User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Is another COVID-19 booster really needed?
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Salt pills for patients with acute decompensated heart failure?
Restriction of dietary salt to alleviate or prevent volume overload in patients with acute decompensated heart failure (ADHF) is common hospital practice, but without a solid evidence base. A trial testing whether taking salt pills might have benefits for patients with ADHF undergoing intensive diuresis, therefore, may seem a bit counterintuitive.
In just such a randomized, placebo-controlled trial, the approach made no difference to weight loss on diuresis, a proxy for volume reduction, or to serum creatinine levels in ADHF patients receiving high-dose intravenous diuretic therapy.
The patients consumed the extra salt during their intravenous therapy in the form of tablets providing 6 g sodium chloride daily on top of their hospital-provided, low-sodium meals.
During that time, serum sodium levels remained stable for the 34 patients assigned to the salt tablets but dropped significantly in the 31 given placebo pills.
They lost about the same weight, averages of 4 kg and 4.6 kg (8.8-10 lb), respectively, and their urine output was also similar. Patients who took the salt tablets showed less of an increase in blood urea nitrogen (BUN) at both 96 hours and at discharge.
The findings “challenge the routine practice of sodium chloride restriction in acute heart failure, something done thousands of times a day, millions of times a year,” Robert A. Montgomery, MD, Cleveland Clinic, said when presenting the study at the annual scientific meeting of the Heart Failure Society of America.
The trial, called OSPREY-AHF (Oral Sodium to Preserve Renal Efficiency in Acute Heart Failure), also may encourage a shift in ADHF management from a preoccupation with salt restriction to focus more on fighting fluid retention.
OSPREY-HF took on “an established practice that doesn’t have much high-quality evidentiary support,” one guided primarily by consensus and observational data, Montgomery said in an interview.
There are also potential downsides to dietary sodium restriction, including some that may complicate or block ADHF therapies.
“Low-sodium diets can be associated with decreased caloric intake and nutritional quality,” Dr. Montgomery observed. And observational studies suggest that “patients who are on a low sodium diet can develop increased neurohormonal activation. The kidney is not sensing salt, and so starts ramping up the hormones,” which promotes diuretic resistance.
But emerging evidence also suggests “that giving sodium chloride in the form of hypertonic saline can help patients who are diuretic resistant.” The intervention, which appears to attenuate the neurohormonal activation associated with high-dose intravenous diuretics, Dr. Montgomery noted, helped inspire the design of OSPREY-AHF.
Edema consists of “a gallon of water and a pinch of salt, so we really should stop being so salt-centric and think much more about water as the problem in decompensated heart failure,” said John G.F. Cleland, MD, PhD, during the question-and-answer period after Montgomery’s presentation. Dr. Cleland, of the University of Glasgow Institute of Health and Wellbeing, is not connected to OSPREY-AHF.
“I think that maybe we overinterpret how important salt is” as a focus of volume management in ADHF, offered David Lanfear, MD, Henry Ford Health System, Detroit, who is also not part of the study.
OSPREY-AHF was well conducted but applies to a “very specific” clinical setting, Dr. Lanfear said in an interview. “These people are getting aggressive diuresis, a big dose and continuous infusion. It’s not everybody that has heart failure.”
Although the study was small, “I think it will fuel interest in this area and, probably, further investigation,” he said. The trial on its own won’t change practice, “but it will raise some eyebrows.”
The trial included patients with ADHF who have been “admitted to a cardiovascular medicine floor, not the intensive care unit” and were receiving at least 10 mg per hour of furosemide. It excluded any who were “hypernatremic or severely hyponatremic,” said Dr. Montgomery when presenting the study. They were required to have an initial estimated glomerular filtration rate (eGFR) of at least 15 mL/min per 1.73 m2.
The patients were randomly assigned double blind at a single center to receive tablets providing 2 g sodium chloride or placebo pills – 34 and 31 patients, respectively – three times daily during intravenous diuresis.
At 96 hours, the two groups showed no difference in change in creatinine levels or change in weight, both primary endpoints. Nor did they differ in urine output or change in eGFR. But serum sodium levels fell further, and BUN levels went up more in those given placebo.
The two groups showed no differences in hospital length of stay, use of renal replacement therapy at 90 days, ICU time during the index hospitalization, 30-day readmission, or 90-day mortality – although the trial wasn’t powered for clinical outcomes, Dr. Montgomery reported.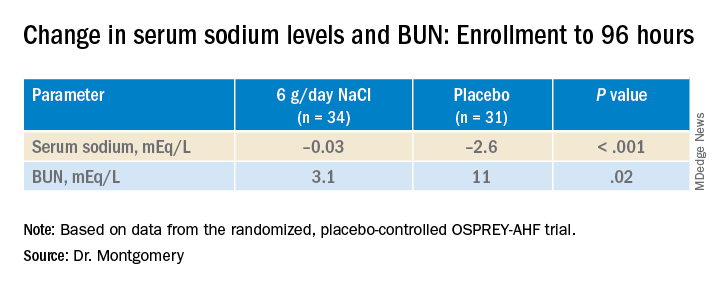
"We have patients who complain about their sodium-restricted diet, we have patients that have cachexia, who have a lot of complaints about provider-ordered meals and recommendations,” Dr. Montgomery explained in an interview.
Clinicians provide education and invest a lot of effort into getting patients with heart failure to start and maintain a low-sodium diet, he said. “But a low-sodium diet, in prior studies – and our study adds to this – is not a lever that actually seems to positively or adversely affect patients.”
Dr. Montgomery pointed to the recently published SODIUM-HF trial comparing low-sodium and unrestricted-sodium diets in outpatients with heart failure. It saw no clinical benefit from the low-sodium intervention.
Until studies show, potentially, that sodium restriction in hospitalized patients with heart failure makes a clinical difference, Dr. Montgomery said, “I’d say we should invest our time in things that we know are the most helpful, like getting them on guideline-directed medical therapy, when instead we spend an enormous amount of time counseling on and enforcing dietary restriction.”
Support for this study was provided by Cleveland Clinic Heart Vascular and Thoracic Institute’s Wilson Grant and Kaufman Center for Heart Failure Treatment and Recovery Grant. Dr. Lanfear disclosed research support from SomaLogic and Lilly; consulting for Abbott Laboratories, AstraZeneca, Janssen, Martin Pharmaceuticals, and Amgen; and serving on advisory panels for Illumina and Cytokinetics. Dr. Montgomery and Dr. Cleland disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Restriction of dietary salt to alleviate or prevent volume overload in patients with acute decompensated heart failure (ADHF) is common hospital practice, but without a solid evidence base. A trial testing whether taking salt pills might have benefits for patients with ADHF undergoing intensive diuresis, therefore, may seem a bit counterintuitive.
In just such a randomized, placebo-controlled trial, the approach made no difference to weight loss on diuresis, a proxy for volume reduction, or to serum creatinine levels in ADHF patients receiving high-dose intravenous diuretic therapy.
The patients consumed the extra salt during their intravenous therapy in the form of tablets providing 6 g sodium chloride daily on top of their hospital-provided, low-sodium meals.
During that time, serum sodium levels remained stable for the 34 patients assigned to the salt tablets but dropped significantly in the 31 given placebo pills.
They lost about the same weight, averages of 4 kg and 4.6 kg (8.8-10 lb), respectively, and their urine output was also similar. Patients who took the salt tablets showed less of an increase in blood urea nitrogen (BUN) at both 96 hours and at discharge.
The findings “challenge the routine practice of sodium chloride restriction in acute heart failure, something done thousands of times a day, millions of times a year,” Robert A. Montgomery, MD, Cleveland Clinic, said when presenting the study at the annual scientific meeting of the Heart Failure Society of America.
The trial, called OSPREY-AHF (Oral Sodium to Preserve Renal Efficiency in Acute Heart Failure), also may encourage a shift in ADHF management from a preoccupation with salt restriction to focus more on fighting fluid retention.
OSPREY-HF took on “an established practice that doesn’t have much high-quality evidentiary support,” one guided primarily by consensus and observational data, Montgomery said in an interview.
There are also potential downsides to dietary sodium restriction, including some that may complicate or block ADHF therapies.
“Low-sodium diets can be associated with decreased caloric intake and nutritional quality,” Dr. Montgomery observed. And observational studies suggest that “patients who are on a low sodium diet can develop increased neurohormonal activation. The kidney is not sensing salt, and so starts ramping up the hormones,” which promotes diuretic resistance.
But emerging evidence also suggests “that giving sodium chloride in the form of hypertonic saline can help patients who are diuretic resistant.” The intervention, which appears to attenuate the neurohormonal activation associated with high-dose intravenous diuretics, Dr. Montgomery noted, helped inspire the design of OSPREY-AHF.
Edema consists of “a gallon of water and a pinch of salt, so we really should stop being so salt-centric and think much more about water as the problem in decompensated heart failure,” said John G.F. Cleland, MD, PhD, during the question-and-answer period after Montgomery’s presentation. Dr. Cleland, of the University of Glasgow Institute of Health and Wellbeing, is not connected to OSPREY-AHF.
“I think that maybe we overinterpret how important salt is” as a focus of volume management in ADHF, offered David Lanfear, MD, Henry Ford Health System, Detroit, who is also not part of the study.
OSPREY-AHF was well conducted but applies to a “very specific” clinical setting, Dr. Lanfear said in an interview. “These people are getting aggressive diuresis, a big dose and continuous infusion. It’s not everybody that has heart failure.”
Although the study was small, “I think it will fuel interest in this area and, probably, further investigation,” he said. The trial on its own won’t change practice, “but it will raise some eyebrows.”
The trial included patients with ADHF who have been “admitted to a cardiovascular medicine floor, not the intensive care unit” and were receiving at least 10 mg per hour of furosemide. It excluded any who were “hypernatremic or severely hyponatremic,” said Dr. Montgomery when presenting the study. They were required to have an initial estimated glomerular filtration rate (eGFR) of at least 15 mL/min per 1.73 m2.
The patients were randomly assigned double blind at a single center to receive tablets providing 2 g sodium chloride or placebo pills – 34 and 31 patients, respectively – three times daily during intravenous diuresis.
At 96 hours, the two groups showed no difference in change in creatinine levels or change in weight, both primary endpoints. Nor did they differ in urine output or change in eGFR. But serum sodium levels fell further, and BUN levels went up more in those given placebo.
The two groups showed no differences in hospital length of stay, use of renal replacement therapy at 90 days, ICU time during the index hospitalization, 30-day readmission, or 90-day mortality – although the trial wasn’t powered for clinical outcomes, Dr. Montgomery reported.
"We have patients who complain about their sodium-restricted diet, we have patients that have cachexia, who have a lot of complaints about provider-ordered meals and recommendations,” Dr. Montgomery explained in an interview.
Clinicians provide education and invest a lot of effort into getting patients with heart failure to start and maintain a low-sodium diet, he said. “But a low-sodium diet, in prior studies – and our study adds to this – is not a lever that actually seems to positively or adversely affect patients.”
Dr. Montgomery pointed to the recently published SODIUM-HF trial comparing low-sodium and unrestricted-sodium diets in outpatients with heart failure. It saw no clinical benefit from the low-sodium intervention.
Until studies show, potentially, that sodium restriction in hospitalized patients with heart failure makes a clinical difference, Dr. Montgomery said, “I’d say we should invest our time in things that we know are the most helpful, like getting them on guideline-directed medical therapy, when instead we spend an enormous amount of time counseling on and enforcing dietary restriction.”
Support for this study was provided by Cleveland Clinic Heart Vascular and Thoracic Institute’s Wilson Grant and Kaufman Center for Heart Failure Treatment and Recovery Grant. Dr. Lanfear disclosed research support from SomaLogic and Lilly; consulting for Abbott Laboratories, AstraZeneca, Janssen, Martin Pharmaceuticals, and Amgen; and serving on advisory panels for Illumina and Cytokinetics. Dr. Montgomery and Dr. Cleland disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Restriction of dietary salt to alleviate or prevent volume overload in patients with acute decompensated heart failure (ADHF) is common hospital practice, but without a solid evidence base. A trial testing whether taking salt pills might have benefits for patients with ADHF undergoing intensive diuresis, therefore, may seem a bit counterintuitive.
In just such a randomized, placebo-controlled trial, the approach made no difference to weight loss on diuresis, a proxy for volume reduction, or to serum creatinine levels in ADHF patients receiving high-dose intravenous diuretic therapy.
The patients consumed the extra salt during their intravenous therapy in the form of tablets providing 6 g sodium chloride daily on top of their hospital-provided, low-sodium meals.
During that time, serum sodium levels remained stable for the 34 patients assigned to the salt tablets but dropped significantly in the 31 given placebo pills.
They lost about the same weight, averages of 4 kg and 4.6 kg (8.8-10 lb), respectively, and their urine output was also similar. Patients who took the salt tablets showed less of an increase in blood urea nitrogen (BUN) at both 96 hours and at discharge.
The findings “challenge the routine practice of sodium chloride restriction in acute heart failure, something done thousands of times a day, millions of times a year,” Robert A. Montgomery, MD, Cleveland Clinic, said when presenting the study at the annual scientific meeting of the Heart Failure Society of America.
The trial, called OSPREY-AHF (Oral Sodium to Preserve Renal Efficiency in Acute Heart Failure), also may encourage a shift in ADHF management from a preoccupation with salt restriction to focus more on fighting fluid retention.
OSPREY-HF took on “an established practice that doesn’t have much high-quality evidentiary support,” one guided primarily by consensus and observational data, Montgomery said in an interview.
There are also potential downsides to dietary sodium restriction, including some that may complicate or block ADHF therapies.
“Low-sodium diets can be associated with decreased caloric intake and nutritional quality,” Dr. Montgomery observed. And observational studies suggest that “patients who are on a low sodium diet can develop increased neurohormonal activation. The kidney is not sensing salt, and so starts ramping up the hormones,” which promotes diuretic resistance.
But emerging evidence also suggests “that giving sodium chloride in the form of hypertonic saline can help patients who are diuretic resistant.” The intervention, which appears to attenuate the neurohormonal activation associated with high-dose intravenous diuretics, Dr. Montgomery noted, helped inspire the design of OSPREY-AHF.
Edema consists of “a gallon of water and a pinch of salt, so we really should stop being so salt-centric and think much more about water as the problem in decompensated heart failure,” said John G.F. Cleland, MD, PhD, during the question-and-answer period after Montgomery’s presentation. Dr. Cleland, of the University of Glasgow Institute of Health and Wellbeing, is not connected to OSPREY-AHF.
“I think that maybe we overinterpret how important salt is” as a focus of volume management in ADHF, offered David Lanfear, MD, Henry Ford Health System, Detroit, who is also not part of the study.
OSPREY-AHF was well conducted but applies to a “very specific” clinical setting, Dr. Lanfear said in an interview. “These people are getting aggressive diuresis, a big dose and continuous infusion. It’s not everybody that has heart failure.”
Although the study was small, “I think it will fuel interest in this area and, probably, further investigation,” he said. The trial on its own won’t change practice, “but it will raise some eyebrows.”
The trial included patients with ADHF who have been “admitted to a cardiovascular medicine floor, not the intensive care unit” and were receiving at least 10 mg per hour of furosemide. It excluded any who were “hypernatremic or severely hyponatremic,” said Dr. Montgomery when presenting the study. They were required to have an initial estimated glomerular filtration rate (eGFR) of at least 15 mL/min per 1.73 m2.
The patients were randomly assigned double blind at a single center to receive tablets providing 2 g sodium chloride or placebo pills – 34 and 31 patients, respectively – three times daily during intravenous diuresis.
At 96 hours, the two groups showed no difference in change in creatinine levels or change in weight, both primary endpoints. Nor did they differ in urine output or change in eGFR. But serum sodium levels fell further, and BUN levels went up more in those given placebo.
The two groups showed no differences in hospital length of stay, use of renal replacement therapy at 90 days, ICU time during the index hospitalization, 30-day readmission, or 90-day mortality – although the trial wasn’t powered for clinical outcomes, Dr. Montgomery reported.
"We have patients who complain about their sodium-restricted diet, we have patients that have cachexia, who have a lot of complaints about provider-ordered meals and recommendations,” Dr. Montgomery explained in an interview.
Clinicians provide education and invest a lot of effort into getting patients with heart failure to start and maintain a low-sodium diet, he said. “But a low-sodium diet, in prior studies – and our study adds to this – is not a lever that actually seems to positively or adversely affect patients.”
Dr. Montgomery pointed to the recently published SODIUM-HF trial comparing low-sodium and unrestricted-sodium diets in outpatients with heart failure. It saw no clinical benefit from the low-sodium intervention.
Until studies show, potentially, that sodium restriction in hospitalized patients with heart failure makes a clinical difference, Dr. Montgomery said, “I’d say we should invest our time in things that we know are the most helpful, like getting them on guideline-directed medical therapy, when instead we spend an enormous amount of time counseling on and enforcing dietary restriction.”
Support for this study was provided by Cleveland Clinic Heart Vascular and Thoracic Institute’s Wilson Grant and Kaufman Center for Heart Failure Treatment and Recovery Grant. Dr. Lanfear disclosed research support from SomaLogic and Lilly; consulting for Abbott Laboratories, AstraZeneca, Janssen, Martin Pharmaceuticals, and Amgen; and serving on advisory panels for Illumina and Cytokinetics. Dr. Montgomery and Dr. Cleland disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HFSA 2022
Analysis of PsA guidelines reveals much room for improvement on conflicts of interest
, according to a retrospective analysis of all authors on the most recent guidelines issued by the American College of Rheumatology (ACR) and the Japanese Dermatological Association (JDA).
In addition to finding that the majority of the authors of psoriatic arthritis (PsA) clinical practice guidelines (CPGs) issued by the JDA and ACR received substantial personal payments from pharmaceutical companies before and during CPG development, researchers led by Hanano Mamada and Anju Murayama of the Medical Governance Research Institute, Tokyo, wrote in Arthritis Care & Research that “several CPG authors self-cited their articles without the disclosure of NFCOI [nonfinancial conflicts of interest], and most of the recommendations were based on low or very low quality of evidence. Although the COI policies used by JDA and ACR are clearly inadequate, no significant revisions have been made for the last 3 years.”
Based on their findings, which were made using payment data from major Japanese pharmaceutical companies and the U.S. Open Payments Database from 2016 to 2018, the researchers suggested that the medical societies should:
- Adopt global standard COI policies from organizations such as the National Academy of Medicine and Guidelines International Network, including a 3-year lookback period for COI declaration.
- Consider a comprehensive definition and rigorous management with full disclosure of NFCOI.
- Publish a list of authors making each recommendation to grasp the implications of COI in clinical practice guidelines.
- Mention the detailed date of the COI disclosure, which should be close to the publication date as much as possible.
Financial conflicts of interest
The researchers used payment data published between 2016 and 2018 for all 83 companies belonging to the Japan Pharmaceutical Manufacturers Association, focusing on personal payments (for lecturing, writing, and consultancy) and excluding research payments, “since in Japan, the name, institution, and position of the author or researcher who received the research payment is not disclosed, which makes assessing research payments difficult.” To evaluate authors’ FCOI in the ACR’s CPG, the researchers analyzed the U.S. Open Payments Database “for all categories of general payments such as speaking, consulting, meals, and travel expenses 3 years from before the guideline’s first online publication on November 30, 2018.”
The 2018 ACR/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis had 36 authors and the JDA’s Clinical Practice Guideline for the Treatment of Psoriatic Arthritis 2019 had 23. Overall, 61% of JDA authors and half of ACR authors voluntarily declared FCOI with pharmaceutical companies; 25 of the ACR authors were U.S. physicians and could be included in the Open Payments Database search.
A total of 21 (91.3%) JDA authors and 21 (84.0%) ACR authors received at least one payment, with the combined total of $3,335,413 and $4,081,629 payments, respectively, over the 3 years. The average and median personal payments were $145,018 and $123,876 for JDA authors and $162,825 and $58,826 for ACR authors. When the payments to ACR authors were limited to lecturing, writing, and consulting fees that are required under the ACR’s COI policy, the mean was $130,102 and median was $39,375. The corresponding payments for JDA authors were $123,876 and $8,170, respectively,
The researchers found undisclosed payments for more than three-quarters of physician authors of the Japanese guideline, and nearly half of the doctors authoring the American guideline had undisclosed payments. These added up to $474,000 for the JDA, which amounted to 38% of the total for personal payments that must be reported to the JDA based on its COI policy for clinical practice guidelines, and $218,000 for the ACR, amounting to 18% of the total for personal payments that must be reported to the society based on its COI policy.
Of the 11 ACR authors who were not eligible for the U.S. Open Payments Database search, 5 declared FCOI with pharmaceutical companies in the guideline, meaning that 26 (72%) of the 36 authors had FCOI with pharmaceutical companies.
The ACR only required authors to declare FCOI covering 1 year before and during guideline development, and although the JDA required authors to declare their FCOI for the past 3 years of guideline development, the study authors noted that the JDA guideline disclosed them for only 2 years (between Jan. 1, 2017, and Dec. 31, 2018).
“It is true that influential doctors such as clinical practice guideline authors tend to receive various types of payments from pharmaceutical companies and that it is difficult to conduct research without funding from pharmaceutical companies. However, our current research mainly focuses on personal payments from pharmaceutical companies such as lecture fees and consulting fees. These payments are recognized as pocket money and are not used for research. Thus, it is questionable that the observed relationships are something evitable,” the researchers wrote.
Nonfinancial conflicts of interest
Many authors of the ACR’s CPG and the JDA’s CPG also had NFCOI, defined objectively in this study as self-citation rate. NFCOI have been more broadly defined by the International Committee of Medical Journal Editors (ICMJE) as “conflicts, such as personal relationships or rivalries, academic competition, and intellectual beliefs”; the ICMJE recommends reporting NFCOI on its COI form.
The JDA guideline included self-citations by 78% of its authors, compared with 32% of the ACR guideline authors, but this weighed differently among the two guidelines in that only 12 of the 354 (3.4%) citations in the JDA guideline were self-cited, compared with 46 of 137 (34%) citations in the ACR guideline.
The researchers noted that while the self-citation rates between JDA and ACR authors “differed remarkably,” the impact of ACR authors on CPG recommendations was much more direct. Three-quarters of JDA authors’ self-cited articles were about observational studies, whereas 52% of the ACR authors’ self-cited articles were clinical trials, most of which were randomized, controlled studies, and these NFCOI were not disclosed in the guideline.
Half of the strong recommendations in the JDA guideline were based on low or very low quality of evidence, whereas the ACR guideline had no strong recommendations based on low or very low quality of evidence.
This study was supported by the nonprofit Medical Governance Research Institute, which receives donations from Ain Pharmacies Inc., other organizations, and private individuals. The study also received support from the Tansa (formerly known as the Waseda Chronicle), an independent nonprofit news organization dedicated to investigative journalism. Three authors reported receiving personal fees from several pharmaceutical companies for work outside of the scope of this study.
, according to a retrospective analysis of all authors on the most recent guidelines issued by the American College of Rheumatology (ACR) and the Japanese Dermatological Association (JDA).
In addition to finding that the majority of the authors of psoriatic arthritis (PsA) clinical practice guidelines (CPGs) issued by the JDA and ACR received substantial personal payments from pharmaceutical companies before and during CPG development, researchers led by Hanano Mamada and Anju Murayama of the Medical Governance Research Institute, Tokyo, wrote in Arthritis Care & Research that “several CPG authors self-cited their articles without the disclosure of NFCOI [nonfinancial conflicts of interest], and most of the recommendations were based on low or very low quality of evidence. Although the COI policies used by JDA and ACR are clearly inadequate, no significant revisions have been made for the last 3 years.”
Based on their findings, which were made using payment data from major Japanese pharmaceutical companies and the U.S. Open Payments Database from 2016 to 2018, the researchers suggested that the medical societies should:
- Adopt global standard COI policies from organizations such as the National Academy of Medicine and Guidelines International Network, including a 3-year lookback period for COI declaration.
- Consider a comprehensive definition and rigorous management with full disclosure of NFCOI.
- Publish a list of authors making each recommendation to grasp the implications of COI in clinical practice guidelines.
- Mention the detailed date of the COI disclosure, which should be close to the publication date as much as possible.
Financial conflicts of interest
The researchers used payment data published between 2016 and 2018 for all 83 companies belonging to the Japan Pharmaceutical Manufacturers Association, focusing on personal payments (for lecturing, writing, and consultancy) and excluding research payments, “since in Japan, the name, institution, and position of the author or researcher who received the research payment is not disclosed, which makes assessing research payments difficult.” To evaluate authors’ FCOI in the ACR’s CPG, the researchers analyzed the U.S. Open Payments Database “for all categories of general payments such as speaking, consulting, meals, and travel expenses 3 years from before the guideline’s first online publication on November 30, 2018.”
The 2018 ACR/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis had 36 authors and the JDA’s Clinical Practice Guideline for the Treatment of Psoriatic Arthritis 2019 had 23. Overall, 61% of JDA authors and half of ACR authors voluntarily declared FCOI with pharmaceutical companies; 25 of the ACR authors were U.S. physicians and could be included in the Open Payments Database search.
A total of 21 (91.3%) JDA authors and 21 (84.0%) ACR authors received at least one payment, with the combined total of $3,335,413 and $4,081,629 payments, respectively, over the 3 years. The average and median personal payments were $145,018 and $123,876 for JDA authors and $162,825 and $58,826 for ACR authors. When the payments to ACR authors were limited to lecturing, writing, and consulting fees that are required under the ACR’s COI policy, the mean was $130,102 and median was $39,375. The corresponding payments for JDA authors were $123,876 and $8,170, respectively,
The researchers found undisclosed payments for more than three-quarters of physician authors of the Japanese guideline, and nearly half of the doctors authoring the American guideline had undisclosed payments. These added up to $474,000 for the JDA, which amounted to 38% of the total for personal payments that must be reported to the JDA based on its COI policy for clinical practice guidelines, and $218,000 for the ACR, amounting to 18% of the total for personal payments that must be reported to the society based on its COI policy.
Of the 11 ACR authors who were not eligible for the U.S. Open Payments Database search, 5 declared FCOI with pharmaceutical companies in the guideline, meaning that 26 (72%) of the 36 authors had FCOI with pharmaceutical companies.
The ACR only required authors to declare FCOI covering 1 year before and during guideline development, and although the JDA required authors to declare their FCOI for the past 3 years of guideline development, the study authors noted that the JDA guideline disclosed them for only 2 years (between Jan. 1, 2017, and Dec. 31, 2018).
“It is true that influential doctors such as clinical practice guideline authors tend to receive various types of payments from pharmaceutical companies and that it is difficult to conduct research without funding from pharmaceutical companies. However, our current research mainly focuses on personal payments from pharmaceutical companies such as lecture fees and consulting fees. These payments are recognized as pocket money and are not used for research. Thus, it is questionable that the observed relationships are something evitable,” the researchers wrote.
Nonfinancial conflicts of interest
Many authors of the ACR’s CPG and the JDA’s CPG also had NFCOI, defined objectively in this study as self-citation rate. NFCOI have been more broadly defined by the International Committee of Medical Journal Editors (ICMJE) as “conflicts, such as personal relationships or rivalries, academic competition, and intellectual beliefs”; the ICMJE recommends reporting NFCOI on its COI form.
The JDA guideline included self-citations by 78% of its authors, compared with 32% of the ACR guideline authors, but this weighed differently among the two guidelines in that only 12 of the 354 (3.4%) citations in the JDA guideline were self-cited, compared with 46 of 137 (34%) citations in the ACR guideline.
The researchers noted that while the self-citation rates between JDA and ACR authors “differed remarkably,” the impact of ACR authors on CPG recommendations was much more direct. Three-quarters of JDA authors’ self-cited articles were about observational studies, whereas 52% of the ACR authors’ self-cited articles were clinical trials, most of which were randomized, controlled studies, and these NFCOI were not disclosed in the guideline.
Half of the strong recommendations in the JDA guideline were based on low or very low quality of evidence, whereas the ACR guideline had no strong recommendations based on low or very low quality of evidence.
This study was supported by the nonprofit Medical Governance Research Institute, which receives donations from Ain Pharmacies Inc., other organizations, and private individuals. The study also received support from the Tansa (formerly known as the Waseda Chronicle), an independent nonprofit news organization dedicated to investigative journalism. Three authors reported receiving personal fees from several pharmaceutical companies for work outside of the scope of this study.
, according to a retrospective analysis of all authors on the most recent guidelines issued by the American College of Rheumatology (ACR) and the Japanese Dermatological Association (JDA).
In addition to finding that the majority of the authors of psoriatic arthritis (PsA) clinical practice guidelines (CPGs) issued by the JDA and ACR received substantial personal payments from pharmaceutical companies before and during CPG development, researchers led by Hanano Mamada and Anju Murayama of the Medical Governance Research Institute, Tokyo, wrote in Arthritis Care & Research that “several CPG authors self-cited their articles without the disclosure of NFCOI [nonfinancial conflicts of interest], and most of the recommendations were based on low or very low quality of evidence. Although the COI policies used by JDA and ACR are clearly inadequate, no significant revisions have been made for the last 3 years.”
Based on their findings, which were made using payment data from major Japanese pharmaceutical companies and the U.S. Open Payments Database from 2016 to 2018, the researchers suggested that the medical societies should:
- Adopt global standard COI policies from organizations such as the National Academy of Medicine and Guidelines International Network, including a 3-year lookback period for COI declaration.
- Consider a comprehensive definition and rigorous management with full disclosure of NFCOI.
- Publish a list of authors making each recommendation to grasp the implications of COI in clinical practice guidelines.
- Mention the detailed date of the COI disclosure, which should be close to the publication date as much as possible.
Financial conflicts of interest
The researchers used payment data published between 2016 and 2018 for all 83 companies belonging to the Japan Pharmaceutical Manufacturers Association, focusing on personal payments (for lecturing, writing, and consultancy) and excluding research payments, “since in Japan, the name, institution, and position of the author or researcher who received the research payment is not disclosed, which makes assessing research payments difficult.” To evaluate authors’ FCOI in the ACR’s CPG, the researchers analyzed the U.S. Open Payments Database “for all categories of general payments such as speaking, consulting, meals, and travel expenses 3 years from before the guideline’s first online publication on November 30, 2018.”
The 2018 ACR/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis had 36 authors and the JDA’s Clinical Practice Guideline for the Treatment of Psoriatic Arthritis 2019 had 23. Overall, 61% of JDA authors and half of ACR authors voluntarily declared FCOI with pharmaceutical companies; 25 of the ACR authors were U.S. physicians and could be included in the Open Payments Database search.
A total of 21 (91.3%) JDA authors and 21 (84.0%) ACR authors received at least one payment, with the combined total of $3,335,413 and $4,081,629 payments, respectively, over the 3 years. The average and median personal payments were $145,018 and $123,876 for JDA authors and $162,825 and $58,826 for ACR authors. When the payments to ACR authors were limited to lecturing, writing, and consulting fees that are required under the ACR’s COI policy, the mean was $130,102 and median was $39,375. The corresponding payments for JDA authors were $123,876 and $8,170, respectively,
The researchers found undisclosed payments for more than three-quarters of physician authors of the Japanese guideline, and nearly half of the doctors authoring the American guideline had undisclosed payments. These added up to $474,000 for the JDA, which amounted to 38% of the total for personal payments that must be reported to the JDA based on its COI policy for clinical practice guidelines, and $218,000 for the ACR, amounting to 18% of the total for personal payments that must be reported to the society based on its COI policy.
Of the 11 ACR authors who were not eligible for the U.S. Open Payments Database search, 5 declared FCOI with pharmaceutical companies in the guideline, meaning that 26 (72%) of the 36 authors had FCOI with pharmaceutical companies.
The ACR only required authors to declare FCOI covering 1 year before and during guideline development, and although the JDA required authors to declare their FCOI for the past 3 years of guideline development, the study authors noted that the JDA guideline disclosed them for only 2 years (between Jan. 1, 2017, and Dec. 31, 2018).
“It is true that influential doctors such as clinical practice guideline authors tend to receive various types of payments from pharmaceutical companies and that it is difficult to conduct research without funding from pharmaceutical companies. However, our current research mainly focuses on personal payments from pharmaceutical companies such as lecture fees and consulting fees. These payments are recognized as pocket money and are not used for research. Thus, it is questionable that the observed relationships are something evitable,” the researchers wrote.
Nonfinancial conflicts of interest
Many authors of the ACR’s CPG and the JDA’s CPG also had NFCOI, defined objectively in this study as self-citation rate. NFCOI have been more broadly defined by the International Committee of Medical Journal Editors (ICMJE) as “conflicts, such as personal relationships or rivalries, academic competition, and intellectual beliefs”; the ICMJE recommends reporting NFCOI on its COI form.
The JDA guideline included self-citations by 78% of its authors, compared with 32% of the ACR guideline authors, but this weighed differently among the two guidelines in that only 12 of the 354 (3.4%) citations in the JDA guideline were self-cited, compared with 46 of 137 (34%) citations in the ACR guideline.
The researchers noted that while the self-citation rates between JDA and ACR authors “differed remarkably,” the impact of ACR authors on CPG recommendations was much more direct. Three-quarters of JDA authors’ self-cited articles were about observational studies, whereas 52% of the ACR authors’ self-cited articles were clinical trials, most of which were randomized, controlled studies, and these NFCOI were not disclosed in the guideline.
Half of the strong recommendations in the JDA guideline were based on low or very low quality of evidence, whereas the ACR guideline had no strong recommendations based on low or very low quality of evidence.
This study was supported by the nonprofit Medical Governance Research Institute, which receives donations from Ain Pharmacies Inc., other organizations, and private individuals. The study also received support from the Tansa (formerly known as the Waseda Chronicle), an independent nonprofit news organization dedicated to investigative journalism. Three authors reported receiving personal fees from several pharmaceutical companies for work outside of the scope of this study.
FROM ARTHRITIS CARE & RESEARCH
Newer drugs not cost effective for first-line diabetes therapy
To be cost effective, compared with metformin, for initial therapy for type 2 diabetes, prices for a sodium-glucose cotransporter-2 (SGLT2) inhibitor or a glucagon-like peptide-1 (GLP-1) agonist would have to fall by at least 70% and at least 90%, respectively, according to estimates.
The study, modeled on U.S. patients, by Jin G. Choi, MD, and colleagues, was published online Oct. 3 in the Annals of Internal Medicine.
The researchers simulated the lifetime incidence, prevalence, mortality, and costs associated with three different first-line treatment strategies – metformin, an SGLT2 inhibitor, or a GLP-1 agonist – in U.S. patients with untreated type 2 diabetes.
Compared with patients who received initial treatment with metformin, those who received one of the newer drugs had 4.4% to 5.2% lower lifetime rates of congestive heart failure, ischemic heart disease, myocardial infarction, and stroke.
However, to be cost-effective at under $150,000 per quality-adjusted life-years (QALY), SGLT2 inhibitors would need to cost less than $5 a day ($1,800 a year), and GLP-1 agonists would have to cost less than $6 a day ($2,100 a year), a lot less than now.
Knowing how expensive these drugs are, “I am not surprised” that the model predicts that the price would have to drop so much to make them cost-effective, compared with first-line treatment with metformin, senior author Neda Laiteerapong, MD, said in an interview.
“But I am disappointed,” she said, because these drugs are very effective, and if the prices were lower, more people could benefit.
“In the interest of improving access to high-quality care in the United States, our study results indicate the need to reduce SGLT2 inhibitor and GLP-1 receptor agonist medication costs substantially for patients with type 2 [diabetes] to improve health outcomes and prevent exacerbating diabetes health disparities,” the researchers conclude.
One way that the newer drugs might be more widely affordable is if the government became involved, possibly by passing a law similar to the Affordable Insulin Now Act, speculated Dr. Laiteerapong, who is associate director at the Center for Chronic Disease Research and Policy, University of Chicago.
‘Current prices too high to encourage first-line adoption’
Guidelines recommend the use of SGLT2 inhibitors and GLP-1 agonists as second-line therapies for patients with type 2 diabetes, but it has not been clear if clinical benefits would outweigh costs for use as first-line therapies.
“Although clinical trials have demonstrated the clinical effectiveness of these newer drugs, they are hundreds of times more expensive than other ... diabetes drugs,” the researchers note.
On the other hand, costs may fall in the coming years when these new drugs come off-patent.
The current study was designed to help inform future clinical guidelines.
The researchers created a population simulation model based on the United Kingdom Prospective Diabetes Study, Outcomes Model version 2 (UKPDS OM2) for diabetes-related complications and mortality, with added information about hypoglycemic events, quality of life, and U.S. costs.
The researchers also identified a nationally representative sample of people who would be eligible to start first-line diabetes therapy when their A1c reached 7% for the model.
Using National Health and Nutrition Examination Survey (NHANES) data (2013-2016), the researchers identified about 7.3 million U.S. adults aged 18 and older with self-reported diabetes or an A1c greater than 6.5% with no reported use of diabetes medications.
Patients were an average age of 55, and 55% were women. They had had diabetes for an average of 4.2 years, and 36% had a history of diabetes complications.
The model projected that patients would have an improved life expectancy of 3.0 and 3.4 months from first-line SGLT2 inhibitors and GLP-1 agonists, respectively, compared with initial therapy with metformin due to reduced rates of macrovascular disease.
“However, the current drug costs would be too high to encourage their adoption as first-line for usual clinical practice,” the researchers report.
‘Disparities could remain for decades’
Generic SGLT2 inhibitors could enter the marketplace shortly, because one of two dapagliflozin patents expired in October 2020 and approval for generic alternatives has been sought from the U.S. Food and Drug Administration, Dr. Choi and colleagues note.
However, it could still take decades for medication prices to drop low enough to become affordable, the group cautions. For example, a generic GLP-1 agonist became available in 2017, but costs remain high.
“Without external incentives,” the group writes, “limited access to these drug classes will likely persist (for example, due to higher copays or requirements for prior authorizations), as will further diabetes disparities – for decades into the future – because of differential access to care due to insurance (for example, private vs. public), which often tracks race and ethnicity.”
The study was supported by the American Diabetes Association. Dr. Choi was supported by a National Institutes of Health, National Institute on Aging grant. Dr. Laiteerapong and other co-authors are members of the National Institute of Diabetes and Digestive and Kidney Diseases Chicago Center for Diabetes Translation Research at the University of Chicago. Dr. Choi and Dr. Laiteerapong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
To be cost effective, compared with metformin, for initial therapy for type 2 diabetes, prices for a sodium-glucose cotransporter-2 (SGLT2) inhibitor or a glucagon-like peptide-1 (GLP-1) agonist would have to fall by at least 70% and at least 90%, respectively, according to estimates.
The study, modeled on U.S. patients, by Jin G. Choi, MD, and colleagues, was published online Oct. 3 in the Annals of Internal Medicine.
The researchers simulated the lifetime incidence, prevalence, mortality, and costs associated with three different first-line treatment strategies – metformin, an SGLT2 inhibitor, or a GLP-1 agonist – in U.S. patients with untreated type 2 diabetes.
Compared with patients who received initial treatment with metformin, those who received one of the newer drugs had 4.4% to 5.2% lower lifetime rates of congestive heart failure, ischemic heart disease, myocardial infarction, and stroke.
However, to be cost-effective at under $150,000 per quality-adjusted life-years (QALY), SGLT2 inhibitors would need to cost less than $5 a day ($1,800 a year), and GLP-1 agonists would have to cost less than $6 a day ($2,100 a year), a lot less than now.
Knowing how expensive these drugs are, “I am not surprised” that the model predicts that the price would have to drop so much to make them cost-effective, compared with first-line treatment with metformin, senior author Neda Laiteerapong, MD, said in an interview.
“But I am disappointed,” she said, because these drugs are very effective, and if the prices were lower, more people could benefit.
“In the interest of improving access to high-quality care in the United States, our study results indicate the need to reduce SGLT2 inhibitor and GLP-1 receptor agonist medication costs substantially for patients with type 2 [diabetes] to improve health outcomes and prevent exacerbating diabetes health disparities,” the researchers conclude.
One way that the newer drugs might be more widely affordable is if the government became involved, possibly by passing a law similar to the Affordable Insulin Now Act, speculated Dr. Laiteerapong, who is associate director at the Center for Chronic Disease Research and Policy, University of Chicago.
‘Current prices too high to encourage first-line adoption’
Guidelines recommend the use of SGLT2 inhibitors and GLP-1 agonists as second-line therapies for patients with type 2 diabetes, but it has not been clear if clinical benefits would outweigh costs for use as first-line therapies.
“Although clinical trials have demonstrated the clinical effectiveness of these newer drugs, they are hundreds of times more expensive than other ... diabetes drugs,” the researchers note.
On the other hand, costs may fall in the coming years when these new drugs come off-patent.
The current study was designed to help inform future clinical guidelines.
The researchers created a population simulation model based on the United Kingdom Prospective Diabetes Study, Outcomes Model version 2 (UKPDS OM2) for diabetes-related complications and mortality, with added information about hypoglycemic events, quality of life, and U.S. costs.
The researchers also identified a nationally representative sample of people who would be eligible to start first-line diabetes therapy when their A1c reached 7% for the model.
Using National Health and Nutrition Examination Survey (NHANES) data (2013-2016), the researchers identified about 7.3 million U.S. adults aged 18 and older with self-reported diabetes or an A1c greater than 6.5% with no reported use of diabetes medications.
Patients were an average age of 55, and 55% were women. They had had diabetes for an average of 4.2 years, and 36% had a history of diabetes complications.
The model projected that patients would have an improved life expectancy of 3.0 and 3.4 months from first-line SGLT2 inhibitors and GLP-1 agonists, respectively, compared with initial therapy with metformin due to reduced rates of macrovascular disease.
“However, the current drug costs would be too high to encourage their adoption as first-line for usual clinical practice,” the researchers report.
‘Disparities could remain for decades’
Generic SGLT2 inhibitors could enter the marketplace shortly, because one of two dapagliflozin patents expired in October 2020 and approval for generic alternatives has been sought from the U.S. Food and Drug Administration, Dr. Choi and colleagues note.
However, it could still take decades for medication prices to drop low enough to become affordable, the group cautions. For example, a generic GLP-1 agonist became available in 2017, but costs remain high.
“Without external incentives,” the group writes, “limited access to these drug classes will likely persist (for example, due to higher copays or requirements for prior authorizations), as will further diabetes disparities – for decades into the future – because of differential access to care due to insurance (for example, private vs. public), which often tracks race and ethnicity.”
The study was supported by the American Diabetes Association. Dr. Choi was supported by a National Institutes of Health, National Institute on Aging grant. Dr. Laiteerapong and other co-authors are members of the National Institute of Diabetes and Digestive and Kidney Diseases Chicago Center for Diabetes Translation Research at the University of Chicago. Dr. Choi and Dr. Laiteerapong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
To be cost effective, compared with metformin, for initial therapy for type 2 diabetes, prices for a sodium-glucose cotransporter-2 (SGLT2) inhibitor or a glucagon-like peptide-1 (GLP-1) agonist would have to fall by at least 70% and at least 90%, respectively, according to estimates.
The study, modeled on U.S. patients, by Jin G. Choi, MD, and colleagues, was published online Oct. 3 in the Annals of Internal Medicine.
The researchers simulated the lifetime incidence, prevalence, mortality, and costs associated with three different first-line treatment strategies – metformin, an SGLT2 inhibitor, or a GLP-1 agonist – in U.S. patients with untreated type 2 diabetes.
Compared with patients who received initial treatment with metformin, those who received one of the newer drugs had 4.4% to 5.2% lower lifetime rates of congestive heart failure, ischemic heart disease, myocardial infarction, and stroke.
However, to be cost-effective at under $150,000 per quality-adjusted life-years (QALY), SGLT2 inhibitors would need to cost less than $5 a day ($1,800 a year), and GLP-1 agonists would have to cost less than $6 a day ($2,100 a year), a lot less than now.
Knowing how expensive these drugs are, “I am not surprised” that the model predicts that the price would have to drop so much to make them cost-effective, compared with first-line treatment with metformin, senior author Neda Laiteerapong, MD, said in an interview.
“But I am disappointed,” she said, because these drugs are very effective, and if the prices were lower, more people could benefit.
“In the interest of improving access to high-quality care in the United States, our study results indicate the need to reduce SGLT2 inhibitor and GLP-1 receptor agonist medication costs substantially for patients with type 2 [diabetes] to improve health outcomes and prevent exacerbating diabetes health disparities,” the researchers conclude.
One way that the newer drugs might be more widely affordable is if the government became involved, possibly by passing a law similar to the Affordable Insulin Now Act, speculated Dr. Laiteerapong, who is associate director at the Center for Chronic Disease Research and Policy, University of Chicago.
‘Current prices too high to encourage first-line adoption’
Guidelines recommend the use of SGLT2 inhibitors and GLP-1 agonists as second-line therapies for patients with type 2 diabetes, but it has not been clear if clinical benefits would outweigh costs for use as first-line therapies.
“Although clinical trials have demonstrated the clinical effectiveness of these newer drugs, they are hundreds of times more expensive than other ... diabetes drugs,” the researchers note.
On the other hand, costs may fall in the coming years when these new drugs come off-patent.
The current study was designed to help inform future clinical guidelines.
The researchers created a population simulation model based on the United Kingdom Prospective Diabetes Study, Outcomes Model version 2 (UKPDS OM2) for diabetes-related complications and mortality, with added information about hypoglycemic events, quality of life, and U.S. costs.
The researchers also identified a nationally representative sample of people who would be eligible to start first-line diabetes therapy when their A1c reached 7% for the model.
Using National Health and Nutrition Examination Survey (NHANES) data (2013-2016), the researchers identified about 7.3 million U.S. adults aged 18 and older with self-reported diabetes or an A1c greater than 6.5% with no reported use of diabetes medications.
Patients were an average age of 55, and 55% were women. They had had diabetes for an average of 4.2 years, and 36% had a history of diabetes complications.
The model projected that patients would have an improved life expectancy of 3.0 and 3.4 months from first-line SGLT2 inhibitors and GLP-1 agonists, respectively, compared with initial therapy with metformin due to reduced rates of macrovascular disease.
“However, the current drug costs would be too high to encourage their adoption as first-line for usual clinical practice,” the researchers report.
‘Disparities could remain for decades’
Generic SGLT2 inhibitors could enter the marketplace shortly, because one of two dapagliflozin patents expired in October 2020 and approval for generic alternatives has been sought from the U.S. Food and Drug Administration, Dr. Choi and colleagues note.
However, it could still take decades for medication prices to drop low enough to become affordable, the group cautions. For example, a generic GLP-1 agonist became available in 2017, but costs remain high.
“Without external incentives,” the group writes, “limited access to these drug classes will likely persist (for example, due to higher copays or requirements for prior authorizations), as will further diabetes disparities – for decades into the future – because of differential access to care due to insurance (for example, private vs. public), which often tracks race and ethnicity.”
The study was supported by the American Diabetes Association. Dr. Choi was supported by a National Institutes of Health, National Institute on Aging grant. Dr. Laiteerapong and other co-authors are members of the National Institute of Diabetes and Digestive and Kidney Diseases Chicago Center for Diabetes Translation Research at the University of Chicago. Dr. Choi and Dr. Laiteerapong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Death of son reinforces flu vaccination message
“It was what the CDC [Centers for Disease Control and Prevention] would call classic influenza-like illness,” Dr. Teichman said. “It was too late to start antivirals, so I gave him advice on symptomatic treatment. We texted the next day, and I was glad to hear that his fever was trending down and that he was feeling a little bit better.”
Two days later, his son called again.
“He said he was having trouble breathing, and over the phone I could hear him hyperventilating.” The retired pediatrician and health care executive told his son to seek medical care.
“Then I got the call that no parent wants to get.”
Brent’s cousin Jake called saying he couldn’t wake Brent up.
“I called Jake back a few minutes later and asked him to hold up the phone,” Dr. Teichman said. “I listened to EMS working on my son, calling for round after round of many medications. He was in arrest and they couldn’t revive him.”
“To this day when I close my eyes at night, I still hear the beeping of those monitors.”
Brent had no health conditions to put him at higher risk for complications of the flu. “Brent was a wonderful son, brother, uncle, and friend. He had a passion for everything he did, and that included his chosen calling of the culinary arts but also included University of Kentucky sports,” Dr. Teichman said.
Brent planned to get a flu vaccine but had not done it yet. “In his obituary, we requested that, in lieu of flowers or donations, people go get their flu shot,” Dr. Teichman said.
“I’m here today to put a face on influenza,” Dr. Teichman said at a news briefing Oct. 4 on preventing the flu and pneumococcal disease, sponsored by the National Foundation for Infectious Diseases.
New survey numbers ‘alarming’
The NFID commissioned a national survey of more than 1,000 U.S. adults to better understand their knowledge and attitudes about the flu, pneumococcal disease, vaccines, and the impact of COVID-19.
“We were alarmed to learn that only 49% of U.S. adults plan to get their flu vaccine this season,” said Patricia A. “Patsy” Stinchfield, a registered nurse, NFID president, and moderator of the news briefing. “That is not good enough.”
In addition, 22% of people at higher risk for flu-related complications do not plan to get vaccinated this season. “That’s a dangerous risk to take,” Ms. Stinchfield said.
An encouraging finding, she said, is that 69% of adults surveyed recognize that an annual flu vaccination is the best way to prevent flu-related hospitalizations and death.
“So, most people know what to do. We just need to do it,” she said.
The top reason for not getting a flu shot in 2022 mentioned by 41% of people surveyed, is they do not think vaccines work very well. Another 39% are concerned about vaccine side effects, and 28% skip the vaccine because they “never get the flu.”
The experts on the panel emphasized the recommendation that all Americans 6 months or older get the flu vaccine, preferably by the end of October. Vaccination is especially important for those at higher risk of complications from the flu, including children under 5, pregnant women, people with one or more health conditions, the immunocompromised, and Americans 65 years and older.
Ms. Stinchfield acknowledged that the effectiveness of the flu vaccine varies season to season, but even if the vaccine does not completely match the circulating viruses, it can help prevent serious outcomes like hospitalization and death. One of the serious potential complications is pneumonia or “pneumococcal disease.”
“Our survey shows that only 29% of those at risk have been advised to receive a pneumococcal vaccine,” Ms. Stinchfield said. “The good news is that, among those who were advised to get the vaccine, 74% did receive their pneumococcal vaccine,” she said. “This underscores a key point to you, my fellow clinicians: As health professionals, our recommendations matter.”
Higher doses for 65+ Americans
The CDC updated recommendations this flu season for adults 65 and older to receive one of three preferentially recommended flu vaccines, said CDC Director Rochelle Walensky, MD. The CDC is recommending higher-dose, stronger vaccines for older Americans “based on a review of the available studies, which suggested that in this age group, these vaccines are potentially more effective than standard-dose ... vaccines.”
During most seasons, people 65 and older bear the greatest burden of severe flu disease, accounting for most flu-related hospitalizations and deaths.
“They are the largest vulnerable segment of our society,” Dr. Walensky said.
What will this flu season be like?
Health officials in the flu vaccine business also tend to be in the flu season prediction business. That includes Dr. Walensky.
“While we will never exactly know what each flu season will hold, we do know that every year, the best way you can protect yourself and those around you is to get your annual flu vaccine,” she said while taking part remotely in the briefing.
How severe will the flu season be in 2022-23? William Schaffner, MD, said he gets that question a lot. “Don’t think about that. Just focus on the fact that flu will be with us each year.
“We were a little bit spoiled. We’ve had two mild influenza seasons,” said Dr. Schaffner, medical director of NFID and a professor of infectious diseases and preventive medicine at Vanderbilt University, Nashville, Tenn. “I think with all the interest in COVID, people have rather forgotten about influenza. I’ve had to remind them that this is yet another serious winter respiratory virus.
“As I like to say, flu is fickle. It’s difficult to predict how serious this next outbreak of influenza this season is going to be. We could look at what happened in the Southern Hemisphere,” he said.
For example, Australia had the worst influenza season in the past 5 years, Schaffner said. “If you want a hint of what might happen here and you want yet another reason to be vaccinated, there it is.”
What we do know, Dr. Walensky said, is that the timing and severity of the past two flu seasons in the U.S. have been different than typical flu seasons. “And this is likely due to the COVID mitigation measures and other changes in circulating respiratory viruses.” Also, although last flu season was “relatively mild,” there was more flu activity than in the prior, 2020-21 season.
Also, Dr. Walensky said, last season’s flu cases began to increase in November and remained elevated until mid-June, “making it the latest season on record.”
The official cause of Brent Teichman’s death was multilobar pneumonia, cause undetermined. “But after 30-plus years as a pediatrician ... I know influenza when I see it,” Dr. Teichman said.
“There’s a hole in our hearts that will never heal. Loss of a child is devastating,” he said. The flu “can take the life of a healthy young person, as it did to my son.
“And for all those listening to my story who are vaccine hesitant, do it for those who love you. So that they won’t walk the path that we and many other families in this country have walked.”
To prove their point, Dr. Teichman and Ms. Stinchfield raised their sleeves and received flu shots during the news briefing.
“This one is for Brent,” Dr. Teichman said.
A version of this article first appeared on WebMD.com.
“It was what the CDC [Centers for Disease Control and Prevention] would call classic influenza-like illness,” Dr. Teichman said. “It was too late to start antivirals, so I gave him advice on symptomatic treatment. We texted the next day, and I was glad to hear that his fever was trending down and that he was feeling a little bit better.”
Two days later, his son called again.
“He said he was having trouble breathing, and over the phone I could hear him hyperventilating.” The retired pediatrician and health care executive told his son to seek medical care.
“Then I got the call that no parent wants to get.”
Brent’s cousin Jake called saying he couldn’t wake Brent up.
“I called Jake back a few minutes later and asked him to hold up the phone,” Dr. Teichman said. “I listened to EMS working on my son, calling for round after round of many medications. He was in arrest and they couldn’t revive him.”
“To this day when I close my eyes at night, I still hear the beeping of those monitors.”
Brent had no health conditions to put him at higher risk for complications of the flu. “Brent was a wonderful son, brother, uncle, and friend. He had a passion for everything he did, and that included his chosen calling of the culinary arts but also included University of Kentucky sports,” Dr. Teichman said.
Brent planned to get a flu vaccine but had not done it yet. “In his obituary, we requested that, in lieu of flowers or donations, people go get their flu shot,” Dr. Teichman said.
“I’m here today to put a face on influenza,” Dr. Teichman said at a news briefing Oct. 4 on preventing the flu and pneumococcal disease, sponsored by the National Foundation for Infectious Diseases.
New survey numbers ‘alarming’
The NFID commissioned a national survey of more than 1,000 U.S. adults to better understand their knowledge and attitudes about the flu, pneumococcal disease, vaccines, and the impact of COVID-19.
“We were alarmed to learn that only 49% of U.S. adults plan to get their flu vaccine this season,” said Patricia A. “Patsy” Stinchfield, a registered nurse, NFID president, and moderator of the news briefing. “That is not good enough.”
In addition, 22% of people at higher risk for flu-related complications do not plan to get vaccinated this season. “That’s a dangerous risk to take,” Ms. Stinchfield said.
An encouraging finding, she said, is that 69% of adults surveyed recognize that an annual flu vaccination is the best way to prevent flu-related hospitalizations and death.
“So, most people know what to do. We just need to do it,” she said.
The top reason for not getting a flu shot in 2022 mentioned by 41% of people surveyed, is they do not think vaccines work very well. Another 39% are concerned about vaccine side effects, and 28% skip the vaccine because they “never get the flu.”
The experts on the panel emphasized the recommendation that all Americans 6 months or older get the flu vaccine, preferably by the end of October. Vaccination is especially important for those at higher risk of complications from the flu, including children under 5, pregnant women, people with one or more health conditions, the immunocompromised, and Americans 65 years and older.
Ms. Stinchfield acknowledged that the effectiveness of the flu vaccine varies season to season, but even if the vaccine does not completely match the circulating viruses, it can help prevent serious outcomes like hospitalization and death. One of the serious potential complications is pneumonia or “pneumococcal disease.”
“Our survey shows that only 29% of those at risk have been advised to receive a pneumococcal vaccine,” Ms. Stinchfield said. “The good news is that, among those who were advised to get the vaccine, 74% did receive their pneumococcal vaccine,” she said. “This underscores a key point to you, my fellow clinicians: As health professionals, our recommendations matter.”
Higher doses for 65+ Americans
The CDC updated recommendations this flu season for adults 65 and older to receive one of three preferentially recommended flu vaccines, said CDC Director Rochelle Walensky, MD. The CDC is recommending higher-dose, stronger vaccines for older Americans “based on a review of the available studies, which suggested that in this age group, these vaccines are potentially more effective than standard-dose ... vaccines.”
During most seasons, people 65 and older bear the greatest burden of severe flu disease, accounting for most flu-related hospitalizations and deaths.
“They are the largest vulnerable segment of our society,” Dr. Walensky said.
What will this flu season be like?
Health officials in the flu vaccine business also tend to be in the flu season prediction business. That includes Dr. Walensky.
“While we will never exactly know what each flu season will hold, we do know that every year, the best way you can protect yourself and those around you is to get your annual flu vaccine,” she said while taking part remotely in the briefing.
How severe will the flu season be in 2022-23? William Schaffner, MD, said he gets that question a lot. “Don’t think about that. Just focus on the fact that flu will be with us each year.
“We were a little bit spoiled. We’ve had two mild influenza seasons,” said Dr. Schaffner, medical director of NFID and a professor of infectious diseases and preventive medicine at Vanderbilt University, Nashville, Tenn. “I think with all the interest in COVID, people have rather forgotten about influenza. I’ve had to remind them that this is yet another serious winter respiratory virus.
“As I like to say, flu is fickle. It’s difficult to predict how serious this next outbreak of influenza this season is going to be. We could look at what happened in the Southern Hemisphere,” he said.
For example, Australia had the worst influenza season in the past 5 years, Schaffner said. “If you want a hint of what might happen here and you want yet another reason to be vaccinated, there it is.”
What we do know, Dr. Walensky said, is that the timing and severity of the past two flu seasons in the U.S. have been different than typical flu seasons. “And this is likely due to the COVID mitigation measures and other changes in circulating respiratory viruses.” Also, although last flu season was “relatively mild,” there was more flu activity than in the prior, 2020-21 season.
Also, Dr. Walensky said, last season’s flu cases began to increase in November and remained elevated until mid-June, “making it the latest season on record.”
The official cause of Brent Teichman’s death was multilobar pneumonia, cause undetermined. “But after 30-plus years as a pediatrician ... I know influenza when I see it,” Dr. Teichman said.
“There’s a hole in our hearts that will never heal. Loss of a child is devastating,” he said. The flu “can take the life of a healthy young person, as it did to my son.
“And for all those listening to my story who are vaccine hesitant, do it for those who love you. So that they won’t walk the path that we and many other families in this country have walked.”
To prove their point, Dr. Teichman and Ms. Stinchfield raised their sleeves and received flu shots during the news briefing.
“This one is for Brent,” Dr. Teichman said.
A version of this article first appeared on WebMD.com.
“It was what the CDC [Centers for Disease Control and Prevention] would call classic influenza-like illness,” Dr. Teichman said. “It was too late to start antivirals, so I gave him advice on symptomatic treatment. We texted the next day, and I was glad to hear that his fever was trending down and that he was feeling a little bit better.”
Two days later, his son called again.
“He said he was having trouble breathing, and over the phone I could hear him hyperventilating.” The retired pediatrician and health care executive told his son to seek medical care.
“Then I got the call that no parent wants to get.”
Brent’s cousin Jake called saying he couldn’t wake Brent up.
“I called Jake back a few minutes later and asked him to hold up the phone,” Dr. Teichman said. “I listened to EMS working on my son, calling for round after round of many medications. He was in arrest and they couldn’t revive him.”
“To this day when I close my eyes at night, I still hear the beeping of those monitors.”
Brent had no health conditions to put him at higher risk for complications of the flu. “Brent was a wonderful son, brother, uncle, and friend. He had a passion for everything he did, and that included his chosen calling of the culinary arts but also included University of Kentucky sports,” Dr. Teichman said.
Brent planned to get a flu vaccine but had not done it yet. “In his obituary, we requested that, in lieu of flowers or donations, people go get their flu shot,” Dr. Teichman said.
“I’m here today to put a face on influenza,” Dr. Teichman said at a news briefing Oct. 4 on preventing the flu and pneumococcal disease, sponsored by the National Foundation for Infectious Diseases.
New survey numbers ‘alarming’
The NFID commissioned a national survey of more than 1,000 U.S. adults to better understand their knowledge and attitudes about the flu, pneumococcal disease, vaccines, and the impact of COVID-19.
“We were alarmed to learn that only 49% of U.S. adults plan to get their flu vaccine this season,” said Patricia A. “Patsy” Stinchfield, a registered nurse, NFID president, and moderator of the news briefing. “That is not good enough.”
In addition, 22% of people at higher risk for flu-related complications do not plan to get vaccinated this season. “That’s a dangerous risk to take,” Ms. Stinchfield said.
An encouraging finding, she said, is that 69% of adults surveyed recognize that an annual flu vaccination is the best way to prevent flu-related hospitalizations and death.
“So, most people know what to do. We just need to do it,” she said.
The top reason for not getting a flu shot in 2022 mentioned by 41% of people surveyed, is they do not think vaccines work very well. Another 39% are concerned about vaccine side effects, and 28% skip the vaccine because they “never get the flu.”
The experts on the panel emphasized the recommendation that all Americans 6 months or older get the flu vaccine, preferably by the end of October. Vaccination is especially important for those at higher risk of complications from the flu, including children under 5, pregnant women, people with one or more health conditions, the immunocompromised, and Americans 65 years and older.
Ms. Stinchfield acknowledged that the effectiveness of the flu vaccine varies season to season, but even if the vaccine does not completely match the circulating viruses, it can help prevent serious outcomes like hospitalization and death. One of the serious potential complications is pneumonia or “pneumococcal disease.”
“Our survey shows that only 29% of those at risk have been advised to receive a pneumococcal vaccine,” Ms. Stinchfield said. “The good news is that, among those who were advised to get the vaccine, 74% did receive their pneumococcal vaccine,” she said. “This underscores a key point to you, my fellow clinicians: As health professionals, our recommendations matter.”
Higher doses for 65+ Americans
The CDC updated recommendations this flu season for adults 65 and older to receive one of three preferentially recommended flu vaccines, said CDC Director Rochelle Walensky, MD. The CDC is recommending higher-dose, stronger vaccines for older Americans “based on a review of the available studies, which suggested that in this age group, these vaccines are potentially more effective than standard-dose ... vaccines.”
During most seasons, people 65 and older bear the greatest burden of severe flu disease, accounting for most flu-related hospitalizations and deaths.
“They are the largest vulnerable segment of our society,” Dr. Walensky said.
What will this flu season be like?
Health officials in the flu vaccine business also tend to be in the flu season prediction business. That includes Dr. Walensky.
“While we will never exactly know what each flu season will hold, we do know that every year, the best way you can protect yourself and those around you is to get your annual flu vaccine,” she said while taking part remotely in the briefing.
How severe will the flu season be in 2022-23? William Schaffner, MD, said he gets that question a lot. “Don’t think about that. Just focus on the fact that flu will be with us each year.
“We were a little bit spoiled. We’ve had two mild influenza seasons,” said Dr. Schaffner, medical director of NFID and a professor of infectious diseases and preventive medicine at Vanderbilt University, Nashville, Tenn. “I think with all the interest in COVID, people have rather forgotten about influenza. I’ve had to remind them that this is yet another serious winter respiratory virus.
“As I like to say, flu is fickle. It’s difficult to predict how serious this next outbreak of influenza this season is going to be. We could look at what happened in the Southern Hemisphere,” he said.
For example, Australia had the worst influenza season in the past 5 years, Schaffner said. “If you want a hint of what might happen here and you want yet another reason to be vaccinated, there it is.”
What we do know, Dr. Walensky said, is that the timing and severity of the past two flu seasons in the U.S. have been different than typical flu seasons. “And this is likely due to the COVID mitigation measures and other changes in circulating respiratory viruses.” Also, although last flu season was “relatively mild,” there was more flu activity than in the prior, 2020-21 season.
Also, Dr. Walensky said, last season’s flu cases began to increase in November and remained elevated until mid-June, “making it the latest season on record.”
The official cause of Brent Teichman’s death was multilobar pneumonia, cause undetermined. “But after 30-plus years as a pediatrician ... I know influenza when I see it,” Dr. Teichman said.
“There’s a hole in our hearts that will never heal. Loss of a child is devastating,” he said. The flu “can take the life of a healthy young person, as it did to my son.
“And for all those listening to my story who are vaccine hesitant, do it for those who love you. So that they won’t walk the path that we and many other families in this country have walked.”
To prove their point, Dr. Teichman and Ms. Stinchfield raised their sleeves and received flu shots during the news briefing.
“This one is for Brent,” Dr. Teichman said.
A version of this article first appeared on WebMD.com.
Recurrent drainage from an old gunshot wound
An x-ray revealed a metal density in the area of concern that was consistent with a bullet fragment or other metallic foreign body. Since there were no lucencies on x-ray or tracking from the area of concern to the metacarpal, the diagnosis was confirmed as an infected foreign body. The history was very concerning for osteomyelitis, given that the patient had sustained a GSW and had undergone surgical repair with hardware. (Shifting hardware can also lead to callus formation and skin breakdown.)
The patient was told that he’d retained a bullet fragment or foreign body that caused a chronic infection and the recurrent drainage. In addition, the hardware spanning the gap between the remnants of his proximal and distal metacarpal had broken as a result of fatigue. He was referred to a surgeon to remove the foreign body and treat the infection. The patient was advised that he might also need replacement hardware and a bone graft.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo.

An x-ray revealed a metal density in the area of concern that was consistent with a bullet fragment or other metallic foreign body. Since there were no lucencies on x-ray or tracking from the area of concern to the metacarpal, the diagnosis was confirmed as an infected foreign body. The history was very concerning for osteomyelitis, given that the patient had sustained a GSW and had undergone surgical repair with hardware. (Shifting hardware can also lead to callus formation and skin breakdown.)
The patient was told that he’d retained a bullet fragment or foreign body that caused a chronic infection and the recurrent drainage. In addition, the hardware spanning the gap between the remnants of his proximal and distal metacarpal had broken as a result of fatigue. He was referred to a surgeon to remove the foreign body and treat the infection. The patient was advised that he might also need replacement hardware and a bone graft.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo.

An x-ray revealed a metal density in the area of concern that was consistent with a bullet fragment or other metallic foreign body. Since there were no lucencies on x-ray or tracking from the area of concern to the metacarpal, the diagnosis was confirmed as an infected foreign body. The history was very concerning for osteomyelitis, given that the patient had sustained a GSW and had undergone surgical repair with hardware. (Shifting hardware can also lead to callus formation and skin breakdown.)
The patient was told that he’d retained a bullet fragment or foreign body that caused a chronic infection and the recurrent drainage. In addition, the hardware spanning the gap between the remnants of his proximal and distal metacarpal had broken as a result of fatigue. He was referred to a surgeon to remove the foreign body and treat the infection. The patient was advised that he might also need replacement hardware and a bone graft.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo.
Children and COVID: Weekly cases dropped by 57% in September
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
Shortage of family physicians in Canada intensified during pandemic
A higher percentage of family physicians quit during the early months of the pandemic than the average yearly percentage that did in the prior decade, according to data from Canada.
The researchers conducted two analyses of billing claims data for family physicians practicing in Ontario. They examined data for a period from 2010 to 2019 – before the onset of the pandemic – and from 2019 through 2020. The findings were published in Annals of Family Medicine.
Overall, the proportion of family physicians who stopped working rose from an average of 1.6% each year for the period between 2010 and 2019 to 3% in the period from 2019 to 2020. The pandemic data set included 12,247 physicians in Ontario. Of these, 385 (3.1%) reported no billings in the first 6 months of the pandemic.
Compared with family physicians billing for work during the pandemic, those reporting no billings were significantly more likely to be 75 years or older (13.0% vs. 3.4%), to have patient panels of less than 500 patients (40.0% vs. 25.8%), and to be eligible for fee-for-service reimbursement (37.7% vs. 24.9%; P less than .001 for all). The family physicians who reported no billing early in the pandemic also had fewer billing days in the previous year (mean of 73 days vs. 101 days, P less than .001).
In a regression analysis, the absolute increase in the percentage of family physicians who stopped working was 0.3% per year from 2010 to 2019, but rose to 1.2% between 2019 and 2020.
Challenges to family physicians in Ontario in the early months of the COVID-19 pandemic included reduced revenue, inability to keep offices fully staffed, and problems obtaining enough personal protective equipment. Such challenges may have prompted some family physicians to stop working prematurely, but more research is needed in other settings, wrote study author Tara Kiran, MD, of the University of Toronto, and colleagues.
“There were a lot of stories and suggestions that more family physicians were choosing to retire due to COVID,” Michael Green, MD, a coauthor of the paper, said in an interview. “Given the preexisting shortages we thought it would be important to see if this was true, and how big of an issue it was,” he said.
Although the absolute number of primary care physicians who stopped working is small, the implications are large given the ongoing shortage of family physicians in Canada, the researchers wrote.
The characteristics of physicians stopping work, such as older age and smaller practice size, were consistent with that of physicians preparing for retirement, the researchers noted. In addition, 56% of the family physicians who stopped working during the pandemic practiced in a patient enrollment model, in which patients are enrolled and between 15% and 70% of payment is based on age and sex. In this study, approximately 80% of physicians worked in this model. The remaining 20% operated in independent, fee-for-service practices.
“Although we cannot directly attribute causation, we hypothesize that some family physicians accelerated their retirement plans because of the pandemic,” the researchers noted. They proposed that possible reasons include health concerns, increased costs of infection prevention and control, reduced revenue from office visits, and burnout. The current study did not examine these issues.
Additional studies are needed to understand the impact on population health, the researchers concluded, but they estimated that the number of family physicians who stopped work during the pandemic would have provided care for approximately 170,000 patients.
The study findings reflect a genuine turnover by family physicians, vs. a departure from family practice to a fellowship and practice in another specialty, Dr. Green said. “We looked at physician billings to determine who stopped practicing, so we report only on those who stopped billing the Ontario Health Insurance Program altogether,” he explained.
The ongoing pandemic accelerated the issue of an upcoming wave of physician retirements and added to an already large number of people without a family physician, Dr. Green noted.
“We know there will be significant shortages of family physicians if we don’t modernize our ways of delivering primary care,” said Dr. Green. More research is needed on how to support family doctors with teams and administrative supports to allow them to provide high quality care to more patients, he said. Better models to estimate health workforce needs in primary care are needed as well, he added.
In the United States, a physician shortage has been growing since before the pandemic, according to a report published in 2021 by the Association of American Medical Colleges. In this report, “The Complexities of Physician Supply and Demand: Projections from 2019 to 2034,” the authors specifically projected a primary care physician shortage of 17,800 to 48,000 by 2034. This projection is in part based on an increase in the percentage of the U.S. population aged 65 years and older, which will increase the demand for care, according to the authors. The report also confirmed that many U.S. physicians are approaching retirement age and that more than two of five active physicians will be 65 years or older within the next 10 years.
However, the authors of this U.S. report acknowledged that the impact of the pandemic on existing primary care shortages remains unclear.
“There are still many unknowns about the direct short-term and long-term impacts of COVID-19 on the physician workforce, and it may be several years before those impacts are clearly understood,” they said in the executive summary of their report.
Alison N. Huffstetler, MD, a coauthor of a recent report that tried to identify the active primary care workforce in Virginia, said, “We know from other research that there are not enough primary care doctors, right now, to do the work that needs to be done – some citations have noted it would take a primary care doc over 20 hours a day just to provide preventive care.
“As our population continues to age, live longer, and need more complex care management, we must ensure we have an accountable, accessible, and knowledgeable primary care network to care for our communities,” she said.
Current state of primary care in Virginia
The study by Dr. Huffstetler, of Virginia Commonwealth University, Richmond, and colleagues was published in Annals of Family Medicine. It used a novel strategy involving the analysis of state all-payer claims data to determine how many physicians were practicing primary care in Virginia.
The researchers used the National Plan and Provider Enumeration System (NPPES) and the Virginia All-Payer Claims Database (VA-APCD) and identified all Virginia physicians and their specialties through the NPPES between 2015 and 2019. Active physicians were defined as those with at least one claim in the VA-APCD during the study period. They identified 20,976 active physicians in Virginia, 28.1% of whom were classified as primary care. Of these, 52% were family medicine physicians, 18.5% were internal medicine physicians, 16.8% were pediatricians, 11.8% were ob.gyns., and 0.5% were other specialists.
Clinician specialties were identified via specialty codes from the NPPES. Physicians were identified as primary care providers in two ways. The first way was by identifying those who had a National Uniform Claim Committee (NUCC) taxonomy of family medicine. The NUCC identifies a provider’s specialty using several levels of classification based on board certification and subspecialty certification data. The second identifier was having been a physician who had billed for at least 10 wellness visit codes from Jan. 1, 2019, through Dec. 31, 2019.
Over the 5-year study period (2015-2019), the counts and percentages of primary care physicians in the workforce remained stable, and the overall number of physicians in the state increased by 3.5%, the researchers noted. A total of 60.45% of all physicians and 60.87% of primary care physicians remained active, and 11.66% of all physicians had a claim in only 1 of the 5 years.
How distribution and access impact patients
In an interview, Dr. Huffstetler said the study she and colleagues authored “offers a transparent and reproducible process for identifying primary care physicians in a state, where they practice, and what changes in staffing occur over time.”
“In Virginia, this is particularly important, as we recently expanded Medicaid, making primary care more affordable for over 500,000 people,” she said. “We also saw the importance of distribution and accessibility to primary care over the past 3 years of COVID. In order to adequately prepare for community needs in the coming years, we must know who is providing primary care, and where they are.”
However, the model used in this study has its limitations, Dr. Huffstetler said, including the lack of a definitive definition of primary care using claims data.
“We used a data-informed wellness visit threshold, but it is likely that primary care is delivered in some locations without claims that are reflected by a wellness visit, and we hope to look at scope in the future to help refine these results,” she said.
Canadian study shows pandemic’s impact on patient care
“The pandemic’s impact on primary care remains palpable, and Dr. Kiran’s team has done an excellent analysis on the practice trends during the past several years,” Dr. Huffstetler said.
“The Canadian analysis uses claims in a similar manner to our study; however, it appears that they already knew who the FPs were in Ontario,” Dr. Huffstetler noted. “Their claims threshold of 50 for active practice was higher than ours, at only 1. Should those FPs have moved to a different specialty, the physicians would still have claims for the patients seen in other subspecialties. As such, I don’t suspect that their analysis miscalculated those that transitioned, rather than stopped practice,” she explained.
The Ontario study was supported by the Initial Credential Evaluation Service, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care, as well as by the Canadian Institutes of Health Research. Additional support came from the INSPIRE Primary Health Care Research Program, which is also funded by the Ontario Ministry of Health and Long-Term Care. The researchers had no financial conflicts to disclose.
The Virginia study was supported by the Department of Medical Assistance Services and the National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
The supply and demand report was conducted for the AAMC by IHS Markit, a global information company.
A higher percentage of family physicians quit during the early months of the pandemic than the average yearly percentage that did in the prior decade, according to data from Canada.
The researchers conducted two analyses of billing claims data for family physicians practicing in Ontario. They examined data for a period from 2010 to 2019 – before the onset of the pandemic – and from 2019 through 2020. The findings were published in Annals of Family Medicine.
Overall, the proportion of family physicians who stopped working rose from an average of 1.6% each year for the period between 2010 and 2019 to 3% in the period from 2019 to 2020. The pandemic data set included 12,247 physicians in Ontario. Of these, 385 (3.1%) reported no billings in the first 6 months of the pandemic.
Compared with family physicians billing for work during the pandemic, those reporting no billings were significantly more likely to be 75 years or older (13.0% vs. 3.4%), to have patient panels of less than 500 patients (40.0% vs. 25.8%), and to be eligible for fee-for-service reimbursement (37.7% vs. 24.9%; P less than .001 for all). The family physicians who reported no billing early in the pandemic also had fewer billing days in the previous year (mean of 73 days vs. 101 days, P less than .001).
In a regression analysis, the absolute increase in the percentage of family physicians who stopped working was 0.3% per year from 2010 to 2019, but rose to 1.2% between 2019 and 2020.
Challenges to family physicians in Ontario in the early months of the COVID-19 pandemic included reduced revenue, inability to keep offices fully staffed, and problems obtaining enough personal protective equipment. Such challenges may have prompted some family physicians to stop working prematurely, but more research is needed in other settings, wrote study author Tara Kiran, MD, of the University of Toronto, and colleagues.
“There were a lot of stories and suggestions that more family physicians were choosing to retire due to COVID,” Michael Green, MD, a coauthor of the paper, said in an interview. “Given the preexisting shortages we thought it would be important to see if this was true, and how big of an issue it was,” he said.
Although the absolute number of primary care physicians who stopped working is small, the implications are large given the ongoing shortage of family physicians in Canada, the researchers wrote.
The characteristics of physicians stopping work, such as older age and smaller practice size, were consistent with that of physicians preparing for retirement, the researchers noted. In addition, 56% of the family physicians who stopped working during the pandemic practiced in a patient enrollment model, in which patients are enrolled and between 15% and 70% of payment is based on age and sex. In this study, approximately 80% of physicians worked in this model. The remaining 20% operated in independent, fee-for-service practices.
“Although we cannot directly attribute causation, we hypothesize that some family physicians accelerated their retirement plans because of the pandemic,” the researchers noted. They proposed that possible reasons include health concerns, increased costs of infection prevention and control, reduced revenue from office visits, and burnout. The current study did not examine these issues.
Additional studies are needed to understand the impact on population health, the researchers concluded, but they estimated that the number of family physicians who stopped work during the pandemic would have provided care for approximately 170,000 patients.
The study findings reflect a genuine turnover by family physicians, vs. a departure from family practice to a fellowship and practice in another specialty, Dr. Green said. “We looked at physician billings to determine who stopped practicing, so we report only on those who stopped billing the Ontario Health Insurance Program altogether,” he explained.
The ongoing pandemic accelerated the issue of an upcoming wave of physician retirements and added to an already large number of people without a family physician, Dr. Green noted.
“We know there will be significant shortages of family physicians if we don’t modernize our ways of delivering primary care,” said Dr. Green. More research is needed on how to support family doctors with teams and administrative supports to allow them to provide high quality care to more patients, he said. Better models to estimate health workforce needs in primary care are needed as well, he added.
In the United States, a physician shortage has been growing since before the pandemic, according to a report published in 2021 by the Association of American Medical Colleges. In this report, “The Complexities of Physician Supply and Demand: Projections from 2019 to 2034,” the authors specifically projected a primary care physician shortage of 17,800 to 48,000 by 2034. This projection is in part based on an increase in the percentage of the U.S. population aged 65 years and older, which will increase the demand for care, according to the authors. The report also confirmed that many U.S. physicians are approaching retirement age and that more than two of five active physicians will be 65 years or older within the next 10 years.
However, the authors of this U.S. report acknowledged that the impact of the pandemic on existing primary care shortages remains unclear.
“There are still many unknowns about the direct short-term and long-term impacts of COVID-19 on the physician workforce, and it may be several years before those impacts are clearly understood,” they said in the executive summary of their report.
Alison N. Huffstetler, MD, a coauthor of a recent report that tried to identify the active primary care workforce in Virginia, said, “We know from other research that there are not enough primary care doctors, right now, to do the work that needs to be done – some citations have noted it would take a primary care doc over 20 hours a day just to provide preventive care.
“As our population continues to age, live longer, and need more complex care management, we must ensure we have an accountable, accessible, and knowledgeable primary care network to care for our communities,” she said.
Current state of primary care in Virginia
The study by Dr. Huffstetler, of Virginia Commonwealth University, Richmond, and colleagues was published in Annals of Family Medicine. It used a novel strategy involving the analysis of state all-payer claims data to determine how many physicians were practicing primary care in Virginia.
The researchers used the National Plan and Provider Enumeration System (NPPES) and the Virginia All-Payer Claims Database (VA-APCD) and identified all Virginia physicians and their specialties through the NPPES between 2015 and 2019. Active physicians were defined as those with at least one claim in the VA-APCD during the study period. They identified 20,976 active physicians in Virginia, 28.1% of whom were classified as primary care. Of these, 52% were family medicine physicians, 18.5% were internal medicine physicians, 16.8% were pediatricians, 11.8% were ob.gyns., and 0.5% were other specialists.
Clinician specialties were identified via specialty codes from the NPPES. Physicians were identified as primary care providers in two ways. The first way was by identifying those who had a National Uniform Claim Committee (NUCC) taxonomy of family medicine. The NUCC identifies a provider’s specialty using several levels of classification based on board certification and subspecialty certification data. The second identifier was having been a physician who had billed for at least 10 wellness visit codes from Jan. 1, 2019, through Dec. 31, 2019.
Over the 5-year study period (2015-2019), the counts and percentages of primary care physicians in the workforce remained stable, and the overall number of physicians in the state increased by 3.5%, the researchers noted. A total of 60.45% of all physicians and 60.87% of primary care physicians remained active, and 11.66% of all physicians had a claim in only 1 of the 5 years.
How distribution and access impact patients
In an interview, Dr. Huffstetler said the study she and colleagues authored “offers a transparent and reproducible process for identifying primary care physicians in a state, where they practice, and what changes in staffing occur over time.”
“In Virginia, this is particularly important, as we recently expanded Medicaid, making primary care more affordable for over 500,000 people,” she said. “We also saw the importance of distribution and accessibility to primary care over the past 3 years of COVID. In order to adequately prepare for community needs in the coming years, we must know who is providing primary care, and where they are.”
However, the model used in this study has its limitations, Dr. Huffstetler said, including the lack of a definitive definition of primary care using claims data.
“We used a data-informed wellness visit threshold, but it is likely that primary care is delivered in some locations without claims that are reflected by a wellness visit, and we hope to look at scope in the future to help refine these results,” she said.
Canadian study shows pandemic’s impact on patient care
“The pandemic’s impact on primary care remains palpable, and Dr. Kiran’s team has done an excellent analysis on the practice trends during the past several years,” Dr. Huffstetler said.
“The Canadian analysis uses claims in a similar manner to our study; however, it appears that they already knew who the FPs were in Ontario,” Dr. Huffstetler noted. “Their claims threshold of 50 for active practice was higher than ours, at only 1. Should those FPs have moved to a different specialty, the physicians would still have claims for the patients seen in other subspecialties. As such, I don’t suspect that their analysis miscalculated those that transitioned, rather than stopped practice,” she explained.
The Ontario study was supported by the Initial Credential Evaluation Service, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care, as well as by the Canadian Institutes of Health Research. Additional support came from the INSPIRE Primary Health Care Research Program, which is also funded by the Ontario Ministry of Health and Long-Term Care. The researchers had no financial conflicts to disclose.
The Virginia study was supported by the Department of Medical Assistance Services and the National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
The supply and demand report was conducted for the AAMC by IHS Markit, a global information company.
A higher percentage of family physicians quit during the early months of the pandemic than the average yearly percentage that did in the prior decade, according to data from Canada.
The researchers conducted two analyses of billing claims data for family physicians practicing in Ontario. They examined data for a period from 2010 to 2019 – before the onset of the pandemic – and from 2019 through 2020. The findings were published in Annals of Family Medicine.
Overall, the proportion of family physicians who stopped working rose from an average of 1.6% each year for the period between 2010 and 2019 to 3% in the period from 2019 to 2020. The pandemic data set included 12,247 physicians in Ontario. Of these, 385 (3.1%) reported no billings in the first 6 months of the pandemic.
Compared with family physicians billing for work during the pandemic, those reporting no billings were significantly more likely to be 75 years or older (13.0% vs. 3.4%), to have patient panels of less than 500 patients (40.0% vs. 25.8%), and to be eligible for fee-for-service reimbursement (37.7% vs. 24.9%; P less than .001 for all). The family physicians who reported no billing early in the pandemic also had fewer billing days in the previous year (mean of 73 days vs. 101 days, P less than .001).
In a regression analysis, the absolute increase in the percentage of family physicians who stopped working was 0.3% per year from 2010 to 2019, but rose to 1.2% between 2019 and 2020.
Challenges to family physicians in Ontario in the early months of the COVID-19 pandemic included reduced revenue, inability to keep offices fully staffed, and problems obtaining enough personal protective equipment. Such challenges may have prompted some family physicians to stop working prematurely, but more research is needed in other settings, wrote study author Tara Kiran, MD, of the University of Toronto, and colleagues.
“There were a lot of stories and suggestions that more family physicians were choosing to retire due to COVID,” Michael Green, MD, a coauthor of the paper, said in an interview. “Given the preexisting shortages we thought it would be important to see if this was true, and how big of an issue it was,” he said.
Although the absolute number of primary care physicians who stopped working is small, the implications are large given the ongoing shortage of family physicians in Canada, the researchers wrote.
The characteristics of physicians stopping work, such as older age and smaller practice size, were consistent with that of physicians preparing for retirement, the researchers noted. In addition, 56% of the family physicians who stopped working during the pandemic practiced in a patient enrollment model, in which patients are enrolled and between 15% and 70% of payment is based on age and sex. In this study, approximately 80% of physicians worked in this model. The remaining 20% operated in independent, fee-for-service practices.
“Although we cannot directly attribute causation, we hypothesize that some family physicians accelerated their retirement plans because of the pandemic,” the researchers noted. They proposed that possible reasons include health concerns, increased costs of infection prevention and control, reduced revenue from office visits, and burnout. The current study did not examine these issues.
Additional studies are needed to understand the impact on population health, the researchers concluded, but they estimated that the number of family physicians who stopped work during the pandemic would have provided care for approximately 170,000 patients.
The study findings reflect a genuine turnover by family physicians, vs. a departure from family practice to a fellowship and practice in another specialty, Dr. Green said. “We looked at physician billings to determine who stopped practicing, so we report only on those who stopped billing the Ontario Health Insurance Program altogether,” he explained.
The ongoing pandemic accelerated the issue of an upcoming wave of physician retirements and added to an already large number of people without a family physician, Dr. Green noted.
“We know there will be significant shortages of family physicians if we don’t modernize our ways of delivering primary care,” said Dr. Green. More research is needed on how to support family doctors with teams and administrative supports to allow them to provide high quality care to more patients, he said. Better models to estimate health workforce needs in primary care are needed as well, he added.
In the United States, a physician shortage has been growing since before the pandemic, according to a report published in 2021 by the Association of American Medical Colleges. In this report, “The Complexities of Physician Supply and Demand: Projections from 2019 to 2034,” the authors specifically projected a primary care physician shortage of 17,800 to 48,000 by 2034. This projection is in part based on an increase in the percentage of the U.S. population aged 65 years and older, which will increase the demand for care, according to the authors. The report also confirmed that many U.S. physicians are approaching retirement age and that more than two of five active physicians will be 65 years or older within the next 10 years.
However, the authors of this U.S. report acknowledged that the impact of the pandemic on existing primary care shortages remains unclear.
“There are still many unknowns about the direct short-term and long-term impacts of COVID-19 on the physician workforce, and it may be several years before those impacts are clearly understood,” they said in the executive summary of their report.
Alison N. Huffstetler, MD, a coauthor of a recent report that tried to identify the active primary care workforce in Virginia, said, “We know from other research that there are not enough primary care doctors, right now, to do the work that needs to be done – some citations have noted it would take a primary care doc over 20 hours a day just to provide preventive care.
“As our population continues to age, live longer, and need more complex care management, we must ensure we have an accountable, accessible, and knowledgeable primary care network to care for our communities,” she said.
Current state of primary care in Virginia
The study by Dr. Huffstetler, of Virginia Commonwealth University, Richmond, and colleagues was published in Annals of Family Medicine. It used a novel strategy involving the analysis of state all-payer claims data to determine how many physicians were practicing primary care in Virginia.
The researchers used the National Plan and Provider Enumeration System (NPPES) and the Virginia All-Payer Claims Database (VA-APCD) and identified all Virginia physicians and their specialties through the NPPES between 2015 and 2019. Active physicians were defined as those with at least one claim in the VA-APCD during the study period. They identified 20,976 active physicians in Virginia, 28.1% of whom were classified as primary care. Of these, 52% were family medicine physicians, 18.5% were internal medicine physicians, 16.8% were pediatricians, 11.8% were ob.gyns., and 0.5% were other specialists.
Clinician specialties were identified via specialty codes from the NPPES. Physicians were identified as primary care providers in two ways. The first way was by identifying those who had a National Uniform Claim Committee (NUCC) taxonomy of family medicine. The NUCC identifies a provider’s specialty using several levels of classification based on board certification and subspecialty certification data. The second identifier was having been a physician who had billed for at least 10 wellness visit codes from Jan. 1, 2019, through Dec. 31, 2019.
Over the 5-year study period (2015-2019), the counts and percentages of primary care physicians in the workforce remained stable, and the overall number of physicians in the state increased by 3.5%, the researchers noted. A total of 60.45% of all physicians and 60.87% of primary care physicians remained active, and 11.66% of all physicians had a claim in only 1 of the 5 years.
How distribution and access impact patients
In an interview, Dr. Huffstetler said the study she and colleagues authored “offers a transparent and reproducible process for identifying primary care physicians in a state, where they practice, and what changes in staffing occur over time.”
“In Virginia, this is particularly important, as we recently expanded Medicaid, making primary care more affordable for over 500,000 people,” she said. “We also saw the importance of distribution and accessibility to primary care over the past 3 years of COVID. In order to adequately prepare for community needs in the coming years, we must know who is providing primary care, and where they are.”
However, the model used in this study has its limitations, Dr. Huffstetler said, including the lack of a definitive definition of primary care using claims data.
“We used a data-informed wellness visit threshold, but it is likely that primary care is delivered in some locations without claims that are reflected by a wellness visit, and we hope to look at scope in the future to help refine these results,” she said.
Canadian study shows pandemic’s impact on patient care
“The pandemic’s impact on primary care remains palpable, and Dr. Kiran’s team has done an excellent analysis on the practice trends during the past several years,” Dr. Huffstetler said.
“The Canadian analysis uses claims in a similar manner to our study; however, it appears that they already knew who the FPs were in Ontario,” Dr. Huffstetler noted. “Their claims threshold of 50 for active practice was higher than ours, at only 1. Should those FPs have moved to a different specialty, the physicians would still have claims for the patients seen in other subspecialties. As such, I don’t suspect that their analysis miscalculated those that transitioned, rather than stopped practice,” she explained.
The Ontario study was supported by the Initial Credential Evaluation Service, which is funded by the Ontario Ministry of Health and the Ministry of Long-Term Care, as well as by the Canadian Institutes of Health Research. Additional support came from the INSPIRE Primary Health Care Research Program, which is also funded by the Ontario Ministry of Health and Long-Term Care. The researchers had no financial conflicts to disclose.
The Virginia study was supported by the Department of Medical Assistance Services and the National Center for Advancing Translational Sciences. The researchers had no financial conflicts to disclose.
The supply and demand report was conducted for the AAMC by IHS Markit, a global information company.
FROM ANNALS OF FAMILY MEDICINE
Retinal imaging can predict cardiovascular mortality
according to a new study using data from the UK Biobank Eye and Vision Consortium and the European Prospective Investigation into Cancer (EPIC)–Norfolk study.
The researchers, from St. George’s University of London, Cambridge University, Kingston University, Moorfields Eye Hospital, and University College London, developed a method of artificial intelligence (AI)–enabled imaging of the retina’s vascular network that could accurately predict CVD and death, without the need for blood tests or blood pressure measurement.
The system “paves the way for a highly effective, noninvasive screening test for people at medium to high risk of circulatory disease that doesn’t have to be done in a clinic,” they said. “In the general population it could be used as a noncontact form of systemic vascular health check, to triage those at medium-high risk of circulatory mortality for further clinical risk assessment and appropriate intervention.” Optometry specialists welcomed the prospect and hailed it as “an exciting development.”
Retinal vessels give an accurate early indicator of CVD
The study, published online in the British Journal of Ophthalmology, was based on previous research showing that the width of retinal arterioles and venules seen on retinal imaging may provide an accurate early indicator of CVD, whereas current risk prediction frameworks aren’t always reliable in identifying people who will go on to develop or die of circulatory diseases.
The researchers developed a fully automated AI-enabled algorithm, called Quantitative Analysis of Retinal vessels Topology and Size (QUARTZ), to assess the potential of retinal vasculature imaging plus known risk factors to predict vascular health and death. They applied QUARTZ to retinal images from 88,052 UK Biobank participants aged 40-69 years, looking specifically at the width, vessel area, and degree of tortuosity of the retinal microvasculature, to develop prediction models for stroke, heart attack, and death from circulatory disease.
They then applied these models to the retinal images of 7,411 participants, aged 48-92 years, in the EPIC-Norfolk study. They then compared the performance of QUARTZ with the widely used Framingham Risk Scores framework.
The participants in the two studies were tracked for an average of 7.7 and 9.1 years, respectively, during which time there were 327 circulatory disease deaths among 64,144 UK Biobank participants (average age, 56.8 years) and 201 circulatory deaths among 5,862 EPIC-Norfolk participants (average age, 67.6 years).
Vessel characteristics important predictors of CVD mortality
Results from the QUARTZ models showed that in all participants, arteriolar and venular width, venular tortuosity, and width variation were important predictors of circulatory disease death. In addition, in women, but not in men, arteriolar and venular area were separate factors that contributed to risk prediction.
Overall, the predictive models, based on age, smoking, and medical history (antihypertensive or cholesterol lowering medication, diabetes, and history of stroke or MI) as well as retinal vasculature, captured between half and two-thirds of circulatory disease deaths in those most at risk, the authors said.
Compared with Framingham Risk Scores (FRS), the retinal vasculature (RV) models captured about 5% more cases of stroke in UK Biobank men, 8% more cases in UK Biobank women, and 3% more cases among EPIC-Norfolk men most at risk, but nearly 2% fewer cases among EPIC-Norfolk women. However, the team said that, while adding RV to FRS resulted in only marginal changes in prediction of stroke or MI, a simpler noninvasive risk score based on age, sex, smoking status, medical history, and RV “yielded comparable performance to FRS, without the need for blood sampling or BP measurement.”
Vasculometry low cost, noninvasive and with high street availability
They concluded: “Retinal imaging is established within clinic and hospital eye care and in optometric practices in the U.S. and U.K. AI-enabled vasculometry risk prediction is fully automated, low cost, noninvasive and has the potential for reaching a higher proportion of the population in the community because of “high street” availability and because blood sampling or sphygmomanometry are not needed.
“[Retinal vasculature] is a microvascular marker, hence offers better prediction for circulatory mortality and stroke, compared with MI, which is more macrovascular, except perhaps in women.
“In the general population it could be used as a noncontact form of systemic vascular health check, to triage those at medium-high risk of circulatory mortality for further clinical risk assessment and appropriate intervention.”
In the United Kingdom, for example, it could be included in the primary care NHS Health Check for those aged 41-74 years, they suggested. In addition, “high street” retinal scanning could directly feed into primary medical services and help achieve greater screening coverage for older age groups, who are likely to attend an optometric practice for visual correction, especially with the onset of presbyopia. “This would offer a novel approach to identify those at high risk of circulatory mortality, which is not currently screened for,” the team said.
Test could help to identify high-risk individuals
In a linked editorial, Ify Mordi, MD, and Emanuele Trucco, MD, of the University of Dundee (Scotland), said that CVD remains a significant cause of mortality and morbidity and the most common cause of death worldwide, accounting for a quarter of all U.K. deaths – and its burden is increasing. “Identification of individuals at high risk is particularly important,” they said, but current clinical risk scores to identify those at risk “are unfortunately not perfect,” so miss some of those who might benefit from preventative therapy.
“The retina is the only location that allows non-invasive direct visualisation of the vasculature, potentially providing a rich source of information.” In the new study, the measurements derived with the software tool, QUARTZ, were significantly associated with CVD, they said, with similar predictive performance to the Framingham clinical risk score.
“The results strengthen the evidence from several similar studies that the retina can be a useful and potentially disruptive source of information for CVD risk in personalised medicine.” However, a number of questions remain about how this knowledge could be integrated into clinical care, including who would conduct such a retinal screening program and who would act on the findings?
The editorial concluded: “What is now needed is for ophthalmologists, cardiologists, primary care physicians, and computer scientists to work together to design studies to determine whether using this information improves clinical outcome, and, if so, to work with regulatory bodies, scientific societies and healthcare systems to optimize clinical work flows and enable practical implementation in routine practice.”
‘Exciting development that could improve outcomes’
Asked to comment, Farah Topia, clinical and regulatory adviser at the Association of Optometrists, said: “This is an exciting development that could improve outcomes for many patients by enabling earlier detection of serious health risks. Patients attend optometric practice for a variety of reasons and this interaction could be used to a greater extent to help detect disease earlier. With optometrists available on every High Street, in the heart of communities, it’s an element of primary care that can be accessed quickly and easily, and optometrists are also already trained to have health and lifestyle discussion with patients.”
She added: “Retinal photographs are regularly taken when patients visit an optometrist, so being able to further enhance this process using AI is exciting.
“We look forward to seeing how this area develops and how optometrists can work together with other healthcare sectors to improve patient outcomes and ease the burden the NHS currently faces.”
The study was funded by the Medical Research Council Population and Systems Medicine Board and the British Heart Foundation.
A version of this article first appeared on Medscape UK.
according to a new study using data from the UK Biobank Eye and Vision Consortium and the European Prospective Investigation into Cancer (EPIC)–Norfolk study.
The researchers, from St. George’s University of London, Cambridge University, Kingston University, Moorfields Eye Hospital, and University College London, developed a method of artificial intelligence (AI)–enabled imaging of the retina’s vascular network that could accurately predict CVD and death, without the need for blood tests or blood pressure measurement.
The system “paves the way for a highly effective, noninvasive screening test for people at medium to high risk of circulatory disease that doesn’t have to be done in a clinic,” they said. “In the general population it could be used as a noncontact form of systemic vascular health check, to triage those at medium-high risk of circulatory mortality for further clinical risk assessment and appropriate intervention.” Optometry specialists welcomed the prospect and hailed it as “an exciting development.”
Retinal vessels give an accurate early indicator of CVD
The study, published online in the British Journal of Ophthalmology, was based on previous research showing that the width of retinal arterioles and venules seen on retinal imaging may provide an accurate early indicator of CVD, whereas current risk prediction frameworks aren’t always reliable in identifying people who will go on to develop or die of circulatory diseases.
The researchers developed a fully automated AI-enabled algorithm, called Quantitative Analysis of Retinal vessels Topology and Size (QUARTZ), to assess the potential of retinal vasculature imaging plus known risk factors to predict vascular health and death. They applied QUARTZ to retinal images from 88,052 UK Biobank participants aged 40-69 years, looking specifically at the width, vessel area, and degree of tortuosity of the retinal microvasculature, to develop prediction models for stroke, heart attack, and death from circulatory disease.
They then applied these models to the retinal images of 7,411 participants, aged 48-92 years, in the EPIC-Norfolk study. They then compared the performance of QUARTZ with the widely used Framingham Risk Scores framework.
The participants in the two studies were tracked for an average of 7.7 and 9.1 years, respectively, during which time there were 327 circulatory disease deaths among 64,144 UK Biobank participants (average age, 56.8 years) and 201 circulatory deaths among 5,862 EPIC-Norfolk participants (average age, 67.6 years).
Vessel characteristics important predictors of CVD mortality
Results from the QUARTZ models showed that in all participants, arteriolar and venular width, venular tortuosity, and width variation were important predictors of circulatory disease death. In addition, in women, but not in men, arteriolar and venular area were separate factors that contributed to risk prediction.
Overall, the predictive models, based on age, smoking, and medical history (antihypertensive or cholesterol lowering medication, diabetes, and history of stroke or MI) as well as retinal vasculature, captured between half and two-thirds of circulatory disease deaths in those most at risk, the authors said.
Compared with Framingham Risk Scores (FRS), the retinal vasculature (RV) models captured about 5% more cases of stroke in UK Biobank men, 8% more cases in UK Biobank women, and 3% more cases among EPIC-Norfolk men most at risk, but nearly 2% fewer cases among EPIC-Norfolk women. However, the team said that, while adding RV to FRS resulted in only marginal changes in prediction of stroke or MI, a simpler noninvasive risk score based on age, sex, smoking status, medical history, and RV “yielded comparable performance to FRS, without the need for blood sampling or BP measurement.”
Vasculometry low cost, noninvasive and with high street availability
They concluded: “Retinal imaging is established within clinic and hospital eye care and in optometric practices in the U.S. and U.K. AI-enabled vasculometry risk prediction is fully automated, low cost, noninvasive and has the potential for reaching a higher proportion of the population in the community because of “high street” availability and because blood sampling or sphygmomanometry are not needed.
“[Retinal vasculature] is a microvascular marker, hence offers better prediction for circulatory mortality and stroke, compared with MI, which is more macrovascular, except perhaps in women.
“In the general population it could be used as a noncontact form of systemic vascular health check, to triage those at medium-high risk of circulatory mortality for further clinical risk assessment and appropriate intervention.”
In the United Kingdom, for example, it could be included in the primary care NHS Health Check for those aged 41-74 years, they suggested. In addition, “high street” retinal scanning could directly feed into primary medical services and help achieve greater screening coverage for older age groups, who are likely to attend an optometric practice for visual correction, especially with the onset of presbyopia. “This would offer a novel approach to identify those at high risk of circulatory mortality, which is not currently screened for,” the team said.
Test could help to identify high-risk individuals
In a linked editorial, Ify Mordi, MD, and Emanuele Trucco, MD, of the University of Dundee (Scotland), said that CVD remains a significant cause of mortality and morbidity and the most common cause of death worldwide, accounting for a quarter of all U.K. deaths – and its burden is increasing. “Identification of individuals at high risk is particularly important,” they said, but current clinical risk scores to identify those at risk “are unfortunately not perfect,” so miss some of those who might benefit from preventative therapy.
“The retina is the only location that allows non-invasive direct visualisation of the vasculature, potentially providing a rich source of information.” In the new study, the measurements derived with the software tool, QUARTZ, were significantly associated with CVD, they said, with similar predictive performance to the Framingham clinical risk score.
“The results strengthen the evidence from several similar studies that the retina can be a useful and potentially disruptive source of information for CVD risk in personalised medicine.” However, a number of questions remain about how this knowledge could be integrated into clinical care, including who would conduct such a retinal screening program and who would act on the findings?
The editorial concluded: “What is now needed is for ophthalmologists, cardiologists, primary care physicians, and computer scientists to work together to design studies to determine whether using this information improves clinical outcome, and, if so, to work with regulatory bodies, scientific societies and healthcare systems to optimize clinical work flows and enable practical implementation in routine practice.”
‘Exciting development that could improve outcomes’
Asked to comment, Farah Topia, clinical and regulatory adviser at the Association of Optometrists, said: “This is an exciting development that could improve outcomes for many patients by enabling earlier detection of serious health risks. Patients attend optometric practice for a variety of reasons and this interaction could be used to a greater extent to help detect disease earlier. With optometrists available on every High Street, in the heart of communities, it’s an element of primary care that can be accessed quickly and easily, and optometrists are also already trained to have health and lifestyle discussion with patients.”
She added: “Retinal photographs are regularly taken when patients visit an optometrist, so being able to further enhance this process using AI is exciting.
“We look forward to seeing how this area develops and how optometrists can work together with other healthcare sectors to improve patient outcomes and ease the burden the NHS currently faces.”
The study was funded by the Medical Research Council Population and Systems Medicine Board and the British Heart Foundation.
A version of this article first appeared on Medscape UK.
according to a new study using data from the UK Biobank Eye and Vision Consortium and the European Prospective Investigation into Cancer (EPIC)–Norfolk study.
The researchers, from St. George’s University of London, Cambridge University, Kingston University, Moorfields Eye Hospital, and University College London, developed a method of artificial intelligence (AI)–enabled imaging of the retina’s vascular network that could accurately predict CVD and death, without the need for blood tests or blood pressure measurement.
The system “paves the way for a highly effective, noninvasive screening test for people at medium to high risk of circulatory disease that doesn’t have to be done in a clinic,” they said. “In the general population it could be used as a noncontact form of systemic vascular health check, to triage those at medium-high risk of circulatory mortality for further clinical risk assessment and appropriate intervention.” Optometry specialists welcomed the prospect and hailed it as “an exciting development.”
Retinal vessels give an accurate early indicator of CVD
The study, published online in the British Journal of Ophthalmology, was based on previous research showing that the width of retinal arterioles and venules seen on retinal imaging may provide an accurate early indicator of CVD, whereas current risk prediction frameworks aren’t always reliable in identifying people who will go on to develop or die of circulatory diseases.
The researchers developed a fully automated AI-enabled algorithm, called Quantitative Analysis of Retinal vessels Topology and Size (QUARTZ), to assess the potential of retinal vasculature imaging plus known risk factors to predict vascular health and death. They applied QUARTZ to retinal images from 88,052 UK Biobank participants aged 40-69 years, looking specifically at the width, vessel area, and degree of tortuosity of the retinal microvasculature, to develop prediction models for stroke, heart attack, and death from circulatory disease.
They then applied these models to the retinal images of 7,411 participants, aged 48-92 years, in the EPIC-Norfolk study. They then compared the performance of QUARTZ with the widely used Framingham Risk Scores framework.
The participants in the two studies were tracked for an average of 7.7 and 9.1 years, respectively, during which time there were 327 circulatory disease deaths among 64,144 UK Biobank participants (average age, 56.8 years) and 201 circulatory deaths among 5,862 EPIC-Norfolk participants (average age, 67.6 years).
Vessel characteristics important predictors of CVD mortality
Results from the QUARTZ models showed that in all participants, arteriolar and venular width, venular tortuosity, and width variation were important predictors of circulatory disease death. In addition, in women, but not in men, arteriolar and venular area were separate factors that contributed to risk prediction.
Overall, the predictive models, based on age, smoking, and medical history (antihypertensive or cholesterol lowering medication, diabetes, and history of stroke or MI) as well as retinal vasculature, captured between half and two-thirds of circulatory disease deaths in those most at risk, the authors said.
Compared with Framingham Risk Scores (FRS), the retinal vasculature (RV) models captured about 5% more cases of stroke in UK Biobank men, 8% more cases in UK Biobank women, and 3% more cases among EPIC-Norfolk men most at risk, but nearly 2% fewer cases among EPIC-Norfolk women. However, the team said that, while adding RV to FRS resulted in only marginal changes in prediction of stroke or MI, a simpler noninvasive risk score based on age, sex, smoking status, medical history, and RV “yielded comparable performance to FRS, without the need for blood sampling or BP measurement.”
Vasculometry low cost, noninvasive and with high street availability
They concluded: “Retinal imaging is established within clinic and hospital eye care and in optometric practices in the U.S. and U.K. AI-enabled vasculometry risk prediction is fully automated, low cost, noninvasive and has the potential for reaching a higher proportion of the population in the community because of “high street” availability and because blood sampling or sphygmomanometry are not needed.
“[Retinal vasculature] is a microvascular marker, hence offers better prediction for circulatory mortality and stroke, compared with MI, which is more macrovascular, except perhaps in women.
“In the general population it could be used as a noncontact form of systemic vascular health check, to triage those at medium-high risk of circulatory mortality for further clinical risk assessment and appropriate intervention.”
In the United Kingdom, for example, it could be included in the primary care NHS Health Check for those aged 41-74 years, they suggested. In addition, “high street” retinal scanning could directly feed into primary medical services and help achieve greater screening coverage for older age groups, who are likely to attend an optometric practice for visual correction, especially with the onset of presbyopia. “This would offer a novel approach to identify those at high risk of circulatory mortality, which is not currently screened for,” the team said.
Test could help to identify high-risk individuals
In a linked editorial, Ify Mordi, MD, and Emanuele Trucco, MD, of the University of Dundee (Scotland), said that CVD remains a significant cause of mortality and morbidity and the most common cause of death worldwide, accounting for a quarter of all U.K. deaths – and its burden is increasing. “Identification of individuals at high risk is particularly important,” they said, but current clinical risk scores to identify those at risk “are unfortunately not perfect,” so miss some of those who might benefit from preventative therapy.
“The retina is the only location that allows non-invasive direct visualisation of the vasculature, potentially providing a rich source of information.” In the new study, the measurements derived with the software tool, QUARTZ, were significantly associated with CVD, they said, with similar predictive performance to the Framingham clinical risk score.
“The results strengthen the evidence from several similar studies that the retina can be a useful and potentially disruptive source of information for CVD risk in personalised medicine.” However, a number of questions remain about how this knowledge could be integrated into clinical care, including who would conduct such a retinal screening program and who would act on the findings?
The editorial concluded: “What is now needed is for ophthalmologists, cardiologists, primary care physicians, and computer scientists to work together to design studies to determine whether using this information improves clinical outcome, and, if so, to work with regulatory bodies, scientific societies and healthcare systems to optimize clinical work flows and enable practical implementation in routine practice.”
‘Exciting development that could improve outcomes’
Asked to comment, Farah Topia, clinical and regulatory adviser at the Association of Optometrists, said: “This is an exciting development that could improve outcomes for many patients by enabling earlier detection of serious health risks. Patients attend optometric practice for a variety of reasons and this interaction could be used to a greater extent to help detect disease earlier. With optometrists available on every High Street, in the heart of communities, it’s an element of primary care that can be accessed quickly and easily, and optometrists are also already trained to have health and lifestyle discussion with patients.”
She added: “Retinal photographs are regularly taken when patients visit an optometrist, so being able to further enhance this process using AI is exciting.
“We look forward to seeing how this area develops and how optometrists can work together with other healthcare sectors to improve patient outcomes and ease the burden the NHS currently faces.”
The study was funded by the Medical Research Council Population and Systems Medicine Board and the British Heart Foundation.
A version of this article first appeared on Medscape UK.
FROM THE BRITISH JOURNAL OF OPHTHALMOLOGY
Sore throat becoming dominant COVID symptom: Reports
according to recent reports in the United Kingdom.
The shift could be a cause of concern for the fall. As the main symptoms of the coronavirus change, people could spread the virus without realizing it.
“Many people are still using the government guidelines about symptoms, which are wrong,” Tim Spector, a professor of genetic epidemiology at King’s College London, told the Independent.
Prof. Spector cofounded the COVID ZOE app, which is part of the world’s largest COVID-19 study. Throughout the pandemic, researchers have used data from the app to track changes in symptoms.
“At the moment, COVID starts in two-thirds of people with a sore throat,” he said. “Fever and loss of smell are really rare now, so many old people may not think they’ve got COVID. They’d say it’s a cold and not be tested.”
COVID-19 infections in the United Kingdom increased 14% at the end of September, according to data from the U.K.’s Office for National Statistics. More than 1.1 million people tested positive during the week ending Sept. 20, up from 927,000 cases the week before. The numbers continue to increase in England and Wales, with an uncertain trend in Northern Ireland and Scotland.
The fall wave of infections has likely arrived in the United Kingdom, Prof. Spector told the Independent. Omicron variants continue to evolve and are escaping immunity from previous infection and vaccination, which he expects to continue into the winter.
But with reduced testing and surveillance of new variants, public health experts have voiced concerns about tracking the latest variants and COVID-19 trends.
“We can only detect variants or know what’s coming by doing sequencing from PCR testing, and that’s not going on anywhere near the extent it was a year ago,” Lawrence Young, a professor of virology at the University of Warwick, Coventry, England, told the Independent.
“People are going to get various infections over the winter but won’t know what they are because free tests aren’t available,” he said. “It’s going to be a problem.”
COVID-19 cases are also increasing across Europe, which could mark the first regional spike since the BA.5 wave, according to the latest data from the European CDC. (In the past, increases in Europe have signaled a trend to come in other regions.)
People aged 65 and older have been hit the hardest, the data shows, with cases rising 9% from the previous week. Hospitalizations remain stable for now, although 14 of 27 countries in the European region have noted an upward trend.
“Changes in population mixing following the summer break are likely to be the main driver of these increases, with no indication of changes in the distribution of circulating variants,” the European CDC said.
For now, most COVID-19 numbers are still falling in the United States, according to a weekly CDC update published Sept. 30. About 47,000 cases are being reported each day, marking a 13% decrease from the week before. Hospitalizations dropped 7%, and deaths dropped 6%.
At the same time, test positivity rose slightly last week, from 9.6% to 9.8%. Wastewater surveillance indicates that 53% of sites in the United States reported a decrease in virus levels, while 41% reported an increase last week.
The CDC encouraged people to get the updated Omicron-targeted booster shot for the fall. About 7.5 million Americans have received the updated vaccine. Half of the eligible population in the United States hasn’t received any booster dose yet.
“Bivalent boosters help restore protection that might have gone down since your last dose – and they also give extra protection for you and those around you against all lineages of the Omicron variant,” the CDC wrote. “The more people who stay up to date on vaccinations, the better chance we have of avoiding a possible surge in COVID-19 illness later this fall and winter.”
A version of this article first appeared on WebMD.com.
according to recent reports in the United Kingdom.
The shift could be a cause of concern for the fall. As the main symptoms of the coronavirus change, people could spread the virus without realizing it.
“Many people are still using the government guidelines about symptoms, which are wrong,” Tim Spector, a professor of genetic epidemiology at King’s College London, told the Independent.
Prof. Spector cofounded the COVID ZOE app, which is part of the world’s largest COVID-19 study. Throughout the pandemic, researchers have used data from the app to track changes in symptoms.
“At the moment, COVID starts in two-thirds of people with a sore throat,” he said. “Fever and loss of smell are really rare now, so many old people may not think they’ve got COVID. They’d say it’s a cold and not be tested.”
COVID-19 infections in the United Kingdom increased 14% at the end of September, according to data from the U.K.’s Office for National Statistics. More than 1.1 million people tested positive during the week ending Sept. 20, up from 927,000 cases the week before. The numbers continue to increase in England and Wales, with an uncertain trend in Northern Ireland and Scotland.
The fall wave of infections has likely arrived in the United Kingdom, Prof. Spector told the Independent. Omicron variants continue to evolve and are escaping immunity from previous infection and vaccination, which he expects to continue into the winter.
But with reduced testing and surveillance of new variants, public health experts have voiced concerns about tracking the latest variants and COVID-19 trends.
“We can only detect variants or know what’s coming by doing sequencing from PCR testing, and that’s not going on anywhere near the extent it was a year ago,” Lawrence Young, a professor of virology at the University of Warwick, Coventry, England, told the Independent.
“People are going to get various infections over the winter but won’t know what they are because free tests aren’t available,” he said. “It’s going to be a problem.”
COVID-19 cases are also increasing across Europe, which could mark the first regional spike since the BA.5 wave, according to the latest data from the European CDC. (In the past, increases in Europe have signaled a trend to come in other regions.)
People aged 65 and older have been hit the hardest, the data shows, with cases rising 9% from the previous week. Hospitalizations remain stable for now, although 14 of 27 countries in the European region have noted an upward trend.
“Changes in population mixing following the summer break are likely to be the main driver of these increases, with no indication of changes in the distribution of circulating variants,” the European CDC said.
For now, most COVID-19 numbers are still falling in the United States, according to a weekly CDC update published Sept. 30. About 47,000 cases are being reported each day, marking a 13% decrease from the week before. Hospitalizations dropped 7%, and deaths dropped 6%.
At the same time, test positivity rose slightly last week, from 9.6% to 9.8%. Wastewater surveillance indicates that 53% of sites in the United States reported a decrease in virus levels, while 41% reported an increase last week.
The CDC encouraged people to get the updated Omicron-targeted booster shot for the fall. About 7.5 million Americans have received the updated vaccine. Half of the eligible population in the United States hasn’t received any booster dose yet.
“Bivalent boosters help restore protection that might have gone down since your last dose – and they also give extra protection for you and those around you against all lineages of the Omicron variant,” the CDC wrote. “The more people who stay up to date on vaccinations, the better chance we have of avoiding a possible surge in COVID-19 illness later this fall and winter.”
A version of this article first appeared on WebMD.com.
according to recent reports in the United Kingdom.
The shift could be a cause of concern for the fall. As the main symptoms of the coronavirus change, people could spread the virus without realizing it.
“Many people are still using the government guidelines about symptoms, which are wrong,” Tim Spector, a professor of genetic epidemiology at King’s College London, told the Independent.
Prof. Spector cofounded the COVID ZOE app, which is part of the world’s largest COVID-19 study. Throughout the pandemic, researchers have used data from the app to track changes in symptoms.
“At the moment, COVID starts in two-thirds of people with a sore throat,” he said. “Fever and loss of smell are really rare now, so many old people may not think they’ve got COVID. They’d say it’s a cold and not be tested.”
COVID-19 infections in the United Kingdom increased 14% at the end of September, according to data from the U.K.’s Office for National Statistics. More than 1.1 million people tested positive during the week ending Sept. 20, up from 927,000 cases the week before. The numbers continue to increase in England and Wales, with an uncertain trend in Northern Ireland and Scotland.
The fall wave of infections has likely arrived in the United Kingdom, Prof. Spector told the Independent. Omicron variants continue to evolve and are escaping immunity from previous infection and vaccination, which he expects to continue into the winter.
But with reduced testing and surveillance of new variants, public health experts have voiced concerns about tracking the latest variants and COVID-19 trends.
“We can only detect variants or know what’s coming by doing sequencing from PCR testing, and that’s not going on anywhere near the extent it was a year ago,” Lawrence Young, a professor of virology at the University of Warwick, Coventry, England, told the Independent.
“People are going to get various infections over the winter but won’t know what they are because free tests aren’t available,” he said. “It’s going to be a problem.”
COVID-19 cases are also increasing across Europe, which could mark the first regional spike since the BA.5 wave, according to the latest data from the European CDC. (In the past, increases in Europe have signaled a trend to come in other regions.)
People aged 65 and older have been hit the hardest, the data shows, with cases rising 9% from the previous week. Hospitalizations remain stable for now, although 14 of 27 countries in the European region have noted an upward trend.
“Changes in population mixing following the summer break are likely to be the main driver of these increases, with no indication of changes in the distribution of circulating variants,” the European CDC said.
For now, most COVID-19 numbers are still falling in the United States, according to a weekly CDC update published Sept. 30. About 47,000 cases are being reported each day, marking a 13% decrease from the week before. Hospitalizations dropped 7%, and deaths dropped 6%.
At the same time, test positivity rose slightly last week, from 9.6% to 9.8%. Wastewater surveillance indicates that 53% of sites in the United States reported a decrease in virus levels, while 41% reported an increase last week.
The CDC encouraged people to get the updated Omicron-targeted booster shot for the fall. About 7.5 million Americans have received the updated vaccine. Half of the eligible population in the United States hasn’t received any booster dose yet.
“Bivalent boosters help restore protection that might have gone down since your last dose – and they also give extra protection for you and those around you against all lineages of the Omicron variant,” the CDC wrote. “The more people who stay up to date on vaccinations, the better chance we have of avoiding a possible surge in COVID-19 illness later this fall and winter.”
A version of this article first appeared on WebMD.com.