User login
No advantages to using ADM in implant-based breast reconstruction
A European study involving 155 women found that the use of acellular dermal matrix (ADM) did not lead to fewer reoperations, nor was it superior in terms of health-related quality of life or patient-reported cosmetic outcomes.
“We feel that women considering implant-based reconstructions for breast cancer should be informed about the lack of evidence supporting its advantage,” said lead author Fredrik Lohmander MD, department of breast and endocrine surgery, section of breast urgery, Karolinska University Hospital, Stockholm.
It is difficult to say generally whether ADM should be used in IBBR, he noted. “We can only conclude from our trial that there is no hard evidence that ADM is beneficial when performing breast reconstructions with implants,” he said in an interview. “In selected patients, ADM might be indicated.”
The study was conducted in Sweden and the United Kingdom. “Mostly because of high costs, ADM in implant-based breast reconstructions in Sweden is not frequently used,” Dr. Lohmander said. “It is slightly more common in the U.K., but much more common in the U.S.A.”
Although biological meshes have received regulatory approval by the U.S. Food and Drug Administration for reconstructive purposes, ADM has not been approved for use in breast reconstruction surgery, and its use in this setting is off label.
The study was published online October 1 in JAMA Network Open.
Any advantage to using mesh device?
Previous studies of ADMs suggested that the mesh device conferred several benefits, including superior cosmetic results, less need for tissue expanders, fewer elective reoperations, and less capsular contracture. The use of a mesh device also enlarges the subpectoral pocket, which allows for larger fixed-volume implants, the authors note.
However, these suggested advantages have not been universally accepted, and the authors note that there have been reports of associated harm, such as higher rates of infection and implant loss.
The new study included 135 women from five centers in Sweden and the United Kingdom. The patients had breast cancer and had planned to undergo mastectomy and immediate IBBR between 2014 and May 2017.
The primary endpoint was the number of repeat surgeries at 2 years.
At the 2-year follow-up, 31 patients (48%) in the ADM group had undergone at least one reoperation on the ipsilateral side, vs 35 (54%) in the control group (P = .54). Results were similar for the contralateral side: 34 (53%) vs 31 (48%).
Two patients in the ADM group and three patients in the control group underwent a risk-reducing mastectomy on the contralateral side. These five surgeries were included in the final analysis.
For nine patients (14%) in the ADM arm, the implant was removed. Four of the removals took place within 6 months after early surgical complications. In the control group, seven patients (11%) underwent implant removal; four were removed within 6 months, owing to early surgical complications.
The secondary endpoint was postoperative health-related quality of life, including perception of body image and satisfaction with cosmetic outcome. There were no significant differences between the two groups.
Some questions remain
Approached for comment on the study, Sameer A. Patel, MD, FACS, chief of plastic and reconstructive surgery at Fox Chase Cancer Center, Philadelphia, noted that the practice of using AMD for breast reconstruction is quite common in the United States, so these data are informative and add to the current understanding of the value of ADM in breast reconstruction. “The study hypothesized that the use of ADM would reduce the number of reoperations within the first 24 months, which it did not,” he said. “This is despite the fact that the ADM group had a significantly higher number of direct-to-implant reconstructions.”
Importantly, the study showed that patient-reported outcomes, as opposed to surgeon’s evaluation of outcomes, were also not different for the most part between the two groups, Dr. Patel pointed out. “The only exception of small favorable advantage in the ADM group was for fitting bras,” he said.
However, there were limitations to the study’s endpoint. “I would add that there are some purported advantages of using ADM, such as reduction in postoperative pain and reduction in length of hospital stay, which are not evaluated by this study,” Patel explained. “Also, I am not certain that they can conclude from this study that capsular contracture is not reduced, because it is not designed to evaluate that.”
But the biggest limitation is one that study authors point out in their discussion at the end of the article, he added. “The use of prepectoral reconstruction is rapidly replacing the dual plane reconstruction that this paper used in the ADM group,” Dr. Patel said. “The role of ADM in prepectoral reconstruction is somewhat different than in the dual plane reconstruction, and so these results may not necessarily be extrapolated to prepectoral reconstruction.”
The study was funded with grants from the Swedish Breast Cancer Association and Stockholm City Council. The trial was initiated by Karolinska University Hospital and Karolinska Institutet. Acelity (an Allergan company) supplied the study with acellular dermal matrix meshes. Dr. Lohmander and Dr. Patel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A European study involving 155 women found that the use of acellular dermal matrix (ADM) did not lead to fewer reoperations, nor was it superior in terms of health-related quality of life or patient-reported cosmetic outcomes.
“We feel that women considering implant-based reconstructions for breast cancer should be informed about the lack of evidence supporting its advantage,” said lead author Fredrik Lohmander MD, department of breast and endocrine surgery, section of breast urgery, Karolinska University Hospital, Stockholm.
It is difficult to say generally whether ADM should be used in IBBR, he noted. “We can only conclude from our trial that there is no hard evidence that ADM is beneficial when performing breast reconstructions with implants,” he said in an interview. “In selected patients, ADM might be indicated.”
The study was conducted in Sweden and the United Kingdom. “Mostly because of high costs, ADM in implant-based breast reconstructions in Sweden is not frequently used,” Dr. Lohmander said. “It is slightly more common in the U.K., but much more common in the U.S.A.”
Although biological meshes have received regulatory approval by the U.S. Food and Drug Administration for reconstructive purposes, ADM has not been approved for use in breast reconstruction surgery, and its use in this setting is off label.
The study was published online October 1 in JAMA Network Open.
Any advantage to using mesh device?
Previous studies of ADMs suggested that the mesh device conferred several benefits, including superior cosmetic results, less need for tissue expanders, fewer elective reoperations, and less capsular contracture. The use of a mesh device also enlarges the subpectoral pocket, which allows for larger fixed-volume implants, the authors note.
However, these suggested advantages have not been universally accepted, and the authors note that there have been reports of associated harm, such as higher rates of infection and implant loss.
The new study included 135 women from five centers in Sweden and the United Kingdom. The patients had breast cancer and had planned to undergo mastectomy and immediate IBBR between 2014 and May 2017.
The primary endpoint was the number of repeat surgeries at 2 years.
At the 2-year follow-up, 31 patients (48%) in the ADM group had undergone at least one reoperation on the ipsilateral side, vs 35 (54%) in the control group (P = .54). Results were similar for the contralateral side: 34 (53%) vs 31 (48%).
Two patients in the ADM group and three patients in the control group underwent a risk-reducing mastectomy on the contralateral side. These five surgeries were included in the final analysis.
For nine patients (14%) in the ADM arm, the implant was removed. Four of the removals took place within 6 months after early surgical complications. In the control group, seven patients (11%) underwent implant removal; four were removed within 6 months, owing to early surgical complications.
The secondary endpoint was postoperative health-related quality of life, including perception of body image and satisfaction with cosmetic outcome. There were no significant differences between the two groups.
Some questions remain
Approached for comment on the study, Sameer A. Patel, MD, FACS, chief of plastic and reconstructive surgery at Fox Chase Cancer Center, Philadelphia, noted that the practice of using AMD for breast reconstruction is quite common in the United States, so these data are informative and add to the current understanding of the value of ADM in breast reconstruction. “The study hypothesized that the use of ADM would reduce the number of reoperations within the first 24 months, which it did not,” he said. “This is despite the fact that the ADM group had a significantly higher number of direct-to-implant reconstructions.”
Importantly, the study showed that patient-reported outcomes, as opposed to surgeon’s evaluation of outcomes, were also not different for the most part between the two groups, Dr. Patel pointed out. “The only exception of small favorable advantage in the ADM group was for fitting bras,” he said.
However, there were limitations to the study’s endpoint. “I would add that there are some purported advantages of using ADM, such as reduction in postoperative pain and reduction in length of hospital stay, which are not evaluated by this study,” Patel explained. “Also, I am not certain that they can conclude from this study that capsular contracture is not reduced, because it is not designed to evaluate that.”
But the biggest limitation is one that study authors point out in their discussion at the end of the article, he added. “The use of prepectoral reconstruction is rapidly replacing the dual plane reconstruction that this paper used in the ADM group,” Dr. Patel said. “The role of ADM in prepectoral reconstruction is somewhat different than in the dual plane reconstruction, and so these results may not necessarily be extrapolated to prepectoral reconstruction.”
The study was funded with grants from the Swedish Breast Cancer Association and Stockholm City Council. The trial was initiated by Karolinska University Hospital and Karolinska Institutet. Acelity (an Allergan company) supplied the study with acellular dermal matrix meshes. Dr. Lohmander and Dr. Patel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A European study involving 155 women found that the use of acellular dermal matrix (ADM) did not lead to fewer reoperations, nor was it superior in terms of health-related quality of life or patient-reported cosmetic outcomes.
“We feel that women considering implant-based reconstructions for breast cancer should be informed about the lack of evidence supporting its advantage,” said lead author Fredrik Lohmander MD, department of breast and endocrine surgery, section of breast urgery, Karolinska University Hospital, Stockholm.
It is difficult to say generally whether ADM should be used in IBBR, he noted. “We can only conclude from our trial that there is no hard evidence that ADM is beneficial when performing breast reconstructions with implants,” he said in an interview. “In selected patients, ADM might be indicated.”
The study was conducted in Sweden and the United Kingdom. “Mostly because of high costs, ADM in implant-based breast reconstructions in Sweden is not frequently used,” Dr. Lohmander said. “It is slightly more common in the U.K., but much more common in the U.S.A.”
Although biological meshes have received regulatory approval by the U.S. Food and Drug Administration for reconstructive purposes, ADM has not been approved for use in breast reconstruction surgery, and its use in this setting is off label.
The study was published online October 1 in JAMA Network Open.
Any advantage to using mesh device?
Previous studies of ADMs suggested that the mesh device conferred several benefits, including superior cosmetic results, less need for tissue expanders, fewer elective reoperations, and less capsular contracture. The use of a mesh device also enlarges the subpectoral pocket, which allows for larger fixed-volume implants, the authors note.
However, these suggested advantages have not been universally accepted, and the authors note that there have been reports of associated harm, such as higher rates of infection and implant loss.
The new study included 135 women from five centers in Sweden and the United Kingdom. The patients had breast cancer and had planned to undergo mastectomy and immediate IBBR between 2014 and May 2017.
The primary endpoint was the number of repeat surgeries at 2 years.
At the 2-year follow-up, 31 patients (48%) in the ADM group had undergone at least one reoperation on the ipsilateral side, vs 35 (54%) in the control group (P = .54). Results were similar for the contralateral side: 34 (53%) vs 31 (48%).
Two patients in the ADM group and three patients in the control group underwent a risk-reducing mastectomy on the contralateral side. These five surgeries were included in the final analysis.
For nine patients (14%) in the ADM arm, the implant was removed. Four of the removals took place within 6 months after early surgical complications. In the control group, seven patients (11%) underwent implant removal; four were removed within 6 months, owing to early surgical complications.
The secondary endpoint was postoperative health-related quality of life, including perception of body image and satisfaction with cosmetic outcome. There were no significant differences between the two groups.
Some questions remain
Approached for comment on the study, Sameer A. Patel, MD, FACS, chief of plastic and reconstructive surgery at Fox Chase Cancer Center, Philadelphia, noted that the practice of using AMD for breast reconstruction is quite common in the United States, so these data are informative and add to the current understanding of the value of ADM in breast reconstruction. “The study hypothesized that the use of ADM would reduce the number of reoperations within the first 24 months, which it did not,” he said. “This is despite the fact that the ADM group had a significantly higher number of direct-to-implant reconstructions.”
Importantly, the study showed that patient-reported outcomes, as opposed to surgeon’s evaluation of outcomes, were also not different for the most part between the two groups, Dr. Patel pointed out. “The only exception of small favorable advantage in the ADM group was for fitting bras,” he said.
However, there were limitations to the study’s endpoint. “I would add that there are some purported advantages of using ADM, such as reduction in postoperative pain and reduction in length of hospital stay, which are not evaluated by this study,” Patel explained. “Also, I am not certain that they can conclude from this study that capsular contracture is not reduced, because it is not designed to evaluate that.”
But the biggest limitation is one that study authors point out in their discussion at the end of the article, he added. “The use of prepectoral reconstruction is rapidly replacing the dual plane reconstruction that this paper used in the ADM group,” Dr. Patel said. “The role of ADM in prepectoral reconstruction is somewhat different than in the dual plane reconstruction, and so these results may not necessarily be extrapolated to prepectoral reconstruction.”
The study was funded with grants from the Swedish Breast Cancer Association and Stockholm City Council. The trial was initiated by Karolinska University Hospital and Karolinska Institutet. Acelity (an Allergan company) supplied the study with acellular dermal matrix meshes. Dr. Lohmander and Dr. Patel have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Childhood vaccination rates up since early pandemic, but few are up to date
The proportion of children caught up on vaccinations is lower than 2019 levels, despite an increase in weekly vaccine administration among children from summer to fall 2020.
The finding, published in JAMA Pediatrics, joins a growing collection of studies examining the COVID-19 pandemic’s effect on routine pediatric vaccine delivery. A 2021 survey from the Urban Institute that found that nearly one in five parents delayed or did not get care for their children in the past 12 months because of fear of exposure to the virus.
“We need to think about what additional interventions are needed to promote catch-up vaccination, especially for those at-risk populations that we saw were undervaccinated even prior to the pandemic,” study author Malini B. DeSilva, MD, MPH, said in an interview. “[That means] working creatively to ensure that all children would have the opportunity to receive these recommended vaccines.”
While examining data on pediatric vaccination of 1.4 million children between Jan. 5, 2020, and Oct. 3, 2020, across eight health systems in California, Oregon, Washington, Colorado, Minnesota, and Wisconsin, Dr. DeSilva and colleagues saw vaccination administration rates return to near prepandemic levels after an initial decline, particularly after the Centers for Disease Control and Prevention and American Academy of Pediatrics guidelines specified that in-person visits for children younger than 2 years should be prioritized.
“I think we’ve all been concerned and aware that people just weren’t bringing their children to their pediatricians as frequently [caused by] the fear of being in medical settings during the heat of the pandemic,” said James Schneider, MD, who was not involved with the study. “So it’s not surprising that we saw lower rates of overall vaccinations in all age groups.”
The current study found that lower vaccination rates persisted among most age groups from March to September 2020. However, during the period of expanded primary care, which took place between May and October 2020, vaccination administration rates in infants younger than 2 years old and children aged 4-6 years approached or were equal to 2019 rates. However, these rebounds were not enough to make up for the missed vaccines.
Still, only 74% of infants reaching 7 months old in September 2020 were caught up on their vaccinations, compared with 81% of infants turning the same age in 2019. Researchers also found that, compared with 61% of infants reaching 18 months in September 2019, only 57% of 18-month-olds were up to date with vaccinations in September 2020. However, the proportion of 6-, 13-, and 18-year-olds up to date on vaccinations were about the same in 2020 and 2019.
Racial disparities also persisted during this time, with Black children having the lowest proportion of up-to-date vaccinations for most ages from January to September 2020. Although these disparities were evident prior to the pandemic, these differences became more pronounced for the 18-month-old age group, where just 41% of Black infants were up to date in vaccinations, compared with 76% of Asian infants, 54% of Hispanics infants, and 56% of White infants.
Dr. Schneider believes Dr. DeSilva’s study is a “robust” one and paints an accurate picture of the pandemic’s effect on pediatric vaccinations, despite examining data from just eight health systems.
“I think it’s a fairly reasonable representation of what we already have been recognizing during the pandemic,” he explained. “Which is that people are really reluctant to go to their physicians’ offices for routine care because of the fear of getting sick. I think the study emphasized the importance of catching these children up to keep them safe in the future.”
The Advisory Committee on Immunization Practices recommends a childhood immunization schedule that protects children against 14 infectious diseases before their second birthday. Since the on-time administration of these vaccines is essential for preventing communicable diseases, many pediatric offices are trying to ensure a safe environment for patients and families, said Dr. Schneider, chief of pediatric critical care at Cohen Children’s Medical Center, New York.
There’s also some concern that COVID-19 vaccine hesitancy my spillover into routine childhood vaccinations, especially for families who were already hesitant toward the routine well-established vaccine schedule for children.
The CDC and AAP recommend that children continue to receive recommended vaccinations during the COVID-19 pandemic.
To boost the number of children caught up on vaccinations, health system and community-level interventions are needed, especially in underserved communities, the researchers wrote. Additionally, enforcing mandates that require vaccination prior to school entry could also increase vaccine administration across populations and reduce disparities.
The study emphasizes the “immediate and lagging” disruptions in the delivery of pediatric health care caused by the pandemic, which will likely have long-term consequences for pediatric health, Brian P. Jenssen, MD, MSHP, who was not involved in the study, wrote in a solicited commentary.
However, interventions tailored to specific age groups could help remedy this. These include increasing the frequency of well-child care during the next year of life for infants younger than 24 months and prioritizing visits with 13-year-old adolescents who are behind on vaccinations.
“Although there is no evidence base for this approach, such a change could create not only catch-up opportunities for vaccination for children delayed at age 7 and 18 months, but also provide opportunities to attend to developmental concerns and social needs that have emerged during COVID-19,” wrote Dr. Jenssen, a researcher and primary care pediatrician at Children’s Hospital of Philadelphia.
Other practices such as reaching out to patients and families directly via text message, email, or phone to “notify them of needed vaccinations,” vaccine mandates, and having pediatric health systems partner with alternative settings to promote vaccination could also get kids back on track, health wise. Furthermore, financial incentives from insurers or primary care practices also may help.
“The COVID-19 pandemic’s lost care may have long-term consequences unless pediatric health care systems and child health advocates are proactive in engaging families to take advantage of every opportunity to catch up,” Dr. Jenssen wrote.
The proportion of children caught up on vaccinations is lower than 2019 levels, despite an increase in weekly vaccine administration among children from summer to fall 2020.
The finding, published in JAMA Pediatrics, joins a growing collection of studies examining the COVID-19 pandemic’s effect on routine pediatric vaccine delivery. A 2021 survey from the Urban Institute that found that nearly one in five parents delayed or did not get care for their children in the past 12 months because of fear of exposure to the virus.
“We need to think about what additional interventions are needed to promote catch-up vaccination, especially for those at-risk populations that we saw were undervaccinated even prior to the pandemic,” study author Malini B. DeSilva, MD, MPH, said in an interview. “[That means] working creatively to ensure that all children would have the opportunity to receive these recommended vaccines.”
While examining data on pediatric vaccination of 1.4 million children between Jan. 5, 2020, and Oct. 3, 2020, across eight health systems in California, Oregon, Washington, Colorado, Minnesota, and Wisconsin, Dr. DeSilva and colleagues saw vaccination administration rates return to near prepandemic levels after an initial decline, particularly after the Centers for Disease Control and Prevention and American Academy of Pediatrics guidelines specified that in-person visits for children younger than 2 years should be prioritized.
“I think we’ve all been concerned and aware that people just weren’t bringing their children to their pediatricians as frequently [caused by] the fear of being in medical settings during the heat of the pandemic,” said James Schneider, MD, who was not involved with the study. “So it’s not surprising that we saw lower rates of overall vaccinations in all age groups.”
The current study found that lower vaccination rates persisted among most age groups from March to September 2020. However, during the period of expanded primary care, which took place between May and October 2020, vaccination administration rates in infants younger than 2 years old and children aged 4-6 years approached or were equal to 2019 rates. However, these rebounds were not enough to make up for the missed vaccines.
Still, only 74% of infants reaching 7 months old in September 2020 were caught up on their vaccinations, compared with 81% of infants turning the same age in 2019. Researchers also found that, compared with 61% of infants reaching 18 months in September 2019, only 57% of 18-month-olds were up to date with vaccinations in September 2020. However, the proportion of 6-, 13-, and 18-year-olds up to date on vaccinations were about the same in 2020 and 2019.
Racial disparities also persisted during this time, with Black children having the lowest proportion of up-to-date vaccinations for most ages from January to September 2020. Although these disparities were evident prior to the pandemic, these differences became more pronounced for the 18-month-old age group, where just 41% of Black infants were up to date in vaccinations, compared with 76% of Asian infants, 54% of Hispanics infants, and 56% of White infants.
Dr. Schneider believes Dr. DeSilva’s study is a “robust” one and paints an accurate picture of the pandemic’s effect on pediatric vaccinations, despite examining data from just eight health systems.
“I think it’s a fairly reasonable representation of what we already have been recognizing during the pandemic,” he explained. “Which is that people are really reluctant to go to their physicians’ offices for routine care because of the fear of getting sick. I think the study emphasized the importance of catching these children up to keep them safe in the future.”
The Advisory Committee on Immunization Practices recommends a childhood immunization schedule that protects children against 14 infectious diseases before their second birthday. Since the on-time administration of these vaccines is essential for preventing communicable diseases, many pediatric offices are trying to ensure a safe environment for patients and families, said Dr. Schneider, chief of pediatric critical care at Cohen Children’s Medical Center, New York.
There’s also some concern that COVID-19 vaccine hesitancy my spillover into routine childhood vaccinations, especially for families who were already hesitant toward the routine well-established vaccine schedule for children.
The CDC and AAP recommend that children continue to receive recommended vaccinations during the COVID-19 pandemic.
To boost the number of children caught up on vaccinations, health system and community-level interventions are needed, especially in underserved communities, the researchers wrote. Additionally, enforcing mandates that require vaccination prior to school entry could also increase vaccine administration across populations and reduce disparities.
The study emphasizes the “immediate and lagging” disruptions in the delivery of pediatric health care caused by the pandemic, which will likely have long-term consequences for pediatric health, Brian P. Jenssen, MD, MSHP, who was not involved in the study, wrote in a solicited commentary.
However, interventions tailored to specific age groups could help remedy this. These include increasing the frequency of well-child care during the next year of life for infants younger than 24 months and prioritizing visits with 13-year-old adolescents who are behind on vaccinations.
“Although there is no evidence base for this approach, such a change could create not only catch-up opportunities for vaccination for children delayed at age 7 and 18 months, but also provide opportunities to attend to developmental concerns and social needs that have emerged during COVID-19,” wrote Dr. Jenssen, a researcher and primary care pediatrician at Children’s Hospital of Philadelphia.
Other practices such as reaching out to patients and families directly via text message, email, or phone to “notify them of needed vaccinations,” vaccine mandates, and having pediatric health systems partner with alternative settings to promote vaccination could also get kids back on track, health wise. Furthermore, financial incentives from insurers or primary care practices also may help.
“The COVID-19 pandemic’s lost care may have long-term consequences unless pediatric health care systems and child health advocates are proactive in engaging families to take advantage of every opportunity to catch up,” Dr. Jenssen wrote.
The proportion of children caught up on vaccinations is lower than 2019 levels, despite an increase in weekly vaccine administration among children from summer to fall 2020.
The finding, published in JAMA Pediatrics, joins a growing collection of studies examining the COVID-19 pandemic’s effect on routine pediatric vaccine delivery. A 2021 survey from the Urban Institute that found that nearly one in five parents delayed or did not get care for their children in the past 12 months because of fear of exposure to the virus.
“We need to think about what additional interventions are needed to promote catch-up vaccination, especially for those at-risk populations that we saw were undervaccinated even prior to the pandemic,” study author Malini B. DeSilva, MD, MPH, said in an interview. “[That means] working creatively to ensure that all children would have the opportunity to receive these recommended vaccines.”
While examining data on pediatric vaccination of 1.4 million children between Jan. 5, 2020, and Oct. 3, 2020, across eight health systems in California, Oregon, Washington, Colorado, Minnesota, and Wisconsin, Dr. DeSilva and colleagues saw vaccination administration rates return to near prepandemic levels after an initial decline, particularly after the Centers for Disease Control and Prevention and American Academy of Pediatrics guidelines specified that in-person visits for children younger than 2 years should be prioritized.
“I think we’ve all been concerned and aware that people just weren’t bringing their children to their pediatricians as frequently [caused by] the fear of being in medical settings during the heat of the pandemic,” said James Schneider, MD, who was not involved with the study. “So it’s not surprising that we saw lower rates of overall vaccinations in all age groups.”
The current study found that lower vaccination rates persisted among most age groups from March to September 2020. However, during the period of expanded primary care, which took place between May and October 2020, vaccination administration rates in infants younger than 2 years old and children aged 4-6 years approached or were equal to 2019 rates. However, these rebounds were not enough to make up for the missed vaccines.
Still, only 74% of infants reaching 7 months old in September 2020 were caught up on their vaccinations, compared with 81% of infants turning the same age in 2019. Researchers also found that, compared with 61% of infants reaching 18 months in September 2019, only 57% of 18-month-olds were up to date with vaccinations in September 2020. However, the proportion of 6-, 13-, and 18-year-olds up to date on vaccinations were about the same in 2020 and 2019.
Racial disparities also persisted during this time, with Black children having the lowest proportion of up-to-date vaccinations for most ages from January to September 2020. Although these disparities were evident prior to the pandemic, these differences became more pronounced for the 18-month-old age group, where just 41% of Black infants were up to date in vaccinations, compared with 76% of Asian infants, 54% of Hispanics infants, and 56% of White infants.
Dr. Schneider believes Dr. DeSilva’s study is a “robust” one and paints an accurate picture of the pandemic’s effect on pediatric vaccinations, despite examining data from just eight health systems.
“I think it’s a fairly reasonable representation of what we already have been recognizing during the pandemic,” he explained. “Which is that people are really reluctant to go to their physicians’ offices for routine care because of the fear of getting sick. I think the study emphasized the importance of catching these children up to keep them safe in the future.”
The Advisory Committee on Immunization Practices recommends a childhood immunization schedule that protects children against 14 infectious diseases before their second birthday. Since the on-time administration of these vaccines is essential for preventing communicable diseases, many pediatric offices are trying to ensure a safe environment for patients and families, said Dr. Schneider, chief of pediatric critical care at Cohen Children’s Medical Center, New York.
There’s also some concern that COVID-19 vaccine hesitancy my spillover into routine childhood vaccinations, especially for families who were already hesitant toward the routine well-established vaccine schedule for children.
The CDC and AAP recommend that children continue to receive recommended vaccinations during the COVID-19 pandemic.
To boost the number of children caught up on vaccinations, health system and community-level interventions are needed, especially in underserved communities, the researchers wrote. Additionally, enforcing mandates that require vaccination prior to school entry could also increase vaccine administration across populations and reduce disparities.
The study emphasizes the “immediate and lagging” disruptions in the delivery of pediatric health care caused by the pandemic, which will likely have long-term consequences for pediatric health, Brian P. Jenssen, MD, MSHP, who was not involved in the study, wrote in a solicited commentary.
However, interventions tailored to specific age groups could help remedy this. These include increasing the frequency of well-child care during the next year of life for infants younger than 24 months and prioritizing visits with 13-year-old adolescents who are behind on vaccinations.
“Although there is no evidence base for this approach, such a change could create not only catch-up opportunities for vaccination for children delayed at age 7 and 18 months, but also provide opportunities to attend to developmental concerns and social needs that have emerged during COVID-19,” wrote Dr. Jenssen, a researcher and primary care pediatrician at Children’s Hospital of Philadelphia.
Other practices such as reaching out to patients and families directly via text message, email, or phone to “notify them of needed vaccinations,” vaccine mandates, and having pediatric health systems partner with alternative settings to promote vaccination could also get kids back on track, health wise. Furthermore, financial incentives from insurers or primary care practices also may help.
“The COVID-19 pandemic’s lost care may have long-term consequences unless pediatric health care systems and child health advocates are proactive in engaging families to take advantage of every opportunity to catch up,” Dr. Jenssen wrote.
FROM JAMA PEDIATRICS
AAN blasts ‘runaway’ costs for neurologic and other prescription drugs
This situation is also taking a toll on neurologists’ mental health, who already have the second-highest burnout rate across medical specialties, the statement adds.
The statement was published online Oct. 5, 2021, in Neurology.
Dramatic price increases
Drafted by the Ethics, Law, and Humanities Committee – a joint committee that includes the AAN, the American Neurological Association, and the Child Neurology Society – the statement was prompted by a 2018 report from the AAN Neurology Drug Pricing Task Force to address challenges associated with high drug costs.
It highlights ethical concerns from high drug costs, policy proposals that might temper the problem, and how clinicians can adjust to the current reality of pharmaceutical pricing and better advocate for changes to the healthcare system.
“Runaway drug costs continue to be a pressing problem with recent dramatic price increases not only for specialty drugs, but also generic ones,” said lead author Amy Tsou, MD, MSc, codirector of the ECRI Evidence-Based Practice Center at the Center for Evidence and Guidelines in Plymouth Meeting, Pa.
She noted that one in four Americans has difficulty paying for medication, and many report going without a medication because of cost.
“Ensuring a fair system for drug pricing and coverage rules that balance the goods of individual patients with the needs of broader populations when resources are limited remains more important than ever,” Dr. Tsou said.
Out-of-pocket costs for neurologic medications have risen dramatically over the past decade, with the fastest rise reported among drugs for multiple sclerosis. Results from a study published in 2019 showed that, between 2004 and 2016, patients’ out-of-pocket expenses skyrocketed from $15 a month to $309 a month.
The steep increases have forced some neurology patients to ration their medication or stop taking it altogether, which is one of the ethical concerns cited in the AAN statement.
Patient self-rationing
Commenting on the statement, Ilana Katz Sand, MD, associate director of the Dickinson Center for Multiple Sclerosis at Mount Sinai Medical Center, New York, noted that clinicians are already acutely aware of the effect high drug costs have on their patients’ medical decisions. However, statements such as the current one bring much-needed outside attention to the problem.
“I’ve definitely had more and more people struggling with deductibles and copays, even among people who are insured,” said Dr. Katz Sand, who was not involved with the AAN paper.
She has a number of patients who have rationed their medication or stopped taking it altogether when their copays increased or they lost access to a copay assistance program because their insurance company chose to cover a still-expensive generic drug with no assistance program over a slightly costlier brand-name medication that comes with patient discounts.
Too often, patients don’t tell her they’re not taking their medication as prescribed. At a recent appointment, Dr. Katz Sand learned about a patient’s drug rationing only after a routine MRI showed new brain lesions that regular treatment might have prevented.
Another patient, new to her clinic, questioned the treatment plan Dr. Katz Sand recommended because they could not cover the copay. This sort of self-rationing happens in patients with and without insurance, she added.
“It’s a terrible thing and it’s happening to all patients,” Dr. Katz Sand said, adding that “the old credo of ‘yeah, the drug prices are high, but they are covered by insurance’ is not a sustainable argument anymore.”
What neurologists can do
Some sort of rationing is an unavoidable outcome of steep treatment costs, the authors noted. But what does that mean in clinical practice?
Neurologists should be aware of the costs involved in ordering diagnostic tests, treatment, or medication – and shouldn’t feel compelled to order treatments or tests that they feel are medically inappropriate just because a patient requests them, the authors wrote.
The statement also encourages clinicians to include financial realities in the shared decision-making process with patients.
However, Dr. Katz Sand said that is not always possible. Drug prices aren’t fixed, with different insurance plans offering different pricing, deductibles, and copays. “It’s hard for us to attempt to incorporate discussions about price in our discussions with patients when we can’t even predict what their out-of-pocket cost is going to be,” she said.
“Every single prescription we write requires prior authorization, and that’s directly related to the fact that the cost of these drugs is so high,” she added.
As do many other clinicians, Dr. Katz Sand spends hours each week on preauthorization forms and haggling with insurance companies on behalf of her patients. To get needed medication at a cost they can afford sometimes takes creative problem solving and almost always takes a lot of time. “It all adds to the administrative burden, patients’ stress, and our stress,” she said.
Physician-advocates needed
The AAN paper identifies a number of policy reforms to address drug pricing at a national level, including giving Medicare officials the power to negotiate drug prices, allowing the safe importation of drugs from other countries, and speeding the Food and Drug Administration approval process for generic drugs.
There is also a need to address systemic problems that, the authors noted, help create and perpetuate health care disparities. Lawmakers at the state and federal level are considering a number of these policy ideas and others that could address the kinds of issues Dr. Katz Sand described. However, the chances of their success are slim at best, said Bruce H. Cohen, MD, chair of the AAN advocacy committee and director of the NeuroDevelopmental Science Center at Akron (Ohio) Children’s Hospital.
“On a federal level, we’re watching in real time how the entrenched divisions, even within parties, are resulting in continued stalemate,” said Dr. Cohen, who is not one of the statement authors.
Those ideas need advocates and the AAN paper suggests neurologists should be among the ones championing these changes, he added.
“One of the most effective strategies is to bring attention to the impact high drug costs have on neurology patients and medical practices,” Dr. Cohen said. “It’s so important to make sure policy makers know the significant impact of high drug costs in neurology and within the context of finite resources.”
One way to do that is to share statements such as the current one with members of Congress working on policy reform, Dr. Cohen said. Another is through programs such as the academy’s annual Neurology on the Hill conference.
A third strategy is to encourage individuals such as Dr. Katz Sand to speak out when and where they can, he added.
While she agrees with the idea, finding time for advocacy work amid patient care, administrative work, and research is challenging. Dr. Katz Sand would like to see groups like the AAN work with health care institutions to implement policies that allocate time and resources to train clinicians in advocacy – and then support their efforts on that front.
“I’m really glad they wrote this and think they did a good job of crystallizing the issues,” Dr. Katz Sand said. “It’s good to put it out there as a document that could help serve as the basis for the requests we make collectively. I just hope that people listen.”
The paper received no funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This situation is also taking a toll on neurologists’ mental health, who already have the second-highest burnout rate across medical specialties, the statement adds.
The statement was published online Oct. 5, 2021, in Neurology.
Dramatic price increases
Drafted by the Ethics, Law, and Humanities Committee – a joint committee that includes the AAN, the American Neurological Association, and the Child Neurology Society – the statement was prompted by a 2018 report from the AAN Neurology Drug Pricing Task Force to address challenges associated with high drug costs.
It highlights ethical concerns from high drug costs, policy proposals that might temper the problem, and how clinicians can adjust to the current reality of pharmaceutical pricing and better advocate for changes to the healthcare system.
“Runaway drug costs continue to be a pressing problem with recent dramatic price increases not only for specialty drugs, but also generic ones,” said lead author Amy Tsou, MD, MSc, codirector of the ECRI Evidence-Based Practice Center at the Center for Evidence and Guidelines in Plymouth Meeting, Pa.
She noted that one in four Americans has difficulty paying for medication, and many report going without a medication because of cost.
“Ensuring a fair system for drug pricing and coverage rules that balance the goods of individual patients with the needs of broader populations when resources are limited remains more important than ever,” Dr. Tsou said.
Out-of-pocket costs for neurologic medications have risen dramatically over the past decade, with the fastest rise reported among drugs for multiple sclerosis. Results from a study published in 2019 showed that, between 2004 and 2016, patients’ out-of-pocket expenses skyrocketed from $15 a month to $309 a month.
The steep increases have forced some neurology patients to ration their medication or stop taking it altogether, which is one of the ethical concerns cited in the AAN statement.
Patient self-rationing
Commenting on the statement, Ilana Katz Sand, MD, associate director of the Dickinson Center for Multiple Sclerosis at Mount Sinai Medical Center, New York, noted that clinicians are already acutely aware of the effect high drug costs have on their patients’ medical decisions. However, statements such as the current one bring much-needed outside attention to the problem.
“I’ve definitely had more and more people struggling with deductibles and copays, even among people who are insured,” said Dr. Katz Sand, who was not involved with the AAN paper.
She has a number of patients who have rationed their medication or stopped taking it altogether when their copays increased or they lost access to a copay assistance program because their insurance company chose to cover a still-expensive generic drug with no assistance program over a slightly costlier brand-name medication that comes with patient discounts.
Too often, patients don’t tell her they’re not taking their medication as prescribed. At a recent appointment, Dr. Katz Sand learned about a patient’s drug rationing only after a routine MRI showed new brain lesions that regular treatment might have prevented.
Another patient, new to her clinic, questioned the treatment plan Dr. Katz Sand recommended because they could not cover the copay. This sort of self-rationing happens in patients with and without insurance, she added.
“It’s a terrible thing and it’s happening to all patients,” Dr. Katz Sand said, adding that “the old credo of ‘yeah, the drug prices are high, but they are covered by insurance’ is not a sustainable argument anymore.”
What neurologists can do
Some sort of rationing is an unavoidable outcome of steep treatment costs, the authors noted. But what does that mean in clinical practice?
Neurologists should be aware of the costs involved in ordering diagnostic tests, treatment, or medication – and shouldn’t feel compelled to order treatments or tests that they feel are medically inappropriate just because a patient requests them, the authors wrote.
The statement also encourages clinicians to include financial realities in the shared decision-making process with patients.
However, Dr. Katz Sand said that is not always possible. Drug prices aren’t fixed, with different insurance plans offering different pricing, deductibles, and copays. “It’s hard for us to attempt to incorporate discussions about price in our discussions with patients when we can’t even predict what their out-of-pocket cost is going to be,” she said.
“Every single prescription we write requires prior authorization, and that’s directly related to the fact that the cost of these drugs is so high,” she added.
As do many other clinicians, Dr. Katz Sand spends hours each week on preauthorization forms and haggling with insurance companies on behalf of her patients. To get needed medication at a cost they can afford sometimes takes creative problem solving and almost always takes a lot of time. “It all adds to the administrative burden, patients’ stress, and our stress,” she said.
Physician-advocates needed
The AAN paper identifies a number of policy reforms to address drug pricing at a national level, including giving Medicare officials the power to negotiate drug prices, allowing the safe importation of drugs from other countries, and speeding the Food and Drug Administration approval process for generic drugs.
There is also a need to address systemic problems that, the authors noted, help create and perpetuate health care disparities. Lawmakers at the state and federal level are considering a number of these policy ideas and others that could address the kinds of issues Dr. Katz Sand described. However, the chances of their success are slim at best, said Bruce H. Cohen, MD, chair of the AAN advocacy committee and director of the NeuroDevelopmental Science Center at Akron (Ohio) Children’s Hospital.
“On a federal level, we’re watching in real time how the entrenched divisions, even within parties, are resulting in continued stalemate,” said Dr. Cohen, who is not one of the statement authors.
Those ideas need advocates and the AAN paper suggests neurologists should be among the ones championing these changes, he added.
“One of the most effective strategies is to bring attention to the impact high drug costs have on neurology patients and medical practices,” Dr. Cohen said. “It’s so important to make sure policy makers know the significant impact of high drug costs in neurology and within the context of finite resources.”
One way to do that is to share statements such as the current one with members of Congress working on policy reform, Dr. Cohen said. Another is through programs such as the academy’s annual Neurology on the Hill conference.
A third strategy is to encourage individuals such as Dr. Katz Sand to speak out when and where they can, he added.
While she agrees with the idea, finding time for advocacy work amid patient care, administrative work, and research is challenging. Dr. Katz Sand would like to see groups like the AAN work with health care institutions to implement policies that allocate time and resources to train clinicians in advocacy – and then support their efforts on that front.
“I’m really glad they wrote this and think they did a good job of crystallizing the issues,” Dr. Katz Sand said. “It’s good to put it out there as a document that could help serve as the basis for the requests we make collectively. I just hope that people listen.”
The paper received no funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This situation is also taking a toll on neurologists’ mental health, who already have the second-highest burnout rate across medical specialties, the statement adds.
The statement was published online Oct. 5, 2021, in Neurology.
Dramatic price increases
Drafted by the Ethics, Law, and Humanities Committee – a joint committee that includes the AAN, the American Neurological Association, and the Child Neurology Society – the statement was prompted by a 2018 report from the AAN Neurology Drug Pricing Task Force to address challenges associated with high drug costs.
It highlights ethical concerns from high drug costs, policy proposals that might temper the problem, and how clinicians can adjust to the current reality of pharmaceutical pricing and better advocate for changes to the healthcare system.
“Runaway drug costs continue to be a pressing problem with recent dramatic price increases not only for specialty drugs, but also generic ones,” said lead author Amy Tsou, MD, MSc, codirector of the ECRI Evidence-Based Practice Center at the Center for Evidence and Guidelines in Plymouth Meeting, Pa.
She noted that one in four Americans has difficulty paying for medication, and many report going without a medication because of cost.
“Ensuring a fair system for drug pricing and coverage rules that balance the goods of individual patients with the needs of broader populations when resources are limited remains more important than ever,” Dr. Tsou said.
Out-of-pocket costs for neurologic medications have risen dramatically over the past decade, with the fastest rise reported among drugs for multiple sclerosis. Results from a study published in 2019 showed that, between 2004 and 2016, patients’ out-of-pocket expenses skyrocketed from $15 a month to $309 a month.
The steep increases have forced some neurology patients to ration their medication or stop taking it altogether, which is one of the ethical concerns cited in the AAN statement.
Patient self-rationing
Commenting on the statement, Ilana Katz Sand, MD, associate director of the Dickinson Center for Multiple Sclerosis at Mount Sinai Medical Center, New York, noted that clinicians are already acutely aware of the effect high drug costs have on their patients’ medical decisions. However, statements such as the current one bring much-needed outside attention to the problem.
“I’ve definitely had more and more people struggling with deductibles and copays, even among people who are insured,” said Dr. Katz Sand, who was not involved with the AAN paper.
She has a number of patients who have rationed their medication or stopped taking it altogether when their copays increased or they lost access to a copay assistance program because their insurance company chose to cover a still-expensive generic drug with no assistance program over a slightly costlier brand-name medication that comes with patient discounts.
Too often, patients don’t tell her they’re not taking their medication as prescribed. At a recent appointment, Dr. Katz Sand learned about a patient’s drug rationing only after a routine MRI showed new brain lesions that regular treatment might have prevented.
Another patient, new to her clinic, questioned the treatment plan Dr. Katz Sand recommended because they could not cover the copay. This sort of self-rationing happens in patients with and without insurance, she added.
“It’s a terrible thing and it’s happening to all patients,” Dr. Katz Sand said, adding that “the old credo of ‘yeah, the drug prices are high, but they are covered by insurance’ is not a sustainable argument anymore.”
What neurologists can do
Some sort of rationing is an unavoidable outcome of steep treatment costs, the authors noted. But what does that mean in clinical practice?
Neurologists should be aware of the costs involved in ordering diagnostic tests, treatment, or medication – and shouldn’t feel compelled to order treatments or tests that they feel are medically inappropriate just because a patient requests them, the authors wrote.
The statement also encourages clinicians to include financial realities in the shared decision-making process with patients.
However, Dr. Katz Sand said that is not always possible. Drug prices aren’t fixed, with different insurance plans offering different pricing, deductibles, and copays. “It’s hard for us to attempt to incorporate discussions about price in our discussions with patients when we can’t even predict what their out-of-pocket cost is going to be,” she said.
“Every single prescription we write requires prior authorization, and that’s directly related to the fact that the cost of these drugs is so high,” she added.
As do many other clinicians, Dr. Katz Sand spends hours each week on preauthorization forms and haggling with insurance companies on behalf of her patients. To get needed medication at a cost they can afford sometimes takes creative problem solving and almost always takes a lot of time. “It all adds to the administrative burden, patients’ stress, and our stress,” she said.
Physician-advocates needed
The AAN paper identifies a number of policy reforms to address drug pricing at a national level, including giving Medicare officials the power to negotiate drug prices, allowing the safe importation of drugs from other countries, and speeding the Food and Drug Administration approval process for generic drugs.
There is also a need to address systemic problems that, the authors noted, help create and perpetuate health care disparities. Lawmakers at the state and federal level are considering a number of these policy ideas and others that could address the kinds of issues Dr. Katz Sand described. However, the chances of their success are slim at best, said Bruce H. Cohen, MD, chair of the AAN advocacy committee and director of the NeuroDevelopmental Science Center at Akron (Ohio) Children’s Hospital.
“On a federal level, we’re watching in real time how the entrenched divisions, even within parties, are resulting in continued stalemate,” said Dr. Cohen, who is not one of the statement authors.
Those ideas need advocates and the AAN paper suggests neurologists should be among the ones championing these changes, he added.
“One of the most effective strategies is to bring attention to the impact high drug costs have on neurology patients and medical practices,” Dr. Cohen said. “It’s so important to make sure policy makers know the significant impact of high drug costs in neurology and within the context of finite resources.”
One way to do that is to share statements such as the current one with members of Congress working on policy reform, Dr. Cohen said. Another is through programs such as the academy’s annual Neurology on the Hill conference.
A third strategy is to encourage individuals such as Dr. Katz Sand to speak out when and where they can, he added.
While she agrees with the idea, finding time for advocacy work amid patient care, administrative work, and research is challenging. Dr. Katz Sand would like to see groups like the AAN work with health care institutions to implement policies that allocate time and resources to train clinicians in advocacy – and then support their efforts on that front.
“I’m really glad they wrote this and think they did a good job of crystallizing the issues,” Dr. Katz Sand said. “It’s good to put it out there as a document that could help serve as the basis for the requests we make collectively. I just hope that people listen.”
The paper received no funding. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New approval in early breast cancer: First advance in 20 years
Abemaciclib had already been approved for use in the treatment of HR+, HER2– advanced or metastatic breast cancer.
Now it is also approved for use in HR+, HER2– early breast cancer for patients who have high-risk, node-positive disease and whose tumors have a Ki-67 score of 20% or higher, as determined by a U.S. Food and Drug Administration–approved test.
The FDA also approved the Ki-67 IHC MIB-1 pharmDx (Dako Omnis) assay for use as a companion diagnostic test.
This is the first CDK4/6 inhibitor to be approved for use in this patient population.
Approximately 70% of all breast cancers are of the HR+, HER2– subtype.
The approval is based on some of the results from the monarchE study, which was presented last year at the annual meeting of the European Society of Medical Oncology and was simultaneously published in the Journal of Clinical Oncology.
The results showed that the addition of abemaciclib to endocrine therapy (tamoxifen or aromatase inhibitors) significantly improved invasive disease-free survival (IDFS), which was defined on the basis of the length of time before breast cancer comes back, any new cancer develops, or death.
The 2-year IDFS rates were 92.2% with the combination vs. 88.7% for endocrine therapy alone for the overall patient population.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust, London, said at the meeting, as reported at the time by this news organization.
Reacting to the findings, Giuseppe Curigliano, MD, PhD, head of the division of early drug development at the European Institute of Oncology, Milan, said, “This is a very important trial and the findings will change practice.”
He predicted that once the drug is approved for use in high-risk HR+, HER2– early breast cancer, “the new standard of care for these patients will be to add 2 years of abemaciclib to endocrine therapy.”
In a press release about the new approval from the manufacturer (Lilly), another investigator on the monarchE study, Sara M. Tolaney, MD, MPH, Harvard Medical School and the Dana-Farber Cancer Institute, Boston, agreed that the results are practice changing. She said that the combination of abemaciclib and endocrine therapy is a potential new standard of care for this patient population. “We are encouraged by the marked reduction in the risk of recurrence even beyond the 2-year treatment period in these patients, and I’m grateful to be able to offer this as a treatment option to my patients,” she said.
On Twitter, she commented that restricting the indication to patients who show Ki67 ≥20% is “interesting,” inasmuch as benefits were seen in patients with both low and high Ki67.
Hal Burstein, MD, from Dana-Farber, also found this detail “interesting, as Ki67 testing remains a very controversial topic and difficult to standardize.”
Replying, Pedro Exman, MD, from the Hospital Alemão Oswaldo Cruz, in São Paulo, said: “Does it make sense to approve only in a subset of patients based in a positive subgroup analysis of a positive ITT study that was not even described in the JCO publication?”
Other experts said they were eagerly awaiting further results, particularly on overall survival, from the monarchE trial. New data are due to be presented on Oct. 14 at an ESMO virtual plenary session.
Commenting late last year about these results, George W. Sledge Jr, MD, professor of medicine at Stanford University Medical Center, Palo Alto, Calif., said that the median follow-up time “is still quite short for a study of ER+ adjuvant therapy, where the majority of recurrences and deaths occur after 5 years in many studies.”
Consequently, “we still have a long way to go to understand the ultimate effects of CDK4/6 inhibition on early-stage ER+ breast cancer, particularly on late recurrences,” he told this news organization at the time.
Agreed, said C. Kent Osborne, MD, codirector of the San Antonio Breast Cancer Symposium and founding director of the Duncan Cancer Center at Baylor College of Medicine, Houston, Tex. The results are “very encouraging, especially in the subgroup of tumors with high proliferation” (identified by the K1-67 score).
However, Dr. Osborne also urged caution in the interpretation of the results, “given the still rather short follow-up, given that that ER+ disease is known for its persistent recurrence rate, even past 10 years.”
He also noted that “this class of inhibitors is likely cytostatic, rather than cytocidal, meaning that it blocks cell proliferation rather than killing the cells.” Questions therefore remain over whether the survival curves for combination therapy will come together with those for endocrine therapy alone once patients stop taking the drug.
Study details
The monarchE trial involved patients with HR+, HER2–, high-risk early breast cancer who had undergone surgery and, as indicated, radiotherapy and/or adjuvant/neoadjuvant chemotherapy. Patients with four or more positive nodes or one to three nodes and either tumors of size ≥5 cm, histologic grade 3, or central Ki-67 ≥20% were eligible; 5,637 patients were randomly assigned in a 1:1 ratio to receive standard-of-care adjuvant endocrine therapy (ET) with or without abemaciclib (150 mg twice daily for 2 years).
A preplanned interim analysis was carried out after 323 IDFS events were observed in the intent-to-treat population. The results, as published last year in the Journal of Clinical Oncology, show that abemaciclib plus ET yielded superior IDFS in comparison with ET alone (P = .01; hazard ratio, 0.75; 95% confidence interval, 0.60-0.93), with 2-year IDFS rates of 92.2% vs. 88.7%.
In the press release announcing the approval of the new indication, the manufacturer notes that the approval was based on the results from a subgroup of 2,003 patients whose tumors had a Ki-67 score of ≥20% and who were also at high risk for recurrence (≥four positive axillary lymph nodes [ALN], or one-three positive ALN with grade 3 disease and/or tumor size ≥5 cm).
There was a statistically significant improvement in IDFS for this prespecified subgroup of patients (HR, 0.643; 95% CI, 0.475-0.872; P = .0042).
With additional follow-up, conducted post hoc, the results showed a 37% decrease in the risk for breast cancer recurrence or death, compared with ET alone (HR, 0.626; 95% CI, 0.49-0.80) and an absolute benefit in IDFS event rate of 7.1% at 3 years. IDFS was 86.1% for abemaciclib plus ET vs. 79.0% for ET alone.
Adverse reactions from monarchE were consistent with the known safety profile for abemaciclib, the company noted. Safety and tolerability were evaluated in 5,591 patients. The most common adverse reactions reported (≥10%) with abemaciclib plus ET vs. ET alone were diarrhea (84% vs. 9%), infections (51% vs. 39%), neutropenia (46% vs. 6%), fatigue (41% vs. 18%), leukopenia (38% vs. 7%), nausea (30% vs. 9%), anemia (24% vs. 4%), headache (20% vs. 15%), vomiting (18% vs. 4.6%), stomatitis (14% vs. 5%), lymphopenia (14% vs. 3%), thrombocytopenia (13% vs. 2%), decreased appetite (12% vs. 2.4%), increased ALT (12% vs. 6%), increased AST (12% vs. 5%), dizziness (11% vs. 7%), rash (11% vs. 4.5%), and alopecia (11% vs. 2.7 %).
A version of this article first appeared on Medscape.com.
Abemaciclib had already been approved for use in the treatment of HR+, HER2– advanced or metastatic breast cancer.
Now it is also approved for use in HR+, HER2– early breast cancer for patients who have high-risk, node-positive disease and whose tumors have a Ki-67 score of 20% or higher, as determined by a U.S. Food and Drug Administration–approved test.
The FDA also approved the Ki-67 IHC MIB-1 pharmDx (Dako Omnis) assay for use as a companion diagnostic test.
This is the first CDK4/6 inhibitor to be approved for use in this patient population.
Approximately 70% of all breast cancers are of the HR+, HER2– subtype.
The approval is based on some of the results from the monarchE study, which was presented last year at the annual meeting of the European Society of Medical Oncology and was simultaneously published in the Journal of Clinical Oncology.
The results showed that the addition of abemaciclib to endocrine therapy (tamoxifen or aromatase inhibitors) significantly improved invasive disease-free survival (IDFS), which was defined on the basis of the length of time before breast cancer comes back, any new cancer develops, or death.
The 2-year IDFS rates were 92.2% with the combination vs. 88.7% for endocrine therapy alone for the overall patient population.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust, London, said at the meeting, as reported at the time by this news organization.
Reacting to the findings, Giuseppe Curigliano, MD, PhD, head of the division of early drug development at the European Institute of Oncology, Milan, said, “This is a very important trial and the findings will change practice.”
He predicted that once the drug is approved for use in high-risk HR+, HER2– early breast cancer, “the new standard of care for these patients will be to add 2 years of abemaciclib to endocrine therapy.”
In a press release about the new approval from the manufacturer (Lilly), another investigator on the monarchE study, Sara M. Tolaney, MD, MPH, Harvard Medical School and the Dana-Farber Cancer Institute, Boston, agreed that the results are practice changing. She said that the combination of abemaciclib and endocrine therapy is a potential new standard of care for this patient population. “We are encouraged by the marked reduction in the risk of recurrence even beyond the 2-year treatment period in these patients, and I’m grateful to be able to offer this as a treatment option to my patients,” she said.
On Twitter, she commented that restricting the indication to patients who show Ki67 ≥20% is “interesting,” inasmuch as benefits were seen in patients with both low and high Ki67.
Hal Burstein, MD, from Dana-Farber, also found this detail “interesting, as Ki67 testing remains a very controversial topic and difficult to standardize.”
Replying, Pedro Exman, MD, from the Hospital Alemão Oswaldo Cruz, in São Paulo, said: “Does it make sense to approve only in a subset of patients based in a positive subgroup analysis of a positive ITT study that was not even described in the JCO publication?”
Other experts said they were eagerly awaiting further results, particularly on overall survival, from the monarchE trial. New data are due to be presented on Oct. 14 at an ESMO virtual plenary session.
Commenting late last year about these results, George W. Sledge Jr, MD, professor of medicine at Stanford University Medical Center, Palo Alto, Calif., said that the median follow-up time “is still quite short for a study of ER+ adjuvant therapy, where the majority of recurrences and deaths occur after 5 years in many studies.”
Consequently, “we still have a long way to go to understand the ultimate effects of CDK4/6 inhibition on early-stage ER+ breast cancer, particularly on late recurrences,” he told this news organization at the time.
Agreed, said C. Kent Osborne, MD, codirector of the San Antonio Breast Cancer Symposium and founding director of the Duncan Cancer Center at Baylor College of Medicine, Houston, Tex. The results are “very encouraging, especially in the subgroup of tumors with high proliferation” (identified by the K1-67 score).
However, Dr. Osborne also urged caution in the interpretation of the results, “given the still rather short follow-up, given that that ER+ disease is known for its persistent recurrence rate, even past 10 years.”
He also noted that “this class of inhibitors is likely cytostatic, rather than cytocidal, meaning that it blocks cell proliferation rather than killing the cells.” Questions therefore remain over whether the survival curves for combination therapy will come together with those for endocrine therapy alone once patients stop taking the drug.
Study details
The monarchE trial involved patients with HR+, HER2–, high-risk early breast cancer who had undergone surgery and, as indicated, radiotherapy and/or adjuvant/neoadjuvant chemotherapy. Patients with four or more positive nodes or one to three nodes and either tumors of size ≥5 cm, histologic grade 3, or central Ki-67 ≥20% were eligible; 5,637 patients were randomly assigned in a 1:1 ratio to receive standard-of-care adjuvant endocrine therapy (ET) with or without abemaciclib (150 mg twice daily for 2 years).
A preplanned interim analysis was carried out after 323 IDFS events were observed in the intent-to-treat population. The results, as published last year in the Journal of Clinical Oncology, show that abemaciclib plus ET yielded superior IDFS in comparison with ET alone (P = .01; hazard ratio, 0.75; 95% confidence interval, 0.60-0.93), with 2-year IDFS rates of 92.2% vs. 88.7%.
In the press release announcing the approval of the new indication, the manufacturer notes that the approval was based on the results from a subgroup of 2,003 patients whose tumors had a Ki-67 score of ≥20% and who were also at high risk for recurrence (≥four positive axillary lymph nodes [ALN], or one-three positive ALN with grade 3 disease and/or tumor size ≥5 cm).
There was a statistically significant improvement in IDFS for this prespecified subgroup of patients (HR, 0.643; 95% CI, 0.475-0.872; P = .0042).
With additional follow-up, conducted post hoc, the results showed a 37% decrease in the risk for breast cancer recurrence or death, compared with ET alone (HR, 0.626; 95% CI, 0.49-0.80) and an absolute benefit in IDFS event rate of 7.1% at 3 years. IDFS was 86.1% for abemaciclib plus ET vs. 79.0% for ET alone.
Adverse reactions from monarchE were consistent with the known safety profile for abemaciclib, the company noted. Safety and tolerability were evaluated in 5,591 patients. The most common adverse reactions reported (≥10%) with abemaciclib plus ET vs. ET alone were diarrhea (84% vs. 9%), infections (51% vs. 39%), neutropenia (46% vs. 6%), fatigue (41% vs. 18%), leukopenia (38% vs. 7%), nausea (30% vs. 9%), anemia (24% vs. 4%), headache (20% vs. 15%), vomiting (18% vs. 4.6%), stomatitis (14% vs. 5%), lymphopenia (14% vs. 3%), thrombocytopenia (13% vs. 2%), decreased appetite (12% vs. 2.4%), increased ALT (12% vs. 6%), increased AST (12% vs. 5%), dizziness (11% vs. 7%), rash (11% vs. 4.5%), and alopecia (11% vs. 2.7 %).
A version of this article first appeared on Medscape.com.
Abemaciclib had already been approved for use in the treatment of HR+, HER2– advanced or metastatic breast cancer.
Now it is also approved for use in HR+, HER2– early breast cancer for patients who have high-risk, node-positive disease and whose tumors have a Ki-67 score of 20% or higher, as determined by a U.S. Food and Drug Administration–approved test.
The FDA also approved the Ki-67 IHC MIB-1 pharmDx (Dako Omnis) assay for use as a companion diagnostic test.
This is the first CDK4/6 inhibitor to be approved for use in this patient population.
Approximately 70% of all breast cancers are of the HR+, HER2– subtype.
The approval is based on some of the results from the monarchE study, which was presented last year at the annual meeting of the European Society of Medical Oncology and was simultaneously published in the Journal of Clinical Oncology.
The results showed that the addition of abemaciclib to endocrine therapy (tamoxifen or aromatase inhibitors) significantly improved invasive disease-free survival (IDFS), which was defined on the basis of the length of time before breast cancer comes back, any new cancer develops, or death.
The 2-year IDFS rates were 92.2% with the combination vs. 88.7% for endocrine therapy alone for the overall patient population.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust, London, said at the meeting, as reported at the time by this news organization.
Reacting to the findings, Giuseppe Curigliano, MD, PhD, head of the division of early drug development at the European Institute of Oncology, Milan, said, “This is a very important trial and the findings will change practice.”
He predicted that once the drug is approved for use in high-risk HR+, HER2– early breast cancer, “the new standard of care for these patients will be to add 2 years of abemaciclib to endocrine therapy.”
In a press release about the new approval from the manufacturer (Lilly), another investigator on the monarchE study, Sara M. Tolaney, MD, MPH, Harvard Medical School and the Dana-Farber Cancer Institute, Boston, agreed that the results are practice changing. She said that the combination of abemaciclib and endocrine therapy is a potential new standard of care for this patient population. “We are encouraged by the marked reduction in the risk of recurrence even beyond the 2-year treatment period in these patients, and I’m grateful to be able to offer this as a treatment option to my patients,” she said.
On Twitter, she commented that restricting the indication to patients who show Ki67 ≥20% is “interesting,” inasmuch as benefits were seen in patients with both low and high Ki67.
Hal Burstein, MD, from Dana-Farber, also found this detail “interesting, as Ki67 testing remains a very controversial topic and difficult to standardize.”
Replying, Pedro Exman, MD, from the Hospital Alemão Oswaldo Cruz, in São Paulo, said: “Does it make sense to approve only in a subset of patients based in a positive subgroup analysis of a positive ITT study that was not even described in the JCO publication?”
Other experts said they were eagerly awaiting further results, particularly on overall survival, from the monarchE trial. New data are due to be presented on Oct. 14 at an ESMO virtual plenary session.
Commenting late last year about these results, George W. Sledge Jr, MD, professor of medicine at Stanford University Medical Center, Palo Alto, Calif., said that the median follow-up time “is still quite short for a study of ER+ adjuvant therapy, where the majority of recurrences and deaths occur after 5 years in many studies.”
Consequently, “we still have a long way to go to understand the ultimate effects of CDK4/6 inhibition on early-stage ER+ breast cancer, particularly on late recurrences,” he told this news organization at the time.
Agreed, said C. Kent Osborne, MD, codirector of the San Antonio Breast Cancer Symposium and founding director of the Duncan Cancer Center at Baylor College of Medicine, Houston, Tex. The results are “very encouraging, especially in the subgroup of tumors with high proliferation” (identified by the K1-67 score).
However, Dr. Osborne also urged caution in the interpretation of the results, “given the still rather short follow-up, given that that ER+ disease is known for its persistent recurrence rate, even past 10 years.”
He also noted that “this class of inhibitors is likely cytostatic, rather than cytocidal, meaning that it blocks cell proliferation rather than killing the cells.” Questions therefore remain over whether the survival curves for combination therapy will come together with those for endocrine therapy alone once patients stop taking the drug.
Study details
The monarchE trial involved patients with HR+, HER2–, high-risk early breast cancer who had undergone surgery and, as indicated, radiotherapy and/or adjuvant/neoadjuvant chemotherapy. Patients with four or more positive nodes or one to three nodes and either tumors of size ≥5 cm, histologic grade 3, or central Ki-67 ≥20% were eligible; 5,637 patients were randomly assigned in a 1:1 ratio to receive standard-of-care adjuvant endocrine therapy (ET) with or without abemaciclib (150 mg twice daily for 2 years).
A preplanned interim analysis was carried out after 323 IDFS events were observed in the intent-to-treat population. The results, as published last year in the Journal of Clinical Oncology, show that abemaciclib plus ET yielded superior IDFS in comparison with ET alone (P = .01; hazard ratio, 0.75; 95% confidence interval, 0.60-0.93), with 2-year IDFS rates of 92.2% vs. 88.7%.
In the press release announcing the approval of the new indication, the manufacturer notes that the approval was based on the results from a subgroup of 2,003 patients whose tumors had a Ki-67 score of ≥20% and who were also at high risk for recurrence (≥four positive axillary lymph nodes [ALN], or one-three positive ALN with grade 3 disease and/or tumor size ≥5 cm).
There was a statistically significant improvement in IDFS for this prespecified subgroup of patients (HR, 0.643; 95% CI, 0.475-0.872; P = .0042).
With additional follow-up, conducted post hoc, the results showed a 37% decrease in the risk for breast cancer recurrence or death, compared with ET alone (HR, 0.626; 95% CI, 0.49-0.80) and an absolute benefit in IDFS event rate of 7.1% at 3 years. IDFS was 86.1% for abemaciclib plus ET vs. 79.0% for ET alone.
Adverse reactions from monarchE were consistent with the known safety profile for abemaciclib, the company noted. Safety and tolerability were evaluated in 5,591 patients. The most common adverse reactions reported (≥10%) with abemaciclib plus ET vs. ET alone were diarrhea (84% vs. 9%), infections (51% vs. 39%), neutropenia (46% vs. 6%), fatigue (41% vs. 18%), leukopenia (38% vs. 7%), nausea (30% vs. 9%), anemia (24% vs. 4%), headache (20% vs. 15%), vomiting (18% vs. 4.6%), stomatitis (14% vs. 5%), lymphopenia (14% vs. 3%), thrombocytopenia (13% vs. 2%), decreased appetite (12% vs. 2.4%), increased ALT (12% vs. 6%), increased AST (12% vs. 5%), dizziness (11% vs. 7%), rash (11% vs. 4.5%), and alopecia (11% vs. 2.7 %).
A version of this article first appeared on Medscape.com.
True or false: Breast density increases breast cancer risk
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).
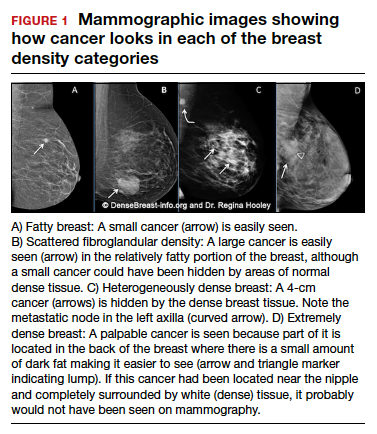
Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8
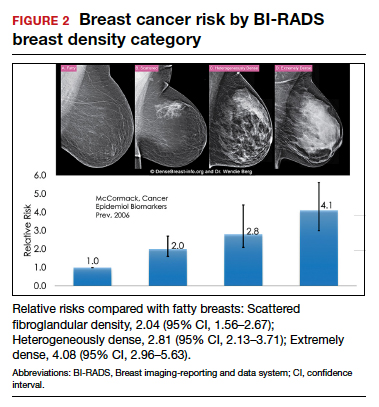
Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).

Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8

Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).

Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8

Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
Quiz developed in collaboration with 
Community mourns nurse knocked over, killed in Times Square
in New York City. The Mass was promoted by the local consulate general of the Philippines.
Maria Ambrocio, 58, was visiting Times Square with a friend on October 1 when she was shoved to the ground by a man who reportedly snatched a cellphone and was running away. He later collided with a police officer before being arrested.
Ms. Ambrosio, of Bayonne, N.J., was taken to Bellevue Hospital in Manhattan with a traumatic brain injury. She was taken off life support a day later.
Jermaine Foster, 26, was charged with murder and robbery and is scheduled to appear in New York Criminal Court Thursday, according to court records.
Ms. Ambrocio, who had been a nurse for 25 years at Bayonne Medical Center in New Jersey, treated cancer patients, even during the height of the pandemic. The medical community at the hospital posted on social media, “Maria’s untimely death is a profound loss to us all, especially those whose lives she touched each day at Bayonne Medical Center.”
New York organizations expressed sympathy after the incident. Tom Harris, president of the Times Square Alliance, said in a statement shared with this news organization, “Our deepest condolences to the family of Maria Ambrocio. The killing of Maria Ambrocio near Times Square highlights one of our city’s greatest public safety challenges, the proliferation of people with untreated mental illness and drug addictions on our streets committing crimes without an effective strategy to address them. Our city needs to come together and solve these problems and those of us who work in these areas are willing and able to help. Let her death not be in vain.”
The New York Philippine consulate reported on its Facebook page that Ms. Ambrocio had just visited its office when the incident occurred. “We grieve with the rest of the Filipino Community over the death of our kababayan [countryman], Maria Ambrocio, a 58-year-old health frontliner from Bayonne, New Jersey....
“Maria’s passing was announced shortly after she was removed from life support a few hours ago. She had been on life support for the head trauma she sustained on Friday afternoon after she was knocked down by someone who was described as a mentally disturbed homeless man. Maria was walking with a kababayan near Times Square after visiting the Philippine consulate general when she was struck by the suspect, who was reportedly being chased after grabbing a mobile phone from someone.”
On the day she passed away, Bayonne Mayor Jimmy Davis shared an emotional message on Facebook: “I’m asking for all Bayonne people to say a prayer for Maria Ambrocio. Maria, an Oncology nurse at Bayonne Medical Center, was viciously attacked in an unprovoked assault by a deranged man in Times Square yesterday.
“Please keep Maria and her family in your thoughts through these difficult days.”
A version of this article first appeared on Medscape.com.
in New York City. The Mass was promoted by the local consulate general of the Philippines.
Maria Ambrocio, 58, was visiting Times Square with a friend on October 1 when she was shoved to the ground by a man who reportedly snatched a cellphone and was running away. He later collided with a police officer before being arrested.
Ms. Ambrosio, of Bayonne, N.J., was taken to Bellevue Hospital in Manhattan with a traumatic brain injury. She was taken off life support a day later.
Jermaine Foster, 26, was charged with murder and robbery and is scheduled to appear in New York Criminal Court Thursday, according to court records.
Ms. Ambrocio, who had been a nurse for 25 years at Bayonne Medical Center in New Jersey, treated cancer patients, even during the height of the pandemic. The medical community at the hospital posted on social media, “Maria’s untimely death is a profound loss to us all, especially those whose lives she touched each day at Bayonne Medical Center.”
New York organizations expressed sympathy after the incident. Tom Harris, president of the Times Square Alliance, said in a statement shared with this news organization, “Our deepest condolences to the family of Maria Ambrocio. The killing of Maria Ambrocio near Times Square highlights one of our city’s greatest public safety challenges, the proliferation of people with untreated mental illness and drug addictions on our streets committing crimes without an effective strategy to address them. Our city needs to come together and solve these problems and those of us who work in these areas are willing and able to help. Let her death not be in vain.”
The New York Philippine consulate reported on its Facebook page that Ms. Ambrocio had just visited its office when the incident occurred. “We grieve with the rest of the Filipino Community over the death of our kababayan [countryman], Maria Ambrocio, a 58-year-old health frontliner from Bayonne, New Jersey....
“Maria’s passing was announced shortly after she was removed from life support a few hours ago. She had been on life support for the head trauma she sustained on Friday afternoon after she was knocked down by someone who was described as a mentally disturbed homeless man. Maria was walking with a kababayan near Times Square after visiting the Philippine consulate general when she was struck by the suspect, who was reportedly being chased after grabbing a mobile phone from someone.”
On the day she passed away, Bayonne Mayor Jimmy Davis shared an emotional message on Facebook: “I’m asking for all Bayonne people to say a prayer for Maria Ambrocio. Maria, an Oncology nurse at Bayonne Medical Center, was viciously attacked in an unprovoked assault by a deranged man in Times Square yesterday.
“Please keep Maria and her family in your thoughts through these difficult days.”
A version of this article first appeared on Medscape.com.
in New York City. The Mass was promoted by the local consulate general of the Philippines.
Maria Ambrocio, 58, was visiting Times Square with a friend on October 1 when she was shoved to the ground by a man who reportedly snatched a cellphone and was running away. He later collided with a police officer before being arrested.
Ms. Ambrosio, of Bayonne, N.J., was taken to Bellevue Hospital in Manhattan with a traumatic brain injury. She was taken off life support a day later.
Jermaine Foster, 26, was charged with murder and robbery and is scheduled to appear in New York Criminal Court Thursday, according to court records.
Ms. Ambrocio, who had been a nurse for 25 years at Bayonne Medical Center in New Jersey, treated cancer patients, even during the height of the pandemic. The medical community at the hospital posted on social media, “Maria’s untimely death is a profound loss to us all, especially those whose lives she touched each day at Bayonne Medical Center.”
New York organizations expressed sympathy after the incident. Tom Harris, president of the Times Square Alliance, said in a statement shared with this news organization, “Our deepest condolences to the family of Maria Ambrocio. The killing of Maria Ambrocio near Times Square highlights one of our city’s greatest public safety challenges, the proliferation of people with untreated mental illness and drug addictions on our streets committing crimes without an effective strategy to address them. Our city needs to come together and solve these problems and those of us who work in these areas are willing and able to help. Let her death not be in vain.”
The New York Philippine consulate reported on its Facebook page that Ms. Ambrocio had just visited its office when the incident occurred. “We grieve with the rest of the Filipino Community over the death of our kababayan [countryman], Maria Ambrocio, a 58-year-old health frontliner from Bayonne, New Jersey....
“Maria’s passing was announced shortly after she was removed from life support a few hours ago. She had been on life support for the head trauma she sustained on Friday afternoon after she was knocked down by someone who was described as a mentally disturbed homeless man. Maria was walking with a kababayan near Times Square after visiting the Philippine consulate general when she was struck by the suspect, who was reportedly being chased after grabbing a mobile phone from someone.”
On the day she passed away, Bayonne Mayor Jimmy Davis shared an emotional message on Facebook: “I’m asking for all Bayonne people to say a prayer for Maria Ambrocio. Maria, an Oncology nurse at Bayonne Medical Center, was viciously attacked in an unprovoked assault by a deranged man in Times Square yesterday.
“Please keep Maria and her family in your thoughts through these difficult days.”
A version of this article first appeared on Medscape.com.
Benzene prompts recalls of spray antifungals and sunscreens
Bayer has voluntarily recalled batches of its Lotrimin and Tinactin products because of benzene detected in some samples, according to an Oct. 1 company announcement, available on the Food and Drug Administration website. “It is important to note that Bayer’s decision to voluntarily recall these products is a precautionary measure and that the levels detected are not expected to cause adverse health consequences in consumers,” the announcement said.
Benzene is classified as a human carcinogen present in the environment from both natural sources and human activity, and it has been shown to cause cancer with long-term exposure.
The products included in the recall – all in aerosol spray cans – are unexpired Lotrimin and Tinactin sprays with lot numbers starting with TN, CV, or NAA that were distributed to consumer venues between September 2018 and September 2021. The over-the-counter products are Lotrimin Anti-Fungal Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal Jock Itch (AFJI) Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal (AF) Athlete’s Foot Deodorant Powder Spray, Lotrimin AF Athlete’s Foot Liquid Spray, Lotrimin AF Athlete’s Foot Daily Prevention Deodorant Powder Spray, Tinactin Jock Itch (JI) Powder Spray, Tinactin Athlete’s Foot Deodorant Powder Spray, Tinactin Athlete’s Foot Powder Spray, and Tinactin Athlete’s Foot Liquid Spray.
Bayer has received no reports of adverse events related to the recall. The company also reported no concerns with its antifungal creams or other products.
In addition, Coppertone has issued a voluntary recall of specific lots of five spray sunscreen products because of the presence of benzene, according to a Sept. 30th company announcement, also posted on the FDA website. The recall includes Pure&Simple spray for babies, children, and adults; Coppertone Sport Mineral Spray; and Travel-sized Coppertone Sport spray. The specific lots were manufactured between January and June 2021, and are listed on the company announcement.
“Daily exposure to benzene at the levels detected in these affected Coppertone aerosol sunscreen spray products would not be expected to cause adverse health consequences based on generally accepted exposure modeling by numerous regulatory agencies,” according to the announcement. Coppertone has received no reports of adverse events related to the recall.
In the announcement, Coppertone advised consumers to discontinue use of the impacted products, dispose of the aerosol cans properly, and contact their physician or health care provider if they experience any problems related to the sunscreen sprays.
In May 2021, online pharmacy Valisure, which routinely tests their medications, petitioned the FDA to recall specific sunscreens after detecting high benzene levels in several brands and batches of sunscreen products. The FDA evaluated the petition, but the agency itself did not issue any recalls of sunscreens.
Clinicians are advised to report any adverse events to the FDA’s MedWatch Adverse Event Reporting program either online or by regular mail or fax using this form.
Bayer has voluntarily recalled batches of its Lotrimin and Tinactin products because of benzene detected in some samples, according to an Oct. 1 company announcement, available on the Food and Drug Administration website. “It is important to note that Bayer’s decision to voluntarily recall these products is a precautionary measure and that the levels detected are not expected to cause adverse health consequences in consumers,” the announcement said.
Benzene is classified as a human carcinogen present in the environment from both natural sources and human activity, and it has been shown to cause cancer with long-term exposure.
The products included in the recall – all in aerosol spray cans – are unexpired Lotrimin and Tinactin sprays with lot numbers starting with TN, CV, or NAA that were distributed to consumer venues between September 2018 and September 2021. The over-the-counter products are Lotrimin Anti-Fungal Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal Jock Itch (AFJI) Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal (AF) Athlete’s Foot Deodorant Powder Spray, Lotrimin AF Athlete’s Foot Liquid Spray, Lotrimin AF Athlete’s Foot Daily Prevention Deodorant Powder Spray, Tinactin Jock Itch (JI) Powder Spray, Tinactin Athlete’s Foot Deodorant Powder Spray, Tinactin Athlete’s Foot Powder Spray, and Tinactin Athlete’s Foot Liquid Spray.
Bayer has received no reports of adverse events related to the recall. The company also reported no concerns with its antifungal creams or other products.
In addition, Coppertone has issued a voluntary recall of specific lots of five spray sunscreen products because of the presence of benzene, according to a Sept. 30th company announcement, also posted on the FDA website. The recall includes Pure&Simple spray for babies, children, and adults; Coppertone Sport Mineral Spray; and Travel-sized Coppertone Sport spray. The specific lots were manufactured between January and June 2021, and are listed on the company announcement.
“Daily exposure to benzene at the levels detected in these affected Coppertone aerosol sunscreen spray products would not be expected to cause adverse health consequences based on generally accepted exposure modeling by numerous regulatory agencies,” according to the announcement. Coppertone has received no reports of adverse events related to the recall.
In the announcement, Coppertone advised consumers to discontinue use of the impacted products, dispose of the aerosol cans properly, and contact their physician or health care provider if they experience any problems related to the sunscreen sprays.
In May 2021, online pharmacy Valisure, which routinely tests their medications, petitioned the FDA to recall specific sunscreens after detecting high benzene levels in several brands and batches of sunscreen products. The FDA evaluated the petition, but the agency itself did not issue any recalls of sunscreens.
Clinicians are advised to report any adverse events to the FDA’s MedWatch Adverse Event Reporting program either online or by regular mail or fax using this form.
Bayer has voluntarily recalled batches of its Lotrimin and Tinactin products because of benzene detected in some samples, according to an Oct. 1 company announcement, available on the Food and Drug Administration website. “It is important to note that Bayer’s decision to voluntarily recall these products is a precautionary measure and that the levels detected are not expected to cause adverse health consequences in consumers,” the announcement said.
Benzene is classified as a human carcinogen present in the environment from both natural sources and human activity, and it has been shown to cause cancer with long-term exposure.
The products included in the recall – all in aerosol spray cans – are unexpired Lotrimin and Tinactin sprays with lot numbers starting with TN, CV, or NAA that were distributed to consumer venues between September 2018 and September 2021. The over-the-counter products are Lotrimin Anti-Fungal Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal Jock Itch (AFJI) Athlete’s Foot Powder Spray, Lotrimin Anti-Fungal (AF) Athlete’s Foot Deodorant Powder Spray, Lotrimin AF Athlete’s Foot Liquid Spray, Lotrimin AF Athlete’s Foot Daily Prevention Deodorant Powder Spray, Tinactin Jock Itch (JI) Powder Spray, Tinactin Athlete’s Foot Deodorant Powder Spray, Tinactin Athlete’s Foot Powder Spray, and Tinactin Athlete’s Foot Liquid Spray.
Bayer has received no reports of adverse events related to the recall. The company also reported no concerns with its antifungal creams or other products.
In addition, Coppertone has issued a voluntary recall of specific lots of five spray sunscreen products because of the presence of benzene, according to a Sept. 30th company announcement, also posted on the FDA website. The recall includes Pure&Simple spray for babies, children, and adults; Coppertone Sport Mineral Spray; and Travel-sized Coppertone Sport spray. The specific lots were manufactured between January and June 2021, and are listed on the company announcement.
“Daily exposure to benzene at the levels detected in these affected Coppertone aerosol sunscreen spray products would not be expected to cause adverse health consequences based on generally accepted exposure modeling by numerous regulatory agencies,” according to the announcement. Coppertone has received no reports of adverse events related to the recall.
In the announcement, Coppertone advised consumers to discontinue use of the impacted products, dispose of the aerosol cans properly, and contact their physician or health care provider if they experience any problems related to the sunscreen sprays.
In May 2021, online pharmacy Valisure, which routinely tests their medications, petitioned the FDA to recall specific sunscreens after detecting high benzene levels in several brands and batches of sunscreen products. The FDA evaluated the petition, but the agency itself did not issue any recalls of sunscreens.
Clinicians are advised to report any adverse events to the FDA’s MedWatch Adverse Event Reporting program either online or by regular mail or fax using this form.
Biomarker testing in metastatic breast cancer management: ‘Essential’
Identifying biomarkers in metastatic breast cancer (MBC) has become an integral part of choosing treatments and understanding disease progression. The American Society of Clinical Oncology Clinical Practice Guideline, published in 2015, recommends an initial biopsy to confirm estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) status as well as repeat biopsies to watch for receptor status changes over time.
“Decisions concerning the initiation of systemic therapy or selection of systemic therapy for metastatic breast cancer should be guided by ER, PR, and HER2 status in conjunction with clinical evaluation, judgment, and the patient’s goals for care,” according to the guideline authors.This news organization reached out to Kelly McCann, MD, PhD, a hematologist and oncologist in the department of medicine at the David Geffen School of Medicine, University of California, Los Angeles, to explore the role biomarker testing plays in managing MBC.
Question: How important is biomarker testing in guiding MBC treatments? Is there a standard or recommended process?
Dr. McCann: Biomarker testing is essential to breast cancer treatment and the development of targeted therapies. Oncologists typically identify a tumor’s canonical biomarkers — ER, PR, and HER2 — using immunohistochemistry or fluorescence in situ hybridization (FISH) testing and then try to match the tumor biology to drugs that target that subtype.
For tumors that lack canonical biomarkers — for example, triple-negative breast cancer (TNBC) — I send the tumor tissue for next-generation sequencing at the time of metastatic diagnosis to identify a wider range of potential targets or oncogenic drivers, such as somatic or germline mutations in homologous recombination repair genes ( BRCA1, BRCA2, and PALB2 ) or mutations in the PI3K/AKT/mTOR pathway.
In our attempts to define tumor biology and design a treatment strategy, two additional issues quickly arise. First, tumors are heterogeneous from the start. Second, tumors evolve.
Let’s start with how we define or subtype a tumor. Would you walk us through this process?
Defining a breast tumor can be tricky because these cancers often don’t fit neatly into predefined categories. Let’s take the estrogen receptor. In clinical trials, we need to define the cutoff for what constitutes ER-positive MBC or TNBC. Some trials define ER-positive as 1% or greater, others define it as 10% or greater.
But is a PR- and HER2-negative tumor with 1% or even 5% ER expression really ER-positive in the biological or prognostic sense? Probably not. A tumor with less than 10% ER expression, for instance, will actually behave like a triple-negative tumor. Instead of choosing a regimen targeting the ER-positive cells, I’ll lean more toward cytotoxic chemotherapy, the standard treatment for TNBC.
Tumors may have multiple drivers as well. What are some aberrations in addition to the main subtypes?
Tumors also often harbor more than one targetable driver. For instance, PIK3CA gene mutations are present in about 40% of hormone receptor–positive, HER2-negative tumors. Activating mutations in ESR1 develop in anywhere from 10% to 50% of MBCs as a resistance mechanism to estrogen deprivation therapy, conferring estrogen independence to the cells. Activating mutations in ERBB2, which essentially turns HER2 into an active receptor, are found in 2%-4% of breast cancers, including ER-positive, HER2-mutant breast cancers, and are enriched in lobular breast cancers, which are typically ER positive, HER2 negative.
What about tumor evolution, given the growing body of evidence that biomarker status in MBC can change over time?
Patients with MBC often have several active areas of cancer, and these areas will evolve differently. During each line of treatment, some metastases will develop resistance and others won’t. For instance, if my patient’s liver metastases start to grow, I will change therapy immediately. If, however, a single bone metastasis begins to grow and the liver metastases have responded well, I might consider local therapy — such as radiation — to target that bone metastasis, though this particular approach hasn’t been formally studied.
Ultimately, we can expect tumors to change over time as they become more biologically aggressive or resistant to current therapy. The most common biomarker change is probably loss of ER or PR expression, but the frequency of ER, PR, or HER2 biomarker changes is still not well understood.
Resistance mutations can also happen. When, for instance, activating mutations in ESR1 occur, the estrogen receptor becomes independent of estrogen and tumors then develop resistance to endocrine therapies. We see a similar problem arise in metastatic prostate cancer. With chronic testosterone deprivation, eventually the androgen receptor evolves to become independent of testosterone in a stage known as castrate-resistant prostate cancer.
Which biomarkers or combinations of biomarkers can be paired with an approved treatment?
We have a range of treatments targeting ER-positive and HER2-positive MBC in particular. For tumors harboring additional targetable mutations, preliminary data suggest that HER2-targeted tyrosine kinase inhibitors (TKIs), such as tucatinib and neratinib, are effective against activating mutations in ERBB2.
The PI3K inhibitor alpelisib in combination with fulvestrant has been approved for patients with ER-positive, HER2-negative MBC and mutations in PIK3CA. The mTOR inhibitor everolimus plus exemestane is an option for patients with ER-positive, HER2-negative. And for those with activating mutations in ESR1, I switch patients to a selective estrogen receptor degrader, such as fulvestrant.
PARP inhibitors, including olaparib or talazoparib, target metastatic HR-positive disease or TNBC with deleterious germline BRCA1 or BRCA2 mutations. Sacituzumab govitecan has been approved for treating metastatic TNBC and targets the cell surface protein TROP2, expressed in almost 90% of TNBC tumors.
What targets, on the other hand, are less informative for treatment choice?
When we order next-generation sequencing, we also will get a list of possible targets for which there are currently no therapeutic options, but there may be in the future. I find this knowledge is helpful. For example, an activating mutation in KRAS tells me that the cancer has a very strong oncogenic driver that I won›t be able to target. I know that activating KRAS mutations in lung cancer and colon cancer portend a poorer prognosis, which helps me to prepare the patient and family.
Atezolizumab in combination with paclitaxel has been FDA-approved for PD-L1 TNBC in the first-line setting, though data show that immune checkpoint inhibitors may be effective even without PD-L1 expression. Although cell surface protein TROP2 has emerged as a target in recent years, its expression is so common in TNBC that confirmatory testing for TROP2 expression is not required to prescribe sacituzumab govitecan.
What factors do you weigh when selecting among the large number of tests available for tumor testing?
We have many biomarker tests available, but the National Comprehensive Cancer Network does not have guidelines for tumor genetics testing in breast cancer. That means insurance does not have to cover the cost, and many companies don’t. Ultimately, though, drug companies and some testing companies have an incentive to cover the cost themselves because a companion diagnostic might be linked to their drug — therascreen PIK3CA RGQ PCR kit for alpelisib, for instance.
I tend not to use a companion diagnostic test because I want more information with a wider panel. The tumor tests I often use are FoundationOne CDx, Caris Molecular Intelligence, and Tempus. I use Tempus because their financial aid is very generous and almost all of my patients qualify to be tested for less than $100. For germline genetic testing, Invitae, Myriad, and Color are also options. Invitae and Color are about $250 out of pocket without insurance. Many academic centers have their own gene panels as well.
How far have we come in identifying biomarkers in MBC?
Targeted treatment for breast cancer has advanced significantly since doing my PhD research in cancer biology about 15 years ago. Of course, targeted therapies for ER-positive and HER2-amplified cancers were available at that point, but many more have been developed. The most significant advance has been the development of efficient and affordable genome sequencing, which has led to these large panels and identification of therapeutic targets. We’ve also expanded our knowledge of genetic predispositions for breast cancer beyond BRCA1 and BRCA2, which not only allows us to preemptively advise patients and their families about cancer risks and recommendations for cancer screening, but also to select a therapy to target a cancer’s DNA repair deficits.
I feel that we are in an exciting discovery phase in oncology. We currently rely on biomarkers to manage MBC and will continue to refine our strategies and develop more effective drug therapies as we identify more oncogenic drivers, tumor-specific proteins, and cancer cell vulnerabilities.
Identifying biomarkers in metastatic breast cancer (MBC) has become an integral part of choosing treatments and understanding disease progression. The American Society of Clinical Oncology Clinical Practice Guideline, published in 2015, recommends an initial biopsy to confirm estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) status as well as repeat biopsies to watch for receptor status changes over time.
“Decisions concerning the initiation of systemic therapy or selection of systemic therapy for metastatic breast cancer should be guided by ER, PR, and HER2 status in conjunction with clinical evaluation, judgment, and the patient’s goals for care,” according to the guideline authors.This news organization reached out to Kelly McCann, MD, PhD, a hematologist and oncologist in the department of medicine at the David Geffen School of Medicine, University of California, Los Angeles, to explore the role biomarker testing plays in managing MBC.
Question: How important is biomarker testing in guiding MBC treatments? Is there a standard or recommended process?
Dr. McCann: Biomarker testing is essential to breast cancer treatment and the development of targeted therapies. Oncologists typically identify a tumor’s canonical biomarkers — ER, PR, and HER2 — using immunohistochemistry or fluorescence in situ hybridization (FISH) testing and then try to match the tumor biology to drugs that target that subtype.
For tumors that lack canonical biomarkers — for example, triple-negative breast cancer (TNBC) — I send the tumor tissue for next-generation sequencing at the time of metastatic diagnosis to identify a wider range of potential targets or oncogenic drivers, such as somatic or germline mutations in homologous recombination repair genes ( BRCA1, BRCA2, and PALB2 ) or mutations in the PI3K/AKT/mTOR pathway.
In our attempts to define tumor biology and design a treatment strategy, two additional issues quickly arise. First, tumors are heterogeneous from the start. Second, tumors evolve.
Let’s start with how we define or subtype a tumor. Would you walk us through this process?
Defining a breast tumor can be tricky because these cancers often don’t fit neatly into predefined categories. Let’s take the estrogen receptor. In clinical trials, we need to define the cutoff for what constitutes ER-positive MBC or TNBC. Some trials define ER-positive as 1% or greater, others define it as 10% or greater.
But is a PR- and HER2-negative tumor with 1% or even 5% ER expression really ER-positive in the biological or prognostic sense? Probably not. A tumor with less than 10% ER expression, for instance, will actually behave like a triple-negative tumor. Instead of choosing a regimen targeting the ER-positive cells, I’ll lean more toward cytotoxic chemotherapy, the standard treatment for TNBC.
Tumors may have multiple drivers as well. What are some aberrations in addition to the main subtypes?
Tumors also often harbor more than one targetable driver. For instance, PIK3CA gene mutations are present in about 40% of hormone receptor–positive, HER2-negative tumors. Activating mutations in ESR1 develop in anywhere from 10% to 50% of MBCs as a resistance mechanism to estrogen deprivation therapy, conferring estrogen independence to the cells. Activating mutations in ERBB2, which essentially turns HER2 into an active receptor, are found in 2%-4% of breast cancers, including ER-positive, HER2-mutant breast cancers, and are enriched in lobular breast cancers, which are typically ER positive, HER2 negative.
What about tumor evolution, given the growing body of evidence that biomarker status in MBC can change over time?
Patients with MBC often have several active areas of cancer, and these areas will evolve differently. During each line of treatment, some metastases will develop resistance and others won’t. For instance, if my patient’s liver metastases start to grow, I will change therapy immediately. If, however, a single bone metastasis begins to grow and the liver metastases have responded well, I might consider local therapy — such as radiation — to target that bone metastasis, though this particular approach hasn’t been formally studied.
Ultimately, we can expect tumors to change over time as they become more biologically aggressive or resistant to current therapy. The most common biomarker change is probably loss of ER or PR expression, but the frequency of ER, PR, or HER2 biomarker changes is still not well understood.
Resistance mutations can also happen. When, for instance, activating mutations in ESR1 occur, the estrogen receptor becomes independent of estrogen and tumors then develop resistance to endocrine therapies. We see a similar problem arise in metastatic prostate cancer. With chronic testosterone deprivation, eventually the androgen receptor evolves to become independent of testosterone in a stage known as castrate-resistant prostate cancer.
Which biomarkers or combinations of biomarkers can be paired with an approved treatment?
We have a range of treatments targeting ER-positive and HER2-positive MBC in particular. For tumors harboring additional targetable mutations, preliminary data suggest that HER2-targeted tyrosine kinase inhibitors (TKIs), such as tucatinib and neratinib, are effective against activating mutations in ERBB2.
The PI3K inhibitor alpelisib in combination with fulvestrant has been approved for patients with ER-positive, HER2-negative MBC and mutations in PIK3CA. The mTOR inhibitor everolimus plus exemestane is an option for patients with ER-positive, HER2-negative. And for those with activating mutations in ESR1, I switch patients to a selective estrogen receptor degrader, such as fulvestrant.
PARP inhibitors, including olaparib or talazoparib, target metastatic HR-positive disease or TNBC with deleterious germline BRCA1 or BRCA2 mutations. Sacituzumab govitecan has been approved for treating metastatic TNBC and targets the cell surface protein TROP2, expressed in almost 90% of TNBC tumors.
What targets, on the other hand, are less informative for treatment choice?
When we order next-generation sequencing, we also will get a list of possible targets for which there are currently no therapeutic options, but there may be in the future. I find this knowledge is helpful. For example, an activating mutation in KRAS tells me that the cancer has a very strong oncogenic driver that I won›t be able to target. I know that activating KRAS mutations in lung cancer and colon cancer portend a poorer prognosis, which helps me to prepare the patient and family.
Atezolizumab in combination with paclitaxel has been FDA-approved for PD-L1 TNBC in the first-line setting, though data show that immune checkpoint inhibitors may be effective even without PD-L1 expression. Although cell surface protein TROP2 has emerged as a target in recent years, its expression is so common in TNBC that confirmatory testing for TROP2 expression is not required to prescribe sacituzumab govitecan.
What factors do you weigh when selecting among the large number of tests available for tumor testing?
We have many biomarker tests available, but the National Comprehensive Cancer Network does not have guidelines for tumor genetics testing in breast cancer. That means insurance does not have to cover the cost, and many companies don’t. Ultimately, though, drug companies and some testing companies have an incentive to cover the cost themselves because a companion diagnostic might be linked to their drug — therascreen PIK3CA RGQ PCR kit for alpelisib, for instance.
I tend not to use a companion diagnostic test because I want more information with a wider panel. The tumor tests I often use are FoundationOne CDx, Caris Molecular Intelligence, and Tempus. I use Tempus because their financial aid is very generous and almost all of my patients qualify to be tested for less than $100. For germline genetic testing, Invitae, Myriad, and Color are also options. Invitae and Color are about $250 out of pocket without insurance. Many academic centers have their own gene panels as well.
How far have we come in identifying biomarkers in MBC?
Targeted treatment for breast cancer has advanced significantly since doing my PhD research in cancer biology about 15 years ago. Of course, targeted therapies for ER-positive and HER2-amplified cancers were available at that point, but many more have been developed. The most significant advance has been the development of efficient and affordable genome sequencing, which has led to these large panels and identification of therapeutic targets. We’ve also expanded our knowledge of genetic predispositions for breast cancer beyond BRCA1 and BRCA2, which not only allows us to preemptively advise patients and their families about cancer risks and recommendations for cancer screening, but also to select a therapy to target a cancer’s DNA repair deficits.
I feel that we are in an exciting discovery phase in oncology. We currently rely on biomarkers to manage MBC and will continue to refine our strategies and develop more effective drug therapies as we identify more oncogenic drivers, tumor-specific proteins, and cancer cell vulnerabilities.
Identifying biomarkers in metastatic breast cancer (MBC) has become an integral part of choosing treatments and understanding disease progression. The American Society of Clinical Oncology Clinical Practice Guideline, published in 2015, recommends an initial biopsy to confirm estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) status as well as repeat biopsies to watch for receptor status changes over time.
“Decisions concerning the initiation of systemic therapy or selection of systemic therapy for metastatic breast cancer should be guided by ER, PR, and HER2 status in conjunction with clinical evaluation, judgment, and the patient’s goals for care,” according to the guideline authors.This news organization reached out to Kelly McCann, MD, PhD, a hematologist and oncologist in the department of medicine at the David Geffen School of Medicine, University of California, Los Angeles, to explore the role biomarker testing plays in managing MBC.
Question: How important is biomarker testing in guiding MBC treatments? Is there a standard or recommended process?
Dr. McCann: Biomarker testing is essential to breast cancer treatment and the development of targeted therapies. Oncologists typically identify a tumor’s canonical biomarkers — ER, PR, and HER2 — using immunohistochemistry or fluorescence in situ hybridization (FISH) testing and then try to match the tumor biology to drugs that target that subtype.
For tumors that lack canonical biomarkers — for example, triple-negative breast cancer (TNBC) — I send the tumor tissue for next-generation sequencing at the time of metastatic diagnosis to identify a wider range of potential targets or oncogenic drivers, such as somatic or germline mutations in homologous recombination repair genes ( BRCA1, BRCA2, and PALB2 ) or mutations in the PI3K/AKT/mTOR pathway.
In our attempts to define tumor biology and design a treatment strategy, two additional issues quickly arise. First, tumors are heterogeneous from the start. Second, tumors evolve.
Let’s start with how we define or subtype a tumor. Would you walk us through this process?
Defining a breast tumor can be tricky because these cancers often don’t fit neatly into predefined categories. Let’s take the estrogen receptor. In clinical trials, we need to define the cutoff for what constitutes ER-positive MBC or TNBC. Some trials define ER-positive as 1% or greater, others define it as 10% or greater.
But is a PR- and HER2-negative tumor with 1% or even 5% ER expression really ER-positive in the biological or prognostic sense? Probably not. A tumor with less than 10% ER expression, for instance, will actually behave like a triple-negative tumor. Instead of choosing a regimen targeting the ER-positive cells, I’ll lean more toward cytotoxic chemotherapy, the standard treatment for TNBC.
Tumors may have multiple drivers as well. What are some aberrations in addition to the main subtypes?
Tumors also often harbor more than one targetable driver. For instance, PIK3CA gene mutations are present in about 40% of hormone receptor–positive, HER2-negative tumors. Activating mutations in ESR1 develop in anywhere from 10% to 50% of MBCs as a resistance mechanism to estrogen deprivation therapy, conferring estrogen independence to the cells. Activating mutations in ERBB2, which essentially turns HER2 into an active receptor, are found in 2%-4% of breast cancers, including ER-positive, HER2-mutant breast cancers, and are enriched in lobular breast cancers, which are typically ER positive, HER2 negative.
What about tumor evolution, given the growing body of evidence that biomarker status in MBC can change over time?
Patients with MBC often have several active areas of cancer, and these areas will evolve differently. During each line of treatment, some metastases will develop resistance and others won’t. For instance, if my patient’s liver metastases start to grow, I will change therapy immediately. If, however, a single bone metastasis begins to grow and the liver metastases have responded well, I might consider local therapy — such as radiation — to target that bone metastasis, though this particular approach hasn’t been formally studied.
Ultimately, we can expect tumors to change over time as they become more biologically aggressive or resistant to current therapy. The most common biomarker change is probably loss of ER or PR expression, but the frequency of ER, PR, or HER2 biomarker changes is still not well understood.
Resistance mutations can also happen. When, for instance, activating mutations in ESR1 occur, the estrogen receptor becomes independent of estrogen and tumors then develop resistance to endocrine therapies. We see a similar problem arise in metastatic prostate cancer. With chronic testosterone deprivation, eventually the androgen receptor evolves to become independent of testosterone in a stage known as castrate-resistant prostate cancer.
Which biomarkers or combinations of biomarkers can be paired with an approved treatment?
We have a range of treatments targeting ER-positive and HER2-positive MBC in particular. For tumors harboring additional targetable mutations, preliminary data suggest that HER2-targeted tyrosine kinase inhibitors (TKIs), such as tucatinib and neratinib, are effective against activating mutations in ERBB2.
The PI3K inhibitor alpelisib in combination with fulvestrant has been approved for patients with ER-positive, HER2-negative MBC and mutations in PIK3CA. The mTOR inhibitor everolimus plus exemestane is an option for patients with ER-positive, HER2-negative. And for those with activating mutations in ESR1, I switch patients to a selective estrogen receptor degrader, such as fulvestrant.
PARP inhibitors, including olaparib or talazoparib, target metastatic HR-positive disease or TNBC with deleterious germline BRCA1 or BRCA2 mutations. Sacituzumab govitecan has been approved for treating metastatic TNBC and targets the cell surface protein TROP2, expressed in almost 90% of TNBC tumors.
What targets, on the other hand, are less informative for treatment choice?
When we order next-generation sequencing, we also will get a list of possible targets for which there are currently no therapeutic options, but there may be in the future. I find this knowledge is helpful. For example, an activating mutation in KRAS tells me that the cancer has a very strong oncogenic driver that I won›t be able to target. I know that activating KRAS mutations in lung cancer and colon cancer portend a poorer prognosis, which helps me to prepare the patient and family.
Atezolizumab in combination with paclitaxel has been FDA-approved for PD-L1 TNBC in the first-line setting, though data show that immune checkpoint inhibitors may be effective even without PD-L1 expression. Although cell surface protein TROP2 has emerged as a target in recent years, its expression is so common in TNBC that confirmatory testing for TROP2 expression is not required to prescribe sacituzumab govitecan.
What factors do you weigh when selecting among the large number of tests available for tumor testing?
We have many biomarker tests available, but the National Comprehensive Cancer Network does not have guidelines for tumor genetics testing in breast cancer. That means insurance does not have to cover the cost, and many companies don’t. Ultimately, though, drug companies and some testing companies have an incentive to cover the cost themselves because a companion diagnostic might be linked to their drug — therascreen PIK3CA RGQ PCR kit for alpelisib, for instance.
I tend not to use a companion diagnostic test because I want more information with a wider panel. The tumor tests I often use are FoundationOne CDx, Caris Molecular Intelligence, and Tempus. I use Tempus because their financial aid is very generous and almost all of my patients qualify to be tested for less than $100. For germline genetic testing, Invitae, Myriad, and Color are also options. Invitae and Color are about $250 out of pocket without insurance. Many academic centers have their own gene panels as well.
How far have we come in identifying biomarkers in MBC?
Targeted treatment for breast cancer has advanced significantly since doing my PhD research in cancer biology about 15 years ago. Of course, targeted therapies for ER-positive and HER2-amplified cancers were available at that point, but many more have been developed. The most significant advance has been the development of efficient and affordable genome sequencing, which has led to these large panels and identification of therapeutic targets. We’ve also expanded our knowledge of genetic predispositions for breast cancer beyond BRCA1 and BRCA2, which not only allows us to preemptively advise patients and their families about cancer risks and recommendations for cancer screening, but also to select a therapy to target a cancer’s DNA repair deficits.
I feel that we are in an exciting discovery phase in oncology. We currently rely on biomarkers to manage MBC and will continue to refine our strategies and develop more effective drug therapies as we identify more oncogenic drivers, tumor-specific proteins, and cancer cell vulnerabilities.
Is AFib a stroke cause or innocent bystander? The debate continues
Discovery of substantial atrial fibrillation (AFib) is usually an indication to start oral anticoagulation (OAC) for stroke prevention, but it’s far from settled whether such AFib is actually a direct cause of thromboembolic stroke. And that has implications for whether patients with occasional bouts of the arrhythmia need to be on continuous OAC.
It’s possible that some with infrequent paroxysmal AFib can get away with OAC maintained only about as long as the arrhythmia persists, and then go off the drugs, say researchers based on their study, which, they caution, would need the support of prospective trials before such a strategy could be considered.
But importantly, in their patients who had been continuously monitored by their cardiac implantable electronic devices (CIEDs) prior to experiencing a stroke, the 30-day risk of that stroke more than tripled if their AFib burden on 1 day reached at least 5-6 hours. The risk jumped especially high within the first few days after accumulating that amount of AFib in a day, but then fell off sharply over the next few days.
Based on the study, “Your risk of stroke goes up acutely when you have an episode of AFib, and it decreases rapidly, back to baseline – certainly by 30 days and it looked like in our data by 5 days,” Daniel E. Singer, MD, of Massachusetts General Hospital, Boston, said in an interview.
Increasingly, he noted, “there’s a widespread belief that AFib is a risk marker, not a causal risk factor.” In that scenario, most embolic strokes are caused by thrombi formed as a result of an atrial myopathy, characterized by fibrosis and inflammation, that also happens to trigger AFib.
But said Dr. Singer, who is lead author on the analysis published online Sept. 29 in JAMA Cardiology.
Some studies have “shown that anticoagulants seem to lower stroke risk even in patients without atrial fib, and even from sources not likely to be coming from the atrium,” Mintu P. Turakhia, MD, of Stanford (Calif.) University, Palo Alto, said in an interview. Collectively they point to “atrial fibrillation as a cause of and a noncausal marker for stroke.”
For example, Dr. Turakhia pointed out in an editorial accompanying the current report that stroke in patients with CIEDs “may occur during prolonged periods of sinus rhythm.”
The current study, he said in an interview, doesn’t preclude atrial myopathy as one direct cause of stroke-associated thrombus, because probably both the myopathy and AFib can be culprits. Still, AFib itself it may bear more responsibility for strokes in patients with fewer competing risks for stroke.
In such patients at lower vascular risk, who may have a CHA2DS2-VASc score of only 1 or 2, for example, “AFib can become a more important cause” of ischemic stroke, Dr. Turakhia said. That’s when AFib is more likely to be temporally related to stroke as the likely culprit, the mechanism addressed by Dr. Singer and associates.
“I think we’re all trying to grapple with what the truth is,” Dr. Singer observed. Still, the current study was unusual for primarily looking at the temporal relationship between AFib and stroke, rather than stroke risk. “And once again, as we found in our earlier study, but now a much larger study, it’s a tight relationship.”
Based on the current results, he said, the risk is “high when you have AFib, and it decreases very rapidly after the AFib is over.” And, “it takes multiple hours of AFib to raise stroke risk.” Inclusion in the analysis required accumulation of at least 5.5 hours of AFib on at least 1 day in a month, the cut point at which stroke risk started to climb significantly in an earlier trial.
In the current analysis, however, the 30-day odds ratio for stroke was a nonsignificant 2.75 for an AFib burden of 6-23 hours in a day and jumped to a significant 5.0 for a burden in excess of 23 hours in a day. “That’s a lot of AFib” before the risk actually goes up, and supports AFib as causative, Dr. Singer said. If it were the myopathy itself triggering stroke in these particular patients, the risk would be ongoing and not subject to a threshold of AFib burden.
Implications for noncontinuous OAC
“The hope is that there are people who have very little AFib: They may have several hours, and then they have nothing for 6 months. Do they have to be anticoagulated or not?” Dr. Singer asked.
“If you believe the risk-marker story, you might say they have to be anticoagulated. But if you believe our results, you would certainly think there’s a good chance they don’t have to be anticoagulated,” he said.
“So it is logical to think, if you have the right people and continuous monitoring, that you could have time-delimited anticoagulation.” That is, patients might start right away on a direct OAC once reaching the AFib threshold in a day, Dr. Singer said, “going on and off anticoagulants in parallel with their episodes of AFib.”
The strategy wouldn’t be feasible in patients who often experience AFib, Dr. Singer noted, “but it might work for people who have infrequent paroxysmal AFib.” It certainly would first have to be tested in prospective trials, he said. Such trials would be more practical than ever to carry out given the growing availability of continuous AFib monitoring by wearables.
“We need a trial to make the case whether it’s safe or not,” Dr. Turakhia said of such a rhythm-guided approach to OAC for AFib. The population to start with, he said, would be patients with paroxysmal AFib and low CHA2DS2-VASc scores. “If you think CHA2DS2-VASc as an integrated score of vascular risk, such patients would have a lot fewer reasons to have strokes. And if they do have a stroke, it’s more reasonable to assume that it’s likely caused by atrial fib and not just a marker.”
Importantly, such a strategy could well be safer than continuous OAC for some patients – those at the lowest vascular risk and with the most occasional AFib and lowest AFib burden “who are otherwise doing fine,” Dr. Turakhia said. In such patients on continuous OAC, he proposed, the risks of bleeding and intracranial hemorrhage could potentially exceed the expected degree of protection from ischemic events.
Discordant periods of AFib burden
Dr. Singer and his colleagues linked a national electronic health record database with Medtronic CareLink records covering 10 years to identify 891 patients who experienced an ischemic stroke preceded by at least 120 days of continuous heart-rhythm monitoring.
The patients were then categorized by their pattern of AFib, if any, within each of two prestroke periods: the most recent 30 days, which was the test period, and the preceding 91-120 days, the control period.
The analysis then excluded any patients who reached an AFib-burden threshold of at least 5.5 hours on any day during both the test and control periods, and those who did not attain that threshold in either period.
“The ones who had AFib in both periods mostly had permanent AFib, and ones that didn’t have AFib in either period mostly were in sinus rhythm,” Dr. Singer said. It was “close to 100%” in both cases.
Those exclusions left 66 patients, 7.4% of the total, who reached the AFib-burden threshold on at least 1 day during either the test or control periods, but not both. They included 52 and 14 patients, respectively, with “discordant” periods, that is, at least that burden of AFib in a day during either the test or control period, but not both.
Comparing AFib burden at test versus control periods among patients for whom the two periods were discordant yielded an OR for stroke of 3.71 (95% confidence interval, 2.06-6.70).
Stroke risk levels were not evenly spread throughout the 24-hour periods that met the AFib-burden threshold or the 30 days preceding the patients’ strokes. The OR for stroke was 5.00 (95% CI, 2.62-9.55) during days 1-5 following the day in which the AFib-burden threshold was met. And it was 5.00 (95% CI, 2.08-12.01) over 30 days if the AFib burden exceeded 23 hours on any day of the test period.
The study’s case-crossover design, in which each patient served as their own control, is one of its advantages, Dr. Singer observed. Most patient features, including CHA2DS2-VASc score and comorbidities, did not change appreciably from earliest to the latest 30-day period, which strengthens the comparison of the two because “you don’t have to worry about long-term confounding.”
Dr. Singer was supported by the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital. He discloses receiving grants from Boehringer Ingelheim and Bristol-Myers Squibb; personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, Fitbit, Johnson & Johnson, Merck, and Pfizer; and royalties from UpToDate.
Dr. Turakhia discloses personal fees from Medtronic, Abbott, Sanofi, Pfizer, Myokardia, Johnson & Johnson, Milestone Pharmaceuticals, InCarda Therapeutics, 100Plus, Forward Pharma, and AliveCor; and grants from Bristol-Myers Squibb, the American Heart Association, Apple, and Bayer.
A version of this article first appeared on Medscape.com.
Discovery of substantial atrial fibrillation (AFib) is usually an indication to start oral anticoagulation (OAC) for stroke prevention, but it’s far from settled whether such AFib is actually a direct cause of thromboembolic stroke. And that has implications for whether patients with occasional bouts of the arrhythmia need to be on continuous OAC.
It’s possible that some with infrequent paroxysmal AFib can get away with OAC maintained only about as long as the arrhythmia persists, and then go off the drugs, say researchers based on their study, which, they caution, would need the support of prospective trials before such a strategy could be considered.
But importantly, in their patients who had been continuously monitored by their cardiac implantable electronic devices (CIEDs) prior to experiencing a stroke, the 30-day risk of that stroke more than tripled if their AFib burden on 1 day reached at least 5-6 hours. The risk jumped especially high within the first few days after accumulating that amount of AFib in a day, but then fell off sharply over the next few days.
Based on the study, “Your risk of stroke goes up acutely when you have an episode of AFib, and it decreases rapidly, back to baseline – certainly by 30 days and it looked like in our data by 5 days,” Daniel E. Singer, MD, of Massachusetts General Hospital, Boston, said in an interview.
Increasingly, he noted, “there’s a widespread belief that AFib is a risk marker, not a causal risk factor.” In that scenario, most embolic strokes are caused by thrombi formed as a result of an atrial myopathy, characterized by fibrosis and inflammation, that also happens to trigger AFib.
But said Dr. Singer, who is lead author on the analysis published online Sept. 29 in JAMA Cardiology.
Some studies have “shown that anticoagulants seem to lower stroke risk even in patients without atrial fib, and even from sources not likely to be coming from the atrium,” Mintu P. Turakhia, MD, of Stanford (Calif.) University, Palo Alto, said in an interview. Collectively they point to “atrial fibrillation as a cause of and a noncausal marker for stroke.”
For example, Dr. Turakhia pointed out in an editorial accompanying the current report that stroke in patients with CIEDs “may occur during prolonged periods of sinus rhythm.”
The current study, he said in an interview, doesn’t preclude atrial myopathy as one direct cause of stroke-associated thrombus, because probably both the myopathy and AFib can be culprits. Still, AFib itself it may bear more responsibility for strokes in patients with fewer competing risks for stroke.
In such patients at lower vascular risk, who may have a CHA2DS2-VASc score of only 1 or 2, for example, “AFib can become a more important cause” of ischemic stroke, Dr. Turakhia said. That’s when AFib is more likely to be temporally related to stroke as the likely culprit, the mechanism addressed by Dr. Singer and associates.
“I think we’re all trying to grapple with what the truth is,” Dr. Singer observed. Still, the current study was unusual for primarily looking at the temporal relationship between AFib and stroke, rather than stroke risk. “And once again, as we found in our earlier study, but now a much larger study, it’s a tight relationship.”
Based on the current results, he said, the risk is “high when you have AFib, and it decreases very rapidly after the AFib is over.” And, “it takes multiple hours of AFib to raise stroke risk.” Inclusion in the analysis required accumulation of at least 5.5 hours of AFib on at least 1 day in a month, the cut point at which stroke risk started to climb significantly in an earlier trial.
In the current analysis, however, the 30-day odds ratio for stroke was a nonsignificant 2.75 for an AFib burden of 6-23 hours in a day and jumped to a significant 5.0 for a burden in excess of 23 hours in a day. “That’s a lot of AFib” before the risk actually goes up, and supports AFib as causative, Dr. Singer said. If it were the myopathy itself triggering stroke in these particular patients, the risk would be ongoing and not subject to a threshold of AFib burden.
Implications for noncontinuous OAC
“The hope is that there are people who have very little AFib: They may have several hours, and then they have nothing for 6 months. Do they have to be anticoagulated or not?” Dr. Singer asked.
“If you believe the risk-marker story, you might say they have to be anticoagulated. But if you believe our results, you would certainly think there’s a good chance they don’t have to be anticoagulated,” he said.
“So it is logical to think, if you have the right people and continuous monitoring, that you could have time-delimited anticoagulation.” That is, patients might start right away on a direct OAC once reaching the AFib threshold in a day, Dr. Singer said, “going on and off anticoagulants in parallel with their episodes of AFib.”
The strategy wouldn’t be feasible in patients who often experience AFib, Dr. Singer noted, “but it might work for people who have infrequent paroxysmal AFib.” It certainly would first have to be tested in prospective trials, he said. Such trials would be more practical than ever to carry out given the growing availability of continuous AFib monitoring by wearables.
“We need a trial to make the case whether it’s safe or not,” Dr. Turakhia said of such a rhythm-guided approach to OAC for AFib. The population to start with, he said, would be patients with paroxysmal AFib and low CHA2DS2-VASc scores. “If you think CHA2DS2-VASc as an integrated score of vascular risk, such patients would have a lot fewer reasons to have strokes. And if they do have a stroke, it’s more reasonable to assume that it’s likely caused by atrial fib and not just a marker.”
Importantly, such a strategy could well be safer than continuous OAC for some patients – those at the lowest vascular risk and with the most occasional AFib and lowest AFib burden “who are otherwise doing fine,” Dr. Turakhia said. In such patients on continuous OAC, he proposed, the risks of bleeding and intracranial hemorrhage could potentially exceed the expected degree of protection from ischemic events.
Discordant periods of AFib burden
Dr. Singer and his colleagues linked a national electronic health record database with Medtronic CareLink records covering 10 years to identify 891 patients who experienced an ischemic stroke preceded by at least 120 days of continuous heart-rhythm monitoring.
The patients were then categorized by their pattern of AFib, if any, within each of two prestroke periods: the most recent 30 days, which was the test period, and the preceding 91-120 days, the control period.
The analysis then excluded any patients who reached an AFib-burden threshold of at least 5.5 hours on any day during both the test and control periods, and those who did not attain that threshold in either period.
“The ones who had AFib in both periods mostly had permanent AFib, and ones that didn’t have AFib in either period mostly were in sinus rhythm,” Dr. Singer said. It was “close to 100%” in both cases.
Those exclusions left 66 patients, 7.4% of the total, who reached the AFib-burden threshold on at least 1 day during either the test or control periods, but not both. They included 52 and 14 patients, respectively, with “discordant” periods, that is, at least that burden of AFib in a day during either the test or control period, but not both.
Comparing AFib burden at test versus control periods among patients for whom the two periods were discordant yielded an OR for stroke of 3.71 (95% confidence interval, 2.06-6.70).
Stroke risk levels were not evenly spread throughout the 24-hour periods that met the AFib-burden threshold or the 30 days preceding the patients’ strokes. The OR for stroke was 5.00 (95% CI, 2.62-9.55) during days 1-5 following the day in which the AFib-burden threshold was met. And it was 5.00 (95% CI, 2.08-12.01) over 30 days if the AFib burden exceeded 23 hours on any day of the test period.
The study’s case-crossover design, in which each patient served as their own control, is one of its advantages, Dr. Singer observed. Most patient features, including CHA2DS2-VASc score and comorbidities, did not change appreciably from earliest to the latest 30-day period, which strengthens the comparison of the two because “you don’t have to worry about long-term confounding.”
Dr. Singer was supported by the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital. He discloses receiving grants from Boehringer Ingelheim and Bristol-Myers Squibb; personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, Fitbit, Johnson & Johnson, Merck, and Pfizer; and royalties from UpToDate.
Dr. Turakhia discloses personal fees from Medtronic, Abbott, Sanofi, Pfizer, Myokardia, Johnson & Johnson, Milestone Pharmaceuticals, InCarda Therapeutics, 100Plus, Forward Pharma, and AliveCor; and grants from Bristol-Myers Squibb, the American Heart Association, Apple, and Bayer.
A version of this article first appeared on Medscape.com.
Discovery of substantial atrial fibrillation (AFib) is usually an indication to start oral anticoagulation (OAC) for stroke prevention, but it’s far from settled whether such AFib is actually a direct cause of thromboembolic stroke. And that has implications for whether patients with occasional bouts of the arrhythmia need to be on continuous OAC.
It’s possible that some with infrequent paroxysmal AFib can get away with OAC maintained only about as long as the arrhythmia persists, and then go off the drugs, say researchers based on their study, which, they caution, would need the support of prospective trials before such a strategy could be considered.
But importantly, in their patients who had been continuously monitored by their cardiac implantable electronic devices (CIEDs) prior to experiencing a stroke, the 30-day risk of that stroke more than tripled if their AFib burden on 1 day reached at least 5-6 hours. The risk jumped especially high within the first few days after accumulating that amount of AFib in a day, but then fell off sharply over the next few days.
Based on the study, “Your risk of stroke goes up acutely when you have an episode of AFib, and it decreases rapidly, back to baseline – certainly by 30 days and it looked like in our data by 5 days,” Daniel E. Singer, MD, of Massachusetts General Hospital, Boston, said in an interview.
Increasingly, he noted, “there’s a widespread belief that AFib is a risk marker, not a causal risk factor.” In that scenario, most embolic strokes are caused by thrombi formed as a result of an atrial myopathy, characterized by fibrosis and inflammation, that also happens to trigger AFib.
But said Dr. Singer, who is lead author on the analysis published online Sept. 29 in JAMA Cardiology.
Some studies have “shown that anticoagulants seem to lower stroke risk even in patients without atrial fib, and even from sources not likely to be coming from the atrium,” Mintu P. Turakhia, MD, of Stanford (Calif.) University, Palo Alto, said in an interview. Collectively they point to “atrial fibrillation as a cause of and a noncausal marker for stroke.”
For example, Dr. Turakhia pointed out in an editorial accompanying the current report that stroke in patients with CIEDs “may occur during prolonged periods of sinus rhythm.”
The current study, he said in an interview, doesn’t preclude atrial myopathy as one direct cause of stroke-associated thrombus, because probably both the myopathy and AFib can be culprits. Still, AFib itself it may bear more responsibility for strokes in patients with fewer competing risks for stroke.
In such patients at lower vascular risk, who may have a CHA2DS2-VASc score of only 1 or 2, for example, “AFib can become a more important cause” of ischemic stroke, Dr. Turakhia said. That’s when AFib is more likely to be temporally related to stroke as the likely culprit, the mechanism addressed by Dr. Singer and associates.
“I think we’re all trying to grapple with what the truth is,” Dr. Singer observed. Still, the current study was unusual for primarily looking at the temporal relationship between AFib and stroke, rather than stroke risk. “And once again, as we found in our earlier study, but now a much larger study, it’s a tight relationship.”
Based on the current results, he said, the risk is “high when you have AFib, and it decreases very rapidly after the AFib is over.” And, “it takes multiple hours of AFib to raise stroke risk.” Inclusion in the analysis required accumulation of at least 5.5 hours of AFib on at least 1 day in a month, the cut point at which stroke risk started to climb significantly in an earlier trial.
In the current analysis, however, the 30-day odds ratio for stroke was a nonsignificant 2.75 for an AFib burden of 6-23 hours in a day and jumped to a significant 5.0 for a burden in excess of 23 hours in a day. “That’s a lot of AFib” before the risk actually goes up, and supports AFib as causative, Dr. Singer said. If it were the myopathy itself triggering stroke in these particular patients, the risk would be ongoing and not subject to a threshold of AFib burden.
Implications for noncontinuous OAC
“The hope is that there are people who have very little AFib: They may have several hours, and then they have nothing for 6 months. Do they have to be anticoagulated or not?” Dr. Singer asked.
“If you believe the risk-marker story, you might say they have to be anticoagulated. But if you believe our results, you would certainly think there’s a good chance they don’t have to be anticoagulated,” he said.
“So it is logical to think, if you have the right people and continuous monitoring, that you could have time-delimited anticoagulation.” That is, patients might start right away on a direct OAC once reaching the AFib threshold in a day, Dr. Singer said, “going on and off anticoagulants in parallel with their episodes of AFib.”
The strategy wouldn’t be feasible in patients who often experience AFib, Dr. Singer noted, “but it might work for people who have infrequent paroxysmal AFib.” It certainly would first have to be tested in prospective trials, he said. Such trials would be more practical than ever to carry out given the growing availability of continuous AFib monitoring by wearables.
“We need a trial to make the case whether it’s safe or not,” Dr. Turakhia said of such a rhythm-guided approach to OAC for AFib. The population to start with, he said, would be patients with paroxysmal AFib and low CHA2DS2-VASc scores. “If you think CHA2DS2-VASc as an integrated score of vascular risk, such patients would have a lot fewer reasons to have strokes. And if they do have a stroke, it’s more reasonable to assume that it’s likely caused by atrial fib and not just a marker.”
Importantly, such a strategy could well be safer than continuous OAC for some patients – those at the lowest vascular risk and with the most occasional AFib and lowest AFib burden “who are otherwise doing fine,” Dr. Turakhia said. In such patients on continuous OAC, he proposed, the risks of bleeding and intracranial hemorrhage could potentially exceed the expected degree of protection from ischemic events.
Discordant periods of AFib burden
Dr. Singer and his colleagues linked a national electronic health record database with Medtronic CareLink records covering 10 years to identify 891 patients who experienced an ischemic stroke preceded by at least 120 days of continuous heart-rhythm monitoring.
The patients were then categorized by their pattern of AFib, if any, within each of two prestroke periods: the most recent 30 days, which was the test period, and the preceding 91-120 days, the control period.
The analysis then excluded any patients who reached an AFib-burden threshold of at least 5.5 hours on any day during both the test and control periods, and those who did not attain that threshold in either period.
“The ones who had AFib in both periods mostly had permanent AFib, and ones that didn’t have AFib in either period mostly were in sinus rhythm,” Dr. Singer said. It was “close to 100%” in both cases.
Those exclusions left 66 patients, 7.4% of the total, who reached the AFib-burden threshold on at least 1 day during either the test or control periods, but not both. They included 52 and 14 patients, respectively, with “discordant” periods, that is, at least that burden of AFib in a day during either the test or control period, but not both.
Comparing AFib burden at test versus control periods among patients for whom the two periods were discordant yielded an OR for stroke of 3.71 (95% confidence interval, 2.06-6.70).
Stroke risk levels were not evenly spread throughout the 24-hour periods that met the AFib-burden threshold or the 30 days preceding the patients’ strokes. The OR for stroke was 5.00 (95% CI, 2.62-9.55) during days 1-5 following the day in which the AFib-burden threshold was met. And it was 5.00 (95% CI, 2.08-12.01) over 30 days if the AFib burden exceeded 23 hours on any day of the test period.
The study’s case-crossover design, in which each patient served as their own control, is one of its advantages, Dr. Singer observed. Most patient features, including CHA2DS2-VASc score and comorbidities, did not change appreciably from earliest to the latest 30-day period, which strengthens the comparison of the two because “you don’t have to worry about long-term confounding.”
Dr. Singer was supported by the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital. He discloses receiving grants from Boehringer Ingelheim and Bristol-Myers Squibb; personal fees from Boehringer Ingelheim, Bristol-Myers Squibb, Fitbit, Johnson & Johnson, Merck, and Pfizer; and royalties from UpToDate.
Dr. Turakhia discloses personal fees from Medtronic, Abbott, Sanofi, Pfizer, Myokardia, Johnson & Johnson, Milestone Pharmaceuticals, InCarda Therapeutics, 100Plus, Forward Pharma, and AliveCor; and grants from Bristol-Myers Squibb, the American Heart Association, Apple, and Bayer.
A version of this article first appeared on Medscape.com.
‘Regionalized’ care tied to better survival in tough cancer
The Kaiser Permanente Northern California (KPNC) Gastric-Esophageal Cancer Program was established in 2016 to consolidate the care of patients with gastric cancer and increase physician specialization and standardization.
In a retrospective study, Kaiser Permanente investigators compared the outcomes of the 942 patients diagnosed before the regional gastric cancer team was established with the outcomes of the 487 patients treated after it was implemented. Overall, the transformation appears to enhance the delivery of care and improves clinical and oncologic outcomes.
For example, among 394 patients who received curative-intent surgery, overall survival at 2 years increased from 72.7% before the program to 85.5% afterward.
“The regionalized gastric cancer program combines the benefits of community-based care, which is local and convenient, with the expertise of a specialized cancer center,” said coauthor Lisa Herrinton, PhD, a research scientist at the Kaiser Permanente division of research, in a statement.
She added that, in their integrated care system, “it is easy for the different physicians that treat cancer patients – including surgical oncologists, medical oncologists, and radiation oncologists, among others – to come together and collaborate and tie their work flows together.”
The study was published in the Journal of Clinical Oncology.
Gastric cancer accounts for about 27,600 cases diagnosed annually in the United States, but the overall 5-year survival is still only 32%. About half of the cases are locoregional (stage I-III) and potentially eligible for curative-intent surgery and adjuvant therapy, the authors pointed out in the study.
As compared with North America, the incidence rate is five to six times higher in East Asia, but they have developed better surveillance for early detection and treatment is highly effective, as the increased incidence allows oncologists and surgeons to achieve greater specialization. Laparoscopic gastrectomy and extended (D2) lymph node dissection are more commonly performed in East Asia as compared with the United States, and surgical outcomes appear to be better in East Asia.
Regionalizing care
The rationale for regionalizing care was that increasing and concentrating the volume of cases to a specific location would make it more possible to introduce new surgical procedures as well as allow medical oncologists to uniformly introduce and standardize the use of the newest chemotherapy regimens.
Prior to regionalization, gastric cancer surgery was performed at 19 medical centers in the Kaiser system. Now, curative-intent laparoscopic gastrectomy with Roux-en-Y gastrojejunostomy or esophagojejunostomy and side-to-side jejunojejunostomy is subsequently performed at only two centers by five surgeons.
“The two centers were selected based on how skilled the surgeons were at performing advanced minimally invasive oncologic surgery,” said lead author Swee H. Teh, MD, surgical director, gastric cancer surgery, Kaiser Permanente Northern California. “We also looked at the center’s retrospective gastrectomy outcomes and the strength of the leadership that would be collaborating with the regional multidisciplinary team.”
Dr. Teh said in an interview that it was imperative that their regional gastric cancer centers have surgeons highly skilled in advanced minimally invasive gastrointestinal surgery. “This is a newer technique and not one of our more senior surgeons had been trained in [it],” he said. “With this change, some surgeons were now no longer performing gastric cancer surgery.”
Not only were the centers selected based on the surgical skills of the surgeons already there, but they also took into account the locations and geographical membership distribution. “We have found that our patients’ traveling distance to receive surgical care has not changed significantly,” Dr. Teh said.
Improved outcomes
The cohort included 1,429 eligible patients all stages of gastric cancer; one-third were treated after regionalization, 650 had stage I-III disease, and 394 underwent curative-intent surgery.
Overall survival at 2 years was 32.8% pre- and 37.3% post regionalization (P = .20) for all stages of cancer; stage I-III cases with or without surgery, 55.6% and 61.1%, respectively (P = .25); and among all surgery patients, 72.7% and 85.5%, respectively (P < .03).
Among patients who underwent surgery, the use of neoadjuvant chemotherapy increased from 35% to 66% (P < .0001), as did laparoscopic gastrectomy from 18% to 92% (P < .0001), and D2 lymphadenectomy from 2% to 80% (P < .0001). In addition, dissection of 15 or more lymph nodes also rose from 61% to 95% (P < .0001). Post regionalization, the resection margin was more often negative, and the resection less often involved other organs.
The median length of hospitalization declined from 7 to 3 days (P < .001) after regionalization but all-cause readmissions and reoperation at 30 and 90 days were similar in both cohorts. The risk of bowel obstruction was less frequent post regionalization (P = .01 at both 30 and 90 days), as was risk of infection (P = .03 at 30 days and P = .01 at 90 days).
The risk of one or more serious adverse events was also lower (P < .01), and 30-day mortality did not change (pre 0.7% and post 0.0%, P = .34).
Generalizability may not be feasible
But although this was successful for Kaiser, the authors note that a key limitation of the study is generalizability – and that regionalization may not be feasible in many U.S. settings.
“There needs to be a standardized workflow that all stakeholders agree upon,” Dr. Teh explained. “For example, in our gastric cancer staging pathway, all patients who are considered candidates for surgery have four staging tests: CT scan, PET scan and endoscopic ultrasound [EUS], and staging laparoscopy.”
Another important aspect is that in order to make sure the workflow is smooth and timely, all subspecialties responsible for the staging modalities need to create a “special access.” What this means, he continued, is for example, that the radiology department must ensure that these patients will have their CT scan and PET scans promptly. “Similarly, the GI department must provide quick access to EUS, and the surgery department must quickly provide a staging laparoscopy,” Dr. Teh said. “We have been extremely successful in achieving this goal.”
Dr. Teh also noted that a skilled patient navigator and a team where each member brings high-level expertise and experience to the table are also necessary. And innovative technology is also needed.
“We use artificial intelligence to identify all newly diagnosed cases of gastric [cancer], and within 24 hours of a patient’s diagnosis, a notification is sent to entire team about this new patient,” he added. “We also use AI to extract data to create a dashboard that will track each patient’s progress and outcomes, so that the results are accessible to every member of the team. The innovative technology has also helped us build a comprehensive survivorship program.”
They also noted in their study that European and Canadian systems, as well as the Department of Veterans Affairs, could probably implement components of this, including enhanced recovery after surgery.
The study had no specific funding. Dr. Teh and Dr. Herrinton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Kaiser Permanente Northern California (KPNC) Gastric-Esophageal Cancer Program was established in 2016 to consolidate the care of patients with gastric cancer and increase physician specialization and standardization.
In a retrospective study, Kaiser Permanente investigators compared the outcomes of the 942 patients diagnosed before the regional gastric cancer team was established with the outcomes of the 487 patients treated after it was implemented. Overall, the transformation appears to enhance the delivery of care and improves clinical and oncologic outcomes.
For example, among 394 patients who received curative-intent surgery, overall survival at 2 years increased from 72.7% before the program to 85.5% afterward.
“The regionalized gastric cancer program combines the benefits of community-based care, which is local and convenient, with the expertise of a specialized cancer center,” said coauthor Lisa Herrinton, PhD, a research scientist at the Kaiser Permanente division of research, in a statement.
She added that, in their integrated care system, “it is easy for the different physicians that treat cancer patients – including surgical oncologists, medical oncologists, and radiation oncologists, among others – to come together and collaborate and tie their work flows together.”
The study was published in the Journal of Clinical Oncology.
Gastric cancer accounts for about 27,600 cases diagnosed annually in the United States, but the overall 5-year survival is still only 32%. About half of the cases are locoregional (stage I-III) and potentially eligible for curative-intent surgery and adjuvant therapy, the authors pointed out in the study.
As compared with North America, the incidence rate is five to six times higher in East Asia, but they have developed better surveillance for early detection and treatment is highly effective, as the increased incidence allows oncologists and surgeons to achieve greater specialization. Laparoscopic gastrectomy and extended (D2) lymph node dissection are more commonly performed in East Asia as compared with the United States, and surgical outcomes appear to be better in East Asia.
Regionalizing care
The rationale for regionalizing care was that increasing and concentrating the volume of cases to a specific location would make it more possible to introduce new surgical procedures as well as allow medical oncologists to uniformly introduce and standardize the use of the newest chemotherapy regimens.
Prior to regionalization, gastric cancer surgery was performed at 19 medical centers in the Kaiser system. Now, curative-intent laparoscopic gastrectomy with Roux-en-Y gastrojejunostomy or esophagojejunostomy and side-to-side jejunojejunostomy is subsequently performed at only two centers by five surgeons.
“The two centers were selected based on how skilled the surgeons were at performing advanced minimally invasive oncologic surgery,” said lead author Swee H. Teh, MD, surgical director, gastric cancer surgery, Kaiser Permanente Northern California. “We also looked at the center’s retrospective gastrectomy outcomes and the strength of the leadership that would be collaborating with the regional multidisciplinary team.”
Dr. Teh said in an interview that it was imperative that their regional gastric cancer centers have surgeons highly skilled in advanced minimally invasive gastrointestinal surgery. “This is a newer technique and not one of our more senior surgeons had been trained in [it],” he said. “With this change, some surgeons were now no longer performing gastric cancer surgery.”
Not only were the centers selected based on the surgical skills of the surgeons already there, but they also took into account the locations and geographical membership distribution. “We have found that our patients’ traveling distance to receive surgical care has not changed significantly,” Dr. Teh said.
Improved outcomes
The cohort included 1,429 eligible patients all stages of gastric cancer; one-third were treated after regionalization, 650 had stage I-III disease, and 394 underwent curative-intent surgery.
Overall survival at 2 years was 32.8% pre- and 37.3% post regionalization (P = .20) for all stages of cancer; stage I-III cases with or without surgery, 55.6% and 61.1%, respectively (P = .25); and among all surgery patients, 72.7% and 85.5%, respectively (P < .03).
Among patients who underwent surgery, the use of neoadjuvant chemotherapy increased from 35% to 66% (P < .0001), as did laparoscopic gastrectomy from 18% to 92% (P < .0001), and D2 lymphadenectomy from 2% to 80% (P < .0001). In addition, dissection of 15 or more lymph nodes also rose from 61% to 95% (P < .0001). Post regionalization, the resection margin was more often negative, and the resection less often involved other organs.
The median length of hospitalization declined from 7 to 3 days (P < .001) after regionalization but all-cause readmissions and reoperation at 30 and 90 days were similar in both cohorts. The risk of bowel obstruction was less frequent post regionalization (P = .01 at both 30 and 90 days), as was risk of infection (P = .03 at 30 days and P = .01 at 90 days).
The risk of one or more serious adverse events was also lower (P < .01), and 30-day mortality did not change (pre 0.7% and post 0.0%, P = .34).
Generalizability may not be feasible
But although this was successful for Kaiser, the authors note that a key limitation of the study is generalizability – and that regionalization may not be feasible in many U.S. settings.
“There needs to be a standardized workflow that all stakeholders agree upon,” Dr. Teh explained. “For example, in our gastric cancer staging pathway, all patients who are considered candidates for surgery have four staging tests: CT scan, PET scan and endoscopic ultrasound [EUS], and staging laparoscopy.”
Another important aspect is that in order to make sure the workflow is smooth and timely, all subspecialties responsible for the staging modalities need to create a “special access.” What this means, he continued, is for example, that the radiology department must ensure that these patients will have their CT scan and PET scans promptly. “Similarly, the GI department must provide quick access to EUS, and the surgery department must quickly provide a staging laparoscopy,” Dr. Teh said. “We have been extremely successful in achieving this goal.”
Dr. Teh also noted that a skilled patient navigator and a team where each member brings high-level expertise and experience to the table are also necessary. And innovative technology is also needed.
“We use artificial intelligence to identify all newly diagnosed cases of gastric [cancer], and within 24 hours of a patient’s diagnosis, a notification is sent to entire team about this new patient,” he added. “We also use AI to extract data to create a dashboard that will track each patient’s progress and outcomes, so that the results are accessible to every member of the team. The innovative technology has also helped us build a comprehensive survivorship program.”
They also noted in their study that European and Canadian systems, as well as the Department of Veterans Affairs, could probably implement components of this, including enhanced recovery after surgery.
The study had no specific funding. Dr. Teh and Dr. Herrinton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Kaiser Permanente Northern California (KPNC) Gastric-Esophageal Cancer Program was established in 2016 to consolidate the care of patients with gastric cancer and increase physician specialization and standardization.
In a retrospective study, Kaiser Permanente investigators compared the outcomes of the 942 patients diagnosed before the regional gastric cancer team was established with the outcomes of the 487 patients treated after it was implemented. Overall, the transformation appears to enhance the delivery of care and improves clinical and oncologic outcomes.
For example, among 394 patients who received curative-intent surgery, overall survival at 2 years increased from 72.7% before the program to 85.5% afterward.
“The regionalized gastric cancer program combines the benefits of community-based care, which is local and convenient, with the expertise of a specialized cancer center,” said coauthor Lisa Herrinton, PhD, a research scientist at the Kaiser Permanente division of research, in a statement.
She added that, in their integrated care system, “it is easy for the different physicians that treat cancer patients – including surgical oncologists, medical oncologists, and radiation oncologists, among others – to come together and collaborate and tie their work flows together.”
The study was published in the Journal of Clinical Oncology.
Gastric cancer accounts for about 27,600 cases diagnosed annually in the United States, but the overall 5-year survival is still only 32%. About half of the cases are locoregional (stage I-III) and potentially eligible for curative-intent surgery and adjuvant therapy, the authors pointed out in the study.
As compared with North America, the incidence rate is five to six times higher in East Asia, but they have developed better surveillance for early detection and treatment is highly effective, as the increased incidence allows oncologists and surgeons to achieve greater specialization. Laparoscopic gastrectomy and extended (D2) lymph node dissection are more commonly performed in East Asia as compared with the United States, and surgical outcomes appear to be better in East Asia.
Regionalizing care
The rationale for regionalizing care was that increasing and concentrating the volume of cases to a specific location would make it more possible to introduce new surgical procedures as well as allow medical oncologists to uniformly introduce and standardize the use of the newest chemotherapy regimens.
Prior to regionalization, gastric cancer surgery was performed at 19 medical centers in the Kaiser system. Now, curative-intent laparoscopic gastrectomy with Roux-en-Y gastrojejunostomy or esophagojejunostomy and side-to-side jejunojejunostomy is subsequently performed at only two centers by five surgeons.
“The two centers were selected based on how skilled the surgeons were at performing advanced minimally invasive oncologic surgery,” said lead author Swee H. Teh, MD, surgical director, gastric cancer surgery, Kaiser Permanente Northern California. “We also looked at the center’s retrospective gastrectomy outcomes and the strength of the leadership that would be collaborating with the regional multidisciplinary team.”
Dr. Teh said in an interview that it was imperative that their regional gastric cancer centers have surgeons highly skilled in advanced minimally invasive gastrointestinal surgery. “This is a newer technique and not one of our more senior surgeons had been trained in [it],” he said. “With this change, some surgeons were now no longer performing gastric cancer surgery.”
Not only were the centers selected based on the surgical skills of the surgeons already there, but they also took into account the locations and geographical membership distribution. “We have found that our patients’ traveling distance to receive surgical care has not changed significantly,” Dr. Teh said.
Improved outcomes
The cohort included 1,429 eligible patients all stages of gastric cancer; one-third were treated after regionalization, 650 had stage I-III disease, and 394 underwent curative-intent surgery.
Overall survival at 2 years was 32.8% pre- and 37.3% post regionalization (P = .20) for all stages of cancer; stage I-III cases with or without surgery, 55.6% and 61.1%, respectively (P = .25); and among all surgery patients, 72.7% and 85.5%, respectively (P < .03).
Among patients who underwent surgery, the use of neoadjuvant chemotherapy increased from 35% to 66% (P < .0001), as did laparoscopic gastrectomy from 18% to 92% (P < .0001), and D2 lymphadenectomy from 2% to 80% (P < .0001). In addition, dissection of 15 or more lymph nodes also rose from 61% to 95% (P < .0001). Post regionalization, the resection margin was more often negative, and the resection less often involved other organs.
The median length of hospitalization declined from 7 to 3 days (P < .001) after regionalization but all-cause readmissions and reoperation at 30 and 90 days were similar in both cohorts. The risk of bowel obstruction was less frequent post regionalization (P = .01 at both 30 and 90 days), as was risk of infection (P = .03 at 30 days and P = .01 at 90 days).
The risk of one or more serious adverse events was also lower (P < .01), and 30-day mortality did not change (pre 0.7% and post 0.0%, P = .34).
Generalizability may not be feasible
But although this was successful for Kaiser, the authors note that a key limitation of the study is generalizability – and that regionalization may not be feasible in many U.S. settings.
“There needs to be a standardized workflow that all stakeholders agree upon,” Dr. Teh explained. “For example, in our gastric cancer staging pathway, all patients who are considered candidates for surgery have four staging tests: CT scan, PET scan and endoscopic ultrasound [EUS], and staging laparoscopy.”
Another important aspect is that in order to make sure the workflow is smooth and timely, all subspecialties responsible for the staging modalities need to create a “special access.” What this means, he continued, is for example, that the radiology department must ensure that these patients will have their CT scan and PET scans promptly. “Similarly, the GI department must provide quick access to EUS, and the surgery department must quickly provide a staging laparoscopy,” Dr. Teh said. “We have been extremely successful in achieving this goal.”
Dr. Teh also noted that a skilled patient navigator and a team where each member brings high-level expertise and experience to the table are also necessary. And innovative technology is also needed.
“We use artificial intelligence to identify all newly diagnosed cases of gastric [cancer], and within 24 hours of a patient’s diagnosis, a notification is sent to entire team about this new patient,” he added. “We also use AI to extract data to create a dashboard that will track each patient’s progress and outcomes, so that the results are accessible to every member of the team. The innovative technology has also helped us build a comprehensive survivorship program.”
They also noted in their study that European and Canadian systems, as well as the Department of Veterans Affairs, could probably implement components of this, including enhanced recovery after surgery.
The study had no specific funding. Dr. Teh and Dr. Herrinton have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.



