User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Do Women With Knee Osteoarthritis Experience Greater Pain Sensitivity Than Men?
Among patients with osteoarthritis of the knee, women experienced greater sensitivity to various pain modalities, such as lower tolerance to heat, cold, and pressure, and greater widespread pain than men, according to a study published online ahead of print October 5 in Arthritis Care & Research.
“Many questions still remain as to why women with knee osteoarthritis are more sensitive to painful stimuli than are men. While therapeutic approaches to control pain are only beginning to take these sex differences into account, there is still quite a bit of research yet to be done to help reduce this gender gap and improve clinical therapies for men and women alike,” said lead author Emily J. Bartley, PhD, a Research Assistant Professor at the University of Florida College of Dentistry in Gainsville.
For this study, 288 participants between the ages of 45 and 85 completed a battery of quantitative sensory pain procedures assessing sensitivity to contact heat, cold pressor, mechanical pressure, and punctate stimuli. Differences in temporal summation were examined, along with measures of clinical pain and functional performance.
When compared to men, women exhibited greater sensitivity to multiple pain modalities (eg, lower heat, cold, pressure thresholds/tolerances, greater temporal summation of pain). There were no sex differences in clinical pain with the exception of greater widespread pain observed in women. Although there were select age-related differences in pain sensitivity, sex differences in pain varied minimally across age cohort.
“Overall, these findings provide evidence for greater overall sensitivity to experimental pain in women with symptomatic knee osteoarthritis compared with men, suggesting that enhanced central sensitivity may be an important contributor to pain in this group,” wrote Dr. Bartley and colleagues.
Suggested Reading
Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken). 2015 Oct 5. [Epub ahead of print].
Among patients with osteoarthritis of the knee, women experienced greater sensitivity to various pain modalities, such as lower tolerance to heat, cold, and pressure, and greater widespread pain than men, according to a study published online ahead of print October 5 in Arthritis Care & Research.
“Many questions still remain as to why women with knee osteoarthritis are more sensitive to painful stimuli than are men. While therapeutic approaches to control pain are only beginning to take these sex differences into account, there is still quite a bit of research yet to be done to help reduce this gender gap and improve clinical therapies for men and women alike,” said lead author Emily J. Bartley, PhD, a Research Assistant Professor at the University of Florida College of Dentistry in Gainsville.
For this study, 288 participants between the ages of 45 and 85 completed a battery of quantitative sensory pain procedures assessing sensitivity to contact heat, cold pressor, mechanical pressure, and punctate stimuli. Differences in temporal summation were examined, along with measures of clinical pain and functional performance.
When compared to men, women exhibited greater sensitivity to multiple pain modalities (eg, lower heat, cold, pressure thresholds/tolerances, greater temporal summation of pain). There were no sex differences in clinical pain with the exception of greater widespread pain observed in women. Although there were select age-related differences in pain sensitivity, sex differences in pain varied minimally across age cohort.
“Overall, these findings provide evidence for greater overall sensitivity to experimental pain in women with symptomatic knee osteoarthritis compared with men, suggesting that enhanced central sensitivity may be an important contributor to pain in this group,” wrote Dr. Bartley and colleagues.
Among patients with osteoarthritis of the knee, women experienced greater sensitivity to various pain modalities, such as lower tolerance to heat, cold, and pressure, and greater widespread pain than men, according to a study published online ahead of print October 5 in Arthritis Care & Research.
“Many questions still remain as to why women with knee osteoarthritis are more sensitive to painful stimuli than are men. While therapeutic approaches to control pain are only beginning to take these sex differences into account, there is still quite a bit of research yet to be done to help reduce this gender gap and improve clinical therapies for men and women alike,” said lead author Emily J. Bartley, PhD, a Research Assistant Professor at the University of Florida College of Dentistry in Gainsville.
For this study, 288 participants between the ages of 45 and 85 completed a battery of quantitative sensory pain procedures assessing sensitivity to contact heat, cold pressor, mechanical pressure, and punctate stimuli. Differences in temporal summation were examined, along with measures of clinical pain and functional performance.
When compared to men, women exhibited greater sensitivity to multiple pain modalities (eg, lower heat, cold, pressure thresholds/tolerances, greater temporal summation of pain). There were no sex differences in clinical pain with the exception of greater widespread pain observed in women. Although there were select age-related differences in pain sensitivity, sex differences in pain varied minimally across age cohort.
“Overall, these findings provide evidence for greater overall sensitivity to experimental pain in women with symptomatic knee osteoarthritis compared with men, suggesting that enhanced central sensitivity may be an important contributor to pain in this group,” wrote Dr. Bartley and colleagues.
Suggested Reading
Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken). 2015 Oct 5. [Epub ahead of print].
Suggested Reading
Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res (Hoboken). 2015 Oct 5. [Epub ahead of print].
Does Arthritis Contribute to Higher Rates of Poverty In Women?
Developing arthritis increases a person’s risk of falling into poverty, especially for women, according to a study published online ahead of print September 8 in Arthritis & Rheumatology.
For this study of more than 4,000 Australian adults, females who developed arthritis were 51% more likely to fall into income poverty than nonarthritic women. In men, having arthritis was associated with a 22% increased risk of poverty.
Women with arthritis also were 87% more likely to fall into “multidimensional poverty,” which includes income, health, and education attainment, while the arthritis-related risk in men was 29%. The investigators noted that given the high prevalence of arthritis, the condition is an overlooked driver of poverty.
“With population ageing occurring in most of the developed nations around the world, health conditions such as arthritis will become increasingly common. That developing arthritis has such a pronounced impact on the risk of falling into poverty should flag to policy makers in welfare departments the influence of the condition on national living standards,” said lead author Emily Callander, PhD, from the Faculty of Pharmacy at the University of Sydney in Australia.
Researchers utilized survey data from the Household Income and Labour Dynamics in Australia (HILDA) Survey. Survival analysis using Cox regression models were applied to the nationally representative, longitudinal data between the years 2007 and 2012 for Australian adults ages 21 and older.
The hazard ratio for falling into income poverty for females who develop arthritis was 1.51, and for males the hazard ratio for falling into income poverty was 1.22, compared with people who never developed arthritis. The hazard ratio for falling into multidimensional poverty for females who develop arthritis was 1.87 and for males the hazard ratio was 1.29.
According to Dr. Callander, “The high risk of poverty should be kept in mind by clinicians seeking the most appropriate treatment for their patients with arthritis, as affordability of out-of-pocket costs may be an important factor.”
Suggested Reading
Callander EJ, Schofield DJ. Arthritis and the risk of falling into poverty: a survival analysis using Australian data. Arthritis Rheumatol. 2015 Sep 8. [Epub ahead of print].
Developing arthritis increases a person’s risk of falling into poverty, especially for women, according to a study published online ahead of print September 8 in Arthritis & Rheumatology.
For this study of more than 4,000 Australian adults, females who developed arthritis were 51% more likely to fall into income poverty than nonarthritic women. In men, having arthritis was associated with a 22% increased risk of poverty.
Women with arthritis also were 87% more likely to fall into “multidimensional poverty,” which includes income, health, and education attainment, while the arthritis-related risk in men was 29%. The investigators noted that given the high prevalence of arthritis, the condition is an overlooked driver of poverty.
“With population ageing occurring in most of the developed nations around the world, health conditions such as arthritis will become increasingly common. That developing arthritis has such a pronounced impact on the risk of falling into poverty should flag to policy makers in welfare departments the influence of the condition on national living standards,” said lead author Emily Callander, PhD, from the Faculty of Pharmacy at the University of Sydney in Australia.
Researchers utilized survey data from the Household Income and Labour Dynamics in Australia (HILDA) Survey. Survival analysis using Cox regression models were applied to the nationally representative, longitudinal data between the years 2007 and 2012 for Australian adults ages 21 and older.
The hazard ratio for falling into income poverty for females who develop arthritis was 1.51, and for males the hazard ratio for falling into income poverty was 1.22, compared with people who never developed arthritis. The hazard ratio for falling into multidimensional poverty for females who develop arthritis was 1.87 and for males the hazard ratio was 1.29.
According to Dr. Callander, “The high risk of poverty should be kept in mind by clinicians seeking the most appropriate treatment for their patients with arthritis, as affordability of out-of-pocket costs may be an important factor.”
Developing arthritis increases a person’s risk of falling into poverty, especially for women, according to a study published online ahead of print September 8 in Arthritis & Rheumatology.
For this study of more than 4,000 Australian adults, females who developed arthritis were 51% more likely to fall into income poverty than nonarthritic women. In men, having arthritis was associated with a 22% increased risk of poverty.
Women with arthritis also were 87% more likely to fall into “multidimensional poverty,” which includes income, health, and education attainment, while the arthritis-related risk in men was 29%. The investigators noted that given the high prevalence of arthritis, the condition is an overlooked driver of poverty.
“With population ageing occurring in most of the developed nations around the world, health conditions such as arthritis will become increasingly common. That developing arthritis has such a pronounced impact on the risk of falling into poverty should flag to policy makers in welfare departments the influence of the condition on national living standards,” said lead author Emily Callander, PhD, from the Faculty of Pharmacy at the University of Sydney in Australia.
Researchers utilized survey data from the Household Income and Labour Dynamics in Australia (HILDA) Survey. Survival analysis using Cox regression models were applied to the nationally representative, longitudinal data between the years 2007 and 2012 for Australian adults ages 21 and older.
The hazard ratio for falling into income poverty for females who develop arthritis was 1.51, and for males the hazard ratio for falling into income poverty was 1.22, compared with people who never developed arthritis. The hazard ratio for falling into multidimensional poverty for females who develop arthritis was 1.87 and for males the hazard ratio was 1.29.
According to Dr. Callander, “The high risk of poverty should be kept in mind by clinicians seeking the most appropriate treatment for their patients with arthritis, as affordability of out-of-pocket costs may be an important factor.”
Suggested Reading
Callander EJ, Schofield DJ. Arthritis and the risk of falling into poverty: a survival analysis using Australian data. Arthritis Rheumatol. 2015 Sep 8. [Epub ahead of print].
Suggested Reading
Callander EJ, Schofield DJ. Arthritis and the risk of falling into poverty: a survival analysis using Australian data. Arthritis Rheumatol. 2015 Sep 8. [Epub ahead of print].
AAOS Guidelines Sum-Up Prevention and Treatment Strategies for ACL Injuries
The American Academy of Orthopaedic Surgeons (AAOS) Board of Directors has approved Appropriate Use Criteria (AUCs) for anterior cruciate ligament (ACL) injury prevention programs and treatment, as well as rehabilitation and function checklists to help guide and ensure a safe return to sports for the treated athlete. The AUCs and checklists are available online at http://www.orthoguidelines.org/go/auc/.
“Both prevention and treatment of ACL injuries can be confusing given the diversity of injured patients—from skeletally immature youth to older adults, low- and high-risk athletes playing a variety of sports, and patients with and without arthritis,” said Robert Quinn, MD, AUC Section Leader on the Committee on Evidence-Based Quality and Value.
Last year, the AAOS released the Clinical Practice Guideline (CPG) titled “Management of Anterior Cruciate Ligament Injuries.” The guideline recommends, with “moderate” supporting evidence, that reconstructive surgery occur within 5 months of an ACL injury to protect the knee joint. In addition, the CPG states that in young adults, ages 18 to 35, use of a patient's own tissue is preferable over donor tissue to repair an ACL tear.
The new “Appropriate Use Guideline for the Treatment of Anterior Cruciate Ligament Injuries" provides more specific guidance to orthopedic surgeons based on a patient’s various indications, including age, activity level, presence of advanced arthritis, and the status of the ACL tear. The guideline recommends specific next steps and procedures to ensure optimal recovery. Each treatment recommendation is ranked by level of appropriateness.
“The good news for patients and practitioners is that ACL reconstruction with autograft or allograft tissue is very successful,” said Dr. Quinn. “What these guidelines do is delineate, in a very easy-to-maneuver way, what the most appropriate treatments are in each category. It actually gives you the specific circumstances to plug in, and highlights where the evidence matches the recommendations.”
Most patients, especially high-level athletes, are eager to return to play following ACL surgery. However, there is a significant amount of post-surgical rehabilitation and functional recovery required before an athlete can resume sports play.
The new ACL Reconstruction Surgery “Return to Play” and “Postoperative Rehabilitation” checklists “are evidence-based lists on what should be going on before an athlete returns to play, and are constructed in a way that realistically sets expectations for what needs to be accomplished,” said Dr. Quinn.
The “Postoperative Rehabilitation” checklist outlines the post-surgical protocol, from early range of motion, weight bearing and closed and open chain quad and hamstring therapy, to optional rehabilitative bracing and neuromuscular stimulation.
According to the “Return to Play” checklist, a patient should feel confident that he or she can return to their sport of interest, and have been advised to participate in an ongoing ACL-prevention/movement-retraining program before resuming activities. In addition, the graft and surgical site have fully healed; and range of motion, balance, knee stability, strength and functional skills, have been restored.
For athletes involved in competitive or recreational athletics with no prior history of ACL reconstruction and no current history of ACL deficiency, the “Appropriate Use Guideline for ACL Injury Prevention Programs” provides advice regarding a supervised ACL injury prevention program, utilizing the best available scientific evidence and expert opinion.
“Injury prevention programs are very successful,” said Dr. Quinn. “This AUC helps alleviate some of the controversy about when these good options are most applicable.”
Like the treatment AUC, the injury prevention guidelines use patient indications and classifications. For example, sex, growth status, activity level, sports participation, and athlete risk can help determine whether or not a particular, supervised ACL injury prevention program is optimal.
The American Academy of Orthopaedic Surgeons (AAOS) Board of Directors has approved Appropriate Use Criteria (AUCs) for anterior cruciate ligament (ACL) injury prevention programs and treatment, as well as rehabilitation and function checklists to help guide and ensure a safe return to sports for the treated athlete. The AUCs and checklists are available online at http://www.orthoguidelines.org/go/auc/.
“Both prevention and treatment of ACL injuries can be confusing given the diversity of injured patients—from skeletally immature youth to older adults, low- and high-risk athletes playing a variety of sports, and patients with and without arthritis,” said Robert Quinn, MD, AUC Section Leader on the Committee on Evidence-Based Quality and Value.
Last year, the AAOS released the Clinical Practice Guideline (CPG) titled “Management of Anterior Cruciate Ligament Injuries.” The guideline recommends, with “moderate” supporting evidence, that reconstructive surgery occur within 5 months of an ACL injury to protect the knee joint. In addition, the CPG states that in young adults, ages 18 to 35, use of a patient's own tissue is preferable over donor tissue to repair an ACL tear.
The new “Appropriate Use Guideline for the Treatment of Anterior Cruciate Ligament Injuries" provides more specific guidance to orthopedic surgeons based on a patient’s various indications, including age, activity level, presence of advanced arthritis, and the status of the ACL tear. The guideline recommends specific next steps and procedures to ensure optimal recovery. Each treatment recommendation is ranked by level of appropriateness.
“The good news for patients and practitioners is that ACL reconstruction with autograft or allograft tissue is very successful,” said Dr. Quinn. “What these guidelines do is delineate, in a very easy-to-maneuver way, what the most appropriate treatments are in each category. It actually gives you the specific circumstances to plug in, and highlights where the evidence matches the recommendations.”
Most patients, especially high-level athletes, are eager to return to play following ACL surgery. However, there is a significant amount of post-surgical rehabilitation and functional recovery required before an athlete can resume sports play.
The new ACL Reconstruction Surgery “Return to Play” and “Postoperative Rehabilitation” checklists “are evidence-based lists on what should be going on before an athlete returns to play, and are constructed in a way that realistically sets expectations for what needs to be accomplished,” said Dr. Quinn.
The “Postoperative Rehabilitation” checklist outlines the post-surgical protocol, from early range of motion, weight bearing and closed and open chain quad and hamstring therapy, to optional rehabilitative bracing and neuromuscular stimulation.
According to the “Return to Play” checklist, a patient should feel confident that he or she can return to their sport of interest, and have been advised to participate in an ongoing ACL-prevention/movement-retraining program before resuming activities. In addition, the graft and surgical site have fully healed; and range of motion, balance, knee stability, strength and functional skills, have been restored.
For athletes involved in competitive or recreational athletics with no prior history of ACL reconstruction and no current history of ACL deficiency, the “Appropriate Use Guideline for ACL Injury Prevention Programs” provides advice regarding a supervised ACL injury prevention program, utilizing the best available scientific evidence and expert opinion.
“Injury prevention programs are very successful,” said Dr. Quinn. “This AUC helps alleviate some of the controversy about when these good options are most applicable.”
Like the treatment AUC, the injury prevention guidelines use patient indications and classifications. For example, sex, growth status, activity level, sports participation, and athlete risk can help determine whether or not a particular, supervised ACL injury prevention program is optimal.
The American Academy of Orthopaedic Surgeons (AAOS) Board of Directors has approved Appropriate Use Criteria (AUCs) for anterior cruciate ligament (ACL) injury prevention programs and treatment, as well as rehabilitation and function checklists to help guide and ensure a safe return to sports for the treated athlete. The AUCs and checklists are available online at http://www.orthoguidelines.org/go/auc/.
“Both prevention and treatment of ACL injuries can be confusing given the diversity of injured patients—from skeletally immature youth to older adults, low- and high-risk athletes playing a variety of sports, and patients with and without arthritis,” said Robert Quinn, MD, AUC Section Leader on the Committee on Evidence-Based Quality and Value.
Last year, the AAOS released the Clinical Practice Guideline (CPG) titled “Management of Anterior Cruciate Ligament Injuries.” The guideline recommends, with “moderate” supporting evidence, that reconstructive surgery occur within 5 months of an ACL injury to protect the knee joint. In addition, the CPG states that in young adults, ages 18 to 35, use of a patient's own tissue is preferable over donor tissue to repair an ACL tear.
The new “Appropriate Use Guideline for the Treatment of Anterior Cruciate Ligament Injuries" provides more specific guidance to orthopedic surgeons based on a patient’s various indications, including age, activity level, presence of advanced arthritis, and the status of the ACL tear. The guideline recommends specific next steps and procedures to ensure optimal recovery. Each treatment recommendation is ranked by level of appropriateness.
“The good news for patients and practitioners is that ACL reconstruction with autograft or allograft tissue is very successful,” said Dr. Quinn. “What these guidelines do is delineate, in a very easy-to-maneuver way, what the most appropriate treatments are in each category. It actually gives you the specific circumstances to plug in, and highlights where the evidence matches the recommendations.”
Most patients, especially high-level athletes, are eager to return to play following ACL surgery. However, there is a significant amount of post-surgical rehabilitation and functional recovery required before an athlete can resume sports play.
The new ACL Reconstruction Surgery “Return to Play” and “Postoperative Rehabilitation” checklists “are evidence-based lists on what should be going on before an athlete returns to play, and are constructed in a way that realistically sets expectations for what needs to be accomplished,” said Dr. Quinn.
The “Postoperative Rehabilitation” checklist outlines the post-surgical protocol, from early range of motion, weight bearing and closed and open chain quad and hamstring therapy, to optional rehabilitative bracing and neuromuscular stimulation.
According to the “Return to Play” checklist, a patient should feel confident that he or she can return to their sport of interest, and have been advised to participate in an ongoing ACL-prevention/movement-retraining program before resuming activities. In addition, the graft and surgical site have fully healed; and range of motion, balance, knee stability, strength and functional skills, have been restored.
For athletes involved in competitive or recreational athletics with no prior history of ACL reconstruction and no current history of ACL deficiency, the “Appropriate Use Guideline for ACL Injury Prevention Programs” provides advice regarding a supervised ACL injury prevention program, utilizing the best available scientific evidence and expert opinion.
“Injury prevention programs are very successful,” said Dr. Quinn. “This AUC helps alleviate some of the controversy about when these good options are most applicable.”
Like the treatment AUC, the injury prevention guidelines use patient indications and classifications. For example, sex, growth status, activity level, sports participation, and athlete risk can help determine whether or not a particular, supervised ACL injury prevention program is optimal.
Is There a Greater Risk of Mortality Following Hip Fracture Surgery Compared With Hip Replacement Surgery?
Hip fracture patients have worse outcomes in comparison with hip replacement surgery patients, and this finding is not entirely explained by age or medical condition, according to a study published September 15 in JAMA.
Researchers studied nearly 700,000 hip surgery patients older than 45 in France between 2010 and 2013 and found that the total hip replacement patients were younger, more commonly men, and had fewer comorbidities than hip fracture patients.
Investigators also found there were more deaths among the hip fracture patients, with 3.4% dying before hospital discharge compared with 0.18% of total hip replacement surgery patients.
Even when the demographics of the patients were matched by gender, age, and medical conditions, study authors found hip fracture patients had a 1.8% chance of dying compared with 0.3% of elective hip replacement patients. People with a hip fracture had a 5.9% chance of major postoperative complications, compared with 2.3% of those patients who underwent an elective hip replacement.
The research team was led by Yannick Le Manach, MD, PhD, an Assistant Professor of Anesthesia for the Michael G. DeGroote School of Medicine of McMaster University and a member of the Population Health Research Institute of McMaster and Hamilton Health Sciences in Hamilton, Ontario.
“The fact that the hip fracture patients were older and had more health problems does account for some of the difference in outcomes,” Dr. Le Manach said. “But it may be that hip fracture is tied to other physiologic processes that are not present in the circumstances of people going for an elective hip replacement. More research is needed.”
Senior author P.J. Devereaux, MD, PhD, Professor of Medicine and Director of Cardiology for the Michael G DeGroote School of Medicine stated, “These results are encouraging that there are likely risk factors specific to a hip fracture that are potentially modifiable.”
Suggested Reading
Le Manach Y, Collins G, Bhandari M, et al. Outcomes after hip fracture surgery compared with elective total hip replacement. JAMA. 2015;314(11):1159-1166.
Hip fracture patients have worse outcomes in comparison with hip replacement surgery patients, and this finding is not entirely explained by age or medical condition, according to a study published September 15 in JAMA.
Researchers studied nearly 700,000 hip surgery patients older than 45 in France between 2010 and 2013 and found that the total hip replacement patients were younger, more commonly men, and had fewer comorbidities than hip fracture patients.
Investigators also found there were more deaths among the hip fracture patients, with 3.4% dying before hospital discharge compared with 0.18% of total hip replacement surgery patients.
Even when the demographics of the patients were matched by gender, age, and medical conditions, study authors found hip fracture patients had a 1.8% chance of dying compared with 0.3% of elective hip replacement patients. People with a hip fracture had a 5.9% chance of major postoperative complications, compared with 2.3% of those patients who underwent an elective hip replacement.
The research team was led by Yannick Le Manach, MD, PhD, an Assistant Professor of Anesthesia for the Michael G. DeGroote School of Medicine of McMaster University and a member of the Population Health Research Institute of McMaster and Hamilton Health Sciences in Hamilton, Ontario.
“The fact that the hip fracture patients were older and had more health problems does account for some of the difference in outcomes,” Dr. Le Manach said. “But it may be that hip fracture is tied to other physiologic processes that are not present in the circumstances of people going for an elective hip replacement. More research is needed.”
Senior author P.J. Devereaux, MD, PhD, Professor of Medicine and Director of Cardiology for the Michael G DeGroote School of Medicine stated, “These results are encouraging that there are likely risk factors specific to a hip fracture that are potentially modifiable.”
Hip fracture patients have worse outcomes in comparison with hip replacement surgery patients, and this finding is not entirely explained by age or medical condition, according to a study published September 15 in JAMA.
Researchers studied nearly 700,000 hip surgery patients older than 45 in France between 2010 and 2013 and found that the total hip replacement patients were younger, more commonly men, and had fewer comorbidities than hip fracture patients.
Investigators also found there were more deaths among the hip fracture patients, with 3.4% dying before hospital discharge compared with 0.18% of total hip replacement surgery patients.
Even when the demographics of the patients were matched by gender, age, and medical conditions, study authors found hip fracture patients had a 1.8% chance of dying compared with 0.3% of elective hip replacement patients. People with a hip fracture had a 5.9% chance of major postoperative complications, compared with 2.3% of those patients who underwent an elective hip replacement.
The research team was led by Yannick Le Manach, MD, PhD, an Assistant Professor of Anesthesia for the Michael G. DeGroote School of Medicine of McMaster University and a member of the Population Health Research Institute of McMaster and Hamilton Health Sciences in Hamilton, Ontario.
“The fact that the hip fracture patients were older and had more health problems does account for some of the difference in outcomes,” Dr. Le Manach said. “But it may be that hip fracture is tied to other physiologic processes that are not present in the circumstances of people going for an elective hip replacement. More research is needed.”
Senior author P.J. Devereaux, MD, PhD, Professor of Medicine and Director of Cardiology for the Michael G DeGroote School of Medicine stated, “These results are encouraging that there are likely risk factors specific to a hip fracture that are potentially modifiable.”
Suggested Reading
Le Manach Y, Collins G, Bhandari M, et al. Outcomes after hip fracture surgery compared with elective total hip replacement. JAMA. 2015;314(11):1159-1166.
Suggested Reading
Le Manach Y, Collins G, Bhandari M, et al. Outcomes after hip fracture surgery compared with elective total hip replacement. JAMA. 2015;314(11):1159-1166.
Changing Paradigms in Short Stay Total Joint Arthroplasty
Reflections on My VA Experience and Why I See the Proverbial Glass as Half Full
Veterans Health Administration (VA) hospitals have received notoriety due to episodes of misdiagnosis, poor management, and negligent care described in many recent reports and news articles.1-3 While veterans are appropriately the primary focus of these investigative reports, physicians are also challenged in this setting, as they often meet resistance when advocating for patients and attempting to improve a flawed system.2 Although my residency training includes 6 months at a VA hospital mired in controversy, the hospital has played a critical role in my training.3
Despite my many frustrations with the VA and the daily stresses incurred because of barriers impeding the timing and quality of care, I have several reasons to see the glass as “half full” when reflecting on my experiences as an orthopedic surgery resident at a VA medical center. This editorial will focus on the most important of these reasons—the special opportunity and pride associated with caring for veterans and these patients’ extremely appreciative nature.
The VA is one of the largest integrated health care systems in the United States, offering both inpatient and outpatient care to eligible veterans. Although eligibility has historically been based on military service–related medical conditions, disability, and financial need, reforms from 1996 to 2002 expanded enrollment to veteran populations previously deemed ineligible for VA care.4,5 Despite this, studies suggest that some uninsured veterans do not seek VA care, even when eligible for VA coverage. This troubling notion is further complicated by research suggesting that veterans who use the VA for all of their health care are more likely to be from poor, less-educated, and minority populations, and are more likely to report fair or poor health and seek more disability days.6
Such disheartening realities can mask the most important attributes of VA patients, which pertain to their selfless commitment to our country. Orthopedic surgery residents must appreciate these attributes as well as the tremendous need for musculoskeletal care in this setting, as musculoskeletal conditions are some of the most common reasons for patient visits at the VA.7 Although combat-related high-energy blast injuries and the reconstructive procedures used to treat them have received a lot of attention, it is the more common musculoskeletal disorders that are most responsible for the tremendous burden of musculoskeletal disease in the VA. In a study by Dominick and colleagues,8 veterans had significantly greater odds of reporting doctor-diagnosed arthritis compared with nonveterans. Furthermore, veterans are also more vulnerable to overuse injuries, a finding attributed to the intense physical activity associated with military training and service.9
The busy orthopedic surgery clinic at my VA hospital is a fulfilling experience and a reminder of the large demand for musculoskeletal care. However, it is the patient population that makes it most gratifying. Most of the veterans seeking care are appreciative, regularly expressing their gratitude. They view me and the other residents as their physicians, not simply as doctors in training, like so many other non-VA patients do. Despite the fact that VA patients sometimes have to wait several hours to be seen in clinic and several months for surgery, I have never been subjected to their inevitable disdain or frustration. This is true in even the most trying and infuriating times, such as when an operation is cancelled on the day of surgery for reasons that many surgeons in non-VA hospitals would consider trivial. And even when witness to my visible irritation with the VA system, the veterans remain respectful and understanding; if they ever share similar feelings, they most certainly never voice them to me.
I cannot refute the notion that the VA must change and that the veterans deserve an improved health care system. However, this editorial is not written as a call to action. Instead, I hope it helps to humanize the patients of the VA, serving as a reminder to residents and other providers that the VA is a unique and extraordinary opportunity to give back and say thank you to veterans.
This editorial is dedicated to CPT David Huskie, USAR (Ret.), a veteran of Operation Desert Storm and orthopedic nurse at my VA hospital. It was he who first reminded me, and the other orthopedic residents, of the importance of our time at the VA. The Figure depicts the letter he gives to orthopedic residents at our program, along with a pewter coin, after their first VA rotation.
1. Pearson M. The VA’s troubled history. Cable News Network (CNN) website. http://www.cnn.com/2014/05/23/politics/va-scandals-timeline. Updated May 30, 2014. Accessed August 28, 2015.
2. Scherz H. Doctors’ war stories from VA hospitals. The Wall Street Journal website. http://www.wsj.com/articles/hal-scherz-doctors-war-stories-from-va-hospitals-1401233147. Published May 27, 2014. Accessed August 28, 2015.
3. Riviello V. Nurse exposes VA hospital: stolen drugs, tortured veterans. New York Post website. http://nypost.com/2014/07/12/nurse-exposes-va-hospital-stolen-drugs-tortured-veterans. Published July 12, 2014. Accessed August 28, 2015.
4. Enrollment—provision of hospital and outpatient care to veterans—VA. Proposed rule. Fed Regist. 1998;63(132):37299-37307.
5. US Department of Veterans Affairs, Veterans Health Administration, Office of Assistant Deputy Under Secretary for Health for Policy and Planning. 2003 Survey of Veteran Enrollees’ Health and Reliance Upon VA With Selected Comparisons to the 1999 and 2002 Surveys. US Department of Veterans Affairs website. www.va.gov/healthpolicyplanning/Docs/SOE2003_Report.pdf. Published December 2004. Accessed August 28, 2015.
6. Nelson KM, Starkebaum GA, Reiber GE. Veterans using and uninsured veterans not using Veterans Affairs (VA) health care. Public Health Rep. 2007;122(1):93-100.
7. Wasserman GM, Martin BL, Hyams KC, Merrill BR, Oaks HG, McAdoo HA. A survey of outpatient visits in a United States Army forward unit during Operation Desert Shield. Mil Med. 1997;162(6):374-379.
8. Dominick KL, Golightly YM, Jackson GL. Arthritis prevalence and symptoms among US non-veterans, veterans, and veterans receiving Department of Veterans Affairs Healthcare. J Rheumatol. 2006;33(2):348-354.
9. West SG. Rheumatic disorders during Operation Desert Storm. Arthritis Rheum. 1993;36(10):1487-1488.
Veterans Health Administration (VA) hospitals have received notoriety due to episodes of misdiagnosis, poor management, and negligent care described in many recent reports and news articles.1-3 While veterans are appropriately the primary focus of these investigative reports, physicians are also challenged in this setting, as they often meet resistance when advocating for patients and attempting to improve a flawed system.2 Although my residency training includes 6 months at a VA hospital mired in controversy, the hospital has played a critical role in my training.3
Despite my many frustrations with the VA and the daily stresses incurred because of barriers impeding the timing and quality of care, I have several reasons to see the glass as “half full” when reflecting on my experiences as an orthopedic surgery resident at a VA medical center. This editorial will focus on the most important of these reasons—the special opportunity and pride associated with caring for veterans and these patients’ extremely appreciative nature.
The VA is one of the largest integrated health care systems in the United States, offering both inpatient and outpatient care to eligible veterans. Although eligibility has historically been based on military service–related medical conditions, disability, and financial need, reforms from 1996 to 2002 expanded enrollment to veteran populations previously deemed ineligible for VA care.4,5 Despite this, studies suggest that some uninsured veterans do not seek VA care, even when eligible for VA coverage. This troubling notion is further complicated by research suggesting that veterans who use the VA for all of their health care are more likely to be from poor, less-educated, and minority populations, and are more likely to report fair or poor health and seek more disability days.6
Such disheartening realities can mask the most important attributes of VA patients, which pertain to their selfless commitment to our country. Orthopedic surgery residents must appreciate these attributes as well as the tremendous need for musculoskeletal care in this setting, as musculoskeletal conditions are some of the most common reasons for patient visits at the VA.7 Although combat-related high-energy blast injuries and the reconstructive procedures used to treat them have received a lot of attention, it is the more common musculoskeletal disorders that are most responsible for the tremendous burden of musculoskeletal disease in the VA. In a study by Dominick and colleagues,8 veterans had significantly greater odds of reporting doctor-diagnosed arthritis compared with nonveterans. Furthermore, veterans are also more vulnerable to overuse injuries, a finding attributed to the intense physical activity associated with military training and service.9
The busy orthopedic surgery clinic at my VA hospital is a fulfilling experience and a reminder of the large demand for musculoskeletal care. However, it is the patient population that makes it most gratifying. Most of the veterans seeking care are appreciative, regularly expressing their gratitude. They view me and the other residents as their physicians, not simply as doctors in training, like so many other non-VA patients do. Despite the fact that VA patients sometimes have to wait several hours to be seen in clinic and several months for surgery, I have never been subjected to their inevitable disdain or frustration. This is true in even the most trying and infuriating times, such as when an operation is cancelled on the day of surgery for reasons that many surgeons in non-VA hospitals would consider trivial. And even when witness to my visible irritation with the VA system, the veterans remain respectful and understanding; if they ever share similar feelings, they most certainly never voice them to me.
I cannot refute the notion that the VA must change and that the veterans deserve an improved health care system. However, this editorial is not written as a call to action. Instead, I hope it helps to humanize the patients of the VA, serving as a reminder to residents and other providers that the VA is a unique and extraordinary opportunity to give back and say thank you to veterans.
This editorial is dedicated to CPT David Huskie, USAR (Ret.), a veteran of Operation Desert Storm and orthopedic nurse at my VA hospital. It was he who first reminded me, and the other orthopedic residents, of the importance of our time at the VA. The Figure depicts the letter he gives to orthopedic residents at our program, along with a pewter coin, after their first VA rotation.
Veterans Health Administration (VA) hospitals have received notoriety due to episodes of misdiagnosis, poor management, and negligent care described in many recent reports and news articles.1-3 While veterans are appropriately the primary focus of these investigative reports, physicians are also challenged in this setting, as they often meet resistance when advocating for patients and attempting to improve a flawed system.2 Although my residency training includes 6 months at a VA hospital mired in controversy, the hospital has played a critical role in my training.3
Despite my many frustrations with the VA and the daily stresses incurred because of barriers impeding the timing and quality of care, I have several reasons to see the glass as “half full” when reflecting on my experiences as an orthopedic surgery resident at a VA medical center. This editorial will focus on the most important of these reasons—the special opportunity and pride associated with caring for veterans and these patients’ extremely appreciative nature.
The VA is one of the largest integrated health care systems in the United States, offering both inpatient and outpatient care to eligible veterans. Although eligibility has historically been based on military service–related medical conditions, disability, and financial need, reforms from 1996 to 2002 expanded enrollment to veteran populations previously deemed ineligible for VA care.4,5 Despite this, studies suggest that some uninsured veterans do not seek VA care, even when eligible for VA coverage. This troubling notion is further complicated by research suggesting that veterans who use the VA for all of their health care are more likely to be from poor, less-educated, and minority populations, and are more likely to report fair or poor health and seek more disability days.6
Such disheartening realities can mask the most important attributes of VA patients, which pertain to their selfless commitment to our country. Orthopedic surgery residents must appreciate these attributes as well as the tremendous need for musculoskeletal care in this setting, as musculoskeletal conditions are some of the most common reasons for patient visits at the VA.7 Although combat-related high-energy blast injuries and the reconstructive procedures used to treat them have received a lot of attention, it is the more common musculoskeletal disorders that are most responsible for the tremendous burden of musculoskeletal disease in the VA. In a study by Dominick and colleagues,8 veterans had significantly greater odds of reporting doctor-diagnosed arthritis compared with nonveterans. Furthermore, veterans are also more vulnerable to overuse injuries, a finding attributed to the intense physical activity associated with military training and service.9
The busy orthopedic surgery clinic at my VA hospital is a fulfilling experience and a reminder of the large demand for musculoskeletal care. However, it is the patient population that makes it most gratifying. Most of the veterans seeking care are appreciative, regularly expressing their gratitude. They view me and the other residents as their physicians, not simply as doctors in training, like so many other non-VA patients do. Despite the fact that VA patients sometimes have to wait several hours to be seen in clinic and several months for surgery, I have never been subjected to their inevitable disdain or frustration. This is true in even the most trying and infuriating times, such as when an operation is cancelled on the day of surgery for reasons that many surgeons in non-VA hospitals would consider trivial. And even when witness to my visible irritation with the VA system, the veterans remain respectful and understanding; if they ever share similar feelings, they most certainly never voice them to me.
I cannot refute the notion that the VA must change and that the veterans deserve an improved health care system. However, this editorial is not written as a call to action. Instead, I hope it helps to humanize the patients of the VA, serving as a reminder to residents and other providers that the VA is a unique and extraordinary opportunity to give back and say thank you to veterans.
This editorial is dedicated to CPT David Huskie, USAR (Ret.), a veteran of Operation Desert Storm and orthopedic nurse at my VA hospital. It was he who first reminded me, and the other orthopedic residents, of the importance of our time at the VA. The Figure depicts the letter he gives to orthopedic residents at our program, along with a pewter coin, after their first VA rotation.
1. Pearson M. The VA’s troubled history. Cable News Network (CNN) website. http://www.cnn.com/2014/05/23/politics/va-scandals-timeline. Updated May 30, 2014. Accessed August 28, 2015.
2. Scherz H. Doctors’ war stories from VA hospitals. The Wall Street Journal website. http://www.wsj.com/articles/hal-scherz-doctors-war-stories-from-va-hospitals-1401233147. Published May 27, 2014. Accessed August 28, 2015.
3. Riviello V. Nurse exposes VA hospital: stolen drugs, tortured veterans. New York Post website. http://nypost.com/2014/07/12/nurse-exposes-va-hospital-stolen-drugs-tortured-veterans. Published July 12, 2014. Accessed August 28, 2015.
4. Enrollment—provision of hospital and outpatient care to veterans—VA. Proposed rule. Fed Regist. 1998;63(132):37299-37307.
5. US Department of Veterans Affairs, Veterans Health Administration, Office of Assistant Deputy Under Secretary for Health for Policy and Planning. 2003 Survey of Veteran Enrollees’ Health and Reliance Upon VA With Selected Comparisons to the 1999 and 2002 Surveys. US Department of Veterans Affairs website. www.va.gov/healthpolicyplanning/Docs/SOE2003_Report.pdf. Published December 2004. Accessed August 28, 2015.
6. Nelson KM, Starkebaum GA, Reiber GE. Veterans using and uninsured veterans not using Veterans Affairs (VA) health care. Public Health Rep. 2007;122(1):93-100.
7. Wasserman GM, Martin BL, Hyams KC, Merrill BR, Oaks HG, McAdoo HA. A survey of outpatient visits in a United States Army forward unit during Operation Desert Shield. Mil Med. 1997;162(6):374-379.
8. Dominick KL, Golightly YM, Jackson GL. Arthritis prevalence and symptoms among US non-veterans, veterans, and veterans receiving Department of Veterans Affairs Healthcare. J Rheumatol. 2006;33(2):348-354.
9. West SG. Rheumatic disorders during Operation Desert Storm. Arthritis Rheum. 1993;36(10):1487-1488.
1. Pearson M. The VA’s troubled history. Cable News Network (CNN) website. http://www.cnn.com/2014/05/23/politics/va-scandals-timeline. Updated May 30, 2014. Accessed August 28, 2015.
2. Scherz H. Doctors’ war stories from VA hospitals. The Wall Street Journal website. http://www.wsj.com/articles/hal-scherz-doctors-war-stories-from-va-hospitals-1401233147. Published May 27, 2014. Accessed August 28, 2015.
3. Riviello V. Nurse exposes VA hospital: stolen drugs, tortured veterans. New York Post website. http://nypost.com/2014/07/12/nurse-exposes-va-hospital-stolen-drugs-tortured-veterans. Published July 12, 2014. Accessed August 28, 2015.
4. Enrollment—provision of hospital and outpatient care to veterans—VA. Proposed rule. Fed Regist. 1998;63(132):37299-37307.
5. US Department of Veterans Affairs, Veterans Health Administration, Office of Assistant Deputy Under Secretary for Health for Policy and Planning. 2003 Survey of Veteran Enrollees’ Health and Reliance Upon VA With Selected Comparisons to the 1999 and 2002 Surveys. US Department of Veterans Affairs website. www.va.gov/healthpolicyplanning/Docs/SOE2003_Report.pdf. Published December 2004. Accessed August 28, 2015.
6. Nelson KM, Starkebaum GA, Reiber GE. Veterans using and uninsured veterans not using Veterans Affairs (VA) health care. Public Health Rep. 2007;122(1):93-100.
7. Wasserman GM, Martin BL, Hyams KC, Merrill BR, Oaks HG, McAdoo HA. A survey of outpatient visits in a United States Army forward unit during Operation Desert Shield. Mil Med. 1997;162(6):374-379.
8. Dominick KL, Golightly YM, Jackson GL. Arthritis prevalence and symptoms among US non-veterans, veterans, and veterans receiving Department of Veterans Affairs Healthcare. J Rheumatol. 2006;33(2):348-354.
9. West SG. Rheumatic disorders during Operation Desert Storm. Arthritis Rheum. 1993;36(10):1487-1488.
Is Skin Tenting Secondary to Displaced Clavicle Fracture More Than a Theoretical Risk? A Report of 2 Adolescent Cases
Fractures of the clavicle, which account for 2.6% of all fractures, are displaced in 70% of cases and are mid-diaphyseal in 80% of cases.1-3 Historically, both displaced and nondisplaced fractures were treated nonoperatively with excellent outcomes reported in the majority of patients.1-3 Traditionally, the indications for surgical fixation of a clavicular fracture include open fractures, which occur infrequently, accounting for only 3.2% of clavicle fractures.4 Other indications include floating shoulder girdle or scapulothoracic dissociation, neurovascular injury, and skin “tenting” by the fracture fragments.3,5 Recently, both meta-analyses and randomized clinical trials have reported reduced malunion rates and improved patient outcomes with open reduction and internal fixation (ORIF).6-9 Consequently, operative fixation could be considered in patients with 100% displacement or greater than 1.5 cm shortening.6-9 Open reduction and internal fixation of the clavicle has been demonstrated to have excellent outcomes in pediatric populations as well.10
The clavicle is subcutaneous for much of its length and, thus, displaced clavicular fractures often result in a visible deformity with a stretch of the soft-tissue envelope over the fracture. While this has been suggested as an operative indication, several recent sources indicate that this concern may only be theoretical. According to the fourth edition of Skeletal Trauma, “It is often stated that open reduction and internal fixation should be considered if the skin is threatened by pressure from a prominent clavicle fracture fragment; however, it is extremely rare of the skin to be perforated from within.”5 The most recent Journal of Bone and Joint Surgery Current Concepts Review on the subject stated that “open fractures or soft-tissue tenting sufficient to produce skin necrosis is uncommon.”3 To the best of our knowledge, there is no reported case of a displaced midshaft clavicle fracture with secondary skin necrosis and conversion into an open fracture, validating the conclusion that this complication may be only theoretical. Given that surgical fixation carries a risk of complications including wound complications, infection, nonunion, malunion, and damage to the nearby neurovascular structures and pleural apices,11 some surgeons may be uncertain how to proceed in cases at risk for disturbance of the soft tissues.
We report 2 adolescent cases of displaced, comminuted clavicle fractures in which the skin was initially intact. Both were managed nonoperatively and both secondarily presented with open lesions at the fracture site requiring urgent irrigation and débridement (I&D) and ORIF. The patients and their guardians provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
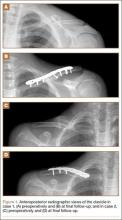
A 15-year-old boy with no significant medical or surgical history flipped over the handlebars of his bicycle the day prior to presentation and sustained a clavicle fracture on his left nondominant upper extremity. This was an isolated injury. On examination, his skin was intact with an area of tender mild osseous protuberance at the midclavicle with associated surrounding edema. He was neurovascularly intact. Radiographs showed a displaced fracture of the midshaft of the clavicle with 20% shortening with a vertically angulated piece of comminution (Figure 1A). After a discussion of the treatment options with the family, the decision was made to pursue nonoperative treatment with sling immobilization as needed and restriction from gym and sports.
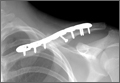
Two and a half weeks later, the patient presented at follow-up with significant reduction but persistence of his pain and a new complaint of drainage from the area of the fracture. On examination, he was found to have a puncture wound of the skin with exposed clavicle protruding through the wound with a 1-cm circumferential area of erythema without purulence present or expressible. The patient denied reinjury and endorsed compliance with sling immobilization. He was taken for urgent I&D and ORIF. After excision of the eschar surrounding the open lesion and full I&D of the soft tissues, the protruding spike was partially excised and the fracture site was débrided. The fracture was reduced and fixated with a lag screw and neutralization plate technique using an anatomically contoured locking clavicle plate (Synthes). Vancomycin powder was sprinkled into the wound at the completion of the procedure to reduce the chance of infection.12
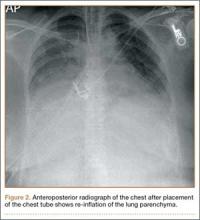
Postoperatively, the patient was prescribed oral clindamycin but was subsequently switched to oral cephalexin because of mild signs of an allergic reaction, for a total course of antibiotics of 1 week. The patient was immobilized in a sling for comfort for the first 9 weeks postoperatively until radiographic union occurred. The patient’s wound healed uneventfully and with acceptable cosmesis. He was released to full activities at 10 weeks postoperatively. At final follow-up 6 months after surgery, the patient had returned to all of his regular activities without pain, and with full range of motion and no demonstrable deficits with radiographic union (Figure 1B).
Case 2
An 11-year-old boy with no significant medical or surgical history fell onto his right dominant upper extremity while doing a jump on his dirt bike 1 week prior to presentation, sustaining a clavicle fracture. This was an isolated injury. He was seen and evaluated by an outside orthopedist who noted that the soft-tissue envelope was intact and the patient was neurovascularly intact. Radiographs showed a displaced fracture of the midshaft of the clavicle with 15% shortening and with a vertically angulated piece of comminution (Figure 1C). Nonoperative treatment with a figure-of-8 brace was recommended. The patient’s discomfort completely resolved.
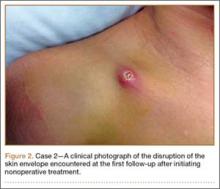
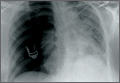
One week later, when he presented to the outside orthopedist for follow-up, the development of a wound overlying the fracture site was noted, and the patient was started on oral trimethoprim/sulfamethoxazole and referred to our office for treatment (Figure 1D). The patient denied reinjury and endorsed compliance with brace immobilization. On examination, the patient was afebrile and was noted to have a puncture wound at the fracture site with a protruding spike of bone and surrounding erythema but without present or expressible discharge (Figure 2). The patient was taken urgently for I&D and ORIF, using a similar technique to case 1, except that no lag screw was employed.
Postoperatively, the patient did well with no complications; he was prescribed oral cephalexin for 1 week. The patient was immobilized in a sling for the first 5 weeks after surgery until radiographic union had occurred, after which the sling was discontinued. The patient’s wound healed uneventfully and with acceptable cosmesis. The patient was released from activity restrictions at 6 weeks postoperatively. At final follow-up 5 weeks after surgery, the patient had full painless range of motion, no tenderness at the fracture site, no signs of infection on examination, and radiographic union (Figure 1D).
Discussion
Optimal treatment of displaced clavicle fractures is controversial. While nonoperative treatment has been recommended,1-3 especially in skeletally immature populations with a capacity for remodeling,7-9 2 recent randomized clinical trials have demonstrated improved patient outcomes with ORIF.6,8,9 Traditionally, ORIF was recommended with tenting of the skin because of concern for an impending open fracture. However, recent review materials have implied that this complication may only be theoretical.3,5 Indeed, in 2 randomized trials, sufficient displacement to cause concern for impending violation of the skin envelope was not listed as an exclusion criteria.8,9 We report 2 cases of displaced comminuted clavicle fractures that were initially managed nonoperatively but developed open lesions at the fracture site. This complication, while rare, is possible, and surgeons must consider it as a possibility when assessing patients with displaced clavicle fractures. To the best of the authors’ knowledge, no guidelines exist to direct antibiotic choice and duration in secondarily open fractures.
These 2 cases have several features in common that may serve as risk factors for impending violation of the skin envelope. Both fractures had a vertically angulated segmental piece of comminution with a sharp spike. This feature has been identified as a potential risk factor for subsequent development of an open fracture in a case report of fragment excision without reduction or fixation to allow rapid return to play in a professional jockey.13 Both patients in these cases presented with high-velocity mechanisms of injury and significant displacement, both of which may serve as risk factors. In the only similar case the authors could identify, Strauss and colleagues14 described a distal clavicle fracture with significant displacement and with secondary ulceration of the skin complicated by infection presenting with purulent discharge, cultured positive for methicillin-sensitive Staphylococcus aureus, requiring management with an external fixator and 6 weeks of intravenous antibiotics. Because both cases presented here occurred in healthy adolescent patients who were taken urgently for I&D and ORIF as soon as the wound was discovered, deep infection was avoided in these cases. Finally, in 1 case, a figure-of-8 brace was employed, which may also have placed pressure on the skin overlying the fracture and may have predisposed this patient to this complication.
Conclusion
In displaced midshaft clavicle fractures, tenting of the skin sufficient to cause subsequent violation of the soft-tissue envelope is possible and is more than a theoretical risk. At-risk patients, ie, those with a vertically angulated sharp fragment of comminution, should be counseled appropriately and observed closely or considered for primary ORIF.
1. Neer CS 2nd. Nonunion of the clavicle. J Am Med Assoc. 1960;172:1006-1011.
2. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
3. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
4. Gottschalk HP, Dumont G, Khanani S, Browne RH, Starr AJ. Open clavicle fractures: patterns of trauma and associated injuries. J Orthop Trauma. 2012;26(2):107-109.
5. Ring D, Jupiter JB. Injuries to the shoulder girdle. In: Browner BD, Jupiter JB, eds. Skeletal Trauma. 4th ed. New York, NY: Elsevier; 2009:1755–1778.
6. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
7. Zlowodzki M, Zelle BA, Cole PA, Jeray K, McKee MD; Evidence-Based Orthopaedic Trauma Working Group. Treatment of acute midshaft clavicle fractures: systematic review of 2144 fractures: on behalf of the Evidence-Based Orthopaedic Trauma Working Group. J Orthop Trauma. 2005;19(7):504-507.
8. Robinson CM, Goudie EB, Murray IR, et al. Open reduction and plate fixation versus nonoperative treatment for displaced midshaft clavicular fractures: a multicenter, randomized, controlled trial. J Bone Joint Surg Am. 2013;95(17):1576-1584.
9. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
10. Mehlman CT, Yihua G, Bochang C, Zhigang W. Operative treatment of completely displaced clavicle shaft fractures in children. J Pediatr Orthop. 2009;29(8):851-855.
11. Gross CE, Chalmers PN, Ellman M, Fernandez JJ, Verma NN. Acute brachial plexopathy after clavicular open reduction and internal fixation. J Shoulder Elbow Surg. 2013;22(5):e6-e9.
12. Pahys JM, Pahys JR, Cho SK, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am. 2013;95(6):549-554.
13. Mandalia V, Shivshanker V, Foy MA. Excision of a bony spike without fixation of the fractured clavicle in a jockey. Clin Orthop Relat Res. 2003;(409):275-277.
14. Strauss EJ, Kaplan KM, Paksima N, Bosco JA. Treatment of an open infected type IIB distal clavicle fracture: case report and review of the literature. Bull NYU Hosp Jt Dis. 2008;66(2):129-133.
Fractures of the clavicle, which account for 2.6% of all fractures, are displaced in 70% of cases and are mid-diaphyseal in 80% of cases.1-3 Historically, both displaced and nondisplaced fractures were treated nonoperatively with excellent outcomes reported in the majority of patients.1-3 Traditionally, the indications for surgical fixation of a clavicular fracture include open fractures, which occur infrequently, accounting for only 3.2% of clavicle fractures.4 Other indications include floating shoulder girdle or scapulothoracic dissociation, neurovascular injury, and skin “tenting” by the fracture fragments.3,5 Recently, both meta-analyses and randomized clinical trials have reported reduced malunion rates and improved patient outcomes with open reduction and internal fixation (ORIF).6-9 Consequently, operative fixation could be considered in patients with 100% displacement or greater than 1.5 cm shortening.6-9 Open reduction and internal fixation of the clavicle has been demonstrated to have excellent outcomes in pediatric populations as well.10
The clavicle is subcutaneous for much of its length and, thus, displaced clavicular fractures often result in a visible deformity with a stretch of the soft-tissue envelope over the fracture. While this has been suggested as an operative indication, several recent sources indicate that this concern may only be theoretical. According to the fourth edition of Skeletal Trauma, “It is often stated that open reduction and internal fixation should be considered if the skin is threatened by pressure from a prominent clavicle fracture fragment; however, it is extremely rare of the skin to be perforated from within.”5 The most recent Journal of Bone and Joint Surgery Current Concepts Review on the subject stated that “open fractures or soft-tissue tenting sufficient to produce skin necrosis is uncommon.”3 To the best of our knowledge, there is no reported case of a displaced midshaft clavicle fracture with secondary skin necrosis and conversion into an open fracture, validating the conclusion that this complication may be only theoretical. Given that surgical fixation carries a risk of complications including wound complications, infection, nonunion, malunion, and damage to the nearby neurovascular structures and pleural apices,11 some surgeons may be uncertain how to proceed in cases at risk for disturbance of the soft tissues.
We report 2 adolescent cases of displaced, comminuted clavicle fractures in which the skin was initially intact. Both were managed nonoperatively and both secondarily presented with open lesions at the fracture site requiring urgent irrigation and débridement (I&D) and ORIF. The patients and their guardians provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 15-year-old boy with no significant medical or surgical history flipped over the handlebars of his bicycle the day prior to presentation and sustained a clavicle fracture on his left nondominant upper extremity. This was an isolated injury. On examination, his skin was intact with an area of tender mild osseous protuberance at the midclavicle with associated surrounding edema. He was neurovascularly intact. Radiographs showed a displaced fracture of the midshaft of the clavicle with 20% shortening with a vertically angulated piece of comminution (Figure 1A). After a discussion of the treatment options with the family, the decision was made to pursue nonoperative treatment with sling immobilization as needed and restriction from gym and sports.
Two and a half weeks later, the patient presented at follow-up with significant reduction but persistence of his pain and a new complaint of drainage from the area of the fracture. On examination, he was found to have a puncture wound of the skin with exposed clavicle protruding through the wound with a 1-cm circumferential area of erythema without purulence present or expressible. The patient denied reinjury and endorsed compliance with sling immobilization. He was taken for urgent I&D and ORIF. After excision of the eschar surrounding the open lesion and full I&D of the soft tissues, the protruding spike was partially excised and the fracture site was débrided. The fracture was reduced and fixated with a lag screw and neutralization plate technique using an anatomically contoured locking clavicle plate (Synthes). Vancomycin powder was sprinkled into the wound at the completion of the procedure to reduce the chance of infection.12
Postoperatively, the patient was prescribed oral clindamycin but was subsequently switched to oral cephalexin because of mild signs of an allergic reaction, for a total course of antibiotics of 1 week. The patient was immobilized in a sling for comfort for the first 9 weeks postoperatively until radiographic union occurred. The patient’s wound healed uneventfully and with acceptable cosmesis. He was released to full activities at 10 weeks postoperatively. At final follow-up 6 months after surgery, the patient had returned to all of his regular activities without pain, and with full range of motion and no demonstrable deficits with radiographic union (Figure 1B).
Case 2
An 11-year-old boy with no significant medical or surgical history fell onto his right dominant upper extremity while doing a jump on his dirt bike 1 week prior to presentation, sustaining a clavicle fracture. This was an isolated injury. He was seen and evaluated by an outside orthopedist who noted that the soft-tissue envelope was intact and the patient was neurovascularly intact. Radiographs showed a displaced fracture of the midshaft of the clavicle with 15% shortening and with a vertically angulated piece of comminution (Figure 1C). Nonoperative treatment with a figure-of-8 brace was recommended. The patient’s discomfort completely resolved.
One week later, when he presented to the outside orthopedist for follow-up, the development of a wound overlying the fracture site was noted, and the patient was started on oral trimethoprim/sulfamethoxazole and referred to our office for treatment (Figure 1D). The patient denied reinjury and endorsed compliance with brace immobilization. On examination, the patient was afebrile and was noted to have a puncture wound at the fracture site with a protruding spike of bone and surrounding erythema but without present or expressible discharge (Figure 2). The patient was taken urgently for I&D and ORIF, using a similar technique to case 1, except that no lag screw was employed.
Postoperatively, the patient did well with no complications; he was prescribed oral cephalexin for 1 week. The patient was immobilized in a sling for the first 5 weeks after surgery until radiographic union had occurred, after which the sling was discontinued. The patient’s wound healed uneventfully and with acceptable cosmesis. The patient was released from activity restrictions at 6 weeks postoperatively. At final follow-up 5 weeks after surgery, the patient had full painless range of motion, no tenderness at the fracture site, no signs of infection on examination, and radiographic union (Figure 1D).
Discussion
Optimal treatment of displaced clavicle fractures is controversial. While nonoperative treatment has been recommended,1-3 especially in skeletally immature populations with a capacity for remodeling,7-9 2 recent randomized clinical trials have demonstrated improved patient outcomes with ORIF.6,8,9 Traditionally, ORIF was recommended with tenting of the skin because of concern for an impending open fracture. However, recent review materials have implied that this complication may only be theoretical.3,5 Indeed, in 2 randomized trials, sufficient displacement to cause concern for impending violation of the skin envelope was not listed as an exclusion criteria.8,9 We report 2 cases of displaced comminuted clavicle fractures that were initially managed nonoperatively but developed open lesions at the fracture site. This complication, while rare, is possible, and surgeons must consider it as a possibility when assessing patients with displaced clavicle fractures. To the best of the authors’ knowledge, no guidelines exist to direct antibiotic choice and duration in secondarily open fractures.
These 2 cases have several features in common that may serve as risk factors for impending violation of the skin envelope. Both fractures had a vertically angulated segmental piece of comminution with a sharp spike. This feature has been identified as a potential risk factor for subsequent development of an open fracture in a case report of fragment excision without reduction or fixation to allow rapid return to play in a professional jockey.13 Both patients in these cases presented with high-velocity mechanisms of injury and significant displacement, both of which may serve as risk factors. In the only similar case the authors could identify, Strauss and colleagues14 described a distal clavicle fracture with significant displacement and with secondary ulceration of the skin complicated by infection presenting with purulent discharge, cultured positive for methicillin-sensitive Staphylococcus aureus, requiring management with an external fixator and 6 weeks of intravenous antibiotics. Because both cases presented here occurred in healthy adolescent patients who were taken urgently for I&D and ORIF as soon as the wound was discovered, deep infection was avoided in these cases. Finally, in 1 case, a figure-of-8 brace was employed, which may also have placed pressure on the skin overlying the fracture and may have predisposed this patient to this complication.
Conclusion
In displaced midshaft clavicle fractures, tenting of the skin sufficient to cause subsequent violation of the soft-tissue envelope is possible and is more than a theoretical risk. At-risk patients, ie, those with a vertically angulated sharp fragment of comminution, should be counseled appropriately and observed closely or considered for primary ORIF.
Fractures of the clavicle, which account for 2.6% of all fractures, are displaced in 70% of cases and are mid-diaphyseal in 80% of cases.1-3 Historically, both displaced and nondisplaced fractures were treated nonoperatively with excellent outcomes reported in the majority of patients.1-3 Traditionally, the indications for surgical fixation of a clavicular fracture include open fractures, which occur infrequently, accounting for only 3.2% of clavicle fractures.4 Other indications include floating shoulder girdle or scapulothoracic dissociation, neurovascular injury, and skin “tenting” by the fracture fragments.3,5 Recently, both meta-analyses and randomized clinical trials have reported reduced malunion rates and improved patient outcomes with open reduction and internal fixation (ORIF).6-9 Consequently, operative fixation could be considered in patients with 100% displacement or greater than 1.5 cm shortening.6-9 Open reduction and internal fixation of the clavicle has been demonstrated to have excellent outcomes in pediatric populations as well.10
The clavicle is subcutaneous for much of its length and, thus, displaced clavicular fractures often result in a visible deformity with a stretch of the soft-tissue envelope over the fracture. While this has been suggested as an operative indication, several recent sources indicate that this concern may only be theoretical. According to the fourth edition of Skeletal Trauma, “It is often stated that open reduction and internal fixation should be considered if the skin is threatened by pressure from a prominent clavicle fracture fragment; however, it is extremely rare of the skin to be perforated from within.”5 The most recent Journal of Bone and Joint Surgery Current Concepts Review on the subject stated that “open fractures or soft-tissue tenting sufficient to produce skin necrosis is uncommon.”3 To the best of our knowledge, there is no reported case of a displaced midshaft clavicle fracture with secondary skin necrosis and conversion into an open fracture, validating the conclusion that this complication may be only theoretical. Given that surgical fixation carries a risk of complications including wound complications, infection, nonunion, malunion, and damage to the nearby neurovascular structures and pleural apices,11 some surgeons may be uncertain how to proceed in cases at risk for disturbance of the soft tissues.
We report 2 adolescent cases of displaced, comminuted clavicle fractures in which the skin was initially intact. Both were managed nonoperatively and both secondarily presented with open lesions at the fracture site requiring urgent irrigation and débridement (I&D) and ORIF. The patients and their guardians provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
A 15-year-old boy with no significant medical or surgical history flipped over the handlebars of his bicycle the day prior to presentation and sustained a clavicle fracture on his left nondominant upper extremity. This was an isolated injury. On examination, his skin was intact with an area of tender mild osseous protuberance at the midclavicle with associated surrounding edema. He was neurovascularly intact. Radiographs showed a displaced fracture of the midshaft of the clavicle with 20% shortening with a vertically angulated piece of comminution (Figure 1A). After a discussion of the treatment options with the family, the decision was made to pursue nonoperative treatment with sling immobilization as needed and restriction from gym and sports.
Two and a half weeks later, the patient presented at follow-up with significant reduction but persistence of his pain and a new complaint of drainage from the area of the fracture. On examination, he was found to have a puncture wound of the skin with exposed clavicle protruding through the wound with a 1-cm circumferential area of erythema without purulence present or expressible. The patient denied reinjury and endorsed compliance with sling immobilization. He was taken for urgent I&D and ORIF. After excision of the eschar surrounding the open lesion and full I&D of the soft tissues, the protruding spike was partially excised and the fracture site was débrided. The fracture was reduced and fixated with a lag screw and neutralization plate technique using an anatomically contoured locking clavicle plate (Synthes). Vancomycin powder was sprinkled into the wound at the completion of the procedure to reduce the chance of infection.12
Postoperatively, the patient was prescribed oral clindamycin but was subsequently switched to oral cephalexin because of mild signs of an allergic reaction, for a total course of antibiotics of 1 week. The patient was immobilized in a sling for comfort for the first 9 weeks postoperatively until radiographic union occurred. The patient’s wound healed uneventfully and with acceptable cosmesis. He was released to full activities at 10 weeks postoperatively. At final follow-up 6 months after surgery, the patient had returned to all of his regular activities without pain, and with full range of motion and no demonstrable deficits with radiographic union (Figure 1B).
Case 2
An 11-year-old boy with no significant medical or surgical history fell onto his right dominant upper extremity while doing a jump on his dirt bike 1 week prior to presentation, sustaining a clavicle fracture. This was an isolated injury. He was seen and evaluated by an outside orthopedist who noted that the soft-tissue envelope was intact and the patient was neurovascularly intact. Radiographs showed a displaced fracture of the midshaft of the clavicle with 15% shortening and with a vertically angulated piece of comminution (Figure 1C). Nonoperative treatment with a figure-of-8 brace was recommended. The patient’s discomfort completely resolved.
One week later, when he presented to the outside orthopedist for follow-up, the development of a wound overlying the fracture site was noted, and the patient was started on oral trimethoprim/sulfamethoxazole and referred to our office for treatment (Figure 1D). The patient denied reinjury and endorsed compliance with brace immobilization. On examination, the patient was afebrile and was noted to have a puncture wound at the fracture site with a protruding spike of bone and surrounding erythema but without present or expressible discharge (Figure 2). The patient was taken urgently for I&D and ORIF, using a similar technique to case 1, except that no lag screw was employed.
Postoperatively, the patient did well with no complications; he was prescribed oral cephalexin for 1 week. The patient was immobilized in a sling for the first 5 weeks after surgery until radiographic union had occurred, after which the sling was discontinued. The patient’s wound healed uneventfully and with acceptable cosmesis. The patient was released from activity restrictions at 6 weeks postoperatively. At final follow-up 5 weeks after surgery, the patient had full painless range of motion, no tenderness at the fracture site, no signs of infection on examination, and radiographic union (Figure 1D).
Discussion
Optimal treatment of displaced clavicle fractures is controversial. While nonoperative treatment has been recommended,1-3 especially in skeletally immature populations with a capacity for remodeling,7-9 2 recent randomized clinical trials have demonstrated improved patient outcomes with ORIF.6,8,9 Traditionally, ORIF was recommended with tenting of the skin because of concern for an impending open fracture. However, recent review materials have implied that this complication may only be theoretical.3,5 Indeed, in 2 randomized trials, sufficient displacement to cause concern for impending violation of the skin envelope was not listed as an exclusion criteria.8,9 We report 2 cases of displaced comminuted clavicle fractures that were initially managed nonoperatively but developed open lesions at the fracture site. This complication, while rare, is possible, and surgeons must consider it as a possibility when assessing patients with displaced clavicle fractures. To the best of the authors’ knowledge, no guidelines exist to direct antibiotic choice and duration in secondarily open fractures.
These 2 cases have several features in common that may serve as risk factors for impending violation of the skin envelope. Both fractures had a vertically angulated segmental piece of comminution with a sharp spike. This feature has been identified as a potential risk factor for subsequent development of an open fracture in a case report of fragment excision without reduction or fixation to allow rapid return to play in a professional jockey.13 Both patients in these cases presented with high-velocity mechanisms of injury and significant displacement, both of which may serve as risk factors. In the only similar case the authors could identify, Strauss and colleagues14 described a distal clavicle fracture with significant displacement and with secondary ulceration of the skin complicated by infection presenting with purulent discharge, cultured positive for methicillin-sensitive Staphylococcus aureus, requiring management with an external fixator and 6 weeks of intravenous antibiotics. Because both cases presented here occurred in healthy adolescent patients who were taken urgently for I&D and ORIF as soon as the wound was discovered, deep infection was avoided in these cases. Finally, in 1 case, a figure-of-8 brace was employed, which may also have placed pressure on the skin overlying the fracture and may have predisposed this patient to this complication.
Conclusion
In displaced midshaft clavicle fractures, tenting of the skin sufficient to cause subsequent violation of the soft-tissue envelope is possible and is more than a theoretical risk. At-risk patients, ie, those with a vertically angulated sharp fragment of comminution, should be counseled appropriately and observed closely or considered for primary ORIF.
1. Neer CS 2nd. Nonunion of the clavicle. J Am Med Assoc. 1960;172:1006-1011.
2. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
3. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
4. Gottschalk HP, Dumont G, Khanani S, Browne RH, Starr AJ. Open clavicle fractures: patterns of trauma and associated injuries. J Orthop Trauma. 2012;26(2):107-109.
5. Ring D, Jupiter JB. Injuries to the shoulder girdle. In: Browner BD, Jupiter JB, eds. Skeletal Trauma. 4th ed. New York, NY: Elsevier; 2009:1755–1778.
6. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
7. Zlowodzki M, Zelle BA, Cole PA, Jeray K, McKee MD; Evidence-Based Orthopaedic Trauma Working Group. Treatment of acute midshaft clavicle fractures: systematic review of 2144 fractures: on behalf of the Evidence-Based Orthopaedic Trauma Working Group. J Orthop Trauma. 2005;19(7):504-507.
8. Robinson CM, Goudie EB, Murray IR, et al. Open reduction and plate fixation versus nonoperative treatment for displaced midshaft clavicular fractures: a multicenter, randomized, controlled trial. J Bone Joint Surg Am. 2013;95(17):1576-1584.
9. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
10. Mehlman CT, Yihua G, Bochang C, Zhigang W. Operative treatment of completely displaced clavicle shaft fractures in children. J Pediatr Orthop. 2009;29(8):851-855.
11. Gross CE, Chalmers PN, Ellman M, Fernandez JJ, Verma NN. Acute brachial plexopathy after clavicular open reduction and internal fixation. J Shoulder Elbow Surg. 2013;22(5):e6-e9.
12. Pahys JM, Pahys JR, Cho SK, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am. 2013;95(6):549-554.
13. Mandalia V, Shivshanker V, Foy MA. Excision of a bony spike without fixation of the fractured clavicle in a jockey. Clin Orthop Relat Res. 2003;(409):275-277.
14. Strauss EJ, Kaplan KM, Paksima N, Bosco JA. Treatment of an open infected type IIB distal clavicle fracture: case report and review of the literature. Bull NYU Hosp Jt Dis. 2008;66(2):129-133.
1. Neer CS 2nd. Nonunion of the clavicle. J Am Med Assoc. 1960;172:1006-1011.
2. Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. J Bone Joint Surg Br. 1998;80(3):476-484.
3. Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. J Bone Joint Surg Am. 2009;91(2):447-460.
4. Gottschalk HP, Dumont G, Khanani S, Browne RH, Starr AJ. Open clavicle fractures: patterns of trauma and associated injuries. J Orthop Trauma. 2012;26(2):107-109.
5. Ring D, Jupiter JB. Injuries to the shoulder girdle. In: Browner BD, Jupiter JB, eds. Skeletal Trauma. 4th ed. New York, NY: Elsevier; 2009:1755–1778.
6. McKee RC, Whelan DB, Schemitsch EH, McKee MD. Operative versus nonoperative care of displaced midshaft clavicular fractures: a meta-analysis of randomized clinical trials. J Bone Joint Surg Am. 2012;94(8):675-684.
7. Zlowodzki M, Zelle BA, Cole PA, Jeray K, McKee MD; Evidence-Based Orthopaedic Trauma Working Group. Treatment of acute midshaft clavicle fractures: systematic review of 2144 fractures: on behalf of the Evidence-Based Orthopaedic Trauma Working Group. J Orthop Trauma. 2005;19(7):504-507.
8. Robinson CM, Goudie EB, Murray IR, et al. Open reduction and plate fixation versus nonoperative treatment for displaced midshaft clavicular fractures: a multicenter, randomized, controlled trial. J Bone Joint Surg Am. 2013;95(17):1576-1584.
9. Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. J Bone Joint Surg Am. 2007;89(1):1-10.
10. Mehlman CT, Yihua G, Bochang C, Zhigang W. Operative treatment of completely displaced clavicle shaft fractures in children. J Pediatr Orthop. 2009;29(8):851-855.
11. Gross CE, Chalmers PN, Ellman M, Fernandez JJ, Verma NN. Acute brachial plexopathy after clavicular open reduction and internal fixation. J Shoulder Elbow Surg. 2013;22(5):e6-e9.
12. Pahys JM, Pahys JR, Cho SK, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am. 2013;95(6):549-554.
13. Mandalia V, Shivshanker V, Foy MA. Excision of a bony spike without fixation of the fractured clavicle in a jockey. Clin Orthop Relat Res. 2003;(409):275-277.
14. Strauss EJ, Kaplan KM, Paksima N, Bosco JA. Treatment of an open infected type IIB distal clavicle fracture: case report and review of the literature. Bull NYU Hosp Jt Dis. 2008;66(2):129-133.
Osteofibrous Dysplasia–like Adamantinoma of the Tibia in a 15-Year-Old Girl
Adamantinomas are rare primary malignant bone tumors (less than 1% of all bone tumors) that arise most commonly in the tibia.1 There is a predilection for adult men aged 20 to 50 years, with rare occurrences in children. These tumors are malignant, highly invasive, and have significant metastatic potential.2 A rarely seen, benign variant, known as osteofibrous dysplasia–like adamantinoma, is described in the literature, with fewer than 135 cases reported.3-5 This variant predominantly has benign characteristics of an osteofibrous dysplasia lesion but has the potential to transform into an adamantinoma.6 Osteofibrous dysplasia–like adamantinoma has been observed to regress with age and is also referred to as a regressing adamantinoma or differentiated adamantinoma.7
We report an uncommon case of an osteofibrous dysplasia–like adamantinoma of the tibia in a 15-year-old girl. We decided to observe the tumor with regular 3- to 6-month follow-ups. Osteofibrous dysplasia–like adamantinoma in our patient has remained stable for 2 years and has an excellent prognosis.8 We report this case for its rarity, its short-term stability, and significant treatment implications due to its potential to regress or develop into a malignant form. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 15-year-old girl was referred to our institution for evaluation of anterior left knee pain. She had sustained a fall while playing basketball 3 months earlier and had been having left knee pain since that time. She did not have any swelling, catching, or locking in her left knee. She denied any recent fever, chills, night sweats, weight loss, nausea, vomiting, or diarrhea. On physical examination, her gait was normal and no swelling, erythema, or tenderness was noticed around the left knee.
Plain radiographs revealed a heterogeneous lesion with sclerosis and thickening of the anteromedial cortex of the proximal left tibia (Figures 1A, 1B). A computed tomography (CT) scan of the abdomen, pelvis, and chest showed no osseous abnormalities. A whole-body bone scan showed activity in the anterior aspect of the left proximal tibia. No other areas of activity were noted. Magnetic resonance imaging of the left leg showed an elongated, multiloculated, enhancing mass arising from the anterolateral cortex and extending from the tibial tuberosity to the mid-diaphysis of the left tibia. Histologic examination of the CT-guided core needle biopsy specimen showed that the lesion was composed of dense fibrocollagenous tissue separating irregular bony trabeculae with osteoblastic and osteoclastic activity. There was no evidence of any atypical cells, necrosis, or significant mitotic activity. No epithelial cells were identified on hematoxylin-eosin (H&E) stain (Figure 2). However, immunohistochemical staining was positive for focal cytokeratin-positive epithelial cells (Figure 3). The lesion was diagnosed as an osteofibrous dysplasia–like adamantinoma on the basis of the radiographic and histologic findings. We elected nonoperative intervention given the benign nature of the lesion and its potential to regress. Given the possibility of sampling error and potential for progression, the patient was followed regularly at 3- to 6-month intervals over a 2-year period without disease progression.
Discussion
Osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma are rare fibro-osseous lesions that largely involve the midshaft of the tibia. Osteofibrous dysplasia accounts for 0.2% of primary bone tumors, whereas adamantinoma accounts for 0.1% to 0.5% of malignant bone tumors.9 Osteofibrous dysplasia is a benign lesion composed primarily of fibro-osseous tissue. Adamantinoma, however, is a slow-growing, low-grade, malignant biphasic tumor with intermingled epithelial and fibro-osseous components. It is an aggressive tumor that is locally invasive and can metastasize.2 Osteofibrous dysplasia–like adamantinoma (also known as differentiated or regressing adamantinoma) is a benign lesion like osteofibrous dysplasia but has features of both osteofibrous dysplasia and adamantinoma. Osteofibrous dysplasia–like adamantinoma may progress and become a malignant adamantinoma.6,10
The radiologic features of the 3 lesions are quite similar. It is not possible to distinguish between osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma based on imaging alone.9 Adamantinoma, being highly invasive, can be distinguished from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma according to the extent of involvement of the medullary cavity seen on magnetic resonance imaging.9 Complete involvement of the medullary cavity is almost always seen in an adamantinoma. Involvement of the medullary cavity is minimal or absent in osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma lesions.
Tissue confirmation through biopsy is crucial for accurate diagnosis. A biopsy should always be performed on any suspicious lesion,3,6 and the fibro-osseous lesion should be treated as an adamantinoma if findings are equivocal. A biopsy also distinctly distinguishes these lesions from benign fibrous cortical defects, which have a similar radiographic appearance. While open biopsy is the gold standard, minimally invasive techniques such as core needle biopsy and fine needle biopsy are increasingly used.6 Because of the higher risk of misdiagnosis with minimally invasive techniques, radiographic confirmation is highly recommended.5
Histologically, both osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma do not stain for cytokeratin on H&E stain. However, they can be differentiated based on immunohistochemical staining for cytokeratin. Osteofibrous dysplasia lesions exhibit diffuse staining whereas osteofibrous dysplasia–like adamantinoma lesions show focal staining of small nests of epithelial cells. Adamantinoma, in comparison, exhibits a biphasic pattern on H&E stain, representing areas of epithelial and osteofibrous cells. Immunohistochemical staining for cytokeratin of an adamantinoma reveals large nests of epithelial cells.
The association between osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma is not clearly established. However, it is widely believed that these 3 lesions represent a spectrum of the same disease and are linearly related in disease progression, with osteofibrous dysplasia at the benign end of the spectrum, osteofibrous dysplasia–like adamantinoma the intermediate form, and adamantinoma at the malignant end of the spectrum.11
Hazelbag and colleagues6 and Springfield and colleagues10 point out that osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma could be precursor lesions of adamantinoma. We found several studies in the literature that support and document progression from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma to an adamantinoma.4,6,10,12 Other studies, however, showed no progression from either a benign osteofibrous dysplasia or an osteofibrous dysplasia–like adamantinoma lesion to a malignant adamantinoma. Park and colleagues13 described 41 cases of osteofibrous dysplasia that did not progress to adamantinoma. Kuruvilla and Steiner8 described 5 cases of osteofibrous dysplasia–like adamantinoma that showed no progression to adamantinoma. Additionally, our case has not progressed and has remained radiographically stable over a 2-year follow-up. Czerniak and colleagues7 and Ueda and colleagues14 postulated, based on histologic and immunohistochemical studies, that osteofibrous dysplasia–like adamantinoma might be a regressing form of an adamantinoma that is undergoing reparative processes that could result in complete elimination of all tumor cells.
In general, any lesion with absent to low malignant potential could be managed nonoperatively with periodic observation and without the need for surgical intervention. Thus, identification of a stable or nonprogressing osteofibrous dysplasia–like adamantinoma lesion has significant treatment implications. Campanacci and Laus15 at the Rizzoli Institute in Milan, through long term follow-up of their patients with osteofibrous dysplasia, found that most lesions had a tendency to regress spontaneously by puberty. They recommended that nonextensive osteofibrous dysplasia lesions should be observed, and surgery should be delayed until puberty. Gleason and colleagues16 also recommended nonoperative management of osteofibrous dysplasia lesions, with surgery used only for large, deforming, and highly invasive lesions. We recommend a similar treatment approach for osteofibrous dysplasia–like adamantinoma lesions.
Adamantinomas, however, are usually symptomatic, are highly invasive, have a high recurrence rate, and can metastasize.9 In these patients, a wide en bloc resection or amputation should be performed as soon as possible.11 Our case highlights that osteofibrous dysplasia–like adamantinoma lesions can occur in children and can remain stable, especially over the short term. Such lesions can be observed without surgical intervention.
1. Kanakaraddi SV, Nagaraj G, Ravinath TM. Adamantinoma of the tibia with late skeletal metastasis: an unusual presentation. J Bone Joint Surg Br. 2007;89(3):388-389.
2. Van Geel AN, Hazelbag HM, Slingerland R, Vermeulen MI. Disseminating adamantinoma of the tibia. Sarcoma. 1997;1(2):109-111.
3. Povysil C, Kohout A, Urban K, Horak M. Differentiated adamantinoma of the fibula: a rhabdoid variant. Skeletal Radiol. 2004;33(8):488-492.
4. Hatori M, Watanabe M, Hosaka M, Sasano H, Narita M, Kokubun S. A classic adamantinoma arising from osteofibrous dysplasia-like adamantinoma in the lower leg: a case report and review of the literature. Tohoku J Exp Med. 2006;209(1):53-59.
5. Khanna M, Delaney D, Tirabosco R, Saifuddin A. Osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma and adamantinoma: correlation of radiological imaging features with surgical histology and assessment of the use of radiology in contributing to needle biopsy diagnosis. Skeletal Radiol. 2008;37(12):1077-1084.
6. Hazelbag HM, Taminiau AH, Fleuren GJ, Hogendoorn PC. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am. 1994;76(10):1482-1499.
7. Czerniak B, Rojas-Corona RR, Dorfman HD. Morphologic diversity of long bone adamantinoma. The concept of differentiated (regressing) adamantinoma and its relationship to osteofibrous dysplasia. Cancer. 1989;64(11):2319-2334.
8. Kuruvilla G, Steiner GC. Osteofibrous dysplasia-like adamantinoma of bone: a report of five cases with immunohistochemical and ultrastructural studies. Hum Pathol. 1998;29(8):809-814.
9. Bethapudi S, Ritchie DA, Macduff E, Straiton J. Imaging in osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma, and classic adamantinoma. Clin Radiol. 2014;69(2):200-208.
10. Springfield DS, Rosenberg AE, Mankin HJ, Mindell ER. Relationship between osteofibrous dysplasia and adamantinoma. Clin Orthop Relat Res. 1994;(309):234-244.
11. Most MJ, Sim FH, Inwards CY. Osteofibrous dysplasia and adamantinoma. J Am Acad Orthop Surg. 2010;18(6):358-366.
12. Lee RS, Weitzel S, Eastwood DM, et al. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J Bone Joint Surg Br. 2006;88(5):658-664.
13. Park YK, Unni KK, McLeod RA, Pritchard DJ. Osteofibrous dysplasia: clinicopathologic study of 80 cases. Hum Pathol. 1993;24(12):1339-1347.
14. Ueda Y, Roessner A, Bosse A, Edel G, Bocker W, Wuisman P. Juvenile intracortical adamantinoma of the tibia with predominant osteofibrous dysplasia-like features. Pathol Res Pract. 1991;187(8):1039-1043; discussion 1043-1034.
15. Campanacci M, Laus M. Osteofibrous dysplasia of the tibia and fibula. J Bone Joint Surg Am. 1981;63(3):367-375.
16. Gleason BC, Liegl-Atzwanger B, Kozakewich HP, et al. Osteofibrous dysplasia and adamantinoma in children and adolescents: a clinicopathologic reappraisal. Am J Surg Pathol. 2008;32(3):363-376.
Adamantinomas are rare primary malignant bone tumors (less than 1% of all bone tumors) that arise most commonly in the tibia.1 There is a predilection for adult men aged 20 to 50 years, with rare occurrences in children. These tumors are malignant, highly invasive, and have significant metastatic potential.2 A rarely seen, benign variant, known as osteofibrous dysplasia–like adamantinoma, is described in the literature, with fewer than 135 cases reported.3-5 This variant predominantly has benign characteristics of an osteofibrous dysplasia lesion but has the potential to transform into an adamantinoma.6 Osteofibrous dysplasia–like adamantinoma has been observed to regress with age and is also referred to as a regressing adamantinoma or differentiated adamantinoma.7
We report an uncommon case of an osteofibrous dysplasia–like adamantinoma of the tibia in a 15-year-old girl. We decided to observe the tumor with regular 3- to 6-month follow-ups. Osteofibrous dysplasia–like adamantinoma in our patient has remained stable for 2 years and has an excellent prognosis.8 We report this case for its rarity, its short-term stability, and significant treatment implications due to its potential to regress or develop into a malignant form. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 15-year-old girl was referred to our institution for evaluation of anterior left knee pain. She had sustained a fall while playing basketball 3 months earlier and had been having left knee pain since that time. She did not have any swelling, catching, or locking in her left knee. She denied any recent fever, chills, night sweats, weight loss, nausea, vomiting, or diarrhea. On physical examination, her gait was normal and no swelling, erythema, or tenderness was noticed around the left knee.
Plain radiographs revealed a heterogeneous lesion with sclerosis and thickening of the anteromedial cortex of the proximal left tibia (Figures 1A, 1B). A computed tomography (CT) scan of the abdomen, pelvis, and chest showed no osseous abnormalities. A whole-body bone scan showed activity in the anterior aspect of the left proximal tibia. No other areas of activity were noted. Magnetic resonance imaging of the left leg showed an elongated, multiloculated, enhancing mass arising from the anterolateral cortex and extending from the tibial tuberosity to the mid-diaphysis of the left tibia. Histologic examination of the CT-guided core needle biopsy specimen showed that the lesion was composed of dense fibrocollagenous tissue separating irregular bony trabeculae with osteoblastic and osteoclastic activity. There was no evidence of any atypical cells, necrosis, or significant mitotic activity. No epithelial cells were identified on hematoxylin-eosin (H&E) stain (Figure 2). However, immunohistochemical staining was positive for focal cytokeratin-positive epithelial cells (Figure 3). The lesion was diagnosed as an osteofibrous dysplasia–like adamantinoma on the basis of the radiographic and histologic findings. We elected nonoperative intervention given the benign nature of the lesion and its potential to regress. Given the possibility of sampling error and potential for progression, the patient was followed regularly at 3- to 6-month intervals over a 2-year period without disease progression.
Discussion
Osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma are rare fibro-osseous lesions that largely involve the midshaft of the tibia. Osteofibrous dysplasia accounts for 0.2% of primary bone tumors, whereas adamantinoma accounts for 0.1% to 0.5% of malignant bone tumors.9 Osteofibrous dysplasia is a benign lesion composed primarily of fibro-osseous tissue. Adamantinoma, however, is a slow-growing, low-grade, malignant biphasic tumor with intermingled epithelial and fibro-osseous components. It is an aggressive tumor that is locally invasive and can metastasize.2 Osteofibrous dysplasia–like adamantinoma (also known as differentiated or regressing adamantinoma) is a benign lesion like osteofibrous dysplasia but has features of both osteofibrous dysplasia and adamantinoma. Osteofibrous dysplasia–like adamantinoma may progress and become a malignant adamantinoma.6,10
The radiologic features of the 3 lesions are quite similar. It is not possible to distinguish between osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma based on imaging alone.9 Adamantinoma, being highly invasive, can be distinguished from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma according to the extent of involvement of the medullary cavity seen on magnetic resonance imaging.9 Complete involvement of the medullary cavity is almost always seen in an adamantinoma. Involvement of the medullary cavity is minimal or absent in osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma lesions.
Tissue confirmation through biopsy is crucial for accurate diagnosis. A biopsy should always be performed on any suspicious lesion,3,6 and the fibro-osseous lesion should be treated as an adamantinoma if findings are equivocal. A biopsy also distinctly distinguishes these lesions from benign fibrous cortical defects, which have a similar radiographic appearance. While open biopsy is the gold standard, minimally invasive techniques such as core needle biopsy and fine needle biopsy are increasingly used.6 Because of the higher risk of misdiagnosis with minimally invasive techniques, radiographic confirmation is highly recommended.5
Histologically, both osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma do not stain for cytokeratin on H&E stain. However, they can be differentiated based on immunohistochemical staining for cytokeratin. Osteofibrous dysplasia lesions exhibit diffuse staining whereas osteofibrous dysplasia–like adamantinoma lesions show focal staining of small nests of epithelial cells. Adamantinoma, in comparison, exhibits a biphasic pattern on H&E stain, representing areas of epithelial and osteofibrous cells. Immunohistochemical staining for cytokeratin of an adamantinoma reveals large nests of epithelial cells.
The association between osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma is not clearly established. However, it is widely believed that these 3 lesions represent a spectrum of the same disease and are linearly related in disease progression, with osteofibrous dysplasia at the benign end of the spectrum, osteofibrous dysplasia–like adamantinoma the intermediate form, and adamantinoma at the malignant end of the spectrum.11
Hazelbag and colleagues6 and Springfield and colleagues10 point out that osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma could be precursor lesions of adamantinoma. We found several studies in the literature that support and document progression from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma to an adamantinoma.4,6,10,12 Other studies, however, showed no progression from either a benign osteofibrous dysplasia or an osteofibrous dysplasia–like adamantinoma lesion to a malignant adamantinoma. Park and colleagues13 described 41 cases of osteofibrous dysplasia that did not progress to adamantinoma. Kuruvilla and Steiner8 described 5 cases of osteofibrous dysplasia–like adamantinoma that showed no progression to adamantinoma. Additionally, our case has not progressed and has remained radiographically stable over a 2-year follow-up. Czerniak and colleagues7 and Ueda and colleagues14 postulated, based on histologic and immunohistochemical studies, that osteofibrous dysplasia–like adamantinoma might be a regressing form of an adamantinoma that is undergoing reparative processes that could result in complete elimination of all tumor cells.
In general, any lesion with absent to low malignant potential could be managed nonoperatively with periodic observation and without the need for surgical intervention. Thus, identification of a stable or nonprogressing osteofibrous dysplasia–like adamantinoma lesion has significant treatment implications. Campanacci and Laus15 at the Rizzoli Institute in Milan, through long term follow-up of their patients with osteofibrous dysplasia, found that most lesions had a tendency to regress spontaneously by puberty. They recommended that nonextensive osteofibrous dysplasia lesions should be observed, and surgery should be delayed until puberty. Gleason and colleagues16 also recommended nonoperative management of osteofibrous dysplasia lesions, with surgery used only for large, deforming, and highly invasive lesions. We recommend a similar treatment approach for osteofibrous dysplasia–like adamantinoma lesions.
Adamantinomas, however, are usually symptomatic, are highly invasive, have a high recurrence rate, and can metastasize.9 In these patients, a wide en bloc resection or amputation should be performed as soon as possible.11 Our case highlights that osteofibrous dysplasia–like adamantinoma lesions can occur in children and can remain stable, especially over the short term. Such lesions can be observed without surgical intervention.
Adamantinomas are rare primary malignant bone tumors (less than 1% of all bone tumors) that arise most commonly in the tibia.1 There is a predilection for adult men aged 20 to 50 years, with rare occurrences in children. These tumors are malignant, highly invasive, and have significant metastatic potential.2 A rarely seen, benign variant, known as osteofibrous dysplasia–like adamantinoma, is described in the literature, with fewer than 135 cases reported.3-5 This variant predominantly has benign characteristics of an osteofibrous dysplasia lesion but has the potential to transform into an adamantinoma.6 Osteofibrous dysplasia–like adamantinoma has been observed to regress with age and is also referred to as a regressing adamantinoma or differentiated adamantinoma.7
We report an uncommon case of an osteofibrous dysplasia–like adamantinoma of the tibia in a 15-year-old girl. We decided to observe the tumor with regular 3- to 6-month follow-ups. Osteofibrous dysplasia–like adamantinoma in our patient has remained stable for 2 years and has an excellent prognosis.8 We report this case for its rarity, its short-term stability, and significant treatment implications due to its potential to regress or develop into a malignant form. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A healthy 15-year-old girl was referred to our institution for evaluation of anterior left knee pain. She had sustained a fall while playing basketball 3 months earlier and had been having left knee pain since that time. She did not have any swelling, catching, or locking in her left knee. She denied any recent fever, chills, night sweats, weight loss, nausea, vomiting, or diarrhea. On physical examination, her gait was normal and no swelling, erythema, or tenderness was noticed around the left knee.
Plain radiographs revealed a heterogeneous lesion with sclerosis and thickening of the anteromedial cortex of the proximal left tibia (Figures 1A, 1B). A computed tomography (CT) scan of the abdomen, pelvis, and chest showed no osseous abnormalities. A whole-body bone scan showed activity in the anterior aspect of the left proximal tibia. No other areas of activity were noted. Magnetic resonance imaging of the left leg showed an elongated, multiloculated, enhancing mass arising from the anterolateral cortex and extending from the tibial tuberosity to the mid-diaphysis of the left tibia. Histologic examination of the CT-guided core needle biopsy specimen showed that the lesion was composed of dense fibrocollagenous tissue separating irregular bony trabeculae with osteoblastic and osteoclastic activity. There was no evidence of any atypical cells, necrosis, or significant mitotic activity. No epithelial cells were identified on hematoxylin-eosin (H&E) stain (Figure 2). However, immunohistochemical staining was positive for focal cytokeratin-positive epithelial cells (Figure 3). The lesion was diagnosed as an osteofibrous dysplasia–like adamantinoma on the basis of the radiographic and histologic findings. We elected nonoperative intervention given the benign nature of the lesion and its potential to regress. Given the possibility of sampling error and potential for progression, the patient was followed regularly at 3- to 6-month intervals over a 2-year period without disease progression.
Discussion
Osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma are rare fibro-osseous lesions that largely involve the midshaft of the tibia. Osteofibrous dysplasia accounts for 0.2% of primary bone tumors, whereas adamantinoma accounts for 0.1% to 0.5% of malignant bone tumors.9 Osteofibrous dysplasia is a benign lesion composed primarily of fibro-osseous tissue. Adamantinoma, however, is a slow-growing, low-grade, malignant biphasic tumor with intermingled epithelial and fibro-osseous components. It is an aggressive tumor that is locally invasive and can metastasize.2 Osteofibrous dysplasia–like adamantinoma (also known as differentiated or regressing adamantinoma) is a benign lesion like osteofibrous dysplasia but has features of both osteofibrous dysplasia and adamantinoma. Osteofibrous dysplasia–like adamantinoma may progress and become a malignant adamantinoma.6,10
The radiologic features of the 3 lesions are quite similar. It is not possible to distinguish between osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma based on imaging alone.9 Adamantinoma, being highly invasive, can be distinguished from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma according to the extent of involvement of the medullary cavity seen on magnetic resonance imaging.9 Complete involvement of the medullary cavity is almost always seen in an adamantinoma. Involvement of the medullary cavity is minimal or absent in osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma lesions.
Tissue confirmation through biopsy is crucial for accurate diagnosis. A biopsy should always be performed on any suspicious lesion,3,6 and the fibro-osseous lesion should be treated as an adamantinoma if findings are equivocal. A biopsy also distinctly distinguishes these lesions from benign fibrous cortical defects, which have a similar radiographic appearance. While open biopsy is the gold standard, minimally invasive techniques such as core needle biopsy and fine needle biopsy are increasingly used.6 Because of the higher risk of misdiagnosis with minimally invasive techniques, radiographic confirmation is highly recommended.5
Histologically, both osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma do not stain for cytokeratin on H&E stain. However, they can be differentiated based on immunohistochemical staining for cytokeratin. Osteofibrous dysplasia lesions exhibit diffuse staining whereas osteofibrous dysplasia–like adamantinoma lesions show focal staining of small nests of epithelial cells. Adamantinoma, in comparison, exhibits a biphasic pattern on H&E stain, representing areas of epithelial and osteofibrous cells. Immunohistochemical staining for cytokeratin of an adamantinoma reveals large nests of epithelial cells.
The association between osteofibrous dysplasia, osteofibrous dysplasia–like adamantinoma, and adamantinoma is not clearly established. However, it is widely believed that these 3 lesions represent a spectrum of the same disease and are linearly related in disease progression, with osteofibrous dysplasia at the benign end of the spectrum, osteofibrous dysplasia–like adamantinoma the intermediate form, and adamantinoma at the malignant end of the spectrum.11
Hazelbag and colleagues6 and Springfield and colleagues10 point out that osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma could be precursor lesions of adamantinoma. We found several studies in the literature that support and document progression from osteofibrous dysplasia and osteofibrous dysplasia–like adamantinoma to an adamantinoma.4,6,10,12 Other studies, however, showed no progression from either a benign osteofibrous dysplasia or an osteofibrous dysplasia–like adamantinoma lesion to a malignant adamantinoma. Park and colleagues13 described 41 cases of osteofibrous dysplasia that did not progress to adamantinoma. Kuruvilla and Steiner8 described 5 cases of osteofibrous dysplasia–like adamantinoma that showed no progression to adamantinoma. Additionally, our case has not progressed and has remained radiographically stable over a 2-year follow-up. Czerniak and colleagues7 and Ueda and colleagues14 postulated, based on histologic and immunohistochemical studies, that osteofibrous dysplasia–like adamantinoma might be a regressing form of an adamantinoma that is undergoing reparative processes that could result in complete elimination of all tumor cells.
In general, any lesion with absent to low malignant potential could be managed nonoperatively with periodic observation and without the need for surgical intervention. Thus, identification of a stable or nonprogressing osteofibrous dysplasia–like adamantinoma lesion has significant treatment implications. Campanacci and Laus15 at the Rizzoli Institute in Milan, through long term follow-up of their patients with osteofibrous dysplasia, found that most lesions had a tendency to regress spontaneously by puberty. They recommended that nonextensive osteofibrous dysplasia lesions should be observed, and surgery should be delayed until puberty. Gleason and colleagues16 also recommended nonoperative management of osteofibrous dysplasia lesions, with surgery used only for large, deforming, and highly invasive lesions. We recommend a similar treatment approach for osteofibrous dysplasia–like adamantinoma lesions.
Adamantinomas, however, are usually symptomatic, are highly invasive, have a high recurrence rate, and can metastasize.9 In these patients, a wide en bloc resection or amputation should be performed as soon as possible.11 Our case highlights that osteofibrous dysplasia–like adamantinoma lesions can occur in children and can remain stable, especially over the short term. Such lesions can be observed without surgical intervention.
1. Kanakaraddi SV, Nagaraj G, Ravinath TM. Adamantinoma of the tibia with late skeletal metastasis: an unusual presentation. J Bone Joint Surg Br. 2007;89(3):388-389.
2. Van Geel AN, Hazelbag HM, Slingerland R, Vermeulen MI. Disseminating adamantinoma of the tibia. Sarcoma. 1997;1(2):109-111.
3. Povysil C, Kohout A, Urban K, Horak M. Differentiated adamantinoma of the fibula: a rhabdoid variant. Skeletal Radiol. 2004;33(8):488-492.
4. Hatori M, Watanabe M, Hosaka M, Sasano H, Narita M, Kokubun S. A classic adamantinoma arising from osteofibrous dysplasia-like adamantinoma in the lower leg: a case report and review of the literature. Tohoku J Exp Med. 2006;209(1):53-59.
5. Khanna M, Delaney D, Tirabosco R, Saifuddin A. Osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma and adamantinoma: correlation of radiological imaging features with surgical histology and assessment of the use of radiology in contributing to needle biopsy diagnosis. Skeletal Radiol. 2008;37(12):1077-1084.
6. Hazelbag HM, Taminiau AH, Fleuren GJ, Hogendoorn PC. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am. 1994;76(10):1482-1499.
7. Czerniak B, Rojas-Corona RR, Dorfman HD. Morphologic diversity of long bone adamantinoma. The concept of differentiated (regressing) adamantinoma and its relationship to osteofibrous dysplasia. Cancer. 1989;64(11):2319-2334.
8. Kuruvilla G, Steiner GC. Osteofibrous dysplasia-like adamantinoma of bone: a report of five cases with immunohistochemical and ultrastructural studies. Hum Pathol. 1998;29(8):809-814.
9. Bethapudi S, Ritchie DA, Macduff E, Straiton J. Imaging in osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma, and classic adamantinoma. Clin Radiol. 2014;69(2):200-208.
10. Springfield DS, Rosenberg AE, Mankin HJ, Mindell ER. Relationship between osteofibrous dysplasia and adamantinoma. Clin Orthop Relat Res. 1994;(309):234-244.
11. Most MJ, Sim FH, Inwards CY. Osteofibrous dysplasia and adamantinoma. J Am Acad Orthop Surg. 2010;18(6):358-366.
12. Lee RS, Weitzel S, Eastwood DM, et al. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J Bone Joint Surg Br. 2006;88(5):658-664.
13. Park YK, Unni KK, McLeod RA, Pritchard DJ. Osteofibrous dysplasia: clinicopathologic study of 80 cases. Hum Pathol. 1993;24(12):1339-1347.
14. Ueda Y, Roessner A, Bosse A, Edel G, Bocker W, Wuisman P. Juvenile intracortical adamantinoma of the tibia with predominant osteofibrous dysplasia-like features. Pathol Res Pract. 1991;187(8):1039-1043; discussion 1043-1034.
15. Campanacci M, Laus M. Osteofibrous dysplasia of the tibia and fibula. J Bone Joint Surg Am. 1981;63(3):367-375.
16. Gleason BC, Liegl-Atzwanger B, Kozakewich HP, et al. Osteofibrous dysplasia and adamantinoma in children and adolescents: a clinicopathologic reappraisal. Am J Surg Pathol. 2008;32(3):363-376.
1. Kanakaraddi SV, Nagaraj G, Ravinath TM. Adamantinoma of the tibia with late skeletal metastasis: an unusual presentation. J Bone Joint Surg Br. 2007;89(3):388-389.
2. Van Geel AN, Hazelbag HM, Slingerland R, Vermeulen MI. Disseminating adamantinoma of the tibia. Sarcoma. 1997;1(2):109-111.
3. Povysil C, Kohout A, Urban K, Horak M. Differentiated adamantinoma of the fibula: a rhabdoid variant. Skeletal Radiol. 2004;33(8):488-492.
4. Hatori M, Watanabe M, Hosaka M, Sasano H, Narita M, Kokubun S. A classic adamantinoma arising from osteofibrous dysplasia-like adamantinoma in the lower leg: a case report and review of the literature. Tohoku J Exp Med. 2006;209(1):53-59.
5. Khanna M, Delaney D, Tirabosco R, Saifuddin A. Osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma and adamantinoma: correlation of radiological imaging features with surgical histology and assessment of the use of radiology in contributing to needle biopsy diagnosis. Skeletal Radiol. 2008;37(12):1077-1084.
6. Hazelbag HM, Taminiau AH, Fleuren GJ, Hogendoorn PC. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am. 1994;76(10):1482-1499.
7. Czerniak B, Rojas-Corona RR, Dorfman HD. Morphologic diversity of long bone adamantinoma. The concept of differentiated (regressing) adamantinoma and its relationship to osteofibrous dysplasia. Cancer. 1989;64(11):2319-2334.
8. Kuruvilla G, Steiner GC. Osteofibrous dysplasia-like adamantinoma of bone: a report of five cases with immunohistochemical and ultrastructural studies. Hum Pathol. 1998;29(8):809-814.
9. Bethapudi S, Ritchie DA, Macduff E, Straiton J. Imaging in osteofibrous dysplasia, osteofibrous dysplasia-like adamantinoma, and classic adamantinoma. Clin Radiol. 2014;69(2):200-208.
10. Springfield DS, Rosenberg AE, Mankin HJ, Mindell ER. Relationship between osteofibrous dysplasia and adamantinoma. Clin Orthop Relat Res. 1994;(309):234-244.
11. Most MJ, Sim FH, Inwards CY. Osteofibrous dysplasia and adamantinoma. J Am Acad Orthop Surg. 2010;18(6):358-366.
12. Lee RS, Weitzel S, Eastwood DM, et al. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J Bone Joint Surg Br. 2006;88(5):658-664.
13. Park YK, Unni KK, McLeod RA, Pritchard DJ. Osteofibrous dysplasia: clinicopathologic study of 80 cases. Hum Pathol. 1993;24(12):1339-1347.
14. Ueda Y, Roessner A, Bosse A, Edel G, Bocker W, Wuisman P. Juvenile intracortical adamantinoma of the tibia with predominant osteofibrous dysplasia-like features. Pathol Res Pract. 1991;187(8):1039-1043; discussion 1043-1034.
15. Campanacci M, Laus M. Osteofibrous dysplasia of the tibia and fibula. J Bone Joint Surg Am. 1981;63(3):367-375.
16. Gleason BC, Liegl-Atzwanger B, Kozakewich HP, et al. Osteofibrous dysplasia and adamantinoma in children and adolescents: a clinicopathologic reappraisal. Am J Surg Pathol. 2008;32(3):363-376.
Tension Pneumothorax After Ultrasound-Guided Interscalene Block and Shoulder Arthroscopy
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
Interscalene brachial plexus anesthesia is commonly used for arthroscopic and open procedures of the shoulder. This regional anesthetic targets the trunks of the brachial plexus and anesthetizes the area about the shoulder and proximal arm. Its use may obviate the need for concomitant general anesthesia, potentially reducing the use of postoperative intravenous and oral pain medication. Furthermore, patients often bypass the acute postoperative anesthesia care unit and proceed directly to the ambulatory unit, permitting earlier hospital discharge. Previous reports in the literature have demonstrated higher rates of neurologic, cardiac, and pulmonary complications from this procedure; in particular, the incidence of pneumothorax was reported as high as 3%.1 Techniques to localize the nerves, such as electrical nerve stimulation and, more recently, ultrasound guidance, have reduced these complication rates.2,3 Successful administration of the block has been shown to result in satisfactory postoperative pain relief.2 However, ultrasound-guided interscalene nerve blocks remain operator-dependent and complications may still occur.
We report a case of tension pneumothorax after arthroscopic rotator cuff repair and subacromial decompression with an ultrasound-guided interscalene block. Immediate recognition and treatment of this complication resulted in a good clinical outcome. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman presented with 3 months of right shoulder pain after a fall. Examination was pertinent for weakness in forward elevation and positive rotator cuff impingement signs. She remained symptomatic despite a course of nonsurgical management that included cortisone injections and physical therapy. Magnetic resonance imaging of the shoulder showed a full-thickness supraspinatus tear with minimal fatty atrophy. After a discussion of her treatment options, she elected to undergo an arthroscopic rotator cuff repair with subacromial decompression. An evaluation by her internist revealed no pertinent medical history apart from obesity (body mass index, 36). Specifically, there was no reported history of chronic obstructive pulmonary disease or asthma. She denied any prior cigarette smoking.
The patient was evaluated by the regional anesthesia team and was classified as a class 2 airway. An interscalene brachial plexus block was performed using a 2-inch, 22-gauge needle inserted into the interscalene groove. Using an out-of-plane technique under direct ultrasound guidance, 30 mL of 0.52% ropivacaine was injected. The block was considered successful, and no complications, such as resistance, paresthesias, pain, or blood on aspiration, were noted during injection. The patient had no complaints of chest pain or shortness of breath immediately afterward, and all vital signs were stable throughout the procedure.
The patient was brought to the operating room and placed in the beach-chair position. Induction for general anesthesia was started 15 minutes after the regional anesthetic, with 2 intubation attempts necessary because of poor airway visualization. After placement of the endotracheal tube, breath sounds were noted to be equal bilaterally. The arthroscopic procedure consisted of double-row rotator cuff repair, subacromial decompression, and débridement of the glenohumeral joint for synovitis, using standard arthroscopic portals. There were no difficulties with trocar placement, and bleeding was minimal throughout the case. The total surgical time was 150 minutes and a pump pressure of 30 mm Hg was maintained during the arthroscopy.
Within the first 60 minutes of the start of the arthroscopic procedure, the patient was noted to be intermittently hypotensive with mean arterial pressure (MAP) ranging from the 30s to 130s mm Hg and pulse in the 70 to 80 beats/min range. FiO2 in the 85% to 95% range was maintained throughout the procedure. During that time, 50 μg phenylephrine was administered on 4 separate occasions to maintain her blood pressure. The labile blood pressure was attributed by the anesthesiologist to the beach-chair position. During an attempted extubation upon conclusion of the surgery, the patient became hypotensive with MAP that ranged from the 40s to 60s mm Hg and tachycardic to 90 beats/min. The oxygen saturation was in the low 90s and tidal volume was poor. Absent lung sounds were noted on the right chest. An urgent portable chest radiograph showed a large right-sided tension pneumothorax with mediastinal shift (Figure 1). After an immediate general surgery consultation, a chest tube was placed in the operating room. The patient’s vital signs improved and a repeat chest radiograph revealed successful re-expansion of the lung (Figure 2). She was transferred to the acute postoperative anesthesia care unit and extubated in the intensive care unit later that day.
The patient’s chest tube was removed 2 days later and she was discharged home on hospital day 5 with a completely resolved pneumothorax. She was seen 1 week later in the office for a postoperative visit and reported feeling well without chest pain or shortness of breath.
Discussion
Interscalene brachial plexus anesthesia was first described by Winnie4 in 1970. This block targets the trunks of the brachial plexus, which are enclosed in a fascial sheath between the anterior and middle scalene muscles. In this region lie several structures at risk: the phrenic nerve superficially and inferiorly; the carotid sheath located superficially and medially; the subclavian artery parallel to the trunks; and the cupula of the lung that lies deep and inferior to the anterior scalene muscle. Recognized complications of the block include vocal hoarseness, Horner syndrome, and hemidiaphragmatic paresis caused by the temporary blockade of the ipsilateral recurrent laryngeal nerve, stellate ganglion, and phrenic nerve, in that order.5 Use of the interscalene block has been associated with minimal risk for pneumothorax, because the needle entry point is superior and directed away from the lung pleura.6 This is in contrast to the more inferiorly placed supraclavicular block, located in closer proximity to the lung cupula.5
Two different approaches are commonly used during ultrasound-guided nerve blocks. The in-plane approach generates a long-axis view of the needle by advancing the needle parallel with the long axis of the ultrasound probe. While this allows direct visualization of the needle tip, it requires deeper needle insertion from lateral to medial, causing puncture of the middle scalene muscle that may increase patient discomfort and risk nerve injury within the muscle.7 The out-of-plane approach used on our patient involves needle insertion parallel to the brachial plexus, but along the short axis of the ultrasound probe. Although this permits the operator to assess the periphery of the nerve, it may lead to poor needle-tip visualization during the procedure. As a result, operators often use a combination of tissue disturbance and “hydrolocation,” in which fluid is injected to indicate the needle-tip location.8,9
Tension pneumothorax represents the accumulation of air in the pleural space that leads to impaired pulmonary and cardiac function. It is often caused by disruption or puncture of the parietal or visceral pleura, creating a connection between the alveoli and pleural cavity. The gradual buildup of air in the pleural cavity results in increased intrapleural pressure, which compresses and ultimately collapses the ipsilateral lung. Venous compression restricts blood return to the heart and reduces cardiac output. Clinical manifestations include dyspnea, hypoxemia, tachycardia, and hypotension.10 Multiple techniques were developed to better localize the brachial plexus while reducing injury to nearby structures, including the lung. These include eliciting needle paresthesias, electrical nerve stimulation, and ultrasound guidance. While nerve stimulation was once the gold standard for brachial plexus localization, ultrasound guidance has gained in popularity because of its noninvasive nature and dynamic capability to identify nerves and surrounding structures.11 Perlas and colleagues12 determined the sensitivity of needle paresthesias and nerve stimulation to be 38% and 75%, respectively, in cases in which plexus localization had been confirmed by ultrasound.
Several studies have reported on the efficacy of interscalene nerve block with either nerve stimulation or ultrasound guidance in the setting of shoulder surgery.2,3 Bishop and colleagues3 reviewed 547 patients who underwent interscalene regional anesthesia with nerve stimulation for both arthroscopic and open-shoulder procedures. They reported a 97% success rate and 12 (2.3%) minor complications, including sensory neuropathy and complex regional pain syndrome. There were no cases of pneumothorax, cardiac events, or other major complications.3 In a prospective study of 1319 patients, Singh and colleagues2 reported a 99.6% success rate using ultrasound-guided interscalene blocks for their shoulder surgeries. A total of 38 adverse events (2.88%) were identified: 14 transient neurologic events, including ear numbness, digital numbness, and brachial plexitis; 1 case of intraoperative bradycardia, and 2 cancellations after the block for chest pain and flank pain, which yielded negative cardiac workups. Other complications included postoperative emergency room visits and hospital admissions for reasons unrelated to the block.2 Interscalene regional anesthesia, therefore, provides effective anesthesia for shoulder surgery with low complication rates.
Pneumothorax after ultrasound-guided interscalene block has rarely been reported.13,14 In a review of 144 ultrasound-guided indwelling interscalene catheter placements, a 98% successful block rate with a single complication of small pneumothorax after total shoulder arthroplasty was reported.13 Mandim and colleagues14 reported a case of pneumothorax in a smoker who underwent an ultrasound-guided brachial plexus block prior to open reduction and internal fixation of an ulnar fracture. While the patient was asymptomatic and vital signs remained stable during the procedure, the patient complained postoperatively of chest pain with hypoxia, tachycardia, and hypotension. A chest radiograph confirmed an ipsilateral pneumothorax, and the patient was treated successfully with chest-tube placement. The authors attributed this complication to a higher pleural dome resulting from a hyperinflated lung caused by chronic smoking. Our patient reported no history of smoking and her preoperative chest radiograph had no evidence of lung disease.
In contrast, several cases of pneumothorax after shoulder surgery have been reported in the absence of nerve block. Oldman and Peng1 reported a 41-year-old nonsmoker who underwent arthroscopic labral repair and subacromial decompression. The preoperative nerve block was cancelled, and the patient received general endotracheal anesthesia alone. Fifty minutes after the case, the patient developed chest pain and hypoxia. A chest radiograph showed a small pneumothorax that was managed conservatively. The pneumothorax was attributed to spontaneous rupture of a preexisting lung bulla, suggesting that blocks are not always the cause of this complication. Furthermore, Dietzel and Ciullo15 reported 4 cases of spontaneous pneumothorax within 24 hours of uncomplicated arthroscopic shoulder procedures under general anesthesia in the lateral decubitus position. The patient ages ranged from 22 to 38 years, and medical histories were all significant for preexisting lung disease, remote history of pneumonia, and heavy smoking. Three of the patients experienced symptoms at home the day after surgery. The authors concluded that these cases were likely caused by rupture of blebs or bullae from underlying lung disease; these ruptured blebs or bullae are difficult to detect and usually located in the upper lung. The pressure gradient from the positive pressure of anesthesia and the ipsilateral upper lung is thought to be highest in the lateral decubitus position, increasing their chance of rupture.15
Finally, Lee and colleagues16 described 3 patients aged 40 to 45 years who underwent uncomplicated subacromial decompression in the beach-chair position under general anesthesia. Significant shoulder, neck, and axillary swelling were noted after surgery, and a chest radiograph showed tension pneumothorax, subcutaneous emphysema, and pneumomediastinum. The authors speculated that pressure in the subacromial space may become negative relative to atmospheric pressure when the shaver and suction are running, drawing in air through other portals. When the suction is discontinued, fluid infusion may push air into the surrounding tissue, leading to subcutaneous emphysema, which may spread to the mediastinum.16
Conclusion
Ultrasound-guided interscalene nerve blocks have successfully provided anesthesia for shoulder surgeries with low complication rates. Although the incidence of pneumothorax has decreased significantly with ultrasound guidance, the success of this procedure is highly operator-dependent. We present the case of an otherwise healthy patient without known pulmonary disease who developed a tension pneumothorax after the administration of ultrasound-guided regional and general anesthesia for arthroscopic shoulder surgery. Orthopedic surgeons and anesthesiologists must remain vigilant for pneumothorax during the perioperative period after shoulder surgery performed under interscalene regional aesthesia, particularly in the setting of hypotension, hypoxia, and/or tachycardia. Risk factors, such as history of smoking and preexisting lung disease, may predispose patients to the development of pneumothorax. Timely recognition and placement of a chest tube result in satisfactory clinical outcomes.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
1. Oldman M, Peng Pi P. Pneumothorax after shoulder arthroscopy: don’t blame it on regional anesthesia. Reg Anesth Pain Med. 2004;29(4):382-383.
2. Singh A, Kelly C, O’Brien T, Wilson J, Warner JJ. Ultrasound-guided interscalene block anesthesia for shoulder arthroscopy: a prospective study of 1319 patients. J Bone Joint Surg Am. 2012;94(22):2040-2046.
3. Bishop JY, Sprague M, Gelber J, et al. Interscalene regional anesthesia for shoulder surgery. J Bone Joint Surg Am. 2005;87(5):974-979.
4. Winnie AP. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455-466.
5. Mian A, Chaudhry I, Huang R, Rizk E, Tubbs RS, Loukas M. Brachial plexus anesthesia: a review of the relevant anatomy, complications, and anatomical variations. Clin Anat. 2014;27(2):210-221.
6. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295-300.
7. Marhofer P, Harrop-Griffiths W, Willschke H, Kirchmair L. Fifteen years of ultrasound guidance in regional anaesthesia: Part 2 - recent developments in block techniques. Br J Anaesth. 2010;104(6):673-683.
8. Sites BD, Spence BC, Gallagher J, et al. Regional anesthesia meets ultrasound: a specialty in transition. Acta Anaesthesiol Scand. 2008;52(4):456-466.
9. Ilfeld BM, Fredrickson MJ, Mariano ER. Ultrasound-guided perineural catheter insertion: three approaches but few illuminating data. Reg Anesth Pain Med. 2010;35(2):123-126.
10. Choi WI. Pneumothorax. Tuberc Respir Dis (Seoul). 2014;76(3):99-104.
11. Klaastad O, Sauter AR, Dodgson MS. Brachial plexus block with or without ultrasound guidance. Curr Opin Anaesthesiol. 2009;22(5):655-660.
12. Perlas A, Niazi A, McCartney C, Chan V, Xu D, Abbas S. The sensitivity of motor response to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med. 2006;31(5):445-450.
13. Bryan NA, Swenson JD, Greis PE, Burks RT. Indwelling interscalene catheter use in an outpatient setting for shoulder surgery: technique, efficacy, and complications. J Shoulder Elbow Surg. 2007;16(4):388-395.
14. Mandim BL, Alves RR, Almeida R, Pontes JP, Arantes LJ, Morais FP. Pneumothorax post brachial plexus block guided by ultrasound: a case report. Rev Bras Anestesiol. 2012;62(5):741-747.
15. Dietzel DP, Ciullo JV. Spontaneous pneumothorax after shoulder arthroscopy: a report of four cases. Arthroscopy. 1996;12(1):99-102.
16. Lee HC, Dewan N, Crosby L. Subcutaneous emphysema, pneumomediastinum, and potentially life-threatening tension pneumothorax. Pulmonary complications from arthroscopic shoulder decompression. Chest. 1992;101(5):1265-1267.
Osteoid Osteoma of the Talar Neck With Subacute Presentation
Osteoid osteoma of the talar neck is an unusual clinical condition that is often overlooked on initial assessment of patients with ankle pain. Here, we present a case report of an adolescent male with talar neck osteoid osteoma who reported persistent pain after an injury. We discuss the differential diagnosis of persistent anterior ankle pain and assess the treatment options for osteoid osteoma of the talar neck. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 13-year-old boy presented to our clinic 3 months after a right ankle sprain. He had visited the emergency department at the time of injury; radiographs of the ankle were reported negative for fractures, dislocations, or bone pathologies. He was treated conservatively with elastic support, icing, rest, elevation, and weight-bearing as tolerated. Upon presentation to our office, his pain involved the entire ankle joint. He had not put weight on it since the injury. On examination, he had a significant limp, anteromedial swelling, and tenderness over the ankle joint anteromedially. His neurologic and vascular examinations were normal.
His plain radiographs showed a cystic mass, located at the dorsal aspect of the talar neck (Figures 1A, 1B). Computed tomography (CT) showed a round lucent lesion involving the superior aspect of the talar neck, measuring 9 mm by 6 mm. A sclerotic radiodense focus was evident in the center (Figures 2A, 2B). Noncontrast multiplanar, multisequence magnetic resonance imaging (MRI) showed abnormal edema throughout the talus and a 9-mm rounded ossicle overlying the superior margin of the neck of the talus (Figures 3A, 3B).
Differential Diagnosis
The differential diagnosis for anterior ankle pain includes ankle sprain, monoarticular arthritis, anterior ankle impingement, and talar neck fractures. Other related findings include the presence of a talar ridge and a talar beak.
Ankle sprains are very common injuries. The mainstay treatment consists of ice, resting, elevation, and elastic or semirigid support, and patients usually recover over the course of a few weeks. These sprains are typically injuries of the lateral or medial ligaments of the ankle. Extension of a ligament tear across the anterior capsule can explain persistent anterior ankle pain. The presence of a bony lesion on plain radiographs, however, makes the diagnosis of an ankle sprain, with or without extension into the anterior capsule, less likely.
Monoarticular arthritis, which may present in the ankle and has a wide differential diagnosis, usually involves the whole joint.
Anterior ankle impingement typically occurs in athletes who participate in sports that involve kicking. It can be a bony or soft-tissue impingent. Clinically, patients present with pain and loss of motion, specifically dorsiflexion.
Talar neck fractures are usually the result of high-energy trauma. Stress fractures of the neck of the talus are uncommon and are associated with a recent sudden increase in physical activity, such as running, dancing, or military training. Radiographs, CT scans, and MRI help define the fracture line.
The talar ridge is the site of capsular and ligamentous attachment on the superior aspect of the talar neck and may become hypertrophic in athletes. A hypertrophic talar ridge is asymptomatic and is not considered a pathologic finding on radiographs.
The talar beak, a flaring of the anterosuperior aspect of the talar head, is an indirect sign of tarsal coalition. When symptomatic, patients complain of subtalar symptoms, typically pain and limitation of motion. It usually does not present acutely.
Treatment
We offered the patient surgical excision, and his guardian consented to left ankle arthroscopy. We performed synovectomy using a combination of 3.5-mm shaver and radiofrequency probe. We identified the mass: round, soft, and located at the superior-medial aspect of the talar neck. We removed it in piecemeal fashion using manual arthroscopic instruments, and cauterized its base using the radiofrequency probe. We allowed the patient weight-bearing as tolerated starting the day after surgery.
We submitted the specimen for pathologic evaluation (Figure 4). It consisted of multiple pieces of tan/brown tissue. Histologic examination showed benign osteoblastic proliferation composed of anastomosing bony trabeculae with variable mineralization, lined by plump osteoblasts, within vascularized connective tissue; benign giant cells were present, consistent with a nidus of an osteoid osteoma.
On the first postoperative visit, the patient was pain-free and bearing weight with crutches. He was gradually weaned from his crutches and returned to full weight-bearing over the next 4 weeks. At 12-month follow-up, he was symptom-free with good range of motion and full return to previous level of activity.
Discussion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in a few case series2-7 and is associated with a typical nidus that can be identified on CT scans. It does not present acutely, however. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. However, this presentation is not universal and is frequently missed.2
Juxta-articular osteoid osteomas in the ankle and foot can be difficult to diagnose. The most common site is the talus.3 The majority of patients link their pain to a remote ankle injury. The time delay to diagnosis is on average 2.5 years, but it can be as long as 10 years.4-6 A CT scan is the best method to identify the nidus; MRI can be misleading if it shows only marrow edema but not a nidus.4,5,7 In our patient, an injury was documented, and the patient denied prior symptoms. We cannot explain how an injury would trigger the formation of an osteoid osteoma or cause a previously asymptomatic osteoid osteoma to become symptomatic.
Medical treatment with nonsteroidal anti-inflammatory drugs has been used but is reported to take 2 to 4 years for resolution of symptoms; many patients may consider the treatment time frame too long when other alternatives are available.8 These include open resection, arthroscopic resection, and image-guided ablation. Open surgical techniques include en bloc resection and curettage. Bone grafting or internal fixation may be performed as needed. Arthroscopic excision of juxta-articular osteoid osteomas offers the advantages of good visualization and avoidance of soft-tissue dissection, and allows for complete excision of the lesion as well as synovectomy.6,9,10 Arthroscopic excision also allows for quicker rehabilitation. Image-guided ablation, such as radionuclide-guided excision, CT-guided thermal ablation, and laser photocoagulation, may be even less invasive but do not allow for direct visualization, complete resection, and biopsy.11
Conclusion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in multiple case series and is associated with a typical nidus that can be identified on CT scans. Usually, it does not present acutely. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. This presentation is not universal, however, and is frequently missed, especially when the pain is associated with a prior injury.2 Arthroscopic exploration of the ankle with resection of subperiosteal osteoid osteoma and the associated synovitis using thermal ablation of the base with radiofrequency offers lasting cure with minimal morbidity.
1. Edeiken J, DePalma AF, Hodes PJ. Osteoid osteoma. Clin Orthop Relat Res. 1966;49:201-206.
2. El Rayes MA, El Kordy S. Osteoid osteoma of the talus. Foot. 2003;13(3):166–168.
3. Capanna R, Van Horn JR, Ayala A, Picci P, Bettelli G. Osteoid osteoma and osteoblastoma of the talus. A report of 40 cases. Skeletal Radiol. 1986;15(5):360-364.
4. Chuang SY, Wang SJ, Au MK, Huang GS. Osteoid osteoma in talar neck: a report of two cases. Foot Ankle Int. 1998;19(1):44-47.
5. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
6. Yercan HS, Okcu G, Őzalp T, Ősiç U. Arthroscopic removal of the osteoid osteoma on the neck of the talus. Knee Surg Sports Traumatol Arthrosc. 2004;12(3):246-249.
7. Mazlout O, Saudan M, Ladeb MF, Garcia JF, Bianchi S. Osteoid osteoma of the talar neck: a diagnostic challenge. Eur J Radiol Extra. 2004;49(2):67-70.
8. Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74(2):179-185.
9. Bojanić I, Orlić D, Ivković A. Arthroscopic removal of a juxtaarticular osteoid osteoma of the talar neck. J Foot Ankle Surg. 2003;42(6):359-362.
10. Tüzüner S, Aydin AT. Arthroscopic removal of an osteoid osteoma at talar neck. Arthroscopy. 1998;14(4):405-409.
11. Amendola A, Vellet D, Willits K. Osteoid osteoma of the neck of the talus: percutaneous, computed tomography-guided technique for complete excision. Foot Ankle Int. 1994;15(8):429-432.
Osteoid osteoma of the talar neck is an unusual clinical condition that is often overlooked on initial assessment of patients with ankle pain. Here, we present a case report of an adolescent male with talar neck osteoid osteoma who reported persistent pain after an injury. We discuss the differential diagnosis of persistent anterior ankle pain and assess the treatment options for osteoid osteoma of the talar neck. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 13-year-old boy presented to our clinic 3 months after a right ankle sprain. He had visited the emergency department at the time of injury; radiographs of the ankle were reported negative for fractures, dislocations, or bone pathologies. He was treated conservatively with elastic support, icing, rest, elevation, and weight-bearing as tolerated. Upon presentation to our office, his pain involved the entire ankle joint. He had not put weight on it since the injury. On examination, he had a significant limp, anteromedial swelling, and tenderness over the ankle joint anteromedially. His neurologic and vascular examinations were normal.
His plain radiographs showed a cystic mass, located at the dorsal aspect of the talar neck (Figures 1A, 1B). Computed tomography (CT) showed a round lucent lesion involving the superior aspect of the talar neck, measuring 9 mm by 6 mm. A sclerotic radiodense focus was evident in the center (Figures 2A, 2B). Noncontrast multiplanar, multisequence magnetic resonance imaging (MRI) showed abnormal edema throughout the talus and a 9-mm rounded ossicle overlying the superior margin of the neck of the talus (Figures 3A, 3B).
Differential Diagnosis
The differential diagnosis for anterior ankle pain includes ankle sprain, monoarticular arthritis, anterior ankle impingement, and talar neck fractures. Other related findings include the presence of a talar ridge and a talar beak.
Ankle sprains are very common injuries. The mainstay treatment consists of ice, resting, elevation, and elastic or semirigid support, and patients usually recover over the course of a few weeks. These sprains are typically injuries of the lateral or medial ligaments of the ankle. Extension of a ligament tear across the anterior capsule can explain persistent anterior ankle pain. The presence of a bony lesion on plain radiographs, however, makes the diagnosis of an ankle sprain, with or without extension into the anterior capsule, less likely.
Monoarticular arthritis, which may present in the ankle and has a wide differential diagnosis, usually involves the whole joint.
Anterior ankle impingement typically occurs in athletes who participate in sports that involve kicking. It can be a bony or soft-tissue impingent. Clinically, patients present with pain and loss of motion, specifically dorsiflexion.
Talar neck fractures are usually the result of high-energy trauma. Stress fractures of the neck of the talus are uncommon and are associated with a recent sudden increase in physical activity, such as running, dancing, or military training. Radiographs, CT scans, and MRI help define the fracture line.
The talar ridge is the site of capsular and ligamentous attachment on the superior aspect of the talar neck and may become hypertrophic in athletes. A hypertrophic talar ridge is asymptomatic and is not considered a pathologic finding on radiographs.
The talar beak, a flaring of the anterosuperior aspect of the talar head, is an indirect sign of tarsal coalition. When symptomatic, patients complain of subtalar symptoms, typically pain and limitation of motion. It usually does not present acutely.
Treatment
We offered the patient surgical excision, and his guardian consented to left ankle arthroscopy. We performed synovectomy using a combination of 3.5-mm shaver and radiofrequency probe. We identified the mass: round, soft, and located at the superior-medial aspect of the talar neck. We removed it in piecemeal fashion using manual arthroscopic instruments, and cauterized its base using the radiofrequency probe. We allowed the patient weight-bearing as tolerated starting the day after surgery.
We submitted the specimen for pathologic evaluation (Figure 4). It consisted of multiple pieces of tan/brown tissue. Histologic examination showed benign osteoblastic proliferation composed of anastomosing bony trabeculae with variable mineralization, lined by plump osteoblasts, within vascularized connective tissue; benign giant cells were present, consistent with a nidus of an osteoid osteoma.
On the first postoperative visit, the patient was pain-free and bearing weight with crutches. He was gradually weaned from his crutches and returned to full weight-bearing over the next 4 weeks. At 12-month follow-up, he was symptom-free with good range of motion and full return to previous level of activity.
Discussion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in a few case series2-7 and is associated with a typical nidus that can be identified on CT scans. It does not present acutely, however. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. However, this presentation is not universal and is frequently missed.2
Juxta-articular osteoid osteomas in the ankle and foot can be difficult to diagnose. The most common site is the talus.3 The majority of patients link their pain to a remote ankle injury. The time delay to diagnosis is on average 2.5 years, but it can be as long as 10 years.4-6 A CT scan is the best method to identify the nidus; MRI can be misleading if it shows only marrow edema but not a nidus.4,5,7 In our patient, an injury was documented, and the patient denied prior symptoms. We cannot explain how an injury would trigger the formation of an osteoid osteoma or cause a previously asymptomatic osteoid osteoma to become symptomatic.
Medical treatment with nonsteroidal anti-inflammatory drugs has been used but is reported to take 2 to 4 years for resolution of symptoms; many patients may consider the treatment time frame too long when other alternatives are available.8 These include open resection, arthroscopic resection, and image-guided ablation. Open surgical techniques include en bloc resection and curettage. Bone grafting or internal fixation may be performed as needed. Arthroscopic excision of juxta-articular osteoid osteomas offers the advantages of good visualization and avoidance of soft-tissue dissection, and allows for complete excision of the lesion as well as synovectomy.6,9,10 Arthroscopic excision also allows for quicker rehabilitation. Image-guided ablation, such as radionuclide-guided excision, CT-guided thermal ablation, and laser photocoagulation, may be even less invasive but do not allow for direct visualization, complete resection, and biopsy.11
Conclusion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in multiple case series and is associated with a typical nidus that can be identified on CT scans. Usually, it does not present acutely. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. This presentation is not universal, however, and is frequently missed, especially when the pain is associated with a prior injury.2 Arthroscopic exploration of the ankle with resection of subperiosteal osteoid osteoma and the associated synovitis using thermal ablation of the base with radiofrequency offers lasting cure with minimal morbidity.
Osteoid osteoma of the talar neck is an unusual clinical condition that is often overlooked on initial assessment of patients with ankle pain. Here, we present a case report of an adolescent male with talar neck osteoid osteoma who reported persistent pain after an injury. We discuss the differential diagnosis of persistent anterior ankle pain and assess the treatment options for osteoid osteoma of the talar neck. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 13-year-old boy presented to our clinic 3 months after a right ankle sprain. He had visited the emergency department at the time of injury; radiographs of the ankle were reported negative for fractures, dislocations, or bone pathologies. He was treated conservatively with elastic support, icing, rest, elevation, and weight-bearing as tolerated. Upon presentation to our office, his pain involved the entire ankle joint. He had not put weight on it since the injury. On examination, he had a significant limp, anteromedial swelling, and tenderness over the ankle joint anteromedially. His neurologic and vascular examinations were normal.
His plain radiographs showed a cystic mass, located at the dorsal aspect of the talar neck (Figures 1A, 1B). Computed tomography (CT) showed a round lucent lesion involving the superior aspect of the talar neck, measuring 9 mm by 6 mm. A sclerotic radiodense focus was evident in the center (Figures 2A, 2B). Noncontrast multiplanar, multisequence magnetic resonance imaging (MRI) showed abnormal edema throughout the talus and a 9-mm rounded ossicle overlying the superior margin of the neck of the talus (Figures 3A, 3B).
Differential Diagnosis
The differential diagnosis for anterior ankle pain includes ankle sprain, monoarticular arthritis, anterior ankle impingement, and talar neck fractures. Other related findings include the presence of a talar ridge and a talar beak.
Ankle sprains are very common injuries. The mainstay treatment consists of ice, resting, elevation, and elastic or semirigid support, and patients usually recover over the course of a few weeks. These sprains are typically injuries of the lateral or medial ligaments of the ankle. Extension of a ligament tear across the anterior capsule can explain persistent anterior ankle pain. The presence of a bony lesion on plain radiographs, however, makes the diagnosis of an ankle sprain, with or without extension into the anterior capsule, less likely.
Monoarticular arthritis, which may present in the ankle and has a wide differential diagnosis, usually involves the whole joint.
Anterior ankle impingement typically occurs in athletes who participate in sports that involve kicking. It can be a bony or soft-tissue impingent. Clinically, patients present with pain and loss of motion, specifically dorsiflexion.
Talar neck fractures are usually the result of high-energy trauma. Stress fractures of the neck of the talus are uncommon and are associated with a recent sudden increase in physical activity, such as running, dancing, or military training. Radiographs, CT scans, and MRI help define the fracture line.
The talar ridge is the site of capsular and ligamentous attachment on the superior aspect of the talar neck and may become hypertrophic in athletes. A hypertrophic talar ridge is asymptomatic and is not considered a pathologic finding on radiographs.
The talar beak, a flaring of the anterosuperior aspect of the talar head, is an indirect sign of tarsal coalition. When symptomatic, patients complain of subtalar symptoms, typically pain and limitation of motion. It usually does not present acutely.
Treatment
We offered the patient surgical excision, and his guardian consented to left ankle arthroscopy. We performed synovectomy using a combination of 3.5-mm shaver and radiofrequency probe. We identified the mass: round, soft, and located at the superior-medial aspect of the talar neck. We removed it in piecemeal fashion using manual arthroscopic instruments, and cauterized its base using the radiofrequency probe. We allowed the patient weight-bearing as tolerated starting the day after surgery.
We submitted the specimen for pathologic evaluation (Figure 4). It consisted of multiple pieces of tan/brown tissue. Histologic examination showed benign osteoblastic proliferation composed of anastomosing bony trabeculae with variable mineralization, lined by plump osteoblasts, within vascularized connective tissue; benign giant cells were present, consistent with a nidus of an osteoid osteoma.
On the first postoperative visit, the patient was pain-free and bearing weight with crutches. He was gradually weaned from his crutches and returned to full weight-bearing over the next 4 weeks. At 12-month follow-up, he was symptom-free with good range of motion and full return to previous level of activity.
Discussion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in a few case series2-7 and is associated with a typical nidus that can be identified on CT scans. It does not present acutely, however. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. However, this presentation is not universal and is frequently missed.2
Juxta-articular osteoid osteomas in the ankle and foot can be difficult to diagnose. The most common site is the talus.3 The majority of patients link their pain to a remote ankle injury. The time delay to diagnosis is on average 2.5 years, but it can be as long as 10 years.4-6 A CT scan is the best method to identify the nidus; MRI can be misleading if it shows only marrow edema but not a nidus.4,5,7 In our patient, an injury was documented, and the patient denied prior symptoms. We cannot explain how an injury would trigger the formation of an osteoid osteoma or cause a previously asymptomatic osteoid osteoma to become symptomatic.
Medical treatment with nonsteroidal anti-inflammatory drugs has been used but is reported to take 2 to 4 years for resolution of symptoms; many patients may consider the treatment time frame too long when other alternatives are available.8 These include open resection, arthroscopic resection, and image-guided ablation. Open surgical techniques include en bloc resection and curettage. Bone grafting or internal fixation may be performed as needed. Arthroscopic excision of juxta-articular osteoid osteomas offers the advantages of good visualization and avoidance of soft-tissue dissection, and allows for complete excision of the lesion as well as synovectomy.6,9,10 Arthroscopic excision also allows for quicker rehabilitation. Image-guided ablation, such as radionuclide-guided excision, CT-guided thermal ablation, and laser photocoagulation, may be even less invasive but do not allow for direct visualization, complete resection, and biopsy.11
Conclusion
Osteoid osteoma is a small, benign, well-circumscribed osteoblastic cortical lesion, typically identified in long bones or, less frequently, in the subperiosteal region.1 It often affects adolescents. Osteoid osteoma has been described in the talus in multiple case series and is associated with a typical nidus that can be identified on CT scans. Usually, it does not present acutely. The typical presentation for osteoid osteoma is bone pain at night that responds to nonsteroidal anti-inflammatory drugs. This presentation is not universal, however, and is frequently missed, especially when the pain is associated with a prior injury.2 Arthroscopic exploration of the ankle with resection of subperiosteal osteoid osteoma and the associated synovitis using thermal ablation of the base with radiofrequency offers lasting cure with minimal morbidity.
1. Edeiken J, DePalma AF, Hodes PJ. Osteoid osteoma. Clin Orthop Relat Res. 1966;49:201-206.
2. El Rayes MA, El Kordy S. Osteoid osteoma of the talus. Foot. 2003;13(3):166–168.
3. Capanna R, Van Horn JR, Ayala A, Picci P, Bettelli G. Osteoid osteoma and osteoblastoma of the talus. A report of 40 cases. Skeletal Radiol. 1986;15(5):360-364.
4. Chuang SY, Wang SJ, Au MK, Huang GS. Osteoid osteoma in talar neck: a report of two cases. Foot Ankle Int. 1998;19(1):44-47.
5. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
6. Yercan HS, Okcu G, Őzalp T, Ősiç U. Arthroscopic removal of the osteoid osteoma on the neck of the talus. Knee Surg Sports Traumatol Arthrosc. 2004;12(3):246-249.
7. Mazlout O, Saudan M, Ladeb MF, Garcia JF, Bianchi S. Osteoid osteoma of the talar neck: a diagnostic challenge. Eur J Radiol Extra. 2004;49(2):67-70.
8. Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74(2):179-185.
9. Bojanić I, Orlić D, Ivković A. Arthroscopic removal of a juxtaarticular osteoid osteoma of the talar neck. J Foot Ankle Surg. 2003;42(6):359-362.
10. Tüzüner S, Aydin AT. Arthroscopic removal of an osteoid osteoma at talar neck. Arthroscopy. 1998;14(4):405-409.
11. Amendola A, Vellet D, Willits K. Osteoid osteoma of the neck of the talus: percutaneous, computed tomography-guided technique for complete excision. Foot Ankle Int. 1994;15(8):429-432.
1. Edeiken J, DePalma AF, Hodes PJ. Osteoid osteoma. Clin Orthop Relat Res. 1966;49:201-206.
2. El Rayes MA, El Kordy S. Osteoid osteoma of the talus. Foot. 2003;13(3):166–168.
3. Capanna R, Van Horn JR, Ayala A, Picci P, Bettelli G. Osteoid osteoma and osteoblastoma of the talus. A report of 40 cases. Skeletal Radiol. 1986;15(5):360-364.
4. Chuang SY, Wang SJ, Au MK, Huang GS. Osteoid osteoma in talar neck: a report of two cases. Foot Ankle Int. 1998;19(1):44-47.
5. Snow SW, Sobel M, DiCarlo EF, Thompson FM, Deland JT. Chronic ankle pain caused by osteoid osteoma of the neck of the talus. Foot Ankle Int. 1997;18(2):98-101.
6. Yercan HS, Okcu G, Őzalp T, Ősiç U. Arthroscopic removal of the osteoid osteoma on the neck of the talus. Knee Surg Sports Traumatol Arthrosc. 2004;12(3):246-249.
7. Mazlout O, Saudan M, Ladeb MF, Garcia JF, Bianchi S. Osteoid osteoma of the talar neck: a diagnostic challenge. Eur J Radiol Extra. 2004;49(2):67-70.
8. Kneisl JS, Simon MA. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74(2):179-185.
9. Bojanić I, Orlić D, Ivković A. Arthroscopic removal of a juxtaarticular osteoid osteoma of the talar neck. J Foot Ankle Surg. 2003;42(6):359-362.
10. Tüzüner S, Aydin AT. Arthroscopic removal of an osteoid osteoma at talar neck. Arthroscopy. 1998;14(4):405-409.
11. Amendola A, Vellet D, Willits K. Osteoid osteoma of the neck of the talus: percutaneous, computed tomography-guided technique for complete excision. Foot Ankle Int. 1994;15(8):429-432.