User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
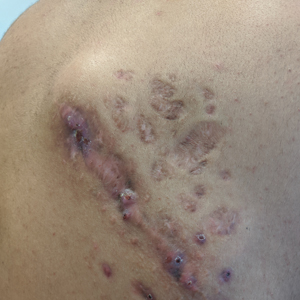
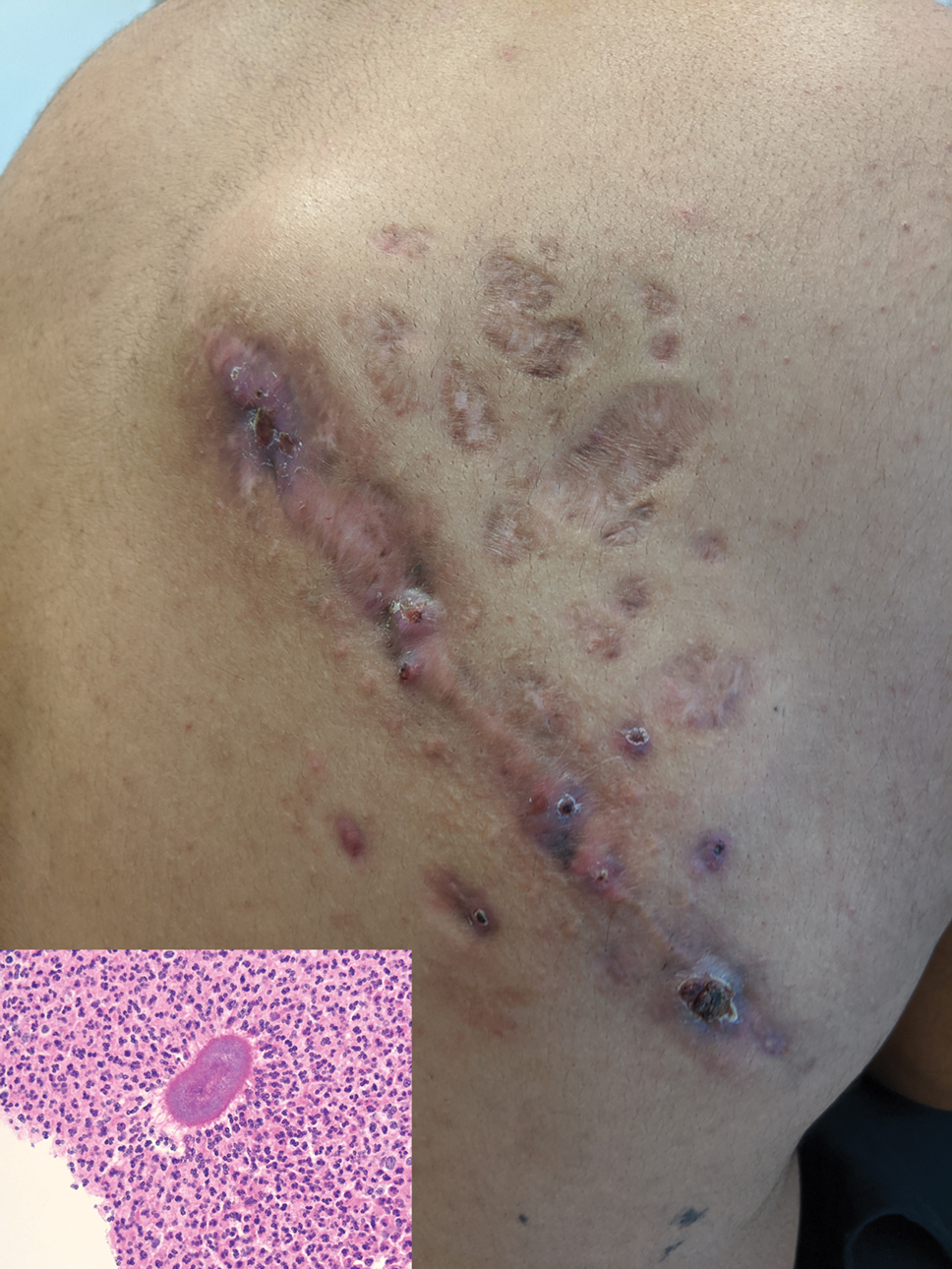
Indurated Mass on the Right Central Back
The Diagnosis: Actinomycetoma
Histopathology revealed evidence of an actinomycete organism within the suppuration, consistent with actinomycosis (quiz image [inset]). Given the clinical presentation and histopathologic findings, our patient was diagnosed with actinomycetoma.
Actinomycetoma is an indolent, progressive, subcutaneous infection characterized by a well-known clinical triad of tumefaction/subcutaneous mass, draining sinuses, and an exudate containing grains on microscopy. The sinus tracts are formed from the chronic infectious process that destroys tissue, creating tunnels. This infectious disease of soft tissue is a clinical subset of mycetoma, which is categorized as eumycetoma (fungal) and actinomycetoma (bacterial). Actinomycetoma resembles the behavior of insidious and chronic fungal infections; however, most mycetoma infections are bacterial.1,2 Actinomycetoma may be confused with actinomycosis, which is caused by Actinomycoses species, commensal organisms commonly located on the teeth and oral mucosa in association with other microorganisms that may pathogenically cause cervicofacial actinomycosis.3,4 Actinomycetoma can be caused by Nocardia, Streptomyces, and Actinomadura. 2,5 The foot is the most common location of involvement followed by the thoracic region. It is more common in tropical or equatorial locations and may be contracted through exposure to soil or wood.5 Mycetoma is considered a neglected tropical disease by the World Health Organization.1 In tropical countries, this disease may go undiagnosed or untreated for so long that surgical amputation may be the only effective treatment.
Actinomycetoma commonly is identifiable by direct microscopy, Gram stain, or bacterial culture, with Gram stain being more sensitive than bacterial culture.3 It is important to indicate the suspected organism to the microbiology laboratory because common bacterial pathogens are detected within 24 to 48 hours, but the causative microorganism in actinomycetoma may require up to 4 weeks for culture,2 leading to possible false negatives due to inadequate culture time.3 Histopathology of actinomycotic infections will demonstrate granulomatous inflammation, focal suppuration, and the presence of grains (ie, a colony of filamentous bacteria in a stellate shaped mass)(quiz image [inset]).
The gold standard of treatment is trimethoprim-sulfamethoxazole for up to several years.4,5 Amoxicillin–clavulanic acid, dapsone, amikacin, streptomycin, and beta-lactams have been used successfully.2,5 The treatment course is dependent on clinical severity and location of the disease. The cure rate with appropriate antibiotics can be as high as 90%,2,5 and thus surgical intervention can be avoided.
In the differential, cutaneous tuberculosis would show tuberculoid granulomas with epithelioid histiocytes with possible caseation on histopathology, typically alongside positive tuberculosis screening. Botryomycosis has a similar clinical presentation of a swollen or indurated lesion with draining sinus tracts, but it less commonly occurs on the trunk. Histopathology also is a close mimic of actinomycetoma with a small grain inside a suppurative infiltrate; however, it has no filamentous bacteria. A foreign body reaction would not histologically present with suppuration or grains, and draining sinuses typically would not be seen on clinical presentation. Sarcoma is a neoplastic process and most commonly would show a proliferation of cells with soft tissue or bone origin on histopathology and not primarily an inflammatory cell process.6
- Verma P, Jha A. Mycetoma: reviewing a neglected disease. Clin Exp Dermatol. 2019;44:123-129.
- Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197.
- Bennhoff DF. Actinomycosis: diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope. 1984;94:1198-1217.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Weedon D. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010.
The Diagnosis: Actinomycetoma
Histopathology revealed evidence of an actinomycete organism within the suppuration, consistent with actinomycosis (quiz image [inset]). Given the clinical presentation and histopathologic findings, our patient was diagnosed with actinomycetoma.
Actinomycetoma is an indolent, progressive, subcutaneous infection characterized by a well-known clinical triad of tumefaction/subcutaneous mass, draining sinuses, and an exudate containing grains on microscopy. The sinus tracts are formed from the chronic infectious process that destroys tissue, creating tunnels. This infectious disease of soft tissue is a clinical subset of mycetoma, which is categorized as eumycetoma (fungal) and actinomycetoma (bacterial). Actinomycetoma resembles the behavior of insidious and chronic fungal infections; however, most mycetoma infections are bacterial.1,2 Actinomycetoma may be confused with actinomycosis, which is caused by Actinomycoses species, commensal organisms commonly located on the teeth and oral mucosa in association with other microorganisms that may pathogenically cause cervicofacial actinomycosis.3,4 Actinomycetoma can be caused by Nocardia, Streptomyces, and Actinomadura. 2,5 The foot is the most common location of involvement followed by the thoracic region. It is more common in tropical or equatorial locations and may be contracted through exposure to soil or wood.5 Mycetoma is considered a neglected tropical disease by the World Health Organization.1 In tropical countries, this disease may go undiagnosed or untreated for so long that surgical amputation may be the only effective treatment.
Actinomycetoma commonly is identifiable by direct microscopy, Gram stain, or bacterial culture, with Gram stain being more sensitive than bacterial culture.3 It is important to indicate the suspected organism to the microbiology laboratory because common bacterial pathogens are detected within 24 to 48 hours, but the causative microorganism in actinomycetoma may require up to 4 weeks for culture,2 leading to possible false negatives due to inadequate culture time.3 Histopathology of actinomycotic infections will demonstrate granulomatous inflammation, focal suppuration, and the presence of grains (ie, a colony of filamentous bacteria in a stellate shaped mass)(quiz image [inset]).
The gold standard of treatment is trimethoprim-sulfamethoxazole for up to several years.4,5 Amoxicillin–clavulanic acid, dapsone, amikacin, streptomycin, and beta-lactams have been used successfully.2,5 The treatment course is dependent on clinical severity and location of the disease. The cure rate with appropriate antibiotics can be as high as 90%,2,5 and thus surgical intervention can be avoided.
In the differential, cutaneous tuberculosis would show tuberculoid granulomas with epithelioid histiocytes with possible caseation on histopathology, typically alongside positive tuberculosis screening. Botryomycosis has a similar clinical presentation of a swollen or indurated lesion with draining sinus tracts, but it less commonly occurs on the trunk. Histopathology also is a close mimic of actinomycetoma with a small grain inside a suppurative infiltrate; however, it has no filamentous bacteria. A foreign body reaction would not histologically present with suppuration or grains, and draining sinuses typically would not be seen on clinical presentation. Sarcoma is a neoplastic process and most commonly would show a proliferation of cells with soft tissue or bone origin on histopathology and not primarily an inflammatory cell process.6
The Diagnosis: Actinomycetoma
Histopathology revealed evidence of an actinomycete organism within the suppuration, consistent with actinomycosis (quiz image [inset]). Given the clinical presentation and histopathologic findings, our patient was diagnosed with actinomycetoma.
Actinomycetoma is an indolent, progressive, subcutaneous infection characterized by a well-known clinical triad of tumefaction/subcutaneous mass, draining sinuses, and an exudate containing grains on microscopy. The sinus tracts are formed from the chronic infectious process that destroys tissue, creating tunnels. This infectious disease of soft tissue is a clinical subset of mycetoma, which is categorized as eumycetoma (fungal) and actinomycetoma (bacterial). Actinomycetoma resembles the behavior of insidious and chronic fungal infections; however, most mycetoma infections are bacterial.1,2 Actinomycetoma may be confused with actinomycosis, which is caused by Actinomycoses species, commensal organisms commonly located on the teeth and oral mucosa in association with other microorganisms that may pathogenically cause cervicofacial actinomycosis.3,4 Actinomycetoma can be caused by Nocardia, Streptomyces, and Actinomadura. 2,5 The foot is the most common location of involvement followed by the thoracic region. It is more common in tropical or equatorial locations and may be contracted through exposure to soil or wood.5 Mycetoma is considered a neglected tropical disease by the World Health Organization.1 In tropical countries, this disease may go undiagnosed or untreated for so long that surgical amputation may be the only effective treatment.
Actinomycetoma commonly is identifiable by direct microscopy, Gram stain, or bacterial culture, with Gram stain being more sensitive than bacterial culture.3 It is important to indicate the suspected organism to the microbiology laboratory because common bacterial pathogens are detected within 24 to 48 hours, but the causative microorganism in actinomycetoma may require up to 4 weeks for culture,2 leading to possible false negatives due to inadequate culture time.3 Histopathology of actinomycotic infections will demonstrate granulomatous inflammation, focal suppuration, and the presence of grains (ie, a colony of filamentous bacteria in a stellate shaped mass)(quiz image [inset]).
The gold standard of treatment is trimethoprim-sulfamethoxazole for up to several years.4,5 Amoxicillin–clavulanic acid, dapsone, amikacin, streptomycin, and beta-lactams have been used successfully.2,5 The treatment course is dependent on clinical severity and location of the disease. The cure rate with appropriate antibiotics can be as high as 90%,2,5 and thus surgical intervention can be avoided.
In the differential, cutaneous tuberculosis would show tuberculoid granulomas with epithelioid histiocytes with possible caseation on histopathology, typically alongside positive tuberculosis screening. Botryomycosis has a similar clinical presentation of a swollen or indurated lesion with draining sinus tracts, but it less commonly occurs on the trunk. Histopathology also is a close mimic of actinomycetoma with a small grain inside a suppurative infiltrate; however, it has no filamentous bacteria. A foreign body reaction would not histologically present with suppuration or grains, and draining sinuses typically would not be seen on clinical presentation. Sarcoma is a neoplastic process and most commonly would show a proliferation of cells with soft tissue or bone origin on histopathology and not primarily an inflammatory cell process.6
- Verma P, Jha A. Mycetoma: reviewing a neglected disease. Clin Exp Dermatol. 2019;44:123-129.
- Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197.
- Bennhoff DF. Actinomycosis: diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope. 1984;94:1198-1217.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Weedon D. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010.
- Verma P, Jha A. Mycetoma: reviewing a neglected disease. Clin Exp Dermatol. 2019;44:123-129.
- Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197.
- Bennhoff DF. Actinomycosis: diagnostic and therapeutic considerations and a review of 32 cases. Laryngoscope. 1984;94:1198-1217.
- Welsh O, Vera-Cabrera L, Welsh E, et al. Actinomycetoma and advances in its treatment. Clin Dermatol. 2012;30:372-381.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Weedon D. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone Elsevier; 2010.
A 26-year-old Guatemalan man who was a former carpenter presented with an indurated, nontender, nonpruritic, subcutaneous mass on the right central back with multiple draining sinus tracts on the surface and several depressed circular atrophic scars on the periphery of the mass. He noticed that the lesion began as a pustule 1.5 years prior and gradually enlarged. He denied any trauma, insect bites, fever, chills, headaches, weight loss, or travel history (he relocated to the United States 3.5 years ago) prior to the skin eruption. A biopsy was performed by an outside dermatologist 1 year prior to the current presentation, with a diagnosis of Pityrosporum folliculitis. Throughout his clinical course, treatment with oral antifungals, oral doxycycline, and topical clindamycin all failed. The mass was removed by plastic surgery 1 year prior.
A tissue biopsy for histology and culture was obtained at presentation to our institution. Laboratory findings showed that the basic metabolic panel was within reference range. Chest radiography indicated no active disease. A tuberculosis screening was negative. A bacterial culture of the lesion identified no growth after 48 hours. Our tissue biopsy revealed fibrosing granulation tissue, but the surgical pathology from a prior mass excision revealed sinus tracts with suppuration, evidence of scarring, foreign body giant cell reaction, and a characteristic finding (inset: H&E, original magnification ×200).
Serious problems rare in ages 5-11 from COVID vaccine
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
The CDC has released two studies that showed vaccine safety for ages 5-11 and emphasized the importance of vaccinating children against the coronavirus to prevent serious illness and hospitalization.
In one study published in the Morbidity and Mortality Weekly Report, researchers found that serious problems were rare among children who had received the Pfizer vaccine.
In another study, researchers looked at hundreds of pediatric hospitalizations from the summer and found that nearly all of children who developed severe COVID-19 weren’t fully vaccinated.
“This study demonstrates that unvaccinated children hospitalized for COVID-19 could experience severe disease and reinforces the importance of vaccination of all eligible children to provide individual protection and to protect those who are not yet eligible to be vaccinated,” the authors of the second study wrote.
Nearly 9 million doses of the Pfizer vaccine have been given to children aged 5-11 in the United States so far, according to The New York Times. By mid-December, or about 6 weeks after the age group became eligible for vaccination in October, the CDC said it had received very few reports of serious problems.
CDC researchers evaluated reports received from doctors and the public, including survey responses from parents and guardians of about 43,000 children between ages 5 and 11. Many children reported nonserious events such as pain at the injection site, fatigue, or a headache, especially after the second dose.
Among more than 4,100 adverse event reports received in November and December, 100 were for serious events, with the most common being fever or vomiting.
The CDC had received 11 verified reports of myocarditis, or inflammation of the heart muscle, which has been noted as a rare side effect of the vaccine among boys and men between ages 12 and 29. Among those, seven children had already recovered and four were still recovering at the time of the report.
The CDC received reports of two deaths – girls who were aged 5 and 6 – who had chronic medical conditions and were in “fragile health” before their shots. The agency said that no data suggested a “causal association between death and vaccination.”
The CDC also received some reports that children between ages 5 and 11 received the larger vaccine dose meant for older children and adults. Most reports said that the children didn’t experience any problems after an incorrect dose.
In a separate study about pediatric hospitalizations, CDC researchers looked at more than 700 children under age 18 who were hospitalized for COVID-19 in July and August at six children’s hospitals in Arkansas, Florida, Illinois, Louisiana, Texas, and Washington, D.C.
Researchers found that only one of the 272 vaccine-eligible patients between ages 12 and 17 had been fully vaccinated, and 12 were partially vaccinated.
In addition, about two-thirds of the hospitalized children between ages 12 and 17 had an underlying condition, with obesity being the most common. About one-third of children under age 5 had more than one viral infection.
Overall, about 30% of the children had to be treated in intensive care units, and 15% needed invasive medical ventilation, CDC researchers found. Nearly 3% had multisystem inflammatory syndrome in children, or MIS-C, which is a rare but serious inflammatory condition associated with COVID-19.
Among all the children hospitalized with COVID-19, about 1.5% died.
“Few vaccine-eligible patients hospitalized for COVID-19 were vaccinated, highlighting the importance of vaccination for those aged ≥5 years and other prevention strategies to protect children and adolescents from COVID-19, particularly those with underlying medical conditions,” study authors wrote.
A version of this article first appeared on WebMD.com.
FDA backs Pfizer booster for 12- to 15-year-olds
Besides updating the authorization for the Pfizer COVID-19 vaccine, the agency also shortened the recommended time between a second dose and the booster to 5 months or more, based on new evidence. In addition, a third primary series dose is now authorized for certain immunocompromised children 5 years to 11 years old. Full details are available in an FDA news release.
The amended emergency use authorization (EUA) only applies to the Pfizer vaccine, said acting FDA Commissioner Janet Woodcock, MD.
“Just to make sure every everyone is clear on this, right now: If you got [Johnson & Johnson’s one-dose vaccine], you get a booster after 2 months. If you got Moderna, you can get a booster at 6 months or beyond,” she said during a media briefing.
What is new, she said, is “if you got Pfizer as your primary series, you can get a booster at 5 months or beyond.”
A lower risk of myocarditis?
Asked about concerns about the risk of myocarditis with vaccination in the 12- to 15-year age group, Dr. Woodcock said they expect it would be “extremely rare with the third dose.”
“We have the real-world evidence from the Israeli experience to help us with that analysis,” she said.
The data so far consistently points to a higher risk of myocarditis after a second mRNA vaccine dose among males, from teenagers to 30-year-olds, with a peak at about 16 to 17 years of age, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said during the media call.
The risk of myocarditis is about 2 to 3 times higher after a second vaccine dose, compared to a booster shot, Dr. Marks said, based on available data. It may be related to the closer dose timing of the second dose versus a third, he added.
“The inference here is that on the risk of myocarditis with third doses in the 12- to 15-year age range is likely to be quite acceptable,” he said.
Dr. Marks also pointed out that most cases of myocarditis clear up quickly.
“We’re not seeing long-lasting effects. That’s not to say that we don’t care about this and that it’s not important,” he said.
“But what it is saying is that in the setting of a tremendous number of Omicron and Delta cases in this country, the potential benefits of getting vaccinated in this age group outweigh that risk,” Dr. Marks said. “We can look at that risk-benefit and still feel comfortable.”
He said that “the really overwhelming majority of these cases, 98%, have been mild” -- shown by a 1-day median hospital stay.
Even so, the FDA plans to continue monitoring for the risk of myocarditis “very closely,” he said.
Interestingly, swollen underarm lymph nodes were seen more frequently after the booster dose than after the second dose of a two-dose primary series, the FDA said.
Reducing the time between primary vaccination with the Pfizer vaccine -- two initial doses -- and the booster shot from 6 months to 5 months is based on decreasing efficacy data that the drugmaker submitted to the FDA.
The 5-month interval was evaluated in a study from Israel published Dec. 21 in the New England Journal of Medicine .
Mixing and matching vaccines
Less clear at the moment is guidance about boosters for people who opted to mix and match their primary vaccine series.
“There was a mix-and-match study that was done which showed that in some cases, the mixing and matching … of an adenoviral record vaccine and an mRNA vaccine seem to give a very good immune response,” Dr. Marks said.
Once more data comes in on mixing and matching, “we’ll analyze them and then potentially make recommendations,” he said.
‘It’s not too late’
No federal government media briefing on COVID-19 would be complete without a plea for the unvaccinated to get immunized.
“We’re talking a lot about boosters right now, but it’s not too late for those who have not gotten a vaccine to get a vaccine,” Dr. Marks said, referring to the tens of millions of Americans who remain unvaccinated at the beginning of 2022.
“We know from our previous studies that even a single dose of the vaccine -- and probably two doses -- can help prevent the worst outcomes from COVID-19, including hospitalization and death.”
A version of this article first appeared on WebMD.com.
Besides updating the authorization for the Pfizer COVID-19 vaccine, the agency also shortened the recommended time between a second dose and the booster to 5 months or more, based on new evidence. In addition, a third primary series dose is now authorized for certain immunocompromised children 5 years to 11 years old. Full details are available in an FDA news release.
The amended emergency use authorization (EUA) only applies to the Pfizer vaccine, said acting FDA Commissioner Janet Woodcock, MD.
“Just to make sure every everyone is clear on this, right now: If you got [Johnson & Johnson’s one-dose vaccine], you get a booster after 2 months. If you got Moderna, you can get a booster at 6 months or beyond,” she said during a media briefing.
What is new, she said, is “if you got Pfizer as your primary series, you can get a booster at 5 months or beyond.”
A lower risk of myocarditis?
Asked about concerns about the risk of myocarditis with vaccination in the 12- to 15-year age group, Dr. Woodcock said they expect it would be “extremely rare with the third dose.”
“We have the real-world evidence from the Israeli experience to help us with that analysis,” she said.
The data so far consistently points to a higher risk of myocarditis after a second mRNA vaccine dose among males, from teenagers to 30-year-olds, with a peak at about 16 to 17 years of age, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said during the media call.
The risk of myocarditis is about 2 to 3 times higher after a second vaccine dose, compared to a booster shot, Dr. Marks said, based on available data. It may be related to the closer dose timing of the second dose versus a third, he added.
“The inference here is that on the risk of myocarditis with third doses in the 12- to 15-year age range is likely to be quite acceptable,” he said.
Dr. Marks also pointed out that most cases of myocarditis clear up quickly.
“We’re not seeing long-lasting effects. That’s not to say that we don’t care about this and that it’s not important,” he said.
“But what it is saying is that in the setting of a tremendous number of Omicron and Delta cases in this country, the potential benefits of getting vaccinated in this age group outweigh that risk,” Dr. Marks said. “We can look at that risk-benefit and still feel comfortable.”
He said that “the really overwhelming majority of these cases, 98%, have been mild” -- shown by a 1-day median hospital stay.
Even so, the FDA plans to continue monitoring for the risk of myocarditis “very closely,” he said.
Interestingly, swollen underarm lymph nodes were seen more frequently after the booster dose than after the second dose of a two-dose primary series, the FDA said.
Reducing the time between primary vaccination with the Pfizer vaccine -- two initial doses -- and the booster shot from 6 months to 5 months is based on decreasing efficacy data that the drugmaker submitted to the FDA.
The 5-month interval was evaluated in a study from Israel published Dec. 21 in the New England Journal of Medicine .
Mixing and matching vaccines
Less clear at the moment is guidance about boosters for people who opted to mix and match their primary vaccine series.
“There was a mix-and-match study that was done which showed that in some cases, the mixing and matching … of an adenoviral record vaccine and an mRNA vaccine seem to give a very good immune response,” Dr. Marks said.
Once more data comes in on mixing and matching, “we’ll analyze them and then potentially make recommendations,” he said.
‘It’s not too late’
No federal government media briefing on COVID-19 would be complete without a plea for the unvaccinated to get immunized.
“We’re talking a lot about boosters right now, but it’s not too late for those who have not gotten a vaccine to get a vaccine,” Dr. Marks said, referring to the tens of millions of Americans who remain unvaccinated at the beginning of 2022.
“We know from our previous studies that even a single dose of the vaccine -- and probably two doses -- can help prevent the worst outcomes from COVID-19, including hospitalization and death.”
A version of this article first appeared on WebMD.com.
Besides updating the authorization for the Pfizer COVID-19 vaccine, the agency also shortened the recommended time between a second dose and the booster to 5 months or more, based on new evidence. In addition, a third primary series dose is now authorized for certain immunocompromised children 5 years to 11 years old. Full details are available in an FDA news release.
The amended emergency use authorization (EUA) only applies to the Pfizer vaccine, said acting FDA Commissioner Janet Woodcock, MD.
“Just to make sure every everyone is clear on this, right now: If you got [Johnson & Johnson’s one-dose vaccine], you get a booster after 2 months. If you got Moderna, you can get a booster at 6 months or beyond,” she said during a media briefing.
What is new, she said, is “if you got Pfizer as your primary series, you can get a booster at 5 months or beyond.”
A lower risk of myocarditis?
Asked about concerns about the risk of myocarditis with vaccination in the 12- to 15-year age group, Dr. Woodcock said they expect it would be “extremely rare with the third dose.”
“We have the real-world evidence from the Israeli experience to help us with that analysis,” she said.
The data so far consistently points to a higher risk of myocarditis after a second mRNA vaccine dose among males, from teenagers to 30-year-olds, with a peak at about 16 to 17 years of age, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said during the media call.
The risk of myocarditis is about 2 to 3 times higher after a second vaccine dose, compared to a booster shot, Dr. Marks said, based on available data. It may be related to the closer dose timing of the second dose versus a third, he added.
“The inference here is that on the risk of myocarditis with third doses in the 12- to 15-year age range is likely to be quite acceptable,” he said.
Dr. Marks also pointed out that most cases of myocarditis clear up quickly.
“We’re not seeing long-lasting effects. That’s not to say that we don’t care about this and that it’s not important,” he said.
“But what it is saying is that in the setting of a tremendous number of Omicron and Delta cases in this country, the potential benefits of getting vaccinated in this age group outweigh that risk,” Dr. Marks said. “We can look at that risk-benefit and still feel comfortable.”
He said that “the really overwhelming majority of these cases, 98%, have been mild” -- shown by a 1-day median hospital stay.
Even so, the FDA plans to continue monitoring for the risk of myocarditis “very closely,” he said.
Interestingly, swollen underarm lymph nodes were seen more frequently after the booster dose than after the second dose of a two-dose primary series, the FDA said.
Reducing the time between primary vaccination with the Pfizer vaccine -- two initial doses -- and the booster shot from 6 months to 5 months is based on decreasing efficacy data that the drugmaker submitted to the FDA.
The 5-month interval was evaluated in a study from Israel published Dec. 21 in the New England Journal of Medicine .
Mixing and matching vaccines
Less clear at the moment is guidance about boosters for people who opted to mix and match their primary vaccine series.
“There was a mix-and-match study that was done which showed that in some cases, the mixing and matching … of an adenoviral record vaccine and an mRNA vaccine seem to give a very good immune response,” Dr. Marks said.
Once more data comes in on mixing and matching, “we’ll analyze them and then potentially make recommendations,” he said.
‘It’s not too late’
No federal government media briefing on COVID-19 would be complete without a plea for the unvaccinated to get immunized.
“We’re talking a lot about boosters right now, but it’s not too late for those who have not gotten a vaccine to get a vaccine,” Dr. Marks said, referring to the tens of millions of Americans who remain unvaccinated at the beginning of 2022.
“We know from our previous studies that even a single dose of the vaccine -- and probably two doses -- can help prevent the worst outcomes from COVID-19, including hospitalization and death.”
A version of this article first appeared on WebMD.com.
Travel/school disruptions as COVID-19 cases grow in 2022
As the United States enters a third year of the COVID-19 pandemic,
The United States is reporting a 7-day average of more than 386,000 cases after several record-breaking days, according to the data tracker by the New York Times. The United States surpassed 585,000 cases on Dec. 30, setting a new record before the New Year’s holiday.
New York, Washington, D.C., and other states along the East Coast are leading the national surge. New York reported more than 85,000 new cases on the last day of 2021, marking the highest 1-day total in the state since the pandemic began.
“As we fight the winter surge, we need to keep the most vulnerable among us in mind – do what you can to keep others in your community safe from COVID-19,” New York Gov. Kathy Hochul said in a statement on Jan. 1, 2022.
“Wear a mask, wash your hands, and take advantage of the best tool we have at our disposal – the vaccine,” she said.
The 2021 winter surge in the United States peaked around Jan. 12, which may suggest that the country has a week or so before the current wave reaches its height and begins to drop, the newspaper reported.
In the meantime, people are dealing with disruptions as they return from holiday travel and begin the new year. Airlines canceled more than 2,700 flights on Jan. 1 and more than 1,900 flights on Jan. 2, bringing the total since Christmas Eve to more than 14,000 canceled flights.
About half of cancellations were connected to wintry weather at key airline hubs in Chicago and Denver, the newspaper reported, as well as ongoing flight crew shortages caused by the Omicron variant.
More disruptions could continue, the Federal Aviation Administration warned, as an increasing number of its air-traffic control employees test positive for COVID-19.
“To maintain safety, traffic volumes at some facilities could be reduced, which might result in delays during busy periods,” an FAA spokesman told The Wall Street Journal.
The current COVID-19 surge will also affect businesses and schools as the new year begins. A growing number of universities are opting to start the next semester with remote instruction.
American University, Duke University, and Michigan State University announced in recent days that they would delay in-person classes to slow the spread of the coronavirus on campus. They will begin classes online on Jan. 10 and return to campus the following week or later.
“I realize that students prefer to be in person, and so do I. But it is important that we do so in a safe manner,” Samuel Stanley Jr., MD, president of Michigan State University, said in a statement on New Year’s Eve.
K-12 school districts are deciding how to adapt as well. Some districts are bringing back mask requirements, and some are ramping up testing. Others are moving to remote learning – and signaling the need for flexibility as the Omicron variant brings new surprises.
“Change has been the only constant in this fight,” Roger Leon, the superintendent for Newark (N.J.) Public Schools, wrote in a note to parents. He announced on Dec. 30, 2021, that students would learn remotely for at least the first 2 weeks of the new year.
This continues “to be a brutal, relentless, and ruthless virus that rears its ugly head at inopportune times,” he said.
A version of this article first appeared on WebMD.com.
As the United States enters a third year of the COVID-19 pandemic,
The United States is reporting a 7-day average of more than 386,000 cases after several record-breaking days, according to the data tracker by the New York Times. The United States surpassed 585,000 cases on Dec. 30, setting a new record before the New Year’s holiday.
New York, Washington, D.C., and other states along the East Coast are leading the national surge. New York reported more than 85,000 new cases on the last day of 2021, marking the highest 1-day total in the state since the pandemic began.
“As we fight the winter surge, we need to keep the most vulnerable among us in mind – do what you can to keep others in your community safe from COVID-19,” New York Gov. Kathy Hochul said in a statement on Jan. 1, 2022.
“Wear a mask, wash your hands, and take advantage of the best tool we have at our disposal – the vaccine,” she said.
The 2021 winter surge in the United States peaked around Jan. 12, which may suggest that the country has a week or so before the current wave reaches its height and begins to drop, the newspaper reported.
In the meantime, people are dealing with disruptions as they return from holiday travel and begin the new year. Airlines canceled more than 2,700 flights on Jan. 1 and more than 1,900 flights on Jan. 2, bringing the total since Christmas Eve to more than 14,000 canceled flights.
About half of cancellations were connected to wintry weather at key airline hubs in Chicago and Denver, the newspaper reported, as well as ongoing flight crew shortages caused by the Omicron variant.
More disruptions could continue, the Federal Aviation Administration warned, as an increasing number of its air-traffic control employees test positive for COVID-19.
“To maintain safety, traffic volumes at some facilities could be reduced, which might result in delays during busy periods,” an FAA spokesman told The Wall Street Journal.
The current COVID-19 surge will also affect businesses and schools as the new year begins. A growing number of universities are opting to start the next semester with remote instruction.
American University, Duke University, and Michigan State University announced in recent days that they would delay in-person classes to slow the spread of the coronavirus on campus. They will begin classes online on Jan. 10 and return to campus the following week or later.
“I realize that students prefer to be in person, and so do I. But it is important that we do so in a safe manner,” Samuel Stanley Jr., MD, president of Michigan State University, said in a statement on New Year’s Eve.
K-12 school districts are deciding how to adapt as well. Some districts are bringing back mask requirements, and some are ramping up testing. Others are moving to remote learning – and signaling the need for flexibility as the Omicron variant brings new surprises.
“Change has been the only constant in this fight,” Roger Leon, the superintendent for Newark (N.J.) Public Schools, wrote in a note to parents. He announced on Dec. 30, 2021, that students would learn remotely for at least the first 2 weeks of the new year.
This continues “to be a brutal, relentless, and ruthless virus that rears its ugly head at inopportune times,” he said.
A version of this article first appeared on WebMD.com.
As the United States enters a third year of the COVID-19 pandemic,
The United States is reporting a 7-day average of more than 386,000 cases after several record-breaking days, according to the data tracker by the New York Times. The United States surpassed 585,000 cases on Dec. 30, setting a new record before the New Year’s holiday.
New York, Washington, D.C., and other states along the East Coast are leading the national surge. New York reported more than 85,000 new cases on the last day of 2021, marking the highest 1-day total in the state since the pandemic began.
“As we fight the winter surge, we need to keep the most vulnerable among us in mind – do what you can to keep others in your community safe from COVID-19,” New York Gov. Kathy Hochul said in a statement on Jan. 1, 2022.
“Wear a mask, wash your hands, and take advantage of the best tool we have at our disposal – the vaccine,” she said.
The 2021 winter surge in the United States peaked around Jan. 12, which may suggest that the country has a week or so before the current wave reaches its height and begins to drop, the newspaper reported.
In the meantime, people are dealing with disruptions as they return from holiday travel and begin the new year. Airlines canceled more than 2,700 flights on Jan. 1 and more than 1,900 flights on Jan. 2, bringing the total since Christmas Eve to more than 14,000 canceled flights.
About half of cancellations were connected to wintry weather at key airline hubs in Chicago and Denver, the newspaper reported, as well as ongoing flight crew shortages caused by the Omicron variant.
More disruptions could continue, the Federal Aviation Administration warned, as an increasing number of its air-traffic control employees test positive for COVID-19.
“To maintain safety, traffic volumes at some facilities could be reduced, which might result in delays during busy periods,” an FAA spokesman told The Wall Street Journal.
The current COVID-19 surge will also affect businesses and schools as the new year begins. A growing number of universities are opting to start the next semester with remote instruction.
American University, Duke University, and Michigan State University announced in recent days that they would delay in-person classes to slow the spread of the coronavirus on campus. They will begin classes online on Jan. 10 and return to campus the following week or later.
“I realize that students prefer to be in person, and so do I. But it is important that we do so in a safe manner,” Samuel Stanley Jr., MD, president of Michigan State University, said in a statement on New Year’s Eve.
K-12 school districts are deciding how to adapt as well. Some districts are bringing back mask requirements, and some are ramping up testing. Others are moving to remote learning – and signaling the need for flexibility as the Omicron variant brings new surprises.
“Change has been the only constant in this fight,” Roger Leon, the superintendent for Newark (N.J.) Public Schools, wrote in a note to parents. He announced on Dec. 30, 2021, that students would learn remotely for at least the first 2 weeks of the new year.
This continues “to be a brutal, relentless, and ruthless virus that rears its ugly head at inopportune times,” he said.
A version of this article first appeared on WebMD.com.
Antiretroviral pill better at suppressing HIV in children
Dolutegravir suppresses HIV by inhibiting integrase, an enzyme that the virus needs to replicate.
The pill-based regimen, which researchers described as easier to take than standard treatment, reduced the chances of treatment failure among children aged 3-18 years by about 40%, compared with other treatments. Dolutegravir is already used for the suppression of HIV in adults.
“About 1.8 million children live with HIV but they have had limited treatment options, with medicines that taste unpalatable, that need to be taken twice a day, or that come in large pills that are difficult to swallow” said lead author Anna Turkova, MD, from the MRC clinical trials unit at UCL. “Dolutegravir is given in small tablets usually once a day and the baby pills can be dispersed in water, meaning it’s a lot easier for young children to take. This is important in encouraging uptake of the treatment and adherence to it over many years.
“Sadly, only about half of children living with HIV are currently receiving treatment, and those who are not treated face high risks of impaired immunity and worsening health.”
Study details
The randomized controlled trial, called ODYSSEY, involved more than 700 children from 29 clinical centers located in Africa, Europe, and Asia. The children were given either dolutegravir or standard anti-HIV drugs, and were followed up for at least 2 years.
The study showed that 14% of children receiving dolutegravir experienced treatment failure over 2 years, compared with 22% of those receiving standard treatment. Treatment failure was deemed to occur if measurable virus appeared in the blood or if the child had symptoms of HIV-related illness.
“Our findings provide strong evidence for the global rollout of dolutegravir for children with HIV,” said Diana Gibb, MD, also from the MRC clinical trials unit at UCL, principal investigator of the trial and one of the senior authors of the paper.
“Medical treatments for children often lag woefully behind those of adults because of the separate formulations and studies that are needed,” she added. “With the evidence from ODYSSEY which used simplified dosing of both adult and baby pills, this treatment gap has been reduced and we hope that countries can quickly scale up access to children globally.”
Simplified dosing
“Simplifying the dosing is crucial,” concurred Cissy Kityo Mutuluuza, MD, from the Joint Clinical Research Centre in Uganda, the country enrolling most children in the trial. “Older children being able to take the same tablets as adults immediately opens access to dolutegravir for the majority of children living with HIV. It greatly simplifies procurement for national health systems in low- and middle-income countries, and lowers costs.”
Evidence from adults shows dolutegravir has a high genetic barrier to resistance, meaning viruses are less likely to become resistant to it over time. This was confirmed in the ODYSSEY trial, with much less resistance occurring among children and adolescents on dolutegravir-based treatment. In addition, past studies of the drug have shown that it may be associated with weight gain in adults, but the findings were reassuring for children. Those given dolutegravir gained on average 1 kg more and grew 1 cm higher over the study period, indicating better growth rather than abnormal weight gain.
Early findings from the trial have informed new guidance by the World Health Organization, recommending the use of dolutegravir for children.
The study was sponsored by the Penta Foundation, an international independent research network, and funded by specialist pharmaceutical company ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Dolutegravir suppresses HIV by inhibiting integrase, an enzyme that the virus needs to replicate.
The pill-based regimen, which researchers described as easier to take than standard treatment, reduced the chances of treatment failure among children aged 3-18 years by about 40%, compared with other treatments. Dolutegravir is already used for the suppression of HIV in adults.
“About 1.8 million children live with HIV but they have had limited treatment options, with medicines that taste unpalatable, that need to be taken twice a day, or that come in large pills that are difficult to swallow” said lead author Anna Turkova, MD, from the MRC clinical trials unit at UCL. “Dolutegravir is given in small tablets usually once a day and the baby pills can be dispersed in water, meaning it’s a lot easier for young children to take. This is important in encouraging uptake of the treatment and adherence to it over many years.
“Sadly, only about half of children living with HIV are currently receiving treatment, and those who are not treated face high risks of impaired immunity and worsening health.”
Study details
The randomized controlled trial, called ODYSSEY, involved more than 700 children from 29 clinical centers located in Africa, Europe, and Asia. The children were given either dolutegravir or standard anti-HIV drugs, and were followed up for at least 2 years.
The study showed that 14% of children receiving dolutegravir experienced treatment failure over 2 years, compared with 22% of those receiving standard treatment. Treatment failure was deemed to occur if measurable virus appeared in the blood or if the child had symptoms of HIV-related illness.
“Our findings provide strong evidence for the global rollout of dolutegravir for children with HIV,” said Diana Gibb, MD, also from the MRC clinical trials unit at UCL, principal investigator of the trial and one of the senior authors of the paper.
“Medical treatments for children often lag woefully behind those of adults because of the separate formulations and studies that are needed,” she added. “With the evidence from ODYSSEY which used simplified dosing of both adult and baby pills, this treatment gap has been reduced and we hope that countries can quickly scale up access to children globally.”
Simplified dosing
“Simplifying the dosing is crucial,” concurred Cissy Kityo Mutuluuza, MD, from the Joint Clinical Research Centre in Uganda, the country enrolling most children in the trial. “Older children being able to take the same tablets as adults immediately opens access to dolutegravir for the majority of children living with HIV. It greatly simplifies procurement for national health systems in low- and middle-income countries, and lowers costs.”
Evidence from adults shows dolutegravir has a high genetic barrier to resistance, meaning viruses are less likely to become resistant to it over time. This was confirmed in the ODYSSEY trial, with much less resistance occurring among children and adolescents on dolutegravir-based treatment. In addition, past studies of the drug have shown that it may be associated with weight gain in adults, but the findings were reassuring for children. Those given dolutegravir gained on average 1 kg more and grew 1 cm higher over the study period, indicating better growth rather than abnormal weight gain.
Early findings from the trial have informed new guidance by the World Health Organization, recommending the use of dolutegravir for children.
The study was sponsored by the Penta Foundation, an international independent research network, and funded by specialist pharmaceutical company ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Dolutegravir suppresses HIV by inhibiting integrase, an enzyme that the virus needs to replicate.
The pill-based regimen, which researchers described as easier to take than standard treatment, reduced the chances of treatment failure among children aged 3-18 years by about 40%, compared with other treatments. Dolutegravir is already used for the suppression of HIV in adults.
“About 1.8 million children live with HIV but they have had limited treatment options, with medicines that taste unpalatable, that need to be taken twice a day, or that come in large pills that are difficult to swallow” said lead author Anna Turkova, MD, from the MRC clinical trials unit at UCL. “Dolutegravir is given in small tablets usually once a day and the baby pills can be dispersed in water, meaning it’s a lot easier for young children to take. This is important in encouraging uptake of the treatment and adherence to it over many years.
“Sadly, only about half of children living with HIV are currently receiving treatment, and those who are not treated face high risks of impaired immunity and worsening health.”
Study details
The randomized controlled trial, called ODYSSEY, involved more than 700 children from 29 clinical centers located in Africa, Europe, and Asia. The children were given either dolutegravir or standard anti-HIV drugs, and were followed up for at least 2 years.
The study showed that 14% of children receiving dolutegravir experienced treatment failure over 2 years, compared with 22% of those receiving standard treatment. Treatment failure was deemed to occur if measurable virus appeared in the blood or if the child had symptoms of HIV-related illness.
“Our findings provide strong evidence for the global rollout of dolutegravir for children with HIV,” said Diana Gibb, MD, also from the MRC clinical trials unit at UCL, principal investigator of the trial and one of the senior authors of the paper.
“Medical treatments for children often lag woefully behind those of adults because of the separate formulations and studies that are needed,” she added. “With the evidence from ODYSSEY which used simplified dosing of both adult and baby pills, this treatment gap has been reduced and we hope that countries can quickly scale up access to children globally.”
Simplified dosing
“Simplifying the dosing is crucial,” concurred Cissy Kityo Mutuluuza, MD, from the Joint Clinical Research Centre in Uganda, the country enrolling most children in the trial. “Older children being able to take the same tablets as adults immediately opens access to dolutegravir for the majority of children living with HIV. It greatly simplifies procurement for national health systems in low- and middle-income countries, and lowers costs.”
Evidence from adults shows dolutegravir has a high genetic barrier to resistance, meaning viruses are less likely to become resistant to it over time. This was confirmed in the ODYSSEY trial, with much less resistance occurring among children and adolescents on dolutegravir-based treatment. In addition, past studies of the drug have shown that it may be associated with weight gain in adults, but the findings were reassuring for children. Those given dolutegravir gained on average 1 kg more and grew 1 cm higher over the study period, indicating better growth rather than abnormal weight gain.
Early findings from the trial have informed new guidance by the World Health Organization, recommending the use of dolutegravir for children.
The study was sponsored by the Penta Foundation, an international independent research network, and funded by specialist pharmaceutical company ViiV Healthcare.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Pandemic screen time linked to anxiety, depression in older kids
However, the study doesn’t definitively prove that screen time is harmful, and an expert challenged the conclusions.
Still, the findings highlight the potential harms of excessive screen time, especially in the context of pandemic-era virtual learning. Clinicians “really need to advocate for policies that would be protective for children to reduce their screen time and social isolation and increase their involvement with school, sports, and academic activities,” Catherine S. Birken, MD, a pediatrician at the University of Toronto and study coauthor said in an interview.
The study appeared Dec. 28, 2021, in the journal JAMA Network Open (doi: 10.1001/jamanetworkopen.2021.40875).
Dr. Birken and colleagues launched the study to examine whether heightened levels of screen time during the pandemic disrupted mental health in kids. In particular, they wanted to break down different types of screen time, such as virtual learning, watching television, and playing video games.
“The bulk of the literature is supportive of a strong relationship between screen time and mental health symptoms like anxiety,” Dr. Birken said.
For the study, the researchers surveyed parents to track the screen time of 2,026 children between May 2020 and April 2021.
In a cohort of 532 younger children (average age, 5.9 years; 52% male; 58% of European ancestry), the researchers linked each extra daily hour of TV or use of digital media to worse behavior, as measured by the Strengths and Difficulties Questionnaire: 0.22 in an adjusted model for children aged 2-4;(95% confidence interval, 0.10-0.35; P < .001) and 0.07 in an adjusted model in those aged 4 and older (95% CI, 0.02-0.11; P = .007).
However, the researchers observed no statistically significant links to more anxiety/depression or hyperactivity/inattention in this group of children.
Among 1,494 older kids (mean age, 11.3; 57% male; 58% of European ancestry), researchers linked greater daily use of TV or digital media to higher levels of depression symptoms in a dose-dependent relationship, Dr. Birken said (1 hour: beta, 0.21; 95% CI, –1.28 to 0.78; 2-3 hours: beta, 1.81; 95% CI, 0.29-3.33; 4-5 hours: beta, 2.80; 95% CI, 1.15-4.44; 6-8 hours: beta, 5.16; 95% CI, 3.32-7.01; 9 hours: beta, 5.42; 95% CI, 3.30-7.54; overall P < .001).
“Similarly, higher TV or digital media time per day was associated with higher levels of anxiety symptoms,” the researchers reported. “TV or digital media time per day was also significantly associated with differences in symptoms of irritability, inattention, and hyperactivity/inattention.”
More time spent learning virtually was associated with higher levels of depression and anxiety in both groups of children, according to the researchers. Whether this finding reflects an effect of screens themselves or because the children most exposed to virtual learning may also have been the most exposed to the stressful disruptiveness of the pandemic is unclear.
The researchers also found “insufficient evidence” to link more virtual learning to irritability, inattention and hyperactivity, inattention, and hyperactivity/impulsivity in adjusted models.
Video chatting did not appear to have a protective effect, Dr. Birken said. The researchers also specifically analyzed children with autism and found no link between more screen time and various mental health/conduct problems.
Is it possible that kids with more anxiety, depression, and isolation simply turn to screens because they’re anxious, depressed, and isolated? Dr. Birken said the researchers adjusted the findings to account for previous mental health problems. And she noted that the study linked more pandemic-era virtual learning to more depression/anxiety. It’s “hard to imagine” how more mental health problems would cause more virtual learning.
Bad news or bad stats?
Chris Ferguson, PhD, a professor of psychology at Stetson University. DeLand, Fla., who studies screen time, criticized the study in an interview. “The observed effects are so tiny, it’s impossible to know if they are real or a false-positive artifact common to social science research,” he said. “Ultimately, this study is better evidence about how many scholars are bad at statistics than anything having to do with kids and screens.”
Dr. Ferguson said that the results may be confounded because kids turn to screens to reduce their anxiety. “For the most part, screens were a godsend during COVID-19,” he said. “They helped kids stay inside and gave them something to do while social distancing and allowed them to keep in touch with friends and families. Honestly, what else were we expecting kids to do, stare at the wallpaper?”
Children with depression and anxiety often retreat into screens or books to escape the unpleasantries of real life. “That doesn’t mean the screens or books are the culprits,” he said.
Instead of focusing on screen time, Dr. Ferguson suggested parents consider these factors: “Keeping in mind not every kid is a genius, is your kid doing about as well in school as you’d expect, given their natural ability? Are they getting at least some exercise every day? Are they getting adequate sleep? Are they able to socialize with friends in some context, either in real life or online? Are they happy?”
The study was funded by the Canadian Institutes of Health Research, the Center for Brain & Mental Health at The Hospital for Sick Children, the Ontario Ministry of Health, and the Miner’s Lamp Innovation Fund in Prevention and Early Detection of Severe Mental Illness at the University of Toronto. The study authors reported various financial relationships. Dr. Ferguson reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
However, the study doesn’t definitively prove that screen time is harmful, and an expert challenged the conclusions.
Still, the findings highlight the potential harms of excessive screen time, especially in the context of pandemic-era virtual learning. Clinicians “really need to advocate for policies that would be protective for children to reduce their screen time and social isolation and increase their involvement with school, sports, and academic activities,” Catherine S. Birken, MD, a pediatrician at the University of Toronto and study coauthor said in an interview.
The study appeared Dec. 28, 2021, in the journal JAMA Network Open (doi: 10.1001/jamanetworkopen.2021.40875).
Dr. Birken and colleagues launched the study to examine whether heightened levels of screen time during the pandemic disrupted mental health in kids. In particular, they wanted to break down different types of screen time, such as virtual learning, watching television, and playing video games.
“The bulk of the literature is supportive of a strong relationship between screen time and mental health symptoms like anxiety,” Dr. Birken said.
For the study, the researchers surveyed parents to track the screen time of 2,026 children between May 2020 and April 2021.
In a cohort of 532 younger children (average age, 5.9 years; 52% male; 58% of European ancestry), the researchers linked each extra daily hour of TV or use of digital media to worse behavior, as measured by the Strengths and Difficulties Questionnaire: 0.22 in an adjusted model for children aged 2-4;(95% confidence interval, 0.10-0.35; P < .001) and 0.07 in an adjusted model in those aged 4 and older (95% CI, 0.02-0.11; P = .007).
However, the researchers observed no statistically significant links to more anxiety/depression or hyperactivity/inattention in this group of children.
Among 1,494 older kids (mean age, 11.3; 57% male; 58% of European ancestry), researchers linked greater daily use of TV or digital media to higher levels of depression symptoms in a dose-dependent relationship, Dr. Birken said (1 hour: beta, 0.21; 95% CI, –1.28 to 0.78; 2-3 hours: beta, 1.81; 95% CI, 0.29-3.33; 4-5 hours: beta, 2.80; 95% CI, 1.15-4.44; 6-8 hours: beta, 5.16; 95% CI, 3.32-7.01; 9 hours: beta, 5.42; 95% CI, 3.30-7.54; overall P < .001).
“Similarly, higher TV or digital media time per day was associated with higher levels of anxiety symptoms,” the researchers reported. “TV or digital media time per day was also significantly associated with differences in symptoms of irritability, inattention, and hyperactivity/inattention.”
More time spent learning virtually was associated with higher levels of depression and anxiety in both groups of children, according to the researchers. Whether this finding reflects an effect of screens themselves or because the children most exposed to virtual learning may also have been the most exposed to the stressful disruptiveness of the pandemic is unclear.
The researchers also found “insufficient evidence” to link more virtual learning to irritability, inattention and hyperactivity, inattention, and hyperactivity/impulsivity in adjusted models.
Video chatting did not appear to have a protective effect, Dr. Birken said. The researchers also specifically analyzed children with autism and found no link between more screen time and various mental health/conduct problems.
Is it possible that kids with more anxiety, depression, and isolation simply turn to screens because they’re anxious, depressed, and isolated? Dr. Birken said the researchers adjusted the findings to account for previous mental health problems. And she noted that the study linked more pandemic-era virtual learning to more depression/anxiety. It’s “hard to imagine” how more mental health problems would cause more virtual learning.
Bad news or bad stats?
Chris Ferguson, PhD, a professor of psychology at Stetson University. DeLand, Fla., who studies screen time, criticized the study in an interview. “The observed effects are so tiny, it’s impossible to know if they are real or a false-positive artifact common to social science research,” he said. “Ultimately, this study is better evidence about how many scholars are bad at statistics than anything having to do with kids and screens.”
Dr. Ferguson said that the results may be confounded because kids turn to screens to reduce their anxiety. “For the most part, screens were a godsend during COVID-19,” he said. “They helped kids stay inside and gave them something to do while social distancing and allowed them to keep in touch with friends and families. Honestly, what else were we expecting kids to do, stare at the wallpaper?”
Children with depression and anxiety often retreat into screens or books to escape the unpleasantries of real life. “That doesn’t mean the screens or books are the culprits,” he said.
Instead of focusing on screen time, Dr. Ferguson suggested parents consider these factors: “Keeping in mind not every kid is a genius, is your kid doing about as well in school as you’d expect, given their natural ability? Are they getting at least some exercise every day? Are they getting adequate sleep? Are they able to socialize with friends in some context, either in real life or online? Are they happy?”
The study was funded by the Canadian Institutes of Health Research, the Center for Brain & Mental Health at The Hospital for Sick Children, the Ontario Ministry of Health, and the Miner’s Lamp Innovation Fund in Prevention and Early Detection of Severe Mental Illness at the University of Toronto. The study authors reported various financial relationships. Dr. Ferguson reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
However, the study doesn’t definitively prove that screen time is harmful, and an expert challenged the conclusions.
Still, the findings highlight the potential harms of excessive screen time, especially in the context of pandemic-era virtual learning. Clinicians “really need to advocate for policies that would be protective for children to reduce their screen time and social isolation and increase their involvement with school, sports, and academic activities,” Catherine S. Birken, MD, a pediatrician at the University of Toronto and study coauthor said in an interview.
The study appeared Dec. 28, 2021, in the journal JAMA Network Open (doi: 10.1001/jamanetworkopen.2021.40875).
Dr. Birken and colleagues launched the study to examine whether heightened levels of screen time during the pandemic disrupted mental health in kids. In particular, they wanted to break down different types of screen time, such as virtual learning, watching television, and playing video games.
“The bulk of the literature is supportive of a strong relationship between screen time and mental health symptoms like anxiety,” Dr. Birken said.
For the study, the researchers surveyed parents to track the screen time of 2,026 children between May 2020 and April 2021.
In a cohort of 532 younger children (average age, 5.9 years; 52% male; 58% of European ancestry), the researchers linked each extra daily hour of TV or use of digital media to worse behavior, as measured by the Strengths and Difficulties Questionnaire: 0.22 in an adjusted model for children aged 2-4;(95% confidence interval, 0.10-0.35; P < .001) and 0.07 in an adjusted model in those aged 4 and older (95% CI, 0.02-0.11; P = .007).
However, the researchers observed no statistically significant links to more anxiety/depression or hyperactivity/inattention in this group of children.
Among 1,494 older kids (mean age, 11.3; 57% male; 58% of European ancestry), researchers linked greater daily use of TV or digital media to higher levels of depression symptoms in a dose-dependent relationship, Dr. Birken said (1 hour: beta, 0.21; 95% CI, –1.28 to 0.78; 2-3 hours: beta, 1.81; 95% CI, 0.29-3.33; 4-5 hours: beta, 2.80; 95% CI, 1.15-4.44; 6-8 hours: beta, 5.16; 95% CI, 3.32-7.01; 9 hours: beta, 5.42; 95% CI, 3.30-7.54; overall P < .001).
“Similarly, higher TV or digital media time per day was associated with higher levels of anxiety symptoms,” the researchers reported. “TV or digital media time per day was also significantly associated with differences in symptoms of irritability, inattention, and hyperactivity/inattention.”
More time spent learning virtually was associated with higher levels of depression and anxiety in both groups of children, according to the researchers. Whether this finding reflects an effect of screens themselves or because the children most exposed to virtual learning may also have been the most exposed to the stressful disruptiveness of the pandemic is unclear.
The researchers also found “insufficient evidence” to link more virtual learning to irritability, inattention and hyperactivity, inattention, and hyperactivity/impulsivity in adjusted models.
Video chatting did not appear to have a protective effect, Dr. Birken said. The researchers also specifically analyzed children with autism and found no link between more screen time and various mental health/conduct problems.
Is it possible that kids with more anxiety, depression, and isolation simply turn to screens because they’re anxious, depressed, and isolated? Dr. Birken said the researchers adjusted the findings to account for previous mental health problems. And she noted that the study linked more pandemic-era virtual learning to more depression/anxiety. It’s “hard to imagine” how more mental health problems would cause more virtual learning.
Bad news or bad stats?
Chris Ferguson, PhD, a professor of psychology at Stetson University. DeLand, Fla., who studies screen time, criticized the study in an interview. “The observed effects are so tiny, it’s impossible to know if they are real or a false-positive artifact common to social science research,” he said. “Ultimately, this study is better evidence about how many scholars are bad at statistics than anything having to do with kids and screens.”
Dr. Ferguson said that the results may be confounded because kids turn to screens to reduce their anxiety. “For the most part, screens were a godsend during COVID-19,” he said. “They helped kids stay inside and gave them something to do while social distancing and allowed them to keep in touch with friends and families. Honestly, what else were we expecting kids to do, stare at the wallpaper?”
Children with depression and anxiety often retreat into screens or books to escape the unpleasantries of real life. “That doesn’t mean the screens or books are the culprits,” he said.
Instead of focusing on screen time, Dr. Ferguson suggested parents consider these factors: “Keeping in mind not every kid is a genius, is your kid doing about as well in school as you’d expect, given their natural ability? Are they getting at least some exercise every day? Are they getting adequate sleep? Are they able to socialize with friends in some context, either in real life or online? Are they happy?”
The study was funded by the Canadian Institutes of Health Research, the Center for Brain & Mental Health at The Hospital for Sick Children, the Ontario Ministry of Health, and the Miner’s Lamp Innovation Fund in Prevention and Early Detection of Severe Mental Illness at the University of Toronto. The study authors reported various financial relationships. Dr. Ferguson reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
More lots of metformin recalled
The drumbeat of U.S. recalls continues for various lots of extended-release metformin because of contamination with unacceptably high levels of a nitrosamine that pose a cancer risk.
On Dec. 28, 2021, Viona Pharmaceuticals voluntarily recalled 33 lots of metformin hydrochloride extended-release tablets, USP 750 mg to the retail level, as a precautionary measure, because of possible contamination with N-nitrosodimethylamine (NDMA).
Metformin is used as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes mellitus. Patients who have received impacted lots of metformin are advised to continue taking their medication and contact their physician for advice regarding an alternative treatment
The product can be identified as white to off-white, capsule shaped, uncoated tablets, debossed with “Z,” “C” on one side and “20” on the other side, and come in bottles of 100 tablets, which have been distributed nationwide. The 33 batch numbers are listed in a company statement.
The affected product was manufactured by Cadila Healthcare, Ahmedabad, India, for U.S. distribution by Viona.
In its statement, Viona said: “NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables.”
This recall is being conducted “with the knowledge of the U.S. Food and Drug Administration,” it added.
Consumers with questions regarding this recall can contact the recall processor Eversana Life Science Services by phone at 1-888-304-5022, option 1; Monday-Friday, 8:00 a.m.–7:00 p.m. CT. Customers with medical-related questions who wish to report an adverse event or quality issues about the products being recalled should contact Viona Pharmaceuticals by phone at 888-304-5011, Monday-Friday, 8:30 p.m.–5:30 p.m., EST.
Latest in a long line of metformin recalls
This is the second time in 2021 that Viona has voluntarily recalled extended-release metformin tablets, 750 mg, because of potential contamination with NDMA. It recalled two lots in June, as reported by this news organization.
And in January 2021, Nostrum Laboratories recalled another lot of metformin extended-release 750-mg tablets, following on from a prior recall in November 2020.
These recalls follows 258 distinct U.S. lot recalls tracked by the FDA during the past 2 years because of unacceptably high NDMA levels in lots of metformin hydrochloride extended-release tablets.
The FDA has issued several statements about NDMA contamination of metformin formulations over the past 2 years, including a review of the methods used to detect NDMA and a summary of the information the agency had collected on excessive levels of NDMA in metformin.
According to the FDA’s 2020 summary, the agency has not yet determined how or why high levels of NDMA turn up so often in multiple batches of metformin hydrochloride extended-release tablets. However, published research attributed the contamination to certain methods of manufacturing metformin tablets.
A version of this article first appeared on Medscape.com.
The drumbeat of U.S. recalls continues for various lots of extended-release metformin because of contamination with unacceptably high levels of a nitrosamine that pose a cancer risk.
On Dec. 28, 2021, Viona Pharmaceuticals voluntarily recalled 33 lots of metformin hydrochloride extended-release tablets, USP 750 mg to the retail level, as a precautionary measure, because of possible contamination with N-nitrosodimethylamine (NDMA).
Metformin is used as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes mellitus. Patients who have received impacted lots of metformin are advised to continue taking their medication and contact their physician for advice regarding an alternative treatment
The product can be identified as white to off-white, capsule shaped, uncoated tablets, debossed with “Z,” “C” on one side and “20” on the other side, and come in bottles of 100 tablets, which have been distributed nationwide. The 33 batch numbers are listed in a company statement.
The affected product was manufactured by Cadila Healthcare, Ahmedabad, India, for U.S. distribution by Viona.
In its statement, Viona said: “NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables.”
This recall is being conducted “with the knowledge of the U.S. Food and Drug Administration,” it added.
Consumers with questions regarding this recall can contact the recall processor Eversana Life Science Services by phone at 1-888-304-5022, option 1; Monday-Friday, 8:00 a.m.–7:00 p.m. CT. Customers with medical-related questions who wish to report an adverse event or quality issues about the products being recalled should contact Viona Pharmaceuticals by phone at 888-304-5011, Monday-Friday, 8:30 p.m.–5:30 p.m., EST.
Latest in a long line of metformin recalls
This is the second time in 2021 that Viona has voluntarily recalled extended-release metformin tablets, 750 mg, because of potential contamination with NDMA. It recalled two lots in June, as reported by this news organization.
And in January 2021, Nostrum Laboratories recalled another lot of metformin extended-release 750-mg tablets, following on from a prior recall in November 2020.
These recalls follows 258 distinct U.S. lot recalls tracked by the FDA during the past 2 years because of unacceptably high NDMA levels in lots of metformin hydrochloride extended-release tablets.
The FDA has issued several statements about NDMA contamination of metformin formulations over the past 2 years, including a review of the methods used to detect NDMA and a summary of the information the agency had collected on excessive levels of NDMA in metformin.
According to the FDA’s 2020 summary, the agency has not yet determined how or why high levels of NDMA turn up so often in multiple batches of metformin hydrochloride extended-release tablets. However, published research attributed the contamination to certain methods of manufacturing metformin tablets.
A version of this article first appeared on Medscape.com.
The drumbeat of U.S. recalls continues for various lots of extended-release metformin because of contamination with unacceptably high levels of a nitrosamine that pose a cancer risk.
On Dec. 28, 2021, Viona Pharmaceuticals voluntarily recalled 33 lots of metformin hydrochloride extended-release tablets, USP 750 mg to the retail level, as a precautionary measure, because of possible contamination with N-nitrosodimethylamine (NDMA).
Metformin is used as an adjunct to diet and exercise to improve blood glucose control in adults with type 2 diabetes mellitus. Patients who have received impacted lots of metformin are advised to continue taking their medication and contact their physician for advice regarding an alternative treatment
The product can be identified as white to off-white, capsule shaped, uncoated tablets, debossed with “Z,” “C” on one side and “20” on the other side, and come in bottles of 100 tablets, which have been distributed nationwide. The 33 batch numbers are listed in a company statement.
The affected product was manufactured by Cadila Healthcare, Ahmedabad, India, for U.S. distribution by Viona.
In its statement, Viona said: “NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests. NDMA is a known environmental contaminant and found in water and foods, including meats, dairy products, and vegetables.”
This recall is being conducted “with the knowledge of the U.S. Food and Drug Administration,” it added.
Consumers with questions regarding this recall can contact the recall processor Eversana Life Science Services by phone at 1-888-304-5022, option 1; Monday-Friday, 8:00 a.m.–7:00 p.m. CT. Customers with medical-related questions who wish to report an adverse event or quality issues about the products being recalled should contact Viona Pharmaceuticals by phone at 888-304-5011, Monday-Friday, 8:30 p.m.–5:30 p.m., EST.
Latest in a long line of metformin recalls
This is the second time in 2021 that Viona has voluntarily recalled extended-release metformin tablets, 750 mg, because of potential contamination with NDMA. It recalled two lots in June, as reported by this news organization.
And in January 2021, Nostrum Laboratories recalled another lot of metformin extended-release 750-mg tablets, following on from a prior recall in November 2020.
These recalls follows 258 distinct U.S. lot recalls tracked by the FDA during the past 2 years because of unacceptably high NDMA levels in lots of metformin hydrochloride extended-release tablets.
The FDA has issued several statements about NDMA contamination of metformin formulations over the past 2 years, including a review of the methods used to detect NDMA and a summary of the information the agency had collected on excessive levels of NDMA in metformin.
According to the FDA’s 2020 summary, the agency has not yet determined how or why high levels of NDMA turn up so often in multiple batches of metformin hydrochloride extended-release tablets. However, published research attributed the contamination to certain methods of manufacturing metformin tablets.
A version of this article first appeared on Medscape.com.
Confusing messages on COVID taking a psychological toll
The Centers for Disease Control and Prevention’s decision to shorten the length of isolation time for asymptomatic Americans with COVID-19, regardless of their vaccination status, to 5 days from 10 days is confusing. I hope the agency reconsiders this decision.
After all, one of the CDC’s key messages during this pandemic has been that even people with asymptomatic COVID who have been vaccinated and boosted can transmit the disease. So it seems to me that the Dec. 27, 2021, recommendation about shortening the isolation time for COVID-19–positive people, like the agency’s earlier guidance encouraging people who are vaccinated to stop wearing masks while in indoor settings, runs contrary to good public health principles.
As an expert in human behavior, I am worried about the impact of these confusing messages on the psyche of people in general, as well as on our patients.
Mental health impact
Soon after the United States went on lockdown in March 2020, I wrote about the likelihood of a pandemic of PTSD, anxiety, and depression that would occur in the wake of rising COVID-19 rates. Well, it happened.
Many people have felt a sense of existential despair, depression, and anxiety. As we head into year No. 3 of disruption of our daily lives – and face the loss of more than 825,000 Americans to COVID – we continue to navigate this uncertainty. And now we must deal with Omicron, a variant that is so highly transmissible that it is apparently able to, in some cases, evade two-dose regimens of mRNA vaccines, boosters, and immunity from past infections, according to a report from Imperial College London. Yet, we are being told by some that Omicron might be less severe, compared with other variants. I worry that this assessment is misleading. In that same report, the Imperial College said it “found no evidence” that Omicron is less virulent than Delta, based on the risk of hospitalization and symptom status.
Meanwhile, animal studies suggest that the Omicron variant might lead to less lung damage than previous variants. A preprint article that is being considered for publication by a Nature Portfolio journal suggests that hamsters and mice infected with the Omicron variant do not have as much lung damage as those infected with other variants. More data need to come in for us to get a true understanding of Omicron’s virulence and transmissibility. We should keep an eye on Israel, which is launching a clinical trial of a second booster, or fourth mRNA shot.
As clinicians, we should give our patients and other people with whom we come in contact a sense of hope. In addition to urging people to get boosters, let’s tell them to err on the side of safety when it comes to this pandemic. That means encouraging them to remain isolated for longer than 5 days – until they test negative for COVID. It also means encouraging patients to wear high-quality face masks while inside public spaces – even in the absence of mandates. I have found it heartbreaking to watch televised broadcasts of sporting events held at some stadiums across the country where masks are not being worn. This absence of face coverings is counterintuitive at a time when some Broadway shows are closing. Even the great Radio City Rockettes shut down their holiday shows early in December 2021 because of COVID.
And, as I’ve argued before, we must not give up on unvaccinated people. I have had success in changing the minds of a few patients and some acquaintances with gentle, respectful prodding and vaccine education.
I would also like to see public health principles implemented in our schools and colleges. To protect the health of our children and young adults, we must continue to be nimble – which means school districts should implement layered prevention strategies, as the CDC recommends. This includes not only encouraging eligible staff members and students to get vaccinated, but requiring face masks inside school facilities, maintaining a physical distance of at least 3 feet, “screening testing, ventilation, handwashing, and staying home when sick.”
Furthermore, in deciding whether schools should remain open or be closed after positive COVID cases are discovered, officials should look at the vaccine demographics of that particular school. For example, if 15% of students are vaccinated in one school and 70% are vaccinated in another, the judgment would be different. Of course, it’s clearly best for schools to remain open, but perhaps closing them temporarily – perhaps for a week or 10 days – should be on the table if infection rates reach a certain level.
Now that we know more and have the benefit of getting more than 200 million Americans fully vaccinated, we can be far more selective about closings and openings. An important part of our strategy must be to communicate honestly with the public about which measures are best for safety. As a key tenet of cognitive-behavioral therapy tells us, “all-or-nothing” thinking is not productive. That should also be the case with our approach to managing COVID-19.
We don’t know the future of the pandemic. Yes, it will end, and possibly COVID will become endemic – like the flu. However, in the meantime, in addition to promoting vaccinations and boosters, we must rigorously encourage our patients to follow public health standards of masking, social distancing, and closing down businesses – and schools – temporarily.
This pandemic has taken a horrendous mental health toll on all of us – especially our patients and frontline health care workers. I’ve spoken with numerous people who were anxious, depressed, and showed signs of PTSD in early 2020; after they got vaccinated, COVID spread diminished, and as public health protocols began to lift, so did their spirits. Clearly for some, the benefit of psychiatric/psychological care centering on the pandemic has proven invaluable. In some ways, the pandemic has brought to the surface the importance of mental health care and removed some of the stigma from mental illness. And that’s a good thing.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
The Centers for Disease Control and Prevention’s decision to shorten the length of isolation time for asymptomatic Americans with COVID-19, regardless of their vaccination status, to 5 days from 10 days is confusing. I hope the agency reconsiders this decision.
After all, one of the CDC’s key messages during this pandemic has been that even people with asymptomatic COVID who have been vaccinated and boosted can transmit the disease. So it seems to me that the Dec. 27, 2021, recommendation about shortening the isolation time for COVID-19–positive people, like the agency’s earlier guidance encouraging people who are vaccinated to stop wearing masks while in indoor settings, runs contrary to good public health principles.
As an expert in human behavior, I am worried about the impact of these confusing messages on the psyche of people in general, as well as on our patients.
Mental health impact
Soon after the United States went on lockdown in March 2020, I wrote about the likelihood of a pandemic of PTSD, anxiety, and depression that would occur in the wake of rising COVID-19 rates. Well, it happened.
Many people have felt a sense of existential despair, depression, and anxiety. As we head into year No. 3 of disruption of our daily lives – and face the loss of more than 825,000 Americans to COVID – we continue to navigate this uncertainty. And now we must deal with Omicron, a variant that is so highly transmissible that it is apparently able to, in some cases, evade two-dose regimens of mRNA vaccines, boosters, and immunity from past infections, according to a report from Imperial College London. Yet, we are being told by some that Omicron might be less severe, compared with other variants. I worry that this assessment is misleading. In that same report, the Imperial College said it “found no evidence” that Omicron is less virulent than Delta, based on the risk of hospitalization and symptom status.
Meanwhile, animal studies suggest that the Omicron variant might lead to less lung damage than previous variants. A preprint article that is being considered for publication by a Nature Portfolio journal suggests that hamsters and mice infected with the Omicron variant do not have as much lung damage as those infected with other variants. More data need to come in for us to get a true understanding of Omicron’s virulence and transmissibility. We should keep an eye on Israel, which is launching a clinical trial of a second booster, or fourth mRNA shot.
As clinicians, we should give our patients and other people with whom we come in contact a sense of hope. In addition to urging people to get boosters, let’s tell them to err on the side of safety when it comes to this pandemic. That means encouraging them to remain isolated for longer than 5 days – until they test negative for COVID. It also means encouraging patients to wear high-quality face masks while inside public spaces – even in the absence of mandates. I have found it heartbreaking to watch televised broadcasts of sporting events held at some stadiums across the country where masks are not being worn. This absence of face coverings is counterintuitive at a time when some Broadway shows are closing. Even the great Radio City Rockettes shut down their holiday shows early in December 2021 because of COVID.
And, as I’ve argued before, we must not give up on unvaccinated people. I have had success in changing the minds of a few patients and some acquaintances with gentle, respectful prodding and vaccine education.
I would also like to see public health principles implemented in our schools and colleges. To protect the health of our children and young adults, we must continue to be nimble – which means school districts should implement layered prevention strategies, as the CDC recommends. This includes not only encouraging eligible staff members and students to get vaccinated, but requiring face masks inside school facilities, maintaining a physical distance of at least 3 feet, “screening testing, ventilation, handwashing, and staying home when sick.”
Furthermore, in deciding whether schools should remain open or be closed after positive COVID cases are discovered, officials should look at the vaccine demographics of that particular school. For example, if 15% of students are vaccinated in one school and 70% are vaccinated in another, the judgment would be different. Of course, it’s clearly best for schools to remain open, but perhaps closing them temporarily – perhaps for a week or 10 days – should be on the table if infection rates reach a certain level.
Now that we know more and have the benefit of getting more than 200 million Americans fully vaccinated, we can be far more selective about closings and openings. An important part of our strategy must be to communicate honestly with the public about which measures are best for safety. As a key tenet of cognitive-behavioral therapy tells us, “all-or-nothing” thinking is not productive. That should also be the case with our approach to managing COVID-19.
We don’t know the future of the pandemic. Yes, it will end, and possibly COVID will become endemic – like the flu. However, in the meantime, in addition to promoting vaccinations and boosters, we must rigorously encourage our patients to follow public health standards of masking, social distancing, and closing down businesses – and schools – temporarily.
This pandemic has taken a horrendous mental health toll on all of us – especially our patients and frontline health care workers. I’ve spoken with numerous people who were anxious, depressed, and showed signs of PTSD in early 2020; after they got vaccinated, COVID spread diminished, and as public health protocols began to lift, so did their spirits. Clearly for some, the benefit of psychiatric/psychological care centering on the pandemic has proven invaluable. In some ways, the pandemic has brought to the surface the importance of mental health care and removed some of the stigma from mental illness. And that’s a good thing.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
The Centers for Disease Control and Prevention’s decision to shorten the length of isolation time for asymptomatic Americans with COVID-19, regardless of their vaccination status, to 5 days from 10 days is confusing. I hope the agency reconsiders this decision.
After all, one of the CDC’s key messages during this pandemic has been that even people with asymptomatic COVID who have been vaccinated and boosted can transmit the disease. So it seems to me that the Dec. 27, 2021, recommendation about shortening the isolation time for COVID-19–positive people, like the agency’s earlier guidance encouraging people who are vaccinated to stop wearing masks while in indoor settings, runs contrary to good public health principles.
As an expert in human behavior, I am worried about the impact of these confusing messages on the psyche of people in general, as well as on our patients.
Mental health impact
Soon after the United States went on lockdown in March 2020, I wrote about the likelihood of a pandemic of PTSD, anxiety, and depression that would occur in the wake of rising COVID-19 rates. Well, it happened.
Many people have felt a sense of existential despair, depression, and anxiety. As we head into year No. 3 of disruption of our daily lives – and face the loss of more than 825,000 Americans to COVID – we continue to navigate this uncertainty. And now we must deal with Omicron, a variant that is so highly transmissible that it is apparently able to, in some cases, evade two-dose regimens of mRNA vaccines, boosters, and immunity from past infections, according to a report from Imperial College London. Yet, we are being told by some that Omicron might be less severe, compared with other variants. I worry that this assessment is misleading. In that same report, the Imperial College said it “found no evidence” that Omicron is less virulent than Delta, based on the risk of hospitalization and symptom status.
Meanwhile, animal studies suggest that the Omicron variant might lead to less lung damage than previous variants. A preprint article that is being considered for publication by a Nature Portfolio journal suggests that hamsters and mice infected with the Omicron variant do not have as much lung damage as those infected with other variants. More data need to come in for us to get a true understanding of Omicron’s virulence and transmissibility. We should keep an eye on Israel, which is launching a clinical trial of a second booster, or fourth mRNA shot.
As clinicians, we should give our patients and other people with whom we come in contact a sense of hope. In addition to urging people to get boosters, let’s tell them to err on the side of safety when it comes to this pandemic. That means encouraging them to remain isolated for longer than 5 days – until they test negative for COVID. It also means encouraging patients to wear high-quality face masks while inside public spaces – even in the absence of mandates. I have found it heartbreaking to watch televised broadcasts of sporting events held at some stadiums across the country where masks are not being worn. This absence of face coverings is counterintuitive at a time when some Broadway shows are closing. Even the great Radio City Rockettes shut down their holiday shows early in December 2021 because of COVID.
And, as I’ve argued before, we must not give up on unvaccinated people. I have had success in changing the minds of a few patients and some acquaintances with gentle, respectful prodding and vaccine education.
I would also like to see public health principles implemented in our schools and colleges. To protect the health of our children and young adults, we must continue to be nimble – which means school districts should implement layered prevention strategies, as the CDC recommends. This includes not only encouraging eligible staff members and students to get vaccinated, but requiring face masks inside school facilities, maintaining a physical distance of at least 3 feet, “screening testing, ventilation, handwashing, and staying home when sick.”
Furthermore, in deciding whether schools should remain open or be closed after positive COVID cases are discovered, officials should look at the vaccine demographics of that particular school. For example, if 15% of students are vaccinated in one school and 70% are vaccinated in another, the judgment would be different. Of course, it’s clearly best for schools to remain open, but perhaps closing them temporarily – perhaps for a week or 10 days – should be on the table if infection rates reach a certain level.
Now that we know more and have the benefit of getting more than 200 million Americans fully vaccinated, we can be far more selective about closings and openings. An important part of our strategy must be to communicate honestly with the public about which measures are best for safety. As a key tenet of cognitive-behavioral therapy tells us, “all-or-nothing” thinking is not productive. That should also be the case with our approach to managing COVID-19.
We don’t know the future of the pandemic. Yes, it will end, and possibly COVID will become endemic – like the flu. However, in the meantime, in addition to promoting vaccinations and boosters, we must rigorously encourage our patients to follow public health standards of masking, social distancing, and closing down businesses – and schools – temporarily.
This pandemic has taken a horrendous mental health toll on all of us – especially our patients and frontline health care workers. I’ve spoken with numerous people who were anxious, depressed, and showed signs of PTSD in early 2020; after they got vaccinated, COVID spread diminished, and as public health protocols began to lift, so did their spirits. Clearly for some, the benefit of psychiatric/psychological care centering on the pandemic has proven invaluable. In some ways, the pandemic has brought to the surface the importance of mental health care and removed some of the stigma from mental illness. And that’s a good thing.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
FDA to review PDE4-inhibitor roflumilast for psoriasis
The , according to a statement from the manufacturer.
Roflumilast cream (also known as ARQ-151) is a small molecule inhibitor of PDE4, an enzyme that increases proinflammatory mediators and decreases anti-inflammatory mediators. PDE4 is an established treatment target in dermatology: The FDA approved PDE-4 inhibitor crisaborole (Eucrisa) as a topical treatment for mild to moderate atopic dermatitis in 2016, and an oral PDE-4 inhibitor, orismilast, is being studied for the treatment of plaque psoriasis.
Topical roflumilast, if approved, would be the first topical PDE4 inhibitor for psoriasis in particular, according to the Arcutis Biotherapeutics statement. The cream is designed for use on the entire body, including the face and sensitive intertriginous areas.
The NDA is based on data from a pair of phase 3 randomized, double-blind 8-week studies known as DERMIS 1 and DERMIS 2 (Trials of PDE4 Inhibition with Roflumilast for the Management of Plaque Psoriasis” One and Two) and a long-term phase 2b open-label study.
DERMIS 1 and DERMIS 2 were identical multinational, multicenter studies designed to assess the safety and efficacy of 0.3% roflumilast cream. In the studies, roflumilast met its primary endpoint and patients treated with it demonstrated an Investigator Global Assessment (IGA) success rate of 42.4% compared with 6.1% for the vehicle control (P < .0001), and 37.5% compared with 6.9% for the vehicle control (P < .0001), in the DERMIS 1 and 2 trials, respectively, according to Arcutis.
In the phase 2b study, the treatment effect lasted for 52-64 weeks. Roflumilast was well tolerated across the three studies.
Overall, the most common adverse events reported in the studies were diarrhea (3%), headache (2%), insomnia (1%), nausea (1%), upper respiratory tract infections (1%), and urinary tract infections (1%).
Roflumilast also showed statistically significant improvement compared to a vehicle on secondary endpoints including Intertriginous IGA (I-IGA) Success, Psoriasis Area Severity Index-75 (PASI-75), reductions in itch as measured by the Worst Itch-Numerical Rating Scale (WI-NRS), and patient perceptions of symptoms based on the Psoriasis Symptoms Diary (PSD).
The FDA has set a Prescription Drug User Fee Act (PDUFA) target action date of July 29, 2022, according to the manufacturer’s statement. An oral formulation of roflumilast was approved by the FDA in 2011, for reducing the risk of exacerbations of chronic obstructive pulmonary disease (COPD) in patients with severe COPD.
The , according to a statement from the manufacturer.
Roflumilast cream (also known as ARQ-151) is a small molecule inhibitor of PDE4, an enzyme that increases proinflammatory mediators and decreases anti-inflammatory mediators. PDE4 is an established treatment target in dermatology: The FDA approved PDE-4 inhibitor crisaborole (Eucrisa) as a topical treatment for mild to moderate atopic dermatitis in 2016, and an oral PDE-4 inhibitor, orismilast, is being studied for the treatment of plaque psoriasis.
Topical roflumilast, if approved, would be the first topical PDE4 inhibitor for psoriasis in particular, according to the Arcutis Biotherapeutics statement. The cream is designed for use on the entire body, including the face and sensitive intertriginous areas.
The NDA is based on data from a pair of phase 3 randomized, double-blind 8-week studies known as DERMIS 1 and DERMIS 2 (Trials of PDE4 Inhibition with Roflumilast for the Management of Plaque Psoriasis” One and Two) and a long-term phase 2b open-label study.
DERMIS 1 and DERMIS 2 were identical multinational, multicenter studies designed to assess the safety and efficacy of 0.3% roflumilast cream. In the studies, roflumilast met its primary endpoint and patients treated with it demonstrated an Investigator Global Assessment (IGA) success rate of 42.4% compared with 6.1% for the vehicle control (P < .0001), and 37.5% compared with 6.9% for the vehicle control (P < .0001), in the DERMIS 1 and 2 trials, respectively, according to Arcutis.
In the phase 2b study, the treatment effect lasted for 52-64 weeks. Roflumilast was well tolerated across the three studies.
Overall, the most common adverse events reported in the studies were diarrhea (3%), headache (2%), insomnia (1%), nausea (1%), upper respiratory tract infections (1%), and urinary tract infections (1%).
Roflumilast also showed statistically significant improvement compared to a vehicle on secondary endpoints including Intertriginous IGA (I-IGA) Success, Psoriasis Area Severity Index-75 (PASI-75), reductions in itch as measured by the Worst Itch-Numerical Rating Scale (WI-NRS), and patient perceptions of symptoms based on the Psoriasis Symptoms Diary (PSD).
The FDA has set a Prescription Drug User Fee Act (PDUFA) target action date of July 29, 2022, according to the manufacturer’s statement. An oral formulation of roflumilast was approved by the FDA in 2011, for reducing the risk of exacerbations of chronic obstructive pulmonary disease (COPD) in patients with severe COPD.
The , according to a statement from the manufacturer.
Roflumilast cream (also known as ARQ-151) is a small molecule inhibitor of PDE4, an enzyme that increases proinflammatory mediators and decreases anti-inflammatory mediators. PDE4 is an established treatment target in dermatology: The FDA approved PDE-4 inhibitor crisaborole (Eucrisa) as a topical treatment for mild to moderate atopic dermatitis in 2016, and an oral PDE-4 inhibitor, orismilast, is being studied for the treatment of plaque psoriasis.
Topical roflumilast, if approved, would be the first topical PDE4 inhibitor for psoriasis in particular, according to the Arcutis Biotherapeutics statement. The cream is designed for use on the entire body, including the face and sensitive intertriginous areas.
The NDA is based on data from a pair of phase 3 randomized, double-blind 8-week studies known as DERMIS 1 and DERMIS 2 (Trials of PDE4 Inhibition with Roflumilast for the Management of Plaque Psoriasis” One and Two) and a long-term phase 2b open-label study.
DERMIS 1 and DERMIS 2 were identical multinational, multicenter studies designed to assess the safety and efficacy of 0.3% roflumilast cream. In the studies, roflumilast met its primary endpoint and patients treated with it demonstrated an Investigator Global Assessment (IGA) success rate of 42.4% compared with 6.1% for the vehicle control (P < .0001), and 37.5% compared with 6.9% for the vehicle control (P < .0001), in the DERMIS 1 and 2 trials, respectively, according to Arcutis.
In the phase 2b study, the treatment effect lasted for 52-64 weeks. Roflumilast was well tolerated across the three studies.
Overall, the most common adverse events reported in the studies were diarrhea (3%), headache (2%), insomnia (1%), nausea (1%), upper respiratory tract infections (1%), and urinary tract infections (1%).
Roflumilast also showed statistically significant improvement compared to a vehicle on secondary endpoints including Intertriginous IGA (I-IGA) Success, Psoriasis Area Severity Index-75 (PASI-75), reductions in itch as measured by the Worst Itch-Numerical Rating Scale (WI-NRS), and patient perceptions of symptoms based on the Psoriasis Symptoms Diary (PSD).
The FDA has set a Prescription Drug User Fee Act (PDUFA) target action date of July 29, 2022, according to the manufacturer’s statement. An oral formulation of roflumilast was approved by the FDA in 2011, for reducing the risk of exacerbations of chronic obstructive pulmonary disease (COPD) in patients with severe COPD.
COVID-19, sure, but what else will we remember 2021 for?
who answered a recent Medscape Medical News poll. Perhaps no surprise there.
Coming in distant second, at 26%, was the new law requiring that patients be granted electronic access to clinical notes. The controversial Food and Drug Administration approval of aducanumab (Aduhelm, Biogen/Eisai) to treat Alzheimer’s disease was next, cited by almost 16% when asked what they would remember most about 2021.
Coming in at 10% or less were the permanent end to the Step 2 Clinical Skills test, the JAMA deputy editor resignation over controversial comments, and an “other” option that allowed for write-in responses.
It should be noted respondents could choose up to three answers to this and other questions in this survey, except for questions about profession and specialty.
Exciting news in 2021
Widespread availability of COVID-19 vaccines was the No. 1 response – chosen by 85% – when asked what medical news or events excited them in 2021.
FDA clearance of a 5-minute test for early dementia was selected by 22%, followed by almost 16% citing approval in October 2021 of abemaciclib (Verzenio, Lilly) “described as the first advance for early breast cancer in 20 years.”
The resignation of JAMA editors over a podcast on race rounded out the list of exciting medical news or events – coming in fourth at 11%. A total 5% of readers chose “other” and were asked to specify what news or events excited them in 2021.
A frustrating year?
Medscape also asked readers what medical news or events frustrated them in 2021. A majority, 81%, chose COVID-19 vaccine hesitancy or refusal. Almost one-third, 31%, chose the effect of climate change on health worldwide.
Some of the most memorable news or events of 2021 were also selected as frustrating by readers. For example, 22% were frustrated by the law requiring that patients be granted electronic access to clinical notes, followed by 19% who referred to the aducanumab approval in June. Furthermore, about 12% selected the JAMA resignations.
A shocking survey question
Asked what medical news or event from 2021 shocked readers, COVID-19 vaccine hesitancy or refusal was the most common answer, at 69%.
The U.S. Preventive Services Task Force ruling out aspirin in people over age 60 for primary prevention of cardiovascular disease shocked 36% of respondents.
Coming in third and fourth on the survey were the two JAMA editors resigning after a podcast on race, chosen by 19%, and the demise of the Step 2 Clinical Skills test, selected by 18%.
Interestingly, almost 96% of respondents were physicians. Less than 1% were residents, physician assistants, or nurses. Respondents also represented a wide range of specialties. From a list of 29 possible specialties, including “other,” family medicine, internal medicine, and psychiatry were the most common.
For more on the year that was 2021, see the Medscape Year in Medicine 2021: News That Made a Difference slideshow. Read Medscape’s full Year in Medicine report.
Wondering what stood out most to our readers in 2020? Here is a story about the results of a similar survey 1 year ago.
A version of this article first appeared on Medscape.com.
who answered a recent Medscape Medical News poll. Perhaps no surprise there.
Coming in distant second, at 26%, was the new law requiring that patients be granted electronic access to clinical notes. The controversial Food and Drug Administration approval of aducanumab (Aduhelm, Biogen/Eisai) to treat Alzheimer’s disease was next, cited by almost 16% when asked what they would remember most about 2021.
Coming in at 10% or less were the permanent end to the Step 2 Clinical Skills test, the JAMA deputy editor resignation over controversial comments, and an “other” option that allowed for write-in responses.
It should be noted respondents could choose up to three answers to this and other questions in this survey, except for questions about profession and specialty.
Exciting news in 2021
Widespread availability of COVID-19 vaccines was the No. 1 response – chosen by 85% – when asked what medical news or events excited them in 2021.
FDA clearance of a 5-minute test for early dementia was selected by 22%, followed by almost 16% citing approval in October 2021 of abemaciclib (Verzenio, Lilly) “described as the first advance for early breast cancer in 20 years.”
The resignation of JAMA editors over a podcast on race rounded out the list of exciting medical news or events – coming in fourth at 11%. A total 5% of readers chose “other” and were asked to specify what news or events excited them in 2021.
A frustrating year?
Medscape also asked readers what medical news or events frustrated them in 2021. A majority, 81%, chose COVID-19 vaccine hesitancy or refusal. Almost one-third, 31%, chose the effect of climate change on health worldwide.
Some of the most memorable news or events of 2021 were also selected as frustrating by readers. For example, 22% were frustrated by the law requiring that patients be granted electronic access to clinical notes, followed by 19% who referred to the aducanumab approval in June. Furthermore, about 12% selected the JAMA resignations.
A shocking survey question
Asked what medical news or event from 2021 shocked readers, COVID-19 vaccine hesitancy or refusal was the most common answer, at 69%.
The U.S. Preventive Services Task Force ruling out aspirin in people over age 60 for primary prevention of cardiovascular disease shocked 36% of respondents.
Coming in third and fourth on the survey were the two JAMA editors resigning after a podcast on race, chosen by 19%, and the demise of the Step 2 Clinical Skills test, selected by 18%.
Interestingly, almost 96% of respondents were physicians. Less than 1% were residents, physician assistants, or nurses. Respondents also represented a wide range of specialties. From a list of 29 possible specialties, including “other,” family medicine, internal medicine, and psychiatry were the most common.
For more on the year that was 2021, see the Medscape Year in Medicine 2021: News That Made a Difference slideshow. Read Medscape’s full Year in Medicine report.
Wondering what stood out most to our readers in 2020? Here is a story about the results of a similar survey 1 year ago.
A version of this article first appeared on Medscape.com.
who answered a recent Medscape Medical News poll. Perhaps no surprise there.
Coming in distant second, at 26%, was the new law requiring that patients be granted electronic access to clinical notes. The controversial Food and Drug Administration approval of aducanumab (Aduhelm, Biogen/Eisai) to treat Alzheimer’s disease was next, cited by almost 16% when asked what they would remember most about 2021.
Coming in at 10% or less were the permanent end to the Step 2 Clinical Skills test, the JAMA deputy editor resignation over controversial comments, and an “other” option that allowed for write-in responses.
It should be noted respondents could choose up to three answers to this and other questions in this survey, except for questions about profession and specialty.
Exciting news in 2021
Widespread availability of COVID-19 vaccines was the No. 1 response – chosen by 85% – when asked what medical news or events excited them in 2021.
FDA clearance of a 5-minute test for early dementia was selected by 22%, followed by almost 16% citing approval in October 2021 of abemaciclib (Verzenio, Lilly) “described as the first advance for early breast cancer in 20 years.”
The resignation of JAMA editors over a podcast on race rounded out the list of exciting medical news or events – coming in fourth at 11%. A total 5% of readers chose “other” and were asked to specify what news or events excited them in 2021.
A frustrating year?
Medscape also asked readers what medical news or events frustrated them in 2021. A majority, 81%, chose COVID-19 vaccine hesitancy or refusal. Almost one-third, 31%, chose the effect of climate change on health worldwide.
Some of the most memorable news or events of 2021 were also selected as frustrating by readers. For example, 22% were frustrated by the law requiring that patients be granted electronic access to clinical notes, followed by 19% who referred to the aducanumab approval in June. Furthermore, about 12% selected the JAMA resignations.
A shocking survey question
Asked what medical news or event from 2021 shocked readers, COVID-19 vaccine hesitancy or refusal was the most common answer, at 69%.
The U.S. Preventive Services Task Force ruling out aspirin in people over age 60 for primary prevention of cardiovascular disease shocked 36% of respondents.
Coming in third and fourth on the survey were the two JAMA editors resigning after a podcast on race, chosen by 19%, and the demise of the Step 2 Clinical Skills test, selected by 18%.
Interestingly, almost 96% of respondents were physicians. Less than 1% were residents, physician assistants, or nurses. Respondents also represented a wide range of specialties. From a list of 29 possible specialties, including “other,” family medicine, internal medicine, and psychiatry were the most common.
For more on the year that was 2021, see the Medscape Year in Medicine 2021: News That Made a Difference slideshow. Read Medscape’s full Year in Medicine report.
Wondering what stood out most to our readers in 2020? Here is a story about the results of a similar survey 1 year ago.
A version of this article first appeared on Medscape.com.