User login
Empowering Culture Change and Safety on the Journey to Zero Harm With Huddle Cards
Empowering Culture Change and Safety on the Journey to Zero Harm With Huddle Cards
Safety event reporting plays a vital role in fostering a culture of safety within a health care organization. The US Department of Veterans Affairs (VA) has shifted its focus from eradicating medical errors to minimizing or eliminating harm to patients.1 The National Center for Patient Safety’s objective is to prevent recurring errors by identifying and addressing systemic problems that may have been overlooked.2
Taking inspiration from industries known for high reliability, such as aviation and nuclear power, the Veterans Health Administration (VHA) patient safety program aims to identify and eliminate system vulnerabilities, such as medical errors. Learning from near misses, which occur more frequently than actual adverse events, is a crucial part of this process.3 By addressing these issues, the VHA can establish safer systems and encourage continuous identification of potential problems with proactive resolution.
All staff should participate actively in event reporting, which involves documenting and communicating details, outcomes, and relevant data about an event to understand what occurred, evaluate success, identify areas for improvement, and inform future decisions. This helps identify system weaknesses, create opportunities to standardize procedures and enhance patient care.
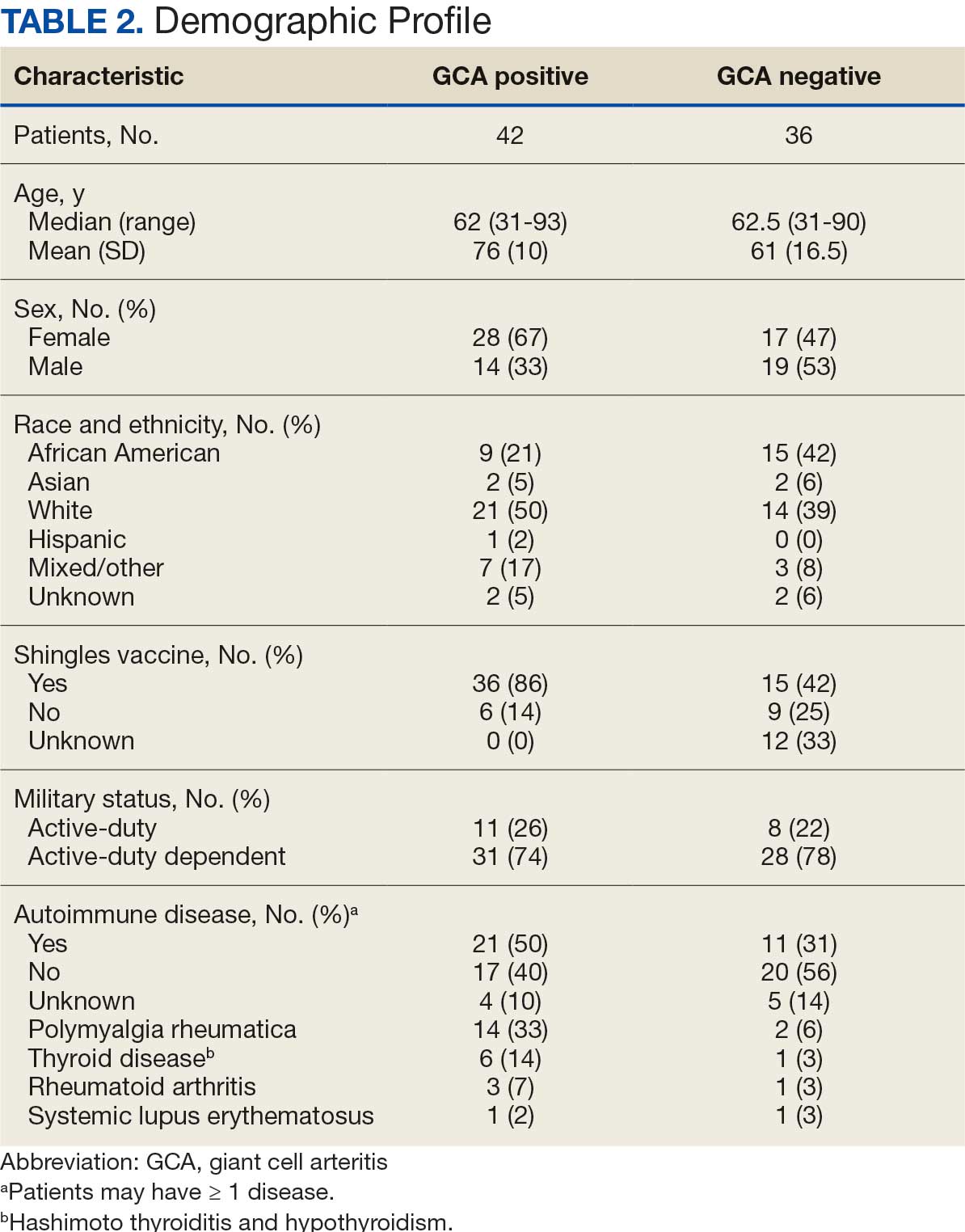
At the high complexity Central Texas Veterans Health Care System (CTVHCS), the fiscal year (FY) 2023 All Employee Survey (AES) found that staff members require additional education and awareness regarding the reporting of patient safety concerns.4 The survey highlighted areas such as lack of education on reporting, doubts about the effectiveness of reporting, confusion about the process after a report is made, and insufficient feedback.
BACKGROUND
To improve the culture of safety and address deficiencies noted in the AES, the CTVHCS patient safety (PS) and high reliability organization (HRO) teams partnered to develop a quality improvement initiative to increase staff understanding of safety event reporting and strengthen the safety culture. The PS and HRO teams developed an innovative education model that integrates Joint Patient Safety Reporting System (JPSR) education into huddles.
This initiative, called the JPSR Huddle Card Toolkit, sought to assess the impact of the toolkit on staff knowledge and behaviors related to patient safety event reporting. The toolkit consisted of educational materials encompassing 6 key areas: (1) reporting incidents; (2) close calls and near misses; (3) identification of root causes; (4) understanding the life cycle of a JPSR; (5) celebrating achievements; and (6) distinguishing between facts and fiction. Each JPSR huddle card included discussion points for the facilitator and was formatted on a 5 × 7-inch card (Figure 1). Topics were addressed during weekly safety huddles conducted in the pilot unit over a 6-week period. To evaluate its effectiveness, a pilot unit was selected and distributed an anonymous questionnaire paired with the JPSR huddle card toolkit to measure staff responses.

The pilot was conducted from November 2023 to January 2024. The participating pilot unit was a 10-bed critical care unit with 42 full-time employees. Nursing leadership, quality safety, and value personnel, and the Veterans Integrated Services Network (VISN) PS Team reviewed and approved the pilot.
Reporting of adverse events and near misses provides an opportunity to learn about latent systems errors.2 In 2018, the VHA began using the JPSR to standardize the capture and data management on medical errors and close calls across the Defense Health Administration (DHA) and VHA.1 The JPSR software is a joint application of the VHA and DHA. It improves the identification and documentation of patient safety-related events for VA medical centers, military hospitals and clinics, active-duty personnel, veterans and their families.
Event reporting is a key element in advancing high reliability and achieving zero preventable harm.1 Teams use these data to identify organizational patient safety trends and preempt common safety issues. All data are protected under 38 USC §5705 and 10 USC §1102.5 The JPSR single-source system standardizes the collection of core data points and increases collaboration between the DHA and VHA. This partnership increases insight into safety-related incidents, allowing for earlier detection and prevention of patient harm or injury incidents.
Numerous studies consistently commend huddles for their effectiveness in promoting teamwork and their positive impact on patient safety.6-8 Huddles facilitate connections between employees who may not typically interact, provide opportunities for discussions, and serve as a platform to encourage employees to voice their opinions. By fostering these interactions, huddles empower employees and create an environment for shared understanding, building trust, and promoting continuous learning.8
OBSERVATIONS
The JPSR huddle card initiative aimed to improve understanding of the JPSR process and promote knowledge and attitudes about patient safety and event reporting, while emphasizing shared responsibility. The goals focused on effective communication, respect for expertise, awareness of operational nuances, voicing concerns, and ensuring zero harm.
The facilitator initiated huddles by announcing their start to cultivate a constructive outcome.8 The JPSR huddle cards used a structured format designed to foster engagement and understanding of the topic. Each card begins with a factual statement or an open-ended question to gauge participants’ awareness or understanding. It then provides essential facts, principles, and relevant information to deepen knowledge. The card concludes with a discussion question, allowing facilitators to assess shared learning and encourage group reflection. This format promotes active participation and ensures that key concepts are both introduced and reinforced through dialogue.
The PS team standardized the format for all huddle cards, allowing 5 to 10 minutes for discussing training materials, receiving feedback, and concluding with a discussion question and call to action. Prior to each huddle, the facilitator would read a scripted remark that reviewed the objectives and ground rules for an effective huddle.
The PS and HRO teams promoted interactive discussions and welcomed ongoing feedback. Huddles provided a psychologically safe environment where individuals were encouraged to voice their thoughts and ideas.
Each weekly huddle card addressed a different patient safety topic. The Week 1 huddle card focuses on event reporting for safety improvement. The card outlines the purpose of JPSR as a tool to identify, manage, and analyze safety events to reduce preventable harm. The card emphasizes 3 core principles: (1) acknowledging mistakes, recognizing that errors happen; (2) no blame, no shame (encouraging a no-blame just culture to raise concerns); and (3) continuous improvement (committing to ongoing learning and prevention). It provides guidance on event details entry, advising staff to include facts in an SBAR (Situation, Background, Assessment, Response) format, avoid assumptions, and exclude personal identifiers. Tips include entering only relevant facts to help reviewers understand the incident. The card ends with discussion questions on reporting barriers and potential improvements in event reporting practices.
The Week 2 huddle card focuses on understanding and reporting near miss events, also known as close calls or good catches. A near miss is an incident where a potential hazard was identified and prevented before it reached the patient, avoiding harm due to timely intervention. The card emphasizes the importance of identifying these events to understand weaknesses and proactively reduce risks. Examples of near misses include discovering expired medication before use, catching a potential wrong-site surgery, and noticing incorrect medication dosages. Staff are encouraged to develop a mindset for anticipating and solving risks. The card ends with a discussion asking participants to share examples of near misses in their area.
The Week 3 huddle card covers root causes in preventing errors. The card highlights that errors in health care often stem from flawed processes rather than individual faults. By identifying root causes, systemic weaknesses can be addressed to reduce mistakes and build more error-tolerant and robust systems. All staff are advised to adopt a mindset of continuous improvement, error trapping behaviors and problem-solving. It concludes with discussion questions prompting reflection on assumptions and identifying weaknesses when something goes wrong.
The Week 4 huddle card covers the life of a JPSR, detailing that after entry JPSR events are viewed by the highest leadership levels at the morning report, and that lessons learned are distributed through frontline managers and chiefs in a monthly report to be shared with frontline staff. Additionally, JPSR trends are shared during monthly HRO safety forums. These practices promote a culture of safety through open communication and problem-solving. Staff and leaders are encouraged to prioritize safety daily. Discussion prompts ask team members if they had seen positive changes from JPSR reporting and what they would like leadership to communicate after investigations.
The Week 5 huddle card covers celebrating safety event reporting called Cue the Confetti. The VHA emphasizes recognizing staff who report safety events as part of their commitment to zero harm. By celebrating these contributions, the VHA fosters respect, joy, and satisfaction in the work. Staff are encouraged to nominate colleagues for recognition, reinforcing a supportive environment. Prompts invite teams to discuss how they celebrate JPSR reporting and how they’d like to enhance this culture of appreciation.
The Week 6 huddle card covers common misconceptions about JPSR. Key facts include that JPSRs are confidential, not for disciplinary action, and can be submitted by any staff member at any time. Only PS can view reporter identities for clarification purposes. The card concludes with prompts to ensure staff know how to access JPSR support and resources.
Measuring the impact on staff was essential to assess effectiveness and gather data for program improvement. To evaluate the impact of the huddle cards on the staff, the team provided a voluntary and anonymous 9 question survey (Figure 2). The survey was completed before the pilot began and again at the end of Week 6.
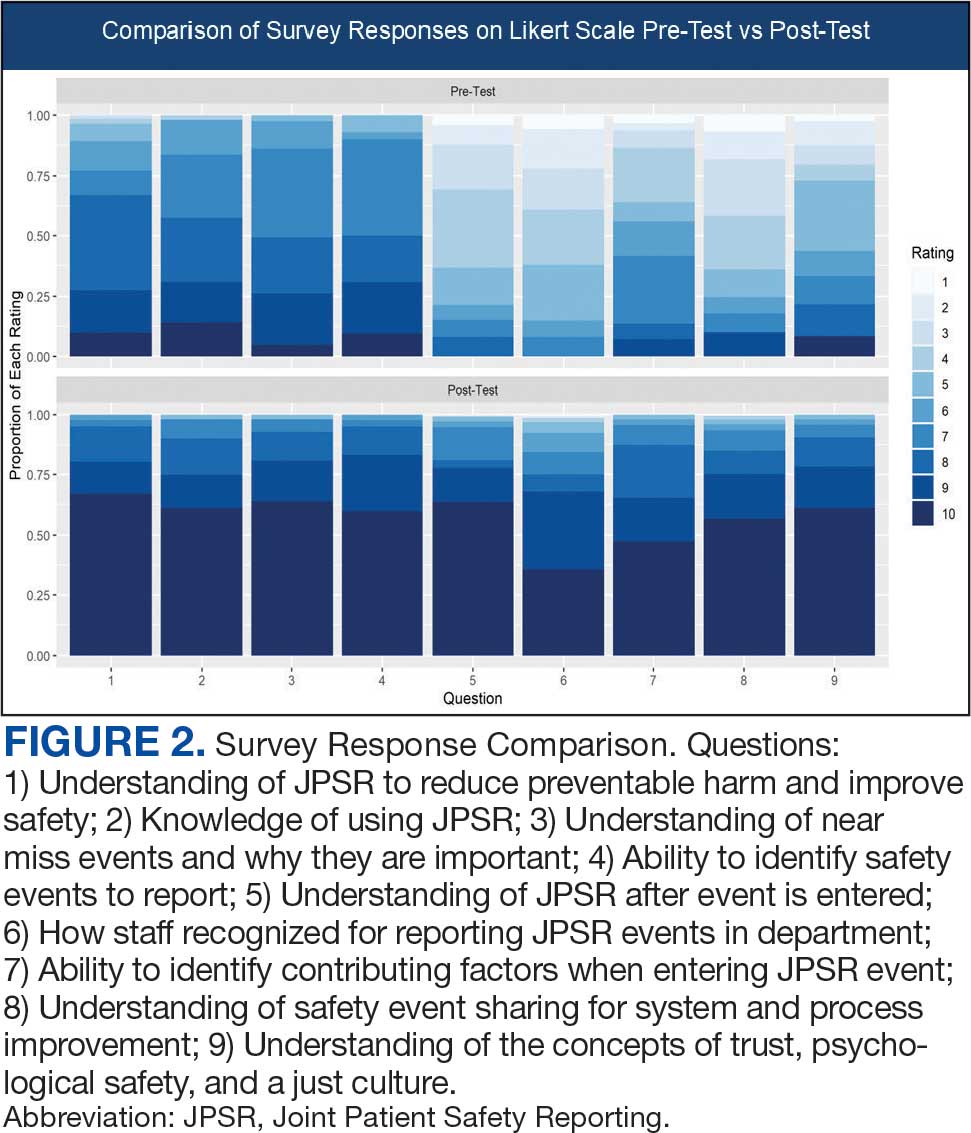
Questions 1 through 5 and 7 through 9 pertained to participants’ perceived knowledge and understanding of aspects of the JPSR. Perceived improvement among intensive care unit (ICU) participants ranged from 15% to 53%. There was a positive increase associated with every question with the top improvements: question 8, “How do you rate your understanding of how we share safety events for system and process improvement?” (53.4% increase); question 5, “How do you rate your understanding of what happens to a JPSR after it is entered?” (51.9% increase), and question 9, “How do you rate your understanding of the concepts of trust, psychological safety and a just culture?” (47.8% increase).
The survey analysis was not able to track individual changes. As a result, the findings reflect an overall change for the entire study group. Moreover, the questions assessed participants’ perceived knowledge rather than actual knowledge gained. It is important to note that there may be a significant gap between the actual knowledge gained and how participants perceive it. Additionally, improvement in knowledge and comprehension does not necessarily translate into behavior changes.
CONCLUSIONS
The use of JPSR huddle cards and direct engagement with staff during safety huddles yielded positive outcomes. On average, participants demonstrated higher scores in posttest questions compared to pretest questions. The posttest scores were consistently higher than the pretest scores, showing an average increase of around 2 standard deviations across all questions. This indicates an improvement in participants’ perceived knowledge and comprehension of the JPSR material.
During the pilot implementation of the huddle cards, there was a notable improvement in team member engagement. The structured format of the cards facilitated focused and meaningful discussions during safety huddles, encouraging open dialogue and fostering a culture of safety. Team members actively participated in identifying potential risks, sharing observations, and proposing actionable solutions, which reflected an enhanced sense of ownership regarding safety practices.
The support dialogue facilitated by the huddle cards highlighted the significance of mutual accountability and a collective commitment to achieving zero harm. This collaborative environment strengthened trust among team members and underscored the importance of shared vigilance in preventing adverse events. The pilot demonstrated the potential of huddle cards as an essential tool for enhancing team-based safety initiatives and promoting a culture of high reliability within the organization.
The total number of JPSR events in the ICU rose from 156 in FY 23 to 170 in FY 24. Adverse events increased from 19 to 31, while close calls saw a slight uptick from 137 to 139. Despite the overall rise in adverse events, a detailed analysis indicated that incidents of moderate harm decreased from 4 in FY 23 to 2 in FY 24. Furthermore, there was 1 reported case of death or severe harm in FY 23, which decreased to 0 in FY 24. This trend is consistent with the overarching objective of a high-reliability organization to achieve zero harm.
The next step is to expand this initiative across CTVHCS. This initiative aims to make this an annual education for all areas. The JPSR huddle card toolkit will be formatted by the media department for easy printing and retrieval. Leaders within units, clinics, and services will be empowered to facilitate the sessions in their safety huddles and reap the same outcomes as in the pilot. CTVHCS PS will monitor the effectiveness of this through ongoing CTVHCS patient safety rounding and future AES.
- Essen K, Villalobos C, Sculli GL, Steinbach L. Establishing a just culture: implications for the Veterans Health Administration journey to high reliability. Fed Pract. 2024;41:290-297. doi:10.12788/fp.0512
- Louis MY, Hussain LR, Dhanraj DN, et al. Improving patient safety event reporting among residents and teaching faculty. Ochsner J. 2016;16:73-80.
- Pimental CB, Snow AL, Carnes SL, et al. Huddles and their effectiveness at the frontlines of clinical care: a scoping review. J Gen Intern Med. 2021;36:2772-2783. doi:10.1007/s11606-021-06632-9
- National Academies of Sciences, Engineering, and Medicine. Appendix C: Nature of Veterans Health Administration Facilities Management (Engineering) Tasks and Staffing. Facilities Staffing Requirements for the Veterans Health Administration-Resource Planning and Methodology for the Future. National Academies Press. 2020:105-116. Accessed August 11, 2025. https://nap.nationalacademies.org/read/25454/chapter/11
- Woodier N, Burnett C, Moppett I. The value of learning from near misses to improve patient safety: a scoping review. J Patient Saf. 2023;19:42-47. doi:10.1097/pts.0000000000001078
- Ismail A, Khalid SNM. Patient safety culture and its determinants among healthcare professionals at a cluster hospital in Malaysia: a cross-sectional study. BMJ Open. 2022;12:e060546. doi:10.1136/bmjopen-2021-060546
- Ngo J, Lau D, Ploquin J, Receveur T, Stassen K, Del Castilho C. Improving incident reporting among physicians at south health campus hospital. BMJ Open Qual. 2022;11:e001945. doi:10.1136/bmjoq-2022-001945
- Oweidat I, Al-Mugheed K, Alsenany SA, et al. Awareness of reporting practices and barriers to incident reporting among nurses. BMC Nurs. 2023;22:231. doi:10.1186/s12912-023-01376-9
Safety event reporting plays a vital role in fostering a culture of safety within a health care organization. The US Department of Veterans Affairs (VA) has shifted its focus from eradicating medical errors to minimizing or eliminating harm to patients.1 The National Center for Patient Safety’s objective is to prevent recurring errors by identifying and addressing systemic problems that may have been overlooked.2
Taking inspiration from industries known for high reliability, such as aviation and nuclear power, the Veterans Health Administration (VHA) patient safety program aims to identify and eliminate system vulnerabilities, such as medical errors. Learning from near misses, which occur more frequently than actual adverse events, is a crucial part of this process.3 By addressing these issues, the VHA can establish safer systems and encourage continuous identification of potential problems with proactive resolution.
All staff should participate actively in event reporting, which involves documenting and communicating details, outcomes, and relevant data about an event to understand what occurred, evaluate success, identify areas for improvement, and inform future decisions. This helps identify system weaknesses, create opportunities to standardize procedures and enhance patient care.
At the high complexity Central Texas Veterans Health Care System (CTVHCS), the fiscal year (FY) 2023 All Employee Survey (AES) found that staff members require additional education and awareness regarding the reporting of patient safety concerns.4 The survey highlighted areas such as lack of education on reporting, doubts about the effectiveness of reporting, confusion about the process after a report is made, and insufficient feedback.
BACKGROUND
To improve the culture of safety and address deficiencies noted in the AES, the CTVHCS patient safety (PS) and high reliability organization (HRO) teams partnered to develop a quality improvement initiative to increase staff understanding of safety event reporting and strengthen the safety culture. The PS and HRO teams developed an innovative education model that integrates Joint Patient Safety Reporting System (JPSR) education into huddles.
This initiative, called the JPSR Huddle Card Toolkit, sought to assess the impact of the toolkit on staff knowledge and behaviors related to patient safety event reporting. The toolkit consisted of educational materials encompassing 6 key areas: (1) reporting incidents; (2) close calls and near misses; (3) identification of root causes; (4) understanding the life cycle of a JPSR; (5) celebrating achievements; and (6) distinguishing between facts and fiction. Each JPSR huddle card included discussion points for the facilitator and was formatted on a 5 × 7-inch card (Figure 1). Topics were addressed during weekly safety huddles conducted in the pilot unit over a 6-week period. To evaluate its effectiveness, a pilot unit was selected and distributed an anonymous questionnaire paired with the JPSR huddle card toolkit to measure staff responses.

The pilot was conducted from November 2023 to January 2024. The participating pilot unit was a 10-bed critical care unit with 42 full-time employees. Nursing leadership, quality safety, and value personnel, and the Veterans Integrated Services Network (VISN) PS Team reviewed and approved the pilot.
Reporting of adverse events and near misses provides an opportunity to learn about latent systems errors.2 In 2018, the VHA began using the JPSR to standardize the capture and data management on medical errors and close calls across the Defense Health Administration (DHA) and VHA.1 The JPSR software is a joint application of the VHA and DHA. It improves the identification and documentation of patient safety-related events for VA medical centers, military hospitals and clinics, active-duty personnel, veterans and their families.
Event reporting is a key element in advancing high reliability and achieving zero preventable harm.1 Teams use these data to identify organizational patient safety trends and preempt common safety issues. All data are protected under 38 USC §5705 and 10 USC §1102.5 The JPSR single-source system standardizes the collection of core data points and increases collaboration between the DHA and VHA. This partnership increases insight into safety-related incidents, allowing for earlier detection and prevention of patient harm or injury incidents.
Numerous studies consistently commend huddles for their effectiveness in promoting teamwork and their positive impact on patient safety.6-8 Huddles facilitate connections between employees who may not typically interact, provide opportunities for discussions, and serve as a platform to encourage employees to voice their opinions. By fostering these interactions, huddles empower employees and create an environment for shared understanding, building trust, and promoting continuous learning.8
OBSERVATIONS
The JPSR huddle card initiative aimed to improve understanding of the JPSR process and promote knowledge and attitudes about patient safety and event reporting, while emphasizing shared responsibility. The goals focused on effective communication, respect for expertise, awareness of operational nuances, voicing concerns, and ensuring zero harm.
The facilitator initiated huddles by announcing their start to cultivate a constructive outcome.8 The JPSR huddle cards used a structured format designed to foster engagement and understanding of the topic. Each card begins with a factual statement or an open-ended question to gauge participants’ awareness or understanding. It then provides essential facts, principles, and relevant information to deepen knowledge. The card concludes with a discussion question, allowing facilitators to assess shared learning and encourage group reflection. This format promotes active participation and ensures that key concepts are both introduced and reinforced through dialogue.
The PS team standardized the format for all huddle cards, allowing 5 to 10 minutes for discussing training materials, receiving feedback, and concluding with a discussion question and call to action. Prior to each huddle, the facilitator would read a scripted remark that reviewed the objectives and ground rules for an effective huddle.
The PS and HRO teams promoted interactive discussions and welcomed ongoing feedback. Huddles provided a psychologically safe environment where individuals were encouraged to voice their thoughts and ideas.
Each weekly huddle card addressed a different patient safety topic. The Week 1 huddle card focuses on event reporting for safety improvement. The card outlines the purpose of JPSR as a tool to identify, manage, and analyze safety events to reduce preventable harm. The card emphasizes 3 core principles: (1) acknowledging mistakes, recognizing that errors happen; (2) no blame, no shame (encouraging a no-blame just culture to raise concerns); and (3) continuous improvement (committing to ongoing learning and prevention). It provides guidance on event details entry, advising staff to include facts in an SBAR (Situation, Background, Assessment, Response) format, avoid assumptions, and exclude personal identifiers. Tips include entering only relevant facts to help reviewers understand the incident. The card ends with discussion questions on reporting barriers and potential improvements in event reporting practices.
The Week 2 huddle card focuses on understanding and reporting near miss events, also known as close calls or good catches. A near miss is an incident where a potential hazard was identified and prevented before it reached the patient, avoiding harm due to timely intervention. The card emphasizes the importance of identifying these events to understand weaknesses and proactively reduce risks. Examples of near misses include discovering expired medication before use, catching a potential wrong-site surgery, and noticing incorrect medication dosages. Staff are encouraged to develop a mindset for anticipating and solving risks. The card ends with a discussion asking participants to share examples of near misses in their area.
The Week 3 huddle card covers root causes in preventing errors. The card highlights that errors in health care often stem from flawed processes rather than individual faults. By identifying root causes, systemic weaknesses can be addressed to reduce mistakes and build more error-tolerant and robust systems. All staff are advised to adopt a mindset of continuous improvement, error trapping behaviors and problem-solving. It concludes with discussion questions prompting reflection on assumptions and identifying weaknesses when something goes wrong.
The Week 4 huddle card covers the life of a JPSR, detailing that after entry JPSR events are viewed by the highest leadership levels at the morning report, and that lessons learned are distributed through frontline managers and chiefs in a monthly report to be shared with frontline staff. Additionally, JPSR trends are shared during monthly HRO safety forums. These practices promote a culture of safety through open communication and problem-solving. Staff and leaders are encouraged to prioritize safety daily. Discussion prompts ask team members if they had seen positive changes from JPSR reporting and what they would like leadership to communicate after investigations.
The Week 5 huddle card covers celebrating safety event reporting called Cue the Confetti. The VHA emphasizes recognizing staff who report safety events as part of their commitment to zero harm. By celebrating these contributions, the VHA fosters respect, joy, and satisfaction in the work. Staff are encouraged to nominate colleagues for recognition, reinforcing a supportive environment. Prompts invite teams to discuss how they celebrate JPSR reporting and how they’d like to enhance this culture of appreciation.
The Week 6 huddle card covers common misconceptions about JPSR. Key facts include that JPSRs are confidential, not for disciplinary action, and can be submitted by any staff member at any time. Only PS can view reporter identities for clarification purposes. The card concludes with prompts to ensure staff know how to access JPSR support and resources.
Measuring the impact on staff was essential to assess effectiveness and gather data for program improvement. To evaluate the impact of the huddle cards on the staff, the team provided a voluntary and anonymous 9 question survey (Figure 2). The survey was completed before the pilot began and again at the end of Week 6.

Questions 1 through 5 and 7 through 9 pertained to participants’ perceived knowledge and understanding of aspects of the JPSR. Perceived improvement among intensive care unit (ICU) participants ranged from 15% to 53%. There was a positive increase associated with every question with the top improvements: question 8, “How do you rate your understanding of how we share safety events for system and process improvement?” (53.4% increase); question 5, “How do you rate your understanding of what happens to a JPSR after it is entered?” (51.9% increase), and question 9, “How do you rate your understanding of the concepts of trust, psychological safety and a just culture?” (47.8% increase).
The survey analysis was not able to track individual changes. As a result, the findings reflect an overall change for the entire study group. Moreover, the questions assessed participants’ perceived knowledge rather than actual knowledge gained. It is important to note that there may be a significant gap between the actual knowledge gained and how participants perceive it. Additionally, improvement in knowledge and comprehension does not necessarily translate into behavior changes.
CONCLUSIONS
The use of JPSR huddle cards and direct engagement with staff during safety huddles yielded positive outcomes. On average, participants demonstrated higher scores in posttest questions compared to pretest questions. The posttest scores were consistently higher than the pretest scores, showing an average increase of around 2 standard deviations across all questions. This indicates an improvement in participants’ perceived knowledge and comprehension of the JPSR material.
During the pilot implementation of the huddle cards, there was a notable improvement in team member engagement. The structured format of the cards facilitated focused and meaningful discussions during safety huddles, encouraging open dialogue and fostering a culture of safety. Team members actively participated in identifying potential risks, sharing observations, and proposing actionable solutions, which reflected an enhanced sense of ownership regarding safety practices.
The support dialogue facilitated by the huddle cards highlighted the significance of mutual accountability and a collective commitment to achieving zero harm. This collaborative environment strengthened trust among team members and underscored the importance of shared vigilance in preventing adverse events. The pilot demonstrated the potential of huddle cards as an essential tool for enhancing team-based safety initiatives and promoting a culture of high reliability within the organization.
The total number of JPSR events in the ICU rose from 156 in FY 23 to 170 in FY 24. Adverse events increased from 19 to 31, while close calls saw a slight uptick from 137 to 139. Despite the overall rise in adverse events, a detailed analysis indicated that incidents of moderate harm decreased from 4 in FY 23 to 2 in FY 24. Furthermore, there was 1 reported case of death or severe harm in FY 23, which decreased to 0 in FY 24. This trend is consistent with the overarching objective of a high-reliability organization to achieve zero harm.
The next step is to expand this initiative across CTVHCS. This initiative aims to make this an annual education for all areas. The JPSR huddle card toolkit will be formatted by the media department for easy printing and retrieval. Leaders within units, clinics, and services will be empowered to facilitate the sessions in their safety huddles and reap the same outcomes as in the pilot. CTVHCS PS will monitor the effectiveness of this through ongoing CTVHCS patient safety rounding and future AES.
Safety event reporting plays a vital role in fostering a culture of safety within a health care organization. The US Department of Veterans Affairs (VA) has shifted its focus from eradicating medical errors to minimizing or eliminating harm to patients.1 The National Center for Patient Safety’s objective is to prevent recurring errors by identifying and addressing systemic problems that may have been overlooked.2
Taking inspiration from industries known for high reliability, such as aviation and nuclear power, the Veterans Health Administration (VHA) patient safety program aims to identify and eliminate system vulnerabilities, such as medical errors. Learning from near misses, which occur more frequently than actual adverse events, is a crucial part of this process.3 By addressing these issues, the VHA can establish safer systems and encourage continuous identification of potential problems with proactive resolution.
All staff should participate actively in event reporting, which involves documenting and communicating details, outcomes, and relevant data about an event to understand what occurred, evaluate success, identify areas for improvement, and inform future decisions. This helps identify system weaknesses, create opportunities to standardize procedures and enhance patient care.
At the high complexity Central Texas Veterans Health Care System (CTVHCS), the fiscal year (FY) 2023 All Employee Survey (AES) found that staff members require additional education and awareness regarding the reporting of patient safety concerns.4 The survey highlighted areas such as lack of education on reporting, doubts about the effectiveness of reporting, confusion about the process after a report is made, and insufficient feedback.
BACKGROUND
To improve the culture of safety and address deficiencies noted in the AES, the CTVHCS patient safety (PS) and high reliability organization (HRO) teams partnered to develop a quality improvement initiative to increase staff understanding of safety event reporting and strengthen the safety culture. The PS and HRO teams developed an innovative education model that integrates Joint Patient Safety Reporting System (JPSR) education into huddles.
This initiative, called the JPSR Huddle Card Toolkit, sought to assess the impact of the toolkit on staff knowledge and behaviors related to patient safety event reporting. The toolkit consisted of educational materials encompassing 6 key areas: (1) reporting incidents; (2) close calls and near misses; (3) identification of root causes; (4) understanding the life cycle of a JPSR; (5) celebrating achievements; and (6) distinguishing between facts and fiction. Each JPSR huddle card included discussion points for the facilitator and was formatted on a 5 × 7-inch card (Figure 1). Topics were addressed during weekly safety huddles conducted in the pilot unit over a 6-week period. To evaluate its effectiveness, a pilot unit was selected and distributed an anonymous questionnaire paired with the JPSR huddle card toolkit to measure staff responses.

The pilot was conducted from November 2023 to January 2024. The participating pilot unit was a 10-bed critical care unit with 42 full-time employees. Nursing leadership, quality safety, and value personnel, and the Veterans Integrated Services Network (VISN) PS Team reviewed and approved the pilot.
Reporting of adverse events and near misses provides an opportunity to learn about latent systems errors.2 In 2018, the VHA began using the JPSR to standardize the capture and data management on medical errors and close calls across the Defense Health Administration (DHA) and VHA.1 The JPSR software is a joint application of the VHA and DHA. It improves the identification and documentation of patient safety-related events for VA medical centers, military hospitals and clinics, active-duty personnel, veterans and their families.
Event reporting is a key element in advancing high reliability and achieving zero preventable harm.1 Teams use these data to identify organizational patient safety trends and preempt common safety issues. All data are protected under 38 USC §5705 and 10 USC §1102.5 The JPSR single-source system standardizes the collection of core data points and increases collaboration between the DHA and VHA. This partnership increases insight into safety-related incidents, allowing for earlier detection and prevention of patient harm or injury incidents.
Numerous studies consistently commend huddles for their effectiveness in promoting teamwork and their positive impact on patient safety.6-8 Huddles facilitate connections between employees who may not typically interact, provide opportunities for discussions, and serve as a platform to encourage employees to voice their opinions. By fostering these interactions, huddles empower employees and create an environment for shared understanding, building trust, and promoting continuous learning.8
OBSERVATIONS
The JPSR huddle card initiative aimed to improve understanding of the JPSR process and promote knowledge and attitudes about patient safety and event reporting, while emphasizing shared responsibility. The goals focused on effective communication, respect for expertise, awareness of operational nuances, voicing concerns, and ensuring zero harm.
The facilitator initiated huddles by announcing their start to cultivate a constructive outcome.8 The JPSR huddle cards used a structured format designed to foster engagement and understanding of the topic. Each card begins with a factual statement or an open-ended question to gauge participants’ awareness or understanding. It then provides essential facts, principles, and relevant information to deepen knowledge. The card concludes with a discussion question, allowing facilitators to assess shared learning and encourage group reflection. This format promotes active participation and ensures that key concepts are both introduced and reinforced through dialogue.
The PS team standardized the format for all huddle cards, allowing 5 to 10 minutes for discussing training materials, receiving feedback, and concluding with a discussion question and call to action. Prior to each huddle, the facilitator would read a scripted remark that reviewed the objectives and ground rules for an effective huddle.
The PS and HRO teams promoted interactive discussions and welcomed ongoing feedback. Huddles provided a psychologically safe environment where individuals were encouraged to voice their thoughts and ideas.
Each weekly huddle card addressed a different patient safety topic. The Week 1 huddle card focuses on event reporting for safety improvement. The card outlines the purpose of JPSR as a tool to identify, manage, and analyze safety events to reduce preventable harm. The card emphasizes 3 core principles: (1) acknowledging mistakes, recognizing that errors happen; (2) no blame, no shame (encouraging a no-blame just culture to raise concerns); and (3) continuous improvement (committing to ongoing learning and prevention). It provides guidance on event details entry, advising staff to include facts in an SBAR (Situation, Background, Assessment, Response) format, avoid assumptions, and exclude personal identifiers. Tips include entering only relevant facts to help reviewers understand the incident. The card ends with discussion questions on reporting barriers and potential improvements in event reporting practices.
The Week 2 huddle card focuses on understanding and reporting near miss events, also known as close calls or good catches. A near miss is an incident where a potential hazard was identified and prevented before it reached the patient, avoiding harm due to timely intervention. The card emphasizes the importance of identifying these events to understand weaknesses and proactively reduce risks. Examples of near misses include discovering expired medication before use, catching a potential wrong-site surgery, and noticing incorrect medication dosages. Staff are encouraged to develop a mindset for anticipating and solving risks. The card ends with a discussion asking participants to share examples of near misses in their area.
The Week 3 huddle card covers root causes in preventing errors. The card highlights that errors in health care often stem from flawed processes rather than individual faults. By identifying root causes, systemic weaknesses can be addressed to reduce mistakes and build more error-tolerant and robust systems. All staff are advised to adopt a mindset of continuous improvement, error trapping behaviors and problem-solving. It concludes with discussion questions prompting reflection on assumptions and identifying weaknesses when something goes wrong.
The Week 4 huddle card covers the life of a JPSR, detailing that after entry JPSR events are viewed by the highest leadership levels at the morning report, and that lessons learned are distributed through frontline managers and chiefs in a monthly report to be shared with frontline staff. Additionally, JPSR trends are shared during monthly HRO safety forums. These practices promote a culture of safety through open communication and problem-solving. Staff and leaders are encouraged to prioritize safety daily. Discussion prompts ask team members if they had seen positive changes from JPSR reporting and what they would like leadership to communicate after investigations.
The Week 5 huddle card covers celebrating safety event reporting called Cue the Confetti. The VHA emphasizes recognizing staff who report safety events as part of their commitment to zero harm. By celebrating these contributions, the VHA fosters respect, joy, and satisfaction in the work. Staff are encouraged to nominate colleagues for recognition, reinforcing a supportive environment. Prompts invite teams to discuss how they celebrate JPSR reporting and how they’d like to enhance this culture of appreciation.
The Week 6 huddle card covers common misconceptions about JPSR. Key facts include that JPSRs are confidential, not for disciplinary action, and can be submitted by any staff member at any time. Only PS can view reporter identities for clarification purposes. The card concludes with prompts to ensure staff know how to access JPSR support and resources.
Measuring the impact on staff was essential to assess effectiveness and gather data for program improvement. To evaluate the impact of the huddle cards on the staff, the team provided a voluntary and anonymous 9 question survey (Figure 2). The survey was completed before the pilot began and again at the end of Week 6.

Questions 1 through 5 and 7 through 9 pertained to participants’ perceived knowledge and understanding of aspects of the JPSR. Perceived improvement among intensive care unit (ICU) participants ranged from 15% to 53%. There was a positive increase associated with every question with the top improvements: question 8, “How do you rate your understanding of how we share safety events for system and process improvement?” (53.4% increase); question 5, “How do you rate your understanding of what happens to a JPSR after it is entered?” (51.9% increase), and question 9, “How do you rate your understanding of the concepts of trust, psychological safety and a just culture?” (47.8% increase).
The survey analysis was not able to track individual changes. As a result, the findings reflect an overall change for the entire study group. Moreover, the questions assessed participants’ perceived knowledge rather than actual knowledge gained. It is important to note that there may be a significant gap between the actual knowledge gained and how participants perceive it. Additionally, improvement in knowledge and comprehension does not necessarily translate into behavior changes.
CONCLUSIONS
The use of JPSR huddle cards and direct engagement with staff during safety huddles yielded positive outcomes. On average, participants demonstrated higher scores in posttest questions compared to pretest questions. The posttest scores were consistently higher than the pretest scores, showing an average increase of around 2 standard deviations across all questions. This indicates an improvement in participants’ perceived knowledge and comprehension of the JPSR material.
During the pilot implementation of the huddle cards, there was a notable improvement in team member engagement. The structured format of the cards facilitated focused and meaningful discussions during safety huddles, encouraging open dialogue and fostering a culture of safety. Team members actively participated in identifying potential risks, sharing observations, and proposing actionable solutions, which reflected an enhanced sense of ownership regarding safety practices.
The support dialogue facilitated by the huddle cards highlighted the significance of mutual accountability and a collective commitment to achieving zero harm. This collaborative environment strengthened trust among team members and underscored the importance of shared vigilance in preventing adverse events. The pilot demonstrated the potential of huddle cards as an essential tool for enhancing team-based safety initiatives and promoting a culture of high reliability within the organization.
The total number of JPSR events in the ICU rose from 156 in FY 23 to 170 in FY 24. Adverse events increased from 19 to 31, while close calls saw a slight uptick from 137 to 139. Despite the overall rise in adverse events, a detailed analysis indicated that incidents of moderate harm decreased from 4 in FY 23 to 2 in FY 24. Furthermore, there was 1 reported case of death or severe harm in FY 23, which decreased to 0 in FY 24. This trend is consistent with the overarching objective of a high-reliability organization to achieve zero harm.
The next step is to expand this initiative across CTVHCS. This initiative aims to make this an annual education for all areas. The JPSR huddle card toolkit will be formatted by the media department for easy printing and retrieval. Leaders within units, clinics, and services will be empowered to facilitate the sessions in their safety huddles and reap the same outcomes as in the pilot. CTVHCS PS will monitor the effectiveness of this through ongoing CTVHCS patient safety rounding and future AES.
- Essen K, Villalobos C, Sculli GL, Steinbach L. Establishing a just culture: implications for the Veterans Health Administration journey to high reliability. Fed Pract. 2024;41:290-297. doi:10.12788/fp.0512
- Louis MY, Hussain LR, Dhanraj DN, et al. Improving patient safety event reporting among residents and teaching faculty. Ochsner J. 2016;16:73-80.
- Pimental CB, Snow AL, Carnes SL, et al. Huddles and their effectiveness at the frontlines of clinical care: a scoping review. J Gen Intern Med. 2021;36:2772-2783. doi:10.1007/s11606-021-06632-9
- National Academies of Sciences, Engineering, and Medicine. Appendix C: Nature of Veterans Health Administration Facilities Management (Engineering) Tasks and Staffing. Facilities Staffing Requirements for the Veterans Health Administration-Resource Planning and Methodology for the Future. National Academies Press. 2020:105-116. Accessed August 11, 2025. https://nap.nationalacademies.org/read/25454/chapter/11
- Woodier N, Burnett C, Moppett I. The value of learning from near misses to improve patient safety: a scoping review. J Patient Saf. 2023;19:42-47. doi:10.1097/pts.0000000000001078
- Ismail A, Khalid SNM. Patient safety culture and its determinants among healthcare professionals at a cluster hospital in Malaysia: a cross-sectional study. BMJ Open. 2022;12:e060546. doi:10.1136/bmjopen-2021-060546
- Ngo J, Lau D, Ploquin J, Receveur T, Stassen K, Del Castilho C. Improving incident reporting among physicians at south health campus hospital. BMJ Open Qual. 2022;11:e001945. doi:10.1136/bmjoq-2022-001945
- Oweidat I, Al-Mugheed K, Alsenany SA, et al. Awareness of reporting practices and barriers to incident reporting among nurses. BMC Nurs. 2023;22:231. doi:10.1186/s12912-023-01376-9
- Essen K, Villalobos C, Sculli GL, Steinbach L. Establishing a just culture: implications for the Veterans Health Administration journey to high reliability. Fed Pract. 2024;41:290-297. doi:10.12788/fp.0512
- Louis MY, Hussain LR, Dhanraj DN, et al. Improving patient safety event reporting among residents and teaching faculty. Ochsner J. 2016;16:73-80.
- Pimental CB, Snow AL, Carnes SL, et al. Huddles and their effectiveness at the frontlines of clinical care: a scoping review. J Gen Intern Med. 2021;36:2772-2783. doi:10.1007/s11606-021-06632-9
- National Academies of Sciences, Engineering, and Medicine. Appendix C: Nature of Veterans Health Administration Facilities Management (Engineering) Tasks and Staffing. Facilities Staffing Requirements for the Veterans Health Administration-Resource Planning and Methodology for the Future. National Academies Press. 2020:105-116. Accessed August 11, 2025. https://nap.nationalacademies.org/read/25454/chapter/11
- Woodier N, Burnett C, Moppett I. The value of learning from near misses to improve patient safety: a scoping review. J Patient Saf. 2023;19:42-47. doi:10.1097/pts.0000000000001078
- Ismail A, Khalid SNM. Patient safety culture and its determinants among healthcare professionals at a cluster hospital in Malaysia: a cross-sectional study. BMJ Open. 2022;12:e060546. doi:10.1136/bmjopen-2021-060546
- Ngo J, Lau D, Ploquin J, Receveur T, Stassen K, Del Castilho C. Improving incident reporting among physicians at south health campus hospital. BMJ Open Qual. 2022;11:e001945. doi:10.1136/bmjoq-2022-001945
- Oweidat I, Al-Mugheed K, Alsenany SA, et al. Awareness of reporting practices and barriers to incident reporting among nurses. BMC Nurs. 2023;22:231. doi:10.1186/s12912-023-01376-9
Empowering Culture Change and Safety on the Journey to Zero Harm With Huddle Cards
Empowering Culture Change and Safety on the Journey to Zero Harm With Huddle Cards
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.
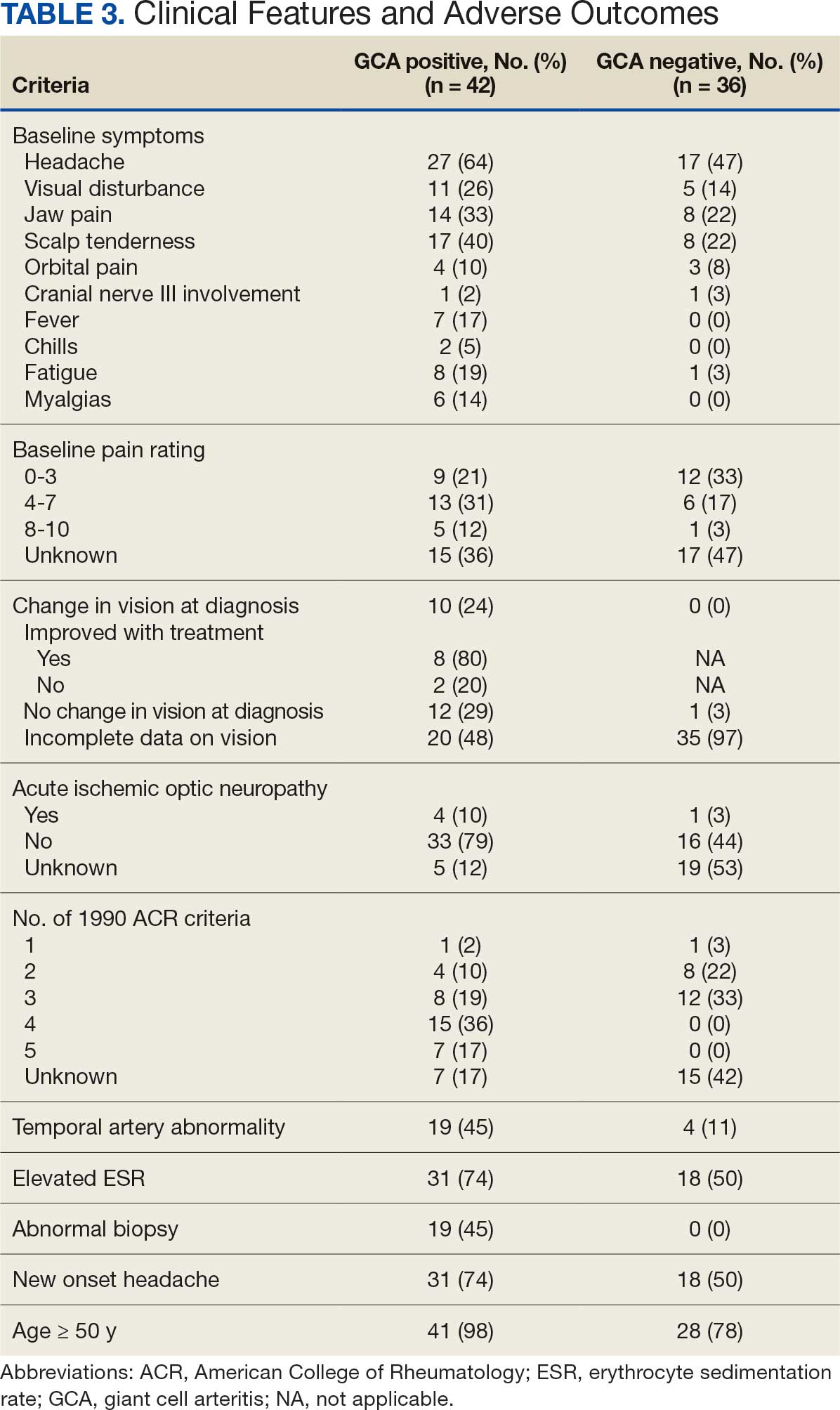
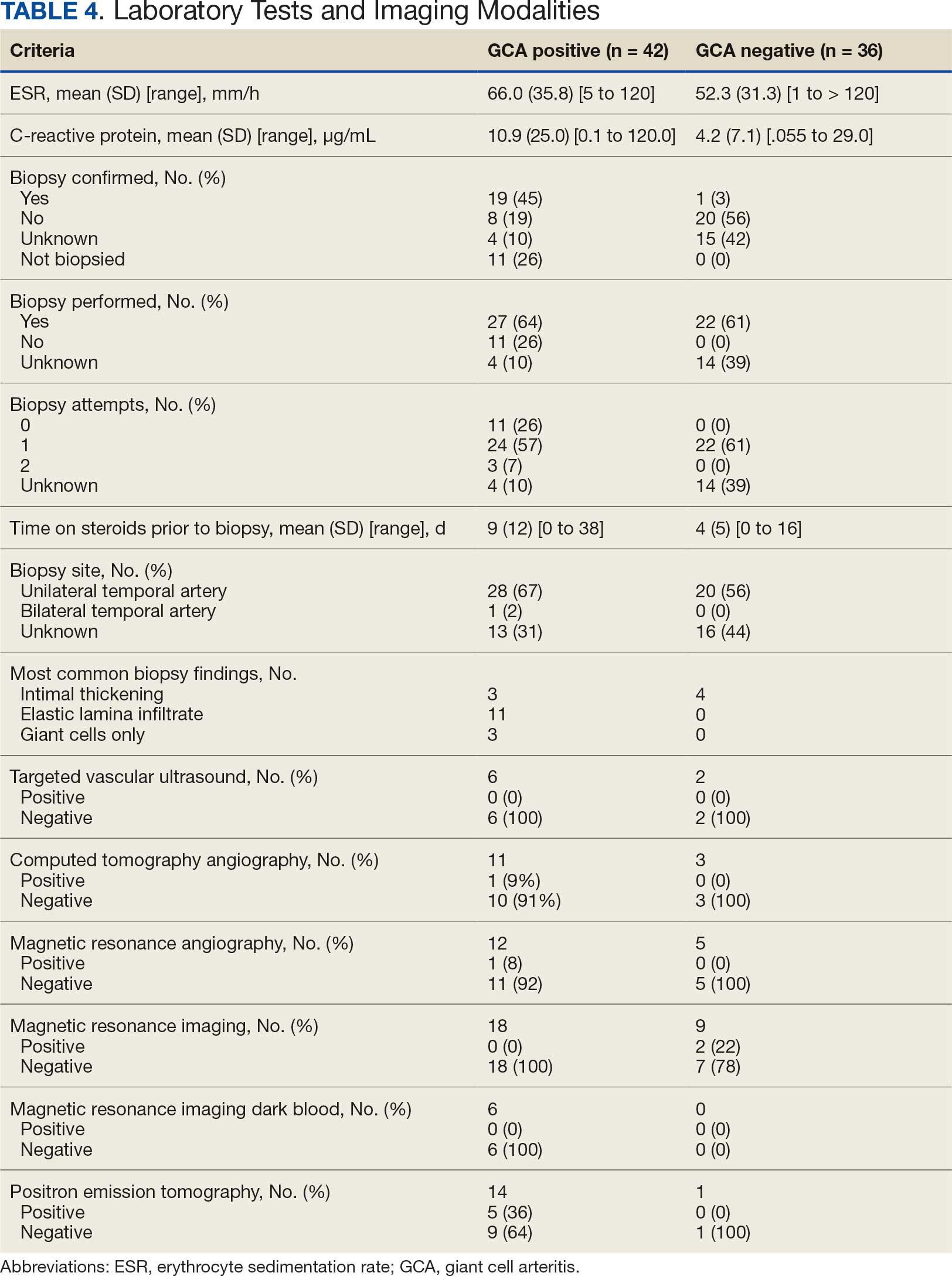
Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.



Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.



Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
If I have seen further, it is by standing on the shoulders of giants.
Sir Isaac Newton (1642-1727) 1
Early in residency, I decided I only wanted to work at the US Department of Veterans Affairs (VA). It was a way to follow the example of service that my parents, an Army doctor and nurse, had set. I spent much of my residency, including all of my last year of training, at a VA medical center, hoping a vacancy would open in the psychiatry service. In those days, VA jobs were hard to come by; doctors spent their entire careers in the system, only retiring after decades of commitment to its unique mission. Finally, close to graduation, one of my favorite attending physicians left his post. After mountains of paperwork and running the human resources obstacle course with the usual stumbles, I arrived at my dream job as a VA psychiatrist.
So, it is with immense sadness and even shock that I read a recent ProPublica article reporting that from January to March 2025 almost 40% of the physicians who received employment offers from the Veterans Health Administration (VHA) declined the positions.2 Medical media rapidly picked up the story, likely further discouraging potential applicants.3
There have always been health care professionals (HCPs) who had zero interest in working for the VA. Medical students and residents often have a love/hate relationship with the VA, with some trainees not having the patience for the behemoth pace of the bureaucracy or finding the old-style physical environment and more relaxed pace antiquated and inefficient.
The reasons doctors are saying no to VA employment at 4 times the previous rate are different and more disturbing. According to ProPublica, VA officials in Texas reported in a June internal presentation that about 90 people had turned down job offers due to the “uncertainty of reorganization.”2 They reported that low morale was causing existing employees to recommend against working at the VA. My own anecdotal experience is similar: contrary to prior years, few residents, if any, are interested in working at the VA because of concerns about the stability of employment and the direction of its organizational culture.
It is fair to question the objectivity of the ProPublica report. However, the latest VA Office of the Inspector General (OIG) analysis of staffing had similar findings. “Despite the ability to make noncompetitive appointments for such occupations, VHA continues to experience severe occupational staffing shortages for these occupations that are fundamental to the delivery of health care.” The 4434 severe occupational shortage figures in fiscal year (FY) 2025 were 50% higher than in FY 2024.4 OIG reported that 57% of facilities noted severe occupational staffing shortages for psychology, making it the most frequently reported clinical shortage.
At this critical juncture, when new health care professional energy is not flowing into the VHA, there is an unprecedented drain of the lifeblood of any system—the departure of the bearers of institutional memory. Early and scheduled retirements, the deferred resignation program, and severance have decimated the ranks of senior HCPs, experienced leaders, and career clinicians. ProPublica noted the loss of 600 doctors and 1900 nurses at the VHA so far in 2025.2 Internal VA data from exit interviews suggest similar motivations. Many cited lack of trust and confidence in senior leaders and job stress/pressure.5
It should be noted the VA has an alternative and plausible explanation for the expected departure of 30,000 employees. They argue that the VHA was overstaffed and the increased workforce decreased the efficiency of service. Voluntary separation from employment, VA contends, has avoided the need for a far more disruptive reduction in force. VA leaders avow that downsizing has not adversely impacted its ability to deliver high-quality health care and benefits and they assert that a reduction in red tape will enable VA to provide easier access to care. VA Secretary Doug Collins has concluded that because of these difficult but necessary changes, “VA is headed in the right direction.”6
What is institutional memory, and why is it important? “The core of institutional memory is collective awareness and understanding of a collective set of facts, concepts, experiences, and know-how,” Bhugra and Ventriglio explain. “These are all held collectively at various levels in any given institution. Thus, collective memory or history can be utilized to build on what has gone before and how we take things forward.”7
The authors of this quote offer a modern twist on what Sir Isaac Newton described in more metaphorical language in the epigraph: to survive, and even more to thrive, an enterprise must have those who have accumulated technical knowledge and professional wisdom as well as those who assume responsibility for appropriating and adding to this storehouse of operational skill, expertise, unique cultural values, and ethical commitments. The VHA is losing its instructors and students of institutional memory which deals a serious blow to the stability and vitality of any learning health system.6 As Bhugra and Ventriglio put it, institutional memory identifies “what has worked in delivering the aims in the past and what has not, thereby ensuring the lessons learnt are remembered and passed on to the next generation.”7
Nearly every week, at all levels of the agency, I have encountered this exodus of builders and bearers of institutional memory. Those who have left did so for many of the same reasons cited by those who declined to come, leaving incalculable gaps at both ends of the career spectrum. Both the old and new are essential for organizational resilience: fresh ideas enable an institution to be agile in responding to challenges, while operational savvy ensures responses are ecologically aligned with the organizational mission.8
The dire shortage of HCPs—especially in mental health and primary care—has opened up unprecedented opportunities.9 Colleagues have noted that with only a little searching they found multiple lucrative positions. Once, HCPs picked the VA because they valued the commitment to public service and being part of a community of education and research more than fame or fortune. Having the best benefits packages in the industry only reinforced its value.
Even so, surpassing a genius such as Sir Isaac Newton, writing to a scientific competitor, Robert Hooke, recognized that progress and discovery in science and medicine are nigh well impossible without the collective achievements housed in institutional memory.1 It was inspiring teachers and attending physicians—Newton’s giants—who attracted the best and brightest in medicine and nursing, other HCPs, and research, to the VA, where they could participate in a transactive organizational learning process from their seniors, and then grow that fund of knowledge to improve patient care, educate their learners, and innovate. What will happen when there are no longer shoulders of giants or anyone to stand on them?
- Chen C. Mapping Scientific Frontiers: The Quest for Knowledge Visualization. Springer; 2013:135.
- Armstrong D, Umansky E, Coleman V. Veterans’ care at risk under Trump as hundreds of doctors and nurses reject working at VA hospitals. ProPublica. August 8, 2025. Accessed August 25, 2025. https://www.propublica.org/article/veterans-affairs-hospital-shortages-trump
- Kuchno K. VA physician job offers rejections up fourfold in 2025: report. Becker’s Hospital Review. August 12, 2025. Accessed August 26, 2025. https://www.beckershospitalreview.com/workforce/va-physician-job-offer-rejections-up-fourfold-in-2025-report/
- US Department of Veterans Affairs, Office of Inspector General. OIG determination of Veterans Health Administration’s severe occupational staffing shortages fiscal year 2025. August 12, 2025. Accessed August 25, 2025. https://www.vaoig.gov/reports/national-healthcare-review/oig-determination-veterans-health-administrations-severe-1
- US Department of Veterans Affairs. VA workforce dashboard. July 25, 2025. Accessed August 25, 2025. https://www.va.gov/EMPLOYEE/docs/workforce/VA-Workforce-Dashboard-Issue-27.pdf
- VA to reduce staff by nearly 30K by end of FY2025. News release. Veterans Affairs News. July 7, 2025. Accessed August 25, 2025. https://news.va.gov/press-room/va-to-reduce-staff-by-nearly-30k-by-end-of-fy2025/
- Bhugra D, Ventriglio A. Institutions, institutional memory, healthcare and research. Int J Soc Psychiatry. 2023;69(8):1843-1844. doi:10.1177/00207640231213905
- Jain A. Is organizational memory a useful capability? An analysis of its effects on productivity, absorptive capacity adaptation. In Argote L, Levine JM. The Oxford Handbook of Group and Organizational Learning. Oxford; 2020.
- Broder J. Ready to pick a specialty? These may have the brightest futures. Medscape. April 21, 2025. Accessed August 25, 2025. https://www.medscape.com/viewarticle/ready-pick-specialty-these-may-have-brightest-futures-2025a10009if
If I have seen further, it is by standing on the shoulders of giants.
Sir Isaac Newton (1642-1727) 1
Early in residency, I decided I only wanted to work at the US Department of Veterans Affairs (VA). It was a way to follow the example of service that my parents, an Army doctor and nurse, had set. I spent much of my residency, including all of my last year of training, at a VA medical center, hoping a vacancy would open in the psychiatry service. In those days, VA jobs were hard to come by; doctors spent their entire careers in the system, only retiring after decades of commitment to its unique mission. Finally, close to graduation, one of my favorite attending physicians left his post. After mountains of paperwork and running the human resources obstacle course with the usual stumbles, I arrived at my dream job as a VA psychiatrist.
So, it is with immense sadness and even shock that I read a recent ProPublica article reporting that from January to March 2025 almost 40% of the physicians who received employment offers from the Veterans Health Administration (VHA) declined the positions.2 Medical media rapidly picked up the story, likely further discouraging potential applicants.3
There have always been health care professionals (HCPs) who had zero interest in working for the VA. Medical students and residents often have a love/hate relationship with the VA, with some trainees not having the patience for the behemoth pace of the bureaucracy or finding the old-style physical environment and more relaxed pace antiquated and inefficient.
The reasons doctors are saying no to VA employment at 4 times the previous rate are different and more disturbing. According to ProPublica, VA officials in Texas reported in a June internal presentation that about 90 people had turned down job offers due to the “uncertainty of reorganization.”2 They reported that low morale was causing existing employees to recommend against working at the VA. My own anecdotal experience is similar: contrary to prior years, few residents, if any, are interested in working at the VA because of concerns about the stability of employment and the direction of its organizational culture.
It is fair to question the objectivity of the ProPublica report. However, the latest VA Office of the Inspector General (OIG) analysis of staffing had similar findings. “Despite the ability to make noncompetitive appointments for such occupations, VHA continues to experience severe occupational staffing shortages for these occupations that are fundamental to the delivery of health care.” The 4434 severe occupational shortage figures in fiscal year (FY) 2025 were 50% higher than in FY 2024.4 OIG reported that 57% of facilities noted severe occupational staffing shortages for psychology, making it the most frequently reported clinical shortage.
At this critical juncture, when new health care professional energy is not flowing into the VHA, there is an unprecedented drain of the lifeblood of any system—the departure of the bearers of institutional memory. Early and scheduled retirements, the deferred resignation program, and severance have decimated the ranks of senior HCPs, experienced leaders, and career clinicians. ProPublica noted the loss of 600 doctors and 1900 nurses at the VHA so far in 2025.2 Internal VA data from exit interviews suggest similar motivations. Many cited lack of trust and confidence in senior leaders and job stress/pressure.5
It should be noted the VA has an alternative and plausible explanation for the expected departure of 30,000 employees. They argue that the VHA was overstaffed and the increased workforce decreased the efficiency of service. Voluntary separation from employment, VA contends, has avoided the need for a far more disruptive reduction in force. VA leaders avow that downsizing has not adversely impacted its ability to deliver high-quality health care and benefits and they assert that a reduction in red tape will enable VA to provide easier access to care. VA Secretary Doug Collins has concluded that because of these difficult but necessary changes, “VA is headed in the right direction.”6
What is institutional memory, and why is it important? “The core of institutional memory is collective awareness and understanding of a collective set of facts, concepts, experiences, and know-how,” Bhugra and Ventriglio explain. “These are all held collectively at various levels in any given institution. Thus, collective memory or history can be utilized to build on what has gone before and how we take things forward.”7
The authors of this quote offer a modern twist on what Sir Isaac Newton described in more metaphorical language in the epigraph: to survive, and even more to thrive, an enterprise must have those who have accumulated technical knowledge and professional wisdom as well as those who assume responsibility for appropriating and adding to this storehouse of operational skill, expertise, unique cultural values, and ethical commitments. The VHA is losing its instructors and students of institutional memory which deals a serious blow to the stability and vitality of any learning health system.6 As Bhugra and Ventriglio put it, institutional memory identifies “what has worked in delivering the aims in the past and what has not, thereby ensuring the lessons learnt are remembered and passed on to the next generation.”7
Nearly every week, at all levels of the agency, I have encountered this exodus of builders and bearers of institutional memory. Those who have left did so for many of the same reasons cited by those who declined to come, leaving incalculable gaps at both ends of the career spectrum. Both the old and new are essential for organizational resilience: fresh ideas enable an institution to be agile in responding to challenges, while operational savvy ensures responses are ecologically aligned with the organizational mission.8
The dire shortage of HCPs—especially in mental health and primary care—has opened up unprecedented opportunities.9 Colleagues have noted that with only a little searching they found multiple lucrative positions. Once, HCPs picked the VA because they valued the commitment to public service and being part of a community of education and research more than fame or fortune. Having the best benefits packages in the industry only reinforced its value.
Even so, surpassing a genius such as Sir Isaac Newton, writing to a scientific competitor, Robert Hooke, recognized that progress and discovery in science and medicine are nigh well impossible without the collective achievements housed in institutional memory.1 It was inspiring teachers and attending physicians—Newton’s giants—who attracted the best and brightest in medicine and nursing, other HCPs, and research, to the VA, where they could participate in a transactive organizational learning process from their seniors, and then grow that fund of knowledge to improve patient care, educate their learners, and innovate. What will happen when there are no longer shoulders of giants or anyone to stand on them?
If I have seen further, it is by standing on the shoulders of giants.
Sir Isaac Newton (1642-1727) 1
Early in residency, I decided I only wanted to work at the US Department of Veterans Affairs (VA). It was a way to follow the example of service that my parents, an Army doctor and nurse, had set. I spent much of my residency, including all of my last year of training, at a VA medical center, hoping a vacancy would open in the psychiatry service. In those days, VA jobs were hard to come by; doctors spent their entire careers in the system, only retiring after decades of commitment to its unique mission. Finally, close to graduation, one of my favorite attending physicians left his post. After mountains of paperwork and running the human resources obstacle course with the usual stumbles, I arrived at my dream job as a VA psychiatrist.
So, it is with immense sadness and even shock that I read a recent ProPublica article reporting that from January to March 2025 almost 40% of the physicians who received employment offers from the Veterans Health Administration (VHA) declined the positions.2 Medical media rapidly picked up the story, likely further discouraging potential applicants.3
There have always been health care professionals (HCPs) who had zero interest in working for the VA. Medical students and residents often have a love/hate relationship with the VA, with some trainees not having the patience for the behemoth pace of the bureaucracy or finding the old-style physical environment and more relaxed pace antiquated and inefficient.
The reasons doctors are saying no to VA employment at 4 times the previous rate are different and more disturbing. According to ProPublica, VA officials in Texas reported in a June internal presentation that about 90 people had turned down job offers due to the “uncertainty of reorganization.”2 They reported that low morale was causing existing employees to recommend against working at the VA. My own anecdotal experience is similar: contrary to prior years, few residents, if any, are interested in working at the VA because of concerns about the stability of employment and the direction of its organizational culture.
It is fair to question the objectivity of the ProPublica report. However, the latest VA Office of the Inspector General (OIG) analysis of staffing had similar findings. “Despite the ability to make noncompetitive appointments for such occupations, VHA continues to experience severe occupational staffing shortages for these occupations that are fundamental to the delivery of health care.” The 4434 severe occupational shortage figures in fiscal year (FY) 2025 were 50% higher than in FY 2024.4 OIG reported that 57% of facilities noted severe occupational staffing shortages for psychology, making it the most frequently reported clinical shortage.
At this critical juncture, when new health care professional energy is not flowing into the VHA, there is an unprecedented drain of the lifeblood of any system—the departure of the bearers of institutional memory. Early and scheduled retirements, the deferred resignation program, and severance have decimated the ranks of senior HCPs, experienced leaders, and career clinicians. ProPublica noted the loss of 600 doctors and 1900 nurses at the VHA so far in 2025.2 Internal VA data from exit interviews suggest similar motivations. Many cited lack of trust and confidence in senior leaders and job stress/pressure.5
It should be noted the VA has an alternative and plausible explanation for the expected departure of 30,000 employees. They argue that the VHA was overstaffed and the increased workforce decreased the efficiency of service. Voluntary separation from employment, VA contends, has avoided the need for a far more disruptive reduction in force. VA leaders avow that downsizing has not adversely impacted its ability to deliver high-quality health care and benefits and they assert that a reduction in red tape will enable VA to provide easier access to care. VA Secretary Doug Collins has concluded that because of these difficult but necessary changes, “VA is headed in the right direction.”6
What is institutional memory, and why is it important? “The core of institutional memory is collective awareness and understanding of a collective set of facts, concepts, experiences, and know-how,” Bhugra and Ventriglio explain. “These are all held collectively at various levels in any given institution. Thus, collective memory or history can be utilized to build on what has gone before and how we take things forward.”7
The authors of this quote offer a modern twist on what Sir Isaac Newton described in more metaphorical language in the epigraph: to survive, and even more to thrive, an enterprise must have those who have accumulated technical knowledge and professional wisdom as well as those who assume responsibility for appropriating and adding to this storehouse of operational skill, expertise, unique cultural values, and ethical commitments. The VHA is losing its instructors and students of institutional memory which deals a serious blow to the stability and vitality of any learning health system.6 As Bhugra and Ventriglio put it, institutional memory identifies “what has worked in delivering the aims in the past and what has not, thereby ensuring the lessons learnt are remembered and passed on to the next generation.”7
Nearly every week, at all levels of the agency, I have encountered this exodus of builders and bearers of institutional memory. Those who have left did so for many of the same reasons cited by those who declined to come, leaving incalculable gaps at both ends of the career spectrum. Both the old and new are essential for organizational resilience: fresh ideas enable an institution to be agile in responding to challenges, while operational savvy ensures responses are ecologically aligned with the organizational mission.8
The dire shortage of HCPs—especially in mental health and primary care—has opened up unprecedented opportunities.9 Colleagues have noted that with only a little searching they found multiple lucrative positions. Once, HCPs picked the VA because they valued the commitment to public service and being part of a community of education and research more than fame or fortune. Having the best benefits packages in the industry only reinforced its value.
Even so, surpassing a genius such as Sir Isaac Newton, writing to a scientific competitor, Robert Hooke, recognized that progress and discovery in science and medicine are nigh well impossible without the collective achievements housed in institutional memory.1 It was inspiring teachers and attending physicians—Newton’s giants—who attracted the best and brightest in medicine and nursing, other HCPs, and research, to the VA, where they could participate in a transactive organizational learning process from their seniors, and then grow that fund of knowledge to improve patient care, educate their learners, and innovate. What will happen when there are no longer shoulders of giants or anyone to stand on them?
- Chen C. Mapping Scientific Frontiers: The Quest for Knowledge Visualization. Springer; 2013:135.
- Armstrong D, Umansky E, Coleman V. Veterans’ care at risk under Trump as hundreds of doctors and nurses reject working at VA hospitals. ProPublica. August 8, 2025. Accessed August 25, 2025. https://www.propublica.org/article/veterans-affairs-hospital-shortages-trump
- Kuchno K. VA physician job offers rejections up fourfold in 2025: report. Becker’s Hospital Review. August 12, 2025. Accessed August 26, 2025. https://www.beckershospitalreview.com/workforce/va-physician-job-offer-rejections-up-fourfold-in-2025-report/
- US Department of Veterans Affairs, Office of Inspector General. OIG determination of Veterans Health Administration’s severe occupational staffing shortages fiscal year 2025. August 12, 2025. Accessed August 25, 2025. https://www.vaoig.gov/reports/national-healthcare-review/oig-determination-veterans-health-administrations-severe-1
- US Department of Veterans Affairs. VA workforce dashboard. July 25, 2025. Accessed August 25, 2025. https://www.va.gov/EMPLOYEE/docs/workforce/VA-Workforce-Dashboard-Issue-27.pdf
- VA to reduce staff by nearly 30K by end of FY2025. News release. Veterans Affairs News. July 7, 2025. Accessed August 25, 2025. https://news.va.gov/press-room/va-to-reduce-staff-by-nearly-30k-by-end-of-fy2025/
- Bhugra D, Ventriglio A. Institutions, institutional memory, healthcare and research. Int J Soc Psychiatry. 2023;69(8):1843-1844. doi:10.1177/00207640231213905
- Jain A. Is organizational memory a useful capability? An analysis of its effects on productivity, absorptive capacity adaptation. In Argote L, Levine JM. The Oxford Handbook of Group and Organizational Learning. Oxford; 2020.
- Broder J. Ready to pick a specialty? These may have the brightest futures. Medscape. April 21, 2025. Accessed August 25, 2025. https://www.medscape.com/viewarticle/ready-pick-specialty-these-may-have-brightest-futures-2025a10009if
- Chen C. Mapping Scientific Frontiers: The Quest for Knowledge Visualization. Springer; 2013:135.
- Armstrong D, Umansky E, Coleman V. Veterans’ care at risk under Trump as hundreds of doctors and nurses reject working at VA hospitals. ProPublica. August 8, 2025. Accessed August 25, 2025. https://www.propublica.org/article/veterans-affairs-hospital-shortages-trump
- Kuchno K. VA physician job offers rejections up fourfold in 2025: report. Becker’s Hospital Review. August 12, 2025. Accessed August 26, 2025. https://www.beckershospitalreview.com/workforce/va-physician-job-offer-rejections-up-fourfold-in-2025-report/
- US Department of Veterans Affairs, Office of Inspector General. OIG determination of Veterans Health Administration’s severe occupational staffing shortages fiscal year 2025. August 12, 2025. Accessed August 25, 2025. https://www.vaoig.gov/reports/national-healthcare-review/oig-determination-veterans-health-administrations-severe-1
- US Department of Veterans Affairs. VA workforce dashboard. July 25, 2025. Accessed August 25, 2025. https://www.va.gov/EMPLOYEE/docs/workforce/VA-Workforce-Dashboard-Issue-27.pdf
- VA to reduce staff by nearly 30K by end of FY2025. News release. Veterans Affairs News. July 7, 2025. Accessed August 25, 2025. https://news.va.gov/press-room/va-to-reduce-staff-by-nearly-30k-by-end-of-fy2025/
- Bhugra D, Ventriglio A. Institutions, institutional memory, healthcare and research. Int J Soc Psychiatry. 2023;69(8):1843-1844. doi:10.1177/00207640231213905
- Jain A. Is organizational memory a useful capability? An analysis of its effects on productivity, absorptive capacity adaptation. In Argote L, Levine JM. The Oxford Handbook of Group and Organizational Learning. Oxford; 2020.
- Broder J. Ready to pick a specialty? These may have the brightest futures. Medscape. April 21, 2025. Accessed August 25, 2025. https://www.medscape.com/viewarticle/ready-pick-specialty-these-may-have-brightest-futures-2025a10009if
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
When The Giants and Those Who Stand on Their Shoulders Are Gone: The Loss of VA Institutional Memory
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
As one of the most competitive specialties in medicine, dermatology presents unique challenges for residency applicants, especially following the shift in United States Medical Licensing Examination (USMLE) Step 1 scoring to a pass/fail format.1,2 Historically, USMLE Step 1 served as a major screening metric for residency programs, with 90% of program directors in 2020 using USMLE Step 1 scores as a primary factor when deciding whether to invite applicants for interviews.1 However, the recent transition to pass/fail has made it much harder for program directors to objectively compare applicants, particularly in dermatology. In a 2020 survey, Patrinely Jr et al2 found that 77.2% of dermatology program directors agreed that this change would make it more difficult to assess candidates objectively. Consequently, research productivity has taken on greater importance as programs seek new ways to distinguish top applicants.1,2
In response to this increased emphasis on research, dermatology applicants have substantially boosted their scholarly output over the past several years. The 2022 and 2024 results from the National Residency Matching Program’s Charting Outcomes survey demonstrated a steady rise in research metrics among applicants across various specialties, with dermatology showing one of the largest increases.3,4 For instance, the average number of abstracts, presentations, and publications for matched allopathic dermatology applicants was 5.7 in 2007.5 This average increased to 20.9 in 20223 and to 27.7 in 2024,4 marking an astonishing 485% increase in 17 years. Interestingly, unmatched dermatology applicants had an average of 19.0 research products in 2024, which was similar to the average of successfully matched applicants just 2 years earlier.3,4
Engaging in research offers benefits beyond building a strong residency application. Specifically, it enhances critical thinking skills and provides hands-on experience in scientific inquiry.6 It allows students to explore dermatology topics of interest and address existing knowledge gaps within the specialty.6 Additionally, it creates opportunities to build meaningful relationships with experienced dermatologists who can guide and support students throughout their careers.7 Despite these benefits, the pursuit of research may be landscaped with obstacles, and the fervent race to obtain high research outputs may overshadow developmental advantages.8 These challenges and demands also could contribute to inequities in the residency selection process, particularly if barriers are influenced by socioeconomic and demographic disparities. As dermatology already ranks as the second least diverse specialty in medicine,9 research requirements that disproportionately disadvantage certain demographic groups risk further widening these concerning representation gaps rather than creating opportunities to address them.
Given these trends in research requirements and their potential impact on applicant success, understanding specific barriers to research engagement is essential for creating equitable opportunities in dermatology. In this study, we aimed to identify barriers to research engagement among dermatology applicants, analyze their relationship with demographic factors, assess their impact on specialty choice and research productivity, and provide actionable solutions to address these obstacles.
Methods
A cross-sectional survey was conducted targeting medical students applying to dermatology residency programs in the United States in the 2025 or 2026 match cycles as well as residents who applied to dermatology residency in the 2021 to 2024 match cycles. The 23-item survey was developed by adapting questions from several validated studies examining research barriers and experiences in medical education.6,7,10,11 Specifically, the survey included questions on demographics and background; research productivity; general research barriers; conference participation accessibility; mentorship access; and quality, career impact, and support needs. Socioeconomic background was measured via a single self-reported item asking participants to select the income class that best reflected their background growing up (low-income, lower-middle, upper-middle, or high-income); no income ranges were provided.
The survey was distributed electronically via Qualtrics between November 11, 2024, and December 30, 2024, through listserves of the Dermatology Interest Group Association (sent directly to medical students) and the Association of Professors of Dermatology (forwarded to residents by program directors). There was no way to determine the number of dermatology applicants and residents reached through either listserve. The surveys were reviewed and approved by the University of Alabama at Birmingham institutional review board (IRB-300013671).
Statistical analyses were conducted using RStudio (Posit, PBC; version 2024.12.0+467). Descriptive statistics characterized participant demographics and quantified barrier scores using frequencies and proportions. We performed regression analyses to examine relationships between demographic factors and barriers using linear regression; the relationship between barriers and research productivity correlation; and the prediction of specialty change consideration using logistic regression. For all analyses, barrier scores were rated on a scale of 0 to 3 (0=not a barrier, 1=minor barrier, 2=moderate barrier, 3=major barrier); R² values were reported to indicate strength of associations, and statistical significance was set at P<.05.
Results
Participant Demographics—A total of 136 participants completed the survey. Among the respondents, 12% identified as from a background of low-income class, 28% lower-middle class, 49% upper-middle class, and 11% high-income class. Additionally, 27% of respondents identified as underrepresented in medicine (URiM). Regarding debt levels (or expected debt levels) upon graduation from medical school, 32% reported no debt, 9% reported $1000 to $49,000 in debt, 5% reported $50,000 to $99,000 in debt, 15% reported $100,000 to $199,000 in debt, 22% reported $200,000 to $299,000 in debt, and 17% reported $300,000 in debt or higher. The majority of respondents (95%) were MD candidates, and the remaining 5% were DO candidates; additionally, 5% were participants in an MD/PhD program (eTable 1).
Respondents represented various stages of training: 13.2% and 16.2% were third- and fourth-year medical students, respectively, while 6.0%, 20.1%, 18.4%, and 22.8% were postgraduate year (PGY) 1, PGY-2, PGY-3, and PGY-4, respectively. A few respondents (2.9%) were participating in a research year or reapplying to dermatology residency (eTable 2).
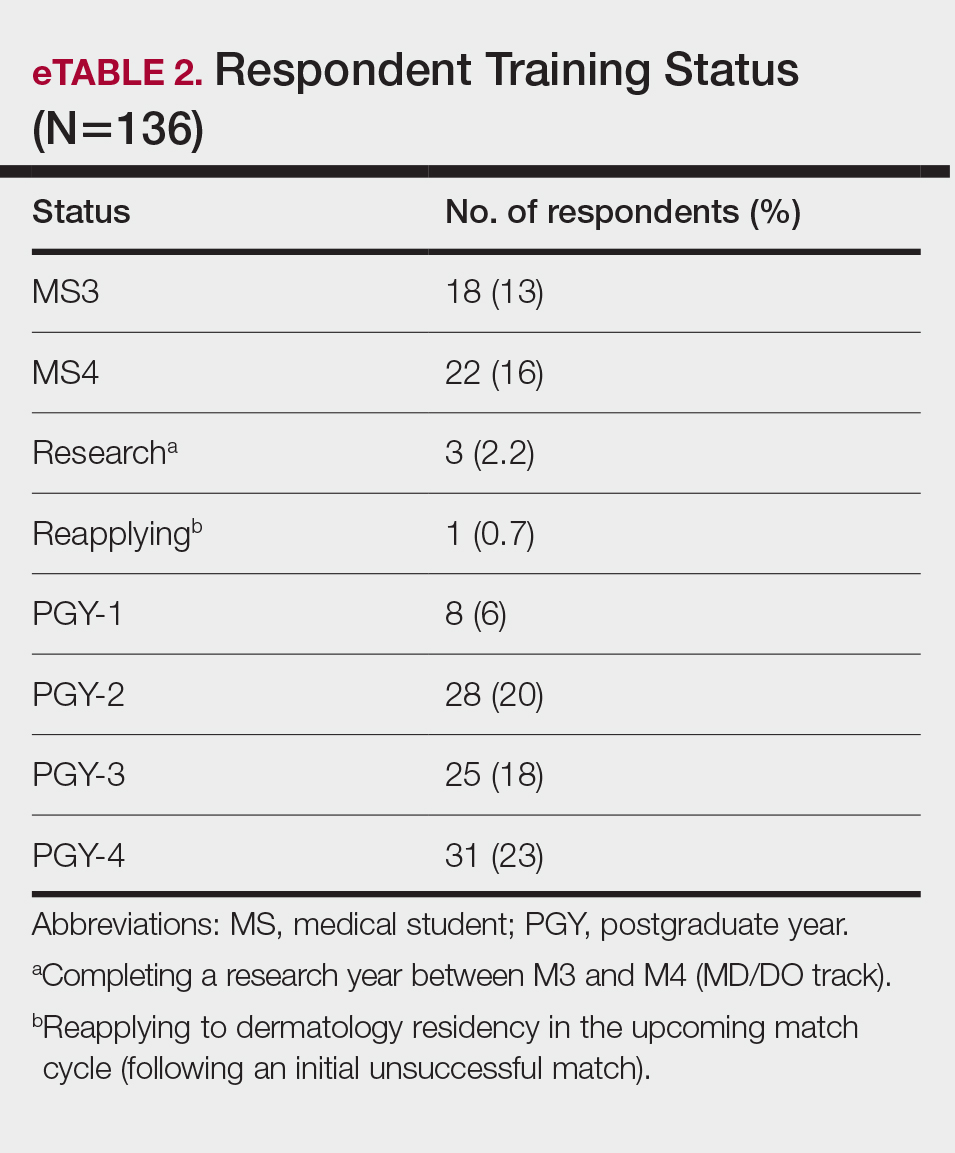
Research Barriers and Productivity—Respondents were presented with a list of potential barriers and asked to rate each as not a barrier, a minor barrier, a moderate barrier, or a major barrier. The most common barriers (ie, those with >50% of respondents rating them as a moderate or major) included lack of time, limited access to research opportunities, not knowing how to begin research, and lack of mentorship or support. Lack of time and not knowing where to begin research were reported most frequently as major barriers, with 32% of participants identifying them as such. In contrast, barriers such as financial costs and personal obligations were less frequently rated as major barriers (10% and 4%, respectively), although they still were identified as obstacles by many respondents. Interestingly, most respondents (58%) indicated that institutional limitations were not a barrier, but a separate and sizeable proportion (25%) of respondents considered it to be a major barrier (eFigure 1).
The distributions for all research metrics were right-skewed. The total range was 0 to 45 (median, 6) for number of publications (excluding abstracts), 0 to 33 (median, 2) for published abstracts, 0 to 40 (median, 5) for poster publications, and 0 to 20 (median, 2) for oral presentations (eTable 3).
Regression Analysis—Linear regression analysis identified significant relationships between demographic variables (socioeconomic status [SES], URiM status, and debt level) and individual research barriers. The heatmap in eFigure 2 illustrates the strength of these relationships. Higher SES was predictive of lower reported financial barriers (R²=.2317; P<.0001) and lower reported institutional limitations (R²=.0884; P=.0006). A URiM status predicted higher reported financial barriers (R²=.1097; P<.0001) and institutional limitations (R²=.04537; P=.013). Also, higher debt level predicted increased financial barriers (R²=.2099; P<.0001), institutional limitations (R2=.1258; P<.0001), and lack of mentorship (R²=.06553; P=.003).
Next, the data were evaluated for correlative relationships between individual research barriers and research productivity metrics including number of publications, published abstracts and presentations (oral and poster) and total research output. While correlations were weak or nonsignificant between barriers and most research productivity metrics (published abstracts, oral and poster presentations, and total research output), the number of publications was significantly correlated with several research barriers, including limited access to research opportunities (P=.002), not knowing how to begin research (P=.025), lack of mentorship or support (P=.011), and institutional limitations (P=.042). Higher ratings for limited access to research opportunities, not knowing where to begin research, lack of mentorship or support, and institutional limitations all were negatively correlated with total number of publications (R2=−.27, –.19, −.22, and –.18, respectively)(eFigure 3).
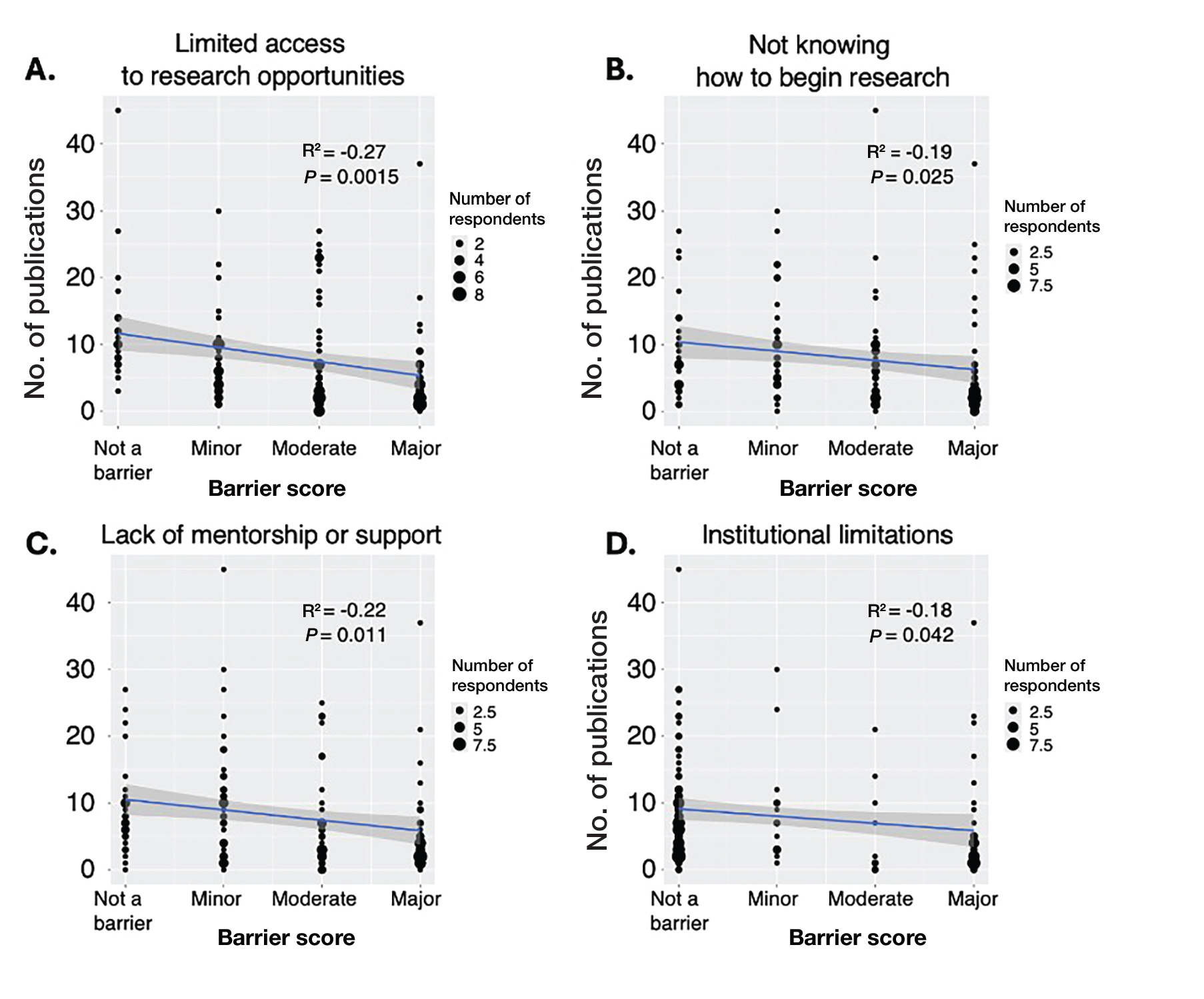
Logistic regression analysis examined the impact of research barriers on the likelihood of specialty change consideration. The results, presented in a forest plot, include odds ratios (ORs) and their corresponding 95% CIs and P values. Lack of time (P=.001) and not knowing where to begin research (P<.001) were the strongest predictors of specialty change consideration (OR, 6.3 and 4.7, respectively). Financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) also were significant predictors of specialty change consideration (OR, 2.2, 3.1, and 3.5, respectively). Institutional limitations and personal obligations did not predict specialty change consideration (eTable 4 and eFigure 4).
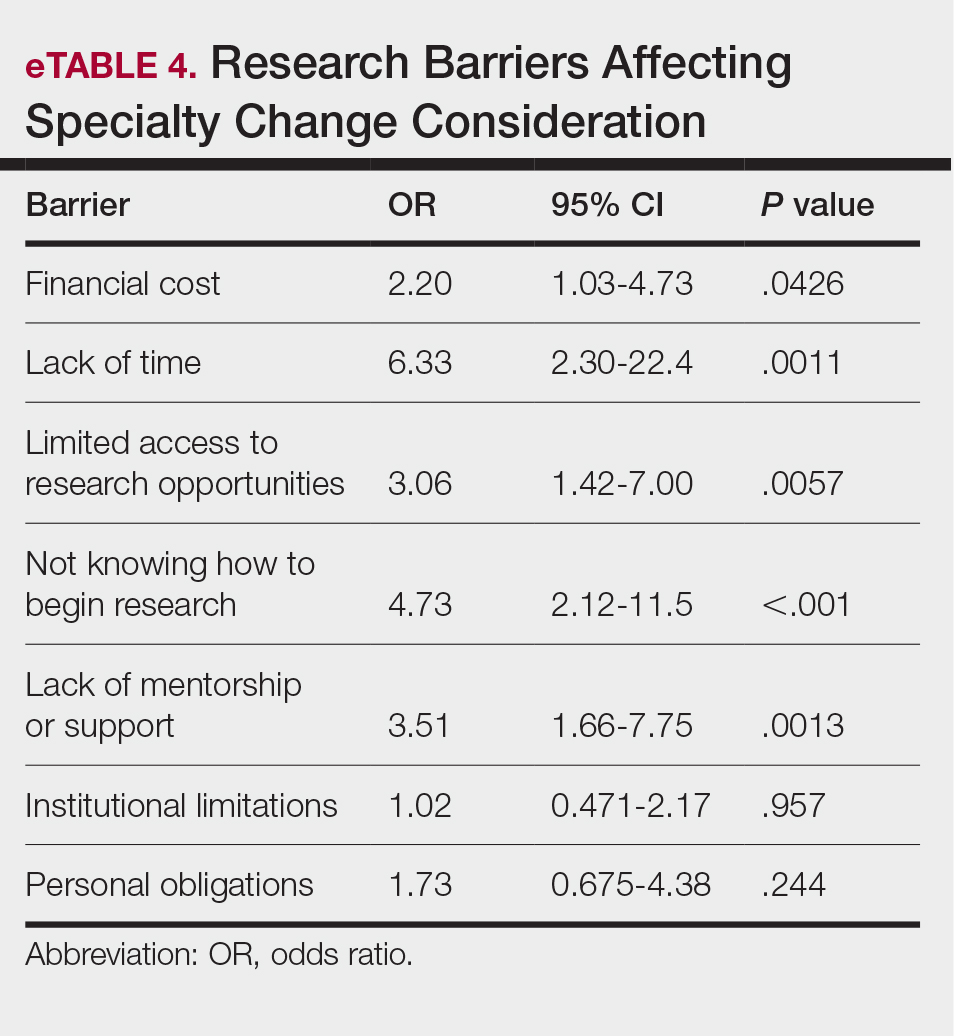
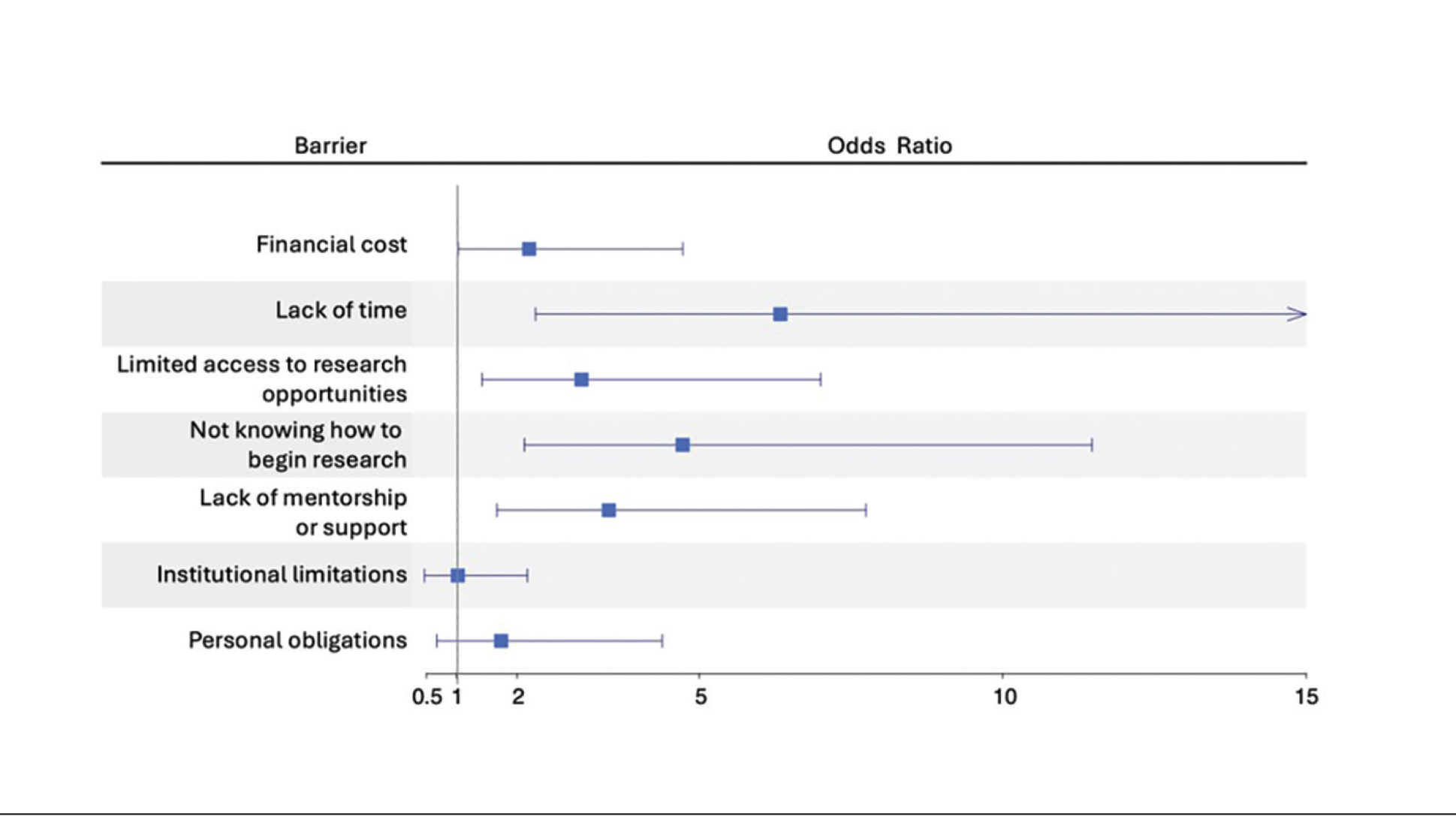
Mitigation Strategies—Mitigation strategies were ranked by respondents based on their perceived importance on a scale of 1 to 7 (1=most important, 7=least important)(eFigure 5). Respondents considered access to engaged mentors to be the most important mitigation strategy by far, with 95% ranking it in the top 3 (47% of respondents ranked it as the top most important mitigation strategy). Financial assistance was the mitigation strategy with the second highest number of respondents (28%) ranking it as the top strategy. Flexible scheduling during rotations, research training programs or discussions, and peer networking and research collaboration opportunities also were considered by respondents to be important mitigation strategies. Time management support/resources frequently was viewed as the least important mitigation strategy, with 38% of respondents ranking it last.
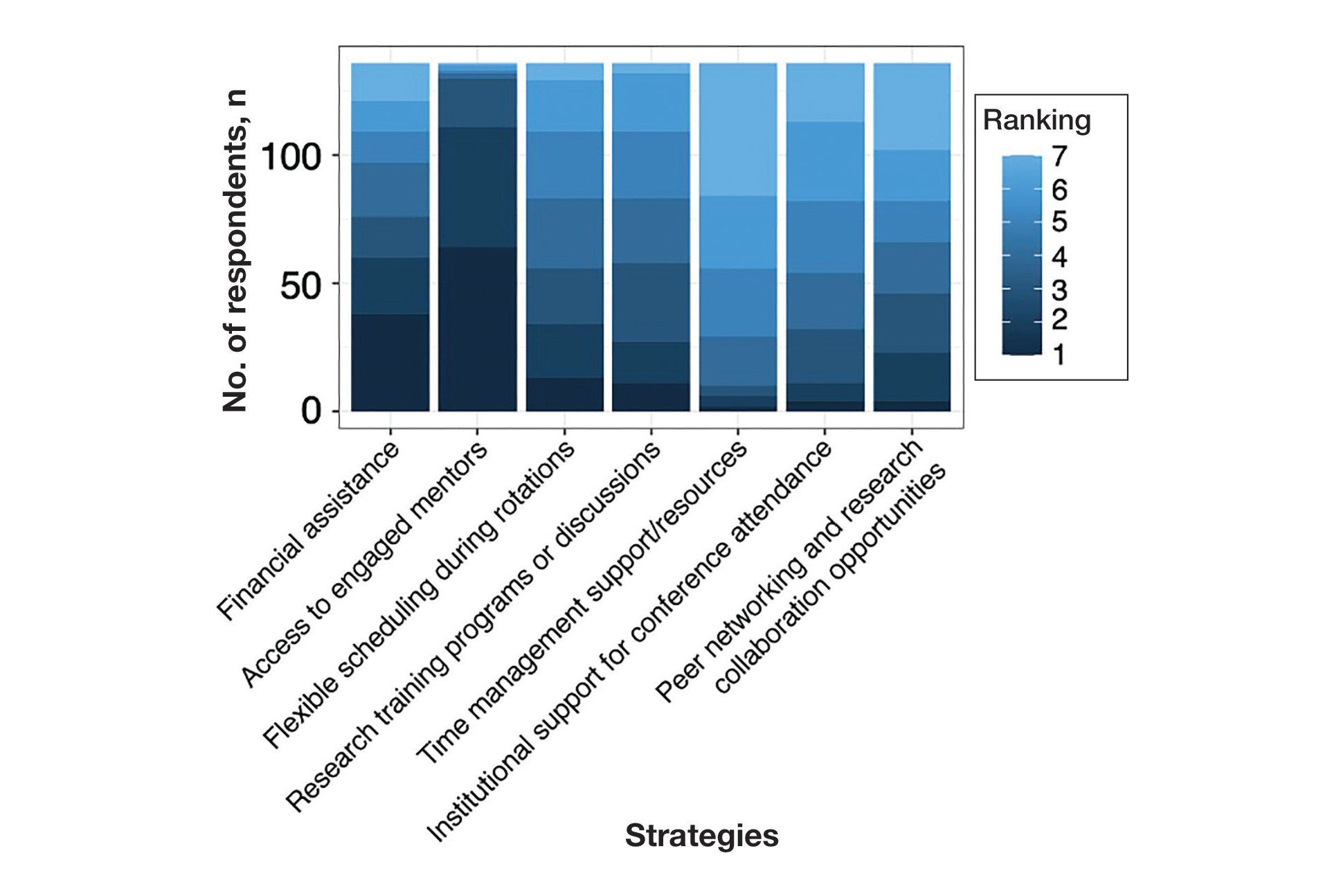
Comment
Our study revealed notable disparities in research barriers among dermatology applicants, with several demonstrating consistent patterns of association with SES, URiM status, and debt burden. Furthermore, the strong relationship between these barriers and decreased research productivity and specialty change consideration suggests that capable candidates may be deterred from pursuing dermatology due to surmountable obstacles rather than lack of interest or ability.
Impact of Demographic Factors on Research Barriers—All 7 general research barriers surveyed were correlated with distinct demographic predictors. Regression analyses indicated that the barrier of financial cost was significantly predicted by lower SES (R²=.2317; P<.001), URiM status (R²=.1097; P<.001), and higher debt levels (R²=.2099; P<.001)(eFigure 2). These findings are particularly concerning given the trend of dermatology applicants pursuing 1-year research fellowships, many of which are unpaid.12 In fact, Jacobson et al11 found that 71.7% (43/60) of dermatology applicants who pursued a year-long research fellowship experienced financial strain during their fellowship, with many requiring additional loans or drawing from personal savings despite already carrying substantial medical school debt of $200,000 or more. Our findings showcase how financial barriers to research disproportionately affect students from lower socioeconomic backgrounds, those who identify as URiM, and those with higher debt, creating systemic inequities in research access at a time when research productivity is increasingly vital for matching into dermatology. To address these financial barriers, institutions may consider establishing more funded research fellowships or expanding grant programs targeting students from economically disadvantaged and/or underrepresented backgrounds.
Institutional limitations (eg, the absence of a dermatology department) also was a notable barrier that was significantly predicted by lower SES (R²=.0884; P<.001) and URiM status (R²=.04537; P=.013)(eFigure 2). Students at institutions lacking dermatology programs face restricted access to mentorship and research opportunities,13 with our results demonstrating that these barriers disproportionately affect students from underresourced and minority groups. These limitations compound disparities in building competitive residency applications.14 The Women’s Dermatologic Society (WDS) has developed a model for addressing these institutional barriers through its summer research fellowship program for medical students who identify as URiM. By pairing students with WDS mentors who guide them through summer research projects, this initiative addresses access and mentorship gaps for students lacking dermatology departments at their home institution.15 The WDS program serves as a model for other organizations to adopt and expand, with particular attention to including students who identify as URiM as well as those from lower socioeconomic backgrounds.
Our results identified time constraints and lack of experience as notable research barriers. Higher debt levels significantly predicted both lack of time (R²=.03915; P=.021) and not knowing how to begin research (R²=.0572; P=.005)(eFigure 2). These statistical relationships may be explained by students with higher debt levels needing to prioritize paid work over unpaid research opportunities, limiting their engagement in research due to the scarcity of funded positions.12 The data further revealed that personal obligations, particularly family care responsibilities, were significantly predicted by both lower SES (R²=.0539; P=.008) and higher debt level (R²=.03237; P=.036)(eFigure 2). These findings demonstrate how students managing academic demands alongside financial and familial responsibilities may face compounded barriers to research engagement. To address these disparities, medical schools may consider implementing protected research time within their curricula; for example, the Emory University School of Medicine (Atlanta, Georgia) has implemented a Discovery Phase program that provides students with 5 months of protected faculty-mentored research time away from academic demands between their third and fourth years of medical school.16 Integrating similarly structured research periods across medical school curricula could help ensure equitable research opportunities for all students pursuing competitive specialties such as dermatology.8
Access to mentorship is a critical determinant of research engagement and productivity, as mentors provide valuable guidance on navigating research processes and professional development.17 Our analysis revealed that lack of mentorship was predicted by both lower SES (R²=.039; P=.023) and higher debt level (R²=.06553; P=.003)(eFigure 2). Several organizations have developed programs to address these mentorship gaps. The Skin of Color Society pairs medical students with skin of color experts while advancing its mission of increasing diversity in dermatology.18 Similarly, the American Academy of Dermatology founded a diversity mentorship program that connects students who identify as URiM with dermatologist mentors for summer research experiences.19 Notably, the Skin of Color Society’s program allows residents to serve as mentors for medical students. Involving residents and community dermatologists as potential dermatology mentors for medical students not only distributes mentorship demands more sustainably but also increases overall access to dermatology mentors. Our findings indicate that similar programs could be expanded to include more residents and community dermatologists as mentors and to target students from disadvantaged backgrounds, those facing financial constraints, and students who identify as URiM.
Impact of Research Barriers on Career Trajectories—Among survey participants, 35% reported considering changing their specialty choice due to research-related barriers. This substantial percentage likely stems from the escalating pressure to achieve increasingly high research output amidst a lack of sufficient support, time, or tools, as our results suggest. The specific barriers that most notably predicted specialty change consideration were lack of time and not knowing how to begin research (P=.001 and P<.001, respectively). Remarkably, our findings revealed that respondents who rated these as moderate or major barriers were 6.3 and 4.7 times more likely to consider changing their specialty choice, respectively. Respondents reporting financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) as at least moderate barriers also were 2.2 to 3.5 times more likely to consider a specialty change (eTable 4 and eFigure 4). Additionally, barriers such as limited access to research opportunities (R²=−.27; P=.002), lack of mentorship (R2=−.22; P=.011), not knowing how to begin research (R2=−.19; P=.025), and institutional limitations (R2=−.18; P=.042) all were associated with lower publication output according to our data (eFigure 3). These findings are especially concerning given current match statistics, where the trajectory of research productivity required for a successful dermatology match continues to rise sharply.3,4
Alarmingly, many of the barriers we identified—linked to both reduced research output and specialty change consideration—are associated with several demographic factors. Higher debt levels predicted greater likelihood of experiencing lack of time, insufficient mentorship, and uncertainty about initiating research, while lower SES was associated with lack of mentorship. These relationships suggest that structural barriers, rather than lack of interest or ability, may create cumulative disadvantages that deter capable candidates from pursuing dermatology or impact their success in the application process.
One potential solution to address the disproportionate emphasis on research quantity would be implementing caps on reportable research products in residency applications (eg, limiting applications to a certain number of publications, abstracts, and presentations). This change could shift applicant focus toward substantive scientific contributions rather than rapid output accumulation.8 The need for such caps was evident in our dataset, which revealed a stark contrast: some respondents reported 30 to 40 publications, while MD/PhD respondents—who dedicate 3 to 5 years to performing quality research—averaged only 7.4 publications. Implementing a research output ceiling could help alleviate barriers for applicants facing institutional and demographic disadvantages while simultaneously boosting the scientific rigor of dermatology research.8
Mitigation Strategies From Applicant Feedback—Our findings emphasize the multifaceted relationship between structural barriers and demographics in dermatology research engagement. While our statistical interpretations have outlined several potential interventions, the applicants’ perspectives on mitigation strategies offer qualitative insight. Although participants did not consistently mark financial cost and lack of mentorship as major barriers (eFigure 1), financial assistance and access to engaged mentors were among the highest-ranked mitigation strategies (eFigure 5), suggesting these resources may be fundamental to overcoming multiple structural challenges. To address these needs comprehensively, we propose a multilevel approach: at the institutional level, dermatology interest groups could establish centralized databases of research opportunities, mentorship programs, and funding sources. At the national level, dermatology organizations could consider expanding grant programs, developing virtual mentorship networks, and creating opportunities for external students through remote research projects or short-term research rotations. These interventions, informed by both our statistical analyses and applicant feedback, could help create more equitable access to research opportunities in dermatology.
Limitations
A major limitation of this study was that potential dermatology candidates who were deterred by barriers and later decided on a different specialty would not be captured in our data. As these candidates may have faced substantial barriers that caused them to choose a different path, their absence from the current data may indicate that the reported results underpredict the effect size of the true population. Another limitation is the absence of a control group, such as applicants to less competitive specialties, which would provide valuable context for whether the barriers identified are unique to dermatology.
Conclusion
Our study provides compelling evidence that research barriers in dermatology residency applications intersect with demographic factors to influence research engagement and career trajectories. Our findings suggest that without targeted intervention, increasing emphasis on research productivity may exacerbate existing disparities in dermatology. Moving forward, a coordinated effort among institutions, dermatology associations, and dermatology residency programs will be fundamental to ensure that research requirements enhance rather than impede the development of a diverse, qualified dermatology workforce.
- Ozair A, Bhat V, Detchou DKE. The US residency selection process after the United States Medical Licensing Examination Step 1 pass/fail change: overview for applicants and educators. JMIR Med Educ. 2023;9:E37069. doi:10.2196/37069
- Patrinely JR Jr, Zakria D, Drolet BC. USMLE Step 1 changes: dermatology program director perspectives and implications. Cutis. 2021;107:293-294. doi:10.12788/cutis.0277
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2022. July 2022. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2022/07/Charting-Outcomes-MD-Seniors-2022_Final.pdf
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2024. August 2024. Accessed February 14, 2024. https://www.nrmp.org/match-data/2024/08/charting-outcomes-characteristics-of-u-s-md-seniors-who-matched-to-their-preferred-specialty-2024-main-residency-match/
- National Resident Matching Program. Charting outcomes in the match: characteristics of applicants who matched to their preferred specialty in the 2007 main residency match. July 2021. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2021/07/chartingoutcomes2007.pdf
- Sanabria-de la Torre R, Quiñones-Vico MI, Ubago-Rodríguez A, et al. Medical students’ interest in research: changing trends during university training. Front Med. 2023;10. doi:10.3389/fmed.2023.1257574
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:7. doi:10.5070/D398p8q1m5
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527. doi:10.4300/JGME-D-23-00262.1
- Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315. doi:10.1016/j.clindermatol.2020.02.005
- Orebi HA, Shahin MR, Awad Allah MT, et al. Medical students’ perceptions, experiences, and barriers towards research implementation at the faculty of medicine, Tanta University. BMC Med Educ. 2023;23:902. doi:10.1186/s12909-023-04884-z
- Jacobsen A, Kabbur G, Freese RL, et al. Socioeconomic factors and financial burdens of research “gap years” for dermatology residency applicants. Int J Womens Dermatol. 2023;9:e099. doi:10.1097/JW9.0000000000000099
- Jung J, Stoff BK, Orenstein LAV. Unpaid research fellowships among dermatology residency applicants. J Am Acad Dermatol. 2022;87:1230-1231. doi:10.1016/j.jaad.2021.12.027
- Rehman R, Shareef SJ, Mohammad TF, et al. Applying to dermatology residency without a home program: advice to medical students in the COVID-19 pandemic and beyond. Clin Dermatol. 2022;40:513-515. doi:10.1016/j.clindermatol.2022.01.003
- Villa NM, Shi VY, Hsiao JL. An underrecognized barrier to the dermatology residency match: lack of a home program. Int J Womens Dermatol. 2021;7:512-513. doi:10.1016/j.ijwd.2021.02.011
- Sekyere NAN, Grimes PE, Roberts WE, et al. Turning the tide: how the Women’s Dermatologic Society leads in diversifying dermatology. Int J Womens Dermatol. 2020;7:135-136. doi:10.1016/j.ijwd.2020.12.012
- Emory School of Medicine. Four phases in four years. Accessed January 17, 2025. https://med.emory.edu/education/programs/md/curriculum/4phases/index.html
- Bhatnagar V, Diaz S, Bucur PA. The need for more mentorship in medical school. Cureus. 2020;12:E7984. doi:10.7759/cureus.7984
- Skin of Color Society. Mentorship. Accessed January 17, 2025. https://skinofcolorsociety.org/what-we-do/mentorship
- American Academy of Dermatology. Diversity Mentorship Program: information for medical students. Accessed January 17, 2025. https://www.aad.org/member/career/awards/diversity
As one of the most competitive specialties in medicine, dermatology presents unique challenges for residency applicants, especially following the shift in United States Medical Licensing Examination (USMLE) Step 1 scoring to a pass/fail format.1,2 Historically, USMLE Step 1 served as a major screening metric for residency programs, with 90% of program directors in 2020 using USMLE Step 1 scores as a primary factor when deciding whether to invite applicants for interviews.1 However, the recent transition to pass/fail has made it much harder for program directors to objectively compare applicants, particularly in dermatology. In a 2020 survey, Patrinely Jr et al2 found that 77.2% of dermatology program directors agreed that this change would make it more difficult to assess candidates objectively. Consequently, research productivity has taken on greater importance as programs seek new ways to distinguish top applicants.1,2
In response to this increased emphasis on research, dermatology applicants have substantially boosted their scholarly output over the past several years. The 2022 and 2024 results from the National Residency Matching Program’s Charting Outcomes survey demonstrated a steady rise in research metrics among applicants across various specialties, with dermatology showing one of the largest increases.3,4 For instance, the average number of abstracts, presentations, and publications for matched allopathic dermatology applicants was 5.7 in 2007.5 This average increased to 20.9 in 20223 and to 27.7 in 2024,4 marking an astonishing 485% increase in 17 years. Interestingly, unmatched dermatology applicants had an average of 19.0 research products in 2024, which was similar to the average of successfully matched applicants just 2 years earlier.3,4
Engaging in research offers benefits beyond building a strong residency application. Specifically, it enhances critical thinking skills and provides hands-on experience in scientific inquiry.6 It allows students to explore dermatology topics of interest and address existing knowledge gaps within the specialty.6 Additionally, it creates opportunities to build meaningful relationships with experienced dermatologists who can guide and support students throughout their careers.7 Despite these benefits, the pursuit of research may be landscaped with obstacles, and the fervent race to obtain high research outputs may overshadow developmental advantages.8 These challenges and demands also could contribute to inequities in the residency selection process, particularly if barriers are influenced by socioeconomic and demographic disparities. As dermatology already ranks as the second least diverse specialty in medicine,9 research requirements that disproportionately disadvantage certain demographic groups risk further widening these concerning representation gaps rather than creating opportunities to address them.
Given these trends in research requirements and their potential impact on applicant success, understanding specific barriers to research engagement is essential for creating equitable opportunities in dermatology. In this study, we aimed to identify barriers to research engagement among dermatology applicants, analyze their relationship with demographic factors, assess their impact on specialty choice and research productivity, and provide actionable solutions to address these obstacles.
Methods
A cross-sectional survey was conducted targeting medical students applying to dermatology residency programs in the United States in the 2025 or 2026 match cycles as well as residents who applied to dermatology residency in the 2021 to 2024 match cycles. The 23-item survey was developed by adapting questions from several validated studies examining research barriers and experiences in medical education.6,7,10,11 Specifically, the survey included questions on demographics and background; research productivity; general research barriers; conference participation accessibility; mentorship access; and quality, career impact, and support needs. Socioeconomic background was measured via a single self-reported item asking participants to select the income class that best reflected their background growing up (low-income, lower-middle, upper-middle, or high-income); no income ranges were provided.
The survey was distributed electronically via Qualtrics between November 11, 2024, and December 30, 2024, through listserves of the Dermatology Interest Group Association (sent directly to medical students) and the Association of Professors of Dermatology (forwarded to residents by program directors). There was no way to determine the number of dermatology applicants and residents reached through either listserve. The surveys were reviewed and approved by the University of Alabama at Birmingham institutional review board (IRB-300013671).
Statistical analyses were conducted using RStudio (Posit, PBC; version 2024.12.0+467). Descriptive statistics characterized participant demographics and quantified barrier scores using frequencies and proportions. We performed regression analyses to examine relationships between demographic factors and barriers using linear regression; the relationship between barriers and research productivity correlation; and the prediction of specialty change consideration using logistic regression. For all analyses, barrier scores were rated on a scale of 0 to 3 (0=not a barrier, 1=minor barrier, 2=moderate barrier, 3=major barrier); R² values were reported to indicate strength of associations, and statistical significance was set at P<.05.
Results
Participant Demographics—A total of 136 participants completed the survey. Among the respondents, 12% identified as from a background of low-income class, 28% lower-middle class, 49% upper-middle class, and 11% high-income class. Additionally, 27% of respondents identified as underrepresented in medicine (URiM). Regarding debt levels (or expected debt levels) upon graduation from medical school, 32% reported no debt, 9% reported $1000 to $49,000 in debt, 5% reported $50,000 to $99,000 in debt, 15% reported $100,000 to $199,000 in debt, 22% reported $200,000 to $299,000 in debt, and 17% reported $300,000 in debt or higher. The majority of respondents (95%) were MD candidates, and the remaining 5% were DO candidates; additionally, 5% were participants in an MD/PhD program (eTable 1).

Respondents represented various stages of training: 13.2% and 16.2% were third- and fourth-year medical students, respectively, while 6.0%, 20.1%, 18.4%, and 22.8% were postgraduate year (PGY) 1, PGY-2, PGY-3, and PGY-4, respectively. A few respondents (2.9%) were participating in a research year or reapplying to dermatology residency (eTable 2).

Research Barriers and Productivity—Respondents were presented with a list of potential barriers and asked to rate each as not a barrier, a minor barrier, a moderate barrier, or a major barrier. The most common barriers (ie, those with >50% of respondents rating them as a moderate or major) included lack of time, limited access to research opportunities, not knowing how to begin research, and lack of mentorship or support. Lack of time and not knowing where to begin research were reported most frequently as major barriers, with 32% of participants identifying them as such. In contrast, barriers such as financial costs and personal obligations were less frequently rated as major barriers (10% and 4%, respectively), although they still were identified as obstacles by many respondents. Interestingly, most respondents (58%) indicated that institutional limitations were not a barrier, but a separate and sizeable proportion (25%) of respondents considered it to be a major barrier (eFigure 1).

The distributions for all research metrics were right-skewed. The total range was 0 to 45 (median, 6) for number of publications (excluding abstracts), 0 to 33 (median, 2) for published abstracts, 0 to 40 (median, 5) for poster publications, and 0 to 20 (median, 2) for oral presentations (eTable 3).

Regression Analysis—Linear regression analysis identified significant relationships between demographic variables (socioeconomic status [SES], URiM status, and debt level) and individual research barriers. The heatmap in eFigure 2 illustrates the strength of these relationships. Higher SES was predictive of lower reported financial barriers (R²=.2317; P<.0001) and lower reported institutional limitations (R²=.0884; P=.0006). A URiM status predicted higher reported financial barriers (R²=.1097; P<.0001) and institutional limitations (R²=.04537; P=.013). Also, higher debt level predicted increased financial barriers (R²=.2099; P<.0001), institutional limitations (R2=.1258; P<.0001), and lack of mentorship (R²=.06553; P=.003).

Next, the data were evaluated for correlative relationships between individual research barriers and research productivity metrics including number of publications, published abstracts and presentations (oral and poster) and total research output. While correlations were weak or nonsignificant between barriers and most research productivity metrics (published abstracts, oral and poster presentations, and total research output), the number of publications was significantly correlated with several research barriers, including limited access to research opportunities (P=.002), not knowing how to begin research (P=.025), lack of mentorship or support (P=.011), and institutional limitations (P=.042). Higher ratings for limited access to research opportunities, not knowing where to begin research, lack of mentorship or support, and institutional limitations all were negatively correlated with total number of publications (R2=−.27, –.19, −.22, and –.18, respectively)(eFigure 3).

Logistic regression analysis examined the impact of research barriers on the likelihood of specialty change consideration. The results, presented in a forest plot, include odds ratios (ORs) and their corresponding 95% CIs and P values. Lack of time (P=.001) and not knowing where to begin research (P<.001) were the strongest predictors of specialty change consideration (OR, 6.3 and 4.7, respectively). Financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) also were significant predictors of specialty change consideration (OR, 2.2, 3.1, and 3.5, respectively). Institutional limitations and personal obligations did not predict specialty change consideration (eTable 4 and eFigure 4).


Mitigation Strategies—Mitigation strategies were ranked by respondents based on their perceived importance on a scale of 1 to 7 (1=most important, 7=least important)(eFigure 5). Respondents considered access to engaged mentors to be the most important mitigation strategy by far, with 95% ranking it in the top 3 (47% of respondents ranked it as the top most important mitigation strategy). Financial assistance was the mitigation strategy with the second highest number of respondents (28%) ranking it as the top strategy. Flexible scheduling during rotations, research training programs or discussions, and peer networking and research collaboration opportunities also were considered by respondents to be important mitigation strategies. Time management support/resources frequently was viewed as the least important mitigation strategy, with 38% of respondents ranking it last.

Comment
Our study revealed notable disparities in research barriers among dermatology applicants, with several demonstrating consistent patterns of association with SES, URiM status, and debt burden. Furthermore, the strong relationship between these barriers and decreased research productivity and specialty change consideration suggests that capable candidates may be deterred from pursuing dermatology due to surmountable obstacles rather than lack of interest or ability.
Impact of Demographic Factors on Research Barriers—All 7 general research barriers surveyed were correlated with distinct demographic predictors. Regression analyses indicated that the barrier of financial cost was significantly predicted by lower SES (R²=.2317; P<.001), URiM status (R²=.1097; P<.001), and higher debt levels (R²=.2099; P<.001)(eFigure 2). These findings are particularly concerning given the trend of dermatology applicants pursuing 1-year research fellowships, many of which are unpaid.12 In fact, Jacobson et al11 found that 71.7% (43/60) of dermatology applicants who pursued a year-long research fellowship experienced financial strain during their fellowship, with many requiring additional loans or drawing from personal savings despite already carrying substantial medical school debt of $200,000 or more. Our findings showcase how financial barriers to research disproportionately affect students from lower socioeconomic backgrounds, those who identify as URiM, and those with higher debt, creating systemic inequities in research access at a time when research productivity is increasingly vital for matching into dermatology. To address these financial barriers, institutions may consider establishing more funded research fellowships or expanding grant programs targeting students from economically disadvantaged and/or underrepresented backgrounds.
Institutional limitations (eg, the absence of a dermatology department) also was a notable barrier that was significantly predicted by lower SES (R²=.0884; P<.001) and URiM status (R²=.04537; P=.013)(eFigure 2). Students at institutions lacking dermatology programs face restricted access to mentorship and research opportunities,13 with our results demonstrating that these barriers disproportionately affect students from underresourced and minority groups. These limitations compound disparities in building competitive residency applications.14 The Women’s Dermatologic Society (WDS) has developed a model for addressing these institutional barriers through its summer research fellowship program for medical students who identify as URiM. By pairing students with WDS mentors who guide them through summer research projects, this initiative addresses access and mentorship gaps for students lacking dermatology departments at their home institution.15 The WDS program serves as a model for other organizations to adopt and expand, with particular attention to including students who identify as URiM as well as those from lower socioeconomic backgrounds.
Our results identified time constraints and lack of experience as notable research barriers. Higher debt levels significantly predicted both lack of time (R²=.03915; P=.021) and not knowing how to begin research (R²=.0572; P=.005)(eFigure 2). These statistical relationships may be explained by students with higher debt levels needing to prioritize paid work over unpaid research opportunities, limiting their engagement in research due to the scarcity of funded positions.12 The data further revealed that personal obligations, particularly family care responsibilities, were significantly predicted by both lower SES (R²=.0539; P=.008) and higher debt level (R²=.03237; P=.036)(eFigure 2). These findings demonstrate how students managing academic demands alongside financial and familial responsibilities may face compounded barriers to research engagement. To address these disparities, medical schools may consider implementing protected research time within their curricula; for example, the Emory University School of Medicine (Atlanta, Georgia) has implemented a Discovery Phase program that provides students with 5 months of protected faculty-mentored research time away from academic demands between their third and fourth years of medical school.16 Integrating similarly structured research periods across medical school curricula could help ensure equitable research opportunities for all students pursuing competitive specialties such as dermatology.8
Access to mentorship is a critical determinant of research engagement and productivity, as mentors provide valuable guidance on navigating research processes and professional development.17 Our analysis revealed that lack of mentorship was predicted by both lower SES (R²=.039; P=.023) and higher debt level (R²=.06553; P=.003)(eFigure 2). Several organizations have developed programs to address these mentorship gaps. The Skin of Color Society pairs medical students with skin of color experts while advancing its mission of increasing diversity in dermatology.18 Similarly, the American Academy of Dermatology founded a diversity mentorship program that connects students who identify as URiM with dermatologist mentors for summer research experiences.19 Notably, the Skin of Color Society’s program allows residents to serve as mentors for medical students. Involving residents and community dermatologists as potential dermatology mentors for medical students not only distributes mentorship demands more sustainably but also increases overall access to dermatology mentors. Our findings indicate that similar programs could be expanded to include more residents and community dermatologists as mentors and to target students from disadvantaged backgrounds, those facing financial constraints, and students who identify as URiM.
Impact of Research Barriers on Career Trajectories—Among survey participants, 35% reported considering changing their specialty choice due to research-related barriers. This substantial percentage likely stems from the escalating pressure to achieve increasingly high research output amidst a lack of sufficient support, time, or tools, as our results suggest. The specific barriers that most notably predicted specialty change consideration were lack of time and not knowing how to begin research (P=.001 and P<.001, respectively). Remarkably, our findings revealed that respondents who rated these as moderate or major barriers were 6.3 and 4.7 times more likely to consider changing their specialty choice, respectively. Respondents reporting financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) as at least moderate barriers also were 2.2 to 3.5 times more likely to consider a specialty change (eTable 4 and eFigure 4). Additionally, barriers such as limited access to research opportunities (R²=−.27; P=.002), lack of mentorship (R2=−.22; P=.011), not knowing how to begin research (R2=−.19; P=.025), and institutional limitations (R2=−.18; P=.042) all were associated with lower publication output according to our data (eFigure 3). These findings are especially concerning given current match statistics, where the trajectory of research productivity required for a successful dermatology match continues to rise sharply.3,4
Alarmingly, many of the barriers we identified—linked to both reduced research output and specialty change consideration—are associated with several demographic factors. Higher debt levels predicted greater likelihood of experiencing lack of time, insufficient mentorship, and uncertainty about initiating research, while lower SES was associated with lack of mentorship. These relationships suggest that structural barriers, rather than lack of interest or ability, may create cumulative disadvantages that deter capable candidates from pursuing dermatology or impact their success in the application process.
One potential solution to address the disproportionate emphasis on research quantity would be implementing caps on reportable research products in residency applications (eg, limiting applications to a certain number of publications, abstracts, and presentations). This change could shift applicant focus toward substantive scientific contributions rather than rapid output accumulation.8 The need for such caps was evident in our dataset, which revealed a stark contrast: some respondents reported 30 to 40 publications, while MD/PhD respondents—who dedicate 3 to 5 years to performing quality research—averaged only 7.4 publications. Implementing a research output ceiling could help alleviate barriers for applicants facing institutional and demographic disadvantages while simultaneously boosting the scientific rigor of dermatology research.8
Mitigation Strategies From Applicant Feedback—Our findings emphasize the multifaceted relationship between structural barriers and demographics in dermatology research engagement. While our statistical interpretations have outlined several potential interventions, the applicants’ perspectives on mitigation strategies offer qualitative insight. Although participants did not consistently mark financial cost and lack of mentorship as major barriers (eFigure 1), financial assistance and access to engaged mentors were among the highest-ranked mitigation strategies (eFigure 5), suggesting these resources may be fundamental to overcoming multiple structural challenges. To address these needs comprehensively, we propose a multilevel approach: at the institutional level, dermatology interest groups could establish centralized databases of research opportunities, mentorship programs, and funding sources. At the national level, dermatology organizations could consider expanding grant programs, developing virtual mentorship networks, and creating opportunities for external students through remote research projects or short-term research rotations. These interventions, informed by both our statistical analyses and applicant feedback, could help create more equitable access to research opportunities in dermatology.
Limitations
A major limitation of this study was that potential dermatology candidates who were deterred by barriers and later decided on a different specialty would not be captured in our data. As these candidates may have faced substantial barriers that caused them to choose a different path, their absence from the current data may indicate that the reported results underpredict the effect size of the true population. Another limitation is the absence of a control group, such as applicants to less competitive specialties, which would provide valuable context for whether the barriers identified are unique to dermatology.
Conclusion
Our study provides compelling evidence that research barriers in dermatology residency applications intersect with demographic factors to influence research engagement and career trajectories. Our findings suggest that without targeted intervention, increasing emphasis on research productivity may exacerbate existing disparities in dermatology. Moving forward, a coordinated effort among institutions, dermatology associations, and dermatology residency programs will be fundamental to ensure that research requirements enhance rather than impede the development of a diverse, qualified dermatology workforce.
As one of the most competitive specialties in medicine, dermatology presents unique challenges for residency applicants, especially following the shift in United States Medical Licensing Examination (USMLE) Step 1 scoring to a pass/fail format.1,2 Historically, USMLE Step 1 served as a major screening metric for residency programs, with 90% of program directors in 2020 using USMLE Step 1 scores as a primary factor when deciding whether to invite applicants for interviews.1 However, the recent transition to pass/fail has made it much harder for program directors to objectively compare applicants, particularly in dermatology. In a 2020 survey, Patrinely Jr et al2 found that 77.2% of dermatology program directors agreed that this change would make it more difficult to assess candidates objectively. Consequently, research productivity has taken on greater importance as programs seek new ways to distinguish top applicants.1,2
In response to this increased emphasis on research, dermatology applicants have substantially boosted their scholarly output over the past several years. The 2022 and 2024 results from the National Residency Matching Program’s Charting Outcomes survey demonstrated a steady rise in research metrics among applicants across various specialties, with dermatology showing one of the largest increases.3,4 For instance, the average number of abstracts, presentations, and publications for matched allopathic dermatology applicants was 5.7 in 2007.5 This average increased to 20.9 in 20223 and to 27.7 in 2024,4 marking an astonishing 485% increase in 17 years. Interestingly, unmatched dermatology applicants had an average of 19.0 research products in 2024, which was similar to the average of successfully matched applicants just 2 years earlier.3,4
Engaging in research offers benefits beyond building a strong residency application. Specifically, it enhances critical thinking skills and provides hands-on experience in scientific inquiry.6 It allows students to explore dermatology topics of interest and address existing knowledge gaps within the specialty.6 Additionally, it creates opportunities to build meaningful relationships with experienced dermatologists who can guide and support students throughout their careers.7 Despite these benefits, the pursuit of research may be landscaped with obstacles, and the fervent race to obtain high research outputs may overshadow developmental advantages.8 These challenges and demands also could contribute to inequities in the residency selection process, particularly if barriers are influenced by socioeconomic and demographic disparities. As dermatology already ranks as the second least diverse specialty in medicine,9 research requirements that disproportionately disadvantage certain demographic groups risk further widening these concerning representation gaps rather than creating opportunities to address them.
Given these trends in research requirements and their potential impact on applicant success, understanding specific barriers to research engagement is essential for creating equitable opportunities in dermatology. In this study, we aimed to identify barriers to research engagement among dermatology applicants, analyze their relationship with demographic factors, assess their impact on specialty choice and research productivity, and provide actionable solutions to address these obstacles.
Methods
A cross-sectional survey was conducted targeting medical students applying to dermatology residency programs in the United States in the 2025 or 2026 match cycles as well as residents who applied to dermatology residency in the 2021 to 2024 match cycles. The 23-item survey was developed by adapting questions from several validated studies examining research barriers and experiences in medical education.6,7,10,11 Specifically, the survey included questions on demographics and background; research productivity; general research barriers; conference participation accessibility; mentorship access; and quality, career impact, and support needs. Socioeconomic background was measured via a single self-reported item asking participants to select the income class that best reflected their background growing up (low-income, lower-middle, upper-middle, or high-income); no income ranges were provided.
The survey was distributed electronically via Qualtrics between November 11, 2024, and December 30, 2024, through listserves of the Dermatology Interest Group Association (sent directly to medical students) and the Association of Professors of Dermatology (forwarded to residents by program directors). There was no way to determine the number of dermatology applicants and residents reached through either listserve. The surveys were reviewed and approved by the University of Alabama at Birmingham institutional review board (IRB-300013671).
Statistical analyses were conducted using RStudio (Posit, PBC; version 2024.12.0+467). Descriptive statistics characterized participant demographics and quantified barrier scores using frequencies and proportions. We performed regression analyses to examine relationships between demographic factors and barriers using linear regression; the relationship between barriers and research productivity correlation; and the prediction of specialty change consideration using logistic regression. For all analyses, barrier scores were rated on a scale of 0 to 3 (0=not a barrier, 1=minor barrier, 2=moderate barrier, 3=major barrier); R² values were reported to indicate strength of associations, and statistical significance was set at P<.05.
Results
Participant Demographics—A total of 136 participants completed the survey. Among the respondents, 12% identified as from a background of low-income class, 28% lower-middle class, 49% upper-middle class, and 11% high-income class. Additionally, 27% of respondents identified as underrepresented in medicine (URiM). Regarding debt levels (or expected debt levels) upon graduation from medical school, 32% reported no debt, 9% reported $1000 to $49,000 in debt, 5% reported $50,000 to $99,000 in debt, 15% reported $100,000 to $199,000 in debt, 22% reported $200,000 to $299,000 in debt, and 17% reported $300,000 in debt or higher. The majority of respondents (95%) were MD candidates, and the remaining 5% were DO candidates; additionally, 5% were participants in an MD/PhD program (eTable 1).

Respondents represented various stages of training: 13.2% and 16.2% were third- and fourth-year medical students, respectively, while 6.0%, 20.1%, 18.4%, and 22.8% were postgraduate year (PGY) 1, PGY-2, PGY-3, and PGY-4, respectively. A few respondents (2.9%) were participating in a research year or reapplying to dermatology residency (eTable 2).

Research Barriers and Productivity—Respondents were presented with a list of potential barriers and asked to rate each as not a barrier, a minor barrier, a moderate barrier, or a major barrier. The most common barriers (ie, those with >50% of respondents rating them as a moderate or major) included lack of time, limited access to research opportunities, not knowing how to begin research, and lack of mentorship or support. Lack of time and not knowing where to begin research were reported most frequently as major barriers, with 32% of participants identifying them as such. In contrast, barriers such as financial costs and personal obligations were less frequently rated as major barriers (10% and 4%, respectively), although they still were identified as obstacles by many respondents. Interestingly, most respondents (58%) indicated that institutional limitations were not a barrier, but a separate and sizeable proportion (25%) of respondents considered it to be a major barrier (eFigure 1).

The distributions for all research metrics were right-skewed. The total range was 0 to 45 (median, 6) for number of publications (excluding abstracts), 0 to 33 (median, 2) for published abstracts, 0 to 40 (median, 5) for poster publications, and 0 to 20 (median, 2) for oral presentations (eTable 3).

Regression Analysis—Linear regression analysis identified significant relationships between demographic variables (socioeconomic status [SES], URiM status, and debt level) and individual research barriers. The heatmap in eFigure 2 illustrates the strength of these relationships. Higher SES was predictive of lower reported financial barriers (R²=.2317; P<.0001) and lower reported institutional limitations (R²=.0884; P=.0006). A URiM status predicted higher reported financial barriers (R²=.1097; P<.0001) and institutional limitations (R²=.04537; P=.013). Also, higher debt level predicted increased financial barriers (R²=.2099; P<.0001), institutional limitations (R2=.1258; P<.0001), and lack of mentorship (R²=.06553; P=.003).

Next, the data were evaluated for correlative relationships between individual research barriers and research productivity metrics including number of publications, published abstracts and presentations (oral and poster) and total research output. While correlations were weak or nonsignificant between barriers and most research productivity metrics (published abstracts, oral and poster presentations, and total research output), the number of publications was significantly correlated with several research barriers, including limited access to research opportunities (P=.002), not knowing how to begin research (P=.025), lack of mentorship or support (P=.011), and institutional limitations (P=.042). Higher ratings for limited access to research opportunities, not knowing where to begin research, lack of mentorship or support, and institutional limitations all were negatively correlated with total number of publications (R2=−.27, –.19, −.22, and –.18, respectively)(eFigure 3).

Logistic regression analysis examined the impact of research barriers on the likelihood of specialty change consideration. The results, presented in a forest plot, include odds ratios (ORs) and their corresponding 95% CIs and P values. Lack of time (P=.001) and not knowing where to begin research (P<.001) were the strongest predictors of specialty change consideration (OR, 6.3 and 4.7, respectively). Financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) also were significant predictors of specialty change consideration (OR, 2.2, 3.1, and 3.5, respectively). Institutional limitations and personal obligations did not predict specialty change consideration (eTable 4 and eFigure 4).


Mitigation Strategies—Mitigation strategies were ranked by respondents based on their perceived importance on a scale of 1 to 7 (1=most important, 7=least important)(eFigure 5). Respondents considered access to engaged mentors to be the most important mitigation strategy by far, with 95% ranking it in the top 3 (47% of respondents ranked it as the top most important mitigation strategy). Financial assistance was the mitigation strategy with the second highest number of respondents (28%) ranking it as the top strategy. Flexible scheduling during rotations, research training programs or discussions, and peer networking and research collaboration opportunities also were considered by respondents to be important mitigation strategies. Time management support/resources frequently was viewed as the least important mitigation strategy, with 38% of respondents ranking it last.

Comment
Our study revealed notable disparities in research barriers among dermatology applicants, with several demonstrating consistent patterns of association with SES, URiM status, and debt burden. Furthermore, the strong relationship between these barriers and decreased research productivity and specialty change consideration suggests that capable candidates may be deterred from pursuing dermatology due to surmountable obstacles rather than lack of interest or ability.
Impact of Demographic Factors on Research Barriers—All 7 general research barriers surveyed were correlated with distinct demographic predictors. Regression analyses indicated that the barrier of financial cost was significantly predicted by lower SES (R²=.2317; P<.001), URiM status (R²=.1097; P<.001), and higher debt levels (R²=.2099; P<.001)(eFigure 2). These findings are particularly concerning given the trend of dermatology applicants pursuing 1-year research fellowships, many of which are unpaid.12 In fact, Jacobson et al11 found that 71.7% (43/60) of dermatology applicants who pursued a year-long research fellowship experienced financial strain during their fellowship, with many requiring additional loans or drawing from personal savings despite already carrying substantial medical school debt of $200,000 or more. Our findings showcase how financial barriers to research disproportionately affect students from lower socioeconomic backgrounds, those who identify as URiM, and those with higher debt, creating systemic inequities in research access at a time when research productivity is increasingly vital for matching into dermatology. To address these financial barriers, institutions may consider establishing more funded research fellowships or expanding grant programs targeting students from economically disadvantaged and/or underrepresented backgrounds.
Institutional limitations (eg, the absence of a dermatology department) also was a notable barrier that was significantly predicted by lower SES (R²=.0884; P<.001) and URiM status (R²=.04537; P=.013)(eFigure 2). Students at institutions lacking dermatology programs face restricted access to mentorship and research opportunities,13 with our results demonstrating that these barriers disproportionately affect students from underresourced and minority groups. These limitations compound disparities in building competitive residency applications.14 The Women’s Dermatologic Society (WDS) has developed a model for addressing these institutional barriers through its summer research fellowship program for medical students who identify as URiM. By pairing students with WDS mentors who guide them through summer research projects, this initiative addresses access and mentorship gaps for students lacking dermatology departments at their home institution.15 The WDS program serves as a model for other organizations to adopt and expand, with particular attention to including students who identify as URiM as well as those from lower socioeconomic backgrounds.
Our results identified time constraints and lack of experience as notable research barriers. Higher debt levels significantly predicted both lack of time (R²=.03915; P=.021) and not knowing how to begin research (R²=.0572; P=.005)(eFigure 2). These statistical relationships may be explained by students with higher debt levels needing to prioritize paid work over unpaid research opportunities, limiting their engagement in research due to the scarcity of funded positions.12 The data further revealed that personal obligations, particularly family care responsibilities, were significantly predicted by both lower SES (R²=.0539; P=.008) and higher debt level (R²=.03237; P=.036)(eFigure 2). These findings demonstrate how students managing academic demands alongside financial and familial responsibilities may face compounded barriers to research engagement. To address these disparities, medical schools may consider implementing protected research time within their curricula; for example, the Emory University School of Medicine (Atlanta, Georgia) has implemented a Discovery Phase program that provides students with 5 months of protected faculty-mentored research time away from academic demands between their third and fourth years of medical school.16 Integrating similarly structured research periods across medical school curricula could help ensure equitable research opportunities for all students pursuing competitive specialties such as dermatology.8
Access to mentorship is a critical determinant of research engagement and productivity, as mentors provide valuable guidance on navigating research processes and professional development.17 Our analysis revealed that lack of mentorship was predicted by both lower SES (R²=.039; P=.023) and higher debt level (R²=.06553; P=.003)(eFigure 2). Several organizations have developed programs to address these mentorship gaps. The Skin of Color Society pairs medical students with skin of color experts while advancing its mission of increasing diversity in dermatology.18 Similarly, the American Academy of Dermatology founded a diversity mentorship program that connects students who identify as URiM with dermatologist mentors for summer research experiences.19 Notably, the Skin of Color Society’s program allows residents to serve as mentors for medical students. Involving residents and community dermatologists as potential dermatology mentors for medical students not only distributes mentorship demands more sustainably but also increases overall access to dermatology mentors. Our findings indicate that similar programs could be expanded to include more residents and community dermatologists as mentors and to target students from disadvantaged backgrounds, those facing financial constraints, and students who identify as URiM.
Impact of Research Barriers on Career Trajectories—Among survey participants, 35% reported considering changing their specialty choice due to research-related barriers. This substantial percentage likely stems from the escalating pressure to achieve increasingly high research output amidst a lack of sufficient support, time, or tools, as our results suggest. The specific barriers that most notably predicted specialty change consideration were lack of time and not knowing how to begin research (P=.001 and P<.001, respectively). Remarkably, our findings revealed that respondents who rated these as moderate or major barriers were 6.3 and 4.7 times more likely to consider changing their specialty choice, respectively. Respondents reporting financial cost (P=.043), limited access to research opportunities (P=.006), and lack of mentorship or support (P=.001) as at least moderate barriers also were 2.2 to 3.5 times more likely to consider a specialty change (eTable 4 and eFigure 4). Additionally, barriers such as limited access to research opportunities (R²=−.27; P=.002), lack of mentorship (R2=−.22; P=.011), not knowing how to begin research (R2=−.19; P=.025), and institutional limitations (R2=−.18; P=.042) all were associated with lower publication output according to our data (eFigure 3). These findings are especially concerning given current match statistics, where the trajectory of research productivity required for a successful dermatology match continues to rise sharply.3,4
Alarmingly, many of the barriers we identified—linked to both reduced research output and specialty change consideration—are associated with several demographic factors. Higher debt levels predicted greater likelihood of experiencing lack of time, insufficient mentorship, and uncertainty about initiating research, while lower SES was associated with lack of mentorship. These relationships suggest that structural barriers, rather than lack of interest or ability, may create cumulative disadvantages that deter capable candidates from pursuing dermatology or impact their success in the application process.
One potential solution to address the disproportionate emphasis on research quantity would be implementing caps on reportable research products in residency applications (eg, limiting applications to a certain number of publications, abstracts, and presentations). This change could shift applicant focus toward substantive scientific contributions rather than rapid output accumulation.8 The need for such caps was evident in our dataset, which revealed a stark contrast: some respondents reported 30 to 40 publications, while MD/PhD respondents—who dedicate 3 to 5 years to performing quality research—averaged only 7.4 publications. Implementing a research output ceiling could help alleviate barriers for applicants facing institutional and demographic disadvantages while simultaneously boosting the scientific rigor of dermatology research.8
Mitigation Strategies From Applicant Feedback—Our findings emphasize the multifaceted relationship between structural barriers and demographics in dermatology research engagement. While our statistical interpretations have outlined several potential interventions, the applicants’ perspectives on mitigation strategies offer qualitative insight. Although participants did not consistently mark financial cost and lack of mentorship as major barriers (eFigure 1), financial assistance and access to engaged mentors were among the highest-ranked mitigation strategies (eFigure 5), suggesting these resources may be fundamental to overcoming multiple structural challenges. To address these needs comprehensively, we propose a multilevel approach: at the institutional level, dermatology interest groups could establish centralized databases of research opportunities, mentorship programs, and funding sources. At the national level, dermatology organizations could consider expanding grant programs, developing virtual mentorship networks, and creating opportunities for external students through remote research projects or short-term research rotations. These interventions, informed by both our statistical analyses and applicant feedback, could help create more equitable access to research opportunities in dermatology.
Limitations
A major limitation of this study was that potential dermatology candidates who were deterred by barriers and later decided on a different specialty would not be captured in our data. As these candidates may have faced substantial barriers that caused them to choose a different path, their absence from the current data may indicate that the reported results underpredict the effect size of the true population. Another limitation is the absence of a control group, such as applicants to less competitive specialties, which would provide valuable context for whether the barriers identified are unique to dermatology.
Conclusion
Our study provides compelling evidence that research barriers in dermatology residency applications intersect with demographic factors to influence research engagement and career trajectories. Our findings suggest that without targeted intervention, increasing emphasis on research productivity may exacerbate existing disparities in dermatology. Moving forward, a coordinated effort among institutions, dermatology associations, and dermatology residency programs will be fundamental to ensure that research requirements enhance rather than impede the development of a diverse, qualified dermatology workforce.
- Ozair A, Bhat V, Detchou DKE. The US residency selection process after the United States Medical Licensing Examination Step 1 pass/fail change: overview for applicants and educators. JMIR Med Educ. 2023;9:E37069. doi:10.2196/37069
- Patrinely JR Jr, Zakria D, Drolet BC. USMLE Step 1 changes: dermatology program director perspectives and implications. Cutis. 2021;107:293-294. doi:10.12788/cutis.0277
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2022. July 2022. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2022/07/Charting-Outcomes-MD-Seniors-2022_Final.pdf
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2024. August 2024. Accessed February 14, 2024. https://www.nrmp.org/match-data/2024/08/charting-outcomes-characteristics-of-u-s-md-seniors-who-matched-to-their-preferred-specialty-2024-main-residency-match/
- National Resident Matching Program. Charting outcomes in the match: characteristics of applicants who matched to their preferred specialty in the 2007 main residency match. July 2021. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2021/07/chartingoutcomes2007.pdf
- Sanabria-de la Torre R, Quiñones-Vico MI, Ubago-Rodríguez A, et al. Medical students’ interest in research: changing trends during university training. Front Med. 2023;10. doi:10.3389/fmed.2023.1257574
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:7. doi:10.5070/D398p8q1m5
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527. doi:10.4300/JGME-D-23-00262.1
- Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315. doi:10.1016/j.clindermatol.2020.02.005
- Orebi HA, Shahin MR, Awad Allah MT, et al. Medical students’ perceptions, experiences, and barriers towards research implementation at the faculty of medicine, Tanta University. BMC Med Educ. 2023;23:902. doi:10.1186/s12909-023-04884-z
- Jacobsen A, Kabbur G, Freese RL, et al. Socioeconomic factors and financial burdens of research “gap years” for dermatology residency applicants. Int J Womens Dermatol. 2023;9:e099. doi:10.1097/JW9.0000000000000099
- Jung J, Stoff BK, Orenstein LAV. Unpaid research fellowships among dermatology residency applicants. J Am Acad Dermatol. 2022;87:1230-1231. doi:10.1016/j.jaad.2021.12.027
- Rehman R, Shareef SJ, Mohammad TF, et al. Applying to dermatology residency without a home program: advice to medical students in the COVID-19 pandemic and beyond. Clin Dermatol. 2022;40:513-515. doi:10.1016/j.clindermatol.2022.01.003
- Villa NM, Shi VY, Hsiao JL. An underrecognized barrier to the dermatology residency match: lack of a home program. Int J Womens Dermatol. 2021;7:512-513. doi:10.1016/j.ijwd.2021.02.011
- Sekyere NAN, Grimes PE, Roberts WE, et al. Turning the tide: how the Women’s Dermatologic Society leads in diversifying dermatology. Int J Womens Dermatol. 2020;7:135-136. doi:10.1016/j.ijwd.2020.12.012
- Emory School of Medicine. Four phases in four years. Accessed January 17, 2025. https://med.emory.edu/education/programs/md/curriculum/4phases/index.html
- Bhatnagar V, Diaz S, Bucur PA. The need for more mentorship in medical school. Cureus. 2020;12:E7984. doi:10.7759/cureus.7984
- Skin of Color Society. Mentorship. Accessed January 17, 2025. https://skinofcolorsociety.org/what-we-do/mentorship
- American Academy of Dermatology. Diversity Mentorship Program: information for medical students. Accessed January 17, 2025. https://www.aad.org/member/career/awards/diversity
- Ozair A, Bhat V, Detchou DKE. The US residency selection process after the United States Medical Licensing Examination Step 1 pass/fail change: overview for applicants and educators. JMIR Med Educ. 2023;9:E37069. doi:10.2196/37069
- Patrinely JR Jr, Zakria D, Drolet BC. USMLE Step 1 changes: dermatology program director perspectives and implications. Cutis. 2021;107:293-294. doi:10.12788/cutis.0277
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2022. July 2022. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2022/07/Charting-Outcomes-MD-Seniors-2022_Final.pdf
- National Resident Matching Program. Charting outcomes in the match: US MD seniors, 2024. August 2024. Accessed February 14, 2024. https://www.nrmp.org/match-data/2024/08/charting-outcomes-characteristics-of-u-s-md-seniors-who-matched-to-their-preferred-specialty-2024-main-residency-match/
- National Resident Matching Program. Charting outcomes in the match: characteristics of applicants who matched to their preferred specialty in the 2007 main residency match. July 2021. Accessed February 14, 2024. https://www.nrmp.org/wp-content/uploads/2021/07/chartingoutcomes2007.pdf
- Sanabria-de la Torre R, Quiñones-Vico MI, Ubago-Rodríguez A, et al. Medical students’ interest in research: changing trends during university training. Front Med. 2023;10. doi:10.3389/fmed.2023.1257574
- Alikhan A, Sivamani RK, Mutizwa MM, et al. Advice for medical students interested in dermatology: perspectives from fourth year students who matched. Dermatol Online J. 2009;15:7. doi:10.5070/D398p8q1m5
- Elliott B, Carmody JB. Publish or perish: the research arms race in residency selection. J Grad Med Educ. 2023;15:524-527. doi:10.4300/JGME-D-23-00262.1
- Akhiyat S, Cardwell L, Sokumbi O. Why dermatology is the second least diverse specialty in medicine: how did we get here? Clin Dermatol. 2020;38:310-315. doi:10.1016/j.clindermatol.2020.02.005
- Orebi HA, Shahin MR, Awad Allah MT, et al. Medical students’ perceptions, experiences, and barriers towards research implementation at the faculty of medicine, Tanta University. BMC Med Educ. 2023;23:902. doi:10.1186/s12909-023-04884-z
- Jacobsen A, Kabbur G, Freese RL, et al. Socioeconomic factors and financial burdens of research “gap years” for dermatology residency applicants. Int J Womens Dermatol. 2023;9:e099. doi:10.1097/JW9.0000000000000099
- Jung J, Stoff BK, Orenstein LAV. Unpaid research fellowships among dermatology residency applicants. J Am Acad Dermatol. 2022;87:1230-1231. doi:10.1016/j.jaad.2021.12.027
- Rehman R, Shareef SJ, Mohammad TF, et al. Applying to dermatology residency without a home program: advice to medical students in the COVID-19 pandemic and beyond. Clin Dermatol. 2022;40:513-515. doi:10.1016/j.clindermatol.2022.01.003
- Villa NM, Shi VY, Hsiao JL. An underrecognized barrier to the dermatology residency match: lack of a home program. Int J Womens Dermatol. 2021;7:512-513. doi:10.1016/j.ijwd.2021.02.011
- Sekyere NAN, Grimes PE, Roberts WE, et al. Turning the tide: how the Women’s Dermatologic Society leads in diversifying dermatology. Int J Womens Dermatol. 2020;7:135-136. doi:10.1016/j.ijwd.2020.12.012
- Emory School of Medicine. Four phases in four years. Accessed January 17, 2025. https://med.emory.edu/education/programs/md/curriculum/4phases/index.html
- Bhatnagar V, Diaz S, Bucur PA. The need for more mentorship in medical school. Cureus. 2020;12:E7984. doi:10.7759/cureus.7984
- Skin of Color Society. Mentorship. Accessed January 17, 2025. https://skinofcolorsociety.org/what-we-do/mentorship
- American Academy of Dermatology. Diversity Mentorship Program: information for medical students. Accessed January 17, 2025. https://www.aad.org/member/career/awards/diversity
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
How Increasing Research Demands Threaten Equity in Dermatology Residency Selection and Strategies for Reform
Practice Points
- Dermatology programs should establish sustainable mentorship networks incorporating faculty, residents, and community dermatologists, as most applicants ranked access to engaged mentors as a top priority for overcoming research barriers.
- Protected research time and funding support for projects are critical, particularly since applicants reporting lack of time and financial barriers were more likely to consider changing their specialty choice.
- Programs should consider implementing caps on reportable research products in residency applications to shift emphasis from quantity to quality while helping address demographic disparities in research access.
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Cydnidae is a family of small to medium-sized shield bugs with spiny legs that commonly are known as burrowing bugs (or burrower bugs). The family Cydnidae includes more than 100 genera and approximately 600 species worldwide.1 These insects are arthropods of the order Hemiptera (suborder: Heteroptera; superfamily: Pentatomoidae) and largely are concentrated in tropical and temperate regions. Approximately 145 species have been recorded in the Neotropical Region and have been included in the subfamilies Amnestinae, Cephalocteinae, and Sehirinae, in addition to Cydnidae.2 Burrowing bugs are ovoid in shape and 2 to 20 mm in length and morphologically are well adapted for burrowing. Their life span is 100 to 300 days. Being phytophagous, they burrow to feed on plants and roots. Adult burrowing bugs have wings and can fly. They have specialized glands located in either the abdomen (nymph) or thorax (adult) that secrete odorous chemicals for self-protection.3 The secretions contain hydrocarbonates that function as repellents and danger signals, can cause paralysis in prey, and act as a chemoattractant for mates.4-6 They also cause hyperpigmentation upon contact with the skin.
In this article, we present a series of cases from the same community to demonstrate the characteristic features of hyperpigmented macules caused by exposure to burrowing bugs. Dermatologists should be aware of this entity to prevent misdiagnosis and unnecessary investigations and treatment.
Case Series
A 36-year-old woman and 6 children (age range, 6-12 years) presented with a widespread, acute, brown-pigmented, macular eruption with lesions that increased in number over a 1-week period. All 7 patients resided in the same locality and were otherwise systemically healthy. Initially, the index case, a 7-year-old girl, was referred to our tertiary care center by a dermatologist with a provisional diagnosis of idiopathic macular eruptive pigmentation. The patient’s mother recalled noticing a tiny black insect on the child's scalp that left pigment on the skin when she crushed it between her fingers. The rest of the patients presented over the next few days: 3 of the children belonged to the same household as the index case, and there was history of all 6 children playing in the neighborhood park during late evening hours. The adult patient was the parent of one of the affected children. The lesions were associated with mild itching and tingling in 3 children but were asymptomatic in the other patients.
Clinical examination of the patients revealed multiple dark- to light-brown, discrete, irregularly shaped macules over the trunk, arms, and soles (eFigure 1). Dermoscopic examination of a pigmented macule showed an irregularly shaped, brownish, structureless area with accentuation of the pigment at skin creases and perieccrine pigmentation (eFigure 2). The pigmentation was unaffected by rubbing with alcohol or water. Clinicoepidemiologic parameters of the patients are summarized in the eTable.
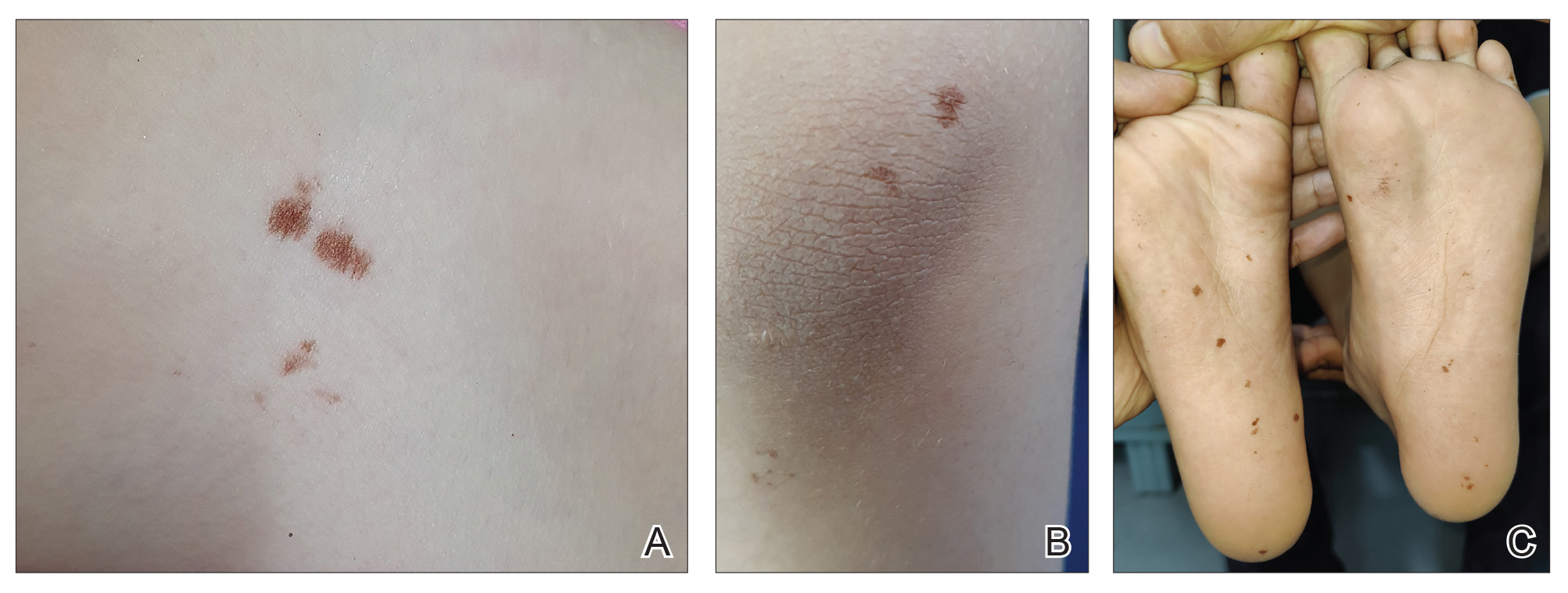
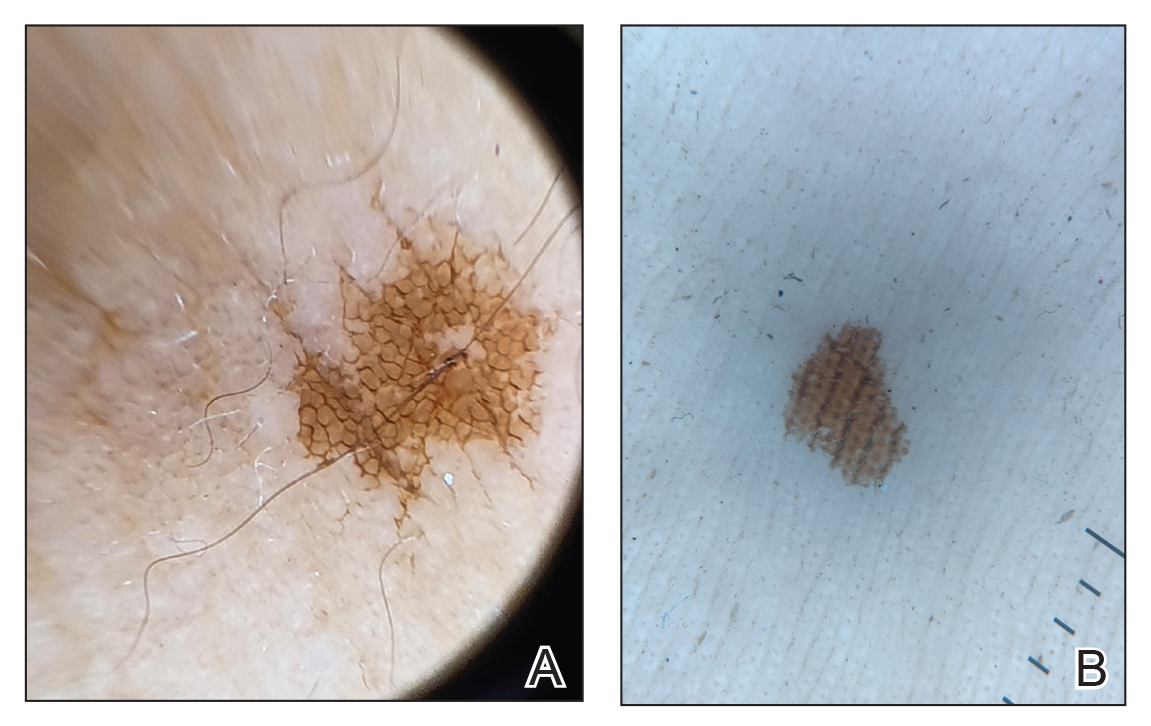
One of the children’s parents conducted a geological examination of the ground in the neighborhood park during evening hours and found tiny burrowing bugs (eFigure 3). When crushed between the fingers, these insects left a similar brownish hyperpigmentation on the skin. The parents were counseled on the nature of the eruption, and the patients were kept under observation for 2 weeks. On follow-up after 5 days, the lesions showed markedly decreased intensity of hyperpigmentation, and no new lesions were observed in any of the 7 patients.

Comment
Pentatomoidae insects generally are benign and harmless to humans. There have been isolated reports of erythematous plaques caused by Antiteuchus mixtus and Edessa maculate.7 Malhotra et al8 reported the first known series of cases with Cydnidae insect–induced hyperpigmented macules. The reported patients presented with asymptomatic, brown, hyperpigmented macules over exposed sites such as the feet, neck, and chest. All the cases occurred during the monsoon season in tropical and temperate regions of the world, and the patients were characteristically clustered in similar geographic areas. The causative insect was identified as Chilocoris assmuthi Breddin, 1904, belonging to the family Cydnidae. When it was crushed between the fingers, the skin became hyperpigmented, confirming the role of the secretions from the insect in the etiology.8
A second case was described by Sonthalia,9 who also described the dermoscopic features of hyperpigmented macules caused by burrowing bugs. The lesions showed a stuck-on, clustered appearance of ovoid and bizarre pigmented clods, globules, and granules.9 Although the lesions occur mainly over exposed sites, pigmented macules occurring over unusual sites such as the abdomen and back also have been reported in association with burrowing bugs.10 Characteristically, the lesions initially are faint and darken with time and usually fade within a week. They can be rubbed off with acetone but persist when washed with soap and water. The fleeting nature of the pigmentation also has led to the term transient pseudo-lentigines sign to describe hyperpigmentation caused by burrowing bugs.11
Soil and plants are burrowing bugs’ natural habitats, and the insects typically are seen in vegetation-rich, moist areas adjoining human dwellings (eg, parks, gardens), where clusters of cases can occur. These insects proliferate during the monsoon season in tropical and temperate areas, leading to more cases occurring during these months.
Compared to prior reports,8,9 a few of our patients had predominant trunk and neck involvement with an occasional tingling sensation or pruritus while the rest were asymptomatic. Dermoscopic features from our patients shared similar reported features of Cydnidae pigmentation.4,5 The accentuation of pigment over skin creases seen on dermoscopy was due to accumulation of Cydnidae secretion at these sites.
The differential diagnosis commonly includes idiopathic macular eruptive pigmentation, which is characterized by an asymptomatic progressive eruption of hyperpigmented macules over the trunk that persists from a few months up to 3 years. Other conditions in the differential include benign conditions such as acral benign melanocytic nevi, lentigines, pigmented purpuric dermatosis, and postinflammatory hyperpigmentation, as well as malignant conditions such as acral melanoma. Dermoscopy is a helpful, easy-to-use tool in differentiating these pigmentation disorders, obviating the need for an invasive investigation such as histopathologic analysis. Simultaneous involvement in a group of people living together or visiting the same place, abrupt onset, predominant involvement of the exposed sites, characteristic clinical and dermoscopic features, self-limiting course, and timing with the monsoon season should suggest a possibility of Cydnidae dermatitis/pigmentation, which can be confirmed by finding the causative bug in the affected locality.
Management
No specific treatment is required for the pigmentation caused by Cydnidae, as it is self-resolving. The macules can, however, be removed with acetone. Patients must be counseled regarding the benign and fleeting nature of this condition, as the abrupt onset may alarm them of a systemic disease. Affected patients should be advised against walking barefoot in areas where the insects can be found. Spraying insecticides in the affected locality also helps to reduce the presence of burrowing bugs.
- Hosokawa T, Kikuchi Y, Nikoh N, et al. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl Environ Microbiol. 2012; 78:4758-4761.
- Schwertner CF, Nardi C. Burrower bugs (Cydnidae). In: Panizzi A, Grazia J, eds. True Bugs (Heteroptera) of the Neotropics. Entomology in Focus, vol 2. Springer; 2015.
- Lis JA. Burrower bugs of the Old World: a catalogue (Hemiptera: Heteroptera: Cydnidae). Genus (Wroclaw). 1999;10:165-249.
- Hayashi N, Yamamura Y, Ôhama S, et al. Defensive substances from stink bugs of Cydnidae. Experientia. 1976;32:418-419.
- Smith RM. The defensive secretion of the bugs Lampropharadifasciata, Adrisanumeensis, and Tectocorisdiophthalmus from Fiji. NZ J Zool. 1978;5:821-822.
- Krall BS, Zilkowski BW, Kight SL, et al. Chemistry and defensive efficacy of secretion of burrowing bugs. J Chem Ecol. 1997;23:1951-1962.
- Haddad V Jr, Cardoso J, Moraes R. Skin lesions caused by stink bugs (Insecta: Heteroptera: Pentatomidae): first report of dermatological injuries in humans. Wilderness Environ Med. 2002;13:48-50.
- Malhotra AK, Lis JA, Ramam M. Cydnidae (burrowing bug) pigmentation: a novel arthropod dermatosis. JAMA Dermatol. 2015;151:232-233.
- Sonthalia S. Dermoscopy of Cydnidae pigmentation: a novel disorder of pigmentation. Dermatol Pract Concept. 2019;9:228-229.
- Poojary S, Baddireddy K. Demystifying the stinking reddish brown stains through the dermoscope: Cydnidae pigmentation. Indian Dermatol Online J. 2019;10:757-758.
- Amrani A, Das A. Cydnidae pigmentation: unusual location on the abdomen and back. Br J Dermatol. 2021;184:E125.
Cydnidae is a family of small to medium-sized shield bugs with spiny legs that commonly are known as burrowing bugs (or burrower bugs). The family Cydnidae includes more than 100 genera and approximately 600 species worldwide.1 These insects are arthropods of the order Hemiptera (suborder: Heteroptera; superfamily: Pentatomoidae) and largely are concentrated in tropical and temperate regions. Approximately 145 species have been recorded in the Neotropical Region and have been included in the subfamilies Amnestinae, Cephalocteinae, and Sehirinae, in addition to Cydnidae.2 Burrowing bugs are ovoid in shape and 2 to 20 mm in length and morphologically are well adapted for burrowing. Their life span is 100 to 300 days. Being phytophagous, they burrow to feed on plants and roots. Adult burrowing bugs have wings and can fly. They have specialized glands located in either the abdomen (nymph) or thorax (adult) that secrete odorous chemicals for self-protection.3 The secretions contain hydrocarbonates that function as repellents and danger signals, can cause paralysis in prey, and act as a chemoattractant for mates.4-6 They also cause hyperpigmentation upon contact with the skin.
In this article, we present a series of cases from the same community to demonstrate the characteristic features of hyperpigmented macules caused by exposure to burrowing bugs. Dermatologists should be aware of this entity to prevent misdiagnosis and unnecessary investigations and treatment.
Case Series
A 36-year-old woman and 6 children (age range, 6-12 years) presented with a widespread, acute, brown-pigmented, macular eruption with lesions that increased in number over a 1-week period. All 7 patients resided in the same locality and were otherwise systemically healthy. Initially, the index case, a 7-year-old girl, was referred to our tertiary care center by a dermatologist with a provisional diagnosis of idiopathic macular eruptive pigmentation. The patient’s mother recalled noticing a tiny black insect on the child's scalp that left pigment on the skin when she crushed it between her fingers. The rest of the patients presented over the next few days: 3 of the children belonged to the same household as the index case, and there was history of all 6 children playing in the neighborhood park during late evening hours. The adult patient was the parent of one of the affected children. The lesions were associated with mild itching and tingling in 3 children but were asymptomatic in the other patients.
Clinical examination of the patients revealed multiple dark- to light-brown, discrete, irregularly shaped macules over the trunk, arms, and soles (eFigure 1). Dermoscopic examination of a pigmented macule showed an irregularly shaped, brownish, structureless area with accentuation of the pigment at skin creases and perieccrine pigmentation (eFigure 2). The pigmentation was unaffected by rubbing with alcohol or water. Clinicoepidemiologic parameters of the patients are summarized in the eTable.



One of the children’s parents conducted a geological examination of the ground in the neighborhood park during evening hours and found tiny burrowing bugs (eFigure 3). When crushed between the fingers, these insects left a similar brownish hyperpigmentation on the skin. The parents were counseled on the nature of the eruption, and the patients were kept under observation for 2 weeks. On follow-up after 5 days, the lesions showed markedly decreased intensity of hyperpigmentation, and no new lesions were observed in any of the 7 patients.

Comment
Pentatomoidae insects generally are benign and harmless to humans. There have been isolated reports of erythematous plaques caused by Antiteuchus mixtus and Edessa maculate.7 Malhotra et al8 reported the first known series of cases with Cydnidae insect–induced hyperpigmented macules. The reported patients presented with asymptomatic, brown, hyperpigmented macules over exposed sites such as the feet, neck, and chest. All the cases occurred during the monsoon season in tropical and temperate regions of the world, and the patients were characteristically clustered in similar geographic areas. The causative insect was identified as Chilocoris assmuthi Breddin, 1904, belonging to the family Cydnidae. When it was crushed between the fingers, the skin became hyperpigmented, confirming the role of the secretions from the insect in the etiology.8
A second case was described by Sonthalia,9 who also described the dermoscopic features of hyperpigmented macules caused by burrowing bugs. The lesions showed a stuck-on, clustered appearance of ovoid and bizarre pigmented clods, globules, and granules.9 Although the lesions occur mainly over exposed sites, pigmented macules occurring over unusual sites such as the abdomen and back also have been reported in association with burrowing bugs.10 Characteristically, the lesions initially are faint and darken with time and usually fade within a week. They can be rubbed off with acetone but persist when washed with soap and water. The fleeting nature of the pigmentation also has led to the term transient pseudo-lentigines sign to describe hyperpigmentation caused by burrowing bugs.11
Soil and plants are burrowing bugs’ natural habitats, and the insects typically are seen in vegetation-rich, moist areas adjoining human dwellings (eg, parks, gardens), where clusters of cases can occur. These insects proliferate during the monsoon season in tropical and temperate areas, leading to more cases occurring during these months.
Compared to prior reports,8,9 a few of our patients had predominant trunk and neck involvement with an occasional tingling sensation or pruritus while the rest were asymptomatic. Dermoscopic features from our patients shared similar reported features of Cydnidae pigmentation.4,5 The accentuation of pigment over skin creases seen on dermoscopy was due to accumulation of Cydnidae secretion at these sites.
The differential diagnosis commonly includes idiopathic macular eruptive pigmentation, which is characterized by an asymptomatic progressive eruption of hyperpigmented macules over the trunk that persists from a few months up to 3 years. Other conditions in the differential include benign conditions such as acral benign melanocytic nevi, lentigines, pigmented purpuric dermatosis, and postinflammatory hyperpigmentation, as well as malignant conditions such as acral melanoma. Dermoscopy is a helpful, easy-to-use tool in differentiating these pigmentation disorders, obviating the need for an invasive investigation such as histopathologic analysis. Simultaneous involvement in a group of people living together or visiting the same place, abrupt onset, predominant involvement of the exposed sites, characteristic clinical and dermoscopic features, self-limiting course, and timing with the monsoon season should suggest a possibility of Cydnidae dermatitis/pigmentation, which can be confirmed by finding the causative bug in the affected locality.
Management
No specific treatment is required for the pigmentation caused by Cydnidae, as it is self-resolving. The macules can, however, be removed with acetone. Patients must be counseled regarding the benign and fleeting nature of this condition, as the abrupt onset may alarm them of a systemic disease. Affected patients should be advised against walking barefoot in areas where the insects can be found. Spraying insecticides in the affected locality also helps to reduce the presence of burrowing bugs.
Cydnidae is a family of small to medium-sized shield bugs with spiny legs that commonly are known as burrowing bugs (or burrower bugs). The family Cydnidae includes more than 100 genera and approximately 600 species worldwide.1 These insects are arthropods of the order Hemiptera (suborder: Heteroptera; superfamily: Pentatomoidae) and largely are concentrated in tropical and temperate regions. Approximately 145 species have been recorded in the Neotropical Region and have been included in the subfamilies Amnestinae, Cephalocteinae, and Sehirinae, in addition to Cydnidae.2 Burrowing bugs are ovoid in shape and 2 to 20 mm in length and morphologically are well adapted for burrowing. Their life span is 100 to 300 days. Being phytophagous, they burrow to feed on plants and roots. Adult burrowing bugs have wings and can fly. They have specialized glands located in either the abdomen (nymph) or thorax (adult) that secrete odorous chemicals for self-protection.3 The secretions contain hydrocarbonates that function as repellents and danger signals, can cause paralysis in prey, and act as a chemoattractant for mates.4-6 They also cause hyperpigmentation upon contact with the skin.
In this article, we present a series of cases from the same community to demonstrate the characteristic features of hyperpigmented macules caused by exposure to burrowing bugs. Dermatologists should be aware of this entity to prevent misdiagnosis and unnecessary investigations and treatment.
Case Series
A 36-year-old woman and 6 children (age range, 6-12 years) presented with a widespread, acute, brown-pigmented, macular eruption with lesions that increased in number over a 1-week period. All 7 patients resided in the same locality and were otherwise systemically healthy. Initially, the index case, a 7-year-old girl, was referred to our tertiary care center by a dermatologist with a provisional diagnosis of idiopathic macular eruptive pigmentation. The patient’s mother recalled noticing a tiny black insect on the child's scalp that left pigment on the skin when she crushed it between her fingers. The rest of the patients presented over the next few days: 3 of the children belonged to the same household as the index case, and there was history of all 6 children playing in the neighborhood park during late evening hours. The adult patient was the parent of one of the affected children. The lesions were associated with mild itching and tingling in 3 children but were asymptomatic in the other patients.
Clinical examination of the patients revealed multiple dark- to light-brown, discrete, irregularly shaped macules over the trunk, arms, and soles (eFigure 1). Dermoscopic examination of a pigmented macule showed an irregularly shaped, brownish, structureless area with accentuation of the pigment at skin creases and perieccrine pigmentation (eFigure 2). The pigmentation was unaffected by rubbing with alcohol or water. Clinicoepidemiologic parameters of the patients are summarized in the eTable.



One of the children’s parents conducted a geological examination of the ground in the neighborhood park during evening hours and found tiny burrowing bugs (eFigure 3). When crushed between the fingers, these insects left a similar brownish hyperpigmentation on the skin. The parents were counseled on the nature of the eruption, and the patients were kept under observation for 2 weeks. On follow-up after 5 days, the lesions showed markedly decreased intensity of hyperpigmentation, and no new lesions were observed in any of the 7 patients.

Comment
Pentatomoidae insects generally are benign and harmless to humans. There have been isolated reports of erythematous plaques caused by Antiteuchus mixtus and Edessa maculate.7 Malhotra et al8 reported the first known series of cases with Cydnidae insect–induced hyperpigmented macules. The reported patients presented with asymptomatic, brown, hyperpigmented macules over exposed sites such as the feet, neck, and chest. All the cases occurred during the monsoon season in tropical and temperate regions of the world, and the patients were characteristically clustered in similar geographic areas. The causative insect was identified as Chilocoris assmuthi Breddin, 1904, belonging to the family Cydnidae. When it was crushed between the fingers, the skin became hyperpigmented, confirming the role of the secretions from the insect in the etiology.8
A second case was described by Sonthalia,9 who also described the dermoscopic features of hyperpigmented macules caused by burrowing bugs. The lesions showed a stuck-on, clustered appearance of ovoid and bizarre pigmented clods, globules, and granules.9 Although the lesions occur mainly over exposed sites, pigmented macules occurring over unusual sites such as the abdomen and back also have been reported in association with burrowing bugs.10 Characteristically, the lesions initially are faint and darken with time and usually fade within a week. They can be rubbed off with acetone but persist when washed with soap and water. The fleeting nature of the pigmentation also has led to the term transient pseudo-lentigines sign to describe hyperpigmentation caused by burrowing bugs.11
Soil and plants are burrowing bugs’ natural habitats, and the insects typically are seen in vegetation-rich, moist areas adjoining human dwellings (eg, parks, gardens), where clusters of cases can occur. These insects proliferate during the monsoon season in tropical and temperate areas, leading to more cases occurring during these months.
Compared to prior reports,8,9 a few of our patients had predominant trunk and neck involvement with an occasional tingling sensation or pruritus while the rest were asymptomatic. Dermoscopic features from our patients shared similar reported features of Cydnidae pigmentation.4,5 The accentuation of pigment over skin creases seen on dermoscopy was due to accumulation of Cydnidae secretion at these sites.
The differential diagnosis commonly includes idiopathic macular eruptive pigmentation, which is characterized by an asymptomatic progressive eruption of hyperpigmented macules over the trunk that persists from a few months up to 3 years. Other conditions in the differential include benign conditions such as acral benign melanocytic nevi, lentigines, pigmented purpuric dermatosis, and postinflammatory hyperpigmentation, as well as malignant conditions such as acral melanoma. Dermoscopy is a helpful, easy-to-use tool in differentiating these pigmentation disorders, obviating the need for an invasive investigation such as histopathologic analysis. Simultaneous involvement in a group of people living together or visiting the same place, abrupt onset, predominant involvement of the exposed sites, characteristic clinical and dermoscopic features, self-limiting course, and timing with the monsoon season should suggest a possibility of Cydnidae dermatitis/pigmentation, which can be confirmed by finding the causative bug in the affected locality.
Management
No specific treatment is required for the pigmentation caused by Cydnidae, as it is self-resolving. The macules can, however, be removed with acetone. Patients must be counseled regarding the benign and fleeting nature of this condition, as the abrupt onset may alarm them of a systemic disease. Affected patients should be advised against walking barefoot in areas where the insects can be found. Spraying insecticides in the affected locality also helps to reduce the presence of burrowing bugs.
- Hosokawa T, Kikuchi Y, Nikoh N, et al. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl Environ Microbiol. 2012; 78:4758-4761.
- Schwertner CF, Nardi C. Burrower bugs (Cydnidae). In: Panizzi A, Grazia J, eds. True Bugs (Heteroptera) of the Neotropics. Entomology in Focus, vol 2. Springer; 2015.
- Lis JA. Burrower bugs of the Old World: a catalogue (Hemiptera: Heteroptera: Cydnidae). Genus (Wroclaw). 1999;10:165-249.
- Hayashi N, Yamamura Y, Ôhama S, et al. Defensive substances from stink bugs of Cydnidae. Experientia. 1976;32:418-419.
- Smith RM. The defensive secretion of the bugs Lampropharadifasciata, Adrisanumeensis, and Tectocorisdiophthalmus from Fiji. NZ J Zool. 1978;5:821-822.
- Krall BS, Zilkowski BW, Kight SL, et al. Chemistry and defensive efficacy of secretion of burrowing bugs. J Chem Ecol. 1997;23:1951-1962.
- Haddad V Jr, Cardoso J, Moraes R. Skin lesions caused by stink bugs (Insecta: Heteroptera: Pentatomidae): first report of dermatological injuries in humans. Wilderness Environ Med. 2002;13:48-50.
- Malhotra AK, Lis JA, Ramam M. Cydnidae (burrowing bug) pigmentation: a novel arthropod dermatosis. JAMA Dermatol. 2015;151:232-233.
- Sonthalia S. Dermoscopy of Cydnidae pigmentation: a novel disorder of pigmentation. Dermatol Pract Concept. 2019;9:228-229.
- Poojary S, Baddireddy K. Demystifying the stinking reddish brown stains through the dermoscope: Cydnidae pigmentation. Indian Dermatol Online J. 2019;10:757-758.
- Amrani A, Das A. Cydnidae pigmentation: unusual location on the abdomen and back. Br J Dermatol. 2021;184:E125.
- Hosokawa T, Kikuchi Y, Nikoh N, et al. Polyphyly of gut symbionts in stinkbugs of the family Cydnidae. Appl Environ Microbiol. 2012; 78:4758-4761.
- Schwertner CF, Nardi C. Burrower bugs (Cydnidae). In: Panizzi A, Grazia J, eds. True Bugs (Heteroptera) of the Neotropics. Entomology in Focus, vol 2. Springer; 2015.
- Lis JA. Burrower bugs of the Old World: a catalogue (Hemiptera: Heteroptera: Cydnidae). Genus (Wroclaw). 1999;10:165-249.
- Hayashi N, Yamamura Y, Ôhama S, et al. Defensive substances from stink bugs of Cydnidae. Experientia. 1976;32:418-419.
- Smith RM. The defensive secretion of the bugs Lampropharadifasciata, Adrisanumeensis, and Tectocorisdiophthalmus from Fiji. NZ J Zool. 1978;5:821-822.
- Krall BS, Zilkowski BW, Kight SL, et al. Chemistry and defensive efficacy of secretion of burrowing bugs. J Chem Ecol. 1997;23:1951-1962.
- Haddad V Jr, Cardoso J, Moraes R. Skin lesions caused by stink bugs (Insecta: Heteroptera: Pentatomidae): first report of dermatological injuries in humans. Wilderness Environ Med. 2002;13:48-50.
- Malhotra AK, Lis JA, Ramam M. Cydnidae (burrowing bug) pigmentation: a novel arthropod dermatosis. JAMA Dermatol. 2015;151:232-233.
- Sonthalia S. Dermoscopy of Cydnidae pigmentation: a novel disorder of pigmentation. Dermatol Pract Concept. 2019;9:228-229.
- Poojary S, Baddireddy K. Demystifying the stinking reddish brown stains through the dermoscope: Cydnidae pigmentation. Indian Dermatol Online J. 2019;10:757-758.
- Amrani A, Das A. Cydnidae pigmentation: unusual location on the abdomen and back. Br J Dermatol. 2021;184:E125.
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Hyperpigmented Macules Caused by Burrowing Bugs (Cydnidae) May Mimic More Serious Conditions
Practice Points
- Burrowing bugs (Cydnidae) are phytophagous and burrow to feed on plants and roots. They are more numerous during the monsoon season in tropical and temperate regions.
- Secretions from burrowing bugs cause asymptomatic, hyperpigmented, irregularly shaped macules suggestive of an exogenous cause that commonly affect clusters of patients from the same geographic locality.
- The lesions are self-limiting and must be differentiated from close mimickers to ensure adequate and appropriate patient counseling.
Epidemiologic and Clinical Evaluation of the Bidirectional Link Between Molluscum Contagiosum and Atopic Dermatitis in Children
Epidemiologic and Clinical Evaluation of the Bidirectional Link Between Molluscum Contagiosum and Atopic Dermatitis in Children
Molluscum contagiosum (MC), which is caused by a DNA virus in the Poxviridae family, is a common viral skin infection that primarily affects children.1-4 The reported incidence and prevalence of MC exhibit notable geographic variation. Worldwide, annual incidence rates per 1000 individuals range from 3.1 to 25, and prevalence ranges from 0.27% to 34.6%.2-7
Molluscum contagiosum is diagnosed clinically and typically manifests as smooth, flesh-colored papules measuring 2 to 6 mm in diameter with central umbilication. It can manifest as a single lesion or multiple clustered lesions, or in a disseminated pattern. The primary mode of transmission is through contact with skin, lesions, or contaminated personal items, or via self-inoculation. The majority of cases are asymptomatic, but in some patients, MC may be associated with pruritus, tenderness, erythema, or irritation. When present, secondary bacterial infections can cause localized inflammation and pain.1,3,4 The pathogenesis hinges on MC virus replication within keratinocytes, disrupting cellular differentiation and keratinization. The virus persists in the host by influencing the immune response through various mechanisms, including interference with signaling pathways, apoptosis inhibition, and antigen presentation disruption.3,4
Molluscum contagiosum typically follows a self-limiting trajectory, resolving over several months to 2 years.3,4 The resolution timeframe is intricately linked to variables such as the patient’s immune profile, lesion burden, and treatment approach. For symptomatic lesions, a variety of treatment options have been described, including physical ablation (eg, cryotherapy, curettage) and topical agents such as potassium hydroxide, cantharidin, imiquimod, and salicylic acid.3,4,8,9
Atopic dermatitis (AD) is a common chronic relapsing inflammatory skin disorder. In the United States, its prevalence ranges from 15% to 30% in children and from 2% to 10% in adults, with ongoing evidence of a growing global incidence.10-14 While AD can emerge at any age, typical onset is during early childhood. The clinical manifestation of AD includes a spectrum of eczematous features, often accompanied by persistent itching. The pathogenesis is multifactorial, involving a complex interplay of genetic, immunologic, and environmental factors. Key contributors to this multifaceted process encompass a compromised epidermal barrier, alterations in the skin microbiome, and an immune dysregulation promoting a type 2 immune response. Epidermal barrier dysfunction can be attributed to various factors, including diminished ceramide production, altered lipid composition, the release of inflammatory mediators, and mechanical damage from the persistent itch-scratch cycle.10-13,15 These factors or their interplay may enhance the susceptibility of patients with AD to infections.
Several studies conducted across various geographic regions examining the relationship between MC and AD have reported variable findings.2,6,7,16-21 Published studies have reported a prevalence of AD in children with MC ranging from 13.2% to 43%.2,6,7,16-21 Although some studies suggest a higher rate of atopy in patients with MC, not all research has confirmed this association.16,21 Dohil et al2 reported a greater number of MC lesions in children with AD than those without an atopic background. Silverberg20 reported that in 10% (5/50) of children with MC, the onset of AD was triggered, and in 22% (11/50) MC was associated with flares of pre-existing AD.
In this study, we aimed to assess MC infection rates in children with AD, analyze the epidemiologic aspects and severity differences between atopic children with and without MC infection, and compare data from atopic and nonatopic children with MC.
Methods
In this retrospective cohort study, we analyzed the medical records of pediatric patients diagnosed with MC, AD, or both conditions at an outpatient dermatology practice in Netanya, HaSharon, Israel, from September 2013 to August 2022. Data were collected from the electronic medical records and included patient demographics, the clinical presentation of MC and/or AD at diagnosis, and the duration of both conditions. Only patients with complete data and at least 6 months of follow-up were included. Key epidemiologic characteristics assessed included patient sex, age at the initial visit, and age at the onset of MC and/or AD. Diagnoses of MC and AD were established through clinical examinations conducted by dermatologists. The clinical evaluation of AD encompassed the assessment of body surface area involvement (categorized as <5%, 5%-10%, or >10%). Atopic dermatitis severity was classified as mild, moderate, or severe using the validated Investigator Global Assessment Scale for Atopic Dermatitis.22 Clinical evaluation of MC included assessment of the number of lesions (categorized as ≤4, 5-9, or ≥10), presence of inflammatory lesions, and resolution times for individual lesions (categorized as <1 week, several weeks, or unknown), as well as the overall resolution time for all lesions (categorized as <6 months, 6-12 months, 13-18 months, or >18 months). The temporal relationship between the appearance of MC and AD also was assessed.
Statistical Analysis—Numbers and percentages were used for categorical variables. Continuous variables were represented by mean and standard deviation. Categorical variables were compared using the χ2 test, and continuous variables between groups were compared using the Student t test. All statistical tests were 2-sided, with statistical significance defined as P≤.05. Statistical analysis was performed using SPSS software version 28 (IBM).
Results
Study Population—A total of 610 children were included in the study; 263 (43%) were female and 347 (57%) were male. The patients ranged in age from 4 months to 10 years, with a mean (SD) age of 4.87 (1.82) years. Five hundred fifty-six (91%) patients had AD, and 336 (55%) had MC. Within this cohort, 274 (45%) children had AD only, 54 (9%) had MC only, and 282 (46%) had both AD and MC. Regarding the temporal sequence, among the 282 children who had both AD and MC, AD preceded MC in 203 (72%) cases, both conditions were diagnosed concomitantly in 43 (15%) cases, and MC preceded AD in 36 (13%) cases. For cases in which the MC diagnosis followed the diagnosis of AD, the mean (SD) time between each diagnosis was 3.17 (1.5) years.
Comparison of Atopic and Nonatopic Children With MC—Although a higher proportion of males were diagnosed with MC (with or without concurrent AD), the differences in sex distribution between the 2 groups did not reach statistical significance. Among all children with MC, the majority (81.5% [274/336]) were aged 1 to 6 years at presentation. Patients with MC as their sole diagnosis had a similar mean age compared with those with concurrent AD. However, a detailed age subgroup analysis revealed a notable distinction: in the group with MC as the sole diagnosis, the majority (95% [51/54]) were younger than 7 years. In contrast, in the combined MC and AD group, MC manifested across a wider age range, with 21% (58/282) of patients being older than 7 years. In MC cases associated with AD, a notably higher lesion count and increased local inflammatory response were observed compared to those without AD. The time for complete resolution of all MC lesions was substantially prolonged in patients with comorbid AD. Specifically, 93% (50/54) of patients with MC without comorbid AD achieved full resolution within 1 year, whereas 52% (146/282) of patients with comorbid AD required more than 1 year for resolution (eTable 1).
Comparison of Atopic Children With and Without MC—Sex, age distribution, and disease duration showed no differences between atopic patients with and without MC. Atopic patients with MC exhibited greater body surface area involvement and higher validated Investigator Global Assessment Scale for Atopic Dermatitis scores compared to atopic patients without MC (eTable 2).
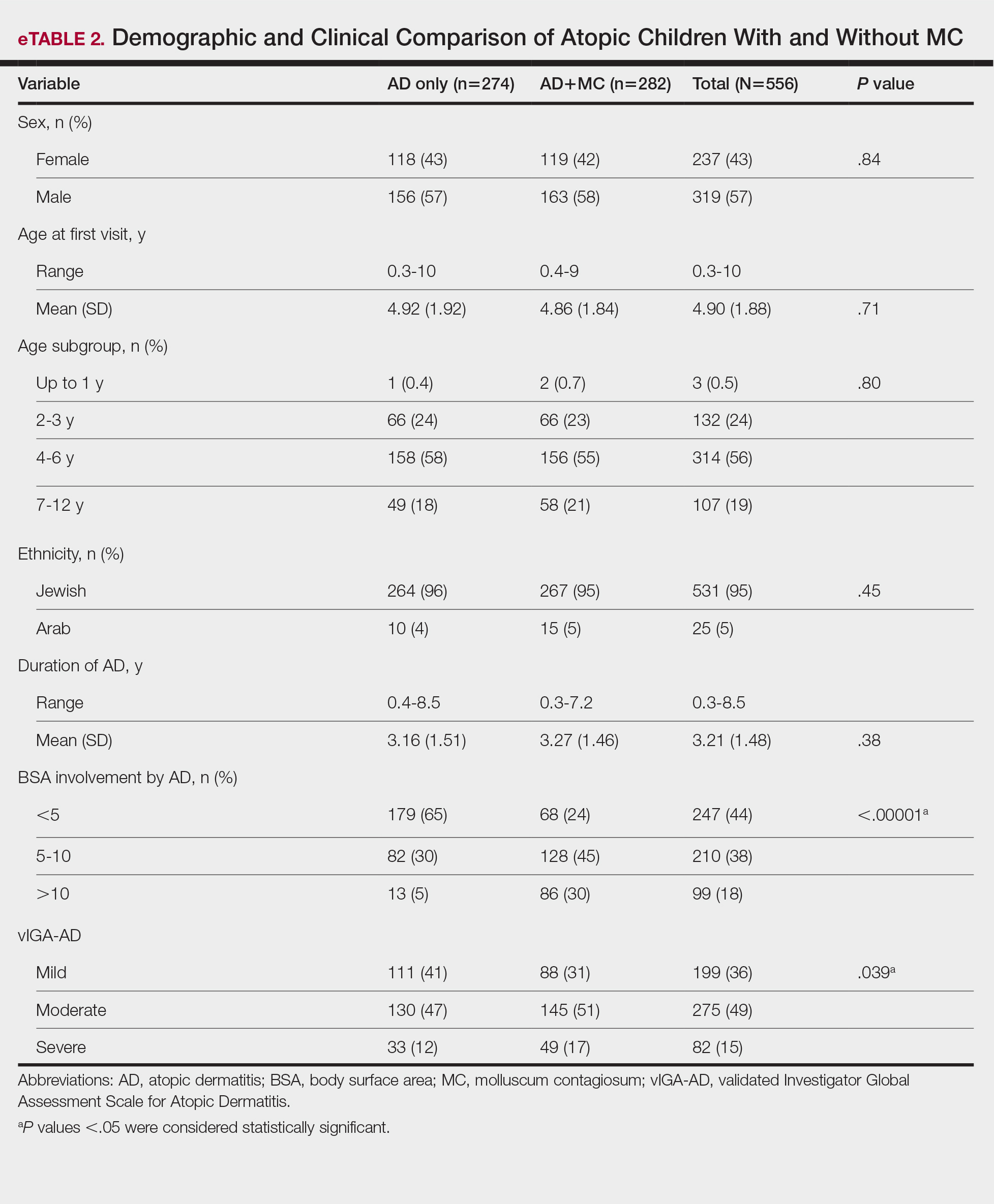
Comment
This study examined the relationship between MC and AD in pediatric patients, revealing a notable correlation and yielding valuable epidemiologic and clinical insights. Consistent with previous research, our study demonstrated a high prevalence of AD in children with MC.2,6,7,16-21 Previous studies indicated AD rates of 13% to 43% in pediatric patients with MC, whereas our study found a higher prevalence (84%), signifying a substantial majority of patients with MC in our cohort had AD. This discrepancy arises from factors such as demographic, genetic, and environmental differences, along with differences in access to medical care, referral practices, and diagnostic approaches across health care systems.14
Our temporal analysis of MC and AD diagnoses offers important insights. In the majority (72% [203/282]) of cases, the diagnosis of AD preceded MC, supporting previous research suggesting that the underlying pathophysiology of AD heightens susceptibility to MC.15,17-20 Less frequently, MC was diagnosed before or concurrently with AD, indicating that MC may occasionally trigger or exacerbate milder or undiagnosed AD, as previously proposed.20
A notable finding in our study was the expanded age range for MC onset in patients with AD, encompassing older age groups compared to patients with MC as their sole diagnosis, possibly due to persistent immune dysregulation. To the best of our knowledge, this specific observation has not been systematically reported or documented in prior cohort studies. Visible skin lesions of MC may have a psychological impact on patients, influencing self-consciousness and causing embarrassment and emotional distress. This may be more pronounced in older children, who are more aware of their appearance and social perceptions.23-25 These considerations should play a role in the management of MC.
Our study revealed that children with AD and MC displayed higher lesion counts, increased local inflammatory responses, and a more protracted resolution period compared to nonatopic children. In more than 50% of children with AD, MC took more than 1 year for resolution, whereas the majority of those without AD achieved resolution within 1 year. These findings may be attributed to AD-related immune dysregulation, influencing the natural course of MC. Consequently, it suggests that while nonatopic children with MC usually are managed through observation, atopic patients may benefit from an intervention-oriented approach.
Comparing atopic patients with and without MC showed a heightened occurrence of severe and extensive AD among those with concurrent MC. Several factors could contribute to this observation. On one hand, there could be a direct association between the extent and severity of AD, leading to an elevated susceptibility to MC. Conversely, MC might exacerbate immunologic dysregulation and intensify skin inflammation in atopic individuals.20 Additionally, itching related to both disorders may exacerbate inflammation and compromise the epidermal barrier, facilitating the spread of MC. This interplay suggests that each condition exacerbates the other in a self-reinforcing cycle. The importance of patient and caregiver education is underscored by recognizing these interactions. To manage both conditions effectively, health care providers should counsel patients and caregivers on maintaining proper skin care practices such as gentle cleansing with mild, fragrance-free products, regular moisturization, and avoidance of irritants, encourage them to avoid scratching, and recommend adopting an active treatment approach.
Our study had notable strengths. Firstly, a substantial sample size enhanced the statistical reliability of our findings. Additionally, valuable insights into the epidemiology and clinical aspects of AD and MC were obtained by utilizing real-world data from an outpatient dermatology practice. In our study, clinical evaluations covered body surface area involvement and disease severity for AD while also assessing lesion counts and the presence of inflammatory lesions for MC. This comprehensive approach facilitated a thorough analysis of both conditions. The extended data collection period not only allowed for observation of their clinical course and duration, but also enabled a detailed assessment of their interplay.
Our study also had several limitations. Primarily, its retrospective design relied on the accuracy and comprehensiveness of medical records, which may have introduced bias. The exclusion of some patients due to incomplete data further increased the potential for selection bias. Additionally, this study was conducted in a single outpatient dermatology practice in Israel, resulting in a study population composed predominantly of Jewish patients (94%), with a minority (6%) of Arab patients. Other ethnic groups, including Black, Asian, and Hispanic populations, were not represented. This reflects the country’s demographic composition rather than an intentional selection bias. However, the limited ethnic diversity reduces the generalizability of our findings. Differences in demographics, coding practices, health care utilization (eg, timeliness of seeking care, access to dermatology services), and treatment strategies also may impact the observed prevalence, clinical characteristics, and patient outcomes. Furthermore, while our study highlighted the potential advantages of a proactive treatment approach for atopic children with MC, it did not evaluate specific treatment protocols. Future research should aim to confirm the most efficacious therapeutic strategies for managing MC in atopic individuals and to include a more diverse population to better understand the applicability of findings across various ethnic groups.
Conclusion
Our study found a high prevalence of AD in children with MC and a strong bidirectional relationship between these conditions. Pediatric patients with AD display a broader age range for MC, greater lesion burden, increased local inflammatory responses, prolonged resolution times, and more extensive and severe AD.
Recognizing the interplay between MC and AD is crucial, highlighting the importance of health care providers educating patients and caregivers. Emphasizing skin hygiene, discouraging scratching, and implementing proactive treatment approaches can enhance the outcomes of both conditions. Further research into the underlying mechanisms of this association and effective therapeutic strategies for MC in atopic individuals is warranted.
Acknowledgments—The authors thank Zvi Segal, MD (Tel Hashomer, Israel) for his insightful contribution to the statistical analysis of the results. We would like to express our appreciation to the dedicated team of the dermatology practice in Netanya for the support throughout the performance of the study. Additionally, we thank all study participants and their parents for their participation and contribution to our research.
- Han H, Smythe C, Yousefian F, et al. Molluscum contagiosum virus evasion of immune surveillance: a review. J Drugs Dermatol. 2023;22182-189.
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305;E1;E2.
- Forbat E, Al-Niaimi F, Ali FR. Molluscum contagiosum: review and update on management. Pediatr Dermatol. 2017;34:504-515.
- Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
- Kakourou T, Zachariades A, Anastasiou T, et al. Molluscum contagiosum in Greek children: a case series. Int J Dermatol. 2005;44:221-223.
- Osio A, Deslandes E, Saada V, et al. Clinical characteristics of molluscum contagiosum in children in a private dermatology practice in the greater Paris area, France: a prospective study in 661 patients. Dermatology. 2011;222:314-320.
- Hebert AA, Bhatia N, Del Rosso JQ. Molluscum contagiosum: epidemiology, considerations, treatment options, and therapeutic gaps. J Clin Aesthet Dermatol. 2023;16(8 Suppl 1):S4-S11.
- Chao YC, Ko MJ, Tsai WC, et al. Comparative efficacy of treatments for molluscum contagiosum: a systematic review and network meta-analysis. J Dtsch Dermatol Ges. 2023;21:587-597.
- Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
- Hale G, Davies E, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2017. part 2: epidemiology, etiology, and risk factors. Clin Exp Dermatol. 2019;44:868-873.
- Tracy A, Bhatti S, Eichenfield LF. Update on pediatric atopic dermatitis. Cutis. 2020;106:143-146.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345-360.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Manti S, Amorini M, Cuppari C, et al. Filaggrin mutations and molluscum contagiosum skin infection in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119446-451.
- Seize M, Ianhez M, Cestari S. A study of the correlation between molluscum contagiosum and atopic dermatitis in children. An Bras Dermatol. 2011;86:663-668.
- Ren Z, Silverberg JI. Association of atopic dermatitis with bacterial, fungal, viral, and sexually transmitted skin infections. Dermatitis. 2020;31:157-164.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Han JH, Yoon JW, Yook HJ, et al. Evaluation of atopic dermatitis and cutaneous infectious disorders using sequential pattern mining: a nationwide population-based cohort study. J Clin Med. 2022;11:3422.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Hayashida S, Furusho N, Uchi H, et al. Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci. 2010;60:173-178.
- Simpson E, Bissonnette R, Eichenfield LF, et al. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): the development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J Am Acad Dermatol. 2020;83:839-846.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Ðurovic´ MR, Jankovic´ J, Spiric´ VT, et al. Does age influence the quality of life in children with atopic dermatitis? PLoS One. 2019;14:E0224618.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
Molluscum contagiosum (MC), which is caused by a DNA virus in the Poxviridae family, is a common viral skin infection that primarily affects children.1-4 The reported incidence and prevalence of MC exhibit notable geographic variation. Worldwide, annual incidence rates per 1000 individuals range from 3.1 to 25, and prevalence ranges from 0.27% to 34.6%.2-7
Molluscum contagiosum is diagnosed clinically and typically manifests as smooth, flesh-colored papules measuring 2 to 6 mm in diameter with central umbilication. It can manifest as a single lesion or multiple clustered lesions, or in a disseminated pattern. The primary mode of transmission is through contact with skin, lesions, or contaminated personal items, or via self-inoculation. The majority of cases are asymptomatic, but in some patients, MC may be associated with pruritus, tenderness, erythema, or irritation. When present, secondary bacterial infections can cause localized inflammation and pain.1,3,4 The pathogenesis hinges on MC virus replication within keratinocytes, disrupting cellular differentiation and keratinization. The virus persists in the host by influencing the immune response through various mechanisms, including interference with signaling pathways, apoptosis inhibition, and antigen presentation disruption.3,4
Molluscum contagiosum typically follows a self-limiting trajectory, resolving over several months to 2 years.3,4 The resolution timeframe is intricately linked to variables such as the patient’s immune profile, lesion burden, and treatment approach. For symptomatic lesions, a variety of treatment options have been described, including physical ablation (eg, cryotherapy, curettage) and topical agents such as potassium hydroxide, cantharidin, imiquimod, and salicylic acid.3,4,8,9
Atopic dermatitis (AD) is a common chronic relapsing inflammatory skin disorder. In the United States, its prevalence ranges from 15% to 30% in children and from 2% to 10% in adults, with ongoing evidence of a growing global incidence.10-14 While AD can emerge at any age, typical onset is during early childhood. The clinical manifestation of AD includes a spectrum of eczematous features, often accompanied by persistent itching. The pathogenesis is multifactorial, involving a complex interplay of genetic, immunologic, and environmental factors. Key contributors to this multifaceted process encompass a compromised epidermal barrier, alterations in the skin microbiome, and an immune dysregulation promoting a type 2 immune response. Epidermal barrier dysfunction can be attributed to various factors, including diminished ceramide production, altered lipid composition, the release of inflammatory mediators, and mechanical damage from the persistent itch-scratch cycle.10-13,15 These factors or their interplay may enhance the susceptibility of patients with AD to infections.
Several studies conducted across various geographic regions examining the relationship between MC and AD have reported variable findings.2,6,7,16-21 Published studies have reported a prevalence of AD in children with MC ranging from 13.2% to 43%.2,6,7,16-21 Although some studies suggest a higher rate of atopy in patients with MC, not all research has confirmed this association.16,21 Dohil et al2 reported a greater number of MC lesions in children with AD than those without an atopic background. Silverberg20 reported that in 10% (5/50) of children with MC, the onset of AD was triggered, and in 22% (11/50) MC was associated with flares of pre-existing AD.
In this study, we aimed to assess MC infection rates in children with AD, analyze the epidemiologic aspects and severity differences between atopic children with and without MC infection, and compare data from atopic and nonatopic children with MC.
Methods
In this retrospective cohort study, we analyzed the medical records of pediatric patients diagnosed with MC, AD, or both conditions at an outpatient dermatology practice in Netanya, HaSharon, Israel, from September 2013 to August 2022. Data were collected from the electronic medical records and included patient demographics, the clinical presentation of MC and/or AD at diagnosis, and the duration of both conditions. Only patients with complete data and at least 6 months of follow-up were included. Key epidemiologic characteristics assessed included patient sex, age at the initial visit, and age at the onset of MC and/or AD. Diagnoses of MC and AD were established through clinical examinations conducted by dermatologists. The clinical evaluation of AD encompassed the assessment of body surface area involvement (categorized as <5%, 5%-10%, or >10%). Atopic dermatitis severity was classified as mild, moderate, or severe using the validated Investigator Global Assessment Scale for Atopic Dermatitis.22 Clinical evaluation of MC included assessment of the number of lesions (categorized as ≤4, 5-9, or ≥10), presence of inflammatory lesions, and resolution times for individual lesions (categorized as <1 week, several weeks, or unknown), as well as the overall resolution time for all lesions (categorized as <6 months, 6-12 months, 13-18 months, or >18 months). The temporal relationship between the appearance of MC and AD also was assessed.
Statistical Analysis—Numbers and percentages were used for categorical variables. Continuous variables were represented by mean and standard deviation. Categorical variables were compared using the χ2 test, and continuous variables between groups were compared using the Student t test. All statistical tests were 2-sided, with statistical significance defined as P≤.05. Statistical analysis was performed using SPSS software version 28 (IBM).
Results
Study Population—A total of 610 children were included in the study; 263 (43%) were female and 347 (57%) were male. The patients ranged in age from 4 months to 10 years, with a mean (SD) age of 4.87 (1.82) years. Five hundred fifty-six (91%) patients had AD, and 336 (55%) had MC. Within this cohort, 274 (45%) children had AD only, 54 (9%) had MC only, and 282 (46%) had both AD and MC. Regarding the temporal sequence, among the 282 children who had both AD and MC, AD preceded MC in 203 (72%) cases, both conditions were diagnosed concomitantly in 43 (15%) cases, and MC preceded AD in 36 (13%) cases. For cases in which the MC diagnosis followed the diagnosis of AD, the mean (SD) time between each diagnosis was 3.17 (1.5) years.
Comparison of Atopic and Nonatopic Children With MC—Although a higher proportion of males were diagnosed with MC (with or without concurrent AD), the differences in sex distribution between the 2 groups did not reach statistical significance. Among all children with MC, the majority (81.5% [274/336]) were aged 1 to 6 years at presentation. Patients with MC as their sole diagnosis had a similar mean age compared with those with concurrent AD. However, a detailed age subgroup analysis revealed a notable distinction: in the group with MC as the sole diagnosis, the majority (95% [51/54]) were younger than 7 years. In contrast, in the combined MC and AD group, MC manifested across a wider age range, with 21% (58/282) of patients being older than 7 years. In MC cases associated with AD, a notably higher lesion count and increased local inflammatory response were observed compared to those without AD. The time for complete resolution of all MC lesions was substantially prolonged in patients with comorbid AD. Specifically, 93% (50/54) of patients with MC without comorbid AD achieved full resolution within 1 year, whereas 52% (146/282) of patients with comorbid AD required more than 1 year for resolution (eTable 1).

Comparison of Atopic Children With and Without MC—Sex, age distribution, and disease duration showed no differences between atopic patients with and without MC. Atopic patients with MC exhibited greater body surface area involvement and higher validated Investigator Global Assessment Scale for Atopic Dermatitis scores compared to atopic patients without MC (eTable 2).

Comment
This study examined the relationship between MC and AD in pediatric patients, revealing a notable correlation and yielding valuable epidemiologic and clinical insights. Consistent with previous research, our study demonstrated a high prevalence of AD in children with MC.2,6,7,16-21 Previous studies indicated AD rates of 13% to 43% in pediatric patients with MC, whereas our study found a higher prevalence (84%), signifying a substantial majority of patients with MC in our cohort had AD. This discrepancy arises from factors such as demographic, genetic, and environmental differences, along with differences in access to medical care, referral practices, and diagnostic approaches across health care systems.14
Our temporal analysis of MC and AD diagnoses offers important insights. In the majority (72% [203/282]) of cases, the diagnosis of AD preceded MC, supporting previous research suggesting that the underlying pathophysiology of AD heightens susceptibility to MC.15,17-20 Less frequently, MC was diagnosed before or concurrently with AD, indicating that MC may occasionally trigger or exacerbate milder or undiagnosed AD, as previously proposed.20
A notable finding in our study was the expanded age range for MC onset in patients with AD, encompassing older age groups compared to patients with MC as their sole diagnosis, possibly due to persistent immune dysregulation. To the best of our knowledge, this specific observation has not been systematically reported or documented in prior cohort studies. Visible skin lesions of MC may have a psychological impact on patients, influencing self-consciousness and causing embarrassment and emotional distress. This may be more pronounced in older children, who are more aware of their appearance and social perceptions.23-25 These considerations should play a role in the management of MC.
Our study revealed that children with AD and MC displayed higher lesion counts, increased local inflammatory responses, and a more protracted resolution period compared to nonatopic children. In more than 50% of children with AD, MC took more than 1 year for resolution, whereas the majority of those without AD achieved resolution within 1 year. These findings may be attributed to AD-related immune dysregulation, influencing the natural course of MC. Consequently, it suggests that while nonatopic children with MC usually are managed through observation, atopic patients may benefit from an intervention-oriented approach.
Comparing atopic patients with and without MC showed a heightened occurrence of severe and extensive AD among those with concurrent MC. Several factors could contribute to this observation. On one hand, there could be a direct association between the extent and severity of AD, leading to an elevated susceptibility to MC. Conversely, MC might exacerbate immunologic dysregulation and intensify skin inflammation in atopic individuals.20 Additionally, itching related to both disorders may exacerbate inflammation and compromise the epidermal barrier, facilitating the spread of MC. This interplay suggests that each condition exacerbates the other in a self-reinforcing cycle. The importance of patient and caregiver education is underscored by recognizing these interactions. To manage both conditions effectively, health care providers should counsel patients and caregivers on maintaining proper skin care practices such as gentle cleansing with mild, fragrance-free products, regular moisturization, and avoidance of irritants, encourage them to avoid scratching, and recommend adopting an active treatment approach.
Our study had notable strengths. Firstly, a substantial sample size enhanced the statistical reliability of our findings. Additionally, valuable insights into the epidemiology and clinical aspects of AD and MC were obtained by utilizing real-world data from an outpatient dermatology practice. In our study, clinical evaluations covered body surface area involvement and disease severity for AD while also assessing lesion counts and the presence of inflammatory lesions for MC. This comprehensive approach facilitated a thorough analysis of both conditions. The extended data collection period not only allowed for observation of their clinical course and duration, but also enabled a detailed assessment of their interplay.
Our study also had several limitations. Primarily, its retrospective design relied on the accuracy and comprehensiveness of medical records, which may have introduced bias. The exclusion of some patients due to incomplete data further increased the potential for selection bias. Additionally, this study was conducted in a single outpatient dermatology practice in Israel, resulting in a study population composed predominantly of Jewish patients (94%), with a minority (6%) of Arab patients. Other ethnic groups, including Black, Asian, and Hispanic populations, were not represented. This reflects the country’s demographic composition rather than an intentional selection bias. However, the limited ethnic diversity reduces the generalizability of our findings. Differences in demographics, coding practices, health care utilization (eg, timeliness of seeking care, access to dermatology services), and treatment strategies also may impact the observed prevalence, clinical characteristics, and patient outcomes. Furthermore, while our study highlighted the potential advantages of a proactive treatment approach for atopic children with MC, it did not evaluate specific treatment protocols. Future research should aim to confirm the most efficacious therapeutic strategies for managing MC in atopic individuals and to include a more diverse population to better understand the applicability of findings across various ethnic groups.
Conclusion
Our study found a high prevalence of AD in children with MC and a strong bidirectional relationship between these conditions. Pediatric patients with AD display a broader age range for MC, greater lesion burden, increased local inflammatory responses, prolonged resolution times, and more extensive and severe AD.
Recognizing the interplay between MC and AD is crucial, highlighting the importance of health care providers educating patients and caregivers. Emphasizing skin hygiene, discouraging scratching, and implementing proactive treatment approaches can enhance the outcomes of both conditions. Further research into the underlying mechanisms of this association and effective therapeutic strategies for MC in atopic individuals is warranted.
Acknowledgments—The authors thank Zvi Segal, MD (Tel Hashomer, Israel) for his insightful contribution to the statistical analysis of the results. We would like to express our appreciation to the dedicated team of the dermatology practice in Netanya for the support throughout the performance of the study. Additionally, we thank all study participants and their parents for their participation and contribution to our research.
Molluscum contagiosum (MC), which is caused by a DNA virus in the Poxviridae family, is a common viral skin infection that primarily affects children.1-4 The reported incidence and prevalence of MC exhibit notable geographic variation. Worldwide, annual incidence rates per 1000 individuals range from 3.1 to 25, and prevalence ranges from 0.27% to 34.6%.2-7
Molluscum contagiosum is diagnosed clinically and typically manifests as smooth, flesh-colored papules measuring 2 to 6 mm in diameter with central umbilication. It can manifest as a single lesion or multiple clustered lesions, or in a disseminated pattern. The primary mode of transmission is through contact with skin, lesions, or contaminated personal items, or via self-inoculation. The majority of cases are asymptomatic, but in some patients, MC may be associated with pruritus, tenderness, erythema, or irritation. When present, secondary bacterial infections can cause localized inflammation and pain.1,3,4 The pathogenesis hinges on MC virus replication within keratinocytes, disrupting cellular differentiation and keratinization. The virus persists in the host by influencing the immune response through various mechanisms, including interference with signaling pathways, apoptosis inhibition, and antigen presentation disruption.3,4
Molluscum contagiosum typically follows a self-limiting trajectory, resolving over several months to 2 years.3,4 The resolution timeframe is intricately linked to variables such as the patient’s immune profile, lesion burden, and treatment approach. For symptomatic lesions, a variety of treatment options have been described, including physical ablation (eg, cryotherapy, curettage) and topical agents such as potassium hydroxide, cantharidin, imiquimod, and salicylic acid.3,4,8,9
Atopic dermatitis (AD) is a common chronic relapsing inflammatory skin disorder. In the United States, its prevalence ranges from 15% to 30% in children and from 2% to 10% in adults, with ongoing evidence of a growing global incidence.10-14 While AD can emerge at any age, typical onset is during early childhood. The clinical manifestation of AD includes a spectrum of eczematous features, often accompanied by persistent itching. The pathogenesis is multifactorial, involving a complex interplay of genetic, immunologic, and environmental factors. Key contributors to this multifaceted process encompass a compromised epidermal barrier, alterations in the skin microbiome, and an immune dysregulation promoting a type 2 immune response. Epidermal barrier dysfunction can be attributed to various factors, including diminished ceramide production, altered lipid composition, the release of inflammatory mediators, and mechanical damage from the persistent itch-scratch cycle.10-13,15 These factors or their interplay may enhance the susceptibility of patients with AD to infections.
Several studies conducted across various geographic regions examining the relationship between MC and AD have reported variable findings.2,6,7,16-21 Published studies have reported a prevalence of AD in children with MC ranging from 13.2% to 43%.2,6,7,16-21 Although some studies suggest a higher rate of atopy in patients with MC, not all research has confirmed this association.16,21 Dohil et al2 reported a greater number of MC lesions in children with AD than those without an atopic background. Silverberg20 reported that in 10% (5/50) of children with MC, the onset of AD was triggered, and in 22% (11/50) MC was associated with flares of pre-existing AD.
In this study, we aimed to assess MC infection rates in children with AD, analyze the epidemiologic aspects and severity differences between atopic children with and without MC infection, and compare data from atopic and nonatopic children with MC.
Methods
In this retrospective cohort study, we analyzed the medical records of pediatric patients diagnosed with MC, AD, or both conditions at an outpatient dermatology practice in Netanya, HaSharon, Israel, from September 2013 to August 2022. Data were collected from the electronic medical records and included patient demographics, the clinical presentation of MC and/or AD at diagnosis, and the duration of both conditions. Only patients with complete data and at least 6 months of follow-up were included. Key epidemiologic characteristics assessed included patient sex, age at the initial visit, and age at the onset of MC and/or AD. Diagnoses of MC and AD were established through clinical examinations conducted by dermatologists. The clinical evaluation of AD encompassed the assessment of body surface area involvement (categorized as <5%, 5%-10%, or >10%). Atopic dermatitis severity was classified as mild, moderate, or severe using the validated Investigator Global Assessment Scale for Atopic Dermatitis.22 Clinical evaluation of MC included assessment of the number of lesions (categorized as ≤4, 5-9, or ≥10), presence of inflammatory lesions, and resolution times for individual lesions (categorized as <1 week, several weeks, or unknown), as well as the overall resolution time for all lesions (categorized as <6 months, 6-12 months, 13-18 months, or >18 months). The temporal relationship between the appearance of MC and AD also was assessed.
Statistical Analysis—Numbers and percentages were used for categorical variables. Continuous variables were represented by mean and standard deviation. Categorical variables were compared using the χ2 test, and continuous variables between groups were compared using the Student t test. All statistical tests were 2-sided, with statistical significance defined as P≤.05. Statistical analysis was performed using SPSS software version 28 (IBM).
Results
Study Population—A total of 610 children were included in the study; 263 (43%) were female and 347 (57%) were male. The patients ranged in age from 4 months to 10 years, with a mean (SD) age of 4.87 (1.82) years. Five hundred fifty-six (91%) patients had AD, and 336 (55%) had MC. Within this cohort, 274 (45%) children had AD only, 54 (9%) had MC only, and 282 (46%) had both AD and MC. Regarding the temporal sequence, among the 282 children who had both AD and MC, AD preceded MC in 203 (72%) cases, both conditions were diagnosed concomitantly in 43 (15%) cases, and MC preceded AD in 36 (13%) cases. For cases in which the MC diagnosis followed the diagnosis of AD, the mean (SD) time between each diagnosis was 3.17 (1.5) years.
Comparison of Atopic and Nonatopic Children With MC—Although a higher proportion of males were diagnosed with MC (with or without concurrent AD), the differences in sex distribution between the 2 groups did not reach statistical significance. Among all children with MC, the majority (81.5% [274/336]) were aged 1 to 6 years at presentation. Patients with MC as their sole diagnosis had a similar mean age compared with those with concurrent AD. However, a detailed age subgroup analysis revealed a notable distinction: in the group with MC as the sole diagnosis, the majority (95% [51/54]) were younger than 7 years. In contrast, in the combined MC and AD group, MC manifested across a wider age range, with 21% (58/282) of patients being older than 7 years. In MC cases associated with AD, a notably higher lesion count and increased local inflammatory response were observed compared to those without AD. The time for complete resolution of all MC lesions was substantially prolonged in patients with comorbid AD. Specifically, 93% (50/54) of patients with MC without comorbid AD achieved full resolution within 1 year, whereas 52% (146/282) of patients with comorbid AD required more than 1 year for resolution (eTable 1).

Comparison of Atopic Children With and Without MC—Sex, age distribution, and disease duration showed no differences between atopic patients with and without MC. Atopic patients with MC exhibited greater body surface area involvement and higher validated Investigator Global Assessment Scale for Atopic Dermatitis scores compared to atopic patients without MC (eTable 2).

Comment
This study examined the relationship between MC and AD in pediatric patients, revealing a notable correlation and yielding valuable epidemiologic and clinical insights. Consistent with previous research, our study demonstrated a high prevalence of AD in children with MC.2,6,7,16-21 Previous studies indicated AD rates of 13% to 43% in pediatric patients with MC, whereas our study found a higher prevalence (84%), signifying a substantial majority of patients with MC in our cohort had AD. This discrepancy arises from factors such as demographic, genetic, and environmental differences, along with differences in access to medical care, referral practices, and diagnostic approaches across health care systems.14
Our temporal analysis of MC and AD diagnoses offers important insights. In the majority (72% [203/282]) of cases, the diagnosis of AD preceded MC, supporting previous research suggesting that the underlying pathophysiology of AD heightens susceptibility to MC.15,17-20 Less frequently, MC was diagnosed before or concurrently with AD, indicating that MC may occasionally trigger or exacerbate milder or undiagnosed AD, as previously proposed.20
A notable finding in our study was the expanded age range for MC onset in patients with AD, encompassing older age groups compared to patients with MC as their sole diagnosis, possibly due to persistent immune dysregulation. To the best of our knowledge, this specific observation has not been systematically reported or documented in prior cohort studies. Visible skin lesions of MC may have a psychological impact on patients, influencing self-consciousness and causing embarrassment and emotional distress. This may be more pronounced in older children, who are more aware of their appearance and social perceptions.23-25 These considerations should play a role in the management of MC.
Our study revealed that children with AD and MC displayed higher lesion counts, increased local inflammatory responses, and a more protracted resolution period compared to nonatopic children. In more than 50% of children with AD, MC took more than 1 year for resolution, whereas the majority of those without AD achieved resolution within 1 year. These findings may be attributed to AD-related immune dysregulation, influencing the natural course of MC. Consequently, it suggests that while nonatopic children with MC usually are managed through observation, atopic patients may benefit from an intervention-oriented approach.
Comparing atopic patients with and without MC showed a heightened occurrence of severe and extensive AD among those with concurrent MC. Several factors could contribute to this observation. On one hand, there could be a direct association between the extent and severity of AD, leading to an elevated susceptibility to MC. Conversely, MC might exacerbate immunologic dysregulation and intensify skin inflammation in atopic individuals.20 Additionally, itching related to both disorders may exacerbate inflammation and compromise the epidermal barrier, facilitating the spread of MC. This interplay suggests that each condition exacerbates the other in a self-reinforcing cycle. The importance of patient and caregiver education is underscored by recognizing these interactions. To manage both conditions effectively, health care providers should counsel patients and caregivers on maintaining proper skin care practices such as gentle cleansing with mild, fragrance-free products, regular moisturization, and avoidance of irritants, encourage them to avoid scratching, and recommend adopting an active treatment approach.
Our study had notable strengths. Firstly, a substantial sample size enhanced the statistical reliability of our findings. Additionally, valuable insights into the epidemiology and clinical aspects of AD and MC were obtained by utilizing real-world data from an outpatient dermatology practice. In our study, clinical evaluations covered body surface area involvement and disease severity for AD while also assessing lesion counts and the presence of inflammatory lesions for MC. This comprehensive approach facilitated a thorough analysis of both conditions. The extended data collection period not only allowed for observation of their clinical course and duration, but also enabled a detailed assessment of their interplay.
Our study also had several limitations. Primarily, its retrospective design relied on the accuracy and comprehensiveness of medical records, which may have introduced bias. The exclusion of some patients due to incomplete data further increased the potential for selection bias. Additionally, this study was conducted in a single outpatient dermatology practice in Israel, resulting in a study population composed predominantly of Jewish patients (94%), with a minority (6%) of Arab patients. Other ethnic groups, including Black, Asian, and Hispanic populations, were not represented. This reflects the country’s demographic composition rather than an intentional selection bias. However, the limited ethnic diversity reduces the generalizability of our findings. Differences in demographics, coding practices, health care utilization (eg, timeliness of seeking care, access to dermatology services), and treatment strategies also may impact the observed prevalence, clinical characteristics, and patient outcomes. Furthermore, while our study highlighted the potential advantages of a proactive treatment approach for atopic children with MC, it did not evaluate specific treatment protocols. Future research should aim to confirm the most efficacious therapeutic strategies for managing MC in atopic individuals and to include a more diverse population to better understand the applicability of findings across various ethnic groups.
Conclusion
Our study found a high prevalence of AD in children with MC and a strong bidirectional relationship between these conditions. Pediatric patients with AD display a broader age range for MC, greater lesion burden, increased local inflammatory responses, prolonged resolution times, and more extensive and severe AD.
Recognizing the interplay between MC and AD is crucial, highlighting the importance of health care providers educating patients and caregivers. Emphasizing skin hygiene, discouraging scratching, and implementing proactive treatment approaches can enhance the outcomes of both conditions. Further research into the underlying mechanisms of this association and effective therapeutic strategies for MC in atopic individuals is warranted.
Acknowledgments—The authors thank Zvi Segal, MD (Tel Hashomer, Israel) for his insightful contribution to the statistical analysis of the results. We would like to express our appreciation to the dedicated team of the dermatology practice in Netanya for the support throughout the performance of the study. Additionally, we thank all study participants and their parents for their participation and contribution to our research.
- Han H, Smythe C, Yousefian F, et al. Molluscum contagiosum virus evasion of immune surveillance: a review. J Drugs Dermatol. 2023;22182-189.
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305;E1;E2.
- Forbat E, Al-Niaimi F, Ali FR. Molluscum contagiosum: review and update on management. Pediatr Dermatol. 2017;34:504-515.
- Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
- Kakourou T, Zachariades A, Anastasiou T, et al. Molluscum contagiosum in Greek children: a case series. Int J Dermatol. 2005;44:221-223.
- Osio A, Deslandes E, Saada V, et al. Clinical characteristics of molluscum contagiosum in children in a private dermatology practice in the greater Paris area, France: a prospective study in 661 patients. Dermatology. 2011;222:314-320.
- Hebert AA, Bhatia N, Del Rosso JQ. Molluscum contagiosum: epidemiology, considerations, treatment options, and therapeutic gaps. J Clin Aesthet Dermatol. 2023;16(8 Suppl 1):S4-S11.
- Chao YC, Ko MJ, Tsai WC, et al. Comparative efficacy of treatments for molluscum contagiosum: a systematic review and network meta-analysis. J Dtsch Dermatol Ges. 2023;21:587-597.
- Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
- Hale G, Davies E, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2017. part 2: epidemiology, etiology, and risk factors. Clin Exp Dermatol. 2019;44:868-873.
- Tracy A, Bhatti S, Eichenfield LF. Update on pediatric atopic dermatitis. Cutis. 2020;106:143-146.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345-360.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Manti S, Amorini M, Cuppari C, et al. Filaggrin mutations and molluscum contagiosum skin infection in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119446-451.
- Seize M, Ianhez M, Cestari S. A study of the correlation between molluscum contagiosum and atopic dermatitis in children. An Bras Dermatol. 2011;86:663-668.
- Ren Z, Silverberg JI. Association of atopic dermatitis with bacterial, fungal, viral, and sexually transmitted skin infections. Dermatitis. 2020;31:157-164.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Han JH, Yoon JW, Yook HJ, et al. Evaluation of atopic dermatitis and cutaneous infectious disorders using sequential pattern mining: a nationwide population-based cohort study. J Clin Med. 2022;11:3422.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Hayashida S, Furusho N, Uchi H, et al. Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci. 2010;60:173-178.
- Simpson E, Bissonnette R, Eichenfield LF, et al. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): the development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J Am Acad Dermatol. 2020;83:839-846.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Ðurovic´ MR, Jankovic´ J, Spiric´ VT, et al. Does age influence the quality of life in children with atopic dermatitis? PLoS One. 2019;14:E0224618.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
- Han H, Smythe C, Yousefian F, et al. Molluscum contagiosum virus evasion of immune surveillance: a review. J Drugs Dermatol. 2023;22182-189.
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54.
- Silverberg NB. Pediatric molluscum: an update. Cutis. 2019;104:301-305;E1;E2.
- Forbat E, Al-Niaimi F, Ali FR. Molluscum contagiosum: review and update on management. Pediatr Dermatol. 2017;34:504-515.
- Olsen JR, Gallacher J, Piguet V, et al. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31:130-136.
- Kakourou T, Zachariades A, Anastasiou T, et al. Molluscum contagiosum in Greek children: a case series. Int J Dermatol. 2005;44:221-223.
- Osio A, Deslandes E, Saada V, et al. Clinical characteristics of molluscum contagiosum in children in a private dermatology practice in the greater Paris area, France: a prospective study in 661 patients. Dermatology. 2011;222:314-320.
- Hebert AA, Bhatia N, Del Rosso JQ. Molluscum contagiosum: epidemiology, considerations, treatment options, and therapeutic gaps. J Clin Aesthet Dermatol. 2023;16(8 Suppl 1):S4-S11.
- Chao YC, Ko MJ, Tsai WC, et al. Comparative efficacy of treatments for molluscum contagiosum: a systematic review and network meta-analysis. J Dtsch Dermatol Ges. 2023;21:587-597.
- Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
- Hale G, Davies E, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2017. part 2: epidemiology, etiology, and risk factors. Clin Exp Dermatol. 2019;44:868-873.
- Tracy A, Bhatti S, Eichenfield LF. Update on pediatric atopic dermatitis. Cutis. 2020;106:143-146.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345-360.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatol Clin. 2017;35:283-289.
- Manti S, Amorini M, Cuppari C, et al. Filaggrin mutations and molluscum contagiosum skin infection in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119446-451.
- Seize M, Ianhez M, Cestari S. A study of the correlation between molluscum contagiosum and atopic dermatitis in children. An Bras Dermatol. 2011;86:663-668.
- Ren Z, Silverberg JI. Association of atopic dermatitis with bacterial, fungal, viral, and sexually transmitted skin infections. Dermatitis. 2020;31:157-164.
- Olsen JR, Piguet V, Gallacher J, et al. Molluscum contagiosum and associations with atopic eczema in children: a retrospective longitudinal study in primary care. Br J Gen Pract. 2016;66:E53-E58.
- Han JH, Yoon JW, Yook HJ, et al. Evaluation of atopic dermatitis and cutaneous infectious disorders using sequential pattern mining: a nationwide population-based cohort study. J Clin Med. 2022;11:3422.
- Silverberg NB. Molluscum contagiosum virus infection can trigger atopic dermatitis disease onset or flare. Cutis. 2018;102:191-194.
- Hayashida S, Furusho N, Uchi H, et al. Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci. 2010;60:173-178.
- Simpson E, Bissonnette R, Eichenfield LF, et al. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD): the development and reliability testing of a novel clinical outcome measurement instrument for the severity of atopic dermatitis. J Am Acad Dermatol. 2020;83:839-846.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195.
- Ðurovic´ MR, Jankovic´ J, Spiric´ VT, et al. Does age influence the quality of life in children with atopic dermatitis? PLoS One. 2019;14:E0224618.
- Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159-166.
Epidemiologic and Clinical Evaluation of the Bidirectional Link Between Molluscum Contagiosum and Atopic Dermatitis in Children
Epidemiologic and Clinical Evaluation of the Bidirectional Link Between Molluscum Contagiosum and Atopic Dermatitis in Children
Practice Points
- There is a high prevalence of atopic dermatitis (AD) in children with molluscum contagiosum, with a strong bidirectional relationship between these conditions.
- Children with AD display a broader age range for molluscum contagiosum, greater lesion burden, increased local inflammatory responses, prolonged resolution time, and more extensive and severe disease.
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.
This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.

This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.

This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Choosing a Job After Graduation: Advice for Residents From Scott Worswick, MD
Choosing a Job After Graduation: Advice for Residents From Scott Worswick, MD
What are the most important things to look at when considering joining a practice after residency?
DR. WORSWICK: When considering a private practice job, I think the most important things to determine might be how much control you will have over your day-to-day work experience (eg, will you be involved in the hiring/ firing of staff, how many rooms will you have in which to see patients, what flexibility exists for your daily schedule), who you will be working with, opportunities for growth and ownership, and the many extraneous things included in your contract (eg, medical insurance, time off, other benefits).
If you are considering joining an academic group, often times many of these things will be out of your control, but you will want to make sure you are finding a program where your teaching or research interests will be supported, that you are choosing a group with people and a mission statement similar to your own, and that you have mentorship available from faculty you want to emulate. There are many fun twists and turns that occur in careers in academic dermatology, so you want to be in a place that will foster your professional interests and allow you to grow and change.
What do academic dermatology programs look for when hiring new junior faculty members?
DR. WORSWICK: I think this depends a lot on time and place. At any given time, a program may need to find a specialist in a particular disease or niche (eg, a mycosis fungoides expert, a pediatric dermatologist, or someone doing hidradenitis suppurativa research). But in general, most academic places are looking to hire people who are excited to care for patients, will work well with the team and support the department’s mission, and enjoy teaching residents and students. For me, much of the fun of being in academics comes from mentorship (as a junior faculty member, this came from being a mentor to residents and students while also being mentored by more senior faculty), teaching, and the ability to care for patients with complicated problems that often require team-based care.
What are some red flags to watch for when considering joining a new practice?
DR. WORSWICK: I think the biggest red flags would be a practice that allows you no control over your schedule and no potential for growth of your compensation. We’ve had many residents choose to work for Kaiser lately, and I think in part that is because Kaiser is very clear regarding what salary, schedule, and expectations are. Fewer and fewer graduating residents are going into solo practice and even dermatologist-owned private practice, but I would encourage residents looking for jobs to consider these models rather than venture capital–funded practices that may not be patient care centered.
How many positions should graduating residents apply for?
DR. WORSWICK: I think this depends a lot on who you are, how specific your preferences are, and what part of the country/world you are looking to practice in. In general, there is a great need for dermatologists, and it shouldn’t be hard to find a job. If you’re in a more saturated urban area, you’re going to want to apply for multiple positions. But if you really know what you want, you may only apply to one practice. I generally advise our residents to consider at least 3 places, if only to compare them to give a better idea of best fit or to ensure that their “top choice” is indeed their top choice.
What are the most important things to look at when considering joining a practice after residency?
DR. WORSWICK: When considering a private practice job, I think the most important things to determine might be how much control you will have over your day-to-day work experience (eg, will you be involved in the hiring/ firing of staff, how many rooms will you have in which to see patients, what flexibility exists for your daily schedule), who you will be working with, opportunities for growth and ownership, and the many extraneous things included in your contract (eg, medical insurance, time off, other benefits).
If you are considering joining an academic group, often times many of these things will be out of your control, but you will want to make sure you are finding a program where your teaching or research interests will be supported, that you are choosing a group with people and a mission statement similar to your own, and that you have mentorship available from faculty you want to emulate. There are many fun twists and turns that occur in careers in academic dermatology, so you want to be in a place that will foster your professional interests and allow you to grow and change.
What do academic dermatology programs look for when hiring new junior faculty members?
DR. WORSWICK: I think this depends a lot on time and place. At any given time, a program may need to find a specialist in a particular disease or niche (eg, a mycosis fungoides expert, a pediatric dermatologist, or someone doing hidradenitis suppurativa research). But in general, most academic places are looking to hire people who are excited to care for patients, will work well with the team and support the department’s mission, and enjoy teaching residents and students. For me, much of the fun of being in academics comes from mentorship (as a junior faculty member, this came from being a mentor to residents and students while also being mentored by more senior faculty), teaching, and the ability to care for patients with complicated problems that often require team-based care.
What are some red flags to watch for when considering joining a new practice?
DR. WORSWICK: I think the biggest red flags would be a practice that allows you no control over your schedule and no potential for growth of your compensation. We’ve had many residents choose to work for Kaiser lately, and I think in part that is because Kaiser is very clear regarding what salary, schedule, and expectations are. Fewer and fewer graduating residents are going into solo practice and even dermatologist-owned private practice, but I would encourage residents looking for jobs to consider these models rather than venture capital–funded practices that may not be patient care centered.
How many positions should graduating residents apply for?
DR. WORSWICK: I think this depends a lot on who you are, how specific your preferences are, and what part of the country/world you are looking to practice in. In general, there is a great need for dermatologists, and it shouldn’t be hard to find a job. If you’re in a more saturated urban area, you’re going to want to apply for multiple positions. But if you really know what you want, you may only apply to one practice. I generally advise our residents to consider at least 3 places, if only to compare them to give a better idea of best fit or to ensure that their “top choice” is indeed their top choice.
What are the most important things to look at when considering joining a practice after residency?
DR. WORSWICK: When considering a private practice job, I think the most important things to determine might be how much control you will have over your day-to-day work experience (eg, will you be involved in the hiring/ firing of staff, how many rooms will you have in which to see patients, what flexibility exists for your daily schedule), who you will be working with, opportunities for growth and ownership, and the many extraneous things included in your contract (eg, medical insurance, time off, other benefits).
If you are considering joining an academic group, often times many of these things will be out of your control, but you will want to make sure you are finding a program where your teaching or research interests will be supported, that you are choosing a group with people and a mission statement similar to your own, and that you have mentorship available from faculty you want to emulate. There are many fun twists and turns that occur in careers in academic dermatology, so you want to be in a place that will foster your professional interests and allow you to grow and change.
What do academic dermatology programs look for when hiring new junior faculty members?
DR. WORSWICK: I think this depends a lot on time and place. At any given time, a program may need to find a specialist in a particular disease or niche (eg, a mycosis fungoides expert, a pediatric dermatologist, or someone doing hidradenitis suppurativa research). But in general, most academic places are looking to hire people who are excited to care for patients, will work well with the team and support the department’s mission, and enjoy teaching residents and students. For me, much of the fun of being in academics comes from mentorship (as a junior faculty member, this came from being a mentor to residents and students while also being mentored by more senior faculty), teaching, and the ability to care for patients with complicated problems that often require team-based care.
What are some red flags to watch for when considering joining a new practice?
DR. WORSWICK: I think the biggest red flags would be a practice that allows you no control over your schedule and no potential for growth of your compensation. We’ve had many residents choose to work for Kaiser lately, and I think in part that is because Kaiser is very clear regarding what salary, schedule, and expectations are. Fewer and fewer graduating residents are going into solo practice and even dermatologist-owned private practice, but I would encourage residents looking for jobs to consider these models rather than venture capital–funded practices that may not be patient care centered.
How many positions should graduating residents apply for?
DR. WORSWICK: I think this depends a lot on who you are, how specific your preferences are, and what part of the country/world you are looking to practice in. In general, there is a great need for dermatologists, and it shouldn’t be hard to find a job. If you’re in a more saturated urban area, you’re going to want to apply for multiple positions. But if you really know what you want, you may only apply to one practice. I generally advise our residents to consider at least 3 places, if only to compare them to give a better idea of best fit or to ensure that their “top choice” is indeed their top choice.
Choosing a Job After Graduation: Advice for Residents From Scott Worswick, MD
Choosing a Job After Graduation: Advice for Residents From Scott Worswick, MD
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
THE DIAGNOSIS: Mpox Virus
The histopathologic features of mpox virus infection may vary depending on the stage of evolution; findings include ballooning degeneration with multinucleated keratinocytes, acanthosis, spongiosis, a neutrophil-rich inflammatory infiltrate, and eosinophilic intracytoplasmic (Guarnieri) inclusion bodies (quiz image inset [arrows]). Prominent neutrophil exocytosis also has been described and may be a characteristic feature in the pustular stage.1,2 A pattern of interface dermatitis also has been observed on histopathology.3 In our patient, the diagnosis of mpox initially was made by clinical and histopathologic correlation and exclusion of other entities in the differential diagnosis. The diagnosis subsequently was confirmed by real-time polymerase chain reaction. The patient received treatment with tecovirimat, but lesions progressed over the following 6 weeks. He subsequently died due to sepsis and multiorgan failure secondary to AIDS.
Mpox is a zoonotic, double-stranded DNA virus of the genus Orthopoxvirus in the family Poxviridae.4 It is transmitted to humans via direct contact with infected animals, most commonly small mammals such as monkeys, squirrels, and rodents. Mpox also may be transmitted between humans through direct contact with bodily fluids, skin and mucosal lesions, respiratory droplets, or fomites. Mpox infection typically begins with a nonspecific flulike prodrome after a 5- to 21-day incubation period, followed by skin lesions of variable morphology affecting any region of the body. Clinically, mpox lesions have been reported to evolve through macular, papular, and vesiculopustular phases, followed by resolution with crusting. Lesions may occur anywhere on the body but frequently manifest on the face then spread centrifugally across the body, with various phases observed simultaneously.5 A worldwide outbreak in 2022 involved larger numbers of cases in nonendemic areas, primarily due to skin-to-skin contact, with predominant anal and genital localization of the lesions as well as fewer prodromal symptoms.6
The differential diagnosis of crusted and umbilicated papules includes disseminated herpesvirus infection, molluscum contagiosum, disseminated cryptococcosis, and histoplasmosis. Additional causative organisms to consider include Penicillium, Mycobacterium tuberculosis and nontuberculous mycobacteria, as well as Sporothrix schenckii.
Herpesvirus infections may have similar clinical and histopathologic findings to mpox. Histopathologically, herpes simplex virus (HSV) and varicella zoster virus (VZV) are essentially identical; both demonstrate ballooning and reticular epidermal degeneration, chromatin condensation, nuclear degeneration, multinucleated keratinocytes with steel-gray nuclei, and prominent epidermal acantholysis with an inflammatory infiltrate (Figure 1). However, involvement of folliculosebaceous units may favor a diagnosis of VZV. Immunohistochemical staining can further differentiate between HSV and VZV.7 While mpox may have features that overlap with both HSV and VZV, including ballooning degeneration and multinucleated keratinocytes with nuclear degeneration, acantholysis is a less commonly reported feature of mpox, and mpox virus infection is characterized by intracytoplasmic (Guarnieri) inclusion bodies rather than the intranuclear inclusion bodies of HSV and VZV.2,5 The presence of Guarnieri bodies in mpox may further help to distinguish mpox from HSV infection on routine histology.
Molluscum contagiosum infection typically manifests as multiple umbilicated papules at sites of inoculation. Large lesions may be seen in the setting of immunosuppression; however, they usually do not progress to vesicular, pustular, or crusted morphologies. Histopathology demonstrates a cup-shaped invagination of the epidermis into the dermis and proliferative rete ridges that descend downward and encircle the dermis with large eosinophilic intracytoplasmic inclusion (Henderson-Patterson) bodies (Figure 2).8
Disseminated cryptococcus infection is caused by the invasive fungus Cryptococcus neoformans and is characterized by meningitis along with fever, malaise, headache, neck stiffness, photophobia, nausea, vomiting, pneumonia with cough and dyspnea, and skin rash, most commonly in immunocompromised individuals.9 Skin lesions are a sign of disseminated infection and can manifest as umbilicated or molluscumlike lesions. Histopathology of cryptococcosis demonstrates a granulomatous dermal infiltrate with neutrophils and pleomorphic yeasts measuring 4 µm to 6 µm with refringent capsules.10 Staining with Grocott methenamine silver and/or mucicarmine for yeast capsules can help to identify organisms (Figure 3).
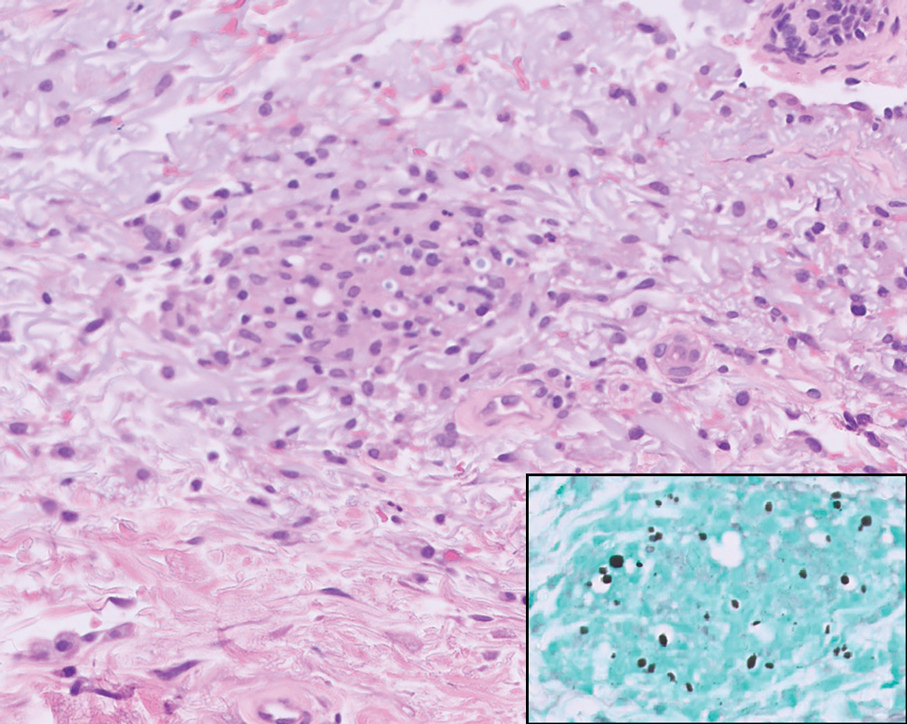
Cutaneous histoplasmosis is caused by Histoplasma capsulatum, a dimorphic fungus that can lead to pulmonary, cutaneous, and disseminated disease, often in immunocompromised patients.11 Cutaneous disease may manifest with molluscumlike or verrucous papules and plaques. Histopathologic examination reveals diffuse suppurative and granulomatous infiltrates with foamy histiocytes and multinucleated giant cells, containing intracellular and extracellular yeasts measuring 1µm to 5µm, surrounded by a clear halo visible with Grocott methenamine silver stain (Figure 4).
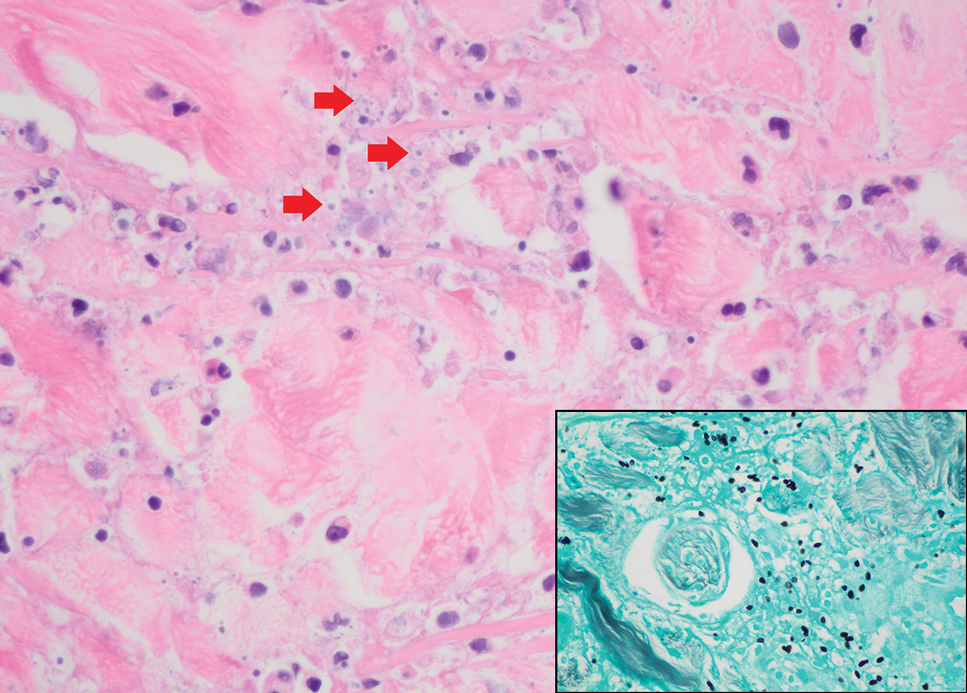
×600). Grocott methenamine silver staining highlights numerous intracellular yeasts (inset, original magnification ×600).
Spreading cutaneous lesions in an immunocompromised individual may be the presentation of multiple infectious etiologies. With the recent rise in mpox cases occurring in nonendemic areas, clinicians should be aware of the spectrum of clinical findings that may occur. Notably, more than one infection may be present in severely immunocompromised individuals, as seen in our patient with chronic orolabial HSV-2 and acute mpox infection. Thorough clinical, histopathologic, and laboratory investigations are necessary for timely diagnosis, appropriate treatment, and exclusion of other life-threatening conditions.
- Moltrasio C, Boggio FL, Romagnuolo M, et al. Monkeypox: a histopathological and transmission electron microscopy study. Microorganisms. 2023;11:1781-1793. doi:10.3390/microorganisms11071781
- Ortins-Pina A, Hegemann B, Saggini A, et al. Histopathological features of human mpox: report of two cases and review of the literature. J Cutan Pathol. 2023;50:706-710. doi:10.1111/cup.14398
- Chalali F, Merlant M, Truong A, et al. Histological features associated with human mpox virus infection in 2022 outbreak in a nonendemic country. Clin Infect Dis. 21;76:1132-1135. doi:10.1093/cid/ciac856.
- Mpox (monkeypox). World Health Organization. https://www.who.int/health-topics/monkeypox/#tab=tab_1. Accessed August 6, 2025.
- Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33:1027-1043. doi:10.1016/j.idc.2019.03.001
- Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018-1022. doi:10.15585 /mmwr.mm7132e3
- Nikkels AF, Debrus S, Sadzot-Delvaux C, et al. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Arch A Pathol Anat Histopathol. 1993;422:121-126. doi:10.1007 /BF01607163
- Badri T, Gandhi GR. Molluscum Contagiosum. StatPearls [Internet]. StatPearls Publishing; 2025. Updated March 27, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK441898/
- Mada PK, Jamil RT, Alam MU. Cryptococcus. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 7, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK431060/
- Hayashida MZ, Seque CA, Pasin VP, et al. Disseminated cryptococcosis with skin lesions: report of a case series. An Bras Dermatol. 2017;92:69-72. doi:10.1590/abd1806-4841.20176343
- Mustari AP, Rao S, Keshavamurthy V, et al. Dermoscopic evaluation of cutaneous histoplasmosis. Indian J Dermatol Venereol Leprol. 2023;19:1-4. doi:10.25259/IJDVL_889_2022
THE DIAGNOSIS: Mpox Virus
The histopathologic features of mpox virus infection may vary depending on the stage of evolution; findings include ballooning degeneration with multinucleated keratinocytes, acanthosis, spongiosis, a neutrophil-rich inflammatory infiltrate, and eosinophilic intracytoplasmic (Guarnieri) inclusion bodies (quiz image inset [arrows]). Prominent neutrophil exocytosis also has been described and may be a characteristic feature in the pustular stage.1,2 A pattern of interface dermatitis also has been observed on histopathology.3 In our patient, the diagnosis of mpox initially was made by clinical and histopathologic correlation and exclusion of other entities in the differential diagnosis. The diagnosis subsequently was confirmed by real-time polymerase chain reaction. The patient received treatment with tecovirimat, but lesions progressed over the following 6 weeks. He subsequently died due to sepsis and multiorgan failure secondary to AIDS.
Mpox is a zoonotic, double-stranded DNA virus of the genus Orthopoxvirus in the family Poxviridae.4 It is transmitted to humans via direct contact with infected animals, most commonly small mammals such as monkeys, squirrels, and rodents. Mpox also may be transmitted between humans through direct contact with bodily fluids, skin and mucosal lesions, respiratory droplets, or fomites. Mpox infection typically begins with a nonspecific flulike prodrome after a 5- to 21-day incubation period, followed by skin lesions of variable morphology affecting any region of the body. Clinically, mpox lesions have been reported to evolve through macular, papular, and vesiculopustular phases, followed by resolution with crusting. Lesions may occur anywhere on the body but frequently manifest on the face then spread centrifugally across the body, with various phases observed simultaneously.5 A worldwide outbreak in 2022 involved larger numbers of cases in nonendemic areas, primarily due to skin-to-skin contact, with predominant anal and genital localization of the lesions as well as fewer prodromal symptoms.6
The differential diagnosis of crusted and umbilicated papules includes disseminated herpesvirus infection, molluscum contagiosum, disseminated cryptococcosis, and histoplasmosis. Additional causative organisms to consider include Penicillium, Mycobacterium tuberculosis and nontuberculous mycobacteria, as well as Sporothrix schenckii.
Herpesvirus infections may have similar clinical and histopathologic findings to mpox. Histopathologically, herpes simplex virus (HSV) and varicella zoster virus (VZV) are essentially identical; both demonstrate ballooning and reticular epidermal degeneration, chromatin condensation, nuclear degeneration, multinucleated keratinocytes with steel-gray nuclei, and prominent epidermal acantholysis with an inflammatory infiltrate (Figure 1). However, involvement of folliculosebaceous units may favor a diagnosis of VZV. Immunohistochemical staining can further differentiate between HSV and VZV.7 While mpox may have features that overlap with both HSV and VZV, including ballooning degeneration and multinucleated keratinocytes with nuclear degeneration, acantholysis is a less commonly reported feature of mpox, and mpox virus infection is characterized by intracytoplasmic (Guarnieri) inclusion bodies rather than the intranuclear inclusion bodies of HSV and VZV.2,5 The presence of Guarnieri bodies in mpox may further help to distinguish mpox from HSV infection on routine histology.

Molluscum contagiosum infection typically manifests as multiple umbilicated papules at sites of inoculation. Large lesions may be seen in the setting of immunosuppression; however, they usually do not progress to vesicular, pustular, or crusted morphologies. Histopathology demonstrates a cup-shaped invagination of the epidermis into the dermis and proliferative rete ridges that descend downward and encircle the dermis with large eosinophilic intracytoplasmic inclusion (Henderson-Patterson) bodies (Figure 2).8

Disseminated cryptococcus infection is caused by the invasive fungus Cryptococcus neoformans and is characterized by meningitis along with fever, malaise, headache, neck stiffness, photophobia, nausea, vomiting, pneumonia with cough and dyspnea, and skin rash, most commonly in immunocompromised individuals.9 Skin lesions are a sign of disseminated infection and can manifest as umbilicated or molluscumlike lesions. Histopathology of cryptococcosis demonstrates a granulomatous dermal infiltrate with neutrophils and pleomorphic yeasts measuring 4 µm to 6 µm with refringent capsules.10 Staining with Grocott methenamine silver and/or mucicarmine for yeast capsules can help to identify organisms (Figure 3).

Cutaneous histoplasmosis is caused by Histoplasma capsulatum, a dimorphic fungus that can lead to pulmonary, cutaneous, and disseminated disease, often in immunocompromised patients.11 Cutaneous disease may manifest with molluscumlike or verrucous papules and plaques. Histopathologic examination reveals diffuse suppurative and granulomatous infiltrates with foamy histiocytes and multinucleated giant cells, containing intracellular and extracellular yeasts measuring 1µm to 5µm, surrounded by a clear halo visible with Grocott methenamine silver stain (Figure 4).

×600). Grocott methenamine silver staining highlights numerous intracellular yeasts (inset, original magnification ×600).
Spreading cutaneous lesions in an immunocompromised individual may be the presentation of multiple infectious etiologies. With the recent rise in mpox cases occurring in nonendemic areas, clinicians should be aware of the spectrum of clinical findings that may occur. Notably, more than one infection may be present in severely immunocompromised individuals, as seen in our patient with chronic orolabial HSV-2 and acute mpox infection. Thorough clinical, histopathologic, and laboratory investigations are necessary for timely diagnosis, appropriate treatment, and exclusion of other life-threatening conditions.
THE DIAGNOSIS: Mpox Virus
The histopathologic features of mpox virus infection may vary depending on the stage of evolution; findings include ballooning degeneration with multinucleated keratinocytes, acanthosis, spongiosis, a neutrophil-rich inflammatory infiltrate, and eosinophilic intracytoplasmic (Guarnieri) inclusion bodies (quiz image inset [arrows]). Prominent neutrophil exocytosis also has been described and may be a characteristic feature in the pustular stage.1,2 A pattern of interface dermatitis also has been observed on histopathology.3 In our patient, the diagnosis of mpox initially was made by clinical and histopathologic correlation and exclusion of other entities in the differential diagnosis. The diagnosis subsequently was confirmed by real-time polymerase chain reaction. The patient received treatment with tecovirimat, but lesions progressed over the following 6 weeks. He subsequently died due to sepsis and multiorgan failure secondary to AIDS.
Mpox is a zoonotic, double-stranded DNA virus of the genus Orthopoxvirus in the family Poxviridae.4 It is transmitted to humans via direct contact with infected animals, most commonly small mammals such as monkeys, squirrels, and rodents. Mpox also may be transmitted between humans through direct contact with bodily fluids, skin and mucosal lesions, respiratory droplets, or fomites. Mpox infection typically begins with a nonspecific flulike prodrome after a 5- to 21-day incubation period, followed by skin lesions of variable morphology affecting any region of the body. Clinically, mpox lesions have been reported to evolve through macular, papular, and vesiculopustular phases, followed by resolution with crusting. Lesions may occur anywhere on the body but frequently manifest on the face then spread centrifugally across the body, with various phases observed simultaneously.5 A worldwide outbreak in 2022 involved larger numbers of cases in nonendemic areas, primarily due to skin-to-skin contact, with predominant anal and genital localization of the lesions as well as fewer prodromal symptoms.6
The differential diagnosis of crusted and umbilicated papules includes disseminated herpesvirus infection, molluscum contagiosum, disseminated cryptococcosis, and histoplasmosis. Additional causative organisms to consider include Penicillium, Mycobacterium tuberculosis and nontuberculous mycobacteria, as well as Sporothrix schenckii.
Herpesvirus infections may have similar clinical and histopathologic findings to mpox. Histopathologically, herpes simplex virus (HSV) and varicella zoster virus (VZV) are essentially identical; both demonstrate ballooning and reticular epidermal degeneration, chromatin condensation, nuclear degeneration, multinucleated keratinocytes with steel-gray nuclei, and prominent epidermal acantholysis with an inflammatory infiltrate (Figure 1). However, involvement of folliculosebaceous units may favor a diagnosis of VZV. Immunohistochemical staining can further differentiate between HSV and VZV.7 While mpox may have features that overlap with both HSV and VZV, including ballooning degeneration and multinucleated keratinocytes with nuclear degeneration, acantholysis is a less commonly reported feature of mpox, and mpox virus infection is characterized by intracytoplasmic (Guarnieri) inclusion bodies rather than the intranuclear inclusion bodies of HSV and VZV.2,5 The presence of Guarnieri bodies in mpox may further help to distinguish mpox from HSV infection on routine histology.

Molluscum contagiosum infection typically manifests as multiple umbilicated papules at sites of inoculation. Large lesions may be seen in the setting of immunosuppression; however, they usually do not progress to vesicular, pustular, or crusted morphologies. Histopathology demonstrates a cup-shaped invagination of the epidermis into the dermis and proliferative rete ridges that descend downward and encircle the dermis with large eosinophilic intracytoplasmic inclusion (Henderson-Patterson) bodies (Figure 2).8

Disseminated cryptococcus infection is caused by the invasive fungus Cryptococcus neoformans and is characterized by meningitis along with fever, malaise, headache, neck stiffness, photophobia, nausea, vomiting, pneumonia with cough and dyspnea, and skin rash, most commonly in immunocompromised individuals.9 Skin lesions are a sign of disseminated infection and can manifest as umbilicated or molluscumlike lesions. Histopathology of cryptococcosis demonstrates a granulomatous dermal infiltrate with neutrophils and pleomorphic yeasts measuring 4 µm to 6 µm with refringent capsules.10 Staining with Grocott methenamine silver and/or mucicarmine for yeast capsules can help to identify organisms (Figure 3).

Cutaneous histoplasmosis is caused by Histoplasma capsulatum, a dimorphic fungus that can lead to pulmonary, cutaneous, and disseminated disease, often in immunocompromised patients.11 Cutaneous disease may manifest with molluscumlike or verrucous papules and plaques. Histopathologic examination reveals diffuse suppurative and granulomatous infiltrates with foamy histiocytes and multinucleated giant cells, containing intracellular and extracellular yeasts measuring 1µm to 5µm, surrounded by a clear halo visible with Grocott methenamine silver stain (Figure 4).

×600). Grocott methenamine silver staining highlights numerous intracellular yeasts (inset, original magnification ×600).
Spreading cutaneous lesions in an immunocompromised individual may be the presentation of multiple infectious etiologies. With the recent rise in mpox cases occurring in nonendemic areas, clinicians should be aware of the spectrum of clinical findings that may occur. Notably, more than one infection may be present in severely immunocompromised individuals, as seen in our patient with chronic orolabial HSV-2 and acute mpox infection. Thorough clinical, histopathologic, and laboratory investigations are necessary for timely diagnosis, appropriate treatment, and exclusion of other life-threatening conditions.
- Moltrasio C, Boggio FL, Romagnuolo M, et al. Monkeypox: a histopathological and transmission electron microscopy study. Microorganisms. 2023;11:1781-1793. doi:10.3390/microorganisms11071781
- Ortins-Pina A, Hegemann B, Saggini A, et al. Histopathological features of human mpox: report of two cases and review of the literature. J Cutan Pathol. 2023;50:706-710. doi:10.1111/cup.14398
- Chalali F, Merlant M, Truong A, et al. Histological features associated with human mpox virus infection in 2022 outbreak in a nonendemic country. Clin Infect Dis. 21;76:1132-1135. doi:10.1093/cid/ciac856.
- Mpox (monkeypox). World Health Organization. https://www.who.int/health-topics/monkeypox/#tab=tab_1. Accessed August 6, 2025.
- Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33:1027-1043. doi:10.1016/j.idc.2019.03.001
- Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018-1022. doi:10.15585 /mmwr.mm7132e3
- Nikkels AF, Debrus S, Sadzot-Delvaux C, et al. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Arch A Pathol Anat Histopathol. 1993;422:121-126. doi:10.1007 /BF01607163
- Badri T, Gandhi GR. Molluscum Contagiosum. StatPearls [Internet]. StatPearls Publishing; 2025. Updated March 27, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK441898/
- Mada PK, Jamil RT, Alam MU. Cryptococcus. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 7, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK431060/
- Hayashida MZ, Seque CA, Pasin VP, et al. Disseminated cryptococcosis with skin lesions: report of a case series. An Bras Dermatol. 2017;92:69-72. doi:10.1590/abd1806-4841.20176343
- Mustari AP, Rao S, Keshavamurthy V, et al. Dermoscopic evaluation of cutaneous histoplasmosis. Indian J Dermatol Venereol Leprol. 2023;19:1-4. doi:10.25259/IJDVL_889_2022
- Moltrasio C, Boggio FL, Romagnuolo M, et al. Monkeypox: a histopathological and transmission electron microscopy study. Microorganisms. 2023;11:1781-1793. doi:10.3390/microorganisms11071781
- Ortins-Pina A, Hegemann B, Saggini A, et al. Histopathological features of human mpox: report of two cases and review of the literature. J Cutan Pathol. 2023;50:706-710. doi:10.1111/cup.14398
- Chalali F, Merlant M, Truong A, et al. Histological features associated with human mpox virus infection in 2022 outbreak in a nonendemic country. Clin Infect Dis. 21;76:1132-1135. doi:10.1093/cid/ciac856.
- Mpox (monkeypox). World Health Organization. https://www.who.int/health-topics/monkeypox/#tab=tab_1. Accessed August 6, 2025.
- Petersen E, Kantele A, Koopmans M, et al. Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect Dis Clin North Am. 2019;33:1027-1043. doi:10.1016/j.idc.2019.03.001
- Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018-1022. doi:10.15585 /mmwr.mm7132e3
- Nikkels AF, Debrus S, Sadzot-Delvaux C, et al. Comparative immunohistochemical study of herpes simplex and varicella-zoster infections. Virchows Arch A Pathol Anat Histopathol. 1993;422:121-126. doi:10.1007 /BF01607163
- Badri T, Gandhi GR. Molluscum Contagiosum. StatPearls [Internet]. StatPearls Publishing; 2025. Updated March 27, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK441898/
- Mada PK, Jamil RT, Alam MU. Cryptococcus. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 7, 2023. Accessed August 8, 2025. https://www.ncbi.nlm.nih.gov/books/NBK431060/
- Hayashida MZ, Seque CA, Pasin VP, et al. Disseminated cryptococcosis with skin lesions: report of a case series. An Bras Dermatol. 2017;92:69-72. doi:10.1590/abd1806-4841.20176343
- Mustari AP, Rao S, Keshavamurthy V, et al. Dermoscopic evaluation of cutaneous histoplasmosis. Indian J Dermatol Venereol Leprol. 2023;19:1-4. doi:10.25259/IJDVL_889_2022
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
Scattered Umbilicated Papules on the Cheek, Neck, and Arms
A 42-year-old man with a history of multidrug-resistant HIV/AIDS presented to the emergency department for evaluation of pruritic, scattered, umbilicated papules on the left cheek, neck, and arms of 3 days’ duration. The patient’s most recent CD4+ T-cell count 6 weeks prior to the development of the rash was 1 cell/mm3. He was noncompliant with antiretroviral therapy. He reported that the lesions had progressed rapidly, starting on the face and extending down the neck and arms. Physical examination revealed scattered umbilicated and centrally crusted papules and plaques on the left cheek, neck, and arms. Erosions involving the oral mucosa also were noted, which the patient reported had been present for several weeks. An oral swab was positive for herpes simplex virus 2 on polymerase chain reaction. A shave biopsy of a lesion from the left cheek was performed.
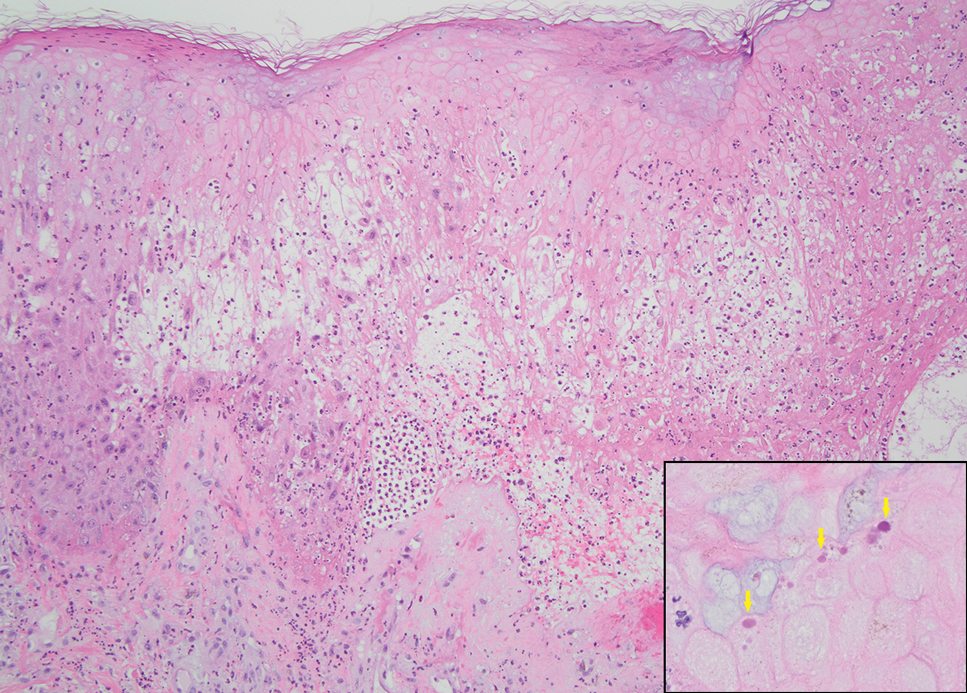
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
To the Editor:
The popularity of the social media platform TikTok, which is known for its short-form videos, has surged in recent years. Viral videos demonstrating skin care routines reach millions of viewers,1 showcasing specific products, detailing beauty regimens, and setting fads that many users eagerly follow. These trends often influence consumer behavior—in 2023, viral videos using the tag #TikTokMadeMeBuy lead to a 14% growth in the sale of skin care products.2 However, they also encourage purchasing decisions that may escalate environmental waste through plastic packaging and single-use products. In this study, we analyzed videos on TikTok to assess the environmental impact of trending skin care routines. By examining the types of products promoted, their packaging, and the frequency with which they appear in viral content, we aimed to investigate how these trends, which may be imitated by users, impact the environment.
A search of TikTok videos using #skincareroutine was conducted on June 21, 2024. Sponsored content, non–English language videos, videos without demonstrated skin care routines, and videos showing makeup routines were excluded from our analysis. Data collected from each video included username, date posted, number of likes, total number of skin care products used, number of single-use skin care products used, average amount of product used, number of skin care applicators used, and number of single-use applicators used. Single-use items, defined as those intended for one-time use and subsequent disposal, were identified visually by packaging, manufacturer intent, and common consumer usage patterns. The amount of product used per application was graded on a scale of 1 to 3 (1=pea-sized amount or less; 2=single full pump/spray; 3=multiple pumps/sprays). Videos were categorized as personal (ie, skin care routine walk-throughs by the creator) or autonomous sensory meridian response (ASMR)(focused on product sounds and aesthetics).3 A Mann-Whitney U test was utilized to statistically compare the 2 groups. Statistical analysis was performed using Microsoft Excel (α=0.05).
A total of 50 videos met the inclusion criteria and were included in the analysis. The average number of likes per video was 499,696.15, with skin care routines featuring an average of 6.4 unique products (Table). There was a weak positive correlation (r=0.1809) between the number of skin care products used and the number of likes. A total of 320 products were used across the videos, 23 of which were single-use (7.2%).On average, single-use skin care items were used 0.46 times per routine, comprising a mean 7.99% of total products per video. The average score for the amount of product used per application was 2.18. There was no difference in personal vs ASMR videos with regard to the total number of skin care products used or the average amount of product used per application (P>.05). Thirty-three (70.2%) of the 47 applicators used across all videos were single-use. An average of 0.94 applicators per routine were utilized, with a mean 68.83% being single-use applicators. Common single-use products were toner wipes and eye patches, and single-use applicators included cotton pads and plastic spatulas.
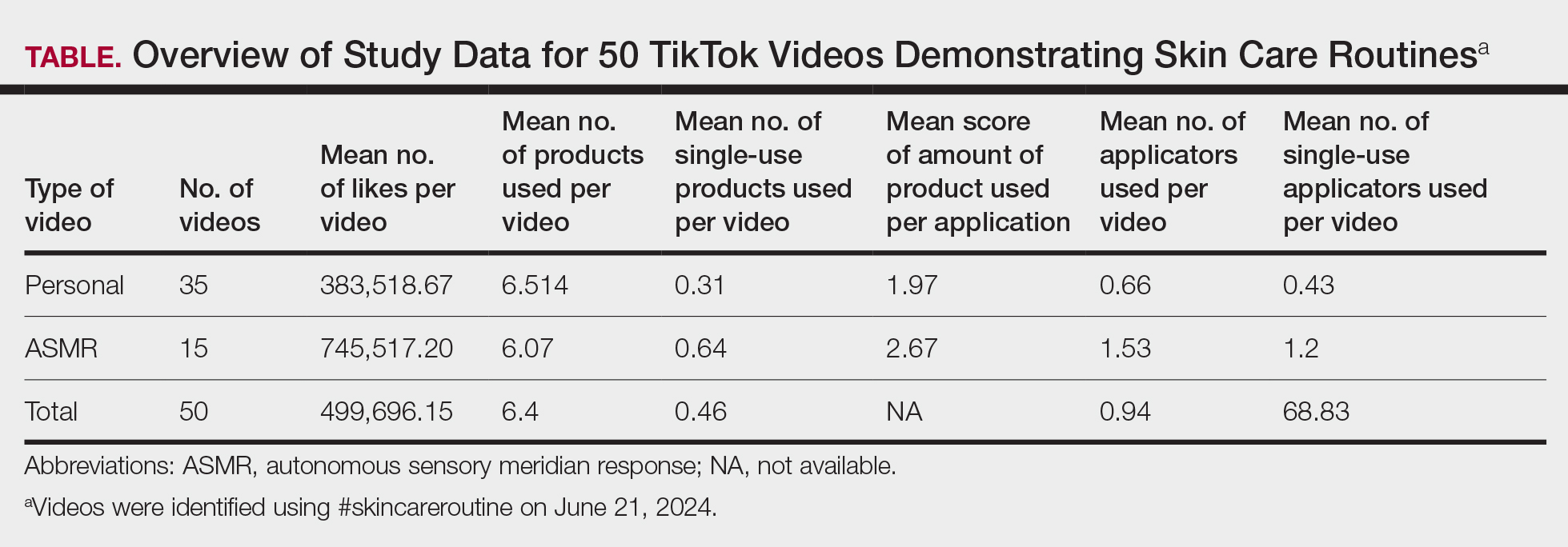
Our findings indicated a prevalence of multiple products and large amount of product used in trending skin care routines, suggesting a shift toward multistep skin care. This implies a high rate of product consumption that may accelerate the carbon footprint associated with skin care products,3 which could contribute to climate change and environmental degradation. Consumers also may feel compelled to purchase and discard numerous partially used products in order to keep up with the latest trends, exacerbating the environmental impact. Furthermore, the utilization of single-use products and applicators contributes to increased plastic waste, pollution, and resource depletion. Single-use items often are difficult to recycle due to their mixed materials and small size,4,5 and therefore they can accumulate in landfills and oceans. This impact can be mitigated by switching to reusable applicators, refillable packaging, and biodegradable materials.
The substantial average number of likes per video indicates high engagement with skin care content among TikTok users. The continued popularity of complex multistep skin care routines, despite a weak correlation between the number of skin care products used and the number of likes per video, likely stems from factors such as aesthetic appeal, ASMR effects, and creators’ established followings, which may drive user engagement to contribute to unsustainable consumption patterns. Factors such as presentation style, aesthetics, or creators’ pre-existing online following may have a major impact on how well a video performs on TikTok. The similarity between personal and ASMR videos, particularly in the number of products used and the amount applied, suggests that both formats employ common approaches to meet audience expectations and align with promotional trends, relying more on sensory and aesthetic strategies than substantive differences in skin care routines.
Our use of only one tag in our search as well as the subjective quantity scale limits the generalizability of these findings to broader TikTok skin care content.
Overall, our study underscores the role of brands and social media influencers in skin care education and promotion of sustainable practices. The extensive number of products used and generous application of each product in skin care routines demonstrated in TikTok videos may mislead viewers into believing that using more product improves outcomes, when often, less is more. We recommend that dermatologists counsel patients about informed skin care regimens that prioritize individual needs over social media fads.
- Pagani K, Lukac D, Martinez R, et al. Slugging: TikTokTM as a source of a viral “harmless” beauty trend. Clin Dermatol. 2022;40:810-812. doi:10.1016/j.clindermatol.2022.08.005
- Stern C. TikTok drives $31.7B in beauty sales: how viral trends are shaping the future of cosmetics. CosmeticsDesign. August 20, 2024. Accessed June 24, 2025. https://www.cosmeticsdesign.com/Article/2024/08/20/tiktok-drives-31.7b-in-beauty-sales-how-viral-trends-are-shaping-the-future-of-cosmetics/
- Fountain C. ASMR content saw huge growth on YouTube, but now creators are flocking to TikTok instead. Business Insider. July 4, 2022. Accessed June 24, 2025. https://www.businessinsider.com/asmr-tiktok-instead-of-youtube-growth-subscribers-2022-7
- Rathore S, Schuler B, Park J. Life cycle assessment of multiple dispensing systems used for cosmetic product packaging. Packaging Technol Sci. 2023;36:533-547. doi:10.1002/pts.2729
- Shaw S. How to actually recycle your empty beauty products. CNN Underscored. Updated April 17, 2024. Accessed June 24, 2025. https://www.cnn.com/cnn-underscored/beauty/how-to-recycle-beauty-products
To the Editor:
The popularity of the social media platform TikTok, which is known for its short-form videos, has surged in recent years. Viral videos demonstrating skin care routines reach millions of viewers,1 showcasing specific products, detailing beauty regimens, and setting fads that many users eagerly follow. These trends often influence consumer behavior—in 2023, viral videos using the tag #TikTokMadeMeBuy lead to a 14% growth in the sale of skin care products.2 However, they also encourage purchasing decisions that may escalate environmental waste through plastic packaging and single-use products. In this study, we analyzed videos on TikTok to assess the environmental impact of trending skin care routines. By examining the types of products promoted, their packaging, and the frequency with which they appear in viral content, we aimed to investigate how these trends, which may be imitated by users, impact the environment.
A search of TikTok videos using #skincareroutine was conducted on June 21, 2024. Sponsored content, non–English language videos, videos without demonstrated skin care routines, and videos showing makeup routines were excluded from our analysis. Data collected from each video included username, date posted, number of likes, total number of skin care products used, number of single-use skin care products used, average amount of product used, number of skin care applicators used, and number of single-use applicators used. Single-use items, defined as those intended for one-time use and subsequent disposal, were identified visually by packaging, manufacturer intent, and common consumer usage patterns. The amount of product used per application was graded on a scale of 1 to 3 (1=pea-sized amount or less; 2=single full pump/spray; 3=multiple pumps/sprays). Videos were categorized as personal (ie, skin care routine walk-throughs by the creator) or autonomous sensory meridian response (ASMR)(focused on product sounds and aesthetics).3 A Mann-Whitney U test was utilized to statistically compare the 2 groups. Statistical analysis was performed using Microsoft Excel (α=0.05).
A total of 50 videos met the inclusion criteria and were included in the analysis. The average number of likes per video was 499,696.15, with skin care routines featuring an average of 6.4 unique products (Table). There was a weak positive correlation (r=0.1809) between the number of skin care products used and the number of likes. A total of 320 products were used across the videos, 23 of which were single-use (7.2%).On average, single-use skin care items were used 0.46 times per routine, comprising a mean 7.99% of total products per video. The average score for the amount of product used per application was 2.18. There was no difference in personal vs ASMR videos with regard to the total number of skin care products used or the average amount of product used per application (P>.05). Thirty-three (70.2%) of the 47 applicators used across all videos were single-use. An average of 0.94 applicators per routine were utilized, with a mean 68.83% being single-use applicators. Common single-use products were toner wipes and eye patches, and single-use applicators included cotton pads and plastic spatulas.

Our findings indicated a prevalence of multiple products and large amount of product used in trending skin care routines, suggesting a shift toward multistep skin care. This implies a high rate of product consumption that may accelerate the carbon footprint associated with skin care products,3 which could contribute to climate change and environmental degradation. Consumers also may feel compelled to purchase and discard numerous partially used products in order to keep up with the latest trends, exacerbating the environmental impact. Furthermore, the utilization of single-use products and applicators contributes to increased plastic waste, pollution, and resource depletion. Single-use items often are difficult to recycle due to their mixed materials and small size,4,5 and therefore they can accumulate in landfills and oceans. This impact can be mitigated by switching to reusable applicators, refillable packaging, and biodegradable materials.
The substantial average number of likes per video indicates high engagement with skin care content among TikTok users. The continued popularity of complex multistep skin care routines, despite a weak correlation between the number of skin care products used and the number of likes per video, likely stems from factors such as aesthetic appeal, ASMR effects, and creators’ established followings, which may drive user engagement to contribute to unsustainable consumption patterns. Factors such as presentation style, aesthetics, or creators’ pre-existing online following may have a major impact on how well a video performs on TikTok. The similarity between personal and ASMR videos, particularly in the number of products used and the amount applied, suggests that both formats employ common approaches to meet audience expectations and align with promotional trends, relying more on sensory and aesthetic strategies than substantive differences in skin care routines.
Our use of only one tag in our search as well as the subjective quantity scale limits the generalizability of these findings to broader TikTok skin care content.
Overall, our study underscores the role of brands and social media influencers in skin care education and promotion of sustainable practices. The extensive number of products used and generous application of each product in skin care routines demonstrated in TikTok videos may mislead viewers into believing that using more product improves outcomes, when often, less is more. We recommend that dermatologists counsel patients about informed skin care regimens that prioritize individual needs over social media fads.
To the Editor:
The popularity of the social media platform TikTok, which is known for its short-form videos, has surged in recent years. Viral videos demonstrating skin care routines reach millions of viewers,1 showcasing specific products, detailing beauty regimens, and setting fads that many users eagerly follow. These trends often influence consumer behavior—in 2023, viral videos using the tag #TikTokMadeMeBuy lead to a 14% growth in the sale of skin care products.2 However, they also encourage purchasing decisions that may escalate environmental waste through plastic packaging and single-use products. In this study, we analyzed videos on TikTok to assess the environmental impact of trending skin care routines. By examining the types of products promoted, their packaging, and the frequency with which they appear in viral content, we aimed to investigate how these trends, which may be imitated by users, impact the environment.
A search of TikTok videos using #skincareroutine was conducted on June 21, 2024. Sponsored content, non–English language videos, videos without demonstrated skin care routines, and videos showing makeup routines were excluded from our analysis. Data collected from each video included username, date posted, number of likes, total number of skin care products used, number of single-use skin care products used, average amount of product used, number of skin care applicators used, and number of single-use applicators used. Single-use items, defined as those intended for one-time use and subsequent disposal, were identified visually by packaging, manufacturer intent, and common consumer usage patterns. The amount of product used per application was graded on a scale of 1 to 3 (1=pea-sized amount or less; 2=single full pump/spray; 3=multiple pumps/sprays). Videos were categorized as personal (ie, skin care routine walk-throughs by the creator) or autonomous sensory meridian response (ASMR)(focused on product sounds and aesthetics).3 A Mann-Whitney U test was utilized to statistically compare the 2 groups. Statistical analysis was performed using Microsoft Excel (α=0.05).
A total of 50 videos met the inclusion criteria and were included in the analysis. The average number of likes per video was 499,696.15, with skin care routines featuring an average of 6.4 unique products (Table). There was a weak positive correlation (r=0.1809) between the number of skin care products used and the number of likes. A total of 320 products were used across the videos, 23 of which were single-use (7.2%).On average, single-use skin care items were used 0.46 times per routine, comprising a mean 7.99% of total products per video. The average score for the amount of product used per application was 2.18. There was no difference in personal vs ASMR videos with regard to the total number of skin care products used or the average amount of product used per application (P>.05). Thirty-three (70.2%) of the 47 applicators used across all videos were single-use. An average of 0.94 applicators per routine were utilized, with a mean 68.83% being single-use applicators. Common single-use products were toner wipes and eye patches, and single-use applicators included cotton pads and plastic spatulas.

Our findings indicated a prevalence of multiple products and large amount of product used in trending skin care routines, suggesting a shift toward multistep skin care. This implies a high rate of product consumption that may accelerate the carbon footprint associated with skin care products,3 which could contribute to climate change and environmental degradation. Consumers also may feel compelled to purchase and discard numerous partially used products in order to keep up with the latest trends, exacerbating the environmental impact. Furthermore, the utilization of single-use products and applicators contributes to increased plastic waste, pollution, and resource depletion. Single-use items often are difficult to recycle due to their mixed materials and small size,4,5 and therefore they can accumulate in landfills and oceans. This impact can be mitigated by switching to reusable applicators, refillable packaging, and biodegradable materials.
The substantial average number of likes per video indicates high engagement with skin care content among TikTok users. The continued popularity of complex multistep skin care routines, despite a weak correlation between the number of skin care products used and the number of likes per video, likely stems from factors such as aesthetic appeal, ASMR effects, and creators’ established followings, which may drive user engagement to contribute to unsustainable consumption patterns. Factors such as presentation style, aesthetics, or creators’ pre-existing online following may have a major impact on how well a video performs on TikTok. The similarity between personal and ASMR videos, particularly in the number of products used and the amount applied, suggests that both formats employ common approaches to meet audience expectations and align with promotional trends, relying more on sensory and aesthetic strategies than substantive differences in skin care routines.
Our use of only one tag in our search as well as the subjective quantity scale limits the generalizability of these findings to broader TikTok skin care content.
Overall, our study underscores the role of brands and social media influencers in skin care education and promotion of sustainable practices. The extensive number of products used and generous application of each product in skin care routines demonstrated in TikTok videos may mislead viewers into believing that using more product improves outcomes, when often, less is more. We recommend that dermatologists counsel patients about informed skin care regimens that prioritize individual needs over social media fads.
- Pagani K, Lukac D, Martinez R, et al. Slugging: TikTokTM as a source of a viral “harmless” beauty trend. Clin Dermatol. 2022;40:810-812. doi:10.1016/j.clindermatol.2022.08.005
- Stern C. TikTok drives $31.7B in beauty sales: how viral trends are shaping the future of cosmetics. CosmeticsDesign. August 20, 2024. Accessed June 24, 2025. https://www.cosmeticsdesign.com/Article/2024/08/20/tiktok-drives-31.7b-in-beauty-sales-how-viral-trends-are-shaping-the-future-of-cosmetics/
- Fountain C. ASMR content saw huge growth on YouTube, but now creators are flocking to TikTok instead. Business Insider. July 4, 2022. Accessed June 24, 2025. https://www.businessinsider.com/asmr-tiktok-instead-of-youtube-growth-subscribers-2022-7
- Rathore S, Schuler B, Park J. Life cycle assessment of multiple dispensing systems used for cosmetic product packaging. Packaging Technol Sci. 2023;36:533-547. doi:10.1002/pts.2729
- Shaw S. How to actually recycle your empty beauty products. CNN Underscored. Updated April 17, 2024. Accessed June 24, 2025. https://www.cnn.com/cnn-underscored/beauty/how-to-recycle-beauty-products
- Pagani K, Lukac D, Martinez R, et al. Slugging: TikTokTM as a source of a viral “harmless” beauty trend. Clin Dermatol. 2022;40:810-812. doi:10.1016/j.clindermatol.2022.08.005
- Stern C. TikTok drives $31.7B in beauty sales: how viral trends are shaping the future of cosmetics. CosmeticsDesign. August 20, 2024. Accessed June 24, 2025. https://www.cosmeticsdesign.com/Article/2024/08/20/tiktok-drives-31.7b-in-beauty-sales-how-viral-trends-are-shaping-the-future-of-cosmetics/
- Fountain C. ASMR content saw huge growth on YouTube, but now creators are flocking to TikTok instead. Business Insider. July 4, 2022. Accessed June 24, 2025. https://www.businessinsider.com/asmr-tiktok-instead-of-youtube-growth-subscribers-2022-7
- Rathore S, Schuler B, Park J. Life cycle assessment of multiple dispensing systems used for cosmetic product packaging. Packaging Technol Sci. 2023;36:533-547. doi:10.1002/pts.2729
- Shaw S. How to actually recycle your empty beauty products. CNN Underscored. Updated April 17, 2024. Accessed June 24, 2025. https://www.cnn.com/cnn-underscored/beauty/how-to-recycle-beauty-products
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
PRACTICE POINTS
- Social media platforms are increasingly influential in shaping consumer skin care habits, particularly among younger demographics.
- Dermatologists should be aware of the aesthetic-driven nature of online skin care trends when advising patients on product use.
- Viral skin care routines often feature multiple products and applicators, potentially encouraging excessive product use and waste.