User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Risankizumab induction therapy safe and effective in moderate-to-severe Crohn’s disease
Key Clinical Point: Intravenous risankizumab induction therapy is safe and effective in patients with moderate-to-severe Crohn’s disease (CD).
Major finding: In the ADVANCE trial, Crohn’s Disease Activity Index clinical remission at week 12 was higher with 600 mg risankizumab (adjusted difference [Δ] 21%) and 1200 mg (Δ 17%) vs. placebo, with the endoscopic response being higher with 600 mg risankizumab (Δ 28%) and 1200 mg (Δ 20%; all P < .0001) vs. placebo. The MOTIVATE trial reported similar findings. The incidence of adverse events was similar across all treatment groups.
Study details: This study included patients with moderate-to-severe CD and intolerance/inadequate response to biologics or conventional therapy from the phase 3 ADVANCE (n=931) and MOTIVATE (n = 618) trials who were randomly assigned to receive risankizumab (600 or 1200 mg) or placebo.
Disclosures: This study was funded by AbbVie. Some authors declared being employees or holding stocks at AbbVie, and other authors reported receiving grants, speaker’s fees, or consulting fees or serving as advisory board members for various sources, including AbbVie.
Source: D’Haens G et al. Risankizumab as induction therapy for Crohn's disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015-2030 (May 28). Doi: 10.1016/S0140-6736(22)00467-6
Key Clinical Point: Intravenous risankizumab induction therapy is safe and effective in patients with moderate-to-severe Crohn’s disease (CD).
Major finding: In the ADVANCE trial, Crohn’s Disease Activity Index clinical remission at week 12 was higher with 600 mg risankizumab (adjusted difference [Δ] 21%) and 1200 mg (Δ 17%) vs. placebo, with the endoscopic response being higher with 600 mg risankizumab (Δ 28%) and 1200 mg (Δ 20%; all P < .0001) vs. placebo. The MOTIVATE trial reported similar findings. The incidence of adverse events was similar across all treatment groups.
Study details: This study included patients with moderate-to-severe CD and intolerance/inadequate response to biologics or conventional therapy from the phase 3 ADVANCE (n=931) and MOTIVATE (n = 618) trials who were randomly assigned to receive risankizumab (600 or 1200 mg) or placebo.
Disclosures: This study was funded by AbbVie. Some authors declared being employees or holding stocks at AbbVie, and other authors reported receiving grants, speaker’s fees, or consulting fees or serving as advisory board members for various sources, including AbbVie.
Source: D’Haens G et al. Risankizumab as induction therapy for Crohn's disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015-2030 (May 28). Doi: 10.1016/S0140-6736(22)00467-6
Key Clinical Point: Intravenous risankizumab induction therapy is safe and effective in patients with moderate-to-severe Crohn’s disease (CD).
Major finding: In the ADVANCE trial, Crohn’s Disease Activity Index clinical remission at week 12 was higher with 600 mg risankizumab (adjusted difference [Δ] 21%) and 1200 mg (Δ 17%) vs. placebo, with the endoscopic response being higher with 600 mg risankizumab (Δ 28%) and 1200 mg (Δ 20%; all P < .0001) vs. placebo. The MOTIVATE trial reported similar findings. The incidence of adverse events was similar across all treatment groups.
Study details: This study included patients with moderate-to-severe CD and intolerance/inadequate response to biologics or conventional therapy from the phase 3 ADVANCE (n=931) and MOTIVATE (n = 618) trials who were randomly assigned to receive risankizumab (600 or 1200 mg) or placebo.
Disclosures: This study was funded by AbbVie. Some authors declared being employees or holding stocks at AbbVie, and other authors reported receiving grants, speaker’s fees, or consulting fees or serving as advisory board members for various sources, including AbbVie.
Source: D’Haens G et al. Risankizumab as induction therapy for Crohn's disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015-2030 (May 28). Doi: 10.1016/S0140-6736(22)00467-6
More evidence the flu vaccine may guard against Alzheimer’s
In a large propensity-matched cohort of older adults, those who had received at least one influenza inoculation were 40% less likely than unvaccinated peers to develop AD over the course of 4 years.
“Influenza infection can cause serious health complications, particularly in adults 65 and older. Our study’s findings – that vaccination against the flu virus may also reduce the risk of Alzheimer’s dementia for at least a few years – adds to the already compelling reasons get the flu vaccine annually,” Avram Bukhbinder, MD, of the University of Texas, Houston, said in an interview.
The new findings support earlier work by the same researchers that also suggested a protective effect of flu vaccination on dementia risk.
The latest study was published online in the Journal of Alzheimer’s Disease.
40% lower risk
Prior studies have found a lower risk of dementia of any etiology following influenza vaccination in selected populations, including veterans and patients with serious chronic health conditions.
However, the effect of influenza vaccination on AD risk in a general cohort of older U.S. adults has not been characterized.
Dr. Bukhbinder and colleagues used claims data to create a propensity-matched cohort of 935,887 influenza-vaccinated adults and a like number of unvaccinated adults aged 65 and older.
The median age of the persons in the matched sample was 73.7 years, and 57% were women. All were free of dementia during the 6-year look-back study period.
During median follow-up of 46 months, 47,889 (5.1%) flu-vaccinated adults and 79,630 (8.5%) unvaccinated adults developed AD.
The risk of AD was 40% lower in the vaccinated group (relative risk, 0.60; 95% confidence interval, 0.59-0.61). The absolute risk reduction was 0.034 (95% CI, 0.033-0.035), corresponding to a number needed to treat of 29.4.
Mechanism unclear
“Our study does not address the mechanism(s) underlying the apparent effect of influenza vaccination on Alzheimer’s risk, but we look forward to future research investigating this important question,” Dr. Bukhbinder said.
“One possible mechanism is that, by helping to prevent or mitigate infection with the flu virus and the systemic inflammation that follows such an infection, the flu vaccine helps to decrease the systemic inflammation that may have otherwise occurred,” he explained.
It’s also possible that influenza vaccination may trigger non–influenza-specific changes in the immune system that help to reduce the damage caused by AD pathology, including amyloid plaques and neurofibrillary tangles, he said.
“For example, the influenza vaccine may alter the brain’s immune cells such that they are better at clearing Alzheimer’s pathologies, an effect that has been seen in mice, or it may reprogram these immune cells to respond to Alzheimer’s pathologies in ways that are less likely to damage nearby healthy brain cells, or it may do both,” Dr. Bukhbinder noted.
Alzheimer’s expert weighs in
Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this study “suggests that flu vaccination may be valuable for maintaining cognition and memory as we age. This is even more relevant today in the COVID-19 environment.
“It is too early to tell if getting flu vaccine, on its own, can reduce risk of Alzheimer’s. More research is needed to understand the biological mechanisms behind the results in this study,” Dr. Snyder said in an interview.
“For example, it is possible that people who are getting vaccinated also take better care of their health in other ways, and these things add up to lower risk of Alzheimer’s and other dementias,” she noted.
“It is also possible that there are issues related to unequal access and/or vaccine hesitancy and how this may influence the study population and the research results,” Dr. Snyder said.
The study had no specific funding. Dr. Bukhbinder and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large propensity-matched cohort of older adults, those who had received at least one influenza inoculation were 40% less likely than unvaccinated peers to develop AD over the course of 4 years.
“Influenza infection can cause serious health complications, particularly in adults 65 and older. Our study’s findings – that vaccination against the flu virus may also reduce the risk of Alzheimer’s dementia for at least a few years – adds to the already compelling reasons get the flu vaccine annually,” Avram Bukhbinder, MD, of the University of Texas, Houston, said in an interview.
The new findings support earlier work by the same researchers that also suggested a protective effect of flu vaccination on dementia risk.
The latest study was published online in the Journal of Alzheimer’s Disease.
40% lower risk
Prior studies have found a lower risk of dementia of any etiology following influenza vaccination in selected populations, including veterans and patients with serious chronic health conditions.
However, the effect of influenza vaccination on AD risk in a general cohort of older U.S. adults has not been characterized.
Dr. Bukhbinder and colleagues used claims data to create a propensity-matched cohort of 935,887 influenza-vaccinated adults and a like number of unvaccinated adults aged 65 and older.
The median age of the persons in the matched sample was 73.7 years, and 57% were women. All were free of dementia during the 6-year look-back study period.
During median follow-up of 46 months, 47,889 (5.1%) flu-vaccinated adults and 79,630 (8.5%) unvaccinated adults developed AD.
The risk of AD was 40% lower in the vaccinated group (relative risk, 0.60; 95% confidence interval, 0.59-0.61). The absolute risk reduction was 0.034 (95% CI, 0.033-0.035), corresponding to a number needed to treat of 29.4.
Mechanism unclear
“Our study does not address the mechanism(s) underlying the apparent effect of influenza vaccination on Alzheimer’s risk, but we look forward to future research investigating this important question,” Dr. Bukhbinder said.
“One possible mechanism is that, by helping to prevent or mitigate infection with the flu virus and the systemic inflammation that follows such an infection, the flu vaccine helps to decrease the systemic inflammation that may have otherwise occurred,” he explained.
It’s also possible that influenza vaccination may trigger non–influenza-specific changes in the immune system that help to reduce the damage caused by AD pathology, including amyloid plaques and neurofibrillary tangles, he said.
“For example, the influenza vaccine may alter the brain’s immune cells such that they are better at clearing Alzheimer’s pathologies, an effect that has been seen in mice, or it may reprogram these immune cells to respond to Alzheimer’s pathologies in ways that are less likely to damage nearby healthy brain cells, or it may do both,” Dr. Bukhbinder noted.
Alzheimer’s expert weighs in
Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this study “suggests that flu vaccination may be valuable for maintaining cognition and memory as we age. This is even more relevant today in the COVID-19 environment.
“It is too early to tell if getting flu vaccine, on its own, can reduce risk of Alzheimer’s. More research is needed to understand the biological mechanisms behind the results in this study,” Dr. Snyder said in an interview.
“For example, it is possible that people who are getting vaccinated also take better care of their health in other ways, and these things add up to lower risk of Alzheimer’s and other dementias,” she noted.
“It is also possible that there are issues related to unequal access and/or vaccine hesitancy and how this may influence the study population and the research results,” Dr. Snyder said.
The study had no specific funding. Dr. Bukhbinder and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large propensity-matched cohort of older adults, those who had received at least one influenza inoculation were 40% less likely than unvaccinated peers to develop AD over the course of 4 years.
“Influenza infection can cause serious health complications, particularly in adults 65 and older. Our study’s findings – that vaccination against the flu virus may also reduce the risk of Alzheimer’s dementia for at least a few years – adds to the already compelling reasons get the flu vaccine annually,” Avram Bukhbinder, MD, of the University of Texas, Houston, said in an interview.
The new findings support earlier work by the same researchers that also suggested a protective effect of flu vaccination on dementia risk.
The latest study was published online in the Journal of Alzheimer’s Disease.
40% lower risk
Prior studies have found a lower risk of dementia of any etiology following influenza vaccination in selected populations, including veterans and patients with serious chronic health conditions.
However, the effect of influenza vaccination on AD risk in a general cohort of older U.S. adults has not been characterized.
Dr. Bukhbinder and colleagues used claims data to create a propensity-matched cohort of 935,887 influenza-vaccinated adults and a like number of unvaccinated adults aged 65 and older.
The median age of the persons in the matched sample was 73.7 years, and 57% were women. All were free of dementia during the 6-year look-back study period.
During median follow-up of 46 months, 47,889 (5.1%) flu-vaccinated adults and 79,630 (8.5%) unvaccinated adults developed AD.
The risk of AD was 40% lower in the vaccinated group (relative risk, 0.60; 95% confidence interval, 0.59-0.61). The absolute risk reduction was 0.034 (95% CI, 0.033-0.035), corresponding to a number needed to treat of 29.4.
Mechanism unclear
“Our study does not address the mechanism(s) underlying the apparent effect of influenza vaccination on Alzheimer’s risk, but we look forward to future research investigating this important question,” Dr. Bukhbinder said.
“One possible mechanism is that, by helping to prevent or mitigate infection with the flu virus and the systemic inflammation that follows such an infection, the flu vaccine helps to decrease the systemic inflammation that may have otherwise occurred,” he explained.
It’s also possible that influenza vaccination may trigger non–influenza-specific changes in the immune system that help to reduce the damage caused by AD pathology, including amyloid plaques and neurofibrillary tangles, he said.
“For example, the influenza vaccine may alter the brain’s immune cells such that they are better at clearing Alzheimer’s pathologies, an effect that has been seen in mice, or it may reprogram these immune cells to respond to Alzheimer’s pathologies in ways that are less likely to damage nearby healthy brain cells, or it may do both,” Dr. Bukhbinder noted.
Alzheimer’s expert weighs in
Heather M. Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, said this study “suggests that flu vaccination may be valuable for maintaining cognition and memory as we age. This is even more relevant today in the COVID-19 environment.
“It is too early to tell if getting flu vaccine, on its own, can reduce risk of Alzheimer’s. More research is needed to understand the biological mechanisms behind the results in this study,” Dr. Snyder said in an interview.
“For example, it is possible that people who are getting vaccinated also take better care of their health in other ways, and these things add up to lower risk of Alzheimer’s and other dementias,” she noted.
“It is also possible that there are issues related to unequal access and/or vaccine hesitancy and how this may influence the study population and the research results,” Dr. Snyder said.
The study had no specific funding. Dr. Bukhbinder and Dr. Snyder disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Acute hepatitis cases in children show declining trend; adenovirus, COVID-19 remain key leads
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2022
Rapidly Evolving Papulonodular Eruption in the Axilla
The Diagnosis: Lymphomatoid Papulosis
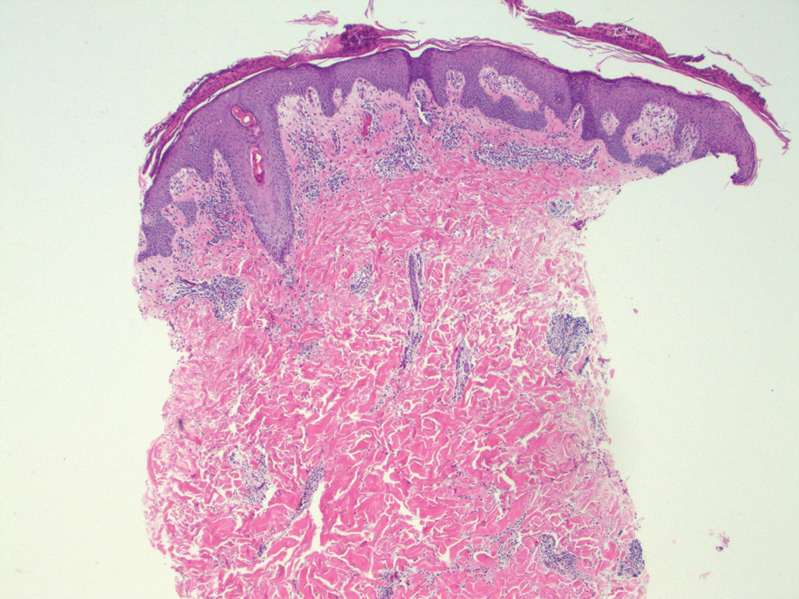
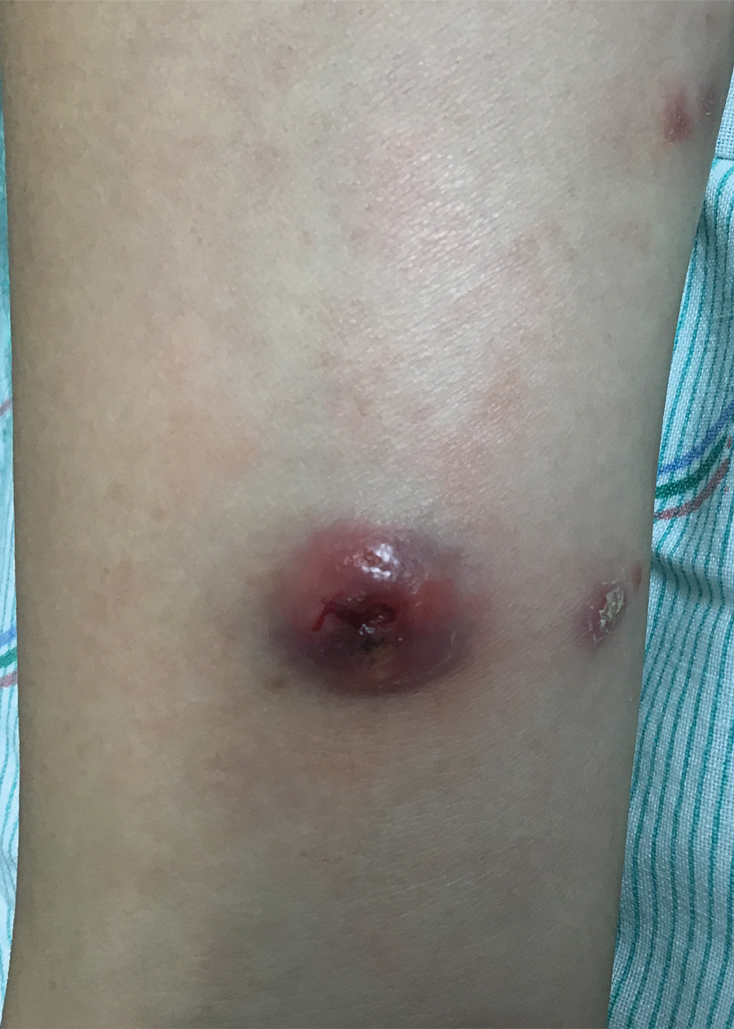
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.
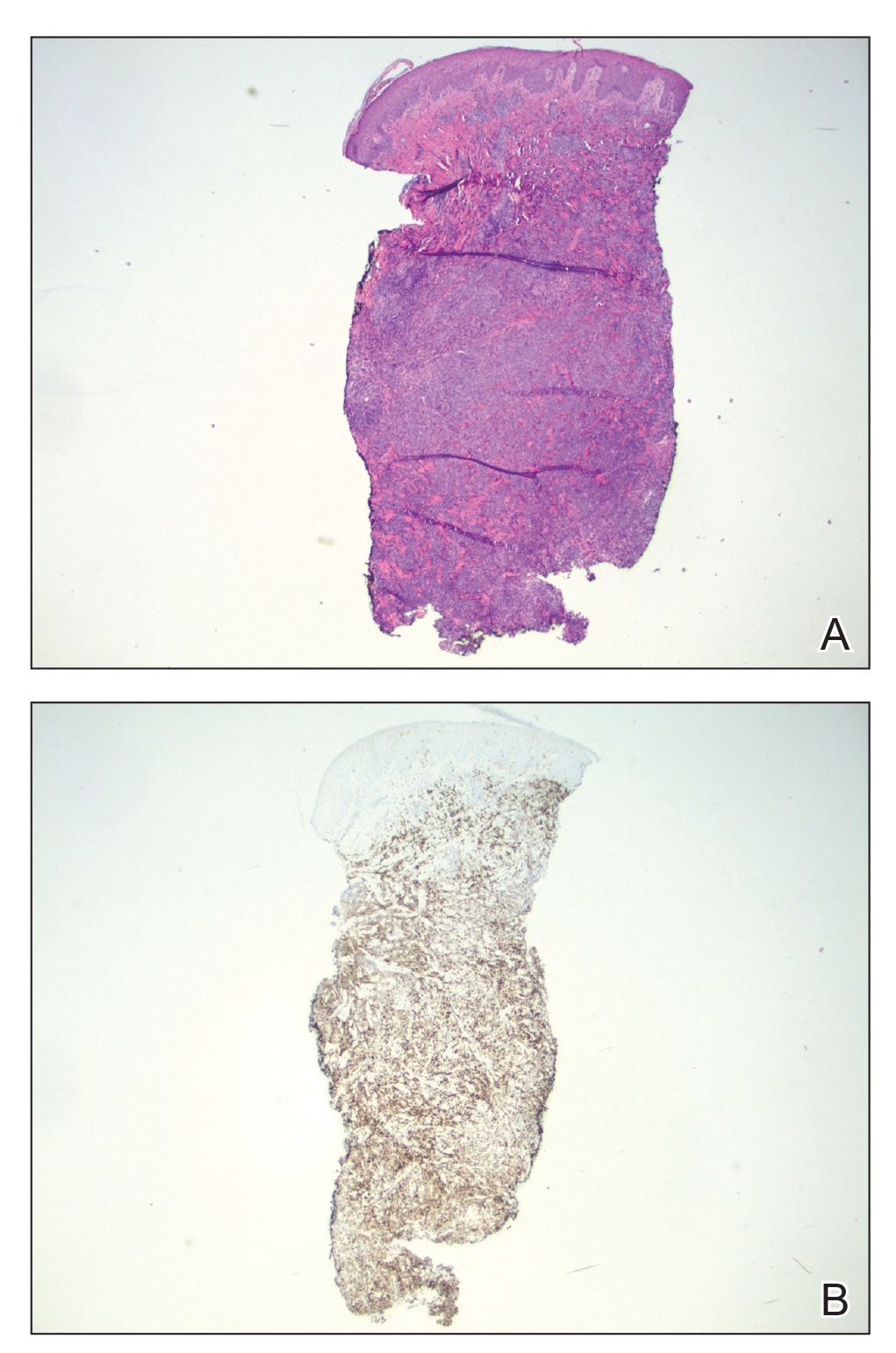
Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.
Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5
Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6
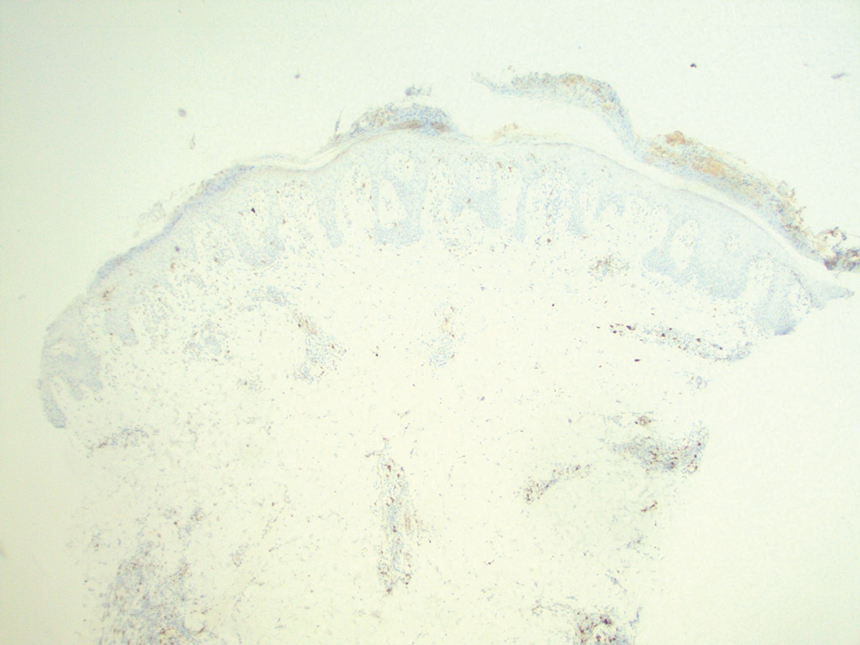
The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
The Diagnosis: Lymphomatoid Papulosis
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.

Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.

Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5

Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6

The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
The Diagnosis: Lymphomatoid Papulosis
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.

Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.

Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5

Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6

The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
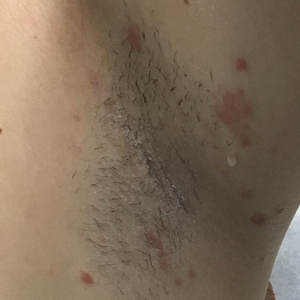
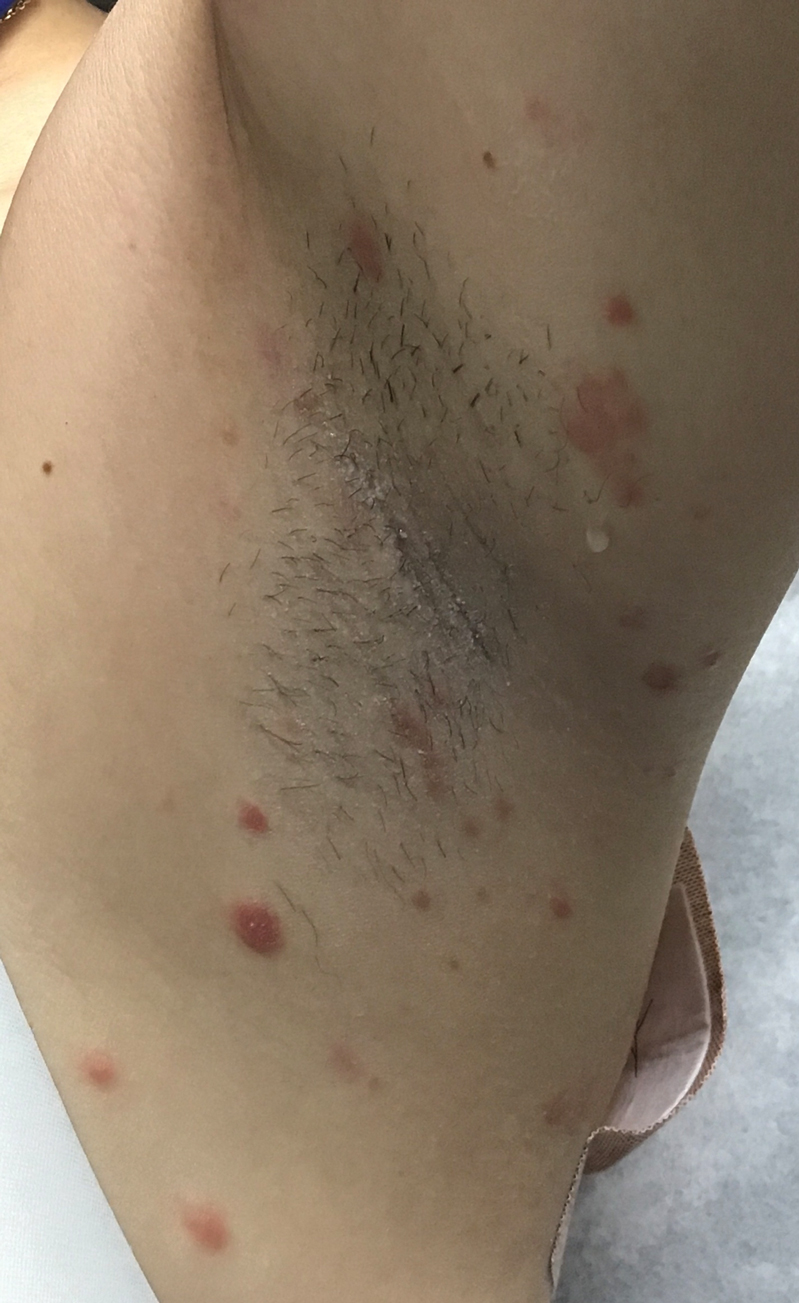
A 37-year-old woman presented to our dermatology clinic with a pruritic erythematous eruption involving the trunk, axillae, and proximal extremities of 10 days’ duration. Her medical history was notable only for eczema, and she denied taking any medications. Physical examination revealed scattered erythematous papules and crusts involving the trunk bilaterally and the extremities. We initially made a clinical diagnosis of bullous impetigo, and the patient was prescribed mupirocin ointment and dicloxacillin. At 1-week follow-up, the patient reported persistent skin lesions that were evolving despite therapy. Physical examination at this visit revealed an evolving eruption of multiple reddish-brown scaly papules involving the axillae, arms, forearms, and thighs, as depicted here.
White House expands access to monkeypox vaccines
The White House is scaling up its response to the monkeypox outbreak, expanding access to vaccines to more at-risk individuals, officials said in a press call. More than 56,000 doses of the monkeypox vaccine JYNNEOS will be made available immediately, and more than 240,000 doses will be allocated in the coming weeks.
“The administration’s current strategy is focused on containing the outbreak by providing vaccines to those most in need to prevent further spread of monkeypox in the communities most impacted,” CDC Director Rochelle Walensky, MD, MPH, said on a June 28 press call. “As additional supply becomes available, we will further expand our efforts making vaccines available to a wider population.”
As of June 28, there were 4,700 detected cases of monkeypox globally in 49 countries. Since the first U.S. case of monkeypox was identified on May 17, there have been 306 confirmed cases across 28 jurisdictions.
Prior to this announcement, vaccination against monkeypox was recommended only for people with known exposures to the virus. Now, the vaccine is available to people who are likely to be exposed to the virus, including:
- People who have had close physical contact with someone diagnosed with monkeypox.
- People with a sexual partner diagnosed with monkeypox.
- Men who have sex with men who have had multiple sex partners in a venue where monkeypox was identified.
The JYNNEOS vaccine is administered in two doses, delivered 28 days apart. People will have maximum immunity 2 weeks after the second dose. People should be vaccinated within 2 weeks of a possible monkeypox exposure, Dr. Walensky said, adding, “The sooner you can get vaccinated after exposure, the better.”
The U.S. Department of Health and Human Services will immediately allocate the 56,000 JYNNEOS doses across the country, prioritizing jurisdictions to areas of high transmission. A second vaccine, ACAM2000, can also be requested, but it has a greater risk for serious side effects and is not appropriate for immunocompromised individuals or people with heart disease. In the coming weeks, 240,000 JYNNEOS doses will be made available for second doses as well as first doses “as the vaccine strategy broadens,” said David Boucher, director of infectious disease preparedness and response for HHS. There are currently 800,000 JYNNEOS doses that have been manufactured and approved for release, he said, and awaiting inspection by the Food and Drug Administration, which should be completed in the beginning of July.
At the same time, the administration is focusing on increasing access to testing. Monkeypox testing is now available in 78 state public health labs in 48 states that can collectively conduct 10,000 tests per week. In addition, the administration announced on June 23 that HHS began shipping monkeypox tests to five commercial lab companies to expand testing capacity as well as make testing more accessible.
“We continue to work very closely with the community and with public health partners and clinicians to increase awareness of the monkey pox outbreak and to facilitate adequate capacity and equitable access to testing,” Dr. Walensky said. “I strongly encourage all health care providers to have a high clinical suspicion for monkeypox among their patients. Patients presenting with a suspicious rash should be tested.”
A version of this article first appeared on Medscape.com.
The White House is scaling up its response to the monkeypox outbreak, expanding access to vaccines to more at-risk individuals, officials said in a press call. More than 56,000 doses of the monkeypox vaccine JYNNEOS will be made available immediately, and more than 240,000 doses will be allocated in the coming weeks.
“The administration’s current strategy is focused on containing the outbreak by providing vaccines to those most in need to prevent further spread of monkeypox in the communities most impacted,” CDC Director Rochelle Walensky, MD, MPH, said on a June 28 press call. “As additional supply becomes available, we will further expand our efforts making vaccines available to a wider population.”
As of June 28, there were 4,700 detected cases of monkeypox globally in 49 countries. Since the first U.S. case of monkeypox was identified on May 17, there have been 306 confirmed cases across 28 jurisdictions.
Prior to this announcement, vaccination against monkeypox was recommended only for people with known exposures to the virus. Now, the vaccine is available to people who are likely to be exposed to the virus, including:
- People who have had close physical contact with someone diagnosed with monkeypox.
- People with a sexual partner diagnosed with monkeypox.
- Men who have sex with men who have had multiple sex partners in a venue where monkeypox was identified.
The JYNNEOS vaccine is administered in two doses, delivered 28 days apart. People will have maximum immunity 2 weeks after the second dose. People should be vaccinated within 2 weeks of a possible monkeypox exposure, Dr. Walensky said, adding, “The sooner you can get vaccinated after exposure, the better.”
The U.S. Department of Health and Human Services will immediately allocate the 56,000 JYNNEOS doses across the country, prioritizing jurisdictions to areas of high transmission. A second vaccine, ACAM2000, can also be requested, but it has a greater risk for serious side effects and is not appropriate for immunocompromised individuals or people with heart disease. In the coming weeks, 240,000 JYNNEOS doses will be made available for second doses as well as first doses “as the vaccine strategy broadens,” said David Boucher, director of infectious disease preparedness and response for HHS. There are currently 800,000 JYNNEOS doses that have been manufactured and approved for release, he said, and awaiting inspection by the Food and Drug Administration, which should be completed in the beginning of July.
At the same time, the administration is focusing on increasing access to testing. Monkeypox testing is now available in 78 state public health labs in 48 states that can collectively conduct 10,000 tests per week. In addition, the administration announced on June 23 that HHS began shipping monkeypox tests to five commercial lab companies to expand testing capacity as well as make testing more accessible.
“We continue to work very closely with the community and with public health partners and clinicians to increase awareness of the monkey pox outbreak and to facilitate adequate capacity and equitable access to testing,” Dr. Walensky said. “I strongly encourage all health care providers to have a high clinical suspicion for monkeypox among their patients. Patients presenting with a suspicious rash should be tested.”
A version of this article first appeared on Medscape.com.
The White House is scaling up its response to the monkeypox outbreak, expanding access to vaccines to more at-risk individuals, officials said in a press call. More than 56,000 doses of the monkeypox vaccine JYNNEOS will be made available immediately, and more than 240,000 doses will be allocated in the coming weeks.
“The administration’s current strategy is focused on containing the outbreak by providing vaccines to those most in need to prevent further spread of monkeypox in the communities most impacted,” CDC Director Rochelle Walensky, MD, MPH, said on a June 28 press call. “As additional supply becomes available, we will further expand our efforts making vaccines available to a wider population.”
As of June 28, there were 4,700 detected cases of monkeypox globally in 49 countries. Since the first U.S. case of monkeypox was identified on May 17, there have been 306 confirmed cases across 28 jurisdictions.
Prior to this announcement, vaccination against monkeypox was recommended only for people with known exposures to the virus. Now, the vaccine is available to people who are likely to be exposed to the virus, including:
- People who have had close physical contact with someone diagnosed with monkeypox.
- People with a sexual partner diagnosed with monkeypox.
- Men who have sex with men who have had multiple sex partners in a venue where monkeypox was identified.
The JYNNEOS vaccine is administered in two doses, delivered 28 days apart. People will have maximum immunity 2 weeks after the second dose. People should be vaccinated within 2 weeks of a possible monkeypox exposure, Dr. Walensky said, adding, “The sooner you can get vaccinated after exposure, the better.”
The U.S. Department of Health and Human Services will immediately allocate the 56,000 JYNNEOS doses across the country, prioritizing jurisdictions to areas of high transmission. A second vaccine, ACAM2000, can also be requested, but it has a greater risk for serious side effects and is not appropriate for immunocompromised individuals or people with heart disease. In the coming weeks, 240,000 JYNNEOS doses will be made available for second doses as well as first doses “as the vaccine strategy broadens,” said David Boucher, director of infectious disease preparedness and response for HHS. There are currently 800,000 JYNNEOS doses that have been manufactured and approved for release, he said, and awaiting inspection by the Food and Drug Administration, which should be completed in the beginning of July.
At the same time, the administration is focusing on increasing access to testing. Monkeypox testing is now available in 78 state public health labs in 48 states that can collectively conduct 10,000 tests per week. In addition, the administration announced on June 23 that HHS began shipping monkeypox tests to five commercial lab companies to expand testing capacity as well as make testing more accessible.
“We continue to work very closely with the community and with public health partners and clinicians to increase awareness of the monkey pox outbreak and to facilitate adequate capacity and equitable access to testing,” Dr. Walensky said. “I strongly encourage all health care providers to have a high clinical suspicion for monkeypox among their patients. Patients presenting with a suspicious rash should be tested.”
A version of this article first appeared on Medscape.com.
FDA panel backs adding Omicron component to COVID boosters
A federal advisory panel on June 28 recommended updating COVID-19 booster vaccines in the United States to include an Omicron component, while urging the need for more information on how well these shots work on emerging strains of the virus.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted 19-2 in favor of a new formulation – although what that formulation will be is yet to be determined. The FDA often incorporates the views of its advisers into its decisions, although it is not bound to do so.
In this case, though, top FDA staff at the meeting seemed inclined to encourage the development of COVID vaccines modified to keep up with an evolving virus. Two Omicron subvariants, BA.4 and BA.5, which first appeared in South Africa in March 2022, have spread to the United States and have begun to increase rapidly in proportion to the virus population, the FDA said in a briefing for the meeting.
New information from the Centers for Disease Control and Prevention shows the two highly infectious subvariants now make up more than half the number of new COVID cases in the US.
Double-duty vaccine
In summarizing the message of the advisory committee, Peter W. Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation & Research, said panelists had lent support to modifying vaccines to protect against both the original, or “ancestral” viral strain, and against Omicron, perhaps emphasizing the newly emerging subvariants.
Dr. Marks emphasized that this is a challenging decision, as no one has a “crystal ball” to forecast how SARS-CoV-2 will evolve.
“We are trying to use every last ounce of what we can from predictive modeling and from the data that we have that’s emerging, to try to get ahead of a virus that has been very crafty,” he said.”It’s pretty darn crafty.”
Limited data
Voting “no” were Paul Offit, MD, of Children’s Hospital of Philadelphia and Henry Bernstein, DO, MHCM, of Hofstra/Northwell Health in New Hyde Park, N.Y.
Both Dr. Offit and Dr. Bernstein earlier in the meeting expressed doubts about the evidence gathered to date in favor of a strain change. Dr. Offit had noted that protection seems to persist from the vaccines now available.
“To date, the current prototypical vaccines, the ancestral strain vaccines do protect against serious illness,” he said. “We don’t yet have a variant that is resistant to protection against serious illness.“
Dr. Bernstein said he was “struggling” with the question as well, given the limited data gathered to date about the vaccines and emerging strains of the virus.
Other panelists also expressed reservations, while supporting the concept of altering vaccines to teach the body to fight the emerging strains as well as the original one.
Panelist Wayne Marasco, MD, PhD, of Harvard Medical School, Boston, who voted yes, noted the difficulties of keeping up with the rapidly evolving virus, saying it’s possible that Omicron strains BA.4 and BA.5 could peak within months. That could be before the vaccines are even distributed – if all goes to plan – in the fall.
“This is a step in the right direction, but we have to reevaluate this as we move forward,” Dr. Marasco said, adding that a good strategy would be to elicit antibody response to bridge more than one variant of the virus.
Even panelists like Dr. Marasco who voted yes stressed the need for further data collection about how vaccines may be adapted to a changing virus. But they also acknowledged a need to give vaccine makers a clear indication of what the medical community expects in terms of changes to these shots.
“With the waning vaccine efficacy and the confluence of risk this fall, we need to make a move sooner rather than later and direct our sponsors in the proper direction,” said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville, said before the vote.
A version of this article first appeared on Medscape.com.
A federal advisory panel on June 28 recommended updating COVID-19 booster vaccines in the United States to include an Omicron component, while urging the need for more information on how well these shots work on emerging strains of the virus.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted 19-2 in favor of a new formulation – although what that formulation will be is yet to be determined. The FDA often incorporates the views of its advisers into its decisions, although it is not bound to do so.
In this case, though, top FDA staff at the meeting seemed inclined to encourage the development of COVID vaccines modified to keep up with an evolving virus. Two Omicron subvariants, BA.4 and BA.5, which first appeared in South Africa in March 2022, have spread to the United States and have begun to increase rapidly in proportion to the virus population, the FDA said in a briefing for the meeting.
New information from the Centers for Disease Control and Prevention shows the two highly infectious subvariants now make up more than half the number of new COVID cases in the US.
Double-duty vaccine
In summarizing the message of the advisory committee, Peter W. Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation & Research, said panelists had lent support to modifying vaccines to protect against both the original, or “ancestral” viral strain, and against Omicron, perhaps emphasizing the newly emerging subvariants.
Dr. Marks emphasized that this is a challenging decision, as no one has a “crystal ball” to forecast how SARS-CoV-2 will evolve.
“We are trying to use every last ounce of what we can from predictive modeling and from the data that we have that’s emerging, to try to get ahead of a virus that has been very crafty,” he said.”It’s pretty darn crafty.”
Limited data
Voting “no” were Paul Offit, MD, of Children’s Hospital of Philadelphia and Henry Bernstein, DO, MHCM, of Hofstra/Northwell Health in New Hyde Park, N.Y.
Both Dr. Offit and Dr. Bernstein earlier in the meeting expressed doubts about the evidence gathered to date in favor of a strain change. Dr. Offit had noted that protection seems to persist from the vaccines now available.
“To date, the current prototypical vaccines, the ancestral strain vaccines do protect against serious illness,” he said. “We don’t yet have a variant that is resistant to protection against serious illness.“
Dr. Bernstein said he was “struggling” with the question as well, given the limited data gathered to date about the vaccines and emerging strains of the virus.
Other panelists also expressed reservations, while supporting the concept of altering vaccines to teach the body to fight the emerging strains as well as the original one.
Panelist Wayne Marasco, MD, PhD, of Harvard Medical School, Boston, who voted yes, noted the difficulties of keeping up with the rapidly evolving virus, saying it’s possible that Omicron strains BA.4 and BA.5 could peak within months. That could be before the vaccines are even distributed – if all goes to plan – in the fall.
“This is a step in the right direction, but we have to reevaluate this as we move forward,” Dr. Marasco said, adding that a good strategy would be to elicit antibody response to bridge more than one variant of the virus.
Even panelists like Dr. Marasco who voted yes stressed the need for further data collection about how vaccines may be adapted to a changing virus. But they also acknowledged a need to give vaccine makers a clear indication of what the medical community expects in terms of changes to these shots.
“With the waning vaccine efficacy and the confluence of risk this fall, we need to make a move sooner rather than later and direct our sponsors in the proper direction,” said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville, said before the vote.
A version of this article first appeared on Medscape.com.
A federal advisory panel on June 28 recommended updating COVID-19 booster vaccines in the United States to include an Omicron component, while urging the need for more information on how well these shots work on emerging strains of the virus.
The Vaccines and Related Biological Products Advisory Committee of the Food and Drug Administration voted 19-2 in favor of a new formulation – although what that formulation will be is yet to be determined. The FDA often incorporates the views of its advisers into its decisions, although it is not bound to do so.
In this case, though, top FDA staff at the meeting seemed inclined to encourage the development of COVID vaccines modified to keep up with an evolving virus. Two Omicron subvariants, BA.4 and BA.5, which first appeared in South Africa in March 2022, have spread to the United States and have begun to increase rapidly in proportion to the virus population, the FDA said in a briefing for the meeting.
New information from the Centers for Disease Control and Prevention shows the two highly infectious subvariants now make up more than half the number of new COVID cases in the US.
Double-duty vaccine
In summarizing the message of the advisory committee, Peter W. Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation & Research, said panelists had lent support to modifying vaccines to protect against both the original, or “ancestral” viral strain, and against Omicron, perhaps emphasizing the newly emerging subvariants.
Dr. Marks emphasized that this is a challenging decision, as no one has a “crystal ball” to forecast how SARS-CoV-2 will evolve.
“We are trying to use every last ounce of what we can from predictive modeling and from the data that we have that’s emerging, to try to get ahead of a virus that has been very crafty,” he said.”It’s pretty darn crafty.”
Limited data
Voting “no” were Paul Offit, MD, of Children’s Hospital of Philadelphia and Henry Bernstein, DO, MHCM, of Hofstra/Northwell Health in New Hyde Park, N.Y.
Both Dr. Offit and Dr. Bernstein earlier in the meeting expressed doubts about the evidence gathered to date in favor of a strain change. Dr. Offit had noted that protection seems to persist from the vaccines now available.
“To date, the current prototypical vaccines, the ancestral strain vaccines do protect against serious illness,” he said. “We don’t yet have a variant that is resistant to protection against serious illness.“
Dr. Bernstein said he was “struggling” with the question as well, given the limited data gathered to date about the vaccines and emerging strains of the virus.
Other panelists also expressed reservations, while supporting the concept of altering vaccines to teach the body to fight the emerging strains as well as the original one.
Panelist Wayne Marasco, MD, PhD, of Harvard Medical School, Boston, who voted yes, noted the difficulties of keeping up with the rapidly evolving virus, saying it’s possible that Omicron strains BA.4 and BA.5 could peak within months. That could be before the vaccines are even distributed – if all goes to plan – in the fall.
“This is a step in the right direction, but we have to reevaluate this as we move forward,” Dr. Marasco said, adding that a good strategy would be to elicit antibody response to bridge more than one variant of the virus.
Even panelists like Dr. Marasco who voted yes stressed the need for further data collection about how vaccines may be adapted to a changing virus. But they also acknowledged a need to give vaccine makers a clear indication of what the medical community expects in terms of changes to these shots.
“With the waning vaccine efficacy and the confluence of risk this fall, we need to make a move sooner rather than later and direct our sponsors in the proper direction,” said FDA panelist Michael Nelson, MD, PhD, of the University of Virginia, Charlottesville, said before the vote.
A version of this article first appeared on Medscape.com.
COVID subvariants could cause ‘substantial’ summer cases
As the coronavirus continues to evolve, Omicron subvariants such as BA.4 and BA.5 are expected to lead to many COVID-19 cases in the coming months.
Researchers recently reported that the subvariants have mutated for better “immune escape,” or the ability to avoid antibodies from vaccination or previous infection.
“That has changed our view for what will happen this summer,” Ali Mokdad, PhD, an epidemiologist who has developed COVID-19 forecasts for the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, told The Boston Globe.
Until recently, Dr. Mokdad expected the United States to have a “very good summer” in terms of cases, hospitalizations, and deaths through September. The U.S. is reporting about 100,000 new cases per day, according to the data tracker by The New York Times, which has remained flat throughout June. Cases will likely decrease this summer, Dr. Mokdad said, though the decline will be slower and smaller than first thought.
As of June 18, BA.4 and BA.5 accounted for about 35% of cases in the United States, according to the latest CDC data, with BA.5 making up 23.5% and BA.4 making up 11.4%. The two subvariants will likely take over BA.2.12.1 as top subvariants in coming weeks.
“I expect that BA.5 will likely become the dominant virus in the United States this summer,” Dan Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston, told the Globe.
Dr. Barouch said the Omicron subvariants will likely create a summer of “substantial infections” but low rates of hospitalization and death. He published a recent study in the New England Journal of Medicine that found BA.4 and BA.5 are better at escaping antibodies than other coronavirus strains – about three times better than the Omicron variants BA.1 and BA.2 and 20 times better than the first coronavirus strain.
“What we’re seeing with each subsequent variant is iteratively higher levels of transmissibility and higher levels of antibody immune escape,” he said. “We’re seeing high levels of infection in populations that are highly vaccinated, as well as populations that have a high level of natural immunity to the prior variants.”
At the same time, current antibodies still appear to protect people against the worst outcomes, Dr. Barouch said.
“If people have vaccine immunity or natural immunity, then they have substantial protection against severe disease,” he said.
So far, researchers have found that Omicron subvariants tend to cause less severe disease than other variants, such as Delta. Dr. Mokdad estimated that 80% of Omicron infections don’t show symptoms.
He said there is a “remote possibility” of another wave during the summer, but he expects cases to rise significantly around the beginning of October, when the seasons change, and most people’s immunity will wane. Other things could play into the predictions this summer, he noted, such as coronavirus mutations and new variants.
“Anybody that models this more than a couple of weeks out is basically just using pixie dust,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told the newspaper.
“There is no pattern whatsoever developing from a seasonality standpoint. It’s all being driven by the variants,” he said. “We just have to be humble and acknowledge that we don’t know.”
A version of this article first appeared on WebMD.com.
As the coronavirus continues to evolve, Omicron subvariants such as BA.4 and BA.5 are expected to lead to many COVID-19 cases in the coming months.
Researchers recently reported that the subvariants have mutated for better “immune escape,” or the ability to avoid antibodies from vaccination or previous infection.
“That has changed our view for what will happen this summer,” Ali Mokdad, PhD, an epidemiologist who has developed COVID-19 forecasts for the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, told The Boston Globe.
Until recently, Dr. Mokdad expected the United States to have a “very good summer” in terms of cases, hospitalizations, and deaths through September. The U.S. is reporting about 100,000 new cases per day, according to the data tracker by The New York Times, which has remained flat throughout June. Cases will likely decrease this summer, Dr. Mokdad said, though the decline will be slower and smaller than first thought.
As of June 18, BA.4 and BA.5 accounted for about 35% of cases in the United States, according to the latest CDC data, with BA.5 making up 23.5% and BA.4 making up 11.4%. The two subvariants will likely take over BA.2.12.1 as top subvariants in coming weeks.
“I expect that BA.5 will likely become the dominant virus in the United States this summer,” Dan Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston, told the Globe.
Dr. Barouch said the Omicron subvariants will likely create a summer of “substantial infections” but low rates of hospitalization and death. He published a recent study in the New England Journal of Medicine that found BA.4 and BA.5 are better at escaping antibodies than other coronavirus strains – about three times better than the Omicron variants BA.1 and BA.2 and 20 times better than the first coronavirus strain.
“What we’re seeing with each subsequent variant is iteratively higher levels of transmissibility and higher levels of antibody immune escape,” he said. “We’re seeing high levels of infection in populations that are highly vaccinated, as well as populations that have a high level of natural immunity to the prior variants.”
At the same time, current antibodies still appear to protect people against the worst outcomes, Dr. Barouch said.
“If people have vaccine immunity or natural immunity, then they have substantial protection against severe disease,” he said.
So far, researchers have found that Omicron subvariants tend to cause less severe disease than other variants, such as Delta. Dr. Mokdad estimated that 80% of Omicron infections don’t show symptoms.
He said there is a “remote possibility” of another wave during the summer, but he expects cases to rise significantly around the beginning of October, when the seasons change, and most people’s immunity will wane. Other things could play into the predictions this summer, he noted, such as coronavirus mutations and new variants.
“Anybody that models this more than a couple of weeks out is basically just using pixie dust,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told the newspaper.
“There is no pattern whatsoever developing from a seasonality standpoint. It’s all being driven by the variants,” he said. “We just have to be humble and acknowledge that we don’t know.”
A version of this article first appeared on WebMD.com.
As the coronavirus continues to evolve, Omicron subvariants such as BA.4 and BA.5 are expected to lead to many COVID-19 cases in the coming months.
Researchers recently reported that the subvariants have mutated for better “immune escape,” or the ability to avoid antibodies from vaccination or previous infection.
“That has changed our view for what will happen this summer,” Ali Mokdad, PhD, an epidemiologist who has developed COVID-19 forecasts for the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, told The Boston Globe.
Until recently, Dr. Mokdad expected the United States to have a “very good summer” in terms of cases, hospitalizations, and deaths through September. The U.S. is reporting about 100,000 new cases per day, according to the data tracker by The New York Times, which has remained flat throughout June. Cases will likely decrease this summer, Dr. Mokdad said, though the decline will be slower and smaller than first thought.
As of June 18, BA.4 and BA.5 accounted for about 35% of cases in the United States, according to the latest CDC data, with BA.5 making up 23.5% and BA.4 making up 11.4%. The two subvariants will likely take over BA.2.12.1 as top subvariants in coming weeks.
“I expect that BA.5 will likely become the dominant virus in the United States this summer,” Dan Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center in Boston, told the Globe.
Dr. Barouch said the Omicron subvariants will likely create a summer of “substantial infections” but low rates of hospitalization and death. He published a recent study in the New England Journal of Medicine that found BA.4 and BA.5 are better at escaping antibodies than other coronavirus strains – about three times better than the Omicron variants BA.1 and BA.2 and 20 times better than the first coronavirus strain.
“What we’re seeing with each subsequent variant is iteratively higher levels of transmissibility and higher levels of antibody immune escape,” he said. “We’re seeing high levels of infection in populations that are highly vaccinated, as well as populations that have a high level of natural immunity to the prior variants.”
At the same time, current antibodies still appear to protect people against the worst outcomes, Dr. Barouch said.
“If people have vaccine immunity or natural immunity, then they have substantial protection against severe disease,” he said.
So far, researchers have found that Omicron subvariants tend to cause less severe disease than other variants, such as Delta. Dr. Mokdad estimated that 80% of Omicron infections don’t show symptoms.
He said there is a “remote possibility” of another wave during the summer, but he expects cases to rise significantly around the beginning of October, when the seasons change, and most people’s immunity will wane. Other things could play into the predictions this summer, he noted, such as coronavirus mutations and new variants.
“Anybody that models this more than a couple of weeks out is basically just using pixie dust,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, Minneapolis, told the newspaper.
“There is no pattern whatsoever developing from a seasonality standpoint. It’s all being driven by the variants,” he said. “We just have to be humble and acknowledge that we don’t know.”
A version of this article first appeared on WebMD.com.
Study finds higher risk of skin cancer after childhood organ transplant
A large study showing an increased risk of keratinocyte carcinoma (KC) in children who receive a solid-organ transplant highlights the need for early education about risk reduction and more research to determine optimal timing for screening, say an investigator and two dermatologists with expertise in transplant-related skin issues.
The increased incidence of KC in pediatric transplant recipients is “really high, so we definitely know there’s risk there,” just as there is for adult recipients of solid-organ transplants, said Cathryn Sibbald, MD, MSc, a dermatologist at the Hospital for Sick Children in Toronto and coauthor of a research letter published in June in JAMA Dermatology.
For their study, Dr. Sibbald and her coinvestigators turned to the Ontario Health Insurance plan database, which covers health care for Canadian citizens and qualified residents in the province. They identified 951 patients younger than the age of 18 who received a solid-organ transplant between 1991 and 2004 at an Ontario hospital
They then used a validated health insurance claims–based algorithm to identify diagnoses of KC for the transplant recipients and for more than 5 million age-matched controls. KC, including squamous and basal cell carcinoma, is the most prevalent skin cancer for people who have had a solid-organ transplant.
Fifteen posttransplant KCs (10 patients, 1.1%) were reported a mean of 13.1 years after transplant, with none reported in the first 4 years. The mean age at transplant was 7.8 years, and the mean age at KC diagnosis was 25.2 years. Kidney transplants were the most common (42.1% of transplantations). Most of the transplants recipients (eight patients) who developed KC had kidney transplantation, and most of them had functional graft at the time of KC diagnosis.
Researchers found an increased incidence of KC compared with that of the general population (standardized incidence ratio, 9.09; 95% confidence interval, 5.48-15.08). And the risk for KC increased with time since transplant, with adjusted hazard ratios for KC of 3.63 (95% CI, 0.51-25.77) for 1-5 years, 5.14 (95% CI, 1.28-20.55) for 5-10 years, and 4.80 (95% CI, 2.29-10.08) for 10 years or more, compared with the control population.
Several years ago, another research team performed a similar population-based cohort study of adult transplant recipients in Ontario and found a 6.6-times increased risk of KC in transplant recipients compared with the general population.
Sun protection and skin cancer screening
In commenting on the study, Sarah Arron, MD, PhD, a San Francisco Bay area dermatologist and immediate past president of the International Immunosuppression and Transplant Skin Cancer Collaborative (www.itscc.org), said she feels “reassured” that young transplant patients tend not to develop the skin cancer until young adulthood.
A ”large study like this is important because the overall rate of KC is low in this age group,” she noted.
The findings “suggest that we can focus our efforts on prevention during childhood, with sun protection and skin cancer education,” she said. “Then, as these children move into adulthood, we can begin screening with skin examinations. Of course, [any child] with a skin lesion or mole that concerns their parents or transplant team should be referred to dermatology for evaluation.”
Pediatric transplant recipients and their parents are most interested in learning about skin cancer prevention either before or immediately after transplantation, according to a survey by other researchers.
Intervention studies needed
The increased risk of KC probably stems largely from immunosuppression, said Dr. Sibbald in an interview. “We know [this is the case] in the older population, and it’s likely true in the younger population as well that it’s one of the primary drivers,” she said.
More research to extensively analyze risk factors should come next, she said. This includes “the granularity of what [immunosuppressants and other] medications are received, and at what dose and for what periods of time, so we can calculate cumulative exposure and its relation to risk,” she said.
Kristin Bibee, MD, PhD, assistant professor of dermatology at Johns Hopkins University in Baltimore, said she’d like to see further studies “evaluate appropriate interventions, like sun-protective behavior in childhood and adolescence or immunosuppression modulation, to prevent malignancy development.”
The optimal time and intensity of screening for young transplant recipients must still be determined, both Dr. Bibee and Dr. Arron said. Patients deemed through further research to be at higher risk may need earlier and/or more intensive surveillance.
The role of race in skin cancer risk in this population is “one question the study leaves open,” said Dr. Arron. U.S. studies have shown that among adult transplant recipients White patients are “at highest risk for the ultraviolet-associated melanoma and squamous cell carcinoma, followed by Asian and Latino patients. African Americans have had the lowest risk, but some still developed skin cancer after transplant,” she said.
Prior studies of cancer in pediatric transplant recipients have reported primarily on internal malignant neoplasms, with limited data on KC, Dr. Sibbald and coauthors wrote. It is possible the incidence of KS is underestimated in the new study because of “undiagnosed or unreported KCs,” they noted.
The new study was funded by a grant from the Pediatric Dermatology Research Alliance and a Hospital for Sick Children grant. In disclosures, Dr. Sibbald reported to JAMA Dermatology receiving grants from the alliance and from Paediatric Consultants Partnership during the conduct of the study. Dr. Arron and Dr. Bibee both said they have no disclosures relevant to the study and its content.
A large study showing an increased risk of keratinocyte carcinoma (KC) in children who receive a solid-organ transplant highlights the need for early education about risk reduction and more research to determine optimal timing for screening, say an investigator and two dermatologists with expertise in transplant-related skin issues.
The increased incidence of KC in pediatric transplant recipients is “really high, so we definitely know there’s risk there,” just as there is for adult recipients of solid-organ transplants, said Cathryn Sibbald, MD, MSc, a dermatologist at the Hospital for Sick Children in Toronto and coauthor of a research letter published in June in JAMA Dermatology.
For their study, Dr. Sibbald and her coinvestigators turned to the Ontario Health Insurance plan database, which covers health care for Canadian citizens and qualified residents in the province. They identified 951 patients younger than the age of 18 who received a solid-organ transplant between 1991 and 2004 at an Ontario hospital
They then used a validated health insurance claims–based algorithm to identify diagnoses of KC for the transplant recipients and for more than 5 million age-matched controls. KC, including squamous and basal cell carcinoma, is the most prevalent skin cancer for people who have had a solid-organ transplant.
Fifteen posttransplant KCs (10 patients, 1.1%) were reported a mean of 13.1 years after transplant, with none reported in the first 4 years. The mean age at transplant was 7.8 years, and the mean age at KC diagnosis was 25.2 years. Kidney transplants were the most common (42.1% of transplantations). Most of the transplants recipients (eight patients) who developed KC had kidney transplantation, and most of them had functional graft at the time of KC diagnosis.
Researchers found an increased incidence of KC compared with that of the general population (standardized incidence ratio, 9.09; 95% confidence interval, 5.48-15.08). And the risk for KC increased with time since transplant, with adjusted hazard ratios for KC of 3.63 (95% CI, 0.51-25.77) for 1-5 years, 5.14 (95% CI, 1.28-20.55) for 5-10 years, and 4.80 (95% CI, 2.29-10.08) for 10 years or more, compared with the control population.
Several years ago, another research team performed a similar population-based cohort study of adult transplant recipients in Ontario and found a 6.6-times increased risk of KC in transplant recipients compared with the general population.
Sun protection and skin cancer screening
In commenting on the study, Sarah Arron, MD, PhD, a San Francisco Bay area dermatologist and immediate past president of the International Immunosuppression and Transplant Skin Cancer Collaborative (www.itscc.org), said she feels “reassured” that young transplant patients tend not to develop the skin cancer until young adulthood.
A ”large study like this is important because the overall rate of KC is low in this age group,” she noted.
The findings “suggest that we can focus our efforts on prevention during childhood, with sun protection and skin cancer education,” she said. “Then, as these children move into adulthood, we can begin screening with skin examinations. Of course, [any child] with a skin lesion or mole that concerns their parents or transplant team should be referred to dermatology for evaluation.”
Pediatric transplant recipients and their parents are most interested in learning about skin cancer prevention either before or immediately after transplantation, according to a survey by other researchers.
Intervention studies needed
The increased risk of KC probably stems largely from immunosuppression, said Dr. Sibbald in an interview. “We know [this is the case] in the older population, and it’s likely true in the younger population as well that it’s one of the primary drivers,” she said.
More research to extensively analyze risk factors should come next, she said. This includes “the granularity of what [immunosuppressants and other] medications are received, and at what dose and for what periods of time, so we can calculate cumulative exposure and its relation to risk,” she said.
Kristin Bibee, MD, PhD, assistant professor of dermatology at Johns Hopkins University in Baltimore, said she’d like to see further studies “evaluate appropriate interventions, like sun-protective behavior in childhood and adolescence or immunosuppression modulation, to prevent malignancy development.”
The optimal time and intensity of screening for young transplant recipients must still be determined, both Dr. Bibee and Dr. Arron said. Patients deemed through further research to be at higher risk may need earlier and/or more intensive surveillance.
The role of race in skin cancer risk in this population is “one question the study leaves open,” said Dr. Arron. U.S. studies have shown that among adult transplant recipients White patients are “at highest risk for the ultraviolet-associated melanoma and squamous cell carcinoma, followed by Asian and Latino patients. African Americans have had the lowest risk, but some still developed skin cancer after transplant,” she said.
Prior studies of cancer in pediatric transplant recipients have reported primarily on internal malignant neoplasms, with limited data on KC, Dr. Sibbald and coauthors wrote. It is possible the incidence of KS is underestimated in the new study because of “undiagnosed or unreported KCs,” they noted.
The new study was funded by a grant from the Pediatric Dermatology Research Alliance and a Hospital for Sick Children grant. In disclosures, Dr. Sibbald reported to JAMA Dermatology receiving grants from the alliance and from Paediatric Consultants Partnership during the conduct of the study. Dr. Arron and Dr. Bibee both said they have no disclosures relevant to the study and its content.
A large study showing an increased risk of keratinocyte carcinoma (KC) in children who receive a solid-organ transplant highlights the need for early education about risk reduction and more research to determine optimal timing for screening, say an investigator and two dermatologists with expertise in transplant-related skin issues.
The increased incidence of KC in pediatric transplant recipients is “really high, so we definitely know there’s risk there,” just as there is for adult recipients of solid-organ transplants, said Cathryn Sibbald, MD, MSc, a dermatologist at the Hospital for Sick Children in Toronto and coauthor of a research letter published in June in JAMA Dermatology.
For their study, Dr. Sibbald and her coinvestigators turned to the Ontario Health Insurance plan database, which covers health care for Canadian citizens and qualified residents in the province. They identified 951 patients younger than the age of 18 who received a solid-organ transplant between 1991 and 2004 at an Ontario hospital
They then used a validated health insurance claims–based algorithm to identify diagnoses of KC for the transplant recipients and for more than 5 million age-matched controls. KC, including squamous and basal cell carcinoma, is the most prevalent skin cancer for people who have had a solid-organ transplant.
Fifteen posttransplant KCs (10 patients, 1.1%) were reported a mean of 13.1 years after transplant, with none reported in the first 4 years. The mean age at transplant was 7.8 years, and the mean age at KC diagnosis was 25.2 years. Kidney transplants were the most common (42.1% of transplantations). Most of the transplants recipients (eight patients) who developed KC had kidney transplantation, and most of them had functional graft at the time of KC diagnosis.
Researchers found an increased incidence of KC compared with that of the general population (standardized incidence ratio, 9.09; 95% confidence interval, 5.48-15.08). And the risk for KC increased with time since transplant, with adjusted hazard ratios for KC of 3.63 (95% CI, 0.51-25.77) for 1-5 years, 5.14 (95% CI, 1.28-20.55) for 5-10 years, and 4.80 (95% CI, 2.29-10.08) for 10 years or more, compared with the control population.
Several years ago, another research team performed a similar population-based cohort study of adult transplant recipients in Ontario and found a 6.6-times increased risk of KC in transplant recipients compared with the general population.
Sun protection and skin cancer screening
In commenting on the study, Sarah Arron, MD, PhD, a San Francisco Bay area dermatologist and immediate past president of the International Immunosuppression and Transplant Skin Cancer Collaborative (www.itscc.org), said she feels “reassured” that young transplant patients tend not to develop the skin cancer until young adulthood.
A ”large study like this is important because the overall rate of KC is low in this age group,” she noted.
The findings “suggest that we can focus our efforts on prevention during childhood, with sun protection and skin cancer education,” she said. “Then, as these children move into adulthood, we can begin screening with skin examinations. Of course, [any child] with a skin lesion or mole that concerns their parents or transplant team should be referred to dermatology for evaluation.”
Pediatric transplant recipients and their parents are most interested in learning about skin cancer prevention either before or immediately after transplantation, according to a survey by other researchers.
Intervention studies needed
The increased risk of KC probably stems largely from immunosuppression, said Dr. Sibbald in an interview. “We know [this is the case] in the older population, and it’s likely true in the younger population as well that it’s one of the primary drivers,” she said.
More research to extensively analyze risk factors should come next, she said. This includes “the granularity of what [immunosuppressants and other] medications are received, and at what dose and for what periods of time, so we can calculate cumulative exposure and its relation to risk,” she said.
Kristin Bibee, MD, PhD, assistant professor of dermatology at Johns Hopkins University in Baltimore, said she’d like to see further studies “evaluate appropriate interventions, like sun-protective behavior in childhood and adolescence or immunosuppression modulation, to prevent malignancy development.”
The optimal time and intensity of screening for young transplant recipients must still be determined, both Dr. Bibee and Dr. Arron said. Patients deemed through further research to be at higher risk may need earlier and/or more intensive surveillance.
The role of race in skin cancer risk in this population is “one question the study leaves open,” said Dr. Arron. U.S. studies have shown that among adult transplant recipients White patients are “at highest risk for the ultraviolet-associated melanoma and squamous cell carcinoma, followed by Asian and Latino patients. African Americans have had the lowest risk, but some still developed skin cancer after transplant,” she said.
Prior studies of cancer in pediatric transplant recipients have reported primarily on internal malignant neoplasms, with limited data on KC, Dr. Sibbald and coauthors wrote. It is possible the incidence of KS is underestimated in the new study because of “undiagnosed or unreported KCs,” they noted.
The new study was funded by a grant from the Pediatric Dermatology Research Alliance and a Hospital for Sick Children grant. In disclosures, Dr. Sibbald reported to JAMA Dermatology receiving grants from the alliance and from Paediatric Consultants Partnership during the conduct of the study. Dr. Arron and Dr. Bibee both said they have no disclosures relevant to the study and its content.
FROM JAMA DERMATOLOGY
Children and COVID: Vaccination off to slow start for the newly eligible
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.
New cases of COVID-19 continue to drop among children, but the vaccination effort in those under age 5 years began with something less than a bang.
In the first 2 days after their respective approvals, almost 99,000 children aged 5-11 years and over 675,000 children aged 12-15 were vaccinated, according to data from the Centers for Disease Control and Prevention. Children aged 0-4 years represent almost 6% of the overall population, compared with 8.7% for the 5- to 11-year-olds and 5.1% for those aged 12-15.
The recent decline in new cases over the past 4 weeks and the substantial decline since the Omicron surge could be a factor in the lack of response, but it is worth noting that the almost 68,000 new child cases reported in the past week, June 17-23, are “far higher than 1 year ago, June 24, 2021, when 8,400 child cases were reported,” the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
That total for June 17-23 was 19% lower than the previous week and down by 40% since new cases hit a spring peak of 112,000 in late May. Regionally, new cases were down in the Midwest, the South, and the West, the AAP/CHA report showed, but the Northeast saw a small increase, which could be a signal of things to come for the summer.
The decline in new cases, however, has not been accompanied by decreases in hospitalizations or emergency department visits. New admissions of children aged 0-17 with confirmed COVID were at 0.31 per 100,000 population on June 24 after reaching that level on June 15, so no drop-off has occurred yet but there are signs of leveling off, based on CDC data.
The ED visit rates have been fairly steady through June, although COVID-related visits were up to 3.4% of all ED visits on June 22 for children aged 0-11 years, after being below 3% for the first 2 weeks of the month. The rate for children aged 12-15 has been between 1.6% and 1.9% for the past 3 weeks and the rate for 16- and 17-year-olds has been hovering between 1.7% and 2.2% for most of June, after going as high as 2.7% in late May, the CDC said on its COVID Data Tracker.
FDA approves Qsymia for treating teens with obesity
The indication is for use as additional therapy along with a reduced-calorie diet and increased physical activity in youth with obesity, defined as a body mass index of the 95th percentile or greater when standardized for age and sex.
Qsymia was first approved in July 2012 for chronic weight management in adults with an initial BMI of 30 kg/m2 or greater (obese) or 27 kg/m2 or greater (overweight) with one or more weight-related comorbidities, as an adjunct to lifestyle modification.
About 1 in 5 adolescents in the United States has obesity, according to the FDA.
The drug is the fourth to be approved for treating obesity in youth, along with liraglutide (Saxenda) and orlistat (Alli, Xenical), both approved down to age 12, and phentermine for those aged 16 and older.
The Qsymia approval was based on data from a phase 4 double-blind, placebo-controlled trial of 223 youth aged 12-16 with obesity who had not lost weight with lifestyle modifications. They were randomly assigned to Qsymia in doses of 7.5 mg phentermine/46 mg topiramate, 15 mg phentermine/92 mg topiramate, or placebo once daily, along with lifestyle counseling for all.
At 56 weeks, those taking the lower Qsymia dose lost an average of 4.8% of their BMI, and those on the higher dose lost 7.1%. In contrast, the placebo group gained about 3.3% of their BMI.
Because Qsymia increases the risk for oral clefts (lip and palate) in a fetus if taken during pregnancy, female patients should obtain negative pregnancy tests before starting the drug, take monthly pregnancy tests while on the drug, and use effective contraception throughout. Also because of the oral cleft risk, Qsymia is available only through an FDA program called a Risk Evaluation and Mitigation Strategy.
Additional potential adverse effects with Qsymia include increased heart rate and suicidal behavior/ideation. Patients should be advised to monitor for mood changes and discontinue the drug if depression or suicidal thoughts develop. The drug has also been linked to slowing of linear growth, so growth should be monitored in adolescents taking the drug, according to the FDA.
Qsymia is also associated with acute myopia, secondary angle closure glaucoma, visual problems, sleep disorders, cognitive impairment, metabolic acidosis, and decreased renal function.
The most common adverse reactions reported in the pediatric clinical trial included depression, dizziness, joint pain, fever, flu, and ankle sprain.
A version of this article first appeared on Medscape.com.
The indication is for use as additional therapy along with a reduced-calorie diet and increased physical activity in youth with obesity, defined as a body mass index of the 95th percentile or greater when standardized for age and sex.
Qsymia was first approved in July 2012 for chronic weight management in adults with an initial BMI of 30 kg/m2 or greater (obese) or 27 kg/m2 or greater (overweight) with one or more weight-related comorbidities, as an adjunct to lifestyle modification.
About 1 in 5 adolescents in the United States has obesity, according to the FDA.
The drug is the fourth to be approved for treating obesity in youth, along with liraglutide (Saxenda) and orlistat (Alli, Xenical), both approved down to age 12, and phentermine for those aged 16 and older.
The Qsymia approval was based on data from a phase 4 double-blind, placebo-controlled trial of 223 youth aged 12-16 with obesity who had not lost weight with lifestyle modifications. They were randomly assigned to Qsymia in doses of 7.5 mg phentermine/46 mg topiramate, 15 mg phentermine/92 mg topiramate, or placebo once daily, along with lifestyle counseling for all.
At 56 weeks, those taking the lower Qsymia dose lost an average of 4.8% of their BMI, and those on the higher dose lost 7.1%. In contrast, the placebo group gained about 3.3% of their BMI.
Because Qsymia increases the risk for oral clefts (lip and palate) in a fetus if taken during pregnancy, female patients should obtain negative pregnancy tests before starting the drug, take monthly pregnancy tests while on the drug, and use effective contraception throughout. Also because of the oral cleft risk, Qsymia is available only through an FDA program called a Risk Evaluation and Mitigation Strategy.
Additional potential adverse effects with Qsymia include increased heart rate and suicidal behavior/ideation. Patients should be advised to monitor for mood changes and discontinue the drug if depression or suicidal thoughts develop. The drug has also been linked to slowing of linear growth, so growth should be monitored in adolescents taking the drug, according to the FDA.
Qsymia is also associated with acute myopia, secondary angle closure glaucoma, visual problems, sleep disorders, cognitive impairment, metabolic acidosis, and decreased renal function.
The most common adverse reactions reported in the pediatric clinical trial included depression, dizziness, joint pain, fever, flu, and ankle sprain.
A version of this article first appeared on Medscape.com.
The indication is for use as additional therapy along with a reduced-calorie diet and increased physical activity in youth with obesity, defined as a body mass index of the 95th percentile or greater when standardized for age and sex.
Qsymia was first approved in July 2012 for chronic weight management in adults with an initial BMI of 30 kg/m2 or greater (obese) or 27 kg/m2 or greater (overweight) with one or more weight-related comorbidities, as an adjunct to lifestyle modification.
About 1 in 5 adolescents in the United States has obesity, according to the FDA.
The drug is the fourth to be approved for treating obesity in youth, along with liraglutide (Saxenda) and orlistat (Alli, Xenical), both approved down to age 12, and phentermine for those aged 16 and older.
The Qsymia approval was based on data from a phase 4 double-blind, placebo-controlled trial of 223 youth aged 12-16 with obesity who had not lost weight with lifestyle modifications. They were randomly assigned to Qsymia in doses of 7.5 mg phentermine/46 mg topiramate, 15 mg phentermine/92 mg topiramate, or placebo once daily, along with lifestyle counseling for all.
At 56 weeks, those taking the lower Qsymia dose lost an average of 4.8% of their BMI, and those on the higher dose lost 7.1%. In contrast, the placebo group gained about 3.3% of their BMI.
Because Qsymia increases the risk for oral clefts (lip and palate) in a fetus if taken during pregnancy, female patients should obtain negative pregnancy tests before starting the drug, take monthly pregnancy tests while on the drug, and use effective contraception throughout. Also because of the oral cleft risk, Qsymia is available only through an FDA program called a Risk Evaluation and Mitigation Strategy.
Additional potential adverse effects with Qsymia include increased heart rate and suicidal behavior/ideation. Patients should be advised to monitor for mood changes and discontinue the drug if depression or suicidal thoughts develop. The drug has also been linked to slowing of linear growth, so growth should be monitored in adolescents taking the drug, according to the FDA.
Qsymia is also associated with acute myopia, secondary angle closure glaucoma, visual problems, sleep disorders, cognitive impairment, metabolic acidosis, and decreased renal function.
The most common adverse reactions reported in the pediatric clinical trial included depression, dizziness, joint pain, fever, flu, and ankle sprain.
A version of this article first appeared on Medscape.com.