User login
Consensus Statement Supporting the Presence of Onsite Radiation Oncology Departments at VHA Medical Centers
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
Radiation therapy, along with surgery and systemic therapy, is a primary therapeutic modality for cancer management. At least half of cancer patients receive radiation as part of their treatment regimen.1 Multiple studies demonstrate that radiotherapy is underutilized worldwide.2 One reason for underutilization of radiotherapy globally is poor access to this treatment modality. Factors that contribute to poor access include long wait times for consultation, delays in treatment initiation, distance to a treatment facility, and poor coordination of care.
Taskforce Findings
The presence of onsite radiation oncology and its impact on utilization of radiotherapy is poorly studied. The Veterans Health Administration (VHA) Palliative Radiotherapy Taskforce recently conducted a survey to determine the barriers to referral and timeliness of treatment for palliative radiotherapy within the VHA.3 Key findings of this study comparing centers with onsite radiation departments with centers without onsite radiation departments include:
a. Radiation consults are more likely to be completed within 1 week of consult request at centers with onsite radiation therapy (68% vs 31%, respectively; P = .01).
b. Centers with onsite radiation therapy more frequently deliver emergent treatment within 24 hours for patients with spinal cord compression, an emergency condition in which prompt radiation can prevent or minimize long-term neurologic disability (94% vs 70%, respectively; P = .01).
c. Referring practitioners with onsite radiation departments are less likely to report difficulty contacting a radiation oncologist as a barrier to referral for palliative radiotherapy (0% vs 20%, respectively; P = .006).
d. Referring practitioners with onsite radiotherapy report patient travel as a barrier to referral for palliative radiotherapy less frequently (28% vs 71%, respectively; P < .001).
e. Practitioners with onsite radiation oncology departments are more likely to have multidisciplinary tumor boards (31% vs 3%, respectively; P = .01) and are more likely to be influenced by radiation oncology recommendations at tumor boards (69% vs 44%, respectively; P = .02).
Based on the findings of this study, the VHA Palliative Radiotherapy Taskforce has prepared this consensus statement regarding the importance of onsite radiation oncology departments at VHA medical centers. More information regarding our 5 key findings and their implications for patient care are as follows:
Timeliness of Radiation Oncology Consultation
Delays in radiation oncology consultation, which can also delay treatment initiation, are associated with poor satisfaction among both patients and referring clinicians.4 Wait times have been identified as a barrier to utilization of radiotherapy by both patients and clinicians.5,6 Furthermore, delays in initiation of definitive therapy have been associated with worse outcomes, including worse overall survival.7,8 Our survey study demonstrates that consults for palliative radiotherapy are occurring in a more timely manner at centers with onsite radiation departments. Radiation oncology consults are more frequently completed within 1 week at centers with onsite radiation oncology departments compared with centers without onsite radiation oncology departments (68% vs 31%, P = .01). This trend would likely be seen for nonpalliative, definitive cases as well. The presence of radiation oncology departments onsite at VHA medical centers is an important component of timely care for veterans to optimize outcomes of cancer treatment.
Timely Delivery of Radiotherapy for Oncologic Emergencies
There are a few scenarios in which emergent radiation treatment, within 24 hours, is indicated. These include malignant spinal cord compression, uncal herniation from brain metastasis, superior vena cava syndrome, and tumor hemorrhage.9 Studies on management of metastatic spinal cord compression demonstrate that delays in treatment are associated with reduced ambulation10 as well as loss of sphincter function and incontinence.11
Our study demonstrates that VHA medical centers with onsite radiotherapy more frequently deliver radiotherapy within 24 hours for patients with metastatic spinal cord compression. This timely delivery of treatment is critical to optimizing functional status and quality of life in patients requiring treatment for oncologic emergencies. Revisiting treatment pathways for such situations at regular intervals is crucial given that residents and staff may rotate and be unfamiliar with emergency protocols.
Communication With Radiation Oncologists
Several studies have demonstrated that the inability to contact a radiation oncologist and poor communication result in decreased referrals for palliative radiotherapy.12,13 Our study demonstrates that onsite radiation oncology is associated with improved ability to contact a radiation oncologist. About 20% of clinicians at facilities without onsite radiation oncology reported difficulty contacting a radiation oncologist, compared with 0% at facilities with onsite radiation departments (P = .006).
It is possible that increased radiation oncology presence at VHA medical centers, through attenuation of barriers related to contacting a radiation oncologist and improved communication, would lead to increased use of radiotherapy. Increased communication between referring clinicians and radiation oncologists also can help with education of those clinicians making the referral. Since knowledge gaps have been identified in multiple studies as a barrier to referral for radiotherapy, such communication and increased education on the role of radiotherapy could increase use.12-14
Patient Travel
Patient ability to travel was the most commonly reported barrier (81%) to referral for palliative radiotherapy in our study. Travel time and transportation difficulties have been established in multiple studies as barriers to radiotherapy for both definitive and palliative management.15-18 Travel for radiotherapy was much less frequently reported as a barrier among respondents with onsite radiation oncology departments compared with those without onsite radiation departments (28% vs 71%, respectively; P < .001).
It is therefore possible that expansion of VHA radiation oncology services, allowing for provision of onsite radiotherapy at more VHA facilities, would reduce travel burden. Increasing travel accommodations for patients and provision of patient lodging on hospital campuses, which is already offered at some VHA medical centers (ie, Fisher House Foundation), could also help attenuate this barrier.
Multidisciplinary Tumor Boards
Our study demonstrates that centers with onsite radiation departments more frequently hold multidisciplinary tumor boards compared with centers without radiation departments (31% vs 3%, respectively; P = .01). Multidisciplinary tumor boards allow subspecialties to meet regularly to communicate about patient care and can help mitigate barriers related to communication and education of the referring health care practitioners.
As cases are discussed in multidisciplinary tumor boards, health care practitioners have the opportunity to make recommendations and provide education on potential benefits and/or downsides of treatments offered by their respective specialties. Several studies have demonstrated that cases discussed at multidisciplinary tumor boards are more likely to be referred for radiation therapy.19-21 Furthermore, multidisciplinary tumor boards have been associated with improved treatment outcomes.22
Conclusions
In this consensus statement the VHA Palliative Radiotherapy Taskforce recommends the optimization of use of radiotherapy within the VHA. Radiation oncology services should be maintained where present in the VHA, with consideration for expansion of services to additional facilities. Telehealth should be used to expedite consults and treatment. Hypofractionation should be used, when appropriate, to ease travel burden. Options for transportation services and onsite housing, or hospitalization, should be understood by practitioners and offered to patients to mitigate barriers related to travel.
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
1. Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140-144. doi:10.1016/j.radonc.2014.03.024
2. Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153-1186. doi:10.1016/S1470-2045(15)00222-3
3. Gutt R, Malhotra S, Hagan MP, et al. Palliative radiotherapy within the Veterans Health Administration: barriers to referral and timeliness of treatment. JCO Oncol Pract. 2021;17(12):e1913-e1922. doi:10.1200/OP.20.00981
4. Agazaryan N, Chow P, Lamb J, et al. The timeliness initiative: continuous process improvement for prompt initiation of radiation therapy treatment. Adv Radiat Oncol. 2020;5(5):1014-1021. Published 2020 Mar 10. doi:10.1016/j.adro.2020.01.007
5. Gillan C, Briggs K, Goytisolo Pazos A, et al. Barriers to accessing radiation therapy in Canada: a systematic review. Radiat Oncol. 2012;7:167. Published 2012 Oct 12. doi:10.1186/1748-717X-7-167
6. Hanna TP, Richardson H, Peng Y, Kong W, Zhang-Salomons J, Mackillop WJ. A population-based study of factors affecting the use of radiotherapy for endometrial cancer. Clin Oncol (R Coll Radiol). 2012;24(8):e113-e124. doi:10.1016/j.clon.2012.01.007
7. Ho AS, Kim S, Tighiouart M, et al. Quantitative survival impact of composite treatment delays in head and neck cancer. Cancer. 2018;124(15):3154-3162. doi:10.1002/cncr.31533
8. Cone EB, Marchese M, Paciotti M, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open. 2020;3(12):e2030072. Published 2020 Dec 1. doi:10.1001/jamanetworkopen.2020.30072
9. Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol. 2009;16(4):55-60. doi:10.3747/co.v16i4.352
10. Laufer I, Zuckerman SL, Bird JE, et al. Predicting neurologic recovery after surgery in patients with deficits secondary to MESCC: systematic review. Spine (Phila Pa 1976). 2016;41 (Suppl 20):S224-S230. doi:10.1097/BRS.0000000000001827
11. Husband DJ. Malignant spinal cord compression: prospective study of delays in referral and treatment. BMJ. 1998;317(7150):18-21. doi:10.1136/bmj.317.7150.18
12. Samant RS, Fitzgibbon E, Meng J, Graham ID. Family physicians’ perspectives regarding palliative radiotherapy. Radiother Oncol. 2006;78(1):101-106. doi:10.1016/j.radonc.2005.11.008
13. McCloskey SA, Tao ML, Rose CM, Fink A, Amadeo AM. National survey of perspectives of palliative radiation therapy: role, barriers, and needs. Cancer J. 2007;13(2):130-137. doi:10.1097/PPO.0b013e31804675d4
14. Chierchini S, Ingrosso G, Saldi S, Stracci F, Aristei C. Physician and patient barriers to radiotherapy service access: treatment referral implications. Cancer Manag Res. 2019;11:8829-8833. Published 2019 Oct 7. doi:10.2147/CMAR.S168941
15. Longacre CF, Neprash HT, Shippee ND, Tuttle TM, Virnig BA. Travel, treatment choice, and survival among breast cancer patients: a population-based analysis. Womens Health Rep (New Rochelle). 2021;2(1):1-10. Published 2021 Jan 11. doi:10.1089/whr.2020.0094
16. Yang DD, Muralidhar V, Mahal BA, et al. Travel distance as a barrier to receipt of adjuvant radiation therapy after radical Prostatectomy. Am J Clin Oncol. 2018;41(10):953-959. doi:10.1097/COC.0000000000000410
17. Sundaresan P, King M, Stockler M, Costa D, Milross C. Barriers to radiotherapy utilization: Consumer perceptions of issues influencing radiotherapy-related decisions. Asia Pac J Clin Oncol. 2017;13(5):e489-e496. doi:10.1111/ajco.12579
18. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. Oncologist. 2015;20(12):1378-1385. doi:10.1634/theoncologist.2015-0110
19. Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838-841. doi:10.1111/j.1445-5994.2009.02019.x
20. Brännström F, Bjerregaard JK, Winbladh A, et al. Multidisciplinary team conferences promote treatment according to guidelines in rectal cancer. Acta Oncol. 2015;54(4):447-453. doi:10.3109/0284186X.2014.952387
21. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. doi:10.1016/j.ctrv.2015.11.007
22. Freytag M, Herrlinger U, Hauser S, et al. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer. 2020;20(1):355. Published 2020 Apr 28. doi:10.1186/s12885-020-06809-1
Agent Orange Exposure, Transformation From MGUS to Multiple Myeloma, and Outcomes in Veterans
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
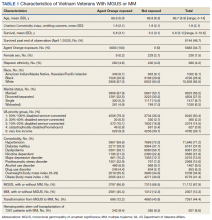
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
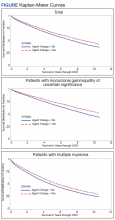
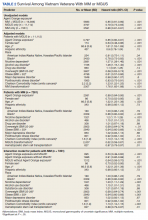
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
Multiple myeloma (MM) accounts for 1% to 2% of all cancers and slightly more than 17% of hematologic malignancies in the United States.1 MM is characterized by the neoplastic proliferation of immunoglobulin (Ig)-producing plasma cells with ≥ 10% clonal plasma cells in the bone marrow or biopsy-proven bony or soft tissue plasmacytoma, plus presence of related organ or tissue impairment or presence of a biomarker associated with near-inevitable progression to end-organ damage.2
Background
Up to 97% of patients with MM will have a monoclonal (M) protein produced and secreted by the malignant plasma cells, which can be detected by protein electrophoresis of the serum and an aliquot of urine from a 24-hour collection combined with immunofixation of the serum and urine. The M protein in MM usually consists of IgG 50% of the time and light chains 16% of the time. Patients who lack detectable M protein are considered to have nonsecretory myeloma. MM presents with end-organ damage, which includes hypercalcemia, renal dysfunction, anemia, or lytic bone lesions. Patients with MM frequently present with renal insufficiency due to cast nephropathy or light chain deposition disease.3
MM is thought to evolve from monoclonal gammopathy of uncertain significance (MGUS), an asymptomatic premalignant stage of clonal plasma cell proliferation with a risk of progression to active myeloma at 1% per year.4,5 Epidemiologic data suggest that people who develop MM have a genetic predisposition, but risk factors may develop or be acquired, such as age, immunosuppression, and environmental exposures. To better assess what causes transformation from MGUS to MM, it is important to identify agents that may cause this second hit.6
In November 1961, President John F. Kennedy authorized the start of Operation Ranch Hand, the US Air Force’s herbicide program during the Vietnam War. Twenty million gallons of various chemicals were sprayed in Vietnam, eastern Laos, and parts of Cambodia to defoliate rural land, depriving guerillas of their support base. Agent Orange (AO) was one of these chemicals; it is a mixed herbicide with traces of dioxin, a compound that has been associated with major health problems among exposed individuals.7 Several studies have evaluated exposure to AO and its potential harmful repercussions. Studies have assessed the link between AO and MGUS as well as AO to various leukemias, such as chronic lymphocytic leukemia.8,9 Other studies have shown the relationship between AO exposure and worse outcomes in persons with MM.10 To date, only a single abstract from a US Department of Veterans Affairs (VA) medical center has investigated the relationships between AO exposure and MGUS, MM, and the rate of transformation. The VA study of patients seen from 2005 to 2015 in Detroit, Michigan, found that AO exposure led to an increase in cumulative incidence rate of MGUS/MM, suggesting possible changes in disease biology and genetics.11
In this study, we aimed to determine the incidence of transformation of MGUS to MM in patients with and without exposure to AO. We then analyzed survival as a function of AO exposure, transformation, and clinical and sociodemographic variables. We also explored the impact of psychosocial variables and hematopoietic stem cell transplantation (HSCT), a standard of treatment for MM.
Methods
This retrospective cohort study assembled electronic health record (EHR) data from the Veterans Health Administration Corporate Data Warehouse (CDW). The VA Central Texas Veterans Healthcare System Institutional Review Board granted a waiver of consent for this record review. Eligible patients were Vietnam-era veterans who were in the military during the time that AO was used (1961-1971). Veterans were included if they were being cared for and received a diagnosis for MGUS or MM between October 1, 2009, and September 30, 2015 (all prevalent cases fiscal years 2010-2015). Cases were excluded if there was illogical death data or if age, race, ethnicity, body mass index (BMI), or prior-year diagnostic data were missing.
Measures
Patients were followed through April 2020. Presence of MGUS was defined by the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code 273.1. MM was identified by ICD-9 diagnosis codes 203.00, 203.01, and 203.02. The study index date was the earliest date of diagnosis of MGUS or MM in fiscal years 2010-2015. It was suspected that some patients with MM may have had a history of MGUS prior to this period. Therefore, for patients with MM, historical diagnosis of MGUS was extracted going back through the earliest data in the CDW (October 1999). Patients diagnosed with both MGUS and MM were considered transformation patients.
Other measures included age at index date, sex, race, ethnicity, VA priority status (a value 1 to 8 summarizing why the veteran qualified for VA care, such as military service-connected disability or very low income), and AO exposure authenticated per VA enrollment files and disability records. Service years were separated into 1961 to 1968 and 1969 to 1971 to match a change in the formulation of AO associated with decreased carcinogenic effect. Comorbidity data from the year prior to first MGUS/MM diagnosis in the observation period were extracted. Lifestyle factors associated with development of MGUS/MM were determined using the following codes: obesity per BMI calculation or diagnosis (ICD-9, 278.0), tobacco use per diagnosis (ICD-9, 305.1, V15.82), and survival from MGUS/MM diagnosis index date to date of death from any cause. Comorbidity was assessed using ICD-9 diagnosis codes to calculate the Charlson Comorbidity Index (CCI), which includes cardiovascular diseases, diabetes mellitus, liver and kidney diseases, cancers, and metastatic solid tumors. Cancers were omitted from our adapted CCI to avoid collinearity in the multivariable models. The theoretical maximum CCI score in this study was 25.12,13 Additional conditions known to be associated with variation in outcomes among veterans using the VA were indicated, including major depressive disorder, posttraumatic stress disorder (PTSD), alcohol use disorder (AUD), substance use disorder (SUD), and common chronic disease (hypertension, lipid disorders).14
Treatment with autologous HSCT was defined by Current Procedural Terminology and ICD-9 Clinical Modification procedure codes for bone marrow and autologous HSCT occurring at any time in the CDW (eAppendix). Days elapsed from MM diagnosis to HSCT were calculated.
Statistical Analysis
Sample characteristics were represented by frequencies and percentages for categorical variables and means and SDs (or medians and ranges where appropriate) for continuous variables. A χ2 test (or Fisher exact test when cell counts were low) assessed associations in bivariate comparisons. A 2-sample t test (or Wilcoxon rank sum test as appropriate) assessed differences in continuous variables between 2 groups. Kaplan-Meier curves depicted the unadjusted relationship of AO exposure to survival. Cox proportional hazards survival models examined an unadjusted model containing only the AO exposure indicator as a predictor and adjusted models were used for demographic and clinical factors for MGUS and patients with MM separately.
Predictors were age in decades, sex, Hispanic ethnicity, race, nicotine dependence, obesity, overweight, AUD, SUD, major depressive disorder, PTSD, and the adapted CCI. When modeling patients with MM, MGUS was added to the model to identify the transformation group. The interaction of AO with transformation was also analyzed for patients with MM. Results were reported as hazard ratios (HR) with their 95% CI.
Results
We identified 18,215 veterans diagnosed with either MGUS or MM during fiscal years 2010-2015 with 16,366 meeting inclusion criteria. Patients were excluded for missing data on exposure (n = 334), age (n = 12), race (n = 1058), ethnicity (n = 164), diagnosis (n = 47), treatment (n = 56), and BMI (n = 178). All were Vietnam War era veterans; 14 also served in other eras.
The cohort was 98.5% male (Table 1). Twenty-nine percent were Black veterans, 65% were White veterans, and 4% of individuals reported Hispanic ethnicity. Patients had a mean (SD) age of 66.7 (5.9) years (range, 52-96). Most patients were married (58%) or divorced/separated (27%). All were VA priority 1 to 5 (no 6, 7, or 8); 50% were priority 1 with 50% to 100% service-connected disability. Another 29% were eligible for VA care by reason of low income, 17% had 10% to 40% service-connected disability, and 4% were otherwise disabled.
During fiscal years 2010 to 2015, 68% of our cohort had a diagnosis of MGUS (n = 11,112; 9105 had MGUS only), 44% had MM (n = 7261; 5254 had MM only), and 12% of these were transformation patients (n = 2007). AO exposure characterized 3102 MGUS-only patients (34%), 1886 MM-only patients (36%), and 695 transformation patients (35%) (χ2 = 4.92, P = .09). Among 5683 AO-exposed patients, 695 (12.2%) underwent MGUS-to-MM transformation. Among 10,683 nonexposed veterans, 1312 (12.3%) experienced transformation.
Comorbidity in the year leading up to the index MGUS/MM date determined using CCI was a mean (SD) of 1.9 (2.1) (range, 0-14). Among disorders not included in the CCI, 71% were diagnosed with hypertension, 57% with lipid disorders, 22% with nicotine dependence, 14% with major depressive disorder, 13% with PTSD, and 9% with AUD. Overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) were common (35% and 41%, respectively). For 98% of patients, weight was measured within 90 days of their index MGUS/MM date. Most of the cohort (70%) were in Vietnam in 1961 to 1968.
HSCT was provided to 632 patients with MM (8.7%), including 441 patients who were treated after their index date and 219 patients treated before their index date. From fiscal years 2010 to 2015, the median (IQR) number of days from MM index date to HSCT receipt was 349 (243-650) days. Historical HSCT occurred a median (IQR) of 857 (353-1592) days before the index date, per data available back to October 1999; this median suggests long histories of MM in this cohort.
The unadjusted survival model found a very small inverse association of mortality with AO exposure in the total sample, meaning patients with documented AO exposure lived longer (HR, 0.85; 95% CI, 0.81-0.89; Table 2; Figure). Among 11,112 MGUS patients, AO was similarly associated with mortality (HR, 0.79; 95% CI, 0.74-0.84). The effect was also seen among 7269 patients with MM (HR, 0.86; 95% CI, 0.81-0.91).
In the adjusted model of the total sample, the mortality hazard was greater for veterans who were older, with AUD and nicotine dependence, greater comorbidity per the CCI, diagnosis of MM, and transformation from MGUS to MM. Protective effects were noted for AO exposure, female sex, Black race, obesity, overweight, PTSD, and HSCT.
After adjusting for covariates, AO exposure was still associated with lower mortality among 11,112 patients with MGUS (HR, 0.85; 95% CI, 0.80-0.91). Risk factors were older age, nicotine dependence, AUD, the adapted CCI score (HR, 1.23 per point increase in the index; 95% CI, 1.22-1.25), and transformation to MM (HR, 1.76; 95% CI, 1.65-1.88). Additional protective factors were female sex, Black race, obesity, overweight, and PTSD.
After adjusting for covariates and limiting the analytic cohort to MM patients, the effect of AO exposure persisted (HR, 0.89; 95% CI, 0.84-0.95). Mortality risk factors were older age, nicotine dependence, AUD, and higher CCI score. Also protective were female sex, Black race, obesity, overweight, diagnosis of MGUS (transformation), and HSCT.
In the final model on patients with MM, the interaction term of AO exposure with transformation was significant. The combination of AO exposure with MGUS transformation had a greater protective effect than either AO exposure alone or MGUS without prior AO exposure. Additional protective factors were female sex, Black race, obesity, overweight, and HSCT. Older age, AUD, nicotine dependence, and greater comorbidity increased mortality risk.
Disscussion
Elucidating the pathophysiology and risk of transformation from MGUS to MM is an ongoing endeavor, even 35 years after the end of US involvement in the Vietnam War. Our study sought to understand a relationship between AO exposure, risk of MGUS transforming to MM, and associated mortality in US Vietnam War veterans. The rate of transformation (MGUS progressing to active MM) is well cited at 1% per year.15 Here, we found 12% of our cohort had undergone this transformation over 10 years.
Vietnam War era veterans who were exposed to AO during the Operation Ranch Hand period had 2.4 times greater risk of developing MGUS compared with veterans not exposed to AO.8 Our study was not designed to look at this association of AO exposure and MGUS/MM as this was a retrospective review to assess the difference in outcomes based on AO exposure. We found that AO exposure is associated with a decrease in mortality in contrast to a prior study showing worse survival with individuals with AO exposure.10 Another single center study found no association between AO exposure and overall survival, but it did identify an increased risk of progression from MGUS to MM.11 Our study did not show increased risk of transformation but did show positive effect on survival.
Black individuals have twice the risk of developing MM compared with White individuals and are diagnosed at a younger age (66 vs 70 years, respectively).16 Interestingly, Black race was a protective factor in our study. Given the length of time (35 years) elapsed since the Vietnam War ended, it is likely that most vulnerable Black veterans did not survive until our observation period.
HSCT, as expected, was a protective factor for veterans undergoing this treatment modality, but it is unclear why such a small number (8%) underwent HSCT as this is a standard of care in the management of MM. Obesity was also found to be a protective factor in a prior study, which was also seen in our study cohort.8
Limitations
This study was limited by its retrospective review of survivors among the Vietnam-era cohort several decades after the exposure of concern. Clinician notes and full historical data, such as date of onset for any disorder, were unavailable. These data also relied on the practitioners caring for the veterans to make the correct diagnosis with the associated code so that the data could be captured. Neither AO exposure nor diagnoses codes were verified against other sources of data; however, validation studies over the years have supported the accuracy of the diagnosis codes recorded in the VA EHR.
Conclusions
Because AO exposure is a nonmodifiable risk factor, focus should be placed on modifiable risk factors (eg, nicotine dependence, alcohol and substance use disorders, underlying comorbid conditions) as these were associated with worse outcomes. Future studies will look at the correlation of AO exposure, cytogenetics, and clinical outcomes in these veterans to learn how best to identify their disease course and optimize their care in the latter part of their life.
Acknowledgments
This research was supported by the Central Texas Veterans Health Care System and Baylor Scott and White Health, both in Temple and Veterans Affairs Central Western Massachusetts Healthcare System, Leeds.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi:10.3322/caac.21442
2. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. doi:10.1016/S1470-2045(14)70442-5
3. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21-33. doi:10.4065/78.1.21
4. Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564- 569. doi:10.1056/NEJMoa01133202
5. International Myeloma Foundation. What Are MGUS, smoldering and active myeloma? Updated June 6, 2021. Accessed June 20, 2022. https://www.myeloma .org/what-are-mgus-smm-mm
6. Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6(2):225-247. doi:10.1016/S0889-8588(18)30341-1
7. Buckingham Jr WA. Operation Ranch Hand: The Air Force and herbicides in southeast Asia, 1961-1971. Washington, DC: Office of Air Force History, United States Air Force; 1982. Accessed June 20, 2022. https://apps.dtic.mil/sti /pdfs/ADA121709.pdf
8. Landgren O, Shim YK, Michalek J, et al. Agent Orange exposure and monoclonal gammopathy of undetermined significance: an Operation Ranch Hand veteran cohort study. JAMA Oncol. 2015;1(8):1061-1068. doi:10.1001/jamaoncol.2015.2938
9. Mescher C, Gilbertson D, Randall NM, et al. The impact of Agent Orange exposure on prognosis and management in patients with chronic lymphocytic leukemia: a National Veteran Affairs Tumor Registry Study. Leuk Lymphoma. 2018;59(6):1348-1355. doi:10.1080/10428194.2017.1375109
10. Callander NS, Freytes CO, Luo S, Carson KR. Previous Agent Orange exposure is correlated with worse outcome in patients with multiple myeloma (MM) [abstract]. Blood. 2015;126(23):4194. doi:10.1182/blood.V126.23.4194.4194
11. Bumma N, Nagasaka M, Kim S, Vankayala HM, Ahmed S, Jasti P. Incidence of monoclonal gammopathy of undetermined significance (MGUS) and subsequent transformation to multiple myeloma (MM) and effect of exposure to Agent Orange (AO): a single center experience from VA Detroit [abstract]. Blood. 2017;130(suppl 1):5383. doi:10.1182/blood.V130.Suppl_1.5383.5383
12. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi:10.1016/0895-4356(92)90133-8
14. Copeland LA, Zeber JE, Sako EY, et al. Serious mental illnesses associated with receipt of surgery in retrospective analysis of patients in the Veterans Health Administration. BMC Surg. 2015;15:74. doi:10.1186/s12893-015-0064-7
15. Younes MA, Perez JD, Alirhayim Z, Ochoa C, Patel R, Dabak VS. MGUS Transformation into multiple myeloma in patients with solid organ transplantation [Abstract presented at American Society of Hematology Annual Meeting, November 15, 2013]. Blood. 2013;122(21):5325. doi:10.1182/blood.V122.21.5325.5325
16. Waxman AJ, Mink PJ, Devesa SS, et al. Racial disparities in incidence and outcome in multiple myeloma: a population- based study. Blood. 2010 Dec 16;116(25):5501-5506. doi:10.1182/blood-2010-07-298760
FDA approves trastuzumab-deruxtecan for HER2-low breast cancer
This is the first therapy approved for HER2-low breast cancer, a newly defined subset of HER2-negative breast cancer in which there are some HER2 proteins on the cell surface, but not enough to warrant classification as HER2-positive cancer, the FDA said in a press release.
The indication is for patients who have received prior chemotherapy in the metastatic setting or for patients whose cancer has returned during adjuvant chemotherapy or within 6 months of completing it.
Approval was based on the DESTINY-Breast04 trial, which included 557 patients with unresectable or metastatic HER2-low breast cancer. The trial had two cohorts: 494 hormone receptor–positive (HR+) patients, and 63 hormone receptor–negative (HR–) patients.
Of these patients, 373 were randomly assigned to received trastuzumab deruxtecan every 3 weeks, and 184 were randomly assigned to receive physician’s choice of chemotherapy (eribulin, capecitabine, gemcitabine, nab paclitaxel, or paclitaxel).
Among patients who received trastuzumab deruxtecan, progression-free survival was longer (10.1 months vs. 5.4 months), as was overall survival (23.9 months vs. 17.5 months), compared with those in the chemotherapy group.
“Overall, these results establish HER2-low metastatic breast cancer as a targetable population of breast cancer with trastuzumab deruxtecan as a new standard of care in this setting,” Shanu Modi, MD, said in June at a press conference held during the annual meeting of the American Society of Clinical Oncology, where she presented the results.
The most common adverse reactions in the trial were nausea, fatigue, alopecia, vomiting, constipation, decreased appetite, musculoskeletal pain, and diarrhea. The agent carries a boxed warning regarding the risk of interstitial lung disease and embryo-fetal toxicity.
The targeted agent is not recommended for women who are pregnant.
A version of this article first appeared on Medscape.com.
This is the first therapy approved for HER2-low breast cancer, a newly defined subset of HER2-negative breast cancer in which there are some HER2 proteins on the cell surface, but not enough to warrant classification as HER2-positive cancer, the FDA said in a press release.
The indication is for patients who have received prior chemotherapy in the metastatic setting or for patients whose cancer has returned during adjuvant chemotherapy or within 6 months of completing it.
Approval was based on the DESTINY-Breast04 trial, which included 557 patients with unresectable or metastatic HER2-low breast cancer. The trial had two cohorts: 494 hormone receptor–positive (HR+) patients, and 63 hormone receptor–negative (HR–) patients.
Of these patients, 373 were randomly assigned to received trastuzumab deruxtecan every 3 weeks, and 184 were randomly assigned to receive physician’s choice of chemotherapy (eribulin, capecitabine, gemcitabine, nab paclitaxel, or paclitaxel).
Among patients who received trastuzumab deruxtecan, progression-free survival was longer (10.1 months vs. 5.4 months), as was overall survival (23.9 months vs. 17.5 months), compared with those in the chemotherapy group.
“Overall, these results establish HER2-low metastatic breast cancer as a targetable population of breast cancer with trastuzumab deruxtecan as a new standard of care in this setting,” Shanu Modi, MD, said in June at a press conference held during the annual meeting of the American Society of Clinical Oncology, where she presented the results.
The most common adverse reactions in the trial were nausea, fatigue, alopecia, vomiting, constipation, decreased appetite, musculoskeletal pain, and diarrhea. The agent carries a boxed warning regarding the risk of interstitial lung disease and embryo-fetal toxicity.
The targeted agent is not recommended for women who are pregnant.
A version of this article first appeared on Medscape.com.
This is the first therapy approved for HER2-low breast cancer, a newly defined subset of HER2-negative breast cancer in which there are some HER2 proteins on the cell surface, but not enough to warrant classification as HER2-positive cancer, the FDA said in a press release.
The indication is for patients who have received prior chemotherapy in the metastatic setting or for patients whose cancer has returned during adjuvant chemotherapy or within 6 months of completing it.
Approval was based on the DESTINY-Breast04 trial, which included 557 patients with unresectable or metastatic HER2-low breast cancer. The trial had two cohorts: 494 hormone receptor–positive (HR+) patients, and 63 hormone receptor–negative (HR–) patients.
Of these patients, 373 were randomly assigned to received trastuzumab deruxtecan every 3 weeks, and 184 were randomly assigned to receive physician’s choice of chemotherapy (eribulin, capecitabine, gemcitabine, nab paclitaxel, or paclitaxel).
Among patients who received trastuzumab deruxtecan, progression-free survival was longer (10.1 months vs. 5.4 months), as was overall survival (23.9 months vs. 17.5 months), compared with those in the chemotherapy group.
“Overall, these results establish HER2-low metastatic breast cancer as a targetable population of breast cancer with trastuzumab deruxtecan as a new standard of care in this setting,” Shanu Modi, MD, said in June at a press conference held during the annual meeting of the American Society of Clinical Oncology, where she presented the results.
The most common adverse reactions in the trial were nausea, fatigue, alopecia, vomiting, constipation, decreased appetite, musculoskeletal pain, and diarrhea. The agent carries a boxed warning regarding the risk of interstitial lung disease and embryo-fetal toxicity.
The targeted agent is not recommended for women who are pregnant.
A version of this article first appeared on Medscape.com.
How retraining your brain could help with lower back pain
Are you among the hundreds of millions of people worldwide with low back pain? If so, you may be familiar with standard treatments like surgery, shots, medications, and spinal manipulations. But new research suggests the solution for the world’s leading cause of disability may lie in fixing how the brain and the body communicate.
Setting out to challenge traditional treatments for chronic back pain, scientists across Australia, Europe, and the United States came together to test the effectiveness of altering how neural networks recognize pain for new research published this week in JAMA.
The randomized clinical trial recruited two groups of 138 participants with chronic low back pain, testing one group with a novel method called graded sensorimotor retraining intervention (RESOLVE) and the other with things like mock laser therapy and noninvasive brain stimulation.
The researchers found the RESOLVE 12-week training course resulted in a statistically significant improvement in pain intensity at 18 weeks.
“What we observed in our trial was a clinically meaningful effect on pain intensity and a clinically meaningful effect on disability. People were happier, they reported their backs felt better, and their quality of life was better,” the study’s lead author, James McAuley, PhD, said in a statement. “This is the first new treatment of its kind for back pain.”
Brainy talk
Communication between your brain and back changes over time when you have chronic lower back pain, leading the brain to interpret signals from the back differently and change how you move. It is thought that these neural changes make recovery from pain slower and more complicated , according to Neuroscience Research Australia (NeuRA), a nonprofit research institute in Sydney.
“Over time, the back becomes less fit, and the way the back and brain communicate is disrupted in ways that seem to reinforce the notion that the back is vulnerable and needs protecting,” said Dr. McAuley, a professor at the University of New South Wales, Sydney, and a NeuRA senior research scientist. “The treatment we devised aims to break this self-sustaining cycle.”
RESOLVE treatment focuses on improving this transformed brain-back communication by slowly retraining the body and the brain without the use of opioids or surgery. People in the study have reported improved quality of life 1 year later, according to Dr. McAuley.
The researchers said the pain improvement was “modest,” and the method will need to be tested on other patients and conditions. They hope to introduce this new treatment to doctors and physiotherapists within the next 6-9 months and have already enlisted partner organizations to start this process, according to NeuRA.
A version of this article first appeared on Webmd.com.
Are you among the hundreds of millions of people worldwide with low back pain? If so, you may be familiar with standard treatments like surgery, shots, medications, and spinal manipulations. But new research suggests the solution for the world’s leading cause of disability may lie in fixing how the brain and the body communicate.
Setting out to challenge traditional treatments for chronic back pain, scientists across Australia, Europe, and the United States came together to test the effectiveness of altering how neural networks recognize pain for new research published this week in JAMA.
The randomized clinical trial recruited two groups of 138 participants with chronic low back pain, testing one group with a novel method called graded sensorimotor retraining intervention (RESOLVE) and the other with things like mock laser therapy and noninvasive brain stimulation.
The researchers found the RESOLVE 12-week training course resulted in a statistically significant improvement in pain intensity at 18 weeks.
“What we observed in our trial was a clinically meaningful effect on pain intensity and a clinically meaningful effect on disability. People were happier, they reported their backs felt better, and their quality of life was better,” the study’s lead author, James McAuley, PhD, said in a statement. “This is the first new treatment of its kind for back pain.”
Brainy talk
Communication between your brain and back changes over time when you have chronic lower back pain, leading the brain to interpret signals from the back differently and change how you move. It is thought that these neural changes make recovery from pain slower and more complicated , according to Neuroscience Research Australia (NeuRA), a nonprofit research institute in Sydney.
“Over time, the back becomes less fit, and the way the back and brain communicate is disrupted in ways that seem to reinforce the notion that the back is vulnerable and needs protecting,” said Dr. McAuley, a professor at the University of New South Wales, Sydney, and a NeuRA senior research scientist. “The treatment we devised aims to break this self-sustaining cycle.”
RESOLVE treatment focuses on improving this transformed brain-back communication by slowly retraining the body and the brain without the use of opioids or surgery. People in the study have reported improved quality of life 1 year later, according to Dr. McAuley.
The researchers said the pain improvement was “modest,” and the method will need to be tested on other patients and conditions. They hope to introduce this new treatment to doctors and physiotherapists within the next 6-9 months and have already enlisted partner organizations to start this process, according to NeuRA.
A version of this article first appeared on Webmd.com.
Are you among the hundreds of millions of people worldwide with low back pain? If so, you may be familiar with standard treatments like surgery, shots, medications, and spinal manipulations. But new research suggests the solution for the world’s leading cause of disability may lie in fixing how the brain and the body communicate.
Setting out to challenge traditional treatments for chronic back pain, scientists across Australia, Europe, and the United States came together to test the effectiveness of altering how neural networks recognize pain for new research published this week in JAMA.
The randomized clinical trial recruited two groups of 138 participants with chronic low back pain, testing one group with a novel method called graded sensorimotor retraining intervention (RESOLVE) and the other with things like mock laser therapy and noninvasive brain stimulation.
The researchers found the RESOLVE 12-week training course resulted in a statistically significant improvement in pain intensity at 18 weeks.
“What we observed in our trial was a clinically meaningful effect on pain intensity and a clinically meaningful effect on disability. People were happier, they reported their backs felt better, and their quality of life was better,” the study’s lead author, James McAuley, PhD, said in a statement. “This is the first new treatment of its kind for back pain.”
Brainy talk
Communication between your brain and back changes over time when you have chronic lower back pain, leading the brain to interpret signals from the back differently and change how you move. It is thought that these neural changes make recovery from pain slower and more complicated , according to Neuroscience Research Australia (NeuRA), a nonprofit research institute in Sydney.
“Over time, the back becomes less fit, and the way the back and brain communicate is disrupted in ways that seem to reinforce the notion that the back is vulnerable and needs protecting,” said Dr. McAuley, a professor at the University of New South Wales, Sydney, and a NeuRA senior research scientist. “The treatment we devised aims to break this self-sustaining cycle.”
RESOLVE treatment focuses on improving this transformed brain-back communication by slowly retraining the body and the brain without the use of opioids or surgery. People in the study have reported improved quality of life 1 year later, according to Dr. McAuley.
The researchers said the pain improvement was “modest,” and the method will need to be tested on other patients and conditions. They hope to introduce this new treatment to doctors and physiotherapists within the next 6-9 months and have already enlisted partner organizations to start this process, according to NeuRA.
A version of this article first appeared on Webmd.com.
New blood test could reshape early CRC screening
A simple blood test that looks for a combination of specific RNA snippets may become a novel way to screen for early-onset colorectal cancer, suggests a new study published online in Gastroenterology.
Researchers identified four microRNAs that together comprise a signature biomarker that can be used to detect and diagnose the presence of colorectal cancer from a liquid biopsy in a younger population.
MicroRNAs, or miRNAs, are small RNA molecules that do not encode proteins but are used instead to regulate gene expression. The study authors developed and validated a panel that detects four miRNAs occurring at higher levels in plasma samples from patients with early-onset colorectal cancer, with high sensitivity and specificity.
“The point would be to use this test as a routine part of annual healthcare, or for people in high-risk families every 6 months,” study senior author Ajay Goel, PhD, MS, chair of the department of molecular diagnostics and experimental therapeutics at the City of Hope Comprehensive Cancer Center, Duarte, Calif., said in an interview.
“It’s affordable, it can be done easily from a small tube of blood, and as long as that test stays negative, you’re good,” Dr. Goel said, because even if patients miss a test, the next one, whether it’s 6 months or a year later, will catch any potential cancer.
“Colon cancer is not going to kill somebody overnight, so this should be used as a precursor to colonoscopy. As long as that test is negative, you can postpone a colonoscopy,” he said.
Andrew T. Chan, MD, MPH, a professor of medicine at Harvard Medical School and vice chair of gastroenterology at Massachusetts General Hospital, both in Boston, who was not involved in the research, said in an interview that the findings are exciting.
“It would be really value-added to have a blood-based screening test,” Dr. Chan said, adding that researchers have pursued multiple different avenues in pursuit of one. “It’s very nice to see that area progress and to actually have some evidence that microRNAs could be a potential biomarker for colorectal cancer.”
Screening now insufficient for early-onset disease
The U.S. Preventive Services Task Force recently lowered the recommended age to 45 years to begin screening for colorectal cancer. Part of the rationale for the change came from the rising rates of early-onset colorectal cancer, a distinct clinical and molecular entity that tends to have poorer survival than late-onset disease, the authors noted.
Early-onset disease, occurring primarily in people under 50 without a family or genetic history of colorectal cancer, now makes up about 10%-15% of all new cases and continues to rise, they write.
“Early-onset colorectal cancer patients are more likely to exhibit an advanced stage tumor at initial presentation, distal tumor localization, signet ring histology, and a disease presentation with concurrent metastasis,” the authors wrote. “This raises the logistical clinical concern that, since the tumors in early-onset colorectal cancer patients are often more aggressive than those with late-onset colorectal cancer, a delayed diagnosis could have a significant adverse impact and can lead to early death.”
Yet current screening strategies are insufficient for detecting enough early-onset cases, the authors assert.
Colonoscopies are invasive, carry a risk for complications, and are cost- and time-prohibitive for people at average risk. Meanwhile, existing fecal and blood tests “lack adequate diagnostic performance for the early detection of colorectal cancer, especially early-onset colorectal cancer, as these assays have yet to be explored or developed in this population,” they wrote.
The ideal “diagnostic modality should preferably be acceptable to healthy individuals, inexpensive, rapid, and preferably noninvasive,” they note.
Finding and validating miRNA
The researchers therefore turned to the concept of a liquid biopsy, focusing on identifying miRNAs associated with colorectal cancer, because their expression tends to be stable in tissues, blood, stool, and other body fluids.
They first analyzed an miRNA expression profiling dataset from 1,061 individuals to look for miRNAs whose expression was higher in colorectal cancer patients. The dataset included 42 patients with stage 1-2 early-onset colorectal cancer, 370 patients with stage 1-2 late-onset colorectal cancer, 62 patients younger than 50 years without cancer, and 587 patients aged 50 years or older without cancer.
The researchers found 28 miRNAs that were significantly unregulated in early-onset colorectal cancer tissue samples, compared with cancer-free samples and 11 miRNAs unregulated specifically in only the early-onset colorectal cancer samples. Four of these 11 miRNAs were adequately distinct from one another and were detectable in the plasma samples that the researchers would use to train and validate them as a combination biomarker.
The researchers used 117 plasma samples from Japan, including 72 from people with early-onset colorectal cancer and 45 from healthy donors, to develop and train an assay detecting the four miRNAs. They then validated the assay using 142 plasma samples from Spain, including 77 with early-onset colorectal cancer and 65 healthy donors.
In the Japan cohort, the four-miRNA assay had a sensitivity of 90% and a specificity of 80%, with a positive predictive value (PPV) of 88% and a negative predictive value (NPV) of 84%. In the Spain cohort used for validation, the assay performed with a sensitivity of 82%, a specificity of 86%, a PPV of 88%, and an NPV of 80%.
“Taken together, the genome-wide transcriptomic profiling approach was indeed robust, as it identified the biomarkers that were successfully trained and validated in plasma specimens from independent cohorts of patients with early-onset colorectal cancer, hence highlighting their translational potential in the clinic for the detection of this malignancy in early stages,” the authors wrote.
By disease stage, the four-miRNA panel identified both early-stage (stage 1-2; sensitivity, 92%; specificity, 80%) and late-stage (stage 3-4; sensitivity, 79%; specificity, 86%) early-onset colorectal cancer in the validation cohort.
Clinical benefit of blood test
The researchers also assessed the benefit-harm trade-off of this liquid biopsy assay compared with other screening modalities, taking into consideration the risk for false positives and false negatives.
A decision curve analysis “revealed that the miRNA panel achieved a higher net benefit regardless of threshold probability in comparison to intervention for all patients or none of the patients,” the researchers reported. “These findings suggest that this miRNA panel might offer more clinical benefit with regards to the avoidance of physical harm and misdiagnosis.”
They also found that expression levels of these four miRNAs significantly decreased after surgical removal of the colorectal cancer, strongly suggesting that the miRNAs do originate with the tumor.
“To have a relatively inexpensive and noninvasive means of screening a younger population is a very important unmet need,” said Dr. Chan.
It’s not feasible to recommend colonoscopies in people younger than 45 years because of resource constraints, he said, so “this is a wonderful new development to actually have the possibility of a blood-based screening test for younger individuals, especially given that rising incidence of young-onset colorectal cancer.”
Dr. Goel pointed out that only half of those recommended to get screened for colorectal cancer actually undergo screening, and a large reason for that is the desire to avoid colonoscopy, a concern echoed in the findings of a recent study by Christopher V. Almario, MD, MSHPM, and colleagues.
Dr. Goel expects that this strategy would increase compliance with screening because it’s less invasive and more affordable, particularly for younger patients. He estimates that a commercial assay using this panel, if approved by the Food and Drug Administration, should cost less than $100.
Dr. Almario, an assistant professor of medicine at the Cedars-Sinai Karsh Division of Gastroenterology and Hepatology in Los Angeles, agreed that an FDA-approved blood-based screening test would be a “game-changer,” as long as it’s accurate and effective.
Though Dr. Almario did not review the data in Goel’s study, he said in an interview that a blood test for colorectal cancer screening would be “the holy grail, so to speak, in terms of really moving the needle on screening uptake.”
Next steps
Dr. Chan noted that one caveat to consider with this study is that it was done in a relatively small population of individuals, even though the test was validated in a second set of plasma samples.
“Additional validation needs to be done in larger numbers of patients to really understand the performance characteristics because it is possible that some of these signatures may, when they’re using a broader group of individuals, not perform as well,” Dr. Chan said.
Dr. Goel said he is working with several companies right now to develop and further test a commercial product. He anticipates it may be shelf-ready in 2-5 years.
“The take-home message is that clinicians need to be more cognizant of the fact that incidence of this disease is rising, and we need to do something about it,” Dr. Goel said, particularly for those younger than 45 years who currently don’t have a screening option.
“Now we have at least a sliver of hope for those who might be suffering from this disease, for those for whom we have zero screening or diagnostic tests,” he said.
The research was funded by the National Cancer Institute and Fundación MAPFRE Guanarteme. Dr. Goel, Dr. Chan, and Dr. Almario reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
A simple blood test that looks for a combination of specific RNA snippets may become a novel way to screen for early-onset colorectal cancer, suggests a new study published online in Gastroenterology.
Researchers identified four microRNAs that together comprise a signature biomarker that can be used to detect and diagnose the presence of colorectal cancer from a liquid biopsy in a younger population.
MicroRNAs, or miRNAs, are small RNA molecules that do not encode proteins but are used instead to regulate gene expression. The study authors developed and validated a panel that detects four miRNAs occurring at higher levels in plasma samples from patients with early-onset colorectal cancer, with high sensitivity and specificity.
“The point would be to use this test as a routine part of annual healthcare, or for people in high-risk families every 6 months,” study senior author Ajay Goel, PhD, MS, chair of the department of molecular diagnostics and experimental therapeutics at the City of Hope Comprehensive Cancer Center, Duarte, Calif., said in an interview.
“It’s affordable, it can be done easily from a small tube of blood, and as long as that test stays negative, you’re good,” Dr. Goel said, because even if patients miss a test, the next one, whether it’s 6 months or a year later, will catch any potential cancer.
“Colon cancer is not going to kill somebody overnight, so this should be used as a precursor to colonoscopy. As long as that test is negative, you can postpone a colonoscopy,” he said.
Andrew T. Chan, MD, MPH, a professor of medicine at Harvard Medical School and vice chair of gastroenterology at Massachusetts General Hospital, both in Boston, who was not involved in the research, said in an interview that the findings are exciting.
“It would be really value-added to have a blood-based screening test,” Dr. Chan said, adding that researchers have pursued multiple different avenues in pursuit of one. “It’s very nice to see that area progress and to actually have some evidence that microRNAs could be a potential biomarker for colorectal cancer.”
Screening now insufficient for early-onset disease
The U.S. Preventive Services Task Force recently lowered the recommended age to 45 years to begin screening for colorectal cancer. Part of the rationale for the change came from the rising rates of early-onset colorectal cancer, a distinct clinical and molecular entity that tends to have poorer survival than late-onset disease, the authors noted.
Early-onset disease, occurring primarily in people under 50 without a family or genetic history of colorectal cancer, now makes up about 10%-15% of all new cases and continues to rise, they write.
“Early-onset colorectal cancer patients are more likely to exhibit an advanced stage tumor at initial presentation, distal tumor localization, signet ring histology, and a disease presentation with concurrent metastasis,” the authors wrote. “This raises the logistical clinical concern that, since the tumors in early-onset colorectal cancer patients are often more aggressive than those with late-onset colorectal cancer, a delayed diagnosis could have a significant adverse impact and can lead to early death.”
Yet current screening strategies are insufficient for detecting enough early-onset cases, the authors assert.
Colonoscopies are invasive, carry a risk for complications, and are cost- and time-prohibitive for people at average risk. Meanwhile, existing fecal and blood tests “lack adequate diagnostic performance for the early detection of colorectal cancer, especially early-onset colorectal cancer, as these assays have yet to be explored or developed in this population,” they wrote.
The ideal “diagnostic modality should preferably be acceptable to healthy individuals, inexpensive, rapid, and preferably noninvasive,” they note.
Finding and validating miRNA
The researchers therefore turned to the concept of a liquid biopsy, focusing on identifying miRNAs associated with colorectal cancer, because their expression tends to be stable in tissues, blood, stool, and other body fluids.
They first analyzed an miRNA expression profiling dataset from 1,061 individuals to look for miRNAs whose expression was higher in colorectal cancer patients. The dataset included 42 patients with stage 1-2 early-onset colorectal cancer, 370 patients with stage 1-2 late-onset colorectal cancer, 62 patients younger than 50 years without cancer, and 587 patients aged 50 years or older without cancer.
The researchers found 28 miRNAs that were significantly unregulated in early-onset colorectal cancer tissue samples, compared with cancer-free samples and 11 miRNAs unregulated specifically in only the early-onset colorectal cancer samples. Four of these 11 miRNAs were adequately distinct from one another and were detectable in the plasma samples that the researchers would use to train and validate them as a combination biomarker.
The researchers used 117 plasma samples from Japan, including 72 from people with early-onset colorectal cancer and 45 from healthy donors, to develop and train an assay detecting the four miRNAs. They then validated the assay using 142 plasma samples from Spain, including 77 with early-onset colorectal cancer and 65 healthy donors.
In the Japan cohort, the four-miRNA assay had a sensitivity of 90% and a specificity of 80%, with a positive predictive value (PPV) of 88% and a negative predictive value (NPV) of 84%. In the Spain cohort used for validation, the assay performed with a sensitivity of 82%, a specificity of 86%, a PPV of 88%, and an NPV of 80%.
“Taken together, the genome-wide transcriptomic profiling approach was indeed robust, as it identified the biomarkers that were successfully trained and validated in plasma specimens from independent cohorts of patients with early-onset colorectal cancer, hence highlighting their translational potential in the clinic for the detection of this malignancy in early stages,” the authors wrote.
By disease stage, the four-miRNA panel identified both early-stage (stage 1-2; sensitivity, 92%; specificity, 80%) and late-stage (stage 3-4; sensitivity, 79%; specificity, 86%) early-onset colorectal cancer in the validation cohort.
Clinical benefit of blood test
The researchers also assessed the benefit-harm trade-off of this liquid biopsy assay compared with other screening modalities, taking into consideration the risk for false positives and false negatives.
A decision curve analysis “revealed that the miRNA panel achieved a higher net benefit regardless of threshold probability in comparison to intervention for all patients or none of the patients,” the researchers reported. “These findings suggest that this miRNA panel might offer more clinical benefit with regards to the avoidance of physical harm and misdiagnosis.”
They also found that expression levels of these four miRNAs significantly decreased after surgical removal of the colorectal cancer, strongly suggesting that the miRNAs do originate with the tumor.
“To have a relatively inexpensive and noninvasive means of screening a younger population is a very important unmet need,” said Dr. Chan.
It’s not feasible to recommend colonoscopies in people younger than 45 years because of resource constraints, he said, so “this is a wonderful new development to actually have the possibility of a blood-based screening test for younger individuals, especially given that rising incidence of young-onset colorectal cancer.”
Dr. Goel pointed out that only half of those recommended to get screened for colorectal cancer actually undergo screening, and a large reason for that is the desire to avoid colonoscopy, a concern echoed in the findings of a recent study by Christopher V. Almario, MD, MSHPM, and colleagues.
Dr. Goel expects that this strategy would increase compliance with screening because it’s less invasive and more affordable, particularly for younger patients. He estimates that a commercial assay using this panel, if approved by the Food and Drug Administration, should cost less than $100.
Dr. Almario, an assistant professor of medicine at the Cedars-Sinai Karsh Division of Gastroenterology and Hepatology in Los Angeles, agreed that an FDA-approved blood-based screening test would be a “game-changer,” as long as it’s accurate and effective.
Though Dr. Almario did not review the data in Goel’s study, he said in an interview that a blood test for colorectal cancer screening would be “the holy grail, so to speak, in terms of really moving the needle on screening uptake.”
Next steps
Dr. Chan noted that one caveat to consider with this study is that it was done in a relatively small population of individuals, even though the test was validated in a second set of plasma samples.
“Additional validation needs to be done in larger numbers of patients to really understand the performance characteristics because it is possible that some of these signatures may, when they’re using a broader group of individuals, not perform as well,” Dr. Chan said.
Dr. Goel said he is working with several companies right now to develop and further test a commercial product. He anticipates it may be shelf-ready in 2-5 years.
“The take-home message is that clinicians need to be more cognizant of the fact that incidence of this disease is rising, and we need to do something about it,” Dr. Goel said, particularly for those younger than 45 years who currently don’t have a screening option.
“Now we have at least a sliver of hope for those who might be suffering from this disease, for those for whom we have zero screening or diagnostic tests,” he said.
The research was funded by the National Cancer Institute and Fundación MAPFRE Guanarteme. Dr. Goel, Dr. Chan, and Dr. Almario reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
A simple blood test that looks for a combination of specific RNA snippets may become a novel way to screen for early-onset colorectal cancer, suggests a new study published online in Gastroenterology.
Researchers identified four microRNAs that together comprise a signature biomarker that can be used to detect and diagnose the presence of colorectal cancer from a liquid biopsy in a younger population.
MicroRNAs, or miRNAs, are small RNA molecules that do not encode proteins but are used instead to regulate gene expression. The study authors developed and validated a panel that detects four miRNAs occurring at higher levels in plasma samples from patients with early-onset colorectal cancer, with high sensitivity and specificity.
“The point would be to use this test as a routine part of annual healthcare, or for people in high-risk families every 6 months,” study senior author Ajay Goel, PhD, MS, chair of the department of molecular diagnostics and experimental therapeutics at the City of Hope Comprehensive Cancer Center, Duarte, Calif., said in an interview.
“It’s affordable, it can be done easily from a small tube of blood, and as long as that test stays negative, you’re good,” Dr. Goel said, because even if patients miss a test, the next one, whether it’s 6 months or a year later, will catch any potential cancer.
“Colon cancer is not going to kill somebody overnight, so this should be used as a precursor to colonoscopy. As long as that test is negative, you can postpone a colonoscopy,” he said.
Andrew T. Chan, MD, MPH, a professor of medicine at Harvard Medical School and vice chair of gastroenterology at Massachusetts General Hospital, both in Boston, who was not involved in the research, said in an interview that the findings are exciting.
“It would be really value-added to have a blood-based screening test,” Dr. Chan said, adding that researchers have pursued multiple different avenues in pursuit of one. “It’s very nice to see that area progress and to actually have some evidence that microRNAs could be a potential biomarker for colorectal cancer.”
Screening now insufficient for early-onset disease
The U.S. Preventive Services Task Force recently lowered the recommended age to 45 years to begin screening for colorectal cancer. Part of the rationale for the change came from the rising rates of early-onset colorectal cancer, a distinct clinical and molecular entity that tends to have poorer survival than late-onset disease, the authors noted.
Early-onset disease, occurring primarily in people under 50 without a family or genetic history of colorectal cancer, now makes up about 10%-15% of all new cases and continues to rise, they write.
“Early-onset colorectal cancer patients are more likely to exhibit an advanced stage tumor at initial presentation, distal tumor localization, signet ring histology, and a disease presentation with concurrent metastasis,” the authors wrote. “This raises the logistical clinical concern that, since the tumors in early-onset colorectal cancer patients are often more aggressive than those with late-onset colorectal cancer, a delayed diagnosis could have a significant adverse impact and can lead to early death.”
Yet current screening strategies are insufficient for detecting enough early-onset cases, the authors assert.
Colonoscopies are invasive, carry a risk for complications, and are cost- and time-prohibitive for people at average risk. Meanwhile, existing fecal and blood tests “lack adequate diagnostic performance for the early detection of colorectal cancer, especially early-onset colorectal cancer, as these assays have yet to be explored or developed in this population,” they wrote.
The ideal “diagnostic modality should preferably be acceptable to healthy individuals, inexpensive, rapid, and preferably noninvasive,” they note.
Finding and validating miRNA
The researchers therefore turned to the concept of a liquid biopsy, focusing on identifying miRNAs associated with colorectal cancer, because their expression tends to be stable in tissues, blood, stool, and other body fluids.
They first analyzed an miRNA expression profiling dataset from 1,061 individuals to look for miRNAs whose expression was higher in colorectal cancer patients. The dataset included 42 patients with stage 1-2 early-onset colorectal cancer, 370 patients with stage 1-2 late-onset colorectal cancer, 62 patients younger than 50 years without cancer, and 587 patients aged 50 years or older without cancer.
The researchers found 28 miRNAs that were significantly unregulated in early-onset colorectal cancer tissue samples, compared with cancer-free samples and 11 miRNAs unregulated specifically in only the early-onset colorectal cancer samples. Four of these 11 miRNAs were adequately distinct from one another and were detectable in the plasma samples that the researchers would use to train and validate them as a combination biomarker.
The researchers used 117 plasma samples from Japan, including 72 from people with early-onset colorectal cancer and 45 from healthy donors, to develop and train an assay detecting the four miRNAs. They then validated the assay using 142 plasma samples from Spain, including 77 with early-onset colorectal cancer and 65 healthy donors.
In the Japan cohort, the four-miRNA assay had a sensitivity of 90% and a specificity of 80%, with a positive predictive value (PPV) of 88% and a negative predictive value (NPV) of 84%. In the Spain cohort used for validation, the assay performed with a sensitivity of 82%, a specificity of 86%, a PPV of 88%, and an NPV of 80%.
“Taken together, the genome-wide transcriptomic profiling approach was indeed robust, as it identified the biomarkers that were successfully trained and validated in plasma specimens from independent cohorts of patients with early-onset colorectal cancer, hence highlighting their translational potential in the clinic for the detection of this malignancy in early stages,” the authors wrote.
By disease stage, the four-miRNA panel identified both early-stage (stage 1-2; sensitivity, 92%; specificity, 80%) and late-stage (stage 3-4; sensitivity, 79%; specificity, 86%) early-onset colorectal cancer in the validation cohort.
Clinical benefit of blood test
The researchers also assessed the benefit-harm trade-off of this liquid biopsy assay compared with other screening modalities, taking into consideration the risk for false positives and false negatives.
A decision curve analysis “revealed that the miRNA panel achieved a higher net benefit regardless of threshold probability in comparison to intervention for all patients or none of the patients,” the researchers reported. “These findings suggest that this miRNA panel might offer more clinical benefit with regards to the avoidance of physical harm and misdiagnosis.”
They also found that expression levels of these four miRNAs significantly decreased after surgical removal of the colorectal cancer, strongly suggesting that the miRNAs do originate with the tumor.
“To have a relatively inexpensive and noninvasive means of screening a younger population is a very important unmet need,” said Dr. Chan.
It’s not feasible to recommend colonoscopies in people younger than 45 years because of resource constraints, he said, so “this is a wonderful new development to actually have the possibility of a blood-based screening test for younger individuals, especially given that rising incidence of young-onset colorectal cancer.”
Dr. Goel pointed out that only half of those recommended to get screened for colorectal cancer actually undergo screening, and a large reason for that is the desire to avoid colonoscopy, a concern echoed in the findings of a recent study by Christopher V. Almario, MD, MSHPM, and colleagues.
Dr. Goel expects that this strategy would increase compliance with screening because it’s less invasive and more affordable, particularly for younger patients. He estimates that a commercial assay using this panel, if approved by the Food and Drug Administration, should cost less than $100.
Dr. Almario, an assistant professor of medicine at the Cedars-Sinai Karsh Division of Gastroenterology and Hepatology in Los Angeles, agreed that an FDA-approved blood-based screening test would be a “game-changer,” as long as it’s accurate and effective.
Though Dr. Almario did not review the data in Goel’s study, he said in an interview that a blood test for colorectal cancer screening would be “the holy grail, so to speak, in terms of really moving the needle on screening uptake.”
Next steps
Dr. Chan noted that one caveat to consider with this study is that it was done in a relatively small population of individuals, even though the test was validated in a second set of plasma samples.
“Additional validation needs to be done in larger numbers of patients to really understand the performance characteristics because it is possible that some of these signatures may, when they’re using a broader group of individuals, not perform as well,” Dr. Chan said.
Dr. Goel said he is working with several companies right now to develop and further test a commercial product. He anticipates it may be shelf-ready in 2-5 years.
“The take-home message is that clinicians need to be more cognizant of the fact that incidence of this disease is rising, and we need to do something about it,” Dr. Goel said, particularly for those younger than 45 years who currently don’t have a screening option.
“Now we have at least a sliver of hope for those who might be suffering from this disease, for those for whom we have zero screening or diagnostic tests,” he said.
The research was funded by the National Cancer Institute and Fundación MAPFRE Guanarteme. Dr. Goel, Dr. Chan, and Dr. Almario reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM GASTROENTEROLOGY
One in eight COVID patients likely to develop long COVID: Large study
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
a large study published in The Lancet indicates.
The researchers determined that percentage by comparing long-term symptoms in people infected by SARS-CoV-2 with similar symptoms in uninfected people over the same time period.
Among the group of infected study participants in the Netherlands, 21.4% had at least one new or severely increased symptom 3-5 months after infection compared with before infection. When that group of 21.4% was compared with 8.7% of uninfected people in the same study, the researchers were able to calculate a prevalence 12.7% with long COVID.
“This finding shows that post–COVID-19 condition is an urgent problem with a mounting human toll,” the study authors wrote.
The research design was novel, two editorialists said in an accompanying commentary.
Christopher Brightling, PhD, and Rachael Evans, MBChB, PhD, of the Institute for Lung Health, University of Leicester (England), noted: “This is a major advance on prior long COVID prevalence estimates as it includes a matched uninfected group and accounts for symptoms before COVID-19 infection.”
Symptoms that persist
The Lancet study found that 3-5 months after COVID (compared with before COVID) and compared with the non-COVID comparison group, the symptoms that persist were chest pain, breathing difficulties, pain when breathing, muscle pain, loss of taste and/or smell, tingling extremities, lump in throat, feeling hot and cold alternately, heavy limbs, and tiredness.
The authors noted that symptoms such as brain fog were found to be relevant to long COVID after the data collection period for this paper and were not included in this research.
Researcher Aranka V. Ballering, MSc, PhD candidate, said in an interview that the researchers found fever is a symptom that is clearly present during the acute phase of the disease and it peaks the day of the COVID-19 diagnosis, but also wears off.
Loss of taste and smell, however, rapidly increases in severity when COVID-19 is diagnosed, but also persists and is still present 3-5 months after COVID.
Ms. Ballering, with the department of psychiatry at the University of Groningen (the Netherlands), said she was surprised by the sex difference made evident in their research: “Women showed more severe persistent symptoms than men.”
Closer to a clearer definition
The authors said their findings also pinpoint symptoms that bring us closer to a better definition of long COVID, which has many different definitions globally.
“These symptoms have the highest discriminative ability to distinguish between post–COVID-19 condition and non–COVID-19–related symptoms,” they wrote.
Researchers collected data by asking participants in the northern Netherlands, who were part of the population-based Lifelines COVID-19 study, to regularly complete digital questionnaires on 23 symptoms commonly associated with long COVID. The questionnaire was sent out 24 times to the same people between March 2020 and August 2021. At that time, people had the Alpha or earlier variants.
Participants were considered COVID-19 positive if they had either a positive test or a doctor’s diagnosis of COVID-19.
Of 76,422 study participants, the 5.5% (4,231) who had COVID were matched to 8,462 controls. Researchers accounted for sex, age, and time of completing questionnaires.
Effect of hospitalization, vaccination unclear
Ms. Ballering said it’s unclear from this data whether vaccination or whether a person was hospitalized would change the prevalence of persistent symptoms.
Because of the period when the data were collected, “the vast majority of our study population was not fully vaccinated,” she said.
However, she pointed to recent research that shows that immunization against COVID is only partially effective against persistent somatic symptoms after COVID.
Also, only 5% of men and 2.5% of women in the study were hospitalized as a result of COVID-19, so the findings can’t easily be generalized to hospitalized patients.
The Lifelines study was an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining 167,729 people in the Netherlands. Almost all were White, a limitation of the study, and 58% were female. Average age was 54.
The editorialists also noted additional limitations of the study were that this research “did not fully consider the impact on mental health” and was conducted in one region in the Netherlands.
Janko Nikolich-Žugich, MD, PhD, director of the Aegis Consortium for Pandemic-Free Future and head of the immunobiology department at University of Arizona, Tucson, said in an interview that he agreed with the editorialists that a primary benefit of this study is that it corrected for symptoms people had before COVID, something other studies have not been able to do.
However, he cautioned about generalizing the results for the United States and other countries because of the lack of diversity in the study population with regard to education level, socioeconomic factors, and race. He pointed out that access issues are also different in the Netherlands, which has universal health care.
He said brain fog as a symptom of long COVID is of high interest and will be important to include in future studies that are able to extend the study period.
The work was funded by ZonMw; the Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; and the provinces of Drenthe, Friesland, and Groningen. The study authors and Dr. Nikolich-Žugich have reported no relevant financial relationships. Dr. Brightling has received consultancy and or grants paid to his institution from GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Novartis, Chiesi, Genentech, Roche, Sanofi, Regeneron, Mologic, and 4DPharma for asthma and chronic obstructive pulmonary disease research. Dr. Evans has received consultancy fees from AstraZeneca on the topic of long COVID and from GlaxoSmithKline on digital health, and speaker’s fees from Boehringer Ingelheim on long COVID.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
‘Staggering’ CVD rise projected in U.S., especially in minorities
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF AMERICAN COLLEGE OF CARDIOLOGY
Waking up at night could be your brain boosting your memory
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
FROM NATURE NEUROSCIENCE
Smell loss may be a biomarker of Alzheimer’s disease risk
, according to new research findings.
Olfactory dysfunction is common in late life and well documented among people with Alzheimer’s disease. However, it was unknown whether faster olfactory decline predicts either onset of Alzheimer’s disease or structural brain changes associated with Alzheimer’s disease.
In a study published online in Alzheimer’s and Dementia, Jayant M. Pinto, MD, and his colleagues at the University of Chicago Medical Center reported that among older adults with normal cognition at baseline, people who experienced rapid loss of sense of smell were more likely to be subsequently diagnosed with mild cognitive impairment (MCI) or dementia, compared with those who did not.
Participants were recruited from Rush University’s Memory and Aging Project, a longitudinal cohort of older adults who undergo yearly cognitive and sensory exams, including a scratch test of 12 common smells to identify. The Rush study “was ahead of the curve in looking at smell,” Dr. Pinto said in an interview. “It gave us a very valuable resource with which to attack these questions.”
Dr. Pinto has long investigated links between smell and accelerated aging; in 2014 his group published the finding that olfactory dysfunction could predict death within 5 years in older adults, and in 2018 they reported that olfactory dysfunction could predict dementia.
Smell and cognition over time
For the current study, Dr. Pinto said, “we were able to look at the question not just using a single point in time, but a more granular trajectory of smell loss. Measuring change year by year showed that the faster people’s sense of smell declined, the more likely they were to be diagnosed with MCI or Alzheimer’s disease.”
Dr. Pinto and his colleagues evaluated results from 515 adults (mean age 76.6, 78% female, 94% White) with no cognitive impairment and at least 3 years of normal results on smell tests at baseline. The subjects were followed for a mean 8 years. One hundred subjects (19%) were diagnosed with MCI or dementia by the end of the study period. A subset of the cohort (n = 121) underwent structural magnetic resonance imaging (MRI) between their final smell tests and the study’s end. Of these, most still had normal cognition; 17 individuals had MCI.
Patients’ individual trajectories of smell loss were mapped as slopes. After adjusting for expected differences in age and sex, the investigators found steeper decline associated with greater risk of incident MCI or dementia (odds ratio, 1.89; 95% confidence interval, 1.26-2.90; P < .01). The risk was comparable to that of carrying an apo E ε4 allele, the key risk variant for late-onset Alzheimer’s disease, but was independent of apo E status. The association was strongest among subjects younger than 76 years.
Olfactory decline and brain volume
Dr. Pinto and his colleagues, including lead author Rachel R. Pacyna, a 4th-year medical student at the University of Chicago, also sought to identify brain volume changes corresponding with olfactory decline and Alzheimer’s disease. The researchers hypothesized that certain brain regions not seen affected in Alzheimer’s disease would remain unchanged regardless of olfactory status, but that regions associated with smell and Alzheimer’s disease would see smaller volumes linked with olfactory decline.
Faster olfactory decline did predict lower gray matter volume in olfactory regions, even after controlling for apo E status and other known risk factors. Conversely, cognitively unimpaired patients undergoing MRI saw more gray matter volume in primary olfactory and temporal brain regions, compared with those with cognitive symptoms.
Taken together, the findings suggest that “change in sense of smell is better than looking at sense of smell at one time point,” Dr. Pinto commented. “There are other reasons people have impaired sense of smell: car accidents, COVID, other viruses and infections. But if you identify on a time course those who are starting to lose it faster, these are the people on whom we need to focus.”
Not yet diagnostic
More work needs to be done to establish thresholds for smell loss that could be useful in clinical or investigative settings as a marker of dementia risk, Dr. Pinto acknowledged. “Everyone gets their hearing tested; everyone gets their vision tested. It’s not as easy to get your sense of smell tested. But this study is telling people that if we were to start measuring it routinely, we could actually use it.”
Smell testing “could become a component of a diagnostic battery that includes things like genotyping and cerebrospinal fluid markers, but adds a little more information. It could be useful in clinical prevention trials to identify people at the highest risk, as smell loss presents quite a few years before MCI or Alzheimer’s disease.”
The investigators acknowledged that their findings need to be replicated in more diverse cohorts that better represent the Alzheimer’s population in the United States. Another limitation of their study, they said, was that the method used to calculate the rate of olfactory decline “was based on slope of measured time points assuming linearity, which may oversimplify the complexity of olfactory changes in normal aging and during the preclinical Alzheimer’s disease period.” The study was funded by the National Institutes of Health. Dr. Pinto disclosed receiving consulting fees from Sanofi/Regeneron, Optinose, and Genentech not related to this work.
, according to new research findings.
Olfactory dysfunction is common in late life and well documented among people with Alzheimer’s disease. However, it was unknown whether faster olfactory decline predicts either onset of Alzheimer’s disease or structural brain changes associated with Alzheimer’s disease.
In a study published online in Alzheimer’s and Dementia, Jayant M. Pinto, MD, and his colleagues at the University of Chicago Medical Center reported that among older adults with normal cognition at baseline, people who experienced rapid loss of sense of smell were more likely to be subsequently diagnosed with mild cognitive impairment (MCI) or dementia, compared with those who did not.
Participants were recruited from Rush University’s Memory and Aging Project, a longitudinal cohort of older adults who undergo yearly cognitive and sensory exams, including a scratch test of 12 common smells to identify. The Rush study “was ahead of the curve in looking at smell,” Dr. Pinto said in an interview. “It gave us a very valuable resource with which to attack these questions.”
Dr. Pinto has long investigated links between smell and accelerated aging; in 2014 his group published the finding that olfactory dysfunction could predict death within 5 years in older adults, and in 2018 they reported that olfactory dysfunction could predict dementia.
Smell and cognition over time
For the current study, Dr. Pinto said, “we were able to look at the question not just using a single point in time, but a more granular trajectory of smell loss. Measuring change year by year showed that the faster people’s sense of smell declined, the more likely they were to be diagnosed with MCI or Alzheimer’s disease.”
Dr. Pinto and his colleagues evaluated results from 515 adults (mean age 76.6, 78% female, 94% White) with no cognitive impairment and at least 3 years of normal results on smell tests at baseline. The subjects were followed for a mean 8 years. One hundred subjects (19%) were diagnosed with MCI or dementia by the end of the study period. A subset of the cohort (n = 121) underwent structural magnetic resonance imaging (MRI) between their final smell tests and the study’s end. Of these, most still had normal cognition; 17 individuals had MCI.
Patients’ individual trajectories of smell loss were mapped as slopes. After adjusting for expected differences in age and sex, the investigators found steeper decline associated with greater risk of incident MCI or dementia (odds ratio, 1.89; 95% confidence interval, 1.26-2.90; P < .01). The risk was comparable to that of carrying an apo E ε4 allele, the key risk variant for late-onset Alzheimer’s disease, but was independent of apo E status. The association was strongest among subjects younger than 76 years.
Olfactory decline and brain volume
Dr. Pinto and his colleagues, including lead author Rachel R. Pacyna, a 4th-year medical student at the University of Chicago, also sought to identify brain volume changes corresponding with olfactory decline and Alzheimer’s disease. The researchers hypothesized that certain brain regions not seen affected in Alzheimer’s disease would remain unchanged regardless of olfactory status, but that regions associated with smell and Alzheimer’s disease would see smaller volumes linked with olfactory decline.
Faster olfactory decline did predict lower gray matter volume in olfactory regions, even after controlling for apo E status and other known risk factors. Conversely, cognitively unimpaired patients undergoing MRI saw more gray matter volume in primary olfactory and temporal brain regions, compared with those with cognitive symptoms.
Taken together, the findings suggest that “change in sense of smell is better than looking at sense of smell at one time point,” Dr. Pinto commented. “There are other reasons people have impaired sense of smell: car accidents, COVID, other viruses and infections. But if you identify on a time course those who are starting to lose it faster, these are the people on whom we need to focus.”
Not yet diagnostic
More work needs to be done to establish thresholds for smell loss that could be useful in clinical or investigative settings as a marker of dementia risk, Dr. Pinto acknowledged. “Everyone gets their hearing tested; everyone gets their vision tested. It’s not as easy to get your sense of smell tested. But this study is telling people that if we were to start measuring it routinely, we could actually use it.”
Smell testing “could become a component of a diagnostic battery that includes things like genotyping and cerebrospinal fluid markers, but adds a little more information. It could be useful in clinical prevention trials to identify people at the highest risk, as smell loss presents quite a few years before MCI or Alzheimer’s disease.”
The investigators acknowledged that their findings need to be replicated in more diverse cohorts that better represent the Alzheimer’s population in the United States. Another limitation of their study, they said, was that the method used to calculate the rate of olfactory decline “was based on slope of measured time points assuming linearity, which may oversimplify the complexity of olfactory changes in normal aging and during the preclinical Alzheimer’s disease period.” The study was funded by the National Institutes of Health. Dr. Pinto disclosed receiving consulting fees from Sanofi/Regeneron, Optinose, and Genentech not related to this work.
, according to new research findings.
Olfactory dysfunction is common in late life and well documented among people with Alzheimer’s disease. However, it was unknown whether faster olfactory decline predicts either onset of Alzheimer’s disease or structural brain changes associated with Alzheimer’s disease.
In a study published online in Alzheimer’s and Dementia, Jayant M. Pinto, MD, and his colleagues at the University of Chicago Medical Center reported that among older adults with normal cognition at baseline, people who experienced rapid loss of sense of smell were more likely to be subsequently diagnosed with mild cognitive impairment (MCI) or dementia, compared with those who did not.
Participants were recruited from Rush University’s Memory and Aging Project, a longitudinal cohort of older adults who undergo yearly cognitive and sensory exams, including a scratch test of 12 common smells to identify. The Rush study “was ahead of the curve in looking at smell,” Dr. Pinto said in an interview. “It gave us a very valuable resource with which to attack these questions.”
Dr. Pinto has long investigated links between smell and accelerated aging; in 2014 his group published the finding that olfactory dysfunction could predict death within 5 years in older adults, and in 2018 they reported that olfactory dysfunction could predict dementia.
Smell and cognition over time
For the current study, Dr. Pinto said, “we were able to look at the question not just using a single point in time, but a more granular trajectory of smell loss. Measuring change year by year showed that the faster people’s sense of smell declined, the more likely they were to be diagnosed with MCI or Alzheimer’s disease.”
Dr. Pinto and his colleagues evaluated results from 515 adults (mean age 76.6, 78% female, 94% White) with no cognitive impairment and at least 3 years of normal results on smell tests at baseline. The subjects were followed for a mean 8 years. One hundred subjects (19%) were diagnosed with MCI or dementia by the end of the study period. A subset of the cohort (n = 121) underwent structural magnetic resonance imaging (MRI) between their final smell tests and the study’s end. Of these, most still had normal cognition; 17 individuals had MCI.
Patients’ individual trajectories of smell loss were mapped as slopes. After adjusting for expected differences in age and sex, the investigators found steeper decline associated with greater risk of incident MCI or dementia (odds ratio, 1.89; 95% confidence interval, 1.26-2.90; P < .01). The risk was comparable to that of carrying an apo E ε4 allele, the key risk variant for late-onset Alzheimer’s disease, but was independent of apo E status. The association was strongest among subjects younger than 76 years.
Olfactory decline and brain volume
Dr. Pinto and his colleagues, including lead author Rachel R. Pacyna, a 4th-year medical student at the University of Chicago, also sought to identify brain volume changes corresponding with olfactory decline and Alzheimer’s disease. The researchers hypothesized that certain brain regions not seen affected in Alzheimer’s disease would remain unchanged regardless of olfactory status, but that regions associated with smell and Alzheimer’s disease would see smaller volumes linked with olfactory decline.
Faster olfactory decline did predict lower gray matter volume in olfactory regions, even after controlling for apo E status and other known risk factors. Conversely, cognitively unimpaired patients undergoing MRI saw more gray matter volume in primary olfactory and temporal brain regions, compared with those with cognitive symptoms.
Taken together, the findings suggest that “change in sense of smell is better than looking at sense of smell at one time point,” Dr. Pinto commented. “There are other reasons people have impaired sense of smell: car accidents, COVID, other viruses and infections. But if you identify on a time course those who are starting to lose it faster, these are the people on whom we need to focus.”
Not yet diagnostic
More work needs to be done to establish thresholds for smell loss that could be useful in clinical or investigative settings as a marker of dementia risk, Dr. Pinto acknowledged. “Everyone gets their hearing tested; everyone gets their vision tested. It’s not as easy to get your sense of smell tested. But this study is telling people that if we were to start measuring it routinely, we could actually use it.”
Smell testing “could become a component of a diagnostic battery that includes things like genotyping and cerebrospinal fluid markers, but adds a little more information. It could be useful in clinical prevention trials to identify people at the highest risk, as smell loss presents quite a few years before MCI or Alzheimer’s disease.”
The investigators acknowledged that their findings need to be replicated in more diverse cohorts that better represent the Alzheimer’s population in the United States. Another limitation of their study, they said, was that the method used to calculate the rate of olfactory decline “was based on slope of measured time points assuming linearity, which may oversimplify the complexity of olfactory changes in normal aging and during the preclinical Alzheimer’s disease period.” The study was funded by the National Institutes of Health. Dr. Pinto disclosed receiving consulting fees from Sanofi/Regeneron, Optinose, and Genentech not related to this work.
FROM ALZHEIMER’S & DEMENTIA
One in four NSCLC patients respond poorly to COVID-19 vaccine
according to a new study.
The study was published in the Journal of Clinical Oncology.
“Booster vaccination increased binding and neutralizing antibody titers to Omicron, but antibody titers declined after 3 months. These data highlight the concern for patients with cancer given the rapid spread of SARS-CoV-2 Omicron variant,” wrote the authors, who were led by Rafi Ahmed, PhD, Emory University, Atlanta.
Researchers found that 18% had no detectable antibody at all and active treatment type had no association with vaccine response.
Researchers examined antibody titers among 82 NSCLC patients and 53 healthy volunteers. They collected blood samples longitudinally for analysis. While most patients had binding and neutralizing antibody titers that were comparable with healthy volunteers, 25% had poor responses, which led to six- to sevenfold lower titers than healthy controls. There was no association between worse vaccine responses and history of programmed death–1 monotherapy, chemotherapy, or both in combination. Receipt of a booster vaccine improved binding and neutralizing antibody titers to both the wild type and the Omicron variant, but 2-4 months after the booster there was a five- to sevenfold decrease in neutralizing titers to both the wild type and Omicron variant.
“This study indicates both the need to monitor our patients with lung cancer for response to COVID-19 mRNA vaccines, identify the nonresponders for follow-up and further attempts at immunization, and continue collecting and analyzing clinicodemographic information and biospecimens from our patients,” wrote the authors of an accompanying editorial.
Although the findings reveal potential concerns, the good news is that most patients NSCLC patients do respond normally to COVID-19 vaccination, said John D. Minna, MD, University of Texas Southwestern Medical Center, Dallas, lead author of the editorial.
He offered some advice to physicians. “You can test your patients using currently available [Clinical Laboratory Improvement Amendments]–approved lab tests to determine what their antibody titers are. This should be done after boosting since titers will go down after time. We know that if a patient has lung cancer and they do get infected with SARS-CoV-2 that potentially they could develop serious COVID-19 disease. Besides giving antiviral treatment, it is important that they be closely monitored for symptoms of progression so if they need to be hospitalized it can be done in a prudent manner,” said Dr. Minna, who is director of the Hamon Center for Therapeutic Oncology Research at the University of Texas Southwestern Medical Center.
No clinical details have emerged that might predict which patients have an insufficient response to vaccination. “When we started these studies, a lot of us thought that anyone who did not develop a good antibody response would be weak or sicker. For example, [patients with] late-stage disease, or having had a lot of therapy, or perhaps immune checkpoint blockade. However, none of these things are correlated. The main advice we are giving our lung cancer patients are to get vaccinated, get boosted (double boosted), and just do the smart thing to protect yourself from exposure,” he said.
For example, when traveling on a plane, patients should wear a mask. They should also avoid large indoor events. He also recommended that, following vaccination and boosters, patients seek out CLIA-certified tests to get their titer checked.
“Upon any COVID infection, even if their titer is at or above 80%, patients should see their physician to consider treatment with Paxlovid (nirmatrelvir/ritonavir), which has emergency use authorization. For patients with a lower titer, it’s important to seek out a physician and consider Paxlovid and possibly antibody therapy. But these are individual decisions to be made with your doctor,” Dr. Minna said.
The next important research question is what happens to T-cell immune response following vaccination. “We know that a good cellular immune response is also important in preventing infection and severe infection, but we don’t yet know which persons (with or without cancer) have good T-cell responses. This information will also likely impact what we tell our patients and will add to the antibody data,” he said.
Studies are ongoing to determine specific T-cell responses to mRNA vaccines, and how well those T-cell responses respond to SARS-CoV-2 infection in the laboratory.
according to a new study.
The study was published in the Journal of Clinical Oncology.
“Booster vaccination increased binding and neutralizing antibody titers to Omicron, but antibody titers declined after 3 months. These data highlight the concern for patients with cancer given the rapid spread of SARS-CoV-2 Omicron variant,” wrote the authors, who were led by Rafi Ahmed, PhD, Emory University, Atlanta.
Researchers found that 18% had no detectable antibody at all and active treatment type had no association with vaccine response.
Researchers examined antibody titers among 82 NSCLC patients and 53 healthy volunteers. They collected blood samples longitudinally for analysis. While most patients had binding and neutralizing antibody titers that were comparable with healthy volunteers, 25% had poor responses, which led to six- to sevenfold lower titers than healthy controls. There was no association between worse vaccine responses and history of programmed death–1 monotherapy, chemotherapy, or both in combination. Receipt of a booster vaccine improved binding and neutralizing antibody titers to both the wild type and the Omicron variant, but 2-4 months after the booster there was a five- to sevenfold decrease in neutralizing titers to both the wild type and Omicron variant.
“This study indicates both the need to monitor our patients with lung cancer for response to COVID-19 mRNA vaccines, identify the nonresponders for follow-up and further attempts at immunization, and continue collecting and analyzing clinicodemographic information and biospecimens from our patients,” wrote the authors of an accompanying editorial.
Although the findings reveal potential concerns, the good news is that most patients NSCLC patients do respond normally to COVID-19 vaccination, said John D. Minna, MD, University of Texas Southwestern Medical Center, Dallas, lead author of the editorial.
He offered some advice to physicians. “You can test your patients using currently available [Clinical Laboratory Improvement Amendments]–approved lab tests to determine what their antibody titers are. This should be done after boosting since titers will go down after time. We know that if a patient has lung cancer and they do get infected with SARS-CoV-2 that potentially they could develop serious COVID-19 disease. Besides giving antiviral treatment, it is important that they be closely monitored for symptoms of progression so if they need to be hospitalized it can be done in a prudent manner,” said Dr. Minna, who is director of the Hamon Center for Therapeutic Oncology Research at the University of Texas Southwestern Medical Center.
No clinical details have emerged that might predict which patients have an insufficient response to vaccination. “When we started these studies, a lot of us thought that anyone who did not develop a good antibody response would be weak or sicker. For example, [patients with] late-stage disease, or having had a lot of therapy, or perhaps immune checkpoint blockade. However, none of these things are correlated. The main advice we are giving our lung cancer patients are to get vaccinated, get boosted (double boosted), and just do the smart thing to protect yourself from exposure,” he said.
For example, when traveling on a plane, patients should wear a mask. They should also avoid large indoor events. He also recommended that, following vaccination and boosters, patients seek out CLIA-certified tests to get their titer checked.
“Upon any COVID infection, even if their titer is at or above 80%, patients should see their physician to consider treatment with Paxlovid (nirmatrelvir/ritonavir), which has emergency use authorization. For patients with a lower titer, it’s important to seek out a physician and consider Paxlovid and possibly antibody therapy. But these are individual decisions to be made with your doctor,” Dr. Minna said.
The next important research question is what happens to T-cell immune response following vaccination. “We know that a good cellular immune response is also important in preventing infection and severe infection, but we don’t yet know which persons (with or without cancer) have good T-cell responses. This information will also likely impact what we tell our patients and will add to the antibody data,” he said.
Studies are ongoing to determine specific T-cell responses to mRNA vaccines, and how well those T-cell responses respond to SARS-CoV-2 infection in the laboratory.
according to a new study.
The study was published in the Journal of Clinical Oncology.
“Booster vaccination increased binding and neutralizing antibody titers to Omicron, but antibody titers declined after 3 months. These data highlight the concern for patients with cancer given the rapid spread of SARS-CoV-2 Omicron variant,” wrote the authors, who were led by Rafi Ahmed, PhD, Emory University, Atlanta.
Researchers found that 18% had no detectable antibody at all and active treatment type had no association with vaccine response.
Researchers examined antibody titers among 82 NSCLC patients and 53 healthy volunteers. They collected blood samples longitudinally for analysis. While most patients had binding and neutralizing antibody titers that were comparable with healthy volunteers, 25% had poor responses, which led to six- to sevenfold lower titers than healthy controls. There was no association between worse vaccine responses and history of programmed death–1 monotherapy, chemotherapy, or both in combination. Receipt of a booster vaccine improved binding and neutralizing antibody titers to both the wild type and the Omicron variant, but 2-4 months after the booster there was a five- to sevenfold decrease in neutralizing titers to both the wild type and Omicron variant.
“This study indicates both the need to monitor our patients with lung cancer for response to COVID-19 mRNA vaccines, identify the nonresponders for follow-up and further attempts at immunization, and continue collecting and analyzing clinicodemographic information and biospecimens from our patients,” wrote the authors of an accompanying editorial.
Although the findings reveal potential concerns, the good news is that most patients NSCLC patients do respond normally to COVID-19 vaccination, said John D. Minna, MD, University of Texas Southwestern Medical Center, Dallas, lead author of the editorial.
He offered some advice to physicians. “You can test your patients using currently available [Clinical Laboratory Improvement Amendments]–approved lab tests to determine what their antibody titers are. This should be done after boosting since titers will go down after time. We know that if a patient has lung cancer and they do get infected with SARS-CoV-2 that potentially they could develop serious COVID-19 disease. Besides giving antiviral treatment, it is important that they be closely monitored for symptoms of progression so if they need to be hospitalized it can be done in a prudent manner,” said Dr. Minna, who is director of the Hamon Center for Therapeutic Oncology Research at the University of Texas Southwestern Medical Center.
No clinical details have emerged that might predict which patients have an insufficient response to vaccination. “When we started these studies, a lot of us thought that anyone who did not develop a good antibody response would be weak or sicker. For example, [patients with] late-stage disease, or having had a lot of therapy, or perhaps immune checkpoint blockade. However, none of these things are correlated. The main advice we are giving our lung cancer patients are to get vaccinated, get boosted (double boosted), and just do the smart thing to protect yourself from exposure,” he said.
For example, when traveling on a plane, patients should wear a mask. They should also avoid large indoor events. He also recommended that, following vaccination and boosters, patients seek out CLIA-certified tests to get their titer checked.
“Upon any COVID infection, even if their titer is at or above 80%, patients should see their physician to consider treatment with Paxlovid (nirmatrelvir/ritonavir), which has emergency use authorization. For patients with a lower titer, it’s important to seek out a physician and consider Paxlovid and possibly antibody therapy. But these are individual decisions to be made with your doctor,” Dr. Minna said.
The next important research question is what happens to T-cell immune response following vaccination. “We know that a good cellular immune response is also important in preventing infection and severe infection, but we don’t yet know which persons (with or without cancer) have good T-cell responses. This information will also likely impact what we tell our patients and will add to the antibody data,” he said.
Studies are ongoing to determine specific T-cell responses to mRNA vaccines, and how well those T-cell responses respond to SARS-CoV-2 infection in the laboratory.
FROM THE JOURNAL OF CLINICAL ONCOLOGY