User login
Guideline: Supplemental, dietary calcium both heart safe
Both dietary and supplemental calcium should be considered safe for the cardiovascular system as long as total intake doesn’t exceed 2,000-2,500 mg/day – the maximal tolerable level defined by the National Academy of Medicine, according to an updated Clinical Practice Guideline published online October 24 in Annals of Internal Medicine.
For generally healthy patients who don’t consume adequate calcium and take supplements, either alone or in combination with vitamin D, to prevent osteoporosis and related fractures, “discontinuation of supplemental calcium for safety reasons is not necessary and may be harmful to bone health,” said Stephen L. Kopecky, MD, of the Mayo Clinic, Rochester Minn., and his associates on the expert panel that wrote the new guideline.
The National Osteoporosis Foundation (NOF) and the American Society for Preventive Cardiology (ASPC) commissioned an independent review of the current evidence to update the Evidence Report and assembled the expert panel to write the guideline based on the new findings (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-1743).
Separately, Mei Chung, PhD, of the department of public health and community medicine, and her associates at Tufts University, Boston, reviewed 4 recent randomized clinical trials, 1 nested case-control study, and 26 cohort studies that assessed the effects of calcium intake on 17 health outcomes in generally healthy adults of all ages. None of the studies evaluated cardiovascular disease risk as a primary outcome. “We conclude that calcium intake (from either food or supplement sources) at levels within the recommended tolerable upper intake range (2,000-2,500 mg/d) are not associated with CVD risks in generally healthy adults,” they said.
“Although a few trials and cohort studies reported increased risks with higher calcium intake, risk estimates in most of those studies were small (10% relative risk) and not considered clinically important, even if they were statistically significant,” Dr. Chung and her associates added (Ann Int Med. 2016 Oct 24. doi: 10.7326/M16-1165).
According to the guideline, “The NOF and the ASPC now adopt the position that there is moderate-quality evidence that calcium with or without vitamin D intake from food or supplements has no relationship (beneficial or harmful) with the risk for cardiovascular or cerebrovascular disease, mortality, or all-cause mortality in generally healthy adults at this time.”
In addition, “Currently, no established biological mechanism supports and association between calcium and cardiovascular disease,” Dr. Kopecky and his associates on the expert panel noted.
The volume of literature on the subject of calcium’s potential harmful cardiovascular disease effects appears to be robust, with the largest meta-analysis to date including 18 studies with 64,000 participants. But this evidence base has some limitations, chief among them the fact that none of the studies was designed to evaluate CVD as a primary outcome.
In addition, concerns about harmful cardiovascular effects arose after most of the trials had already been initiated, so unpublished data on those outcomes were collected and adjudicated retrospectively. In addition, many of the participants showed poor long-term treatment adherence, making it difficult to interpret the data.
Karen L. Margolis, MD, of HealthPartners Institute in Minneapolis and JoAnn E. Manson, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, made these remarks in an editorial accompanying the new Clinical Practice Guideline (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-2193). Their financial disclosures are available at www.acponline.org.
The volume of literature on the subject of calcium’s potential harmful cardiovascular disease effects appears to be robust, with the largest meta-analysis to date including 18 studies with 64,000 participants. But this evidence base has some limitations, chief among them the fact that none of the studies was designed to evaluate CVD as a primary outcome.
In addition, concerns about harmful cardiovascular effects arose after most of the trials had already been initiated, so unpublished data on those outcomes were collected and adjudicated retrospectively. In addition, many of the participants showed poor long-term treatment adherence, making it difficult to interpret the data.
Karen L. Margolis, MD, of HealthPartners Institute in Minneapolis and JoAnn E. Manson, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, made these remarks in an editorial accompanying the new Clinical Practice Guideline (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-2193). Their financial disclosures are available at www.acponline.org.
The volume of literature on the subject of calcium’s potential harmful cardiovascular disease effects appears to be robust, with the largest meta-analysis to date including 18 studies with 64,000 participants. But this evidence base has some limitations, chief among them the fact that none of the studies was designed to evaluate CVD as a primary outcome.
In addition, concerns about harmful cardiovascular effects arose after most of the trials had already been initiated, so unpublished data on those outcomes were collected and adjudicated retrospectively. In addition, many of the participants showed poor long-term treatment adherence, making it difficult to interpret the data.
Karen L. Margolis, MD, of HealthPartners Institute in Minneapolis and JoAnn E. Manson, MD, DrPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, made these remarks in an editorial accompanying the new Clinical Practice Guideline (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-2193). Their financial disclosures are available at www.acponline.org.
Both dietary and supplemental calcium should be considered safe for the cardiovascular system as long as total intake doesn’t exceed 2,000-2,500 mg/day – the maximal tolerable level defined by the National Academy of Medicine, according to an updated Clinical Practice Guideline published online October 24 in Annals of Internal Medicine.
For generally healthy patients who don’t consume adequate calcium and take supplements, either alone or in combination with vitamin D, to prevent osteoporosis and related fractures, “discontinuation of supplemental calcium for safety reasons is not necessary and may be harmful to bone health,” said Stephen L. Kopecky, MD, of the Mayo Clinic, Rochester Minn., and his associates on the expert panel that wrote the new guideline.
The National Osteoporosis Foundation (NOF) and the American Society for Preventive Cardiology (ASPC) commissioned an independent review of the current evidence to update the Evidence Report and assembled the expert panel to write the guideline based on the new findings (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-1743).
Separately, Mei Chung, PhD, of the department of public health and community medicine, and her associates at Tufts University, Boston, reviewed 4 recent randomized clinical trials, 1 nested case-control study, and 26 cohort studies that assessed the effects of calcium intake on 17 health outcomes in generally healthy adults of all ages. None of the studies evaluated cardiovascular disease risk as a primary outcome. “We conclude that calcium intake (from either food or supplement sources) at levels within the recommended tolerable upper intake range (2,000-2,500 mg/d) are not associated with CVD risks in generally healthy adults,” they said.
“Although a few trials and cohort studies reported increased risks with higher calcium intake, risk estimates in most of those studies were small (10% relative risk) and not considered clinically important, even if they were statistically significant,” Dr. Chung and her associates added (Ann Int Med. 2016 Oct 24. doi: 10.7326/M16-1165).
According to the guideline, “The NOF and the ASPC now adopt the position that there is moderate-quality evidence that calcium with or without vitamin D intake from food or supplements has no relationship (beneficial or harmful) with the risk for cardiovascular or cerebrovascular disease, mortality, or all-cause mortality in generally healthy adults at this time.”
In addition, “Currently, no established biological mechanism supports and association between calcium and cardiovascular disease,” Dr. Kopecky and his associates on the expert panel noted.
Both dietary and supplemental calcium should be considered safe for the cardiovascular system as long as total intake doesn’t exceed 2,000-2,500 mg/day – the maximal tolerable level defined by the National Academy of Medicine, according to an updated Clinical Practice Guideline published online October 24 in Annals of Internal Medicine.
For generally healthy patients who don’t consume adequate calcium and take supplements, either alone or in combination with vitamin D, to prevent osteoporosis and related fractures, “discontinuation of supplemental calcium for safety reasons is not necessary and may be harmful to bone health,” said Stephen L. Kopecky, MD, of the Mayo Clinic, Rochester Minn., and his associates on the expert panel that wrote the new guideline.
The National Osteoporosis Foundation (NOF) and the American Society for Preventive Cardiology (ASPC) commissioned an independent review of the current evidence to update the Evidence Report and assembled the expert panel to write the guideline based on the new findings (Ann Intern Med. 2016 Oct 24. doi: 10.7326/M16-1743).
Separately, Mei Chung, PhD, of the department of public health and community medicine, and her associates at Tufts University, Boston, reviewed 4 recent randomized clinical trials, 1 nested case-control study, and 26 cohort studies that assessed the effects of calcium intake on 17 health outcomes in generally healthy adults of all ages. None of the studies evaluated cardiovascular disease risk as a primary outcome. “We conclude that calcium intake (from either food or supplement sources) at levels within the recommended tolerable upper intake range (2,000-2,500 mg/d) are not associated with CVD risks in generally healthy adults,” they said.
“Although a few trials and cohort studies reported increased risks with higher calcium intake, risk estimates in most of those studies were small (10% relative risk) and not considered clinically important, even if they were statistically significant,” Dr. Chung and her associates added (Ann Int Med. 2016 Oct 24. doi: 10.7326/M16-1165).
According to the guideline, “The NOF and the ASPC now adopt the position that there is moderate-quality evidence that calcium with or without vitamin D intake from food or supplements has no relationship (beneficial or harmful) with the risk for cardiovascular or cerebrovascular disease, mortality, or all-cause mortality in generally healthy adults at this time.”
In addition, “Currently, no established biological mechanism supports and association between calcium and cardiovascular disease,” Dr. Kopecky and his associates on the expert panel noted.
CMS offering educational webinars on MACRA
The Centers for Medicare & Medicaid Services is offering a pair of webinars aimed at helping physicians navigate the new regulation that operationalizes the Medicare Access and CHIP Reauthorization Act (MACRA).
The first webinar, scheduled for Oct. 26, will provide an overview of the two components of the Quality Payment Program – the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs).
The second webinar, scheduled for Nov. 15, is targeted to Medicare Part B fee-for-service clinicians, office managers and administrators, state and national associations that represent health care providers, and other stakeholders and will feature a question-and-answer session.
The webinars are part of the agency’s ongoing efforts to help educate practitioners on the provisions of the final MACRA regulation, which was issued on Oct. 14. CMS also recently launched a website to help in that regard.
The Centers for Medicare & Medicaid Services is offering a pair of webinars aimed at helping physicians navigate the new regulation that operationalizes the Medicare Access and CHIP Reauthorization Act (MACRA).
The first webinar, scheduled for Oct. 26, will provide an overview of the two components of the Quality Payment Program – the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs).
The second webinar, scheduled for Nov. 15, is targeted to Medicare Part B fee-for-service clinicians, office managers and administrators, state and national associations that represent health care providers, and other stakeholders and will feature a question-and-answer session.
The webinars are part of the agency’s ongoing efforts to help educate practitioners on the provisions of the final MACRA regulation, which was issued on Oct. 14. CMS also recently launched a website to help in that regard.
The Centers for Medicare & Medicaid Services is offering a pair of webinars aimed at helping physicians navigate the new regulation that operationalizes the Medicare Access and CHIP Reauthorization Act (MACRA).
The first webinar, scheduled for Oct. 26, will provide an overview of the two components of the Quality Payment Program – the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs).
The second webinar, scheduled for Nov. 15, is targeted to Medicare Part B fee-for-service clinicians, office managers and administrators, state and national associations that represent health care providers, and other stakeholders and will feature a question-and-answer session.
The webinars are part of the agency’s ongoing efforts to help educate practitioners on the provisions of the final MACRA regulation, which was issued on Oct. 14. CMS also recently launched a website to help in that regard.
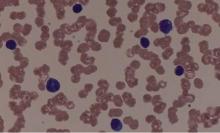
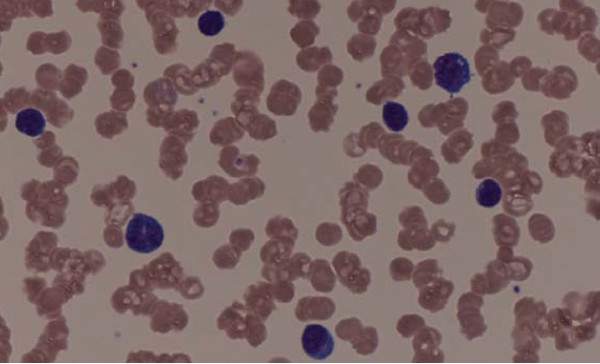
More restrictive hemoglobin threshold recommended for transfusion
New guidelines on red blood cell blood transfusion recommend a restrictive threshold in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL for most patients, finding that it is safe in most clinical settings.
The updated clinical practice guidelines on transfusion thresholds and storage from the AABB (formerly known as the American Association of Blood Banks), also note that red blood cell units can be used at any time within their licensed dating period, rather than a preference being given to fresher units less than 10 days old.
The guidelines, published online Oct. 12 in JAMA, are an update of the 2012 transfusion guidelines, and are a response to a more than doubling of the number of patients since enrolled in randomized controlled trials of red blood cell transfusions.
The AABB’s clinical transfusion medicine committee, led by Jeffrey L. Carson, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J., analyzed data from 31 randomized controlled trials of 12,587 participants, which compared restrictive transfusion thresholds of 7-8 g/dL to more liberal thresholds of 9-10 g/dL.
This analysis showed that the use of restrictive transfusion protocols was associated with an absolute difference in 30-day mortality of three fewer deaths compared to the more liberal thresholds. There was no significant difference in 30-day mortality in trials that compared a threshold of 8-9 g/dL to a threshold of less than 7 g/dL (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.9185).
“For all other outcomes evaluated, there was no evidence to suggest that patients were harmed by restrictive transfusion protocols, although the quality of the evidence was low for the outcomes of congestive heart failure and rebleeding,” the authors reported.
Based on these findings, they recommended a restrictive red blood cell transfusion threshold, in which transfusion is not indicated until the hemoglobin level is 7 g/dL for hospitalized adult patients who are hemodynamically stable, including critically ill patients.
However for patients undergoing orthopedic or cardiac surgery, or those with preexisting cardiovascular disease, they advised a threshold of 8 g/dL for initiating a red blood cell transfusion.
They also stressed that these recommendations did not apply to patients with acute coronary syndrome, those with severe thrombocytopenia, those treated for hematologic or oncologic disorders who at risk of bleeding, and those with chronic transfusion–dependent anemia, citing a lack of quality randomized controlled trial evidence.
The guideline authors examined the issue of the optimal length of time that red blood cell units should be stored, pointing out that there is currently no formal guidance on the optimal period of red blood cell storage prior to transfusion.
While units of red blood cells can be stored for up to 42 days, the committee said there was some evidence that longer storage may be associated with adverse transfusion outcomes.
“The RBCs stored for longer periods have decreased ability to deliver oxygen due to decreased levels of 2,3-diphsophoglycerate, decreased nitric oxide metabolism, alterations of the RBC membrane leading to increased rigidity, and increased RBC endothelial adherence,” they wrote.
Despite this, the review of 13 randomized controlled trials examining the effect of storage duration found no evidence that fresher units had any impact on mortality compared to standard issue units, nor were there any more adverse events with the standard issue units.
The absolute difference in 30-day mortality was four more deaths per 1,000 with fresher blood, and there was a higher risk of nosocomial infections among patients who received fresher red blood cell units although the authors said the quality of evidence was low.
They therefore recommended that no preference be given to fresher red blood cell units, and that all patients be treated with units chosen at any point within their licensed dating period.
Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies, but no other conflicts of interest were declared.
The two-tiered approach of this important update to the red blood cell transfusion guidelines acknowledges the current state of the evidence and also provides support for making more individualized transfusion decisions.
These new guidelines represent medicine at its best in that they are evidence based, derived from randomized controlled trials, reflect important clinical perspectives, and are definitive for conditions in which data are substantial, but provide greater flexibility for conditions in which data are less certain.
One major limitation of these guidelines is that they are based on hemoglobin level as the transfusion trigger, when good clinical practice dictates that the decision to transfuse should also be based on clinical factors, availability of alternative therapies, and patient preferences.
Mark H. Yazer, MD and Darrell J. Triulzi, MD, are in the division of transfusion medicine at the University of Pittsburgh Medical Center. These comments are adapted from an editorial (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.10887 ). Dr Triulzi reported receiving grants from the National Heart, Lung, and Blood Institute; and receiving personal fees for serving on an advisory board for Fresenius Kabi.
The two-tiered approach of this important update to the red blood cell transfusion guidelines acknowledges the current state of the evidence and also provides support for making more individualized transfusion decisions.
These new guidelines represent medicine at its best in that they are evidence based, derived from randomized controlled trials, reflect important clinical perspectives, and are definitive for conditions in which data are substantial, but provide greater flexibility for conditions in which data are less certain.
One major limitation of these guidelines is that they are based on hemoglobin level as the transfusion trigger, when good clinical practice dictates that the decision to transfuse should also be based on clinical factors, availability of alternative therapies, and patient preferences.
Mark H. Yazer, MD and Darrell J. Triulzi, MD, are in the division of transfusion medicine at the University of Pittsburgh Medical Center. These comments are adapted from an editorial (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.10887 ). Dr Triulzi reported receiving grants from the National Heart, Lung, and Blood Institute; and receiving personal fees for serving on an advisory board for Fresenius Kabi.
The two-tiered approach of this important update to the red blood cell transfusion guidelines acknowledges the current state of the evidence and also provides support for making more individualized transfusion decisions.
These new guidelines represent medicine at its best in that they are evidence based, derived from randomized controlled trials, reflect important clinical perspectives, and are definitive for conditions in which data are substantial, but provide greater flexibility for conditions in which data are less certain.
One major limitation of these guidelines is that they are based on hemoglobin level as the transfusion trigger, when good clinical practice dictates that the decision to transfuse should also be based on clinical factors, availability of alternative therapies, and patient preferences.
Mark H. Yazer, MD and Darrell J. Triulzi, MD, are in the division of transfusion medicine at the University of Pittsburgh Medical Center. These comments are adapted from an editorial (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.10887 ). Dr Triulzi reported receiving grants from the National Heart, Lung, and Blood Institute; and receiving personal fees for serving on an advisory board for Fresenius Kabi.
New guidelines on red blood cell blood transfusion recommend a restrictive threshold in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL for most patients, finding that it is safe in most clinical settings.
The updated clinical practice guidelines on transfusion thresholds and storage from the AABB (formerly known as the American Association of Blood Banks), also note that red blood cell units can be used at any time within their licensed dating period, rather than a preference being given to fresher units less than 10 days old.
The guidelines, published online Oct. 12 in JAMA, are an update of the 2012 transfusion guidelines, and are a response to a more than doubling of the number of patients since enrolled in randomized controlled trials of red blood cell transfusions.
The AABB’s clinical transfusion medicine committee, led by Jeffrey L. Carson, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J., analyzed data from 31 randomized controlled trials of 12,587 participants, which compared restrictive transfusion thresholds of 7-8 g/dL to more liberal thresholds of 9-10 g/dL.
This analysis showed that the use of restrictive transfusion protocols was associated with an absolute difference in 30-day mortality of three fewer deaths compared to the more liberal thresholds. There was no significant difference in 30-day mortality in trials that compared a threshold of 8-9 g/dL to a threshold of less than 7 g/dL (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.9185).
“For all other outcomes evaluated, there was no evidence to suggest that patients were harmed by restrictive transfusion protocols, although the quality of the evidence was low for the outcomes of congestive heart failure and rebleeding,” the authors reported.
Based on these findings, they recommended a restrictive red blood cell transfusion threshold, in which transfusion is not indicated until the hemoglobin level is 7 g/dL for hospitalized adult patients who are hemodynamically stable, including critically ill patients.
However for patients undergoing orthopedic or cardiac surgery, or those with preexisting cardiovascular disease, they advised a threshold of 8 g/dL for initiating a red blood cell transfusion.
They also stressed that these recommendations did not apply to patients with acute coronary syndrome, those with severe thrombocytopenia, those treated for hematologic or oncologic disorders who at risk of bleeding, and those with chronic transfusion–dependent anemia, citing a lack of quality randomized controlled trial evidence.
The guideline authors examined the issue of the optimal length of time that red blood cell units should be stored, pointing out that there is currently no formal guidance on the optimal period of red blood cell storage prior to transfusion.
While units of red blood cells can be stored for up to 42 days, the committee said there was some evidence that longer storage may be associated with adverse transfusion outcomes.
“The RBCs stored for longer periods have decreased ability to deliver oxygen due to decreased levels of 2,3-diphsophoglycerate, decreased nitric oxide metabolism, alterations of the RBC membrane leading to increased rigidity, and increased RBC endothelial adherence,” they wrote.
Despite this, the review of 13 randomized controlled trials examining the effect of storage duration found no evidence that fresher units had any impact on mortality compared to standard issue units, nor were there any more adverse events with the standard issue units.
The absolute difference in 30-day mortality was four more deaths per 1,000 with fresher blood, and there was a higher risk of nosocomial infections among patients who received fresher red blood cell units although the authors said the quality of evidence was low.
They therefore recommended that no preference be given to fresher red blood cell units, and that all patients be treated with units chosen at any point within their licensed dating period.
Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies, but no other conflicts of interest were declared.
New guidelines on red blood cell blood transfusion recommend a restrictive threshold in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL for most patients, finding that it is safe in most clinical settings.
The updated clinical practice guidelines on transfusion thresholds and storage from the AABB (formerly known as the American Association of Blood Banks), also note that red blood cell units can be used at any time within their licensed dating period, rather than a preference being given to fresher units less than 10 days old.
The guidelines, published online Oct. 12 in JAMA, are an update of the 2012 transfusion guidelines, and are a response to a more than doubling of the number of patients since enrolled in randomized controlled trials of red blood cell transfusions.
The AABB’s clinical transfusion medicine committee, led by Jeffrey L. Carson, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J., analyzed data from 31 randomized controlled trials of 12,587 participants, which compared restrictive transfusion thresholds of 7-8 g/dL to more liberal thresholds of 9-10 g/dL.
This analysis showed that the use of restrictive transfusion protocols was associated with an absolute difference in 30-day mortality of three fewer deaths compared to the more liberal thresholds. There was no significant difference in 30-day mortality in trials that compared a threshold of 8-9 g/dL to a threshold of less than 7 g/dL (JAMA 2016, Oct 12. doi: 10.1001/jama.2016.9185).
“For all other outcomes evaluated, there was no evidence to suggest that patients were harmed by restrictive transfusion protocols, although the quality of the evidence was low for the outcomes of congestive heart failure and rebleeding,” the authors reported.
Based on these findings, they recommended a restrictive red blood cell transfusion threshold, in which transfusion is not indicated until the hemoglobin level is 7 g/dL for hospitalized adult patients who are hemodynamically stable, including critically ill patients.
However for patients undergoing orthopedic or cardiac surgery, or those with preexisting cardiovascular disease, they advised a threshold of 8 g/dL for initiating a red blood cell transfusion.
They also stressed that these recommendations did not apply to patients with acute coronary syndrome, those with severe thrombocytopenia, those treated for hematologic or oncologic disorders who at risk of bleeding, and those with chronic transfusion–dependent anemia, citing a lack of quality randomized controlled trial evidence.
The guideline authors examined the issue of the optimal length of time that red blood cell units should be stored, pointing out that there is currently no formal guidance on the optimal period of red blood cell storage prior to transfusion.
While units of red blood cells can be stored for up to 42 days, the committee said there was some evidence that longer storage may be associated with adverse transfusion outcomes.
“The RBCs stored for longer periods have decreased ability to deliver oxygen due to decreased levels of 2,3-diphsophoglycerate, decreased nitric oxide metabolism, alterations of the RBC membrane leading to increased rigidity, and increased RBC endothelial adherence,” they wrote.
Despite this, the review of 13 randomized controlled trials examining the effect of storage duration found no evidence that fresher units had any impact on mortality compared to standard issue units, nor were there any more adverse events with the standard issue units.
The absolute difference in 30-day mortality was four more deaths per 1,000 with fresher blood, and there was a higher risk of nosocomial infections among patients who received fresher red blood cell units although the authors said the quality of evidence was low.
They therefore recommended that no preference be given to fresher red blood cell units, and that all patients be treated with units chosen at any point within their licensed dating period.
Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies, but no other conflicts of interest were declared.
FROM JAMA
Key clinical point: A restrictive threshold for red blood cell transfusion, in which transfusion is not indicated until the hemoglobin level is 7-8 g/dL, is now recommended for most patients.
Major finding: A more restrictive threshold for red blood cell transfusion is not associated with an increased risk of mortality or other adverse outcomes from transfusion.
Data source: Updated guidelines from the AABB (formerly known as the American Association of Blood Banks).
Disclosures: Guideline development was supported by AABB. Four authors declared grants, fees, stock options or consultancies from pharmaceutical companies including CSL and Fresenius Kabi, but no other conflicts of interest were declared.
Expert panel offers treatment recommendations in Waldenström macroglobulinemia
Treatment recommendations for Waldenström macroglobulinemia have been updated based on the advice of a task force convened at the Eighth International Workshop on Waldenström Macroglobulinemia; the guidelines have been published in Blood.
The task force was impaneled to review recently published and ongoing clinical trial data as well as the impact of the newly recognized mutations MYD88 and CXCR4 on treatment decisions, indications for B-cell receptor and proteasome inhibitors, and future clinical trial initiatives.
The panel reiterated that the criteria for initiating therapy include immunoglobulin M (IgM)-related complications and/or symptoms that are related to direct involvement of the bone marrow by tumor cells, constitutional symptoms, and bulky extramedullary disease. Patients presenting with symptoms that include symptomatic hyperviscosity, moderate to severe hemolytic anemia, and symptomatic cryoglobulinemia need immediate treatment.
Close observation is recommended for the subgroup of patients who do not really fulfill the criteria for a diagnosis of Waldenström macroglobulinemia (WM), and whose laboratory findings may be the only indicator of the presence of a progressive disease.
Treatment recommendations
For symptomatic patients in the first-line setting, “anti-CD20 monoclonal antibody therapy alone or in combination with chemotherapy is an important standard of care for most patients with WM,” the authors, led by Veronique Leblond, MD, of Pitié-Salpêtrière Hôpital, Paris, wrote.
Rituximab is frequently used in WM, either as monotherapy or in combination with chemotherapeutic agents. The panel cautions that rituximab as monotherapy should be avoided in patients with high IgM levels, because of a lower chance of response and the risk of an IgM flare.
In patients with high IgM levels (typically around 4,000 mg/dL), plasmapheresis can be initiated before rituximab therapy, and plasmapheresis should always and immediately be used when symptomatic hyperviscosity is present. However, plasmapheresis alone is not an effective treatment for WM and must be followed by a rapidly acting cytoreductive regimen.
Several rituximab combinations are recommended by the panel. These include:
• Dexamethasone-rituximab-cyclophosphamide, which is an active and safe option, has a manageable toxicity, and can be considered for frail patients who need combination therapy.
• Bendamustine-rituximab is effective for front-line treatment and is well tolerated even in elderly patients who experience limited episodes of myelosuppression and infections.
Other therapeutic regimens include bortezomib-based therapy, which is recommended for patients with high IgM levels, symptomatic hyperviscosity, cryoglobulinemia or cold agglutinemia, amyloidosis, and renal impairment or in young patients who prefer to avoid alkylator or nucleoside analogue therapy.
Another option is carfilzomib-based therapy, which is an emerging “neuropathy-sparing” regimen for proteasome-inhibitor–based therapy, although it may not be the best choice for elderly patients with preexisting cardiac conditions due to potential cardiac toxicity.
Ibrutinib has been approved as a primary therapy for patients who are not candidates for chemoimmunotherapy, but the authors point out that the optimal use of this agent is still being investigated.
“The aim of the first-line treatments is to reach a high response rate with a prolonged progression-free survival,” write the authors. “The panel agrees that there is need to perform clinical trials with chemotherapy-free combinations with new compounds alone or in combination with anti-CD20 antibodies.”
For symptomatic previously treated patients
The panel also offered recommendations for previously treated symptomatic patients who have relapsed or are refractory to treatment.
Any of the interventions recommended for symptomatic, untreated patients can be considered for those who have already gone through first line therapy. Retreatment can be considered with a specific intervention if a response was achieved for 2 or more years with that therapy, although they caution that patients who have progressed on first-line ibrutinib should not use it again.
Ofatumumab is a potential option for patients who are unable to tolerate rituximab, and nucleoside analogues can be considered in fit patients who have not responded to less-toxic treatments.
Another option in this setting is everolimus, although since it is associated with considerable toxicities, the best candidates for this drug are those who have not responded to or have progressed after multiple lines of other better-tolerated regimens.
Immunomodulatory agents can also be considered, but in the context of a clinical trial only, because of their potential adverse events.
Finally, the panel also agreed that stem cell transplantation should be discussed with select patients, and while it is a feasible and effective treatment option for high-risk WM patients, it should be ideally offered at early relapse.
Investigating B-cell receptor (BCR) pathway inhibitors along with existing and novel compounds in patients in the relapsed/refractory setting should be a priority, according to the panel.
“BCR inhibitors, combined with proteasome inhibitors, would be of interest for overcoming resistance by interfering with the two key pathways that are affected by MYD88,” wrote Dr. Leblond and coauthors.
Treatment recommendations for Waldenström macroglobulinemia have been updated based on the advice of a task force convened at the Eighth International Workshop on Waldenström Macroglobulinemia; the guidelines have been published in Blood.
The task force was impaneled to review recently published and ongoing clinical trial data as well as the impact of the newly recognized mutations MYD88 and CXCR4 on treatment decisions, indications for B-cell receptor and proteasome inhibitors, and future clinical trial initiatives.
The panel reiterated that the criteria for initiating therapy include immunoglobulin M (IgM)-related complications and/or symptoms that are related to direct involvement of the bone marrow by tumor cells, constitutional symptoms, and bulky extramedullary disease. Patients presenting with symptoms that include symptomatic hyperviscosity, moderate to severe hemolytic anemia, and symptomatic cryoglobulinemia need immediate treatment.
Close observation is recommended for the subgroup of patients who do not really fulfill the criteria for a diagnosis of Waldenström macroglobulinemia (WM), and whose laboratory findings may be the only indicator of the presence of a progressive disease.
Treatment recommendations
For symptomatic patients in the first-line setting, “anti-CD20 monoclonal antibody therapy alone or in combination with chemotherapy is an important standard of care for most patients with WM,” the authors, led by Veronique Leblond, MD, of Pitié-Salpêtrière Hôpital, Paris, wrote.
Rituximab is frequently used in WM, either as monotherapy or in combination with chemotherapeutic agents. The panel cautions that rituximab as monotherapy should be avoided in patients with high IgM levels, because of a lower chance of response and the risk of an IgM flare.
In patients with high IgM levels (typically around 4,000 mg/dL), plasmapheresis can be initiated before rituximab therapy, and plasmapheresis should always and immediately be used when symptomatic hyperviscosity is present. However, plasmapheresis alone is not an effective treatment for WM and must be followed by a rapidly acting cytoreductive regimen.
Several rituximab combinations are recommended by the panel. These include:
• Dexamethasone-rituximab-cyclophosphamide, which is an active and safe option, has a manageable toxicity, and can be considered for frail patients who need combination therapy.
• Bendamustine-rituximab is effective for front-line treatment and is well tolerated even in elderly patients who experience limited episodes of myelosuppression and infections.
Other therapeutic regimens include bortezomib-based therapy, which is recommended for patients with high IgM levels, symptomatic hyperviscosity, cryoglobulinemia or cold agglutinemia, amyloidosis, and renal impairment or in young patients who prefer to avoid alkylator or nucleoside analogue therapy.
Another option is carfilzomib-based therapy, which is an emerging “neuropathy-sparing” regimen for proteasome-inhibitor–based therapy, although it may not be the best choice for elderly patients with preexisting cardiac conditions due to potential cardiac toxicity.
Ibrutinib has been approved as a primary therapy for patients who are not candidates for chemoimmunotherapy, but the authors point out that the optimal use of this agent is still being investigated.
“The aim of the first-line treatments is to reach a high response rate with a prolonged progression-free survival,” write the authors. “The panel agrees that there is need to perform clinical trials with chemotherapy-free combinations with new compounds alone or in combination with anti-CD20 antibodies.”
For symptomatic previously treated patients
The panel also offered recommendations for previously treated symptomatic patients who have relapsed or are refractory to treatment.
Any of the interventions recommended for symptomatic, untreated patients can be considered for those who have already gone through first line therapy. Retreatment can be considered with a specific intervention if a response was achieved for 2 or more years with that therapy, although they caution that patients who have progressed on first-line ibrutinib should not use it again.
Ofatumumab is a potential option for patients who are unable to tolerate rituximab, and nucleoside analogues can be considered in fit patients who have not responded to less-toxic treatments.
Another option in this setting is everolimus, although since it is associated with considerable toxicities, the best candidates for this drug are those who have not responded to or have progressed after multiple lines of other better-tolerated regimens.
Immunomodulatory agents can also be considered, but in the context of a clinical trial only, because of their potential adverse events.
Finally, the panel also agreed that stem cell transplantation should be discussed with select patients, and while it is a feasible and effective treatment option for high-risk WM patients, it should be ideally offered at early relapse.
Investigating B-cell receptor (BCR) pathway inhibitors along with existing and novel compounds in patients in the relapsed/refractory setting should be a priority, according to the panel.
“BCR inhibitors, combined with proteasome inhibitors, would be of interest for overcoming resistance by interfering with the two key pathways that are affected by MYD88,” wrote Dr. Leblond and coauthors.
Treatment recommendations for Waldenström macroglobulinemia have been updated based on the advice of a task force convened at the Eighth International Workshop on Waldenström Macroglobulinemia; the guidelines have been published in Blood.
The task force was impaneled to review recently published and ongoing clinical trial data as well as the impact of the newly recognized mutations MYD88 and CXCR4 on treatment decisions, indications for B-cell receptor and proteasome inhibitors, and future clinical trial initiatives.
The panel reiterated that the criteria for initiating therapy include immunoglobulin M (IgM)-related complications and/or symptoms that are related to direct involvement of the bone marrow by tumor cells, constitutional symptoms, and bulky extramedullary disease. Patients presenting with symptoms that include symptomatic hyperviscosity, moderate to severe hemolytic anemia, and symptomatic cryoglobulinemia need immediate treatment.
Close observation is recommended for the subgroup of patients who do not really fulfill the criteria for a diagnosis of Waldenström macroglobulinemia (WM), and whose laboratory findings may be the only indicator of the presence of a progressive disease.
Treatment recommendations
For symptomatic patients in the first-line setting, “anti-CD20 monoclonal antibody therapy alone or in combination with chemotherapy is an important standard of care for most patients with WM,” the authors, led by Veronique Leblond, MD, of Pitié-Salpêtrière Hôpital, Paris, wrote.
Rituximab is frequently used in WM, either as monotherapy or in combination with chemotherapeutic agents. The panel cautions that rituximab as monotherapy should be avoided in patients with high IgM levels, because of a lower chance of response and the risk of an IgM flare.
In patients with high IgM levels (typically around 4,000 mg/dL), plasmapheresis can be initiated before rituximab therapy, and plasmapheresis should always and immediately be used when symptomatic hyperviscosity is present. However, plasmapheresis alone is not an effective treatment for WM and must be followed by a rapidly acting cytoreductive regimen.
Several rituximab combinations are recommended by the panel. These include:
• Dexamethasone-rituximab-cyclophosphamide, which is an active and safe option, has a manageable toxicity, and can be considered for frail patients who need combination therapy.
• Bendamustine-rituximab is effective for front-line treatment and is well tolerated even in elderly patients who experience limited episodes of myelosuppression and infections.
Other therapeutic regimens include bortezomib-based therapy, which is recommended for patients with high IgM levels, symptomatic hyperviscosity, cryoglobulinemia or cold agglutinemia, amyloidosis, and renal impairment or in young patients who prefer to avoid alkylator or nucleoside analogue therapy.
Another option is carfilzomib-based therapy, which is an emerging “neuropathy-sparing” regimen for proteasome-inhibitor–based therapy, although it may not be the best choice for elderly patients with preexisting cardiac conditions due to potential cardiac toxicity.
Ibrutinib has been approved as a primary therapy for patients who are not candidates for chemoimmunotherapy, but the authors point out that the optimal use of this agent is still being investigated.
“The aim of the first-line treatments is to reach a high response rate with a prolonged progression-free survival,” write the authors. “The panel agrees that there is need to perform clinical trials with chemotherapy-free combinations with new compounds alone or in combination with anti-CD20 antibodies.”
For symptomatic previously treated patients
The panel also offered recommendations for previously treated symptomatic patients who have relapsed or are refractory to treatment.
Any of the interventions recommended for symptomatic, untreated patients can be considered for those who have already gone through first line therapy. Retreatment can be considered with a specific intervention if a response was achieved for 2 or more years with that therapy, although they caution that patients who have progressed on first-line ibrutinib should not use it again.
Ofatumumab is a potential option for patients who are unable to tolerate rituximab, and nucleoside analogues can be considered in fit patients who have not responded to less-toxic treatments.
Another option in this setting is everolimus, although since it is associated with considerable toxicities, the best candidates for this drug are those who have not responded to or have progressed after multiple lines of other better-tolerated regimens.
Immunomodulatory agents can also be considered, but in the context of a clinical trial only, because of their potential adverse events.
Finally, the panel also agreed that stem cell transplantation should be discussed with select patients, and while it is a feasible and effective treatment option for high-risk WM patients, it should be ideally offered at early relapse.
Investigating B-cell receptor (BCR) pathway inhibitors along with existing and novel compounds in patients in the relapsed/refractory setting should be a priority, according to the panel.
“BCR inhibitors, combined with proteasome inhibitors, would be of interest for overcoming resistance by interfering with the two key pathways that are affected by MYD88,” wrote Dr. Leblond and coauthors.
FROM BLOOD
Need-to-know information for the 2016-2017 flu season
The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
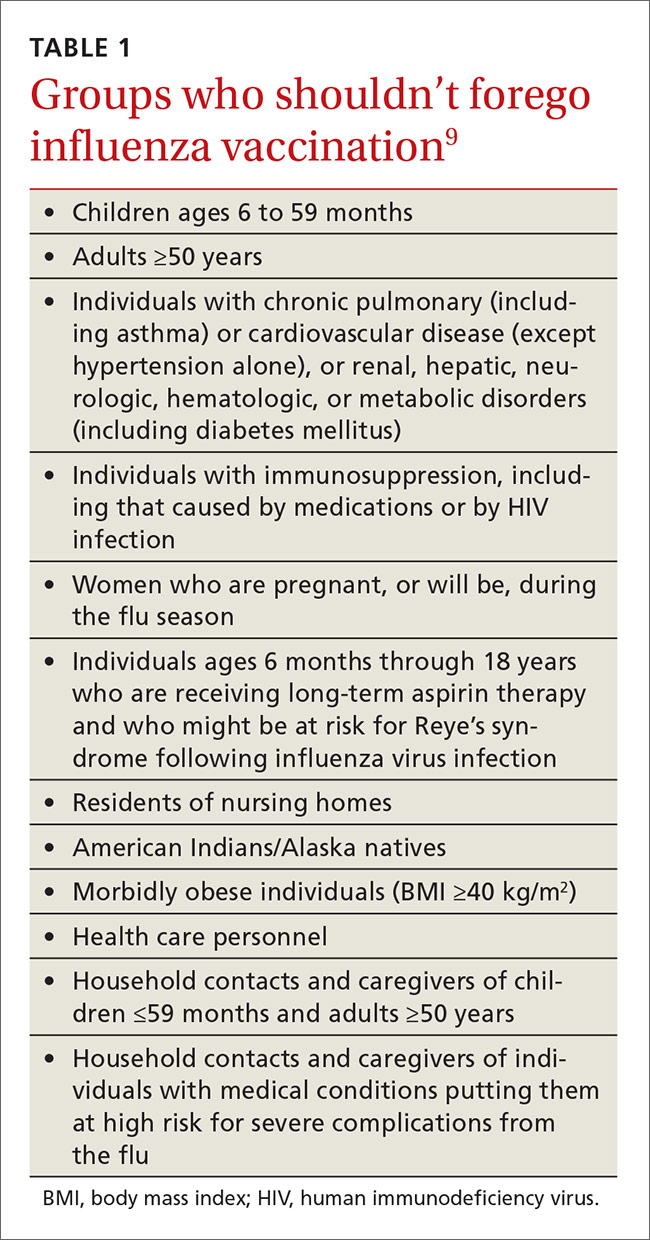
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9
There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
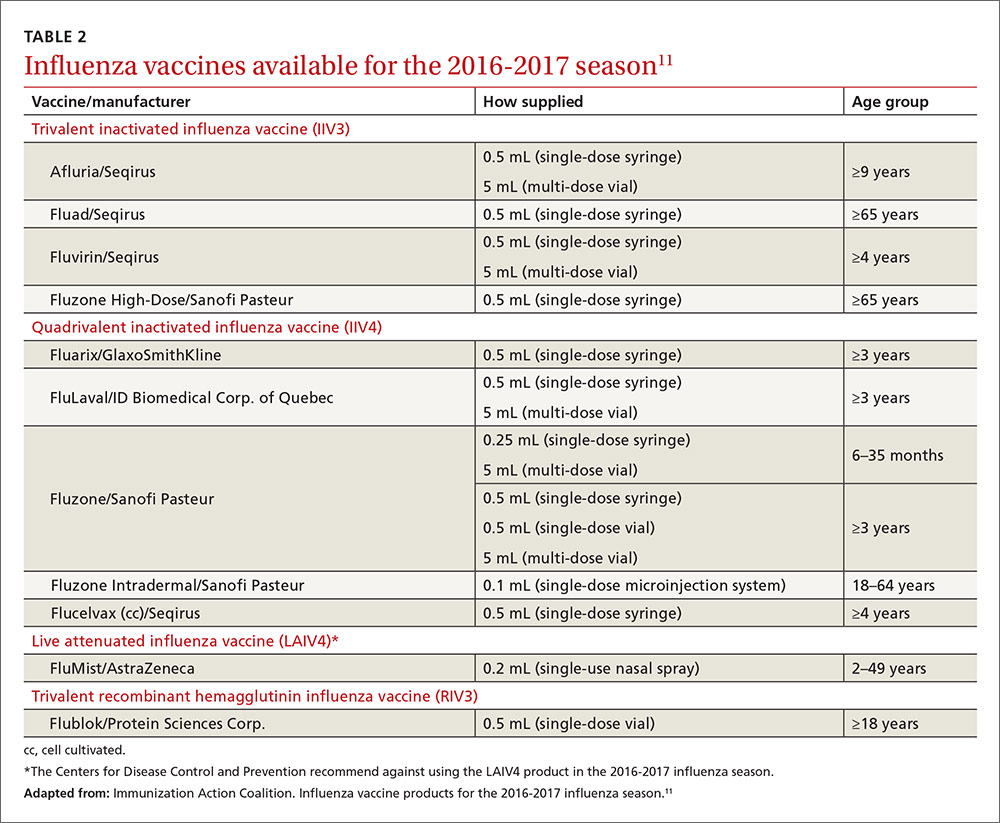
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.
Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.
Antiviral medications for treatment or prevention
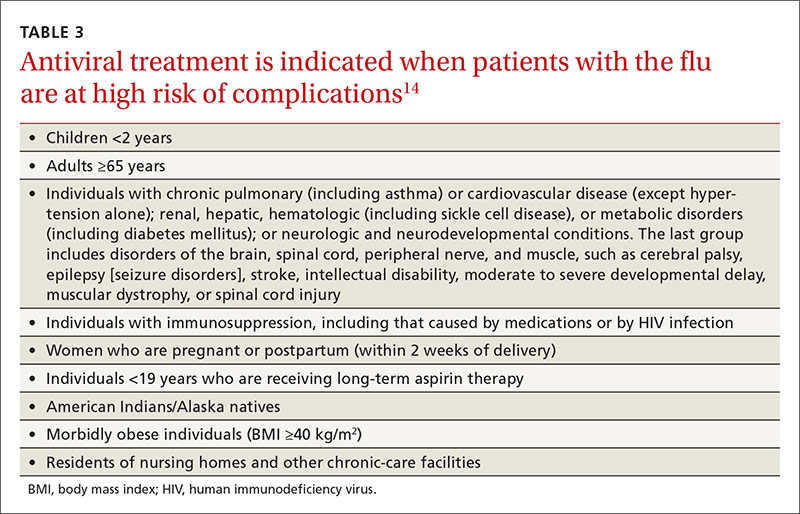
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15

1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.
The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9

There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.

Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.

Antiviral medications for treatment or prevention
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15

The Advisory Committee on Immunization Practices (ACIP) took the unusual step at its June 2016 meeting of recommending against using a currently licensed vaccine, live attenuated influenza vaccine (LAIV), in the 2016-2017 influenza season.1 ACIP based its recommendation on surveillance data collected by the US Influenza Vaccine Effectiveness Network of the Centers for Disease Control and Prevention (CDC), which showed poor effectiveness by the LAIV vaccine among children and adolescents during the past 3 years.
The US Food and Drug Administration (FDA), however, has chosen not to take any action on this matter, saying on its Web site it “has determined that specific regulatory action is not warranted at this time. This determination is based on FDA’s review of manufacturing and clinical data supporting licensure … the totality of the evidence presented at the ACIP meeting, taking into account the inherent limitations of observational studies conducted to evaluate influenza vaccine effectiveness, as well as the well-known variability of influenza vaccine effectiveness across influenza seasons.”2
CDC data for the 2015-2016 flu season showed the effectiveness of LAIV to be just 3% among children 2 years through 17 years of age.3 The reason for this apparent lack of effectiveness is unknown. Other LAIV-effectiveness studies conducted in the 2015-2016 season—one each, in the United States, United Kingdom, and Finland—had results that differed from the CDC surveillance data, with effectiveness ranging from 46% to 58% against all strains combined.2 These results are comparable to vaccine effectiveness found in observational studies in children for both LAIV and inactivated influenza vaccines (IIV) in prior seasons.2
Vaccine manufacturers had projected that 171 to 176 million doses of flu vaccine, in all forms, would be available in the United States during the 2016-2017 season.3 LAIV accounts for about 8% of the total supply of influenza vaccine in the United States,3 and ACIP’s recommendation is not expected to create shortages of other options for the upcoming season. However, the LAIV accounts for one-third of flu vaccines administered to children, and clinicians who provide vaccinations to children have already ordered their vaccine supplies for the upcoming season. Also, it is not clear if children who have previously received the LAIV product will now accept other options for influenza vaccination—all of which involve an injection.
Whether the recommendation against LAIV will continue after this season is also unknown.
What happened during the 2015-2016 influenza season?
The 2015-2016 influenza season was relatively mild with the peak activity occurring in March, somewhat later than in previous years. The circulating influenza strains matched closely to those in the vaccine, making it more effective than the previous year’s vaccine. The predominant circulating strain was A (H1N1), accounting for 58% of illness; A (H3N2) caused 6% of cases and all B types together accounted for 34%.4 The hospitalization rate for all ages was 31.3/100,000 compared with 64.1 the year before.5 There were 85 pediatric deaths compared with 148 in 2014-2015.6
Vaccine effectiveness among all age groups and against all circulating strains was 47%.4 No major vaccine safety concerns were detected. Among those who received IIV3, there was a slight increase in the incidence of Guillain-Barré syndrome of 2.6 cases per one million vaccines.7
Other recommendations for 2016-2017
Once again, ACIP recommends influenza vaccine for all individuals 6 months and older.8 The CDC additionally specifies particular groups that should not skip vaccination given that they are at high risk of complications from influenza infection or because they could expose high-risk individuals to infection (TABLE 1).9

There will continue to be a selection of trivalent and quadrivalent influenza vaccine products in 2016-2017. Trivalent products will contain 3 viral strains: A/California/7/2009 (H1N1), A/Hong Kong/4801/2014 (H3N2) and B/Brisbane/60/2008.10 The quadrivalent products will contain those 3 antigens plus B/Phuket/3073/2013.10 The H3N2 strain is different from the one in last year’s vaccine. Each year, influenza experts analyze surveillance data to predict which circulating strains will predominate in North America, and these antigens constitute the vaccine formulation. The accuracy of this prediction in large part determines how effective the vaccine will be that season.
Two new vaccines have been approved for use in the United States. A quadrivalent cell culture inactivated vaccine (CCIV4), Flucelvax, was licensed in May 2016. It is prepared from virus propagated in canine kidney cells, not with an egg-based production process. It is approved for use in individuals 4 years of age and older.8 Fluad, an adjuvanted trivalent inactivated influenza vaccine, was licensed in late 2015 for individuals 65 years of age and older.8 This is the first adjuvanted influenza vaccine licensed in the United States and will compete with high-dose quadrivalent vaccine for use in older adults. ACIP does not express a preference for any vaccine in this age group.
Two other vaccines should also be available by this fall: Flublok, a quadrivalent recombinant influenza vaccine for individuals 18 years and older, and Flulaval, a quadrivalent inactivated influenza vaccine, for individuals 6 months of age and older. TABLE 211 lists approved influenza vaccines.

Issues specific to children
Deciding how many vaccine doses children need has been further simplified. Children younger than 9 years need 2 doses if they have received fewer than 2 doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The interval between the 2 doses should be at least 4 weeks. The 2 doses do not have to be the same product; importantly, do not delay a second dose just to obtain the same product used for the first dose. Also, one dose can be trivalent and the other one quadrivalent, although this offers less-than-optimal protection against the B-virus that is only in the quadrivalent product.
Children younger than 9 years require only one dose if they have received 2 or more total doses of trivalent or quadrivalent influenza vaccine before July 1, 2016. The 2 previous doses need not have been received during the same influenza season or consecutive influenza seasons.
In children ages 6 through 23 months there is a slight increased risk of febrile seizure if the influenza vaccine is co-administered with other vaccines, specifically pneumococcal conjugate vaccine (PCV 13) and diphtheria-tetanus-acellular-pertussis (DTaP). The 3 vaccines administered at the same time result in 30 febrile seizures per 100,000 children;12 the rate is lower when influenza vaccine is co-administered with only one of the others. ACIP believes that the risk of a febrile seizure, which does no long-term harm, does not warrant delaying vaccines that could be co-administered.13
Egg allergy requires no special precautions
Evidence continues to grow that influenza vaccine products do not contain enough egg protein to cause significant problems in those with a history of egg allergies. This year’s recommendations state that no special precautions are needed regarding the anatomic site of immunization or the length of observation after administering influenza vaccine in those with a history of allergies to eggs, no matter how severe. All vaccine-administration facilities should be able to respond to any hypersensitivity reaction, and the standard waiting time for observation after all vaccinations is 15 minutes.

Antiviral medications for treatment or prevention
Most influenza strains circulating in 2016-2017 are expected to remain sensitive to oseltamivir and zanamivir, which can be used for treatment or disease prevention. A third neuraminidase inhibitor, peramivir, is available for intravenous use in adults 18 and older. Treatment is recommended for those who have confirmed or suspected influenza and are at high risk for complications (TABLE 3).14 Consideration of antiviral chemoprevention is recommended under certain circumstances (TABLE 4).15,16 The CDC influenza Web site lists recommended doses and duration for each antiviral for treatment and chemoprevention.15

1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.
1. Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2016-17 influenza season. MMWR Recomm Rep. 2016;65:1-54.
2. U.S. Food and Drug Administration. FDA information regarding FluMist quadrivalent vaccine. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm508761.htm. Accessed July 13, 2016.
3. Centers for Disease Control and Prevention. ACIP votes down use of LAIV for 2016-2017 flu season. Available at: http://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed July 13, 2016.
4. Flannery B, Chung J. Influenza vaccine effectiveness, including LAIV vs IIV in children and adolescents, US Flu VE Network, 2015-2016. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-05-flannery.pdf. Accessed July 22, 2016.
5. Centers for Disease Control and Prevention. FluView. Laboratory-confirmed influenza hospitalizations. Available at: http://gis.cdc.gov/GRASP/Fluview/FluHospRates.html. Accessed July 25, 2016.
6. Centers for Disease Control and Prevention. FluView. Number of influenza-associated pediatric deaths by week of death. Available at: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html. Accessed July 25, 2016.
7. Shimabukuro T. End-of-season update: 2015-2016 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-04-shimabukuro.pdf. Accessed July 22, 2016.
8. Grohskopf L. Proposed recommendations 2016-2017 influenza season. Presented at: meeting of the Advisory Committee on Immunization Practices; June 22, 2016; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2016-06/influenza-08-grohskopf.pdf. Accessed July 22, 2016.
9. Centers for Disease Control and Prevention. Influenza vaccination: a summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/vaccination/vax-summary.htm. Accessed July 13, 2016.
10. Centers for Disease Control and Prevention. What you should know for the 2016-2017 influenza season. Available at: http://www.cdc.gov/flu/about/season/flu-season-2016-2017.htm. Accessed July 13, 2016.
11. Immunization Action Coalition. Influenza vaccine products for the 2016-2017 influenza season. Available at: http://www.immunize.org/catg.d/p4072.pdf. Accessed July 13, 2016.
12. Duffy J, Weintraub E, Hambidge SJ, et al. Febrile seizure risk after vaccination in children 6 to 23 months. Pediatrics. 2016;138.
13. Centers for Disease Control and Prevention. Childhood vaccines and febrile seizures. Available at: http://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html. Accessed August 11, 2016.
14. Centers for Disease Control and Prevention. Use of antivirals. Background and guidance on the use of influenza antiviral agents. Available at: http://www.cdc.gov/flu/professionals/antivirals/antiviral-use-influenza.htm. Accessed July 13, 2016.
15. Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at: http://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed July 13, 2016.
16. American Academy of Pediatrics. Recommendations for prevention and control of influenza in children, 2015-2016. Pediatrics. 2015;136:792-808.
ASCO: Always screen cancer survivors for chronic pain
All adult cancer survivors should be screened for chronic pain at every visit, according to the American Society of Clinical Oncology’s first clinical practice guideline for managing this patient population, published July 25 in the Journal of Clinical Oncology.
An estimated 14 million adults with a history of cancer are living in the United States alone, and the prevalence of chronic pain related to their malignancy is reported to be as high as 40%. Yet most health care providers “haven’t been trained to recognize or treat long-term pain associated with cancer,” Judith A. Paice, PhD, RN, said in a press statement accompanying the release of the new guideline.
Existing guidelines focus on the acute pain of cancer and its treatment, or on acute pain related to advanced cancer, said Dr. Paice, cochair of the expert panel that developed the guideline and research professor of hematology/oncology at Northwestern University, Chicago.
The chronic pain addressed in the new guideline document can be related to the malignancy itself or to its treatment. Surgery, chemotherapy, hormone therapy, radiation treatment, and stem-cell transplantation can induce dozens of pain-producing complications, including osteonecrosis, bone fractures, peripheral or central nerve damage, fistulas, lymphedema, arthralgias, and myelopathy. The pain can develop months or years after diagnosis, significantly impacting patients’ quality of life well after treatment is completed.
“This guideline will help clinicians identify pain early and develop comprehensive treatment plans using a broad range of approaches,” Dr. Paice said in the statement.
The panel that developed the guideline comprised experts in medical oncology, hematology, pain medicine, palliative care, hospice, radiation oncology, social work, rehabilitation, psychology, and anesthesiology. They performed a systematic review of the literature and based their recommendations on 35 meta-analyses, 9 randomized controlled trials, 19 comparative studies, and expert consensus.
Some key recommendations include the following:
• Screen all survivors of adult cancers for chronic pain using both a physical exam and an in-depth interview that covers physical, functional, psychological, social, and spiritual aspects of pain, as well as the patient’s treatment history and comorbid conditions. The guideline includes a list of 36 common pain syndromes resulting from cancer treatments.
• Monitor patients at every visit for late-onset treatment effects that can produce pain, just as they are monitored for recurrent disease and secondary malignancy. Some cancer survivors may not recognize that their current pain is related to past disease or its treatments, or may consider it an untreatable complication that they have to endure.
• Consider the entire range of pain medicines – not just opioid analgesics – including NSAIDs, acetaminophen, and selected antidepressants and anticonvulsants with analgesic efficacy. The panel noted that some cancer survivors with chronic pain may benefit from other drugs, including muscle relaxants, benzodiazepines, N-methyl-D-aspartate receptor blockers, alpha-2 agonists, and neutraceuticals and botanicals. But they urged caution since the efficacy of these agents has not been fully established.
Consider nonmedical treatments such as physical, occupational, and recreation therapy; exercise; orthotics; ultrasound; massage; acupuncture; cognitive behavioral therapy; relaxation therapy; and TENS or other types of nerve stimulation.
Consider medical cannabis or cannabinoids as an adjuvant but not a first-line pain treatment, being sure to follow specific state regulations.
Consider a trial of opioids only for carefully selected patients who don’t respond to more conservative management. Weigh potential risks against benefits and employ universal precautions to minimize abuse and addiction, and be cautious in coprescribing other centrally-acting agents (J Clin Oncol. 2016 July 25. doi: 10.1200/JCO.2016.68.5206).
The full clinical practice guideline and other clinical tools and resources are available at www.asco.org/chronic-pai-guideline.
The guideline development process was supported by ASCO. Dr. Paice reported having no relevant financial disclosures; some of her associates reported financial ties to industry sources.
All adult cancer survivors should be screened for chronic pain at every visit, according to the American Society of Clinical Oncology’s first clinical practice guideline for managing this patient population, published July 25 in the Journal of Clinical Oncology.
An estimated 14 million adults with a history of cancer are living in the United States alone, and the prevalence of chronic pain related to their malignancy is reported to be as high as 40%. Yet most health care providers “haven’t been trained to recognize or treat long-term pain associated with cancer,” Judith A. Paice, PhD, RN, said in a press statement accompanying the release of the new guideline.
Existing guidelines focus on the acute pain of cancer and its treatment, or on acute pain related to advanced cancer, said Dr. Paice, cochair of the expert panel that developed the guideline and research professor of hematology/oncology at Northwestern University, Chicago.
The chronic pain addressed in the new guideline document can be related to the malignancy itself or to its treatment. Surgery, chemotherapy, hormone therapy, radiation treatment, and stem-cell transplantation can induce dozens of pain-producing complications, including osteonecrosis, bone fractures, peripheral or central nerve damage, fistulas, lymphedema, arthralgias, and myelopathy. The pain can develop months or years after diagnosis, significantly impacting patients’ quality of life well after treatment is completed.
“This guideline will help clinicians identify pain early and develop comprehensive treatment plans using a broad range of approaches,” Dr. Paice said in the statement.
The panel that developed the guideline comprised experts in medical oncology, hematology, pain medicine, palliative care, hospice, radiation oncology, social work, rehabilitation, psychology, and anesthesiology. They performed a systematic review of the literature and based their recommendations on 35 meta-analyses, 9 randomized controlled trials, 19 comparative studies, and expert consensus.
Some key recommendations include the following:
• Screen all survivors of adult cancers for chronic pain using both a physical exam and an in-depth interview that covers physical, functional, psychological, social, and spiritual aspects of pain, as well as the patient’s treatment history and comorbid conditions. The guideline includes a list of 36 common pain syndromes resulting from cancer treatments.
• Monitor patients at every visit for late-onset treatment effects that can produce pain, just as they are monitored for recurrent disease and secondary malignancy. Some cancer survivors may not recognize that their current pain is related to past disease or its treatments, or may consider it an untreatable complication that they have to endure.
• Consider the entire range of pain medicines – not just opioid analgesics – including NSAIDs, acetaminophen, and selected antidepressants and anticonvulsants with analgesic efficacy. The panel noted that some cancer survivors with chronic pain may benefit from other drugs, including muscle relaxants, benzodiazepines, N-methyl-D-aspartate receptor blockers, alpha-2 agonists, and neutraceuticals and botanicals. But they urged caution since the efficacy of these agents has not been fully established.
Consider nonmedical treatments such as physical, occupational, and recreation therapy; exercise; orthotics; ultrasound; massage; acupuncture; cognitive behavioral therapy; relaxation therapy; and TENS or other types of nerve stimulation.
Consider medical cannabis or cannabinoids as an adjuvant but not a first-line pain treatment, being sure to follow specific state regulations.
Consider a trial of opioids only for carefully selected patients who don’t respond to more conservative management. Weigh potential risks against benefits and employ universal precautions to minimize abuse and addiction, and be cautious in coprescribing other centrally-acting agents (J Clin Oncol. 2016 July 25. doi: 10.1200/JCO.2016.68.5206).
The full clinical practice guideline and other clinical tools and resources are available at www.asco.org/chronic-pai-guideline.
The guideline development process was supported by ASCO. Dr. Paice reported having no relevant financial disclosures; some of her associates reported financial ties to industry sources.
All adult cancer survivors should be screened for chronic pain at every visit, according to the American Society of Clinical Oncology’s first clinical practice guideline for managing this patient population, published July 25 in the Journal of Clinical Oncology.
An estimated 14 million adults with a history of cancer are living in the United States alone, and the prevalence of chronic pain related to their malignancy is reported to be as high as 40%. Yet most health care providers “haven’t been trained to recognize or treat long-term pain associated with cancer,” Judith A. Paice, PhD, RN, said in a press statement accompanying the release of the new guideline.
Existing guidelines focus on the acute pain of cancer and its treatment, or on acute pain related to advanced cancer, said Dr. Paice, cochair of the expert panel that developed the guideline and research professor of hematology/oncology at Northwestern University, Chicago.
The chronic pain addressed in the new guideline document can be related to the malignancy itself or to its treatment. Surgery, chemotherapy, hormone therapy, radiation treatment, and stem-cell transplantation can induce dozens of pain-producing complications, including osteonecrosis, bone fractures, peripheral or central nerve damage, fistulas, lymphedema, arthralgias, and myelopathy. The pain can develop months or years after diagnosis, significantly impacting patients’ quality of life well after treatment is completed.
“This guideline will help clinicians identify pain early and develop comprehensive treatment plans using a broad range of approaches,” Dr. Paice said in the statement.
The panel that developed the guideline comprised experts in medical oncology, hematology, pain medicine, palliative care, hospice, radiation oncology, social work, rehabilitation, psychology, and anesthesiology. They performed a systematic review of the literature and based their recommendations on 35 meta-analyses, 9 randomized controlled trials, 19 comparative studies, and expert consensus.
Some key recommendations include the following:
• Screen all survivors of adult cancers for chronic pain using both a physical exam and an in-depth interview that covers physical, functional, psychological, social, and spiritual aspects of pain, as well as the patient’s treatment history and comorbid conditions. The guideline includes a list of 36 common pain syndromes resulting from cancer treatments.
• Monitor patients at every visit for late-onset treatment effects that can produce pain, just as they are monitored for recurrent disease and secondary malignancy. Some cancer survivors may not recognize that their current pain is related to past disease or its treatments, or may consider it an untreatable complication that they have to endure.
• Consider the entire range of pain medicines – not just opioid analgesics – including NSAIDs, acetaminophen, and selected antidepressants and anticonvulsants with analgesic efficacy. The panel noted that some cancer survivors with chronic pain may benefit from other drugs, including muscle relaxants, benzodiazepines, N-methyl-D-aspartate receptor blockers, alpha-2 agonists, and neutraceuticals and botanicals. But they urged caution since the efficacy of these agents has not been fully established.
Consider nonmedical treatments such as physical, occupational, and recreation therapy; exercise; orthotics; ultrasound; massage; acupuncture; cognitive behavioral therapy; relaxation therapy; and TENS or other types of nerve stimulation.
Consider medical cannabis or cannabinoids as an adjuvant but not a first-line pain treatment, being sure to follow specific state regulations.
Consider a trial of opioids only for carefully selected patients who don’t respond to more conservative management. Weigh potential risks against benefits and employ universal precautions to minimize abuse and addiction, and be cautious in coprescribing other centrally-acting agents (J Clin Oncol. 2016 July 25. doi: 10.1200/JCO.2016.68.5206).
The full clinical practice guideline and other clinical tools and resources are available at www.asco.org/chronic-pai-guideline.
The guideline development process was supported by ASCO. Dr. Paice reported having no relevant financial disclosures; some of her associates reported financial ties to industry sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Screen all survivors of adult cancers for chronic pain at every visit.
Major finding: An estimated 14 million adults with a history of cancer are living in the United States alone, and the prevalence of chronic pain related to their malignancy is reported to be as high as 40%.
Data source: The first ASCO clinical practice guideline for managing chronic pain in survivors of adult cancers.
Disclosures: This work was supported by ASCO. Dr. Paice reported having no relevant financial disclosures; some of her associates reported financial ties to industry.
USPSTF update: Screening for abnormal blood glucose, diabetes
In December 2015, the United States Preventive Services Task Force updated its recommendation on screening for abnormal blood glucose and diabetes to say that clinicians should screen all adults ages 40 to 70 years who are overweight or obese as part of a cardiovascular risk assessment.1 This recommendation carries a B grade signifying a moderate certainty that a moderate net benefit will be gained by detecting impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or diabetes, and by implementing intensive lifestyle interventions. In this article, as in the Task Force recommendation, the term diabetes means type 2 diabetes. Obesity is defined as a body mass index (BMI) of ≥30 kg/m2, and overweight as a BMI >25.
How the Task Force recommendation evolved
The previous Task Force recommendation on this topic, made in 2008, advised screening only adults with hypertension because there was no evidence that any other group benefited from screening. In subsequent years, there were calls for the Task Force to revise its recommendation to bring it more in line with that of the American Diabetes Association (ADA).2 While this new recommendation does add more adults to the cohort of those the Task Force believes should be screened, it is still not totally in concert with the ADA, which recommends screening all adults 45 years or older and those who are younger if they have multiple risk factors.3
Both the Task Force and the ADA acknowledge there is no direct evidence for any benefit in screening for diabetes in the general, asymptomatic population. The Task Force, with its standard of making recommendations only when good evidence supports them, has opted to address screening for abnormal glucose levels in the context of cardiovascular risk reduction and persuasive evidence that lifestyle interventions can reduce cardiovascular risks and slow progression to diabetes.
The ADA is willing to rely on less rigorous evidence of benefit in screening, diagnosing, and treating undetected diabetes. It believes that morbidity and mortality from this pervasive chronic disease can be reduced with early detection and treatment.
Still the Task Force and ADA agree more than they differ
While it appears that significant differences exist between the recommendations of the Task Force and the ADA, a closer look shows they actually have much in common; and, as they pertain to daily practice, any remaining differences are primarily ones of emphasis. For instance, the Clinical Considerations section of the Task Force recommendation acknowledges that certain people are at increased risk for diabetes at younger ages and at a lower BMI, and that clinicians should “consider” screening them earlier than at age 40 years. The risks listed include a family history of diabetes or a personal history of gestational diabetes or polycystic ovarian syndrome; or being African American, Hispanic, Asian American, American Indian, Alaskan Native, or Native Hawaiian.
The Task Force statement seems to imply—although this is not entirely clear—that those who have these risks should also be screened if they are older than age 40 years even if they are not obese. So, although the ADA would screen everyone ages 45 and older, the Task Force would screen everyone ages 40 and older, except for non-Hispanic whites who are not overweight or obese, and who have no other risk factors. TABLE 11,3 details the Task Force and the ADA screening criteria and how they differ.
The Task Force and the ADA also agree on the 3 tests acceptable for screening and the test values that define normal glucose, IGT, IFG, and diabetes (TABLE 2).1,3 The tests are a randomly measured glycated hemoglobin level, a fasting plasma glucose level, and an oral glucose tolerance test performed in the morning after an overnight fast, with glucose measured 2 hours after a 75-g oral glucose load. If a screening result is abnormal, confirmation should be sought by repeating the same test. And both organizations suggest that, following a normal test result, the optimal interval for retesting is 3 years.
Intervening to delay progression to diabetes
For anyone with a confirmed abnormal blood glucose level, the Task Force advises referral for intensive behavioral interventions—ie, multiple counseling sessions over an extended period on a healthy diet and optimal physical activity. These types of interventions can reduce blood glucose levels and lower the risk of progression to diabetes, and can help with lowering weight, blood pressure, and lipid levels. The evidence report that preceded the recommendation pooled the results from 10 studies on lifestyle modification.4 The length of follow-up in these studies ranged from 3 to 23 years, and the number needed to treat to prevent one case of progression to diabetes ranged from about 5 to 20.4
Medications such as metformin, thiazolidinediones, and alpha-glucosidase inhibitors can also reduce blood glucose levels and slow progression to diabetes. However, the Task Force says there is insufficient evidence that pharmacologic interventions have the same multifactorial benefits—weight loss or reductions in glucose levels, blood pressure, and lipid levels—as behavioral interventions.1
As for the other modifiable risk factors for cardiovascular disease—obesity, lack of physical activity, high lipid levels, high blood pressure, and smoking—the Task Force has developed recommendations on screening for and treating each of them,5 which supplement the recommendations discussed in this article.
1. U.S. Preventive Services Task Force. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed May 20, 2016.
2. Casagrande SS, Cowie CC, Fradkin JE. Utility of the US Preventive Services Task Force criteria for diabetes screening. Am J Prev Med. 2013;45:167-174.
3. American Diabetes Association. Standards of medical care in diabetes - 2016. Diabetes Care. 2016;39(Suppl 1):S1–S112.
4. Selph S, Dana T, Bougatsos C, et al. A systematic review to update the 2008 U.S. Preventive Services Task Force recommendation [Agency for Healthcare Research and Quality]. 2015. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed May 20, 2016.
5. U.S. Preventive Services Task Force. Healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd. Accessed May 20,
2016.
In December 2015, the United States Preventive Services Task Force updated its recommendation on screening for abnormal blood glucose and diabetes to say that clinicians should screen all adults ages 40 to 70 years who are overweight or obese as part of a cardiovascular risk assessment.1 This recommendation carries a B grade signifying a moderate certainty that a moderate net benefit will be gained by detecting impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or diabetes, and by implementing intensive lifestyle interventions. In this article, as in the Task Force recommendation, the term diabetes means type 2 diabetes. Obesity is defined as a body mass index (BMI) of ≥30 kg/m2, and overweight as a BMI >25.
How the Task Force recommendation evolved
The previous Task Force recommendation on this topic, made in 2008, advised screening only adults with hypertension because there was no evidence that any other group benefited from screening. In subsequent years, there were calls for the Task Force to revise its recommendation to bring it more in line with that of the American Diabetes Association (ADA).2 While this new recommendation does add more adults to the cohort of those the Task Force believes should be screened, it is still not totally in concert with the ADA, which recommends screening all adults 45 years or older and those who are younger if they have multiple risk factors.3
Both the Task Force and the ADA acknowledge there is no direct evidence for any benefit in screening for diabetes in the general, asymptomatic population. The Task Force, with its standard of making recommendations only when good evidence supports them, has opted to address screening for abnormal glucose levels in the context of cardiovascular risk reduction and persuasive evidence that lifestyle interventions can reduce cardiovascular risks and slow progression to diabetes.
The ADA is willing to rely on less rigorous evidence of benefit in screening, diagnosing, and treating undetected diabetes. It believes that morbidity and mortality from this pervasive chronic disease can be reduced with early detection and treatment.
Still the Task Force and ADA agree more than they differ
While it appears that significant differences exist between the recommendations of the Task Force and the ADA, a closer look shows they actually have much in common; and, as they pertain to daily practice, any remaining differences are primarily ones of emphasis. For instance, the Clinical Considerations section of the Task Force recommendation acknowledges that certain people are at increased risk for diabetes at younger ages and at a lower BMI, and that clinicians should “consider” screening them earlier than at age 40 years. The risks listed include a family history of diabetes or a personal history of gestational diabetes or polycystic ovarian syndrome; or being African American, Hispanic, Asian American, American Indian, Alaskan Native, or Native Hawaiian.
The Task Force statement seems to imply—although this is not entirely clear—that those who have these risks should also be screened if they are older than age 40 years even if they are not obese. So, although the ADA would screen everyone ages 45 and older, the Task Force would screen everyone ages 40 and older, except for non-Hispanic whites who are not overweight or obese, and who have no other risk factors. TABLE 11,3 details the Task Force and the ADA screening criteria and how they differ.
The Task Force and the ADA also agree on the 3 tests acceptable for screening and the test values that define normal glucose, IGT, IFG, and diabetes (TABLE 2).1,3 The tests are a randomly measured glycated hemoglobin level, a fasting plasma glucose level, and an oral glucose tolerance test performed in the morning after an overnight fast, with glucose measured 2 hours after a 75-g oral glucose load. If a screening result is abnormal, confirmation should be sought by repeating the same test. And both organizations suggest that, following a normal test result, the optimal interval for retesting is 3 years.
Intervening to delay progression to diabetes
For anyone with a confirmed abnormal blood glucose level, the Task Force advises referral for intensive behavioral interventions—ie, multiple counseling sessions over an extended period on a healthy diet and optimal physical activity. These types of interventions can reduce blood glucose levels and lower the risk of progression to diabetes, and can help with lowering weight, blood pressure, and lipid levels. The evidence report that preceded the recommendation pooled the results from 10 studies on lifestyle modification.4 The length of follow-up in these studies ranged from 3 to 23 years, and the number needed to treat to prevent one case of progression to diabetes ranged from about 5 to 20.4
Medications such as metformin, thiazolidinediones, and alpha-glucosidase inhibitors can also reduce blood glucose levels and slow progression to diabetes. However, the Task Force says there is insufficient evidence that pharmacologic interventions have the same multifactorial benefits—weight loss or reductions in glucose levels, blood pressure, and lipid levels—as behavioral interventions.1
As for the other modifiable risk factors for cardiovascular disease—obesity, lack of physical activity, high lipid levels, high blood pressure, and smoking—the Task Force has developed recommendations on screening for and treating each of them,5 which supplement the recommendations discussed in this article.
In December 2015, the United States Preventive Services Task Force updated its recommendation on screening for abnormal blood glucose and diabetes to say that clinicians should screen all adults ages 40 to 70 years who are overweight or obese as part of a cardiovascular risk assessment.1 This recommendation carries a B grade signifying a moderate certainty that a moderate net benefit will be gained by detecting impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or diabetes, and by implementing intensive lifestyle interventions. In this article, as in the Task Force recommendation, the term diabetes means type 2 diabetes. Obesity is defined as a body mass index (BMI) of ≥30 kg/m2, and overweight as a BMI >25.
How the Task Force recommendation evolved
The previous Task Force recommendation on this topic, made in 2008, advised screening only adults with hypertension because there was no evidence that any other group benefited from screening. In subsequent years, there were calls for the Task Force to revise its recommendation to bring it more in line with that of the American Diabetes Association (ADA).2 While this new recommendation does add more adults to the cohort of those the Task Force believes should be screened, it is still not totally in concert with the ADA, which recommends screening all adults 45 years or older and those who are younger if they have multiple risk factors.3
Both the Task Force and the ADA acknowledge there is no direct evidence for any benefit in screening for diabetes in the general, asymptomatic population. The Task Force, with its standard of making recommendations only when good evidence supports them, has opted to address screening for abnormal glucose levels in the context of cardiovascular risk reduction and persuasive evidence that lifestyle interventions can reduce cardiovascular risks and slow progression to diabetes.
The ADA is willing to rely on less rigorous evidence of benefit in screening, diagnosing, and treating undetected diabetes. It believes that morbidity and mortality from this pervasive chronic disease can be reduced with early detection and treatment.
Still the Task Force and ADA agree more than they differ
While it appears that significant differences exist between the recommendations of the Task Force and the ADA, a closer look shows they actually have much in common; and, as they pertain to daily practice, any remaining differences are primarily ones of emphasis. For instance, the Clinical Considerations section of the Task Force recommendation acknowledges that certain people are at increased risk for diabetes at younger ages and at a lower BMI, and that clinicians should “consider” screening them earlier than at age 40 years. The risks listed include a family history of diabetes or a personal history of gestational diabetes or polycystic ovarian syndrome; or being African American, Hispanic, Asian American, American Indian, Alaskan Native, or Native Hawaiian.
The Task Force statement seems to imply—although this is not entirely clear—that those who have these risks should also be screened if they are older than age 40 years even if they are not obese. So, although the ADA would screen everyone ages 45 and older, the Task Force would screen everyone ages 40 and older, except for non-Hispanic whites who are not overweight or obese, and who have no other risk factors. TABLE 11,3 details the Task Force and the ADA screening criteria and how they differ.
The Task Force and the ADA also agree on the 3 tests acceptable for screening and the test values that define normal glucose, IGT, IFG, and diabetes (TABLE 2).1,3 The tests are a randomly measured glycated hemoglobin level, a fasting plasma glucose level, and an oral glucose tolerance test performed in the morning after an overnight fast, with glucose measured 2 hours after a 75-g oral glucose load. If a screening result is abnormal, confirmation should be sought by repeating the same test. And both organizations suggest that, following a normal test result, the optimal interval for retesting is 3 years.
Intervening to delay progression to diabetes
For anyone with a confirmed abnormal blood glucose level, the Task Force advises referral for intensive behavioral interventions—ie, multiple counseling sessions over an extended period on a healthy diet and optimal physical activity. These types of interventions can reduce blood glucose levels and lower the risk of progression to diabetes, and can help with lowering weight, blood pressure, and lipid levels. The evidence report that preceded the recommendation pooled the results from 10 studies on lifestyle modification.4 The length of follow-up in these studies ranged from 3 to 23 years, and the number needed to treat to prevent one case of progression to diabetes ranged from about 5 to 20.4
Medications such as metformin, thiazolidinediones, and alpha-glucosidase inhibitors can also reduce blood glucose levels and slow progression to diabetes. However, the Task Force says there is insufficient evidence that pharmacologic interventions have the same multifactorial benefits—weight loss or reductions in glucose levels, blood pressure, and lipid levels—as behavioral interventions.1
As for the other modifiable risk factors for cardiovascular disease—obesity, lack of physical activity, high lipid levels, high blood pressure, and smoking—the Task Force has developed recommendations on screening for and treating each of them,5 which supplement the recommendations discussed in this article.
1. U.S. Preventive Services Task Force. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed May 20, 2016.
2. Casagrande SS, Cowie CC, Fradkin JE. Utility of the US Preventive Services Task Force criteria for diabetes screening. Am J Prev Med. 2013;45:167-174.
3. American Diabetes Association. Standards of medical care in diabetes - 2016. Diabetes Care. 2016;39(Suppl 1):S1–S112.
4. Selph S, Dana T, Bougatsos C, et al. A systematic review to update the 2008 U.S. Preventive Services Task Force recommendation [Agency for Healthcare Research and Quality]. 2015. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed May 20, 2016.
5. U.S. Preventive Services Task Force. Healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd. Accessed May 20,
2016.
1. U.S. Preventive Services Task Force. Abnormal blood glucose and type 2 diabetes mellitus: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed May 20, 2016.
2. Casagrande SS, Cowie CC, Fradkin JE. Utility of the US Preventive Services Task Force criteria for diabetes screening. Am J Prev Med. 2013;45:167-174.
3. American Diabetes Association. Standards of medical care in diabetes - 2016. Diabetes Care. 2016;39(Suppl 1):S1–S112.
4. Selph S, Dana T, Bougatsos C, et al. A systematic review to update the 2008 U.S. Preventive Services Task Force recommendation [Agency for Healthcare Research and Quality]. 2015. Available at: http://www.ncbi.nlm.nih.gov/books/NBK293871/. Accessed May 20, 2016.
5. U.S. Preventive Services Task Force. Healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: behavioral counseling. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/healthy-diet-and-physical-activity-counseling-adults-with-high-risk-of-cvd. Accessed May 20,
2016.
8 USPSTF recommendations FPs need to know about
The US Preventive Services Task Force made 8 recommendations in 2015 that family physicians should implement in their practices (TABLE 11). The conditions addressed are high blood pressure, abnormal blood glucose, breast cancer, depression, and tobacco use. The Task Force also issued 13 “I” statements (TABLE 21) reflecting insufficient evidence to recommend for or against a particular intervention—once again underscoring the inadequate evidence base for many commonly-accepted practices aimed at prevention. Four such interventions were targeted toward children.
High blood pressure: Verify before starting treatment
The Task Force continues to give strong backing to the practice of screening for high blood pressure (HBP) and treating those with HBP to prevent cardiovascular and renal disease. The new recommendation, however, recognizes there is significant over-diagnosis of this condition and advises that, before starting treatment, HBP found with office measurement be confirmed with either ambulatory blood pressure monitoring or home blood pressure monitoring. This topic was covered in more depth in a recent Practice Alert.2
Since cardiovascular disease is the leading cause of death in the United States and much of this mortality is preventable, the Task Force also has recommendations in place for screening and treatment of other risks for cardiovascular disease, including obesity, hyperlipidemia, elevated blood glucose (discussed below), and tobacco use.1
Blood glucose: Focus is now on overweight/obese individuals
The Task Force’s new recommendation for diabetes screening differs from the one made in 2008, which recommended screening for type 2 diabetes (T2DM) only in adults with hypertension. The Task Force now recommends screening for abnormal blood glucose in all obese and overweight adults between the ages of 40 and 70. The Task Force analysis is detailed3 and will be the subject of the next Practice Alert, with only the highlights described here.
The recommendation is limited to overweight and obese adults because they are most likely to have abnormal blood glucose and to benefit from interventions. Screening can be done by measuring fasting blood glucose levels, performing a glucose tolerance test, or measuring glycated hemoglobin levels. The optimal screening frequency is unknown but suggested to be every 3 years. Refer patients with abnormal screen results to an intensive behavioral counseling program that promotes healthy eating and physical activity. Those with T2DM should also receive these services and consider pharmacotherapy.
Breast cancer: Mammography advice is age dependent
The Task Force breast cancer screening recommendations, first proposed in 2015 and finalized in early 2016, essentially reaffirm those made in 2009. Women ages 50 through 74 should be screened with mammography every 2 years, and individuals younger than age 50 should make a decision to receive screening—or not—based on the known benefits and risks of mammography at their age and their personal risks and preferences.
Insufficient evidence exists to make recommendations regarding mammography for women ages 75 and up, the use of digital breast tomosynthesis as a primary screening tool, and the use of any modality to augment screening in women with dense breasts who have normal mammogram results. Details of these recommendations were described in a Practice Alert last year.4
Depression: Use screening tools designed for specific patients
The 2015 updates on screening for depression essentially reconfirm the Task Force’s previous findings and recommendations on this topic. Screening for depression is recommended for all adults, including pregnant and postpartum women,5 and adolescents starting at age 12.6 Once again, the evidence is insufficient to make a recommendation on screening for depression in children younger than age 12.
Both recommendations emphasize the importance of follow-up steps after screening to ensure accurate diagnosis, adequate treatment, and appropriate follow-up. Treatment for adults and adolescents can include pharmacotherapy, cognitive-behavioral therapy, and/or psychosocial counseling. However, pharmacotherapy is not recommended for pregnant and breastfeeding women because of potential harms to the fetus and newborn.
The Task Force deems a number of screening tools acceptable. For adolescents, it suggests the Patient Health Questionnaire for Adolescents and the primary care version of the Beck Depression Inventory.6 For adults, the Task Force suggests the Patient Health Questionnaire, the Hospital Anxiety and Depression Scales, the Geriatric Depression Scale for older adults, and the Edinburgh Postnatal Depression Scale for postpartum and pregnant women.5
There is no known optimal frequency of screening or evidence on the value of repeated screening. The Task Force suggests one initial screen with repeated screening based on individual characteristics.
Tobacco use: Ask every adult patient about it
Preventing the harms from tobacco use is one of the most important and productive primary care interventions. The Task Force has affirmed its previous recommendation to ask all adults about tobacco use, encourage those that use tobacco to quit, and to offer behavioral and pharmacologic interventions to assist with quitting.7 The new recommendations emphasize the importance of smoking cessation during pregnancy; however, because of concern about the unknown potential harms from pharmacologic interventions, they advise only behavioral therapy to assist pregnant women to quit smoking.
The Task Force also examined the potential of electronic nicotine delivery systems for smoking cessation and concluded the evidence is insufficient to make a recommendation. It also concluded that the availability of other proven methods of smoking cessation make them the preferred alternatives.
Services with insufficient evidence
TABLE 21 lists the interventions that the Task Force studied this past year and found insufficient evidence to support a recommendation for or against. For adults, these “I” recommendations include screening for visual acuity disorders in older adults, screening for thyroid disorders, screening for iron deficiency anemia during pregnancy, and routinely providing iron supplementation during pregnancy.
The persistent inadequate evidence for the effectiveness of preventive services in infants and children was highlighted by the results of last year’s examination of 4 screening tests, all recommended by the American Academy of Pediatrics, but given an “I” recommendation by the Task Force. These included screening for autism spectrum disorder (ASD) in young children (18-30 months), iron deficiency anemia in children ages 6 to 24 months, depression in those ages 11 and younger, and speech and language delay and disorders in children ages 5 or younger. (Ages noted are from the Task Force.)
The Task Force is careful to emphasize that the statement about ASD screening refers to infants and children who appear normal and for whom no concerns of ASD have been raised by their parents. Screening all young children for this disorder is problematic, according to the Task Force, because of possible over-diagnosis and unclear benefits of early intervention.8
1. US Preventive Services Task Force. Published recommendations. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 18, 2016.
2. Campos-Outcalt D. USPSTF urges extra step before treating hypertension. J Fam Pract. 2016;65:41-44.
3. US Preventive Services Task Force. Abnormal blood glucose and diabetes type 2: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed March 18, 2016.
4. Campos-Outcalt D. Breast cancer screening: the latest from the USPSTF. J Fam Pract. 2015;64:407-410.
5. US Preventive Services Task Force. Depression in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening1. Accessed March 18, 2016.
6. US Preventive Services Task Force. Depression in children and adolescents: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-children-and-adolescents-screening1. Accessed March 18, 2016.
7. US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed April 7, 2016.
8. US Preventive Services Task Force. Autism spectrum disorder in young children: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/autism-spectrum-disorder-in-young-children-screening. Accessed March 18, 2016.
The US Preventive Services Task Force made 8 recommendations in 2015 that family physicians should implement in their practices (TABLE 11). The conditions addressed are high blood pressure, abnormal blood glucose, breast cancer, depression, and tobacco use. The Task Force also issued 13 “I” statements (TABLE 21) reflecting insufficient evidence to recommend for or against a particular intervention—once again underscoring the inadequate evidence base for many commonly-accepted practices aimed at prevention. Four such interventions were targeted toward children.
High blood pressure: Verify before starting treatment
The Task Force continues to give strong backing to the practice of screening for high blood pressure (HBP) and treating those with HBP to prevent cardiovascular and renal disease. The new recommendation, however, recognizes there is significant over-diagnosis of this condition and advises that, before starting treatment, HBP found with office measurement be confirmed with either ambulatory blood pressure monitoring or home blood pressure monitoring. This topic was covered in more depth in a recent Practice Alert.2
Since cardiovascular disease is the leading cause of death in the United States and much of this mortality is preventable, the Task Force also has recommendations in place for screening and treatment of other risks for cardiovascular disease, including obesity, hyperlipidemia, elevated blood glucose (discussed below), and tobacco use.1
Blood glucose: Focus is now on overweight/obese individuals
The Task Force’s new recommendation for diabetes screening differs from the one made in 2008, which recommended screening for type 2 diabetes (T2DM) only in adults with hypertension. The Task Force now recommends screening for abnormal blood glucose in all obese and overweight adults between the ages of 40 and 70. The Task Force analysis is detailed3 and will be the subject of the next Practice Alert, with only the highlights described here.
The recommendation is limited to overweight and obese adults because they are most likely to have abnormal blood glucose and to benefit from interventions. Screening can be done by measuring fasting blood glucose levels, performing a glucose tolerance test, or measuring glycated hemoglobin levels. The optimal screening frequency is unknown but suggested to be every 3 years. Refer patients with abnormal screen results to an intensive behavioral counseling program that promotes healthy eating and physical activity. Those with T2DM should also receive these services and consider pharmacotherapy.
Breast cancer: Mammography advice is age dependent
The Task Force breast cancer screening recommendations, first proposed in 2015 and finalized in early 2016, essentially reaffirm those made in 2009. Women ages 50 through 74 should be screened with mammography every 2 years, and individuals younger than age 50 should make a decision to receive screening—or not—based on the known benefits and risks of mammography at their age and their personal risks and preferences.
Insufficient evidence exists to make recommendations regarding mammography for women ages 75 and up, the use of digital breast tomosynthesis as a primary screening tool, and the use of any modality to augment screening in women with dense breasts who have normal mammogram results. Details of these recommendations were described in a Practice Alert last year.4
Depression: Use screening tools designed for specific patients
The 2015 updates on screening for depression essentially reconfirm the Task Force’s previous findings and recommendations on this topic. Screening for depression is recommended for all adults, including pregnant and postpartum women,5 and adolescents starting at age 12.6 Once again, the evidence is insufficient to make a recommendation on screening for depression in children younger than age 12.
Both recommendations emphasize the importance of follow-up steps after screening to ensure accurate diagnosis, adequate treatment, and appropriate follow-up. Treatment for adults and adolescents can include pharmacotherapy, cognitive-behavioral therapy, and/or psychosocial counseling. However, pharmacotherapy is not recommended for pregnant and breastfeeding women because of potential harms to the fetus and newborn.
The Task Force deems a number of screening tools acceptable. For adolescents, it suggests the Patient Health Questionnaire for Adolescents and the primary care version of the Beck Depression Inventory.6 For adults, the Task Force suggests the Patient Health Questionnaire, the Hospital Anxiety and Depression Scales, the Geriatric Depression Scale for older adults, and the Edinburgh Postnatal Depression Scale for postpartum and pregnant women.5
There is no known optimal frequency of screening or evidence on the value of repeated screening. The Task Force suggests one initial screen with repeated screening based on individual characteristics.
Tobacco use: Ask every adult patient about it
Preventing the harms from tobacco use is one of the most important and productive primary care interventions. The Task Force has affirmed its previous recommendation to ask all adults about tobacco use, encourage those that use tobacco to quit, and to offer behavioral and pharmacologic interventions to assist with quitting.7 The new recommendations emphasize the importance of smoking cessation during pregnancy; however, because of concern about the unknown potential harms from pharmacologic interventions, they advise only behavioral therapy to assist pregnant women to quit smoking.
The Task Force also examined the potential of electronic nicotine delivery systems for smoking cessation and concluded the evidence is insufficient to make a recommendation. It also concluded that the availability of other proven methods of smoking cessation make them the preferred alternatives.
Services with insufficient evidence
TABLE 21 lists the interventions that the Task Force studied this past year and found insufficient evidence to support a recommendation for or against. For adults, these “I” recommendations include screening for visual acuity disorders in older adults, screening for thyroid disorders, screening for iron deficiency anemia during pregnancy, and routinely providing iron supplementation during pregnancy.
The persistent inadequate evidence for the effectiveness of preventive services in infants and children was highlighted by the results of last year’s examination of 4 screening tests, all recommended by the American Academy of Pediatrics, but given an “I” recommendation by the Task Force. These included screening for autism spectrum disorder (ASD) in young children (18-30 months), iron deficiency anemia in children ages 6 to 24 months, depression in those ages 11 and younger, and speech and language delay and disorders in children ages 5 or younger. (Ages noted are from the Task Force.)
The Task Force is careful to emphasize that the statement about ASD screening refers to infants and children who appear normal and for whom no concerns of ASD have been raised by their parents. Screening all young children for this disorder is problematic, according to the Task Force, because of possible over-diagnosis and unclear benefits of early intervention.8
The US Preventive Services Task Force made 8 recommendations in 2015 that family physicians should implement in their practices (TABLE 11). The conditions addressed are high blood pressure, abnormal blood glucose, breast cancer, depression, and tobacco use. The Task Force also issued 13 “I” statements (TABLE 21) reflecting insufficient evidence to recommend for or against a particular intervention—once again underscoring the inadequate evidence base for many commonly-accepted practices aimed at prevention. Four such interventions were targeted toward children.
High blood pressure: Verify before starting treatment
The Task Force continues to give strong backing to the practice of screening for high blood pressure (HBP) and treating those with HBP to prevent cardiovascular and renal disease. The new recommendation, however, recognizes there is significant over-diagnosis of this condition and advises that, before starting treatment, HBP found with office measurement be confirmed with either ambulatory blood pressure monitoring or home blood pressure monitoring. This topic was covered in more depth in a recent Practice Alert.2
Since cardiovascular disease is the leading cause of death in the United States and much of this mortality is preventable, the Task Force also has recommendations in place for screening and treatment of other risks for cardiovascular disease, including obesity, hyperlipidemia, elevated blood glucose (discussed below), and tobacco use.1
Blood glucose: Focus is now on overweight/obese individuals
The Task Force’s new recommendation for diabetes screening differs from the one made in 2008, which recommended screening for type 2 diabetes (T2DM) only in adults with hypertension. The Task Force now recommends screening for abnormal blood glucose in all obese and overweight adults between the ages of 40 and 70. The Task Force analysis is detailed3 and will be the subject of the next Practice Alert, with only the highlights described here.
The recommendation is limited to overweight and obese adults because they are most likely to have abnormal blood glucose and to benefit from interventions. Screening can be done by measuring fasting blood glucose levels, performing a glucose tolerance test, or measuring glycated hemoglobin levels. The optimal screening frequency is unknown but suggested to be every 3 years. Refer patients with abnormal screen results to an intensive behavioral counseling program that promotes healthy eating and physical activity. Those with T2DM should also receive these services and consider pharmacotherapy.
Breast cancer: Mammography advice is age dependent
The Task Force breast cancer screening recommendations, first proposed in 2015 and finalized in early 2016, essentially reaffirm those made in 2009. Women ages 50 through 74 should be screened with mammography every 2 years, and individuals younger than age 50 should make a decision to receive screening—or not—based on the known benefits and risks of mammography at their age and their personal risks and preferences.
Insufficient evidence exists to make recommendations regarding mammography for women ages 75 and up, the use of digital breast tomosynthesis as a primary screening tool, and the use of any modality to augment screening in women with dense breasts who have normal mammogram results. Details of these recommendations were described in a Practice Alert last year.4
Depression: Use screening tools designed for specific patients
The 2015 updates on screening for depression essentially reconfirm the Task Force’s previous findings and recommendations on this topic. Screening for depression is recommended for all adults, including pregnant and postpartum women,5 and adolescents starting at age 12.6 Once again, the evidence is insufficient to make a recommendation on screening for depression in children younger than age 12.
Both recommendations emphasize the importance of follow-up steps after screening to ensure accurate diagnosis, adequate treatment, and appropriate follow-up. Treatment for adults and adolescents can include pharmacotherapy, cognitive-behavioral therapy, and/or psychosocial counseling. However, pharmacotherapy is not recommended for pregnant and breastfeeding women because of potential harms to the fetus and newborn.
The Task Force deems a number of screening tools acceptable. For adolescents, it suggests the Patient Health Questionnaire for Adolescents and the primary care version of the Beck Depression Inventory.6 For adults, the Task Force suggests the Patient Health Questionnaire, the Hospital Anxiety and Depression Scales, the Geriatric Depression Scale for older adults, and the Edinburgh Postnatal Depression Scale for postpartum and pregnant women.5
There is no known optimal frequency of screening or evidence on the value of repeated screening. The Task Force suggests one initial screen with repeated screening based on individual characteristics.
Tobacco use: Ask every adult patient about it
Preventing the harms from tobacco use is one of the most important and productive primary care interventions. The Task Force has affirmed its previous recommendation to ask all adults about tobacco use, encourage those that use tobacco to quit, and to offer behavioral and pharmacologic interventions to assist with quitting.7 The new recommendations emphasize the importance of smoking cessation during pregnancy; however, because of concern about the unknown potential harms from pharmacologic interventions, they advise only behavioral therapy to assist pregnant women to quit smoking.
The Task Force also examined the potential of electronic nicotine delivery systems for smoking cessation and concluded the evidence is insufficient to make a recommendation. It also concluded that the availability of other proven methods of smoking cessation make them the preferred alternatives.
Services with insufficient evidence
TABLE 21 lists the interventions that the Task Force studied this past year and found insufficient evidence to support a recommendation for or against. For adults, these “I” recommendations include screening for visual acuity disorders in older adults, screening for thyroid disorders, screening for iron deficiency anemia during pregnancy, and routinely providing iron supplementation during pregnancy.
The persistent inadequate evidence for the effectiveness of preventive services in infants and children was highlighted by the results of last year’s examination of 4 screening tests, all recommended by the American Academy of Pediatrics, but given an “I” recommendation by the Task Force. These included screening for autism spectrum disorder (ASD) in young children (18-30 months), iron deficiency anemia in children ages 6 to 24 months, depression in those ages 11 and younger, and speech and language delay and disorders in children ages 5 or younger. (Ages noted are from the Task Force.)
The Task Force is careful to emphasize that the statement about ASD screening refers to infants and children who appear normal and for whom no concerns of ASD have been raised by their parents. Screening all young children for this disorder is problematic, according to the Task Force, because of possible over-diagnosis and unclear benefits of early intervention.8
1. US Preventive Services Task Force. Published recommendations. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 18, 2016.
2. Campos-Outcalt D. USPSTF urges extra step before treating hypertension. J Fam Pract. 2016;65:41-44.
3. US Preventive Services Task Force. Abnormal blood glucose and diabetes type 2: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed March 18, 2016.
4. Campos-Outcalt D. Breast cancer screening: the latest from the USPSTF. J Fam Pract. 2015;64:407-410.
5. US Preventive Services Task Force. Depression in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening1. Accessed March 18, 2016.
6. US Preventive Services Task Force. Depression in children and adolescents: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-children-and-adolescents-screening1. Accessed March 18, 2016.
7. US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed April 7, 2016.
8. US Preventive Services Task Force. Autism spectrum disorder in young children: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/autism-spectrum-disorder-in-young-children-screening. Accessed March 18, 2016.
1. US Preventive Services Task Force. Published recommendations. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 18, 2016.
2. Campos-Outcalt D. USPSTF urges extra step before treating hypertension. J Fam Pract. 2016;65:41-44.
3. US Preventive Services Task Force. Abnormal blood glucose and diabetes type 2: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed March 18, 2016.
4. Campos-Outcalt D. Breast cancer screening: the latest from the USPSTF. J Fam Pract. 2015;64:407-410.
5. US Preventive Services Task Force. Depression in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening1. Accessed March 18, 2016.
6. US Preventive Services Task Force. Depression in children and adolescents: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-children-and-adolescents-screening1. Accessed March 18, 2016.
7. US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed April 7, 2016.
8. US Preventive Services Task Force. Autism spectrum disorder in young children: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/autism-spectrum-disorder-in-young-children-screening. Accessed March 18, 2016.
From The Journal of Family Practice | 2016;65(5):338-341.
Immunization update: This year’s changes
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
The annual update of immunization schedules by the Centers for Disease Control and Prevention (CDC)—one for adults and one for infants, children, and adolescents—was published recently in Morbidity and Mortality Weekly Report.1,2 The Advisory Committee on Immunization Practices (ACIP) made a few new recommendations in 2015 (although no major changes from the previous year), which are summarized in this Practice Alert.
HPV vaccine: 9-valent formulation available
While the recommended recipients of the human papillomavirus (HPV) vaccine have not changed (TABLE 1),3 the 9-valent human papillomavirus vaccine (HPV9) has been added to the immunization schedule. Licensed in December 2014, HPV9 added 5 high-risk HPV antigens to the quadrivalent HPV vaccine (HPV4). The antigen types in HPV4 cause 66% of cervical cancers, while those in HPV9 cause 81%.3
Three HPV vaccines are available for use in the United States (TABLE 2).3 All require 3 doses, given on a schedule of 0, 1 to 2, and 6 months, beginning at 11 through 12 years of age. HPV4 will likely become unavailable as its supply is used up in the transition to HPV9.
Although HPV9 offers wider protection than HPV4, the recommendation is to start or continue a series of HPV vaccine, as indicated, without waiting for HPV9 if it is not immediately available. Those who are in the middle of a 3-dose HPV4 schedule can finish the remaining doses with HPV9. ACIP has not recommended that HPV9 be administered to those who have completed a series of HPV4 or HPV2.
Pneumococcal vaccines: Give one year apart, regardless of sequence
There are 2 pneumococcal vaccines in the United States: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent conjugate vaccine (PCV13). Adults ages 65 years or older should receive both vaccines. The preferred order of administration is PCV13 first, then PPSV23. The recommended interval between injections in this order had been 6 to 12 months. If the vaccines were given in the reverse order, PCV13 was to be administered at least one year later. Thus, the timing interval differed depending on the order of administration.4 However, to complicate matters, Medicare will pay for 2 pneumococcal vaccinations only if they are separated by a year.
ACIP reexamined the data and found little evidence to support any specific interval, regardless of the order of administration. Therefore, to simplify the schedule and reconcile with Medicare, the new recommendation states it is best to administer PCV13 first, but, regardless of the order, to separate the 2 vaccines by one year. If, for logistical reasons or error, the interval is less than one year, neither vaccine needs to be repeated.
Meningococcal B vaccine
ACIP’s immunization schedule now recommends giving meningococcal B vaccine to individuals in high-risk groups and those exposed to community outbreaks. It gives a “B” recommendation (can be provided if an individual wants it) for vaccine use in all adolescents. These recommendations were described in greater detail in a recent Practice Alert.5
Smallpox vaccine recommendations are reaffirmed
In June 2015, ACIP, having reviewed recent clinical data, reaffirmed the CDC’s standing recommendations that the live vaccinia virus smallpox vaccine ACAM2000 (which replaced Dryvax in 2008) be administered routinely to those with occupational exposure to orthopox viruses (eg, laboratory personnel who work with monkeypox, variola, or smallpox viruses).6 Health care workers who administer the vaccine or care for someone who might be infected with an orthopox virus may be offered the vaccine.6 And some members of the Armed Forces are required to receive it.7
Information about smallpox vaccination, including potential adverse reactions to the vaccine and what to do about them, can be found on the CDC Web site at http://www.emergency.cdc.gov/agent/smallpox/clinicians.asp.
Yellow fever vaccine: Boosters needed only for some
Yellow fever vaccine is required for travelers who are visiting areas where the disease is endemic. After reviewing data on the duration of protection provided by the current vaccine, ACIP changed its recommendation in June 2015 to bring it in line with that of the World Health Organization, which states that one dose of vaccine provides long-lasting protection and that a booster is no longer recommended for most travelers.
Three exceptions to the booster exemption are noted: women who are pregnant when they receive their first dose of vaccine; those who undergo stem-cell transplantation following vaccination; and HIV-positive individuals, who should be vaccinated every 10 years.8
A “B” recommendation for the vaccine applies to those who were vaccinated 10 or more years previously and who will be traveling to highly endemic areas for prolonged periods. Laboratory personnel who work with yellow fever virus should have their antibody titers checked every 10 years and receive a booster dose if the titers are low.8
New vaccines coming soon
No cholera vaccine is licensed for use in the United States, but a new single-dose, live attenuated oral cholera vaccine will likely be licensed this year.
A new adjuvanted herpes zoster vaccine has completed a phase-3 study and the results were presented to ACIP in June 2015. It is expected to be approved sometime this year.
Finally, a new combination vaccine for infants is being developed cooperatively between Sanofi Pasteur and Merck & Co. It will offer protection against diphtheria, pertussis, tetanus, polio, Haemophilus influenzae type B, and hepatitis B. When available, it will offer an option that means fewer injections than current combination products (TABLE 3).9
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
1. Centers for Disease Control and Prevention. Recommended Immunization Schedules for Persons Aged 0 through 18 years— United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed February 9, 2016.
2. Centers for Disease Control and Prevention. Recommended Adult Immunization Schedule: United States, 2016. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule.pdf. Accessed February 9, 2016.
3. Petrosky E, Bocchini JA Jr, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64;300-304.
4. Campos-Outcalt D. Pneumococcal vaccines for older adults: getting the timing right. J Fam Pract. 2014;63:730-732.
5. Campos-Outcalt D. ACIP weighs in on meningococcal B vaccines. J Fam Pract. 2015;64:787-789.
6. Petersen BW. Use of smallpox vaccine in laboratory and health-care workers at risk for occupational exposure to orthopoxviruses. Presented at: Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/smallpox-02-petersen.pdf. Accessed February 13, 2016.
7. Defense Health Agency. Smallpox. Available at: https://www.vaccines.mil/smallpox. Accessed February 16, 2016.
8. Centers for Disease Control and Prevention (CDC). Yellow fever vaccine information for healthcare providers. Available at: http://www.cdc.gov/yellowfever/healthcareproviders/vaccine-info.html. Accessed January 27, 2016.
9. Lee AW. Immunogenicity and safety of DTaP5-IPV-Hib-HepB, a pediatric hexavalent combination vaccine. Presentation at: Advisory Committee on Immunization Practices; October 2015; Atlanta, GA. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-10/comb-vax-02-lee.pdf. Accessed January 22, 2015.
How new dietary guidelines affect health care providers
The U.S. Department of Health and Human Services and the U.S. Department of Agriculture have released the eighth iteration of the Dietary Guidelines for Americans, a set of recommendations for healthy eating habits Americans should adopt to prevent development of hypertension, heart disease, and type 2 diabetes, among other conditions.
The new guidelines, effective through 2020, highlight the importance of eating a wide variety of fruits, vegetables, grains, and dairy products, while staying away from processed foods heavy in saturated fats, sugar, and cholesterol as much as possible. The difference between this and earlier editions of the guidelines, the health agencies say, is to promote the importance of a wider variety of foods Americans should be consuming, rather than focusing on just a few isolated foods that should be integrated into an otherwise inadequate diet.
“Protecting the health of the American public includes empowering them with the tools they need to make healthy choices in their daily lives,” Secretary of Health and Human Services Sylvia M. Burwell said in a statement. “By focusing on small shifts in what we eat and drink, eating healthy becomes more manageable. The Dietary Guidelines provide science-based recommendations on food and nutrition so people can make decisions that may help keep their weight under control, and prevent chronic conditions, like Type 2 diabetes, hypertension, and heart disease.”
The American Medical Association voiced their support of the new guidelines, saying that they are “extremely pleased that the new recommendations call for significantly reducing the amount of added sugars and sugar sweetened beverages from the American diet.” Similarly, the American College of Cardiology issued a statement saying that the existence of “a source of clear science-based information about diet” is more important than ever for Americans in the face of increasingly omnipresent and often confusing information available; the college also lauded the recommendations to limit the intake of added sugars, saturated and trans fats, and sodium. The American Heart Association released a new Authoritative Review of data on the topic of nutritional balance as it related to chronic diseases.
While the recommendations may seem common sense and geared more towards patients and laymen, they are of equal importance to health care providers. Dr. Carolyn Lopez – a Chicago-area family physician and adjunct professor of medicine at Northwestern University – called the guidelines an important resource for physicians, with several elements that will be of particular benefit to physicians and clinicians looking to improve the quality of care given to their patients.
“People [should] understand that while individual food choices are important, the pattern of eating is paramount,” Dr. Lopez explained in an interview. “It’s not impossible to bring vegetables into breakfast – veggie omelettes are great – but it’s hard to imagine bringing a vegetable into the whole grain cereals with skim milk breakfast.”
As a family practitioner, however, Dr. Lopez stressed the difficulty of any doctor having a significant enough amount of time with each patient to really go in-depth into what needs to be done to enact meaningful nutritional and lifestyle changes. “These guidelines can only be effective if the whole team is talking to patients,” she explained. “On its own, it would be extraordinarily difficult [or] impossible to accomplish.
Dr. Nazrat Mirza, a pediatrician who is medical director of the IDEAL Pediatric Weight Management Clinic at Children’s National Health System, Washington, said in an interview that the guidelines are “significant in that they are leading us even closer to healthier dietary living – and from my pediatric perspective, healthier children who will grow into healthier adults. We must keep in mind that these guidelines are not prescriptive – but generalized to relatively healthy people. In particular, the recommendation to reduce sugar intake with specification of an upper limit of 10% of calories from added sugar factored into the suggested daily nutritional intake is an excellent update. Added sugar consumption is linked to diabetes, so any reduction in recommended intake will steer people away from potentially developing diabetes.”
She continued, “In my clinic and day-to-day counseling of patients, being able to point to helpful resources such as these guidelines is crucial. These guidelines will continue to serve as yet another way to reach parents and affect the daily dietary habits they practice at home. I was happy to see resources such as MyPlate.gov referenced in the guidelines; that is a tool I use regularly. When educating families on what a healthy plate looks like, I’m able to point directly to the MyPlate.gov posters hanging in the clinic. Comprehensive resources such as these guidelines, [which] give parents actual examples of foods, and deliver the information in a clear, concise, implementable way, are the best methods to reach parents and kids.”
“As a medical provider, such guidelines provide us with tools and reinforcement to say ‘this is policy,’ when approaching schools about the food and drink options they offer children on a daily basis – because the schools are going to have to follow these guidelines. If these guidelines can enforce public policy for the kids to eat healthy, ... and we can implement changes according to the guidelines, that would help greatly in the prevention of chronic disorders such as obesity, hypertension and diabetes – which is a step in the right direction,” said Dr. Mirza, also of George Washington University, Washington.
“Most physicians are so rushed nowadays by the health care system that they typically do not have adequate time to discuss these very important issues,” Dr. Rodbard said in an interview. “It takes time and perseverance to educate the patient and provide individualized care, assessing their current diet, assessing their willingness or potential for changing their diet or increasing their physical activity.”
On that last point. Dr. Rodbard criticized the new guidelines for not emphasizing the importance of exercise enough. “More clarity and emphasis on physical activity should have been provided,” she said, adding that “people need continual, gentle reminders with repetition and long-term follow-up.” Dr. Rodbard summed up the guidelines as “marginally adequate” but “a step in the right direction.”
An endocrinologist who practices in Winter Park, Fla., Dr. Victor L. Roberts – a Fellow of the ACP and ACE – agreed that time with the patients is critical in order for these guidelines to have any effect, calling the guidelines “just the GPS, not the destination.”
Preaching an ABC strategy – accountability, behavior, and calories, the latter of which he stressed is the most important part of any dietary strategy – Dr. Roberts explained that clinicians and physicians should leave the more specific dietary advice to nutritional experts and focus on telling their patients to watch calories, watch the amount of food they’re eating of any given type, and to exercise more.
“These guidelines crystallize and summarize what we’ve already known, and what we should have been practicing and advocating, for decades,” Dr. Roberts said in an interview, adding that “the difficulty is putting these recommendations into practice and having patients and doctors accept responsibility.”
Ultimately, the three doctors who treat adult patients concurred that the new guidelines are imperfect, and likely won’t result in any automatic widespread change. Dr. Rodbard admitted that she does not anticipate the guidelines affecting the way she treats her patients very much, if at all, while Dr. Lopez said that she’s “not sure it’s going to have a major effect.” Furthermore, the sheer volume of data and information available often leads to patients simply not knowing what to believe, which Dr. Roberts described as a “glazed over” effect of being told so many different things from so many different sources, patients just tune everything out.
Ultimately, while it’s important for health care providers to give advice and recommendations as necessary, these should be done on an individual basis. And, if a patient requires extensive dietary and nutritional intervention, that should be left in the hands of specialist who can accurately determine what the patient is capable of, what their goals should be, and how to tailor a plan specifically for them.
“I don’t know many clinicians, even in my own specialty, who know much about what specifically to eat,” said Dr. Roberts. “That should come from nutritional medicine people, not us.”
Dr. Lopez, Dr. Rodbard, and Dr. Roberts did not report any relevant financial disclosures.
*This article was updated 1/12/2016.
The U.S. Department of Health and Human Services and the U.S. Department of Agriculture have released the eighth iteration of the Dietary Guidelines for Americans, a set of recommendations for healthy eating habits Americans should adopt to prevent development of hypertension, heart disease, and type 2 diabetes, among other conditions.
The new guidelines, effective through 2020, highlight the importance of eating a wide variety of fruits, vegetables, grains, and dairy products, while staying away from processed foods heavy in saturated fats, sugar, and cholesterol as much as possible. The difference between this and earlier editions of the guidelines, the health agencies say, is to promote the importance of a wider variety of foods Americans should be consuming, rather than focusing on just a few isolated foods that should be integrated into an otherwise inadequate diet.
“Protecting the health of the American public includes empowering them with the tools they need to make healthy choices in their daily lives,” Secretary of Health and Human Services Sylvia M. Burwell said in a statement. “By focusing on small shifts in what we eat and drink, eating healthy becomes more manageable. The Dietary Guidelines provide science-based recommendations on food and nutrition so people can make decisions that may help keep their weight under control, and prevent chronic conditions, like Type 2 diabetes, hypertension, and heart disease.”
The American Medical Association voiced their support of the new guidelines, saying that they are “extremely pleased that the new recommendations call for significantly reducing the amount of added sugars and sugar sweetened beverages from the American diet.” Similarly, the American College of Cardiology issued a statement saying that the existence of “a source of clear science-based information about diet” is more important than ever for Americans in the face of increasingly omnipresent and often confusing information available; the college also lauded the recommendations to limit the intake of added sugars, saturated and trans fats, and sodium. The American Heart Association released a new Authoritative Review of data on the topic of nutritional balance as it related to chronic diseases.
While the recommendations may seem common sense and geared more towards patients and laymen, they are of equal importance to health care providers. Dr. Carolyn Lopez – a Chicago-area family physician and adjunct professor of medicine at Northwestern University – called the guidelines an important resource for physicians, with several elements that will be of particular benefit to physicians and clinicians looking to improve the quality of care given to their patients.
“People [should] understand that while individual food choices are important, the pattern of eating is paramount,” Dr. Lopez explained in an interview. “It’s not impossible to bring vegetables into breakfast – veggie omelettes are great – but it’s hard to imagine bringing a vegetable into the whole grain cereals with skim milk breakfast.”
As a family practitioner, however, Dr. Lopez stressed the difficulty of any doctor having a significant enough amount of time with each patient to really go in-depth into what needs to be done to enact meaningful nutritional and lifestyle changes. “These guidelines can only be effective if the whole team is talking to patients,” she explained. “On its own, it would be extraordinarily difficult [or] impossible to accomplish.
Dr. Nazrat Mirza, a pediatrician who is medical director of the IDEAL Pediatric Weight Management Clinic at Children’s National Health System, Washington, said in an interview that the guidelines are “significant in that they are leading us even closer to healthier dietary living – and from my pediatric perspective, healthier children who will grow into healthier adults. We must keep in mind that these guidelines are not prescriptive – but generalized to relatively healthy people. In particular, the recommendation to reduce sugar intake with specification of an upper limit of 10% of calories from added sugar factored into the suggested daily nutritional intake is an excellent update. Added sugar consumption is linked to diabetes, so any reduction in recommended intake will steer people away from potentially developing diabetes.”
She continued, “In my clinic and day-to-day counseling of patients, being able to point to helpful resources such as these guidelines is crucial. These guidelines will continue to serve as yet another way to reach parents and affect the daily dietary habits they practice at home. I was happy to see resources such as MyPlate.gov referenced in the guidelines; that is a tool I use regularly. When educating families on what a healthy plate looks like, I’m able to point directly to the MyPlate.gov posters hanging in the clinic. Comprehensive resources such as these guidelines, [which] give parents actual examples of foods, and deliver the information in a clear, concise, implementable way, are the best methods to reach parents and kids.”
“As a medical provider, such guidelines provide us with tools and reinforcement to say ‘this is policy,’ when approaching schools about the food and drink options they offer children on a daily basis – because the schools are going to have to follow these guidelines. If these guidelines can enforce public policy for the kids to eat healthy, ... and we can implement changes according to the guidelines, that would help greatly in the prevention of chronic disorders such as obesity, hypertension and diabetes – which is a step in the right direction,” said Dr. Mirza, also of George Washington University, Washington.
“Most physicians are so rushed nowadays by the health care system that they typically do not have adequate time to discuss these very important issues,” Dr. Rodbard said in an interview. “It takes time and perseverance to educate the patient and provide individualized care, assessing their current diet, assessing their willingness or potential for changing their diet or increasing their physical activity.”
On that last point. Dr. Rodbard criticized the new guidelines for not emphasizing the importance of exercise enough. “More clarity and emphasis on physical activity should have been provided,” she said, adding that “people need continual, gentle reminders with repetition and long-term follow-up.” Dr. Rodbard summed up the guidelines as “marginally adequate” but “a step in the right direction.”
An endocrinologist who practices in Winter Park, Fla., Dr. Victor L. Roberts – a Fellow of the ACP and ACE – agreed that time with the patients is critical in order for these guidelines to have any effect, calling the guidelines “just the GPS, not the destination.”
Preaching an ABC strategy – accountability, behavior, and calories, the latter of which he stressed is the most important part of any dietary strategy – Dr. Roberts explained that clinicians and physicians should leave the more specific dietary advice to nutritional experts and focus on telling their patients to watch calories, watch the amount of food they’re eating of any given type, and to exercise more.
“These guidelines crystallize and summarize what we’ve already known, and what we should have been practicing and advocating, for decades,” Dr. Roberts said in an interview, adding that “the difficulty is putting these recommendations into practice and having patients and doctors accept responsibility.”
Ultimately, the three doctors who treat adult patients concurred that the new guidelines are imperfect, and likely won’t result in any automatic widespread change. Dr. Rodbard admitted that she does not anticipate the guidelines affecting the way she treats her patients very much, if at all, while Dr. Lopez said that she’s “not sure it’s going to have a major effect.” Furthermore, the sheer volume of data and information available often leads to patients simply not knowing what to believe, which Dr. Roberts described as a “glazed over” effect of being told so many different things from so many different sources, patients just tune everything out.
Ultimately, while it’s important for health care providers to give advice and recommendations as necessary, these should be done on an individual basis. And, if a patient requires extensive dietary and nutritional intervention, that should be left in the hands of specialist who can accurately determine what the patient is capable of, what their goals should be, and how to tailor a plan specifically for them.
“I don’t know many clinicians, even in my own specialty, who know much about what specifically to eat,” said Dr. Roberts. “That should come from nutritional medicine people, not us.”
Dr. Lopez, Dr. Rodbard, and Dr. Roberts did not report any relevant financial disclosures.
*This article was updated 1/12/2016.
The U.S. Department of Health and Human Services and the U.S. Department of Agriculture have released the eighth iteration of the Dietary Guidelines for Americans, a set of recommendations for healthy eating habits Americans should adopt to prevent development of hypertension, heart disease, and type 2 diabetes, among other conditions.
The new guidelines, effective through 2020, highlight the importance of eating a wide variety of fruits, vegetables, grains, and dairy products, while staying away from processed foods heavy in saturated fats, sugar, and cholesterol as much as possible. The difference between this and earlier editions of the guidelines, the health agencies say, is to promote the importance of a wider variety of foods Americans should be consuming, rather than focusing on just a few isolated foods that should be integrated into an otherwise inadequate diet.
“Protecting the health of the American public includes empowering them with the tools they need to make healthy choices in their daily lives,” Secretary of Health and Human Services Sylvia M. Burwell said in a statement. “By focusing on small shifts in what we eat and drink, eating healthy becomes more manageable. The Dietary Guidelines provide science-based recommendations on food and nutrition so people can make decisions that may help keep their weight under control, and prevent chronic conditions, like Type 2 diabetes, hypertension, and heart disease.”
The American Medical Association voiced their support of the new guidelines, saying that they are “extremely pleased that the new recommendations call for significantly reducing the amount of added sugars and sugar sweetened beverages from the American diet.” Similarly, the American College of Cardiology issued a statement saying that the existence of “a source of clear science-based information about diet” is more important than ever for Americans in the face of increasingly omnipresent and often confusing information available; the college also lauded the recommendations to limit the intake of added sugars, saturated and trans fats, and sodium. The American Heart Association released a new Authoritative Review of data on the topic of nutritional balance as it related to chronic diseases.
While the recommendations may seem common sense and geared more towards patients and laymen, they are of equal importance to health care providers. Dr. Carolyn Lopez – a Chicago-area family physician and adjunct professor of medicine at Northwestern University – called the guidelines an important resource for physicians, with several elements that will be of particular benefit to physicians and clinicians looking to improve the quality of care given to their patients.
“People [should] understand that while individual food choices are important, the pattern of eating is paramount,” Dr. Lopez explained in an interview. “It’s not impossible to bring vegetables into breakfast – veggie omelettes are great – but it’s hard to imagine bringing a vegetable into the whole grain cereals with skim milk breakfast.”
As a family practitioner, however, Dr. Lopez stressed the difficulty of any doctor having a significant enough amount of time with each patient to really go in-depth into what needs to be done to enact meaningful nutritional and lifestyle changes. “These guidelines can only be effective if the whole team is talking to patients,” she explained. “On its own, it would be extraordinarily difficult [or] impossible to accomplish.
Dr. Nazrat Mirza, a pediatrician who is medical director of the IDEAL Pediatric Weight Management Clinic at Children’s National Health System, Washington, said in an interview that the guidelines are “significant in that they are leading us even closer to healthier dietary living – and from my pediatric perspective, healthier children who will grow into healthier adults. We must keep in mind that these guidelines are not prescriptive – but generalized to relatively healthy people. In particular, the recommendation to reduce sugar intake with specification of an upper limit of 10% of calories from added sugar factored into the suggested daily nutritional intake is an excellent update. Added sugar consumption is linked to diabetes, so any reduction in recommended intake will steer people away from potentially developing diabetes.”
She continued, “In my clinic and day-to-day counseling of patients, being able to point to helpful resources such as these guidelines is crucial. These guidelines will continue to serve as yet another way to reach parents and affect the daily dietary habits they practice at home. I was happy to see resources such as MyPlate.gov referenced in the guidelines; that is a tool I use regularly. When educating families on what a healthy plate looks like, I’m able to point directly to the MyPlate.gov posters hanging in the clinic. Comprehensive resources such as these guidelines, [which] give parents actual examples of foods, and deliver the information in a clear, concise, implementable way, are the best methods to reach parents and kids.”
“As a medical provider, such guidelines provide us with tools and reinforcement to say ‘this is policy,’ when approaching schools about the food and drink options they offer children on a daily basis – because the schools are going to have to follow these guidelines. If these guidelines can enforce public policy for the kids to eat healthy, ... and we can implement changes according to the guidelines, that would help greatly in the prevention of chronic disorders such as obesity, hypertension and diabetes – which is a step in the right direction,” said Dr. Mirza, also of George Washington University, Washington.
“Most physicians are so rushed nowadays by the health care system that they typically do not have adequate time to discuss these very important issues,” Dr. Rodbard said in an interview. “It takes time and perseverance to educate the patient and provide individualized care, assessing their current diet, assessing their willingness or potential for changing their diet or increasing their physical activity.”
On that last point. Dr. Rodbard criticized the new guidelines for not emphasizing the importance of exercise enough. “More clarity and emphasis on physical activity should have been provided,” she said, adding that “people need continual, gentle reminders with repetition and long-term follow-up.” Dr. Rodbard summed up the guidelines as “marginally adequate” but “a step in the right direction.”
An endocrinologist who practices in Winter Park, Fla., Dr. Victor L. Roberts – a Fellow of the ACP and ACE – agreed that time with the patients is critical in order for these guidelines to have any effect, calling the guidelines “just the GPS, not the destination.”
Preaching an ABC strategy – accountability, behavior, and calories, the latter of which he stressed is the most important part of any dietary strategy – Dr. Roberts explained that clinicians and physicians should leave the more specific dietary advice to nutritional experts and focus on telling their patients to watch calories, watch the amount of food they’re eating of any given type, and to exercise more.
“These guidelines crystallize and summarize what we’ve already known, and what we should have been practicing and advocating, for decades,” Dr. Roberts said in an interview, adding that “the difficulty is putting these recommendations into practice and having patients and doctors accept responsibility.”
Ultimately, the three doctors who treat adult patients concurred that the new guidelines are imperfect, and likely won’t result in any automatic widespread change. Dr. Rodbard admitted that she does not anticipate the guidelines affecting the way she treats her patients very much, if at all, while Dr. Lopez said that she’s “not sure it’s going to have a major effect.” Furthermore, the sheer volume of data and information available often leads to patients simply not knowing what to believe, which Dr. Roberts described as a “glazed over” effect of being told so many different things from so many different sources, patients just tune everything out.
Ultimately, while it’s important for health care providers to give advice and recommendations as necessary, these should be done on an individual basis. And, if a patient requires extensive dietary and nutritional intervention, that should be left in the hands of specialist who can accurately determine what the patient is capable of, what their goals should be, and how to tailor a plan specifically for them.
“I don’t know many clinicians, even in my own specialty, who know much about what specifically to eat,” said Dr. Roberts. “That should come from nutritional medicine people, not us.”
Dr. Lopez, Dr. Rodbard, and Dr. Roberts did not report any relevant financial disclosures.
*This article was updated 1/12/2016.
FROM THE USDA AND HHS