User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Coronavirus has infected over 2% of U.S. children
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.
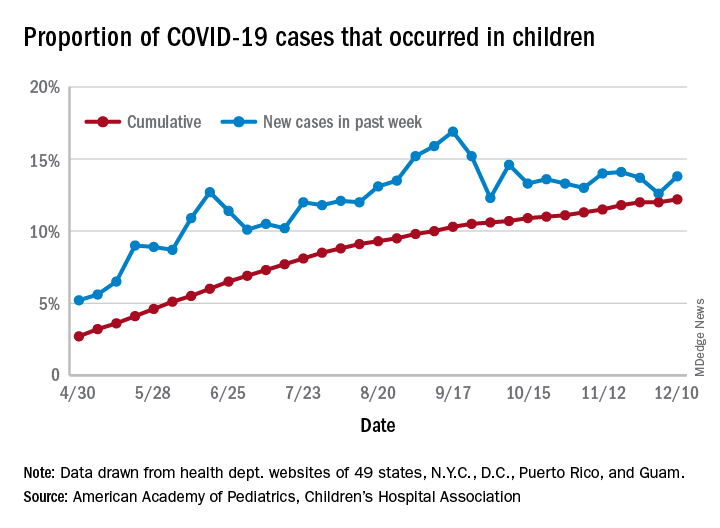
For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.

For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
After last week’s ever-so-slightly positive news, the COVID-19 numbers in children have gone back to their old ways.

For the week ending Dec. 10, there were 178,823 new COVID-19 cases reported in U.S. children, the highest weekly total yet during the pandemic. The number of new cases had dropped the week before after setting a new high of almost 154,000 during the last full week of November, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
A new weekly high has been seen in 9 of the last 10 weeks, during which time the weekly total of child cases has gone from just over 40,000 (week ending Oct. 8) to almost 179,000, the two organizations said.
and that 2.1% of all children (2,179 per 100,000) in the United States have been infected with the coronavirus, the AAP and CHA said in their weekly report, which includes health department data from 49 states (New York does not report age distribution), the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative proportion of 12.2% has been exceeded in 27 states, as well as Puerto Rico and Guam, with the highest coming in Wyoming (21.3%), South Carolina (18.1%), and Tennessee (18.1%) and the lowest in Florida (6.7%, but the state uses an age range of 0-14 years) and New Jersey (7.6%), the AAP/CHA data show.
In a separate statement, AAP president Sally Goza, MD, welcomed the approval of the Pfizer-BioNTech COVID-19 vaccine but noted that the “virus is at unprecedented levels in nearly every community in the U.S., and in many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight. I urge everyone to continue to practice social distancing, and wear masks or cloth face coverings, and get a flu shot, so we can protect the people we care about.”
Dr. Goza continued: “We applaud Pfizer-BioNTech for including children ages 12 through 17 in their clinical trials and we look forward to learning more about the data from children aged 12-15. We also want to acknowledge the discussion during the committee meeting on including 16- to 17-year-olds in the EUA [emergency-use authorization]. We believe that discussion underscores the need to keep expanding these trials to the pediatric population so we can collect robust data on this age group.”
[email protected]
PTSD, depression combo tied to high risk for early death in women
Middle-aged women with PTSD and comorbid depression have a nearly fourfold increased risk for early death from a variety of causes in comparison with their peers who do not have those conditions, new research shows.
“Women with more severe symptoms of depression and PTSD were more at risk, compared with those with fewer symptoms or women with symptoms of only PTSD or only depression,” lead investigator Andrea Roberts, PhD, Harvard School of Public Health, Boston, said in an interview.
Health care providers “should be aware that mental health is a critical component of overall health and is tightly entwined with physical health. Identifying and treating mental health issues should be a foundational part of general health practice,” said Dr. Roberts.
The study was published online Dec. 4 in JAMA Network Open.
Mental health fundamental to survival
The researchers studied more than 51,000 mostly White women from the Nurses Health Study II who were followed for 9 years (2008-2017). At baseline in 2008, the women were aged between 43 and 64 years (mean age, 53.3 years).
Women with high levels of PTSD (six or seven symptoms) and probable depression were nearly four times more likely to die during follow-up than their peers who did not have these conditions (hazard ratio, 3.8; 95% confidence interval, 2.65-5.45; P < .001).
With adjustment for health factors such as smoking and body mass index, women with a high level of PTSD and depression remained at increased risk for early death (HR, 3.11; 95% CI, 2.16-4.47; P < .001).
The risk for early death was also elevated among women with moderate PTSD (four or five symptoms) and depression (HR, 2.03; 95% CI, 1.35-3.03; P < .001) and women with subclinical PTSD and depression (HR, 2.85; 95% CI, 1.99-4.07; P < .001) compared with those who did not have PTSD or depression.
Among women with PTSD symptoms and depression, the incidence of death from nearly all major causes was increased, including death from cardiovascular disease, respiratory disease, type 2 diabetes, unintentional injury, suicide, and other causes.
“These findings provide further evidence that mental health is fundamental to physical health – and to our very survival. We ignore our emotional well-being at our peril,” senior author Karestan Koenen, PhD, said in a news release.
New knowledge
Commenting on the findings, Jennifer Sumner, PhD, said that it’s “critical to appreciate the physical health consequences of psychopathology in individuals who have experienced trauma. This study adds to a growing literature demonstrating that the impact extends far beyond emotional health.
“Furthermore, these results highlight the potential value of promoting healthy lifestyle changes in order to reduce the elevated mortality risk in trauma-exposed individuals with co-occurring PTSD and depression,” said Dr. Sumner, who is with the department of psychology, University of California, Los Angeles.
She noted that this study builds on other work that links PTSD to mortality in men.
“Most work on posttraumatic psychopathology and physical health has actually been conducted in predominantly male samples of veterans, so said Dr. Sumner.
“It’s also important to note that PTSD and depression are more prevalent in women than in men, so demonstrating these associations in women is particularly relevant,” she added.
Funding for the study was provided by the National Institutes of Heath. The authors disclosed no relevant financial relationships. Dr. Sumner has collaborated with the study investigators on prior studies.
A version of this article originally appeared on Medscape.com.
Middle-aged women with PTSD and comorbid depression have a nearly fourfold increased risk for early death from a variety of causes in comparison with their peers who do not have those conditions, new research shows.
“Women with more severe symptoms of depression and PTSD were more at risk, compared with those with fewer symptoms or women with symptoms of only PTSD or only depression,” lead investigator Andrea Roberts, PhD, Harvard School of Public Health, Boston, said in an interview.
Health care providers “should be aware that mental health is a critical component of overall health and is tightly entwined with physical health. Identifying and treating mental health issues should be a foundational part of general health practice,” said Dr. Roberts.
The study was published online Dec. 4 in JAMA Network Open.
Mental health fundamental to survival
The researchers studied more than 51,000 mostly White women from the Nurses Health Study II who were followed for 9 years (2008-2017). At baseline in 2008, the women were aged between 43 and 64 years (mean age, 53.3 years).
Women with high levels of PTSD (six or seven symptoms) and probable depression were nearly four times more likely to die during follow-up than their peers who did not have these conditions (hazard ratio, 3.8; 95% confidence interval, 2.65-5.45; P < .001).
With adjustment for health factors such as smoking and body mass index, women with a high level of PTSD and depression remained at increased risk for early death (HR, 3.11; 95% CI, 2.16-4.47; P < .001).
The risk for early death was also elevated among women with moderate PTSD (four or five symptoms) and depression (HR, 2.03; 95% CI, 1.35-3.03; P < .001) and women with subclinical PTSD and depression (HR, 2.85; 95% CI, 1.99-4.07; P < .001) compared with those who did not have PTSD or depression.
Among women with PTSD symptoms and depression, the incidence of death from nearly all major causes was increased, including death from cardiovascular disease, respiratory disease, type 2 diabetes, unintentional injury, suicide, and other causes.
“These findings provide further evidence that mental health is fundamental to physical health – and to our very survival. We ignore our emotional well-being at our peril,” senior author Karestan Koenen, PhD, said in a news release.
New knowledge
Commenting on the findings, Jennifer Sumner, PhD, said that it’s “critical to appreciate the physical health consequences of psychopathology in individuals who have experienced trauma. This study adds to a growing literature demonstrating that the impact extends far beyond emotional health.
“Furthermore, these results highlight the potential value of promoting healthy lifestyle changes in order to reduce the elevated mortality risk in trauma-exposed individuals with co-occurring PTSD and depression,” said Dr. Sumner, who is with the department of psychology, University of California, Los Angeles.
She noted that this study builds on other work that links PTSD to mortality in men.
“Most work on posttraumatic psychopathology and physical health has actually been conducted in predominantly male samples of veterans, so said Dr. Sumner.
“It’s also important to note that PTSD and depression are more prevalent in women than in men, so demonstrating these associations in women is particularly relevant,” she added.
Funding for the study was provided by the National Institutes of Heath. The authors disclosed no relevant financial relationships. Dr. Sumner has collaborated with the study investigators on prior studies.
A version of this article originally appeared on Medscape.com.
Middle-aged women with PTSD and comorbid depression have a nearly fourfold increased risk for early death from a variety of causes in comparison with their peers who do not have those conditions, new research shows.
“Women with more severe symptoms of depression and PTSD were more at risk, compared with those with fewer symptoms or women with symptoms of only PTSD or only depression,” lead investigator Andrea Roberts, PhD, Harvard School of Public Health, Boston, said in an interview.
Health care providers “should be aware that mental health is a critical component of overall health and is tightly entwined with physical health. Identifying and treating mental health issues should be a foundational part of general health practice,” said Dr. Roberts.
The study was published online Dec. 4 in JAMA Network Open.
Mental health fundamental to survival
The researchers studied more than 51,000 mostly White women from the Nurses Health Study II who were followed for 9 years (2008-2017). At baseline in 2008, the women were aged between 43 and 64 years (mean age, 53.3 years).
Women with high levels of PTSD (six or seven symptoms) and probable depression were nearly four times more likely to die during follow-up than their peers who did not have these conditions (hazard ratio, 3.8; 95% confidence interval, 2.65-5.45; P < .001).
With adjustment for health factors such as smoking and body mass index, women with a high level of PTSD and depression remained at increased risk for early death (HR, 3.11; 95% CI, 2.16-4.47; P < .001).
The risk for early death was also elevated among women with moderate PTSD (four or five symptoms) and depression (HR, 2.03; 95% CI, 1.35-3.03; P < .001) and women with subclinical PTSD and depression (HR, 2.85; 95% CI, 1.99-4.07; P < .001) compared with those who did not have PTSD or depression.
Among women with PTSD symptoms and depression, the incidence of death from nearly all major causes was increased, including death from cardiovascular disease, respiratory disease, type 2 diabetes, unintentional injury, suicide, and other causes.
“These findings provide further evidence that mental health is fundamental to physical health – and to our very survival. We ignore our emotional well-being at our peril,” senior author Karestan Koenen, PhD, said in a news release.
New knowledge
Commenting on the findings, Jennifer Sumner, PhD, said that it’s “critical to appreciate the physical health consequences of psychopathology in individuals who have experienced trauma. This study adds to a growing literature demonstrating that the impact extends far beyond emotional health.
“Furthermore, these results highlight the potential value of promoting healthy lifestyle changes in order to reduce the elevated mortality risk in trauma-exposed individuals with co-occurring PTSD and depression,” said Dr. Sumner, who is with the department of psychology, University of California, Los Angeles.
She noted that this study builds on other work that links PTSD to mortality in men.
“Most work on posttraumatic psychopathology and physical health has actually been conducted in predominantly male samples of veterans, so said Dr. Sumner.
“It’s also important to note that PTSD and depression are more prevalent in women than in men, so demonstrating these associations in women is particularly relevant,” she added.
Funding for the study was provided by the National Institutes of Heath. The authors disclosed no relevant financial relationships. Dr. Sumner has collaborated with the study investigators on prior studies.
A version of this article originally appeared on Medscape.com.
ADA 2021 standards address financial hardship in diabetes
For 2021, the American Diabetes Association offers new guidance on assessing patients’ financial and social barriers to care, especially given the COVID-19 pandemic, individualizing treatment of patients with type 2 diabetes, and use of diabetes technology.
As it does every year, the annual update incorporates new clinical information that has become available since the last guideline, with occasional revisions during the year as needed. “Standards of Medical Care in Diabetes – 2021,” was published online as a supplement to Diabetes Care.
The new standards advise that patients be assessed for food and housing insecurity, social support, and “cost-related medication nonadherence,” and those found to have difficulty referred to appropriate community resources.
“Clinicians need to be sensitive to the fact that patients may have very good reasons for not taking their medication, [as in] if they can’t afford it,” ADA chief science & medical officer Robert A. Gabbay, MD, PhD, said in an interview.
Dr. Gabbay noted that “a heightened awareness” of social determinants of health is weaved throughout the 2021 standards because of the pandemic, with information on the topic derived from a July 2020 joint consensus statement in Diabetes Care, endorsed by a number of other societies, as well as a November publication also in Diabetes Care.
“We made several recommendations that speak to social determinants of health, placing an emphasis on engaging in conversations around this subject and screening for related issues such as food insecurity that weren’t there previously,” he said.
“Screening tools are suggested. It helped us to have an in-depth scientific review of the literature to know the prevalence of this in people with diabetes. ... Having the science to put it in was a key step,” Dr. Gabbay noted.
Consider kidney, heart disease in type 2 treatment individualization
Recent data from trials such as CREDENCE and DAPA-HF, among others, have been added to inform the choice of pharmacologic treatment in patients with type 2 diabetes with comorbid diabetic kidney disease and chronic heart failure.
“ADA has been advocating individualization of treatment based on comorbidities for a while, but we’ve taken more steps in that direction. Beyond lifestyle for all individuals with type 2 diabetes, clinicians want to think early on about which comorbidities patients have and then think about the appropriate treatment based on that,” Dr. Gabbay said.
And for the third year in a row, the section on cardiovascular disease and risk management has been endorsed by the American College of Cardiology.
“All the things in that section are very much aligned with ACC and that’s been a great partnership,” Dr. Gabbay said.
Now, ADA is in discussions with other professional societies representing relevant specialties to create further such unified messages.
“What we all want to avoid is having multiple different guidelines. We want to speak with one voice and find common ground as much as possible. … It makes it much easier for clinicians to know what to do. That’s the goal of all this,” he noted.
Diabetes technology: The rise of CGM during pandemic and beyond
New information about continuous glucose monitoring (CGM) has been added to the diabetes technology section. Use of CGM is now recommended for anyone with diabetes who takes multiple daily injections or uses an insulin pump, regardless of age or diabetes type. The document provides expanded advice on use of time in range data for glycemic monitoring, particularly during the COVID-19 pandemic when remote monitoring is preferable.
Insurers are increasingly covering CGM for patients on insulin, but it’s far from universal. While the ultimate goal is to ensure access to CGM for everyone with diabetes, those treated with multiple daily insulin doses are the priority for now.
“Our hope is that as there’s greater evidence there will be more movement towards coverage. There are still so many people for whom it’s quite clear they would benefit because they’re on insulin but don’t have access to it. That’s an important area that ADA is advocating for, and it’s reflected in the standards of care,” Dr. Gabbay said.
In another technology-related revision, the term “blinded” CGM has been replaced with “professional CGM,” because clinic-based use of the devices can be “blinded” to the patient or monitored in real-time by both the patient and clinician. Also, a new recommendation has been added to address skin reactions associated with diabetes technology use.
Information about use of CGM in hospital settings during the COVID-19 pandemic has also been added in the technology section.
The COVID-19 pandemic comes up again in the section on vaccines.
“We mention that people with diabetes should be considered high priority [for COVID-19 vaccines], and that’s something that ADA is strongly advocating for because 40% of COVID-19 deaths have been in people with diabetes,” Dr. Gabbay said.
Dr. Gabbay reported being on the advisory boards of Onduo, Health Reveal, Vida Health, Lark, and Form Health.
A version of this article originally appeared on Medscape.com.
For 2021, the American Diabetes Association offers new guidance on assessing patients’ financial and social barriers to care, especially given the COVID-19 pandemic, individualizing treatment of patients with type 2 diabetes, and use of diabetes technology.
As it does every year, the annual update incorporates new clinical information that has become available since the last guideline, with occasional revisions during the year as needed. “Standards of Medical Care in Diabetes – 2021,” was published online as a supplement to Diabetes Care.
The new standards advise that patients be assessed for food and housing insecurity, social support, and “cost-related medication nonadherence,” and those found to have difficulty referred to appropriate community resources.
“Clinicians need to be sensitive to the fact that patients may have very good reasons for not taking their medication, [as in] if they can’t afford it,” ADA chief science & medical officer Robert A. Gabbay, MD, PhD, said in an interview.
Dr. Gabbay noted that “a heightened awareness” of social determinants of health is weaved throughout the 2021 standards because of the pandemic, with information on the topic derived from a July 2020 joint consensus statement in Diabetes Care, endorsed by a number of other societies, as well as a November publication also in Diabetes Care.
“We made several recommendations that speak to social determinants of health, placing an emphasis on engaging in conversations around this subject and screening for related issues such as food insecurity that weren’t there previously,” he said.
“Screening tools are suggested. It helped us to have an in-depth scientific review of the literature to know the prevalence of this in people with diabetes. ... Having the science to put it in was a key step,” Dr. Gabbay noted.
Consider kidney, heart disease in type 2 treatment individualization
Recent data from trials such as CREDENCE and DAPA-HF, among others, have been added to inform the choice of pharmacologic treatment in patients with type 2 diabetes with comorbid diabetic kidney disease and chronic heart failure.
“ADA has been advocating individualization of treatment based on comorbidities for a while, but we’ve taken more steps in that direction. Beyond lifestyle for all individuals with type 2 diabetes, clinicians want to think early on about which comorbidities patients have and then think about the appropriate treatment based on that,” Dr. Gabbay said.
And for the third year in a row, the section on cardiovascular disease and risk management has been endorsed by the American College of Cardiology.
“All the things in that section are very much aligned with ACC and that’s been a great partnership,” Dr. Gabbay said.
Now, ADA is in discussions with other professional societies representing relevant specialties to create further such unified messages.
“What we all want to avoid is having multiple different guidelines. We want to speak with one voice and find common ground as much as possible. … It makes it much easier for clinicians to know what to do. That’s the goal of all this,” he noted.
Diabetes technology: The rise of CGM during pandemic and beyond
New information about continuous glucose monitoring (CGM) has been added to the diabetes technology section. Use of CGM is now recommended for anyone with diabetes who takes multiple daily injections or uses an insulin pump, regardless of age or diabetes type. The document provides expanded advice on use of time in range data for glycemic monitoring, particularly during the COVID-19 pandemic when remote monitoring is preferable.
Insurers are increasingly covering CGM for patients on insulin, but it’s far from universal. While the ultimate goal is to ensure access to CGM for everyone with diabetes, those treated with multiple daily insulin doses are the priority for now.
“Our hope is that as there’s greater evidence there will be more movement towards coverage. There are still so many people for whom it’s quite clear they would benefit because they’re on insulin but don’t have access to it. That’s an important area that ADA is advocating for, and it’s reflected in the standards of care,” Dr. Gabbay said.
In another technology-related revision, the term “blinded” CGM has been replaced with “professional CGM,” because clinic-based use of the devices can be “blinded” to the patient or monitored in real-time by both the patient and clinician. Also, a new recommendation has been added to address skin reactions associated with diabetes technology use.
Information about use of CGM in hospital settings during the COVID-19 pandemic has also been added in the technology section.
The COVID-19 pandemic comes up again in the section on vaccines.
“We mention that people with diabetes should be considered high priority [for COVID-19 vaccines], and that’s something that ADA is strongly advocating for because 40% of COVID-19 deaths have been in people with diabetes,” Dr. Gabbay said.
Dr. Gabbay reported being on the advisory boards of Onduo, Health Reveal, Vida Health, Lark, and Form Health.
A version of this article originally appeared on Medscape.com.
For 2021, the American Diabetes Association offers new guidance on assessing patients’ financial and social barriers to care, especially given the COVID-19 pandemic, individualizing treatment of patients with type 2 diabetes, and use of diabetes technology.
As it does every year, the annual update incorporates new clinical information that has become available since the last guideline, with occasional revisions during the year as needed. “Standards of Medical Care in Diabetes – 2021,” was published online as a supplement to Diabetes Care.
The new standards advise that patients be assessed for food and housing insecurity, social support, and “cost-related medication nonadherence,” and those found to have difficulty referred to appropriate community resources.
“Clinicians need to be sensitive to the fact that patients may have very good reasons for not taking their medication, [as in] if they can’t afford it,” ADA chief science & medical officer Robert A. Gabbay, MD, PhD, said in an interview.
Dr. Gabbay noted that “a heightened awareness” of social determinants of health is weaved throughout the 2021 standards because of the pandemic, with information on the topic derived from a July 2020 joint consensus statement in Diabetes Care, endorsed by a number of other societies, as well as a November publication also in Diabetes Care.
“We made several recommendations that speak to social determinants of health, placing an emphasis on engaging in conversations around this subject and screening for related issues such as food insecurity that weren’t there previously,” he said.
“Screening tools are suggested. It helped us to have an in-depth scientific review of the literature to know the prevalence of this in people with diabetes. ... Having the science to put it in was a key step,” Dr. Gabbay noted.
Consider kidney, heart disease in type 2 treatment individualization
Recent data from trials such as CREDENCE and DAPA-HF, among others, have been added to inform the choice of pharmacologic treatment in patients with type 2 diabetes with comorbid diabetic kidney disease and chronic heart failure.
“ADA has been advocating individualization of treatment based on comorbidities for a while, but we’ve taken more steps in that direction. Beyond lifestyle for all individuals with type 2 diabetes, clinicians want to think early on about which comorbidities patients have and then think about the appropriate treatment based on that,” Dr. Gabbay said.
And for the third year in a row, the section on cardiovascular disease and risk management has been endorsed by the American College of Cardiology.
“All the things in that section are very much aligned with ACC and that’s been a great partnership,” Dr. Gabbay said.
Now, ADA is in discussions with other professional societies representing relevant specialties to create further such unified messages.
“What we all want to avoid is having multiple different guidelines. We want to speak with one voice and find common ground as much as possible. … It makes it much easier for clinicians to know what to do. That’s the goal of all this,” he noted.
Diabetes technology: The rise of CGM during pandemic and beyond
New information about continuous glucose monitoring (CGM) has been added to the diabetes technology section. Use of CGM is now recommended for anyone with diabetes who takes multiple daily injections or uses an insulin pump, regardless of age or diabetes type. The document provides expanded advice on use of time in range data for glycemic monitoring, particularly during the COVID-19 pandemic when remote monitoring is preferable.
Insurers are increasingly covering CGM for patients on insulin, but it’s far from universal. While the ultimate goal is to ensure access to CGM for everyone with diabetes, those treated with multiple daily insulin doses are the priority for now.
“Our hope is that as there’s greater evidence there will be more movement towards coverage. There are still so many people for whom it’s quite clear they would benefit because they’re on insulin but don’t have access to it. That’s an important area that ADA is advocating for, and it’s reflected in the standards of care,” Dr. Gabbay said.
In another technology-related revision, the term “blinded” CGM has been replaced with “professional CGM,” because clinic-based use of the devices can be “blinded” to the patient or monitored in real-time by both the patient and clinician. Also, a new recommendation has been added to address skin reactions associated with diabetes technology use.
Information about use of CGM in hospital settings during the COVID-19 pandemic has also been added in the technology section.
The COVID-19 pandemic comes up again in the section on vaccines.
“We mention that people with diabetes should be considered high priority [for COVID-19 vaccines], and that’s something that ADA is strongly advocating for because 40% of COVID-19 deaths have been in people with diabetes,” Dr. Gabbay said.
Dr. Gabbay reported being on the advisory boards of Onduo, Health Reveal, Vida Health, Lark, and Form Health.
A version of this article originally appeared on Medscape.com.
Sac/val heart failure benefit extends to diabetes patients
The beneficial effects of sacubitril/valsartan on reverse cardiac remodeling in patients with heart failure and reduced ejection fraction have been well established, but those benefits haven’t been as clearly demonstrated to carry over to HFrEF patients who also have type 2 diabetes mellitus (T2DM).
Now, a post-hoc analysis of a pivotal clinical trial reports that those benefits do extend to patients with HFrEF and T2DM.
“It’s really not about a Sophie’s choice of whether you give this or that drug in these patients,” said corresponding author Javed Butler, MD, MPH, MBA. “We really ought to be giving all of these drugs – the angiotensin receptor neprilysin inhibitors (ARNIs) and sodium-glucose transporter 2 (SGLT-2) inhibitors – to our patients for the best outcomes.”
The post-hoc analysis, published in JACC: Heart Failure, evaluated 361 patients with T2DM who were enrolled in the PROVE-HF trial of sac/val therapy for HF, published in JAMA.
PROVE-HF evaluated biomarkers, myocardial remodeling, and outcomes through a year of treatment with sac/val. The primary endpoint was the level of changes in natriuretic peptide (NT-proBNP) concentrations, left-ventricle ejection fraction (LVEF) and overall Kansas City Cardiomyopathy Questionnaire (KCCQ)-23 scores through 12 months of treatment.
The post hoc study reported that baseline NT-proBNP concentrations were higher in the DM patients (854 pg/mL vs. 706 pg/mL), but at 12 months those levels were 513 and 441 pg/mL, respectively.
LVEF changed similarly from baseline to 12 months in both groups: from 28.3% to 37% in the DM patients and from 28.1% to 38.34% in non-DM patients. Overall KCCQ-23 scores improved similarly in both groups, but longitudinal analyses found modestly higher gains in the T2DM group, 9.3 vs. 8.6 points (P = .07).
“The real reason I wanted to do this study is that I’m a huge fan of all the SGLT-2 inhibitors, and I’m very involved in those trials, and there is right now so much momentum behind SGLT-2 inhibitors that I don’t want people to forget that ARNI is still the base therapy for HF,” said Dr. Butler, chair of cardiovascular research and the department of medicine at the University of Mississippi in Jackson.
He noted that the size of the diabetes cohort in PROVE-HF “is a nonissue” for evaluating power of the post hoc analysis because it tracked key measures in the study population continuously at eight intervals over the 12 months.
The analysis further demonstrates the synergistic effects of ARNI and SGLT-2 inhibitors in patients with T2DM and HF that were also reported in the PARADIGM-HF study, Dr. Butler said.
“We have sort of moved on, saying that SGLT-2 inhibitors have a benefit on the heart, but the reverse is also true: ARNIs are still heart failure drugs, and we don’t think of them as diabetes drugs, but the PARADIGM-HF data showed that there was a substantial reduction in hemoglobin A1c in those who had diabetes,” he said.
The researchers noted that an absence of a control group may contribute to an overestimation of reverse cardiac remodeling in the T2DM patients, and that the PROVE-HF study wasn’t prospectively powered to delineate differences in how sac/val therapy affected patients with or without diabetes. “Future investigations seeking to evaluate differences by T2DM status after sacubitril/valsartan initiation may use our findings to plan prospective sample sizes,” the researchers wrote.
Dr. Butler disclosed financial relationships with Abbott, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, Eli Lilly, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana, StealthPeptide and Vifor. Lead author Muhammad Shahzeb Khan, MD, MSc, a professor at the University of Mississippi, has no relevant financial relationships to disclose.
SOURCE: Kahn MS et al. JACC: HF. 2020 Dec 9. doi: 10.1016/j.jchf.2020.09.014.
The beneficial effects of sacubitril/valsartan on reverse cardiac remodeling in patients with heart failure and reduced ejection fraction have been well established, but those benefits haven’t been as clearly demonstrated to carry over to HFrEF patients who also have type 2 diabetes mellitus (T2DM).
Now, a post-hoc analysis of a pivotal clinical trial reports that those benefits do extend to patients with HFrEF and T2DM.
“It’s really not about a Sophie’s choice of whether you give this or that drug in these patients,” said corresponding author Javed Butler, MD, MPH, MBA. “We really ought to be giving all of these drugs – the angiotensin receptor neprilysin inhibitors (ARNIs) and sodium-glucose transporter 2 (SGLT-2) inhibitors – to our patients for the best outcomes.”
The post-hoc analysis, published in JACC: Heart Failure, evaluated 361 patients with T2DM who were enrolled in the PROVE-HF trial of sac/val therapy for HF, published in JAMA.
PROVE-HF evaluated biomarkers, myocardial remodeling, and outcomes through a year of treatment with sac/val. The primary endpoint was the level of changes in natriuretic peptide (NT-proBNP) concentrations, left-ventricle ejection fraction (LVEF) and overall Kansas City Cardiomyopathy Questionnaire (KCCQ)-23 scores through 12 months of treatment.
The post hoc study reported that baseline NT-proBNP concentrations were higher in the DM patients (854 pg/mL vs. 706 pg/mL), but at 12 months those levels were 513 and 441 pg/mL, respectively.
LVEF changed similarly from baseline to 12 months in both groups: from 28.3% to 37% in the DM patients and from 28.1% to 38.34% in non-DM patients. Overall KCCQ-23 scores improved similarly in both groups, but longitudinal analyses found modestly higher gains in the T2DM group, 9.3 vs. 8.6 points (P = .07).
“The real reason I wanted to do this study is that I’m a huge fan of all the SGLT-2 inhibitors, and I’m very involved in those trials, and there is right now so much momentum behind SGLT-2 inhibitors that I don’t want people to forget that ARNI is still the base therapy for HF,” said Dr. Butler, chair of cardiovascular research and the department of medicine at the University of Mississippi in Jackson.
He noted that the size of the diabetes cohort in PROVE-HF “is a nonissue” for evaluating power of the post hoc analysis because it tracked key measures in the study population continuously at eight intervals over the 12 months.
The analysis further demonstrates the synergistic effects of ARNI and SGLT-2 inhibitors in patients with T2DM and HF that were also reported in the PARADIGM-HF study, Dr. Butler said.
“We have sort of moved on, saying that SGLT-2 inhibitors have a benefit on the heart, but the reverse is also true: ARNIs are still heart failure drugs, and we don’t think of them as diabetes drugs, but the PARADIGM-HF data showed that there was a substantial reduction in hemoglobin A1c in those who had diabetes,” he said.
The researchers noted that an absence of a control group may contribute to an overestimation of reverse cardiac remodeling in the T2DM patients, and that the PROVE-HF study wasn’t prospectively powered to delineate differences in how sac/val therapy affected patients with or without diabetes. “Future investigations seeking to evaluate differences by T2DM status after sacubitril/valsartan initiation may use our findings to plan prospective sample sizes,” the researchers wrote.
Dr. Butler disclosed financial relationships with Abbott, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, Eli Lilly, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana, StealthPeptide and Vifor. Lead author Muhammad Shahzeb Khan, MD, MSc, a professor at the University of Mississippi, has no relevant financial relationships to disclose.
SOURCE: Kahn MS et al. JACC: HF. 2020 Dec 9. doi: 10.1016/j.jchf.2020.09.014.
The beneficial effects of sacubitril/valsartan on reverse cardiac remodeling in patients with heart failure and reduced ejection fraction have been well established, but those benefits haven’t been as clearly demonstrated to carry over to HFrEF patients who also have type 2 diabetes mellitus (T2DM).
Now, a post-hoc analysis of a pivotal clinical trial reports that those benefits do extend to patients with HFrEF and T2DM.
“It’s really not about a Sophie’s choice of whether you give this or that drug in these patients,” said corresponding author Javed Butler, MD, MPH, MBA. “We really ought to be giving all of these drugs – the angiotensin receptor neprilysin inhibitors (ARNIs) and sodium-glucose transporter 2 (SGLT-2) inhibitors – to our patients for the best outcomes.”
The post-hoc analysis, published in JACC: Heart Failure, evaluated 361 patients with T2DM who were enrolled in the PROVE-HF trial of sac/val therapy for HF, published in JAMA.
PROVE-HF evaluated biomarkers, myocardial remodeling, and outcomes through a year of treatment with sac/val. The primary endpoint was the level of changes in natriuretic peptide (NT-proBNP) concentrations, left-ventricle ejection fraction (LVEF) and overall Kansas City Cardiomyopathy Questionnaire (KCCQ)-23 scores through 12 months of treatment.
The post hoc study reported that baseline NT-proBNP concentrations were higher in the DM patients (854 pg/mL vs. 706 pg/mL), but at 12 months those levels were 513 and 441 pg/mL, respectively.
LVEF changed similarly from baseline to 12 months in both groups: from 28.3% to 37% in the DM patients and from 28.1% to 38.34% in non-DM patients. Overall KCCQ-23 scores improved similarly in both groups, but longitudinal analyses found modestly higher gains in the T2DM group, 9.3 vs. 8.6 points (P = .07).
“The real reason I wanted to do this study is that I’m a huge fan of all the SGLT-2 inhibitors, and I’m very involved in those trials, and there is right now so much momentum behind SGLT-2 inhibitors that I don’t want people to forget that ARNI is still the base therapy for HF,” said Dr. Butler, chair of cardiovascular research and the department of medicine at the University of Mississippi in Jackson.
He noted that the size of the diabetes cohort in PROVE-HF “is a nonissue” for evaluating power of the post hoc analysis because it tracked key measures in the study population continuously at eight intervals over the 12 months.
The analysis further demonstrates the synergistic effects of ARNI and SGLT-2 inhibitors in patients with T2DM and HF that were also reported in the PARADIGM-HF study, Dr. Butler said.
“We have sort of moved on, saying that SGLT-2 inhibitors have a benefit on the heart, but the reverse is also true: ARNIs are still heart failure drugs, and we don’t think of them as diabetes drugs, but the PARADIGM-HF data showed that there was a substantial reduction in hemoglobin A1c in those who had diabetes,” he said.
The researchers noted that an absence of a control group may contribute to an overestimation of reverse cardiac remodeling in the T2DM patients, and that the PROVE-HF study wasn’t prospectively powered to delineate differences in how sac/val therapy affected patients with or without diabetes. “Future investigations seeking to evaluate differences by T2DM status after sacubitril/valsartan initiation may use our findings to plan prospective sample sizes,” the researchers wrote.
Dr. Butler disclosed financial relationships with Abbott, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, Eli Lilly, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana, StealthPeptide and Vifor. Lead author Muhammad Shahzeb Khan, MD, MSc, a professor at the University of Mississippi, has no relevant financial relationships to disclose.
SOURCE: Kahn MS et al. JACC: HF. 2020 Dec 9. doi: 10.1016/j.jchf.2020.09.014.
FROM JACC: HEART FAILURE
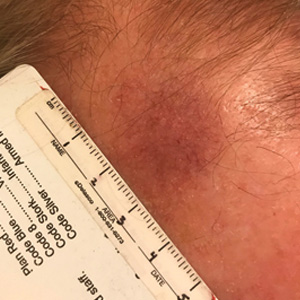
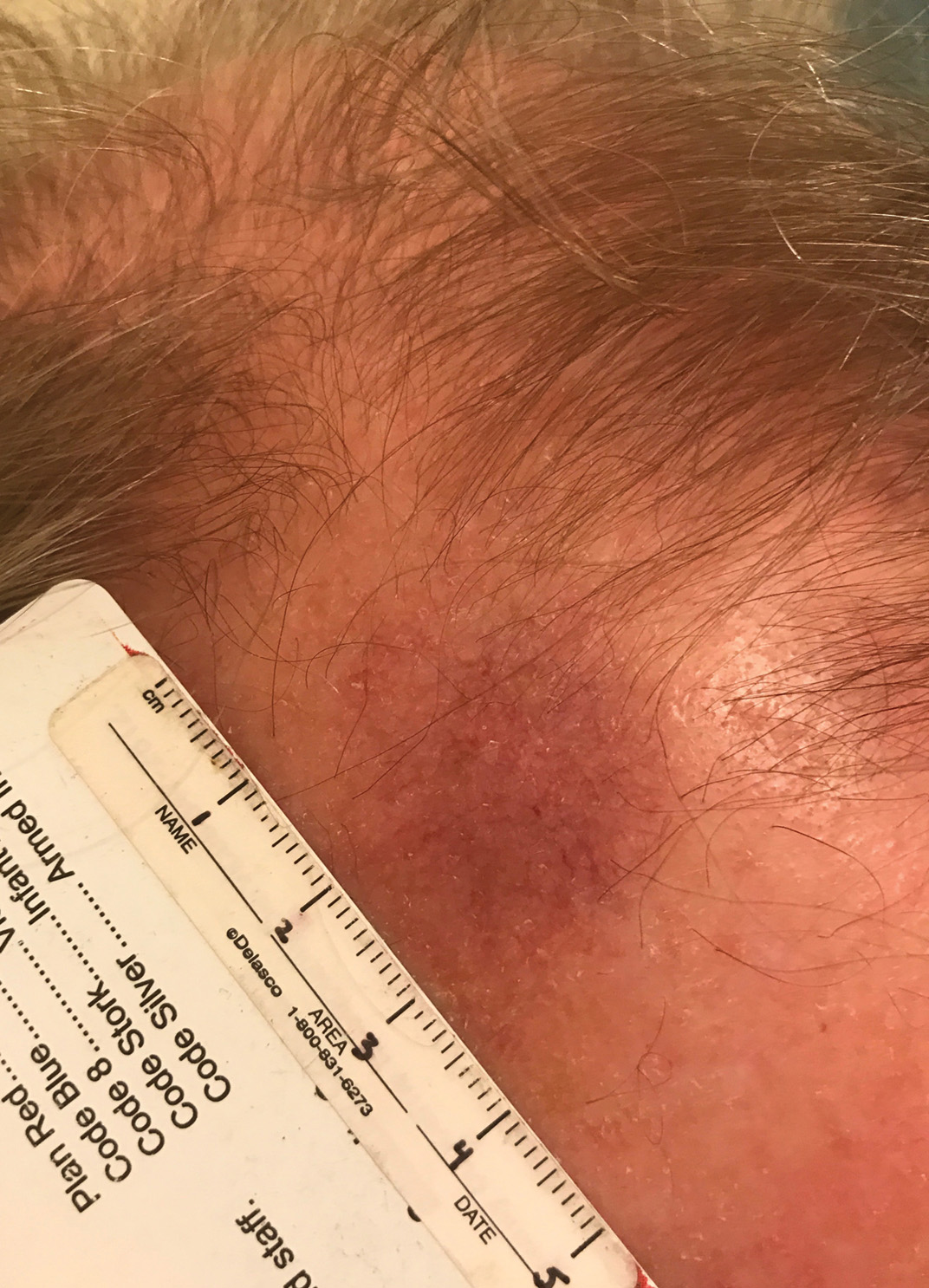
Telangiectatic Patch on the Forehead
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3
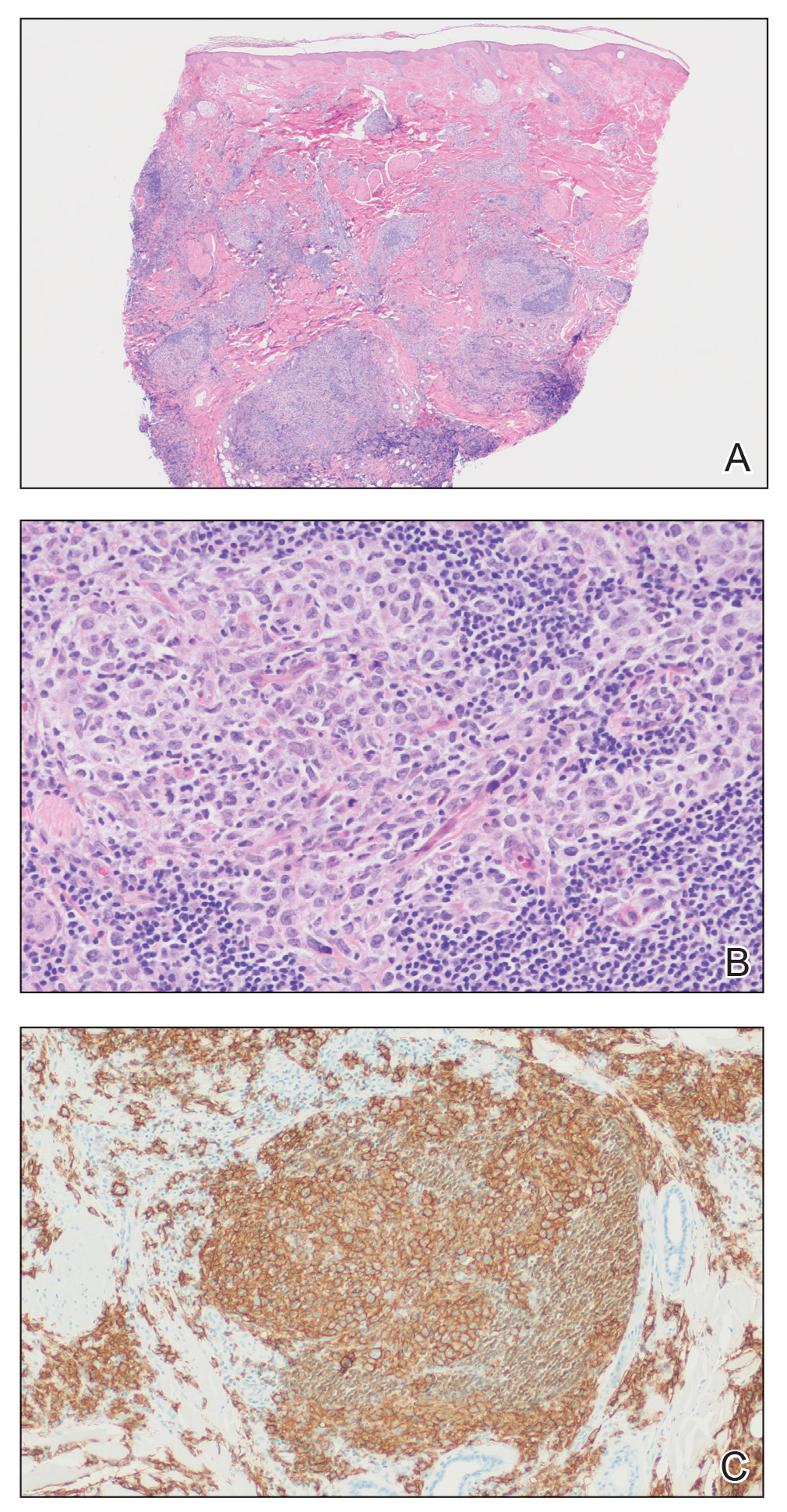
Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3

Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3

Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
FDA gives guidance on allergy, pregnancy concerns for Pfizer COVID vaccine
stating that it is safe for people with any history of allergies, but not for those who might have a known history of severe allergic reaction to any component of the vaccine.
The warning is included in the FDA’s information sheet for health care providers, but questions are arising as to whether the vaccine – which was authorized for emergency use by the FDA on Friday – should not be given to anyone with a history of allergies.
Sara Oliver, MD, an epidemic intelligence service officer with the Centers for Disease Control and Prevention reported at a Dec. 11 meeting of the agency’s Advisory Committee on Immunization Practices that two U.K. health care workers with a history of significant allergic reactions had a reaction to the Pfizer vaccine. A third health care worker with no history of allergies developed tachycardia, Dr. Oliver said.
“I want to reassure the public that although there were these few reactions in Great Britain, these were not seen in the larger clinical trial datasets,” said Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA, during a press briefing on Dec. 12.
The Pfizer vaccine “is one that we’re comfortable giving to patients who have had other allergic reactions besides those other than severe allergic reactions to a vaccine or one of its components,” he said.
Dr. Marks suggested that individuals let their physicians know about any history of allergic reactions. He also noted that the federal government will be supplying vaccine administration sites, at least initially, with epinephrine, diphenhydramine, hydrocortisone, and other medications needed to manage allergic reactions.
The FDA is going to monitor side effects such as allergic reactions very closely, “but I think we still need to learn more and that’s why we’re going to be taking precautions. We may have to modify things as we move forward,” said Dr. Marks.
Dr. Oliver said that on Dec. 12 the CDC convened an external panel with experience in vaccine safety, immunology, and allergies “to collate expert knowledge regarding possible cases,” and that the FDA is getting more data from U.K. regulatory authorities.
Pregnancy concerns
Agency officials had little to say, however, about the safety or efficacy of the vaccine for pregnant or breastfeeding women.
The FDA’s information to health care professionals noted that “available data on Pfizer-BioNTech COVID-19 vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.”
Additionally, the agency stated, “data are not available to assess the effects of Pfizer-BioNTech COVID-19 vaccine on the breastfed infant or on milk production/excretion.”
Dr. Marks said that, for pregnant women and people who are immunocompromised, “it will be something that providers will need to consider on an individual basis.” He suggested that individuals consult with physicians to weigh the potential benefits and potential risks.
“Certainly, COVID-19 in a pregnant woman is not a good thing,” Dr. Marks said.
An individual might decide to go ahead with vaccination. “But that’s not something we’re recommending, that’s something we’re leaving up to the individual,” he said.
A version of this article originally appeared on Medscape.com.
stating that it is safe for people with any history of allergies, but not for those who might have a known history of severe allergic reaction to any component of the vaccine.
The warning is included in the FDA’s information sheet for health care providers, but questions are arising as to whether the vaccine – which was authorized for emergency use by the FDA on Friday – should not be given to anyone with a history of allergies.
Sara Oliver, MD, an epidemic intelligence service officer with the Centers for Disease Control and Prevention reported at a Dec. 11 meeting of the agency’s Advisory Committee on Immunization Practices that two U.K. health care workers with a history of significant allergic reactions had a reaction to the Pfizer vaccine. A third health care worker with no history of allergies developed tachycardia, Dr. Oliver said.
“I want to reassure the public that although there were these few reactions in Great Britain, these were not seen in the larger clinical trial datasets,” said Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA, during a press briefing on Dec. 12.
The Pfizer vaccine “is one that we’re comfortable giving to patients who have had other allergic reactions besides those other than severe allergic reactions to a vaccine or one of its components,” he said.
Dr. Marks suggested that individuals let their physicians know about any history of allergic reactions. He also noted that the federal government will be supplying vaccine administration sites, at least initially, with epinephrine, diphenhydramine, hydrocortisone, and other medications needed to manage allergic reactions.
The FDA is going to monitor side effects such as allergic reactions very closely, “but I think we still need to learn more and that’s why we’re going to be taking precautions. We may have to modify things as we move forward,” said Dr. Marks.
Dr. Oliver said that on Dec. 12 the CDC convened an external panel with experience in vaccine safety, immunology, and allergies “to collate expert knowledge regarding possible cases,” and that the FDA is getting more data from U.K. regulatory authorities.
Pregnancy concerns
Agency officials had little to say, however, about the safety or efficacy of the vaccine for pregnant or breastfeeding women.
The FDA’s information to health care professionals noted that “available data on Pfizer-BioNTech COVID-19 vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.”
Additionally, the agency stated, “data are not available to assess the effects of Pfizer-BioNTech COVID-19 vaccine on the breastfed infant or on milk production/excretion.”
Dr. Marks said that, for pregnant women and people who are immunocompromised, “it will be something that providers will need to consider on an individual basis.” He suggested that individuals consult with physicians to weigh the potential benefits and potential risks.
“Certainly, COVID-19 in a pregnant woman is not a good thing,” Dr. Marks said.
An individual might decide to go ahead with vaccination. “But that’s not something we’re recommending, that’s something we’re leaving up to the individual,” he said.
A version of this article originally appeared on Medscape.com.
stating that it is safe for people with any history of allergies, but not for those who might have a known history of severe allergic reaction to any component of the vaccine.
The warning is included in the FDA’s information sheet for health care providers, but questions are arising as to whether the vaccine – which was authorized for emergency use by the FDA on Friday – should not be given to anyone with a history of allergies.
Sara Oliver, MD, an epidemic intelligence service officer with the Centers for Disease Control and Prevention reported at a Dec. 11 meeting of the agency’s Advisory Committee on Immunization Practices that two U.K. health care workers with a history of significant allergic reactions had a reaction to the Pfizer vaccine. A third health care worker with no history of allergies developed tachycardia, Dr. Oliver said.
“I want to reassure the public that although there were these few reactions in Great Britain, these were not seen in the larger clinical trial datasets,” said Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA, during a press briefing on Dec. 12.
The Pfizer vaccine “is one that we’re comfortable giving to patients who have had other allergic reactions besides those other than severe allergic reactions to a vaccine or one of its components,” he said.
Dr. Marks suggested that individuals let their physicians know about any history of allergic reactions. He also noted that the federal government will be supplying vaccine administration sites, at least initially, with epinephrine, diphenhydramine, hydrocortisone, and other medications needed to manage allergic reactions.
The FDA is going to monitor side effects such as allergic reactions very closely, “but I think we still need to learn more and that’s why we’re going to be taking precautions. We may have to modify things as we move forward,” said Dr. Marks.
Dr. Oliver said that on Dec. 12 the CDC convened an external panel with experience in vaccine safety, immunology, and allergies “to collate expert knowledge regarding possible cases,” and that the FDA is getting more data from U.K. regulatory authorities.
Pregnancy concerns
Agency officials had little to say, however, about the safety or efficacy of the vaccine for pregnant or breastfeeding women.
The FDA’s information to health care professionals noted that “available data on Pfizer-BioNTech COVID-19 vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.”
Additionally, the agency stated, “data are not available to assess the effects of Pfizer-BioNTech COVID-19 vaccine on the breastfed infant or on milk production/excretion.”
Dr. Marks said that, for pregnant women and people who are immunocompromised, “it will be something that providers will need to consider on an individual basis.” He suggested that individuals consult with physicians to weigh the potential benefits and potential risks.
“Certainly, COVID-19 in a pregnant woman is not a good thing,” Dr. Marks said.
An individual might decide to go ahead with vaccination. “But that’s not something we’re recommending, that’s something we’re leaving up to the individual,” he said.
A version of this article originally appeared on Medscape.com.
Twincretin ‘impressive’: Topline data from phase 3 trial in diabetes
Tirzepatide, a novel subcutaneously injected drug that acts via two related but separate pathways of glucose control, produced strikingly positive effects in top-line results from the phase 3, placebo-controlled study SURPASS-1 in 478 adults with type 2 diabetes, according to a Dec. 9 press release from the manufacturer, Lilly.
The tirzepatide molecule exerts agonist effects at both the glucose-dependent insulinotropic polypeptide (GIP) receptor and the glucagon-like peptide-1 (GLP-1) receptor, and has been called a “twincretin” for its activity encompassing two different incretins. Phase 2 trial results caused excitement, with one physician calling the data “unbelievable” when reported in 2018.
SURPASS-1 enrolled patients who were very early in the course of their disease, had on average relatively mild elevation in glucose levels, and few metabolic comorbidities. They took one of three doses of the agent (5, 10, or 15 mg) as monotherapy or placebo for 40 weeks.
Julio Rosenstock, MD, said in the Lilly statement: “The study took a bold approach in assessing A1c targets. Not only did nearly 90% of all participants taking tirzepatide meet the standard A1c goal of less than 7%, more than half taking the highest dose also achieved an A1c less than 5.7%, the level seen in people without diabetes.”
Dr. Rosenstock is principal investigator of SURPASS-1 and director of the Dallas Diabetes Research Center in Texas.
The discontinuation rate in the high-dose group was 21.5% compared with less than 10% in the two lower-dose cohorts. Lilly said most of the dropouts “were due to the pandemic and family or work reasons.” The dropout rate in the placebo group was 14.8%.
These data were not included in the efficacy analysis, however, which “muddied” the analysis somewhat, one pharma analyst told BioPharma Dive.
Commenting on the new trial data, Ildiko Lingvay, MD, said in an interview: “I am very impressed with these results,” which are “unprecedented for any glucose-lowering medication that has ever been tested.”
Dr. Lingvay, of the department of internal medicine/endocrinology, and medical director, office of clinical trials management at UT Southwestern Medical Center, Dallas, was not involved in the study.
She added that the weight loss seen with tirzepatide “is equally impressive with greater than 10% of body weight loss above placebo achieved within 40 weeks of treatment and without any directed weight loss efforts.”
If the agent is eventually approved, “I am enthusiastic about the prospect of having another very powerful tool to address both diabetes and obesity,” she added.
The full results of SURPASS-1 will be presented at the American Diabetes Association 81st Scientific Sessions and published in a peer-reviewed journal in 2021.
SURPASS-1 is one of eight phase 3 studies of the drug, including five registration studies and one large 12,500-patient cardiovascular outcomes trial.
Tirzepatide patients lost up to 20 lb, side effect profile ‘reassuring’
In the study, patients had been recently diagnosed with type 2 diabetes (average duration, 4.8 years) and 54% were treatment-naive. Average baseline hemoglobin A1c was 7.9% and mean weight was 85.9 kg (189 pounds).
Patients started on a subcutaneous injectable dose of tirzepatide of 2.5 mg per week, which was titrated up to the final dose – 5, 10, or 15 mg – in 2.5-mg increments given as monotherapy for 40 weeks and compared with placebo.
Treatment with tirzepatide resulted in average reductions in A1c from baseline that ranged from 1.87% to 2.07%, depending on the dose, and were all significant compared with an increase of 0.4% with placebo.
The percentage of patients whose A1c fell to normal levels (less than 5.7%) ranged from 30.5% to 51.7%, compared with 0.9% among controls, and again, was significant for all doses.
Patients treated with tirzepatide also lost weight. Average weight reductions after 40 weeks were significant and ranged from 7.0 to 9.5 kg (15-21 pounds) compared with an average loss of 0.7 kg (1.5 pounds) among patients who received placebo.
The most common adverse events were gastrointestinal-related and mild to moderate in severity, and usually occurred during dose escalation.
Dr. Lingvay said the safety data reported are “reassuring, with side effects in the anticipated range and comparable with other medications in the GLP-1 agonist class.”
And no hypoglycemic (level 2, < 54 mg/dL) events were reported, “which is impressive considering the overall glucose level achieved,” she noted.
“I am eagerly awaiting the results of the other studies within the SURPASS program and hope those will confirm these initial findings and provide additional safety and efficacy information in a wider range of patients with type 2 diabetes,” she concluded.
Dr. Lingvay has reported receiving research funding, advisory/consulting fees, and/or other support from Novo Nordisk, Eli Lilly, Sanofi, AstraZeneca, Boehringer Ingelheim, Janssen, Intercept, Intarcia, Target Pharma, Merck, Pfizer, Novartis, GI Dynamics, Mylan, MannKind, Valeritas, Bayer, and Zealand Pharma.
A version of this article originally appeared on Medscape.com.
Tirzepatide, a novel subcutaneously injected drug that acts via two related but separate pathways of glucose control, produced strikingly positive effects in top-line results from the phase 3, placebo-controlled study SURPASS-1 in 478 adults with type 2 diabetes, according to a Dec. 9 press release from the manufacturer, Lilly.
The tirzepatide molecule exerts agonist effects at both the glucose-dependent insulinotropic polypeptide (GIP) receptor and the glucagon-like peptide-1 (GLP-1) receptor, and has been called a “twincretin” for its activity encompassing two different incretins. Phase 2 trial results caused excitement, with one physician calling the data “unbelievable” when reported in 2018.
SURPASS-1 enrolled patients who were very early in the course of their disease, had on average relatively mild elevation in glucose levels, and few metabolic comorbidities. They took one of three doses of the agent (5, 10, or 15 mg) as monotherapy or placebo for 40 weeks.
Julio Rosenstock, MD, said in the Lilly statement: “The study took a bold approach in assessing A1c targets. Not only did nearly 90% of all participants taking tirzepatide meet the standard A1c goal of less than 7%, more than half taking the highest dose also achieved an A1c less than 5.7%, the level seen in people without diabetes.”
Dr. Rosenstock is principal investigator of SURPASS-1 and director of the Dallas Diabetes Research Center in Texas.
The discontinuation rate in the high-dose group was 21.5% compared with less than 10% in the two lower-dose cohorts. Lilly said most of the dropouts “were due to the pandemic and family or work reasons.” The dropout rate in the placebo group was 14.8%.
These data were not included in the efficacy analysis, however, which “muddied” the analysis somewhat, one pharma analyst told BioPharma Dive.
Commenting on the new trial data, Ildiko Lingvay, MD, said in an interview: “I am very impressed with these results,” which are “unprecedented for any glucose-lowering medication that has ever been tested.”
Dr. Lingvay, of the department of internal medicine/endocrinology, and medical director, office of clinical trials management at UT Southwestern Medical Center, Dallas, was not involved in the study.
She added that the weight loss seen with tirzepatide “is equally impressive with greater than 10% of body weight loss above placebo achieved within 40 weeks of treatment and without any directed weight loss efforts.”
If the agent is eventually approved, “I am enthusiastic about the prospect of having another very powerful tool to address both diabetes and obesity,” she added.
The full results of SURPASS-1 will be presented at the American Diabetes Association 81st Scientific Sessions and published in a peer-reviewed journal in 2021.
SURPASS-1 is one of eight phase 3 studies of the drug, including five registration studies and one large 12,500-patient cardiovascular outcomes trial.
Tirzepatide patients lost up to 20 lb, side effect profile ‘reassuring’
In the study, patients had been recently diagnosed with type 2 diabetes (average duration, 4.8 years) and 54% were treatment-naive. Average baseline hemoglobin A1c was 7.9% and mean weight was 85.9 kg (189 pounds).
Patients started on a subcutaneous injectable dose of tirzepatide of 2.5 mg per week, which was titrated up to the final dose – 5, 10, or 15 mg – in 2.5-mg increments given as monotherapy for 40 weeks and compared with placebo.
Treatment with tirzepatide resulted in average reductions in A1c from baseline that ranged from 1.87% to 2.07%, depending on the dose, and were all significant compared with an increase of 0.4% with placebo.
The percentage of patients whose A1c fell to normal levels (less than 5.7%) ranged from 30.5% to 51.7%, compared with 0.9% among controls, and again, was significant for all doses.
Patients treated with tirzepatide also lost weight. Average weight reductions after 40 weeks were significant and ranged from 7.0 to 9.5 kg (15-21 pounds) compared with an average loss of 0.7 kg (1.5 pounds) among patients who received placebo.
The most common adverse events were gastrointestinal-related and mild to moderate in severity, and usually occurred during dose escalation.
Dr. Lingvay said the safety data reported are “reassuring, with side effects in the anticipated range and comparable with other medications in the GLP-1 agonist class.”
And no hypoglycemic (level 2, < 54 mg/dL) events were reported, “which is impressive considering the overall glucose level achieved,” she noted.
“I am eagerly awaiting the results of the other studies within the SURPASS program and hope those will confirm these initial findings and provide additional safety and efficacy information in a wider range of patients with type 2 diabetes,” she concluded.
Dr. Lingvay has reported receiving research funding, advisory/consulting fees, and/or other support from Novo Nordisk, Eli Lilly, Sanofi, AstraZeneca, Boehringer Ingelheim, Janssen, Intercept, Intarcia, Target Pharma, Merck, Pfizer, Novartis, GI Dynamics, Mylan, MannKind, Valeritas, Bayer, and Zealand Pharma.
A version of this article originally appeared on Medscape.com.
Tirzepatide, a novel subcutaneously injected drug that acts via two related but separate pathways of glucose control, produced strikingly positive effects in top-line results from the phase 3, placebo-controlled study SURPASS-1 in 478 adults with type 2 diabetes, according to a Dec. 9 press release from the manufacturer, Lilly.
The tirzepatide molecule exerts agonist effects at both the glucose-dependent insulinotropic polypeptide (GIP) receptor and the glucagon-like peptide-1 (GLP-1) receptor, and has been called a “twincretin” for its activity encompassing two different incretins. Phase 2 trial results caused excitement, with one physician calling the data “unbelievable” when reported in 2018.
SURPASS-1 enrolled patients who were very early in the course of their disease, had on average relatively mild elevation in glucose levels, and few metabolic comorbidities. They took one of three doses of the agent (5, 10, or 15 mg) as monotherapy or placebo for 40 weeks.
Julio Rosenstock, MD, said in the Lilly statement: “The study took a bold approach in assessing A1c targets. Not only did nearly 90% of all participants taking tirzepatide meet the standard A1c goal of less than 7%, more than half taking the highest dose also achieved an A1c less than 5.7%, the level seen in people without diabetes.”
Dr. Rosenstock is principal investigator of SURPASS-1 and director of the Dallas Diabetes Research Center in Texas.
The discontinuation rate in the high-dose group was 21.5% compared with less than 10% in the two lower-dose cohorts. Lilly said most of the dropouts “were due to the pandemic and family or work reasons.” The dropout rate in the placebo group was 14.8%.
These data were not included in the efficacy analysis, however, which “muddied” the analysis somewhat, one pharma analyst told BioPharma Dive.
Commenting on the new trial data, Ildiko Lingvay, MD, said in an interview: “I am very impressed with these results,” which are “unprecedented for any glucose-lowering medication that has ever been tested.”
Dr. Lingvay, of the department of internal medicine/endocrinology, and medical director, office of clinical trials management at UT Southwestern Medical Center, Dallas, was not involved in the study.
She added that the weight loss seen with tirzepatide “is equally impressive with greater than 10% of body weight loss above placebo achieved within 40 weeks of treatment and without any directed weight loss efforts.”
If the agent is eventually approved, “I am enthusiastic about the prospect of having another very powerful tool to address both diabetes and obesity,” she added.
The full results of SURPASS-1 will be presented at the American Diabetes Association 81st Scientific Sessions and published in a peer-reviewed journal in 2021.
SURPASS-1 is one of eight phase 3 studies of the drug, including five registration studies and one large 12,500-patient cardiovascular outcomes trial.
Tirzepatide patients lost up to 20 lb, side effect profile ‘reassuring’
In the study, patients had been recently diagnosed with type 2 diabetes (average duration, 4.8 years) and 54% were treatment-naive. Average baseline hemoglobin A1c was 7.9% and mean weight was 85.9 kg (189 pounds).
Patients started on a subcutaneous injectable dose of tirzepatide of 2.5 mg per week, which was titrated up to the final dose – 5, 10, or 15 mg – in 2.5-mg increments given as monotherapy for 40 weeks and compared with placebo.
Treatment with tirzepatide resulted in average reductions in A1c from baseline that ranged from 1.87% to 2.07%, depending on the dose, and were all significant compared with an increase of 0.4% with placebo.
The percentage of patients whose A1c fell to normal levels (less than 5.7%) ranged from 30.5% to 51.7%, compared with 0.9% among controls, and again, was significant for all doses.
Patients treated with tirzepatide also lost weight. Average weight reductions after 40 weeks were significant and ranged from 7.0 to 9.5 kg (15-21 pounds) compared with an average loss of 0.7 kg (1.5 pounds) among patients who received placebo.
The most common adverse events were gastrointestinal-related and mild to moderate in severity, and usually occurred during dose escalation.
Dr. Lingvay said the safety data reported are “reassuring, with side effects in the anticipated range and comparable with other medications in the GLP-1 agonist class.”
And no hypoglycemic (level 2, < 54 mg/dL) events were reported, “which is impressive considering the overall glucose level achieved,” she noted.
“I am eagerly awaiting the results of the other studies within the SURPASS program and hope those will confirm these initial findings and provide additional safety and efficacy information in a wider range of patients with type 2 diabetes,” she concluded.
Dr. Lingvay has reported receiving research funding, advisory/consulting fees, and/or other support from Novo Nordisk, Eli Lilly, Sanofi, AstraZeneca, Boehringer Ingelheim, Janssen, Intercept, Intarcia, Target Pharma, Merck, Pfizer, Novartis, GI Dynamics, Mylan, MannKind, Valeritas, Bayer, and Zealand Pharma.
A version of this article originally appeared on Medscape.com.
Type 2 Diabetes 2021
This supplement to Clinician Reviews brings together key updates in the field of T2D to help care for patients who have not only T2D, but also other interconnected diseases.
Supplementary Materials:
Chapter 1: Evolution of Type 2 Diabetes Treatment
Plain Language Patient Summary:

Infographic:
Chapter 2: A Practical Approach to Managing Kidney Disease in Type 2 Diabetes
Plain Language Patient Summary:

Infographic:
Chapter 3: Heart Failure in Patients with Type 2 Diabetes
Plain Language Patient Summary:
Infographic:
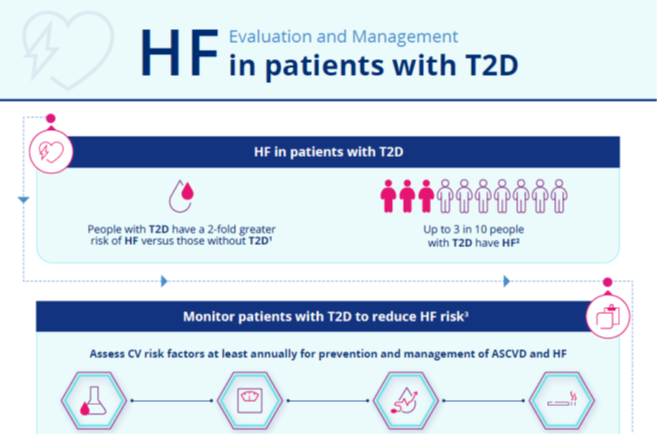
Chapter 4: Overcoming Therapeutic Inertia: Practical Approaches
Plain Language Patient Summary:
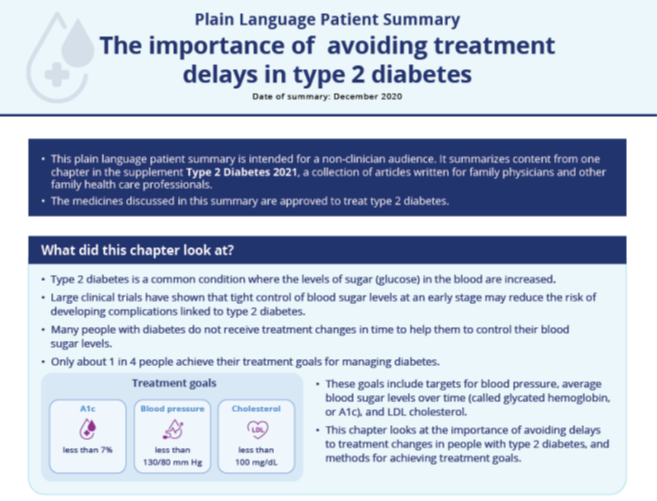
Infographic:
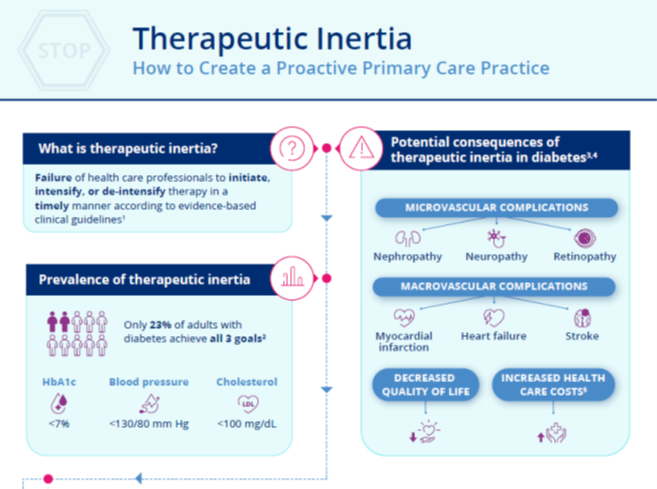
Supplemental Materials are joint copyright © 2020 Frontline Medical Communications and Boehringer Ingelheim Pharmaceuticals, Inc.
This supplement to Clinician Reviews brings together key updates in the field of T2D to help care for patients who have not only T2D, but also other interconnected diseases.
Supplementary Materials:
Chapter 1: Evolution of Type 2 Diabetes Treatment
Plain Language Patient Summary:

Infographic:

Chapter 2: A Practical Approach to Managing Kidney Disease in Type 2 Diabetes
Plain Language Patient Summary:

Infographic:

Chapter 3: Heart Failure in Patients with Type 2 Diabetes
Plain Language Patient Summary:

Infographic:

Chapter 4: Overcoming Therapeutic Inertia: Practical Approaches
Plain Language Patient Summary:

Infographic:

Supplemental Materials are joint copyright © 2020 Frontline Medical Communications and Boehringer Ingelheim Pharmaceuticals, Inc.
This supplement to Clinician Reviews brings together key updates in the field of T2D to help care for patients who have not only T2D, but also other interconnected diseases.
Supplementary Materials:
Chapter 1: Evolution of Type 2 Diabetes Treatment
Plain Language Patient Summary:

Infographic:

Chapter 2: A Practical Approach to Managing Kidney Disease in Type 2 Diabetes
Plain Language Patient Summary:

Infographic:

Chapter 3: Heart Failure in Patients with Type 2 Diabetes
Plain Language Patient Summary:

Infographic:

Chapter 4: Overcoming Therapeutic Inertia: Practical Approaches
Plain Language Patient Summary:

Infographic:

Supplemental Materials are joint copyright © 2020 Frontline Medical Communications and Boehringer Ingelheim Pharmaceuticals, Inc.
ACC/AHA update two atrial fibrillation performance measures
The American College of Cardiology and American Heart Association Task Force on Performance Measures have made two changes to performance measures for adults with atrial fibrillation or atrial flutter.
The 2020 Update to the 2016 ACC/AHA Clinical Performance and Quality Measures for Adults With Atrial Fibrillation or Atrial Flutter was published online Dec. 7 in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes. It was developed in collaboration with the Heart Rhythm Society.
Both performance measure changes were prompted by, and are in accordance with, the 2019 ACC/AHA/Heart Rhythm Society atrial fibrillation guideline focused update issued in January 2019, and reported by this news organization at that time.
The first change is the clarification that valvular atrial fibrillation is atrial fibrillation with either moderate or severe mitral stenosis or a mechanical heart valve. This change is incorporated into all the performance measures.
The second change, which only applies to the performance measure of anticoagulation prescribed, is the separation of a male and female threshold for the CHA2DS2-VASc score.
This threshold is now a score higher than 1 for men and higher than 2 for women, further demonstrating that the risk for stroke differs for men and women with atrial fibrillation or atrial flutter, the ACC/AHA noted in a press release.
“Successful implementation of these updated performance measures by clinicians and healthcare organizations will lead to quality improvement for adult patients with atrial fibrillation or atrial flutter,” they said.
A version of this article originally appeared on Medscape.com.
The American College of Cardiology and American Heart Association Task Force on Performance Measures have made two changes to performance measures for adults with atrial fibrillation or atrial flutter.
The 2020 Update to the 2016 ACC/AHA Clinical Performance and Quality Measures for Adults With Atrial Fibrillation or Atrial Flutter was published online Dec. 7 in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes. It was developed in collaboration with the Heart Rhythm Society.
Both performance measure changes were prompted by, and are in accordance with, the 2019 ACC/AHA/Heart Rhythm Society atrial fibrillation guideline focused update issued in January 2019, and reported by this news organization at that time.
The first change is the clarification that valvular atrial fibrillation is atrial fibrillation with either moderate or severe mitral stenosis or a mechanical heart valve. This change is incorporated into all the performance measures.
The second change, which only applies to the performance measure of anticoagulation prescribed, is the separation of a male and female threshold for the CHA2DS2-VASc score.
This threshold is now a score higher than 1 for men and higher than 2 for women, further demonstrating that the risk for stroke differs for men and women with atrial fibrillation or atrial flutter, the ACC/AHA noted in a press release.
“Successful implementation of these updated performance measures by clinicians and healthcare organizations will lead to quality improvement for adult patients with atrial fibrillation or atrial flutter,” they said.
A version of this article originally appeared on Medscape.com.
The American College of Cardiology and American Heart Association Task Force on Performance Measures have made two changes to performance measures for adults with atrial fibrillation or atrial flutter.
The 2020 Update to the 2016 ACC/AHA Clinical Performance and Quality Measures for Adults With Atrial Fibrillation or Atrial Flutter was published online Dec. 7 in the Journal of the American College of Cardiology and Circulation: Cardiovascular Quality and Outcomes. It was developed in collaboration with the Heart Rhythm Society.
Both performance measure changes were prompted by, and are in accordance with, the 2019 ACC/AHA/Heart Rhythm Society atrial fibrillation guideline focused update issued in January 2019, and reported by this news organization at that time.
The first change is the clarification that valvular atrial fibrillation is atrial fibrillation with either moderate or severe mitral stenosis or a mechanical heart valve. This change is incorporated into all the performance measures.
The second change, which only applies to the performance measure of anticoagulation prescribed, is the separation of a male and female threshold for the CHA2DS2-VASc score.
This threshold is now a score higher than 1 for men and higher than 2 for women, further demonstrating that the risk for stroke differs for men and women with atrial fibrillation or atrial flutter, the ACC/AHA noted in a press release.
“Successful implementation of these updated performance measures by clinicians and healthcare organizations will lead to quality improvement for adult patients with atrial fibrillation or atrial flutter,” they said.
A version of this article originally appeared on Medscape.com.
CDC panel recommends Pfizer’s COVID-19 vaccine for people 16 and over
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.