4 additional cancers per 1,000 examinations. However, the false-positive rate with whole breast ultrasonography is higher; the positive predictive value of combined mammography and ultrasonography is 11.2% vs 22.6% for mammography alone.22 Therefore, we do not generally recommend whole breast ultrasonography as a supplement to mammography in women with dense breast tissue unless other studies are not an option.
Molecular breast imaging is not widely available because it requires special equipment, injection of a radiopharamceutical (technetium Tc 99m sestamibi), and a radiologist who specializes in breast imaging to interpret the results. When it is available, however, it increases the cancer detection rate by 8.8 in 1,000 examinations; the positive predictive value is similar to that of screening mammography alone.21 It is particularly useful in patients with dense breasts who do not qualify for screening magnetic resonance imaging (lifetime risk of < 20% to 25%).
Technetium sestamibi is associated with a minimal amount of radiation exposure (2.4 mSv vs 1.2 mSV with standard mammography). However, this exposure is much less than background radiation exposure and is considered safe.21
IF THE PATIENT HAS AN ABNORMAL SCREENING MAMMOGRAM
Screening mammography can disclose abnormalities such as calcifications, masses, asymmetry, or architectural distortion.27 Abnormalities are reported using standardized BI-RADS categories designated with the numbers 0 through 6 (Table 3).23
A report of BI-RADS category 0 (incomplete), 4 (suspicious), or 5 (highly suspicious) requires additional workup.
Category 1 (negative) requires no further follow-up, and the patient should resume age-appropriate screening.
For patients with category 2 (benign) findings, routine screening is recommended, whereas patients with category 3 (probably benign) are advised to come back in 6 months for follow-up imaging.
Diagnostic mammography includes additional assessments for focal symptoms or areas of abnormality noted on screening imaging or clinical examination. These may include spot magnification views of areas of asymmetry, mass, architectural distortion, or calcifications. Ultrasonography of focal breast abnormalities can help determine if there is an underlying cyst or solid mass.
MANAGEMENT OF BENIGN FINDINGS ON BREAST BIOPSY
Benign breast disease is diagnosed when a patient with a palpable or radiographic abnormality undergoes breast biopsy with benign findings.28,29 It can be largely grouped into 3 categories: nonproliferative, proliferative without atypia, and proliferative with atypia (Table 4).28,29
If core-needle biopsy study results are benign, the next step is to establish radiologic-pathologic and clinical-pathologic concordance. If the findings on clinical examination or imaging are not consistent with those on pathologic study, excisional biopsy should be performed, as imaging-directed biopsy may not have adequately sampled the lesion.30
Nonproliferative lesions account for about 65% of findings on core-needle biopsy and include simple cysts, fibroadenomas, columnar cell changes, apocrine metaplasia, and mild ductal hyperplasia of the usual type. These lesions do not significantly increase the risk of breast cancer; the relative risk is 1.2 to 1.4.28,29 Additionally, the risk of “upstaging” after excisional biopsy—ie, to a higher-risk lesion or to malignancy—is minimal. Therefore, no additional action is necessary when these findings alone are noted on core-needle biopsy.
Proliferative lesions without atypia account for about 30% of biopsy results and include usual ductal hyperplasia, sclerosing adenosis, columnar hyperplasia, papilloma, and radial scar. Generally, there is a slightly increased risk of subsequent breast cancer, with a relative risk of 1.7 to 2.1.28 Usual ductal hyperplasia and columnar hyperplasia have little risk of upstaging with excision, and therefore, surgical consultation is not recommended.
Previously, surgical excision was recommended for any intraductal papilloma due to risk of upgrade in pathologic diagnosis at the time of excision. However, more recent data suggest that the upgrade rate is about 2.2% for a solitary papilloma that is less than 1 cm in diameter and without associated mass lesion (either clinically or radiographically), is concordant with radiographic findings, and has no associated atypical cells on biopsy.31 In this case, observation and short-interval clinical follow-up are reasonable. If there are multiple papillomas, the patient has symptoms such as persistent bloody nipple discharge, or any of the above criteria are not met, surgical excision is recommended.28
Similarly, radial scars and complex sclerosing lesions are increasingly likely to be associated with malignancy based on size. Upstaging ranges from 0% to 12%. It is again important when evaluating radial scars that there is pathologic concordance and that there were no associated high-risk lesions on pathology. If this is the case, it is reasonable to clinically monitor patients with small radial scars, particularly in those who do not have an elevated risk of developing breast cancer.30
For all patients who have undergone biopsy and whose pathology study results are benign, a thorough risk evaluation should be performed, including calculation of their lifetime risk of breast cancer. This can be done with the National Cancer Institute Breast Cancer Risk Assessment Tool, the International Breast Cancer Intervention Study (IBIS) risk calculator, or other model using family history as a basis for calculations. Patients found to have a lifetime risk of breast cancer of greater than 20% to 25% should be offered annual screening with magnetic resonance imaging in addition to mammography.
ATYPICAL HYPERPLASIA: INCREASED RISK
When biopsy study shows atypical ductal hyperplasia or atypical lobular hyperplasia, there is an increased risk of breast cancer.28,32 The absolute overall risk of developing breast cancer in 25 years is 30%, and that risk is further stratified based on the number of foci of atypia noted in the specimen.29
When core-needle biopsy study reveals atypical ductal hyperplasia in the tissue, there is a 15% to 30% risk of finding breast cancer with surgical excision.28 Surgical excision is therefore recommended for atypical ductal hyperplasia noted on core-needle biopsy.28
In contrast, when atypical lobular hyperplasia alone is noted, the risk of upstagingto malignancy varies widely—from 0% to 67%—although recent studies have noted risks of 1% to 3%.33,34 Thus, the decision for surgical excision is more variable. Generally, if the atypical lobular hyperplasia is noted incidentally, is not associated with a higher grade lesion, and is concordant with imaging, it is reasonable to closely monitor with serial imaging and physical examination. Excision is unnecessary.35
Patients found to have atypical hyperplasia on breast biopsy should receive counseling about risk-reducing medications. Selective estrogen receptor modulators such as tamoxifen and raloxifene have been shown to reduce the risk of breast cancer by as much as 86% in patients with atypical hyperplasia.36 Similarly, aromatase inhibitors such as exemestane and anastrozole reduce breast cancer risk by approximately 65%.37
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
- Eberl MM, Phillips RL Jr, Lamberts H, Okkes I, Mahoney MC. Characterizing breast symptoms in family practice. Ann Fam Med 2008; 6(6):528–533. doi:10.1370/afm.905
- Harvey JA, Mahoney MC, Newell MS, et al. ACR appropriateness criteria palpable breast masses. J Am Coll Radiol 2013; 10(10):742–749.e3. doi:10.1016/j.jacr.2013.06.013
- Ha R, Kim H, Mango V, Wynn R, Comstock C. Ultrasonographic features and clinical implications of benign palpable breast lesions in young women. Ultrasonography 2015; 34(1):66–70. doi:10.14366/usg.14043
- Provencher L, Hogue JC, Desbiens C, et al. Is clinical breast examination important for breast cancer detection? Curr Oncol 2016; 23(4):e332–e339. doi:10.3747/co.23.2881
- Scurr J, Hedger W, Morris P, Brown N. The prevalence, severity, and impact of breast pain in the general population. Breast J 2014; 20(5):508–513. doi:10.1111/tbj.12305
- Leddy R, Irshad A, Zerwas E, et al. Role of breast ultrasound and mammography in evaluating patients presenting with focal breast pain in the absence of a palpable lump. Breast J 2013; 19(6):582–589. doi:10.1111/tbj.12178
- Noroozian M, Stein LF, Gaetke-Udager K, Helvie MA. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: mammography remains indicated. Breast Cancer Res Treat 2015; 149(2):417–424. doi:10.1007/s10549-014-3257-3
- Gateley CA, Miers M, Mansel RE, Hughes LE. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J R Soc Med 1992; 85(1):12–15. pmid:1548647
- Fentiman IS, Caleffi M, Hamed H, Chaudary MA. Dosage and duration of tamoxifen treatment for mastalgia: a controlled trial. Br J Surg 1988; 75(9):845–846. pmid:3052691
- Oksa S, Luukkaala T, Mäenpää J. Toremifene for premenstrual mastalgia: a randomised, placebo-controlled crossover study. BJOG 2006; 113(6):713–718. doi:10.1111/j.1471-0528.2006.00943.x
- Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y. Effects of Vitex agnus and flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 2016; 24:90–95. doi:10.1016/j.ctim.2015.12.009
- Shobeiri F, Oshvandi K, Nazari M. Clinical effectiveness of vitamin E and vitamin B6 for improving pain severity in cyclic mastalgia. Iran J Nurs Midwifery Res 2015; 20(6):723–727. doi:10.4103/1735-9066.170003
- Thicke LA, Hazelton JK, Bauer BA, et al. Acupuncture for treatment of noncyclic breast pain: a pilot study. Am J Chin Med 2011; 39(6):1117–1129. doi:10.1142/S0192415X11009445
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med 2005; 353(3):275–285. doi:10.1056/NEJMra035692
- Gülay H, Bora S, Kìlìçturgay S, Hamaloglu E, Göksel HA. Management of nipple discharge. J Am Coll Surg 1994; 178(5):471–474. pmid:8167884
- Murad TM, Contesso G, Mouriesse H. Nipple discharge from the breast. Ann Surg 1982; 195(3):259–264. pmid:6277258
- Sakorafas GH. Nipple discharge: current diagnostic and therapeutic approaches. Cancer Treat Rev 2001; 27(5):275–282. doi:10.1053/ctrv.2001.0234
- Ashfaq A, Senior D, Pockaj BA, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg 2014; 208(2):222–227. doi:10.1016/j.amjsurg.2013.12.035
- Chen CY, Sun LM, Anderson BO. Paget disease of the breast: changing patterns of incidence, clinical presentation, and treatment in the US. Cancer 2006; 107(7):1448–1458. doi:10.1002/cncr.22137
- Kollmorgen DR, Varanasi JS, Edge SB, Carson WE 3rd. Paget's disease of the breast: a 33-year experience. J Am Coll Surg 1998; 187(2):171–177. pmid:9704964
- Hruska CB. Molecular breast imaging for screening in dense breasts: state of the art and future directions. AJR Am J Roentgenol 2017; 208(2):275–283. doi:10.2214/AJR.16.17131
- Melnikow J, Fenton JJ, Whitlock EP, et al. Supplemental screening for breast cancer in women with dense breasts: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2016; 164(4):268–278. doi:10.7326/M15-1789
- American College of Radiology. Breast imaging reporting and data system (BI-RADS). Reston, VA: American College of Radiology; 2013.
- Brentnall AR, Harkness EF, Astley SM, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res 2015; 17(1):147. doi:10.1186/s13058-015-0653-5
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016; 315(16):1784–1786. doi:10.1001/jama.2016.1708
- Venkatesan A, Chu P, Kerlikowske K, Sickles EA, Smith-Bindman R. Positive predictive value of specific mammographic findings according to reader and patient variables. Radiology 2009; 250(3):648–657. doi:10.1148/radiol.2503080541
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med 2005; 353(3):229–237. doi:10.1056/NEJMoa044383
- Hartmann LC, Degnim AC, Santen RJ, DuPont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med 2015; 372(1):78–89. doi:10.1056/NEJMsr1407164
- Neal L, Sandhu NP, Hieken TJ, et al. Diagnosis and management of benign, atypical, and indeterminate breast lesions detected on core needle biopsy. Mayo Clin Proc 2014; 89(4):536–547. doi:10.1016/j.mayocp.2014.02.004
- Nakhlis F, Ahmadiyeh N, Lester S, Raza S, Lotfi P, Golshan M. Papilloma on core biopsy: excision vs observation. Ann Surg Oncol 2015; 22(5):1479–1482. doi:10.1245/s10434-014-4091-x
- Degnim AC, Dupont WE, Radisky DC, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer 2016; 122(19):2971-2978. doi:10.1002/cncr.30153
- Sen LQ, Berg WA, Hooley RJ, Carter GJ, Desouki MM, Sumkin JH. Core breast biopsies showing lobular carcinoma in situ should be excised and surveillance is reasonable for atypical lobular hyperplasia. AJR Am J Roentgenol 2016; 207(5):1132–1145. doi:10.2214/AJR.15.15425
- Nakhlis F, Gilmore L, Gelman R, et al. Incidence of adjacent synchronous invasive carcinoma and/or ductal carcinoma in situ in patient with lobular neoplasia on core biopsy: results from a prospective multi-institutional registry (TBCRC 020). Ann Surg Oncol 2016; 23(3):722–728. doi:10.1245/s10434-015-4922-4
- Racz JM, Carter JM, Degnim AC. Lobular neoplasia and atypical ductal hyperplasia on core biopsy: current surgical management recommendations. Ann Surg Oncol 2017; 24(10):2848–2854. doi:10.1245/s10434-017-5978-0
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388. doi:10.1093/jnci/dji372
- Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 2011; 364(25):2381–2391. doi:10.1056/NEJMoa1103507
KEY POINTS
- The two most common breast symptoms are lumps and pain.
- Most breast problems are not caused by cancer.
- Evaluation of any breast problem begins with a focused history followed by a breast examination and, when necessary, imaging.
- If the results of the breast examination and imaging suggest a benign cause, no further follow-up is necessary.
- If there is discordance between imaging and breast examination results, or if there is a high clinical suspicion of cancer, then consider serial follow-up examinations at short intervals, referral to a breast surgeon for excision, or both.
Miscarriage after myomectomy depends on fibroid number, uterine incisions
LAS VEGAS – according to a review of 252 cases at Northwestern University, Chicago.
Surgeons feel terrible when a woman loses a pregnancy after fibroid treatment, and wonder if they “caused it, or if it was just a bad uterus or a bad initial pathology,” said lead investigator Laura M. Glaser, MD, an ob.gyn. in private practice in Lake Forest, Ill.
Her study, which was presented at a meeting sponsored by AAGL, suggests that miscarriage occurs mostly from complex pathology, as indicated by the number of fibroids and the degree of uterine cutting needed to remove them. The team reviewed outcomes among women who conceived after treatment; 28 had robotic-assisted myomectomies; 208 had open, abdominal myomectomies; and 16 had uterine fibroid embolization (UFE). Miscarriage was defined as pregnancy loss before 24 weeks.
After the researchers adjusted for age, body mass index, and parity, there were no statistically significant differences in miscarriage rates among the three groups (31% after UFE, 29% after robotic myomectomy, and 22% after abdominal myomectomy).
Open cases had the largest dominant fibroid at a mean of 8.5 cm, the most fibroids removed at 4.5, and the highest rate of cavity entry, 42%. Even so, at 22%, open cases were the least likely to miscarry.
Uterine size, specimen weight, time from procedure to pregnancy, and fibroid location didn’t seem to matter otherwise. The only risk factors that reached statistical significance were among women who had myomectomies; an increasing number of uterine cuts (odds ratio, 1.558; P = .004) and fibroids removed (OR, 1.11; P = .033) increased the odds of miscarriage.
More than 40% of women in the UFE group had previous fibroid surgery, versus 5% among women who had myomectomies. UFE women also were far more likely to have had a previous birth (50% versus 17%), but less likely to have subserosal fibroids (13% versus 33%), and their dominant fibroid was a few centimeters smaller.
Subjects were in their mid-30s, on average, with a mean body mass index of about 28 kg/m2. Just over 40% of the women who had myomectomies were white, versus 19% of women who had UFE.
There was no outside funding for the work, and the investigators didn’t have any disclosures.
SOURCE: Glaser LM et al. 2018 AAGL Global Congress, Abstract 160
LAS VEGAS – according to a review of 252 cases at Northwestern University, Chicago.
Surgeons feel terrible when a woman loses a pregnancy after fibroid treatment, and wonder if they “caused it, or if it was just a bad uterus or a bad initial pathology,” said lead investigator Laura M. Glaser, MD, an ob.gyn. in private practice in Lake Forest, Ill.
Her study, which was presented at a meeting sponsored by AAGL, suggests that miscarriage occurs mostly from complex pathology, as indicated by the number of fibroids and the degree of uterine cutting needed to remove them. The team reviewed outcomes among women who conceived after treatment; 28 had robotic-assisted myomectomies; 208 had open, abdominal myomectomies; and 16 had uterine fibroid embolization (UFE). Miscarriage was defined as pregnancy loss before 24 weeks.
After the researchers adjusted for age, body mass index, and parity, there were no statistically significant differences in miscarriage rates among the three groups (31% after UFE, 29% after robotic myomectomy, and 22% after abdominal myomectomy).
Open cases had the largest dominant fibroid at a mean of 8.5 cm, the most fibroids removed at 4.5, and the highest rate of cavity entry, 42%. Even so, at 22%, open cases were the least likely to miscarry.
Uterine size, specimen weight, time from procedure to pregnancy, and fibroid location didn’t seem to matter otherwise. The only risk factors that reached statistical significance were among women who had myomectomies; an increasing number of uterine cuts (odds ratio, 1.558; P = .004) and fibroids removed (OR, 1.11; P = .033) increased the odds of miscarriage.
More than 40% of women in the UFE group had previous fibroid surgery, versus 5% among women who had myomectomies. UFE women also were far more likely to have had a previous birth (50% versus 17%), but less likely to have subserosal fibroids (13% versus 33%), and their dominant fibroid was a few centimeters smaller.
Subjects were in their mid-30s, on average, with a mean body mass index of about 28 kg/m2. Just over 40% of the women who had myomectomies were white, versus 19% of women who had UFE.
There was no outside funding for the work, and the investigators didn’t have any disclosures.
SOURCE: Glaser LM et al. 2018 AAGL Global Congress, Abstract 160
LAS VEGAS – according to a review of 252 cases at Northwestern University, Chicago.
Surgeons feel terrible when a woman loses a pregnancy after fibroid treatment, and wonder if they “caused it, or if it was just a bad uterus or a bad initial pathology,” said lead investigator Laura M. Glaser, MD, an ob.gyn. in private practice in Lake Forest, Ill.
Her study, which was presented at a meeting sponsored by AAGL, suggests that miscarriage occurs mostly from complex pathology, as indicated by the number of fibroids and the degree of uterine cutting needed to remove them. The team reviewed outcomes among women who conceived after treatment; 28 had robotic-assisted myomectomies; 208 had open, abdominal myomectomies; and 16 had uterine fibroid embolization (UFE). Miscarriage was defined as pregnancy loss before 24 weeks.
After the researchers adjusted for age, body mass index, and parity, there were no statistically significant differences in miscarriage rates among the three groups (31% after UFE, 29% after robotic myomectomy, and 22% after abdominal myomectomy).
Open cases had the largest dominant fibroid at a mean of 8.5 cm, the most fibroids removed at 4.5, and the highest rate of cavity entry, 42%. Even so, at 22%, open cases were the least likely to miscarry.
Uterine size, specimen weight, time from procedure to pregnancy, and fibroid location didn’t seem to matter otherwise. The only risk factors that reached statistical significance were among women who had myomectomies; an increasing number of uterine cuts (odds ratio, 1.558; P = .004) and fibroids removed (OR, 1.11; P = .033) increased the odds of miscarriage.
More than 40% of women in the UFE group had previous fibroid surgery, versus 5% among women who had myomectomies. UFE women also were far more likely to have had a previous birth (50% versus 17%), but less likely to have subserosal fibroids (13% versus 33%), and their dominant fibroid was a few centimeters smaller.
Subjects were in their mid-30s, on average, with a mean body mass index of about 28 kg/m2. Just over 40% of the women who had myomectomies were white, versus 19% of women who had UFE.
There was no outside funding for the work, and the investigators didn’t have any disclosures.
SOURCE: Glaser LM et al. 2018 AAGL Global Congress, Abstract 160
REPORTING FROM AAGL GLOBAL CONGRESS
Key clinical point: The number of uterine incisions and fibroids removed increase the risk of miscarriage after fibroid treatment, not the type of procedure.
Major finding: After adjusting for age, body mass index, and parity, there were no statistically significant differences in miscarriage rates between the three groups (31% after uterine fibroid embolization; 29% after robotic myomectomy, and 22% after open abdominal myomectomy).
Study details: Review of 252 cases
Disclosures: There was no outside funding for the work, and the investigators didn’t have any disclosures.
Source: Glaser LM et al. 2018 AAGL Global Congress, Abstract 160
Aspirin appears underused to prevent preeclampsia in SLE patients
Women with systemic lupus erythematosus (SLE) were not more likely to take aspirin during pregnancy than when not pregnant, despite the potential to reduce preeclampsia risk, based on data from 300 women.
Although aspirin is recommended to reduce preeclampsia risk in pregnant SLE patients, data on current practice patterns are limited, wrote Arielle Mendel, MD, of McGill University, Montreal, and colleagues in Annals of the Rheumatic Diseases.
The researchers identified 475 pregnancies among 300 women aged 18-45 years who were pregnant during the study period from 2000 to 2017. The average duration of SLE duration at the time of pregnancy was 5.6 years, and approximately half (51%) of pregnancies had one or more traditional preeclampsia risk factors. In addition, 33% of the women had positive antiphospholipid antibodies (aPL).
Overall, 25% of the pregnancies included aspirin use, with no significant difference among those with one or more risk factors, any individual risk factor, or nephritis.
The study population was 44% white, 19% black, 14% Asian, 13% Hispanic, 5% from the Indian subcontinent, 1% Native American, and 5% other ethnicities.
Approximately 34% of white patients and 32% of Hispanic patients were exposed to aspirin, compared with 18% and 20% of black and Asian patients, respectively. Aspirin use did not increase over the study period, although there was a trend for increased use in patients with a positive aPL, compared with those with no aPL.
“The low aspirin use among black SLE subjects is noteworthy given the worse reproductive outcomes observed in this population,” the researchers wrote.
The findings were limited by several factors, including a lack of data on gestational age and pregnancy outcomes, the researchers noted. However, the results highlight the gap between recommendations and practice, and the need for additional research on aspirin use in pregnant SLE patients.
The study was supported in part by a McGill University Health Centre Research Award; the researchers reported no financial conflicts.
SOURCE: Mendel A et al. Ann Rheum Dis. 2018 Dec 20. doi: 10.1136/annrheumdis-2018-214434.
Women with systemic lupus erythematosus (SLE) were not more likely to take aspirin during pregnancy than when not pregnant, despite the potential to reduce preeclampsia risk, based on data from 300 women.
Although aspirin is recommended to reduce preeclampsia risk in pregnant SLE patients, data on current practice patterns are limited, wrote Arielle Mendel, MD, of McGill University, Montreal, and colleagues in Annals of the Rheumatic Diseases.
The researchers identified 475 pregnancies among 300 women aged 18-45 years who were pregnant during the study period from 2000 to 2017. The average duration of SLE duration at the time of pregnancy was 5.6 years, and approximately half (51%) of pregnancies had one or more traditional preeclampsia risk factors. In addition, 33% of the women had positive antiphospholipid antibodies (aPL).
Overall, 25% of the pregnancies included aspirin use, with no significant difference among those with one or more risk factors, any individual risk factor, or nephritis.
The study population was 44% white, 19% black, 14% Asian, 13% Hispanic, 5% from the Indian subcontinent, 1% Native American, and 5% other ethnicities.
Approximately 34% of white patients and 32% of Hispanic patients were exposed to aspirin, compared with 18% and 20% of black and Asian patients, respectively. Aspirin use did not increase over the study period, although there was a trend for increased use in patients with a positive aPL, compared with those with no aPL.
“The low aspirin use among black SLE subjects is noteworthy given the worse reproductive outcomes observed in this population,” the researchers wrote.
The findings were limited by several factors, including a lack of data on gestational age and pregnancy outcomes, the researchers noted. However, the results highlight the gap between recommendations and practice, and the need for additional research on aspirin use in pregnant SLE patients.
The study was supported in part by a McGill University Health Centre Research Award; the researchers reported no financial conflicts.
SOURCE: Mendel A et al. Ann Rheum Dis. 2018 Dec 20. doi: 10.1136/annrheumdis-2018-214434.
Women with systemic lupus erythematosus (SLE) were not more likely to take aspirin during pregnancy than when not pregnant, despite the potential to reduce preeclampsia risk, based on data from 300 women.
Although aspirin is recommended to reduce preeclampsia risk in pregnant SLE patients, data on current practice patterns are limited, wrote Arielle Mendel, MD, of McGill University, Montreal, and colleagues in Annals of the Rheumatic Diseases.
The researchers identified 475 pregnancies among 300 women aged 18-45 years who were pregnant during the study period from 2000 to 2017. The average duration of SLE duration at the time of pregnancy was 5.6 years, and approximately half (51%) of pregnancies had one or more traditional preeclampsia risk factors. In addition, 33% of the women had positive antiphospholipid antibodies (aPL).
Overall, 25% of the pregnancies included aspirin use, with no significant difference among those with one or more risk factors, any individual risk factor, or nephritis.
The study population was 44% white, 19% black, 14% Asian, 13% Hispanic, 5% from the Indian subcontinent, 1% Native American, and 5% other ethnicities.
Approximately 34% of white patients and 32% of Hispanic patients were exposed to aspirin, compared with 18% and 20% of black and Asian patients, respectively. Aspirin use did not increase over the study period, although there was a trend for increased use in patients with a positive aPL, compared with those with no aPL.
“The low aspirin use among black SLE subjects is noteworthy given the worse reproductive outcomes observed in this population,” the researchers wrote.
The findings were limited by several factors, including a lack of data on gestational age and pregnancy outcomes, the researchers noted. However, the results highlight the gap between recommendations and practice, and the need for additional research on aspirin use in pregnant SLE patients.
The study was supported in part by a McGill University Health Centre Research Award; the researchers reported no financial conflicts.
SOURCE: Mendel A et al. Ann Rheum Dis. 2018 Dec 20. doi: 10.1136/annrheumdis-2018-214434.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Aspirin use was low among pregnant systemic lupus erythematosus patients despite risk factors for preeclampsia.
Major finding: Approximately 25% of women with systemic lupus erythematosus took aspirin during pregnancy.
Study details: The data come from a prospective study of 300 women and 475 pregnancies.
Disclosures: The study was supported in part by a McGill University Health Centre Research Award; the researchers reported no financial conflicts.
Source: Mendel A et al. Ann Rheum Dis. 2018 Dec 20. doi: 10.1136/annrheumdis-2018-214434.