User login
Nonopioid med promising for neuropathic pain
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Premature menopause a ‘warning sign’ for greater ASCVD risk
Premature menopause is well known to be linked to cardiovascular disease in women, but it may not carry as much weight as more traditional cardiovascular risk factors in determining a patient’s 10-year risk of having a heart attack or stroke in this population, a cohort study that evaluated the veracity of premature menopause found.
Premature menopause can serve as a “marker or warning sign” that cardiologists should pay closer attention to traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, lead study author Sadiya S. Khan, MD, MS, said in an interview. “When we looked at the addition of premature menopause into the risk-prediction equation, we did not see that it meaningfully improved the ability of the risk predictions of pooled cohort equations [PCEs] to identify who developed cardiovascular disease,” said Dr. Khan, a cardiologist at Northwestern University, Chicago.
The cohort study included 5,466 Black women and 10,584 White women from seven U.S. population-based cohorts, including the Women’s Health Initiative, of whom 951 and 1,039, respectively, self-reported early menopause. The cohort study researchers noted that the 2019 American College of Cardiology/American Heart Association guideline for prevention of CVD acknowledged premature menopause as risk-enhancing factor in the CVD assessment in women younger than 40.
The cohort study found that Black women had almost twice the rate of premature menopause than White women, 17.4% and 9.8%, respectively. And it found that premature menopause was significantly linked with ASCVD in both populations independent of traditional risk factors – a 24% greater risk for Black women and 28% greater risk for White women.
‘Surprising’ finding
However, when premature menopause was added to the pooled cohort equations per the 2013 ACC/AHA guideline, the researchers found no incremental benefit, a finding Dr. Khan called “really surprising to us.”
She added, “If we look at the differences in the characteristics of women who have premature menopause, compared with those who didn’t, there were slight differences in terms of higher blood pressure, higher body mass index, and slightly higher glucose. So maybe what we’re seeing – and this is more speculative – is that risk factors are developing after early menopause, and the focus should be earlier in the patient’s life course to try to prevent hypertension, diabetes, and obesity.”
Dr. Khan emphasized that the findings don’t obviate the value of premature menopause in assessing ASCVD risk in women. “We still know that this is an important marker for women and their risk for heart disease, and it should be a warning sign to pay close attention to those other risk factors and what other preventive measures can be taken,” she said.
Christie Ballantyne, MD, said it’s important to note that the study did not dismiss the relevance of premature menopause in shared decision-making for postmenopausal women. “It certainly doesn’t mean that premature menopause is not a risk,” Dr. Ballantyne said in an interview. “Premature menopause may cause a worsening of traditional CVD risk factors, so that’s one possible explanation for it. The other possible explanation is that women with worse ASCVD risk factors – who are more overweight, have higher blood pressure, and have more diabetes and insulin resistance – are more likely to have earlier menopause.” Dr. Ballantyne is chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston.
“You should still look very carefully at the patient’s risk factors, calculate the pooled cohort equations, and make sure you get a recommendation,” he said. “If their risks are up, give recommendations on how to improve diet and exercise. Consider if you need to treat lipids or treat blood pressure with more than diet and exercise because there’s nothing magical about 7.5%”, the threshold for lipid-lowering therapy in the ASCVD risk calculator.
Dr. Khan and coauthors disclosed receiving grants from the National Institutes of Health and the American Heart Association. One coauthor reported a financial relationship with HGM Biopharmaceuticals. Dr. Ballantyne is a lead investigator of the Atherosclerosis Risk in Communities study, one of the population-based cohorts used in the cohort study. He has no other relevant relationships to disclose.
Premature menopause is well known to be linked to cardiovascular disease in women, but it may not carry as much weight as more traditional cardiovascular risk factors in determining a patient’s 10-year risk of having a heart attack or stroke in this population, a cohort study that evaluated the veracity of premature menopause found.
Premature menopause can serve as a “marker or warning sign” that cardiologists should pay closer attention to traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, lead study author Sadiya S. Khan, MD, MS, said in an interview. “When we looked at the addition of premature menopause into the risk-prediction equation, we did not see that it meaningfully improved the ability of the risk predictions of pooled cohort equations [PCEs] to identify who developed cardiovascular disease,” said Dr. Khan, a cardiologist at Northwestern University, Chicago.
The cohort study included 5,466 Black women and 10,584 White women from seven U.S. population-based cohorts, including the Women’s Health Initiative, of whom 951 and 1,039, respectively, self-reported early menopause. The cohort study researchers noted that the 2019 American College of Cardiology/American Heart Association guideline for prevention of CVD acknowledged premature menopause as risk-enhancing factor in the CVD assessment in women younger than 40.
The cohort study found that Black women had almost twice the rate of premature menopause than White women, 17.4% and 9.8%, respectively. And it found that premature menopause was significantly linked with ASCVD in both populations independent of traditional risk factors – a 24% greater risk for Black women and 28% greater risk for White women.
‘Surprising’ finding
However, when premature menopause was added to the pooled cohort equations per the 2013 ACC/AHA guideline, the researchers found no incremental benefit, a finding Dr. Khan called “really surprising to us.”
She added, “If we look at the differences in the characteristics of women who have premature menopause, compared with those who didn’t, there were slight differences in terms of higher blood pressure, higher body mass index, and slightly higher glucose. So maybe what we’re seeing – and this is more speculative – is that risk factors are developing after early menopause, and the focus should be earlier in the patient’s life course to try to prevent hypertension, diabetes, and obesity.”
Dr. Khan emphasized that the findings don’t obviate the value of premature menopause in assessing ASCVD risk in women. “We still know that this is an important marker for women and their risk for heart disease, and it should be a warning sign to pay close attention to those other risk factors and what other preventive measures can be taken,” she said.
Christie Ballantyne, MD, said it’s important to note that the study did not dismiss the relevance of premature menopause in shared decision-making for postmenopausal women. “It certainly doesn’t mean that premature menopause is not a risk,” Dr. Ballantyne said in an interview. “Premature menopause may cause a worsening of traditional CVD risk factors, so that’s one possible explanation for it. The other possible explanation is that women with worse ASCVD risk factors – who are more overweight, have higher blood pressure, and have more diabetes and insulin resistance – are more likely to have earlier menopause.” Dr. Ballantyne is chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston.
“You should still look very carefully at the patient’s risk factors, calculate the pooled cohort equations, and make sure you get a recommendation,” he said. “If their risks are up, give recommendations on how to improve diet and exercise. Consider if you need to treat lipids or treat blood pressure with more than diet and exercise because there’s nothing magical about 7.5%”, the threshold for lipid-lowering therapy in the ASCVD risk calculator.
Dr. Khan and coauthors disclosed receiving grants from the National Institutes of Health and the American Heart Association. One coauthor reported a financial relationship with HGM Biopharmaceuticals. Dr. Ballantyne is a lead investigator of the Atherosclerosis Risk in Communities study, one of the population-based cohorts used in the cohort study. He has no other relevant relationships to disclose.
Premature menopause is well known to be linked to cardiovascular disease in women, but it may not carry as much weight as more traditional cardiovascular risk factors in determining a patient’s 10-year risk of having a heart attack or stroke in this population, a cohort study that evaluated the veracity of premature menopause found.
Premature menopause can serve as a “marker or warning sign” that cardiologists should pay closer attention to traditional atherosclerotic cardiovascular disease (ASCVD) risk factors, lead study author Sadiya S. Khan, MD, MS, said in an interview. “When we looked at the addition of premature menopause into the risk-prediction equation, we did not see that it meaningfully improved the ability of the risk predictions of pooled cohort equations [PCEs] to identify who developed cardiovascular disease,” said Dr. Khan, a cardiologist at Northwestern University, Chicago.
The cohort study included 5,466 Black women and 10,584 White women from seven U.S. population-based cohorts, including the Women’s Health Initiative, of whom 951 and 1,039, respectively, self-reported early menopause. The cohort study researchers noted that the 2019 American College of Cardiology/American Heart Association guideline for prevention of CVD acknowledged premature menopause as risk-enhancing factor in the CVD assessment in women younger than 40.
The cohort study found that Black women had almost twice the rate of premature menopause than White women, 17.4% and 9.8%, respectively. And it found that premature menopause was significantly linked with ASCVD in both populations independent of traditional risk factors – a 24% greater risk for Black women and 28% greater risk for White women.
‘Surprising’ finding
However, when premature menopause was added to the pooled cohort equations per the 2013 ACC/AHA guideline, the researchers found no incremental benefit, a finding Dr. Khan called “really surprising to us.”
She added, “If we look at the differences in the characteristics of women who have premature menopause, compared with those who didn’t, there were slight differences in terms of higher blood pressure, higher body mass index, and slightly higher glucose. So maybe what we’re seeing – and this is more speculative – is that risk factors are developing after early menopause, and the focus should be earlier in the patient’s life course to try to prevent hypertension, diabetes, and obesity.”
Dr. Khan emphasized that the findings don’t obviate the value of premature menopause in assessing ASCVD risk in women. “We still know that this is an important marker for women and their risk for heart disease, and it should be a warning sign to pay close attention to those other risk factors and what other preventive measures can be taken,” she said.
Christie Ballantyne, MD, said it’s important to note that the study did not dismiss the relevance of premature menopause in shared decision-making for postmenopausal women. “It certainly doesn’t mean that premature menopause is not a risk,” Dr. Ballantyne said in an interview. “Premature menopause may cause a worsening of traditional CVD risk factors, so that’s one possible explanation for it. The other possible explanation is that women with worse ASCVD risk factors – who are more overweight, have higher blood pressure, and have more diabetes and insulin resistance – are more likely to have earlier menopause.” Dr. Ballantyne is chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston.
“You should still look very carefully at the patient’s risk factors, calculate the pooled cohort equations, and make sure you get a recommendation,” he said. “If their risks are up, give recommendations on how to improve diet and exercise. Consider if you need to treat lipids or treat blood pressure with more than diet and exercise because there’s nothing magical about 7.5%”, the threshold for lipid-lowering therapy in the ASCVD risk calculator.
Dr. Khan and coauthors disclosed receiving grants from the National Institutes of Health and the American Heart Association. One coauthor reported a financial relationship with HGM Biopharmaceuticals. Dr. Ballantyne is a lead investigator of the Atherosclerosis Risk in Communities study, one of the population-based cohorts used in the cohort study. He has no other relevant relationships to disclose.
FROM JAMA CARDIOLOGY
Researchers warn young adults are at highest risk of obesity
Individuals aged 18-24 years are at the highest risk of weight gain and developing overweight or obesity over the next 10 years, compared with all other adults, and should be a target for obesity prevention policies, say U.K. researchers.
The research, published online Sept. 2, 2021, in The Lancet Diabetes and Endocrinology, showed that factors more traditionally associated with obesity – such as socioeconomic status and ethnicity – play less of a role than age.
“Our results show clearly that age is the most important sociodemographic factor for BMI [body mass index] change,” lead author Michail Katsoulis, PhD, Institute of Health Informatics, University College London, said in a press release.
Cosenior author Claudia Langenberg, PhD, agreed, adding young people “go through big life changes. They may start work, go to university, or leave home for the first time,” and the habits formed during these years “may stick through adulthood.”
Current obesity prevention guidelines are mainly directed at individuals who already have obesity, the researchers said in their article.
“As the evidence presented in our study suggests, the opportunity to modify weight gain is greatest in individuals who are young and do not yet have obesity,” they observed.
“If we are serious about preventing obesity, then we should develop interventions that can be targeted and are relevant for young adults,” added Dr. Langenberg, of the MRC Epidemiology Unit, University of Cambridge, (England), and Berlin Institute of Health.
Risks for higher BMI substantially greater in the youngest adults
The researchers gathered data on more than 2 million adults aged 18-74 years registered with general practitioners in England. Participants had BMI and weight measurements recorded between Jan. 1, 1998, and June 30, 2016, with at least 1 year of follow-up. Overall, 58% were women, 76% were White, 9% had prevalent cardiovascular disease, and 4% had prevalent cancer.
Changes in BMI were assessed at 1 year, 5 years, and 10 years.
At 10 years, adults aged 18-24 years had the highest risk of transitioning from normal weight to overweight or obesity, compared with adults aged 65-74 years, at a greatest absolute risk of 37% versus 24% (odds ratio, 4.22).
Moreover, the results showed that adults aged 18-24 years who were already overweight or obese had a greater risk of transitioning to a higher BMI category during follow-up versus the oldest participants.
They had an absolute risk of 42% versus 18% of transitioning from overweight to class 1 and 2 obesity (OR, 4.60), and an absolute risk of transitioning from class 1 and 2 obesity to class 3 obesity of 22% versus 5% (OR, 5.87).
Online risk calculator and YouTube video help explain findings
While factors other than age were associated with transitioning to a higher BMI category, the association was less pronounced.
For example, the OR of transitioning from normal weight to overweight or obesity in the most socially deprived versus the least deprived areas was 1.23 in men and 1.12 in women. The OR for making the same transition in Black versus White individuals was 1.13.
The findings allowed the researchers to develop a series of nomograms to determine an individual’s absolute risk of transitioning to a higher BMI category over 10 years based on their baseline BMI category, age, sex, and Index of Multiple Deprivation quintile.
“We show that, within each stratum, the risks for transitioning to higher BMI categories were substantially higher in the youngest adult age group than in older age groups,” the team writes.
From this, they developed an open-access online risk calculator to help individuals calculate their risk of weight change over the next 1, 5, and 10 years. The calculator takes into account current weight, height, age, sex, ethnicity, and socioeconomic-area characteristics.
They have also posted a video on YouTube to help explain their findings.
COVID and obesity pandemics collide
Cosenior author Harry Hemingway, MD, PhD, also of University College London, believes that focusing on this young age group is especially critical now because of the COVID-19 pandemic.
“Calculating personal risk of transitioning to a higher weight category is important” as COVID-19 “collides with the obesity pandemic,” he said, noting that “people are exercising less and finding it harder to eat healthy diets during lockdowns.
“Health systems like the NHS [National Health Service] need to identify new ways to prevent obesity and its consequences,” he continued. “This study demonstrates that NHS data collected over time in primary care holds an important key to unlocking new insights for public health action.”
The study was funded by the British Heart Foundation, Health Data Research UK, the UK Medical Research Council, and the National Institute for Health Research. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals aged 18-24 years are at the highest risk of weight gain and developing overweight or obesity over the next 10 years, compared with all other adults, and should be a target for obesity prevention policies, say U.K. researchers.
The research, published online Sept. 2, 2021, in The Lancet Diabetes and Endocrinology, showed that factors more traditionally associated with obesity – such as socioeconomic status and ethnicity – play less of a role than age.
“Our results show clearly that age is the most important sociodemographic factor for BMI [body mass index] change,” lead author Michail Katsoulis, PhD, Institute of Health Informatics, University College London, said in a press release.
Cosenior author Claudia Langenberg, PhD, agreed, adding young people “go through big life changes. They may start work, go to university, or leave home for the first time,” and the habits formed during these years “may stick through adulthood.”
Current obesity prevention guidelines are mainly directed at individuals who already have obesity, the researchers said in their article.
“As the evidence presented in our study suggests, the opportunity to modify weight gain is greatest in individuals who are young and do not yet have obesity,” they observed.
“If we are serious about preventing obesity, then we should develop interventions that can be targeted and are relevant for young adults,” added Dr. Langenberg, of the MRC Epidemiology Unit, University of Cambridge, (England), and Berlin Institute of Health.
Risks for higher BMI substantially greater in the youngest adults
The researchers gathered data on more than 2 million adults aged 18-74 years registered with general practitioners in England. Participants had BMI and weight measurements recorded between Jan. 1, 1998, and June 30, 2016, with at least 1 year of follow-up. Overall, 58% were women, 76% were White, 9% had prevalent cardiovascular disease, and 4% had prevalent cancer.
Changes in BMI were assessed at 1 year, 5 years, and 10 years.
At 10 years, adults aged 18-24 years had the highest risk of transitioning from normal weight to overweight or obesity, compared with adults aged 65-74 years, at a greatest absolute risk of 37% versus 24% (odds ratio, 4.22).
Moreover, the results showed that adults aged 18-24 years who were already overweight or obese had a greater risk of transitioning to a higher BMI category during follow-up versus the oldest participants.
They had an absolute risk of 42% versus 18% of transitioning from overweight to class 1 and 2 obesity (OR, 4.60), and an absolute risk of transitioning from class 1 and 2 obesity to class 3 obesity of 22% versus 5% (OR, 5.87).
Online risk calculator and YouTube video help explain findings
While factors other than age were associated with transitioning to a higher BMI category, the association was less pronounced.
For example, the OR of transitioning from normal weight to overweight or obesity in the most socially deprived versus the least deprived areas was 1.23 in men and 1.12 in women. The OR for making the same transition in Black versus White individuals was 1.13.
The findings allowed the researchers to develop a series of nomograms to determine an individual’s absolute risk of transitioning to a higher BMI category over 10 years based on their baseline BMI category, age, sex, and Index of Multiple Deprivation quintile.
“We show that, within each stratum, the risks for transitioning to higher BMI categories were substantially higher in the youngest adult age group than in older age groups,” the team writes.
From this, they developed an open-access online risk calculator to help individuals calculate their risk of weight change over the next 1, 5, and 10 years. The calculator takes into account current weight, height, age, sex, ethnicity, and socioeconomic-area characteristics.
They have also posted a video on YouTube to help explain their findings.
COVID and obesity pandemics collide
Cosenior author Harry Hemingway, MD, PhD, also of University College London, believes that focusing on this young age group is especially critical now because of the COVID-19 pandemic.
“Calculating personal risk of transitioning to a higher weight category is important” as COVID-19 “collides with the obesity pandemic,” he said, noting that “people are exercising less and finding it harder to eat healthy diets during lockdowns.
“Health systems like the NHS [National Health Service] need to identify new ways to prevent obesity and its consequences,” he continued. “This study demonstrates that NHS data collected over time in primary care holds an important key to unlocking new insights for public health action.”
The study was funded by the British Heart Foundation, Health Data Research UK, the UK Medical Research Council, and the National Institute for Health Research. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals aged 18-24 years are at the highest risk of weight gain and developing overweight or obesity over the next 10 years, compared with all other adults, and should be a target for obesity prevention policies, say U.K. researchers.
The research, published online Sept. 2, 2021, in The Lancet Diabetes and Endocrinology, showed that factors more traditionally associated with obesity – such as socioeconomic status and ethnicity – play less of a role than age.
“Our results show clearly that age is the most important sociodemographic factor for BMI [body mass index] change,” lead author Michail Katsoulis, PhD, Institute of Health Informatics, University College London, said in a press release.
Cosenior author Claudia Langenberg, PhD, agreed, adding young people “go through big life changes. They may start work, go to university, or leave home for the first time,” and the habits formed during these years “may stick through adulthood.”
Current obesity prevention guidelines are mainly directed at individuals who already have obesity, the researchers said in their article.
“As the evidence presented in our study suggests, the opportunity to modify weight gain is greatest in individuals who are young and do not yet have obesity,” they observed.
“If we are serious about preventing obesity, then we should develop interventions that can be targeted and are relevant for young adults,” added Dr. Langenberg, of the MRC Epidemiology Unit, University of Cambridge, (England), and Berlin Institute of Health.
Risks for higher BMI substantially greater in the youngest adults
The researchers gathered data on more than 2 million adults aged 18-74 years registered with general practitioners in England. Participants had BMI and weight measurements recorded between Jan. 1, 1998, and June 30, 2016, with at least 1 year of follow-up. Overall, 58% were women, 76% were White, 9% had prevalent cardiovascular disease, and 4% had prevalent cancer.
Changes in BMI were assessed at 1 year, 5 years, and 10 years.
At 10 years, adults aged 18-24 years had the highest risk of transitioning from normal weight to overweight or obesity, compared with adults aged 65-74 years, at a greatest absolute risk of 37% versus 24% (odds ratio, 4.22).
Moreover, the results showed that adults aged 18-24 years who were already overweight or obese had a greater risk of transitioning to a higher BMI category during follow-up versus the oldest participants.
They had an absolute risk of 42% versus 18% of transitioning from overweight to class 1 and 2 obesity (OR, 4.60), and an absolute risk of transitioning from class 1 and 2 obesity to class 3 obesity of 22% versus 5% (OR, 5.87).
Online risk calculator and YouTube video help explain findings
While factors other than age were associated with transitioning to a higher BMI category, the association was less pronounced.
For example, the OR of transitioning from normal weight to overweight or obesity in the most socially deprived versus the least deprived areas was 1.23 in men and 1.12 in women. The OR for making the same transition in Black versus White individuals was 1.13.
The findings allowed the researchers to develop a series of nomograms to determine an individual’s absolute risk of transitioning to a higher BMI category over 10 years based on their baseline BMI category, age, sex, and Index of Multiple Deprivation quintile.
“We show that, within each stratum, the risks for transitioning to higher BMI categories were substantially higher in the youngest adult age group than in older age groups,” the team writes.
From this, they developed an open-access online risk calculator to help individuals calculate their risk of weight change over the next 1, 5, and 10 years. The calculator takes into account current weight, height, age, sex, ethnicity, and socioeconomic-area characteristics.
They have also posted a video on YouTube to help explain their findings.
COVID and obesity pandemics collide
Cosenior author Harry Hemingway, MD, PhD, also of University College London, believes that focusing on this young age group is especially critical now because of the COVID-19 pandemic.
“Calculating personal risk of transitioning to a higher weight category is important” as COVID-19 “collides with the obesity pandemic,” he said, noting that “people are exercising less and finding it harder to eat healthy diets during lockdowns.
“Health systems like the NHS [National Health Service] need to identify new ways to prevent obesity and its consequences,” he continued. “This study demonstrates that NHS data collected over time in primary care holds an important key to unlocking new insights for public health action.”
The study was funded by the British Heart Foundation, Health Data Research UK, the UK Medical Research Council, and the National Institute for Health Research. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Texts boost activity, quality of life in patients with heart failure and diabetes
A 3-month lifestyle intervention that used a step counter and regular, personalized text messages to encourage increased mobility and adherence to medications led to a substantial rise in the quality of life in a randomized controlled study with 187 U.S. patients with heart failure and diabetes.
The TARGET-HF-DM study supplied a wrist-worn step counting device to adults with any type of heart failure and any type of diabetes at six U.S. sites and collected data on daily step counts and medication adherence through smartphone-based apps. Researchers randomized the patients to an intervention of thrice-weekly text messages that gave them personalized feedback on their recent activity and adherence and updated activity and adherence goals, or to a control group that only received a once-weekly generic message to wear the step counter.
After 3 months, patients in the intervention arm had an average incremental gain of 313 steps per day from baseline, compared with the controls, a significant difference for the study’s primary endpoint, G. Michael Felker, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
A ‘quite large’ increase in quality of life.
Perhaps more importantly, a secondary analysis assessed quality of life with the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, which showed after 3 months a 5.5-point average increased improvement among patients in the intervention arm, compared with controls. Score increases of 5 of more points on the KCCQ represent clinically meaningful changes.
This average, incremental KCCQ score improvement was “quite large relative to what we typically see in placebo-controlled trials of effective drugs,” said Dr. Felker, professor of medicine at Duke University, Durham, N.C., and director of cardiovascular research at the Duke Clinical Research Institute. If a similar magnitude change in KCCQ was associated with a drug treatment “we would say it was an incredibly large signal in terms of quality of life, so I think the patients are telling us that [the intervention] is making a clinically important difference.”
But Dr. Felker cautioned that the study was not blinded, raising the possibility that the change in quality of life could have been partially explained by “patients feeling more engaged about doing something for their health.”
His report omitted data on the medication adherence facet of the study, which will come out in a subsequent report, raising the possibility that some of the quality of life benefit as well as the ability of patients to boost their step count was related to more consistent treatment with their prescribed medications, but Dr. Felker discounted this possibility.
“The adherence intervention was basically a digital tool that helped people better remember their medication regimen. While it is possible that this could have influenced the KCCQ data this seems quite unlikely to me,” he said in an interview.
‘Exercise is the new magic’
“Exercise is the new magic,” commented Mariann R. Piano, PhD, a professor at Vanderbilt University, Nashville, Tenn., and cochair of the session where Dr. Felker gave his report. “I love that the trial was pragmatic, randomized, and ran at six sites so the generalizability of the findings is really strong.” Dr. Piano also gave the study high marks for recruiting many African American patients, 47% of the study population, and its assessment of a patient-reported outcome, the KCCQ score.
Patients enrolled in TARGET-HF-DM averaged 59 years of age, about a third were women, and two-thirds had heart failure with a reduced ejection fraction of 40% or less. Eighty percent of participants had New York Heart Association class II functional limitations, and a third also had atrial fibrillation. Their average serum level of the N-terminal of the prohormone brain natriuretic peptide at baseline was 1,309 pg/mL. Most patients were on standard heart failure and diabetes medications, with 88% receiving an ACE inhibitor or angiotensin-receptor blocker (in some cases coupled with sacubitril), 90% were on a beta-blocker, 50% were on a mineralocorticoid receptor antagonist, 54% were on insulin, 47% were on a biguanidine, 25% were on a sulfonylurea, and 7% were on a sodium-glucose cotransporter inhibitor. About half the patients also had an implantable cardioverter defibrillator.
Dr. Felker acknowledged that the 313 average increment in steps per day among patients in the intervention group, compared with controls was modest, but it represented about a 10% increase from baseline among patients who in general had a very sedentary life. All patients had received at the start of the study guidelines from the American Heart Association on appropriate types and levels of physical activity for patients with heart failure and diabetes. The researcher previously published a description of the design and rationale of the study.
The study followed patients for an additional 3 months beyond the end of the intervention period, and the excess step count among people in the intervention arm persisted, although the between-group difference was no longer significant. The researchers also analyzed changes during the intervention phase in abnormal fatty acid metabolites among a subgroup of 110 patients and found that these levels tended to decline among those in the intervention group but not among the controls. These metabolites have been associated with disordered metabolism in patient with heart failure, so the observed reduced levels were consistent with the other outcomes. “The signals all went in the direction of reduced metabolic dysregulation,” said Dr. Felker.
Despite the positive outcomes of the intervention studied, Dr. Felker said that this type of approach needs further refinement and study before it’s ready for widespread use. “I think TARGET-HF-DM is another piece of the puzzle, but like all small trials it needs replication in larger trials before adoption into practice guidelines,” he added.
The study received no commercial funding. Dr. Felker has been a consultant to Amgen, Bristol-Myers Squibb, Cytokinetics, Medtronic, Novartis, Reprieve, and Sequana, and he has received research funding from several companies. Dr. Piano had no disclosures.
A 3-month lifestyle intervention that used a step counter and regular, personalized text messages to encourage increased mobility and adherence to medications led to a substantial rise in the quality of life in a randomized controlled study with 187 U.S. patients with heart failure and diabetes.
The TARGET-HF-DM study supplied a wrist-worn step counting device to adults with any type of heart failure and any type of diabetes at six U.S. sites and collected data on daily step counts and medication adherence through smartphone-based apps. Researchers randomized the patients to an intervention of thrice-weekly text messages that gave them personalized feedback on their recent activity and adherence and updated activity and adherence goals, or to a control group that only received a once-weekly generic message to wear the step counter.
After 3 months, patients in the intervention arm had an average incremental gain of 313 steps per day from baseline, compared with the controls, a significant difference for the study’s primary endpoint, G. Michael Felker, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
A ‘quite large’ increase in quality of life.
Perhaps more importantly, a secondary analysis assessed quality of life with the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, which showed after 3 months a 5.5-point average increased improvement among patients in the intervention arm, compared with controls. Score increases of 5 of more points on the KCCQ represent clinically meaningful changes.
This average, incremental KCCQ score improvement was “quite large relative to what we typically see in placebo-controlled trials of effective drugs,” said Dr. Felker, professor of medicine at Duke University, Durham, N.C., and director of cardiovascular research at the Duke Clinical Research Institute. If a similar magnitude change in KCCQ was associated with a drug treatment “we would say it was an incredibly large signal in terms of quality of life, so I think the patients are telling us that [the intervention] is making a clinically important difference.”
But Dr. Felker cautioned that the study was not blinded, raising the possibility that the change in quality of life could have been partially explained by “patients feeling more engaged about doing something for their health.”
His report omitted data on the medication adherence facet of the study, which will come out in a subsequent report, raising the possibility that some of the quality of life benefit as well as the ability of patients to boost their step count was related to more consistent treatment with their prescribed medications, but Dr. Felker discounted this possibility.
“The adherence intervention was basically a digital tool that helped people better remember their medication regimen. While it is possible that this could have influenced the KCCQ data this seems quite unlikely to me,” he said in an interview.
‘Exercise is the new magic’
“Exercise is the new magic,” commented Mariann R. Piano, PhD, a professor at Vanderbilt University, Nashville, Tenn., and cochair of the session where Dr. Felker gave his report. “I love that the trial was pragmatic, randomized, and ran at six sites so the generalizability of the findings is really strong.” Dr. Piano also gave the study high marks for recruiting many African American patients, 47% of the study population, and its assessment of a patient-reported outcome, the KCCQ score.
Patients enrolled in TARGET-HF-DM averaged 59 years of age, about a third were women, and two-thirds had heart failure with a reduced ejection fraction of 40% or less. Eighty percent of participants had New York Heart Association class II functional limitations, and a third also had atrial fibrillation. Their average serum level of the N-terminal of the prohormone brain natriuretic peptide at baseline was 1,309 pg/mL. Most patients were on standard heart failure and diabetes medications, with 88% receiving an ACE inhibitor or angiotensin-receptor blocker (in some cases coupled with sacubitril), 90% were on a beta-blocker, 50% were on a mineralocorticoid receptor antagonist, 54% were on insulin, 47% were on a biguanidine, 25% were on a sulfonylurea, and 7% were on a sodium-glucose cotransporter inhibitor. About half the patients also had an implantable cardioverter defibrillator.
Dr. Felker acknowledged that the 313 average increment in steps per day among patients in the intervention group, compared with controls was modest, but it represented about a 10% increase from baseline among patients who in general had a very sedentary life. All patients had received at the start of the study guidelines from the American Heart Association on appropriate types and levels of physical activity for patients with heart failure and diabetes. The researcher previously published a description of the design and rationale of the study.
The study followed patients for an additional 3 months beyond the end of the intervention period, and the excess step count among people in the intervention arm persisted, although the between-group difference was no longer significant. The researchers also analyzed changes during the intervention phase in abnormal fatty acid metabolites among a subgroup of 110 patients and found that these levels tended to decline among those in the intervention group but not among the controls. These metabolites have been associated with disordered metabolism in patient with heart failure, so the observed reduced levels were consistent with the other outcomes. “The signals all went in the direction of reduced metabolic dysregulation,” said Dr. Felker.
Despite the positive outcomes of the intervention studied, Dr. Felker said that this type of approach needs further refinement and study before it’s ready for widespread use. “I think TARGET-HF-DM is another piece of the puzzle, but like all small trials it needs replication in larger trials before adoption into practice guidelines,” he added.
The study received no commercial funding. Dr. Felker has been a consultant to Amgen, Bristol-Myers Squibb, Cytokinetics, Medtronic, Novartis, Reprieve, and Sequana, and he has received research funding from several companies. Dr. Piano had no disclosures.
A 3-month lifestyle intervention that used a step counter and regular, personalized text messages to encourage increased mobility and adherence to medications led to a substantial rise in the quality of life in a randomized controlled study with 187 U.S. patients with heart failure and diabetes.
The TARGET-HF-DM study supplied a wrist-worn step counting device to adults with any type of heart failure and any type of diabetes at six U.S. sites and collected data on daily step counts and medication adherence through smartphone-based apps. Researchers randomized the patients to an intervention of thrice-weekly text messages that gave them personalized feedback on their recent activity and adherence and updated activity and adherence goals, or to a control group that only received a once-weekly generic message to wear the step counter.
After 3 months, patients in the intervention arm had an average incremental gain of 313 steps per day from baseline, compared with the controls, a significant difference for the study’s primary endpoint, G. Michael Felker, MD, reported at the annual scientific meeting of the Heart Failure Society of America.
A ‘quite large’ increase in quality of life.
Perhaps more importantly, a secondary analysis assessed quality of life with the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, which showed after 3 months a 5.5-point average increased improvement among patients in the intervention arm, compared with controls. Score increases of 5 of more points on the KCCQ represent clinically meaningful changes.
This average, incremental KCCQ score improvement was “quite large relative to what we typically see in placebo-controlled trials of effective drugs,” said Dr. Felker, professor of medicine at Duke University, Durham, N.C., and director of cardiovascular research at the Duke Clinical Research Institute. If a similar magnitude change in KCCQ was associated with a drug treatment “we would say it was an incredibly large signal in terms of quality of life, so I think the patients are telling us that [the intervention] is making a clinically important difference.”
But Dr. Felker cautioned that the study was not blinded, raising the possibility that the change in quality of life could have been partially explained by “patients feeling more engaged about doing something for their health.”
His report omitted data on the medication adherence facet of the study, which will come out in a subsequent report, raising the possibility that some of the quality of life benefit as well as the ability of patients to boost their step count was related to more consistent treatment with their prescribed medications, but Dr. Felker discounted this possibility.
“The adherence intervention was basically a digital tool that helped people better remember their medication regimen. While it is possible that this could have influenced the KCCQ data this seems quite unlikely to me,” he said in an interview.
‘Exercise is the new magic’
“Exercise is the new magic,” commented Mariann R. Piano, PhD, a professor at Vanderbilt University, Nashville, Tenn., and cochair of the session where Dr. Felker gave his report. “I love that the trial was pragmatic, randomized, and ran at six sites so the generalizability of the findings is really strong.” Dr. Piano also gave the study high marks for recruiting many African American patients, 47% of the study population, and its assessment of a patient-reported outcome, the KCCQ score.
Patients enrolled in TARGET-HF-DM averaged 59 years of age, about a third were women, and two-thirds had heart failure with a reduced ejection fraction of 40% or less. Eighty percent of participants had New York Heart Association class II functional limitations, and a third also had atrial fibrillation. Their average serum level of the N-terminal of the prohormone brain natriuretic peptide at baseline was 1,309 pg/mL. Most patients were on standard heart failure and diabetes medications, with 88% receiving an ACE inhibitor or angiotensin-receptor blocker (in some cases coupled with sacubitril), 90% were on a beta-blocker, 50% were on a mineralocorticoid receptor antagonist, 54% were on insulin, 47% were on a biguanidine, 25% were on a sulfonylurea, and 7% were on a sodium-glucose cotransporter inhibitor. About half the patients also had an implantable cardioverter defibrillator.
Dr. Felker acknowledged that the 313 average increment in steps per day among patients in the intervention group, compared with controls was modest, but it represented about a 10% increase from baseline among patients who in general had a very sedentary life. All patients had received at the start of the study guidelines from the American Heart Association on appropriate types and levels of physical activity for patients with heart failure and diabetes. The researcher previously published a description of the design and rationale of the study.
The study followed patients for an additional 3 months beyond the end of the intervention period, and the excess step count among people in the intervention arm persisted, although the between-group difference was no longer significant. The researchers also analyzed changes during the intervention phase in abnormal fatty acid metabolites among a subgroup of 110 patients and found that these levels tended to decline among those in the intervention group but not among the controls. These metabolites have been associated with disordered metabolism in patient with heart failure, so the observed reduced levels were consistent with the other outcomes. “The signals all went in the direction of reduced metabolic dysregulation,” said Dr. Felker.
Despite the positive outcomes of the intervention studied, Dr. Felker said that this type of approach needs further refinement and study before it’s ready for widespread use. “I think TARGET-HF-DM is another piece of the puzzle, but like all small trials it needs replication in larger trials before adoption into practice guidelines,” he added.
The study received no commercial funding. Dr. Felker has been a consultant to Amgen, Bristol-Myers Squibb, Cytokinetics, Medtronic, Novartis, Reprieve, and Sequana, and he has received research funding from several companies. Dr. Piano had no disclosures.
FROM HFSA 2021
Weight-loss surgery linked to fewer cardiovascular events, more so with RYGB
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
Those are the key findings of a retrospective analysis of a large group of patients who received care at the Cleveland Clinic between 1998 and 2017. MACE is defined as first occurrence of coronary artery events, cerebrovascular events, heart failure, nephropathy, atrial fibrillation, and all-cause mortality.
“I think what it tells us is that, in making these choices and in counseling patients about the potential advantages of undergoing bariatric surgery for their obesity and diabetes, that they should know that they’re more likely to be protected by a Roux-en-Y gastric bypass, although certainly sleeve gastrectomy is effective,” said study coauthor Steven E. Nissen, MD, who is the chief academic officer of the Heart and Vascular Institute at the Cleveland Clinic.
Previous studies have shown a benefit to metabolic surgery in patients with type 2 diabetes and obesity, improving diabetes control and altering cardiometabolic risk factors. Others have shown a link between surgery and reduced mortality. Most studies examined the impact of RYGB. SG is a newer procedure, but its relative simplicity and lower complication rate have helped it become the most commonly performed metabolic surgery in the world.
“There was no study to compare gastric bypass and sleeve gastrectomy head to head in terms of reduction in risk of cardiovascular disease. There are studies comparing these two procedures for diabetes control and weight loss, but not specifically in terms of effects on their risk of developing cardiovascular disease. That’s the unique feature of this study,” said lead author Ali Aminian, MD, who is director of the Bariatric and Metabolic Institute at the Cleveland Clinic.
The researchers included 2,287 adults with type 2 diabetes and a body mass index of at least 30 kg/m2, with no history of solid organ transplant, severe heart failure, or active cancer. 1,362 underwent RYGB, and 693 SG. Outcomes were compared with 11,435 matched nonsurgical patients.
At 5 years, 13.7% of the RYGB group experienced a MACE (95% confidence interval, 11.4-15.9), compared with 24.7% of the SG group for a relative reduction of 33% (95% CI, 19.0-30.0; adjusted hazard ratio, 0.77; P = .035). The nonsurgical group had a 5-year MACE incidence of 30.4% (95% CI, 29.4-31.5). Compared with usual care, the risk of MACE was lower in both the RYGB group (HR, 0.53; P < .001) and the SG group (HR, 0.69; P < .001). The researchers also analyzed the cumulative incidence of all-cause mortality, myocardial infarction, and ischemic stroke (three-component MACE) at 5 years. The cumulative incidence of three-component MACE at 5 years was 15.5% in the usual care group, 6.4% in the RYGB group (HR, 0.53 versus usual care; P < .001) and 11.8% in the SG group (HR vs. usual care, 0.65; P = .006).
The RYGB group had less nephropathy at 5 years (2.8% vs. 8.3%; HR, 0.47; P = .005), and experienced a greater reduction in weight, glycated hemoglobin, and diabetes and cardiovascular medication use. At 5 years, RYGB was associated with a higher frequency of upper endoscopy (45.8% vs. 35.6%, P < .001) and abdominal surgical procedures (10.8% vs. 5.4%, P = .001), compared with SG.
“Both procedures are extremely safe and extremely effective,” said Dr. Aminian. He pointed out the need to consider multiple factors when choosing between the procedures, including overall health, weight, comorbidities, and the patient’s values and goals.
A few factors may be contraindicated for one procedure or another. The sleeve may worsen severe reflux disease, while the gastric bypass may interfere more with absorption of psychiatric medications. Some patients may have multiple comorbidities that could point to a less risky procedure. “Decision-making should not be solely based on findings of this study. All these conditions need to be considered when patients and surgeons make a final decision about the most appropriate procedure,” said Dr. Aminian.
Dr. Nissen noted that the associations were wide ranging, including classic outcomes like death, stroke, and heart failure, but also extending to heart failure, coronary events, cerebral vascular events, nephropathy, and atrial fibrillation. “I found the nephropathy results to be amongst the most striking, that Roux-en-Y really dramatically reduced the risk of neuropathy,” he added. That’s a particularly important point because end-stage renal disease is a common cause of diabetes mortality.
Dr. Nissen acknowledged the limitations of the retrospective nature of the study, though he feels confident that the relationships are causal. “Bariatric surgery desperately needs a randomized, controlled trial, where both groups get intensive dietary and lifestyle counseling, but one group gets metabolic surgery and the other doesn’t. Given the dramatic effects in diabetic patients of reducing their hemoglobin A1c in a sustained way, reducing their body weight. We think these are very strong data to suggest that we have a major reduction in all the endpoints. If we’re right about this, the randomized controlled trial will show that dramatic effect, and will convince even the skeptics that metabolic surgery is the best way to go.”
FROM DIABETES CARE
The benefits—and inequities—of improved diabetes care
Primary care clinicians care for the vast majority of the 34 million individuals in the United States with type 2 diabetes; these patients make up about 11% of visits in most practices.1,2 Maximizing their health requires that we make the most of the ever-growing number of medications and devices that can be used to manage diabetes, while being sensitive to the health care inequities that limit patient access to the best care we have to offer.
A growing number of effective Tx options. In the past few years, we have seen the number of new drug classes for treating type 2 diabetes climb steadily. Within-class effects and adverse effects vary widely, demanding familiarity with the proven benefits of each individual drug. The advent of oral and injectable agents that include glucagon-like peptide 1 (GLP-1) receptor agonists and sodium glucose cotransporter 2 (SGLT2) inhibitors now supplement an expanding list of reliable basal insulins. Never before have we had such effective drugs with fewer adverse effects to manage glycemic control. New evidence supports adding selected medicines from these categories to reduce the risk of cardiovascular disease, heart failure, or chronic kidney disease in patients at risk—regardless of the level of glucose control.
The benefit of more achievable goals. When the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial began in 1999, the UKPDS (United Kingdom Prospective Diabetes Study) had just demonstrated that lower blood sugars resulted in lower morbidity in patients with type 2 diabetes. Colleagues insisted that an extrapolation of UKPDS results suggested that low blood sugars were better, and that it would be unethical to allow a patient to maintain an A1C of 7.5% if less than 6.0% was possible.
By 2008, the ACCORD trial demonstrated that more lives were saved with a less aggressive approach, and family physicians could breathe a sigh of relief as they addressed other important comorbidities of diabetes. However, the tools we used in ACCORD were rudimentary compared to today’s approaches. As glycemic control becomes safer and more effective, demands for further normalizing glycemic control to minimize complications are inevitable.
Devices have transformed care, too. A wide variety of new continuous monitoring devices, delivery systems, and self-management tools provide more options for ensuring that treatment is less disruptive and more effective than ever before. Inevitably, the advent of these major advances also brings new and serious challenges. Practices will need to transform to support the demands and the needs of our patients.
Practice transformation is necessary if primary care is to continue the delivery of high-quality diabetes care. The link between practice diabetes performance measures and the introduction of enhanced patient-centered care teams providing proactive outreach is clear.3
Our biggest challenge. Despite advances in the science, perhaps the biggest challenge in diabetes care is the inevitable inequity in access to new medications. The average wholesale price of glargine has soared to $340 per month, while the most effective new GLP-1 receptor agonists are close to $1000 per month.4
Continue to: Although primary care doctors...
Although primary care doctors have always tried to accommodate the uninsured, the stark differences between new and old medicines now resembles a 2-tiered system. We can all celebrate advances in diabetes care and work hard to learn when and how to best use them, but those advances are accompanied by an uncomfortable awareness of the enormous inequity of prescribing regimens that haven't been considered best practice since the 1990s to patients who simply can’t afford better medicine.
We can expect amplified inequities in diabetes clinical outcomes to continue unless we develop a better system of distributing these life-changing medicines to those Americans who need them. Some state legislatures have made progress by supporting limited access to affordable insulin. However, ensuring that all patients with diabetes have access to modern insulin and effective medications is a national responsibility that needs a national response. Universal access to the modern tools of basic health care is a long-overdue treatment for an expanding epidemic of inequity.
1. CDC. National Diabetes Statistics Report, 2020. Accessed August 30, 2021. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
2. Ashman JJ, Talwalkar A, Taylor SA. Age differences in visits to office-based physicians by patients with diabetes: United States, 2010. NCHS data brief, no 161; July 2014. Accessed August 30, 2021. www.cdc.gov/nchs/data/databriefs/db161.pdf
3. Solberg LI, Peterson KA, Fu H, et al. Strategies and factors associated with top performance in primary care for diabetes: insights from a mixed methods study. Ann Fam Med. 2021;19:110-116. doi: 10.1370/afm.2646
4. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl. 1):S111–S124.
Primary care clinicians care for the vast majority of the 34 million individuals in the United States with type 2 diabetes; these patients make up about 11% of visits in most practices.1,2 Maximizing their health requires that we make the most of the ever-growing number of medications and devices that can be used to manage diabetes, while being sensitive to the health care inequities that limit patient access to the best care we have to offer.
A growing number of effective Tx options. In the past few years, we have seen the number of new drug classes for treating type 2 diabetes climb steadily. Within-class effects and adverse effects vary widely, demanding familiarity with the proven benefits of each individual drug. The advent of oral and injectable agents that include glucagon-like peptide 1 (GLP-1) receptor agonists and sodium glucose cotransporter 2 (SGLT2) inhibitors now supplement an expanding list of reliable basal insulins. Never before have we had such effective drugs with fewer adverse effects to manage glycemic control. New evidence supports adding selected medicines from these categories to reduce the risk of cardiovascular disease, heart failure, or chronic kidney disease in patients at risk—regardless of the level of glucose control.
The benefit of more achievable goals. When the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial began in 1999, the UKPDS (United Kingdom Prospective Diabetes Study) had just demonstrated that lower blood sugars resulted in lower morbidity in patients with type 2 diabetes. Colleagues insisted that an extrapolation of UKPDS results suggested that low blood sugars were better, and that it would be unethical to allow a patient to maintain an A1C of 7.5% if less than 6.0% was possible.
By 2008, the ACCORD trial demonstrated that more lives were saved with a less aggressive approach, and family physicians could breathe a sigh of relief as they addressed other important comorbidities of diabetes. However, the tools we used in ACCORD were rudimentary compared to today’s approaches. As glycemic control becomes safer and more effective, demands for further normalizing glycemic control to minimize complications are inevitable.
Devices have transformed care, too. A wide variety of new continuous monitoring devices, delivery systems, and self-management tools provide more options for ensuring that treatment is less disruptive and more effective than ever before. Inevitably, the advent of these major advances also brings new and serious challenges. Practices will need to transform to support the demands and the needs of our patients.
Practice transformation is necessary if primary care is to continue the delivery of high-quality diabetes care. The link between practice diabetes performance measures and the introduction of enhanced patient-centered care teams providing proactive outreach is clear.3
Our biggest challenge. Despite advances in the science, perhaps the biggest challenge in diabetes care is the inevitable inequity in access to new medications. The average wholesale price of glargine has soared to $340 per month, while the most effective new GLP-1 receptor agonists are close to $1000 per month.4
Continue to: Although primary care doctors...
Although primary care doctors have always tried to accommodate the uninsured, the stark differences between new and old medicines now resembles a 2-tiered system. We can all celebrate advances in diabetes care and work hard to learn when and how to best use them, but those advances are accompanied by an uncomfortable awareness of the enormous inequity of prescribing regimens that haven't been considered best practice since the 1990s to patients who simply can’t afford better medicine.
We can expect amplified inequities in diabetes clinical outcomes to continue unless we develop a better system of distributing these life-changing medicines to those Americans who need them. Some state legislatures have made progress by supporting limited access to affordable insulin. However, ensuring that all patients with diabetes have access to modern insulin and effective medications is a national responsibility that needs a national response. Universal access to the modern tools of basic health care is a long-overdue treatment for an expanding epidemic of inequity.
Primary care clinicians care for the vast majority of the 34 million individuals in the United States with type 2 diabetes; these patients make up about 11% of visits in most practices.1,2 Maximizing their health requires that we make the most of the ever-growing number of medications and devices that can be used to manage diabetes, while being sensitive to the health care inequities that limit patient access to the best care we have to offer.
A growing number of effective Tx options. In the past few years, we have seen the number of new drug classes for treating type 2 diabetes climb steadily. Within-class effects and adverse effects vary widely, demanding familiarity with the proven benefits of each individual drug. The advent of oral and injectable agents that include glucagon-like peptide 1 (GLP-1) receptor agonists and sodium glucose cotransporter 2 (SGLT2) inhibitors now supplement an expanding list of reliable basal insulins. Never before have we had such effective drugs with fewer adverse effects to manage glycemic control. New evidence supports adding selected medicines from these categories to reduce the risk of cardiovascular disease, heart failure, or chronic kidney disease in patients at risk—regardless of the level of glucose control.
The benefit of more achievable goals. When the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial began in 1999, the UKPDS (United Kingdom Prospective Diabetes Study) had just demonstrated that lower blood sugars resulted in lower morbidity in patients with type 2 diabetes. Colleagues insisted that an extrapolation of UKPDS results suggested that low blood sugars were better, and that it would be unethical to allow a patient to maintain an A1C of 7.5% if less than 6.0% was possible.
By 2008, the ACCORD trial demonstrated that more lives were saved with a less aggressive approach, and family physicians could breathe a sigh of relief as they addressed other important comorbidities of diabetes. However, the tools we used in ACCORD were rudimentary compared to today’s approaches. As glycemic control becomes safer and more effective, demands for further normalizing glycemic control to minimize complications are inevitable.
Devices have transformed care, too. A wide variety of new continuous monitoring devices, delivery systems, and self-management tools provide more options for ensuring that treatment is less disruptive and more effective than ever before. Inevitably, the advent of these major advances also brings new and serious challenges. Practices will need to transform to support the demands and the needs of our patients.
Practice transformation is necessary if primary care is to continue the delivery of high-quality diabetes care. The link between practice diabetes performance measures and the introduction of enhanced patient-centered care teams providing proactive outreach is clear.3
Our biggest challenge. Despite advances in the science, perhaps the biggest challenge in diabetes care is the inevitable inequity in access to new medications. The average wholesale price of glargine has soared to $340 per month, while the most effective new GLP-1 receptor agonists are close to $1000 per month.4
Continue to: Although primary care doctors...
Although primary care doctors have always tried to accommodate the uninsured, the stark differences between new and old medicines now resembles a 2-tiered system. We can all celebrate advances in diabetes care and work hard to learn when and how to best use them, but those advances are accompanied by an uncomfortable awareness of the enormous inequity of prescribing regimens that haven't been considered best practice since the 1990s to patients who simply can’t afford better medicine.
We can expect amplified inequities in diabetes clinical outcomes to continue unless we develop a better system of distributing these life-changing medicines to those Americans who need them. Some state legislatures have made progress by supporting limited access to affordable insulin. However, ensuring that all patients with diabetes have access to modern insulin and effective medications is a national responsibility that needs a national response. Universal access to the modern tools of basic health care is a long-overdue treatment for an expanding epidemic of inequity.
1. CDC. National Diabetes Statistics Report, 2020. Accessed August 30, 2021. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
2. Ashman JJ, Talwalkar A, Taylor SA. Age differences in visits to office-based physicians by patients with diabetes: United States, 2010. NCHS data brief, no 161; July 2014. Accessed August 30, 2021. www.cdc.gov/nchs/data/databriefs/db161.pdf
3. Solberg LI, Peterson KA, Fu H, et al. Strategies and factors associated with top performance in primary care for diabetes: insights from a mixed methods study. Ann Fam Med. 2021;19:110-116. doi: 10.1370/afm.2646
4. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl. 1):S111–S124.
1. CDC. National Diabetes Statistics Report, 2020. Accessed August 30, 2021. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
2. Ashman JJ, Talwalkar A, Taylor SA. Age differences in visits to office-based physicians by patients with diabetes: United States, 2010. NCHS data brief, no 161; July 2014. Accessed August 30, 2021. www.cdc.gov/nchs/data/databriefs/db161.pdf
3. Solberg LI, Peterson KA, Fu H, et al. Strategies and factors associated with top performance in primary care for diabetes: insights from a mixed methods study. Ann Fam Med. 2021;19:110-116. doi: 10.1370/afm.2646
4. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl. 1):S111–S124.
PRESERVED-HF: Dapagliflozin improves physical limitations in patients with HFpEF
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
The SGLT2 inhibitor dapagliflozin scored a clear win in a randomized, controlled trial with more than 300 U.S. patients with heart failure with preserved ejection fraction (HFpEF), showing a significant and clinically meaningful benefit for the primary endpoint, a KCCQ measure of symptoms and physical limitations, after 12 weeks of treatment.
These results in the PRESERVED-HF study follow closely on the heals of the initial report from the EMPEROR-Preserved trial that showed a benefit from a different sodium-glucose cotransporter 2 (SGLT2) inhibitor, empagliflozin (Jardiance) in nearly 6,000 randomized patients for the primary endpoint of preventing cardiovascular death or hospitalizations for heart failure.
In PRESERVED-HF, patients with HFpEF who received a standard, once-daily dose of dapagliflozin (Farxiga) had an average 5.8-point improvement in their condition as measured by the Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CS), the study’s primary endpoint.
This is “the first study to demonstrate that an SGLT2 inhibitor dapagliflozin significantly improves symptoms, physical limitations, and 6-minute walking distance in patients with HFpEF,” Mikhail N. Kosiborod, MD, reported at the annual scientific meeting of the Heart Failure Society of America. The secondary endpoint of 6-minute walking distance “has been very difficult to improve in many previous studies of other treatments” tested in patients with HFpEF, noted Dr. Kosiborod, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute.
The results are “highly complementary” to the findings from large outcome trials, such as the findings from EMPEROR-Preserved, he said, and collectively the recent findings from these studies of SGLT2 inhibitors in patients with HFpEF identify drugs in this class as a “new treatment option” for patients with a disorder that until now had no treatment with unequivocally proven efficacy and safety.
‘Impressive and unprecedented’ findings
The findings are “really impressive and unprecedented,” said Milton Packer, MD, a cardiologist at Baylor University Medical Center in Dallas who was not involved in the study. “This is the largest KCCQ benefit ever seen in either patients with HFpEF or in patients with heart failure with reduced ejection fraction,” said Dr. Packer, one of the investigators who led the EMPEROR-Preserved trial.
PRESERVED-HF randomized 324 patients diagnosed with heart failure and with a left ventricular ejection fraction of 45% or higher at any of 26 U.S. centers, with 304 patients completing the planned final analysis after 12 weeks on treatment. Patients could be in New York Heart Association (NYHA) functional class II-IV, they had to have a baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) level of at least 225 pg/mL (or higher if they also had atrial fibrillation), and they required at least one of three markers of established heart failure: recent hospitalization for heart failure or an urgent outpatient visit that required treatment with an IV diuretic, elevated filling pressure measured by left or right catheterization, or structural heart disease detected by echocardiography.
The average age of the enrolled patients was 70 years, and they had been diagnosed with heart failure for about 3 years; 57% were women, 30% were African American, and their median body mass index was 35 kg/m2. Roughly 42% had NYHA class III or IV disease, 56% had type 2 diabetes, their median estimated glomerular filtration rate was about 55 mL/min per 1.73m2, their median KCCQ-CS score at baseline was about 62, and their average 6-minute walk distance was 244 m.
These and other features of the enrolled population define a distinctly U.S. patient population, stressed Dr. Kosiborod, professor of medicine at the University of Missouri–Kansas City.
“The patients we enrolled are the patients we see in U.S. clinical practice,” he said in an interview. Importantly, the patient profile of a median BMI of 35 kg/m2, a median KCCQ-CS score of 62 – “quite low,” noted Dr. Kosiborod – and having more than 40% of patients in NYHA functional class III defines a study population with a substantially greater burden of obesity, symptoms, and functional impairment compared with those enrolled in prior trials involving patients with HFpEF such as EMPEROR-Preserved.
Results complement findings from larger trials
PRESERVED-HF was an investigator-initiated study designed to inform clinical practice, not as a pivotal trial like EMPEROR-Preserved, which aims to gather evidence to support a new indication for regulatory approval. (On Sept. 9, 2021, the Food and Drug Administration granted empagliflozin “breakthrough therapy” status for treating HFpEF based on the EMPEROR-Preserved results, which will fast-track the agency’s decision on this indication.)
Dr. Kosiborod noted that he and his associates designed PRESERVED-HF with adequate patient numbers to power a statistically valid assessment of effect on KCCQ-CS score. While the new findings will not by themselves lead to a new indication for dapagliflozin to treat patients with HFpEF, they will potentially complement the pending results of another trial, DELIVER, by showing efficacy and safety in a uniquely U.S. patient population. DELIVER is a pivotal, global trial of dapagliflozin in more than 6,000 patients with HFpEF that’s on track to report findings in 2022.
Dr. Kosiborod also stressed that dapagliflozin has U.S.-approved indications for treating patients with type 2 diabetes, and for patients with chronic kidney disease, and that a majority of patients enrolled in PRESERVED-HF had one or both of these conditions. That makes the new findings especially compelling for patients with either type 2 diabetes or chronic kidney disease and HFpEF who are not already receiving an SGLT2 inhibitor.
Other findings that he reported showed a range of benefits consistent with the primary endpoint, including the KCCQ overall summary score, which also showed a significant 4.5-point average increase over placebo after 12 weeks. Analysis by the percentage of patients achieving at least a 5-point improvement in the KCCQ clinical summary score (the threshold for a clinically meaningful improvement) showed that about 45% of patients treated with dapagliflozin reached this mark compared with roughly 35% of patients in the placebo arm, indicating a number needed to treat of nine to have one additional patient achieve this threshold after 12 weeks. Average improvement in 6-minute walk distance was about 20 m with dapagliflozin compared with placebo.
No heterogeneity of effect by baseline ejection fraction.
Subgroup analyses showed no heterogeneity of response across 12 different ways of subdividing the study population, including age, sex, race, diabetes status, and BMI. The median left ventricular ejection fraction among enrolled patients was 60%, and the findings showed identical KCCQ improvements among patients with ejection fractions less than the median and those with an ejection fraction above the median.
This last finding was especially relevant because the EMPEROR-Preserved results showed a possible signal of heterogeneity by ejection fraction and an attenuated effect among patients with HFpEF and an ejection fraction above the 60%-65% range, although the certainty of this finding is currently controversial.
The impact of empagliflozin on KCCQ clinical summary score in EMPEROR-Preserved showed an average incremental improvement of 1.32 points compared with placebo, a significant difference, but more modest than the increment from dapagliflozin treatment seen in PRESERVED-HF. Dr. Kosiborod hypothesized that this difference might be mostly because of the different patient populations enrolled in the two studies.
Dr. Kosiborod noted that a report on the PRESERVED-HF results will soon appear in Nature Medicine.
PRESERVED-HF was funded by AstraZeneca, which markets dapagliflozin (Farxiga), but the trials’ design and conduct were independent of this funding source. Dr. Kosiborod has been a consultant to AstraZeneca and numerous other companies, and he has received research funding from AstraZeneca and Boehringer Ingelheim. Dr. Packer has had financial relationships with AstraZeneca and numerous other companies.
FROM HFSA 2021
Are ESC’s new heart failure guidelines already outdated?
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”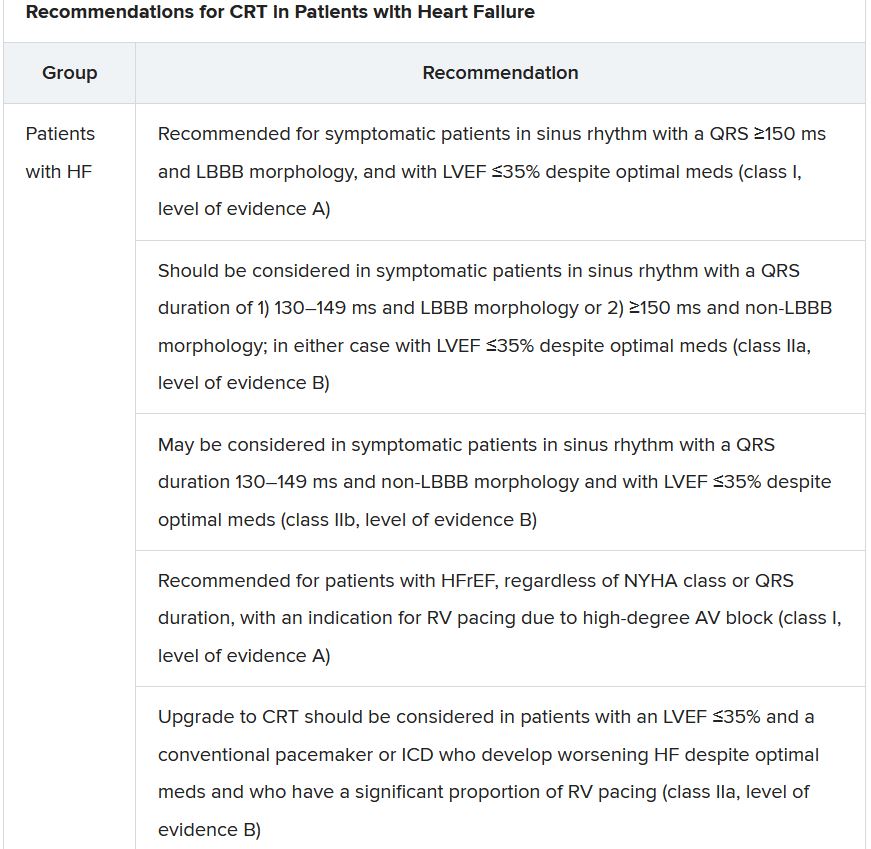
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.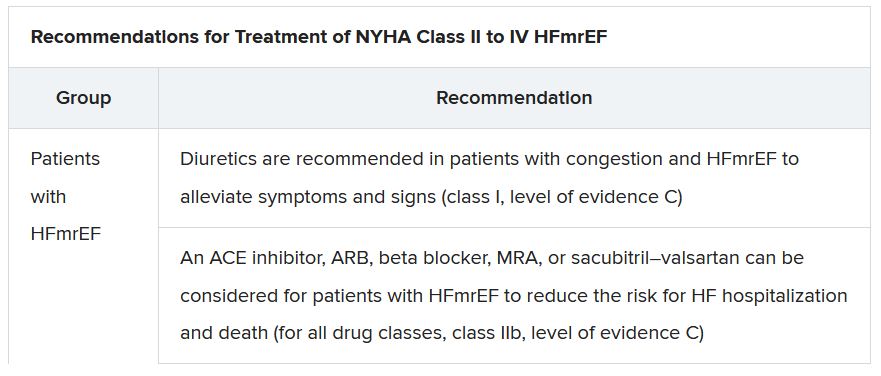
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
The new guideline on management of heart failure (HF) from the European Society of Cardiology seemed to bear an asterisk or footnote even before its full unveiling in the early hours of ESC Congress 2021.
The document would offer little new in the arena of HF with preserved ejection fraction (HFpEF), so understandably the fast-approaching presentation of a major HFpEF trial – arguably the conference’s marquee event – would feel to some like the elephant in the room.
“I’d like to highlight this unfortunate timing of the guideline, because it’s an hour or 2 before we hear the full story from EMPEROR-Preserved, which I’m sure will change the guidelines,” Faiez Zannad, MD, PhD, University of Lorraine, Vandoeuvre-Les-Nancy, France, said wryly.
Anticipation of the trial’s full presentation was intense as the ESC congress got underway, in part because the top-line and incomplete message from EMPEROR-Preserved had already been released: Patients with HFpEF treated with the sodium-glucose cotransporter 2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) showed a significant benefit for the primary endpoint of cardiovascular (CV) death or HF hospitalization.
Although empagliflozin is the first medication to achieve that status in a major HFpEF trial, conspicuously absent from the early announcement were the magnitude of “benefit” and any data. Still, the tantalizing top-line results mean that technically, at least, “we have a drug which is effective in reduced and preserved ejection fraction,” Dr. Zannad said.
But the new guideline, published online Aug. 27, 2021, in the European Heart Journal and comprehensively described that day at the congress, was never really expected to consider results from EMPEROR-Reduced. “These new indications do need to go through the regulatory authorities,” such as the European Medicines Agency and the U.S. Food and Drug Administration, observed Carlos Aguiar, MD, Hospital Santa Cruz, Carnaxide, Portugal.
“It does take some time for the whole process to be concluded and, finally, as physicians, being able to implement it in clinical practice,” Dr. Aguiar said as moderator of press briefing prior to the ESC congress.
The ESC guideline’s next iteration or update could well include an SGLT2 inhibitor recommendation that applies beyond the ejection fraction limits of HFrEF. Still, the document summarized that day reflects a number of pivotal concepts with profound treatment implications. Among them are the field’s latest paradigm for medical therapy of HFrEF and the increasingly accepted division of traditional HFpEF into two entities: HF with mildly reduced ejection fraction (HFmrEF); and HFpEF, with its left ventricular ejection fraction (LVEF) threshold raised to 50%.
In fact, HFmrEF in the new document is a drug-therapy indication that barely existed a few years ago but grew in prominence after secondary findings from trials like TOPCAT for spironolactone and PARAGON-HF for sacubitril-valsartan (Entresto, Novartis), an angiotensin-receptor/neprilysin inhibitor (ARNI). Still, the HFmrEF recommendations come with different class and level-of-evidence designations.
Those new guideline features and others in the realm of pharmacologic therapy were summarized by the document’s authors at the 2021 Heart Failure Association of the European Society of Cardiology (ESC-HFA) meeting, and covered at the time by this news organization
The ‘fantastic four’
One of the document’s central recommendations specifies which contemporary drug classes should be initiated, and when, in patients with HFrEF. An ACE inhibitor or ARNI, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor collectively earned a class I recommendation, “given the importance of these key HFrEF therapies, some of which have been shown to improve outcomes within a month of initiation,” observed Roy S. Gardner, MBChB, MD.
An agent from each of the four classes is to be “commenced and up-titrated as quickly and as safely as possible, whilst using the lowest effective dose of loop diuretic to relieve congestion,” said Dr. Gardner, from Golden Jubilee National Hospital, Clydebank, Scotland, when presenting the full HFrEF portion of the guidelines.
The oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo, Merck), which recently emerged from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization, gained a class IIb recommendation.
The document’s “simplified algorithm” for managing such patients overall and the advent of SGLT2 inhibitors are new twists in ESC guidelines for HF. But the way the four drug classes are started in patients is key and could take some practitioners time to get used to. There is no prespecified order of initiation.
“We’ve left the door open for clinicians to evaluate the evidence to make sure these four drugs are started, and to tailor how to do it according to the patient,” based on clinical considerations such as blood pressure or renal function, said Theresa A. McDonagh, MD, King’s College London, cochair of the guideline task force.
“The SGLT2 inhibitor trials were done on top of therapy with ACE inhibitors or ARNI, beta-blockers, and MRAs, so some people no doubt will choose to follow a sequenced approach,” Dr. McDonagh said. Other practitioners will consider each patient and attempt to get all four started “as quickly and safely as possible based on the phenotype.”
Importantly, clinicians “should not wait for weeks, months, or years until you have the four drugs in the patient, but you should do this within weeks,” cautioned Johann Bauersachs, MD, Hannover (Germany) Medical School, a discussant for the guideline presentation who is listed as a reviewer on the document.
Although angiotensin-receptor blockers (ARBs) and ACE inhibitors are sometimes thought of as interchangeable, the new guideline does not give them the same weight. “The angiotensin-receptor blocker valsartan is a constituent of the ARNI,” Dr. McDonagh noted. “So, the place of ARBs in heart failure has been downgraded in HFrEF. They are really for those who are intolerant of an ACE inhibitor or an ARNI.”
In practice, ARBs are likely to be used as first-line therapy in some circumstances, observed Dr. Bauersachs. They are “the default option in, unfortunately, many low-income countries that may not afford sacubitril-valsartan. And I know that there are many of them.”
Tweaks to device recommendations
The new document contains several new wrinkles in the recommendations for HF device therapy, which should usually be considered only if still appropriate after at least 3 months of optimal medical therapy, Dr. Gardner said.
For example, use of an implantable cardioverter-defibrillator (ICD) has been demoted from its previous class I recommendation to class II, level of evidence A, in patients with nonischemic cardiomyopathy “in light of the data from the DANISH study,” Dr. Gardner said.
The 2016 DANISH trial was noteworthy for questioning the survival benefits of ICDs in patients with nonischemic cardiomyopathy, whether or not they were also receiving cardiac resynchronization therapy (CRT).
The new document also puts greater emphasis on a range of specific CRT patient-selection criteria. Beyond the conventional recommended standards of an LVEF of 35% or less, QRS of at least 150 ms, and left-bundle-branch block on optimal meds, consideration can be given to CRT if the QRS is only 130 ms or greater. “And where it’s appropriate to do so, an ICD could be an option,” Dr. Gardner said.
It also recommends CRT as a replacement for right ventricular pacing in patients with high-degree atrioventricular block. “And this, for the first time, includes patients with atrial fibrillation,” he said. “The previous indications for CRT were in individuals in sinus rhythm.”
The new document recommends that HF in any patient be classified as HFrEF, defined by an LVEF of ≤40%; HFmrEF, defined by an LVEF of 41%-49%; or HFpEF, defined by an LVEF of at least 50%. “Importantly, for all forms, the presence of the clinical syndrome of heart failure is a prerequisite,” observed Carolyn S.P. Lam, MBBS, PhD, Duke-NUS Graduate Medical School, Singapore, at the presentation.
In a critical update from previous guidelines, the term HF with “mid-range” ejection fraction was replaced by the term specifying “mildly reduced” ejection fraction, Dr. Lam noted. The shift retains the acronym but now reflects growing appreciation that HFmrEF patients can benefit from treatments also used in HFrEF, including ACE inhibitors, ARBs, beta-blockers, MRAs, and sacubitril-valsartan, she said.
Support for that relationship comes largely from post hoc subgroup analyses of trials that featured some patients with LVEF 40%-49%. That includes most HFpEF trials represented in the guideline document, but also EMPEROR-Preserved, which saw gains for the primary outcome across the entire range of LVEF above 40%.
The LVEF-based definitions are consistent with a recent HF classification proposal endorsed by the ESC and subspecialty societies in Europe, North America, Japan, India, Australia, New Zealand, and China.
The document doesn’t update recommendations for HFpEF, in which “no treatment has been shown to convincingly reduce mortality or morbidity,” Dr. Lam observed. Still, she noted, the guideline task force “acknowledges that treatment options for HFpEF are being revised even as the guidelines have been published.”
That could be a reference to empagliflozin in EMPEROR-Preserved, but it also refers to the strikingly broad wording of an expanded indication for sacubitril-valsartan in the United States – “to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure” – without specific restrictions on the basis of LVEF. The new indication was announced in early 2021, too late to be considered in the new guidelines.
Whither LVEF-based definitions?
During discussion after the guideline presentation, Dr. Zannad speculated on the future of HF classifications based on ventricular function, given trial evidence in recent years that some agents – notably spironolactone, sacubitril-valsartan, and now, apparently, empagliflozin – might be effective in HFpEF as well as HFrEF.
Will the field continue with “LVEF-centric” distinctions across the range of HF, or transition to “some definition in which drug therapies can be used independently across the full spectrum of ejection fraction?” Dr. Zannad posed.
“I think we need to wait and see what some of these trials with the SGLT2 inhibitors are going to show in heart failure with preserved ejection fraction,” Dr. McDonagh replied. “And I think that will be a step for the next guideline, completely redefining heart failure.”
A version of this article first appeared on Medscape.com.
Novel diabetic foot ulcer cream shows promise in phase 3 trial
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ON101 (Fespixon, Oneness Biotech), a first-in-class, macrophage-regulating, wound-healing cream for diabetic foot ulcers has shown benefit over absorbent dressings in a phase 3 trial, with another trial ongoing.
The product became available in Taiwan on July 4, 2021, after receiving regulatory approval from the Taiwan Food and Drug Administration based on efficacy and safety findings in a three-country phase 3 clinical trial.
Oneness Biotech has also just started a second phase 3 trial in the United States, with a planned enrollment of 208 patients with diabetic foot ulcers, which will compare ON101 cream versus placebo cream, in addition to standard care, over 20 weeks.
The company expects to complete that trial and file a new drug application with the U.S. Food and Drug Administration in 2023, and a global launch is planned for 2025, said Oneness Biotech founder and CEO William Lu.
Current and upcoming trials
The Taiwan FDA approval of ON101 was based on a 236-patient clinical trial conducted in Taiwan, China, and the United States by Yu-Yao Huang MD, PhD, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, and colleagues, which was published online Sept. 3, 2021, in JAMA Network Open.
The study results will also be presented during an oral session at the European Association for the Study of Diabetes meeting on Sept. 30.
The published trial showed that foot ulcers treated with ON101 cream were almost three times more likely to be completely healed at 16 weeks than those treated with standard care with an absorbent dressing (Aquacel Hydrofiber, ConvaTec) (odds ratio, 2.84; P < .001).
“The findings of this study suggest that ON101, a macrophage regulator that behaves differently from moisture-retaining dressings, represents an active-healing alternative for home and primary care of patients with chronic [diabetic foot ulcers],” the researchers concluded.
“ON101 was also granted a fast track designation by the U.S. FDA in March this year,” senior author Shun-Chen Chang, MD, Taipei Medical University–Shuang Ho Hospital, New Taipei City, Taiwan, said in an interview.
“Patients in the United States can access this new drug via the expanded access program or by participating in the second phase 3 trial in the United States,” added coauthor Shawn M. Cazzell, DPM, chief medical officer, Limb Preservation Platform, Fresno, Calif., who is involved with both trials.
It is “exciting” to have a new therapy for diabetic foot ulcers, said Dr. Cazzell, because they are serious and life-threatening.
Could cream with plant extracts surpass current care?
Current standard clinical care for diabetic foot ulcer consists of debridement, off-loading, infection control, and maintaining a moist environment with dressings, Huang and colleagues explain. If the foot ulcer does not respond, growth factors, tissue-engineering products, hyperbaric oxygen, or negative pressure wound therapies may be used.
However, the number of amputations from chronic diabetic foot ulcers that do not heal is increasing, pointing to a need for better treatment options.
Hyperglycemia increases the ratio of M1 proinflammatory macrophages to M2 proregenerative macrophages, and accumulating evidence suggests this might be a potential treatment target.
Researchers at Oneness Biotech showed that ON101, which is comprised of extracts from two plants, Plectranthus amboinicus and Centella asiatica, exerts a wound-healing effect by regulating the balance between M1 and M2 macrophages.
An extract of one plant suppresses inflammation, while an extract of the other increases collagen synthesis.
In preclinical studies, these two plant extracts had a synergistic effect on balancing the ratio of M1 to M2 macrophages and accelerating wound healing in a mouse model. This was followed by promising efficacy and safety results in two trials of 24 patients and 30 patients.
Significantly better healing with ON101 than standard care
For the current phase 3, randomized clinical trial, researchers enrolled patients in 21 clinics from November 2012 to May 2020.
To be eligible for the study, patients had to be 20-80 years old, with a hemoglobin A1c less than 12%. They also had to have a Wagner grade 1 or 2 foot ulcer that was 1-25 cm2 after debridement, had been treated with standard care, and was present for at least 4 weeks.
Patients were a mean age of 57 years and 74% were men. They had a mean A1c of 8.1%, and 61% had had diabetes for more than 10 years.
Most (78%) of the diabetic foot ulcers were Wagner grade 2. The wounds had a mean area of 4.8 cm2 and had been present for a mean of 7 months.
Patients were instructed on how to self-administer ON101 cream twice a day (treatment group, n = 122) or how to apply an absorbent dressing and change it daily or two or three times a week (standard care group, n = 114). All patients were allowed to apply a sterile gauze dressing.
They visited the clinic every 2 weeks during the 16-week treatment phase and 12-week observation phase.
In the full analysis set, 74 patients (61%) in the ON101 group and 40 patients (35%) in the standard care group had complete wound healing after 16 weeks of treatment.
The subgroup of patients at higher risk of poor wound healing (A1c >9%, ulcer area >5 cm2, and diabetic foot ulcer duration >6 months) also had significantly better healing with the ON101 cream than standard care.
There were seven (5.7%) treatment-emergent adverse events in the ON101 group versus five (4.4%) in the standard care group.
There were no treatment-related serious adverse events in the ON101 group versus one (0.9%) in the comparator group.
The study was funded by Oneness Biotech, Microbio Group, and Shanghai Haihe Pharmaceutical. One author has reported receiving fees from Oneness Biotech, and Dr. Chang has reported receiving a speakers fee from Oneness Biotech. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At 18 months, much still unknown about diabetes and COVID-19
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.












