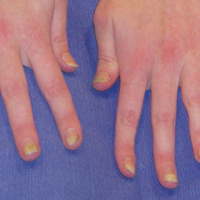
User login
Increasing Skin of Color Publications in the Dermatology Literature: A Call to Action
The US population is becoming more diverse. By 2044, it is predicted that there will be a majority minority population in the United States.1 Therefore, it is imperative to continue to develop educational mechanisms for all dermatologists to increase and maintain competency in skin of color dermatology, which will contribute to the achievement of health equity for patients with all skin tones and hair types.
Not only is clinical skin of color education necessary, but diversity, equity, and inclusion (DEI) education for dermatologists also is critical. Clinical examination,2 diagnosis, and treatment of skin and hair disorders across the skin of color spectrum with cultural humility is essential to achieve health equity. If trainees, dermatologists, other specialists, and primary care clinicians are not frequently exposed to patients with darker skin tones and coily hair, the nuances in diagnosing and treating these patients must be learned in alternate ways.
To ready the nation’s physicians and clinicians to care for the growing diverse population, exposure to more images of dermatologic diseases in those with darker skin tones in journal articles, textbooks, conference lectures, and online dermatology image libraries is necessary to help close the skin of color training and practice gap.3,4 The following initiatives demonstrate how Cutis has sought to address these educational gaps and remains committed to improving DEI education in dermatology.
Collaboration With the Skin of Color Society
The Skin of Color Society (SOCS), which was founded in 2004 by Dr. Susan C. Taylor, is a dermatologic organization with more than 800 members representing 32 countries. Its mission includes promoting awareness and excellence within skin of color dermatology through research, education, and mentorship. The SOCS has utilized strategic partnerships with national and international dermatologists, as well as professional medical organizations and community, industry, and corporate groups, to ultimately ensure that patients with skin of color receive the expert care they deserve.5 In 2017, Cutis published the inaugural article in its collaboration with the SOCS,6 and more articles, which undergo regular peer review, continue to be published quarterly (https://www.mdedge.com/dermatology/skin-color).
Increase Number of Journal Articles on Skin of Color Topics
Increasing the number of journal articles on skin of color–related topics needs to be intentional, as it is a tool that has been identified as a necessary part of enhancing awareness and subsequently improving patient care. Wilson et al7 used stringent criteria to review all articles published from January 2018 to October 2020 in 52 dermatology journals for inclusion of topics on skin of color, hair in patients with skin of color, diversity and inclusion, and socioeconomic and health care disparities in the skin of color population. The journals they reviewed included publications based on continents with majority skin of color populations, such as Asia, as well as those with minority skin of color populations, such as Europe. During the study period, the percentage of articles covering skin of color ranged from 2.04% to 61.8%, with an average of 16.8%.7
The total number of Cutis articles published during the study period was 709, with 132 (18.62%) meeting the investigators’ criteria for articles on skin of color; these included case reports in which at least 1 patient with skin of color was featured.7 Overall, Cutis ranked 16th of the 52 journals for inclusion of skin of color content. Cutis was one of only a few journals based in North America, a non–skin-of-color–predominant continent, to make the top 16 in this study.7
Some of the 132 skin of color articles published in Cutis were the result of the journal’s collaboration with the SOCS. Through this collaboration, articles were published on a variety of skin of color topics, including DEI (6), alopecia and hair care (5), dermoscopy/optical coherence tomography imaging (1), atopic dermatitis (1), cosmetics (1), hidradenitis suppurativa (1), pigmentation (1), rosacea (1), and skin cancer (2). These articles also resulted in a number of podcast discussions (https://www.mdedge.com/podcasts/dermatology-weekly), including one on dealing with DEI, one on pigmentation, and one on dermoscopy/optical coherence tomography imaging. The latter featured the SOCS Scientific Symposium poster winners in 2020.
The number of articles published specifically through Cutis’s collaboration with the SOCS accounted for only a small part of the journal’s 132 skin of color articles identified in the study by Wilson et al.7 We speculate that Cutis’s display of intentional commitment to supporting the inclusion of skin of color articles in the journal may in turn encourage its broader readership to submit more skin of color–focused articles for peer review.
Wilson et al7 specifically remarked that “Cutis’s [Skin of Color] section in each issue is a promising idea.” They also highlighted Clinics in Dermatology for committing an entire issue to skin of color; however, despite this initiative, Clinics in Dermatology still ranked 35th of 52 journals with regard to the overall percentage of skin of color articles published.7 This suggests that a journal publishing one special issue on skin of color annually is a helpful addition to the literature, but increasing the number of articles related to skin of color in each journal issue, similar to Cutis, will ultimately result in a higher overall number of skin of color articles in the dermatology literature.
Both Amuzie et al4 and Wilson et al7 concluded that the higher a journal’s impact factor, the lower the number of skin of color articles published.However, skin of color articles published in high-impact journals received a higher number of citations than those in other lower-impact journals.4 High-impact journals may use Cutis as a model for increasing the number of skin of color articles they publish, which will have a notable impact on increasing skin of color knowledge and educating dermatologists.
Coverage of Diversity, Equity, and Inclusion
In another study, Bray et al8 conducted a PubMed search of articles indexed for MEDLINE from January 2008 to July 2019 to quantify the number of articles specifically focused on DEI in a variety of medical specialties. The field of dermatology had the highest number of articles published on DEI (25) compared to the other specialties, including family medicine (23), orthopedic surgery (12), internal medicine (9), general surgery (7), radiology (6), ophthalmology (2), and anesthesiology (2).8 However, Wilson et al7 found that, out of all the categories of skin of color articles published in dermatology journals during their study period, those focused on DEI made up less than 1% of the total number of articles. Dermatology is off to a great start compared to other specialties, but there is still more work to do in dermatology for DEI. Cutis’s collaboration with the SOCS has resulted in 6 DEI articles published since 2017.
Think Beyond Dermatology Education
The collaboration between Cutis and the SOCS was established to create a series of articles dedicated to increasing the skin of color dermatology knowledge base of the Cutis readership and beyond; however, increased readership and more citations are needed to amplify the reach of the articles published by these skin of color experts. Cutis’s collaboration with SOCS is one mechanism to increase the skin of color literature, but skin of color and DEI articles outside of this collaboration should continue to be published in each issue of Cutis.
The collaboration between SOCS and Cutis was and continues to be a forward-thinking step toward improving skin of color dermatology education, but there is still work to be done across the medical literature with regard to increasing intentional publication of skin of color articles. Nondermatologist clinicians in the Cutis readership benefit from knowledge of skin of color, as all specialties and primary care will see increased patient diversity in their examination rooms.
To further ensure that primary care is not left behind, Cutis has partnered with The Journal of Family Practice to produce a new column called Dx Across the Skin of Color Spectrum (https://www.mdedge.com/dermatology/dx-across-skin-color-spectrum), which is co-published in both journals.9,10 These one-page fact sheets highlight images of dermatologic conditions in skin of color as well as images of the same condition in lighter skin, a concept suggested by Cutis Associate Editor, Dr. Candrice R. Heath. The goal of this new column is to increase the accurate diagnosis of dermatologic conditions in skin of color and to highlight health disparities related to a particular condition in an easy-to-understand format. Uniquely, Dr. Heath co-authors this content with family physician Dr. Richard P. Usatine.
Final Thoughts
The entire community of medical journals should continue to develop creative ways to educate their readership. Medical professionals stay up-to-date on best practices through journal articles, textbooks, conferences, and even podcasts. Therefore, it is best to incorporate skin of color knowledge throughout all educational programming, particularly through enduring materials such as journal articles. Wilson et al7 suggested that a minimum of 16.8% of a dermatology journal’s articles in each issue should focus on skin of color in addition to special focus issues, as this will work toward more equitable dermatologic care.
Knowledge is only part of the equation; compassionate care with cultural humility is the other part. Publishing scientific facts about biology and structure, diagnosis, and treatment selection in skin of color, as well as committing to lifelong learning about the differences in our patients despite the absence of shared life or cultural experiences, may be the key to truly impacting health equity.11 We believe that together we will get there one journal article and one citation at a time.
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed August 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Amuzie AU, Jia JL, Taylor SC, et al. Skin-of-color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Learn more about SOCS. Skin of Color Society website. Accessed August 11, 2021. https://skinofcolorsociety.org/about-socs/
- Subash J, Tull R, McMichael A. Diversity in dermatology: a society devoted to skin of color. Cutis. 2017;99:322-324.
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of colorand diversity and inclusion content of dermatologic published literature: an analysis and call to action [published online April 20, 2021]. Int J Womens Dermatol. https://doi.org/10.1016/j.ijwd.2021.04.001
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:13030/qt094243gp.
- Heath CR, Usatine R. Atopic dermatitis. Cutis. 2021;107:332. doi:10.12788/cutis.0274
- Heath CR, Usatine R. Psoriasis. Cutis. 2021;108:56. doi:10.12788/cutis.0298
- Jones N, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships [published online August 3, 2021]. Pediatr Dermatol. doi:10.1111/pde.14721
The US population is becoming more diverse. By 2044, it is predicted that there will be a majority minority population in the United States.1 Therefore, it is imperative to continue to develop educational mechanisms for all dermatologists to increase and maintain competency in skin of color dermatology, which will contribute to the achievement of health equity for patients with all skin tones and hair types.
Not only is clinical skin of color education necessary, but diversity, equity, and inclusion (DEI) education for dermatologists also is critical. Clinical examination,2 diagnosis, and treatment of skin and hair disorders across the skin of color spectrum with cultural humility is essential to achieve health equity. If trainees, dermatologists, other specialists, and primary care clinicians are not frequently exposed to patients with darker skin tones and coily hair, the nuances in diagnosing and treating these patients must be learned in alternate ways.
To ready the nation’s physicians and clinicians to care for the growing diverse population, exposure to more images of dermatologic diseases in those with darker skin tones in journal articles, textbooks, conference lectures, and online dermatology image libraries is necessary to help close the skin of color training and practice gap.3,4 The following initiatives demonstrate how Cutis has sought to address these educational gaps and remains committed to improving DEI education in dermatology.
Collaboration With the Skin of Color Society
The Skin of Color Society (SOCS), which was founded in 2004 by Dr. Susan C. Taylor, is a dermatologic organization with more than 800 members representing 32 countries. Its mission includes promoting awareness and excellence within skin of color dermatology through research, education, and mentorship. The SOCS has utilized strategic partnerships with national and international dermatologists, as well as professional medical organizations and community, industry, and corporate groups, to ultimately ensure that patients with skin of color receive the expert care they deserve.5 In 2017, Cutis published the inaugural article in its collaboration with the SOCS,6 and more articles, which undergo regular peer review, continue to be published quarterly (https://www.mdedge.com/dermatology/skin-color).
Increase Number of Journal Articles on Skin of Color Topics
Increasing the number of journal articles on skin of color–related topics needs to be intentional, as it is a tool that has been identified as a necessary part of enhancing awareness and subsequently improving patient care. Wilson et al7 used stringent criteria to review all articles published from January 2018 to October 2020 in 52 dermatology journals for inclusion of topics on skin of color, hair in patients with skin of color, diversity and inclusion, and socioeconomic and health care disparities in the skin of color population. The journals they reviewed included publications based on continents with majority skin of color populations, such as Asia, as well as those with minority skin of color populations, such as Europe. During the study period, the percentage of articles covering skin of color ranged from 2.04% to 61.8%, with an average of 16.8%.7
The total number of Cutis articles published during the study period was 709, with 132 (18.62%) meeting the investigators’ criteria for articles on skin of color; these included case reports in which at least 1 patient with skin of color was featured.7 Overall, Cutis ranked 16th of the 52 journals for inclusion of skin of color content. Cutis was one of only a few journals based in North America, a non–skin-of-color–predominant continent, to make the top 16 in this study.7
Some of the 132 skin of color articles published in Cutis were the result of the journal’s collaboration with the SOCS. Through this collaboration, articles were published on a variety of skin of color topics, including DEI (6), alopecia and hair care (5), dermoscopy/optical coherence tomography imaging (1), atopic dermatitis (1), cosmetics (1), hidradenitis suppurativa (1), pigmentation (1), rosacea (1), and skin cancer (2). These articles also resulted in a number of podcast discussions (https://www.mdedge.com/podcasts/dermatology-weekly), including one on dealing with DEI, one on pigmentation, and one on dermoscopy/optical coherence tomography imaging. The latter featured the SOCS Scientific Symposium poster winners in 2020.
The number of articles published specifically through Cutis’s collaboration with the SOCS accounted for only a small part of the journal’s 132 skin of color articles identified in the study by Wilson et al.7 We speculate that Cutis’s display of intentional commitment to supporting the inclusion of skin of color articles in the journal may in turn encourage its broader readership to submit more skin of color–focused articles for peer review.
Wilson et al7 specifically remarked that “Cutis’s [Skin of Color] section in each issue is a promising idea.” They also highlighted Clinics in Dermatology for committing an entire issue to skin of color; however, despite this initiative, Clinics in Dermatology still ranked 35th of 52 journals with regard to the overall percentage of skin of color articles published.7 This suggests that a journal publishing one special issue on skin of color annually is a helpful addition to the literature, but increasing the number of articles related to skin of color in each journal issue, similar to Cutis, will ultimately result in a higher overall number of skin of color articles in the dermatology literature.
Both Amuzie et al4 and Wilson et al7 concluded that the higher a journal’s impact factor, the lower the number of skin of color articles published.However, skin of color articles published in high-impact journals received a higher number of citations than those in other lower-impact journals.4 High-impact journals may use Cutis as a model for increasing the number of skin of color articles they publish, which will have a notable impact on increasing skin of color knowledge and educating dermatologists.
Coverage of Diversity, Equity, and Inclusion
In another study, Bray et al8 conducted a PubMed search of articles indexed for MEDLINE from January 2008 to July 2019 to quantify the number of articles specifically focused on DEI in a variety of medical specialties. The field of dermatology had the highest number of articles published on DEI (25) compared to the other specialties, including family medicine (23), orthopedic surgery (12), internal medicine (9), general surgery (7), radiology (6), ophthalmology (2), and anesthesiology (2).8 However, Wilson et al7 found that, out of all the categories of skin of color articles published in dermatology journals during their study period, those focused on DEI made up less than 1% of the total number of articles. Dermatology is off to a great start compared to other specialties, but there is still more work to do in dermatology for DEI. Cutis’s collaboration with the SOCS has resulted in 6 DEI articles published since 2017.
Think Beyond Dermatology Education
The collaboration between Cutis and the SOCS was established to create a series of articles dedicated to increasing the skin of color dermatology knowledge base of the Cutis readership and beyond; however, increased readership and more citations are needed to amplify the reach of the articles published by these skin of color experts. Cutis’s collaboration with SOCS is one mechanism to increase the skin of color literature, but skin of color and DEI articles outside of this collaboration should continue to be published in each issue of Cutis.
The collaboration between SOCS and Cutis was and continues to be a forward-thinking step toward improving skin of color dermatology education, but there is still work to be done across the medical literature with regard to increasing intentional publication of skin of color articles. Nondermatologist clinicians in the Cutis readership benefit from knowledge of skin of color, as all specialties and primary care will see increased patient diversity in their examination rooms.
To further ensure that primary care is not left behind, Cutis has partnered with The Journal of Family Practice to produce a new column called Dx Across the Skin of Color Spectrum (https://www.mdedge.com/dermatology/dx-across-skin-color-spectrum), which is co-published in both journals.9,10 These one-page fact sheets highlight images of dermatologic conditions in skin of color as well as images of the same condition in lighter skin, a concept suggested by Cutis Associate Editor, Dr. Candrice R. Heath. The goal of this new column is to increase the accurate diagnosis of dermatologic conditions in skin of color and to highlight health disparities related to a particular condition in an easy-to-understand format. Uniquely, Dr. Heath co-authors this content with family physician Dr. Richard P. Usatine.
Final Thoughts
The entire community of medical journals should continue to develop creative ways to educate their readership. Medical professionals stay up-to-date on best practices through journal articles, textbooks, conferences, and even podcasts. Therefore, it is best to incorporate skin of color knowledge throughout all educational programming, particularly through enduring materials such as journal articles. Wilson et al7 suggested that a minimum of 16.8% of a dermatology journal’s articles in each issue should focus on skin of color in addition to special focus issues, as this will work toward more equitable dermatologic care.
Knowledge is only part of the equation; compassionate care with cultural humility is the other part. Publishing scientific facts about biology and structure, diagnosis, and treatment selection in skin of color, as well as committing to lifelong learning about the differences in our patients despite the absence of shared life or cultural experiences, may be the key to truly impacting health equity.11 We believe that together we will get there one journal article and one citation at a time.
The US population is becoming more diverse. By 2044, it is predicted that there will be a majority minority population in the United States.1 Therefore, it is imperative to continue to develop educational mechanisms for all dermatologists to increase and maintain competency in skin of color dermatology, which will contribute to the achievement of health equity for patients with all skin tones and hair types.
Not only is clinical skin of color education necessary, but diversity, equity, and inclusion (DEI) education for dermatologists also is critical. Clinical examination,2 diagnosis, and treatment of skin and hair disorders across the skin of color spectrum with cultural humility is essential to achieve health equity. If trainees, dermatologists, other specialists, and primary care clinicians are not frequently exposed to patients with darker skin tones and coily hair, the nuances in diagnosing and treating these patients must be learned in alternate ways.
To ready the nation’s physicians and clinicians to care for the growing diverse population, exposure to more images of dermatologic diseases in those with darker skin tones in journal articles, textbooks, conference lectures, and online dermatology image libraries is necessary to help close the skin of color training and practice gap.3,4 The following initiatives demonstrate how Cutis has sought to address these educational gaps and remains committed to improving DEI education in dermatology.
Collaboration With the Skin of Color Society
The Skin of Color Society (SOCS), which was founded in 2004 by Dr. Susan C. Taylor, is a dermatologic organization with more than 800 members representing 32 countries. Its mission includes promoting awareness and excellence within skin of color dermatology through research, education, and mentorship. The SOCS has utilized strategic partnerships with national and international dermatologists, as well as professional medical organizations and community, industry, and corporate groups, to ultimately ensure that patients with skin of color receive the expert care they deserve.5 In 2017, Cutis published the inaugural article in its collaboration with the SOCS,6 and more articles, which undergo regular peer review, continue to be published quarterly (https://www.mdedge.com/dermatology/skin-color).
Increase Number of Journal Articles on Skin of Color Topics
Increasing the number of journal articles on skin of color–related topics needs to be intentional, as it is a tool that has been identified as a necessary part of enhancing awareness and subsequently improving patient care. Wilson et al7 used stringent criteria to review all articles published from January 2018 to October 2020 in 52 dermatology journals for inclusion of topics on skin of color, hair in patients with skin of color, diversity and inclusion, and socioeconomic and health care disparities in the skin of color population. The journals they reviewed included publications based on continents with majority skin of color populations, such as Asia, as well as those with minority skin of color populations, such as Europe. During the study period, the percentage of articles covering skin of color ranged from 2.04% to 61.8%, with an average of 16.8%.7
The total number of Cutis articles published during the study period was 709, with 132 (18.62%) meeting the investigators’ criteria for articles on skin of color; these included case reports in which at least 1 patient with skin of color was featured.7 Overall, Cutis ranked 16th of the 52 journals for inclusion of skin of color content. Cutis was one of only a few journals based in North America, a non–skin-of-color–predominant continent, to make the top 16 in this study.7
Some of the 132 skin of color articles published in Cutis were the result of the journal’s collaboration with the SOCS. Through this collaboration, articles were published on a variety of skin of color topics, including DEI (6), alopecia and hair care (5), dermoscopy/optical coherence tomography imaging (1), atopic dermatitis (1), cosmetics (1), hidradenitis suppurativa (1), pigmentation (1), rosacea (1), and skin cancer (2). These articles also resulted in a number of podcast discussions (https://www.mdedge.com/podcasts/dermatology-weekly), including one on dealing with DEI, one on pigmentation, and one on dermoscopy/optical coherence tomography imaging. The latter featured the SOCS Scientific Symposium poster winners in 2020.
The number of articles published specifically through Cutis’s collaboration with the SOCS accounted for only a small part of the journal’s 132 skin of color articles identified in the study by Wilson et al.7 We speculate that Cutis’s display of intentional commitment to supporting the inclusion of skin of color articles in the journal may in turn encourage its broader readership to submit more skin of color–focused articles for peer review.
Wilson et al7 specifically remarked that “Cutis’s [Skin of Color] section in each issue is a promising idea.” They also highlighted Clinics in Dermatology for committing an entire issue to skin of color; however, despite this initiative, Clinics in Dermatology still ranked 35th of 52 journals with regard to the overall percentage of skin of color articles published.7 This suggests that a journal publishing one special issue on skin of color annually is a helpful addition to the literature, but increasing the number of articles related to skin of color in each journal issue, similar to Cutis, will ultimately result in a higher overall number of skin of color articles in the dermatology literature.
Both Amuzie et al4 and Wilson et al7 concluded that the higher a journal’s impact factor, the lower the number of skin of color articles published.However, skin of color articles published in high-impact journals received a higher number of citations than those in other lower-impact journals.4 High-impact journals may use Cutis as a model for increasing the number of skin of color articles they publish, which will have a notable impact on increasing skin of color knowledge and educating dermatologists.
Coverage of Diversity, Equity, and Inclusion
In another study, Bray et al8 conducted a PubMed search of articles indexed for MEDLINE from January 2008 to July 2019 to quantify the number of articles specifically focused on DEI in a variety of medical specialties. The field of dermatology had the highest number of articles published on DEI (25) compared to the other specialties, including family medicine (23), orthopedic surgery (12), internal medicine (9), general surgery (7), radiology (6), ophthalmology (2), and anesthesiology (2).8 However, Wilson et al7 found that, out of all the categories of skin of color articles published in dermatology journals during their study period, those focused on DEI made up less than 1% of the total number of articles. Dermatology is off to a great start compared to other specialties, but there is still more work to do in dermatology for DEI. Cutis’s collaboration with the SOCS has resulted in 6 DEI articles published since 2017.
Think Beyond Dermatology Education
The collaboration between Cutis and the SOCS was established to create a series of articles dedicated to increasing the skin of color dermatology knowledge base of the Cutis readership and beyond; however, increased readership and more citations are needed to amplify the reach of the articles published by these skin of color experts. Cutis’s collaboration with SOCS is one mechanism to increase the skin of color literature, but skin of color and DEI articles outside of this collaboration should continue to be published in each issue of Cutis.
The collaboration between SOCS and Cutis was and continues to be a forward-thinking step toward improving skin of color dermatology education, but there is still work to be done across the medical literature with regard to increasing intentional publication of skin of color articles. Nondermatologist clinicians in the Cutis readership benefit from knowledge of skin of color, as all specialties and primary care will see increased patient diversity in their examination rooms.
To further ensure that primary care is not left behind, Cutis has partnered with The Journal of Family Practice to produce a new column called Dx Across the Skin of Color Spectrum (https://www.mdedge.com/dermatology/dx-across-skin-color-spectrum), which is co-published in both journals.9,10 These one-page fact sheets highlight images of dermatologic conditions in skin of color as well as images of the same condition in lighter skin, a concept suggested by Cutis Associate Editor, Dr. Candrice R. Heath. The goal of this new column is to increase the accurate diagnosis of dermatologic conditions in skin of color and to highlight health disparities related to a particular condition in an easy-to-understand format. Uniquely, Dr. Heath co-authors this content with family physician Dr. Richard P. Usatine.
Final Thoughts
The entire community of medical journals should continue to develop creative ways to educate their readership. Medical professionals stay up-to-date on best practices through journal articles, textbooks, conferences, and even podcasts. Therefore, it is best to incorporate skin of color knowledge throughout all educational programming, particularly through enduring materials such as journal articles. Wilson et al7 suggested that a minimum of 16.8% of a dermatology journal’s articles in each issue should focus on skin of color in addition to special focus issues, as this will work toward more equitable dermatologic care.
Knowledge is only part of the equation; compassionate care with cultural humility is the other part. Publishing scientific facts about biology and structure, diagnosis, and treatment selection in skin of color, as well as committing to lifelong learning about the differences in our patients despite the absence of shared life or cultural experiences, may be the key to truly impacting health equity.11 We believe that together we will get there one journal article and one citation at a time.
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed August 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Amuzie AU, Jia JL, Taylor SC, et al. Skin-of-color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Learn more about SOCS. Skin of Color Society website. Accessed August 11, 2021. https://skinofcolorsociety.org/about-socs/
- Subash J, Tull R, McMichael A. Diversity in dermatology: a society devoted to skin of color. Cutis. 2017;99:322-324.
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of colorand diversity and inclusion content of dermatologic published literature: an analysis and call to action [published online April 20, 2021]. Int J Womens Dermatol. https://doi.org/10.1016/j.ijwd.2021.04.001
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:13030/qt094243gp.
- Heath CR, Usatine R. Atopic dermatitis. Cutis. 2021;107:332. doi:10.12788/cutis.0274
- Heath CR, Usatine R. Psoriasis. Cutis. 2021;108:56. doi:10.12788/cutis.0298
- Jones N, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships [published online August 3, 2021]. Pediatr Dermatol. doi:10.1111/pde.14721
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed August 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Amuzie AU, Jia JL, Taylor SC, et al. Skin-of-color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Learn more about SOCS. Skin of Color Society website. Accessed August 11, 2021. https://skinofcolorsociety.org/about-socs/
- Subash J, Tull R, McMichael A. Diversity in dermatology: a society devoted to skin of color. Cutis. 2017;99:322-324.
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of colorand diversity and inclusion content of dermatologic published literature: an analysis and call to action [published online April 20, 2021]. Int J Womens Dermatol. https://doi.org/10.1016/j.ijwd.2021.04.001
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:13030/qt094243gp.
- Heath CR, Usatine R. Atopic dermatitis. Cutis. 2021;107:332. doi:10.12788/cutis.0274
- Heath CR, Usatine R. Psoriasis. Cutis. 2021;108:56. doi:10.12788/cutis.0298
- Jones N, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships [published online August 3, 2021]. Pediatr Dermatol. doi:10.1111/pde.14721
Practice Points
- Submitting more articles related to skin of color for peer review and publication will increase educational opportunities.
- Journals that publish skin of color articles play a critical role in reducing educational gaps and ultimately help improve patient care for those with skin of color.
Sunscreen Regulations and Advice for Your Patients
If by now you have not had a patient ask, “Doctor, what sunscreen should I use NOW?” you will soon.
The US Food and Drug Administration (FDA) recently published a press release detailing a proposed rule on how manufacturers will be required to test and label sunscreens in the United States.1,2 Although the press release was complicated and contained much information, the media specifically latched onto the FDA’s consideration of only 2 active sunscreen ingredients—zinc oxide and titanium dioxide—as generally recognized as safe and effective (GRASE). In response, some patients may assume that most sunscreens on the market are dangerous.
How did this new proposed rule come about? To understand the process, it takes some explanation of the history of the FDA’s regulation of sunscreens.
How are sunscreens regulated by the FDA?
The regulatory process for sunscreens in the United States is complicated. The FDA regulates sunscreens as over-the-counter (OTC) drugs rather than as cosmetics, which is how they are regulated in most of the rest of the world.
The US sunscreen regulation process began in 1978 with an advance notice of proposed rulemaking from the FDA that included recommendations from an advisory review panel on the safe and effective use of OTC sunscreen products.3 At that time, 21 active sunscreen ingredients and their maximum use concentrations were listed and determined to be safe, or GRASE. It also gave manufacturers guidance on how to test for efficacy with the methodology for determining the sun protection factor (SPF) as well as various labeling requirements. Over the years, the FDA has issued a number of other sunscreen guidelines, such as removing padimate A and adding avobenzone and zinc oxide to the list of GRASE ingredients in the 1990s.4,5
In 1999, the FDA issued a final rule that listed 16 active sunscreen ingredients and concentrations as GRASE.6 There were some restrictions as to certain combinations of ingredients that could not be used in a finished product. Labeling requirements, including a maximum SPF of 30, also were put in place. This final rule established a final sunscreen monograph that was supposed to have been effective by 2002; however, in 2001 the agency delayed the effective date indefinitely because they had not yet established broad-spectrum (UVA) protection testing and labeling.7
The FDA published a proposed rule in 2007 as well as a final rule in 2011 that again listed the same 16 ingredients as GRASE and specified labeling and testing methods for establishing SPF, broad-spectrum protection, and water-resistance claims.8,9 The final rule limited product labels to a maximum SPF of 50+; provided directions for use with regard to other labeling elements (eg, warnings); and identified specific claims that would not be allowed on product labels, such as “waterproof” and “all-day protection.”9
Nevertheless, an effective final OTC monograph for sunscreen products has not yet been published.
What is the Sunscreen Innovation Act?
In 2014, the US Congress enacted the Sunscreen Innovation Act10 primarily to mandate that the FDA develop a more efficient way to determine the safety and efficacy of new active sunscreen ingredients that were commonly used in Europe and other parts of the world at the time. Many of these agents were thought to be more protective in the UVA and/or UVB spectrum, and if added to the list of GRASE ingredients available to US manufacturers, they would lead to the development of products that would improve the protection offered by sunscreens marketed to US consumers. The time and extent application (TEA) was established, a method that allowed manufacturers to apply for FDA approval of specific agents. The TEA also suggested allowing data generated in other countries where these agents were already in use for years to be considered in the FDA’s evaluation of the agents as GRASE. In addition, Congress mandated that a final monograph on OTC sunscreens be published by the end of 2019. A number of manufacturers have submitted TEAs for new active sunscreen ingredients, and so far, all have been rejected.
Why is the FDA interested in more safety data?
Since then, the FDA has become concerned not only with the safety and efficacy of newly proposed agents through the TEA but also with the original 16 active sunscreen ingredients listed as GRASE in the 2011 final rule. In the 1970s and 1980s, sunscreen use was limited to beach vacations or outdoor sporting events, but sun-protective behaviors have changed dramatically since that time, with health care providers now becoming cognizant of the growing threats of skin cancer and melanoma as well as the cosmetic concerns of photoaging, thereby recommending daily sunscreen use to their patients. In addition, the science behind sunscreens with higher concentrations of active ingredients intended to achieve higher and higher SPFs and their respective penetration of the skin has evolved, leading to new concerns about systemic toxicity. Early limited research frequently touted by the lay media has suggested that some of these agents might lead to hormonal changes, reproductive toxicity, and carcinogenicity.
In November 2016, the FDA issued a guidance for manufacturers that outlined the safety data that would be required to establish an OTC sunscreen active ingredient as GRASE.11 It also provided detailed information about both clinical and nonclinical safety testing, including human irritation and sensitization studies as well as human photosafety studies. In vitro dermal and systemic carcinogenicity studies and animal developmental and reproductive toxicity studies also were required as well studies regarding safety in children.
Many of these recommendations were already being utilized by manufacturers; however, one important change was the requirement for human absorption studies by a maximal usage trial, which more accurately addresses the absorption of sunscreen agents according to actual use. Such studies will be required at the highest allowable concentration of an agent in multiple vehicles and over large body surface areas for considerable exposure times.
This guidance to sunscreen manufacturers was announced to the public in a press release in May 2018.12
What are the new regulations?
All of this has culminated in the recent proposed rule, which includes several important proposals2:
- Of the 16 currently marketed active sunscreen ingredients, only 2—zinc oxide and titanium dioxide—are considered GRASE. Two ingredients—trolamine salicylate and para-aminobenzoic acid—are considered non-GRASE, but there is not enough information at this time to determine if the remaining 12 ingredients are GRASE. The FDA is working with manufacturers to obtain sufficient information to make this determination.
- Approved dosage formulations include sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks. Further information is needed regarding powders before they can be considered.
- The maximum SPF will be increased from 50+ to 60+.
- Sunscreens with an SPF of 15 or higher are required to provide broad-spectrum protection commensurate with the SPF, expanding on critical wavelength testing.
- There are new labeling changes, including a requirement that active ingredients be listed on the front of the packaging.
- Sunscreen products that contain insect repellents are considered non-GRASE.
What’s next?
The process for the proposed final rule has now entered a 90-day public comment period that will end on May 27, 2019; however, it is unlikely that a final monograph as mandated by Congress will be produced by the end of this year.
Sunscreen manufacturers currently are coordinating a response to the proposed rule through the Personal Care Products Council and the Consumer Healthcare Products Association Sunscreen Task Force. It is likely that the new required testing will be costly, with estimates exceeding tens or even hundreds of millions of dollars. In all likelihood, the number of active ingredients that the industry will agree to support with costly testing will be fewer than the 12 that are now on the list. It also is likely that this process will lead to fewer sunscreen products for consumers to choose from and almost certainly at a higher cost.
What do we tell patients in the meantime?
According to the FDA’s rules, it was necessary that this process was made public, but it will almost certainly concern our patients as to the safety of the sunscreen products they have been using. We should be concerned that some of our patients may limit their use of sunscreens because of safety concerns.
There is no question that, as physicians, we want to “first, do no harm,” so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data. The good news is that when this process is completed, a large number of agents will likely be found to be GRASE. When the FDA finally gives its imprimatur to sunscreens, it will hopefully help to silence those naysayers who report that sunscreens are dangerous for consumers; however, it has been suggested by some in industry that the new testing required may take at least 5 years.
What should dermatologists do when we are asked, “What sunscreen should I use NOW?” For most patients, I would explain the regulatory process and assure them that the risk-benefit ratio at this point suggests they should continue using the same sunscreens that they are currently using. For special situations such as pregnant women and children, it may be best to suggest products that contain only the 2 GRASE inorganic agents.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective [news release]. Silver Spring, MD: US Food and Drug Administration; February 21, 2019. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm631736.htm. Accessed April 4, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 1978;43(166):38206-38269. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph. Fed Registr. 1996;60(180):48645-48655. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph; enforcement policy. Fed Registr. 1998;63(204):56584-56589. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; final monograph. Fed Registr. 1999;64(98):27666-27693. To be codified at 21 CFR §310, 352, 700, and 740.
- Sunscreen drug products for over-the-counter human use; final monograph; partial stay; final rule. Fed Registr. 2001;66:67485-67487. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; proposed amendment of final monograph. Fed Registr. 2007;72(165):49069-49122. To be codified at 21 CFR §347 and 352.
- Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed Registr. 2011;76(117):35619-35665. To be codified at 21 CFR §201 and 310.
- Sunscreen Innovation Act, S 2141, 113th Cong, 2nd Sess (2014).
- Nonprescription sunscreen drug products-safety and effectiveness data; guidance for industry; availability. Fed Registr. 2016;81(226):84594-84595.
- Statement from Commissioner Scott Gottlieb, MD, on new FDA actions to keep consumers safe from the harmful effects of sun exposure, and ensure the long-term safety and benefits of sunscreens [news release]. Silver Spring, MD: US Food and Drug Administration; May 22, 2018. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm608499.htm. Accessed April 5, 2019.
If by now you have not had a patient ask, “Doctor, what sunscreen should I use NOW?” you will soon.
The US Food and Drug Administration (FDA) recently published a press release detailing a proposed rule on how manufacturers will be required to test and label sunscreens in the United States.1,2 Although the press release was complicated and contained much information, the media specifically latched onto the FDA’s consideration of only 2 active sunscreen ingredients—zinc oxide and titanium dioxide—as generally recognized as safe and effective (GRASE). In response, some patients may assume that most sunscreens on the market are dangerous.
How did this new proposed rule come about? To understand the process, it takes some explanation of the history of the FDA’s regulation of sunscreens.
How are sunscreens regulated by the FDA?
The regulatory process for sunscreens in the United States is complicated. The FDA regulates sunscreens as over-the-counter (OTC) drugs rather than as cosmetics, which is how they are regulated in most of the rest of the world.
The US sunscreen regulation process began in 1978 with an advance notice of proposed rulemaking from the FDA that included recommendations from an advisory review panel on the safe and effective use of OTC sunscreen products.3 At that time, 21 active sunscreen ingredients and their maximum use concentrations were listed and determined to be safe, or GRASE. It also gave manufacturers guidance on how to test for efficacy with the methodology for determining the sun protection factor (SPF) as well as various labeling requirements. Over the years, the FDA has issued a number of other sunscreen guidelines, such as removing padimate A and adding avobenzone and zinc oxide to the list of GRASE ingredients in the 1990s.4,5
In 1999, the FDA issued a final rule that listed 16 active sunscreen ingredients and concentrations as GRASE.6 There were some restrictions as to certain combinations of ingredients that could not be used in a finished product. Labeling requirements, including a maximum SPF of 30, also were put in place. This final rule established a final sunscreen monograph that was supposed to have been effective by 2002; however, in 2001 the agency delayed the effective date indefinitely because they had not yet established broad-spectrum (UVA) protection testing and labeling.7
The FDA published a proposed rule in 2007 as well as a final rule in 2011 that again listed the same 16 ingredients as GRASE and specified labeling and testing methods for establishing SPF, broad-spectrum protection, and water-resistance claims.8,9 The final rule limited product labels to a maximum SPF of 50+; provided directions for use with regard to other labeling elements (eg, warnings); and identified specific claims that would not be allowed on product labels, such as “waterproof” and “all-day protection.”9
Nevertheless, an effective final OTC monograph for sunscreen products has not yet been published.
What is the Sunscreen Innovation Act?
In 2014, the US Congress enacted the Sunscreen Innovation Act10 primarily to mandate that the FDA develop a more efficient way to determine the safety and efficacy of new active sunscreen ingredients that were commonly used in Europe and other parts of the world at the time. Many of these agents were thought to be more protective in the UVA and/or UVB spectrum, and if added to the list of GRASE ingredients available to US manufacturers, they would lead to the development of products that would improve the protection offered by sunscreens marketed to US consumers. The time and extent application (TEA) was established, a method that allowed manufacturers to apply for FDA approval of specific agents. The TEA also suggested allowing data generated in other countries where these agents were already in use for years to be considered in the FDA’s evaluation of the agents as GRASE. In addition, Congress mandated that a final monograph on OTC sunscreens be published by the end of 2019. A number of manufacturers have submitted TEAs for new active sunscreen ingredients, and so far, all have been rejected.
Why is the FDA interested in more safety data?
Since then, the FDA has become concerned not only with the safety and efficacy of newly proposed agents through the TEA but also with the original 16 active sunscreen ingredients listed as GRASE in the 2011 final rule. In the 1970s and 1980s, sunscreen use was limited to beach vacations or outdoor sporting events, but sun-protective behaviors have changed dramatically since that time, with health care providers now becoming cognizant of the growing threats of skin cancer and melanoma as well as the cosmetic concerns of photoaging, thereby recommending daily sunscreen use to their patients. In addition, the science behind sunscreens with higher concentrations of active ingredients intended to achieve higher and higher SPFs and their respective penetration of the skin has evolved, leading to new concerns about systemic toxicity. Early limited research frequently touted by the lay media has suggested that some of these agents might lead to hormonal changes, reproductive toxicity, and carcinogenicity.
In November 2016, the FDA issued a guidance for manufacturers that outlined the safety data that would be required to establish an OTC sunscreen active ingredient as GRASE.11 It also provided detailed information about both clinical and nonclinical safety testing, including human irritation and sensitization studies as well as human photosafety studies. In vitro dermal and systemic carcinogenicity studies and animal developmental and reproductive toxicity studies also were required as well studies regarding safety in children.
Many of these recommendations were already being utilized by manufacturers; however, one important change was the requirement for human absorption studies by a maximal usage trial, which more accurately addresses the absorption of sunscreen agents according to actual use. Such studies will be required at the highest allowable concentration of an agent in multiple vehicles and over large body surface areas for considerable exposure times.
This guidance to sunscreen manufacturers was announced to the public in a press release in May 2018.12
What are the new regulations?
All of this has culminated in the recent proposed rule, which includes several important proposals2:
- Of the 16 currently marketed active sunscreen ingredients, only 2—zinc oxide and titanium dioxide—are considered GRASE. Two ingredients—trolamine salicylate and para-aminobenzoic acid—are considered non-GRASE, but there is not enough information at this time to determine if the remaining 12 ingredients are GRASE. The FDA is working with manufacturers to obtain sufficient information to make this determination.
- Approved dosage formulations include sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks. Further information is needed regarding powders before they can be considered.
- The maximum SPF will be increased from 50+ to 60+.
- Sunscreens with an SPF of 15 or higher are required to provide broad-spectrum protection commensurate with the SPF, expanding on critical wavelength testing.
- There are new labeling changes, including a requirement that active ingredients be listed on the front of the packaging.
- Sunscreen products that contain insect repellents are considered non-GRASE.
What’s next?
The process for the proposed final rule has now entered a 90-day public comment period that will end on May 27, 2019; however, it is unlikely that a final monograph as mandated by Congress will be produced by the end of this year.
Sunscreen manufacturers currently are coordinating a response to the proposed rule through the Personal Care Products Council and the Consumer Healthcare Products Association Sunscreen Task Force. It is likely that the new required testing will be costly, with estimates exceeding tens or even hundreds of millions of dollars. In all likelihood, the number of active ingredients that the industry will agree to support with costly testing will be fewer than the 12 that are now on the list. It also is likely that this process will lead to fewer sunscreen products for consumers to choose from and almost certainly at a higher cost.
What do we tell patients in the meantime?
According to the FDA’s rules, it was necessary that this process was made public, but it will almost certainly concern our patients as to the safety of the sunscreen products they have been using. We should be concerned that some of our patients may limit their use of sunscreens because of safety concerns.
There is no question that, as physicians, we want to “first, do no harm,” so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data. The good news is that when this process is completed, a large number of agents will likely be found to be GRASE. When the FDA finally gives its imprimatur to sunscreens, it will hopefully help to silence those naysayers who report that sunscreens are dangerous for consumers; however, it has been suggested by some in industry that the new testing required may take at least 5 years.
What should dermatologists do when we are asked, “What sunscreen should I use NOW?” For most patients, I would explain the regulatory process and assure them that the risk-benefit ratio at this point suggests they should continue using the same sunscreens that they are currently using. For special situations such as pregnant women and children, it may be best to suggest products that contain only the 2 GRASE inorganic agents.
If by now you have not had a patient ask, “Doctor, what sunscreen should I use NOW?” you will soon.
The US Food and Drug Administration (FDA) recently published a press release detailing a proposed rule on how manufacturers will be required to test and label sunscreens in the United States.1,2 Although the press release was complicated and contained much information, the media specifically latched onto the FDA’s consideration of only 2 active sunscreen ingredients—zinc oxide and titanium dioxide—as generally recognized as safe and effective (GRASE). In response, some patients may assume that most sunscreens on the market are dangerous.
How did this new proposed rule come about? To understand the process, it takes some explanation of the history of the FDA’s regulation of sunscreens.
How are sunscreens regulated by the FDA?
The regulatory process for sunscreens in the United States is complicated. The FDA regulates sunscreens as over-the-counter (OTC) drugs rather than as cosmetics, which is how they are regulated in most of the rest of the world.
The US sunscreen regulation process began in 1978 with an advance notice of proposed rulemaking from the FDA that included recommendations from an advisory review panel on the safe and effective use of OTC sunscreen products.3 At that time, 21 active sunscreen ingredients and their maximum use concentrations were listed and determined to be safe, or GRASE. It also gave manufacturers guidance on how to test for efficacy with the methodology for determining the sun protection factor (SPF) as well as various labeling requirements. Over the years, the FDA has issued a number of other sunscreen guidelines, such as removing padimate A and adding avobenzone and zinc oxide to the list of GRASE ingredients in the 1990s.4,5
In 1999, the FDA issued a final rule that listed 16 active sunscreen ingredients and concentrations as GRASE.6 There were some restrictions as to certain combinations of ingredients that could not be used in a finished product. Labeling requirements, including a maximum SPF of 30, also were put in place. This final rule established a final sunscreen monograph that was supposed to have been effective by 2002; however, in 2001 the agency delayed the effective date indefinitely because they had not yet established broad-spectrum (UVA) protection testing and labeling.7
The FDA published a proposed rule in 2007 as well as a final rule in 2011 that again listed the same 16 ingredients as GRASE and specified labeling and testing methods for establishing SPF, broad-spectrum protection, and water-resistance claims.8,9 The final rule limited product labels to a maximum SPF of 50+; provided directions for use with regard to other labeling elements (eg, warnings); and identified specific claims that would not be allowed on product labels, such as “waterproof” and “all-day protection.”9
Nevertheless, an effective final OTC monograph for sunscreen products has not yet been published.
What is the Sunscreen Innovation Act?
In 2014, the US Congress enacted the Sunscreen Innovation Act10 primarily to mandate that the FDA develop a more efficient way to determine the safety and efficacy of new active sunscreen ingredients that were commonly used in Europe and other parts of the world at the time. Many of these agents were thought to be more protective in the UVA and/or UVB spectrum, and if added to the list of GRASE ingredients available to US manufacturers, they would lead to the development of products that would improve the protection offered by sunscreens marketed to US consumers. The time and extent application (TEA) was established, a method that allowed manufacturers to apply for FDA approval of specific agents. The TEA also suggested allowing data generated in other countries where these agents were already in use for years to be considered in the FDA’s evaluation of the agents as GRASE. In addition, Congress mandated that a final monograph on OTC sunscreens be published by the end of 2019. A number of manufacturers have submitted TEAs for new active sunscreen ingredients, and so far, all have been rejected.
Why is the FDA interested in more safety data?
Since then, the FDA has become concerned not only with the safety and efficacy of newly proposed agents through the TEA but also with the original 16 active sunscreen ingredients listed as GRASE in the 2011 final rule. In the 1970s and 1980s, sunscreen use was limited to beach vacations or outdoor sporting events, but sun-protective behaviors have changed dramatically since that time, with health care providers now becoming cognizant of the growing threats of skin cancer and melanoma as well as the cosmetic concerns of photoaging, thereby recommending daily sunscreen use to their patients. In addition, the science behind sunscreens with higher concentrations of active ingredients intended to achieve higher and higher SPFs and their respective penetration of the skin has evolved, leading to new concerns about systemic toxicity. Early limited research frequently touted by the lay media has suggested that some of these agents might lead to hormonal changes, reproductive toxicity, and carcinogenicity.
In November 2016, the FDA issued a guidance for manufacturers that outlined the safety data that would be required to establish an OTC sunscreen active ingredient as GRASE.11 It also provided detailed information about both clinical and nonclinical safety testing, including human irritation and sensitization studies as well as human photosafety studies. In vitro dermal and systemic carcinogenicity studies and animal developmental and reproductive toxicity studies also were required as well studies regarding safety in children.
Many of these recommendations were already being utilized by manufacturers; however, one important change was the requirement for human absorption studies by a maximal usage trial, which more accurately addresses the absorption of sunscreen agents according to actual use. Such studies will be required at the highest allowable concentration of an agent in multiple vehicles and over large body surface areas for considerable exposure times.
This guidance to sunscreen manufacturers was announced to the public in a press release in May 2018.12
What are the new regulations?
All of this has culminated in the recent proposed rule, which includes several important proposals2:
- Of the 16 currently marketed active sunscreen ingredients, only 2—zinc oxide and titanium dioxide—are considered GRASE. Two ingredients—trolamine salicylate and para-aminobenzoic acid—are considered non-GRASE, but there is not enough information at this time to determine if the remaining 12 ingredients are GRASE. The FDA is working with manufacturers to obtain sufficient information to make this determination.
- Approved dosage formulations include sprays, oils, lotions, creams, gels, butters, pastes, ointments, and sticks. Further information is needed regarding powders before they can be considered.
- The maximum SPF will be increased from 50+ to 60+.
- Sunscreens with an SPF of 15 or higher are required to provide broad-spectrum protection commensurate with the SPF, expanding on critical wavelength testing.
- There are new labeling changes, including a requirement that active ingredients be listed on the front of the packaging.
- Sunscreen products that contain insect repellents are considered non-GRASE.
What’s next?
The process for the proposed final rule has now entered a 90-day public comment period that will end on May 27, 2019; however, it is unlikely that a final monograph as mandated by Congress will be produced by the end of this year.
Sunscreen manufacturers currently are coordinating a response to the proposed rule through the Personal Care Products Council and the Consumer Healthcare Products Association Sunscreen Task Force. It is likely that the new required testing will be costly, with estimates exceeding tens or even hundreds of millions of dollars. In all likelihood, the number of active ingredients that the industry will agree to support with costly testing will be fewer than the 12 that are now on the list. It also is likely that this process will lead to fewer sunscreen products for consumers to choose from and almost certainly at a higher cost.
What do we tell patients in the meantime?
According to the FDA’s rules, it was necessary that this process was made public, but it will almost certainly concern our patients as to the safety of the sunscreen products they have been using. We should be concerned that some of our patients may limit their use of sunscreens because of safety concerns.
There is no question that, as physicians, we want to “first, do no harm,” so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data. The good news is that when this process is completed, a large number of agents will likely be found to be GRASE. When the FDA finally gives its imprimatur to sunscreens, it will hopefully help to silence those naysayers who report that sunscreens are dangerous for consumers; however, it has been suggested by some in industry that the new testing required may take at least 5 years.
What should dermatologists do when we are asked, “What sunscreen should I use NOW?” For most patients, I would explain the regulatory process and assure them that the risk-benefit ratio at this point suggests they should continue using the same sunscreens that they are currently using. For special situations such as pregnant women and children, it may be best to suggest products that contain only the 2 GRASE inorganic agents.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective [news release]. Silver Spring, MD: US Food and Drug Administration; February 21, 2019. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm631736.htm. Accessed April 4, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 1978;43(166):38206-38269. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph. Fed Registr. 1996;60(180):48645-48655. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph; enforcement policy. Fed Registr. 1998;63(204):56584-56589. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; final monograph. Fed Registr. 1999;64(98):27666-27693. To be codified at 21 CFR §310, 352, 700, and 740.
- Sunscreen drug products for over-the-counter human use; final monograph; partial stay; final rule. Fed Registr. 2001;66:67485-67487. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; proposed amendment of final monograph. Fed Registr. 2007;72(165):49069-49122. To be codified at 21 CFR §347 and 352.
- Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed Registr. 2011;76(117):35619-35665. To be codified at 21 CFR §201 and 310.
- Sunscreen Innovation Act, S 2141, 113th Cong, 2nd Sess (2014).
- Nonprescription sunscreen drug products-safety and effectiveness data; guidance for industry; availability. Fed Registr. 2016;81(226):84594-84595.
- Statement from Commissioner Scott Gottlieb, MD, on new FDA actions to keep consumers safe from the harmful effects of sun exposure, and ensure the long-term safety and benefits of sunscreens [news release]. Silver Spring, MD: US Food and Drug Administration; May 22, 2018. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm608499.htm. Accessed April 5, 2019.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective [news release]. Silver Spring, MD: US Food and Drug Administration; February 21, 2019. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm631736.htm. Accessed April 4, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 1978;43(166):38206-38269. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph. Fed Registr. 1996;60(180):48645-48655. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; amendment to the tentative final monograph; enforcement policy. Fed Registr. 1998;63(204):56584-56589. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; final monograph. Fed Registr. 1999;64(98):27666-27693. To be codified at 21 CFR §310, 352, 700, and 740.
- Sunscreen drug products for over-the-counter human use; final monograph; partial stay; final rule. Fed Registr. 2001;66:67485-67487. To be codified at 21 CFR §352.
- Sunscreen drug products for over-the-counter human use; proposed amendment of final monograph. Fed Registr. 2007;72(165):49069-49122. To be codified at 21 CFR §347 and 352.
- Labeling and effectiveness testing; sunscreen drug products for over-the-counter human use. Fed Registr. 2011;76(117):35619-35665. To be codified at 21 CFR §201 and 310.
- Sunscreen Innovation Act, S 2141, 113th Cong, 2nd Sess (2014).
- Nonprescription sunscreen drug products-safety and effectiveness data; guidance for industry; availability. Fed Registr. 2016;81(226):84594-84595.
- Statement from Commissioner Scott Gottlieb, MD, on new FDA actions to keep consumers safe from the harmful effects of sun exposure, and ensure the long-term safety and benefits of sunscreens [news release]. Silver Spring, MD: US Food and Drug Administration; May 22, 2018. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm608499.htm. Accessed April 5, 2019.
Digital Revolution: Dermatology Is on the Edge
The Digital Revolution has invaded the House of Medicine, which is not really news since the invasion has been long-standing, but it seems to be generating more interest and concern in recent months. The American Medical Association recently created the Digital Health Implementation Playbook to lend guidance in developing technologies that are fundamentally altering the manner in which patients interact with health care providers.1 The playbook lays out specific steps for developing and implementing digital health technologies such as remote patient monitoring devices. The goal of the playbook is to make certain that such devices are accurate, reliable, and validated as valuable additions to high-quality patient care.1
In the February 2018 issue of Cutis, Masud et al2 evaluated 44 patient-directed mobile applications (apps) for dermatologic conditions and developed a schematic for evaluating their value in providing valid usable information for patients. They found that most of the apps failed to live up to their purported usefulness in patient education.2 I am certain we have all seen numerous examples on the Internet, many times brought to us by patients, of fallacious and inaccurate information about the diagnosis and treatment of dermatologic conditions that are actually harmful to the care of our patients.
A more upsetting trend in recent years is the proliferation of open-access journals. Although such digital journals can result in more rapid dissemination of valid scientific information, many of them do not follow a true peer-review process. So-called predatory journals from for-profit unethical publishers are increasing at an alarming rate.3
Furthermore, there is a need to present data more accurately and in formats that provide more meaningful interpretation, according to a recent Letter From the Editor in the Journal of the American Academy of Dermatology.4 Elston4 wrote: “Be honest about your data and the limitations of the study design.” He cautioned further about the proper use of statistical analysis.
As dermatologists, how do we make certain that the Digital Revolution results in better care of our patients? The answer, of course, is in education of the practitioner and our young colleagues in training. Although most Cutis readers still access the print version of our journal, more and more readers are accessing our digital format. Online we are able to offer readers the peer-reviewed content you have known to trust and rely on to improve your care of patients as well as other educational tools. Furthermore, we can provide readers access to additional charts and tables pertaining to research published in the print journal.
In January 2019 the Cutis website will merge with Dermatology News, our sister news publication, to become MDedge Dermatology (www.mdedge.com/dermatology). This site will be your new one-stop destination for timely news and clinical content you can trust from both publications. This interactive site is designed to help clinicians quickly find the information they need to improve the treatment and care of patients with conditions affecting the hair, skin, and nails. You will have free access to digital resources such as procedural videos, podcasts, image quizzes, board review, and resident resources, as well as an archive of Cutis content dating back to 2000.
As we at Cutis broaden our digital footprint, we look forward to providing our readers with a larger volume of clinically relevant content in more easily accessed formats while maintaining our commitment to valid trustworthy information. In the coming months we look forward to joining with you in this new digital endeavor, and as always, we appreciate the input of our readers during this process.
1. AMA announces playbook to successfully adopt digital health [press release]. Boston, MA: American Medical Association; October 16, 2018. https://www.ama-assn.org/press-center/press-releases/ama-announces-playbook-successfully-adopt-digital-health. Accessed December 14, 2018.
2. Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
3. Shahrivar N, Grant-Kels JM, Payette MJ. Predatory journals: how to recognize and avoid the threat of involvement with these unethical “publishers.” J Am Acad Dermatol. 2016;75:658-659.
4. Elston DM. Presentation of data. J Am Acad Dermatol. 2019;80:55.
The Digital Revolution has invaded the House of Medicine, which is not really news since the invasion has been long-standing, but it seems to be generating more interest and concern in recent months. The American Medical Association recently created the Digital Health Implementation Playbook to lend guidance in developing technologies that are fundamentally altering the manner in which patients interact with health care providers.1 The playbook lays out specific steps for developing and implementing digital health technologies such as remote patient monitoring devices. The goal of the playbook is to make certain that such devices are accurate, reliable, and validated as valuable additions to high-quality patient care.1
In the February 2018 issue of Cutis, Masud et al2 evaluated 44 patient-directed mobile applications (apps) for dermatologic conditions and developed a schematic for evaluating their value in providing valid usable information for patients. They found that most of the apps failed to live up to their purported usefulness in patient education.2 I am certain we have all seen numerous examples on the Internet, many times brought to us by patients, of fallacious and inaccurate information about the diagnosis and treatment of dermatologic conditions that are actually harmful to the care of our patients.
A more upsetting trend in recent years is the proliferation of open-access journals. Although such digital journals can result in more rapid dissemination of valid scientific information, many of them do not follow a true peer-review process. So-called predatory journals from for-profit unethical publishers are increasing at an alarming rate.3
Furthermore, there is a need to present data more accurately and in formats that provide more meaningful interpretation, according to a recent Letter From the Editor in the Journal of the American Academy of Dermatology.4 Elston4 wrote: “Be honest about your data and the limitations of the study design.” He cautioned further about the proper use of statistical analysis.
As dermatologists, how do we make certain that the Digital Revolution results in better care of our patients? The answer, of course, is in education of the practitioner and our young colleagues in training. Although most Cutis readers still access the print version of our journal, more and more readers are accessing our digital format. Online we are able to offer readers the peer-reviewed content you have known to trust and rely on to improve your care of patients as well as other educational tools. Furthermore, we can provide readers access to additional charts and tables pertaining to research published in the print journal.
In January 2019 the Cutis website will merge with Dermatology News, our sister news publication, to become MDedge Dermatology (www.mdedge.com/dermatology). This site will be your new one-stop destination for timely news and clinical content you can trust from both publications. This interactive site is designed to help clinicians quickly find the information they need to improve the treatment and care of patients with conditions affecting the hair, skin, and nails. You will have free access to digital resources such as procedural videos, podcasts, image quizzes, board review, and resident resources, as well as an archive of Cutis content dating back to 2000.
As we at Cutis broaden our digital footprint, we look forward to providing our readers with a larger volume of clinically relevant content in more easily accessed formats while maintaining our commitment to valid trustworthy information. In the coming months we look forward to joining with you in this new digital endeavor, and as always, we appreciate the input of our readers during this process.
The Digital Revolution has invaded the House of Medicine, which is not really news since the invasion has been long-standing, but it seems to be generating more interest and concern in recent months. The American Medical Association recently created the Digital Health Implementation Playbook to lend guidance in developing technologies that are fundamentally altering the manner in which patients interact with health care providers.1 The playbook lays out specific steps for developing and implementing digital health technologies such as remote patient monitoring devices. The goal of the playbook is to make certain that such devices are accurate, reliable, and validated as valuable additions to high-quality patient care.1
In the February 2018 issue of Cutis, Masud et al2 evaluated 44 patient-directed mobile applications (apps) for dermatologic conditions and developed a schematic for evaluating their value in providing valid usable information for patients. They found that most of the apps failed to live up to their purported usefulness in patient education.2 I am certain we have all seen numerous examples on the Internet, many times brought to us by patients, of fallacious and inaccurate information about the diagnosis and treatment of dermatologic conditions that are actually harmful to the care of our patients.
A more upsetting trend in recent years is the proliferation of open-access journals. Although such digital journals can result in more rapid dissemination of valid scientific information, many of them do not follow a true peer-review process. So-called predatory journals from for-profit unethical publishers are increasing at an alarming rate.3
Furthermore, there is a need to present data more accurately and in formats that provide more meaningful interpretation, according to a recent Letter From the Editor in the Journal of the American Academy of Dermatology.4 Elston4 wrote: “Be honest about your data and the limitations of the study design.” He cautioned further about the proper use of statistical analysis.
As dermatologists, how do we make certain that the Digital Revolution results in better care of our patients? The answer, of course, is in education of the practitioner and our young colleagues in training. Although most Cutis readers still access the print version of our journal, more and more readers are accessing our digital format. Online we are able to offer readers the peer-reviewed content you have known to trust and rely on to improve your care of patients as well as other educational tools. Furthermore, we can provide readers access to additional charts and tables pertaining to research published in the print journal.
In January 2019 the Cutis website will merge with Dermatology News, our sister news publication, to become MDedge Dermatology (www.mdedge.com/dermatology). This site will be your new one-stop destination for timely news and clinical content you can trust from both publications. This interactive site is designed to help clinicians quickly find the information they need to improve the treatment and care of patients with conditions affecting the hair, skin, and nails. You will have free access to digital resources such as procedural videos, podcasts, image quizzes, board review, and resident resources, as well as an archive of Cutis content dating back to 2000.
As we at Cutis broaden our digital footprint, we look forward to providing our readers with a larger volume of clinically relevant content in more easily accessed formats while maintaining our commitment to valid trustworthy information. In the coming months we look forward to joining with you in this new digital endeavor, and as always, we appreciate the input of our readers during this process.
1. AMA announces playbook to successfully adopt digital health [press release]. Boston, MA: American Medical Association; October 16, 2018. https://www.ama-assn.org/press-center/press-releases/ama-announces-playbook-successfully-adopt-digital-health. Accessed December 14, 2018.
2. Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
3. Shahrivar N, Grant-Kels JM, Payette MJ. Predatory journals: how to recognize and avoid the threat of involvement with these unethical “publishers.” J Am Acad Dermatol. 2016;75:658-659.
4. Elston DM. Presentation of data. J Am Acad Dermatol. 2019;80:55.
1. AMA announces playbook to successfully adopt digital health [press release]. Boston, MA: American Medical Association; October 16, 2018. https://www.ama-assn.org/press-center/press-releases/ama-announces-playbook-successfully-adopt-digital-health. Accessed December 14, 2018.
2. Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
3. Shahrivar N, Grant-Kels JM, Payette MJ. Predatory journals: how to recognize and avoid the threat of involvement with these unethical “publishers.” J Am Acad Dermatol. 2016;75:658-659.
4. Elston DM. Presentation of data. J Am Acad Dermatol. 2019;80:55.
Bilateral Symmetric Onycholysis of Distal Fingernails
The Diagnosis: Allergic Contact Dermatitis
An allergic contact dermatitis (ACD) to acrylates was suspected and 4 patches were applied to the forearm (the North American Standard Series of the North American Contact Dermatitis Group). The patches were 2-hydroxyethyl methacrylate (2-HEMA) 2.0% permissible exposure limit (peL), ethyl acrylate 0.1% peL, tosylamide formaldehyde resin 10.0% peL, and methyl methacrylate 2.0% peL. A reading at 72 hours was performed and showed a positive reaction to hydroxyethyl methacrylate, ethyl acrylate, and methyl methacrylate, and a negative patch test to tosylamide formaldehyde resin (nail polish)(Figure). The patient was diagnosed with an allergic contact hypersensitivity to the aforementioned acrylates and instructed to avoid artificial nails and acrylate glues. She also was started on oral biotin supplements. On 6-month follow-up the patient had regrowth of all 10 fingernails without brittleness or splitting. She was able to use nail polishes but avoided all acrylic artificial nails and acrylate-containing personal care products.
Acrylate Allergy and Artificial Nails
Acrylates are plastic materials formed by polymerization of acrylic or methacrylic acid monomers and have been cited as a major cause of occupational and nonoccupational contact dermatitis. Contact dermatitis to acrylates in artificial nails was first reported in the 1950s.1,2 Products containing 100% methyl methacrylate monomers in acrylic nails were banned by the US Food and Drug Administration in the early 1970s after receiving a number of complaints.3 However, no regulation prohibits the use of methyl methacrylate monomer in cosmetic products, and various methacrylate and acrylate monomers remain widely used.4 With a growing popularity in artificial nails, it is expected the number of sensitized persons will increase.
Acrylate allergy from sculptured nails concern self-curing resins made from a polymer powder and a liquid monomer solution. Advantages of new UV-cured products include the lack of unpleasant smell and simplified modeling. They also do not require an irritant, such as methacrylic acid, as a bonding agent. Instead, 2-HEMA and 2-hydroxypropyl methacrylate are added. These photobonded nails colloquially are called gel nails (acid free) as opposed to acrylic nails (using methacrylic acid as a primer). It is important to note that the esters of acrylic acid but not the acid itself sensitize patients, and sensitization is not caused by the uncured gel or the monomer solution but by the remaining monomers in the cured plastic nail and the dust filings that are produced during the finishing process.
Clinical Presentation
Symptoms of an ACD to nail acrylates include pruritus and fingertip dermatitis along with nail plate dystrophy. There may be pruritus at the nail base, with subsequent dryness, thickening, and onycholysis. The brittle nails may become split, discolored, and develop paronychia. Inadvertent contact with glue monomers or other acrylate-containing substances may cause eczematous lesions at distant sites. Avoidance of the allergen often results in complete restoration of the normal nail and fingertip within months.
Sensitization
Acrylates and methacrylates are ubiquitous materials used for both industrial and commercial applications. Due to their widespread industrial use, contact allergies to acrylates including 2-HEMA, 2-hydroxypropyl methacrylate, and triethyleneglycol diacrylate (TREGDA) are common. Cross-reaction of these compounds has been observed and is postulated to be due to reaction of the (meth)acrylate carboxyethyl group with the receptors of antigen-presenting cells.5 As a result, an individual with an acrylate allergy sensitized to one allergen often is allergic to its similar compounds and cross-reactors and must avoid the assortment of compounds containing these ingredients, which is important for individuals with occupational sensitization to a particular acrylate who is subsequently susceptible to other acrylate-containing compounds triggering allergic reactions when reexposure occurs in different settings.
Allergens and Occupational Exposure
Acrylates in cosmetic nail products are a source of ACD for not only the customer but also the manicurist.6 The most frequently cited sources of ACD in beauticians are acrylate chemicals.7 However, acrylate compounds are an occupational hazard for a number of other specialists, including dentists and dental technicians, histology technicians, and individuals in the printing industry.8,9 Other individuals may be sensitized to acrylates through their inclusion in adhesives, dental bonding agents, hearing aids, electrocardiogram electrodes, artificial bone cement, and a myriad of other medical and nonmedical applications.4,10-12 For workers who cannot avoid occupational exposure to these allergens, polyvinyl alcohol and multilayer laminate gloves are recommended, as natural rubber latex gloves do not always provide adequate protection from many of these agents.10
Testing for Suspected Acrylate Allergy
Cross-reactivity among acrylates is widely considered in the literature but remains enigmatic and is an important consideration with regard to routine patch test screening.13 In the case of an acrylate allergy to nail products, using 2-HEMA and ethylene glycol dimethacrylate is effective in detecting sensitization by photobonded nails and in patients sensitized by powder liquid products.14 One study showed a patch test panel including 2-HEMA, ethylene glycol dimethacrylate, and TREGDA was effective in identifying the majority of individuals with an allergy to acrylates in nail products and nail technicians.15 Another study has shown the most commonly positive testing allergens to be HEMA, ethyl acrylate, and methyl methacrylate.16 If one is patch testing only one chemical, it appears 2-HEMA is preferred.17 However, broader panels of screening allergens are necessary to achieve an accurate diagnosis. Furthermore, different panels of test allergens have been shown to vary in their ability to detect an acrylate allergy in different occupational exposures.12
The time to patch test read also is important. A standard read at 72 hours is warranted; however, one study showed if only one read at day 3 was done without a subsequent day 7 read, then 25% of TREGDA and 50% of 2-HEMA allergies would have been missed in patients with occupational acrylate allergy.15 Other studies have reported late-appearing and long-lasting test reactions when testing for an acrylate allergy.18,19 Clinicians should be cognizant that an acrylate allergy may be present even if initial screening is negative but the history and clinical picture are suggestive.
- Canizares O. Contact dermatitis due to the acrylic materials used in artificial nails. AMA Arch Derm. 1956;74:141-143.
- Fisher AA, Franks A, Glick H. Allergic sensitization of the skin and nails to acrylic plastic nails. J Allergy. 1957;28:84-88.
- US Food and Drug Administration. Nail care products. http://www.fda.gov/Cosmetics/ProductsIngredients/Products/ucm127068.htm. Updated October 26, 2016. Accessed December 27, 2016.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59(5 suppl):S123-S124.
- Kanerva L. Cross-reactions of multifunctional methacrylates and acrylates. Acta Odontol Scand. 2001;59:320-329.
- Tammaro A, Narcisi A, Abruzzese C, et al. Fingertip dermatitis: occupational acrylate cross reaction. Allergol Int. 2014;63:609-610.
- Kwok C, Money A, Carder M, et al. Cases of occupational dermatitis and asthma in beauticians that were reported to The Health and Occupation Research (THOR) network from 1996 to 2011. Clin Exp Dermatol. 2014;39:590-595.
- Aalto-Korte K, Alanko K, Kuuliala O, et al. Methacrylate and acrylate allergy in dental personnel. Contact Dermatitis. 2007;57:324-330.
- Molina L, Amado A, Mattei PL 4th, et al. Contact dermatitis from acrylics in a histology laboratory assistant. Dermatitis. 2009;20:E11-E12.
- Prasad Hunasehally RY, Hughes TM, Stone NM. Atypical pattern of (meth)acrylate allergic contact dermatitis in dental professionals. Br Dent J. 2012;213:223-224.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes [published online January 27, 2015]. Contact Dermatitis. 2015;73:44-48.
- Sasseville D. Acrylates in contact dermatitis. Dermatitis. 2012;23:6-16.
- Fisher AA. Cross reactions between methyl methacrylate monomer and acrylic monomers presently used in acrylic nail preparations. Contact Dermatitis. 1980;6:345-347.
- Hemmer W, Focke M, Wantke F, et al. Allergic contact dermatitis to artificial fingernails prepared from UV light-cured acrylates. J Am Acad Dermatol. 1996;35(3, pt 1):377-380.
- Teik-Jin Goon A, Bruze M, Zimerson E, et al. Contact allergy to acrylates/methacrylates in the acrylate and nail acrylics series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2007;57:21-27.
- Drucker AM, Pratt MD. Acrylate contact allergy: patient characteristics and evaluation of screening allergens. Dermatitis. 2011;22:98-101.
- Ramos L, Cabral R, Goncalo M. Allergic contact dermatitis caused by acrylates and methacrylates--a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Goon AT, Isaksson M, Zimerson E, et al. Contact allergy to (meth)acrylates in the dental series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2006;55:219-226.
- Isaksson M, Lindberg M, Sundberg K, et al. The development and course of patch-test reactions to 2-hydroxyethyl methacrylate and ethyleneglycol dimethacrylate. Contact Dermatitis. 2005;53:292-297.
The Diagnosis: Allergic Contact Dermatitis
An allergic contact dermatitis (ACD) to acrylates was suspected and 4 patches were applied to the forearm (the North American Standard Series of the North American Contact Dermatitis Group). The patches were 2-hydroxyethyl methacrylate (2-HEMA) 2.0% permissible exposure limit (peL), ethyl acrylate 0.1% peL, tosylamide formaldehyde resin 10.0% peL, and methyl methacrylate 2.0% peL. A reading at 72 hours was performed and showed a positive reaction to hydroxyethyl methacrylate, ethyl acrylate, and methyl methacrylate, and a negative patch test to tosylamide formaldehyde resin (nail polish)(Figure). The patient was diagnosed with an allergic contact hypersensitivity to the aforementioned acrylates and instructed to avoid artificial nails and acrylate glues. She also was started on oral biotin supplements. On 6-month follow-up the patient had regrowth of all 10 fingernails without brittleness or splitting. She was able to use nail polishes but avoided all acrylic artificial nails and acrylate-containing personal care products.

Acrylate Allergy and Artificial Nails
Acrylates are plastic materials formed by polymerization of acrylic or methacrylic acid monomers and have been cited as a major cause of occupational and nonoccupational contact dermatitis. Contact dermatitis to acrylates in artificial nails was first reported in the 1950s.1,2 Products containing 100% methyl methacrylate monomers in acrylic nails were banned by the US Food and Drug Administration in the early 1970s after receiving a number of complaints.3 However, no regulation prohibits the use of methyl methacrylate monomer in cosmetic products, and various methacrylate and acrylate monomers remain widely used.4 With a growing popularity in artificial nails, it is expected the number of sensitized persons will increase.
Acrylate allergy from sculptured nails concern self-curing resins made from a polymer powder and a liquid monomer solution. Advantages of new UV-cured products include the lack of unpleasant smell and simplified modeling. They also do not require an irritant, such as methacrylic acid, as a bonding agent. Instead, 2-HEMA and 2-hydroxypropyl methacrylate are added. These photobonded nails colloquially are called gel nails (acid free) as opposed to acrylic nails (using methacrylic acid as a primer). It is important to note that the esters of acrylic acid but not the acid itself sensitize patients, and sensitization is not caused by the uncured gel or the monomer solution but by the remaining monomers in the cured plastic nail and the dust filings that are produced during the finishing process.
Clinical Presentation
Symptoms of an ACD to nail acrylates include pruritus and fingertip dermatitis along with nail plate dystrophy. There may be pruritus at the nail base, with subsequent dryness, thickening, and onycholysis. The brittle nails may become split, discolored, and develop paronychia. Inadvertent contact with glue monomers or other acrylate-containing substances may cause eczematous lesions at distant sites. Avoidance of the allergen often results in complete restoration of the normal nail and fingertip within months.
Sensitization
Acrylates and methacrylates are ubiquitous materials used for both industrial and commercial applications. Due to their widespread industrial use, contact allergies to acrylates including 2-HEMA, 2-hydroxypropyl methacrylate, and triethyleneglycol diacrylate (TREGDA) are common. Cross-reaction of these compounds has been observed and is postulated to be due to reaction of the (meth)acrylate carboxyethyl group with the receptors of antigen-presenting cells.5 As a result, an individual with an acrylate allergy sensitized to one allergen often is allergic to its similar compounds and cross-reactors and must avoid the assortment of compounds containing these ingredients, which is important for individuals with occupational sensitization to a particular acrylate who is subsequently susceptible to other acrylate-containing compounds triggering allergic reactions when reexposure occurs in different settings.
Allergens and Occupational Exposure
Acrylates in cosmetic nail products are a source of ACD for not only the customer but also the manicurist.6 The most frequently cited sources of ACD in beauticians are acrylate chemicals.7 However, acrylate compounds are an occupational hazard for a number of other specialists, including dentists and dental technicians, histology technicians, and individuals in the printing industry.8,9 Other individuals may be sensitized to acrylates through their inclusion in adhesives, dental bonding agents, hearing aids, electrocardiogram electrodes, artificial bone cement, and a myriad of other medical and nonmedical applications.4,10-12 For workers who cannot avoid occupational exposure to these allergens, polyvinyl alcohol and multilayer laminate gloves are recommended, as natural rubber latex gloves do not always provide adequate protection from many of these agents.10
Testing for Suspected Acrylate Allergy
Cross-reactivity among acrylates is widely considered in the literature but remains enigmatic and is an important consideration with regard to routine patch test screening.13 In the case of an acrylate allergy to nail products, using 2-HEMA and ethylene glycol dimethacrylate is effective in detecting sensitization by photobonded nails and in patients sensitized by powder liquid products.14 One study showed a patch test panel including 2-HEMA, ethylene glycol dimethacrylate, and TREGDA was effective in identifying the majority of individuals with an allergy to acrylates in nail products and nail technicians.15 Another study has shown the most commonly positive testing allergens to be HEMA, ethyl acrylate, and methyl methacrylate.16 If one is patch testing only one chemical, it appears 2-HEMA is preferred.17 However, broader panels of screening allergens are necessary to achieve an accurate diagnosis. Furthermore, different panels of test allergens have been shown to vary in their ability to detect an acrylate allergy in different occupational exposures.12
The time to patch test read also is important. A standard read at 72 hours is warranted; however, one study showed if only one read at day 3 was done without a subsequent day 7 read, then 25% of TREGDA and 50% of 2-HEMA allergies would have been missed in patients with occupational acrylate allergy.15 Other studies have reported late-appearing and long-lasting test reactions when testing for an acrylate allergy.18,19 Clinicians should be cognizant that an acrylate allergy may be present even if initial screening is negative but the history and clinical picture are suggestive.
The Diagnosis: Allergic Contact Dermatitis
An allergic contact dermatitis (ACD) to acrylates was suspected and 4 patches were applied to the forearm (the North American Standard Series of the North American Contact Dermatitis Group). The patches were 2-hydroxyethyl methacrylate (2-HEMA) 2.0% permissible exposure limit (peL), ethyl acrylate 0.1% peL, tosylamide formaldehyde resin 10.0% peL, and methyl methacrylate 2.0% peL. A reading at 72 hours was performed and showed a positive reaction to hydroxyethyl methacrylate, ethyl acrylate, and methyl methacrylate, and a negative patch test to tosylamide formaldehyde resin (nail polish)(Figure). The patient was diagnosed with an allergic contact hypersensitivity to the aforementioned acrylates and instructed to avoid artificial nails and acrylate glues. She also was started on oral biotin supplements. On 6-month follow-up the patient had regrowth of all 10 fingernails without brittleness or splitting. She was able to use nail polishes but avoided all acrylic artificial nails and acrylate-containing personal care products.

Acrylate Allergy and Artificial Nails
Acrylates are plastic materials formed by polymerization of acrylic or methacrylic acid monomers and have been cited as a major cause of occupational and nonoccupational contact dermatitis. Contact dermatitis to acrylates in artificial nails was first reported in the 1950s.1,2 Products containing 100% methyl methacrylate monomers in acrylic nails were banned by the US Food and Drug Administration in the early 1970s after receiving a number of complaints.3 However, no regulation prohibits the use of methyl methacrylate monomer in cosmetic products, and various methacrylate and acrylate monomers remain widely used.4 With a growing popularity in artificial nails, it is expected the number of sensitized persons will increase.
Acrylate allergy from sculptured nails concern self-curing resins made from a polymer powder and a liquid monomer solution. Advantages of new UV-cured products include the lack of unpleasant smell and simplified modeling. They also do not require an irritant, such as methacrylic acid, as a bonding agent. Instead, 2-HEMA and 2-hydroxypropyl methacrylate are added. These photobonded nails colloquially are called gel nails (acid free) as opposed to acrylic nails (using methacrylic acid as a primer). It is important to note that the esters of acrylic acid but not the acid itself sensitize patients, and sensitization is not caused by the uncured gel or the monomer solution but by the remaining monomers in the cured plastic nail and the dust filings that are produced during the finishing process.
Clinical Presentation
Symptoms of an ACD to nail acrylates include pruritus and fingertip dermatitis along with nail plate dystrophy. There may be pruritus at the nail base, with subsequent dryness, thickening, and onycholysis. The brittle nails may become split, discolored, and develop paronychia. Inadvertent contact with glue monomers or other acrylate-containing substances may cause eczematous lesions at distant sites. Avoidance of the allergen often results in complete restoration of the normal nail and fingertip within months.
Sensitization
Acrylates and methacrylates are ubiquitous materials used for both industrial and commercial applications. Due to their widespread industrial use, contact allergies to acrylates including 2-HEMA, 2-hydroxypropyl methacrylate, and triethyleneglycol diacrylate (TREGDA) are common. Cross-reaction of these compounds has been observed and is postulated to be due to reaction of the (meth)acrylate carboxyethyl group with the receptors of antigen-presenting cells.5 As a result, an individual with an acrylate allergy sensitized to one allergen often is allergic to its similar compounds and cross-reactors and must avoid the assortment of compounds containing these ingredients, which is important for individuals with occupational sensitization to a particular acrylate who is subsequently susceptible to other acrylate-containing compounds triggering allergic reactions when reexposure occurs in different settings.
Allergens and Occupational Exposure
Acrylates in cosmetic nail products are a source of ACD for not only the customer but also the manicurist.6 The most frequently cited sources of ACD in beauticians are acrylate chemicals.7 However, acrylate compounds are an occupational hazard for a number of other specialists, including dentists and dental technicians, histology technicians, and individuals in the printing industry.8,9 Other individuals may be sensitized to acrylates through their inclusion in adhesives, dental bonding agents, hearing aids, electrocardiogram electrodes, artificial bone cement, and a myriad of other medical and nonmedical applications.4,10-12 For workers who cannot avoid occupational exposure to these allergens, polyvinyl alcohol and multilayer laminate gloves are recommended, as natural rubber latex gloves do not always provide adequate protection from many of these agents.10
Testing for Suspected Acrylate Allergy
Cross-reactivity among acrylates is widely considered in the literature but remains enigmatic and is an important consideration with regard to routine patch test screening.13 In the case of an acrylate allergy to nail products, using 2-HEMA and ethylene glycol dimethacrylate is effective in detecting sensitization by photobonded nails and in patients sensitized by powder liquid products.14 One study showed a patch test panel including 2-HEMA, ethylene glycol dimethacrylate, and TREGDA was effective in identifying the majority of individuals with an allergy to acrylates in nail products and nail technicians.15 Another study has shown the most commonly positive testing allergens to be HEMA, ethyl acrylate, and methyl methacrylate.16 If one is patch testing only one chemical, it appears 2-HEMA is preferred.17 However, broader panels of screening allergens are necessary to achieve an accurate diagnosis. Furthermore, different panels of test allergens have been shown to vary in their ability to detect an acrylate allergy in different occupational exposures.12
The time to patch test read also is important. A standard read at 72 hours is warranted; however, one study showed if only one read at day 3 was done without a subsequent day 7 read, then 25% of TREGDA and 50% of 2-HEMA allergies would have been missed in patients with occupational acrylate allergy.15 Other studies have reported late-appearing and long-lasting test reactions when testing for an acrylate allergy.18,19 Clinicians should be cognizant that an acrylate allergy may be present even if initial screening is negative but the history and clinical picture are suggestive.
- Canizares O. Contact dermatitis due to the acrylic materials used in artificial nails. AMA Arch Derm. 1956;74:141-143.
- Fisher AA, Franks A, Glick H. Allergic sensitization of the skin and nails to acrylic plastic nails. J Allergy. 1957;28:84-88.
- US Food and Drug Administration. Nail care products. http://www.fda.gov/Cosmetics/ProductsIngredients/Products/ucm127068.htm. Updated October 26, 2016. Accessed December 27, 2016.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59(5 suppl):S123-S124.
- Kanerva L. Cross-reactions of multifunctional methacrylates and acrylates. Acta Odontol Scand. 2001;59:320-329.
- Tammaro A, Narcisi A, Abruzzese C, et al. Fingertip dermatitis: occupational acrylate cross reaction. Allergol Int. 2014;63:609-610.
- Kwok C, Money A, Carder M, et al. Cases of occupational dermatitis and asthma in beauticians that were reported to The Health and Occupation Research (THOR) network from 1996 to 2011. Clin Exp Dermatol. 2014;39:590-595.
- Aalto-Korte K, Alanko K, Kuuliala O, et al. Methacrylate and acrylate allergy in dental personnel. Contact Dermatitis. 2007;57:324-330.
- Molina L, Amado A, Mattei PL 4th, et al. Contact dermatitis from acrylics in a histology laboratory assistant. Dermatitis. 2009;20:E11-E12.
- Prasad Hunasehally RY, Hughes TM, Stone NM. Atypical pattern of (meth)acrylate allergic contact dermatitis in dental professionals. Br Dent J. 2012;213:223-224.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes [published online January 27, 2015]. Contact Dermatitis. 2015;73:44-48.
- Sasseville D. Acrylates in contact dermatitis. Dermatitis. 2012;23:6-16.
- Fisher AA. Cross reactions between methyl methacrylate monomer and acrylic monomers presently used in acrylic nail preparations. Contact Dermatitis. 1980;6:345-347.
- Hemmer W, Focke M, Wantke F, et al. Allergic contact dermatitis to artificial fingernails prepared from UV light-cured acrylates. J Am Acad Dermatol. 1996;35(3, pt 1):377-380.
- Teik-Jin Goon A, Bruze M, Zimerson E, et al. Contact allergy to acrylates/methacrylates in the acrylate and nail acrylics series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2007;57:21-27.
- Drucker AM, Pratt MD. Acrylate contact allergy: patient characteristics and evaluation of screening allergens. Dermatitis. 2011;22:98-101.
- Ramos L, Cabral R, Goncalo M. Allergic contact dermatitis caused by acrylates and methacrylates--a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Goon AT, Isaksson M, Zimerson E, et al. Contact allergy to (meth)acrylates in the dental series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2006;55:219-226.
- Isaksson M, Lindberg M, Sundberg K, et al. The development and course of patch-test reactions to 2-hydroxyethyl methacrylate and ethyleneglycol dimethacrylate. Contact Dermatitis. 2005;53:292-297.
- Canizares O. Contact dermatitis due to the acrylic materials used in artificial nails. AMA Arch Derm. 1956;74:141-143.
- Fisher AA, Franks A, Glick H. Allergic sensitization of the skin and nails to acrylic plastic nails. J Allergy. 1957;28:84-88.
- US Food and Drug Administration. Nail care products. http://www.fda.gov/Cosmetics/ProductsIngredients/Products/ucm127068.htm. Updated October 26, 2016. Accessed December 27, 2016.
- Haughton AM, Belsito DV. Acrylate allergy induced by acrylic nails resulting in prosthesis failure. J Am Acad Dermatol. 2008;59(5 suppl):S123-S124.
- Kanerva L. Cross-reactions of multifunctional methacrylates and acrylates. Acta Odontol Scand. 2001;59:320-329.
- Tammaro A, Narcisi A, Abruzzese C, et al. Fingertip dermatitis: occupational acrylate cross reaction. Allergol Int. 2014;63:609-610.
- Kwok C, Money A, Carder M, et al. Cases of occupational dermatitis and asthma in beauticians that were reported to The Health and Occupation Research (THOR) network from 1996 to 2011. Clin Exp Dermatol. 2014;39:590-595.
- Aalto-Korte K, Alanko K, Kuuliala O, et al. Methacrylate and acrylate allergy in dental personnel. Contact Dermatitis. 2007;57:324-330.
- Molina L, Amado A, Mattei PL 4th, et al. Contact dermatitis from acrylics in a histology laboratory assistant. Dermatitis. 2009;20:E11-E12.
- Prasad Hunasehally RY, Hughes TM, Stone NM. Atypical pattern of (meth)acrylate allergic contact dermatitis in dental professionals. Br Dent J. 2012;213:223-224.
- Stingeni L, Cerulli E, Spalletti A, et al. The role of acrylic acid impurity as a sensitizing component in electrocardiogram electrodes [published online January 27, 2015]. Contact Dermatitis. 2015;73:44-48.
- Sasseville D. Acrylates in contact dermatitis. Dermatitis. 2012;23:6-16.
- Fisher AA. Cross reactions between methyl methacrylate monomer and acrylic monomers presently used in acrylic nail preparations. Contact Dermatitis. 1980;6:345-347.
- Hemmer W, Focke M, Wantke F, et al. Allergic contact dermatitis to artificial fingernails prepared from UV light-cured acrylates. J Am Acad Dermatol. 1996;35(3, pt 1):377-380.
- Teik-Jin Goon A, Bruze M, Zimerson E, et al. Contact allergy to acrylates/methacrylates in the acrylate and nail acrylics series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2007;57:21-27.
- Drucker AM, Pratt MD. Acrylate contact allergy: patient characteristics and evaluation of screening allergens. Dermatitis. 2011;22:98-101.
- Ramos L, Cabral R, Goncalo M. Allergic contact dermatitis caused by acrylates and methacrylates--a 7-year study. Contact Dermatitis. 2014;71:102-107.
- Goon AT, Isaksson M, Zimerson E, et al. Contact allergy to (meth)acrylates in the dental series in southern Sweden: simultaneous positive patch test reaction patterns and possible screening allergens. Contact Dermatitis. 2006;55:219-226.
- Isaksson M, Lindberg M, Sundberg K, et al. The development and course of patch-test reactions to 2-hydroxyethyl methacrylate and ethyleneglycol dimethacrylate. Contact Dermatitis. 2005;53:292-297.
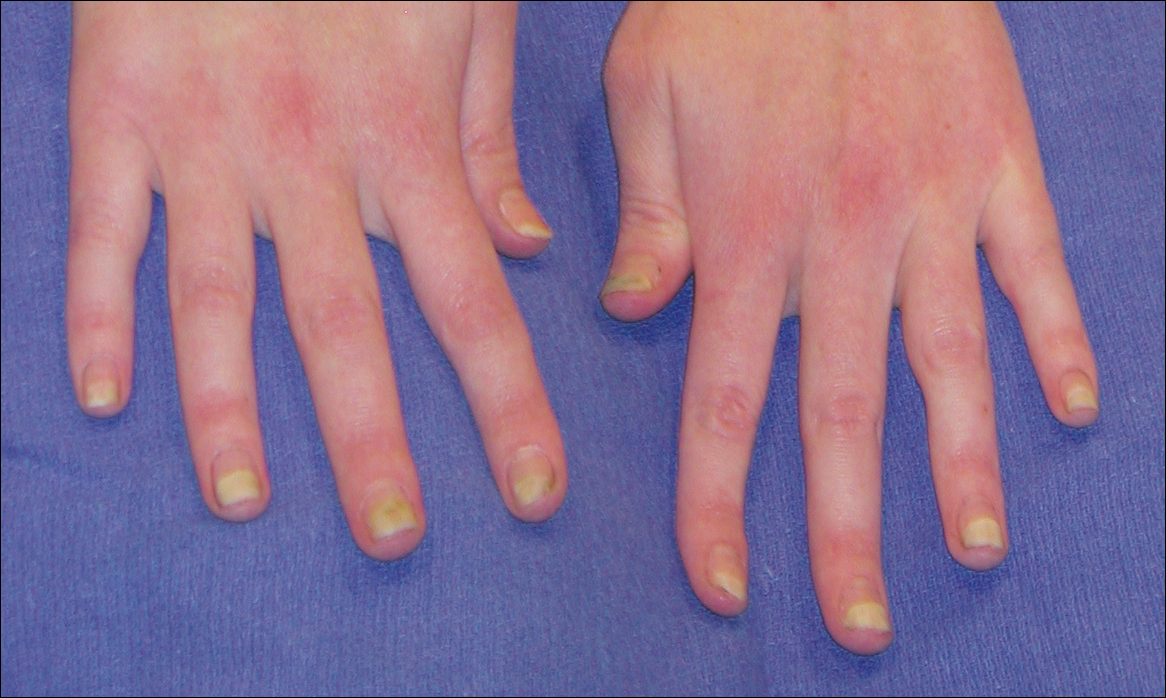
A 28-year-old woman presented with distal onycholysis of all 10 fingernails. The patient started to notice brittleness in the first, second, and third fingernails of the right hand 2 months prior. She had a 10-year history of wearing acrylic nails and reported a history of periungual eczema. On physical examination, all 10 fingernails had distal onycholysis and there was a green discoloration of the first fingernail on the left hand. On blood analysis, thyroid-stimulating hormone and free thyroxine were within reference range. A nail clipping showed onychodystrophy and a negative periodic acid-Schiff stain.
Direct-to-Consumer Pharmaceutical Advertising
The American Medical Association (AMA) recently adopted a new policy supporting a ban on direct-to-consumer pharmaceutical advertising (DTCPA) of prescription drugs and medical devices in an effort to make prescription drugs more affordable.1 Dr. Patrice A. Harris, MD, MA, chair-elect of the AMA Board of Trustees, stated that the vote “reflects concerns among physicians about the negative impact of commercially-driven promotions, and the role that marketing costs play in fueling escalating drug prices.” She added, “[it] also inflates demand for new and more expensive drugs, even when these drugs may not be appropriate.”1
The United States and New Zealand are the only 2 countries that allow DTCPA that includes product claims.1 There are 3 basic types of DTCPA, all of which are regulated by the US Food and Drug Administration (FDA): (1) help-seeking advertisements, which provide information about a disease but do not recommend specific drugs or devices; (2) reminder advertisements, which mention specific drugs and provide some information (eg, strength, dosage form, price) but do not mention the indication or make efficacy claims; and (3) product claim advertisements, which are the most common type and mention drug names, indications, and efficacy and/or safety data.2
The FDA’s Division of Drug Marketing, Advertising, and Communications is responsible for regulating DTCPA. The FDA has had authority to approve pharmaceutical products in the United States since 1938 and has regulated labeling and advertising of these products since 1962. In 1969, the FDA issued regulations stipulating that drug advertisements should not be false or misleading and should provide information about risks and benefits, facts about the uses of the drug, and a list of all risks in the product’s labeling. At that time, drug advertisements were directed at health care providers—not the general public—and were mainly found in medical journals and other print sources aimed directly as physicians.
When a number of pharmaceutical manufacturers ran direct-to-consumer advertisements in print and broadcast media, the FDA had to consider how to regulate a new area of advertising. In 1985, the FDA issued a notice claiming regulatory jurisdiction over DTCPA. Believing that DTCPA was beneficial to the general health of consumers, the FDA gradually eased its regulations in recognition of the prohibitive time and expense that the older rules required. Advertisers now only had to list major risks rather than all risks and direct consumers to sources of further information.
In 1980, total spending on DTCPA by industry was $12 million; this figure reached $1.2 billion by 1998, topped out at $5 billion in 2006 and 2007, and dropped to $4.5 billion in 2009.2 Today, most DTCPA spending goes toward television commercials, with the average American viewer watching as many as 9 drug advertisements per day; however, spending on Internet advertising is increasing since the return on investment in that medium is greater.
Is DTCPA beneficial or detrimental to the health of US consumers and, specifically, to patients with skin disease? Unfortunately, there are no quick answers. In a review by Ventola,2 data showed that DTCPA informs, educates, and empowers patients and encourages them to seek medical care as well as to make appointments with their doctors to discuss conditions they had not previously discussed. Data also showed that DTCPA strengthens patients’ relationships with health care providers and improves patient compliance with treatments. Importantly, DTCPA has been shown to reduce underdiagnosis and undertreatment of medical conditions and removes the stigma associated with certain diseases. Finally, DTCPA also has been shown to encourage product competition and lower drug prices.2
In contrast, data also have shown that DTCPA can lead to patient misinformation and may damage the patient-physician relationship.2 Many advertisements may overemphasize a drug’s benefits while downplaying associated risks, while others may promote an unnecessary fear of side effects. These advertisements have been criticized for causing beliefs about diseases in patients that lead to overutilization of drugs and inappropriate prescribing. Some fear DTCPA may promote new drugs before their safety profiles are fully known, which may be particularly true for first-in-class drugs. Finally, DTCPA may increase the cost of drugs in general, not only because of the amount spent on the advertisements themselves, but also because DTCPAs promote copycat drugs that do not offer any increased benefit over older and cheaper medications.
How does all of this relate to dermatology? In the last few years, we have seen the development of drugs (eg, psoriasis treatments) that offer real improvement for patients who only had access to minimally effective therapies in the past. The research pipeline is full of agents for other diseases for which we lack adequate treatments, such as atopic eczema and certain forms of skin cancer. Additionally, for patients with diseases like psoriasis and eczema who may have given up on dermatologists to provide adequate treatments, DTCPAs may give them hope and renewed interest in seeking our help.
It comes as no surprise to dermatologists and their patients that the costs for drugs used to treat dermatologic diseases have skyrocketed. Rosenberg and Rosenberg3 recently evaluated the cost of 19 dermatologic drugs from 2009 to 2015 and noted increases ranging from 60% to 1698%, the majority of which may be passed on directly to our patients.
Ultimately, there are no easy answers. Hopefully, studies evaluating the pros and cons of DTCPAs—specifically for dermatology patients—that can help dermatologists make rational decisions about how to best serve our patients in a cost-efficient manner will be forthcoming. For the time being, it is unlikely that DTCPA will be banned in the United States, as such action would surely lead to claims of unconstitutional infringement on free speech. Nevertheless, increased oversight and more stringent regulations might improve the acceptability of such advertising to those that oppose DTCPA.
- AMA calls for ban on direct to consumer advertising of prescription drugs and medical devices [press release]. Atlanta, GA: American Medical Association; November 17, 2105.
- Ventola CL. Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? P T. 2011;36:669-684.
- Rosenberg ME, Rosenberg SP. Changes in retail prices of prescription dermatologic drugs from 2009 to 2015 [published online November 25, 2015]. JAMA Dermatol. doi: 10.1001/jamadermatol.2015.3897.
The American Medical Association (AMA) recently adopted a new policy supporting a ban on direct-to-consumer pharmaceutical advertising (DTCPA) of prescription drugs and medical devices in an effort to make prescription drugs more affordable.1 Dr. Patrice A. Harris, MD, MA, chair-elect of the AMA Board of Trustees, stated that the vote “reflects concerns among physicians about the negative impact of commercially-driven promotions, and the role that marketing costs play in fueling escalating drug prices.” She added, “[it] also inflates demand for new and more expensive drugs, even when these drugs may not be appropriate.”1
The United States and New Zealand are the only 2 countries that allow DTCPA that includes product claims.1 There are 3 basic types of DTCPA, all of which are regulated by the US Food and Drug Administration (FDA): (1) help-seeking advertisements, which provide information about a disease but do not recommend specific drugs or devices; (2) reminder advertisements, which mention specific drugs and provide some information (eg, strength, dosage form, price) but do not mention the indication or make efficacy claims; and (3) product claim advertisements, which are the most common type and mention drug names, indications, and efficacy and/or safety data.2
The FDA’s Division of Drug Marketing, Advertising, and Communications is responsible for regulating DTCPA. The FDA has had authority to approve pharmaceutical products in the United States since 1938 and has regulated labeling and advertising of these products since 1962. In 1969, the FDA issued regulations stipulating that drug advertisements should not be false or misleading and should provide information about risks and benefits, facts about the uses of the drug, and a list of all risks in the product’s labeling. At that time, drug advertisements were directed at health care providers—not the general public—and were mainly found in medical journals and other print sources aimed directly as physicians.
When a number of pharmaceutical manufacturers ran direct-to-consumer advertisements in print and broadcast media, the FDA had to consider how to regulate a new area of advertising. In 1985, the FDA issued a notice claiming regulatory jurisdiction over DTCPA. Believing that DTCPA was beneficial to the general health of consumers, the FDA gradually eased its regulations in recognition of the prohibitive time and expense that the older rules required. Advertisers now only had to list major risks rather than all risks and direct consumers to sources of further information.
In 1980, total spending on DTCPA by industry was $12 million; this figure reached $1.2 billion by 1998, topped out at $5 billion in 2006 and 2007, and dropped to $4.5 billion in 2009.2 Today, most DTCPA spending goes toward television commercials, with the average American viewer watching as many as 9 drug advertisements per day; however, spending on Internet advertising is increasing since the return on investment in that medium is greater.
Is DTCPA beneficial or detrimental to the health of US consumers and, specifically, to patients with skin disease? Unfortunately, there are no quick answers. In a review by Ventola,2 data showed that DTCPA informs, educates, and empowers patients and encourages them to seek medical care as well as to make appointments with their doctors to discuss conditions they had not previously discussed. Data also showed that DTCPA strengthens patients’ relationships with health care providers and improves patient compliance with treatments. Importantly, DTCPA has been shown to reduce underdiagnosis and undertreatment of medical conditions and removes the stigma associated with certain diseases. Finally, DTCPA also has been shown to encourage product competition and lower drug prices.2
In contrast, data also have shown that DTCPA can lead to patient misinformation and may damage the patient-physician relationship.2 Many advertisements may overemphasize a drug’s benefits while downplaying associated risks, while others may promote an unnecessary fear of side effects. These advertisements have been criticized for causing beliefs about diseases in patients that lead to overutilization of drugs and inappropriate prescribing. Some fear DTCPA may promote new drugs before their safety profiles are fully known, which may be particularly true for first-in-class drugs. Finally, DTCPA may increase the cost of drugs in general, not only because of the amount spent on the advertisements themselves, but also because DTCPAs promote copycat drugs that do not offer any increased benefit over older and cheaper medications.
How does all of this relate to dermatology? In the last few years, we have seen the development of drugs (eg, psoriasis treatments) that offer real improvement for patients who only had access to minimally effective therapies in the past. The research pipeline is full of agents for other diseases for which we lack adequate treatments, such as atopic eczema and certain forms of skin cancer. Additionally, for patients with diseases like psoriasis and eczema who may have given up on dermatologists to provide adequate treatments, DTCPAs may give them hope and renewed interest in seeking our help.
It comes as no surprise to dermatologists and their patients that the costs for drugs used to treat dermatologic diseases have skyrocketed. Rosenberg and Rosenberg3 recently evaluated the cost of 19 dermatologic drugs from 2009 to 2015 and noted increases ranging from 60% to 1698%, the majority of which may be passed on directly to our patients.
Ultimately, there are no easy answers. Hopefully, studies evaluating the pros and cons of DTCPAs—specifically for dermatology patients—that can help dermatologists make rational decisions about how to best serve our patients in a cost-efficient manner will be forthcoming. For the time being, it is unlikely that DTCPA will be banned in the United States, as such action would surely lead to claims of unconstitutional infringement on free speech. Nevertheless, increased oversight and more stringent regulations might improve the acceptability of such advertising to those that oppose DTCPA.
The American Medical Association (AMA) recently adopted a new policy supporting a ban on direct-to-consumer pharmaceutical advertising (DTCPA) of prescription drugs and medical devices in an effort to make prescription drugs more affordable.1 Dr. Patrice A. Harris, MD, MA, chair-elect of the AMA Board of Trustees, stated that the vote “reflects concerns among physicians about the negative impact of commercially-driven promotions, and the role that marketing costs play in fueling escalating drug prices.” She added, “[it] also inflates demand for new and more expensive drugs, even when these drugs may not be appropriate.”1
The United States and New Zealand are the only 2 countries that allow DTCPA that includes product claims.1 There are 3 basic types of DTCPA, all of which are regulated by the US Food and Drug Administration (FDA): (1) help-seeking advertisements, which provide information about a disease but do not recommend specific drugs or devices; (2) reminder advertisements, which mention specific drugs and provide some information (eg, strength, dosage form, price) but do not mention the indication or make efficacy claims; and (3) product claim advertisements, which are the most common type and mention drug names, indications, and efficacy and/or safety data.2
The FDA’s Division of Drug Marketing, Advertising, and Communications is responsible for regulating DTCPA. The FDA has had authority to approve pharmaceutical products in the United States since 1938 and has regulated labeling and advertising of these products since 1962. In 1969, the FDA issued regulations stipulating that drug advertisements should not be false or misleading and should provide information about risks and benefits, facts about the uses of the drug, and a list of all risks in the product’s labeling. At that time, drug advertisements were directed at health care providers—not the general public—and were mainly found in medical journals and other print sources aimed directly as physicians.
When a number of pharmaceutical manufacturers ran direct-to-consumer advertisements in print and broadcast media, the FDA had to consider how to regulate a new area of advertising. In 1985, the FDA issued a notice claiming regulatory jurisdiction over DTCPA. Believing that DTCPA was beneficial to the general health of consumers, the FDA gradually eased its regulations in recognition of the prohibitive time and expense that the older rules required. Advertisers now only had to list major risks rather than all risks and direct consumers to sources of further information.
In 1980, total spending on DTCPA by industry was $12 million; this figure reached $1.2 billion by 1998, topped out at $5 billion in 2006 and 2007, and dropped to $4.5 billion in 2009.2 Today, most DTCPA spending goes toward television commercials, with the average American viewer watching as many as 9 drug advertisements per day; however, spending on Internet advertising is increasing since the return on investment in that medium is greater.
Is DTCPA beneficial or detrimental to the health of US consumers and, specifically, to patients with skin disease? Unfortunately, there are no quick answers. In a review by Ventola,2 data showed that DTCPA informs, educates, and empowers patients and encourages them to seek medical care as well as to make appointments with their doctors to discuss conditions they had not previously discussed. Data also showed that DTCPA strengthens patients’ relationships with health care providers and improves patient compliance with treatments. Importantly, DTCPA has been shown to reduce underdiagnosis and undertreatment of medical conditions and removes the stigma associated with certain diseases. Finally, DTCPA also has been shown to encourage product competition and lower drug prices.2
In contrast, data also have shown that DTCPA can lead to patient misinformation and may damage the patient-physician relationship.2 Many advertisements may overemphasize a drug’s benefits while downplaying associated risks, while others may promote an unnecessary fear of side effects. These advertisements have been criticized for causing beliefs about diseases in patients that lead to overutilization of drugs and inappropriate prescribing. Some fear DTCPA may promote new drugs before their safety profiles are fully known, which may be particularly true for first-in-class drugs. Finally, DTCPA may increase the cost of drugs in general, not only because of the amount spent on the advertisements themselves, but also because DTCPAs promote copycat drugs that do not offer any increased benefit over older and cheaper medications.
How does all of this relate to dermatology? In the last few years, we have seen the development of drugs (eg, psoriasis treatments) that offer real improvement for patients who only had access to minimally effective therapies in the past. The research pipeline is full of agents for other diseases for which we lack adequate treatments, such as atopic eczema and certain forms of skin cancer. Additionally, for patients with diseases like psoriasis and eczema who may have given up on dermatologists to provide adequate treatments, DTCPAs may give them hope and renewed interest in seeking our help.
It comes as no surprise to dermatologists and their patients that the costs for drugs used to treat dermatologic diseases have skyrocketed. Rosenberg and Rosenberg3 recently evaluated the cost of 19 dermatologic drugs from 2009 to 2015 and noted increases ranging from 60% to 1698%, the majority of which may be passed on directly to our patients.
Ultimately, there are no easy answers. Hopefully, studies evaluating the pros and cons of DTCPAs—specifically for dermatology patients—that can help dermatologists make rational decisions about how to best serve our patients in a cost-efficient manner will be forthcoming. For the time being, it is unlikely that DTCPA will be banned in the United States, as such action would surely lead to claims of unconstitutional infringement on free speech. Nevertheless, increased oversight and more stringent regulations might improve the acceptability of such advertising to those that oppose DTCPA.
- AMA calls for ban on direct to consumer advertising of prescription drugs and medical devices [press release]. Atlanta, GA: American Medical Association; November 17, 2105.
- Ventola CL. Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? P T. 2011;36:669-684.
- Rosenberg ME, Rosenberg SP. Changes in retail prices of prescription dermatologic drugs from 2009 to 2015 [published online November 25, 2015]. JAMA Dermatol. doi: 10.1001/jamadermatol.2015.3897.
- AMA calls for ban on direct to consumer advertising of prescription drugs and medical devices [press release]. Atlanta, GA: American Medical Association; November 17, 2105.
- Ventola CL. Direct-to-consumer pharmaceutical advertising: therapeutic or toxic? P T. 2011;36:669-684.
- Rosenberg ME, Rosenberg SP. Changes in retail prices of prescription dermatologic drugs from 2009 to 2015 [published online November 25, 2015]. JAMA Dermatol. doi: 10.1001/jamadermatol.2015.3897.
Patient Compliance With Photoprotection
What does your patient need to know at the first visit?
Patients need a realistic approach to photoprotection based on their genetics, including Fitzpatrick skin type and family history of melanoma and nonmelanoma skin cancer; skin examination for photodamage and photoaging as well as number and type of pigmented lesions; and lifestyle history, which should include location of residence as well as occupation and recreational pursuits. This discussion should, as usual, include questions about general health, systemic and skin disease, and medication usage, with particular focus on photoaggravated diseases such as lupus and melasma as well as ongoing use of topical agents and systemic photosensitizers. These inquiries should lead to a frank discussion of the patient’s risk for developing photodamage and skin cancer and other specific conditions that alter the advice you would give.
What are your go-to treatments? Is your recommendation anecdotal or evidence based? What are the side effects?
I always recommend that my patients use a product that they like, which may sound simplistic. But if the patient doesn’t like the feel and look of the sunscreen, he/she won’t use it. Patients routinely should use a sunscreen with a sun protection factor (SPF) of 30 or higher that also carries a “broad spectrum” label. At the beach or during sweaty sports, patients should use one with a water-resistant SPF.
I prefer spray sunscreens for application on the back if the patient is alone without someone to help apply sunscreen to hard-to-reach areas and for male scalps. But you never know how much spray to use, so use a lot!
If patients are at the beach, playing sports, or watching sports outside, then they should reapply sunscreen every 2 hours. If patients work indoors and use a facial sunscreen in the morning, that’s sufficient.
Although there is no evidence that sunscreens are harmful for children older than 6 months of age and pregnant women, if patients in these special populations have concerns, I recommend using agents with inorganic compounds (physical blockers) such as titanium dioxide and zinc oxide only. Children are best protected with clothing and hats.
The evidence supports this approach. Patients really don’t need SPF 30 protection, but no one uses the amount of product that will result in the SPF listed on the bottle. So if patients use an SPF 30 or greater, they will get at least an SPF 15, which is sufficient everywhere but at the equator. Using SPF 30 the way we all apply it will give SPF 15–level protection.
There is evidence that sunscreens prevent squamous cell carcinoma, actinic keratosis, and photoaging. Early evidence, less strong but positive, also suggests protection against basal cell carcinoma and melanoma.
The biggest side effect is not using the sunscreen. Others include irritation and allergy. Irritation is common, but finding a product to use without irritation should be easy. Allergy is rarer, and when it occurs, it is usually due to the preservative or fragrance, not the active ingredients. If allergy does occur, patch testing by a dermatologist is necessary to determine the allergen.
Although it is still controversial, wearing sunscreens religiously can lead to vitamin D insufficiency or deficiency, which is particularly true for individuals with skin of color—Fitzpatrick skin types IV, V, and VI—and those cancer patients who adhere to rigorous photoprotection. These patients should be encouraged to take supplemental vitamin D3 and I suggest 2000 IU; this recommendation is my opinion and is not evidence based.
As to the literature in the laypress about hormonal changes from benzophenone, cancer from retinoids, and nanoparticle toxicity: There is no evidence to support those claims.
How do you keep patients compliant with treatment?
Keep telling them, and then tell them again.
What do you do if they refuse treatment?
Tell them to see someone else.
What resources do you recommend to patients for more information?
Consult the American Academy of Dermatology Web site (www.aad.org) and the Skin Cancer Foundation (www.skincancer.org).
Editorial Note
Practical Pearls From the Cutis® Board is a new feature that will appear in print and online (www.cutis.com). Each month a member of the Cutis Editorial Board will provide pearls relating to the practice needs of dermatologists. Future topics will include:
- Electronic Medical Record Implementation
- Injection Technique With Fillers
- Psoriasis Treatment in Pregnancy
- Technology to Aid in Melanoma Diagnosis
- Plus more
Looking for pearls on a specific topic? The Editorial Board welcomes your feedback on potential topics. Send an e-mail to the Editorial Office ([email protected]) with your suggestions.
What does your patient need to know at the first visit?
Patients need a realistic approach to photoprotection based on their genetics, including Fitzpatrick skin type and family history of melanoma and nonmelanoma skin cancer; skin examination for photodamage and photoaging as well as number and type of pigmented lesions; and lifestyle history, which should include location of residence as well as occupation and recreational pursuits. This discussion should, as usual, include questions about general health, systemic and skin disease, and medication usage, with particular focus on photoaggravated diseases such as lupus and melasma as well as ongoing use of topical agents and systemic photosensitizers. These inquiries should lead to a frank discussion of the patient’s risk for developing photodamage and skin cancer and other specific conditions that alter the advice you would give.
What are your go-to treatments? Is your recommendation anecdotal or evidence based? What are the side effects?
I always recommend that my patients use a product that they like, which may sound simplistic. But if the patient doesn’t like the feel and look of the sunscreen, he/she won’t use it. Patients routinely should use a sunscreen with a sun protection factor (SPF) of 30 or higher that also carries a “broad spectrum” label. At the beach or during sweaty sports, patients should use one with a water-resistant SPF.
I prefer spray sunscreens for application on the back if the patient is alone without someone to help apply sunscreen to hard-to-reach areas and for male scalps. But you never know how much spray to use, so use a lot!
If patients are at the beach, playing sports, or watching sports outside, then they should reapply sunscreen every 2 hours. If patients work indoors and use a facial sunscreen in the morning, that’s sufficient.
Although there is no evidence that sunscreens are harmful for children older than 6 months of age and pregnant women, if patients in these special populations have concerns, I recommend using agents with inorganic compounds (physical blockers) such as titanium dioxide and zinc oxide only. Children are best protected with clothing and hats.
The evidence supports this approach. Patients really don’t need SPF 30 protection, but no one uses the amount of product that will result in the SPF listed on the bottle. So if patients use an SPF 30 or greater, they will get at least an SPF 15, which is sufficient everywhere but at the equator. Using SPF 30 the way we all apply it will give SPF 15–level protection.
There is evidence that sunscreens prevent squamous cell carcinoma, actinic keratosis, and photoaging. Early evidence, less strong but positive, also suggests protection against basal cell carcinoma and melanoma.
The biggest side effect is not using the sunscreen. Others include irritation and allergy. Irritation is common, but finding a product to use without irritation should be easy. Allergy is rarer, and when it occurs, it is usually due to the preservative or fragrance, not the active ingredients. If allergy does occur, patch testing by a dermatologist is necessary to determine the allergen.
Although it is still controversial, wearing sunscreens religiously can lead to vitamin D insufficiency or deficiency, which is particularly true for individuals with skin of color—Fitzpatrick skin types IV, V, and VI—and those cancer patients who adhere to rigorous photoprotection. These patients should be encouraged to take supplemental vitamin D3 and I suggest 2000 IU; this recommendation is my opinion and is not evidence based.
As to the literature in the laypress about hormonal changes from benzophenone, cancer from retinoids, and nanoparticle toxicity: There is no evidence to support those claims.
How do you keep patients compliant with treatment?
Keep telling them, and then tell them again.
What do you do if they refuse treatment?
Tell them to see someone else.
What resources do you recommend to patients for more information?
Consult the American Academy of Dermatology Web site (www.aad.org) and the Skin Cancer Foundation (www.skincancer.org).
Editorial Note
Practical Pearls From the Cutis® Board is a new feature that will appear in print and online (www.cutis.com). Each month a member of the Cutis Editorial Board will provide pearls relating to the practice needs of dermatologists. Future topics will include:
- Electronic Medical Record Implementation
- Injection Technique With Fillers
- Psoriasis Treatment in Pregnancy
- Technology to Aid in Melanoma Diagnosis
- Plus more
Looking for pearls on a specific topic? The Editorial Board welcomes your feedback on potential topics. Send an e-mail to the Editorial Office ([email protected]) with your suggestions.
What does your patient need to know at the first visit?
Patients need a realistic approach to photoprotection based on their genetics, including Fitzpatrick skin type and family history of melanoma and nonmelanoma skin cancer; skin examination for photodamage and photoaging as well as number and type of pigmented lesions; and lifestyle history, which should include location of residence as well as occupation and recreational pursuits. This discussion should, as usual, include questions about general health, systemic and skin disease, and medication usage, with particular focus on photoaggravated diseases such as lupus and melasma as well as ongoing use of topical agents and systemic photosensitizers. These inquiries should lead to a frank discussion of the patient’s risk for developing photodamage and skin cancer and other specific conditions that alter the advice you would give.
What are your go-to treatments? Is your recommendation anecdotal or evidence based? What are the side effects?
I always recommend that my patients use a product that they like, which may sound simplistic. But if the patient doesn’t like the feel and look of the sunscreen, he/she won’t use it. Patients routinely should use a sunscreen with a sun protection factor (SPF) of 30 or higher that also carries a “broad spectrum” label. At the beach or during sweaty sports, patients should use one with a water-resistant SPF.
I prefer spray sunscreens for application on the back if the patient is alone without someone to help apply sunscreen to hard-to-reach areas and for male scalps. But you never know how much spray to use, so use a lot!
If patients are at the beach, playing sports, or watching sports outside, then they should reapply sunscreen every 2 hours. If patients work indoors and use a facial sunscreen in the morning, that’s sufficient.
Although there is no evidence that sunscreens are harmful for children older than 6 months of age and pregnant women, if patients in these special populations have concerns, I recommend using agents with inorganic compounds (physical blockers) such as titanium dioxide and zinc oxide only. Children are best protected with clothing and hats.
The evidence supports this approach. Patients really don’t need SPF 30 protection, but no one uses the amount of product that will result in the SPF listed on the bottle. So if patients use an SPF 30 or greater, they will get at least an SPF 15, which is sufficient everywhere but at the equator. Using SPF 30 the way we all apply it will give SPF 15–level protection.
There is evidence that sunscreens prevent squamous cell carcinoma, actinic keratosis, and photoaging. Early evidence, less strong but positive, also suggests protection against basal cell carcinoma and melanoma.
The biggest side effect is not using the sunscreen. Others include irritation and allergy. Irritation is common, but finding a product to use without irritation should be easy. Allergy is rarer, and when it occurs, it is usually due to the preservative or fragrance, not the active ingredients. If allergy does occur, patch testing by a dermatologist is necessary to determine the allergen.
Although it is still controversial, wearing sunscreens religiously can lead to vitamin D insufficiency or deficiency, which is particularly true for individuals with skin of color—Fitzpatrick skin types IV, V, and VI—and those cancer patients who adhere to rigorous photoprotection. These patients should be encouraged to take supplemental vitamin D3 and I suggest 2000 IU; this recommendation is my opinion and is not evidence based.
As to the literature in the laypress about hormonal changes from benzophenone, cancer from retinoids, and nanoparticle toxicity: There is no evidence to support those claims.
How do you keep patients compliant with treatment?
Keep telling them, and then tell them again.
What do you do if they refuse treatment?
Tell them to see someone else.
What resources do you recommend to patients for more information?
Consult the American Academy of Dermatology Web site (www.aad.org) and the Skin Cancer Foundation (www.skincancer.org).
Editorial Note
Practical Pearls From the Cutis® Board is a new feature that will appear in print and online (www.cutis.com). Each month a member of the Cutis Editorial Board will provide pearls relating to the practice needs of dermatologists. Future topics will include:
- Electronic Medical Record Implementation
- Injection Technique With Fillers
- Psoriasis Treatment in Pregnancy
- Technology to Aid in Melanoma Diagnosis
- Plus more
Looking for pearls on a specific topic? The Editorial Board welcomes your feedback on potential topics. Send an e-mail to the Editorial Office ([email protected]) with your suggestions.
50 Years of Helping Dermatologists Improve Patient Care
Fifty years! It might not have been easy to imagine that a journal that is not supported by a medical group or society and is supplied free of charge to its audience could survive for half a century. But here we are, thanks to the continued interest of our readers and the hundreds of clinicians and scientists who have committed to the task of composing articles to educate their fellow physicians in the field of dermatology.
In the first issue of Cutis® (Figure), Chief Editor Eugene F. Traub, MD, outlined what were, and still are, the goals of the journal: to provide articles “dealing with common dermatoses or those rarer diseases of great interest to all practitioners.”1 Dr. Traub chose John T. McCarthy, MD, to conduct the day-to-day business of the journal as the Assistant Chief Editor. Dr. McCarthy then became Editor of Cutis in 1983 following Dr. Traub’s retirement and led the journal until his death in 2000. Dr. McCarthy loved his job and the journal, serving for an amazing 35 years, and could rightly be called “the father of Cutis.” In his 25th anniversary editorial entitled “Thank You,” he emphasized both the struggles and successes he experienced during his leadership and concluded by thanking the readers of Cutis for their support.2 During this time, his great friend and colleague Joseph W. Burnett, MD, served as Senior Associate Editor.
|

|
In 2001, along with my colleagues Jeffrey M. Weinberg, MD, and Nanette B. Silverberg, MD, I was honored to join the staff of Cutis as the new Editor-in-Chief. On that occasion, we laid out what we hoped would be some changes in the journal’s structure, aesthetics, and content, but we also stated our intention to maintain what we considered to be the most important aspect of the journal: “to publish ORIGINAL and PRACTICAL articles.”3 We have continued to emphasize the publication of articles that describe the “clinical presentation, diagnosis, histopathology, therapy, and management of the more common entities.”3
Medicine and the specialty of dermatology have changed in our 13 years at the helm of Cutis. With the changes brought by the digital revolution, the ways physicians, both young and old, can access information have been broadened. Our editorial staff has expanded our reach with online exclusives that comprise the digital component of the journal (http://www.cutis.com). Our digital archive dates back to 2000. We have greatly increased our outreach to our young colleagues in training with a Resident Resources section on our Web site, and we also have expanded our online presence with our popular Photo Challenge as well as audio and video commentaries.
Because our specialty has become more and more complex, Cutis will be taking a new route in 2015, focusing solely on practicing dermatologists, dermatopathologists, dermatologic surgeons, dermatology nurse practitioners and physician assistants, and our resident colleagues. Loyal readers from other areas of medicine will still have full online access to the journal.
At this juncture, we look forward to a reinvigoration of our efforts and another 50 years! We wish to thank all of our Editorial Board members for their continued dedication and support. Finally, my colleagues and I would like to thank all of the behind-the-scenes professionals that make the publication of this journal possible, with special thanks to the tireless efforts of our Senior Vice President/Group Publisher Sharon Finch and our Group Editor Melissa Steiger Sears. I would be remiss if I did not also thank our advertisers in the pharmaceutical industry; without their support publication would not be possible. And finally, in the words of Dr. McCarthy, “Most important of all is you, the reader.”2
1. Traub EF. Our editorial objectives. Cutis. 1965;1:9.
2. McCarthy JT. Thank you. 1990;45:80.
3. DeLeo VA, Weinberg JM, Silverberg NB. Original and practical. 2001;67:191.
Fifty years! It might not have been easy to imagine that a journal that is not supported by a medical group or society and is supplied free of charge to its audience could survive for half a century. But here we are, thanks to the continued interest of our readers and the hundreds of clinicians and scientists who have committed to the task of composing articles to educate their fellow physicians in the field of dermatology.
In the first issue of Cutis® (Figure), Chief Editor Eugene F. Traub, MD, outlined what were, and still are, the goals of the journal: to provide articles “dealing with common dermatoses or those rarer diseases of great interest to all practitioners.”1 Dr. Traub chose John T. McCarthy, MD, to conduct the day-to-day business of the journal as the Assistant Chief Editor. Dr. McCarthy then became Editor of Cutis in 1983 following Dr. Traub’s retirement and led the journal until his death in 2000. Dr. McCarthy loved his job and the journal, serving for an amazing 35 years, and could rightly be called “the father of Cutis.” In his 25th anniversary editorial entitled “Thank You,” he emphasized both the struggles and successes he experienced during his leadership and concluded by thanking the readers of Cutis for their support.2 During this time, his great friend and colleague Joseph W. Burnett, MD, served as Senior Associate Editor.

|

|
In 2001, along with my colleagues Jeffrey M. Weinberg, MD, and Nanette B. Silverberg, MD, I was honored to join the staff of Cutis as the new Editor-in-Chief. On that occasion, we laid out what we hoped would be some changes in the journal’s structure, aesthetics, and content, but we also stated our intention to maintain what we considered to be the most important aspect of the journal: “to publish ORIGINAL and PRACTICAL articles.”3 We have continued to emphasize the publication of articles that describe the “clinical presentation, diagnosis, histopathology, therapy, and management of the more common entities.”3
Medicine and the specialty of dermatology have changed in our 13 years at the helm of Cutis. With the changes brought by the digital revolution, the ways physicians, both young and old, can access information have been broadened. Our editorial staff has expanded our reach with online exclusives that comprise the digital component of the journal (http://www.cutis.com). Our digital archive dates back to 2000. We have greatly increased our outreach to our young colleagues in training with a Resident Resources section on our Web site, and we also have expanded our online presence with our popular Photo Challenge as well as audio and video commentaries.
Because our specialty has become more and more complex, Cutis will be taking a new route in 2015, focusing solely on practicing dermatologists, dermatopathologists, dermatologic surgeons, dermatology nurse practitioners and physician assistants, and our resident colleagues. Loyal readers from other areas of medicine will still have full online access to the journal.
At this juncture, we look forward to a reinvigoration of our efforts and another 50 years! We wish to thank all of our Editorial Board members for their continued dedication and support. Finally, my colleagues and I would like to thank all of the behind-the-scenes professionals that make the publication of this journal possible, with special thanks to the tireless efforts of our Senior Vice President/Group Publisher Sharon Finch and our Group Editor Melissa Steiger Sears. I would be remiss if I did not also thank our advertisers in the pharmaceutical industry; without their support publication would not be possible. And finally, in the words of Dr. McCarthy, “Most important of all is you, the reader.”2
Fifty years! It might not have been easy to imagine that a journal that is not supported by a medical group or society and is supplied free of charge to its audience could survive for half a century. But here we are, thanks to the continued interest of our readers and the hundreds of clinicians and scientists who have committed to the task of composing articles to educate their fellow physicians in the field of dermatology.
In the first issue of Cutis® (Figure), Chief Editor Eugene F. Traub, MD, outlined what were, and still are, the goals of the journal: to provide articles “dealing with common dermatoses or those rarer diseases of great interest to all practitioners.”1 Dr. Traub chose John T. McCarthy, MD, to conduct the day-to-day business of the journal as the Assistant Chief Editor. Dr. McCarthy then became Editor of Cutis in 1983 following Dr. Traub’s retirement and led the journal until his death in 2000. Dr. McCarthy loved his job and the journal, serving for an amazing 35 years, and could rightly be called “the father of Cutis.” In his 25th anniversary editorial entitled “Thank You,” he emphasized both the struggles and successes he experienced during his leadership and concluded by thanking the readers of Cutis for their support.2 During this time, his great friend and colleague Joseph W. Burnett, MD, served as Senior Associate Editor.

|

|
In 2001, along with my colleagues Jeffrey M. Weinberg, MD, and Nanette B. Silverberg, MD, I was honored to join the staff of Cutis as the new Editor-in-Chief. On that occasion, we laid out what we hoped would be some changes in the journal’s structure, aesthetics, and content, but we also stated our intention to maintain what we considered to be the most important aspect of the journal: “to publish ORIGINAL and PRACTICAL articles.”3 We have continued to emphasize the publication of articles that describe the “clinical presentation, diagnosis, histopathology, therapy, and management of the more common entities.”3
Medicine and the specialty of dermatology have changed in our 13 years at the helm of Cutis. With the changes brought by the digital revolution, the ways physicians, both young and old, can access information have been broadened. Our editorial staff has expanded our reach with online exclusives that comprise the digital component of the journal (http://www.cutis.com). Our digital archive dates back to 2000. We have greatly increased our outreach to our young colleagues in training with a Resident Resources section on our Web site, and we also have expanded our online presence with our popular Photo Challenge as well as audio and video commentaries.
Because our specialty has become more and more complex, Cutis will be taking a new route in 2015, focusing solely on practicing dermatologists, dermatopathologists, dermatologic surgeons, dermatology nurse practitioners and physician assistants, and our resident colleagues. Loyal readers from other areas of medicine will still have full online access to the journal.
At this juncture, we look forward to a reinvigoration of our efforts and another 50 years! We wish to thank all of our Editorial Board members for their continued dedication and support. Finally, my colleagues and I would like to thank all of the behind-the-scenes professionals that make the publication of this journal possible, with special thanks to the tireless efforts of our Senior Vice President/Group Publisher Sharon Finch and our Group Editor Melissa Steiger Sears. I would be remiss if I did not also thank our advertisers in the pharmaceutical industry; without their support publication would not be possible. And finally, in the words of Dr. McCarthy, “Most important of all is you, the reader.”2
1. Traub EF. Our editorial objectives. Cutis. 1965;1:9.
2. McCarthy JT. Thank you. 1990;45:80.
3. DeLeo VA, Weinberg JM, Silverberg NB. Original and practical. 2001;67:191.
1. Traub EF. Our editorial objectives. Cutis. 1965;1:9.
2. McCarthy JT. Thank you. 1990;45:80.
3. DeLeo VA, Weinberg JM, Silverberg NB. Original and practical. 2001;67:191.