User login
What should you tell your patients about the risks of ART?
CORONADO, CALIF. –
In addition, multiples conceived using ART – including twins – continue to be the biggest preventable risk factor for adverse pregnancy and fetal outcomes.
Those are key points that Joseph C. Gambone, DO, MPH, made during a wide-ranging talk about the adverse pregnancy and fetal outcomes related to ART at a meeting on in vitro fertilization (IVF) and embryo transfer sponsored by the University of California, San Diego.
In 2016, Barbara Luke, ScD, MPH, and her colleagues published results from the ongoing Massachusetts Outcomes Study of Reproductive Technologies (J Reprod Med. 2016 Mar-Apr;61[3-4]:114-27). They found that pregnancy plurality is the predominant risk factor for infants and mothers. Of 8,948 birth outcomes resulting from ART, risks for pregnancy-induced hypertension, cesarean delivery, gestational diabetes, preterm birth, birth defects, and small for gestational age were significantly increased among twins.
“Lowering the plurality rate, including twins, should substantially reduce morbidity with ART,” said Dr. Gambone, professor emeritus at the David Geffen School of Medicine at the University of California, Los Angeles, who was not affiliated with the study. Thawed embryos were associated with a higher risk for pregnancy-induced hypertension and large for gestational age offspring, but a lower risk for low birth weight and small for gestational age.
According to data from the Society for Assisted Reproductive Technology, elective singleton embryo transfer increased from 35% of all cycles in 2015 to 42% in 2016, while singleton births increased from 80.5% to 84% during the same time period. In addition, the proportion of twins born in 2015 was 19% and declined to 16% in 2016, while the percentage of triplets or greater born was the same in both years (0.4%).
Meanwhile, in an analysis of more than 1.1 million cycles between 2000 and 2011 drawn from Centers for Disease Control and Prevention surveillance data, researchers found that the most commonly reported patient complication was ovarian hyperstimulation syndrome (a peak of 154 per 10,000 autologous cycles) and hospitalization (a peak of 35 per 10,000 autologous cycles; JAMA. 2015 Jan 6;313[1]:88-90). Other complications remained below 10 per 10,000 cycles and included infection, hemorrhage with transfusion, adverse event from medication, adverse event to anesthesia, and patient death. In all, 58 deaths were reported: 18 because of stimulation and 40 during pregnancy. “Some deaths were due to potentially preventable complications because of unrecognized comorbidities or conditions,” said Dr. Gambone, who was not affiliated with the study. “Women with Turner syndrome who receive donor embryos could be an example.”
Today, the most feared maternal and pregnancy outcome from ART is breast and ovarian cancer from treatment, he said, while the most feared outcome in offspring is birth defects from treatment. On the breast cancer front, an analysis of nearly 2 million women provided some reassurance (Fertil Steril. 2017 Jul;108:137-44). It found no increased risk of breast cancer in women who have birth after ART, compared with women who gave birth after spontaneous conception (adjusted hazard ratio, 0.84). It also found no increased risk in women who received ovarian stimulation or other hormonal treatment for infertility (HRs, 0.86 and 0.79, respectively). A smaller study with a median follow-up of 21 years found no difference in the rate of invasive and in situ breast cancer between women who received IVF treatment and those who did not (JAMA. 2016 Jul 19;316[3]:300-12). However, a recent analysis from Great Britain found a slight increase for in situ breast cancer that was associated with women who had a higher number of treatment cycles (BMJ. 2018;362:k2644).
On the ovarian cancer front, a case-control analysis of 1,900 women conducted by researchers at Mayo Clinic found that infertile women who used fertility drugs were not at increased risk of developing ovarian tumors, compared with infertile women who did not use fertility drugs (adjusted odds ratio, 0.64; Fertil Steril. 2013;99[7]:2031-6). There also was no increased risk because of underlying infertility or any increase in borderline tumors. More recently, an abstract presented at the annual meeting of the European Society of Human Reproduction and Embryology, based on a large cohort study from Denmark, found a slightly higher overall risk of ovarian cancer among the ART women (0.11%), compared with non-ART controls (0.06%). However, the analysis also showed comparably higher rates of ovarian cancer in women who were nulliparous (a risk factor for ovarian cancer) and in the ART women who had a female cause of infertility. In an analysis from Great Britain, increased ovarian tumor risk was limited to women with endometriosis, low parity, or both (BMJ. 2018;362:k2644). Dr. Gambone noted that an article published online Oct. 23, 2017 in Nature Communication implicates the fallopian tube, rather than the ovaries, as a probable source of papillary serous cancers.
Women who are subfertile should be counseled about the increased risk of birth defects, irrespective of whether they undergo IVF or not, said Dr. Gambone, who also runs a private infertility practice in Durango, Colo. Studies consistently show an increased risk associated with subfertility. A large, retrospective cohort analysis of live and stillbirths from 2004 to 2010 in Massachusetts found that congenital anomalies were reported in 2% of ART births, 1.7% of subfertile births, and 1.4% of fertile births (Birth Defects Res. 2017 Aug 15;109[14]:1144-53). The adjusted prevalence ratios for birth defects were 1.5 for ART births and 1.3 for subfertile births, compared with fertile mother births. The researchers observed elevated rates of several birth defects with ART, including tetralogy of Fallot and hypospadias. Subfertility and multiple births affect these associations, with multiple births explaining 36% of the relative effect of ART on nonchromosomal birth defects.
“The absolute risk of birth defects is small with ART,” Dr. Gambone said. “A significant portion [but not all] is related to multiple births and underlying subfertility.” A more recent analysis found that subfertile women were 21% more likely to have babies born with birth defects, compared with fertile women (Pediatrics. 2018 Jul; e20174069).
In a study of the overall risk and etiology of major birth defects, researchers from Utah determined that they affect 1 in 33 babies in the United States at an annual direct cost of $2.6 billion per year (BMJ. 2017;357:j2249). Although major birth defects are the leading cause of infant mortality (20%), a known cause of the defect was established in only 20.2% of cases. “Of that percentage, the majority are chromosome or genetic causes,” Dr. Gambone said. “Interestingly, ART and/or subfertility were not listed in this analysis as causes of birth defects. However, the authors speculated that as genetic technology improves, both genetic and epigenetic causes will be identified.”
Dr. Gambone reported no relevant financial disclosures.
[email protected]
CORONADO, CALIF. –
In addition, multiples conceived using ART – including twins – continue to be the biggest preventable risk factor for adverse pregnancy and fetal outcomes.
Those are key points that Joseph C. Gambone, DO, MPH, made during a wide-ranging talk about the adverse pregnancy and fetal outcomes related to ART at a meeting on in vitro fertilization (IVF) and embryo transfer sponsored by the University of California, San Diego.
In 2016, Barbara Luke, ScD, MPH, and her colleagues published results from the ongoing Massachusetts Outcomes Study of Reproductive Technologies (J Reprod Med. 2016 Mar-Apr;61[3-4]:114-27). They found that pregnancy plurality is the predominant risk factor for infants and mothers. Of 8,948 birth outcomes resulting from ART, risks for pregnancy-induced hypertension, cesarean delivery, gestational diabetes, preterm birth, birth defects, and small for gestational age were significantly increased among twins.
“Lowering the plurality rate, including twins, should substantially reduce morbidity with ART,” said Dr. Gambone, professor emeritus at the David Geffen School of Medicine at the University of California, Los Angeles, who was not affiliated with the study. Thawed embryos were associated with a higher risk for pregnancy-induced hypertension and large for gestational age offspring, but a lower risk for low birth weight and small for gestational age.
According to data from the Society for Assisted Reproductive Technology, elective singleton embryo transfer increased from 35% of all cycles in 2015 to 42% in 2016, while singleton births increased from 80.5% to 84% during the same time period. In addition, the proportion of twins born in 2015 was 19% and declined to 16% in 2016, while the percentage of triplets or greater born was the same in both years (0.4%).
Meanwhile, in an analysis of more than 1.1 million cycles between 2000 and 2011 drawn from Centers for Disease Control and Prevention surveillance data, researchers found that the most commonly reported patient complication was ovarian hyperstimulation syndrome (a peak of 154 per 10,000 autologous cycles) and hospitalization (a peak of 35 per 10,000 autologous cycles; JAMA. 2015 Jan 6;313[1]:88-90). Other complications remained below 10 per 10,000 cycles and included infection, hemorrhage with transfusion, adverse event from medication, adverse event to anesthesia, and patient death. In all, 58 deaths were reported: 18 because of stimulation and 40 during pregnancy. “Some deaths were due to potentially preventable complications because of unrecognized comorbidities or conditions,” said Dr. Gambone, who was not affiliated with the study. “Women with Turner syndrome who receive donor embryos could be an example.”
Today, the most feared maternal and pregnancy outcome from ART is breast and ovarian cancer from treatment, he said, while the most feared outcome in offspring is birth defects from treatment. On the breast cancer front, an analysis of nearly 2 million women provided some reassurance (Fertil Steril. 2017 Jul;108:137-44). It found no increased risk of breast cancer in women who have birth after ART, compared with women who gave birth after spontaneous conception (adjusted hazard ratio, 0.84). It also found no increased risk in women who received ovarian stimulation or other hormonal treatment for infertility (HRs, 0.86 and 0.79, respectively). A smaller study with a median follow-up of 21 years found no difference in the rate of invasive and in situ breast cancer between women who received IVF treatment and those who did not (JAMA. 2016 Jul 19;316[3]:300-12). However, a recent analysis from Great Britain found a slight increase for in situ breast cancer that was associated with women who had a higher number of treatment cycles (BMJ. 2018;362:k2644).
On the ovarian cancer front, a case-control analysis of 1,900 women conducted by researchers at Mayo Clinic found that infertile women who used fertility drugs were not at increased risk of developing ovarian tumors, compared with infertile women who did not use fertility drugs (adjusted odds ratio, 0.64; Fertil Steril. 2013;99[7]:2031-6). There also was no increased risk because of underlying infertility or any increase in borderline tumors. More recently, an abstract presented at the annual meeting of the European Society of Human Reproduction and Embryology, based on a large cohort study from Denmark, found a slightly higher overall risk of ovarian cancer among the ART women (0.11%), compared with non-ART controls (0.06%). However, the analysis also showed comparably higher rates of ovarian cancer in women who were nulliparous (a risk factor for ovarian cancer) and in the ART women who had a female cause of infertility. In an analysis from Great Britain, increased ovarian tumor risk was limited to women with endometriosis, low parity, or both (BMJ. 2018;362:k2644). Dr. Gambone noted that an article published online Oct. 23, 2017 in Nature Communication implicates the fallopian tube, rather than the ovaries, as a probable source of papillary serous cancers.
Women who are subfertile should be counseled about the increased risk of birth defects, irrespective of whether they undergo IVF or not, said Dr. Gambone, who also runs a private infertility practice in Durango, Colo. Studies consistently show an increased risk associated with subfertility. A large, retrospective cohort analysis of live and stillbirths from 2004 to 2010 in Massachusetts found that congenital anomalies were reported in 2% of ART births, 1.7% of subfertile births, and 1.4% of fertile births (Birth Defects Res. 2017 Aug 15;109[14]:1144-53). The adjusted prevalence ratios for birth defects were 1.5 for ART births and 1.3 for subfertile births, compared with fertile mother births. The researchers observed elevated rates of several birth defects with ART, including tetralogy of Fallot and hypospadias. Subfertility and multiple births affect these associations, with multiple births explaining 36% of the relative effect of ART on nonchromosomal birth defects.
“The absolute risk of birth defects is small with ART,” Dr. Gambone said. “A significant portion [but not all] is related to multiple births and underlying subfertility.” A more recent analysis found that subfertile women were 21% more likely to have babies born with birth defects, compared with fertile women (Pediatrics. 2018 Jul; e20174069).
In a study of the overall risk and etiology of major birth defects, researchers from Utah determined that they affect 1 in 33 babies in the United States at an annual direct cost of $2.6 billion per year (BMJ. 2017;357:j2249). Although major birth defects are the leading cause of infant mortality (20%), a known cause of the defect was established in only 20.2% of cases. “Of that percentage, the majority are chromosome or genetic causes,” Dr. Gambone said. “Interestingly, ART and/or subfertility were not listed in this analysis as causes of birth defects. However, the authors speculated that as genetic technology improves, both genetic and epigenetic causes will be identified.”
Dr. Gambone reported no relevant financial disclosures.
[email protected]
CORONADO, CALIF. –
In addition, multiples conceived using ART – including twins – continue to be the biggest preventable risk factor for adverse pregnancy and fetal outcomes.
Those are key points that Joseph C. Gambone, DO, MPH, made during a wide-ranging talk about the adverse pregnancy and fetal outcomes related to ART at a meeting on in vitro fertilization (IVF) and embryo transfer sponsored by the University of California, San Diego.
In 2016, Barbara Luke, ScD, MPH, and her colleagues published results from the ongoing Massachusetts Outcomes Study of Reproductive Technologies (J Reprod Med. 2016 Mar-Apr;61[3-4]:114-27). They found that pregnancy plurality is the predominant risk factor for infants and mothers. Of 8,948 birth outcomes resulting from ART, risks for pregnancy-induced hypertension, cesarean delivery, gestational diabetes, preterm birth, birth defects, and small for gestational age were significantly increased among twins.
“Lowering the plurality rate, including twins, should substantially reduce morbidity with ART,” said Dr. Gambone, professor emeritus at the David Geffen School of Medicine at the University of California, Los Angeles, who was not affiliated with the study. Thawed embryos were associated with a higher risk for pregnancy-induced hypertension and large for gestational age offspring, but a lower risk for low birth weight and small for gestational age.
According to data from the Society for Assisted Reproductive Technology, elective singleton embryo transfer increased from 35% of all cycles in 2015 to 42% in 2016, while singleton births increased from 80.5% to 84% during the same time period. In addition, the proportion of twins born in 2015 was 19% and declined to 16% in 2016, while the percentage of triplets or greater born was the same in both years (0.4%).
Meanwhile, in an analysis of more than 1.1 million cycles between 2000 and 2011 drawn from Centers for Disease Control and Prevention surveillance data, researchers found that the most commonly reported patient complication was ovarian hyperstimulation syndrome (a peak of 154 per 10,000 autologous cycles) and hospitalization (a peak of 35 per 10,000 autologous cycles; JAMA. 2015 Jan 6;313[1]:88-90). Other complications remained below 10 per 10,000 cycles and included infection, hemorrhage with transfusion, adverse event from medication, adverse event to anesthesia, and patient death. In all, 58 deaths were reported: 18 because of stimulation and 40 during pregnancy. “Some deaths were due to potentially preventable complications because of unrecognized comorbidities or conditions,” said Dr. Gambone, who was not affiliated with the study. “Women with Turner syndrome who receive donor embryos could be an example.”
Today, the most feared maternal and pregnancy outcome from ART is breast and ovarian cancer from treatment, he said, while the most feared outcome in offspring is birth defects from treatment. On the breast cancer front, an analysis of nearly 2 million women provided some reassurance (Fertil Steril. 2017 Jul;108:137-44). It found no increased risk of breast cancer in women who have birth after ART, compared with women who gave birth after spontaneous conception (adjusted hazard ratio, 0.84). It also found no increased risk in women who received ovarian stimulation or other hormonal treatment for infertility (HRs, 0.86 and 0.79, respectively). A smaller study with a median follow-up of 21 years found no difference in the rate of invasive and in situ breast cancer between women who received IVF treatment and those who did not (JAMA. 2016 Jul 19;316[3]:300-12). However, a recent analysis from Great Britain found a slight increase for in situ breast cancer that was associated with women who had a higher number of treatment cycles (BMJ. 2018;362:k2644).
On the ovarian cancer front, a case-control analysis of 1,900 women conducted by researchers at Mayo Clinic found that infertile women who used fertility drugs were not at increased risk of developing ovarian tumors, compared with infertile women who did not use fertility drugs (adjusted odds ratio, 0.64; Fertil Steril. 2013;99[7]:2031-6). There also was no increased risk because of underlying infertility or any increase in borderline tumors. More recently, an abstract presented at the annual meeting of the European Society of Human Reproduction and Embryology, based on a large cohort study from Denmark, found a slightly higher overall risk of ovarian cancer among the ART women (0.11%), compared with non-ART controls (0.06%). However, the analysis also showed comparably higher rates of ovarian cancer in women who were nulliparous (a risk factor for ovarian cancer) and in the ART women who had a female cause of infertility. In an analysis from Great Britain, increased ovarian tumor risk was limited to women with endometriosis, low parity, or both (BMJ. 2018;362:k2644). Dr. Gambone noted that an article published online Oct. 23, 2017 in Nature Communication implicates the fallopian tube, rather than the ovaries, as a probable source of papillary serous cancers.
Women who are subfertile should be counseled about the increased risk of birth defects, irrespective of whether they undergo IVF or not, said Dr. Gambone, who also runs a private infertility practice in Durango, Colo. Studies consistently show an increased risk associated with subfertility. A large, retrospective cohort analysis of live and stillbirths from 2004 to 2010 in Massachusetts found that congenital anomalies were reported in 2% of ART births, 1.7% of subfertile births, and 1.4% of fertile births (Birth Defects Res. 2017 Aug 15;109[14]:1144-53). The adjusted prevalence ratios for birth defects were 1.5 for ART births and 1.3 for subfertile births, compared with fertile mother births. The researchers observed elevated rates of several birth defects with ART, including tetralogy of Fallot and hypospadias. Subfertility and multiple births affect these associations, with multiple births explaining 36% of the relative effect of ART on nonchromosomal birth defects.
“The absolute risk of birth defects is small with ART,” Dr. Gambone said. “A significant portion [but not all] is related to multiple births and underlying subfertility.” A more recent analysis found that subfertile women were 21% more likely to have babies born with birth defects, compared with fertile women (Pediatrics. 2018 Jul; e20174069).
In a study of the overall risk and etiology of major birth defects, researchers from Utah determined that they affect 1 in 33 babies in the United States at an annual direct cost of $2.6 billion per year (BMJ. 2017;357:j2249). Although major birth defects are the leading cause of infant mortality (20%), a known cause of the defect was established in only 20.2% of cases. “Of that percentage, the majority are chromosome or genetic causes,” Dr. Gambone said. “Interestingly, ART and/or subfertility were not listed in this analysis as causes of birth defects. However, the authors speculated that as genetic technology improves, both genetic and epigenetic causes will be identified.”
Dr. Gambone reported no relevant financial disclosures.
[email protected]
EXPERT ANALYSIS FROM A CME MEETING SPONSORED BY UCSD
Two approaches lowered opioid use after cesarean deliveries
An individual prescribing plan based on inpatient use for women who delivered by cesarean section was associated with a lower number of unused oxycodone tablets, according to research published in Obstetrics & Gynecology.
In a second study, there was a significantly lower number of opioid tablets prescribed for women who delivered by cesarean section after a shared decision-making quality improvement plan was implemented on an inpatient basis.
Sarah S. Osmundson, MD, MS, of Vanderbilt University Medical Center in Nashville, Tenn., and her colleagues conducted a randomized, controlled trial of women who underwent cesarean delivery between June and August 2017. Of these women, 85 were randomized to receive standard care of 30 oxycodone tablets (5 mg) and 87 received an individualized care plan based on inpatient use of oxycodone. Patients were a minimum of 18 years old with similar baseline characteristics and inpatient pain scores.
The investigators found that women who were randomized to the individual care group received 14 oxycodone tablets (interquartile range, 12-16) and had 5 tablets (IQR, 1-8) left 2 weeks after discharge, compared with women who were prescribed 30 tablets in the standard care group (IQR, 30-30; P less than .001) and left 10 tablets unused (IQR, 0-22; P less than .001). There were no significant differences regarding patient-reported pain outcomes in either group, and women used about half as many prescribed tablets in the individualized group (8; IQR, 4-14), compared with women in the standard care group (15; IQR, 6-30; P less than .001).
Most women reported that they used opioids to treat pain and 30% said they believed they were supposed to finish all the tablets that were prescribed, the researchers noted. There were 11 patients (13%) in the standard care group and 12 (14%) in the individualized group who did not use any opioids; similarly, 23 patients (27%) in the standard care group and 21 (24%) in the individual care group used all tablets prescribed.
“If pain were the only determinant of opioid use, randomization should yield similar opioid use in both study groups,” Dr. Osmundson and her colleagues wrote. “These findings suggest that factors other than pain influence opioid use patterns.”
In a second study, Malavika Prabhu, MD, of Massachusetts General Hospital in Boston, and her colleagues employed a prospective quality improvement (QI) initiative for women prescribed opioids after cesarean delivery. They evaluated the opioid use of 624 women and counseled them at discharge on the need for prescription opioids, using shared decision-making to determine the number of opioid tablets, with a maximum number of 30 oxycodone tablets (5 mg). The investigators also changed their protocol for multimodal analgesia to scheduled acetaminophen and NSAIDs. Patients were a mean of 30 years old and more than 50% were white. Median gestational age was 39 weeks at delivery. In a second phase of the study, the investigators lowered the maximum number of opioid tablets prescribed to 25 tablets.
At discharge, the mean number of prescribed opioid tablets decreased to 27 tablets from 33 tablets in phase 1 (P less than .01). In phase 2, the number of tablets further decreased to 22 tablets from 25 tablets (P less than .01). The investigators noted no significant difference in the refill rate during phase 1 (8% vs. 9%; P less than .79) and phase 2 (6% vs. 6%; P = .72). There was a significant increase in prescribing of acetaminophen from 33% at baseline to 77% at the end of phase 1 and an increase at the beginning of phase 2 at 91% to 92% at the end of phase 2. There were no statistically significant differences among prescriptions of ibuprofen at any phase time point.
“As a result of our success in decreasing opioid prescribing after cesarean delivery, our current protocol has again been amended to recommend a maximum of 20 tablets of 5-mg oxycodone (or equivalent opioid) prescribed at the time of discharge,” Dr. Prabhu and her colleagues wrote. “This decrease, which represents a 50% decrease in the departmental standard before this study, has been successfully implemented in 18 months. In addition, efforts to optimize multimodal analgesic use continue both inpatient and on discharge.”
Dr. Osmundson was supported by a grant from the National Institutes of Health, and the research also was supported by an award from the National Center for Advancing Translational Sciences; the authors of the study reported no relevant conflicts of interest. The authors led by Dr. Prabhu had no relevant financial disclosures.
SOURCE: Prabhu M et al. Obstet Gyncol. 2018 Aug 4; doi: 10.1097/AOG.0000000000002789, and Osmundson SS et al. Obstet Gynecol. 2018 Aug 6; doi: 10.1097/AOG.0000000000002782.
While overprescribing opioids for women after cesarean delivery will probably have little effect on the opioid crisis, reducing prescription rates for these women post partum still has benefits for mother and child, Bankole Johnson, MD, DSc, said in an interview.
“The larger problem is with mothers who are dependent on opiates who get pregnant,” Dr. Johnson said. “These mothers give birth to children who need significant support and weaning off opiates, sometime in the NICU, and the mother also has to be treated by the addiction services. Sometimes, the mother simply abandons the baby who is difficult to nurse and comfort because he or she is weaning off opiates.”
Dr. Johnson said overprescribing has three main implications or risks: a risk of developing dependence in newborn babies that are breastfed, an increased risk of dependence for the mother, and a decrease in nurturing skills and bonding from the mother due to high opioid use.
He said these studies had “notable caveats” and noted there may not be much clinical significance because of the small numeric difference in opioid use.
“The best take-home message from these papers is that individualized care with supportive services decreases opioid use and optimizes the care of mothers and their babies after a cesarean section,” he said.
Dr. Johnson is the Dr. Irving J. Taylor Professor and Chair in the department of psychiatry; professor of both neurology and pharmacology, among others; and director of the Brain Science Research Consortium Unit at the University of Maryland, Baltimore. He had no relevant financial disclosures.
While overprescribing opioids for women after cesarean delivery will probably have little effect on the opioid crisis, reducing prescription rates for these women post partum still has benefits for mother and child, Bankole Johnson, MD, DSc, said in an interview.
“The larger problem is with mothers who are dependent on opiates who get pregnant,” Dr. Johnson said. “These mothers give birth to children who need significant support and weaning off opiates, sometime in the NICU, and the mother also has to be treated by the addiction services. Sometimes, the mother simply abandons the baby who is difficult to nurse and comfort because he or she is weaning off opiates.”
Dr. Johnson said overprescribing has three main implications or risks: a risk of developing dependence in newborn babies that are breastfed, an increased risk of dependence for the mother, and a decrease in nurturing skills and bonding from the mother due to high opioid use.
He said these studies had “notable caveats” and noted there may not be much clinical significance because of the small numeric difference in opioid use.
“The best take-home message from these papers is that individualized care with supportive services decreases opioid use and optimizes the care of mothers and their babies after a cesarean section,” he said.
Dr. Johnson is the Dr. Irving J. Taylor Professor and Chair in the department of psychiatry; professor of both neurology and pharmacology, among others; and director of the Brain Science Research Consortium Unit at the University of Maryland, Baltimore. He had no relevant financial disclosures.
While overprescribing opioids for women after cesarean delivery will probably have little effect on the opioid crisis, reducing prescription rates for these women post partum still has benefits for mother and child, Bankole Johnson, MD, DSc, said in an interview.
“The larger problem is with mothers who are dependent on opiates who get pregnant,” Dr. Johnson said. “These mothers give birth to children who need significant support and weaning off opiates, sometime in the NICU, and the mother also has to be treated by the addiction services. Sometimes, the mother simply abandons the baby who is difficult to nurse and comfort because he or she is weaning off opiates.”
Dr. Johnson said overprescribing has three main implications or risks: a risk of developing dependence in newborn babies that are breastfed, an increased risk of dependence for the mother, and a decrease in nurturing skills and bonding from the mother due to high opioid use.
He said these studies had “notable caveats” and noted there may not be much clinical significance because of the small numeric difference in opioid use.
“The best take-home message from these papers is that individualized care with supportive services decreases opioid use and optimizes the care of mothers and their babies after a cesarean section,” he said.
Dr. Johnson is the Dr. Irving J. Taylor Professor and Chair in the department of psychiatry; professor of both neurology and pharmacology, among others; and director of the Brain Science Research Consortium Unit at the University of Maryland, Baltimore. He had no relevant financial disclosures.
An individual prescribing plan based on inpatient use for women who delivered by cesarean section was associated with a lower number of unused oxycodone tablets, according to research published in Obstetrics & Gynecology.
In a second study, there was a significantly lower number of opioid tablets prescribed for women who delivered by cesarean section after a shared decision-making quality improvement plan was implemented on an inpatient basis.
Sarah S. Osmundson, MD, MS, of Vanderbilt University Medical Center in Nashville, Tenn., and her colleagues conducted a randomized, controlled trial of women who underwent cesarean delivery between June and August 2017. Of these women, 85 were randomized to receive standard care of 30 oxycodone tablets (5 mg) and 87 received an individualized care plan based on inpatient use of oxycodone. Patients were a minimum of 18 years old with similar baseline characteristics and inpatient pain scores.
The investigators found that women who were randomized to the individual care group received 14 oxycodone tablets (interquartile range, 12-16) and had 5 tablets (IQR, 1-8) left 2 weeks after discharge, compared with women who were prescribed 30 tablets in the standard care group (IQR, 30-30; P less than .001) and left 10 tablets unused (IQR, 0-22; P less than .001). There were no significant differences regarding patient-reported pain outcomes in either group, and women used about half as many prescribed tablets in the individualized group (8; IQR, 4-14), compared with women in the standard care group (15; IQR, 6-30; P less than .001).
Most women reported that they used opioids to treat pain and 30% said they believed they were supposed to finish all the tablets that were prescribed, the researchers noted. There were 11 patients (13%) in the standard care group and 12 (14%) in the individualized group who did not use any opioids; similarly, 23 patients (27%) in the standard care group and 21 (24%) in the individual care group used all tablets prescribed.
“If pain were the only determinant of opioid use, randomization should yield similar opioid use in both study groups,” Dr. Osmundson and her colleagues wrote. “These findings suggest that factors other than pain influence opioid use patterns.”
In a second study, Malavika Prabhu, MD, of Massachusetts General Hospital in Boston, and her colleagues employed a prospective quality improvement (QI) initiative for women prescribed opioids after cesarean delivery. They evaluated the opioid use of 624 women and counseled them at discharge on the need for prescription opioids, using shared decision-making to determine the number of opioid tablets, with a maximum number of 30 oxycodone tablets (5 mg). The investigators also changed their protocol for multimodal analgesia to scheduled acetaminophen and NSAIDs. Patients were a mean of 30 years old and more than 50% were white. Median gestational age was 39 weeks at delivery. In a second phase of the study, the investigators lowered the maximum number of opioid tablets prescribed to 25 tablets.
At discharge, the mean number of prescribed opioid tablets decreased to 27 tablets from 33 tablets in phase 1 (P less than .01). In phase 2, the number of tablets further decreased to 22 tablets from 25 tablets (P less than .01). The investigators noted no significant difference in the refill rate during phase 1 (8% vs. 9%; P less than .79) and phase 2 (6% vs. 6%; P = .72). There was a significant increase in prescribing of acetaminophen from 33% at baseline to 77% at the end of phase 1 and an increase at the beginning of phase 2 at 91% to 92% at the end of phase 2. There were no statistically significant differences among prescriptions of ibuprofen at any phase time point.
“As a result of our success in decreasing opioid prescribing after cesarean delivery, our current protocol has again been amended to recommend a maximum of 20 tablets of 5-mg oxycodone (or equivalent opioid) prescribed at the time of discharge,” Dr. Prabhu and her colleagues wrote. “This decrease, which represents a 50% decrease in the departmental standard before this study, has been successfully implemented in 18 months. In addition, efforts to optimize multimodal analgesic use continue both inpatient and on discharge.”
Dr. Osmundson was supported by a grant from the National Institutes of Health, and the research also was supported by an award from the National Center for Advancing Translational Sciences; the authors of the study reported no relevant conflicts of interest. The authors led by Dr. Prabhu had no relevant financial disclosures.
SOURCE: Prabhu M et al. Obstet Gyncol. 2018 Aug 4; doi: 10.1097/AOG.0000000000002789, and Osmundson SS et al. Obstet Gynecol. 2018 Aug 6; doi: 10.1097/AOG.0000000000002782.
An individual prescribing plan based on inpatient use for women who delivered by cesarean section was associated with a lower number of unused oxycodone tablets, according to research published in Obstetrics & Gynecology.
In a second study, there was a significantly lower number of opioid tablets prescribed for women who delivered by cesarean section after a shared decision-making quality improvement plan was implemented on an inpatient basis.
Sarah S. Osmundson, MD, MS, of Vanderbilt University Medical Center in Nashville, Tenn., and her colleagues conducted a randomized, controlled trial of women who underwent cesarean delivery between June and August 2017. Of these women, 85 were randomized to receive standard care of 30 oxycodone tablets (5 mg) and 87 received an individualized care plan based on inpatient use of oxycodone. Patients were a minimum of 18 years old with similar baseline characteristics and inpatient pain scores.
The investigators found that women who were randomized to the individual care group received 14 oxycodone tablets (interquartile range, 12-16) and had 5 tablets (IQR, 1-8) left 2 weeks after discharge, compared with women who were prescribed 30 tablets in the standard care group (IQR, 30-30; P less than .001) and left 10 tablets unused (IQR, 0-22; P less than .001). There were no significant differences regarding patient-reported pain outcomes in either group, and women used about half as many prescribed tablets in the individualized group (8; IQR, 4-14), compared with women in the standard care group (15; IQR, 6-30; P less than .001).
Most women reported that they used opioids to treat pain and 30% said they believed they were supposed to finish all the tablets that were prescribed, the researchers noted. There were 11 patients (13%) in the standard care group and 12 (14%) in the individualized group who did not use any opioids; similarly, 23 patients (27%) in the standard care group and 21 (24%) in the individual care group used all tablets prescribed.
“If pain were the only determinant of opioid use, randomization should yield similar opioid use in both study groups,” Dr. Osmundson and her colleagues wrote. “These findings suggest that factors other than pain influence opioid use patterns.”
In a second study, Malavika Prabhu, MD, of Massachusetts General Hospital in Boston, and her colleagues employed a prospective quality improvement (QI) initiative for women prescribed opioids after cesarean delivery. They evaluated the opioid use of 624 women and counseled them at discharge on the need for prescription opioids, using shared decision-making to determine the number of opioid tablets, with a maximum number of 30 oxycodone tablets (5 mg). The investigators also changed their protocol for multimodal analgesia to scheduled acetaminophen and NSAIDs. Patients were a mean of 30 years old and more than 50% were white. Median gestational age was 39 weeks at delivery. In a second phase of the study, the investigators lowered the maximum number of opioid tablets prescribed to 25 tablets.
At discharge, the mean number of prescribed opioid tablets decreased to 27 tablets from 33 tablets in phase 1 (P less than .01). In phase 2, the number of tablets further decreased to 22 tablets from 25 tablets (P less than .01). The investigators noted no significant difference in the refill rate during phase 1 (8% vs. 9%; P less than .79) and phase 2 (6% vs. 6%; P = .72). There was a significant increase in prescribing of acetaminophen from 33% at baseline to 77% at the end of phase 1 and an increase at the beginning of phase 2 at 91% to 92% at the end of phase 2. There were no statistically significant differences among prescriptions of ibuprofen at any phase time point.
“As a result of our success in decreasing opioid prescribing after cesarean delivery, our current protocol has again been amended to recommend a maximum of 20 tablets of 5-mg oxycodone (or equivalent opioid) prescribed at the time of discharge,” Dr. Prabhu and her colleagues wrote. “This decrease, which represents a 50% decrease in the departmental standard before this study, has been successfully implemented in 18 months. In addition, efforts to optimize multimodal analgesic use continue both inpatient and on discharge.”
Dr. Osmundson was supported by a grant from the National Institutes of Health, and the research also was supported by an award from the National Center for Advancing Translational Sciences; the authors of the study reported no relevant conflicts of interest. The authors led by Dr. Prabhu had no relevant financial disclosures.
SOURCE: Prabhu M et al. Obstet Gyncol. 2018 Aug 4; doi: 10.1097/AOG.0000000000002789, and Osmundson SS et al. Obstet Gynecol. 2018 Aug 6; doi: 10.1097/AOG.0000000000002782.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Individualized opioid prescription and a quality improvement plan for opioid prescribing among women who underwent cesarean delivery lowered the number of overall tablets used among these patients.
Major finding: Patients who were prescribed at discharge the number of inpatient opioid tablets used had fewer leftover tablets than did patients prescribed the standard of care; there was a mean lower number of opioid tablets prescribed after delivery in the study with a quality improvement plan, from 33 tablets to 27 tablets.
Data source: A randomized controlled trial of 323 women who underwent cesarean delivery and were prescribed opioid tablets, and a quality improvement trial of 624 women.
Disclosures: Dr. Osmundson was supported by a grant from the National Institutes of Health, and the research also was supported by an award from the National Center for Advancing Translational Sciences; the authors of the study reported no relevant conflicts of interest. The authors led by Dr. Prabhu had no relevant financial disclosures.
Source: Prabhu M et al. Obstet Gyncol. 2018 Aug 4. doi: 10.1097/AOG.0000000000002789 and Osmundson SS et al. Obstet Gynecol. 2018 Aug 6. doi: 10.1097/AOG.0000000000002782.
Breast arterial calcification, low bone mass predict CAD in women
Breast arterial calcification and low bone mass (LBM) were strongly linked to risk of coronary artery disease in asymptomatic women, according to a cross-sectional registry study.
The results suggest that breast arterial calcification – easily visible on every mammogram – “provides an independent and incremental predictive value over conventional risk factors,” wrote Yeonyee E. Yoon, MD, and colleagues from Seoul National University Bundang Hospital in Seongnam, South Korea.
In addition, they show that “atherosclerosis imaging allows a more direct visualization of the cumulative effects of all risk determinants in an individual patient,” they noted. The report is in JACC: Cardiovascular Imaging.
The researchers evaluated 2,100 asymptomatic women aged at least 40 years in the Women Health Registry Study for Bone, Breast, and Coronary Artery Disease (BBC study). All underwent a self-referred health evaluation that included simultaneous dual-energy x-ray absorptiometry (DXA), coronary computed tomography angiography (CCTA), and digital mammography. They predicted the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) with the Korean Risk Prediction Model (KRPM) and Pooled Cohort Equation (PCE).
Overall, Dr. Yoon and colleagues found that 199 patients (9.5%) had BAC and 716 patients (34.1%) had LBM, with 235 patients (11.2%) having coronary artery calcification (CAC) and 328 with coronary artery plaque (CAP, 15.6%). BAC presence was associated with CAC (unadjusted odds ratio, 3.54), with mild (OR, 2.84) and severe BAC scores (OR, 5.50) having a greater association with CAC. All associations were statistically significant at P less than .001.
LBM also had a positive link with CAC that grew with its severity. Specifically, the odds ratio of CAC with osteopenia, defined as a T-score between –1.0 and –2.5, was 2.06, and that for osteoporosis, defined as a T-score at or below –2.5, was 3.21. All links were significant at P less than .001.
Similarly, BAC and LBM were also significantly tied to coronary artery plaque, with mild (OR, 2.61) and moderate (OR, 4.15) BAC severity as well as osteopenia (OR, 1.76) and osteoporosis (OR 2.82) being significantly associated with CAP (all P less than .001).
In a multivariable analysis, BAC presence and BAC score were significantly associated with CAC and CAP after adjustment for 10-year ASCVD risk. Predictions for CAC and CAP under the KRPM and PCE curve analyses showed a significant increase of areas under the curve (0.71 vs. 0.64), while adding BAC presence significantly increased the AUCs for the KRPM curve analysis (0.61 vs 0.64).
“Being able to predict CAC or CAP presence in an individual patient based on the presence and severity of BAC in addition to the use of conventional risk stratification algorithms may help clinicians decide when to recommend further cardiac tests and how aggressive interventions to prescribe in order to prevent the onset of clinical CAD,” the researchers noted.
They added that patients were all self-referred women, and results may not be generalizable to a larger population. The study’s retrospective nature also is a limitation, the researchers wrote. However, they said they are planning a future trial that will attempt to determine whether there is a long-term clinical benefit to identifying BAC and LBM in women without symptoms of CAD.
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea; the Ministry of Science, ICT, and Future Planning; and the Seoul National University Bundang Hospital Research Fund. The authors report no relevant financial disclosures.
SOURCE: Yoon YE et al. JACC: Cardiovasc Imaging. 2018 Aug 15. doi: 10.1016/j.jcmg.2018.07.004.
Breast arterial calcification and low bone mass (LBM) were strongly linked to risk of coronary artery disease in asymptomatic women, according to a cross-sectional registry study.
The results suggest that breast arterial calcification – easily visible on every mammogram – “provides an independent and incremental predictive value over conventional risk factors,” wrote Yeonyee E. Yoon, MD, and colleagues from Seoul National University Bundang Hospital in Seongnam, South Korea.
In addition, they show that “atherosclerosis imaging allows a more direct visualization of the cumulative effects of all risk determinants in an individual patient,” they noted. The report is in JACC: Cardiovascular Imaging.
The researchers evaluated 2,100 asymptomatic women aged at least 40 years in the Women Health Registry Study for Bone, Breast, and Coronary Artery Disease (BBC study). All underwent a self-referred health evaluation that included simultaneous dual-energy x-ray absorptiometry (DXA), coronary computed tomography angiography (CCTA), and digital mammography. They predicted the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) with the Korean Risk Prediction Model (KRPM) and Pooled Cohort Equation (PCE).
Overall, Dr. Yoon and colleagues found that 199 patients (9.5%) had BAC and 716 patients (34.1%) had LBM, with 235 patients (11.2%) having coronary artery calcification (CAC) and 328 with coronary artery plaque (CAP, 15.6%). BAC presence was associated with CAC (unadjusted odds ratio, 3.54), with mild (OR, 2.84) and severe BAC scores (OR, 5.50) having a greater association with CAC. All associations were statistically significant at P less than .001.
LBM also had a positive link with CAC that grew with its severity. Specifically, the odds ratio of CAC with osteopenia, defined as a T-score between –1.0 and –2.5, was 2.06, and that for osteoporosis, defined as a T-score at or below –2.5, was 3.21. All links were significant at P less than .001.
Similarly, BAC and LBM were also significantly tied to coronary artery plaque, with mild (OR, 2.61) and moderate (OR, 4.15) BAC severity as well as osteopenia (OR, 1.76) and osteoporosis (OR 2.82) being significantly associated with CAP (all P less than .001).
In a multivariable analysis, BAC presence and BAC score were significantly associated with CAC and CAP after adjustment for 10-year ASCVD risk. Predictions for CAC and CAP under the KRPM and PCE curve analyses showed a significant increase of areas under the curve (0.71 vs. 0.64), while adding BAC presence significantly increased the AUCs for the KRPM curve analysis (0.61 vs 0.64).
“Being able to predict CAC or CAP presence in an individual patient based on the presence and severity of BAC in addition to the use of conventional risk stratification algorithms may help clinicians decide when to recommend further cardiac tests and how aggressive interventions to prescribe in order to prevent the onset of clinical CAD,” the researchers noted.
They added that patients were all self-referred women, and results may not be generalizable to a larger population. The study’s retrospective nature also is a limitation, the researchers wrote. However, they said they are planning a future trial that will attempt to determine whether there is a long-term clinical benefit to identifying BAC and LBM in women without symptoms of CAD.
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea; the Ministry of Science, ICT, and Future Planning; and the Seoul National University Bundang Hospital Research Fund. The authors report no relevant financial disclosures.
SOURCE: Yoon YE et al. JACC: Cardiovasc Imaging. 2018 Aug 15. doi: 10.1016/j.jcmg.2018.07.004.
Breast arterial calcification and low bone mass (LBM) were strongly linked to risk of coronary artery disease in asymptomatic women, according to a cross-sectional registry study.
The results suggest that breast arterial calcification – easily visible on every mammogram – “provides an independent and incremental predictive value over conventional risk factors,” wrote Yeonyee E. Yoon, MD, and colleagues from Seoul National University Bundang Hospital in Seongnam, South Korea.
In addition, they show that “atherosclerosis imaging allows a more direct visualization of the cumulative effects of all risk determinants in an individual patient,” they noted. The report is in JACC: Cardiovascular Imaging.
The researchers evaluated 2,100 asymptomatic women aged at least 40 years in the Women Health Registry Study for Bone, Breast, and Coronary Artery Disease (BBC study). All underwent a self-referred health evaluation that included simultaneous dual-energy x-ray absorptiometry (DXA), coronary computed tomography angiography (CCTA), and digital mammography. They predicted the 10-year risk of atherosclerotic cardiovascular disease (ASCVD) with the Korean Risk Prediction Model (KRPM) and Pooled Cohort Equation (PCE).
Overall, Dr. Yoon and colleagues found that 199 patients (9.5%) had BAC and 716 patients (34.1%) had LBM, with 235 patients (11.2%) having coronary artery calcification (CAC) and 328 with coronary artery plaque (CAP, 15.6%). BAC presence was associated with CAC (unadjusted odds ratio, 3.54), with mild (OR, 2.84) and severe BAC scores (OR, 5.50) having a greater association with CAC. All associations were statistically significant at P less than .001.
LBM also had a positive link with CAC that grew with its severity. Specifically, the odds ratio of CAC with osteopenia, defined as a T-score between –1.0 and –2.5, was 2.06, and that for osteoporosis, defined as a T-score at or below –2.5, was 3.21. All links were significant at P less than .001.
Similarly, BAC and LBM were also significantly tied to coronary artery plaque, with mild (OR, 2.61) and moderate (OR, 4.15) BAC severity as well as osteopenia (OR, 1.76) and osteoporosis (OR 2.82) being significantly associated with CAP (all P less than .001).
In a multivariable analysis, BAC presence and BAC score were significantly associated with CAC and CAP after adjustment for 10-year ASCVD risk. Predictions for CAC and CAP under the KRPM and PCE curve analyses showed a significant increase of areas under the curve (0.71 vs. 0.64), while adding BAC presence significantly increased the AUCs for the KRPM curve analysis (0.61 vs 0.64).
“Being able to predict CAC or CAP presence in an individual patient based on the presence and severity of BAC in addition to the use of conventional risk stratification algorithms may help clinicians decide when to recommend further cardiac tests and how aggressive interventions to prescribe in order to prevent the onset of clinical CAD,” the researchers noted.
They added that patients were all self-referred women, and results may not be generalizable to a larger population. The study’s retrospective nature also is a limitation, the researchers wrote. However, they said they are planning a future trial that will attempt to determine whether there is a long-term clinical benefit to identifying BAC and LBM in women without symptoms of CAD.
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea; the Ministry of Science, ICT, and Future Planning; and the Seoul National University Bundang Hospital Research Fund. The authors report no relevant financial disclosures.
SOURCE: Yoon YE et al. JACC: Cardiovasc Imaging. 2018 Aug 15. doi: 10.1016/j.jcmg.2018.07.004.
FROM JACC: CARDIOVASCULAR IMAGING
Key clinical point:
Major finding: Breast arterial calcification (BAC) carried an odds ratio of 3.54 and low bone mass (LBM) carried an OR of 2.22 for the associated presence of coronary artery calcification, while BAC had an OR of 3.02 and LBM had an OR of 1.92 for associated presence of coronary atherosclerotic plaque.
Study details: A cross-sectional study of 2,100 women analyzed for subclinical coronary artery disease in the Bone, Breast, and CAD health registry study.
Disclosures: This study was supported by the Basic Science Research Program through the National Research Foundation of Korea; the Ministry of Science, ICT, and Future Planning; and the Seoul National University Bundang Hospital Research Fund. The authors report no relevant financial disclosures.
Source: Yoon YE et al. JACC: Cardiovasc Imaging. 2018 Aug 15; doi: 10.1016/j.jcmg.2018.07.004.
Sexual minorities seeking abortion report high levels of male violence
Pregnant lesbian and bisexual women who seek abortions are more likely than are their heterosexual counterparts to be the victims of violence by the men who impregnated them, a new study finds.
Rachel K. Jones, PhD, of the Guttmacher Institute, New York, and her associates also found that these sexual minority women, plus a group of individuals who described their sexual orientation as “something else,” were much more likely to report exposure to sexual and physical violence.
“No patient should be presumed to be heterosexual for any reason, including a pregnancy history. All pregnancies – like all patients – should be treated as unique and operating within the dynamic and interconnected circumstances of peoples’ lives, which may encompass differences in sexual orientation and exposure to violence,” the researchers wrote. Their report is in Obstetrics & Gynecology.
Previous research has suggested that nonheterosexual women are more likely than are straight women to become pregnant unintentionally. There also are signs suggesting that they have more abortions, too, although the findings are iffy, the study authors wrote.
For this study, Dr. Jones and her associates examined questionnaire answers of 8,380 women who responded to the Guttmacher Institute’s 2014 Abortion Patient Survey. All were undergoing abortions at 87 U.S. nonhospital facilities that performed 30 or more abortions each per year.
Of the sample, about 9% declined to describe their sexual orientation. Of the rest, 94% described themselves as heterosexual; of those, 41% were white, 28% were black, and 22% were Hispanic. Most were in their 20s, 47% were never married, and 48% had incomes below the federal poverty level.
Women also described themselves as bisexual (4%), “something else” (1%), and lesbian (0.4%). All these groups were more likely than were heterosexuals to be below the federal poverty level; more than half of the lesbian and bisexual respondents said they had previously given birth.
Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals and 3% of bisexuals. (P less than .001).
Bisexuals (9%) and lesbians (33%) were more likely than were heterosexuals (4%) to say the men who impregnated them had physically abused them. The same was true for sexual abuse, which was reported by 7% of bisexuals, 35% of lesbians, and 2% of heterosexuals. After the researchers controlled for various factors including age and race, lesbians remained much more likely to report physical abuse, sexual abuse, and forced sex at the hands of the men who impregnated them (odds ratios = 15, 25, and 10, respectively, P less than .001).
“Exposure to physical and sexual violence was substantially higher among each of the sexual minority groups compared with their heterosexual counterparts, sometimes by a factor of 15 or more,” the study authors wrote. “We found that lesbian respondents had the highest levels of exposure to violence, perhaps because this population was more likely to have had sex with a man only in the context of forced sex.”
The researchers noted that their study has various limitations, such as low numbers of sexual minority women and the 4-year gap since the data were collected.
Still, Dr. Jones and her associates wrote, the study has strengths. “Health care providers, including those working in abortion settings, need to be aware that a proportion of their patient population identifies as something other than heterosexual,” they wrote.
The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
SOURCE: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
Pregnant lesbian and bisexual women who seek abortions are more likely than are their heterosexual counterparts to be the victims of violence by the men who impregnated them, a new study finds.
Rachel K. Jones, PhD, of the Guttmacher Institute, New York, and her associates also found that these sexual minority women, plus a group of individuals who described their sexual orientation as “something else,” were much more likely to report exposure to sexual and physical violence.
“No patient should be presumed to be heterosexual for any reason, including a pregnancy history. All pregnancies – like all patients – should be treated as unique and operating within the dynamic and interconnected circumstances of peoples’ lives, which may encompass differences in sexual orientation and exposure to violence,” the researchers wrote. Their report is in Obstetrics & Gynecology.
Previous research has suggested that nonheterosexual women are more likely than are straight women to become pregnant unintentionally. There also are signs suggesting that they have more abortions, too, although the findings are iffy, the study authors wrote.
For this study, Dr. Jones and her associates examined questionnaire answers of 8,380 women who responded to the Guttmacher Institute’s 2014 Abortion Patient Survey. All were undergoing abortions at 87 U.S. nonhospital facilities that performed 30 or more abortions each per year.
Of the sample, about 9% declined to describe their sexual orientation. Of the rest, 94% described themselves as heterosexual; of those, 41% were white, 28% were black, and 22% were Hispanic. Most were in their 20s, 47% were never married, and 48% had incomes below the federal poverty level.
Women also described themselves as bisexual (4%), “something else” (1%), and lesbian (0.4%). All these groups were more likely than were heterosexuals to be below the federal poverty level; more than half of the lesbian and bisexual respondents said they had previously given birth.
Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals and 3% of bisexuals. (P less than .001).
Bisexuals (9%) and lesbians (33%) were more likely than were heterosexuals (4%) to say the men who impregnated them had physically abused them. The same was true for sexual abuse, which was reported by 7% of bisexuals, 35% of lesbians, and 2% of heterosexuals. After the researchers controlled for various factors including age and race, lesbians remained much more likely to report physical abuse, sexual abuse, and forced sex at the hands of the men who impregnated them (odds ratios = 15, 25, and 10, respectively, P less than .001).
“Exposure to physical and sexual violence was substantially higher among each of the sexual minority groups compared with their heterosexual counterparts, sometimes by a factor of 15 or more,” the study authors wrote. “We found that lesbian respondents had the highest levels of exposure to violence, perhaps because this population was more likely to have had sex with a man only in the context of forced sex.”
The researchers noted that their study has various limitations, such as low numbers of sexual minority women and the 4-year gap since the data were collected.
Still, Dr. Jones and her associates wrote, the study has strengths. “Health care providers, including those working in abortion settings, need to be aware that a proportion of their patient population identifies as something other than heterosexual,” they wrote.
The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
SOURCE: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
Pregnant lesbian and bisexual women who seek abortions are more likely than are their heterosexual counterparts to be the victims of violence by the men who impregnated them, a new study finds.
Rachel K. Jones, PhD, of the Guttmacher Institute, New York, and her associates also found that these sexual minority women, plus a group of individuals who described their sexual orientation as “something else,” were much more likely to report exposure to sexual and physical violence.
“No patient should be presumed to be heterosexual for any reason, including a pregnancy history. All pregnancies – like all patients – should be treated as unique and operating within the dynamic and interconnected circumstances of peoples’ lives, which may encompass differences in sexual orientation and exposure to violence,” the researchers wrote. Their report is in Obstetrics & Gynecology.
Previous research has suggested that nonheterosexual women are more likely than are straight women to become pregnant unintentionally. There also are signs suggesting that they have more abortions, too, although the findings are iffy, the study authors wrote.
For this study, Dr. Jones and her associates examined questionnaire answers of 8,380 women who responded to the Guttmacher Institute’s 2014 Abortion Patient Survey. All were undergoing abortions at 87 U.S. nonhospital facilities that performed 30 or more abortions each per year.
Of the sample, about 9% declined to describe their sexual orientation. Of the rest, 94% described themselves as heterosexual; of those, 41% were white, 28% were black, and 22% were Hispanic. Most were in their 20s, 47% were never married, and 48% had incomes below the federal poverty level.
Women also described themselves as bisexual (4%), “something else” (1%), and lesbian (0.4%). All these groups were more likely than were heterosexuals to be below the federal poverty level; more than half of the lesbian and bisexual respondents said they had previously given birth.
Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals and 3% of bisexuals. (P less than .001).
Bisexuals (9%) and lesbians (33%) were more likely than were heterosexuals (4%) to say the men who impregnated them had physically abused them. The same was true for sexual abuse, which was reported by 7% of bisexuals, 35% of lesbians, and 2% of heterosexuals. After the researchers controlled for various factors including age and race, lesbians remained much more likely to report physical abuse, sexual abuse, and forced sex at the hands of the men who impregnated them (odds ratios = 15, 25, and 10, respectively, P less than .001).
“Exposure to physical and sexual violence was substantially higher among each of the sexual minority groups compared with their heterosexual counterparts, sometimes by a factor of 15 or more,” the study authors wrote. “We found that lesbian respondents had the highest levels of exposure to violence, perhaps because this population was more likely to have had sex with a man only in the context of forced sex.”
The researchers noted that their study has various limitations, such as low numbers of sexual minority women and the 4-year gap since the data were collected.
Still, Dr. Jones and her associates wrote, the study has strengths. “Health care providers, including those working in abortion settings, need to be aware that a proportion of their patient population identifies as something other than heterosexual,” they wrote.
The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
SOURCE: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: Fifteen percent of lesbians said their current pregnancy was caused by forced sex, compared with 1% of heterosexuals (P less than .001), and 3% of bisexuals. Lesbians (33%) were more likely than were heterosexuals (4%) to say the man who impregnated them had physically and/or sexually abused them.
Study details: A 2014 survey of 8,380 women seeking abortions at 87 U.S. nonhospital facilities.
Disclosures: The study was funded by the Susan Thompson Buffett Foundation with support from the National Institutes of Health via a grant to the Guttmacher Center for Population Research Innovation and Dissemination. The study authors reported no relevant financial disclosures.
Source: Jones R et al. Obstet Gynecol. 2018 Sep;132(3):605-11.
No increase in autism risk with prenatal Tdap
A retrospective cohort study in more than 80,000 children has found no evidence of an increased risk of autism spectrum disorder associated with prenatal tetanus, diphtheria, and acellular pertussis (Tdap) immunization.
Of 81,993 children born between 2011 and 2014, 1,341 children (1.6%) were diagnosed with autism spectrum disorder. The incidence of autism spectrum disorder was 3.78 per 1,000 person-years in the Tdap-vaccinated group, and 4.05 per 1,000 person years in the unvaccinated group, representing an unadjusted hazard ratio of 0.98 and an adjusted hazard ratio of 0.85. This was consistent across all the birth cohorts.
Prenatal immunization rates with the prenatal Tdap vaccine ranged from 26% of the 2012 birth cohort to 79% of the 2014 birth cohort, and mean gestational age at vaccination was 28 weeks.
Tracy A. Becerra-Culqui, PhD, MPH, and colleagues of the department of research and evaluation at Kaiser Permanente Southern California, Pasadena, said this was the first study to look at the risk of autism spectrum disorder after maternal exposure to the Tdap vaccine, to their knowledge. “Our results potentially indicate that the maternal Tdap vaccine affects immune trajectories protecting infants against infections that would otherwise lead to neurodevelopmental alterations.”
They highlighted several strengths of their study. One was that maternal Tdap vaccination and information on autism spectrum disorder both were derived from EHRs and therefore not subject to recall bias. The study, published online in Pediatrics, also included children diagnosed with autism spectrum disorder from age 1 year onwards, reflecting the latest evidence on screening and diagnosis of autism.
“Our weighting procedures enabled us to balance the Tdap-exposed and -unexposed groups to compare two populations that were comparable in important measured confounding factors,” Dr. Becerra-Culqui and associates noted.
The investigators found that women who received the Tdap vaccine during pregnancy were more likely to be Asian American or Pacific Islander, to have a bachelor’s degree or higher, be nulliparous, to have also been vaccinated prenatally against influenza, and to deliver at term, compared with unvaccinated women.
However the authors did note that their follow-up was limited to 6.5 years for the earliest birth cohort, and 3.5 years for the latest cohort, so they may not have picked up children who received a later diagnosis of autism spectrum disorder.
The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
SOURCE: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.
A retrospective cohort study in more than 80,000 children has found no evidence of an increased risk of autism spectrum disorder associated with prenatal tetanus, diphtheria, and acellular pertussis (Tdap) immunization.
Of 81,993 children born between 2011 and 2014, 1,341 children (1.6%) were diagnosed with autism spectrum disorder. The incidence of autism spectrum disorder was 3.78 per 1,000 person-years in the Tdap-vaccinated group, and 4.05 per 1,000 person years in the unvaccinated group, representing an unadjusted hazard ratio of 0.98 and an adjusted hazard ratio of 0.85. This was consistent across all the birth cohorts.
Prenatal immunization rates with the prenatal Tdap vaccine ranged from 26% of the 2012 birth cohort to 79% of the 2014 birth cohort, and mean gestational age at vaccination was 28 weeks.
Tracy A. Becerra-Culqui, PhD, MPH, and colleagues of the department of research and evaluation at Kaiser Permanente Southern California, Pasadena, said this was the first study to look at the risk of autism spectrum disorder after maternal exposure to the Tdap vaccine, to their knowledge. “Our results potentially indicate that the maternal Tdap vaccine affects immune trajectories protecting infants against infections that would otherwise lead to neurodevelopmental alterations.”
They highlighted several strengths of their study. One was that maternal Tdap vaccination and information on autism spectrum disorder both were derived from EHRs and therefore not subject to recall bias. The study, published online in Pediatrics, also included children diagnosed with autism spectrum disorder from age 1 year onwards, reflecting the latest evidence on screening and diagnosis of autism.
“Our weighting procedures enabled us to balance the Tdap-exposed and -unexposed groups to compare two populations that were comparable in important measured confounding factors,” Dr. Becerra-Culqui and associates noted.
The investigators found that women who received the Tdap vaccine during pregnancy were more likely to be Asian American or Pacific Islander, to have a bachelor’s degree or higher, be nulliparous, to have also been vaccinated prenatally against influenza, and to deliver at term, compared with unvaccinated women.
However the authors did note that their follow-up was limited to 6.5 years for the earliest birth cohort, and 3.5 years for the latest cohort, so they may not have picked up children who received a later diagnosis of autism spectrum disorder.
The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
SOURCE: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.
A retrospective cohort study in more than 80,000 children has found no evidence of an increased risk of autism spectrum disorder associated with prenatal tetanus, diphtheria, and acellular pertussis (Tdap) immunization.
Of 81,993 children born between 2011 and 2014, 1,341 children (1.6%) were diagnosed with autism spectrum disorder. The incidence of autism spectrum disorder was 3.78 per 1,000 person-years in the Tdap-vaccinated group, and 4.05 per 1,000 person years in the unvaccinated group, representing an unadjusted hazard ratio of 0.98 and an adjusted hazard ratio of 0.85. This was consistent across all the birth cohorts.
Prenatal immunization rates with the prenatal Tdap vaccine ranged from 26% of the 2012 birth cohort to 79% of the 2014 birth cohort, and mean gestational age at vaccination was 28 weeks.
Tracy A. Becerra-Culqui, PhD, MPH, and colleagues of the department of research and evaluation at Kaiser Permanente Southern California, Pasadena, said this was the first study to look at the risk of autism spectrum disorder after maternal exposure to the Tdap vaccine, to their knowledge. “Our results potentially indicate that the maternal Tdap vaccine affects immune trajectories protecting infants against infections that would otherwise lead to neurodevelopmental alterations.”
They highlighted several strengths of their study. One was that maternal Tdap vaccination and information on autism spectrum disorder both were derived from EHRs and therefore not subject to recall bias. The study, published online in Pediatrics, also included children diagnosed with autism spectrum disorder from age 1 year onwards, reflecting the latest evidence on screening and diagnosis of autism.
“Our weighting procedures enabled us to balance the Tdap-exposed and -unexposed groups to compare two populations that were comparable in important measured confounding factors,” Dr. Becerra-Culqui and associates noted.
The investigators found that women who received the Tdap vaccine during pregnancy were more likely to be Asian American or Pacific Islander, to have a bachelor’s degree or higher, be nulliparous, to have also been vaccinated prenatally against influenza, and to deliver at term, compared with unvaccinated women.
However the authors did note that their follow-up was limited to 6.5 years for the earliest birth cohort, and 3.5 years for the latest cohort, so they may not have picked up children who received a later diagnosis of autism spectrum disorder.
The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
SOURCE: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.
FROM PEDIATRICS
Key clinical point:
Major finding: The adjusted hazard ratio for autism spectrum disorder in children exposed to the prenatal Tdap vaccine is 0.98, compared with unvaccinated children.
Study details: A retrospective cohort study in 81,993 children exposed to the prenatal Tdap vaccine.
Disclosures: The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
Source: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.
FDA approves vaginal ring contraceptive Annovera
The first vaginal ring contraceptive that can be used for 1 year has been approved by the Food and Drug Administration.
The FDA granted approval of Annovera (segesterone acetate and ethinyl estradiol vaginal system), a reusable donut-shaped ring, to the Population Council. The nonbiodegradable, flexible vaginal system is placed in the vagina for 3 weeks followed by 1 week out of the vagina, at which time women may experience a menstrual period. This schedule is repeated every 4 weeks for 13 28-day menstrual cycles.
The contraceptive ring is washed and stored in a compact case for the 7 days when it is not in use. Annovera does not require refrigeration prior to dispensing and can withstand storage temperatures up to 30° C (86° F).
“Today’s approval builds on available birth control options,” said Victor Crentsil, MD, acting deputy director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research.
The efficacy and safety of Annovera were studied in three, open-label clinical trials with healthy women ranging from 18 to 40 years of age. Based on the results, about 2%-4% of women may get pregnant during the first year they use Annovera.
Annovera carries a boxed warning regarding cigarette smoking and serious cardiovascular events. Women over age 35 who smoke should not use Annovera. Cigarette smoking increases the risk of serious cardiovascular events from combination hormonal contraceptive use.
Annovera is contraindicated for women with a high risk of arterial or venous thrombotic diseases; a history of breast cancer or another estrogen- or progestin-sensitive cancer; liver tumors, acute hepatitis, or severe (decompensated) cirrhosis; undiagnosed abnormal uterine bleeding; hypersensitivity to any of the components of Annovera; and use of hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, according to an FDA press release.
The most common side effects of Annovera are similar to those of other combined hormonal contraceptive products and include headache, nausea and vomiting, yeast infections, abdominal pain, dysmenorrhea, breast tenderness, irregular bleeding, diarrhea, and genital itching.
The FDA is requiring postmarketing studies to further evaluate the risks of venous thromboembolism and the effects of CYP3A-modulating drugs and tampon use on the pharmacokinetics of Annovera.
It is very exciting to see ongoing research and development for new contraceptives and approval of these new methods! We know that each person’s contraceptive needs are unique and having more options from which to choose will help us as providers connect our patients to methods that meet their goals and individual needs. These two methods fill important gaps in our current contraceptive portfolio: a patient-controlled, long-acting method and a facilitated non-hormonal, non-prescription method (the Natural Cycles app). I am looking forward to hearing feedback from patients about their thoughts and experiences with these new methods.
Melissa Kottke, MD, MPH, MBA, is an associate professor of obstetrics and gynecology and director of the Jane Fonda Center for Adolescent Reproductive Health, Emory University, Atlanta.
It is very exciting to see ongoing research and development for new contraceptives and approval of these new methods! We know that each person’s contraceptive needs are unique and having more options from which to choose will help us as providers connect our patients to methods that meet their goals and individual needs. These two methods fill important gaps in our current contraceptive portfolio: a patient-controlled, long-acting method and a facilitated non-hormonal, non-prescription method (the Natural Cycles app). I am looking forward to hearing feedback from patients about their thoughts and experiences with these new methods.
Melissa Kottke, MD, MPH, MBA, is an associate professor of obstetrics and gynecology and director of the Jane Fonda Center for Adolescent Reproductive Health, Emory University, Atlanta.
It is very exciting to see ongoing research and development for new contraceptives and approval of these new methods! We know that each person’s contraceptive needs are unique and having more options from which to choose will help us as providers connect our patients to methods that meet their goals and individual needs. These two methods fill important gaps in our current contraceptive portfolio: a patient-controlled, long-acting method and a facilitated non-hormonal, non-prescription method (the Natural Cycles app). I am looking forward to hearing feedback from patients about their thoughts and experiences with these new methods.
Melissa Kottke, MD, MPH, MBA, is an associate professor of obstetrics and gynecology and director of the Jane Fonda Center for Adolescent Reproductive Health, Emory University, Atlanta.
The first vaginal ring contraceptive that can be used for 1 year has been approved by the Food and Drug Administration.
The FDA granted approval of Annovera (segesterone acetate and ethinyl estradiol vaginal system), a reusable donut-shaped ring, to the Population Council. The nonbiodegradable, flexible vaginal system is placed in the vagina for 3 weeks followed by 1 week out of the vagina, at which time women may experience a menstrual period. This schedule is repeated every 4 weeks for 13 28-day menstrual cycles.
The contraceptive ring is washed and stored in a compact case for the 7 days when it is not in use. Annovera does not require refrigeration prior to dispensing and can withstand storage temperatures up to 30° C (86° F).
“Today’s approval builds on available birth control options,” said Victor Crentsil, MD, acting deputy director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research.
The efficacy and safety of Annovera were studied in three, open-label clinical trials with healthy women ranging from 18 to 40 years of age. Based on the results, about 2%-4% of women may get pregnant during the first year they use Annovera.
Annovera carries a boxed warning regarding cigarette smoking and serious cardiovascular events. Women over age 35 who smoke should not use Annovera. Cigarette smoking increases the risk of serious cardiovascular events from combination hormonal contraceptive use.
Annovera is contraindicated for women with a high risk of arterial or venous thrombotic diseases; a history of breast cancer or another estrogen- or progestin-sensitive cancer; liver tumors, acute hepatitis, or severe (decompensated) cirrhosis; undiagnosed abnormal uterine bleeding; hypersensitivity to any of the components of Annovera; and use of hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, according to an FDA press release.
The most common side effects of Annovera are similar to those of other combined hormonal contraceptive products and include headache, nausea and vomiting, yeast infections, abdominal pain, dysmenorrhea, breast tenderness, irregular bleeding, diarrhea, and genital itching.
The FDA is requiring postmarketing studies to further evaluate the risks of venous thromboembolism and the effects of CYP3A-modulating drugs and tampon use on the pharmacokinetics of Annovera.
The first vaginal ring contraceptive that can be used for 1 year has been approved by the Food and Drug Administration.
The FDA granted approval of Annovera (segesterone acetate and ethinyl estradiol vaginal system), a reusable donut-shaped ring, to the Population Council. The nonbiodegradable, flexible vaginal system is placed in the vagina for 3 weeks followed by 1 week out of the vagina, at which time women may experience a menstrual period. This schedule is repeated every 4 weeks for 13 28-day menstrual cycles.
The contraceptive ring is washed and stored in a compact case for the 7 days when it is not in use. Annovera does not require refrigeration prior to dispensing and can withstand storage temperatures up to 30° C (86° F).
“Today’s approval builds on available birth control options,” said Victor Crentsil, MD, acting deputy director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research.
The efficacy and safety of Annovera were studied in three, open-label clinical trials with healthy women ranging from 18 to 40 years of age. Based on the results, about 2%-4% of women may get pregnant during the first year they use Annovera.
Annovera carries a boxed warning regarding cigarette smoking and serious cardiovascular events. Women over age 35 who smoke should not use Annovera. Cigarette smoking increases the risk of serious cardiovascular events from combination hormonal contraceptive use.
Annovera is contraindicated for women with a high risk of arterial or venous thrombotic diseases; a history of breast cancer or another estrogen- or progestin-sensitive cancer; liver tumors, acute hepatitis, or severe (decompensated) cirrhosis; undiagnosed abnormal uterine bleeding; hypersensitivity to any of the components of Annovera; and use of hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, according to an FDA press release.
The most common side effects of Annovera are similar to those of other combined hormonal contraceptive products and include headache, nausea and vomiting, yeast infections, abdominal pain, dysmenorrhea, breast tenderness, irregular bleeding, diarrhea, and genital itching.
The FDA is requiring postmarketing studies to further evaluate the risks of venous thromboembolism and the effects of CYP3A-modulating drugs and tampon use on the pharmacokinetics of Annovera.
Continuation, complication rates similar for implants, IUDs
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
FROM CONTRACEPTION
Key clinical point:
Major finding: For implants and IUDS, respectively, 1-year continuation rates were similar (adjusted 81% vs. 77%, P = .01), as were complication rates (unadjusted 8% for implants, 7% for IUDs).
Study details: Retrospective analysis of 3,305 Medicaid-covered women treated with the two types of contraceptives during 2012-2015 in the District of Columbia and Maryland.
Disclosures: MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen discloses serving on the Diclegis speakers bureau.
Source: Romano MJ et al. Contraception. 2018 Aug;98:125-9.
New IUD expelled less often after C-section than older device
that’s inserted the same way, according to results of a Turkish study.
The study authors, led by Ceren Unal, MD, of Zeynep Kamil Women’s and Children’s Disease Training and Research Hospital, Istanbul, wrote that the new device, the frameless copper-releasing Gyn-CS IUD, “could be a major advance, potentially suitable for general use due to the ease and safety of the insertion procedure,” which requires limited training.
According to the study authors, most IUDs are retained in the uterus, and most have a T-shape configuration. Some are inserted immediately following expulsion of the placenta at birth, although techniques “are far from being optimal,” and devices are frequently displaced and sometimes expelled.
The newly developed Gyn-CS IUD, a redesigned version of a previous model, has an alternative “anchor” design.
The investigators tracked 140 pregnant women – 106 who underwent elective cesarean section and 34 who underwent emergency cesarean section – who had the Gyn-CS or the TCu380A IUD inserted shortly after they gave birth. Their median age was around 30 years , all were white, and all were married.
The TCu380A IUD, a decades-old model that’s been recommended by the World Health Organization, and the Gyn-CS IUD were each inserted in 70 women. The researchers then followed them for 3 months. Three women (one in the Gyn-CS IUD group and two in the TCu380A IUD group) were lost to follow-up.
In total, 61 of 70 IUDs (88%) remained in place in the Gyn-CS group, and 54 of 70 (79%) in the TCu380A group (P = .30). One (1%) Gyn-CS IUD was expelled , possibly because it was incorrectly anchored, and eight (11%) TCu380A IUDs were expelled (P = .04). There were equal numbers of medical removals (four) and nonmedical removals (two) in the two groups, and one “other medical removal” in the Gyn-CS IUD group.
“The very low expulsion rate of Gyn-CS, compared with TCu380A, is a very strong argument in favor of the anchored IUD, preventing expulsion and displacement,” Dr. Unal and colleagues reported in Contraception.
They noted that the study has a limited 3-month tracking period, but they also pointed out that most expulsions inserted post partum occurred in the initial 6 weeks.
The low expulsion rate of the Gyn-CS device “will prevent more women becoming pregnant too soon which constitutes an important safety issue during future pregnancy,” the authors wrote. “The device, preferably its high-load 10-year version, could also interest many women as a reversible alternative for tubal sterilization. Additional clinical experience with the Gyn-CS IUD is, therefore, urgently warranted.”
No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
that’s inserted the same way, according to results of a Turkish study.
The study authors, led by Ceren Unal, MD, of Zeynep Kamil Women’s and Children’s Disease Training and Research Hospital, Istanbul, wrote that the new device, the frameless copper-releasing Gyn-CS IUD, “could be a major advance, potentially suitable for general use due to the ease and safety of the insertion procedure,” which requires limited training.
According to the study authors, most IUDs are retained in the uterus, and most have a T-shape configuration. Some are inserted immediately following expulsion of the placenta at birth, although techniques “are far from being optimal,” and devices are frequently displaced and sometimes expelled.
The newly developed Gyn-CS IUD, a redesigned version of a previous model, has an alternative “anchor” design.
The investigators tracked 140 pregnant women – 106 who underwent elective cesarean section and 34 who underwent emergency cesarean section – who had the Gyn-CS or the TCu380A IUD inserted shortly after they gave birth. Their median age was around 30 years , all were white, and all were married.
The TCu380A IUD, a decades-old model that’s been recommended by the World Health Organization, and the Gyn-CS IUD were each inserted in 70 women. The researchers then followed them for 3 months. Three women (one in the Gyn-CS IUD group and two in the TCu380A IUD group) were lost to follow-up.
In total, 61 of 70 IUDs (88%) remained in place in the Gyn-CS group, and 54 of 70 (79%) in the TCu380A group (P = .30). One (1%) Gyn-CS IUD was expelled , possibly because it was incorrectly anchored, and eight (11%) TCu380A IUDs were expelled (P = .04). There were equal numbers of medical removals (four) and nonmedical removals (two) in the two groups, and one “other medical removal” in the Gyn-CS IUD group.
“The very low expulsion rate of Gyn-CS, compared with TCu380A, is a very strong argument in favor of the anchored IUD, preventing expulsion and displacement,” Dr. Unal and colleagues reported in Contraception.
They noted that the study has a limited 3-month tracking period, but they also pointed out that most expulsions inserted post partum occurred in the initial 6 weeks.
The low expulsion rate of the Gyn-CS device “will prevent more women becoming pregnant too soon which constitutes an important safety issue during future pregnancy,” the authors wrote. “The device, preferably its high-load 10-year version, could also interest many women as a reversible alternative for tubal sterilization. Additional clinical experience with the Gyn-CS IUD is, therefore, urgently warranted.”
No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
that’s inserted the same way, according to results of a Turkish study.
The study authors, led by Ceren Unal, MD, of Zeynep Kamil Women’s and Children’s Disease Training and Research Hospital, Istanbul, wrote that the new device, the frameless copper-releasing Gyn-CS IUD, “could be a major advance, potentially suitable for general use due to the ease and safety of the insertion procedure,” which requires limited training.
According to the study authors, most IUDs are retained in the uterus, and most have a T-shape configuration. Some are inserted immediately following expulsion of the placenta at birth, although techniques “are far from being optimal,” and devices are frequently displaced and sometimes expelled.
The newly developed Gyn-CS IUD, a redesigned version of a previous model, has an alternative “anchor” design.
The investigators tracked 140 pregnant women – 106 who underwent elective cesarean section and 34 who underwent emergency cesarean section – who had the Gyn-CS or the TCu380A IUD inserted shortly after they gave birth. Their median age was around 30 years , all were white, and all were married.
The TCu380A IUD, a decades-old model that’s been recommended by the World Health Organization, and the Gyn-CS IUD were each inserted in 70 women. The researchers then followed them for 3 months. Three women (one in the Gyn-CS IUD group and two in the TCu380A IUD group) were lost to follow-up.
In total, 61 of 70 IUDs (88%) remained in place in the Gyn-CS group, and 54 of 70 (79%) in the TCu380A group (P = .30). One (1%) Gyn-CS IUD was expelled , possibly because it was incorrectly anchored, and eight (11%) TCu380A IUDs were expelled (P = .04). There were equal numbers of medical removals (four) and nonmedical removals (two) in the two groups, and one “other medical removal” in the Gyn-CS IUD group.
“The very low expulsion rate of Gyn-CS, compared with TCu380A, is a very strong argument in favor of the anchored IUD, preventing expulsion and displacement,” Dr. Unal and colleagues reported in Contraception.
They noted that the study has a limited 3-month tracking period, but they also pointed out that most expulsions inserted post partum occurred in the initial 6 weeks.
The low expulsion rate of the Gyn-CS device “will prevent more women becoming pregnant too soon which constitutes an important safety issue during future pregnancy,” the authors wrote. “The device, preferably its high-load 10-year version, could also interest many women as a reversible alternative for tubal sterilization. Additional clinical experience with the Gyn-CS IUD is, therefore, urgently warranted.”
No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
FROM CONTRACEPTION
Key clinical point: A newly developed IUD appears to be expelled less frequently than an older device after insertion following cesarean section births.
Major finding: 61 of 70 IUDs (88%) remained in place in women who received the frameless copper-releasing Gyn-CS device, while 54 of 70 (79%) did so in those who received the TCu380A device (P = .30).
Study details: Randomized trial of 140 women who underwent cesarean section followed by insertion of one of the two IUD types (n = 70 for each).
Disclosures: No study funding is reported, although Contrel Research provided the Gyn-CS devices at no charge. The study authors reported no relevant disclosures.
Source: Unal C et al. Contraception. 2018 Aug;98:135-40.
FDA approves first mobile app for contraceptive use
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
The Food and Drug Administration has approved marketing of the first medical mobile application that can be used as a contraceptive, the agency announced in a written statement.
The app, called Natural Cycles, contains an algorithm that calculates the days of the month women are likely to be fertile, based on daily body temperature readings and menstrual cycle information, and is intended for premenopausal women aged 18 years and older. App users are instructed to take their daily body temperatures with a basal body thermometer and enter the information into the app. The more-sensitive basal body thermometer can detect temperature elevations during ovulation, and the app will display a warning on days when users are most fertile. During these days, women should either abstain from sex or use protection.
In clinical studies comprising more than 15,000 women, Natural Cycles had a “perfect use” failure rate of 1.8% and a “normal use” failure rate of 6.5%. This compares favorably with other forms of contraception and birth control: Male condoms have a perfect use failure rate of 2.0% and a normal use failure rate of 18.0%, and combined oral contraceptives have a perfect use failure rate of 0.3% and a normal use failure rate of 9.0%, according to the Association of Reproductive Care Professionals.
“Consumers are increasingly using digital health technologies to inform their everyday health decisions, and this new app can provide an effective method of contraception if it’s used carefully and correctly,” Terri Cornelison, MD, PhD, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health, said in the statement.
The app should not be used by women with a condition in which a pregnancy would present risk to the mother or fetus or by women who are using a birth control or hormonal treatment that inhibits ovulation. Natural Cycles does not protect against sexually transmitted infections.
More deliveries now include opioid use disorder
according to the Centers for Disease Control and Prevention.
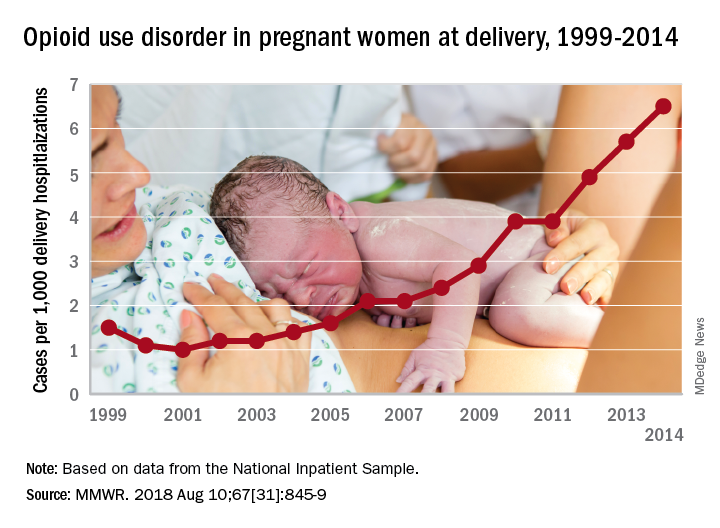
The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
according to the Centers for Disease Control and Prevention.

The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
according to the Centers for Disease Control and Prevention.

The national prevalence of opioid use disorder increased by 333% as it went from 1.5 cases per 1,000 delivery hospitalizations in 1999 to 6.5 cases per 1,000 in 2014. At the state level, there were significant increases in all 28 states with data available for at least 3 consecutive years during the study period, Sarah C. Haight, MPH, and her associates at the CDC in Atlanta said in the Morbidity and Mortality Weekly Report.
Average annual rate changes for those states ranged from a low of 0.01 per 1,000 delivery hospitalizations per year in California to 5.37 per year in Vermont, with the national rate change coming in at 0.39 per year. Of the 14 states with data available in 1999, Iowa had the lowest rate at 0.1 per 1,000 deliveries and Maryland had the highest at 8.2. In 2014, when data were available for 26 states and the District of Columbia, the highest rate was Vermont’s 48.6 per 1,000 deliveries and the lowest was 0.7 in Washington, D.C., the investigators reported.
Although “increasing trends might represent actual increases in prevalence or improved screening and diagnosis,” Ms. Haight and her associates added that “these estimates also correlate with state opioid prescribing rates in the general population. West Virginia, for example, had a prescribing rate estimated at 138 opioid prescriptions per 100 persons in 2012.”
“These findings illustrate the devastating impact of the opioid epidemic on families across the U.S., including on the very youngest,” said CDC Director Robert R. Redfield, MD. “Untreated opioid use disorder during pregnancy can lead to heartbreaking results. Each case represents a mother, a child, and a family in need of continued treatment and support.”
Data for the analysis came from the Agency for Healthcare Research and Quality’s National Inpatient Sample and State Inpatient Databases.
SOURCE: Haight SC et al. MMWR. 2018 Aug 10;67[31]:845-9.
FROM MMWR