User login
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.
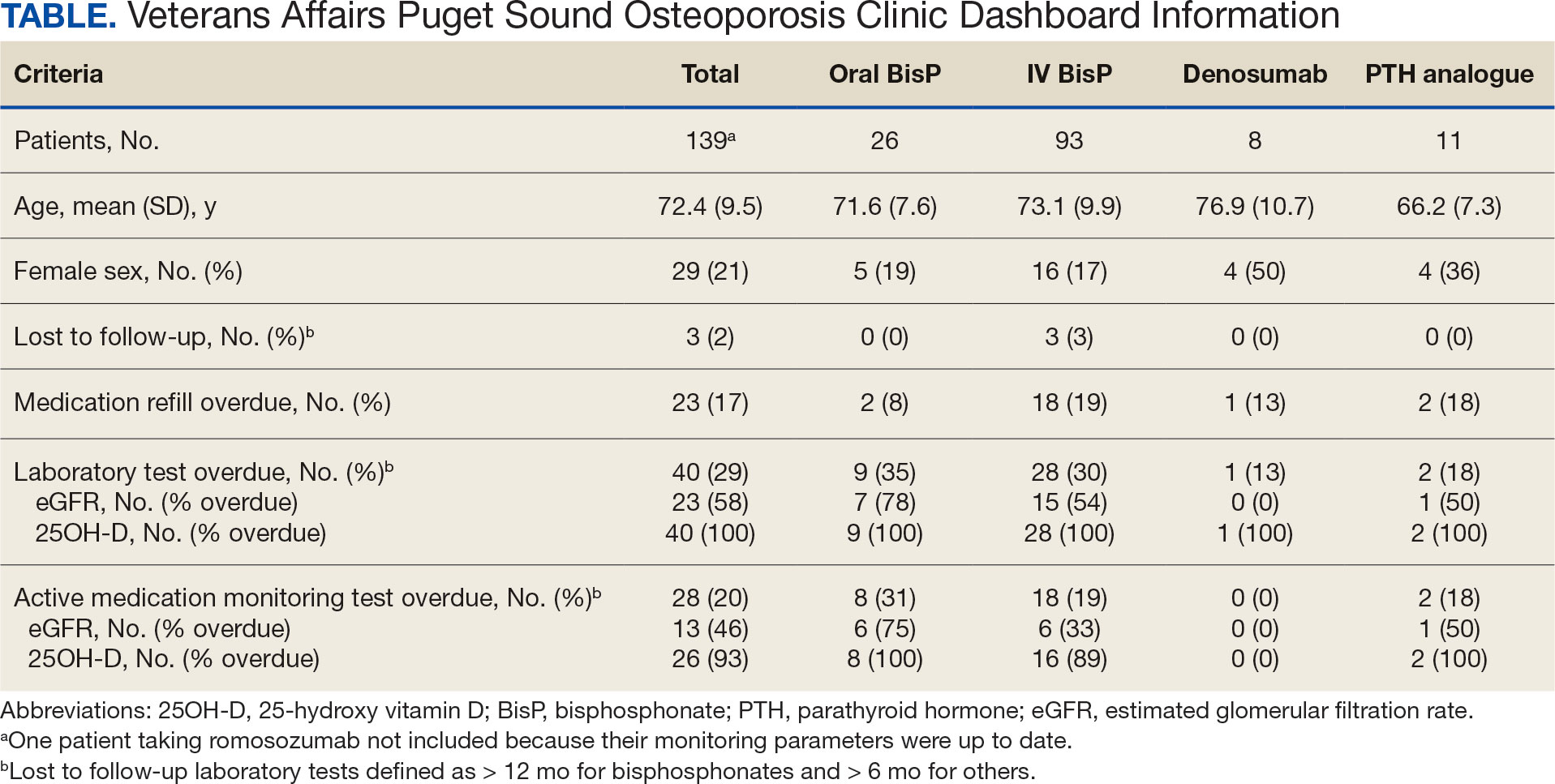
The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.

The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.

The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
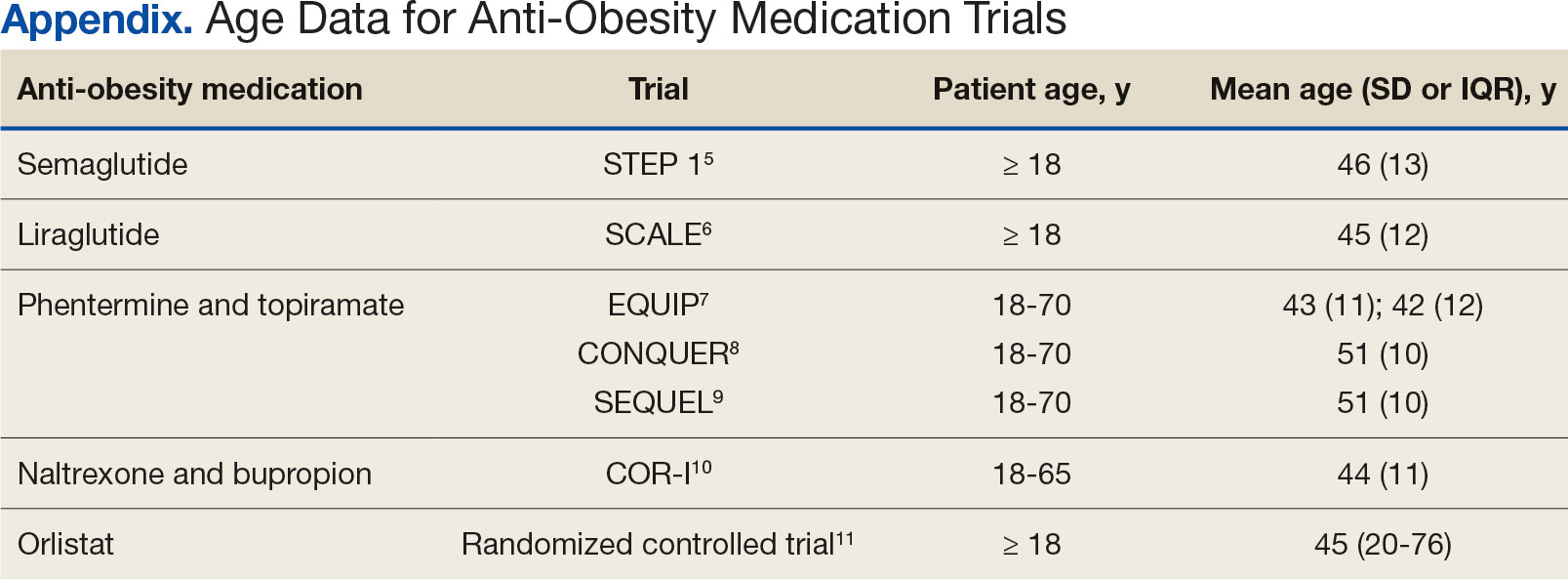
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.
Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
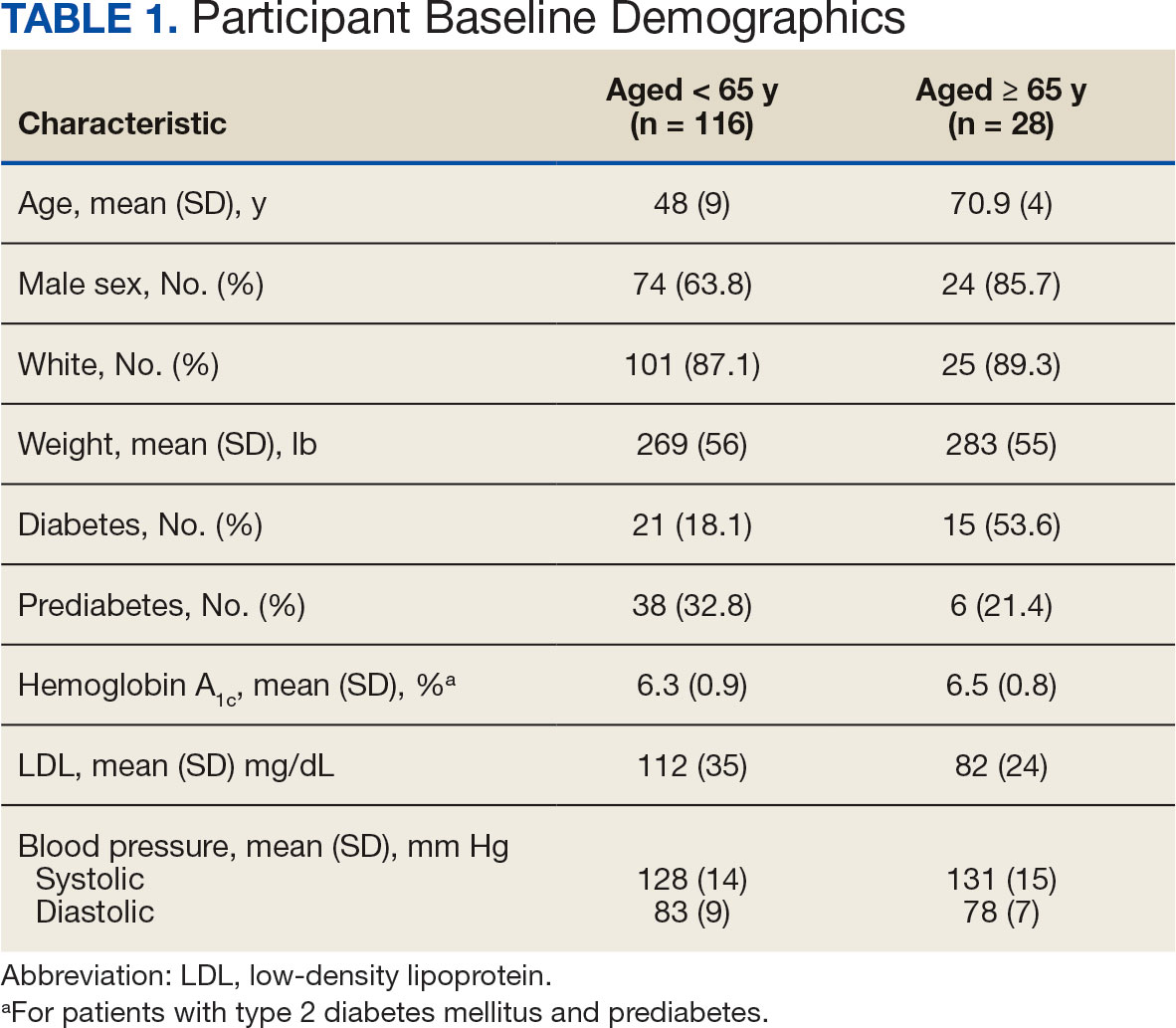
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).
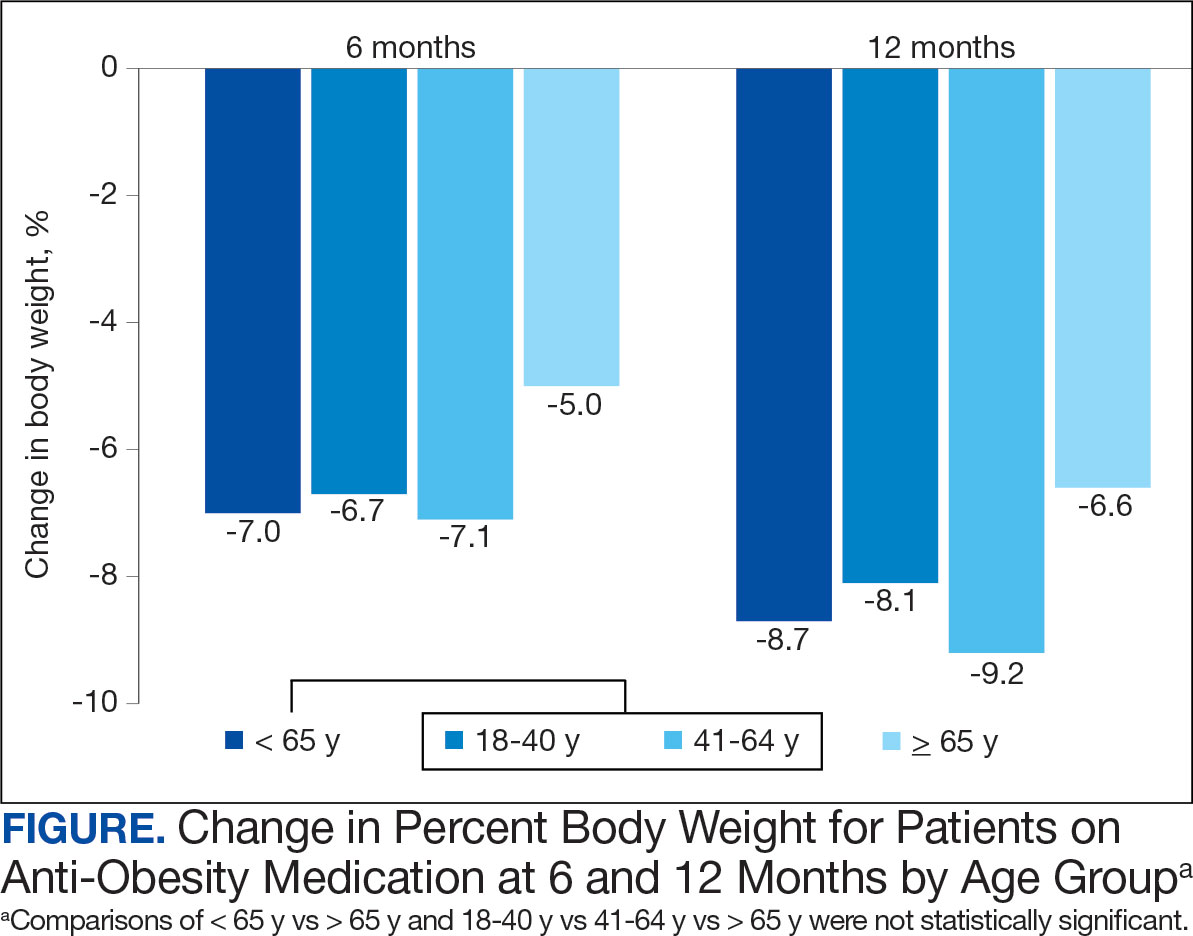
Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).
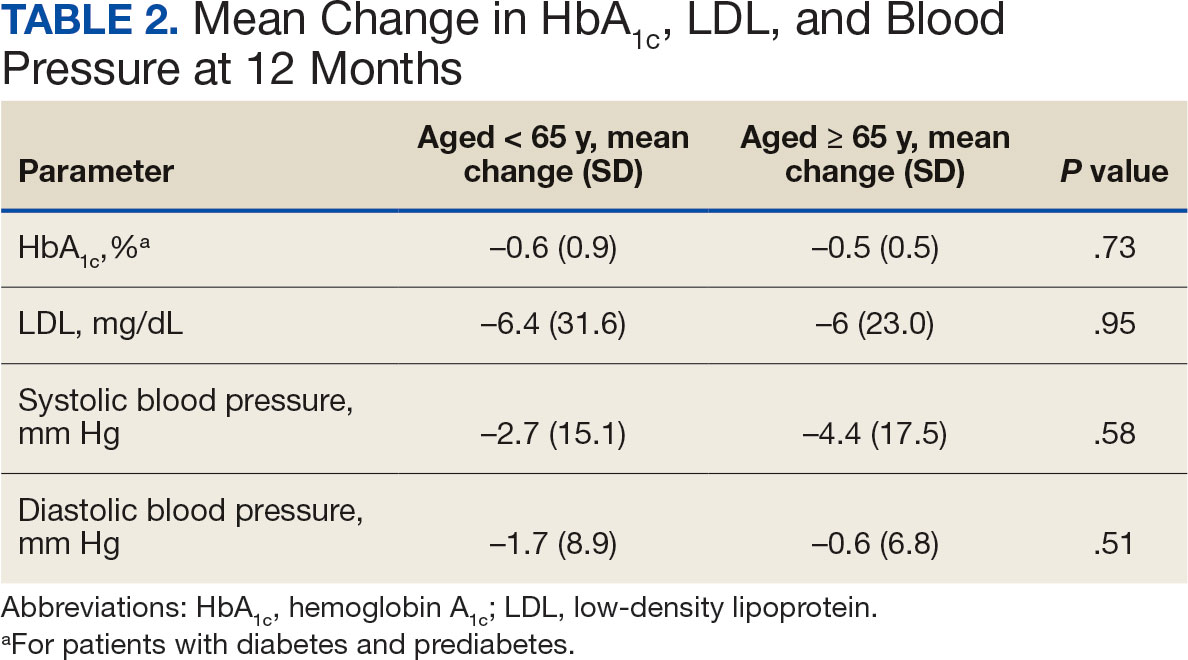
At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).
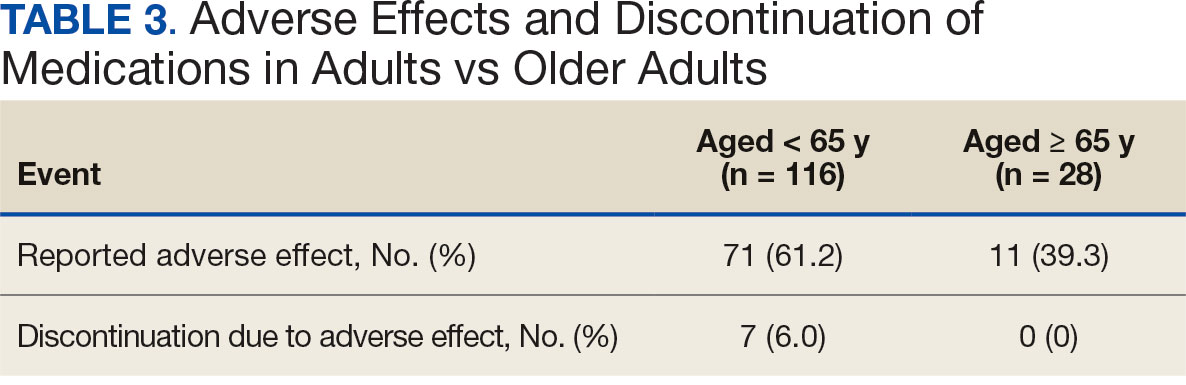
For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).
Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.

Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).

Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).

At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).

For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).

Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.

Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).

Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).

At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).

For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).

Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).
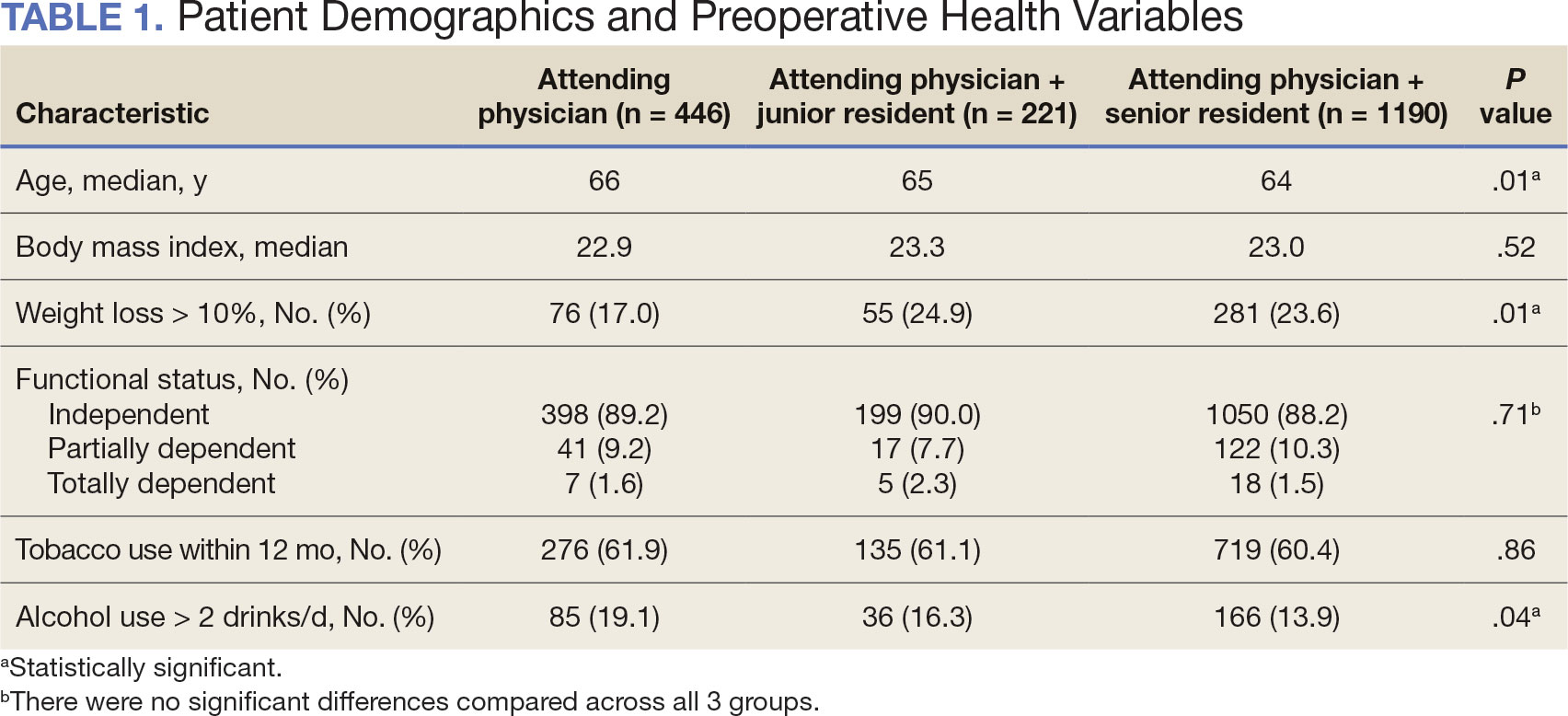
When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.
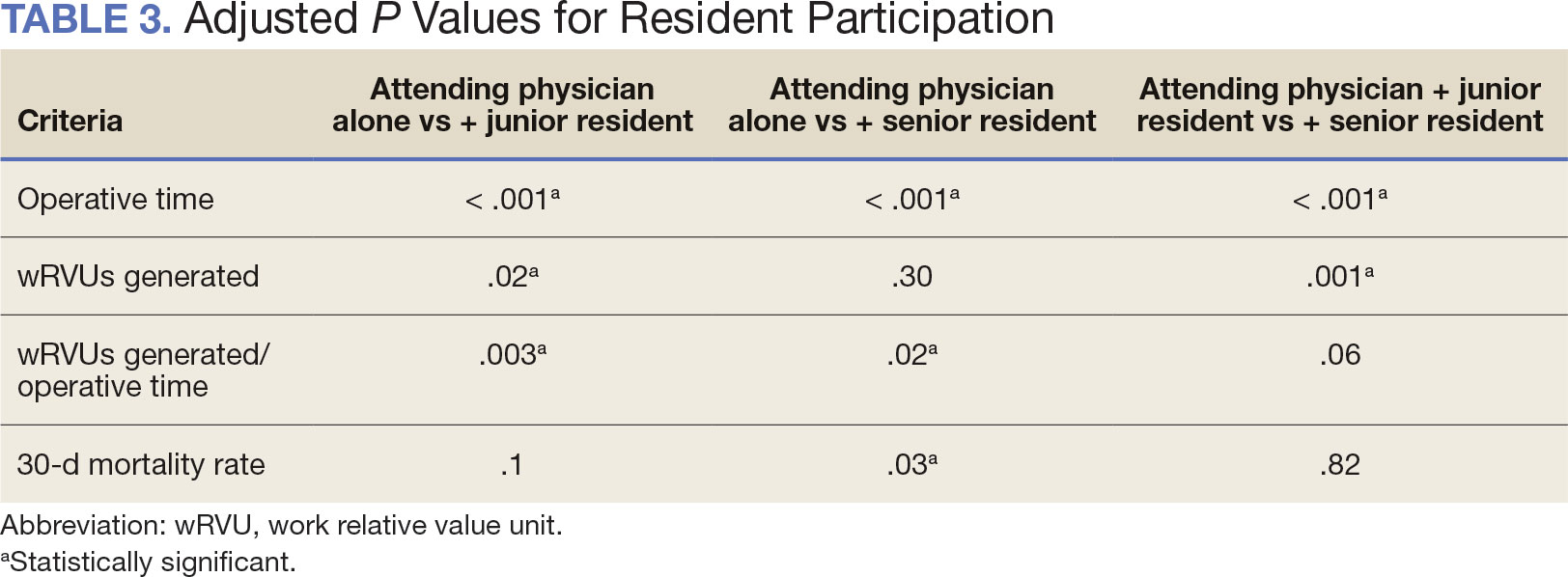
The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).
The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).
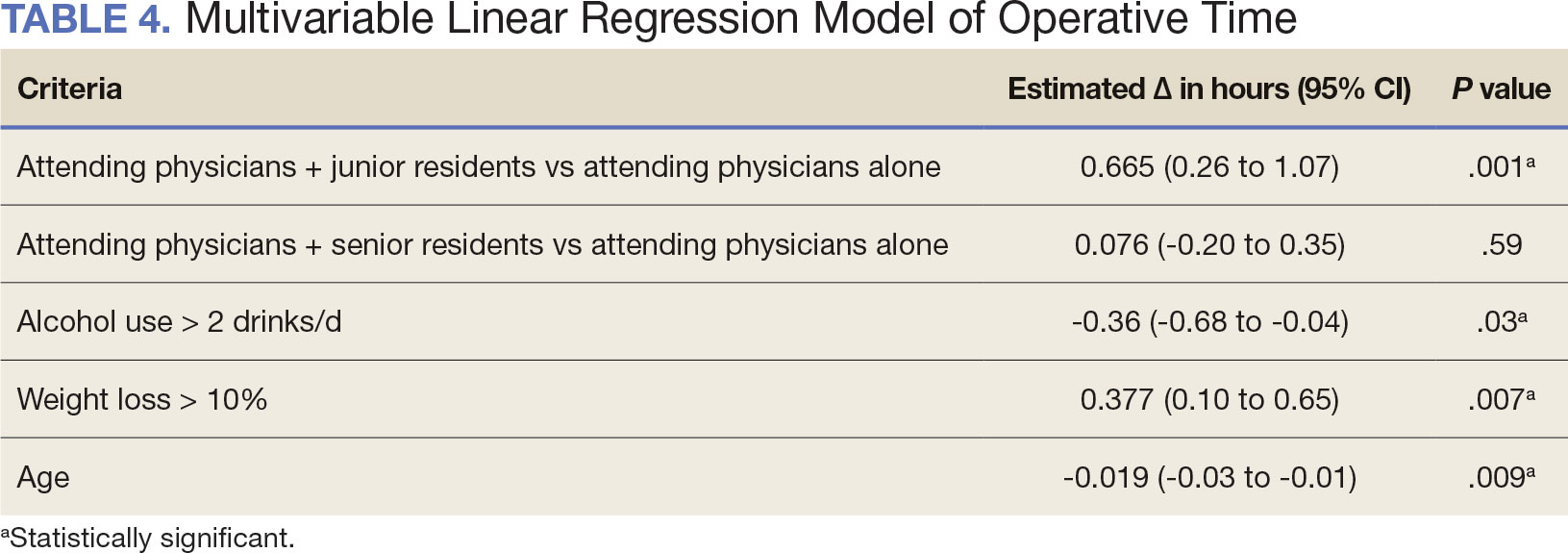
A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.
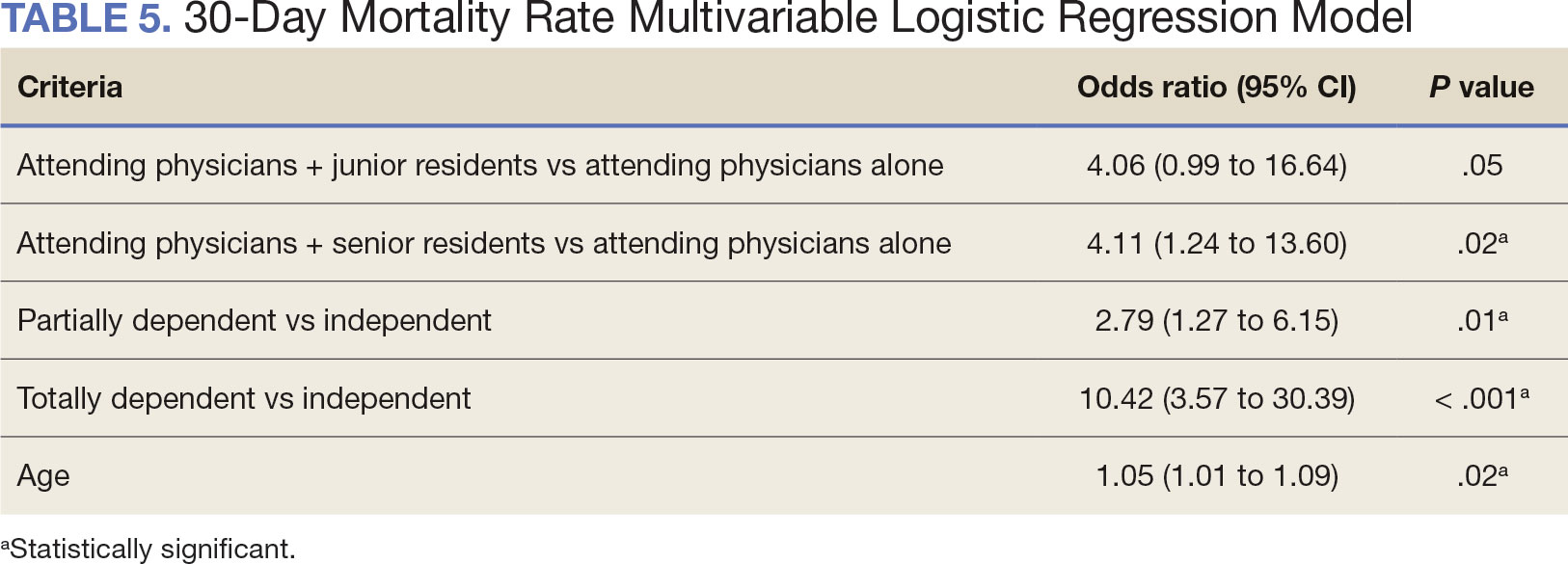
Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).


When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.

The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).


The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).

A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.

Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
The US Department of Veterans Affairs (VA) has been integral in resident training. Resident surgical training requires a balance of supervision and autonomy, along with procedure repetition and appropriate feedback.1-3 Non-VA research has found that resident participation across various otolaryngology procedures, including thyroidectomy, neck dissection, and laryngectomy, does not increase patient morbidity.4-7 However, resident involvement in private and academic settings that included nonhead and neck procedures was linked to increased operative time and reduced productivity, as determined by work relative value units (wRVUs).7-13 This has also been identified in other specialties, including general surgery, orthopedics, and ophthalmology.14-16
Unlike the private sector, surgeon compensation at the VA is not as closely linked to operative productivity, offering a unique setting for resident training. While VA integration in otolaryngology residency programs increases resident case numbers, particularly in head and neck cases, the impact on VA patient outcomes and productivity is unknown.17 The use of larynxpreserving treatment modalities for laryngeal cancer has led to a decline in the number of total laryngectomies performed, which could potentially impact resident operative training for laryngectomies.18-20
This study sought to determine the impact of resident participation on operative time, wRVUs, and patient outcomes in veterans who underwent a total laryngectomy. This study was reviewed and approved by the MedStar Georgetown University Hospital Institutional Review Board and Research and Development Committee (#1595672).
Methods
A retrospective cohort of veterans nationwide who underwent total laryngectomy between 2001 and 2021, with or without neck dissection, was identified from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Data were extracted via the VA Informatics and Computing Infrastructure and patients were included based on Current Procedural Terminology codes for total laryngectomy, with or without neck dissection (31320, 31360, 31365). Laryngopharyngectomies, partial laryngectomies, and minimally invasive laryngectomies were excluded. VASQIP nurse data managers reviewed patient data for operative data, postoperative outcomes (including 30- day morbidity and mortality), and preoperative risk factors (Appendix).21
The VASQIP data provide the highest resident or postgraduate year (PGY) per surgery. PGY 1, 2, and 3 were considered junior residents and PGY ≥4, surgical fellows, and individuals who took research years during residency were considered senior residents. Cases performed by attending physicians alone were compared with those involving junior or senior residents.
Patient demographic data included age, body mass index, smoking and alcohol use, weight loss, and functional status. Consumption of any tobacco products within 12 months of surgery was considered tobacco use. Drinking on average ≥2 alcoholic beverages daily was considered alcohol use. Weight loss was defined as a 10% reduction in body weight within the 6 months before surgery, excluding patients enrolled in a weight loss program. Functional status was categorized as independent, partially dependent, totally dependent, and unknown.
Primary outcomes included operative time, wRVUs generated, and wRVUs generated per hour of operative time. Postoperative complications were recorded both as a continuous variable and as a binary variable for presence or absence of a complication. Additional outcome variables included length of postoperative hospital stay, return to the operating room (OR), and death within 30 days of surgery.
Statistical Analysis
Data were summarized using frequency and percentage for categorical variables and median with IQR for continuous variables. Data were also summarized based on resident involvement in the surgery and the PGY level of the residents involved. The occurrence of total laryngectomy, rate of complications, and patient return to the OR were summarized by year.
Univariate associations between resident involvement and surgical outcomes were analyzed using the Kruskal-Wallis test for continuous variables and the ÷2 test for categorical variables. A Fisher exact test was used when the cell count in the contingency table was < 5. The univariate associations between surgical outcomes and demographic/preoperative variables were examined using 2-sided Wilcoxon ranksum tests or Kruskal-Wallis tests between continuous variables and categorical variables, X2 or Fisher exact test between 2 categorical variables, and 2-sided Spearman correlation test between 2 continuous variables. A false-discovery rate approach was used for simultaneous posthoc tests to determine the adjusted P values for wRVUs generated/operative time for attending physicians alone vs with junior residents and for attending physicians alone vs with senior residents. Models were used to evaluate the effects of resident involvement on surgical outcomes, adjusting for variables that showed significant univariate associations. Linear regression models were used for operative time, wRVUs generated, wRVUs generated/operative time, and length of postoperative stay. A logistic regression model was used for death within 30 days. Models were not built for postoperative complications or patient return to the OR, as these were only statistically significantly associated with the patient’s preoperative functional status. A finding was considered significant if P < .05. All analyses were performed using statistical software RStudio Version 2023.03.0.
Results
Between 2001 and 2021, 1857 patients who underwent total laryngectomy were identified from the VASQIP database nationwide. Most of the total laryngectomies were staffed by an attending physician with a senior resident (n = 1190, 64%), 446 (24%) were conducted by the attending physician alone, and 221 (12%) by an attending physician with a junior resident (Table 1). The mean operating time for an attending physician alone was 378 minutes, 384 minutes for an attending physician with a senior resident, and 432 minutes for an attending physician with a junior resident (Table 2). There was a statistically significant increase in operating time for laryngectomies with resident participation compared to attending physicians operating alone (P < .001).


When the wRVUs generated/operative time was analyzed, there was a statistically significant difference between comparison groups. Total laryngectomies performed by attending physicians alone had the highest wRVUs generated/operative time (5.5), followed by laryngectomies performed by attending physicians with senior residents and laryngectomies performed by attending physicians with junior residents (5.2 and 4.8, respectively; P = .002). Table 3 describes adjusted P values for wRVUs generated/ operative time for total laryngectomies performed by attending physicians alone vs with junior residents (P = .003) and for attending physicians alone vs with senior residents (P = .02). Resident participation in total laryngectomies did not significantly impact the development or number of postoperative complications or the rate of return to the OR.

The number of laryngectomies performed in a single fiscal year peaked in 2010 at 170 cases (Figure 1). Between 2001 and 2021, the mean rates of postoperative complications (21.3%) and patient return to the OR (14.6%) did not significantly change. Resident participation in total laryngectomies also peaked in 2010 at 89.0% but has significantly declined, falling to a low of 43.6% in 2021 (Figure 2). From 2001 to 2011, the mean resident participation rate in total laryngectomies was 80.6%, compared with 68.3% from 2012 to 2021 (P < .001).


The effect of various demographic and preoperative characteristics on surgical outcomes was also analyzed. A linear regression model accounted for each variable significantly associated with operative time. On multivariable analysis, when all other variables were held constant, Table 4 shows the estimated change in operative time based on certain criteria. For instance, the operative time for attendings with junior residents surgeries was 40 minutes longer (95% CI, 16 to 64) than that of attending alone surgeries (P = .001). Furthermore, operative time decreased by 1.1 minutes (95% CI, 0.30 to 2.04) for each 1-year increase in patient age (P = .009).

A multivariable logistic regression model evaluated the effect of resident involvement on 30-day mortality rates. Senior resident involvement (P = .02), partially dependent functional status (P = .01), totally dependent functional status (P < .001), and advanced age (P = .02) all were significantly associated with 30-day mortality (Table 5). When other variables remained constant, the odds of death for totally dependent patients were 10.4 times higher than that of patients with independent functional status. Thus, totally dependent functional status appeared to have a greater impact on this outcome than resident participation. The linear regression model for postoperative length of stay demonstrated that senior resident involvement (P = .04), functional status (partially dependent vs independent P < .001), and age (P = .03) were significantly associated with prolonged length of stay.

Discussion
Otolaryngology residency training is designed to educate future otolaryngologists through hands-on learning, adequate feedback, and supervision.1 Although this exposure is paramount for resident education, balancing appropriate supervision and autonomy while mitigating patient risk has been difficult. Numerous non-VA studies have reviewed the impact of resident participation on patient outcomes in various specialties, ranging from a single institution to the National Surgical Quality Improvement Program (NSQIP).4,5,7,22 This study is the first to describe the nationwide impact of resident participation on outcomes in veterans undergoing total laryngectomy.
This study found that resident participation increases operative time and decreases wRVUs generated/operative time without impacting complication rates or patient return to the OR. This reinforces the notion that under close supervision, resident participation does not negatively impact patient outcomes. Resident operative training requires time and dedication by the attending physician and surgical team, thereby increasing operative time. Because VA physician compensation is not linked with productivity as closely as it is in other private and academic settings, surgeons can dedicate more time to operative teaching. This study found that a total laryngectomy involving a junior resident took about 45 minutes longer than an attending physician working alone.
As expected, with longer operative times, the wRVUs generated/operative time ratio was lower in cases with resident participation. Even though resident participation leads to lower OR efficiency, their participation may not significantly impact ancillary costs.23 However, a recent study from NSQIP found an opportunity cost of $60.44 per hour for surgeons operating with a resident in head and neck cases.13
Postoperative complications and mortality are key measures of surgical outcomes in addition to operative time and efficiency. This study found that neither junior nor senior resident participation significantly increased complication rates or patient return to the OR. Despite declining resident involvement and the number of total laryngectomy surgeries in the VA, the complication rate has remained steady. The 30-day mortality rate was significantly higher in cases involving senior residents compared to cases with attending physicians alone. This could be a result of senior resident participation in more challenging cases, such as laryngectomies performed as salvage surgery following radiation. Residents are more often involved in cases with greater complexity at teaching institutions.24-26 Therefore, the higher mortality seen among laryngectomies with senior resident involvement is likely due to the higher complexity of those cases.
The proportion of resident involvement in laryngectomies at VA medical centers has been decreasing over time. Due to the single payer nature of the VA health care system and the number of complex and comorbid patients, the VA offers an invaluable space for resident education in the OR. The fact that less than half of laryngectomies in 2021 involved resident participation is noteworthy for residency training programs. As wRVU compensation models evolve, VA attending surgeons may face less pressure to move the case along, leading to a high potential for operative teaching. Therefore, complex cases, such as laryngectomies, are often ideal for resident participation in the VA.
The steady decline in total laryngectomies at the VA parallels the recent decrease seen in non-VA settings.20 This is due in part to the use of larynx-preserving treatment modalities for laryngeal cancer as well as decreases in the incidence of laryngeal cancer due to population level changes in smoking behaviors. 18,19 Although a laryngectomy is not a key indicator case as determined by the Accreditation Council for Graduate Medical Education, it is important for otolaryngology residents to be exposed to these cases and have a thorough understanding of the operative technique.27 Total laryngectomy was selected for this study because it is a complex and time-consuming surgery with somewhat standardized surgical steps. Unlike microvascular surgery that is very rarely performed by an attending physician alone, laryngectomies can be performed by attending physicians alone or with a resident.28
Limitations
Since this was a retrospective study, it was susceptible to errors in data entry and data extraction from the VASQIP database. Another limitation is the lack of preoperative treatment data on tumor stage and prior nonoperative treatment. For example, a salvage laryngectomy after treatment with radiation and/or chemoradiation is a higher risk procedure than an upfront laryngectomy. Senior resident involvement may be more common in patients undergoing salvage laryngectomy due to the high risk of postoperative fistula and other complications. This may have contributed to the association identified between senior resident participation and 30-day mortality.
Since we could not account for residents who took research years or were fellows, a senior resident may have been mislabeled as a junior resident or vice versa. However, because most research years occur following the third year of residency. We are confident that PGY-1, PGY-2, and PGY-3 is likely to capture junior residents. Other factors, such as coattending surgeon cases, medical student assistance, and fellow involvement may have also impacted the results of this study.
Conclusions
This study is the first to investigate the impact of resident participation on operative time, wRVUs generated, and complication rates in head and neck surgery at VA medical centers. It found that resident participation in total laryngectomies among veterans increased operative time and reduced wRVUs generated per hour but did not impact complication rate or patient return to the OR. The VA offers a unique and invaluable space for resident education and operative training, and the recent decline in resident participation among laryngectomies is important for residency programs to acknowledge and potentially address moving forward.
In contrast to oral cavity resections which can vary from partial glossectomies to composite resections, laryngectomy represents a homogenous procedure from which to draw meaningful conclusions about complication rates, operative time, and outcome. Future directions should include studying other types of head and neck surgery in the VA to determine whether the impact of resident participation mirrors the findings of this study.
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
- Chung RS. How much time do surgical residents need to learn operative surgery? Am J Surg. 2005;190(3):351-353. doi:10.1016/j.amjsurg.2005.06.035
- S, Darzi A. Defining quality in surgical training: perceptions of the profession. Am J Surg. 2014;207(4):628-636. doi:10.1016/j.amjsurg.2013.07.044
- Bhatti NI, Ahmed A, Choi SS. Identifying quality indicators of surgical of surgical training: a national survey. Laryngoscope. 2015;125(12):2685-2689. doi:10.1002/lary.25262
- Abt NB, Reh DD, Eisele DW, Francis HW, Gourin CG. Does resident participation influence otolaryngology-head and neck surgery morbidity and mortality? Laryngoscope. 2016;126(10):2263-2269. doi:10.1002/lary.25973
- Jubbal KT, Chang D, Izaddoost SA, Pederson W, Zavlin D, Echo A. Resident involvement in microsurgery: an American College of Surgeons national surgical quality improvement program analysis. J Surg Educ. 2017;74(6):1124-1132. doi:10.1016/j.jsurg.2017.05.017
- Kshirsagar RS, Chandy Z, Mahboubi H, Verma SP. Does resident involvement in thyroid surgery lead to increased postoperative complications? Laryngoscope. 2017;127(5):1242-1246. doi:10.1002/lary.26176
- Vieira BL, Hernandez DJ, Qin C, Smith SS, Kim JY, Dutra JC. The impact of resident involvement on otolaryngology surgical outcomes. Laryngoscope. 2016;126(3):602-607. doi:10.1002/lary.25046
- Advani V, Ahad S, Gonczy C, Markwell S, Hassan I. Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg. 2012;203(3):347-352. doi:10.1016/j.amjsurg.2011.08.015
- Quinn NA, Alt JA, Ashby S, Orlandi RR. Time, resident involvement, and supply drive cost variability in septoplasty with turbinate reduction. Otolaryngol Head Neck Surg. 2018;159(2):310-314. doi:10.1177/0194599818765099
- Leader BA, Wiebracht ND, Meinzen-Derr J, Ishman SL. The impact of resident involvement on tonsillectomy outcomes and surgical time. Laryngoscope. 2020;130(10):2481-2486. doi:10.1002/lary.28427
- Muelleman T, Shew M, Muelleman RJ, et al. Impact of resident participation on operative time and outcomes in otologic surgery. Otolaryngol Head Neck Surg. 2018;158(1):151-154. doi:10.1177/0194599817737270
- Puram SV, Kozin ED, Sethi R, et al. Impact of resident surgeons on procedure length based on common pediatric otolaryngology cases. Laryngoscope. 2015;125(4):991 -997. doi:10.1002/lary.24912
- Chow MS, Gordon AJ, Talwar A, Lydiatt WM, Yueh B, Givi B. The RVU compensation model and head and neck surgical education. Laryngoscope. 2024;134(1):113-119. doi:10.1002/lary.30807
- Papandria D, Rhee D, Ortega G, et al. Assessing trainee impact on operative time for common general surgical procedures in ACS-NSQIP. J Surg Educ. 2012;69(2):149-155. doi:10.1016/j.jsurg.2011.08.003
- Pugely AJ, Gao Y, Martin CT, Callagh JJ, Weinstein SL, Marsh JL. The effect of resident participation on short-term outcomes after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(7):2290-2300. doi:10.1007/s11999-014-3567-0
- Hosler MR, Scott IU, Kunselman AR, Wolford KR, Oltra EZ, Murray WB. Impact of resident participation in cataract surgery on operative time and cost. Ophthalmology. 2012;119(1):95-98. doi:10.1016/j.ophtha.2011.06.026
- Lanigan A, Spaw M, Donaghe C, Brennan J. The impact of the Veteran’s Affairs medical system on an otolaryngology residency training program. Mil Med. 2018;183(11-12):e671-e675. doi:10.1093/milmed/usy041
- American Society of Clinical Oncology, Pfister DG, Laurie SA, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24(22):3693-3704. doi:10.1200/JCO.2006.07.4559
- Forastiere AA, Ismaila N, Lewin JS, et al. Use of larynxpreservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(11):1143-1169. doi:10.1200/JCO.2017.75.7385
- Verma SP, Mahboubi H. The changing landscape of total laryngectomy surgery. Otolaryngol Head Neck Surg. 2014;150(3):413-418. doi:10.1177/0194599813514515
- Habermann EB, Harris AHS, Giori NJ. Large surgical databases with direct data abstraction: VASQIP and ACSNSQIP. J Bone Joint Surg Am. 2022;104(suppl 3):9-14. doi:10.2106/JBJS.22.00596
- Benito DA, Mamidi I, Pasick LJ, et al. Evaluating resident involvement and the ‘July effect’ in parotidectomy. J Laryngol Otol. 2021;135(5):452-457. doi:10.1017/S0022215121000578
- Hwang CS, Wichterman KA, Alfrey EJ. The cost of resident education. J Surg Res. 2010;163(1):18-23. doi:10.1016/j.jss.2010.03.013
- Saliba AN, Taher AT, Tamim H, et al. Impact of resident involvement in surgery (IRIS-NSQIP): looking at the bigger picture based on the American College of Surgeons- NSQIP database. J Am Coll Surg. 2016; 222(1):30-40. doi:10.1016/j.jamcollsurg.2015.10.011
- Khuri SF, Najjar SF, Daley J, et al. Comparison of surgical outcomes between teaching and nonteaching hospitals in the Department of Veterans Affairs. Ann Surg. 2001;234(3):370-383. doi:10.1097/00000658-200109000-00011
- Relles DM, Burkhart RA, Pucci MJ et al. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279-285. doi:10.1007/s11605-013-2372-5
- Accreditation Council for Graduate Medical Education. Required minimum number of key indicator procedures for graduating residents. June 2019. Accessed January 2, 2025. https://www.acgme.org/globalassets/pfassets/programresources/280_core_case_log_minimums.pdf
- Brady JS, Crippen MM, Filimonov A, et al. The effect of training level on complications after free flap surgery of the head and neck. Am J Otolaryngol. 2017;38(5):560-564. doi:10.1016/j.amjoto.2017.06.001
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
Resident Participation Impact on Operative Time and Outcomes in Veterans Undergoing Total Laryngectomy
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
PTSD Guidelines
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.
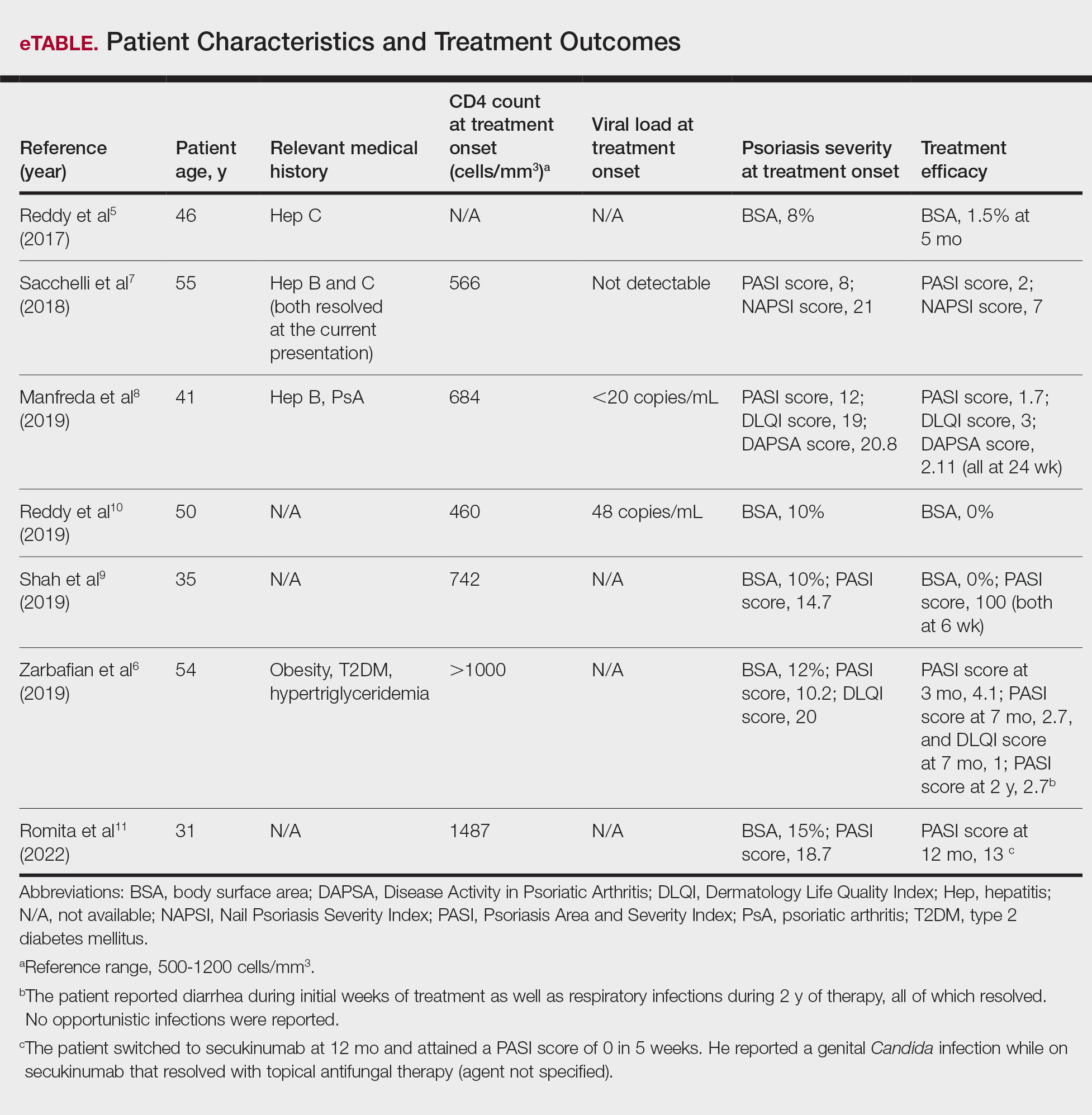
The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.

The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.

The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
PRACTICE POINT
- For patients with HIV who require systemic therapy for psoriasis, apremilast may provide an effective and safe therapeutic option, with minimal immunosuppressive adverse effects.
Oral Biologics: The New Wave for Treating Psoriasis
Oral Biologics: The New Wave for Treating Psoriasis
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.
A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.

A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
Biologic therapies have transformed the treatment of psoriasis. Current biologics approved for psoriasis include monoclonal antibodies targeting various pathways: tumor necrosis factor α (TNF-α) inhibitors (infliximab, adalimumab, certolizumab, etanercept), the p40 subunit common to IL-12 and IL-23 (ustekinumab), the p19 subunit of IL-23 (guselkumab, tildrakizumab, risankizumab), IL-17A (secukinumab, ixekizumab), IL-17 receptor A (brodalumab), and dual IL-17A/IL-17F inhibition (bimekizumab). Recent research showed that risankizumab achieved the highest Psoriasis Area and Severity Index (PASI) 90 scores in short- and long-term treatment periods (4 and 16 weeks, respectively) compared to other biologics, and IL-23 inhibitors demonstrated the lowest short- and long-term adverse event rates and the most favorable long-term risk-benefit profile compared to IL-17, IL-12/23, and TNF-α inhibitors.1
Although these monoclonal antibodies have revolutionized psoriasis treatment, they are large proteins that must be administered subcutaneously or via intravenous injection. Emerging biologics are smaller proteins administered orally via a tablet or pill. In clinical trials, oral biologics have demonstrated efficacy (eTable), suggesting that oral biologics may be the future for psoriasis treatment, as this noninvasive delivery method may help improve patient compliance with treatment.

A major inflammatory pathway in psoriasis, IL-23 has been an effective and safe drug target. The novel oral IL-23 inhibitor, JNJ-2113, was discovered in 2017 and currently is being compared to deucravacitinib in the phase III ICONIC-LEAD trial (ClinicalTrials. gov Identifier NCT06095115) in patients with moderate to severe plaque psoriasis.2,3 In the phase IIb FRONTIER 1 trial, treatment with either 3 once-daily (25 mg, 50 mg, 100 mg) and 2 twice-daily (25 mg, 100 mg) doses of JNJ-2113 led to significant improvements in PASI 75 response at 16 weeks compared to placebo (P<.001).4 In the phase IIb long-term extension FRONTIER 2 trial, JNJ-2113 maintained high rates of skin clearance through 52 weeks in adults with moderate to severe plaque psoriasis, with the highest PASI 75 response observed in the 100-mg twice-daily group (32/42 [76.2%]).5 Responses were maintained through week 52 for all JNJ-2113 treatment groups for PASI 90 and PASI 100 endpoints. In addition to ICONIC-LEAD, JNJ-2113 is being evaluated in the phase III multicenter, randomized, double-blind, placebo-controlled trial ICONIC-TOTAL (NCT06095102) in patients with special area psoriasis and ANTHEM-UC (NCT06049017) in patients with ulcerative colitis to evaluate its efficacy and safety. The most common adverse events associated with JNJ-77242113 were mild to moderate and included COVID-19 infection and nasopharyngitis.6 Higher rates of COVID-19 infection likely were due to immune compromise in the setting of the recent pandemic. Similar percentages of at least 1 adverse event were found in JNJ-77242113 and placebo groups (52%-58.6% and 51%-65.7%, respectively).4,5,7
An orally administered small-molecule inhibitor of IL-17A, LY3509754, may represent a convenient alternative to IL-17A–
The small potent molecule SAR441566 inhibits TNF-α by stabilizing an asymmetrical form of the soluble TNF trimer. As the asymmetrical trimer is the biologically active form of TNF-α, stabilization of the trimer compromises downstream signaling and inhibits the functions of TNF-α in vitro and in vivo. Recently, SAR441566 was found to be safe and well tolerated in healthy participants, showing efficacy in mild to moderate psoriasis in a phase Ib trial.9 A phase II trial of SAR441566 (NCT06073119) is being developed to create a more convenient orally bioavailable treatment option for patients with psoriasis compared to established biologic drugs targeting TNF-α.10
Few trials have focused on investigating the antipsoriatic effects of orally administered small molecules. Some of these small molecules can enter cells and inhibit the activation of T lymphocytes, leukocyte trafficking, leukotriene activity/production and angiogenesis, and promote apoptosis. Oral administration of small molecules is the future of effective and affordable psoriasis treatment, but safety and efficacy must first be assessed in clinical trials. JNJ-77242113 has shown a more promising safety profile, has recently undergone phase III trials, and may represent the newest wave for psoriasis treatment. While LY3509754 had a strong pharmacokinetics profile, it was poorly tolerated, and study participants' laboratory results suggested the drug to be hepatotoxic.8 SAR441566 has been shown to be safe and well tolerated in treating psoriasis, and phase II readouts are expected later in 2025. We can expect a new wave of psoriasis treatments with emerging oral therapies.
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
- Wride AM, Chen GF, Spaulding SL, et al. Biologics for psoriasis. Dermatol Clin. 2024;42:339-355. doi:10.1016/j.det.2024.02.001
- New data shows JNJ-2113, the first and only investigational targeted oral peptide, maintained skin clearance in moderate-to-severe plaque psoriasis through one year. Johnson & Johnson website. March 9, 2024. Accessed August 29, 2024. https://www.jnj.com/media-center/press-releases/new-data-shows-jnj-2113-the-first-and-only-investigational-targeted-oral-peptide-maintained-skin-clearance-in-moderate-to-severe-plaque-psoriasis-through-one-year
- Drakos A, Torres T, Vender R. Emerging oral therapies for the treatment of psoriasis: a review of pipeline agents. Pharmaceutics. 2024;16:111. doi:10.3390/pharmaceutics16010111
- Bissonnette R. A phase 2, randomized, placebo-controlled, dose -ranging study of oral JNJ-77242113 for the treatment of moderate -to-severe plaque psoriasis: FRONTIER 1. Presented at: 25th World Congress of Dermatology; July 3, 2023; Suntec City, Singapore.
- Ferris L. S026. A phase 2b, long-term extension, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severeplaque psoriasis: FRONTIER 2. Presented at: Annual Meeting of the American Academy of Dermatology; San Diego, California; March 8-12, 2024.
- Inc PT. Protagonist announces two new phase 3 ICONIC studies in psoriasis evaluating JNJ-2113 in head-to-head comparisons with deucravacitinib. ACCESSWIRE website. November 27, 2023. Accessed August 29, 2024. https://www.accesswire.com/810075/protagonist-announces-two-new-phase-3-iconic-studies-in-psoriasis-evaluating-jnj-2113-in-head-to-head-comparisons-with-deucravacitinib
- Bissonnette R, Pinter A, Ferris LK, et al. An oral interleukin-23-receptor antagonist peptide for plaque psoriasis. N Engl J Med. 2024;390:510-521. doi:10.1056/NEJMoa2308713
- Datta-Mannan A, Regev A, Coutant DE, et al. Safety, tolerability, and pharmacokinetics of an oral small molecule inhibitor of IL-17A (LY3509754): a phase I randomized placebo-controlled study. Clin Pharmacol Ther. 2024;115:1152-1161. doi:10.1002/cpt.3185
- Vugler A, O’Connell J, Nguyen MA, et al. An orally available small molecule that targets soluble TNF to deliver anti-TNF biologic-like efficacy in rheumatoid arthritis. Front Pharmacol. 2022;13:1037983. doi:10.3389/fphar.2022.1037983
- Sanofi pipeline transformation to accelerate growth driven by record number of potential blockbuster launches, paving the way to industry leadership in immunology. News release. Sanofi; New York: Sanofi; Dec 7, 2023. https://www.sanofi.com/en/media-room/press-releases/2023/2023-12-07-02-30-00-2792186
Oral Biologics: The New Wave for Treating Psoriasis
Oral Biologics: The New Wave for Treating Psoriasis
PRACTICE POINTS
- The biologics that currently are approved for psoriasis are expensive and must be administered via injection due to their large molecule size.
- Emerging small-molecule oral therapies for psoriasis are effective and affordable and may represent the future for psoriasis patients.
Legislative, Practice Management, and Coding Updates for 2025
Legislative, Practice Management, and Coding Updates for 2025
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
Update on Excimer Laser CPT Codes
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11
Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13
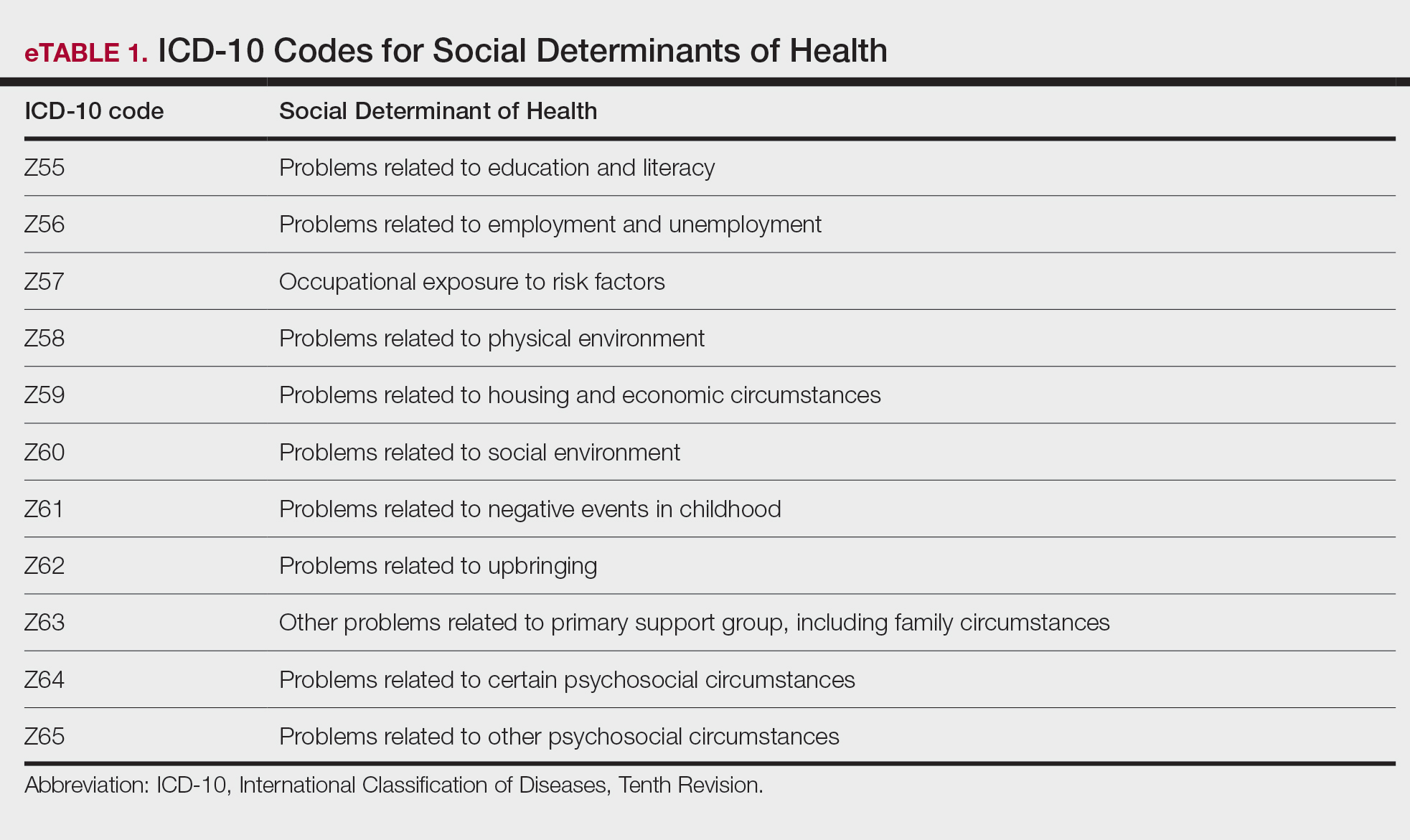
New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14
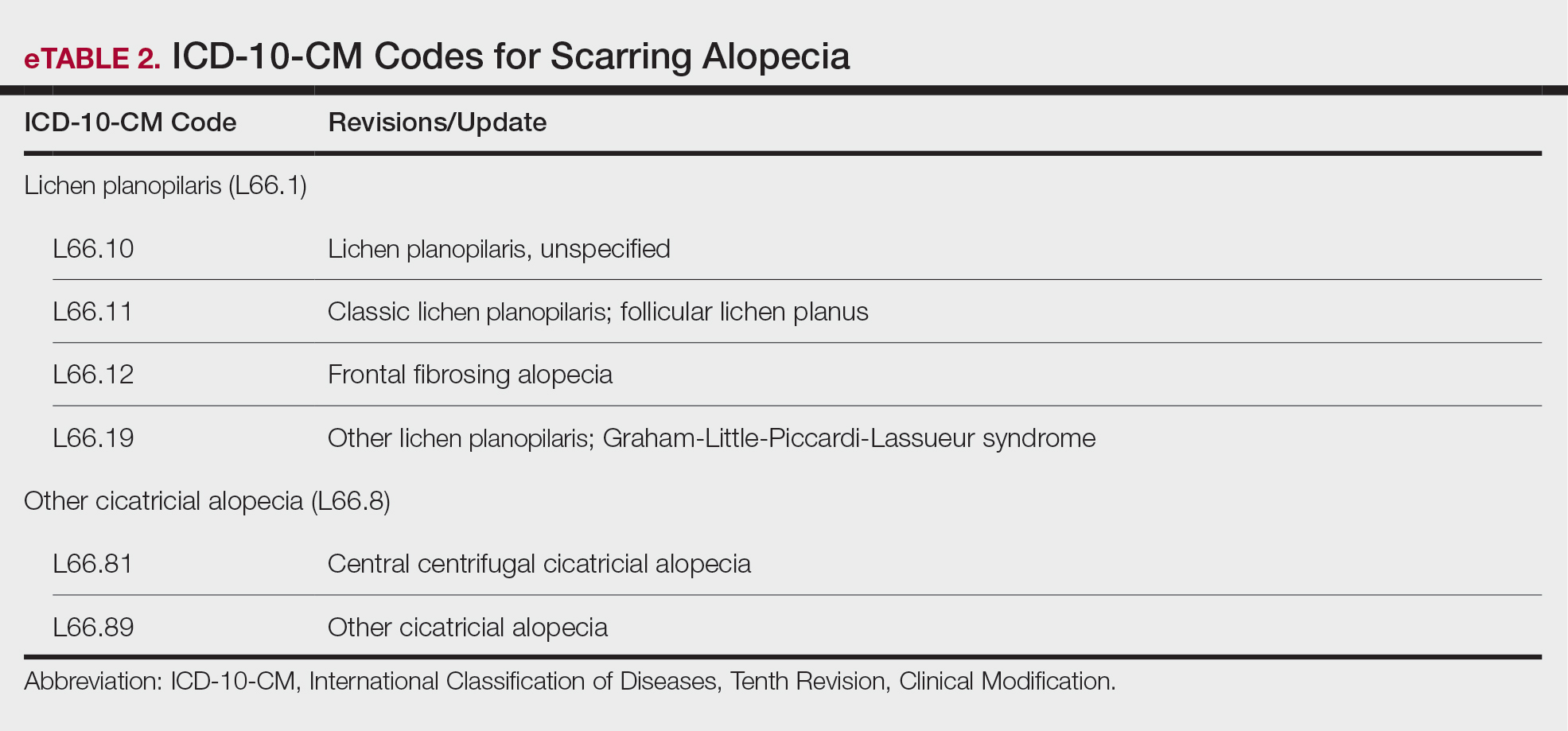
Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
Update on Excimer Laser CPT Codes
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11

Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13

New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14

Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
Health care costs continue to increase in 2025 while physician reimbursement continues to decrease. Of the $4.5 trillion spent on health care in 2022, only 20% was spent on physician and clinical services.1 Since 2001, practice expense has risen 47%, while the Consumer Price Index has risen 73%; adjusted for inflation, physician reimbursement has declined 30% since 2001.2
The formula for Medicare payments for physician services, calculated by multiplying the conversion factor (CF) by the relative value unit (RVU), was developed by the Centers for Medicare & Medicaid Services (CMS) in 1992. The combination of the physician’s work, the practice’s expense, and the cost of professional liability insurance make up RVUs, which are aligned by geographic index adjustments.3 The 2024 CF was $32.75, compared to $32.00 in 1992. The proposed 2025 CF is $32.35, which is a 10% decrease since 2019 and a 2.8% decrease relative to the 2024 Medicare Physician Fee Schedule (MPFS). The 2.8% cut is due to expiration of the 2.93% temporary payment increase for services provided by the Consolidated Appropriations Act 2024 and the supplemental relief provided from March 9, 2024, to December 31, 2024.4 If the CF had increased with inflation, it would have been $71.15 in 2024.4
Declining reimbursement rates for physician services undermine the ability of physician practices to keep their doors open in the face of increased operating costs. Faced with the widening gap between what Medicare pays for physician services and the cost of delivering value-based, quality care, physicians are urging Congress to pass a reform package to permanently strengthen Medicare.
Herein, an overview of key coding updates and changes, telehealth flexibilities, and a new dermatologyfocused Merit-based Incentive Payment System (MIPS) Value Pathways is provided.
Update on the Medicare Economic Index Postponement
Developed in 1975, the Medicare Economic Index (MEI) is a measure of practice cost inflation. It is a yearly calculation that estimates the annual changes in physicians’ operating costs to determine appropriate Medicare physician payment updates.5 The MEI is composed of physician practice costs (eg, staff salaries, office space, malpractice insurance) and physician compensation (direct earnings by the physician). Both are used to calculate adjustments to Medicare physician payments to account for inflationary increases in health care costs. The MEI for 2025 is projected to increase by 3.5%, while physician payment continues to dwindle.5 This disparity between rising costs and declining physician payments will impact patient access to medical care. Physicians may choose to stop accepting Medicare and other health insurance, face the possibility of closing or selling their practices, or even decide to leave the profession.
The CMS has continued to delay implementation of the 2017 MEI cost weights (which currently are based on 2006 data5) for RVUs in the MPFS rate setting for 2025 pending completion of the American Medical Association (AMA) Physician Practice Information Survey.6 The AMA contracted with an independent research company to conduct the survey, which will be used to update the MEI. Survey data will be shared with the CMS in early 2025.6
Future of Telehealth is Uncertain
On January 1, 2025, many telehealth flexibilities were set to expire; however, Congress passed an extension of the current telehealth policy flexibilities that have been in place since the COVID-19 pandemic through March 31, 2025.7 The CMS recognizes concerns about maintaining access to Medicare telehealth services once the statutory flexibilities expire; however, it maintains that it has limited statutory authority to extend these Medicare telehealth flexibilities.8 There will be originating site requirements and geographic location restrictions. Clinicians working in a federally qualified health center or a rural health clinic would not be affected.8
The CMS rejected adoption of 16 of 17 new Current Procedural Terminology (CPT) codes (98000–98016) for telemedicine evaluation and management (E/M) services, rendering them nonreimbursable.8 Physicians should continue to use the standard E/M codes 99202 through 99215 for telehealth visits. The CMS only approved code 99016, which will replace Healthcare Common Procedure Coding System code G2012, for brief virtual check-in encounters. The CMS specified that CPT codes 99441 through 99443, which describe telephone E/M services, have been removed and are no longer valid for billing. Asynchronous communication (eg, store-and-forward technology via an electronic health record portal) will continue to be reported using the online digital E/M service codes 99421, 99422, and 99423.8
Practitioners can use their enrolled practice location instead of their home address when providing telehealth services from home.8 Teaching physicians will continue to be allowed to have a virtual presence for purposes of billing for services involving residents in all teaching settings, but only when the service is furnished remotely (ie, the patient, resident, and teaching physician all are in separate locations). The use of real-time audio and video technology for direct supervision has been extended through December 31, 2025, allowing practitioners to be immediately available virtually. The CMS also plans to permanently allow virtual supervision for lower-risk services that typically do not require the billing practitioner’s physical presence or extensive direction (eg, diagnostic tests, behavioral health, dermatology, therapy).8
It is essential to verify the reimbursement policies and billing guidelines of individual payers, as some may adopt policies that differ from the AMA and CMS guidelines.
When to Use Modifiers -59 and -76
Modifiers -59 and -76 are used when billing for multiple procedures on the same day and can be confused. These modifiers help clarify situations in which procedures might appear redundant or improperly coded, reducing the risk for claim denials and ensuring compliance with coding guidelines. Use modifier -59 when a procedure or service is distinct or separate from other services performed on the same day (eg, cryosurgery of 4 actinic keratoses and a tangential biopsy of a nevus). Use modifier -76 when a physician performs the exact same procedure multiple times on the same patient on the same day (eg, removing 2 nevi on the face with the same excision code or performing multiple biopsies on different areas on the skin).9
What Are the Medical Team Conference CPT Codes?
Dermatologists frequently manage complex medical and surgical cases and actively participate in tumor boards and multidisciplinary teams conferences. It is essential to be familiar with the relevant CPT codes that can be used in these scenarios: CPT code 99366 can be used when the medical team conference occurs face-to-face with the patient present, and CPT code 99367 can be used for a medical team conference with an interdisciplinary group of health care professionals from different specialties, each of whom provides direct care to the patient.10 For CPT code 99367, the patient and/or family are not present during the meeting, which lasts a minimum of 30 minutes or more and requires participation by a physician. Current Procedural Terminology code 99368 can be used for participation in the medical team conference by a nonphysician qualified health care professional. The reporting participants need to document their participation in the medical team conference as well as their contributed information that explains the case and subsequent treatment recommendations.10
No more than 1 individual from the same specialty may report CPT codes 99366 through 99368 at the same encounter.10 Codes 99366 through 99368 should not be reported when participation in the medical team conference is part of a facility or contractually provided by the facility such as group therapy.10 The medical team conference starts at the beginning of the review of an individual patient and ends at the conclusion of the review for coding purposes. Time related to record-keeping or report generation does not need to be reported. The reporting participant needs to be present for the entire conference. The time reported is not limited to the time that the participant is communicating with other team members or the patient and/or their family/ caregiver(s). Time reported for medical team conferences may not be used in the determination for other services, such as care plan oversight (99374-99380), prolonged services (99358, 99359), psychotherapy, or any E/M service. When the patient is present for any part of the duration of the team conference, nonphysician qualified health care professionals (eg, speech-language pathologists, physical therapists, occupational therapists, social workers, dietitians) report the medical team conference face-to-face with code 99366.10
Update on Excimer Laser CPT Codes
The CMS rejected values recommended for CPT codes (96920-96922) by the Relative Value Scale Update Committee, proposing lower work RVUs of 0.83, 0.90, and 1.15, respectively (Table).2,11 The CPT panel did not recognize the strength of the literature supporting the expanded use of the codes for conditions other than psoriasis. Report the use of excimer laser for treatment of vitiligo, atopic dermatitis, and alopecia areata using CPT code 96999 (unlisted special dermatological service or procedure).11

Update on the New G2211 Code
Healthcare Common Procedure Coding System code G2211 is an add-on complexity code that can be reported with all outpatient E/M visits to better account for additional resources associated with primary care or similarly ongoing medical care related to a patient’s single serious condition or complex condition.12 It can be billed if the physician is serving as the continuing focal point for all the patient's health care service needs, acting as the central point of contact for the patient’s ongoing medical care, and managing all aspects of their health needs over time. It is not restricted based on specialty, but it is determined based on the nature of the physician-patient relationship.12
Code G2211 should not be used for the following scenarios: (1) care provided by a clinician with a discrete, routine, or time-limited relationship with the patient, such as a routine skin examination or an acute allergic contact dermatitis; (2) conditions in which comorbidities are not present or addressed; (3) when the billing clinician has not assumed responsibility for ongoing medical care with consistency and continuity over time; and (4) visits billed with modifier -25.12 In the 2025 MPFS, the CMS is proposing to allow payment of G2211 when the code is reported by the same practitioner on the same day as an annual wellness visit, vaccine administration, or any Medicare Part B preventive service furnished in the office or outpatient setting (ie, creating a limited exception to the prohibition of using this code with modifier -25).2
Documentation in the medical record must support reporting code G2211 and indicate a medically reasonable and necessary reason for the additional RVUs (0.33 and additional payment of $16.05).12
Underutilization of Z Codes for Social Determinants of Health
Barriers to documentation of social determinants of health (SDOH)–related International Classification of Diseases, Tenth Revision, Z codes (Z55-Z66)(eTable 1), include lack of clarity on who can document patients’ social needs, lack of systems and processes for documenting and coding SDOH, unfamiliarity with these Z codes, and a low prioritization of collecting these data.13 Documentation of a SDOH-related Z code relevant to a patient encounter is considered moderate risk and can have a major impact on a patient’s overall health, unmet social needs, and outcomes.13 If the other 2 medical decision-making elements (ie, number and complexity of problems addressed along with amount and/or complexity of data to be reviewed and analyzed) for the E/M visit also are moderate, then the encounter can be coded as level 4.13

New Codes for Alopecia and Acne Surgery
New International Classification of Diseases, Tenth Revision, Clinical Modification, codes for alopecia have been developed through collaboration of the American Academy of Dermatology Association and the Scarring Alopecia Foundation (eTable 2). Cutaneous extraction—previously coded as acne surgery (CPT code 10040)—will now be listed in the 2026 CPT coding manual as “extraction” (eg, marsupialization, opening of multiple milia, acne comedones, cysts, pustules).14

Quality Payment Program Update
The MIPS performance threshold will remain at 75 for the 2025 performance period, impacting the 2027 payment year.15 The MIPS Value Pathways will be available but optional in 2025, and the CMS plans to fully replace MIPS by 2029. The goal for the MVPs is to reduce the administrative burden of MIPS for physicians and their staff while simplifying reporting; however, there are several concerns. The MIPS Value Pathways build on the MIPS’s flawed processes; compare the cost for one condition to the quality of another; continue to be burdensome to physicians; have not demonstrated improved patient care; are a broad, one-size-fits-all model that could lead to inequity based on practice mix; and are not clinically relevant to physicians and patients.15
Beginning in 2025, dermatologists also will have access to a new high-priority quality measure—Melanoma: Tracking and Evaluation of Recurrence—and the Melanoma: Continuity of Care–Recall System measure (MIPS measure 137) will be removed starting in 2025.15
What Can Dermatologists Do?
With the fifth consecutive year of payment cuts, the cumulative reduction to physician payments has reached an untenable level, and physicians cannot continue to absorb the reductions, which impact access and ability to provide patient care. Members of the American Academy of Dermatology Association must urge members of Congress to stop the cuts and find a permanent solution to fix Medicare physician payment by asking their representatives to cosponsor the following bills in the US House of Representatives and Senate16:
- HR 10073—The Medicare Patient Access and Practice Stabilization Act of 2024 would stop the 2.8% cut to the 2025 MPFS and provide a positive inflationary adjustment for physician practices equal to 50% of the 2025 MEI, which comes down to an increase of approximately 1.8%.17
- HR 2424—The Strengthening Medicare for Patients and Providers Act would provide an annual inflation update equal to the MEI for Medicare physician payments.18
- HR 6371—The Provider Reimbursement Stability Act would revise budget neutrality policies that contribute to eroding Medicare physician reimbursement.19
- S 4935—The Physician Fee Stabilization Act would increase the budget neutrality trigger from $20 million to $53 million.20
Advocacy is critically important: be engaged and get involved in grassroots efforts to protect access to health care, as these cuts do nothing to curb health care costs.
Final Thoughts
Congress has failed to address declining Medicare reimbursement rates, allowing cuts that jeopardize patient access to care as physicians close or sell their practices. It is important for dermatologists to attend the American Medical Association’s National Advocacy Conference in February 2025, which will feature an event on fixing Medicare. Dermatologists also can join prominent House members in urging Congress to reverse Medicare cuts and reform the physician payment system as well as write to their representatives and share how these cuts impact their practices and patients.
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
- Centers for Medicare & Medicaid Services. Office of the Actuary. National Health Statistics Group. Accessed January 10, 2025. https://www.cms.gov/files/document/nations-health-dollar-where-it-came-where-it-went.pdf
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare Physician Fee Schedule proposed rule. July 10, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-proposed-rule
- RVS Update Committee (RUC). RBRVS overview. American Medical Association. Updated November 8, 2024. Accessed January 10, 2025. https://www.ama-assn.org/about/rvs-update-committee-ruc/rbrvs-overview
- American Medical Association. History of Medicare conversion charts. Accessed January 10, 2025. https://www.ama-assn.org/system/files/cf-history.pdf
- American Medical Association. Medicare basics series: the Medicare Economic Index. June 3, 2024. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/medicare-basics-series-medicare-economic-index
- O’Reilly KB. Physician answers on this survey will shape future Medicare pay. American Medical Association. November 3, 2023. Accessed January 10, 2025. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future-medicare-pay
- Solis E. Stopgap spending bill extends telehealth flexibility, Medicare payment relief still awaits. American Academy of Family Physicians. December 3, 2024. Accessed January 10, 2025. https://www.aafp.org/pubs/fpm/blogs/gettingpaid/entry/2024-shutdown-averted.html
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2025 Medicare physician fee schedule final rule. November 1, 2024. Accessed January 10, 2025. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2025-medicare-physician-fee-schedule-final-rulen
- Novitas Solutions. Other CPT modifiers. Accessed January 10, 2025. https://www.novitas-solutions.com/webcenter/portal/MedicareJH/pagebyid?contentId=00144515
- Medical team conference, without direct (face-to-face) contact with patient and/or family CPT® code range 99367-99368. Codify by AAPC. Accessed January 10, 2025. https://www.aapc.com/codes/cpt-codes-range/99367-99368/
- McNichols FCM. Cracking the code. DermWorld. November 2023. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=806167&article_id=4666988
- McNichols FCM. Coding Consult. Derm World. Published April 2024. https://www.aad.org/dw/monthly/2024/may/dcc-hcpcs-add-on-code-g2211
- Venkatesh KP, Jothishankar B, Nambudiri VE. Incorporating social determinants of health into medical decision-making -implications for dermatology. JAMA Dermatol. 2023;159:367-368.
- McNichols FCM. Coding consult. DermWorld. October 2024. Accessed January 10, 2025. https://digitaleditions.walsworth.com/publication/?i=832260&article_id=4863646
- Centers for Medicare and Medicaid Services. Quality Payment Program. Dermatologic care MVP candidate. December 1, 2023. Updated December 15, 2023. Accessed January 10, 2025. https://qpp.cms.gov/resources/document/78e999ba-3690-4e02-9b35-6cc7c98d840b
- American Academy of Dermatology Association. AADA advocacy action center. Accessed January 10, 2025. https://www.aad.org/member/advocacy/take-action
- Medicare Patient Access and Practice Stabilization Act of 2024, HR 10073, 118th Congress (NC 2024).
- Strengthening Medicare for Patients and Providers Act, HR 2424, 118th Congress (CA 2023).
- Provider Reimbursement Stability Act, HR 6371, 118th Congress (NC 2023).
- Physician Fee Stabilization Act. S 4935. 2023-2024 Session (AR 2024).
Legislative, Practice Management, and Coding Updates for 2025
Legislative, Practice Management, and Coding Updates for 2025
PRACTICE POINTS
- The Centers for Medicare & Medicaid Services released the 2025 Medicare Physician Fee Schedule final rule on November 1, 2024, setting the 2025 conversion factor at $32.35—a 2.83% reduction from 2024.
- With this change, dermatology practices may see an overall 2.83% reduction in payments in 2025 compared to 2024, although individual outcomes will vary based on practice mix.
- The American Academy of Dermatology Association continues to advocate for change, and members need to urge their federal legislators to support critical bills aimed at reforming Medicare physician payment.
Painful Ulcers on the Elbows, Knees, and Ankles
Painful Ulcers on the Elbows, Knees, and Ankles
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.
The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6
Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9
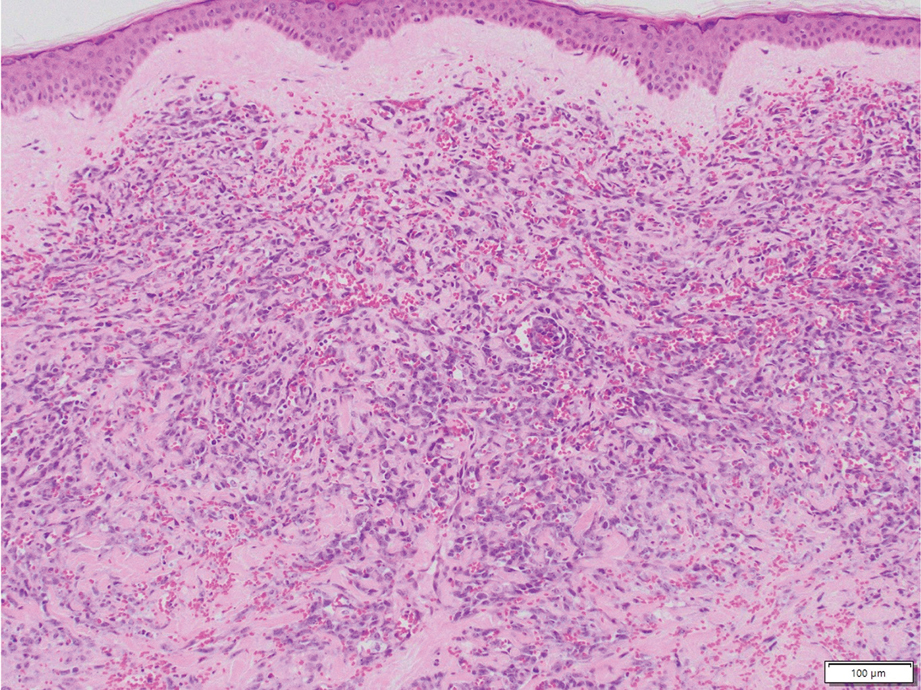
Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2
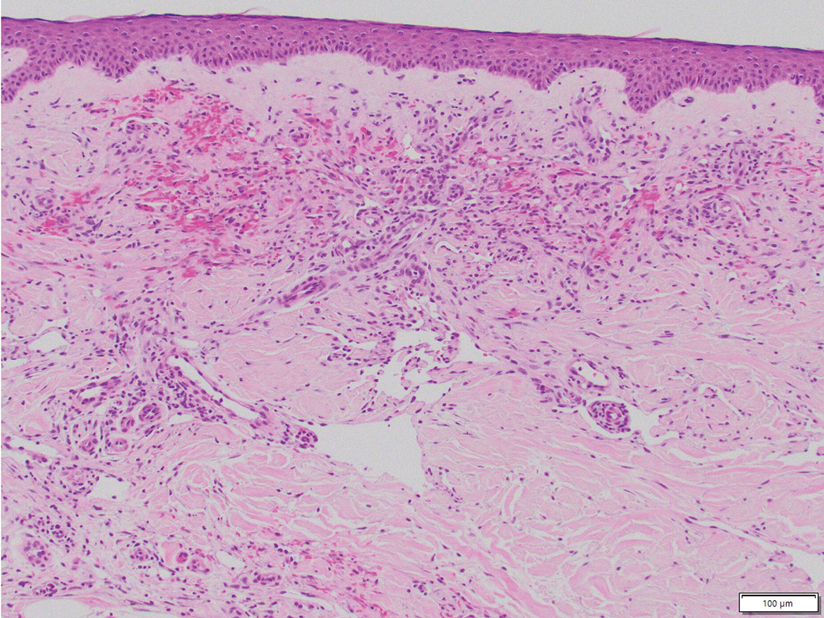
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15
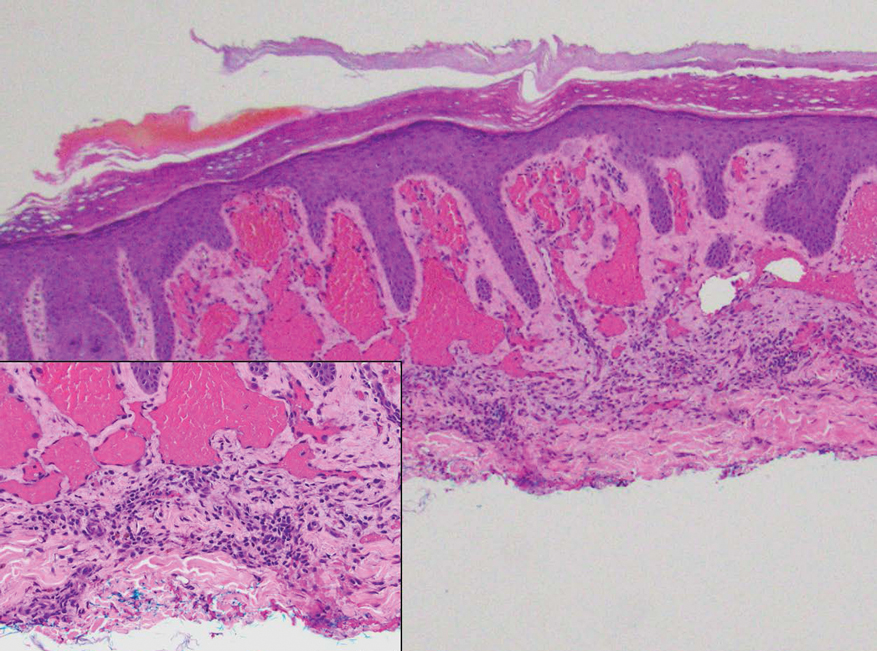
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

THE DIAGNOSIS: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is a rare benign condition that manifests as tender, indurated, erythematous or violaceous plaques that can develop ulceration and necrosis. It typically occurs in areas susceptible to chronic hypoxia, such as the arms and legs, as was seen in our patient, as well as on large pendulous breasts in females. This condition is a distinct variant of reactive angioendotheliomatosis associated with smoking, trauma, underlying vaso-occlusion, and hypercoagulability.1,2 Risk factors include a history of smoking as well as conditions associated with chronic hypoxia, such as severe peripheral vascular disease, subclavian artery stenosis, hypercoagulable states, monoclonal gammopathy, steal syndrome from an arteriovenous fistula, end-stage renal failure, calciphylaxis, and obesity.1
Histopathology of DDA reveals a diffuse dermal proliferation of capillaries due to upregulation of vascular endothelial growth factor secondary to chronic ischemia and hypoxia.1,2 Small, well-formed capillaries surrounded by pericytes dissect through dermal collagen into the subcutis (eFigure 1). Spindle-shaped cells with vacuolated cytoplasm and scattered extravasated erythrocytes with hemosiderin may be observed.2 Cellular atypia generally is not seen.2,3 Diffuse dermal angiomatosis is characterized by positive CD31, CD34, and ERG immunostaining1 and HHV-8 and D2-40 negativity.2 In our patient, the areas suggestive of connective tissue calciumlike depositions were concerning for dystrophic calcification related to end-stage renal disease. Although Von Kossa staining failed to highlight vascular calcifications, early calciphylaxis from end-stage renal disease could not be excluded.

The main goal of DDA treatment is to target tissue hypoxia, and primary preventive measures aim to reduce risk factors associated with atherosclerosis.1 Treatment options for DDA include revascularization, reduction mammoplasty, excision, isotretinoin, oral corticosteroids, smoking cessation, pentoxifylline plus aspirin, and management of underlying calciphylaxis.1,2 Spontaneous resolution of DDA rarely has been reported.1
Acroangiodermatitis, also known as pseudo–Kaposi sarcoma (KS), is a rare angioproliferative disorder that often is associated with vascular anomalies.4,5 It is divided into 2 main variants: Mali type, which is associated with chronic venous insufficiency, and Stewart-Bluefarb type, associated with arteriovenous malformations.4 This condition is characterized by red to violaceous macules, papules, or plaques that may become ulcerated or coalesce to form larger confluent patches, typically arising on the lower extremities.4,6,7 Histopathology of acroangiodermatitis reveals circumscribed lobular proliferation of thick-walled dermal vessels (eFigure 2), in contrast to the diffuse dermal proliferation of endothelial cells between collagen bundles seen in DDA.2,3,6

Angiosarcoma is a rare, highly aggressive vascular tumor that originates from vascular or lymphatic endothelial cells. It typically manifests with raised, bruiselike, erythematous to violaceous papules or plaques.8,9 Histopathologically, the hallmark feature of angiosarcoma is abnormal, pleomorphic, malignant endothelial cells with pale, light, eosinophilic cytoplasm and hyperchromatic nuclei (eFigure 3).2,9 In poorly differentiated cases, malignant endothelial cells may exhibit an epithelioid morphology with areas of hemorrhage and necrosis.9 Immunohistochemistry is positive for ERG, CD34, CD31, vascular endothelial growth factor, and D2-40.2,9

Kaposi sarcoma is a soft tissue malignancy known to occur in immunosuppressed patients such as individuals with AIDS or those undergoing immunosuppressive therapy for organ transplantation.10 There are 4 major forms of KS: classic (appearing on the lower extremities in elderly men of Mediterranean and Eastern European descent), endemic (occurring in children specifically in Africa with generalized lymph node involvement), HIV/ AIDS–related (occurring in patients not taking highly active antiretroviral therapy with diffuse involvement of the skin and internal organs), and iatrogenic (occurring in immunosuppressed patients with diffuse involvement of the skin and internal organs).10,11 Kaposi sarcoma presents as multiple reddish brown, raised or flat, painless, nonblanching mucocutaneous lesions that occasionally can ulcerate and bleed.11 Histopathologic features of KS include vascular proliferation in the dermis with diffuse slitlike lumen formation with the promontory sign, hyaline globules, hemosiderin accumulation, and an inflammatory component that often contains plasma cells (eFigure 4).2,11 Kaposi sarcoma is characterized by positive staining for CD31, CD34, D2-40, and HHV-8; the last 2 are an important distinction from DDA.2

Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a benign vascular lesion that typically manifests as a solitary, brown to violaceous papule or plaque on the trunk or extremities.12 It is sometimes surrounded by a pale area and a peripheral ecchymotic ring, giving the lesion a targetoid appearance.12,13 Histopathologic features include dilated, thin-walled vessels with prominent endothelial hobnailing in the papillary dermis, slit-shaped vascular channels between collagen bundles in the deeper dermis, and an interstitial lymphocytic infiltrate with extravasated erythrocytes and hemosiderin deposits (eFigure 5).12,14 The etiology of targetoid hemosiderotic hemangioma remains unclear. Chronic inflammation, trauma, exposure to ionizing radiation, and vascular obstruction have been suggested as inciting factors, though many cases have been reported without a history of cutaneous injury.12,13 Studies suggest a lymphatic origin instead of its original classification as a hemangioma.13,15 The endothelial cells stain positive with CD31 and may stain with D2-40 and CD34.13,15

- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Proc Bayl Univ Med Cent. 2020;33:273-275. doi:10.1080/08998280.2020.1722052
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7
- Yang H, Ahmed I, Mathew V, et al. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142:343-347. doi:10.1001 /archderm.142.3.343
- Chhabra G, Verma P, Khullar G, et al. Acroangiodermatitis, Mali and Stewart-Bluefarb type: two additional cases in adolescents. Australas J Dermatol. 2021;62:E156-E157. doi:10.1111/ajd.13386
- Ramírez-Marín HA, Ruben-Castillo C, Barrera-Godínez A, et al. Acroangiodermatitis of the hand secondary to a dysfunctional a rteriovenous fistula. Ann Vasc Surg. 2021;77:350.e13-350.e17. doi:10.1016/j.avsg.2021.05.042
- Sun L, Duarte S, Soares-de-Almeida L. Acroangiodermatitis of Mali—an unusual cause of painful ulcer. Actas Dermo-Sifiliográficas. 2023;114:546. doi:10.1016/j.ad.2022.07.013
- Parsi K, O’Connor A, Bester L. Stewart–Bluefarb syndrome: report of five cases and a review of literature. Phlebology. 2015;30:505-514. doi:10.1177/0268355514548090
- Alharbi A, Kim YC, AlShomer F, et al. Utility of multimodal treatment protocols in the management of scalp cutaneous angiosarcoma. Plast Reconstr Surg Glob Open. 2023;11:E4827. doi:10.1097 /GOX.0000000000004827
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991. doi:10.1016/S1470-2045(10)70023-1
- Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls [Internet]. StatPearls Publishing; 2024. Updated June 5, 2023. Accessed January 7, 2024. http://www.ncbi.nlm.nih.gov/books/NBK534839/
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primer. 2019;5:1-21. doi:10.1038/s41572-019-0060-9
- AbuHilal M, Breslavet M, Ho N, et al. Hobnail hemangioma (superficial hemosiderotic lymphovascular malformation) in children: a series of 6 pediatric cases and review of the literature. J Cutan Med Surg. 2016;20:216-220. doi:10.1177/1203475415612421
- Kakizaki P, Valente NYS, Paiva DLM, et al. Targetoid hemosiderotic hemangioma—case report. An Bras Dermatol. 2014;89:956-959. doi:10.1590/abd1806-4841.20143264
- Trindade F, Kutzner H, Tellechea Ó, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115. doi:10.1016/j.jaad.2011.05.019
- Hejnold M, Dyduch G, Mojsa I, et al. Hobnail hemangioma: a immunohistochemical study and literature review. Pol J Pathol. 2012;63:189-192. doi:10.5114/pjp.2012.31504
Painful Ulcers on the Elbows, Knees, and Ankles
Painful Ulcers on the Elbows, Knees, and Ankles
A 46-year-old woman with a history of systemic lupus erythematosus and end-stage renal disease presented to the dermatology department with painful ulcers on the extensor surfaces of the elbows, knees, and ankles of 2 months’ duration. Physical examination revealed angulated ulcers with surrounding pink erythema. A 4-mm punch biopsy and CD31 immunostaining of the left knee revealed dystrophic elastic fibers and purplish calciumlike depositions on connective tissue fibers in the mid to deep dermis.
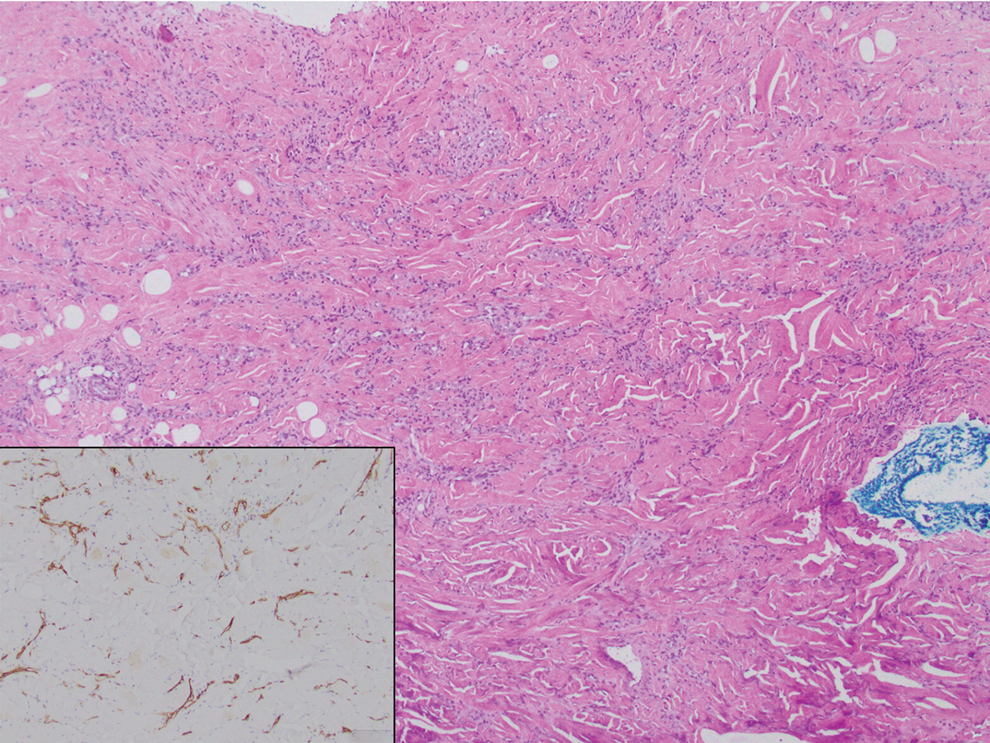
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents
Dermatopathology education, which comprises approximately 30% of the dermatology residency curriculum, is crucial for the holistic training of dermatology residents to diagnose and manage a range of dermatologic conditions.1 Additionally, dermatopathology is the topic of one of the 4 American Board of Dermatology CORE Exam modules, further highlighting the need for comprehensive education in this area. A variety of resources including virtual dermatopathology and conventional microscopy training currently are used in residency programs for dermatopathology education.2,3 Although used less frequently, social media platforms such as Instagram also are used to aid in dermatopathology education for a wider audience.4 Other online resources, including the American Society of Dermatopathology website (www.asdp.org) and DermpathAtlas.com, are excellent tools for medical students, residents, and fellows to develop their knowledge.5 While these resources are accessible, they must be directly sought out by the student and utilized on their own time. Additionally, if medical students do not have a strong understanding of the basics of dermatopathology, they may not have the foundation required to benefit from these resources.
Dermatopathology education is critical for the overall practice of dermatology, yet most dermatology residency programs may not be incorporating dermatopathology education early enough in training. One study evaluating the timing and length of dermatopathology education during residency reported that fewer than 40% (20/51) of dermatology residency programs allocate 3 or more weeks to dermatopathology education during the second postgraduate year.1 Despite Ackerman6 advocating for early dermatopathology exposure to best prepare medical students to recognize and manage certain dermatologic conditions, the majority of exposure still seems to occur during postgraduate year 4.1 Furthermore, current primary care residents feel that their medical school training did not sufficiently prepare them to diagnose and manage dermatologic conditions, with only 37% (93/252) reporting feeling adequately prepared.7,8 Medical students also reported a lack of confidence in overall dermatology knowledge, with 89% (72/81) reporting they felt neutral, slightly confident, or not at all confident when asked to diagnose skin lesions.9 In the same study, the average score was 46.6% (7/15 questions answered correctly) when 74 participants were assessed via a multiple choice quiz on dermatologic diagnosis and treatment, further demonstrating the lack of general dermatology comfort among medical students.9 This likely stems from limited dermatology curriculum in medical schools, demonstrating the need for further dermatology education as a whole in medical school.10
Ensuring robust dermatopathology education in medical school and the first year of dermatology residency has the potential to better prepare medical students for the transition into dermatology residency and clinical practice. We created an introductory dermatopathology lecture and presented it to medical students and first year dermatology residents to improve dermatopathology knowledge and confidence in learners early in their dermatology training.
Structure of the Lecture
Participants included first-year dermatology residents and fourth-year medical students rotating with the Wayne State University Department of Dermatology (Detroit, Michigan). The same facilitator (H.O.) taught each of the lectures, and all lectures were conducted via Zoom at the beginning of the month from May 2024 through November 2024. A total of 7 lectures were given. The lecture was formatted so that a histologic image was shown, then learners expressed their thoughts about what the image was showing before the answer was given. This format allowed participants to view the images on their own device screen and allowed the facilitator to annotate the images. The lecture was divided into 3 sections: (1) cell types and basic structures, (2) anatomic slides, and (3) common diagnoses. Each session lasted approximately 45 minutes.
Section 1: Cell Types and Basic Structures—The first section covered the fundamental cell types (neutrophils, lymphocytes, plasma cells, melanocytes, and eosinophils) along with glandular structures (apocrine, eccrine, and sebaceous). The session was designed to follow a retention and allow learners to think through each slide. First, participants were shown histologic images of each cell type and were asked to identify what type of cell was being shown. On the following slide, key features of each cell type were highlighted. Next, participants similarly were shown images of the glandular structures followed by key features of each. The section concluded with a review of the layers of the skin (stratum corneum, stratum granulosum, stratum lucidum, stratum spinosum, and stratum basale). A histologic image was shown, and the facilitator discussed how to distinguish the layers.
Section 2: Anatomic Sites—This section focused on key pathologic features for differentiating body surfaces, including the scalp, face, eyelids, ears, areolae, palms and soles, and mucosae. Participants initially were shown an image of a hematoxylin and eosin–stained slide from a specific body surface and then were asked to identify structures that may serve as a clue to the anatomic location. If the participants were not sure, they were given hints; for example, when participants were shown an image of the ear and were unsure of the location, the facilitator circled cartilage and asked them to identify the structure. In most cases, once participants named this structure, they were able to recognize that the location was the ear.
Section 3: Common Diagnoses—This section addressed frequently encountered diagnoses in dermatopathology, including basal cell carcinoma, squamous cell carcinoma, squamous cell carcinoma in situ, epidermoid cyst, pilar cyst, seborrheic keratosis, solar lentigo, melanocytic nevus, melanoma, verruca vulgaris, spongiotic dermatitis, psoriasis, and lichen planus. It followed the same format of the first section: participants were shown an hemotoxyllin and eosin–stained image and then were asked to discuss what the diagnosis could be and why. Hints were given if participants struggled to come up with the correct diagnosis. A few slides also were dedicated to distinguishing benign nevi, dysplastic nevi, and melanoma.
Pretest and Posttest Results
Residents participated in the lecture as part of their first-year orientation, and medical students participated during their dermatology rotation. All participants were invited to complete a pretest and a posttest before and after the lecture, respectively. Both assessments were optional and anonymous. The pretest was completed electronically and consisted of 10 knowledge-based, multiple-choice questions that included a histopathologic image and asked, “What is the most likely diagnosis?,” “What is the predominant cell type?,” and “Where was this specimen taken from?” In addition to the knowledge-based questions, participants also were asked to rate their confidence in dermatopathology on a 5-point Likert scale ranging from 1 (not confident at all) to 5 (extremely confident). Participants completed the entire pretest before any information on the topic was provided. After the lecture, participants were asked to complete a posttest identical to the pretest and to rate their confidence in dermatopathology again on the same scale. The posttest included an additional question asking participants to rate the helpfulness of the lecture on a Likert scale ranging from 1 (not helpful at all) to 5 (extremely helpful). Participants completed the posttest within 48 hours of the lecture.
Overall, 15 learners participated in the pretest and 12 in the posttest. Of the 15 pretest participants, 3 were first-year residents and 12 were medical students. Similarly, in the posttest, 2 respondents were first-year residents and 10 were medical students. All responses contained complete pretests and posttests. The mean score on the pretest was 62%, whereas the mean score on the posttest was 75%. A paired t test indicated a statistically significant improvement (P=.017). In addition, the mean rating for confidence in dermatopathology knowledge before the lecture was 1.5 prior to the lecture and 2.6 after the lecture. A paired t test demonstrated statistical significance (P=.010). The mean rating of the helpfulness of the lecture was 4.67. The majority (91.7% [11/12]) of the participants gave a rating of 4 or 5.
Impact of the Lecture on Dermatopathology Knowledge
There is a gap in dermatopathology education early in medical training. Our introductory lecture led to higher post test scores and increased confidence in dermatopathology among medical students and dermatology residents, demonstrating the effectiveness of this kind of program in bridging this education gap. The majority of participants in our lecture said they found the session helpful. A previously published article called for early implementation of dermatology education as a whole in the medical curriculum due to lack of knowledge and confidence, and our introductory lecture may help to bridge this gap.8 Increasing dermatopathology content for medical students and first-year dermatology residents can expand knowledge, as shown by the increased scores on the posttest, and better supports learners transitioning to dermatology residency, where dermatopathology constitutes a large part of the overall curriculum.2 More comprehensive knowledge of dermatopathology early in dermatology training also may help to better prepare residents to accurately diagnose and manage dermatologic conditions.
Pretest scores showed that the average confidence rating in dermatopathology among participants in our lecture was 1.5, which is rather low. This is consistent with prior studies that have found that residents feel that medical school inadequately prepared them for dermatology residency.7,8 More than 87% (71/81) of medical students surveyed felt they received inadequate general dermatology training in medical school.9 This supports the proposed educational gap that is impacting confidence in overall dermatology knowledge, which includes dermatopathology. In our study, the average confidence rating increased by 1.1 points after the lecture, which was statistically significant (P=.010) and demonstrates that an introductory lecture serves as a feasible intervention to improve confidence in this area.
The feedback we received from participants in our lecture shows the benefits of an introductory interactive lecture with virtual dermatopathology images. Ngo et al2 highlighted how residents perceive virtual images to be superior to conventional microscopy for dermatopathology, which we utilized in our lecture. This method is not only cost effective but also provides a simple way for learners and facilitators to point out key findings on histopathology slides.2
Final Thoughts
Overall, implementing dermatopathology education early in training has a measurable impact on dermatopathology knowledge and confidence among medical students and first-year dermatology residents. An interactive lecture with virtual images similar to the one we describe here may better prepare learners for the transition to dermatology residency by addressing the educational gap in dermatopathology early in training.
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826, vii.
- Ngo TB, Niu W, Fang Z, et al. Dermatology residents’ perspectives on virtual dermatopathology education. J Cutan Pathol. 2024;51:530-537.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Hubbard G, Saal R, Wintringham J, et al. Utilizing Instagram as a novel method for dermatopathology instruction. Clin Exp Dermatol. 2023;49:89-91.
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14.
- Ackerman AB. Training residents in dermatopathology: why, when, where, and how. J Am Acad Dermatol. 1990;22(6 Pt 1):1104-1106.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.e1.
- Murase JE. Understanding the importance of dermatology training in undergraduate medical education. Dermatol Pract Concept. 2015;5:95-96.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
Dermatopathology education, which comprises approximately 30% of the dermatology residency curriculum, is crucial for the holistic training of dermatology residents to diagnose and manage a range of dermatologic conditions.1 Additionally, dermatopathology is the topic of one of the 4 American Board of Dermatology CORE Exam modules, further highlighting the need for comprehensive education in this area. A variety of resources including virtual dermatopathology and conventional microscopy training currently are used in residency programs for dermatopathology education.2,3 Although used less frequently, social media platforms such as Instagram also are used to aid in dermatopathology education for a wider audience.4 Other online resources, including the American Society of Dermatopathology website (www.asdp.org) and DermpathAtlas.com, are excellent tools for medical students, residents, and fellows to develop their knowledge.5 While these resources are accessible, they must be directly sought out by the student and utilized on their own time. Additionally, if medical students do not have a strong understanding of the basics of dermatopathology, they may not have the foundation required to benefit from these resources.
Dermatopathology education is critical for the overall practice of dermatology, yet most dermatology residency programs may not be incorporating dermatopathology education early enough in training. One study evaluating the timing and length of dermatopathology education during residency reported that fewer than 40% (20/51) of dermatology residency programs allocate 3 or more weeks to dermatopathology education during the second postgraduate year.1 Despite Ackerman6 advocating for early dermatopathology exposure to best prepare medical students to recognize and manage certain dermatologic conditions, the majority of exposure still seems to occur during postgraduate year 4.1 Furthermore, current primary care residents feel that their medical school training did not sufficiently prepare them to diagnose and manage dermatologic conditions, with only 37% (93/252) reporting feeling adequately prepared.7,8 Medical students also reported a lack of confidence in overall dermatology knowledge, with 89% (72/81) reporting they felt neutral, slightly confident, or not at all confident when asked to diagnose skin lesions.9 In the same study, the average score was 46.6% (7/15 questions answered correctly) when 74 participants were assessed via a multiple choice quiz on dermatologic diagnosis and treatment, further demonstrating the lack of general dermatology comfort among medical students.9 This likely stems from limited dermatology curriculum in medical schools, demonstrating the need for further dermatology education as a whole in medical school.10
Ensuring robust dermatopathology education in medical school and the first year of dermatology residency has the potential to better prepare medical students for the transition into dermatology residency and clinical practice. We created an introductory dermatopathology lecture and presented it to medical students and first year dermatology residents to improve dermatopathology knowledge and confidence in learners early in their dermatology training.
Structure of the Lecture
Participants included first-year dermatology residents and fourth-year medical students rotating with the Wayne State University Department of Dermatology (Detroit, Michigan). The same facilitator (H.O.) taught each of the lectures, and all lectures were conducted via Zoom at the beginning of the month from May 2024 through November 2024. A total of 7 lectures were given. The lecture was formatted so that a histologic image was shown, then learners expressed their thoughts about what the image was showing before the answer was given. This format allowed participants to view the images on their own device screen and allowed the facilitator to annotate the images. The lecture was divided into 3 sections: (1) cell types and basic structures, (2) anatomic slides, and (3) common diagnoses. Each session lasted approximately 45 minutes.
Section 1: Cell Types and Basic Structures—The first section covered the fundamental cell types (neutrophils, lymphocytes, plasma cells, melanocytes, and eosinophils) along with glandular structures (apocrine, eccrine, and sebaceous). The session was designed to follow a retention and allow learners to think through each slide. First, participants were shown histologic images of each cell type and were asked to identify what type of cell was being shown. On the following slide, key features of each cell type were highlighted. Next, participants similarly were shown images of the glandular structures followed by key features of each. The section concluded with a review of the layers of the skin (stratum corneum, stratum granulosum, stratum lucidum, stratum spinosum, and stratum basale). A histologic image was shown, and the facilitator discussed how to distinguish the layers.
Section 2: Anatomic Sites—This section focused on key pathologic features for differentiating body surfaces, including the scalp, face, eyelids, ears, areolae, palms and soles, and mucosae. Participants initially were shown an image of a hematoxylin and eosin–stained slide from a specific body surface and then were asked to identify structures that may serve as a clue to the anatomic location. If the participants were not sure, they were given hints; for example, when participants were shown an image of the ear and were unsure of the location, the facilitator circled cartilage and asked them to identify the structure. In most cases, once participants named this structure, they were able to recognize that the location was the ear.
Section 3: Common Diagnoses—This section addressed frequently encountered diagnoses in dermatopathology, including basal cell carcinoma, squamous cell carcinoma, squamous cell carcinoma in situ, epidermoid cyst, pilar cyst, seborrheic keratosis, solar lentigo, melanocytic nevus, melanoma, verruca vulgaris, spongiotic dermatitis, psoriasis, and lichen planus. It followed the same format of the first section: participants were shown an hemotoxyllin and eosin–stained image and then were asked to discuss what the diagnosis could be and why. Hints were given if participants struggled to come up with the correct diagnosis. A few slides also were dedicated to distinguishing benign nevi, dysplastic nevi, and melanoma.
Pretest and Posttest Results
Residents participated in the lecture as part of their first-year orientation, and medical students participated during their dermatology rotation. All participants were invited to complete a pretest and a posttest before and after the lecture, respectively. Both assessments were optional and anonymous. The pretest was completed electronically and consisted of 10 knowledge-based, multiple-choice questions that included a histopathologic image and asked, “What is the most likely diagnosis?,” “What is the predominant cell type?,” and “Where was this specimen taken from?” In addition to the knowledge-based questions, participants also were asked to rate their confidence in dermatopathology on a 5-point Likert scale ranging from 1 (not confident at all) to 5 (extremely confident). Participants completed the entire pretest before any information on the topic was provided. After the lecture, participants were asked to complete a posttest identical to the pretest and to rate their confidence in dermatopathology again on the same scale. The posttest included an additional question asking participants to rate the helpfulness of the lecture on a Likert scale ranging from 1 (not helpful at all) to 5 (extremely helpful). Participants completed the posttest within 48 hours of the lecture.
Overall, 15 learners participated in the pretest and 12 in the posttest. Of the 15 pretest participants, 3 were first-year residents and 12 were medical students. Similarly, in the posttest, 2 respondents were first-year residents and 10 were medical students. All responses contained complete pretests and posttests. The mean score on the pretest was 62%, whereas the mean score on the posttest was 75%. A paired t test indicated a statistically significant improvement (P=.017). In addition, the mean rating for confidence in dermatopathology knowledge before the lecture was 1.5 prior to the lecture and 2.6 after the lecture. A paired t test demonstrated statistical significance (P=.010). The mean rating of the helpfulness of the lecture was 4.67. The majority (91.7% [11/12]) of the participants gave a rating of 4 or 5.
Impact of the Lecture on Dermatopathology Knowledge
There is a gap in dermatopathology education early in medical training. Our introductory lecture led to higher post test scores and increased confidence in dermatopathology among medical students and dermatology residents, demonstrating the effectiveness of this kind of program in bridging this education gap. The majority of participants in our lecture said they found the session helpful. A previously published article called for early implementation of dermatology education as a whole in the medical curriculum due to lack of knowledge and confidence, and our introductory lecture may help to bridge this gap.8 Increasing dermatopathology content for medical students and first-year dermatology residents can expand knowledge, as shown by the increased scores on the posttest, and better supports learners transitioning to dermatology residency, where dermatopathology constitutes a large part of the overall curriculum.2 More comprehensive knowledge of dermatopathology early in dermatology training also may help to better prepare residents to accurately diagnose and manage dermatologic conditions.
Pretest scores showed that the average confidence rating in dermatopathology among participants in our lecture was 1.5, which is rather low. This is consistent with prior studies that have found that residents feel that medical school inadequately prepared them for dermatology residency.7,8 More than 87% (71/81) of medical students surveyed felt they received inadequate general dermatology training in medical school.9 This supports the proposed educational gap that is impacting confidence in overall dermatology knowledge, which includes dermatopathology. In our study, the average confidence rating increased by 1.1 points after the lecture, which was statistically significant (P=.010) and demonstrates that an introductory lecture serves as a feasible intervention to improve confidence in this area.
The feedback we received from participants in our lecture shows the benefits of an introductory interactive lecture with virtual dermatopathology images. Ngo et al2 highlighted how residents perceive virtual images to be superior to conventional microscopy for dermatopathology, which we utilized in our lecture. This method is not only cost effective but also provides a simple way for learners and facilitators to point out key findings on histopathology slides.2
Final Thoughts
Overall, implementing dermatopathology education early in training has a measurable impact on dermatopathology knowledge and confidence among medical students and first-year dermatology residents. An interactive lecture with virtual images similar to the one we describe here may better prepare learners for the transition to dermatology residency by addressing the educational gap in dermatopathology early in training.
Dermatopathology education, which comprises approximately 30% of the dermatology residency curriculum, is crucial for the holistic training of dermatology residents to diagnose and manage a range of dermatologic conditions.1 Additionally, dermatopathology is the topic of one of the 4 American Board of Dermatology CORE Exam modules, further highlighting the need for comprehensive education in this area. A variety of resources including virtual dermatopathology and conventional microscopy training currently are used in residency programs for dermatopathology education.2,3 Although used less frequently, social media platforms such as Instagram also are used to aid in dermatopathology education for a wider audience.4 Other online resources, including the American Society of Dermatopathology website (www.asdp.org) and DermpathAtlas.com, are excellent tools for medical students, residents, and fellows to develop their knowledge.5 While these resources are accessible, they must be directly sought out by the student and utilized on their own time. Additionally, if medical students do not have a strong understanding of the basics of dermatopathology, they may not have the foundation required to benefit from these resources.
Dermatopathology education is critical for the overall practice of dermatology, yet most dermatology residency programs may not be incorporating dermatopathology education early enough in training. One study evaluating the timing and length of dermatopathology education during residency reported that fewer than 40% (20/51) of dermatology residency programs allocate 3 or more weeks to dermatopathology education during the second postgraduate year.1 Despite Ackerman6 advocating for early dermatopathology exposure to best prepare medical students to recognize and manage certain dermatologic conditions, the majority of exposure still seems to occur during postgraduate year 4.1 Furthermore, current primary care residents feel that their medical school training did not sufficiently prepare them to diagnose and manage dermatologic conditions, with only 37% (93/252) reporting feeling adequately prepared.7,8 Medical students also reported a lack of confidence in overall dermatology knowledge, with 89% (72/81) reporting they felt neutral, slightly confident, or not at all confident when asked to diagnose skin lesions.9 In the same study, the average score was 46.6% (7/15 questions answered correctly) when 74 participants were assessed via a multiple choice quiz on dermatologic diagnosis and treatment, further demonstrating the lack of general dermatology comfort among medical students.9 This likely stems from limited dermatology curriculum in medical schools, demonstrating the need for further dermatology education as a whole in medical school.10
Ensuring robust dermatopathology education in medical school and the first year of dermatology residency has the potential to better prepare medical students for the transition into dermatology residency and clinical practice. We created an introductory dermatopathology lecture and presented it to medical students and first year dermatology residents to improve dermatopathology knowledge and confidence in learners early in their dermatology training.
Structure of the Lecture
Participants included first-year dermatology residents and fourth-year medical students rotating with the Wayne State University Department of Dermatology (Detroit, Michigan). The same facilitator (H.O.) taught each of the lectures, and all lectures were conducted via Zoom at the beginning of the month from May 2024 through November 2024. A total of 7 lectures were given. The lecture was formatted so that a histologic image was shown, then learners expressed their thoughts about what the image was showing before the answer was given. This format allowed participants to view the images on their own device screen and allowed the facilitator to annotate the images. The lecture was divided into 3 sections: (1) cell types and basic structures, (2) anatomic slides, and (3) common diagnoses. Each session lasted approximately 45 minutes.
Section 1: Cell Types and Basic Structures—The first section covered the fundamental cell types (neutrophils, lymphocytes, plasma cells, melanocytes, and eosinophils) along with glandular structures (apocrine, eccrine, and sebaceous). The session was designed to follow a retention and allow learners to think through each slide. First, participants were shown histologic images of each cell type and were asked to identify what type of cell was being shown. On the following slide, key features of each cell type were highlighted. Next, participants similarly were shown images of the glandular structures followed by key features of each. The section concluded with a review of the layers of the skin (stratum corneum, stratum granulosum, stratum lucidum, stratum spinosum, and stratum basale). A histologic image was shown, and the facilitator discussed how to distinguish the layers.
Section 2: Anatomic Sites—This section focused on key pathologic features for differentiating body surfaces, including the scalp, face, eyelids, ears, areolae, palms and soles, and mucosae. Participants initially were shown an image of a hematoxylin and eosin–stained slide from a specific body surface and then were asked to identify structures that may serve as a clue to the anatomic location. If the participants were not sure, they were given hints; for example, when participants were shown an image of the ear and were unsure of the location, the facilitator circled cartilage and asked them to identify the structure. In most cases, once participants named this structure, they were able to recognize that the location was the ear.
Section 3: Common Diagnoses—This section addressed frequently encountered diagnoses in dermatopathology, including basal cell carcinoma, squamous cell carcinoma, squamous cell carcinoma in situ, epidermoid cyst, pilar cyst, seborrheic keratosis, solar lentigo, melanocytic nevus, melanoma, verruca vulgaris, spongiotic dermatitis, psoriasis, and lichen planus. It followed the same format of the first section: participants were shown an hemotoxyllin and eosin–stained image and then were asked to discuss what the diagnosis could be and why. Hints were given if participants struggled to come up with the correct diagnosis. A few slides also were dedicated to distinguishing benign nevi, dysplastic nevi, and melanoma.
Pretest and Posttest Results
Residents participated in the lecture as part of their first-year orientation, and medical students participated during their dermatology rotation. All participants were invited to complete a pretest and a posttest before and after the lecture, respectively. Both assessments were optional and anonymous. The pretest was completed electronically and consisted of 10 knowledge-based, multiple-choice questions that included a histopathologic image and asked, “What is the most likely diagnosis?,” “What is the predominant cell type?,” and “Where was this specimen taken from?” In addition to the knowledge-based questions, participants also were asked to rate their confidence in dermatopathology on a 5-point Likert scale ranging from 1 (not confident at all) to 5 (extremely confident). Participants completed the entire pretest before any information on the topic was provided. After the lecture, participants were asked to complete a posttest identical to the pretest and to rate their confidence in dermatopathology again on the same scale. The posttest included an additional question asking participants to rate the helpfulness of the lecture on a Likert scale ranging from 1 (not helpful at all) to 5 (extremely helpful). Participants completed the posttest within 48 hours of the lecture.
Overall, 15 learners participated in the pretest and 12 in the posttest. Of the 15 pretest participants, 3 were first-year residents and 12 were medical students. Similarly, in the posttest, 2 respondents were first-year residents and 10 were medical students. All responses contained complete pretests and posttests. The mean score on the pretest was 62%, whereas the mean score on the posttest was 75%. A paired t test indicated a statistically significant improvement (P=.017). In addition, the mean rating for confidence in dermatopathology knowledge before the lecture was 1.5 prior to the lecture and 2.6 after the lecture. A paired t test demonstrated statistical significance (P=.010). The mean rating of the helpfulness of the lecture was 4.67. The majority (91.7% [11/12]) of the participants gave a rating of 4 or 5.
Impact of the Lecture on Dermatopathology Knowledge
There is a gap in dermatopathology education early in medical training. Our introductory lecture led to higher post test scores and increased confidence in dermatopathology among medical students and dermatology residents, demonstrating the effectiveness of this kind of program in bridging this education gap. The majority of participants in our lecture said they found the session helpful. A previously published article called for early implementation of dermatology education as a whole in the medical curriculum due to lack of knowledge and confidence, and our introductory lecture may help to bridge this gap.8 Increasing dermatopathology content for medical students and first-year dermatology residents can expand knowledge, as shown by the increased scores on the posttest, and better supports learners transitioning to dermatology residency, where dermatopathology constitutes a large part of the overall curriculum.2 More comprehensive knowledge of dermatopathology early in dermatology training also may help to better prepare residents to accurately diagnose and manage dermatologic conditions.
Pretest scores showed that the average confidence rating in dermatopathology among participants in our lecture was 1.5, which is rather low. This is consistent with prior studies that have found that residents feel that medical school inadequately prepared them for dermatology residency.7,8 More than 87% (71/81) of medical students surveyed felt they received inadequate general dermatology training in medical school.9 This supports the proposed educational gap that is impacting confidence in overall dermatology knowledge, which includes dermatopathology. In our study, the average confidence rating increased by 1.1 points after the lecture, which was statistically significant (P=.010) and demonstrates that an introductory lecture serves as a feasible intervention to improve confidence in this area.
The feedback we received from participants in our lecture shows the benefits of an introductory interactive lecture with virtual dermatopathology images. Ngo et al2 highlighted how residents perceive virtual images to be superior to conventional microscopy for dermatopathology, which we utilized in our lecture. This method is not only cost effective but also provides a simple way for learners and facilitators to point out key findings on histopathology slides.2
Final Thoughts
Overall, implementing dermatopathology education early in training has a measurable impact on dermatopathology knowledge and confidence among medical students and first-year dermatology residents. An interactive lecture with virtual images similar to the one we describe here may better prepare learners for the transition to dermatology residency by addressing the educational gap in dermatopathology early in training.
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826, vii.
- Ngo TB, Niu W, Fang Z, et al. Dermatology residents’ perspectives on virtual dermatopathology education. J Cutan Pathol. 2024;51:530-537.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Hubbard G, Saal R, Wintringham J, et al. Utilizing Instagram as a novel method for dermatopathology instruction. Clin Exp Dermatol. 2023;49:89-91.
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14.
- Ackerman AB. Training residents in dermatopathology: why, when, where, and how. J Am Acad Dermatol. 1990;22(6 Pt 1):1104-1106.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.e1.
- Murase JE. Understanding the importance of dermatology training in undergraduate medical education. Dermatol Pract Concept. 2015;5:95-96.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826, vii.
- Ngo TB, Niu W, Fang Z, et al. Dermatology residents’ perspectives on virtual dermatopathology education. J Cutan Pathol. 2024;51:530-537.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Hubbard G, Saal R, Wintringham J, et al. Utilizing Instagram as a novel method for dermatopathology instruction. Clin Exp Dermatol. 2023;49:89-91.
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14.
- Ackerman AB. Training residents in dermatopathology: why, when, where, and how. J Am Acad Dermatol. 1990;22(6 Pt 1):1104-1106.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.e1.
- Murase JE. Understanding the importance of dermatology training in undergraduate medical education. Dermatol Pract Concept. 2015;5:95-96.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents