User login
Warts difficult to eradicate in immunocompromised children
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
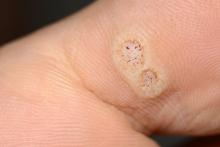
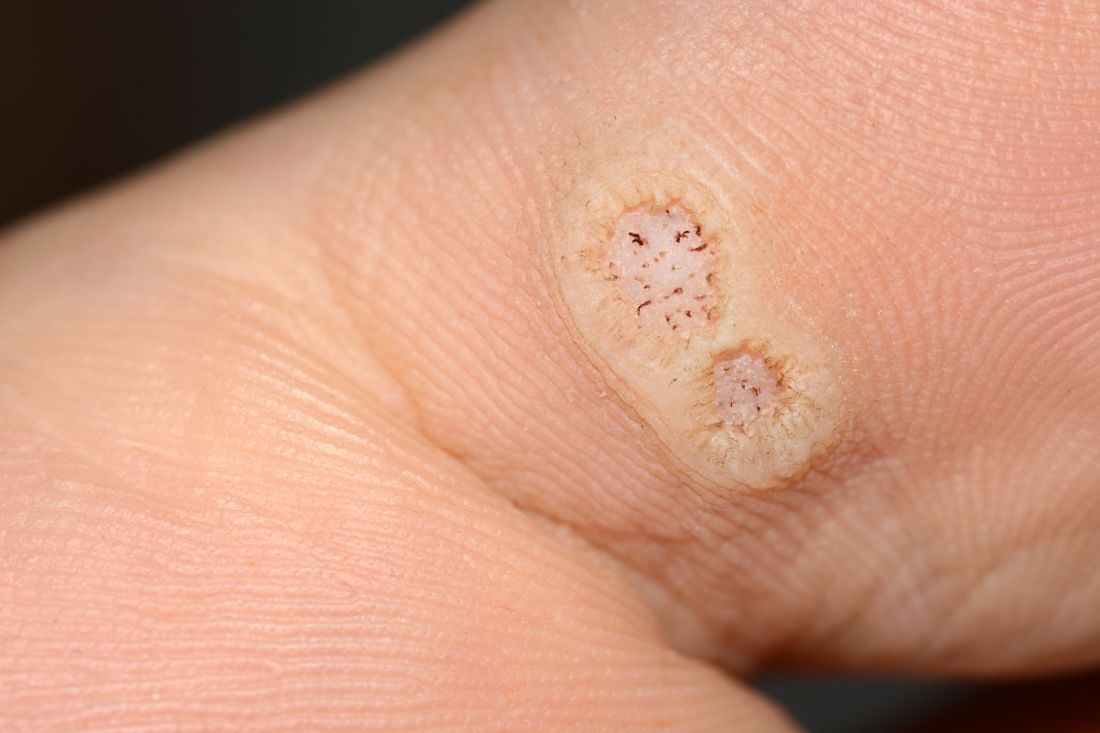
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
FROM PEDIATRIC DERMATOLOGY
Doctors treat osteoporosis with hormone therapy against guidelines
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
This type of hormone therapy (HT) can be given as estrogen or a combination of hormones including estrogen. The physicians interviewed for this piece who prescribe HT for osteoporosis suggest the benefits outweigh the downsides to its use for some of their patients. But such doctors may be a minority group, suggests Michael R. McClung, MD, founding director of the Oregon Osteoporosis Center, Portland.
According to Dr. McClung, HT is now rarely prescribed as treatment – as opposed to prevention – for osteoporosis in the absence of additional benefits such as reducing vasomotor symptoms.
Researchers’ findings on HT use in women with osteoporosis are complex. While HT is approved for menopausal prevention of osteoporosis, it is not indicated as a treatment for the disease by the Food and Drug Administration. See the prescribing information for Premarin tablets, which contain a mixture of estrogen hormones, for an example of the FDA’s indications and usage for the type of HT addressed in this article.
Women’s Health Initiative findings
The Women’s Health Initiative (WHI) hormone therapy trials showed that HT reduces the incidence of all osteoporosis-related fractures in postmenopausal women, even those at low risk of fracture, but osteoporosis-related fractures was not a study endpoint. These trials also revealed that HT was associated with increased risks of cardiovascular and cerebrovascular events, an increased risk of breast cancer, and other adverse health outcomes.
The release of the interim results of the WHI trials in 2002 led to a fair amount of fear and confusion about the use of HT after menopause. After the WHI findings were published, estrogen use dropped dramatically, but for everything, including for vasomotor symptoms and the prevention and treatment of osteoporosis.
Prior to the WHI study, it was very common for hormone therapy to be prescribed as women neared or entered menopause, said Risa Kagan MD, clinical professor of obstetrics, gynecology, and reproductive sciences, University of California, San Francisco.
“When a woman turned 50, that was one of the first things we did – was to put her on hormone therapy. All that changed with the WHI, but now we are coming full circle,” noted Dr. Kagan, who currently prescribes HT as first line treatment for osteoporosis to some women.
Hormone therapy’s complex history
HT’s ability to reduce bone loss in postmenopausal women is well-documented in many papers, including one published March 8, 2018, in Osteoporosis International, by Dr. Kagan and colleagues. This reduced bone loss has been shown to significantly reduce fractures in patients with low bone mass and osteoporosis.
While a growing number of therapies are now available to treat osteoporosis, HT was traditionally viewed as a standard method of preventing fractures in this population. It was also widely used to prevent other types of symptoms associated with the menopause, such as hot flashes, night sweats, and sleep disturbances, and multiple observational studies had demonstrated that its use appeared to reduce the incidence of cardiovascular disease (CVD) in symptomatic menopausal women who initiated HT in early menopause.
Even though the WHI studies were the largest randomized trials ever performed in postmenopausal women, they had notable limitations, according to Dr. Kagan.
“The women were older – the average age was 63 years,” she said. “And they only investigated one route and one dose of estrogen.”
Since then, many different formulations and routes of administration with more favorable safety profiles than what was used in the WHI have become available.
It’s both scientifically and clinically unsound to extrapolate the unfavorable risk-benefit profile of HT seen in the WHI trials to all women regardless of age, HT dosage or formulation, or the length of time they’re on it, she added.
Today’s use of HT in women with osteoporosis
Re-analyses and follow-up studies from the WHI trials, along with data from other studies, have suggested that the benefit-risk profiles of HT are affected by a variety of factors. These include the timing of use in relation to menopause and chronological age and the type of hormone regimen.
“Clinically, many advocate for [hormone therapy] use, especially in the newer younger postmenopausal women to prevent bone loss, but also in younger women who are diagnosed with osteoporosis and then as they get older transition to more bone specific agents,” noted Dr. Kagan.
“Some advocate preserving bone mass and preventing osteoporosis and even treating the younger newly postmenopausal women who have no contraindications with hormone therapy initially, and then gradually transitioning them to a bone specific agent as they get older and at risk for fracture.
“If a woman is already fractured and/or has very low bone density with no other obvious secondary metabolic reason, we also often advocate anabolic agents for 1-2 years then consider estrogen for maintenance – again, if [there is] no contraindication to using HT,” she added.
Thus, an individualized approach is recommended to determine a woman’s risk-benefit ratio of HT use based on the absolute risk of adverse effects, Dr. Kagan noted.
“Transdermal and low/ultra-low doses of HT, have a favorable risk profile, and are effective in preserving bone mineral density and bone quality in many women,” she said.
According to Dr. McClung, HT “is most often used for treatment in women in whom hormone therapy was begun for hot flashes and then, when osteoporosis was found later, was simply continued.
“Society guidelines are cautious about recommending hormone therapy for osteoporosis treatment since estrogen is not approved for treatment, despite the clear fracture protection benefit observed in the WHI study,” he said. “Since [women in the WHI trials] were not recruited as having osteoporosis, those results do not meet the FDA requirement for treatment approval, namely the reduction in fracture risk in patients with osteoporosis. However, knowing what we know about the salutary skeletal effects of estrogen, many of us do use them in our patients with osteoporosis – although not prescribed for that purpose.”
Additional scenarios when doctors may advise HT
“I often recommend – and I think colleagues do as well – that women with recent menopause and menopausal symptoms who also have low bone mineral density or even scores showing osteoporosis see their gynecologist to discuss HT for a few years, perhaps until age 60 if no contraindications, and if it is well tolerated,” said Ethel S. Siris, MD, professor of medicine at Columbia University Medical Center in New York.
“Once they stop it we can then give one of our other bone drugs, but it delays the need to start them since on adequate estrogen the bone density should remain stable while they take it,” added Dr. Siris, an endocrinologist and internist, and director of the Toni Stabile Osteoporosis Center in New York. “They may need a bisphosphonate or another bone drug to further protect them from bone loss and future fracture [after stopping HT].”
Victor L. Roberts, MD, founder of Endocrine Associates of Florida, Lake Mary, pointed out that women now have many options for treatment of osteoporosis.
“If a woman is in early menopause and is having other symptoms, then estrogen is warranted,” he said. “If she has osteoporosis, then it’s a bonus.”
“We have better agents that are bone specific,” for a patient who presents with osteoporosis and no other symptoms, he said.
“If a woman is intolerant of alendronate or other similar drugs, or chooses not to have an injectable, then estrogen or a SERM [selective estrogen receptor modulator] would be an option.”
Dr. Roberts added that HT would be more of a niche drug.
“It has a role and documented benefit and works,” he said. “There is good scientific data for the use of estrogen.”
Dr. Kagan is a consultant for Pfizer, Therapeutics MD, Amgen, on the Medical and Scientific Advisory Board of American Bone Health. The other experts interviewed for this piece reported no conflicts.
NY radiation oncologist loses license, poses ‘potential danger’
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
Twitter storm over ‘reprehensible behavior’ at conference podium
, held in San Francisco and also online. One of the panelists was accused of being less than professional in handling questions from the floor.
It began with a tweet from Sumanta Pal, MD, of the City of Hope, when he called out the behavior, although he did not identify the perpetrator.
“Yesterday we saw some reprehensible behavior emanating from the @ASCO #GU22 podium, with a well known investigator insulting (in a manner deeply imbued w microaggressions) an esteemed colleague at the mic. It’s a teachable moment & the lesson is simple: be kind to ur colleagues.”
It was not immediately clear what had occurred, and several people asked him to identify the people involved. One physician responded, “If it was bad enough to infer and share on Twitter, name the name.”
But even without details, the post provoked a reaction.
Erika Hamilton, MD, wrote, “I am not aware of this incident, but I always wonder ... if someone would do that from the podium, what would they/have they done and said when they didn’t think everyone was watching?”
Another tweet questioned why there were no codes of conduct in place, so that situations like this one wouldn’t happen.
“[It is] about time professional societies had codes of conduct in place for invited speakers, moderators of sessions etc,” wrote Deborah Verran, MD. “Then if it is breached the individual concerned is not invited back. This kind of incident also needs to be mentioned as part of the conference feedback process.”
As the number of clinicians chiming in increased, it soon became apparent that others were aware of the incident, and one posted a video clip, putting the mystery to rest.
Moderator quips, ‘Behave ... children’
The exchange occurred immediately following the oral abstract presentations on prostate cancer on Feb. 17. Two of these abstracts featured new data from large clinical trials investigating the use of PARP inhibitors in metastatic prostate cancer, which had slightly different results, leading to some debate. The PROpel trial with olaparib showed a benefit in all patients, irrespective of gene mutation status, but the MAGNITUDE trial with niraparib showed a benefit only in patients with gene alterations, and especially in those with BRCA1/2 mutations.
The invited discussant, Celestia Higano, MD, from the University of British Columbia, Vancouver, compared and contrasted the two trials and the differing results.
During the question-and-answer session that followed, Neeraj Agarwal, MD, from the Huntsman Cancer Institute, University of Utah, directed a question to Fred Saad, MD, who had presented the results of the PROpel trial.*
“As a practicing oncologist, I am very intrigued by the different results of the MAGNITUDE and PROpel trials. I am trying to figure out what do I do in my practice,” Dr. Agarwal began.
He continued on, explaining that he didn’t think that olaparib and niraparib were that different from one another for the two studies to have such differing results, when both of the drugs were given in combination with abiraterone.
The inclusion criteria for PROpel stipulated that patients undergo testing by ctDNA to determine if they were biomarker positive or not, but he pointed out that it is possible for testing to miss patients who might otherwise be positive for homologous recombination repair (HRR) gene alterations. He posed the question, could some of the patients deemed biomarker negative in fact have been positive but whose status was not detected by ctDNA testing?
At that point, Dr. Higano interrupted him before he had completed his question and asked, “Can I make a comment? Were you listening to my discussion?”
She then continued, pointing out that “you can’t talk about comparing olaparib and niraparib – these two trials had two very different populations.”
She emphasized that this was about the combinations in the populations being studied and not about olaparib and niraparib. “I clearly wasn’t very clear,” she said.
Dr. Agarwal then repeated that he wanted to know what to do with his patients and asked again about the accuracy of ctDNA testing.
“That’s a good question,” Dr. Higano said, “But the other comments you made weren’t.”
At that point, the moderator chimed in. “Let’s all calm down ... children.”
After a brief applause following the moderator’s comment, Dr. Saad then addressed the question.
When the video clip of this exchange was posted, a flood of tweets poured in, supporting Dr. Pal’s initial summation of the situation.
Alison Birtle, MD, tweeted, “I’m even more appalled having seen this exchange. Unacceptable. You don’t humiliate either the speaker or the delegate and the questions were entirely valid in my opinion. Basically what do I do tomorrow with these data so why ask were you listening. That’s just rude.”
Jason Kovac, MD, tweeted: “It’s absolutely not a proper way to behave. Whether it’s behind the scenes or to your face. You can hear the arrogance from everyone including the moderator with his children comment. This is the true face of medicine.”
Jarey Wang, MD, PhD, however, liked the moderator’s input. “Love it. ‘Let’s all calm down children.’ Good for the moderator.”
Several of the physicians who commented thought that the issue should have been addressed at the time it happened. Don S. Dizon, MD, wrote, “Calling out microagressions as they occur is so important. And very difficult. Agree, the teachable moment must be met with bravery. But it should happen in real time too.”
After 2 days of bantering on Twitter, with the thread growing increasingly longer and with the vast majority of posts supporting Dr. Pal’s initial assessment, Dr. Higano finally entered the discussion and defended her comments: “What you do not know is that the questioner and I are good colleagues,” she tweeted. “I have been involved in two of his academic promotions. My main concern was his comment re: how the two trials could have ‘so different results with the same combination.’ I sought to rectify wrong message.”
There were no direct replies to Dr. Higano’s tweet.
Perhaps the line to draw under this affair is the tweet from Simon Kim, MD, MPH, who wrote: “We can do better!”
A version of this article first appeared on Medscape.com.
Correction, 2/24/22: Dr. Fred Saad's name was misstated in an earlier version of this article.
, held in San Francisco and also online. One of the panelists was accused of being less than professional in handling questions from the floor.
It began with a tweet from Sumanta Pal, MD, of the City of Hope, when he called out the behavior, although he did not identify the perpetrator.
“Yesterday we saw some reprehensible behavior emanating from the @ASCO #GU22 podium, with a well known investigator insulting (in a manner deeply imbued w microaggressions) an esteemed colleague at the mic. It’s a teachable moment & the lesson is simple: be kind to ur colleagues.”
It was not immediately clear what had occurred, and several people asked him to identify the people involved. One physician responded, “If it was bad enough to infer and share on Twitter, name the name.”
But even without details, the post provoked a reaction.
Erika Hamilton, MD, wrote, “I am not aware of this incident, but I always wonder ... if someone would do that from the podium, what would they/have they done and said when they didn’t think everyone was watching?”
Another tweet questioned why there were no codes of conduct in place, so that situations like this one wouldn’t happen.
“[It is] about time professional societies had codes of conduct in place for invited speakers, moderators of sessions etc,” wrote Deborah Verran, MD. “Then if it is breached the individual concerned is not invited back. This kind of incident also needs to be mentioned as part of the conference feedback process.”
As the number of clinicians chiming in increased, it soon became apparent that others were aware of the incident, and one posted a video clip, putting the mystery to rest.
Moderator quips, ‘Behave ... children’
The exchange occurred immediately following the oral abstract presentations on prostate cancer on Feb. 17. Two of these abstracts featured new data from large clinical trials investigating the use of PARP inhibitors in metastatic prostate cancer, which had slightly different results, leading to some debate. The PROpel trial with olaparib showed a benefit in all patients, irrespective of gene mutation status, but the MAGNITUDE trial with niraparib showed a benefit only in patients with gene alterations, and especially in those with BRCA1/2 mutations.
The invited discussant, Celestia Higano, MD, from the University of British Columbia, Vancouver, compared and contrasted the two trials and the differing results.
During the question-and-answer session that followed, Neeraj Agarwal, MD, from the Huntsman Cancer Institute, University of Utah, directed a question to Fred Saad, MD, who had presented the results of the PROpel trial.*
“As a practicing oncologist, I am very intrigued by the different results of the MAGNITUDE and PROpel trials. I am trying to figure out what do I do in my practice,” Dr. Agarwal began.
He continued on, explaining that he didn’t think that olaparib and niraparib were that different from one another for the two studies to have such differing results, when both of the drugs were given in combination with abiraterone.
The inclusion criteria for PROpel stipulated that patients undergo testing by ctDNA to determine if they were biomarker positive or not, but he pointed out that it is possible for testing to miss patients who might otherwise be positive for homologous recombination repair (HRR) gene alterations. He posed the question, could some of the patients deemed biomarker negative in fact have been positive but whose status was not detected by ctDNA testing?
At that point, Dr. Higano interrupted him before he had completed his question and asked, “Can I make a comment? Were you listening to my discussion?”
She then continued, pointing out that “you can’t talk about comparing olaparib and niraparib – these two trials had two very different populations.”
She emphasized that this was about the combinations in the populations being studied and not about olaparib and niraparib. “I clearly wasn’t very clear,” she said.
Dr. Agarwal then repeated that he wanted to know what to do with his patients and asked again about the accuracy of ctDNA testing.
“That’s a good question,” Dr. Higano said, “But the other comments you made weren’t.”
At that point, the moderator chimed in. “Let’s all calm down ... children.”
After a brief applause following the moderator’s comment, Dr. Saad then addressed the question.
When the video clip of this exchange was posted, a flood of tweets poured in, supporting Dr. Pal’s initial summation of the situation.
Alison Birtle, MD, tweeted, “I’m even more appalled having seen this exchange. Unacceptable. You don’t humiliate either the speaker or the delegate and the questions were entirely valid in my opinion. Basically what do I do tomorrow with these data so why ask were you listening. That’s just rude.”
Jason Kovac, MD, tweeted: “It’s absolutely not a proper way to behave. Whether it’s behind the scenes or to your face. You can hear the arrogance from everyone including the moderator with his children comment. This is the true face of medicine.”
Jarey Wang, MD, PhD, however, liked the moderator’s input. “Love it. ‘Let’s all calm down children.’ Good for the moderator.”
Several of the physicians who commented thought that the issue should have been addressed at the time it happened. Don S. Dizon, MD, wrote, “Calling out microagressions as they occur is so important. And very difficult. Agree, the teachable moment must be met with bravery. But it should happen in real time too.”
After 2 days of bantering on Twitter, with the thread growing increasingly longer and with the vast majority of posts supporting Dr. Pal’s initial assessment, Dr. Higano finally entered the discussion and defended her comments: “What you do not know is that the questioner and I are good colleagues,” she tweeted. “I have been involved in two of his academic promotions. My main concern was his comment re: how the two trials could have ‘so different results with the same combination.’ I sought to rectify wrong message.”
There were no direct replies to Dr. Higano’s tweet.
Perhaps the line to draw under this affair is the tweet from Simon Kim, MD, MPH, who wrote: “We can do better!”
A version of this article first appeared on Medscape.com.
Correction, 2/24/22: Dr. Fred Saad's name was misstated in an earlier version of this article.
, held in San Francisco and also online. One of the panelists was accused of being less than professional in handling questions from the floor.
It began with a tweet from Sumanta Pal, MD, of the City of Hope, when he called out the behavior, although he did not identify the perpetrator.
“Yesterday we saw some reprehensible behavior emanating from the @ASCO #GU22 podium, with a well known investigator insulting (in a manner deeply imbued w microaggressions) an esteemed colleague at the mic. It’s a teachable moment & the lesson is simple: be kind to ur colleagues.”
It was not immediately clear what had occurred, and several people asked him to identify the people involved. One physician responded, “If it was bad enough to infer and share on Twitter, name the name.”
But even without details, the post provoked a reaction.
Erika Hamilton, MD, wrote, “I am not aware of this incident, but I always wonder ... if someone would do that from the podium, what would they/have they done and said when they didn’t think everyone was watching?”
Another tweet questioned why there were no codes of conduct in place, so that situations like this one wouldn’t happen.
“[It is] about time professional societies had codes of conduct in place for invited speakers, moderators of sessions etc,” wrote Deborah Verran, MD. “Then if it is breached the individual concerned is not invited back. This kind of incident also needs to be mentioned as part of the conference feedback process.”
As the number of clinicians chiming in increased, it soon became apparent that others were aware of the incident, and one posted a video clip, putting the mystery to rest.
Moderator quips, ‘Behave ... children’
The exchange occurred immediately following the oral abstract presentations on prostate cancer on Feb. 17. Two of these abstracts featured new data from large clinical trials investigating the use of PARP inhibitors in metastatic prostate cancer, which had slightly different results, leading to some debate. The PROpel trial with olaparib showed a benefit in all patients, irrespective of gene mutation status, but the MAGNITUDE trial with niraparib showed a benefit only in patients with gene alterations, and especially in those with BRCA1/2 mutations.
The invited discussant, Celestia Higano, MD, from the University of British Columbia, Vancouver, compared and contrasted the two trials and the differing results.
During the question-and-answer session that followed, Neeraj Agarwal, MD, from the Huntsman Cancer Institute, University of Utah, directed a question to Fred Saad, MD, who had presented the results of the PROpel trial.*
“As a practicing oncologist, I am very intrigued by the different results of the MAGNITUDE and PROpel trials. I am trying to figure out what do I do in my practice,” Dr. Agarwal began.
He continued on, explaining that he didn’t think that olaparib and niraparib were that different from one another for the two studies to have such differing results, when both of the drugs were given in combination with abiraterone.
The inclusion criteria for PROpel stipulated that patients undergo testing by ctDNA to determine if they were biomarker positive or not, but he pointed out that it is possible for testing to miss patients who might otherwise be positive for homologous recombination repair (HRR) gene alterations. He posed the question, could some of the patients deemed biomarker negative in fact have been positive but whose status was not detected by ctDNA testing?
At that point, Dr. Higano interrupted him before he had completed his question and asked, “Can I make a comment? Were you listening to my discussion?”
She then continued, pointing out that “you can’t talk about comparing olaparib and niraparib – these two trials had two very different populations.”
She emphasized that this was about the combinations in the populations being studied and not about olaparib and niraparib. “I clearly wasn’t very clear,” she said.
Dr. Agarwal then repeated that he wanted to know what to do with his patients and asked again about the accuracy of ctDNA testing.
“That’s a good question,” Dr. Higano said, “But the other comments you made weren’t.”
At that point, the moderator chimed in. “Let’s all calm down ... children.”
After a brief applause following the moderator’s comment, Dr. Saad then addressed the question.
When the video clip of this exchange was posted, a flood of tweets poured in, supporting Dr. Pal’s initial summation of the situation.
Alison Birtle, MD, tweeted, “I’m even more appalled having seen this exchange. Unacceptable. You don’t humiliate either the speaker or the delegate and the questions were entirely valid in my opinion. Basically what do I do tomorrow with these data so why ask were you listening. That’s just rude.”
Jason Kovac, MD, tweeted: “It’s absolutely not a proper way to behave. Whether it’s behind the scenes or to your face. You can hear the arrogance from everyone including the moderator with his children comment. This is the true face of medicine.”
Jarey Wang, MD, PhD, however, liked the moderator’s input. “Love it. ‘Let’s all calm down children.’ Good for the moderator.”
Several of the physicians who commented thought that the issue should have been addressed at the time it happened. Don S. Dizon, MD, wrote, “Calling out microagressions as they occur is so important. And very difficult. Agree, the teachable moment must be met with bravery. But it should happen in real time too.”
After 2 days of bantering on Twitter, with the thread growing increasingly longer and with the vast majority of posts supporting Dr. Pal’s initial assessment, Dr. Higano finally entered the discussion and defended her comments: “What you do not know is that the questioner and I are good colleagues,” she tweeted. “I have been involved in two of his academic promotions. My main concern was his comment re: how the two trials could have ‘so different results with the same combination.’ I sought to rectify wrong message.”
There were no direct replies to Dr. Higano’s tweet.
Perhaps the line to draw under this affair is the tweet from Simon Kim, MD, MPH, who wrote: “We can do better!”
A version of this article first appeared on Medscape.com.
Correction, 2/24/22: Dr. Fred Saad's name was misstated in an earlier version of this article.
FROM ASCO GU 2022
City of Hope completes acquisition of CTCA
The combined group now has 575 physicians and more than 11,000 employees and is expected to care for approximately 115,000 patients each year.
City of Hope, a National Cancer Institute–designated comprehensive cancer center, is located near Los Angeles. It currently comprises its main campus and a network of clinical care locations across Southern California. A new campus is scheduled to open this year in Irvine, California, about 50 miles south of the main center.
The acquisition of CTCA expands its reach into three new states – Arizona, Illinois, and Georgia – with an additional 41 clinical network locations.
Commenting on the completion of the deal, Robert Stone, president and CEO of City of Hope, said in a statement: “With the completion of this acquisition, City of Hope and Cancer Treatment Centers of America are combining complementary strengths. ... Together, we are creating a new model for how cancer care is delivered, leveraging real-world cancer care experience to inform scientific innovation and making tomorrow’s new discoveries available to the people who need them today.”
City of Hope announced in December 2020 that it would acquire CTCA for $390 million, as previously reported by this news organization.
At the time, Pat Basu MD, MBA, president and CEO of CTCA, said that they were excited about the deal. “Through the shared, patient-centric values of both organizations and expanded access as a result of the collaboration, cancer patients across the nation will be the ultimate beneficiaries of this relationship,” he said. Dr. Basu will remain CEO of CTCA and report to Robert Stone.
Controversies and closures
CTCA is a national oncology network of hospitals and outpatient care centers that offers an integrated approach to care, including surgery, radiotherapy, chemotherapy, immunotherapy, and advancements in precision medicine with supportive therapies to manage side effects and enhance quality of life during treatment and into survivorship.
However, it appears to have run into financial problems. During 2021, CTCA closed a center in Tulsa, Oklahoma, and sold off assets from a Philadelphia-based hospital.
In addition, for the past 10 years, CTCA had been involved in a series of controversies. These include a 2013 investigation into alleged questionable practices designed to boost its mortality statistics, as well as an analysis of cancer center advertising practices that showed that CTCA spent $101.7 million on advertising in 2014. More recently, a 2019 report showed that CTCA’s high advertising expenditures did not correlate with better patient outcomes in comparison with other centers.
Now that it has been acquired, CTCA will transition from a private for-profit company to a nonprofit organization, according to City of Hope.
A version of this article first appeared on Medscape.com.
The combined group now has 575 physicians and more than 11,000 employees and is expected to care for approximately 115,000 patients each year.
City of Hope, a National Cancer Institute–designated comprehensive cancer center, is located near Los Angeles. It currently comprises its main campus and a network of clinical care locations across Southern California. A new campus is scheduled to open this year in Irvine, California, about 50 miles south of the main center.
The acquisition of CTCA expands its reach into three new states – Arizona, Illinois, and Georgia – with an additional 41 clinical network locations.
Commenting on the completion of the deal, Robert Stone, president and CEO of City of Hope, said in a statement: “With the completion of this acquisition, City of Hope and Cancer Treatment Centers of America are combining complementary strengths. ... Together, we are creating a new model for how cancer care is delivered, leveraging real-world cancer care experience to inform scientific innovation and making tomorrow’s new discoveries available to the people who need them today.”
City of Hope announced in December 2020 that it would acquire CTCA for $390 million, as previously reported by this news organization.
At the time, Pat Basu MD, MBA, president and CEO of CTCA, said that they were excited about the deal. “Through the shared, patient-centric values of both organizations and expanded access as a result of the collaboration, cancer patients across the nation will be the ultimate beneficiaries of this relationship,” he said. Dr. Basu will remain CEO of CTCA and report to Robert Stone.
Controversies and closures
CTCA is a national oncology network of hospitals and outpatient care centers that offers an integrated approach to care, including surgery, radiotherapy, chemotherapy, immunotherapy, and advancements in precision medicine with supportive therapies to manage side effects and enhance quality of life during treatment and into survivorship.
However, it appears to have run into financial problems. During 2021, CTCA closed a center in Tulsa, Oklahoma, and sold off assets from a Philadelphia-based hospital.
In addition, for the past 10 years, CTCA had been involved in a series of controversies. These include a 2013 investigation into alleged questionable practices designed to boost its mortality statistics, as well as an analysis of cancer center advertising practices that showed that CTCA spent $101.7 million on advertising in 2014. More recently, a 2019 report showed that CTCA’s high advertising expenditures did not correlate with better patient outcomes in comparison with other centers.
Now that it has been acquired, CTCA will transition from a private for-profit company to a nonprofit organization, according to City of Hope.
A version of this article first appeared on Medscape.com.
The combined group now has 575 physicians and more than 11,000 employees and is expected to care for approximately 115,000 patients each year.
City of Hope, a National Cancer Institute–designated comprehensive cancer center, is located near Los Angeles. It currently comprises its main campus and a network of clinical care locations across Southern California. A new campus is scheduled to open this year in Irvine, California, about 50 miles south of the main center.
The acquisition of CTCA expands its reach into three new states – Arizona, Illinois, and Georgia – with an additional 41 clinical network locations.
Commenting on the completion of the deal, Robert Stone, president and CEO of City of Hope, said in a statement: “With the completion of this acquisition, City of Hope and Cancer Treatment Centers of America are combining complementary strengths. ... Together, we are creating a new model for how cancer care is delivered, leveraging real-world cancer care experience to inform scientific innovation and making tomorrow’s new discoveries available to the people who need them today.”
City of Hope announced in December 2020 that it would acquire CTCA for $390 million, as previously reported by this news organization.
At the time, Pat Basu MD, MBA, president and CEO of CTCA, said that they were excited about the deal. “Through the shared, patient-centric values of both organizations and expanded access as a result of the collaboration, cancer patients across the nation will be the ultimate beneficiaries of this relationship,” he said. Dr. Basu will remain CEO of CTCA and report to Robert Stone.
Controversies and closures
CTCA is a national oncology network of hospitals and outpatient care centers that offers an integrated approach to care, including surgery, radiotherapy, chemotherapy, immunotherapy, and advancements in precision medicine with supportive therapies to manage side effects and enhance quality of life during treatment and into survivorship.
However, it appears to have run into financial problems. During 2021, CTCA closed a center in Tulsa, Oklahoma, and sold off assets from a Philadelphia-based hospital.
In addition, for the past 10 years, CTCA had been involved in a series of controversies. These include a 2013 investigation into alleged questionable practices designed to boost its mortality statistics, as well as an analysis of cancer center advertising practices that showed that CTCA spent $101.7 million on advertising in 2014. More recently, a 2019 report showed that CTCA’s high advertising expenditures did not correlate with better patient outcomes in comparison with other centers.
Now that it has been acquired, CTCA will transition from a private for-profit company to a nonprofit organization, according to City of Hope.
A version of this article first appeared on Medscape.com.
President Biden’s ‘Cancer Moonshot’ to be relaunched
The “Cancer Moonshot” is about to be relaunched.
In a White House briefing, President Joe Biden announced that he is “reigniting” the initiative he spearheaded when he was vice president during the Obama administration.
During the livestreamed event, the president discussed his plans to bring a “fierce sense of urgency” to the fight against cancer and better support patients with cancer and their families.
He emphasized that cancer is one of the truly bipartisan issues. There is strong support from both “sides of the aisle,” he said, and he sees it as an issue that can bring the country together.
“We can do this. I promise you, we can do this. For all those we lost, for all those we miss. We can end cancer as we know it,” he said. “This is a presidential White House priority.”
The aim is to reduce the death rate from cancer by at least 50% over the next 25 years.
There is also a proposal to create the Advanced Research Projects Agency for Health, which would focus on driving cutting-edge innovation in health research.
Part of the plan is to assemble a “cancer cabinet” that includes 18 federal departments, agencies, and offices, including leaders from the departments of Health & Human Services, Veterans Affairs, Defense, Energy, and Agriculture.
At present, there are few details about the new program or how it will be funded.
Presumably more will be revealed at the Cancer Moonshot Summit being planned, as well as on a planned new website where people can track its progress.
President priority
Cancer Moonshot began back in 2016, when during his last State of the Union Address, former President Barack Obama announced the ambitious initiative. A few days later, Obama asked Congress for $1 billion to send cancer to the moon, and he put Biden, then vice president, in charge of “mission control” in the remaining months of the administration.
The new initiative will be headed by Danielle Carnival, PhD, who serves in the White House Office of Science and Technology Policy and has been appointed as White House Cancer Moonshot coordinator.
At the briefing, Mr. Biden and Vice President Kamala Harris spoke about losing family members to cancer. The president spoke about his eldest son, Beau, who died from brain cancer when he was 46 years old, while Ms. Harris spoke about her mother, Shyamala Gopalan, a breast cancer researcher who died of colon cancer in 2009.
Accolades but a bit of caution
The president’s speech was applauded by many cancer groups, both professional organizations and patient advocacy groups.
Karen E. Knudsen, PhD, chief executive officer of the American Cancer Society and its advocacy affiliate, the American Cancer Society Cancer Action Network, commended Mr. Biden for reigniting Cancer Moonshot.
“In 2022 alone, there will be an estimated 1.9 million people diagnosed with cancer and more than 600,000 people in the U.S. will die. Marshaling the resources of the federal government will be critical in our ability to reduce death and suffering from this disease,” she said.
The American Society for Radiation Oncology issued a press release, saying: “On behalf of radiation oncologists who treat people with cancer every day, we support the Biden-Harris administration’s move to drastically reduce the number of cancer deaths in the United States and improve the lives of people diagnosed with this disease.
“We believe the administration’s commitment to expand cancer prevention efforts and to increase equitable access to screenings and treatments will help mitigate some of the negative impact of the COVID-19 pandemic,” the society added.
At the American Association for Cancer Research, Chief Executive Officer Margaret Foti, MD, PhD, said she was thrilled to hear the announcement after the devastating interruptions in cancer research and patient care over the past 2 years.
“The reignited Cancer Moonshot will provide an important framework to help improve cancer prevention strategies, increase cancer screenings and early detection, reduce cancer disparities, and propel new lifesaving cures for patients with cancer,” she said.
However, increased funding from Congress will be needed for these goals to be achieved, she emphasized.
A version of this article first appeared on Medscape.com.
The “Cancer Moonshot” is about to be relaunched.
In a White House briefing, President Joe Biden announced that he is “reigniting” the initiative he spearheaded when he was vice president during the Obama administration.
During the livestreamed event, the president discussed his plans to bring a “fierce sense of urgency” to the fight against cancer and better support patients with cancer and their families.
He emphasized that cancer is one of the truly bipartisan issues. There is strong support from both “sides of the aisle,” he said, and he sees it as an issue that can bring the country together.
“We can do this. I promise you, we can do this. For all those we lost, for all those we miss. We can end cancer as we know it,” he said. “This is a presidential White House priority.”
The aim is to reduce the death rate from cancer by at least 50% over the next 25 years.
There is also a proposal to create the Advanced Research Projects Agency for Health, which would focus on driving cutting-edge innovation in health research.
Part of the plan is to assemble a “cancer cabinet” that includes 18 federal departments, agencies, and offices, including leaders from the departments of Health & Human Services, Veterans Affairs, Defense, Energy, and Agriculture.
At present, there are few details about the new program or how it will be funded.
Presumably more will be revealed at the Cancer Moonshot Summit being planned, as well as on a planned new website where people can track its progress.
President priority
Cancer Moonshot began back in 2016, when during his last State of the Union Address, former President Barack Obama announced the ambitious initiative. A few days later, Obama asked Congress for $1 billion to send cancer to the moon, and he put Biden, then vice president, in charge of “mission control” in the remaining months of the administration.
The new initiative will be headed by Danielle Carnival, PhD, who serves in the White House Office of Science and Technology Policy and has been appointed as White House Cancer Moonshot coordinator.
At the briefing, Mr. Biden and Vice President Kamala Harris spoke about losing family members to cancer. The president spoke about his eldest son, Beau, who died from brain cancer when he was 46 years old, while Ms. Harris spoke about her mother, Shyamala Gopalan, a breast cancer researcher who died of colon cancer in 2009.
Accolades but a bit of caution
The president’s speech was applauded by many cancer groups, both professional organizations and patient advocacy groups.
Karen E. Knudsen, PhD, chief executive officer of the American Cancer Society and its advocacy affiliate, the American Cancer Society Cancer Action Network, commended Mr. Biden for reigniting Cancer Moonshot.
“In 2022 alone, there will be an estimated 1.9 million people diagnosed with cancer and more than 600,000 people in the U.S. will die. Marshaling the resources of the federal government will be critical in our ability to reduce death and suffering from this disease,” she said.
The American Society for Radiation Oncology issued a press release, saying: “On behalf of radiation oncologists who treat people with cancer every day, we support the Biden-Harris administration’s move to drastically reduce the number of cancer deaths in the United States and improve the lives of people diagnosed with this disease.
“We believe the administration’s commitment to expand cancer prevention efforts and to increase equitable access to screenings and treatments will help mitigate some of the negative impact of the COVID-19 pandemic,” the society added.
At the American Association for Cancer Research, Chief Executive Officer Margaret Foti, MD, PhD, said she was thrilled to hear the announcement after the devastating interruptions in cancer research and patient care over the past 2 years.
“The reignited Cancer Moonshot will provide an important framework to help improve cancer prevention strategies, increase cancer screenings and early detection, reduce cancer disparities, and propel new lifesaving cures for patients with cancer,” she said.
However, increased funding from Congress will be needed for these goals to be achieved, she emphasized.
A version of this article first appeared on Medscape.com.
The “Cancer Moonshot” is about to be relaunched.
In a White House briefing, President Joe Biden announced that he is “reigniting” the initiative he spearheaded when he was vice president during the Obama administration.
During the livestreamed event, the president discussed his plans to bring a “fierce sense of urgency” to the fight against cancer and better support patients with cancer and their families.
He emphasized that cancer is one of the truly bipartisan issues. There is strong support from both “sides of the aisle,” he said, and he sees it as an issue that can bring the country together.
“We can do this. I promise you, we can do this. For all those we lost, for all those we miss. We can end cancer as we know it,” he said. “This is a presidential White House priority.”
The aim is to reduce the death rate from cancer by at least 50% over the next 25 years.
There is also a proposal to create the Advanced Research Projects Agency for Health, which would focus on driving cutting-edge innovation in health research.
Part of the plan is to assemble a “cancer cabinet” that includes 18 federal departments, agencies, and offices, including leaders from the departments of Health & Human Services, Veterans Affairs, Defense, Energy, and Agriculture.
At present, there are few details about the new program or how it will be funded.
Presumably more will be revealed at the Cancer Moonshot Summit being planned, as well as on a planned new website where people can track its progress.
President priority
Cancer Moonshot began back in 2016, when during his last State of the Union Address, former President Barack Obama announced the ambitious initiative. A few days later, Obama asked Congress for $1 billion to send cancer to the moon, and he put Biden, then vice president, in charge of “mission control” in the remaining months of the administration.
The new initiative will be headed by Danielle Carnival, PhD, who serves in the White House Office of Science and Technology Policy and has been appointed as White House Cancer Moonshot coordinator.
At the briefing, Mr. Biden and Vice President Kamala Harris spoke about losing family members to cancer. The president spoke about his eldest son, Beau, who died from brain cancer when he was 46 years old, while Ms. Harris spoke about her mother, Shyamala Gopalan, a breast cancer researcher who died of colon cancer in 2009.
Accolades but a bit of caution
The president’s speech was applauded by many cancer groups, both professional organizations and patient advocacy groups.
Karen E. Knudsen, PhD, chief executive officer of the American Cancer Society and its advocacy affiliate, the American Cancer Society Cancer Action Network, commended Mr. Biden for reigniting Cancer Moonshot.
“In 2022 alone, there will be an estimated 1.9 million people diagnosed with cancer and more than 600,000 people in the U.S. will die. Marshaling the resources of the federal government will be critical in our ability to reduce death and suffering from this disease,” she said.
The American Society for Radiation Oncology issued a press release, saying: “On behalf of radiation oncologists who treat people with cancer every day, we support the Biden-Harris administration’s move to drastically reduce the number of cancer deaths in the United States and improve the lives of people diagnosed with this disease.
“We believe the administration’s commitment to expand cancer prevention efforts and to increase equitable access to screenings and treatments will help mitigate some of the negative impact of the COVID-19 pandemic,” the society added.
At the American Association for Cancer Research, Chief Executive Officer Margaret Foti, MD, PhD, said she was thrilled to hear the announcement after the devastating interruptions in cancer research and patient care over the past 2 years.
“The reignited Cancer Moonshot will provide an important framework to help improve cancer prevention strategies, increase cancer screenings and early detection, reduce cancer disparities, and propel new lifesaving cures for patients with cancer,” she said.
However, increased funding from Congress will be needed for these goals to be achieved, she emphasized.
A version of this article first appeared on Medscape.com.
Medicaid expansion curbs disparities, increases immigrant access, in postpartum care
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
FROM JAMA HEALTH FORUM AND JAMA NETWORK OPEN
Chicago oncologist charged with insider trading
, according to a Dec. 20 press release issued by the U.S. Department of Justice.
Daniel V.T. Catenacci, MD, PhD, a gastrointestinal medical oncologist and associate professor of medicine at the University of Chicago, is alleged to have used confidential information to purchase shares of California-based biotechnology company Five Prime Therapeutics before it publicly announced positive results from a clinical trial of bemarituzumab, an experimental cancer drug.
Dr. Catenacci served as the lead investigator of the clinical trial that evaluated bemarituzumab. The drug, which earned breakthrough therapy designation from the Food and Drug Administration earlier this year, is designed to target fibroblast growth factor receptor 2b (FGFR2b), overexpressed in about 30% of patients with HER2-negative gastric cancer and other solid tumors.
Bemarituzumab is being positioned as a potential frontline therapy for advanced gastric or gastroesophageal junction cancer. A recent phase 2 trial found that adding bemarituzumab to chemotherapy in this patient population improved survival over chemotherapy alone.
According to the criminal information, filed on Dec. 17 in U.S. District Court in Chicago, the charges state that, in November 2020, Dr. Catenacci “used material, non-public information about the trial results to make more than $134,000 in illegal profits from the purchase and sale of securities in the company.”
More specifically, the SEC’s complaint alleges that Dr. Catenacci received confidential information about the company and its positive clinical trial results through his position as principal investigator. Dr. Catenacci then purchased almost 8,800 shares of Five Prime Therapeutics before the company announced the positive results. Dr. Catenacci subsequently sold those shares shortly after the trial results were announced. In the interim, the shares tripled or quadrupled in value.
He has been charged with one count of securities fraud, punishable by up to 20 years in federal prison. Arraignment in federal court in Chicago has yet to be scheduled.
In addition, the federal complaint alleges that Dr. Catenacci violated the antifraud provisions of the federal securities laws. According to a press release, “Catenacci has agreed to be permanently enjoined from violations of these provisions, and to pay a civil penalty in an amount to be determined by the court later.”
Erin E. Schneider, regional director of the SEC’s San Francisco Regional Office, stated in the press release that clinical drug trials typically involve sensitive and valuable information about the viability of an experimental drug.
“As alleged in our complaint, Catenacci was required to safeguard the material nonpublic information he learned about Five Prime’s clinical trial, and not trade on it,” said Mr. Schneider.
A version of this article first appeared on Medscape.com.
, according to a Dec. 20 press release issued by the U.S. Department of Justice.
Daniel V.T. Catenacci, MD, PhD, a gastrointestinal medical oncologist and associate professor of medicine at the University of Chicago, is alleged to have used confidential information to purchase shares of California-based biotechnology company Five Prime Therapeutics before it publicly announced positive results from a clinical trial of bemarituzumab, an experimental cancer drug.
Dr. Catenacci served as the lead investigator of the clinical trial that evaluated bemarituzumab. The drug, which earned breakthrough therapy designation from the Food and Drug Administration earlier this year, is designed to target fibroblast growth factor receptor 2b (FGFR2b), overexpressed in about 30% of patients with HER2-negative gastric cancer and other solid tumors.
Bemarituzumab is being positioned as a potential frontline therapy for advanced gastric or gastroesophageal junction cancer. A recent phase 2 trial found that adding bemarituzumab to chemotherapy in this patient population improved survival over chemotherapy alone.
According to the criminal information, filed on Dec. 17 in U.S. District Court in Chicago, the charges state that, in November 2020, Dr. Catenacci “used material, non-public information about the trial results to make more than $134,000 in illegal profits from the purchase and sale of securities in the company.”
More specifically, the SEC’s complaint alleges that Dr. Catenacci received confidential information about the company and its positive clinical trial results through his position as principal investigator. Dr. Catenacci then purchased almost 8,800 shares of Five Prime Therapeutics before the company announced the positive results. Dr. Catenacci subsequently sold those shares shortly after the trial results were announced. In the interim, the shares tripled or quadrupled in value.
He has been charged with one count of securities fraud, punishable by up to 20 years in federal prison. Arraignment in federal court in Chicago has yet to be scheduled.
In addition, the federal complaint alleges that Dr. Catenacci violated the antifraud provisions of the federal securities laws. According to a press release, “Catenacci has agreed to be permanently enjoined from violations of these provisions, and to pay a civil penalty in an amount to be determined by the court later.”
Erin E. Schneider, regional director of the SEC’s San Francisco Regional Office, stated in the press release that clinical drug trials typically involve sensitive and valuable information about the viability of an experimental drug.
“As alleged in our complaint, Catenacci was required to safeguard the material nonpublic information he learned about Five Prime’s clinical trial, and not trade on it,” said Mr. Schneider.
A version of this article first appeared on Medscape.com.
, according to a Dec. 20 press release issued by the U.S. Department of Justice.
Daniel V.T. Catenacci, MD, PhD, a gastrointestinal medical oncologist and associate professor of medicine at the University of Chicago, is alleged to have used confidential information to purchase shares of California-based biotechnology company Five Prime Therapeutics before it publicly announced positive results from a clinical trial of bemarituzumab, an experimental cancer drug.
Dr. Catenacci served as the lead investigator of the clinical trial that evaluated bemarituzumab. The drug, which earned breakthrough therapy designation from the Food and Drug Administration earlier this year, is designed to target fibroblast growth factor receptor 2b (FGFR2b), overexpressed in about 30% of patients with HER2-negative gastric cancer and other solid tumors.
Bemarituzumab is being positioned as a potential frontline therapy for advanced gastric or gastroesophageal junction cancer. A recent phase 2 trial found that adding bemarituzumab to chemotherapy in this patient population improved survival over chemotherapy alone.
According to the criminal information, filed on Dec. 17 in U.S. District Court in Chicago, the charges state that, in November 2020, Dr. Catenacci “used material, non-public information about the trial results to make more than $134,000 in illegal profits from the purchase and sale of securities in the company.”
More specifically, the SEC’s complaint alleges that Dr. Catenacci received confidential information about the company and its positive clinical trial results through his position as principal investigator. Dr. Catenacci then purchased almost 8,800 shares of Five Prime Therapeutics before the company announced the positive results. Dr. Catenacci subsequently sold those shares shortly after the trial results were announced. In the interim, the shares tripled or quadrupled in value.
He has been charged with one count of securities fraud, punishable by up to 20 years in federal prison. Arraignment in federal court in Chicago has yet to be scheduled.
In addition, the federal complaint alleges that Dr. Catenacci violated the antifraud provisions of the federal securities laws. According to a press release, “Catenacci has agreed to be permanently enjoined from violations of these provisions, and to pay a civil penalty in an amount to be determined by the court later.”
Erin E. Schneider, regional director of the SEC’s San Francisco Regional Office, stated in the press release that clinical drug trials typically involve sensitive and valuable information about the viability of an experimental drug.
“As alleged in our complaint, Catenacci was required to safeguard the material nonpublic information he learned about Five Prime’s clinical trial, and not trade on it,” said Mr. Schneider.
A version of this article first appeared on Medscape.com.
Racial, other disparities in blood cancer treatment
As compared with White individuals, minorities often face higher barriers to cancer care. Racial and ethnic disparities in patients with solid tumors, particularly those of the prostate and breast, have been well documented. Hematologic malignancies are less common, but an increasing number of studies have documented disparities within this subgroup of cancer, particularly among Black and non-White Hispanics. An increasing armamentarium of therapeutics, including novel chemotherapy agents and targeted molecular, cellular, and immunologic therapies, has highlighted the need for understanding and exploring the differences in care as well as biology, which may lead to disparate outcomes.
Overall, an estimated 186,400 people living in the United States are expected to be diagnosed with leukemia, lymphoma, or myeloma in 2021, and new cases of hematologic malignancies are expected to account for 9.8% of the estimated 1,898,160 new cancer cases diagnosed this year.1
The underlying reasons for disparities are highly complex and multifactorial, and clinicians must consider how the biologic, clinical, demographic, and socioeconomic characteristics of their patients interact. All of these factors can play a role in prognosis and/or access to care.
Disparities in leukemia
Leukemia is a heterogeneous group of diseases affecting both children and adults, but during the past few decades survival rates have steadily improved, particularly among children. Response to therapy and prognosis do vary among leukemia types, but one large analysis reported that there were overall improvements in survival seen across racial/ethnic groups, most age groups, and genders during a 40-year period.2
From 1973 through 2014, survival trends were assessed across four leukemia types: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and chronic lymphoid leukemia (CLL). After stratifying survival for each leukemia type by race/ethnicity, improvement rates were not uniform among all groups.
For example, there were substantial improvements of leukemia-specific survival in 2010-2014 among Black (81.0%) and Asian (80.0%) patients with CML, as well as younger patients (20-49 years) with CLL (96.0%). But in contrast, Black patients, those with AML, and individuals over the age of 75 years experienced the lowest improvement in survival.
Studies have found that Hispanics have increased rates of ALL and acute promyelocytic leukemia (APL), but lower rates of AML, as compared to Whites. They also tend to be diagnosed at a younger age and have poorer overall survival.3
Demographics may also play a role, as Hispanics born outside the United States had a higher incidence rate of APL versus U.S.-born Hispanics (incidence rate ratio, 1.79; 1.11-2.94). Thus, the higher incidence rates of increased B-cell ALL may be due to heritable genetic factors, while APL may also be attributable to environmental exposures.4
Hispanics living on the Texas-Mexico border were also found to have a higher incidence of chronic myeloid leukemia (RR, 1.28; 95% CI, 1.07-1.51; P = .02) and acute myeloid leukemia (RR, 1.17; 95% CI, 1.04-1.33; P = .0009) as compared with Hispanics living elsewhere in Texas5 AML and CML were more likely to be observed in patients who resided in this border region, and those with ALL, AML, and CML had worse outcomes compared with Hispanics living elsewhere in Texas. In addition, both Hispanic and non-Hispanic patients along the border have a worse prognosis for ALL than patients in other areas of Texas.
“We don’t yet understand if the differences are due to nonbiologic factors, or if biology plays a role because of the more aggressive disease that we’re seeing,” said study author Anna Eiring, PhD, an assistant professor at Texas Tech University, El Paso. “This is a medically underserved region, and even though we are a safety net hospital, many of the Hispanic patients don’t have health insurance.”
They also tend to have worse socioeconomic status compared with non-Hispanic populations, and there may also be lifestyle and environmental factors. “Exposure to environmental toxins may also play a role, as many work in jobs that could put them at risk,” she said. “Lifestyle factors may also play a role.”
AML is a hematopoietic disorder that is characterized by numerous cytogenetic and molecular aberrations, with poor overall survival. Researchers found that Black patients had shorter survival than White patients, based on an analysis of Surveillance Epidemiology and End Results (SEER) Program data, and performing and performed mutational profiling of 1,339 patients with AML treated on frontline Alliance for Clinical Trials in Oncology (Alliance) protocols.6 The disparity was especially pronounced in Black patients under 60 years old, after adjustment for socioeconomic (SEER) and molecular (Alliance) factors. Black race was an independent prognosticator of poor survival.
“Based on our analyses in Black and White AML patients under the age of 60 years, we believe that a differential impact of molecular aberrations, specifically AML-associated gene mutations, contribute to the observed survival disparities,” said study author Ann-Kathrin Eisfeld, MD, an assistant professor in the division of hematology at the Ohio State University, Columbus, and a member of the leukemia research program at the university’s comprehensive cancer center, the James. “For example, NPM1 mutations seem to lack the known positive prognostic impact we are used to seeing in previous studies with White AML patients.”
She noted that when looking at molecular prognosticators just within Black AML patients, researchers found that FLT3-ITD and also IDH2 mutations were associated with poor overall survival. “While FLT3-ITD is a known adverse prognosticator, the significant impact of IDH2 mutations was surprising to us and is currently being further explored,” said Dr. Eisfeld.
“In general, however, it can’t be highlighted enough that while this study suggests an impact of somatic tumor genomics that needs a lot more attention and investigation and ideally, also prospective studies, structural racism and its impact is still the problem,” she emphasized. “It’s the ‘elephant in the room’ and the major factor that needs to be addressed in order to improve and overcome these survival disparities.”
Disparities in lymphoma
Similar to leukemia, lymphomas are a heterogenous and diverse group of malignancies that range from indolent to highly aggressive. The two main types are listed below:
Non-Hodgkin lymphoma (NHL), the most common subtype, with about 80,000 new cases a year in the United States. There are more than 90 types of NHL, the most common being B-cell lymphomas, which include diffuse large B cell, primary mediastinal B cell, follicular, small lymphocytic lymphoma, and chronic lymphocytic leukemia; marginal zone, mantle zone, and Burkitt lymphomas; and Waldenström macroglobulinemia.
Hodgkin lymphoma (HL), less common than NHL, with about 9,000 people diagnosed every year. There are five types of HL, and it is primarily seen in children and young adults.
Disparities in incidence, age at diagnosis, and overall survival have been observed in lymphoma, which, aside from marginal zone and follicular lymphoma, are more common among men. The incidence of most lymphoma subtypes is generally lower in racial and ethnic minority groups, although Black and Hispanic patients tend to be diagnosed at a younger age, and in Black patients, at a more advanced stage and the lymphomas have higher risk features at initial presentation.7
One study that looked at racial disparities in Hodgkin lymphoma found that HL was significantly more common in Hispanics versus Whites under the age of 65 years. The 5-, 10-, and 15-year overall survival rates were also inferior for Blacks and Hispanics compared with Whites (P less than 0.005 and P less than 0.001, respectively).8
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma in the United States, comprising approximately one-third of lymphomas diagnosed in adults (Lee et al. 2020). In one study that examined ancestry and tumor genomics, recurrent somatic mutations in established driver genes, such as ATM, MGA, SETD2, TET2, DNMT3A, and MLL3, were observed more frequently in patients with African ancestry versus those of European ancestry.9 Other data suggest a variety of disparities in receipt of treatment. For example, patients with localized disease who were Black, uninsured/Medicaid insured, or of lower socioeconomic status were less likely to receive any form of chemotherapy (all P less than 0.0001), and Black race was also associated with being less likely to receive chemoimmunotherapy.
Leveling the field of disparities is complex and requires a multifaceted approach. But one facility found that they could help minority patients overcome some of the hurdles related to nonbiologic factors by the support of a nurse navigator in addition to therapy.10 Their study included 204 patients with DLBCL (47 minority patients and 157 White patients) and following the initiation of the nurse navigator program, virtually all patients received frontline chemotherapy (98% versus 96%). The incidence of relapsed/refractory disease was similar (40% versus 38%) and in the relapsed/refractory population, similar proportions of patients underwent hematopoietic stem cell transplantation (32% versus 29%) or received chimeric antigen receptor T-cell therapy (16% versus 19%). The 2-year overall survival rates were 81% and 76% for minorities and Whites, respectively, and 2-year progression-free survival rates were 62% and 65%, respectively.
“We found that the minority patients often needed more help to get care, and they utilized the nurse navigator more intensively,” said study author Bei Hu, MD, who is with the department of hematologic oncology and blood disorders, Levine Cancer Institute/Atrium Health, Charlotte, N.C. “The nurse navigator was able to help them with things like finances, transportation, and insurance.”
Minorities tended to face more barriers than White patients. “Even something as simple as needing money for gas to get to the clinic can be a barrier to care,” said Dr. Hu. “And many of the patients are often uncomfortable discussing these things with their physician – plus a lot is covered in our appointments and we focus on the cancer. So, they may be more comfortable discussing these issues with the nurse.”
Disparities in multiple myeloma
Multiple myeloma is the malignant clonal proliferation of plasma B cells in the bone marrow and, despite the advent of new therapies, remains incurable and generally fatal. It progresses from the more common but often subclinical precursor states of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM) to overt and symptomatic multiple myeloma. Racial disparities have been observed in all stages of the disease, and as compared with Whites, individuals who are Black have a higher risk of MGUS and myeloma and a higher mortality rate.11 They have not experienced the same survival gains seen in White patients.
Some research suggests that these disparities may be more related to socioeconomic status as opposed to race. One analysis of 562 patients found that those with higher socioeconomic status had a median overall survival of 62.8 months compared with 53.7 and 48.6 months for middle and low socioeconomic status (P = 0.015).12
After controlling for confounders including race, patients with low socioeconomic status had a 54% increase in mortality rate relative to those with high status. The authors then performed a similar analysis of 45,505 patients with multiple myeloma from the S
“In some homogeneous health systems, such as the VA, we are seeing that Black patients do as well or better than White patients,” said Catherine Marinac, PhD, an assistant professor of medicine, Harvard Medical School, Boston. “Survival is equal or better, as long as treatment is not delayed and they receive the standard of care.”
Black patients generally have a more indolent disease subtype and may experience less aggressive disease, but they have not experienced the same magnitude in survival as White patients following the introduction of new therapeutics. This disparity lends support to the influence of socioeconomic factors, such as unequal access to novel therapies and/or differences in treatment response, and lower rates of autologous stem cell transplantation.13
However, there are racial/ethnic differences in risk for both myeloma and its premalignant conditions, as well as incidence. Blacks have a twofold increased risk of myeloma compared with White individuals and are diagnosed at younger ages. Differences in myeloma incidence is less marked in other racial/ethnic groups, such as Hispanics, where it is only slightly higher than in Whites at 6.7 per 100,00.11 In contrast, the incidence of myeloma is markedly lower in Asians as compared with non-Hispanic Whites (incidence rate of 3.8 versus 6.2 per 100,000). Black persons also have a markedly higher prevalence of MGUS, and these differences suggest that biology, and clinical characteristics, differ by race or ancestry.
“Mortality among Black patients is also higher,” said Dr. Marinac, who is also on the faculty in the division of population sciences at the Dana Farber Cancer Institute, also in Boston. “The higher mortality rate is driven by the higher incidence.”
There are also differences in the prevalence of immunoglobulin isotypes observed across racial/ethnic groups of MGUS patients, Dr. Marinac explained, which is consistent with the hypothesis that there is a biological basis for disparities arising in precursor lesions.
“What we are looking at now is cancer prevention and early intervention,” she said. “There are well-defined precursors to myeloma, and Blacks are three times more likely to have a precursor condition.”
Early detection of precursors followed by preventing progression to full-blown multiple myeloma is one way of addressing disparities, but right now, there are no real screening guidelines. “Most patients now are diagnosed incidentally, and then the only intervention is to monitor them,” Dr. Marinac said. “At Dana Farber, we are now looking to see if we can refine screening, and then see who may need additional monitoring.”
The Promise study, being conducted at Dana Farber, is recruiting participants to examine the molecular changes that occur when precursor conditions develop into full-blown multiple myeloma and is open to individuals considered to be at high risk: Black race and/or have a first-degree relative with multiple myeloma or one of its precursor conditions.
Dr. Marinac also pointed out that there are ongoing clinical trials that are looking at low-risk early interventions in patients with precursor conditions. “We are now looking at lifestyle and metformin,” she said. “The thought is that if we treat them now, we can prevent myeloma from developing.”
Lessening barriers to care
When trying to tease out the strongest/most prominent reasons for the disparities that have been observed in the care of patients with blood cancers, Stephanie Lee, M.D., M.P.H, professor and associate director of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle, thinks that the problem is truly multifactorial.
“Access has been cited many times because some studies show that if access is equitable, sometimes racial/ethnic minorities do the same as non-Hispanic Whites,” she said. “Same thing with quality of care – if all people are treated on clinical trials, sometimes the outcomes are the same.”
That said, many things have to go right to get the best outcomes, and if one factor isn’t optimal, then treatment may never achieve the success that is possible, she noted.
Considering how complex the issue of disparities is, addressing it can seem daunting. Dr. Lee points out that the place to begin is with clinical trials. “I would like to see more studies that test interventions to correct disparities,” said Dr. Lee. “But I have actually seen in my own work that racial and ethnic minorities are less likely to participate in studies, even survey and observational studies where physical risks are low or nonexistent.”
People are studying how to increase minority participation in clinical trials, but thus far, there isn’t one solution. “As with routine care, there are probably a lot of logistical barriers to trial participation that disproportionately affect minority populations,” she noted. “There is also greater distrust of studies.”
But for now, there are some steps that clinicians can take to start to improve these disparities. “I think we can start inquiring about and documenting barriers to care and clinical trial participation, just like we document other aspects of the social history,” Dr. Lee explained. “Truly understanding the problem is the first step toward trying to solve it.”
References
1. Leukemia & Lymphoma Society. 2021. www.lls.org/facts-and-statistics/facts-and-statistics-overview.
2. Utuama O et al. PLoS One. 2019 Aug 19;14(8):e0220864.
3. Pollyea DA et al. J Cancer Prev Curr Res. 2014;1(1):14-19.
4. Bencomo-Alvarez AE et al. Cancer. 2021 Apr 1;127(7):1068-79.
5. Nabhan C et al. Cancer. 2012 Oct 1;118(19):4842-50.
6. Bhatnagar B et al. Blood. 2020;136(Suppl 1):5-7.
7. Shenoy PJ et al. Cancer. 2011;117:2530-40.
8. Evens AM et al. Ann Oncol. 2012 Aug 1;23(8):2128-37.
9. Lee MJ et al. Cancer. 2020;126:3493-3503.
10. Hu B et al. Cancer. 2021 Jul 21. doi: 10.1002/cncr.33779.
11. Marinac CR et al. Blood Cancer J. 2020 Feb 17;10(2):19.
12. Fiala MA et al. Leuk Lymphoma. 2015;56(9):2643-9.
13. Costa LJ et al. Biol Blood Marrow Transplant. 2015 Apr;21(4):701-6.
As compared with White individuals, minorities often face higher barriers to cancer care. Racial and ethnic disparities in patients with solid tumors, particularly those of the prostate and breast, have been well documented. Hematologic malignancies are less common, but an increasing number of studies have documented disparities within this subgroup of cancer, particularly among Black and non-White Hispanics. An increasing armamentarium of therapeutics, including novel chemotherapy agents and targeted molecular, cellular, and immunologic therapies, has highlighted the need for understanding and exploring the differences in care as well as biology, which may lead to disparate outcomes.
Overall, an estimated 186,400 people living in the United States are expected to be diagnosed with leukemia, lymphoma, or myeloma in 2021, and new cases of hematologic malignancies are expected to account for 9.8% of the estimated 1,898,160 new cancer cases diagnosed this year.1
The underlying reasons for disparities are highly complex and multifactorial, and clinicians must consider how the biologic, clinical, demographic, and socioeconomic characteristics of their patients interact. All of these factors can play a role in prognosis and/or access to care.
Disparities in leukemia
Leukemia is a heterogeneous group of diseases affecting both children and adults, but during the past few decades survival rates have steadily improved, particularly among children. Response to therapy and prognosis do vary among leukemia types, but one large analysis reported that there were overall improvements in survival seen across racial/ethnic groups, most age groups, and genders during a 40-year period.2
From 1973 through 2014, survival trends were assessed across four leukemia types: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and chronic lymphoid leukemia (CLL). After stratifying survival for each leukemia type by race/ethnicity, improvement rates were not uniform among all groups.
For example, there were substantial improvements of leukemia-specific survival in 2010-2014 among Black (81.0%) and Asian (80.0%) patients with CML, as well as younger patients (20-49 years) with CLL (96.0%). But in contrast, Black patients, those with AML, and individuals over the age of 75 years experienced the lowest improvement in survival.
Studies have found that Hispanics have increased rates of ALL and acute promyelocytic leukemia (APL), but lower rates of AML, as compared to Whites. They also tend to be diagnosed at a younger age and have poorer overall survival.3
Demographics may also play a role, as Hispanics born outside the United States had a higher incidence rate of APL versus U.S.-born Hispanics (incidence rate ratio, 1.79; 1.11-2.94). Thus, the higher incidence rates of increased B-cell ALL may be due to heritable genetic factors, while APL may also be attributable to environmental exposures.4
Hispanics living on the Texas-Mexico border were also found to have a higher incidence of chronic myeloid leukemia (RR, 1.28; 95% CI, 1.07-1.51; P = .02) and acute myeloid leukemia (RR, 1.17; 95% CI, 1.04-1.33; P = .0009) as compared with Hispanics living elsewhere in Texas5 AML and CML were more likely to be observed in patients who resided in this border region, and those with ALL, AML, and CML had worse outcomes compared with Hispanics living elsewhere in Texas. In addition, both Hispanic and non-Hispanic patients along the border have a worse prognosis for ALL than patients in other areas of Texas.
“We don’t yet understand if the differences are due to nonbiologic factors, or if biology plays a role because of the more aggressive disease that we’re seeing,” said study author Anna Eiring, PhD, an assistant professor at Texas Tech University, El Paso. “This is a medically underserved region, and even though we are a safety net hospital, many of the Hispanic patients don’t have health insurance.”
They also tend to have worse socioeconomic status compared with non-Hispanic populations, and there may also be lifestyle and environmental factors. “Exposure to environmental toxins may also play a role, as many work in jobs that could put them at risk,” she said. “Lifestyle factors may also play a role.”
AML is a hematopoietic disorder that is characterized by numerous cytogenetic and molecular aberrations, with poor overall survival. Researchers found that Black patients had shorter survival than White patients, based on an analysis of Surveillance Epidemiology and End Results (SEER) Program data, and performing and performed mutational profiling of 1,339 patients with AML treated on frontline Alliance for Clinical Trials in Oncology (Alliance) protocols.6 The disparity was especially pronounced in Black patients under 60 years old, after adjustment for socioeconomic (SEER) and molecular (Alliance) factors. Black race was an independent prognosticator of poor survival.
“Based on our analyses in Black and White AML patients under the age of 60 years, we believe that a differential impact of molecular aberrations, specifically AML-associated gene mutations, contribute to the observed survival disparities,” said study author Ann-Kathrin Eisfeld, MD, an assistant professor in the division of hematology at the Ohio State University, Columbus, and a member of the leukemia research program at the university’s comprehensive cancer center, the James. “For example, NPM1 mutations seem to lack the known positive prognostic impact we are used to seeing in previous studies with White AML patients.”
She noted that when looking at molecular prognosticators just within Black AML patients, researchers found that FLT3-ITD and also IDH2 mutations were associated with poor overall survival. “While FLT3-ITD is a known adverse prognosticator, the significant impact of IDH2 mutations was surprising to us and is currently being further explored,” said Dr. Eisfeld.
“In general, however, it can’t be highlighted enough that while this study suggests an impact of somatic tumor genomics that needs a lot more attention and investigation and ideally, also prospective studies, structural racism and its impact is still the problem,” she emphasized. “It’s the ‘elephant in the room’ and the major factor that needs to be addressed in order to improve and overcome these survival disparities.”
Disparities in lymphoma
Similar to leukemia, lymphomas are a heterogenous and diverse group of malignancies that range from indolent to highly aggressive. The two main types are listed below:
Non-Hodgkin lymphoma (NHL), the most common subtype, with about 80,000 new cases a year in the United States. There are more than 90 types of NHL, the most common being B-cell lymphomas, which include diffuse large B cell, primary mediastinal B cell, follicular, small lymphocytic lymphoma, and chronic lymphocytic leukemia; marginal zone, mantle zone, and Burkitt lymphomas; and Waldenström macroglobulinemia.
Hodgkin lymphoma (HL), less common than NHL, with about 9,000 people diagnosed every year. There are five types of HL, and it is primarily seen in children and young adults.
Disparities in incidence, age at diagnosis, and overall survival have been observed in lymphoma, which, aside from marginal zone and follicular lymphoma, are more common among men. The incidence of most lymphoma subtypes is generally lower in racial and ethnic minority groups, although Black and Hispanic patients tend to be diagnosed at a younger age, and in Black patients, at a more advanced stage and the lymphomas have higher risk features at initial presentation.7
One study that looked at racial disparities in Hodgkin lymphoma found that HL was significantly more common in Hispanics versus Whites under the age of 65 years. The 5-, 10-, and 15-year overall survival rates were also inferior for Blacks and Hispanics compared with Whites (P less than 0.005 and P less than 0.001, respectively).8
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma in the United States, comprising approximately one-third of lymphomas diagnosed in adults (Lee et al. 2020). In one study that examined ancestry and tumor genomics, recurrent somatic mutations in established driver genes, such as ATM, MGA, SETD2, TET2, DNMT3A, and MLL3, were observed more frequently in patients with African ancestry versus those of European ancestry.9 Other data suggest a variety of disparities in receipt of treatment. For example, patients with localized disease who were Black, uninsured/Medicaid insured, or of lower socioeconomic status were less likely to receive any form of chemotherapy (all P less than 0.0001), and Black race was also associated with being less likely to receive chemoimmunotherapy.
Leveling the field of disparities is complex and requires a multifaceted approach. But one facility found that they could help minority patients overcome some of the hurdles related to nonbiologic factors by the support of a nurse navigator in addition to therapy.10 Their study included 204 patients with DLBCL (47 minority patients and 157 White patients) and following the initiation of the nurse navigator program, virtually all patients received frontline chemotherapy (98% versus 96%). The incidence of relapsed/refractory disease was similar (40% versus 38%) and in the relapsed/refractory population, similar proportions of patients underwent hematopoietic stem cell transplantation (32% versus 29%) or received chimeric antigen receptor T-cell therapy (16% versus 19%). The 2-year overall survival rates were 81% and 76% for minorities and Whites, respectively, and 2-year progression-free survival rates were 62% and 65%, respectively.
“We found that the minority patients often needed more help to get care, and they utilized the nurse navigator more intensively,” said study author Bei Hu, MD, who is with the department of hematologic oncology and blood disorders, Levine Cancer Institute/Atrium Health, Charlotte, N.C. “The nurse navigator was able to help them with things like finances, transportation, and insurance.”
Minorities tended to face more barriers than White patients. “Even something as simple as needing money for gas to get to the clinic can be a barrier to care,” said Dr. Hu. “And many of the patients are often uncomfortable discussing these things with their physician – plus a lot is covered in our appointments and we focus on the cancer. So, they may be more comfortable discussing these issues with the nurse.”
Disparities in multiple myeloma
Multiple myeloma is the malignant clonal proliferation of plasma B cells in the bone marrow and, despite the advent of new therapies, remains incurable and generally fatal. It progresses from the more common but often subclinical precursor states of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM) to overt and symptomatic multiple myeloma. Racial disparities have been observed in all stages of the disease, and as compared with Whites, individuals who are Black have a higher risk of MGUS and myeloma and a higher mortality rate.11 They have not experienced the same survival gains seen in White patients.
Some research suggests that these disparities may be more related to socioeconomic status as opposed to race. One analysis of 562 patients found that those with higher socioeconomic status had a median overall survival of 62.8 months compared with 53.7 and 48.6 months for middle and low socioeconomic status (P = 0.015).12
After controlling for confounders including race, patients with low socioeconomic status had a 54% increase in mortality rate relative to those with high status. The authors then performed a similar analysis of 45,505 patients with multiple myeloma from the S
“In some homogeneous health systems, such as the VA, we are seeing that Black patients do as well or better than White patients,” said Catherine Marinac, PhD, an assistant professor of medicine, Harvard Medical School, Boston. “Survival is equal or better, as long as treatment is not delayed and they receive the standard of care.”
Black patients generally have a more indolent disease subtype and may experience less aggressive disease, but they have not experienced the same magnitude in survival as White patients following the introduction of new therapeutics. This disparity lends support to the influence of socioeconomic factors, such as unequal access to novel therapies and/or differences in treatment response, and lower rates of autologous stem cell transplantation.13
However, there are racial/ethnic differences in risk for both myeloma and its premalignant conditions, as well as incidence. Blacks have a twofold increased risk of myeloma compared with White individuals and are diagnosed at younger ages. Differences in myeloma incidence is less marked in other racial/ethnic groups, such as Hispanics, where it is only slightly higher than in Whites at 6.7 per 100,00.11 In contrast, the incidence of myeloma is markedly lower in Asians as compared with non-Hispanic Whites (incidence rate of 3.8 versus 6.2 per 100,000). Black persons also have a markedly higher prevalence of MGUS, and these differences suggest that biology, and clinical characteristics, differ by race or ancestry.
“Mortality among Black patients is also higher,” said Dr. Marinac, who is also on the faculty in the division of population sciences at the Dana Farber Cancer Institute, also in Boston. “The higher mortality rate is driven by the higher incidence.”
There are also differences in the prevalence of immunoglobulin isotypes observed across racial/ethnic groups of MGUS patients, Dr. Marinac explained, which is consistent with the hypothesis that there is a biological basis for disparities arising in precursor lesions.
“What we are looking at now is cancer prevention and early intervention,” she said. “There are well-defined precursors to myeloma, and Blacks are three times more likely to have a precursor condition.”
Early detection of precursors followed by preventing progression to full-blown multiple myeloma is one way of addressing disparities, but right now, there are no real screening guidelines. “Most patients now are diagnosed incidentally, and then the only intervention is to monitor them,” Dr. Marinac said. “At Dana Farber, we are now looking to see if we can refine screening, and then see who may need additional monitoring.”
The Promise study, being conducted at Dana Farber, is recruiting participants to examine the molecular changes that occur when precursor conditions develop into full-blown multiple myeloma and is open to individuals considered to be at high risk: Black race and/or have a first-degree relative with multiple myeloma or one of its precursor conditions.
Dr. Marinac also pointed out that there are ongoing clinical trials that are looking at low-risk early interventions in patients with precursor conditions. “We are now looking at lifestyle and metformin,” she said. “The thought is that if we treat them now, we can prevent myeloma from developing.”
Lessening barriers to care
When trying to tease out the strongest/most prominent reasons for the disparities that have been observed in the care of patients with blood cancers, Stephanie Lee, M.D., M.P.H, professor and associate director of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle, thinks that the problem is truly multifactorial.
“Access has been cited many times because some studies show that if access is equitable, sometimes racial/ethnic minorities do the same as non-Hispanic Whites,” she said. “Same thing with quality of care – if all people are treated on clinical trials, sometimes the outcomes are the same.”
That said, many things have to go right to get the best outcomes, and if one factor isn’t optimal, then treatment may never achieve the success that is possible, she noted.
Considering how complex the issue of disparities is, addressing it can seem daunting. Dr. Lee points out that the place to begin is with clinical trials. “I would like to see more studies that test interventions to correct disparities,” said Dr. Lee. “But I have actually seen in my own work that racial and ethnic minorities are less likely to participate in studies, even survey and observational studies where physical risks are low or nonexistent.”
People are studying how to increase minority participation in clinical trials, but thus far, there isn’t one solution. “As with routine care, there are probably a lot of logistical barriers to trial participation that disproportionately affect minority populations,” she noted. “There is also greater distrust of studies.”
But for now, there are some steps that clinicians can take to start to improve these disparities. “I think we can start inquiring about and documenting barriers to care and clinical trial participation, just like we document other aspects of the social history,” Dr. Lee explained. “Truly understanding the problem is the first step toward trying to solve it.”
References
1. Leukemia & Lymphoma Society. 2021. www.lls.org/facts-and-statistics/facts-and-statistics-overview.
2. Utuama O et al. PLoS One. 2019 Aug 19;14(8):e0220864.
3. Pollyea DA et al. J Cancer Prev Curr Res. 2014;1(1):14-19.
4. Bencomo-Alvarez AE et al. Cancer. 2021 Apr 1;127(7):1068-79.
5. Nabhan C et al. Cancer. 2012 Oct 1;118(19):4842-50.
6. Bhatnagar B et al. Blood. 2020;136(Suppl 1):5-7.
7. Shenoy PJ et al. Cancer. 2011;117:2530-40.
8. Evens AM et al. Ann Oncol. 2012 Aug 1;23(8):2128-37.
9. Lee MJ et al. Cancer. 2020;126:3493-3503.
10. Hu B et al. Cancer. 2021 Jul 21. doi: 10.1002/cncr.33779.
11. Marinac CR et al. Blood Cancer J. 2020 Feb 17;10(2):19.
12. Fiala MA et al. Leuk Lymphoma. 2015;56(9):2643-9.
13. Costa LJ et al. Biol Blood Marrow Transplant. 2015 Apr;21(4):701-6.
As compared with White individuals, minorities often face higher barriers to cancer care. Racial and ethnic disparities in patients with solid tumors, particularly those of the prostate and breast, have been well documented. Hematologic malignancies are less common, but an increasing number of studies have documented disparities within this subgroup of cancer, particularly among Black and non-White Hispanics. An increasing armamentarium of therapeutics, including novel chemotherapy agents and targeted molecular, cellular, and immunologic therapies, has highlighted the need for understanding and exploring the differences in care as well as biology, which may lead to disparate outcomes.
Overall, an estimated 186,400 people living in the United States are expected to be diagnosed with leukemia, lymphoma, or myeloma in 2021, and new cases of hematologic malignancies are expected to account for 9.8% of the estimated 1,898,160 new cancer cases diagnosed this year.1
The underlying reasons for disparities are highly complex and multifactorial, and clinicians must consider how the biologic, clinical, demographic, and socioeconomic characteristics of their patients interact. All of these factors can play a role in prognosis and/or access to care.
Disparities in leukemia
Leukemia is a heterogeneous group of diseases affecting both children and adults, but during the past few decades survival rates have steadily improved, particularly among children. Response to therapy and prognosis do vary among leukemia types, but one large analysis reported that there were overall improvements in survival seen across racial/ethnic groups, most age groups, and genders during a 40-year period.2
From 1973 through 2014, survival trends were assessed across four leukemia types: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), and chronic lymphoid leukemia (CLL). After stratifying survival for each leukemia type by race/ethnicity, improvement rates were not uniform among all groups.
For example, there were substantial improvements of leukemia-specific survival in 2010-2014 among Black (81.0%) and Asian (80.0%) patients with CML, as well as younger patients (20-49 years) with CLL (96.0%). But in contrast, Black patients, those with AML, and individuals over the age of 75 years experienced the lowest improvement in survival.
Studies have found that Hispanics have increased rates of ALL and acute promyelocytic leukemia (APL), but lower rates of AML, as compared to Whites. They also tend to be diagnosed at a younger age and have poorer overall survival.3
Demographics may also play a role, as Hispanics born outside the United States had a higher incidence rate of APL versus U.S.-born Hispanics (incidence rate ratio, 1.79; 1.11-2.94). Thus, the higher incidence rates of increased B-cell ALL may be due to heritable genetic factors, while APL may also be attributable to environmental exposures.4
Hispanics living on the Texas-Mexico border were also found to have a higher incidence of chronic myeloid leukemia (RR, 1.28; 95% CI, 1.07-1.51; P = .02) and acute myeloid leukemia (RR, 1.17; 95% CI, 1.04-1.33; P = .0009) as compared with Hispanics living elsewhere in Texas5 AML and CML were more likely to be observed in patients who resided in this border region, and those with ALL, AML, and CML had worse outcomes compared with Hispanics living elsewhere in Texas. In addition, both Hispanic and non-Hispanic patients along the border have a worse prognosis for ALL than patients in other areas of Texas.
“We don’t yet understand if the differences are due to nonbiologic factors, or if biology plays a role because of the more aggressive disease that we’re seeing,” said study author Anna Eiring, PhD, an assistant professor at Texas Tech University, El Paso. “This is a medically underserved region, and even though we are a safety net hospital, many of the Hispanic patients don’t have health insurance.”
They also tend to have worse socioeconomic status compared with non-Hispanic populations, and there may also be lifestyle and environmental factors. “Exposure to environmental toxins may also play a role, as many work in jobs that could put them at risk,” she said. “Lifestyle factors may also play a role.”
AML is a hematopoietic disorder that is characterized by numerous cytogenetic and molecular aberrations, with poor overall survival. Researchers found that Black patients had shorter survival than White patients, based on an analysis of Surveillance Epidemiology and End Results (SEER) Program data, and performing and performed mutational profiling of 1,339 patients with AML treated on frontline Alliance for Clinical Trials in Oncology (Alliance) protocols.6 The disparity was especially pronounced in Black patients under 60 years old, after adjustment for socioeconomic (SEER) and molecular (Alliance) factors. Black race was an independent prognosticator of poor survival.
“Based on our analyses in Black and White AML patients under the age of 60 years, we believe that a differential impact of molecular aberrations, specifically AML-associated gene mutations, contribute to the observed survival disparities,” said study author Ann-Kathrin Eisfeld, MD, an assistant professor in the division of hematology at the Ohio State University, Columbus, and a member of the leukemia research program at the university’s comprehensive cancer center, the James. “For example, NPM1 mutations seem to lack the known positive prognostic impact we are used to seeing in previous studies with White AML patients.”
She noted that when looking at molecular prognosticators just within Black AML patients, researchers found that FLT3-ITD and also IDH2 mutations were associated with poor overall survival. “While FLT3-ITD is a known adverse prognosticator, the significant impact of IDH2 mutations was surprising to us and is currently being further explored,” said Dr. Eisfeld.
“In general, however, it can’t be highlighted enough that while this study suggests an impact of somatic tumor genomics that needs a lot more attention and investigation and ideally, also prospective studies, structural racism and its impact is still the problem,” she emphasized. “It’s the ‘elephant in the room’ and the major factor that needs to be addressed in order to improve and overcome these survival disparities.”
Disparities in lymphoma
Similar to leukemia, lymphomas are a heterogenous and diverse group of malignancies that range from indolent to highly aggressive. The two main types are listed below:
Non-Hodgkin lymphoma (NHL), the most common subtype, with about 80,000 new cases a year in the United States. There are more than 90 types of NHL, the most common being B-cell lymphomas, which include diffuse large B cell, primary mediastinal B cell, follicular, small lymphocytic lymphoma, and chronic lymphocytic leukemia; marginal zone, mantle zone, and Burkitt lymphomas; and Waldenström macroglobulinemia.
Hodgkin lymphoma (HL), less common than NHL, with about 9,000 people diagnosed every year. There are five types of HL, and it is primarily seen in children and young adults.
Disparities in incidence, age at diagnosis, and overall survival have been observed in lymphoma, which, aside from marginal zone and follicular lymphoma, are more common among men. The incidence of most lymphoma subtypes is generally lower in racial and ethnic minority groups, although Black and Hispanic patients tend to be diagnosed at a younger age, and in Black patients, at a more advanced stage and the lymphomas have higher risk features at initial presentation.7
One study that looked at racial disparities in Hodgkin lymphoma found that HL was significantly more common in Hispanics versus Whites under the age of 65 years. The 5-, 10-, and 15-year overall survival rates were also inferior for Blacks and Hispanics compared with Whites (P less than 0.005 and P less than 0.001, respectively).8
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma in the United States, comprising approximately one-third of lymphomas diagnosed in adults (Lee et al. 2020). In one study that examined ancestry and tumor genomics, recurrent somatic mutations in established driver genes, such as ATM, MGA, SETD2, TET2, DNMT3A, and MLL3, were observed more frequently in patients with African ancestry versus those of European ancestry.9 Other data suggest a variety of disparities in receipt of treatment. For example, patients with localized disease who were Black, uninsured/Medicaid insured, or of lower socioeconomic status were less likely to receive any form of chemotherapy (all P less than 0.0001), and Black race was also associated with being less likely to receive chemoimmunotherapy.
Leveling the field of disparities is complex and requires a multifaceted approach. But one facility found that they could help minority patients overcome some of the hurdles related to nonbiologic factors by the support of a nurse navigator in addition to therapy.10 Their study included 204 patients with DLBCL (47 minority patients and 157 White patients) and following the initiation of the nurse navigator program, virtually all patients received frontline chemotherapy (98% versus 96%). The incidence of relapsed/refractory disease was similar (40% versus 38%) and in the relapsed/refractory population, similar proportions of patients underwent hematopoietic stem cell transplantation (32% versus 29%) or received chimeric antigen receptor T-cell therapy (16% versus 19%). The 2-year overall survival rates were 81% and 76% for minorities and Whites, respectively, and 2-year progression-free survival rates were 62% and 65%, respectively.
“We found that the minority patients often needed more help to get care, and they utilized the nurse navigator more intensively,” said study author Bei Hu, MD, who is with the department of hematologic oncology and blood disorders, Levine Cancer Institute/Atrium Health, Charlotte, N.C. “The nurse navigator was able to help them with things like finances, transportation, and insurance.”
Minorities tended to face more barriers than White patients. “Even something as simple as needing money for gas to get to the clinic can be a barrier to care,” said Dr. Hu. “And many of the patients are often uncomfortable discussing these things with their physician – plus a lot is covered in our appointments and we focus on the cancer. So, they may be more comfortable discussing these issues with the nurse.”
Disparities in multiple myeloma
Multiple myeloma is the malignant clonal proliferation of plasma B cells in the bone marrow and, despite the advent of new therapies, remains incurable and generally fatal. It progresses from the more common but often subclinical precursor states of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM) to overt and symptomatic multiple myeloma. Racial disparities have been observed in all stages of the disease, and as compared with Whites, individuals who are Black have a higher risk of MGUS and myeloma and a higher mortality rate.11 They have not experienced the same survival gains seen in White patients.
Some research suggests that these disparities may be more related to socioeconomic status as opposed to race. One analysis of 562 patients found that those with higher socioeconomic status had a median overall survival of 62.8 months compared with 53.7 and 48.6 months for middle and low socioeconomic status (P = 0.015).12
After controlling for confounders including race, patients with low socioeconomic status had a 54% increase in mortality rate relative to those with high status. The authors then performed a similar analysis of 45,505 patients with multiple myeloma from the S
“In some homogeneous health systems, such as the VA, we are seeing that Black patients do as well or better than White patients,” said Catherine Marinac, PhD, an assistant professor of medicine, Harvard Medical School, Boston. “Survival is equal or better, as long as treatment is not delayed and they receive the standard of care.”
Black patients generally have a more indolent disease subtype and may experience less aggressive disease, but they have not experienced the same magnitude in survival as White patients following the introduction of new therapeutics. This disparity lends support to the influence of socioeconomic factors, such as unequal access to novel therapies and/or differences in treatment response, and lower rates of autologous stem cell transplantation.13
However, there are racial/ethnic differences in risk for both myeloma and its premalignant conditions, as well as incidence. Blacks have a twofold increased risk of myeloma compared with White individuals and are diagnosed at younger ages. Differences in myeloma incidence is less marked in other racial/ethnic groups, such as Hispanics, where it is only slightly higher than in Whites at 6.7 per 100,00.11 In contrast, the incidence of myeloma is markedly lower in Asians as compared with non-Hispanic Whites (incidence rate of 3.8 versus 6.2 per 100,000). Black persons also have a markedly higher prevalence of MGUS, and these differences suggest that biology, and clinical characteristics, differ by race or ancestry.
“Mortality among Black patients is also higher,” said Dr. Marinac, who is also on the faculty in the division of population sciences at the Dana Farber Cancer Institute, also in Boston. “The higher mortality rate is driven by the higher incidence.”
There are also differences in the prevalence of immunoglobulin isotypes observed across racial/ethnic groups of MGUS patients, Dr. Marinac explained, which is consistent with the hypothesis that there is a biological basis for disparities arising in precursor lesions.
“What we are looking at now is cancer prevention and early intervention,” she said. “There are well-defined precursors to myeloma, and Blacks are three times more likely to have a precursor condition.”
Early detection of precursors followed by preventing progression to full-blown multiple myeloma is one way of addressing disparities, but right now, there are no real screening guidelines. “Most patients now are diagnosed incidentally, and then the only intervention is to monitor them,” Dr. Marinac said. “At Dana Farber, we are now looking to see if we can refine screening, and then see who may need additional monitoring.”
The Promise study, being conducted at Dana Farber, is recruiting participants to examine the molecular changes that occur when precursor conditions develop into full-blown multiple myeloma and is open to individuals considered to be at high risk: Black race and/or have a first-degree relative with multiple myeloma or one of its precursor conditions.
Dr. Marinac also pointed out that there are ongoing clinical trials that are looking at low-risk early interventions in patients with precursor conditions. “We are now looking at lifestyle and metformin,” she said. “The thought is that if we treat them now, we can prevent myeloma from developing.”
Lessening barriers to care
When trying to tease out the strongest/most prominent reasons for the disparities that have been observed in the care of patients with blood cancers, Stephanie Lee, M.D., M.P.H, professor and associate director of the clinical research division at Fred Hutchinson Cancer Research Center, Seattle, thinks that the problem is truly multifactorial.
“Access has been cited many times because some studies show that if access is equitable, sometimes racial/ethnic minorities do the same as non-Hispanic Whites,” she said. “Same thing with quality of care – if all people are treated on clinical trials, sometimes the outcomes are the same.”
That said, many things have to go right to get the best outcomes, and if one factor isn’t optimal, then treatment may never achieve the success that is possible, she noted.
Considering how complex the issue of disparities is, addressing it can seem daunting. Dr. Lee points out that the place to begin is with clinical trials. “I would like to see more studies that test interventions to correct disparities,” said Dr. Lee. “But I have actually seen in my own work that racial and ethnic minorities are less likely to participate in studies, even survey and observational studies where physical risks are low or nonexistent.”
People are studying how to increase minority participation in clinical trials, but thus far, there isn’t one solution. “As with routine care, there are probably a lot of logistical barriers to trial participation that disproportionately affect minority populations,” she noted. “There is also greater distrust of studies.”
But for now, there are some steps that clinicians can take to start to improve these disparities. “I think we can start inquiring about and documenting barriers to care and clinical trial participation, just like we document other aspects of the social history,” Dr. Lee explained. “Truly understanding the problem is the first step toward trying to solve it.”
References
1. Leukemia & Lymphoma Society. 2021. www.lls.org/facts-and-statistics/facts-and-statistics-overview.
2. Utuama O et al. PLoS One. 2019 Aug 19;14(8):e0220864.
3. Pollyea DA et al. J Cancer Prev Curr Res. 2014;1(1):14-19.
4. Bencomo-Alvarez AE et al. Cancer. 2021 Apr 1;127(7):1068-79.
5. Nabhan C et al. Cancer. 2012 Oct 1;118(19):4842-50.
6. Bhatnagar B et al. Blood. 2020;136(Suppl 1):5-7.
7. Shenoy PJ et al. Cancer. 2011;117:2530-40.
8. Evens AM et al. Ann Oncol. 2012 Aug 1;23(8):2128-37.
9. Lee MJ et al. Cancer. 2020;126:3493-3503.
10. Hu B et al. Cancer. 2021 Jul 21. doi: 10.1002/cncr.33779.
11. Marinac CR et al. Blood Cancer J. 2020 Feb 17;10(2):19.
12. Fiala MA et al. Leuk Lymphoma. 2015;56(9):2643-9.
13. Costa LJ et al. Biol Blood Marrow Transplant. 2015 Apr;21(4):701-6.
Health care unaffordability common for pregnant/postpartum women
Financial hardship remains prevalent among pregnant and postpartum women, despite the implementation of the Affordable Care Act (ACA), according to new findings published in JAMA Network Open.
Nearly a quarter (24%) of pregnant and postpartum women reported having unmet health care needs, 60% had health care unaffordability, and 54% reported general financial stress. Notably, the type of insurance was associated with the ability to afford health care.
Those with private insurance, along with women with lower incomes, were more likely to experience unaffordable health care, compared to those covered by public insurance or who had higher incomes.
Senior study author Michelle H. Moniz, MD, assistant professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor, was surprised by multiple study findings. “The prevalence of financial hardship overall, and the three individual indicators of hardship, did not change over time from 2013 to 2018,” she said. “The ACA was enacted just prior to the study period, and while this policy had many benefits for women – especially around increasing insurance coverage – it does not seem to have improved financial hardship among pregnant and postpartum women.”
She emphasized that two groups were at the highest risk of health care unaffordability: those with private insurance and those living on low incomes. “This is notable, as we often think of private insurance as offering ‘Cadillac coverage,’ but our prior work suggests that privately insured women have strikingly high out-of-pocket costs for pregnancy and childbirth-related care,” Dr. Moniz said.
These expenses include deductibles, copays, and coinsurance payments, which come to about $4,500 on average. Medicaid plans, in contrast, have exceedingly low out-of-pocket costs for pregnant and postpartum women. “Findings from the current study call for targeted policy interventions to alleviate financial strain and remove financial barriers to health care access for privately insured families,” she said. “Similarly, families living on lower incomes were also at high risk of health care unaffordability. This may be because even small out-of-pocket costs, or health care–associated costs, account for a larger share of the family’s income.”
This finding for lower-income women calls for targeted policy interventions. “Sliding-scale deductibles, for example, are one solution that might mitigate economic hardship and remove cost-related barriers to health care for pregnant and postpartum women,” Dr. Moniz added.
Health care unaffordability high
In this study, Dr. Moniz and colleagues evaluated the prevalence of financial hardship among peripartum women over time, and how it was affected by their income level and the type of insurance coverage.
They conducted a cross-sectional study that included peripartum women between the ages of 18 and 45 years who reported being currently pregnant or pregnant in the past 12 months. The women were all participants in the National Health Interview Survey, which covers the period from 2013 to 2018, and the data were analyzed from January to May 2021.
The cohort included 3,509 peripartum women, and was weighted to represent 1,050,789 women, with a mean age of 29 years. In 2018, an estimated 39,017 of 184,018 (21.2%) were Black; 36,045 (19.6%) were Hispanic; and 97,366 (52.9%) were White. In the latter years of the study period, the participants tended to be older, more highly educated, and less likely to lack insurance.
When the authors compared the unadjusted reported financial hardship outcome by each study year, unmet health care need (2013: 27.9% [95% confidence interval, 24.4%-31.7%]; 2018: 23.7% [95% CI, 19.5%-28.6%]), health care unaffordability (2013: 65.7% [95% CI, 61.1%-70.0%]; 2018: 58.8% [95% CI, 53.4%-64.0%]), and general financial stress (2013: 60.6% [95% CI, 55.2%-65.8%]; 2018: 53.8% [95% CI, 47.8%-59.8%]) remained largely unchanged between 2013 and 2018.
When they looked at the relationship between insurance type, income, and financial difficulties, some degree of financial hardship was common across all groups; private insurance: 63.8% [95% CI, 61.1%-66.6%]; with public insurance: 49.9% [95% CI, 46.4%-53.4%]; with no insurance: 81.8% [95% CI, 76.4%-87.3%]; with income < 400% of the federal poverty level (FPL): 65.5% [95% CI, 62.1%-66.9%]; with income at least 400% of the FPL: 49.3% [95% CI,44.7%-53.9%]).
Those without any insurance had the highest odds of reporting unmet health care needs (adjusted OR [aOR], 4.40; 95% CI, 3.23-6.00) and health care unaffordability (aOR, 5.18; 95% CI, 3.49-7.70) compared with women who received public insurance.
But while women with private insurance had lower odds of reporting unmet health care needs (aOR, 0.67; 95% CI, 0.52-0.87), they faced higher odds of reporting health care unaffordability (aOR, 1.88; 95% CI, 1.49-2.36) compared to women who had public insurance.
Those with household incomes of less than 400% of the FPL had higher odds of reporting unmet health care need (aOR,1.50; 95% CI, 1.08-2.08) and health care unaffordability (aOR, 1.98; 95% CI, 1.54-2.55) versus women whose household incomes were at least 400% of FPL. The odds of general financial stress did not significantly differ by insurance status/type or income level.
Weighing in on the data
Jamie Daw, PhD, assistant professor of health policy and management, Columbia University Mailman School of Public Health, New York, noted that many people think of private insurance as “good coverage.”
“But the portion of medical costs that patients are required to pay under private plans has risen dramatically over the past decade,” she said. “Over half of the U.S. workforce is now enrolled in high-deductible plans, where the average deductible was $4,500 in 2020. The private insurance of today does not provide sufficient financial protection for most families, who would need to have the liquid assets to cover childbirth.”
Another expert agreed that the high out-of-pocket costs for women with private health insurance were probably responsible for making peripartum health care more unaffordable. These included costs for pregnancy care as well as for maternal and infant care during and after childbirth.
“This study reporting the high unmet medical needs and unaffordability of health care for peripartum women further underscores that the U.S. health care system is not meeting the needs of pregnant women, mothers, and their newborn infants,” said Lois K. Lee, MD, associate professor of pediatrics and emergency medicine at Harvard Medical School and associate director for public policy at the Sandra L. Fenwick Institute for Pediatric Health Equity and Inclusion, Boston.
“It is imperative to optimize the health of pregnant mothers to optimize the health of infants, who are our future society,” she said. “Policies which would expand Medicaid coverage to a full 1-year postpartum across all states is one important strategy to improve health care access and affordability to peripartum women. However, this must be part of a multipronged approach addressing the social determinants of health, as insurance coverage alone will not fully address this important health issue of peripartum women, and their children.”
Dr Moniz reported receiving personal fees from the RAND Corporation, the Society of Family Planning outside the submitted work and grant K08 HS025465 from the Agency for Healthcare Research and Quality. Dr. Daw has no disclosures. Dr. Lee reports speaker fees from the American Academy of Pediatrics and SUNY Upstate Medical University. Coauthor Dr. Taylor was supported by the National Clinician Scholars Program at the University of Michigan. Dr Dalton was supported by grant R01 HS023784 from the Agency for Healthcare Research and Quality.
Financial hardship remains prevalent among pregnant and postpartum women, despite the implementation of the Affordable Care Act (ACA), according to new findings published in JAMA Network Open.
Nearly a quarter (24%) of pregnant and postpartum women reported having unmet health care needs, 60% had health care unaffordability, and 54% reported general financial stress. Notably, the type of insurance was associated with the ability to afford health care.
Those with private insurance, along with women with lower incomes, were more likely to experience unaffordable health care, compared to those covered by public insurance or who had higher incomes.
Senior study author Michelle H. Moniz, MD, assistant professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor, was surprised by multiple study findings. “The prevalence of financial hardship overall, and the three individual indicators of hardship, did not change over time from 2013 to 2018,” she said. “The ACA was enacted just prior to the study period, and while this policy had many benefits for women – especially around increasing insurance coverage – it does not seem to have improved financial hardship among pregnant and postpartum women.”
She emphasized that two groups were at the highest risk of health care unaffordability: those with private insurance and those living on low incomes. “This is notable, as we often think of private insurance as offering ‘Cadillac coverage,’ but our prior work suggests that privately insured women have strikingly high out-of-pocket costs for pregnancy and childbirth-related care,” Dr. Moniz said.
These expenses include deductibles, copays, and coinsurance payments, which come to about $4,500 on average. Medicaid plans, in contrast, have exceedingly low out-of-pocket costs for pregnant and postpartum women. “Findings from the current study call for targeted policy interventions to alleviate financial strain and remove financial barriers to health care access for privately insured families,” she said. “Similarly, families living on lower incomes were also at high risk of health care unaffordability. This may be because even small out-of-pocket costs, or health care–associated costs, account for a larger share of the family’s income.”
This finding for lower-income women calls for targeted policy interventions. “Sliding-scale deductibles, for example, are one solution that might mitigate economic hardship and remove cost-related barriers to health care for pregnant and postpartum women,” Dr. Moniz added.
Health care unaffordability high
In this study, Dr. Moniz and colleagues evaluated the prevalence of financial hardship among peripartum women over time, and how it was affected by their income level and the type of insurance coverage.
They conducted a cross-sectional study that included peripartum women between the ages of 18 and 45 years who reported being currently pregnant or pregnant in the past 12 months. The women were all participants in the National Health Interview Survey, which covers the period from 2013 to 2018, and the data were analyzed from January to May 2021.
The cohort included 3,509 peripartum women, and was weighted to represent 1,050,789 women, with a mean age of 29 years. In 2018, an estimated 39,017 of 184,018 (21.2%) were Black; 36,045 (19.6%) were Hispanic; and 97,366 (52.9%) were White. In the latter years of the study period, the participants tended to be older, more highly educated, and less likely to lack insurance.
When the authors compared the unadjusted reported financial hardship outcome by each study year, unmet health care need (2013: 27.9% [95% confidence interval, 24.4%-31.7%]; 2018: 23.7% [95% CI, 19.5%-28.6%]), health care unaffordability (2013: 65.7% [95% CI, 61.1%-70.0%]; 2018: 58.8% [95% CI, 53.4%-64.0%]), and general financial stress (2013: 60.6% [95% CI, 55.2%-65.8%]; 2018: 53.8% [95% CI, 47.8%-59.8%]) remained largely unchanged between 2013 and 2018.
When they looked at the relationship between insurance type, income, and financial difficulties, some degree of financial hardship was common across all groups; private insurance: 63.8% [95% CI, 61.1%-66.6%]; with public insurance: 49.9% [95% CI, 46.4%-53.4%]; with no insurance: 81.8% [95% CI, 76.4%-87.3%]; with income < 400% of the federal poverty level (FPL): 65.5% [95% CI, 62.1%-66.9%]; with income at least 400% of the FPL: 49.3% [95% CI,44.7%-53.9%]).
Those without any insurance had the highest odds of reporting unmet health care needs (adjusted OR [aOR], 4.40; 95% CI, 3.23-6.00) and health care unaffordability (aOR, 5.18; 95% CI, 3.49-7.70) compared with women who received public insurance.
But while women with private insurance had lower odds of reporting unmet health care needs (aOR, 0.67; 95% CI, 0.52-0.87), they faced higher odds of reporting health care unaffordability (aOR, 1.88; 95% CI, 1.49-2.36) compared to women who had public insurance.
Those with household incomes of less than 400% of the FPL had higher odds of reporting unmet health care need (aOR,1.50; 95% CI, 1.08-2.08) and health care unaffordability (aOR, 1.98; 95% CI, 1.54-2.55) versus women whose household incomes were at least 400% of FPL. The odds of general financial stress did not significantly differ by insurance status/type or income level.
Weighing in on the data
Jamie Daw, PhD, assistant professor of health policy and management, Columbia University Mailman School of Public Health, New York, noted that many people think of private insurance as “good coverage.”
“But the portion of medical costs that patients are required to pay under private plans has risen dramatically over the past decade,” she said. “Over half of the U.S. workforce is now enrolled in high-deductible plans, where the average deductible was $4,500 in 2020. The private insurance of today does not provide sufficient financial protection for most families, who would need to have the liquid assets to cover childbirth.”
Another expert agreed that the high out-of-pocket costs for women with private health insurance were probably responsible for making peripartum health care more unaffordable. These included costs for pregnancy care as well as for maternal and infant care during and after childbirth.
“This study reporting the high unmet medical needs and unaffordability of health care for peripartum women further underscores that the U.S. health care system is not meeting the needs of pregnant women, mothers, and their newborn infants,” said Lois K. Lee, MD, associate professor of pediatrics and emergency medicine at Harvard Medical School and associate director for public policy at the Sandra L. Fenwick Institute for Pediatric Health Equity and Inclusion, Boston.
“It is imperative to optimize the health of pregnant mothers to optimize the health of infants, who are our future society,” she said. “Policies which would expand Medicaid coverage to a full 1-year postpartum across all states is one important strategy to improve health care access and affordability to peripartum women. However, this must be part of a multipronged approach addressing the social determinants of health, as insurance coverage alone will not fully address this important health issue of peripartum women, and their children.”
Dr Moniz reported receiving personal fees from the RAND Corporation, the Society of Family Planning outside the submitted work and grant K08 HS025465 from the Agency for Healthcare Research and Quality. Dr. Daw has no disclosures. Dr. Lee reports speaker fees from the American Academy of Pediatrics and SUNY Upstate Medical University. Coauthor Dr. Taylor was supported by the National Clinician Scholars Program at the University of Michigan. Dr Dalton was supported by grant R01 HS023784 from the Agency for Healthcare Research and Quality.
Financial hardship remains prevalent among pregnant and postpartum women, despite the implementation of the Affordable Care Act (ACA), according to new findings published in JAMA Network Open.
Nearly a quarter (24%) of pregnant and postpartum women reported having unmet health care needs, 60% had health care unaffordability, and 54% reported general financial stress. Notably, the type of insurance was associated with the ability to afford health care.
Those with private insurance, along with women with lower incomes, were more likely to experience unaffordable health care, compared to those covered by public insurance or who had higher incomes.
Senior study author Michelle H. Moniz, MD, assistant professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor, was surprised by multiple study findings. “The prevalence of financial hardship overall, and the three individual indicators of hardship, did not change over time from 2013 to 2018,” she said. “The ACA was enacted just prior to the study period, and while this policy had many benefits for women – especially around increasing insurance coverage – it does not seem to have improved financial hardship among pregnant and postpartum women.”
She emphasized that two groups were at the highest risk of health care unaffordability: those with private insurance and those living on low incomes. “This is notable, as we often think of private insurance as offering ‘Cadillac coverage,’ but our prior work suggests that privately insured women have strikingly high out-of-pocket costs for pregnancy and childbirth-related care,” Dr. Moniz said.
These expenses include deductibles, copays, and coinsurance payments, which come to about $4,500 on average. Medicaid plans, in contrast, have exceedingly low out-of-pocket costs for pregnant and postpartum women. “Findings from the current study call for targeted policy interventions to alleviate financial strain and remove financial barriers to health care access for privately insured families,” she said. “Similarly, families living on lower incomes were also at high risk of health care unaffordability. This may be because even small out-of-pocket costs, or health care–associated costs, account for a larger share of the family’s income.”
This finding for lower-income women calls for targeted policy interventions. “Sliding-scale deductibles, for example, are one solution that might mitigate economic hardship and remove cost-related barriers to health care for pregnant and postpartum women,” Dr. Moniz added.
Health care unaffordability high
In this study, Dr. Moniz and colleagues evaluated the prevalence of financial hardship among peripartum women over time, and how it was affected by their income level and the type of insurance coverage.
They conducted a cross-sectional study that included peripartum women between the ages of 18 and 45 years who reported being currently pregnant or pregnant in the past 12 months. The women were all participants in the National Health Interview Survey, which covers the period from 2013 to 2018, and the data were analyzed from January to May 2021.
The cohort included 3,509 peripartum women, and was weighted to represent 1,050,789 women, with a mean age of 29 years. In 2018, an estimated 39,017 of 184,018 (21.2%) were Black; 36,045 (19.6%) were Hispanic; and 97,366 (52.9%) were White. In the latter years of the study period, the participants tended to be older, more highly educated, and less likely to lack insurance.
When the authors compared the unadjusted reported financial hardship outcome by each study year, unmet health care need (2013: 27.9% [95% confidence interval, 24.4%-31.7%]; 2018: 23.7% [95% CI, 19.5%-28.6%]), health care unaffordability (2013: 65.7% [95% CI, 61.1%-70.0%]; 2018: 58.8% [95% CI, 53.4%-64.0%]), and general financial stress (2013: 60.6% [95% CI, 55.2%-65.8%]; 2018: 53.8% [95% CI, 47.8%-59.8%]) remained largely unchanged between 2013 and 2018.
When they looked at the relationship between insurance type, income, and financial difficulties, some degree of financial hardship was common across all groups; private insurance: 63.8% [95% CI, 61.1%-66.6%]; with public insurance: 49.9% [95% CI, 46.4%-53.4%]; with no insurance: 81.8% [95% CI, 76.4%-87.3%]; with income < 400% of the federal poverty level (FPL): 65.5% [95% CI, 62.1%-66.9%]; with income at least 400% of the FPL: 49.3% [95% CI,44.7%-53.9%]).
Those without any insurance had the highest odds of reporting unmet health care needs (adjusted OR [aOR], 4.40; 95% CI, 3.23-6.00) and health care unaffordability (aOR, 5.18; 95% CI, 3.49-7.70) compared with women who received public insurance.
But while women with private insurance had lower odds of reporting unmet health care needs (aOR, 0.67; 95% CI, 0.52-0.87), they faced higher odds of reporting health care unaffordability (aOR, 1.88; 95% CI, 1.49-2.36) compared to women who had public insurance.
Those with household incomes of less than 400% of the FPL had higher odds of reporting unmet health care need (aOR,1.50; 95% CI, 1.08-2.08) and health care unaffordability (aOR, 1.98; 95% CI, 1.54-2.55) versus women whose household incomes were at least 400% of FPL. The odds of general financial stress did not significantly differ by insurance status/type or income level.
Weighing in on the data
Jamie Daw, PhD, assistant professor of health policy and management, Columbia University Mailman School of Public Health, New York, noted that many people think of private insurance as “good coverage.”
“But the portion of medical costs that patients are required to pay under private plans has risen dramatically over the past decade,” she said. “Over half of the U.S. workforce is now enrolled in high-deductible plans, where the average deductible was $4,500 in 2020. The private insurance of today does not provide sufficient financial protection for most families, who would need to have the liquid assets to cover childbirth.”
Another expert agreed that the high out-of-pocket costs for women with private health insurance were probably responsible for making peripartum health care more unaffordable. These included costs for pregnancy care as well as for maternal and infant care during and after childbirth.
“This study reporting the high unmet medical needs and unaffordability of health care for peripartum women further underscores that the U.S. health care system is not meeting the needs of pregnant women, mothers, and their newborn infants,” said Lois K. Lee, MD, associate professor of pediatrics and emergency medicine at Harvard Medical School and associate director for public policy at the Sandra L. Fenwick Institute for Pediatric Health Equity and Inclusion, Boston.
“It is imperative to optimize the health of pregnant mothers to optimize the health of infants, who are our future society,” she said. “Policies which would expand Medicaid coverage to a full 1-year postpartum across all states is one important strategy to improve health care access and affordability to peripartum women. However, this must be part of a multipronged approach addressing the social determinants of health, as insurance coverage alone will not fully address this important health issue of peripartum women, and their children.”
Dr Moniz reported receiving personal fees from the RAND Corporation, the Society of Family Planning outside the submitted work and grant K08 HS025465 from the Agency for Healthcare Research and Quality. Dr. Daw has no disclosures. Dr. Lee reports speaker fees from the American Academy of Pediatrics and SUNY Upstate Medical University. Coauthor Dr. Taylor was supported by the National Clinician Scholars Program at the University of Michigan. Dr Dalton was supported by grant R01 HS023784 from the Agency for Healthcare Research and Quality.
FROM JAMA NETWORK OPEN