User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Tips and Techniques to Boost Colonoscopy Quality
This transcript has been edited for clarity.
When it comes to the use of colonoscopy to reduce the risk for cancer, quality is key.
There are a number of performance improvements we can make in our practices so that we can do better. This is evident in several recently published studies and a recent review article on the topic, which I’d like to profile for you; many of these key quality indicators you can implement now.
Even though it may take more time before they’re supported in the guidelines, you’ll see that the evidence behind these is extraordinarily strong.
Increasing the Adenoma Detection Rate
Certainly, we all do what we can to increase the adenoma detection rate (ADR).
However, at the moment, the nationally recommended benchmark is to achieve an ADR of 25%, which is inordinately low. The ADR rate reported in the GIQuIC registry data is closer to 39%, and in high-level detectors, it’s actually in the greater-than-50% range.
There’s no question that we can do more, and there are a number of ways to do that.
First, This may actually decrease your withdrawal time because you don’t spend so much time trying to face these folds.
In considering tools to aid ADR, don’t forget electronic chromoendoscopy (eg, narrow-band imaging).
There are a number of new artificial intelligence options out there as well, which have been reported to increase the ADR by approximately 10%. Of importance, this improvement even occurs among expert endoscopists.
There’s also important emerging data about ADR in fecal immunochemical test (FIT)–positive patients. FIT-positive status increases the ADR threshold by 15%-20%. This places you in an ADR range of approximately 50%, which is really the norm when screening patients that present for colonoscopy because of FIT positivity.
Adenoma Per Colonoscopy: A Possible ADR Substitute
Growing evidence supports the use of adenoma per colonoscopy (APC) as a substitute to ADR. This would allow you to record every adenoma and attribute it to that index colonoscopy.
A high-quality paper showed that the APC value should be around 0.6 to achieve the current ADR minimum threshold of 25%. Having the APC < 0.6 seems to be associated with an increased risk for residual polyp. Sessile serrated lesions also increased the hazard ratio for interval colorectal cancer. This was evaluated recently with data from the New Hampshire Colonoscopy Registry, which Dr Joseph Anderson has led for so long. They showed that 21% of endoscopists had an ADR of 25% or greater but still had APCs < 0.6.
Therefore, when it comes to remedial corrective work, doctors need to be reevaluated, retrained, and educated in the ways that they can incorporate this. The APC in high-level detectors is > 1.0.
APC may be something you want to consider using internally. It does require that you place each polyp into an individual jar, which can increase incremental cost. Nonetheless, there is clear evidence that APC positively changes outcomes.
Including Sessile Serrated Lesions in ADR Detectors
Unfortunately, some of the high-level ADR detectors aren’t so “high level” when it comes to detecting sessile serrated lesions. It’s not quite as concordant as we had previously thought.
Nonetheless, there are many adjunctive things you can do with sessile serrated lesions, including narrow-band imaging and chromoendoscopy.
When it comes to establishing a discriminant, the numbers should be 5%-6% if we’re going to set a quality ratio and an index. However, this is somewhat dependent on your pathologist because they have to read these correctly. Lesions that are ≥ 6 mm above the sigmoid colon and anything in the right colon should be evaluated really closely as a sessile serrated lesion.
I’ve had indications where the pathologist says the lesion is hyperplastic, to which I say, “I’m going to follow as a sessile serrated lesion.” This is because it’s apparent to me in the endoscopic appearance and the narrow-band imaging appearance that it was characteristic of sessile serrated lesions.
Best Practices in Bowel Preparations
The US Multi-Society Task Force recommends that adequate bowel preparation should occur in 85% or more of outpatients, and for the European Society for Gastrointestinal Endoscopy, it’s 90% or more.
I’ll pass along a tip I use in my patients undergoing bowel preparation: I make them aware that during this process, they want to see a clear, yellow, urine-like color to their stool. Otherwise, many patients will think if they’ve had some diarrhea, they don’t need to finish prep. Setting that expectation for them upfront is really important.
The nurses also should be aware of this because if there’s a murky brown effluent upon presentation for the colonoscopy, there’s a greater than 50% chance that they’re going to have had an inadequate preparation. In such cases, you would want to preempt the colonoscopy and perhaps send them out for a re-prep that day or bring them back for a rescheduled appointment.
Resection Considerations
There is substantial variation when it comes to lesion resection, which makes it an important quality indicator on which to focus: High-level detectors aren’t always high-level resectors.
There are two validated instruments that you can use to gauge the adequacy of resection. Those aren’t really ready for prime time in every practice, though they may be seen in fellowship programs.
The idea here is that you want to get a ≥ 2 mm margin for cold snare polypectomy in lesions 1-10 mm in size. This has been a challenge, as findings indicate we don’t do this that well.
Joseph Anderson and colleagues recently published a study using a 2-mm resection margin. They reported that this was only possible in approximately 28% of polyps. For a 1-mm margin, the rate was 84%.
We simply need to set clearer margins when setting our snare. Make sure you’re close enough to the polyp, push down on the snare, and get a good margin of tissue.
When the sample contracts are placed into the formalin, it’s not quite so simple to define the margin at the time of the surgical resection. This often requires an audit evaluation by the pathologist.
There are two other considerations regarding resection.
The first is about the referral for surgery. Referral should not occur for any benign lesions ascribed by your endoscopic advanced imaging techniques and classifications that are not thought to have intramucosal carcinoma. These should be referred to an expert endoscopic evaluation. If you can’t do it, then somebody else should. And you shouldn’t attempt it unless you can get it totally because resection of partially resected lesions is much more complicated. The European Society of Gastrointestinal Endoscopy says this applies to any benign lesion of any size, which I think really is the emerging standard of care. You should consider and offer that to the patient. It may require a referral for outside of your institution.
The second additional consideration is around the minimization of cold forceps for removal of polyps. The US Multi-Society Task Force says cold forceps shouldn’t be used for any lesions > 2 mm, whereas for the European Society of Gastrointestinal Endoscopy, it is > 3 mm. However, it’s still done very commonly in clinical practice. Nibbling the polyp is not an option. Cold snare is actually quicker, more effective, has better outcomes, and is something that you can bill for when you look at the coding.
In summary, there are a lot of things that we can do now to improve colonoscopy. Quality indicators continue to emerge with a compelling, excellent evidence base that strongly supports their use. Given that, I think most of these are actionable now, and it’s not necessary to wait for the guidelines to begin using them. I’d therefore challenge all of us to incorporate them in our continual efforts to do better.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When it comes to the use of colonoscopy to reduce the risk for cancer, quality is key.
There are a number of performance improvements we can make in our practices so that we can do better. This is evident in several recently published studies and a recent review article on the topic, which I’d like to profile for you; many of these key quality indicators you can implement now.
Even though it may take more time before they’re supported in the guidelines, you’ll see that the evidence behind these is extraordinarily strong.
Increasing the Adenoma Detection Rate
Certainly, we all do what we can to increase the adenoma detection rate (ADR).
However, at the moment, the nationally recommended benchmark is to achieve an ADR of 25%, which is inordinately low. The ADR rate reported in the GIQuIC registry data is closer to 39%, and in high-level detectors, it’s actually in the greater-than-50% range.
There’s no question that we can do more, and there are a number of ways to do that.
First, This may actually decrease your withdrawal time because you don’t spend so much time trying to face these folds.
In considering tools to aid ADR, don’t forget electronic chromoendoscopy (eg, narrow-band imaging).
There are a number of new artificial intelligence options out there as well, which have been reported to increase the ADR by approximately 10%. Of importance, this improvement even occurs among expert endoscopists.
There’s also important emerging data about ADR in fecal immunochemical test (FIT)–positive patients. FIT-positive status increases the ADR threshold by 15%-20%. This places you in an ADR range of approximately 50%, which is really the norm when screening patients that present for colonoscopy because of FIT positivity.
Adenoma Per Colonoscopy: A Possible ADR Substitute
Growing evidence supports the use of adenoma per colonoscopy (APC) as a substitute to ADR. This would allow you to record every adenoma and attribute it to that index colonoscopy.
A high-quality paper showed that the APC value should be around 0.6 to achieve the current ADR minimum threshold of 25%. Having the APC < 0.6 seems to be associated with an increased risk for residual polyp. Sessile serrated lesions also increased the hazard ratio for interval colorectal cancer. This was evaluated recently with data from the New Hampshire Colonoscopy Registry, which Dr Joseph Anderson has led for so long. They showed that 21% of endoscopists had an ADR of 25% or greater but still had APCs < 0.6.
Therefore, when it comes to remedial corrective work, doctors need to be reevaluated, retrained, and educated in the ways that they can incorporate this. The APC in high-level detectors is > 1.0.
APC may be something you want to consider using internally. It does require that you place each polyp into an individual jar, which can increase incremental cost. Nonetheless, there is clear evidence that APC positively changes outcomes.
Including Sessile Serrated Lesions in ADR Detectors
Unfortunately, some of the high-level ADR detectors aren’t so “high level” when it comes to detecting sessile serrated lesions. It’s not quite as concordant as we had previously thought.
Nonetheless, there are many adjunctive things you can do with sessile serrated lesions, including narrow-band imaging and chromoendoscopy.
When it comes to establishing a discriminant, the numbers should be 5%-6% if we’re going to set a quality ratio and an index. However, this is somewhat dependent on your pathologist because they have to read these correctly. Lesions that are ≥ 6 mm above the sigmoid colon and anything in the right colon should be evaluated really closely as a sessile serrated lesion.
I’ve had indications where the pathologist says the lesion is hyperplastic, to which I say, “I’m going to follow as a sessile serrated lesion.” This is because it’s apparent to me in the endoscopic appearance and the narrow-band imaging appearance that it was characteristic of sessile serrated lesions.
Best Practices in Bowel Preparations
The US Multi-Society Task Force recommends that adequate bowel preparation should occur in 85% or more of outpatients, and for the European Society for Gastrointestinal Endoscopy, it’s 90% or more.
I’ll pass along a tip I use in my patients undergoing bowel preparation: I make them aware that during this process, they want to see a clear, yellow, urine-like color to their stool. Otherwise, many patients will think if they’ve had some diarrhea, they don’t need to finish prep. Setting that expectation for them upfront is really important.
The nurses also should be aware of this because if there’s a murky brown effluent upon presentation for the colonoscopy, there’s a greater than 50% chance that they’re going to have had an inadequate preparation. In such cases, you would want to preempt the colonoscopy and perhaps send them out for a re-prep that day or bring them back for a rescheduled appointment.
Resection Considerations
There is substantial variation when it comes to lesion resection, which makes it an important quality indicator on which to focus: High-level detectors aren’t always high-level resectors.
There are two validated instruments that you can use to gauge the adequacy of resection. Those aren’t really ready for prime time in every practice, though they may be seen in fellowship programs.
The idea here is that you want to get a ≥ 2 mm margin for cold snare polypectomy in lesions 1-10 mm in size. This has been a challenge, as findings indicate we don’t do this that well.
Joseph Anderson and colleagues recently published a study using a 2-mm resection margin. They reported that this was only possible in approximately 28% of polyps. For a 1-mm margin, the rate was 84%.
We simply need to set clearer margins when setting our snare. Make sure you’re close enough to the polyp, push down on the snare, and get a good margin of tissue.
When the sample contracts are placed into the formalin, it’s not quite so simple to define the margin at the time of the surgical resection. This often requires an audit evaluation by the pathologist.
There are two other considerations regarding resection.
The first is about the referral for surgery. Referral should not occur for any benign lesions ascribed by your endoscopic advanced imaging techniques and classifications that are not thought to have intramucosal carcinoma. These should be referred to an expert endoscopic evaluation. If you can’t do it, then somebody else should. And you shouldn’t attempt it unless you can get it totally because resection of partially resected lesions is much more complicated. The European Society of Gastrointestinal Endoscopy says this applies to any benign lesion of any size, which I think really is the emerging standard of care. You should consider and offer that to the patient. It may require a referral for outside of your institution.
The second additional consideration is around the minimization of cold forceps for removal of polyps. The US Multi-Society Task Force says cold forceps shouldn’t be used for any lesions > 2 mm, whereas for the European Society of Gastrointestinal Endoscopy, it is > 3 mm. However, it’s still done very commonly in clinical practice. Nibbling the polyp is not an option. Cold snare is actually quicker, more effective, has better outcomes, and is something that you can bill for when you look at the coding.
In summary, there are a lot of things that we can do now to improve colonoscopy. Quality indicators continue to emerge with a compelling, excellent evidence base that strongly supports their use. Given that, I think most of these are actionable now, and it’s not necessary to wait for the guidelines to begin using them. I’d therefore challenge all of us to incorporate them in our continual efforts to do better.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When it comes to the use of colonoscopy to reduce the risk for cancer, quality is key.
There are a number of performance improvements we can make in our practices so that we can do better. This is evident in several recently published studies and a recent review article on the topic, which I’d like to profile for you; many of these key quality indicators you can implement now.
Even though it may take more time before they’re supported in the guidelines, you’ll see that the evidence behind these is extraordinarily strong.
Increasing the Adenoma Detection Rate
Certainly, we all do what we can to increase the adenoma detection rate (ADR).
However, at the moment, the nationally recommended benchmark is to achieve an ADR of 25%, which is inordinately low. The ADR rate reported in the GIQuIC registry data is closer to 39%, and in high-level detectors, it’s actually in the greater-than-50% range.
There’s no question that we can do more, and there are a number of ways to do that.
First, This may actually decrease your withdrawal time because you don’t spend so much time trying to face these folds.
In considering tools to aid ADR, don’t forget electronic chromoendoscopy (eg, narrow-band imaging).
There are a number of new artificial intelligence options out there as well, which have been reported to increase the ADR by approximately 10%. Of importance, this improvement even occurs among expert endoscopists.
There’s also important emerging data about ADR in fecal immunochemical test (FIT)–positive patients. FIT-positive status increases the ADR threshold by 15%-20%. This places you in an ADR range of approximately 50%, which is really the norm when screening patients that present for colonoscopy because of FIT positivity.
Adenoma Per Colonoscopy: A Possible ADR Substitute
Growing evidence supports the use of adenoma per colonoscopy (APC) as a substitute to ADR. This would allow you to record every adenoma and attribute it to that index colonoscopy.
A high-quality paper showed that the APC value should be around 0.6 to achieve the current ADR minimum threshold of 25%. Having the APC < 0.6 seems to be associated with an increased risk for residual polyp. Sessile serrated lesions also increased the hazard ratio for interval colorectal cancer. This was evaluated recently with data from the New Hampshire Colonoscopy Registry, which Dr Joseph Anderson has led for so long. They showed that 21% of endoscopists had an ADR of 25% or greater but still had APCs < 0.6.
Therefore, when it comes to remedial corrective work, doctors need to be reevaluated, retrained, and educated in the ways that they can incorporate this. The APC in high-level detectors is > 1.0.
APC may be something you want to consider using internally. It does require that you place each polyp into an individual jar, which can increase incremental cost. Nonetheless, there is clear evidence that APC positively changes outcomes.
Including Sessile Serrated Lesions in ADR Detectors
Unfortunately, some of the high-level ADR detectors aren’t so “high level” when it comes to detecting sessile serrated lesions. It’s not quite as concordant as we had previously thought.
Nonetheless, there are many adjunctive things you can do with sessile serrated lesions, including narrow-band imaging and chromoendoscopy.
When it comes to establishing a discriminant, the numbers should be 5%-6% if we’re going to set a quality ratio and an index. However, this is somewhat dependent on your pathologist because they have to read these correctly. Lesions that are ≥ 6 mm above the sigmoid colon and anything in the right colon should be evaluated really closely as a sessile serrated lesion.
I’ve had indications where the pathologist says the lesion is hyperplastic, to which I say, “I’m going to follow as a sessile serrated lesion.” This is because it’s apparent to me in the endoscopic appearance and the narrow-band imaging appearance that it was characteristic of sessile serrated lesions.
Best Practices in Bowel Preparations
The US Multi-Society Task Force recommends that adequate bowel preparation should occur in 85% or more of outpatients, and for the European Society for Gastrointestinal Endoscopy, it’s 90% or more.
I’ll pass along a tip I use in my patients undergoing bowel preparation: I make them aware that during this process, they want to see a clear, yellow, urine-like color to their stool. Otherwise, many patients will think if they’ve had some diarrhea, they don’t need to finish prep. Setting that expectation for them upfront is really important.
The nurses also should be aware of this because if there’s a murky brown effluent upon presentation for the colonoscopy, there’s a greater than 50% chance that they’re going to have had an inadequate preparation. In such cases, you would want to preempt the colonoscopy and perhaps send them out for a re-prep that day or bring them back for a rescheduled appointment.
Resection Considerations
There is substantial variation when it comes to lesion resection, which makes it an important quality indicator on which to focus: High-level detectors aren’t always high-level resectors.
There are two validated instruments that you can use to gauge the adequacy of resection. Those aren’t really ready for prime time in every practice, though they may be seen in fellowship programs.
The idea here is that you want to get a ≥ 2 mm margin for cold snare polypectomy in lesions 1-10 mm in size. This has been a challenge, as findings indicate we don’t do this that well.
Joseph Anderson and colleagues recently published a study using a 2-mm resection margin. They reported that this was only possible in approximately 28% of polyps. For a 1-mm margin, the rate was 84%.
We simply need to set clearer margins when setting our snare. Make sure you’re close enough to the polyp, push down on the snare, and get a good margin of tissue.
When the sample contracts are placed into the formalin, it’s not quite so simple to define the margin at the time of the surgical resection. This often requires an audit evaluation by the pathologist.
There are two other considerations regarding resection.
The first is about the referral for surgery. Referral should not occur for any benign lesions ascribed by your endoscopic advanced imaging techniques and classifications that are not thought to have intramucosal carcinoma. These should be referred to an expert endoscopic evaluation. If you can’t do it, then somebody else should. And you shouldn’t attempt it unless you can get it totally because resection of partially resected lesions is much more complicated. The European Society of Gastrointestinal Endoscopy says this applies to any benign lesion of any size, which I think really is the emerging standard of care. You should consider and offer that to the patient. It may require a referral for outside of your institution.
The second additional consideration is around the minimization of cold forceps for removal of polyps. The US Multi-Society Task Force says cold forceps shouldn’t be used for any lesions > 2 mm, whereas for the European Society of Gastrointestinal Endoscopy, it is > 3 mm. However, it’s still done very commonly in clinical practice. Nibbling the polyp is not an option. Cold snare is actually quicker, more effective, has better outcomes, and is something that you can bill for when you look at the coding.
In summary, there are a lot of things that we can do now to improve colonoscopy. Quality indicators continue to emerge with a compelling, excellent evidence base that strongly supports their use. Given that, I think most of these are actionable now, and it’s not necessary to wait for the guidelines to begin using them. I’d therefore challenge all of us to incorporate them in our continual efforts to do better.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He has disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Continued Caution Needed Combining Nitrates With ED Drugs
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.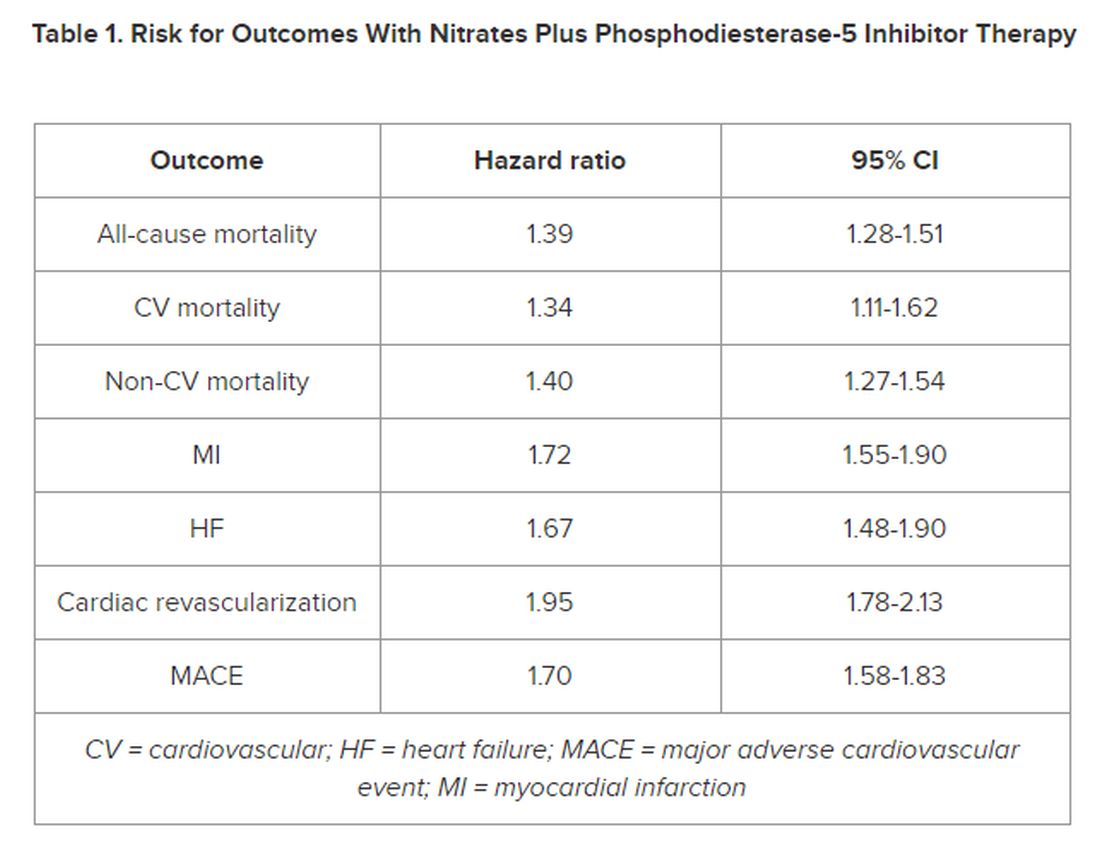
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
New research supports continued caution in prescribing a phosphodiesterase-5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in men with heart disease using nitrate medications.
In a large Swedish population study of men with stable coronary artery disease (CAD), the combined use of a PDE5i and nitrates was associated with a higher risk for cardiovascular (CV) morbidity and mortality.
“According to current recommendations, PDE5i are contraindicated in patients taking organic nitrates; however, in clinical practice, both are commonly prescribed, and concomitant use has increased,” first author Ylva Trolle Lagerros, MD, PhD, with Karolinska Institutet, Stockholm, Sweden, told this news organization.
and weigh the benefits of the medication against the possible increased risk for cardiovascular morbidity and mortality given by this combination,” Dr. Lagerros said.
The study was published online in the Journal of the American College of Cardiology (JACC).
The researchers used the Swedish Patient Register and the Prescribed Drug Register to assess the association between PDE5i treatment and CV outcomes in men with stable CAD treated with nitrate medication.
Among 55,777 men with a history of previous myocardial infarction (MI) or coronary revascularization who had filled at least two nitrate prescriptions (sublingual, oral, or both), 5710 also had at least two filled prescriptions of a PDE5i.
In multivariate-adjusted analysis, the combined use of PDE5i treatment with nitrates was associated with an increased relative risk for all studied outcomes, including all-cause mortality, CV and non-CV mortality, MI, heart failure, cardiac revascularization (hazard ratio), and major adverse cardiovascular events.
However, the number of events 28 days following a PDE5i prescription fill was “few, with lower incidence rates than in subjects taking nitrates only, indicating a low immediate risk for any event,” the authors noted in their article.
‘Common Bedfellows’
In a JACC editorial, Glenn N. Levine, MD, with Baylor College of Medicine, Houston, Texas, noted that, “ED and CAD are unfortunate, and all too common, bedfellows. But, as with most relationships, assuming proper precautions and care, they can coexist together for many years, perhaps even a lifetime.”
Dr. Levine noted that PDE5is are “reasonably safe” in most patients with stable CAD and only mild angina if not on chronic nitrate therapy. For those on chronic oral nitrate therapy, the use of PDE5is should continue to be regarded as “ill-advised at best and generally contraindicated.”
In some patients on oral nitrate therapy who want to use a PDE5i, particularly those who have undergone revascularization and have minimal or no angina, Dr. Levine said it may be reasonable to initiate a several-week trial of the nitrate therapy (or on a different class of antianginal therapy) and assess if the patient remains relatively angina-free.
In those patients with just rare exertional angina at generally higher levels of activity or those prescribed sublingual nitroglycerin “just in case,” it may be reasonable to prescribe PDE5i after a “clear and detailed” discussion with the patient of the risks for temporarily combining PDE5i and sublingual nitroglycerin.
Dr. Levine said these patients should be instructed not to take nitroglycerin within 24 hours of using a shorter-acting PDE5i and within 48 hours of using the longer-acting PDE5i tadalafil.
They should also be told to call 9-1-1 if angina develops during sexual intercourse and does not resolve upon cessation of such sexual activity, as well as to make medical personnel aware that they have recently used a PDE5i.
The study was funded by Region Stockholm, the Center for Innovative Medicine, and Karolinska Institutet. The researchers and editorial writer had declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
FDA Clears AI-Powered Device for Noninvasive Skin Cancer Testing
The handheld wireless tool, which was developed by Miami-based DermaSensor Inc., operates on battery power, uses spectroscopy and algorithms to evaluate skin lesions for potential cancer in a matter of seconds, and is intended for use by primary care physicians. After the device completes the scan of a lesion, a result of “investigate further” (positive result) suggests further evaluation through a referral to a dermatologist, while “monitor” (negative result) suggests that there is no immediate need for a referral to a dermatologist.
In a pivotal trial of the device that evaluated 224 high risk lesions at 18 primary care study sites in the United States and 4 in Australia, the device had an overall sensitivity of 95.5% for detecting malignancy.
In a more recent validation study funded by DermaSensor, investigators tested 333 lesions at four U.S. dermatology offices and found that the overall device sensitivity was 97.04%, with subgroup sensitivity of 96.67% for melanoma, 97.22% for basal cell carcinoma, and 97.01% for squamous cell carcinoma. Overall specificity of the device was 26.22%.
The study authors, led by Tallahassee, Fla.–based dermatologist Armand B. Cognetta Jr., MD, concluded that DermaSensor’s rapid clinical analysis of lesions “allows for its easy integration into clinical practice infrastructures. Proper use of this device may aid in the reduction of morbidity and mortality associated with skin cancer through expedited and enhanced detection and intervention.”
According to marketing material from the DermaSensor website, the device’s AI algorithm was developed and validated with more than 20,000 scans, composed of more than 4,000 benign and malignant lesions. In a statement about the clearance, the FDA emphasized that the device “should not be used as the sole diagnostic criterion nor to confirm a diagnosis of skin cancer.” The agency is requiring that the manufacturer “conduct additional post-market clinical validation performance testing of the DermaSensor device in patients from demographic groups representative of the U.S. population, including populations who had limited representation of melanomas in the premarket studies, due to their having a relatively low incidence of the disease.”
According to a spokesperson for DermaSensor, pricing for the device is based on a subscription model: $199 per month for five patients or $399 per month for unlimited use. DermaSensor is currently commercially available in Europe and Australia.
Asked to comment, Vishal A. Patel, MD, director of cutaneous oncology at the George Washington Cancer Center, Washington, said that the FDA clearance of DermaSensor highlights the growing appreciation of AI-driven diagnostic support for primary care providers and dermatologists. "Skin cancers are a growing epidemic in the US and the ability to accurately identify potential suspicious lesions without immediately reaching for the scalpel is invaluable," Patel told this news organization. He was not involved with DermSensor studies.
"Furthermore, this tool can help address the shortage of dermatologists and long wait times by helping primary care providers accurately risk-stratify patients and identify those who need to be seen immediately for potential biopsy and expert care," he added. "However, just like with any new technology, we must use caution to not overutilize this tool," which he said, could "lead to overdiagnosis and overtreatment of early or innocuous lesions that are better managed with empiric field treatments."
Dr. Cognetta was a paid investigator for the study.
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
The handheld wireless tool, which was developed by Miami-based DermaSensor Inc., operates on battery power, uses spectroscopy and algorithms to evaluate skin lesions for potential cancer in a matter of seconds, and is intended for use by primary care physicians. After the device completes the scan of a lesion, a result of “investigate further” (positive result) suggests further evaluation through a referral to a dermatologist, while “monitor” (negative result) suggests that there is no immediate need for a referral to a dermatologist.
In a pivotal trial of the device that evaluated 224 high risk lesions at 18 primary care study sites in the United States and 4 in Australia, the device had an overall sensitivity of 95.5% for detecting malignancy.
In a more recent validation study funded by DermaSensor, investigators tested 333 lesions at four U.S. dermatology offices and found that the overall device sensitivity was 97.04%, with subgroup sensitivity of 96.67% for melanoma, 97.22% for basal cell carcinoma, and 97.01% for squamous cell carcinoma. Overall specificity of the device was 26.22%.
The study authors, led by Tallahassee, Fla.–based dermatologist Armand B. Cognetta Jr., MD, concluded that DermaSensor’s rapid clinical analysis of lesions “allows for its easy integration into clinical practice infrastructures. Proper use of this device may aid in the reduction of morbidity and mortality associated with skin cancer through expedited and enhanced detection and intervention.”
According to marketing material from the DermaSensor website, the device’s AI algorithm was developed and validated with more than 20,000 scans, composed of more than 4,000 benign and malignant lesions. In a statement about the clearance, the FDA emphasized that the device “should not be used as the sole diagnostic criterion nor to confirm a diagnosis of skin cancer.” The agency is requiring that the manufacturer “conduct additional post-market clinical validation performance testing of the DermaSensor device in patients from demographic groups representative of the U.S. population, including populations who had limited representation of melanomas in the premarket studies, due to their having a relatively low incidence of the disease.”
According to a spokesperson for DermaSensor, pricing for the device is based on a subscription model: $199 per month for five patients or $399 per month for unlimited use. DermaSensor is currently commercially available in Europe and Australia.
Asked to comment, Vishal A. Patel, MD, director of cutaneous oncology at the George Washington Cancer Center, Washington, said that the FDA clearance of DermaSensor highlights the growing appreciation of AI-driven diagnostic support for primary care providers and dermatologists. "Skin cancers are a growing epidemic in the US and the ability to accurately identify potential suspicious lesions without immediately reaching for the scalpel is invaluable," Patel told this news organization. He was not involved with DermSensor studies.
"Furthermore, this tool can help address the shortage of dermatologists and long wait times by helping primary care providers accurately risk-stratify patients and identify those who need to be seen immediately for potential biopsy and expert care," he added. "However, just like with any new technology, we must use caution to not overutilize this tool," which he said, could "lead to overdiagnosis and overtreatment of early or innocuous lesions that are better managed with empiric field treatments."
Dr. Cognetta was a paid investigator for the study.
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
The handheld wireless tool, which was developed by Miami-based DermaSensor Inc., operates on battery power, uses spectroscopy and algorithms to evaluate skin lesions for potential cancer in a matter of seconds, and is intended for use by primary care physicians. After the device completes the scan of a lesion, a result of “investigate further” (positive result) suggests further evaluation through a referral to a dermatologist, while “monitor” (negative result) suggests that there is no immediate need for a referral to a dermatologist.
In a pivotal trial of the device that evaluated 224 high risk lesions at 18 primary care study sites in the United States and 4 in Australia, the device had an overall sensitivity of 95.5% for detecting malignancy.
In a more recent validation study funded by DermaSensor, investigators tested 333 lesions at four U.S. dermatology offices and found that the overall device sensitivity was 97.04%, with subgroup sensitivity of 96.67% for melanoma, 97.22% for basal cell carcinoma, and 97.01% for squamous cell carcinoma. Overall specificity of the device was 26.22%.
The study authors, led by Tallahassee, Fla.–based dermatologist Armand B. Cognetta Jr., MD, concluded that DermaSensor’s rapid clinical analysis of lesions “allows for its easy integration into clinical practice infrastructures. Proper use of this device may aid in the reduction of morbidity and mortality associated with skin cancer through expedited and enhanced detection and intervention.”
According to marketing material from the DermaSensor website, the device’s AI algorithm was developed and validated with more than 20,000 scans, composed of more than 4,000 benign and malignant lesions. In a statement about the clearance, the FDA emphasized that the device “should not be used as the sole diagnostic criterion nor to confirm a diagnosis of skin cancer.” The agency is requiring that the manufacturer “conduct additional post-market clinical validation performance testing of the DermaSensor device in patients from demographic groups representative of the U.S. population, including populations who had limited representation of melanomas in the premarket studies, due to their having a relatively low incidence of the disease.”
According to a spokesperson for DermaSensor, pricing for the device is based on a subscription model: $199 per month for five patients or $399 per month for unlimited use. DermaSensor is currently commercially available in Europe and Australia.
Asked to comment, Vishal A. Patel, MD, director of cutaneous oncology at the George Washington Cancer Center, Washington, said that the FDA clearance of DermaSensor highlights the growing appreciation of AI-driven diagnostic support for primary care providers and dermatologists. "Skin cancers are a growing epidemic in the US and the ability to accurately identify potential suspicious lesions without immediately reaching for the scalpel is invaluable," Patel told this news organization. He was not involved with DermSensor studies.
"Furthermore, this tool can help address the shortage of dermatologists and long wait times by helping primary care providers accurately risk-stratify patients and identify those who need to be seen immediately for potential biopsy and expert care," he added. "However, just like with any new technology, we must use caution to not overutilize this tool," which he said, could "lead to overdiagnosis and overtreatment of early or innocuous lesions that are better managed with empiric field treatments."
Dr. Cognetta was a paid investigator for the study.
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
Direct Measurement of T3 Is Likely Vital, Say Researchers
To assess thyroid function, clinicians typically determine blood levels of thyroid-stimulating hormone (TSH) and thyroxine (T4) and do not directly measure triiodothyronine (T3).
However, linking socioeconomic forces, human biology, and aging,” researchers reported.
The study, with lead author Ralph I. Lawton, an MD-PhD student at Harvard Medical School, Boston, was published online on January 4, 2024, in Proceedings of the National Academy of Sciences. In this national sample of healthy adults older than 20, free T3 was negatively related to household income. At age 40 and older, free T3 was significantly linked to being employed. Among adults from age 51 to 80, free T3 levels were inversely related to mortality.
“It is important to note that the more typically measured biomarkers for thyroid function (T4 and TSH) are poorly linked to free T3 levels,” Dr. Lawton and colleagues wrote.
The ratio of TSH:T4 explained only 1.7% of variation in T3 levels, they noted, which suggested that TSH and T4 may not be accurate surrogates of free T3. Thus, “direct measurement of free T3 is likely vital to properly stratify the effects of HPT-axis variation,” they maintained. “Improved methods for measurement [of T3] and further investigation of the role of free T3 in clinical conditions may be high yield,” according to the researchers.
Further studies of the relationship between the action of deiodinase in converting T4 to T3 and social forces and aging could elucidate critical biology. “New therapeutics targeting T3/T4 ratios,” they suggested, “may be more beneficial for a significant cohort of older individuals [with hypothyroidism] currently treated with levothyroxine [(LT4)].”
‘Cherry on Top’
“I think this is a really important paper,” thyroidology expert Antonio Bianco, MD, PhD, professor of medicine, The University of Chicago, who was not involved with this research, told this news organization.
His research group and others “have slowly been providing evidence of the relevance of measuring T3 levels,” he said. “This paper is sort of a cherry on top. It is really the most recent strong evidence that looking at T3 levels in plasma is really important. We need to do a better job at developing a much more precise assay for T3,” he said, “because now we know T3 is a very important parameter that endocrinologists should be looking for. We know that about 20 million Americans have hypothyroidism, and are being treated with T4 (levothyroxine),” Dr. Bianco continued.
“As endocrinologists, we believe that if you treat someone with levothyroxine, you normalize T3 levels. However, many times, with standard of care with levothyroxine, T3 levels are not normalized.”
Larger Variations in T3
Thyroid hormones (TSH, free T4, and free T3) influence many aspects of human physiology and behavior, but it is not known how variations in these levels in a population free of thyroid disease are associated with mortality and socioeconomic factors.
The researchers analyzed data from 7626 adults over age 20 up to age 80 who participated in the National Health and Nutrition Examination Survey during 2007-2012 and had blood tests to determine TSH, free T4, and free T3 and were not being actively treated for thyroid conditions. Among the 3603 older adults (over age 50), there were 981 deaths up until the end of 2019. TSH levels were not linked to mortality, but increasing levels of free T3 and decreasing levels of free T4 were protective for mortality (hazard ratios, 0.88 and 1.26, respectively; P < .01).
There was a robust negative relationship between household income and employment and free T3 but no relationship between these factors and T4. There was a much larger variation across age in levels of free T3 than in levels of TSH or free T4. Free T4 and free T3 diverged at older ages, when free T4 increased and free T3 continually decreased.
Older adults with symptoms of hypothyroidism may have high free T4 levels despite low free T3, the researchers noted.
“In a nonclinical sample representative of the US population,” they summarized, “we find that free T3 is much more strongly related to all domains studied — age, sex, seasonality, household income, employment, and longevity — than the other molecules of the HPT axis.”
This work was supported by the Burroughs Wellcome Fund Career at Scientific Interface award, a Brain and Behavior Research Foundation young investigator award, the Howard Hughes Medical Institute, the National Institute of Aging, and the National Institute of General Medical Sciences. Dr. Bianco served as a consultant for AbbVie, Avion Pharmaceuticals, Synthonics, Synthion, and Thyron and recently published the book Rethinking Hypothyroidism: Why Treatment Must Change and What Patients Can Do.
A version of this article appeared on Medscape.com.
To assess thyroid function, clinicians typically determine blood levels of thyroid-stimulating hormone (TSH) and thyroxine (T4) and do not directly measure triiodothyronine (T3).
However, linking socioeconomic forces, human biology, and aging,” researchers reported.
The study, with lead author Ralph I. Lawton, an MD-PhD student at Harvard Medical School, Boston, was published online on January 4, 2024, in Proceedings of the National Academy of Sciences. In this national sample of healthy adults older than 20, free T3 was negatively related to household income. At age 40 and older, free T3 was significantly linked to being employed. Among adults from age 51 to 80, free T3 levels were inversely related to mortality.
“It is important to note that the more typically measured biomarkers for thyroid function (T4 and TSH) are poorly linked to free T3 levels,” Dr. Lawton and colleagues wrote.
The ratio of TSH:T4 explained only 1.7% of variation in T3 levels, they noted, which suggested that TSH and T4 may not be accurate surrogates of free T3. Thus, “direct measurement of free T3 is likely vital to properly stratify the effects of HPT-axis variation,” they maintained. “Improved methods for measurement [of T3] and further investigation of the role of free T3 in clinical conditions may be high yield,” according to the researchers.
Further studies of the relationship between the action of deiodinase in converting T4 to T3 and social forces and aging could elucidate critical biology. “New therapeutics targeting T3/T4 ratios,” they suggested, “may be more beneficial for a significant cohort of older individuals [with hypothyroidism] currently treated with levothyroxine [(LT4)].”
‘Cherry on Top’
“I think this is a really important paper,” thyroidology expert Antonio Bianco, MD, PhD, professor of medicine, The University of Chicago, who was not involved with this research, told this news organization.
His research group and others “have slowly been providing evidence of the relevance of measuring T3 levels,” he said. “This paper is sort of a cherry on top. It is really the most recent strong evidence that looking at T3 levels in plasma is really important. We need to do a better job at developing a much more precise assay for T3,” he said, “because now we know T3 is a very important parameter that endocrinologists should be looking for. We know that about 20 million Americans have hypothyroidism, and are being treated with T4 (levothyroxine),” Dr. Bianco continued.
“As endocrinologists, we believe that if you treat someone with levothyroxine, you normalize T3 levels. However, many times, with standard of care with levothyroxine, T3 levels are not normalized.”
Larger Variations in T3
Thyroid hormones (TSH, free T4, and free T3) influence many aspects of human physiology and behavior, but it is not known how variations in these levels in a population free of thyroid disease are associated with mortality and socioeconomic factors.
The researchers analyzed data from 7626 adults over age 20 up to age 80 who participated in the National Health and Nutrition Examination Survey during 2007-2012 and had blood tests to determine TSH, free T4, and free T3 and were not being actively treated for thyroid conditions. Among the 3603 older adults (over age 50), there were 981 deaths up until the end of 2019. TSH levels were not linked to mortality, but increasing levels of free T3 and decreasing levels of free T4 were protective for mortality (hazard ratios, 0.88 and 1.26, respectively; P < .01).
There was a robust negative relationship between household income and employment and free T3 but no relationship between these factors and T4. There was a much larger variation across age in levels of free T3 than in levels of TSH or free T4. Free T4 and free T3 diverged at older ages, when free T4 increased and free T3 continually decreased.
Older adults with symptoms of hypothyroidism may have high free T4 levels despite low free T3, the researchers noted.
“In a nonclinical sample representative of the US population,” they summarized, “we find that free T3 is much more strongly related to all domains studied — age, sex, seasonality, household income, employment, and longevity — than the other molecules of the HPT axis.”
This work was supported by the Burroughs Wellcome Fund Career at Scientific Interface award, a Brain and Behavior Research Foundation young investigator award, the Howard Hughes Medical Institute, the National Institute of Aging, and the National Institute of General Medical Sciences. Dr. Bianco served as a consultant for AbbVie, Avion Pharmaceuticals, Synthonics, Synthion, and Thyron and recently published the book Rethinking Hypothyroidism: Why Treatment Must Change and What Patients Can Do.
A version of this article appeared on Medscape.com.
To assess thyroid function, clinicians typically determine blood levels of thyroid-stimulating hormone (TSH) and thyroxine (T4) and do not directly measure triiodothyronine (T3).
However, linking socioeconomic forces, human biology, and aging,” researchers reported.
The study, with lead author Ralph I. Lawton, an MD-PhD student at Harvard Medical School, Boston, was published online on January 4, 2024, in Proceedings of the National Academy of Sciences. In this national sample of healthy adults older than 20, free T3 was negatively related to household income. At age 40 and older, free T3 was significantly linked to being employed. Among adults from age 51 to 80, free T3 levels were inversely related to mortality.
“It is important to note that the more typically measured biomarkers for thyroid function (T4 and TSH) are poorly linked to free T3 levels,” Dr. Lawton and colleagues wrote.
The ratio of TSH:T4 explained only 1.7% of variation in T3 levels, they noted, which suggested that TSH and T4 may not be accurate surrogates of free T3. Thus, “direct measurement of free T3 is likely vital to properly stratify the effects of HPT-axis variation,” they maintained. “Improved methods for measurement [of T3] and further investigation of the role of free T3 in clinical conditions may be high yield,” according to the researchers.
Further studies of the relationship between the action of deiodinase in converting T4 to T3 and social forces and aging could elucidate critical biology. “New therapeutics targeting T3/T4 ratios,” they suggested, “may be more beneficial for a significant cohort of older individuals [with hypothyroidism] currently treated with levothyroxine [(LT4)].”
‘Cherry on Top’
“I think this is a really important paper,” thyroidology expert Antonio Bianco, MD, PhD, professor of medicine, The University of Chicago, who was not involved with this research, told this news organization.
His research group and others “have slowly been providing evidence of the relevance of measuring T3 levels,” he said. “This paper is sort of a cherry on top. It is really the most recent strong evidence that looking at T3 levels in plasma is really important. We need to do a better job at developing a much more precise assay for T3,” he said, “because now we know T3 is a very important parameter that endocrinologists should be looking for. We know that about 20 million Americans have hypothyroidism, and are being treated with T4 (levothyroxine),” Dr. Bianco continued.
“As endocrinologists, we believe that if you treat someone with levothyroxine, you normalize T3 levels. However, many times, with standard of care with levothyroxine, T3 levels are not normalized.”
Larger Variations in T3
Thyroid hormones (TSH, free T4, and free T3) influence many aspects of human physiology and behavior, but it is not known how variations in these levels in a population free of thyroid disease are associated with mortality and socioeconomic factors.
The researchers analyzed data from 7626 adults over age 20 up to age 80 who participated in the National Health and Nutrition Examination Survey during 2007-2012 and had blood tests to determine TSH, free T4, and free T3 and were not being actively treated for thyroid conditions. Among the 3603 older adults (over age 50), there were 981 deaths up until the end of 2019. TSH levels were not linked to mortality, but increasing levels of free T3 and decreasing levels of free T4 were protective for mortality (hazard ratios, 0.88 and 1.26, respectively; P < .01).
There was a robust negative relationship between household income and employment and free T3 but no relationship between these factors and T4. There was a much larger variation across age in levels of free T3 than in levels of TSH or free T4. Free T4 and free T3 diverged at older ages, when free T4 increased and free T3 continually decreased.
Older adults with symptoms of hypothyroidism may have high free T4 levels despite low free T3, the researchers noted.
“In a nonclinical sample representative of the US population,” they summarized, “we find that free T3 is much more strongly related to all domains studied — age, sex, seasonality, household income, employment, and longevity — than the other molecules of the HPT axis.”
This work was supported by the Burroughs Wellcome Fund Career at Scientific Interface award, a Brain and Behavior Research Foundation young investigator award, the Howard Hughes Medical Institute, the National Institute of Aging, and the National Institute of General Medical Sciences. Dr. Bianco served as a consultant for AbbVie, Avion Pharmaceuticals, Synthonics, Synthion, and Thyron and recently published the book Rethinking Hypothyroidism: Why Treatment Must Change and What Patients Can Do.
A version of this article appeared on Medscape.com.
Coming Soon: The First mRNA Vaccine for Melanoma?
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Efficacy of Topical Clascoterone for Acne Increased Over Time, Analysis Shows
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
New Insights Into Mortality in Takotsubo Syndrome
TOPLINE:
Mortality in patients with takotsubo syndrome (TTS), sometimes called broken heart syndrome or stress-induced cardiomyopathy is substantially higher than that in the general population and comparable with that in patients having myocardial infarction (MI), results of a new case-control study showed. The rates of medication use are similar for TTS and MI, despite no current clinical trials or recommendations to guide such therapies, the authors noted.
METHODOLOGY:
- The study included 620 Scottish patients (mean age, 66 years; 91% women) with TTS, a potentially fatal condition that mimics MI, predominantly affects middle-aged women, and is often triggered by stress.
- The analysis also included two age-, sex-, and geographically matched control groups: Representative participants from the general Scottish population (1:4) and patients with acute MI (1:1).
- Using comprehensive national data sets, researchers extracted information for all three cohorts on prescribing of cardiovascular and noncardiovascular medications, including the duration of dispensing and causes of death, and clustered the major causes of death into 17 major groups.
- At a median follow-up of 5.5 years, there were 722 deaths (153 in patients with TTS, 195 in those with MI, and 374 in the general population cohort).
TAKEAWAY:
- and slightly lower than that in patients having MI (HR, 0.76; 95% CI, 0.62-0.94; P = .012), with cardiovascular causes, particularly heart failure, being the most strongly associated with TTS (HR, 2.47; 95% CI, 1.81-3.39; P < .0001 vs general population), followed by pulmonary causes. Noncardiovascular mortality was similar in TTS and MI.
- Prescription rates of cardiovascular and noncardiovascular medications were similar between patients with TTS and MI.
- The only cardiovascular therapy associated with lower mortality in patients with TTS was angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy (P = .0056); in contrast, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, antiplatelet agents, and statins were all associated with improved survival in patients with MI.
- Diuretics were associated with worse outcomes in both patients with TTS and MI, as was psychotropic therapy.
IN PRACTICE:
“These findings may help to lay the foundations for further exploration of potential mechanisms and treatments” for TTS, an “increasingly recognized and potentially fatal condition,” the authors concluded.
In an accompanying comment, Rodolfo Citro, MD, PHD, Cardiovascular and Thoracic Department, San Giovanni di Dio e Ruggi d’ Aragona University Hospital, Salerno, Italy, and colleagues said the authors should be commended for providing data on cardiovascular mortality “during one of the longest available follow-ups in TTS,” adding the study “suggests the importance of further research for more appropriate management of patients with acute and long-term TTS.”
SOURCE:
The research was led by Amelia E. Rudd, MSC, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen and NHS Grampian, Aberdeen, Scotland. It was published online in the Journal of the American College of Cardiology.
LIMITATIONS:
Complete alignment of all variables related to clinical characteristics of patients with TTS and MI wasn’t feasible. During the study, TTS was still relatively unfamiliar to clinicians and underdiagnosed. As the study used a national data set of routinely collected data, not all desirable information was available, including indications of why drugs were prescribed or discontinued, which could have led to imprecise results. As the study used nonrandomized data, causality can’t be assumed.
DISCLOSURES:
Dr. Rudd had no relevant conflicts of interest. Study author Dana K. Dawson, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen, Scotland, declared receiving the Chief Scientist Office Scotland award CGA-16-4 and the BHF Research Training Fellowship. Commentary authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Mortality in patients with takotsubo syndrome (TTS), sometimes called broken heart syndrome or stress-induced cardiomyopathy is substantially higher than that in the general population and comparable with that in patients having myocardial infarction (MI), results of a new case-control study showed. The rates of medication use are similar for TTS and MI, despite no current clinical trials or recommendations to guide such therapies, the authors noted.
METHODOLOGY:
- The study included 620 Scottish patients (mean age, 66 years; 91% women) with TTS, a potentially fatal condition that mimics MI, predominantly affects middle-aged women, and is often triggered by stress.
- The analysis also included two age-, sex-, and geographically matched control groups: Representative participants from the general Scottish population (1:4) and patients with acute MI (1:1).
- Using comprehensive national data sets, researchers extracted information for all three cohorts on prescribing of cardiovascular and noncardiovascular medications, including the duration of dispensing and causes of death, and clustered the major causes of death into 17 major groups.
- At a median follow-up of 5.5 years, there were 722 deaths (153 in patients with TTS, 195 in those with MI, and 374 in the general population cohort).
TAKEAWAY:
- and slightly lower than that in patients having MI (HR, 0.76; 95% CI, 0.62-0.94; P = .012), with cardiovascular causes, particularly heart failure, being the most strongly associated with TTS (HR, 2.47; 95% CI, 1.81-3.39; P < .0001 vs general population), followed by pulmonary causes. Noncardiovascular mortality was similar in TTS and MI.
- Prescription rates of cardiovascular and noncardiovascular medications were similar between patients with TTS and MI.
- The only cardiovascular therapy associated with lower mortality in patients with TTS was angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy (P = .0056); in contrast, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, antiplatelet agents, and statins were all associated with improved survival in patients with MI.
- Diuretics were associated with worse outcomes in both patients with TTS and MI, as was psychotropic therapy.
IN PRACTICE:
“These findings may help to lay the foundations for further exploration of potential mechanisms and treatments” for TTS, an “increasingly recognized and potentially fatal condition,” the authors concluded.
In an accompanying comment, Rodolfo Citro, MD, PHD, Cardiovascular and Thoracic Department, San Giovanni di Dio e Ruggi d’ Aragona University Hospital, Salerno, Italy, and colleagues said the authors should be commended for providing data on cardiovascular mortality “during one of the longest available follow-ups in TTS,” adding the study “suggests the importance of further research for more appropriate management of patients with acute and long-term TTS.”
SOURCE:
The research was led by Amelia E. Rudd, MSC, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen and NHS Grampian, Aberdeen, Scotland. It was published online in the Journal of the American College of Cardiology.
LIMITATIONS:
Complete alignment of all variables related to clinical characteristics of patients with TTS and MI wasn’t feasible. During the study, TTS was still relatively unfamiliar to clinicians and underdiagnosed. As the study used a national data set of routinely collected data, not all desirable information was available, including indications of why drugs were prescribed or discontinued, which could have led to imprecise results. As the study used nonrandomized data, causality can’t be assumed.
DISCLOSURES:
Dr. Rudd had no relevant conflicts of interest. Study author Dana K. Dawson, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen, Scotland, declared receiving the Chief Scientist Office Scotland award CGA-16-4 and the BHF Research Training Fellowship. Commentary authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Mortality in patients with takotsubo syndrome (TTS), sometimes called broken heart syndrome or stress-induced cardiomyopathy is substantially higher than that in the general population and comparable with that in patients having myocardial infarction (MI), results of a new case-control study showed. The rates of medication use are similar for TTS and MI, despite no current clinical trials or recommendations to guide such therapies, the authors noted.
METHODOLOGY:
- The study included 620 Scottish patients (mean age, 66 years; 91% women) with TTS, a potentially fatal condition that mimics MI, predominantly affects middle-aged women, and is often triggered by stress.
- The analysis also included two age-, sex-, and geographically matched control groups: Representative participants from the general Scottish population (1:4) and patients with acute MI (1:1).
- Using comprehensive national data sets, researchers extracted information for all three cohorts on prescribing of cardiovascular and noncardiovascular medications, including the duration of dispensing and causes of death, and clustered the major causes of death into 17 major groups.
- At a median follow-up of 5.5 years, there were 722 deaths (153 in patients with TTS, 195 in those with MI, and 374 in the general population cohort).
TAKEAWAY:
- and slightly lower than that in patients having MI (HR, 0.76; 95% CI, 0.62-0.94; P = .012), with cardiovascular causes, particularly heart failure, being the most strongly associated with TTS (HR, 2.47; 95% CI, 1.81-3.39; P < .0001 vs general population), followed by pulmonary causes. Noncardiovascular mortality was similar in TTS and MI.
- Prescription rates of cardiovascular and noncardiovascular medications were similar between patients with TTS and MI.
- The only cardiovascular therapy associated with lower mortality in patients with TTS was angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy (P = .0056); in contrast, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, antiplatelet agents, and statins were all associated with improved survival in patients with MI.
- Diuretics were associated with worse outcomes in both patients with TTS and MI, as was psychotropic therapy.
IN PRACTICE:
“These findings may help to lay the foundations for further exploration of potential mechanisms and treatments” for TTS, an “increasingly recognized and potentially fatal condition,” the authors concluded.
In an accompanying comment, Rodolfo Citro, MD, PHD, Cardiovascular and Thoracic Department, San Giovanni di Dio e Ruggi d’ Aragona University Hospital, Salerno, Italy, and colleagues said the authors should be commended for providing data on cardiovascular mortality “during one of the longest available follow-ups in TTS,” adding the study “suggests the importance of further research for more appropriate management of patients with acute and long-term TTS.”
SOURCE:
The research was led by Amelia E. Rudd, MSC, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen and NHS Grampian, Aberdeen, Scotland. It was published online in the Journal of the American College of Cardiology.
LIMITATIONS:
Complete alignment of all variables related to clinical characteristics of patients with TTS and MI wasn’t feasible. During the study, TTS was still relatively unfamiliar to clinicians and underdiagnosed. As the study used a national data set of routinely collected data, not all desirable information was available, including indications of why drugs were prescribed or discontinued, which could have led to imprecise results. As the study used nonrandomized data, causality can’t be assumed.
DISCLOSURES:
Dr. Rudd had no relevant conflicts of interest. Study author Dana K. Dawson, Aberdeen Cardiovascular and Diabetes Centre, University of Aberdeen, Scotland, declared receiving the Chief Scientist Office Scotland award CGA-16-4 and the BHF Research Training Fellowship. Commentary authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Why GLP-1 Drugs Stop Working, and What to Do About It
There’s no question that glucagon-like peptide 1 (GLP-1) agonists represent a major advance in the treatment of obesity for patients with or without diabetes. In clinical trials, participants lost 15%-20% of their body weight, depending on the drug.
But studies also have shown that once people stop taking these drugs — either by choice, because of shortage, or lack of access — they regain most, if not all, the weight they lost.
Arguably more frustrating is the fact that those who continue on the drug eventually reach a plateau, at which point, the body seemingly stubbornly refuses to lose more weight. Essentially, it stabilizes at its set point, said Fatima Cody Stanford, MD, MPH, MPA, MBA, an obesity medicine physician at Massachusetts General Hospital and associate professor at Harvard Medical School in Boston.
‘Tug of War’
Every study of weight loss drugs done over the past 40 years or so shows a plateau, Dr. Stanford told this news organization. “If you look at the phentermine/topiramate studies, there’s a plateau. If you look at the bupropion/naltrexone studies, there’s a plateau. Or if we look at bariatric surgery, there’s a plateau. And it’s the same for the newer GLP-1 drugs.”
The reason? “It really depends on where the body gets to,” Dr. Stanford said. “The body knows what it needs to do to maintain itself, and the brain knows where it’s supposed to be. And when you lose weight and reach what you feel is a lower set point, the body resists.”
When the body goes below its set point, the hunger hormone ghrelin, which is housed in the brain, gets reactivated and gradually starts to reemerge, she explained. GLP-1, which is housed in the distal portion of the small intestine and in the colon, also starts to reemerge over time.
“It becomes kind of a tug of war” between the body and whatever weight loss strategy is being implemented, from drugs to surgery to lifestyle changes, Dr. Stanford said. “The patient will start to notice changes in how their body is responding. Usually, they’ll say they don’t feel like the treatment is working the same. But the treatment is working the same as it’s always been working — except their body is now acclimated to it.”
Anne L. Peters, MD, CDE, professor and clinical scholar, Keck School of Medicine of the University of Southern California, and director, agreed that in the simplest terms, a plateau occurs because “the body becomes more and more used to” the weight loss intervention.
However, when you lose weight, you lose both fat mass and lean body mass, and lean body mass is the metabolically active part of your body, explained Dr. Peters. “That’s what burns and basically makes up your basal metabolic rate.”
With weight loss, the metabolism slows down, she said. If patients need 2000 calories a day to survive at a certain weight and then lose 50 pounds, they may then need only a 1000 calories a day. “With any obesity treatment, you reach a point at which your metabolic rate and your daily caloric requirements become equal, and you stop losing weight, even though your daily caloric requirement is less than it was when your weight was higher.”
Managing the Plateau
Several strategies can be used to help patients break through a plateau. One is to try multiple weight loss agents with different targets — something often done in the real world, Dr. Stanford said. “You don’t see this in the studies, which are focused on just one drug, but many of our patients are on combination therapy. They’re on a GLP-1 drug plus phentermine/topiramate plus metformin, and more. They’re usually on three, four, five drugs, similar to what we would see with resistant hypertension.”
If a patient plateaus on a GLP-1 drug, Dr. Stanford might add phentermine. When the patient reaches a plateau on phentermine, she would switch again to another agent. “The goal is to use agents that treat different receptors in the brain,” she said. “You would never use two GLP-1 agonists; you would use the GLP-1, and then something that treats norepinephrine, for example.”
At the same time, Dr. Peters noted, “try to get them off the drugs that cause weight gain, like insulin and sulfonylurea agents.”
Tapering the GLP-1 dose can also help, Dr. Peters said. However, she added, “If I’m using a GLP-1 drug for type 2 diabetes, it’s different than if I’m using it just for weight loss. With type 2 diabetes, if you taper too much, the blood sugar and weight will go back up, so you need to reach a balance.”
Dr. Peters has successfully tapered patients from a 2-mg dose down to 1 mg. She has also changed the strategy for some — ie, the patient takes the drug every other week instead of every week. “I even have a patient or two who just take it once a month and that seems to be enough,” she said. “You want to help them be at the dose that maintains their weight and keeps them healthy with the least possible medication.”
Emphasizing lifestyle changes is also important, she said. Although resistance training won’t necessarily help with weight loss, “it’s critical to maintaining lean body mass. If people keep losing and regaining weight, they’re going to lose more and more lean body mass and gain the weight back primarily as fat mass. So, their exercise should include about half aerobic activity and half resistance training.”
Long-term Journey
Setting appropriate expectations is a key part of helping patients accept and deal with a plateau. “This is long-term, lifelong journey,” Dr. Stanford said. “We need to think about obesity as a complex, multifactorial chronic disease, like we think about hypertension or type 2 diabetes or hyperlipidemia.”
Furthermore, and in keeping with that perspective, emerging evidence is demonstrating that GLP-1 drugs also have important nonglycemic benefits that can be achieved and maintained, Dr. Peters said. “Obviously weight loss matters, and weight loss is good for you if you’re overweight or obese. But now we know that GLP-1 drugs have wonderful benefits for the heart as well as renal function.” These are reasons to continue the drugs even in the face of a plateau.
One of Dr. Peters’ patients, a physician with type 2 diabetes, had “fought with her weight her whole life. She’s been on one or another GLP-1 drug for more than 15 years, and while none seem to impact her weight, she’s gone from having relatively poorly controlled to now beautifully controlled diabetes,” Dr. Peters said. “Even if she hasn’t lost, she’s maintained her weight, a benefit since people tend to gain weight as they get older, and she hasn’t gained.”
Another patient was disabled, on oxygen, and had recurrent pulmonary embolisms. “She weighed 420 pounds, and I put her on semaglutide because she was too sick to be considered for bariatric surgery.” When that didn’t work, Dr. Peters switched her to tirzepatide, gradually increasing the dose; the patient lost 80 pounds, her emboli are gone, she can walk down the street, and went back to work.
“Part of why she could do that is that she started exercising,” Dr. Peters noted. “She felt so much better from the drug-related weight loss that she began to do things that help enhance weight loss. She became happier because she was no longer homebound.”
This points to another element that can help patients break through a plateau over time, Dr. Peters said — namely, behavioral health. “The more people lose weight, the more they feel better about themselves, and that may mean that they take better care of themselves. The psychological part of this journey is as important as anything else. Not everyone has the same response to these agents, and there are all sorts of issues behind why people are overweight that physicians can’t ignore.
“So, in addition to managing the drugs and lifestyle, it’s important to make sure that people access the behavioral health help they need, and that once they break through a plateau, they don’t develop an eating disorder or go to the opposite extreme and become too thin, which has happened with some of my patients,” she said. “We need to remember that we’re not just giving patients a miraculous weight loss. We’re helping them to be healthier, mentally as well as physically.”
Dr. Stanford disclosed that she had been a consultant for Calibrate, GoodRx, Pfizer, Eli Lilly, Boehringer Ingelheim, Gelesis, Vida Health, Life Force, Ilant Health, Melli Cell, and Novo Nordisk. Dr. Peters disclosed that she had been a consultant for Vertex, Medscape Medical News, and Lilly; received funding from Abbott and Insulet; and had stock options in Omada Health.
A version of this article appeared on Medscape.com.
There’s no question that glucagon-like peptide 1 (GLP-1) agonists represent a major advance in the treatment of obesity for patients with or without diabetes. In clinical trials, participants lost 15%-20% of their body weight, depending on the drug.
But studies also have shown that once people stop taking these drugs — either by choice, because of shortage, or lack of access — they regain most, if not all, the weight they lost.
Arguably more frustrating is the fact that those who continue on the drug eventually reach a plateau, at which point, the body seemingly stubbornly refuses to lose more weight. Essentially, it stabilizes at its set point, said Fatima Cody Stanford, MD, MPH, MPA, MBA, an obesity medicine physician at Massachusetts General Hospital and associate professor at Harvard Medical School in Boston.
‘Tug of War’
Every study of weight loss drugs done over the past 40 years or so shows a plateau, Dr. Stanford told this news organization. “If you look at the phentermine/topiramate studies, there’s a plateau. If you look at the bupropion/naltrexone studies, there’s a plateau. Or if we look at bariatric surgery, there’s a plateau. And it’s the same for the newer GLP-1 drugs.”
The reason? “It really depends on where the body gets to,” Dr. Stanford said. “The body knows what it needs to do to maintain itself, and the brain knows where it’s supposed to be. And when you lose weight and reach what you feel is a lower set point, the body resists.”
When the body goes below its set point, the hunger hormone ghrelin, which is housed in the brain, gets reactivated and gradually starts to reemerge, she explained. GLP-1, which is housed in the distal portion of the small intestine and in the colon, also starts to reemerge over time.
“It becomes kind of a tug of war” between the body and whatever weight loss strategy is being implemented, from drugs to surgery to lifestyle changes, Dr. Stanford said. “The patient will start to notice changes in how their body is responding. Usually, they’ll say they don’t feel like the treatment is working the same. But the treatment is working the same as it’s always been working — except their body is now acclimated to it.”
Anne L. Peters, MD, CDE, professor and clinical scholar, Keck School of Medicine of the University of Southern California, and director, agreed that in the simplest terms, a plateau occurs because “the body becomes more and more used to” the weight loss intervention.
However, when you lose weight, you lose both fat mass and lean body mass, and lean body mass is the metabolically active part of your body, explained Dr. Peters. “That’s what burns and basically makes up your basal metabolic rate.”
With weight loss, the metabolism slows down, she said. If patients need 2000 calories a day to survive at a certain weight and then lose 50 pounds, they may then need only a 1000 calories a day. “With any obesity treatment, you reach a point at which your metabolic rate and your daily caloric requirements become equal, and you stop losing weight, even though your daily caloric requirement is less than it was when your weight was higher.”
Managing the Plateau
Several strategies can be used to help patients break through a plateau. One is to try multiple weight loss agents with different targets — something often done in the real world, Dr. Stanford said. “You don’t see this in the studies, which are focused on just one drug, but many of our patients are on combination therapy. They’re on a GLP-1 drug plus phentermine/topiramate plus metformin, and more. They’re usually on three, four, five drugs, similar to what we would see with resistant hypertension.”
If a patient plateaus on a GLP-1 drug, Dr. Stanford might add phentermine. When the patient reaches a plateau on phentermine, she would switch again to another agent. “The goal is to use agents that treat different receptors in the brain,” she said. “You would never use two GLP-1 agonists; you would use the GLP-1, and then something that treats norepinephrine, for example.”
At the same time, Dr. Peters noted, “try to get them off the drugs that cause weight gain, like insulin and sulfonylurea agents.”
Tapering the GLP-1 dose can also help, Dr. Peters said. However, she added, “If I’m using a GLP-1 drug for type 2 diabetes, it’s different than if I’m using it just for weight loss. With type 2 diabetes, if you taper too much, the blood sugar and weight will go back up, so you need to reach a balance.”
Dr. Peters has successfully tapered patients from a 2-mg dose down to 1 mg. She has also changed the strategy for some — ie, the patient takes the drug every other week instead of every week. “I even have a patient or two who just take it once a month and that seems to be enough,” she said. “You want to help them be at the dose that maintains their weight and keeps them healthy with the least possible medication.”
Emphasizing lifestyle changes is also important, she said. Although resistance training won’t necessarily help with weight loss, “it’s critical to maintaining lean body mass. If people keep losing and regaining weight, they’re going to lose more and more lean body mass and gain the weight back primarily as fat mass. So, their exercise should include about half aerobic activity and half resistance training.”
Long-term Journey
Setting appropriate expectations is a key part of helping patients accept and deal with a plateau. “This is long-term, lifelong journey,” Dr. Stanford said. “We need to think about obesity as a complex, multifactorial chronic disease, like we think about hypertension or type 2 diabetes or hyperlipidemia.”
Furthermore, and in keeping with that perspective, emerging evidence is demonstrating that GLP-1 drugs also have important nonglycemic benefits that can be achieved and maintained, Dr. Peters said. “Obviously weight loss matters, and weight loss is good for you if you’re overweight or obese. But now we know that GLP-1 drugs have wonderful benefits for the heart as well as renal function.” These are reasons to continue the drugs even in the face of a plateau.
One of Dr. Peters’ patients, a physician with type 2 diabetes, had “fought with her weight her whole life. She’s been on one or another GLP-1 drug for more than 15 years, and while none seem to impact her weight, she’s gone from having relatively poorly controlled to now beautifully controlled diabetes,” Dr. Peters said. “Even if she hasn’t lost, she’s maintained her weight, a benefit since people tend to gain weight as they get older, and she hasn’t gained.”
Another patient was disabled, on oxygen, and had recurrent pulmonary embolisms. “She weighed 420 pounds, and I put her on semaglutide because she was too sick to be considered for bariatric surgery.” When that didn’t work, Dr. Peters switched her to tirzepatide, gradually increasing the dose; the patient lost 80 pounds, her emboli are gone, she can walk down the street, and went back to work.
“Part of why she could do that is that she started exercising,” Dr. Peters noted. “She felt so much better from the drug-related weight loss that she began to do things that help enhance weight loss. She became happier because she was no longer homebound.”
This points to another element that can help patients break through a plateau over time, Dr. Peters said — namely, behavioral health. “The more people lose weight, the more they feel better about themselves, and that may mean that they take better care of themselves. The psychological part of this journey is as important as anything else. Not everyone has the same response to these agents, and there are all sorts of issues behind why people are overweight that physicians can’t ignore.
“So, in addition to managing the drugs and lifestyle, it’s important to make sure that people access the behavioral health help they need, and that once they break through a plateau, they don’t develop an eating disorder or go to the opposite extreme and become too thin, which has happened with some of my patients,” she said. “We need to remember that we’re not just giving patients a miraculous weight loss. We’re helping them to be healthier, mentally as well as physically.”
Dr. Stanford disclosed that she had been a consultant for Calibrate, GoodRx, Pfizer, Eli Lilly, Boehringer Ingelheim, Gelesis, Vida Health, Life Force, Ilant Health, Melli Cell, and Novo Nordisk. Dr. Peters disclosed that she had been a consultant for Vertex, Medscape Medical News, and Lilly; received funding from Abbott and Insulet; and had stock options in Omada Health.
A version of this article appeared on Medscape.com.
There’s no question that glucagon-like peptide 1 (GLP-1) agonists represent a major advance in the treatment of obesity for patients with or without diabetes. In clinical trials, participants lost 15%-20% of their body weight, depending on the drug.
But studies also have shown that once people stop taking these drugs — either by choice, because of shortage, or lack of access — they regain most, if not all, the weight they lost.
Arguably more frustrating is the fact that those who continue on the drug eventually reach a plateau, at which point, the body seemingly stubbornly refuses to lose more weight. Essentially, it stabilizes at its set point, said Fatima Cody Stanford, MD, MPH, MPA, MBA, an obesity medicine physician at Massachusetts General Hospital and associate professor at Harvard Medical School in Boston.
‘Tug of War’
Every study of weight loss drugs done over the past 40 years or so shows a plateau, Dr. Stanford told this news organization. “If you look at the phentermine/topiramate studies, there’s a plateau. If you look at the bupropion/naltrexone studies, there’s a plateau. Or if we look at bariatric surgery, there’s a plateau. And it’s the same for the newer GLP-1 drugs.”
The reason? “It really depends on where the body gets to,” Dr. Stanford said. “The body knows what it needs to do to maintain itself, and the brain knows where it’s supposed to be. And when you lose weight and reach what you feel is a lower set point, the body resists.”
When the body goes below its set point, the hunger hormone ghrelin, which is housed in the brain, gets reactivated and gradually starts to reemerge, she explained. GLP-1, which is housed in the distal portion of the small intestine and in the colon, also starts to reemerge over time.
“It becomes kind of a tug of war” between the body and whatever weight loss strategy is being implemented, from drugs to surgery to lifestyle changes, Dr. Stanford said. “The patient will start to notice changes in how their body is responding. Usually, they’ll say they don’t feel like the treatment is working the same. But the treatment is working the same as it’s always been working — except their body is now acclimated to it.”
Anne L. Peters, MD, CDE, professor and clinical scholar, Keck School of Medicine of the University of Southern California, and director, agreed that in the simplest terms, a plateau occurs because “the body becomes more and more used to” the weight loss intervention.
However, when you lose weight, you lose both fat mass and lean body mass, and lean body mass is the metabolically active part of your body, explained Dr. Peters. “That’s what burns and basically makes up your basal metabolic rate.”
With weight loss, the metabolism slows down, she said. If patients need 2000 calories a day to survive at a certain weight and then lose 50 pounds, they may then need only a 1000 calories a day. “With any obesity treatment, you reach a point at which your metabolic rate and your daily caloric requirements become equal, and you stop losing weight, even though your daily caloric requirement is less than it was when your weight was higher.”
Managing the Plateau
Several strategies can be used to help patients break through a plateau. One is to try multiple weight loss agents with different targets — something often done in the real world, Dr. Stanford said. “You don’t see this in the studies, which are focused on just one drug, but many of our patients are on combination therapy. They’re on a GLP-1 drug plus phentermine/topiramate plus metformin, and more. They’re usually on three, four, five drugs, similar to what we would see with resistant hypertension.”
If a patient plateaus on a GLP-1 drug, Dr. Stanford might add phentermine. When the patient reaches a plateau on phentermine, she would switch again to another agent. “The goal is to use agents that treat different receptors in the brain,” she said. “You would never use two GLP-1 agonists; you would use the GLP-1, and then something that treats norepinephrine, for example.”
At the same time, Dr. Peters noted, “try to get them off the drugs that cause weight gain, like insulin and sulfonylurea agents.”
Tapering the GLP-1 dose can also help, Dr. Peters said. However, she added, “If I’m using a GLP-1 drug for type 2 diabetes, it’s different than if I’m using it just for weight loss. With type 2 diabetes, if you taper too much, the blood sugar and weight will go back up, so you need to reach a balance.”
Dr. Peters has successfully tapered patients from a 2-mg dose down to 1 mg. She has also changed the strategy for some — ie, the patient takes the drug every other week instead of every week. “I even have a patient or two who just take it once a month and that seems to be enough,” she said. “You want to help them be at the dose that maintains their weight and keeps them healthy with the least possible medication.”
Emphasizing lifestyle changes is also important, she said. Although resistance training won’t necessarily help with weight loss, “it’s critical to maintaining lean body mass. If people keep losing and regaining weight, they’re going to lose more and more lean body mass and gain the weight back primarily as fat mass. So, their exercise should include about half aerobic activity and half resistance training.”
Long-term Journey
Setting appropriate expectations is a key part of helping patients accept and deal with a plateau. “This is long-term, lifelong journey,” Dr. Stanford said. “We need to think about obesity as a complex, multifactorial chronic disease, like we think about hypertension or type 2 diabetes or hyperlipidemia.”
Furthermore, and in keeping with that perspective, emerging evidence is demonstrating that GLP-1 drugs also have important nonglycemic benefits that can be achieved and maintained, Dr. Peters said. “Obviously weight loss matters, and weight loss is good for you if you’re overweight or obese. But now we know that GLP-1 drugs have wonderful benefits for the heart as well as renal function.” These are reasons to continue the drugs even in the face of a plateau.
One of Dr. Peters’ patients, a physician with type 2 diabetes, had “fought with her weight her whole life. She’s been on one or another GLP-1 drug for more than 15 years, and while none seem to impact her weight, she’s gone from having relatively poorly controlled to now beautifully controlled diabetes,” Dr. Peters said. “Even if she hasn’t lost, she’s maintained her weight, a benefit since people tend to gain weight as they get older, and she hasn’t gained.”
Another patient was disabled, on oxygen, and had recurrent pulmonary embolisms. “She weighed 420 pounds, and I put her on semaglutide because she was too sick to be considered for bariatric surgery.” When that didn’t work, Dr. Peters switched her to tirzepatide, gradually increasing the dose; the patient lost 80 pounds, her emboli are gone, she can walk down the street, and went back to work.
“Part of why she could do that is that she started exercising,” Dr. Peters noted. “She felt so much better from the drug-related weight loss that she began to do things that help enhance weight loss. She became happier because she was no longer homebound.”
This points to another element that can help patients break through a plateau over time, Dr. Peters said — namely, behavioral health. “The more people lose weight, the more they feel better about themselves, and that may mean that they take better care of themselves. The psychological part of this journey is as important as anything else. Not everyone has the same response to these agents, and there are all sorts of issues behind why people are overweight that physicians can’t ignore.
“So, in addition to managing the drugs and lifestyle, it’s important to make sure that people access the behavioral health help they need, and that once they break through a plateau, they don’t develop an eating disorder or go to the opposite extreme and become too thin, which has happened with some of my patients,” she said. “We need to remember that we’re not just giving patients a miraculous weight loss. We’re helping them to be healthier, mentally as well as physically.”
Dr. Stanford disclosed that she had been a consultant for Calibrate, GoodRx, Pfizer, Eli Lilly, Boehringer Ingelheim, Gelesis, Vida Health, Life Force, Ilant Health, Melli Cell, and Novo Nordisk. Dr. Peters disclosed that she had been a consultant for Vertex, Medscape Medical News, and Lilly; received funding from Abbott and Insulet; and had stock options in Omada Health.
A version of this article appeared on Medscape.com.
Ozempic is Appealing, but Not Cost-Effective, for Obesity Treatment
, according to a modeling study that compared the drugs with surgery and endoscopy.
Sleeve gastrectomy (SG) for moderate to severe (class II/III) obesity and the less invasive endoscopic sleeve gastroplasty (ESG) for mild (class I) obesity were both cost effective strategies to reduce obesity, the researchers report.
“SG should be offered as the first-line treatment for class II and class III obesity,” write Monica Saumoy, MD, of the Center for Digestive Health, Penn Medicine Princeton Medical Center, Plainsboro, N.J., and coauthors. “ESG is an effective and cost-effective nonsurgical treatment for class I, class II and class III obesity, and more efforts are needed to ensure that patients have access to this procedure.
“While semaglutide is highly effective for weight loss, and there is substantial patient interest, it is not currently cost-effective due to its high cost,” they add. “With methods to reduce semaglutide’s annual cost, it may provide an effective and cost-effective method to reduce the morbidity related to obesity.”
The study was published in Gut.
Cost Concerns
One in two Americans will likely be obese by 2030, according to current models, and nearly one in four adults will be severely obese.
Several weight-loss therapies exist to treat obesity. Evidence shows bariatric surgery is effective in reducing weight, metabolic comorbidities, and mortality in people with obesity compared with lifestyle intervention alone, but surgery has risks, adverse events, and poor national uptake. Patients are likely more interested in less invasive options, the authors write.
Recent trials have reported effective weight loss from less invasive options. A five-year follow-up of the randomized controlled MERIT trial found that ESG was associated with a 13.6% total body weight loss for people with mild to moderate obesity.
On the pharmaceutical front, other randomized controlled trials have shown that semaglutide is linked with as much as 17% total body weight loss at two years. Also, recent guidance from the American Gastroenterological Association (AGA) states that long-term treatment with a semaglutide is the preferred strategy for weight loss.
“However, concerns about the cost and the cost-effectiveness of these [less invasive] interventions have limited their usage in the USA,” the study authors write.
The aim of the study was to perform a cost-effectiveness analysis comparing SG, ESG, semaglutide, and lifestyle interventions (LI) for patients with obesity in class I (defined as BMI 30-34.9 kg/m2), class II (35-29.9 kg/m2), and class III (>40kg/m2) obesity.
Researchers used a state-transition, semi-Markov microsimulation model to analyze the effectiveness of ESG, SG, semaglutide, and LI in a simulated 40-year-old with three different base-case scenarios of class I, II, or III obesity. They then performed a detailed threshold and sensitivity analysis to change the cost of treatment modalities and the semaglutide adherence rate. Outcome measures included a willingness-to-pay threshold of US $100,000/quality-adjusted life years (QALY) and incremental cost-effectiveness ratios (ICERs).
Cost-Effectiveness of Treatments
When the treatment modalities were compared with each other, findings showed that for class I obesity, ESG was cost effective (US $4,105/QALY). For class II and III obesity, SG was cost-effective as well (US $5,883/QALY) and (US $7,821/QALY), respectively.
In all classes of obesity, SG and ESG were cost-effective compared with LI. Semaglutide was not cost-effective compared with LI for class I, II, and III obesity (ICER US $508,414/QALY, $420,483/QALY, and $350,637/QALY, respectively).
“For semaglutide to be cost-effective when compared with ESG, it would have to cost less than US $1,879 (class III), US $1,204 (class II), or US $297 (class I) annually,” the authors note.
The authors addressed recent guidelines to consider bariatric surgery in all obese patients. They recommend SG remain the standard of care for patients with severe obesity.
But national projections show that SG would address only 0.5% of life-years lost due to obesity.
“Barring a dramatic increase in patient adherence, bariatric surgery will not likely successfully mitigate the harm from the obesity epidemic,” they write.
“ESG may fill this gap and provide an additional option for patients with obesity as it demonstrated sustained weight loss at 2-5 years.” While insurance coverage is limited, they write, “our model demonstrates that payer coverage for ESG would provide an alternative tool to combat the obesity epidemic as part of a multidisciplinary approach.”
Semaglutide shows sustained weight loss in trials for up to two years but has a substantial annual cost, the authors note.
At lower prices, semaglutide can make a “major impact on the obesity pandemic as it can be prescribed in multiple healthcare settings and due to increased patient interested in non-invasive obesity treatment,” they write.
One limitation to the study is a lack of long-term data available for ESG and semaglutide. Authors were also not able to use a lifetime horizon because of a lack of long-term weight loss.
One study author reports financial relationships with BSC, Cook Medical, Surgical Intuitive, and Olympus America. Another author reports relationships with ACI, AGA-Varia, BSC, Dark Canyon Labs, Endiatx, Medtronic, Olympus, Virgo Systems; equity: AGA-Varia, Dark Canyon Labs, Endiatx, EndoSound, and Virgo Systems. The rest of the authors have no conflicts to disclose.
, according to a modeling study that compared the drugs with surgery and endoscopy.
Sleeve gastrectomy (SG) for moderate to severe (class II/III) obesity and the less invasive endoscopic sleeve gastroplasty (ESG) for mild (class I) obesity were both cost effective strategies to reduce obesity, the researchers report.
“SG should be offered as the first-line treatment for class II and class III obesity,” write Monica Saumoy, MD, of the Center for Digestive Health, Penn Medicine Princeton Medical Center, Plainsboro, N.J., and coauthors. “ESG is an effective and cost-effective nonsurgical treatment for class I, class II and class III obesity, and more efforts are needed to ensure that patients have access to this procedure.
“While semaglutide is highly effective for weight loss, and there is substantial patient interest, it is not currently cost-effective due to its high cost,” they add. “With methods to reduce semaglutide’s annual cost, it may provide an effective and cost-effective method to reduce the morbidity related to obesity.”
The study was published in Gut.
Cost Concerns
One in two Americans will likely be obese by 2030, according to current models, and nearly one in four adults will be severely obese.
Several weight-loss therapies exist to treat obesity. Evidence shows bariatric surgery is effective in reducing weight, metabolic comorbidities, and mortality in people with obesity compared with lifestyle intervention alone, but surgery has risks, adverse events, and poor national uptake. Patients are likely more interested in less invasive options, the authors write.
Recent trials have reported effective weight loss from less invasive options. A five-year follow-up of the randomized controlled MERIT trial found that ESG was associated with a 13.6% total body weight loss for people with mild to moderate obesity.
On the pharmaceutical front, other randomized controlled trials have shown that semaglutide is linked with as much as 17% total body weight loss at two years. Also, recent guidance from the American Gastroenterological Association (AGA) states that long-term treatment with a semaglutide is the preferred strategy for weight loss.
“However, concerns about the cost and the cost-effectiveness of these [less invasive] interventions have limited their usage in the USA,” the study authors write.
The aim of the study was to perform a cost-effectiveness analysis comparing SG, ESG, semaglutide, and lifestyle interventions (LI) for patients with obesity in class I (defined as BMI 30-34.9 kg/m2), class II (35-29.9 kg/m2), and class III (>40kg/m2) obesity.
Researchers used a state-transition, semi-Markov microsimulation model to analyze the effectiveness of ESG, SG, semaglutide, and LI in a simulated 40-year-old with three different base-case scenarios of class I, II, or III obesity. They then performed a detailed threshold and sensitivity analysis to change the cost of treatment modalities and the semaglutide adherence rate. Outcome measures included a willingness-to-pay threshold of US $100,000/quality-adjusted life years (QALY) and incremental cost-effectiveness ratios (ICERs).
Cost-Effectiveness of Treatments
When the treatment modalities were compared with each other, findings showed that for class I obesity, ESG was cost effective (US $4,105/QALY). For class II and III obesity, SG was cost-effective as well (US $5,883/QALY) and (US $7,821/QALY), respectively.
In all classes of obesity, SG and ESG were cost-effective compared with LI. Semaglutide was not cost-effective compared with LI for class I, II, and III obesity (ICER US $508,414/QALY, $420,483/QALY, and $350,637/QALY, respectively).
“For semaglutide to be cost-effective when compared with ESG, it would have to cost less than US $1,879 (class III), US $1,204 (class II), or US $297 (class I) annually,” the authors note.
The authors addressed recent guidelines to consider bariatric surgery in all obese patients. They recommend SG remain the standard of care for patients with severe obesity.
But national projections show that SG would address only 0.5% of life-years lost due to obesity.
“Barring a dramatic increase in patient adherence, bariatric surgery will not likely successfully mitigate the harm from the obesity epidemic,” they write.
“ESG may fill this gap and provide an additional option for patients with obesity as it demonstrated sustained weight loss at 2-5 years.” While insurance coverage is limited, they write, “our model demonstrates that payer coverage for ESG would provide an alternative tool to combat the obesity epidemic as part of a multidisciplinary approach.”
Semaglutide shows sustained weight loss in trials for up to two years but has a substantial annual cost, the authors note.
At lower prices, semaglutide can make a “major impact on the obesity pandemic as it can be prescribed in multiple healthcare settings and due to increased patient interested in non-invasive obesity treatment,” they write.
One limitation to the study is a lack of long-term data available for ESG and semaglutide. Authors were also not able to use a lifetime horizon because of a lack of long-term weight loss.
One study author reports financial relationships with BSC, Cook Medical, Surgical Intuitive, and Olympus America. Another author reports relationships with ACI, AGA-Varia, BSC, Dark Canyon Labs, Endiatx, Medtronic, Olympus, Virgo Systems; equity: AGA-Varia, Dark Canyon Labs, Endiatx, EndoSound, and Virgo Systems. The rest of the authors have no conflicts to disclose.
, according to a modeling study that compared the drugs with surgery and endoscopy.
Sleeve gastrectomy (SG) for moderate to severe (class II/III) obesity and the less invasive endoscopic sleeve gastroplasty (ESG) for mild (class I) obesity were both cost effective strategies to reduce obesity, the researchers report.
“SG should be offered as the first-line treatment for class II and class III obesity,” write Monica Saumoy, MD, of the Center for Digestive Health, Penn Medicine Princeton Medical Center, Plainsboro, N.J., and coauthors. “ESG is an effective and cost-effective nonsurgical treatment for class I, class II and class III obesity, and more efforts are needed to ensure that patients have access to this procedure.
“While semaglutide is highly effective for weight loss, and there is substantial patient interest, it is not currently cost-effective due to its high cost,” they add. “With methods to reduce semaglutide’s annual cost, it may provide an effective and cost-effective method to reduce the morbidity related to obesity.”
The study was published in Gut.
Cost Concerns
One in two Americans will likely be obese by 2030, according to current models, and nearly one in four adults will be severely obese.
Several weight-loss therapies exist to treat obesity. Evidence shows bariatric surgery is effective in reducing weight, metabolic comorbidities, and mortality in people with obesity compared with lifestyle intervention alone, but surgery has risks, adverse events, and poor national uptake. Patients are likely more interested in less invasive options, the authors write.
Recent trials have reported effective weight loss from less invasive options. A five-year follow-up of the randomized controlled MERIT trial found that ESG was associated with a 13.6% total body weight loss for people with mild to moderate obesity.
On the pharmaceutical front, other randomized controlled trials have shown that semaglutide is linked with as much as 17% total body weight loss at two years. Also, recent guidance from the American Gastroenterological Association (AGA) states that long-term treatment with a semaglutide is the preferred strategy for weight loss.
“However, concerns about the cost and the cost-effectiveness of these [less invasive] interventions have limited their usage in the USA,” the study authors write.
The aim of the study was to perform a cost-effectiveness analysis comparing SG, ESG, semaglutide, and lifestyle interventions (LI) for patients with obesity in class I (defined as BMI 30-34.9 kg/m2), class II (35-29.9 kg/m2), and class III (>40kg/m2) obesity.
Researchers used a state-transition, semi-Markov microsimulation model to analyze the effectiveness of ESG, SG, semaglutide, and LI in a simulated 40-year-old with three different base-case scenarios of class I, II, or III obesity. They then performed a detailed threshold and sensitivity analysis to change the cost of treatment modalities and the semaglutide adherence rate. Outcome measures included a willingness-to-pay threshold of US $100,000/quality-adjusted life years (QALY) and incremental cost-effectiveness ratios (ICERs).
Cost-Effectiveness of Treatments
When the treatment modalities were compared with each other, findings showed that for class I obesity, ESG was cost effective (US $4,105/QALY). For class II and III obesity, SG was cost-effective as well (US $5,883/QALY) and (US $7,821/QALY), respectively.
In all classes of obesity, SG and ESG were cost-effective compared with LI. Semaglutide was not cost-effective compared with LI for class I, II, and III obesity (ICER US $508,414/QALY, $420,483/QALY, and $350,637/QALY, respectively).
“For semaglutide to be cost-effective when compared with ESG, it would have to cost less than US $1,879 (class III), US $1,204 (class II), or US $297 (class I) annually,” the authors note.
The authors addressed recent guidelines to consider bariatric surgery in all obese patients. They recommend SG remain the standard of care for patients with severe obesity.
But national projections show that SG would address only 0.5% of life-years lost due to obesity.
“Barring a dramatic increase in patient adherence, bariatric surgery will not likely successfully mitigate the harm from the obesity epidemic,” they write.
“ESG may fill this gap and provide an additional option for patients with obesity as it demonstrated sustained weight loss at 2-5 years.” While insurance coverage is limited, they write, “our model demonstrates that payer coverage for ESG would provide an alternative tool to combat the obesity epidemic as part of a multidisciplinary approach.”
Semaglutide shows sustained weight loss in trials for up to two years but has a substantial annual cost, the authors note.
At lower prices, semaglutide can make a “major impact on the obesity pandemic as it can be prescribed in multiple healthcare settings and due to increased patient interested in non-invasive obesity treatment,” they write.
One limitation to the study is a lack of long-term data available for ESG and semaglutide. Authors were also not able to use a lifetime horizon because of a lack of long-term weight loss.
One study author reports financial relationships with BSC, Cook Medical, Surgical Intuitive, and Olympus America. Another author reports relationships with ACI, AGA-Varia, BSC, Dark Canyon Labs, Endiatx, Medtronic, Olympus, Virgo Systems; equity: AGA-Varia, Dark Canyon Labs, Endiatx, EndoSound, and Virgo Systems. The rest of the authors have no conflicts to disclose.
FROM GUT
Severely Irregular Sleep Patterns and OSA Prompt Increased Odds of Hypertension
TOPLINE:
Severe sleep irregularity often occurs with obstructive sleep apnea (OSA), and this combination approximately doubled the odds of hypertension in middle-aged individuals.
METHODOLOGY:
- OSA has demonstrated an association with hypertension, but data on the impact of sleep irregularity on this relationship are lacking.
- The researchers used the recently developed sleep regularity index (SRI) to determine sleep patterns using a scale of 0-100 (with higher numbers indicating greater regularity) to assess relationships between OSA, sleep patterns, and hypertension in 602 adults with a mean age of 57 years.
- The study’s goal was an assessment of the associations between sleep regularity, OSA, and hypertension in a community sample of adults with normal circadian patterns.
TAKEAWAY:
- The odds of OSA were significantly greater for individuals with mildly irregular or severely irregular sleep than for regular sleepers (odds ratios, 1.97 and 2.06, respectively).
- Individuals with OSA and severely irregular sleep had the highest odds of hypertension compared with individuals with no OSA and regular sleep (OR, 2.34).
- However, participants with OSA and regular sleep or mildly irregular sleep had no significant increase in hypertension risk.
IN PRACTICE:
“Irregular sleep may be an important marker of OSA-related sleep disruption and may be an important modifiable health target,” the researchers wrote.
SOURCE:
The study was led by Kelly Sansom, a PhD candidate at the Centre for Sleep Science at the University of Western Australia, Albany. The study was published online in the journal Sleep.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality, and the SRI is a nonspecific measure that may capture a range of phenotypes with one score; other limitations included the small sample sizes of sleep regularity groups and the use of actigraphy to collect sleep times.
DISCLOSURES:
The study was supported by an Australian Government Research Training Program Scholarship and the Raine Study PhD Top-up Scholarship; the Raine Study Scholarship is supported by the NHMRC, the Centre for Sleep Science, School of Anatomy, Physiology & Human Biology of the University of Western Australia, and the Lions Eye Institute. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Severe sleep irregularity often occurs with obstructive sleep apnea (OSA), and this combination approximately doubled the odds of hypertension in middle-aged individuals.
METHODOLOGY:
- OSA has demonstrated an association with hypertension, but data on the impact of sleep irregularity on this relationship are lacking.
- The researchers used the recently developed sleep regularity index (SRI) to determine sleep patterns using a scale of 0-100 (with higher numbers indicating greater regularity) to assess relationships between OSA, sleep patterns, and hypertension in 602 adults with a mean age of 57 years.
- The study’s goal was an assessment of the associations between sleep regularity, OSA, and hypertension in a community sample of adults with normal circadian patterns.
TAKEAWAY:
- The odds of OSA were significantly greater for individuals with mildly irregular or severely irregular sleep than for regular sleepers (odds ratios, 1.97 and 2.06, respectively).
- Individuals with OSA and severely irregular sleep had the highest odds of hypertension compared with individuals with no OSA and regular sleep (OR, 2.34).
- However, participants with OSA and regular sleep or mildly irregular sleep had no significant increase in hypertension risk.
IN PRACTICE:
“Irregular sleep may be an important marker of OSA-related sleep disruption and may be an important modifiable health target,” the researchers wrote.
SOURCE:
The study was led by Kelly Sansom, a PhD candidate at the Centre for Sleep Science at the University of Western Australia, Albany. The study was published online in the journal Sleep.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality, and the SRI is a nonspecific measure that may capture a range of phenotypes with one score; other limitations included the small sample sizes of sleep regularity groups and the use of actigraphy to collect sleep times.
DISCLOSURES:
The study was supported by an Australian Government Research Training Program Scholarship and the Raine Study PhD Top-up Scholarship; the Raine Study Scholarship is supported by the NHMRC, the Centre for Sleep Science, School of Anatomy, Physiology & Human Biology of the University of Western Australia, and the Lions Eye Institute. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Severe sleep irregularity often occurs with obstructive sleep apnea (OSA), and this combination approximately doubled the odds of hypertension in middle-aged individuals.
METHODOLOGY:
- OSA has demonstrated an association with hypertension, but data on the impact of sleep irregularity on this relationship are lacking.
- The researchers used the recently developed sleep regularity index (SRI) to determine sleep patterns using a scale of 0-100 (with higher numbers indicating greater regularity) to assess relationships between OSA, sleep patterns, and hypertension in 602 adults with a mean age of 57 years.
- The study’s goal was an assessment of the associations between sleep regularity, OSA, and hypertension in a community sample of adults with normal circadian patterns.
TAKEAWAY:
- The odds of OSA were significantly greater for individuals with mildly irregular or severely irregular sleep than for regular sleepers (odds ratios, 1.97 and 2.06, respectively).
- Individuals with OSA and severely irregular sleep had the highest odds of hypertension compared with individuals with no OSA and regular sleep (OR, 2.34).
- However, participants with OSA and regular sleep or mildly irregular sleep had no significant increase in hypertension risk.
IN PRACTICE:
“Irregular sleep may be an important marker of OSA-related sleep disruption and may be an important modifiable health target,” the researchers wrote.
SOURCE:
The study was led by Kelly Sansom, a PhD candidate at the Centre for Sleep Science at the University of Western Australia, Albany. The study was published online in the journal Sleep.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality, and the SRI is a nonspecific measure that may capture a range of phenotypes with one score; other limitations included the small sample sizes of sleep regularity groups and the use of actigraphy to collect sleep times.
DISCLOSURES:
The study was supported by an Australian Government Research Training Program Scholarship and the Raine Study PhD Top-up Scholarship; the Raine Study Scholarship is supported by the NHMRC, the Centre for Sleep Science, School of Anatomy, Physiology & Human Biology of the University of Western Australia, and the Lions Eye Institute. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.

