User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Positive top-line results for cannabinoid-based med for nerve pain
, new top-line results released by Zelira Therapeutics suggest.
“The implications of these results for patients are incredibly promising,” principal investigator Bryan Doner, DO, medical director of HealthyWays Integrated Wellness Solutions, Gibsonia, Pa., said in a news release.
“Through this rigorously designed study, we have demonstrated that ZLT-L-007 is a safe, effective, and well-tolerated alternative for patients who would typically seek a Lyrica-level of pain relief,” he added.
The observational, nonblinded trial tested the efficacy, safety, and tolerability of ZLT-L-007 against pregabalin in 60 adults with diabetic nerve pain.
The study had three groups with 20 patients each (pregabalin alone, pregabalin plus ZLT-L-007, and ZLT-L-007 alone).
Top-line results show the study met its primary endpoint for change in daily pain severity as measured by the percent change from baseline at 30, 60, and 90 days on the Numerical Rating Scale.
For the pregabalin-only group, there was a reduction in symptom severity at all follow-up points, ranging from 20% to 35% (median percent change from baseline), the company said.
For the ZLT-L-007 only group, there was about a 33% reduction in symptom severity at 30 days, and 71% and 78% reduction, respectively, at 60 and 90 days, suggesting a larger improvement in symptom severity than with pregabalin alone, the company said.
For the pregabalin plus ZLT-L-007 group, there was a moderate 20% reduction in symptom severity at 30 days, but a larger reduction at 60 and 90 days (50% and 72%, respectively), which indicates substantially greater improvement in symptom severity than with pregabalin alone, the company said.
The study also met secondary endpoints, including significant decreases in daily pain severity as measured by the Visual Analog Scale and measurable changes in the short-form McGill Pain Questionnaire and Neuropathic Pain Symptom Inventory.
Dr. Doner noted that the top-line data showed “no serious adverse events, and participants’ blood pressure and other safety vitals remained unaffected throughout. This confirms that ZLT-L-007 is a well-tolerated product that delivers statistically significant pain relief, surpassing the levels achieved by Lyrica.”
The company plans to report additional insights from the full study, as they become available, during fiscal year 2023-2024.
A version of this article first appeared on Medscape.com.
, new top-line results released by Zelira Therapeutics suggest.
“The implications of these results for patients are incredibly promising,” principal investigator Bryan Doner, DO, medical director of HealthyWays Integrated Wellness Solutions, Gibsonia, Pa., said in a news release.
“Through this rigorously designed study, we have demonstrated that ZLT-L-007 is a safe, effective, and well-tolerated alternative for patients who would typically seek a Lyrica-level of pain relief,” he added.
The observational, nonblinded trial tested the efficacy, safety, and tolerability of ZLT-L-007 against pregabalin in 60 adults with diabetic nerve pain.
The study had three groups with 20 patients each (pregabalin alone, pregabalin plus ZLT-L-007, and ZLT-L-007 alone).
Top-line results show the study met its primary endpoint for change in daily pain severity as measured by the percent change from baseline at 30, 60, and 90 days on the Numerical Rating Scale.
For the pregabalin-only group, there was a reduction in symptom severity at all follow-up points, ranging from 20% to 35% (median percent change from baseline), the company said.
For the ZLT-L-007 only group, there was about a 33% reduction in symptom severity at 30 days, and 71% and 78% reduction, respectively, at 60 and 90 days, suggesting a larger improvement in symptom severity than with pregabalin alone, the company said.
For the pregabalin plus ZLT-L-007 group, there was a moderate 20% reduction in symptom severity at 30 days, but a larger reduction at 60 and 90 days (50% and 72%, respectively), which indicates substantially greater improvement in symptom severity than with pregabalin alone, the company said.
The study also met secondary endpoints, including significant decreases in daily pain severity as measured by the Visual Analog Scale and measurable changes in the short-form McGill Pain Questionnaire and Neuropathic Pain Symptom Inventory.
Dr. Doner noted that the top-line data showed “no serious adverse events, and participants’ blood pressure and other safety vitals remained unaffected throughout. This confirms that ZLT-L-007 is a well-tolerated product that delivers statistically significant pain relief, surpassing the levels achieved by Lyrica.”
The company plans to report additional insights from the full study, as they become available, during fiscal year 2023-2024.
A version of this article first appeared on Medscape.com.
, new top-line results released by Zelira Therapeutics suggest.
“The implications of these results for patients are incredibly promising,” principal investigator Bryan Doner, DO, medical director of HealthyWays Integrated Wellness Solutions, Gibsonia, Pa., said in a news release.
“Through this rigorously designed study, we have demonstrated that ZLT-L-007 is a safe, effective, and well-tolerated alternative for patients who would typically seek a Lyrica-level of pain relief,” he added.
The observational, nonblinded trial tested the efficacy, safety, and tolerability of ZLT-L-007 against pregabalin in 60 adults with diabetic nerve pain.
The study had three groups with 20 patients each (pregabalin alone, pregabalin plus ZLT-L-007, and ZLT-L-007 alone).
Top-line results show the study met its primary endpoint for change in daily pain severity as measured by the percent change from baseline at 30, 60, and 90 days on the Numerical Rating Scale.
For the pregabalin-only group, there was a reduction in symptom severity at all follow-up points, ranging from 20% to 35% (median percent change from baseline), the company said.
For the ZLT-L-007 only group, there was about a 33% reduction in symptom severity at 30 days, and 71% and 78% reduction, respectively, at 60 and 90 days, suggesting a larger improvement in symptom severity than with pregabalin alone, the company said.
For the pregabalin plus ZLT-L-007 group, there was a moderate 20% reduction in symptom severity at 30 days, but a larger reduction at 60 and 90 days (50% and 72%, respectively), which indicates substantially greater improvement in symptom severity than with pregabalin alone, the company said.
The study also met secondary endpoints, including significant decreases in daily pain severity as measured by the Visual Analog Scale and measurable changes in the short-form McGill Pain Questionnaire and Neuropathic Pain Symptom Inventory.
Dr. Doner noted that the top-line data showed “no serious adverse events, and participants’ blood pressure and other safety vitals remained unaffected throughout. This confirms that ZLT-L-007 is a well-tolerated product that delivers statistically significant pain relief, surpassing the levels achieved by Lyrica.”
The company plans to report additional insights from the full study, as they become available, during fiscal year 2023-2024.
A version of this article first appeared on Medscape.com.
Troponin to ID diabetes patients with silent heart disease?
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
COVID boosters effective, but not for long
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.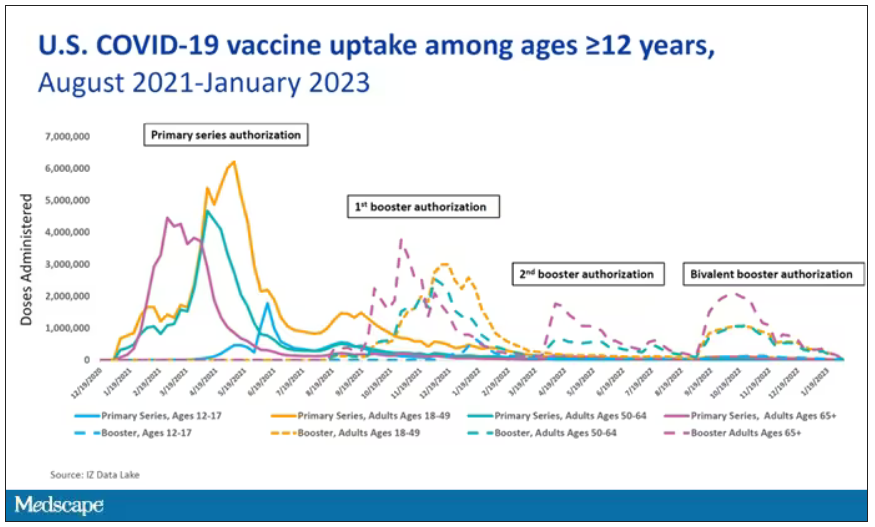
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.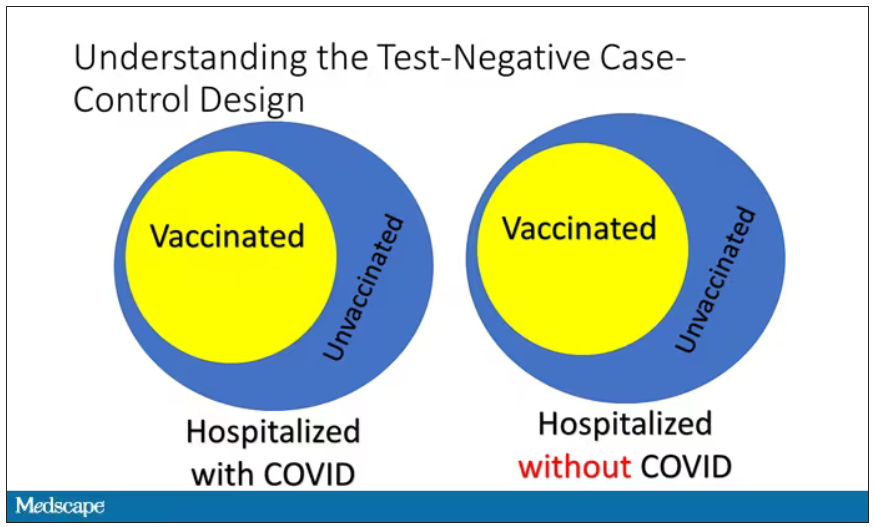
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.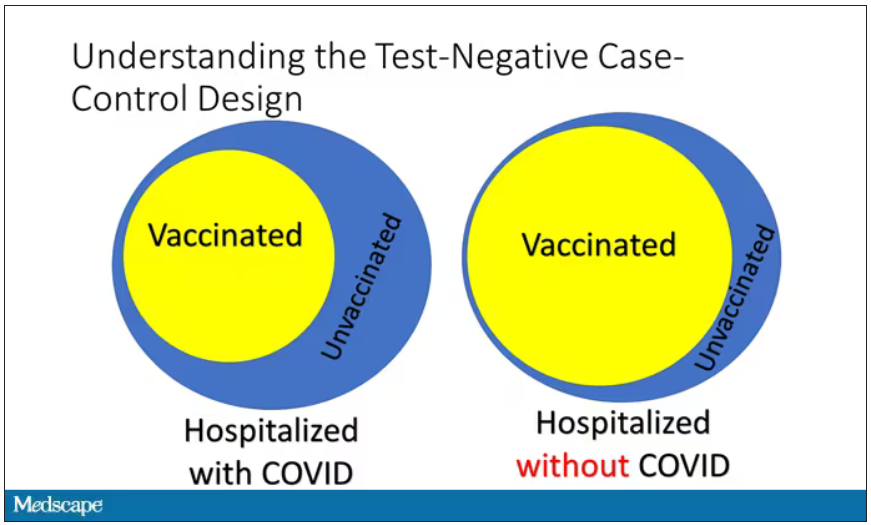
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.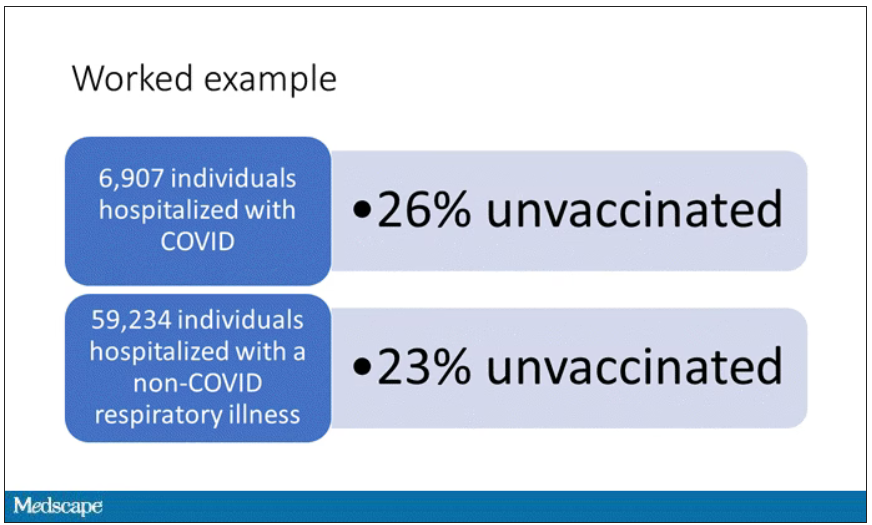
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.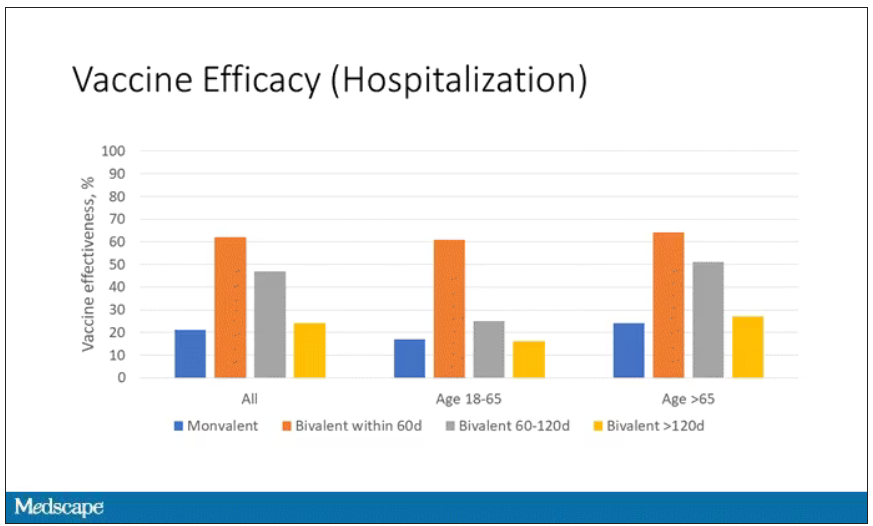
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.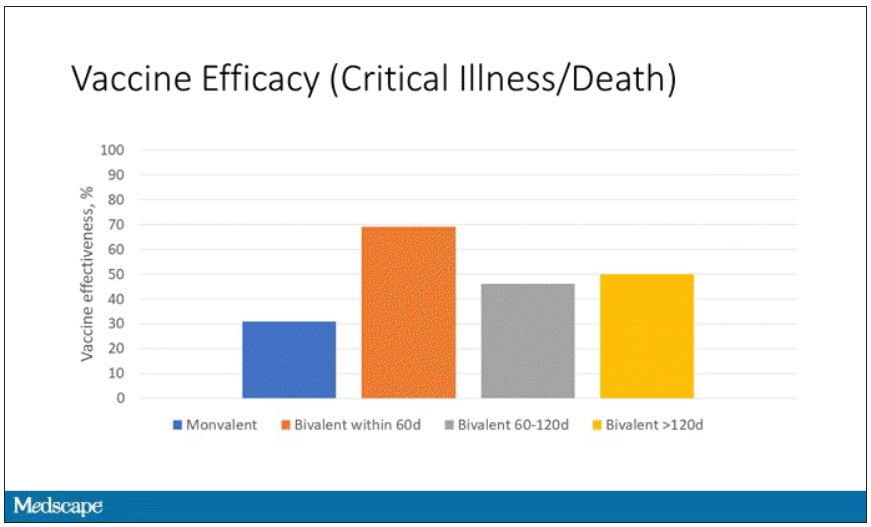
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.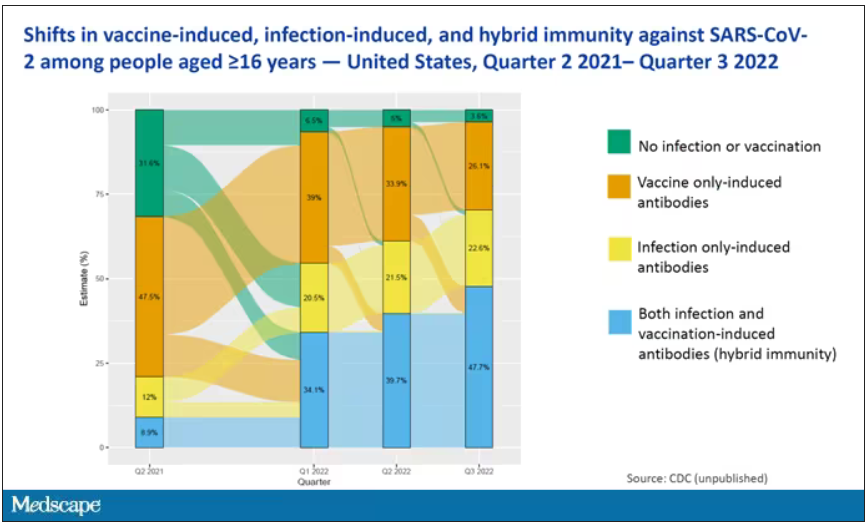
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Overweight in heterozygous FH tied to even higher CAD risk
MANNHEIM, GERMANY – – rates that appear to have a substantial impact on these patients’ already increased risk of coronary artery disease, a registry analysis suggests.
Data on almost 36,000 individuals with FH were collated from an international registry, revealing that 55% of adults and 25% of children and adolescents with the homozygous form of FH had overweight or obesity. The figures for heterozygous FH were 52% and 27%, respectively.
Crucially, overweight or obesity was associated with substantially increased rates of coronary artery disease, particularly in persons with heterozygous FH, among whom adults with obesity faced a twofold increased risk, rising to more than sixfold in children and adolescents.
Moreover, “obesity is associated with a worse lipid profile, even from childhood, regardless of whether a patient is on medication,” said study presenter Amany Elshorbagy, DPhil, Cardiovascular Epidemiologist, department of primary care and public health, Imperial College London.
She added that, with the increased risk of coronary artery disease associated with heterozygous FH, the results showed that “together with lipid-lowering medication, weight management is needed.”
The research was presented at the annual meeting of the European Atherosclerosis Society.
Tended to be thin
Alberico L. Catapano, MD, PhD, director of cardiovascular research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, and past president of the EAS, said in an interview that, historically, few FH patients were overweight or obese; rather, they tended to be thin.
However, there is now “a trend for people with FH to show more diabetes and obesity,” with the “bottom line” being that, as they are already at increased risk of coronary artery disease, it pushes their risk up even further.
In other words, if a risk factor such as obesity is added “on top of the strongest risk factor, that is LDL cholesterol, it is not one plus one makes two, it is one plus one makes three,” he said.
As such, Dr. Catapano believes that the study is “very interesting,” because it further underlines the importance of weight management for individuals with increased LDL cholesterol, “especially when you have genetic forms, like FH.”
Dr. Catapano’s comments were echoed by session co-chair Ulrike Schatz, MD, leader of the lipidology specialty department at the University Hospital Carl Gustav Carus, Technical University of Dresden (Germany).
Indeed, she told Dr. Elshorbagy before her presentation that she finds “a lot of my FH patients have a tendency towards anorexia.”
In an interview, Dr. Elshorbagy said that that reaction was typical of “most of the clinicians” she had spoken to. Upon seeing her data, especially for homozygous FH patients, they say, “They are on the lean side.”
Consequently, the research team went into the study “with the expectation that they might have a lower prevalence of obesity and overweight than the general population,” but “that’s not what we’re seeing.”
Dr. Elshorbagy noted that it would be helpful to have longitudinal data to determine whether, 50 years ago, patients with HF “were leaner, along with the rest of the population.”
The registry data are cross-sectional, and the team is now reaching out to the respective national lead investigators to submit follow-up data on their patients, with the aim of looking at changes in body weight and the impact on outcomes over time.
Another key question for the researchers is in regard to fat distribution, as body mass index “is not the best predictor of heart disease,” Dr. Elshorbagy said, but is rather central obesity.
Although they have also asked investigators to share waist circumference data, she conceded that it is a measurement that “is a lot harder to standardize across centers and countries; it’s not like putting patients on a scale.”
Overall, Dr. Elshorbagy believes that her findings indicate that clinicians should take a broader, more holistic approach toward their patients – in other words, an approach in which lipid lowering medication is “key but is just one of several things we need to do to make sure the coronary event rate goes down.”
More with than without
Dr. Elshorbagy began her presentation by highlighting that the prevalence of overweight and obesity ranges from 50% to 70% and that it is “the only health condition where you’ve got more people worldwide with the condition than without.”
Crucially, overweight increases the risk of coronary artery disease by approximately 20%. Among patients with obesity, the risk rises to 50%.
Given that FH patients “already have a very high risk of cardiovascular disease from their high cholesterol levels,” the team set out to determine rates of obesity and overweight in this population and their impact on coronary artery disease risk.
They used cross-sectional data from the EAS FH Studies Collaboration Global Registry, which involves 29,262 adults aged greater than or equal to 18 years and 6,275 children and adolescents aged 5 to 17 years with heterozygous FH, and 325 adults and 57 children with homozygous FH.
Dividing the adults into standard BMI categories, they found that 16% of heterozygous and 23% of homozygous FH patients had obesity, while 52% and 55%, respectively, had overweight or obesity.
For children, the team used World Health Organization z score cutoffs, which indicated that 9% of patients with heterozygous FH and 7% of patients with homozygous FH had obesity. Rates of overweight or obesity were 27% and 25%, respectively.
Among patients with heterozygous FH, rates of overweight or obesity among adults were 50% in high-income countries and 63% in other countries; among children, the rates were and 27% and 29%, respectively.
Stratified by region, the team found that the lowest rate of overweight or obesity among adult patients with heterozygous FH was in Eastern Asia, at 27%, while the highest was in Northern Africa/Western Asia (the Middle East), at 82%.
In North America, 56% of adult patients had overweight or obesity. The prevalence of coronary artery disease rose with increasing BMI.
Among adult patients with heterozygous FH, 11.3% of those with normal weight had coronary artery disease; the percentage rose to 22.9% among those with overweight, and 30.9% among those with obesity. Among children, the corresponding figures were 0.1%, 0.2%, and 0.7%.
Putting adults and children with homozygous FH together, the researchers found that 29.0% of patients with normal weight had coronary artery disease, compared with 31.3% of those with overweight and 49.3% of those with obesity.
Moreover, the results showed that levels of LDL and remnant cholesterol were significantly associated with BMI in adults and children with heterozygous FH, even after adjusting for age, sex, and lipid-lowering medication (P < .001 for all).
Multivariate analysis that took into account age, sex, lipid-lowering medication, and LDL cholesterol revealed that having obesity, compared with not having obesity, was associated with a substantial increase in the risk of coronary artery disease among patients with heterozygous FH.
Among adults with the condition, the odds ratio was 2.16 (95% confidence interval, 1.97-2.36), while among children and adolescents, it was 6.87 (95% CI, 1.55-30.46).
The results remained similar after further adjustment for the presence of diabetes and when considering peripheral artery disease and stroke.
No funding for the study was declared. Dr. Elshorbagy has relationships with Amgen, Daiichi Sankyo, and Regeneron.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY – – rates that appear to have a substantial impact on these patients’ already increased risk of coronary artery disease, a registry analysis suggests.
Data on almost 36,000 individuals with FH were collated from an international registry, revealing that 55% of adults and 25% of children and adolescents with the homozygous form of FH had overweight or obesity. The figures for heterozygous FH were 52% and 27%, respectively.
Crucially, overweight or obesity was associated with substantially increased rates of coronary artery disease, particularly in persons with heterozygous FH, among whom adults with obesity faced a twofold increased risk, rising to more than sixfold in children and adolescents.
Moreover, “obesity is associated with a worse lipid profile, even from childhood, regardless of whether a patient is on medication,” said study presenter Amany Elshorbagy, DPhil, Cardiovascular Epidemiologist, department of primary care and public health, Imperial College London.
She added that, with the increased risk of coronary artery disease associated with heterozygous FH, the results showed that “together with lipid-lowering medication, weight management is needed.”
The research was presented at the annual meeting of the European Atherosclerosis Society.
Tended to be thin
Alberico L. Catapano, MD, PhD, director of cardiovascular research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, and past president of the EAS, said in an interview that, historically, few FH patients were overweight or obese; rather, they tended to be thin.
However, there is now “a trend for people with FH to show more diabetes and obesity,” with the “bottom line” being that, as they are already at increased risk of coronary artery disease, it pushes their risk up even further.
In other words, if a risk factor such as obesity is added “on top of the strongest risk factor, that is LDL cholesterol, it is not one plus one makes two, it is one plus one makes three,” he said.
As such, Dr. Catapano believes that the study is “very interesting,” because it further underlines the importance of weight management for individuals with increased LDL cholesterol, “especially when you have genetic forms, like FH.”
Dr. Catapano’s comments were echoed by session co-chair Ulrike Schatz, MD, leader of the lipidology specialty department at the University Hospital Carl Gustav Carus, Technical University of Dresden (Germany).
Indeed, she told Dr. Elshorbagy before her presentation that she finds “a lot of my FH patients have a tendency towards anorexia.”
In an interview, Dr. Elshorbagy said that that reaction was typical of “most of the clinicians” she had spoken to. Upon seeing her data, especially for homozygous FH patients, they say, “They are on the lean side.”
Consequently, the research team went into the study “with the expectation that they might have a lower prevalence of obesity and overweight than the general population,” but “that’s not what we’re seeing.”
Dr. Elshorbagy noted that it would be helpful to have longitudinal data to determine whether, 50 years ago, patients with HF “were leaner, along with the rest of the population.”
The registry data are cross-sectional, and the team is now reaching out to the respective national lead investigators to submit follow-up data on their patients, with the aim of looking at changes in body weight and the impact on outcomes over time.
Another key question for the researchers is in regard to fat distribution, as body mass index “is not the best predictor of heart disease,” Dr. Elshorbagy said, but is rather central obesity.
Although they have also asked investigators to share waist circumference data, she conceded that it is a measurement that “is a lot harder to standardize across centers and countries; it’s not like putting patients on a scale.”
Overall, Dr. Elshorbagy believes that her findings indicate that clinicians should take a broader, more holistic approach toward their patients – in other words, an approach in which lipid lowering medication is “key but is just one of several things we need to do to make sure the coronary event rate goes down.”
More with than without
Dr. Elshorbagy began her presentation by highlighting that the prevalence of overweight and obesity ranges from 50% to 70% and that it is “the only health condition where you’ve got more people worldwide with the condition than without.”
Crucially, overweight increases the risk of coronary artery disease by approximately 20%. Among patients with obesity, the risk rises to 50%.
Given that FH patients “already have a very high risk of cardiovascular disease from their high cholesterol levels,” the team set out to determine rates of obesity and overweight in this population and their impact on coronary artery disease risk.
They used cross-sectional data from the EAS FH Studies Collaboration Global Registry, which involves 29,262 adults aged greater than or equal to 18 years and 6,275 children and adolescents aged 5 to 17 years with heterozygous FH, and 325 adults and 57 children with homozygous FH.
Dividing the adults into standard BMI categories, they found that 16% of heterozygous and 23% of homozygous FH patients had obesity, while 52% and 55%, respectively, had overweight or obesity.
For children, the team used World Health Organization z score cutoffs, which indicated that 9% of patients with heterozygous FH and 7% of patients with homozygous FH had obesity. Rates of overweight or obesity were 27% and 25%, respectively.
Among patients with heterozygous FH, rates of overweight or obesity among adults were 50% in high-income countries and 63% in other countries; among children, the rates were and 27% and 29%, respectively.
Stratified by region, the team found that the lowest rate of overweight or obesity among adult patients with heterozygous FH was in Eastern Asia, at 27%, while the highest was in Northern Africa/Western Asia (the Middle East), at 82%.
In North America, 56% of adult patients had overweight or obesity. The prevalence of coronary artery disease rose with increasing BMI.
Among adult patients with heterozygous FH, 11.3% of those with normal weight had coronary artery disease; the percentage rose to 22.9% among those with overweight, and 30.9% among those with obesity. Among children, the corresponding figures were 0.1%, 0.2%, and 0.7%.
Putting adults and children with homozygous FH together, the researchers found that 29.0% of patients with normal weight had coronary artery disease, compared with 31.3% of those with overweight and 49.3% of those with obesity.
Moreover, the results showed that levels of LDL and remnant cholesterol were significantly associated with BMI in adults and children with heterozygous FH, even after adjusting for age, sex, and lipid-lowering medication (P < .001 for all).
Multivariate analysis that took into account age, sex, lipid-lowering medication, and LDL cholesterol revealed that having obesity, compared with not having obesity, was associated with a substantial increase in the risk of coronary artery disease among patients with heterozygous FH.
Among adults with the condition, the odds ratio was 2.16 (95% confidence interval, 1.97-2.36), while among children and adolescents, it was 6.87 (95% CI, 1.55-30.46).
The results remained similar after further adjustment for the presence of diabetes and when considering peripheral artery disease and stroke.
No funding for the study was declared. Dr. Elshorbagy has relationships with Amgen, Daiichi Sankyo, and Regeneron.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY – – rates that appear to have a substantial impact on these patients’ already increased risk of coronary artery disease, a registry analysis suggests.
Data on almost 36,000 individuals with FH were collated from an international registry, revealing that 55% of adults and 25% of children and adolescents with the homozygous form of FH had overweight or obesity. The figures for heterozygous FH were 52% and 27%, respectively.
Crucially, overweight or obesity was associated with substantially increased rates of coronary artery disease, particularly in persons with heterozygous FH, among whom adults with obesity faced a twofold increased risk, rising to more than sixfold in children and adolescents.
Moreover, “obesity is associated with a worse lipid profile, even from childhood, regardless of whether a patient is on medication,” said study presenter Amany Elshorbagy, DPhil, Cardiovascular Epidemiologist, department of primary care and public health, Imperial College London.
She added that, with the increased risk of coronary artery disease associated with heterozygous FH, the results showed that “together with lipid-lowering medication, weight management is needed.”
The research was presented at the annual meeting of the European Atherosclerosis Society.
Tended to be thin
Alberico L. Catapano, MD, PhD, director of cardiovascular research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, and past president of the EAS, said in an interview that, historically, few FH patients were overweight or obese; rather, they tended to be thin.
However, there is now “a trend for people with FH to show more diabetes and obesity,” with the “bottom line” being that, as they are already at increased risk of coronary artery disease, it pushes their risk up even further.
In other words, if a risk factor such as obesity is added “on top of the strongest risk factor, that is LDL cholesterol, it is not one plus one makes two, it is one plus one makes three,” he said.
As such, Dr. Catapano believes that the study is “very interesting,” because it further underlines the importance of weight management for individuals with increased LDL cholesterol, “especially when you have genetic forms, like FH.”
Dr. Catapano’s comments were echoed by session co-chair Ulrike Schatz, MD, leader of the lipidology specialty department at the University Hospital Carl Gustav Carus, Technical University of Dresden (Germany).
Indeed, she told Dr. Elshorbagy before her presentation that she finds “a lot of my FH patients have a tendency towards anorexia.”
In an interview, Dr. Elshorbagy said that that reaction was typical of “most of the clinicians” she had spoken to. Upon seeing her data, especially for homozygous FH patients, they say, “They are on the lean side.”
Consequently, the research team went into the study “with the expectation that they might have a lower prevalence of obesity and overweight than the general population,” but “that’s not what we’re seeing.”
Dr. Elshorbagy noted that it would be helpful to have longitudinal data to determine whether, 50 years ago, patients with HF “were leaner, along with the rest of the population.”
The registry data are cross-sectional, and the team is now reaching out to the respective national lead investigators to submit follow-up data on their patients, with the aim of looking at changes in body weight and the impact on outcomes over time.
Another key question for the researchers is in regard to fat distribution, as body mass index “is not the best predictor of heart disease,” Dr. Elshorbagy said, but is rather central obesity.
Although they have also asked investigators to share waist circumference data, she conceded that it is a measurement that “is a lot harder to standardize across centers and countries; it’s not like putting patients on a scale.”
Overall, Dr. Elshorbagy believes that her findings indicate that clinicians should take a broader, more holistic approach toward their patients – in other words, an approach in which lipid lowering medication is “key but is just one of several things we need to do to make sure the coronary event rate goes down.”
More with than without
Dr. Elshorbagy began her presentation by highlighting that the prevalence of overweight and obesity ranges from 50% to 70% and that it is “the only health condition where you’ve got more people worldwide with the condition than without.”
Crucially, overweight increases the risk of coronary artery disease by approximately 20%. Among patients with obesity, the risk rises to 50%.
Given that FH patients “already have a very high risk of cardiovascular disease from their high cholesterol levels,” the team set out to determine rates of obesity and overweight in this population and their impact on coronary artery disease risk.
They used cross-sectional data from the EAS FH Studies Collaboration Global Registry, which involves 29,262 adults aged greater than or equal to 18 years and 6,275 children and adolescents aged 5 to 17 years with heterozygous FH, and 325 adults and 57 children with homozygous FH.
Dividing the adults into standard BMI categories, they found that 16% of heterozygous and 23% of homozygous FH patients had obesity, while 52% and 55%, respectively, had overweight or obesity.
For children, the team used World Health Organization z score cutoffs, which indicated that 9% of patients with heterozygous FH and 7% of patients with homozygous FH had obesity. Rates of overweight or obesity were 27% and 25%, respectively.
Among patients with heterozygous FH, rates of overweight or obesity among adults were 50% in high-income countries and 63% in other countries; among children, the rates were and 27% and 29%, respectively.
Stratified by region, the team found that the lowest rate of overweight or obesity among adult patients with heterozygous FH was in Eastern Asia, at 27%, while the highest was in Northern Africa/Western Asia (the Middle East), at 82%.
In North America, 56% of adult patients had overweight or obesity. The prevalence of coronary artery disease rose with increasing BMI.
Among adult patients with heterozygous FH, 11.3% of those with normal weight had coronary artery disease; the percentage rose to 22.9% among those with overweight, and 30.9% among those with obesity. Among children, the corresponding figures were 0.1%, 0.2%, and 0.7%.
Putting adults and children with homozygous FH together, the researchers found that 29.0% of patients with normal weight had coronary artery disease, compared with 31.3% of those with overweight and 49.3% of those with obesity.
Moreover, the results showed that levels of LDL and remnant cholesterol were significantly associated with BMI in adults and children with heterozygous FH, even after adjusting for age, sex, and lipid-lowering medication (P < .001 for all).
Multivariate analysis that took into account age, sex, lipid-lowering medication, and LDL cholesterol revealed that having obesity, compared with not having obesity, was associated with a substantial increase in the risk of coronary artery disease among patients with heterozygous FH.
Among adults with the condition, the odds ratio was 2.16 (95% confidence interval, 1.97-2.36), while among children and adolescents, it was 6.87 (95% CI, 1.55-30.46).
The results remained similar after further adjustment for the presence of diabetes and when considering peripheral artery disease and stroke.
No funding for the study was declared. Dr. Elshorbagy has relationships with Amgen, Daiichi Sankyo, and Regeneron.
A version of this article first appeared on Medscape.com.
AT EAS 2023
Itchy scaling rash
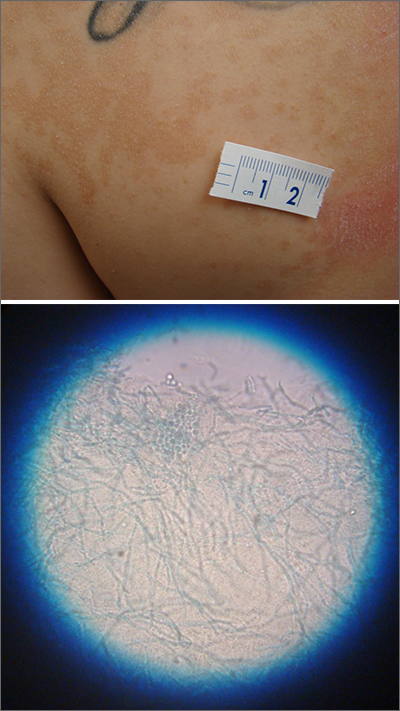
A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013

A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013
PTSD, anxiety linked to out-of-hospital cardiac arrest
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.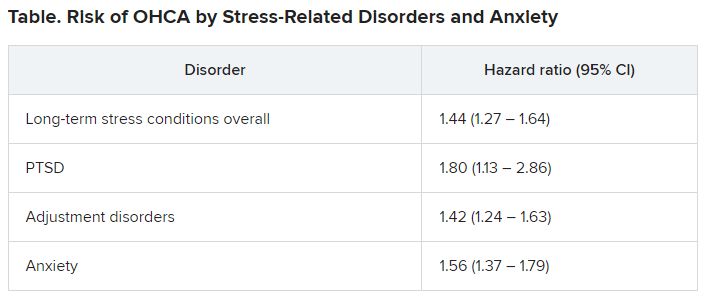
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN HEART
Can a saliva test predict the best way to manage obesity?
It sounds like a simple solution to a complicated problem: Find out what kind of obesity someone has based on a one-time genetic saliva test. Then patients and their doctor can get a better idea if antiobesity drugs or other treatments are more likely to work for them.
It’s what Mayo Clinic researchers had in mind when they created four phenotypes of obesity.
Obesity experts not affiliated with the research have some concerns and say independent studies are needed to verify the potential of this strategy.
This research could help predict who will respond best to popular antiobesity medications, said Andres Acosta, MD, PhD, cofounder of Phenomix Sciences, the company behind the tests. These medications include glucagonlike peptide–1 (GLP-1) receptor agonists like liraglutide (Saxenda, Victoza) and semaglutide (Ozempic, Wegovy).
“We know that not everyone on a GLP-1 will respond. In reality, about a third of the patients don’t do well with GLP-1s,” said Dr. Acosta, an assistant professor of medicine and researcher in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
Furthest along in development is the “My Phenome Hungry Gut” test for predicting GLP-1 response. People in this Hungry Gut group tend to empty their stomach after a meal faster and are more likely to feel hungry again a short time later, as explained on the company’s website.
A pilot study to test how well it works started in April at three primary care practices. Plans are to expand real-world testing for this and other obesity types later in 2023.
The other obesity categories are:
- “Hungry brain,” where the brain does not recognize signals that the stomach is full
- “Emotional hunger,” where cravings to eat are driven by emotions, anxiety, and negative feelings
- “Slow burn,” where people have a slow metabolism and low energy level
People in these categories might be more likely to benefit from other obesity management strategies, like changes to their diet or placement of an intragastric balloon.
Some things to consider
While applauding their efforts to be more precise in treating people with obesity, not all experts are convinced this saliva test will be the answer. The company’s research might look promising, but verification of results is warranted.
“Can we get better outcomes with things like this? Well, that’s the hope,” said Jaime Almandoz, MD, medical director of weight wellness at the University of Texas Southwestern Medical Center, Dallas.
“We still don’t have randomized trials where we’re looking at obesity phenotyping yet,” said Dr. Almandoz, who is also a spokesperson for the Obesity Society, a professional group of clinicians, researchers, educators, and others focused on obesity science, treatment, and prevention.
There is always concern when a diagnostic test is being developed for commercial use, said Daniel Bessesen, MD, a professor of medicine–endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora. “What they’re talking about doing is super important. But this is a company. This is a company that is, I think, selling a product.”
In an online search, Dr. Bessesen did not find any external studies that showed how well the saliva testing worked. But referring to work by Dr. Acosta and Michael Camilleri, MD, the other cofounder of Phenomix, he said, “I found some papers that they did that I hadn’t read before that are good.”
“These guys are smart guys. And they’ve done a lot of work on [the movement of food through the gut] and how that correlates with obesity and response to some therapies,” said Dr. Bessesen, who is also a spokesperson for the Obesity Society. “So their scientific work does line up with this area.”
Validation of any research is important because the obesity industry has been known for a lot of lose-weight-quick strategies, some with little or no science behind them, he said.
It is also essential, he said, because “anytime you do something commercial in the area of obesity, you have to acknowledge that people with obesity are a vulnerable population. These people face stigma and bias all the time.”
Removing the stigma
If knowing your obesity type ends up making a difference, it could change the conversation people have with their medical provider, Dr. Acosta said. It could also help remove some of the stigma around obesity.
“We’re going to change the conversation because now we can say: ‘Hey, you have obesity because you have ‘Hungry Gut’ phenotype. And because of that, you’re going to respond to this medication,” Dr. Acosta said. The phenotyping suggests a strong genetic tendency – a biologic basis for obesity.
“So it’s not only a way of taking the blame out, but it’s also way of explaining that there’s a reason why you have obesity,” Dr. Acosta said. It tells people: “You’re not a failure.”
More cost-effective treatment?
Targeting obesity treatment could also save on overall health care costs, Dr. Almandoz said. He estimated a cost of $1,400 per month “for forever and ever semaglutide” or at least $1,400 a month for a 3-month trial to see if this medication works in a particular person with obesity.
“That’s a lot of money when you extrapolate that out over the number of people who probably meet the criteria for treatment,” he said. A total 42% of Americans meet the Centers for Disease Control and Prevention definition for obesity.
“You can imagine the potential cost if we were to provide antiobesity therapies to everybody and we were to use what is the most effective class of medication, which is more than a thousand dollars per month, indefinitely,” Dr. Almandoz said. “Not that we should not treat everybody. That’s not the message I’m saying. But if we’re looking at yield or value in terms of treating obesity in a setting with limited resources, it may be best to start with who is most likely to benefit.”
How they created four obesity types
Starting in 2015, Dr. Acosta and colleagues started comparing tests in people with normal weight versus obesity. They used artificial intelligence and machine learning to classify obesity into 11 types at first. They realized this many obesity types were not practical for doctors and people with obesity, so they combined them into four phenotypes.
“The AI machine learning was followed by, as I like to call, HI, or human intelligence,” he said.
The saliva test checks for about 6,000 relevant genetic single-nucleotide polymorphisms. Six thousand genetic changes may sound like a large number to check; however, the average individual carries 5 million and 6 million SNPs in their DNA.
The results are translated to a score that yields a low risk or high risk for Hungry Gut or other types of obesity. “You can have all six thousand genetic mutations, or you can have zero,” Dr. Acosta said.
Moving forward
After the soft launch of Hungry Gut testing in April, Phenomix plans to continue studying their saliva test on other obesity types.
Dr. Acosta is not aware of any direct competitors to Phenomix, although that could change. “I think we’re the only diagnostic company in the space right now. But if it’s really a $14.8 billion market, we’re going to see a lot of diagnostic companies trying to do what we’re doing – if we’re successful,” he said.
An October 2022 report from Polaris Market Research estimates that the global market for obesity treatment – medications, surgery, and all others – was about $14 billion in 2021. The same report predicts the market will grow to $32 billion by 2030.
A version of this article first appeared on WebMD.com.
It sounds like a simple solution to a complicated problem: Find out what kind of obesity someone has based on a one-time genetic saliva test. Then patients and their doctor can get a better idea if antiobesity drugs or other treatments are more likely to work for them.
It’s what Mayo Clinic researchers had in mind when they created four phenotypes of obesity.
Obesity experts not affiliated with the research have some concerns and say independent studies are needed to verify the potential of this strategy.
This research could help predict who will respond best to popular antiobesity medications, said Andres Acosta, MD, PhD, cofounder of Phenomix Sciences, the company behind the tests. These medications include glucagonlike peptide–1 (GLP-1) receptor agonists like liraglutide (Saxenda, Victoza) and semaglutide (Ozempic, Wegovy).
“We know that not everyone on a GLP-1 will respond. In reality, about a third of the patients don’t do well with GLP-1s,” said Dr. Acosta, an assistant professor of medicine and researcher in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
Furthest along in development is the “My Phenome Hungry Gut” test for predicting GLP-1 response. People in this Hungry Gut group tend to empty their stomach after a meal faster and are more likely to feel hungry again a short time later, as explained on the company’s website.
A pilot study to test how well it works started in April at three primary care practices. Plans are to expand real-world testing for this and other obesity types later in 2023.
The other obesity categories are:
- “Hungry brain,” where the brain does not recognize signals that the stomach is full
- “Emotional hunger,” where cravings to eat are driven by emotions, anxiety, and negative feelings
- “Slow burn,” where people have a slow metabolism and low energy level
People in these categories might be more likely to benefit from other obesity management strategies, like changes to their diet or placement of an intragastric balloon.
Some things to consider
While applauding their efforts to be more precise in treating people with obesity, not all experts are convinced this saliva test will be the answer. The company’s research might look promising, but verification of results is warranted.
“Can we get better outcomes with things like this? Well, that’s the hope,” said Jaime Almandoz, MD, medical director of weight wellness at the University of Texas Southwestern Medical Center, Dallas.
“We still don’t have randomized trials where we’re looking at obesity phenotyping yet,” said Dr. Almandoz, who is also a spokesperson for the Obesity Society, a professional group of clinicians, researchers, educators, and others focused on obesity science, treatment, and prevention.
There is always concern when a diagnostic test is being developed for commercial use, said Daniel Bessesen, MD, a professor of medicine–endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora. “What they’re talking about doing is super important. But this is a company. This is a company that is, I think, selling a product.”
In an online search, Dr. Bessesen did not find any external studies that showed how well the saliva testing worked. But referring to work by Dr. Acosta and Michael Camilleri, MD, the other cofounder of Phenomix, he said, “I found some papers that they did that I hadn’t read before that are good.”
“These guys are smart guys. And they’ve done a lot of work on [the movement of food through the gut] and how that correlates with obesity and response to some therapies,” said Dr. Bessesen, who is also a spokesperson for the Obesity Society. “So their scientific work does line up with this area.”
Validation of any research is important because the obesity industry has been known for a lot of lose-weight-quick strategies, some with little or no science behind them, he said.
It is also essential, he said, because “anytime you do something commercial in the area of obesity, you have to acknowledge that people with obesity are a vulnerable population. These people face stigma and bias all the time.”
Removing the stigma
If knowing your obesity type ends up making a difference, it could change the conversation people have with their medical provider, Dr. Acosta said. It could also help remove some of the stigma around obesity.
“We’re going to change the conversation because now we can say: ‘Hey, you have obesity because you have ‘Hungry Gut’ phenotype. And because of that, you’re going to respond to this medication,” Dr. Acosta said. The phenotyping suggests a strong genetic tendency – a biologic basis for obesity.
“So it’s not only a way of taking the blame out, but it’s also way of explaining that there’s a reason why you have obesity,” Dr. Acosta said. It tells people: “You’re not a failure.”
More cost-effective treatment?
Targeting obesity treatment could also save on overall health care costs, Dr. Almandoz said. He estimated a cost of $1,400 per month “for forever and ever semaglutide” or at least $1,400 a month for a 3-month trial to see if this medication works in a particular person with obesity.
“That’s a lot of money when you extrapolate that out over the number of people who probably meet the criteria for treatment,” he said. A total 42% of Americans meet the Centers for Disease Control and Prevention definition for obesity.
“You can imagine the potential cost if we were to provide antiobesity therapies to everybody and we were to use what is the most effective class of medication, which is more than a thousand dollars per month, indefinitely,” Dr. Almandoz said. “Not that we should not treat everybody. That’s not the message I’m saying. But if we’re looking at yield or value in terms of treating obesity in a setting with limited resources, it may be best to start with who is most likely to benefit.”
How they created four obesity types
Starting in 2015, Dr. Acosta and colleagues started comparing tests in people with normal weight versus obesity. They used artificial intelligence and machine learning to classify obesity into 11 types at first. They realized this many obesity types were not practical for doctors and people with obesity, so they combined them into four phenotypes.
“The AI machine learning was followed by, as I like to call, HI, or human intelligence,” he said.
The saliva test checks for about 6,000 relevant genetic single-nucleotide polymorphisms. Six thousand genetic changes may sound like a large number to check; however, the average individual carries 5 million and 6 million SNPs in their DNA.
The results are translated to a score that yields a low risk or high risk for Hungry Gut or other types of obesity. “You can have all six thousand genetic mutations, or you can have zero,” Dr. Acosta said.
Moving forward
After the soft launch of Hungry Gut testing in April, Phenomix plans to continue studying their saliva test on other obesity types.
Dr. Acosta is not aware of any direct competitors to Phenomix, although that could change. “I think we’re the only diagnostic company in the space right now. But if it’s really a $14.8 billion market, we’re going to see a lot of diagnostic companies trying to do what we’re doing – if we’re successful,” he said.
An October 2022 report from Polaris Market Research estimates that the global market for obesity treatment – medications, surgery, and all others – was about $14 billion in 2021. The same report predicts the market will grow to $32 billion by 2030.
A version of this article first appeared on WebMD.com.
It sounds like a simple solution to a complicated problem: Find out what kind of obesity someone has based on a one-time genetic saliva test. Then patients and their doctor can get a better idea if antiobesity drugs or other treatments are more likely to work for them.
It’s what Mayo Clinic researchers had in mind when they created four phenotypes of obesity.
Obesity experts not affiliated with the research have some concerns and say independent studies are needed to verify the potential of this strategy.
This research could help predict who will respond best to popular antiobesity medications, said Andres Acosta, MD, PhD, cofounder of Phenomix Sciences, the company behind the tests. These medications include glucagonlike peptide–1 (GLP-1) receptor agonists like liraglutide (Saxenda, Victoza) and semaglutide (Ozempic, Wegovy).
“We know that not everyone on a GLP-1 will respond. In reality, about a third of the patients don’t do well with GLP-1s,” said Dr. Acosta, an assistant professor of medicine and researcher in the division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn.
Furthest along in development is the “My Phenome Hungry Gut” test for predicting GLP-1 response. People in this Hungry Gut group tend to empty their stomach after a meal faster and are more likely to feel hungry again a short time later, as explained on the company’s website.
A pilot study to test how well it works started in April at three primary care practices. Plans are to expand real-world testing for this and other obesity types later in 2023.
The other obesity categories are:
- “Hungry brain,” where the brain does not recognize signals that the stomach is full
- “Emotional hunger,” where cravings to eat are driven by emotions, anxiety, and negative feelings
- “Slow burn,” where people have a slow metabolism and low energy level
People in these categories might be more likely to benefit from other obesity management strategies, like changes to their diet or placement of an intragastric balloon.
Some things to consider
While applauding their efforts to be more precise in treating people with obesity, not all experts are convinced this saliva test will be the answer. The company’s research might look promising, but verification of results is warranted.
“Can we get better outcomes with things like this? Well, that’s the hope,” said Jaime Almandoz, MD, medical director of weight wellness at the University of Texas Southwestern Medical Center, Dallas.
“We still don’t have randomized trials where we’re looking at obesity phenotyping yet,” said Dr. Almandoz, who is also a spokesperson for the Obesity Society, a professional group of clinicians, researchers, educators, and others focused on obesity science, treatment, and prevention.
There is always concern when a diagnostic test is being developed for commercial use, said Daniel Bessesen, MD, a professor of medicine–endocrinology, metabolism, and diabetes at the University of Colorado at Denver, Aurora. “What they’re talking about doing is super important. But this is a company. This is a company that is, I think, selling a product.”
In an online search, Dr. Bessesen did not find any external studies that showed how well the saliva testing worked. But referring to work by Dr. Acosta and Michael Camilleri, MD, the other cofounder of Phenomix, he said, “I found some papers that they did that I hadn’t read before that are good.”
“These guys are smart guys. And they’ve done a lot of work on [the movement of food through the gut] and how that correlates with obesity and response to some therapies,” said Dr. Bessesen, who is also a spokesperson for the Obesity Society. “So their scientific work does line up with this area.”
Validation of any research is important because the obesity industry has been known for a lot of lose-weight-quick strategies, some with little or no science behind them, he said.
It is also essential, he said, because “anytime you do something commercial in the area of obesity, you have to acknowledge that people with obesity are a vulnerable population. These people face stigma and bias all the time.”
Removing the stigma
If knowing your obesity type ends up making a difference, it could change the conversation people have with their medical provider, Dr. Acosta said. It could also help remove some of the stigma around obesity.
“We’re going to change the conversation because now we can say: ‘Hey, you have obesity because you have ‘Hungry Gut’ phenotype. And because of that, you’re going to respond to this medication,” Dr. Acosta said. The phenotyping suggests a strong genetic tendency – a biologic basis for obesity.
“So it’s not only a way of taking the blame out, but it’s also way of explaining that there’s a reason why you have obesity,” Dr. Acosta said. It tells people: “You’re not a failure.”
More cost-effective treatment?
Targeting obesity treatment could also save on overall health care costs, Dr. Almandoz said. He estimated a cost of $1,400 per month “for forever and ever semaglutide” or at least $1,400 a month for a 3-month trial to see if this medication works in a particular person with obesity.
“That’s a lot of money when you extrapolate that out over the number of people who probably meet the criteria for treatment,” he said. A total 42% of Americans meet the Centers for Disease Control and Prevention definition for obesity.
“You can imagine the potential cost if we were to provide antiobesity therapies to everybody and we were to use what is the most effective class of medication, which is more than a thousand dollars per month, indefinitely,” Dr. Almandoz said. “Not that we should not treat everybody. That’s not the message I’m saying. But if we’re looking at yield or value in terms of treating obesity in a setting with limited resources, it may be best to start with who is most likely to benefit.”
How they created four obesity types
Starting in 2015, Dr. Acosta and colleagues started comparing tests in people with normal weight versus obesity. They used artificial intelligence and machine learning to classify obesity into 11 types at first. They realized this many obesity types were not practical for doctors and people with obesity, so they combined them into four phenotypes.
“The AI machine learning was followed by, as I like to call, HI, or human intelligence,” he said.
The saliva test checks for about 6,000 relevant genetic single-nucleotide polymorphisms. Six thousand genetic changes may sound like a large number to check; however, the average individual carries 5 million and 6 million SNPs in their DNA.
The results are translated to a score that yields a low risk or high risk for Hungry Gut or other types of obesity. “You can have all six thousand genetic mutations, or you can have zero,” Dr. Acosta said.
Moving forward
After the soft launch of Hungry Gut testing in April, Phenomix plans to continue studying their saliva test on other obesity types.
Dr. Acosta is not aware of any direct competitors to Phenomix, although that could change. “I think we’re the only diagnostic company in the space right now. But if it’s really a $14.8 billion market, we’re going to see a lot of diagnostic companies trying to do what we’re doing – if we’re successful,” he said.
An October 2022 report from Polaris Market Research estimates that the global market for obesity treatment – medications, surgery, and all others – was about $14 billion in 2021. The same report predicts the market will grow to $32 billion by 2030.
A version of this article first appeared on WebMD.com.
FDA approves new drug, sotagliflozin, for heart failure
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Plant-based diet tied to healthier blood lipid levels
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN HEART JOURNAL
JAK-inhibitor safety in adolescents with AD: Long-term analyses reported
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
AT RAD 2023