User login
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey
On March 11, 2020, the World Health Organization designated COVID- 19 as a pandemic.1 Pandemics have historically impacted physical and mental health across all populations, but especially health care workers (HCWs).2 Nurses and other HCWs were profoundly impacted by the pandemic.3-8
Throughout the pandemic, nurses continued to provide care while working in short-staffed workplaces, facing increased exposure to COVID-19, and witnessing COVID—19–related morbidity and mortality.9 Many nurses were mandated to cross-train in unfamiliar clinical settings and adjust to new and prolonged shift schedules. Physical and emotional exhaustion associated with managing care for individuals with COVID-19, shortage of personal protective equipment (PPE), risk of infection, fear of secondary transmission to family members, feelings of being rejected by others, and social isolation, led to HCWs’ increased vulnerability to psychological impacts of the pandemic.8,10
A meta-analysis of 65 studies with > 79,000 participants found HCWs experienced significant levels of anxiety, depression, stress, insomnia, and other mental health issues, such as posttraumatic stress disorder (PTSD). Female HCWs, nurses, and frontline responders experienced a higher incidence of psychological impact.11 Other meta-analyses revealed that nurses’ compassion satisfaction, compassion fatigue, and burnout levels were significantly impacted with increased levels of burnout among nurses who had a friend or family member diagnosed with COVID- 19 or experienced prolonged threat of exposure to the virus.12,13 A study of 350 nurses found high rates of perceived transgressions by others, and betrayal.8 Nurse leaders and staff nurses had to persevere as moral distress became pervasive among nursing staff, which led to complex and often unsustainable circumstances. 14 The themes identified in the literature about the pandemic’s impact as well as witnessing nurse colleagues’ distress with patient mortality and death of coworkers during the early phase of the COVID-19 pandemic compelled a group of Veterans Health Administration (VHA) nurses to form a research team to understand the scope of impact and identify possible solutions.
Since published studies on the impact of pandemics on HCWs, including nurses, primarily focused on inpatient settings, the investigators of this study sought to capture the experiences of outpatient and inpatient nurses providing care in the US Department of Veterans Affairs (VA) Sierra Pacific Network (Veterans Integrated Service Network [VISN] 21), which has facilities in northern California, Hawaii, and Nevada.15-19 The purpose of this study was to identify the impact of COVID-19 on nurses caring for veterans in both outpatient and inpatient settings at VISN 21 facilities from March 2020 to September 2022, to inform leadership about the extent the virus affected nurses, and identify strategies that address current and future impacts of pandemics.
METHODS
This retrospective descriptive survey adapted the Pandemic Impact Survey by Purcell et al, which included the Moral Injury Events Scale, Primary Care PTSD Screener, the Patient Health Questionnaire-2 for depression, and a modified burnout scale.20-24 The survey of 70 Likert-scale questions was intended to measure nurses’ needs, burnout, moral distress, depression and stress symptoms, work-related factors, and intent to remain working in their current position. A nurse was defined broadly and included those employed as licensed vocational nurses (LVN), licensed practical nurses (LPN), registered nurses (RN), nurses with advanced degrees, advanced practice registered nurses (APRNs), and nurses with other certifications or licenses.
The VA Pacific Islands Research and Development Committee reviewed and approved the institutional review board-exempted study. The VISN 21 union was notified; only limited demographic information and broad VA tenure categories were collected to protect privacy. The principal investigator redacted facility identifier data after each facility had participated.
The survey was placed in REDCAP and a confidential link was emailed to all VISN 21 inpatient and outpatient nurses during March 2023. Because a comprehensive VISN 21 list of nurse email addresses was unavailable, the email was distributed by nursing leadership at each facility. Nurses received an email reminder at the 2-week halfway point, prompting them to complete the survey. The email indicated the purpose and voluntary nature of the study and cautioned nurses that they might experience stress while answering survey questions. Stress management resources were provided.
Descriptive statistics were used to report the results. Data were aggregated for analyzing and reporting purposes.
RESULTS
In March 2023, 860 of 5586 nurses (15%) responded to the survey. Respondents included 344 clinical inpatient nurses (40%) and 516 clinical outpatient nurses (60%); 688 (80%) were RNs, 129 (15%) were LPNs/LVNs, and 43 (5%) were APRNs. Of 849 respondents to provide their age, 15 (2%) were < 30 years, 163 (19%) were 30 to 39 years, 232 (27%) were 40 to 49 years, 259 (30%) were 50 to 59 years, and 180 (21%) were ≥ 60 years.
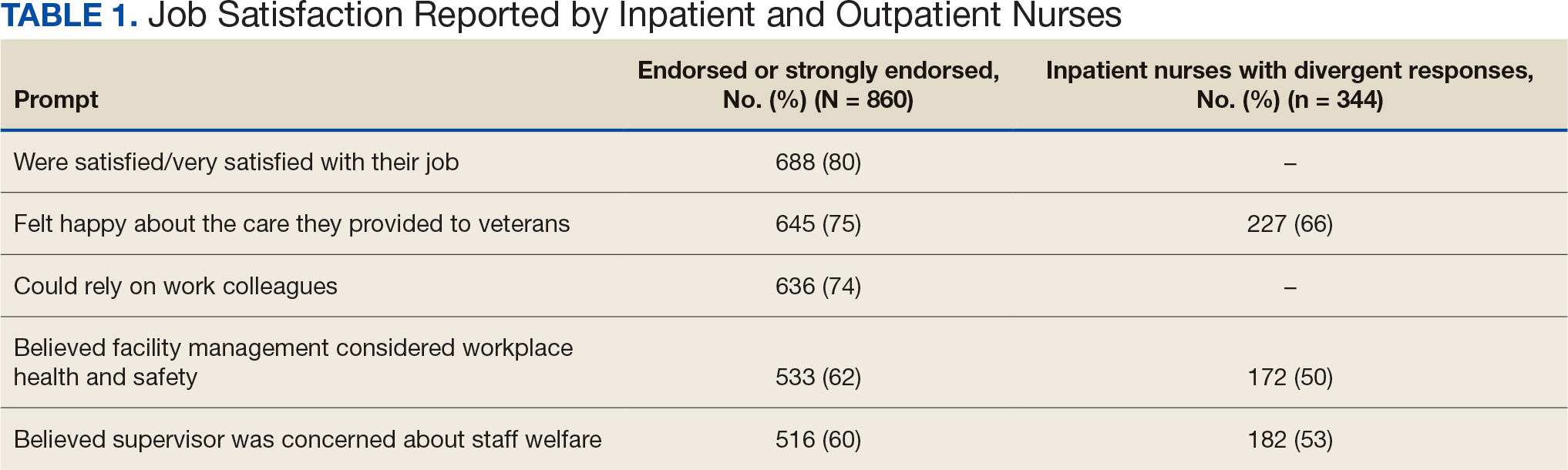
The survey found that 688 nurses reported job satisfaction (80%) and 75% of all respondents (66% among inpatient nurses) reported feeling happy with the care they delivered. Both inpatient and outpatient nurses indicated they could rely on staff. Sixty percent (n = 516) of the nurses indicated that facility management considered workplace health and safety and supervisors showed concern for subordinates, although inpatient nurses reported a lower percentage (Table 1).
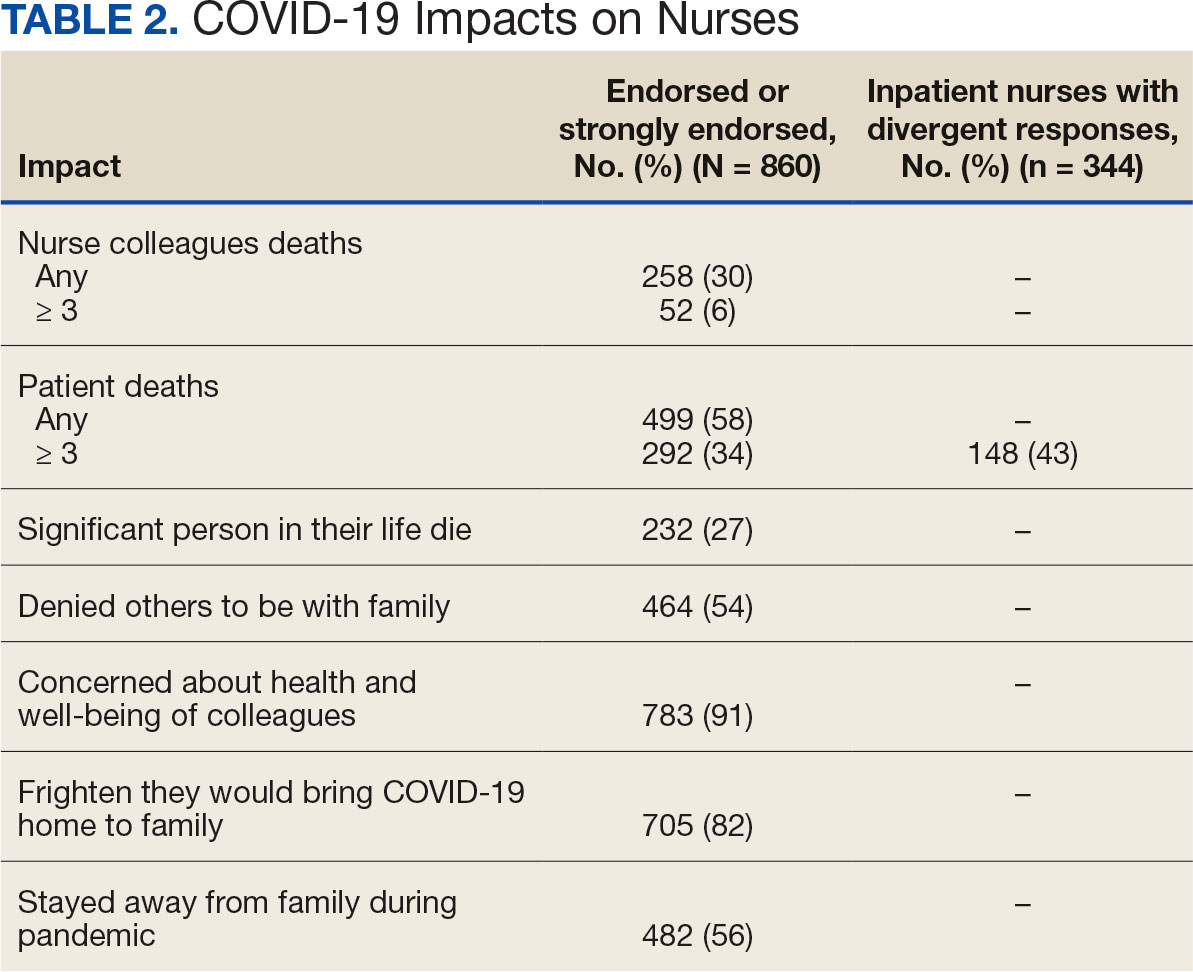
Two hundred fifty-eight nurses (30%) reported having nurse colleagues who died and 52 (6%) had ≥ 3 colleagues who died. Among respondents, 292 had ≥ 3 patients who died after contracting COVID-19 and 232 (27%) had a significant person in their life die. More than one-half (54%; n = 464) of nurses had to limit contact with a family member who had COVID-19. Most nurses reported concerns about their colleagues (91%), were concerned about bringing COVID-19 home (82%), and stayed away from family during the pandemic (56%) (Table 2).
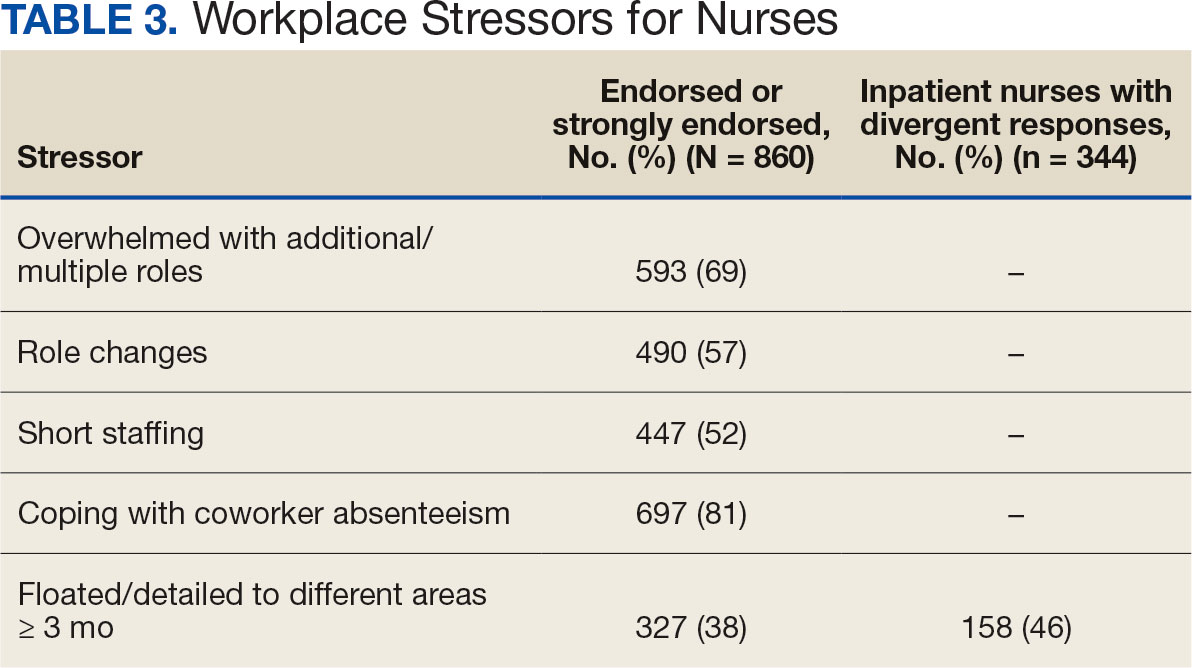
A total of 593 nurses (69%) reported feeling overwhelmed from the workload associated with the pandemic, 490 (57%) felt frustrated with role changes, 447 (52%) were stressed because of short staffing, and 327 (38%) felt stressed because of being assigned or floated to different patient care areas. Among inpatient nurses, 158 (46%) reported stress related to being floated. Coworker absenteeism caused challenges for 697 nurses (81%) (Table 3).
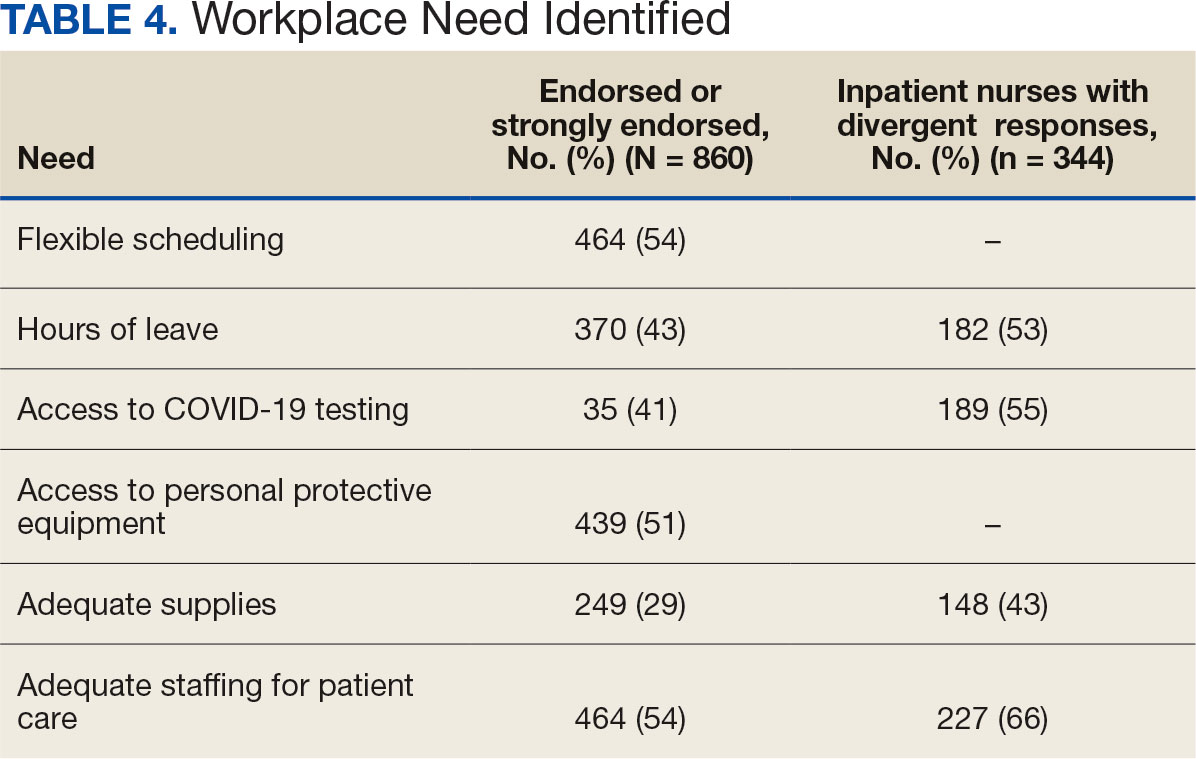
Nurses suggested a number of changes that could improve working conditions, including flexible scheduling (54%) and more hours of leave, which was requested by 43% of outpatient/inpatient nurses and 53% of inpatient alone nurses. Access to COVID-19 testing and PPE was endorsed as a workplace need by 439 nurses; the need for access to PPE was reported by 43% of inpatient-only nurses vs 29% of outpatient/inpatient nurses. The need for adequate staffing was reported by 54% of nurses although the rate was higher among those working inpatient settings (66%) (Table 4).
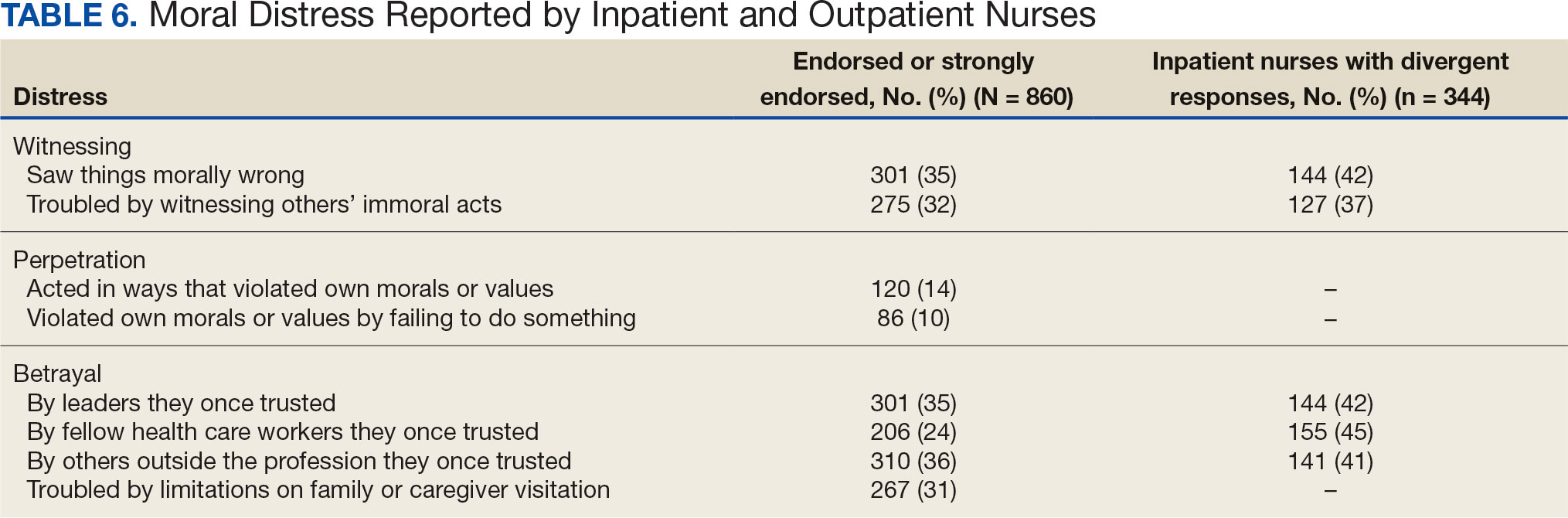
Four hundred sixty-four nurses (54%) felt tense and irritable at home because of work and 447 had ≥ 1 symptoms of burnout (Table 5). In terms of moral distress, > 30% of nurses witnessed morally incongruent situations, 10% felt their own moral code was violated, and > 30% felt betrayed by others (Table 6). Among respondents, 16% to 21% of nurses reported depressive symptoms (eAppendix). About 50% of nurses intended to stay in their current position while 20% indicated an intention to leave for another VA position.


DISCUSSION
This study identified the impact of COVID-19 on nurses who work in VISN 21. The survey included a significant number of nurses who work in outpatient settings, which differed from most other published studies to date.15-19 This study found that inpatient and outpatient nurses were similarly impacted by the COVID-19 pandemic, although there were differences. A high percentage of nurses reported job satisfaction despite the personal and professional impact of the pandemic.
Caring for veterans can result in a therapeutic relationship with a deep appreciation of veterans’ service and sensitivity to their needs.25 Some nurses reported that they feel it is a privilege to care for veterans.
Most nurses who participated in this study felt they could rely on their colleagues and were concerned about their health and wellbeing. Kissel et al explored protective factors for nurses during the pandemic and found participants often reported that their coworkers were positive safeguards.17 At least 50% of respondents reported that management considered workplace safety and was concerned about their welfare. Previous research has found that a positive working organization that promoted safety and concern for staff were protective factors against stress among HCWs.26 A literature review of 3 coronavirus outbreaks illustrated the support from supervisors and colleagues promoted resiliency and reduced stress disorders.3
Similar to other studies, study respondents experienced profound losses, including the deaths of colleagues, patients, and family. In 2021 Howell reported that HCWs experienced increased stress, fear, anxiety, and other negative emotions following news of colleagues’ deaths from COVID-19.27 Kissel et al reported that nurses frequently described pandemic-related physical and psychological harm and witnessing distress that they had not been previously exposed to.17
Our findings illustrate the tightrope nurses walked while caring for patients and concerns about the health of their colleagues and family. Consistent with our findings, Howell found that HCWs were afraid of contracting the infection at work and then unknowingly giving it to others such as patients, coworkers, and household members. 27 Murat et al reported that some nurses chose to live separately during the pandemic to avoid spreading COVID-19 to relatives.19 Several researchers found that concerns about family and children were prevalent and led to fear, anxiety, and burnout among nurses.18,28,29 Shah et al suggested that nurses experiencing death in the workplace and within their family may have resulted in fear and anxiety about returning to work.29 Garcia and Calvo argued that nurses may have been stigmatized as carriers of COVID-19.16 In addition, the loss of prepandemic workplace rituals may have impacted performance, team connection, and functioning, and led to increased turnover and decreased attachment to the organization.30
This study described the significant workplace issues nurses endured during the pandemic, including being overwhelmed with additional and/or multiple roles and frustrated and stressed with role changes and short staffing. Nurses endorsed workplace challenges in the context of coworker absenteeism and reassignments to different areas, such as intensive care units (ICUs).17 Researchers also reported that displaced team members experienced loneliness and isolation when they were removed from their usual place of work and experienced distress caring for patients beyond their perceived competency or comfort.17,31 Nurses also experienced rapid organizational changes, resource scarcity, high patient-to-nurse ratios, inconsistent or limited communications, and the absence of protocols for prolonged mass casualty events.17 These challenges, such as significant uncertainty and rapidly changing working conditions, were shared experiences suggested to be similar to “tumbling into chaos,” and likened to the overwhelming situations faced during patient surges to a medical “war zone.”17
Study respondents indicated that nurses wanted better access to critical supplies, PPE, and COVID-19 testing; more flexible scheduling; longer leave times; and staffing that was appropriate to the patient volumes. These findings aligned with previous research. Howell found that HCWs, especially nurses, worried about childcare because of school closures and increased work hours.27 Nurses felt that hospital support was inaccessible or inadequate and worried about access to essential resources.17-19,27 Studies also found excessive workloads, and many nurses needed mental or financial assistance from the hospital in addition to more rest and less work.18,28 An editorial highlighted the potential adverse effects that a lack of PPE could have on staff ’s mental health because of perceptions of institutional betrayal, which occurs when trusted and powerful organizations seemingly act in ways that can harm those dependent on them for safety and well-being.32
Consistent with other research, this study found that a majority of nurses experienced significant burnout symptoms. The number of nurses reporting symptoms of burnout increased during the pandemic with ICU nurses reporting the highest levels.17,33 Soto-Rubio et al emphasized that working conditions experienced by nurses, such as interpersonal conflict, lack of trust in administration, workload, and role conflict, contributed to burnout during COVID-19.34 Other studies found that nurses experienced burnout caused by uncertainty, intense work, and extra duties contributed to higher burnout scores.18,19 It is not surprising that researchers have indicated that nurses experiencing burnout might display depressive and stress-related symptoms, insomnia, and concentration and memory problems.19
The results of this study indicate that one-third of participating nurses were experiencing moral distress. Burton et al described COVID-19 as an environment in which nurses witnessed, experienced, and at times had to participate in acts that involved ethical violations in care, institutional betrayal, and traumatic strain.9 Of note, our findings revealed that both inpatient and outpatient nurses experienced moral distress. Interestingly, Mantri et al found that COVID-19 increased moral injury but not burnout among health professionals, which differed from the results of this study.35
The findings of this study indicate that many nurses experienced depressive symptoms. A systematic review found a similar percentage of HCWs experienced depression while caring for patients with COVID- 19, though a Chinese study found a higher percentage.36,37 Previous research also found that the most difficult aspect of the COVID- 19 pandemic for nurses was coping with mental disorders such as depression, and that many experienced difficulty sleeping/ had poor sleep quality, believed a similar disaster would occur in the future, were irritated or angered easily, and experienced emotional exhaustion.15,19 The long-term mental and physical ramifications of caring for individuals with COVID-19 remain unknown. However, previous research suggests a high prevalence of depression, insomnia, anxiety, and distress, which could impair nurses’ professional performance.29
This study reported that a majority of nurses intended to stay in their current position and about 20% intended to leave for another position within the VA. Similar findings conducted early in the pandemic indicated that most participants did not intend to quit nursing.19
This study’s findings suggest the COVID-19 pandemic had an adverse impact on VISN 21 nurses. It is critical to develop, implement, and adopt adequate measures as early as possible to support the health care system, especially nurses.18
Implications
Before the COVID-19 pandemic, discussing burnout and moral anguish was common, primarily in critical care.14 However, these experiences became more widespread throughout nursing settings during the pandemic. Nurse leaders have been identified as responsible for ensuring the environmental safety and personal well-being of their colleagues during and after pandemics.14
Studies of HCW experiences during COVID-19 provide many insights into future preparedness, strategies to best handle another pandemic during its acute stage, and techniques to address issues that might persist. This study and others suggest that comprehensive interventions in preparation for, during, and after a pandemic are needed. We break down strategies into pandemic and postpandemic interventions based on a synthesis of the literature and the research team’s knowledge and expertise.3,14-16,27,29,36,38-44
Pandemic interventions. During a pandemic, it is important that nurses are adequately cared for to ensure they can continue to provide quality care for others. Resources supporting emotional well-being and addressing moral distress offered during a pandemic are essential. Implementing meaningful strategies could enhance nurses’ health and wellbeing. It is essential that leaders provide nurses a safe work environment/experience during a pandemic by instituting meaningful resources. In addition, developing best practices for leadership are critical.
Postpandemic interventions. Personal experiences of depression, burnout, and moral distress have not spontaneously resolved as the pandemic receded. Providing postpandemic interventions to lessen ongoing and lingering depressive, burnout, and moral distress symptoms experienced by frontline workers are critical. These interventions might prevent long-term health issues and the exodus of nurses.
Postpandemic interventions should include the integration of pandemic planning into new or existing educational or training programs for staff. Promotion and support of mental health services by health system leadership for nursing personnel implemented as a usual service will play an important role in preparing for future pandemics. A key role in preparation is developing and maintaining cooperation and ongoing mutual understanding, respect, and communication between leadership and nursing staff.
Future Research
This study’s findings inform VHA leadership and society about how a large group of nurses were impacted by COVID-19 while caring for patients in inpatient and outpatient settings and could provide a basis for extending this research to other groups of nurses or health care personnel. Future research might be helpful in identifying the impact of COVID-19 on nursing leadership. During conversations with nursing leadership, a common theme identified was that nurses did not feel that leadership was fully prepared for the level of emergency the pandemic created both personally and professionally; leadership expressed experiences similar to nurses providing direct care and felt powerless to help their nursing staff. Other areas of research could include identifying underlying factors contributing to burnout and moral distress and describing nurses’ expectations of or needs from leadership to best manage burnout and moral distress.
Limitations
Experiences of nurses who stopped working were not captured and information about their experiences might have different results. The survey distribution was limited to 2 emails (an initial email and a second at midpoint) sent at the discretion of the nurse executive of each facility. The study timeline was long because of complex regulatory protective processes inherent in the VHA system for researchers to include initial institutional review board review process, union notifications, and each facility’s response to the survey. Although 860 nurses participated, this was 15% of the 5586 VISN 21 nurses at the time of the study. Many clinical inpatient nurses do not have regular access to email, which might have impacted participation rate.
CONCLUSIONS
This study identified the impact COVID-19 had on nurses who worked in a large hospital system. The research team outlined strategies to be employed during and after the pandemic, such as preplanning for future pandemics to provide a framework for a comprehensive pandemic response protocol.
This study adds to generalized knowledge because it captured voices of inpatient and outpatient nurses, the latter had not been previously studied. As nurses and health care organizations move beyond the pandemic with a significant number of nurses continuing to experience effects, there is a need to institute interventions to assist nurses in healing and begin preparations for future pandemics.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
- Liu X, Kakade M, Fuller CJ, et al. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53(1):15-23. doi:10.1016/j.comppsych.2011.02.003
- Carmassi C, Foghi C, Dell’Oste V, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: What can we expect after the COVID-19 pandemic. Psychiatry Res. 2020;292:113312. doi:10.1016/j.psychres.2020.113312
- De Kock JH, Latham HA, Leslie SJ, et al. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21(1):104. doi:10.1186/s12889-020-10070-3
- Gualano MR, Sinigaglia T, Lo Moro G, et al. The burden of burnout among healthcare professionals of intensive care units and emergency departments during the covid-19 pandemic: a systematic review. Int J Environ Res Public Health. 2021;18(15):8172. doi:10.3390/ijerph18158172
- Sirois FM, Owens J. Factors associated with psychological distress in health-care workers during an infectious disease outbreak: a rapid systematic review of the evidence. Front Psychiatry. 2020;11;589545. doi:10.3389/fpsyt.2020.589545
- Talevi D, Socci V, Carai M, et al. Mental health outcomes of the COVID-19 pandemic. Riv Psichiatr. 2020;55(3);137-144. doi:10.1708/3382.33569
- Amsalem D, Lazarov A, Markowitz JC, et al. Psychiatric symptoms and moral injury among US healthcare workers in the COVID-19 era. BMC Psychiatry. 2021;21(1):546. doi:10.1186/s12888-021-03565-9
- Burton CW, Jenkins DK, Chan G.K, Zellner KL, Zalta AK. A mixed methods study of moral distress among frontline nurses during the COVID-19 pandemic. Psychol Trauma. 2023;16(4):568-575. doi:10.1037/tra0001493
- Stawicki SP, Jeanmonod R, Miller AC, et al. The 2019- 2020 novel coronavirus (Severe acute respiratory syndrome coronavirus 2) Pandemic:a Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group consensus paper. J Glob Infect Dis. 2020;12(2):47- 93. doi:10.4103/jgid.jgid_86_20
- Batra K, Singh TP, Sharma M, Batra R, Schvaneveldt N. Investigating the psychological impact of COVID- 19 among healthcare workers: a meta-analysis. Int J Environ Res Public Health. 2020;17(23):9096. doi:10.3390/ijerph17239096
- Xie W, Chen L, Feng F, et al. The prevalence of compassion satisfaction and compassion fatigue among nurses: a systematic review and meta-analysis. Int J Nurs Stud. 2021;120:103973. doi:10.1016/j.ijnurstu.2021.103973
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Nurses’ burnout and associated risk factors during the COVID-19 pandemic: a systematic review and meta-analysis. J Adv Nurs. 2021;77(8):3286-3302. doi:10.1111/jan.14839
- Hofmeyer A, Taylor R. Strategies and resources for nurse leaders to use to lead with empathy and prudence so they understand and address sources of anxiety among nurses practicing in the era of COVID-19. J Clin Nurs. 2021;30(1- 2):298-305. doi:10.1111/jocn.15520
- Chen R, Sun C, Chen JJ, et al. A large-scale survey on trauma, burnout, and posttraumatic growth among nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(1):102-116. doi:10.1111/inm.12796
- García G, Calvo J. The threat of COVID-19 and its influence on nursing staff burnout. J Adv Nurs. 2021;77(2):832-844. doi:10.1111/jan.14642
- Kissel KA, Filipek C, Jenkins J. Impact of the COVID- 19 pandemic on nurses working in intensive care units: a scoping review. Crit Care Nurse. 2023;43(2):55-63. doi:10.4037/ccn2023196
- Lin YY, Pan YA, Hsieh YL, et al. COVID-19 pandemic is associated with an adverse impact on burnout and mood disorder in healthcare professionals. Int J Environ Res and Public Health. 2021;18(7):3654. doi:10.3390/ijerph18073654
- Murat M, Köse S, Savas¸er S. Determination of stress, depression and burnout levels of front-line nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(2):533-543. doi:10.1111/inm.12818
- Purcell N, Bertenthal D, Usman H, et al. Moral injury and mental health in healthcare workers are linked to organizational culture and modifiable workplace conditions: results of a national, mixed-methods study conducted at Veterans Affairs (VA) medical centers during the COVID- 19 pandemic. PLOS Ment Health. 2024;1(7):e0000085. doi:10.1371/journal.pmen.0000085
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the Moral Injury Events Scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Prins A, Bovin MJ, Smolenski DJ, et al. The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. doi:10.1007/s11606-016-3703-5
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi:10.1097/01.MLR.0000093487.78664.3C
- Rohland BM, Kruse GR, Rohrer JE. Validation of a single- item measure of burnout against the Maslach Burnout Inventory among physicians. Stress and Health. 2004;20(2):75-79. doi:10.1002/smi.1002
- Carlson J. Baccalaureate nursing faculty competencies and teaching strategies to enhance the care of the veteran population: perspectives of Veteran Affairs Nursing Academy (VANA) faculty. J Prof Nurs. 2016;32(4):314-323. doi:10.1016/j.profnurs.2016.01.006
- Denning M, Goh ET, Tan B, et al. Determinants of burnout and other aspects of psychological well-being in healthcare workers during the Covid-19 pandemic: a multinational cross-sectional study. PloS One. 2021;16(4):e0238666. doi:10.1371/journal.pone.0238666
- Howell BAM. Battling burnout at the frontlines of health care amid COVID-19. AACN Adv Crit Care. 2021;32(2):195- 203. doi:10.4037/aacnacc2021454
- Afshari D, Nourollahi-Darabad M, Chinisaz N. Demographic predictors of resilience among nurses during the COVID-19 pandemic. Work. 2021;68(2):297-303. doi:10.3233/WOR-203376
- Shah M, Roggenkamp M, Ferrer L, Burger V, Brassil KJ. Mental health and COVID-19: the psychological implications of a pandemic for nurses. Clin J Oncol Nurs. 2021;25(1), 69-75. doi:10.1188/21.CJON.69-75
- Griner T, Souza M, Girard A, Hain P, High H, Williams M. COVID-19’s impact on nurses’ workplace rituals. Nurs Lead. 2021;19(4):425-430. doi:10.1016/j.mnl.2021.06.008
- Koren A, Alam MAU, Koneru S, DeVito A, Abdallah L, Liu B. Nursing perspectives on the impacts of COVID- 19: social media content analysis. JMIR Form Res. 2021;5(12):e31358. doi:10.2196/31358
- Gold JA. Covid-19: adverse mental health outcomes for healthcare workers. BMJ. 2020;5:369:m1815. doi: 10.1136/bmj.m1815. doi:10.1136/bmj.m1815
- Slusarz R, Cwiekala-Lewis K, Wysokinski M, Filipska- Blejder K, Fidecki W, Biercewicz M. Characteristics of occupational burnout among nurses of various specialties and in the time of the COVID-19 pandemic-review. Int J Environ Res Public Health. 2022;19(21):13775. doi:10.3390/ijerph192113775
- Soto-Rubio A, Giménez-Espert MDC, Prado-Gascó V. Effect of emotional intelligence and psychosocial risks on burnout, job satisfaction, and nurses’ health during the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(21):7998. doi:10.3390/ijerph17217998
- Mantri S, Song YK, Lawson JM, Berger EJ, Koenig HG. Moral injury and burnout in health care professionals during the COVID-19 pandemic. J Nerv Ment Dis. 2021;209(10):720-726. doi:10.1097/NMD.0000000000001367
- Salari N, Khazaie H, Hosseinian-Far A, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health 2020;18(1):100. doi:10.1186/s12960-020-00544-1
- Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. doi:10.1001/jamanetworkopen.2020.3976
- Chesak SS, Cutshall SM, Bowe CL, Montanari KM, Bhagra A. Stress management interventions for nurses: critical literature review. J Holist Nurs. 2019;37(3):288-295. doi:10.1177/0898010119842693
- Cooper AL, Brown JA, Leslie GD. Nurse resilience for clinical practice: an integrative review. J Adv Nurs. 2021;77(6):2623-2640. doi:10.1111/jan.14763
- Melnyk BM, Kelly SA, Stephens J, et al. Interventions to improve mental health, well-being, physical health, and lifestyle behaviors in physicians and nurses: a systematic review. Am J Health Promot. 2020;34(8):929-941. doi:10.1177/0890117120920451
- Cho H, Sagherian K, Steege LM. Hospital staff nurse perceptions of resources and resource needs during the COVID-19 pandemic. Nurs Outlook. 2023;71(3):101984. doi:10.1016/j.outlook.2023.101984
- Bachem R, Tsur N, Levin Y, Abu-Raiya H, Maercker A. Negative affect, fatalism, and perceived institutional betrayal in times of the coronavirus pandemic: a cross-cultural investigation of control beliefs. Front Psychiatry. 2020;11:589914. doi:10.3389/fpsyt.2020.589914
- Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323(21):2133. doi:10.1001/jama.2020.5893
- Schuster M, Dwyer PA. Post-traumatic stress disorder in nurses: an integrative review. J Clin Nurs. 2020;29(15- 16):2769-2787. doi:10.1111/jocn.15288
On March 11, 2020, the World Health Organization designated COVID- 19 as a pandemic.1 Pandemics have historically impacted physical and mental health across all populations, but especially health care workers (HCWs).2 Nurses and other HCWs were profoundly impacted by the pandemic.3-8
Throughout the pandemic, nurses continued to provide care while working in short-staffed workplaces, facing increased exposure to COVID-19, and witnessing COVID—19–related morbidity and mortality.9 Many nurses were mandated to cross-train in unfamiliar clinical settings and adjust to new and prolonged shift schedules. Physical and emotional exhaustion associated with managing care for individuals with COVID-19, shortage of personal protective equipment (PPE), risk of infection, fear of secondary transmission to family members, feelings of being rejected by others, and social isolation, led to HCWs’ increased vulnerability to psychological impacts of the pandemic.8,10
A meta-analysis of 65 studies with > 79,000 participants found HCWs experienced significant levels of anxiety, depression, stress, insomnia, and other mental health issues, such as posttraumatic stress disorder (PTSD). Female HCWs, nurses, and frontline responders experienced a higher incidence of psychological impact.11 Other meta-analyses revealed that nurses’ compassion satisfaction, compassion fatigue, and burnout levels were significantly impacted with increased levels of burnout among nurses who had a friend or family member diagnosed with COVID- 19 or experienced prolonged threat of exposure to the virus.12,13 A study of 350 nurses found high rates of perceived transgressions by others, and betrayal.8 Nurse leaders and staff nurses had to persevere as moral distress became pervasive among nursing staff, which led to complex and often unsustainable circumstances. 14 The themes identified in the literature about the pandemic’s impact as well as witnessing nurse colleagues’ distress with patient mortality and death of coworkers during the early phase of the COVID-19 pandemic compelled a group of Veterans Health Administration (VHA) nurses to form a research team to understand the scope of impact and identify possible solutions.
Since published studies on the impact of pandemics on HCWs, including nurses, primarily focused on inpatient settings, the investigators of this study sought to capture the experiences of outpatient and inpatient nurses providing care in the US Department of Veterans Affairs (VA) Sierra Pacific Network (Veterans Integrated Service Network [VISN] 21), which has facilities in northern California, Hawaii, and Nevada.15-19 The purpose of this study was to identify the impact of COVID-19 on nurses caring for veterans in both outpatient and inpatient settings at VISN 21 facilities from March 2020 to September 2022, to inform leadership about the extent the virus affected nurses, and identify strategies that address current and future impacts of pandemics.
METHODS
This retrospective descriptive survey adapted the Pandemic Impact Survey by Purcell et al, which included the Moral Injury Events Scale, Primary Care PTSD Screener, the Patient Health Questionnaire-2 for depression, and a modified burnout scale.20-24 The survey of 70 Likert-scale questions was intended to measure nurses’ needs, burnout, moral distress, depression and stress symptoms, work-related factors, and intent to remain working in their current position. A nurse was defined broadly and included those employed as licensed vocational nurses (LVN), licensed practical nurses (LPN), registered nurses (RN), nurses with advanced degrees, advanced practice registered nurses (APRNs), and nurses with other certifications or licenses.
The VA Pacific Islands Research and Development Committee reviewed and approved the institutional review board-exempted study. The VISN 21 union was notified; only limited demographic information and broad VA tenure categories were collected to protect privacy. The principal investigator redacted facility identifier data after each facility had participated.
The survey was placed in REDCAP and a confidential link was emailed to all VISN 21 inpatient and outpatient nurses during March 2023. Because a comprehensive VISN 21 list of nurse email addresses was unavailable, the email was distributed by nursing leadership at each facility. Nurses received an email reminder at the 2-week halfway point, prompting them to complete the survey. The email indicated the purpose and voluntary nature of the study and cautioned nurses that they might experience stress while answering survey questions. Stress management resources were provided.
Descriptive statistics were used to report the results. Data were aggregated for analyzing and reporting purposes.
RESULTS
In March 2023, 860 of 5586 nurses (15%) responded to the survey. Respondents included 344 clinical inpatient nurses (40%) and 516 clinical outpatient nurses (60%); 688 (80%) were RNs, 129 (15%) were LPNs/LVNs, and 43 (5%) were APRNs. Of 849 respondents to provide their age, 15 (2%) were < 30 years, 163 (19%) were 30 to 39 years, 232 (27%) were 40 to 49 years, 259 (30%) were 50 to 59 years, and 180 (21%) were ≥ 60 years.
The survey found that 688 nurses reported job satisfaction (80%) and 75% of all respondents (66% among inpatient nurses) reported feeling happy with the care they delivered. Both inpatient and outpatient nurses indicated they could rely on staff. Sixty percent (n = 516) of the nurses indicated that facility management considered workplace health and safety and supervisors showed concern for subordinates, although inpatient nurses reported a lower percentage (Table 1).

Two hundred fifty-eight nurses (30%) reported having nurse colleagues who died and 52 (6%) had ≥ 3 colleagues who died. Among respondents, 292 had ≥ 3 patients who died after contracting COVID-19 and 232 (27%) had a significant person in their life die. More than one-half (54%; n = 464) of nurses had to limit contact with a family member who had COVID-19. Most nurses reported concerns about their colleagues (91%), were concerned about bringing COVID-19 home (82%), and stayed away from family during the pandemic (56%) (Table 2).

A total of 593 nurses (69%) reported feeling overwhelmed from the workload associated with the pandemic, 490 (57%) felt frustrated with role changes, 447 (52%) were stressed because of short staffing, and 327 (38%) felt stressed because of being assigned or floated to different patient care areas. Among inpatient nurses, 158 (46%) reported stress related to being floated. Coworker absenteeism caused challenges for 697 nurses (81%) (Table 3).

Nurses suggested a number of changes that could improve working conditions, including flexible scheduling (54%) and more hours of leave, which was requested by 43% of outpatient/inpatient nurses and 53% of inpatient alone nurses. Access to COVID-19 testing and PPE was endorsed as a workplace need by 439 nurses; the need for access to PPE was reported by 43% of inpatient-only nurses vs 29% of outpatient/inpatient nurses. The need for adequate staffing was reported by 54% of nurses although the rate was higher among those working inpatient settings (66%) (Table 4).

Four hundred sixty-four nurses (54%) felt tense and irritable at home because of work and 447 had ≥ 1 symptoms of burnout (Table 5). In terms of moral distress, > 30% of nurses witnessed morally incongruent situations, 10% felt their own moral code was violated, and > 30% felt betrayed by others (Table 6). Among respondents, 16% to 21% of nurses reported depressive symptoms (eAppendix). About 50% of nurses intended to stay in their current position while 20% indicated an intention to leave for another VA position.



DISCUSSION
This study identified the impact of COVID-19 on nurses who work in VISN 21. The survey included a significant number of nurses who work in outpatient settings, which differed from most other published studies to date.15-19 This study found that inpatient and outpatient nurses were similarly impacted by the COVID-19 pandemic, although there were differences. A high percentage of nurses reported job satisfaction despite the personal and professional impact of the pandemic.
Caring for veterans can result in a therapeutic relationship with a deep appreciation of veterans’ service and sensitivity to their needs.25 Some nurses reported that they feel it is a privilege to care for veterans.
Most nurses who participated in this study felt they could rely on their colleagues and were concerned about their health and wellbeing. Kissel et al explored protective factors for nurses during the pandemic and found participants often reported that their coworkers were positive safeguards.17 At least 50% of respondents reported that management considered workplace safety and was concerned about their welfare. Previous research has found that a positive working organization that promoted safety and concern for staff were protective factors against stress among HCWs.26 A literature review of 3 coronavirus outbreaks illustrated the support from supervisors and colleagues promoted resiliency and reduced stress disorders.3
Similar to other studies, study respondents experienced profound losses, including the deaths of colleagues, patients, and family. In 2021 Howell reported that HCWs experienced increased stress, fear, anxiety, and other negative emotions following news of colleagues’ deaths from COVID-19.27 Kissel et al reported that nurses frequently described pandemic-related physical and psychological harm and witnessing distress that they had not been previously exposed to.17
Our findings illustrate the tightrope nurses walked while caring for patients and concerns about the health of their colleagues and family. Consistent with our findings, Howell found that HCWs were afraid of contracting the infection at work and then unknowingly giving it to others such as patients, coworkers, and household members. 27 Murat et al reported that some nurses chose to live separately during the pandemic to avoid spreading COVID-19 to relatives.19 Several researchers found that concerns about family and children were prevalent and led to fear, anxiety, and burnout among nurses.18,28,29 Shah et al suggested that nurses experiencing death in the workplace and within their family may have resulted in fear and anxiety about returning to work.29 Garcia and Calvo argued that nurses may have been stigmatized as carriers of COVID-19.16 In addition, the loss of prepandemic workplace rituals may have impacted performance, team connection, and functioning, and led to increased turnover and decreased attachment to the organization.30
This study described the significant workplace issues nurses endured during the pandemic, including being overwhelmed with additional and/or multiple roles and frustrated and stressed with role changes and short staffing. Nurses endorsed workplace challenges in the context of coworker absenteeism and reassignments to different areas, such as intensive care units (ICUs).17 Researchers also reported that displaced team members experienced loneliness and isolation when they were removed from their usual place of work and experienced distress caring for patients beyond their perceived competency or comfort.17,31 Nurses also experienced rapid organizational changes, resource scarcity, high patient-to-nurse ratios, inconsistent or limited communications, and the absence of protocols for prolonged mass casualty events.17 These challenges, such as significant uncertainty and rapidly changing working conditions, were shared experiences suggested to be similar to “tumbling into chaos,” and likened to the overwhelming situations faced during patient surges to a medical “war zone.”17
Study respondents indicated that nurses wanted better access to critical supplies, PPE, and COVID-19 testing; more flexible scheduling; longer leave times; and staffing that was appropriate to the patient volumes. These findings aligned with previous research. Howell found that HCWs, especially nurses, worried about childcare because of school closures and increased work hours.27 Nurses felt that hospital support was inaccessible or inadequate and worried about access to essential resources.17-19,27 Studies also found excessive workloads, and many nurses needed mental or financial assistance from the hospital in addition to more rest and less work.18,28 An editorial highlighted the potential adverse effects that a lack of PPE could have on staff ’s mental health because of perceptions of institutional betrayal, which occurs when trusted and powerful organizations seemingly act in ways that can harm those dependent on them for safety and well-being.32
Consistent with other research, this study found that a majority of nurses experienced significant burnout symptoms. The number of nurses reporting symptoms of burnout increased during the pandemic with ICU nurses reporting the highest levels.17,33 Soto-Rubio et al emphasized that working conditions experienced by nurses, such as interpersonal conflict, lack of trust in administration, workload, and role conflict, contributed to burnout during COVID-19.34 Other studies found that nurses experienced burnout caused by uncertainty, intense work, and extra duties contributed to higher burnout scores.18,19 It is not surprising that researchers have indicated that nurses experiencing burnout might display depressive and stress-related symptoms, insomnia, and concentration and memory problems.19
The results of this study indicate that one-third of participating nurses were experiencing moral distress. Burton et al described COVID-19 as an environment in which nurses witnessed, experienced, and at times had to participate in acts that involved ethical violations in care, institutional betrayal, and traumatic strain.9 Of note, our findings revealed that both inpatient and outpatient nurses experienced moral distress. Interestingly, Mantri et al found that COVID-19 increased moral injury but not burnout among health professionals, which differed from the results of this study.35
The findings of this study indicate that many nurses experienced depressive symptoms. A systematic review found a similar percentage of HCWs experienced depression while caring for patients with COVID- 19, though a Chinese study found a higher percentage.36,37 Previous research also found that the most difficult aspect of the COVID- 19 pandemic for nurses was coping with mental disorders such as depression, and that many experienced difficulty sleeping/ had poor sleep quality, believed a similar disaster would occur in the future, were irritated or angered easily, and experienced emotional exhaustion.15,19 The long-term mental and physical ramifications of caring for individuals with COVID-19 remain unknown. However, previous research suggests a high prevalence of depression, insomnia, anxiety, and distress, which could impair nurses’ professional performance.29
This study reported that a majority of nurses intended to stay in their current position and about 20% intended to leave for another position within the VA. Similar findings conducted early in the pandemic indicated that most participants did not intend to quit nursing.19
This study’s findings suggest the COVID-19 pandemic had an adverse impact on VISN 21 nurses. It is critical to develop, implement, and adopt adequate measures as early as possible to support the health care system, especially nurses.18
Implications
Before the COVID-19 pandemic, discussing burnout and moral anguish was common, primarily in critical care.14 However, these experiences became more widespread throughout nursing settings during the pandemic. Nurse leaders have been identified as responsible for ensuring the environmental safety and personal well-being of their colleagues during and after pandemics.14
Studies of HCW experiences during COVID-19 provide many insights into future preparedness, strategies to best handle another pandemic during its acute stage, and techniques to address issues that might persist. This study and others suggest that comprehensive interventions in preparation for, during, and after a pandemic are needed. We break down strategies into pandemic and postpandemic interventions based on a synthesis of the literature and the research team’s knowledge and expertise.3,14-16,27,29,36,38-44
Pandemic interventions. During a pandemic, it is important that nurses are adequately cared for to ensure they can continue to provide quality care for others. Resources supporting emotional well-being and addressing moral distress offered during a pandemic are essential. Implementing meaningful strategies could enhance nurses’ health and wellbeing. It is essential that leaders provide nurses a safe work environment/experience during a pandemic by instituting meaningful resources. In addition, developing best practices for leadership are critical.
Postpandemic interventions. Personal experiences of depression, burnout, and moral distress have not spontaneously resolved as the pandemic receded. Providing postpandemic interventions to lessen ongoing and lingering depressive, burnout, and moral distress symptoms experienced by frontline workers are critical. These interventions might prevent long-term health issues and the exodus of nurses.
Postpandemic interventions should include the integration of pandemic planning into new or existing educational or training programs for staff. Promotion and support of mental health services by health system leadership for nursing personnel implemented as a usual service will play an important role in preparing for future pandemics. A key role in preparation is developing and maintaining cooperation and ongoing mutual understanding, respect, and communication between leadership and nursing staff.
Future Research
This study’s findings inform VHA leadership and society about how a large group of nurses were impacted by COVID-19 while caring for patients in inpatient and outpatient settings and could provide a basis for extending this research to other groups of nurses or health care personnel. Future research might be helpful in identifying the impact of COVID-19 on nursing leadership. During conversations with nursing leadership, a common theme identified was that nurses did not feel that leadership was fully prepared for the level of emergency the pandemic created both personally and professionally; leadership expressed experiences similar to nurses providing direct care and felt powerless to help their nursing staff. Other areas of research could include identifying underlying factors contributing to burnout and moral distress and describing nurses’ expectations of or needs from leadership to best manage burnout and moral distress.
Limitations
Experiences of nurses who stopped working were not captured and information about their experiences might have different results. The survey distribution was limited to 2 emails (an initial email and a second at midpoint) sent at the discretion of the nurse executive of each facility. The study timeline was long because of complex regulatory protective processes inherent in the VHA system for researchers to include initial institutional review board review process, union notifications, and each facility’s response to the survey. Although 860 nurses participated, this was 15% of the 5586 VISN 21 nurses at the time of the study. Many clinical inpatient nurses do not have regular access to email, which might have impacted participation rate.
CONCLUSIONS
This study identified the impact COVID-19 had on nurses who worked in a large hospital system. The research team outlined strategies to be employed during and after the pandemic, such as preplanning for future pandemics to provide a framework for a comprehensive pandemic response protocol.
This study adds to generalized knowledge because it captured voices of inpatient and outpatient nurses, the latter had not been previously studied. As nurses and health care organizations move beyond the pandemic with a significant number of nurses continuing to experience effects, there is a need to institute interventions to assist nurses in healing and begin preparations for future pandemics.
On March 11, 2020, the World Health Organization designated COVID- 19 as a pandemic.1 Pandemics have historically impacted physical and mental health across all populations, but especially health care workers (HCWs).2 Nurses and other HCWs were profoundly impacted by the pandemic.3-8
Throughout the pandemic, nurses continued to provide care while working in short-staffed workplaces, facing increased exposure to COVID-19, and witnessing COVID—19–related morbidity and mortality.9 Many nurses were mandated to cross-train in unfamiliar clinical settings and adjust to new and prolonged shift schedules. Physical and emotional exhaustion associated with managing care for individuals with COVID-19, shortage of personal protective equipment (PPE), risk of infection, fear of secondary transmission to family members, feelings of being rejected by others, and social isolation, led to HCWs’ increased vulnerability to psychological impacts of the pandemic.8,10
A meta-analysis of 65 studies with > 79,000 participants found HCWs experienced significant levels of anxiety, depression, stress, insomnia, and other mental health issues, such as posttraumatic stress disorder (PTSD). Female HCWs, nurses, and frontline responders experienced a higher incidence of psychological impact.11 Other meta-analyses revealed that nurses’ compassion satisfaction, compassion fatigue, and burnout levels were significantly impacted with increased levels of burnout among nurses who had a friend or family member diagnosed with COVID- 19 or experienced prolonged threat of exposure to the virus.12,13 A study of 350 nurses found high rates of perceived transgressions by others, and betrayal.8 Nurse leaders and staff nurses had to persevere as moral distress became pervasive among nursing staff, which led to complex and often unsustainable circumstances. 14 The themes identified in the literature about the pandemic’s impact as well as witnessing nurse colleagues’ distress with patient mortality and death of coworkers during the early phase of the COVID-19 pandemic compelled a group of Veterans Health Administration (VHA) nurses to form a research team to understand the scope of impact and identify possible solutions.
Since published studies on the impact of pandemics on HCWs, including nurses, primarily focused on inpatient settings, the investigators of this study sought to capture the experiences of outpatient and inpatient nurses providing care in the US Department of Veterans Affairs (VA) Sierra Pacific Network (Veterans Integrated Service Network [VISN] 21), which has facilities in northern California, Hawaii, and Nevada.15-19 The purpose of this study was to identify the impact of COVID-19 on nurses caring for veterans in both outpatient and inpatient settings at VISN 21 facilities from March 2020 to September 2022, to inform leadership about the extent the virus affected nurses, and identify strategies that address current and future impacts of pandemics.
METHODS
This retrospective descriptive survey adapted the Pandemic Impact Survey by Purcell et al, which included the Moral Injury Events Scale, Primary Care PTSD Screener, the Patient Health Questionnaire-2 for depression, and a modified burnout scale.20-24 The survey of 70 Likert-scale questions was intended to measure nurses’ needs, burnout, moral distress, depression and stress symptoms, work-related factors, and intent to remain working in their current position. A nurse was defined broadly and included those employed as licensed vocational nurses (LVN), licensed practical nurses (LPN), registered nurses (RN), nurses with advanced degrees, advanced practice registered nurses (APRNs), and nurses with other certifications or licenses.
The VA Pacific Islands Research and Development Committee reviewed and approved the institutional review board-exempted study. The VISN 21 union was notified; only limited demographic information and broad VA tenure categories were collected to protect privacy. The principal investigator redacted facility identifier data after each facility had participated.
The survey was placed in REDCAP and a confidential link was emailed to all VISN 21 inpatient and outpatient nurses during March 2023. Because a comprehensive VISN 21 list of nurse email addresses was unavailable, the email was distributed by nursing leadership at each facility. Nurses received an email reminder at the 2-week halfway point, prompting them to complete the survey. The email indicated the purpose and voluntary nature of the study and cautioned nurses that they might experience stress while answering survey questions. Stress management resources were provided.
Descriptive statistics were used to report the results. Data were aggregated for analyzing and reporting purposes.
RESULTS
In March 2023, 860 of 5586 nurses (15%) responded to the survey. Respondents included 344 clinical inpatient nurses (40%) and 516 clinical outpatient nurses (60%); 688 (80%) were RNs, 129 (15%) were LPNs/LVNs, and 43 (5%) were APRNs. Of 849 respondents to provide their age, 15 (2%) were < 30 years, 163 (19%) were 30 to 39 years, 232 (27%) were 40 to 49 years, 259 (30%) were 50 to 59 years, and 180 (21%) were ≥ 60 years.
The survey found that 688 nurses reported job satisfaction (80%) and 75% of all respondents (66% among inpatient nurses) reported feeling happy with the care they delivered. Both inpatient and outpatient nurses indicated they could rely on staff. Sixty percent (n = 516) of the nurses indicated that facility management considered workplace health and safety and supervisors showed concern for subordinates, although inpatient nurses reported a lower percentage (Table 1).

Two hundred fifty-eight nurses (30%) reported having nurse colleagues who died and 52 (6%) had ≥ 3 colleagues who died. Among respondents, 292 had ≥ 3 patients who died after contracting COVID-19 and 232 (27%) had a significant person in their life die. More than one-half (54%; n = 464) of nurses had to limit contact with a family member who had COVID-19. Most nurses reported concerns about their colleagues (91%), were concerned about bringing COVID-19 home (82%), and stayed away from family during the pandemic (56%) (Table 2).

A total of 593 nurses (69%) reported feeling overwhelmed from the workload associated with the pandemic, 490 (57%) felt frustrated with role changes, 447 (52%) were stressed because of short staffing, and 327 (38%) felt stressed because of being assigned or floated to different patient care areas. Among inpatient nurses, 158 (46%) reported stress related to being floated. Coworker absenteeism caused challenges for 697 nurses (81%) (Table 3).

Nurses suggested a number of changes that could improve working conditions, including flexible scheduling (54%) and more hours of leave, which was requested by 43% of outpatient/inpatient nurses and 53% of inpatient alone nurses. Access to COVID-19 testing and PPE was endorsed as a workplace need by 439 nurses; the need for access to PPE was reported by 43% of inpatient-only nurses vs 29% of outpatient/inpatient nurses. The need for adequate staffing was reported by 54% of nurses although the rate was higher among those working inpatient settings (66%) (Table 4).

Four hundred sixty-four nurses (54%) felt tense and irritable at home because of work and 447 had ≥ 1 symptoms of burnout (Table 5). In terms of moral distress, > 30% of nurses witnessed morally incongruent situations, 10% felt their own moral code was violated, and > 30% felt betrayed by others (Table 6). Among respondents, 16% to 21% of nurses reported depressive symptoms (eAppendix). About 50% of nurses intended to stay in their current position while 20% indicated an intention to leave for another VA position.



DISCUSSION
This study identified the impact of COVID-19 on nurses who work in VISN 21. The survey included a significant number of nurses who work in outpatient settings, which differed from most other published studies to date.15-19 This study found that inpatient and outpatient nurses were similarly impacted by the COVID-19 pandemic, although there were differences. A high percentage of nurses reported job satisfaction despite the personal and professional impact of the pandemic.
Caring for veterans can result in a therapeutic relationship with a deep appreciation of veterans’ service and sensitivity to their needs.25 Some nurses reported that they feel it is a privilege to care for veterans.
Most nurses who participated in this study felt they could rely on their colleagues and were concerned about their health and wellbeing. Kissel et al explored protective factors for nurses during the pandemic and found participants often reported that their coworkers were positive safeguards.17 At least 50% of respondents reported that management considered workplace safety and was concerned about their welfare. Previous research has found that a positive working organization that promoted safety and concern for staff were protective factors against stress among HCWs.26 A literature review of 3 coronavirus outbreaks illustrated the support from supervisors and colleagues promoted resiliency and reduced stress disorders.3
Similar to other studies, study respondents experienced profound losses, including the deaths of colleagues, patients, and family. In 2021 Howell reported that HCWs experienced increased stress, fear, anxiety, and other negative emotions following news of colleagues’ deaths from COVID-19.27 Kissel et al reported that nurses frequently described pandemic-related physical and psychological harm and witnessing distress that they had not been previously exposed to.17
Our findings illustrate the tightrope nurses walked while caring for patients and concerns about the health of their colleagues and family. Consistent with our findings, Howell found that HCWs were afraid of contracting the infection at work and then unknowingly giving it to others such as patients, coworkers, and household members. 27 Murat et al reported that some nurses chose to live separately during the pandemic to avoid spreading COVID-19 to relatives.19 Several researchers found that concerns about family and children were prevalent and led to fear, anxiety, and burnout among nurses.18,28,29 Shah et al suggested that nurses experiencing death in the workplace and within their family may have resulted in fear and anxiety about returning to work.29 Garcia and Calvo argued that nurses may have been stigmatized as carriers of COVID-19.16 In addition, the loss of prepandemic workplace rituals may have impacted performance, team connection, and functioning, and led to increased turnover and decreased attachment to the organization.30
This study described the significant workplace issues nurses endured during the pandemic, including being overwhelmed with additional and/or multiple roles and frustrated and stressed with role changes and short staffing. Nurses endorsed workplace challenges in the context of coworker absenteeism and reassignments to different areas, such as intensive care units (ICUs).17 Researchers also reported that displaced team members experienced loneliness and isolation when they were removed from their usual place of work and experienced distress caring for patients beyond their perceived competency or comfort.17,31 Nurses also experienced rapid organizational changes, resource scarcity, high patient-to-nurse ratios, inconsistent or limited communications, and the absence of protocols for prolonged mass casualty events.17 These challenges, such as significant uncertainty and rapidly changing working conditions, were shared experiences suggested to be similar to “tumbling into chaos,” and likened to the overwhelming situations faced during patient surges to a medical “war zone.”17
Study respondents indicated that nurses wanted better access to critical supplies, PPE, and COVID-19 testing; more flexible scheduling; longer leave times; and staffing that was appropriate to the patient volumes. These findings aligned with previous research. Howell found that HCWs, especially nurses, worried about childcare because of school closures and increased work hours.27 Nurses felt that hospital support was inaccessible or inadequate and worried about access to essential resources.17-19,27 Studies also found excessive workloads, and many nurses needed mental or financial assistance from the hospital in addition to more rest and less work.18,28 An editorial highlighted the potential adverse effects that a lack of PPE could have on staff ’s mental health because of perceptions of institutional betrayal, which occurs when trusted and powerful organizations seemingly act in ways that can harm those dependent on them for safety and well-being.32
Consistent with other research, this study found that a majority of nurses experienced significant burnout symptoms. The number of nurses reporting symptoms of burnout increased during the pandemic with ICU nurses reporting the highest levels.17,33 Soto-Rubio et al emphasized that working conditions experienced by nurses, such as interpersonal conflict, lack of trust in administration, workload, and role conflict, contributed to burnout during COVID-19.34 Other studies found that nurses experienced burnout caused by uncertainty, intense work, and extra duties contributed to higher burnout scores.18,19 It is not surprising that researchers have indicated that nurses experiencing burnout might display depressive and stress-related symptoms, insomnia, and concentration and memory problems.19
The results of this study indicate that one-third of participating nurses were experiencing moral distress. Burton et al described COVID-19 as an environment in which nurses witnessed, experienced, and at times had to participate in acts that involved ethical violations in care, institutional betrayal, and traumatic strain.9 Of note, our findings revealed that both inpatient and outpatient nurses experienced moral distress. Interestingly, Mantri et al found that COVID-19 increased moral injury but not burnout among health professionals, which differed from the results of this study.35
The findings of this study indicate that many nurses experienced depressive symptoms. A systematic review found a similar percentage of HCWs experienced depression while caring for patients with COVID- 19, though a Chinese study found a higher percentage.36,37 Previous research also found that the most difficult aspect of the COVID- 19 pandemic for nurses was coping with mental disorders such as depression, and that many experienced difficulty sleeping/ had poor sleep quality, believed a similar disaster would occur in the future, were irritated or angered easily, and experienced emotional exhaustion.15,19 The long-term mental and physical ramifications of caring for individuals with COVID-19 remain unknown. However, previous research suggests a high prevalence of depression, insomnia, anxiety, and distress, which could impair nurses’ professional performance.29
This study reported that a majority of nurses intended to stay in their current position and about 20% intended to leave for another position within the VA. Similar findings conducted early in the pandemic indicated that most participants did not intend to quit nursing.19
This study’s findings suggest the COVID-19 pandemic had an adverse impact on VISN 21 nurses. It is critical to develop, implement, and adopt adequate measures as early as possible to support the health care system, especially nurses.18
Implications
Before the COVID-19 pandemic, discussing burnout and moral anguish was common, primarily in critical care.14 However, these experiences became more widespread throughout nursing settings during the pandemic. Nurse leaders have been identified as responsible for ensuring the environmental safety and personal well-being of their colleagues during and after pandemics.14
Studies of HCW experiences during COVID-19 provide many insights into future preparedness, strategies to best handle another pandemic during its acute stage, and techniques to address issues that might persist. This study and others suggest that comprehensive interventions in preparation for, during, and after a pandemic are needed. We break down strategies into pandemic and postpandemic interventions based on a synthesis of the literature and the research team’s knowledge and expertise.3,14-16,27,29,36,38-44
Pandemic interventions. During a pandemic, it is important that nurses are adequately cared for to ensure they can continue to provide quality care for others. Resources supporting emotional well-being and addressing moral distress offered during a pandemic are essential. Implementing meaningful strategies could enhance nurses’ health and wellbeing. It is essential that leaders provide nurses a safe work environment/experience during a pandemic by instituting meaningful resources. In addition, developing best practices for leadership are critical.
Postpandemic interventions. Personal experiences of depression, burnout, and moral distress have not spontaneously resolved as the pandemic receded. Providing postpandemic interventions to lessen ongoing and lingering depressive, burnout, and moral distress symptoms experienced by frontline workers are critical. These interventions might prevent long-term health issues and the exodus of nurses.
Postpandemic interventions should include the integration of pandemic planning into new or existing educational or training programs for staff. Promotion and support of mental health services by health system leadership for nursing personnel implemented as a usual service will play an important role in preparing for future pandemics. A key role in preparation is developing and maintaining cooperation and ongoing mutual understanding, respect, and communication between leadership and nursing staff.
Future Research
This study’s findings inform VHA leadership and society about how a large group of nurses were impacted by COVID-19 while caring for patients in inpatient and outpatient settings and could provide a basis for extending this research to other groups of nurses or health care personnel. Future research might be helpful in identifying the impact of COVID-19 on nursing leadership. During conversations with nursing leadership, a common theme identified was that nurses did not feel that leadership was fully prepared for the level of emergency the pandemic created both personally and professionally; leadership expressed experiences similar to nurses providing direct care and felt powerless to help their nursing staff. Other areas of research could include identifying underlying factors contributing to burnout and moral distress and describing nurses’ expectations of or needs from leadership to best manage burnout and moral distress.
Limitations
Experiences of nurses who stopped working were not captured and information about their experiences might have different results. The survey distribution was limited to 2 emails (an initial email and a second at midpoint) sent at the discretion of the nurse executive of each facility. The study timeline was long because of complex regulatory protective processes inherent in the VHA system for researchers to include initial institutional review board review process, union notifications, and each facility’s response to the survey. Although 860 nurses participated, this was 15% of the 5586 VISN 21 nurses at the time of the study. Many clinical inpatient nurses do not have regular access to email, which might have impacted participation rate.
CONCLUSIONS
This study identified the impact COVID-19 had on nurses who worked in a large hospital system. The research team outlined strategies to be employed during and after the pandemic, such as preplanning for future pandemics to provide a framework for a comprehensive pandemic response protocol.
This study adds to generalized knowledge because it captured voices of inpatient and outpatient nurses, the latter had not been previously studied. As nurses and health care organizations move beyond the pandemic with a significant number of nurses continuing to experience effects, there is a need to institute interventions to assist nurses in healing and begin preparations for future pandemics.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
- Liu X, Kakade M, Fuller CJ, et al. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53(1):15-23. doi:10.1016/j.comppsych.2011.02.003
- Carmassi C, Foghi C, Dell’Oste V, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: What can we expect after the COVID-19 pandemic. Psychiatry Res. 2020;292:113312. doi:10.1016/j.psychres.2020.113312
- De Kock JH, Latham HA, Leslie SJ, et al. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21(1):104. doi:10.1186/s12889-020-10070-3
- Gualano MR, Sinigaglia T, Lo Moro G, et al. The burden of burnout among healthcare professionals of intensive care units and emergency departments during the covid-19 pandemic: a systematic review. Int J Environ Res Public Health. 2021;18(15):8172. doi:10.3390/ijerph18158172
- Sirois FM, Owens J. Factors associated with psychological distress in health-care workers during an infectious disease outbreak: a rapid systematic review of the evidence. Front Psychiatry. 2020;11;589545. doi:10.3389/fpsyt.2020.589545
- Talevi D, Socci V, Carai M, et al. Mental health outcomes of the COVID-19 pandemic. Riv Psichiatr. 2020;55(3);137-144. doi:10.1708/3382.33569
- Amsalem D, Lazarov A, Markowitz JC, et al. Psychiatric symptoms and moral injury among US healthcare workers in the COVID-19 era. BMC Psychiatry. 2021;21(1):546. doi:10.1186/s12888-021-03565-9
- Burton CW, Jenkins DK, Chan G.K, Zellner KL, Zalta AK. A mixed methods study of moral distress among frontline nurses during the COVID-19 pandemic. Psychol Trauma. 2023;16(4):568-575. doi:10.1037/tra0001493
- Stawicki SP, Jeanmonod R, Miller AC, et al. The 2019- 2020 novel coronavirus (Severe acute respiratory syndrome coronavirus 2) Pandemic:a Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group consensus paper. J Glob Infect Dis. 2020;12(2):47- 93. doi:10.4103/jgid.jgid_86_20
- Batra K, Singh TP, Sharma M, Batra R, Schvaneveldt N. Investigating the psychological impact of COVID- 19 among healthcare workers: a meta-analysis. Int J Environ Res Public Health. 2020;17(23):9096. doi:10.3390/ijerph17239096
- Xie W, Chen L, Feng F, et al. The prevalence of compassion satisfaction and compassion fatigue among nurses: a systematic review and meta-analysis. Int J Nurs Stud. 2021;120:103973. doi:10.1016/j.ijnurstu.2021.103973
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Nurses’ burnout and associated risk factors during the COVID-19 pandemic: a systematic review and meta-analysis. J Adv Nurs. 2021;77(8):3286-3302. doi:10.1111/jan.14839
- Hofmeyer A, Taylor R. Strategies and resources for nurse leaders to use to lead with empathy and prudence so they understand and address sources of anxiety among nurses practicing in the era of COVID-19. J Clin Nurs. 2021;30(1- 2):298-305. doi:10.1111/jocn.15520
- Chen R, Sun C, Chen JJ, et al. A large-scale survey on trauma, burnout, and posttraumatic growth among nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(1):102-116. doi:10.1111/inm.12796
- García G, Calvo J. The threat of COVID-19 and its influence on nursing staff burnout. J Adv Nurs. 2021;77(2):832-844. doi:10.1111/jan.14642
- Kissel KA, Filipek C, Jenkins J. Impact of the COVID- 19 pandemic on nurses working in intensive care units: a scoping review. Crit Care Nurse. 2023;43(2):55-63. doi:10.4037/ccn2023196
- Lin YY, Pan YA, Hsieh YL, et al. COVID-19 pandemic is associated with an adverse impact on burnout and mood disorder in healthcare professionals. Int J Environ Res and Public Health. 2021;18(7):3654. doi:10.3390/ijerph18073654
- Murat M, Köse S, Savas¸er S. Determination of stress, depression and burnout levels of front-line nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(2):533-543. doi:10.1111/inm.12818
- Purcell N, Bertenthal D, Usman H, et al. Moral injury and mental health in healthcare workers are linked to organizational culture and modifiable workplace conditions: results of a national, mixed-methods study conducted at Veterans Affairs (VA) medical centers during the COVID- 19 pandemic. PLOS Ment Health. 2024;1(7):e0000085. doi:10.1371/journal.pmen.0000085
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the Moral Injury Events Scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Prins A, Bovin MJ, Smolenski DJ, et al. The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. doi:10.1007/s11606-016-3703-5
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi:10.1097/01.MLR.0000093487.78664.3C
- Rohland BM, Kruse GR, Rohrer JE. Validation of a single- item measure of burnout against the Maslach Burnout Inventory among physicians. Stress and Health. 2004;20(2):75-79. doi:10.1002/smi.1002
- Carlson J. Baccalaureate nursing faculty competencies and teaching strategies to enhance the care of the veteran population: perspectives of Veteran Affairs Nursing Academy (VANA) faculty. J Prof Nurs. 2016;32(4):314-323. doi:10.1016/j.profnurs.2016.01.006
- Denning M, Goh ET, Tan B, et al. Determinants of burnout and other aspects of psychological well-being in healthcare workers during the Covid-19 pandemic: a multinational cross-sectional study. PloS One. 2021;16(4):e0238666. doi:10.1371/journal.pone.0238666
- Howell BAM. Battling burnout at the frontlines of health care amid COVID-19. AACN Adv Crit Care. 2021;32(2):195- 203. doi:10.4037/aacnacc2021454
- Afshari D, Nourollahi-Darabad M, Chinisaz N. Demographic predictors of resilience among nurses during the COVID-19 pandemic. Work. 2021;68(2):297-303. doi:10.3233/WOR-203376
- Shah M, Roggenkamp M, Ferrer L, Burger V, Brassil KJ. Mental health and COVID-19: the psychological implications of a pandemic for nurses. Clin J Oncol Nurs. 2021;25(1), 69-75. doi:10.1188/21.CJON.69-75
- Griner T, Souza M, Girard A, Hain P, High H, Williams M. COVID-19’s impact on nurses’ workplace rituals. Nurs Lead. 2021;19(4):425-430. doi:10.1016/j.mnl.2021.06.008
- Koren A, Alam MAU, Koneru S, DeVito A, Abdallah L, Liu B. Nursing perspectives on the impacts of COVID- 19: social media content analysis. JMIR Form Res. 2021;5(12):e31358. doi:10.2196/31358
- Gold JA. Covid-19: adverse mental health outcomes for healthcare workers. BMJ. 2020;5:369:m1815. doi: 10.1136/bmj.m1815. doi:10.1136/bmj.m1815
- Slusarz R, Cwiekala-Lewis K, Wysokinski M, Filipska- Blejder K, Fidecki W, Biercewicz M. Characteristics of occupational burnout among nurses of various specialties and in the time of the COVID-19 pandemic-review. Int J Environ Res Public Health. 2022;19(21):13775. doi:10.3390/ijerph192113775
- Soto-Rubio A, Giménez-Espert MDC, Prado-Gascó V. Effect of emotional intelligence and psychosocial risks on burnout, job satisfaction, and nurses’ health during the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(21):7998. doi:10.3390/ijerph17217998
- Mantri S, Song YK, Lawson JM, Berger EJ, Koenig HG. Moral injury and burnout in health care professionals during the COVID-19 pandemic. J Nerv Ment Dis. 2021;209(10):720-726. doi:10.1097/NMD.0000000000001367
- Salari N, Khazaie H, Hosseinian-Far A, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health 2020;18(1):100. doi:10.1186/s12960-020-00544-1
- Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. doi:10.1001/jamanetworkopen.2020.3976
- Chesak SS, Cutshall SM, Bowe CL, Montanari KM, Bhagra A. Stress management interventions for nurses: critical literature review. J Holist Nurs. 2019;37(3):288-295. doi:10.1177/0898010119842693
- Cooper AL, Brown JA, Leslie GD. Nurse resilience for clinical practice: an integrative review. J Adv Nurs. 2021;77(6):2623-2640. doi:10.1111/jan.14763
- Melnyk BM, Kelly SA, Stephens J, et al. Interventions to improve mental health, well-being, physical health, and lifestyle behaviors in physicians and nurses: a systematic review. Am J Health Promot. 2020;34(8):929-941. doi:10.1177/0890117120920451
- Cho H, Sagherian K, Steege LM. Hospital staff nurse perceptions of resources and resource needs during the COVID-19 pandemic. Nurs Outlook. 2023;71(3):101984. doi:10.1016/j.outlook.2023.101984
- Bachem R, Tsur N, Levin Y, Abu-Raiya H, Maercker A. Negative affect, fatalism, and perceived institutional betrayal in times of the coronavirus pandemic: a cross-cultural investigation of control beliefs. Front Psychiatry. 2020;11:589914. doi:10.3389/fpsyt.2020.589914
- Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323(21):2133. doi:10.1001/jama.2020.5893
- Schuster M, Dwyer PA. Post-traumatic stress disorder in nurses: an integrative review. J Clin Nurs. 2020;29(15- 16):2769-2787. doi:10.1111/jocn.15288
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
- Liu X, Kakade M, Fuller CJ, et al. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53(1):15-23. doi:10.1016/j.comppsych.2011.02.003
- Carmassi C, Foghi C, Dell’Oste V, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: What can we expect after the COVID-19 pandemic. Psychiatry Res. 2020;292:113312. doi:10.1016/j.psychres.2020.113312
- De Kock JH, Latham HA, Leslie SJ, et al. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21(1):104. doi:10.1186/s12889-020-10070-3
- Gualano MR, Sinigaglia T, Lo Moro G, et al. The burden of burnout among healthcare professionals of intensive care units and emergency departments during the covid-19 pandemic: a systematic review. Int J Environ Res Public Health. 2021;18(15):8172. doi:10.3390/ijerph18158172
- Sirois FM, Owens J. Factors associated with psychological distress in health-care workers during an infectious disease outbreak: a rapid systematic review of the evidence. Front Psychiatry. 2020;11;589545. doi:10.3389/fpsyt.2020.589545
- Talevi D, Socci V, Carai M, et al. Mental health outcomes of the COVID-19 pandemic. Riv Psichiatr. 2020;55(3);137-144. doi:10.1708/3382.33569
- Amsalem D, Lazarov A, Markowitz JC, et al. Psychiatric symptoms and moral injury among US healthcare workers in the COVID-19 era. BMC Psychiatry. 2021;21(1):546. doi:10.1186/s12888-021-03565-9
- Burton CW, Jenkins DK, Chan G.K, Zellner KL, Zalta AK. A mixed methods study of moral distress among frontline nurses during the COVID-19 pandemic. Psychol Trauma. 2023;16(4):568-575. doi:10.1037/tra0001493
- Stawicki SP, Jeanmonod R, Miller AC, et al. The 2019- 2020 novel coronavirus (Severe acute respiratory syndrome coronavirus 2) Pandemic:a Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group consensus paper. J Glob Infect Dis. 2020;12(2):47- 93. doi:10.4103/jgid.jgid_86_20
- Batra K, Singh TP, Sharma M, Batra R, Schvaneveldt N. Investigating the psychological impact of COVID- 19 among healthcare workers: a meta-analysis. Int J Environ Res Public Health. 2020;17(23):9096. doi:10.3390/ijerph17239096
- Xie W, Chen L, Feng F, et al. The prevalence of compassion satisfaction and compassion fatigue among nurses: a systematic review and meta-analysis. Int J Nurs Stud. 2021;120:103973. doi:10.1016/j.ijnurstu.2021.103973
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Nurses’ burnout and associated risk factors during the COVID-19 pandemic: a systematic review and meta-analysis. J Adv Nurs. 2021;77(8):3286-3302. doi:10.1111/jan.14839
- Hofmeyer A, Taylor R. Strategies and resources for nurse leaders to use to lead with empathy and prudence so they understand and address sources of anxiety among nurses practicing in the era of COVID-19. J Clin Nurs. 2021;30(1- 2):298-305. doi:10.1111/jocn.15520
- Chen R, Sun C, Chen JJ, et al. A large-scale survey on trauma, burnout, and posttraumatic growth among nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(1):102-116. doi:10.1111/inm.12796
- García G, Calvo J. The threat of COVID-19 and its influence on nursing staff burnout. J Adv Nurs. 2021;77(2):832-844. doi:10.1111/jan.14642
- Kissel KA, Filipek C, Jenkins J. Impact of the COVID- 19 pandemic on nurses working in intensive care units: a scoping review. Crit Care Nurse. 2023;43(2):55-63. doi:10.4037/ccn2023196
- Lin YY, Pan YA, Hsieh YL, et al. COVID-19 pandemic is associated with an adverse impact on burnout and mood disorder in healthcare professionals. Int J Environ Res and Public Health. 2021;18(7):3654. doi:10.3390/ijerph18073654
- Murat M, Köse S, Savas¸er S. Determination of stress, depression and burnout levels of front-line nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(2):533-543. doi:10.1111/inm.12818
- Purcell N, Bertenthal D, Usman H, et al. Moral injury and mental health in healthcare workers are linked to organizational culture and modifiable workplace conditions: results of a national, mixed-methods study conducted at Veterans Affairs (VA) medical centers during the COVID- 19 pandemic. PLOS Ment Health. 2024;1(7):e0000085. doi:10.1371/journal.pmen.0000085
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the Moral Injury Events Scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Prins A, Bovin MJ, Smolenski DJ, et al. The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. doi:10.1007/s11606-016-3703-5
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi:10.1097/01.MLR.0000093487.78664.3C
- Rohland BM, Kruse GR, Rohrer JE. Validation of a single- item measure of burnout against the Maslach Burnout Inventory among physicians. Stress and Health. 2004;20(2):75-79. doi:10.1002/smi.1002
- Carlson J. Baccalaureate nursing faculty competencies and teaching strategies to enhance the care of the veteran population: perspectives of Veteran Affairs Nursing Academy (VANA) faculty. J Prof Nurs. 2016;32(4):314-323. doi:10.1016/j.profnurs.2016.01.006
- Denning M, Goh ET, Tan B, et al. Determinants of burnout and other aspects of psychological well-being in healthcare workers during the Covid-19 pandemic: a multinational cross-sectional study. PloS One. 2021;16(4):e0238666. doi:10.1371/journal.pone.0238666
- Howell BAM. Battling burnout at the frontlines of health care amid COVID-19. AACN Adv Crit Care. 2021;32(2):195- 203. doi:10.4037/aacnacc2021454
- Afshari D, Nourollahi-Darabad M, Chinisaz N. Demographic predictors of resilience among nurses during the COVID-19 pandemic. Work. 2021;68(2):297-303. doi:10.3233/WOR-203376
- Shah M, Roggenkamp M, Ferrer L, Burger V, Brassil KJ. Mental health and COVID-19: the psychological implications of a pandemic for nurses. Clin J Oncol Nurs. 2021;25(1), 69-75. doi:10.1188/21.CJON.69-75
- Griner T, Souza M, Girard A, Hain P, High H, Williams M. COVID-19’s impact on nurses’ workplace rituals. Nurs Lead. 2021;19(4):425-430. doi:10.1016/j.mnl.2021.06.008
- Koren A, Alam MAU, Koneru S, DeVito A, Abdallah L, Liu B. Nursing perspectives on the impacts of COVID- 19: social media content analysis. JMIR Form Res. 2021;5(12):e31358. doi:10.2196/31358
- Gold JA. Covid-19: adverse mental health outcomes for healthcare workers. BMJ. 2020;5:369:m1815. doi: 10.1136/bmj.m1815. doi:10.1136/bmj.m1815
- Slusarz R, Cwiekala-Lewis K, Wysokinski M, Filipska- Blejder K, Fidecki W, Biercewicz M. Characteristics of occupational burnout among nurses of various specialties and in the time of the COVID-19 pandemic-review. Int J Environ Res Public Health. 2022;19(21):13775. doi:10.3390/ijerph192113775
- Soto-Rubio A, Giménez-Espert MDC, Prado-Gascó V. Effect of emotional intelligence and psychosocial risks on burnout, job satisfaction, and nurses’ health during the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(21):7998. doi:10.3390/ijerph17217998
- Mantri S, Song YK, Lawson JM, Berger EJ, Koenig HG. Moral injury and burnout in health care professionals during the COVID-19 pandemic. J Nerv Ment Dis. 2021;209(10):720-726. doi:10.1097/NMD.0000000000001367
- Salari N, Khazaie H, Hosseinian-Far A, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health 2020;18(1):100. doi:10.1186/s12960-020-00544-1
- Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. doi:10.1001/jamanetworkopen.2020.3976
- Chesak SS, Cutshall SM, Bowe CL, Montanari KM, Bhagra A. Stress management interventions for nurses: critical literature review. J Holist Nurs. 2019;37(3):288-295. doi:10.1177/0898010119842693
- Cooper AL, Brown JA, Leslie GD. Nurse resilience for clinical practice: an integrative review. J Adv Nurs. 2021;77(6):2623-2640. doi:10.1111/jan.14763
- Melnyk BM, Kelly SA, Stephens J, et al. Interventions to improve mental health, well-being, physical health, and lifestyle behaviors in physicians and nurses: a systematic review. Am J Health Promot. 2020;34(8):929-941. doi:10.1177/0890117120920451
- Cho H, Sagherian K, Steege LM. Hospital staff nurse perceptions of resources and resource needs during the COVID-19 pandemic. Nurs Outlook. 2023;71(3):101984. doi:10.1016/j.outlook.2023.101984
- Bachem R, Tsur N, Levin Y, Abu-Raiya H, Maercker A. Negative affect, fatalism, and perceived institutional betrayal in times of the coronavirus pandemic: a cross-cultural investigation of control beliefs. Front Psychiatry. 2020;11:589914. doi:10.3389/fpsyt.2020.589914
- Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323(21):2133. doi:10.1001/jama.2020.5893
- Schuster M, Dwyer PA. Post-traumatic stress disorder in nurses: an integrative review. J Clin Nurs. 2020;29(15- 16):2769-2787. doi:10.1111/jocn.15288
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey
Progressive cognitive decline
Individuals with DS are at significantly increased risk of developing Alzheimer’s disease (AD) because of the overexpression of the amyloid precursor protein (APP) gene on chromosome 21.
The patient exhibits hallmark symptoms of AD, including progressive memory loss, disorientation, difficulty performing daily tasks, and behavioral changes such as irritability and social withdrawal. The MRI findings of ventricular enlargement and cortical atrophy are consistent with brain changes commonly seen in AD, particularly in individuals with DS who often develop these changes earlier in life (typically in their thirties or forties).
Frontotemporal dementia primarily causes behavioral and language changes with relative memory sparing early on, making it inconsistent with this patient's prominent memory loss, disorientation, and generalized cortical atrophy — features more typical of AD.
Vascular dementia often presents with stepwise decline and focal neurologic deficits; it is unlikely in this patient, given the absence of cerebrovascular events or risk factors like hypertension or diabetes.
While normal pressure hydrocephalus can cause ventricular enlargement, its classic triad of gait disturbance, urinary incontinence, and dementia is incomplete here, making this answer unlikely.
DS is the most common genetic cause of intellectual disability, occurring in approximately 1 in 700 live births worldwide. Nearly all adults with DS develop neuropathologic changes associated with AD by age 40, and the lifetime risk of developing dementia exceeds 90%; by the age of 55-60, at least 70% of individuals with DS exhibit clinical signs of dementia.
The link between DS and neurodegeneration is largely attributed to the triplication of chromosome 21, which includes the APP gene. Overexpression of APP leads to excessive production and accumulation of amyloid-β (Aβ), which forms the hallmark plaques seen in AD. In DS, amyloid plaques begin to form as early as the teenage years, and by the fourth decade, neurofibrillary tangles (tau protein aggregates) and widespread neurodegeneration are nearly universal.
Initial symptoms of AD often include memory impairment, particularly short-term memory deficits, and executive dysfunction, difficulty with planning and problem-solving, and visuospatial deficits. Behavioral and personality changes, such as irritability, withdrawal, or apathy, are also commonly reported. Compared with sporadic AD, individuals with DS often show earlier behavioral symptoms, including impulsivity and changes in social interactions, which may precede noticeable memory deficits. Additionally, late-onset myoclonic epilepsy is common in individuals with DS and dementia, further complicating diagnosis and care.
The diagnosis of dementia in DS is challenging because of baseline intellectual disability, which makes it difficult to assess cognitive decline using standard neuropsychological tests. However, a combination of clinical history, caregiver reports, neuroimaging, and biomarker analysis can aid in early detection. Structural MRI of the brain often reveals ventricular enlargement and cortical atrophy, while PET imaging can detect early amyloid and tau accumulation.
Because AD is now the leading cause of death in individuals with DS, the lack of effective disease-modifying treatments highlights an important need for clinical trials focused on this population. Current research aims to explore the role of anti-amyloid monoclonal antibodies, neuroprotective agents, and lifestyle interventions to delay or prevent neurodegeneration.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Individuals with DS are at significantly increased risk of developing Alzheimer’s disease (AD) because of the overexpression of the amyloid precursor protein (APP) gene on chromosome 21.
The patient exhibits hallmark symptoms of AD, including progressive memory loss, disorientation, difficulty performing daily tasks, and behavioral changes such as irritability and social withdrawal. The MRI findings of ventricular enlargement and cortical atrophy are consistent with brain changes commonly seen in AD, particularly in individuals with DS who often develop these changes earlier in life (typically in their thirties or forties).
Frontotemporal dementia primarily causes behavioral and language changes with relative memory sparing early on, making it inconsistent with this patient's prominent memory loss, disorientation, and generalized cortical atrophy — features more typical of AD.
Vascular dementia often presents with stepwise decline and focal neurologic deficits; it is unlikely in this patient, given the absence of cerebrovascular events or risk factors like hypertension or diabetes.
While normal pressure hydrocephalus can cause ventricular enlargement, its classic triad of gait disturbance, urinary incontinence, and dementia is incomplete here, making this answer unlikely.
DS is the most common genetic cause of intellectual disability, occurring in approximately 1 in 700 live births worldwide. Nearly all adults with DS develop neuropathologic changes associated with AD by age 40, and the lifetime risk of developing dementia exceeds 90%; by the age of 55-60, at least 70% of individuals with DS exhibit clinical signs of dementia.
The link between DS and neurodegeneration is largely attributed to the triplication of chromosome 21, which includes the APP gene. Overexpression of APP leads to excessive production and accumulation of amyloid-β (Aβ), which forms the hallmark plaques seen in AD. In DS, amyloid plaques begin to form as early as the teenage years, and by the fourth decade, neurofibrillary tangles (tau protein aggregates) and widespread neurodegeneration are nearly universal.
Initial symptoms of AD often include memory impairment, particularly short-term memory deficits, and executive dysfunction, difficulty with planning and problem-solving, and visuospatial deficits. Behavioral and personality changes, such as irritability, withdrawal, or apathy, are also commonly reported. Compared with sporadic AD, individuals with DS often show earlier behavioral symptoms, including impulsivity and changes in social interactions, which may precede noticeable memory deficits. Additionally, late-onset myoclonic epilepsy is common in individuals with DS and dementia, further complicating diagnosis and care.
The diagnosis of dementia in DS is challenging because of baseline intellectual disability, which makes it difficult to assess cognitive decline using standard neuropsychological tests. However, a combination of clinical history, caregiver reports, neuroimaging, and biomarker analysis can aid in early detection. Structural MRI of the brain often reveals ventricular enlargement and cortical atrophy, while PET imaging can detect early amyloid and tau accumulation.
Because AD is now the leading cause of death in individuals with DS, the lack of effective disease-modifying treatments highlights an important need for clinical trials focused on this population. Current research aims to explore the role of anti-amyloid monoclonal antibodies, neuroprotective agents, and lifestyle interventions to delay or prevent neurodegeneration.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Individuals with DS are at significantly increased risk of developing Alzheimer’s disease (AD) because of the overexpression of the amyloid precursor protein (APP) gene on chromosome 21.
The patient exhibits hallmark symptoms of AD, including progressive memory loss, disorientation, difficulty performing daily tasks, and behavioral changes such as irritability and social withdrawal. The MRI findings of ventricular enlargement and cortical atrophy are consistent with brain changes commonly seen in AD, particularly in individuals with DS who often develop these changes earlier in life (typically in their thirties or forties).
Frontotemporal dementia primarily causes behavioral and language changes with relative memory sparing early on, making it inconsistent with this patient's prominent memory loss, disorientation, and generalized cortical atrophy — features more typical of AD.
Vascular dementia often presents with stepwise decline and focal neurologic deficits; it is unlikely in this patient, given the absence of cerebrovascular events or risk factors like hypertension or diabetes.
While normal pressure hydrocephalus can cause ventricular enlargement, its classic triad of gait disturbance, urinary incontinence, and dementia is incomplete here, making this answer unlikely.
DS is the most common genetic cause of intellectual disability, occurring in approximately 1 in 700 live births worldwide. Nearly all adults with DS develop neuropathologic changes associated with AD by age 40, and the lifetime risk of developing dementia exceeds 90%; by the age of 55-60, at least 70% of individuals with DS exhibit clinical signs of dementia.
The link between DS and neurodegeneration is largely attributed to the triplication of chromosome 21, which includes the APP gene. Overexpression of APP leads to excessive production and accumulation of amyloid-β (Aβ), which forms the hallmark plaques seen in AD. In DS, amyloid plaques begin to form as early as the teenage years, and by the fourth decade, neurofibrillary tangles (tau protein aggregates) and widespread neurodegeneration are nearly universal.
Initial symptoms of AD often include memory impairment, particularly short-term memory deficits, and executive dysfunction, difficulty with planning and problem-solving, and visuospatial deficits. Behavioral and personality changes, such as irritability, withdrawal, or apathy, are also commonly reported. Compared with sporadic AD, individuals with DS often show earlier behavioral symptoms, including impulsivity and changes in social interactions, which may precede noticeable memory deficits. Additionally, late-onset myoclonic epilepsy is common in individuals with DS and dementia, further complicating diagnosis and care.
The diagnosis of dementia in DS is challenging because of baseline intellectual disability, which makes it difficult to assess cognitive decline using standard neuropsychological tests. However, a combination of clinical history, caregiver reports, neuroimaging, and biomarker analysis can aid in early detection. Structural MRI of the brain often reveals ventricular enlargement and cortical atrophy, while PET imaging can detect early amyloid and tau accumulation.
Because AD is now the leading cause of death in individuals with DS, the lack of effective disease-modifying treatments highlights an important need for clinical trials focused on this population. Current research aims to explore the role of anti-amyloid monoclonal antibodies, neuroprotective agents, and lifestyle interventions to delay or prevent neurodegeneration.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 40-year-old man with Down syndrome (DS) presented with progressive cognitive decline over 2 years, characterized by memory impairment, difficulty performing familiar tasks, and increasing disorientation. His caregivers noted mood changes, including irritability and withdrawal, and occasional episodes of agitation. Clinical history revealed congenital heart disease (surgically repaired in childhood) and hypothyroidism, which was well controlled with levothyroxine. Physical examination showed no focal neurologic deficits, but he exhibited mild hypotonia, a characteristic feature of DS, and a shuffling gait. Routine blood tests revealed normal thyroid function, no evidence of vitamin B12 deficiency, and unremarkable metabolic panels. An MRI scan (as shown in the image) demonstrated marked ventricular enlargement and generalized cortical atrophy.
Violaceous Papules on Face
Violaceous Papules on Face
Discussion
The patient’s violaceous papule on the nose with an apple jelly appearance is consistent with lupus pernio—a cutaneous form of sarcoidosis associated with respiratory involvement. Lupus pernio disproportionately affects African Americans, which further supports this diagnosis.1 Lupus pernio is characterized by violaceous, indurated plaques predominantly on the face. It has a strong association with systemic sarcoidosis and often involves the lungs and other organs, as seen in this case. The laboratory results support this diagnosis. Hypercalcemia is a common systemic manifestation of sarcoidosis due to increased production of 1,25-dihydroxyvitamin D by activated macrophages with granulomas.2 Elevated chitotriosidase, an enzyme produced by macrophages, is another biomarker of sarcoidosis reflecting granuloma burden.3
The differential diagnoses included Langerhans cell histiocytosis (LCH), discoid lupus erythematosus, granulomatosis with polyangiitis, and granuloma annulare. However, these diagnoses did not fully align with the entirety of the patient’s clinical presentation and laboratory findings. LCH is a rare neoplastic disorder characterized by the abnormal proliferation and accumulation of Langerhans cells, a type of dendritic cell involved in immune response, in various tissues such as the skin and bone. Dermatologic findings in LCH include brown/purple papules and an erythematous papular rash rather than the violaceous plaques/papules in lupus pernio. LCH can have lung involvement; it typically presents with nodular or cystic changes in the upper lobes as opposed to the bibasilar opacities seen in this case.
Discoid lupus erythematosus presents with characteristic round, erythematous, scaly plaques on the cheeks, scalp, and ears. This is different from the apple jelly appearance seen in this case and does not present with systemic granulomatous involvement.
Typical manifestations of granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis, include renal disease, upper and lower respiratory tract involvement, or necrotizing vasculitis. Cutaneous manifestions of granulomatosis with polyangiitis typically include purpura or ulcers rather than the violaceous plaques seen in lupus pernio. Patients with granulomatosis with polyangiitis would also present with nonspecific systemic symptoms such as fever, weight loss, and malaise, which are not depicted in this case.4
Granuloma annulare is a benign condition that often presents with annular plaques that are skin-colored rather than violaceous. These plaques are often found on the hands and feet rather than the face. This condition also lacks the systemic manifestations seen in this case.
In primary care, encountering violaceous papule and plaques on the face, especially on the nasal alae or ear, should be concerning for possible lupus pernio, particularly in high-risk populations such as young African Americans. These lesions generally have a more indurated “deep” and “doughy” appearance and can result in scarring, distinguishing them from other types of cutaneous sarcoidosis. An apple jelly appearance seen on diascopy with a glass slide can further support the diagnosis. While the lesions are typically asymptomatic, patients may be concerned about potential cosmetic disfigurement. Given the potential for scarring and the association with systemic sarcoidosis, a dermatology referral is recommended for further evaluation and management.
A detailed patient history, physical examination, and laboratory exams are essential to accurately diagnose lupus pernio. Biopsy of a skin lesion, serum markers, and imaging studies were utilized to help assess systemic involvement and further confirm diagnosis in this patient. Following the diagnosis, the patient was started on his current regimen of prednisone, methotrexate, and hydroxychloroquine, which are standard therapies for managing both cutaneous and systemic sarcoidosis.
This case shows the importance of recognizing lupus pernio, a distinct form of cutaneous sarcoidosis, in patients presenting with characteristic skin lesions and systemic involvement. It is essential to differentiate it from other granulomatous and inflammatory skin conditions to ensure appropriate management and prevent complications.
Federal Practitioner thanks the Association of Military Dermatologists (militaryderm.org) for their assistance in developing the Image Challenge. Submissions based on photographs, radiography, or any other visual medium are welcomed.
- Lai J, Almazan E, Le T, Taylor MT, Alhariri J, Kwatra SG. Demographics, cutaneous manifestations, and comorbidities associated with progressive cutaneous sarcoidosis: a retrospective cohort study. Medicines (Basel). 2023;10(10):57. doi:10.3390/medicines10100057
- Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31(4):474-484. doi:10.1055/s-0030-1262215
- Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration. 2008;76(2):234-238. doi:10.1159/000134009
- Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener’s disease): An updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5(2):61-69. doi:10.5582/irdr.2016.01014
Discussion
The patient’s violaceous papule on the nose with an apple jelly appearance is consistent with lupus pernio—a cutaneous form of sarcoidosis associated with respiratory involvement. Lupus pernio disproportionately affects African Americans, which further supports this diagnosis.1 Lupus pernio is characterized by violaceous, indurated plaques predominantly on the face. It has a strong association with systemic sarcoidosis and often involves the lungs and other organs, as seen in this case. The laboratory results support this diagnosis. Hypercalcemia is a common systemic manifestation of sarcoidosis due to increased production of 1,25-dihydroxyvitamin D by activated macrophages with granulomas.2 Elevated chitotriosidase, an enzyme produced by macrophages, is another biomarker of sarcoidosis reflecting granuloma burden.3
The differential diagnoses included Langerhans cell histiocytosis (LCH), discoid lupus erythematosus, granulomatosis with polyangiitis, and granuloma annulare. However, these diagnoses did not fully align with the entirety of the patient’s clinical presentation and laboratory findings. LCH is a rare neoplastic disorder characterized by the abnormal proliferation and accumulation of Langerhans cells, a type of dendritic cell involved in immune response, in various tissues such as the skin and bone. Dermatologic findings in LCH include brown/purple papules and an erythematous papular rash rather than the violaceous plaques/papules in lupus pernio. LCH can have lung involvement; it typically presents with nodular or cystic changes in the upper lobes as opposed to the bibasilar opacities seen in this case.
Discoid lupus erythematosus presents with characteristic round, erythematous, scaly plaques on the cheeks, scalp, and ears. This is different from the apple jelly appearance seen in this case and does not present with systemic granulomatous involvement.
Typical manifestations of granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis, include renal disease, upper and lower respiratory tract involvement, or necrotizing vasculitis. Cutaneous manifestions of granulomatosis with polyangiitis typically include purpura or ulcers rather than the violaceous plaques seen in lupus pernio. Patients with granulomatosis with polyangiitis would also present with nonspecific systemic symptoms such as fever, weight loss, and malaise, which are not depicted in this case.4
Granuloma annulare is a benign condition that often presents with annular plaques that are skin-colored rather than violaceous. These plaques are often found on the hands and feet rather than the face. This condition also lacks the systemic manifestations seen in this case.
In primary care, encountering violaceous papule and plaques on the face, especially on the nasal alae or ear, should be concerning for possible lupus pernio, particularly in high-risk populations such as young African Americans. These lesions generally have a more indurated “deep” and “doughy” appearance and can result in scarring, distinguishing them from other types of cutaneous sarcoidosis. An apple jelly appearance seen on diascopy with a glass slide can further support the diagnosis. While the lesions are typically asymptomatic, patients may be concerned about potential cosmetic disfigurement. Given the potential for scarring and the association with systemic sarcoidosis, a dermatology referral is recommended for further evaluation and management.
A detailed patient history, physical examination, and laboratory exams are essential to accurately diagnose lupus pernio. Biopsy of a skin lesion, serum markers, and imaging studies were utilized to help assess systemic involvement and further confirm diagnosis in this patient. Following the diagnosis, the patient was started on his current regimen of prednisone, methotrexate, and hydroxychloroquine, which are standard therapies for managing both cutaneous and systemic sarcoidosis.
This case shows the importance of recognizing lupus pernio, a distinct form of cutaneous sarcoidosis, in patients presenting with characteristic skin lesions and systemic involvement. It is essential to differentiate it from other granulomatous and inflammatory skin conditions to ensure appropriate management and prevent complications.
Federal Practitioner thanks the Association of Military Dermatologists (militaryderm.org) for their assistance in developing the Image Challenge. Submissions based on photographs, radiography, or any other visual medium are welcomed.
Discussion
The patient’s violaceous papule on the nose with an apple jelly appearance is consistent with lupus pernio—a cutaneous form of sarcoidosis associated with respiratory involvement. Lupus pernio disproportionately affects African Americans, which further supports this diagnosis.1 Lupus pernio is characterized by violaceous, indurated plaques predominantly on the face. It has a strong association with systemic sarcoidosis and often involves the lungs and other organs, as seen in this case. The laboratory results support this diagnosis. Hypercalcemia is a common systemic manifestation of sarcoidosis due to increased production of 1,25-dihydroxyvitamin D by activated macrophages with granulomas.2 Elevated chitotriosidase, an enzyme produced by macrophages, is another biomarker of sarcoidosis reflecting granuloma burden.3
The differential diagnoses included Langerhans cell histiocytosis (LCH), discoid lupus erythematosus, granulomatosis with polyangiitis, and granuloma annulare. However, these diagnoses did not fully align with the entirety of the patient’s clinical presentation and laboratory findings. LCH is a rare neoplastic disorder characterized by the abnormal proliferation and accumulation of Langerhans cells, a type of dendritic cell involved in immune response, in various tissues such as the skin and bone. Dermatologic findings in LCH include brown/purple papules and an erythematous papular rash rather than the violaceous plaques/papules in lupus pernio. LCH can have lung involvement; it typically presents with nodular or cystic changes in the upper lobes as opposed to the bibasilar opacities seen in this case.
Discoid lupus erythematosus presents with characteristic round, erythematous, scaly plaques on the cheeks, scalp, and ears. This is different from the apple jelly appearance seen in this case and does not present with systemic granulomatous involvement.
Typical manifestations of granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis, include renal disease, upper and lower respiratory tract involvement, or necrotizing vasculitis. Cutaneous manifestions of granulomatosis with polyangiitis typically include purpura or ulcers rather than the violaceous plaques seen in lupus pernio. Patients with granulomatosis with polyangiitis would also present with nonspecific systemic symptoms such as fever, weight loss, and malaise, which are not depicted in this case.4
Granuloma annulare is a benign condition that often presents with annular plaques that are skin-colored rather than violaceous. These plaques are often found on the hands and feet rather than the face. This condition also lacks the systemic manifestations seen in this case.
In primary care, encountering violaceous papule and plaques on the face, especially on the nasal alae or ear, should be concerning for possible lupus pernio, particularly in high-risk populations such as young African Americans. These lesions generally have a more indurated “deep” and “doughy” appearance and can result in scarring, distinguishing them from other types of cutaneous sarcoidosis. An apple jelly appearance seen on diascopy with a glass slide can further support the diagnosis. While the lesions are typically asymptomatic, patients may be concerned about potential cosmetic disfigurement. Given the potential for scarring and the association with systemic sarcoidosis, a dermatology referral is recommended for further evaluation and management.
A detailed patient history, physical examination, and laboratory exams are essential to accurately diagnose lupus pernio. Biopsy of a skin lesion, serum markers, and imaging studies were utilized to help assess systemic involvement and further confirm diagnosis in this patient. Following the diagnosis, the patient was started on his current regimen of prednisone, methotrexate, and hydroxychloroquine, which are standard therapies for managing both cutaneous and systemic sarcoidosis.
This case shows the importance of recognizing lupus pernio, a distinct form of cutaneous sarcoidosis, in patients presenting with characteristic skin lesions and systemic involvement. It is essential to differentiate it from other granulomatous and inflammatory skin conditions to ensure appropriate management and prevent complications.
Federal Practitioner thanks the Association of Military Dermatologists (militaryderm.org) for their assistance in developing the Image Challenge. Submissions based on photographs, radiography, or any other visual medium are welcomed.
- Lai J, Almazan E, Le T, Taylor MT, Alhariri J, Kwatra SG. Demographics, cutaneous manifestations, and comorbidities associated with progressive cutaneous sarcoidosis: a retrospective cohort study. Medicines (Basel). 2023;10(10):57. doi:10.3390/medicines10100057
- Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31(4):474-484. doi:10.1055/s-0030-1262215
- Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration. 2008;76(2):234-238. doi:10.1159/000134009
- Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener’s disease): An updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5(2):61-69. doi:10.5582/irdr.2016.01014
- Lai J, Almazan E, Le T, Taylor MT, Alhariri J, Kwatra SG. Demographics, cutaneous manifestations, and comorbidities associated with progressive cutaneous sarcoidosis: a retrospective cohort study. Medicines (Basel). 2023;10(10):57. doi:10.3390/medicines10100057
- Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med. 2010;31(4):474-484. doi:10.1055/s-0030-1262215
- Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration. 2008;76(2):234-238. doi:10.1159/000134009
- Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener’s disease): An updated review of ocular disease manifestations. Intractable Rare Dis Res. 2016;5(2):61-69. doi:10.5582/irdr.2016.01014
Violaceous Papules on Face
Violaceous Papules on Face
A 40-year-old man with no significant medical history or comorbidities presented with a violaceous papule involving his nasal tip and scaly, violaceous plaques with associated alopecia involving his beard (Figure). Skin biopsy confirmed granulomatous dermatitis. Additional workup was notable for hypercalcemia (10.5 mg/dL; reference range, 8.4-10.2 mg/dL), elevated chitotriosidase (317 nmol/h/mL; reference range, < 150 nmol/h/mL), and bibasilar opacities with left perihilar consolidation on chest X-ray. The patient had a prolonged PR interval (207 ms; reference range, 120-200 ms) on electrocardiogram. A cardiac positron emission tomography revealed low level fluorodeoxyglucose uptake in the left ventricle. No ocular involvement was noted on evaluation by ophthalmology. The patient’s pharmacotherapy included prednisone 10 mg daily, methotrexate 7.5 mg weekly, and hydroxychloroquine 200 mg daily.
Alzheimer's Disease & Down Syndrome
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
Express Scripts, the contractor that manages the pharmacy benefit for Tricare, the military health insurance program, announced in 2021 that after a 5-year absence, CVS Pharmacy was once more in the network. In 2023, CVS had the largest profits of any pharmacy chain in the United States, about $159 billion, and generated a quarter of the overall revenue of the US pharmacy industry.1 Tricare officials heralded the return of CVS as a move that would offer US Department of Defense (DoD) beneficiaries more competitive prices, convenient access, and overall quality.2
DOJ Files Lawsuit Against CVS
In December 2024, the US Department of Justice (DoJ) filed a lawsuit alleging that CVS violated both the Controlled Substances Act (CSA) and the False Claims Act (FCA).3,4 The United States ex rel. Estright v Health Corporation, et al, filed in Rhode Island, charged that CVS “routinely” and “knowingly” filled invalid prescriptions for controlled substances violating the CSA and then billed federal health care programs for payment for these prescriptions, a breach of the FCA.5 The DoJ alleged that CVS pharmacies and pharmacists filled prescriptions for controlled substances that (1) lacked a legitimate medical purpose; (2) were not legally valid; and/or (3) were not issued in the usual course of medical practice. 6 CVS contests the charges and issued an official response, stating that it disputes the allegations as false, plans to disprove them in litigation, and has nonetheless fully cooperated with the investigation.7
The allegations involved prescriptions for drugs like opioids and benzodiazepines, primary culprits in the American overdose epidemic.8 The complaint notes that the prescriptions were early refills in excessive quantities and included what has been called the “holy trinity” of dangerous medications: opioids, benzodiazepines, and muscle relaxants. 5,8 Even worse (if that is possible), as the complaint outlines, CVS had access to data from both inside and outside the company that these prescriptions came from notorious pill mills and were hence unlawful and yet continued to fill them, leading the DoJ to file the more serious charge that the corporation “knowingly” violated the CSA and “prioritized profits over safety in dispensing controlled substances.”5,6
The Unholy Trinity
The infamous members of what I prefer to call the “unholy trinity” are a benzodiazepine, often alprazolam, an opioid, and the muscle relaxant carisoprodol. The combination amplifies each agent’s independent risk of respiratory depression. The latter is a schedule IV medication with an active metabolite, meprobamate, that also has this adverse effect. All 3 drugs have high abuse potential and, when combined, increase the risk of fatal overdose. The colloquial name holy trinity derives from the synergistic euphoria experienced when taking this triple cocktail of sedative agents.9 This pharmacological recipe for disaster is the house specialty of pill mills: infamous storefront practices that generate high profits and exploit persons with chronic pain and addiction by handing out controlled substances with little clinical assessment and even less oversight.10
When the Means Become the End
The DoJ allegations suggest that the violations resulted from “corporate-mandated performance metrics, incentive compensation, and staffing policies that prioritized corporate profits over patient safety.”6 If the allegations are true, why would a company reinvited by Tricare to serve the nation’s heroes seemingly engage in illegal practices? While CVS has not responded in court, their statement argued that “too often, we have seen government agencies and trial lawyers question the good-faith decisions made by pharmacists while a patient waits at the pharmacy counter, often in pain.”6
The DoJ complaint offers a cautionary warning for the US health care system, which is increasingly being micromanaged in the pursuit of efficiency. Like many practitioners in and out of the federal system, I get a cold chill when I read the word productivity. “CVS pharmacists described working at CVS as ‘soul crushing’ because it was impossible to meet the company’s expectations,” the complaint alleges, because “CVS set staffing levels so low that it was impossible for pharmacists to comply with their legal obligations and meet CVS’s demanding metrics.”5 Did top-down mandates drive the alleged activities by imposing unattainable performance metrics on pharmacists, offering incentives that encouraged and rewarded corner-cutting, and refusing to fund sufficient staffing to ensure patient safety? This may be what happens when the means (efficiency) become the end rather than a mechanism to achieve the goal of more accessible, affordable, high-quality health care.
Ethically, what is most concerning is that leadership intentionally “deprived its pharmacists of crucial information” about specific practitioners known to engage in illegal prescribing practices.6 CVS did not provide pharmacists with “information about prescribers’ prescribing habits that CVS routinely collected and reviewed at the corporate level,” and even removed prescriber blocks that were implemented at Target pharmacies before it was acquired by CVS.5 The first element of informed consent is providing patients with adequate information upon which to decide whether to accept or decline treatment. 11 In this situation, however, CVS allegedly prevented “pharmacists from warning one another about certain prescribers.”6
If true, the company deprived frontline pharmacists of the information they needed to safely and responsibly dispense medications: “The practices alleged contributed to the opioid crisis and opioid-related deaths, and today’s complaint seeks to hold CVS accountable for its misconduct.”6 Though the cost in human life that may have resulted from CSA violations must absolutely and always outweigh financial considerations, the economic damage to Tricare from fraudulent billing and the betrayal of its fiduciary responsinility cannot be underestimated.
A Corporate Morality Play
CVS is not the only company, nor is pharmacy the only industry in health care, that has been the subject of watchdog agency lawsuits or variegated forms of wrongdoing, including violations of the CSA and FCA.10,12 As of this writing, the DoJ case against CVS has not been heard, much less adjudicated in a court of law. It is ironic that both the DoJ claims and the CVS rebuttal describe the manifest conflict of obligation that pharmacists confront between protecting their livelihood and safeguarding patients’ lives as suggested in the epigraph that has been attributed to the 19th-century British physician and medical educator Peter Mere Latham. It is a dilemma that a growing number of health care practitioners face daily in a vocation becoming increasingly commercialized. It is all too easy for an individual physician, nurse, or pharmacist to feel hopeless and helpless before the behemoth might of a large and looming entity. Yet, it was a whistleblower whose moral courage led to the DoJ investigation and subsequent charges.13 We must all never doubt the power of a committed person of conscience to withstand the pressure to mutate medications into poison and stand up for the principles of our professions and inspire a community of colleagues to follow their example.
- Fein AJ. The Top U.S. pharmacy markets of 2023: market shares and revenues at the biggest chains and PBMs. Drug Channels. March 12, 2024. Accessed February 24, 2025. https://www.drugchannels.net/2024/03/the-top-15-us-pharmacies-of-2023-market.html
- Jowers K. CVS returns to the military Tricare network. Walmart’s out. Military Times. October 18, 2021. Accessed February 24, 2025. https://www.militarytimes.com/pay-benefits/mil-money/2021/10/28/cvs-returns-to-the-military-tricare-pharmacy-network-walmarts-out/
- False Claims, 31 USC § 3729 (2009). Accessed February 24, 2025. https://www.govinfo.gov/content/pkg/USCODE-2011-title31/pdf/USCODE-2011-title31-subtitleIII-chap37-subchapIII-sec3729.pdf
- Drug Abuse Prevention and Control, Control and Enforcement, 21 USC 13 § 801 (2022). Accessed February 24, 2025. https://www.govinfo.gov/app/details/USCODE-2021-title21/USCODE-2021-title21-chap13-subchapI-partA-sec801
- United States ex rel. Estright v Health Corporation, et al. Accessed February 26, 2025. https://www.justice.gov/archives/opa/media/1381111/dl
- US Department of Justice. Justice Department files nationwide lawsuit alleging CVS knowingly dispensed controlled substances in violation of the Controlled Substances ACT and the False Claims Act. News release. December 18, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/justice-department-files-nationwide-lawsuit-alleging-cvs-knowingly-dispensed-controlled
- CVS Health. CVS Health statement regarding the U.S. Department of Justice’s lawsuit against CVS pharmacy. News release. December 18, 2024. Accessed February 24, 2025. https://www.cvshealth.com/impact/healthy-community/our-opioid-response.html
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi:10.1136/bmj.h2698
- Wang Y, Delcher C, Li Y, Goldberger BA, Reisfield GM. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019;205:107693. doi:10.1016/j.drugalcdep.2019.107693
- Wolf AA. The perfect storm: opioid risks and ‘The Holy Trinity’. Pharmacy Times. September 24, 2014. Accessed February 24, 2025. https://www.pharmacytimes.com/view/the-perfect-storm-opioid-risks-and-the-holy-trinity
- The meaning and justification of informed consent. In: Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Eighth Edition. Oxford University Press; 2019:118-123.
- US Department of Justice. OptumRX agrees to pay $20M to resolve allegations that it filled certain opioid prescriptions in violation of the Controlled Substances Act. News release. June 27, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/optumrx-agrees-pay-20m-resolve-allegations-it-filled-certain-opioid-prescriptions-violation
- US Department of Justice. False Claims Act settlements and judgments exceed $2.9B in fiscal year 2024. News release. January 15, 2025. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/false-claims-act-settlements-and-judgments-exceed-29b-fiscal-year-2024
Express Scripts, the contractor that manages the pharmacy benefit for Tricare, the military health insurance program, announced in 2021 that after a 5-year absence, CVS Pharmacy was once more in the network. In 2023, CVS had the largest profits of any pharmacy chain in the United States, about $159 billion, and generated a quarter of the overall revenue of the US pharmacy industry.1 Tricare officials heralded the return of CVS as a move that would offer US Department of Defense (DoD) beneficiaries more competitive prices, convenient access, and overall quality.2
DOJ Files Lawsuit Against CVS
In December 2024, the US Department of Justice (DoJ) filed a lawsuit alleging that CVS violated both the Controlled Substances Act (CSA) and the False Claims Act (FCA).3,4 The United States ex rel. Estright v Health Corporation, et al, filed in Rhode Island, charged that CVS “routinely” and “knowingly” filled invalid prescriptions for controlled substances violating the CSA and then billed federal health care programs for payment for these prescriptions, a breach of the FCA.5 The DoJ alleged that CVS pharmacies and pharmacists filled prescriptions for controlled substances that (1) lacked a legitimate medical purpose; (2) were not legally valid; and/or (3) were not issued in the usual course of medical practice. 6 CVS contests the charges and issued an official response, stating that it disputes the allegations as false, plans to disprove them in litigation, and has nonetheless fully cooperated with the investigation.7
The allegations involved prescriptions for drugs like opioids and benzodiazepines, primary culprits in the American overdose epidemic.8 The complaint notes that the prescriptions were early refills in excessive quantities and included what has been called the “holy trinity” of dangerous medications: opioids, benzodiazepines, and muscle relaxants. 5,8 Even worse (if that is possible), as the complaint outlines, CVS had access to data from both inside and outside the company that these prescriptions came from notorious pill mills and were hence unlawful and yet continued to fill them, leading the DoJ to file the more serious charge that the corporation “knowingly” violated the CSA and “prioritized profits over safety in dispensing controlled substances.”5,6
The Unholy Trinity
The infamous members of what I prefer to call the “unholy trinity” are a benzodiazepine, often alprazolam, an opioid, and the muscle relaxant carisoprodol. The combination amplifies each agent’s independent risk of respiratory depression. The latter is a schedule IV medication with an active metabolite, meprobamate, that also has this adverse effect. All 3 drugs have high abuse potential and, when combined, increase the risk of fatal overdose. The colloquial name holy trinity derives from the synergistic euphoria experienced when taking this triple cocktail of sedative agents.9 This pharmacological recipe for disaster is the house specialty of pill mills: infamous storefront practices that generate high profits and exploit persons with chronic pain and addiction by handing out controlled substances with little clinical assessment and even less oversight.10
When the Means Become the End
The DoJ allegations suggest that the violations resulted from “corporate-mandated performance metrics, incentive compensation, and staffing policies that prioritized corporate profits over patient safety.”6 If the allegations are true, why would a company reinvited by Tricare to serve the nation’s heroes seemingly engage in illegal practices? While CVS has not responded in court, their statement argued that “too often, we have seen government agencies and trial lawyers question the good-faith decisions made by pharmacists while a patient waits at the pharmacy counter, often in pain.”6
The DoJ complaint offers a cautionary warning for the US health care system, which is increasingly being micromanaged in the pursuit of efficiency. Like many practitioners in and out of the federal system, I get a cold chill when I read the word productivity. “CVS pharmacists described working at CVS as ‘soul crushing’ because it was impossible to meet the company’s expectations,” the complaint alleges, because “CVS set staffing levels so low that it was impossible for pharmacists to comply with their legal obligations and meet CVS’s demanding metrics.”5 Did top-down mandates drive the alleged activities by imposing unattainable performance metrics on pharmacists, offering incentives that encouraged and rewarded corner-cutting, and refusing to fund sufficient staffing to ensure patient safety? This may be what happens when the means (efficiency) become the end rather than a mechanism to achieve the goal of more accessible, affordable, high-quality health care.
Ethically, what is most concerning is that leadership intentionally “deprived its pharmacists of crucial information” about specific practitioners known to engage in illegal prescribing practices.6 CVS did not provide pharmacists with “information about prescribers’ prescribing habits that CVS routinely collected and reviewed at the corporate level,” and even removed prescriber blocks that were implemented at Target pharmacies before it was acquired by CVS.5 The first element of informed consent is providing patients with adequate information upon which to decide whether to accept or decline treatment. 11 In this situation, however, CVS allegedly prevented “pharmacists from warning one another about certain prescribers.”6
If true, the company deprived frontline pharmacists of the information they needed to safely and responsibly dispense medications: “The practices alleged contributed to the opioid crisis and opioid-related deaths, and today’s complaint seeks to hold CVS accountable for its misconduct.”6 Though the cost in human life that may have resulted from CSA violations must absolutely and always outweigh financial considerations, the economic damage to Tricare from fraudulent billing and the betrayal of its fiduciary responsinility cannot be underestimated.
A Corporate Morality Play
CVS is not the only company, nor is pharmacy the only industry in health care, that has been the subject of watchdog agency lawsuits or variegated forms of wrongdoing, including violations of the CSA and FCA.10,12 As of this writing, the DoJ case against CVS has not been heard, much less adjudicated in a court of law. It is ironic that both the DoJ claims and the CVS rebuttal describe the manifest conflict of obligation that pharmacists confront between protecting their livelihood and safeguarding patients’ lives as suggested in the epigraph that has been attributed to the 19th-century British physician and medical educator Peter Mere Latham. It is a dilemma that a growing number of health care practitioners face daily in a vocation becoming increasingly commercialized. It is all too easy for an individual physician, nurse, or pharmacist to feel hopeless and helpless before the behemoth might of a large and looming entity. Yet, it was a whistleblower whose moral courage led to the DoJ investigation and subsequent charges.13 We must all never doubt the power of a committed person of conscience to withstand the pressure to mutate medications into poison and stand up for the principles of our professions and inspire a community of colleagues to follow their example.
Express Scripts, the contractor that manages the pharmacy benefit for Tricare, the military health insurance program, announced in 2021 that after a 5-year absence, CVS Pharmacy was once more in the network. In 2023, CVS had the largest profits of any pharmacy chain in the United States, about $159 billion, and generated a quarter of the overall revenue of the US pharmacy industry.1 Tricare officials heralded the return of CVS as a move that would offer US Department of Defense (DoD) beneficiaries more competitive prices, convenient access, and overall quality.2
DOJ Files Lawsuit Against CVS
In December 2024, the US Department of Justice (DoJ) filed a lawsuit alleging that CVS violated both the Controlled Substances Act (CSA) and the False Claims Act (FCA).3,4 The United States ex rel. Estright v Health Corporation, et al, filed in Rhode Island, charged that CVS “routinely” and “knowingly” filled invalid prescriptions for controlled substances violating the CSA and then billed federal health care programs for payment for these prescriptions, a breach of the FCA.5 The DoJ alleged that CVS pharmacies and pharmacists filled prescriptions for controlled substances that (1) lacked a legitimate medical purpose; (2) were not legally valid; and/or (3) were not issued in the usual course of medical practice. 6 CVS contests the charges and issued an official response, stating that it disputes the allegations as false, plans to disprove them in litigation, and has nonetheless fully cooperated with the investigation.7
The allegations involved prescriptions for drugs like opioids and benzodiazepines, primary culprits in the American overdose epidemic.8 The complaint notes that the prescriptions were early refills in excessive quantities and included what has been called the “holy trinity” of dangerous medications: opioids, benzodiazepines, and muscle relaxants. 5,8 Even worse (if that is possible), as the complaint outlines, CVS had access to data from both inside and outside the company that these prescriptions came from notorious pill mills and were hence unlawful and yet continued to fill them, leading the DoJ to file the more serious charge that the corporation “knowingly” violated the CSA and “prioritized profits over safety in dispensing controlled substances.”5,6
The Unholy Trinity
The infamous members of what I prefer to call the “unholy trinity” are a benzodiazepine, often alprazolam, an opioid, and the muscle relaxant carisoprodol. The combination amplifies each agent’s independent risk of respiratory depression. The latter is a schedule IV medication with an active metabolite, meprobamate, that also has this adverse effect. All 3 drugs have high abuse potential and, when combined, increase the risk of fatal overdose. The colloquial name holy trinity derives from the synergistic euphoria experienced when taking this triple cocktail of sedative agents.9 This pharmacological recipe for disaster is the house specialty of pill mills: infamous storefront practices that generate high profits and exploit persons with chronic pain and addiction by handing out controlled substances with little clinical assessment and even less oversight.10
When the Means Become the End
The DoJ allegations suggest that the violations resulted from “corporate-mandated performance metrics, incentive compensation, and staffing policies that prioritized corporate profits over patient safety.”6 If the allegations are true, why would a company reinvited by Tricare to serve the nation’s heroes seemingly engage in illegal practices? While CVS has not responded in court, their statement argued that “too often, we have seen government agencies and trial lawyers question the good-faith decisions made by pharmacists while a patient waits at the pharmacy counter, often in pain.”6
The DoJ complaint offers a cautionary warning for the US health care system, which is increasingly being micromanaged in the pursuit of efficiency. Like many practitioners in and out of the federal system, I get a cold chill when I read the word productivity. “CVS pharmacists described working at CVS as ‘soul crushing’ because it was impossible to meet the company’s expectations,” the complaint alleges, because “CVS set staffing levels so low that it was impossible for pharmacists to comply with their legal obligations and meet CVS’s demanding metrics.”5 Did top-down mandates drive the alleged activities by imposing unattainable performance metrics on pharmacists, offering incentives that encouraged and rewarded corner-cutting, and refusing to fund sufficient staffing to ensure patient safety? This may be what happens when the means (efficiency) become the end rather than a mechanism to achieve the goal of more accessible, affordable, high-quality health care.
Ethically, what is most concerning is that leadership intentionally “deprived its pharmacists of crucial information” about specific practitioners known to engage in illegal prescribing practices.6 CVS did not provide pharmacists with “information about prescribers’ prescribing habits that CVS routinely collected and reviewed at the corporate level,” and even removed prescriber blocks that were implemented at Target pharmacies before it was acquired by CVS.5 The first element of informed consent is providing patients with adequate information upon which to decide whether to accept or decline treatment. 11 In this situation, however, CVS allegedly prevented “pharmacists from warning one another about certain prescribers.”6
If true, the company deprived frontline pharmacists of the information they needed to safely and responsibly dispense medications: “The practices alleged contributed to the opioid crisis and opioid-related deaths, and today’s complaint seeks to hold CVS accountable for its misconduct.”6 Though the cost in human life that may have resulted from CSA violations must absolutely and always outweigh financial considerations, the economic damage to Tricare from fraudulent billing and the betrayal of its fiduciary responsinility cannot be underestimated.
A Corporate Morality Play
CVS is not the only company, nor is pharmacy the only industry in health care, that has been the subject of watchdog agency lawsuits or variegated forms of wrongdoing, including violations of the CSA and FCA.10,12 As of this writing, the DoJ case against CVS has not been heard, much less adjudicated in a court of law. It is ironic that both the DoJ claims and the CVS rebuttal describe the manifest conflict of obligation that pharmacists confront between protecting their livelihood and safeguarding patients’ lives as suggested in the epigraph that has been attributed to the 19th-century British physician and medical educator Peter Mere Latham. It is a dilemma that a growing number of health care practitioners face daily in a vocation becoming increasingly commercialized. It is all too easy for an individual physician, nurse, or pharmacist to feel hopeless and helpless before the behemoth might of a large and looming entity. Yet, it was a whistleblower whose moral courage led to the DoJ investigation and subsequent charges.13 We must all never doubt the power of a committed person of conscience to withstand the pressure to mutate medications into poison and stand up for the principles of our professions and inspire a community of colleagues to follow their example.
- Fein AJ. The Top U.S. pharmacy markets of 2023: market shares and revenues at the biggest chains and PBMs. Drug Channels. March 12, 2024. Accessed February 24, 2025. https://www.drugchannels.net/2024/03/the-top-15-us-pharmacies-of-2023-market.html
- Jowers K. CVS returns to the military Tricare network. Walmart’s out. Military Times. October 18, 2021. Accessed February 24, 2025. https://www.militarytimes.com/pay-benefits/mil-money/2021/10/28/cvs-returns-to-the-military-tricare-pharmacy-network-walmarts-out/
- False Claims, 31 USC § 3729 (2009). Accessed February 24, 2025. https://www.govinfo.gov/content/pkg/USCODE-2011-title31/pdf/USCODE-2011-title31-subtitleIII-chap37-subchapIII-sec3729.pdf
- Drug Abuse Prevention and Control, Control and Enforcement, 21 USC 13 § 801 (2022). Accessed February 24, 2025. https://www.govinfo.gov/app/details/USCODE-2021-title21/USCODE-2021-title21-chap13-subchapI-partA-sec801
- United States ex rel. Estright v Health Corporation, et al. Accessed February 26, 2025. https://www.justice.gov/archives/opa/media/1381111/dl
- US Department of Justice. Justice Department files nationwide lawsuit alleging CVS knowingly dispensed controlled substances in violation of the Controlled Substances ACT and the False Claims Act. News release. December 18, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/justice-department-files-nationwide-lawsuit-alleging-cvs-knowingly-dispensed-controlled
- CVS Health. CVS Health statement regarding the U.S. Department of Justice’s lawsuit against CVS pharmacy. News release. December 18, 2024. Accessed February 24, 2025. https://www.cvshealth.com/impact/healthy-community/our-opioid-response.html
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi:10.1136/bmj.h2698
- Wang Y, Delcher C, Li Y, Goldberger BA, Reisfield GM. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019;205:107693. doi:10.1016/j.drugalcdep.2019.107693
- Wolf AA. The perfect storm: opioid risks and ‘The Holy Trinity’. Pharmacy Times. September 24, 2014. Accessed February 24, 2025. https://www.pharmacytimes.com/view/the-perfect-storm-opioid-risks-and-the-holy-trinity
- The meaning and justification of informed consent. In: Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Eighth Edition. Oxford University Press; 2019:118-123.
- US Department of Justice. OptumRX agrees to pay $20M to resolve allegations that it filled certain opioid prescriptions in violation of the Controlled Substances Act. News release. June 27, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/optumrx-agrees-pay-20m-resolve-allegations-it-filled-certain-opioid-prescriptions-violation
- US Department of Justice. False Claims Act settlements and judgments exceed $2.9B in fiscal year 2024. News release. January 15, 2025. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/false-claims-act-settlements-and-judgments-exceed-29b-fiscal-year-2024
- Fein AJ. The Top U.S. pharmacy markets of 2023: market shares and revenues at the biggest chains and PBMs. Drug Channels. March 12, 2024. Accessed February 24, 2025. https://www.drugchannels.net/2024/03/the-top-15-us-pharmacies-of-2023-market.html
- Jowers K. CVS returns to the military Tricare network. Walmart’s out. Military Times. October 18, 2021. Accessed February 24, 2025. https://www.militarytimes.com/pay-benefits/mil-money/2021/10/28/cvs-returns-to-the-military-tricare-pharmacy-network-walmarts-out/
- False Claims, 31 USC § 3729 (2009). Accessed February 24, 2025. https://www.govinfo.gov/content/pkg/USCODE-2011-title31/pdf/USCODE-2011-title31-subtitleIII-chap37-subchapIII-sec3729.pdf
- Drug Abuse Prevention and Control, Control and Enforcement, 21 USC 13 § 801 (2022). Accessed February 24, 2025. https://www.govinfo.gov/app/details/USCODE-2021-title21/USCODE-2021-title21-chap13-subchapI-partA-sec801
- United States ex rel. Estright v Health Corporation, et al. Accessed February 26, 2025. https://www.justice.gov/archives/opa/media/1381111/dl
- US Department of Justice. Justice Department files nationwide lawsuit alleging CVS knowingly dispensed controlled substances in violation of the Controlled Substances ACT and the False Claims Act. News release. December 18, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/justice-department-files-nationwide-lawsuit-alleging-cvs-knowingly-dispensed-controlled
- CVS Health. CVS Health statement regarding the U.S. Department of Justice’s lawsuit against CVS pharmacy. News release. December 18, 2024. Accessed February 24, 2025. https://www.cvshealth.com/impact/healthy-community/our-opioid-response.html
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi:10.1136/bmj.h2698
- Wang Y, Delcher C, Li Y, Goldberger BA, Reisfield GM. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019;205:107693. doi:10.1016/j.drugalcdep.2019.107693
- Wolf AA. The perfect storm: opioid risks and ‘The Holy Trinity’. Pharmacy Times. September 24, 2014. Accessed February 24, 2025. https://www.pharmacytimes.com/view/the-perfect-storm-opioid-risks-and-the-holy-trinity
- The meaning and justification of informed consent. In: Beauchamp TL, Childress JF. Principles of Biomedical Ethics. Eighth Edition. Oxford University Press; 2019:118-123.
- US Department of Justice. OptumRX agrees to pay $20M to resolve allegations that it filled certain opioid prescriptions in violation of the Controlled Substances Act. News release. June 27, 2024. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/optumrx-agrees-pay-20m-resolve-allegations-it-filled-certain-opioid-prescriptions-violation
- US Department of Justice. False Claims Act settlements and judgments exceed $2.9B in fiscal year 2024. News release. January 15, 2025. Accessed February 24, 2025. https://www.justice.gov/archives/opa/pr/false-claims-act-settlements-and-judgments-exceed-29b-fiscal-year-2024
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
The Unholy Trinity: Unlawful Prescriptions, False Claims, and Dangerous Drugs
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
Patients often ask dermatologists how to best care for their specific hair type; however, there are no formal recommendations that apply to the many different hair care practices utilized by Black patients, as hair types in this community can range from wavy to tightly coiled.1 Understanding the the history of hair care in those of African ancestry and various styling practices in this population is necessary to adequately counsel patients and gain trust in the doctor-patient relationship. In this article, we provide an overview of hair care recommendations based on common styling practices in Black women.
A PubMed search of articles indexed for MEDLINE using the terms Black hair care, African American hair management, hair loss prevention, hair care practices, natural hair, natural-hair styles, alopecia, hairdressing, hair breakage, hair fragility, heat-stressed hair, traction alopecia, and natural hair care yielded 305 results; 107 duplicates were identified and removed, leaving 198 articles to be screened for eligibility (ie, English-language studies created in the past 15 years). Sixty-eight full-text articles were screened against the exclusion criteria, which included case reports and case series, articles not focused on Afro-textured hair, and cancer-related hair loss. Three additional fulltext articles were identified via resources from Wayne State University library (Detroit, Michigan) that were not available on PubMed. A total of 29 full-text articles were included in our review.
Background on Hair Care and Styling in African Populations
It is difficult to understand the history of hair in those of African ancestry in the United States.2 Prior to slavery, hair styling was considered a way of identification, classification, and communication as well as a medium through which to connect with the spiritual world in many parts of Africa. Hair-styling practices in Africa included elaborate cornrows, threading, and braiding with many accessories. Notable hair-styling products included natural butters, herbs, and powders to assist with moisture retention. Scarves also were used during this time for ceremonies or protection.3 During the mass enslavement of African populations and their transportation to the Americas by Europeans, slaveholders routinely cut off all the hair of both men and women in order to objectify and erase the culture of African hair styling passed down through generations.4,5 Hair texture then was weaponized to create a caste system in plantation life, in which Black slaves with straight hair textures were granted the “privilege” of domestic work, while those with kinky hair were relegated to arduous manual labor in the fields.4 Years later, during the 1800s, laws were enacted in the United States to prohibit Black women from wearing tightly coiled natural hair in public places.5 Over the next few centuries from the 1800s to the early 2000s, various hair-styling trends such as the use of hot combs, perms, afros, and Jheri curls developed as a means for Black individuals to conform to societal pressure to adopt more European features; however, as time progressed, afros, braids, locs, and natural hair would become more dominant as statements against these same societal pressures.5
The natural hair movement, which emerged in the United States in the 2000s, encouraged Black women to abandon the use of toxic chemical hair straighteners, cultivate healthier hair care practices, disrupt Eurocentric standards of wearing straightened hair, and facilitate self-definition of beauty ideals from the Civil Rights Movement of the 1960s.4,5 It is estimated that between 30% and 70% of all Black women in the United States wear natural hair, including 79% of millennial Black women younger than 30 years6; however, several new trends such as wigs and weaves have grown in popularity since the early 2000s due to mainstream pop culture and improvements in creating natural hairlines.7,8
Key Features of Afro-Textured Hair
Individuals of African descent have the most diverse hair texture phenotypes, ranging from straight to tightly coiled.9 Although hair is chemically similar across various racial groups, differences are noted mainly in the shape of the hair shaft, with elliptical and curved shapes seen in Afrotextured hair. These differences yield more tightly curled strands than in other hair types; however, these features also contribute to fragility, as it creates points of weakness and decreases the tensile strength of the hair shaft.10 This inherent fragility leads to higher rates of hair breakage as well as lower moisture content and slower growth rates, which is why Afro-textured hair requires special care.9
Afro-textured hair generally falls into 2 main categories of the Andre Walker hair typing system: 4A-4C and 3A-3C.11 In the 4A-4C category, hair is described as coily or kinky. Common concerns related to this hair type include dryness and brittleness with increased susceptibility to breakage. The 3A-3C category is described as loose to corkscrew curls, with a common concern of dryness.11,12 Additionally, Loussouarn et al13 established a method to further define natural hair curliness using curve diameter and curl meters on glass plates to measure the curvature of hair strands. This method allows for assessing diversity and range of curliness within various races without relying on ethnic origin.13
Common Hair Care Practices
A description of each hair type and recommended styling practices with their levels of evidence can be found in the eTable.
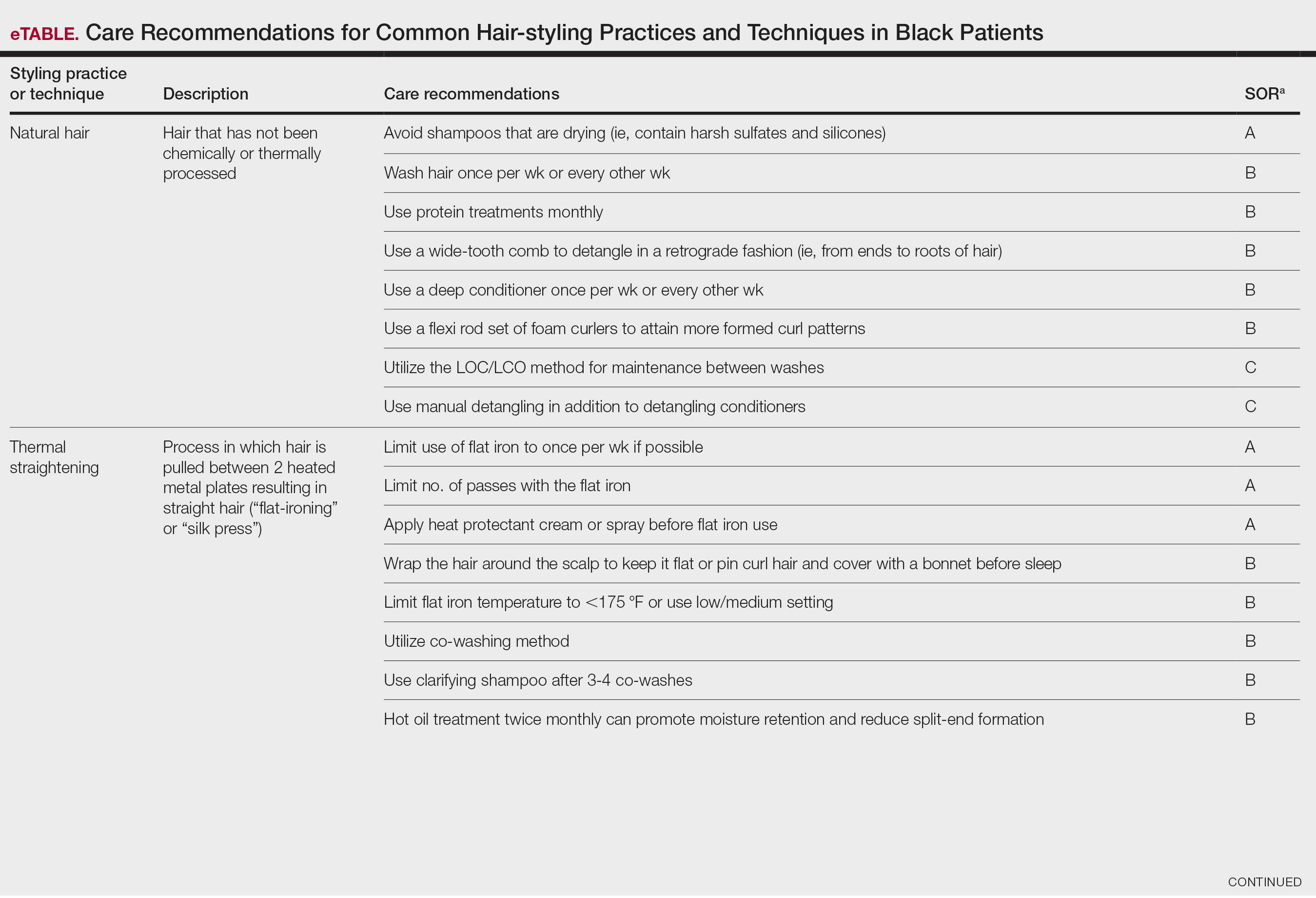
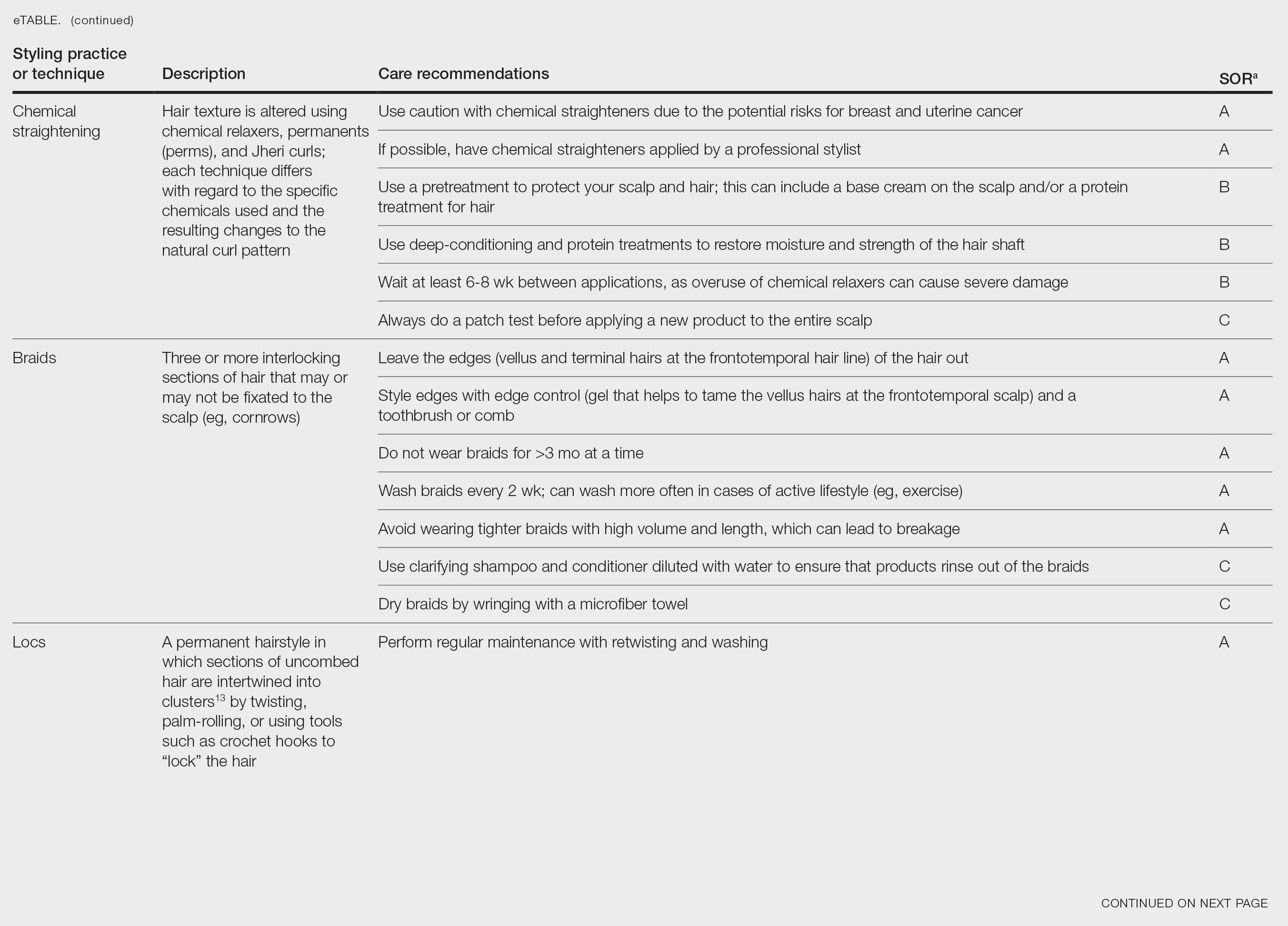
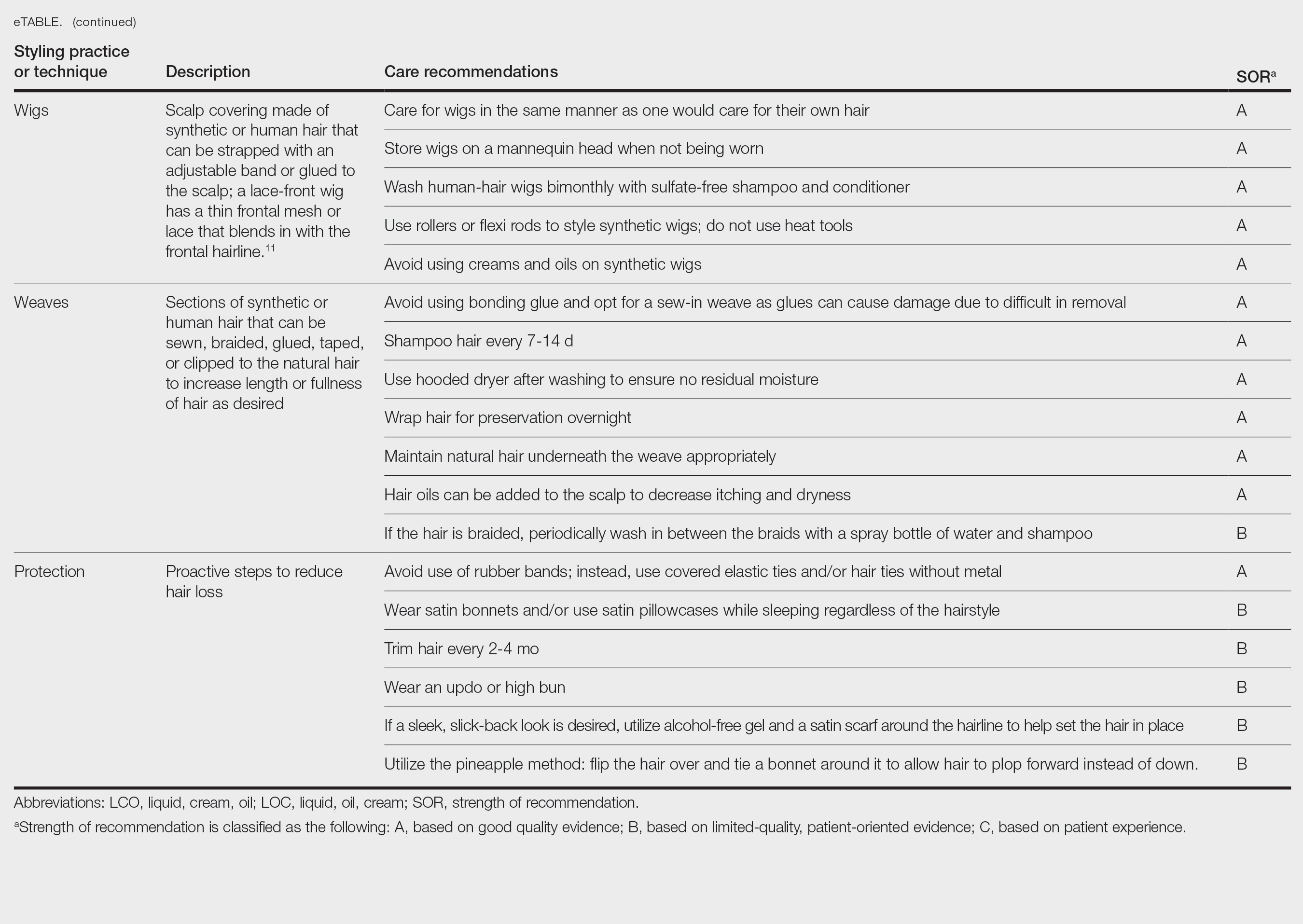
Natural Hair—Natural hair is classified as hair that has not been chemically changed by perms, heat, or other straightening treatments.12,14 For natural hair, retaining the moisture of the hair shaft should be the main focus, as moisture loss leads to considerable dryness.14 Generally, it is recommended to wash natural hair once per week or every other week; however, this can change based on hair length and oil production on the scalp. Washing daily may be ideal for shorter hair and monthly for longer hair to help prevent product build-up that can have a drying effect.15 Avoid shampoos that are drying (eg, sulfate and silicone products). The co-washing method also can be utilized, which entails washing the hair with a conditioning cleanser instead of shampoo and conditioner. However, this technique is not meant to completely replace shampoo.16 In fact, a clarifying shampoo is recommended after co-washing 3 or 4 times.16 The use of a hot oil treatment twice per month can promote moisture retention and reduce split-end formation.17 For maintenance between washes, many utilize the liquid, oil, cream (LOC) or liquid, cream, oil (LCO) methods, which describe regimens that utilize water, an oil of choice, and cream such as shea butter to lock in moisture.18 This method can be used as often as needed for dry hair.
Due to the susceptibility of Afro-textured hair to tangle and knot, using a wide-tooth comb, detangling brush, or detangling conditioners is a grade B recommendation for care (eTable). Though not widely documented in the literature, many of our patients have had anecdotal success detangling their hair simply by pulling hair strands apart by hand or “finger detangling” as well as using wide-tooth combs. Although both hair types are healthier in their natural states, kinky hair (type 4A-4C) is extremely fragile and more difficult to manage than less kinky hair (type 3A-3C).18
Special care is needed when detangling due to strands being weaker when wet.19 Detangling should be performed in a retrograde fashion. Deep conditioning can aid in moisture retention and should be performed weekly or biweekly.17-20 Depending on the health of the hair, protein treatments can be considered on a monthly basis to help preserve the cuticle. Styling with braids, twists, or other protective styles can then be completed on an individual basis.
Thermal Straightening—A blowout involves straightening the hair after a wash with the use of a hair dryer.21 This common hair-styling method does not employ the use of chemicals beyond light hair oils and heat-protectant creams or sprays, typically resulting in a less kinky afro or semi-straight hair. Thermal straightening utilizes heat to temporarily straighten hair strands. Flat irons with heated metal plates then can be used after blow-drying the hair to fully straighten and smooth the strands. These processes combined commonly are known as a silk press.21-22
For thermally straightened hair, it is recommended to either wrap the hair around the scalp to keep it flat or pin curl the hair and cover with a bonnet to sleep. Safe straightening techniques with the use of a flat iron include setting the temperature no higher than 175 °F or a low/medium setting while also limiting use to once per week if possible.23 The number of passes of the flat iron also should be limited to 1 to 2 to reduce breakage. A heat-protectant cream or spray also can be applied to the hair before flat ironing to minimize damage. Applying heat protectant to the hair prior to styling will help minimize heat damage by distributing the heat along the hair fiber surface, avoiding water boiling in the hair shaft and the development of bubble hair leading to damage.24
Chemical Straightening—Similar to how relaxers, perms, and Jheri curl treatments chemically modify hair texture using distinct chemicals yielding different curl patterns, the Brazilian blowout similarly straightens hair using a hair dryer and chemicals applied to hair strands after washing.21-24 Relaxers utilize sodium or guanidine hydroxide for straightening, perms use ammonium thioglycolate for curling, and Jheri curl treatments employ thioglycolates or mercaptans for defined curls. However, these treatments generally are cautioned against due to potential hair damage and recent associations with uterine and breast cancer in Black women. Research has suggested that endocrine disrupters in these products, especially those marketed to Black women, contribute to hormone-related disease processes.25,26 One study found higher concentrations of alkylphenols, the fragrance marker diethyl phthalate, and parabens in relaxers27; however, more research is needed to determine specific chemicals associated with these cancers.
Braids and Locs—Braiding is a technique that involves interlocking 3 or more sections of hair that may or may not be fixated to the scalp like a cornrow,11 and one can utilize extensions or natural hair depending on the desired outcome. Intended for long-term wear (ie, weeks to months), braids minimize breakage and reduce daily styling needs. Two popular styles—cornrows and individual braids—differ in preparation and weaving techniques. Cornrows are an Afro-centric style involving uniform, tightly woven braids that are close to the scalp, creating distinct patterns. Conversely, individual braids weave separate hair sections, offering diverse styling possibilities. Braiding practices should exclude hairline edges—often termed baby hairs—to prevent traction alopecia. Minimal use of edge gel, which helps to tame the vellus hairs at the frontotemporal scalp, as well as mindful weave volume, weight, and length are recommended to avert breakage. Braids that cause pain are too tight, can damage hair, and may cause traction alopecia.11 Braids should not be worn for longer than 3 months at a time and require biweekly washing with diluted shampoo and conditioner. Proper drying by wringing the hair with a microfiber towel is essential to avoid frizz and mold formation.
Locs are a low-maintenance hairstyle considered permanent until cut.28 This style involves twisting, palm rolling, or using tools such as crochet hooks to “lock” the hair. Regular maintenance with retwisting and cleaning is vital for loc health. Increased weight and tight twisting of locs can cause damage to the scalp and hair strands; however, locs are known to increase hair volume over time, often due to the accumulation of hairs that would otherwise have been shed in the telogen phase.28
Wigs and Weaves—Wigs consist of synthetic or human hair that can be strapped to the head with an adjustable band or glued to the scalp depending on the desired style.29 Wigs are removed daily, which allows for quick access to hair for cleansing and moisturizing. In contrast, weaves typically are sewn into the natural hair, which may make it difficult to reach the scalp for cleansing, leading to dryness and product build-up.29 Notably, there is evidence of a relationship between long-term use of weaves and traction alopecia.30
Wigs can have a fully synthetic hair line or lace hair line and can range from very affordable to expensive. When applied correctly, both styles offer an easy way to cover and protect the natural hair by reducing the amount of physical trauma related to daily hair styling. A lace-front wig contains a frontal thin mesh or lace that camouflages the natural frontal hairline.29,30 A risk of lace-front wigs is that they can cause friction alopecia secondary to repeated use of adhesives and repeated friction against the hairline. Generally, wigs and weaves should be cared for as one would care for one’s own hair.
Hair Care in Black Children—Children’s hair care begins with washing the hair and scalp with shampoo, applying conditioner, and detangling as needed.31 After rinsing out the conditioner, a leave-in conditioner can assist with moisture retention and further detangling. The hair is then styled, either wet or dry. Recommendations for hair care practices in Black children include loose hairstyles that do not strain hair roots and nightly removal of root-securing accessories (eg, barrettes, elastic hairbeads). Frequent cornrow styling and friction on chemically straightened hair were identified by a survey as considerable traction alopecia risk factors.32 Thus, educating caregivers on appropriate hair-grooming practices for children is important.
Hair Protection—Proactive steps to reduce hair loss include wearing satin bonnets and/or using satin pillowcases while sleeping regardless of hairstyle. Although evidence is limited, it is thought that satin and silk allow the hair to retain its moisture and natural oils, preventing breakage and friction.33,34 Frequent hair trimming every 2 to 4 months can reduce breakage when doing thermal treatments.35,36 When prolonged or repetitive styles are used, it is encouraged to give the hair a break between styles to recover from the repeated stress. Wearing an intermittent updo or high bun—a hairstyle in which the hair is pulled upward—can prevent breakage by reducing heavy strain on the hair; however, it is important to avoid the use of rubber bands due to friction and risk for tangling of hair strands. Instead, the use of covered elastic ties and/or those without metal is preferred.11 Alternatively, if a polished and neat appearance with slicked-back hair is desired, the practice of tautly pulling the hair is not recommended. Instead, use of an alcohol-free gel is suggested along with a satin scarf wrapped around the hairline to facilitate the setting of the hair in place.11
A common practice to preserve curly hairstyles while sleeping is known as the pineapple method, which protects the hair and aids in preserving the freshness and style of the curls.37 It consists of a loosely tied high ponytail at the top of the head allowing the curls to fall forward. This minimizes frizz and prevents the curls from forming knots.
Conclusion
Hair care recommendations in Black women can be complex due to a wide range of personal care preferences and styling techniques in this population. While evidence in the literature is limited, it still is important for dermatologists to be familiar with the different hair care practices utilized by Black women so they can effectively counsel patients and improve hair health. Knowledge of optimal hair care practices can aid in the prevention of common hair disorders that disproportionately affect this patient population, such as traction alopecia and trichorrhexis nodosa or breakage.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314. doi:10.1001/jamadermatol.2013.1946
- Johnson T, Bankhead T. Hair it is: examining the experiences of Black women with natural hair. Open J Soc Sci. 2014;02:86-100. doi:10.4236/jss.2014.21010
- Byrd AD, Tharps LL. Hair Story: Untangling the Roots of Black Hair in America. 2nd ed. St Martin’s Griffin; 2014.
- Mbilishaka AM, Clemons K, Hudlin M, et al. Don’t get it twisted: untangling the psychology of hair discrimination within Black communities. Am J Orthopsychiatry. 2020;90:590-599. doi:10.1037 /ort0000468
- Khumalo NP. On the history of African hair care: more treasures await discovery. J Cosmet Dermatol. 2008;7:231. doi:10.1111/j.1473- 2165.2008.00396.x
- Johnson AM, Godsil RD, MacFarlane J, et al. The “good hair” study: explicit and implicit attitudes toward Black women’s hair. Perception Institute. February 2017. Accessed February 11, 2025. https://perception.org/publications/goodhairstudy/
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Roseborough IE, McMichael AJ. Hair care practices in African- American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Menkart J Wolfram LJ Mao I. Caucasian hair, Negro hair and wool: similarities and differences. J Soc Cosmet Chem. 1996;17:769-787.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Mayo TT, Callender VD. The art of prevention: it’s too tight-loosen up and let your hair down. Int J Womens Dermatol. 2021;7:174-179. doi:10.1016/j.ijwd.2021.01.019
- De Sá Dias TC, Baby AR, Kaneko TM, et al. Relaxing/straightening of Afro-ethnic hair: historical overview. J Cosmet Dermatol. 2007;6:2-5. doi:10.1111/j.1473-2165.2007.00294.x
- Loussouarn G, Garcel AL, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46 (suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- Barba C, Mendez S, Marti M, et al. Water content of hair and nails. Thermochimica Acta. 2009;494:136-140. doi:10.1016/j.tca.2009.05.005
- Gray J. Hair care and hair care products. Clin Dermatol. 2001;19:227-236. doi:10.1016/s0738-081x(00)00133-4
- Gavazzoni Dias MFR. Pro and contra of cleansing conditioners. Skin Appendage Disord. 2019;5:131-134. doi:10.1159/000493588
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15. doi:10.4103/0974-7753.153450
- Beal AC, Villarosa L, Abner A. The Black Parenting Book. 1999.
- Davis-Sivasothy A. The Science of Black Hair: A Comprehensive Guide to Textured Care. Saga Publishing; 2011.
- Robbins CR. The Physical Properties and Cosmetic Behavior of Hair. In: Robbins CR. Chemical and Physical Behavior of Human Hair. 3rd ed. Springer Nature; 1994:299-370. doi:10.1007/978-1-4757-3898-8_8
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Barreto T, Weffort F, Frattini S, et al. Straight to the point: what do we know so far on hair straightening? Skin Appendage Disord. 2021;7:265-271. doi:10.1159/000514367
- Dussaud A, Rana B, Lam HT. Progressive hair straightening using an automated flat iron: function of silicones. J Cosmet Sci. 2013;64:119-131.
- Zhou Y, Rigoletto R, Koelmel D, et al. The effect of various cosmetic pretreatments on protecting hair from thermal damage by hot flat ironing. J Cosmet Sci. 2011;62:265-282.
- Chang CJ, O’Brien KM, Keil AP, et al. Use of straighteners and other hair products and incident uterine cancer. J Natl Cancer Inst. 2022;114:1636-1645. doi:10.1093/jnci/djac165
- White AJ, Gregoire AM, Taylor KW, et al. Adolescent use of hair dyes, straighteners and perms in relation to breast cancer risk. Int J Cancer. 2021;148:2255-2263. doi:10.1002/ijc.33413
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018;165:448-458.
- Asbeck S, Riley-Prescott C, Glaser E, et al. Afro-ethnic hairstyling trends, risks, and recommendations. Cosmetics. 2022;9:17. doi:10.3390 /cosmetics9010017
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127. doi:10.1016 /j.ijwd.2016.09.002
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID .S137296
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160. doi:10.1111/pde.14721
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j.jaad.2010.05.037
- Carefoot H. Silk pillowcases for better hair and skin: what to know. The Washington Post. April 6, 2021. Accessed February 10, 2025. https://www.washingtonpost.com/lifestyle/wellness/silk-pillowcases-hair-skin-benefits-myths/2021/04/05/a7dcad7c-866a-11eb-82bc-e58213caa38e_story.html
- Samrao A, McMichael A, Mirmirani P. Nocturnal traction: techniques used for hair style maintenance while sleeping may be a risk factor for traction alopecia. Skin Appendage Disord. 2021;7:220-223. doi:10.1159/000513088
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- McMichael AJ. Hair breakage in normal and weathered hair: focus on the Black patient. J Investig Dermatol Symp Proc. 2007;12:6-9. doi:10.1038/sj.jidsymp.5650047
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80,106.
Patients often ask dermatologists how to best care for their specific hair type; however, there are no formal recommendations that apply to the many different hair care practices utilized by Black patients, as hair types in this community can range from wavy to tightly coiled.1 Understanding the the history of hair care in those of African ancestry and various styling practices in this population is necessary to adequately counsel patients and gain trust in the doctor-patient relationship. In this article, we provide an overview of hair care recommendations based on common styling practices in Black women.
A PubMed search of articles indexed for MEDLINE using the terms Black hair care, African American hair management, hair loss prevention, hair care practices, natural hair, natural-hair styles, alopecia, hairdressing, hair breakage, hair fragility, heat-stressed hair, traction alopecia, and natural hair care yielded 305 results; 107 duplicates were identified and removed, leaving 198 articles to be screened for eligibility (ie, English-language studies created in the past 15 years). Sixty-eight full-text articles were screened against the exclusion criteria, which included case reports and case series, articles not focused on Afro-textured hair, and cancer-related hair loss. Three additional fulltext articles were identified via resources from Wayne State University library (Detroit, Michigan) that were not available on PubMed. A total of 29 full-text articles were included in our review.
Background on Hair Care and Styling in African Populations
It is difficult to understand the history of hair in those of African ancestry in the United States.2 Prior to slavery, hair styling was considered a way of identification, classification, and communication as well as a medium through which to connect with the spiritual world in many parts of Africa. Hair-styling practices in Africa included elaborate cornrows, threading, and braiding with many accessories. Notable hair-styling products included natural butters, herbs, and powders to assist with moisture retention. Scarves also were used during this time for ceremonies or protection.3 During the mass enslavement of African populations and their transportation to the Americas by Europeans, slaveholders routinely cut off all the hair of both men and women in order to objectify and erase the culture of African hair styling passed down through generations.4,5 Hair texture then was weaponized to create a caste system in plantation life, in which Black slaves with straight hair textures were granted the “privilege” of domestic work, while those with kinky hair were relegated to arduous manual labor in the fields.4 Years later, during the 1800s, laws were enacted in the United States to prohibit Black women from wearing tightly coiled natural hair in public places.5 Over the next few centuries from the 1800s to the early 2000s, various hair-styling trends such as the use of hot combs, perms, afros, and Jheri curls developed as a means for Black individuals to conform to societal pressure to adopt more European features; however, as time progressed, afros, braids, locs, and natural hair would become more dominant as statements against these same societal pressures.5
The natural hair movement, which emerged in the United States in the 2000s, encouraged Black women to abandon the use of toxic chemical hair straighteners, cultivate healthier hair care practices, disrupt Eurocentric standards of wearing straightened hair, and facilitate self-definition of beauty ideals from the Civil Rights Movement of the 1960s.4,5 It is estimated that between 30% and 70% of all Black women in the United States wear natural hair, including 79% of millennial Black women younger than 30 years6; however, several new trends such as wigs and weaves have grown in popularity since the early 2000s due to mainstream pop culture and improvements in creating natural hairlines.7,8
Key Features of Afro-Textured Hair
Individuals of African descent have the most diverse hair texture phenotypes, ranging from straight to tightly coiled.9 Although hair is chemically similar across various racial groups, differences are noted mainly in the shape of the hair shaft, with elliptical and curved shapes seen in Afrotextured hair. These differences yield more tightly curled strands than in other hair types; however, these features also contribute to fragility, as it creates points of weakness and decreases the tensile strength of the hair shaft.10 This inherent fragility leads to higher rates of hair breakage as well as lower moisture content and slower growth rates, which is why Afro-textured hair requires special care.9
Afro-textured hair generally falls into 2 main categories of the Andre Walker hair typing system: 4A-4C and 3A-3C.11 In the 4A-4C category, hair is described as coily or kinky. Common concerns related to this hair type include dryness and brittleness with increased susceptibility to breakage. The 3A-3C category is described as loose to corkscrew curls, with a common concern of dryness.11,12 Additionally, Loussouarn et al13 established a method to further define natural hair curliness using curve diameter and curl meters on glass plates to measure the curvature of hair strands. This method allows for assessing diversity and range of curliness within various races without relying on ethnic origin.13
Common Hair Care Practices
A description of each hair type and recommended styling practices with their levels of evidence can be found in the eTable.



Natural Hair—Natural hair is classified as hair that has not been chemically changed by perms, heat, or other straightening treatments.12,14 For natural hair, retaining the moisture of the hair shaft should be the main focus, as moisture loss leads to considerable dryness.14 Generally, it is recommended to wash natural hair once per week or every other week; however, this can change based on hair length and oil production on the scalp. Washing daily may be ideal for shorter hair and monthly for longer hair to help prevent product build-up that can have a drying effect.15 Avoid shampoos that are drying (eg, sulfate and silicone products). The co-washing method also can be utilized, which entails washing the hair with a conditioning cleanser instead of shampoo and conditioner. However, this technique is not meant to completely replace shampoo.16 In fact, a clarifying shampoo is recommended after co-washing 3 or 4 times.16 The use of a hot oil treatment twice per month can promote moisture retention and reduce split-end formation.17 For maintenance between washes, many utilize the liquid, oil, cream (LOC) or liquid, cream, oil (LCO) methods, which describe regimens that utilize water, an oil of choice, and cream such as shea butter to lock in moisture.18 This method can be used as often as needed for dry hair.
Due to the susceptibility of Afro-textured hair to tangle and knot, using a wide-tooth comb, detangling brush, or detangling conditioners is a grade B recommendation for care (eTable). Though not widely documented in the literature, many of our patients have had anecdotal success detangling their hair simply by pulling hair strands apart by hand or “finger detangling” as well as using wide-tooth combs. Although both hair types are healthier in their natural states, kinky hair (type 4A-4C) is extremely fragile and more difficult to manage than less kinky hair (type 3A-3C).18
Special care is needed when detangling due to strands being weaker when wet.19 Detangling should be performed in a retrograde fashion. Deep conditioning can aid in moisture retention and should be performed weekly or biweekly.17-20 Depending on the health of the hair, protein treatments can be considered on a monthly basis to help preserve the cuticle. Styling with braids, twists, or other protective styles can then be completed on an individual basis.
Thermal Straightening—A blowout involves straightening the hair after a wash with the use of a hair dryer.21 This common hair-styling method does not employ the use of chemicals beyond light hair oils and heat-protectant creams or sprays, typically resulting in a less kinky afro or semi-straight hair. Thermal straightening utilizes heat to temporarily straighten hair strands. Flat irons with heated metal plates then can be used after blow-drying the hair to fully straighten and smooth the strands. These processes combined commonly are known as a silk press.21-22
For thermally straightened hair, it is recommended to either wrap the hair around the scalp to keep it flat or pin curl the hair and cover with a bonnet to sleep. Safe straightening techniques with the use of a flat iron include setting the temperature no higher than 175 °F or a low/medium setting while also limiting use to once per week if possible.23 The number of passes of the flat iron also should be limited to 1 to 2 to reduce breakage. A heat-protectant cream or spray also can be applied to the hair before flat ironing to minimize damage. Applying heat protectant to the hair prior to styling will help minimize heat damage by distributing the heat along the hair fiber surface, avoiding water boiling in the hair shaft and the development of bubble hair leading to damage.24
Chemical Straightening—Similar to how relaxers, perms, and Jheri curl treatments chemically modify hair texture using distinct chemicals yielding different curl patterns, the Brazilian blowout similarly straightens hair using a hair dryer and chemicals applied to hair strands after washing.21-24 Relaxers utilize sodium or guanidine hydroxide for straightening, perms use ammonium thioglycolate for curling, and Jheri curl treatments employ thioglycolates or mercaptans for defined curls. However, these treatments generally are cautioned against due to potential hair damage and recent associations with uterine and breast cancer in Black women. Research has suggested that endocrine disrupters in these products, especially those marketed to Black women, contribute to hormone-related disease processes.25,26 One study found higher concentrations of alkylphenols, the fragrance marker diethyl phthalate, and parabens in relaxers27; however, more research is needed to determine specific chemicals associated with these cancers.
Braids and Locs—Braiding is a technique that involves interlocking 3 or more sections of hair that may or may not be fixated to the scalp like a cornrow,11 and one can utilize extensions or natural hair depending on the desired outcome. Intended for long-term wear (ie, weeks to months), braids minimize breakage and reduce daily styling needs. Two popular styles—cornrows and individual braids—differ in preparation and weaving techniques. Cornrows are an Afro-centric style involving uniform, tightly woven braids that are close to the scalp, creating distinct patterns. Conversely, individual braids weave separate hair sections, offering diverse styling possibilities. Braiding practices should exclude hairline edges—often termed baby hairs—to prevent traction alopecia. Minimal use of edge gel, which helps to tame the vellus hairs at the frontotemporal scalp, as well as mindful weave volume, weight, and length are recommended to avert breakage. Braids that cause pain are too tight, can damage hair, and may cause traction alopecia.11 Braids should not be worn for longer than 3 months at a time and require biweekly washing with diluted shampoo and conditioner. Proper drying by wringing the hair with a microfiber towel is essential to avoid frizz and mold formation.
Locs are a low-maintenance hairstyle considered permanent until cut.28 This style involves twisting, palm rolling, or using tools such as crochet hooks to “lock” the hair. Regular maintenance with retwisting and cleaning is vital for loc health. Increased weight and tight twisting of locs can cause damage to the scalp and hair strands; however, locs are known to increase hair volume over time, often due to the accumulation of hairs that would otherwise have been shed in the telogen phase.28
Wigs and Weaves—Wigs consist of synthetic or human hair that can be strapped to the head with an adjustable band or glued to the scalp depending on the desired style.29 Wigs are removed daily, which allows for quick access to hair for cleansing and moisturizing. In contrast, weaves typically are sewn into the natural hair, which may make it difficult to reach the scalp for cleansing, leading to dryness and product build-up.29 Notably, there is evidence of a relationship between long-term use of weaves and traction alopecia.30
Wigs can have a fully synthetic hair line or lace hair line and can range from very affordable to expensive. When applied correctly, both styles offer an easy way to cover and protect the natural hair by reducing the amount of physical trauma related to daily hair styling. A lace-front wig contains a frontal thin mesh or lace that camouflages the natural frontal hairline.29,30 A risk of lace-front wigs is that they can cause friction alopecia secondary to repeated use of adhesives and repeated friction against the hairline. Generally, wigs and weaves should be cared for as one would care for one’s own hair.
Hair Care in Black Children—Children’s hair care begins with washing the hair and scalp with shampoo, applying conditioner, and detangling as needed.31 After rinsing out the conditioner, a leave-in conditioner can assist with moisture retention and further detangling. The hair is then styled, either wet or dry. Recommendations for hair care practices in Black children include loose hairstyles that do not strain hair roots and nightly removal of root-securing accessories (eg, barrettes, elastic hairbeads). Frequent cornrow styling and friction on chemically straightened hair were identified by a survey as considerable traction alopecia risk factors.32 Thus, educating caregivers on appropriate hair-grooming practices for children is important.
Hair Protection—Proactive steps to reduce hair loss include wearing satin bonnets and/or using satin pillowcases while sleeping regardless of hairstyle. Although evidence is limited, it is thought that satin and silk allow the hair to retain its moisture and natural oils, preventing breakage and friction.33,34 Frequent hair trimming every 2 to 4 months can reduce breakage when doing thermal treatments.35,36 When prolonged or repetitive styles are used, it is encouraged to give the hair a break between styles to recover from the repeated stress. Wearing an intermittent updo or high bun—a hairstyle in which the hair is pulled upward—can prevent breakage by reducing heavy strain on the hair; however, it is important to avoid the use of rubber bands due to friction and risk for tangling of hair strands. Instead, the use of covered elastic ties and/or those without metal is preferred.11 Alternatively, if a polished and neat appearance with slicked-back hair is desired, the practice of tautly pulling the hair is not recommended. Instead, use of an alcohol-free gel is suggested along with a satin scarf wrapped around the hairline to facilitate the setting of the hair in place.11
A common practice to preserve curly hairstyles while sleeping is known as the pineapple method, which protects the hair and aids in preserving the freshness and style of the curls.37 It consists of a loosely tied high ponytail at the top of the head allowing the curls to fall forward. This minimizes frizz and prevents the curls from forming knots.
Conclusion
Hair care recommendations in Black women can be complex due to a wide range of personal care preferences and styling techniques in this population. While evidence in the literature is limited, it still is important for dermatologists to be familiar with the different hair care practices utilized by Black women so they can effectively counsel patients and improve hair health. Knowledge of optimal hair care practices can aid in the prevention of common hair disorders that disproportionately affect this patient population, such as traction alopecia and trichorrhexis nodosa or breakage.
Patients often ask dermatologists how to best care for their specific hair type; however, there are no formal recommendations that apply to the many different hair care practices utilized by Black patients, as hair types in this community can range from wavy to tightly coiled.1 Understanding the the history of hair care in those of African ancestry and various styling practices in this population is necessary to adequately counsel patients and gain trust in the doctor-patient relationship. In this article, we provide an overview of hair care recommendations based on common styling practices in Black women.
A PubMed search of articles indexed for MEDLINE using the terms Black hair care, African American hair management, hair loss prevention, hair care practices, natural hair, natural-hair styles, alopecia, hairdressing, hair breakage, hair fragility, heat-stressed hair, traction alopecia, and natural hair care yielded 305 results; 107 duplicates were identified and removed, leaving 198 articles to be screened for eligibility (ie, English-language studies created in the past 15 years). Sixty-eight full-text articles were screened against the exclusion criteria, which included case reports and case series, articles not focused on Afro-textured hair, and cancer-related hair loss. Three additional fulltext articles were identified via resources from Wayne State University library (Detroit, Michigan) that were not available on PubMed. A total of 29 full-text articles were included in our review.
Background on Hair Care and Styling in African Populations
It is difficult to understand the history of hair in those of African ancestry in the United States.2 Prior to slavery, hair styling was considered a way of identification, classification, and communication as well as a medium through which to connect with the spiritual world in many parts of Africa. Hair-styling practices in Africa included elaborate cornrows, threading, and braiding with many accessories. Notable hair-styling products included natural butters, herbs, and powders to assist with moisture retention. Scarves also were used during this time for ceremonies or protection.3 During the mass enslavement of African populations and their transportation to the Americas by Europeans, slaveholders routinely cut off all the hair of both men and women in order to objectify and erase the culture of African hair styling passed down through generations.4,5 Hair texture then was weaponized to create a caste system in plantation life, in which Black slaves with straight hair textures were granted the “privilege” of domestic work, while those with kinky hair were relegated to arduous manual labor in the fields.4 Years later, during the 1800s, laws were enacted in the United States to prohibit Black women from wearing tightly coiled natural hair in public places.5 Over the next few centuries from the 1800s to the early 2000s, various hair-styling trends such as the use of hot combs, perms, afros, and Jheri curls developed as a means for Black individuals to conform to societal pressure to adopt more European features; however, as time progressed, afros, braids, locs, and natural hair would become more dominant as statements against these same societal pressures.5
The natural hair movement, which emerged in the United States in the 2000s, encouraged Black women to abandon the use of toxic chemical hair straighteners, cultivate healthier hair care practices, disrupt Eurocentric standards of wearing straightened hair, and facilitate self-definition of beauty ideals from the Civil Rights Movement of the 1960s.4,5 It is estimated that between 30% and 70% of all Black women in the United States wear natural hair, including 79% of millennial Black women younger than 30 years6; however, several new trends such as wigs and weaves have grown in popularity since the early 2000s due to mainstream pop culture and improvements in creating natural hairlines.7,8
Key Features of Afro-Textured Hair
Individuals of African descent have the most diverse hair texture phenotypes, ranging from straight to tightly coiled.9 Although hair is chemically similar across various racial groups, differences are noted mainly in the shape of the hair shaft, with elliptical and curved shapes seen in Afrotextured hair. These differences yield more tightly curled strands than in other hair types; however, these features also contribute to fragility, as it creates points of weakness and decreases the tensile strength of the hair shaft.10 This inherent fragility leads to higher rates of hair breakage as well as lower moisture content and slower growth rates, which is why Afro-textured hair requires special care.9
Afro-textured hair generally falls into 2 main categories of the Andre Walker hair typing system: 4A-4C and 3A-3C.11 In the 4A-4C category, hair is described as coily or kinky. Common concerns related to this hair type include dryness and brittleness with increased susceptibility to breakage. The 3A-3C category is described as loose to corkscrew curls, with a common concern of dryness.11,12 Additionally, Loussouarn et al13 established a method to further define natural hair curliness using curve diameter and curl meters on glass plates to measure the curvature of hair strands. This method allows for assessing diversity and range of curliness within various races without relying on ethnic origin.13
Common Hair Care Practices
A description of each hair type and recommended styling practices with their levels of evidence can be found in the eTable.



Natural Hair—Natural hair is classified as hair that has not been chemically changed by perms, heat, or other straightening treatments.12,14 For natural hair, retaining the moisture of the hair shaft should be the main focus, as moisture loss leads to considerable dryness.14 Generally, it is recommended to wash natural hair once per week or every other week; however, this can change based on hair length and oil production on the scalp. Washing daily may be ideal for shorter hair and monthly for longer hair to help prevent product build-up that can have a drying effect.15 Avoid shampoos that are drying (eg, sulfate and silicone products). The co-washing method also can be utilized, which entails washing the hair with a conditioning cleanser instead of shampoo and conditioner. However, this technique is not meant to completely replace shampoo.16 In fact, a clarifying shampoo is recommended after co-washing 3 or 4 times.16 The use of a hot oil treatment twice per month can promote moisture retention and reduce split-end formation.17 For maintenance between washes, many utilize the liquid, oil, cream (LOC) or liquid, cream, oil (LCO) methods, which describe regimens that utilize water, an oil of choice, and cream such as shea butter to lock in moisture.18 This method can be used as often as needed for dry hair.
Due to the susceptibility of Afro-textured hair to tangle and knot, using a wide-tooth comb, detangling brush, or detangling conditioners is a grade B recommendation for care (eTable). Though not widely documented in the literature, many of our patients have had anecdotal success detangling their hair simply by pulling hair strands apart by hand or “finger detangling” as well as using wide-tooth combs. Although both hair types are healthier in their natural states, kinky hair (type 4A-4C) is extremely fragile and more difficult to manage than less kinky hair (type 3A-3C).18
Special care is needed when detangling due to strands being weaker when wet.19 Detangling should be performed in a retrograde fashion. Deep conditioning can aid in moisture retention and should be performed weekly or biweekly.17-20 Depending on the health of the hair, protein treatments can be considered on a monthly basis to help preserve the cuticle. Styling with braids, twists, or other protective styles can then be completed on an individual basis.
Thermal Straightening—A blowout involves straightening the hair after a wash with the use of a hair dryer.21 This common hair-styling method does not employ the use of chemicals beyond light hair oils and heat-protectant creams or sprays, typically resulting in a less kinky afro or semi-straight hair. Thermal straightening utilizes heat to temporarily straighten hair strands. Flat irons with heated metal plates then can be used after blow-drying the hair to fully straighten and smooth the strands. These processes combined commonly are known as a silk press.21-22
For thermally straightened hair, it is recommended to either wrap the hair around the scalp to keep it flat or pin curl the hair and cover with a bonnet to sleep. Safe straightening techniques with the use of a flat iron include setting the temperature no higher than 175 °F or a low/medium setting while also limiting use to once per week if possible.23 The number of passes of the flat iron also should be limited to 1 to 2 to reduce breakage. A heat-protectant cream or spray also can be applied to the hair before flat ironing to minimize damage. Applying heat protectant to the hair prior to styling will help minimize heat damage by distributing the heat along the hair fiber surface, avoiding water boiling in the hair shaft and the development of bubble hair leading to damage.24
Chemical Straightening—Similar to how relaxers, perms, and Jheri curl treatments chemically modify hair texture using distinct chemicals yielding different curl patterns, the Brazilian blowout similarly straightens hair using a hair dryer and chemicals applied to hair strands after washing.21-24 Relaxers utilize sodium or guanidine hydroxide for straightening, perms use ammonium thioglycolate for curling, and Jheri curl treatments employ thioglycolates or mercaptans for defined curls. However, these treatments generally are cautioned against due to potential hair damage and recent associations with uterine and breast cancer in Black women. Research has suggested that endocrine disrupters in these products, especially those marketed to Black women, contribute to hormone-related disease processes.25,26 One study found higher concentrations of alkylphenols, the fragrance marker diethyl phthalate, and parabens in relaxers27; however, more research is needed to determine specific chemicals associated with these cancers.
Braids and Locs—Braiding is a technique that involves interlocking 3 or more sections of hair that may or may not be fixated to the scalp like a cornrow,11 and one can utilize extensions or natural hair depending on the desired outcome. Intended for long-term wear (ie, weeks to months), braids minimize breakage and reduce daily styling needs. Two popular styles—cornrows and individual braids—differ in preparation and weaving techniques. Cornrows are an Afro-centric style involving uniform, tightly woven braids that are close to the scalp, creating distinct patterns. Conversely, individual braids weave separate hair sections, offering diverse styling possibilities. Braiding practices should exclude hairline edges—often termed baby hairs—to prevent traction alopecia. Minimal use of edge gel, which helps to tame the vellus hairs at the frontotemporal scalp, as well as mindful weave volume, weight, and length are recommended to avert breakage. Braids that cause pain are too tight, can damage hair, and may cause traction alopecia.11 Braids should not be worn for longer than 3 months at a time and require biweekly washing with diluted shampoo and conditioner. Proper drying by wringing the hair with a microfiber towel is essential to avoid frizz and mold formation.
Locs are a low-maintenance hairstyle considered permanent until cut.28 This style involves twisting, palm rolling, or using tools such as crochet hooks to “lock” the hair. Regular maintenance with retwisting and cleaning is vital for loc health. Increased weight and tight twisting of locs can cause damage to the scalp and hair strands; however, locs are known to increase hair volume over time, often due to the accumulation of hairs that would otherwise have been shed in the telogen phase.28
Wigs and Weaves—Wigs consist of synthetic or human hair that can be strapped to the head with an adjustable band or glued to the scalp depending on the desired style.29 Wigs are removed daily, which allows for quick access to hair for cleansing and moisturizing. In contrast, weaves typically are sewn into the natural hair, which may make it difficult to reach the scalp for cleansing, leading to dryness and product build-up.29 Notably, there is evidence of a relationship between long-term use of weaves and traction alopecia.30
Wigs can have a fully synthetic hair line or lace hair line and can range from very affordable to expensive. When applied correctly, both styles offer an easy way to cover and protect the natural hair by reducing the amount of physical trauma related to daily hair styling. A lace-front wig contains a frontal thin mesh or lace that camouflages the natural frontal hairline.29,30 A risk of lace-front wigs is that they can cause friction alopecia secondary to repeated use of adhesives and repeated friction against the hairline. Generally, wigs and weaves should be cared for as one would care for one’s own hair.
Hair Care in Black Children—Children’s hair care begins with washing the hair and scalp with shampoo, applying conditioner, and detangling as needed.31 After rinsing out the conditioner, a leave-in conditioner can assist with moisture retention and further detangling. The hair is then styled, either wet or dry. Recommendations for hair care practices in Black children include loose hairstyles that do not strain hair roots and nightly removal of root-securing accessories (eg, barrettes, elastic hairbeads). Frequent cornrow styling and friction on chemically straightened hair were identified by a survey as considerable traction alopecia risk factors.32 Thus, educating caregivers on appropriate hair-grooming practices for children is important.
Hair Protection—Proactive steps to reduce hair loss include wearing satin bonnets and/or using satin pillowcases while sleeping regardless of hairstyle. Although evidence is limited, it is thought that satin and silk allow the hair to retain its moisture and natural oils, preventing breakage and friction.33,34 Frequent hair trimming every 2 to 4 months can reduce breakage when doing thermal treatments.35,36 When prolonged or repetitive styles are used, it is encouraged to give the hair a break between styles to recover from the repeated stress. Wearing an intermittent updo or high bun—a hairstyle in which the hair is pulled upward—can prevent breakage by reducing heavy strain on the hair; however, it is important to avoid the use of rubber bands due to friction and risk for tangling of hair strands. Instead, the use of covered elastic ties and/or those without metal is preferred.11 Alternatively, if a polished and neat appearance with slicked-back hair is desired, the practice of tautly pulling the hair is not recommended. Instead, use of an alcohol-free gel is suggested along with a satin scarf wrapped around the hairline to facilitate the setting of the hair in place.11
A common practice to preserve curly hairstyles while sleeping is known as the pineapple method, which protects the hair and aids in preserving the freshness and style of the curls.37 It consists of a loosely tied high ponytail at the top of the head allowing the curls to fall forward. This minimizes frizz and prevents the curls from forming knots.
Conclusion
Hair care recommendations in Black women can be complex due to a wide range of personal care preferences and styling techniques in this population. While evidence in the literature is limited, it still is important for dermatologists to be familiar with the different hair care practices utilized by Black women so they can effectively counsel patients and improve hair health. Knowledge of optimal hair care practices can aid in the prevention of common hair disorders that disproportionately affect this patient population, such as traction alopecia and trichorrhexis nodosa or breakage.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314. doi:10.1001/jamadermatol.2013.1946
- Johnson T, Bankhead T. Hair it is: examining the experiences of Black women with natural hair. Open J Soc Sci. 2014;02:86-100. doi:10.4236/jss.2014.21010
- Byrd AD, Tharps LL. Hair Story: Untangling the Roots of Black Hair in America. 2nd ed. St Martin’s Griffin; 2014.
- Mbilishaka AM, Clemons K, Hudlin M, et al. Don’t get it twisted: untangling the psychology of hair discrimination within Black communities. Am J Orthopsychiatry. 2020;90:590-599. doi:10.1037 /ort0000468
- Khumalo NP. On the history of African hair care: more treasures await discovery. J Cosmet Dermatol. 2008;7:231. doi:10.1111/j.1473- 2165.2008.00396.x
- Johnson AM, Godsil RD, MacFarlane J, et al. The “good hair” study: explicit and implicit attitudes toward Black women’s hair. Perception Institute. February 2017. Accessed February 11, 2025. https://perception.org/publications/goodhairstudy/
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Roseborough IE, McMichael AJ. Hair care practices in African- American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Menkart J Wolfram LJ Mao I. Caucasian hair, Negro hair and wool: similarities and differences. J Soc Cosmet Chem. 1996;17:769-787.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Mayo TT, Callender VD. The art of prevention: it’s too tight-loosen up and let your hair down. Int J Womens Dermatol. 2021;7:174-179. doi:10.1016/j.ijwd.2021.01.019
- De Sá Dias TC, Baby AR, Kaneko TM, et al. Relaxing/straightening of Afro-ethnic hair: historical overview. J Cosmet Dermatol. 2007;6:2-5. doi:10.1111/j.1473-2165.2007.00294.x
- Loussouarn G, Garcel AL, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46 (suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- Barba C, Mendez S, Marti M, et al. Water content of hair and nails. Thermochimica Acta. 2009;494:136-140. doi:10.1016/j.tca.2009.05.005
- Gray J. Hair care and hair care products. Clin Dermatol. 2001;19:227-236. doi:10.1016/s0738-081x(00)00133-4
- Gavazzoni Dias MFR. Pro and contra of cleansing conditioners. Skin Appendage Disord. 2019;5:131-134. doi:10.1159/000493588
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15. doi:10.4103/0974-7753.153450
- Beal AC, Villarosa L, Abner A. The Black Parenting Book. 1999.
- Davis-Sivasothy A. The Science of Black Hair: A Comprehensive Guide to Textured Care. Saga Publishing; 2011.
- Robbins CR. The Physical Properties and Cosmetic Behavior of Hair. In: Robbins CR. Chemical and Physical Behavior of Human Hair. 3rd ed. Springer Nature; 1994:299-370. doi:10.1007/978-1-4757-3898-8_8
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Barreto T, Weffort F, Frattini S, et al. Straight to the point: what do we know so far on hair straightening? Skin Appendage Disord. 2021;7:265-271. doi:10.1159/000514367
- Dussaud A, Rana B, Lam HT. Progressive hair straightening using an automated flat iron: function of silicones. J Cosmet Sci. 2013;64:119-131.
- Zhou Y, Rigoletto R, Koelmel D, et al. The effect of various cosmetic pretreatments on protecting hair from thermal damage by hot flat ironing. J Cosmet Sci. 2011;62:265-282.
- Chang CJ, O’Brien KM, Keil AP, et al. Use of straighteners and other hair products and incident uterine cancer. J Natl Cancer Inst. 2022;114:1636-1645. doi:10.1093/jnci/djac165
- White AJ, Gregoire AM, Taylor KW, et al. Adolescent use of hair dyes, straighteners and perms in relation to breast cancer risk. Int J Cancer. 2021;148:2255-2263. doi:10.1002/ijc.33413
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018;165:448-458.
- Asbeck S, Riley-Prescott C, Glaser E, et al. Afro-ethnic hairstyling trends, risks, and recommendations. Cosmetics. 2022;9:17. doi:10.3390 /cosmetics9010017
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127. doi:10.1016 /j.ijwd.2016.09.002
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID .S137296
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160. doi:10.1111/pde.14721
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j.jaad.2010.05.037
- Carefoot H. Silk pillowcases for better hair and skin: what to know. The Washington Post. April 6, 2021. Accessed February 10, 2025. https://www.washingtonpost.com/lifestyle/wellness/silk-pillowcases-hair-skin-benefits-myths/2021/04/05/a7dcad7c-866a-11eb-82bc-e58213caa38e_story.html
- Samrao A, McMichael A, Mirmirani P. Nocturnal traction: techniques used for hair style maintenance while sleeping may be a risk factor for traction alopecia. Skin Appendage Disord. 2021;7:220-223. doi:10.1159/000513088
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- McMichael AJ. Hair breakage in normal and weathered hair: focus on the Black patient. J Investig Dermatol Symp Proc. 2007;12:6-9. doi:10.1038/sj.jidsymp.5650047
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80,106.
- Hall RR, Francis S, Whitt-Glover M, et al. Hair care practices as a barrier to physical activity in African American women. JAMA Dermatol. 2013;149:310-314. doi:10.1001/jamadermatol.2013.1946
- Johnson T, Bankhead T. Hair it is: examining the experiences of Black women with natural hair. Open J Soc Sci. 2014;02:86-100. doi:10.4236/jss.2014.21010
- Byrd AD, Tharps LL. Hair Story: Untangling the Roots of Black Hair in America. 2nd ed. St Martin’s Griffin; 2014.
- Mbilishaka AM, Clemons K, Hudlin M, et al. Don’t get it twisted: untangling the psychology of hair discrimination within Black communities. Am J Orthopsychiatry. 2020;90:590-599. doi:10.1037 /ort0000468
- Khumalo NP. On the history of African hair care: more treasures await discovery. J Cosmet Dermatol. 2008;7:231. doi:10.1111/j.1473- 2165.2008.00396.x
- Johnson AM, Godsil RD, MacFarlane J, et al. The “good hair” study: explicit and implicit attitudes toward Black women’s hair. Perception Institute. February 2017. Accessed February 11, 2025. https://perception.org/publications/goodhairstudy/
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Roseborough IE, McMichael AJ. Hair care practices in African- American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Menkart J Wolfram LJ Mao I. Caucasian hair, Negro hair and wool: similarities and differences. J Soc Cosmet Chem. 1996;17:769-787.
- Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
- Mayo TT, Callender VD. The art of prevention: it’s too tight-loosen up and let your hair down. Int J Womens Dermatol. 2021;7:174-179. doi:10.1016/j.ijwd.2021.01.019
- De Sá Dias TC, Baby AR, Kaneko TM, et al. Relaxing/straightening of Afro-ethnic hair: historical overview. J Cosmet Dermatol. 2007;6:2-5. doi:10.1111/j.1473-2165.2007.00294.x
- Loussouarn G, Garcel AL, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46 (suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- Barba C, Mendez S, Marti M, et al. Water content of hair and nails. Thermochimica Acta. 2009;494:136-140. doi:10.1016/j.tca.2009.05.005
- Gray J. Hair care and hair care products. Clin Dermatol. 2001;19:227-236. doi:10.1016/s0738-081x(00)00133-4
- Gavazzoni Dias MFR. Pro and contra of cleansing conditioners. Skin Appendage Disord. 2019;5:131-134. doi:10.1159/000493588
- Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2-15. doi:10.4103/0974-7753.153450
- Beal AC, Villarosa L, Abner A. The Black Parenting Book. 1999.
- Davis-Sivasothy A. The Science of Black Hair: A Comprehensive Guide to Textured Care. Saga Publishing; 2011.
- Robbins CR. The Physical Properties and Cosmetic Behavior of Hair. In: Robbins CR. Chemical and Physical Behavior of Human Hair. 3rd ed. Springer Nature; 1994:299-370. doi:10.1007/978-1-4757-3898-8_8
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Barreto T, Weffort F, Frattini S, et al. Straight to the point: what do we know so far on hair straightening? Skin Appendage Disord. 2021;7:265-271. doi:10.1159/000514367
- Dussaud A, Rana B, Lam HT. Progressive hair straightening using an automated flat iron: function of silicones. J Cosmet Sci. 2013;64:119-131.
- Zhou Y, Rigoletto R, Koelmel D, et al. The effect of various cosmetic pretreatments on protecting hair from thermal damage by hot flat ironing. J Cosmet Sci. 2011;62:265-282.
- Chang CJ, O’Brien KM, Keil AP, et al. Use of straighteners and other hair products and incident uterine cancer. J Natl Cancer Inst. 2022;114:1636-1645. doi:10.1093/jnci/djac165
- White AJ, Gregoire AM, Taylor KW, et al. Adolescent use of hair dyes, straighteners and perms in relation to breast cancer risk. Int J Cancer. 2021;148:2255-2263. doi:10.1002/ijc.33413
- Helm JS, Nishioka M, Brody JG, et al. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environ Res. 2018;165:448-458.
- Asbeck S, Riley-Prescott C, Glaser E, et al. Afro-ethnic hairstyling trends, risks, and recommendations. Cosmetics. 2022;9:17. doi:10.3390 /cosmetics9010017
- Saed S, Ibrahim O, Bergfeld WF. Hair camouflage: a comprehensive review. Int J Womens Dermatol. 2016;2:122-127. doi:10.1016 /j.ijwd.2016.09.002
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID .S137296
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160. doi:10.1111/pde.14721
- Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262. doi:10.1016/j.jaad.2010.05.037
- Carefoot H. Silk pillowcases for better hair and skin: what to know. The Washington Post. April 6, 2021. Accessed February 10, 2025. https://www.washingtonpost.com/lifestyle/wellness/silk-pillowcases-hair-skin-benefits-myths/2021/04/05/a7dcad7c-866a-11eb-82bc-e58213caa38e_story.html
- Samrao A, McMichael A, Mirmirani P. Nocturnal traction: techniques used for hair style maintenance while sleeping may be a risk factor for traction alopecia. Skin Appendage Disord. 2021;7:220-223. doi:10.1159/000513088
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- McMichael AJ. Hair breakage in normal and weathered hair: focus on the Black patient. J Investig Dermatol Symp Proc. 2007;12:6-9. doi:10.1038/sj.jidsymp.5650047
- Bosley RE, Daveluy S. A primer to natural hair care practices in black patients. Cutis. 2015;95:78-80,106.
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
Historical Perspectives on Hair Care and Common Styling Practices in Black Women
PRACTICE POINTS
- There is a dearth in understanding of hair care practices in Black women among health care professionals.
- Increased knowledge and cultural understanding of past and present hair care practices in Black women enhances patient care.
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening immunologic phenomenon characterized by a systemic inflammatory response syndrome—like clinical picture with additional features, including hepatosplenomegaly, hyperferritinemia, and increased natural killer cell activity. Clinical manifestations of HLH often are nonspecific, making HLH diagnosis challenging. High persistent fever is a key feature of HLH; patients also may report gastrointestinal distress, lethargy, and/or widespread rash.1
Hemophagocytic lymphohistiocytosis is believed to stem from inherited defects in several genes, such as perforin (PRF1), as well as immune dysregulation due to infections, rheumatologic diseases, hematologic malignancies, or drug reactions.2 The primary mechanism of HLH is hypothesized to be driven by aberrant immune activation, interferon gamma released from CD8+ T cells, and uncontrolled phagocytosis by activated macrophages. The cytokine cascade results in tissue injury and multiorgan dysfunction.3,4
Although HLH historically has been categorized as primary (familial) or secondary (acquired), the most recent guidelines suggest the etiology is not always binary.3,5 That said, the concept of secondary causes is useful in understanding risk factors for developing HLH. Both forms of the disease are thought to be elicited by a trigger (eg, infection), even when inherited genetic mutations exist.6 The primary form commonly affects the pediatric population,4,6-8 whereas the secondary form is more common in adults.7
Several sets of diagnostic criteria for HLH have been developed, the most well-known being the HLH-2004 criteria.1,3 The HLH-2009 modified criteria were developed after further evidence provided a refined sense of how the HLH-2004 criteria should be stratified.9 Finally, Fardet et al10 presented the HScore as an estimation of likelihood of diagnosis of HLH. These sets of HLH criteria include clinical and laboratory features that demonstrate inflammation, natrual killer cell activity, hemophagocytosis, end-organ damage, and cell lineage effects. The HScore differs from the other sets of HLH criteria in that it is designed to estimate an individual patient’s risk of having reactive hemophagocytic syndrome, which likely is equivalent to secondary HLH, although the authors do not use this exact terminology.10
While these criteria provide a framework for diagnosing HLH, they may fail to distinguish between HLH disease and HLH disease mimics, a concept described by the North American Consortium for Histiocytosis that may impact the success of immunosuppressive treatment.3 Individuals with HLH syndrome meet the aforementioned diagnostic criteria; HLH syndrome is further divided into HLH disease and HLH disease mimics (Figure 1). The “disease” label describes the traditional concept of HLH, driven by aberrant immune overactivation, in which patients benefit from immunosuppression. In contrast, HLH mimics include a subset of patients who meet the HLH criteria but are unlikely to benefit from immunosuppression because the primary mechanism driving their condition is not owed to immune overactivation, as is the case with HLH disease. Examples of HLH mimics include certain infections, such as Epstein-Barr virus (EBV), that may demonstrate clinical findings consistent with HLH but would not benefit from immunosuppression. Ironically, infections (including EBV) also are known triggers of HLH disease, making this concept difficult to understand and adopt. In this study, we refer to HLH disease simply as HLH.
Although cutaneous manifestations of HLH are not included in the diagnostic criteria, skin findings are common and may coincide with the severity and progression of the disease.11 Despite the fact that HLH can manifest with rash,1 comprehensive reviews of reported cutaneous findings in adult HLH are lacking. Thus, the goal of this study was to provide an organized characterization of reported cutaneous findings in adults with HLH and context for how the dermatologic examination may support the diagnosis or uncover the underlying etiology of this condition.
Methods
A search of PubMed articles indexed for MEDLINE using the phrase (cutaneous OR dermatologic OR skin) findings) AND hemophagocytic lymphohistiocytosis performed on September 20, 2023, yielded 423 results (Figure 2). Filters to exclude non–English language publications and pediatric populations were applied, resulting in 161 articles. Other exclusion criteria included the absence of a description of dermatologic findings. Seventy-five articles remained after screening titles and abstracts, and full-text review yielded 55 articles that were deemed appropriate for inclusion in the study. Subsequent reference searches and use of the online resource Litmaps revealed 45 additional publications that underwent full-text screening; of these articles, 5 were included in the final review.
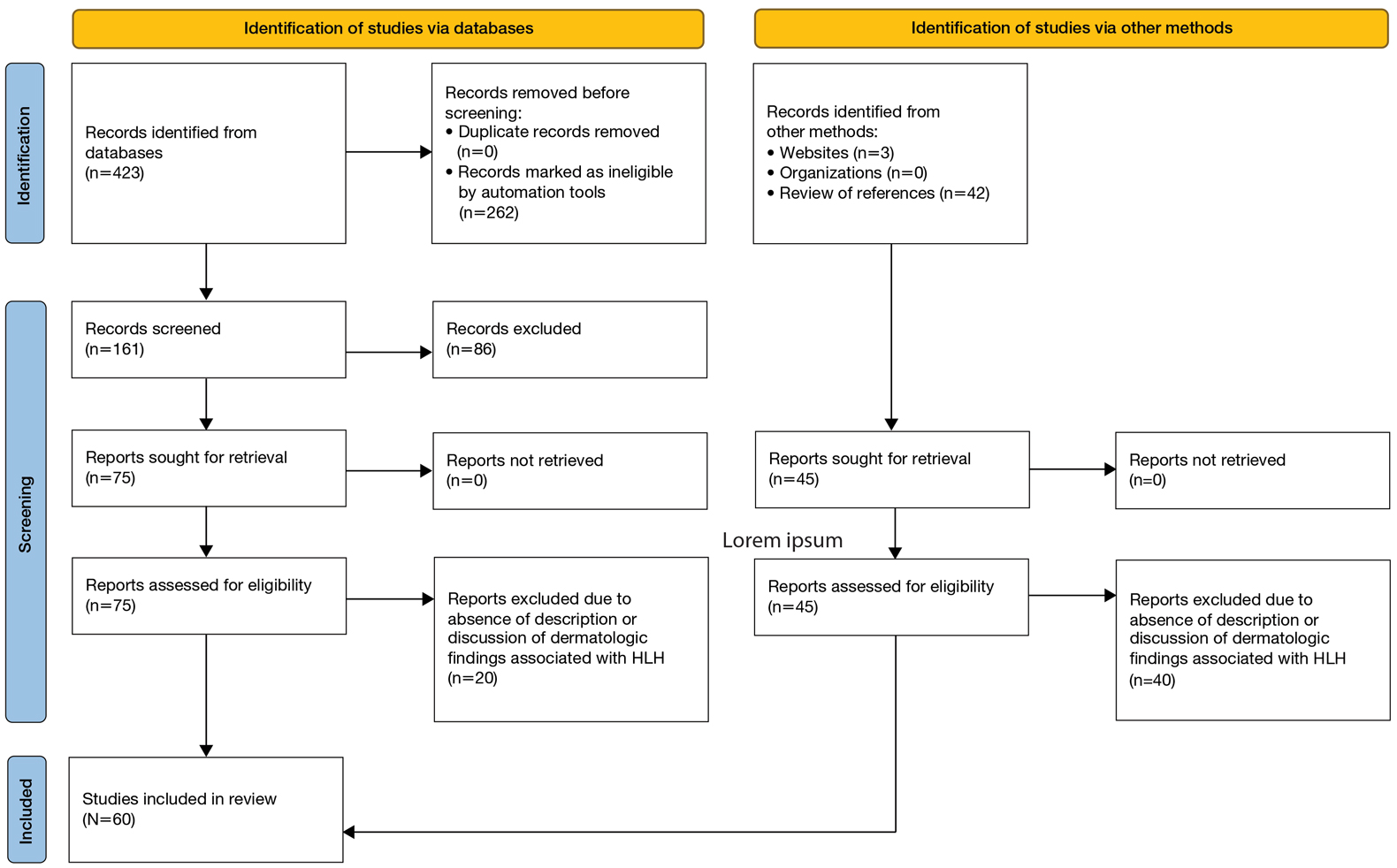
Results
Sixty studies were included in this systematic review.5,7,11-68 The reported prevalence of skin findings among patients with HLH from the included retrospective studies ranged from 15% to 85%.12-15 Several literature reviews reported similarly varied prevalence among adult patients with HLH.7,16 Fardet et al14 categorized cutaneous manifestations of HLH into 3 types: direct manifestations of HLH not explained by systemic features (eg, generalized maculopapular eruption), indirect manifestations of HLH that are explained by systemic features of the disease (eg, purpura due to HLH-induced coagulopathy), and findings specific to the underlying etiology of HLH (eg, malar rash seen in systemic lupus erythematosus [SLE]–associated HLH). This categorization served as the outline for the results below, providing an organized review of cutaneous findings and context for how they may support the diagnosis or uncover the underlying etiology of HLH.
Category I: Direct Manifestations of HLH
Several articles reported cutaneous findings that seemed to be the direct result of HLH and not attributed to an underlying trigger or sequalae of HLH.11,14,16-31 The most common descriptions were a generalized, morbilliform, or nonspecific eruption that encompasses large areas of the skin, commonly the trunk and extremities, sometimes extending to the face and scalp.14,16-23,25,31,32 There were variations in secondary features such as pruritus and tenderness; some studies also described violaceous discoloration in addition to erythema.16,23
Other skin findings thought to be a direct result of HLH were described in detail by Zerah and DeWitt11 in their retrospective study, including pyoderma gangrenosum, panniculitis, Stevens-Johnson syndrome, atypical targetoid lesions, and bullous eruptions. The authors also analyzed dermatopathologic data that ultimately revealed that pathologic analysis was largely inconsistent and nondescript.11 There was a single case report of purpura fulminans arising alongside signs and symptoms of HLH,26 and several case reports described Sweet syndrome developing around the same time as HLH.27-29 Lastly, Collins et al30 described a case of HLH manifesting with violaceous ulcerating papules and nodules scattered across the legs, abdomen, and arms. Biopsy of this patient’s lesions showed a diffuse dermal infiltrate of histiocytes and hemophagocytosis.
Category II: Secondary Complications and Sequelae of HLH
This was the smallest group among the 3 categories, comprising a few case reports and retrospective cohort studies primarily reporting jaundice/icterus and hemorrhagic lesions such as purpura, petechiae, and scleral hemorrhage.11,21,23,33-35 Several literature reviews described these conditions as nonspecific findings in HLH.16,20 The cause of jaundice in HLH likely can be attributed to its characteristic hepatic dysfunction, whereas hemorrhagic lesions likely are the result of both hepatic and bone marrow dysfunction resulting in coagulopathy.
Category III: Manifestations of Underlying Etiology or Triggers of HLH
Infectious—Infection is known to be one of the most common triggers of HLH, with several retrospective studies reporting infectious triggers in approximately 20% of cases.13,15 Although many pathogens have been implicated, only a few of these infection-induced HLH reports described cutaneous findings, which included a case of varicella zoster virus, Escherichia coli necrotizing fasciitis, leprosy, EBV reactivation, parvovirus B19, and both focal and disseminated herpes simplex virus 2.36-42 Most of these patients presented with classic findings of each disease. The case of varicella zoster virus exhibited pruritic erythematous papules on the face, trunk, and limbs.36 The necrotizing fasciitis case presented with tender erythematous swelling of the lower extremity.37 The patient with leprosy exhibited leonine facies and numerous erythematous nodules, plaques, and superficial ulcerating plaques over the trunk and limbs with palmoplantar involvement,39 and both cases of herpes simplex virus 2 reported small bullae either diffusely over the face, trunk, and extremities or over the genitalia.38,40 Interestingly, the cases of parvovirus B19 and EBV reactivation both exhibited polyarteritis nodosa and occurred in patients with underlying autoimmune conditions, raising the question of whether these cases of HLH had a single trigger or were the result of the overall immunologic dysregulation induced by both infection and autoimmunity.41,42
Rheumatologic—Several articles reported dermatologic findings associated with macrophage activation syndrome, a term that often is used to describe HLH associated with autoimmune conditions. Cases of HLH in adult-onset Still disease, dermatomyositis, polyarteritis nodosa, and SLE described skin findings characteristic of the underlying rheumatologic disease, sometimes with acutely worse dermatologic findings at the time of HLH presentation.35,41-48 With regard to SLE, the acute manifestation of classic findings of the disease with HLH has sometimes been described as acute lupus hemophagocytic syndrome (HPS).48 Lambotte at al48 described common findings of acute lupus hemophagocytic syndrome in their retrospective study as malar rash, weight loss, polyarthralgia, and nephritis in addition to classic HLH findings including fever, lymphadenopathy, and hepatosplenomegaly. Many other rheumatologic conditions have been associated with HLH, including rheumatoid arthritis, mixed connective tissue disease, systemic sclerosis, and Sjögren disease. All these conditions can have dermatologic manifestations; however, no descriptions of dermatologic findings in cases of HLH associated with these diseases were found.13
Malignancy—Several cases of malignancy-induced HLH described cutaneous findings, the majority being cutaneous lymphomas, namely subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Other less commonly reported malignancies in this group included Kaposi sarcoma, intravascular lymphoma, Sézary syndrome, mycosis fungoides, cutaneous diffuse large B-cell lymphoma, and several subtypes of primary cutaneous T-cell lymphoma.2,32,49-60 The most common description of SPTCL included multiple scattered plaques and subcutaneous nodules, some associated with tenderness, induration, drainage, or hemorrhagic features.32,50,52,55,57,60 Cases of mycosis fungoides and Sézary syndrome presented with variations in size and distribution of erythroderma with associated lymphadenopathy.2 A unique case of HLH developing in a patient with intravascular lymphoma described an eruption of multiple telangiectasias and petechial hemorrhages on the trunk,58 while one case associated with primary cutaneous anaplastic large cell lymphoma presented with a rapidly enlarging tumor with central ulceration and eschar.59
Drug Induced—Interestingly, most of the drug-induced cases of HLH identified in our search were secondary to biologic therapies used in the treatment of metastatic melanoma, specifically the immune checkpoint inhibitors (ICIs), which have been reported to have an association with HLH in prior literature reviews.61-65 Choi et al66 described an interesting case of ICI-induced HLH presenting with a concurrent severe lichenoid drug eruption that progressed from a pruritic truncal rash to mucocutaneous bullae, erosions, and desquamation resembling a Stevens-Johnson syndrome–type picture. This patient had treatment-refractory, HIV-negative Kaposi sarcoma, where the underlying immunologic dysregulation may explain the more severe cutaneous presentation not observed in other reported cases of ICI-induced HLH.
Yang et al’s67 review of 23 cases with concurrent diagnoses of HLH and DIHS found that 61% (14/23) of cases were diagnosed initially as DIHS before failing treatment and receiving a diagnosis of HLH several weeks later. Additionally, the authors found that several cases met criteria for one diagnosis while clinically presenting strongly for the other.67 This overlap in clinical presentation also was demonstrated in Zerah and DeWitt’s11 retrospective study regarding cutaneous findings in HLH, in which several of the morbilliform eruptions thought to be contributed to HLH ultimately were decided to be drug reactions.
Comment
Regarding direct (or primary) cutaneous findings in HLH (category I), there seem to be 2 groups of features associated with the onset of HLH that are not related to its characteristic hepatic dysfunction (category II) nor its underlying triggers (category III): a nonspecific, generalized, erythematous eruption; and dermatologic conditions separate from HLH itself (eg, Sweet syndrome, pyoderma gangrenosum). Whether the latter group truly is a direct manifestation of HLH is difficult to discern with the evidence available. Nevertheless, we can conclude that there is some type of association between these dermatologic diseases and HLH, and this association can serve as both a diagnostic tool for clinicians and a point of interest for further clinical research.
The relatively low number of articles identified through our systematic review that specifically reported secondary findings, such as jaundice or coagulopathy-associated hemorrhagic lesions, may lead one to believe that these are not common findings in HLH; however, it is possible that these are not regularly reported in the literature simply because these findings are nonspecific and can be considered expected results of the characteristic organ dysfunction in HLH.
As suspected, the skin findings in category III were the most broad given the variety of underlying etiologies that have been associated with HLH. Like the other 2 categories, these skin findings generally are nonspecific to HLH; however, the ones in category III are specific to underlying etiology of HLH and may aid in identifying and treating the underlying cause of a patient’s HLH when indicated.
Most of the rheumatologic diseases seem to have been known at the time of HLH development and diagnosis, which may highlight the importance of considering a diagnosis of HLH early on in patients with known autoimmune disease and systemic signs of illness or acutely worsening signs and symptoms of their underlying autoimmune disease.
Interestingly, several cases of malignancy-associated HLH reported signs and symptoms of HLH at initial presentation of the malignant disease.32,50,59 This situation seems to be somewhat common, as Go and Wester’s68 systematic analysis of 156 patients with SPTCL found HLH was the presenting feature in 37% of patients included in their study. This may call attention to the importance of considering cutaneous lymphomas as the cause of skin lesions in patients with signs and symptoms of HLH, where it may be easy to assume that skin findings are a result of their systemic disease.
In highlighting cases of HLH related to medication use, we found it pertinent to include and discuss the complex relationship between drug-induced hypersensitivity syndrome (DIHS [formerly known as drug rash with eosinophilia and systemic symptoms [DRESS] syndrome) and HLH. The results of this study suggest that DIHS may have considerable clinical overlap with HLH11 and may even lead to development of HLH,67 creating difficulty in distinguishing between these conditions where there may be similar findings, such as cutaneous eruptions, fever, and hepatic or other internal organ involvement. We agree with Yang et al67 that there can be large overlap in symptomology between these two conditions and that more investigation is necessary to explore the relationship between them.
Conclusion
Diagnosis of HLH in adults continues to be challenging, with several diagnostic tools but no true gold standard. In addition to the nonspecific symptomology, there is a myriad of cutaneous findings that can be present in adults with HLH (eTable), all of which are also nonspecific. Even so, awareness of which dermatologic findings have been associated with HLH may provide a cue to consider HLH in the systemically ill patient with a notable dermatologic examination. Furthermore, there are several avenues for further investigation that can be drawn, including further dermatologic analysis among nonspecific eruptions attributed to HLH, clinical and pathologic differentiation between DIHS/DRESS and HLH, and correlation between severity of skin manifestations and severity of HLH disease.
Limitations of this study included a lack of clarity in diagnosis of HLH in patients described in the included articles, as some reports use variable terminology (HLH vs hemophagocytic syndrome vs macrophage activation syndrome, etc), and it is impossible to know if all authors used the same diagnostic criteria—or any validated diagnostic criteria—unless specifically stated. Additionally, including case reports in our study limited the amount and quality of information described in each report. Despite its limitations, this systematic review outlines the cutaneous manifestations associated with HLH. These data will promote clinical awareness of this complex condition and allow for consideration of HLH in patients meeting criteria for HLH syndrome. More studies ultimately are needed to differentiate HLH from its mimics.
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. doi:10.1002/pbc.21039
- Blom A, Beylot-Barry M, D’Incan M, et al. Lymphoma-associated hemophagocytic syndrome (LAHS) in advanced-stage mycosis fungoides/ Sézary syndrome cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;65:404-410. doi:10.1016/j.jaad.2010.05.029
- Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66:e27929. doi:10.1002/pbc.27929
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515. doi:10.1016/j.berh.2020.101515
- Tomasini D, Berti E. Subcutaneous panniculitis-like T-cell lymphoma. G Ital Dermatol Venereol. 2013;148:395-411.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. doi:10.1016/s0140-6736(13)61048-x
- Sieni E, Cetica V, Piccin A, et al. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. PLoS One. 2012;7:e44649. doi:10.1371/journal.pone.0044649
- Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology. 2009:127-131. doi:10.1182 /asheducation-2009.1.127
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613-2620. doi:10.1002/art.38690
- Zerah ML, DeWitt CA. Cutaneous findings in hemophagocytic lymphohistiocytosis. Dermatology. 2015;230:234-243. doi:10.1159/000368552
- Fardet L, Galicier L, Vignon-Pennamen MD, et al. Frequency, clinical features and prognosis of cutaneous manifestations in adult patients with reactive haemophagocytic syndrome. Br J Dermatol. 2010;162:547-553. doi:10.1111/j.1365-2133.2009.09549.x
- Dhote R, Simon J, Papo T, et al. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum. 2003;49:633-639. doi:10.1002/art.11368
- Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93:100-105. doi:10.1097/md.0000000000000022
- Tudesq JJ, Valade S, Galicier L, et al. Diagnostic strategy for trigger identification in severe reactive hemophagocytic lymphohistiocytosis: a diagnostic accuracy study. Hematol Oncol. 2021;39:114-122. doi:10.1002 /hon.2819
- Sakai H, Otsubo S, Miura T, et al. Hemophagocytic syndrome presenting with a facial erythema in a patient with systemic lupus erythematosus. J Am Acad Dermatol. 2007;57(5 Suppl):S111-S114. doi:10.1016/j .jaad.2006.11.024
- Chung SM, Song JY, Kim W, et al. Dengue-associated hemophagocytic lymphohistiocytosis in an adult: a case report and literature review. Medicine (Baltimore). 2017;96:e6159. doi:10.1097/md.0000000000006159
- Esmaili H, Rahmani O, Fouladi RF. Hemophagocytic syndrome in patients with unexplained cytopenia: report of 15 cases. Turk Patoloji Derg. 2013;29:15-18. doi:10.5146/tjpath.2013.01142
- Jiwnani S, Karimundackal G, Kulkarni A, et al. Hemophagocytic syndrome complicating lung resection. Asian Cardiovasc Thorac Ann. 2012;20:341-343. doi:10.1177/0218492311435686
- Lee WJ, Lee DW, Kim CH, et al. Dermatopathic lymphadenitis with generalized erythroderma in a patient with Epstein-Barr virusassociated hemophagocytic lymphohistiocytosis. Am J Dermatopathol. 2010;32:357-361. doi:10.1097/DAD.0b013e3181b2a50f
- Lovisari F, Terzi V, Lippi MG, et al. Hemophagocytic lymphohistiocytosis complicated by multiorgan failure: a case report. Medicine (Baltimore). 2017;96:e9198. doi:10.1097/md.0000000000009198
- Miechowiecki J, Stainer W, Wallner G, et al. Severe complication during remission of Crohn’s disease: hemophagocytic lymphohistiocytosis due to acute cytomegalovirus infection. Z Gastroenterol. 2018;56:259-263. doi:10.1055/s-0043-123999
- Ochoa S, Cheng K, Fleury CM, et al. A 28-year-old woman with fever, rash, and pancytopenia. Allergy Asthma Proc. 2017;38:322-327. doi:10.2500/aap.2017.38.4042
- Tokoro S, Namiki T, Miura K, et al. Chronic active Epstein-Barr virus infection with cutaneous lymphoproliferation: haemophagocytosis in the skin and haemophagocytic syndrome. J Eur Acad Dermatol Venereol. 2018;32:e116-e117. doi:10.1111/jdv.14640
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitislike T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106 (2 Suppl):S55-S59. doi:10.1016/s0929-6646(09)60354-5
- Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer. 2019;7:97. doi:10.1186/s40425- 019-0582-4
- Kao RL, Jacobsen AA, Billington CJ Jr, et al. A case of VEXAS syndrome associated with EBV-associated hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2022;93:102636. doi:10.1016/j .bcmd.2021.102636
- Koga T, Takano K, Horai Y, et al. Sweet’s syndrome complicated by Kikuchi’s disease and hemophagocytic syndrome which presented with retinoic acid-inducible gene-I in both the skin epidermal basal layer and the cervical lymph nodes. Intern Med. 2013;52:1839-1843. doi:10.2169 /internalmedicine.52.9542
- Lin WL, Lin WC, Chiu CS, et al. Paraneoplastic Sweet’s syndrome in a patient with hemophagocytic syndrome. Int J Dermatol. 2008;3:305-307.
- Collins MK, Ho J, Akilov OE. Case 52. A unique presentation of hemophagocytic lymphohistiocytosis with ulcerating papulonodules. In: Akilov OE, ed. Cutaneous Lymphomas: Unusual Cases 3. Springer International Publishing; 2021:126-127.
- Chakrapani A, Avery A, Warnke R. Primary cutaneous gamma delta T-cell lymphoma with brain involvement and hemophagocytic syndrome. Am J Dermatopathol. 2013;35:270-272. doi:10.1097 /DAD.0b013e3182624e98
- Sullivan C, Loghmani A, Thomas K, et al. Hemophagocytic lymphohistiocytosis as the initial presentation of subcutaneous panniculitis-like T-cell lymphoma: a rare case responding to cyclosporine A and steroids. J Investig Med High Impact Case Rep. 2020;8:2324709620981531. doi:10.1177/2324709620981531
- Darmawan G, Salido EO, Concepcion ML, et al. Hemophagocytic lymphohistiocytosis: “a dreadful mimic.” Int J Rheum Dis. 2015; 18:810-812. doi:10.1111/1756-185x.12506
- Maus MV, Leick MB, Cornejo KM, et al. Case 35-2019: a 66-year-old man with pancytopenia and rash. N Engl J Med. 2019;381:1951-1960. doi:10.1056/NEJMcpc1909627
- Chamseddin B, Marks E, Dominguez A, et al. Refractory macrophage activation syndrome in the setting of adult-onset Still disease with hemophagocytic lymphohistiocytosis detected on skin biopsy treated with canakinumab and tacrolimus. J Cutan Pathol. 2019;46:528-531. doi:10.1111/cup.13466
- Bérar A, Ardois S, Walter-Moraux P, et al. Primary varicella-zoster virus infection of the immunocompromised associated with acute pancreatitis and hemophagocytic lymphohistiocytosis: a case report. Medicine (Baltimore). 2021;100:e25351. doi:10.1097 /md.0000000000025351
- Chang CC, Hsiao PJ, Chiu CC, et al. Catastrophic hemophagocytic lymphohistiocytosis in a young man with nephrotic syndrome. Clin Chim Acta. 2015;439:168-171. doi:10.1016/j.cca.2014.10.025
- Kurosawa S, Sekiya N, Fukushima K, et al. Unusual manifestation of disseminated herpes simplex virus type 2 infection associated with pharyngotonsilitis, esophagitis, and hemophagocytic lymphohisitocytosis without genital involvement. BMC Infect Dis. 2019;19:65. doi:10.1186/s12879-019-3721-0
- Saidi W, Gammoudi R, Korbi M, et al. Hemophagocytic lymphohistiocytosis: an unusual complication of leprosy. Int J Dermatol. 2015;54: 1054-1059. doi:10.1111/ijd.12792
- Yamaguchi K, Yamamoto A, Hisano M, et al. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol. 2005;105(5 Pt 2):1241-1244. doi:10.1097 /01.AOG.0000157757.54948.9b
- Hayakawa I, Shirasaki F, Ikeda H, et al. Reactive hemophagocytic syndrome in a patient with polyarteritis nodosa associated with Epstein- Barr virus reactivation. Rheumatol Int. 2006;26:573-576. doi:10.1007 /s00296-005-0024-0
- Jeong JY, Park JY, Ham JY, et al. Molecular evidence of parvovirus B19 in the cutaneous polyarteritis nodosa tissue from a patient with parvovirus-associated hemophagocytic syndrome: case report. Medicine (Baltimore). 2020;99:e22079. doi:10.1097 /md.0000000000022079
- Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478. doi:10.2169 /internalmedicine.1121-18
- Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune- associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246. doi:10 .1080/03009742.2019.1653493
- Jung SY. Hemophagocytic syndrome diagnosed by liver biopsy in a female patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:449-451. doi:10.1097/rhu.0000000000000040
- Kerl K, Wolf IH, Cerroni L, et al. Hemophagocytosis in cutaneous autoimmune disease. Am J Dermatopathol. 2015;37:539-543. doi:10.1097 /dad.0000000000000166
- Komiya Y, Saito T, Mizoguchi F, et al. Hemophagocytic syndrome complicated with dermatomyositis controlled successfully with infliximab and conventional therapies. Intern Med. 2017;56:3237-3241. doi:10.2169 /internalmedicine.7966-16
- Lambotte O, Khellaf M, Harmouche H, et al. Characteristics and long-term outcome of 15 episodes of systemic lupus erythematosusassociated hemophagocytic syndrome. Medicine (Baltimore). 2006;85: 169-182. doi:10.1097/01.md.0000224708.62510.d1
- Guitart J, Mangold AR, Martinez-Escala ME, et al. Clinical and pathological characteristics and outcomes among patients with subcutaneous panniculitis-like T-cell lymphoma and related adipotropic lymphoproliferative disorders. JAMA Dermatol. 2022;158:1167-1174. doi:10.1001/jamadermatol.2022.3347
- Hung GD, Chen YH, Chen DY, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with hemophagocytic lymphohistiocytosis and skin lesions with characteristic high-resolution ultrasonographic findings. Clin Rheumatol. 2007;26:775-778. doi:10.1007/s10067 -005-0193-y
- Jamil A, Nadzri N, Harun N, et al. Primary cutaneous diffuse large B-cell lymphoma leg type presenting with hemophagocytic syndrome. J Am Acad Dermatol. 2012;67:e222-3. doi:10.1016/j.jaad.2012.04.021
- LeBlanc RE, Lansigan F. Unraveling subcutaneous panniculitis-like T-cell lymphoma: an association between subcutaneous panniculitislike T-cell lymphoma, autoimmune lymphoproliferative syndrome, and familial hemophagocytic lymphohistiocytosis. J Cutan Pathol. 2021;48:572-577. doi:10.1111/cup.13863
- Lee DE, Martinez-Escala ME, Serrano LM, et al. Hemophagocytic lymphohistiocytosis in cutaneous T-cell lymphoma. JAMA Dermatol. 2018;154:828-831. doi:10.1001/jamadermatol.2018.1264
- Maejima H, Tanei R, Morioka T, et al. Haemophagocytosis-related intravascular large B-cell lymphoma associated with skin eruption. Acta Derm Venereol. 2011;91:339-340. doi:10.2340/00015555-0981
- Mody A, Cherry D, Georgescu G, et al. A rare case of subcutaneous panniculitis-like T cell lymphoma with hemophagocytic lymphohistiocytosis mimicking cellulitis. Am J Case Rep. 2021;22:E927142. doi:10.12659/ajcr.927142
- Park YJ, Bae HJ, Chang JY, et al. Development of Kaposi sarcoma and hemophagocytic lymphohistiocytosis associated with human herpesvirus 8 in a renal transplant recipient. Korean J Intern Med. 2017;4:750-752.
- Phatak S, Gupta L, Aggarwal A. A young woman with panniculitis and cytopenia who later developed coagulopathy. J Assoc Physicians India. 2016;64:65-67.
- Pongpairoj K, Rerknimitr P, Wititsuwannakul J, et al. Eruptive telangiectasia in a patient with fever and haemophagocytic syndrome. Clin Exp Dermatol. 2016;41:696-698. doi:10.1111/ced.12859
- Shimizu Y, Tanae K, Takahashi N, et al. Primary cutaneous anaplastic large-cell lymphoma presenting with hemophagocytic syndrome: a case report and review of the literature. Leuk Res. 2010;34:263-266. doi:10.1016/j.leukres.2009.07.001
- Sirka CS, Pradhan S, Patra S, et al. Hemophagocytic lymphohistiocytosis: a rare, potentially fatal complication in subcutaneous panniculitis like T cell lymphoma. Indian J Dermatol Venereol Leprol. 2019;5:481-485.
- Chin CK, Hall S, Green C, et al. Secondary haemophagocytic lymphohistiocytosis due to checkpoint inhibitor therapy. Eur J Cancer. 2019;115: 84-87. doi:10.1016/j.ejca.2019.04.026
- Dudda M, Mann C, Heinz J, et al. Hemophagocytic lymphohistiocytosis of a melanoma patient under BRAF/MEK-inhibitor therapy following anti-PD1 inhibitor treatment: a case report and review to the literature. Melanoma Res. 2021;31:81-84. doi:10.1097 /cmr.0000000000000703
- Mizuta H, Nakano E, Takahashi A, et al. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: a case report and literature review. Dermatol Ther. 2020;33:e13321. doi:10.1111/dth.13321
- Satzger I, Ivanyi P, Länger F, et al. Treatment-related hemophagocytic lymphohistiocytosis secondary to checkpoint inhibition with nivolumab plus ipilimumab. Eur J Cancer. 2018;93:150-153. doi:10.1016/j.ejca.2018.01.063
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72-90. doi:10.1016/J.EJCA.2019.07.014
- Choi S, Zhou M, Bahrani E, et al. Rare and fatal complication of immune checkpoint inhibition: a case report of haemophagocytic lymphohistiocytosis with severe lichenoid dermatitis. Br J Haematol. 2021;193:e44-e47. doi:10.1111/BJH.17442
- Yang JJ, Lei DK, Ravi V, et al. Overlap between hemophagocytic lymphohistiocytosis and drug reaction and eosinophilia with systemic symptoms: a review. Int J Dermatol. 2021;60:925-932. doi:10.1111 /ijd.15196
- Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. doi:10.1002/cncr.20502
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening immunologic phenomenon characterized by a systemic inflammatory response syndrome—like clinical picture with additional features, including hepatosplenomegaly, hyperferritinemia, and increased natural killer cell activity. Clinical manifestations of HLH often are nonspecific, making HLH diagnosis challenging. High persistent fever is a key feature of HLH; patients also may report gastrointestinal distress, lethargy, and/or widespread rash.1
Hemophagocytic lymphohistiocytosis is believed to stem from inherited defects in several genes, such as perforin (PRF1), as well as immune dysregulation due to infections, rheumatologic diseases, hematologic malignancies, or drug reactions.2 The primary mechanism of HLH is hypothesized to be driven by aberrant immune activation, interferon gamma released from CD8+ T cells, and uncontrolled phagocytosis by activated macrophages. The cytokine cascade results in tissue injury and multiorgan dysfunction.3,4
Although HLH historically has been categorized as primary (familial) or secondary (acquired), the most recent guidelines suggest the etiology is not always binary.3,5 That said, the concept of secondary causes is useful in understanding risk factors for developing HLH. Both forms of the disease are thought to be elicited by a trigger (eg, infection), even when inherited genetic mutations exist.6 The primary form commonly affects the pediatric population,4,6-8 whereas the secondary form is more common in adults.7
Several sets of diagnostic criteria for HLH have been developed, the most well-known being the HLH-2004 criteria.1,3 The HLH-2009 modified criteria were developed after further evidence provided a refined sense of how the HLH-2004 criteria should be stratified.9 Finally, Fardet et al10 presented the HScore as an estimation of likelihood of diagnosis of HLH. These sets of HLH criteria include clinical and laboratory features that demonstrate inflammation, natrual killer cell activity, hemophagocytosis, end-organ damage, and cell lineage effects. The HScore differs from the other sets of HLH criteria in that it is designed to estimate an individual patient’s risk of having reactive hemophagocytic syndrome, which likely is equivalent to secondary HLH, although the authors do not use this exact terminology.10
While these criteria provide a framework for diagnosing HLH, they may fail to distinguish between HLH disease and HLH disease mimics, a concept described by the North American Consortium for Histiocytosis that may impact the success of immunosuppressive treatment.3 Individuals with HLH syndrome meet the aforementioned diagnostic criteria; HLH syndrome is further divided into HLH disease and HLH disease mimics (Figure 1). The “disease” label describes the traditional concept of HLH, driven by aberrant immune overactivation, in which patients benefit from immunosuppression. In contrast, HLH mimics include a subset of patients who meet the HLH criteria but are unlikely to benefit from immunosuppression because the primary mechanism driving their condition is not owed to immune overactivation, as is the case with HLH disease. Examples of HLH mimics include certain infections, such as Epstein-Barr virus (EBV), that may demonstrate clinical findings consistent with HLH but would not benefit from immunosuppression. Ironically, infections (including EBV) also are known triggers of HLH disease, making this concept difficult to understand and adopt. In this study, we refer to HLH disease simply as HLH.

Although cutaneous manifestations of HLH are not included in the diagnostic criteria, skin findings are common and may coincide with the severity and progression of the disease.11 Despite the fact that HLH can manifest with rash,1 comprehensive reviews of reported cutaneous findings in adult HLH are lacking. Thus, the goal of this study was to provide an organized characterization of reported cutaneous findings in adults with HLH and context for how the dermatologic examination may support the diagnosis or uncover the underlying etiology of this condition.
Methods
A search of PubMed articles indexed for MEDLINE using the phrase (cutaneous OR dermatologic OR skin) findings) AND hemophagocytic lymphohistiocytosis performed on September 20, 2023, yielded 423 results (Figure 2). Filters to exclude non–English language publications and pediatric populations were applied, resulting in 161 articles. Other exclusion criteria included the absence of a description of dermatologic findings. Seventy-five articles remained after screening titles and abstracts, and full-text review yielded 55 articles that were deemed appropriate for inclusion in the study. Subsequent reference searches and use of the online resource Litmaps revealed 45 additional publications that underwent full-text screening; of these articles, 5 were included in the final review.

Results
Sixty studies were included in this systematic review.5,7,11-68 The reported prevalence of skin findings among patients with HLH from the included retrospective studies ranged from 15% to 85%.12-15 Several literature reviews reported similarly varied prevalence among adult patients with HLH.7,16 Fardet et al14 categorized cutaneous manifestations of HLH into 3 types: direct manifestations of HLH not explained by systemic features (eg, generalized maculopapular eruption), indirect manifestations of HLH that are explained by systemic features of the disease (eg, purpura due to HLH-induced coagulopathy), and findings specific to the underlying etiology of HLH (eg, malar rash seen in systemic lupus erythematosus [SLE]–associated HLH). This categorization served as the outline for the results below, providing an organized review of cutaneous findings and context for how they may support the diagnosis or uncover the underlying etiology of HLH.
Category I: Direct Manifestations of HLH
Several articles reported cutaneous findings that seemed to be the direct result of HLH and not attributed to an underlying trigger or sequalae of HLH.11,14,16-31 The most common descriptions were a generalized, morbilliform, or nonspecific eruption that encompasses large areas of the skin, commonly the trunk and extremities, sometimes extending to the face and scalp.14,16-23,25,31,32 There were variations in secondary features such as pruritus and tenderness; some studies also described violaceous discoloration in addition to erythema.16,23
Other skin findings thought to be a direct result of HLH were described in detail by Zerah and DeWitt11 in their retrospective study, including pyoderma gangrenosum, panniculitis, Stevens-Johnson syndrome, atypical targetoid lesions, and bullous eruptions. The authors also analyzed dermatopathologic data that ultimately revealed that pathologic analysis was largely inconsistent and nondescript.11 There was a single case report of purpura fulminans arising alongside signs and symptoms of HLH,26 and several case reports described Sweet syndrome developing around the same time as HLH.27-29 Lastly, Collins et al30 described a case of HLH manifesting with violaceous ulcerating papules and nodules scattered across the legs, abdomen, and arms. Biopsy of this patient’s lesions showed a diffuse dermal infiltrate of histiocytes and hemophagocytosis.
Category II: Secondary Complications and Sequelae of HLH
This was the smallest group among the 3 categories, comprising a few case reports and retrospective cohort studies primarily reporting jaundice/icterus and hemorrhagic lesions such as purpura, petechiae, and scleral hemorrhage.11,21,23,33-35 Several literature reviews described these conditions as nonspecific findings in HLH.16,20 The cause of jaundice in HLH likely can be attributed to its characteristic hepatic dysfunction, whereas hemorrhagic lesions likely are the result of both hepatic and bone marrow dysfunction resulting in coagulopathy.
Category III: Manifestations of Underlying Etiology or Triggers of HLH
Infectious—Infection is known to be one of the most common triggers of HLH, with several retrospective studies reporting infectious triggers in approximately 20% of cases.13,15 Although many pathogens have been implicated, only a few of these infection-induced HLH reports described cutaneous findings, which included a case of varicella zoster virus, Escherichia coli necrotizing fasciitis, leprosy, EBV reactivation, parvovirus B19, and both focal and disseminated herpes simplex virus 2.36-42 Most of these patients presented with classic findings of each disease. The case of varicella zoster virus exhibited pruritic erythematous papules on the face, trunk, and limbs.36 The necrotizing fasciitis case presented with tender erythematous swelling of the lower extremity.37 The patient with leprosy exhibited leonine facies and numerous erythematous nodules, plaques, and superficial ulcerating plaques over the trunk and limbs with palmoplantar involvement,39 and both cases of herpes simplex virus 2 reported small bullae either diffusely over the face, trunk, and extremities or over the genitalia.38,40 Interestingly, the cases of parvovirus B19 and EBV reactivation both exhibited polyarteritis nodosa and occurred in patients with underlying autoimmune conditions, raising the question of whether these cases of HLH had a single trigger or were the result of the overall immunologic dysregulation induced by both infection and autoimmunity.41,42
Rheumatologic—Several articles reported dermatologic findings associated with macrophage activation syndrome, a term that often is used to describe HLH associated with autoimmune conditions. Cases of HLH in adult-onset Still disease, dermatomyositis, polyarteritis nodosa, and SLE described skin findings characteristic of the underlying rheumatologic disease, sometimes with acutely worse dermatologic findings at the time of HLH presentation.35,41-48 With regard to SLE, the acute manifestation of classic findings of the disease with HLH has sometimes been described as acute lupus hemophagocytic syndrome (HPS).48 Lambotte at al48 described common findings of acute lupus hemophagocytic syndrome in their retrospective study as malar rash, weight loss, polyarthralgia, and nephritis in addition to classic HLH findings including fever, lymphadenopathy, and hepatosplenomegaly. Many other rheumatologic conditions have been associated with HLH, including rheumatoid arthritis, mixed connective tissue disease, systemic sclerosis, and Sjögren disease. All these conditions can have dermatologic manifestations; however, no descriptions of dermatologic findings in cases of HLH associated with these diseases were found.13
Malignancy—Several cases of malignancy-induced HLH described cutaneous findings, the majority being cutaneous lymphomas, namely subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Other less commonly reported malignancies in this group included Kaposi sarcoma, intravascular lymphoma, Sézary syndrome, mycosis fungoides, cutaneous diffuse large B-cell lymphoma, and several subtypes of primary cutaneous T-cell lymphoma.2,32,49-60 The most common description of SPTCL included multiple scattered plaques and subcutaneous nodules, some associated with tenderness, induration, drainage, or hemorrhagic features.32,50,52,55,57,60 Cases of mycosis fungoides and Sézary syndrome presented with variations in size and distribution of erythroderma with associated lymphadenopathy.2 A unique case of HLH developing in a patient with intravascular lymphoma described an eruption of multiple telangiectasias and petechial hemorrhages on the trunk,58 while one case associated with primary cutaneous anaplastic large cell lymphoma presented with a rapidly enlarging tumor with central ulceration and eschar.59
Drug Induced—Interestingly, most of the drug-induced cases of HLH identified in our search were secondary to biologic therapies used in the treatment of metastatic melanoma, specifically the immune checkpoint inhibitors (ICIs), which have been reported to have an association with HLH in prior literature reviews.61-65 Choi et al66 described an interesting case of ICI-induced HLH presenting with a concurrent severe lichenoid drug eruption that progressed from a pruritic truncal rash to mucocutaneous bullae, erosions, and desquamation resembling a Stevens-Johnson syndrome–type picture. This patient had treatment-refractory, HIV-negative Kaposi sarcoma, where the underlying immunologic dysregulation may explain the more severe cutaneous presentation not observed in other reported cases of ICI-induced HLH.
Yang et al’s67 review of 23 cases with concurrent diagnoses of HLH and DIHS found that 61% (14/23) of cases were diagnosed initially as DIHS before failing treatment and receiving a diagnosis of HLH several weeks later. Additionally, the authors found that several cases met criteria for one diagnosis while clinically presenting strongly for the other.67 This overlap in clinical presentation also was demonstrated in Zerah and DeWitt’s11 retrospective study regarding cutaneous findings in HLH, in which several of the morbilliform eruptions thought to be contributed to HLH ultimately were decided to be drug reactions.
Comment
Regarding direct (or primary) cutaneous findings in HLH (category I), there seem to be 2 groups of features associated with the onset of HLH that are not related to its characteristic hepatic dysfunction (category II) nor its underlying triggers (category III): a nonspecific, generalized, erythematous eruption; and dermatologic conditions separate from HLH itself (eg, Sweet syndrome, pyoderma gangrenosum). Whether the latter group truly is a direct manifestation of HLH is difficult to discern with the evidence available. Nevertheless, we can conclude that there is some type of association between these dermatologic diseases and HLH, and this association can serve as both a diagnostic tool for clinicians and a point of interest for further clinical research.
The relatively low number of articles identified through our systematic review that specifically reported secondary findings, such as jaundice or coagulopathy-associated hemorrhagic lesions, may lead one to believe that these are not common findings in HLH; however, it is possible that these are not regularly reported in the literature simply because these findings are nonspecific and can be considered expected results of the characteristic organ dysfunction in HLH.
As suspected, the skin findings in category III were the most broad given the variety of underlying etiologies that have been associated with HLH. Like the other 2 categories, these skin findings generally are nonspecific to HLH; however, the ones in category III are specific to underlying etiology of HLH and may aid in identifying and treating the underlying cause of a patient’s HLH when indicated.
Most of the rheumatologic diseases seem to have been known at the time of HLH development and diagnosis, which may highlight the importance of considering a diagnosis of HLH early on in patients with known autoimmune disease and systemic signs of illness or acutely worsening signs and symptoms of their underlying autoimmune disease.
Interestingly, several cases of malignancy-associated HLH reported signs and symptoms of HLH at initial presentation of the malignant disease.32,50,59 This situation seems to be somewhat common, as Go and Wester’s68 systematic analysis of 156 patients with SPTCL found HLH was the presenting feature in 37% of patients included in their study. This may call attention to the importance of considering cutaneous lymphomas as the cause of skin lesions in patients with signs and symptoms of HLH, where it may be easy to assume that skin findings are a result of their systemic disease.
In highlighting cases of HLH related to medication use, we found it pertinent to include and discuss the complex relationship between drug-induced hypersensitivity syndrome (DIHS [formerly known as drug rash with eosinophilia and systemic symptoms [DRESS] syndrome) and HLH. The results of this study suggest that DIHS may have considerable clinical overlap with HLH11 and may even lead to development of HLH,67 creating difficulty in distinguishing between these conditions where there may be similar findings, such as cutaneous eruptions, fever, and hepatic or other internal organ involvement. We agree with Yang et al67 that there can be large overlap in symptomology between these two conditions and that more investigation is necessary to explore the relationship between them.
Conclusion
Diagnosis of HLH in adults continues to be challenging, with several diagnostic tools but no true gold standard. In addition to the nonspecific symptomology, there is a myriad of cutaneous findings that can be present in adults with HLH (eTable), all of which are also nonspecific. Even so, awareness of which dermatologic findings have been associated with HLH may provide a cue to consider HLH in the systemically ill patient with a notable dermatologic examination. Furthermore, there are several avenues for further investigation that can be drawn, including further dermatologic analysis among nonspecific eruptions attributed to HLH, clinical and pathologic differentiation between DIHS/DRESS and HLH, and correlation between severity of skin manifestations and severity of HLH disease.

Limitations of this study included a lack of clarity in diagnosis of HLH in patients described in the included articles, as some reports use variable terminology (HLH vs hemophagocytic syndrome vs macrophage activation syndrome, etc), and it is impossible to know if all authors used the same diagnostic criteria—or any validated diagnostic criteria—unless specifically stated. Additionally, including case reports in our study limited the amount and quality of information described in each report. Despite its limitations, this systematic review outlines the cutaneous manifestations associated with HLH. These data will promote clinical awareness of this complex condition and allow for consideration of HLH in patients meeting criteria for HLH syndrome. More studies ultimately are needed to differentiate HLH from its mimics.
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening immunologic phenomenon characterized by a systemic inflammatory response syndrome—like clinical picture with additional features, including hepatosplenomegaly, hyperferritinemia, and increased natural killer cell activity. Clinical manifestations of HLH often are nonspecific, making HLH diagnosis challenging. High persistent fever is a key feature of HLH; patients also may report gastrointestinal distress, lethargy, and/or widespread rash.1
Hemophagocytic lymphohistiocytosis is believed to stem from inherited defects in several genes, such as perforin (PRF1), as well as immune dysregulation due to infections, rheumatologic diseases, hematologic malignancies, or drug reactions.2 The primary mechanism of HLH is hypothesized to be driven by aberrant immune activation, interferon gamma released from CD8+ T cells, and uncontrolled phagocytosis by activated macrophages. The cytokine cascade results in tissue injury and multiorgan dysfunction.3,4
Although HLH historically has been categorized as primary (familial) or secondary (acquired), the most recent guidelines suggest the etiology is not always binary.3,5 That said, the concept of secondary causes is useful in understanding risk factors for developing HLH. Both forms of the disease are thought to be elicited by a trigger (eg, infection), even when inherited genetic mutations exist.6 The primary form commonly affects the pediatric population,4,6-8 whereas the secondary form is more common in adults.7
Several sets of diagnostic criteria for HLH have been developed, the most well-known being the HLH-2004 criteria.1,3 The HLH-2009 modified criteria were developed after further evidence provided a refined sense of how the HLH-2004 criteria should be stratified.9 Finally, Fardet et al10 presented the HScore as an estimation of likelihood of diagnosis of HLH. These sets of HLH criteria include clinical and laboratory features that demonstrate inflammation, natrual killer cell activity, hemophagocytosis, end-organ damage, and cell lineage effects. The HScore differs from the other sets of HLH criteria in that it is designed to estimate an individual patient’s risk of having reactive hemophagocytic syndrome, which likely is equivalent to secondary HLH, although the authors do not use this exact terminology.10
While these criteria provide a framework for diagnosing HLH, they may fail to distinguish between HLH disease and HLH disease mimics, a concept described by the North American Consortium for Histiocytosis that may impact the success of immunosuppressive treatment.3 Individuals with HLH syndrome meet the aforementioned diagnostic criteria; HLH syndrome is further divided into HLH disease and HLH disease mimics (Figure 1). The “disease” label describes the traditional concept of HLH, driven by aberrant immune overactivation, in which patients benefit from immunosuppression. In contrast, HLH mimics include a subset of patients who meet the HLH criteria but are unlikely to benefit from immunosuppression because the primary mechanism driving their condition is not owed to immune overactivation, as is the case with HLH disease. Examples of HLH mimics include certain infections, such as Epstein-Barr virus (EBV), that may demonstrate clinical findings consistent with HLH but would not benefit from immunosuppression. Ironically, infections (including EBV) also are known triggers of HLH disease, making this concept difficult to understand and adopt. In this study, we refer to HLH disease simply as HLH.

Although cutaneous manifestations of HLH are not included in the diagnostic criteria, skin findings are common and may coincide with the severity and progression of the disease.11 Despite the fact that HLH can manifest with rash,1 comprehensive reviews of reported cutaneous findings in adult HLH are lacking. Thus, the goal of this study was to provide an organized characterization of reported cutaneous findings in adults with HLH and context for how the dermatologic examination may support the diagnosis or uncover the underlying etiology of this condition.
Methods
A search of PubMed articles indexed for MEDLINE using the phrase (cutaneous OR dermatologic OR skin) findings) AND hemophagocytic lymphohistiocytosis performed on September 20, 2023, yielded 423 results (Figure 2). Filters to exclude non–English language publications and pediatric populations were applied, resulting in 161 articles. Other exclusion criteria included the absence of a description of dermatologic findings. Seventy-five articles remained after screening titles and abstracts, and full-text review yielded 55 articles that were deemed appropriate for inclusion in the study. Subsequent reference searches and use of the online resource Litmaps revealed 45 additional publications that underwent full-text screening; of these articles, 5 were included in the final review.

Results
Sixty studies were included in this systematic review.5,7,11-68 The reported prevalence of skin findings among patients with HLH from the included retrospective studies ranged from 15% to 85%.12-15 Several literature reviews reported similarly varied prevalence among adult patients with HLH.7,16 Fardet et al14 categorized cutaneous manifestations of HLH into 3 types: direct manifestations of HLH not explained by systemic features (eg, generalized maculopapular eruption), indirect manifestations of HLH that are explained by systemic features of the disease (eg, purpura due to HLH-induced coagulopathy), and findings specific to the underlying etiology of HLH (eg, malar rash seen in systemic lupus erythematosus [SLE]–associated HLH). This categorization served as the outline for the results below, providing an organized review of cutaneous findings and context for how they may support the diagnosis or uncover the underlying etiology of HLH.
Category I: Direct Manifestations of HLH
Several articles reported cutaneous findings that seemed to be the direct result of HLH and not attributed to an underlying trigger or sequalae of HLH.11,14,16-31 The most common descriptions were a generalized, morbilliform, or nonspecific eruption that encompasses large areas of the skin, commonly the trunk and extremities, sometimes extending to the face and scalp.14,16-23,25,31,32 There were variations in secondary features such as pruritus and tenderness; some studies also described violaceous discoloration in addition to erythema.16,23
Other skin findings thought to be a direct result of HLH were described in detail by Zerah and DeWitt11 in their retrospective study, including pyoderma gangrenosum, panniculitis, Stevens-Johnson syndrome, atypical targetoid lesions, and bullous eruptions. The authors also analyzed dermatopathologic data that ultimately revealed that pathologic analysis was largely inconsistent and nondescript.11 There was a single case report of purpura fulminans arising alongside signs and symptoms of HLH,26 and several case reports described Sweet syndrome developing around the same time as HLH.27-29 Lastly, Collins et al30 described a case of HLH manifesting with violaceous ulcerating papules and nodules scattered across the legs, abdomen, and arms. Biopsy of this patient’s lesions showed a diffuse dermal infiltrate of histiocytes and hemophagocytosis.
Category II: Secondary Complications and Sequelae of HLH
This was the smallest group among the 3 categories, comprising a few case reports and retrospective cohort studies primarily reporting jaundice/icterus and hemorrhagic lesions such as purpura, petechiae, and scleral hemorrhage.11,21,23,33-35 Several literature reviews described these conditions as nonspecific findings in HLH.16,20 The cause of jaundice in HLH likely can be attributed to its characteristic hepatic dysfunction, whereas hemorrhagic lesions likely are the result of both hepatic and bone marrow dysfunction resulting in coagulopathy.
Category III: Manifestations of Underlying Etiology or Triggers of HLH
Infectious—Infection is known to be one of the most common triggers of HLH, with several retrospective studies reporting infectious triggers in approximately 20% of cases.13,15 Although many pathogens have been implicated, only a few of these infection-induced HLH reports described cutaneous findings, which included a case of varicella zoster virus, Escherichia coli necrotizing fasciitis, leprosy, EBV reactivation, parvovirus B19, and both focal and disseminated herpes simplex virus 2.36-42 Most of these patients presented with classic findings of each disease. The case of varicella zoster virus exhibited pruritic erythematous papules on the face, trunk, and limbs.36 The necrotizing fasciitis case presented with tender erythematous swelling of the lower extremity.37 The patient with leprosy exhibited leonine facies and numerous erythematous nodules, plaques, and superficial ulcerating plaques over the trunk and limbs with palmoplantar involvement,39 and both cases of herpes simplex virus 2 reported small bullae either diffusely over the face, trunk, and extremities or over the genitalia.38,40 Interestingly, the cases of parvovirus B19 and EBV reactivation both exhibited polyarteritis nodosa and occurred in patients with underlying autoimmune conditions, raising the question of whether these cases of HLH had a single trigger or were the result of the overall immunologic dysregulation induced by both infection and autoimmunity.41,42
Rheumatologic—Several articles reported dermatologic findings associated with macrophage activation syndrome, a term that often is used to describe HLH associated with autoimmune conditions. Cases of HLH in adult-onset Still disease, dermatomyositis, polyarteritis nodosa, and SLE described skin findings characteristic of the underlying rheumatologic disease, sometimes with acutely worse dermatologic findings at the time of HLH presentation.35,41-48 With regard to SLE, the acute manifestation of classic findings of the disease with HLH has sometimes been described as acute lupus hemophagocytic syndrome (HPS).48 Lambotte at al48 described common findings of acute lupus hemophagocytic syndrome in their retrospective study as malar rash, weight loss, polyarthralgia, and nephritis in addition to classic HLH findings including fever, lymphadenopathy, and hepatosplenomegaly. Many other rheumatologic conditions have been associated with HLH, including rheumatoid arthritis, mixed connective tissue disease, systemic sclerosis, and Sjögren disease. All these conditions can have dermatologic manifestations; however, no descriptions of dermatologic findings in cases of HLH associated with these diseases were found.13
Malignancy—Several cases of malignancy-induced HLH described cutaneous findings, the majority being cutaneous lymphomas, namely subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Other less commonly reported malignancies in this group included Kaposi sarcoma, intravascular lymphoma, Sézary syndrome, mycosis fungoides, cutaneous diffuse large B-cell lymphoma, and several subtypes of primary cutaneous T-cell lymphoma.2,32,49-60 The most common description of SPTCL included multiple scattered plaques and subcutaneous nodules, some associated with tenderness, induration, drainage, or hemorrhagic features.32,50,52,55,57,60 Cases of mycosis fungoides and Sézary syndrome presented with variations in size and distribution of erythroderma with associated lymphadenopathy.2 A unique case of HLH developing in a patient with intravascular lymphoma described an eruption of multiple telangiectasias and petechial hemorrhages on the trunk,58 while one case associated with primary cutaneous anaplastic large cell lymphoma presented with a rapidly enlarging tumor with central ulceration and eschar.59
Drug Induced—Interestingly, most of the drug-induced cases of HLH identified in our search were secondary to biologic therapies used in the treatment of metastatic melanoma, specifically the immune checkpoint inhibitors (ICIs), which have been reported to have an association with HLH in prior literature reviews.61-65 Choi et al66 described an interesting case of ICI-induced HLH presenting with a concurrent severe lichenoid drug eruption that progressed from a pruritic truncal rash to mucocutaneous bullae, erosions, and desquamation resembling a Stevens-Johnson syndrome–type picture. This patient had treatment-refractory, HIV-negative Kaposi sarcoma, where the underlying immunologic dysregulation may explain the more severe cutaneous presentation not observed in other reported cases of ICI-induced HLH.
Yang et al’s67 review of 23 cases with concurrent diagnoses of HLH and DIHS found that 61% (14/23) of cases were diagnosed initially as DIHS before failing treatment and receiving a diagnosis of HLH several weeks later. Additionally, the authors found that several cases met criteria for one diagnosis while clinically presenting strongly for the other.67 This overlap in clinical presentation also was demonstrated in Zerah and DeWitt’s11 retrospective study regarding cutaneous findings in HLH, in which several of the morbilliform eruptions thought to be contributed to HLH ultimately were decided to be drug reactions.
Comment
Regarding direct (or primary) cutaneous findings in HLH (category I), there seem to be 2 groups of features associated with the onset of HLH that are not related to its characteristic hepatic dysfunction (category II) nor its underlying triggers (category III): a nonspecific, generalized, erythematous eruption; and dermatologic conditions separate from HLH itself (eg, Sweet syndrome, pyoderma gangrenosum). Whether the latter group truly is a direct manifestation of HLH is difficult to discern with the evidence available. Nevertheless, we can conclude that there is some type of association between these dermatologic diseases and HLH, and this association can serve as both a diagnostic tool for clinicians and a point of interest for further clinical research.
The relatively low number of articles identified through our systematic review that specifically reported secondary findings, such as jaundice or coagulopathy-associated hemorrhagic lesions, may lead one to believe that these are not common findings in HLH; however, it is possible that these are not regularly reported in the literature simply because these findings are nonspecific and can be considered expected results of the characteristic organ dysfunction in HLH.
As suspected, the skin findings in category III were the most broad given the variety of underlying etiologies that have been associated with HLH. Like the other 2 categories, these skin findings generally are nonspecific to HLH; however, the ones in category III are specific to underlying etiology of HLH and may aid in identifying and treating the underlying cause of a patient’s HLH when indicated.
Most of the rheumatologic diseases seem to have been known at the time of HLH development and diagnosis, which may highlight the importance of considering a diagnosis of HLH early on in patients with known autoimmune disease and systemic signs of illness or acutely worsening signs and symptoms of their underlying autoimmune disease.
Interestingly, several cases of malignancy-associated HLH reported signs and symptoms of HLH at initial presentation of the malignant disease.32,50,59 This situation seems to be somewhat common, as Go and Wester’s68 systematic analysis of 156 patients with SPTCL found HLH was the presenting feature in 37% of patients included in their study. This may call attention to the importance of considering cutaneous lymphomas as the cause of skin lesions in patients with signs and symptoms of HLH, where it may be easy to assume that skin findings are a result of their systemic disease.
In highlighting cases of HLH related to medication use, we found it pertinent to include and discuss the complex relationship between drug-induced hypersensitivity syndrome (DIHS [formerly known as drug rash with eosinophilia and systemic symptoms [DRESS] syndrome) and HLH. The results of this study suggest that DIHS may have considerable clinical overlap with HLH11 and may even lead to development of HLH,67 creating difficulty in distinguishing between these conditions where there may be similar findings, such as cutaneous eruptions, fever, and hepatic or other internal organ involvement. We agree with Yang et al67 that there can be large overlap in symptomology between these two conditions and that more investigation is necessary to explore the relationship between them.
Conclusion
Diagnosis of HLH in adults continues to be challenging, with several diagnostic tools but no true gold standard. In addition to the nonspecific symptomology, there is a myriad of cutaneous findings that can be present in adults with HLH (eTable), all of which are also nonspecific. Even so, awareness of which dermatologic findings have been associated with HLH may provide a cue to consider HLH in the systemically ill patient with a notable dermatologic examination. Furthermore, there are several avenues for further investigation that can be drawn, including further dermatologic analysis among nonspecific eruptions attributed to HLH, clinical and pathologic differentiation between DIHS/DRESS and HLH, and correlation between severity of skin manifestations and severity of HLH disease.

Limitations of this study included a lack of clarity in diagnosis of HLH in patients described in the included articles, as some reports use variable terminology (HLH vs hemophagocytic syndrome vs macrophage activation syndrome, etc), and it is impossible to know if all authors used the same diagnostic criteria—or any validated diagnostic criteria—unless specifically stated. Additionally, including case reports in our study limited the amount and quality of information described in each report. Despite its limitations, this systematic review outlines the cutaneous manifestations associated with HLH. These data will promote clinical awareness of this complex condition and allow for consideration of HLH in patients meeting criteria for HLH syndrome. More studies ultimately are needed to differentiate HLH from its mimics.
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. doi:10.1002/pbc.21039
- Blom A, Beylot-Barry M, D’Incan M, et al. Lymphoma-associated hemophagocytic syndrome (LAHS) in advanced-stage mycosis fungoides/ Sézary syndrome cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;65:404-410. doi:10.1016/j.jaad.2010.05.029
- Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66:e27929. doi:10.1002/pbc.27929
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515. doi:10.1016/j.berh.2020.101515
- Tomasini D, Berti E. Subcutaneous panniculitis-like T-cell lymphoma. G Ital Dermatol Venereol. 2013;148:395-411.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. doi:10.1016/s0140-6736(13)61048-x
- Sieni E, Cetica V, Piccin A, et al. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. PLoS One. 2012;7:e44649. doi:10.1371/journal.pone.0044649
- Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology. 2009:127-131. doi:10.1182 /asheducation-2009.1.127
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613-2620. doi:10.1002/art.38690
- Zerah ML, DeWitt CA. Cutaneous findings in hemophagocytic lymphohistiocytosis. Dermatology. 2015;230:234-243. doi:10.1159/000368552
- Fardet L, Galicier L, Vignon-Pennamen MD, et al. Frequency, clinical features and prognosis of cutaneous manifestations in adult patients with reactive haemophagocytic syndrome. Br J Dermatol. 2010;162:547-553. doi:10.1111/j.1365-2133.2009.09549.x
- Dhote R, Simon J, Papo T, et al. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum. 2003;49:633-639. doi:10.1002/art.11368
- Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93:100-105. doi:10.1097/md.0000000000000022
- Tudesq JJ, Valade S, Galicier L, et al. Diagnostic strategy for trigger identification in severe reactive hemophagocytic lymphohistiocytosis: a diagnostic accuracy study. Hematol Oncol. 2021;39:114-122. doi:10.1002 /hon.2819
- Sakai H, Otsubo S, Miura T, et al. Hemophagocytic syndrome presenting with a facial erythema in a patient with systemic lupus erythematosus. J Am Acad Dermatol. 2007;57(5 Suppl):S111-S114. doi:10.1016/j .jaad.2006.11.024
- Chung SM, Song JY, Kim W, et al. Dengue-associated hemophagocytic lymphohistiocytosis in an adult: a case report and literature review. Medicine (Baltimore). 2017;96:e6159. doi:10.1097/md.0000000000006159
- Esmaili H, Rahmani O, Fouladi RF. Hemophagocytic syndrome in patients with unexplained cytopenia: report of 15 cases. Turk Patoloji Derg. 2013;29:15-18. doi:10.5146/tjpath.2013.01142
- Jiwnani S, Karimundackal G, Kulkarni A, et al. Hemophagocytic syndrome complicating lung resection. Asian Cardiovasc Thorac Ann. 2012;20:341-343. doi:10.1177/0218492311435686
- Lee WJ, Lee DW, Kim CH, et al. Dermatopathic lymphadenitis with generalized erythroderma in a patient with Epstein-Barr virusassociated hemophagocytic lymphohistiocytosis. Am J Dermatopathol. 2010;32:357-361. doi:10.1097/DAD.0b013e3181b2a50f
- Lovisari F, Terzi V, Lippi MG, et al. Hemophagocytic lymphohistiocytosis complicated by multiorgan failure: a case report. Medicine (Baltimore). 2017;96:e9198. doi:10.1097/md.0000000000009198
- Miechowiecki J, Stainer W, Wallner G, et al. Severe complication during remission of Crohn’s disease: hemophagocytic lymphohistiocytosis due to acute cytomegalovirus infection. Z Gastroenterol. 2018;56:259-263. doi:10.1055/s-0043-123999
- Ochoa S, Cheng K, Fleury CM, et al. A 28-year-old woman with fever, rash, and pancytopenia. Allergy Asthma Proc. 2017;38:322-327. doi:10.2500/aap.2017.38.4042
- Tokoro S, Namiki T, Miura K, et al. Chronic active Epstein-Barr virus infection with cutaneous lymphoproliferation: haemophagocytosis in the skin and haemophagocytic syndrome. J Eur Acad Dermatol Venereol. 2018;32:e116-e117. doi:10.1111/jdv.14640
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitislike T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106 (2 Suppl):S55-S59. doi:10.1016/s0929-6646(09)60354-5
- Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer. 2019;7:97. doi:10.1186/s40425- 019-0582-4
- Kao RL, Jacobsen AA, Billington CJ Jr, et al. A case of VEXAS syndrome associated with EBV-associated hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2022;93:102636. doi:10.1016/j .bcmd.2021.102636
- Koga T, Takano K, Horai Y, et al. Sweet’s syndrome complicated by Kikuchi’s disease and hemophagocytic syndrome which presented with retinoic acid-inducible gene-I in both the skin epidermal basal layer and the cervical lymph nodes. Intern Med. 2013;52:1839-1843. doi:10.2169 /internalmedicine.52.9542
- Lin WL, Lin WC, Chiu CS, et al. Paraneoplastic Sweet’s syndrome in a patient with hemophagocytic syndrome. Int J Dermatol. 2008;3:305-307.
- Collins MK, Ho J, Akilov OE. Case 52. A unique presentation of hemophagocytic lymphohistiocytosis with ulcerating papulonodules. In: Akilov OE, ed. Cutaneous Lymphomas: Unusual Cases 3. Springer International Publishing; 2021:126-127.
- Chakrapani A, Avery A, Warnke R. Primary cutaneous gamma delta T-cell lymphoma with brain involvement and hemophagocytic syndrome. Am J Dermatopathol. 2013;35:270-272. doi:10.1097 /DAD.0b013e3182624e98
- Sullivan C, Loghmani A, Thomas K, et al. Hemophagocytic lymphohistiocytosis as the initial presentation of subcutaneous panniculitis-like T-cell lymphoma: a rare case responding to cyclosporine A and steroids. J Investig Med High Impact Case Rep. 2020;8:2324709620981531. doi:10.1177/2324709620981531
- Darmawan G, Salido EO, Concepcion ML, et al. Hemophagocytic lymphohistiocytosis: “a dreadful mimic.” Int J Rheum Dis. 2015; 18:810-812. doi:10.1111/1756-185x.12506
- Maus MV, Leick MB, Cornejo KM, et al. Case 35-2019: a 66-year-old man with pancytopenia and rash. N Engl J Med. 2019;381:1951-1960. doi:10.1056/NEJMcpc1909627
- Chamseddin B, Marks E, Dominguez A, et al. Refractory macrophage activation syndrome in the setting of adult-onset Still disease with hemophagocytic lymphohistiocytosis detected on skin biopsy treated with canakinumab and tacrolimus. J Cutan Pathol. 2019;46:528-531. doi:10.1111/cup.13466
- Bérar A, Ardois S, Walter-Moraux P, et al. Primary varicella-zoster virus infection of the immunocompromised associated with acute pancreatitis and hemophagocytic lymphohistiocytosis: a case report. Medicine (Baltimore). 2021;100:e25351. doi:10.1097 /md.0000000000025351
- Chang CC, Hsiao PJ, Chiu CC, et al. Catastrophic hemophagocytic lymphohistiocytosis in a young man with nephrotic syndrome. Clin Chim Acta. 2015;439:168-171. doi:10.1016/j.cca.2014.10.025
- Kurosawa S, Sekiya N, Fukushima K, et al. Unusual manifestation of disseminated herpes simplex virus type 2 infection associated with pharyngotonsilitis, esophagitis, and hemophagocytic lymphohisitocytosis without genital involvement. BMC Infect Dis. 2019;19:65. doi:10.1186/s12879-019-3721-0
- Saidi W, Gammoudi R, Korbi M, et al. Hemophagocytic lymphohistiocytosis: an unusual complication of leprosy. Int J Dermatol. 2015;54: 1054-1059. doi:10.1111/ijd.12792
- Yamaguchi K, Yamamoto A, Hisano M, et al. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol. 2005;105(5 Pt 2):1241-1244. doi:10.1097 /01.AOG.0000157757.54948.9b
- Hayakawa I, Shirasaki F, Ikeda H, et al. Reactive hemophagocytic syndrome in a patient with polyarteritis nodosa associated with Epstein- Barr virus reactivation. Rheumatol Int. 2006;26:573-576. doi:10.1007 /s00296-005-0024-0
- Jeong JY, Park JY, Ham JY, et al. Molecular evidence of parvovirus B19 in the cutaneous polyarteritis nodosa tissue from a patient with parvovirus-associated hemophagocytic syndrome: case report. Medicine (Baltimore). 2020;99:e22079. doi:10.1097 /md.0000000000022079
- Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478. doi:10.2169 /internalmedicine.1121-18
- Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune- associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246. doi:10 .1080/03009742.2019.1653493
- Jung SY. Hemophagocytic syndrome diagnosed by liver biopsy in a female patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:449-451. doi:10.1097/rhu.0000000000000040
- Kerl K, Wolf IH, Cerroni L, et al. Hemophagocytosis in cutaneous autoimmune disease. Am J Dermatopathol. 2015;37:539-543. doi:10.1097 /dad.0000000000000166
- Komiya Y, Saito T, Mizoguchi F, et al. Hemophagocytic syndrome complicated with dermatomyositis controlled successfully with infliximab and conventional therapies. Intern Med. 2017;56:3237-3241. doi:10.2169 /internalmedicine.7966-16
- Lambotte O, Khellaf M, Harmouche H, et al. Characteristics and long-term outcome of 15 episodes of systemic lupus erythematosusassociated hemophagocytic syndrome. Medicine (Baltimore). 2006;85: 169-182. doi:10.1097/01.md.0000224708.62510.d1
- Guitart J, Mangold AR, Martinez-Escala ME, et al. Clinical and pathological characteristics and outcomes among patients with subcutaneous panniculitis-like T-cell lymphoma and related adipotropic lymphoproliferative disorders. JAMA Dermatol. 2022;158:1167-1174. doi:10.1001/jamadermatol.2022.3347
- Hung GD, Chen YH, Chen DY, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with hemophagocytic lymphohistiocytosis and skin lesions with characteristic high-resolution ultrasonographic findings. Clin Rheumatol. 2007;26:775-778. doi:10.1007/s10067 -005-0193-y
- Jamil A, Nadzri N, Harun N, et al. Primary cutaneous diffuse large B-cell lymphoma leg type presenting with hemophagocytic syndrome. J Am Acad Dermatol. 2012;67:e222-3. doi:10.1016/j.jaad.2012.04.021
- LeBlanc RE, Lansigan F. Unraveling subcutaneous panniculitis-like T-cell lymphoma: an association between subcutaneous panniculitislike T-cell lymphoma, autoimmune lymphoproliferative syndrome, and familial hemophagocytic lymphohistiocytosis. J Cutan Pathol. 2021;48:572-577. doi:10.1111/cup.13863
- Lee DE, Martinez-Escala ME, Serrano LM, et al. Hemophagocytic lymphohistiocytosis in cutaneous T-cell lymphoma. JAMA Dermatol. 2018;154:828-831. doi:10.1001/jamadermatol.2018.1264
- Maejima H, Tanei R, Morioka T, et al. Haemophagocytosis-related intravascular large B-cell lymphoma associated with skin eruption. Acta Derm Venereol. 2011;91:339-340. doi:10.2340/00015555-0981
- Mody A, Cherry D, Georgescu G, et al. A rare case of subcutaneous panniculitis-like T cell lymphoma with hemophagocytic lymphohistiocytosis mimicking cellulitis. Am J Case Rep. 2021;22:E927142. doi:10.12659/ajcr.927142
- Park YJ, Bae HJ, Chang JY, et al. Development of Kaposi sarcoma and hemophagocytic lymphohistiocytosis associated with human herpesvirus 8 in a renal transplant recipient. Korean J Intern Med. 2017;4:750-752.
- Phatak S, Gupta L, Aggarwal A. A young woman with panniculitis and cytopenia who later developed coagulopathy. J Assoc Physicians India. 2016;64:65-67.
- Pongpairoj K, Rerknimitr P, Wititsuwannakul J, et al. Eruptive telangiectasia in a patient with fever and haemophagocytic syndrome. Clin Exp Dermatol. 2016;41:696-698. doi:10.1111/ced.12859
- Shimizu Y, Tanae K, Takahashi N, et al. Primary cutaneous anaplastic large-cell lymphoma presenting with hemophagocytic syndrome: a case report and review of the literature. Leuk Res. 2010;34:263-266. doi:10.1016/j.leukres.2009.07.001
- Sirka CS, Pradhan S, Patra S, et al. Hemophagocytic lymphohistiocytosis: a rare, potentially fatal complication in subcutaneous panniculitis like T cell lymphoma. Indian J Dermatol Venereol Leprol. 2019;5:481-485.
- Chin CK, Hall S, Green C, et al. Secondary haemophagocytic lymphohistiocytosis due to checkpoint inhibitor therapy. Eur J Cancer. 2019;115: 84-87. doi:10.1016/j.ejca.2019.04.026
- Dudda M, Mann C, Heinz J, et al. Hemophagocytic lymphohistiocytosis of a melanoma patient under BRAF/MEK-inhibitor therapy following anti-PD1 inhibitor treatment: a case report and review to the literature. Melanoma Res. 2021;31:81-84. doi:10.1097 /cmr.0000000000000703
- Mizuta H, Nakano E, Takahashi A, et al. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: a case report and literature review. Dermatol Ther. 2020;33:e13321. doi:10.1111/dth.13321
- Satzger I, Ivanyi P, Länger F, et al. Treatment-related hemophagocytic lymphohistiocytosis secondary to checkpoint inhibition with nivolumab plus ipilimumab. Eur J Cancer. 2018;93:150-153. doi:10.1016/j.ejca.2018.01.063
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72-90. doi:10.1016/J.EJCA.2019.07.014
- Choi S, Zhou M, Bahrani E, et al. Rare and fatal complication of immune checkpoint inhibition: a case report of haemophagocytic lymphohistiocytosis with severe lichenoid dermatitis. Br J Haematol. 2021;193:e44-e47. doi:10.1111/BJH.17442
- Yang JJ, Lei DK, Ravi V, et al. Overlap between hemophagocytic lymphohistiocytosis and drug reaction and eosinophilia with systemic symptoms: a review. Int J Dermatol. 2021;60:925-932. doi:10.1111 /ijd.15196
- Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. doi:10.1002/cncr.20502
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. doi:10.1002/pbc.21039
- Blom A, Beylot-Barry M, D’Incan M, et al. Lymphoma-associated hemophagocytic syndrome (LAHS) in advanced-stage mycosis fungoides/ Sézary syndrome cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;65:404-410. doi:10.1016/j.jaad.2010.05.029
- Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66:e27929. doi:10.1002/pbc.27929
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515. doi:10.1016/j.berh.2020.101515
- Tomasini D, Berti E. Subcutaneous panniculitis-like T-cell lymphoma. G Ital Dermatol Venereol. 2013;148:395-411.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. doi:10.1016/s0140-6736(13)61048-x
- Sieni E, Cetica V, Piccin A, et al. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. PLoS One. 2012;7:e44649. doi:10.1371/journal.pone.0044649
- Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology. 2009:127-131. doi:10.1182 /asheducation-2009.1.127
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613-2620. doi:10.1002/art.38690
- Zerah ML, DeWitt CA. Cutaneous findings in hemophagocytic lymphohistiocytosis. Dermatology. 2015;230:234-243. doi:10.1159/000368552
- Fardet L, Galicier L, Vignon-Pennamen MD, et al. Frequency, clinical features and prognosis of cutaneous manifestations in adult patients with reactive haemophagocytic syndrome. Br J Dermatol. 2010;162:547-553. doi:10.1111/j.1365-2133.2009.09549.x
- Dhote R, Simon J, Papo T, et al. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum. 2003;49:633-639. doi:10.1002/art.11368
- Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93:100-105. doi:10.1097/md.0000000000000022
- Tudesq JJ, Valade S, Galicier L, et al. Diagnostic strategy for trigger identification in severe reactive hemophagocytic lymphohistiocytosis: a diagnostic accuracy study. Hematol Oncol. 2021;39:114-122. doi:10.1002 /hon.2819
- Sakai H, Otsubo S, Miura T, et al. Hemophagocytic syndrome presenting with a facial erythema in a patient with systemic lupus erythematosus. J Am Acad Dermatol. 2007;57(5 Suppl):S111-S114. doi:10.1016/j .jaad.2006.11.024
- Chung SM, Song JY, Kim W, et al. Dengue-associated hemophagocytic lymphohistiocytosis in an adult: a case report and literature review. Medicine (Baltimore). 2017;96:e6159. doi:10.1097/md.0000000000006159
- Esmaili H, Rahmani O, Fouladi RF. Hemophagocytic syndrome in patients with unexplained cytopenia: report of 15 cases. Turk Patoloji Derg. 2013;29:15-18. doi:10.5146/tjpath.2013.01142
- Jiwnani S, Karimundackal G, Kulkarni A, et al. Hemophagocytic syndrome complicating lung resection. Asian Cardiovasc Thorac Ann. 2012;20:341-343. doi:10.1177/0218492311435686
- Lee WJ, Lee DW, Kim CH, et al. Dermatopathic lymphadenitis with generalized erythroderma in a patient with Epstein-Barr virusassociated hemophagocytic lymphohistiocytosis. Am J Dermatopathol. 2010;32:357-361. doi:10.1097/DAD.0b013e3181b2a50f
- Lovisari F, Terzi V, Lippi MG, et al. Hemophagocytic lymphohistiocytosis complicated by multiorgan failure: a case report. Medicine (Baltimore). 2017;96:e9198. doi:10.1097/md.0000000000009198
- Miechowiecki J, Stainer W, Wallner G, et al. Severe complication during remission of Crohn’s disease: hemophagocytic lymphohistiocytosis due to acute cytomegalovirus infection. Z Gastroenterol. 2018;56:259-263. doi:10.1055/s-0043-123999
- Ochoa S, Cheng K, Fleury CM, et al. A 28-year-old woman with fever, rash, and pancytopenia. Allergy Asthma Proc. 2017;38:322-327. doi:10.2500/aap.2017.38.4042
- Tokoro S, Namiki T, Miura K, et al. Chronic active Epstein-Barr virus infection with cutaneous lymphoproliferation: haemophagocytosis in the skin and haemophagocytic syndrome. J Eur Acad Dermatol Venereol. 2018;32:e116-e117. doi:10.1111/jdv.14640
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitislike T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106 (2 Suppl):S55-S59. doi:10.1016/s0929-6646(09)60354-5
- Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer. 2019;7:97. doi:10.1186/s40425- 019-0582-4
- Kao RL, Jacobsen AA, Billington CJ Jr, et al. A case of VEXAS syndrome associated with EBV-associated hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2022;93:102636. doi:10.1016/j .bcmd.2021.102636
- Koga T, Takano K, Horai Y, et al. Sweet’s syndrome complicated by Kikuchi’s disease and hemophagocytic syndrome which presented with retinoic acid-inducible gene-I in both the skin epidermal basal layer and the cervical lymph nodes. Intern Med. 2013;52:1839-1843. doi:10.2169 /internalmedicine.52.9542
- Lin WL, Lin WC, Chiu CS, et al. Paraneoplastic Sweet’s syndrome in a patient with hemophagocytic syndrome. Int J Dermatol. 2008;3:305-307.
- Collins MK, Ho J, Akilov OE. Case 52. A unique presentation of hemophagocytic lymphohistiocytosis with ulcerating papulonodules. In: Akilov OE, ed. Cutaneous Lymphomas: Unusual Cases 3. Springer International Publishing; 2021:126-127.
- Chakrapani A, Avery A, Warnke R. Primary cutaneous gamma delta T-cell lymphoma with brain involvement and hemophagocytic syndrome. Am J Dermatopathol. 2013;35:270-272. doi:10.1097 /DAD.0b013e3182624e98
- Sullivan C, Loghmani A, Thomas K, et al. Hemophagocytic lymphohistiocytosis as the initial presentation of subcutaneous panniculitis-like T-cell lymphoma: a rare case responding to cyclosporine A and steroids. J Investig Med High Impact Case Rep. 2020;8:2324709620981531. doi:10.1177/2324709620981531
- Darmawan G, Salido EO, Concepcion ML, et al. Hemophagocytic lymphohistiocytosis: “a dreadful mimic.” Int J Rheum Dis. 2015; 18:810-812. doi:10.1111/1756-185x.12506
- Maus MV, Leick MB, Cornejo KM, et al. Case 35-2019: a 66-year-old man with pancytopenia and rash. N Engl J Med. 2019;381:1951-1960. doi:10.1056/NEJMcpc1909627
- Chamseddin B, Marks E, Dominguez A, et al. Refractory macrophage activation syndrome in the setting of adult-onset Still disease with hemophagocytic lymphohistiocytosis detected on skin biopsy treated with canakinumab and tacrolimus. J Cutan Pathol. 2019;46:528-531. doi:10.1111/cup.13466
- Bérar A, Ardois S, Walter-Moraux P, et al. Primary varicella-zoster virus infection of the immunocompromised associated with acute pancreatitis and hemophagocytic lymphohistiocytosis: a case report. Medicine (Baltimore). 2021;100:e25351. doi:10.1097 /md.0000000000025351
- Chang CC, Hsiao PJ, Chiu CC, et al. Catastrophic hemophagocytic lymphohistiocytosis in a young man with nephrotic syndrome. Clin Chim Acta. 2015;439:168-171. doi:10.1016/j.cca.2014.10.025
- Kurosawa S, Sekiya N, Fukushima K, et al. Unusual manifestation of disseminated herpes simplex virus type 2 infection associated with pharyngotonsilitis, esophagitis, and hemophagocytic lymphohisitocytosis without genital involvement. BMC Infect Dis. 2019;19:65. doi:10.1186/s12879-019-3721-0
- Saidi W, Gammoudi R, Korbi M, et al. Hemophagocytic lymphohistiocytosis: an unusual complication of leprosy. Int J Dermatol. 2015;54: 1054-1059. doi:10.1111/ijd.12792
- Yamaguchi K, Yamamoto A, Hisano M, et al. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol. 2005;105(5 Pt 2):1241-1244. doi:10.1097 /01.AOG.0000157757.54948.9b
- Hayakawa I, Shirasaki F, Ikeda H, et al. Reactive hemophagocytic syndrome in a patient with polyarteritis nodosa associated with Epstein- Barr virus reactivation. Rheumatol Int. 2006;26:573-576. doi:10.1007 /s00296-005-0024-0
- Jeong JY, Park JY, Ham JY, et al. Molecular evidence of parvovirus B19 in the cutaneous polyarteritis nodosa tissue from a patient with parvovirus-associated hemophagocytic syndrome: case report. Medicine (Baltimore). 2020;99:e22079. doi:10.1097 /md.0000000000022079
- Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478. doi:10.2169 /internalmedicine.1121-18
- Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune- associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246. doi:10 .1080/03009742.2019.1653493
- Jung SY. Hemophagocytic syndrome diagnosed by liver biopsy in a female patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:449-451. doi:10.1097/rhu.0000000000000040
- Kerl K, Wolf IH, Cerroni L, et al. Hemophagocytosis in cutaneous autoimmune disease. Am J Dermatopathol. 2015;37:539-543. doi:10.1097 /dad.0000000000000166
- Komiya Y, Saito T, Mizoguchi F, et al. Hemophagocytic syndrome complicated with dermatomyositis controlled successfully with infliximab and conventional therapies. Intern Med. 2017;56:3237-3241. doi:10.2169 /internalmedicine.7966-16
- Lambotte O, Khellaf M, Harmouche H, et al. Characteristics and long-term outcome of 15 episodes of systemic lupus erythematosusassociated hemophagocytic syndrome. Medicine (Baltimore). 2006;85: 169-182. doi:10.1097/01.md.0000224708.62510.d1
- Guitart J, Mangold AR, Martinez-Escala ME, et al. Clinical and pathological characteristics and outcomes among patients with subcutaneous panniculitis-like T-cell lymphoma and related adipotropic lymphoproliferative disorders. JAMA Dermatol. 2022;158:1167-1174. doi:10.1001/jamadermatol.2022.3347
- Hung GD, Chen YH, Chen DY, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with hemophagocytic lymphohistiocytosis and skin lesions with characteristic high-resolution ultrasonographic findings. Clin Rheumatol. 2007;26:775-778. doi:10.1007/s10067 -005-0193-y
- Jamil A, Nadzri N, Harun N, et al. Primary cutaneous diffuse large B-cell lymphoma leg type presenting with hemophagocytic syndrome. J Am Acad Dermatol. 2012;67:e222-3. doi:10.1016/j.jaad.2012.04.021
- LeBlanc RE, Lansigan F. Unraveling subcutaneous panniculitis-like T-cell lymphoma: an association between subcutaneous panniculitislike T-cell lymphoma, autoimmune lymphoproliferative syndrome, and familial hemophagocytic lymphohistiocytosis. J Cutan Pathol. 2021;48:572-577. doi:10.1111/cup.13863
- Lee DE, Martinez-Escala ME, Serrano LM, et al. Hemophagocytic lymphohistiocytosis in cutaneous T-cell lymphoma. JAMA Dermatol. 2018;154:828-831. doi:10.1001/jamadermatol.2018.1264
- Maejima H, Tanei R, Morioka T, et al. Haemophagocytosis-related intravascular large B-cell lymphoma associated with skin eruption. Acta Derm Venereol. 2011;91:339-340. doi:10.2340/00015555-0981
- Mody A, Cherry D, Georgescu G, et al. A rare case of subcutaneous panniculitis-like T cell lymphoma with hemophagocytic lymphohistiocytosis mimicking cellulitis. Am J Case Rep. 2021;22:E927142. doi:10.12659/ajcr.927142
- Park YJ, Bae HJ, Chang JY, et al. Development of Kaposi sarcoma and hemophagocytic lymphohistiocytosis associated with human herpesvirus 8 in a renal transplant recipient. Korean J Intern Med. 2017;4:750-752.
- Phatak S, Gupta L, Aggarwal A. A young woman with panniculitis and cytopenia who later developed coagulopathy. J Assoc Physicians India. 2016;64:65-67.
- Pongpairoj K, Rerknimitr P, Wititsuwannakul J, et al. Eruptive telangiectasia in a patient with fever and haemophagocytic syndrome. Clin Exp Dermatol. 2016;41:696-698. doi:10.1111/ced.12859
- Shimizu Y, Tanae K, Takahashi N, et al. Primary cutaneous anaplastic large-cell lymphoma presenting with hemophagocytic syndrome: a case report and review of the literature. Leuk Res. 2010;34:263-266. doi:10.1016/j.leukres.2009.07.001
- Sirka CS, Pradhan S, Patra S, et al. Hemophagocytic lymphohistiocytosis: a rare, potentially fatal complication in subcutaneous panniculitis like T cell lymphoma. Indian J Dermatol Venereol Leprol. 2019;5:481-485.
- Chin CK, Hall S, Green C, et al. Secondary haemophagocytic lymphohistiocytosis due to checkpoint inhibitor therapy. Eur J Cancer. 2019;115: 84-87. doi:10.1016/j.ejca.2019.04.026
- Dudda M, Mann C, Heinz J, et al. Hemophagocytic lymphohistiocytosis of a melanoma patient under BRAF/MEK-inhibitor therapy following anti-PD1 inhibitor treatment: a case report and review to the literature. Melanoma Res. 2021;31:81-84. doi:10.1097 /cmr.0000000000000703
- Mizuta H, Nakano E, Takahashi A, et al. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: a case report and literature review. Dermatol Ther. 2020;33:e13321. doi:10.1111/dth.13321
- Satzger I, Ivanyi P, Länger F, et al. Treatment-related hemophagocytic lymphohistiocytosis secondary to checkpoint inhibition with nivolumab plus ipilimumab. Eur J Cancer. 2018;93:150-153. doi:10.1016/j.ejca.2018.01.063
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72-90. doi:10.1016/J.EJCA.2019.07.014
- Choi S, Zhou M, Bahrani E, et al. Rare and fatal complication of immune checkpoint inhibition: a case report of haemophagocytic lymphohistiocytosis with severe lichenoid dermatitis. Br J Haematol. 2021;193:e44-e47. doi:10.1111/BJH.17442
- Yang JJ, Lei DK, Ravi V, et al. Overlap between hemophagocytic lymphohistiocytosis and drug reaction and eosinophilia with systemic symptoms: a review. Int J Dermatol. 2021;60:925-932. doi:10.1111 /ijd.15196
- Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. doi:10.1002/cncr.20502
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
PRACTICE POINTS
- Hemophagocytic lymphohistiocytosis (HLH) is a complex, life-threatening immunologic condition that is associated with various diagnostic tools.
- Physicians who care for patients with HLH should know that skin findings are not uncommon but are largely nonspecific and can be a direct result of HLH itself, systemic complications, or the underlying etiology of the condition.
- There is a myriad of cutaneous findings that can manifest in adult patients with HLH. Awareness of HLH-associated dermatologic conditions and available diagnostic tools among multidisciplinary teams will aid in diagnosis.
Cyclically Bleeding Umbilical Papules
Cyclically Bleeding Umbilical Papules
THE DIAGNOSIS: Cutaneous Endometriosis
On histopathology, a biopsy specimen of an umbilical papule showed a dermal lymphohistiocyticrich infiltrate, hemorrhage, and ectopic endometrial glands consistent with cutaneous endometriosis (CE)(Figure). Cutaneous endometriosis is a rare condition that typically affects females of reproductive potential and is characterized by endometrial glands and stroma within the dermis and hypodermis. Cutaneous endometriosis is classified as primary or secondary. There is no surgical history of the abdomen or pelvis in primary CE. In contrast, a history of abdominopelvic surgery is the defining characteristic of secondary CE, which is more common than primary CE and typically manifests as painful red, brown, or purple papules along preexisting surgical scars of the umbilicus, lower abdomen, or pelvic region.1 Our patient may have developed secondary CE related to the laparoscopic cholecystectomy performed 10 years prior. Surgical excision is considered the definitive treatment for CE, and hormonal therapy with danazol or leuprolide may help ameliorate symptoms.1 Our patient deferred any hormonal or surgical interventions to undergo fertility treatments for pregnancy.
Cyclical bleeding and pain that coincides with menstruation is consistent with CE; however, cyclical symptoms are not always present, which can lead to delayed or incorrect diagnosis. Biopsy and histopathologic analysis are required for definitive diagnosis and are critical for distinguishing CE from other conditions. The differential diagnosis in our patient included pyogenic granuloma, dermatofibrosarcoma protuberans, keloid, and cutaneous metastasis of a primary malignancy. Vascular lesions such as pyogenic granuloma can manifest with bleeding but have a characteristic histopathologic lobular capillary arrangement that was not present in our patient.
Dermatofibrosarcoma protuberans is a rare, slow-growing, malignant soft-tissue sarcoma that most commonly manifests on the trunk, arms, and legs.2 It is characterized by a slow-growing, indurated plaque that often is present for years and may suddenly progress into a smooth, red-brown, multinodular mass. Histopathology typically shows spindle cells infiltrating the dermis and subcutaneous tissue in storiform or whorled pattern with variations based on the tumor stage, as well as diffuse CD34 immunoreactivity.2
Keloids are dense, raised, hyperpigmented, fibrous nodules—sometimes with accompanying telangiectasias—that typically grow secondary to trauma and project past the boundaries of the initial trauma site.1 Keloids are more commonly seen in individuals with darker skin types and tend to grow larger in this population. Histopathology reveals thickened hyalinized collagen bundles, which were not seen in our patient.1
Metastatic skin lesions of the umbilicus are rare but can arise from internal malignancies including cancers of the lung, colon, and breast.3 We considered Sister Mary Joseph nodule, which is caused most commonly by metastasis of a primary gastrointestinal cancer and signifies poor prognosis. The histopathology of metastatic lesions would reveal the presence of atypical cells with cancer-specific markers. Histopathology along with the patient’s personal and family history, a comprehensive review of symptoms, and cancer screening may help with reaching the correct diagnosis.
The average duration between abdominopelvic surgery and onset of secondary CE symptoms is 3.7 to 5.3 years.4 Our patient presented 10 years post surgery and after cessation of oral contraception, which may suggest a potential role of hormonal contraception in delayed CE onset. Diagnosis of CE can be challenging due to atypical signs or symptoms, delayed onset, and lack of awareness among health care professionals. Patients with delayed diagnosis may endure multiple procedures, prolonged physical pain, and emotional distress. Furthermore, 30% to 50% of females with endometriosis experience infertility. Delayed diagnosis of CE compounded with associated age-related increase in oocyte atresia could potentially worsen fecundity as patients age.5 It is important to consider CE in the differential diagnosis of females of reproductive age who present with cyclical bleeding and abdominal or umbilical nodules.
- James WD, Elston D, Treat JR, et al. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2019. Accessed March 19, 2024. https://search.worldcat.org/title/1084979207
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Komurcugil I, Arslan Z, Bal ZI, et al. Cutaneous metastases different clinical presentations: case series and review of the literature. Dermatol Reports. 2022;15:9553.
- Marras S, Pluchino N, Petignat P, et al. Abdominal wall endometriosis: an 11-year retrospective observational cohort study. Published online September 16, 2019. Eur J Obstet Gynecol Reprod Biol X.
- Missmer SA, Hankinson SE, Spiegelman D, et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
THE DIAGNOSIS: Cutaneous Endometriosis
On histopathology, a biopsy specimen of an umbilical papule showed a dermal lymphohistiocyticrich infiltrate, hemorrhage, and ectopic endometrial glands consistent with cutaneous endometriosis (CE)(Figure). Cutaneous endometriosis is a rare condition that typically affects females of reproductive potential and is characterized by endometrial glands and stroma within the dermis and hypodermis. Cutaneous endometriosis is classified as primary or secondary. There is no surgical history of the abdomen or pelvis in primary CE. In contrast, a history of abdominopelvic surgery is the defining characteristic of secondary CE, which is more common than primary CE and typically manifests as painful red, brown, or purple papules along preexisting surgical scars of the umbilicus, lower abdomen, or pelvic region.1 Our patient may have developed secondary CE related to the laparoscopic cholecystectomy performed 10 years prior. Surgical excision is considered the definitive treatment for CE, and hormonal therapy with danazol or leuprolide may help ameliorate symptoms.1 Our patient deferred any hormonal or surgical interventions to undergo fertility treatments for pregnancy.

Cyclical bleeding and pain that coincides with menstruation is consistent with CE; however, cyclical symptoms are not always present, which can lead to delayed or incorrect diagnosis. Biopsy and histopathologic analysis are required for definitive diagnosis and are critical for distinguishing CE from other conditions. The differential diagnosis in our patient included pyogenic granuloma, dermatofibrosarcoma protuberans, keloid, and cutaneous metastasis of a primary malignancy. Vascular lesions such as pyogenic granuloma can manifest with bleeding but have a characteristic histopathologic lobular capillary arrangement that was not present in our patient.
Dermatofibrosarcoma protuberans is a rare, slow-growing, malignant soft-tissue sarcoma that most commonly manifests on the trunk, arms, and legs.2 It is characterized by a slow-growing, indurated plaque that often is present for years and may suddenly progress into a smooth, red-brown, multinodular mass. Histopathology typically shows spindle cells infiltrating the dermis and subcutaneous tissue in storiform or whorled pattern with variations based on the tumor stage, as well as diffuse CD34 immunoreactivity.2
Keloids are dense, raised, hyperpigmented, fibrous nodules—sometimes with accompanying telangiectasias—that typically grow secondary to trauma and project past the boundaries of the initial trauma site.1 Keloids are more commonly seen in individuals with darker skin types and tend to grow larger in this population. Histopathology reveals thickened hyalinized collagen bundles, which were not seen in our patient.1
Metastatic skin lesions of the umbilicus are rare but can arise from internal malignancies including cancers of the lung, colon, and breast.3 We considered Sister Mary Joseph nodule, which is caused most commonly by metastasis of a primary gastrointestinal cancer and signifies poor prognosis. The histopathology of metastatic lesions would reveal the presence of atypical cells with cancer-specific markers. Histopathology along with the patient’s personal and family history, a comprehensive review of symptoms, and cancer screening may help with reaching the correct diagnosis.
The average duration between abdominopelvic surgery and onset of secondary CE symptoms is 3.7 to 5.3 years.4 Our patient presented 10 years post surgery and after cessation of oral contraception, which may suggest a potential role of hormonal contraception in delayed CE onset. Diagnosis of CE can be challenging due to atypical signs or symptoms, delayed onset, and lack of awareness among health care professionals. Patients with delayed diagnosis may endure multiple procedures, prolonged physical pain, and emotional distress. Furthermore, 30% to 50% of females with endometriosis experience infertility. Delayed diagnosis of CE compounded with associated age-related increase in oocyte atresia could potentially worsen fecundity as patients age.5 It is important to consider CE in the differential diagnosis of females of reproductive age who present with cyclical bleeding and abdominal or umbilical nodules.
THE DIAGNOSIS: Cutaneous Endometriosis
On histopathology, a biopsy specimen of an umbilical papule showed a dermal lymphohistiocyticrich infiltrate, hemorrhage, and ectopic endometrial glands consistent with cutaneous endometriosis (CE)(Figure). Cutaneous endometriosis is a rare condition that typically affects females of reproductive potential and is characterized by endometrial glands and stroma within the dermis and hypodermis. Cutaneous endometriosis is classified as primary or secondary. There is no surgical history of the abdomen or pelvis in primary CE. In contrast, a history of abdominopelvic surgery is the defining characteristic of secondary CE, which is more common than primary CE and typically manifests as painful red, brown, or purple papules along preexisting surgical scars of the umbilicus, lower abdomen, or pelvic region.1 Our patient may have developed secondary CE related to the laparoscopic cholecystectomy performed 10 years prior. Surgical excision is considered the definitive treatment for CE, and hormonal therapy with danazol or leuprolide may help ameliorate symptoms.1 Our patient deferred any hormonal or surgical interventions to undergo fertility treatments for pregnancy.

Cyclical bleeding and pain that coincides with menstruation is consistent with CE; however, cyclical symptoms are not always present, which can lead to delayed or incorrect diagnosis. Biopsy and histopathologic analysis are required for definitive diagnosis and are critical for distinguishing CE from other conditions. The differential diagnosis in our patient included pyogenic granuloma, dermatofibrosarcoma protuberans, keloid, and cutaneous metastasis of a primary malignancy. Vascular lesions such as pyogenic granuloma can manifest with bleeding but have a characteristic histopathologic lobular capillary arrangement that was not present in our patient.
Dermatofibrosarcoma protuberans is a rare, slow-growing, malignant soft-tissue sarcoma that most commonly manifests on the trunk, arms, and legs.2 It is characterized by a slow-growing, indurated plaque that often is present for years and may suddenly progress into a smooth, red-brown, multinodular mass. Histopathology typically shows spindle cells infiltrating the dermis and subcutaneous tissue in storiform or whorled pattern with variations based on the tumor stage, as well as diffuse CD34 immunoreactivity.2
Keloids are dense, raised, hyperpigmented, fibrous nodules—sometimes with accompanying telangiectasias—that typically grow secondary to trauma and project past the boundaries of the initial trauma site.1 Keloids are more commonly seen in individuals with darker skin types and tend to grow larger in this population. Histopathology reveals thickened hyalinized collagen bundles, which were not seen in our patient.1
Metastatic skin lesions of the umbilicus are rare but can arise from internal malignancies including cancers of the lung, colon, and breast.3 We considered Sister Mary Joseph nodule, which is caused most commonly by metastasis of a primary gastrointestinal cancer and signifies poor prognosis. The histopathology of metastatic lesions would reveal the presence of atypical cells with cancer-specific markers. Histopathology along with the patient’s personal and family history, a comprehensive review of symptoms, and cancer screening may help with reaching the correct diagnosis.
The average duration between abdominopelvic surgery and onset of secondary CE symptoms is 3.7 to 5.3 years.4 Our patient presented 10 years post surgery and after cessation of oral contraception, which may suggest a potential role of hormonal contraception in delayed CE onset. Diagnosis of CE can be challenging due to atypical signs or symptoms, delayed onset, and lack of awareness among health care professionals. Patients with delayed diagnosis may endure multiple procedures, prolonged physical pain, and emotional distress. Furthermore, 30% to 50% of females with endometriosis experience infertility. Delayed diagnosis of CE compounded with associated age-related increase in oocyte atresia could potentially worsen fecundity as patients age.5 It is important to consider CE in the differential diagnosis of females of reproductive age who present with cyclical bleeding and abdominal or umbilical nodules.
- James WD, Elston D, Treat JR, et al. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2019. Accessed March 19, 2024. https://search.worldcat.org/title/1084979207
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Komurcugil I, Arslan Z, Bal ZI, et al. Cutaneous metastases different clinical presentations: case series and review of the literature. Dermatol Reports. 2022;15:9553.
- Marras S, Pluchino N, Petignat P, et al. Abdominal wall endometriosis: an 11-year retrospective observational cohort study. Published online September 16, 2019. Eur J Obstet Gynecol Reprod Biol X.
- Missmer SA, Hankinson SE, Spiegelman D, et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- James WD, Elston D, Treat JR, et al. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2019. Accessed March 19, 2024. https://search.worldcat.org/title/1084979207
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Komurcugil I, Arslan Z, Bal ZI, et al. Cutaneous metastases different clinical presentations: case series and review of the literature. Dermatol Reports. 2022;15:9553.
- Marras S, Pluchino N, Petignat P, et al. Abdominal wall endometriosis: an 11-year retrospective observational cohort study. Published online September 16, 2019. Eur J Obstet Gynecol Reprod Biol X.
- Missmer SA, Hankinson SE, Spiegelman D, et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
Cyclically Bleeding Umbilical Papules
Cyclically Bleeding Umbilical Papules
A 38-year-old nulligravid female with menorrhagia and dysmenorrhea presented with cyclical umbilical bleeding of 1 year’s duration. Shortly before the onset of symptoms, the patient had discontinued oral contraceptive therapy with the intent to become pregnant. She had an uncomplicated laparoscopic cholecystectomy 10 years prior, but her medical history was otherwise unremarkable. At the current presentation, physical examination revealed multilobular brown papules with serosanguineous crusting in the umbilicus.
Dupilumab in the Treatment of Pemphigoid Gestationis
Dupilumab in the Treatment of Pemphigoid Gestationis
Pemphigoid gestationis (PG), which manifests in the second or third trimester of pregnancy, is thought to result from an excessive type 2 inflammatory response that leads to the formation of antibodies primarily targeting BP180 antigens with resultant damage to the skin basement membrane.1 Maternal antibodies can be transferred to the fetus, resulting in neonatal pemphigoid with the development of widespread vesicles and bullae.2 Maternal morbidity from placental insufficiency, intrauterine growth restriction, and premature labor are common comorbidities of PG, underscoring the critical need for safe and effective treatments for this condition.3
Systemic corticosteroids currently are the first-line treatment for moderate to severe PG but carry considerable risks to both the mother and fetus, including preterm labor and intrauterine growth restriction.4,5 Dupilumab is approved by the US Food and Drug Administration for moderate to severe atopic dermatitis in children aged 6 months and older. Dupilumab inhibits downstream signaling of IL-4Rα, reducing IL-4 and IL-13. Use of dupilumab to target the type 2 inflammatory response has shown significant promise in the treatment of BP, where it met primary and secondary endpoints in adults with moderate to severe disease, but studies in PG are limited.6-8 There are multiple reports in the literature demonstrating the safety of dupilumab in pregnancy and postpartum,9-27 including a pharmacovigilance report that found no adverse drug reactions from dupilumab reported during pregnancy.9 There also are 4 reports of pregnant patients who were diagnosed with PG and treated with dupilumab, all of whom were initially started on prednisone prior to treatment initiation.9-12 In this article, we report 2 additional cases of dupilumab treatment in patients with PG.
Case Reports
Patient 1—A 39-year-old G5P1 woman presented to the dermatology department at 27.5 weeks’ gestation with a widespread eruption of erythematous, annular, urticarial, edematous papules and plaques on the abdomen of 4 weeks’ duration (Figure 1A). Direct immunofluorescence was positive, indirect immunofluorescence confirmed an IgG-positive epidermal pattern, and serum BP180 levels were elevated, supporting a diagnosis of PG. The patient was prescribed prednisone (60 mg/d) but developed type 1 diabetes mellitus after 1 week of treatment. Following insurance approval, dupilumab therapy was initiated 3 weeks later at a dose of 300 mg subcutaneously every 2 weeks. Rapid and complete resolution of papules and plaques as well as symptomatic relief from pruritus was noted within 2 weeks of treatment (Figure 1B). The prednisone dose was tapered to 2.5 mg every other day at 6 weeks prior to induction of labor; the diabetes resolved 7 weeks after initiation of dupilumab.
At the recommendation of the patient’s high-risk maternal-fetal medicine team, 100 mg of stress-dose hydrocortisone was administered intravenously just prior to delivery to prevent flaring of PG. She delivered a healthy infant at 37 weeks and 3 days’ gestation without bullous disease and was discharged from the hospital the day after delivery on a prednisone dose of 2.5 mg every other day.
The patient subsequently developed localized pruritic papules on the hands and feet at 2 weeks postpartum. Based on shared decision-making and the patient’s concern for the severity of the previous pruritic eruption, prednisone was increased to 10 mg daily for 5 days and then was tapered over 2 weeks without flaring. Dupilumab was continued until 12 weeks postpartum with complete resolution of PG and no further sequelae.
Patient 2—A 30-year-old G1P0 woman presented to the dermatology department at 25 weeks’ gestation with a widespread eruption of 1 week’s duration on the abdomen, hands, thighs, legs, buttocks, and feet that was clinically consistent with PG (Figure 2A). Direct immunofluorescence was positive, indirect immunofluorescence showed an IgG-positive epidermal pattern, and an enzyme-linked immunosorbent assay for BP180 was elevated, confirming a diagnosis of PG. The patient was started on 40 mg of prednisone and topical steroids daily, with improvement of the pruritus but persistence of the eruption after 3 to 4 days. Five days after the initial presentation following expedited insurance approval, dupilumab 300 mg was initiated subcutaneously every 2 weeks along with a slow taper of prednisone to 5 mg, with complete clearance of the eruption within 4 weeks (Figure 2B). She delivered a healthy infant at 38 weeks’ gestation without bullous disease.
In contrast to patient 1, this patient did not receive corticosteroids at the time of delivery and did not experience flaring of her disease. The patient remained on dupilumab 5 weeks postpartum without subsequent recurrence after treatment discontinuation.
Comment
Although a myriad of effective treatments exist for bullous pemphigoid, there are very few options for PG due to the need for treatment during pregnancy. Systemic corticosteroids—the treatment of choice in severe PG disease—are not without risk in pregnancy and complicate assessment of morbidity, as both PG and chronic steroid exposure are associated with preterm labor and intrauterine growth restriction.3
Dupilumab currently is undergoing phase III trials (Clinicaltrials.gov identifiers NCT02277743 and NCT02277769) for the treatment of bullous pemphigoid, with interim reports suggesting efficacy across all primary and key secondary endpoints in moderate to severe disease, including notable steroid-sparing effects.8 In our patients, treatment with dupilumab resulted in resolution of cutaneous disease and was well tolerated, facilitating the tapering of corticosteroids and resolution of type 1 diabetes in patient 1. Although the response to dupilumab in both cases may have been confounded by concomitant steroid administration, which was started due to the severity of symptoms and uncertainty regarding insurance approval, the dose was tapered in both patients after initiation of dupilumab. Patient 1 was given a stress dose of hydrocortisone during delivery and developed a mild flare following delivery, consistent with previous literature.28, 29 Because the flare was localized to the hands and feet, she might have responded to clobetasol in addition to dupilumab, but given the severity of disease at presentation and her concern that it might worsen, low-dose prednisone was added with resolution of the flare within 2 weeks.
Dupilumab dosing regimens have not been studied in a controlled prospective manner for PG. We acknowledge that dupilumab (at least using the conventional atopic dermatitis dosing regimen) may be insufficient as monotherapy to control PG, as both patients received steroids prior to initiation of dupilumab, in part due to concern that the insurance might delay or deny approval. Previous World Health Organization vigilance reporting has suggested that dupilumab appears safe during pregnancy although it lacks pregnancy categorization in the United States due to limited studies in this population.9-28 This observation supports the conclusion that, like bullous pemphigoid, PG also is driven by Th2–mediated inflammation. Treatment with dupilumab may be safe and effective in pregnancy, reducing maternal complications from long-term corticosteroids. Additional studies are needed to confirm these hypotheses.
- Vičić M, MarinoviĆ B. Autoimmune bullous diseases in pregnancy: an overview of pathogenesis, clinical presentations, diagnostics and available therapies. Ital J Dermatol Venerol. 2023;158:99-109. doi:10.23736/ S2784-8671.23.07553-9
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly follow-up of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168- 1172. doi:10.1001/archderm.143.9.1168
- Patsatsi A, Marinovic B, Murrell D. Autoimmune bullous diseases during pregnancy: solving common and uncommon issues. Int J Womens Dermatol. 2019;5:166-170. doi:10.1016/j.ijwd.2019.01.003
- Genovese G, Derlino F, Cerri A, et al. A systematic review of treatment options and clinical outcomes in pemphigoid gestationis. Front Med (Lausanne). 2020;7:604945. doi:10.3389/fmed.2020.604945
- Tavakolpour S, Mirsafaei HS, Delshad S. Management of pemphigus disease in pregnancy. Am J Reprod Immunol. 2017;77. doi:10.1111/aji.12601
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. 2022;13:928621. doi:10.3389/fimmu.2022.928621
- Zhang Y, Xu Q, Chen L, et al. Efficacy and safety of dupilumab in moderate- to-severe bullous pemphigoid. Front Immunol. 2021;12: 738907. doi:10.3389/fimmu.2021.738907
- Dupixent is the first and only biologic to achieve significant improvements in disease remission and symptoms in bullous pemphigoid positive pivotal study. News release. Sanofi. September 11, 2024. Accessed February 17, 2025. https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-11-05-00-00-2944237
- Khamisy-Farah R, Damiani G, Kong JD, et al. Safety profile of dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBase™). Eur Rev Med Pharmacol Sci. 2021;25:5448-5451. doi:10.26355/eurrev_202109_26652
- Avallone G, Cavallo F, Tancredi A, et al. Association between maternal dupilumab exposure and pregnancy outcomes in patients with moderate-to-severe atopic dermatitis: a nationwide retrospective cohort study. J Eur Acad Dermatol Venereol. 2024;38:1799 -1808. doi:10.1111/jdv.19794
- Chen RE, Yokoyama CC, Anadkat MJ. Pemphigoid gestationis treated with dupilumab. JAAD Case Rep. 2023;41:10-12. doi:10.1016/ j.jdcr.2023.08.013
- Liu Y, Yuan J, Xia Y, et al. A case of pemphigoid gestationis successfully treated with dupilumab. J Eur Acad Dermatol Venereol. 2023;37:E1164-E1165. doi:10.1111/jdv.19171
- Alvarez Martinez D, Russo G, Fontao L, et al. Successful therapy of pemphigoid gestationis with dupilumab—a new case. J Eur Acad Dermatol Venereol. 2023;37:E752-E753. doi:10.1111/jdv.18911
- Riquelme-Mc Loughlin C, Mascaró JM Jr. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46:1578-1579. doi:10.1111/ced.14765
- Adam DN, Gooderham MJ, Beecker JR, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatol Venereol. 2023;37:1135-1148. doi:10.1111/jdv.18922
- Akhtar NH, Khosravi-Hafshejani T, Akhtar D, et al. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol. 2022;18:9. doi:10.1186 /s13223-022-00650-w
- Bosma AL, Gerbens LAA, Middelkamp-Hup MA, et al. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol. 2021;46:1089-1092. doi:10.1111 /ced.14725
- Chan TC, Wu NL, Wong LS, et al. Taiwanese dermatological association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. 2021;120:429-442. doi:10.101 6/j.jfma.2020.06.008
- Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022;47:960-961. doi:10.1111/ced.15049
- Gracia-Darder I, Pons De Ves J, Reyero Cortina M, et al. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022;35:E15237. doi:10.1111/dth.15237
- Heilskov S, Deleuran MS, Vestergaard C. Immunosuppressive and immunomodulating therapy for atopic dermatitis in pregnancy: an appraisal of the literature. Dermatol Ther (Heidelb). 2020;10:1215-1228. doi:10.1007/s13555-020-00457-w
- Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34:E256-E257. doi:10.1111/jdv.16235
- Kage P, Simon JC, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021;48:E484-E485. doi:10.1111/1346-8138.16033
- Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13:248-256. doi:10.1159/000515246
- Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020;6:1051-1052. doi:10.1016/j.jdcr.2020.08.001
- Napolitano M, Ruggiero A, Fontanella G, et al. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther. 2021;34:E14475. doi:10.1111/dth.14475
- Vestergaard C, Wollenberg A, Barbarot S, et al. European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol. 2019;33:1644-1659. doi:10.1111/jdv.15709
- Minakawa S, Kaneko T, Rokunohe D, et al. Pemphigoid gestationis with prepartum flare. J Dermatol. 2014;41:850-851. doi:10.1111 /1346-8138.12576
- Baxi LV, Kovilam OP, Collins MH, et al. Recurrent herpes gestationis with postpartum flare: a case report. Am J Obstet Gynecol. 1991;164: 778-780. doi:10.1016/0002-9378(91)90514-r
Pemphigoid gestationis (PG), which manifests in the second or third trimester of pregnancy, is thought to result from an excessive type 2 inflammatory response that leads to the formation of antibodies primarily targeting BP180 antigens with resultant damage to the skin basement membrane.1 Maternal antibodies can be transferred to the fetus, resulting in neonatal pemphigoid with the development of widespread vesicles and bullae.2 Maternal morbidity from placental insufficiency, intrauterine growth restriction, and premature labor are common comorbidities of PG, underscoring the critical need for safe and effective treatments for this condition.3
Systemic corticosteroids currently are the first-line treatment for moderate to severe PG but carry considerable risks to both the mother and fetus, including preterm labor and intrauterine growth restriction.4,5 Dupilumab is approved by the US Food and Drug Administration for moderate to severe atopic dermatitis in children aged 6 months and older. Dupilumab inhibits downstream signaling of IL-4Rα, reducing IL-4 and IL-13. Use of dupilumab to target the type 2 inflammatory response has shown significant promise in the treatment of BP, where it met primary and secondary endpoints in adults with moderate to severe disease, but studies in PG are limited.6-8 There are multiple reports in the literature demonstrating the safety of dupilumab in pregnancy and postpartum,9-27 including a pharmacovigilance report that found no adverse drug reactions from dupilumab reported during pregnancy.9 There also are 4 reports of pregnant patients who were diagnosed with PG and treated with dupilumab, all of whom were initially started on prednisone prior to treatment initiation.9-12 In this article, we report 2 additional cases of dupilumab treatment in patients with PG.
Case Reports
Patient 1—A 39-year-old G5P1 woman presented to the dermatology department at 27.5 weeks’ gestation with a widespread eruption of erythematous, annular, urticarial, edematous papules and plaques on the abdomen of 4 weeks’ duration (Figure 1A). Direct immunofluorescence was positive, indirect immunofluorescence confirmed an IgG-positive epidermal pattern, and serum BP180 levels were elevated, supporting a diagnosis of PG. The patient was prescribed prednisone (60 mg/d) but developed type 1 diabetes mellitus after 1 week of treatment. Following insurance approval, dupilumab therapy was initiated 3 weeks later at a dose of 300 mg subcutaneously every 2 weeks. Rapid and complete resolution of papules and plaques as well as symptomatic relief from pruritus was noted within 2 weeks of treatment (Figure 1B). The prednisone dose was tapered to 2.5 mg every other day at 6 weeks prior to induction of labor; the diabetes resolved 7 weeks after initiation of dupilumab.

At the recommendation of the patient’s high-risk maternal-fetal medicine team, 100 mg of stress-dose hydrocortisone was administered intravenously just prior to delivery to prevent flaring of PG. She delivered a healthy infant at 37 weeks and 3 days’ gestation without bullous disease and was discharged from the hospital the day after delivery on a prednisone dose of 2.5 mg every other day.
The patient subsequently developed localized pruritic papules on the hands and feet at 2 weeks postpartum. Based on shared decision-making and the patient’s concern for the severity of the previous pruritic eruption, prednisone was increased to 10 mg daily for 5 days and then was tapered over 2 weeks without flaring. Dupilumab was continued until 12 weeks postpartum with complete resolution of PG and no further sequelae.
Patient 2—A 30-year-old G1P0 woman presented to the dermatology department at 25 weeks’ gestation with a widespread eruption of 1 week’s duration on the abdomen, hands, thighs, legs, buttocks, and feet that was clinically consistent with PG (Figure 2A). Direct immunofluorescence was positive, indirect immunofluorescence showed an IgG-positive epidermal pattern, and an enzyme-linked immunosorbent assay for BP180 was elevated, confirming a diagnosis of PG. The patient was started on 40 mg of prednisone and topical steroids daily, with improvement of the pruritus but persistence of the eruption after 3 to 4 days. Five days after the initial presentation following expedited insurance approval, dupilumab 300 mg was initiated subcutaneously every 2 weeks along with a slow taper of prednisone to 5 mg, with complete clearance of the eruption within 4 weeks (Figure 2B). She delivered a healthy infant at 38 weeks’ gestation without bullous disease.

In contrast to patient 1, this patient did not receive corticosteroids at the time of delivery and did not experience flaring of her disease. The patient remained on dupilumab 5 weeks postpartum without subsequent recurrence after treatment discontinuation.
Comment
Although a myriad of effective treatments exist for bullous pemphigoid, there are very few options for PG due to the need for treatment during pregnancy. Systemic corticosteroids—the treatment of choice in severe PG disease—are not without risk in pregnancy and complicate assessment of morbidity, as both PG and chronic steroid exposure are associated with preterm labor and intrauterine growth restriction.3
Dupilumab currently is undergoing phase III trials (Clinicaltrials.gov identifiers NCT02277743 and NCT02277769) for the treatment of bullous pemphigoid, with interim reports suggesting efficacy across all primary and key secondary endpoints in moderate to severe disease, including notable steroid-sparing effects.8 In our patients, treatment with dupilumab resulted in resolution of cutaneous disease and was well tolerated, facilitating the tapering of corticosteroids and resolution of type 1 diabetes in patient 1. Although the response to dupilumab in both cases may have been confounded by concomitant steroid administration, which was started due to the severity of symptoms and uncertainty regarding insurance approval, the dose was tapered in both patients after initiation of dupilumab. Patient 1 was given a stress dose of hydrocortisone during delivery and developed a mild flare following delivery, consistent with previous literature.28, 29 Because the flare was localized to the hands and feet, she might have responded to clobetasol in addition to dupilumab, but given the severity of disease at presentation and her concern that it might worsen, low-dose prednisone was added with resolution of the flare within 2 weeks.
Dupilumab dosing regimens have not been studied in a controlled prospective manner for PG. We acknowledge that dupilumab (at least using the conventional atopic dermatitis dosing regimen) may be insufficient as monotherapy to control PG, as both patients received steroids prior to initiation of dupilumab, in part due to concern that the insurance might delay or deny approval. Previous World Health Organization vigilance reporting has suggested that dupilumab appears safe during pregnancy although it lacks pregnancy categorization in the United States due to limited studies in this population.9-28 This observation supports the conclusion that, like bullous pemphigoid, PG also is driven by Th2–mediated inflammation. Treatment with dupilumab may be safe and effective in pregnancy, reducing maternal complications from long-term corticosteroids. Additional studies are needed to confirm these hypotheses.
Pemphigoid gestationis (PG), which manifests in the second or third trimester of pregnancy, is thought to result from an excessive type 2 inflammatory response that leads to the formation of antibodies primarily targeting BP180 antigens with resultant damage to the skin basement membrane.1 Maternal antibodies can be transferred to the fetus, resulting in neonatal pemphigoid with the development of widespread vesicles and bullae.2 Maternal morbidity from placental insufficiency, intrauterine growth restriction, and premature labor are common comorbidities of PG, underscoring the critical need for safe and effective treatments for this condition.3
Systemic corticosteroids currently are the first-line treatment for moderate to severe PG but carry considerable risks to both the mother and fetus, including preterm labor and intrauterine growth restriction.4,5 Dupilumab is approved by the US Food and Drug Administration for moderate to severe atopic dermatitis in children aged 6 months and older. Dupilumab inhibits downstream signaling of IL-4Rα, reducing IL-4 and IL-13. Use of dupilumab to target the type 2 inflammatory response has shown significant promise in the treatment of BP, where it met primary and secondary endpoints in adults with moderate to severe disease, but studies in PG are limited.6-8 There are multiple reports in the literature demonstrating the safety of dupilumab in pregnancy and postpartum,9-27 including a pharmacovigilance report that found no adverse drug reactions from dupilumab reported during pregnancy.9 There also are 4 reports of pregnant patients who were diagnosed with PG and treated with dupilumab, all of whom were initially started on prednisone prior to treatment initiation.9-12 In this article, we report 2 additional cases of dupilumab treatment in patients with PG.
Case Reports
Patient 1—A 39-year-old G5P1 woman presented to the dermatology department at 27.5 weeks’ gestation with a widespread eruption of erythematous, annular, urticarial, edematous papules and plaques on the abdomen of 4 weeks’ duration (Figure 1A). Direct immunofluorescence was positive, indirect immunofluorescence confirmed an IgG-positive epidermal pattern, and serum BP180 levels were elevated, supporting a diagnosis of PG. The patient was prescribed prednisone (60 mg/d) but developed type 1 diabetes mellitus after 1 week of treatment. Following insurance approval, dupilumab therapy was initiated 3 weeks later at a dose of 300 mg subcutaneously every 2 weeks. Rapid and complete resolution of papules and plaques as well as symptomatic relief from pruritus was noted within 2 weeks of treatment (Figure 1B). The prednisone dose was tapered to 2.5 mg every other day at 6 weeks prior to induction of labor; the diabetes resolved 7 weeks after initiation of dupilumab.

At the recommendation of the patient’s high-risk maternal-fetal medicine team, 100 mg of stress-dose hydrocortisone was administered intravenously just prior to delivery to prevent flaring of PG. She delivered a healthy infant at 37 weeks and 3 days’ gestation without bullous disease and was discharged from the hospital the day after delivery on a prednisone dose of 2.5 mg every other day.
The patient subsequently developed localized pruritic papules on the hands and feet at 2 weeks postpartum. Based on shared decision-making and the patient’s concern for the severity of the previous pruritic eruption, prednisone was increased to 10 mg daily for 5 days and then was tapered over 2 weeks without flaring. Dupilumab was continued until 12 weeks postpartum with complete resolution of PG and no further sequelae.
Patient 2—A 30-year-old G1P0 woman presented to the dermatology department at 25 weeks’ gestation with a widespread eruption of 1 week’s duration on the abdomen, hands, thighs, legs, buttocks, and feet that was clinically consistent with PG (Figure 2A). Direct immunofluorescence was positive, indirect immunofluorescence showed an IgG-positive epidermal pattern, and an enzyme-linked immunosorbent assay for BP180 was elevated, confirming a diagnosis of PG. The patient was started on 40 mg of prednisone and topical steroids daily, with improvement of the pruritus but persistence of the eruption after 3 to 4 days. Five days after the initial presentation following expedited insurance approval, dupilumab 300 mg was initiated subcutaneously every 2 weeks along with a slow taper of prednisone to 5 mg, with complete clearance of the eruption within 4 weeks (Figure 2B). She delivered a healthy infant at 38 weeks’ gestation without bullous disease.

In contrast to patient 1, this patient did not receive corticosteroids at the time of delivery and did not experience flaring of her disease. The patient remained on dupilumab 5 weeks postpartum without subsequent recurrence after treatment discontinuation.
Comment
Although a myriad of effective treatments exist for bullous pemphigoid, there are very few options for PG due to the need for treatment during pregnancy. Systemic corticosteroids—the treatment of choice in severe PG disease—are not without risk in pregnancy and complicate assessment of morbidity, as both PG and chronic steroid exposure are associated with preterm labor and intrauterine growth restriction.3
Dupilumab currently is undergoing phase III trials (Clinicaltrials.gov identifiers NCT02277743 and NCT02277769) for the treatment of bullous pemphigoid, with interim reports suggesting efficacy across all primary and key secondary endpoints in moderate to severe disease, including notable steroid-sparing effects.8 In our patients, treatment with dupilumab resulted in resolution of cutaneous disease and was well tolerated, facilitating the tapering of corticosteroids and resolution of type 1 diabetes in patient 1. Although the response to dupilumab in both cases may have been confounded by concomitant steroid administration, which was started due to the severity of symptoms and uncertainty regarding insurance approval, the dose was tapered in both patients after initiation of dupilumab. Patient 1 was given a stress dose of hydrocortisone during delivery and developed a mild flare following delivery, consistent with previous literature.28, 29 Because the flare was localized to the hands and feet, she might have responded to clobetasol in addition to dupilumab, but given the severity of disease at presentation and her concern that it might worsen, low-dose prednisone was added with resolution of the flare within 2 weeks.
Dupilumab dosing regimens have not been studied in a controlled prospective manner for PG. We acknowledge that dupilumab (at least using the conventional atopic dermatitis dosing regimen) may be insufficient as monotherapy to control PG, as both patients received steroids prior to initiation of dupilumab, in part due to concern that the insurance might delay or deny approval. Previous World Health Organization vigilance reporting has suggested that dupilumab appears safe during pregnancy although it lacks pregnancy categorization in the United States due to limited studies in this population.9-28 This observation supports the conclusion that, like bullous pemphigoid, PG also is driven by Th2–mediated inflammation. Treatment with dupilumab may be safe and effective in pregnancy, reducing maternal complications from long-term corticosteroids. Additional studies are needed to confirm these hypotheses.
- Vičić M, MarinoviĆ B. Autoimmune bullous diseases in pregnancy: an overview of pathogenesis, clinical presentations, diagnostics and available therapies. Ital J Dermatol Venerol. 2023;158:99-109. doi:10.23736/ S2784-8671.23.07553-9
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly follow-up of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168- 1172. doi:10.1001/archderm.143.9.1168
- Patsatsi A, Marinovic B, Murrell D. Autoimmune bullous diseases during pregnancy: solving common and uncommon issues. Int J Womens Dermatol. 2019;5:166-170. doi:10.1016/j.ijwd.2019.01.003
- Genovese G, Derlino F, Cerri A, et al. A systematic review of treatment options and clinical outcomes in pemphigoid gestationis. Front Med (Lausanne). 2020;7:604945. doi:10.3389/fmed.2020.604945
- Tavakolpour S, Mirsafaei HS, Delshad S. Management of pemphigus disease in pregnancy. Am J Reprod Immunol. 2017;77. doi:10.1111/aji.12601
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. 2022;13:928621. doi:10.3389/fimmu.2022.928621
- Zhang Y, Xu Q, Chen L, et al. Efficacy and safety of dupilumab in moderate- to-severe bullous pemphigoid. Front Immunol. 2021;12: 738907. doi:10.3389/fimmu.2021.738907
- Dupixent is the first and only biologic to achieve significant improvements in disease remission and symptoms in bullous pemphigoid positive pivotal study. News release. Sanofi. September 11, 2024. Accessed February 17, 2025. https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-11-05-00-00-2944237
- Khamisy-Farah R, Damiani G, Kong JD, et al. Safety profile of dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBase™). Eur Rev Med Pharmacol Sci. 2021;25:5448-5451. doi:10.26355/eurrev_202109_26652
- Avallone G, Cavallo F, Tancredi A, et al. Association between maternal dupilumab exposure and pregnancy outcomes in patients with moderate-to-severe atopic dermatitis: a nationwide retrospective cohort study. J Eur Acad Dermatol Venereol. 2024;38:1799 -1808. doi:10.1111/jdv.19794
- Chen RE, Yokoyama CC, Anadkat MJ. Pemphigoid gestationis treated with dupilumab. JAAD Case Rep. 2023;41:10-12. doi:10.1016/ j.jdcr.2023.08.013
- Liu Y, Yuan J, Xia Y, et al. A case of pemphigoid gestationis successfully treated with dupilumab. J Eur Acad Dermatol Venereol. 2023;37:E1164-E1165. doi:10.1111/jdv.19171
- Alvarez Martinez D, Russo G, Fontao L, et al. Successful therapy of pemphigoid gestationis with dupilumab—a new case. J Eur Acad Dermatol Venereol. 2023;37:E752-E753. doi:10.1111/jdv.18911
- Riquelme-Mc Loughlin C, Mascaró JM Jr. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46:1578-1579. doi:10.1111/ced.14765
- Adam DN, Gooderham MJ, Beecker JR, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatol Venereol. 2023;37:1135-1148. doi:10.1111/jdv.18922
- Akhtar NH, Khosravi-Hafshejani T, Akhtar D, et al. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol. 2022;18:9. doi:10.1186 /s13223-022-00650-w
- Bosma AL, Gerbens LAA, Middelkamp-Hup MA, et al. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol. 2021;46:1089-1092. doi:10.1111 /ced.14725
- Chan TC, Wu NL, Wong LS, et al. Taiwanese dermatological association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. 2021;120:429-442. doi:10.101 6/j.jfma.2020.06.008
- Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022;47:960-961. doi:10.1111/ced.15049
- Gracia-Darder I, Pons De Ves J, Reyero Cortina M, et al. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022;35:E15237. doi:10.1111/dth.15237
- Heilskov S, Deleuran MS, Vestergaard C. Immunosuppressive and immunomodulating therapy for atopic dermatitis in pregnancy: an appraisal of the literature. Dermatol Ther (Heidelb). 2020;10:1215-1228. doi:10.1007/s13555-020-00457-w
- Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34:E256-E257. doi:10.1111/jdv.16235
- Kage P, Simon JC, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021;48:E484-E485. doi:10.1111/1346-8138.16033
- Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13:248-256. doi:10.1159/000515246
- Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020;6:1051-1052. doi:10.1016/j.jdcr.2020.08.001
- Napolitano M, Ruggiero A, Fontanella G, et al. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther. 2021;34:E14475. doi:10.1111/dth.14475
- Vestergaard C, Wollenberg A, Barbarot S, et al. European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol. 2019;33:1644-1659. doi:10.1111/jdv.15709
- Minakawa S, Kaneko T, Rokunohe D, et al. Pemphigoid gestationis with prepartum flare. J Dermatol. 2014;41:850-851. doi:10.1111 /1346-8138.12576
- Baxi LV, Kovilam OP, Collins MH, et al. Recurrent herpes gestationis with postpartum flare: a case report. Am J Obstet Gynecol. 1991;164: 778-780. doi:10.1016/0002-9378(91)90514-r
- Vičić M, MarinoviĆ B. Autoimmune bullous diseases in pregnancy: an overview of pathogenesis, clinical presentations, diagnostics and available therapies. Ital J Dermatol Venerol. 2023;158:99-109. doi:10.23736/ S2784-8671.23.07553-9
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly follow-up of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168- 1172. doi:10.1001/archderm.143.9.1168
- Patsatsi A, Marinovic B, Murrell D. Autoimmune bullous diseases during pregnancy: solving common and uncommon issues. Int J Womens Dermatol. 2019;5:166-170. doi:10.1016/j.ijwd.2019.01.003
- Genovese G, Derlino F, Cerri A, et al. A systematic review of treatment options and clinical outcomes in pemphigoid gestationis. Front Med (Lausanne). 2020;7:604945. doi:10.3389/fmed.2020.604945
- Tavakolpour S, Mirsafaei HS, Delshad S. Management of pemphigus disease in pregnancy. Am J Reprod Immunol. 2017;77. doi:10.1111/aji.12601
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. 2022;13:928621. doi:10.3389/fimmu.2022.928621
- Zhang Y, Xu Q, Chen L, et al. Efficacy and safety of dupilumab in moderate- to-severe bullous pemphigoid. Front Immunol. 2021;12: 738907. doi:10.3389/fimmu.2021.738907
- Dupixent is the first and only biologic to achieve significant improvements in disease remission and symptoms in bullous pemphigoid positive pivotal study. News release. Sanofi. September 11, 2024. Accessed February 17, 2025. https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-11-05-00-00-2944237
- Khamisy-Farah R, Damiani G, Kong JD, et al. Safety profile of dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBase™). Eur Rev Med Pharmacol Sci. 2021;25:5448-5451. doi:10.26355/eurrev_202109_26652
- Avallone G, Cavallo F, Tancredi A, et al. Association between maternal dupilumab exposure and pregnancy outcomes in patients with moderate-to-severe atopic dermatitis: a nationwide retrospective cohort study. J Eur Acad Dermatol Venereol. 2024;38:1799 -1808. doi:10.1111/jdv.19794
- Chen RE, Yokoyama CC, Anadkat MJ. Pemphigoid gestationis treated with dupilumab. JAAD Case Rep. 2023;41:10-12. doi:10.1016/ j.jdcr.2023.08.013
- Liu Y, Yuan J, Xia Y, et al. A case of pemphigoid gestationis successfully treated with dupilumab. J Eur Acad Dermatol Venereol. 2023;37:E1164-E1165. doi:10.1111/jdv.19171
- Alvarez Martinez D, Russo G, Fontao L, et al. Successful therapy of pemphigoid gestationis with dupilumab—a new case. J Eur Acad Dermatol Venereol. 2023;37:E752-E753. doi:10.1111/jdv.18911
- Riquelme-Mc Loughlin C, Mascaró JM Jr. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46:1578-1579. doi:10.1111/ced.14765
- Adam DN, Gooderham MJ, Beecker JR, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatol Venereol. 2023;37:1135-1148. doi:10.1111/jdv.18922
- Akhtar NH, Khosravi-Hafshejani T, Akhtar D, et al. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol. 2022;18:9. doi:10.1186 /s13223-022-00650-w
- Bosma AL, Gerbens LAA, Middelkamp-Hup MA, et al. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol. 2021;46:1089-1092. doi:10.1111 /ced.14725
- Chan TC, Wu NL, Wong LS, et al. Taiwanese dermatological association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. 2021;120:429-442. doi:10.101 6/j.jfma.2020.06.008
- Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022;47:960-961. doi:10.1111/ced.15049
- Gracia-Darder I, Pons De Ves J, Reyero Cortina M, et al. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022;35:E15237. doi:10.1111/dth.15237
- Heilskov S, Deleuran MS, Vestergaard C. Immunosuppressive and immunomodulating therapy for atopic dermatitis in pregnancy: an appraisal of the literature. Dermatol Ther (Heidelb). 2020;10:1215-1228. doi:10.1007/s13555-020-00457-w
- Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34:E256-E257. doi:10.1111/jdv.16235
- Kage P, Simon JC, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021;48:E484-E485. doi:10.1111/1346-8138.16033
- Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13:248-256. doi:10.1159/000515246
- Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020;6:1051-1052. doi:10.1016/j.jdcr.2020.08.001
- Napolitano M, Ruggiero A, Fontanella G, et al. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther. 2021;34:E14475. doi:10.1111/dth.14475
- Vestergaard C, Wollenberg A, Barbarot S, et al. European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol. 2019;33:1644-1659. doi:10.1111/jdv.15709
- Minakawa S, Kaneko T, Rokunohe D, et al. Pemphigoid gestationis with prepartum flare. J Dermatol. 2014;41:850-851. doi:10.1111 /1346-8138.12576
- Baxi LV, Kovilam OP, Collins MH, et al. Recurrent herpes gestationis with postpartum flare: a case report. Am J Obstet Gynecol. 1991;164: 778-780. doi:10.1016/0002-9378(91)90514-r
Dupilumab in the Treatment of Pemphigoid Gestationis
Dupilumab in the Treatment of Pemphigoid Gestationis
PRACTICE POINTS
- Dupilumab inhibits the IL-4Rα subunit, which is bound by IL‐4 and IL‐13, thereby reducing type 2 inflammation associated with pemphigoid gestationis (PG).
- Dupilumab may reduce the dose and duration of systemic corticosteroid therapy for PG, and its use in the second and third trimesters of pregnancy has been supported by emerging safety data.
Managing Contact Dermatitis Related to Amputee Care
Managing Contact Dermatitis Related to Amputee Care
Amputees who use prosthetic devices are particularly susceptible to contact dermatitis due to moisture, irritation, and prolonged contact with components of the device. Contact dermatitis accounts for approximately one-third of the dermatoses encountered by amputees who wear a prosthesis.1 Diagnosing allergic contact dermatitis (ACD) and irritant contact dermatitis (ICD) is challenging due to errors of omission from the differential and the substantial clinical overlap with other eczematous dermatoses. Diagnosis relies on patient history, clinical examination, exposure assessment, diagnostic testing, and a high index of suspicion. Conventionally, ACD comprises approximately 20% of all contact dermatitis cases, whereas ICD accounts for 80%.2 Symptoms vary between the 2 conditions, with pruritus more common in ACD and burning and soreness more common in ICD.3 Onset of dermatitis relative to exposure is crucial, with ICD often manifesting more quickly and ACD requiring an initial sensitization phase.4 Additionally, the complexity of ICD as a condition with variable features adds to the diagnostic difficulty, especially when allergens also have irritant effects.
Understanding these 2 primary types of contact dermatitis is crucial for effective management and prevention strategies in amputees who use prosthetics. In this article, we describe common causes of ACD and ICD related to amputee prosthetics and propose a tailored patch testing panel in order to better diagnose ACD in this patient population.
Allergic Contact Dermatitis
Allergic contact dermatitis occurs when the skin comes into contact with a substance to which the individual is sensitized. In amputees who use prosthetics, the socket and sock liner materials are frequent culprits for triggering allergic reactions. Components such as rubber, metals (eg, nickel), adhesives, and various plastic monomers can induce ACD in susceptible individuals. Additionally, chronic friction and sweat augment hapten penetration, increasing the risk of developing ACD.5
Contact allergens (typically small molecules under 500 Da) penetrate the skin, engage dendritic cells, activate T lymphocytes, and trigger the immune response and memory.6 The skin contains a substantial population of memory T cells, with CD8+ T cells in the epidermis and CD4+ T cells in the dermis, expressing markers that facilitate skin reactivity. The balance between effector and regulatory T cells, which can produce suppressive cytokines such as IL-10, promotes clinical tolerance to allergens such as nickel.
Textile-driven ACD presents with a distinct clinical pattern, often manifesting as patchy generalized dermatitis that coincides with sites where garments fit most snugly. This presentation can mimic other forms of dermatitis, such as nummular or asteatotic dermatitis. The skin beneath undergarments such as underwear or prosthetic socks may be spared, as these act as shields from contact allergens. Notably, the face and hands typically are spared unless the patient has a cross-reaction to formaldehyde-based preservatives found in personal care products.4
Allergy to Components of the Prosthetic Socket and Sock Liner
A prosthesis consists of several key components, including a socket, sleeve, liner, and stump shrinker (eFigure 1). The prosthetic socket, custom-made to fit the residual limb, is the upper part of the prosthesis, while the lower part consists of prosthetic components such as joints and terminal devices ordered to meet individual needs. Prosthetic sleeves provide suspension by securely holding the prosthetic limb in place, while liners offer cushioning and protection to the residual limb, enhancing comfort and reducing friction. Stump shrinkers aid in reducing swelling and shaping the residual limb, facilitating a better fit for the prosthetic socket. Together, these components work in harmony to optimize stability, comfort, and functionality for the user, enabling them to navigate daily activities with greater ease and confidence. Common allergens found in components of the socket and sock liner include rubbers and other elastomers, metals, plastics, adhesives, and textiles.
Rubbers and Other Elastomers—Consumables, including liners, knee sleeves, and socks, are tailored to each client and utilize materials such as silicone and natural and synthetic rubbers for comfort and secure fit. Allergic reactions to natural rubber latex, more commonly used in earlier prosthetics, are associated with both type I and type IV hypersensitivity reactions.4 Proteins inherent to natural rubber are overwhelmingly associated with an immediate urticarial eruption, whereas chemical additives used to produce latex are mostly linked to delayed hypersensitivity reactions, manifesting as allergic reactions ranging from mild itching to severe skin blistering.4
Vulcanization is the process of using heat and other accelerators to manufacture rubber. Common rubber accelerators include thiurams (the most common allergen associated with rubbers and other elastomers), carbamates/carba mix, 1,3-diphenylguanidine, and mercaptobenzothiazole.4 Thiourea is an implicated cause of ACD to neoprene rubber.7 These sensitizing chemicals are all included in the North American 80 Comprehensive Series; only thiuram mix, carba mix, and mercaptobenzothiazole are available in the T.R.U.E. TEST (SmartPractice). Sensitization often occurs due to repeated exposure, particularly in individuals who have undergone multiple prosthetic fittings. Many modern prospective liners utilize a medical-grade silicone as an elastomer for its high flexibility; silicone is considered biologically nonreactive and generally is considered a rare cause of ACD.8
Metals—Nickel, a ubiquitous allergen found in metal alloys used in prosthetic hardware, can cause localized itching, redness, and even blistering upon contact with the skin. Other metals, such as cobalt and chromium, also may trigger allergic reactions in susceptible individuals. Though many elastic fitting prosthetic socks contain silver fibers to reduce odors and friction-causing blisters, pure silver used in clothing or jewelry rarely causes dermatitis.4
Plastics and Adhesives—Leg prosthesis sockets typically are finished with the application of varnish, plastics, and/or resins—all potential allergens—to improve the appearance of the device and protect it from external agents.9 Polyester plastics themselves can cause ICD, only rarely leading to ACD.4 Incomplete curing during their manufacture may result in inadvertent exposure to epoxy resins or other phenol- formaldehyde resins such as 4-tert-butylcatechol and 4-tert-butylphenol formaldehyde, demonstrated causes of ACD in amputees.10 Adhesives used in sock liners or tapes to secure prosthetic devices can contain ingredients such as acrylates (a well-known cause of nail allergens) and other formaldehyde resins.4 Additionally, benzophenone commonly is added to paints and rubbers as a UV light absorber, reducing UV degradation and enhancing the material’s durability under light exposure.11
Textiles—Cotton, a common component in prosthetic sock liners, is almost 100% cellulose and typically does not cause ACD; however, synthetic fibers such as polypropylene and elastane (spandex) can elicit allergic reactions.4 Allergy to textiles often is driven by the chemicals used in the manufacturing process, particularly textile finishes, dyes, and formaldehyde resins, which are commonly used as fabric treatments. Disperse dyes are another common cause of allergic reactions. Para-phenylenediamine, a dye found in permanent hair dye and other darkly colored fabrics, is a potent sensitizer that may cross-react with other compounds that also contain similar amine groups, such as ester anesthetics, sunscreens containing para-aminobenzoic acid, other para dyes, and sulfonamides.12 Sweat can exacerbate these reactions by causing allergens to leach out of textiles, increasing skin exposure. Additionally, prosthetics containing leather may trigger allergies to potassium dichromate and other chromium compounds used in the leather-tanning process.12
Allergy to Personal Care Products
Skin protectants and prosthetic cleansers are crucial in dermatologic care for amputees, working together to safeguard the skin and maintain prosthetic hygiene. Skin protectants form a barrier against irritation, friction, and moisture, protecting the residual limb from damage and enhancing comfort and mobility. Meanwhile, prosthetic cleansers remove sweat, oils, and bacteria from the prosthetic socket, reducing the risk of infections and odors and ensuring the longevity and optimal function of the prosthetic device. Together, they support skin health, comfort, and overall quality of life for amputees.
The socket should be cleaned with warm water prior to use, but more importantly, immediately after removing the prosthesis. If cleaning products are used at night, residual haptens may remain on the device, increasing the risk of sensitization. Common contact irritants found in personal care products utilized in amputee care include sulfates, surfactants, preservatives, and fragrances (eTable 1).4 Additionally, common household cleaners and disinfectants can damage the prosthesis, leading to breakdown and the release of the monomers, precipitating ACD.
Patch Testing to Identify Causative Allergens
Patch testing is a valuable tool for identifying specific allergens responsible for ACD in amputees. This procedure involves applying small amounts of suspected allergens to the patient’s skin under occlusion and leaving the patches in place for 48 hours. After removal, the skin is assessed for reactions at 48 hours, with additional assessments conducted according to International Contact Dermatitis Research Group guidelines, typically at 72 and 96 hours, to identify delayed responses. This diagnostic approach helps pinpoint the substances to which the individual is allergic, enabling targeted avoidance strategies and treatment recommendations. Two widely used patch tests—the T.R.U.E. TEST, a preassembled patch test encompassing 35 allergens, and the North American 80 Comprehensive Series, which includes 80 allergens—demonstrate a sensitivity range between 70% and 80%.13,14 eTable 2 shows a recommended custom contact dermatitis panel to assess the most common causes of ACD related to amputee care.
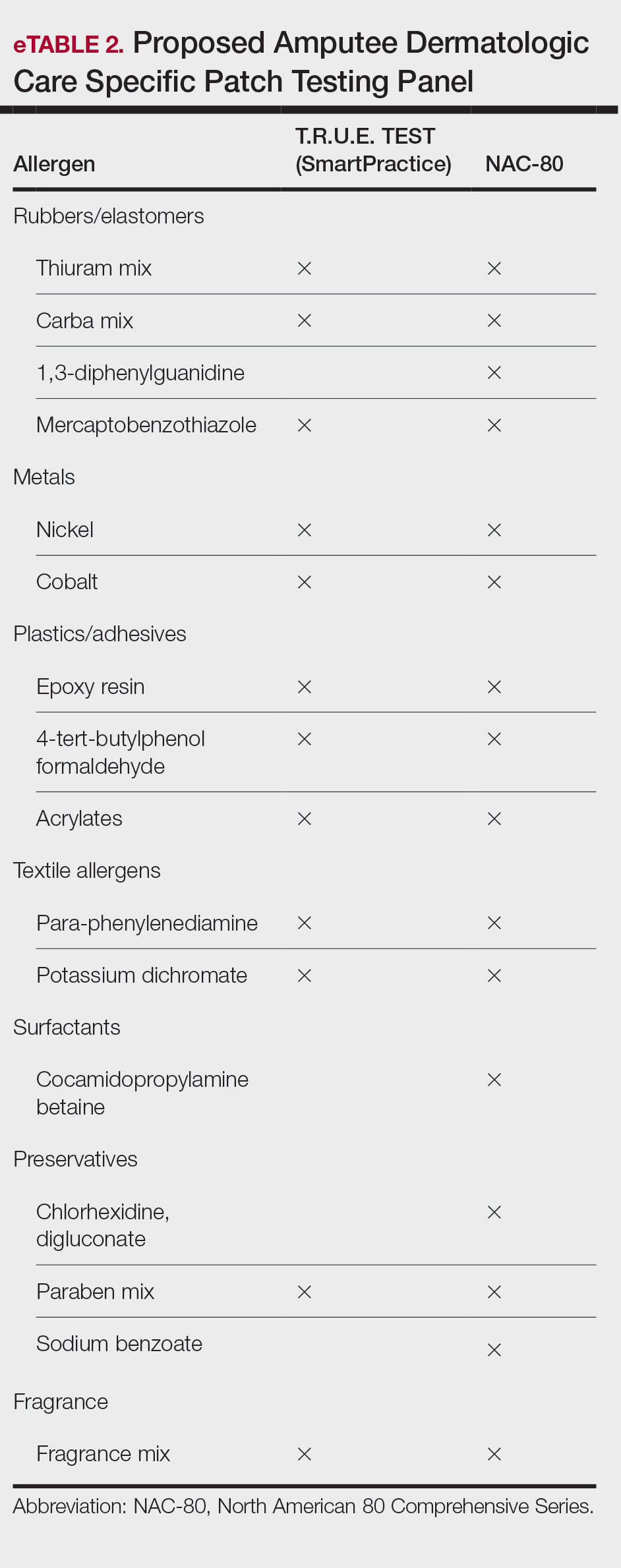
Irritant Contact Dermatitis
Irritant contact dermatitis occurs when the skin’s protective barrier is damaged by repeated exposure to a particular irritant. In amputees, perspiration, friction, and pressure from prosthetic devices can exacerbate irritant reactions, leading to skin maceration, breakdown, and increased transepidermal penetration. Sweat accumulation within the prosthetic socket creates a moist environment conducive to ICD. The combination of sweat and friction can strip the skin of its natural oils, leading to dryness, chafing, and maceration. Continuous exposure to moisture also can exacerbate existing dermatitis and compromise skin integrity.4 Additionally, chronic irritation may increase transepidermal penetration of haptens, potentiating the development of ACD.15
Management of ICD in amputees involves a combination of treatments aimed at reducing friction, reducing sweating, and restoring barrier protection. Strategies to minimize mechanical trauma to the skin include ensuring proper socket fit, managing moisture, and protecting the skin. Using moisture-wicking sock liners and breathable prosthetic materials can help keep the skin dry. Topical antiperspirants containing aluminum chloride or similar compounds that help to block sweat glands often are the first line of treatment. Oral anticholinergics may be prescribed to reduce overall sweating, though they can have systemic side effects. Iontophoresis, a procedure where the affected area is exposed to a mild electrical current, can also be effective, especially for sweating of the hands and feet, though its application in amputees might be more limited.14
Recently, 2 treatments have emerged as options for managing excessive sweating (hyperhidrosis) in amputees: botulinum toxin injections and laser hair removal. By inhibiting the release of acetylcholine from sweat glands, botulinum toxin effectively reduces sweat production, thereby alleviating perspiration-induced skin irritation. Approximately 2 to 3 units of botulinum toxin at a dilution of 100 units in 1 mL of bacteriostatic saline 0.9% are injected transdermally at 1-cm intervals in a circumferential pattern on the skin covered by the prosthesis socket (typically a total of 300-500 units are utilized in the procedure)(eFigure 2).16 Laser hair removal can assist amputees with hyperhidrosis by reducing hair in the residual limb area, which decreases sweat retention and the potential for skin irritation due to friction.
Final Thoughts
In amputee dermatologic care, individuals with limb loss are particularly prone to contact dermatitis due to moisture, friction, and prolonged contact with prosthetic components. Diagnosing ACD and ICD is challenging due to overlapping symptoms and the potential for simultaneous occurrence. Distinguishing between these conditions is crucial for effective management. Understanding their causes, particularly in relation to prosthetic use, is essential for developing targeted prevention and treatment strategies, including the use of tailored patch testing panels to better diagnose ACD in amputees.
- Lyon CC, Kulkarni J, Zimersonc E, et al. Skin disorders in amputees. J Am Acad Dermatol. 2000;42:501-507.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2018;56:99-109.
- Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis: A Practical Approach. Springer; 2021:57-92.
- Fisher AA, Rietschel RL, Fowler JF. Fisher’s Contact Dermatitis. BC Decker Inc; 2008.
- Johansen JD, Frosch PJ, Lepoittevin JP. Contact Dermatitis. Springer; 2010:43-90.
- Eisen HN, Orris L, Belman S. Elicitation of delayed allergic skin reactions with haptens: the dependence of elicitation on hapten combination with protein. J Exp Med. 1952;95:473-487.
- Johnson R. Wrist dermatitis: contact allergy to neoprene in a keyboard wrist rest. Am J Contact Dermat. 1997;8:172-174.
- Adams RM. Occupational Skin Disease. WB Saunders; 1999:501-551.
- Requena L, Vázquez F, Requena C, et al. Epoxy dermatitis of an amputation stump. Contact Dermatitis. 1986;14:320.
- Freeman S. Contact dermatitis of a limb stump caused by p-tertiary butyl catechol in the artificial limb. Contact Dermatitis. 1986;14:68-69.
- Heurung AR, Raju SI, Warshaw EM. Benzophenones. Dermatitis. 2014;25:3-10.
- Manneschi V, Palmerio B, Pauluzzi P, et al. Contact dermatitis from myoelectric prostheses. Contact Dermatitis. 1989;21:116-117.
- Heinrich D, Altmeyer P, Brasch J. “New” techniques for more sensitive patch testing? J Dtsch Dermatol Ges. 2011;9:889-896.
- James WD. Contact dermatitis update. Presented at: Walter Reed National Military Medical Center; April 18, 2024.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486.
Amputees who use prosthetic devices are particularly susceptible to contact dermatitis due to moisture, irritation, and prolonged contact with components of the device. Contact dermatitis accounts for approximately one-third of the dermatoses encountered by amputees who wear a prosthesis.1 Diagnosing allergic contact dermatitis (ACD) and irritant contact dermatitis (ICD) is challenging due to errors of omission from the differential and the substantial clinical overlap with other eczematous dermatoses. Diagnosis relies on patient history, clinical examination, exposure assessment, diagnostic testing, and a high index of suspicion. Conventionally, ACD comprises approximately 20% of all contact dermatitis cases, whereas ICD accounts for 80%.2 Symptoms vary between the 2 conditions, with pruritus more common in ACD and burning and soreness more common in ICD.3 Onset of dermatitis relative to exposure is crucial, with ICD often manifesting more quickly and ACD requiring an initial sensitization phase.4 Additionally, the complexity of ICD as a condition with variable features adds to the diagnostic difficulty, especially when allergens also have irritant effects.
Understanding these 2 primary types of contact dermatitis is crucial for effective management and prevention strategies in amputees who use prosthetics. In this article, we describe common causes of ACD and ICD related to amputee prosthetics and propose a tailored patch testing panel in order to better diagnose ACD in this patient population.
Allergic Contact Dermatitis
Allergic contact dermatitis occurs when the skin comes into contact with a substance to which the individual is sensitized. In amputees who use prosthetics, the socket and sock liner materials are frequent culprits for triggering allergic reactions. Components such as rubber, metals (eg, nickel), adhesives, and various plastic monomers can induce ACD in susceptible individuals. Additionally, chronic friction and sweat augment hapten penetration, increasing the risk of developing ACD.5
Contact allergens (typically small molecules under 500 Da) penetrate the skin, engage dendritic cells, activate T lymphocytes, and trigger the immune response and memory.6 The skin contains a substantial population of memory T cells, with CD8+ T cells in the epidermis and CD4+ T cells in the dermis, expressing markers that facilitate skin reactivity. The balance between effector and regulatory T cells, which can produce suppressive cytokines such as IL-10, promotes clinical tolerance to allergens such as nickel.
Textile-driven ACD presents with a distinct clinical pattern, often manifesting as patchy generalized dermatitis that coincides with sites where garments fit most snugly. This presentation can mimic other forms of dermatitis, such as nummular or asteatotic dermatitis. The skin beneath undergarments such as underwear or prosthetic socks may be spared, as these act as shields from contact allergens. Notably, the face and hands typically are spared unless the patient has a cross-reaction to formaldehyde-based preservatives found in personal care products.4
Allergy to Components of the Prosthetic Socket and Sock Liner
A prosthesis consists of several key components, including a socket, sleeve, liner, and stump shrinker (eFigure 1). The prosthetic socket, custom-made to fit the residual limb, is the upper part of the prosthesis, while the lower part consists of prosthetic components such as joints and terminal devices ordered to meet individual needs. Prosthetic sleeves provide suspension by securely holding the prosthetic limb in place, while liners offer cushioning and protection to the residual limb, enhancing comfort and reducing friction. Stump shrinkers aid in reducing swelling and shaping the residual limb, facilitating a better fit for the prosthetic socket. Together, these components work in harmony to optimize stability, comfort, and functionality for the user, enabling them to navigate daily activities with greater ease and confidence. Common allergens found in components of the socket and sock liner include rubbers and other elastomers, metals, plastics, adhesives, and textiles.

Rubbers and Other Elastomers—Consumables, including liners, knee sleeves, and socks, are tailored to each client and utilize materials such as silicone and natural and synthetic rubbers for comfort and secure fit. Allergic reactions to natural rubber latex, more commonly used in earlier prosthetics, are associated with both type I and type IV hypersensitivity reactions.4 Proteins inherent to natural rubber are overwhelmingly associated with an immediate urticarial eruption, whereas chemical additives used to produce latex are mostly linked to delayed hypersensitivity reactions, manifesting as allergic reactions ranging from mild itching to severe skin blistering.4
Vulcanization is the process of using heat and other accelerators to manufacture rubber. Common rubber accelerators include thiurams (the most common allergen associated with rubbers and other elastomers), carbamates/carba mix, 1,3-diphenylguanidine, and mercaptobenzothiazole.4 Thiourea is an implicated cause of ACD to neoprene rubber.7 These sensitizing chemicals are all included in the North American 80 Comprehensive Series; only thiuram mix, carba mix, and mercaptobenzothiazole are available in the T.R.U.E. TEST (SmartPractice). Sensitization often occurs due to repeated exposure, particularly in individuals who have undergone multiple prosthetic fittings. Many modern prospective liners utilize a medical-grade silicone as an elastomer for its high flexibility; silicone is considered biologically nonreactive and generally is considered a rare cause of ACD.8
Metals—Nickel, a ubiquitous allergen found in metal alloys used in prosthetic hardware, can cause localized itching, redness, and even blistering upon contact with the skin. Other metals, such as cobalt and chromium, also may trigger allergic reactions in susceptible individuals. Though many elastic fitting prosthetic socks contain silver fibers to reduce odors and friction-causing blisters, pure silver used in clothing or jewelry rarely causes dermatitis.4
Plastics and Adhesives—Leg prosthesis sockets typically are finished with the application of varnish, plastics, and/or resins—all potential allergens—to improve the appearance of the device and protect it from external agents.9 Polyester plastics themselves can cause ICD, only rarely leading to ACD.4 Incomplete curing during their manufacture may result in inadvertent exposure to epoxy resins or other phenol- formaldehyde resins such as 4-tert-butylcatechol and 4-tert-butylphenol formaldehyde, demonstrated causes of ACD in amputees.10 Adhesives used in sock liners or tapes to secure prosthetic devices can contain ingredients such as acrylates (a well-known cause of nail allergens) and other formaldehyde resins.4 Additionally, benzophenone commonly is added to paints and rubbers as a UV light absorber, reducing UV degradation and enhancing the material’s durability under light exposure.11
Textiles—Cotton, a common component in prosthetic sock liners, is almost 100% cellulose and typically does not cause ACD; however, synthetic fibers such as polypropylene and elastane (spandex) can elicit allergic reactions.4 Allergy to textiles often is driven by the chemicals used in the manufacturing process, particularly textile finishes, dyes, and formaldehyde resins, which are commonly used as fabric treatments. Disperse dyes are another common cause of allergic reactions. Para-phenylenediamine, a dye found in permanent hair dye and other darkly colored fabrics, is a potent sensitizer that may cross-react with other compounds that also contain similar amine groups, such as ester anesthetics, sunscreens containing para-aminobenzoic acid, other para dyes, and sulfonamides.12 Sweat can exacerbate these reactions by causing allergens to leach out of textiles, increasing skin exposure. Additionally, prosthetics containing leather may trigger allergies to potassium dichromate and other chromium compounds used in the leather-tanning process.12
Allergy to Personal Care Products
Skin protectants and prosthetic cleansers are crucial in dermatologic care for amputees, working together to safeguard the skin and maintain prosthetic hygiene. Skin protectants form a barrier against irritation, friction, and moisture, protecting the residual limb from damage and enhancing comfort and mobility. Meanwhile, prosthetic cleansers remove sweat, oils, and bacteria from the prosthetic socket, reducing the risk of infections and odors and ensuring the longevity and optimal function of the prosthetic device. Together, they support skin health, comfort, and overall quality of life for amputees.
The socket should be cleaned with warm water prior to use, but more importantly, immediately after removing the prosthesis. If cleaning products are used at night, residual haptens may remain on the device, increasing the risk of sensitization. Common contact irritants found in personal care products utilized in amputee care include sulfates, surfactants, preservatives, and fragrances (eTable 1).4 Additionally, common household cleaners and disinfectants can damage the prosthesis, leading to breakdown and the release of the monomers, precipitating ACD.

Patch Testing to Identify Causative Allergens
Patch testing is a valuable tool for identifying specific allergens responsible for ACD in amputees. This procedure involves applying small amounts of suspected allergens to the patient’s skin under occlusion and leaving the patches in place for 48 hours. After removal, the skin is assessed for reactions at 48 hours, with additional assessments conducted according to International Contact Dermatitis Research Group guidelines, typically at 72 and 96 hours, to identify delayed responses. This diagnostic approach helps pinpoint the substances to which the individual is allergic, enabling targeted avoidance strategies and treatment recommendations. Two widely used patch tests—the T.R.U.E. TEST, a preassembled patch test encompassing 35 allergens, and the North American 80 Comprehensive Series, which includes 80 allergens—demonstrate a sensitivity range between 70% and 80%.13,14 eTable 2 shows a recommended custom contact dermatitis panel to assess the most common causes of ACD related to amputee care.

Irritant Contact Dermatitis
Irritant contact dermatitis occurs when the skin’s protective barrier is damaged by repeated exposure to a particular irritant. In amputees, perspiration, friction, and pressure from prosthetic devices can exacerbate irritant reactions, leading to skin maceration, breakdown, and increased transepidermal penetration. Sweat accumulation within the prosthetic socket creates a moist environment conducive to ICD. The combination of sweat and friction can strip the skin of its natural oils, leading to dryness, chafing, and maceration. Continuous exposure to moisture also can exacerbate existing dermatitis and compromise skin integrity.4 Additionally, chronic irritation may increase transepidermal penetration of haptens, potentiating the development of ACD.15
Management of ICD in amputees involves a combination of treatments aimed at reducing friction, reducing sweating, and restoring barrier protection. Strategies to minimize mechanical trauma to the skin include ensuring proper socket fit, managing moisture, and protecting the skin. Using moisture-wicking sock liners and breathable prosthetic materials can help keep the skin dry. Topical antiperspirants containing aluminum chloride or similar compounds that help to block sweat glands often are the first line of treatment. Oral anticholinergics may be prescribed to reduce overall sweating, though they can have systemic side effects. Iontophoresis, a procedure where the affected area is exposed to a mild electrical current, can also be effective, especially for sweating of the hands and feet, though its application in amputees might be more limited.14
Recently, 2 treatments have emerged as options for managing excessive sweating (hyperhidrosis) in amputees: botulinum toxin injections and laser hair removal. By inhibiting the release of acetylcholine from sweat glands, botulinum toxin effectively reduces sweat production, thereby alleviating perspiration-induced skin irritation. Approximately 2 to 3 units of botulinum toxin at a dilution of 100 units in 1 mL of bacteriostatic saline 0.9% are injected transdermally at 1-cm intervals in a circumferential pattern on the skin covered by the prosthesis socket (typically a total of 300-500 units are utilized in the procedure)(eFigure 2).16 Laser hair removal can assist amputees with hyperhidrosis by reducing hair in the residual limb area, which decreases sweat retention and the potential for skin irritation due to friction.

Final Thoughts
In amputee dermatologic care, individuals with limb loss are particularly prone to contact dermatitis due to moisture, friction, and prolonged contact with prosthetic components. Diagnosing ACD and ICD is challenging due to overlapping symptoms and the potential for simultaneous occurrence. Distinguishing between these conditions is crucial for effective management. Understanding their causes, particularly in relation to prosthetic use, is essential for developing targeted prevention and treatment strategies, including the use of tailored patch testing panels to better diagnose ACD in amputees.
Amputees who use prosthetic devices are particularly susceptible to contact dermatitis due to moisture, irritation, and prolonged contact with components of the device. Contact dermatitis accounts for approximately one-third of the dermatoses encountered by amputees who wear a prosthesis.1 Diagnosing allergic contact dermatitis (ACD) and irritant contact dermatitis (ICD) is challenging due to errors of omission from the differential and the substantial clinical overlap with other eczematous dermatoses. Diagnosis relies on patient history, clinical examination, exposure assessment, diagnostic testing, and a high index of suspicion. Conventionally, ACD comprises approximately 20% of all contact dermatitis cases, whereas ICD accounts for 80%.2 Symptoms vary between the 2 conditions, with pruritus more common in ACD and burning and soreness more common in ICD.3 Onset of dermatitis relative to exposure is crucial, with ICD often manifesting more quickly and ACD requiring an initial sensitization phase.4 Additionally, the complexity of ICD as a condition with variable features adds to the diagnostic difficulty, especially when allergens also have irritant effects.
Understanding these 2 primary types of contact dermatitis is crucial for effective management and prevention strategies in amputees who use prosthetics. In this article, we describe common causes of ACD and ICD related to amputee prosthetics and propose a tailored patch testing panel in order to better diagnose ACD in this patient population.
Allergic Contact Dermatitis
Allergic contact dermatitis occurs when the skin comes into contact with a substance to which the individual is sensitized. In amputees who use prosthetics, the socket and sock liner materials are frequent culprits for triggering allergic reactions. Components such as rubber, metals (eg, nickel), adhesives, and various plastic monomers can induce ACD in susceptible individuals. Additionally, chronic friction and sweat augment hapten penetration, increasing the risk of developing ACD.5
Contact allergens (typically small molecules under 500 Da) penetrate the skin, engage dendritic cells, activate T lymphocytes, and trigger the immune response and memory.6 The skin contains a substantial population of memory T cells, with CD8+ T cells in the epidermis and CD4+ T cells in the dermis, expressing markers that facilitate skin reactivity. The balance between effector and regulatory T cells, which can produce suppressive cytokines such as IL-10, promotes clinical tolerance to allergens such as nickel.
Textile-driven ACD presents with a distinct clinical pattern, often manifesting as patchy generalized dermatitis that coincides with sites where garments fit most snugly. This presentation can mimic other forms of dermatitis, such as nummular or asteatotic dermatitis. The skin beneath undergarments such as underwear or prosthetic socks may be spared, as these act as shields from contact allergens. Notably, the face and hands typically are spared unless the patient has a cross-reaction to formaldehyde-based preservatives found in personal care products.4
Allergy to Components of the Prosthetic Socket and Sock Liner
A prosthesis consists of several key components, including a socket, sleeve, liner, and stump shrinker (eFigure 1). The prosthetic socket, custom-made to fit the residual limb, is the upper part of the prosthesis, while the lower part consists of prosthetic components such as joints and terminal devices ordered to meet individual needs. Prosthetic sleeves provide suspension by securely holding the prosthetic limb in place, while liners offer cushioning and protection to the residual limb, enhancing comfort and reducing friction. Stump shrinkers aid in reducing swelling and shaping the residual limb, facilitating a better fit for the prosthetic socket. Together, these components work in harmony to optimize stability, comfort, and functionality for the user, enabling them to navigate daily activities with greater ease and confidence. Common allergens found in components of the socket and sock liner include rubbers and other elastomers, metals, plastics, adhesives, and textiles.

Rubbers and Other Elastomers—Consumables, including liners, knee sleeves, and socks, are tailored to each client and utilize materials such as silicone and natural and synthetic rubbers for comfort and secure fit. Allergic reactions to natural rubber latex, more commonly used in earlier prosthetics, are associated with both type I and type IV hypersensitivity reactions.4 Proteins inherent to natural rubber are overwhelmingly associated with an immediate urticarial eruption, whereas chemical additives used to produce latex are mostly linked to delayed hypersensitivity reactions, manifesting as allergic reactions ranging from mild itching to severe skin blistering.4
Vulcanization is the process of using heat and other accelerators to manufacture rubber. Common rubber accelerators include thiurams (the most common allergen associated with rubbers and other elastomers), carbamates/carba mix, 1,3-diphenylguanidine, and mercaptobenzothiazole.4 Thiourea is an implicated cause of ACD to neoprene rubber.7 These sensitizing chemicals are all included in the North American 80 Comprehensive Series; only thiuram mix, carba mix, and mercaptobenzothiazole are available in the T.R.U.E. TEST (SmartPractice). Sensitization often occurs due to repeated exposure, particularly in individuals who have undergone multiple prosthetic fittings. Many modern prospective liners utilize a medical-grade silicone as an elastomer for its high flexibility; silicone is considered biologically nonreactive and generally is considered a rare cause of ACD.8
Metals—Nickel, a ubiquitous allergen found in metal alloys used in prosthetic hardware, can cause localized itching, redness, and even blistering upon contact with the skin. Other metals, such as cobalt and chromium, also may trigger allergic reactions in susceptible individuals. Though many elastic fitting prosthetic socks contain silver fibers to reduce odors and friction-causing blisters, pure silver used in clothing or jewelry rarely causes dermatitis.4
Plastics and Adhesives—Leg prosthesis sockets typically are finished with the application of varnish, plastics, and/or resins—all potential allergens—to improve the appearance of the device and protect it from external agents.9 Polyester plastics themselves can cause ICD, only rarely leading to ACD.4 Incomplete curing during their manufacture may result in inadvertent exposure to epoxy resins or other phenol- formaldehyde resins such as 4-tert-butylcatechol and 4-tert-butylphenol formaldehyde, demonstrated causes of ACD in amputees.10 Adhesives used in sock liners or tapes to secure prosthetic devices can contain ingredients such as acrylates (a well-known cause of nail allergens) and other formaldehyde resins.4 Additionally, benzophenone commonly is added to paints and rubbers as a UV light absorber, reducing UV degradation and enhancing the material’s durability under light exposure.11
Textiles—Cotton, a common component in prosthetic sock liners, is almost 100% cellulose and typically does not cause ACD; however, synthetic fibers such as polypropylene and elastane (spandex) can elicit allergic reactions.4 Allergy to textiles often is driven by the chemicals used in the manufacturing process, particularly textile finishes, dyes, and formaldehyde resins, which are commonly used as fabric treatments. Disperse dyes are another common cause of allergic reactions. Para-phenylenediamine, a dye found in permanent hair dye and other darkly colored fabrics, is a potent sensitizer that may cross-react with other compounds that also contain similar amine groups, such as ester anesthetics, sunscreens containing para-aminobenzoic acid, other para dyes, and sulfonamides.12 Sweat can exacerbate these reactions by causing allergens to leach out of textiles, increasing skin exposure. Additionally, prosthetics containing leather may trigger allergies to potassium dichromate and other chromium compounds used in the leather-tanning process.12
Allergy to Personal Care Products
Skin protectants and prosthetic cleansers are crucial in dermatologic care for amputees, working together to safeguard the skin and maintain prosthetic hygiene. Skin protectants form a barrier against irritation, friction, and moisture, protecting the residual limb from damage and enhancing comfort and mobility. Meanwhile, prosthetic cleansers remove sweat, oils, and bacteria from the prosthetic socket, reducing the risk of infections and odors and ensuring the longevity and optimal function of the prosthetic device. Together, they support skin health, comfort, and overall quality of life for amputees.
The socket should be cleaned with warm water prior to use, but more importantly, immediately after removing the prosthesis. If cleaning products are used at night, residual haptens may remain on the device, increasing the risk of sensitization. Common contact irritants found in personal care products utilized in amputee care include sulfates, surfactants, preservatives, and fragrances (eTable 1).4 Additionally, common household cleaners and disinfectants can damage the prosthesis, leading to breakdown and the release of the monomers, precipitating ACD.

Patch Testing to Identify Causative Allergens
Patch testing is a valuable tool for identifying specific allergens responsible for ACD in amputees. This procedure involves applying small amounts of suspected allergens to the patient’s skin under occlusion and leaving the patches in place for 48 hours. After removal, the skin is assessed for reactions at 48 hours, with additional assessments conducted according to International Contact Dermatitis Research Group guidelines, typically at 72 and 96 hours, to identify delayed responses. This diagnostic approach helps pinpoint the substances to which the individual is allergic, enabling targeted avoidance strategies and treatment recommendations. Two widely used patch tests—the T.R.U.E. TEST, a preassembled patch test encompassing 35 allergens, and the North American 80 Comprehensive Series, which includes 80 allergens—demonstrate a sensitivity range between 70% and 80%.13,14 eTable 2 shows a recommended custom contact dermatitis panel to assess the most common causes of ACD related to amputee care.

Irritant Contact Dermatitis
Irritant contact dermatitis occurs when the skin’s protective barrier is damaged by repeated exposure to a particular irritant. In amputees, perspiration, friction, and pressure from prosthetic devices can exacerbate irritant reactions, leading to skin maceration, breakdown, and increased transepidermal penetration. Sweat accumulation within the prosthetic socket creates a moist environment conducive to ICD. The combination of sweat and friction can strip the skin of its natural oils, leading to dryness, chafing, and maceration. Continuous exposure to moisture also can exacerbate existing dermatitis and compromise skin integrity.4 Additionally, chronic irritation may increase transepidermal penetration of haptens, potentiating the development of ACD.15
Management of ICD in amputees involves a combination of treatments aimed at reducing friction, reducing sweating, and restoring barrier protection. Strategies to minimize mechanical trauma to the skin include ensuring proper socket fit, managing moisture, and protecting the skin. Using moisture-wicking sock liners and breathable prosthetic materials can help keep the skin dry. Topical antiperspirants containing aluminum chloride or similar compounds that help to block sweat glands often are the first line of treatment. Oral anticholinergics may be prescribed to reduce overall sweating, though they can have systemic side effects. Iontophoresis, a procedure where the affected area is exposed to a mild electrical current, can also be effective, especially for sweating of the hands and feet, though its application in amputees might be more limited.14
Recently, 2 treatments have emerged as options for managing excessive sweating (hyperhidrosis) in amputees: botulinum toxin injections and laser hair removal. By inhibiting the release of acetylcholine from sweat glands, botulinum toxin effectively reduces sweat production, thereby alleviating perspiration-induced skin irritation. Approximately 2 to 3 units of botulinum toxin at a dilution of 100 units in 1 mL of bacteriostatic saline 0.9% are injected transdermally at 1-cm intervals in a circumferential pattern on the skin covered by the prosthesis socket (typically a total of 300-500 units are utilized in the procedure)(eFigure 2).16 Laser hair removal can assist amputees with hyperhidrosis by reducing hair in the residual limb area, which decreases sweat retention and the potential for skin irritation due to friction.

Final Thoughts
In amputee dermatologic care, individuals with limb loss are particularly prone to contact dermatitis due to moisture, friction, and prolonged contact with prosthetic components. Diagnosing ACD and ICD is challenging due to overlapping symptoms and the potential for simultaneous occurrence. Distinguishing between these conditions is crucial for effective management. Understanding their causes, particularly in relation to prosthetic use, is essential for developing targeted prevention and treatment strategies, including the use of tailored patch testing panels to better diagnose ACD in amputees.
- Lyon CC, Kulkarni J, Zimersonc E, et al. Skin disorders in amputees. J Am Acad Dermatol. 2000;42:501-507.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2018;56:99-109.
- Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis: A Practical Approach. Springer; 2021:57-92.
- Fisher AA, Rietschel RL, Fowler JF. Fisher’s Contact Dermatitis. BC Decker Inc; 2008.
- Johansen JD, Frosch PJ, Lepoittevin JP. Contact Dermatitis. Springer; 2010:43-90.
- Eisen HN, Orris L, Belman S. Elicitation of delayed allergic skin reactions with haptens: the dependence of elicitation on hapten combination with protein. J Exp Med. 1952;95:473-487.
- Johnson R. Wrist dermatitis: contact allergy to neoprene in a keyboard wrist rest. Am J Contact Dermat. 1997;8:172-174.
- Adams RM. Occupational Skin Disease. WB Saunders; 1999:501-551.
- Requena L, Vázquez F, Requena C, et al. Epoxy dermatitis of an amputation stump. Contact Dermatitis. 1986;14:320.
- Freeman S. Contact dermatitis of a limb stump caused by p-tertiary butyl catechol in the artificial limb. Contact Dermatitis. 1986;14:68-69.
- Heurung AR, Raju SI, Warshaw EM. Benzophenones. Dermatitis. 2014;25:3-10.
- Manneschi V, Palmerio B, Pauluzzi P, et al. Contact dermatitis from myoelectric prostheses. Contact Dermatitis. 1989;21:116-117.
- Heinrich D, Altmeyer P, Brasch J. “New” techniques for more sensitive patch testing? J Dtsch Dermatol Ges. 2011;9:889-896.
- James WD. Contact dermatitis update. Presented at: Walter Reed National Military Medical Center; April 18, 2024.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486.
- Lyon CC, Kulkarni J, Zimersonc E, et al. Skin disorders in amputees. J Am Acad Dermatol. 2000;42:501-507.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2018;56:99-109.
- Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis: A Practical Approach. Springer; 2021:57-92.
- Fisher AA, Rietschel RL, Fowler JF. Fisher’s Contact Dermatitis. BC Decker Inc; 2008.
- Johansen JD, Frosch PJ, Lepoittevin JP. Contact Dermatitis. Springer; 2010:43-90.
- Eisen HN, Orris L, Belman S. Elicitation of delayed allergic skin reactions with haptens: the dependence of elicitation on hapten combination with protein. J Exp Med. 1952;95:473-487.
- Johnson R. Wrist dermatitis: contact allergy to neoprene in a keyboard wrist rest. Am J Contact Dermat. 1997;8:172-174.
- Adams RM. Occupational Skin Disease. WB Saunders; 1999:501-551.
- Requena L, Vázquez F, Requena C, et al. Epoxy dermatitis of an amputation stump. Contact Dermatitis. 1986;14:320.
- Freeman S. Contact dermatitis of a limb stump caused by p-tertiary butyl catechol in the artificial limb. Contact Dermatitis. 1986;14:68-69.
- Heurung AR, Raju SI, Warshaw EM. Benzophenones. Dermatitis. 2014;25:3-10.
- Manneschi V, Palmerio B, Pauluzzi P, et al. Contact dermatitis from myoelectric prostheses. Contact Dermatitis. 1989;21:116-117.
- Heinrich D, Altmeyer P, Brasch J. “New” techniques for more sensitive patch testing? J Dtsch Dermatol Ges. 2011;9:889-896.
- James WD. Contact dermatitis update. Presented at: Walter Reed National Military Medical Center; April 18, 2024.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486.
Managing Contact Dermatitis Related to Amputee Care
Managing Contact Dermatitis Related to Amputee Care
PRACTICE POINTS
- Incorporating a tailored patch testing panel that includes common prosthetic-related allergens (eg, rubber, metals, adhesives) can greatly improve the diagnosis and treatment of allergic vs irritant contact dermatitis in amputees.
- Effective management of irritant contact dermatitis in amputees involves reducing moisture and friction in the prosthetic socket with moisture-wicking liners, ensuring proper fit, and utilizing treatments such as topical antiperspirants and botulinum toxin injections.