User login
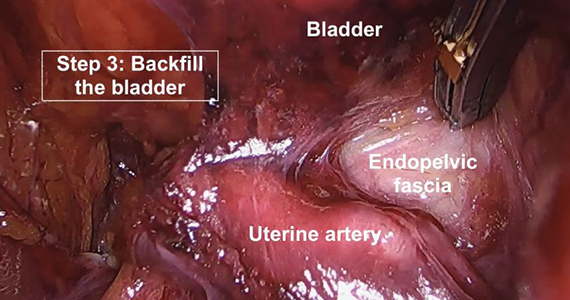
A stepwise approach to the difficult bladder flap to prevent urinary tract injury during laparoscopic hysterectomy
Efforts toward producing CNO/CRMO classification criteria show first results
MADRID – according to recent findings from international surveys of pediatric rheumatologists that were presented at the European Congress of Rheumatology.
Melissa Oliver, MD, a pediatric rheumatologist at Riley Hospital for Children, Indianapolis, and colleagues recently undertook the multiphase study as part of an international collaborative effort led by the Childhood Arthritis and Rheumatology Research Alliance to establish consensus-based diagnostic and classification criteria for CNO, an autoinflammatory bone disease of unknown cause that primarily affects children and adolescents. CNO is also known as chronic recurrent multifocal osteomyelitis (CRMO). If this disease is not diagnosed and treated appropriately in a timely fashion, damage and long-term disability is possible. In the absence of widely accepted, consensus-driven criteria, treatment is based largely on expert opinion, Dr. Oliver explained in an interview.
“There is an urgent need for a new and more robust set of classification criteria for CRMO, based on large expert consensus and the analysis of a large sample of patients and controls,” she said.
There are two proposed diagnostic criteria, the 2007 classification of nonbacterial osteitis and the 2016 Bristol diagnostic criteria for CRMO, but both are derived from single-center cohort studies and have not been validated, Dr. Oliver explained.
The list of candidate items that have come out of the study is moving clinicians a step closer toward the design of a practical patient data collection form that appropriately weighs each item included in the classification criteria.
The study employed anonymous survey and nominal group techniques with the goal of developing a set of classification criteria sensitive and specific enough to identify CRMO/CNO patients. In phase 1, a Delphi survey was administered among international rheumatologists to generate candidate criteria items. Phase 2 sought to reduce candidate criteria items through consensus processes via input from physicians managing CNO and patients or caregivers of children with CNO.
Altogether, 259 of 865 pediatric rheumatologists (30%) completed an online questionnaire addressing features key to the classification of CNO, including 77 who practice in Europe (30%), 132 in North America (51%), and 50 on other continents (19%). Of these, 138 (53%) had greater than 10 years of clinical practice experience, and 108 (42%) had managed more than 10 CNO patients.
Initially, Dr. Oliver and colleagues identified 33 candidate criteria items that fell into six domains: clinical presentation, physical exam, laboratory findings, imaging findings, bone biopsy, and treatment response. The top eight weighted items that increased the likelihood of CNO/CRMO were exclusion of malignancy by bone biopsy; multifocal bone lesions; presence of bone pain, swelling, and/or warmth; signs of fibrosis and/or inflammation on bone biopsy; typical location of CNO/CRMO lesion, such as the clavicle, metaphysis of long bones, the mandible, and vertebrae; presence of CNO/CRMO–related comorbidities; normal C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR); and typical MRI findings of CNO/CRMO.
By phase 2, candidate items, which were presented to 39 rheumatologists and 7 parents, were refined or eliminated using item-reduction techniques. A second survey was issued to 77 of 82 members of a work group so that the remaining items could be ranked by their power of distinguishing CNO from conditions that merely mimicked the disease. The greatest mean discriminatory scores were identified with multifocal lesions (ruling out malignancy and infection) and typical location on imaging. Normal C-reactive protein and/or an erythrocyte sedimentation rate more than three times the upper limit of normal had the greatest negative mean discriminatory scores.
The next steps will be to form an expert panel who will use 1000minds software to determine the final criteria and identify a threshold for disease. The investigators hope to build a large multinational case repository of at least 500 patients with CNO/CRMO and 500 patients with mimicking conditions from which to derive a development cohort and an external validation cohort. So far, 10 sites, including 4 in Europe, have obtained approval from an institutional review board. The group has also submitted a proposal for classification criteria to the American College of Rheumatology and the European League Against Rheumatism, Dr. Oliver said.
Dr. Oliver had no disclosures to report, but several coauthors reported financial ties to industry.
SOURCE: Oliver M et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):254-5, Abstract OP0342. doi: 10.1136/annrheumdis-2019-eular.1539.
MADRID – according to recent findings from international surveys of pediatric rheumatologists that were presented at the European Congress of Rheumatology.
Melissa Oliver, MD, a pediatric rheumatologist at Riley Hospital for Children, Indianapolis, and colleagues recently undertook the multiphase study as part of an international collaborative effort led by the Childhood Arthritis and Rheumatology Research Alliance to establish consensus-based diagnostic and classification criteria for CNO, an autoinflammatory bone disease of unknown cause that primarily affects children and adolescents. CNO is also known as chronic recurrent multifocal osteomyelitis (CRMO). If this disease is not diagnosed and treated appropriately in a timely fashion, damage and long-term disability is possible. In the absence of widely accepted, consensus-driven criteria, treatment is based largely on expert opinion, Dr. Oliver explained in an interview.
“There is an urgent need for a new and more robust set of classification criteria for CRMO, based on large expert consensus and the analysis of a large sample of patients and controls,” she said.
There are two proposed diagnostic criteria, the 2007 classification of nonbacterial osteitis and the 2016 Bristol diagnostic criteria for CRMO, but both are derived from single-center cohort studies and have not been validated, Dr. Oliver explained.
The list of candidate items that have come out of the study is moving clinicians a step closer toward the design of a practical patient data collection form that appropriately weighs each item included in the classification criteria.
The study employed anonymous survey and nominal group techniques with the goal of developing a set of classification criteria sensitive and specific enough to identify CRMO/CNO patients. In phase 1, a Delphi survey was administered among international rheumatologists to generate candidate criteria items. Phase 2 sought to reduce candidate criteria items through consensus processes via input from physicians managing CNO and patients or caregivers of children with CNO.
Altogether, 259 of 865 pediatric rheumatologists (30%) completed an online questionnaire addressing features key to the classification of CNO, including 77 who practice in Europe (30%), 132 in North America (51%), and 50 on other continents (19%). Of these, 138 (53%) had greater than 10 years of clinical practice experience, and 108 (42%) had managed more than 10 CNO patients.
Initially, Dr. Oliver and colleagues identified 33 candidate criteria items that fell into six domains: clinical presentation, physical exam, laboratory findings, imaging findings, bone biopsy, and treatment response. The top eight weighted items that increased the likelihood of CNO/CRMO were exclusion of malignancy by bone biopsy; multifocal bone lesions; presence of bone pain, swelling, and/or warmth; signs of fibrosis and/or inflammation on bone biopsy; typical location of CNO/CRMO lesion, such as the clavicle, metaphysis of long bones, the mandible, and vertebrae; presence of CNO/CRMO–related comorbidities; normal C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR); and typical MRI findings of CNO/CRMO.
By phase 2, candidate items, which were presented to 39 rheumatologists and 7 parents, were refined or eliminated using item-reduction techniques. A second survey was issued to 77 of 82 members of a work group so that the remaining items could be ranked by their power of distinguishing CNO from conditions that merely mimicked the disease. The greatest mean discriminatory scores were identified with multifocal lesions (ruling out malignancy and infection) and typical location on imaging. Normal C-reactive protein and/or an erythrocyte sedimentation rate more than three times the upper limit of normal had the greatest negative mean discriminatory scores.
The next steps will be to form an expert panel who will use 1000minds software to determine the final criteria and identify a threshold for disease. The investigators hope to build a large multinational case repository of at least 500 patients with CNO/CRMO and 500 patients with mimicking conditions from which to derive a development cohort and an external validation cohort. So far, 10 sites, including 4 in Europe, have obtained approval from an institutional review board. The group has also submitted a proposal for classification criteria to the American College of Rheumatology and the European League Against Rheumatism, Dr. Oliver said.
Dr. Oliver had no disclosures to report, but several coauthors reported financial ties to industry.
SOURCE: Oliver M et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):254-5, Abstract OP0342. doi: 10.1136/annrheumdis-2019-eular.1539.
MADRID – according to recent findings from international surveys of pediatric rheumatologists that were presented at the European Congress of Rheumatology.
Melissa Oliver, MD, a pediatric rheumatologist at Riley Hospital for Children, Indianapolis, and colleagues recently undertook the multiphase study as part of an international collaborative effort led by the Childhood Arthritis and Rheumatology Research Alliance to establish consensus-based diagnostic and classification criteria for CNO, an autoinflammatory bone disease of unknown cause that primarily affects children and adolescents. CNO is also known as chronic recurrent multifocal osteomyelitis (CRMO). If this disease is not diagnosed and treated appropriately in a timely fashion, damage and long-term disability is possible. In the absence of widely accepted, consensus-driven criteria, treatment is based largely on expert opinion, Dr. Oliver explained in an interview.
“There is an urgent need for a new and more robust set of classification criteria for CRMO, based on large expert consensus and the analysis of a large sample of patients and controls,” she said.
There are two proposed diagnostic criteria, the 2007 classification of nonbacterial osteitis and the 2016 Bristol diagnostic criteria for CRMO, but both are derived from single-center cohort studies and have not been validated, Dr. Oliver explained.
The list of candidate items that have come out of the study is moving clinicians a step closer toward the design of a practical patient data collection form that appropriately weighs each item included in the classification criteria.
The study employed anonymous survey and nominal group techniques with the goal of developing a set of classification criteria sensitive and specific enough to identify CRMO/CNO patients. In phase 1, a Delphi survey was administered among international rheumatologists to generate candidate criteria items. Phase 2 sought to reduce candidate criteria items through consensus processes via input from physicians managing CNO and patients or caregivers of children with CNO.
Altogether, 259 of 865 pediatric rheumatologists (30%) completed an online questionnaire addressing features key to the classification of CNO, including 77 who practice in Europe (30%), 132 in North America (51%), and 50 on other continents (19%). Of these, 138 (53%) had greater than 10 years of clinical practice experience, and 108 (42%) had managed more than 10 CNO patients.
Initially, Dr. Oliver and colleagues identified 33 candidate criteria items that fell into six domains: clinical presentation, physical exam, laboratory findings, imaging findings, bone biopsy, and treatment response. The top eight weighted items that increased the likelihood of CNO/CRMO were exclusion of malignancy by bone biopsy; multifocal bone lesions; presence of bone pain, swelling, and/or warmth; signs of fibrosis and/or inflammation on bone biopsy; typical location of CNO/CRMO lesion, such as the clavicle, metaphysis of long bones, the mandible, and vertebrae; presence of CNO/CRMO–related comorbidities; normal C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR); and typical MRI findings of CNO/CRMO.
By phase 2, candidate items, which were presented to 39 rheumatologists and 7 parents, were refined or eliminated using item-reduction techniques. A second survey was issued to 77 of 82 members of a work group so that the remaining items could be ranked by their power of distinguishing CNO from conditions that merely mimicked the disease. The greatest mean discriminatory scores were identified with multifocal lesions (ruling out malignancy and infection) and typical location on imaging. Normal C-reactive protein and/or an erythrocyte sedimentation rate more than three times the upper limit of normal had the greatest negative mean discriminatory scores.
The next steps will be to form an expert panel who will use 1000minds software to determine the final criteria and identify a threshold for disease. The investigators hope to build a large multinational case repository of at least 500 patients with CNO/CRMO and 500 patients with mimicking conditions from which to derive a development cohort and an external validation cohort. So far, 10 sites, including 4 in Europe, have obtained approval from an institutional review board. The group has also submitted a proposal for classification criteria to the American College of Rheumatology and the European League Against Rheumatism, Dr. Oliver said.
Dr. Oliver had no disclosures to report, but several coauthors reported financial ties to industry.
SOURCE: Oliver M et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):254-5, Abstract OP0342. doi: 10.1136/annrheumdis-2019-eular.1539.
REPORTING FROM EULAR 2019 CONGRESS
Bisphosphonates before denosumab may prevent postdenosumab BMD rebound effect
MADRID – Results from an ongoing study of postmenopausal women who discontinue osteoporosis treatment with denosumab (Prolia) so far support the use of denosumab as a second-line therapy after a bisphosphonate, unless otherwise indicated, in order to reduce the loss of bone mineral density (BMD) after its discontinuation and also to support treatment to reduce bone turnover biomarkers as much as possible after stopping denosumab.
“We saw in our study that, even if you give bisphosphonates after denosumab discontinuation, [patients] could lose bone, and the group that controlled the loss of bone had very high control of bone turnover markers,” study author and presenter Bérengère Rozier Aubry, MD, said in an interview at the European Congress of Rheumatology.
She and her colleagues at the Center of Bone Diseases at Lausanne (Switzerland) University Hospital are conducting the ReoLaus (Rebound Effect Observatory in Lausanne) Bone Project to determine whether giving a bisphosphonate to postmenopausal women with osteoporosis after they have discontinued denosumab can stop the loss of bone mineral density (BMD) observed in many patients up to 2 years after stopping denosumab. This postdenosumab BMD loss has also been observed to occur with multiple spontaneous vertebral fractures.
Nearly half of patients who start denosumab discontinue it within 1 year, and 64% by 2 years, according to U.S. administrative claims data (Osteoporos Int. 2017 Apr. doi: 10.1007/s00198-016-3886-y), even though it can be taken for up to 10 years. The discontinuation is either because the patient wishes to do so or there’s a medical indication such as stopping aromatase inhibitor treatment, resolution of osteoporosis, or side effects, Dr. Rozier Aubry said in a press conference at the European Congress of Rheumatology.
Upon discontinuing denosumab, there’s a marked rebound effect in which levels of bone turnover markers rise for 2 years, and some or all of the BMD that was gained is lost (J Clin Endocrinol Metab. 2011 Apr. doi: 10.1210/jc.2010-1502). Multiple spontaneous vertebral fractures also have been reported in 5%-7%, as Dr. Rozier Aubry and colleagues first described in 2016 (Osteoporos Int. 2016 May. doi: 10.1007/s00198-015-3380-y) and others have reported subsequently.
Recommendations from the Endocrine Society in March 2019, a 2017 position statement from the European Calcified Tissue Society, and guidelines from other groups advise giving antiresorptive treatment (bisphosphonates, hormone therapy, or selective estrogen-receptor modulators) but do not say which one, in what dose, when, or for how long, Dr. Rozier Aubry noted.
Treatment with zoledronate 6 months after the last denosumab injection achieves partial preservation of BMD, but multiple vertebral fractures have still been reported when raloxifene, ibandronate, or alendronate have been given after stopping denosumab, she said.
In the ReoLaus Bone Project, Dr. Rozier Aubry and associates are following 170 postmenopausal women with osteoporosis at Lausanne University Hospital who are taking denosumab therapy. At the congress, she reported on the first 71 women in the cohort with 1 year of follow-up. They had a mean age of 64 years, had fewer than one prevalent fracture before starting denosumab, and stopped denosumab after a mean of 7.7 injections. Overall, 8% took glucocorticoids, and 22% took aromatase inhibitors.
The investigators collected data on what treatment was used after denosumab, how bone turnover markers changed 1-3 months after the last denosumab injection and then regularly afterward, how bone mineral density changed after 1 year, and any new osteoporotic fractures.
At the time of denosumab discontinuation, 59% received zoledronate, 24% alendronate, 3% other drugs, and 14% nothing. At a mean of about 17 months after the last denosumab injection, the investigators classified 30 patients as BMD losers (losing at least 3.96%), and 41 had stable BMD. The researchers found that BMD losers were younger (61.4 years vs. 65.5 years), were less likely to use zoledronate before starting denosumab (0% vs. 12%), and had greater serum CTX (C-telopeptide cross-linked type 1 collagen) levels at denosumab initiation (644 ng/mL vs. 474 ng/mL) and 12.8 months after stopping denosumab (592 ng/mL vs. 336 ng/mL) than did those with stable BMD. All differences were statistically significant.
“Our results support the use of denosumab in second line after bisphosphonate therapy to restrain the BMD loss at its discontinuation ... and a strategy to maintain the bone turnover marker serum CTX as low as possible after denosumab discontinuation,” she concluded.
“Our proposition is to start with 1 or 2 years of bisphosphonates, and if the osteoporosis is severe, to switch to denosumab treatment for 4, 6 years. … We can use denosumab for 10 years without side effects, and after that we give bisphosphonates to consolidate the treatment,” she said.
Dr. Rozier Aubry and her associates plan to follow patients in their study for 2 years.
Dr. Rozier Aubry disclosed serving on speakers bureaus for Eli Lilly, Pfizer, Amgen, and Novartis.
SOURCE: Rozier Aubry B et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):115; Abstract OP0085. doi: 10.1136/annrheumdis-2019-eular.4175.
MADRID – Results from an ongoing study of postmenopausal women who discontinue osteoporosis treatment with denosumab (Prolia) so far support the use of denosumab as a second-line therapy after a bisphosphonate, unless otherwise indicated, in order to reduce the loss of bone mineral density (BMD) after its discontinuation and also to support treatment to reduce bone turnover biomarkers as much as possible after stopping denosumab.
“We saw in our study that, even if you give bisphosphonates after denosumab discontinuation, [patients] could lose bone, and the group that controlled the loss of bone had very high control of bone turnover markers,” study author and presenter Bérengère Rozier Aubry, MD, said in an interview at the European Congress of Rheumatology.
She and her colleagues at the Center of Bone Diseases at Lausanne (Switzerland) University Hospital are conducting the ReoLaus (Rebound Effect Observatory in Lausanne) Bone Project to determine whether giving a bisphosphonate to postmenopausal women with osteoporosis after they have discontinued denosumab can stop the loss of bone mineral density (BMD) observed in many patients up to 2 years after stopping denosumab. This postdenosumab BMD loss has also been observed to occur with multiple spontaneous vertebral fractures.
Nearly half of patients who start denosumab discontinue it within 1 year, and 64% by 2 years, according to U.S. administrative claims data (Osteoporos Int. 2017 Apr. doi: 10.1007/s00198-016-3886-y), even though it can be taken for up to 10 years. The discontinuation is either because the patient wishes to do so or there’s a medical indication such as stopping aromatase inhibitor treatment, resolution of osteoporosis, or side effects, Dr. Rozier Aubry said in a press conference at the European Congress of Rheumatology.
Upon discontinuing denosumab, there’s a marked rebound effect in which levels of bone turnover markers rise for 2 years, and some or all of the BMD that was gained is lost (J Clin Endocrinol Metab. 2011 Apr. doi: 10.1210/jc.2010-1502). Multiple spontaneous vertebral fractures also have been reported in 5%-7%, as Dr. Rozier Aubry and colleagues first described in 2016 (Osteoporos Int. 2016 May. doi: 10.1007/s00198-015-3380-y) and others have reported subsequently.
Recommendations from the Endocrine Society in March 2019, a 2017 position statement from the European Calcified Tissue Society, and guidelines from other groups advise giving antiresorptive treatment (bisphosphonates, hormone therapy, or selective estrogen-receptor modulators) but do not say which one, in what dose, when, or for how long, Dr. Rozier Aubry noted.
Treatment with zoledronate 6 months after the last denosumab injection achieves partial preservation of BMD, but multiple vertebral fractures have still been reported when raloxifene, ibandronate, or alendronate have been given after stopping denosumab, she said.
In the ReoLaus Bone Project, Dr. Rozier Aubry and associates are following 170 postmenopausal women with osteoporosis at Lausanne University Hospital who are taking denosumab therapy. At the congress, she reported on the first 71 women in the cohort with 1 year of follow-up. They had a mean age of 64 years, had fewer than one prevalent fracture before starting denosumab, and stopped denosumab after a mean of 7.7 injections. Overall, 8% took glucocorticoids, and 22% took aromatase inhibitors.
The investigators collected data on what treatment was used after denosumab, how bone turnover markers changed 1-3 months after the last denosumab injection and then regularly afterward, how bone mineral density changed after 1 year, and any new osteoporotic fractures.
At the time of denosumab discontinuation, 59% received zoledronate, 24% alendronate, 3% other drugs, and 14% nothing. At a mean of about 17 months after the last denosumab injection, the investigators classified 30 patients as BMD losers (losing at least 3.96%), and 41 had stable BMD. The researchers found that BMD losers were younger (61.4 years vs. 65.5 years), were less likely to use zoledronate before starting denosumab (0% vs. 12%), and had greater serum CTX (C-telopeptide cross-linked type 1 collagen) levels at denosumab initiation (644 ng/mL vs. 474 ng/mL) and 12.8 months after stopping denosumab (592 ng/mL vs. 336 ng/mL) than did those with stable BMD. All differences were statistically significant.
“Our results support the use of denosumab in second line after bisphosphonate therapy to restrain the BMD loss at its discontinuation ... and a strategy to maintain the bone turnover marker serum CTX as low as possible after denosumab discontinuation,” she concluded.
“Our proposition is to start with 1 or 2 years of bisphosphonates, and if the osteoporosis is severe, to switch to denosumab treatment for 4, 6 years. … We can use denosumab for 10 years without side effects, and after that we give bisphosphonates to consolidate the treatment,” she said.
Dr. Rozier Aubry and her associates plan to follow patients in their study for 2 years.
Dr. Rozier Aubry disclosed serving on speakers bureaus for Eli Lilly, Pfizer, Amgen, and Novartis.
SOURCE: Rozier Aubry B et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):115; Abstract OP0085. doi: 10.1136/annrheumdis-2019-eular.4175.
MADRID – Results from an ongoing study of postmenopausal women who discontinue osteoporosis treatment with denosumab (Prolia) so far support the use of denosumab as a second-line therapy after a bisphosphonate, unless otherwise indicated, in order to reduce the loss of bone mineral density (BMD) after its discontinuation and also to support treatment to reduce bone turnover biomarkers as much as possible after stopping denosumab.
“We saw in our study that, even if you give bisphosphonates after denosumab discontinuation, [patients] could lose bone, and the group that controlled the loss of bone had very high control of bone turnover markers,” study author and presenter Bérengère Rozier Aubry, MD, said in an interview at the European Congress of Rheumatology.
She and her colleagues at the Center of Bone Diseases at Lausanne (Switzerland) University Hospital are conducting the ReoLaus (Rebound Effect Observatory in Lausanne) Bone Project to determine whether giving a bisphosphonate to postmenopausal women with osteoporosis after they have discontinued denosumab can stop the loss of bone mineral density (BMD) observed in many patients up to 2 years after stopping denosumab. This postdenosumab BMD loss has also been observed to occur with multiple spontaneous vertebral fractures.
Nearly half of patients who start denosumab discontinue it within 1 year, and 64% by 2 years, according to U.S. administrative claims data (Osteoporos Int. 2017 Apr. doi: 10.1007/s00198-016-3886-y), even though it can be taken for up to 10 years. The discontinuation is either because the patient wishes to do so or there’s a medical indication such as stopping aromatase inhibitor treatment, resolution of osteoporosis, or side effects, Dr. Rozier Aubry said in a press conference at the European Congress of Rheumatology.
Upon discontinuing denosumab, there’s a marked rebound effect in which levels of bone turnover markers rise for 2 years, and some or all of the BMD that was gained is lost (J Clin Endocrinol Metab. 2011 Apr. doi: 10.1210/jc.2010-1502). Multiple spontaneous vertebral fractures also have been reported in 5%-7%, as Dr. Rozier Aubry and colleagues first described in 2016 (Osteoporos Int. 2016 May. doi: 10.1007/s00198-015-3380-y) and others have reported subsequently.
Recommendations from the Endocrine Society in March 2019, a 2017 position statement from the European Calcified Tissue Society, and guidelines from other groups advise giving antiresorptive treatment (bisphosphonates, hormone therapy, or selective estrogen-receptor modulators) but do not say which one, in what dose, when, or for how long, Dr. Rozier Aubry noted.
Treatment with zoledronate 6 months after the last denosumab injection achieves partial preservation of BMD, but multiple vertebral fractures have still been reported when raloxifene, ibandronate, or alendronate have been given after stopping denosumab, she said.
In the ReoLaus Bone Project, Dr. Rozier Aubry and associates are following 170 postmenopausal women with osteoporosis at Lausanne University Hospital who are taking denosumab therapy. At the congress, she reported on the first 71 women in the cohort with 1 year of follow-up. They had a mean age of 64 years, had fewer than one prevalent fracture before starting denosumab, and stopped denosumab after a mean of 7.7 injections. Overall, 8% took glucocorticoids, and 22% took aromatase inhibitors.
The investigators collected data on what treatment was used after denosumab, how bone turnover markers changed 1-3 months after the last denosumab injection and then regularly afterward, how bone mineral density changed after 1 year, and any new osteoporotic fractures.
At the time of denosumab discontinuation, 59% received zoledronate, 24% alendronate, 3% other drugs, and 14% nothing. At a mean of about 17 months after the last denosumab injection, the investigators classified 30 patients as BMD losers (losing at least 3.96%), and 41 had stable BMD. The researchers found that BMD losers were younger (61.4 years vs. 65.5 years), were less likely to use zoledronate before starting denosumab (0% vs. 12%), and had greater serum CTX (C-telopeptide cross-linked type 1 collagen) levels at denosumab initiation (644 ng/mL vs. 474 ng/mL) and 12.8 months after stopping denosumab (592 ng/mL vs. 336 ng/mL) than did those with stable BMD. All differences were statistically significant.
“Our results support the use of denosumab in second line after bisphosphonate therapy to restrain the BMD loss at its discontinuation ... and a strategy to maintain the bone turnover marker serum CTX as low as possible after denosumab discontinuation,” she concluded.
“Our proposition is to start with 1 or 2 years of bisphosphonates, and if the osteoporosis is severe, to switch to denosumab treatment for 4, 6 years. … We can use denosumab for 10 years without side effects, and after that we give bisphosphonates to consolidate the treatment,” she said.
Dr. Rozier Aubry and her associates plan to follow patients in their study for 2 years.
Dr. Rozier Aubry disclosed serving on speakers bureaus for Eli Lilly, Pfizer, Amgen, and Novartis.
SOURCE: Rozier Aubry B et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):115; Abstract OP0085. doi: 10.1136/annrheumdis-2019-eular.4175.
REPORTING FROM EULAR 2019 CONGRESS
Study finds differences for HCC in women
SAN DIEGO – Hepatocellular carcinoma is the third leading cause of cancer-related death in the United States and its incidence is increasing worldwide. While it affects men much more frequently than women, approximately 4 to 1, the differences in risk factors between men and women have never been studied.
At the annual Digestive Disease Week, Meaghan Phipps, MD, of New York–Presbyterian Hospital, described in a video interview how she and her colleagues set up a retrospective study of these differences in 5,327 patients at five large academic centers around the country. She and her colleagues found that women tended to present later, and with less severe disease, which was more likely to be treated with resection than transplantation. Women had better overall survival. Women were significantly more likely to present without cirrhosis and with nonalcoholic fatty liver disease than were men. Dr. Phipps noted that they did not characterize the women in their study by menopausal status, and suggested that this would be an important thing to look at in a future prospective study because it has long been thought that estrogen confers some protection against hepatocellular carcinoma.
SAN DIEGO – Hepatocellular carcinoma is the third leading cause of cancer-related death in the United States and its incidence is increasing worldwide. While it affects men much more frequently than women, approximately 4 to 1, the differences in risk factors between men and women have never been studied.
At the annual Digestive Disease Week, Meaghan Phipps, MD, of New York–Presbyterian Hospital, described in a video interview how she and her colleagues set up a retrospective study of these differences in 5,327 patients at five large academic centers around the country. She and her colleagues found that women tended to present later, and with less severe disease, which was more likely to be treated with resection than transplantation. Women had better overall survival. Women were significantly more likely to present without cirrhosis and with nonalcoholic fatty liver disease than were men. Dr. Phipps noted that they did not characterize the women in their study by menopausal status, and suggested that this would be an important thing to look at in a future prospective study because it has long been thought that estrogen confers some protection against hepatocellular carcinoma.
SAN DIEGO – Hepatocellular carcinoma is the third leading cause of cancer-related death in the United States and its incidence is increasing worldwide. While it affects men much more frequently than women, approximately 4 to 1, the differences in risk factors between men and women have never been studied.
At the annual Digestive Disease Week, Meaghan Phipps, MD, of New York–Presbyterian Hospital, described in a video interview how she and her colleagues set up a retrospective study of these differences in 5,327 patients at five large academic centers around the country. She and her colleagues found that women tended to present later, and with less severe disease, which was more likely to be treated with resection than transplantation. Women had better overall survival. Women were significantly more likely to present without cirrhosis and with nonalcoholic fatty liver disease than were men. Dr. Phipps noted that they did not characterize the women in their study by menopausal status, and suggested that this would be an important thing to look at in a future prospective study because it has long been thought that estrogen confers some protection against hepatocellular carcinoma.
REPORTING FROM DDW 2019
Patient registry sheds light on the economic impact of MS
SEATTLE –
“MS seems to prevent people with MS from realizing their full potential at work or home,” said study coauthor Kottil Rammohan, MD, who summarized the study results in a video interview. Dr. Rammohan is professor of clinical neurology, director of the MS center of excellence, and chief of the multiple sclerosis division at the University of Miami. The study findings were presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
The North American Registry for Care and Research in Multiple Sclerosis (NARCRMS) prospectively collects information about the health care economics of patients with MS and its effects on daily life. In 2017, NARCRMS established the health care economics outcomes research (HEOR) advisory group. NARCRMS developed a Health-Related Productivity Questionnaire and Health Resource Utilization Questionnaire. The questionnaires were incorporated into the existing case report forms that are completed by patients at enrollment, annual, and exacerbation visits.
This analysis was based on 480 patients who had completed HEOR case report forms. Among those, 77% are employed either full or part time; however, of those 15% were underemployed, meaning they wanted to work more than their current work levels. About 13% are on disability.
“What we found was there was a significant impact at home as well,” said Dr. Rammohan. Patients reported that MS kept them from completing household chores. “MS is a disease that seems to impact not only the work environment, but also the home environment.”
When polled to determine the main reason why these MS patients are not able to function, “what we found was that it was not because of gait or immobility, it was difficulty related to fatigue,” Dr. Rammohan said. The second most common impairment was related to cognition.
“These are what we call the silent or the transparent symptoms of MS.”
Dr. Rammohan disclosed consulting fees from EMD Serono, Biogen, Sanofi-Aventis, Genzyme, Novartis, Teva Neurosciences, Acorda, and Roche/Genentech.
SEATTLE –
“MS seems to prevent people with MS from realizing their full potential at work or home,” said study coauthor Kottil Rammohan, MD, who summarized the study results in a video interview. Dr. Rammohan is professor of clinical neurology, director of the MS center of excellence, and chief of the multiple sclerosis division at the University of Miami. The study findings were presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
The North American Registry for Care and Research in Multiple Sclerosis (NARCRMS) prospectively collects information about the health care economics of patients with MS and its effects on daily life. In 2017, NARCRMS established the health care economics outcomes research (HEOR) advisory group. NARCRMS developed a Health-Related Productivity Questionnaire and Health Resource Utilization Questionnaire. The questionnaires were incorporated into the existing case report forms that are completed by patients at enrollment, annual, and exacerbation visits.
This analysis was based on 480 patients who had completed HEOR case report forms. Among those, 77% are employed either full or part time; however, of those 15% were underemployed, meaning they wanted to work more than their current work levels. About 13% are on disability.
“What we found was there was a significant impact at home as well,” said Dr. Rammohan. Patients reported that MS kept them from completing household chores. “MS is a disease that seems to impact not only the work environment, but also the home environment.”
When polled to determine the main reason why these MS patients are not able to function, “what we found was that it was not because of gait or immobility, it was difficulty related to fatigue,” Dr. Rammohan said. The second most common impairment was related to cognition.
“These are what we call the silent or the transparent symptoms of MS.”
Dr. Rammohan disclosed consulting fees from EMD Serono, Biogen, Sanofi-Aventis, Genzyme, Novartis, Teva Neurosciences, Acorda, and Roche/Genentech.
SEATTLE –
“MS seems to prevent people with MS from realizing their full potential at work or home,” said study coauthor Kottil Rammohan, MD, who summarized the study results in a video interview. Dr. Rammohan is professor of clinical neurology, director of the MS center of excellence, and chief of the multiple sclerosis division at the University of Miami. The study findings were presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
The North American Registry for Care and Research in Multiple Sclerosis (NARCRMS) prospectively collects information about the health care economics of patients with MS and its effects on daily life. In 2017, NARCRMS established the health care economics outcomes research (HEOR) advisory group. NARCRMS developed a Health-Related Productivity Questionnaire and Health Resource Utilization Questionnaire. The questionnaires were incorporated into the existing case report forms that are completed by patients at enrollment, annual, and exacerbation visits.
This analysis was based on 480 patients who had completed HEOR case report forms. Among those, 77% are employed either full or part time; however, of those 15% were underemployed, meaning they wanted to work more than their current work levels. About 13% are on disability.
“What we found was there was a significant impact at home as well,” said Dr. Rammohan. Patients reported that MS kept them from completing household chores. “MS is a disease that seems to impact not only the work environment, but also the home environment.”
When polled to determine the main reason why these MS patients are not able to function, “what we found was that it was not because of gait or immobility, it was difficulty related to fatigue,” Dr. Rammohan said. The second most common impairment was related to cognition.
“These are what we call the silent or the transparent symptoms of MS.”
Dr. Rammohan disclosed consulting fees from EMD Serono, Biogen, Sanofi-Aventis, Genzyme, Novartis, Teva Neurosciences, Acorda, and Roche/Genentech.
EXPERT ANALYSIS FROM CMSC 2019
VIDEO: Dr. Lihi Eder on the diagnostic challenges of psoriatic arthritis

How strong are the links between psoriasis and psoriatic arthritis? What are the biggest challenges that keep clinicians from diagnosing PsA? And which approaches show promise in helping prevent PsA? In this expert analysis, Lihi Eder, MD, PhD, of Women’s College Research Institute, Toronto, and the University of Toronto offers answers to these questions and more.

How strong are the links between psoriasis and psoriatic arthritis? What are the biggest challenges that keep clinicians from diagnosing PsA? And which approaches show promise in helping prevent PsA? In this expert analysis, Lihi Eder, MD, PhD, of Women’s College Research Institute, Toronto, and the University of Toronto offers answers to these questions and more.

How strong are the links between psoriasis and psoriatic arthritis? What are the biggest challenges that keep clinicians from diagnosing PsA? And which approaches show promise in helping prevent PsA? In this expert analysis, Lihi Eder, MD, PhD, of Women’s College Research Institute, Toronto, and the University of Toronto offers answers to these questions and more.
Ultra low-dose rituximab retains promise in rheumatoid arthritis
MADRID – Retreatment of rheumatoid arthritis (RA) with an ultra low-dose of rituximab failed to meet the predefined noninferiority endpoint relative to a higher dose in a double-blind randomized trial, but the investigators still think this strategy may be viable in selected patients.
Lise M. Verhoef, MSc, a researcher in rheumatology at the Sint Maartenskliniek in Nijmegen, the Netherlands, who presented the data as a late-breaker at the European Congress of Rheumatology, explains in a video interview why the negative trial still might support an ultra low-dose strategy.
This trial, called REDO, was conceived after it was observed that most patients with RA are well controlled on a single injection of 1,000 mg of rituximab even though this is half the standard dose of two 1,000 mg doses given 15 days apart. The study was designed to determine whether even lower doses could be used.
The study enrolled 142 patients with RA who were being retreated with rituximab after responding previously to this therapy. In a 1:2:2 ratio, patients were randomized to single rituximab injections of 1,000 mg, 500 mg, or 200 mg. Outcome then were compared at the end of 6 months.
Noninferiority was defined as 0.5 difference in DAS28-CRP score (disease activity score using C-reactive protein instead of erythrocyte sedimentation rate) score adjusted for baseline disease status and use of conventional disease-modifying antirheumatic drugs (DMARDs).
Although noninferior at 3 months, the 500 mg dose did not meet the noninferiority criteria at 6 months. Due to a hierarchical design, evaluation of the 200 mg dose was precluded by the negative result with the 500 mg dose.
However, the majority of patients did respond to both the 500 mg and 200 mg dose. The failure to meet noninferiority was due to a limited number of patients who required rescue therapy for a flare. As a result, the investigators believe a trial of ultra low-dose therapy still might be reasonable.
In this interview, Ms. Verhoef explains that at her center patients who are well controlled on a 1,000 mg dose of rituximab now are being offered a 500 mg dose for retreatment. If they continue to respond, further retreatment with a 200 mg dose is considered.
Ms. Verhoef had no relevant financial disclosures.
MADRID – Retreatment of rheumatoid arthritis (RA) with an ultra low-dose of rituximab failed to meet the predefined noninferiority endpoint relative to a higher dose in a double-blind randomized trial, but the investigators still think this strategy may be viable in selected patients.
Lise M. Verhoef, MSc, a researcher in rheumatology at the Sint Maartenskliniek in Nijmegen, the Netherlands, who presented the data as a late-breaker at the European Congress of Rheumatology, explains in a video interview why the negative trial still might support an ultra low-dose strategy.
This trial, called REDO, was conceived after it was observed that most patients with RA are well controlled on a single injection of 1,000 mg of rituximab even though this is half the standard dose of two 1,000 mg doses given 15 days apart. The study was designed to determine whether even lower doses could be used.
The study enrolled 142 patients with RA who were being retreated with rituximab after responding previously to this therapy. In a 1:2:2 ratio, patients were randomized to single rituximab injections of 1,000 mg, 500 mg, or 200 mg. Outcome then were compared at the end of 6 months.
Noninferiority was defined as 0.5 difference in DAS28-CRP score (disease activity score using C-reactive protein instead of erythrocyte sedimentation rate) score adjusted for baseline disease status and use of conventional disease-modifying antirheumatic drugs (DMARDs).
Although noninferior at 3 months, the 500 mg dose did not meet the noninferiority criteria at 6 months. Due to a hierarchical design, evaluation of the 200 mg dose was precluded by the negative result with the 500 mg dose.
However, the majority of patients did respond to both the 500 mg and 200 mg dose. The failure to meet noninferiority was due to a limited number of patients who required rescue therapy for a flare. As a result, the investigators believe a trial of ultra low-dose therapy still might be reasonable.
In this interview, Ms. Verhoef explains that at her center patients who are well controlled on a 1,000 mg dose of rituximab now are being offered a 500 mg dose for retreatment. If they continue to respond, further retreatment with a 200 mg dose is considered.
Ms. Verhoef had no relevant financial disclosures.
MADRID – Retreatment of rheumatoid arthritis (RA) with an ultra low-dose of rituximab failed to meet the predefined noninferiority endpoint relative to a higher dose in a double-blind randomized trial, but the investigators still think this strategy may be viable in selected patients.
Lise M. Verhoef, MSc, a researcher in rheumatology at the Sint Maartenskliniek in Nijmegen, the Netherlands, who presented the data as a late-breaker at the European Congress of Rheumatology, explains in a video interview why the negative trial still might support an ultra low-dose strategy.
This trial, called REDO, was conceived after it was observed that most patients with RA are well controlled on a single injection of 1,000 mg of rituximab even though this is half the standard dose of two 1,000 mg doses given 15 days apart. The study was designed to determine whether even lower doses could be used.
The study enrolled 142 patients with RA who were being retreated with rituximab after responding previously to this therapy. In a 1:2:2 ratio, patients were randomized to single rituximab injections of 1,000 mg, 500 mg, or 200 mg. Outcome then were compared at the end of 6 months.
Noninferiority was defined as 0.5 difference in DAS28-CRP score (disease activity score using C-reactive protein instead of erythrocyte sedimentation rate) score adjusted for baseline disease status and use of conventional disease-modifying antirheumatic drugs (DMARDs).
Although noninferior at 3 months, the 500 mg dose did not meet the noninferiority criteria at 6 months. Due to a hierarchical design, evaluation of the 200 mg dose was precluded by the negative result with the 500 mg dose.
However, the majority of patients did respond to both the 500 mg and 200 mg dose. The failure to meet noninferiority was due to a limited number of patients who required rescue therapy for a flare. As a result, the investigators believe a trial of ultra low-dose therapy still might be reasonable.
In this interview, Ms. Verhoef explains that at her center patients who are well controlled on a 1,000 mg dose of rituximab now are being offered a 500 mg dose for retreatment. If they continue to respond, further retreatment with a 200 mg dose is considered.
Ms. Verhoef had no relevant financial disclosures.
REPORTING FROM EULAR 2019 Congress
Systemic sclerosis gastrointestinal symptoms helped by gut microbiota transplant
MADRID – compared with control subjects in a 16-week randomized, double-blind, placebo-controlled pilot study presented at the European Congress of Rheumatology.
The effects were most pronounced on lower GI symptoms, including bloating, diarrhea, and fecal incontinence, with improvement reported by three of five of the patients given the gut microbiota transplant, compared with two of the five patients who received placebo.
“We were surprised by the effect the patients reported, as all had longstanding SSc with GI symptoms,” Anna-Maria Hoffmann-Vold, MD, PhD, of Oslo University Hospital, said in an interview ahead of the congress. “We were especially surprised at the strong effect FMT had on fecal incontinence.”“Patients with systemic sclerosis are very prone to having gastrointestinal involvement – up to 90% of patients have GI symptoms, and it’s associated with very high morbidity and mortality,” she observed during her presentation at the Congress. Despite that, there currently are no disease-modifying treatments that specifically addresses GI involvement in SSc.
It’s been known for a while that patients with SSc have a different intestinal microbiota composition, or dysbiosis, compared with healthy controls, and the possibility of permanent modification of the microbiome through fecal microbiota transplant (FMT) from healthy to ill individuals has become a subject of increased attention in the scientific literature in recent years,. Dr. Hoffmann-Vold said.
In particular, FMT has shown promising results in the treatment of Clostridium difficile infections. While the current study did not focus on mechanistic pathways by which FMT might be exerting its effects, such studies are definitely warranted, she said. “One could speculate that there is a mechanistic link between dysmotility and dysbiosis in SSc, and that the manipulation of gut microbiota with FMT primarily affects motility patterns, which in turn leads to improvement of GI symptoms.”
Together with colleagues at the Oslo University Hospital, Dr. Hoffmann-Vold randomly assigned 10 patients – all women – with limited cutaneous SSc either to treatment with a commercially-available gut microbiota preparation known as anaerobic cultivated human intestinal microbiota (ACHIM) or to placebo. Both ACHIM and placebo were given via gastroduodenoscopy. Their aim was to determine the safety of the approach, as well as to obtain preliminary data on its therapeutic potential.
The UCLA GIT 2.0 score questionnaire was used to assess GI symptoms, with patients defined as responders if they met the questionnaire’s definition of a minimally clinically important difference.
Primary endpoints were safety and clinical efficacy on GI symptoms assessed at weeks 4 and 16, and safety was assessed by observation, interviews, and a standardized safety form.
Results showed improvement in GI symptoms (total UCLA GIT score) in three of the five patients who received the gut microbiota transplant versus two of the five placebo-treated patients at 16 weeks. Two patients in the active treatment versus one in the placebo group had unchanged symptoms, and one patient in the placebo group had worsening symptoms.
Adverse events associated with treatment were “transient and mild”. However, one procedure-related serious adverse event occurred in a placebo-treated patient, which was a duodenal perforation.
Concluding her presentation, Dr. Hoffman-Vold said: “FMT of commercially-available ACHIM in patients with SSc appeared safe, had beneficial effects on lower GI symptoms, altered gut microbiota composition – richness and diversity – and appeared to affect the mucosal immune system.”
The research team has just received national funding for a larger randomized clinical trial that will involve 70 SSc patients and should start towards the end of the year.
The study was sponsored by Helse Sør-øst and NKS. Dr. Hoffmann-Vold has received research funding, consulting fees, or other remuneration from Boehringer Ingelheim, GlaxoSmithKline, and Actelion. A coauthor is the owner of the company that provided the gut microbiota.
SOURCE: Hoffmann-Vold AM et al., Ann Rheum Dis. 2019 Jun. doi: 10.1136/annrheumdis-2019-eular.4684 .
MADRID – compared with control subjects in a 16-week randomized, double-blind, placebo-controlled pilot study presented at the European Congress of Rheumatology.
The effects were most pronounced on lower GI symptoms, including bloating, diarrhea, and fecal incontinence, with improvement reported by three of five of the patients given the gut microbiota transplant, compared with two of the five patients who received placebo.
“We were surprised by the effect the patients reported, as all had longstanding SSc with GI symptoms,” Anna-Maria Hoffmann-Vold, MD, PhD, of Oslo University Hospital, said in an interview ahead of the congress. “We were especially surprised at the strong effect FMT had on fecal incontinence.”“Patients with systemic sclerosis are very prone to having gastrointestinal involvement – up to 90% of patients have GI symptoms, and it’s associated with very high morbidity and mortality,” she observed during her presentation at the Congress. Despite that, there currently are no disease-modifying treatments that specifically addresses GI involvement in SSc.
It’s been known for a while that patients with SSc have a different intestinal microbiota composition, or dysbiosis, compared with healthy controls, and the possibility of permanent modification of the microbiome through fecal microbiota transplant (FMT) from healthy to ill individuals has become a subject of increased attention in the scientific literature in recent years,. Dr. Hoffmann-Vold said.
In particular, FMT has shown promising results in the treatment of Clostridium difficile infections. While the current study did not focus on mechanistic pathways by which FMT might be exerting its effects, such studies are definitely warranted, she said. “One could speculate that there is a mechanistic link between dysmotility and dysbiosis in SSc, and that the manipulation of gut microbiota with FMT primarily affects motility patterns, which in turn leads to improvement of GI symptoms.”
Together with colleagues at the Oslo University Hospital, Dr. Hoffmann-Vold randomly assigned 10 patients – all women – with limited cutaneous SSc either to treatment with a commercially-available gut microbiota preparation known as anaerobic cultivated human intestinal microbiota (ACHIM) or to placebo. Both ACHIM and placebo were given via gastroduodenoscopy. Their aim was to determine the safety of the approach, as well as to obtain preliminary data on its therapeutic potential.
The UCLA GIT 2.0 score questionnaire was used to assess GI symptoms, with patients defined as responders if they met the questionnaire’s definition of a minimally clinically important difference.
Primary endpoints were safety and clinical efficacy on GI symptoms assessed at weeks 4 and 16, and safety was assessed by observation, interviews, and a standardized safety form.
Results showed improvement in GI symptoms (total UCLA GIT score) in three of the five patients who received the gut microbiota transplant versus two of the five placebo-treated patients at 16 weeks. Two patients in the active treatment versus one in the placebo group had unchanged symptoms, and one patient in the placebo group had worsening symptoms.
Adverse events associated with treatment were “transient and mild”. However, one procedure-related serious adverse event occurred in a placebo-treated patient, which was a duodenal perforation.
Concluding her presentation, Dr. Hoffman-Vold said: “FMT of commercially-available ACHIM in patients with SSc appeared safe, had beneficial effects on lower GI symptoms, altered gut microbiota composition – richness and diversity – and appeared to affect the mucosal immune system.”
The research team has just received national funding for a larger randomized clinical trial that will involve 70 SSc patients and should start towards the end of the year.
The study was sponsored by Helse Sør-øst and NKS. Dr. Hoffmann-Vold has received research funding, consulting fees, or other remuneration from Boehringer Ingelheim, GlaxoSmithKline, and Actelion. A coauthor is the owner of the company that provided the gut microbiota.
SOURCE: Hoffmann-Vold AM et al., Ann Rheum Dis. 2019 Jun. doi: 10.1136/annrheumdis-2019-eular.4684 .
MADRID – compared with control subjects in a 16-week randomized, double-blind, placebo-controlled pilot study presented at the European Congress of Rheumatology.
The effects were most pronounced on lower GI symptoms, including bloating, diarrhea, and fecal incontinence, with improvement reported by three of five of the patients given the gut microbiota transplant, compared with two of the five patients who received placebo.
“We were surprised by the effect the patients reported, as all had longstanding SSc with GI symptoms,” Anna-Maria Hoffmann-Vold, MD, PhD, of Oslo University Hospital, said in an interview ahead of the congress. “We were especially surprised at the strong effect FMT had on fecal incontinence.”“Patients with systemic sclerosis are very prone to having gastrointestinal involvement – up to 90% of patients have GI symptoms, and it’s associated with very high morbidity and mortality,” she observed during her presentation at the Congress. Despite that, there currently are no disease-modifying treatments that specifically addresses GI involvement in SSc.
It’s been known for a while that patients with SSc have a different intestinal microbiota composition, or dysbiosis, compared with healthy controls, and the possibility of permanent modification of the microbiome through fecal microbiota transplant (FMT) from healthy to ill individuals has become a subject of increased attention in the scientific literature in recent years,. Dr. Hoffmann-Vold said.
In particular, FMT has shown promising results in the treatment of Clostridium difficile infections. While the current study did not focus on mechanistic pathways by which FMT might be exerting its effects, such studies are definitely warranted, she said. “One could speculate that there is a mechanistic link between dysmotility and dysbiosis in SSc, and that the manipulation of gut microbiota with FMT primarily affects motility patterns, which in turn leads to improvement of GI symptoms.”
Together with colleagues at the Oslo University Hospital, Dr. Hoffmann-Vold randomly assigned 10 patients – all women – with limited cutaneous SSc either to treatment with a commercially-available gut microbiota preparation known as anaerobic cultivated human intestinal microbiota (ACHIM) or to placebo. Both ACHIM and placebo were given via gastroduodenoscopy. Their aim was to determine the safety of the approach, as well as to obtain preliminary data on its therapeutic potential.
The UCLA GIT 2.0 score questionnaire was used to assess GI symptoms, with patients defined as responders if they met the questionnaire’s definition of a minimally clinically important difference.
Primary endpoints were safety and clinical efficacy on GI symptoms assessed at weeks 4 and 16, and safety was assessed by observation, interviews, and a standardized safety form.
Results showed improvement in GI symptoms (total UCLA GIT score) in three of the five patients who received the gut microbiota transplant versus two of the five placebo-treated patients at 16 weeks. Two patients in the active treatment versus one in the placebo group had unchanged symptoms, and one patient in the placebo group had worsening symptoms.
Adverse events associated with treatment were “transient and mild”. However, one procedure-related serious adverse event occurred in a placebo-treated patient, which was a duodenal perforation.
Concluding her presentation, Dr. Hoffman-Vold said: “FMT of commercially-available ACHIM in patients with SSc appeared safe, had beneficial effects on lower GI symptoms, altered gut microbiota composition – richness and diversity – and appeared to affect the mucosal immune system.”
The research team has just received national funding for a larger randomized clinical trial that will involve 70 SSc patients and should start towards the end of the year.
The study was sponsored by Helse Sør-øst and NKS. Dr. Hoffmann-Vold has received research funding, consulting fees, or other remuneration from Boehringer Ingelheim, GlaxoSmithKline, and Actelion. A coauthor is the owner of the company that provided the gut microbiota.
SOURCE: Hoffmann-Vold AM et al., Ann Rheum Dis. 2019 Jun. doi: 10.1136/annrheumdis-2019-eular.4684 .
REPORTING FROM THE EULAR 2019 CONGRESS
Retention rates comparable for biosimilars, original drug in spondyloarthritis
MADRID – judging from data drawn from registries in five Scandinavian countries in a study that evaluated retention rates after 1 year of therapy.
Bente Glintborg, MD, PhD, from the Center for Rheumatology and Spine Diseases, Rigshospitalet, Glostrup, Denmark, explains in a video interview that the indication provided to biosimilars for spondyloarthritis was extended from comparisons conducted in rheumatoid arthritis (RA).
In the absence of a randomized trial in spondyloarthritis, she suggested that this comparison might be the best opportunity to evaluate whether biosimilars perform as well as their biologic originator. This is an important aim based on the theoretical possibility that equivalence in RA does not translate into equivalence in other rheumatic conditions where biologics are indicated.
As she explains, 1,015 biologic-naïve patients initiating etanercept, a tumor necrosis factor (TNF) inhibitor, or a biosimilar were assessed at baseline and at the end of 1 year of therapy. The patients were enrolled in biologic registries maintained in Denmark, Finland, Iceland, Norway, or Sweden.
Retention rates at 1 year were numerically lower on etanercept than the biosimilars, but the difference was not significant (66% vs. 73%; P = 0.18). There also were no significant differences between the biosimilars and etanercept when disease activity was compared at 6 months.
Retention rates are a reasonable surrogate for both efficacy and tolerability based on the expectation that more patients would switch or discontinue agents in the event of lack of efficacy or unacceptable side effects, Dr. Glintborg said at the European Congress of Rheumatology.
In this interview, she notes that a similar study from the Nordic registries led by a coinvestigator also showed equivalent retention rates among spondyloarthritis patients when biosimilars and infliximab were compared at 2 years.
Dr. Glintborg received research support from Biogen, Pfizer, and Abbievie.
MADRID – judging from data drawn from registries in five Scandinavian countries in a study that evaluated retention rates after 1 year of therapy.
Bente Glintborg, MD, PhD, from the Center for Rheumatology and Spine Diseases, Rigshospitalet, Glostrup, Denmark, explains in a video interview that the indication provided to biosimilars for spondyloarthritis was extended from comparisons conducted in rheumatoid arthritis (RA).
In the absence of a randomized trial in spondyloarthritis, she suggested that this comparison might be the best opportunity to evaluate whether biosimilars perform as well as their biologic originator. This is an important aim based on the theoretical possibility that equivalence in RA does not translate into equivalence in other rheumatic conditions where biologics are indicated.
As she explains, 1,015 biologic-naïve patients initiating etanercept, a tumor necrosis factor (TNF) inhibitor, or a biosimilar were assessed at baseline and at the end of 1 year of therapy. The patients were enrolled in biologic registries maintained in Denmark, Finland, Iceland, Norway, or Sweden.
Retention rates at 1 year were numerically lower on etanercept than the biosimilars, but the difference was not significant (66% vs. 73%; P = 0.18). There also were no significant differences between the biosimilars and etanercept when disease activity was compared at 6 months.
Retention rates are a reasonable surrogate for both efficacy and tolerability based on the expectation that more patients would switch or discontinue agents in the event of lack of efficacy or unacceptable side effects, Dr. Glintborg said at the European Congress of Rheumatology.
In this interview, she notes that a similar study from the Nordic registries led by a coinvestigator also showed equivalent retention rates among spondyloarthritis patients when biosimilars and infliximab were compared at 2 years.
Dr. Glintborg received research support from Biogen, Pfizer, and Abbievie.
MADRID – judging from data drawn from registries in five Scandinavian countries in a study that evaluated retention rates after 1 year of therapy.
Bente Glintborg, MD, PhD, from the Center for Rheumatology and Spine Diseases, Rigshospitalet, Glostrup, Denmark, explains in a video interview that the indication provided to biosimilars for spondyloarthritis was extended from comparisons conducted in rheumatoid arthritis (RA).
In the absence of a randomized trial in spondyloarthritis, she suggested that this comparison might be the best opportunity to evaluate whether biosimilars perform as well as their biologic originator. This is an important aim based on the theoretical possibility that equivalence in RA does not translate into equivalence in other rheumatic conditions where biologics are indicated.
As she explains, 1,015 biologic-naïve patients initiating etanercept, a tumor necrosis factor (TNF) inhibitor, or a biosimilar were assessed at baseline and at the end of 1 year of therapy. The patients were enrolled in biologic registries maintained in Denmark, Finland, Iceland, Norway, or Sweden.
Retention rates at 1 year were numerically lower on etanercept than the biosimilars, but the difference was not significant (66% vs. 73%; P = 0.18). There also were no significant differences between the biosimilars and etanercept when disease activity was compared at 6 months.
Retention rates are a reasonable surrogate for both efficacy and tolerability based on the expectation that more patients would switch or discontinue agents in the event of lack of efficacy or unacceptable side effects, Dr. Glintborg said at the European Congress of Rheumatology.
In this interview, she notes that a similar study from the Nordic registries led by a coinvestigator also showed equivalent retention rates among spondyloarthritis patients when biosimilars and infliximab were compared at 2 years.
Dr. Glintborg received research support from Biogen, Pfizer, and Abbievie.
REPORTING FROM EULAR 2019 Congress
Video program engages patients in treat-to-target concept
MADRID – according to data generated from a randomized trial.
One of the major goals of the video program is to inform patients about the treat-to-target concept of RA management, explained Maria I. Danila, MD, a rheumatologist at the University of Alabama at Birmingham.
Although physicians know this to be a guiding tenet for RA management, she explains in this video interview that 50% or more of patients are unaware of this therapeutic goal.
For patients who resist treatment escalation for fear of side effects, this lack of awareness might provide one explanation for failing to adhere to guideline-recommended therapy, Dr. Danila said at the European Congress of Rheumatology. She believes that patients need more information about the potential for treatment escalation to improve function.
To address this issue, a short video was developed to explain the treat-to-target concept. It was then tested in a randomized trial. Those who viewed the video expressed greater willingness to change intervention on the advice of their rheumatologist relative to those who did not (P = 0.01).
Further studies are planned, including studies to test whether willingness to escalate treatment results in better outcomes and whether linking patient behavioral goals such as being able to play golf again will enhance treatment adherence. Dr. Danila envisions wide distribution of this video if further studies demonstrate that it helps patients cooperate with treatment escalation when needed.
Dr. Danila received research support from Pfizer.
MADRID – according to data generated from a randomized trial.
One of the major goals of the video program is to inform patients about the treat-to-target concept of RA management, explained Maria I. Danila, MD, a rheumatologist at the University of Alabama at Birmingham.
Although physicians know this to be a guiding tenet for RA management, she explains in this video interview that 50% or more of patients are unaware of this therapeutic goal.
For patients who resist treatment escalation for fear of side effects, this lack of awareness might provide one explanation for failing to adhere to guideline-recommended therapy, Dr. Danila said at the European Congress of Rheumatology. She believes that patients need more information about the potential for treatment escalation to improve function.
To address this issue, a short video was developed to explain the treat-to-target concept. It was then tested in a randomized trial. Those who viewed the video expressed greater willingness to change intervention on the advice of their rheumatologist relative to those who did not (P = 0.01).
Further studies are planned, including studies to test whether willingness to escalate treatment results in better outcomes and whether linking patient behavioral goals such as being able to play golf again will enhance treatment adherence. Dr. Danila envisions wide distribution of this video if further studies demonstrate that it helps patients cooperate with treatment escalation when needed.
Dr. Danila received research support from Pfizer.
MADRID – according to data generated from a randomized trial.
One of the major goals of the video program is to inform patients about the treat-to-target concept of RA management, explained Maria I. Danila, MD, a rheumatologist at the University of Alabama at Birmingham.
Although physicians know this to be a guiding tenet for RA management, she explains in this video interview that 50% or more of patients are unaware of this therapeutic goal.
For patients who resist treatment escalation for fear of side effects, this lack of awareness might provide one explanation for failing to adhere to guideline-recommended therapy, Dr. Danila said at the European Congress of Rheumatology. She believes that patients need more information about the potential for treatment escalation to improve function.
To address this issue, a short video was developed to explain the treat-to-target concept. It was then tested in a randomized trial. Those who viewed the video expressed greater willingness to change intervention on the advice of their rheumatologist relative to those who did not (P = 0.01).
Further studies are planned, including studies to test whether willingness to escalate treatment results in better outcomes and whether linking patient behavioral goals such as being able to play golf again will enhance treatment adherence. Dr. Danila envisions wide distribution of this video if further studies demonstrate that it helps patients cooperate with treatment escalation when needed.
Dr. Danila received research support from Pfizer.
REPORTING FROM EULAR 2019 Congress