User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Some, not all, ultraprocessed foods linked to type 2 diabetes
High total intake of ultraprocessed food (UPF) is associated with an increased risk of developing type 2 diabetes, suggests a large-scale analysis that nevertheless revealed that the risk applies only to certain such foods.
The research was recently published in Diabetes Care by Zhangling Chen, PhD, Erasmus MC Rotterdam, Netherlands, and colleagues.
Examining almost 200,000 participants in three U.S. studies, yielding more than 5 million person-years of follow-up, the scientists found that high intake of UPF was associated with a 28% increased risk of type 2 diabetes, after statistical adjustments.
However, the increased risk was restricted to certain UPFs, including ready meals, refined breads, sweetened beverages, and sauces and condiments, with other foods considered UPFs, such as cereals, dark- and whole grain breads, and packaged sweet and savory snacks, among others, associated with a reduced risk of diabetes.
Senior author Jean-Philippe Drouin-Chartier, PhD, Nutrition Center, Laval University, Quebec City, told this news organization: “While whole grain breads can be considered as ultraprocessed foods, their consumption should not be discouraged. In our study, we observed that whole grain breads consumption is inversely associated with type 2 diabetes risk. This is supported by many studies linking dietary fiber consumption to better cardiometabolic health.”
Ultraprocessed food intake higher in the U.S. than in Europe
The researchers note that a handful of European studies have also reported an association between UPF consumption and increased type 2 diabetes risk, with the effect ranging from 15% to 53%, depending on the level of intake and the cohort of patients studied.
They note, however, that total UPF intake in the U.S. is “much higher than in Europe,” particularly in the case of ultraprocessed breads and cereals and artificially or sugar-sweetened beverages.
In the current study, they examined data on 71,781 women from the Nurses’ Health Study, 87,918 women from the NHS II, and 38,847 men from the Health Professional Follow-up Study, none of whom had cardiovascular disease, cancer, or diabetes at baseline.
In all three studies, questionnaires were administered every 2 years to collect demographic, lifestyle, and medical information, and a validated food frequency questionnaire was used every 2-4 years to assess participants’ diets over 30 years of follow-up.
Using the NOVA Food Classification system, the items on the food frequency questionnaire were categorized into one of four groups: unprocessed or minimally processed foods; processed culinary ingredients; processed foods; or UPFs, which were subdivided into nine mutually exclusive subgroups.
Servings per day were then used to determine individual UPF intake.
Higher total UPF intake was associated with a greater total energy intake, body mass index, and prevalence of hypercholesterolemia and/or hypertension, as well as lower healthy eating scores and physical activity.
The researchers calculated that, over 5,187,678 person-years of follow-up, there were 19,503 cases of type 2 diabetes across the three study cohorts.
Multivariate analysis taking into consideration a range of potential risk factors, including BMI, revealed that, across the three study cohorts, the highest quintile of UPF intake was associated with a significantly increased risk of type 2 diabetes.
Compared with the lowest quintile of UPF intake, the hazard ratio for incident type 2 diabetes was 1.28 (P < .0001), with an increase in risk per additional serving per day of 3%.
The UPFs associated with a higher type 2 diabetes risk were as previously described and also included animal-based products and ready-to-eat mixed dishes.
In contrast, intake of UPFs including cereals, dark and whole grain breads, packaged sweet and savory snacks, fruit-based products, and yogurt and dairy-based desserts were linked to a reduced risk of type 2 diabetes.
Then to further validate their findings, the researchers conducted a meta-analysis of their own and four additional studies, comprising 415,554 participants and 21,932 events, with a follow-up of 3.4-32.0 years.
They determined that the pooled relative risk of type 2 diabetes with the highest versus lowest levels of UPF consumption was 1.40, with each 10% increase in total UPF intake associated with a 12% increase in diabetes risk.
Ideal is to have access to minimally processed foods
The NOVA food classification system states that UPFs are industrial formulations “made mostly or entirely with substances extracted from foods, often chemically modified, with additives and with little, if any, whole foods added.”
A recent study questioned the value of the NOVA classification after finding that it had “low consistency” when assigning foods.
Previous studies have nevertheless revealed that UPFs and their constituents negatively affect the gut microbiota and can cause systemic inflammation, insulin resistance, and increased body weight.
Dr. Drouin-Chartier concluded: “There is a need to facilitate ... access to minimally processed foods. This encompasses [appropriate] pricing and physical access [to such foods], that is, addressing the issue of food deserts.”
The NHS I and II and HPFS studies are supported by National Institutes of Health. Dr. Drouin-Chartier has reported a relationship with the Dairy Farmers of Canada. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
High total intake of ultraprocessed food (UPF) is associated with an increased risk of developing type 2 diabetes, suggests a large-scale analysis that nevertheless revealed that the risk applies only to certain such foods.
The research was recently published in Diabetes Care by Zhangling Chen, PhD, Erasmus MC Rotterdam, Netherlands, and colleagues.
Examining almost 200,000 participants in three U.S. studies, yielding more than 5 million person-years of follow-up, the scientists found that high intake of UPF was associated with a 28% increased risk of type 2 diabetes, after statistical adjustments.
However, the increased risk was restricted to certain UPFs, including ready meals, refined breads, sweetened beverages, and sauces and condiments, with other foods considered UPFs, such as cereals, dark- and whole grain breads, and packaged sweet and savory snacks, among others, associated with a reduced risk of diabetes.
Senior author Jean-Philippe Drouin-Chartier, PhD, Nutrition Center, Laval University, Quebec City, told this news organization: “While whole grain breads can be considered as ultraprocessed foods, their consumption should not be discouraged. In our study, we observed that whole grain breads consumption is inversely associated with type 2 diabetes risk. This is supported by many studies linking dietary fiber consumption to better cardiometabolic health.”
Ultraprocessed food intake higher in the U.S. than in Europe
The researchers note that a handful of European studies have also reported an association between UPF consumption and increased type 2 diabetes risk, with the effect ranging from 15% to 53%, depending on the level of intake and the cohort of patients studied.
They note, however, that total UPF intake in the U.S. is “much higher than in Europe,” particularly in the case of ultraprocessed breads and cereals and artificially or sugar-sweetened beverages.
In the current study, they examined data on 71,781 women from the Nurses’ Health Study, 87,918 women from the NHS II, and 38,847 men from the Health Professional Follow-up Study, none of whom had cardiovascular disease, cancer, or diabetes at baseline.
In all three studies, questionnaires were administered every 2 years to collect demographic, lifestyle, and medical information, and a validated food frequency questionnaire was used every 2-4 years to assess participants’ diets over 30 years of follow-up.
Using the NOVA Food Classification system, the items on the food frequency questionnaire were categorized into one of four groups: unprocessed or minimally processed foods; processed culinary ingredients; processed foods; or UPFs, which were subdivided into nine mutually exclusive subgroups.
Servings per day were then used to determine individual UPF intake.
Higher total UPF intake was associated with a greater total energy intake, body mass index, and prevalence of hypercholesterolemia and/or hypertension, as well as lower healthy eating scores and physical activity.
The researchers calculated that, over 5,187,678 person-years of follow-up, there were 19,503 cases of type 2 diabetes across the three study cohorts.
Multivariate analysis taking into consideration a range of potential risk factors, including BMI, revealed that, across the three study cohorts, the highest quintile of UPF intake was associated with a significantly increased risk of type 2 diabetes.
Compared with the lowest quintile of UPF intake, the hazard ratio for incident type 2 diabetes was 1.28 (P < .0001), with an increase in risk per additional serving per day of 3%.
The UPFs associated with a higher type 2 diabetes risk were as previously described and also included animal-based products and ready-to-eat mixed dishes.
In contrast, intake of UPFs including cereals, dark and whole grain breads, packaged sweet and savory snacks, fruit-based products, and yogurt and dairy-based desserts were linked to a reduced risk of type 2 diabetes.
Then to further validate their findings, the researchers conducted a meta-analysis of their own and four additional studies, comprising 415,554 participants and 21,932 events, with a follow-up of 3.4-32.0 years.
They determined that the pooled relative risk of type 2 diabetes with the highest versus lowest levels of UPF consumption was 1.40, with each 10% increase in total UPF intake associated with a 12% increase in diabetes risk.
Ideal is to have access to minimally processed foods
The NOVA food classification system states that UPFs are industrial formulations “made mostly or entirely with substances extracted from foods, often chemically modified, with additives and with little, if any, whole foods added.”
A recent study questioned the value of the NOVA classification after finding that it had “low consistency” when assigning foods.
Previous studies have nevertheless revealed that UPFs and their constituents negatively affect the gut microbiota and can cause systemic inflammation, insulin resistance, and increased body weight.
Dr. Drouin-Chartier concluded: “There is a need to facilitate ... access to minimally processed foods. This encompasses [appropriate] pricing and physical access [to such foods], that is, addressing the issue of food deserts.”
The NHS I and II and HPFS studies are supported by National Institutes of Health. Dr. Drouin-Chartier has reported a relationship with the Dairy Farmers of Canada. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
High total intake of ultraprocessed food (UPF) is associated with an increased risk of developing type 2 diabetes, suggests a large-scale analysis that nevertheless revealed that the risk applies only to certain such foods.
The research was recently published in Diabetes Care by Zhangling Chen, PhD, Erasmus MC Rotterdam, Netherlands, and colleagues.
Examining almost 200,000 participants in three U.S. studies, yielding more than 5 million person-years of follow-up, the scientists found that high intake of UPF was associated with a 28% increased risk of type 2 diabetes, after statistical adjustments.
However, the increased risk was restricted to certain UPFs, including ready meals, refined breads, sweetened beverages, and sauces and condiments, with other foods considered UPFs, such as cereals, dark- and whole grain breads, and packaged sweet and savory snacks, among others, associated with a reduced risk of diabetes.
Senior author Jean-Philippe Drouin-Chartier, PhD, Nutrition Center, Laval University, Quebec City, told this news organization: “While whole grain breads can be considered as ultraprocessed foods, their consumption should not be discouraged. In our study, we observed that whole grain breads consumption is inversely associated with type 2 diabetes risk. This is supported by many studies linking dietary fiber consumption to better cardiometabolic health.”
Ultraprocessed food intake higher in the U.S. than in Europe
The researchers note that a handful of European studies have also reported an association between UPF consumption and increased type 2 diabetes risk, with the effect ranging from 15% to 53%, depending on the level of intake and the cohort of patients studied.
They note, however, that total UPF intake in the U.S. is “much higher than in Europe,” particularly in the case of ultraprocessed breads and cereals and artificially or sugar-sweetened beverages.
In the current study, they examined data on 71,781 women from the Nurses’ Health Study, 87,918 women from the NHS II, and 38,847 men from the Health Professional Follow-up Study, none of whom had cardiovascular disease, cancer, or diabetes at baseline.
In all three studies, questionnaires were administered every 2 years to collect demographic, lifestyle, and medical information, and a validated food frequency questionnaire was used every 2-4 years to assess participants’ diets over 30 years of follow-up.
Using the NOVA Food Classification system, the items on the food frequency questionnaire were categorized into one of four groups: unprocessed or minimally processed foods; processed culinary ingredients; processed foods; or UPFs, which were subdivided into nine mutually exclusive subgroups.
Servings per day were then used to determine individual UPF intake.
Higher total UPF intake was associated with a greater total energy intake, body mass index, and prevalence of hypercholesterolemia and/or hypertension, as well as lower healthy eating scores and physical activity.
The researchers calculated that, over 5,187,678 person-years of follow-up, there were 19,503 cases of type 2 diabetes across the three study cohorts.
Multivariate analysis taking into consideration a range of potential risk factors, including BMI, revealed that, across the three study cohorts, the highest quintile of UPF intake was associated with a significantly increased risk of type 2 diabetes.
Compared with the lowest quintile of UPF intake, the hazard ratio for incident type 2 diabetes was 1.28 (P < .0001), with an increase in risk per additional serving per day of 3%.
The UPFs associated with a higher type 2 diabetes risk were as previously described and also included animal-based products and ready-to-eat mixed dishes.
In contrast, intake of UPFs including cereals, dark and whole grain breads, packaged sweet and savory snacks, fruit-based products, and yogurt and dairy-based desserts were linked to a reduced risk of type 2 diabetes.
Then to further validate their findings, the researchers conducted a meta-analysis of their own and four additional studies, comprising 415,554 participants and 21,932 events, with a follow-up of 3.4-32.0 years.
They determined that the pooled relative risk of type 2 diabetes with the highest versus lowest levels of UPF consumption was 1.40, with each 10% increase in total UPF intake associated with a 12% increase in diabetes risk.
Ideal is to have access to minimally processed foods
The NOVA food classification system states that UPFs are industrial formulations “made mostly or entirely with substances extracted from foods, often chemically modified, with additives and with little, if any, whole foods added.”
A recent study questioned the value of the NOVA classification after finding that it had “low consistency” when assigning foods.
Previous studies have nevertheless revealed that UPFs and their constituents negatively affect the gut microbiota and can cause systemic inflammation, insulin resistance, and increased body weight.
Dr. Drouin-Chartier concluded: “There is a need to facilitate ... access to minimally processed foods. This encompasses [appropriate] pricing and physical access [to such foods], that is, addressing the issue of food deserts.”
The NHS I and II and HPFS studies are supported by National Institutes of Health. Dr. Drouin-Chartier has reported a relationship with the Dairy Farmers of Canada. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Spironolactone: an ‘inexpensive, effective’ option for acne in women
HONOLULU – In the clinical experience of Julie C. Harper, MD, an increasing number of women with acne are turning to off-label, long-term treatment with spironolactone.
“Spironolactone is fairly accessible, inexpensive, and effective for our patients,” Dr. Harper, a dermatologist who practices in Birmingham, Ala., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
An aldosterone receptor antagonist commonly used to treat high blood pressure and heart failure, spironolactone also has antiandrogenic properties with a proven track record for treating acne and hirsutism. It reduces androgen production, inhibits 5-alpha reductase, and increases sex hormone binding globulin. The dosing range for treating acne is 25 mg to 200 mg per day, but Dr. Harper prefers a maximum dose of 100 mg per day.
According to a systematic review of its use for acne in adult women, the most common side effect is menstrual irregularity, while other common side effects include breast tenderness/swelling, fatigue, and headaches.
“The higher the dose, the higher the rate of side effects,” she said. Concomitant use of an oral contraceptive lessens menstrual irregularities and prevents pregnancies, to avoid exposure during pregnancy and the hypothetical risk of feminization of the male fetus with exposure late in the first trimester. “Early in my career, I used to say if you’re going to be on spironolactone you’re also going to be on an oral contraceptive. But the longer I’ve practiced, I’ve learned that women who have a contraindication to birth control pills or who don’t want to take it can still benefit from an oral antiandrogen by being on spironolactone.”
A large retrospective analysis of 14-year data concluded that routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. “If you’re between the ages of 18 and 45, healthy, and not taking other medications where I’m worried about potassium levels, I’m not checking those levels at all,” Dr. Harper said.
Spironolactone labeling includes a boxed warning regarding the potential for tumorigenicity based on rat studies, but the dosages used in those studies were 25-250 times higher than the exposure dose in humans, Dr. Harper said.
Results from a systematic review and meta-analysis of seven studies in the medical literature found no evidence of an increased risk of breast cancer in women with exposure to spironolactone. “However, the certainty of the evidence was low and future studies are needed, including among diverse populations such as younger individuals and those with acne or hirsutism,” the study authors wrote.
In a separate study, researchers drew from patients in the Humana Insurance database from 2005 to 2017 to address whether spironolactone is associated with an increased risk of recurrence of breast cancer. Recurrent breast cancer was examined in 29,146 women with continuous health insurance for 2 years after a diagnosis of breast cancer. Of these, 746 were prescribed spironolactone, and the remainder were not. The researchers found that 123 women (16.5%) who were prescribed spironolactone had a breast cancer recurrence, compared with 3,649 women (12.8%) with a breast cancer recurrence who had not been prescribed spironolactone (P = .004). Adjusted Cox regression analysis following propensity matching showed no association between spironolactone and increased breast cancer recurrence (adjusted hazard ratio, 0.966; P = .953).
According to Dr. Harper, spironolactone may take about 3 months to kick in. “Likely this is a long-term treatment, and most of the time we’re going to be using it in combination with other acne treatments such as topical retinoids or topical benzoyl peroxide, oral antibiotics, or even isotretinoin.”
A study of long-term spironolactone use in 403 women found that the most common dose prescribed was 100 mg/day, and 68% of the women were concurrently prescribed a topical retinoid, 2.2% an oral antibiotic, and 40.7% an oral contraceptive.
The study population included 32 patients with a history of polycystic ovarian syndrome, 1 with a history of breast cancer, and 5 were hypercoagulable. Patients took the drug for a mean of 471 days. “As opposed to our antibiotics, where the course for patients is generally 3-4 months, when you start someone on spironolactone, they may end up staying on it,” Dr. Harper said.
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, Cassiopeia, Cutera, EPI, Galderma, L’Oreal, Ortho Dermatologics, Sol Gel, and Vyne. She also serves as a speaker or member of a speaker’s bureau for Almirall, Cassiopeia, Cutera, EPI, Galderma, Journey Almirall, L’Oreal, Ortho Dermatologics, Sun Pharmaceutical Industries, and Vyne.
Medscape and this news organization are owned by the same parent company.
HONOLULU – In the clinical experience of Julie C. Harper, MD, an increasing number of women with acne are turning to off-label, long-term treatment with spironolactone.
“Spironolactone is fairly accessible, inexpensive, and effective for our patients,” Dr. Harper, a dermatologist who practices in Birmingham, Ala., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
An aldosterone receptor antagonist commonly used to treat high blood pressure and heart failure, spironolactone also has antiandrogenic properties with a proven track record for treating acne and hirsutism. It reduces androgen production, inhibits 5-alpha reductase, and increases sex hormone binding globulin. The dosing range for treating acne is 25 mg to 200 mg per day, but Dr. Harper prefers a maximum dose of 100 mg per day.
According to a systematic review of its use for acne in adult women, the most common side effect is menstrual irregularity, while other common side effects include breast tenderness/swelling, fatigue, and headaches.
“The higher the dose, the higher the rate of side effects,” she said. Concomitant use of an oral contraceptive lessens menstrual irregularities and prevents pregnancies, to avoid exposure during pregnancy and the hypothetical risk of feminization of the male fetus with exposure late in the first trimester. “Early in my career, I used to say if you’re going to be on spironolactone you’re also going to be on an oral contraceptive. But the longer I’ve practiced, I’ve learned that women who have a contraindication to birth control pills or who don’t want to take it can still benefit from an oral antiandrogen by being on spironolactone.”
A large retrospective analysis of 14-year data concluded that routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. “If you’re between the ages of 18 and 45, healthy, and not taking other medications where I’m worried about potassium levels, I’m not checking those levels at all,” Dr. Harper said.
Spironolactone labeling includes a boxed warning regarding the potential for tumorigenicity based on rat studies, but the dosages used in those studies were 25-250 times higher than the exposure dose in humans, Dr. Harper said.
Results from a systematic review and meta-analysis of seven studies in the medical literature found no evidence of an increased risk of breast cancer in women with exposure to spironolactone. “However, the certainty of the evidence was low and future studies are needed, including among diverse populations such as younger individuals and those with acne or hirsutism,” the study authors wrote.
In a separate study, researchers drew from patients in the Humana Insurance database from 2005 to 2017 to address whether spironolactone is associated with an increased risk of recurrence of breast cancer. Recurrent breast cancer was examined in 29,146 women with continuous health insurance for 2 years after a diagnosis of breast cancer. Of these, 746 were prescribed spironolactone, and the remainder were not. The researchers found that 123 women (16.5%) who were prescribed spironolactone had a breast cancer recurrence, compared with 3,649 women (12.8%) with a breast cancer recurrence who had not been prescribed spironolactone (P = .004). Adjusted Cox regression analysis following propensity matching showed no association between spironolactone and increased breast cancer recurrence (adjusted hazard ratio, 0.966; P = .953).
According to Dr. Harper, spironolactone may take about 3 months to kick in. “Likely this is a long-term treatment, and most of the time we’re going to be using it in combination with other acne treatments such as topical retinoids or topical benzoyl peroxide, oral antibiotics, or even isotretinoin.”
A study of long-term spironolactone use in 403 women found that the most common dose prescribed was 100 mg/day, and 68% of the women were concurrently prescribed a topical retinoid, 2.2% an oral antibiotic, and 40.7% an oral contraceptive.
The study population included 32 patients with a history of polycystic ovarian syndrome, 1 with a history of breast cancer, and 5 were hypercoagulable. Patients took the drug for a mean of 471 days. “As opposed to our antibiotics, where the course for patients is generally 3-4 months, when you start someone on spironolactone, they may end up staying on it,” Dr. Harper said.
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, Cassiopeia, Cutera, EPI, Galderma, L’Oreal, Ortho Dermatologics, Sol Gel, and Vyne. She also serves as a speaker or member of a speaker’s bureau for Almirall, Cassiopeia, Cutera, EPI, Galderma, Journey Almirall, L’Oreal, Ortho Dermatologics, Sun Pharmaceutical Industries, and Vyne.
Medscape and this news organization are owned by the same parent company.
HONOLULU – In the clinical experience of Julie C. Harper, MD, an increasing number of women with acne are turning to off-label, long-term treatment with spironolactone.
“Spironolactone is fairly accessible, inexpensive, and effective for our patients,” Dr. Harper, a dermatologist who practices in Birmingham, Ala., said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!
An aldosterone receptor antagonist commonly used to treat high blood pressure and heart failure, spironolactone also has antiandrogenic properties with a proven track record for treating acne and hirsutism. It reduces androgen production, inhibits 5-alpha reductase, and increases sex hormone binding globulin. The dosing range for treating acne is 25 mg to 200 mg per day, but Dr. Harper prefers a maximum dose of 100 mg per day.
According to a systematic review of its use for acne in adult women, the most common side effect is menstrual irregularity, while other common side effects include breast tenderness/swelling, fatigue, and headaches.
“The higher the dose, the higher the rate of side effects,” she said. Concomitant use of an oral contraceptive lessens menstrual irregularities and prevents pregnancies, to avoid exposure during pregnancy and the hypothetical risk of feminization of the male fetus with exposure late in the first trimester. “Early in my career, I used to say if you’re going to be on spironolactone you’re also going to be on an oral contraceptive. But the longer I’ve practiced, I’ve learned that women who have a contraindication to birth control pills or who don’t want to take it can still benefit from an oral antiandrogen by being on spironolactone.”
A large retrospective analysis of 14-year data concluded that routine potassium monitoring is unnecessary for healthy women taking spironolactone for acne. “If you’re between the ages of 18 and 45, healthy, and not taking other medications where I’m worried about potassium levels, I’m not checking those levels at all,” Dr. Harper said.
Spironolactone labeling includes a boxed warning regarding the potential for tumorigenicity based on rat studies, but the dosages used in those studies were 25-250 times higher than the exposure dose in humans, Dr. Harper said.
Results from a systematic review and meta-analysis of seven studies in the medical literature found no evidence of an increased risk of breast cancer in women with exposure to spironolactone. “However, the certainty of the evidence was low and future studies are needed, including among diverse populations such as younger individuals and those with acne or hirsutism,” the study authors wrote.
In a separate study, researchers drew from patients in the Humana Insurance database from 2005 to 2017 to address whether spironolactone is associated with an increased risk of recurrence of breast cancer. Recurrent breast cancer was examined in 29,146 women with continuous health insurance for 2 years after a diagnosis of breast cancer. Of these, 746 were prescribed spironolactone, and the remainder were not. The researchers found that 123 women (16.5%) who were prescribed spironolactone had a breast cancer recurrence, compared with 3,649 women (12.8%) with a breast cancer recurrence who had not been prescribed spironolactone (P = .004). Adjusted Cox regression analysis following propensity matching showed no association between spironolactone and increased breast cancer recurrence (adjusted hazard ratio, 0.966; P = .953).
According to Dr. Harper, spironolactone may take about 3 months to kick in. “Likely this is a long-term treatment, and most of the time we’re going to be using it in combination with other acne treatments such as topical retinoids or topical benzoyl peroxide, oral antibiotics, or even isotretinoin.”
A study of long-term spironolactone use in 403 women found that the most common dose prescribed was 100 mg/day, and 68% of the women were concurrently prescribed a topical retinoid, 2.2% an oral antibiotic, and 40.7% an oral contraceptive.
The study population included 32 patients with a history of polycystic ovarian syndrome, 1 with a history of breast cancer, and 5 were hypercoagulable. Patients took the drug for a mean of 471 days. “As opposed to our antibiotics, where the course for patients is generally 3-4 months, when you start someone on spironolactone, they may end up staying on it,” Dr. Harper said.
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, Cassiopeia, Cutera, EPI, Galderma, L’Oreal, Ortho Dermatologics, Sol Gel, and Vyne. She also serves as a speaker or member of a speaker’s bureau for Almirall, Cassiopeia, Cutera, EPI, Galderma, Journey Almirall, L’Oreal, Ortho Dermatologics, Sun Pharmaceutical Industries, and Vyne.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
TikTok’s fave weight loss drugs: Link to thyroid cancer?
With #Ozempic and #ozempicweightloss continuing to trend on social media, along with the mainstream media focusing on celebrities who rely on Ozempic (semaglutide) for weight loss, the daily requests for this new medication have been increasing.
Accompanying these requests are concerns and questions about potential risks, including this most recent message from one of my patients: “Dr. P – I saw the warnings. Is this medication going to make me get thyroid cancer? Please let me know!”
Let’s look at what we know to date, including recent studies, and how to advise our patients on this very hot topic.
Using GLP-1 receptor agonists for obesity
We have extensive prior experience with glucagon-like peptide 1 (GLP-1) receptor agonists, such as semaglutide, for treating type 2 diabetes and now recently as agents for weight loss.
Large clinical trials have documented the benefits of this medication class not only for weight reduction but also for cardiovascular and renal benefits in patients with diabetes. The subcutaneously injectable medications work by promoting insulin secretion, slowing gastric emptying, and suppressing glucagon secretion, with a low risk for hypoglycemia.
The Food and Drug Administration approved daily-injection GLP-1 agonist liraglutide for weight loss in 2014, and weekly-injection semaglutide for chronic weight management in 2021, in patients with a body mass index ≥ 27 with at least one weight-related condition or a BMI ≥ 30.
The brand name for semaglutide approved for weight loss is Wegovy, and the dose is slightly higher (maximum 2.4 mg/wk) than that of Ozempic (maximum 2.0 mg/wk), which is semaglutide approved for type 2 diabetes.
In trials for weight loss, data showed a mean change in body weight of almost 15% in the semaglutide group at week 68 compared with placebo, which is very impressive, particularly compared with other FDA-approved oral long-term weight loss medications.
The newest synthetic dual-acting agent is tirzepatide, which targets GLP-1 but is also a glucose-dependent insulinotropic polypeptide (GIP) agonist. The weekly subcutaneous injection was approved in May 2022 as Mounjaro for treating type 2 diabetes and produced even greater weight loss than semaglutide in clinical trials. Tirzepatide is now in trials for obesity and is under expedited review by the FDA for weight loss.
Why the concern about thyroid cancer?
Early on with the FDA approvals of GLP-1 agonists, a warning accompanied the products’ labels to not use this class of medications in patients with medullary thyroid cancer, a family history of medullary thyroid cancer, or multiple endocrine neoplasia syndrome type 2. This warning was based on data from animal studies.
Human pancreatic cells aren’t the only cells that express GLP-1 receptors. These receptors are also expressed by parafollicular cells (C cells) of the thyroid, which secrete calcitonin and are the cells involved in medullary thyroid cancer. A dose-related and duration-dependent increase in thyroid C-cell tumor incidence was noted in rodents. The same relationship was not demonstrated in monkeys. Humans have far fewer C cells than rats, and human C cells have very low expression of the GLP-1 receptor.
Over a decade ago, a study examining the FDA’s database of reported adverse events found an increased risk for thyroid cancer in patients treated with exenatide, another GLP-1 agonist. The reporting system wasn’t designed to distinguish thyroid cancer subtypes.
Numerous subsequent studies didn’t confirm this relationship. The LEADER trial looked at liraglutide in patients with type 2 diabetes and showed no effect of GLP-1 receptor activation on human serum calcitonin levels, C-cell proliferation, or C-cell malignancy. Similarly, a large meta-analysis in patients with type 2 diabetes didn’t find a statistically increased risk for thyroid cancer with liraglutide, and no thyroid malignancies were reported with exenatide.
Two U.S. administrative databases from commercial health plans (a retrospective cohort study and a nested case-control study) compared type 2 diabetes patients who were taking exenatide vs. other antidiabetic drugs and found that exenatide was not significantly associated with an increased risk for thyroid cancer.
And a recent meta-analysis of 45 trials showed no significant effects on the occurrence of thyroid cancer with GLP-1 receptor agonists. Of note, it did find an increased risk for overall thyroid disorders, although there was no clear statistically significant finding pointing to a specific thyroid disorder.
Differing from prior studies, a recent nationwide French health care system study provided newer data suggesting a moderate increased risk for thyroid cancer in a cohort of patients with type 2 diabetes who were taking GLP-1 agonists. The increase in relative risk was noted for all types of thyroid cancer in patients using GLP-1 receptor agonists for 1-3 years.
An accompanying commentary by Caroline A. Thompson, PhD, and Til Stürmer, MD, provides perspective on this study’s potential limitations. These include detection bias, as the study results focused only on the statistically significant data. Also discussed were limitations to the case-control design, issues with claims-based tumor type classification (unavailability of surgical pathology), and an inability to adjust for family history and obesity, which is a risk factor alone for thyroid cancer. There was also no adjustment for exposure to head/neck radiation.
While this study has important findings to consider, it deserves further investigation, with future studies linking data to tumor registry data before a change is made in clinical practice.
No clear relationship has been drawn between GLP-1 receptor agonists and thyroid cancer in humans. Numerous confounding factors limit the data. Studies generally don’t specify the type of thyroid cancer, and they lump medullary thyroid cancer, the rarest form, with papillary thyroid cancer.
Is a detection bias present where weight loss makes nodules more visible on the neck among those treated with GLP-1 agonists? And/or are patients treated with GLP-1 agonists being screened more stringently for thyroid nodules and/or cancer?
How to advise our patients and respond to the EMR messages
The TikTok videos may continue, the celebrity chatter may increase, and we, as physicians, will continue to look to real-world data with randomized controlled trials to tailor our decision-making and guide our patients.
Thyroid cancer remains a rare outcome, and GLP-1 receptor agonists remain a very important and beneficial treatment option for the right patient.
A version of this article first appeared on Medscape.com.
With #Ozempic and #ozempicweightloss continuing to trend on social media, along with the mainstream media focusing on celebrities who rely on Ozempic (semaglutide) for weight loss, the daily requests for this new medication have been increasing.
Accompanying these requests are concerns and questions about potential risks, including this most recent message from one of my patients: “Dr. P – I saw the warnings. Is this medication going to make me get thyroid cancer? Please let me know!”
Let’s look at what we know to date, including recent studies, and how to advise our patients on this very hot topic.
Using GLP-1 receptor agonists for obesity
We have extensive prior experience with glucagon-like peptide 1 (GLP-1) receptor agonists, such as semaglutide, for treating type 2 diabetes and now recently as agents for weight loss.
Large clinical trials have documented the benefits of this medication class not only for weight reduction but also for cardiovascular and renal benefits in patients with diabetes. The subcutaneously injectable medications work by promoting insulin secretion, slowing gastric emptying, and suppressing glucagon secretion, with a low risk for hypoglycemia.
The Food and Drug Administration approved daily-injection GLP-1 agonist liraglutide for weight loss in 2014, and weekly-injection semaglutide for chronic weight management in 2021, in patients with a body mass index ≥ 27 with at least one weight-related condition or a BMI ≥ 30.
The brand name for semaglutide approved for weight loss is Wegovy, and the dose is slightly higher (maximum 2.4 mg/wk) than that of Ozempic (maximum 2.0 mg/wk), which is semaglutide approved for type 2 diabetes.
In trials for weight loss, data showed a mean change in body weight of almost 15% in the semaglutide group at week 68 compared with placebo, which is very impressive, particularly compared with other FDA-approved oral long-term weight loss medications.
The newest synthetic dual-acting agent is tirzepatide, which targets GLP-1 but is also a glucose-dependent insulinotropic polypeptide (GIP) agonist. The weekly subcutaneous injection was approved in May 2022 as Mounjaro for treating type 2 diabetes and produced even greater weight loss than semaglutide in clinical trials. Tirzepatide is now in trials for obesity and is under expedited review by the FDA for weight loss.
Why the concern about thyroid cancer?
Early on with the FDA approvals of GLP-1 agonists, a warning accompanied the products’ labels to not use this class of medications in patients with medullary thyroid cancer, a family history of medullary thyroid cancer, or multiple endocrine neoplasia syndrome type 2. This warning was based on data from animal studies.
Human pancreatic cells aren’t the only cells that express GLP-1 receptors. These receptors are also expressed by parafollicular cells (C cells) of the thyroid, which secrete calcitonin and are the cells involved in medullary thyroid cancer. A dose-related and duration-dependent increase in thyroid C-cell tumor incidence was noted in rodents. The same relationship was not demonstrated in monkeys. Humans have far fewer C cells than rats, and human C cells have very low expression of the GLP-1 receptor.
Over a decade ago, a study examining the FDA’s database of reported adverse events found an increased risk for thyroid cancer in patients treated with exenatide, another GLP-1 agonist. The reporting system wasn’t designed to distinguish thyroid cancer subtypes.
Numerous subsequent studies didn’t confirm this relationship. The LEADER trial looked at liraglutide in patients with type 2 diabetes and showed no effect of GLP-1 receptor activation on human serum calcitonin levels, C-cell proliferation, or C-cell malignancy. Similarly, a large meta-analysis in patients with type 2 diabetes didn’t find a statistically increased risk for thyroid cancer with liraglutide, and no thyroid malignancies were reported with exenatide.
Two U.S. administrative databases from commercial health plans (a retrospective cohort study and a nested case-control study) compared type 2 diabetes patients who were taking exenatide vs. other antidiabetic drugs and found that exenatide was not significantly associated with an increased risk for thyroid cancer.
And a recent meta-analysis of 45 trials showed no significant effects on the occurrence of thyroid cancer with GLP-1 receptor agonists. Of note, it did find an increased risk for overall thyroid disorders, although there was no clear statistically significant finding pointing to a specific thyroid disorder.
Differing from prior studies, a recent nationwide French health care system study provided newer data suggesting a moderate increased risk for thyroid cancer in a cohort of patients with type 2 diabetes who were taking GLP-1 agonists. The increase in relative risk was noted for all types of thyroid cancer in patients using GLP-1 receptor agonists for 1-3 years.
An accompanying commentary by Caroline A. Thompson, PhD, and Til Stürmer, MD, provides perspective on this study’s potential limitations. These include detection bias, as the study results focused only on the statistically significant data. Also discussed were limitations to the case-control design, issues with claims-based tumor type classification (unavailability of surgical pathology), and an inability to adjust for family history and obesity, which is a risk factor alone for thyroid cancer. There was also no adjustment for exposure to head/neck radiation.
While this study has important findings to consider, it deserves further investigation, with future studies linking data to tumor registry data before a change is made in clinical practice.
No clear relationship has been drawn between GLP-1 receptor agonists and thyroid cancer in humans. Numerous confounding factors limit the data. Studies generally don’t specify the type of thyroid cancer, and they lump medullary thyroid cancer, the rarest form, with papillary thyroid cancer.
Is a detection bias present where weight loss makes nodules more visible on the neck among those treated with GLP-1 agonists? And/or are patients treated with GLP-1 agonists being screened more stringently for thyroid nodules and/or cancer?
How to advise our patients and respond to the EMR messages
The TikTok videos may continue, the celebrity chatter may increase, and we, as physicians, will continue to look to real-world data with randomized controlled trials to tailor our decision-making and guide our patients.
Thyroid cancer remains a rare outcome, and GLP-1 receptor agonists remain a very important and beneficial treatment option for the right patient.
A version of this article first appeared on Medscape.com.
With #Ozempic and #ozempicweightloss continuing to trend on social media, along with the mainstream media focusing on celebrities who rely on Ozempic (semaglutide) for weight loss, the daily requests for this new medication have been increasing.
Accompanying these requests are concerns and questions about potential risks, including this most recent message from one of my patients: “Dr. P – I saw the warnings. Is this medication going to make me get thyroid cancer? Please let me know!”
Let’s look at what we know to date, including recent studies, and how to advise our patients on this very hot topic.
Using GLP-1 receptor agonists for obesity
We have extensive prior experience with glucagon-like peptide 1 (GLP-1) receptor agonists, such as semaglutide, for treating type 2 diabetes and now recently as agents for weight loss.
Large clinical trials have documented the benefits of this medication class not only for weight reduction but also for cardiovascular and renal benefits in patients with diabetes. The subcutaneously injectable medications work by promoting insulin secretion, slowing gastric emptying, and suppressing glucagon secretion, with a low risk for hypoglycemia.
The Food and Drug Administration approved daily-injection GLP-1 agonist liraglutide for weight loss in 2014, and weekly-injection semaglutide for chronic weight management in 2021, in patients with a body mass index ≥ 27 with at least one weight-related condition or a BMI ≥ 30.
The brand name for semaglutide approved for weight loss is Wegovy, and the dose is slightly higher (maximum 2.4 mg/wk) than that of Ozempic (maximum 2.0 mg/wk), which is semaglutide approved for type 2 diabetes.
In trials for weight loss, data showed a mean change in body weight of almost 15% in the semaglutide group at week 68 compared with placebo, which is very impressive, particularly compared with other FDA-approved oral long-term weight loss medications.
The newest synthetic dual-acting agent is tirzepatide, which targets GLP-1 but is also a glucose-dependent insulinotropic polypeptide (GIP) agonist. The weekly subcutaneous injection was approved in May 2022 as Mounjaro for treating type 2 diabetes and produced even greater weight loss than semaglutide in clinical trials. Tirzepatide is now in trials for obesity and is under expedited review by the FDA for weight loss.
Why the concern about thyroid cancer?
Early on with the FDA approvals of GLP-1 agonists, a warning accompanied the products’ labels to not use this class of medications in patients with medullary thyroid cancer, a family history of medullary thyroid cancer, or multiple endocrine neoplasia syndrome type 2. This warning was based on data from animal studies.
Human pancreatic cells aren’t the only cells that express GLP-1 receptors. These receptors are also expressed by parafollicular cells (C cells) of the thyroid, which secrete calcitonin and are the cells involved in medullary thyroid cancer. A dose-related and duration-dependent increase in thyroid C-cell tumor incidence was noted in rodents. The same relationship was not demonstrated in monkeys. Humans have far fewer C cells than rats, and human C cells have very low expression of the GLP-1 receptor.
Over a decade ago, a study examining the FDA’s database of reported adverse events found an increased risk for thyroid cancer in patients treated with exenatide, another GLP-1 agonist. The reporting system wasn’t designed to distinguish thyroid cancer subtypes.
Numerous subsequent studies didn’t confirm this relationship. The LEADER trial looked at liraglutide in patients with type 2 diabetes and showed no effect of GLP-1 receptor activation on human serum calcitonin levels, C-cell proliferation, or C-cell malignancy. Similarly, a large meta-analysis in patients with type 2 diabetes didn’t find a statistically increased risk for thyroid cancer with liraglutide, and no thyroid malignancies were reported with exenatide.
Two U.S. administrative databases from commercial health plans (a retrospective cohort study and a nested case-control study) compared type 2 diabetes patients who were taking exenatide vs. other antidiabetic drugs and found that exenatide was not significantly associated with an increased risk for thyroid cancer.
And a recent meta-analysis of 45 trials showed no significant effects on the occurrence of thyroid cancer with GLP-1 receptor agonists. Of note, it did find an increased risk for overall thyroid disorders, although there was no clear statistically significant finding pointing to a specific thyroid disorder.
Differing from prior studies, a recent nationwide French health care system study provided newer data suggesting a moderate increased risk for thyroid cancer in a cohort of patients with type 2 diabetes who were taking GLP-1 agonists. The increase in relative risk was noted for all types of thyroid cancer in patients using GLP-1 receptor agonists for 1-3 years.
An accompanying commentary by Caroline A. Thompson, PhD, and Til Stürmer, MD, provides perspective on this study’s potential limitations. These include detection bias, as the study results focused only on the statistically significant data. Also discussed were limitations to the case-control design, issues with claims-based tumor type classification (unavailability of surgical pathology), and an inability to adjust for family history and obesity, which is a risk factor alone for thyroid cancer. There was also no adjustment for exposure to head/neck radiation.
While this study has important findings to consider, it deserves further investigation, with future studies linking data to tumor registry data before a change is made in clinical practice.
No clear relationship has been drawn between GLP-1 receptor agonists and thyroid cancer in humans. Numerous confounding factors limit the data. Studies generally don’t specify the type of thyroid cancer, and they lump medullary thyroid cancer, the rarest form, with papillary thyroid cancer.
Is a detection bias present where weight loss makes nodules more visible on the neck among those treated with GLP-1 agonists? And/or are patients treated with GLP-1 agonists being screened more stringently for thyroid nodules and/or cancer?
How to advise our patients and respond to the EMR messages
The TikTok videos may continue, the celebrity chatter may increase, and we, as physicians, will continue to look to real-world data with randomized controlled trials to tailor our decision-making and guide our patients.
Thyroid cancer remains a rare outcome, and GLP-1 receptor agonists remain a very important and beneficial treatment option for the right patient.
A version of this article first appeared on Medscape.com.
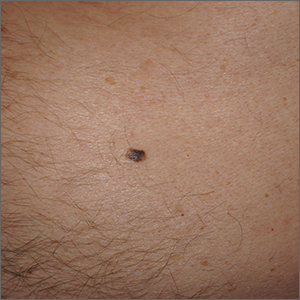
Solitary abdominal papule
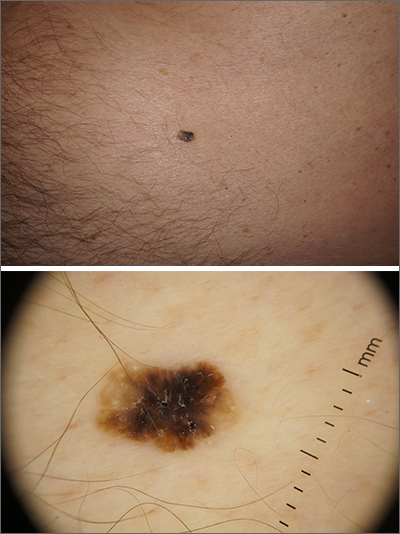
Dermoscopy revealed an 8-mm scaly brown-black papule that lacked melanocytic features (pigment network, globules, streaks, or homogeneous blue or brown color) but had milia-like cysts and so-called “fat fingers” (short, straight to curved radial projections1). These findings were consistent with a diagnosis of seborrheic keratosis (SK).
SKs go by many names and are often confused with nevi. Some patients might know them by such names as “age spots” or “liver spots.” Patients often have many SKs on their body; the back and skin folds are common locations. Patients may be unhappy about the way they look and may describe occasional discomfort when the SKs rub against clothes and inflammation that occurs spontaneously or with trauma.
Classic SKs have a well-demarcated border and waxy, stuck-on appearance. There are times when it is difficult to distinguish between an SK and a melanocytic lesion. Thus, a biopsy may be necessary. In addition, SKs are so common that collision lesions may occur. (Collision lesions result when 2 histologically distinct neoplasms occur adjacent to each other and cause an unusual clinical appearance with features of each lesion.) The atypical clinical features in a collision lesion may prompt a biopsy to exclude malignancy.
Dermoscopic features of SKs include well-demarcated borders, milia-like cysts (white circular inclusions), comedo-like openings (brown/black circular inclusions), fissures and ridges, hairpin vessels, and fat fingers.
Cryotherapy is a quick and efficient treatment when a patient would like the lesions removed. Curettage or light electrodessication may be less likely to cause post-inflammatory hypopigmentation in patients with darker skin types. These various destructive therapies are often considered cosmetic and are unlikely to be covered by insurance unless there is documentation of significant inflammation or discomfort. In this case, the lesion was not treated.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Wang S, Rabinovitz H, Oliviero M, et al. Solar lentigines, seborrheic keratoses, and lichen planus-like keratoses. In: Marghoob A, Malvehy J, Braun, R, eds. Atlas of Dermoscopy. 2nd ed. Informa Healthcare; 2012: 58-69.

Dermoscopy revealed an 8-mm scaly brown-black papule that lacked melanocytic features (pigment network, globules, streaks, or homogeneous blue or brown color) but had milia-like cysts and so-called “fat fingers” (short, straight to curved radial projections1). These findings were consistent with a diagnosis of seborrheic keratosis (SK).
SKs go by many names and are often confused with nevi. Some patients might know them by such names as “age spots” or “liver spots.” Patients often have many SKs on their body; the back and skin folds are common locations. Patients may be unhappy about the way they look and may describe occasional discomfort when the SKs rub against clothes and inflammation that occurs spontaneously or with trauma.
Classic SKs have a well-demarcated border and waxy, stuck-on appearance. There are times when it is difficult to distinguish between an SK and a melanocytic lesion. Thus, a biopsy may be necessary. In addition, SKs are so common that collision lesions may occur. (Collision lesions result when 2 histologically distinct neoplasms occur adjacent to each other and cause an unusual clinical appearance with features of each lesion.) The atypical clinical features in a collision lesion may prompt a biopsy to exclude malignancy.
Dermoscopic features of SKs include well-demarcated borders, milia-like cysts (white circular inclusions), comedo-like openings (brown/black circular inclusions), fissures and ridges, hairpin vessels, and fat fingers.
Cryotherapy is a quick and efficient treatment when a patient would like the lesions removed. Curettage or light electrodessication may be less likely to cause post-inflammatory hypopigmentation in patients with darker skin types. These various destructive therapies are often considered cosmetic and are unlikely to be covered by insurance unless there is documentation of significant inflammation or discomfort. In this case, the lesion was not treated.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Dermoscopy revealed an 8-mm scaly brown-black papule that lacked melanocytic features (pigment network, globules, streaks, or homogeneous blue or brown color) but had milia-like cysts and so-called “fat fingers” (short, straight to curved radial projections1). These findings were consistent with a diagnosis of seborrheic keratosis (SK).
SKs go by many names and are often confused with nevi. Some patients might know them by such names as “age spots” or “liver spots.” Patients often have many SKs on their body; the back and skin folds are common locations. Patients may be unhappy about the way they look and may describe occasional discomfort when the SKs rub against clothes and inflammation that occurs spontaneously or with trauma.
Classic SKs have a well-demarcated border and waxy, stuck-on appearance. There are times when it is difficult to distinguish between an SK and a melanocytic lesion. Thus, a biopsy may be necessary. In addition, SKs are so common that collision lesions may occur. (Collision lesions result when 2 histologically distinct neoplasms occur adjacent to each other and cause an unusual clinical appearance with features of each lesion.) The atypical clinical features in a collision lesion may prompt a biopsy to exclude malignancy.
Dermoscopic features of SKs include well-demarcated borders, milia-like cysts (white circular inclusions), comedo-like openings (brown/black circular inclusions), fissures and ridges, hairpin vessels, and fat fingers.
Cryotherapy is a quick and efficient treatment when a patient would like the lesions removed. Curettage or light electrodessication may be less likely to cause post-inflammatory hypopigmentation in patients with darker skin types. These various destructive therapies are often considered cosmetic and are unlikely to be covered by insurance unless there is documentation of significant inflammation or discomfort. In this case, the lesion was not treated.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Wang S, Rabinovitz H, Oliviero M, et al. Solar lentigines, seborrheic keratoses, and lichen planus-like keratoses. In: Marghoob A, Malvehy J, Braun, R, eds. Atlas of Dermoscopy. 2nd ed. Informa Healthcare; 2012: 58-69.
1. Wang S, Rabinovitz H, Oliviero M, et al. Solar lentigines, seborrheic keratoses, and lichen planus-like keratoses. In: Marghoob A, Malvehy J, Braun, R, eds. Atlas of Dermoscopy. 2nd ed. Informa Healthcare; 2012: 58-69.
High caffeine levels may lower body fat, type 2 diabetes risks
the results of a new study suggest.
Explaining that caffeine has thermogenic effects, the researchers note that previous short-term studies have linked caffeine intake with reductions in weight and fat mass. And observational data have shown associations between coffee consumption and lower risks of type 2 diabetes and cardiovascular disease.
In an effort to isolate the effects of caffeine from those of other food and drink components, Susanna C. Larsson, PhD, of the Karolinska Institute, Stockholm, and colleagues used data from studies of mainly European populations to examine two specific genetic mutations that have been linked to a slower speed of caffeine metabolism.
The two gene variants resulted in “genetically predicted, lifelong, higher plasma caffeine concentrations,” the researchers note “and were associated with lower body mass index and fat mass, as well as a lower risk of type 2 diabetes.”
Approximately half of the effect of caffeine on type 2 diabetes was estimated to be mediated through body mass index (BMI) reduction.
The work was published online March 14 in BMJ Medicine.
“This publication supports existing studies suggesting a link between caffeine consumption and increased fat burn,” notes Stephen Lawrence, MBChB, Warwick (England) University. “The big leap of faith that the authors have made is to assume that the weight loss brought about by increased caffeine consumption is sufficient to reduce the risk of developing type 2 diabetes,” he told the UK Science Media Centre.
“It does not, however, prove cause and effect.”
The researchers agree, noting: “Further clinical study is warranted to investigate the translational potential of these findings towards reducing the burden of metabolic disease.”
Katarina Kos, MD, PhD, a senior lecturer in diabetes and obesity at the University of Exeter (England), emphasized that this genetic study “shows links and potential health benefits for people with certain genes attributed to a faster [caffeine] metabolism as a hereditary trait and potentially a better metabolism.”
“It does not study or recommend drinking more coffee, which was not the purpose of this research,” she told the UK Science Media Centre.
Using Mendelian randomization, Dr. Larsson and colleagues examined data that came from a genomewide association meta-analysis of 9,876 individuals of European ancestry from six population-based studies.
Genetically predicted higher plasma caffeine concentrations in those carrying the two gene variants were associated with a lower BMI, with one standard deviation increase in predicted plasma caffeine equaling about 4.8 kg/m2 in BMI (P < .001).
For whole-body fat mass, one standard deviation increase in plasma caffeine equaled a reduction of about 9.5 kg (P < .001). However, there was no significant association with fat-free body mass (P = .17).
Genetically predicted higher plasma caffeine concentrations were also associated with a lower risk for type 2 diabetes in the FinnGen study (odds ratio, 0.77 per standard deviation increase; P < .001) and the DIAMANTE consortia (0.84, P < .001).
Combined, the odds ratio of type 2 diabetes per standard deviation of plasma caffeine increase was 0.81 (P < .001).
Dr. Larsson and colleagues calculated that approximately 43% of the protective effect of plasma caffeine on type 2 diabetes was mediated through BMI.
They did not find any strong associations between genetically predicted plasma caffeine concentrations and risk of any of the studied cardiovascular disease outcomes (ischemic heart disease, atrial fibrillation, heart failure, and stroke).
The thermogenic response to caffeine has been previously quantified as an approximate 100 kcal increase in energy expenditure per 100 mg daily caffeine intake, an amount that could result in reduced obesity risk. Another possible mechanism is enhanced satiety and suppressed energy intake with higher caffeine levels, the researchers say.
“Long-term clinical studies investigating the effect of caffeine intake on fat mass and type 2 diabetes risk are warranted,” they note. “Randomized controlled trials are warranted to assess whether noncaloric caffeine-containing beverages might play a role in reducing the risk of obesity and type 2 diabetes.”
The study was supported by the Swedish Research Council for Health, Working Life and Welfare, Swedish Heart Lung Foundation, and Swedish Research Council. Dr. Larsson, Dr. Lawrence, and Dr. Kos have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
the results of a new study suggest.
Explaining that caffeine has thermogenic effects, the researchers note that previous short-term studies have linked caffeine intake with reductions in weight and fat mass. And observational data have shown associations between coffee consumption and lower risks of type 2 diabetes and cardiovascular disease.
In an effort to isolate the effects of caffeine from those of other food and drink components, Susanna C. Larsson, PhD, of the Karolinska Institute, Stockholm, and colleagues used data from studies of mainly European populations to examine two specific genetic mutations that have been linked to a slower speed of caffeine metabolism.
The two gene variants resulted in “genetically predicted, lifelong, higher plasma caffeine concentrations,” the researchers note “and were associated with lower body mass index and fat mass, as well as a lower risk of type 2 diabetes.”
Approximately half of the effect of caffeine on type 2 diabetes was estimated to be mediated through body mass index (BMI) reduction.
The work was published online March 14 in BMJ Medicine.
“This publication supports existing studies suggesting a link between caffeine consumption and increased fat burn,” notes Stephen Lawrence, MBChB, Warwick (England) University. “The big leap of faith that the authors have made is to assume that the weight loss brought about by increased caffeine consumption is sufficient to reduce the risk of developing type 2 diabetes,” he told the UK Science Media Centre.
“It does not, however, prove cause and effect.”
The researchers agree, noting: “Further clinical study is warranted to investigate the translational potential of these findings towards reducing the burden of metabolic disease.”
Katarina Kos, MD, PhD, a senior lecturer in diabetes and obesity at the University of Exeter (England), emphasized that this genetic study “shows links and potential health benefits for people with certain genes attributed to a faster [caffeine] metabolism as a hereditary trait and potentially a better metabolism.”
“It does not study or recommend drinking more coffee, which was not the purpose of this research,” she told the UK Science Media Centre.
Using Mendelian randomization, Dr. Larsson and colleagues examined data that came from a genomewide association meta-analysis of 9,876 individuals of European ancestry from six population-based studies.
Genetically predicted higher plasma caffeine concentrations in those carrying the two gene variants were associated with a lower BMI, with one standard deviation increase in predicted plasma caffeine equaling about 4.8 kg/m2 in BMI (P < .001).
For whole-body fat mass, one standard deviation increase in plasma caffeine equaled a reduction of about 9.5 kg (P < .001). However, there was no significant association with fat-free body mass (P = .17).
Genetically predicted higher plasma caffeine concentrations were also associated with a lower risk for type 2 diabetes in the FinnGen study (odds ratio, 0.77 per standard deviation increase; P < .001) and the DIAMANTE consortia (0.84, P < .001).
Combined, the odds ratio of type 2 diabetes per standard deviation of plasma caffeine increase was 0.81 (P < .001).
Dr. Larsson and colleagues calculated that approximately 43% of the protective effect of plasma caffeine on type 2 diabetes was mediated through BMI.
They did not find any strong associations between genetically predicted plasma caffeine concentrations and risk of any of the studied cardiovascular disease outcomes (ischemic heart disease, atrial fibrillation, heart failure, and stroke).
The thermogenic response to caffeine has been previously quantified as an approximate 100 kcal increase in energy expenditure per 100 mg daily caffeine intake, an amount that could result in reduced obesity risk. Another possible mechanism is enhanced satiety and suppressed energy intake with higher caffeine levels, the researchers say.
“Long-term clinical studies investigating the effect of caffeine intake on fat mass and type 2 diabetes risk are warranted,” they note. “Randomized controlled trials are warranted to assess whether noncaloric caffeine-containing beverages might play a role in reducing the risk of obesity and type 2 diabetes.”
The study was supported by the Swedish Research Council for Health, Working Life and Welfare, Swedish Heart Lung Foundation, and Swedish Research Council. Dr. Larsson, Dr. Lawrence, and Dr. Kos have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
the results of a new study suggest.
Explaining that caffeine has thermogenic effects, the researchers note that previous short-term studies have linked caffeine intake with reductions in weight and fat mass. And observational data have shown associations between coffee consumption and lower risks of type 2 diabetes and cardiovascular disease.
In an effort to isolate the effects of caffeine from those of other food and drink components, Susanna C. Larsson, PhD, of the Karolinska Institute, Stockholm, and colleagues used data from studies of mainly European populations to examine two specific genetic mutations that have been linked to a slower speed of caffeine metabolism.
The two gene variants resulted in “genetically predicted, lifelong, higher plasma caffeine concentrations,” the researchers note “and were associated with lower body mass index and fat mass, as well as a lower risk of type 2 diabetes.”
Approximately half of the effect of caffeine on type 2 diabetes was estimated to be mediated through body mass index (BMI) reduction.
The work was published online March 14 in BMJ Medicine.
“This publication supports existing studies suggesting a link between caffeine consumption and increased fat burn,” notes Stephen Lawrence, MBChB, Warwick (England) University. “The big leap of faith that the authors have made is to assume that the weight loss brought about by increased caffeine consumption is sufficient to reduce the risk of developing type 2 diabetes,” he told the UK Science Media Centre.
“It does not, however, prove cause and effect.”
The researchers agree, noting: “Further clinical study is warranted to investigate the translational potential of these findings towards reducing the burden of metabolic disease.”
Katarina Kos, MD, PhD, a senior lecturer in diabetes and obesity at the University of Exeter (England), emphasized that this genetic study “shows links and potential health benefits for people with certain genes attributed to a faster [caffeine] metabolism as a hereditary trait and potentially a better metabolism.”
“It does not study or recommend drinking more coffee, which was not the purpose of this research,” she told the UK Science Media Centre.
Using Mendelian randomization, Dr. Larsson and colleagues examined data that came from a genomewide association meta-analysis of 9,876 individuals of European ancestry from six population-based studies.
Genetically predicted higher plasma caffeine concentrations in those carrying the two gene variants were associated with a lower BMI, with one standard deviation increase in predicted plasma caffeine equaling about 4.8 kg/m2 in BMI (P < .001).
For whole-body fat mass, one standard deviation increase in plasma caffeine equaled a reduction of about 9.5 kg (P < .001). However, there was no significant association with fat-free body mass (P = .17).
Genetically predicted higher plasma caffeine concentrations were also associated with a lower risk for type 2 diabetes in the FinnGen study (odds ratio, 0.77 per standard deviation increase; P < .001) and the DIAMANTE consortia (0.84, P < .001).
Combined, the odds ratio of type 2 diabetes per standard deviation of plasma caffeine increase was 0.81 (P < .001).
Dr. Larsson and colleagues calculated that approximately 43% of the protective effect of plasma caffeine on type 2 diabetes was mediated through BMI.
They did not find any strong associations between genetically predicted plasma caffeine concentrations and risk of any of the studied cardiovascular disease outcomes (ischemic heart disease, atrial fibrillation, heart failure, and stroke).
The thermogenic response to caffeine has been previously quantified as an approximate 100 kcal increase in energy expenditure per 100 mg daily caffeine intake, an amount that could result in reduced obesity risk. Another possible mechanism is enhanced satiety and suppressed energy intake with higher caffeine levels, the researchers say.
“Long-term clinical studies investigating the effect of caffeine intake on fat mass and type 2 diabetes risk are warranted,” they note. “Randomized controlled trials are warranted to assess whether noncaloric caffeine-containing beverages might play a role in reducing the risk of obesity and type 2 diabetes.”
The study was supported by the Swedish Research Council for Health, Working Life and Welfare, Swedish Heart Lung Foundation, and Swedish Research Council. Dr. Larsson, Dr. Lawrence, and Dr. Kos have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ MEDICINE
Pandemic hit Black children harder, study shows
Black children had almost three times as many COVID-related deaths as White children and about twice as many hospitalizations, according to a new study.
The study said that 1,556 children have died from the start of the pandemic until Nov. 30, 2022, with 593 of those children being 4 and under. Black children died of COVID-related causes 2.7 times more often than White children and were hospitalized 2.2 times more often than White children, the study said.
Lower vaccination rates for Black people may be a factor. The study said 43.6% of White children have received two or more vaccinations, compared with 40.2% of Black children.
“First and foremost, this study repudiates the misunderstanding that COVID-19 has not been of consequence to children who have had more than 15.5 million reported cases, representing 18 percent of all cases in the United States,” Reed Tuckson, MD, a member of the Black Coalition Against COVID board of directors and former District of Columbia public health commissioner, said in a news release.
“And second, our research shows that like their adult counterparts, Black and other children of color have shouldered more of the burden of COVID-19 than the White population.”
The study was commissioned by BCAC and conducted by the Satcher Health Leadership Institute of the Morehouse School of Medicine, Atlanta. It’s based on studies conducted by other agencies over 2 years.
Black and Hispanic children also had more severe COVID cases, the study said. Among 281 pediatric patients in New York, New Jersey, and Connecticut, 23.3% of severe cases were Black and 51% of severe cases were Hispanic.
The study says 1 in 310 Black children lost a parent or caregiver to COVID between April 2020 and June 2012, compared with 1 in 738 White children.
Economic and health-related hardships were experienced by 31% of Black households, 29% of Latino households, and 16% of White households, the study said.
“Children with COVID-19 in communities of color were sicker, [were] hospitalized and died at higher rates than White children,” Sandra Harris-Hooker, the interim executive director at the Satcher Health Leadership Institute of Morehouse School, said in the release. “We can now fully understand the devastating impact the virus had on communities of color across generations.”
The study recommends several changes, such as modifying eligibility requirements for the Children’s Health Insurance Program to help more children who fall into coverage gaps and expanding the Child Tax Credit.
A version of this article first appeared on WebMD.com.
Black children had almost three times as many COVID-related deaths as White children and about twice as many hospitalizations, according to a new study.
The study said that 1,556 children have died from the start of the pandemic until Nov. 30, 2022, with 593 of those children being 4 and under. Black children died of COVID-related causes 2.7 times more often than White children and were hospitalized 2.2 times more often than White children, the study said.
Lower vaccination rates for Black people may be a factor. The study said 43.6% of White children have received two or more vaccinations, compared with 40.2% of Black children.
“First and foremost, this study repudiates the misunderstanding that COVID-19 has not been of consequence to children who have had more than 15.5 million reported cases, representing 18 percent of all cases in the United States,” Reed Tuckson, MD, a member of the Black Coalition Against COVID board of directors and former District of Columbia public health commissioner, said in a news release.
“And second, our research shows that like their adult counterparts, Black and other children of color have shouldered more of the burden of COVID-19 than the White population.”
The study was commissioned by BCAC and conducted by the Satcher Health Leadership Institute of the Morehouse School of Medicine, Atlanta. It’s based on studies conducted by other agencies over 2 years.
Black and Hispanic children also had more severe COVID cases, the study said. Among 281 pediatric patients in New York, New Jersey, and Connecticut, 23.3% of severe cases were Black and 51% of severe cases were Hispanic.
The study says 1 in 310 Black children lost a parent or caregiver to COVID between April 2020 and June 2012, compared with 1 in 738 White children.
Economic and health-related hardships were experienced by 31% of Black households, 29% of Latino households, and 16% of White households, the study said.
“Children with COVID-19 in communities of color were sicker, [were] hospitalized and died at higher rates than White children,” Sandra Harris-Hooker, the interim executive director at the Satcher Health Leadership Institute of Morehouse School, said in the release. “We can now fully understand the devastating impact the virus had on communities of color across generations.”
The study recommends several changes, such as modifying eligibility requirements for the Children’s Health Insurance Program to help more children who fall into coverage gaps and expanding the Child Tax Credit.
A version of this article first appeared on WebMD.com.
Black children had almost three times as many COVID-related deaths as White children and about twice as many hospitalizations, according to a new study.
The study said that 1,556 children have died from the start of the pandemic until Nov. 30, 2022, with 593 of those children being 4 and under. Black children died of COVID-related causes 2.7 times more often than White children and were hospitalized 2.2 times more often than White children, the study said.
Lower vaccination rates for Black people may be a factor. The study said 43.6% of White children have received two or more vaccinations, compared with 40.2% of Black children.
“First and foremost, this study repudiates the misunderstanding that COVID-19 has not been of consequence to children who have had more than 15.5 million reported cases, representing 18 percent of all cases in the United States,” Reed Tuckson, MD, a member of the Black Coalition Against COVID board of directors and former District of Columbia public health commissioner, said in a news release.
“And second, our research shows that like their adult counterparts, Black and other children of color have shouldered more of the burden of COVID-19 than the White population.”
The study was commissioned by BCAC and conducted by the Satcher Health Leadership Institute of the Morehouse School of Medicine, Atlanta. It’s based on studies conducted by other agencies over 2 years.
Black and Hispanic children also had more severe COVID cases, the study said. Among 281 pediatric patients in New York, New Jersey, and Connecticut, 23.3% of severe cases were Black and 51% of severe cases were Hispanic.
The study says 1 in 310 Black children lost a parent or caregiver to COVID between April 2020 and June 2012, compared with 1 in 738 White children.
Economic and health-related hardships were experienced by 31% of Black households, 29% of Latino households, and 16% of White households, the study said.
“Children with COVID-19 in communities of color were sicker, [were] hospitalized and died at higher rates than White children,” Sandra Harris-Hooker, the interim executive director at the Satcher Health Leadership Institute of Morehouse School, said in the release. “We can now fully understand the devastating impact the virus had on communities of color across generations.”
The study recommends several changes, such as modifying eligibility requirements for the Children’s Health Insurance Program to help more children who fall into coverage gaps and expanding the Child Tax Credit.
A version of this article first appeared on WebMD.com.
Children and COVID: A look back as the fourth year begins
With 3 years of the COVID-19 experience now past, it’s safe to say that SARS-CoV-2 changed American society in ways that could not have been predicted when the first U.S. cases were reported in January of 2020.
Who would have guessed back then that not one but two vaccines would be developed, approved, and widely distributed before the end of the year? Or that those vaccines would be rejected by large segments of the population on ideological grounds? Could anyone have predicted in early 2020 that schools in 21 states would be forbidden by law to require COVID-19 vaccination in students?
Vaccination is generally considered to be an activity of childhood, but that practice has been turned upside down with COVID-19. Among Americans aged 65 years and older, 95% have received at least one dose of vaccine, versus 27.9% of children younger than 12 years old, according to the Centers for Disease Control and Prevention.
The vaccine situation for children mirrors that of the population as a whole. The oldest children have the highest vaccination rates, and the rates decline along with age: 72.0% of those aged 12-17 years have received at least one dose, compared with 39.8% of 5- to 11-year-olds, 10.5% of 2- to 4-year-olds, and 8.0% of children under age 2, the CDC said on its COVID Data Tracker.
The youngest children were, of course, the last ones to be eligible for the vaccine, but their uptake has been much slower since emergency use was authorized in June of 2022. In the nearly 9 months since then, 9.5% of children aged 4 and under have received at least one dose, versus 66% of children aged 12-15 years in the first 9 months (May 2021 to March 2022).
Altogether, a total of 31.7 million, or 43%, of all children under age 18 had received at least one dose of COVID-19 vaccine as of March 8, 2023, according to the most recent CDC data.
Incidence: Counting COVID
Vaccination and other prevention efforts have tried to stem the tide, but what has COVID actually done to children since the Trump administration declared a nationwide emergency on March 13, 2020?
- 16.6 million cases.
- 186,035 new hospital admissions.
- 2,122 deaths.
Even the proportion of total COVID cases in children, 17.2%, is less than might be expected, given their relatively undervaccinated status.
Seroprevalence estimates seem to support the undercounting of pediatric cases. A survey of commercial laboratories working with the CDC put the seroprevalance of SARS-CoV-2 antibodies in children at 96.3% as of late 2022, based on tests of almost 27,000 specimens performed over an 8-week period from mid-October to mid-December. That would put the number of infected children at 65.7 million children.
Since Omicron
There has not been another major COVID-19 surge since the winter of 2021-2022, when the weekly rate of new cases reached 1,900 per 100,000 population in children aged 16-17 years in early January 2022 – the highest seen among children of any of the CDC’s age groups (0-4, 5-11, 12-15, 16-17) during the entire pandemic. Since the Omicron surge, the highest weekly rate was 221 per 100,000 during the week of May 15-21, again in 16- to 17-year-olds, the CDC reports.
The widely anticipated surge of COVID in the fall and winter of 2022 and 2023 – the so-called “tripledemic” involving influenza and respiratory syncytial virus – did not occur, possibly because so many Americans were vaccinated or previously infected, experts suggested. New-case rates, emergency room visits, and hospitalizations in children have continued to drop as winter comes to a close, CDC data show.
With 3 years of the COVID-19 experience now past, it’s safe to say that SARS-CoV-2 changed American society in ways that could not have been predicted when the first U.S. cases were reported in January of 2020.
Who would have guessed back then that not one but two vaccines would be developed, approved, and widely distributed before the end of the year? Or that those vaccines would be rejected by large segments of the population on ideological grounds? Could anyone have predicted in early 2020 that schools in 21 states would be forbidden by law to require COVID-19 vaccination in students?
Vaccination is generally considered to be an activity of childhood, but that practice has been turned upside down with COVID-19. Among Americans aged 65 years and older, 95% have received at least one dose of vaccine, versus 27.9% of children younger than 12 years old, according to the Centers for Disease Control and Prevention.
The vaccine situation for children mirrors that of the population as a whole. The oldest children have the highest vaccination rates, and the rates decline along with age: 72.0% of those aged 12-17 years have received at least one dose, compared with 39.8% of 5- to 11-year-olds, 10.5% of 2- to 4-year-olds, and 8.0% of children under age 2, the CDC said on its COVID Data Tracker.
The youngest children were, of course, the last ones to be eligible for the vaccine, but their uptake has been much slower since emergency use was authorized in June of 2022. In the nearly 9 months since then, 9.5% of children aged 4 and under have received at least one dose, versus 66% of children aged 12-15 years in the first 9 months (May 2021 to March 2022).
Altogether, a total of 31.7 million, or 43%, of all children under age 18 had received at least one dose of COVID-19 vaccine as of March 8, 2023, according to the most recent CDC data.
Incidence: Counting COVID
Vaccination and other prevention efforts have tried to stem the tide, but what has COVID actually done to children since the Trump administration declared a nationwide emergency on March 13, 2020?
- 16.6 million cases.
- 186,035 new hospital admissions.
- 2,122 deaths.
Even the proportion of total COVID cases in children, 17.2%, is less than might be expected, given their relatively undervaccinated status.
Seroprevalence estimates seem to support the undercounting of pediatric cases. A survey of commercial laboratories working with the CDC put the seroprevalance of SARS-CoV-2 antibodies in children at 96.3% as of late 2022, based on tests of almost 27,000 specimens performed over an 8-week period from mid-October to mid-December. That would put the number of infected children at 65.7 million children.
Since Omicron
There has not been another major COVID-19 surge since the winter of 2021-2022, when the weekly rate of new cases reached 1,900 per 100,000 population in children aged 16-17 years in early January 2022 – the highest seen among children of any of the CDC’s age groups (0-4, 5-11, 12-15, 16-17) during the entire pandemic. Since the Omicron surge, the highest weekly rate was 221 per 100,000 during the week of May 15-21, again in 16- to 17-year-olds, the CDC reports.
The widely anticipated surge of COVID in the fall and winter of 2022 and 2023 – the so-called “tripledemic” involving influenza and respiratory syncytial virus – did not occur, possibly because so many Americans were vaccinated or previously infected, experts suggested. New-case rates, emergency room visits, and hospitalizations in children have continued to drop as winter comes to a close, CDC data show.
With 3 years of the COVID-19 experience now past, it’s safe to say that SARS-CoV-2 changed American society in ways that could not have been predicted when the first U.S. cases were reported in January of 2020.
Who would have guessed back then that not one but two vaccines would be developed, approved, and widely distributed before the end of the year? Or that those vaccines would be rejected by large segments of the population on ideological grounds? Could anyone have predicted in early 2020 that schools in 21 states would be forbidden by law to require COVID-19 vaccination in students?
Vaccination is generally considered to be an activity of childhood, but that practice has been turned upside down with COVID-19. Among Americans aged 65 years and older, 95% have received at least one dose of vaccine, versus 27.9% of children younger than 12 years old, according to the Centers for Disease Control and Prevention.
The vaccine situation for children mirrors that of the population as a whole. The oldest children have the highest vaccination rates, and the rates decline along with age: 72.0% of those aged 12-17 years have received at least one dose, compared with 39.8% of 5- to 11-year-olds, 10.5% of 2- to 4-year-olds, and 8.0% of children under age 2, the CDC said on its COVID Data Tracker.
The youngest children were, of course, the last ones to be eligible for the vaccine, but their uptake has been much slower since emergency use was authorized in June of 2022. In the nearly 9 months since then, 9.5% of children aged 4 and under have received at least one dose, versus 66% of children aged 12-15 years in the first 9 months (May 2021 to March 2022).
Altogether, a total of 31.7 million, or 43%, of all children under age 18 had received at least one dose of COVID-19 vaccine as of March 8, 2023, according to the most recent CDC data.
Incidence: Counting COVID
Vaccination and other prevention efforts have tried to stem the tide, but what has COVID actually done to children since the Trump administration declared a nationwide emergency on March 13, 2020?
- 16.6 million cases.
- 186,035 new hospital admissions.
- 2,122 deaths.
Even the proportion of total COVID cases in children, 17.2%, is less than might be expected, given their relatively undervaccinated status.
Seroprevalence estimates seem to support the undercounting of pediatric cases. A survey of commercial laboratories working with the CDC put the seroprevalance of SARS-CoV-2 antibodies in children at 96.3% as of late 2022, based on tests of almost 27,000 specimens performed over an 8-week period from mid-October to mid-December. That would put the number of infected children at 65.7 million children.
Since Omicron
There has not been another major COVID-19 surge since the winter of 2021-2022, when the weekly rate of new cases reached 1,900 per 100,000 population in children aged 16-17 years in early January 2022 – the highest seen among children of any of the CDC’s age groups (0-4, 5-11, 12-15, 16-17) during the entire pandemic. Since the Omicron surge, the highest weekly rate was 221 per 100,000 during the week of May 15-21, again in 16- to 17-year-olds, the CDC reports.
The widely anticipated surge of COVID in the fall and winter of 2022 and 2023 – the so-called “tripledemic” involving influenza and respiratory syncytial virus – did not occur, possibly because so many Americans were vaccinated or previously infected, experts suggested. New-case rates, emergency room visits, and hospitalizations in children have continued to drop as winter comes to a close, CDC data show.
Will new guidelines widen the gap in treating childhood obesity?
In the United States, the Centers for Disease Control and Prevention estimates that nearly one in five children have obesity. Since the 1980s, the number of children with obesity has been increasing, with each generation reaching higher rates and greater weights at earlier ages. Even with extensive efforts from parents, clinicians, educators, and policymakers to limit the excessive weight gain among children, the number of obesity and severe obesity diagnoses keeps rising.
In response to this critical public health challenge, the American Academy of Pediatrics (AAP) introduced new clinical practice guidelines for the evaluation and management of obesity in children and adolescents. Developed by an expert panel, the new AAP guidelines present a departure in the conceptualization of obesity, recognizing the role that social determinants of health play in contributing to excessive weight gain.
As a community health researcher who investigates disparities in childhood obesity, I applaud the paradigm shift from the AAP. I specifically endorse the recognition that obesity is a very serious metabolic disease that won’t go away unless we introduce systemic changes and effective treatments.
However, I, like so many of my colleagues and anyone aware of the access barriers to the recommended treatments, worry about the consequences that the new guidelines will have in the context of current and future health disparities.
A recent study, published in Pediatrics, showed that childhood obesity disparities are widening. Younger generations of children are reaching higher weights at younger ages. These alarming trends are greater among Black children and children growing up with the greatest socioeconomic disadvantages. The new AAP guidelines – even while driven by good intentions – can exacerbate these differences and set children who are able to live healthy lives further apart from those with disproportionate obesity risks, who lack access to the treatments recommended by the AAP.
Rather than “watchful waiting,” to see if children outgrow obesity, the new guidelines call for “aggressive treatment,” as reported by this news organization. At least 26 hours of in-person intensive health behavior and lifestyle counseling and treatment are recommended for children aged 2 years old or older who meet the obesity criteria. For children aged 12 years or older, the AAP recommends complementing lifestyle counseling with pharmacotherapy. This breakthrough welcomes the use of promising antiobesity medications (for example, orlistat, Wegovy [semaglutide], Saxenda [liraglutide], Qsymia (phentermine and topiramate]) approved by the Food and Drug Administration for long-term use in children aged 12 and up. For children 13 years or older with severe obesity, bariatric surgery should be considered.
Will cost barriers continue to increase disparity?
The very promising semaglutide (Wegovy) is a GLP-1–based medication currently offered for about $1,000 per month. As with other chronic diseases, children should be prepared to take obesity medications for prolonged periods of time. A study conducted in adults found that when the medication is suspended, any weight loss can be regained. The costs of bariatric surgery total over $20,000.
In the U.S. health care system, at current prices, very few of the children in need of the medications or surgical treatments have access to them. Most private health insurance companies and Medicaid reject coverage for childhood obesity treatments. Barriers to treatment access are greater for Black and Hispanic children, children growing up in poverty, and children living in the U.S. South region, all of whom are more likely to develop obesity earlier in life than their White and wealthier counterparts.
The AAP recognized that a substantial time and financial commitment is required to follow the new treatment recommendations. Members of the AAP Expert Committee that developed the guidelines stated that they are “aware of the multitude of barriers to treatment that patients and their families face.”
Nevertheless, the recognition of the role of social determinants of health in the development of childhood obesity didn’t motivate the introduction of treatment options that aren’t unattainable for most U.S. families.
It’s important to step away from the conclusion that because of the price tag, at the population level, the new AAP guidelines will be inconsequential. This conclusion fails to recognize the potential harm that the guidelines may introduce. In the context of childhood obesity disparities, the new treatment recommendations probably will widen the childhood obesity prevalence gap between the haves – who will benefit from the options available to reduce childhood obesity – and the have-nots, whose obesity rates will continue with their growth.
We live in a world of the haves and have-nots. This applies to financial resources as well as obesity rates. In the long term, the optimists hope that the GLP-1 medications will become ubiquitous, generics will be developed, and insurance companies will expand coverage and grant access to most children in need of effective obesity treatment options. Until this happens, unless intentional policies are promptly introduced, childhood obesity disparities will continue to widen.
To avoid the increasing disparities, brave and intentional actions are required. A lack of attention dealt to this known problem will result in a lost opportunity for the AAP, legislators, and others in a position to help U.S. children.
Liliana Aguayo, PhD, MPH, is assistant professor, Clinical Research Track, Hubert Department of Global Health, Emory University, Atlanta. A version of this article first appeared on Medscape.com.
In the United States, the Centers for Disease Control and Prevention estimates that nearly one in five children have obesity. Since the 1980s, the number of children with obesity has been increasing, with each generation reaching higher rates and greater weights at earlier ages. Even with extensive efforts from parents, clinicians, educators, and policymakers to limit the excessive weight gain among children, the number of obesity and severe obesity diagnoses keeps rising.
In response to this critical public health challenge, the American Academy of Pediatrics (AAP) introduced new clinical practice guidelines for the evaluation and management of obesity in children and adolescents. Developed by an expert panel, the new AAP guidelines present a departure in the conceptualization of obesity, recognizing the role that social determinants of health play in contributing to excessive weight gain.
As a community health researcher who investigates disparities in childhood obesity, I applaud the paradigm shift from the AAP. I specifically endorse the recognition that obesity is a very serious metabolic disease that won’t go away unless we introduce systemic changes and effective treatments.
However, I, like so many of my colleagues and anyone aware of the access barriers to the recommended treatments, worry about the consequences that the new guidelines will have in the context of current and future health disparities.
A recent study, published in Pediatrics, showed that childhood obesity disparities are widening. Younger generations of children are reaching higher weights at younger ages. These alarming trends are greater among Black children and children growing up with the greatest socioeconomic disadvantages. The new AAP guidelines – even while driven by good intentions – can exacerbate these differences and set children who are able to live healthy lives further apart from those with disproportionate obesity risks, who lack access to the treatments recommended by the AAP.
Rather than “watchful waiting,” to see if children outgrow obesity, the new guidelines call for “aggressive treatment,” as reported by this news organization. At least 26 hours of in-person intensive health behavior and lifestyle counseling and treatment are recommended for children aged 2 years old or older who meet the obesity criteria. For children aged 12 years or older, the AAP recommends complementing lifestyle counseling with pharmacotherapy. This breakthrough welcomes the use of promising antiobesity medications (for example, orlistat, Wegovy [semaglutide], Saxenda [liraglutide], Qsymia (phentermine and topiramate]) approved by the Food and Drug Administration for long-term use in children aged 12 and up. For children 13 years or older with severe obesity, bariatric surgery should be considered.
Will cost barriers continue to increase disparity?
The very promising semaglutide (Wegovy) is a GLP-1–based medication currently offered for about $1,000 per month. As with other chronic diseases, children should be prepared to take obesity medications for prolonged periods of time. A study conducted in adults found that when the medication is suspended, any weight loss can be regained. The costs of bariatric surgery total over $20,000.
In the U.S. health care system, at current prices, very few of the children in need of the medications or surgical treatments have access to them. Most private health insurance companies and Medicaid reject coverage for childhood obesity treatments. Barriers to treatment access are greater for Black and Hispanic children, children growing up in poverty, and children living in the U.S. South region, all of whom are more likely to develop obesity earlier in life than their White and wealthier counterparts.
The AAP recognized that a substantial time and financial commitment is required to follow the new treatment recommendations. Members of the AAP Expert Committee that developed the guidelines stated that they are “aware of the multitude of barriers to treatment that patients and their families face.”
Nevertheless, the recognition of the role of social determinants of health in the development of childhood obesity didn’t motivate the introduction of treatment options that aren’t unattainable for most U.S. families.
It’s important to step away from the conclusion that because of the price tag, at the population level, the new AAP guidelines will be inconsequential. This conclusion fails to recognize the potential harm that the guidelines may introduce. In the context of childhood obesity disparities, the new treatment recommendations probably will widen the childhood obesity prevalence gap between the haves – who will benefit from the options available to reduce childhood obesity – and the have-nots, whose obesity rates will continue with their growth.
We live in a world of the haves and have-nots. This applies to financial resources as well as obesity rates. In the long term, the optimists hope that the GLP-1 medications will become ubiquitous, generics will be developed, and insurance companies will expand coverage and grant access to most children in need of effective obesity treatment options. Until this happens, unless intentional policies are promptly introduced, childhood obesity disparities will continue to widen.
To avoid the increasing disparities, brave and intentional actions are required. A lack of attention dealt to this known problem will result in a lost opportunity for the AAP, legislators, and others in a position to help U.S. children.
Liliana Aguayo, PhD, MPH, is assistant professor, Clinical Research Track, Hubert Department of Global Health, Emory University, Atlanta. A version of this article first appeared on Medscape.com.
In the United States, the Centers for Disease Control and Prevention estimates that nearly one in five children have obesity. Since the 1980s, the number of children with obesity has been increasing, with each generation reaching higher rates and greater weights at earlier ages. Even with extensive efforts from parents, clinicians, educators, and policymakers to limit the excessive weight gain among children, the number of obesity and severe obesity diagnoses keeps rising.
In response to this critical public health challenge, the American Academy of Pediatrics (AAP) introduced new clinical practice guidelines for the evaluation and management of obesity in children and adolescents. Developed by an expert panel, the new AAP guidelines present a departure in the conceptualization of obesity, recognizing the role that social determinants of health play in contributing to excessive weight gain.
As a community health researcher who investigates disparities in childhood obesity, I applaud the paradigm shift from the AAP. I specifically endorse the recognition that obesity is a very serious metabolic disease that won’t go away unless we introduce systemic changes and effective treatments.
However, I, like so many of my colleagues and anyone aware of the access barriers to the recommended treatments, worry about the consequences that the new guidelines will have in the context of current and future health disparities.
A recent study, published in Pediatrics, showed that childhood obesity disparities are widening. Younger generations of children are reaching higher weights at younger ages. These alarming trends are greater among Black children and children growing up with the greatest socioeconomic disadvantages. The new AAP guidelines – even while driven by good intentions – can exacerbate these differences and set children who are able to live healthy lives further apart from those with disproportionate obesity risks, who lack access to the treatments recommended by the AAP.
Rather than “watchful waiting,” to see if children outgrow obesity, the new guidelines call for “aggressive treatment,” as reported by this news organization. At least 26 hours of in-person intensive health behavior and lifestyle counseling and treatment are recommended for children aged 2 years old or older who meet the obesity criteria. For children aged 12 years or older, the AAP recommends complementing lifestyle counseling with pharmacotherapy. This breakthrough welcomes the use of promising antiobesity medications (for example, orlistat, Wegovy [semaglutide], Saxenda [liraglutide], Qsymia (phentermine and topiramate]) approved by the Food and Drug Administration for long-term use in children aged 12 and up. For children 13 years or older with severe obesity, bariatric surgery should be considered.
Will cost barriers continue to increase disparity?
The very promising semaglutide (Wegovy) is a GLP-1–based medication currently offered for about $1,000 per month. As with other chronic diseases, children should be prepared to take obesity medications for prolonged periods of time. A study conducted in adults found that when the medication is suspended, any weight loss can be regained. The costs of bariatric surgery total over $20,000.
In the U.S. health care system, at current prices, very few of the children in need of the medications or surgical treatments have access to them. Most private health insurance companies and Medicaid reject coverage for childhood obesity treatments. Barriers to treatment access are greater for Black and Hispanic children, children growing up in poverty, and children living in the U.S. South region, all of whom are more likely to develop obesity earlier in life than their White and wealthier counterparts.
The AAP recognized that a substantial time and financial commitment is required to follow the new treatment recommendations. Members of the AAP Expert Committee that developed the guidelines stated that they are “aware of the multitude of barriers to treatment that patients and their families face.”
Nevertheless, the recognition of the role of social determinants of health in the development of childhood obesity didn’t motivate the introduction of treatment options that aren’t unattainable for most U.S. families.
It’s important to step away from the conclusion that because of the price tag, at the population level, the new AAP guidelines will be inconsequential. This conclusion fails to recognize the potential harm that the guidelines may introduce. In the context of childhood obesity disparities, the new treatment recommendations probably will widen the childhood obesity prevalence gap between the haves – who will benefit from the options available to reduce childhood obesity – and the have-nots, whose obesity rates will continue with their growth.
We live in a world of the haves and have-nots. This applies to financial resources as well as obesity rates. In the long term, the optimists hope that the GLP-1 medications will become ubiquitous, generics will be developed, and insurance companies will expand coverage and grant access to most children in need of effective obesity treatment options. Until this happens, unless intentional policies are promptly introduced, childhood obesity disparities will continue to widen.
To avoid the increasing disparities, brave and intentional actions are required. A lack of attention dealt to this known problem will result in a lost opportunity for the AAP, legislators, and others in a position to help U.S. children.
Liliana Aguayo, PhD, MPH, is assistant professor, Clinical Research Track, Hubert Department of Global Health, Emory University, Atlanta. A version of this article first appeared on Medscape.com.
Nearly one in three patients with IBD affected by skin lesions
People with inflammatory bowel disease (IBD) commonly develop skin lesions linked to their condition, but until now few researchers looked at how common they are.
, according to the prospective, single-center study.
“Skin lesions in IBD patients are much more prevalent than it is generally accepted. The lesions may be related to the pathogenesis of IBD, but it is very important to know that the modern biological therapies may also cause skin lesions,” said senior study author Laimas Jonaitis, MD, PhD, professor in the department of gastroenterology at Lithuanian University of Health Sciences in Kaunas.
“If the gastroenterologist is experienced and has enough competence, he or she may establish the diagnosis, but in all other cases it is wise and advisable to refer the patient to the dermatologist,” Dr. Jonaitis said. A referral should include the history and full treatment for IBD.
The results were presented as a poster at the annual congress of the European Crohn’s and Colitis Organisation, held in Copenhagen and virtually.
Dr. Jonaitis and colleagues conducted a literature analysis to determine the prevalence of extra-abdominal manifestations of IBD. The lack of published data prompted them to survey 152 consecutive patients with IBD receiving outpatient treatment at their institution. The patients completed questionnaires from January to October 2022 about any cutaneous lesions.
The mean age of patients was 42 years, and 58% were men. A majority, 72%, had ulcerative colitis, and 28% had Crohn’s disease.
Prevalence of skin lesions
A total of 43% of participants reported skin lesions, but only 30% of patients had lesions considered related to IBD or IBD therapy due to their emergence after the patient’s IBD diagnosis.
By IBD diagnosis, 29% of patients with ulcerative colitis and 33% of patients with Crohn’s disease had lesions related to their condition. The difference in skin lesion prevalence between the two groups was not significant (P > .05), the researchers noted.
The team further investigated the types of skin lesions deemed to be associated with IBD or IBD therapy.
Overall, they found psoriasis in nine patients, eczema in nine, erythema nodosum in six, pyoderma gangrenosum in five, allergic rash in four, and vitiligo in two. They found acne, epidermolysis bullosa acquisita, and hemorrhagic vasculitis in one patient each.
Specifically, among patients with ulcerative colitis, skin lesions were reported in 8 of 27 with left-sided colitis, 2 of 15 with ulcerative colitis proctitis, and 22 of 67 patients with pancolitis. The difference between the groups of proctitis and pancolitis was significant (P = .03).
Within the group with Crohn’s disease, skin lesions were reported in 3 of 15 patients with ileitis, 4 of 10 with colitis, and 7 of 17 with ileocolitis. The difference among these groups was not significant (P > .05).
The most common skin lesions observed in Crohn’s disease were erythema nodosum and eczema, and in ulcerative colitis, psoriasis and eczema, the researchers reported.
They also noted that the cutaneous lesions were significantly more prevalent in extensive ulcerative colitis compared with distal disease.
Skin lesions add to patient misery
“Skin lesions are considered a burden to patients with IBD and add to their suffering,” said Sara Mesilhy, MBBS, a gastroenterologist with the Royal College of Physicians in the United Kingdom, who was not affiliated with the research.
The severity and location of the disease appears to play a role because researchers found extensive ulcerative colitis may carry a higher risk for the development of skin lesions, Dr. Mesilhy noted.
The first step when facing skin lesions is to control the disease activity via the best treatment option, Dr. Mesilhy suggested.
The study was independently supported. Dr. Jonaitis and Dr. Mesilhy have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
People with inflammatory bowel disease (IBD) commonly develop skin lesions linked to their condition, but until now few researchers looked at how common they are.
, according to the prospective, single-center study.
“Skin lesions in IBD patients are much more prevalent than it is generally accepted. The lesions may be related to the pathogenesis of IBD, but it is very important to know that the modern biological therapies may also cause skin lesions,” said senior study author Laimas Jonaitis, MD, PhD, professor in the department of gastroenterology at Lithuanian University of Health Sciences in Kaunas.
“If the gastroenterologist is experienced and has enough competence, he or she may establish the diagnosis, but in all other cases it is wise and advisable to refer the patient to the dermatologist,” Dr. Jonaitis said. A referral should include the history and full treatment for IBD.
The results were presented as a poster at the annual congress of the European Crohn’s and Colitis Organisation, held in Copenhagen and virtually.
Dr. Jonaitis and colleagues conducted a literature analysis to determine the prevalence of extra-abdominal manifestations of IBD. The lack of published data prompted them to survey 152 consecutive patients with IBD receiving outpatient treatment at their institution. The patients completed questionnaires from January to October 2022 about any cutaneous lesions.
The mean age of patients was 42 years, and 58% were men. A majority, 72%, had ulcerative colitis, and 28% had Crohn’s disease.
Prevalence of skin lesions
A total of 43% of participants reported skin lesions, but only 30% of patients had lesions considered related to IBD or IBD therapy due to their emergence after the patient’s IBD diagnosis.
By IBD diagnosis, 29% of patients with ulcerative colitis and 33% of patients with Crohn’s disease had lesions related to their condition. The difference in skin lesion prevalence between the two groups was not significant (P > .05), the researchers noted.
The team further investigated the types of skin lesions deemed to be associated with IBD or IBD therapy.
Overall, they found psoriasis in nine patients, eczema in nine, erythema nodosum in six, pyoderma gangrenosum in five, allergic rash in four, and vitiligo in two. They found acne, epidermolysis bullosa acquisita, and hemorrhagic vasculitis in one patient each.
Specifically, among patients with ulcerative colitis, skin lesions were reported in 8 of 27 with left-sided colitis, 2 of 15 with ulcerative colitis proctitis, and 22 of 67 patients with pancolitis. The difference between the groups of proctitis and pancolitis was significant (P = .03).
Within the group with Crohn’s disease, skin lesions were reported in 3 of 15 patients with ileitis, 4 of 10 with colitis, and 7 of 17 with ileocolitis. The difference among these groups was not significant (P > .05).
The most common skin lesions observed in Crohn’s disease were erythema nodosum and eczema, and in ulcerative colitis, psoriasis and eczema, the researchers reported.
They also noted that the cutaneous lesions were significantly more prevalent in extensive ulcerative colitis compared with distal disease.
Skin lesions add to patient misery
“Skin lesions are considered a burden to patients with IBD and add to their suffering,” said Sara Mesilhy, MBBS, a gastroenterologist with the Royal College of Physicians in the United Kingdom, who was not affiliated with the research.
The severity and location of the disease appears to play a role because researchers found extensive ulcerative colitis may carry a higher risk for the development of skin lesions, Dr. Mesilhy noted.
The first step when facing skin lesions is to control the disease activity via the best treatment option, Dr. Mesilhy suggested.
The study was independently supported. Dr. Jonaitis and Dr. Mesilhy have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
People with inflammatory bowel disease (IBD) commonly develop skin lesions linked to their condition, but until now few researchers looked at how common they are.
, according to the prospective, single-center study.
“Skin lesions in IBD patients are much more prevalent than it is generally accepted. The lesions may be related to the pathogenesis of IBD, but it is very important to know that the modern biological therapies may also cause skin lesions,” said senior study author Laimas Jonaitis, MD, PhD, professor in the department of gastroenterology at Lithuanian University of Health Sciences in Kaunas.
“If the gastroenterologist is experienced and has enough competence, he or she may establish the diagnosis, but in all other cases it is wise and advisable to refer the patient to the dermatologist,” Dr. Jonaitis said. A referral should include the history and full treatment for IBD.
The results were presented as a poster at the annual congress of the European Crohn’s and Colitis Organisation, held in Copenhagen and virtually.
Dr. Jonaitis and colleagues conducted a literature analysis to determine the prevalence of extra-abdominal manifestations of IBD. The lack of published data prompted them to survey 152 consecutive patients with IBD receiving outpatient treatment at their institution. The patients completed questionnaires from January to October 2022 about any cutaneous lesions.
The mean age of patients was 42 years, and 58% were men. A majority, 72%, had ulcerative colitis, and 28% had Crohn’s disease.
Prevalence of skin lesions
A total of 43% of participants reported skin lesions, but only 30% of patients had lesions considered related to IBD or IBD therapy due to their emergence after the patient’s IBD diagnosis.
By IBD diagnosis, 29% of patients with ulcerative colitis and 33% of patients with Crohn’s disease had lesions related to their condition. The difference in skin lesion prevalence between the two groups was not significant (P > .05), the researchers noted.
The team further investigated the types of skin lesions deemed to be associated with IBD or IBD therapy.
Overall, they found psoriasis in nine patients, eczema in nine, erythema nodosum in six, pyoderma gangrenosum in five, allergic rash in four, and vitiligo in two. They found acne, epidermolysis bullosa acquisita, and hemorrhagic vasculitis in one patient each.
Specifically, among patients with ulcerative colitis, skin lesions were reported in 8 of 27 with left-sided colitis, 2 of 15 with ulcerative colitis proctitis, and 22 of 67 patients with pancolitis. The difference between the groups of proctitis and pancolitis was significant (P = .03).
Within the group with Crohn’s disease, skin lesions were reported in 3 of 15 patients with ileitis, 4 of 10 with colitis, and 7 of 17 with ileocolitis. The difference among these groups was not significant (P > .05).
The most common skin lesions observed in Crohn’s disease were erythema nodosum and eczema, and in ulcerative colitis, psoriasis and eczema, the researchers reported.
They also noted that the cutaneous lesions were significantly more prevalent in extensive ulcerative colitis compared with distal disease.
Skin lesions add to patient misery
“Skin lesions are considered a burden to patients with IBD and add to their suffering,” said Sara Mesilhy, MBBS, a gastroenterologist with the Royal College of Physicians in the United Kingdom, who was not affiliated with the research.
The severity and location of the disease appears to play a role because researchers found extensive ulcerative colitis may carry a higher risk for the development of skin lesions, Dr. Mesilhy noted.
The first step when facing skin lesions is to control the disease activity via the best treatment option, Dr. Mesilhy suggested.
The study was independently supported. Dr. Jonaitis and Dr. Mesilhy have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ECCO 2023
COVID raises risk for long-term GI complications
, a large new study indicates.
The researchers estimate that, so far, SARS-CoV-2 infections have contributed to more than 6 million new cases of GI disorders in the United States and 42 million new cases worldwide.
The diagnoses more common among patients who’ve had COVID ranged from stomach upset to acute pancreatitis, say the researchers, led by Evan Xu, a data analyst at the Clinical Epidemiology Center, Research and Development Service, VA St. Louis Health Care System.
Signs and symptoms of GI problems, such as constipation and diarrhea, also were more common among patients who had had the virus, the study found.
“Altogether, our results show that people with SARS-CoV-2 infection are at increased risk of gastrointestinal disorders in the post-acute phase of COVID-19,” the researchers write. “Post-COVID care should involve attention to gastrointestinal health and disease.”
The results were published online in Nature Communications.
Disease risks jump
The researchers used data from the U.S. Department of Veterans Affairs national health care databases to identify 154,068 people with confirmed COVID-19 from March 1, 2020, through Jan. 15, 2021. They used statistical modeling to compare those patients with 5.6 million patients with similar characteristics who had not been infected during the same period and an historical control group of 5.9 million patients from March 1, 2018, to Dec. 31, 2019, before the virus began to spread across the globe.
The study included hospitalized and nonhospitalized COVID patients. The majority of the study population was male, but the study included almost 1.2 million female patients.
Compared with control persons, post-COVID patients’ increased risk of a GI diagnosis and the excess disease burden at 1 year, respectively, were as follows.
- 102% for cholangitis; 0.22 per 1,000 persons
- 62% for peptic ulcer disease; 1.57 per 1,000 persons
- 54% for irritable bowel syndrome; 0.44 per 1,000 persons
- 47% for acute gastritis; 0.47 per 1,000 persons
- 46% for acute pancreatitis; 0.6 per 1,000 persons
- 36% for functional dyspepsia; 0.63 per 1,000 persons
- 35% for gastroesophageal reflux disease; 15.5 per 1,000 persons
Patients who’d had the virus were also at higher risk for GI symptoms than their COVID-free peers. Their risk was 60% higher for constipation, 58% for diarrhea, 52% for vomiting, 46% for bloating, and 44% for abdominal pain, the investigators found.
The risk of developing GI symptoms increased with COVID-19 severity and was highest for those who received intensive care because of the virus, the researchers note.
Subgroup analyses found that the risks of composite gastrointestinal outcome were evident in all subgroups based on age, race, sex, obesity, smoking, cardiovascular disease, chronic kidney disease, diabetes, hyperlipidemia, and hypertension, the authors write.
Disease burden rises
The increased numbers of GI patients with prior SARS-CoV-2 infection are altering the burden on the health care system, senior author Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University, St. Louis, said in an interview.
The shift may be pronounced in primary care, where GI concerns should be seen as a trigger for questions about prior SARS-CoV-2 infection, Dr. Al-Aly said.
Patients may encounter longer wait times at GI clinics or may give up on trying to schedule appointments if waits become too long, he said. They may also present to emergency departments if they can’t get an outpatient appointment, he added.
Simon C. Mathews, MD, assistant professor of medicine, division of gastroenterology, Johns Hopkins Medicine, Baltimore, told this news organization that he’s seeing increased wait times since COVID emerged.
“We know that the pandemic impacted patients’ ability and willingness to seek GI care. There continues to be a long backlog for patients who are only now getting reconnected to care. As a result, our clinics are busier than ever, and our wait times for appointments are unfortunately longer than we would like,” said Dr. Mathews, who was not involved in the research.
Abdominal pain, bloating, diarrhea, and constipation continue to be among the most common symptoms Dr. Mathews sees in clinic, he said.
Kyle Staller, MD, a Massachusetts General Brigham gastroenterologist, said in an interview that it’s important to distinguish symptoms from eventual diagnoses, which lag behind.
“Are patients attributing their symptoms to COVID, or is COVID itself creating a background of inflammation or changes in the nerves that are making these symptoms more common? My suspicion is a little bit of both,” said Dr. Staller, who is director of the Gastrointestinal Motility Laboratory at Mass General, Boston.
Although his clinic is seeing patients with the GI signs and symptoms listed in the article, “we’re not seeing as much of some of the diagnoses, like peptic ulcer disease and pancreatitis,” he said. “I wonder if those may be related to some of the consequences of being critically ill in general, rather than COVID specifically. Those diagnoses I would be more skeptical about.”
Duration of symptoms unclear
It’s hard to tell patients how long their GI symptoms might last after COVID, given the relatively short time researchers have had to study the virus, said Dr. Staller, who was not involved in the research.
The symptoms he’s seeing in patients after COVID mimic those of postinfectious IBS, which literature says could last for months or years, Dr. Staller said. “But they should improve over time,” he added.
Senior author Dr. Al-Aly agreed that the duration of post-COVID GI symptoms is unclear.
“What I can tell you is that even people who got SARS-CoV-2 infection from March 2020 are still coming back for GI problems,” he said.
Unlike other symptoms of long COVID, such as brain fog, gastroenterologists fortunately know how to treat the GI disorders that evolve from SARS-CoV-2 infection, said Dr. Al-Aly, who has studied the long-term effects of the virus on the brain, kidneys, heart, and other organs.
All health care providers “need to be thinking about COVID as a risk factor for all these diseases” and should ask patients about SARS-CoV-2 infection when they take their histories, he said.
The authors, Dr. Staller, and Dr. Mathews report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large new study indicates.
The researchers estimate that, so far, SARS-CoV-2 infections have contributed to more than 6 million new cases of GI disorders in the United States and 42 million new cases worldwide.
The diagnoses more common among patients who’ve had COVID ranged from stomach upset to acute pancreatitis, say the researchers, led by Evan Xu, a data analyst at the Clinical Epidemiology Center, Research and Development Service, VA St. Louis Health Care System.
Signs and symptoms of GI problems, such as constipation and diarrhea, also were more common among patients who had had the virus, the study found.
“Altogether, our results show that people with SARS-CoV-2 infection are at increased risk of gastrointestinal disorders in the post-acute phase of COVID-19,” the researchers write. “Post-COVID care should involve attention to gastrointestinal health and disease.”
The results were published online in Nature Communications.
Disease risks jump
The researchers used data from the U.S. Department of Veterans Affairs national health care databases to identify 154,068 people with confirmed COVID-19 from March 1, 2020, through Jan. 15, 2021. They used statistical modeling to compare those patients with 5.6 million patients with similar characteristics who had not been infected during the same period and an historical control group of 5.9 million patients from March 1, 2018, to Dec. 31, 2019, before the virus began to spread across the globe.
The study included hospitalized and nonhospitalized COVID patients. The majority of the study population was male, but the study included almost 1.2 million female patients.
Compared with control persons, post-COVID patients’ increased risk of a GI diagnosis and the excess disease burden at 1 year, respectively, were as follows.
- 102% for cholangitis; 0.22 per 1,000 persons
- 62% for peptic ulcer disease; 1.57 per 1,000 persons
- 54% for irritable bowel syndrome; 0.44 per 1,000 persons
- 47% for acute gastritis; 0.47 per 1,000 persons
- 46% for acute pancreatitis; 0.6 per 1,000 persons
- 36% for functional dyspepsia; 0.63 per 1,000 persons
- 35% for gastroesophageal reflux disease; 15.5 per 1,000 persons
Patients who’d had the virus were also at higher risk for GI symptoms than their COVID-free peers. Their risk was 60% higher for constipation, 58% for diarrhea, 52% for vomiting, 46% for bloating, and 44% for abdominal pain, the investigators found.
The risk of developing GI symptoms increased with COVID-19 severity and was highest for those who received intensive care because of the virus, the researchers note.
Subgroup analyses found that the risks of composite gastrointestinal outcome were evident in all subgroups based on age, race, sex, obesity, smoking, cardiovascular disease, chronic kidney disease, diabetes, hyperlipidemia, and hypertension, the authors write.
Disease burden rises
The increased numbers of GI patients with prior SARS-CoV-2 infection are altering the burden on the health care system, senior author Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University, St. Louis, said in an interview.
The shift may be pronounced in primary care, where GI concerns should be seen as a trigger for questions about prior SARS-CoV-2 infection, Dr. Al-Aly said.
Patients may encounter longer wait times at GI clinics or may give up on trying to schedule appointments if waits become too long, he said. They may also present to emergency departments if they can’t get an outpatient appointment, he added.
Simon C. Mathews, MD, assistant professor of medicine, division of gastroenterology, Johns Hopkins Medicine, Baltimore, told this news organization that he’s seeing increased wait times since COVID emerged.
“We know that the pandemic impacted patients’ ability and willingness to seek GI care. There continues to be a long backlog for patients who are only now getting reconnected to care. As a result, our clinics are busier than ever, and our wait times for appointments are unfortunately longer than we would like,” said Dr. Mathews, who was not involved in the research.
Abdominal pain, bloating, diarrhea, and constipation continue to be among the most common symptoms Dr. Mathews sees in clinic, he said.
Kyle Staller, MD, a Massachusetts General Brigham gastroenterologist, said in an interview that it’s important to distinguish symptoms from eventual diagnoses, which lag behind.
“Are patients attributing their symptoms to COVID, or is COVID itself creating a background of inflammation or changes in the nerves that are making these symptoms more common? My suspicion is a little bit of both,” said Dr. Staller, who is director of the Gastrointestinal Motility Laboratory at Mass General, Boston.
Although his clinic is seeing patients with the GI signs and symptoms listed in the article, “we’re not seeing as much of some of the diagnoses, like peptic ulcer disease and pancreatitis,” he said. “I wonder if those may be related to some of the consequences of being critically ill in general, rather than COVID specifically. Those diagnoses I would be more skeptical about.”
Duration of symptoms unclear
It’s hard to tell patients how long their GI symptoms might last after COVID, given the relatively short time researchers have had to study the virus, said Dr. Staller, who was not involved in the research.
The symptoms he’s seeing in patients after COVID mimic those of postinfectious IBS, which literature says could last for months or years, Dr. Staller said. “But they should improve over time,” he added.
Senior author Dr. Al-Aly agreed that the duration of post-COVID GI symptoms is unclear.
“What I can tell you is that even people who got SARS-CoV-2 infection from March 2020 are still coming back for GI problems,” he said.
Unlike other symptoms of long COVID, such as brain fog, gastroenterologists fortunately know how to treat the GI disorders that evolve from SARS-CoV-2 infection, said Dr. Al-Aly, who has studied the long-term effects of the virus on the brain, kidneys, heart, and other organs.
All health care providers “need to be thinking about COVID as a risk factor for all these diseases” and should ask patients about SARS-CoV-2 infection when they take their histories, he said.
The authors, Dr. Staller, and Dr. Mathews report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a large new study indicates.
The researchers estimate that, so far, SARS-CoV-2 infections have contributed to more than 6 million new cases of GI disorders in the United States and 42 million new cases worldwide.
The diagnoses more common among patients who’ve had COVID ranged from stomach upset to acute pancreatitis, say the researchers, led by Evan Xu, a data analyst at the Clinical Epidemiology Center, Research and Development Service, VA St. Louis Health Care System.
Signs and symptoms of GI problems, such as constipation and diarrhea, also were more common among patients who had had the virus, the study found.
“Altogether, our results show that people with SARS-CoV-2 infection are at increased risk of gastrointestinal disorders in the post-acute phase of COVID-19,” the researchers write. “Post-COVID care should involve attention to gastrointestinal health and disease.”
The results were published online in Nature Communications.
Disease risks jump
The researchers used data from the U.S. Department of Veterans Affairs national health care databases to identify 154,068 people with confirmed COVID-19 from March 1, 2020, through Jan. 15, 2021. They used statistical modeling to compare those patients with 5.6 million patients with similar characteristics who had not been infected during the same period and an historical control group of 5.9 million patients from March 1, 2018, to Dec. 31, 2019, before the virus began to spread across the globe.
The study included hospitalized and nonhospitalized COVID patients. The majority of the study population was male, but the study included almost 1.2 million female patients.
Compared with control persons, post-COVID patients’ increased risk of a GI diagnosis and the excess disease burden at 1 year, respectively, were as follows.
- 102% for cholangitis; 0.22 per 1,000 persons
- 62% for peptic ulcer disease; 1.57 per 1,000 persons
- 54% for irritable bowel syndrome; 0.44 per 1,000 persons
- 47% for acute gastritis; 0.47 per 1,000 persons
- 46% for acute pancreatitis; 0.6 per 1,000 persons
- 36% for functional dyspepsia; 0.63 per 1,000 persons
- 35% for gastroesophageal reflux disease; 15.5 per 1,000 persons
Patients who’d had the virus were also at higher risk for GI symptoms than their COVID-free peers. Their risk was 60% higher for constipation, 58% for diarrhea, 52% for vomiting, 46% for bloating, and 44% for abdominal pain, the investigators found.
The risk of developing GI symptoms increased with COVID-19 severity and was highest for those who received intensive care because of the virus, the researchers note.
Subgroup analyses found that the risks of composite gastrointestinal outcome were evident in all subgroups based on age, race, sex, obesity, smoking, cardiovascular disease, chronic kidney disease, diabetes, hyperlipidemia, and hypertension, the authors write.
Disease burden rises
The increased numbers of GI patients with prior SARS-CoV-2 infection are altering the burden on the health care system, senior author Ziyad Al-Aly, MD, a clinical epidemiologist at Washington University, St. Louis, said in an interview.
The shift may be pronounced in primary care, where GI concerns should be seen as a trigger for questions about prior SARS-CoV-2 infection, Dr. Al-Aly said.
Patients may encounter longer wait times at GI clinics or may give up on trying to schedule appointments if waits become too long, he said. They may also present to emergency departments if they can’t get an outpatient appointment, he added.
Simon C. Mathews, MD, assistant professor of medicine, division of gastroenterology, Johns Hopkins Medicine, Baltimore, told this news organization that he’s seeing increased wait times since COVID emerged.
“We know that the pandemic impacted patients’ ability and willingness to seek GI care. There continues to be a long backlog for patients who are only now getting reconnected to care. As a result, our clinics are busier than ever, and our wait times for appointments are unfortunately longer than we would like,” said Dr. Mathews, who was not involved in the research.
Abdominal pain, bloating, diarrhea, and constipation continue to be among the most common symptoms Dr. Mathews sees in clinic, he said.
Kyle Staller, MD, a Massachusetts General Brigham gastroenterologist, said in an interview that it’s important to distinguish symptoms from eventual diagnoses, which lag behind.
“Are patients attributing their symptoms to COVID, or is COVID itself creating a background of inflammation or changes in the nerves that are making these symptoms more common? My suspicion is a little bit of both,” said Dr. Staller, who is director of the Gastrointestinal Motility Laboratory at Mass General, Boston.
Although his clinic is seeing patients with the GI signs and symptoms listed in the article, “we’re not seeing as much of some of the diagnoses, like peptic ulcer disease and pancreatitis,” he said. “I wonder if those may be related to some of the consequences of being critically ill in general, rather than COVID specifically. Those diagnoses I would be more skeptical about.”
Duration of symptoms unclear
It’s hard to tell patients how long their GI symptoms might last after COVID, given the relatively short time researchers have had to study the virus, said Dr. Staller, who was not involved in the research.
The symptoms he’s seeing in patients after COVID mimic those of postinfectious IBS, which literature says could last for months or years, Dr. Staller said. “But they should improve over time,” he added.
Senior author Dr. Al-Aly agreed that the duration of post-COVID GI symptoms is unclear.
“What I can tell you is that even people who got SARS-CoV-2 infection from March 2020 are still coming back for GI problems,” he said.
Unlike other symptoms of long COVID, such as brain fog, gastroenterologists fortunately know how to treat the GI disorders that evolve from SARS-CoV-2 infection, said Dr. Al-Aly, who has studied the long-term effects of the virus on the brain, kidneys, heart, and other organs.
All health care providers “need to be thinking about COVID as a risk factor for all these diseases” and should ask patients about SARS-CoV-2 infection when they take their histories, he said.
The authors, Dr. Staller, and Dr. Mathews report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS