User login
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
To the Editor:
Patients with nonmelanoma skin cancers exhibit high quality-of-life satisfaction after treatment with Mohs micrographic surgery (MMS) or excision.1,2 However, perioperative anxiety in patients undergoing MMS is common, especially during the immediate preoperative period.3 Anxiety activates the sympathetic nervous system, resulting in physiologic changes such as tachycardia and hypertension.4,5 These sequelae may not only increase patient distress but also increase intraoperative bleeding, complication rates, and recovery times.4,5 Thus, the preoperative period represents a critical window for interventions aimed at reducing anxiety. Anxiety peaks during the perioperative period for a myriad of reasons, including anticipation of pain or potential complications. Enhancing patient comfort and well-being during the procedure may help reduce negative emotional sequelae, alleviate fear during procedures, and increase patient satisfaction.3
Weighted blankets (WBs) frequently are utilized in occupational and physical therapy as a deep pressure stimulation tool to alleviate anxiety by mimicking the experience of being massaged or swaddled.6 Deep pressure tools increase parasympathetic tone, help reduce anxiety, and provide a calming effect.7,8 Nonhospitalized individuals were more relaxed during mental health evaluations when using a WB, and deep pressure tools have frequently been used to calm individuals with autism spectrum disorders or attention-deficit/hyperactivity disorders.6 Furthermore, WBs have successfully been used to reduce anxiety in mental health care settings, as well as during chemotherapy infusions.6,9 The literature is sparse regarding the use of WB in the perioperative setting. Potential benefit has been demonstrated in the setting of dental cleanings and wisdom teeth extractions.7,8 In the current study, we investigated whether use of a WB could reduce preoperative anxiety in the setting of MMS.
Institutional review board approval was obtained from the University of Virginia (Charlottesville, Virginia), and adult patients undergoing MMS to the head or neck were recruited to participate in a single-blind randomized controlled trial in the spring of 2023. Patients undergoing MMS on other areas of the body were excluded because the placement of the WB could interfere with the procedure. Other exclusion criteria included pregnancy, dementia, or current treatment with an anxiolytic medication.
Twenty-seven patients were included in the study, and informed consent was obtained. Patients were randomized to use a WB or standard hospital towel (control). The medical-grade WBs weighed 8.5 pounds, while the cotton hospital towels weighed less than 1 pound. The WBs were cleaned in between patients with standard germicidal disposable wipes.
Patient data were collected from electronic medical records including age, sex, weight, history of prior MMS, and current use of antihypertensives and/or beta-blockers. Data also were collected on the presence of anxiety disorders, major depression, fibromyalgia, tobacco and alcohol use, hyperthyroidism, hyperhidrosis, cardiac arrhythmias (including atrial fibrillation), chronic obstructive pulmonary disease, asthma, coronary artery disease, diabetes mellitus, peripheral neuropathy, and menopausal symptoms.
During the procedure, anxiety was monitored using the State-Trait Anxiety Inventory (STAI) Form Y-1, the visual analogue scale for anxiety (VAS-A), and vital signs including heart rate, blood pressure, and respiratory rate. Vital signs were evaluated by nursing staff with the patient sitting up and the WB or hospital towel removed. Using these assessments, anxiety was measured at 3 different timepoints: upon arrival to the clinic (timepoint A), after the patient rested in a reclined beach-chair position with the WB or hospital towel placed over them for 10 minutes before administration of local anesthetic and starting the procedure (timepoint B), and after the first MMS stage was taken (timepoint C).
A power analysis was not completed due to a lack of previous studies on the use of WBs during MMS. Group means were analyzed using two-tailed t-tests and one-way analysis of variance. A P value of .05 indicated statistical significance.
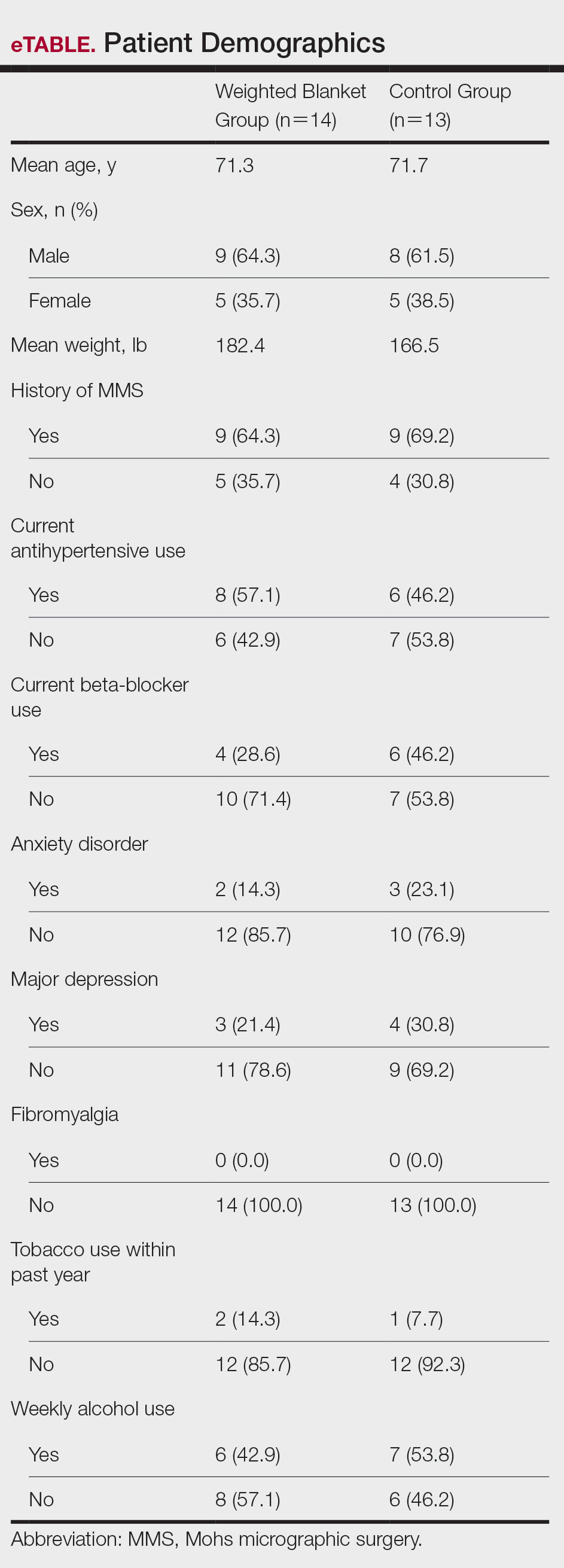
Fourteen patients were randomized to the WB group and 13 were randomized to the control group. Patient demographics are outlined in the eTable. In the WB group, mean STAI scores progressively decreased at each timepoint (A: 15.3, B: 13.6, C: 12.7) and mean VAS-A scores followed a similar trend (A: 24.2, B: 19.3, C: 10.5). In the control group, the mean STAI scores remained stable at timepoints A and B (17.7) and then decreased at timepoint C (14.8). The mean VAS-A scores in the control group followed a similar pattern, remaining stable at timepoints A (22.9) and B (22.8) and then decreasing at timepoint C (14.4). These changes were not statistically significant.
Mean vital signs for both the WB and control groups were relatively stable across all timepoints, although they tended to decrease by timepoint C. In the WB group, mean heart rates were 69, 69, and 67 beats per minute at timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 136 mm Hg and mean diastolic pressures were 71 mm Hg, 68 mm Hg, and 66 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 20, 19, and 18 breaths per minute at timepoints A, B, and C, respectively. In the control group, mean heart rates were 70, 69, and 68 beats per minute across timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 133 mm Hg and mean diastolic pressures were 71 mm Hg, 74 mm Hg, and 68 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 19, 18, and 18 breaths per minute at timepoints A, B, and C, respectively. These changes were not statistically significant.
Our pilot study examined the effects of using a WB to alleviate preoperative anxiety during MMS. Our results suggest that WBs may modestly improve subjective anxiety immediately prior to undergoing MMS. Mean STAI and VAS-A scores decreased from timepoint A to timepoint B in the WB group vs the control group in which these scores remained stable. Although our study was not powered to determine statistical differences and significance was not reached, our results suggest a favorable trend in decreased anxiety scores. Our analysis was limited by a small sample size; therefore, additional larger-scale studies will be needed to confirm this trend.
Our results are broadly consistent with earlier studies that found improvement in physiologic proxies of anxiety with the use of WBs during chemotherapy infusions, dental procedures, and acute inpatient mental health hospitalizations.7-10 During periods of high anxiety, use of WBs shifts the autonomic nervous system from a sympathetic to a parasympathetic state, as demonstrated by increased high-frequency heart rate variability, a marker of parasympathetic activity.6,11 While the exact mechanism of how WBs and other deep pressure stimulation tools affect high-frequency heart rate variability is unclear, one study showed that patients undergoing dental extractions were better equipped when using deep pressure stimulation tools to utilize calming techniques and regulate stress.12 The use of WBs and other deep pressure stimulation tools may extend beyond the perioperative setting and also may be an effective tool for clinicians in other settings (eg, clinic visits, physical examinations).
In our study, all participants demonstrated the greatest reduction in anxiety at timepoint C after the first MMS stage, likely related to patients relaxing more after knowing what to expect from the surgery; this also may have been reflected somewhat in the slight downward trend noted in vital signs across both study groups. One concern regarding WB use in surgical settings is whether the added pressure could trigger unfavorable circulatory effects, such as elevated blood pressure. In our study, with the exception of diastolic blood pressure, vital signs appeared unaffected by the type of blanket used and remained relatively stable from timepoint A to timepoint B and decreased at timepoint C. Diastolic blood pressure in the WB group decreased from timepoint A to timepoint B, then decreased further from timepoint B to timepoint C. This mirrored the decreasing STAI score trend, compared to the control group who increased from timepoint A to timepoint B and reached a nadir at timepoint C. Consistent with prior WB studies, there were no adverse effects from WBs, including adverse impacts on vital signs.6,9
The original recruitment goal was not met due to staffing issues related to the COVID-19 pandemic, and subgroup analyses were deferred as a result of sample size limitations. It is possible that the WB intervention may have a larger impact on subpopulations more prone to perioperative anxiety (eg, patients undergoing MMS for the first time). However, the results of our pilot study suggest a beneficial effect from the use of WBs. While these preliminary data are promising, additional studies in the perioperative setting are needed to more accurately determine the clinical utility of WBs during MMS and other procedures.
- Eberle FC, Schippert W, Trilling B, et al. Cosmetic results of histographically controlled excision of non-melanoma skin cancer in the head and neck region. J Dtsch Dermatol Ges. 2005;3:109-112. doi:10.1111/j.1610-0378.2005.04738.x
- Chren MM, Sahay AP, Bertenthal DS, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351-1357. doi:10.1038/sj.jid.5700740
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035. doi:10.1097 /DSS.0000000000001152
- Pritchard MJ. Identifying and assessing anxiety in pre-operative patients. Nurs Stand. 2009;23:35-40. doi:10.7748/ns2009.08.23.51.35.c7222.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Mullen B, Champagne T, Krishnamurty S, et al. Exploring the safety and therapeutic effects of deep pressure stimulation using a weighted blanket. Occup Ther Ment Health. 2008;24:65-89. doi:10.1300/ J004v24n01_05
- Chen HY, Yang H, Chi HJ, et al. Physiological effects of deep touch pressure on anxiety alleviation: the weighted blanket approach. J Med Biol Eng. 2013;33:463-470. doi:10.5405/jmbe.1043
- Chen HY, Yang H, Meng LF, et al. Effect of deep pressure input on parasympathetic system in patients with wisdom tooth surgery. J Formos Med Assoc. 2016;115:853-859. doi:10.1016 /j.jfma.2016.07.008
- Vinson J, Powers J, Mosesso K. Weighted blankets: anxiety reduction in adult patients receiving chemotherapy. Clin J Oncol Nurs. 2020; 24:360-368. doi:10.1188/20.CJON.360-368
- Champagne T, Mullen B, Dickson D, et al. Evaluating the safety and effectiveness of the weighted blanket with adults during an inpatient mental health hospitalization. Occup Ther Ment Health. 2015;31:211-233. doi:10.1080/0164212X.2015.1066220
- Lane RD, McRae K, Reiman EM, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213-222. doi: 10.1016/j.neuroimage.2008.07.056
- Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130:3-18. doi: 10.1037 /0033-2909.130.1.3
To the Editor:
Patients with nonmelanoma skin cancers exhibit high quality-of-life satisfaction after treatment with Mohs micrographic surgery (MMS) or excision.1,2 However, perioperative anxiety in patients undergoing MMS is common, especially during the immediate preoperative period.3 Anxiety activates the sympathetic nervous system, resulting in physiologic changes such as tachycardia and hypertension.4,5 These sequelae may not only increase patient distress but also increase intraoperative bleeding, complication rates, and recovery times.4,5 Thus, the preoperative period represents a critical window for interventions aimed at reducing anxiety. Anxiety peaks during the perioperative period for a myriad of reasons, including anticipation of pain or potential complications. Enhancing patient comfort and well-being during the procedure may help reduce negative emotional sequelae, alleviate fear during procedures, and increase patient satisfaction.3
Weighted blankets (WBs) frequently are utilized in occupational and physical therapy as a deep pressure stimulation tool to alleviate anxiety by mimicking the experience of being massaged or swaddled.6 Deep pressure tools increase parasympathetic tone, help reduce anxiety, and provide a calming effect.7,8 Nonhospitalized individuals were more relaxed during mental health evaluations when using a WB, and deep pressure tools have frequently been used to calm individuals with autism spectrum disorders or attention-deficit/hyperactivity disorders.6 Furthermore, WBs have successfully been used to reduce anxiety in mental health care settings, as well as during chemotherapy infusions.6,9 The literature is sparse regarding the use of WB in the perioperative setting. Potential benefit has been demonstrated in the setting of dental cleanings and wisdom teeth extractions.7,8 In the current study, we investigated whether use of a WB could reduce preoperative anxiety in the setting of MMS.
Institutional review board approval was obtained from the University of Virginia (Charlottesville, Virginia), and adult patients undergoing MMS to the head or neck were recruited to participate in a single-blind randomized controlled trial in the spring of 2023. Patients undergoing MMS on other areas of the body were excluded because the placement of the WB could interfere with the procedure. Other exclusion criteria included pregnancy, dementia, or current treatment with an anxiolytic medication.
Twenty-seven patients were included in the study, and informed consent was obtained. Patients were randomized to use a WB or standard hospital towel (control). The medical-grade WBs weighed 8.5 pounds, while the cotton hospital towels weighed less than 1 pound. The WBs were cleaned in between patients with standard germicidal disposable wipes.
Patient data were collected from electronic medical records including age, sex, weight, history of prior MMS, and current use of antihypertensives and/or beta-blockers. Data also were collected on the presence of anxiety disorders, major depression, fibromyalgia, tobacco and alcohol use, hyperthyroidism, hyperhidrosis, cardiac arrhythmias (including atrial fibrillation), chronic obstructive pulmonary disease, asthma, coronary artery disease, diabetes mellitus, peripheral neuropathy, and menopausal symptoms.
During the procedure, anxiety was monitored using the State-Trait Anxiety Inventory (STAI) Form Y-1, the visual analogue scale for anxiety (VAS-A), and vital signs including heart rate, blood pressure, and respiratory rate. Vital signs were evaluated by nursing staff with the patient sitting up and the WB or hospital towel removed. Using these assessments, anxiety was measured at 3 different timepoints: upon arrival to the clinic (timepoint A), after the patient rested in a reclined beach-chair position with the WB or hospital towel placed over them for 10 minutes before administration of local anesthetic and starting the procedure (timepoint B), and after the first MMS stage was taken (timepoint C).
A power analysis was not completed due to a lack of previous studies on the use of WBs during MMS. Group means were analyzed using two-tailed t-tests and one-way analysis of variance. A P value of .05 indicated statistical significance.
Fourteen patients were randomized to the WB group and 13 were randomized to the control group. Patient demographics are outlined in the eTable. In the WB group, mean STAI scores progressively decreased at each timepoint (A: 15.3, B: 13.6, C: 12.7) and mean VAS-A scores followed a similar trend (A: 24.2, B: 19.3, C: 10.5). In the control group, the mean STAI scores remained stable at timepoints A and B (17.7) and then decreased at timepoint C (14.8). The mean VAS-A scores in the control group followed a similar pattern, remaining stable at timepoints A (22.9) and B (22.8) and then decreasing at timepoint C (14.4). These changes were not statistically significant.

Mean vital signs for both the WB and control groups were relatively stable across all timepoints, although they tended to decrease by timepoint C. In the WB group, mean heart rates were 69, 69, and 67 beats per minute at timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 136 mm Hg and mean diastolic pressures were 71 mm Hg, 68 mm Hg, and 66 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 20, 19, and 18 breaths per minute at timepoints A, B, and C, respectively. In the control group, mean heart rates were 70, 69, and 68 beats per minute across timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 133 mm Hg and mean diastolic pressures were 71 mm Hg, 74 mm Hg, and 68 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 19, 18, and 18 breaths per minute at timepoints A, B, and C, respectively. These changes were not statistically significant.
Our pilot study examined the effects of using a WB to alleviate preoperative anxiety during MMS. Our results suggest that WBs may modestly improve subjective anxiety immediately prior to undergoing MMS. Mean STAI and VAS-A scores decreased from timepoint A to timepoint B in the WB group vs the control group in which these scores remained stable. Although our study was not powered to determine statistical differences and significance was not reached, our results suggest a favorable trend in decreased anxiety scores. Our analysis was limited by a small sample size; therefore, additional larger-scale studies will be needed to confirm this trend.
Our results are broadly consistent with earlier studies that found improvement in physiologic proxies of anxiety with the use of WBs during chemotherapy infusions, dental procedures, and acute inpatient mental health hospitalizations.7-10 During periods of high anxiety, use of WBs shifts the autonomic nervous system from a sympathetic to a parasympathetic state, as demonstrated by increased high-frequency heart rate variability, a marker of parasympathetic activity.6,11 While the exact mechanism of how WBs and other deep pressure stimulation tools affect high-frequency heart rate variability is unclear, one study showed that patients undergoing dental extractions were better equipped when using deep pressure stimulation tools to utilize calming techniques and regulate stress.12 The use of WBs and other deep pressure stimulation tools may extend beyond the perioperative setting and also may be an effective tool for clinicians in other settings (eg, clinic visits, physical examinations).
In our study, all participants demonstrated the greatest reduction in anxiety at timepoint C after the first MMS stage, likely related to patients relaxing more after knowing what to expect from the surgery; this also may have been reflected somewhat in the slight downward trend noted in vital signs across both study groups. One concern regarding WB use in surgical settings is whether the added pressure could trigger unfavorable circulatory effects, such as elevated blood pressure. In our study, with the exception of diastolic blood pressure, vital signs appeared unaffected by the type of blanket used and remained relatively stable from timepoint A to timepoint B and decreased at timepoint C. Diastolic blood pressure in the WB group decreased from timepoint A to timepoint B, then decreased further from timepoint B to timepoint C. This mirrored the decreasing STAI score trend, compared to the control group who increased from timepoint A to timepoint B and reached a nadir at timepoint C. Consistent with prior WB studies, there were no adverse effects from WBs, including adverse impacts on vital signs.6,9
The original recruitment goal was not met due to staffing issues related to the COVID-19 pandemic, and subgroup analyses were deferred as a result of sample size limitations. It is possible that the WB intervention may have a larger impact on subpopulations more prone to perioperative anxiety (eg, patients undergoing MMS for the first time). However, the results of our pilot study suggest a beneficial effect from the use of WBs. While these preliminary data are promising, additional studies in the perioperative setting are needed to more accurately determine the clinical utility of WBs during MMS and other procedures.
To the Editor:
Patients with nonmelanoma skin cancers exhibit high quality-of-life satisfaction after treatment with Mohs micrographic surgery (MMS) or excision.1,2 However, perioperative anxiety in patients undergoing MMS is common, especially during the immediate preoperative period.3 Anxiety activates the sympathetic nervous system, resulting in physiologic changes such as tachycardia and hypertension.4,5 These sequelae may not only increase patient distress but also increase intraoperative bleeding, complication rates, and recovery times.4,5 Thus, the preoperative period represents a critical window for interventions aimed at reducing anxiety. Anxiety peaks during the perioperative period for a myriad of reasons, including anticipation of pain or potential complications. Enhancing patient comfort and well-being during the procedure may help reduce negative emotional sequelae, alleviate fear during procedures, and increase patient satisfaction.3
Weighted blankets (WBs) frequently are utilized in occupational and physical therapy as a deep pressure stimulation tool to alleviate anxiety by mimicking the experience of being massaged or swaddled.6 Deep pressure tools increase parasympathetic tone, help reduce anxiety, and provide a calming effect.7,8 Nonhospitalized individuals were more relaxed during mental health evaluations when using a WB, and deep pressure tools have frequently been used to calm individuals with autism spectrum disorders or attention-deficit/hyperactivity disorders.6 Furthermore, WBs have successfully been used to reduce anxiety in mental health care settings, as well as during chemotherapy infusions.6,9 The literature is sparse regarding the use of WB in the perioperative setting. Potential benefit has been demonstrated in the setting of dental cleanings and wisdom teeth extractions.7,8 In the current study, we investigated whether use of a WB could reduce preoperative anxiety in the setting of MMS.
Institutional review board approval was obtained from the University of Virginia (Charlottesville, Virginia), and adult patients undergoing MMS to the head or neck were recruited to participate in a single-blind randomized controlled trial in the spring of 2023. Patients undergoing MMS on other areas of the body were excluded because the placement of the WB could interfere with the procedure. Other exclusion criteria included pregnancy, dementia, or current treatment with an anxiolytic medication.
Twenty-seven patients were included in the study, and informed consent was obtained. Patients were randomized to use a WB or standard hospital towel (control). The medical-grade WBs weighed 8.5 pounds, while the cotton hospital towels weighed less than 1 pound. The WBs were cleaned in between patients with standard germicidal disposable wipes.
Patient data were collected from electronic medical records including age, sex, weight, history of prior MMS, and current use of antihypertensives and/or beta-blockers. Data also were collected on the presence of anxiety disorders, major depression, fibromyalgia, tobacco and alcohol use, hyperthyroidism, hyperhidrosis, cardiac arrhythmias (including atrial fibrillation), chronic obstructive pulmonary disease, asthma, coronary artery disease, diabetes mellitus, peripheral neuropathy, and menopausal symptoms.
During the procedure, anxiety was monitored using the State-Trait Anxiety Inventory (STAI) Form Y-1, the visual analogue scale for anxiety (VAS-A), and vital signs including heart rate, blood pressure, and respiratory rate. Vital signs were evaluated by nursing staff with the patient sitting up and the WB or hospital towel removed. Using these assessments, anxiety was measured at 3 different timepoints: upon arrival to the clinic (timepoint A), after the patient rested in a reclined beach-chair position with the WB or hospital towel placed over them for 10 minutes before administration of local anesthetic and starting the procedure (timepoint B), and after the first MMS stage was taken (timepoint C).
A power analysis was not completed due to a lack of previous studies on the use of WBs during MMS. Group means were analyzed using two-tailed t-tests and one-way analysis of variance. A P value of .05 indicated statistical significance.
Fourteen patients were randomized to the WB group and 13 were randomized to the control group. Patient demographics are outlined in the eTable. In the WB group, mean STAI scores progressively decreased at each timepoint (A: 15.3, B: 13.6, C: 12.7) and mean VAS-A scores followed a similar trend (A: 24.2, B: 19.3, C: 10.5). In the control group, the mean STAI scores remained stable at timepoints A and B (17.7) and then decreased at timepoint C (14.8). The mean VAS-A scores in the control group followed a similar pattern, remaining stable at timepoints A (22.9) and B (22.8) and then decreasing at timepoint C (14.4). These changes were not statistically significant.

Mean vital signs for both the WB and control groups were relatively stable across all timepoints, although they tended to decrease by timepoint C. In the WB group, mean heart rates were 69, 69, and 67 beats per minute at timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 136 mm Hg and mean diastolic pressures were 71 mm Hg, 68 mm Hg, and 66 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 20, 19, and 18 breaths per minute at timepoints A, B, and C, respectively. In the control group, mean heart rates were 70, 69, and 68 beats per minute across timepoints A, B, and C, respectively. Mean systolic blood pressures were 137 mm Hg, 138 mm Hg, and 133 mm Hg and mean diastolic pressures were 71 mm Hg, 74 mm Hg, and 68 mm Hg at timepoints A, B, and C, respectively. Mean respiratory rates were 19, 18, and 18 breaths per minute at timepoints A, B, and C, respectively. These changes were not statistically significant.
Our pilot study examined the effects of using a WB to alleviate preoperative anxiety during MMS. Our results suggest that WBs may modestly improve subjective anxiety immediately prior to undergoing MMS. Mean STAI and VAS-A scores decreased from timepoint A to timepoint B in the WB group vs the control group in which these scores remained stable. Although our study was not powered to determine statistical differences and significance was not reached, our results suggest a favorable trend in decreased anxiety scores. Our analysis was limited by a small sample size; therefore, additional larger-scale studies will be needed to confirm this trend.
Our results are broadly consistent with earlier studies that found improvement in physiologic proxies of anxiety with the use of WBs during chemotherapy infusions, dental procedures, and acute inpatient mental health hospitalizations.7-10 During periods of high anxiety, use of WBs shifts the autonomic nervous system from a sympathetic to a parasympathetic state, as demonstrated by increased high-frequency heart rate variability, a marker of parasympathetic activity.6,11 While the exact mechanism of how WBs and other deep pressure stimulation tools affect high-frequency heart rate variability is unclear, one study showed that patients undergoing dental extractions were better equipped when using deep pressure stimulation tools to utilize calming techniques and regulate stress.12 The use of WBs and other deep pressure stimulation tools may extend beyond the perioperative setting and also may be an effective tool for clinicians in other settings (eg, clinic visits, physical examinations).
In our study, all participants demonstrated the greatest reduction in anxiety at timepoint C after the first MMS stage, likely related to patients relaxing more after knowing what to expect from the surgery; this also may have been reflected somewhat in the slight downward trend noted in vital signs across both study groups. One concern regarding WB use in surgical settings is whether the added pressure could trigger unfavorable circulatory effects, such as elevated blood pressure. In our study, with the exception of diastolic blood pressure, vital signs appeared unaffected by the type of blanket used and remained relatively stable from timepoint A to timepoint B and decreased at timepoint C. Diastolic blood pressure in the WB group decreased from timepoint A to timepoint B, then decreased further from timepoint B to timepoint C. This mirrored the decreasing STAI score trend, compared to the control group who increased from timepoint A to timepoint B and reached a nadir at timepoint C. Consistent with prior WB studies, there were no adverse effects from WBs, including adverse impacts on vital signs.6,9
The original recruitment goal was not met due to staffing issues related to the COVID-19 pandemic, and subgroup analyses were deferred as a result of sample size limitations. It is possible that the WB intervention may have a larger impact on subpopulations more prone to perioperative anxiety (eg, patients undergoing MMS for the first time). However, the results of our pilot study suggest a beneficial effect from the use of WBs. While these preliminary data are promising, additional studies in the perioperative setting are needed to more accurately determine the clinical utility of WBs during MMS and other procedures.
- Eberle FC, Schippert W, Trilling B, et al. Cosmetic results of histographically controlled excision of non-melanoma skin cancer in the head and neck region. J Dtsch Dermatol Ges. 2005;3:109-112. doi:10.1111/j.1610-0378.2005.04738.x
- Chren MM, Sahay AP, Bertenthal DS, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351-1357. doi:10.1038/sj.jid.5700740
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035. doi:10.1097 /DSS.0000000000001152
- Pritchard MJ. Identifying and assessing anxiety in pre-operative patients. Nurs Stand. 2009;23:35-40. doi:10.7748/ns2009.08.23.51.35.c7222.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Mullen B, Champagne T, Krishnamurty S, et al. Exploring the safety and therapeutic effects of deep pressure stimulation using a weighted blanket. Occup Ther Ment Health. 2008;24:65-89. doi:10.1300/ J004v24n01_05
- Chen HY, Yang H, Chi HJ, et al. Physiological effects of deep touch pressure on anxiety alleviation: the weighted blanket approach. J Med Biol Eng. 2013;33:463-470. doi:10.5405/jmbe.1043
- Chen HY, Yang H, Meng LF, et al. Effect of deep pressure input on parasympathetic system in patients with wisdom tooth surgery. J Formos Med Assoc. 2016;115:853-859. doi:10.1016 /j.jfma.2016.07.008
- Vinson J, Powers J, Mosesso K. Weighted blankets: anxiety reduction in adult patients receiving chemotherapy. Clin J Oncol Nurs. 2020; 24:360-368. doi:10.1188/20.CJON.360-368
- Champagne T, Mullen B, Dickson D, et al. Evaluating the safety and effectiveness of the weighted blanket with adults during an inpatient mental health hospitalization. Occup Ther Ment Health. 2015;31:211-233. doi:10.1080/0164212X.2015.1066220
- Lane RD, McRae K, Reiman EM, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213-222. doi: 10.1016/j.neuroimage.2008.07.056
- Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130:3-18. doi: 10.1037 /0033-2909.130.1.3
- Eberle FC, Schippert W, Trilling B, et al. Cosmetic results of histographically controlled excision of non-melanoma skin cancer in the head and neck region. J Dtsch Dermatol Ges. 2005;3:109-112. doi:10.1111/j.1610-0378.2005.04738.x
- Chren MM, Sahay AP, Bertenthal DS, et al. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127:1351-1357. doi:10.1038/sj.jid.5700740
- Kossintseva I, Zloty D. Determinants and timeline of perioperative anxiety in Mohs surgery. Dermatol Surg. 2017;43:1029-1035. doi:10.1097 /DSS.0000000000001152
- Pritchard MJ. Identifying and assessing anxiety in pre-operative patients. Nurs Stand. 2009;23:35-40. doi:10.7748/ns2009.08.23.51.35.c7222.
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Mullen B, Champagne T, Krishnamurty S, et al. Exploring the safety and therapeutic effects of deep pressure stimulation using a weighted blanket. Occup Ther Ment Health. 2008;24:65-89. doi:10.1300/ J004v24n01_05
- Chen HY, Yang H, Chi HJ, et al. Physiological effects of deep touch pressure on anxiety alleviation: the weighted blanket approach. J Med Biol Eng. 2013;33:463-470. doi:10.5405/jmbe.1043
- Chen HY, Yang H, Meng LF, et al. Effect of deep pressure input on parasympathetic system in patients with wisdom tooth surgery. J Formos Med Assoc. 2016;115:853-859. doi:10.1016 /j.jfma.2016.07.008
- Vinson J, Powers J, Mosesso K. Weighted blankets: anxiety reduction in adult patients receiving chemotherapy. Clin J Oncol Nurs. 2020; 24:360-368. doi:10.1188/20.CJON.360-368
- Champagne T, Mullen B, Dickson D, et al. Evaluating the safety and effectiveness of the weighted blanket with adults during an inpatient mental health hospitalization. Occup Ther Ment Health. 2015;31:211-233. doi:10.1080/0164212X.2015.1066220
- Lane RD, McRae K, Reiman EM, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213-222. doi: 10.1016/j.neuroimage.2008.07.056
- Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130:3-18. doi: 10.1037 /0033-2909.130.1.3
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
Weighted Blankets May Help Reduce Preoperative Anxiety During Mohs Micrographic Surgery
PRACTICE POINTS
- Preoperative anxiety in patients during Mohs micrographic surgery (MMS) may increase intraoperative bleeding, complication rates, and recovery times.
- Using weighted blankets may reduce anxiety in patients undergoing MMS of the head and neck.
Bullous Pemphigoid Masquerading as a Prosthesis Allergy
To the Editor:
Bullous pemphigoid (BP) is an autoimmune bullous dermatosis characterized by tense subepidermal blisters. It primarily affects older individuals who typically report pruritus in the affected area. Subepidermal blisters are caused by a humoral and cellular autoimmune attack directed against 2 BP antigens—BP180 and BP230—which are 2 critical components of the hemidesmosome whose primary function is to anchor the epidermis to the underlying dermis. Although tense bullae typically prompt immediate consideration of BP in the differential diagnosis, early disease often is characterized by urticarial plaques that require a high degree of suspicion to make the appropriate diagnosis. Locus minoris resistentiae is a term used to describe the phenomenon of skin disease occurring at the point of least resistance.1
A 79-year-old woman with type 2 diabetes mellitus, peptic ulcer disease, and hypertension was referred to the dermatology clinic due to concern for allergic contact dermatitis limited to the area of and adjacent to a well-healed surgical wound. History and examination revealed that the patient had sustained a left femoral neck fracture 10 months prior to presentation that required closed reduction and surgical pinning. The surgical site healed well postoperatively; however, 7 months after surgery, she began to develop edema and erythema within and immediately adjacent to the surgical scar. She subsequently developed areas of superficial erosion within the erythema and was evaluated by her surgeon who was concerned for suture granuloma. Superficial wound debridement of the area was performed without improvement. Approximately 9 months after surgery, the patient developed bullae along the old surgical site, which raised concern for an allergic reaction to the implanted screws. Orthopedics elected to remove the hardware but also sent intraoperative tissue for pathologic examination, which revealed subepidermal bullae containing eosinophils and neutrophils, most consistent with a bullous drug eruption. During the ensuing weeks after hardware removal, the plaque spread along the old surgical wound, and several bullous lesions began to appear. The patient’s primary care physician became concerned for allergic contact dermatitis, possibly to the surgical scrub employed during hardware removal. He prescribed triamcinolone ointment 0.1% and referred the patient to dermatology.
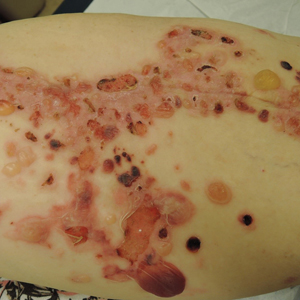
Upon presentation to dermatology, the patient noted stinging pain and intense pruritus of the affected area. Examination revealed a pink edematous plaque distributed along a well-healed surgical wound (Figure). Numerous fluid-filled tense bullae were superimposed on this plaque as well as areas of superficial erosion with serum crust. An expanded examination revealed similar smaller lesions on the upper arms, inner thighs, and lateral breasts. A 4-mm punch biopsy of lesional and perilesional skin was sent for hematoxylin and eosin staining and direct immunofluorescence, which demonstrated a subepidermal bullous dermatosis with a predominance of neutrophilic inflammation as well as a band of linear IgG deposition at the dermal-epidermal junction. The patient was diagnosed with BP exhibiting a locus minoris resistentiae phenomenon within the surgical site. She was started on prednisone 1 mg/kg daily and doxycycline 100 mg twice daily and demonstrated rapid improvement.

Although the tense bullae seen in well-developed BP are fairly characteristic, the prodromal phase of this disease can present with urticarial plaques that are nonspecific. This progression is well described, but our case demonstrates the difficulty of considering BP when a patient presents with an urticarial plaque. As lesions progress to the bullous phase, they may be inappropriately diagnosed as allergic contact dermatitis, an error that may lead to unnecessary interventions (eg, removal of an implicated prosthesis). This case is a reminder that not all cutaneous eruptions in and around postsurgical scars are allergic in nature.
This case also depicts BP appearing in the locus minoris resistentiae, a well-healed surgical wound in our patient. Although many diseases have been shown to exhibit this type of isomorphic response, this phenomenon may pose diagnostic and management conundrums. Locus minoris resistentiae has been reported in many different diseases, both cutaneous and otherwise, but there likely are distinct disease- and case-specific mechanisms via which this occurs. Local phenomena reported to trigger BP include contact dermatitis, vaccination, radiation therapy, phototherapy, infection, and surgery.2 We suspect that the mechanism of locus minoris resistentiae in our patient was disruption of the architecture of the dermal-epidermal basement membrane zone due to surgical trauma. Disruption of this architecture may have resulted in exposure of previously occult antigens, recognition by T cells, T-cell stimulation of autoantibody production by B cells, binding of autoantibodies to BP180, complement deposition, recruitment of inflammatory cells, release of proteinases, and degradation of BP180 and extracellular matrix proteins.2
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
To the Editor:
Bullous pemphigoid (BP) is an autoimmune bullous dermatosis characterized by tense subepidermal blisters. It primarily affects older individuals who typically report pruritus in the affected area. Subepidermal blisters are caused by a humoral and cellular autoimmune attack directed against 2 BP antigens—BP180 and BP230—which are 2 critical components of the hemidesmosome whose primary function is to anchor the epidermis to the underlying dermis. Although tense bullae typically prompt immediate consideration of BP in the differential diagnosis, early disease often is characterized by urticarial plaques that require a high degree of suspicion to make the appropriate diagnosis. Locus minoris resistentiae is a term used to describe the phenomenon of skin disease occurring at the point of least resistance.1
A 79-year-old woman with type 2 diabetes mellitus, peptic ulcer disease, and hypertension was referred to the dermatology clinic due to concern for allergic contact dermatitis limited to the area of and adjacent to a well-healed surgical wound. History and examination revealed that the patient had sustained a left femoral neck fracture 10 months prior to presentation that required closed reduction and surgical pinning. The surgical site healed well postoperatively; however, 7 months after surgery, she began to develop edema and erythema within and immediately adjacent to the surgical scar. She subsequently developed areas of superficial erosion within the erythema and was evaluated by her surgeon who was concerned for suture granuloma. Superficial wound debridement of the area was performed without improvement. Approximately 9 months after surgery, the patient developed bullae along the old surgical site, which raised concern for an allergic reaction to the implanted screws. Orthopedics elected to remove the hardware but also sent intraoperative tissue for pathologic examination, which revealed subepidermal bullae containing eosinophils and neutrophils, most consistent with a bullous drug eruption. During the ensuing weeks after hardware removal, the plaque spread along the old surgical wound, and several bullous lesions began to appear. The patient’s primary care physician became concerned for allergic contact dermatitis, possibly to the surgical scrub employed during hardware removal. He prescribed triamcinolone ointment 0.1% and referred the patient to dermatology.
Upon presentation to dermatology, the patient noted stinging pain and intense pruritus of the affected area. Examination revealed a pink edematous plaque distributed along a well-healed surgical wound (Figure). Numerous fluid-filled tense bullae were superimposed on this plaque as well as areas of superficial erosion with serum crust. An expanded examination revealed similar smaller lesions on the upper arms, inner thighs, and lateral breasts. A 4-mm punch biopsy of lesional and perilesional skin was sent for hematoxylin and eosin staining and direct immunofluorescence, which demonstrated a subepidermal bullous dermatosis with a predominance of neutrophilic inflammation as well as a band of linear IgG deposition at the dermal-epidermal junction. The patient was diagnosed with BP exhibiting a locus minoris resistentiae phenomenon within the surgical site. She was started on prednisone 1 mg/kg daily and doxycycline 100 mg twice daily and demonstrated rapid improvement.

Although the tense bullae seen in well-developed BP are fairly characteristic, the prodromal phase of this disease can present with urticarial plaques that are nonspecific. This progression is well described, but our case demonstrates the difficulty of considering BP when a patient presents with an urticarial plaque. As lesions progress to the bullous phase, they may be inappropriately diagnosed as allergic contact dermatitis, an error that may lead to unnecessary interventions (eg, removal of an implicated prosthesis). This case is a reminder that not all cutaneous eruptions in and around postsurgical scars are allergic in nature.
This case also depicts BP appearing in the locus minoris resistentiae, a well-healed surgical wound in our patient. Although many diseases have been shown to exhibit this type of isomorphic response, this phenomenon may pose diagnostic and management conundrums. Locus minoris resistentiae has been reported in many different diseases, both cutaneous and otherwise, but there likely are distinct disease- and case-specific mechanisms via which this occurs. Local phenomena reported to trigger BP include contact dermatitis, vaccination, radiation therapy, phototherapy, infection, and surgery.2 We suspect that the mechanism of locus minoris resistentiae in our patient was disruption of the architecture of the dermal-epidermal basement membrane zone due to surgical trauma. Disruption of this architecture may have resulted in exposure of previously occult antigens, recognition by T cells, T-cell stimulation of autoantibody production by B cells, binding of autoantibodies to BP180, complement deposition, recruitment of inflammatory cells, release of proteinases, and degradation of BP180 and extracellular matrix proteins.2
To the Editor:
Bullous pemphigoid (BP) is an autoimmune bullous dermatosis characterized by tense subepidermal blisters. It primarily affects older individuals who typically report pruritus in the affected area. Subepidermal blisters are caused by a humoral and cellular autoimmune attack directed against 2 BP antigens—BP180 and BP230—which are 2 critical components of the hemidesmosome whose primary function is to anchor the epidermis to the underlying dermis. Although tense bullae typically prompt immediate consideration of BP in the differential diagnosis, early disease often is characterized by urticarial plaques that require a high degree of suspicion to make the appropriate diagnosis. Locus minoris resistentiae is a term used to describe the phenomenon of skin disease occurring at the point of least resistance.1
A 79-year-old woman with type 2 diabetes mellitus, peptic ulcer disease, and hypertension was referred to the dermatology clinic due to concern for allergic contact dermatitis limited to the area of and adjacent to a well-healed surgical wound. History and examination revealed that the patient had sustained a left femoral neck fracture 10 months prior to presentation that required closed reduction and surgical pinning. The surgical site healed well postoperatively; however, 7 months after surgery, she began to develop edema and erythema within and immediately adjacent to the surgical scar. She subsequently developed areas of superficial erosion within the erythema and was evaluated by her surgeon who was concerned for suture granuloma. Superficial wound debridement of the area was performed without improvement. Approximately 9 months after surgery, the patient developed bullae along the old surgical site, which raised concern for an allergic reaction to the implanted screws. Orthopedics elected to remove the hardware but also sent intraoperative tissue for pathologic examination, which revealed subepidermal bullae containing eosinophils and neutrophils, most consistent with a bullous drug eruption. During the ensuing weeks after hardware removal, the plaque spread along the old surgical wound, and several bullous lesions began to appear. The patient’s primary care physician became concerned for allergic contact dermatitis, possibly to the surgical scrub employed during hardware removal. He prescribed triamcinolone ointment 0.1% and referred the patient to dermatology.
Upon presentation to dermatology, the patient noted stinging pain and intense pruritus of the affected area. Examination revealed a pink edematous plaque distributed along a well-healed surgical wound (Figure). Numerous fluid-filled tense bullae were superimposed on this plaque as well as areas of superficial erosion with serum crust. An expanded examination revealed similar smaller lesions on the upper arms, inner thighs, and lateral breasts. A 4-mm punch biopsy of lesional and perilesional skin was sent for hematoxylin and eosin staining and direct immunofluorescence, which demonstrated a subepidermal bullous dermatosis with a predominance of neutrophilic inflammation as well as a band of linear IgG deposition at the dermal-epidermal junction. The patient was diagnosed with BP exhibiting a locus minoris resistentiae phenomenon within the surgical site. She was started on prednisone 1 mg/kg daily and doxycycline 100 mg twice daily and demonstrated rapid improvement.

Although the tense bullae seen in well-developed BP are fairly characteristic, the prodromal phase of this disease can present with urticarial plaques that are nonspecific. This progression is well described, but our case demonstrates the difficulty of considering BP when a patient presents with an urticarial plaque. As lesions progress to the bullous phase, they may be inappropriately diagnosed as allergic contact dermatitis, an error that may lead to unnecessary interventions (eg, removal of an implicated prosthesis). This case is a reminder that not all cutaneous eruptions in and around postsurgical scars are allergic in nature.
This case also depicts BP appearing in the locus minoris resistentiae, a well-healed surgical wound in our patient. Although many diseases have been shown to exhibit this type of isomorphic response, this phenomenon may pose diagnostic and management conundrums. Locus minoris resistentiae has been reported in many different diseases, both cutaneous and otherwise, but there likely are distinct disease- and case-specific mechanisms via which this occurs. Local phenomena reported to trigger BP include contact dermatitis, vaccination, radiation therapy, phototherapy, infection, and surgery.2 We suspect that the mechanism of locus minoris resistentiae in our patient was disruption of the architecture of the dermal-epidermal basement membrane zone due to surgical trauma. Disruption of this architecture may have resulted in exposure of previously occult antigens, recognition by T cells, T-cell stimulation of autoantibody production by B cells, binding of autoantibodies to BP180, complement deposition, recruitment of inflammatory cells, release of proteinases, and degradation of BP180 and extracellular matrix proteins.2
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
Practice Points
- Bullous pemphigoid frequently presents with urticarial plaques without classic tense blisters in the early phase of disease.
- The phenomenon of locus minoris resistentiae can lead to the presentation of bullous pemphigoid in locations traumatized by surgery.
- Bullous pemphigoid can present as urticarial plaques at surgery sites mimicking allergic contact dermatitis or reaction to surgical sutures or hardware.
Atrophic Lesions in a Pregnant Woman
The Diagnosis: Degos Disease
The pathophysiology of Degos disease (malignant atrophic papulosis) is unknown.1 Histopathology demonstrates a wedge-shaped area of dermal necrosis with edema and mucin deposition extending from the papillary dermis to the deep reticular dermis. Occluded vessels, thrombosis, and perivascular lymphocytic infiltrates also may be seen, particularly at the dermal subcutaneous junction and at the periphery of the wedge-shaped infarction. The vascular damage that occurs may be the result of vasculitis, coagulopathy, or endothelial cell dysfunction.1
Patients typically present with small, round, erythematous papules that eventually develop atrophic porcelain white centers and telangiectatic rims. These lesions most commonly occur on the trunk and arms. In the benign form of atrophic papulosis, only the skin is involved; however, systemic involvement of the gastrointestinal tract and central nervous system can occur, resulting in bowel perforation and stroke, respectively.1 Although there is no definitive treatment of Degos disease, successful therapy with aspirin or dipyridamole has been reported.1 Eculizumab, a monoclonal antibody that binds C5, and treprostinil, a prostacyclin analog, are emerging treatment options.2,3 The differential diagnosis of Degos disease may include granuloma annulare, guttate extragenital lichen sclerosus, livedoid vasculopathy, and lymphomatoid papulosis.
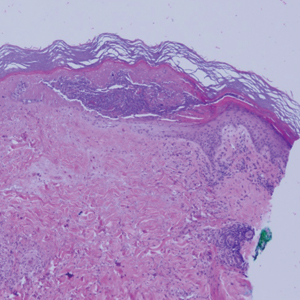
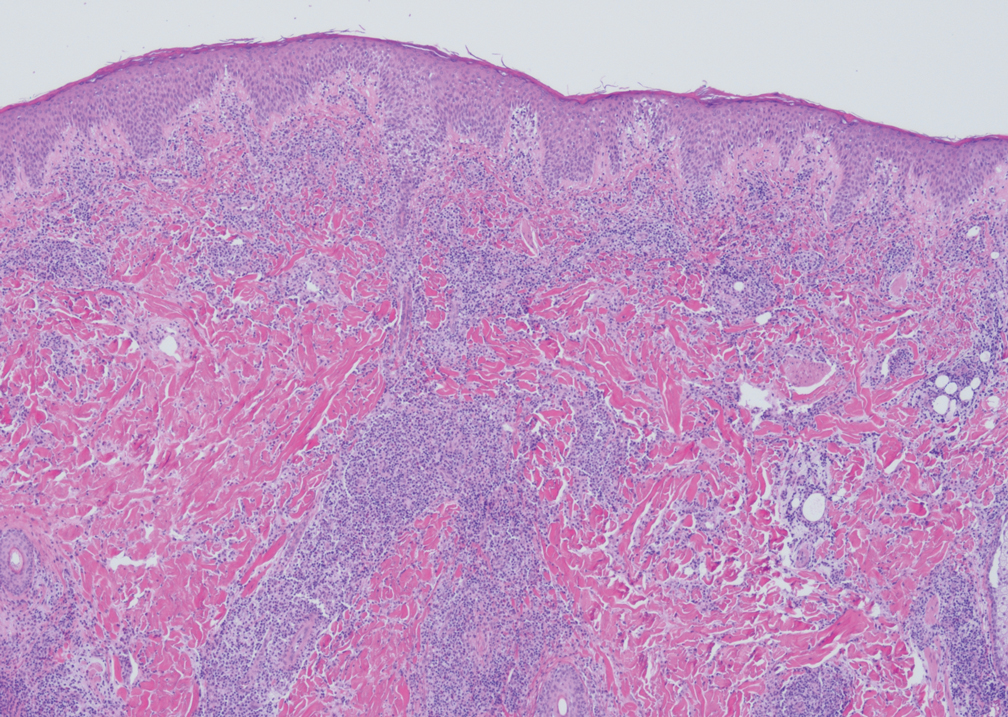
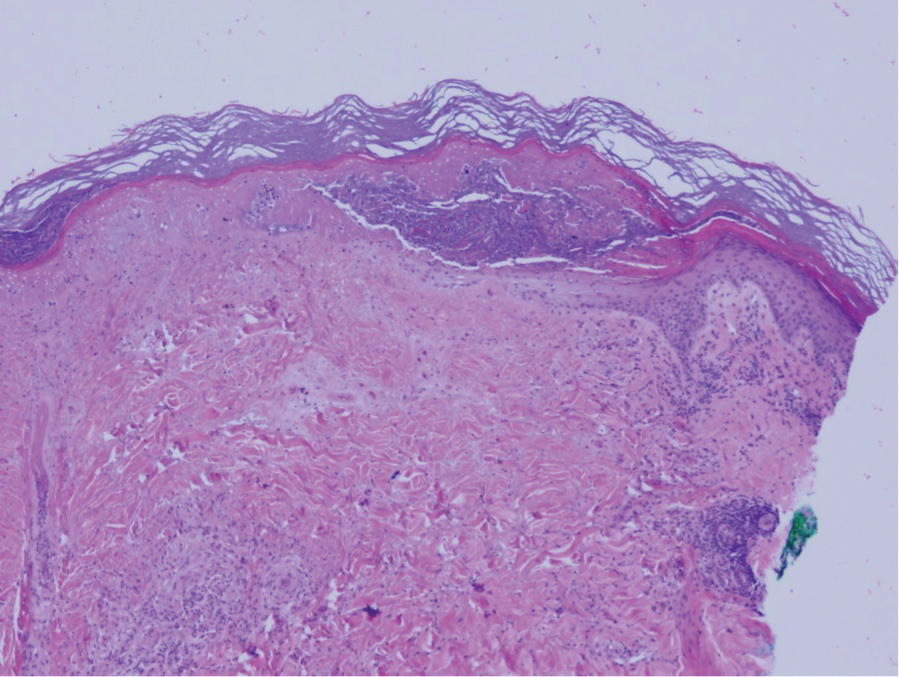
Granuloma annulare may clinically mimic the erythematous papules seen in early Degos disease, and histopathology can be used to distinguish between these two disease processes. Localized granuloma annulare is the most common variant and clinically presents as pink papules and plaques in an annular configuration.4 Histopathology demonstrates an unremarkable epidermis; however, the dermis contains degenerated collagen surrounded by palisading histiocytes as well as lymphocytes. Similar to Degos disease, increased mucin is seen within these areas of degeneration, but occluded vessels and thrombosis typically are not seen (Figure 1).4,5
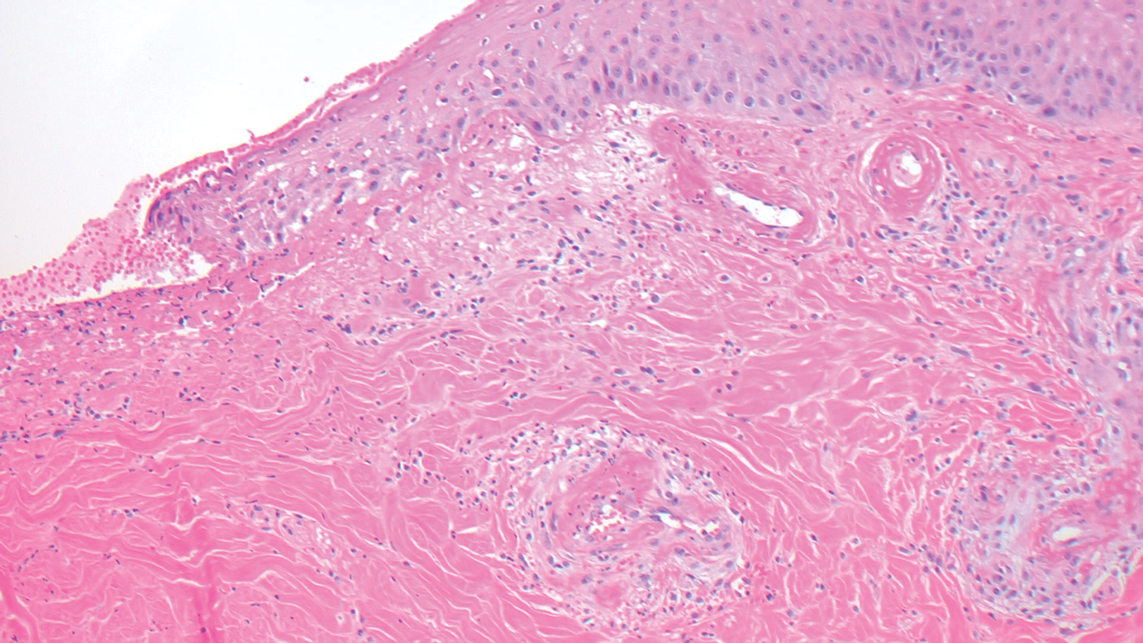
Guttate extragenital lichen sclerosus initially presents as polygonal, bluish white papules that coalesce into plaques.6 Over time, these lesions become more atrophic and may mimic Degos disease but appear differently on histopathology. Histopathology of lichen sclerosus classically demonstrates atrophy of the epidermis with loss of the rete ridges and vacuolar surface changes. Homogenization of the superficial/papillary dermis with an underlying bandlike lymphocytic infiltrate also is seen (Figure 2).6
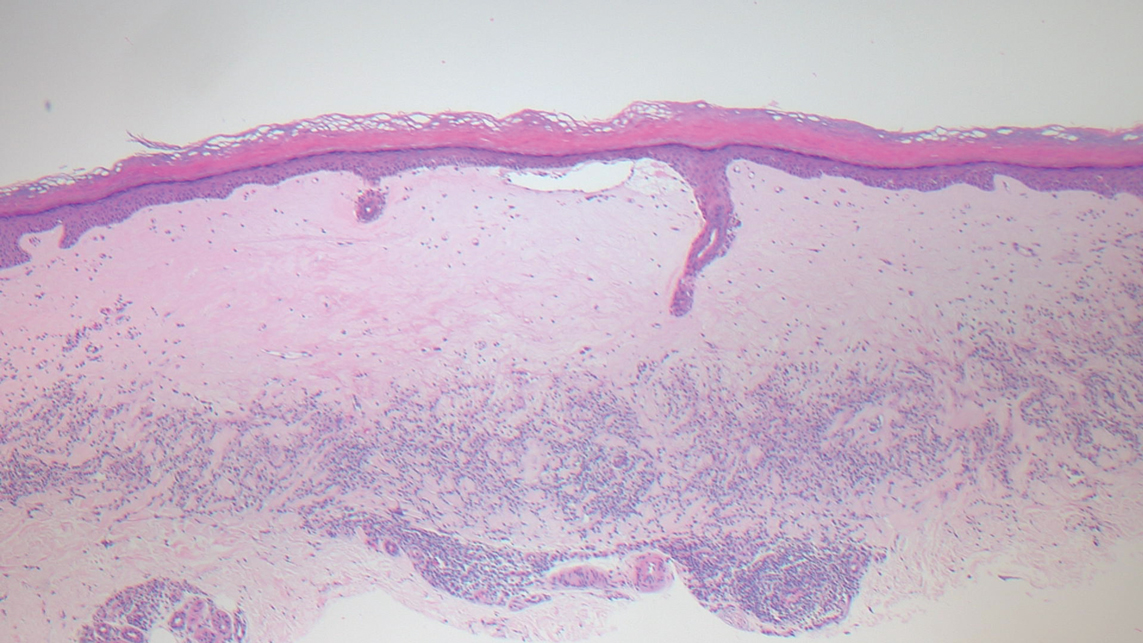
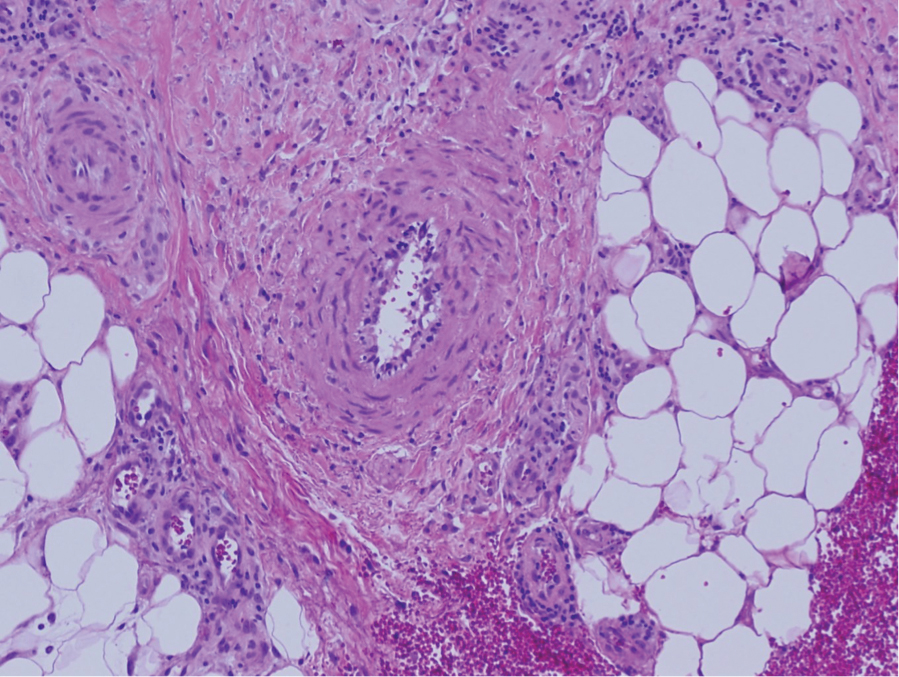
Livedoid vasculopathy is characterized by chronic recurrent ulceration of the legs secondary to thrombosis and subsequent ischemia. In the initial phase of this disease, livedo reticularis is seen followed by the development of ulcerations. As these ulcerations heal, they leave behind porcelain white scars referred to as atrophie blanche.7 The areas of scarring in livedoid vasculopathy are broad and angulated, differentiating them from the small, round, porcelain white macules in end-stage Degos disease. Histopathology demonstrates thrombosis and fibrin occlusion of the upper and mid dermal vessels. Very minimal perivascular infiltrate typically is seen, but when it is present, the infiltrate mostly is lymphocytic. Hyalinization of the vessel walls also is seen, particularly in the atrophie blanche stage (Figure 3).7
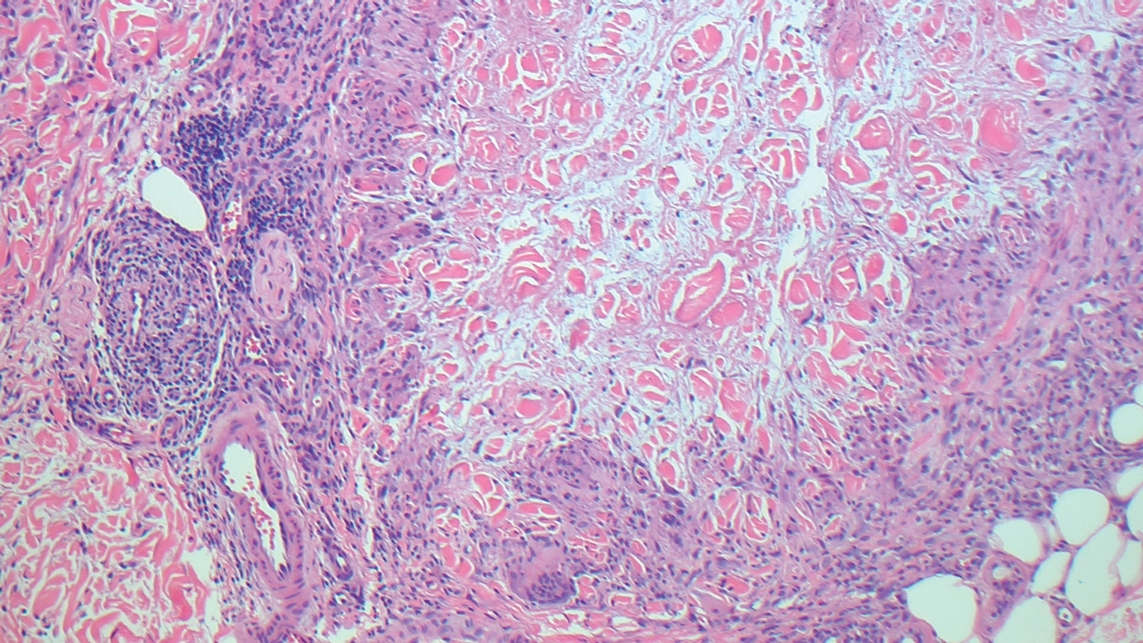
Lymphomatoid papulosis classically presents with pruritic red papules that often spontaneously involute. After resolution of the primary lesions, atrophic varioliform scars may be left behind that can resemble Degos disease.8 Classically, there are 5 histopathologic subtypes: A, B, C, D, and E. Type A is the most common type of lymphomatoid papulosis, and histopathology demonstrates a dermal lymphocytic infiltrate that consists of cells arranged in small clusters. Numerous medium- to large-sized atypical lymphocytes with prominent nucleoli and abundant cytoplasm are seen, and mitotic figures are common (Figure 4).8
Our case was particularly interesting because the patient was 2 to 3 weeks pregnant. Degos disease in pregnancy appears to be quite exceptional. A PubMed search of articles indexed for MEDLINE using the terms Degos disease and pregnancy revealed only 4 other cases reported in the literature.9-12 With the exception of a single case that was complicated by severe abdominal pain requiring labor induction, the other reported cases resulted in uncomplicated pregnancies.9-12 Conversely, our patient's pregnancy was complicated by gestational hypertension and fetal hydrops requiring a preterm cesarean delivery. Furthermore, the infant had multiple complications, which were attributed to both placental insufficiency and a coagulopathic state.
Our patient also was found to have a heterozygous factor V Leiden mutation on workup. A PubMed search using the terms factor V Leiden mutation and Degos disease revealed 2 other cases of factor V Leiden mutation-associated Degos disease.13,14 The importance of factor V Leiden mutations in patients with Degos disease currently is unclear.
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)--a review. Orphanet J Rare Dis. 2013;8:10.
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277.
- Magro CM, Wang X, Garrett-Bakelman F, et al. The effects of eculizumab on the pathology of malignant atrophic papulosis. Orphanet J Rare Dis. 2013;8:185.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Tronnier M, Mitteldorf C. Histologic features of granulomatous skin diseases. part 1: non-infectious granulomatous disorders. J Dtsch Dermatol Ges. 2015;13:211-216.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478‐488.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73.
- Moulin G, Barrut D, Franc MP, et al. Familial Degos' atrophic papulosis (mother-daughter). Ann Dermatol Venereol. 1984;111:149-155.
- Bogenrieder T, Kuske M, Landthaler M, et al. Benign Degos' disease developing during pregnancy and followed for 10 years. Acta Derm Venereol. 2002;82:284-287.
- Sharma S, Brennan B, Naden R, et al. A case of Degos disease in pregnancy. Obstet Med. 2016;9:167-168.
- Zhao Q, Zhang S, Dong A. An unusual case of abdominal pain. Gastroenterology. 2018;154:E1-E2.
- Darwich E, Guilabert A, Mascaró JM Jr, et al. Dermoscopic description of a patient with thrombocythemia and factor V Leiden mutation-associated Degos' disease. Int J Dermatol. 2011;50:604-606.
- Hohwy T, Jensen MG, Tøttrup A, et al. A fatal case of malignant atrophic papulosis (Degos' disease) in a man with factor V Leiden mutation and lupus anticoagulant. Acta Derm Venereol. 2006;86:245-247.
The Diagnosis: Degos Disease
The pathophysiology of Degos disease (malignant atrophic papulosis) is unknown.1 Histopathology demonstrates a wedge-shaped area of dermal necrosis with edema and mucin deposition extending from the papillary dermis to the deep reticular dermis. Occluded vessels, thrombosis, and perivascular lymphocytic infiltrates also may be seen, particularly at the dermal subcutaneous junction and at the periphery of the wedge-shaped infarction. The vascular damage that occurs may be the result of vasculitis, coagulopathy, or endothelial cell dysfunction.1
Patients typically present with small, round, erythematous papules that eventually develop atrophic porcelain white centers and telangiectatic rims. These lesions most commonly occur on the trunk and arms. In the benign form of atrophic papulosis, only the skin is involved; however, systemic involvement of the gastrointestinal tract and central nervous system can occur, resulting in bowel perforation and stroke, respectively.1 Although there is no definitive treatment of Degos disease, successful therapy with aspirin or dipyridamole has been reported.1 Eculizumab, a monoclonal antibody that binds C5, and treprostinil, a prostacyclin analog, are emerging treatment options.2,3 The differential diagnosis of Degos disease may include granuloma annulare, guttate extragenital lichen sclerosus, livedoid vasculopathy, and lymphomatoid papulosis.
Granuloma annulare may clinically mimic the erythematous papules seen in early Degos disease, and histopathology can be used to distinguish between these two disease processes. Localized granuloma annulare is the most common variant and clinically presents as pink papules and plaques in an annular configuration.4 Histopathology demonstrates an unremarkable epidermis; however, the dermis contains degenerated collagen surrounded by palisading histiocytes as well as lymphocytes. Similar to Degos disease, increased mucin is seen within these areas of degeneration, but occluded vessels and thrombosis typically are not seen (Figure 1).4,5

Guttate extragenital lichen sclerosus initially presents as polygonal, bluish white papules that coalesce into plaques.6 Over time, these lesions become more atrophic and may mimic Degos disease but appear differently on histopathology. Histopathology of lichen sclerosus classically demonstrates atrophy of the epidermis with loss of the rete ridges and vacuolar surface changes. Homogenization of the superficial/papillary dermis with an underlying bandlike lymphocytic infiltrate also is seen (Figure 2).6

Livedoid vasculopathy is characterized by chronic recurrent ulceration of the legs secondary to thrombosis and subsequent ischemia. In the initial phase of this disease, livedo reticularis is seen followed by the development of ulcerations. As these ulcerations heal, they leave behind porcelain white scars referred to as atrophie blanche.7 The areas of scarring in livedoid vasculopathy are broad and angulated, differentiating them from the small, round, porcelain white macules in end-stage Degos disease. Histopathology demonstrates thrombosis and fibrin occlusion of the upper and mid dermal vessels. Very minimal perivascular infiltrate typically is seen, but when it is present, the infiltrate mostly is lymphocytic. Hyalinization of the vessel walls also is seen, particularly in the atrophie blanche stage (Figure 3).7

Lymphomatoid papulosis classically presents with pruritic red papules that often spontaneously involute. After resolution of the primary lesions, atrophic varioliform scars may be left behind that can resemble Degos disease.8 Classically, there are 5 histopathologic subtypes: A, B, C, D, and E. Type A is the most common type of lymphomatoid papulosis, and histopathology demonstrates a dermal lymphocytic infiltrate that consists of cells arranged in small clusters. Numerous medium- to large-sized atypical lymphocytes with prominent nucleoli and abundant cytoplasm are seen, and mitotic figures are common (Figure 4).8

Our case was particularly interesting because the patient was 2 to 3 weeks pregnant. Degos disease in pregnancy appears to be quite exceptional. A PubMed search of articles indexed for MEDLINE using the terms Degos disease and pregnancy revealed only 4 other cases reported in the literature.9-12 With the exception of a single case that was complicated by severe abdominal pain requiring labor induction, the other reported cases resulted in uncomplicated pregnancies.9-12 Conversely, our patient's pregnancy was complicated by gestational hypertension and fetal hydrops requiring a preterm cesarean delivery. Furthermore, the infant had multiple complications, which were attributed to both placental insufficiency and a coagulopathic state.
Our patient also was found to have a heterozygous factor V Leiden mutation on workup. A PubMed search using the terms factor V Leiden mutation and Degos disease revealed 2 other cases of factor V Leiden mutation-associated Degos disease.13,14 The importance of factor V Leiden mutations in patients with Degos disease currently is unclear.
The Diagnosis: Degos Disease
The pathophysiology of Degos disease (malignant atrophic papulosis) is unknown.1 Histopathology demonstrates a wedge-shaped area of dermal necrosis with edema and mucin deposition extending from the papillary dermis to the deep reticular dermis. Occluded vessels, thrombosis, and perivascular lymphocytic infiltrates also may be seen, particularly at the dermal subcutaneous junction and at the periphery of the wedge-shaped infarction. The vascular damage that occurs may be the result of vasculitis, coagulopathy, or endothelial cell dysfunction.1
Patients typically present with small, round, erythematous papules that eventually develop atrophic porcelain white centers and telangiectatic rims. These lesions most commonly occur on the trunk and arms. In the benign form of atrophic papulosis, only the skin is involved; however, systemic involvement of the gastrointestinal tract and central nervous system can occur, resulting in bowel perforation and stroke, respectively.1 Although there is no definitive treatment of Degos disease, successful therapy with aspirin or dipyridamole has been reported.1 Eculizumab, a monoclonal antibody that binds C5, and treprostinil, a prostacyclin analog, are emerging treatment options.2,3 The differential diagnosis of Degos disease may include granuloma annulare, guttate extragenital lichen sclerosus, livedoid vasculopathy, and lymphomatoid papulosis.
Granuloma annulare may clinically mimic the erythematous papules seen in early Degos disease, and histopathology can be used to distinguish between these two disease processes. Localized granuloma annulare is the most common variant and clinically presents as pink papules and plaques in an annular configuration.4 Histopathology demonstrates an unremarkable epidermis; however, the dermis contains degenerated collagen surrounded by palisading histiocytes as well as lymphocytes. Similar to Degos disease, increased mucin is seen within these areas of degeneration, but occluded vessels and thrombosis typically are not seen (Figure 1).4,5

Guttate extragenital lichen sclerosus initially presents as polygonal, bluish white papules that coalesce into plaques.6 Over time, these lesions become more atrophic and may mimic Degos disease but appear differently on histopathology. Histopathology of lichen sclerosus classically demonstrates atrophy of the epidermis with loss of the rete ridges and vacuolar surface changes. Homogenization of the superficial/papillary dermis with an underlying bandlike lymphocytic infiltrate also is seen (Figure 2).6

Livedoid vasculopathy is characterized by chronic recurrent ulceration of the legs secondary to thrombosis and subsequent ischemia. In the initial phase of this disease, livedo reticularis is seen followed by the development of ulcerations. As these ulcerations heal, they leave behind porcelain white scars referred to as atrophie blanche.7 The areas of scarring in livedoid vasculopathy are broad and angulated, differentiating them from the small, round, porcelain white macules in end-stage Degos disease. Histopathology demonstrates thrombosis and fibrin occlusion of the upper and mid dermal vessels. Very minimal perivascular infiltrate typically is seen, but when it is present, the infiltrate mostly is lymphocytic. Hyalinization of the vessel walls also is seen, particularly in the atrophie blanche stage (Figure 3).7

Lymphomatoid papulosis classically presents with pruritic red papules that often spontaneously involute. After resolution of the primary lesions, atrophic varioliform scars may be left behind that can resemble Degos disease.8 Classically, there are 5 histopathologic subtypes: A, B, C, D, and E. Type A is the most common type of lymphomatoid papulosis, and histopathology demonstrates a dermal lymphocytic infiltrate that consists of cells arranged in small clusters. Numerous medium- to large-sized atypical lymphocytes with prominent nucleoli and abundant cytoplasm are seen, and mitotic figures are common (Figure 4).8

Our case was particularly interesting because the patient was 2 to 3 weeks pregnant. Degos disease in pregnancy appears to be quite exceptional. A PubMed search of articles indexed for MEDLINE using the terms Degos disease and pregnancy revealed only 4 other cases reported in the literature.9-12 With the exception of a single case that was complicated by severe abdominal pain requiring labor induction, the other reported cases resulted in uncomplicated pregnancies.9-12 Conversely, our patient's pregnancy was complicated by gestational hypertension and fetal hydrops requiring a preterm cesarean delivery. Furthermore, the infant had multiple complications, which were attributed to both placental insufficiency and a coagulopathic state.
Our patient also was found to have a heterozygous factor V Leiden mutation on workup. A PubMed search using the terms factor V Leiden mutation and Degos disease revealed 2 other cases of factor V Leiden mutation-associated Degos disease.13,14 The importance of factor V Leiden mutations in patients with Degos disease currently is unclear.
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)--a review. Orphanet J Rare Dis. 2013;8:10.
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277.
- Magro CM, Wang X, Garrett-Bakelman F, et al. The effects of eculizumab on the pathology of malignant atrophic papulosis. Orphanet J Rare Dis. 2013;8:185.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Tronnier M, Mitteldorf C. Histologic features of granulomatous skin diseases. part 1: non-infectious granulomatous disorders. J Dtsch Dermatol Ges. 2015;13:211-216.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478‐488.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73.
- Moulin G, Barrut D, Franc MP, et al. Familial Degos' atrophic papulosis (mother-daughter). Ann Dermatol Venereol. 1984;111:149-155.
- Bogenrieder T, Kuske M, Landthaler M, et al. Benign Degos' disease developing during pregnancy and followed for 10 years. Acta Derm Venereol. 2002;82:284-287.
- Sharma S, Brennan B, Naden R, et al. A case of Degos disease in pregnancy. Obstet Med. 2016;9:167-168.
- Zhao Q, Zhang S, Dong A. An unusual case of abdominal pain. Gastroenterology. 2018;154:E1-E2.
- Darwich E, Guilabert A, Mascaró JM Jr, et al. Dermoscopic description of a patient with thrombocythemia and factor V Leiden mutation-associated Degos' disease. Int J Dermatol. 2011;50:604-606.
- Hohwy T, Jensen MG, Tøttrup A, et al. A fatal case of malignant atrophic papulosis (Degos' disease) in a man with factor V Leiden mutation and lupus anticoagulant. Acta Derm Venereol. 2006;86:245-247.
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)--a review. Orphanet J Rare Dis. 2013;8:10.
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277.
- Magro CM, Wang X, Garrett-Bakelman F, et al. The effects of eculizumab on the pathology of malignant atrophic papulosis. Orphanet J Rare Dis. 2013;8:185.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Tronnier M, Mitteldorf C. Histologic features of granulomatous skin diseases. part 1: non-infectious granulomatous disorders. J Dtsch Dermatol Ges. 2015;13:211-216.
- Fistarol SK, Itin PH. Diagnosis and treatment of lichen sclerosus: an update. Am J Clin Dermatol. 2013;14:27-47.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478‐488.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73.
- Moulin G, Barrut D, Franc MP, et al. Familial Degos' atrophic papulosis (mother-daughter). Ann Dermatol Venereol. 1984;111:149-155.
- Bogenrieder T, Kuske M, Landthaler M, et al. Benign Degos' disease developing during pregnancy and followed for 10 years. Acta Derm Venereol. 2002;82:284-287.
- Sharma S, Brennan B, Naden R, et al. A case of Degos disease in pregnancy. Obstet Med. 2016;9:167-168.
- Zhao Q, Zhang S, Dong A. An unusual case of abdominal pain. Gastroenterology. 2018;154:E1-E2.
- Darwich E, Guilabert A, Mascaró JM Jr, et al. Dermoscopic description of a patient with thrombocythemia and factor V Leiden mutation-associated Degos' disease. Int J Dermatol. 2011;50:604-606.
- Hohwy T, Jensen MG, Tøttrup A, et al. A fatal case of malignant atrophic papulosis (Degos' disease) in a man with factor V Leiden mutation and lupus anticoagulant. Acta Derm Venereol. 2006;86:245-247.
A 36-year-old pregnant woman presented with painful erythematous papules on the palms and fingers of 2 months’ duration. Similar lesions developed on the thighs and feet several weeks later. Two tender macules with central areas of porcelain white scarring rimmed by telangiectases on the right foot also were present. A punch biopsy of these lesions demonstrated a wedge-shaped area of ischemic necrosis associated with dermal mucin without associated necrobiosis. Fibrin thrombi were seen within several small dermal vessels and were associated with a perivascular lymphocytic infiltrate. Endotheliitis was observed within a deep dermal vessel. Laboratory workup including syphilis IgG, antinuclear antibodies, extractable nuclear antigen antibodies, anti–double-stranded DNA, antistreptolysin O antibodies, Russell viper venom time, cryoglobulin, hepatitis screening, perinuclear antineutrophil cytoplasmic antibodies (ANCA), and cytoplasmic ANCA was unremarkable. Hypercoagulable studies including prothrombin gene mutation, factor V Leiden, plasminogen, proteins C and S, antithrombin III, homocysteine, and antiphospholipid IgM and IgG antibodies were notable only for heterozygosity for factor V Leiden.
Herpes Zoster Following Varicella Vaccination in Children
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
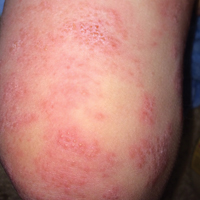
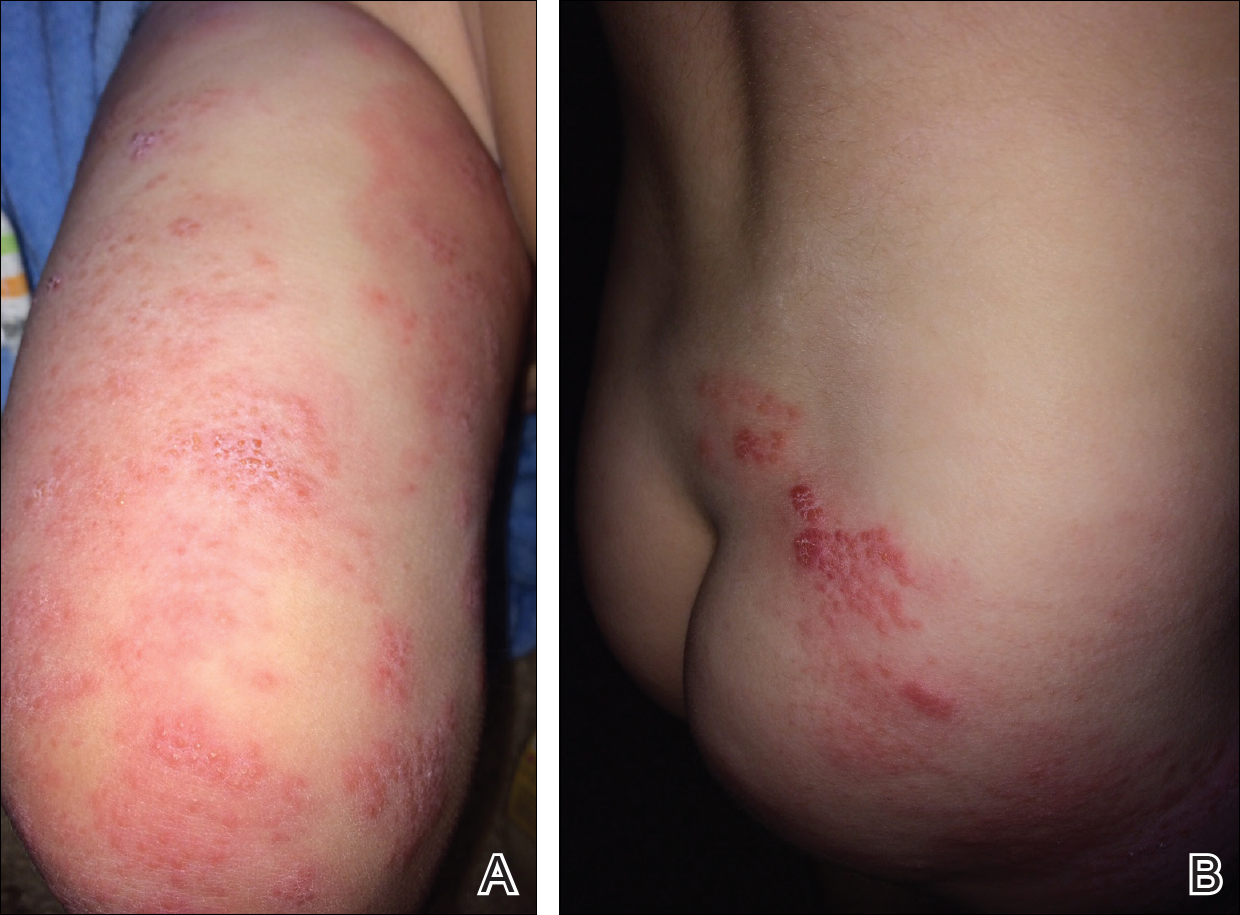
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.
Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.
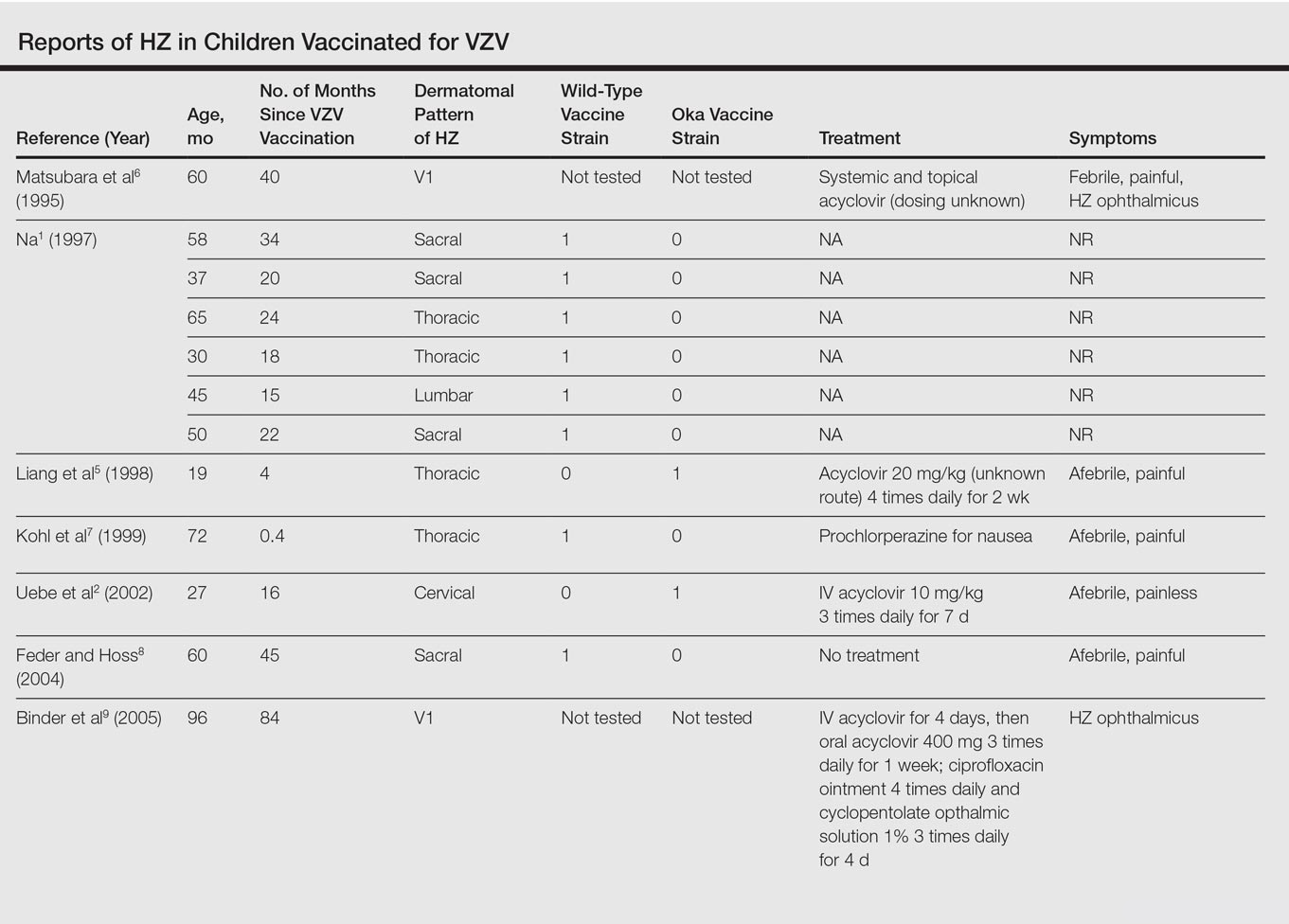

The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.

Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.


The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
Varicella-zoster virus (VZV) causes varicella as a primary infection. It is a highly contagious disease characterized by a widespread papulovesicular eruption with fever and malaise.1,2 After the primary infection, the virus remains latent within the sensory dorsal root ganglia and can reactivate as herpes zoster (HZ).1-5 Herpes zoster is characterized by unilateral radicular pain and a vesicular rash in a dermatomal pattern.1,2 It is most common in adults, especially elderly and immunocompromised patients, but rarely occurs in children. Herpes zoster is most often seen in individuals previously infected with VZV, but it also has occurred in individuals without known varicella infection,1-17 possibly because these individuals had a prior subclinical VZV infection.
A live attenuated VZV vaccine was created after isolation of the virus from a child in Japan.2 Since the introduction of the vaccine in 1995 in the United States, the incidence of VZV and HZ has declined.5 Herpes zoster rates after vaccination vary from 14 to 19 per 100,000 individuals.3,5 Breakthrough disease with the wild-type strain does occur in vaccinated children, but vaccine-strain HZ also has been reported.1-5 The risk for HZ caused by reactivated VZV vaccine in healthy children is unknown. We present a case of HZ in an otherwise healthy 19-month-old boy with no known varicella exposure who received the VZV vaccine at 13 months of age.
Case Report
An otherwise healthy 19-month-old boy presented to the dermatology clinic with a rash that began 2 days prior on the right groin and spread to the right leg. The patient’s mother denied that the child had been febrile and noted that the rash did not appear to bother him in any way. The patient was up-to-date on his vaccinations and received the first dose of the varicella series 6 months prior to presentation. He had no personal history of varicella, no exposure to sick contacts with varicella, and no known exposure to the virus. He was otherwise completely healthy with no signs or symptoms of immunocompromise.
Physical examination revealed grouped vesicles on an erythematous base on the right thigh, right sacrum, and lower abdomen that did not cross the midline (Figure). There were no other pertinent physical examination findings. The eruption was most consistent with HZ but concern remained for herpes simplex virus (HSV) or impetigo. A bacterial culture and polymerase chain reaction assay for VZV and HSV from skin swabs was ordered. The patient was prescribed acyclovir 20 mg/kg every 6 hours for 5 days. Laboratory testing revealed a positive result for VZV on polymerase chain reaction and a negative result for HSV. The majority of the patient’s lesions had crusted after 2 days of treatment with acyclovir, and the rash had nearly resolved 1 week after presentation. Subsequent evaluation with a complete blood cell count with differential and basic metabolic profile was normal. Levels of IgG, IgA, and IgM also were normal; IgE was slightly elevated.

Comment
Herpes zoster in children is an uncommon clinical entity. Most children with HZ are immunocompromised, have a history of varicella, or were exposed to varicella during gestation.8 With the introduction of the live VZV vaccine, the incidence of HZ has declined, but reactivation of the live vaccine leading to HZ infection is possible. The vaccine is 90% effective, and breakthrough varicella has been reported in 15% to 20% of vaccinated patients.1-17 The cause of HZ in vaccinated children is unclear due to the potential for either wild-type or vaccine-strain VZV to induce HZ.
Twenty-two cases of HZ in healthy children after vaccination were identified with a PubMed search of articles indexed for MEDLINE using the search terms herpes zoster infection after vaccination and herpes zoster infection AND immunocompetent AND vaccination in separate searches for all English-language studies (Table). The search was limited to immunocompetent children and adolescents who were 18 years or younger with no history of varicella or exposure to varicella during gestation.


The mean age for HZ infection was 5.3 years. The average time between vaccination and HZ infection was 3.3 years. There was a spread of dermatomal patterns with cases in the first division of the trigeminal nerve, cervical, thoracic, lumbar, and sacral distributions. Of the 22 cases of HZ we reviewed, 16 underwent genotype testing to determine the source of the infection. The Oka vaccine strain virus was identified in 8 (50%) cases, while wild-type virus was found in 8 (50%) cases.1,2,4,5,7,8,10,11,13,14,16 Twelve cases were treated with acyclovir.2,3,5,6,9-12,14-17 The method of delivery, either oral or intravenous, and the length of treatment depended on the severity of the disease. Patients with meningoencephalitis and HZ ophthalmicus received intravenous acyclovir more often and also had a longer course of acyclovir compared to those individuals with involvement limited to the skin.
This review found HZ occurs from reactivation of wild-type or Oka vaccine-strain VZV in immunocompetent children.1-17 It shows that subclinical varicella infection is not the only explanation for HZ in a healthy vaccinated child. It is currently not clear why some healthy children experience HZ from vaccine-strain VZV. When HZ presents in a vaccinated immunocompetent child without a history of varicella infection or exposure, the possibility for vaccine strain–induced HZ should be considered.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
- . Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br J Dermatol. 1997;137:255-258.
- Uebe B, Sauerbrei A, Burdach S, et al. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child [published online June 25, 2002]. Eur J Pediatr. 2002;161:442-444.
- Obieta MP, Jacinto SS. Herpes zoster after varicella vaccination in a healthy young child. Int J Dermatol. 2008;47:640-641.
- Ota K, Kim V, Lavi S, et al. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr Infect Dis J. 2008;27:847-848.
- Liang GL, Heidelberg KA, Jacobson RM, et al. Herpes zoster after varicella vaccination. J Am Acad Dermatol. 1998;38:761-763.
- Matsubara K, Nigami H, Harigaya H, et al. Herpes zoster in a normal child after varicella vaccination. Acta Paediatr Jpn. 1995;37:648-650.
- Kohl S, Rapp J, Larussa P, et al. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr Infect Dis J. 1999;18:1112-1113.
- Feder HM Jr, Hoss DM. Herpes zoster in otherwise healthy children. Pediatr Infect Dis J. 2004;23:451-457; quiz 458-460.
- Binder NR, Holland GN, Hosea S, et al. Herpes zoster ophthalmicus in an otherwise-healthy child. J AAPOS. 2005;9:597-598.
- Levin MJ, DeBiasi RL, Bostik V, et al. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J Infect Dis. 2008;198:1444-1447.
- Iyer S, Mittal MK, Hodinka RL. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann Emerg Med. 2009;53:792-795.
- Lin P, Yoon MK, Chiu CS. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul Immunol Inflamm. 2009;17:33-35.
- Chouliaras G, Spoulou V, Quinlivan M, et al. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child [published online March 1, 2010]. Pediatrics. 2010;125:E969-E972.
- Han JY, Hanson DC, Way SS. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr Infect Dis J. 2011;30:266-268.
- Ryu WY, Kim NY, Kwon YH, et al. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J AAPOS. 2014;18:193-195.
- Iwasaki S, Motokura K, Honda Y, et al. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: a case report [published online November 3, 2016]. J Clin Virol. 2016;85:44-47.
- Peterson N, Goodman S, Peterson M, et al. Herpes zoster in children. Cutis. 2016;98:94-95.
Practice Points
- Most children with herpes zoster are immunocompromised, have a history of varicella, or were exposed to varicella in utero.
- Herpes zoster has been reported in immunocompetent children due to either wild-type or vaccine-strain varicella-zoster virus.