User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
U.S. tops 10,000 confirmed monkeypox cases: CDC
The United States passed the 10,000 mark on Aug. 10, with the number climbing to 10,768 by the morning of Aug. 12, according to the latest CDC data. Monkeypox cases have been found in every state except Wyoming. New York (2,187), California (1,892), and Florida (1,053) have reported the most cases. So far, no monkeypox deaths have been reported in the United States.
The numbers are increasing, with 1,391 cases reported in the United States on Aug. 12 alone, by far the most in 1 day since the current outbreak began.
“We are still operating under a containment goal, although I know many states are starting to wonder if we’re shifting to more of a mitigation phase right now, given that our case counts are still rising rapidly,” Jennifer McQuiston, DVM, the CDC’s top monkeypox official, told a group of the agency’s advisers on Aug. 9, according to CBS News.
Since late July, the United States has reported more monkeypox cases than any other nation. After the United States, Spain has reported 5,162 cases, the United Kingdom 3,017, and France 2,423, according to the World Health Organization.
Globally, 31,655 cases have been recorded, with 5,108 of those cases coming in the last 7 days, according to the WHO. There have been 12 deaths attributed to monkeypox, with one coming in the last week.
The smallpox-like disease was first found in humans in the Democratic Republic of the Congo in 1970 and has become more common in West and Central Africa. It began spreading to European and other Western nations in May 2022.
The WHO declared it a global public health emergency in late July, and the Biden administration declared it a national health emergency Aug. 4.
To fight the spread of monkeypox, the Biden administration is buying $26 million worth of SIGA Technologies Inc.’s IV version of the antiviral drug TPOXX, the company announced on Aug. 9.
U.S. health officials also modified monkeypox vaccine dosing instructions to stretch the supply of vaccine. Instead of sticking with a standard shot that would enter deep into tissue, the FDA now encourages a new way: just under the skin at one-fifth the usual dose.
A version of this article first appeared on WebMD.com.
The United States passed the 10,000 mark on Aug. 10, with the number climbing to 10,768 by the morning of Aug. 12, according to the latest CDC data. Monkeypox cases have been found in every state except Wyoming. New York (2,187), California (1,892), and Florida (1,053) have reported the most cases. So far, no monkeypox deaths have been reported in the United States.
The numbers are increasing, with 1,391 cases reported in the United States on Aug. 12 alone, by far the most in 1 day since the current outbreak began.
“We are still operating under a containment goal, although I know many states are starting to wonder if we’re shifting to more of a mitigation phase right now, given that our case counts are still rising rapidly,” Jennifer McQuiston, DVM, the CDC’s top monkeypox official, told a group of the agency’s advisers on Aug. 9, according to CBS News.
Since late July, the United States has reported more monkeypox cases than any other nation. After the United States, Spain has reported 5,162 cases, the United Kingdom 3,017, and France 2,423, according to the World Health Organization.
Globally, 31,655 cases have been recorded, with 5,108 of those cases coming in the last 7 days, according to the WHO. There have been 12 deaths attributed to monkeypox, with one coming in the last week.
The smallpox-like disease was first found in humans in the Democratic Republic of the Congo in 1970 and has become more common in West and Central Africa. It began spreading to European and other Western nations in May 2022.
The WHO declared it a global public health emergency in late July, and the Biden administration declared it a national health emergency Aug. 4.
To fight the spread of monkeypox, the Biden administration is buying $26 million worth of SIGA Technologies Inc.’s IV version of the antiviral drug TPOXX, the company announced on Aug. 9.
U.S. health officials also modified monkeypox vaccine dosing instructions to stretch the supply of vaccine. Instead of sticking with a standard shot that would enter deep into tissue, the FDA now encourages a new way: just under the skin at one-fifth the usual dose.
A version of this article first appeared on WebMD.com.
The United States passed the 10,000 mark on Aug. 10, with the number climbing to 10,768 by the morning of Aug. 12, according to the latest CDC data. Monkeypox cases have been found in every state except Wyoming. New York (2,187), California (1,892), and Florida (1,053) have reported the most cases. So far, no monkeypox deaths have been reported in the United States.
The numbers are increasing, with 1,391 cases reported in the United States on Aug. 12 alone, by far the most in 1 day since the current outbreak began.
“We are still operating under a containment goal, although I know many states are starting to wonder if we’re shifting to more of a mitigation phase right now, given that our case counts are still rising rapidly,” Jennifer McQuiston, DVM, the CDC’s top monkeypox official, told a group of the agency’s advisers on Aug. 9, according to CBS News.
Since late July, the United States has reported more monkeypox cases than any other nation. After the United States, Spain has reported 5,162 cases, the United Kingdom 3,017, and France 2,423, according to the World Health Organization.
Globally, 31,655 cases have been recorded, with 5,108 of those cases coming in the last 7 days, according to the WHO. There have been 12 deaths attributed to monkeypox, with one coming in the last week.
The smallpox-like disease was first found in humans in the Democratic Republic of the Congo in 1970 and has become more common in West and Central Africa. It began spreading to European and other Western nations in May 2022.
The WHO declared it a global public health emergency in late July, and the Biden administration declared it a national health emergency Aug. 4.
To fight the spread of monkeypox, the Biden administration is buying $26 million worth of SIGA Technologies Inc.’s IV version of the antiviral drug TPOXX, the company announced on Aug. 9.
U.S. health officials also modified monkeypox vaccine dosing instructions to stretch the supply of vaccine. Instead of sticking with a standard shot that would enter deep into tissue, the FDA now encourages a new way: just under the skin at one-fifth the usual dose.
A version of this article first appeared on WebMD.com.
Dermatologists share vitiligo breakthrough news with patients
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
For the first time, patients with vitiligo who have long lived with patches of skin that are without pigment can now have even skin tones on their faces and other bodily regions with a Food and Drug Administration–approved, easy-to-use topical treatment.
In July, , the most common form of the disease.
Topical ruxolitinib was first approved in September 2021 for atopic dermatitis, and dermatologists are already writing prescriptions for its new vitiligo indication.
“The FDA approval of ruxolitinib for repigmentation of vitiligo is historic and groundbreaking,” Seemal R. Desai, MD, a dermatologist at the University of Texas Southwestern Medical Center, Dallas, told this news organization.
The news brings hope to patients 12 years and older who suffer from the psychosocial effects of the disease, which is estimated to affect 1.9 million to 2.8 million adults in the United States.
The announcement followed FDA approval a month earlier of another dermatologic milestone – an oral JAK inhibitor, baricitinib, which became the first treatment for patients with alopecia areata.
For Dr. Desai, the ruxolitinib news is personal. His brother, also a physician, has lived a lifetime with vitiligo. His family experience, Dr. Desai said, showed him “what a disease like this can do to a person psychologically.”
Dr. Desai said his early exposure helped lead to his own decision to dedicate his career to pigmentary diseases.
His brother won’t personally benefit from the cream because his skin has been completely depigmented and repigmentation is not of interest to him, Dr. Desai said. But both brothers are excited as physicians. “It’s really quite an emotional moment,” he said.
Getting the news to patients
As dermatologists introduce the topical treatment to patients, common questions center on why this cream is different and whether it is safe.
David Rosmarin, MD, vice chair of research and education, department of dermatology, Tufts Medical Center, Boston, led the Topical Ruxolitinib Evaluation in Vitiligo Study 1 and 2 (TruE-V1, TruE-V2), conducted in North America and Europe.
He summarized some key findings.
“If patients have involvement on the face, trunk, or extremities, the data show that about half the patients at 52 weeks will get half or more of their pigment back,” he said in an interview. Results for the face alone are even better. “Half the patients will get 75% or more pigment back in the face,” Dr. Rosmarin said.
In addition, analysis of subgroups shows benefit for all patients. “Patients seem to respond similarly well across all subgroups – across gender, sex, age, ethnicity, and race,” Dr. Rosmarin said.
However, anatomic region matters, he pointed out. Skin of the head and neck responds the best, followed by skin of the trunk and extremities. The hands and feet are the most difficult to repigment because there are few hair follicles, which help enable repigmentation.
He added that it’s important to understand patients’ goals, and dermatologists shouldn’t assume that all who have vitiligo will want to undergo repigmentation. They may be interested in the new treatment but may not want it for themselves, he explained.
Explaining risks
Patients may ask about the boxed warning on the label that lists risk of heart attack, stroke, cancer, infections, blood clots, and death. Dermatologists can explain that the warning pertains to the whole JAK class and was based on patients with rheumatoid arthritis, Dr. Rosmarin said.
He added, “We didn’t see a signal for heart attack and stroke for patients using the topical. But it’s still important to discuss the label as the FDA states it.”
There are two main side effects, Dr. Rosmarin said: acne (about 6% of treated patients get it, and it’s usually mild) and application-site reactions. “Luckily, the medication has a tendency not to sting or burn, which is not the case with some of our other treatments. It’s very well tolerated,” he said.
Patients should also know that repigmentation can take time, because initially, the immune system is directed to calm down with treatment, and then pigment must travel back to the affected sites.
Some patients may have a response in as early as 2-3 months, and others need more time, Dr. Rosmarin said.
Treatment responses among adolescents have been particularly good. Responses regarding the skin of the face have been similar to those of adults. “However, on the body, they respond even better,” Dr. Rosmarin said. “About 60% achieve 50% or more repigmentation on the whole body.”
It’s important that ruxolitinib has been approved for persons aged 12 years and older, he said, because “about half the patients will develop vitiligo by the age of 20.”
Approval and insurance coverage
FDA approval will help with reimbursement for the expensive treatment.
The label indicates that patients should not use more than one 60-g tube a week. Currently, the out-of-pocket cost for one tube can be close to $2,000, according to GoodRx.
Raj Chovatiya, MD, PhD, assistant professor of dermatology and director of the Center for Eczema and Itch at Northwestern University, Chicago, said that in recent years, vitiligo patients, aware that their condition could be treated by JAK inhibitors, have been paying out of pocket at compounding pharmacies, which take oral versions of the medication and compound them into topical formulations.
Unlike baricitinib, which is used to treat severe alopecia areata, and other oral JAK inhibitors, testing for TB and hepatitis is not required for initiating treatment with ruxolitinib, so no delay is necessary, Dr. Chovatiya said.
He noted, however, that patients with vitiligo may have given up on effective care after experiencing little or no improvement with topical corticosteroids, phototherapy, or topical calcineurin inhibitors.
“They end up losing steam, are less motivated on therapy, and are lost to care,” he said.
Dermatologists, he said, may need to proactively find these patients and tell them the good news. “Now that we have really good targeted therapeutic options, it’s really up to us to figure out how to bring these people back to the clinic and educate them,” Dr. Chovatiya said.
Unanswered questions to address
Some questions are still unanswered, lead study author Dr. Rosmarin said.
Two big questions are how long people will need to continue using ruxolitinib cream and whether depigmentation will recur if people stop using it.
Another aspect of therapy being studied is whether the cream will be even more effective in combination with other treatments.
“The main combination we think about is ruxolitinib with phototherapy – a light treatment – because light could stimulate those pigment cells,” Dr. Rosmarin said,
He noted that light therapy was included in phase 2 testing and that patients did respond. “What we need and what’s planned is a larger study looking at the combination to see whether it is synergistic or not. The longer patients use the cream, the more benefit we see,” Dr. Rosmarin said.
Dr. Desai has served as an investigator and/or consultant to several companies, including Incyte. Dr. Rosmarin has received honoraria as a consultant and has received research support from Incyte, and has served as a paid speaker for Incyte, as well as other companies.. Dr. Chovatiya has served as an advisory board member, consultant, and/or investigator for companies that include Incyte.
Study suggests psoriasis and PsA are underdiagnosed in underserved groups
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, a study based on national registry data suggests.
“Using the All of Us dataset, we identified lower rates of psoriasis and psoriatic arthritis in participants with skin of color, lower education levels, and no health insurance,” lead author Megan M. Tran said in her oral presentation at the annual meeting of the Society for Investigative Dermatology.
“This suggests psoriasis and psoriatic arthritis underdiagnosis in these underserved populations, possibly due to limited dermatologic care access,” added Ms. Tran, a second-year medical student at Brown University in Providence, R.I.
Ms. Tran and colleagues used the ongoing National Institutes of Health All of Us Research Program registry that contains a large proportion of participants from groups in the United States who have historically been underrepresented in biomedical research, she said in her talk.
Of the 329,038 participants with data in version 5 (released this past March) of the All of Us database, 150,158 (45.6%) had skin of color, and 251,597 (76.5%) had available electronic health records (EHRs).
Underserved groups need better access to health care
Linking data from EHRs, surveys, and physical measurements at enrollment, the researchers used several variables to estimate psoriasis and psoriatic arthritis (PsA) prevalence, and they used multivariate logistic regression to adjust for the variables. They found:
- Twenty-two percent of patients with psoriasis had PsA. Odds of psoriasis and PsA were lower among Black (psoriasis odds ratio [OR], 0.32, 95% confidence interval [CI], 0.28-0.36; PsA OR, 0.20, 95% CI, 0.15-0.26) and Hispanic participants (psoriasis OR, 0.77, 95% CI, 0.71-0.84; PsA OR, 0.74, 95% CI, 0.61-0.89) compared with White participants.
- Psoriasis prevalence increased linearly with age (topping off at age 70 and older [OR, 3.35, 95% CI, 2.91-3.88], with 18-29 years as the reference). The same trend was found with PsA (70 years and above [OR, 4.41, 95% CI, 3.07-6.55] compared with those aged 18-29 years).
- Psoriasis prevalence increased linearly with body mass index (BMI 40 and above [OR, 1.71, 95% CI, 1.54-1.90], with 20-24.9 as the reference). The same trend was found with PsA (BMI 40 and above [OR, 2.09, 95% CI, 1.68-2.59], with 20-24.9 as the reference).
- Former smokers were at increased risk for disease, compared with people who had never smoked (psoriasis OR, 1.30, 95% CI, 1.22-1.39; PsA OR, 2.15, 95% CI, 1.33-3.78).
- Lower odds were found in uninsured adults (psoriasis OR, 0.43, 95% CI, 0.35-0.52; PsA OR, 0.37, 95% CI, 0.22-0.58) compared with those who were insured, and in those with less than a high school degree (psoriasis OR, 0.72, 95% CI, 0.63-0.82; PsA OR, 0.65, 95% CI, 0.47-0.87) compared with those with a college degree.
“The All of Us research program has demonstrated to be a valuable resource to gain unique dermatologic insights on diverse participant populations,” Ms. Tran said.
“There needs to be improvement in access to quality dermatologic care, as this may help to reduce underdiagnosis of psoriasis and psoriatic arthritis,” she added. Access can be increased in various ways, including “outreach to underserved communities, equitable distribution of resources, and increased awareness of clinical variations in skin of color.”
Laura Korb Ferris, MD, PhD, professor of dermatology and director of clinical trials for the department of dermatology at University of Pittsburgh Medical Center, said the study is interesting.
“Because All of Us uses electronic health records to identify cases, while these findings could suggest that these patients are less likely to develop psoriasis and psoriatic arthritis, it more likely shows that they are less likely to receive care for these conditions,” she told this news organization.
“This is concerning, as psoriasis is associated with other comorbidities such as cardiovascular disease and depression, and psoriatic arthritis if left untreated can cause irreversible joint damage that limits function,” she explained in an email. “Both conditions profoundly impact a patient’s quality of life.
“It is important to know whether the diagnoses are simply being missed in these patients or are being neglected,” noted Dr. Ferris, who was not involved in the study and was asked to comment on the results. “It is also important to find strategies to improve diagnosis and treatment, improve quality of life, and allow for interventions to improve long-term sequelae of these diseases and their comorbid conditions.”
The NIH All of Us Research Program, which aims to build a diverse database from at least 1 million adult participants in the United States as a part of the agency’s precision medicine initiative, is open to researchers and to the public. Researchers can access All of Us data and tools to conduct studies at the All of Us Research Hub, and adults who live in the United States can contribute their health data at the All of Us Research Program website and at participating health care provider organizations.
Ms. Tran, study coauthors, and Dr. Ferris reported no relevant relationships. The All of Us Research Program is supported by the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM SID 2022
More evidence salt substitutes lower risk of CVD and death
Dietary salt substitutes not only lower blood pressure but also have a clear impact on hard clinical endpoints, lowering the risk of myocardial infarction (MI), stroke, and death from all causes and cardiovascular disease (CVD), a meta-analysis shows.
The blood pressure–mediated protective effects of salt substitutes on CVD and death are likely to apply to the roughly 1.28 billion people around the world who have high blood pressure, the researchers say.
“These findings are unlikely to reflect the play of chance and support the adoption of salt substitutes in clinical practice and public health policy as a strategy to reduce dietary sodium intake, increase dietary potassium intake, lower blood pressure, and prevent major cardiovascular events,” they write.
The study was published online in Heart.
Strong support for landmark study
In salt substitutes, a proportion of sodium chloride is replaced with potassium chloride. They are known to help lower blood pressure, but less is known about their impact on hard clinical endpoints, Maoyi Tian, PhD, with Harbin Medical University, China, and the George Institute for Global Health, Sydney, and colleagues note in their article.
In the landmark Salt Substitute and Stroke Study (SSaSS), salt substitutes cut the risk of MI, stroke, and early death, as reported previously by this news organization.
But SSaSS was conducted in China, and it was unclear whether these benefits would apply to people in other parts of the world.
To investigate, Dr. Tian and colleagues pooled data from 21 relevant parallel-group, step-wedge, or cluster randomized controlled trials published through August 2021, with 31,949 participants. The trials were conducted in Europe, the Western Pacific Region, the Americas, and South East Asia and reported the effect of a salt substitute on blood pressure or clinical outcomes.
A meta-analysis of blood pressure data from 19 trials that included 29,528 participants showed that salt substitutes lowered systolic blood pressure (SBP) by 4.61 mm Hg (95% confidence interval, −6.07 to −3.14) and diastolic blood pressure (DBP) by 1.61 mm Hg (95% CI, −2.42 to −0.79).
The proportion of sodium chloride in the salt substitutes varied from 33% to 75%; the proportion of potassium ranged from 25% to 65%.
Each 10% lower proportion of sodium chloride in the salt substitute was associated with a 1.53 mm Hg (95% CI, −3.02 to −0.03; P = .045) greater reduction in SBP and a 0.95 mm Hg (95% CI, −1.78 to −0.12; P = .025) greater reduction in DBP.
Reductions in blood pressure appeared consistent, irrespective of country, age, sex, history of high blood pressure, weight, baseline blood pressure, and baseline levels of urinary sodium and potassium.
Clear benefit on hard outcomes
Pooled data on clinical outcomes from five trials that included 24,306 participants, mostly from the SSaSS, showed clear protective effects of salt substitutes on total mortality (risk ratio, 0.89; 95% CI, 0.85-0.94), CV mortality (RR, 0.87; 95% CI, 0.81-0.94), and CV events (RR, 0.89; 95% CI, 0.85-0.94).
Dr. Tian and colleagues say that “broader population use of salt substitute is supported by the absence of any detectable adverse effect of salt substitutes on hyperkalemia in this review.”
They note, however, that all of the trials took “pragmatic steps to exclude participants at elevated risk of hyperkalemia, seeking to exclude those with chronic kidney disease or using medications that elevate serum potassium.”
Offering perspective on the study, Harlan Krumholz, MD, with Yale New Haven Hospital and Yale School of Medicine, both in New Haven, Conn., said it provides “useful information by bringing together the trial evidence on salt substitutes. The evidence is dominated by the SSaSS, but the others add context.”
Dr. Krumholz said that at this point, he thinks salt substitutes “could be included in recommendations to patients.”
“SSaSS was conducted in villages in China, so that is where the evidence is strongest and most relevant, but this is a low-cost and seemingly safe strategy that could be tried by anyone without contraindications, such as kidney disease or taking a potassium-sparing medication or potassium supplement,” Dr. Krumholz told this news organization.
Johanna Contreras, MD, heart failure and transplant cardiologist at the Mount Sinai Hospital, New York, agrees that in the absence of contraindications, salt substitutes should be recommended.
“Americans put salt on everything and don’t even think about it. The salt substitutes are very helpful,” Dr. Contreras said in an interview.
“People who don’t have high blood pressure should limit salt intake, because what we have seen is that if you have high blood pressure in your family – even if you don’t have high blood pressure in your 20s or 30s – you’re likely to develop high blood pressure,” Dr. Contreras said.
“Therefore, it’s wise early on to start protecting yourself and using low salt and salt substitutes,” she added.
The study had no specific funding. Dr. Tian, Dr. Krumholz, and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dietary salt substitutes not only lower blood pressure but also have a clear impact on hard clinical endpoints, lowering the risk of myocardial infarction (MI), stroke, and death from all causes and cardiovascular disease (CVD), a meta-analysis shows.
The blood pressure–mediated protective effects of salt substitutes on CVD and death are likely to apply to the roughly 1.28 billion people around the world who have high blood pressure, the researchers say.
“These findings are unlikely to reflect the play of chance and support the adoption of salt substitutes in clinical practice and public health policy as a strategy to reduce dietary sodium intake, increase dietary potassium intake, lower blood pressure, and prevent major cardiovascular events,” they write.
The study was published online in Heart.
Strong support for landmark study
In salt substitutes, a proportion of sodium chloride is replaced with potassium chloride. They are known to help lower blood pressure, but less is known about their impact on hard clinical endpoints, Maoyi Tian, PhD, with Harbin Medical University, China, and the George Institute for Global Health, Sydney, and colleagues note in their article.
In the landmark Salt Substitute and Stroke Study (SSaSS), salt substitutes cut the risk of MI, stroke, and early death, as reported previously by this news organization.
But SSaSS was conducted in China, and it was unclear whether these benefits would apply to people in other parts of the world.
To investigate, Dr. Tian and colleagues pooled data from 21 relevant parallel-group, step-wedge, or cluster randomized controlled trials published through August 2021, with 31,949 participants. The trials were conducted in Europe, the Western Pacific Region, the Americas, and South East Asia and reported the effect of a salt substitute on blood pressure or clinical outcomes.
A meta-analysis of blood pressure data from 19 trials that included 29,528 participants showed that salt substitutes lowered systolic blood pressure (SBP) by 4.61 mm Hg (95% confidence interval, −6.07 to −3.14) and diastolic blood pressure (DBP) by 1.61 mm Hg (95% CI, −2.42 to −0.79).
The proportion of sodium chloride in the salt substitutes varied from 33% to 75%; the proportion of potassium ranged from 25% to 65%.
Each 10% lower proportion of sodium chloride in the salt substitute was associated with a 1.53 mm Hg (95% CI, −3.02 to −0.03; P = .045) greater reduction in SBP and a 0.95 mm Hg (95% CI, −1.78 to −0.12; P = .025) greater reduction in DBP.
Reductions in blood pressure appeared consistent, irrespective of country, age, sex, history of high blood pressure, weight, baseline blood pressure, and baseline levels of urinary sodium and potassium.
Clear benefit on hard outcomes
Pooled data on clinical outcomes from five trials that included 24,306 participants, mostly from the SSaSS, showed clear protective effects of salt substitutes on total mortality (risk ratio, 0.89; 95% CI, 0.85-0.94), CV mortality (RR, 0.87; 95% CI, 0.81-0.94), and CV events (RR, 0.89; 95% CI, 0.85-0.94).
Dr. Tian and colleagues say that “broader population use of salt substitute is supported by the absence of any detectable adverse effect of salt substitutes on hyperkalemia in this review.”
They note, however, that all of the trials took “pragmatic steps to exclude participants at elevated risk of hyperkalemia, seeking to exclude those with chronic kidney disease or using medications that elevate serum potassium.”
Offering perspective on the study, Harlan Krumholz, MD, with Yale New Haven Hospital and Yale School of Medicine, both in New Haven, Conn., said it provides “useful information by bringing together the trial evidence on salt substitutes. The evidence is dominated by the SSaSS, but the others add context.”
Dr. Krumholz said that at this point, he thinks salt substitutes “could be included in recommendations to patients.”
“SSaSS was conducted in villages in China, so that is where the evidence is strongest and most relevant, but this is a low-cost and seemingly safe strategy that could be tried by anyone without contraindications, such as kidney disease or taking a potassium-sparing medication or potassium supplement,” Dr. Krumholz told this news organization.
Johanna Contreras, MD, heart failure and transplant cardiologist at the Mount Sinai Hospital, New York, agrees that in the absence of contraindications, salt substitutes should be recommended.
“Americans put salt on everything and don’t even think about it. The salt substitutes are very helpful,” Dr. Contreras said in an interview.
“People who don’t have high blood pressure should limit salt intake, because what we have seen is that if you have high blood pressure in your family – even if you don’t have high blood pressure in your 20s or 30s – you’re likely to develop high blood pressure,” Dr. Contreras said.
“Therefore, it’s wise early on to start protecting yourself and using low salt and salt substitutes,” she added.
The study had no specific funding. Dr. Tian, Dr. Krumholz, and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dietary salt substitutes not only lower blood pressure but also have a clear impact on hard clinical endpoints, lowering the risk of myocardial infarction (MI), stroke, and death from all causes and cardiovascular disease (CVD), a meta-analysis shows.
The blood pressure–mediated protective effects of salt substitutes on CVD and death are likely to apply to the roughly 1.28 billion people around the world who have high blood pressure, the researchers say.
“These findings are unlikely to reflect the play of chance and support the adoption of salt substitutes in clinical practice and public health policy as a strategy to reduce dietary sodium intake, increase dietary potassium intake, lower blood pressure, and prevent major cardiovascular events,” they write.
The study was published online in Heart.
Strong support for landmark study
In salt substitutes, a proportion of sodium chloride is replaced with potassium chloride. They are known to help lower blood pressure, but less is known about their impact on hard clinical endpoints, Maoyi Tian, PhD, with Harbin Medical University, China, and the George Institute for Global Health, Sydney, and colleagues note in their article.
In the landmark Salt Substitute and Stroke Study (SSaSS), salt substitutes cut the risk of MI, stroke, and early death, as reported previously by this news organization.
But SSaSS was conducted in China, and it was unclear whether these benefits would apply to people in other parts of the world.
To investigate, Dr. Tian and colleagues pooled data from 21 relevant parallel-group, step-wedge, or cluster randomized controlled trials published through August 2021, with 31,949 participants. The trials were conducted in Europe, the Western Pacific Region, the Americas, and South East Asia and reported the effect of a salt substitute on blood pressure or clinical outcomes.
A meta-analysis of blood pressure data from 19 trials that included 29,528 participants showed that salt substitutes lowered systolic blood pressure (SBP) by 4.61 mm Hg (95% confidence interval, −6.07 to −3.14) and diastolic blood pressure (DBP) by 1.61 mm Hg (95% CI, −2.42 to −0.79).
The proportion of sodium chloride in the salt substitutes varied from 33% to 75%; the proportion of potassium ranged from 25% to 65%.
Each 10% lower proportion of sodium chloride in the salt substitute was associated with a 1.53 mm Hg (95% CI, −3.02 to −0.03; P = .045) greater reduction in SBP and a 0.95 mm Hg (95% CI, −1.78 to −0.12; P = .025) greater reduction in DBP.
Reductions in blood pressure appeared consistent, irrespective of country, age, sex, history of high blood pressure, weight, baseline blood pressure, and baseline levels of urinary sodium and potassium.
Clear benefit on hard outcomes
Pooled data on clinical outcomes from five trials that included 24,306 participants, mostly from the SSaSS, showed clear protective effects of salt substitutes on total mortality (risk ratio, 0.89; 95% CI, 0.85-0.94), CV mortality (RR, 0.87; 95% CI, 0.81-0.94), and CV events (RR, 0.89; 95% CI, 0.85-0.94).
Dr. Tian and colleagues say that “broader population use of salt substitute is supported by the absence of any detectable adverse effect of salt substitutes on hyperkalemia in this review.”
They note, however, that all of the trials took “pragmatic steps to exclude participants at elevated risk of hyperkalemia, seeking to exclude those with chronic kidney disease or using medications that elevate serum potassium.”
Offering perspective on the study, Harlan Krumholz, MD, with Yale New Haven Hospital and Yale School of Medicine, both in New Haven, Conn., said it provides “useful information by bringing together the trial evidence on salt substitutes. The evidence is dominated by the SSaSS, but the others add context.”
Dr. Krumholz said that at this point, he thinks salt substitutes “could be included in recommendations to patients.”
“SSaSS was conducted in villages in China, so that is where the evidence is strongest and most relevant, but this is a low-cost and seemingly safe strategy that could be tried by anyone without contraindications, such as kidney disease or taking a potassium-sparing medication or potassium supplement,” Dr. Krumholz told this news organization.
Johanna Contreras, MD, heart failure and transplant cardiologist at the Mount Sinai Hospital, New York, agrees that in the absence of contraindications, salt substitutes should be recommended.
“Americans put salt on everything and don’t even think about it. The salt substitutes are very helpful,” Dr. Contreras said in an interview.
“People who don’t have high blood pressure should limit salt intake, because what we have seen is that if you have high blood pressure in your family – even if you don’t have high blood pressure in your 20s or 30s – you’re likely to develop high blood pressure,” Dr. Contreras said.
“Therefore, it’s wise early on to start protecting yourself and using low salt and salt substitutes,” she added.
The study had no specific funding. Dr. Tian, Dr. Krumholz, and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low-level light therapy cap shows subtle effects on CCCA
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
though the treatment effects from a small prospective trial appear to be subtle.
Central centrifugal cicatricial alopecia (CCCA) is a form of scarring hair loss with unknown etiology and no known cure that affects mainly women of African descent.
“The low-level light therapy (LLLT) cap does indeed seem to help with symptoms and mild regrowth in CCCA,” senior study author Amy J. McMichael, MD, told this news organization. “The dual-wavelength cap we used appears to have anti-inflammatory properties, and that makes sense for a primarily inflammatory scarring from of alopecia.
“Quality of life improved with the treatment and there were no reported side effects,” added Dr. McMichael, professor of dermatology at Wake Forest University, Winston-Salem, N.C.
The results of the study were presented in a poster at the annual meeting of the Society for Investigative Dermatology.
The REVIAN RED cap (REVIAN Inc.) used in the study contains 119 light-emitting diodes (LEDs) arrayed on the cap’s interior surface that emit orange (620 nm) and red (660 nm) light.
The hypothesis for how the dual-wavelength lights work is that light is absorbed by the chromophore cytochrome c oxidase in the mitochondrial membrane. This induces the release of nitric oxide and the production of adenosine triphosphate (ATP), which leads to vasodilation, cytokine regulation, and increased transcription and release of growth factors.
LLLT is approved to treat androgenetic alopecia, the authors wrote, but has not been studied as a treatment for CCCA.
To assess the effects of LLLT on CCCA, Dr. McMichael and her colleagues at Wake Forest followed the condition’s progress in five Black women over their 6-month course of treatment. Four participants completed the study.
At baseline, all participants had been on individual stable CCCA treatment regimens for at least 3 months. They continued those treatments along with LLLT therapy throughout the study. The women ranged in age from 38 to 69 years, had had CCCA for an average of 12 years, and their disease severity ranged from stage IIB to IVA.
They were instructed to wear the REVIAN RED cap with the LEDs activated for 10 minutes each day.
At 2, 4, and 6 months, participants self-assessed their symptoms, a clinician evaluated the condition’s severity, and digital photographs were taken.
At 6 months:
- Three patients showed improved Dermatology Life Quality Index (DLQI).
- Three patients showed decreased loss of follicular openings and breakage.
- A dermoscopic image of the scalp of one patient revealed short, regrowing vellus hairs and minimal interfollicular and perifollicular scale.
- No patients reported side effects.
Small study raises big questions
“I hope this study will lead to a larger study that will look at the long-term outcomes of CCCA,” Dr. McMichael said. “This is a nice treatment that does not require application of something to the scalp that may affect hair styling, and it has no systemic side effects.”
Dr. McMichael acknowledges that the small sample size, participants continuing with their individual stable treatments while also undergoing light therapy, and the lack of patients with stage I disease, are weaknesses in the study.
“However, the strength is that none of the patients had side effects or stopped using the treatment due to difficulty with the system,” she added.
Dr. McMichael said she would like to investigate the effects of longer use of the cap and whether the cap can be used to prevent CCCA.
Chesahna Kindred, MD, assistant professor of dermatology at Howard University, Washington, D.C., and founder of Kindred Hair & Skin Center in Columbia, Md., told this news organization that she uses LLLT in her practice.
“I find that LLLT is mildly helpful, or at least does not worsen, androgenetic alopecia,” she said.
“Interestingly, while all four patients had stable disease upon initiating the study, it appears as though two of the four worsened after the use of LLLT, one improved, and one remained relatively stable,” noted Dr. Kindred, who was not involved in the study. “This is important because once there is complete destruction of the follicle, CCCA is difficult to improve.
“Given that there are several options to address inflammation and follicular damage in CCCA, more studies are needed before I would incorporate LLLT into my regular treatment algorithms,” she added.
“Studies like this are important and remind us to not lump all forms of hair loss together,” she said.
REVIAN Inc. provided the caps, but the study received no additional funding. Dr. McMichael and Dr. Kindred report relevant financial relationships with the pharmaceutical industry. Study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Early PT for lower back pain sends fewer patients to specialists
The study found that patients who were referred to physical therapists within 2 weeks of seeing their physicians for LBP were significantly less likely to make visits to a chiropractor, pain specialist, or orthopedist.
Patients also filed fewer claims for advanced imaging or epidural steroid injections and were half as likely to visit an emergency department (ED) within 30 days compared with those who did not start early physical therapy (PT), according to the study, published in BMC Health Services Research.
“Some lower back pain resolves itself, but often, that recovery is incomplete, leading to increased health care and opioid use,” said Richard L. Skolasky Jr., ScD, director of the Spine Outcomes Research Center at Johns Hopkins Medicine, Baltimore, and a coauthor of the study. “Our hope is this study helps more primary care physicians embrace nonpharmacologic, first-line treatments.”
LBP accounts for an estimated $1.8 billion annually in health care costs among the patients who do not receive surgery for the condition, according to a 2019 JAMA analysis of commercial insurance and Medicare claims. In addition, LBP accounts for approximately 2.7 million ED visits annually, a 2010 study published in Spine showed.
Dr. Skolasky and his colleagues assessed 980,000 outpatient claims over a period of almost 4 years that ended in 2014. The researchers used Truven MarketScan, a group of U.S.-based administrative commercial health care insurance claims databases. Patients who had a history of conditions that cause LBP, such as endometriosis and spinal fracture, were excluded from the analysis. Approximately 11% of patients in the total sample received early PT, defined as PT received within 2 weeks of their initial visit to a primary care clinician.
After adjustment for sex, age, and Charlson Morbidity Index, patients who received PT were about half as likely as were those who didn’t to see chiropractor or a pain specialist or have an ED visit within 30 days of their initial appointment. They were about one-third as likely to receive an epidural steroid injection, and they were 43% less likely to have claims for advanced imaging, according to the researchers (P < .001 for all).
In addition, the cost of claims was lower for patients who received early PT ($747 vs. $799), the researchers found.
The effects diminished somewhat over time but remained statistically significant.
At 1 year, patients who received early PT had slightly higher health care costs than did those who did not undergo PT ($2,588 vs. $2,510). Dr. Skolasky hypothesized that the increase was attributable to therapy visits and not having as many specialist visits. He said additional research could investigate whether early PT reduces the health care costs associated with LBP over a longer period.
“Physical therapy addresses a patient’s current pain and physical limitations and arms them with resources, exercises, and nonpharmacologic ways to deal with recurrences,” Dr. Skolasky said in an interview. “If we can follow patients even longer than a year, we may see a longer-term reduction in cost.”
Michael Knight, MD, associate chief quality and population health officer at George Washington University Medical Faculty Associates, Washington, said he refers patients to physical therapists if their pain has not resolved within 2 weeks of stretching at home and taking over-the-counter analgesics.
Dr. Knight recalled one patient who had strained her back doing yard work. When home exercises did not help, Dr. Knight referred her to a physical therapist, who created a customized treatment plan. Within 4 weeks, her condition had improved.
“She was then able to take what she learned and continue those exercises at home,” Dr. Knight said. “She got better, and we avoided MRI costs for her and the health care system.”
Dr. Skolasky and his fellow researchers found significant regional differences in the number of patients referred for early PT. The odds of PT utilization within 90 days after the onset of LBP were 1.6 times higher in the Northeast and 0.82 times lower in the South.
“There are health care deserts,” Dr. Skolasky said. “This study should spark a conversation about the inadequacy of distribution of physical resources to meet the needs of patients with LBP.”
Dr. Skolasky said telehealth could be one option for serving patients in these health care deserts – including those with LBP. He has conducted several studies that concluded that patients benefit from and are happy with telehealth PT.
Dr. Knight said Dr. Skolasky’s study will help patients better understand their options.
“Sometimes patients have an expectation – they want an MRI or pain medication when it’s not necessary,” he said. “This kind of evidence helps strengthen our recommendation for early intervention that really can help.”
The study was supported by a grant from the National Institutes of Health’s National Institute on Aging. Dr. Skolasky reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study found that patients who were referred to physical therapists within 2 weeks of seeing their physicians for LBP were significantly less likely to make visits to a chiropractor, pain specialist, or orthopedist.
Patients also filed fewer claims for advanced imaging or epidural steroid injections and were half as likely to visit an emergency department (ED) within 30 days compared with those who did not start early physical therapy (PT), according to the study, published in BMC Health Services Research.
“Some lower back pain resolves itself, but often, that recovery is incomplete, leading to increased health care and opioid use,” said Richard L. Skolasky Jr., ScD, director of the Spine Outcomes Research Center at Johns Hopkins Medicine, Baltimore, and a coauthor of the study. “Our hope is this study helps more primary care physicians embrace nonpharmacologic, first-line treatments.”
LBP accounts for an estimated $1.8 billion annually in health care costs among the patients who do not receive surgery for the condition, according to a 2019 JAMA analysis of commercial insurance and Medicare claims. In addition, LBP accounts for approximately 2.7 million ED visits annually, a 2010 study published in Spine showed.
Dr. Skolasky and his colleagues assessed 980,000 outpatient claims over a period of almost 4 years that ended in 2014. The researchers used Truven MarketScan, a group of U.S.-based administrative commercial health care insurance claims databases. Patients who had a history of conditions that cause LBP, such as endometriosis and spinal fracture, were excluded from the analysis. Approximately 11% of patients in the total sample received early PT, defined as PT received within 2 weeks of their initial visit to a primary care clinician.
After adjustment for sex, age, and Charlson Morbidity Index, patients who received PT were about half as likely as were those who didn’t to see chiropractor or a pain specialist or have an ED visit within 30 days of their initial appointment. They were about one-third as likely to receive an epidural steroid injection, and they were 43% less likely to have claims for advanced imaging, according to the researchers (P < .001 for all).
In addition, the cost of claims was lower for patients who received early PT ($747 vs. $799), the researchers found.
The effects diminished somewhat over time but remained statistically significant.
At 1 year, patients who received early PT had slightly higher health care costs than did those who did not undergo PT ($2,588 vs. $2,510). Dr. Skolasky hypothesized that the increase was attributable to therapy visits and not having as many specialist visits. He said additional research could investigate whether early PT reduces the health care costs associated with LBP over a longer period.
“Physical therapy addresses a patient’s current pain and physical limitations and arms them with resources, exercises, and nonpharmacologic ways to deal with recurrences,” Dr. Skolasky said in an interview. “If we can follow patients even longer than a year, we may see a longer-term reduction in cost.”
Michael Knight, MD, associate chief quality and population health officer at George Washington University Medical Faculty Associates, Washington, said he refers patients to physical therapists if their pain has not resolved within 2 weeks of stretching at home and taking over-the-counter analgesics.
Dr. Knight recalled one patient who had strained her back doing yard work. When home exercises did not help, Dr. Knight referred her to a physical therapist, who created a customized treatment plan. Within 4 weeks, her condition had improved.
“She was then able to take what she learned and continue those exercises at home,” Dr. Knight said. “She got better, and we avoided MRI costs for her and the health care system.”
Dr. Skolasky and his fellow researchers found significant regional differences in the number of patients referred for early PT. The odds of PT utilization within 90 days after the onset of LBP were 1.6 times higher in the Northeast and 0.82 times lower in the South.
“There are health care deserts,” Dr. Skolasky said. “This study should spark a conversation about the inadequacy of distribution of physical resources to meet the needs of patients with LBP.”
Dr. Skolasky said telehealth could be one option for serving patients in these health care deserts – including those with LBP. He has conducted several studies that concluded that patients benefit from and are happy with telehealth PT.
Dr. Knight said Dr. Skolasky’s study will help patients better understand their options.
“Sometimes patients have an expectation – they want an MRI or pain medication when it’s not necessary,” he said. “This kind of evidence helps strengthen our recommendation for early intervention that really can help.”
The study was supported by a grant from the National Institutes of Health’s National Institute on Aging. Dr. Skolasky reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study found that patients who were referred to physical therapists within 2 weeks of seeing their physicians for LBP were significantly less likely to make visits to a chiropractor, pain specialist, or orthopedist.
Patients also filed fewer claims for advanced imaging or epidural steroid injections and were half as likely to visit an emergency department (ED) within 30 days compared with those who did not start early physical therapy (PT), according to the study, published in BMC Health Services Research.
“Some lower back pain resolves itself, but often, that recovery is incomplete, leading to increased health care and opioid use,” said Richard L. Skolasky Jr., ScD, director of the Spine Outcomes Research Center at Johns Hopkins Medicine, Baltimore, and a coauthor of the study. “Our hope is this study helps more primary care physicians embrace nonpharmacologic, first-line treatments.”
LBP accounts for an estimated $1.8 billion annually in health care costs among the patients who do not receive surgery for the condition, according to a 2019 JAMA analysis of commercial insurance and Medicare claims. In addition, LBP accounts for approximately 2.7 million ED visits annually, a 2010 study published in Spine showed.
Dr. Skolasky and his colleagues assessed 980,000 outpatient claims over a period of almost 4 years that ended in 2014. The researchers used Truven MarketScan, a group of U.S.-based administrative commercial health care insurance claims databases. Patients who had a history of conditions that cause LBP, such as endometriosis and spinal fracture, were excluded from the analysis. Approximately 11% of patients in the total sample received early PT, defined as PT received within 2 weeks of their initial visit to a primary care clinician.
After adjustment for sex, age, and Charlson Morbidity Index, patients who received PT were about half as likely as were those who didn’t to see chiropractor or a pain specialist or have an ED visit within 30 days of their initial appointment. They were about one-third as likely to receive an epidural steroid injection, and they were 43% less likely to have claims for advanced imaging, according to the researchers (P < .001 for all).
In addition, the cost of claims was lower for patients who received early PT ($747 vs. $799), the researchers found.
The effects diminished somewhat over time but remained statistically significant.
At 1 year, patients who received early PT had slightly higher health care costs than did those who did not undergo PT ($2,588 vs. $2,510). Dr. Skolasky hypothesized that the increase was attributable to therapy visits and not having as many specialist visits. He said additional research could investigate whether early PT reduces the health care costs associated with LBP over a longer period.
“Physical therapy addresses a patient’s current pain and physical limitations and arms them with resources, exercises, and nonpharmacologic ways to deal with recurrences,” Dr. Skolasky said in an interview. “If we can follow patients even longer than a year, we may see a longer-term reduction in cost.”
Michael Knight, MD, associate chief quality and population health officer at George Washington University Medical Faculty Associates, Washington, said he refers patients to physical therapists if their pain has not resolved within 2 weeks of stretching at home and taking over-the-counter analgesics.
Dr. Knight recalled one patient who had strained her back doing yard work. When home exercises did not help, Dr. Knight referred her to a physical therapist, who created a customized treatment plan. Within 4 weeks, her condition had improved.
“She was then able to take what she learned and continue those exercises at home,” Dr. Knight said. “She got better, and we avoided MRI costs for her and the health care system.”
Dr. Skolasky and his fellow researchers found significant regional differences in the number of patients referred for early PT. The odds of PT utilization within 90 days after the onset of LBP were 1.6 times higher in the Northeast and 0.82 times lower in the South.
“There are health care deserts,” Dr. Skolasky said. “This study should spark a conversation about the inadequacy of distribution of physical resources to meet the needs of patients with LBP.”
Dr. Skolasky said telehealth could be one option for serving patients in these health care deserts – including those with LBP. He has conducted several studies that concluded that patients benefit from and are happy with telehealth PT.
Dr. Knight said Dr. Skolasky’s study will help patients better understand their options.
“Sometimes patients have an expectation – they want an MRI or pain medication when it’s not necessary,” he said. “This kind of evidence helps strengthen our recommendation for early intervention that really can help.”
The study was supported by a grant from the National Institutes of Health’s National Institute on Aging. Dr. Skolasky reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vitamin D supplements during pregnancy may protect infants from atopic eczema
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Meet a champion climber with type 1 diabetes
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Short walks after meals can cut diabetes risk
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Taking a brief walk after eating can help lower the risk of type 2 diabetes, according to a recent study published in Sports Medicine (2022 Aug;52:1765-87).
Light walking after a meal – even for 2-5 minutes – can reduce blood sugar and insulin levels, the researchers found.
Blood sugar levels spike after eating, and the insulin produced to control them can lead to diabetes and cardiovascular issues, the researchers explained.
“With standing and walking, there are contractions of your muscles” that use glucose and lower blood sugar levels, Aidan Buffey, the lead study author and a PhD student in physical education and sport sciences at the University of Limerick (Ireland), told The Times.
“If you can do physical activity before the glucose peak, typically 60-90 minutes [after eating], that is when you’re going to have the benefit of not having the glucose spike,” he said.
Mr. Buffey and colleagues looked at seven studies to understand what would happen if you used standing or easy walking to interrupt prolonged sitting.
In five of the studies, none of the participants had prediabetes or type 2 diabetes. The other two studies included people with and without diabetes. The people in the studies were asked to either stand or walk for 2-5 minutes every 20-30 minutes over the course of a full day.
All seven studies showed that standing after a meal is better than sitting, and taking a short walk offered even better health benefits. Those who stood up for a short period of time after a meal had improved blood sugar levels but not insulin, while those who took a brief walk after a meal had lower blood sugar and insulin levels. Those who walked also had blood sugar levels that rose and fell more gradually, which is critical for managing diabetes.
Going for a walk, doing housework, or finding other ways to move your body within 60-90 minutes after eating could offer the best results, the study authors concluded.
These “mini-walks” could also be useful during the workday to break up prolonged periods of sitting at a desk.
“People are not going to get up and run on a treadmill or run around the office,” Mr. Buffey told The New York Times.
But making mini-walks a normal thing during the workday could be easy and acceptable at the office, he said. Even if people can’t take walks, standing up will help somewhat.
“Each small thing you do will have benefits, even if it is a small step,” Kershaw Patel, MD, a preventive cardiologist at Houston Methodist Hospital, told the newspaper. Dr. Patel wasn’t involved with the study.
“It’s a gradual effect of more activity, better health,” he said. “Each incremental step, each incremental stand or brisk walk appears to have a benefit.”
A version of this article first appeared on WebMD.com.
Experts: EPA should assess risk of sunscreens’ UV filters
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.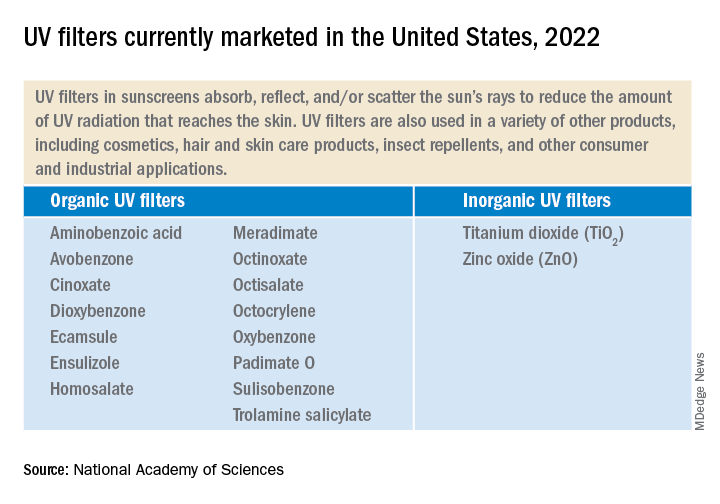
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.