User login
Study: High rate of medical errors in postop drug administrations
Medication errors or adverse drug events after surgery occur in as many as one in twenty perioperative medication administrations, according to data published online in the journal Anesthesiology.
A prospective observational study of 277 surgical operations and 3,671 medication administrations found 193 cases (5.3%) involved a medication error or adverse drug event, nearly four-fifths (79.3%) of which were preventable and 68.9% of which were serious (Anesthesiology. 2015 Oct. doi:10.1097/ALN.0000000000000904).
Among the 51 medication errors that led to adverse reactions, nearly half were the result of inappropriate medication doses and 31.4% were due to omitted medications or failure to act, but the most common overall error type was a labeling error.
The medications most commonly associated with errors were propofol, phenylephrine, and fentanyl, and operations greater than 6 hours in duration or with 13 or more medication administrations were associated with a significantly greater risk of errors.
“Examples of technology-based interventions [to minimize perioperative MEs and/or ADEs] include bar code–assisted syringe labeling systems, point-of-care bar code–assisted anesthesia documentation systems, specific drug decision support, and alerts,” wrote the study’s lead author Dr. Karen C. Nanji of Massachusetts General Hospital in Boston, and her coauthors.
The study was supported by the Doctors Company Foundation and the National Institute of General Medical Sciences of the National Institutes of Health. One coauthor – Dr. David Bates – declared financial interests in medical decision support software, as well as funding and positions with a variety of medical technology companies.
Medication errors or adverse drug events after surgery occur in as many as one in twenty perioperative medication administrations, according to data published online in the journal Anesthesiology.
A prospective observational study of 277 surgical operations and 3,671 medication administrations found 193 cases (5.3%) involved a medication error or adverse drug event, nearly four-fifths (79.3%) of which were preventable and 68.9% of which were serious (Anesthesiology. 2015 Oct. doi:10.1097/ALN.0000000000000904).
Among the 51 medication errors that led to adverse reactions, nearly half were the result of inappropriate medication doses and 31.4% were due to omitted medications or failure to act, but the most common overall error type was a labeling error.
The medications most commonly associated with errors were propofol, phenylephrine, and fentanyl, and operations greater than 6 hours in duration or with 13 or more medication administrations were associated with a significantly greater risk of errors.
“Examples of technology-based interventions [to minimize perioperative MEs and/or ADEs] include bar code–assisted syringe labeling systems, point-of-care bar code–assisted anesthesia documentation systems, specific drug decision support, and alerts,” wrote the study’s lead author Dr. Karen C. Nanji of Massachusetts General Hospital in Boston, and her coauthors.
The study was supported by the Doctors Company Foundation and the National Institute of General Medical Sciences of the National Institutes of Health. One coauthor – Dr. David Bates – declared financial interests in medical decision support software, as well as funding and positions with a variety of medical technology companies.
Medication errors or adverse drug events after surgery occur in as many as one in twenty perioperative medication administrations, according to data published online in the journal Anesthesiology.
A prospective observational study of 277 surgical operations and 3,671 medication administrations found 193 cases (5.3%) involved a medication error or adverse drug event, nearly four-fifths (79.3%) of which were preventable and 68.9% of which were serious (Anesthesiology. 2015 Oct. doi:10.1097/ALN.0000000000000904).
Among the 51 medication errors that led to adverse reactions, nearly half were the result of inappropriate medication doses and 31.4% were due to omitted medications or failure to act, but the most common overall error type was a labeling error.
The medications most commonly associated with errors were propofol, phenylephrine, and fentanyl, and operations greater than 6 hours in duration or with 13 or more medication administrations were associated with a significantly greater risk of errors.
“Examples of technology-based interventions [to minimize perioperative MEs and/or ADEs] include bar code–assisted syringe labeling systems, point-of-care bar code–assisted anesthesia documentation systems, specific drug decision support, and alerts,” wrote the study’s lead author Dr. Karen C. Nanji of Massachusetts General Hospital in Boston, and her coauthors.
The study was supported by the Doctors Company Foundation and the National Institute of General Medical Sciences of the National Institutes of Health. One coauthor – Dr. David Bates – declared financial interests in medical decision support software, as well as funding and positions with a variety of medical technology companies.
FROM ANESTHESIOLOGY
Key clinical point: One in twenty perioperative medication administrations may involve a medication error and/or adverse drug event.
Major finding: Nearly four-fifths of perioperative medication errors or adverse events are preventable.
Data source: A prospective observational study of 277 surgical operations and 3,671 medication administrations.
Disclosures: The study was supported by the Doctors Company Foundation and the National Institute of General Medical Sciences of the National Institutes of Health. One coauthor – Dr. David Bates – declared financial interests in medical decision support software, as well as funding and positions with a variety of medical technology companies.
Simultaneous equal to sequential treatment for actinic keratoses
Patients with multiple actinic keratoses (AKs) may be treated either sequentially or simultaneously with ingenol mebutate gel, according to the authors of a study that found no difference in outcomes or adverse effects from either treatment approach.
The phase IIIb study conducted in Italy and Spain enrolled 199 patients with two separate areas of clinically visible, nonhyperkeratotic AKs. Subjects were randomized to have the two areas (face/scalp and trunk/extremities) treated simultaneously (101 patients) or sequentially (98 patients) with 0.015% and 0.05% ingenol mebutate gel.
There were no significant differences in localized skin responses between the simultaneous and sequential treatment groups, based on the mean composite local skin response scores 3 days after treatment started, which were similar between the groups for the face/scalp and trunk/extremities applications. About 22% of patients in each group experienced adverse events.
At 8 weeks, the complete clearance rates also were not statistically different between the simultaneous and sequential groups (52.7% and 46.9%, respectively; P = .34), and patient satisfaction with treatment was similar for both treatment approaches. At that time, the number of AKs had dropped by a mean of 83.4% among those in the simultaneous group and 79.1% in the sequential group (P = .20).
“The favorable rate of complete clearance in the simultaneous treatment group means that patients can receive their treatment for both areas in one visit, rather than having to return to the clinic for a second cycle of treatment,” wrote Dr. Giovanni Pellacani of the department of dermatology at the University of Modena (Italy) and Reggio Emilia, and his coauthors (J Eur Acad Dermatol Venereol. 2015;29[11]:2192-8).
“Ultimately, the treatment schedule is based on agreement between the physician and the patient; this study helps to support the selection of the most appropriate regimen to treat AK in individual patients,” they commented.
The study was funded by LEO Pharma, the manufacturer of ingenol mebutate gel (Picato). Dr. Pellacani has received consultant fees from the company; three authors are employees of the company; and the other authors declared consultancies, honoraria and, grants from LEO Pharma and other pharmaceutical companies.
Patients with multiple actinic keratoses (AKs) may be treated either sequentially or simultaneously with ingenol mebutate gel, according to the authors of a study that found no difference in outcomes or adverse effects from either treatment approach.
The phase IIIb study conducted in Italy and Spain enrolled 199 patients with two separate areas of clinically visible, nonhyperkeratotic AKs. Subjects were randomized to have the two areas (face/scalp and trunk/extremities) treated simultaneously (101 patients) or sequentially (98 patients) with 0.015% and 0.05% ingenol mebutate gel.
There were no significant differences in localized skin responses between the simultaneous and sequential treatment groups, based on the mean composite local skin response scores 3 days after treatment started, which were similar between the groups for the face/scalp and trunk/extremities applications. About 22% of patients in each group experienced adverse events.
At 8 weeks, the complete clearance rates also were not statistically different between the simultaneous and sequential groups (52.7% and 46.9%, respectively; P = .34), and patient satisfaction with treatment was similar for both treatment approaches. At that time, the number of AKs had dropped by a mean of 83.4% among those in the simultaneous group and 79.1% in the sequential group (P = .20).
“The favorable rate of complete clearance in the simultaneous treatment group means that patients can receive their treatment for both areas in one visit, rather than having to return to the clinic for a second cycle of treatment,” wrote Dr. Giovanni Pellacani of the department of dermatology at the University of Modena (Italy) and Reggio Emilia, and his coauthors (J Eur Acad Dermatol Venereol. 2015;29[11]:2192-8).
“Ultimately, the treatment schedule is based on agreement between the physician and the patient; this study helps to support the selection of the most appropriate regimen to treat AK in individual patients,” they commented.
The study was funded by LEO Pharma, the manufacturer of ingenol mebutate gel (Picato). Dr. Pellacani has received consultant fees from the company; three authors are employees of the company; and the other authors declared consultancies, honoraria and, grants from LEO Pharma and other pharmaceutical companies.
Patients with multiple actinic keratoses (AKs) may be treated either sequentially or simultaneously with ingenol mebutate gel, according to the authors of a study that found no difference in outcomes or adverse effects from either treatment approach.
The phase IIIb study conducted in Italy and Spain enrolled 199 patients with two separate areas of clinically visible, nonhyperkeratotic AKs. Subjects were randomized to have the two areas (face/scalp and trunk/extremities) treated simultaneously (101 patients) or sequentially (98 patients) with 0.015% and 0.05% ingenol mebutate gel.
There were no significant differences in localized skin responses between the simultaneous and sequential treatment groups, based on the mean composite local skin response scores 3 days after treatment started, which were similar between the groups for the face/scalp and trunk/extremities applications. About 22% of patients in each group experienced adverse events.
At 8 weeks, the complete clearance rates also were not statistically different between the simultaneous and sequential groups (52.7% and 46.9%, respectively; P = .34), and patient satisfaction with treatment was similar for both treatment approaches. At that time, the number of AKs had dropped by a mean of 83.4% among those in the simultaneous group and 79.1% in the sequential group (P = .20).
“The favorable rate of complete clearance in the simultaneous treatment group means that patients can receive their treatment for both areas in one visit, rather than having to return to the clinic for a second cycle of treatment,” wrote Dr. Giovanni Pellacani of the department of dermatology at the University of Modena (Italy) and Reggio Emilia, and his coauthors (J Eur Acad Dermatol Venereol. 2015;29[11]:2192-8).
“Ultimately, the treatment schedule is based on agreement between the physician and the patient; this study helps to support the selection of the most appropriate regimen to treat AK in individual patients,” they commented.
The study was funded by LEO Pharma, the manufacturer of ingenol mebutate gel (Picato). Dr. Pellacani has received consultant fees from the company; three authors are employees of the company; and the other authors declared consultancies, honoraria and, grants from LEO Pharma and other pharmaceutical companies.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: Patients with multiple AKs may be treated either sequentially or simultaneously with ingenol mebutate gel, with similar efficacy and safety outcomes.
Major finding: The incidence of localized skin responses, complete clearance rates, and patient treatment satisfaction were similar for simultaneous and sequential treatment approaches.
Data source: A phase IIIb randomized, multicenter, open-label, parallel-group study evaluated 199 patients with two separate areas of clinically visible, nonhyperkeratotic AKs.
Disclosures: The study was funded by ingenol mebutate gel manufacturer LEO Pharma. Three authors are employees of the company; the other authors declared consultancies, honoraria, and/or grants from LEO Pharma and other pharmaceutical companies.
Infections from endemic fungi, mycobacteria rare in patients on TNFIs
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: The incidence of select mycobacterial and fungal infections in patients taking TNFIs is low.
Major finding: Only 0.51% of patients taking TNFIs developed the mycobacterial and fungal infections targeted in this study.
Data source: A case-control study of 30,772 patients taking TNFIs.
Disclosures: The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
Serious infection risk rose with opioid use in rheumatoid arthritis
Patients taking opioids for rheumatoid arthritis are at significantly higher risk of serious infection, particularly those taking long-acting formulations or opioids known to have immunosuppressive properties, new data suggest.
While it has been known for some time that opioids have wide-ranging adverse effects, this is the first convincing evidence of an increased risk of infection associated with opioid use in patients with rheumatoid arthritis, Dr. Samuel Whittle, consultant rheumatologist at the Queen Elizabeth Hospital in Adelaide, Australia, said when asked to comment on the study.
Dr. Whittle, who was not involved in the study, said that these data add to the growing body of evidence that the adverse effects of opioids outweigh the benefits.
“It fits with our general sense these days that opioids really are a poor choice of analgesics in people with rheumatoid arthritis, but people continue to prescribe them because our analgesic armory is not that good,” he said.
Andrew D. Wiese of Vanderbilt University, Nashville, Tenn., and his colleagues analyzed a retrospective cohort of 1,790 patients with rheumatoid arthritis who were enrolled in Tennessee Medicaid during 1995-2009 and experienced at least one hospitalization for serious infection. The patients served as their own controls during periods of opioid nonuse. The rate of infection in these patients was 39% higher when they were taking opioids, compared with periods of nonuse, after adjusting for age, season, nursing home residency, and medication use.
The rate of infection was twice as high when they were using long-acting opioids, compared with nonuse, and 72% higher for the use of immunosuppressive opioids. New opioid use also more than doubled the risk of serious infection in the study (Arthritis Rheumatol. 2015 Oct 16. doi: 10.1002/art.39462)
Researchers also found evidence of a dose-response effect, as patients taking 60 mg or more of morphine equivalent per day had a 73% greater rate of infection than in periods of nonuse, compared with a 24% higher rate in those taking less than 15 mg morphine equivalent per day.
Patients who experienced infections were most likely to get a nonpneumonia infection, while the rates of pneumonia infection were not significantly different between periods of opioid use and nonuse.
To assess whether pain could have been a confounding factor, the researchers looked at nonsteroidal anti-inflammatory medication use as the exposure of interest and found no significant increase in the rate of hospitalizations for serious infections when compared with nonuse.
“The potential association between the risk of infection and opioid use is supported by the literature regarding the immunosuppressive effects of certain opioids from in vitro experiments and animal models, including morphine, methadone, and fentanyl (fentanyl [was] not included in our study, which is restricted to oral formulations),” the investigators wrote.
“Although our study lends support to the idea that opioids might cause further immunosuppression in patients with RA, these patients are already at higher risk of infection and possibly more likely to receive opioid therapy compared to other patient populations. Further studies are needed to determine whether this association exists in other patient populations.”
Another big research challenge, Dr. Whittle said, is “to learn better the mechanisms for persistent pain in people with rheumatoid arthritis, and hopefully through that we might be able to target therapies a little bit better.”
The study was supported by the National Institute on Aging and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. There were no conflicts of interest declared.
Patients taking opioids for rheumatoid arthritis are at significantly higher risk of serious infection, particularly those taking long-acting formulations or opioids known to have immunosuppressive properties, new data suggest.
While it has been known for some time that opioids have wide-ranging adverse effects, this is the first convincing evidence of an increased risk of infection associated with opioid use in patients with rheumatoid arthritis, Dr. Samuel Whittle, consultant rheumatologist at the Queen Elizabeth Hospital in Adelaide, Australia, said when asked to comment on the study.
Dr. Whittle, who was not involved in the study, said that these data add to the growing body of evidence that the adverse effects of opioids outweigh the benefits.
“It fits with our general sense these days that opioids really are a poor choice of analgesics in people with rheumatoid arthritis, but people continue to prescribe them because our analgesic armory is not that good,” he said.
Andrew D. Wiese of Vanderbilt University, Nashville, Tenn., and his colleagues analyzed a retrospective cohort of 1,790 patients with rheumatoid arthritis who were enrolled in Tennessee Medicaid during 1995-2009 and experienced at least one hospitalization for serious infection. The patients served as their own controls during periods of opioid nonuse. The rate of infection in these patients was 39% higher when they were taking opioids, compared with periods of nonuse, after adjusting for age, season, nursing home residency, and medication use.
The rate of infection was twice as high when they were using long-acting opioids, compared with nonuse, and 72% higher for the use of immunosuppressive opioids. New opioid use also more than doubled the risk of serious infection in the study (Arthritis Rheumatol. 2015 Oct 16. doi: 10.1002/art.39462)
Researchers also found evidence of a dose-response effect, as patients taking 60 mg or more of morphine equivalent per day had a 73% greater rate of infection than in periods of nonuse, compared with a 24% higher rate in those taking less than 15 mg morphine equivalent per day.
Patients who experienced infections were most likely to get a nonpneumonia infection, while the rates of pneumonia infection were not significantly different between periods of opioid use and nonuse.
To assess whether pain could have been a confounding factor, the researchers looked at nonsteroidal anti-inflammatory medication use as the exposure of interest and found no significant increase in the rate of hospitalizations for serious infections when compared with nonuse.
“The potential association between the risk of infection and opioid use is supported by the literature regarding the immunosuppressive effects of certain opioids from in vitro experiments and animal models, including morphine, methadone, and fentanyl (fentanyl [was] not included in our study, which is restricted to oral formulations),” the investigators wrote.
“Although our study lends support to the idea that opioids might cause further immunosuppression in patients with RA, these patients are already at higher risk of infection and possibly more likely to receive opioid therapy compared to other patient populations. Further studies are needed to determine whether this association exists in other patient populations.”
Another big research challenge, Dr. Whittle said, is “to learn better the mechanisms for persistent pain in people with rheumatoid arthritis, and hopefully through that we might be able to target therapies a little bit better.”
The study was supported by the National Institute on Aging and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. There were no conflicts of interest declared.
Patients taking opioids for rheumatoid arthritis are at significantly higher risk of serious infection, particularly those taking long-acting formulations or opioids known to have immunosuppressive properties, new data suggest.
While it has been known for some time that opioids have wide-ranging adverse effects, this is the first convincing evidence of an increased risk of infection associated with opioid use in patients with rheumatoid arthritis, Dr. Samuel Whittle, consultant rheumatologist at the Queen Elizabeth Hospital in Adelaide, Australia, said when asked to comment on the study.
Dr. Whittle, who was not involved in the study, said that these data add to the growing body of evidence that the adverse effects of opioids outweigh the benefits.
“It fits with our general sense these days that opioids really are a poor choice of analgesics in people with rheumatoid arthritis, but people continue to prescribe them because our analgesic armory is not that good,” he said.
Andrew D. Wiese of Vanderbilt University, Nashville, Tenn., and his colleagues analyzed a retrospective cohort of 1,790 patients with rheumatoid arthritis who were enrolled in Tennessee Medicaid during 1995-2009 and experienced at least one hospitalization for serious infection. The patients served as their own controls during periods of opioid nonuse. The rate of infection in these patients was 39% higher when they were taking opioids, compared with periods of nonuse, after adjusting for age, season, nursing home residency, and medication use.
The rate of infection was twice as high when they were using long-acting opioids, compared with nonuse, and 72% higher for the use of immunosuppressive opioids. New opioid use also more than doubled the risk of serious infection in the study (Arthritis Rheumatol. 2015 Oct 16. doi: 10.1002/art.39462)
Researchers also found evidence of a dose-response effect, as patients taking 60 mg or more of morphine equivalent per day had a 73% greater rate of infection than in periods of nonuse, compared with a 24% higher rate in those taking less than 15 mg morphine equivalent per day.
Patients who experienced infections were most likely to get a nonpneumonia infection, while the rates of pneumonia infection were not significantly different between periods of opioid use and nonuse.
To assess whether pain could have been a confounding factor, the researchers looked at nonsteroidal anti-inflammatory medication use as the exposure of interest and found no significant increase in the rate of hospitalizations for serious infections when compared with nonuse.
“The potential association between the risk of infection and opioid use is supported by the literature regarding the immunosuppressive effects of certain opioids from in vitro experiments and animal models, including morphine, methadone, and fentanyl (fentanyl [was] not included in our study, which is restricted to oral formulations),” the investigators wrote.
“Although our study lends support to the idea that opioids might cause further immunosuppression in patients with RA, these patients are already at higher risk of infection and possibly more likely to receive opioid therapy compared to other patient populations. Further studies are needed to determine whether this association exists in other patient populations.”
Another big research challenge, Dr. Whittle said, is “to learn better the mechanisms for persistent pain in people with rheumatoid arthritis, and hopefully through that we might be able to target therapies a little bit better.”
The study was supported by the National Institute on Aging and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. There were no conflicts of interest declared.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:This is the first convincing evidence of an increased risk of infection associated with opioid use in patients with rheumatoid arthritis.
Major finding: The rate of infection in patients currently taking opioids was 39% higher than during periods of nonuse.
Data source: A self-controlled case series study of a retrospective cohort of 1,790 patients with rheumatoid arthritis.
Disclosures: The study was supported by the National Institute on Aging and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. There were no conflicts of interest declared.
Botox suppresses atrial fibrillation after CABG
Injections of botulinum toxin into epicardial fat pads during cardiac surgery may provide long-term suppression of atrial fibrillation, according to results from a randomized, placebo-controlled trial published Oct. 20.
Sixty patients with a history of atrial fibrillation, who were undergoing coronary artery bypass graft surgery, were randomized to an injection of botulinum toxin or saline into each epicardial fat pad during surgery.
Data from 12 months of monitoring via an implantable loop recorder showed patients who received the botulinum toxin injections had a significantly reduced incidence of atrial fibrillation, compared with the placebo group in the 30 days after surgery (7% vs. 30%, P = .024) and at 12 months (0% vs. 27%, P = .002).
Both groups showed significant decreases in heart rate variability after surgery, but at 3 months, these had largely recovered in the placebo group and remained depressed in the botulinum toxin group until 6 months (Circ Arrhythm Electrophysiol. 2015 Oct 20. doi: 10.1161/CIRCEP.115.003199).
No patients in the botulinum group developed persistent atrial fibrillation or required antiarrhythmic therapy or interventions, and there were no significant differences between the two groups in other outcomes such as hospital length of stay or postoperative complications.
“The blocking effects are temporary and recover in 1 to 6 months, depending on the injection site [but] for patients with a high short-term risk of postoperative AF after cardiac surgery, temporary suppression of AF without any destruction of the anatomic structures is clinically desirable,” wrote Dr. Evgeny Pokushalov of State Research Institute of Circulation Pathology, Novosibirsk, Russia, and coauthors.
No conflicts of interest were declared.
Injections of botulinum toxin into epicardial fat pads during cardiac surgery may provide long-term suppression of atrial fibrillation, according to results from a randomized, placebo-controlled trial published Oct. 20.
Sixty patients with a history of atrial fibrillation, who were undergoing coronary artery bypass graft surgery, were randomized to an injection of botulinum toxin or saline into each epicardial fat pad during surgery.
Data from 12 months of monitoring via an implantable loop recorder showed patients who received the botulinum toxin injections had a significantly reduced incidence of atrial fibrillation, compared with the placebo group in the 30 days after surgery (7% vs. 30%, P = .024) and at 12 months (0% vs. 27%, P = .002).
Both groups showed significant decreases in heart rate variability after surgery, but at 3 months, these had largely recovered in the placebo group and remained depressed in the botulinum toxin group until 6 months (Circ Arrhythm Electrophysiol. 2015 Oct 20. doi: 10.1161/CIRCEP.115.003199).
No patients in the botulinum group developed persistent atrial fibrillation or required antiarrhythmic therapy or interventions, and there were no significant differences between the two groups in other outcomes such as hospital length of stay or postoperative complications.
“The blocking effects are temporary and recover in 1 to 6 months, depending on the injection site [but] for patients with a high short-term risk of postoperative AF after cardiac surgery, temporary suppression of AF without any destruction of the anatomic structures is clinically desirable,” wrote Dr. Evgeny Pokushalov of State Research Institute of Circulation Pathology, Novosibirsk, Russia, and coauthors.
No conflicts of interest were declared.
Injections of botulinum toxin into epicardial fat pads during cardiac surgery may provide long-term suppression of atrial fibrillation, according to results from a randomized, placebo-controlled trial published Oct. 20.
Sixty patients with a history of atrial fibrillation, who were undergoing coronary artery bypass graft surgery, were randomized to an injection of botulinum toxin or saline into each epicardial fat pad during surgery.
Data from 12 months of monitoring via an implantable loop recorder showed patients who received the botulinum toxin injections had a significantly reduced incidence of atrial fibrillation, compared with the placebo group in the 30 days after surgery (7% vs. 30%, P = .024) and at 12 months (0% vs. 27%, P = .002).
Both groups showed significant decreases in heart rate variability after surgery, but at 3 months, these had largely recovered in the placebo group and remained depressed in the botulinum toxin group until 6 months (Circ Arrhythm Electrophysiol. 2015 Oct 20. doi: 10.1161/CIRCEP.115.003199).
No patients in the botulinum group developed persistent atrial fibrillation or required antiarrhythmic therapy or interventions, and there were no significant differences between the two groups in other outcomes such as hospital length of stay or postoperative complications.
“The blocking effects are temporary and recover in 1 to 6 months, depending on the injection site [but] for patients with a high short-term risk of postoperative AF after cardiac surgery, temporary suppression of AF without any destruction of the anatomic structures is clinically desirable,” wrote Dr. Evgeny Pokushalov of State Research Institute of Circulation Pathology, Novosibirsk, Russia, and coauthors.
No conflicts of interest were declared.
FROM CIRCULATION: ARRHYTHMIA AND ELECTROPHYSIOLOGY
Key clinical point:Injections of botulinum toxin into epicardial fat pads during cardiac surgery may provide long-term suppression of atrial fibrillation.
Major finding: Patients who received the botulinum injections into epicardial fat pads had a significantly reduced incidence of atrial fibrillation (7%), compared with placebo (30%).
Data source: Randomized placebo-controlled trial of 60 patients with a history of atrial fibrillation who were undergoing coronary artery bypass graft surgery.
Disclosures: No conflicts of interest were declared.
Glutamate-based neuroimaging identifies epileptic foci
High-resolution glutamate-based neuroimaging could help identify epileptic foci in individuals with epilepsy who have been assessed as nonlesional via conventional brain MRI, a study has found.
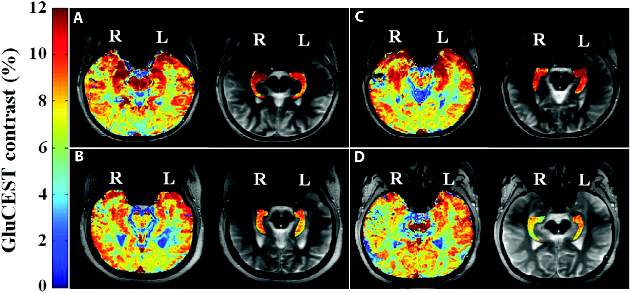
Researchers used glutamate chemical exchange saturation transfer imaging (GluCEST) in four patients with drug-resistant epilepsy who did not show lesions on MRI, and in 11 healthy controls, according to a paper published in Science Translational Medicine.
The glutamate-based imaging showed higher concentrations of glutamate in the ipsilateral hippocampus than in the contralateral hippocampus, and two independent, blinded epilepsy specialists both accurately lateralized the seizure onset in all four patients (Sci Transl Med. 2015 Oct 14. doi: 10.1126/scitranslmed.aaa7095).
Patients with drug-resistant epilepsy currently undergo a range of presurgical imaging, including 3-T MRI and single-photon emission computed tomography (SPECT), but in many patients this still fails to identify a seizure focus, despite the fact that 87% of patients in this group have previously been found to have abnormal histopathology.
“Because it is also well established that patients with lesional epilepsy have better surgical outcomes than those with nonlesional epilepsy, new neuroimaging techniques capable of detecting subtle lesions could potentially improve patient care and increase the chance of seizure freedom after surgery,” wrote Dr. Kathryn Adamiak Davis of Penn Epilepsy Center at the Hospital of the University of Pennsylvania, Philadelphia, and her coauthors.
The National Institutes of Health and the University of Pennsylvania supported the study. There were no conflicts of interest declared.
High-resolution glutamate-based neuroimaging could help identify epileptic foci in individuals with epilepsy who have been assessed as nonlesional via conventional brain MRI, a study has found.
Researchers used glutamate chemical exchange saturation transfer imaging (GluCEST) in four patients with drug-resistant epilepsy who did not show lesions on MRI, and in 11 healthy controls, according to a paper published in Science Translational Medicine.
The glutamate-based imaging showed higher concentrations of glutamate in the ipsilateral hippocampus than in the contralateral hippocampus, and two independent, blinded epilepsy specialists both accurately lateralized the seizure onset in all four patients (Sci Transl Med. 2015 Oct 14. doi: 10.1126/scitranslmed.aaa7095).
Patients with drug-resistant epilepsy currently undergo a range of presurgical imaging, including 3-T MRI and single-photon emission computed tomography (SPECT), but in many patients this still fails to identify a seizure focus, despite the fact that 87% of patients in this group have previously been found to have abnormal histopathology.
“Because it is also well established that patients with lesional epilepsy have better surgical outcomes than those with nonlesional epilepsy, new neuroimaging techniques capable of detecting subtle lesions could potentially improve patient care and increase the chance of seizure freedom after surgery,” wrote Dr. Kathryn Adamiak Davis of Penn Epilepsy Center at the Hospital of the University of Pennsylvania, Philadelphia, and her coauthors.
The National Institutes of Health and the University of Pennsylvania supported the study. There were no conflicts of interest declared.
High-resolution glutamate-based neuroimaging could help identify epileptic foci in individuals with epilepsy who have been assessed as nonlesional via conventional brain MRI, a study has found.
Researchers used glutamate chemical exchange saturation transfer imaging (GluCEST) in four patients with drug-resistant epilepsy who did not show lesions on MRI, and in 11 healthy controls, according to a paper published in Science Translational Medicine.
The glutamate-based imaging showed higher concentrations of glutamate in the ipsilateral hippocampus than in the contralateral hippocampus, and two independent, blinded epilepsy specialists both accurately lateralized the seizure onset in all four patients (Sci Transl Med. 2015 Oct 14. doi: 10.1126/scitranslmed.aaa7095).
Patients with drug-resistant epilepsy currently undergo a range of presurgical imaging, including 3-T MRI and single-photon emission computed tomography (SPECT), but in many patients this still fails to identify a seizure focus, despite the fact that 87% of patients in this group have previously been found to have abnormal histopathology.
“Because it is also well established that patients with lesional epilepsy have better surgical outcomes than those with nonlesional epilepsy, new neuroimaging techniques capable of detecting subtle lesions could potentially improve patient care and increase the chance of seizure freedom after surgery,” wrote Dr. Kathryn Adamiak Davis of Penn Epilepsy Center at the Hospital of the University of Pennsylvania, Philadelphia, and her coauthors.
The National Institutes of Health and the University of Pennsylvania supported the study. There were no conflicts of interest declared.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:Glutamate-based neuroimaging could identify epileptic foci in epilepsy patients who are nonlesional on conventional MRI.
Major finding: Glutamate chemical exchange saturation transfer imaging enables lateralization of seizure onset in previously nonlesional patients.
Data source: Imaging study in four individuals with drug-resistant epilepsy.
Disclosures: The National Institutes of Health and the University of Pennsylvania supported the study. There were no conflicts of interest declared.
Coccidioidomycosis a respiratory threat to construction workers in Southwest
The expansion of the solar energy industry in Coccidioides-endemic areas of the southwestern United States is exposing more workers to the infection, say the authors of a study that found an attack rate of 1.2 cases per 100 workers.
A study among 3,572 workers at two solar power–generating facilities in California identified 44 individuals with the infection between October 2011 and April 2014, 9 of whom were hospitalized, according to a paper published in the Oct. 14 edition of Emerging Infectious Diseases.
The disease is acquired through inhalation of the soil-dwelling Coccidioides fungus spores and while the majority of the patients said they had received safety training about the risk of coccidioidomycosis, only six of those who regularly performed soil-disruptive work reported regularly using respiratory protection (Emerg Infect Dis. 2015 Oct 14; doi: ).
“Large-scale construction, including solar farm construction, might involve substantial soil disturbance for months, and many employees, particularly from non–Coccidioides-endemic areas, probably lack immunity to Coccidioides,” wrote Jason A. Wilken, Ph.D., of the Centers for Disease Control and Prevention, and his coauthors.
“Medical providers should consider work-related coccidioidomycosis when evaluating construction workers with prolonged febrile respiratory illness, particularly after work in Central or Southern California or in Arizona, and medical providers should follow all statutory requirements for documenting and reporting occupational illness,” Dr. Wilken concluded.
The study was supported by the Centers for Disease Control and Prevention. No conflicts of interest were declared.
The expansion of the solar energy industry in Coccidioides-endemic areas of the southwestern United States is exposing more workers to the infection, say the authors of a study that found an attack rate of 1.2 cases per 100 workers.
A study among 3,572 workers at two solar power–generating facilities in California identified 44 individuals with the infection between October 2011 and April 2014, 9 of whom were hospitalized, according to a paper published in the Oct. 14 edition of Emerging Infectious Diseases.
The disease is acquired through inhalation of the soil-dwelling Coccidioides fungus spores and while the majority of the patients said they had received safety training about the risk of coccidioidomycosis, only six of those who regularly performed soil-disruptive work reported regularly using respiratory protection (Emerg Infect Dis. 2015 Oct 14; doi: ).
“Large-scale construction, including solar farm construction, might involve substantial soil disturbance for months, and many employees, particularly from non–Coccidioides-endemic areas, probably lack immunity to Coccidioides,” wrote Jason A. Wilken, Ph.D., of the Centers for Disease Control and Prevention, and his coauthors.
“Medical providers should consider work-related coccidioidomycosis when evaluating construction workers with prolonged febrile respiratory illness, particularly after work in Central or Southern California or in Arizona, and medical providers should follow all statutory requirements for documenting and reporting occupational illness,” Dr. Wilken concluded.
The study was supported by the Centers for Disease Control and Prevention. No conflicts of interest were declared.
The expansion of the solar energy industry in Coccidioides-endemic areas of the southwestern United States is exposing more workers to the infection, say the authors of a study that found an attack rate of 1.2 cases per 100 workers.
A study among 3,572 workers at two solar power–generating facilities in California identified 44 individuals with the infection between October 2011 and April 2014, 9 of whom were hospitalized, according to a paper published in the Oct. 14 edition of Emerging Infectious Diseases.
The disease is acquired through inhalation of the soil-dwelling Coccidioides fungus spores and while the majority of the patients said they had received safety training about the risk of coccidioidomycosis, only six of those who regularly performed soil-disruptive work reported regularly using respiratory protection (Emerg Infect Dis. 2015 Oct 14; doi: ).
“Large-scale construction, including solar farm construction, might involve substantial soil disturbance for months, and many employees, particularly from non–Coccidioides-endemic areas, probably lack immunity to Coccidioides,” wrote Jason A. Wilken, Ph.D., of the Centers for Disease Control and Prevention, and his coauthors.
“Medical providers should consider work-related coccidioidomycosis when evaluating construction workers with prolonged febrile respiratory illness, particularly after work in Central or Southern California or in Arizona, and medical providers should follow all statutory requirements for documenting and reporting occupational illness,” Dr. Wilken concluded.
The study was supported by the Centers for Disease Control and Prevention. No conflicts of interest were declared.
FROM EMERGING INFECTIOUS DISEASES
Key clinical point:Coccidioidomycosis is a significant risk in workers on solar power–generating facilities in Coccidioides-endemic areas of the Southwestern United States.
Major finding: The attack rate of Coccidioides could be as high 1.2 cases per 100 workers involved in constructing solar power–generating facilities.
Data source: A study among 3,572 workers at two solar power–generating facilities in California.
Disclosures: The study was supported by the Centers for Disease Control and Prevention. No conflicts of interest were declared.
Carbapenem resistance on the rise in children
The prevalence of carbapenem-resistant Enterobacteriaceae (CRE) in children is low but has increased significantly since 1999, particularly among isolates from intensive care units and from blood and lower respiratory tract cultures, new data suggest.
Analysis of 316,253 Enterobacteriaceae isolates reported to 300 U.S. laboratories participating in the Surveillance Network-USA database between 1999 and 2012 showed 0.08% of isolates were carbapenem resistant, with the most common resistant isolates being Enterobacter species isolated from urinary sources and from the inpatient non-ICU setting.
“Unlike for adults, where increases were greater than for children, we did not find that the increase in CRE in children appeared to be related to residence in long-term care facilities, because only 0.1% of CRE isolates came from this setting,” wrote Dr. Latania K. Logan, director of pediatric infectious diseases at Rush University Medical Center, Chicago, and her coauthors.
The study, published Oct. 14 in Emerging Infectious Diseases, showed a significant overall increase from 0% to 0.47% in carbapenem-resistant Enterobacteriaceae over the 12-year study period; among ICU isolates, the prevalence increased from 0% to 4.5% over the same period.
Many of the carbapenem-resistant isolates also were resistant to other antimicrobial drugs, such as trimethoprim/sulfamethoxazole and ciprofloxacin, and nearly half (48.3%) were resistant to more than three antimicrobial drug classes (Emerg Infect Dis. 2015 Oct 14. doi: 10.3201/eid2111.150548).
The study was supported by the National Institutes of Health, the Children’s Foundation, the Global Antibiotic Resistance Partnership, the Bill and Melinda Gates Foundation, and the Health Grand Challenges Program at Princeton University. No conflicts of interest were declared.
The prevalence of carbapenem-resistant Enterobacteriaceae (CRE) in children is low but has increased significantly since 1999, particularly among isolates from intensive care units and from blood and lower respiratory tract cultures, new data suggest.
Analysis of 316,253 Enterobacteriaceae isolates reported to 300 U.S. laboratories participating in the Surveillance Network-USA database between 1999 and 2012 showed 0.08% of isolates were carbapenem resistant, with the most common resistant isolates being Enterobacter species isolated from urinary sources and from the inpatient non-ICU setting.
“Unlike for adults, where increases were greater than for children, we did not find that the increase in CRE in children appeared to be related to residence in long-term care facilities, because only 0.1% of CRE isolates came from this setting,” wrote Dr. Latania K. Logan, director of pediatric infectious diseases at Rush University Medical Center, Chicago, and her coauthors.
The study, published Oct. 14 in Emerging Infectious Diseases, showed a significant overall increase from 0% to 0.47% in carbapenem-resistant Enterobacteriaceae over the 12-year study period; among ICU isolates, the prevalence increased from 0% to 4.5% over the same period.
Many of the carbapenem-resistant isolates also were resistant to other antimicrobial drugs, such as trimethoprim/sulfamethoxazole and ciprofloxacin, and nearly half (48.3%) were resistant to more than three antimicrobial drug classes (Emerg Infect Dis. 2015 Oct 14. doi: 10.3201/eid2111.150548).
The study was supported by the National Institutes of Health, the Children’s Foundation, the Global Antibiotic Resistance Partnership, the Bill and Melinda Gates Foundation, and the Health Grand Challenges Program at Princeton University. No conflicts of interest were declared.
The prevalence of carbapenem-resistant Enterobacteriaceae (CRE) in children is low but has increased significantly since 1999, particularly among isolates from intensive care units and from blood and lower respiratory tract cultures, new data suggest.
Analysis of 316,253 Enterobacteriaceae isolates reported to 300 U.S. laboratories participating in the Surveillance Network-USA database between 1999 and 2012 showed 0.08% of isolates were carbapenem resistant, with the most common resistant isolates being Enterobacter species isolated from urinary sources and from the inpatient non-ICU setting.
“Unlike for adults, where increases were greater than for children, we did not find that the increase in CRE in children appeared to be related to residence in long-term care facilities, because only 0.1% of CRE isolates came from this setting,” wrote Dr. Latania K. Logan, director of pediatric infectious diseases at Rush University Medical Center, Chicago, and her coauthors.
The study, published Oct. 14 in Emerging Infectious Diseases, showed a significant overall increase from 0% to 0.47% in carbapenem-resistant Enterobacteriaceae over the 12-year study period; among ICU isolates, the prevalence increased from 0% to 4.5% over the same period.
Many of the carbapenem-resistant isolates also were resistant to other antimicrobial drugs, such as trimethoprim/sulfamethoxazole and ciprofloxacin, and nearly half (48.3%) were resistant to more than three antimicrobial drug classes (Emerg Infect Dis. 2015 Oct 14. doi: 10.3201/eid2111.150548).
The study was supported by the National Institutes of Health, the Children’s Foundation, the Global Antibiotic Resistance Partnership, the Bill and Melinda Gates Foundation, and the Health Grand Challenges Program at Princeton University. No conflicts of interest were declared.
FROM EMERGING INFECTIOUS DISEASES
Key clinical point: The prevalence of carbapenem-resistant Enterobacteriaceae in children has increased significantly since 1999.
Major finding: The prevalence of carbapenem-resistant Enterobacteriaceae isolates increased from 0% to 0.47% during 1999-2012.
Data source: Analysis of 316,253 Enterobacteriaceae isolates reported to 300 U.S. laboratories.
Disclosures: The study was supported by the National Institutes of Health, the Children’s Foundation, the Global Antibiotic Resistance Partnership, the Bill and Melinda Gates Foundation, and the Health Grand Challenges Program at Princeton University. No conflicts of interest were declared.
Concussionlike symptoms prevalent in uninjured teen athletes
Ensuring a high school athlete has returned to an asymptomatic state after a concussion can be challenging, according to the authors of a study that found a significant proportion of uninjured adolescent athletes report at least one symptom of concussion.
A cross-sectional observational study of 31,958 high school athletes, none of whom had experienced a concussion in the prior 6 months, showed 19% of boys and 28% of girls reported a symptom burden resembling an ICD-10 diagnosis of mild postconcussional syndrome, according to a paper published online Oct. 12 in JAMA Pediatrics.
Concussion symptoms were particularly prevalent among students with preexisting conditions such as psychiatric problems, learning disorders, migraine, attention deficit/hyperactivity disorder, or substance abuse. Students who had experienced a concussion previously were the least likely to show concussion symptoms (JAMA Pediatrics. 2015 Oct. 12 [doi: 10.1001/jamapediatrics.2015.2374]).
Boys most commonly reported symptoms such as fatigue, sleep troubles, and difficult concentration, while girls were more likely to report fatigue, sleep troubles, headaches, sadness, feeling emotional, and difficulty concentrating.
“When managing a student athlete with a concussion, it has been widely noted that the athlete should be ‘asymptomatic’ at rest and with exercise before returning to sports, and sometimes athletes are kept out of school for prolonged periods while they wait for symptoms to resolve, which could have negative consequences for their academic, social, and emotional functioning and contribute to symptom reporting,” wrote Grant L. Iverson, Ph.D., from Harvard Medical School, Boston, and his coauthors.
“These results reinforce that ‘asymptomatic’ status after concussion can be difficult to define,” they added.
The study was supported by the Goldfarb Center for Public Policy and Civic Engagement/Colby College, the Bill and Joan Alfond Foundation, the Harvard Integrated Program to Protect and Improve the Health of National Football League Players Association Members, and the Mooney-Reed Charitable Foundation. The lead author declared speakers fees from private industry, but there were no other conflicts of interest declared.
Ensuring a high school athlete has returned to an asymptomatic state after a concussion can be challenging, according to the authors of a study that found a significant proportion of uninjured adolescent athletes report at least one symptom of concussion.
A cross-sectional observational study of 31,958 high school athletes, none of whom had experienced a concussion in the prior 6 months, showed 19% of boys and 28% of girls reported a symptom burden resembling an ICD-10 diagnosis of mild postconcussional syndrome, according to a paper published online Oct. 12 in JAMA Pediatrics.
Concussion symptoms were particularly prevalent among students with preexisting conditions such as psychiatric problems, learning disorders, migraine, attention deficit/hyperactivity disorder, or substance abuse. Students who had experienced a concussion previously were the least likely to show concussion symptoms (JAMA Pediatrics. 2015 Oct. 12 [doi: 10.1001/jamapediatrics.2015.2374]).
Boys most commonly reported symptoms such as fatigue, sleep troubles, and difficult concentration, while girls were more likely to report fatigue, sleep troubles, headaches, sadness, feeling emotional, and difficulty concentrating.
“When managing a student athlete with a concussion, it has been widely noted that the athlete should be ‘asymptomatic’ at rest and with exercise before returning to sports, and sometimes athletes are kept out of school for prolonged periods while they wait for symptoms to resolve, which could have negative consequences for their academic, social, and emotional functioning and contribute to symptom reporting,” wrote Grant L. Iverson, Ph.D., from Harvard Medical School, Boston, and his coauthors.
“These results reinforce that ‘asymptomatic’ status after concussion can be difficult to define,” they added.
The study was supported by the Goldfarb Center for Public Policy and Civic Engagement/Colby College, the Bill and Joan Alfond Foundation, the Harvard Integrated Program to Protect and Improve the Health of National Football League Players Association Members, and the Mooney-Reed Charitable Foundation. The lead author declared speakers fees from private industry, but there were no other conflicts of interest declared.
Ensuring a high school athlete has returned to an asymptomatic state after a concussion can be challenging, according to the authors of a study that found a significant proportion of uninjured adolescent athletes report at least one symptom of concussion.
A cross-sectional observational study of 31,958 high school athletes, none of whom had experienced a concussion in the prior 6 months, showed 19% of boys and 28% of girls reported a symptom burden resembling an ICD-10 diagnosis of mild postconcussional syndrome, according to a paper published online Oct. 12 in JAMA Pediatrics.
Concussion symptoms were particularly prevalent among students with preexisting conditions such as psychiatric problems, learning disorders, migraine, attention deficit/hyperactivity disorder, or substance abuse. Students who had experienced a concussion previously were the least likely to show concussion symptoms (JAMA Pediatrics. 2015 Oct. 12 [doi: 10.1001/jamapediatrics.2015.2374]).
Boys most commonly reported symptoms such as fatigue, sleep troubles, and difficult concentration, while girls were more likely to report fatigue, sleep troubles, headaches, sadness, feeling emotional, and difficulty concentrating.
“When managing a student athlete with a concussion, it has been widely noted that the athlete should be ‘asymptomatic’ at rest and with exercise before returning to sports, and sometimes athletes are kept out of school for prolonged periods while they wait for symptoms to resolve, which could have negative consequences for their academic, social, and emotional functioning and contribute to symptom reporting,” wrote Grant L. Iverson, Ph.D., from Harvard Medical School, Boston, and his coauthors.
“These results reinforce that ‘asymptomatic’ status after concussion can be difficult to define,” they added.
The study was supported by the Goldfarb Center for Public Policy and Civic Engagement/Colby College, the Bill and Joan Alfond Foundation, the Harvard Integrated Program to Protect and Improve the Health of National Football League Players Association Members, and the Mooney-Reed Charitable Foundation. The lead author declared speakers fees from private industry, but there were no other conflicts of interest declared.
FROM JAMA PEDIATRICS
Key clinical point: A significant proportion of uninjured high school athletes reported at least one symptom of concussion.
Major finding: About one in five male high school athletes and one in four female high school athletes reported a symptom burden resembling an ICD-10 diagnosis of mild postconcussional syndrome.
Data source: A cross-sectional observational study of 31,958 high school athletes who had not experienced a concussion in the previous 6 months.
Disclosures: The study was supported by the Goldfarb Center for Public Policy and Civic Engagement/Colby College, the Bill and Joan Alfond Foundation, the Harvard Integrated Program to Protect and Improve the Health of National Football League Players Association Members, and the Mooney-Reed Charitable Foundation. The lead author declared speakers fees from private industry, but there were no other conflicts of interest declared.
High ALT/AST ratio linked to fatty liver risk in HCV
Individuals infected with the hepatitis C virus who have a higher ratio of alanine aminotransferase to aspartate aminotransferase may be at greater risk of developing nonalcoholic fatty liver disease and hepatosteatosis, new data suggest.
A community-based observational study in 1,354 Taiwanese individuals seropositive for hepatitis C virus – including 433 with nonalcoholic fatty liver disease – found a high alanine aminotransferase to aspartate aminotransferase ratio was significantly and independently associated with nonalcoholic fatty liver disease (OR, 1.90; 95% CI, 1.37 to 2.65; P less than .001) and high-degree nonalcoholic fatty liver disease (OR, 2.44; 95% CI, 1.58 to 3.77; P less than .001).
This effect was observed even after researchers accounted for potential confounders: age, body mass index, metabolic syndrome, cholesterol level, hepatitis B virus infection, and smoking.
The study found the ALT/AST ratio was significantly higher among patients with nonalcoholic fatty liver disease (1.2 ± 0.4 vs. 1.1 ± 0.4; P less than .001) – defined as hepatic steatosis by echogenic imaging – according to a paper published online Sept. 14 in BMJ Open.
Nonalcoholic fatty liver disease is a particular issue because not only can it progress to severe liver disease but it is also associated with a lower likelihood of achieving a sustained virologic response to antiviral therapy. In addition, the majority of cases of nonalcoholic fatty liver disease are silent and are discovered incidentally, the authors wrote.
“This is the first study to reveal a strong relationship between the ALT/AST ratio and NAFLD in patients with HCV, and the ALT/AST ratio was also an independent risk factor apart from the conventional risk factors for hepatosteatosis including the MetS [metabolic syndrome], LDL, TC, waist/hip ratio, and body mass index,” wrote Dr. Ming-Shyan Lin of Chang Gung Memorial Hospital, Taiwan, and coauthors.
While the AST/ALT ratio is a marker of liver cirrhosis and advanced liver disease, the ALT/AST ratio is also a marker for insulin resistance and metabolic syndrome.
Researchers also noted that individuals with hepatitis C infection and nonalcoholic fatty liver disease had a significantly higher incidence of metabolic syndrome, significantly higher fasting glucose, uric acid, and triglycerides, and a lower HDL than did those with low-degree nonalcoholic fatty liver disease.
“The prevalence of hepatosteatosis in chronic hepatitis C infection has been reported in up to 31%-72%, which is significantly higher than that in participants with other chronic liver disease such as hepatitis B or autoimmune hepatitis, suggesting a direct effect of HCV replication in the development of excess fat accumulation in the liver,” the authors wrote.
In this cohort, the prevalence of nonalcoholic fatty liver disease was 31.9%, and 19.6% of participants had moderate to severe hepatosteatosis (BMJ Open 2015, Sep 14. doi:10.1136/bmjopen-2015-008797).
Given the silent nature of nonalcoholic fatty liver disease, the authors suggested that the findings could help clinicians identify individuals with hepatosteatosis and implement interventions such as weight loss to reduce their risk of further progression.
No conflicts of interest were declared.
Individuals infected with the hepatitis C virus who have a higher ratio of alanine aminotransferase to aspartate aminotransferase may be at greater risk of developing nonalcoholic fatty liver disease and hepatosteatosis, new data suggest.
A community-based observational study in 1,354 Taiwanese individuals seropositive for hepatitis C virus – including 433 with nonalcoholic fatty liver disease – found a high alanine aminotransferase to aspartate aminotransferase ratio was significantly and independently associated with nonalcoholic fatty liver disease (OR, 1.90; 95% CI, 1.37 to 2.65; P less than .001) and high-degree nonalcoholic fatty liver disease (OR, 2.44; 95% CI, 1.58 to 3.77; P less than .001).
This effect was observed even after researchers accounted for potential confounders: age, body mass index, metabolic syndrome, cholesterol level, hepatitis B virus infection, and smoking.
The study found the ALT/AST ratio was significantly higher among patients with nonalcoholic fatty liver disease (1.2 ± 0.4 vs. 1.1 ± 0.4; P less than .001) – defined as hepatic steatosis by echogenic imaging – according to a paper published online Sept. 14 in BMJ Open.
Nonalcoholic fatty liver disease is a particular issue because not only can it progress to severe liver disease but it is also associated with a lower likelihood of achieving a sustained virologic response to antiviral therapy. In addition, the majority of cases of nonalcoholic fatty liver disease are silent and are discovered incidentally, the authors wrote.
“This is the first study to reveal a strong relationship between the ALT/AST ratio and NAFLD in patients with HCV, and the ALT/AST ratio was also an independent risk factor apart from the conventional risk factors for hepatosteatosis including the MetS [metabolic syndrome], LDL, TC, waist/hip ratio, and body mass index,” wrote Dr. Ming-Shyan Lin of Chang Gung Memorial Hospital, Taiwan, and coauthors.
While the AST/ALT ratio is a marker of liver cirrhosis and advanced liver disease, the ALT/AST ratio is also a marker for insulin resistance and metabolic syndrome.
Researchers also noted that individuals with hepatitis C infection and nonalcoholic fatty liver disease had a significantly higher incidence of metabolic syndrome, significantly higher fasting glucose, uric acid, and triglycerides, and a lower HDL than did those with low-degree nonalcoholic fatty liver disease.
“The prevalence of hepatosteatosis in chronic hepatitis C infection has been reported in up to 31%-72%, which is significantly higher than that in participants with other chronic liver disease such as hepatitis B or autoimmune hepatitis, suggesting a direct effect of HCV replication in the development of excess fat accumulation in the liver,” the authors wrote.
In this cohort, the prevalence of nonalcoholic fatty liver disease was 31.9%, and 19.6% of participants had moderate to severe hepatosteatosis (BMJ Open 2015, Sep 14. doi:10.1136/bmjopen-2015-008797).
Given the silent nature of nonalcoholic fatty liver disease, the authors suggested that the findings could help clinicians identify individuals with hepatosteatosis and implement interventions such as weight loss to reduce their risk of further progression.
No conflicts of interest were declared.
Individuals infected with the hepatitis C virus who have a higher ratio of alanine aminotransferase to aspartate aminotransferase may be at greater risk of developing nonalcoholic fatty liver disease and hepatosteatosis, new data suggest.
A community-based observational study in 1,354 Taiwanese individuals seropositive for hepatitis C virus – including 433 with nonalcoholic fatty liver disease – found a high alanine aminotransferase to aspartate aminotransferase ratio was significantly and independently associated with nonalcoholic fatty liver disease (OR, 1.90; 95% CI, 1.37 to 2.65; P less than .001) and high-degree nonalcoholic fatty liver disease (OR, 2.44; 95% CI, 1.58 to 3.77; P less than .001).
This effect was observed even after researchers accounted for potential confounders: age, body mass index, metabolic syndrome, cholesterol level, hepatitis B virus infection, and smoking.
The study found the ALT/AST ratio was significantly higher among patients with nonalcoholic fatty liver disease (1.2 ± 0.4 vs. 1.1 ± 0.4; P less than .001) – defined as hepatic steatosis by echogenic imaging – according to a paper published online Sept. 14 in BMJ Open.
Nonalcoholic fatty liver disease is a particular issue because not only can it progress to severe liver disease but it is also associated with a lower likelihood of achieving a sustained virologic response to antiviral therapy. In addition, the majority of cases of nonalcoholic fatty liver disease are silent and are discovered incidentally, the authors wrote.
“This is the first study to reveal a strong relationship between the ALT/AST ratio and NAFLD in patients with HCV, and the ALT/AST ratio was also an independent risk factor apart from the conventional risk factors for hepatosteatosis including the MetS [metabolic syndrome], LDL, TC, waist/hip ratio, and body mass index,” wrote Dr. Ming-Shyan Lin of Chang Gung Memorial Hospital, Taiwan, and coauthors.
While the AST/ALT ratio is a marker of liver cirrhosis and advanced liver disease, the ALT/AST ratio is also a marker for insulin resistance and metabolic syndrome.
Researchers also noted that individuals with hepatitis C infection and nonalcoholic fatty liver disease had a significantly higher incidence of metabolic syndrome, significantly higher fasting glucose, uric acid, and triglycerides, and a lower HDL than did those with low-degree nonalcoholic fatty liver disease.
“The prevalence of hepatosteatosis in chronic hepatitis C infection has been reported in up to 31%-72%, which is significantly higher than that in participants with other chronic liver disease such as hepatitis B or autoimmune hepatitis, suggesting a direct effect of HCV replication in the development of excess fat accumulation in the liver,” the authors wrote.
In this cohort, the prevalence of nonalcoholic fatty liver disease was 31.9%, and 19.6% of participants had moderate to severe hepatosteatosis (BMJ Open 2015, Sep 14. doi:10.1136/bmjopen-2015-008797).
Given the silent nature of nonalcoholic fatty liver disease, the authors suggested that the findings could help clinicians identify individuals with hepatosteatosis and implement interventions such as weight loss to reduce their risk of further progression.
No conflicts of interest were declared.
FROM BMJ OPEN
Key clinical point: A higher ratio of alanine aminotransferase to aspartate aminotransferase in individuals seropositive for hepatitis C virus may be associated with nonalcoholic fatty liver disease.
Major finding: A high ALT/AST ratio was significantly and independently associated with nonalcoholic fatty liver disease.
Data source: A community-based observational study in 1354 Taiwanese individuals seropositive for hepatitis C virus.
Disclosures: No conflicts of interest were declared.