User login
Poor night’s sleep impairs glucose control the next morning
Going to bed later than usual and/or getting a poor night’s sleep are both associated with impaired glycemic response to breakfast the following morning in healthy adults, according to a multiple test-meal challenge study conducted over 14 days.
“Our data suggest that sleep duration, efficiency, and midpoint are important determinants of postprandial glycemic control at a population level,” Neil Tsereteli, MD, Lund University Diabetes Centre, Malmo, Sweden, and colleagues wrote in their article, published online Nov. 30, 2021, in Diabetologia.
“And [the results] suggest that one-size-fits-all sleep recommendations are suboptimal, particularly in the context of postprandial glycemic control, a key component of diabetes prevention,” they added.
Prior research on sleep quality and control of glucose lacking
Diet, exercise, and sleep are fundamental components of a healthy lifestyle; however, the role that sleep plays in affecting blood glucose control in generally healthy people has been studied little so far, the researchers wrote.
Sleep disorders can act as a measure of general health as they often occur alongside other health problems. Sleep quality also has a direct causal effect on many conditions such as cardiovascular disease, obesity, and type 2 diabetes. And disturbed sleep caused by conditions such as obstructive sleep apnea is associated with the prevalence of type 2 diabetes and risk of associated complications.
This and other evidence suggest a strong link between glucose regulation and the quality and duration of sleep.
Dr. Tsereteli and colleagues set out to examine this further in the Personalized Responses to Dietary Composition Trial 1, which involved 953 healthy adults who consumed standardized meals over 2 weeks in a clinic setting and at home.
“The meals were consumed either for breakfast or lunch in a randomized meal order and consisted of eight different standardized meals,” the researchers wrote.
Activity and sleep were monitored using a wearable device with an accelerometer. Postprandial blood glucose levels were measured using a continuous glucose monitor.
Sleep variables including quality, duration, and timing and their impact on glycemic response to breakfast the following morning, and were compared between participants and within each individual.
Better sleep efficiency, better glucose control
The study found that, although there was no significant association between length of sleep period and postmeal glycemic response, there was a significant interaction when the nutritional content of the breakfast meal was also considered.
Longer sleep periods were associated with lower blood glucose following high-carbohydrate and high-fat breakfasts, indicating better blood glucose control.
Additionally, the researchers observed a within-person effect in which a study participant who slept for longer than they typically would was likely to have reduced postprandial blood glucose following a high-carbohydrate or high-fat breakfast the next day.
The authors also found a significant link between sleep efficiency (ratio of time asleep to total length of sleep period) and glycemic control. When a participant slept more efficiently than usual, their postprandial blood glucose also tended to be lower than usual.
“This effect was largely driven by sleep onset (going to bed later) rather than sleep offset (waking up later),” Dr. Tsereteli and colleagues noted.
Sleep a key pillar of health
Asked whether these particular sleep effects might be exacerbated in patients with diabetes, senior author Paul Franks, MD, also from the Lund University Diabetes Centre, felt they could not meaningfully extrapolate results to people with diabetes, given that many take glucose-lowering medications.
“However, it is likely that these results would be similar or exacerbated in people with prediabetes, as glucose fluctuations in this subgroup of patients are generally greater than in people with normoglycemia,” he noted in an interview.
“Sleep is a key pillar of health, and focusing on both sleep and diet is key for healthy blood glucose control,” he added.
“Compensating for a bad night’s sleep by consuming a very sugary breakfast or energy drinks is likely to be especially detrimental for blood glucose control,” Dr. Franks said.
The study was funded by Lund University. Dr. Tsereteli and Dr. Franks reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Going to bed later than usual and/or getting a poor night’s sleep are both associated with impaired glycemic response to breakfast the following morning in healthy adults, according to a multiple test-meal challenge study conducted over 14 days.
“Our data suggest that sleep duration, efficiency, and midpoint are important determinants of postprandial glycemic control at a population level,” Neil Tsereteli, MD, Lund University Diabetes Centre, Malmo, Sweden, and colleagues wrote in their article, published online Nov. 30, 2021, in Diabetologia.
“And [the results] suggest that one-size-fits-all sleep recommendations are suboptimal, particularly in the context of postprandial glycemic control, a key component of diabetes prevention,” they added.
Prior research on sleep quality and control of glucose lacking
Diet, exercise, and sleep are fundamental components of a healthy lifestyle; however, the role that sleep plays in affecting blood glucose control in generally healthy people has been studied little so far, the researchers wrote.
Sleep disorders can act as a measure of general health as they often occur alongside other health problems. Sleep quality also has a direct causal effect on many conditions such as cardiovascular disease, obesity, and type 2 diabetes. And disturbed sleep caused by conditions such as obstructive sleep apnea is associated with the prevalence of type 2 diabetes and risk of associated complications.
This and other evidence suggest a strong link between glucose regulation and the quality and duration of sleep.
Dr. Tsereteli and colleagues set out to examine this further in the Personalized Responses to Dietary Composition Trial 1, which involved 953 healthy adults who consumed standardized meals over 2 weeks in a clinic setting and at home.
“The meals were consumed either for breakfast or lunch in a randomized meal order and consisted of eight different standardized meals,” the researchers wrote.
Activity and sleep were monitored using a wearable device with an accelerometer. Postprandial blood glucose levels were measured using a continuous glucose monitor.
Sleep variables including quality, duration, and timing and their impact on glycemic response to breakfast the following morning, and were compared between participants and within each individual.
Better sleep efficiency, better glucose control
The study found that, although there was no significant association between length of sleep period and postmeal glycemic response, there was a significant interaction when the nutritional content of the breakfast meal was also considered.
Longer sleep periods were associated with lower blood glucose following high-carbohydrate and high-fat breakfasts, indicating better blood glucose control.
Additionally, the researchers observed a within-person effect in which a study participant who slept for longer than they typically would was likely to have reduced postprandial blood glucose following a high-carbohydrate or high-fat breakfast the next day.
The authors also found a significant link between sleep efficiency (ratio of time asleep to total length of sleep period) and glycemic control. When a participant slept more efficiently than usual, their postprandial blood glucose also tended to be lower than usual.
“This effect was largely driven by sleep onset (going to bed later) rather than sleep offset (waking up later),” Dr. Tsereteli and colleagues noted.
Sleep a key pillar of health
Asked whether these particular sleep effects might be exacerbated in patients with diabetes, senior author Paul Franks, MD, also from the Lund University Diabetes Centre, felt they could not meaningfully extrapolate results to people with diabetes, given that many take glucose-lowering medications.
“However, it is likely that these results would be similar or exacerbated in people with prediabetes, as glucose fluctuations in this subgroup of patients are generally greater than in people with normoglycemia,” he noted in an interview.
“Sleep is a key pillar of health, and focusing on both sleep and diet is key for healthy blood glucose control,” he added.
“Compensating for a bad night’s sleep by consuming a very sugary breakfast or energy drinks is likely to be especially detrimental for blood glucose control,” Dr. Franks said.
The study was funded by Lund University. Dr. Tsereteli and Dr. Franks reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Going to bed later than usual and/or getting a poor night’s sleep are both associated with impaired glycemic response to breakfast the following morning in healthy adults, according to a multiple test-meal challenge study conducted over 14 days.
“Our data suggest that sleep duration, efficiency, and midpoint are important determinants of postprandial glycemic control at a population level,” Neil Tsereteli, MD, Lund University Diabetes Centre, Malmo, Sweden, and colleagues wrote in their article, published online Nov. 30, 2021, in Diabetologia.
“And [the results] suggest that one-size-fits-all sleep recommendations are suboptimal, particularly in the context of postprandial glycemic control, a key component of diabetes prevention,” they added.
Prior research on sleep quality and control of glucose lacking
Diet, exercise, and sleep are fundamental components of a healthy lifestyle; however, the role that sleep plays in affecting blood glucose control in generally healthy people has been studied little so far, the researchers wrote.
Sleep disorders can act as a measure of general health as they often occur alongside other health problems. Sleep quality also has a direct causal effect on many conditions such as cardiovascular disease, obesity, and type 2 diabetes. And disturbed sleep caused by conditions such as obstructive sleep apnea is associated with the prevalence of type 2 diabetes and risk of associated complications.
This and other evidence suggest a strong link between glucose regulation and the quality and duration of sleep.
Dr. Tsereteli and colleagues set out to examine this further in the Personalized Responses to Dietary Composition Trial 1, which involved 953 healthy adults who consumed standardized meals over 2 weeks in a clinic setting and at home.
“The meals were consumed either for breakfast or lunch in a randomized meal order and consisted of eight different standardized meals,” the researchers wrote.
Activity and sleep were monitored using a wearable device with an accelerometer. Postprandial blood glucose levels were measured using a continuous glucose monitor.
Sleep variables including quality, duration, and timing and their impact on glycemic response to breakfast the following morning, and were compared between participants and within each individual.
Better sleep efficiency, better glucose control
The study found that, although there was no significant association between length of sleep period and postmeal glycemic response, there was a significant interaction when the nutritional content of the breakfast meal was also considered.
Longer sleep periods were associated with lower blood glucose following high-carbohydrate and high-fat breakfasts, indicating better blood glucose control.
Additionally, the researchers observed a within-person effect in which a study participant who slept for longer than they typically would was likely to have reduced postprandial blood glucose following a high-carbohydrate or high-fat breakfast the next day.
The authors also found a significant link between sleep efficiency (ratio of time asleep to total length of sleep period) and glycemic control. When a participant slept more efficiently than usual, their postprandial blood glucose also tended to be lower than usual.
“This effect was largely driven by sleep onset (going to bed later) rather than sleep offset (waking up later),” Dr. Tsereteli and colleagues noted.
Sleep a key pillar of health
Asked whether these particular sleep effects might be exacerbated in patients with diabetes, senior author Paul Franks, MD, also from the Lund University Diabetes Centre, felt they could not meaningfully extrapolate results to people with diabetes, given that many take glucose-lowering medications.
“However, it is likely that these results would be similar or exacerbated in people with prediabetes, as glucose fluctuations in this subgroup of patients are generally greater than in people with normoglycemia,” he noted in an interview.
“Sleep is a key pillar of health, and focusing on both sleep and diet is key for healthy blood glucose control,” he added.
“Compensating for a bad night’s sleep by consuming a very sugary breakfast or energy drinks is likely to be especially detrimental for blood glucose control,” Dr. Franks said.
The study was funded by Lund University. Dr. Tsereteli and Dr. Franks reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Make cholesterol control a greater priority in diabetes
, a new population-based study in Finland suggests.
In the study, recently published online in Scientific Reports , the authors showed that LDL-C control and statin prescriptions remain suboptimal in this patient population in clinical practice.
They identified four 5-year trajectories of LDL-C along with concurrent levels of statin treatment. The percentages of patients in each group were:
- Moderately stable LDL-C: 2.3 mmol/L (90 mg/dL): 86%
- High stable LDL-C: 3.9 mmol/L (152 mg/dL): 7.7%
- Decreasing LDL-C: 3.8%
- Increasing LDL-C: 2.5%
“The second-largest group consisted of predominantly untreated patients (7.7%) with alarmingly ‘high stable’ LDL-C levels around 3.9 mmol/L,” the researchers noted.
And among patients with “increasing” LDL-C cholesterol, statin treatment “declined drastically.”
Moreover, 42% of patients had no statins prescribed at the end of follow-up.
These findings show that “efforts to control LDL-C should be increased – especially in patients with continuously elevated levels – by initiating and intensifying statin treatment earlier and reinitiating the treatment after discontinuation, if possible,” lead author Laura Inglin, MPH, told this news organization.
Discuss risks vs. benefits of statins with patients
Patients may not understand the benefits versus potential side effects of statins, said Ms. Inglin, of the Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio.
To improve management of cholesterol levels, she said, “clinician-patient discussions are crucial, addressing risk/benefits and treatment goals, and offering reputable sources” of information about statins.
When patients discontinue statin treatment, Ms. Inglin continued, “physicians should try to reinitiate another statin or to lower the dose if possible, following guidelines on how to do that,” as other research has reported that more than 70% of patients who stopped a statin because of side effects tolerated it when it was restarted.
The study also identified gender differences, she continued. Compared with men, women had significantly higher average LDL-C levels, but were less likely to be prescribed a statin or were prescribed a lower-dose statin, and they were more likely to discontinue statin therapy.
Four LDL-C trajectories with statin treatment differences
Suboptimal lipid profiles, especially elevated LDL-C, are strongly associated with atherosclerotic CVD in individuals with type 2 diabetes, Ms. Inglin and colleagues write.
“To prevent or at least delay complications, regular follow-up visits and good control of A1c, LDL-C, blood pressure, and other CVD risk factors are vital in diabetes management,” they continued. “Guidelines have consistently identified statins as the principal lipid-lowering therapy, recommended particularly at moderate- to high-intensity.”
The researchers aimed to identify LDL-C level trajectories and concomitant statin treatment in patients with type 2 diabetes.
They identified 8,592 patients – 4,622 men (54%) and 3,970 women (46%) – with type 2 diabetes seen by primary care physicians or specialists in North Karelia, Eastern Finland, during 2011-2017.
As with other international guidelines, the Finnish Current Care Guideline recommended assessing LDL-C levels every 1-3 years in patients with type 2 diabetes, with LDL-C treatment targets of < 2.5 mmol/L (< 100 mg/dL) for those at high CVD risk due to diabetes, and targets of < 1.8 mmol/L (< 70 mg/dL) or a 50% reduction from baseline in those at very high CVD risk due to additional risk factors.
At baseline, on average, men in the current study were aged 66 years and had had diabetes for 8 years; 60% were receiving a statin and 56% had an LDL-C < 2.5 mmol/L.
Women were, on average, age 69 years and had had diabetes for 8 years; 56% were receiving a statin and 51% had an LDL-C < 2.5 mmol/L.
The researchers identified the four distinct LDL-C trajectories, each with differences in statin treatment.
In the “moderate-stable” LDL-C group, 67% of men and 64% of women were receiving a statin, and the rates of high-intensity statin increased in both men and women.
In the “high-stable” LDL-C group, rates of statin use decreased from 42% to 27% among men and from 34% to 23% among women.
In the “decreasing” LDL-C group, the proportion of patients who received a statin increased; the percentage of patients who received a high-intensity statin also increased among men (6.2% to 29%) and women (7.7% to 14%).
In the “increasing” LDL-C group, the percentage of patients receiving a statin decreased from more than 64% to less than 43%.
“Physicians should increase efforts to achieve the LDL-C treatment targets – especially in the patient group with constantly elevated LDL-C levels – by paying attention to earlier initiation of statin treatment, intensification of treatments when necessary, and reinitiating if possible,” the researchers reiterated.
“The results of our study may support physicians to identify patients who need to be monitored more closely beyond a single time point measurement,” they concluded.
The study was partly funded by the Strategic Research Council of the Academy of Finland (project IMPRO), the Finnish Diabetes Association, and the Research Committee of the Kuopio University Hospital Catchment Area for the State Research Funding (project QCARE). The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new population-based study in Finland suggests.
In the study, recently published online in Scientific Reports , the authors showed that LDL-C control and statin prescriptions remain suboptimal in this patient population in clinical practice.
They identified four 5-year trajectories of LDL-C along with concurrent levels of statin treatment. The percentages of patients in each group were:
- Moderately stable LDL-C: 2.3 mmol/L (90 mg/dL): 86%
- High stable LDL-C: 3.9 mmol/L (152 mg/dL): 7.7%
- Decreasing LDL-C: 3.8%
- Increasing LDL-C: 2.5%
“The second-largest group consisted of predominantly untreated patients (7.7%) with alarmingly ‘high stable’ LDL-C levels around 3.9 mmol/L,” the researchers noted.
And among patients with “increasing” LDL-C cholesterol, statin treatment “declined drastically.”
Moreover, 42% of patients had no statins prescribed at the end of follow-up.
These findings show that “efforts to control LDL-C should be increased – especially in patients with continuously elevated levels – by initiating and intensifying statin treatment earlier and reinitiating the treatment after discontinuation, if possible,” lead author Laura Inglin, MPH, told this news organization.
Discuss risks vs. benefits of statins with patients
Patients may not understand the benefits versus potential side effects of statins, said Ms. Inglin, of the Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio.
To improve management of cholesterol levels, she said, “clinician-patient discussions are crucial, addressing risk/benefits and treatment goals, and offering reputable sources” of information about statins.
When patients discontinue statin treatment, Ms. Inglin continued, “physicians should try to reinitiate another statin or to lower the dose if possible, following guidelines on how to do that,” as other research has reported that more than 70% of patients who stopped a statin because of side effects tolerated it when it was restarted.
The study also identified gender differences, she continued. Compared with men, women had significantly higher average LDL-C levels, but were less likely to be prescribed a statin or were prescribed a lower-dose statin, and they were more likely to discontinue statin therapy.
Four LDL-C trajectories with statin treatment differences
Suboptimal lipid profiles, especially elevated LDL-C, are strongly associated with atherosclerotic CVD in individuals with type 2 diabetes, Ms. Inglin and colleagues write.
“To prevent or at least delay complications, regular follow-up visits and good control of A1c, LDL-C, blood pressure, and other CVD risk factors are vital in diabetes management,” they continued. “Guidelines have consistently identified statins as the principal lipid-lowering therapy, recommended particularly at moderate- to high-intensity.”
The researchers aimed to identify LDL-C level trajectories and concomitant statin treatment in patients with type 2 diabetes.
They identified 8,592 patients – 4,622 men (54%) and 3,970 women (46%) – with type 2 diabetes seen by primary care physicians or specialists in North Karelia, Eastern Finland, during 2011-2017.
As with other international guidelines, the Finnish Current Care Guideline recommended assessing LDL-C levels every 1-3 years in patients with type 2 diabetes, with LDL-C treatment targets of < 2.5 mmol/L (< 100 mg/dL) for those at high CVD risk due to diabetes, and targets of < 1.8 mmol/L (< 70 mg/dL) or a 50% reduction from baseline in those at very high CVD risk due to additional risk factors.
At baseline, on average, men in the current study were aged 66 years and had had diabetes for 8 years; 60% were receiving a statin and 56% had an LDL-C < 2.5 mmol/L.
Women were, on average, age 69 years and had had diabetes for 8 years; 56% were receiving a statin and 51% had an LDL-C < 2.5 mmol/L.
The researchers identified the four distinct LDL-C trajectories, each with differences in statin treatment.
In the “moderate-stable” LDL-C group, 67% of men and 64% of women were receiving a statin, and the rates of high-intensity statin increased in both men and women.
In the “high-stable” LDL-C group, rates of statin use decreased from 42% to 27% among men and from 34% to 23% among women.
In the “decreasing” LDL-C group, the proportion of patients who received a statin increased; the percentage of patients who received a high-intensity statin also increased among men (6.2% to 29%) and women (7.7% to 14%).
In the “increasing” LDL-C group, the percentage of patients receiving a statin decreased from more than 64% to less than 43%.
“Physicians should increase efforts to achieve the LDL-C treatment targets – especially in the patient group with constantly elevated LDL-C levels – by paying attention to earlier initiation of statin treatment, intensification of treatments when necessary, and reinitiating if possible,” the researchers reiterated.
“The results of our study may support physicians to identify patients who need to be monitored more closely beyond a single time point measurement,” they concluded.
The study was partly funded by the Strategic Research Council of the Academy of Finland (project IMPRO), the Finnish Diabetes Association, and the Research Committee of the Kuopio University Hospital Catchment Area for the State Research Funding (project QCARE). The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new population-based study in Finland suggests.
In the study, recently published online in Scientific Reports , the authors showed that LDL-C control and statin prescriptions remain suboptimal in this patient population in clinical practice.
They identified four 5-year trajectories of LDL-C along with concurrent levels of statin treatment. The percentages of patients in each group were:
- Moderately stable LDL-C: 2.3 mmol/L (90 mg/dL): 86%
- High stable LDL-C: 3.9 mmol/L (152 mg/dL): 7.7%
- Decreasing LDL-C: 3.8%
- Increasing LDL-C: 2.5%
“The second-largest group consisted of predominantly untreated patients (7.7%) with alarmingly ‘high stable’ LDL-C levels around 3.9 mmol/L,” the researchers noted.
And among patients with “increasing” LDL-C cholesterol, statin treatment “declined drastically.”
Moreover, 42% of patients had no statins prescribed at the end of follow-up.
These findings show that “efforts to control LDL-C should be increased – especially in patients with continuously elevated levels – by initiating and intensifying statin treatment earlier and reinitiating the treatment after discontinuation, if possible,” lead author Laura Inglin, MPH, told this news organization.
Discuss risks vs. benefits of statins with patients
Patients may not understand the benefits versus potential side effects of statins, said Ms. Inglin, of the Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Kuopio.
To improve management of cholesterol levels, she said, “clinician-patient discussions are crucial, addressing risk/benefits and treatment goals, and offering reputable sources” of information about statins.
When patients discontinue statin treatment, Ms. Inglin continued, “physicians should try to reinitiate another statin or to lower the dose if possible, following guidelines on how to do that,” as other research has reported that more than 70% of patients who stopped a statin because of side effects tolerated it when it was restarted.
The study also identified gender differences, she continued. Compared with men, women had significantly higher average LDL-C levels, but were less likely to be prescribed a statin or were prescribed a lower-dose statin, and they were more likely to discontinue statin therapy.
Four LDL-C trajectories with statin treatment differences
Suboptimal lipid profiles, especially elevated LDL-C, are strongly associated with atherosclerotic CVD in individuals with type 2 diabetes, Ms. Inglin and colleagues write.
“To prevent or at least delay complications, regular follow-up visits and good control of A1c, LDL-C, blood pressure, and other CVD risk factors are vital in diabetes management,” they continued. “Guidelines have consistently identified statins as the principal lipid-lowering therapy, recommended particularly at moderate- to high-intensity.”
The researchers aimed to identify LDL-C level trajectories and concomitant statin treatment in patients with type 2 diabetes.
They identified 8,592 patients – 4,622 men (54%) and 3,970 women (46%) – with type 2 diabetes seen by primary care physicians or specialists in North Karelia, Eastern Finland, during 2011-2017.
As with other international guidelines, the Finnish Current Care Guideline recommended assessing LDL-C levels every 1-3 years in patients with type 2 diabetes, with LDL-C treatment targets of < 2.5 mmol/L (< 100 mg/dL) for those at high CVD risk due to diabetes, and targets of < 1.8 mmol/L (< 70 mg/dL) or a 50% reduction from baseline in those at very high CVD risk due to additional risk factors.
At baseline, on average, men in the current study were aged 66 years and had had diabetes for 8 years; 60% were receiving a statin and 56% had an LDL-C < 2.5 mmol/L.
Women were, on average, age 69 years and had had diabetes for 8 years; 56% were receiving a statin and 51% had an LDL-C < 2.5 mmol/L.
The researchers identified the four distinct LDL-C trajectories, each with differences in statin treatment.
In the “moderate-stable” LDL-C group, 67% of men and 64% of women were receiving a statin, and the rates of high-intensity statin increased in both men and women.
In the “high-stable” LDL-C group, rates of statin use decreased from 42% to 27% among men and from 34% to 23% among women.
In the “decreasing” LDL-C group, the proportion of patients who received a statin increased; the percentage of patients who received a high-intensity statin also increased among men (6.2% to 29%) and women (7.7% to 14%).
In the “increasing” LDL-C group, the percentage of patients receiving a statin decreased from more than 64% to less than 43%.
“Physicians should increase efforts to achieve the LDL-C treatment targets – especially in the patient group with constantly elevated LDL-C levels – by paying attention to earlier initiation of statin treatment, intensification of treatments when necessary, and reinitiating if possible,” the researchers reiterated.
“The results of our study may support physicians to identify patients who need to be monitored more closely beyond a single time point measurement,” they concluded.
The study was partly funded by the Strategic Research Council of the Academy of Finland (project IMPRO), the Finnish Diabetes Association, and the Research Committee of the Kuopio University Hospital Catchment Area for the State Research Funding (project QCARE). The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCIENTIFIC REPORTS
Retinopathy risk in children higher in T2D than T1D
Children diagnosed with type 2 diabetes (T2D) appear significantly more likely to develop retinopathy and other ocular complications over time than children who are diagnosed with type 1 diabetes (T1D), researchers report.
Among a population-based cohort of children (defined as younger than 22 years), the risk of diabetic retinopathy was 88% greater in those with T2D than T1D within the first 15 years of disease diagnosis.
“The purpose of this study was to assess the risk of developing diabetes-associated ocular complications among a population-based cohort of children diagnosed with either T1D or T2D during a 50-year period,” lead author Patricia Bai, BA, of Mayo Clinic, Phoenix, and colleagues reported in JAMA Ophthalmology.
The researchers retrospectively reviewed medical records from all residents of Olmsted County, Minn., from 1970 to 2019. The study cohort included 606 children with a confirmed a diagnosis of T1D or T2D, 525 (87%) of whom had at least one ocular examination.
The mean age at diabetes diagnosis was 12 years (standard deviation, 5.4 years); most participants were White (95.7% in 1990), and half (50%) were male. Diabetes-associated ocular complications occurred in 31.9% and 26.6% of children with T1D and T2D, respectively.
The hazard ratios illustrating the risk between T2D and T1D rates were 1.88 (95% confidence interval, 1.13-3.12; P = .02) for any diabetic retinopathy, 2.33 (95% CI, 0.99-5.50; P = .048) for proliferative diabetic retinopathy, 1.49 (95% CI, 0.46-4.89; P = .50) for diabetic macular edema, 2.43 (95% CI, 0.54-11.07; P = .24) for a visually significant cataract, and 4.06 (95% CI, 1.34-12.33; P = .007) for requiring pars plana vitrectomy within the first 15 years of diagnosis.
These results suggest that earlier surveillance and intervention may help prevent vision-threatening complications, the researchers explained.
“After adjusting for race using self-identified categories of White or not White, the adjusted HR of developing any retinopathy was 1.63 (95% CI, 0.96-2.79; P = .07), and the adjusted HR of developing proliferative diabetic retinopathy was 2.02 (95% CI, 0.79-5.16; P = .14)” in T2D versus T1D patients, the researchers wrote.
“We would expect the reported rate of type 2 diabetes to be potentially underestimated in our study cohort,” Ms. Bai commented in an interview. “Race has been suggested to be a surrogate for other social determinants of health, such as lower rates of optimal follow-up care received by racial and ethnic minorities, which could influence subsequent retinopathy rates.”
Understanding retinopathy outcomes in youth
In an accompanying editorial, Jennifer K. Sun, MD, MPH, from Harvard Medical School, Boston, wrote that the present study indicates the natural history of retinopathy may differ between patients with T1D and T2D.
While the pathophysiology of diabetic retinopathy in T1D and T2D appears similar, other patient-related factors such as lipid profiles, the presence of hypertension, and body mass index may differ between the two disease states.
She wrote that “there is a particular need to document retinopathy outcomes and risk factors for advanced disease in youth with T2D, for whom there is a paucity of information.”
Ms. Bai and colleagues acknowledged that a key limitation of the study was the retrospective design. As a result, irregular follow-up and incomplete data may limit the applicability of the findings.
“Some children with milder forms of diabetes may have eluded detection, a limitation that is more likely to affect T2D, which may exist undetected for years before a diagnosis,” Bai explained.
Dr. Sun recommended that further epidemiologic studies are needed to help optimize guidelines for screening and follow-up for young people diagnosed with diabetes. “Such efforts may potentially lead to increased understanding of the mechanistic differences between pathology in T1D versus T2D,” she concluded.
This study used the resources of the Rochester Epidemiology Project (REP) medical records linkage system, which is supported by grant funding from the National Institute on Aging, the Mayo Clinic Research Committee, and by fees paid annually by REP users. The study authors disclosed no conflicts of interest.
Children diagnosed with type 2 diabetes (T2D) appear significantly more likely to develop retinopathy and other ocular complications over time than children who are diagnosed with type 1 diabetes (T1D), researchers report.
Among a population-based cohort of children (defined as younger than 22 years), the risk of diabetic retinopathy was 88% greater in those with T2D than T1D within the first 15 years of disease diagnosis.
“The purpose of this study was to assess the risk of developing diabetes-associated ocular complications among a population-based cohort of children diagnosed with either T1D or T2D during a 50-year period,” lead author Patricia Bai, BA, of Mayo Clinic, Phoenix, and colleagues reported in JAMA Ophthalmology.
The researchers retrospectively reviewed medical records from all residents of Olmsted County, Minn., from 1970 to 2019. The study cohort included 606 children with a confirmed a diagnosis of T1D or T2D, 525 (87%) of whom had at least one ocular examination.
The mean age at diabetes diagnosis was 12 years (standard deviation, 5.4 years); most participants were White (95.7% in 1990), and half (50%) were male. Diabetes-associated ocular complications occurred in 31.9% and 26.6% of children with T1D and T2D, respectively.
The hazard ratios illustrating the risk between T2D and T1D rates were 1.88 (95% confidence interval, 1.13-3.12; P = .02) for any diabetic retinopathy, 2.33 (95% CI, 0.99-5.50; P = .048) for proliferative diabetic retinopathy, 1.49 (95% CI, 0.46-4.89; P = .50) for diabetic macular edema, 2.43 (95% CI, 0.54-11.07; P = .24) for a visually significant cataract, and 4.06 (95% CI, 1.34-12.33; P = .007) for requiring pars plana vitrectomy within the first 15 years of diagnosis.
These results suggest that earlier surveillance and intervention may help prevent vision-threatening complications, the researchers explained.
“After adjusting for race using self-identified categories of White or not White, the adjusted HR of developing any retinopathy was 1.63 (95% CI, 0.96-2.79; P = .07), and the adjusted HR of developing proliferative diabetic retinopathy was 2.02 (95% CI, 0.79-5.16; P = .14)” in T2D versus T1D patients, the researchers wrote.
“We would expect the reported rate of type 2 diabetes to be potentially underestimated in our study cohort,” Ms. Bai commented in an interview. “Race has been suggested to be a surrogate for other social determinants of health, such as lower rates of optimal follow-up care received by racial and ethnic minorities, which could influence subsequent retinopathy rates.”
Understanding retinopathy outcomes in youth
In an accompanying editorial, Jennifer K. Sun, MD, MPH, from Harvard Medical School, Boston, wrote that the present study indicates the natural history of retinopathy may differ between patients with T1D and T2D.
While the pathophysiology of diabetic retinopathy in T1D and T2D appears similar, other patient-related factors such as lipid profiles, the presence of hypertension, and body mass index may differ between the two disease states.
She wrote that “there is a particular need to document retinopathy outcomes and risk factors for advanced disease in youth with T2D, for whom there is a paucity of information.”
Ms. Bai and colleagues acknowledged that a key limitation of the study was the retrospective design. As a result, irregular follow-up and incomplete data may limit the applicability of the findings.
“Some children with milder forms of diabetes may have eluded detection, a limitation that is more likely to affect T2D, which may exist undetected for years before a diagnosis,” Bai explained.
Dr. Sun recommended that further epidemiologic studies are needed to help optimize guidelines for screening and follow-up for young people diagnosed with diabetes. “Such efforts may potentially lead to increased understanding of the mechanistic differences between pathology in T1D versus T2D,” she concluded.
This study used the resources of the Rochester Epidemiology Project (REP) medical records linkage system, which is supported by grant funding from the National Institute on Aging, the Mayo Clinic Research Committee, and by fees paid annually by REP users. The study authors disclosed no conflicts of interest.
Children diagnosed with type 2 diabetes (T2D) appear significantly more likely to develop retinopathy and other ocular complications over time than children who are diagnosed with type 1 diabetes (T1D), researchers report.
Among a population-based cohort of children (defined as younger than 22 years), the risk of diabetic retinopathy was 88% greater in those with T2D than T1D within the first 15 years of disease diagnosis.
“The purpose of this study was to assess the risk of developing diabetes-associated ocular complications among a population-based cohort of children diagnosed with either T1D or T2D during a 50-year period,” lead author Patricia Bai, BA, of Mayo Clinic, Phoenix, and colleagues reported in JAMA Ophthalmology.
The researchers retrospectively reviewed medical records from all residents of Olmsted County, Minn., from 1970 to 2019. The study cohort included 606 children with a confirmed a diagnosis of T1D or T2D, 525 (87%) of whom had at least one ocular examination.
The mean age at diabetes diagnosis was 12 years (standard deviation, 5.4 years); most participants were White (95.7% in 1990), and half (50%) were male. Diabetes-associated ocular complications occurred in 31.9% and 26.6% of children with T1D and T2D, respectively.
The hazard ratios illustrating the risk between T2D and T1D rates were 1.88 (95% confidence interval, 1.13-3.12; P = .02) for any diabetic retinopathy, 2.33 (95% CI, 0.99-5.50; P = .048) for proliferative diabetic retinopathy, 1.49 (95% CI, 0.46-4.89; P = .50) for diabetic macular edema, 2.43 (95% CI, 0.54-11.07; P = .24) for a visually significant cataract, and 4.06 (95% CI, 1.34-12.33; P = .007) for requiring pars plana vitrectomy within the first 15 years of diagnosis.
These results suggest that earlier surveillance and intervention may help prevent vision-threatening complications, the researchers explained.
“After adjusting for race using self-identified categories of White or not White, the adjusted HR of developing any retinopathy was 1.63 (95% CI, 0.96-2.79; P = .07), and the adjusted HR of developing proliferative diabetic retinopathy was 2.02 (95% CI, 0.79-5.16; P = .14)” in T2D versus T1D patients, the researchers wrote.
“We would expect the reported rate of type 2 diabetes to be potentially underestimated in our study cohort,” Ms. Bai commented in an interview. “Race has been suggested to be a surrogate for other social determinants of health, such as lower rates of optimal follow-up care received by racial and ethnic minorities, which could influence subsequent retinopathy rates.”
Understanding retinopathy outcomes in youth
In an accompanying editorial, Jennifer K. Sun, MD, MPH, from Harvard Medical School, Boston, wrote that the present study indicates the natural history of retinopathy may differ between patients with T1D and T2D.
While the pathophysiology of diabetic retinopathy in T1D and T2D appears similar, other patient-related factors such as lipid profiles, the presence of hypertension, and body mass index may differ between the two disease states.
She wrote that “there is a particular need to document retinopathy outcomes and risk factors for advanced disease in youth with T2D, for whom there is a paucity of information.”
Ms. Bai and colleagues acknowledged that a key limitation of the study was the retrospective design. As a result, irregular follow-up and incomplete data may limit the applicability of the findings.
“Some children with milder forms of diabetes may have eluded detection, a limitation that is more likely to affect T2D, which may exist undetected for years before a diagnosis,” Bai explained.
Dr. Sun recommended that further epidemiologic studies are needed to help optimize guidelines for screening and follow-up for young people diagnosed with diabetes. “Such efforts may potentially lead to increased understanding of the mechanistic differences between pathology in T1D versus T2D,” she concluded.
This study used the resources of the Rochester Epidemiology Project (REP) medical records linkage system, which is supported by grant funding from the National Institute on Aging, the Mayo Clinic Research Committee, and by fees paid annually by REP users. The study authors disclosed no conflicts of interest.
FROM JAMA OPHTHALMOLOGY
SGLT2 inhibitor use tied to fewer atrial arrhythmias
Patients with cardiac implantable electronic devices (CIEDs) who received treatment with an sodium-glucose cotransporter 2 inhibitor had significantly fewer atrial arrhythmia events, compared with those who never received such a drug, in a prospective analysis of nearly 14,000 patients with a device who were followed for an average of nearly 2 years.
The findings suggest that use of an agent from the class of SGLT2 inhibitors “is associated with a pronounced reduction in atrial arrhythmia burden and all-cause mortality in patients with a CIED in a real-world setting,” said Ilan Goldenberg, MD, at the American Heart Association scientific sessions. “These data indicate possible antiarrhythmic properties of SGLT2 inhibitors that are incremental to the beneficial effects of the drug on heart failure outcomes,” added Dr. Goldenberg, director of the Clinical Cardiovascular Research Center at the University of Rochester (N.Y.).
In a propensity score–matched analysis that included more than 5,000 of the enrolled patients with a CIED, treatment with an SGLT2 inhibitor was tied to a significant 23% relative reduction in atrial arrhythmia events and a 44% relative drop in all-cause death, he reported.
Effect mediated by reduced left atrial pressure?
“Other heart failure drugs have shown some decrease in the rate of sudden cardiac death, but this is the first [heart failure] drug to associate with a reduction in atrial arrhythmias,” Dr. Goldenberg noted. “We think that a reduction in left atrial pressure” produced by treatment with an SGLT2 inhibitor “may be linked to the reduction in atrial arrhythmias.”
The study did not show an association of SGLT2-inhibitor use and a change in ventricular arrhythmias, compared with patients with CIEDs who did not receive an agent from this class.
The findings suggest “expanding the possible indications for SGLT2 inhibitors,” commented Harriette G.C. Van Spall, MD, a cardiologist at McMaster University, Hamilton, Ont., who moderated the session where Dr. Goldenberg gave his report.
The study included 13,890 consecutive, prospectively enrolled patients who received a CIED during January 2015–April 2020 at any of five hospitals operated by either of two tertiary health care systems, one run by the University of Rochester and the second based at Sheba Medical Center in Tel HaShomer, Israel. The devices that made patients eligible for the study included permanent pacemakers, implantable cardioverter defibrillators, cardiac resynchronization therapy devices, and implantable cardiac monitors. A blinded adjudication committee composed of electrophysiologists identified the arrhythmic episodes.
At entry into the study (the time of device implantation), 12,992 patients were not receiving an SGLT2 inhibitor (94%) and 898 (6%) were receiving a drug from this class. Of those, 39% were on dapagliflozin (Farxiga), 35% were on empagliflozin (Jardiance), and 26% were on canagliflozin (Invokana).
Patients receiving an SGLT2 inhibitor at baseline were on average substantially younger than the patients not on this drug class (59 years vs. 69 years); they had a substantially higher prevalence of diabetes (78% vs. 25%), and ischemic cardiomyopathy (63% vs. 39%). Patients on an SGLT2 inhibitor at baseline also had more modestly higher prevalence rates of prior heart failure (38% vs. 31%), and hypertension (69% vs. 63%). Prevalence of a history of atrial fibrillation (AFib) was nearly the same in both groups: 31% in patients on an SGLT2 inhibitor and 35% in those not on these drugs.
The study’s primary endpoint was the total number of arrhythmia events during follow-up of 24,442 patient-years, during which patients exhibited 19,633 atrial arrhythmia events and 3,231 ventricular arrhythmia events.
1% absolute reduction in atrial arrhythmias
A multivariate analysis of the entire population – adjusted for baseline differences in age, diabetes, sex, and history of AFib – showed that treatment with an SGLT2 inhibitor at baseline was linked with a significant 24% relative reduction in incident atrial arrhythmia events, a significant 24% reduction in both atrial and ventricular arrhythmia events, and a 42% relative reduction in all-cause deaths, compared with no SGLT2-inhibitor treatment.
The only analyzed endpoint that showed no significant between-group difference was incidence of ventricular arrhythmias, which was a relative 7% lower in the SGLT2-inhibitor group.
On an absolute basis, treatment with an SGLT2 inhibitor was tied to about a 1% lower rate of atrial arrhythmia events per year, a reduction from a 2.5% rate in those not on an SGLT2 inhibitor to about a 1.5% rate in those taking this drug class.
A second, confirmatory analysis used propensity score matching to identify 5,323 patients not on an SGLT2 inhibitor at baseline who closely matched the 898 patients on an SGLT2 inhibitor. The multivariate modeling for this analysis also adjusted for age, diabetes, sex, and history of AFib.
The results of these analyses closely matched the calculations that used the entire study population. Relative to patients not on an SGLT2 inhibitor those on a drug from this class had 23% fewer atrial arrhythmias, 44% fewer total death, and 22% fewer atrial or ventricular arrhythmias, all significant differences. However, ventricular arrhythmias only reduced by a relative 5%, a nonsignificant difference.
In the propensity score–matched analysis, the absolute reduction in atrial arrhythmias in those on an SGLT2 inhibitor at baseline was roughly 1.3% fewer per year, compared with those not on this drug class.
The study was funded by an unrestricted grant to the University of Rochester from AstraZeneca, the company that markets the SGLT2 inhibitor dapagliflozin (Farxiga). Dr. Goldenberg and Dr. Van Spall had no disclosures.
Patients with cardiac implantable electronic devices (CIEDs) who received treatment with an sodium-glucose cotransporter 2 inhibitor had significantly fewer atrial arrhythmia events, compared with those who never received such a drug, in a prospective analysis of nearly 14,000 patients with a device who were followed for an average of nearly 2 years.
The findings suggest that use of an agent from the class of SGLT2 inhibitors “is associated with a pronounced reduction in atrial arrhythmia burden and all-cause mortality in patients with a CIED in a real-world setting,” said Ilan Goldenberg, MD, at the American Heart Association scientific sessions. “These data indicate possible antiarrhythmic properties of SGLT2 inhibitors that are incremental to the beneficial effects of the drug on heart failure outcomes,” added Dr. Goldenberg, director of the Clinical Cardiovascular Research Center at the University of Rochester (N.Y.).
In a propensity score–matched analysis that included more than 5,000 of the enrolled patients with a CIED, treatment with an SGLT2 inhibitor was tied to a significant 23% relative reduction in atrial arrhythmia events and a 44% relative drop in all-cause death, he reported.
Effect mediated by reduced left atrial pressure?
“Other heart failure drugs have shown some decrease in the rate of sudden cardiac death, but this is the first [heart failure] drug to associate with a reduction in atrial arrhythmias,” Dr. Goldenberg noted. “We think that a reduction in left atrial pressure” produced by treatment with an SGLT2 inhibitor “may be linked to the reduction in atrial arrhythmias.”
The study did not show an association of SGLT2-inhibitor use and a change in ventricular arrhythmias, compared with patients with CIEDs who did not receive an agent from this class.
The findings suggest “expanding the possible indications for SGLT2 inhibitors,” commented Harriette G.C. Van Spall, MD, a cardiologist at McMaster University, Hamilton, Ont., who moderated the session where Dr. Goldenberg gave his report.
The study included 13,890 consecutive, prospectively enrolled patients who received a CIED during January 2015–April 2020 at any of five hospitals operated by either of two tertiary health care systems, one run by the University of Rochester and the second based at Sheba Medical Center in Tel HaShomer, Israel. The devices that made patients eligible for the study included permanent pacemakers, implantable cardioverter defibrillators, cardiac resynchronization therapy devices, and implantable cardiac monitors. A blinded adjudication committee composed of electrophysiologists identified the arrhythmic episodes.
At entry into the study (the time of device implantation), 12,992 patients were not receiving an SGLT2 inhibitor (94%) and 898 (6%) were receiving a drug from this class. Of those, 39% were on dapagliflozin (Farxiga), 35% were on empagliflozin (Jardiance), and 26% were on canagliflozin (Invokana).
Patients receiving an SGLT2 inhibitor at baseline were on average substantially younger than the patients not on this drug class (59 years vs. 69 years); they had a substantially higher prevalence of diabetes (78% vs. 25%), and ischemic cardiomyopathy (63% vs. 39%). Patients on an SGLT2 inhibitor at baseline also had more modestly higher prevalence rates of prior heart failure (38% vs. 31%), and hypertension (69% vs. 63%). Prevalence of a history of atrial fibrillation (AFib) was nearly the same in both groups: 31% in patients on an SGLT2 inhibitor and 35% in those not on these drugs.
The study’s primary endpoint was the total number of arrhythmia events during follow-up of 24,442 patient-years, during which patients exhibited 19,633 atrial arrhythmia events and 3,231 ventricular arrhythmia events.
1% absolute reduction in atrial arrhythmias
A multivariate analysis of the entire population – adjusted for baseline differences in age, diabetes, sex, and history of AFib – showed that treatment with an SGLT2 inhibitor at baseline was linked with a significant 24% relative reduction in incident atrial arrhythmia events, a significant 24% reduction in both atrial and ventricular arrhythmia events, and a 42% relative reduction in all-cause deaths, compared with no SGLT2-inhibitor treatment.
The only analyzed endpoint that showed no significant between-group difference was incidence of ventricular arrhythmias, which was a relative 7% lower in the SGLT2-inhibitor group.
On an absolute basis, treatment with an SGLT2 inhibitor was tied to about a 1% lower rate of atrial arrhythmia events per year, a reduction from a 2.5% rate in those not on an SGLT2 inhibitor to about a 1.5% rate in those taking this drug class.
A second, confirmatory analysis used propensity score matching to identify 5,323 patients not on an SGLT2 inhibitor at baseline who closely matched the 898 patients on an SGLT2 inhibitor. The multivariate modeling for this analysis also adjusted for age, diabetes, sex, and history of AFib.
The results of these analyses closely matched the calculations that used the entire study population. Relative to patients not on an SGLT2 inhibitor those on a drug from this class had 23% fewer atrial arrhythmias, 44% fewer total death, and 22% fewer atrial or ventricular arrhythmias, all significant differences. However, ventricular arrhythmias only reduced by a relative 5%, a nonsignificant difference.
In the propensity score–matched analysis, the absolute reduction in atrial arrhythmias in those on an SGLT2 inhibitor at baseline was roughly 1.3% fewer per year, compared with those not on this drug class.
The study was funded by an unrestricted grant to the University of Rochester from AstraZeneca, the company that markets the SGLT2 inhibitor dapagliflozin (Farxiga). Dr. Goldenberg and Dr. Van Spall had no disclosures.
Patients with cardiac implantable electronic devices (CIEDs) who received treatment with an sodium-glucose cotransporter 2 inhibitor had significantly fewer atrial arrhythmia events, compared with those who never received such a drug, in a prospective analysis of nearly 14,000 patients with a device who were followed for an average of nearly 2 years.
The findings suggest that use of an agent from the class of SGLT2 inhibitors “is associated with a pronounced reduction in atrial arrhythmia burden and all-cause mortality in patients with a CIED in a real-world setting,” said Ilan Goldenberg, MD, at the American Heart Association scientific sessions. “These data indicate possible antiarrhythmic properties of SGLT2 inhibitors that are incremental to the beneficial effects of the drug on heart failure outcomes,” added Dr. Goldenberg, director of the Clinical Cardiovascular Research Center at the University of Rochester (N.Y.).
In a propensity score–matched analysis that included more than 5,000 of the enrolled patients with a CIED, treatment with an SGLT2 inhibitor was tied to a significant 23% relative reduction in atrial arrhythmia events and a 44% relative drop in all-cause death, he reported.
Effect mediated by reduced left atrial pressure?
“Other heart failure drugs have shown some decrease in the rate of sudden cardiac death, but this is the first [heart failure] drug to associate with a reduction in atrial arrhythmias,” Dr. Goldenberg noted. “We think that a reduction in left atrial pressure” produced by treatment with an SGLT2 inhibitor “may be linked to the reduction in atrial arrhythmias.”
The study did not show an association of SGLT2-inhibitor use and a change in ventricular arrhythmias, compared with patients with CIEDs who did not receive an agent from this class.
The findings suggest “expanding the possible indications for SGLT2 inhibitors,” commented Harriette G.C. Van Spall, MD, a cardiologist at McMaster University, Hamilton, Ont., who moderated the session where Dr. Goldenberg gave his report.
The study included 13,890 consecutive, prospectively enrolled patients who received a CIED during January 2015–April 2020 at any of five hospitals operated by either of two tertiary health care systems, one run by the University of Rochester and the second based at Sheba Medical Center in Tel HaShomer, Israel. The devices that made patients eligible for the study included permanent pacemakers, implantable cardioverter defibrillators, cardiac resynchronization therapy devices, and implantable cardiac monitors. A blinded adjudication committee composed of electrophysiologists identified the arrhythmic episodes.
At entry into the study (the time of device implantation), 12,992 patients were not receiving an SGLT2 inhibitor (94%) and 898 (6%) were receiving a drug from this class. Of those, 39% were on dapagliflozin (Farxiga), 35% were on empagliflozin (Jardiance), and 26% were on canagliflozin (Invokana).
Patients receiving an SGLT2 inhibitor at baseline were on average substantially younger than the patients not on this drug class (59 years vs. 69 years); they had a substantially higher prevalence of diabetes (78% vs. 25%), and ischemic cardiomyopathy (63% vs. 39%). Patients on an SGLT2 inhibitor at baseline also had more modestly higher prevalence rates of prior heart failure (38% vs. 31%), and hypertension (69% vs. 63%). Prevalence of a history of atrial fibrillation (AFib) was nearly the same in both groups: 31% in patients on an SGLT2 inhibitor and 35% in those not on these drugs.
The study’s primary endpoint was the total number of arrhythmia events during follow-up of 24,442 patient-years, during which patients exhibited 19,633 atrial arrhythmia events and 3,231 ventricular arrhythmia events.
1% absolute reduction in atrial arrhythmias
A multivariate analysis of the entire population – adjusted for baseline differences in age, diabetes, sex, and history of AFib – showed that treatment with an SGLT2 inhibitor at baseline was linked with a significant 24% relative reduction in incident atrial arrhythmia events, a significant 24% reduction in both atrial and ventricular arrhythmia events, and a 42% relative reduction in all-cause deaths, compared with no SGLT2-inhibitor treatment.
The only analyzed endpoint that showed no significant between-group difference was incidence of ventricular arrhythmias, which was a relative 7% lower in the SGLT2-inhibitor group.
On an absolute basis, treatment with an SGLT2 inhibitor was tied to about a 1% lower rate of atrial arrhythmia events per year, a reduction from a 2.5% rate in those not on an SGLT2 inhibitor to about a 1.5% rate in those taking this drug class.
A second, confirmatory analysis used propensity score matching to identify 5,323 patients not on an SGLT2 inhibitor at baseline who closely matched the 898 patients on an SGLT2 inhibitor. The multivariate modeling for this analysis also adjusted for age, diabetes, sex, and history of AFib.
The results of these analyses closely matched the calculations that used the entire study population. Relative to patients not on an SGLT2 inhibitor those on a drug from this class had 23% fewer atrial arrhythmias, 44% fewer total death, and 22% fewer atrial or ventricular arrhythmias, all significant differences. However, ventricular arrhythmias only reduced by a relative 5%, a nonsignificant difference.
In the propensity score–matched analysis, the absolute reduction in atrial arrhythmias in those on an SGLT2 inhibitor at baseline was roughly 1.3% fewer per year, compared with those not on this drug class.
The study was funded by an unrestricted grant to the University of Rochester from AstraZeneca, the company that markets the SGLT2 inhibitor dapagliflozin (Farxiga). Dr. Goldenberg and Dr. Van Spall had no disclosures.
FROM AHA 2021
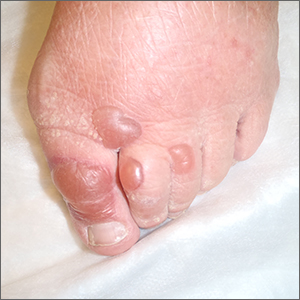
Bullae on elderly woman’s toes
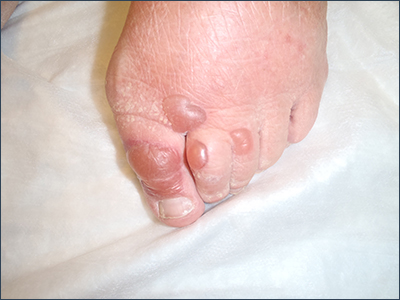
A biopsy was performed and sent for immunofluorescence; the results were negative. This, along with the patient’s history of diabetes, led us to the diagnosis of bullosis diabeticorum (BD). This condition, also known as bullous disease of diabetes, is characterized by abrupt development of noninflammatory bullae on acral areas in patients with diabetes.
The etiology of BD is unknown. The acral location suggests that trauma may be a contributing factor. Although electron microscopy has suggested an abnormality in anchoring fibrils, this cellular change does not fully explain the development of multiple blisters at varying sites.
A diagnosis of BD can be made when biopsy with immunofluorescence excludes other histologically similar diagnoses such as epidermolysis bullosa, noninflammatory bullous pemphigoid, and porphyria cutanea tarda. And, while immunofluorescence findings are typically negative, elevated levels of immunoglobulin M and C3 have, on occasion, been reported.1,2 Cultures are warranted only if a secondary infection is suspected.
The distribution of lesions and the presence—or absence—of systemic symptoms go a long way toward narrowing the differential of blistering diseases. The presence of generalized blistering and systemic symptoms would suggest conditions related to medication exposure, such as Stevens-Johnson syndrome or toxic epidermal necrolysis; infectious etiologies (eg, staphylococcal scalded skin syndrome); autoimmune causes; or underlying malignancy.3 Generalized blistering in the absence of systemic symptoms would support diagnoses such as bullous impetigo and pemphigoid.3
The blisters associated with BD spontaneously resolve over several weeks without treatment but tend to recur. The lesions typically heal without significant scarring, although they may have a darker pigmentation after the first occurrence. Treatment may be warranted if a patient develops a secondary infection. For this patient, the bullae resolved within 2 weeks without treatment, although mild hyperpigmentation remained.
This case was adapted from: Mims L, Savage A, Chessman A. Blisters on an elderly woman’s toes. J Fam Pract. 2014;63:273-274.
1. James WD, Odom RB, Goette DK. Bullous eruption of diabetes. A case with positive immunofluorescence microscopy findings. Arch Dermatol. 1980;116:1191-1192.
2. Basarab T, Munn SE, McGrath J, et al. Bullous diabeticorum. A case report and literature review. Clin Exp Dermatol. 1995;20:218-220. doi: 10.1111/j.1365-2230.1995.tb01305.x
3. Hull C, Zone JJ. Approach to the patient with cutaneous blisters. UpToDate. Updated July 30, 2019. Accessed September 14, 2021. www.uptodate.com/contents/approach-to-the-patient-with-cutaneous-blisters

A biopsy was performed and sent for immunofluorescence; the results were negative. This, along with the patient’s history of diabetes, led us to the diagnosis of bullosis diabeticorum (BD). This condition, also known as bullous disease of diabetes, is characterized by abrupt development of noninflammatory bullae on acral areas in patients with diabetes.
The etiology of BD is unknown. The acral location suggests that trauma may be a contributing factor. Although electron microscopy has suggested an abnormality in anchoring fibrils, this cellular change does not fully explain the development of multiple blisters at varying sites.
A diagnosis of BD can be made when biopsy with immunofluorescence excludes other histologically similar diagnoses such as epidermolysis bullosa, noninflammatory bullous pemphigoid, and porphyria cutanea tarda. And, while immunofluorescence findings are typically negative, elevated levels of immunoglobulin M and C3 have, on occasion, been reported.1,2 Cultures are warranted only if a secondary infection is suspected.
The distribution of lesions and the presence—or absence—of systemic symptoms go a long way toward narrowing the differential of blistering diseases. The presence of generalized blistering and systemic symptoms would suggest conditions related to medication exposure, such as Stevens-Johnson syndrome or toxic epidermal necrolysis; infectious etiologies (eg, staphylococcal scalded skin syndrome); autoimmune causes; or underlying malignancy.3 Generalized blistering in the absence of systemic symptoms would support diagnoses such as bullous impetigo and pemphigoid.3
The blisters associated with BD spontaneously resolve over several weeks without treatment but tend to recur. The lesions typically heal without significant scarring, although they may have a darker pigmentation after the first occurrence. Treatment may be warranted if a patient develops a secondary infection. For this patient, the bullae resolved within 2 weeks without treatment, although mild hyperpigmentation remained.
This case was adapted from: Mims L, Savage A, Chessman A. Blisters on an elderly woman’s toes. J Fam Pract. 2014;63:273-274.

A biopsy was performed and sent for immunofluorescence; the results were negative. This, along with the patient’s history of diabetes, led us to the diagnosis of bullosis diabeticorum (BD). This condition, also known as bullous disease of diabetes, is characterized by abrupt development of noninflammatory bullae on acral areas in patients with diabetes.
The etiology of BD is unknown. The acral location suggests that trauma may be a contributing factor. Although electron microscopy has suggested an abnormality in anchoring fibrils, this cellular change does not fully explain the development of multiple blisters at varying sites.
A diagnosis of BD can be made when biopsy with immunofluorescence excludes other histologically similar diagnoses such as epidermolysis bullosa, noninflammatory bullous pemphigoid, and porphyria cutanea tarda. And, while immunofluorescence findings are typically negative, elevated levels of immunoglobulin M and C3 have, on occasion, been reported.1,2 Cultures are warranted only if a secondary infection is suspected.
The distribution of lesions and the presence—or absence—of systemic symptoms go a long way toward narrowing the differential of blistering diseases. The presence of generalized blistering and systemic symptoms would suggest conditions related to medication exposure, such as Stevens-Johnson syndrome or toxic epidermal necrolysis; infectious etiologies (eg, staphylococcal scalded skin syndrome); autoimmune causes; or underlying malignancy.3 Generalized blistering in the absence of systemic symptoms would support diagnoses such as bullous impetigo and pemphigoid.3
The blisters associated with BD spontaneously resolve over several weeks without treatment but tend to recur. The lesions typically heal without significant scarring, although they may have a darker pigmentation after the first occurrence. Treatment may be warranted if a patient develops a secondary infection. For this patient, the bullae resolved within 2 weeks without treatment, although mild hyperpigmentation remained.
This case was adapted from: Mims L, Savage A, Chessman A. Blisters on an elderly woman’s toes. J Fam Pract. 2014;63:273-274.
1. James WD, Odom RB, Goette DK. Bullous eruption of diabetes. A case with positive immunofluorescence microscopy findings. Arch Dermatol. 1980;116:1191-1192.
2. Basarab T, Munn SE, McGrath J, et al. Bullous diabeticorum. A case report and literature review. Clin Exp Dermatol. 1995;20:218-220. doi: 10.1111/j.1365-2230.1995.tb01305.x
3. Hull C, Zone JJ. Approach to the patient with cutaneous blisters. UpToDate. Updated July 30, 2019. Accessed September 14, 2021. www.uptodate.com/contents/approach-to-the-patient-with-cutaneous-blisters
1. James WD, Odom RB, Goette DK. Bullous eruption of diabetes. A case with positive immunofluorescence microscopy findings. Arch Dermatol. 1980;116:1191-1192.
2. Basarab T, Munn SE, McGrath J, et al. Bullous diabeticorum. A case report and literature review. Clin Exp Dermatol. 1995;20:218-220. doi: 10.1111/j.1365-2230.1995.tb01305.x
3. Hull C, Zone JJ. Approach to the patient with cutaneous blisters. UpToDate. Updated July 30, 2019. Accessed September 14, 2021. www.uptodate.com/contents/approach-to-the-patient-with-cutaneous-blisters
Could an oral PCSK9 inhibitor be on the horizon?
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Swell in off-label antipsychotic prescribing ‘not harmless’
A growing trend of off-label, low-dose antipsychotic prescribing to treat disorders such as anxiety and insomnia has been tied to an increased risk of cardiometabolic death, new research shows.
Investigators studied data from large Swedish registries on over 420,000 individuals without previous psychotic, bipolar, or cardiometabolic disorders and found that off-label treatment with olanzapine or quetiapine for 6 to 12 months – even at a low dose – was associated with an almost twofold higher risk of cardiometabolic mortality, compared to no treatment. The risk remained elevated after 12 months, but the finding was not deemed significant.
“Clinicians should be made aware that low-dose treatment with these drugs is probably not a harmless choice for insomnia and anxiety, and while they have the benefit of not being addictive and [are] seemingly effective, they might come at a cost of shortening patients’ life span,” study investigator Jonas Berge, MD, PhD, associate professor and resident psychiatrist, Lund University, Sweden, said in an interview.
“Clinicians should take this information into account when prescribing the drugs and also monitor the patients with regular physical examinations and blood tests in the same way as when treating patients with psychosis with higher doses of these drugs,” he said.
The study was published online Nov. 9 in the Journal of Psychiatric Research.
A growing trend
Use of low-dose antipsychotics to treat a variety of psychiatric and behavioral disturbances, including anxiety, insomnia, and agitation, has “surged in popularity,” the authors wrote.
Quetiapine and olanzapine “rank as two of the most frequently prescribed second-generation antipsychotics and, next to clozapine, are considered to exhort the highest risk for cardiometabolic sequelae, including components of metabolic syndrome,” they added.
Previous research examining the association between second-generation antipsychotics and placebo has either not focused on cardiometabolic-specific causes or has examined only cohorts with severe mental illness, so those findings “do not necessarily generalize to others treated off-label,” they noted.
“The motivation for the study came from my work as a psychiatrist, in which I’ve noticed that the off-label use of these medications [olanzapine and quetiapine] for anxiety and insomnia seems highly prevalent, and that many patients seem to gain a lot of weight, despite low doses,” Dr. Berge said.
There is “evidence to suggest that clinicians may underappreciate cardiometabolic risks owing to antipsychotic treatment, as routine screening is often incomplete or inconsistent,” the authors noted.
“To do a risk-benefit analysis of these drugs in low doses, the risks involved – as well as the effects, of course – need to be studied,” Dr. Berge stated.
To investigate the question, the researchers turned to three large cross-linked Swedish registers: the National Patient Register, containing demographic and medical data; the Prescribed Drug Register; and the Cause of Death Register.
They identified all individuals aged 18 years and older with at least one psychiatric visit (inpatient or outpatient) between July 1, 2006, and Dec. 31, 2016, to see how many were prescribed low-dose olanzapine or quetiapine (defined as ≤ 5 mg/day of olanzapine or olanzapine equivalent [OE]), which was used as a proxy marker for off-label treatment, since this dose is considered subtherapeutic for severe mental illness.
They calculated two time-dependent variables – cumulative dose and past annual average dose – and then used those to compute three different exposure valuables: those treated with low-dose OE; cumulative exposure (i.e., period treated with an average 5 mg/day); and a continuous variable “corresponding to each year exposed OE 5 mg/day.”
The primary outcome was set as mortality from cardiometabolic-related disorders, while secondary outcomes were disease-specific and all-cause mortality.
‘Weak’ association
The final cohort consisted of 428,525 individuals (mean [SD] age, 36.8 [15.4] years, 52.7% female) at baseline, with observation taking place over a mean of 4.8 years [range, 1 day to 10.5 years]) or a total of over 2 million (2,062,241) person-years.
Of the cohort, 4.3% (n = 18,317) had at least two prescriptions for either olanzapine or quetiapine (although subsequently, 86.5% were censored for exceeding the average OE dose of 5 mg/day).
By the end of the study, 3.1% of the cohort had died during the observation time, and of these, 69.5% were from disease-specific causes, while close to one-fifth (19.5%) were from cardiometabolic-specific causes.
On the whole, treatment status (i.e., treated vs. untreated) was not significantly associated with cardiometabolic mortality (adjusted hazard ratio [HR], .86 [95% confidence interval, 0.64-1.15]; P = .307).
Compared to no treatment, treatment with olanzapine or quetiapine for less than 6 months was significantly associated with a reduced risk of cardiovascular mortality (adjusted HR, .56 [.37 – .87]; P = .010). On the other hand, treatment for 6-12 months was significantly associated with an almost twofold increased risk (adjusted HR, 1.89 [1.22-2.92]; P = .004). The increased risk continued beyond 12 months, although the difference no longer remained significant.
“In the subgroup analysis consisting of individuals who had ever been treated with olanzapine/quetiapine, starting at the date of their first prescription, the hazard for cardiometabolic mortality increased significantly by 45% (6%-99%; P = .019) for every year exposed to an average 5 mg/day,” the authors reported.
The authors concluded that the association between low-dose olanzapine/quetiapine treatment and cardiometabolic mortality was present, but “weak.”
The hazard for disease-specific mortality also significantly increased with each year exposed to an average of 5 mg/day of OE (HR, 1.24 [1.03-1.50]; P = .026).
Treatment status similarly was associated with all-cause mortality (HR, 1.16 [1.03-1.30]; P = .012), although the increased hazard for all-cause mortality with each year of exposure was not considered significant.
“The findings of this study are consistent with the hypothesis that continuous low-dose treatment with these drugs is associated with increased cardiometabolic mortality, but ,” Dr. Berge said.
Seek alternatives
Commenting on the study for this news organization, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, called it a “timely paper” and “an important concept [because] low-doses of these antipsychotics are frequently prescribed across America and there has been less data on the safety [of these antipsychotics at lower doses].”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, said that this “important report reminds us that there are metabolic safety concerns, even at low doses, where these medications are often used off label.”
He advised clinicians to “seek alternatives, and alternatives that are on-label, for conditions like anxiety and sleep disturbances.”
This work was supported by the South Region Board ALF, Sweden. Dr. Berge and coauthors have disclosed no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is CEO of AltMed.
A version of this article first appeared on Medscape.com.
A growing trend of off-label, low-dose antipsychotic prescribing to treat disorders such as anxiety and insomnia has been tied to an increased risk of cardiometabolic death, new research shows.
Investigators studied data from large Swedish registries on over 420,000 individuals without previous psychotic, bipolar, or cardiometabolic disorders and found that off-label treatment with olanzapine or quetiapine for 6 to 12 months – even at a low dose – was associated with an almost twofold higher risk of cardiometabolic mortality, compared to no treatment. The risk remained elevated after 12 months, but the finding was not deemed significant.
“Clinicians should be made aware that low-dose treatment with these drugs is probably not a harmless choice for insomnia and anxiety, and while they have the benefit of not being addictive and [are] seemingly effective, they might come at a cost of shortening patients’ life span,” study investigator Jonas Berge, MD, PhD, associate professor and resident psychiatrist, Lund University, Sweden, said in an interview.
“Clinicians should take this information into account when prescribing the drugs and also monitor the patients with regular physical examinations and blood tests in the same way as when treating patients with psychosis with higher doses of these drugs,” he said.
The study was published online Nov. 9 in the Journal of Psychiatric Research.
A growing trend
Use of low-dose antipsychotics to treat a variety of psychiatric and behavioral disturbances, including anxiety, insomnia, and agitation, has “surged in popularity,” the authors wrote.
Quetiapine and olanzapine “rank as two of the most frequently prescribed second-generation antipsychotics and, next to clozapine, are considered to exhort the highest risk for cardiometabolic sequelae, including components of metabolic syndrome,” they added.
Previous research examining the association between second-generation antipsychotics and placebo has either not focused on cardiometabolic-specific causes or has examined only cohorts with severe mental illness, so those findings “do not necessarily generalize to others treated off-label,” they noted.
“The motivation for the study came from my work as a psychiatrist, in which I’ve noticed that the off-label use of these medications [olanzapine and quetiapine] for anxiety and insomnia seems highly prevalent, and that many patients seem to gain a lot of weight, despite low doses,” Dr. Berge said.
There is “evidence to suggest that clinicians may underappreciate cardiometabolic risks owing to antipsychotic treatment, as routine screening is often incomplete or inconsistent,” the authors noted.
“To do a risk-benefit analysis of these drugs in low doses, the risks involved – as well as the effects, of course – need to be studied,” Dr. Berge stated.
To investigate the question, the researchers turned to three large cross-linked Swedish registers: the National Patient Register, containing demographic and medical data; the Prescribed Drug Register; and the Cause of Death Register.
They identified all individuals aged 18 years and older with at least one psychiatric visit (inpatient or outpatient) between July 1, 2006, and Dec. 31, 2016, to see how many were prescribed low-dose olanzapine or quetiapine (defined as ≤ 5 mg/day of olanzapine or olanzapine equivalent [OE]), which was used as a proxy marker for off-label treatment, since this dose is considered subtherapeutic for severe mental illness.
They calculated two time-dependent variables – cumulative dose and past annual average dose – and then used those to compute three different exposure valuables: those treated with low-dose OE; cumulative exposure (i.e., period treated with an average 5 mg/day); and a continuous variable “corresponding to each year exposed OE 5 mg/day.”
The primary outcome was set as mortality from cardiometabolic-related disorders, while secondary outcomes were disease-specific and all-cause mortality.
‘Weak’ association
The final cohort consisted of 428,525 individuals (mean [SD] age, 36.8 [15.4] years, 52.7% female) at baseline, with observation taking place over a mean of 4.8 years [range, 1 day to 10.5 years]) or a total of over 2 million (2,062,241) person-years.
Of the cohort, 4.3% (n = 18,317) had at least two prescriptions for either olanzapine or quetiapine (although subsequently, 86.5% were censored for exceeding the average OE dose of 5 mg/day).
By the end of the study, 3.1% of the cohort had died during the observation time, and of these, 69.5% were from disease-specific causes, while close to one-fifth (19.5%) were from cardiometabolic-specific causes.
On the whole, treatment status (i.e., treated vs. untreated) was not significantly associated with cardiometabolic mortality (adjusted hazard ratio [HR], .86 [95% confidence interval, 0.64-1.15]; P = .307).
Compared to no treatment, treatment with olanzapine or quetiapine for less than 6 months was significantly associated with a reduced risk of cardiovascular mortality (adjusted HR, .56 [.37 – .87]; P = .010). On the other hand, treatment for 6-12 months was significantly associated with an almost twofold increased risk (adjusted HR, 1.89 [1.22-2.92]; P = .004). The increased risk continued beyond 12 months, although the difference no longer remained significant.
“In the subgroup analysis consisting of individuals who had ever been treated with olanzapine/quetiapine, starting at the date of their first prescription, the hazard for cardiometabolic mortality increased significantly by 45% (6%-99%; P = .019) for every year exposed to an average 5 mg/day,” the authors reported.
The authors concluded that the association between low-dose olanzapine/quetiapine treatment and cardiometabolic mortality was present, but “weak.”
The hazard for disease-specific mortality also significantly increased with each year exposed to an average of 5 mg/day of OE (HR, 1.24 [1.03-1.50]; P = .026).
Treatment status similarly was associated with all-cause mortality (HR, 1.16 [1.03-1.30]; P = .012), although the increased hazard for all-cause mortality with each year of exposure was not considered significant.
“The findings of this study are consistent with the hypothesis that continuous low-dose treatment with these drugs is associated with increased cardiometabolic mortality, but ,” Dr. Berge said.
Seek alternatives
Commenting on the study for this news organization, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, called it a “timely paper” and “an important concept [because] low-doses of these antipsychotics are frequently prescribed across America and there has been less data on the safety [of these antipsychotics at lower doses].”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, said that this “important report reminds us that there are metabolic safety concerns, even at low doses, where these medications are often used off label.”
He advised clinicians to “seek alternatives, and alternatives that are on-label, for conditions like anxiety and sleep disturbances.”
This work was supported by the South Region Board ALF, Sweden. Dr. Berge and coauthors have disclosed no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is CEO of AltMed.
A version of this article first appeared on Medscape.com.
A growing trend of off-label, low-dose antipsychotic prescribing to treat disorders such as anxiety and insomnia has been tied to an increased risk of cardiometabolic death, new research shows.
Investigators studied data from large Swedish registries on over 420,000 individuals without previous psychotic, bipolar, or cardiometabolic disorders and found that off-label treatment with olanzapine or quetiapine for 6 to 12 months – even at a low dose – was associated with an almost twofold higher risk of cardiometabolic mortality, compared to no treatment. The risk remained elevated after 12 months, but the finding was not deemed significant.
“Clinicians should be made aware that low-dose treatment with these drugs is probably not a harmless choice for insomnia and anxiety, and while they have the benefit of not being addictive and [are] seemingly effective, they might come at a cost of shortening patients’ life span,” study investigator Jonas Berge, MD, PhD, associate professor and resident psychiatrist, Lund University, Sweden, said in an interview.
“Clinicians should take this information into account when prescribing the drugs and also monitor the patients with regular physical examinations and blood tests in the same way as when treating patients with psychosis with higher doses of these drugs,” he said.
The study was published online Nov. 9 in the Journal of Psychiatric Research.
A growing trend
Use of low-dose antipsychotics to treat a variety of psychiatric and behavioral disturbances, including anxiety, insomnia, and agitation, has “surged in popularity,” the authors wrote.
Quetiapine and olanzapine “rank as two of the most frequently prescribed second-generation antipsychotics and, next to clozapine, are considered to exhort the highest risk for cardiometabolic sequelae, including components of metabolic syndrome,” they added.
Previous research examining the association between second-generation antipsychotics and placebo has either not focused on cardiometabolic-specific causes or has examined only cohorts with severe mental illness, so those findings “do not necessarily generalize to others treated off-label,” they noted.
“The motivation for the study came from my work as a psychiatrist, in which I’ve noticed that the off-label use of these medications [olanzapine and quetiapine] for anxiety and insomnia seems highly prevalent, and that many patients seem to gain a lot of weight, despite low doses,” Dr. Berge said.
There is “evidence to suggest that clinicians may underappreciate cardiometabolic risks owing to antipsychotic treatment, as routine screening is often incomplete or inconsistent,” the authors noted.
“To do a risk-benefit analysis of these drugs in low doses, the risks involved – as well as the effects, of course – need to be studied,” Dr. Berge stated.
To investigate the question, the researchers turned to three large cross-linked Swedish registers: the National Patient Register, containing demographic and medical data; the Prescribed Drug Register; and the Cause of Death Register.
They identified all individuals aged 18 years and older with at least one psychiatric visit (inpatient or outpatient) between July 1, 2006, and Dec. 31, 2016, to see how many were prescribed low-dose olanzapine or quetiapine (defined as ≤ 5 mg/day of olanzapine or olanzapine equivalent [OE]), which was used as a proxy marker for off-label treatment, since this dose is considered subtherapeutic for severe mental illness.
They calculated two time-dependent variables – cumulative dose and past annual average dose – and then used those to compute three different exposure valuables: those treated with low-dose OE; cumulative exposure (i.e., period treated with an average 5 mg/day); and a continuous variable “corresponding to each year exposed OE 5 mg/day.”
The primary outcome was set as mortality from cardiometabolic-related disorders, while secondary outcomes were disease-specific and all-cause mortality.
‘Weak’ association
The final cohort consisted of 428,525 individuals (mean [SD] age, 36.8 [15.4] years, 52.7% female) at baseline, with observation taking place over a mean of 4.8 years [range, 1 day to 10.5 years]) or a total of over 2 million (2,062,241) person-years.
Of the cohort, 4.3% (n = 18,317) had at least two prescriptions for either olanzapine or quetiapine (although subsequently, 86.5% were censored for exceeding the average OE dose of 5 mg/day).
By the end of the study, 3.1% of the cohort had died during the observation time, and of these, 69.5% were from disease-specific causes, while close to one-fifth (19.5%) were from cardiometabolic-specific causes.
On the whole, treatment status (i.e., treated vs. untreated) was not significantly associated with cardiometabolic mortality (adjusted hazard ratio [HR], .86 [95% confidence interval, 0.64-1.15]; P = .307).
Compared to no treatment, treatment with olanzapine or quetiapine for less than 6 months was significantly associated with a reduced risk of cardiovascular mortality (adjusted HR, .56 [.37 – .87]; P = .010). On the other hand, treatment for 6-12 months was significantly associated with an almost twofold increased risk (adjusted HR, 1.89 [1.22-2.92]; P = .004). The increased risk continued beyond 12 months, although the difference no longer remained significant.
“In the subgroup analysis consisting of individuals who had ever been treated with olanzapine/quetiapine, starting at the date of their first prescription, the hazard for cardiometabolic mortality increased significantly by 45% (6%-99%; P = .019) for every year exposed to an average 5 mg/day,” the authors reported.
The authors concluded that the association between low-dose olanzapine/quetiapine treatment and cardiometabolic mortality was present, but “weak.”
The hazard for disease-specific mortality also significantly increased with each year exposed to an average of 5 mg/day of OE (HR, 1.24 [1.03-1.50]; P = .026).
Treatment status similarly was associated with all-cause mortality (HR, 1.16 [1.03-1.30]; P = .012), although the increased hazard for all-cause mortality with each year of exposure was not considered significant.
“The findings of this study are consistent with the hypothesis that continuous low-dose treatment with these drugs is associated with increased cardiometabolic mortality, but ,” Dr. Berge said.
Seek alternatives
Commenting on the study for this news organization, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, called it a “timely paper” and “an important concept [because] low-doses of these antipsychotics are frequently prescribed across America and there has been less data on the safety [of these antipsychotics at lower doses].”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, said that this “important report reminds us that there are metabolic safety concerns, even at low doses, where these medications are often used off label.”
He advised clinicians to “seek alternatives, and alternatives that are on-label, for conditions like anxiety and sleep disturbances.”
This work was supported by the South Region Board ALF, Sweden. Dr. Berge and coauthors have disclosed no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; and speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Eisai, Minerva, Intra-Cellular, and AbbVie. Dr. McIntyre is CEO of AltMed.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Type 2 diabetes remission can happen naturally in 1 in 20
Roughly 5% of adults with type 2 diabetes achieve remission of their disease, often unbeknownst to the patient and without aggressive weight-loss interventions, according to a new analysis of data from more than 160,000 people in a national diabetes registry in Scotland.
“One of our key new findings is that a reasonably large proportion of people [with type 2 diabetes] were able to achieve remission in routine care, without undergoing bariatric surgery and prior to the introduction of very-low-calorie interventions in routine care,” said Mireille Captieux, MBChB, lead author of the report, in an interview.
The findings “support previous reports that weight loss is associated with type 2 diabetes remission,” said Dr. Captieux, a diabetes researcher at the University of Edinburgh (Scotland).
In her analysis, two of the strongest correlates of remission related to weight loss.
First, a history of bariatric surgery, which included a scant 488 people (0.3% of the study cohort), was associated with a 13-fold increase in the rate of remission, compared with those who did not undergo bariatric surgery. Second, weight loss of 15 kg (33 lb) or more at the time of remission detection in 2019, in comparison with their weight at initial diabetes diagnosis, was linked with a greater than fourfold increase in the rate of remission, compared with those who did not have this amount of weight loss.
But “even losing a small amount of weight increased the chances of remission,” highlights Dr. Captieux. “This finding offers a counterbalance to the pessimistic assumption that almost all people find it very difficult to lose weight.”
Hopeful message, but which people achieve diabetes remission?
“What’s encouraging here is that you have people who probably did not do anything radical, and yet they went into remission. The next step is to find out who these people are and what they did to go into remission,” commented Julia Lawton, PhD, a professor of health and social science at the University of Edinburgh whose research focuses on how patients with diabetes manage their disorder.
“If we can understand who the patients are who can achieve remission without taking extreme measures, it could help people in the health professions get beyond their presumptions about who is, or is not, a good candidate for achieving diabetes remission,” said Dr. Lawton, who was not involved with the study.
The message from this study is “very hopeful,” Dr. Lawton said in an interview. “How can we make this opportunity [for diabetes remission] available to more people? What can we learn from these patients that we could then apply to other patients?”
Dr. Captieux agrees. Given her findings, an important next step is to find out more about the population in remission to better understand “their perspectives on the challenges and benefits of supporting weight loss.
“Obesity is a complex issue, and therefore weight loss interventions that target individual actions and behaviors are much more likely to be effective if they are accompanied by multiple interventions at different levels,” Dr. Captieux said.
In addition, “more evidence is needed to assess the sustainability of diabetes remission and the effect of different durations of remission for a clinically relevant definition.”
Duration, definition of diabetes remission
Dr. Captieux noted that the new international consensus definition of type 2 diabetes remission – which specifies a minimum 3-month duration of glycemic control to qualify as remission – means that people with diabetes “may frequently oscillate” between remission and active disease.
This makes it important to better define the effect of duration of diabetes remission regarding various diabetes complications.
Another issue raised by the new findings is the importance of distinguishing people who lose weight because of a healthier diet and increased activity from those who lose weight because of chronic illness or frailty that’s followed by long-term adverse outcomes.
If these two populations are not distinguished in an observational cohort study – such as the one run by Dr. Captieux and her associates – then the people with chronic illness might appear to have worse outcomes following diabetes remission.
Dr. Captieux and her coauthors used data collected in the Scottish Care Information–Diabetes registry, which includes almost all people diagnosed with diabetes in Scotland. They focused on people with diabetes who had first been diagnosed with diabetes during 2004-2018, who were at least 30 years old at the time of their initial diagnosis, and who had received care in the national health system during 2019.
This yielded a study cohort of 162,316 people, of whom 7,710 (4.8%) were identified by the researchers as being in remission in 2019.
Patients in remission were defined as those whose hemoglobin A1c level was less than 6.5% at their index reading in 2019 and whose A1c level could be documented as being lower than 6.5% for at least 1 year prior to the 2019 measurement.
In a primary logistic regression analysis, the authors identified five variables that were significantly linked with remission: age of at least 65 years (the association was even stronger for age older than 75 years), a lower A1c level at the time of initial diabetes diagnosis, weight loss, prior bariatric surgery, and no prior treatment with a glucose-lowering therapy.
The strongest association was with having had no prior treatment with a glucose-lowering therapy in 2019. People who met this criterion were nearly 15 times more likely to be in remission in 2019, compared with those who had received at least one of these agents.
The study received no commercial funding. Dr. Captieux and Dr. Lawton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Roughly 5% of adults with type 2 diabetes achieve remission of their disease, often unbeknownst to the patient and without aggressive weight-loss interventions, according to a new analysis of data from more than 160,000 people in a national diabetes registry in Scotland.
“One of our key new findings is that a reasonably large proportion of people [with type 2 diabetes] were able to achieve remission in routine care, without undergoing bariatric surgery and prior to the introduction of very-low-calorie interventions in routine care,” said Mireille Captieux, MBChB, lead author of the report, in an interview.
The findings “support previous reports that weight loss is associated with type 2 diabetes remission,” said Dr. Captieux, a diabetes researcher at the University of Edinburgh (Scotland).
In her analysis, two of the strongest correlates of remission related to weight loss.
First, a history of bariatric surgery, which included a scant 488 people (0.3% of the study cohort), was associated with a 13-fold increase in the rate of remission, compared with those who did not undergo bariatric surgery. Second, weight loss of 15 kg (33 lb) or more at the time of remission detection in 2019, in comparison with their weight at initial diabetes diagnosis, was linked with a greater than fourfold increase in the rate of remission, compared with those who did not have this amount of weight loss.
But “even losing a small amount of weight increased the chances of remission,” highlights Dr. Captieux. “This finding offers a counterbalance to the pessimistic assumption that almost all people find it very difficult to lose weight.”
Hopeful message, but which people achieve diabetes remission?
“What’s encouraging here is that you have people who probably did not do anything radical, and yet they went into remission. The next step is to find out who these people are and what they did to go into remission,” commented Julia Lawton, PhD, a professor of health and social science at the University of Edinburgh whose research focuses on how patients with diabetes manage their disorder.
“If we can understand who the patients are who can achieve remission without taking extreme measures, it could help people in the health professions get beyond their presumptions about who is, or is not, a good candidate for achieving diabetes remission,” said Dr. Lawton, who was not involved with the study.
The message from this study is “very hopeful,” Dr. Lawton said in an interview. “How can we make this opportunity [for diabetes remission] available to more people? What can we learn from these patients that we could then apply to other patients?”
Dr. Captieux agrees. Given her findings, an important next step is to find out more about the population in remission to better understand “their perspectives on the challenges and benefits of supporting weight loss.
“Obesity is a complex issue, and therefore weight loss interventions that target individual actions and behaviors are much more likely to be effective if they are accompanied by multiple interventions at different levels,” Dr. Captieux said.
In addition, “more evidence is needed to assess the sustainability of diabetes remission and the effect of different durations of remission for a clinically relevant definition.”
Duration, definition of diabetes remission
Dr. Captieux noted that the new international consensus definition of type 2 diabetes remission – which specifies a minimum 3-month duration of glycemic control to qualify as remission – means that people with diabetes “may frequently oscillate” between remission and active disease.
This makes it important to better define the effect of duration of diabetes remission regarding various diabetes complications.
Another issue raised by the new findings is the importance of distinguishing people who lose weight because of a healthier diet and increased activity from those who lose weight because of chronic illness or frailty that’s followed by long-term adverse outcomes.
If these two populations are not distinguished in an observational cohort study – such as the one run by Dr. Captieux and her associates – then the people with chronic illness might appear to have worse outcomes following diabetes remission.
Dr. Captieux and her coauthors used data collected in the Scottish Care Information–Diabetes registry, which includes almost all people diagnosed with diabetes in Scotland. They focused on people with diabetes who had first been diagnosed with diabetes during 2004-2018, who were at least 30 years old at the time of their initial diagnosis, and who had received care in the national health system during 2019.
This yielded a study cohort of 162,316 people, of whom 7,710 (4.8%) were identified by the researchers as being in remission in 2019.
Patients in remission were defined as those whose hemoglobin A1c level was less than 6.5% at their index reading in 2019 and whose A1c level could be documented as being lower than 6.5% for at least 1 year prior to the 2019 measurement.
In a primary logistic regression analysis, the authors identified five variables that were significantly linked with remission: age of at least 65 years (the association was even stronger for age older than 75 years), a lower A1c level at the time of initial diabetes diagnosis, weight loss, prior bariatric surgery, and no prior treatment with a glucose-lowering therapy.
The strongest association was with having had no prior treatment with a glucose-lowering therapy in 2019. People who met this criterion were nearly 15 times more likely to be in remission in 2019, compared with those who had received at least one of these agents.
The study received no commercial funding. Dr. Captieux and Dr. Lawton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Roughly 5% of adults with type 2 diabetes achieve remission of their disease, often unbeknownst to the patient and without aggressive weight-loss interventions, according to a new analysis of data from more than 160,000 people in a national diabetes registry in Scotland.
“One of our key new findings is that a reasonably large proportion of people [with type 2 diabetes] were able to achieve remission in routine care, without undergoing bariatric surgery and prior to the introduction of very-low-calorie interventions in routine care,” said Mireille Captieux, MBChB, lead author of the report, in an interview.
The findings “support previous reports that weight loss is associated with type 2 diabetes remission,” said Dr. Captieux, a diabetes researcher at the University of Edinburgh (Scotland).
In her analysis, two of the strongest correlates of remission related to weight loss.
First, a history of bariatric surgery, which included a scant 488 people (0.3% of the study cohort), was associated with a 13-fold increase in the rate of remission, compared with those who did not undergo bariatric surgery. Second, weight loss of 15 kg (33 lb) or more at the time of remission detection in 2019, in comparison with their weight at initial diabetes diagnosis, was linked with a greater than fourfold increase in the rate of remission, compared with those who did not have this amount of weight loss.
But “even losing a small amount of weight increased the chances of remission,” highlights Dr. Captieux. “This finding offers a counterbalance to the pessimistic assumption that almost all people find it very difficult to lose weight.”
Hopeful message, but which people achieve diabetes remission?
“What’s encouraging here is that you have people who probably did not do anything radical, and yet they went into remission. The next step is to find out who these people are and what they did to go into remission,” commented Julia Lawton, PhD, a professor of health and social science at the University of Edinburgh whose research focuses on how patients with diabetes manage their disorder.
“If we can understand who the patients are who can achieve remission without taking extreme measures, it could help people in the health professions get beyond their presumptions about who is, or is not, a good candidate for achieving diabetes remission,” said Dr. Lawton, who was not involved with the study.
The message from this study is “very hopeful,” Dr. Lawton said in an interview. “How can we make this opportunity [for diabetes remission] available to more people? What can we learn from these patients that we could then apply to other patients?”
Dr. Captieux agrees. Given her findings, an important next step is to find out more about the population in remission to better understand “their perspectives on the challenges and benefits of supporting weight loss.
“Obesity is a complex issue, and therefore weight loss interventions that target individual actions and behaviors are much more likely to be effective if they are accompanied by multiple interventions at different levels,” Dr. Captieux said.
In addition, “more evidence is needed to assess the sustainability of diabetes remission and the effect of different durations of remission for a clinically relevant definition.”
Duration, definition of diabetes remission
Dr. Captieux noted that the new international consensus definition of type 2 diabetes remission – which specifies a minimum 3-month duration of glycemic control to qualify as remission – means that people with diabetes “may frequently oscillate” between remission and active disease.
This makes it important to better define the effect of duration of diabetes remission regarding various diabetes complications.
Another issue raised by the new findings is the importance of distinguishing people who lose weight because of a healthier diet and increased activity from those who lose weight because of chronic illness or frailty that’s followed by long-term adverse outcomes.
If these two populations are not distinguished in an observational cohort study – such as the one run by Dr. Captieux and her associates – then the people with chronic illness might appear to have worse outcomes following diabetes remission.
Dr. Captieux and her coauthors used data collected in the Scottish Care Information–Diabetes registry, which includes almost all people diagnosed with diabetes in Scotland. They focused on people with diabetes who had first been diagnosed with diabetes during 2004-2018, who were at least 30 years old at the time of their initial diagnosis, and who had received care in the national health system during 2019.
This yielded a study cohort of 162,316 people, of whom 7,710 (4.8%) were identified by the researchers as being in remission in 2019.
Patients in remission were defined as those whose hemoglobin A1c level was less than 6.5% at their index reading in 2019 and whose A1c level could be documented as being lower than 6.5% for at least 1 year prior to the 2019 measurement.
In a primary logistic regression analysis, the authors identified five variables that were significantly linked with remission: age of at least 65 years (the association was even stronger for age older than 75 years), a lower A1c level at the time of initial diabetes diagnosis, weight loss, prior bariatric surgery, and no prior treatment with a glucose-lowering therapy.
The strongest association was with having had no prior treatment with a glucose-lowering therapy in 2019. People who met this criterion were nearly 15 times more likely to be in remission in 2019, compared with those who had received at least one of these agents.
The study received no commercial funding. Dr. Captieux and Dr. Lawton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Empagliflozin a winner in challenging arena of stabilized acute HF
The sodium-glucose transporter 2 inhibitors, relative newcomers among first-line agents for chronic heart failure (HF), could well attain the same go-to status in patients hospitalized with acute HF if the EMPULSE trial has anything to say about it.
Of the study’s 530 such patients, those started on daily empagliflozin (Jardiance) soon after they were stabilized, compared with a control group, were less likely to die or be rehospitalized for HF over the next 3 months.
Also, “we saw an improvement in quality of life, we saw a greater reduction in body weight, and we didn’t see any safety concerns in this very vulnerable and sick patient population,” Adriaan A. Voors, MD, University Medical Center Groningen (the Netherlands), said when presenting the trial at the American Heart Association scientific sessions.
Patients assigned to empagliflozin had a 36% greater likelihood of showing a benefit as reflected in the treatment’s win ratio when opposed by placebo, an emerging way to express outcomes in cardiovascular clinical trials. The SGLT2 inhibitor’s win ratio for the primary endpoint was 1.36 (95% confidence interval, 1.09-1.68, P = .0054), Dr. Voors reported. The outcome consisted of death, number of HF events, time to first HF event, and 90-day change in quality of life scores.
There is reluctance in practice to start patients that early after decompensation on drugs used in chronic HF, Dr. Voors said in an interview. Empagliflozin in the trial was initiated in the stabilized setting an average of 3 days after hospital admission, he said. The trial should reassure physicians that the drug “is not only safe to start early in hospital, but it’s also beneficial to start early in hospital.”
EMPULSE, combined with support from other recent trials, “should be clinical practice changing, with early in-hospital initiation of SGLT2 inhibitors in patients hospitalized with HF being the expectation, along with clear recognition that delaying SGLT2 inhibitor initiation may expose patients to unnecessary harms and delays in improved health status,” Gregg C. Fonarow, MD, University of California Los Angeles Medical Center, told this news organization.
“For patients with HF, irrespective of ejection fraction, early in-hospital initiation of SGLT2 inhibitors – once stabilized and in the absence of contraindications – should be considered a new standard of care,” said Fonarow, who was not part of EMPULSE.
The trial also lends new weight to the strategy of “simultaneous or rapid-sequence initiation” of the so-called four pillars of guideline-directed medical therapy of HF with reduced ejection fraction in patients hospitalized with HFrEF, once they are stabilized, Dr. Fonarow said. The four-pronged approach, he noted, consists of sacubitril/valsartan (Entresto), a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor.
Indeed, the new findings “fill an important gap and are clearly practice changing,” agreed Nancy K. Sweitzer, MD, PhD, University of Arizona Sarver Heart Center, Tucson, as an invited discussant following Dr. Voors’ presentation. “Few therapies have been shown to impact the course of those hospitalized with acute decompensated heart failure.”
Of note in the trial, Dr. Sweitzer continued, patients were started on empagliflozin regardless of any drug therapy they might already be on for chronic HF. “Because patients in the EMPULSE trial could be enrolled with a new diagnosis of heart failure, they were, by definition, not all on chronic guideline-directed heart failure therapy. Nevertheless, such patients benefited equally from the study intervention,” she said.
“This is crucial, as it tells us these drugs have immediate and important effects and should not be withheld while other drug classes are initiated and optimized.”
EMPULSE entered patients hospitalized for acute HF, which could be de novo or a decompensation of chronic HF, without regard to ejection fraction or whether they had diabetes, and who were clinically stable after at least one dose of loop diuretics. Their ejection fractions averaged 35% and exceeded 40% in about one-third of the total cohort.
At 90 days in the win ratio analysis, the 265 patients assigned to empagliflozin 10 mg once daily were the “winners”; that is, they were more likely to show a clinical benefit about 54% of the time in paired match-ups of patient outcomes, compared with about 40% for the 265 in the control group. The match-ups were a tie 6.4% of the time.
The empagliflozin group also benefited significantly for the endpoint of death from any cause or first HF event, with a hazard ratio of 0.65 (95% CI, 0.43-0.99; P = .042). They also were less likely to experience acute renal failure (7.7% vs. 12.1% for the control group) or serious adverse events (32.3% vs. 43.6%), Dr. Voors reported.
Tempting as it might be, the findings can’t necessarily be generalized to other SGLT2 inhibitors without an evidence base. But as Dr. Voors observed, several ongoing trials are exploring dapagliflozin (Farxiga) in a similar clinical setting.
They include DICTATE-AHF in patients with diabetes admitted with acute HF, and DAPA ACT HF-TIMI 68, which is entering patients stabilized during hospitalization with acute decompensated HFrEF. The trials are scheduled for completion in 2022 and 2023, respectively.
EMPULSE was supported by the Boehringer Ingelheim–Eli Lilly Diabetes Alliance. Dr. Voors disclosed research support and consulting for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novo Nordisk, Novartis, and Roche Diagnostics. Dr. Sweitzer disclosed honoraria from Acorda and Myokardia, and reported receiving research support from Novartis and Merck. Dr. Fonarow cited honoraria from Abbott, Amgen, Janssen, Medtronic, Bayer, Merck, AstraZeneca, Cytokinetics, and Novartis.
A version of this article first appeared on Medscape.com.
The sodium-glucose transporter 2 inhibitors, relative newcomers among first-line agents for chronic heart failure (HF), could well attain the same go-to status in patients hospitalized with acute HF if the EMPULSE trial has anything to say about it.
Of the study’s 530 such patients, those started on daily empagliflozin (Jardiance) soon after they were stabilized, compared with a control group, were less likely to die or be rehospitalized for HF over the next 3 months.
Also, “we saw an improvement in quality of life, we saw a greater reduction in body weight, and we didn’t see any safety concerns in this very vulnerable and sick patient population,” Adriaan A. Voors, MD, University Medical Center Groningen (the Netherlands), said when presenting the trial at the American Heart Association scientific sessions.
Patients assigned to empagliflozin had a 36% greater likelihood of showing a benefit as reflected in the treatment’s win ratio when opposed by placebo, an emerging way to express outcomes in cardiovascular clinical trials. The SGLT2 inhibitor’s win ratio for the primary endpoint was 1.36 (95% confidence interval, 1.09-1.68, P = .0054), Dr. Voors reported. The outcome consisted of death, number of HF events, time to first HF event, and 90-day change in quality of life scores.
There is reluctance in practice to start patients that early after decompensation on drugs used in chronic HF, Dr. Voors said in an interview. Empagliflozin in the trial was initiated in the stabilized setting an average of 3 days after hospital admission, he said. The trial should reassure physicians that the drug “is not only safe to start early in hospital, but it’s also beneficial to start early in hospital.”
EMPULSE, combined with support from other recent trials, “should be clinical practice changing, with early in-hospital initiation of SGLT2 inhibitors in patients hospitalized with HF being the expectation, along with clear recognition that delaying SGLT2 inhibitor initiation may expose patients to unnecessary harms and delays in improved health status,” Gregg C. Fonarow, MD, University of California Los Angeles Medical Center, told this news organization.
“For patients with HF, irrespective of ejection fraction, early in-hospital initiation of SGLT2 inhibitors – once stabilized and in the absence of contraindications – should be considered a new standard of care,” said Fonarow, who was not part of EMPULSE.
The trial also lends new weight to the strategy of “simultaneous or rapid-sequence initiation” of the so-called four pillars of guideline-directed medical therapy of HF with reduced ejection fraction in patients hospitalized with HFrEF, once they are stabilized, Dr. Fonarow said. The four-pronged approach, he noted, consists of sacubitril/valsartan (Entresto), a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor.
Indeed, the new findings “fill an important gap and are clearly practice changing,” agreed Nancy K. Sweitzer, MD, PhD, University of Arizona Sarver Heart Center, Tucson, as an invited discussant following Dr. Voors’ presentation. “Few therapies have been shown to impact the course of those hospitalized with acute decompensated heart failure.”
Of note in the trial, Dr. Sweitzer continued, patients were started on empagliflozin regardless of any drug therapy they might already be on for chronic HF. “Because patients in the EMPULSE trial could be enrolled with a new diagnosis of heart failure, they were, by definition, not all on chronic guideline-directed heart failure therapy. Nevertheless, such patients benefited equally from the study intervention,” she said.
“This is crucial, as it tells us these drugs have immediate and important effects and should not be withheld while other drug classes are initiated and optimized.”
EMPULSE entered patients hospitalized for acute HF, which could be de novo or a decompensation of chronic HF, without regard to ejection fraction or whether they had diabetes, and who were clinically stable after at least one dose of loop diuretics. Their ejection fractions averaged 35% and exceeded 40% in about one-third of the total cohort.
At 90 days in the win ratio analysis, the 265 patients assigned to empagliflozin 10 mg once daily were the “winners”; that is, they were more likely to show a clinical benefit about 54% of the time in paired match-ups of patient outcomes, compared with about 40% for the 265 in the control group. The match-ups were a tie 6.4% of the time.
The empagliflozin group also benefited significantly for the endpoint of death from any cause or first HF event, with a hazard ratio of 0.65 (95% CI, 0.43-0.99; P = .042). They also were less likely to experience acute renal failure (7.7% vs. 12.1% for the control group) or serious adverse events (32.3% vs. 43.6%), Dr. Voors reported.
Tempting as it might be, the findings can’t necessarily be generalized to other SGLT2 inhibitors without an evidence base. But as Dr. Voors observed, several ongoing trials are exploring dapagliflozin (Farxiga) in a similar clinical setting.
They include DICTATE-AHF in patients with diabetes admitted with acute HF, and DAPA ACT HF-TIMI 68, which is entering patients stabilized during hospitalization with acute decompensated HFrEF. The trials are scheduled for completion in 2022 and 2023, respectively.
EMPULSE was supported by the Boehringer Ingelheim–Eli Lilly Diabetes Alliance. Dr. Voors disclosed research support and consulting for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novo Nordisk, Novartis, and Roche Diagnostics. Dr. Sweitzer disclosed honoraria from Acorda and Myokardia, and reported receiving research support from Novartis and Merck. Dr. Fonarow cited honoraria from Abbott, Amgen, Janssen, Medtronic, Bayer, Merck, AstraZeneca, Cytokinetics, and Novartis.
A version of this article first appeared on Medscape.com.
The sodium-glucose transporter 2 inhibitors, relative newcomers among first-line agents for chronic heart failure (HF), could well attain the same go-to status in patients hospitalized with acute HF if the EMPULSE trial has anything to say about it.
Of the study’s 530 such patients, those started on daily empagliflozin (Jardiance) soon after they were stabilized, compared with a control group, were less likely to die or be rehospitalized for HF over the next 3 months.
Also, “we saw an improvement in quality of life, we saw a greater reduction in body weight, and we didn’t see any safety concerns in this very vulnerable and sick patient population,” Adriaan A. Voors, MD, University Medical Center Groningen (the Netherlands), said when presenting the trial at the American Heart Association scientific sessions.
Patients assigned to empagliflozin had a 36% greater likelihood of showing a benefit as reflected in the treatment’s win ratio when opposed by placebo, an emerging way to express outcomes in cardiovascular clinical trials. The SGLT2 inhibitor’s win ratio for the primary endpoint was 1.36 (95% confidence interval, 1.09-1.68, P = .0054), Dr. Voors reported. The outcome consisted of death, number of HF events, time to first HF event, and 90-day change in quality of life scores.
There is reluctance in practice to start patients that early after decompensation on drugs used in chronic HF, Dr. Voors said in an interview. Empagliflozin in the trial was initiated in the stabilized setting an average of 3 days after hospital admission, he said. The trial should reassure physicians that the drug “is not only safe to start early in hospital, but it’s also beneficial to start early in hospital.”
EMPULSE, combined with support from other recent trials, “should be clinical practice changing, with early in-hospital initiation of SGLT2 inhibitors in patients hospitalized with HF being the expectation, along with clear recognition that delaying SGLT2 inhibitor initiation may expose patients to unnecessary harms and delays in improved health status,” Gregg C. Fonarow, MD, University of California Los Angeles Medical Center, told this news organization.
“For patients with HF, irrespective of ejection fraction, early in-hospital initiation of SGLT2 inhibitors – once stabilized and in the absence of contraindications – should be considered a new standard of care,” said Fonarow, who was not part of EMPULSE.
The trial also lends new weight to the strategy of “simultaneous or rapid-sequence initiation” of the so-called four pillars of guideline-directed medical therapy of HF with reduced ejection fraction in patients hospitalized with HFrEF, once they are stabilized, Dr. Fonarow said. The four-pronged approach, he noted, consists of sacubitril/valsartan (Entresto), a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor.
Indeed, the new findings “fill an important gap and are clearly practice changing,” agreed Nancy K. Sweitzer, MD, PhD, University of Arizona Sarver Heart Center, Tucson, as an invited discussant following Dr. Voors’ presentation. “Few therapies have been shown to impact the course of those hospitalized with acute decompensated heart failure.”
Of note in the trial, Dr. Sweitzer continued, patients were started on empagliflozin regardless of any drug therapy they might already be on for chronic HF. “Because patients in the EMPULSE trial could be enrolled with a new diagnosis of heart failure, they were, by definition, not all on chronic guideline-directed heart failure therapy. Nevertheless, such patients benefited equally from the study intervention,” she said.
“This is crucial, as it tells us these drugs have immediate and important effects and should not be withheld while other drug classes are initiated and optimized.”
EMPULSE entered patients hospitalized for acute HF, which could be de novo or a decompensation of chronic HF, without regard to ejection fraction or whether they had diabetes, and who were clinically stable after at least one dose of loop diuretics. Their ejection fractions averaged 35% and exceeded 40% in about one-third of the total cohort.
At 90 days in the win ratio analysis, the 265 patients assigned to empagliflozin 10 mg once daily were the “winners”; that is, they were more likely to show a clinical benefit about 54% of the time in paired match-ups of patient outcomes, compared with about 40% for the 265 in the control group. The match-ups were a tie 6.4% of the time.
The empagliflozin group also benefited significantly for the endpoint of death from any cause or first HF event, with a hazard ratio of 0.65 (95% CI, 0.43-0.99; P = .042). They also were less likely to experience acute renal failure (7.7% vs. 12.1% for the control group) or serious adverse events (32.3% vs. 43.6%), Dr. Voors reported.
Tempting as it might be, the findings can’t necessarily be generalized to other SGLT2 inhibitors without an evidence base. But as Dr. Voors observed, several ongoing trials are exploring dapagliflozin (Farxiga) in a similar clinical setting.
They include DICTATE-AHF in patients with diabetes admitted with acute HF, and DAPA ACT HF-TIMI 68, which is entering patients stabilized during hospitalization with acute decompensated HFrEF. The trials are scheduled for completion in 2022 and 2023, respectively.
EMPULSE was supported by the Boehringer Ingelheim–Eli Lilly Diabetes Alliance. Dr. Voors disclosed research support and consulting for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novo Nordisk, Novartis, and Roche Diagnostics. Dr. Sweitzer disclosed honoraria from Acorda and Myokardia, and reported receiving research support from Novartis and Merck. Dr. Fonarow cited honoraria from Abbott, Amgen, Janssen, Medtronic, Bayer, Merck, AstraZeneca, Cytokinetics, and Novartis.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Case: Older patient with T2D has recurrent flushing
He has had no other symptoms. His only abnormalities on physical exam are a blood pressure of 160/100 and mild peripheral edema.
His current medications include: Famotidine 20 mg b.i.d., Pseudoephedrine/guaifenesin SR b.i.d., Metformin 1,000 mg twice a day, Nifedipine 60 mg XL once a day, and Atorvastatin 20 mg once a day.
His laboratory work up includes: blood urea nitrogen: 20, creatinine: 1.3, sodium: 140, Chloride: 104, potassium: 3.9, glucose: 205, white blood cell count: 6,000, hematocrit: 41, 24-hour urine 5-hydroxyindoleacetic acid (5HIAA) test: 12 mg/day (normal 2-8 mg/day), free catecholamines: 80 mg/24 hours (normal less than 100 mg/24 hours).
What is the most likely diagnosis?
A. Drug effect
B. Pheochromocytoma
C. Carcinoid syndrome
D. Mastocytosis
E. Medullary thyroid cancer
The most likely diagnosis is a drug effect. His flushing is likely caused by nifedipine.
Flushing is one of the most common side effects of this drug.1 This patient had lab testing done for carcinoid (urine 5HIAA), presumably because he had flushing. This lab test result was a false positive, likely because of guaifenesin ingestion, which can cause false-positive 5HIAA results.2
Carcinoid syndrome is very rare (estimates from less than 1 patient/100,000), and the vast majority of patients who have it present with metastatic disease at presentation. Drug side effects are common, and usually are much more likely than rare diseases.
Four principles for assisting with making a diagnosis
This case points out the following four principles that I will touch on to help us make diagnoses in challenging cases.
1. Trigger symptoms: These are symptoms that make us think of a rare disease. In this case, the symptom is flushing, which may make you think of carcinoid syndrome.
Another good example of a trigger symptom is night sweats, where you may think of tuberculosis or lymphoma. These symptoms almost always have a much more common and likely cause, which in this case is a common drug side effect.
Trigger symptoms are great to pay attention to, but do not jump to working up the rare diagnosis without more evidence that it is a plausible diagnosis. Working up rare diseases without a reasonable pretest probability will lead to significant false-positive results.
2. Distinguishing features: These are findings, or combinations of findings, that make rarer diseases more likely. For example, flushing, although seen in many patients with carcinoid syndrome, is much more commonly caused by rosacea, medications, or estrogen/testosterone deficiency.
If a patient presents with flushing plus diarrhea, carcinoid syndrome becomes more likely in differentials. An example of a specific distinguishing feature is transient visual obstructions in patients with idiopathic intracranial hypertension (IIH or pseudotumor cerebri).
Sudden transient visual loss is not a symptom we see often, but headaches and obesity are problems we see every day. A patient with headaches and obesity is very likely to have IIH if they have transient visual obstructions along with headaches and obesity.
3. Intentional physical exams: Do the physical exam focusing on what findings will change your diagnostic probabilities. For example, in this case, if you are considering carcinoid, do a careful abdominal exam, with close attention to the liver, as 75% of patients with carcinoid syndrome have liver metastases.
If you are thinking about IIH, a fundoscopic exam is mandatory, as papilledema is a key feature of this diagnosis.
Read about the rare diagnosis you are considering, this will help with targeting your exam.
4. Remember the unusual presentation of a common disease is more common than the common presentation of a rare disease: Good examples of this are sleep apnea and gastroesophageal reflux disease causing night sweats more commonly than finding lymphomas or active tuberculosis (in the United States) as the cause.3
Pearl: Trigger symptoms help us think of rare diseases, but distinguishing features are most helpful in including or excluding the diagnosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Gueret P et al. Drugs. 1990;39 Suppl 2:67-72.
2. Corcuff J et al. Endocr Connect. 2017;6:R87.
3. Smith CS and Paauw DS. J Am Board Fam Pract. 2000;13:424-9.
He has had no other symptoms. His only abnormalities on physical exam are a blood pressure of 160/100 and mild peripheral edema.
His current medications include: Famotidine 20 mg b.i.d., Pseudoephedrine/guaifenesin SR b.i.d., Metformin 1,000 mg twice a day, Nifedipine 60 mg XL once a day, and Atorvastatin 20 mg once a day.
His laboratory work up includes: blood urea nitrogen: 20, creatinine: 1.3, sodium: 140, Chloride: 104, potassium: 3.9, glucose: 205, white blood cell count: 6,000, hematocrit: 41, 24-hour urine 5-hydroxyindoleacetic acid (5HIAA) test: 12 mg/day (normal 2-8 mg/day), free catecholamines: 80 mg/24 hours (normal less than 100 mg/24 hours).
What is the most likely diagnosis?
A. Drug effect
B. Pheochromocytoma
C. Carcinoid syndrome
D. Mastocytosis
E. Medullary thyroid cancer
The most likely diagnosis is a drug effect. His flushing is likely caused by nifedipine.
Flushing is one of the most common side effects of this drug.1 This patient had lab testing done for carcinoid (urine 5HIAA), presumably because he had flushing. This lab test result was a false positive, likely because of guaifenesin ingestion, which can cause false-positive 5HIAA results.2
Carcinoid syndrome is very rare (estimates from less than 1 patient/100,000), and the vast majority of patients who have it present with metastatic disease at presentation. Drug side effects are common, and usually are much more likely than rare diseases.
Four principles for assisting with making a diagnosis
This case points out the following four principles that I will touch on to help us make diagnoses in challenging cases.
1. Trigger symptoms: These are symptoms that make us think of a rare disease. In this case, the symptom is flushing, which may make you think of carcinoid syndrome.
Another good example of a trigger symptom is night sweats, where you may think of tuberculosis or lymphoma. These symptoms almost always have a much more common and likely cause, which in this case is a common drug side effect.
Trigger symptoms are great to pay attention to, but do not jump to working up the rare diagnosis without more evidence that it is a plausible diagnosis. Working up rare diseases without a reasonable pretest probability will lead to significant false-positive results.
2. Distinguishing features: These are findings, or combinations of findings, that make rarer diseases more likely. For example, flushing, although seen in many patients with carcinoid syndrome, is much more commonly caused by rosacea, medications, or estrogen/testosterone deficiency.
If a patient presents with flushing plus diarrhea, carcinoid syndrome becomes more likely in differentials. An example of a specific distinguishing feature is transient visual obstructions in patients with idiopathic intracranial hypertension (IIH or pseudotumor cerebri).
Sudden transient visual loss is not a symptom we see often, but headaches and obesity are problems we see every day. A patient with headaches and obesity is very likely to have IIH if they have transient visual obstructions along with headaches and obesity.
3. Intentional physical exams: Do the physical exam focusing on what findings will change your diagnostic probabilities. For example, in this case, if you are considering carcinoid, do a careful abdominal exam, with close attention to the liver, as 75% of patients with carcinoid syndrome have liver metastases.
If you are thinking about IIH, a fundoscopic exam is mandatory, as papilledema is a key feature of this diagnosis.
Read about the rare diagnosis you are considering, this will help with targeting your exam.
4. Remember the unusual presentation of a common disease is more common than the common presentation of a rare disease: Good examples of this are sleep apnea and gastroesophageal reflux disease causing night sweats more commonly than finding lymphomas or active tuberculosis (in the United States) as the cause.3
Pearl: Trigger symptoms help us think of rare diseases, but distinguishing features are most helpful in including or excluding the diagnosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Gueret P et al. Drugs. 1990;39 Suppl 2:67-72.
2. Corcuff J et al. Endocr Connect. 2017;6:R87.
3. Smith CS and Paauw DS. J Am Board Fam Pract. 2000;13:424-9.
He has had no other symptoms. His only abnormalities on physical exam are a blood pressure of 160/100 and mild peripheral edema.
His current medications include: Famotidine 20 mg b.i.d., Pseudoephedrine/guaifenesin SR b.i.d., Metformin 1,000 mg twice a day, Nifedipine 60 mg XL once a day, and Atorvastatin 20 mg once a day.
His laboratory work up includes: blood urea nitrogen: 20, creatinine: 1.3, sodium: 140, Chloride: 104, potassium: 3.9, glucose: 205, white blood cell count: 6,000, hematocrit: 41, 24-hour urine 5-hydroxyindoleacetic acid (5HIAA) test: 12 mg/day (normal 2-8 mg/day), free catecholamines: 80 mg/24 hours (normal less than 100 mg/24 hours).
What is the most likely diagnosis?
A. Drug effect
B. Pheochromocytoma
C. Carcinoid syndrome
D. Mastocytosis
E. Medullary thyroid cancer
The most likely diagnosis is a drug effect. His flushing is likely caused by nifedipine.
Flushing is one of the most common side effects of this drug.1 This patient had lab testing done for carcinoid (urine 5HIAA), presumably because he had flushing. This lab test result was a false positive, likely because of guaifenesin ingestion, which can cause false-positive 5HIAA results.2
Carcinoid syndrome is very rare (estimates from less than 1 patient/100,000), and the vast majority of patients who have it present with metastatic disease at presentation. Drug side effects are common, and usually are much more likely than rare diseases.
Four principles for assisting with making a diagnosis
This case points out the following four principles that I will touch on to help us make diagnoses in challenging cases.
1. Trigger symptoms: These are symptoms that make us think of a rare disease. In this case, the symptom is flushing, which may make you think of carcinoid syndrome.
Another good example of a trigger symptom is night sweats, where you may think of tuberculosis or lymphoma. These symptoms almost always have a much more common and likely cause, which in this case is a common drug side effect.
Trigger symptoms are great to pay attention to, but do not jump to working up the rare diagnosis without more evidence that it is a plausible diagnosis. Working up rare diseases without a reasonable pretest probability will lead to significant false-positive results.
2. Distinguishing features: These are findings, or combinations of findings, that make rarer diseases more likely. For example, flushing, although seen in many patients with carcinoid syndrome, is much more commonly caused by rosacea, medications, or estrogen/testosterone deficiency.
If a patient presents with flushing plus diarrhea, carcinoid syndrome becomes more likely in differentials. An example of a specific distinguishing feature is transient visual obstructions in patients with idiopathic intracranial hypertension (IIH or pseudotumor cerebri).
Sudden transient visual loss is not a symptom we see often, but headaches and obesity are problems we see every day. A patient with headaches and obesity is very likely to have IIH if they have transient visual obstructions along with headaches and obesity.
3. Intentional physical exams: Do the physical exam focusing on what findings will change your diagnostic probabilities. For example, in this case, if you are considering carcinoid, do a careful abdominal exam, with close attention to the liver, as 75% of patients with carcinoid syndrome have liver metastases.
If you are thinking about IIH, a fundoscopic exam is mandatory, as papilledema is a key feature of this diagnosis.
Read about the rare diagnosis you are considering, this will help with targeting your exam.
4. Remember the unusual presentation of a common disease is more common than the common presentation of a rare disease: Good examples of this are sleep apnea and gastroesophageal reflux disease causing night sweats more commonly than finding lymphomas or active tuberculosis (in the United States) as the cause.3
Pearl: Trigger symptoms help us think of rare diseases, but distinguishing features are most helpful in including or excluding the diagnosis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Gueret P et al. Drugs. 1990;39 Suppl 2:67-72.
2. Corcuff J et al. Endocr Connect. 2017;6:R87.
3. Smith CS and Paauw DS. J Am Board Fam Pract. 2000;13:424-9.