User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Angiotensin drugs and COVID-19: More reassuring data
Initial data from one Chinese center on the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in patients hospitalized with COVID-19 appear to give some further reassurance about continued use of these drugs.
The report from one hospital in Wuhan found that among patients with hypertension hospitalized with the COVID-19 virus, there was no difference in disease severity or death rate in patients taking ACE inhibitors or ARBs and those not taking such medications.
The data were published online April 23 in JAMA Cardiology.
The study adds to another recent report in a larger number of COVID-19 patients from nine Chinese hospitals that suggested a beneficial effect of ACE inhibitors or ARBs on mortality.
Additional studies
Two other similar studies have also been recently released. Another study from China, published online March 31 in Emerging Microbes & Infections, included a small sample of 42 hospitalized patients with COVID-19 on antihypertensive therapy. Those on ACE inhibitor/ARB therapy had a lower rate of severe disease and a trend toward a lower level of IL-6 in peripheral blood. In addition, patients on ACE inhibitor/ARB therapy had increased CD3+ and CD8+ T-cell counts in peripheral blood and decreased peak viral load compared with other antihypertensive drugs.
And a preliminary study from the UK, which has not yet been peer reviewed, found that treatment with ACE inhibitors was associated with a reduced risk of rapidly deteriorating severe COVID-19 disease.
The study, available online on MedRxiv, a preprint server for health sciences, reports on 205 acute inpatients with COVID-19 at King’s College Hospital and Princess Royal University Hospital, London.
Of these, 51.2% had hypertension, 30.2% had diabetes, and 14.6% had ischemic heart disease or heart failure. Of the 37 patients on ACE inhibitors, five (14%) died or required critical care support compared with 29% (48/168) of patients not taking an ACE inhibitor.
New Wuhan study
The authors of the new article published in JAMA Cardiology, led by Juyi Li, MD, reported on a case series of 1,178 patients hospitalized with COVID-19 at the Central Hospital of Wuhan, Hubei, China, between Jan. 15 and March 15, 2020.
Patients were a median age of 55 years, and 46% were men. They had an overall in-hospital mortality rate of 11%.
Of the 1,178 patients, 362 (30.7%) had a diagnosis of hypertension. These patients were older (median age, 66 years) and had a greater prevalence of chronic diseases. Patients with hypertension also had more severe manifestations of COVID-19 compared to those without hypertension, including higher rates of acute respiratory distress syndrome and in-hospital mortality (21.3% vs. 6.5%).
Of the 362 patients with hypertension, 31.8% were taking ACE inhibitors or ARBs.
Apart from a greater prevalence of coronary artery disease, patients taking ACE inhibitors or ARBs had similar comorbidities to those not taking these medications, and also similar laboratory profile results including blood counts, inflammatory markers, renal and liver function tests, and cardiac biomarkers, although those taking ACE inhibitors/ARBs had higher levels of alkaline phosphatase.
The most commonly used antihypertensive drugs were calcium blockers. The percentage of patients with hypertension taking any drug or drug combination did not differ between those with severe and nonsevere infections and between those who survived and those who died.
Specifically regarding ACE inhibitors/ARBs, there was no difference between those with severe versus nonsevere illness in the use of ACE inhibitors (9.2% vs. 10.1%; P = .80), ARBs (24.9% vs. 21.2%; P = .40), or the composite of ACE inhibitors or ARBs (32.9% vs. 30.7%; P = .65).
Similarly, there were no differences in nonsurvivors and survivors in the use of ACE inhibitors (9.1% vs. 9.8%; P = .85); ARBs (19.5% vs. 23.9%; P = .42), or the composite of ACE inhibitors or ARBs (27.3% vs. 33.0%; P = .34).
The frequency of severe illness and death also did not differ between those treated with and without ACE inhibitors/ARBs in patients with hypertension and other various chronic conditions including coronary heart disease, cerebrovascular disease, diabetes, neurological disease, and chronic renal disease.
The authors noted that these data confirm previous reports showing that patients with hypertension have more severe illness and higher mortality rates associated with COVID-19 than those without hypertension.
But they added: “Our data provide some reassurance that ACE inhibitors/ARBs are not associated with the progression or outcome of COVID-19 hospitalizations in patients with hypertension.”
They also noted that these results support the recommendations from almost all major cardiovascular societies that patients do not discontinue ACE inhibitors or ARBs because of worries about COVID-19.
However, the authors did point out some limitations of their study, which included a small number of patients with hypertension taking ACE inhibitors or ARBs and the fact that a nonsevere disease course was still severe enough to require hospitalization. In addition, it was not clear whether ACE inhibitor/ARB treatment at baseline was maintained throughout hospitalization for all patients.
This was also an observational comparison and may be biased by differences in patients taking versus not taking ACE inhibitors or ARBs at the time of hospitalization, although the measured baseline characteristics were similar in both groups.
But the authors also highlighted the finding that, in this cohort, patients with hypertension had three times the mortality rate of all other patients hospitalized with COVID-19.
“Hypertension combined with cardiovascular and cerebrovascular disease, diabetes, and chronic kidney disease would predispose patients to an increased risk of severity and mortality of COVID-19. Therefore, patients with these underlying conditions who develop COVID-19 require particularly intensive surveillance and care,” they wrote.
Experts cautiously optimistic
Some cardiovascular experts were cautiously optimistic about these latest results.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, and editor-in-chief of the Journal of Clinical Hypertension, said: “This new report from Wuhan, China, gives modest reassurance that the use of ACE inhibitors or ARBs in hypertensive patients with COVID-19 disease does not increase the risk of clinical deterioration or death.
“Ongoing, more definitive studies should help resolve competing hypotheses regarding the effects of these agents: whether the increased ACE2 enzyme levels they produce can worsen outcomes by increasing access of the COVID virus to lung tissue; or whether there is a benefit linked to a protective effect of increased ACE2 on alveolar cell function,” Dr. Weber noted.
“Though the number of patients included in this new report is small, it is startling that hypertensive patients were three times as likely as nonhypertensives to have a fatal outcome, presumably reflecting vulnerability due to the cardiovascular and metabolic comorbidities associated with hypertension,” he added.
“In any case, for now, clinicians should continue treating hypertensive patients with whichever drugs, including ACE inhibitors and ARBs, best provide protection from adverse outcomes,” Dr. Weber concluded.
John McMurray, MD, professor of medical cardiology, University of Glasgow, Scotland, commented: “This study from Wuhan provides some reassurance about one of the two questions about ACEI/ARBs: Do these drugs increase susceptibility to infection? And if [the patient is] infected, do they increase the severity of infection? This study addresses the latter question and appears to suggest no increased severity.”
However, Dr. McMurray pointed out that the study had many limitations. There were only small patient numbers and the data were unadjusted, “although it looks like the ACE inhibitor/ARB treated patients were higher risk to start with.” It was an observational study, and patients were not randomized and were predominantly treated with ARBs, and not ACE inhibitors, so “we don’t know if the concerns apply equally to these two classes of drug.
“Other data published and unpublished supporting this (even showing better outcomes in patients treated with an ACE inhibitor/ARB), and, to date, any concerns about these drugs remain unsubstantiated and the guidance from medical societies to continue treatment with these agents in patients prescribed them seems wise,” Dr. McMurray added.
Franz H. Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “The study from Wuhan is not a great study. They didn’t even do a multivariable analysis. They could have done a bit more with the data, but it still gives some reassurance.”
Dr. Messerli said it was “interesting” that 30% of the patients hospitalized with COVID-19 in the sample had hypertension. “That corresponds to the general population, so does not suggest that having hypertension increases susceptibility to infection – but it does seem to increase the risk of a bad outcome.”
Dr. Messerli noted that there are two more similar studies due to be published soon, both said to suggest either a beneficial or neutral effect of ACE inhibitors/ARBs on COVID-19 outcomes in hospitalized patients.
“This does help with confidence in prescribing these agents and reinforces the recommendations for patients to stay on these drugs,” he said.
“However, none of these studies address the infectivity issue – whether their use upregulates the ACE2 receptor, which the virus uses to gain entry to cells, thereby increasing susceptibility to the infection,” Dr. Messerli cautioned. “But the similar or better outcomes on these drugs are encouraging,” he added.
The Wuhan study was supported by the Health and Family Planning Commission of Wuhan City, China. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Initial data from one Chinese center on the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in patients hospitalized with COVID-19 appear to give some further reassurance about continued use of these drugs.
The report from one hospital in Wuhan found that among patients with hypertension hospitalized with the COVID-19 virus, there was no difference in disease severity or death rate in patients taking ACE inhibitors or ARBs and those not taking such medications.
The data were published online April 23 in JAMA Cardiology.
The study adds to another recent report in a larger number of COVID-19 patients from nine Chinese hospitals that suggested a beneficial effect of ACE inhibitors or ARBs on mortality.
Additional studies
Two other similar studies have also been recently released. Another study from China, published online March 31 in Emerging Microbes & Infections, included a small sample of 42 hospitalized patients with COVID-19 on antihypertensive therapy. Those on ACE inhibitor/ARB therapy had a lower rate of severe disease and a trend toward a lower level of IL-6 in peripheral blood. In addition, patients on ACE inhibitor/ARB therapy had increased CD3+ and CD8+ T-cell counts in peripheral blood and decreased peak viral load compared with other antihypertensive drugs.
And a preliminary study from the UK, which has not yet been peer reviewed, found that treatment with ACE inhibitors was associated with a reduced risk of rapidly deteriorating severe COVID-19 disease.
The study, available online on MedRxiv, a preprint server for health sciences, reports on 205 acute inpatients with COVID-19 at King’s College Hospital and Princess Royal University Hospital, London.
Of these, 51.2% had hypertension, 30.2% had diabetes, and 14.6% had ischemic heart disease or heart failure. Of the 37 patients on ACE inhibitors, five (14%) died or required critical care support compared with 29% (48/168) of patients not taking an ACE inhibitor.
New Wuhan study
The authors of the new article published in JAMA Cardiology, led by Juyi Li, MD, reported on a case series of 1,178 patients hospitalized with COVID-19 at the Central Hospital of Wuhan, Hubei, China, between Jan. 15 and March 15, 2020.
Patients were a median age of 55 years, and 46% were men. They had an overall in-hospital mortality rate of 11%.
Of the 1,178 patients, 362 (30.7%) had a diagnosis of hypertension. These patients were older (median age, 66 years) and had a greater prevalence of chronic diseases. Patients with hypertension also had more severe manifestations of COVID-19 compared to those without hypertension, including higher rates of acute respiratory distress syndrome and in-hospital mortality (21.3% vs. 6.5%).
Of the 362 patients with hypertension, 31.8% were taking ACE inhibitors or ARBs.
Apart from a greater prevalence of coronary artery disease, patients taking ACE inhibitors or ARBs had similar comorbidities to those not taking these medications, and also similar laboratory profile results including blood counts, inflammatory markers, renal and liver function tests, and cardiac biomarkers, although those taking ACE inhibitors/ARBs had higher levels of alkaline phosphatase.
The most commonly used antihypertensive drugs were calcium blockers. The percentage of patients with hypertension taking any drug or drug combination did not differ between those with severe and nonsevere infections and between those who survived and those who died.
Specifically regarding ACE inhibitors/ARBs, there was no difference between those with severe versus nonsevere illness in the use of ACE inhibitors (9.2% vs. 10.1%; P = .80), ARBs (24.9% vs. 21.2%; P = .40), or the composite of ACE inhibitors or ARBs (32.9% vs. 30.7%; P = .65).
Similarly, there were no differences in nonsurvivors and survivors in the use of ACE inhibitors (9.1% vs. 9.8%; P = .85); ARBs (19.5% vs. 23.9%; P = .42), or the composite of ACE inhibitors or ARBs (27.3% vs. 33.0%; P = .34).
The frequency of severe illness and death also did not differ between those treated with and without ACE inhibitors/ARBs in patients with hypertension and other various chronic conditions including coronary heart disease, cerebrovascular disease, diabetes, neurological disease, and chronic renal disease.
The authors noted that these data confirm previous reports showing that patients with hypertension have more severe illness and higher mortality rates associated with COVID-19 than those without hypertension.
But they added: “Our data provide some reassurance that ACE inhibitors/ARBs are not associated with the progression or outcome of COVID-19 hospitalizations in patients with hypertension.”
They also noted that these results support the recommendations from almost all major cardiovascular societies that patients do not discontinue ACE inhibitors or ARBs because of worries about COVID-19.
However, the authors did point out some limitations of their study, which included a small number of patients with hypertension taking ACE inhibitors or ARBs and the fact that a nonsevere disease course was still severe enough to require hospitalization. In addition, it was not clear whether ACE inhibitor/ARB treatment at baseline was maintained throughout hospitalization for all patients.
This was also an observational comparison and may be biased by differences in patients taking versus not taking ACE inhibitors or ARBs at the time of hospitalization, although the measured baseline characteristics were similar in both groups.
But the authors also highlighted the finding that, in this cohort, patients with hypertension had three times the mortality rate of all other patients hospitalized with COVID-19.
“Hypertension combined with cardiovascular and cerebrovascular disease, diabetes, and chronic kidney disease would predispose patients to an increased risk of severity and mortality of COVID-19. Therefore, patients with these underlying conditions who develop COVID-19 require particularly intensive surveillance and care,” they wrote.
Experts cautiously optimistic
Some cardiovascular experts were cautiously optimistic about these latest results.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, and editor-in-chief of the Journal of Clinical Hypertension, said: “This new report from Wuhan, China, gives modest reassurance that the use of ACE inhibitors or ARBs in hypertensive patients with COVID-19 disease does not increase the risk of clinical deterioration or death.
“Ongoing, more definitive studies should help resolve competing hypotheses regarding the effects of these agents: whether the increased ACE2 enzyme levels they produce can worsen outcomes by increasing access of the COVID virus to lung tissue; or whether there is a benefit linked to a protective effect of increased ACE2 on alveolar cell function,” Dr. Weber noted.
“Though the number of patients included in this new report is small, it is startling that hypertensive patients were three times as likely as nonhypertensives to have a fatal outcome, presumably reflecting vulnerability due to the cardiovascular and metabolic comorbidities associated with hypertension,” he added.
“In any case, for now, clinicians should continue treating hypertensive patients with whichever drugs, including ACE inhibitors and ARBs, best provide protection from adverse outcomes,” Dr. Weber concluded.
John McMurray, MD, professor of medical cardiology, University of Glasgow, Scotland, commented: “This study from Wuhan provides some reassurance about one of the two questions about ACEI/ARBs: Do these drugs increase susceptibility to infection? And if [the patient is] infected, do they increase the severity of infection? This study addresses the latter question and appears to suggest no increased severity.”
However, Dr. McMurray pointed out that the study had many limitations. There were only small patient numbers and the data were unadjusted, “although it looks like the ACE inhibitor/ARB treated patients were higher risk to start with.” It was an observational study, and patients were not randomized and were predominantly treated with ARBs, and not ACE inhibitors, so “we don’t know if the concerns apply equally to these two classes of drug.
“Other data published and unpublished supporting this (even showing better outcomes in patients treated with an ACE inhibitor/ARB), and, to date, any concerns about these drugs remain unsubstantiated and the guidance from medical societies to continue treatment with these agents in patients prescribed them seems wise,” Dr. McMurray added.
Franz H. Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “The study from Wuhan is not a great study. They didn’t even do a multivariable analysis. They could have done a bit more with the data, but it still gives some reassurance.”
Dr. Messerli said it was “interesting” that 30% of the patients hospitalized with COVID-19 in the sample had hypertension. “That corresponds to the general population, so does not suggest that having hypertension increases susceptibility to infection – but it does seem to increase the risk of a bad outcome.”
Dr. Messerli noted that there are two more similar studies due to be published soon, both said to suggest either a beneficial or neutral effect of ACE inhibitors/ARBs on COVID-19 outcomes in hospitalized patients.
“This does help with confidence in prescribing these agents and reinforces the recommendations for patients to stay on these drugs,” he said.
“However, none of these studies address the infectivity issue – whether their use upregulates the ACE2 receptor, which the virus uses to gain entry to cells, thereby increasing susceptibility to the infection,” Dr. Messerli cautioned. “But the similar or better outcomes on these drugs are encouraging,” he added.
The Wuhan study was supported by the Health and Family Planning Commission of Wuhan City, China. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Initial data from one Chinese center on the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) in patients hospitalized with COVID-19 appear to give some further reassurance about continued use of these drugs.
The report from one hospital in Wuhan found that among patients with hypertension hospitalized with the COVID-19 virus, there was no difference in disease severity or death rate in patients taking ACE inhibitors or ARBs and those not taking such medications.
The data were published online April 23 in JAMA Cardiology.
The study adds to another recent report in a larger number of COVID-19 patients from nine Chinese hospitals that suggested a beneficial effect of ACE inhibitors or ARBs on mortality.
Additional studies
Two other similar studies have also been recently released. Another study from China, published online March 31 in Emerging Microbes & Infections, included a small sample of 42 hospitalized patients with COVID-19 on antihypertensive therapy. Those on ACE inhibitor/ARB therapy had a lower rate of severe disease and a trend toward a lower level of IL-6 in peripheral blood. In addition, patients on ACE inhibitor/ARB therapy had increased CD3+ and CD8+ T-cell counts in peripheral blood and decreased peak viral load compared with other antihypertensive drugs.
And a preliminary study from the UK, which has not yet been peer reviewed, found that treatment with ACE inhibitors was associated with a reduced risk of rapidly deteriorating severe COVID-19 disease.
The study, available online on MedRxiv, a preprint server for health sciences, reports on 205 acute inpatients with COVID-19 at King’s College Hospital and Princess Royal University Hospital, London.
Of these, 51.2% had hypertension, 30.2% had diabetes, and 14.6% had ischemic heart disease or heart failure. Of the 37 patients on ACE inhibitors, five (14%) died or required critical care support compared with 29% (48/168) of patients not taking an ACE inhibitor.
New Wuhan study
The authors of the new article published in JAMA Cardiology, led by Juyi Li, MD, reported on a case series of 1,178 patients hospitalized with COVID-19 at the Central Hospital of Wuhan, Hubei, China, between Jan. 15 and March 15, 2020.
Patients were a median age of 55 years, and 46% were men. They had an overall in-hospital mortality rate of 11%.
Of the 1,178 patients, 362 (30.7%) had a diagnosis of hypertension. These patients were older (median age, 66 years) and had a greater prevalence of chronic diseases. Patients with hypertension also had more severe manifestations of COVID-19 compared to those without hypertension, including higher rates of acute respiratory distress syndrome and in-hospital mortality (21.3% vs. 6.5%).
Of the 362 patients with hypertension, 31.8% were taking ACE inhibitors or ARBs.
Apart from a greater prevalence of coronary artery disease, patients taking ACE inhibitors or ARBs had similar comorbidities to those not taking these medications, and also similar laboratory profile results including blood counts, inflammatory markers, renal and liver function tests, and cardiac biomarkers, although those taking ACE inhibitors/ARBs had higher levels of alkaline phosphatase.
The most commonly used antihypertensive drugs were calcium blockers. The percentage of patients with hypertension taking any drug or drug combination did not differ between those with severe and nonsevere infections and between those who survived and those who died.
Specifically regarding ACE inhibitors/ARBs, there was no difference between those with severe versus nonsevere illness in the use of ACE inhibitors (9.2% vs. 10.1%; P = .80), ARBs (24.9% vs. 21.2%; P = .40), or the composite of ACE inhibitors or ARBs (32.9% vs. 30.7%; P = .65).
Similarly, there were no differences in nonsurvivors and survivors in the use of ACE inhibitors (9.1% vs. 9.8%; P = .85); ARBs (19.5% vs. 23.9%; P = .42), or the composite of ACE inhibitors or ARBs (27.3% vs. 33.0%; P = .34).
The frequency of severe illness and death also did not differ between those treated with and without ACE inhibitors/ARBs in patients with hypertension and other various chronic conditions including coronary heart disease, cerebrovascular disease, diabetes, neurological disease, and chronic renal disease.
The authors noted that these data confirm previous reports showing that patients with hypertension have more severe illness and higher mortality rates associated with COVID-19 than those without hypertension.
But they added: “Our data provide some reassurance that ACE inhibitors/ARBs are not associated with the progression or outcome of COVID-19 hospitalizations in patients with hypertension.”
They also noted that these results support the recommendations from almost all major cardiovascular societies that patients do not discontinue ACE inhibitors or ARBs because of worries about COVID-19.
However, the authors did point out some limitations of their study, which included a small number of patients with hypertension taking ACE inhibitors or ARBs and the fact that a nonsevere disease course was still severe enough to require hospitalization. In addition, it was not clear whether ACE inhibitor/ARB treatment at baseline was maintained throughout hospitalization for all patients.
This was also an observational comparison and may be biased by differences in patients taking versus not taking ACE inhibitors or ARBs at the time of hospitalization, although the measured baseline characteristics were similar in both groups.
But the authors also highlighted the finding that, in this cohort, patients with hypertension had three times the mortality rate of all other patients hospitalized with COVID-19.
“Hypertension combined with cardiovascular and cerebrovascular disease, diabetes, and chronic kidney disease would predispose patients to an increased risk of severity and mortality of COVID-19. Therefore, patients with these underlying conditions who develop COVID-19 require particularly intensive surveillance and care,” they wrote.
Experts cautiously optimistic
Some cardiovascular experts were cautiously optimistic about these latest results.
Michael A. Weber, MD, professor of medicine at the State University of New York, Brooklyn, and editor-in-chief of the Journal of Clinical Hypertension, said: “This new report from Wuhan, China, gives modest reassurance that the use of ACE inhibitors or ARBs in hypertensive patients with COVID-19 disease does not increase the risk of clinical deterioration or death.
“Ongoing, more definitive studies should help resolve competing hypotheses regarding the effects of these agents: whether the increased ACE2 enzyme levels they produce can worsen outcomes by increasing access of the COVID virus to lung tissue; or whether there is a benefit linked to a protective effect of increased ACE2 on alveolar cell function,” Dr. Weber noted.
“Though the number of patients included in this new report is small, it is startling that hypertensive patients were three times as likely as nonhypertensives to have a fatal outcome, presumably reflecting vulnerability due to the cardiovascular and metabolic comorbidities associated with hypertension,” he added.
“In any case, for now, clinicians should continue treating hypertensive patients with whichever drugs, including ACE inhibitors and ARBs, best provide protection from adverse outcomes,” Dr. Weber concluded.
John McMurray, MD, professor of medical cardiology, University of Glasgow, Scotland, commented: “This study from Wuhan provides some reassurance about one of the two questions about ACEI/ARBs: Do these drugs increase susceptibility to infection? And if [the patient is] infected, do they increase the severity of infection? This study addresses the latter question and appears to suggest no increased severity.”
However, Dr. McMurray pointed out that the study had many limitations. There were only small patient numbers and the data were unadjusted, “although it looks like the ACE inhibitor/ARB treated patients were higher risk to start with.” It was an observational study, and patients were not randomized and were predominantly treated with ARBs, and not ACE inhibitors, so “we don’t know if the concerns apply equally to these two classes of drug.
“Other data published and unpublished supporting this (even showing better outcomes in patients treated with an ACE inhibitor/ARB), and, to date, any concerns about these drugs remain unsubstantiated and the guidance from medical societies to continue treatment with these agents in patients prescribed them seems wise,” Dr. McMurray added.
Franz H. Messerli, MD, professor of medicine at the University of Bern, Switzerland, commented: “The study from Wuhan is not a great study. They didn’t even do a multivariable analysis. They could have done a bit more with the data, but it still gives some reassurance.”
Dr. Messerli said it was “interesting” that 30% of the patients hospitalized with COVID-19 in the sample had hypertension. “That corresponds to the general population, so does not suggest that having hypertension increases susceptibility to infection – but it does seem to increase the risk of a bad outcome.”
Dr. Messerli noted that there are two more similar studies due to be published soon, both said to suggest either a beneficial or neutral effect of ACE inhibitors/ARBs on COVID-19 outcomes in hospitalized patients.
“This does help with confidence in prescribing these agents and reinforces the recommendations for patients to stay on these drugs,” he said.
“However, none of these studies address the infectivity issue – whether their use upregulates the ACE2 receptor, which the virus uses to gain entry to cells, thereby increasing susceptibility to the infection,” Dr. Messerli cautioned. “But the similar or better outcomes on these drugs are encouraging,” he added.
The Wuhan study was supported by the Health and Family Planning Commission of Wuhan City, China. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Dermatomyositis without dermatitis correlates with autoantibodies
The prevalence of dermatomyositis without dermatitis among patients with biopsy-confirmed dermatomyositis was approximately 8% in a Japanese cohort study. “Dermatomyositis sine dermatitis does exist and is significantly associated with anti–nuclear matrix protein 2 [anti-NXP-2] autoantibodies,” the researchers reported in JAMA Neurology.
Few case reports of dermatomyositis sine dermatitis have been documented. To confirm the existence of the condition, study its prevalence, and characterize its serologic features, Michio Inoue, MD, PhD, of the National Center of Neurology and Psychiatry in Tokyo, and colleagues conducted a cohort study of patients seen at the center between January 2009 and August 2019.
Of more than 8,800 patients whose muscle biopsies were examined for diagnostic purposes, 199 were tested for dermatomyositis-specific autoantibodies. The investigators excluded patients who did not have myxovirus resistance protein A expression in myofibers on muscle biopsy. In all, 182 patients with dermatomyositis were enrolled in the study (51% women; median age at biopsy, 56 years). Fourteen patients without a skin rash at the time of muscle biopsy received a diagnosis of dermatomyositis sine dermatitis. Before the muscle biopsy, most patients without a rash had a diagnosis of polymyositis.
Association with anti-NXP-2 autoantibodies
Anti-NXP-2 autoantibodies were detected in 86% of the patients without a rash at the time of biopsy, compared with 28% of the patients with rashes. “No other clinical or pathological characteristics were associated with [dermatomyositis sine dermatitis] except increased probability of developing perifascicular atrophy (71% vs. 43%),” Dr. Inoue and colleagues said.
During a median follow-up of 34 months, patients with dermatomyositis sine dermatitis received oral prednisolone with or without additional immunotherapy, and two patients had subcutaneous edema. Calcification was not seen during follow-up. “One patient with ... anti-NXP-2 autoantibodies had severe interstitial lung disease and needed noninvasive positive-pressure ventilation support,” the researchers said.
Four of the 14 patients with dermatomyositis sine dermatitis “developed skin rashes after muscle biopsy,” the researchers noted. “Similarly, a patient with [dermatomyositis sine dermatitis] was reported to have developed a skin rash 2 years after muscle biopsy.”
Potential therapies for refractory dermatomyositis, such as Janus kinase inhibitors, may not be effective for other types of myositis, so identifying patients with dermatomyositis may be “more essential than ever,” the authors said.
Effects on organ systems vary
The study is the first to systematically examine dermatomyositis sine dermatitis, said David Fiorentino, MD, PhD, professor of dermatology and director of the multidisciplinary rheumatic skin disease clinic at the Stanford (Calif.) University.
On the one hand, the results are not surprising because dermatomyositis is a systemic autoimmune disease. “There are no rules about which organs it will or won’t affect in a given individual,” Dr. Fiorentino said in an interview.
At the same time, dermatomyositis’s historical association with rash persists even though there is “no biological reason why that would have to be the case.”
Some patients with dermatomyositis have skin-predominant disease without clinically significant muscle involvement. Lung-predominant disease also may exist, although it has not been carefully studied, he said.
The findings remind clinicians that they need to consider the diagnosis of dermatomyositis “even if they do not have the skin findings,” he said. Dr. Fiorentino cautioned against interpreting the results to mean that certain patients never have signs of cutaneous inflammation. In the study, about a one-third of patients without dermatitis at the time of biopsy developed a rash. In addition, clinicians often miss subtle disease under the fingernails or on the scalp, or mild rash on the elbows.
The cohort of patients who underwent muscle biopsy may not be representative of the spectrum of patients with dermatomyositis, and the findings need to be verified in other populations, Dr. Fiorentino said.
The study was supported by an intramural research grant of the National Center of Neurology and Psychiatry and a grant from the Japan Society for the Promotion of Science. Authors disclosed personal fees from pharmaceutical companies and government and corporate grants outside the submitted work. Dr. Fiorentino had no relevant disclosures.
SOURCE: Inoue M et al. JAMA Neurol. 2020 Apr 20. doi: 10.1001/jamaneurol.2020.0673.
The prevalence of dermatomyositis without dermatitis among patients with biopsy-confirmed dermatomyositis was approximately 8% in a Japanese cohort study. “Dermatomyositis sine dermatitis does exist and is significantly associated with anti–nuclear matrix protein 2 [anti-NXP-2] autoantibodies,” the researchers reported in JAMA Neurology.
Few case reports of dermatomyositis sine dermatitis have been documented. To confirm the existence of the condition, study its prevalence, and characterize its serologic features, Michio Inoue, MD, PhD, of the National Center of Neurology and Psychiatry in Tokyo, and colleagues conducted a cohort study of patients seen at the center between January 2009 and August 2019.
Of more than 8,800 patients whose muscle biopsies were examined for diagnostic purposes, 199 were tested for dermatomyositis-specific autoantibodies. The investigators excluded patients who did not have myxovirus resistance protein A expression in myofibers on muscle biopsy. In all, 182 patients with dermatomyositis were enrolled in the study (51% women; median age at biopsy, 56 years). Fourteen patients without a skin rash at the time of muscle biopsy received a diagnosis of dermatomyositis sine dermatitis. Before the muscle biopsy, most patients without a rash had a diagnosis of polymyositis.
Association with anti-NXP-2 autoantibodies
Anti-NXP-2 autoantibodies were detected in 86% of the patients without a rash at the time of biopsy, compared with 28% of the patients with rashes. “No other clinical or pathological characteristics were associated with [dermatomyositis sine dermatitis] except increased probability of developing perifascicular atrophy (71% vs. 43%),” Dr. Inoue and colleagues said.
During a median follow-up of 34 months, patients with dermatomyositis sine dermatitis received oral prednisolone with or without additional immunotherapy, and two patients had subcutaneous edema. Calcification was not seen during follow-up. “One patient with ... anti-NXP-2 autoantibodies had severe interstitial lung disease and needed noninvasive positive-pressure ventilation support,” the researchers said.
Four of the 14 patients with dermatomyositis sine dermatitis “developed skin rashes after muscle biopsy,” the researchers noted. “Similarly, a patient with [dermatomyositis sine dermatitis] was reported to have developed a skin rash 2 years after muscle biopsy.”
Potential therapies for refractory dermatomyositis, such as Janus kinase inhibitors, may not be effective for other types of myositis, so identifying patients with dermatomyositis may be “more essential than ever,” the authors said.
Effects on organ systems vary
The study is the first to systematically examine dermatomyositis sine dermatitis, said David Fiorentino, MD, PhD, professor of dermatology and director of the multidisciplinary rheumatic skin disease clinic at the Stanford (Calif.) University.
On the one hand, the results are not surprising because dermatomyositis is a systemic autoimmune disease. “There are no rules about which organs it will or won’t affect in a given individual,” Dr. Fiorentino said in an interview.
At the same time, dermatomyositis’s historical association with rash persists even though there is “no biological reason why that would have to be the case.”
Some patients with dermatomyositis have skin-predominant disease without clinically significant muscle involvement. Lung-predominant disease also may exist, although it has not been carefully studied, he said.
The findings remind clinicians that they need to consider the diagnosis of dermatomyositis “even if they do not have the skin findings,” he said. Dr. Fiorentino cautioned against interpreting the results to mean that certain patients never have signs of cutaneous inflammation. In the study, about a one-third of patients without dermatitis at the time of biopsy developed a rash. In addition, clinicians often miss subtle disease under the fingernails or on the scalp, or mild rash on the elbows.
The cohort of patients who underwent muscle biopsy may not be representative of the spectrum of patients with dermatomyositis, and the findings need to be verified in other populations, Dr. Fiorentino said.
The study was supported by an intramural research grant of the National Center of Neurology and Psychiatry and a grant from the Japan Society for the Promotion of Science. Authors disclosed personal fees from pharmaceutical companies and government and corporate grants outside the submitted work. Dr. Fiorentino had no relevant disclosures.
SOURCE: Inoue M et al. JAMA Neurol. 2020 Apr 20. doi: 10.1001/jamaneurol.2020.0673.
The prevalence of dermatomyositis without dermatitis among patients with biopsy-confirmed dermatomyositis was approximately 8% in a Japanese cohort study. “Dermatomyositis sine dermatitis does exist and is significantly associated with anti–nuclear matrix protein 2 [anti-NXP-2] autoantibodies,” the researchers reported in JAMA Neurology.
Few case reports of dermatomyositis sine dermatitis have been documented. To confirm the existence of the condition, study its prevalence, and characterize its serologic features, Michio Inoue, MD, PhD, of the National Center of Neurology and Psychiatry in Tokyo, and colleagues conducted a cohort study of patients seen at the center between January 2009 and August 2019.
Of more than 8,800 patients whose muscle biopsies were examined for diagnostic purposes, 199 were tested for dermatomyositis-specific autoantibodies. The investigators excluded patients who did not have myxovirus resistance protein A expression in myofibers on muscle biopsy. In all, 182 patients with dermatomyositis were enrolled in the study (51% women; median age at biopsy, 56 years). Fourteen patients without a skin rash at the time of muscle biopsy received a diagnosis of dermatomyositis sine dermatitis. Before the muscle biopsy, most patients without a rash had a diagnosis of polymyositis.
Association with anti-NXP-2 autoantibodies
Anti-NXP-2 autoantibodies were detected in 86% of the patients without a rash at the time of biopsy, compared with 28% of the patients with rashes. “No other clinical or pathological characteristics were associated with [dermatomyositis sine dermatitis] except increased probability of developing perifascicular atrophy (71% vs. 43%),” Dr. Inoue and colleagues said.
During a median follow-up of 34 months, patients with dermatomyositis sine dermatitis received oral prednisolone with or without additional immunotherapy, and two patients had subcutaneous edema. Calcification was not seen during follow-up. “One patient with ... anti-NXP-2 autoantibodies had severe interstitial lung disease and needed noninvasive positive-pressure ventilation support,” the researchers said.
Four of the 14 patients with dermatomyositis sine dermatitis “developed skin rashes after muscle biopsy,” the researchers noted. “Similarly, a patient with [dermatomyositis sine dermatitis] was reported to have developed a skin rash 2 years after muscle biopsy.”
Potential therapies for refractory dermatomyositis, such as Janus kinase inhibitors, may not be effective for other types of myositis, so identifying patients with dermatomyositis may be “more essential than ever,” the authors said.
Effects on organ systems vary
The study is the first to systematically examine dermatomyositis sine dermatitis, said David Fiorentino, MD, PhD, professor of dermatology and director of the multidisciplinary rheumatic skin disease clinic at the Stanford (Calif.) University.
On the one hand, the results are not surprising because dermatomyositis is a systemic autoimmune disease. “There are no rules about which organs it will or won’t affect in a given individual,” Dr. Fiorentino said in an interview.
At the same time, dermatomyositis’s historical association with rash persists even though there is “no biological reason why that would have to be the case.”
Some patients with dermatomyositis have skin-predominant disease without clinically significant muscle involvement. Lung-predominant disease also may exist, although it has not been carefully studied, he said.
The findings remind clinicians that they need to consider the diagnosis of dermatomyositis “even if they do not have the skin findings,” he said. Dr. Fiorentino cautioned against interpreting the results to mean that certain patients never have signs of cutaneous inflammation. In the study, about a one-third of patients without dermatitis at the time of biopsy developed a rash. In addition, clinicians often miss subtle disease under the fingernails or on the scalp, or mild rash on the elbows.
The cohort of patients who underwent muscle biopsy may not be representative of the spectrum of patients with dermatomyositis, and the findings need to be verified in other populations, Dr. Fiorentino said.
The study was supported by an intramural research grant of the National Center of Neurology and Psychiatry and a grant from the Japan Society for the Promotion of Science. Authors disclosed personal fees from pharmaceutical companies and government and corporate grants outside the submitted work. Dr. Fiorentino had no relevant disclosures.
SOURCE: Inoue M et al. JAMA Neurol. 2020 Apr 20. doi: 10.1001/jamaneurol.2020.0673.
FROM JAMA NEUROLOGY
Hydroxychloroquine ineffective for COVID-19, VA study suggests
Hydroxychloroquine (HCQ) with or without azithromycin (AZ) is not associated with a lower risk of requiring mechanical ventilation, according to a retrospective study of Veterans Affairs patients hospitalized with COVID-19.
The study, which was posted on a preprint server April 21 and has not been peer reviewed, also showed an increased risk of death associated with COVID-19 patients treated with HCQ alone.
“These findings highlight the importance of awaiting the results of ongoing prospective, randomized controlled studies before widespread adoption of these drugs,” write Joseph Magagnoli with Dorn Research Institute at the Columbia (S.C.) VA Health Care System and the department of clinical pharmacy & outcomes sciences, University of South Carolina, and colleagues.
A spokesperson with the University of Virginia, Charlottesville, where several of coauthors practice, said that the authors declined to comment for this article before peer review is completed.
The new data are not the first to suggest no benefit with HCQ among patients with COVID-19. A randomized trial showed no benefit and more side effects among 75 patients in China treated with HCQ, compared with 75 who received standard of care alone, according to a preprint posted online April 14.
No benefit in ventilation, death rates
The current analysis included data from all 368 male patients hospitalized with confirmed COVID-19 and treated at Veterans Health Administration medical centers in the United States through April 11.
Patients were categorized into three groups: those treated with HCQ in addition to standard of care (n = 97); those treated with HCQ and the antibiotic azithromycin plus standard of care (n = 113); and those who received standard supportive care only (n = 158).
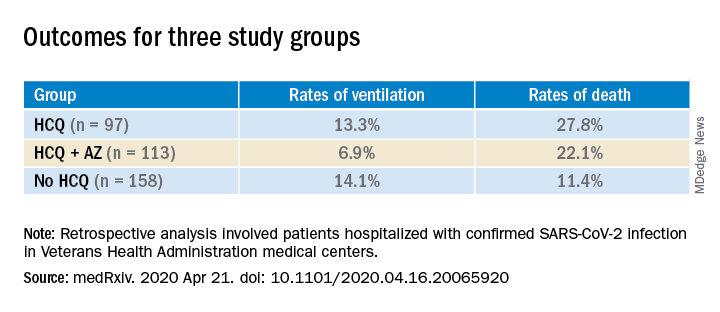
Compared with the no HCQ group, the risk of death from any cause was higher in the HCQ group (adjusted hazard ratio, 2.61; 95% confidence interval, 1.10-6.17; P = .03) but not in the HCQ+AZ group (aHR, 1.14; 95% CI, 0.56-2.32; P = .72).
The risk of ventilation was similar in the HCQ group (aHR, 1.43; 95% CI, 0.53-3.79; P = .48) and in the HCQ+AZ group (aHR, 0.43; 95% CI, 0.16-1.12; P = .09), compared with the no-HCQ group.
This study provides another counterbalance to claims of HCQ efficacy, David R. Wessner, PhD, professor of biology and chair of the department of health and human values at Davidson (N.C.) College, said in an interview.
Interest in HCQ spiked after an open-label, nonrandomized, single-center study of COVID-19 patients in France suggested that hydroxychloroquine helped clear the virus and had a potential enhanced effect when combined with azithromycin.
But the 36-patient trial has since been called into question.
Wait for convincing data
Dr. Wessner, whose research focuses on viral pathogenesis, says that, although the current data don’t definitively answer the question of whether HCQ is effective in treating COVID-19, taking a “let’s try it and see” approach is not reasonable.
“Until we have good, prospective randomized trials, it’s hard to know what to make of this. But this is more evidence that there’s not a good reason to use [HCQ],” Dr. Wessner said. He points out that the small randomized trial from China shows that HCQ comes with potential harms.
Anecdotal evidence is often cited by those who promote HCQ as a potential treatment, but “those are one-off examples,” Wessner continued. “That doesn’t really tell us anything.”
Some HCQ proponents have said that trials finding no benefit are flawed in that the drug is given too late. However, Dr. Wessner says, there’s no way to prove or disprove that claim without randomized controlled trials.
Conflicting messages
Despite lack of clear evidence of benefit for patients with COVID-19, HCQ is recommended off-label by the Chinese National guideline, and the U.S. Food and Drug Administration has issued an emergency-use authorization for the treatment of adult patients with COVID-19.
Conversely, the Infectious Diseases Society of America and a guideline panel convened by the National Institutes of Health each concluded recently that because of insufficient data, they could not recommend any specific treatments for patients with COVID-19.
The VA data for the current study came from the Veterans Affairs Informatics and Computing Infrastructure, which includes inpatient, outpatient and laboratory data and pharmacy claims.
The authors acknowledge some limitations, “including those inherent to all retrospective analyses such as nonrandomization of treatments.”
However, they note that they did adjust for potential confounders, including comorbidities, medications, and clinical and laboratory factors.
A coauthor, Jayakrishna Ambati, MD, is a cofounder of iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics, and has received consultancy fees from Allergan, Biogen, Boehringer Ingelheim, Immunovant, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences, all unrelated to this work. Dr. Ambati is named as an inventor on a patent application filed by the University of Virginia relating to COVID-19 but unrelated to this work. Another coauthor has received research grants from Boehringer Ingelheim, Gilead Sciences, Portola Pharmaceuticals, and United Therapeutics, all unrelated to this work. The other authors and Dr. Wessner have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hydroxychloroquine (HCQ) with or without azithromycin (AZ) is not associated with a lower risk of requiring mechanical ventilation, according to a retrospective study of Veterans Affairs patients hospitalized with COVID-19.
The study, which was posted on a preprint server April 21 and has not been peer reviewed, also showed an increased risk of death associated with COVID-19 patients treated with HCQ alone.
“These findings highlight the importance of awaiting the results of ongoing prospective, randomized controlled studies before widespread adoption of these drugs,” write Joseph Magagnoli with Dorn Research Institute at the Columbia (S.C.) VA Health Care System and the department of clinical pharmacy & outcomes sciences, University of South Carolina, and colleagues.
A spokesperson with the University of Virginia, Charlottesville, where several of coauthors practice, said that the authors declined to comment for this article before peer review is completed.
The new data are not the first to suggest no benefit with HCQ among patients with COVID-19. A randomized trial showed no benefit and more side effects among 75 patients in China treated with HCQ, compared with 75 who received standard of care alone, according to a preprint posted online April 14.
No benefit in ventilation, death rates
The current analysis included data from all 368 male patients hospitalized with confirmed COVID-19 and treated at Veterans Health Administration medical centers in the United States through April 11.
Patients were categorized into three groups: those treated with HCQ in addition to standard of care (n = 97); those treated with HCQ and the antibiotic azithromycin plus standard of care (n = 113); and those who received standard supportive care only (n = 158).

Compared with the no HCQ group, the risk of death from any cause was higher in the HCQ group (adjusted hazard ratio, 2.61; 95% confidence interval, 1.10-6.17; P = .03) but not in the HCQ+AZ group (aHR, 1.14; 95% CI, 0.56-2.32; P = .72).
The risk of ventilation was similar in the HCQ group (aHR, 1.43; 95% CI, 0.53-3.79; P = .48) and in the HCQ+AZ group (aHR, 0.43; 95% CI, 0.16-1.12; P = .09), compared with the no-HCQ group.
This study provides another counterbalance to claims of HCQ efficacy, David R. Wessner, PhD, professor of biology and chair of the department of health and human values at Davidson (N.C.) College, said in an interview.
Interest in HCQ spiked after an open-label, nonrandomized, single-center study of COVID-19 patients in France suggested that hydroxychloroquine helped clear the virus and had a potential enhanced effect when combined with azithromycin.
But the 36-patient trial has since been called into question.
Wait for convincing data
Dr. Wessner, whose research focuses on viral pathogenesis, says that, although the current data don’t definitively answer the question of whether HCQ is effective in treating COVID-19, taking a “let’s try it and see” approach is not reasonable.
“Until we have good, prospective randomized trials, it’s hard to know what to make of this. But this is more evidence that there’s not a good reason to use [HCQ],” Dr. Wessner said. He points out that the small randomized trial from China shows that HCQ comes with potential harms.
Anecdotal evidence is often cited by those who promote HCQ as a potential treatment, but “those are one-off examples,” Wessner continued. “That doesn’t really tell us anything.”
Some HCQ proponents have said that trials finding no benefit are flawed in that the drug is given too late. However, Dr. Wessner says, there’s no way to prove or disprove that claim without randomized controlled trials.
Conflicting messages
Despite lack of clear evidence of benefit for patients with COVID-19, HCQ is recommended off-label by the Chinese National guideline, and the U.S. Food and Drug Administration has issued an emergency-use authorization for the treatment of adult patients with COVID-19.
Conversely, the Infectious Diseases Society of America and a guideline panel convened by the National Institutes of Health each concluded recently that because of insufficient data, they could not recommend any specific treatments for patients with COVID-19.
The VA data for the current study came from the Veterans Affairs Informatics and Computing Infrastructure, which includes inpatient, outpatient and laboratory data and pharmacy claims.
The authors acknowledge some limitations, “including those inherent to all retrospective analyses such as nonrandomization of treatments.”
However, they note that they did adjust for potential confounders, including comorbidities, medications, and clinical and laboratory factors.
A coauthor, Jayakrishna Ambati, MD, is a cofounder of iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics, and has received consultancy fees from Allergan, Biogen, Boehringer Ingelheim, Immunovant, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences, all unrelated to this work. Dr. Ambati is named as an inventor on a patent application filed by the University of Virginia relating to COVID-19 but unrelated to this work. Another coauthor has received research grants from Boehringer Ingelheim, Gilead Sciences, Portola Pharmaceuticals, and United Therapeutics, all unrelated to this work. The other authors and Dr. Wessner have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hydroxychloroquine (HCQ) with or without azithromycin (AZ) is not associated with a lower risk of requiring mechanical ventilation, according to a retrospective study of Veterans Affairs patients hospitalized with COVID-19.
The study, which was posted on a preprint server April 21 and has not been peer reviewed, also showed an increased risk of death associated with COVID-19 patients treated with HCQ alone.
“These findings highlight the importance of awaiting the results of ongoing prospective, randomized controlled studies before widespread adoption of these drugs,” write Joseph Magagnoli with Dorn Research Institute at the Columbia (S.C.) VA Health Care System and the department of clinical pharmacy & outcomes sciences, University of South Carolina, and colleagues.
A spokesperson with the University of Virginia, Charlottesville, where several of coauthors practice, said that the authors declined to comment for this article before peer review is completed.
The new data are not the first to suggest no benefit with HCQ among patients with COVID-19. A randomized trial showed no benefit and more side effects among 75 patients in China treated with HCQ, compared with 75 who received standard of care alone, according to a preprint posted online April 14.
No benefit in ventilation, death rates
The current analysis included data from all 368 male patients hospitalized with confirmed COVID-19 and treated at Veterans Health Administration medical centers in the United States through April 11.
Patients were categorized into three groups: those treated with HCQ in addition to standard of care (n = 97); those treated with HCQ and the antibiotic azithromycin plus standard of care (n = 113); and those who received standard supportive care only (n = 158).

Compared with the no HCQ group, the risk of death from any cause was higher in the HCQ group (adjusted hazard ratio, 2.61; 95% confidence interval, 1.10-6.17; P = .03) but not in the HCQ+AZ group (aHR, 1.14; 95% CI, 0.56-2.32; P = .72).
The risk of ventilation was similar in the HCQ group (aHR, 1.43; 95% CI, 0.53-3.79; P = .48) and in the HCQ+AZ group (aHR, 0.43; 95% CI, 0.16-1.12; P = .09), compared with the no-HCQ group.
This study provides another counterbalance to claims of HCQ efficacy, David R. Wessner, PhD, professor of biology and chair of the department of health and human values at Davidson (N.C.) College, said in an interview.
Interest in HCQ spiked after an open-label, nonrandomized, single-center study of COVID-19 patients in France suggested that hydroxychloroquine helped clear the virus and had a potential enhanced effect when combined with azithromycin.
But the 36-patient trial has since been called into question.
Wait for convincing data
Dr. Wessner, whose research focuses on viral pathogenesis, says that, although the current data don’t definitively answer the question of whether HCQ is effective in treating COVID-19, taking a “let’s try it and see” approach is not reasonable.
“Until we have good, prospective randomized trials, it’s hard to know what to make of this. But this is more evidence that there’s not a good reason to use [HCQ],” Dr. Wessner said. He points out that the small randomized trial from China shows that HCQ comes with potential harms.
Anecdotal evidence is often cited by those who promote HCQ as a potential treatment, but “those are one-off examples,” Wessner continued. “That doesn’t really tell us anything.”
Some HCQ proponents have said that trials finding no benefit are flawed in that the drug is given too late. However, Dr. Wessner says, there’s no way to prove or disprove that claim without randomized controlled trials.
Conflicting messages
Despite lack of clear evidence of benefit for patients with COVID-19, HCQ is recommended off-label by the Chinese National guideline, and the U.S. Food and Drug Administration has issued an emergency-use authorization for the treatment of adult patients with COVID-19.
Conversely, the Infectious Diseases Society of America and a guideline panel convened by the National Institutes of Health each concluded recently that because of insufficient data, they could not recommend any specific treatments for patients with COVID-19.
The VA data for the current study came from the Veterans Affairs Informatics and Computing Infrastructure, which includes inpatient, outpatient and laboratory data and pharmacy claims.
The authors acknowledge some limitations, “including those inherent to all retrospective analyses such as nonrandomization of treatments.”
However, they note that they did adjust for potential confounders, including comorbidities, medications, and clinical and laboratory factors.
A coauthor, Jayakrishna Ambati, MD, is a cofounder of iVeena Holdings, iVeena Delivery Systems and Inflammasome Therapeutics, and has received consultancy fees from Allergan, Biogen, Boehringer Ingelheim, Immunovant, Janssen, Olix Pharmaceuticals, Retinal Solutions, and Saksin LifeSciences, all unrelated to this work. Dr. Ambati is named as an inventor on a patent application filed by the University of Virginia relating to COVID-19 but unrelated to this work. Another coauthor has received research grants from Boehringer Ingelheim, Gilead Sciences, Portola Pharmaceuticals, and United Therapeutics, all unrelated to this work. The other authors and Dr. Wessner have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Prioritizing ambulatory gynecology care during COVID-19: The latest guidance
What exactly constitutes appropriate ambulatory gynecology during this time of social distancing?
On March 30, 2020, the American College of Obstetricians and Gynecologists (ACOG) weighed in, releasing COVID-19 FAQs for Obstetrician-Gynecologists. These recommendations, which include information about obstetric and gynecologic surgery, are available to everyone, including the general public. They are intended to supplement guidance from the Centers for Disease Control and Prevention, as well as previously released ACOG guidance.
The recommendations include examples of patients needing in-person appointments, telehealth visits, or visits that should be deferred.
In-person appointments. Examples of patients for whom in-person appointments are appropriate include those with suspected ectopic pregnancy or profuse vaginal bleeding. With respect to contraceptive services, ACOG suggests that placement of IUDs and implants should continue whenever possible. If placement of the contraceptive device is deferred, use of self-administered hormonal contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring) should be encouraged as a bridge to later initiation of long-acting methods.
Telehealth visits. Video or telephone visits are advised for women desiring counseling and prescribing for contraception or menopausal symptoms.
Deferred. Deferral of office visits until after COVID-19 lockdowns is advised for average-risk women wishing routine well-woman visits. Other situations in which deferral should be considered include the following:
- For patients with abnormal cervical cancer screening results, ACOG suggests that colposcopy with cervical biopsies could be deferred for 6-12 months for patients with low-grade test results. In contrast, for patients with high-grade results, ACOG recommends that evaluation be performed within 3 months.
- For women who wish to discontinue their contraceptive, ACOG advises that removal of IUDs and implants be postponed when possible. These women should be counseled regarding extended use of these devices.
ACOG emphasizes that decisions regarding ambulatory gynecology should be individualized and take into consideration such issues as availability of local and regional resources, staffing, personal protective equipment, and the local prevalence of COVID-19.
As a gynecologist focused on ambulatory care, I believe that many clinicians will welcome this guidance from ACOG, which helps us provide optimal care during these challenging times.
Dr. Kaunitz is professor and associate chairman in the department of obstetrics and gynecology at the University of Florida, Jacksonville. He has disclosed receiving royalties from UpToDate, serving on the safety monitoring board for Femasys, and serving as a consultant for AMAG Pharmaceuticals, Merck & Co, Mithra, and Pfizer. His institution has received funding from pharmaceutical companies and nonprofits.
A version of this article originally appeared on Medscape.com.
What exactly constitutes appropriate ambulatory gynecology during this time of social distancing?
On March 30, 2020, the American College of Obstetricians and Gynecologists (ACOG) weighed in, releasing COVID-19 FAQs for Obstetrician-Gynecologists. These recommendations, which include information about obstetric and gynecologic surgery, are available to everyone, including the general public. They are intended to supplement guidance from the Centers for Disease Control and Prevention, as well as previously released ACOG guidance.
The recommendations include examples of patients needing in-person appointments, telehealth visits, or visits that should be deferred.
In-person appointments. Examples of patients for whom in-person appointments are appropriate include those with suspected ectopic pregnancy or profuse vaginal bleeding. With respect to contraceptive services, ACOG suggests that placement of IUDs and implants should continue whenever possible. If placement of the contraceptive device is deferred, use of self-administered hormonal contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring) should be encouraged as a bridge to later initiation of long-acting methods.
Telehealth visits. Video or telephone visits are advised for women desiring counseling and prescribing for contraception or menopausal symptoms.
Deferred. Deferral of office visits until after COVID-19 lockdowns is advised for average-risk women wishing routine well-woman visits. Other situations in which deferral should be considered include the following:
- For patients with abnormal cervical cancer screening results, ACOG suggests that colposcopy with cervical biopsies could be deferred for 6-12 months for patients with low-grade test results. In contrast, for patients with high-grade results, ACOG recommends that evaluation be performed within 3 months.
- For women who wish to discontinue their contraceptive, ACOG advises that removal of IUDs and implants be postponed when possible. These women should be counseled regarding extended use of these devices.
ACOG emphasizes that decisions regarding ambulatory gynecology should be individualized and take into consideration such issues as availability of local and regional resources, staffing, personal protective equipment, and the local prevalence of COVID-19.
As a gynecologist focused on ambulatory care, I believe that many clinicians will welcome this guidance from ACOG, which helps us provide optimal care during these challenging times.
Dr. Kaunitz is professor and associate chairman in the department of obstetrics and gynecology at the University of Florida, Jacksonville. He has disclosed receiving royalties from UpToDate, serving on the safety monitoring board for Femasys, and serving as a consultant for AMAG Pharmaceuticals, Merck & Co, Mithra, and Pfizer. His institution has received funding from pharmaceutical companies and nonprofits.
A version of this article originally appeared on Medscape.com.
What exactly constitutes appropriate ambulatory gynecology during this time of social distancing?
On March 30, 2020, the American College of Obstetricians and Gynecologists (ACOG) weighed in, releasing COVID-19 FAQs for Obstetrician-Gynecologists. These recommendations, which include information about obstetric and gynecologic surgery, are available to everyone, including the general public. They are intended to supplement guidance from the Centers for Disease Control and Prevention, as well as previously released ACOG guidance.
The recommendations include examples of patients needing in-person appointments, telehealth visits, or visits that should be deferred.
In-person appointments. Examples of patients for whom in-person appointments are appropriate include those with suspected ectopic pregnancy or profuse vaginal bleeding. With respect to contraceptive services, ACOG suggests that placement of IUDs and implants should continue whenever possible. If placement of the contraceptive device is deferred, use of self-administered hormonal contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring) should be encouraged as a bridge to later initiation of long-acting methods.
Telehealth visits. Video or telephone visits are advised for women desiring counseling and prescribing for contraception or menopausal symptoms.
Deferred. Deferral of office visits until after COVID-19 lockdowns is advised for average-risk women wishing routine well-woman visits. Other situations in which deferral should be considered include the following:
- For patients with abnormal cervical cancer screening results, ACOG suggests that colposcopy with cervical biopsies could be deferred for 6-12 months for patients with low-grade test results. In contrast, for patients with high-grade results, ACOG recommends that evaluation be performed within 3 months.
- For women who wish to discontinue their contraceptive, ACOG advises that removal of IUDs and implants be postponed when possible. These women should be counseled regarding extended use of these devices.
ACOG emphasizes that decisions regarding ambulatory gynecology should be individualized and take into consideration such issues as availability of local and regional resources, staffing, personal protective equipment, and the local prevalence of COVID-19.
As a gynecologist focused on ambulatory care, I believe that many clinicians will welcome this guidance from ACOG, which helps us provide optimal care during these challenging times.
Dr. Kaunitz is professor and associate chairman in the department of obstetrics and gynecology at the University of Florida, Jacksonville. He has disclosed receiving royalties from UpToDate, serving on the safety monitoring board for Femasys, and serving as a consultant for AMAG Pharmaceuticals, Merck & Co, Mithra, and Pfizer. His institution has received funding from pharmaceutical companies and nonprofits.
A version of this article originally appeared on Medscape.com.
European COVID-19 insights: Try helmet CPAP
Noninvasive ventilation with helmet continuous positive air pressure (CPAP) deserves to be embraced as an effective strategy in preventing self-induced lung injury, often a key factor in progression from the early milder expression of COVID-19 disease to classic severe acute respiratory distress syndrome, according to European physicians who have been through what they hope are the worst days of the pandemic in the Lombardy region of Northern Italy.
Helmet CPAP is a relatively inexpensive, convenient, well-tolerated intervention. It allows patients to remain conscious and responsive to commands such as “Time to roll over,” which in turn frees up nursing staff. The purpose of helmet CPAP is to curb the huge inspiratory drive that’s a defining feature of this disease and which, unchecked, can lead to self-induced lung injury (SILI), Luciano Gattinoni, MD, explained at a webinar hosted by the European Society of Anaesthesiology.
“Paranoid attention to inspiratory effort – checking it and correcting it – is something where we can make the difference between death and life. It’s extremely important,” said Dr. Gattinoni, guest professor of anesthesiology and intensive care at the University of Gottingen (Germany).
He and his fellow panelists were in accord regarding the merits of helmet CPAP as the premier method of noninvasive ventilatory assistance. They also addressed the importance of monitoring for hypercoagulation, as well as what they’ve come to see as the essential role of pronation in what they define as Type H disease, and the need to have detailed respiratory physiotherapy protocols in place.
“COVID-19 doesn’t like physiotherapy,” explained Paolo Pelosi, MD, professor of anesthesiology and intensive care medicine at the University of Genoa (Italy).
Dr. Gattinoni is credited for identification of two polar phenotypes of what he considers to be a single COVID-19 disease. Early on, many patients present with an atypical form of acute respiratory distress syndrome (ARDS), distinguished by an often-unexpected degree of hypoxia accompanied by high pulmonary compliance and surprisingly little shortness of breath. Dr. Gattinoni and colleagues call this Type L disease, which stands for low elastane, low ventilation to perfusion ratio, low lung weight on CT, and low lung recruitability, which means the patient has a high proportion of aerated lung tissue. Over time, because of either the natural history of the disease or SILI, this may shift to Type H disease, marked by high elastane, high right-to-left shunt, high lung weight, and high recruitability.
“If the pulmonary compliance is above 60 [mL/cm H2O], I’m pretty sure it’s Type L. If it’s 30 [mL/cm H2O] or less, I’m pretty sure it’s Type H. Don’t ask me about 45-55 [mL/cm H2O]; it’s a grey zone,” Dr. Gattinoni said.
Giuseppe Foti, MD, said helmet CPAP in patients with COVID-19 should be free flow, not attached to a ventilator, and the gas flow should be set high – at least 50 L/min – in order to prevent CO2 rebreathing. Although noninvasive ventilation is well accepted for patients with chronic obstructive pulmonary disease or acute cardiogenic pulmonary edema, it hasn’t been extensively studied in the setting of ARDS. A notable exception is a single-center randomized trial in which 83 patients with ARDS at the University of Chicago were assigned to noninvasive ventilation delivered by helmet or face mask (JAMA. 2016 Jun 14;315[22]:2435-41). The endotracheal intubation rate was just 18% in the helmet group, compared with 62% in the face mask group. The 90-day mortality rate was significantly lower in the helmet group as well, noted Dr. Foti, director of the department of anesthesia and intensive care at Monza University Hospital in Milan.
Christian Putensen, MD, said he views intubation for mechanical ventilation as wise in moderate or severe ARDS with an arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio below 150. But in milder, Type L COVID-19 disease, he also likes helmet CPAP. It spares the patient from the traumatic compressive stress to the lung induced by mechanical ventilation, which may cause alveolar edema and SILI.
There is, however, a caveat: “Watch carefully and do not delay intubation if you see helmet CPAP is not working; that is, if the blood gas analysis doesn’t improve, the respiratory rate increases, tidal volume increases, and there is still increased respiratory drive,” advised Dr. Putensen, an anesthesiologist at the University of Bonn (Germany).
There is no agreed-upon practical quantitative measure of respiratory drive. A clinical evaluation of the patient’s depth of inspiration is the best guide, he added.
Dr. Gattinoni said that, when helmet CPAP can’t control respiratory drive in a patient with early-stage disease, he feels the only way to interrupt this destructive process is through early intubation and what he termed “gentle mechanical ventilation,” not with a positive end expiratory pressure of 20 cm H2O, but more like 4-5.
Watch for hypercoagulation
Thromboembolic complications are a common feature in COVID-19 disease.
“I’ve had occasion to see the autopsy results in more than 100 patients. It’s devastating to see the number of thromboses and microthromboses in the lungs, the liver, the kidney, and in the brain,” Dr. Gattinoni said.
“COVID-19 is a serial killer, no doubt,” Dr. Pelosi agreed. “He has no mercy for anyone. And he has two bullets: The first one is for the lung, the second is on the vascular side.”
Dr. Putensen is aggressive in utilizing prophylactic high-dose anticoagulation with heparin. He carefully monitors levels of fibrinogen, Factors V and VIII, and d-dimers. In the setting of COVID-19, he has found thromboelastography to be more reliable than partial thromboplastin time in guiding heparin titration.
Pronation
Panelists agreed that pronation is an especially valuable means of enhancing oxygenation in patients with Type H disease. Dr. Putensen tries for more than 16 hours per day. Dr. Foti is preparing a study of the impact of pronation in 50 awake, nonintubated patients, most of whom were on helmet CPAP. Seven of them couldn’t tolerate pronation for even an hour at a time; for the others, the median duration was 3.5 hours at a time.
“We saw a dramatic improvement, a nearly doubling in the PaO2/FiO2 ratio,” Dr. Foti said.
The helmet CPAP study was done outside of the ICU because, in March 2020, the Milan hospital was utterly overwhelmed by COVID-19. The university hospital ordinarily has 25 ICU beds. This was expanded to 100 ICU beds in an effort to meet the emergency, but that still wasn’t sufficient. Indeed, COVID-19 patients occupied 600 of the hospital’s 650 beds. Physicians were forced to do something formerly unthinkable: triage patients for intubation and mechanical ventilation based upon age, comorbidities, and survival prospects.
“We felt schizophrenic. I completely agree with Luciano’s idea to intubate early when we cannot control the respiratory drive that’s due to the disease. But we couldn’t do it because we had too many patients. So we had to triage,” Dr. Foti recalled, breaking off with a sob as other panelists wiped away their own tears during the webcast.
Respiratory physical therapy
Dr. Pelosi said he believes that optimal care of patients with COVID-19 disease requires a major commitment to physical therapy. He strongly recommends having thoughtfully designed separate written protocols in place for respiratory physiotherapy during mechanical ventilation, weaning, and postextubation. COVID-19 patients typically require 7-10 days of assisted ventilation before weaning, and weaning is a protracted process as well.
“I like to say COVID-19 always requires patience. You have to be very, very patient with this disease,” he emphasized. “These patients have a long and difficult weaning. If the patient isn’t improving during weaning, look at two issues: superinfection and thrombembolism, macro and micro.” The physical therapy measures routinely utilized at his hospital during mechanical ventilation include elevation of the bed head greater than 30 degrees, neuromuscular electrical stimulation, subglottic secretion suctioning, tracheal and oral aspiration, and cough assistance. Separate physical therapy menus are used during before and after extubation.
Dr. Gattinoni offered a final word: “We can do almost nothing with this disease. We try our best to keep the patient alive. What we can do is avoid excessive ventilation of the patient. Applying the typical treatment of ARDS in atypical [Type L] ARDS does not make sense and may be extremely harmful.”
Noninvasive ventilation with helmet continuous positive air pressure (CPAP) deserves to be embraced as an effective strategy in preventing self-induced lung injury, often a key factor in progression from the early milder expression of COVID-19 disease to classic severe acute respiratory distress syndrome, according to European physicians who have been through what they hope are the worst days of the pandemic in the Lombardy region of Northern Italy.
Helmet CPAP is a relatively inexpensive, convenient, well-tolerated intervention. It allows patients to remain conscious and responsive to commands such as “Time to roll over,” which in turn frees up nursing staff. The purpose of helmet CPAP is to curb the huge inspiratory drive that’s a defining feature of this disease and which, unchecked, can lead to self-induced lung injury (SILI), Luciano Gattinoni, MD, explained at a webinar hosted by the European Society of Anaesthesiology.
“Paranoid attention to inspiratory effort – checking it and correcting it – is something where we can make the difference between death and life. It’s extremely important,” said Dr. Gattinoni, guest professor of anesthesiology and intensive care at the University of Gottingen (Germany).
He and his fellow panelists were in accord regarding the merits of helmet CPAP as the premier method of noninvasive ventilatory assistance. They also addressed the importance of monitoring for hypercoagulation, as well as what they’ve come to see as the essential role of pronation in what they define as Type H disease, and the need to have detailed respiratory physiotherapy protocols in place.
“COVID-19 doesn’t like physiotherapy,” explained Paolo Pelosi, MD, professor of anesthesiology and intensive care medicine at the University of Genoa (Italy).
Dr. Gattinoni is credited for identification of two polar phenotypes of what he considers to be a single COVID-19 disease. Early on, many patients present with an atypical form of acute respiratory distress syndrome (ARDS), distinguished by an often-unexpected degree of hypoxia accompanied by high pulmonary compliance and surprisingly little shortness of breath. Dr. Gattinoni and colleagues call this Type L disease, which stands for low elastane, low ventilation to perfusion ratio, low lung weight on CT, and low lung recruitability, which means the patient has a high proportion of aerated lung tissue. Over time, because of either the natural history of the disease or SILI, this may shift to Type H disease, marked by high elastane, high right-to-left shunt, high lung weight, and high recruitability.
“If the pulmonary compliance is above 60 [mL/cm H2O], I’m pretty sure it’s Type L. If it’s 30 [mL/cm H2O] or less, I’m pretty sure it’s Type H. Don’t ask me about 45-55 [mL/cm H2O]; it’s a grey zone,” Dr. Gattinoni said.
Giuseppe Foti, MD, said helmet CPAP in patients with COVID-19 should be free flow, not attached to a ventilator, and the gas flow should be set high – at least 50 L/min – in order to prevent CO2 rebreathing. Although noninvasive ventilation is well accepted for patients with chronic obstructive pulmonary disease or acute cardiogenic pulmonary edema, it hasn’t been extensively studied in the setting of ARDS. A notable exception is a single-center randomized trial in which 83 patients with ARDS at the University of Chicago were assigned to noninvasive ventilation delivered by helmet or face mask (JAMA. 2016 Jun 14;315[22]:2435-41). The endotracheal intubation rate was just 18% in the helmet group, compared with 62% in the face mask group. The 90-day mortality rate was significantly lower in the helmet group as well, noted Dr. Foti, director of the department of anesthesia and intensive care at Monza University Hospital in Milan.
Christian Putensen, MD, said he views intubation for mechanical ventilation as wise in moderate or severe ARDS with an arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio below 150. But in milder, Type L COVID-19 disease, he also likes helmet CPAP. It spares the patient from the traumatic compressive stress to the lung induced by mechanical ventilation, which may cause alveolar edema and SILI.
There is, however, a caveat: “Watch carefully and do not delay intubation if you see helmet CPAP is not working; that is, if the blood gas analysis doesn’t improve, the respiratory rate increases, tidal volume increases, and there is still increased respiratory drive,” advised Dr. Putensen, an anesthesiologist at the University of Bonn (Germany).
There is no agreed-upon practical quantitative measure of respiratory drive. A clinical evaluation of the patient’s depth of inspiration is the best guide, he added.
Dr. Gattinoni said that, when helmet CPAP can’t control respiratory drive in a patient with early-stage disease, he feels the only way to interrupt this destructive process is through early intubation and what he termed “gentle mechanical ventilation,” not with a positive end expiratory pressure of 20 cm H2O, but more like 4-5.
Watch for hypercoagulation
Thromboembolic complications are a common feature in COVID-19 disease.
“I’ve had occasion to see the autopsy results in more than 100 patients. It’s devastating to see the number of thromboses and microthromboses in the lungs, the liver, the kidney, and in the brain,” Dr. Gattinoni said.
“COVID-19 is a serial killer, no doubt,” Dr. Pelosi agreed. “He has no mercy for anyone. And he has two bullets: The first one is for the lung, the second is on the vascular side.”
Dr. Putensen is aggressive in utilizing prophylactic high-dose anticoagulation with heparin. He carefully monitors levels of fibrinogen, Factors V and VIII, and d-dimers. In the setting of COVID-19, he has found thromboelastography to be more reliable than partial thromboplastin time in guiding heparin titration.
Pronation
Panelists agreed that pronation is an especially valuable means of enhancing oxygenation in patients with Type H disease. Dr. Putensen tries for more than 16 hours per day. Dr. Foti is preparing a study of the impact of pronation in 50 awake, nonintubated patients, most of whom were on helmet CPAP. Seven of them couldn’t tolerate pronation for even an hour at a time; for the others, the median duration was 3.5 hours at a time.
“We saw a dramatic improvement, a nearly doubling in the PaO2/FiO2 ratio,” Dr. Foti said.
The helmet CPAP study was done outside of the ICU because, in March 2020, the Milan hospital was utterly overwhelmed by COVID-19. The university hospital ordinarily has 25 ICU beds. This was expanded to 100 ICU beds in an effort to meet the emergency, but that still wasn’t sufficient. Indeed, COVID-19 patients occupied 600 of the hospital’s 650 beds. Physicians were forced to do something formerly unthinkable: triage patients for intubation and mechanical ventilation based upon age, comorbidities, and survival prospects.
“We felt schizophrenic. I completely agree with Luciano’s idea to intubate early when we cannot control the respiratory drive that’s due to the disease. But we couldn’t do it because we had too many patients. So we had to triage,” Dr. Foti recalled, breaking off with a sob as other panelists wiped away their own tears during the webcast.
Respiratory physical therapy
Dr. Pelosi said he believes that optimal care of patients with COVID-19 disease requires a major commitment to physical therapy. He strongly recommends having thoughtfully designed separate written protocols in place for respiratory physiotherapy during mechanical ventilation, weaning, and postextubation. COVID-19 patients typically require 7-10 days of assisted ventilation before weaning, and weaning is a protracted process as well.
“I like to say COVID-19 always requires patience. You have to be very, very patient with this disease,” he emphasized. “These patients have a long and difficult weaning. If the patient isn’t improving during weaning, look at two issues: superinfection and thrombembolism, macro and micro.” The physical therapy measures routinely utilized at his hospital during mechanical ventilation include elevation of the bed head greater than 30 degrees, neuromuscular electrical stimulation, subglottic secretion suctioning, tracheal and oral aspiration, and cough assistance. Separate physical therapy menus are used during before and after extubation.
Dr. Gattinoni offered a final word: “We can do almost nothing with this disease. We try our best to keep the patient alive. What we can do is avoid excessive ventilation of the patient. Applying the typical treatment of ARDS in atypical [Type L] ARDS does not make sense and may be extremely harmful.”
Noninvasive ventilation with helmet continuous positive air pressure (CPAP) deserves to be embraced as an effective strategy in preventing self-induced lung injury, often a key factor in progression from the early milder expression of COVID-19 disease to classic severe acute respiratory distress syndrome, according to European physicians who have been through what they hope are the worst days of the pandemic in the Lombardy region of Northern Italy.
Helmet CPAP is a relatively inexpensive, convenient, well-tolerated intervention. It allows patients to remain conscious and responsive to commands such as “Time to roll over,” which in turn frees up nursing staff. The purpose of helmet CPAP is to curb the huge inspiratory drive that’s a defining feature of this disease and which, unchecked, can lead to self-induced lung injury (SILI), Luciano Gattinoni, MD, explained at a webinar hosted by the European Society of Anaesthesiology.
“Paranoid attention to inspiratory effort – checking it and correcting it – is something where we can make the difference between death and life. It’s extremely important,” said Dr. Gattinoni, guest professor of anesthesiology and intensive care at the University of Gottingen (Germany).
He and his fellow panelists were in accord regarding the merits of helmet CPAP as the premier method of noninvasive ventilatory assistance. They also addressed the importance of monitoring for hypercoagulation, as well as what they’ve come to see as the essential role of pronation in what they define as Type H disease, and the need to have detailed respiratory physiotherapy protocols in place.
“COVID-19 doesn’t like physiotherapy,” explained Paolo Pelosi, MD, professor of anesthesiology and intensive care medicine at the University of Genoa (Italy).
Dr. Gattinoni is credited for identification of two polar phenotypes of what he considers to be a single COVID-19 disease. Early on, many patients present with an atypical form of acute respiratory distress syndrome (ARDS), distinguished by an often-unexpected degree of hypoxia accompanied by high pulmonary compliance and surprisingly little shortness of breath. Dr. Gattinoni and colleagues call this Type L disease, which stands for low elastane, low ventilation to perfusion ratio, low lung weight on CT, and low lung recruitability, which means the patient has a high proportion of aerated lung tissue. Over time, because of either the natural history of the disease or SILI, this may shift to Type H disease, marked by high elastane, high right-to-left shunt, high lung weight, and high recruitability.
“If the pulmonary compliance is above 60 [mL/cm H2O], I’m pretty sure it’s Type L. If it’s 30 [mL/cm H2O] or less, I’m pretty sure it’s Type H. Don’t ask me about 45-55 [mL/cm H2O]; it’s a grey zone,” Dr. Gattinoni said.
Giuseppe Foti, MD, said helmet CPAP in patients with COVID-19 should be free flow, not attached to a ventilator, and the gas flow should be set high – at least 50 L/min – in order to prevent CO2 rebreathing. Although noninvasive ventilation is well accepted for patients with chronic obstructive pulmonary disease or acute cardiogenic pulmonary edema, it hasn’t been extensively studied in the setting of ARDS. A notable exception is a single-center randomized trial in which 83 patients with ARDS at the University of Chicago were assigned to noninvasive ventilation delivered by helmet or face mask (JAMA. 2016 Jun 14;315[22]:2435-41). The endotracheal intubation rate was just 18% in the helmet group, compared with 62% in the face mask group. The 90-day mortality rate was significantly lower in the helmet group as well, noted Dr. Foti, director of the department of anesthesia and intensive care at Monza University Hospital in Milan.
Christian Putensen, MD, said he views intubation for mechanical ventilation as wise in moderate or severe ARDS with an arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio below 150. But in milder, Type L COVID-19 disease, he also likes helmet CPAP. It spares the patient from the traumatic compressive stress to the lung induced by mechanical ventilation, which may cause alveolar edema and SILI.
There is, however, a caveat: “Watch carefully and do not delay intubation if you see helmet CPAP is not working; that is, if the blood gas analysis doesn’t improve, the respiratory rate increases, tidal volume increases, and there is still increased respiratory drive,” advised Dr. Putensen, an anesthesiologist at the University of Bonn (Germany).
There is no agreed-upon practical quantitative measure of respiratory drive. A clinical evaluation of the patient’s depth of inspiration is the best guide, he added.
Dr. Gattinoni said that, when helmet CPAP can’t control respiratory drive in a patient with early-stage disease, he feels the only way to interrupt this destructive process is through early intubation and what he termed “gentle mechanical ventilation,” not with a positive end expiratory pressure of 20 cm H2O, but more like 4-5.
Watch for hypercoagulation
Thromboembolic complications are a common feature in COVID-19 disease.
“I’ve had occasion to see the autopsy results in more than 100 patients. It’s devastating to see the number of thromboses and microthromboses in the lungs, the liver, the kidney, and in the brain,” Dr. Gattinoni said.
“COVID-19 is a serial killer, no doubt,” Dr. Pelosi agreed. “He has no mercy for anyone. And he has two bullets: The first one is for the lung, the second is on the vascular side.”
Dr. Putensen is aggressive in utilizing prophylactic high-dose anticoagulation with heparin. He carefully monitors levels of fibrinogen, Factors V and VIII, and d-dimers. In the setting of COVID-19, he has found thromboelastography to be more reliable than partial thromboplastin time in guiding heparin titration.
Pronation
Panelists agreed that pronation is an especially valuable means of enhancing oxygenation in patients with Type H disease. Dr. Putensen tries for more than 16 hours per day. Dr. Foti is preparing a study of the impact of pronation in 50 awake, nonintubated patients, most of whom were on helmet CPAP. Seven of them couldn’t tolerate pronation for even an hour at a time; for the others, the median duration was 3.5 hours at a time.
“We saw a dramatic improvement, a nearly doubling in the PaO2/FiO2 ratio,” Dr. Foti said.
The helmet CPAP study was done outside of the ICU because, in March 2020, the Milan hospital was utterly overwhelmed by COVID-19. The university hospital ordinarily has 25 ICU beds. This was expanded to 100 ICU beds in an effort to meet the emergency, but that still wasn’t sufficient. Indeed, COVID-19 patients occupied 600 of the hospital’s 650 beds. Physicians were forced to do something formerly unthinkable: triage patients for intubation and mechanical ventilation based upon age, comorbidities, and survival prospects.
“We felt schizophrenic. I completely agree with Luciano’s idea to intubate early when we cannot control the respiratory drive that’s due to the disease. But we couldn’t do it because we had too many patients. So we had to triage,” Dr. Foti recalled, breaking off with a sob as other panelists wiped away their own tears during the webcast.
Respiratory physical therapy
Dr. Pelosi said he believes that optimal care of patients with COVID-19 disease requires a major commitment to physical therapy. He strongly recommends having thoughtfully designed separate written protocols in place for respiratory physiotherapy during mechanical ventilation, weaning, and postextubation. COVID-19 patients typically require 7-10 days of assisted ventilation before weaning, and weaning is a protracted process as well.
“I like to say COVID-19 always requires patience. You have to be very, very patient with this disease,” he emphasized. “These patients have a long and difficult weaning. If the patient isn’t improving during weaning, look at two issues: superinfection and thrombembolism, macro and micro.” The physical therapy measures routinely utilized at his hospital during mechanical ventilation include elevation of the bed head greater than 30 degrees, neuromuscular electrical stimulation, subglottic secretion suctioning, tracheal and oral aspiration, and cough assistance. Separate physical therapy menus are used during before and after extubation.
Dr. Gattinoni offered a final word: “We can do almost nothing with this disease. We try our best to keep the patient alive. What we can do is avoid excessive ventilation of the patient. Applying the typical treatment of ARDS in atypical [Type L] ARDS does not make sense and may be extremely harmful.”
Are patients with epilepsy at increased risk of COVID-19 infection?
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
COVID-19: Experts hasten to head off mental health crisis
The COVID-19 pandemic is already affecting mental health at a population level, with increased anxiety, feelings of isolation, and concerns about access to mental health care.
Two U.K. surveys were conducted to inform research priorities for mental health research and in an effort to head off a mental health crisis. The U.K. charity MQ conducted a “stakeholder” survey of 2,198 individuals who had a lived experience of mental illness, while Ipsos MORI conducted a poll of 1,099 members of the public.
The online surveys were conducted in late March, the same week the U.K.’s nationwide lockdown measures were announced. Respondents were asked about their biggest mental health and well-being concerns and coping strategies as they relate to the COVID-19 pandemic.
Results showed that across the two surveys, respondents’ primary concern was anxiety, which was cited in 750 responses.
In addition, respondents were worried about being social isolated, becoming mentally unwell, and having a lack of access to mental health services, as well as the impact of the pandemic on personal relationships.
The findings were used by a panel of experts to inform a position paper published in the Lancet Psychiatry. The paper outlines a proposed government response to curb the long-term “profound” and “pervasive” impact of the pandemic on mental health.
‘Unprecedented response’ needed
“Governments must find evidence-based ways to boost the resilience of our societies and ... to treat those with mental ill health remotely to come out of this pandemic in good mental health,” coauthor of the paper Emily A. Holmes, PhD, of the department of psychology at Uppsala (Sweden) University, said in a press release.
“Frontline medical staff and vulnerable groups such as the elderly and those with serious mental health conditions must be prioritized for rapid mental health support,” she added.
The position paper authors call for “moment-to-moment” monitoring of anxiety, depression, self-harm, and suicide, as well as using digital technology and rapid deployment of evidence-based programs and treatments.
Patients will need to be accessible via computer, cell phone, and other remote technologies in order to receive treatment during physical isolation. However, they noted that there is no “one-size-fits-all” approach, and novel approaches custom tailored to particular populations, including frontline health care workers, are necessary.
“To make a real difference we will need to harness the tools of our digital age, finding smart new ways to measure the mental health of individuals remotely, finding creative ways to boost resilience, and finding ways to treat people in their homes. This effort must be considered central to our global response to the pandemic,” coauthor Ed Bullmore, PhD, of the department of psychiatry at the University of Cambridge (England), said in a statement.
Dr. Bullmore added that it will take “unprecedented research response if we are to limit the negative consequences of this pandemic on the mental health of our society now and in the future.”
Most vulnerable will bear the brunt
During a webinar held to discuss the paper, Matthew Hotopf, PhD, of the Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, cautioned that society’s most vulnerable citizens will bear the brunt of the pandemic’s mental health consequences.
“These individuals often have unstable housing, unstable work, and are disadvantaged in terms of their physical health and their mental health,” with a “very significant gap” in life expectancy versus the rest of the population, he said. The COVID-19 pandemic will widen the gap between “the haves and the have nots.”
“People with established and significant mental disorders are one version of the ‘have nots’ but actually it applies to a lot of people,” said Dr. Hotopf, noting that his experience of lockdown is “very different” from that of someone “living in overcrowded, unstable accommodation, with kids running around and maybe a partner who has problems with anger control.”
The authors of the position paper noted that the COVID-19 pandemic highlights several important research priorities that need to be addressed in the coming weeks and months. These include:
- Understanding the effect of COVID-19 on risk of anxiety, depression, and other outcomes, such as self-harm and suicide
- Understanding how to create physical and social supports to ensure mental health in a climate of physical distancing
- Determining the mental health consequences of social isolation for vulnerable groups, and how can these be mitigated under pandemic conditions
- Understanding the mental health impact of media reporting of COVID-19 in traditional and social media
- Determining the best methods for promoting successful adherence to behavioral advice about COVID-19 while enabling mental well-being and minimizing distress
Another area highlighted by the experts is the potential for neuropsychiatric sequelae in individuals infected with COVID-19. They called for “experimental medicine studies to validate clinical biomarkers and repurpose new treatments for the potentially neurotoxic effects of the virus.”
The authors/investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic is already affecting mental health at a population level, with increased anxiety, feelings of isolation, and concerns about access to mental health care.
Two U.K. surveys were conducted to inform research priorities for mental health research and in an effort to head off a mental health crisis. The U.K. charity MQ conducted a “stakeholder” survey of 2,198 individuals who had a lived experience of mental illness, while Ipsos MORI conducted a poll of 1,099 members of the public.
The online surveys were conducted in late March, the same week the U.K.’s nationwide lockdown measures were announced. Respondents were asked about their biggest mental health and well-being concerns and coping strategies as they relate to the COVID-19 pandemic.
Results showed that across the two surveys, respondents’ primary concern was anxiety, which was cited in 750 responses.
In addition, respondents were worried about being social isolated, becoming mentally unwell, and having a lack of access to mental health services, as well as the impact of the pandemic on personal relationships.
The findings were used by a panel of experts to inform a position paper published in the Lancet Psychiatry. The paper outlines a proposed government response to curb the long-term “profound” and “pervasive” impact of the pandemic on mental health.
‘Unprecedented response’ needed
“Governments must find evidence-based ways to boost the resilience of our societies and ... to treat those with mental ill health remotely to come out of this pandemic in good mental health,” coauthor of the paper Emily A. Holmes, PhD, of the department of psychology at Uppsala (Sweden) University, said in a press release.
“Frontline medical staff and vulnerable groups such as the elderly and those with serious mental health conditions must be prioritized for rapid mental health support,” she added.
The position paper authors call for “moment-to-moment” monitoring of anxiety, depression, self-harm, and suicide, as well as using digital technology and rapid deployment of evidence-based programs and treatments.
Patients will need to be accessible via computer, cell phone, and other remote technologies in order to receive treatment during physical isolation. However, they noted that there is no “one-size-fits-all” approach, and novel approaches custom tailored to particular populations, including frontline health care workers, are necessary.
“To make a real difference we will need to harness the tools of our digital age, finding smart new ways to measure the mental health of individuals remotely, finding creative ways to boost resilience, and finding ways to treat people in their homes. This effort must be considered central to our global response to the pandemic,” coauthor Ed Bullmore, PhD, of the department of psychiatry at the University of Cambridge (England), said in a statement.
Dr. Bullmore added that it will take “unprecedented research response if we are to limit the negative consequences of this pandemic on the mental health of our society now and in the future.”
Most vulnerable will bear the brunt
During a webinar held to discuss the paper, Matthew Hotopf, PhD, of the Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, cautioned that society’s most vulnerable citizens will bear the brunt of the pandemic’s mental health consequences.
“These individuals often have unstable housing, unstable work, and are disadvantaged in terms of their physical health and their mental health,” with a “very significant gap” in life expectancy versus the rest of the population, he said. The COVID-19 pandemic will widen the gap between “the haves and the have nots.”
“People with established and significant mental disorders are one version of the ‘have nots’ but actually it applies to a lot of people,” said Dr. Hotopf, noting that his experience of lockdown is “very different” from that of someone “living in overcrowded, unstable accommodation, with kids running around and maybe a partner who has problems with anger control.”
The authors of the position paper noted that the COVID-19 pandemic highlights several important research priorities that need to be addressed in the coming weeks and months. These include:
- Understanding the effect of COVID-19 on risk of anxiety, depression, and other outcomes, such as self-harm and suicide
- Understanding how to create physical and social supports to ensure mental health in a climate of physical distancing
- Determining the mental health consequences of social isolation for vulnerable groups, and how can these be mitigated under pandemic conditions
- Understanding the mental health impact of media reporting of COVID-19 in traditional and social media
- Determining the best methods for promoting successful adherence to behavioral advice about COVID-19 while enabling mental well-being and minimizing distress
Another area highlighted by the experts is the potential for neuropsychiatric sequelae in individuals infected with COVID-19. They called for “experimental medicine studies to validate clinical biomarkers and repurpose new treatments for the potentially neurotoxic effects of the virus.”
The authors/investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic is already affecting mental health at a population level, with increased anxiety, feelings of isolation, and concerns about access to mental health care.
Two U.K. surveys were conducted to inform research priorities for mental health research and in an effort to head off a mental health crisis. The U.K. charity MQ conducted a “stakeholder” survey of 2,198 individuals who had a lived experience of mental illness, while Ipsos MORI conducted a poll of 1,099 members of the public.
The online surveys were conducted in late March, the same week the U.K.’s nationwide lockdown measures were announced. Respondents were asked about their biggest mental health and well-being concerns and coping strategies as they relate to the COVID-19 pandemic.
Results showed that across the two surveys, respondents’ primary concern was anxiety, which was cited in 750 responses.
In addition, respondents were worried about being social isolated, becoming mentally unwell, and having a lack of access to mental health services, as well as the impact of the pandemic on personal relationships.
The findings were used by a panel of experts to inform a position paper published in the Lancet Psychiatry. The paper outlines a proposed government response to curb the long-term “profound” and “pervasive” impact of the pandemic on mental health.
‘Unprecedented response’ needed
“Governments must find evidence-based ways to boost the resilience of our societies and ... to treat those with mental ill health remotely to come out of this pandemic in good mental health,” coauthor of the paper Emily A. Holmes, PhD, of the department of psychology at Uppsala (Sweden) University, said in a press release.
“Frontline medical staff and vulnerable groups such as the elderly and those with serious mental health conditions must be prioritized for rapid mental health support,” she added.
The position paper authors call for “moment-to-moment” monitoring of anxiety, depression, self-harm, and suicide, as well as using digital technology and rapid deployment of evidence-based programs and treatments.
Patients will need to be accessible via computer, cell phone, and other remote technologies in order to receive treatment during physical isolation. However, they noted that there is no “one-size-fits-all” approach, and novel approaches custom tailored to particular populations, including frontline health care workers, are necessary.
“To make a real difference we will need to harness the tools of our digital age, finding smart new ways to measure the mental health of individuals remotely, finding creative ways to boost resilience, and finding ways to treat people in their homes. This effort must be considered central to our global response to the pandemic,” coauthor Ed Bullmore, PhD, of the department of psychiatry at the University of Cambridge (England), said in a statement.
Dr. Bullmore added that it will take “unprecedented research response if we are to limit the negative consequences of this pandemic on the mental health of our society now and in the future.”
Most vulnerable will bear the brunt
During a webinar held to discuss the paper, Matthew Hotopf, PhD, of the Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, cautioned that society’s most vulnerable citizens will bear the brunt of the pandemic’s mental health consequences.
“These individuals often have unstable housing, unstable work, and are disadvantaged in terms of their physical health and their mental health,” with a “very significant gap” in life expectancy versus the rest of the population, he said. The COVID-19 pandemic will widen the gap between “the haves and the have nots.”
“People with established and significant mental disorders are one version of the ‘have nots’ but actually it applies to a lot of people,” said Dr. Hotopf, noting that his experience of lockdown is “very different” from that of someone “living in overcrowded, unstable accommodation, with kids running around and maybe a partner who has problems with anger control.”
The authors of the position paper noted that the COVID-19 pandemic highlights several important research priorities that need to be addressed in the coming weeks and months. These include:
- Understanding the effect of COVID-19 on risk of anxiety, depression, and other outcomes, such as self-harm and suicide
- Understanding how to create physical and social supports to ensure mental health in a climate of physical distancing
- Determining the mental health consequences of social isolation for vulnerable groups, and how can these be mitigated under pandemic conditions
- Understanding the mental health impact of media reporting of COVID-19 in traditional and social media
- Determining the best methods for promoting successful adherence to behavioral advice about COVID-19 while enabling mental well-being and minimizing distress
Another area highlighted by the experts is the potential for neuropsychiatric sequelae in individuals infected with COVID-19. They called for “experimental medicine studies to validate clinical biomarkers and repurpose new treatments for the potentially neurotoxic effects of the virus.”
The authors/investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
During a pandemic, infusion center nursing team pitches in to keep patients on track
How do you run a chemotherapy infusion center during a pandemic?
Quick action, innovative staffing solutions, and nimble leadership are allowing one cancer center to continue providing care for the most vulnerable patients, while keeping patients and staff safe.
When nursing leaders at Atrium Health’s Levine Cancer Institute in Charlotte, N.C., realized that business was not going to continue as usual for American health care during the COVID-19 pandemic, they knew they had to act quickly to keep the institute’s 82-chair infusion center up and running.
North Carolina had already imposed restrictions on mass gatherings and closed educational facilities and some businesses by mid-March. Stay-at-home orders were being issued in surrounding states (North Carolina came under a statewide order on March 30). Physical distancing and a healthy, resilient team were prerequisites to an effective COVID-19 solution for the infusion clinic, said Angela Hosking, MBA, MSN, RN, director of nursing for Levine Cancer Institute. In an interview, she said that, at meetings on Monday, March 23, “we divided the team exactly in half.”
Infusion center staff members were broken into an “A” and a “B” rotation, with each team either on site or remotely for a 14-day stretch, and then switching at the 2-week mark. The 14-day rotation, she said, was chosen so that each cohort would have a full 2 weeks away after having been in clinic to ensure they were symptom free before returning. The cohorting scheme also serves to minimize between-staff exposure and risk of transmission.
These changes were implemented immediately, said Ms. Hosking, and included all but the most senior leadership – Ms. Hosking alternates days on site with another senior colleague to help with continuity.
Infusion center patients were triaged to determine “who absolutely needed to be seen,” and clinic staff started making phone calls and reshuffling the schedule so the clinic could continue at half-strength staffing.
The clinic was rearranged to ensure each infusion chair had appropriate space but the nursing work flow was still safe with reduced staff, said Jessica Stewart, MSN, RN, Levine Cancer Institute’s hematology–sickle cell nurse manager.
Patients were receptive, said Ms. Stewart. The team that was working remotely made sure all patients were called the day before their appointments, so they could understand what to expect when they arrived. Any needed updates to the medical history and patient teaching can also be done over the phone the day before the visit, she said, noting that patients are also queried about any concerning symptoms such as fever or cough.
In the spirit of providing information and managing expectations, patients are also informed that they will not be able to bring a visitor along and are advised to expect additional screening when they arrive. In addition to a repeat of symptom screening, patients are checked for fever with a temporal thermometer.
Any patient who arrives reporting symptoms or who has a fever is then subject to additional screening. Physician phone consultation is available, if needed, and patients may be routed to a drive-through screening and testing setup, or to the ED if there are concerns the patient may be seriously ill.
Several weeks into the new operations, Ms. Stewart said, “we’ve fine-tuned the processes we currently have in place. There’s new practices with virtual visits to make reaching our patients easier. Our senior leadership is communicating in a weekly video sent to all [Levine Cancer Institute] teammates for updates; it’s very transparent and the team is appreciative of being kept in the loop.”
Thus far, said Ms. Hosking, “it’s gone well – we’ve successfully operationalized this plan. … I think it shows that people that care about each other and their mission can collaborate with each other” to make change happen in a hurry.
Though it’s too soon to know exactly what the future holds once the pandemic has passed, some aspects of the new way of doing things may carry forward, said Ms. Stewart. “Communication has been massively streamlined,” and staff has found the previsit phone calls an efficient and effective way to gather and impart information.
A staff nurse at the infusion center, Whitney Hollifield, RN, added that patients have seen – and appreciate – the added precautions taken by all. “I feel that we have done well with protecting our patients from unneeded exposure and patients have expressed this to me,” said Ms. Hollifield. “They have said: ‘Thank you for doing this because I am scared to come in right now so I appreciate that your office is thinking of protecting us.’ ”
Ms. Hollifield added that “patients have been very responsive to our strategy for their care because we are truly concerned for them and I think that this shows. I believe that we are doing everything we can to keep them safe during a tumultuous time, and they feel genuine care for them during a frightening time is reassuring.”
On the practical side of things, Ms. Stewart noted, patients and families have provided infusion center staff with a seemingly endless supply of food: “We have never been more well fed!”
Rhonda Davis, RN, is a nurse at the Levine Cancer Institute. Speaking of the changes that have been made in recent weeks, she said, “Some of the changes that I think have been meaningful these last 3 weeks are making sure that the patients are the No. 1 priority. We are doing this by allowing patients options such as phone and virtual visits. This helps patients have some control over their health during this scary time for all.”
Ms. Davis acknowledged her own feelings about the uncertain times ahead. “As an individual with good health, I am scared, so to imagine the fear that these patients are facing must be overwhelming to them. Along that line, one of the most meaningful things that has happened for me is calling patients and having them concerned about my health and telling me to be safe.”
Despite her trepidation, she said, it’s meaningful for her to hear from patients who are in the clinic that they appreciate her presence. She found it heartening “that they are also considering our safety as well as their own.”
The two-cohort scheme has been well received by nursing staff, both administrators and clinic staff agreed. “I think that allowing staff to work 2 weeks on and 2 weeks at home helps keep patients and teammates safe,” Ms. Davis said.
Another infusion nurse, Ursel Wallace, RN, said that she appreciated the speed and efficiency with which the pandemic adaptations were made, including the nuts and bolts of reshuffling a complicated infusion schedule. “I know there were many different moving parts and it took a village” to move with such alacrity without dropping balls, she said.
The infusion nursing team’s spirit was summed up by Patricia Ashworth, RN: “Together, we will prevail!”
How do you run a chemotherapy infusion center during a pandemic?
Quick action, innovative staffing solutions, and nimble leadership are allowing one cancer center to continue providing care for the most vulnerable patients, while keeping patients and staff safe.
When nursing leaders at Atrium Health’s Levine Cancer Institute in Charlotte, N.C., realized that business was not going to continue as usual for American health care during the COVID-19 pandemic, they knew they had to act quickly to keep the institute’s 82-chair infusion center up and running.
North Carolina had already imposed restrictions on mass gatherings and closed educational facilities and some businesses by mid-March. Stay-at-home orders were being issued in surrounding states (North Carolina came under a statewide order on March 30). Physical distancing and a healthy, resilient team were prerequisites to an effective COVID-19 solution for the infusion clinic, said Angela Hosking, MBA, MSN, RN, director of nursing for Levine Cancer Institute. In an interview, she said that, at meetings on Monday, March 23, “we divided the team exactly in half.”
Infusion center staff members were broken into an “A” and a “B” rotation, with each team either on site or remotely for a 14-day stretch, and then switching at the 2-week mark. The 14-day rotation, she said, was chosen so that each cohort would have a full 2 weeks away after having been in clinic to ensure they were symptom free before returning. The cohorting scheme also serves to minimize between-staff exposure and risk of transmission.
These changes were implemented immediately, said Ms. Hosking, and included all but the most senior leadership – Ms. Hosking alternates days on site with another senior colleague to help with continuity.
Infusion center patients were triaged to determine “who absolutely needed to be seen,” and clinic staff started making phone calls and reshuffling the schedule so the clinic could continue at half-strength staffing.
The clinic was rearranged to ensure each infusion chair had appropriate space but the nursing work flow was still safe with reduced staff, said Jessica Stewart, MSN, RN, Levine Cancer Institute’s hematology–sickle cell nurse manager.
Patients were receptive, said Ms. Stewart. The team that was working remotely made sure all patients were called the day before their appointments, so they could understand what to expect when they arrived. Any needed updates to the medical history and patient teaching can also be done over the phone the day before the visit, she said, noting that patients are also queried about any concerning symptoms such as fever or cough.
In the spirit of providing information and managing expectations, patients are also informed that they will not be able to bring a visitor along and are advised to expect additional screening when they arrive. In addition to a repeat of symptom screening, patients are checked for fever with a temporal thermometer.
Any patient who arrives reporting symptoms or who has a fever is then subject to additional screening. Physician phone consultation is available, if needed, and patients may be routed to a drive-through screening and testing setup, or to the ED if there are concerns the patient may be seriously ill.
Several weeks into the new operations, Ms. Stewart said, “we’ve fine-tuned the processes we currently have in place. There’s new practices with virtual visits to make reaching our patients easier. Our senior leadership is communicating in a weekly video sent to all [Levine Cancer Institute] teammates for updates; it’s very transparent and the team is appreciative of being kept in the loop.”
Thus far, said Ms. Hosking, “it’s gone well – we’ve successfully operationalized this plan. … I think it shows that people that care about each other and their mission can collaborate with each other” to make change happen in a hurry.
Though it’s too soon to know exactly what the future holds once the pandemic has passed, some aspects of the new way of doing things may carry forward, said Ms. Stewart. “Communication has been massively streamlined,” and staff has found the previsit phone calls an efficient and effective way to gather and impart information.
A staff nurse at the infusion center, Whitney Hollifield, RN, added that patients have seen – and appreciate – the added precautions taken by all. “I feel that we have done well with protecting our patients from unneeded exposure and patients have expressed this to me,” said Ms. Hollifield. “They have said: ‘Thank you for doing this because I am scared to come in right now so I appreciate that your office is thinking of protecting us.’ ”
Ms. Hollifield added that “patients have been very responsive to our strategy for their care because we are truly concerned for them and I think that this shows. I believe that we are doing everything we can to keep them safe during a tumultuous time, and they feel genuine care for them during a frightening time is reassuring.”
On the practical side of things, Ms. Stewart noted, patients and families have provided infusion center staff with a seemingly endless supply of food: “We have never been more well fed!”
Rhonda Davis, RN, is a nurse at the Levine Cancer Institute. Speaking of the changes that have been made in recent weeks, she said, “Some of the changes that I think have been meaningful these last 3 weeks are making sure that the patients are the No. 1 priority. We are doing this by allowing patients options such as phone and virtual visits. This helps patients have some control over their health during this scary time for all.”
Ms. Davis acknowledged her own feelings about the uncertain times ahead. “As an individual with good health, I am scared, so to imagine the fear that these patients are facing must be overwhelming to them. Along that line, one of the most meaningful things that has happened for me is calling patients and having them concerned about my health and telling me to be safe.”
Despite her trepidation, she said, it’s meaningful for her to hear from patients who are in the clinic that they appreciate her presence. She found it heartening “that they are also considering our safety as well as their own.”
The two-cohort scheme has been well received by nursing staff, both administrators and clinic staff agreed. “I think that allowing staff to work 2 weeks on and 2 weeks at home helps keep patients and teammates safe,” Ms. Davis said.
Another infusion nurse, Ursel Wallace, RN, said that she appreciated the speed and efficiency with which the pandemic adaptations were made, including the nuts and bolts of reshuffling a complicated infusion schedule. “I know there were many different moving parts and it took a village” to move with such alacrity without dropping balls, she said.
The infusion nursing team’s spirit was summed up by Patricia Ashworth, RN: “Together, we will prevail!”
How do you run a chemotherapy infusion center during a pandemic?
Quick action, innovative staffing solutions, and nimble leadership are allowing one cancer center to continue providing care for the most vulnerable patients, while keeping patients and staff safe.
When nursing leaders at Atrium Health’s Levine Cancer Institute in Charlotte, N.C., realized that business was not going to continue as usual for American health care during the COVID-19 pandemic, they knew they had to act quickly to keep the institute’s 82-chair infusion center up and running.
North Carolina had already imposed restrictions on mass gatherings and closed educational facilities and some businesses by mid-March. Stay-at-home orders were being issued in surrounding states (North Carolina came under a statewide order on March 30). Physical distancing and a healthy, resilient team were prerequisites to an effective COVID-19 solution for the infusion clinic, said Angela Hosking, MBA, MSN, RN, director of nursing for Levine Cancer Institute. In an interview, she said that, at meetings on Monday, March 23, “we divided the team exactly in half.”
Infusion center staff members were broken into an “A” and a “B” rotation, with each team either on site or remotely for a 14-day stretch, and then switching at the 2-week mark. The 14-day rotation, she said, was chosen so that each cohort would have a full 2 weeks away after having been in clinic to ensure they were symptom free before returning. The cohorting scheme also serves to minimize between-staff exposure and risk of transmission.
These changes were implemented immediately, said Ms. Hosking, and included all but the most senior leadership – Ms. Hosking alternates days on site with another senior colleague to help with continuity.
Infusion center patients were triaged to determine “who absolutely needed to be seen,” and clinic staff started making phone calls and reshuffling the schedule so the clinic could continue at half-strength staffing.
The clinic was rearranged to ensure each infusion chair had appropriate space but the nursing work flow was still safe with reduced staff, said Jessica Stewart, MSN, RN, Levine Cancer Institute’s hematology–sickle cell nurse manager.
Patients were receptive, said Ms. Stewart. The team that was working remotely made sure all patients were called the day before their appointments, so they could understand what to expect when they arrived. Any needed updates to the medical history and patient teaching can also be done over the phone the day before the visit, she said, noting that patients are also queried about any concerning symptoms such as fever or cough.
In the spirit of providing information and managing expectations, patients are also informed that they will not be able to bring a visitor along and are advised to expect additional screening when they arrive. In addition to a repeat of symptom screening, patients are checked for fever with a temporal thermometer.
Any patient who arrives reporting symptoms or who has a fever is then subject to additional screening. Physician phone consultation is available, if needed, and patients may be routed to a drive-through screening and testing setup, or to the ED if there are concerns the patient may be seriously ill.
Several weeks into the new operations, Ms. Stewart said, “we’ve fine-tuned the processes we currently have in place. There’s new practices with virtual visits to make reaching our patients easier. Our senior leadership is communicating in a weekly video sent to all [Levine Cancer Institute] teammates for updates; it’s very transparent and the team is appreciative of being kept in the loop.”
Thus far, said Ms. Hosking, “it’s gone well – we’ve successfully operationalized this plan. … I think it shows that people that care about each other and their mission can collaborate with each other” to make change happen in a hurry.
Though it’s too soon to know exactly what the future holds once the pandemic has passed, some aspects of the new way of doing things may carry forward, said Ms. Stewart. “Communication has been massively streamlined,” and staff has found the previsit phone calls an efficient and effective way to gather and impart information.
A staff nurse at the infusion center, Whitney Hollifield, RN, added that patients have seen – and appreciate – the added precautions taken by all. “I feel that we have done well with protecting our patients from unneeded exposure and patients have expressed this to me,” said Ms. Hollifield. “They have said: ‘Thank you for doing this because I am scared to come in right now so I appreciate that your office is thinking of protecting us.’ ”
Ms. Hollifield added that “patients have been very responsive to our strategy for their care because we are truly concerned for them and I think that this shows. I believe that we are doing everything we can to keep them safe during a tumultuous time, and they feel genuine care for them during a frightening time is reassuring.”
On the practical side of things, Ms. Stewart noted, patients and families have provided infusion center staff with a seemingly endless supply of food: “We have never been more well fed!”
Rhonda Davis, RN, is a nurse at the Levine Cancer Institute. Speaking of the changes that have been made in recent weeks, she said, “Some of the changes that I think have been meaningful these last 3 weeks are making sure that the patients are the No. 1 priority. We are doing this by allowing patients options such as phone and virtual visits. This helps patients have some control over their health during this scary time for all.”
Ms. Davis acknowledged her own feelings about the uncertain times ahead. “As an individual with good health, I am scared, so to imagine the fear that these patients are facing must be overwhelming to them. Along that line, one of the most meaningful things that has happened for me is calling patients and having them concerned about my health and telling me to be safe.”
Despite her trepidation, she said, it’s meaningful for her to hear from patients who are in the clinic that they appreciate her presence. She found it heartening “that they are also considering our safety as well as their own.”
The two-cohort scheme has been well received by nursing staff, both administrators and clinic staff agreed. “I think that allowing staff to work 2 weeks on and 2 weeks at home helps keep patients and teammates safe,” Ms. Davis said.
Another infusion nurse, Ursel Wallace, RN, said that she appreciated the speed and efficiency with which the pandemic adaptations were made, including the nuts and bolts of reshuffling a complicated infusion schedule. “I know there were many different moving parts and it took a village” to move with such alacrity without dropping balls, she said.
The infusion nursing team’s spirit was summed up by Patricia Ashworth, RN: “Together, we will prevail!”
Latest data on COVID-19 patients with rheumatic diseases revealed in registry
An international registry of adult and pediatric rheumatology patients is beginning to identify trends in the types of patients with COVID-19 and who is recovering.
The COVID-19 Global Rheumatology Alliance (GRA) has created pediatric and adult registries for health care providers to enter information on their rheumatology patients with COVID-19. The adult registry is hosted by the University of California, San Francisco, Research Electronic Data Capture system, while the Childhood Arthritis and Rheumatology Research Alliance is supporting the pediatric registry. A separate path for data entry of both adult and pediatric cases has been established through the European League Against Rheumatism for European countries and countries with EULAR member organizations.
Prior to the creation of the registries, there were no data available to guide rheumatologists in clinical decision making for their patients, noted Jinoos Yazdany, MD, MPH, COVID-19 GRA steering committee member and chief of the division of rheumatology at Zuckerberg San Francisco General Hospital. “COVID-19 has potential to severely affect those with rheumatologic diseases or those taking immunosuppressive drugs,” she said in an interview. “The GRA registries were designed to answer critical questions that will inform the medical care of this population.”
The GRA began on Twitter, with conversations between Leonard H. Calabrese, DO, of the Cleveland Clinic; Paul Sufka, MD, of HealthPartners in St. Paul, Minn.; Philip Robinson, MBChB, PhD, of the Royal Brisbane (Australia) Hospital; and herself, Dr. Yazdany said. Dr. Robinson started work on the governance of the GRA, Dr. Yazdany designed the data infrastructure, and Dr. Sufka approached his professional networks and social media followings to promote the effort and ask for support. The COVID-19 GRA steering committee representatives include patients, private practice rheumatologists, and international investigators. Listed among official supporters of the alliance are the American College of Rheumatology and EULAR along with more than 290 medical societies, institutions, journals, and other organizations in rheumatology.
The goal of the registries is to examine the health outcomes of patients with rheumatic diseases and COVID-19 based on sociodemographic factors, comorbidities, and clinical presentations of COVID-19 as well as what role taking immunosuppressive drugs prior to a COVID-19 infection play in helping or hindering outcomes. Hydroxychloroquine, used to treat lupus and arthritis, is a potential treatment candidate for COVID-19. Biologics such as tocilizumab (Actemra) and sarilumab (Kevzara), which target interleukin-6, and anakinra (Kineret), which targets IL-1, are treatment candidates for patients who have experienced COVID-related cytokine storm syndrome, which researchers believe may contribute to worsening or fatal cases.
Dr. Yazdany, who is also vice chair of real-world data infrastructure, registry, and institutional review board/ethics for the GRA, said that there are some important high-level trends in the data thus far. “People with lupus and those taking hydroxychloroquine are becoming infected with SARS-CoV-2, which is counter to misinformation on social media. Most people with rheumatic diseases on immunosuppression are recovering, which is great news for our patients.”
One of the major strengths of the registries is that each case is entered by the rheumatologist treating the patient and contains detailed clinical information, Dr. Yazdany said. However, the registry has no control group, it is not a population surveillance study, and it may contain selection bias through rheumatologists omitting milder, undiagnosed cases.
“The Global Alliance case reporting registry represents the collective effort of hundreds of rheumatologists across the world. I have never been more inspired by the strength and collaboration of the rheumatology community,” Dr. Yazdany said.
According to a paper published in the Lancet Rheumatology, which references data on 110 cases from the combined databases up to April 1, about three-fourths of cases presented with fever (79%) and cough (77%), and about half presented with shortness of breath (50%) and myalgia (45%).
Results from the global and UCSF registries
As of April 18, 334 cases were in the global and UCSF registries, with 121 patients (36%) in the database having both COVID-19 and RA, 33 patients (10%) with psoriatic arthritis, 58 patients (17%) with systemic lupus erythematosus, 28 patients (8%) with axial spondyloarthritis, 27 patients (8%) with vasculitis, and 19 patients (6%) with Sjögren’s syndrome. There were less than five cases reported for patients with the following rheumatic diseases: inflammatory myopathy, ocular inflammation, other inflammatory arthritis, polymyalgia rheumatica, sarcoidosis, systemic sclerosis, osteoporosis, psoriasis, isolated pulmonary capillaritis, gout, and autoinflammatory disease. A majority of the patients in the registries are women (74%) aged younger than 65 years (78%) and are white (52%).
The most common comorbid conditions among patients in the registry are hypertension (33%), lung disease (18%), diabetes (11%), cardiovascular disease (10%), chronic renal insufficiency or end-stage renal disease (7%), morbid obesity (7%), and cancer (4%). Before being diagnosed with COVID-19, 219 patients (66%) in the registry were taking conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), which included antimalarials, azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, and tacrolimus. A total of 122 patients (37%) were taking biologic DMARDs, 101 patients were taking glucocorticoids (30%), 86 patients (26%) were taking hydroxychloroquine, 41 patients (12%) were taking NSAIDs, and 18 patients (5%) were taking a Janus kinase inhibitor.
The most recent data from the registry show that 128 patients (38%) have been hospitalized for COVID-19, and 19 patients (6%) have died. Although 104 patients (31%) resolved their infections, 177 patients (53%) have a COVID-19 infection status of “unresolved,” and 53 patients (16%) have an unknown infection status.
EULAR registry results
As of April 21, 249 cases were in the EULAR registry, including 110 hospitalizations (44%) and 37 deaths (15%). Overall, 64% of these patients were women, and they had a median age of 60 years.
The top five diagnoses of these patients were RA (39%), psoriatic arthritis (15%), spondyloarthritis (9%), systemic lupus erythematosus (9%), and gout (5%). A total of 27% had no reported comorbidities, while lung disease occurred in 26%, hypertension in 34%, diabetes in 11%, and cardiovascular disease on 11%. The registry also reported use of any DMARD in 80%, including 62% on csDMARDs, 31% on biologics, and 2% on targeted synthetic DMARDs.
Ten authors in the Lancet Rheumatology paper reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speaker’s bureau positions, personal fees, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. The other authors reported no relevant conflicts of interest.
An international registry of adult and pediatric rheumatology patients is beginning to identify trends in the types of patients with COVID-19 and who is recovering.
The COVID-19 Global Rheumatology Alliance (GRA) has created pediatric and adult registries for health care providers to enter information on their rheumatology patients with COVID-19. The adult registry is hosted by the University of California, San Francisco, Research Electronic Data Capture system, while the Childhood Arthritis and Rheumatology Research Alliance is supporting the pediatric registry. A separate path for data entry of both adult and pediatric cases has been established through the European League Against Rheumatism for European countries and countries with EULAR member organizations.
Prior to the creation of the registries, there were no data available to guide rheumatologists in clinical decision making for their patients, noted Jinoos Yazdany, MD, MPH, COVID-19 GRA steering committee member and chief of the division of rheumatology at Zuckerberg San Francisco General Hospital. “COVID-19 has potential to severely affect those with rheumatologic diseases or those taking immunosuppressive drugs,” she said in an interview. “The GRA registries were designed to answer critical questions that will inform the medical care of this population.”
The GRA began on Twitter, with conversations between Leonard H. Calabrese, DO, of the Cleveland Clinic; Paul Sufka, MD, of HealthPartners in St. Paul, Minn.; Philip Robinson, MBChB, PhD, of the Royal Brisbane (Australia) Hospital; and herself, Dr. Yazdany said. Dr. Robinson started work on the governance of the GRA, Dr. Yazdany designed the data infrastructure, and Dr. Sufka approached his professional networks and social media followings to promote the effort and ask for support. The COVID-19 GRA steering committee representatives include patients, private practice rheumatologists, and international investigators. Listed among official supporters of the alliance are the American College of Rheumatology and EULAR along with more than 290 medical societies, institutions, journals, and other organizations in rheumatology.
The goal of the registries is to examine the health outcomes of patients with rheumatic diseases and COVID-19 based on sociodemographic factors, comorbidities, and clinical presentations of COVID-19 as well as what role taking immunosuppressive drugs prior to a COVID-19 infection play in helping or hindering outcomes. Hydroxychloroquine, used to treat lupus and arthritis, is a potential treatment candidate for COVID-19. Biologics such as tocilizumab (Actemra) and sarilumab (Kevzara), which target interleukin-6, and anakinra (Kineret), which targets IL-1, are treatment candidates for patients who have experienced COVID-related cytokine storm syndrome, which researchers believe may contribute to worsening or fatal cases.
Dr. Yazdany, who is also vice chair of real-world data infrastructure, registry, and institutional review board/ethics for the GRA, said that there are some important high-level trends in the data thus far. “People with lupus and those taking hydroxychloroquine are becoming infected with SARS-CoV-2, which is counter to misinformation on social media. Most people with rheumatic diseases on immunosuppression are recovering, which is great news for our patients.”
One of the major strengths of the registries is that each case is entered by the rheumatologist treating the patient and contains detailed clinical information, Dr. Yazdany said. However, the registry has no control group, it is not a population surveillance study, and it may contain selection bias through rheumatologists omitting milder, undiagnosed cases.
“The Global Alliance case reporting registry represents the collective effort of hundreds of rheumatologists across the world. I have never been more inspired by the strength and collaboration of the rheumatology community,” Dr. Yazdany said.
According to a paper published in the Lancet Rheumatology, which references data on 110 cases from the combined databases up to April 1, about three-fourths of cases presented with fever (79%) and cough (77%), and about half presented with shortness of breath (50%) and myalgia (45%).
Results from the global and UCSF registries
As of April 18, 334 cases were in the global and UCSF registries, with 121 patients (36%) in the database having both COVID-19 and RA, 33 patients (10%) with psoriatic arthritis, 58 patients (17%) with systemic lupus erythematosus, 28 patients (8%) with axial spondyloarthritis, 27 patients (8%) with vasculitis, and 19 patients (6%) with Sjögren’s syndrome. There were less than five cases reported for patients with the following rheumatic diseases: inflammatory myopathy, ocular inflammation, other inflammatory arthritis, polymyalgia rheumatica, sarcoidosis, systemic sclerosis, osteoporosis, psoriasis, isolated pulmonary capillaritis, gout, and autoinflammatory disease. A majority of the patients in the registries are women (74%) aged younger than 65 years (78%) and are white (52%).
The most common comorbid conditions among patients in the registry are hypertension (33%), lung disease (18%), diabetes (11%), cardiovascular disease (10%), chronic renal insufficiency or end-stage renal disease (7%), morbid obesity (7%), and cancer (4%). Before being diagnosed with COVID-19, 219 patients (66%) in the registry were taking conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), which included antimalarials, azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, and tacrolimus. A total of 122 patients (37%) were taking biologic DMARDs, 101 patients were taking glucocorticoids (30%), 86 patients (26%) were taking hydroxychloroquine, 41 patients (12%) were taking NSAIDs, and 18 patients (5%) were taking a Janus kinase inhibitor.
The most recent data from the registry show that 128 patients (38%) have been hospitalized for COVID-19, and 19 patients (6%) have died. Although 104 patients (31%) resolved their infections, 177 patients (53%) have a COVID-19 infection status of “unresolved,” and 53 patients (16%) have an unknown infection status.
EULAR registry results
As of April 21, 249 cases were in the EULAR registry, including 110 hospitalizations (44%) and 37 deaths (15%). Overall, 64% of these patients were women, and they had a median age of 60 years.
The top five diagnoses of these patients were RA (39%), psoriatic arthritis (15%), spondyloarthritis (9%), systemic lupus erythematosus (9%), and gout (5%). A total of 27% had no reported comorbidities, while lung disease occurred in 26%, hypertension in 34%, diabetes in 11%, and cardiovascular disease on 11%. The registry also reported use of any DMARD in 80%, including 62% on csDMARDs, 31% on biologics, and 2% on targeted synthetic DMARDs.
Ten authors in the Lancet Rheumatology paper reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speaker’s bureau positions, personal fees, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. The other authors reported no relevant conflicts of interest.
An international registry of adult and pediatric rheumatology patients is beginning to identify trends in the types of patients with COVID-19 and who is recovering.
The COVID-19 Global Rheumatology Alliance (GRA) has created pediatric and adult registries for health care providers to enter information on their rheumatology patients with COVID-19. The adult registry is hosted by the University of California, San Francisco, Research Electronic Data Capture system, while the Childhood Arthritis and Rheumatology Research Alliance is supporting the pediatric registry. A separate path for data entry of both adult and pediatric cases has been established through the European League Against Rheumatism for European countries and countries with EULAR member organizations.
Prior to the creation of the registries, there were no data available to guide rheumatologists in clinical decision making for their patients, noted Jinoos Yazdany, MD, MPH, COVID-19 GRA steering committee member and chief of the division of rheumatology at Zuckerberg San Francisco General Hospital. “COVID-19 has potential to severely affect those with rheumatologic diseases or those taking immunosuppressive drugs,” she said in an interview. “The GRA registries were designed to answer critical questions that will inform the medical care of this population.”
The GRA began on Twitter, with conversations between Leonard H. Calabrese, DO, of the Cleveland Clinic; Paul Sufka, MD, of HealthPartners in St. Paul, Minn.; Philip Robinson, MBChB, PhD, of the Royal Brisbane (Australia) Hospital; and herself, Dr. Yazdany said. Dr. Robinson started work on the governance of the GRA, Dr. Yazdany designed the data infrastructure, and Dr. Sufka approached his professional networks and social media followings to promote the effort and ask for support. The COVID-19 GRA steering committee representatives include patients, private practice rheumatologists, and international investigators. Listed among official supporters of the alliance are the American College of Rheumatology and EULAR along with more than 290 medical societies, institutions, journals, and other organizations in rheumatology.
The goal of the registries is to examine the health outcomes of patients with rheumatic diseases and COVID-19 based on sociodemographic factors, comorbidities, and clinical presentations of COVID-19 as well as what role taking immunosuppressive drugs prior to a COVID-19 infection play in helping or hindering outcomes. Hydroxychloroquine, used to treat lupus and arthritis, is a potential treatment candidate for COVID-19. Biologics such as tocilizumab (Actemra) and sarilumab (Kevzara), which target interleukin-6, and anakinra (Kineret), which targets IL-1, are treatment candidates for patients who have experienced COVID-related cytokine storm syndrome, which researchers believe may contribute to worsening or fatal cases.
Dr. Yazdany, who is also vice chair of real-world data infrastructure, registry, and institutional review board/ethics for the GRA, said that there are some important high-level trends in the data thus far. “People with lupus and those taking hydroxychloroquine are becoming infected with SARS-CoV-2, which is counter to misinformation on social media. Most people with rheumatic diseases on immunosuppression are recovering, which is great news for our patients.”
One of the major strengths of the registries is that each case is entered by the rheumatologist treating the patient and contains detailed clinical information, Dr. Yazdany said. However, the registry has no control group, it is not a population surveillance study, and it may contain selection bias through rheumatologists omitting milder, undiagnosed cases.
“The Global Alliance case reporting registry represents the collective effort of hundreds of rheumatologists across the world. I have never been more inspired by the strength and collaboration of the rheumatology community,” Dr. Yazdany said.
According to a paper published in the Lancet Rheumatology, which references data on 110 cases from the combined databases up to April 1, about three-fourths of cases presented with fever (79%) and cough (77%), and about half presented with shortness of breath (50%) and myalgia (45%).
Results from the global and UCSF registries
As of April 18, 334 cases were in the global and UCSF registries, with 121 patients (36%) in the database having both COVID-19 and RA, 33 patients (10%) with psoriatic arthritis, 58 patients (17%) with systemic lupus erythematosus, 28 patients (8%) with axial spondyloarthritis, 27 patients (8%) with vasculitis, and 19 patients (6%) with Sjögren’s syndrome. There were less than five cases reported for patients with the following rheumatic diseases: inflammatory myopathy, ocular inflammation, other inflammatory arthritis, polymyalgia rheumatica, sarcoidosis, systemic sclerosis, osteoporosis, psoriasis, isolated pulmonary capillaritis, gout, and autoinflammatory disease. A majority of the patients in the registries are women (74%) aged younger than 65 years (78%) and are white (52%).
The most common comorbid conditions among patients in the registry are hypertension (33%), lung disease (18%), diabetes (11%), cardiovascular disease (10%), chronic renal insufficiency or end-stage renal disease (7%), morbid obesity (7%), and cancer (4%). Before being diagnosed with COVID-19, 219 patients (66%) in the registry were taking conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), which included antimalarials, azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine, and tacrolimus. A total of 122 patients (37%) were taking biologic DMARDs, 101 patients were taking glucocorticoids (30%), 86 patients (26%) were taking hydroxychloroquine, 41 patients (12%) were taking NSAIDs, and 18 patients (5%) were taking a Janus kinase inhibitor.
The most recent data from the registry show that 128 patients (38%) have been hospitalized for COVID-19, and 19 patients (6%) have died. Although 104 patients (31%) resolved their infections, 177 patients (53%) have a COVID-19 infection status of “unresolved,” and 53 patients (16%) have an unknown infection status.
EULAR registry results
As of April 21, 249 cases were in the EULAR registry, including 110 hospitalizations (44%) and 37 deaths (15%). Overall, 64% of these patients were women, and they had a median age of 60 years.
The top five diagnoses of these patients were RA (39%), psoriatic arthritis (15%), spondyloarthritis (9%), systemic lupus erythematosus (9%), and gout (5%). A total of 27% had no reported comorbidities, while lung disease occurred in 26%, hypertension in 34%, diabetes in 11%, and cardiovascular disease on 11%. The registry also reported use of any DMARD in 80%, including 62% on csDMARDs, 31% on biologics, and 2% on targeted synthetic DMARDs.
Ten authors in the Lancet Rheumatology paper reported personal and institutional relationships in the form of grants, corporate sponsorships, advisory board memberships, investigator appointments, speaker’s bureau positions, personal fees, and consultancies for a variety of pharmaceutical companies, agencies, societies, and other organizations. The other authors reported no relevant conflicts of interest.
COVID-19 & Mental Health

Join us on Thursday, April 23, at 8:30 p.m. EST as we discuss how the COVID-19 pandemic is affecting the mental health of psychiatry patients and providers around the world.
Our special guests include two psychiatry educators with expertise in therapeutic psychotherapy and solutions on how to help our most vulnerable populations being affected by COVID-19, Dinah Miller, MD (@shrinkraphdinah), and Elizabeth Ryznar, MD (@RyznarMD). We hope you will participate in our Twitter chat this evening, at 8:30 p.m. EST on #MDedgeChats.
Public health emergencies can affect the well-being of individuals and communities causing possible feelings of insecurity, confusion, and emotional isolation. These effects may translate into a range of emotional reactions affecting current psychiatric conditions and creating new ones, according to the New England Journal of Medicine.
According to the Kaiser Family Foundation, “nearly 45% of adults across the country say that worry and stress related to the coronavirus pandemic are hurting their mental health.”
In this chat, we will discuss this topic and how COVID-19 is affecting your patients and practice. Join us tonight and feel free to share what you have experienced during this pandemic at 8:30 pm EST on #MDedgeChats.
Topics of Conversation
Question 1: How are pre-pandemic patients doing during the crisis?
Question 2: How has COVID-19 affected inpatient and outpatient care for you?
Question 3: How are our most vulnerable populations being affected by COVID-19?
Question 4: How are you doing personally and professionally as a medical professional and psychiatrist amidst this pandemic?
Question 5: What psychiatric manifestations are you seeing in your patients who have had COVID-19?

About Dr. Dinah Miller
Dr. Dinah Miller (@shrinkrapdinah) a psychiatrist with a private practice and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore. Dr. Miller is also a columnist for and a member of the Editorial Advisory Board of Clinical Psychiatry News. She is the co-author of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016) and “Shrink Rap: Three Psychiatrists Explain Their Work” (Baltimore: Johns Hopkins University, 2011).
About Dr. Elizabeth Ryznar
Dr. Elizabeth Ryznar (@RyznarMD) is a psychiatry clinician-educator research fellow and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. She completed her medical training at Harvard Medical School in Boston and her residency training at Northwestern University in Chicago. Dr. Ryznar is co-editor of the new book “Landmark Papers in Psychiatry,” published by Oxford University Press, and has several peer-reviewed articles.
Resources
- Mental health and the COVID-19 pandemic
- Brief examines the COVID-19 crisis’ implications for Americans’ mental health
- COVID-19: Helping health care workers on the front lines
- Overcoming COVID-related stress
- Confessions of an outpatient psychiatrist during the pandemic
- Psychiatric patients may be among the hardest hit
- Experts hasten to head off mental health crisis

Join us on Thursday, April 23, at 8:30 p.m. EST as we discuss how the COVID-19 pandemic is affecting the mental health of psychiatry patients and providers around the world.
Our special guests include two psychiatry educators with expertise in therapeutic psychotherapy and solutions on how to help our most vulnerable populations being affected by COVID-19, Dinah Miller, MD (@shrinkraphdinah), and Elizabeth Ryznar, MD (@RyznarMD). We hope you will participate in our Twitter chat this evening, at 8:30 p.m. EST on #MDedgeChats.
Public health emergencies can affect the well-being of individuals and communities causing possible feelings of insecurity, confusion, and emotional isolation. These effects may translate into a range of emotional reactions affecting current psychiatric conditions and creating new ones, according to the New England Journal of Medicine.
According to the Kaiser Family Foundation, “nearly 45% of adults across the country say that worry and stress related to the coronavirus pandemic are hurting their mental health.”
In this chat, we will discuss this topic and how COVID-19 is affecting your patients and practice. Join us tonight and feel free to share what you have experienced during this pandemic at 8:30 pm EST on #MDedgeChats.
Topics of Conversation
Question 1: How are pre-pandemic patients doing during the crisis?
Question 2: How has COVID-19 affected inpatient and outpatient care for you?
Question 3: How are our most vulnerable populations being affected by COVID-19?
Question 4: How are you doing personally and professionally as a medical professional and psychiatrist amidst this pandemic?
Question 5: What psychiatric manifestations are you seeing in your patients who have had COVID-19?

About Dr. Dinah Miller
Dr. Dinah Miller (@shrinkrapdinah) a psychiatrist with a private practice and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore. Dr. Miller is also a columnist for and a member of the Editorial Advisory Board of Clinical Psychiatry News. She is the co-author of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016) and “Shrink Rap: Three Psychiatrists Explain Their Work” (Baltimore: Johns Hopkins University, 2011).
About Dr. Elizabeth Ryznar
Dr. Elizabeth Ryznar (@RyznarMD) is a psychiatry clinician-educator research fellow and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. She completed her medical training at Harvard Medical School in Boston and her residency training at Northwestern University in Chicago. Dr. Ryznar is co-editor of the new book “Landmark Papers in Psychiatry,” published by Oxford University Press, and has several peer-reviewed articles.
Resources
- Mental health and the COVID-19 pandemic
- Brief examines the COVID-19 crisis’ implications for Americans’ mental health
- COVID-19: Helping health care workers on the front lines
- Overcoming COVID-related stress
- Confessions of an outpatient psychiatrist during the pandemic
- Psychiatric patients may be among the hardest hit
- Experts hasten to head off mental health crisis

Join us on Thursday, April 23, at 8:30 p.m. EST as we discuss how the COVID-19 pandemic is affecting the mental health of psychiatry patients and providers around the world.
Our special guests include two psychiatry educators with expertise in therapeutic psychotherapy and solutions on how to help our most vulnerable populations being affected by COVID-19, Dinah Miller, MD (@shrinkraphdinah), and Elizabeth Ryznar, MD (@RyznarMD). We hope you will participate in our Twitter chat this evening, at 8:30 p.m. EST on #MDedgeChats.
Public health emergencies can affect the well-being of individuals and communities causing possible feelings of insecurity, confusion, and emotional isolation. These effects may translate into a range of emotional reactions affecting current psychiatric conditions and creating new ones, according to the New England Journal of Medicine.
According to the Kaiser Family Foundation, “nearly 45% of adults across the country say that worry and stress related to the coronavirus pandemic are hurting their mental health.”
In this chat, we will discuss this topic and how COVID-19 is affecting your patients and practice. Join us tonight and feel free to share what you have experienced during this pandemic at 8:30 pm EST on #MDedgeChats.
Topics of Conversation
Question 1: How are pre-pandemic patients doing during the crisis?
Question 2: How has COVID-19 affected inpatient and outpatient care for you?
Question 3: How are our most vulnerable populations being affected by COVID-19?
Question 4: How are you doing personally and professionally as a medical professional and psychiatrist amidst this pandemic?
Question 5: What psychiatric manifestations are you seeing in your patients who have had COVID-19?

About Dr. Dinah Miller
Dr. Dinah Miller (@shrinkrapdinah) a psychiatrist with a private practice and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, both in Baltimore. Dr. Miller is also a columnist for and a member of the Editorial Advisory Board of Clinical Psychiatry News. She is the co-author of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016) and “Shrink Rap: Three Psychiatrists Explain Their Work” (Baltimore: Johns Hopkins University, 2011).
About Dr. Elizabeth Ryznar
Dr. Elizabeth Ryznar (@RyznarMD) is a psychiatry clinician-educator research fellow and an assistant professor of psychiatry and behavioral sciences at Johns Hopkins in Baltimore. She completed her medical training at Harvard Medical School in Boston and her residency training at Northwestern University in Chicago. Dr. Ryznar is co-editor of the new book “Landmark Papers in Psychiatry,” published by Oxford University Press, and has several peer-reviewed articles.
Resources
- Mental health and the COVID-19 pandemic
- Brief examines the COVID-19 crisis’ implications for Americans’ mental health
- COVID-19: Helping health care workers on the front lines
- Overcoming COVID-related stress
- Confessions of an outpatient psychiatrist during the pandemic
- Psychiatric patients may be among the hardest hit
- Experts hasten to head off mental health crisis









