User login
Postop incentive spirometry had minimal impact on hypoxemia in bariatric surgery patients
The effect of incentive spirometry (IS) on postoperative hypoxemia in bariatric surgery patients was found to be insignificant, according to a randomized cohort study published in JAMA Surgery.
“At present, postoperative IS is considered the standard of care and is incorporated into standardized bariatric surgery recovery protocols,” wrote the authors of the study, led by Haddon Pantel, MD, of the Lahey Hospital and Medical Center in Burlington, Mass. “However, despite the ubiquitous use of IS in the postoperative period, data on its efficacy are conflicting, and high-quality evidence is lacking.” (JAMA Surg. doi:10.1001/jamasurg.2016.4981)
A total of 224 patients were evenly randomized into one of two cohorts; one cohort received no postoperative IS and acted as the control, while the other received postoperative IS. Patients from each of these cohorts were followed up at 6, 12, and 24 hours to measure SaO2 levels as a sign of hypoxemia, which was defined as a level of under 92%.
No significant differences were observed between the two cohorts at any of the three follow-up periods in terms of SaO2 levels. At 6 hours, hypoxemia incidence rates were 11.9% in the control group and 10.4% in the IS group (P = .72). At the 12-hour follow-up, the control group registered a 5.4% incidence rate, compared with 8.2% for those receiving postoperative IS (P = .40). And finally, at 24-hour follow-up, the control group had a 3.7% rate of hypoxemia, while those in the IS cohort had a 4.6% rate (P = .73). In addition, there were no significant differences observed in the average SaO2 levels between the two cohorts (P = .99, P = .40, and P = .69 at 6, 12, and 24 hours, respectively) nor was there a significantly higher rate of pulmonary complications in one cohort versus the other (P = .24).
The authors concluded, “With health care moving toward a more evidence-based, economically driven, and environmentally sustainable field, this study adds evidence to the concept that IS should not be universally used in all patients undergoing surgery and does not appear to be necessary in elective bariatric surgical procedures.”
The study was funded by Lahey Hospital and Medical Center’s department of general surgery; the authors reported no relevant financial disclosures.
The effect of incentive spirometry (IS) on postoperative hypoxemia in bariatric surgery patients was found to be insignificant, according to a randomized cohort study published in JAMA Surgery.
“At present, postoperative IS is considered the standard of care and is incorporated into standardized bariatric surgery recovery protocols,” wrote the authors of the study, led by Haddon Pantel, MD, of the Lahey Hospital and Medical Center in Burlington, Mass. “However, despite the ubiquitous use of IS in the postoperative period, data on its efficacy are conflicting, and high-quality evidence is lacking.” (JAMA Surg. doi:10.1001/jamasurg.2016.4981)
A total of 224 patients were evenly randomized into one of two cohorts; one cohort received no postoperative IS and acted as the control, while the other received postoperative IS. Patients from each of these cohorts were followed up at 6, 12, and 24 hours to measure SaO2 levels as a sign of hypoxemia, which was defined as a level of under 92%.
No significant differences were observed between the two cohorts at any of the three follow-up periods in terms of SaO2 levels. At 6 hours, hypoxemia incidence rates were 11.9% in the control group and 10.4% in the IS group (P = .72). At the 12-hour follow-up, the control group registered a 5.4% incidence rate, compared with 8.2% for those receiving postoperative IS (P = .40). And finally, at 24-hour follow-up, the control group had a 3.7% rate of hypoxemia, while those in the IS cohort had a 4.6% rate (P = .73). In addition, there were no significant differences observed in the average SaO2 levels between the two cohorts (P = .99, P = .40, and P = .69 at 6, 12, and 24 hours, respectively) nor was there a significantly higher rate of pulmonary complications in one cohort versus the other (P = .24).
The authors concluded, “With health care moving toward a more evidence-based, economically driven, and environmentally sustainable field, this study adds evidence to the concept that IS should not be universally used in all patients undergoing surgery and does not appear to be necessary in elective bariatric surgical procedures.”
The study was funded by Lahey Hospital and Medical Center’s department of general surgery; the authors reported no relevant financial disclosures.
The effect of incentive spirometry (IS) on postoperative hypoxemia in bariatric surgery patients was found to be insignificant, according to a randomized cohort study published in JAMA Surgery.
“At present, postoperative IS is considered the standard of care and is incorporated into standardized bariatric surgery recovery protocols,” wrote the authors of the study, led by Haddon Pantel, MD, of the Lahey Hospital and Medical Center in Burlington, Mass. “However, despite the ubiquitous use of IS in the postoperative period, data on its efficacy are conflicting, and high-quality evidence is lacking.” (JAMA Surg. doi:10.1001/jamasurg.2016.4981)
A total of 224 patients were evenly randomized into one of two cohorts; one cohort received no postoperative IS and acted as the control, while the other received postoperative IS. Patients from each of these cohorts were followed up at 6, 12, and 24 hours to measure SaO2 levels as a sign of hypoxemia, which was defined as a level of under 92%.
No significant differences were observed between the two cohorts at any of the three follow-up periods in terms of SaO2 levels. At 6 hours, hypoxemia incidence rates were 11.9% in the control group and 10.4% in the IS group (P = .72). At the 12-hour follow-up, the control group registered a 5.4% incidence rate, compared with 8.2% for those receiving postoperative IS (P = .40). And finally, at 24-hour follow-up, the control group had a 3.7% rate of hypoxemia, while those in the IS cohort had a 4.6% rate (P = .73). In addition, there were no significant differences observed in the average SaO2 levels between the two cohorts (P = .99, P = .40, and P = .69 at 6, 12, and 24 hours, respectively) nor was there a significantly higher rate of pulmonary complications in one cohort versus the other (P = .24).
The authors concluded, “With health care moving toward a more evidence-based, economically driven, and environmentally sustainable field, this study adds evidence to the concept that IS should not be universally used in all patients undergoing surgery and does not appear to be necessary in elective bariatric surgical procedures.”
The study was funded by Lahey Hospital and Medical Center’s department of general surgery; the authors reported no relevant financial disclosures.
FROM JAMA SURGERY
Key clinical point:
Major finding: No significant difference in hypoxemia frequency was found between postoperative IS and control cohorts at 6, 12, and 24-hour follow-ups (P = .72, .40, and .73, respectively).
Data source: A randomized, noninferiority cohort study of 224 bariatric surgery patients during May 2015 through June 2016.
Disclosures: Study funded by Lahey Hospital and Medical Center; authors reported no relevant financial disclosures.
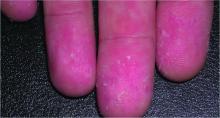
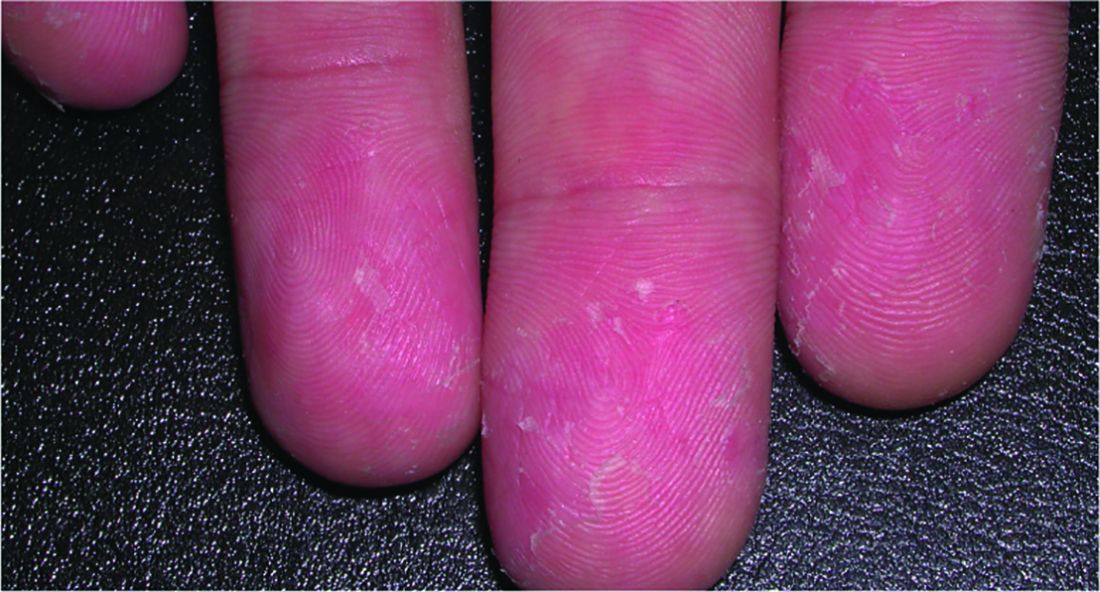
Watch for cutaneous manifestations of tropical infectious diseases
Treatments designed to combat tropical infectious diseases are lacking, so the best thing travelers to these regions of the world can do is defend themselves against mosquito bites, according to Stephen K. Tyring, MD.
“We have no specific therapies for these infections,” explained Dr. Tyring of the University of Texas in Houston.
“Therefore, the best management is to avoid mosquito bites [by using] DEET, protective clothing, etc.,” he said in an interview prior to the Caribbean Dermatology Symposium.
“Treatment [of Zika virus infections] is supportive,” he said, because currently, there are no vaccine and no antiviral therapy aimed specifically at treating Zika virus infections. It’s also important for clinicians to rule out dengue and chikungunya when testing for Zika virus, and to avoid prescribing NSAIDs and aspirin until a definitive diagnosis is made, to avoid causing hemorrhaging.
Dr. Tyring also advised refraining from sexual contact with any individuals who have been to tropical areas and may have been exposed to the Zika virus.
Dr. Tyring also discussed the cutaneous manifestations and other symptoms of the flavivirus infections dengue and chikungunya.
“[About] 40% of the world’s population live in areas where there is a risk of dengue transmission, [and] the World Health Organization estimates that 50 to 100 million infections occur yearly, including 500,000 DHF [dengue hemorrhagic fever] cases and 22,000 deaths,” mostly in children, he said in his presentation at the meeting provided by Global Academy for Medical Education.
The tourniquet test is a useful tool to determine if a patient has dengue fever. This involves taking the patient’s blood pressure, then inflating the cuff to a point midway between the systolic and diastolic blood pressure, and maintaining it for 5 minutes. Deflate the cuff and wait for 2 minutes; then count the petechiae below the antecubital fossa. A positive test result is 10 or more petechiae per square inch, according to the CDC definition.
A relative of dengue, the chikungunya virus can present in the form of a morbilliform rash, nasal hyperpigmentation, purpuric macules, and erythema, the latter of which can sometimes be accompanied by ulcers. In addition to dermatologic symptoms (occurring in 40%-75% of patients), joint pain and fever also are associated with a chikungunya virus infection.
“Redness, swelling, and pain of the scrotum and groin region” also can occur, while “ulceration on the vulva in women has occasionally been reported in other outbreaks,” Dr. Tyring explained.
For further reading on this matter, Dr. Tyring recommended a report by Nawas et al. entitled, “Emerging infectious diseases with cutaneous manifestations” (J Am Acad Dermatol. 2016 Jul;75[1]:1-16) and a 2008 JAMA study on dengue and DHF coauthored by Anthony S. Fauci, MD (299[2]:214-6).
Dr. Tyring reported no relevant financial disclosures. Global Academy and this news organization are owned by the same parent company.
Treatments designed to combat tropical infectious diseases are lacking, so the best thing travelers to these regions of the world can do is defend themselves against mosquito bites, according to Stephen K. Tyring, MD.
“We have no specific therapies for these infections,” explained Dr. Tyring of the University of Texas in Houston.
“Therefore, the best management is to avoid mosquito bites [by using] DEET, protective clothing, etc.,” he said in an interview prior to the Caribbean Dermatology Symposium.
“Treatment [of Zika virus infections] is supportive,” he said, because currently, there are no vaccine and no antiviral therapy aimed specifically at treating Zika virus infections. It’s also important for clinicians to rule out dengue and chikungunya when testing for Zika virus, and to avoid prescribing NSAIDs and aspirin until a definitive diagnosis is made, to avoid causing hemorrhaging.
Dr. Tyring also advised refraining from sexual contact with any individuals who have been to tropical areas and may have been exposed to the Zika virus.
Dr. Tyring also discussed the cutaneous manifestations and other symptoms of the flavivirus infections dengue and chikungunya.
“[About] 40% of the world’s population live in areas where there is a risk of dengue transmission, [and] the World Health Organization estimates that 50 to 100 million infections occur yearly, including 500,000 DHF [dengue hemorrhagic fever] cases and 22,000 deaths,” mostly in children, he said in his presentation at the meeting provided by Global Academy for Medical Education.
The tourniquet test is a useful tool to determine if a patient has dengue fever. This involves taking the patient’s blood pressure, then inflating the cuff to a point midway between the systolic and diastolic blood pressure, and maintaining it for 5 minutes. Deflate the cuff and wait for 2 minutes; then count the petechiae below the antecubital fossa. A positive test result is 10 or more petechiae per square inch, according to the CDC definition.
A relative of dengue, the chikungunya virus can present in the form of a morbilliform rash, nasal hyperpigmentation, purpuric macules, and erythema, the latter of which can sometimes be accompanied by ulcers. In addition to dermatologic symptoms (occurring in 40%-75% of patients), joint pain and fever also are associated with a chikungunya virus infection.
“Redness, swelling, and pain of the scrotum and groin region” also can occur, while “ulceration on the vulva in women has occasionally been reported in other outbreaks,” Dr. Tyring explained.
For further reading on this matter, Dr. Tyring recommended a report by Nawas et al. entitled, “Emerging infectious diseases with cutaneous manifestations” (J Am Acad Dermatol. 2016 Jul;75[1]:1-16) and a 2008 JAMA study on dengue and DHF coauthored by Anthony S. Fauci, MD (299[2]:214-6).
Dr. Tyring reported no relevant financial disclosures. Global Academy and this news organization are owned by the same parent company.
Treatments designed to combat tropical infectious diseases are lacking, so the best thing travelers to these regions of the world can do is defend themselves against mosquito bites, according to Stephen K. Tyring, MD.
“We have no specific therapies for these infections,” explained Dr. Tyring of the University of Texas in Houston.
“Therefore, the best management is to avoid mosquito bites [by using] DEET, protective clothing, etc.,” he said in an interview prior to the Caribbean Dermatology Symposium.
“Treatment [of Zika virus infections] is supportive,” he said, because currently, there are no vaccine and no antiviral therapy aimed specifically at treating Zika virus infections. It’s also important for clinicians to rule out dengue and chikungunya when testing for Zika virus, and to avoid prescribing NSAIDs and aspirin until a definitive diagnosis is made, to avoid causing hemorrhaging.
Dr. Tyring also advised refraining from sexual contact with any individuals who have been to tropical areas and may have been exposed to the Zika virus.
Dr. Tyring also discussed the cutaneous manifestations and other symptoms of the flavivirus infections dengue and chikungunya.
“[About] 40% of the world’s population live in areas where there is a risk of dengue transmission, [and] the World Health Organization estimates that 50 to 100 million infections occur yearly, including 500,000 DHF [dengue hemorrhagic fever] cases and 22,000 deaths,” mostly in children, he said in his presentation at the meeting provided by Global Academy for Medical Education.
The tourniquet test is a useful tool to determine if a patient has dengue fever. This involves taking the patient’s blood pressure, then inflating the cuff to a point midway between the systolic and diastolic blood pressure, and maintaining it for 5 minutes. Deflate the cuff and wait for 2 minutes; then count the petechiae below the antecubital fossa. A positive test result is 10 or more petechiae per square inch, according to the CDC definition.
A relative of dengue, the chikungunya virus can present in the form of a morbilliform rash, nasal hyperpigmentation, purpuric macules, and erythema, the latter of which can sometimes be accompanied by ulcers. In addition to dermatologic symptoms (occurring in 40%-75% of patients), joint pain and fever also are associated with a chikungunya virus infection.
“Redness, swelling, and pain of the scrotum and groin region” also can occur, while “ulceration on the vulva in women has occasionally been reported in other outbreaks,” Dr. Tyring explained.
For further reading on this matter, Dr. Tyring recommended a report by Nawas et al. entitled, “Emerging infectious diseases with cutaneous manifestations” (J Am Acad Dermatol. 2016 Jul;75[1]:1-16) and a 2008 JAMA study on dengue and DHF coauthored by Anthony S. Fauci, MD (299[2]:214-6).
Dr. Tyring reported no relevant financial disclosures. Global Academy and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
Bone fractures more likely to occur in psoriasis, PsA patients
Individuals who have psoriasis or psoriatic arthritis are at a significantly higher risk of also suffering bone fractures, particularly in their hip and vertebrae, according to a new study published in the Annals of the Rheumatic Diseases.
“To our knowledge, these are the first population-based estimates of the risk for incident fracture and osteoporosis in patients with psoriasis and/or PsA [psoriatic arthritis] and the first longitudinal cohort study to address this issue,” wrote the authors of the study, led by Alexis Ogdie-Beatty, MD, of the University of Pennsylvania in Philadelphia (Ann Rheum Dis. 2017 Jan 16. doi: 10.1136/annrheumdis-2016-210441).
A total of 9,788 PsA and 158,323 psoriasis patients were included in the study, along with 39,306 RA patients and 821,834 individuals from the general population. Psoriasis patients were divided into groups classified as mild (n = 149,809) or severe (n = 8,514). The average age of each cohort ranged from nearly 47 years to almost 59 years, with all cohorts comprising mostly females, ranging from about 51% to 69%.
“We found that the risk for any fracture in patients with PsA and severe psoriasis was similar to RA [but] patients with PsA and psoriasis had an increased incidence of fracture compared with the general population by 7%-26%,” the authors explained. “The incidence of vertebral fracture was also increased in patients with severe psoriasis and while hip fracture was elevated in both psoriasis groups, it was only statistically significant in patients with mild psoriasis relative to matched controls after adjusting for risk factors for osteoporosis.”
Dr. Ogdie-Beatty and her colleagues found that all of the conditions conferred an elevated risk for fractures anywhere in the body when compared with the general population, reaching hazard ratios of 1.16 (95% confidence interval, 1.06-1.27) for people with PsA, 1.07 (95% CI, 1.05-1.10) for mild psoriasis, 1.26 for severe psoriasis (95% CI, 1.15-1.39), and 1.23 for RA (95% CI, 1.18-1.28). The risk for hip fractures was only significantly higher for mild (hazard ratio, 1.13; 95% CI, 1.04-1.22) and severe psoriasis (HR, 1.21; 95% CI, 0.88-1.66), and RA (HR, 1.55; 95% CI, 1.40-1.72). Individuals with PsA did not have a significantly higher risk for vertebral fractures (HR, 1.07; 95% CI, 0.66-1.72), whereas those with mild psoriasis (HR, 1.17; 95% CI, 1.03-1.33), severe psoriasis (HR, 2.23; 95% CI, 1.54-3.22), or RA did (HR, 1.53; 95% CI, 1.30-1.80). Each of these models were fully adjusted for multiple different osteoporosis risk factors, although they were all commonly adjusted for age, sex, atrial fibrillation, diabetes, chronic obstructive pulmonary disease, stroke, SSRI use, tricyclic antidepressant use, oral steroids, smoking, and categorical body mass index.
Individual coauthors disclosed receiving funding for their work from the National Institutes of Health, as well as grants from the Department of Veterans Affairs and the Rheumatology Research Foundation. Three of the authors reported receiving payment for continuing medical education work related to psoriatic arthritis or psoriasis.
Individuals who have psoriasis or psoriatic arthritis are at a significantly higher risk of also suffering bone fractures, particularly in their hip and vertebrae, according to a new study published in the Annals of the Rheumatic Diseases.
“To our knowledge, these are the first population-based estimates of the risk for incident fracture and osteoporosis in patients with psoriasis and/or PsA [psoriatic arthritis] and the first longitudinal cohort study to address this issue,” wrote the authors of the study, led by Alexis Ogdie-Beatty, MD, of the University of Pennsylvania in Philadelphia (Ann Rheum Dis. 2017 Jan 16. doi: 10.1136/annrheumdis-2016-210441).
A total of 9,788 PsA and 158,323 psoriasis patients were included in the study, along with 39,306 RA patients and 821,834 individuals from the general population. Psoriasis patients were divided into groups classified as mild (n = 149,809) or severe (n = 8,514). The average age of each cohort ranged from nearly 47 years to almost 59 years, with all cohorts comprising mostly females, ranging from about 51% to 69%.
“We found that the risk for any fracture in patients with PsA and severe psoriasis was similar to RA [but] patients with PsA and psoriasis had an increased incidence of fracture compared with the general population by 7%-26%,” the authors explained. “The incidence of vertebral fracture was also increased in patients with severe psoriasis and while hip fracture was elevated in both psoriasis groups, it was only statistically significant in patients with mild psoriasis relative to matched controls after adjusting for risk factors for osteoporosis.”
Dr. Ogdie-Beatty and her colleagues found that all of the conditions conferred an elevated risk for fractures anywhere in the body when compared with the general population, reaching hazard ratios of 1.16 (95% confidence interval, 1.06-1.27) for people with PsA, 1.07 (95% CI, 1.05-1.10) for mild psoriasis, 1.26 for severe psoriasis (95% CI, 1.15-1.39), and 1.23 for RA (95% CI, 1.18-1.28). The risk for hip fractures was only significantly higher for mild (hazard ratio, 1.13; 95% CI, 1.04-1.22) and severe psoriasis (HR, 1.21; 95% CI, 0.88-1.66), and RA (HR, 1.55; 95% CI, 1.40-1.72). Individuals with PsA did not have a significantly higher risk for vertebral fractures (HR, 1.07; 95% CI, 0.66-1.72), whereas those with mild psoriasis (HR, 1.17; 95% CI, 1.03-1.33), severe psoriasis (HR, 2.23; 95% CI, 1.54-3.22), or RA did (HR, 1.53; 95% CI, 1.30-1.80). Each of these models were fully adjusted for multiple different osteoporosis risk factors, although they were all commonly adjusted for age, sex, atrial fibrillation, diabetes, chronic obstructive pulmonary disease, stroke, SSRI use, tricyclic antidepressant use, oral steroids, smoking, and categorical body mass index.
Individual coauthors disclosed receiving funding for their work from the National Institutes of Health, as well as grants from the Department of Veterans Affairs and the Rheumatology Research Foundation. Three of the authors reported receiving payment for continuing medical education work related to psoriatic arthritis or psoriasis.
Individuals who have psoriasis or psoriatic arthritis are at a significantly higher risk of also suffering bone fractures, particularly in their hip and vertebrae, according to a new study published in the Annals of the Rheumatic Diseases.
“To our knowledge, these are the first population-based estimates of the risk for incident fracture and osteoporosis in patients with psoriasis and/or PsA [psoriatic arthritis] and the first longitudinal cohort study to address this issue,” wrote the authors of the study, led by Alexis Ogdie-Beatty, MD, of the University of Pennsylvania in Philadelphia (Ann Rheum Dis. 2017 Jan 16. doi: 10.1136/annrheumdis-2016-210441).
A total of 9,788 PsA and 158,323 psoriasis patients were included in the study, along with 39,306 RA patients and 821,834 individuals from the general population. Psoriasis patients were divided into groups classified as mild (n = 149,809) or severe (n = 8,514). The average age of each cohort ranged from nearly 47 years to almost 59 years, with all cohorts comprising mostly females, ranging from about 51% to 69%.
“We found that the risk for any fracture in patients with PsA and severe psoriasis was similar to RA [but] patients with PsA and psoriasis had an increased incidence of fracture compared with the general population by 7%-26%,” the authors explained. “The incidence of vertebral fracture was also increased in patients with severe psoriasis and while hip fracture was elevated in both psoriasis groups, it was only statistically significant in patients with mild psoriasis relative to matched controls after adjusting for risk factors for osteoporosis.”
Dr. Ogdie-Beatty and her colleagues found that all of the conditions conferred an elevated risk for fractures anywhere in the body when compared with the general population, reaching hazard ratios of 1.16 (95% confidence interval, 1.06-1.27) for people with PsA, 1.07 (95% CI, 1.05-1.10) for mild psoriasis, 1.26 for severe psoriasis (95% CI, 1.15-1.39), and 1.23 for RA (95% CI, 1.18-1.28). The risk for hip fractures was only significantly higher for mild (hazard ratio, 1.13; 95% CI, 1.04-1.22) and severe psoriasis (HR, 1.21; 95% CI, 0.88-1.66), and RA (HR, 1.55; 95% CI, 1.40-1.72). Individuals with PsA did not have a significantly higher risk for vertebral fractures (HR, 1.07; 95% CI, 0.66-1.72), whereas those with mild psoriasis (HR, 1.17; 95% CI, 1.03-1.33), severe psoriasis (HR, 2.23; 95% CI, 1.54-3.22), or RA did (HR, 1.53; 95% CI, 1.30-1.80). Each of these models were fully adjusted for multiple different osteoporosis risk factors, although they were all commonly adjusted for age, sex, atrial fibrillation, diabetes, chronic obstructive pulmonary disease, stroke, SSRI use, tricyclic antidepressant use, oral steroids, smoking, and categorical body mass index.
Individual coauthors disclosed receiving funding for their work from the National Institutes of Health, as well as grants from the Department of Veterans Affairs and the Rheumatology Research Foundation. Three of the authors reported receiving payment for continuing medical education work related to psoriatic arthritis or psoriasis.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: The risk of fracture for patients with PsA and psoriasis had a risk of fracture that was increased by 7%-26% in comparison with the general U.K. population.
Data source: Population-based, longitudinal cohort study of 9,788 PsA patients and 158,323 psoriasis patients in the United Kingdom during 1994-2014.
Disclosures: Individual coauthors disclosed receiving funding for their work from the National Institutes of Health, as well as grants from the Department of Veterans Affairs and the Rheumatology Research Foundation. Three of the authors reported receiving payment for continuing medical education work related to psoriatic arthritis or psoriasis.
New report highlights gaps in knowledge on marijuana use
A new report by the National Academies of Sciences, Engineering and Medicine shines a light on what the existing literature says about the perceived health benefits and dangers of using cannabis and cannabinoids.
“What little we know for certain about the effects of marijuana on human health — and all that we have reason to suspect — justifies serious national concern,” wrote the authors of the report, the evidence and research review of which was chaired by Marie McCormick, MD, of Harvard University in Boston. “The committee’s major recommendation called for an intensification and more comprehensive research effort into the effects of marijuana on the health of the American people.”
The authors concluded current literature shows substantial evidence stating that cannabis is effective at managing chronic pain in adults, while oral cannabinoid use is effective in mitigating nausea or vomiting induced by chemotherapy and improving patient-reported spasticity in patients with multiple sclerosis. Additionally, cannabinoids – specifically, nabiximols – are moderately effective in the short term for improving sleep disturbances brought on by obstructive sleep apnea syndrome, fibromyalgia, chronic pain, and multiple sclerosis.
However, there is limited evidence to support cannabis or cannabinoid use for reducing weight loss or inducing appetite in HIV/AIDS patients, improving clinician-measured spasicity or Tourette syndrome symptoms, reducing anxiety, improving symptoms brought on by post-traumatic stress, and improving outcomes in patients who have suffered traumatic brain injury or intracranial hemorrhage.
Additionally, there is no evidence to support the use of cannabis or cannabinoids in treating cancers or cancer-related anorexia, irritable bowel syndrome symptoms, epilepsy, spinal cord-related spasticity, symptoms of amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease, schizophrenia, and dystonia.
“Present data on drug use progression neither support nor refute the suggestion that medical availability would increase drug abuse,” the authors noted. “However, this question is beyond the issues normally considered for medical uses of drugs and should not be a factor in evaluating the therapeutic potential of marijuana or cannabinoids.”
From a mental health standpoint, suicidal thoughts were found to be more likely in individuals who frequently used cannabis or cannabinoids. Symptoms like depression and anxiety are also more likely in those who smoke marijuana and have bipolar disorder. There is also “limited evidence of a statistical association between sustained abstinence from cannabis use [and] impairments in the cognitive domains of learning, memory, and attention.”
Among the other significant findings of the report, children who live in states where marijuana has been legalized are significantly more likely to ingest cannabis or cannabinoids; so far, marijuana use in some form – either recreational or medical – has been approved in 28 states and Washington, DC. Furthermore, adolescents who use marijuana are more likely to experience difficulties in social and educational development. And individuals of any age who smoke marijuana and drive are more likely to be involved in a car accident.
Also noteworthy is the lack of evidence pointing to marijuana use causing cancer. While chronic marijuana smoking was found to be linked to bronchitis, it was not found to cause cancers that are most commonly associated with chronic smoking of tobacco.
“This report highlights that there are critical gaps in our understanding of the health effects of cannabis,” explained John H. Krystal, MD, of Yale University in New Haven, Connecticut.
A reviewer of the report, Dr. Krystal elaborated on the gaps that exist in the current literature, saying “One reason for these gaps has been regulatory and practical challenges facing those who attempted to conduct this research. For example, what supply of cannabis should they use? Where, in the typical hospital settings where research is conducted, should patients participating in research be permitted to smoke cannabis? What standards should the institutional review committees employ when evaluating studies that involve the administration of cannabis or other cannabinoids?”
Ultimately, Dr. Krystal stated, “what should be evident from this summary is that only a few of the many publicized clinical applications for cannabis are adequately supported by acceptable research standards for determining safety or efficacy.” Specifically, states that have approved cannabis use for managing PTSD symptoms are doing so based off “meager” evidence, and in some cases, are circumventing FDA regulatory processes in a way. This could not only compromise patient care, but muddy the waters for physicians who want to treat their patients safely while also following legal avenues.
“Physicians may face a tension between their roles as physicians [and] their wish to provide a legal path for access to cannabinoids for their patients,” Dr. Krystal said, adding that “the endorsement of particular cannabis prescription practices by the states, even for clinical indications where cannabis has not been shown to be safe and effective, may create pressure for physicians to engage in ineffective or unsafe cannabis prescription practices.”
Ultimately, the report underlines areas of need in terms of understanding and effectively using cannabis and cannabinoid in treating patients. Calling the report a “call to arms” for those in the health care – and, specifically, the public health – arena, Dr. Krystal added that he hopes the findings of the report will be used for educating “legislators, physicians, and consumers [about the] potential benefits and risks of cannabis and thereby help to guide both legislation, clinical practice, and perhaps recreational use.”
A new report by the National Academies of Sciences, Engineering and Medicine shines a light on what the existing literature says about the perceived health benefits and dangers of using cannabis and cannabinoids.
“What little we know for certain about the effects of marijuana on human health — and all that we have reason to suspect — justifies serious national concern,” wrote the authors of the report, the evidence and research review of which was chaired by Marie McCormick, MD, of Harvard University in Boston. “The committee’s major recommendation called for an intensification and more comprehensive research effort into the effects of marijuana on the health of the American people.”
The authors concluded current literature shows substantial evidence stating that cannabis is effective at managing chronic pain in adults, while oral cannabinoid use is effective in mitigating nausea or vomiting induced by chemotherapy and improving patient-reported spasticity in patients with multiple sclerosis. Additionally, cannabinoids – specifically, nabiximols – are moderately effective in the short term for improving sleep disturbances brought on by obstructive sleep apnea syndrome, fibromyalgia, chronic pain, and multiple sclerosis.
However, there is limited evidence to support cannabis or cannabinoid use for reducing weight loss or inducing appetite in HIV/AIDS patients, improving clinician-measured spasicity or Tourette syndrome symptoms, reducing anxiety, improving symptoms brought on by post-traumatic stress, and improving outcomes in patients who have suffered traumatic brain injury or intracranial hemorrhage.
Additionally, there is no evidence to support the use of cannabis or cannabinoids in treating cancers or cancer-related anorexia, irritable bowel syndrome symptoms, epilepsy, spinal cord-related spasticity, symptoms of amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease, schizophrenia, and dystonia.
“Present data on drug use progression neither support nor refute the suggestion that medical availability would increase drug abuse,” the authors noted. “However, this question is beyond the issues normally considered for medical uses of drugs and should not be a factor in evaluating the therapeutic potential of marijuana or cannabinoids.”
From a mental health standpoint, suicidal thoughts were found to be more likely in individuals who frequently used cannabis or cannabinoids. Symptoms like depression and anxiety are also more likely in those who smoke marijuana and have bipolar disorder. There is also “limited evidence of a statistical association between sustained abstinence from cannabis use [and] impairments in the cognitive domains of learning, memory, and attention.”
Among the other significant findings of the report, children who live in states where marijuana has been legalized are significantly more likely to ingest cannabis or cannabinoids; so far, marijuana use in some form – either recreational or medical – has been approved in 28 states and Washington, DC. Furthermore, adolescents who use marijuana are more likely to experience difficulties in social and educational development. And individuals of any age who smoke marijuana and drive are more likely to be involved in a car accident.
Also noteworthy is the lack of evidence pointing to marijuana use causing cancer. While chronic marijuana smoking was found to be linked to bronchitis, it was not found to cause cancers that are most commonly associated with chronic smoking of tobacco.
“This report highlights that there are critical gaps in our understanding of the health effects of cannabis,” explained John H. Krystal, MD, of Yale University in New Haven, Connecticut.
A reviewer of the report, Dr. Krystal elaborated on the gaps that exist in the current literature, saying “One reason for these gaps has been regulatory and practical challenges facing those who attempted to conduct this research. For example, what supply of cannabis should they use? Where, in the typical hospital settings where research is conducted, should patients participating in research be permitted to smoke cannabis? What standards should the institutional review committees employ when evaluating studies that involve the administration of cannabis or other cannabinoids?”
Ultimately, Dr. Krystal stated, “what should be evident from this summary is that only a few of the many publicized clinical applications for cannabis are adequately supported by acceptable research standards for determining safety or efficacy.” Specifically, states that have approved cannabis use for managing PTSD symptoms are doing so based off “meager” evidence, and in some cases, are circumventing FDA regulatory processes in a way. This could not only compromise patient care, but muddy the waters for physicians who want to treat their patients safely while also following legal avenues.
“Physicians may face a tension between their roles as physicians [and] their wish to provide a legal path for access to cannabinoids for their patients,” Dr. Krystal said, adding that “the endorsement of particular cannabis prescription practices by the states, even for clinical indications where cannabis has not been shown to be safe and effective, may create pressure for physicians to engage in ineffective or unsafe cannabis prescription practices.”
Ultimately, the report underlines areas of need in terms of understanding and effectively using cannabis and cannabinoid in treating patients. Calling the report a “call to arms” for those in the health care – and, specifically, the public health – arena, Dr. Krystal added that he hopes the findings of the report will be used for educating “legislators, physicians, and consumers [about the] potential benefits and risks of cannabis and thereby help to guide both legislation, clinical practice, and perhaps recreational use.”
A new report by the National Academies of Sciences, Engineering and Medicine shines a light on what the existing literature says about the perceived health benefits and dangers of using cannabis and cannabinoids.
“What little we know for certain about the effects of marijuana on human health — and all that we have reason to suspect — justifies serious national concern,” wrote the authors of the report, the evidence and research review of which was chaired by Marie McCormick, MD, of Harvard University in Boston. “The committee’s major recommendation called for an intensification and more comprehensive research effort into the effects of marijuana on the health of the American people.”
The authors concluded current literature shows substantial evidence stating that cannabis is effective at managing chronic pain in adults, while oral cannabinoid use is effective in mitigating nausea or vomiting induced by chemotherapy and improving patient-reported spasticity in patients with multiple sclerosis. Additionally, cannabinoids – specifically, nabiximols – are moderately effective in the short term for improving sleep disturbances brought on by obstructive sleep apnea syndrome, fibromyalgia, chronic pain, and multiple sclerosis.
However, there is limited evidence to support cannabis or cannabinoid use for reducing weight loss or inducing appetite in HIV/AIDS patients, improving clinician-measured spasicity or Tourette syndrome symptoms, reducing anxiety, improving symptoms brought on by post-traumatic stress, and improving outcomes in patients who have suffered traumatic brain injury or intracranial hemorrhage.
Additionally, there is no evidence to support the use of cannabis or cannabinoids in treating cancers or cancer-related anorexia, irritable bowel syndrome symptoms, epilepsy, spinal cord-related spasticity, symptoms of amyotrophic lateral sclerosis, Huntington’s disease, Parkinson’s disease, schizophrenia, and dystonia.
“Present data on drug use progression neither support nor refute the suggestion that medical availability would increase drug abuse,” the authors noted. “However, this question is beyond the issues normally considered for medical uses of drugs and should not be a factor in evaluating the therapeutic potential of marijuana or cannabinoids.”
From a mental health standpoint, suicidal thoughts were found to be more likely in individuals who frequently used cannabis or cannabinoids. Symptoms like depression and anxiety are also more likely in those who smoke marijuana and have bipolar disorder. There is also “limited evidence of a statistical association between sustained abstinence from cannabis use [and] impairments in the cognitive domains of learning, memory, and attention.”
Among the other significant findings of the report, children who live in states where marijuana has been legalized are significantly more likely to ingest cannabis or cannabinoids; so far, marijuana use in some form – either recreational or medical – has been approved in 28 states and Washington, DC. Furthermore, adolescents who use marijuana are more likely to experience difficulties in social and educational development. And individuals of any age who smoke marijuana and drive are more likely to be involved in a car accident.
Also noteworthy is the lack of evidence pointing to marijuana use causing cancer. While chronic marijuana smoking was found to be linked to bronchitis, it was not found to cause cancers that are most commonly associated with chronic smoking of tobacco.
“This report highlights that there are critical gaps in our understanding of the health effects of cannabis,” explained John H. Krystal, MD, of Yale University in New Haven, Connecticut.
A reviewer of the report, Dr. Krystal elaborated on the gaps that exist in the current literature, saying “One reason for these gaps has been regulatory and practical challenges facing those who attempted to conduct this research. For example, what supply of cannabis should they use? Where, in the typical hospital settings where research is conducted, should patients participating in research be permitted to smoke cannabis? What standards should the institutional review committees employ when evaluating studies that involve the administration of cannabis or other cannabinoids?”
Ultimately, Dr. Krystal stated, “what should be evident from this summary is that only a few of the many publicized clinical applications for cannabis are adequately supported by acceptable research standards for determining safety or efficacy.” Specifically, states that have approved cannabis use for managing PTSD symptoms are doing so based off “meager” evidence, and in some cases, are circumventing FDA regulatory processes in a way. This could not only compromise patient care, but muddy the waters for physicians who want to treat their patients safely while also following legal avenues.
“Physicians may face a tension between their roles as physicians [and] their wish to provide a legal path for access to cannabinoids for their patients,” Dr. Krystal said, adding that “the endorsement of particular cannabis prescription practices by the states, even for clinical indications where cannabis has not been shown to be safe and effective, may create pressure for physicians to engage in ineffective or unsafe cannabis prescription practices.”
Ultimately, the report underlines areas of need in terms of understanding and effectively using cannabis and cannabinoid in treating patients. Calling the report a “call to arms” for those in the health care – and, specifically, the public health – arena, Dr. Krystal added that he hopes the findings of the report will be used for educating “legislators, physicians, and consumers [about the] potential benefits and risks of cannabis and thereby help to guide both legislation, clinical practice, and perhaps recreational use.”
FROM THE NATIONAL ACADEMIES OF SCIENCES, ENGINEERING AND MEDICINE
Double-dose influenza vaccine effective against type B strains
A double-dose inactivated quadrivalent influenza vaccine (IIV4) could be administered to all children aged 6-35 months, as it not only offers the best protection against influenza type B, but it also allows for simplifying the current vaccination schedule considerably.
“The introduction of IIV4 provides an opportunity to review long-accepted practices in administration of influenza vaccines,” explained Varsha K. Jain, MD, formerly employed by GlaxoSmithKline Vaccines, King of Prussia, Pa., and associates.
Giving a lower dose to young children was planned to reduce reactogenicity and febrile convulsions observed with the whole virus vaccines that were in use in the 1970s. But young children have a variable immune response to lower doses, especially against vaccine B strains, they noted (J Ped Infect Dis. 2017 Jan 6. doi: 10.1093/jpids/piw068).
Dr. Jain and coauthors enrolled 2,430 children aged 6-35 months during the 2014-2015 influenza season in the United States and Mexico in this phase III study. Children were randomized into one of two cohorts: one cohort received a standard-doze IIV4 vaccination, while the other received a double-dose. Data on age (6-17 months, 18-35 months), health care center, and influenza primer status also were taken into consideration.
The standard-dose vaccine contained 7.5 mcg of A/California/7/2009 (A/H1N1), A/Texas/50/2012 (A/H3N2), B/Brisbane/60/2008 (B/Victoria), and B/Massachusetts/2/2012 (B/Yamagata), while the double-dose vaccine contained 15 mcg, or twice the amount each, of the same strains. The former was developed by Sanofi Pasteur and the latter by GSK Vaccines.
Primed children who completed the study numbered 1,173; 586 received the standard-dose and 587 received the double-dose. On the unprimed side, 868 completed the study: 442 standard-dose and 426 double-dose. Each dose’s immunogenic noninferiority was quantified by calculating the geometric mean titer (GMT) ratio.
“Immunogenicity was higher in the double-dose group compared with the standard-dose group, particularly against vaccine B strains in children 6-17 months of age and unprimed children,” Dr. Vain and associates said. Both vaccines performed well against the influenza B strain, with the double-dose yielding a GMT of 1.89 against the B/Yamagata strain and 2.13 against the B/Victoria in children aged 6-17 months. Across the entire age spectrum of the study population, unprimed children registered a GMT of 1.85 and 2.04 against the same strains, respectively. For comparison, none of the A strains in any cohort based on age or primed/unprimed registered a GMT above 1.5.
“Increased protection against influenza B [would] be a beneficial clinical outcome [and] use of the same vaccine dose for all eligible ages would also simplify the annual influenza vaccine campaign and reduce cost and logistic complexity,” the authors concluded. “This study provides evidence to support a change in clinical practice to use [double-dose IIV4] in all children 6 months of age and older, once that dosing for a vaccine product has been approved.”
Dr. Jain now is employed by the Bill and Melinda Gates Foundation.
Dr. Jain and several coauthors disclosed ties to GlaxoSmithKline, which funded the study.
A double-dose inactivated quadrivalent influenza vaccine (IIV4) could be administered to all children aged 6-35 months, as it not only offers the best protection against influenza type B, but it also allows for simplifying the current vaccination schedule considerably.
“The introduction of IIV4 provides an opportunity to review long-accepted practices in administration of influenza vaccines,” explained Varsha K. Jain, MD, formerly employed by GlaxoSmithKline Vaccines, King of Prussia, Pa., and associates.
Giving a lower dose to young children was planned to reduce reactogenicity and febrile convulsions observed with the whole virus vaccines that were in use in the 1970s. But young children have a variable immune response to lower doses, especially against vaccine B strains, they noted (J Ped Infect Dis. 2017 Jan 6. doi: 10.1093/jpids/piw068).
Dr. Jain and coauthors enrolled 2,430 children aged 6-35 months during the 2014-2015 influenza season in the United States and Mexico in this phase III study. Children were randomized into one of two cohorts: one cohort received a standard-doze IIV4 vaccination, while the other received a double-dose. Data on age (6-17 months, 18-35 months), health care center, and influenza primer status also were taken into consideration.
The standard-dose vaccine contained 7.5 mcg of A/California/7/2009 (A/H1N1), A/Texas/50/2012 (A/H3N2), B/Brisbane/60/2008 (B/Victoria), and B/Massachusetts/2/2012 (B/Yamagata), while the double-dose vaccine contained 15 mcg, or twice the amount each, of the same strains. The former was developed by Sanofi Pasteur and the latter by GSK Vaccines.
Primed children who completed the study numbered 1,173; 586 received the standard-dose and 587 received the double-dose. On the unprimed side, 868 completed the study: 442 standard-dose and 426 double-dose. Each dose’s immunogenic noninferiority was quantified by calculating the geometric mean titer (GMT) ratio.
“Immunogenicity was higher in the double-dose group compared with the standard-dose group, particularly against vaccine B strains in children 6-17 months of age and unprimed children,” Dr. Vain and associates said. Both vaccines performed well against the influenza B strain, with the double-dose yielding a GMT of 1.89 against the B/Yamagata strain and 2.13 against the B/Victoria in children aged 6-17 months. Across the entire age spectrum of the study population, unprimed children registered a GMT of 1.85 and 2.04 against the same strains, respectively. For comparison, none of the A strains in any cohort based on age or primed/unprimed registered a GMT above 1.5.
“Increased protection against influenza B [would] be a beneficial clinical outcome [and] use of the same vaccine dose for all eligible ages would also simplify the annual influenza vaccine campaign and reduce cost and logistic complexity,” the authors concluded. “This study provides evidence to support a change in clinical practice to use [double-dose IIV4] in all children 6 months of age and older, once that dosing for a vaccine product has been approved.”
Dr. Jain now is employed by the Bill and Melinda Gates Foundation.
Dr. Jain and several coauthors disclosed ties to GlaxoSmithKline, which funded the study.
A double-dose inactivated quadrivalent influenza vaccine (IIV4) could be administered to all children aged 6-35 months, as it not only offers the best protection against influenza type B, but it also allows for simplifying the current vaccination schedule considerably.
“The introduction of IIV4 provides an opportunity to review long-accepted practices in administration of influenza vaccines,” explained Varsha K. Jain, MD, formerly employed by GlaxoSmithKline Vaccines, King of Prussia, Pa., and associates.
Giving a lower dose to young children was planned to reduce reactogenicity and febrile convulsions observed with the whole virus vaccines that were in use in the 1970s. But young children have a variable immune response to lower doses, especially against vaccine B strains, they noted (J Ped Infect Dis. 2017 Jan 6. doi: 10.1093/jpids/piw068).
Dr. Jain and coauthors enrolled 2,430 children aged 6-35 months during the 2014-2015 influenza season in the United States and Mexico in this phase III study. Children were randomized into one of two cohorts: one cohort received a standard-doze IIV4 vaccination, while the other received a double-dose. Data on age (6-17 months, 18-35 months), health care center, and influenza primer status also were taken into consideration.
The standard-dose vaccine contained 7.5 mcg of A/California/7/2009 (A/H1N1), A/Texas/50/2012 (A/H3N2), B/Brisbane/60/2008 (B/Victoria), and B/Massachusetts/2/2012 (B/Yamagata), while the double-dose vaccine contained 15 mcg, or twice the amount each, of the same strains. The former was developed by Sanofi Pasteur and the latter by GSK Vaccines.
Primed children who completed the study numbered 1,173; 586 received the standard-dose and 587 received the double-dose. On the unprimed side, 868 completed the study: 442 standard-dose and 426 double-dose. Each dose’s immunogenic noninferiority was quantified by calculating the geometric mean titer (GMT) ratio.
“Immunogenicity was higher in the double-dose group compared with the standard-dose group, particularly against vaccine B strains in children 6-17 months of age and unprimed children,” Dr. Vain and associates said. Both vaccines performed well against the influenza B strain, with the double-dose yielding a GMT of 1.89 against the B/Yamagata strain and 2.13 against the B/Victoria in children aged 6-17 months. Across the entire age spectrum of the study population, unprimed children registered a GMT of 1.85 and 2.04 against the same strains, respectively. For comparison, none of the A strains in any cohort based on age or primed/unprimed registered a GMT above 1.5.
“Increased protection against influenza B [would] be a beneficial clinical outcome [and] use of the same vaccine dose for all eligible ages would also simplify the annual influenza vaccine campaign and reduce cost and logistic complexity,” the authors concluded. “This study provides evidence to support a change in clinical practice to use [double-dose IIV4] in all children 6 months of age and older, once that dosing for a vaccine product has been approved.”
Dr. Jain now is employed by the Bill and Melinda Gates Foundation.
Dr. Jain and several coauthors disclosed ties to GlaxoSmithKline, which funded the study.
FROM THE JOURNAL OF THE PEDIATRIC INFECTIOUS DISEASES SOCIETY
Key clinical point:
Major finding: Geometric mean titer (GMT) ratios showed that the double-dose IIV4 vaccine was the most effective against influenza type B in children aged 6-17 months (GMT = 1.89) and in unprimed children aged 6-35 months (GMT = 1.85).
Data source: Phase III, randomized trial of 2,041 children aged 6-35 months.
Disclosures: Dr. Jain and several coauthors disclosed ties to GlaxoSmithKline, which funded the study.
Assay testing accurate in distinguishing bacterial from viral respiratory tract infections
An assay designed to distinguish between bacterial and viral infections of the lower respiratory tract appears effective and shows promise for helping hospital physicians reduce overprescribing of antibiotics to children, a study showed.
“It is often not possible to differentiate between bacterial and nonbacterial disease on the basis of clinical judgment alone, [so] antibiotics are prescribed almost twice as often as required in children with acute respiratory tract infections in the USA,” wrote Chantal B. van Houten of the University Medical Centre Utrecht (the Netherlands) and associates in a study published in the Lancet Infectious Diseases.
The assay in question is called ImmunoXpert, which uses three biomarkers – tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), interferon-gamma–induced protein-10 (IP-10), and C-reactive protein (CRP) – to determine if a lower respiratory tract infection has a viral or bacterial origin. A total of 777 subjects, aged 2-60 months, were recruited from four hospitals in the Netherlands and two hospitals in Israel between October 16, 2013, and March 1, 2015 (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30519-9).
The patients all had fevers with unidentified sources when they presented, and had a follow-up assessment carried out 28 days after baseline. Blood samples and nasal swabs were collected within 24 hours of presentation for assay analysis. Additionally, every subject was diagnosed as “bacterial” or “viral” by a three-member panel of pediatricians, whose diagnoses were based on the data available from the follow-up assessment and from clinical and laboratory data. The panel diagnosis for each subject was used as the reference standard.
Of the 777 subjects initially recruited, 200 were excluded from the final analysis for various reasons. Of the 577 who remained, the panel diagnosed 435 as having a viral infection and 71 as having a bacterial infection; 71 were deemed “inconclusive.” The panel was unanimous in 354 of these cases, and a majority of the panel (two of the three experts) agreed in 443 of these cases. In unanimous cases, the sensitivity of distinguishing between viral and bacterial cases correctly was 87.8%, with a specificity of 93.0%. The panel’s positive and negative predictive value were 62.1% and 98.3%, respectively.
The assay’s sensitivity rate in distinguishing between viral and bacterial infections was very close: 86.7%, with a specificity of 91.1%, which the authors noted was “promising diagnostic accuracy.” The positive predictive value of the assay was 60.5%, while the negative predictive value was found to be 97.8%.
Regarding the 71 cases that were deemed “inconclusive,” Dr. van Houten and coauthors acknowledged that “such inconclusive cases are inherent to studies without a gold standard, and this was taken into account when calculating the sample size.” Additionally, they noted that follow-up studies should take into consideration the costs of utilizing assay testing like ImmunoXpert, in order to better assess the financial implications that adopting the technology would have on a health care facility.
Nevertheless, the investigators concluded, “our findings [support] the need for implementation research to examine the added clinical utility of ImmunoXpert to diagnose bacterial infection in clinical care for children with lower respiratory tract infection and fever without source presenting at the hospital.”
Funding for this study was provided by MeMed Diagnostics. Dr. van Houten and coauthors did not report any relevant financial disclosures.
“A study by Chantal van Houten and colleagues in this issue of the Lancet Infectious Diseases tested the combined measurement of CRP, TRAIL, and IP-10, and found that this test distinguished bacterial from viral infections with a sensitivity of 86.7% and a specificity of 91.1%. This assay is significantly more effective than procalcitonin determinations in identifying the cause of infection because it improves the diagnostic classification of bacterial infections by 6.3% and of viral infections by 5.4%. However, by comparison with CRP, the CRP, TRAIL, and IP-10 combined assay is as effective at classifying bacterial cases, although it does improve the identification of patients with viral infections by 8.6%. Furthermore, it still has some limitations that currently preclude its routine use in clinical practice.
“First, the test requires advanced laboratory techniques and cannot be used outside hospitals. Second, the collected data came from a relatively small number of children, none of whom had an underlying disease that might modify host response to infection. Third – as in the case of all of the studies that have tried to differentiate bacterial and viral infection – the definition of cause of infection used in these studies varies. Finally, respiratory infections are frequently classified on the basis of clinical and radiological findings, and the results of a microbiological assessment of nasopharyngeal swabs.
“However, it is well known that the investigation into upper respiratory secretions in children can be confounding and lead to the erroneous classification of a lower respiratory disease, and that bacteria and viruses can simply be carried and could have no association with the cause of a disease. This means that future studies should confirm the results of host protein-based assays in larger study populations with various characteristics, and consider their cost to benefit ratios in relation to their real effect on reducing antibiotic use.”
Susanna Esposito, MD, and Nicola Principi, MD, are with the University of Milan. Their opinions are excerpted from a commentary on the article by Dr. van Houten et al. (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30536-9). They had no relevant financial disclosures.
“A study by Chantal van Houten and colleagues in this issue of the Lancet Infectious Diseases tested the combined measurement of CRP, TRAIL, and IP-10, and found that this test distinguished bacterial from viral infections with a sensitivity of 86.7% and a specificity of 91.1%. This assay is significantly more effective than procalcitonin determinations in identifying the cause of infection because it improves the diagnostic classification of bacterial infections by 6.3% and of viral infections by 5.4%. However, by comparison with CRP, the CRP, TRAIL, and IP-10 combined assay is as effective at classifying bacterial cases, although it does improve the identification of patients with viral infections by 8.6%. Furthermore, it still has some limitations that currently preclude its routine use in clinical practice.
“First, the test requires advanced laboratory techniques and cannot be used outside hospitals. Second, the collected data came from a relatively small number of children, none of whom had an underlying disease that might modify host response to infection. Third – as in the case of all of the studies that have tried to differentiate bacterial and viral infection – the definition of cause of infection used in these studies varies. Finally, respiratory infections are frequently classified on the basis of clinical and radiological findings, and the results of a microbiological assessment of nasopharyngeal swabs.
“However, it is well known that the investigation into upper respiratory secretions in children can be confounding and lead to the erroneous classification of a lower respiratory disease, and that bacteria and viruses can simply be carried and could have no association with the cause of a disease. This means that future studies should confirm the results of host protein-based assays in larger study populations with various characteristics, and consider their cost to benefit ratios in relation to their real effect on reducing antibiotic use.”
Susanna Esposito, MD, and Nicola Principi, MD, are with the University of Milan. Their opinions are excerpted from a commentary on the article by Dr. van Houten et al. (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30536-9). They had no relevant financial disclosures.
“A study by Chantal van Houten and colleagues in this issue of the Lancet Infectious Diseases tested the combined measurement of CRP, TRAIL, and IP-10, and found that this test distinguished bacterial from viral infections with a sensitivity of 86.7% and a specificity of 91.1%. This assay is significantly more effective than procalcitonin determinations in identifying the cause of infection because it improves the diagnostic classification of bacterial infections by 6.3% and of viral infections by 5.4%. However, by comparison with CRP, the CRP, TRAIL, and IP-10 combined assay is as effective at classifying bacterial cases, although it does improve the identification of patients with viral infections by 8.6%. Furthermore, it still has some limitations that currently preclude its routine use in clinical practice.
“First, the test requires advanced laboratory techniques and cannot be used outside hospitals. Second, the collected data came from a relatively small number of children, none of whom had an underlying disease that might modify host response to infection. Third – as in the case of all of the studies that have tried to differentiate bacterial and viral infection – the definition of cause of infection used in these studies varies. Finally, respiratory infections are frequently classified on the basis of clinical and radiological findings, and the results of a microbiological assessment of nasopharyngeal swabs.
“However, it is well known that the investigation into upper respiratory secretions in children can be confounding and lead to the erroneous classification of a lower respiratory disease, and that bacteria and viruses can simply be carried and could have no association with the cause of a disease. This means that future studies should confirm the results of host protein-based assays in larger study populations with various characteristics, and consider their cost to benefit ratios in relation to their real effect on reducing antibiotic use.”
Susanna Esposito, MD, and Nicola Principi, MD, are with the University of Milan. Their opinions are excerpted from a commentary on the article by Dr. van Houten et al. (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30536-9). They had no relevant financial disclosures.
An assay designed to distinguish between bacterial and viral infections of the lower respiratory tract appears effective and shows promise for helping hospital physicians reduce overprescribing of antibiotics to children, a study showed.
“It is often not possible to differentiate between bacterial and nonbacterial disease on the basis of clinical judgment alone, [so] antibiotics are prescribed almost twice as often as required in children with acute respiratory tract infections in the USA,” wrote Chantal B. van Houten of the University Medical Centre Utrecht (the Netherlands) and associates in a study published in the Lancet Infectious Diseases.
The assay in question is called ImmunoXpert, which uses three biomarkers – tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), interferon-gamma–induced protein-10 (IP-10), and C-reactive protein (CRP) – to determine if a lower respiratory tract infection has a viral or bacterial origin. A total of 777 subjects, aged 2-60 months, were recruited from four hospitals in the Netherlands and two hospitals in Israel between October 16, 2013, and March 1, 2015 (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30519-9).
The patients all had fevers with unidentified sources when they presented, and had a follow-up assessment carried out 28 days after baseline. Blood samples and nasal swabs were collected within 24 hours of presentation for assay analysis. Additionally, every subject was diagnosed as “bacterial” or “viral” by a three-member panel of pediatricians, whose diagnoses were based on the data available from the follow-up assessment and from clinical and laboratory data. The panel diagnosis for each subject was used as the reference standard.
Of the 777 subjects initially recruited, 200 were excluded from the final analysis for various reasons. Of the 577 who remained, the panel diagnosed 435 as having a viral infection and 71 as having a bacterial infection; 71 were deemed “inconclusive.” The panel was unanimous in 354 of these cases, and a majority of the panel (two of the three experts) agreed in 443 of these cases. In unanimous cases, the sensitivity of distinguishing between viral and bacterial cases correctly was 87.8%, with a specificity of 93.0%. The panel’s positive and negative predictive value were 62.1% and 98.3%, respectively.
The assay’s sensitivity rate in distinguishing between viral and bacterial infections was very close: 86.7%, with a specificity of 91.1%, which the authors noted was “promising diagnostic accuracy.” The positive predictive value of the assay was 60.5%, while the negative predictive value was found to be 97.8%.
Regarding the 71 cases that were deemed “inconclusive,” Dr. van Houten and coauthors acknowledged that “such inconclusive cases are inherent to studies without a gold standard, and this was taken into account when calculating the sample size.” Additionally, they noted that follow-up studies should take into consideration the costs of utilizing assay testing like ImmunoXpert, in order to better assess the financial implications that adopting the technology would have on a health care facility.
Nevertheless, the investigators concluded, “our findings [support] the need for implementation research to examine the added clinical utility of ImmunoXpert to diagnose bacterial infection in clinical care for children with lower respiratory tract infection and fever without source presenting at the hospital.”
Funding for this study was provided by MeMed Diagnostics. Dr. van Houten and coauthors did not report any relevant financial disclosures.
An assay designed to distinguish between bacterial and viral infections of the lower respiratory tract appears effective and shows promise for helping hospital physicians reduce overprescribing of antibiotics to children, a study showed.
“It is often not possible to differentiate between bacterial and nonbacterial disease on the basis of clinical judgment alone, [so] antibiotics are prescribed almost twice as often as required in children with acute respiratory tract infections in the USA,” wrote Chantal B. van Houten of the University Medical Centre Utrecht (the Netherlands) and associates in a study published in the Lancet Infectious Diseases.
The assay in question is called ImmunoXpert, which uses three biomarkers – tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), interferon-gamma–induced protein-10 (IP-10), and C-reactive protein (CRP) – to determine if a lower respiratory tract infection has a viral or bacterial origin. A total of 777 subjects, aged 2-60 months, were recruited from four hospitals in the Netherlands and two hospitals in Israel between October 16, 2013, and March 1, 2015 (Lancet Inf Dis. 2016 Dec. doi: 10.1016/S1473-3099(16)30519-9).
The patients all had fevers with unidentified sources when they presented, and had a follow-up assessment carried out 28 days after baseline. Blood samples and nasal swabs were collected within 24 hours of presentation for assay analysis. Additionally, every subject was diagnosed as “bacterial” or “viral” by a three-member panel of pediatricians, whose diagnoses were based on the data available from the follow-up assessment and from clinical and laboratory data. The panel diagnosis for each subject was used as the reference standard.
Of the 777 subjects initially recruited, 200 were excluded from the final analysis for various reasons. Of the 577 who remained, the panel diagnosed 435 as having a viral infection and 71 as having a bacterial infection; 71 were deemed “inconclusive.” The panel was unanimous in 354 of these cases, and a majority of the panel (two of the three experts) agreed in 443 of these cases. In unanimous cases, the sensitivity of distinguishing between viral and bacterial cases correctly was 87.8%, with a specificity of 93.0%. The panel’s positive and negative predictive value were 62.1% and 98.3%, respectively.
The assay’s sensitivity rate in distinguishing between viral and bacterial infections was very close: 86.7%, with a specificity of 91.1%, which the authors noted was “promising diagnostic accuracy.” The positive predictive value of the assay was 60.5%, while the negative predictive value was found to be 97.8%.
Regarding the 71 cases that were deemed “inconclusive,” Dr. van Houten and coauthors acknowledged that “such inconclusive cases are inherent to studies without a gold standard, and this was taken into account when calculating the sample size.” Additionally, they noted that follow-up studies should take into consideration the costs of utilizing assay testing like ImmunoXpert, in order to better assess the financial implications that adopting the technology would have on a health care facility.
Nevertheless, the investigators concluded, “our findings [support] the need for implementation research to examine the added clinical utility of ImmunoXpert to diagnose bacterial infection in clinical care for children with lower respiratory tract infection and fever without source presenting at the hospital.”
Funding for this study was provided by MeMed Diagnostics. Dr. van Houten and coauthors did not report any relevant financial disclosures.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point:
Major finding: The assay distinguished between bacterial and viral infections with 86.7% sensitivity, compared with unanimous panel diagnosis, which did so with 87.8% sensitivity.
Data source: A double-blind, multicenter, validation study of 577 children aged 2-60 months from October 2013 through March 2015.
Disclosures: The study was funded by MeMed Diagnostics. The authors reported no relevant financial disclosures.
Genetic studies link JIA subtypes to adult diseases, show uniqueness of systemic disease
Two new studies of the genetic relationships between the seven designated categories of juvenile idiopathic arthritis (JIA) provide compelling support for reclassification of the categories, particularly for systemic JIA, and give evidence that some of the categories have clear equivalents in the realm of adult-onset diseases.
The International League of Associations for Rheumatology (ILAR) classification system (J Rheumatol. 2004;31:390-2) that defined the seven juvenile idiopathic arthritis (JIA) categories – systemic arthritis, oligoarticular arthritis, rheumatoid factor (RF)–negative polyarticular arthritis, RF-positive polyarticular arthritis, psoriatic arthritis (PsA), enthesitis-related arthritis (ERA), and undifferentiated arthritis – is problematic because the long-term outcome and response to treatment varies not only between subtypes but also within the subtypes, suggesting that these subgroups do not yet represent uniform groups of patients,” Wendy Thomson, PhD, professor of genetic epidemiology at the University of Manchester (England) and a senior author on both studies, explained in an interview.
“Despite the differences between the subtypes, current treatments of this disease often involve using the same drugs across all subtypes of JIA,” said Dr. Thomson, who is also deputy director of the Arthritis Research UK Centre for Genetics and Genomics at the university. “Understanding the genetic basis of the subtypes of this disease could help understand the cause of this disease and identify more appropriate treatment options [because] the current classification is largely based on clinical data and it is proposed that the addition of genetic data could improve classification.”
Interrelationships between JIA categories and adult disease
The first of these studies found that particular alleles in the human leukocyte antigen (HLA) region that have been associated with the different JIA categories strongly correlate some of the categories with one another, and that each JIA category potentially has an adult-onset counterpart based on shared HLA associations. It is the largest investigation of association of the HLA region with JIA and its categories to date, according to the researchers (Ann Rheum Dis. 2016 Dec 20. doi: 10.1136/annrheumdis-2016-210025).
In particular, the investigators demonstrated for the first time that RF-negative polyarticular JIA and oligoarticular JIA are genetically similar in their HLA associations. They also reported that RF-positive polyarthritis shares an association with adult seropositive rheumatoid arthritis and that combined data for oligoarthritis and RF-negative polyarthritis share the same association with adult seronegative RA. In addition, the researchers generated support for genetic associations between the particular JIA categories that are most commonly thought to have adult counterparts, such as ERA and adult ankylosing spondylitis (AS) as well as juvenile PsA and adult PsA.
Dr. Thomson and her coinvestigators used ImmunoChip genotype array data for 191,494 single nucleotide polymorphism (SNP) markers from the HLA region of 5,737 JIA patients and 16,403 controls in the United States, United Kingdom, Canada, Norway, and Germany to impute classical HLA alleles as well as specific amino acid positions within HLA alleles that may play an important functional role. After quality-control measures for the data were put in place, comparisons between 5,043 JIA cases and 14,390 controls showed that oligoarthritis and RF-negative polyarthritis were the most common and homogeneous JIA categories investigated (2,409 and 1,525 patients, respectively), and that they share a significant association across the HLA spectrum, meaning that they are genetically similar. When combined, the data from these two JIA categories correspond with seronegative rheumatoid arthritis in adults, while RF-positive polyarthritis on its own has an association with seropositive rheumatoid arthritis involving histidine at position 13 of the HLA-DRB1 allele. As expected, the most significant association between the ERA category and AS was for HLA-B*27. For juvenile PsA, no associations reached genome-wide level of significance, although HLA-C*0602 was modestly associated with juvenile PsA, and it is known to be associated with adult-onset PsA and is the primary HLA association in psoriasis.
“The results of this study have important implications for understanding disease pathogenesis, etiology, and potential future therapeutic strategies for JIA categories,” Dr. Thomson and her coauthors wrote, adding that “heterogeneity of JIA remains a key challenge to pediatric rheumatologists; however, these results may inform the debate on classification and help define a more biological-driven and molecular-driven classification system.”
Uniqueness of systemic JIA
In the second of the two studies, investigators from many different childhood arthritis study groups focused on systemic JIA (sJIA), also known in the past as Still’s disease. According to the authors, it is the first large-scale genomic study of sJIA ever published (Ann Rheum Dis. 2016 Dec 7. doi: 10.1136/annrheumdis-2016-210324).
“[sJIA] is characterized by prominent systemic inflammation and has a rare adult-onset counterpart; and undifferentiated arthritis includes arthritis that does not fit into any single category,” the authors wrote, adding that the “unique clinical characteristics of sJIA suggest that it is distinct from other forms of JIA, leading to the contention by some that sJIA should be separated from other forms of JIA and labeled as an autoinflammatory disease.”
The investigators carried out SNP genotyping on 982 children with sJIA in the United States, United Kingdom, Germany, Turkey, Italy, Brazil, Argentina, Canada, and Spain, along with 431 healthy children without sJIA who came from the same countries. A total of 7,579 control patients also underwent genotyping. Ultimately, 770 sJIA and 6,947 control subjects completed the study. sJIA was then compared with other JIA subtypes by using weighted genetic risk scores.
Comparison of the data sets for sJIA with those for the combined categories of RF-negative polyarticular JIA and oligoarticular JIA, as well as for the individual category of RF-positive polyarticular JIA, showed that sJIA has a distinct genetic architecture that separates it from those JIA categories, providing further evidence that sJIA should be classified separately from other forms of JIA and, potentially, treated differently as well.
According to Dr. Thomson, the finding “provides important evidence that sJIA should be considered a unique disease with its own specific disease mechanisms.” Dr. Thomson explained that “knowing more about the genetic risk factors of this disease might give a greater understanding of the disease processes involved in this condition and ultimately lead to novel therapies for this severely disabling disease.”
Funding for the first study was provided by the Wellcome Trust, the National Institutes of Health, the Doris Duke Charitable Foundation, the Medical Research Council, the Canadian Institutes of Health Research, the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology, the Juvenile Diabetes Research Foundation International, and the Texas Scottish Rite Hospital for Children. Funding for the second study was provided by intramural research programs of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Human Genome Research Institute, individual NIH grants, and grants from charitable foundations, advocacy organizations, and the governments of individual researchers’ countries. Dr. Thomson did not report any relevant financial disclosures; however, a number of coauthors reported potential conflicts of interest.
These two excellent multi-institutional genetic studies demonstrate once and for all the error promulgated by the International League of Associations for Rheumatology (ILAR) criteria formulated in the 1990s. Despite contrary voices, the ILAR committee grouped all childhood arthritis that was not due to sepsis or trauma as “juvenile idiopathic arthritis.” And despite the lack of clarity of the subtypes and the many cases that are “unclassifiable,” pediatric rheumatologists have continued to use these criteria in both drug trials and scientific studies.
Thomas Lehman, MD, is chief of the division of pediatric rheumatology at the Hospital for Special Surgery in New York. He has no relevant financial disclosures.
These two excellent multi-institutional genetic studies demonstrate once and for all the error promulgated by the International League of Associations for Rheumatology (ILAR) criteria formulated in the 1990s. Despite contrary voices, the ILAR committee grouped all childhood arthritis that was not due to sepsis or trauma as “juvenile idiopathic arthritis.” And despite the lack of clarity of the subtypes and the many cases that are “unclassifiable,” pediatric rheumatologists have continued to use these criteria in both drug trials and scientific studies.
Thomas Lehman, MD, is chief of the division of pediatric rheumatology at the Hospital for Special Surgery in New York. He has no relevant financial disclosures.
These two excellent multi-institutional genetic studies demonstrate once and for all the error promulgated by the International League of Associations for Rheumatology (ILAR) criteria formulated in the 1990s. Despite contrary voices, the ILAR committee grouped all childhood arthritis that was not due to sepsis or trauma as “juvenile idiopathic arthritis.” And despite the lack of clarity of the subtypes and the many cases that are “unclassifiable,” pediatric rheumatologists have continued to use these criteria in both drug trials and scientific studies.
Thomas Lehman, MD, is chief of the division of pediatric rheumatology at the Hospital for Special Surgery in New York. He has no relevant financial disclosures.
Two new studies of the genetic relationships between the seven designated categories of juvenile idiopathic arthritis (JIA) provide compelling support for reclassification of the categories, particularly for systemic JIA, and give evidence that some of the categories have clear equivalents in the realm of adult-onset diseases.
The International League of Associations for Rheumatology (ILAR) classification system (J Rheumatol. 2004;31:390-2) that defined the seven juvenile idiopathic arthritis (JIA) categories – systemic arthritis, oligoarticular arthritis, rheumatoid factor (RF)–negative polyarticular arthritis, RF-positive polyarticular arthritis, psoriatic arthritis (PsA), enthesitis-related arthritis (ERA), and undifferentiated arthritis – is problematic because the long-term outcome and response to treatment varies not only between subtypes but also within the subtypes, suggesting that these subgroups do not yet represent uniform groups of patients,” Wendy Thomson, PhD, professor of genetic epidemiology at the University of Manchester (England) and a senior author on both studies, explained in an interview.
“Despite the differences between the subtypes, current treatments of this disease often involve using the same drugs across all subtypes of JIA,” said Dr. Thomson, who is also deputy director of the Arthritis Research UK Centre for Genetics and Genomics at the university. “Understanding the genetic basis of the subtypes of this disease could help understand the cause of this disease and identify more appropriate treatment options [because] the current classification is largely based on clinical data and it is proposed that the addition of genetic data could improve classification.”
Interrelationships between JIA categories and adult disease
The first of these studies found that particular alleles in the human leukocyte antigen (HLA) region that have been associated with the different JIA categories strongly correlate some of the categories with one another, and that each JIA category potentially has an adult-onset counterpart based on shared HLA associations. It is the largest investigation of association of the HLA region with JIA and its categories to date, according to the researchers (Ann Rheum Dis. 2016 Dec 20. doi: 10.1136/annrheumdis-2016-210025).
In particular, the investigators demonstrated for the first time that RF-negative polyarticular JIA and oligoarticular JIA are genetically similar in their HLA associations. They also reported that RF-positive polyarthritis shares an association with adult seropositive rheumatoid arthritis and that combined data for oligoarthritis and RF-negative polyarthritis share the same association with adult seronegative RA. In addition, the researchers generated support for genetic associations between the particular JIA categories that are most commonly thought to have adult counterparts, such as ERA and adult ankylosing spondylitis (AS) as well as juvenile PsA and adult PsA.
Dr. Thomson and her coinvestigators used ImmunoChip genotype array data for 191,494 single nucleotide polymorphism (SNP) markers from the HLA region of 5,737 JIA patients and 16,403 controls in the United States, United Kingdom, Canada, Norway, and Germany to impute classical HLA alleles as well as specific amino acid positions within HLA alleles that may play an important functional role. After quality-control measures for the data were put in place, comparisons between 5,043 JIA cases and 14,390 controls showed that oligoarthritis and RF-negative polyarthritis were the most common and homogeneous JIA categories investigated (2,409 and 1,525 patients, respectively), and that they share a significant association across the HLA spectrum, meaning that they are genetically similar. When combined, the data from these two JIA categories correspond with seronegative rheumatoid arthritis in adults, while RF-positive polyarthritis on its own has an association with seropositive rheumatoid arthritis involving histidine at position 13 of the HLA-DRB1 allele. As expected, the most significant association between the ERA category and AS was for HLA-B*27. For juvenile PsA, no associations reached genome-wide level of significance, although HLA-C*0602 was modestly associated with juvenile PsA, and it is known to be associated with adult-onset PsA and is the primary HLA association in psoriasis.
“The results of this study have important implications for understanding disease pathogenesis, etiology, and potential future therapeutic strategies for JIA categories,” Dr. Thomson and her coauthors wrote, adding that “heterogeneity of JIA remains a key challenge to pediatric rheumatologists; however, these results may inform the debate on classification and help define a more biological-driven and molecular-driven classification system.”
Uniqueness of systemic JIA
In the second of the two studies, investigators from many different childhood arthritis study groups focused on systemic JIA (sJIA), also known in the past as Still’s disease. According to the authors, it is the first large-scale genomic study of sJIA ever published (Ann Rheum Dis. 2016 Dec 7. doi: 10.1136/annrheumdis-2016-210324).
“[sJIA] is characterized by prominent systemic inflammation and has a rare adult-onset counterpart; and undifferentiated arthritis includes arthritis that does not fit into any single category,” the authors wrote, adding that the “unique clinical characteristics of sJIA suggest that it is distinct from other forms of JIA, leading to the contention by some that sJIA should be separated from other forms of JIA and labeled as an autoinflammatory disease.”
The investigators carried out SNP genotyping on 982 children with sJIA in the United States, United Kingdom, Germany, Turkey, Italy, Brazil, Argentina, Canada, and Spain, along with 431 healthy children without sJIA who came from the same countries. A total of 7,579 control patients also underwent genotyping. Ultimately, 770 sJIA and 6,947 control subjects completed the study. sJIA was then compared with other JIA subtypes by using weighted genetic risk scores.
Comparison of the data sets for sJIA with those for the combined categories of RF-negative polyarticular JIA and oligoarticular JIA, as well as for the individual category of RF-positive polyarticular JIA, showed that sJIA has a distinct genetic architecture that separates it from those JIA categories, providing further evidence that sJIA should be classified separately from other forms of JIA and, potentially, treated differently as well.
According to Dr. Thomson, the finding “provides important evidence that sJIA should be considered a unique disease with its own specific disease mechanisms.” Dr. Thomson explained that “knowing more about the genetic risk factors of this disease might give a greater understanding of the disease processes involved in this condition and ultimately lead to novel therapies for this severely disabling disease.”
Funding for the first study was provided by the Wellcome Trust, the National Institutes of Health, the Doris Duke Charitable Foundation, the Medical Research Council, the Canadian Institutes of Health Research, the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology, the Juvenile Diabetes Research Foundation International, and the Texas Scottish Rite Hospital for Children. Funding for the second study was provided by intramural research programs of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Human Genome Research Institute, individual NIH grants, and grants from charitable foundations, advocacy organizations, and the governments of individual researchers’ countries. Dr. Thomson did not report any relevant financial disclosures; however, a number of coauthors reported potential conflicts of interest.
Two new studies of the genetic relationships between the seven designated categories of juvenile idiopathic arthritis (JIA) provide compelling support for reclassification of the categories, particularly for systemic JIA, and give evidence that some of the categories have clear equivalents in the realm of adult-onset diseases.
The International League of Associations for Rheumatology (ILAR) classification system (J Rheumatol. 2004;31:390-2) that defined the seven juvenile idiopathic arthritis (JIA) categories – systemic arthritis, oligoarticular arthritis, rheumatoid factor (RF)–negative polyarticular arthritis, RF-positive polyarticular arthritis, psoriatic arthritis (PsA), enthesitis-related arthritis (ERA), and undifferentiated arthritis – is problematic because the long-term outcome and response to treatment varies not only between subtypes but also within the subtypes, suggesting that these subgroups do not yet represent uniform groups of patients,” Wendy Thomson, PhD, professor of genetic epidemiology at the University of Manchester (England) and a senior author on both studies, explained in an interview.
“Despite the differences between the subtypes, current treatments of this disease often involve using the same drugs across all subtypes of JIA,” said Dr. Thomson, who is also deputy director of the Arthritis Research UK Centre for Genetics and Genomics at the university. “Understanding the genetic basis of the subtypes of this disease could help understand the cause of this disease and identify more appropriate treatment options [because] the current classification is largely based on clinical data and it is proposed that the addition of genetic data could improve classification.”
Interrelationships between JIA categories and adult disease
The first of these studies found that particular alleles in the human leukocyte antigen (HLA) region that have been associated with the different JIA categories strongly correlate some of the categories with one another, and that each JIA category potentially has an adult-onset counterpart based on shared HLA associations. It is the largest investigation of association of the HLA region with JIA and its categories to date, according to the researchers (Ann Rheum Dis. 2016 Dec 20. doi: 10.1136/annrheumdis-2016-210025).
In particular, the investigators demonstrated for the first time that RF-negative polyarticular JIA and oligoarticular JIA are genetically similar in their HLA associations. They also reported that RF-positive polyarthritis shares an association with adult seropositive rheumatoid arthritis and that combined data for oligoarthritis and RF-negative polyarthritis share the same association with adult seronegative RA. In addition, the researchers generated support for genetic associations between the particular JIA categories that are most commonly thought to have adult counterparts, such as ERA and adult ankylosing spondylitis (AS) as well as juvenile PsA and adult PsA.
Dr. Thomson and her coinvestigators used ImmunoChip genotype array data for 191,494 single nucleotide polymorphism (SNP) markers from the HLA region of 5,737 JIA patients and 16,403 controls in the United States, United Kingdom, Canada, Norway, and Germany to impute classical HLA alleles as well as specific amino acid positions within HLA alleles that may play an important functional role. After quality-control measures for the data were put in place, comparisons between 5,043 JIA cases and 14,390 controls showed that oligoarthritis and RF-negative polyarthritis were the most common and homogeneous JIA categories investigated (2,409 and 1,525 patients, respectively), and that they share a significant association across the HLA spectrum, meaning that they are genetically similar. When combined, the data from these two JIA categories correspond with seronegative rheumatoid arthritis in adults, while RF-positive polyarthritis on its own has an association with seropositive rheumatoid arthritis involving histidine at position 13 of the HLA-DRB1 allele. As expected, the most significant association between the ERA category and AS was for HLA-B*27. For juvenile PsA, no associations reached genome-wide level of significance, although HLA-C*0602 was modestly associated with juvenile PsA, and it is known to be associated with adult-onset PsA and is the primary HLA association in psoriasis.
“The results of this study have important implications for understanding disease pathogenesis, etiology, and potential future therapeutic strategies for JIA categories,” Dr. Thomson and her coauthors wrote, adding that “heterogeneity of JIA remains a key challenge to pediatric rheumatologists; however, these results may inform the debate on classification and help define a more biological-driven and molecular-driven classification system.”
Uniqueness of systemic JIA
In the second of the two studies, investigators from many different childhood arthritis study groups focused on systemic JIA (sJIA), also known in the past as Still’s disease. According to the authors, it is the first large-scale genomic study of sJIA ever published (Ann Rheum Dis. 2016 Dec 7. doi: 10.1136/annrheumdis-2016-210324).
“[sJIA] is characterized by prominent systemic inflammation and has a rare adult-onset counterpart; and undifferentiated arthritis includes arthritis that does not fit into any single category,” the authors wrote, adding that the “unique clinical characteristics of sJIA suggest that it is distinct from other forms of JIA, leading to the contention by some that sJIA should be separated from other forms of JIA and labeled as an autoinflammatory disease.”
The investigators carried out SNP genotyping on 982 children with sJIA in the United States, United Kingdom, Germany, Turkey, Italy, Brazil, Argentina, Canada, and Spain, along with 431 healthy children without sJIA who came from the same countries. A total of 7,579 control patients also underwent genotyping. Ultimately, 770 sJIA and 6,947 control subjects completed the study. sJIA was then compared with other JIA subtypes by using weighted genetic risk scores.
Comparison of the data sets for sJIA with those for the combined categories of RF-negative polyarticular JIA and oligoarticular JIA, as well as for the individual category of RF-positive polyarticular JIA, showed that sJIA has a distinct genetic architecture that separates it from those JIA categories, providing further evidence that sJIA should be classified separately from other forms of JIA and, potentially, treated differently as well.
According to Dr. Thomson, the finding “provides important evidence that sJIA should be considered a unique disease with its own specific disease mechanisms.” Dr. Thomson explained that “knowing more about the genetic risk factors of this disease might give a greater understanding of the disease processes involved in this condition and ultimately lead to novel therapies for this severely disabling disease.”
Funding for the first study was provided by the Wellcome Trust, the National Institutes of Health, the Doris Duke Charitable Foundation, the Medical Research Council, the Canadian Institutes of Health Research, the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology, the Juvenile Diabetes Research Foundation International, and the Texas Scottish Rite Hospital for Children. Funding for the second study was provided by intramural research programs of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Human Genome Research Institute, individual NIH grants, and grants from charitable foundations, advocacy organizations, and the governments of individual researchers’ countries. Dr. Thomson did not report any relevant financial disclosures; however, a number of coauthors reported potential conflicts of interest.
FROM ANNALS OF THE RHEUMATIC DISEASES
High consumption of red meat increases diverticulitis risk in men
Men who consume higher quantities of red meat are at an increased risk of developing diverticulitis, especially if they’re eating unprocessed red meat, according to a new study published in Gut.
“In our prior analysis from a large prospective cohort study, the Health Professionals Follow-Up Study (HPFS), we found that red meat intake, independent of fiber, may be associated with a composite outcome of symptomatic diverticular disease, which included 385 incident cases over 4 years of follow-up,” wrote the authors, led by Andrew T. Chan, MD, of Massachusetts General Hospital, Boston. Dr. Chan added that “in the present study, we updated this analysis, which allowed us to prospectively examine the association between consumption of meat (total red meat, red unprocessed meat, red processed meat, poultry, and fish) with risk of incident diverticulitis in 764 cases over 26 years of follow-up.”
Dr. Chan and his coinvestigators conducted a prospective cohort study using subjects from the ongoing HPFS. Men who already had a diagnosis of diverticulitis, associated complications, inflammatory bowel disease, or a GI-related cancer at baseline were excluded from this analysis, leaving 46,461 eligible subjects. Of those, 764 developed diverticulitis.
The entirety of the follow-up period constituted 651,970 person-years. Average servings of total red meat per week were 1.2 in quintile 1, compared to 5.3 in quintile 3 and 13.5 in quintile 5. Those in the highest quintile had a multivariable risk ratio of 1.58 (95% CI, 1.19-2.11; P = .01), indicating a significantly higher risk for developing diverticulitis. In terms of unprocessed red meat, the average number of servings per week were 0.8 for the lower quintile, 3.2 for quintile 3, and 8.6 for quintile 5, yielding a risk ratio of 1.51 (95% CI, 1.12-2.03, P = .03) when comparing the highest and lowest cohorts. The increase in risk, however, leveled off after about 6 servings of red meat per week, and was found to be nonlinear (P = .002). Those who ate more servings of poultry or fish did not have a higher risk of diverticulitis.
“We also observed that unprocessed red meat, but not processed red meat, was the primary driver for the association between total red meat and risk of diverticulitis,” the authors explained. “Compared with processed meat, unprocessed meat (e.g., steak) is usually consumed in larger portions, which could lead to a larger undigested piece in the large bowel and induce different changes in colonic microbiota [and] higher cooking temperatures used in the preparation of unprocessed meat may influence bacterial composition or proinflammatory mediators in the colon.”
Although medical information and self-reports were validated, there are inherent possible limitations to self-reported data, such as misremembering the amount of meat consumed or reporting incorrect amounts. Residual confounding may have occurred despite adjustment of the data to account for it.
The National Institutes of Health funded the study. The authors reported no conflicts of interest.
Men who consume higher quantities of red meat are at an increased risk of developing diverticulitis, especially if they’re eating unprocessed red meat, according to a new study published in Gut.
“In our prior analysis from a large prospective cohort study, the Health Professionals Follow-Up Study (HPFS), we found that red meat intake, independent of fiber, may be associated with a composite outcome of symptomatic diverticular disease, which included 385 incident cases over 4 years of follow-up,” wrote the authors, led by Andrew T. Chan, MD, of Massachusetts General Hospital, Boston. Dr. Chan added that “in the present study, we updated this analysis, which allowed us to prospectively examine the association between consumption of meat (total red meat, red unprocessed meat, red processed meat, poultry, and fish) with risk of incident diverticulitis in 764 cases over 26 years of follow-up.”
Dr. Chan and his coinvestigators conducted a prospective cohort study using subjects from the ongoing HPFS. Men who already had a diagnosis of diverticulitis, associated complications, inflammatory bowel disease, or a GI-related cancer at baseline were excluded from this analysis, leaving 46,461 eligible subjects. Of those, 764 developed diverticulitis.
The entirety of the follow-up period constituted 651,970 person-years. Average servings of total red meat per week were 1.2 in quintile 1, compared to 5.3 in quintile 3 and 13.5 in quintile 5. Those in the highest quintile had a multivariable risk ratio of 1.58 (95% CI, 1.19-2.11; P = .01), indicating a significantly higher risk for developing diverticulitis. In terms of unprocessed red meat, the average number of servings per week were 0.8 for the lower quintile, 3.2 for quintile 3, and 8.6 for quintile 5, yielding a risk ratio of 1.51 (95% CI, 1.12-2.03, P = .03) when comparing the highest and lowest cohorts. The increase in risk, however, leveled off after about 6 servings of red meat per week, and was found to be nonlinear (P = .002). Those who ate more servings of poultry or fish did not have a higher risk of diverticulitis.
“We also observed that unprocessed red meat, but not processed red meat, was the primary driver for the association between total red meat and risk of diverticulitis,” the authors explained. “Compared with processed meat, unprocessed meat (e.g., steak) is usually consumed in larger portions, which could lead to a larger undigested piece in the large bowel and induce different changes in colonic microbiota [and] higher cooking temperatures used in the preparation of unprocessed meat may influence bacterial composition or proinflammatory mediators in the colon.”
Although medical information and self-reports were validated, there are inherent possible limitations to self-reported data, such as misremembering the amount of meat consumed or reporting incorrect amounts. Residual confounding may have occurred despite adjustment of the data to account for it.
The National Institutes of Health funded the study. The authors reported no conflicts of interest.
Men who consume higher quantities of red meat are at an increased risk of developing diverticulitis, especially if they’re eating unprocessed red meat, according to a new study published in Gut.
“In our prior analysis from a large prospective cohort study, the Health Professionals Follow-Up Study (HPFS), we found that red meat intake, independent of fiber, may be associated with a composite outcome of symptomatic diverticular disease, which included 385 incident cases over 4 years of follow-up,” wrote the authors, led by Andrew T. Chan, MD, of Massachusetts General Hospital, Boston. Dr. Chan added that “in the present study, we updated this analysis, which allowed us to prospectively examine the association between consumption of meat (total red meat, red unprocessed meat, red processed meat, poultry, and fish) with risk of incident diverticulitis in 764 cases over 26 years of follow-up.”
Dr. Chan and his coinvestigators conducted a prospective cohort study using subjects from the ongoing HPFS. Men who already had a diagnosis of diverticulitis, associated complications, inflammatory bowel disease, or a GI-related cancer at baseline were excluded from this analysis, leaving 46,461 eligible subjects. Of those, 764 developed diverticulitis.
The entirety of the follow-up period constituted 651,970 person-years. Average servings of total red meat per week were 1.2 in quintile 1, compared to 5.3 in quintile 3 and 13.5 in quintile 5. Those in the highest quintile had a multivariable risk ratio of 1.58 (95% CI, 1.19-2.11; P = .01), indicating a significantly higher risk for developing diverticulitis. In terms of unprocessed red meat, the average number of servings per week were 0.8 for the lower quintile, 3.2 for quintile 3, and 8.6 for quintile 5, yielding a risk ratio of 1.51 (95% CI, 1.12-2.03, P = .03) when comparing the highest and lowest cohorts. The increase in risk, however, leveled off after about 6 servings of red meat per week, and was found to be nonlinear (P = .002). Those who ate more servings of poultry or fish did not have a higher risk of diverticulitis.
“We also observed that unprocessed red meat, but not processed red meat, was the primary driver for the association between total red meat and risk of diverticulitis,” the authors explained. “Compared with processed meat, unprocessed meat (e.g., steak) is usually consumed in larger portions, which could lead to a larger undigested piece in the large bowel and induce different changes in colonic microbiota [and] higher cooking temperatures used in the preparation of unprocessed meat may influence bacterial composition or proinflammatory mediators in the colon.”
Although medical information and self-reports were validated, there are inherent possible limitations to self-reported data, such as misremembering the amount of meat consumed or reporting incorrect amounts. Residual confounding may have occurred despite adjustment of the data to account for it.
The National Institutes of Health funded the study. The authors reported no conflicts of interest.
Key clinical point:
Major finding: Men with the highest consumption of red meat per week had a risk ratio of 1.58 (95% CI, 1.19-2.11, P = .01) compared to those with the lowest consumption, with an RR of 1.51 (95% CI, 1.12-2.03, P = .03) when comparing unprocessed red meat consumption.
Data source: Prospective cohort study of 51,529 men aged 40-75 years, in the United States.
Disclosures: The National Institutes of Health funded the study. The authors reported no conflicts of interest.
Understanding SSTI admission, treatment crucial to reducing disease burden
Decreasing the burden of treating skin and soft tissue infections is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Conn.) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous (IV) antibiotic therapy or treated as outpatients.”
Dr. Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average length-of-stay (LOS) and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June of 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion (Hosp Pract. 2017 Jan 5. doi: 10.1080/21548331.2017.1279519).
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall – 22.6% for inpatients and 28.3% for outpatients (P greater than .05) – or for SSTI-related re-presentation: 10.4% for inpatients and 15.1% for outpatients (P greater than .05). For those patients who did get admitted, mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of zero were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of one, and 60.9% of those with a CCI score of two or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus (MSSA) to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S. aureus (MRSA) was the most common (66.7%). According to the investigators, Gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
This study was not funded, according to the authors. Dr. Linder did not report any relevant financial disclosures, but her coauthors disclosed receiving speakers’ and consultants’ fees from Astellas, Theravance. Bayer, Merck and Pfizer.
Decreasing the burden of treating skin and soft tissue infections is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Conn.) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous (IV) antibiotic therapy or treated as outpatients.”
Dr. Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average length-of-stay (LOS) and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June of 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion (Hosp Pract. 2017 Jan 5. doi: 10.1080/21548331.2017.1279519).
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall – 22.6% for inpatients and 28.3% for outpatients (P greater than .05) – or for SSTI-related re-presentation: 10.4% for inpatients and 15.1% for outpatients (P greater than .05). For those patients who did get admitted, mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of zero were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of one, and 60.9% of those with a CCI score of two or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus (MSSA) to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S. aureus (MRSA) was the most common (66.7%). According to the investigators, Gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
This study was not funded, according to the authors. Dr. Linder did not report any relevant financial disclosures, but her coauthors disclosed receiving speakers’ and consultants’ fees from Astellas, Theravance. Bayer, Merck and Pfizer.
Decreasing the burden of treating skin and soft tissue infections is critical to improving care and reducing the costs that SSTIs place on health care facilities, according to a study published in Hospital Practice.
“Despite expert panel recommendations and treatment guidelines, there is no widely accepted classification system for grading SSTIs to outcomes,” wrote the study’s lead author, Kristin E. Linder, PharmD, of Hartford (Conn.) Hospital. “This leads to a considerable variation in treatment approach on initial presentation when deciding which patients should be admitted to receive intravenous (IV) antibiotic therapy or treated as outpatients.”
Dr. Linder and her coinvestigators conducted a single-center retrospective cohort study with the primary objective of determining rates of admission and re-presentation, along with average length-of-stay (LOS) and cost of care for both inpatients and outpatients with SSTIs. Patients aged 18 years and older who received a primary diagnosis of an SSTI during May and June of 2015 at Hartford Hospital were screened; 446 were deemed eligible, with 357 ultimately selected for inclusion (Hosp Pract. 2017 Jan 5. doi: 10.1080/21548331.2017.1279519).
Of the 357 patients included for analysis, 106 (29.7%) were admitted as inpatients while the remaining 251 (70.3%) were treated as outpatients. However, there were no significant differences found in re-presentation rates, either overall – 22.6% for inpatients and 28.3% for outpatients (P greater than .05) – or for SSTI-related re-presentation: 10.4% for inpatients and 15.1% for outpatients (P greater than .05). For those patients who did get admitted, mean LOS was 7.3 days.
Patients who presented with a Charlson Comorbidity Index (CCI) score of zero were admitted at a rate of 14.1%, compared to 30.1% of those with a CCI score of one, and 60.9% of those with a CCI score of two or higher. The biggest disparity, however, was in terms of cost of care; while outpatient care cost an average of $413 per patient, inpatient care cost an average of $13,313 per patient.
Wound and abscess cultures that were tested found methicillin-susceptible Staphylococcus aureus (MSSA) to be the most prevalent gram-positive organism (37.1%) found in inpatients, while for outpatients, methicillin-resistant S. aureus (MRSA) was the most common (66.7%). According to the investigators, Gram-negative bacteria were not isolated in every case, so “prevalent use of combination therapy in this setting may not be warranted.
“Understanding how and where patients with SSTI are treated and their re-presentation rate is important to understand to direct resources for this high frequency disease,” the authors concluded. “This study demonstrated that approximately 70% of patients presenting to the ED with SSTI were treated as outpatients [and] while 30-day re-presentation was similar for inpatient and outpatients, readmission was more likely in those previously admitted.”
This study was not funded, according to the authors. Dr. Linder did not report any relevant financial disclosures, but her coauthors disclosed receiving speakers’ and consultants’ fees from Astellas, Theravance. Bayer, Merck and Pfizer.
FROM HOSPITAL PRACTICE
Key clinical point:
Major finding: Re-presentation rates between inpatients and outpatients with SSTIs were not significantly different – 10.4% versus 15.1%, respectively (P greater than .05) – but cost of care was much higher for inpatients than outpatients: $13,313 versus $413, respectively.
Data source: Retrospective cohort study of 357 SSTI patients during May and June of 2015.
Disclosures: The study was not funded. Two authors reported potential financial conflicts.
Statin use increases risk of herpes zoster
Exposure to statins can significantly increase the odds of developing herpes zoster (HZ), according to a study published in the British Journal of Dermatology.
“The mechanisms by which statins may increase the risk of HZ are not established. As statins are commonly prescribed worldwide, any adverse effects may have substantial public health implications,” wrote Anthony Matthews of the London School of Hygiene and Tropical Medicine, and his colleagues. “An increased risk of HZ would not only have an impact on the quality of life of affected patients, but could also add to the burden on health services, given the high cost of treatment for PHN [postherpetic neuralgia].”
Each subject was matched with no more than four control patients – all of whom had no prior history of HZ or PHN – selected from the same databases. Matching was based on the following criteria: subject’s general practitioner (GP) practice, age (within 1 year of the HZ patient), sex, and lack of prior statin exposure. Ultimately 144,959 subjects were matched with 549,336 controls (Br J Dermatol. 2016 Dec;175[6]:1183-94).
The primary outcome was defined as incident diagnoses of HZ, which was measured via odds ratios (ORs) calculated from conditional logistic regression models and adjusted for any potential confounding factors. In addition, ORs were calculated to “explore the association between the risk of HZ and the dosage of the latest prescription (low, medium, high), stratified by time since last exposure,” the investigators noted.
Results showed an adjusted OR of 1.13 for HZ after any kind of statin treatment, indicating a significant increase in risk. There was also a higher adjusted OR for those who had stopped using statins 12-36 months prior to the study than those whose last exposure was greater than 36 months prior: 1.11 and 1.06, respectively (P less than .001). Those whose current statin usage was “high” had an adjusted OR of 1.27 versus 1.16 for those whose usage was “medium,” and 1.14 for those whose usage was “low” (P less than .001).
“It is clear that the preventive benefits of statin therapy are likely to outweigh the limited increase in HZ risk in many cases [but] this evidence should be taken into account by GPs when prescribing statins to those at high risk of HZ,” Mr. Matthews and his associates concluded. “We would also suggest that there may be an extra motivation to maximize HZ vaccine uptake among eligible patients who are also receiving a statin.”
Three coauthors disclosed individual funding sources: the Wellcome Trust/Royal Society Sir Henry Dale fellowship, the Senior Wellcome Fellowship in Clinical Science, and the National Institute for Health Research Clinician Scientist fellowship. The authors did not report any relevant financial disclosures.
“The present study by Matthews et al. observed a dose-response relationship between statin exposure and zoster risk. [It] is the first to explore the association between time since last statin use and the occurrence of zoster. As the time since last statin exposure increased, the risk of zoster decreased, suggesting that the risk for zoster is reduced after termination of statin treatment.
“Zoster is a significant contributor to morbidity, disability, and chronic pain, and constitutes a major public health concern. Zoster vaccination is recommended in most persons who are 50 years of age or older. Vaccination reduces the risk of zoster and postherpetic neuralgia. However, the price of zoster vaccine limits its use worldwide.
“The ‘take home’ message from the article is that in the setting of older age or immune suppression, dermatologists should consider recommending to their patients the use of a zoster vaccine, particularly in patients who receive statin therapy.”
Guy Shalom, MD, is a member of the department of dermatology and venereology, Soroka Medical Center in Beer-Sheva, Israel, and reported no relevant financial conflicts. Arnon D. Cohen, MD, is a member of the Siaal Research Center for Family Medicine and Primary Care at Ben-Gurion University of the Negev, Beer-Sheva, and reported financial ties to AbbVie, Dexcel Pharma, Janssen, Novartis, Perrigo, Pfizer, and Rafa as a consultant, adviser, or speaker. These comments were excerpted from their commentary accompanying Mr. Matthews’ article ( Br J Dermatol. 2017;175:1137-8 ).
“The present study by Matthews et al. observed a dose-response relationship between statin exposure and zoster risk. [It] is the first to explore the association between time since last statin use and the occurrence of zoster. As the time since last statin exposure increased, the risk of zoster decreased, suggesting that the risk for zoster is reduced after termination of statin treatment.
“Zoster is a significant contributor to morbidity, disability, and chronic pain, and constitutes a major public health concern. Zoster vaccination is recommended in most persons who are 50 years of age or older. Vaccination reduces the risk of zoster and postherpetic neuralgia. However, the price of zoster vaccine limits its use worldwide.
“The ‘take home’ message from the article is that in the setting of older age or immune suppression, dermatologists should consider recommending to their patients the use of a zoster vaccine, particularly in patients who receive statin therapy.”
Guy Shalom, MD, is a member of the department of dermatology and venereology, Soroka Medical Center in Beer-Sheva, Israel, and reported no relevant financial conflicts. Arnon D. Cohen, MD, is a member of the Siaal Research Center for Family Medicine and Primary Care at Ben-Gurion University of the Negev, Beer-Sheva, and reported financial ties to AbbVie, Dexcel Pharma, Janssen, Novartis, Perrigo, Pfizer, and Rafa as a consultant, adviser, or speaker. These comments were excerpted from their commentary accompanying Mr. Matthews’ article ( Br J Dermatol. 2017;175:1137-8 ).
“The present study by Matthews et al. observed a dose-response relationship between statin exposure and zoster risk. [It] is the first to explore the association between time since last statin use and the occurrence of zoster. As the time since last statin exposure increased, the risk of zoster decreased, suggesting that the risk for zoster is reduced after termination of statin treatment.
“Zoster is a significant contributor to morbidity, disability, and chronic pain, and constitutes a major public health concern. Zoster vaccination is recommended in most persons who are 50 years of age or older. Vaccination reduces the risk of zoster and postherpetic neuralgia. However, the price of zoster vaccine limits its use worldwide.
“The ‘take home’ message from the article is that in the setting of older age or immune suppression, dermatologists should consider recommending to their patients the use of a zoster vaccine, particularly in patients who receive statin therapy.”
Guy Shalom, MD, is a member of the department of dermatology and venereology, Soroka Medical Center in Beer-Sheva, Israel, and reported no relevant financial conflicts. Arnon D. Cohen, MD, is a member of the Siaal Research Center for Family Medicine and Primary Care at Ben-Gurion University of the Negev, Beer-Sheva, and reported financial ties to AbbVie, Dexcel Pharma, Janssen, Novartis, Perrigo, Pfizer, and Rafa as a consultant, adviser, or speaker. These comments were excerpted from their commentary accompanying Mr. Matthews’ article ( Br J Dermatol. 2017;175:1137-8 ).
Exposure to statins can significantly increase the odds of developing herpes zoster (HZ), according to a study published in the British Journal of Dermatology.
“The mechanisms by which statins may increase the risk of HZ are not established. As statins are commonly prescribed worldwide, any adverse effects may have substantial public health implications,” wrote Anthony Matthews of the London School of Hygiene and Tropical Medicine, and his colleagues. “An increased risk of HZ would not only have an impact on the quality of life of affected patients, but could also add to the burden on health services, given the high cost of treatment for PHN [postherpetic neuralgia].”
Each subject was matched with no more than four control patients – all of whom had no prior history of HZ or PHN – selected from the same databases. Matching was based on the following criteria: subject’s general practitioner (GP) practice, age (within 1 year of the HZ patient), sex, and lack of prior statin exposure. Ultimately 144,959 subjects were matched with 549,336 controls (Br J Dermatol. 2016 Dec;175[6]:1183-94).
The primary outcome was defined as incident diagnoses of HZ, which was measured via odds ratios (ORs) calculated from conditional logistic regression models and adjusted for any potential confounding factors. In addition, ORs were calculated to “explore the association between the risk of HZ and the dosage of the latest prescription (low, medium, high), stratified by time since last exposure,” the investigators noted.
Results showed an adjusted OR of 1.13 for HZ after any kind of statin treatment, indicating a significant increase in risk. There was also a higher adjusted OR for those who had stopped using statins 12-36 months prior to the study than those whose last exposure was greater than 36 months prior: 1.11 and 1.06, respectively (P less than .001). Those whose current statin usage was “high” had an adjusted OR of 1.27 versus 1.16 for those whose usage was “medium,” and 1.14 for those whose usage was “low” (P less than .001).
“It is clear that the preventive benefits of statin therapy are likely to outweigh the limited increase in HZ risk in many cases [but] this evidence should be taken into account by GPs when prescribing statins to those at high risk of HZ,” Mr. Matthews and his associates concluded. “We would also suggest that there may be an extra motivation to maximize HZ vaccine uptake among eligible patients who are also receiving a statin.”
Three coauthors disclosed individual funding sources: the Wellcome Trust/Royal Society Sir Henry Dale fellowship, the Senior Wellcome Fellowship in Clinical Science, and the National Institute for Health Research Clinician Scientist fellowship. The authors did not report any relevant financial disclosures.
Exposure to statins can significantly increase the odds of developing herpes zoster (HZ), according to a study published in the British Journal of Dermatology.
“The mechanisms by which statins may increase the risk of HZ are not established. As statins are commonly prescribed worldwide, any adverse effects may have substantial public health implications,” wrote Anthony Matthews of the London School of Hygiene and Tropical Medicine, and his colleagues. “An increased risk of HZ would not only have an impact on the quality of life of affected patients, but could also add to the burden on health services, given the high cost of treatment for PHN [postherpetic neuralgia].”
Each subject was matched with no more than four control patients – all of whom had no prior history of HZ or PHN – selected from the same databases. Matching was based on the following criteria: subject’s general practitioner (GP) practice, age (within 1 year of the HZ patient), sex, and lack of prior statin exposure. Ultimately 144,959 subjects were matched with 549,336 controls (Br J Dermatol. 2016 Dec;175[6]:1183-94).
The primary outcome was defined as incident diagnoses of HZ, which was measured via odds ratios (ORs) calculated from conditional logistic regression models and adjusted for any potential confounding factors. In addition, ORs were calculated to “explore the association between the risk of HZ and the dosage of the latest prescription (low, medium, high), stratified by time since last exposure,” the investigators noted.
Results showed an adjusted OR of 1.13 for HZ after any kind of statin treatment, indicating a significant increase in risk. There was also a higher adjusted OR for those who had stopped using statins 12-36 months prior to the study than those whose last exposure was greater than 36 months prior: 1.11 and 1.06, respectively (P less than .001). Those whose current statin usage was “high” had an adjusted OR of 1.27 versus 1.16 for those whose usage was “medium,” and 1.14 for those whose usage was “low” (P less than .001).
“It is clear that the preventive benefits of statin therapy are likely to outweigh the limited increase in HZ risk in many cases [but] this evidence should be taken into account by GPs when prescribing statins to those at high risk of HZ,” Mr. Matthews and his associates concluded. “We would also suggest that there may be an extra motivation to maximize HZ vaccine uptake among eligible patients who are also receiving a statin.”
Three coauthors disclosed individual funding sources: the Wellcome Trust/Royal Society Sir Henry Dale fellowship, the Senior Wellcome Fellowship in Clinical Science, and the National Institute for Health Research Clinician Scientist fellowship. The authors did not report any relevant financial disclosures.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point:
Major finding: There is an odds ratio of 1.13 of contracting HZ if given a statin previously, with a significant increase (P less than .001) in risk in patients who used statins more recently.
Data source: Matched case-control study of 144,959 adults with HZ from 2000 through 2011.
Disclosures: Dr. Matthews and colleagues reported no relevant financial disclosures.