User login
PTSD in Children
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
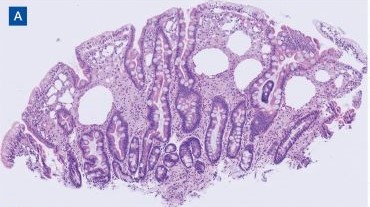
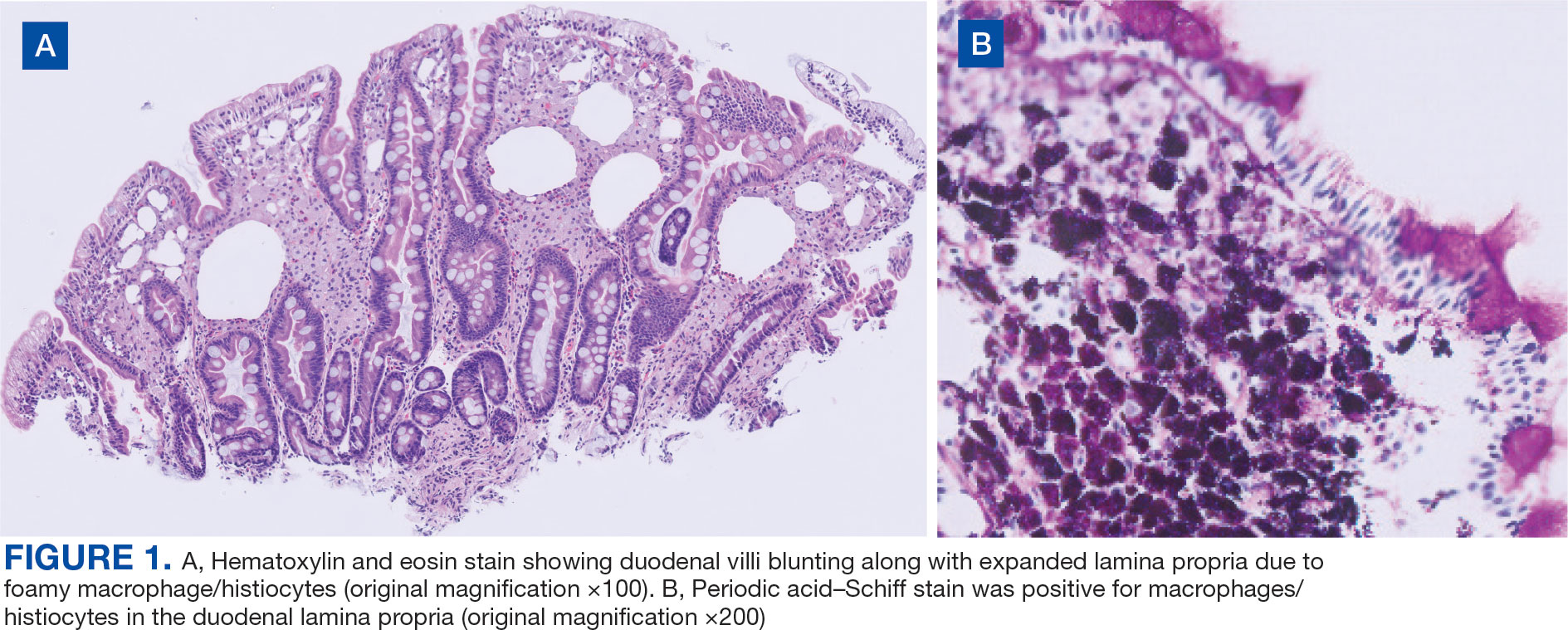
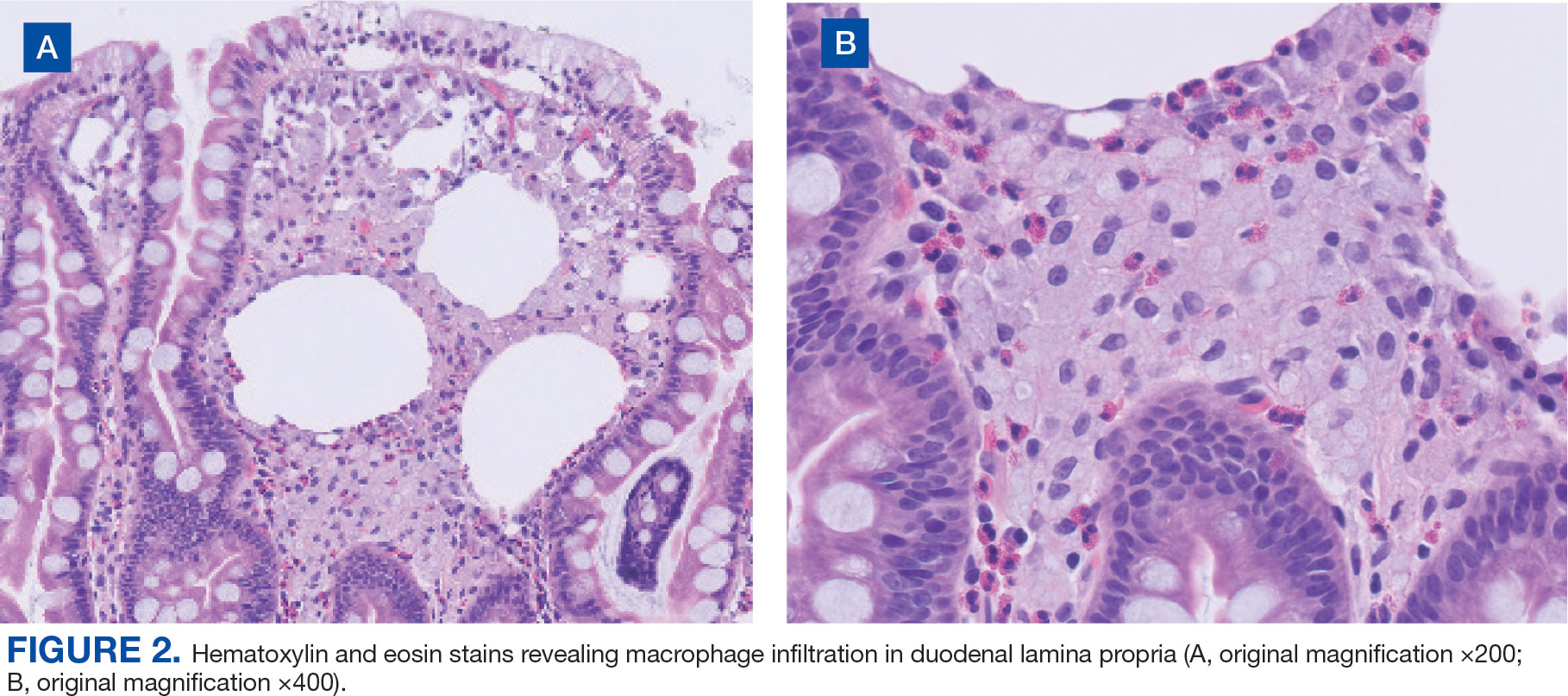
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.
We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
Whipple disease is a chronic, rare, infectious disease that manifests with systemic symptoms. This disease is caused by the gram-positive bacterium Tropheryma whipplei (T. whipplei). Common manifestations include gastrointestinal symptoms indicative of malabsorption, such as chronic diarrhea, unintentional weight loss (despite normal nutrient intake), and greasy, voluminous, foul-smelling stool. Other, less common manifestations include cardiovascular, endocrine, musculoskeletal, neurologic, and renal signs and symptoms. The prevalence of the disease is rare, affecting 3 in 1 million patients.1 This case highlights the importance of considering Whipple disease when treating patients with multiple symptoms and concurrent disease processes.
Case Presentation
A 53-year-old male with a medical history of hypertension, hyperlipidemia, hypothyroidism, and microcytic anemia presented with an 8-month history of persistent diarrhea associated with abdominal bloating, abdominal discomfort, and a 30-lb weight loss. He also reported fatigue, headaches, inability to concentrate, memory distortion, and visual disturbances involving flashes and floaters. The patient reported no fever, chills, nuchal rigidity, or prior neurologic symptoms. He reported intermittent bilateral hand and knee arthralgias. An autoimmune evaluation for arthralgia was negative, and a prior colonoscopy had been normal.
The patient’s hobbies included gardening, hiking, fishing, and deer hunting in Wyoming and Texas. He had spent time around cattle, dogs, and cats. He consumed alcohol twice weekly but reported no tobacco or illicit drug use or recent international travel. The patient’s family history was positive for rheumatoid arthritis, diabetes mellitus, and hypertension.
The patient’s vital signs were all within reference ranges, and lung auscultation revealed clear breathing sounds with no cardiac murmurs, gallops, or rubs. An abdominal examination revealed decreased bowel sounds, while the rest of the physical examination was otherwise normal.
Initial laboratory results showed that his sodium was 134 mEq/L (reference range, 136-145 mEq/L), hemoglobin was 9.3 g/dL (reference range for men, 14.0-18.0 g/dL), and hematocrit was 30.7% (reference range for men 42%-52%). His white blood cell (WBC) count and thyroid-stimulating hormone level were within normal limits. A cerebrospinal fluid (CSF) analysis revealed the following: WBCs 1.0/μL (0-5/μL), segmented neutrophils 10% (reference range, 7%), lymphocytes 80% (reference range, 40-80%), macrophages 10% (reference range, 2%), red blood cells 3 × 106 /μL (reference range, 4.3- 5.9 × 106 /µL), protein 23.5 mg/dL (reference range, 15-60 mg/dL), and glucose 44 mg/dL (reference range, 50-80 mg/dL).
Upper endoscopy with duodenal biopsy showed benign duodenal mucosa. Histopathologic evaluation revealed abundant foamy macrophages within lamina propria. Periodic acid–Schiff (PAS) stain was positive, diastase-resistant material was visualized within the macrophages (Figures 1 and 2). Polymerase chain reaction (PCR) testing of duodenal biopsy tissue was positive for T. whipplei. A lumbar puncture was performed, and PCR testing of CSF for T. whipplei was also positive. A stool PCR test was positive for Giardia. Transthoracic echocardiogram and brain magnetic resonance imaging were normal.


We treated the patient’s giardiasis with a single dose of oral tinidazole 2 g. To treat Whipple disease with central nervous system (CNS) involvement, we started the patient on ceftriaxone 2 g intravenous every 24 hours for 4 weeks, followed by oral trimethoprim and sulfamethoxazole (TMPSMX) 160/800 mg twice daily with an expected 1-year course.
Two months into TMP-SMX therapy, the patient developed an acute kidney injury with hyperkalemia (potassium, 5.5 mEq/L). We transitioned the therapy to doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily to complete 18 months of therapy. A lumbar puncture for CSF PCR and duodenal biopsy was planned for 6 months and 1 year after diagnosis.
Discussion
Whipple disease is often overlooked when making a diagnosis due to the nonspecific nature of its associated signs and symptoms. Classic Whipple disease has 2 stages: an initial prodromal stage marked by intermittent arthralgias, followed by a second gastrointestinal stage that involves chronic diarrhea, abdominal pain, and weight loss.1-3 Infection can sometimes be misdiagnosed as seronegative rheumatoid arthritis and a definite diagnosis can be missed for extended periods, with 1 case taking up to 8 years to diagnose after the first joint manifestations.2,4,5 Blood culture-negative endocarditis has also been well documented.1-5
The most common CNS clinical manifestations of Whipple disease include cognitive changes (eg, dementia), ocular movement disturbances (eg, oculomasticatory myorhythmia, which is pathognomonic for Whipple disease), involuntary movements, and hypothalamic dysfunction.1,6 Other neurologic symptoms include seizures, ataxia, meningitis, and myelopathy. Cerebrospinal fluid studies vary, with some results being normal and others revealing elevated protein counts.1
Disease Course
A retrospective study by Compain and colleagues reports that Whipple disease follows 3 patterns of clinical CNS involvement: classic Whipple disease with neurologic involvement, Whipple disease with isolated neurologic involvement, and neurologic relapse of previously treated Whipple disease.6 Isolated neurologic involvement is roughly 4% to 8%.6-8 Previous studies showed that the average delay from the presentation of neurologic symptoms to diagnosis is about 30 months.9
Diagnosis can be made with histologic evaluation of duodenal tissue using hematoxylin-eosin and PAS stains, which reveal foamy macrophages in expanded duodenal lamina propria, along with a positive tissue PCR.1,5 The slow replication rate of T. whipplei limits the effectiveness of bacterial cultures. After adequate treatment, relapses are still possible and regularly involve the CNS.1,4
Treatment typically involves blood-brain barrier-crossing agents, such as 2 weeks of meropenem 1 g every 24 hours or 2 to 4 weeks of ceftriaxone 2 g every 24 hours, followed by 1 year of TMP-SMX 160/800 mg twice daily. Doxycycline 100 mg twice daily and hydroxychloroquine 200 mg orally 3 times daily have also been shown to be effective, as seen in our patient.
Mortality rates vary for patients with Whipple disease and CNS involvement. One study reported poor overall prognosis in patients with CNS involvement, with mortality rates as high as 27%.10 However, rates of early detection and appropriate treatment may be improving, with 1 case series reporting 11% mortality in 18 patients with Whipple disease.6
Diagnosis
Because Whipple disease mimics many other diseases, misdiagnosis as infectious and noninfectious etiologies is common. PAS stain and tissue PCR helped uncover Whipple disease in a patient erroneously diagnosed with refractory Crohn disease.11
Weight loss, diarrhea, arthralgias, and cognitive impairment can also be seen in celiac disease. However, dermatologic manifestations, metabolic bone disease, and vitamin deficiencies are characteristics of celiac disease and can help distinguish it from T. whipplei infection.12
Whipple disease can also be mistaken for tropical sprue. Both can manifest with chronic diarrhea and duodenal villous atrophy; however, tropical sprue is more prevalent in specific geographic areas, and clinical manifestations are primarily gastrointestinal. Weight loss, diarrhea, steatorrhea, and folate deficiency are unique findings in tropical sprue that help differentiate it from Whipple disease.13 Likewise, other infectious diseases can be misdiagnosed as Whipple disease. Duodenal villi blunting and positive PAS staining have been reported in a Mycobacterium avium complex intestinal infection in a patient with AIDS, leading to a misdiagnosis of Whipple disease.14
Some parasitic infections have gastrointestinal symptoms similar to those of Whipple disease and others, such as giardiasis, are known to occur concurrently with Whipple disease.15-17 Giardiasis can also account for weight loss, malabsorptive symptoms, and greasy diarrhea. One case report hypothesized that 1 disease may predispose individuals to the other, as they both affect villous architecture.17 Additional research is needed to determine where the case reports have left off and to explore the connection between the 2 conditions.
Conclusions
The diagnosis of Whipple disease is challenging and frequently missed due to the rare and protean nature of the disease. This case highlights the importance of clinical suspicion for Whipple disease, especially in patients presenting with chronic seronegative arthritis, gastrointestinal abnormalities, and cognitive changes. Furthermore, this case points to the importance of additional testing for Whipple disease, even when a concurrent infection, such as giardiasis, has been identified.
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
- Biagi F, Balduzzi D, Delvino P, Schiepatti A, Klersy C, Corazza GR. Prevalence of Whipple’s disease in north-western Italy. Eur J Clin Microbiol Infect Dis. 2015;34(7):1347-1348. doi:10.1007/s10096-015-2357-2
- Fenollar F, Puéchal X, Raoult D. Whipple’s disease. N Engl J Med. 2007;356(1):55-66. doi:10.1056/NEJMra062477
- El-Abassi R, Soliman MY, Williams F, England JD. Whipple’s disease. J Neurol Sci. 2017;377:197-206. doi:10.1016/j.jns.2017.01.048
- Melas N, Amin R, Gyllemark P, Younes AH, Almer S. Whipple’s disease: the great masquerader-a high level of suspicion is the key to diagnosis. BMC Gastroenterol. 2021;21(1):128. doi:10.1186/s12876-021-01664-1
- Boumaza A, Azzouz EB, Arrindell J, Lepidi H, Mezouar S, Desnues B. Whipple’s disease and Tropheryma whipplei infections: from bench to bedside. Lancet Infect Dis. 2022;22(10):e280-e291. doi:10.1016/S1473-3099(22)00128-1
- Compain C, Sacre K, Puéchal X, et al. Central nervous system involvement in Whipple disease: clinical study of 18 patients and long-term follow-up. Medicine (Baltimore). 2013;92(6):324-330. doi:10.1097/MD.0000000000000010
- Anderson M. Neurology of Whipple’s disease. J Neurol Neurosurg Psychiatry. 2000;68(1):2-5. doi:10.1136/jnnp.68.1.2
- Gerard A, Sarrot-Reynauld F, Liozon E, et al. Neurologic presentation of Whipple disease: report of 12 cases and review of the literature. Medicine (Baltimore). 2002;81(6):443-457. doi:10.1097/00005792-200211000-00005
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple Disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore). 1997;76(3):170-184. doi:10.1097/00005792-199705000-00003
- Schnider PJ, Reisinger EC, Gerschlager W, et al. Long-term follow-up in cerebral Whipple’s disease. Eur J Gastroenterol Hepatol. 1996;8(9):899-903.
- Klochan C, Anderson TA, Rose D, Dimitrov RK, Johnson RM. Nearly fatal case of Whipple’s disease in a patient mistakenly on anti-TNF therapy. ACG Case Rep J. 2013;1(1):25-28. doi:10.14309/crj.2013.11
- . Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. 2020;54(1):8-21. doi:10.1097/MCG.0000000000001267
- Murray JA, Rubio-Tapia A. Diarrhoea due to small bowel diseases. Best Pract Res Clin Gastroenterol. 2012;26(5):581-600. doi:10.1016/j.bpg.2012.11.013
- Chirayath S, Bin Liaquat H, Bahirwani J, Labeeb A, Chaput K, Kaza C. Mycobacterium avium complex infection imitating Whipple disease in an immunocompromised patient with newly diagnosed acquired immunodeficiency syn - drome. ACG Case Rep J. 2021;8(5):e00588. doi:10.14309/crj.0000000000000588
- Fenollar F, Lepidi H, Gérolami R, Drancourt M, Raoult D. Whipple disease associated with giardiasis. J Infect Dis. 2003;188(6):828-834. doi:10.1086/378093
- Ruiz JAG, Simón PG, Aparicio Duque R, Mayor Jerez JL. Association between Whipple’s disease and Giardia lamblia infection. Rev Esp Enferm Dig. 2005;97(7)521-526. doi:10.4321/s1130-01082005000700007
- Gisbertz IA, Bergmans DC, van Marion-Kievit JA, Haak HR. Concurrent Whipple’s disease and Giardia lamblia infection in a patient presenting with weight loss. Eur J Intern Med. 2001;12(6):525-528. doi:10.1016/s0953-6205(01)00165-0
Whipple Disease With Central Nervous System Involvement
Whipple Disease With Central Nervous System Involvement
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
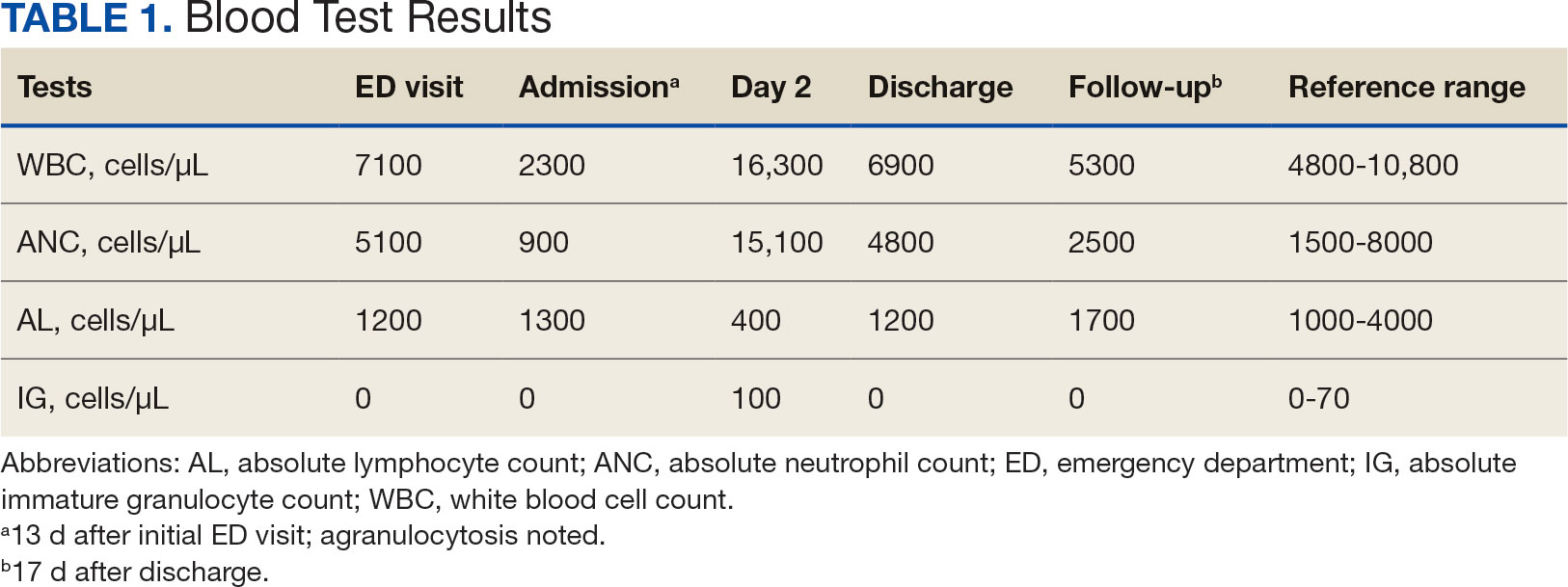
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.
Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.

Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
Acute agranulocytosis and aseptic meningitis are serious adverse effects (AEs) associated with sulfamethoxazole-trimethoprim. Acute agranulocytosis is a rare, potentially life-threatening blood dyscrasia characterized by a neutrophil count of < 500 cells per μL, with no relevant decrease in hemoglobin or platelet levels.1 Patients with agranulocytosis may be asymptomatic or experience severe sore throat, pharyngitis, or tonsillitis in combination with high fever, rigors, headaches, or malaise. These AEs are commonly classified as idiosyncratic and, in most cases, attributable to medications. If drug-induced agranulocytosis is suspected, the patient should discontinue the medication immediately.1
Meningitis is an inflammatory disease typically caused by viral or bacterial infections; however, it may also be attributed to medications or malignancy. Inflammation of the meninges with a negative bacterial cerebrospinal fluid culture is classified as aseptic meningitis. Distinguishing between aseptic and bacterial meningitis is crucial due to differences in illness severity, treatment options, and prognosis.2 Symptoms of meningitis may include fever, headache, nuchal rigidity, nausea, or vomiting.3 Several classes of medications can cause drug-induced aseptic meningitis (DIAM), but the most commonly reported antibiotic is sulfamethoxazole-trimethoprim.
DIAM is more prevalent in immunocompromised patients, such as those with a history of HIV/AIDS, organ transplant, collagen vascular disease, or malignancy, who may be prescribed sulfamethoxazoletrimethoprim for prophylaxis or treatment of infection.2 The case described in this article serves as a distinctive example of acute agranulocytosis complicated with aseptic meningitis caused by sulfamethoxazole-trimethoprim in an immunocompetent patient.
Case Presentation
A healthy male veteran aged 39 years presented to the Fargo Veterans Affairs Medical Center emergency department (ED) for worsening left testicular pain and increased urinary urgency and frequency for about 48 hours. The patient had no known medication allergies, was current on vaccinations, and his only relevant prescription was valacyclovir for herpes labialis. The evaluation included urinalysis, blood tests, and scrotal ultrasound. The urinalysis, blood tests, and vitals were unremarkable for any signs of systemic infection. The scrotal ultrasound was significant for left focal area of abnormal echogenicity with absent blood flow in the superior left testicle and a significant increase in blood flow around the left epididymis. Mild swelling in the left epididymis was present, with no significant testicular or scrotal swelling or skin changes observed. Urology was consulted and prescribed oral sulfamethoxazole-trimethoprim 800-160 mg every 12 hours for 30 days for the treatment of left epididymo-orchitis.
The patient returned to the ED 2 weeks later with fever, chills, headache, generalized body aches, urinary retention, loose stools, and nonspecific chest pressure. A serum blood test revealed significant neutropenia and leukopenia. The patient was admitted for observation, and sulfamethoxazole-trimethoprim was discontinued. The patient received sodium chloride intravenous (IV) fluid, oral potassium chloride supplementation, IV ondansetron, and analgesics, including oral acetaminophen, oral ibuprofen, and IV hydromorphone as needed. Repeated laboratory tests were completed with no specific findings; serum laboratory work, urinalysis, chest and abdominal X-rays, and echocardiogram were all unremarkable. The patient’s neutrophil count dropped from 5100 cells/µL at the initial ED presentation to 900 cells/µL (reference range, 1500-8000 cells/µL) (Table 1). Agranulocytosis quickly resolved after antibiotic discontinuation.

Upon further investigation, the patient took the prescribed sulfamethoxazole-trimethoprim for 10 days before stopping due to the resolution of testicular pain and epididymal swelling. The patient reported initial AEs of loose stools and generalized myalgias when first taking the medication. The patient restarted the antibiotic to complete the course of therapy after not taking it for 2 days. Within 20 minutes of restarting the medication, he experienced myalgias with pruritus, prompting him to return to the ED. Agranulocytosis and aseptic meningitis developed within 12 days after he was prescribed sulfamethoxazole-trimethoprim, though the exact timeframe is unknown.
The patient’s symptoms, except for a persistent headache, resolved during hospitalization. Infectious disease was consulted, and a lumbar puncture was performed due to prior fever and ongoing headaches to rule out a treatable cause of meningitis. The lumbar puncture showed clear spinal fluid, an elevated white blood cell count with neutrophil predominance, and normal protein and glucose levels. Cultures showed no aerobic, anaerobic, or fungal organisms. Herpes virus simplex and Lyme disease testing was not completed during hospitalization. Respiratory panel, legionella, and hepatitis A, B, and C tests were negative (Table 2). The negative laboratory test results strengthened the suspicion of aseptic meningitis caused by sulfamethoxazole-trimethoprim. The neurology consult recommended no additional treatments or tests.

The patient spontaneously recovered 2 days later, with a normalized complete blood count and resolution of headache. Repeat scrotal ultrasounds showed resolution of the left testicle findings. The patient was discharged and seen for a follow-up 14 days later. The final diagnosis requiring hospitalization was aseptic meningitis secondary to a sulfamethoxazole-trimethoprim.
Discussion
Sulfamethoxazole-trimethoprim is a commonly prescribed antibiotic for urinary tract infections, pneumocystis pneumonia, pneumocystis pneumonia prophylaxis, and methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Empiric antibiotics for epididymo-orchitis caused by enteric organisms include levofloxacin or ofloxacin; however sulfamethoxazole-trimethoprim may be considered as alternative.5,6 Agranulocytosis induced by sulfamethoxazole-trimethoprim may occur due to the inhibition on folic acid metabolism, which makes the highly proliferating cells of the hematopoietic system more susceptible to neutropenia. Agranulocytosis typically occurs within 7 days of treatment initiation and generally resolves within a month after discontinuation of the offending agent.7 In this case, agranulocytosis resolved overnight, resulting in leukocytosis. One explanation for the rapid increase in white blood cell count may be the concurrent diagnosis of aseptic meningitis. Alternatively, the patient’s health and immunocompetence may have helped generate an adequate immune response. Medication-induced agranulocytosis is often a diagnosis of exclusion because it is typically difficult to diagnose.7 In more severe or complicated cases of agranulocytosis, filgrastim may be indicated.1
Sulfamethoxazole-trimethoprim is a lipophilic small-molecule medication that can cross the blood-brain barrier and penetrate the tissues of the bone, prostate, and central nervous system.8 Specifically, the medication can pass into the cerebrospinal fluid regardless of meningeal inflammation.9 The exact mechanism for aseptic meningitis caused by sulfamethoxazole-trimethoprim is unknown; however, it may accumulate in the choroid plexus, causing destructive inflammation of small blood vessels and resulting in aseptic meningitis.10 The onset of aseptic meningitis can vary from 10 minutes to 10 days after initiation of the medication. Pre-exposure to the medication typically results in earlier onset of symptoms, though patients do not need to have previously taken the medication to develop aseptic meningitis. Patients generally become afebrile with resolution of headache and mental status changes within 48 to 72 hours after stopping the medication or after about 5 to 7 half-lives of the medication are eliminated.11 Some patients may continue to experience worsening symptoms after discontinuation because the medication is already absorbed into the serum and tissues.
DIAM is an uncommon drug-induced hypersensitivity AE of the central nervous system. Diagnosing aseptic meningitis caused by sulfamethoxazole-trimethoprim can be challenging, as antibiotics are given to treat suspected infections, and the symptoms of aseptic meningitis can be hard to differentiate from those of infectious meningitis.11 Close monitoring between the initiation of the medication and the onset of clinical symptoms is necessary to assist in distinguishing between aseptic and infectious meningitis.3 If the causative agent is not discontinued, symptoms can quickly worsen, progressing from fever and headache to confusion, coma, and respiratory depression. A DIAM diagnosis can only be made with resolution of aseptic meningitis after stopping the contributory agent. If appropriate, this can be proven by rechallenging the medication in a controlled setting. The usual treatment for aseptic meningitis is supportive care, including hydration, antiemetics, electrolyte supplementation, and adequate analgesia.3
Differential diagnoses in this case included viral infection, meningitis, and allergic reaction to sulfamethoxazole-trimethoprim. The patient reported history of experiencing symptoms after restarting his antibiotic, raising strong suspicion for DIAM. Initiation of this antibiotic was the only recent medication change noted. Laboratory testing for infectious agents yielded negative results, including tests for aerobic and anaerobic bacteria as well as viral and fungal infections. A lumbar puncture and cerebrospinal fluid culture was clear, with no organisms shown on gram stain. Bacterial or viral meningitis was presumed less likely due to the duration of symptoms, correlation of symptoms coinciding with restarting the antibiotic, and negative cerebrospinal fluid culture results.
It was concluded that agranulocytosis and aseptic meningitis were likely induced by sulfamethoxazole-trimethoprim as supported by the improvement upon discontinuing the medication and subsequent worsening upon restarting it. Concurrent agranulocytosis and aseptic meningitis is rare, and there is typically no correlation between the 2 reactions. Since agranulocytosis may be asymptomatic, this case highlights the need to monitor blood cell counts in patients using sulfamethoxazole-trimethoprim. The possibility of DIAM should be considered in patients presenting with flu-like symptoms, and a lumbar puncture may be collected to rule out aseptic meningitis if the patient’s AEs are severe following the initiation of an antibiotic, particularly in immunosuppressed patients taking sulfamethoxazole-trimethoprim. This case is unusual because the patient was healthy and immunocompetent.
This case may not be generalizable and may be difficult to compare to other cases. Every case has patient-specific factors affecting subjective information, including the patient’s baseline, severity of symptoms, and treatment options. This report was based on a single patient case and corresponding results may be found in similar patient cases.
Conclusions
This case emphasizes the rare but serious AEs of acute agranulocytosis complicated with aseptic meningitis after prescribed sulfamethoxazole-trimethoprim. This is a unique case of an immunocompetent patient developing both agranulocytosis and aseptic meningitis after restarting the antibiotic to complete therapy. This case highlights the importance of monitoring blood cell counts and monitoring for signs and symptoms of aseptic meningitis, even during short courses of therapy. Further research is needed to recognize characteristics that may increase the risk for these AEs and to develop strategies for their prevention.
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
- Garbe E. Non-chemotherapy drug-induced agranulocytosis. Expert Opin Drug Saf. 2007;6(3):323-335. doi:10.1517/14740338.6.3.323
- Jha P, Stromich J, Cohen M, Wainaina JN. A rare complication of trimethoprim-sulfamethoxazole: drug induced aseptic meningitis. Case Rep Infect Dis. 2016;2016:3879406. doi:10.1155/2016/3879406
- Hopkins S, Jolles S. Drug-induced aseptic meningitis. Expert Opin Drug Saf. 2005;4(2):285-297. doi:10.1517/14740338.4.2.285
- Jarrin I, Sellier P, Lopes A, et al. Etiologies and management of aseptic meningitis in patients admitted to an internal medicine department. Medicine (Baltimore). 2016;95(2):e2372. doi:10.1097/MD.0000000000002372
- Street EJ, Justice ED, Kopa Z, et al. The 2016 European guideline on the management of epididymo-orchitis. Int J STD AIDS. 2017;28(8):744-749. doi:10.1177/0956462417699356
- Kbirou A, Alafifi M, Sayah M, Dakir M, Debbagh A, Aboutaieb R. Acute orchiepididymitis: epidemiological and clinical aspects: an analysis of 152 cases. Ann Med Surg (Lond). 2022;75:103335. doi:10.1016/j.amsu.2022.103335
- Rattay B, Benndorf RA. Drug-induced idiosyncratic agranulocytosis - infrequent but dangerous. Front Pharmacol. 2021;12:727717. doi:10.3389/fphar.2021.727717
- Elmedani S, Albayati A, Udongwo N, Odak M, Khawaja S. Trimethoprim-sulfamethoxazole-induced aseptic meningitis: a new approach. Cureus. 2021;13(6):e15869. doi:10.7759/cureus.15869
- Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858-883. doi:10.1128/CMR.00007-10
- Moris G, Garcia-Monco JC. The challenge of drug-induced aseptic meningitis. Arch Intern Med. 1999;159(11):1185- 1194. doi:10.1001/archinte.159.11.1185
- Bruner KE, Coop CA, White KM. Trimethoprim-sulfamethoxazole-induced aseptic meningitis-not just another sulfa allergy. Ann Allergy Asthma Immunol. 2014;113(5):520-526. doi:10.1016/j.anai.2014.08.006
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Agranulocytosis and Aseptic Meningitis Induced by Sulfamethoxazole-Trimethoprim
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
Chronic pain is one of the most prevalent public health concerns in the United States, affecting > 51 million adults with about $500 billion in health care costs.1 Military veterans are among the most vulnerable subpopulations, with 65% of veterans reporting chronic pain in the last 3 months.2 Chronic pain is complex, affecting the biopsychosocial-spiritual levels of human health, and requires multimodal and comprehensive treatment approaches.3 Hence, chronic pain treatment can be best delivered via interdisciplinary teams (IDTs) that use a patient-centered approach.4,5
The Veterans Healthcare Administration (VHA) is a leader in developing and delivering interdisciplinary pain care.6,7 VHA Directive 2009-053 requires every US Department of Veterans Affairs (VA) medical center to offer an IDT for chronic pain. However, VHA and non-VHA IDT programs vary significantly.8-11 A recent systematic review found a median of 5 disciplines included on IDTs (range, 2-8), and program content often included exercise and education; only 11% of included IDTs met simultaneously with patients.11 The heterogeneity of IDT programs has made determining best practices challenging.8,11 The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials has denoted several core measures and measurement domains that were critical for determining the success of pain management interventions, including patient satisfaction.12,13 Nevertheless, the association of IDTs with high patient satisfaction and improvement in pain measures has been documented.5,11
The VHA has worked to implement the Whole Health System into health care, which considers well-being across physical, behavioral, spiritual, and socioeconomic domains. As such, the Whole Health System involves an interpersonal, team-based approach, “anchored in trusting longitudinal relationships to promote resilience, prevent disease, and restore health.”14 It aligns with the patient’s mission, aspiration, and purpose. Surgeon General VADM Vivek H. Murthy, MD, MBA, recently endorsed this approach.15,16 Other health care systems adopting whole health tend to have higher patient satisfaction, increased access to care, and improved patient-reported outcomes.15 Within the VHA, the Whole Health System has shifted the conversation between clinicians and patients from “What is the matter with you?” to “What is important to you?” while emphasizing a proactive and personalized approach to health care.17 Rather than emphasizing passive modalities such as medications and clinician-led services (eg, interventional pain service), the Whole Health System highlights self-care.3,17 Initial research findings within the VHA have been promising.18-21 Whole health peer coaching calls appear to be an effective approach for veterans diagnosed with PTSD, and the use of whole health services is associated with a decrease in opioid use.19,22 However, there are negligible data on patient experiences after meeting with a whole health-focused pain IDT, and studies to date have focused on urban populations.23 One approach to IDT that has shown promise for other health issues involves a patient meeting simultaneously with all members of the IDT.24-27 With the integration of the Whole Health System and the VHA priorities to provide veterans with the “soonest and best care,” more data are needed on the experiences of patients and support persons with various approaches to IDT pain care.28 This study aimed to evaluate patient and support person experiences with a whole health-focused pain IDT that met simultaneously with the patient and support person during an initial evaluation. This study was approved by the institutional review board at the Salem VA Health Care System (SVAHCS) in Virginia.
Methods
The PREVAIL IDT Track is a clinical program offered at SVAHCS with a whole health-focused approach that involves patients and their support persons meeting simultaneously with a pain IDT. PREVAIL IDT Track is designed to help veterans more effectively self-manage chronic pain (Table 1).6,29 Health care practitioners (HCPs) at SVAHCS recommended that veterans with pain persisting for > 3 months participate in PREVAIL IDT Track. After meeting with an advanced practice clinician for an intake, veterans elected to participate in the PREVAIL IDT Track program and completed the initial 6 weeks of pain education. Veterans were then invited to be evaluated by the pain IDT. A team including HCPs from interventional pain, psychology, pharmacy, nutrition, and physical therapy services met with the veteran for 60 minutes. Veterans were also invited to bring a support person to the IDT initial evaluation.
During the IDT initial evaluation, HCPs inquired about the patient’s mission, aspiration, and purpose (“If you were in less pain, what would you be doing more of?”) and about whole health self-care and wellness factors that may contribute to their chronic pain using the Personal Health Inventory.30,31 Veterans were then invited to select 3 whole health self-care areas to focus on during the 6-month program.3 The IDT HCPs worked with the veteran to establish the treatment plan for the first month in the areas of self-care selected by the patient and made recommendations for additional treatments. If the veteran brought a support person to the IDT initial evaluation, their feedback was elicited throughout and at the end to ensure the final treatment plan and first month’s goals were realistic. At the end of the appointment, the veteran and their support person were asked to complete a program-specific satisfaction survey. The HCPs on the team and the veteran executed the treatment plan developed during the appointment, except for medication prescribing. Recommendations for medication changes are included in clinical notes. Veterans then received 5 monthly coaching calls from a nurse navigator with training in whole health and a 6-month follow-up appointment with the IDT HCPs to discuss a plan for continuity of care.
Participant demographic information was not collected, and participants were not compensated for completing the survey. Veterans in PREVAIL IDT Track are predominantly residents of central Appalachia, White, male, unemployed, have ≥ 1 mental and physical health comorbidity, and have a history of mental health treatment.32 Veterans participating in PREVAIL IDT had a mean age of 57 years, and about 1 in 3 have opioid prescriptions.32
A program-specific 17-question satisfaction survey was developed, which included questions related to satisfaction with previous SVAHCS pain care and staff interactions. To assess the overall impression of the IDT initial evaluation, 3 yes/no questions and a 0 to 10-point scale were used. The 5 remaining open-ended questions allowed participants to give feedback about the IDT initial evaluation.
Data Analysis
A convergent mixed-methods approach was used to evaluate participant satisfaction with the initial IDT evaluation. The study team collected and analyzed quantitative and qualitative survey data and triangulated the findings.33 For quantitative responses, frequencies and means were calculated using Python. For qualitative responses, thematic data analysis was conducted by systematic coding, using inductive methods and allowing themes to emerge. Study team members performed a line-by-line analysis of responses using NVivo to identify important codes and reach a consensus. This study adhered to the Consolidated Criteria for Reporting Qualitative Research and followed the National Institute for Health Care Excellence checklist.34,35
Results
Quantitative Responses
In 2022, 168 veterans completed the initial IDT evaluation, and 144 (85.2%) completed the satisfaction survey and were included in this study. Thirty-two support persons who attended the initial IDT evaluation and completed the survey also were included. Of the 12 quantitative questions, 4 had a 100% completion rate, while 8 had ≤ 3% missing responses. When describing care prior to participating in PREVAIL, participants indicated a mean (SD) response of 4.6 (1.4) with the health care they received at SVAHCS and 4.3 (1.4) with SVAHCS pain management services, both on 6-point scales. All but 2 participants (98.9%) reported always being treated with courtesy and respect by PREVAIL HCPs during the initial IDT evaluation, with a mean (SD) score of 4.0 (0.2) on a 4-point scale. Most respondents (96.6%) reported that PREVAIL HCPs always listened carefully during the initial IDT evaluation, with a mean (SD) 4.0 (0.3) on a 4-point scale. Similarly, 92.6% reported that PREVAIL HCPs explained things clearly during the initial IDT evaluation, with a mean (SD) 3.9 (0.3) on a 4-point scale.
All respondents agreed that PREVAIL HCPs considered veteran preferences and those of their support persons in deciding their health care needs during the initial IDT evaluation, with a mean (SD) 3.7 (0.5) on a 4-point scale. Most respondents left the appointment with a good understanding of their responsibilities for chronic pain management with 99.4% (n = 169) strongly agreeing or agreeing (mean [SD] 3.6 [0.5]). A total of 135 respondents (79.4%) reported they left appointments with written information on their treatment plan. All 170 respondents reported that they would recommend PREVAIL to a friend, and 169 respondents (98.8%) felt that the initial PREVAIL IDT evaluation was a valuable use of time. Eighty-seven respondents (50.9%) rated the initial IDT evaluation as the “best clinical experience possible” with a mean (SD) score of 9.2 (1.1) on a 10-point scale (Table 2).
Qualitative Responses
Respondents provided complementary feedback on the program, with many participants stating that they enjoyed every aspect (eAppendix, available at doi:10.12788/fp.0503). In terms of positive aspects of the program, several themes emerged: participants appreciated meeting as an IDT, feeling cared for and listened to, learning more about their pain and ways to manage it, and specific services offered. Thirty-three of 144 respondents wanted longer appointment times. Twenty-two respondents suggested logistics improvements (eg, meeting in a larger room, having a written plan at the end, sending paperwork ahead of time, and later appointment times).
Discussion
Veterans and support persons were satisfied with the initial IDT evaluation for the PREVAIL whole health-focused pain clinical program for veterans predominantly residing in central Appalachia. These satisfaction findings are noteworthy since 20% of this same sample reported dissatisfaction with prior pain services, which could affect engagement and outcomes in pain care. In addition to high satisfaction levels, the PREVAIL IDT model may benefit veterans with limited resources. Rather than needing to attend several individual appointments, the PREVAIL IDT Track provides a 1-stop shop approach that decreases patient burden and barriers to care (eg, travel, transportation, and time) as well as health care system burden. For instance, schedulers need only to make 1 appointment for the veteran rather than several. This approach was highly acceptable to veterans served at SVAHCS and may increase the reach and impact of VHA IDT pain care.
The PREVAIL model may foster rapport with HCPs and encourage an active role in self-managing pain.36,37 Participants noted that their preferences were considered and that they had a good understanding of their responsibilities for managing their chronic pain. This patient-centered approach, emphasizing an active role for the patient, is a hallmark of the VHA Whole Health System and aligns with the overarching PREVAIL IDT Track goal to enhance self-management skills, thus improving functioning through decreased pain interference.14,38-41
Participants in PREVAIL provided substantial open-ended feedback that has contributed to the program’s improvement and may provide information into preferred components of pain IDT programs, particularly for rural veterans. When asked about their favorite component of the initial IDT evaluation, the most emergent theme was meeting simultaneously with HCPs on the IDT. This finding is significant, given that only 11% of IDTs involve direct patient interaction.
Furthermore, unlike most IDTs, PREVAIL IDT includes a dietitian.11,42 IDT programs may benefit from dietitian involvement given the importance of the anti-inflammatory diet on chronic pain.43-46 Participants recommended improvements, (eg, changes to the location and timing, adding a written treatment plan at the end of the appointment, and completing paperwork prior to the appointment) many of which have been addressed. The program now uses validated measures to track progress and comprehensive assessments of pain in response to calls for measurement-based care.13,47 These process improvement suggestions may be instructive for other VA medical centers with rural populations.
Limitations
This study used a program-specific satisfaction survey with open-ended questions to allow for rich responses; however, the survey has not been validated. It also sought to minimize bias by asking participants to give completed surveys to staff members who were not HCPs on the IDT. However, participants’ responses may still have been influenced by this process. Response rate and demographics for support persons were impossible to determine. The results analyzed the responses of veterans and support persons together, which may have skewed the data. Future studies of pain IDT programs should consider analyzing responses from veterans and their support persons separately and identifying factors (eg, demographics or clinical characteristics) that influence the patients’ experiences while participating.
Conclusions
The initial PREVAIL IDT evaluation at SVAHCS is associated with high-levels of satisfaction. These veterans living in rural Appalachia, similar to the 4.4 million rural US veterans, are more likely to encounter barriers to care (eg, drive time, or transportation concerns) and be prescribed opioids.48 These veterans are also at high risk of chronic physical and mental health comorbidities, drug misuse, overdose, and suicide.49,50 Providing veterans in rural communities the opportunity to attend a single appointment with a pain IDT instead of requiring several individual appointments could improve the reach of evidence-based pain care.
This model of meeting simultaneously with all HCPs on the pain IDT may connect all veterans to the most available and best care, something prioritized by the VHA.28 The initial PREVAIL IDT evaluation also utilizes the Personal Health Inventory and the VHA Whole Health System Circle of Health to design patient-centered treatment plans. Integration of the Whole Health System is currently a high priority within VHA. The PREVAIL IDT Track model warrants additional efficacy research.
- Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults - United States, 2019-2021. MMWR Morb Mortal Wkly Rep. 2023;72(15):379-385. doi:10.15585/mmwr.mm7215a1
- Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Mackey SC, Pearl RG. Pain management: optimizing patient care through comprehensive, interdisciplinary models and continuous innovations. Anesthesiol Clin. 2023;41(2):xv-xvii. doi:10.1016/j.anclin.2023.03.011
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130. doi:10.1037/a0035514
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the veterans administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
- Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi:10.1136/bmj.h444
- Waterschoot FPC, Dijkstra PU, Hollak N, De Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain. 2014;155(1):179-189. doi:10.1016/j.pain.2013.10.006
- Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678. doi:10.1093/rheumatology/ken021
- Elbers S, Wittink H, Konings S, et al. Longitudinal outcome evaluations of interdisciplinary multimodal pain Treatment programmes for patients with chronic primary musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. 2022;26(2):310-335. doi:10.1002/ejp.1875
- Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337-345. doi:10.1016/j.pain.2003.08.001
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi:10.1016/j.pain.2004.09.012
- Kligler B, Hyde J, Gantt C, Bokhour B. The whole health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?”. Med Care. 2022;60(5):387-391. doi:10.1097/mlr.0000000000001706
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Transforming Health Care to Create Whole Health: Strategies to Assess, Scale, and Spread the Whole Person Approach to Health, Meisnere M, SouthPaul J, Krist AH, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. National Academies Press (US); February 15, 2023.
- The time Is now for a whole-person health approach to public health. Public Health Rep. 2023;138(4):561-564. doi:10.1177/00333549231154583
- Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12 Suppl 5):S5-S8. doi:10.1097/mlr.0000000000000226
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the whole health system of care. Health Serv Res. 2022;57 Suppl 1(Suppl 1):53-65. doi:10.1111/1475-6773.13938
- Zeliadt SB, Douglas JH, Gelman H, et al. Effectiveness of a whole health model of care emphasizing complementary and integrative health on reducing opioid use among patients with chronic pain. BMC Health Serv Res. 2022;22(1):1053. doi:10.1186/s12913-022-08388-2
- Reed DE 2nd, Bokhour BG, Gaj L, et al. Whole health use and interest across veterans with cooccurring chronic pain and PTSD: an examination of the 18 VA medical center flagship sites. Glob Adv Health Med. 2022;11:21649561211065374. doi:10.1177/21649561211065374
- Etingen B, Smith BM, Zeliadt SB, et al. VHA whole health services and complementary and integrative health therapies: a gateway to evidence-based mental health treatment. J Gen Intern Med. 2023;38(14):3144-3151. doi:10.1007/s11606-023-08296-z
- Johnson EM, Possemato K, Khan S, Chinman M, Maisto SA. Engagement, experience, and satisfaction with peerdelivered whole health coaching for veterans with PTSD: a mixed methods process evaluation. Psychol Serv. 2021;19(2):305-316. doi:10.1037/ser0000529
- Purcell N, Zamora K, Gibson C, et al. Patient experiences with integrated pain care: a qualitative evaluation of one VA’s biopsychosocial approach to chronic pain treatment and opioid safety. Glob Adv Health Med. 2019;8:2164956119838845. doi:10.1177/2164956119838845
- Will KK, Johnson ML, Lamb G. Team-based care and patient satisfaction in the hospital setting: a systematic review. J Patient Cent Res Rev. 2019;6(2):158-171. doi:10.17294/2330-0698.1695
- van Dongen JJJ, Habets IGJ, Beurskens A, van Bokhoven MA. Successful participation of patients in interprofessional team meetings: a qualitative study. Health Expect. 2017;20(4):724-733. doi:10.1111/hex.12511
- Oliver DP, Albright DL, Kruse RL, Wittenberg-Lyles E, Washington K, Demiris G. Caregiver evaluation of the ACTIVE intervention: “it was like we were sitting at the table with everyone.” Am J Hosp Palliat Care. 2014;31(4):444-453. doi:10.1177/1049909113490823
- Ansmann L, Heuser C, Diekmann A, et al. Patient participation in multidisciplinary tumor conferences: how is it implemented? What is the patients’ role? What are patients’ experiences? Cancer Med. 2021;10(19):6714-6724. doi:10.1002/cam4.4213
- US Department of Veterans Affairs, Veterans Health Administration. Updated March 20, 2023. Accessed June 11, 2024. https://www.va.gov/health/priorities/index.asp
- Darnall BD, Edwards KA, Courtney RE, Ziadni MS, Simons LE, Harrison LE. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res (Lausanne). 2023;4:1223172. doi:10.3389/fpain.2023.1223172
- Kligler B. Whole health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Howe RJ, Poulin LM, Federman DG. The personal health inventory: current use, perceived barriers, and benefits. Fed Pract. 2017;34(5):23-26. doi:10.1177/2164957X221077214
- Hicks N, Harden S, Oursler KA, Courtney RE. Determining the representativeness of participants in a whole health interdisciplinary chronic pain program (PREVAIL) in a VA medical center: who did we reach? Presented at: PAINWeek 2022; September 6-9, 2022; Las Vegas, Nevada. Accessed September 10, 2024. https://www.tandfonline.com/doi/full/10.1080/00325481.2022.2116839
- Creswell JW, Creswell JD. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. SAGE Publications; 2018.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual in Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance, 3rd edition. Published September 26, 2012. Accessed June 11, 2024. https://www.nice.org.uk/process/pmg4/chapter/introduction
- Alexander JA, Hearld LR, Mittler JN, Harvey J. Patient-physician role relationships and patient activation among individuals with chronic illness. Health Serv Res. 2012;47(3 PART 1):1201-1223. doi:10.1111/j.1475-6773.2011.01354.x
- Fu Y, Yu G, McNichol E, Marczewski K, Closs SJ. The association between patient-professional partnerships and self-management of chronic back pain: a mixed methods study. Eur J Pain. 2018;22(7):1229-1244. doi:10.1002/ejp.1210
- Nicholas MK, Asghari A, Blyth FM, et al. Self-management intervention for chronic pain in older adults: a randomised controlled trial. Pain. 2013;154(6):824-835. doi:10.1016/j.pain.2013.02.009
- Nøst TH, Steinsbekk A, Bratås O, Grønning K. Twelvemonth effect of chronic pain self-management intervention delivered in an easily accessible primary healthcare service - a randomised controlled trial. BMC Health Serv Res. 2018;18(1):1012. doi:10.1186/s12913-018-3843-x
- Blyth FM, March LM, Nicholas MK, Cousins MJ. Selfmanagement of chronic pain: a population-based study. Pain. 2005;113(3):285-292. doi:10.1016/j.pain.2004.12.004
- Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(7):1070-1078. doi:10.1002/ejp.830
- Murphy JL, Palyo SA, Schmidt ZS, et al. The resurrection of interdisciplinary pain rehabilitation: outcomes across a veterans affairs collaborative. Pain Med. 2021;22(2):430- 443. doi:10.1093/pm/pnaa417
- Brain K, Burrows TL, Bruggink L, et al. Diet and chronic non-cancer pain: the state of the art and future directions. J Clin Med. 2021;10(21):5203. doi:10.3390/jcm10215203
- Field R, Pourkazemi F, Turton J, Rooney K. Dietary interventions are beneficial for patients with chronic pain: a systematic review with meta-analysis. Pain Med). 2021;22(3):694-714. doi:10.1093/pm/pnaa378
- Bjørklund G, Aaseth J, Do§a MD, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153-165. doi:10.1016/j.nut.2019.04.007
- Kaushik AS, Strath LJ, Sorge RE. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. 2020;9(2):487-498. doi:10.1007/s40122-020-00200-5
- Boswell JF, Hepner KA, Lysell K, et al. The need for a measurement-based care professional practice guideline. Psychotherapy (Chic). 2023;60(1):1-16. doi:10.1037/pst0000439
- Lund BC, Ohl ME, Hadlandsmyth K, Mosher HJ. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894- 900. doi:10.1093/milmed/usz104
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated May 14, 2024. Accessed June 11, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- McCarthy JF, Blow FC, Ignacio R V., Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs health system: rural-urban differences in rates, risks, and methods. Am J Public Health. 2012;102 Suppl 1(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
Chronic pain is one of the most prevalent public health concerns in the United States, affecting > 51 million adults with about $500 billion in health care costs.1 Military veterans are among the most vulnerable subpopulations, with 65% of veterans reporting chronic pain in the last 3 months.2 Chronic pain is complex, affecting the biopsychosocial-spiritual levels of human health, and requires multimodal and comprehensive treatment approaches.3 Hence, chronic pain treatment can be best delivered via interdisciplinary teams (IDTs) that use a patient-centered approach.4,5
The Veterans Healthcare Administration (VHA) is a leader in developing and delivering interdisciplinary pain care.6,7 VHA Directive 2009-053 requires every US Department of Veterans Affairs (VA) medical center to offer an IDT for chronic pain. However, VHA and non-VHA IDT programs vary significantly.8-11 A recent systematic review found a median of 5 disciplines included on IDTs (range, 2-8), and program content often included exercise and education; only 11% of included IDTs met simultaneously with patients.11 The heterogeneity of IDT programs has made determining best practices challenging.8,11 The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials has denoted several core measures and measurement domains that were critical for determining the success of pain management interventions, including patient satisfaction.12,13 Nevertheless, the association of IDTs with high patient satisfaction and improvement in pain measures has been documented.5,11
The VHA has worked to implement the Whole Health System into health care, which considers well-being across physical, behavioral, spiritual, and socioeconomic domains. As such, the Whole Health System involves an interpersonal, team-based approach, “anchored in trusting longitudinal relationships to promote resilience, prevent disease, and restore health.”14 It aligns with the patient’s mission, aspiration, and purpose. Surgeon General VADM Vivek H. Murthy, MD, MBA, recently endorsed this approach.15,16 Other health care systems adopting whole health tend to have higher patient satisfaction, increased access to care, and improved patient-reported outcomes.15 Within the VHA, the Whole Health System has shifted the conversation between clinicians and patients from “What is the matter with you?” to “What is important to you?” while emphasizing a proactive and personalized approach to health care.17 Rather than emphasizing passive modalities such as medications and clinician-led services (eg, interventional pain service), the Whole Health System highlights self-care.3,17 Initial research findings within the VHA have been promising.18-21 Whole health peer coaching calls appear to be an effective approach for veterans diagnosed with PTSD, and the use of whole health services is associated with a decrease in opioid use.19,22 However, there are negligible data on patient experiences after meeting with a whole health-focused pain IDT, and studies to date have focused on urban populations.23 One approach to IDT that has shown promise for other health issues involves a patient meeting simultaneously with all members of the IDT.24-27 With the integration of the Whole Health System and the VHA priorities to provide veterans with the “soonest and best care,” more data are needed on the experiences of patients and support persons with various approaches to IDT pain care.28 This study aimed to evaluate patient and support person experiences with a whole health-focused pain IDT that met simultaneously with the patient and support person during an initial evaluation. This study was approved by the institutional review board at the Salem VA Health Care System (SVAHCS) in Virginia.
Methods
The PREVAIL IDT Track is a clinical program offered at SVAHCS with a whole health-focused approach that involves patients and their support persons meeting simultaneously with a pain IDT. PREVAIL IDT Track is designed to help veterans more effectively self-manage chronic pain (Table 1).6,29 Health care practitioners (HCPs) at SVAHCS recommended that veterans with pain persisting for > 3 months participate in PREVAIL IDT Track. After meeting with an advanced practice clinician for an intake, veterans elected to participate in the PREVAIL IDT Track program and completed the initial 6 weeks of pain education. Veterans were then invited to be evaluated by the pain IDT. A team including HCPs from interventional pain, psychology, pharmacy, nutrition, and physical therapy services met with the veteran for 60 minutes. Veterans were also invited to bring a support person to the IDT initial evaluation.

During the IDT initial evaluation, HCPs inquired about the patient’s mission, aspiration, and purpose (“If you were in less pain, what would you be doing more of?”) and about whole health self-care and wellness factors that may contribute to their chronic pain using the Personal Health Inventory.30,31 Veterans were then invited to select 3 whole health self-care areas to focus on during the 6-month program.3 The IDT HCPs worked with the veteran to establish the treatment plan for the first month in the areas of self-care selected by the patient and made recommendations for additional treatments. If the veteran brought a support person to the IDT initial evaluation, their feedback was elicited throughout and at the end to ensure the final treatment plan and first month’s goals were realistic. At the end of the appointment, the veteran and their support person were asked to complete a program-specific satisfaction survey. The HCPs on the team and the veteran executed the treatment plan developed during the appointment, except for medication prescribing. Recommendations for medication changes are included in clinical notes. Veterans then received 5 monthly coaching calls from a nurse navigator with training in whole health and a 6-month follow-up appointment with the IDT HCPs to discuss a plan for continuity of care.
Participant demographic information was not collected, and participants were not compensated for completing the survey. Veterans in PREVAIL IDT Track are predominantly residents of central Appalachia, White, male, unemployed, have ≥ 1 mental and physical health comorbidity, and have a history of mental health treatment.32 Veterans participating in PREVAIL IDT had a mean age of 57 years, and about 1 in 3 have opioid prescriptions.32
A program-specific 17-question satisfaction survey was developed, which included questions related to satisfaction with previous SVAHCS pain care and staff interactions. To assess the overall impression of the IDT initial evaluation, 3 yes/no questions and a 0 to 10-point scale were used. The 5 remaining open-ended questions allowed participants to give feedback about the IDT initial evaluation.
Data Analysis
A convergent mixed-methods approach was used to evaluate participant satisfaction with the initial IDT evaluation. The study team collected and analyzed quantitative and qualitative survey data and triangulated the findings.33 For quantitative responses, frequencies and means were calculated using Python. For qualitative responses, thematic data analysis was conducted by systematic coding, using inductive methods and allowing themes to emerge. Study team members performed a line-by-line analysis of responses using NVivo to identify important codes and reach a consensus. This study adhered to the Consolidated Criteria for Reporting Qualitative Research and followed the National Institute for Health Care Excellence checklist.34,35
Results
Quantitative Responses
In 2022, 168 veterans completed the initial IDT evaluation, and 144 (85.2%) completed the satisfaction survey and were included in this study. Thirty-two support persons who attended the initial IDT evaluation and completed the survey also were included. Of the 12 quantitative questions, 4 had a 100% completion rate, while 8 had ≤ 3% missing responses. When describing care prior to participating in PREVAIL, participants indicated a mean (SD) response of 4.6 (1.4) with the health care they received at SVAHCS and 4.3 (1.4) with SVAHCS pain management services, both on 6-point scales. All but 2 participants (98.9%) reported always being treated with courtesy and respect by PREVAIL HCPs during the initial IDT evaluation, with a mean (SD) score of 4.0 (0.2) on a 4-point scale. Most respondents (96.6%) reported that PREVAIL HCPs always listened carefully during the initial IDT evaluation, with a mean (SD) 4.0 (0.3) on a 4-point scale. Similarly, 92.6% reported that PREVAIL HCPs explained things clearly during the initial IDT evaluation, with a mean (SD) 3.9 (0.3) on a 4-point scale.
All respondents agreed that PREVAIL HCPs considered veteran preferences and those of their support persons in deciding their health care needs during the initial IDT evaluation, with a mean (SD) 3.7 (0.5) on a 4-point scale. Most respondents left the appointment with a good understanding of their responsibilities for chronic pain management with 99.4% (n = 169) strongly agreeing or agreeing (mean [SD] 3.6 [0.5]). A total of 135 respondents (79.4%) reported they left appointments with written information on their treatment plan. All 170 respondents reported that they would recommend PREVAIL to a friend, and 169 respondents (98.8%) felt that the initial PREVAIL IDT evaluation was a valuable use of time. Eighty-seven respondents (50.9%) rated the initial IDT evaluation as the “best clinical experience possible” with a mean (SD) score of 9.2 (1.1) on a 10-point scale (Table 2).

Qualitative Responses
Respondents provided complementary feedback on the program, with many participants stating that they enjoyed every aspect (eAppendix, available at doi:10.12788/fp.0503). In terms of positive aspects of the program, several themes emerged: participants appreciated meeting as an IDT, feeling cared for and listened to, learning more about their pain and ways to manage it, and specific services offered. Thirty-three of 144 respondents wanted longer appointment times. Twenty-two respondents suggested logistics improvements (eg, meeting in a larger room, having a written plan at the end, sending paperwork ahead of time, and later appointment times).
Discussion
Veterans and support persons were satisfied with the initial IDT evaluation for the PREVAIL whole health-focused pain clinical program for veterans predominantly residing in central Appalachia. These satisfaction findings are noteworthy since 20% of this same sample reported dissatisfaction with prior pain services, which could affect engagement and outcomes in pain care. In addition to high satisfaction levels, the PREVAIL IDT model may benefit veterans with limited resources. Rather than needing to attend several individual appointments, the PREVAIL IDT Track provides a 1-stop shop approach that decreases patient burden and barriers to care (eg, travel, transportation, and time) as well as health care system burden. For instance, schedulers need only to make 1 appointment for the veteran rather than several. This approach was highly acceptable to veterans served at SVAHCS and may increase the reach and impact of VHA IDT pain care.
The PREVAIL model may foster rapport with HCPs and encourage an active role in self-managing pain.36,37 Participants noted that their preferences were considered and that they had a good understanding of their responsibilities for managing their chronic pain. This patient-centered approach, emphasizing an active role for the patient, is a hallmark of the VHA Whole Health System and aligns with the overarching PREVAIL IDT Track goal to enhance self-management skills, thus improving functioning through decreased pain interference.14,38-41
Participants in PREVAIL provided substantial open-ended feedback that has contributed to the program’s improvement and may provide information into preferred components of pain IDT programs, particularly for rural veterans. When asked about their favorite component of the initial IDT evaluation, the most emergent theme was meeting simultaneously with HCPs on the IDT. This finding is significant, given that only 11% of IDTs involve direct patient interaction.
Furthermore, unlike most IDTs, PREVAIL IDT includes a dietitian.11,42 IDT programs may benefit from dietitian involvement given the importance of the anti-inflammatory diet on chronic pain.43-46 Participants recommended improvements, (eg, changes to the location and timing, adding a written treatment plan at the end of the appointment, and completing paperwork prior to the appointment) many of which have been addressed. The program now uses validated measures to track progress and comprehensive assessments of pain in response to calls for measurement-based care.13,47 These process improvement suggestions may be instructive for other VA medical centers with rural populations.
Limitations
This study used a program-specific satisfaction survey with open-ended questions to allow for rich responses; however, the survey has not been validated. It also sought to minimize bias by asking participants to give completed surveys to staff members who were not HCPs on the IDT. However, participants’ responses may still have been influenced by this process. Response rate and demographics for support persons were impossible to determine. The results analyzed the responses of veterans and support persons together, which may have skewed the data. Future studies of pain IDT programs should consider analyzing responses from veterans and their support persons separately and identifying factors (eg, demographics or clinical characteristics) that influence the patients’ experiences while participating.
Conclusions
The initial PREVAIL IDT evaluation at SVAHCS is associated with high-levels of satisfaction. These veterans living in rural Appalachia, similar to the 4.4 million rural US veterans, are more likely to encounter barriers to care (eg, drive time, or transportation concerns) and be prescribed opioids.48 These veterans are also at high risk of chronic physical and mental health comorbidities, drug misuse, overdose, and suicide.49,50 Providing veterans in rural communities the opportunity to attend a single appointment with a pain IDT instead of requiring several individual appointments could improve the reach of evidence-based pain care.
This model of meeting simultaneously with all HCPs on the pain IDT may connect all veterans to the most available and best care, something prioritized by the VHA.28 The initial PREVAIL IDT evaluation also utilizes the Personal Health Inventory and the VHA Whole Health System Circle of Health to design patient-centered treatment plans. Integration of the Whole Health System is currently a high priority within VHA. The PREVAIL IDT Track model warrants additional efficacy research.
Chronic pain is one of the most prevalent public health concerns in the United States, affecting > 51 million adults with about $500 billion in health care costs.1 Military veterans are among the most vulnerable subpopulations, with 65% of veterans reporting chronic pain in the last 3 months.2 Chronic pain is complex, affecting the biopsychosocial-spiritual levels of human health, and requires multimodal and comprehensive treatment approaches.3 Hence, chronic pain treatment can be best delivered via interdisciplinary teams (IDTs) that use a patient-centered approach.4,5
The Veterans Healthcare Administration (VHA) is a leader in developing and delivering interdisciplinary pain care.6,7 VHA Directive 2009-053 requires every US Department of Veterans Affairs (VA) medical center to offer an IDT for chronic pain. However, VHA and non-VHA IDT programs vary significantly.8-11 A recent systematic review found a median of 5 disciplines included on IDTs (range, 2-8), and program content often included exercise and education; only 11% of included IDTs met simultaneously with patients.11 The heterogeneity of IDT programs has made determining best practices challenging.8,11 The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials has denoted several core measures and measurement domains that were critical for determining the success of pain management interventions, including patient satisfaction.12,13 Nevertheless, the association of IDTs with high patient satisfaction and improvement in pain measures has been documented.5,11
The VHA has worked to implement the Whole Health System into health care, which considers well-being across physical, behavioral, spiritual, and socioeconomic domains. As such, the Whole Health System involves an interpersonal, team-based approach, “anchored in trusting longitudinal relationships to promote resilience, prevent disease, and restore health.”14 It aligns with the patient’s mission, aspiration, and purpose. Surgeon General VADM Vivek H. Murthy, MD, MBA, recently endorsed this approach.15,16 Other health care systems adopting whole health tend to have higher patient satisfaction, increased access to care, and improved patient-reported outcomes.15 Within the VHA, the Whole Health System has shifted the conversation between clinicians and patients from “What is the matter with you?” to “What is important to you?” while emphasizing a proactive and personalized approach to health care.17 Rather than emphasizing passive modalities such as medications and clinician-led services (eg, interventional pain service), the Whole Health System highlights self-care.3,17 Initial research findings within the VHA have been promising.18-21 Whole health peer coaching calls appear to be an effective approach for veterans diagnosed with PTSD, and the use of whole health services is associated with a decrease in opioid use.19,22 However, there are negligible data on patient experiences after meeting with a whole health-focused pain IDT, and studies to date have focused on urban populations.23 One approach to IDT that has shown promise for other health issues involves a patient meeting simultaneously with all members of the IDT.24-27 With the integration of the Whole Health System and the VHA priorities to provide veterans with the “soonest and best care,” more data are needed on the experiences of patients and support persons with various approaches to IDT pain care.28 This study aimed to evaluate patient and support person experiences with a whole health-focused pain IDT that met simultaneously with the patient and support person during an initial evaluation. This study was approved by the institutional review board at the Salem VA Health Care System (SVAHCS) in Virginia.
Methods
The PREVAIL IDT Track is a clinical program offered at SVAHCS with a whole health-focused approach that involves patients and their support persons meeting simultaneously with a pain IDT. PREVAIL IDT Track is designed to help veterans more effectively self-manage chronic pain (Table 1).6,29 Health care practitioners (HCPs) at SVAHCS recommended that veterans with pain persisting for > 3 months participate in PREVAIL IDT Track. After meeting with an advanced practice clinician for an intake, veterans elected to participate in the PREVAIL IDT Track program and completed the initial 6 weeks of pain education. Veterans were then invited to be evaluated by the pain IDT. A team including HCPs from interventional pain, psychology, pharmacy, nutrition, and physical therapy services met with the veteran for 60 minutes. Veterans were also invited to bring a support person to the IDT initial evaluation.

During the IDT initial evaluation, HCPs inquired about the patient’s mission, aspiration, and purpose (“If you were in less pain, what would you be doing more of?”) and about whole health self-care and wellness factors that may contribute to their chronic pain using the Personal Health Inventory.30,31 Veterans were then invited to select 3 whole health self-care areas to focus on during the 6-month program.3 The IDT HCPs worked with the veteran to establish the treatment plan for the first month in the areas of self-care selected by the patient and made recommendations for additional treatments. If the veteran brought a support person to the IDT initial evaluation, their feedback was elicited throughout and at the end to ensure the final treatment plan and first month’s goals were realistic. At the end of the appointment, the veteran and their support person were asked to complete a program-specific satisfaction survey. The HCPs on the team and the veteran executed the treatment plan developed during the appointment, except for medication prescribing. Recommendations for medication changes are included in clinical notes. Veterans then received 5 monthly coaching calls from a nurse navigator with training in whole health and a 6-month follow-up appointment with the IDT HCPs to discuss a plan for continuity of care.
Participant demographic information was not collected, and participants were not compensated for completing the survey. Veterans in PREVAIL IDT Track are predominantly residents of central Appalachia, White, male, unemployed, have ≥ 1 mental and physical health comorbidity, and have a history of mental health treatment.32 Veterans participating in PREVAIL IDT had a mean age of 57 years, and about 1 in 3 have opioid prescriptions.32
A program-specific 17-question satisfaction survey was developed, which included questions related to satisfaction with previous SVAHCS pain care and staff interactions. To assess the overall impression of the IDT initial evaluation, 3 yes/no questions and a 0 to 10-point scale were used. The 5 remaining open-ended questions allowed participants to give feedback about the IDT initial evaluation.
Data Analysis
A convergent mixed-methods approach was used to evaluate participant satisfaction with the initial IDT evaluation. The study team collected and analyzed quantitative and qualitative survey data and triangulated the findings.33 For quantitative responses, frequencies and means were calculated using Python. For qualitative responses, thematic data analysis was conducted by systematic coding, using inductive methods and allowing themes to emerge. Study team members performed a line-by-line analysis of responses using NVivo to identify important codes and reach a consensus. This study adhered to the Consolidated Criteria for Reporting Qualitative Research and followed the National Institute for Health Care Excellence checklist.34,35
Results
Quantitative Responses
In 2022, 168 veterans completed the initial IDT evaluation, and 144 (85.2%) completed the satisfaction survey and were included in this study. Thirty-two support persons who attended the initial IDT evaluation and completed the survey also were included. Of the 12 quantitative questions, 4 had a 100% completion rate, while 8 had ≤ 3% missing responses. When describing care prior to participating in PREVAIL, participants indicated a mean (SD) response of 4.6 (1.4) with the health care they received at SVAHCS and 4.3 (1.4) with SVAHCS pain management services, both on 6-point scales. All but 2 participants (98.9%) reported always being treated with courtesy and respect by PREVAIL HCPs during the initial IDT evaluation, with a mean (SD) score of 4.0 (0.2) on a 4-point scale. Most respondents (96.6%) reported that PREVAIL HCPs always listened carefully during the initial IDT evaluation, with a mean (SD) 4.0 (0.3) on a 4-point scale. Similarly, 92.6% reported that PREVAIL HCPs explained things clearly during the initial IDT evaluation, with a mean (SD) 3.9 (0.3) on a 4-point scale.
All respondents agreed that PREVAIL HCPs considered veteran preferences and those of their support persons in deciding their health care needs during the initial IDT evaluation, with a mean (SD) 3.7 (0.5) on a 4-point scale. Most respondents left the appointment with a good understanding of their responsibilities for chronic pain management with 99.4% (n = 169) strongly agreeing or agreeing (mean [SD] 3.6 [0.5]). A total of 135 respondents (79.4%) reported they left appointments with written information on their treatment plan. All 170 respondents reported that they would recommend PREVAIL to a friend, and 169 respondents (98.8%) felt that the initial PREVAIL IDT evaluation was a valuable use of time. Eighty-seven respondents (50.9%) rated the initial IDT evaluation as the “best clinical experience possible” with a mean (SD) score of 9.2 (1.1) on a 10-point scale (Table 2).

Qualitative Responses
Respondents provided complementary feedback on the program, with many participants stating that they enjoyed every aspect (eAppendix, available at doi:10.12788/fp.0503). In terms of positive aspects of the program, several themes emerged: participants appreciated meeting as an IDT, feeling cared for and listened to, learning more about their pain and ways to manage it, and specific services offered. Thirty-three of 144 respondents wanted longer appointment times. Twenty-two respondents suggested logistics improvements (eg, meeting in a larger room, having a written plan at the end, sending paperwork ahead of time, and later appointment times).
Discussion
Veterans and support persons were satisfied with the initial IDT evaluation for the PREVAIL whole health-focused pain clinical program for veterans predominantly residing in central Appalachia. These satisfaction findings are noteworthy since 20% of this same sample reported dissatisfaction with prior pain services, which could affect engagement and outcomes in pain care. In addition to high satisfaction levels, the PREVAIL IDT model may benefit veterans with limited resources. Rather than needing to attend several individual appointments, the PREVAIL IDT Track provides a 1-stop shop approach that decreases patient burden and barriers to care (eg, travel, transportation, and time) as well as health care system burden. For instance, schedulers need only to make 1 appointment for the veteran rather than several. This approach was highly acceptable to veterans served at SVAHCS and may increase the reach and impact of VHA IDT pain care.
The PREVAIL model may foster rapport with HCPs and encourage an active role in self-managing pain.36,37 Participants noted that their preferences were considered and that they had a good understanding of their responsibilities for managing their chronic pain. This patient-centered approach, emphasizing an active role for the patient, is a hallmark of the VHA Whole Health System and aligns with the overarching PREVAIL IDT Track goal to enhance self-management skills, thus improving functioning through decreased pain interference.14,38-41
Participants in PREVAIL provided substantial open-ended feedback that has contributed to the program’s improvement and may provide information into preferred components of pain IDT programs, particularly for rural veterans. When asked about their favorite component of the initial IDT evaluation, the most emergent theme was meeting simultaneously with HCPs on the IDT. This finding is significant, given that only 11% of IDTs involve direct patient interaction.
Furthermore, unlike most IDTs, PREVAIL IDT includes a dietitian.11,42 IDT programs may benefit from dietitian involvement given the importance of the anti-inflammatory diet on chronic pain.43-46 Participants recommended improvements, (eg, changes to the location and timing, adding a written treatment plan at the end of the appointment, and completing paperwork prior to the appointment) many of which have been addressed. The program now uses validated measures to track progress and comprehensive assessments of pain in response to calls for measurement-based care.13,47 These process improvement suggestions may be instructive for other VA medical centers with rural populations.
Limitations
This study used a program-specific satisfaction survey with open-ended questions to allow for rich responses; however, the survey has not been validated. It also sought to minimize bias by asking participants to give completed surveys to staff members who were not HCPs on the IDT. However, participants’ responses may still have been influenced by this process. Response rate and demographics for support persons were impossible to determine. The results analyzed the responses of veterans and support persons together, which may have skewed the data. Future studies of pain IDT programs should consider analyzing responses from veterans and their support persons separately and identifying factors (eg, demographics or clinical characteristics) that influence the patients’ experiences while participating.
Conclusions
The initial PREVAIL IDT evaluation at SVAHCS is associated with high-levels of satisfaction. These veterans living in rural Appalachia, similar to the 4.4 million rural US veterans, are more likely to encounter barriers to care (eg, drive time, or transportation concerns) and be prescribed opioids.48 These veterans are also at high risk of chronic physical and mental health comorbidities, drug misuse, overdose, and suicide.49,50 Providing veterans in rural communities the opportunity to attend a single appointment with a pain IDT instead of requiring several individual appointments could improve the reach of evidence-based pain care.
This model of meeting simultaneously with all HCPs on the pain IDT may connect all veterans to the most available and best care, something prioritized by the VHA.28 The initial PREVAIL IDT evaluation also utilizes the Personal Health Inventory and the VHA Whole Health System Circle of Health to design patient-centered treatment plans. Integration of the Whole Health System is currently a high priority within VHA. The PREVAIL IDT Track model warrants additional efficacy research.
- Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults - United States, 2019-2021. MMWR Morb Mortal Wkly Rep. 2023;72(15):379-385. doi:10.15585/mmwr.mm7215a1
- Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Mackey SC, Pearl RG. Pain management: optimizing patient care through comprehensive, interdisciplinary models and continuous innovations. Anesthesiol Clin. 2023;41(2):xv-xvii. doi:10.1016/j.anclin.2023.03.011
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130. doi:10.1037/a0035514
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the veterans administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
- Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi:10.1136/bmj.h444
- Waterschoot FPC, Dijkstra PU, Hollak N, De Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain. 2014;155(1):179-189. doi:10.1016/j.pain.2013.10.006
- Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678. doi:10.1093/rheumatology/ken021
- Elbers S, Wittink H, Konings S, et al. Longitudinal outcome evaluations of interdisciplinary multimodal pain Treatment programmes for patients with chronic primary musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. 2022;26(2):310-335. doi:10.1002/ejp.1875
- Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337-345. doi:10.1016/j.pain.2003.08.001
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi:10.1016/j.pain.2004.09.012
- Kligler B, Hyde J, Gantt C, Bokhour B. The whole health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?”. Med Care. 2022;60(5):387-391. doi:10.1097/mlr.0000000000001706
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Transforming Health Care to Create Whole Health: Strategies to Assess, Scale, and Spread the Whole Person Approach to Health, Meisnere M, SouthPaul J, Krist AH, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. National Academies Press (US); February 15, 2023.
- The time Is now for a whole-person health approach to public health. Public Health Rep. 2023;138(4):561-564. doi:10.1177/00333549231154583
- Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12 Suppl 5):S5-S8. doi:10.1097/mlr.0000000000000226
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the whole health system of care. Health Serv Res. 2022;57 Suppl 1(Suppl 1):53-65. doi:10.1111/1475-6773.13938
- Zeliadt SB, Douglas JH, Gelman H, et al. Effectiveness of a whole health model of care emphasizing complementary and integrative health on reducing opioid use among patients with chronic pain. BMC Health Serv Res. 2022;22(1):1053. doi:10.1186/s12913-022-08388-2
- Reed DE 2nd, Bokhour BG, Gaj L, et al. Whole health use and interest across veterans with cooccurring chronic pain and PTSD: an examination of the 18 VA medical center flagship sites. Glob Adv Health Med. 2022;11:21649561211065374. doi:10.1177/21649561211065374
- Etingen B, Smith BM, Zeliadt SB, et al. VHA whole health services and complementary and integrative health therapies: a gateway to evidence-based mental health treatment. J Gen Intern Med. 2023;38(14):3144-3151. doi:10.1007/s11606-023-08296-z
- Johnson EM, Possemato K, Khan S, Chinman M, Maisto SA. Engagement, experience, and satisfaction with peerdelivered whole health coaching for veterans with PTSD: a mixed methods process evaluation. Psychol Serv. 2021;19(2):305-316. doi:10.1037/ser0000529
- Purcell N, Zamora K, Gibson C, et al. Patient experiences with integrated pain care: a qualitative evaluation of one VA’s biopsychosocial approach to chronic pain treatment and opioid safety. Glob Adv Health Med. 2019;8:2164956119838845. doi:10.1177/2164956119838845
- Will KK, Johnson ML, Lamb G. Team-based care and patient satisfaction in the hospital setting: a systematic review. J Patient Cent Res Rev. 2019;6(2):158-171. doi:10.17294/2330-0698.1695
- van Dongen JJJ, Habets IGJ, Beurskens A, van Bokhoven MA. Successful participation of patients in interprofessional team meetings: a qualitative study. Health Expect. 2017;20(4):724-733. doi:10.1111/hex.12511
- Oliver DP, Albright DL, Kruse RL, Wittenberg-Lyles E, Washington K, Demiris G. Caregiver evaluation of the ACTIVE intervention: “it was like we were sitting at the table with everyone.” Am J Hosp Palliat Care. 2014;31(4):444-453. doi:10.1177/1049909113490823
- Ansmann L, Heuser C, Diekmann A, et al. Patient participation in multidisciplinary tumor conferences: how is it implemented? What is the patients’ role? What are patients’ experiences? Cancer Med. 2021;10(19):6714-6724. doi:10.1002/cam4.4213
- US Department of Veterans Affairs, Veterans Health Administration. Updated March 20, 2023. Accessed June 11, 2024. https://www.va.gov/health/priorities/index.asp
- Darnall BD, Edwards KA, Courtney RE, Ziadni MS, Simons LE, Harrison LE. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res (Lausanne). 2023;4:1223172. doi:10.3389/fpain.2023.1223172
- Kligler B. Whole health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Howe RJ, Poulin LM, Federman DG. The personal health inventory: current use, perceived barriers, and benefits. Fed Pract. 2017;34(5):23-26. doi:10.1177/2164957X221077214
- Hicks N, Harden S, Oursler KA, Courtney RE. Determining the representativeness of participants in a whole health interdisciplinary chronic pain program (PREVAIL) in a VA medical center: who did we reach? Presented at: PAINWeek 2022; September 6-9, 2022; Las Vegas, Nevada. Accessed September 10, 2024. https://www.tandfonline.com/doi/full/10.1080/00325481.2022.2116839
- Creswell JW, Creswell JD. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. SAGE Publications; 2018.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual in Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance, 3rd edition. Published September 26, 2012. Accessed June 11, 2024. https://www.nice.org.uk/process/pmg4/chapter/introduction
- Alexander JA, Hearld LR, Mittler JN, Harvey J. Patient-physician role relationships and patient activation among individuals with chronic illness. Health Serv Res. 2012;47(3 PART 1):1201-1223. doi:10.1111/j.1475-6773.2011.01354.x
- Fu Y, Yu G, McNichol E, Marczewski K, Closs SJ. The association between patient-professional partnerships and self-management of chronic back pain: a mixed methods study. Eur J Pain. 2018;22(7):1229-1244. doi:10.1002/ejp.1210
- Nicholas MK, Asghari A, Blyth FM, et al. Self-management intervention for chronic pain in older adults: a randomised controlled trial. Pain. 2013;154(6):824-835. doi:10.1016/j.pain.2013.02.009
- Nøst TH, Steinsbekk A, Bratås O, Grønning K. Twelvemonth effect of chronic pain self-management intervention delivered in an easily accessible primary healthcare service - a randomised controlled trial. BMC Health Serv Res. 2018;18(1):1012. doi:10.1186/s12913-018-3843-x
- Blyth FM, March LM, Nicholas MK, Cousins MJ. Selfmanagement of chronic pain: a population-based study. Pain. 2005;113(3):285-292. doi:10.1016/j.pain.2004.12.004
- Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(7):1070-1078. doi:10.1002/ejp.830
- Murphy JL, Palyo SA, Schmidt ZS, et al. The resurrection of interdisciplinary pain rehabilitation: outcomes across a veterans affairs collaborative. Pain Med. 2021;22(2):430- 443. doi:10.1093/pm/pnaa417
- Brain K, Burrows TL, Bruggink L, et al. Diet and chronic non-cancer pain: the state of the art and future directions. J Clin Med. 2021;10(21):5203. doi:10.3390/jcm10215203
- Field R, Pourkazemi F, Turton J, Rooney K. Dietary interventions are beneficial for patients with chronic pain: a systematic review with meta-analysis. Pain Med). 2021;22(3):694-714. doi:10.1093/pm/pnaa378
- Bjørklund G, Aaseth J, Do§a MD, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153-165. doi:10.1016/j.nut.2019.04.007
- Kaushik AS, Strath LJ, Sorge RE. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. 2020;9(2):487-498. doi:10.1007/s40122-020-00200-5
- Boswell JF, Hepner KA, Lysell K, et al. The need for a measurement-based care professional practice guideline. Psychotherapy (Chic). 2023;60(1):1-16. doi:10.1037/pst0000439
- Lund BC, Ohl ME, Hadlandsmyth K, Mosher HJ. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894- 900. doi:10.1093/milmed/usz104
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated May 14, 2024. Accessed June 11, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- McCarthy JF, Blow FC, Ignacio R V., Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs health system: rural-urban differences in rates, risks, and methods. Am J Public Health. 2012;102 Suppl 1(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
- Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults - United States, 2019-2021. MMWR Morb Mortal Wkly Rep. 2023;72(15):379-385. doi:10.15585/mmwr.mm7215a1
- Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
- Courtney RE, Schadegg MJ, Bolton R, Smith S, Harden SM. Using a whole health approach to build biopsychosocial-spiritual personal health plans for veterans with chronic pain. Pain Manag Nurs. 2024;25(1):69-74. doi:10.1016/j.pmn.2023.09.010
- Mackey SC, Pearl RG. Pain management: optimizing patient care through comprehensive, interdisciplinary models and continuous innovations. Anesthesiol Clin. 2023;41(2):xv-xvii. doi:10.1016/j.anclin.2023.03.011
- Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119-130. doi:10.1037/a0035514
- Courtney RE, Schadegg MJ. Chronic, noncancer pain care in the veterans administration: current trends and future directions. Anesthesiol Clin. 2023;41(2):519-529. doi:10.1016/j.anclin.2023.02.004
- Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378. doi:10.1016/j.anclin.2016.01.003
- Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ. 2015;350:h444. doi:10.1136/bmj.h444
- Waterschoot FPC, Dijkstra PU, Hollak N, De Vries HJ, Geertzen JHB, Reneman MF. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain. 2014;155(1):179-189. doi:10.1016/j.pain.2013.10.006
- Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford). 2008;47(5):670-678. doi:10.1093/rheumatology/ken021
- Elbers S, Wittink H, Konings S, et al. Longitudinal outcome evaluations of interdisciplinary multimodal pain Treatment programmes for patients with chronic primary musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. 2022;26(2):310-335. doi:10.1002/ejp.1875
- Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337-345. doi:10.1016/j.pain.2003.08.001
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi:10.1016/j.pain.2004.09.012
- Kligler B, Hyde J, Gantt C, Bokhour B. The whole health transformation at the Veterans Health Administration: moving from “what’s the matter with you?” to “what matters to you?”. Med Care. 2022;60(5):387-391. doi:10.1097/mlr.0000000000001706
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Transforming Health Care to Create Whole Health: Strategies to Assess, Scale, and Spread the Whole Person Approach to Health, Meisnere M, SouthPaul J, Krist AH, eds. Achieving Whole Health: A New Approach for Veterans and the Nation. National Academies Press (US); February 15, 2023.
- The time Is now for a whole-person health approach to public health. Public Health Rep. 2023;138(4):561-564. doi:10.1177/00333549231154583
- Krejci LP, Carter K, Gaudet T. Whole health: the vision and implementation of personalized, proactive, patient-driven health care for veterans. Med Care. 2014;52(12 Suppl 5):S5-S8. doi:10.1097/mlr.0000000000000226
- Bokhour BG, Hyde J, Kligler B, et al. From patient outcomes to system change: evaluating the impact of VHA’s implementation of the whole health system of care. Health Serv Res. 2022;57 Suppl 1(Suppl 1):53-65. doi:10.1111/1475-6773.13938
- Zeliadt SB, Douglas JH, Gelman H, et al. Effectiveness of a whole health model of care emphasizing complementary and integrative health on reducing opioid use among patients with chronic pain. BMC Health Serv Res. 2022;22(1):1053. doi:10.1186/s12913-022-08388-2
- Reed DE 2nd, Bokhour BG, Gaj L, et al. Whole health use and interest across veterans with cooccurring chronic pain and PTSD: an examination of the 18 VA medical center flagship sites. Glob Adv Health Med. 2022;11:21649561211065374. doi:10.1177/21649561211065374
- Etingen B, Smith BM, Zeliadt SB, et al. VHA whole health services and complementary and integrative health therapies: a gateway to evidence-based mental health treatment. J Gen Intern Med. 2023;38(14):3144-3151. doi:10.1007/s11606-023-08296-z
- Johnson EM, Possemato K, Khan S, Chinman M, Maisto SA. Engagement, experience, and satisfaction with peerdelivered whole health coaching for veterans with PTSD: a mixed methods process evaluation. Psychol Serv. 2021;19(2):305-316. doi:10.1037/ser0000529
- Purcell N, Zamora K, Gibson C, et al. Patient experiences with integrated pain care: a qualitative evaluation of one VA’s biopsychosocial approach to chronic pain treatment and opioid safety. Glob Adv Health Med. 2019;8:2164956119838845. doi:10.1177/2164956119838845
- Will KK, Johnson ML, Lamb G. Team-based care and patient satisfaction in the hospital setting: a systematic review. J Patient Cent Res Rev. 2019;6(2):158-171. doi:10.17294/2330-0698.1695
- van Dongen JJJ, Habets IGJ, Beurskens A, van Bokhoven MA. Successful participation of patients in interprofessional team meetings: a qualitative study. Health Expect. 2017;20(4):724-733. doi:10.1111/hex.12511
- Oliver DP, Albright DL, Kruse RL, Wittenberg-Lyles E, Washington K, Demiris G. Caregiver evaluation of the ACTIVE intervention: “it was like we were sitting at the table with everyone.” Am J Hosp Palliat Care. 2014;31(4):444-453. doi:10.1177/1049909113490823
- Ansmann L, Heuser C, Diekmann A, et al. Patient participation in multidisciplinary tumor conferences: how is it implemented? What is the patients’ role? What are patients’ experiences? Cancer Med. 2021;10(19):6714-6724. doi:10.1002/cam4.4213
- US Department of Veterans Affairs, Veterans Health Administration. Updated March 20, 2023. Accessed June 11, 2024. https://www.va.gov/health/priorities/index.asp
- Darnall BD, Edwards KA, Courtney RE, Ziadni MS, Simons LE, Harrison LE. Innovative treatment formats, technologies, and clinician trainings that improve access to behavioral pain treatment for youth and adults. Front Pain Res (Lausanne). 2023;4:1223172. doi:10.3389/fpain.2023.1223172
- Kligler B. Whole health in the Veterans Health Administration. Glob Adv Health Med. 2022;11:2164957X221077214.
- Howe RJ, Poulin LM, Federman DG. The personal health inventory: current use, perceived barriers, and benefits. Fed Pract. 2017;34(5):23-26. doi:10.1177/2164957X221077214
- Hicks N, Harden S, Oursler KA, Courtney RE. Determining the representativeness of participants in a whole health interdisciplinary chronic pain program (PREVAIL) in a VA medical center: who did we reach? Presented at: PAINWeek 2022; September 6-9, 2022; Las Vegas, Nevada. Accessed September 10, 2024. https://www.tandfonline.com/doi/full/10.1080/00325481.2022.2116839
- Creswell JW, Creswell JD. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. SAGE Publications; 2018.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual in Health Care. 2007;19(6):349-357. doi:10.1093/intqhc/mzm042
- National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance, 3rd edition. Published September 26, 2012. Accessed June 11, 2024. https://www.nice.org.uk/process/pmg4/chapter/introduction
- Alexander JA, Hearld LR, Mittler JN, Harvey J. Patient-physician role relationships and patient activation among individuals with chronic illness. Health Serv Res. 2012;47(3 PART 1):1201-1223. doi:10.1111/j.1475-6773.2011.01354.x
- Fu Y, Yu G, McNichol E, Marczewski K, Closs SJ. The association between patient-professional partnerships and self-management of chronic back pain: a mixed methods study. Eur J Pain. 2018;22(7):1229-1244. doi:10.1002/ejp.1210
- Nicholas MK, Asghari A, Blyth FM, et al. Self-management intervention for chronic pain in older adults: a randomised controlled trial. Pain. 2013;154(6):824-835. doi:10.1016/j.pain.2013.02.009
- Nøst TH, Steinsbekk A, Bratås O, Grønning K. Twelvemonth effect of chronic pain self-management intervention delivered in an easily accessible primary healthcare service - a randomised controlled trial. BMC Health Serv Res. 2018;18(1):1012. doi:10.1186/s12913-018-3843-x
- Blyth FM, March LM, Nicholas MK, Cousins MJ. Selfmanagement of chronic pain: a population-based study. Pain. 2005;113(3):285-292. doi:10.1016/j.pain.2004.12.004
- Damush TM, Kroenke K, Bair MJ, et al. Pain self-management training increases self-efficacy, self-management behaviours and pain and depression outcomes. Eur J Pain. 2016;20(7):1070-1078. doi:10.1002/ejp.830
- Murphy JL, Palyo SA, Schmidt ZS, et al. The resurrection of interdisciplinary pain rehabilitation: outcomes across a veterans affairs collaborative. Pain Med. 2021;22(2):430- 443. doi:10.1093/pm/pnaa417
- Brain K, Burrows TL, Bruggink L, et al. Diet and chronic non-cancer pain: the state of the art and future directions. J Clin Med. 2021;10(21):5203. doi:10.3390/jcm10215203
- Field R, Pourkazemi F, Turton J, Rooney K. Dietary interventions are beneficial for patients with chronic pain: a systematic review with meta-analysis. Pain Med). 2021;22(3):694-714. doi:10.1093/pm/pnaa378
- Bjørklund G, Aaseth J, Do§a MD, et al. Does diet play a role in reducing nociception related to inflammation and chronic pain? Nutrition. 2019;66:153-165. doi:10.1016/j.nut.2019.04.007
- Kaushik AS, Strath LJ, Sorge RE. Dietary interventions for treatment of chronic pain: oxidative stress and inflammation. Pain Ther. 2020;9(2):487-498. doi:10.1007/s40122-020-00200-5
- Boswell JF, Hepner KA, Lysell K, et al. The need for a measurement-based care professional practice guideline. Psychotherapy (Chic). 2023;60(1):1-16. doi:10.1037/pst0000439
- Lund BC, Ohl ME, Hadlandsmyth K, Mosher HJ. Regional and rural-urban variation in opioid prescribing in the Veterans Health Administration. Mil Med. 2019;184(11-12):894- 900. doi:10.1093/milmed/usz104
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated May 14, 2024. Accessed June 11, 2024. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- McCarthy JF, Blow FC, Ignacio R V., Ilgen MA, Austin KL, Valenstein M. Suicide among patients in the Veterans Affairs health system: rural-urban differences in rates, risks, and methods. Am J Public Health. 2012;102 Suppl 1(suppl 1):S111-S117. doi:10.2105/AJPH.2011.300463
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
Patient and Support Person Satisfaction Following a Whole Health-Informed Interdisciplinary Pain Team Meeting
The Year of AI: Learning With Machines to Improve Veteran Health Care
The Year of AI: Learning With Machines to Improve Veteran Health Care
We have a tradition at Federal Practitioner where the December editorial usually features some version of the “best and worst” of the last 12 months in government health care. As we close out a difficult year, instead I offer a cautionary yet promising story that epitomizes both risk and benefit.
In some quarters, 2024 has been the year of AI (artificial intelligence).2 While in science fiction, superhuman machines, like the Terminator, are often associated with apocalyptic threats, we often forget the positive models of human-technology interaction, such as the protective robot in Lost in Space. While AI is not yet as advanced as what has already been depicted on the screen, it is inextricably interwoven into the daily fabric of our lives. Almost any website you go to for business or pleasure has a chatbot waiting to help (or frustrate) you. Most of us have Alexa, Siri, or another digital assistant organizing our homes and schedules. When I Google “everyday uses of artificial intelligence,” it is AI that responds with an overview.
Medicine is not immune. Renowned physician and scientist Eric Topol, MD, suggests that AI represents a “fourth industrial revolution in medicine” that can dramatically improve health care.3 The US Department of Veterans Affairs (VA) has been at the forefront of this new space.4 The story recounted below encapsulates the enormous benefits AI can bring to health care and the vigilance we must exercise to anticipate and mitigate risk for this to be an overall positive transition.
The story begins with a key element of AI change—the machine learning predictive algorithm. In this case, the algorithm was designed to predict—and thereby prevent—the top public health priority in federal practice: suicide. The Recovery Engagement and Coordination for Health-Veterans Enhanced Treatment (REACH VET) program was launched in 2017 to assist in identifying the top 0.1% of veterans at the highest risk for suicide.5
At least at this stage of AI in medicine, the safest and most ethical efforts come from collaborations between health care professionals and AI developers that maximize the very different strengths of each partner. REACH VET is an exemplar of this kind of teamwork. Once the algorithm analyzes > 60 variables to identify veterans at high risk for suicide, data are communicated to a REACH VET program coordinator, who then notifies the practitioner responsible for the veteran’s care so they can put into action evidence-based suicide prevention strategies.5
VA researchers in 2021 published a study of 173,313 veterans comparing outcomes before and after entry into the program using a triple differences design. Veterans participating in the program reported an increase in outpatient visits and documentation of safety plans, and a decrease in emergency department visits, inpatient mental health admissions, and recorded suicide attempts.6
A US Government Accounting Office analysis found that “REACH VET had identified veterans who had not been identified through other methods.”7 This was not just an example of AI hype: as a relatively rare and statistically complicated phenomenon, suicide is notoriously difficult to predict and model. Machine learning algorithms like REACH VET have unprecedented potential to assist and augment suicide prevention.8
In 2023, veteran service organizations and journalists raised concerns that the AI algorithm was biased and ignored critical risk factors that put some veterans at increased risk. Based on their analysis, they claimed that the algorithm did not account for risk factors uniquely associated with women veterans, namely military sexual trauma and intimate partner violence.9 Women are the most rapidly growing VA population, yet too often they encounter health care disparities, harassment, and stigmatization when seeking care. The Congressional Veterans Affairs committees investigated and introduced legislation to update the algorithm.10
VA experts dispute these claims, and a computer science PhD may be required to understand the debate. But as the history of medicine has shown us, every treatment and procedure has benefits and risks. No matter how bright and shiny the technology initially appears, a soft scientific underbelly emerges sooner or later. Just as with REACH VET, algorithm bias is often discovered during deployment when the logic of the laboratory encounters the unpredictable variety of humankind.11 Frequently, those problems are—as with REACH VET— not solely or even primarily technical ones. The data mirror society and reflect its biases.
For learning organizations like the VA and the US Department of Defense (DoD), the criticisms of REACH VET signal the need to engage in continuous performance improvement. AI requires the human trainers and supervisors who teach the machines to continuously revise and update their lesson plans. The most recent VA data show that in 2021, 6392 veterans died by suicide.12 In Congressional testimony, VA leaders reported that as of May 2024, REACH VET was operating in 28 VA facilities and had identified 6700 high-risk veterans.13 REACH VET can save veteran’s lives, which is the sine qua non for our federal health care systems.
The algorithm should be improved to identify ALL veterans so they receive lifesaving interventions. Every veteran’s life is sacred; the algorithm that may prevent suicide must be continuously improved. That is why our representatives did not propose to ban REACH VET or enforce an AI winter on the VA and DoD. Instead, they called for an update to the algorithm, underscoring the value of machine learning for suicide prediction and prevention.
The epigraph from one of the top AI ethicists and scientists in the world makes the point that AI is not the moral agent here: it is fallible humans who must keep learning along with machines. That is why, at the end of 2024, VA experts are revising the algorithm so REACH VET can help prevent even more veteran suicides in 2025 and beyond.14
- Waikar S. Health care’s AI future: a conversation with Fei Fei Li and Andrew Ng. HAI Stanford University. May 10, 2021. Accessed November 13, 2024. https://hai.stanford.edu/news/health-cares-ai-future-conversation-fei-fei-li-and-andrew-ng
- Johnson E, Forbes Technology Council. 2023 Was the Year of AI Hype—2024 is the Year of AI Practicality. Forbes. April 2, 2024. Accessed November 13, 2024. https://www.forbes.com/councils/forbestechcouncil/2024/04/02/2023-was-the-year-of-ai-hype-2024-is-the-year-of-ai-practicality/
- Topol E. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again. Basic Books; 2019.
- Perlis R. The VA was an early adopter of artificial intelligence to improve care-here’s what they learned. JAMA. 2024;332(17):1411-1414. doi:10.1001/jama.2024.20563
- VA REACH VET initiative helps save lives [press release]. April 3, 2017. Accessed November 13, 2024. https://news.va.gov/36714/va-reach-vet-initiative-helps-save-veterans-lives/
- McCarthy JF, Cooper SA, Dent KR, et al. Evaluation of the recovery engagement and coordination for health-veterans enhanced treatment suicide risk modeling clinical program in the Veterans Health Administration. JAMA Netw Open. 2021;4(10):e2129900. doi:10.1001/jamanetworkopen.2021.29900
- US Government Office of Accountability. Veteran suicide: VA efforts to identify veterans at risk through analysis of health record information. September 14, 2022. Accessed November 13, 2024. https://www.gao.gov/products/gao-22-105165
- Pigoni A, Delvecchio G, Turtulici N, et al. Machine learning and the prediction of suicide in psychiatric populations: a systematic review. Transl Psychiatry. 2024;14(1):140. doi:10.1038/s41398-024-02852-9
- Glantz A. VA veteran suicide prevention algorithm favors men. Military.com. May 23, 2024. Accessed November 13, 2024. https://www.military.com/daily-news/2024/05/23/vas-veteran-suicide-prevention-algorithm-favors-men.html
- S.5210 BRAVE Act of 2024. 118th Congress. https://www.congress.gov/bill/118th-congress/senate-bill/5210/text
- Ratwani RM, Sutton K, and Galarrga JE. Addressing algorithmic bias in health care. JAMA. 2024;332(13):1051-1052. doi:10.1001/jama.2024.1348/
- US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2023 national veteran suicide prevention annual report. November 2023 Accessed November 13, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- House Committee on Veterans Affairs. Health Chairwoman Miller-Meeks opens Iowa field hearing on breakthroughs in VA healthcare. May 13, 2024. Accessed November 13, 2024. https://veterans.house.gov/news/documentsingle.aspx?DocumentID=6452
- Graham E. VA is updating its AI suicide risk model to reach more women. NEXTGOV/FCW. October 18, 2024. Accessed November 13, 2024. https://www.nextgov.com/artificial-intelligence/2024/10/va-updating-its-ai-suicide-risk-model-reach-more-women/400377/
We have a tradition at Federal Practitioner where the December editorial usually features some version of the “best and worst” of the last 12 months in government health care. As we close out a difficult year, instead I offer a cautionary yet promising story that epitomizes both risk and benefit.
In some quarters, 2024 has been the year of AI (artificial intelligence).2 While in science fiction, superhuman machines, like the Terminator, are often associated with apocalyptic threats, we often forget the positive models of human-technology interaction, such as the protective robot in Lost in Space. While AI is not yet as advanced as what has already been depicted on the screen, it is inextricably interwoven into the daily fabric of our lives. Almost any website you go to for business or pleasure has a chatbot waiting to help (or frustrate) you. Most of us have Alexa, Siri, or another digital assistant organizing our homes and schedules. When I Google “everyday uses of artificial intelligence,” it is AI that responds with an overview.
Medicine is not immune. Renowned physician and scientist Eric Topol, MD, suggests that AI represents a “fourth industrial revolution in medicine” that can dramatically improve health care.3 The US Department of Veterans Affairs (VA) has been at the forefront of this new space.4 The story recounted below encapsulates the enormous benefits AI can bring to health care and the vigilance we must exercise to anticipate and mitigate risk for this to be an overall positive transition.
The story begins with a key element of AI change—the machine learning predictive algorithm. In this case, the algorithm was designed to predict—and thereby prevent—the top public health priority in federal practice: suicide. The Recovery Engagement and Coordination for Health-Veterans Enhanced Treatment (REACH VET) program was launched in 2017 to assist in identifying the top 0.1% of veterans at the highest risk for suicide.5
At least at this stage of AI in medicine, the safest and most ethical efforts come from collaborations between health care professionals and AI developers that maximize the very different strengths of each partner. REACH VET is an exemplar of this kind of teamwork. Once the algorithm analyzes > 60 variables to identify veterans at high risk for suicide, data are communicated to a REACH VET program coordinator, who then notifies the practitioner responsible for the veteran’s care so they can put into action evidence-based suicide prevention strategies.5
VA researchers in 2021 published a study of 173,313 veterans comparing outcomes before and after entry into the program using a triple differences design. Veterans participating in the program reported an increase in outpatient visits and documentation of safety plans, and a decrease in emergency department visits, inpatient mental health admissions, and recorded suicide attempts.6
A US Government Accounting Office analysis found that “REACH VET had identified veterans who had not been identified through other methods.”7 This was not just an example of AI hype: as a relatively rare and statistically complicated phenomenon, suicide is notoriously difficult to predict and model. Machine learning algorithms like REACH VET have unprecedented potential to assist and augment suicide prevention.8
In 2023, veteran service organizations and journalists raised concerns that the AI algorithm was biased and ignored critical risk factors that put some veterans at increased risk. Based on their analysis, they claimed that the algorithm did not account for risk factors uniquely associated with women veterans, namely military sexual trauma and intimate partner violence.9 Women are the most rapidly growing VA population, yet too often they encounter health care disparities, harassment, and stigmatization when seeking care. The Congressional Veterans Affairs committees investigated and introduced legislation to update the algorithm.10
VA experts dispute these claims, and a computer science PhD may be required to understand the debate. But as the history of medicine has shown us, every treatment and procedure has benefits and risks. No matter how bright and shiny the technology initially appears, a soft scientific underbelly emerges sooner or later. Just as with REACH VET, algorithm bias is often discovered during deployment when the logic of the laboratory encounters the unpredictable variety of humankind.11 Frequently, those problems are—as with REACH VET— not solely or even primarily technical ones. The data mirror society and reflect its biases.
For learning organizations like the VA and the US Department of Defense (DoD), the criticisms of REACH VET signal the need to engage in continuous performance improvement. AI requires the human trainers and supervisors who teach the machines to continuously revise and update their lesson plans. The most recent VA data show that in 2021, 6392 veterans died by suicide.12 In Congressional testimony, VA leaders reported that as of May 2024, REACH VET was operating in 28 VA facilities and had identified 6700 high-risk veterans.13 REACH VET can save veteran’s lives, which is the sine qua non for our federal health care systems.
The algorithm should be improved to identify ALL veterans so they receive lifesaving interventions. Every veteran’s life is sacred; the algorithm that may prevent suicide must be continuously improved. That is why our representatives did not propose to ban REACH VET or enforce an AI winter on the VA and DoD. Instead, they called for an update to the algorithm, underscoring the value of machine learning for suicide prediction and prevention.
The epigraph from one of the top AI ethicists and scientists in the world makes the point that AI is not the moral agent here: it is fallible humans who must keep learning along with machines. That is why, at the end of 2024, VA experts are revising the algorithm so REACH VET can help prevent even more veteran suicides in 2025 and beyond.14
We have a tradition at Federal Practitioner where the December editorial usually features some version of the “best and worst” of the last 12 months in government health care. As we close out a difficult year, instead I offer a cautionary yet promising story that epitomizes both risk and benefit.
In some quarters, 2024 has been the year of AI (artificial intelligence).2 While in science fiction, superhuman machines, like the Terminator, are often associated with apocalyptic threats, we often forget the positive models of human-technology interaction, such as the protective robot in Lost in Space. While AI is not yet as advanced as what has already been depicted on the screen, it is inextricably interwoven into the daily fabric of our lives. Almost any website you go to for business or pleasure has a chatbot waiting to help (or frustrate) you. Most of us have Alexa, Siri, or another digital assistant organizing our homes and schedules. When I Google “everyday uses of artificial intelligence,” it is AI that responds with an overview.
Medicine is not immune. Renowned physician and scientist Eric Topol, MD, suggests that AI represents a “fourth industrial revolution in medicine” that can dramatically improve health care.3 The US Department of Veterans Affairs (VA) has been at the forefront of this new space.4 The story recounted below encapsulates the enormous benefits AI can bring to health care and the vigilance we must exercise to anticipate and mitigate risk for this to be an overall positive transition.
The story begins with a key element of AI change—the machine learning predictive algorithm. In this case, the algorithm was designed to predict—and thereby prevent—the top public health priority in federal practice: suicide. The Recovery Engagement and Coordination for Health-Veterans Enhanced Treatment (REACH VET) program was launched in 2017 to assist in identifying the top 0.1% of veterans at the highest risk for suicide.5
At least at this stage of AI in medicine, the safest and most ethical efforts come from collaborations between health care professionals and AI developers that maximize the very different strengths of each partner. REACH VET is an exemplar of this kind of teamwork. Once the algorithm analyzes > 60 variables to identify veterans at high risk for suicide, data are communicated to a REACH VET program coordinator, who then notifies the practitioner responsible for the veteran’s care so they can put into action evidence-based suicide prevention strategies.5
VA researchers in 2021 published a study of 173,313 veterans comparing outcomes before and after entry into the program using a triple differences design. Veterans participating in the program reported an increase in outpatient visits and documentation of safety plans, and a decrease in emergency department visits, inpatient mental health admissions, and recorded suicide attempts.6
A US Government Accounting Office analysis found that “REACH VET had identified veterans who had not been identified through other methods.”7 This was not just an example of AI hype: as a relatively rare and statistically complicated phenomenon, suicide is notoriously difficult to predict and model. Machine learning algorithms like REACH VET have unprecedented potential to assist and augment suicide prevention.8
In 2023, veteran service organizations and journalists raised concerns that the AI algorithm was biased and ignored critical risk factors that put some veterans at increased risk. Based on their analysis, they claimed that the algorithm did not account for risk factors uniquely associated with women veterans, namely military sexual trauma and intimate partner violence.9 Women are the most rapidly growing VA population, yet too often they encounter health care disparities, harassment, and stigmatization when seeking care. The Congressional Veterans Affairs committees investigated and introduced legislation to update the algorithm.10
VA experts dispute these claims, and a computer science PhD may be required to understand the debate. But as the history of medicine has shown us, every treatment and procedure has benefits and risks. No matter how bright and shiny the technology initially appears, a soft scientific underbelly emerges sooner or later. Just as with REACH VET, algorithm bias is often discovered during deployment when the logic of the laboratory encounters the unpredictable variety of humankind.11 Frequently, those problems are—as with REACH VET— not solely or even primarily technical ones. The data mirror society and reflect its biases.
For learning organizations like the VA and the US Department of Defense (DoD), the criticisms of REACH VET signal the need to engage in continuous performance improvement. AI requires the human trainers and supervisors who teach the machines to continuously revise and update their lesson plans. The most recent VA data show that in 2021, 6392 veterans died by suicide.12 In Congressional testimony, VA leaders reported that as of May 2024, REACH VET was operating in 28 VA facilities and had identified 6700 high-risk veterans.13 REACH VET can save veteran’s lives, which is the sine qua non for our federal health care systems.
The algorithm should be improved to identify ALL veterans so they receive lifesaving interventions. Every veteran’s life is sacred; the algorithm that may prevent suicide must be continuously improved. That is why our representatives did not propose to ban REACH VET or enforce an AI winter on the VA and DoD. Instead, they called for an update to the algorithm, underscoring the value of machine learning for suicide prediction and prevention.
The epigraph from one of the top AI ethicists and scientists in the world makes the point that AI is not the moral agent here: it is fallible humans who must keep learning along with machines. That is why, at the end of 2024, VA experts are revising the algorithm so REACH VET can help prevent even more veteran suicides in 2025 and beyond.14
- Waikar S. Health care’s AI future: a conversation with Fei Fei Li and Andrew Ng. HAI Stanford University. May 10, 2021. Accessed November 13, 2024. https://hai.stanford.edu/news/health-cares-ai-future-conversation-fei-fei-li-and-andrew-ng
- Johnson E, Forbes Technology Council. 2023 Was the Year of AI Hype—2024 is the Year of AI Practicality. Forbes. April 2, 2024. Accessed November 13, 2024. https://www.forbes.com/councils/forbestechcouncil/2024/04/02/2023-was-the-year-of-ai-hype-2024-is-the-year-of-ai-practicality/
- Topol E. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again. Basic Books; 2019.
- Perlis R. The VA was an early adopter of artificial intelligence to improve care-here’s what they learned. JAMA. 2024;332(17):1411-1414. doi:10.1001/jama.2024.20563
- VA REACH VET initiative helps save lives [press release]. April 3, 2017. Accessed November 13, 2024. https://news.va.gov/36714/va-reach-vet-initiative-helps-save-veterans-lives/
- McCarthy JF, Cooper SA, Dent KR, et al. Evaluation of the recovery engagement and coordination for health-veterans enhanced treatment suicide risk modeling clinical program in the Veterans Health Administration. JAMA Netw Open. 2021;4(10):e2129900. doi:10.1001/jamanetworkopen.2021.29900
- US Government Office of Accountability. Veteran suicide: VA efforts to identify veterans at risk through analysis of health record information. September 14, 2022. Accessed November 13, 2024. https://www.gao.gov/products/gao-22-105165
- Pigoni A, Delvecchio G, Turtulici N, et al. Machine learning and the prediction of suicide in psychiatric populations: a systematic review. Transl Psychiatry. 2024;14(1):140. doi:10.1038/s41398-024-02852-9
- Glantz A. VA veteran suicide prevention algorithm favors men. Military.com. May 23, 2024. Accessed November 13, 2024. https://www.military.com/daily-news/2024/05/23/vas-veteran-suicide-prevention-algorithm-favors-men.html
- S.5210 BRAVE Act of 2024. 118th Congress. https://www.congress.gov/bill/118th-congress/senate-bill/5210/text
- Ratwani RM, Sutton K, and Galarrga JE. Addressing algorithmic bias in health care. JAMA. 2024;332(13):1051-1052. doi:10.1001/jama.2024.1348/
- US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2023 national veteran suicide prevention annual report. November 2023 Accessed November 13, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- House Committee on Veterans Affairs. Health Chairwoman Miller-Meeks opens Iowa field hearing on breakthroughs in VA healthcare. May 13, 2024. Accessed November 13, 2024. https://veterans.house.gov/news/documentsingle.aspx?DocumentID=6452
- Graham E. VA is updating its AI suicide risk model to reach more women. NEXTGOV/FCW. October 18, 2024. Accessed November 13, 2024. https://www.nextgov.com/artificial-intelligence/2024/10/va-updating-its-ai-suicide-risk-model-reach-more-women/400377/
- Waikar S. Health care’s AI future: a conversation with Fei Fei Li and Andrew Ng. HAI Stanford University. May 10, 2021. Accessed November 13, 2024. https://hai.stanford.edu/news/health-cares-ai-future-conversation-fei-fei-li-and-andrew-ng
- Johnson E, Forbes Technology Council. 2023 Was the Year of AI Hype—2024 is the Year of AI Practicality. Forbes. April 2, 2024. Accessed November 13, 2024. https://www.forbes.com/councils/forbestechcouncil/2024/04/02/2023-was-the-year-of-ai-hype-2024-is-the-year-of-ai-practicality/
- Topol E. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again. Basic Books; 2019.
- Perlis R. The VA was an early adopter of artificial intelligence to improve care-here’s what they learned. JAMA. 2024;332(17):1411-1414. doi:10.1001/jama.2024.20563
- VA REACH VET initiative helps save lives [press release]. April 3, 2017. Accessed November 13, 2024. https://news.va.gov/36714/va-reach-vet-initiative-helps-save-veterans-lives/
- McCarthy JF, Cooper SA, Dent KR, et al. Evaluation of the recovery engagement and coordination for health-veterans enhanced treatment suicide risk modeling clinical program in the Veterans Health Administration. JAMA Netw Open. 2021;4(10):e2129900. doi:10.1001/jamanetworkopen.2021.29900
- US Government Office of Accountability. Veteran suicide: VA efforts to identify veterans at risk through analysis of health record information. September 14, 2022. Accessed November 13, 2024. https://www.gao.gov/products/gao-22-105165
- Pigoni A, Delvecchio G, Turtulici N, et al. Machine learning and the prediction of suicide in psychiatric populations: a systematic review. Transl Psychiatry. 2024;14(1):140. doi:10.1038/s41398-024-02852-9
- Glantz A. VA veteran suicide prevention algorithm favors men. Military.com. May 23, 2024. Accessed November 13, 2024. https://www.military.com/daily-news/2024/05/23/vas-veteran-suicide-prevention-algorithm-favors-men.html
- S.5210 BRAVE Act of 2024. 118th Congress. https://www.congress.gov/bill/118th-congress/senate-bill/5210/text
- Ratwani RM, Sutton K, and Galarrga JE. Addressing algorithmic bias in health care. JAMA. 2024;332(13):1051-1052. doi:10.1001/jama.2024.1348/
- US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. 2023 national veteran suicide prevention annual report. November 2023 Accessed November 13, 2024. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- House Committee on Veterans Affairs. Health Chairwoman Miller-Meeks opens Iowa field hearing on breakthroughs in VA healthcare. May 13, 2024. Accessed November 13, 2024. https://veterans.house.gov/news/documentsingle.aspx?DocumentID=6452
- Graham E. VA is updating its AI suicide risk model to reach more women. NEXTGOV/FCW. October 18, 2024. Accessed November 13, 2024. https://www.nextgov.com/artificial-intelligence/2024/10/va-updating-its-ai-suicide-risk-model-reach-more-women/400377/
The Year of AI: Learning With Machines to Improve Veteran Health Care
The Year of AI: Learning With Machines to Improve Veteran Health Care
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10
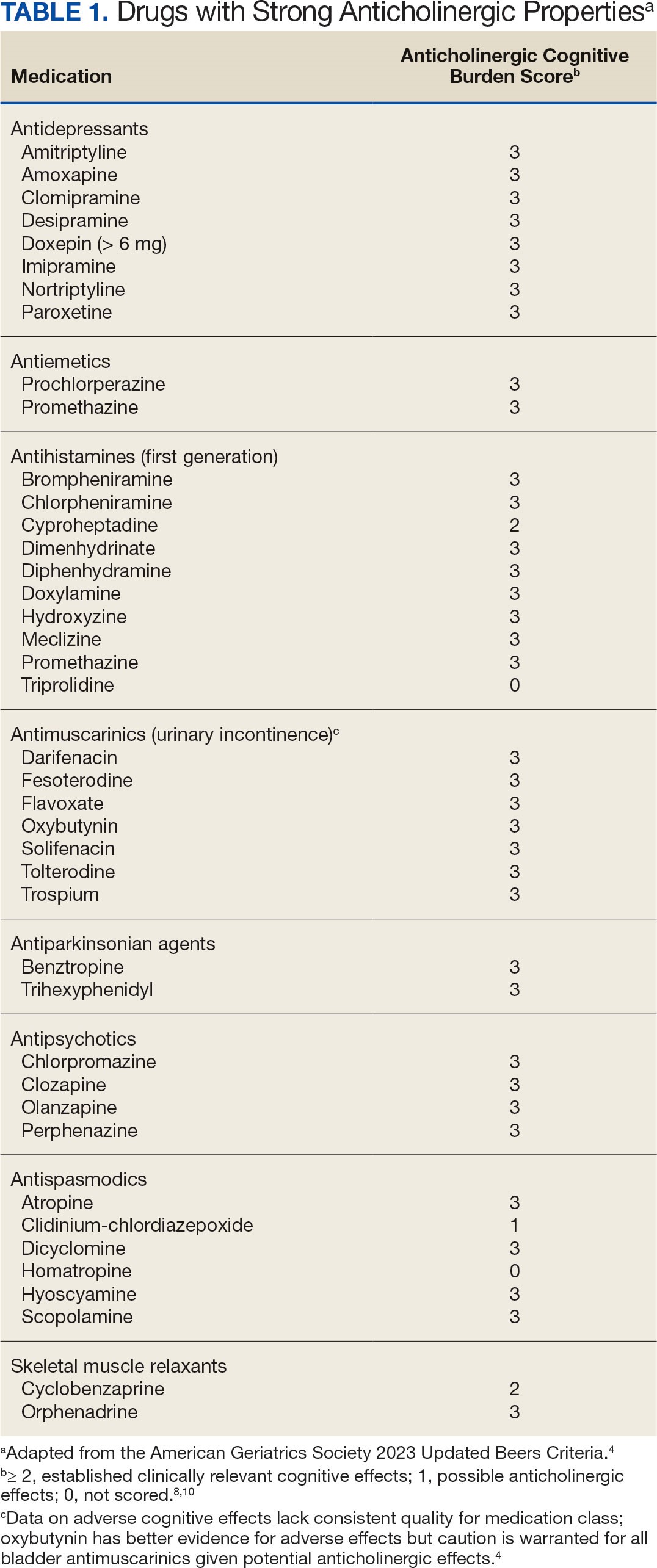
Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.
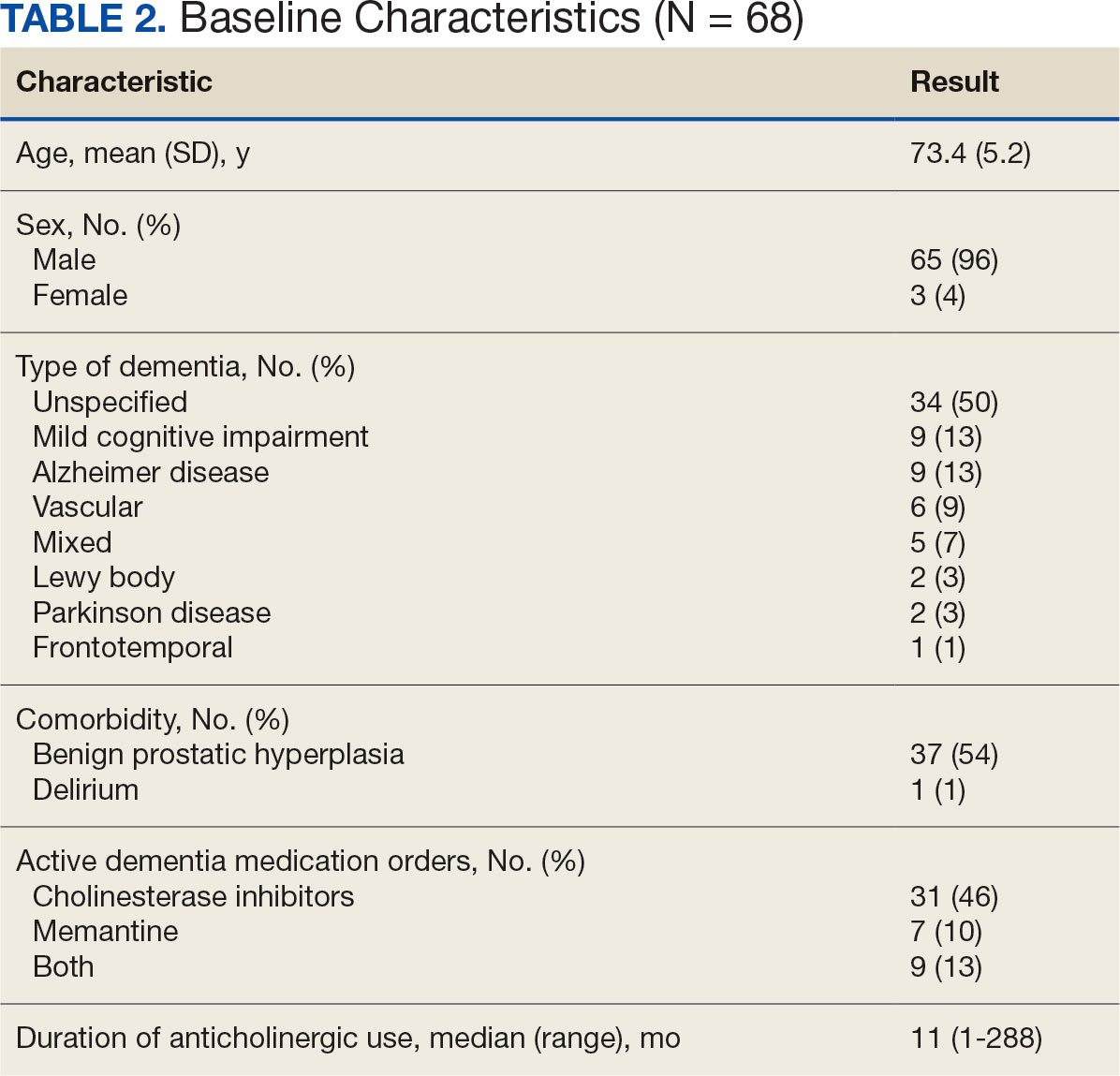
Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.
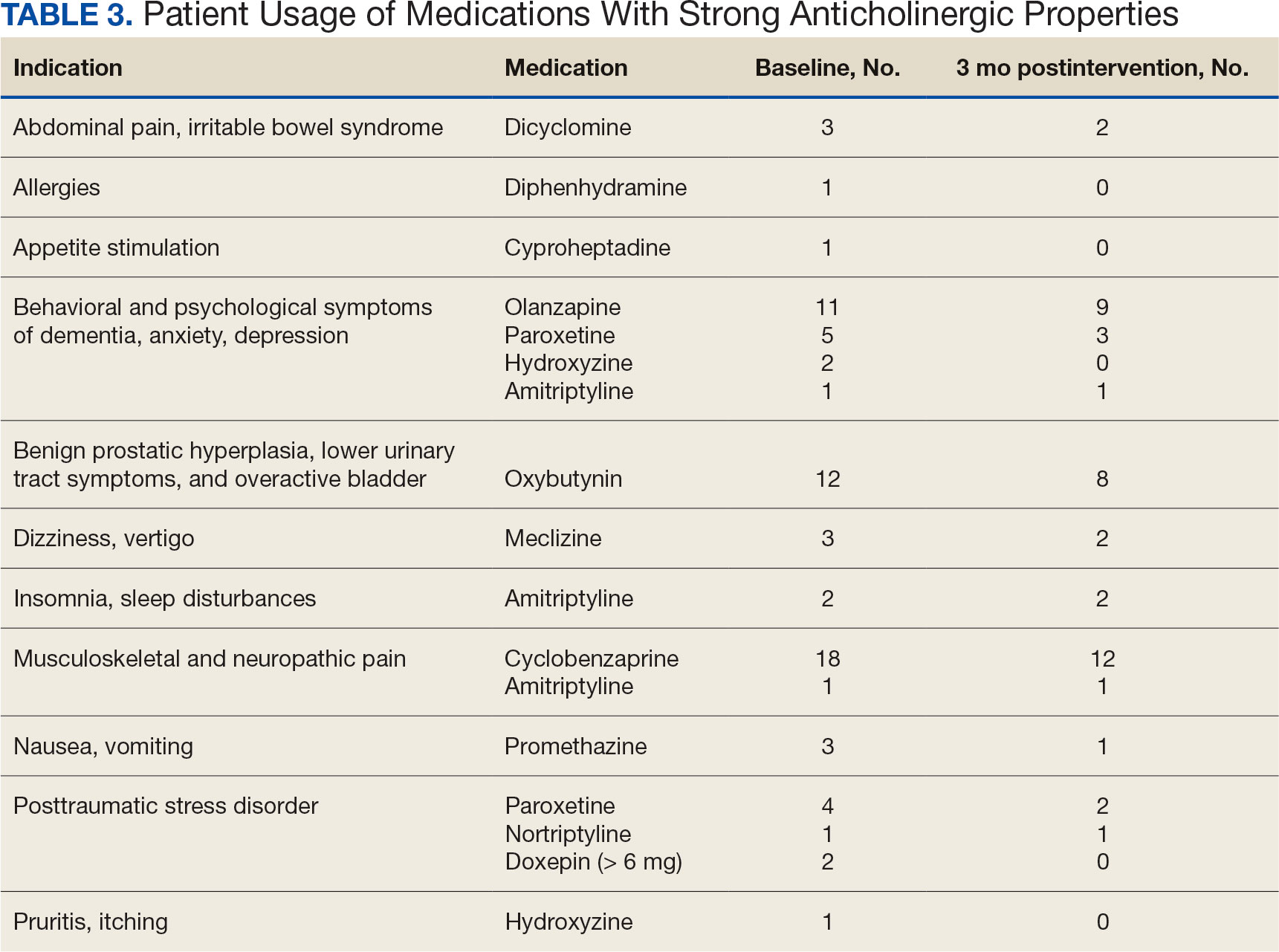
Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10

Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.

Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.

Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
Anticholinergic medications block the activity of the neurotransmitter acetylcholine by binding to either muscarinic or nicotinic receptors in both the peripheral and central nervous system. Anticholinergic medications typically refer to antimuscarinic medications and have been prescribed to treat a variety of conditions common in older adults, including overactive bladder, allergies, muscle spasms, and sleep disorders.1,2 Since muscarinic receptors are present throughout the body, anticholinergic medications are associated with many adverse effects (AEs), including constipation, urinary retention, xerostomia, and delirium. Older adults are more sensitive to these AEs due to physiological changes associated with aging.1
The American Geriatric Society Beers Criteria for Potentially Inappropriate Medications Use in Older Adults identifies drugs with strong anticholinergic properties. The Beers Criteria strongly recommends avoiding these medications in patients with dementia or cognitive impairment due to the risk of central nervous system AEs. In the updated 2023 Beers Criteria, the rationale was expanded to recognize the risks of the cumulative anticholinergic burden associated with concurrent anticholinergic use.3,4
Given the prevalent use of anticholinergic medications in older adults, there has been significant research demonstrating their AEs, specifically delirium and cognitive impairment in geriatric patients. A systematic review of 14 articles conducted in 7 different countries of patients with median age of 76.4 to 86.1 years reviewed clinical outcomes of anticholinergic use in patients with dementia. Five studies found anticholinergics were associated with increased all-cause mortality in patients with dementia, and 3 studies found anticholinergics were associated with longer hospital stays. Other studies found that anticholinergics were associated with delirium and reduced health-related quality of life.5
About 35% of veterans with dementia have been prescribed a medication regimen with a high anticholinergic burden.6 In 2018, the US Department of Veterans Affairs (VA) Pharmacy Benfits Management Center for Medical Safety completed a centrally aggregated medication use evaluation (CAMUE) to assess the appropriateness of anticholinergic medication use in patients with dementia. The retrospective chart review included 1094 veterans from 19 sites. Overall, about 15% of the veterans experienced new falls, delirium, or worsening dementia within 30 days of starting an anticholinergic medication. Furthermore, < 40% had documentation of a nonanticholinergic alternative medication trial, and < 20% had documented nonpharmacologic therapy. The documentation of risk-benefit assessment acknowledging the risks of anticholinergic medication use in veterans with dementia occurred only about 13% of the time. The CAMUE concluded that the risks of initiating an anticholinergic medication in veterans with dementia are likely underdocumented and possibly under considered by prescribers.7
Developed within the Veterans Health Administration (VHA), VIONE (Vital, Important, Optional, Not Indicated, Every medication has an indication) is a medication management methodology that aims to reduce polypharmacy and improve patient safety consistent with high-reliability organizations. Since it launched in 2016, VIONE has gradually been implemented at many VHA facilities. The VIONE deprescribing dashboard had not been used at the VA Louisville Healthcare System prior to this quality improvement project.
This dashboard uses the Beers Criteria to identify potentially inappropriate anticholinergic medications. It uses the Anticholinergic Cognitive Burden (ACB) scale to calculate the cumulative anticholinergic risk for each patient. Medications with an ACB score of 2 or 3 have clinically relevant cognitive effects such as delirium and dementia (Table 1). For each point increase in total ACB score, a decline in mini-mental state examination score of 0.33 points over 2 years has been shown. Each point increase has also been correlated with a 26% increase in risk of death.8-10

Methods
The purpose of this quality improvement project was to determine the impact of pharmacist-driven deprescribing on the anticholinergic burden in veterans with dementia at VA Louisville Healthcare System. Data were obtained through the Computerized Patient Record System (CPRS) and VIONE deprescribing dashboard and entered in a secure Microsoft Excel spreadsheet. Pharmacist deprescribing steps were entered as CPRS progress notes. A deprescribing note template was created, and 11 templates with indication-specific recommendations were created for each anticholinergic indication identified (contact authors for deprescribing note template examples). Usage of anticholinergic medications was reexamined 3 months after the deprescribing note was entered.
Eligible patients identified in the VIONE deprescribing dashboard had an outpatient order for a medication with strong anticholinergic properties as identified using the Beers Criteria and were aged ≥ 65 years. Patients also had to be diagnosed with dementia or cognitive impairment. Patients were excluded if they were receiving hospice care or if the anticholinergic medication was from a non-VA prescriber or filled at a non-VA pharmacy. The VIONE deprescribing dashboard also excluded skeletal muscle relaxants if the patient had a spinal cord-related visit in the previous 2 years, first-generation antihistamines if the patient had a vertigo diagnosis, hydroxyzine if the indication was for anxiety, trospium if the indication was for overactive bladder, and antipsychotics if the patient had been diagnosed with schizophrenia or bipolar disorder. The following were included in the deprescribing recommendations if the dashboard identified the patient due to receiving a second strongly anticholinergic medication: first generation antihistamines if the patient was diagnosed with vertigo and hydroxyzine if the indication is for anxiety.
Each eligible patient received a focused medication review by a pharmacist via electronic chart review and a templated CPRS progress note with patient-specific recommendations. The prescriber and the patient’s primary care practitioner were recommended to perform a patient-specific risk-benefit assessment, deprescribe potentially inappropriate anticholinergic medications, and consider nonanticholinergic alternatives (both pharmacologic and nonpharmacologic). Data collected included baseline age, sex, prespecified comorbidities (type of dementia, cognitive impairment, delirium, benign prostatic hyperplasia/lower urinary tract symptoms), duration of prescribed anticholinergic medication, indication and deprescribing rate for each anticholinergic agent, and concurrent dementia medications (acetylcholinesterase inhibitors, memantine, or both).
The primary outcome was the number of patients that had = 1 medication with strong anticholinergic properties deprescribed. Deprescribing was defined as medication discontinuation or reduction of total daily dose. Secondary outcomes were the mean change in ACB scale, the number of patients with dose tapering, documented patient-specific risk-benefit assessment, and initiated nonanticholinergic alternative per pharmacist recommendation.
Results
The VIONE deprescribing dashboard identified 121 patients; 45 were excluded for non-VA prescriber or pharmacy, and 8 patients were excluded for other reasons. Sixty-eight patients were included in the deprescribing initiative. The mean age was 73.4 years (range, 67-93), 65 (96%) were male, and 34 (50%) had unspecified dementia (Table 2). Thirty-one patients (46%) had concurrent cholinesterase inhibitor prescriptions for dementia. The median duration of use of a strong anticholinergic medication was 11 months.

Twenty-nine patients (43%) had ≥ 1 medication with strong anticholinergic properties deprescribed. Anticholinergic medication was discontinued for 26 patients, and the dose was decreased for 3 patients. ACB score fell by a mean of 1.1 per patient. There was an increase in the documented risk-benefit assessment for anticholinergic medications from a baseline of 4 (6%) to 19 (28%) 3 months after the deprescribing note. Cyclobenzaprine, paroxetine, and oxybutynin were deprescribed the most, and amitriptyline had the lowest rate of deprescribing (Table 3). Thirty patients (44%) had a pharmacologic, nonanticholinergic alternative initiated per pharmacist recommendation, and 6 patients (9%) had a nonpharmacologic alternative initiated per pharmacist recommendation.

Discussion
This quality improvement project suggests that with the use of population health management tools such as the VIONE deprescribing dashboard, pharmacists can help identify and deprescribe strong anticholinergic medications in patients with cognitive impairment or dementia. Pharmacists can also aid in deprescribing through evidence-based recommendations to guide risk-benefit discussion and consider safer, nonanticholinergic alternatives. The authors were able to help reduce anticholinergic cognitive burden in 43% of patients in this sample. The mean 1.1 ACB score reduction was considered clinically significant based on prior studies that found that each 1-point increase in ACB score correlated with declined cognition and increased mortality.8,10 The VIONE deprescribing dashboard provided real-time patient data and helped target patients at the highest risk of anticholinergic AEs. The creation of the note templates based on the indication helped streamline recommendations. Typically, the prescriber addressed the recommendations at a routine follow-up appointment. The deprescribing method used in this project was time-efficient and could be easily replicated once the CPRS note templates were created. Future deprescribing projects could consider more direct pharmacist intervention and medication management.
Limitations
There was no direct assessment of clinical outcomes such as change in cognition using cognitive function tests. However, multiple studies have demonstrated AEs associated with strong anticholinergic medication use and additive anticholinergic burden in patients with dementia or cognitive impairment.1,5 Also, the 3-month follow-up period was relatively short. The pharmacist’s deprescribing recommendations may have been accepted after 3 months, or patients could have restarted their anticholinergic medications. Longer follow-up time could provide more robust results and conclusions. Thirdly, there was no formal definition of what constituted a risk-benefit assessment of anticholinergic medications. The risk-benefit assessment was determined at the discretion of the authors, which was subjective and allowed for bias. Finally, 6 patients died during the 3-month follow-up. The data for these patients were included in the baseline characteristics but not in the study outcomes. If these patients had been excluded from the results, a higher percentage of patients (47%) would have had ≥ 1 anticholinergic medication deprescribed.
Conclusions
In collaboration with the interdisciplinary team, pharmacist recommendations resulted in deprescribing of anticholinergic medications in veterans with dementia or cognitive impairment. The VIONE deprescribing dashboard, an easily accessible population health management tool, can identify patients prescribed potentially inappropriate medications and help target patients at the highest risk of anticholinergic AEs. To prevent worsening cognitive impairment, delirium, falls, and other AEs, this deprescribing initiative can be replicated at other VHA facilities. Future projects could have a longer follow-up period, incorporate more direct pharmacist intervention, and assess clinical outcomes of deprescribing.
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
- Gray SL, Hanlon JT. Anticholinergic medication use and dementia: latest evidence and clinical implications. Ther Adv Drug Saf. 2016;7(5):217-224. doi:10.1177/2042098616658399
- Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain - how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151-159. doi:10.1111/bcpt.12140
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
- By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults J Am Geriatr Soc. 2023;71(7):2052-2081. doi:10.1111/jgs.18372
- Wang K, Alan J, Page AT, Dimopoulos E, Etherton-Beer C. Anticholinergics and clinical outcomes amongst people with pre-existing dementia: a systematic review. Maturitas. 2021;151:1-14. doi:10.1016/j.maturitas.2021.06.004
- Thorpe JM, Thorpe CT, Gellad WF, et al. Dual health care system use and high-risk prescribing in patients with dementia: a national cohort study. Ann Intern Med. 2017;166(3):157-163. doi:10.7326/M16-0551
- McCarren M, Burk M, Carico R, Glassman P, Good CB, Cunningham F. Design of a centrally aggregated medication use evaluation (CAMUE): anticholinergics in dementia. Presented at: 2019 HSR&D/QUERI National Conference; October 29-31, 2019; Washington, DC. https://www.hsrd.research.va.gov/meetings/2019/abstract-display.cfm?AbsNum=4027
- Boustani, M, Campbell, N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311-320. doi:10.2217/1745509.x
- Constantino-Corpuz JK, Alonso MTD. Assessment of a medication deprescribing tool on polypharmacy and cost avoidance. Fed Pract. 2021;38(7):332-336. doi:10.12788/fp.0146
- Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477-1483. doi:10.1111/j.1532-5415.2011.03491.x
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Pharmacist-Driven Deprescribing to Reduce Anticholinergic Burden in Veterans With Dementia
Commentary: Health-Related Consequences of Migraine, December 2024
It is known that there are health-related consequences of migraine, as well as migraine-related comorbidities. Three recent studies examined the relationship between migraine and stroke, with nuanced results, suggesting that migraine does not necessarily increase stroke risk for all populations and may even be associated with a decreased risk for stroke for some patients. But it is clear that migraine is associated with an increased stroke risk for some specific populations, including during pregnancy.
Migraine is also known to have a negative impact on quality of life, affecting many different areas of well-being, including relationships, work productivity, and emotional health. Results from a recent study published in Cephalgia provided evidence that migraine can also increase the risk for occupational burnout.1
Yet, there’s some good news for migraine patients who have a genetic predisposition for migraine. Results of a recent study published in the Journal of Clinical Medicine showed that hereditary predisposition to migraine does not necessarily correlate with development of chronic migraine.2
An observational study, with results published in Cephalgia in November 2024, included 646 patients aged 18-54 years who were hospitalized with their first stroke.3 It showed no significant association between cerebral small-vessel disease and migraine with aura among the study population. Interestingly, migraine with aura is generally more closely linked with stroke risk than migraine without aura, so the results do not align with previously held beliefs about migraine and stroke risk.4
A larger study examined the relationship between migraine and cardiovascular risk scores.4 This cohort study included 140,915 Dutch adults with a mean age of 44 years. Results, published in JAMA Network Open in October 2024, revealed that the odds of having prevalent or incident migraine decreased with increasing cardiovascular risk score categories, especially for women. The authors suggested that having migraine could be associated with a healthier cardiovascular system and suggested several potential mechanisms for this inverse relationship, including alterations in the activity of calcitonin gene–related peptide activity, changes in nitric oxide effects, or cortical spreading depression in response to atherosclerosis.
Although the results of these studies, which were focused on young patients, are interesting and could provide a sense of relief for patients with migraine, the authors of the JAMA Network Open article acknowledged that these results should not be extrapolated to other populations.4 Specifically, they noted that it has been established in other studies that older patients with migraine have an increased cardiovascular risk.
The relationship between migraine and stroke risk is important for pregnant women. Results of a large analysis including 19,825,525 pregnant patients, with data obtained from 2016 to 2020, were published in November 2024 in the Journal of Women’s Health.5 The analysis revealed that a history of migraine substantially increases the risk for hemorrhagic or ischemic stroke during pregnancy. They reported that “acute ischemic stroke was most strongly associated with migraine with aura (odds ratio [OR], 23.26; 95% confidence interval [CI], 18.46-29.31), followed by migraine without aura (OR, 8.15; 95% CI, 4.79-13.88).” The authors advised that stroke risk should be addressed in pregnant women who have migraine or who have a migraine history, especially if they have migraine with aura.
It is well known that migraine risk has a hereditary component, but hereditary factors might not play a role in the time of onset of migraines. In a retrospective clinical genetic case-control study that included over 15,000 participants, researchers identified migraine polygenic risk scores using genome-wide association studies.2 The results were published in October 2024 in the Journal of Clinical Medicine. The study authors noted “a higher genetic risk was associated with earlier onset and increased risk for migraine well into adulthood, but not with chronification.” These results support the benefits of a diligent pursuit of effective migraine treatment, even for patients who might feel hopeless about achieving migraine control due to their own family history of migraine. As migraine therapies have evolved over the past decades, patients who had parents or other older family members with migraine may have a pessimistic outlook on the potential for effective treatment. However, newer therapies are far more effective than migraine treatments of the past, and patients should be informed and given encouragement that they can have a better prognosis and better migraine control than past generations.
The value of effective treatment cannot be underestimated. A study, with results published in Cephalgia in October 2024, included data from a subset of participants from the Negev Migraine Cohort, including 675 migraine patients and 232 control participants without migraine.1 The authors reported that migraine patients reported “significantly higher levels of occupational burnout, with a mean burnout score of 3.46 vs a mean of 2.82 among controls.” They also noted that migraine patients worked longer hours, with 40 hours of work weekly vs 36 for controls. The authors suggested accommodations for migraine patients, such as working from home or flexible scheduling. Although this could be beneficial, achieving migraine relief would be even better for patients, who could eventually be able to enjoy having a 36-hour work week rather than a 40-hour work week. Admittedly, this potential outcome is an overly literal interpretation of the research results, but it emphasizes the potential value of having “more time” in patients’ lives as a result of effective migraine relief.
References
1. Peles I, Sharvit S, Zlotnik Y, et al. Migraine and work — beyond absenteeism: Migraine severity and occupational burnout — a cohort study. Cephalalgia. October 18, 2024. Source
2. Chase BA, Frigerio R, Rubin S, et al. An integrative migraine polygenic risk score is associated with age at onset but not with chronification. J Clin Med. October 29, 2024. Source
3. Cloet F, Gueyraud G, Lerebours F, Munio M, Larrue V, Gollion C. Stroke due to small-vessel disease and migraine: a case-control study of a young adult with ischemic stroke population. Cephalalgia. 2024;44:1-8. Source
4. Al-Hassany L, MaassenVanDenBrink A, Kurth T. Cardiovascular risk scores and migraine status. JAMA Netw Open. October 22, 2024. Source
5. Reddy M, Vazquez S, Nolan B, et al. Migraine and its association with stroke in pregnancy: A national examination. J Womens Health. 2024;33:1476-1481. Source
It is known that there are health-related consequences of migraine, as well as migraine-related comorbidities. Three recent studies examined the relationship between migraine and stroke, with nuanced results, suggesting that migraine does not necessarily increase stroke risk for all populations and may even be associated with a decreased risk for stroke for some patients. But it is clear that migraine is associated with an increased stroke risk for some specific populations, including during pregnancy.
Migraine is also known to have a negative impact on quality of life, affecting many different areas of well-being, including relationships, work productivity, and emotional health. Results from a recent study published in Cephalgia provided evidence that migraine can also increase the risk for occupational burnout.1
Yet, there’s some good news for migraine patients who have a genetic predisposition for migraine. Results of a recent study published in the Journal of Clinical Medicine showed that hereditary predisposition to migraine does not necessarily correlate with development of chronic migraine.2
An observational study, with results published in Cephalgia in November 2024, included 646 patients aged 18-54 years who were hospitalized with their first stroke.3 It showed no significant association between cerebral small-vessel disease and migraine with aura among the study population. Interestingly, migraine with aura is generally more closely linked with stroke risk than migraine without aura, so the results do not align with previously held beliefs about migraine and stroke risk.4
A larger study examined the relationship between migraine and cardiovascular risk scores.4 This cohort study included 140,915 Dutch adults with a mean age of 44 years. Results, published in JAMA Network Open in October 2024, revealed that the odds of having prevalent or incident migraine decreased with increasing cardiovascular risk score categories, especially for women. The authors suggested that having migraine could be associated with a healthier cardiovascular system and suggested several potential mechanisms for this inverse relationship, including alterations in the activity of calcitonin gene–related peptide activity, changes in nitric oxide effects, or cortical spreading depression in response to atherosclerosis.
Although the results of these studies, which were focused on young patients, are interesting and could provide a sense of relief for patients with migraine, the authors of the JAMA Network Open article acknowledged that these results should not be extrapolated to other populations.4 Specifically, they noted that it has been established in other studies that older patients with migraine have an increased cardiovascular risk.
The relationship between migraine and stroke risk is important for pregnant women. Results of a large analysis including 19,825,525 pregnant patients, with data obtained from 2016 to 2020, were published in November 2024 in the Journal of Women’s Health.5 The analysis revealed that a history of migraine substantially increases the risk for hemorrhagic or ischemic stroke during pregnancy. They reported that “acute ischemic stroke was most strongly associated with migraine with aura (odds ratio [OR], 23.26; 95% confidence interval [CI], 18.46-29.31), followed by migraine without aura (OR, 8.15; 95% CI, 4.79-13.88).” The authors advised that stroke risk should be addressed in pregnant women who have migraine or who have a migraine history, especially if they have migraine with aura.
It is well known that migraine risk has a hereditary component, but hereditary factors might not play a role in the time of onset of migraines. In a retrospective clinical genetic case-control study that included over 15,000 participants, researchers identified migraine polygenic risk scores using genome-wide association studies.2 The results were published in October 2024 in the Journal of Clinical Medicine. The study authors noted “a higher genetic risk was associated with earlier onset and increased risk for migraine well into adulthood, but not with chronification.” These results support the benefits of a diligent pursuit of effective migraine treatment, even for patients who might feel hopeless about achieving migraine control due to their own family history of migraine. As migraine therapies have evolved over the past decades, patients who had parents or other older family members with migraine may have a pessimistic outlook on the potential for effective treatment. However, newer therapies are far more effective than migraine treatments of the past, and patients should be informed and given encouragement that they can have a better prognosis and better migraine control than past generations.
The value of effective treatment cannot be underestimated. A study, with results published in Cephalgia in October 2024, included data from a subset of participants from the Negev Migraine Cohort, including 675 migraine patients and 232 control participants without migraine.1 The authors reported that migraine patients reported “significantly higher levels of occupational burnout, with a mean burnout score of 3.46 vs a mean of 2.82 among controls.” They also noted that migraine patients worked longer hours, with 40 hours of work weekly vs 36 for controls. The authors suggested accommodations for migraine patients, such as working from home or flexible scheduling. Although this could be beneficial, achieving migraine relief would be even better for patients, who could eventually be able to enjoy having a 36-hour work week rather than a 40-hour work week. Admittedly, this potential outcome is an overly literal interpretation of the research results, but it emphasizes the potential value of having “more time” in patients’ lives as a result of effective migraine relief.
References
1. Peles I, Sharvit S, Zlotnik Y, et al. Migraine and work — beyond absenteeism: Migraine severity and occupational burnout — a cohort study. Cephalalgia. October 18, 2024. Source
2. Chase BA, Frigerio R, Rubin S, et al. An integrative migraine polygenic risk score is associated with age at onset but not with chronification. J Clin Med. October 29, 2024. Source
3. Cloet F, Gueyraud G, Lerebours F, Munio M, Larrue V, Gollion C. Stroke due to small-vessel disease and migraine: a case-control study of a young adult with ischemic stroke population. Cephalalgia. 2024;44:1-8. Source
4. Al-Hassany L, MaassenVanDenBrink A, Kurth T. Cardiovascular risk scores and migraine status. JAMA Netw Open. October 22, 2024. Source
5. Reddy M, Vazquez S, Nolan B, et al. Migraine and its association with stroke in pregnancy: A national examination. J Womens Health. 2024;33:1476-1481. Source
It is known that there are health-related consequences of migraine, as well as migraine-related comorbidities. Three recent studies examined the relationship between migraine and stroke, with nuanced results, suggesting that migraine does not necessarily increase stroke risk for all populations and may even be associated with a decreased risk for stroke for some patients. But it is clear that migraine is associated with an increased stroke risk for some specific populations, including during pregnancy.
Migraine is also known to have a negative impact on quality of life, affecting many different areas of well-being, including relationships, work productivity, and emotional health. Results from a recent study published in Cephalgia provided evidence that migraine can also increase the risk for occupational burnout.1
Yet, there’s some good news for migraine patients who have a genetic predisposition for migraine. Results of a recent study published in the Journal of Clinical Medicine showed that hereditary predisposition to migraine does not necessarily correlate with development of chronic migraine.2
An observational study, with results published in Cephalgia in November 2024, included 646 patients aged 18-54 years who were hospitalized with their first stroke.3 It showed no significant association between cerebral small-vessel disease and migraine with aura among the study population. Interestingly, migraine with aura is generally more closely linked with stroke risk than migraine without aura, so the results do not align with previously held beliefs about migraine and stroke risk.4
A larger study examined the relationship between migraine and cardiovascular risk scores.4 This cohort study included 140,915 Dutch adults with a mean age of 44 years. Results, published in JAMA Network Open in October 2024, revealed that the odds of having prevalent or incident migraine decreased with increasing cardiovascular risk score categories, especially for women. The authors suggested that having migraine could be associated with a healthier cardiovascular system and suggested several potential mechanisms for this inverse relationship, including alterations in the activity of calcitonin gene–related peptide activity, changes in nitric oxide effects, or cortical spreading depression in response to atherosclerosis.
Although the results of these studies, which were focused on young patients, are interesting and could provide a sense of relief for patients with migraine, the authors of the JAMA Network Open article acknowledged that these results should not be extrapolated to other populations.4 Specifically, they noted that it has been established in other studies that older patients with migraine have an increased cardiovascular risk.
The relationship between migraine and stroke risk is important for pregnant women. Results of a large analysis including 19,825,525 pregnant patients, with data obtained from 2016 to 2020, were published in November 2024 in the Journal of Women’s Health.5 The analysis revealed that a history of migraine substantially increases the risk for hemorrhagic or ischemic stroke during pregnancy. They reported that “acute ischemic stroke was most strongly associated with migraine with aura (odds ratio [OR], 23.26; 95% confidence interval [CI], 18.46-29.31), followed by migraine without aura (OR, 8.15; 95% CI, 4.79-13.88).” The authors advised that stroke risk should be addressed in pregnant women who have migraine or who have a migraine history, especially if they have migraine with aura.
It is well known that migraine risk has a hereditary component, but hereditary factors might not play a role in the time of onset of migraines. In a retrospective clinical genetic case-control study that included over 15,000 participants, researchers identified migraine polygenic risk scores using genome-wide association studies.2 The results were published in October 2024 in the Journal of Clinical Medicine. The study authors noted “a higher genetic risk was associated with earlier onset and increased risk for migraine well into adulthood, but not with chronification.” These results support the benefits of a diligent pursuit of effective migraine treatment, even for patients who might feel hopeless about achieving migraine control due to their own family history of migraine. As migraine therapies have evolved over the past decades, patients who had parents or other older family members with migraine may have a pessimistic outlook on the potential for effective treatment. However, newer therapies are far more effective than migraine treatments of the past, and patients should be informed and given encouragement that they can have a better prognosis and better migraine control than past generations.
The value of effective treatment cannot be underestimated. A study, with results published in Cephalgia in October 2024, included data from a subset of participants from the Negev Migraine Cohort, including 675 migraine patients and 232 control participants without migraine.1 The authors reported that migraine patients reported “significantly higher levels of occupational burnout, with a mean burnout score of 3.46 vs a mean of 2.82 among controls.” They also noted that migraine patients worked longer hours, with 40 hours of work weekly vs 36 for controls. The authors suggested accommodations for migraine patients, such as working from home or flexible scheduling. Although this could be beneficial, achieving migraine relief would be even better for patients, who could eventually be able to enjoy having a 36-hour work week rather than a 40-hour work week. Admittedly, this potential outcome is an overly literal interpretation of the research results, but it emphasizes the potential value of having “more time” in patients’ lives as a result of effective migraine relief.
References
1. Peles I, Sharvit S, Zlotnik Y, et al. Migraine and work — beyond absenteeism: Migraine severity and occupational burnout — a cohort study. Cephalalgia. October 18, 2024. Source
2. Chase BA, Frigerio R, Rubin S, et al. An integrative migraine polygenic risk score is associated with age at onset but not with chronification. J Clin Med. October 29, 2024. Source
3. Cloet F, Gueyraud G, Lerebours F, Munio M, Larrue V, Gollion C. Stroke due to small-vessel disease and migraine: a case-control study of a young adult with ischemic stroke population. Cephalalgia. 2024;44:1-8. Source
4. Al-Hassany L, MaassenVanDenBrink A, Kurth T. Cardiovascular risk scores and migraine status. JAMA Netw Open. October 22, 2024. Source
5. Reddy M, Vazquez S, Nolan B, et al. Migraine and its association with stroke in pregnancy: A national examination. J Womens Health. 2024;33:1476-1481. Source
Demarcated Nonpruritic Lesions Following Antibiotic Therapy
Demarcated Nonpruritic Lesions Following Antibiotic Therapy
THE DIAGNOSIS: Fixed Drug Eruption
Based on the patient’s clinical presentation and history of similar eruptions, a diagnosis of levofloxacin-induced fixed drug eruption (FDE) was made. After cessation of the drug, the lesions resolved within 1 week without any residual postinflammatory hyperpigmentation.
Fixed drug eruption is an adverse cutaneous reaction characterized by the onset of a rash at a fixed location each time a specific medication is administered. Patients typically report a history of similar eruptions, often involving the upper and lower extremities, genital area, or mucous membranes. The most common causative agents vary, but retrospective analyses primarily implicate nonsteroidal anti-inflammatory drugs followed by antibiotics (eg, amoxicillin, levofloxacin, doxycycline) and antiepileptics.1,2
While FDE can be solitary or scattered, most patients have 5 or fewer lesions, with a mean interval of 48 hours from exposure to the causative agent to onset of the rash.1 The lesions can be differentiated by their typically solitary, well-demarcated, round or oval appearance; they also are erythematous to purple with a dusky center. The lesions may increase in size and number with each additional exposure to the offending medication.1,3 Postinflammatory hyperpigmentation may last for weeks to months after the acute inflammatory response has resolved.
The high risk for recurrence of FDE may be explained by the presence of tissue resident memory T (TRM) cells in the affected skin that evoke a characteristic clinical manifestation upon administration of a causative agent.2,3 Intraepidermal CD8+ TRM cells, which have an effectormemory phenotype, may contribute to the development of localized tissue damage; these cells demonstrate their effector function by the rapid increase in interferon gamma after challenge.2 Within 24 hours of administration of the offending medication, CD8+ TRM cells migrate upward in the epidermis, and their activity leads to the epidermal necrosis observed with FDE. The self-limiting nature of FDE can be explained by the action of CD4+ Foxp3+ regulatory T cells that migrate similarly and induce the production of IL-10, which limits the damage inflicted by the CD8+ T cells.1
Type I hypersensitivity reactions are IgE mediated; typically occur much more rapidly than FDE; and involve a raised urticarial rash, pruritus, and flushing. Urticaria is useful in identifying IgE-mediated reactions and mast cell degranulation. Previous exposure to the drug in question is required for diagnosis.4
Type IV delayed hypersensitivity reactions—including contact dermatitis and FDE—are mediated by T cells rather than IgE. These reactions occur at least 48 to 72 hours after drug exposure.4 Contact dermatitis follows exposure to an irritant but generally is limited to the site of contact and manifests with burning or stinging. Chronic contact dermatitis is characterized by erythema, scaling, and lichenification that may be associated with burning pain.
The target lesions of erythema multiforme are associated with the use of medications such as nonsteroidal anti-inflammatory drugs, antiepileptics, and antibiotics in fewer than 10% of cases. Infections are the predominant cause, with herpes simplex virus 1 being the most common etiology.5 Erythema multiforme lesions have 3 concentric segments: a dark red inflammatory zone surrounded by a pale ring of edema, both of which are surrounded by an erythematous halo. Lesions initially are distributed symmetrically on the extensor surfaces of the upper and lower extremities, but mucosal involvement may be present.5
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, involves fever and peripheral neutrophilia in addition to cutaneous erythematous eruptions and dermal neutrophilic infiltration on histopathology.6 Most cases are idiopathic but may occur in the setting of malignancy or drug administration. A major criterion for drug-induced Sweet syndrome is abrupt onset of painful erythematous plaques or nodules with pyrexia.6
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Tokura Y, Phadungsaksawasdi P, Kurihara K, et al. Pathophysiology of skin resident memory T cells. Front Immunol. 2021;11:618897. doi:10.3389/fimmu.2020.618897
- Mockenhaupt M. Bullous drug reactions. Acta Derm Venereol. 2020;100:adv00057. doi:10.2340/00015555-3408
- Böhm R, Proksch E, Schwarz T, et al. Drug hypersensitivity. Dtsch Arztebl Int. 2018;115:501-512. doi:10.3238/arztebl.2018.0501
- Trayes KP, Love G, Studdiford JS. Erythema multiforme: recognition and management. Am Fam Physician. 2019;100:82-88.
- Joshi TP, Friske SK, Hsiou DA, et al. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi:10.1007 /s40257-022-00673-4
THE DIAGNOSIS: Fixed Drug Eruption
Based on the patient’s clinical presentation and history of similar eruptions, a diagnosis of levofloxacin-induced fixed drug eruption (FDE) was made. After cessation of the drug, the lesions resolved within 1 week without any residual postinflammatory hyperpigmentation.
Fixed drug eruption is an adverse cutaneous reaction characterized by the onset of a rash at a fixed location each time a specific medication is administered. Patients typically report a history of similar eruptions, often involving the upper and lower extremities, genital area, or mucous membranes. The most common causative agents vary, but retrospective analyses primarily implicate nonsteroidal anti-inflammatory drugs followed by antibiotics (eg, amoxicillin, levofloxacin, doxycycline) and antiepileptics.1,2
While FDE can be solitary or scattered, most patients have 5 or fewer lesions, with a mean interval of 48 hours from exposure to the causative agent to onset of the rash.1 The lesions can be differentiated by their typically solitary, well-demarcated, round or oval appearance; they also are erythematous to purple with a dusky center. The lesions may increase in size and number with each additional exposure to the offending medication.1,3 Postinflammatory hyperpigmentation may last for weeks to months after the acute inflammatory response has resolved.
The high risk for recurrence of FDE may be explained by the presence of tissue resident memory T (TRM) cells in the affected skin that evoke a characteristic clinical manifestation upon administration of a causative agent.2,3 Intraepidermal CD8+ TRM cells, which have an effectormemory phenotype, may contribute to the development of localized tissue damage; these cells demonstrate their effector function by the rapid increase in interferon gamma after challenge.2 Within 24 hours of administration of the offending medication, CD8+ TRM cells migrate upward in the epidermis, and their activity leads to the epidermal necrosis observed with FDE. The self-limiting nature of FDE can be explained by the action of CD4+ Foxp3+ regulatory T cells that migrate similarly and induce the production of IL-10, which limits the damage inflicted by the CD8+ T cells.1
Type I hypersensitivity reactions are IgE mediated; typically occur much more rapidly than FDE; and involve a raised urticarial rash, pruritus, and flushing. Urticaria is useful in identifying IgE-mediated reactions and mast cell degranulation. Previous exposure to the drug in question is required for diagnosis.4
Type IV delayed hypersensitivity reactions—including contact dermatitis and FDE—are mediated by T cells rather than IgE. These reactions occur at least 48 to 72 hours after drug exposure.4 Contact dermatitis follows exposure to an irritant but generally is limited to the site of contact and manifests with burning or stinging. Chronic contact dermatitis is characterized by erythema, scaling, and lichenification that may be associated with burning pain.
The target lesions of erythema multiforme are associated with the use of medications such as nonsteroidal anti-inflammatory drugs, antiepileptics, and antibiotics in fewer than 10% of cases. Infections are the predominant cause, with herpes simplex virus 1 being the most common etiology.5 Erythema multiforme lesions have 3 concentric segments: a dark red inflammatory zone surrounded by a pale ring of edema, both of which are surrounded by an erythematous halo. Lesions initially are distributed symmetrically on the extensor surfaces of the upper and lower extremities, but mucosal involvement may be present.5
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, involves fever and peripheral neutrophilia in addition to cutaneous erythematous eruptions and dermal neutrophilic infiltration on histopathology.6 Most cases are idiopathic but may occur in the setting of malignancy or drug administration. A major criterion for drug-induced Sweet syndrome is abrupt onset of painful erythematous plaques or nodules with pyrexia.6
THE DIAGNOSIS: Fixed Drug Eruption
Based on the patient’s clinical presentation and history of similar eruptions, a diagnosis of levofloxacin-induced fixed drug eruption (FDE) was made. After cessation of the drug, the lesions resolved within 1 week without any residual postinflammatory hyperpigmentation.
Fixed drug eruption is an adverse cutaneous reaction characterized by the onset of a rash at a fixed location each time a specific medication is administered. Patients typically report a history of similar eruptions, often involving the upper and lower extremities, genital area, or mucous membranes. The most common causative agents vary, but retrospective analyses primarily implicate nonsteroidal anti-inflammatory drugs followed by antibiotics (eg, amoxicillin, levofloxacin, doxycycline) and antiepileptics.1,2
While FDE can be solitary or scattered, most patients have 5 or fewer lesions, with a mean interval of 48 hours from exposure to the causative agent to onset of the rash.1 The lesions can be differentiated by their typically solitary, well-demarcated, round or oval appearance; they also are erythematous to purple with a dusky center. The lesions may increase in size and number with each additional exposure to the offending medication.1,3 Postinflammatory hyperpigmentation may last for weeks to months after the acute inflammatory response has resolved.
The high risk for recurrence of FDE may be explained by the presence of tissue resident memory T (TRM) cells in the affected skin that evoke a characteristic clinical manifestation upon administration of a causative agent.2,3 Intraepidermal CD8+ TRM cells, which have an effectormemory phenotype, may contribute to the development of localized tissue damage; these cells demonstrate their effector function by the rapid increase in interferon gamma after challenge.2 Within 24 hours of administration of the offending medication, CD8+ TRM cells migrate upward in the epidermis, and their activity leads to the epidermal necrosis observed with FDE. The self-limiting nature of FDE can be explained by the action of CD4+ Foxp3+ regulatory T cells that migrate similarly and induce the production of IL-10, which limits the damage inflicted by the CD8+ T cells.1
Type I hypersensitivity reactions are IgE mediated; typically occur much more rapidly than FDE; and involve a raised urticarial rash, pruritus, and flushing. Urticaria is useful in identifying IgE-mediated reactions and mast cell degranulation. Previous exposure to the drug in question is required for diagnosis.4
Type IV delayed hypersensitivity reactions—including contact dermatitis and FDE—are mediated by T cells rather than IgE. These reactions occur at least 48 to 72 hours after drug exposure.4 Contact dermatitis follows exposure to an irritant but generally is limited to the site of contact and manifests with burning or stinging. Chronic contact dermatitis is characterized by erythema, scaling, and lichenification that may be associated with burning pain.
The target lesions of erythema multiforme are associated with the use of medications such as nonsteroidal anti-inflammatory drugs, antiepileptics, and antibiotics in fewer than 10% of cases. Infections are the predominant cause, with herpes simplex virus 1 being the most common etiology.5 Erythema multiforme lesions have 3 concentric segments: a dark red inflammatory zone surrounded by a pale ring of edema, both of which are surrounded by an erythematous halo. Lesions initially are distributed symmetrically on the extensor surfaces of the upper and lower extremities, but mucosal involvement may be present.5
Sweet syndrome, also known as acute febrile neutrophilic dermatosis, involves fever and peripheral neutrophilia in addition to cutaneous erythematous eruptions and dermal neutrophilic infiltration on histopathology.6 Most cases are idiopathic but may occur in the setting of malignancy or drug administration. A major criterion for drug-induced Sweet syndrome is abrupt onset of painful erythematous plaques or nodules with pyrexia.6
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Tokura Y, Phadungsaksawasdi P, Kurihara K, et al. Pathophysiology of skin resident memory T cells. Front Immunol. 2021;11:618897. doi:10.3389/fimmu.2020.618897
- Mockenhaupt M. Bullous drug reactions. Acta Derm Venereol. 2020;100:adv00057. doi:10.2340/00015555-3408
- Böhm R, Proksch E, Schwarz T, et al. Drug hypersensitivity. Dtsch Arztebl Int. 2018;115:501-512. doi:10.3238/arztebl.2018.0501
- Trayes KP, Love G, Studdiford JS. Erythema multiforme: recognition and management. Am Fam Physician. 2019;100:82-88.
- Joshi TP, Friske SK, Hsiou DA, et al. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi:10.1007 /s40257-022-00673-4
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925. doi:10.3390/medicina57090925
- Tokura Y, Phadungsaksawasdi P, Kurihara K, et al. Pathophysiology of skin resident memory T cells. Front Immunol. 2021;11:618897. doi:10.3389/fimmu.2020.618897
- Mockenhaupt M. Bullous drug reactions. Acta Derm Venereol. 2020;100:adv00057. doi:10.2340/00015555-3408
- Böhm R, Proksch E, Schwarz T, et al. Drug hypersensitivity. Dtsch Arztebl Int. 2018;115:501-512. doi:10.3238/arztebl.2018.0501
- Trayes KP, Love G, Studdiford JS. Erythema multiforme: recognition and management. Am Fam Physician. 2019;100:82-88.
- Joshi TP, Friske SK, Hsiou DA, et al. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi:10.1007 /s40257-022-00673-4
Demarcated Nonpruritic Lesions Following Antibiotic Therapy
Demarcated Nonpruritic Lesions Following Antibiotic Therapy
A 35-year-old man was admitted to the hospital for treatment of cellulitis that required antibiotic therapy. Two days after administration of a single dose of intravenous levofloxacin, he developed demarcated nonpruritic and painless lesions on the abdomen (top) and right upper extremity (bottom). He was afebrile through the entire 1-week hospital course and denied use of any topical products prior to hospitalization. The patient reported a history of similar rashes associated with the use of levofloxacin.
Why Hidradenitis Suppurativa Should Be on Your Radar
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
Formaldehyde (FA) is a colorless, flammable, highly pungent gas that remains ubiquitous in the environment despite being a known carcinogen and allergen.1 In the cosmetic industry, FA commonly is used as both a preservative and active ingredient in hairstraightening products. Due to its toxicity and the thermal instability of FA releasers (ie, the release of FA at high temperatures), the US Food and Drug Administration has proposed a ban on formaldehyde and other FA-releasing chemicals (eg, methylene glycol) as an ingredient in hairsmoothing or hair-straightening products marketed in the United States.2 However, the implementation of this ban is not yet in effect.
Hair-straightening products that are referred to as chemical relaxers typically contain alkaline derivatives. Alkaline hair straighteners—which include lye relaxers (active ingredient: sodium hydroxide), nolye relaxers (active ingredients: potassium hydroxide, lithium hydroxide, calcium hydroxide, guanidine hydroxide, or ammonium thioglycolate), and the Japanese hair straightening process (active ingredient: ammonium thioglycolate)—do not contain FA or FA-derivatives as active ingredients.3 Alternatively, acidic hair straighteners—popularly known as keratin treatments—contain either FA or FA-releasers and will be the primary focus of this discussion. As many patients are exposed to these products, we aim to highlight the cutaneous and systemic manifestations of acute and chronic exposure.
How Hair-Straightening Products Work
Hair straighteners that include FA or its derivatives generally contain high and low molecular weights of keratin peptides. The keratin peptides with high molecular weights diffuse into the cuticle while the low-molecular-weight peptides can penetrate further into the cortex of the hair shaft.4 Formaldehyde forms cross-links with the keratin amino acids (eg, tyrosine, arginine), and the application of heat via blow-drying enhances its ability to cross-link the hydrolyzed keratin from the straightening product to the natural keratin in the hair fibers; the use of a heated flat iron further enhances the cross-linking and seals the cuticle.5 The same mechanism of action applies for “safe keratin” (marketing terminology used for FA releasers) treatments, whereby the hydrogen and salt bonds of the hair are weakened, allowing for interconversion of the cysteine bonds of the hair fibers. This chemical conversion allows for the hair shafts to have a stable straight configuration. Of note, this mechanism of action differs from the action of chemical relaxers, which have a high pH and straighten the hair by opening the cuticles and permanently breaking the disulfide bonds in the cortex of the hair shaft—a process that restructures the keratin bonds without requiring heat application.5
The outcome of a keratin treatment, as seen on light microscopy, is the replenishment of gaps in the hair’s cuticle, therefore increasing its mechanical and thermal properties.6 This can give the appearance of increased shine, softness, and tensile strength. However, Sanad et al6 report that, as viewed on transmission electron microscopy, these keratin treatments do not repair lost cuticles, cuticle splitting, or detached cuticle layers from damaged strands.
Lastly, some patients notice lightening of their hair color after a hair-straightening treatment, which is possibly due to inhibition of the enzymatic synthesis of melanin, decomposition of melanin granules, or a direct reaction from chemical neutralizers with a high pH.6 Knowledge of the mechanism of action of hair-straightening treatments will aid dermatologists in educating patients about their immediate and long-term effects. This education subsequently will help patients avoid inappropriate hair care techniques that further damage the hair.
Environmental Distribution and Systemic Absorption of Formaldehyde
Atmospheric FA is absorbed via cutaneous and mucosal surfaces. Atmospheric FA concentrations produced when hair-straightening products are used cannot routinely be predicted because the amount generated depends on factors such as the pH of the preparation, the temperature to which the product is heated during straightening, duration of storage, and aeration and size of the environment in which the product is being used, among others.7
Peteffi et al7 and Aglan et al8 detected a moderate positive correlation between environmental FA concentrations and those in cosmetic products, particularly after blow-drying the hair or using other heat applications; however, the products examined by Peteffi et al7 contained exceedingly high concentrations of FA (up to 5.9%, which is higher than the legal limit of 0.1% in the United States).9 Of note, some products in this study were labelled as “formaldehyde free” but still contained high concentrations of FA.7 This is consistent with data published by the Occupational Health and Safety Administration, which citied salons with exposure limits outside the national recommendations (2.0 FA ppm/air).10 These findings highlight the inadvertent exposure that consumers face from products that are not regulated consistently.
Interestingly, Henault et al11 observed that products with a high concentration of FA dispersed more airborne particles during hair brushing than hair straightening/ironing.11 Further studies are needed to clarify the different routes and methods contributing to FA dispersion and the molecular instability of FA-releasers.
Clinical Correlation
Products that contain low (ie, less than the legal limit) levels of FA are not mandated to declare its presence on the product label; however, many products are contaminated with FA or inappropriately omit FA from the ingredient list, even at elevated concentrations. Consumers therefore may be inadvertently exposed to FA particles. Additionally, occupations with frequent exposure to FA include hairdressers, barbers, beauticians and related workers (33.6% exposure rate); sewers and embroiderers (26.1%); and cooks (19.1%).12
Adverse health effects associated with acute FA exposure include but are not limited to headache, eye irritation, allergic/irritant contact dermatitis, psoriasiform reactions, and acute kidney and respiratory tract injuries. Frontal fibrosing alopecia; non-Hodgkin lymphoma; and cancers of the upper digestive tract, lungs, and bladder also have been associated with chronic FA exposure.7,13 In a cohort of female hairdressers, a longer duration of FA exposure (>8 years) as well as cumulative exposure were associated with an increase in ovarian cancer (OR, 1.48 [0.88 to 2.51]).12 Formalin, the aqueous derivative of FA, also contains phenolic products that can mediate inflammatory response, DNA methylation, and carcinogenesis even with chronic low-level exposure.14 However, evidence supporting a direct correlation of FA exposure with breast carcinoma in both hairstylists and consumers remains controversial.7
Sanchez-Duenas et al15 described a case series of patients who were found to have psoriasiform scalp reactions after exposure to keratin treatments containing FA. The time to development of the lesions was inversely correlated with the number of treatments received, although the mean time to development was 12 months postprocedure.15 These researchers also identified no allergies to the substance on contact testing, which suggests an alternate pathogenesis as a consequence of FA exposure, resulting in the development of a psoriasiform reaction.15
Following adjustment for sex, age, menopause status, and skin color, frontal fibrosing alopecia also has been associated with the use of formalin and FA in hair straighteners.14 This is possibly related to the ability of FA and many phenolic products to induce chronic inflammation; however, a cumulative effect has not been noted consistently across the literature.
Future Directives
Continuous industry regulation is needed to ensure that use of FA is reduced and it is eventually eliminated from consumer products. Additionally, strict regulations are required to ensure products containing FA and FA-releasers are accurately labeled. Physicians and consumers should be aware of the potential health hazards associated with FA and advocate for effective legislation. While there is controversy regarding the level of absorption from environmental exposure and the subsequent biologic effects of absorption, both consumers and workers in industries such as hairdressing and barbering should reduce exposure time to FA and limit the application of heat and contact with products containing FA and FA releasers.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832. doi:10.1016/j.ad.2013.12.018
- Department of Health and Human Services. Use of formaldehyde and formaldehyde-releasing chemicals as an ingredient in hair smoothing products or hair straightening products (RIN: 0910-AI83). Spring 2023. Accessed November 11, 2024. https://www.reginfo.gov/public/do/eAgendaViewRule?pubId=202304&RIN=0910-AI83
- Velasco MVR, de Sá-Dias TC, Dario MF, et al. Impact of acid (“progressive brush”) and alkaline straightening on the hair fiber: differential effects on the cuticle and cortex properties. Int J Trichology. 2022;14:197-203. doi:10.4103/ijt.ijt_158_20
- Malinauskyte E, Shrestha R, Cornwell P, et al. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int J Cosmet Sci. 2021;43:26-37. doi:10.1111/ics.12663
- Weathersby C, McMichael A. Brazilian keratin hair treatment: a review. J Cosmet Dermatol. 2013;12:144-148. doi:10.1111/jocd.12030
- Sanad EM, El]Esawy FM, Mustafa AI, et al. Structural changes of hair shaft after application of chemical hair straighteners: clinical and histopathological study. J Cosmet Dermatol. 2019;18:929-935. doi:10.1111/jocd.12752
- Peteffi GP, Antunes MV, Carrer C, et al. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ Sci Pollut Res Int. 2016;23:908-917. doi:10.1007/s11356-015-5343-4
- Aglan MA, Mansour GN. Hair straightening products and the risk of occupational formaldehyde exposure in hairstylists. Drug Chem Toxicol. 2020;43:488-495. doi: 10.1080/01480545.2018 .1508215
- Occupational Safety and Health Administration. Hair smoothing products that could release formaldehyde. Hazard Alert Update. September 2011. Accessed November 11, 2024. https://www.osha.gov/sites/default/files/hazard_alert.pdf
- US Department of Labor. US Department of Labor continues to cite beauty salons and manufacturers for formaldehyde exposure from hair smoothing products. December 8, 2011. Accessed November 11, 2024. https://www.dol.gov/newsroom/releases/osha/osha20111208
- Henault P, Lemaire R, Salzedo A, et al. A methodological approach for quantifying aerial formaldehyde released by some hair treatmentsmodeling a hair-salon environment. J Air Waste Manage. 2021;71: 754-760. doi:10.1080/10962247.2021.1893238
- Leung L, Lavoué J, Siemiatycki J, et al. Occupational environment and ovarian cancer risk. Occup Environ Med. 2023;80:489-497. doi:10.1136/oemed-2022-108557
- Bnaya A, Abu-Amer N, Beckerman P, et al. Acute kidney injury and hair-straightening products: a case series. Am J Kidney Dis. 2023;82:43-52.E1. doi:10.1053/j.ajkd.2022.11.016
- Ramos PM, Anzai A, Duque-Estrada B, et al. Risk factors for frontal fibrosing alopecia: a case-control study in a multiracial population. J Am Acad Dermatol. 2021;84:712-718. doi:10.1016/j.jaad.2020.08.076
- Sanchez-Duenas LE, Ruiz-Dueñas A, Guevara-Gutiérrez E, et al. Psoriasiform skin reaction due to Brazilian keratin treatment: a clinicaldermatoscopic study of 43 patients. Int J Trichology. 2022;14:103-108. doi:10.4103/ijt.ijt_62_21
Hidden Risks of Formaldehyde in Hair-Straightening Products
Hidden Risks of Formaldehyde in Hair-Straightening Products