User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Dermatologic changes with COVID-19: What we know and don’t know
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
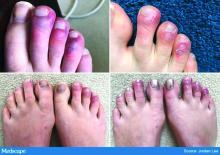
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
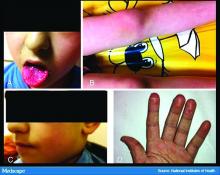
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
Planning for a psychiatric COVID-19–positive unit
Identifying key decision points is critical
Reports have emerged about the unique vulnerability of psychiatric hospitals to the ravages of COVID-19.
In a South Korea psychiatric hospital, 101 of 103 patients contracted SARS-CoV-2 during an outbreak; 7 eventually died.1,2 This report, among a few others, have led to the development of psychiatric COVID-19–positive units (PCU). However, it remains highly unclear how many are currently open, where they are located, or what their operations are like.
We knew that we could not allow a medically asymptomatic “covertly” COVID-19–positive patient to be introduced to the social community of our inpatient units because of the risks of transmission to other patients and staff.
In coordination with our health system infection prevention experts, we have therefore required a confirmed negative COVID-19 polymerase chain reaction nasal swab performed no more than 48 hours prior to the time/date of acute psychiatric inpatient admission. Furthermore, as part of the broad health system response and surge planning, we were asked by our respective incident command centers to begin planning for a Psychiatric COVID-19–positive Unit (PCU) that might allow us to safely care for a cohort of patients needing such hospitalization.
It is worth emphasizing that the typical patient who is a candidate for a PCU is so acutely psychiatrically ill that they cannot be managed in a less restrictive environment than an inpatient psychiatric unit and, at the same time, is likely to not be medically ill enough to warrant admission to an internal medicine service in a general acute care hospital.
We have identified eight principles and critical decision points that can help inpatient units plan for the safe care of COVID-19–positive patients on a PCU.
1. Triage: Patients admitted to a PCU should be medically stable, particularly with regard to COVID-19 and respiratory symptomatology. PCUs should establish clear criteria for admission and discharge (or medical transfer). Examples of potential exclusionary criteria to a PCU include:
- Respiratory distress, shortness of breath, hypoxia, requirement for supplemental oxygen, or requirement for respiratory therapy breathing treatments.
- Fever, or signs of sepsis, or systemic inflammatory response syndrome.
- Medical frailty, significant medical comorbidities, delirium, or altered mental status;
- Requirements for continuous vital sign monitoring or of a monitoring frequency beyond the capacity of the PCU.
Discharge criteria may also include a symptom-based strategy because emerging evidence suggests that patients may be less infectious by day 10-14 of the disease course,3 and viral lab testing is very sensitive and will be positive for periods of time after individuals are no longer infectious. The symptom-based strategy allows for patients to not require retesting prior to discharge. However, some receiving facilities (for example residential or skilled nursing facilities) may necessitate testing, in which case a testing-based strategy can be used. The Centers for Disease Control and Prevention provides guidelines for both types of strategies.4
2. Infection control and personal protective equipment: PCUs require modifications or departures from the typical inpatient free-ranging environment in which common areas are provided for patients to engage in a community of care, including group therapy (such as occupational, recreational, Alcoholics Anonymous, and social work groups).
- Isolation: PCUs must consider whether they will require patients to isolate to their rooms or to allow modified or limited access to “public” or “community” areas. While there do not appear to be standard recommendations from the CDC or other public health entities regarding negative pressure or any specific room ventilation requirements, it is prudent to work with local infectious disease experts on protocols. Important considerations include spatial planning for infection control areas to don and doff appropriate personal protective equipment (PPE) and appropriate workspace to prevent contamination of non–COVID-19 work areas. Approaches can include establishing clearly identified and visually demarcated infection control “zones” (often referred to as “hot, warm, and cold zones”) that correspond to specific PPE requirements for staff. In addition, individuals should eat in their own rooms or designated areas because use of common areas for meals can potentially lead to aerosolized spread of the virus.
- Cohorting: Generally, PCUs should consider admitting only COVID-19–positive patients to a PCU to avoid exposure to other patients. Hospitals and health systems should determine protocols and locations for testing and managing “patients under investigation” for COVID-19, which should precede admission to the PCU.
- PPE: It is important to clearly establish and communicate PPE requirements and procedures for direct physical contact versus no physical contact (for example, visual safety checks). Identify clear supply chains for PPE and hand sanitizer.
3. Medical management and consultation: PCUs should establish clear pathways for accessing consultation from medical consultants. It may be ideal, in addition to standard daily psychiatric physician rounding, to have daily internal medicine rounding and/or medical nursing staff working on the unit. Given the potential of COVID-19–positive patients to rapidly devolve from asymptomatic to acutely ill, it is necessary to establish protocols for the provision of urgent medical care 24/7 and streamlined processes for transfer to a medical unit.
Clear protocols should be established to address any potential signs of decompensation in the respiratory status of a PCU unit, including administration of oxygen and restrictions (or appropriate precautions) related to aerosolizing treatment such as nebulizers or positive airway pressure.
4. Code blue protocol: Any emergent medical issues, including acute respiratory decompensation, should trigger a Code Blue response that has been specifically designed for COVID-19–positive patients, including considerations for proper PPE during resuscitation efforts.
5. Psychiatric staffing and workflows: When possible, it may be preferable to engage volunteer medical and nursing staff for the PCU, as opposed to mandating participation. Take into consideration support needs, including education and training about safe PPE practices, processes for testing health care workers, return-to-work guidance, and potential alternate housing.
- Telehealth: Clinicians (such as physicians, social workers, occupational therapists) should leverage and maximize the use of telemedicine to minimize direct or prolonged exposure to infectious disease risks.
- Nursing: It is important to establish appropriate ratios of nursing and support staff for a COVID-19–positive psychiatry unit given the unique work flows related to isolation precautions and to ensure patient and staff safety. These ratios may take into account patient-specific needs, including the need for additional staff to perform constant observation for high-risk patients, management of agitated patients, and sufficient staff to allow for relief and break-time from PPE. Admission and routine care processes should be adapted in order to limit equipment entering the room, such as computer workstations on wheels.
- Medication administration procedures: Develop work flows related to PPE and infection control when retrieving and administering medications.
- Workspace: Designate appropriate workspace for PCU clinicians to access computers and documents and to minimize use of non–COVID-19 unit work areas.
6. Restraints and management of agitated patients: PCUs should develop plans for addressing agitated patients, including contingency plans for whether seclusion or restraints should be administered in the patient’s individual room or in a dedicated restraint room in the PCU. Staff training should include protocols specifically designed for managing agitated patients in the PCU.
7. Discharge processes: If patients remain medically well and clear their COVID-19 PCR tests, it is conceivable that they might be transferred to a non–COVID-19 psychiatric unit if sufficient isolation time has passed and the infectious disease consultants deem it appropriate. It is also possible that patients would be discharged from a PCU to home or other residential setting. Such patients should be assessed for ability to comply with continued self-quarantine if necessary. Discharge planning must take into consideration follow-up plans for COVID-19 illness and primary care appointments, as well as needed psychiatric follow-up.
8. Patients’ rights: The apparently highly infectious and transmissible nature of SARS-CoV-2 creates novel tensions between a wide range of individual rights and the rights of others. In addition to manifesting in our general society, there are potentially unique tensions in acute inpatient psychiatric settings. Certain patients’ rights may require modification in a PCU (for example, access to outdoor space, personal belongings, visitors, and possibly civil commitment judicial hearings). These discussions may require input from hospital compliance officers, ethics committees, risk managers, and the local department of mental health and also may be partly solved by using video communication platforms.
A few other “pearls” may be of value: Psychiatric hospitals that are colocated with a general acute care hospital or ED might be better situated to develop protocols to safely care for COVID-19–positive psychiatric patients, by virtue of the close proximity of full-spectrum acute general hospital services. Direct engagement by a command center and hospital or health system senior leadership also seems crucial as a means for assuring authorization to proceed with planning what may be a frightening or controversial (but necessary) adaptation of inpatient psychiatric unit(s) to the exigencies of the COVID-19 pandemic.
The resources of a robust community hospital or academic health system (including infection prevention leaders who engage in continuous liaison with local, county, state, and federal public health expertise) are crucial to the “learning health system” model, which requires flexibility, rapid adaptation to new knowledge, and accessibility to infectious disease and other consultation for special situations. Frequent and open communication with all professional stakeholders (through town halls, Q&A sessions, group discussions, and so on) is important in the planning process to socialize the principles and concepts that are critical for providing care in a PCU, reducing anxiety, and bolstering collegiality and staff morale.
References
1. Kim MJ. “ ‘It was a medical disaster’: The psychiatric ward that saw 100 patients with new coronavirus.” Independent. 2020 Mar 1.
2. Korean Society of Infectious Diseases et al. J Korean Med Sci. 2020 Mar 16;35(10):e112.
3. Centers for Disease Control and Prevention. Symptom-based strategy to discontinue isolation for persons with COVID-19. Decision Memo. 2020 May 3.
4. He X et al. Nature Medicine. 2020. 26:672-5.
Dr. Cheung is associate medical director and chief quality officer at the Stewart and Lynda Resnick Neuropsychiatric Hospital at the University of California, Los Angeles. He has no conflicts of interest. Dr. Strouse is medical director, UCLA Stewart and Lynda Resnick Neuropsychiatric Hospital and Maddie Katz Professor at the UCLA department of psychiatry/Semel Institute. He has no conflicts of interest. Dr. Li is associate medical director of quality improvement at Yale-New Haven Psychiatric Hospital in Connecticut. She also serves as medical director of clinical operations at the Yale-New Haven Health System. Dr. Li is a 2019-2020 Health and Aging Policy Fellow and receives funding support from the program.
Identifying key decision points is critical
Identifying key decision points is critical
Reports have emerged about the unique vulnerability of psychiatric hospitals to the ravages of COVID-19.
In a South Korea psychiatric hospital, 101 of 103 patients contracted SARS-CoV-2 during an outbreak; 7 eventually died.1,2 This report, among a few others, have led to the development of psychiatric COVID-19–positive units (PCU). However, it remains highly unclear how many are currently open, where they are located, or what their operations are like.
We knew that we could not allow a medically asymptomatic “covertly” COVID-19–positive patient to be introduced to the social community of our inpatient units because of the risks of transmission to other patients and staff.
In coordination with our health system infection prevention experts, we have therefore required a confirmed negative COVID-19 polymerase chain reaction nasal swab performed no more than 48 hours prior to the time/date of acute psychiatric inpatient admission. Furthermore, as part of the broad health system response and surge planning, we were asked by our respective incident command centers to begin planning for a Psychiatric COVID-19–positive Unit (PCU) that might allow us to safely care for a cohort of patients needing such hospitalization.
It is worth emphasizing that the typical patient who is a candidate for a PCU is so acutely psychiatrically ill that they cannot be managed in a less restrictive environment than an inpatient psychiatric unit and, at the same time, is likely to not be medically ill enough to warrant admission to an internal medicine service in a general acute care hospital.
We have identified eight principles and critical decision points that can help inpatient units plan for the safe care of COVID-19–positive patients on a PCU.
1. Triage: Patients admitted to a PCU should be medically stable, particularly with regard to COVID-19 and respiratory symptomatology. PCUs should establish clear criteria for admission and discharge (or medical transfer). Examples of potential exclusionary criteria to a PCU include:
- Respiratory distress, shortness of breath, hypoxia, requirement for supplemental oxygen, or requirement for respiratory therapy breathing treatments.
- Fever, or signs of sepsis, or systemic inflammatory response syndrome.
- Medical frailty, significant medical comorbidities, delirium, or altered mental status;
- Requirements for continuous vital sign monitoring or of a monitoring frequency beyond the capacity of the PCU.
Discharge criteria may also include a symptom-based strategy because emerging evidence suggests that patients may be less infectious by day 10-14 of the disease course,3 and viral lab testing is very sensitive and will be positive for periods of time after individuals are no longer infectious. The symptom-based strategy allows for patients to not require retesting prior to discharge. However, some receiving facilities (for example residential or skilled nursing facilities) may necessitate testing, in which case a testing-based strategy can be used. The Centers for Disease Control and Prevention provides guidelines for both types of strategies.4
2. Infection control and personal protective equipment: PCUs require modifications or departures from the typical inpatient free-ranging environment in which common areas are provided for patients to engage in a community of care, including group therapy (such as occupational, recreational, Alcoholics Anonymous, and social work groups).
- Isolation: PCUs must consider whether they will require patients to isolate to their rooms or to allow modified or limited access to “public” or “community” areas. While there do not appear to be standard recommendations from the CDC or other public health entities regarding negative pressure or any specific room ventilation requirements, it is prudent to work with local infectious disease experts on protocols. Important considerations include spatial planning for infection control areas to don and doff appropriate personal protective equipment (PPE) and appropriate workspace to prevent contamination of non–COVID-19 work areas. Approaches can include establishing clearly identified and visually demarcated infection control “zones” (often referred to as “hot, warm, and cold zones”) that correspond to specific PPE requirements for staff. In addition, individuals should eat in their own rooms or designated areas because use of common areas for meals can potentially lead to aerosolized spread of the virus.
- Cohorting: Generally, PCUs should consider admitting only COVID-19–positive patients to a PCU to avoid exposure to other patients. Hospitals and health systems should determine protocols and locations for testing and managing “patients under investigation” for COVID-19, which should precede admission to the PCU.
- PPE: It is important to clearly establish and communicate PPE requirements and procedures for direct physical contact versus no physical contact (for example, visual safety checks). Identify clear supply chains for PPE and hand sanitizer.
3. Medical management and consultation: PCUs should establish clear pathways for accessing consultation from medical consultants. It may be ideal, in addition to standard daily psychiatric physician rounding, to have daily internal medicine rounding and/or medical nursing staff working on the unit. Given the potential of COVID-19–positive patients to rapidly devolve from asymptomatic to acutely ill, it is necessary to establish protocols for the provision of urgent medical care 24/7 and streamlined processes for transfer to a medical unit.
Clear protocols should be established to address any potential signs of decompensation in the respiratory status of a PCU unit, including administration of oxygen and restrictions (or appropriate precautions) related to aerosolizing treatment such as nebulizers or positive airway pressure.
4. Code blue protocol: Any emergent medical issues, including acute respiratory decompensation, should trigger a Code Blue response that has been specifically designed for COVID-19–positive patients, including considerations for proper PPE during resuscitation efforts.
5. Psychiatric staffing and workflows: When possible, it may be preferable to engage volunteer medical and nursing staff for the PCU, as opposed to mandating participation. Take into consideration support needs, including education and training about safe PPE practices, processes for testing health care workers, return-to-work guidance, and potential alternate housing.
- Telehealth: Clinicians (such as physicians, social workers, occupational therapists) should leverage and maximize the use of telemedicine to minimize direct or prolonged exposure to infectious disease risks.
- Nursing: It is important to establish appropriate ratios of nursing and support staff for a COVID-19–positive psychiatry unit given the unique work flows related to isolation precautions and to ensure patient and staff safety. These ratios may take into account patient-specific needs, including the need for additional staff to perform constant observation for high-risk patients, management of agitated patients, and sufficient staff to allow for relief and break-time from PPE. Admission and routine care processes should be adapted in order to limit equipment entering the room, such as computer workstations on wheels.
- Medication administration procedures: Develop work flows related to PPE and infection control when retrieving and administering medications.
- Workspace: Designate appropriate workspace for PCU clinicians to access computers and documents and to minimize use of non–COVID-19 unit work areas.
6. Restraints and management of agitated patients: PCUs should develop plans for addressing agitated patients, including contingency plans for whether seclusion or restraints should be administered in the patient’s individual room or in a dedicated restraint room in the PCU. Staff training should include protocols specifically designed for managing agitated patients in the PCU.
7. Discharge processes: If patients remain medically well and clear their COVID-19 PCR tests, it is conceivable that they might be transferred to a non–COVID-19 psychiatric unit if sufficient isolation time has passed and the infectious disease consultants deem it appropriate. It is also possible that patients would be discharged from a PCU to home or other residential setting. Such patients should be assessed for ability to comply with continued self-quarantine if necessary. Discharge planning must take into consideration follow-up plans for COVID-19 illness and primary care appointments, as well as needed psychiatric follow-up.
8. Patients’ rights: The apparently highly infectious and transmissible nature of SARS-CoV-2 creates novel tensions between a wide range of individual rights and the rights of others. In addition to manifesting in our general society, there are potentially unique tensions in acute inpatient psychiatric settings. Certain patients’ rights may require modification in a PCU (for example, access to outdoor space, personal belongings, visitors, and possibly civil commitment judicial hearings). These discussions may require input from hospital compliance officers, ethics committees, risk managers, and the local department of mental health and also may be partly solved by using video communication platforms.
A few other “pearls” may be of value: Psychiatric hospitals that are colocated with a general acute care hospital or ED might be better situated to develop protocols to safely care for COVID-19–positive psychiatric patients, by virtue of the close proximity of full-spectrum acute general hospital services. Direct engagement by a command center and hospital or health system senior leadership also seems crucial as a means for assuring authorization to proceed with planning what may be a frightening or controversial (but necessary) adaptation of inpatient psychiatric unit(s) to the exigencies of the COVID-19 pandemic.
The resources of a robust community hospital or academic health system (including infection prevention leaders who engage in continuous liaison with local, county, state, and federal public health expertise) are crucial to the “learning health system” model, which requires flexibility, rapid adaptation to new knowledge, and accessibility to infectious disease and other consultation for special situations. Frequent and open communication with all professional stakeholders (through town halls, Q&A sessions, group discussions, and so on) is important in the planning process to socialize the principles and concepts that are critical for providing care in a PCU, reducing anxiety, and bolstering collegiality and staff morale.
References
1. Kim MJ. “ ‘It was a medical disaster’: The psychiatric ward that saw 100 patients with new coronavirus.” Independent. 2020 Mar 1.
2. Korean Society of Infectious Diseases et al. J Korean Med Sci. 2020 Mar 16;35(10):e112.
3. Centers for Disease Control and Prevention. Symptom-based strategy to discontinue isolation for persons with COVID-19. Decision Memo. 2020 May 3.
4. He X et al. Nature Medicine. 2020. 26:672-5.
Dr. Cheung is associate medical director and chief quality officer at the Stewart and Lynda Resnick Neuropsychiatric Hospital at the University of California, Los Angeles. He has no conflicts of interest. Dr. Strouse is medical director, UCLA Stewart and Lynda Resnick Neuropsychiatric Hospital and Maddie Katz Professor at the UCLA department of psychiatry/Semel Institute. He has no conflicts of interest. Dr. Li is associate medical director of quality improvement at Yale-New Haven Psychiatric Hospital in Connecticut. She also serves as medical director of clinical operations at the Yale-New Haven Health System. Dr. Li is a 2019-2020 Health and Aging Policy Fellow and receives funding support from the program.
Reports have emerged about the unique vulnerability of psychiatric hospitals to the ravages of COVID-19.
In a South Korea psychiatric hospital, 101 of 103 patients contracted SARS-CoV-2 during an outbreak; 7 eventually died.1,2 This report, among a few others, have led to the development of psychiatric COVID-19–positive units (PCU). However, it remains highly unclear how many are currently open, where they are located, or what their operations are like.
We knew that we could not allow a medically asymptomatic “covertly” COVID-19–positive patient to be introduced to the social community of our inpatient units because of the risks of transmission to other patients and staff.
In coordination with our health system infection prevention experts, we have therefore required a confirmed negative COVID-19 polymerase chain reaction nasal swab performed no more than 48 hours prior to the time/date of acute psychiatric inpatient admission. Furthermore, as part of the broad health system response and surge planning, we were asked by our respective incident command centers to begin planning for a Psychiatric COVID-19–positive Unit (PCU) that might allow us to safely care for a cohort of patients needing such hospitalization.
It is worth emphasizing that the typical patient who is a candidate for a PCU is so acutely psychiatrically ill that they cannot be managed in a less restrictive environment than an inpatient psychiatric unit and, at the same time, is likely to not be medically ill enough to warrant admission to an internal medicine service in a general acute care hospital.
We have identified eight principles and critical decision points that can help inpatient units plan for the safe care of COVID-19–positive patients on a PCU.
1. Triage: Patients admitted to a PCU should be medically stable, particularly with regard to COVID-19 and respiratory symptomatology. PCUs should establish clear criteria for admission and discharge (or medical transfer). Examples of potential exclusionary criteria to a PCU include:
- Respiratory distress, shortness of breath, hypoxia, requirement for supplemental oxygen, or requirement for respiratory therapy breathing treatments.
- Fever, or signs of sepsis, or systemic inflammatory response syndrome.
- Medical frailty, significant medical comorbidities, delirium, or altered mental status;
- Requirements for continuous vital sign monitoring or of a monitoring frequency beyond the capacity of the PCU.
Discharge criteria may also include a symptom-based strategy because emerging evidence suggests that patients may be less infectious by day 10-14 of the disease course,3 and viral lab testing is very sensitive and will be positive for periods of time after individuals are no longer infectious. The symptom-based strategy allows for patients to not require retesting prior to discharge. However, some receiving facilities (for example residential or skilled nursing facilities) may necessitate testing, in which case a testing-based strategy can be used. The Centers for Disease Control and Prevention provides guidelines for both types of strategies.4
2. Infection control and personal protective equipment: PCUs require modifications or departures from the typical inpatient free-ranging environment in which common areas are provided for patients to engage in a community of care, including group therapy (such as occupational, recreational, Alcoholics Anonymous, and social work groups).
- Isolation: PCUs must consider whether they will require patients to isolate to their rooms or to allow modified or limited access to “public” or “community” areas. While there do not appear to be standard recommendations from the CDC or other public health entities regarding negative pressure or any specific room ventilation requirements, it is prudent to work with local infectious disease experts on protocols. Important considerations include spatial planning for infection control areas to don and doff appropriate personal protective equipment (PPE) and appropriate workspace to prevent contamination of non–COVID-19 work areas. Approaches can include establishing clearly identified and visually demarcated infection control “zones” (often referred to as “hot, warm, and cold zones”) that correspond to specific PPE requirements for staff. In addition, individuals should eat in their own rooms or designated areas because use of common areas for meals can potentially lead to aerosolized spread of the virus.
- Cohorting: Generally, PCUs should consider admitting only COVID-19–positive patients to a PCU to avoid exposure to other patients. Hospitals and health systems should determine protocols and locations for testing and managing “patients under investigation” for COVID-19, which should precede admission to the PCU.
- PPE: It is important to clearly establish and communicate PPE requirements and procedures for direct physical contact versus no physical contact (for example, visual safety checks). Identify clear supply chains for PPE and hand sanitizer.
3. Medical management and consultation: PCUs should establish clear pathways for accessing consultation from medical consultants. It may be ideal, in addition to standard daily psychiatric physician rounding, to have daily internal medicine rounding and/or medical nursing staff working on the unit. Given the potential of COVID-19–positive patients to rapidly devolve from asymptomatic to acutely ill, it is necessary to establish protocols for the provision of urgent medical care 24/7 and streamlined processes for transfer to a medical unit.
Clear protocols should be established to address any potential signs of decompensation in the respiratory status of a PCU unit, including administration of oxygen and restrictions (or appropriate precautions) related to aerosolizing treatment such as nebulizers or positive airway pressure.
4. Code blue protocol: Any emergent medical issues, including acute respiratory decompensation, should trigger a Code Blue response that has been specifically designed for COVID-19–positive patients, including considerations for proper PPE during resuscitation efforts.
5. Psychiatric staffing and workflows: When possible, it may be preferable to engage volunteer medical and nursing staff for the PCU, as opposed to mandating participation. Take into consideration support needs, including education and training about safe PPE practices, processes for testing health care workers, return-to-work guidance, and potential alternate housing.
- Telehealth: Clinicians (such as physicians, social workers, occupational therapists) should leverage and maximize the use of telemedicine to minimize direct or prolonged exposure to infectious disease risks.
- Nursing: It is important to establish appropriate ratios of nursing and support staff for a COVID-19–positive psychiatry unit given the unique work flows related to isolation precautions and to ensure patient and staff safety. These ratios may take into account patient-specific needs, including the need for additional staff to perform constant observation for high-risk patients, management of agitated patients, and sufficient staff to allow for relief and break-time from PPE. Admission and routine care processes should be adapted in order to limit equipment entering the room, such as computer workstations on wheels.
- Medication administration procedures: Develop work flows related to PPE and infection control when retrieving and administering medications.
- Workspace: Designate appropriate workspace for PCU clinicians to access computers and documents and to minimize use of non–COVID-19 unit work areas.
6. Restraints and management of agitated patients: PCUs should develop plans for addressing agitated patients, including contingency plans for whether seclusion or restraints should be administered in the patient’s individual room or in a dedicated restraint room in the PCU. Staff training should include protocols specifically designed for managing agitated patients in the PCU.
7. Discharge processes: If patients remain medically well and clear their COVID-19 PCR tests, it is conceivable that they might be transferred to a non–COVID-19 psychiatric unit if sufficient isolation time has passed and the infectious disease consultants deem it appropriate. It is also possible that patients would be discharged from a PCU to home or other residential setting. Such patients should be assessed for ability to comply with continued self-quarantine if necessary. Discharge planning must take into consideration follow-up plans for COVID-19 illness and primary care appointments, as well as needed psychiatric follow-up.
8. Patients’ rights: The apparently highly infectious and transmissible nature of SARS-CoV-2 creates novel tensions between a wide range of individual rights and the rights of others. In addition to manifesting in our general society, there are potentially unique tensions in acute inpatient psychiatric settings. Certain patients’ rights may require modification in a PCU (for example, access to outdoor space, personal belongings, visitors, and possibly civil commitment judicial hearings). These discussions may require input from hospital compliance officers, ethics committees, risk managers, and the local department of mental health and also may be partly solved by using video communication platforms.
A few other “pearls” may be of value: Psychiatric hospitals that are colocated with a general acute care hospital or ED might be better situated to develop protocols to safely care for COVID-19–positive psychiatric patients, by virtue of the close proximity of full-spectrum acute general hospital services. Direct engagement by a command center and hospital or health system senior leadership also seems crucial as a means for assuring authorization to proceed with planning what may be a frightening or controversial (but necessary) adaptation of inpatient psychiatric unit(s) to the exigencies of the COVID-19 pandemic.
The resources of a robust community hospital or academic health system (including infection prevention leaders who engage in continuous liaison with local, county, state, and federal public health expertise) are crucial to the “learning health system” model, which requires flexibility, rapid adaptation to new knowledge, and accessibility to infectious disease and other consultation for special situations. Frequent and open communication with all professional stakeholders (through town halls, Q&A sessions, group discussions, and so on) is important in the planning process to socialize the principles and concepts that are critical for providing care in a PCU, reducing anxiety, and bolstering collegiality and staff morale.
References
1. Kim MJ. “ ‘It was a medical disaster’: The psychiatric ward that saw 100 patients with new coronavirus.” Independent. 2020 Mar 1.
2. Korean Society of Infectious Diseases et al. J Korean Med Sci. 2020 Mar 16;35(10):e112.
3. Centers for Disease Control and Prevention. Symptom-based strategy to discontinue isolation for persons with COVID-19. Decision Memo. 2020 May 3.
4. He X et al. Nature Medicine. 2020. 26:672-5.
Dr. Cheung is associate medical director and chief quality officer at the Stewart and Lynda Resnick Neuropsychiatric Hospital at the University of California, Los Angeles. He has no conflicts of interest. Dr. Strouse is medical director, UCLA Stewart and Lynda Resnick Neuropsychiatric Hospital and Maddie Katz Professor at the UCLA department of psychiatry/Semel Institute. He has no conflicts of interest. Dr. Li is associate medical director of quality improvement at Yale-New Haven Psychiatric Hospital in Connecticut. She also serves as medical director of clinical operations at the Yale-New Haven Health System. Dr. Li is a 2019-2020 Health and Aging Policy Fellow and receives funding support from the program.
Glucose control linked to COVID-19 outcomes in largest-yet study
The strong link between glucose control and COVID-19 outcomes has been reaffirmed in the largest study thus far of hospitalized patients with preexisting type 2 diabetes.
The retrospective, multicenter study, from 7,337 hospitalized patients with COVID-19, was published online in Cell Metabolism by Lihua Zhu, Renmin Hospital of Wuhan University, China, and colleagues.
The study finds that, while the presence of type 2 diabetes per se is a risk factor for worse COVID-19 outcomes, better glycemic control among those with preexisting type 2 diabetes appears to be associated with significant reductions in adverse outcomes and death.
“We were surprised to see such favorable outcomes in the well-controlled blood glucose group among patients with COVID-19 and preexisting type 2 diabetes,” senior author Hongliang Li, also of Renmin Hospital, said in a statement.
“Considering that people with diabetes had much higher risk for death and various complications, and there are no specific drugs for COVID-19, our findings indicate that controlling blood glucose well may act as an effective auxiliary approach to improve the prognosis of patients with COVID-19 and preexisting diabetes,” Dr. Li added.
Asked to comment on the findings, David Klonoff, MD, medical director of the Diabetes Research Institute at Mills–Peninsula Medical Center, San Mateo, Calif., cautioned that the way in which the “well-controlled” diabetes group was distinguished from the “poorly controlled” one in this study used a “nonstandard method for distinguishing these groups based on variability.”
So “there was a great deal of overlap between the two groups,” he observed.
Diabetes itself was associated with worse COVID-19 outcomes
Of the 7,337 participants with confirmed COVID-19 in the Chinese study, 13% (952) had preexisting type 2 diabetes while the other 6,385 did not have diabetes.
Median ages were 62 years for those with and 53 years for those without diabetes. As has been reported several times since the pandemic began, the presence of diabetes was associated with a worse COVID-19 prognosis.
Those with preexisting diabetes received significantly more antibiotics, antifungals, systemic corticosteroids, immunoglobulin, antihypertensive drugs, and vasoactive drugs than did those without diabetes. They were also more likely to receive oxygen inhalation (76.9% vs. 61.2%), noninvasive ventilation (10.2% vs. 3.9%), and invasive ventilation (3.6% vs. 0.7%).
Over 28 days starting with the day of admission, the type 2 diabetes group was significantly more likely to die compared with those without diabetes (7.8% vs. 2.7%; P < .001), with a crude hazard ratio of 2.90 (P < .001). After adjustments for age, gender, and COVID-19 severity, the diabetes group was still significantly more likely to die, with a hazard ratio of 1.49 (P = .005).
Those with diabetes were also significantly more likely to develop acute respiratory distress syndrome (adjusted hazard ratio, 1.44), acute kidney injury (3.01), and septic shock (1.95).
“The results were unequivocal to implicate diabetes mellitus in higher risk of death and other detrimental outcomes of COVID-19,” the authors wrote, although they caution “there were notable differences in the covariate distributions between the two groups.”
With T2D, tighter glycemic control predicted better outcome
Among the 952 with COVID-19 and type 2 diabetes, 282 individuals had “well-controlled” blood glucose, ranging from 3.9 to 10.0 mmol/L (~70 - 180 mg/dL) with median 6.4 mmol/L (115 mg/dL) and hemoglobin A1c of 7.3%.
The other 528 were “poorly controlled,” defined as the lowest fasting glucose level 3.9 mmol/L or above and the highest 2-hour postprandial glucose exceeding 10.0 mmol/L, with median 10.9 mmol/L (196 mg/dL) and HbA1c of 8.1%.
Just as with the diabetes vs. no diabetes comparison, those in the “well-controlled” blood glucose group had lower use of antivirals, antibiotics, antifungals, systemic corticosteroids, immunoglobulin, and vasoactive drugs.
They also were less likely to require oxygen inhalation (70.2% vs. 83.5%), non-invasive ventilation (4.6% vs. 11.9%), invasive ventilation (0% vs. 4.2%), and extracorporeal membrane oxygenation (0% vs. 0.8%).
In-hospital death was significantly lower in the “well-controlled” group (1.1% vs. 11.0%; crude hazard ratio, 0.09; P < .001). After adjustments for the previous factors plus site effect, the difference remained significant (0.13; P < .001). Adjusted hazard ratio for acute respiratory distress syndrome was 0.41 (P < .001) and for acute heart injury it was 0.21 (P = .003).
Stress hyperglycemia in COVID-19 associated with greater mortality
Klonoff was senior author on a previous study from the United States that showed that both diabetes and uncontrolled hyperglycemia among people without prior diabetes – the latter “presumably due to stress,” he said – were strong predictors of mortality among hospitalized patients with COVID-19.
The new Chinese research only looks at individuals with previously diagnosed type 2 diabetes, Klonoff pointed out in an interview.
“The article by Zhu et al. did not look at outcomes of hospitalized COVID-19 patients with uncontrolled hyperglycemia. Per [the U.S. study], in COVID-19 stress hyperglycemia, compared to diabetes, was associated with greater mortality.”
In addition, although international guidance now advises optimizing blood glucose levels in all patients with hyperglycemia and COVID-19, it’s actually not yet totally clear which in-target range improves COVID-19 prognosis the best, Dr. Klonoff said.
He is now working on a study aimed at answering that question.
The researchers have disclosed no relevant financial relationships. Dr. Klonoff is a consultant to Abbott, Ascensia, Dexcom, EOFlow, Fractyl, Lifecare, Novo, Roche, and ThirdWayv.
A version of this article originally appeared on Medscape.com.
The strong link between glucose control and COVID-19 outcomes has been reaffirmed in the largest study thus far of hospitalized patients with preexisting type 2 diabetes.
The retrospective, multicenter study, from 7,337 hospitalized patients with COVID-19, was published online in Cell Metabolism by Lihua Zhu, Renmin Hospital of Wuhan University, China, and colleagues.
The study finds that, while the presence of type 2 diabetes per se is a risk factor for worse COVID-19 outcomes, better glycemic control among those with preexisting type 2 diabetes appears to be associated with significant reductions in adverse outcomes and death.
“We were surprised to see such favorable outcomes in the well-controlled blood glucose group among patients with COVID-19 and preexisting type 2 diabetes,” senior author Hongliang Li, also of Renmin Hospital, said in a statement.
“Considering that people with diabetes had much higher risk for death and various complications, and there are no specific drugs for COVID-19, our findings indicate that controlling blood glucose well may act as an effective auxiliary approach to improve the prognosis of patients with COVID-19 and preexisting diabetes,” Dr. Li added.
Asked to comment on the findings, David Klonoff, MD, medical director of the Diabetes Research Institute at Mills–Peninsula Medical Center, San Mateo, Calif., cautioned that the way in which the “well-controlled” diabetes group was distinguished from the “poorly controlled” one in this study used a “nonstandard method for distinguishing these groups based on variability.”
So “there was a great deal of overlap between the two groups,” he observed.
Diabetes itself was associated with worse COVID-19 outcomes
Of the 7,337 participants with confirmed COVID-19 in the Chinese study, 13% (952) had preexisting type 2 diabetes while the other 6,385 did not have diabetes.
Median ages were 62 years for those with and 53 years for those without diabetes. As has been reported several times since the pandemic began, the presence of diabetes was associated with a worse COVID-19 prognosis.
Those with preexisting diabetes received significantly more antibiotics, antifungals, systemic corticosteroids, immunoglobulin, antihypertensive drugs, and vasoactive drugs than did those without diabetes. They were also more likely to receive oxygen inhalation (76.9% vs. 61.2%), noninvasive ventilation (10.2% vs. 3.9%), and invasive ventilation (3.6% vs. 0.7%).
Over 28 days starting with the day of admission, the type 2 diabetes group was significantly more likely to die compared with those without diabetes (7.8% vs. 2.7%; P < .001), with a crude hazard ratio of 2.90 (P < .001). After adjustments for age, gender, and COVID-19 severity, the diabetes group was still significantly more likely to die, with a hazard ratio of 1.49 (P = .005).
Those with diabetes were also significantly more likely to develop acute respiratory distress syndrome (adjusted hazard ratio, 1.44), acute kidney injury (3.01), and septic shock (1.95).
“The results were unequivocal to implicate diabetes mellitus in higher risk of death and other detrimental outcomes of COVID-19,” the authors wrote, although they caution “there were notable differences in the covariate distributions between the two groups.”
With T2D, tighter glycemic control predicted better outcome
Among the 952 with COVID-19 and type 2 diabetes, 282 individuals had “well-controlled” blood glucose, ranging from 3.9 to 10.0 mmol/L (~70 - 180 mg/dL) with median 6.4 mmol/L (115 mg/dL) and hemoglobin A1c of 7.3%.
The other 528 were “poorly controlled,” defined as the lowest fasting glucose level 3.9 mmol/L or above and the highest 2-hour postprandial glucose exceeding 10.0 mmol/L, with median 10.9 mmol/L (196 mg/dL) and HbA1c of 8.1%.
Just as with the diabetes vs. no diabetes comparison, those in the “well-controlled” blood glucose group had lower use of antivirals, antibiotics, antifungals, systemic corticosteroids, immunoglobulin, and vasoactive drugs.
They also were less likely to require oxygen inhalation (70.2% vs. 83.5%), non-invasive ventilation (4.6% vs. 11.9%), invasive ventilation (0% vs. 4.2%), and extracorporeal membrane oxygenation (0% vs. 0.8%).
In-hospital death was significantly lower in the “well-controlled” group (1.1% vs. 11.0%; crude hazard ratio, 0.09; P < .001). After adjustments for the previous factors plus site effect, the difference remained significant (0.13; P < .001). Adjusted hazard ratio for acute respiratory distress syndrome was 0.41 (P < .001) and for acute heart injury it was 0.21 (P = .003).
Stress hyperglycemia in COVID-19 associated with greater mortality
Klonoff was senior author on a previous study from the United States that showed that both diabetes and uncontrolled hyperglycemia among people without prior diabetes – the latter “presumably due to stress,” he said – were strong predictors of mortality among hospitalized patients with COVID-19.
The new Chinese research only looks at individuals with previously diagnosed type 2 diabetes, Klonoff pointed out in an interview.
“The article by Zhu et al. did not look at outcomes of hospitalized COVID-19 patients with uncontrolled hyperglycemia. Per [the U.S. study], in COVID-19 stress hyperglycemia, compared to diabetes, was associated with greater mortality.”
In addition, although international guidance now advises optimizing blood glucose levels in all patients with hyperglycemia and COVID-19, it’s actually not yet totally clear which in-target range improves COVID-19 prognosis the best, Dr. Klonoff said.
He is now working on a study aimed at answering that question.
The researchers have disclosed no relevant financial relationships. Dr. Klonoff is a consultant to Abbott, Ascensia, Dexcom, EOFlow, Fractyl, Lifecare, Novo, Roche, and ThirdWayv.
A version of this article originally appeared on Medscape.com.
The strong link between glucose control and COVID-19 outcomes has been reaffirmed in the largest study thus far of hospitalized patients with preexisting type 2 diabetes.
The retrospective, multicenter study, from 7,337 hospitalized patients with COVID-19, was published online in Cell Metabolism by Lihua Zhu, Renmin Hospital of Wuhan University, China, and colleagues.
The study finds that, while the presence of type 2 diabetes per se is a risk factor for worse COVID-19 outcomes, better glycemic control among those with preexisting type 2 diabetes appears to be associated with significant reductions in adverse outcomes and death.
“We were surprised to see such favorable outcomes in the well-controlled blood glucose group among patients with COVID-19 and preexisting type 2 diabetes,” senior author Hongliang Li, also of Renmin Hospital, said in a statement.
“Considering that people with diabetes had much higher risk for death and various complications, and there are no specific drugs for COVID-19, our findings indicate that controlling blood glucose well may act as an effective auxiliary approach to improve the prognosis of patients with COVID-19 and preexisting diabetes,” Dr. Li added.
Asked to comment on the findings, David Klonoff, MD, medical director of the Diabetes Research Institute at Mills–Peninsula Medical Center, San Mateo, Calif., cautioned that the way in which the “well-controlled” diabetes group was distinguished from the “poorly controlled” one in this study used a “nonstandard method for distinguishing these groups based on variability.”
So “there was a great deal of overlap between the two groups,” he observed.
Diabetes itself was associated with worse COVID-19 outcomes
Of the 7,337 participants with confirmed COVID-19 in the Chinese study, 13% (952) had preexisting type 2 diabetes while the other 6,385 did not have diabetes.
Median ages were 62 years for those with and 53 years for those without diabetes. As has been reported several times since the pandemic began, the presence of diabetes was associated with a worse COVID-19 prognosis.
Those with preexisting diabetes received significantly more antibiotics, antifungals, systemic corticosteroids, immunoglobulin, antihypertensive drugs, and vasoactive drugs than did those without diabetes. They were also more likely to receive oxygen inhalation (76.9% vs. 61.2%), noninvasive ventilation (10.2% vs. 3.9%), and invasive ventilation (3.6% vs. 0.7%).
Over 28 days starting with the day of admission, the type 2 diabetes group was significantly more likely to die compared with those without diabetes (7.8% vs. 2.7%; P < .001), with a crude hazard ratio of 2.90 (P < .001). After adjustments for age, gender, and COVID-19 severity, the diabetes group was still significantly more likely to die, with a hazard ratio of 1.49 (P = .005).
Those with diabetes were also significantly more likely to develop acute respiratory distress syndrome (adjusted hazard ratio, 1.44), acute kidney injury (3.01), and septic shock (1.95).
“The results were unequivocal to implicate diabetes mellitus in higher risk of death and other detrimental outcomes of COVID-19,” the authors wrote, although they caution “there were notable differences in the covariate distributions between the two groups.”
With T2D, tighter glycemic control predicted better outcome
Among the 952 with COVID-19 and type 2 diabetes, 282 individuals had “well-controlled” blood glucose, ranging from 3.9 to 10.0 mmol/L (~70 - 180 mg/dL) with median 6.4 mmol/L (115 mg/dL) and hemoglobin A1c of 7.3%.
The other 528 were “poorly controlled,” defined as the lowest fasting glucose level 3.9 mmol/L or above and the highest 2-hour postprandial glucose exceeding 10.0 mmol/L, with median 10.9 mmol/L (196 mg/dL) and HbA1c of 8.1%.
Just as with the diabetes vs. no diabetes comparison, those in the “well-controlled” blood glucose group had lower use of antivirals, antibiotics, antifungals, systemic corticosteroids, immunoglobulin, and vasoactive drugs.
They also were less likely to require oxygen inhalation (70.2% vs. 83.5%), non-invasive ventilation (4.6% vs. 11.9%), invasive ventilation (0% vs. 4.2%), and extracorporeal membrane oxygenation (0% vs. 0.8%).
In-hospital death was significantly lower in the “well-controlled” group (1.1% vs. 11.0%; crude hazard ratio, 0.09; P < .001). After adjustments for the previous factors plus site effect, the difference remained significant (0.13; P < .001). Adjusted hazard ratio for acute respiratory distress syndrome was 0.41 (P < .001) and for acute heart injury it was 0.21 (P = .003).
Stress hyperglycemia in COVID-19 associated with greater mortality
Klonoff was senior author on a previous study from the United States that showed that both diabetes and uncontrolled hyperglycemia among people without prior diabetes – the latter “presumably due to stress,” he said – were strong predictors of mortality among hospitalized patients with COVID-19.
The new Chinese research only looks at individuals with previously diagnosed type 2 diabetes, Klonoff pointed out in an interview.
“The article by Zhu et al. did not look at outcomes of hospitalized COVID-19 patients with uncontrolled hyperglycemia. Per [the U.S. study], in COVID-19 stress hyperglycemia, compared to diabetes, was associated with greater mortality.”
In addition, although international guidance now advises optimizing blood glucose levels in all patients with hyperglycemia and COVID-19, it’s actually not yet totally clear which in-target range improves COVID-19 prognosis the best, Dr. Klonoff said.
He is now working on a study aimed at answering that question.
The researchers have disclosed no relevant financial relationships. Dr. Klonoff is a consultant to Abbott, Ascensia, Dexcom, EOFlow, Fractyl, Lifecare, Novo, Roche, and ThirdWayv.
A version of this article originally appeared on Medscape.com.
Hazard pay included in new COVID-19 relief bill
Hazard pay for frontline health care workers – an idea that has been championed by President Donald J. Trump and Senate Minority Leader Chuck Schumer, among others – is included in a just-released COVID-19 relief package assembled by Democrats in the House of Representatives.
according to a report in the Washington Post.
But it is far from a done deal. “The Democrats’ spending bill is a Pelosi-led pipe dream written in private,” said House Republican Leader Kevin McCarthy (Calif.) in a Fox News interview posted May 12 on Facebook.
Senate Majority Leader Mitch McConnell condemned the package. “This is exactly the wrong approach,” he said in a prepared statement that instead laid out a variety of liability protections, which he said should be the first priority.
“We are not going to let health care heroes emerge from this crisis facing a tidal wave of medical malpractice lawsuits so that trial lawyers can line their pockets,” said Sen. McConnell, adding that his plan would “raise the liability threshold for COVID-related malpractice lawsuits.”
Ingrida Lusis, vice president of government affairs and health policy at the American Nurses Association, said in an interview that the ANA had lobbied for hazard pay and was told it would be in the next relief package.
“Though there is an inherent risk in the nursing profession, we think that this is really critical to ensuring that we have a workforce to meet the intense demands of this pandemic,” said Ms. Lusis.
“If health care workers are not treated and compensated appropriately for what they’re going through right now, then we may not have a next generation that will want to enter the field,” she said.
Various nursing organizations, nurses’ unions, and health care unions, such as the American Federation of State, County and Municipal Employees (AFSCME) and the Service Employees International Union, have advocated for hazard pay.
Physicians’ organizations have not been vocal on the issue, however. The American Medical Association, for instance, pushed for hazard pay for residents but has not made any further public statements. An AMA spokesman said that the group was monitoring the situation but declined further comment.
Multiple online petitions seeking hazard pay for health care workers have been circulated, including one seeking the same $600 bump for essential workers that was given out as part of unemployment benefits in the first COVID-19 relief package. More than 1.2 million had signed the petition as of May 12.
‘Heroes fund’
The president first suggested hazard pay for health care workers on March 30 Fox News broadcast. “These are really brave people,” he said, adding that the administration was considering different ways of boosting pay, primarily through hospitals.
“We are asking the hospitals to do it and to consider something, including bonuses,” said Trump. “If anybody’s entitled to it, they are.”
On April 7, Sen. Schumer proposed a “Heroes Fund.” It would give public, private, and tribal frontline employees – including doctors, nurses, first responders, and transit, grocery, and postal workers – a $13 per hour raise up to $25,000 in additional pay through Dec. 31 for workers earning up to $200,000 and $5,000 in additional pay for those earning more than $200,000. It would also provide a $15,000 signing bonus to those who agree to take on such a position.
Rep. Matt Cartwright (D-Pa.) introduced a bill in mid-April, the Coronavirus Frontline Workers Fair Pay Act (HR 6709), that would provide similar pay increases. Health care workers would receive an additional $13 per hour. It would be retroactive to Jan. 31, 2020, and would be available through the end of 2020.
Molly Kinder of the Brookings Institution, a self-described nonpartisan Washington policy institute, estimates that Sen. Schumer’s proposal would represent the equivalent of double-time pay for the average low-wage worker, a 50% pay increase for a mail carrier, a 20% boost for a pharmacist, and less than a 15% increase for a surgeon, as determined from median 2018 wages.
Before the House Democrats unveiled their bill, Isabel Soto of the center-right group American Action Forum estimated that a $13 per hour wage increase could cost $398.9 billion just from the end of March to the end of September. A great proportion of that amount – $264 billion – would go to some 10 million health care workers, Ms. Soto calculated.
Some already offering pay boost
A few states and hospital systems are already offering hazard pay.
On April 12, Massachusetts agreed to give about 6,500 AFSCME union members who work at state human services facilities and group homes a $5 or a $10 per hour pay increase, depending on duties. It was to stay in effect until at least May 30.
Maine Governor Janet Mills (D) also agreed to increase pay by $3-$5 an hour for AFSCME workers in state correctional and mental health facilities beginning March 29.
In New York City, the biggest hospital network, Northwell Health, in late April gave 45,000 workers – including nurses, physicians, respiratory therapists, environmental services workers, housekeepers, and people in outpatient and corporate roles – a lump sum bonus payment of up to $2,500 and 1 week of paid time off. The money came out of the system’s general fund.
“As an organization, we want to continue to support, motivate and inspire our team members,” said Northwell President and CEO Michael Dowling in a statement at the time.
On April 2, New York–Presbyterian Hospital’s chair of the department of surgery, Craig Smith, MD, announced that the facility was “providing a $1,250 bonus for everyone who has worked in or supported the COVID-19 front lines, for at least 1 week.”
Advocate Aurora, with 15 hospitals and 32,000 employees in Wisconsin, said in early April that it was giving increases of $6.25-$15.00 an hour at least through the end of May.
A version of this article originally appeared on Medscape.com.
Hazard pay for frontline health care workers – an idea that has been championed by President Donald J. Trump and Senate Minority Leader Chuck Schumer, among others – is included in a just-released COVID-19 relief package assembled by Democrats in the House of Representatives.
according to a report in the Washington Post.
But it is far from a done deal. “The Democrats’ spending bill is a Pelosi-led pipe dream written in private,” said House Republican Leader Kevin McCarthy (Calif.) in a Fox News interview posted May 12 on Facebook.
Senate Majority Leader Mitch McConnell condemned the package. “This is exactly the wrong approach,” he said in a prepared statement that instead laid out a variety of liability protections, which he said should be the first priority.
“We are not going to let health care heroes emerge from this crisis facing a tidal wave of medical malpractice lawsuits so that trial lawyers can line their pockets,” said Sen. McConnell, adding that his plan would “raise the liability threshold for COVID-related malpractice lawsuits.”
Ingrida Lusis, vice president of government affairs and health policy at the American Nurses Association, said in an interview that the ANA had lobbied for hazard pay and was told it would be in the next relief package.
“Though there is an inherent risk in the nursing profession, we think that this is really critical to ensuring that we have a workforce to meet the intense demands of this pandemic,” said Ms. Lusis.
“If health care workers are not treated and compensated appropriately for what they’re going through right now, then we may not have a next generation that will want to enter the field,” she said.
Various nursing organizations, nurses’ unions, and health care unions, such as the American Federation of State, County and Municipal Employees (AFSCME) and the Service Employees International Union, have advocated for hazard pay.
Physicians’ organizations have not been vocal on the issue, however. The American Medical Association, for instance, pushed for hazard pay for residents but has not made any further public statements. An AMA spokesman said that the group was monitoring the situation but declined further comment.
Multiple online petitions seeking hazard pay for health care workers have been circulated, including one seeking the same $600 bump for essential workers that was given out as part of unemployment benefits in the first COVID-19 relief package. More than 1.2 million had signed the petition as of May 12.
‘Heroes fund’
The president first suggested hazard pay for health care workers on March 30 Fox News broadcast. “These are really brave people,” he said, adding that the administration was considering different ways of boosting pay, primarily through hospitals.
“We are asking the hospitals to do it and to consider something, including bonuses,” said Trump. “If anybody’s entitled to it, they are.”
On April 7, Sen. Schumer proposed a “Heroes Fund.” It would give public, private, and tribal frontline employees – including doctors, nurses, first responders, and transit, grocery, and postal workers – a $13 per hour raise up to $25,000 in additional pay through Dec. 31 for workers earning up to $200,000 and $5,000 in additional pay for those earning more than $200,000. It would also provide a $15,000 signing bonus to those who agree to take on such a position.
Rep. Matt Cartwright (D-Pa.) introduced a bill in mid-April, the Coronavirus Frontline Workers Fair Pay Act (HR 6709), that would provide similar pay increases. Health care workers would receive an additional $13 per hour. It would be retroactive to Jan. 31, 2020, and would be available through the end of 2020.
Molly Kinder of the Brookings Institution, a self-described nonpartisan Washington policy institute, estimates that Sen. Schumer’s proposal would represent the equivalent of double-time pay for the average low-wage worker, a 50% pay increase for a mail carrier, a 20% boost for a pharmacist, and less than a 15% increase for a surgeon, as determined from median 2018 wages.
Before the House Democrats unveiled their bill, Isabel Soto of the center-right group American Action Forum estimated that a $13 per hour wage increase could cost $398.9 billion just from the end of March to the end of September. A great proportion of that amount – $264 billion – would go to some 10 million health care workers, Ms. Soto calculated.
Some already offering pay boost
A few states and hospital systems are already offering hazard pay.
On April 12, Massachusetts agreed to give about 6,500 AFSCME union members who work at state human services facilities and group homes a $5 or a $10 per hour pay increase, depending on duties. It was to stay in effect until at least May 30.
Maine Governor Janet Mills (D) also agreed to increase pay by $3-$5 an hour for AFSCME workers in state correctional and mental health facilities beginning March 29.
In New York City, the biggest hospital network, Northwell Health, in late April gave 45,000 workers – including nurses, physicians, respiratory therapists, environmental services workers, housekeepers, and people in outpatient and corporate roles – a lump sum bonus payment of up to $2,500 and 1 week of paid time off. The money came out of the system’s general fund.
“As an organization, we want to continue to support, motivate and inspire our team members,” said Northwell President and CEO Michael Dowling in a statement at the time.
On April 2, New York–Presbyterian Hospital’s chair of the department of surgery, Craig Smith, MD, announced that the facility was “providing a $1,250 bonus for everyone who has worked in or supported the COVID-19 front lines, for at least 1 week.”
Advocate Aurora, with 15 hospitals and 32,000 employees in Wisconsin, said in early April that it was giving increases of $6.25-$15.00 an hour at least through the end of May.
A version of this article originally appeared on Medscape.com.
Hazard pay for frontline health care workers – an idea that has been championed by President Donald J. Trump and Senate Minority Leader Chuck Schumer, among others – is included in a just-released COVID-19 relief package assembled by Democrats in the House of Representatives.
according to a report in the Washington Post.
But it is far from a done deal. “The Democrats’ spending bill is a Pelosi-led pipe dream written in private,” said House Republican Leader Kevin McCarthy (Calif.) in a Fox News interview posted May 12 on Facebook.
Senate Majority Leader Mitch McConnell condemned the package. “This is exactly the wrong approach,” he said in a prepared statement that instead laid out a variety of liability protections, which he said should be the first priority.
“We are not going to let health care heroes emerge from this crisis facing a tidal wave of medical malpractice lawsuits so that trial lawyers can line their pockets,” said Sen. McConnell, adding that his plan would “raise the liability threshold for COVID-related malpractice lawsuits.”
Ingrida Lusis, vice president of government affairs and health policy at the American Nurses Association, said in an interview that the ANA had lobbied for hazard pay and was told it would be in the next relief package.
“Though there is an inherent risk in the nursing profession, we think that this is really critical to ensuring that we have a workforce to meet the intense demands of this pandemic,” said Ms. Lusis.
“If health care workers are not treated and compensated appropriately for what they’re going through right now, then we may not have a next generation that will want to enter the field,” she said.
Various nursing organizations, nurses’ unions, and health care unions, such as the American Federation of State, County and Municipal Employees (AFSCME) and the Service Employees International Union, have advocated for hazard pay.
Physicians’ organizations have not been vocal on the issue, however. The American Medical Association, for instance, pushed for hazard pay for residents but has not made any further public statements. An AMA spokesman said that the group was monitoring the situation but declined further comment.
Multiple online petitions seeking hazard pay for health care workers have been circulated, including one seeking the same $600 bump for essential workers that was given out as part of unemployment benefits in the first COVID-19 relief package. More than 1.2 million had signed the petition as of May 12.
‘Heroes fund’
The president first suggested hazard pay for health care workers on March 30 Fox News broadcast. “These are really brave people,” he said, adding that the administration was considering different ways of boosting pay, primarily through hospitals.
“We are asking the hospitals to do it and to consider something, including bonuses,” said Trump. “If anybody’s entitled to it, they are.”
On April 7, Sen. Schumer proposed a “Heroes Fund.” It would give public, private, and tribal frontline employees – including doctors, nurses, first responders, and transit, grocery, and postal workers – a $13 per hour raise up to $25,000 in additional pay through Dec. 31 for workers earning up to $200,000 and $5,000 in additional pay for those earning more than $200,000. It would also provide a $15,000 signing bonus to those who agree to take on such a position.
Rep. Matt Cartwright (D-Pa.) introduced a bill in mid-April, the Coronavirus Frontline Workers Fair Pay Act (HR 6709), that would provide similar pay increases. Health care workers would receive an additional $13 per hour. It would be retroactive to Jan. 31, 2020, and would be available through the end of 2020.
Molly Kinder of the Brookings Institution, a self-described nonpartisan Washington policy institute, estimates that Sen. Schumer’s proposal would represent the equivalent of double-time pay for the average low-wage worker, a 50% pay increase for a mail carrier, a 20% boost for a pharmacist, and less than a 15% increase for a surgeon, as determined from median 2018 wages.
Before the House Democrats unveiled their bill, Isabel Soto of the center-right group American Action Forum estimated that a $13 per hour wage increase could cost $398.9 billion just from the end of March to the end of September. A great proportion of that amount – $264 billion – would go to some 10 million health care workers, Ms. Soto calculated.
Some already offering pay boost
A few states and hospital systems are already offering hazard pay.
On April 12, Massachusetts agreed to give about 6,500 AFSCME union members who work at state human services facilities and group homes a $5 or a $10 per hour pay increase, depending on duties. It was to stay in effect until at least May 30.
Maine Governor Janet Mills (D) also agreed to increase pay by $3-$5 an hour for AFSCME workers in state correctional and mental health facilities beginning March 29.
In New York City, the biggest hospital network, Northwell Health, in late April gave 45,000 workers – including nurses, physicians, respiratory therapists, environmental services workers, housekeepers, and people in outpatient and corporate roles – a lump sum bonus payment of up to $2,500 and 1 week of paid time off. The money came out of the system’s general fund.
“As an organization, we want to continue to support, motivate and inspire our team members,” said Northwell President and CEO Michael Dowling in a statement at the time.
On April 2, New York–Presbyterian Hospital’s chair of the department of surgery, Craig Smith, MD, announced that the facility was “providing a $1,250 bonus for everyone who has worked in or supported the COVID-19 front lines, for at least 1 week.”
Advocate Aurora, with 15 hospitals and 32,000 employees in Wisconsin, said in early April that it was giving increases of $6.25-$15.00 an hour at least through the end of May.
A version of this article originally appeared on Medscape.com.
Summary of the IDSA guidelines on the diagnosis of COVID-19
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
ER docs ask, “Where are our patients?”
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
Comparing COVID-19, flu death tolls ‘extremely dangerous’
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.
Doctors advise asthmatics to continue therapy during pandemic
“In fact, there’s no data to support this at this time. Maintaining adequate asthma control is the current CDC recommendation,” said pediatric pulmonologist John Carl, MD, of Cleveland Clinic Children’s Hospital. Patients, he said, should be advised to “follow your asthma action plan as outlined by your primary care or specialty clinician and communicate about evolving symptoms, such as fever rather than just congestion, wheezing, and coughing, etc.”
Dr. Carl spoke in a May 7 webinar about asthma and COVID-19 with Lakiea Wright, M.D., a physician specializing in internal medicine and allergy and immunology at Brigham and Women’s Hospital in Boston and medical director of clinical affairs for Thermo Fisher Scientific’s ImmunoDiagnostics division. The webinar, sponsored by Thermo Fisher Scientific, included discussion of COVID-19 risks, disease management, and distinguishing between the virus and asthma.
In a follow-up interview, Dr. Wright said she’s hearing from patients and parents who are concerned about whether people with asthma face a higher risk of COVID-19 infection. There’s no evidence that they do, she said, but “the CDC states that individuals with moderate to severe asthma may be higher risk for moderate to severe disease from COVID-19 if they were to become infected.”
Indeed, she said, “it is well established that viruses can trigger asthma.” But, as she also noted, early research about the risk in patients with asthma is conflicting.
“Some studies suggest asthma may be a risk factor for hospitalization while other data suggests asthma is not a common risk factor for those hospitalized,” Dr. Wright said.
She highlighted a recent study that suggests people with allergic asthma have “a reduced ACE2 gene expression in airway cells and thus decreased susceptibility to infection” by the novel coronavirus (J Allergy Clin Immunol. 2020 Apr 22. doi: 10.1016/j.jaci.2020.04.009).
Dr. Wright cautioned, however, that “this is a hypothesis and will need to be studied more.”
For now, she said, patients “should follow their asthma action plan and take their inhalers, including inhaled corticosteroids, as prescribed by their health care providers.”
Most patients are reasonable and do comply when their physicians explain why they should take a medication,” she noted.
Dr. Carl agreed, and added that a short course of oral corticosteroids are also recommended to manage minor exacerbations and “prevent patients from having to arrive as inpatients in more acute settings and risk health system–related exposures to the current pandemic.”
He cautioned, however, that metered-dose inhalers are preferable to nebulizers, and side vent ports should be avoided since they can aerosolize infectious agents and put health care providers and family members at risk.
Unfortunately, he said, there’s been a shortage of short-acting beta agonist albuterol inhalers. This has been linked to hospitals trying to avoid the use of nebulizers.
Dr. Wright advised colleagues to focus on unique symptoms first, then address overlapping symptoms and other symptoms to differentiate between COVID-19 and asthma/allergy.
She noted that environmental allergy symptoms alone do not cause fever, a hallmark of COVID-19. Shortness of breath can be a distinguishing symptom for the virus, because this is not a common symptom of environmental allergies unless the patient has asthma, Dr. Wright said.
Cough can be an overlapping symptom because in environmental allergies, postnasal drip from allergic rhinitis can trigger cough, she explained. Nasal congestion and/or runny nose can develop with viral illnesses in general, but these are symptoms not included in the CDC’s list of the most common COVID-19 symptoms. Severe fatigue and body aches aren’t symptoms consistent with environmental allergies, Dr. Wright said.
Both Dr. Carl and Dr. Wright emphasized the importance of continuing routine asthma therapy during the pandemic.
“When discussing the importance of taking their inhaled steroids with patients, I also remind patients that asthma management is comprehensive,” Dr. Wright said. “I want them to take their medications, but I also want them avoid or minimize exposure to triggers. Allergic and nonallergic triggers such as environmental tobacco smoke can exacerbate asthma.”
In addition, she said, “it’s important to take a detailed medical history to identify triggers. And it’s important to conduct allergy testing to common environmental allergens to help identify allergic triggers and tailor environmental allergen control strategies based on the results. All of these strategies help patients keep their asthma well-controlled.”
Dr. Carl and Dr. Wright report having no relevant disclosures.
“In fact, there’s no data to support this at this time. Maintaining adequate asthma control is the current CDC recommendation,” said pediatric pulmonologist John Carl, MD, of Cleveland Clinic Children’s Hospital. Patients, he said, should be advised to “follow your asthma action plan as outlined by your primary care or specialty clinician and communicate about evolving symptoms, such as fever rather than just congestion, wheezing, and coughing, etc.”
Dr. Carl spoke in a May 7 webinar about asthma and COVID-19 with Lakiea Wright, M.D., a physician specializing in internal medicine and allergy and immunology at Brigham and Women’s Hospital in Boston and medical director of clinical affairs for Thermo Fisher Scientific’s ImmunoDiagnostics division. The webinar, sponsored by Thermo Fisher Scientific, included discussion of COVID-19 risks, disease management, and distinguishing between the virus and asthma.
In a follow-up interview, Dr. Wright said she’s hearing from patients and parents who are concerned about whether people with asthma face a higher risk of COVID-19 infection. There’s no evidence that they do, she said, but “the CDC states that individuals with moderate to severe asthma may be higher risk for moderate to severe disease from COVID-19 if they were to become infected.”
Indeed, she said, “it is well established that viruses can trigger asthma.” But, as she also noted, early research about the risk in patients with asthma is conflicting.
“Some studies suggest asthma may be a risk factor for hospitalization while other data suggests asthma is not a common risk factor for those hospitalized,” Dr. Wright said.
She highlighted a recent study that suggests people with allergic asthma have “a reduced ACE2 gene expression in airway cells and thus decreased susceptibility to infection” by the novel coronavirus (J Allergy Clin Immunol. 2020 Apr 22. doi: 10.1016/j.jaci.2020.04.009).
Dr. Wright cautioned, however, that “this is a hypothesis and will need to be studied more.”
For now, she said, patients “should follow their asthma action plan and take their inhalers, including inhaled corticosteroids, as prescribed by their health care providers.”
Most patients are reasonable and do comply when their physicians explain why they should take a medication,” she noted.
Dr. Carl agreed, and added that a short course of oral corticosteroids are also recommended to manage minor exacerbations and “prevent patients from having to arrive as inpatients in more acute settings and risk health system–related exposures to the current pandemic.”
He cautioned, however, that metered-dose inhalers are preferable to nebulizers, and side vent ports should be avoided since they can aerosolize infectious agents and put health care providers and family members at risk.
Unfortunately, he said, there’s been a shortage of short-acting beta agonist albuterol inhalers. This has been linked to hospitals trying to avoid the use of nebulizers.
Dr. Wright advised colleagues to focus on unique symptoms first, then address overlapping symptoms and other symptoms to differentiate between COVID-19 and asthma/allergy.
She noted that environmental allergy symptoms alone do not cause fever, a hallmark of COVID-19. Shortness of breath can be a distinguishing symptom for the virus, because this is not a common symptom of environmental allergies unless the patient has asthma, Dr. Wright said.
Cough can be an overlapping symptom because in environmental allergies, postnasal drip from allergic rhinitis can trigger cough, she explained. Nasal congestion and/or runny nose can develop with viral illnesses in general, but these are symptoms not included in the CDC’s list of the most common COVID-19 symptoms. Severe fatigue and body aches aren’t symptoms consistent with environmental allergies, Dr. Wright said.
Both Dr. Carl and Dr. Wright emphasized the importance of continuing routine asthma therapy during the pandemic.
“When discussing the importance of taking their inhaled steroids with patients, I also remind patients that asthma management is comprehensive,” Dr. Wright said. “I want them to take their medications, but I also want them avoid or minimize exposure to triggers. Allergic and nonallergic triggers such as environmental tobacco smoke can exacerbate asthma.”
In addition, she said, “it’s important to take a detailed medical history to identify triggers. And it’s important to conduct allergy testing to common environmental allergens to help identify allergic triggers and tailor environmental allergen control strategies based on the results. All of these strategies help patients keep their asthma well-controlled.”
Dr. Carl and Dr. Wright report having no relevant disclosures.
“In fact, there’s no data to support this at this time. Maintaining adequate asthma control is the current CDC recommendation,” said pediatric pulmonologist John Carl, MD, of Cleveland Clinic Children’s Hospital. Patients, he said, should be advised to “follow your asthma action plan as outlined by your primary care or specialty clinician and communicate about evolving symptoms, such as fever rather than just congestion, wheezing, and coughing, etc.”
Dr. Carl spoke in a May 7 webinar about asthma and COVID-19 with Lakiea Wright, M.D., a physician specializing in internal medicine and allergy and immunology at Brigham and Women’s Hospital in Boston and medical director of clinical affairs for Thermo Fisher Scientific’s ImmunoDiagnostics division. The webinar, sponsored by Thermo Fisher Scientific, included discussion of COVID-19 risks, disease management, and distinguishing between the virus and asthma.
In a follow-up interview, Dr. Wright said she’s hearing from patients and parents who are concerned about whether people with asthma face a higher risk of COVID-19 infection. There’s no evidence that they do, she said, but “the CDC states that individuals with moderate to severe asthma may be higher risk for moderate to severe disease from COVID-19 if they were to become infected.”
Indeed, she said, “it is well established that viruses can trigger asthma.” But, as she also noted, early research about the risk in patients with asthma is conflicting.
“Some studies suggest asthma may be a risk factor for hospitalization while other data suggests asthma is not a common risk factor for those hospitalized,” Dr. Wright said.
She highlighted a recent study that suggests people with allergic asthma have “a reduced ACE2 gene expression in airway cells and thus decreased susceptibility to infection” by the novel coronavirus (J Allergy Clin Immunol. 2020 Apr 22. doi: 10.1016/j.jaci.2020.04.009).
Dr. Wright cautioned, however, that “this is a hypothesis and will need to be studied more.”
For now, she said, patients “should follow their asthma action plan and take their inhalers, including inhaled corticosteroids, as prescribed by their health care providers.”
Most patients are reasonable and do comply when their physicians explain why they should take a medication,” she noted.
Dr. Carl agreed, and added that a short course of oral corticosteroids are also recommended to manage minor exacerbations and “prevent patients from having to arrive as inpatients in more acute settings and risk health system–related exposures to the current pandemic.”
He cautioned, however, that metered-dose inhalers are preferable to nebulizers, and side vent ports should be avoided since they can aerosolize infectious agents and put health care providers and family members at risk.
Unfortunately, he said, there’s been a shortage of short-acting beta agonist albuterol inhalers. This has been linked to hospitals trying to avoid the use of nebulizers.
Dr. Wright advised colleagues to focus on unique symptoms first, then address overlapping symptoms and other symptoms to differentiate between COVID-19 and asthma/allergy.
She noted that environmental allergy symptoms alone do not cause fever, a hallmark of COVID-19. Shortness of breath can be a distinguishing symptom for the virus, because this is not a common symptom of environmental allergies unless the patient has asthma, Dr. Wright said.
Cough can be an overlapping symptom because in environmental allergies, postnasal drip from allergic rhinitis can trigger cough, she explained. Nasal congestion and/or runny nose can develop with viral illnesses in general, but these are symptoms not included in the CDC’s list of the most common COVID-19 symptoms. Severe fatigue and body aches aren’t symptoms consistent with environmental allergies, Dr. Wright said.
Both Dr. Carl and Dr. Wright emphasized the importance of continuing routine asthma therapy during the pandemic.
“When discussing the importance of taking their inhaled steroids with patients, I also remind patients that asthma management is comprehensive,” Dr. Wright said. “I want them to take their medications, but I also want them avoid or minimize exposure to triggers. Allergic and nonallergic triggers such as environmental tobacco smoke can exacerbate asthma.”
In addition, she said, “it’s important to take a detailed medical history to identify triggers. And it’s important to conduct allergy testing to common environmental allergens to help identify allergic triggers and tailor environmental allergen control strategies based on the results. All of these strategies help patients keep their asthma well-controlled.”
Dr. Carl and Dr. Wright report having no relevant disclosures.
Consider COVID-19–associated multisystem hyperinflammatory syndrome
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
COVID-19 fears tied to dangerous drop in child vaccinations
The social distancing and sheltering in place mandated because of the COVID-19 pandemic are keeping parents and kids out of their doctors’ offices, and that has prompted a steep decline in recommended routine vaccinations for U.S. children, according to Centers for Disease Control and Prevention researchers.
Pediatric vaccinations dropped sharply after the national emergency was declared on March 13, suggesting that some children may be at increased risk for other serious infectious diseases, such as measles.
The researchers compared weekly orders for federally funded vaccines from Jan. 6 to April 19, 2020, with those during the same period in 2019.
They noted that, by the end of the study period, there was a cumulative COVID-19–related decline of 2.5 million doses in orders for routine noninfluenza pediatric childhood vaccines recommended by the Advisory Committee on Immunization Practices, as well as a cumulative decline in orders of 250,000 doses of measles vaccines.
Although the overall decrease in vaccinations during the study period was larger, according to CDC spokesperson Richard Quartarone, the above figures represent declines clearly associated with the pandemic.
The weekly number of measles vaccines ordered for children aged 24 months or older fell dramatically to about 500 during the week beginning March 16, 2020, and fell further to approximately 250 during the week beginning March 23. It stayed at that level until the week beginning April 13. By comparison, more than 2,500 were ordered during the week starting March 2, before the emergency was declared.
The decline was notably less for children younger than 2 years. For those children, orders dropped to about 750 during the week starting March 23 and climbed slightly for 3 weeks. By comparison, during the week of March 2, about 2,000 vaccines were ordered.
The findings, which were published in the CDC’s Morbidity and Mortality Weekly Report, stem from an analysis of ordering data from the federal Vaccines for Children (VFC) Program, as well as from vaccine administration data from the CDC’s Vaccine Tracking System and the collaborative Vaccine Safety Datalink (VSD).
The VFC provides federally purchased vaccines at no cost to about half of persons aged 18 years or younger. The VSD collaborates on vaccine coverage with the CDC’s Immunization Safety Office and eight large health care organizations across the country. Vaccination coverage is the usual metric for assessing vaccine usage; providers’ orders and the number of doses administered are two proxy measures, the authors explained.
“The substantial reduction in VFC-funded pediatric vaccine ordering after the COVID-19 emergency declaration is consistent with changes in vaccine administration among children in the VSD population receiving care through eight large U.S. health care organizations,” wrote Jeanne M. Santoli, MD, and colleagues, of the immunization services division at the National Center for Immunization and Respiratory Diseases. “The smaller decline in measles-containing vaccine administration among children aged ≤24 months suggests that system-level strategies to prioritize well child care and immunization for this age group are being implemented.”
Dr. Santoli, who is an Atlanta-based pediatrician, and associates stressed the importance of maintaining regular vaccinations during the pandemic. “The identified declines in routine pediatric vaccine ordering and doses administered might indicate that U.S. children and their communities face increased risks for outbreaks of vaccine-preventable diseases,” they wrote. “Parental concerns about potentially exposing their children to COVID-19 during well child visits might contribute to the declines observed.” Parents should therefore be reminded of the necessity of protecting their children against vaccine-preventable diseases.
In 2019, a Gallup survey reported that overall support for vaccination continued to decline in the United States.
The researchers predicted that, as social distancing relaxes, unvaccinated children will be more susceptible to other serious diseases. “In response, continued coordinated efforts between health care providers and public health officials at the local, state, and federal levels will be necessary to achieve rapid catch-up vaccination,” they concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The social distancing and sheltering in place mandated because of the COVID-19 pandemic are keeping parents and kids out of their doctors’ offices, and that has prompted a steep decline in recommended routine vaccinations for U.S. children, according to Centers for Disease Control and Prevention researchers.
Pediatric vaccinations dropped sharply after the national emergency was declared on March 13, suggesting that some children may be at increased risk for other serious infectious diseases, such as measles.
The researchers compared weekly orders for federally funded vaccines from Jan. 6 to April 19, 2020, with those during the same period in 2019.
They noted that, by the end of the study period, there was a cumulative COVID-19–related decline of 2.5 million doses in orders for routine noninfluenza pediatric childhood vaccines recommended by the Advisory Committee on Immunization Practices, as well as a cumulative decline in orders of 250,000 doses of measles vaccines.
Although the overall decrease in vaccinations during the study period was larger, according to CDC spokesperson Richard Quartarone, the above figures represent declines clearly associated with the pandemic.
The weekly number of measles vaccines ordered for children aged 24 months or older fell dramatically to about 500 during the week beginning March 16, 2020, and fell further to approximately 250 during the week beginning March 23. It stayed at that level until the week beginning April 13. By comparison, more than 2,500 were ordered during the week starting March 2, before the emergency was declared.
The decline was notably less for children younger than 2 years. For those children, orders dropped to about 750 during the week starting March 23 and climbed slightly for 3 weeks. By comparison, during the week of March 2, about 2,000 vaccines were ordered.
The findings, which were published in the CDC’s Morbidity and Mortality Weekly Report, stem from an analysis of ordering data from the federal Vaccines for Children (VFC) Program, as well as from vaccine administration data from the CDC’s Vaccine Tracking System and the collaborative Vaccine Safety Datalink (VSD).
The VFC provides federally purchased vaccines at no cost to about half of persons aged 18 years or younger. The VSD collaborates on vaccine coverage with the CDC’s Immunization Safety Office and eight large health care organizations across the country. Vaccination coverage is the usual metric for assessing vaccine usage; providers’ orders and the number of doses administered are two proxy measures, the authors explained.
“The substantial reduction in VFC-funded pediatric vaccine ordering after the COVID-19 emergency declaration is consistent with changes in vaccine administration among children in the VSD population receiving care through eight large U.S. health care organizations,” wrote Jeanne M. Santoli, MD, and colleagues, of the immunization services division at the National Center for Immunization and Respiratory Diseases. “The smaller decline in measles-containing vaccine administration among children aged ≤24 months suggests that system-level strategies to prioritize well child care and immunization for this age group are being implemented.”
Dr. Santoli, who is an Atlanta-based pediatrician, and associates stressed the importance of maintaining regular vaccinations during the pandemic. “The identified declines in routine pediatric vaccine ordering and doses administered might indicate that U.S. children and their communities face increased risks for outbreaks of vaccine-preventable diseases,” they wrote. “Parental concerns about potentially exposing their children to COVID-19 during well child visits might contribute to the declines observed.” Parents should therefore be reminded of the necessity of protecting their children against vaccine-preventable diseases.
In 2019, a Gallup survey reported that overall support for vaccination continued to decline in the United States.
The researchers predicted that, as social distancing relaxes, unvaccinated children will be more susceptible to other serious diseases. “In response, continued coordinated efforts between health care providers and public health officials at the local, state, and federal levels will be necessary to achieve rapid catch-up vaccination,” they concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The social distancing and sheltering in place mandated because of the COVID-19 pandemic are keeping parents and kids out of their doctors’ offices, and that has prompted a steep decline in recommended routine vaccinations for U.S. children, according to Centers for Disease Control and Prevention researchers.
Pediatric vaccinations dropped sharply after the national emergency was declared on March 13, suggesting that some children may be at increased risk for other serious infectious diseases, such as measles.
The researchers compared weekly orders for federally funded vaccines from Jan. 6 to April 19, 2020, with those during the same period in 2019.
They noted that, by the end of the study period, there was a cumulative COVID-19–related decline of 2.5 million doses in orders for routine noninfluenza pediatric childhood vaccines recommended by the Advisory Committee on Immunization Practices, as well as a cumulative decline in orders of 250,000 doses of measles vaccines.
Although the overall decrease in vaccinations during the study period was larger, according to CDC spokesperson Richard Quartarone, the above figures represent declines clearly associated with the pandemic.
The weekly number of measles vaccines ordered for children aged 24 months or older fell dramatically to about 500 during the week beginning March 16, 2020, and fell further to approximately 250 during the week beginning March 23. It stayed at that level until the week beginning April 13. By comparison, more than 2,500 were ordered during the week starting March 2, before the emergency was declared.
The decline was notably less for children younger than 2 years. For those children, orders dropped to about 750 during the week starting March 23 and climbed slightly for 3 weeks. By comparison, during the week of March 2, about 2,000 vaccines were ordered.
The findings, which were published in the CDC’s Morbidity and Mortality Weekly Report, stem from an analysis of ordering data from the federal Vaccines for Children (VFC) Program, as well as from vaccine administration data from the CDC’s Vaccine Tracking System and the collaborative Vaccine Safety Datalink (VSD).
The VFC provides federally purchased vaccines at no cost to about half of persons aged 18 years or younger. The VSD collaborates on vaccine coverage with the CDC’s Immunization Safety Office and eight large health care organizations across the country. Vaccination coverage is the usual metric for assessing vaccine usage; providers’ orders and the number of doses administered are two proxy measures, the authors explained.
“The substantial reduction in VFC-funded pediatric vaccine ordering after the COVID-19 emergency declaration is consistent with changes in vaccine administration among children in the VSD population receiving care through eight large U.S. health care organizations,” wrote Jeanne M. Santoli, MD, and colleagues, of the immunization services division at the National Center for Immunization and Respiratory Diseases. “The smaller decline in measles-containing vaccine administration among children aged ≤24 months suggests that system-level strategies to prioritize well child care and immunization for this age group are being implemented.”
Dr. Santoli, who is an Atlanta-based pediatrician, and associates stressed the importance of maintaining regular vaccinations during the pandemic. “The identified declines in routine pediatric vaccine ordering and doses administered might indicate that U.S. children and their communities face increased risks for outbreaks of vaccine-preventable diseases,” they wrote. “Parental concerns about potentially exposing their children to COVID-19 during well child visits might contribute to the declines observed.” Parents should therefore be reminded of the necessity of protecting their children against vaccine-preventable diseases.
In 2019, a Gallup survey reported that overall support for vaccination continued to decline in the United States.
The researchers predicted that, as social distancing relaxes, unvaccinated children will be more susceptible to other serious diseases. “In response, continued coordinated efforts between health care providers and public health officials at the local, state, and federal levels will be necessary to achieve rapid catch-up vaccination,” they concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.