User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Bariatric Surgery Doesn’t Improve Mental Health in Teens
TOPLINE:
Adolescents with severe obesity who undergo bariatric surgery may have a continuing need for mental health treatment and an increased risk for alcohol use disorder after the procedure.
METHODOLOGY:
- Researchers evaluated the long-term effects of bariatric surgery on the mental health of 1554 adolescents (75% women) with severe obesity who underwent bariatric surgery in Sweden between 2007 and 2017.
- At the time of surgery, the mean age was 19.0 years, and the mean body mass index was 43.7.
- A general population reference group of 15,540 adolescents was created by matching 10 comparators each to adolescents in the surgery group by age, sex, and country of residence.
- Information on psychiatric healthcare use and filled psychiatric drug prescriptions for 5 years before surgery and the first 10 years after surgery were obtained from national registers.
- The number of visits for self-harm and substance use disorder and the number of filled prescriptions for any psychiatric drug, antidepressants, and anxiolytics were other outcomes of interest.
TAKEAWAY:
- At 5 years before surgery, the prevalence of psychiatric healthcare visits (prevalence difference [Δ], 3.7%) and of psychiatric drug use (Δ, 6.2%) was higher in the surgery vs reference group.
- The preoperative trajectories continued and grew post-surgery, with the differences in psychiatric healthcare visits (Δ, ~12%) and psychiatric drug use (Δ, 20.4%) between the groups peaking at 9 and 10 years post surgery, respectively.
- A low prevalence of healthcare visits for substance use disorder in both groups grew to about 5% of adolescents in the surgery group after 10 years, driven primarily by alcohol use, compared with about 1% of adolescents in the reference group (Δ, 4.3%).
- Surgery is an obesity treatment, leading to sustainable weight loss, cardiometabolic health, and physical quality of life, but mental health improvements cannot be expected at the group level.
IN PRACTICE:
“Adolescent patients should be informed of the increased risk for alcohol use disorder and that they might continue needing mental health treatment,” the authors wrote.
SOURCE:
Gustaf Bruze, PhD, from the Department of Medicine, Clinical Epidemiology Division, Karolinska Institutet, Solna, and Kajsa Jarvholm, PhD, from the Department of Psychology, Lund University, Lund, Sweden, led this study, which was published online in The Lancet Child & Adolescent Health.
LIMITATIONS:
The findings may have limited generalizability to other settings, as the study was performed in Sweden with a predominantly White population undergoing Roux-en-Y gastric bypass in a universally accessible healthcare system. Moreover, there was a shortage of nonsurgically treated adolescents with severe obesity for comparison. Patients undergoing surgery may have easier access to healthcare than the general population, which could account for an increase in healthcare visits.
DISCLOSURES:
This study was supported by the Swedish Research Council and the Swedish Research Council for Health, Working Life, and Welfare. Two authors were the current or previous director of the Scandinavian Obesity Surgery Registry. Several authors declared receiving personal fees, participating in advisory boards and educational activities, and having other ties with Ethicon Johnson & Johnson, and Novo Nordisk.
A version of this article appeared on Medscape.com.
TOPLINE:
Adolescents with severe obesity who undergo bariatric surgery may have a continuing need for mental health treatment and an increased risk for alcohol use disorder after the procedure.
METHODOLOGY:
- Researchers evaluated the long-term effects of bariatric surgery on the mental health of 1554 adolescents (75% women) with severe obesity who underwent bariatric surgery in Sweden between 2007 and 2017.
- At the time of surgery, the mean age was 19.0 years, and the mean body mass index was 43.7.
- A general population reference group of 15,540 adolescents was created by matching 10 comparators each to adolescents in the surgery group by age, sex, and country of residence.
- Information on psychiatric healthcare use and filled psychiatric drug prescriptions for 5 years before surgery and the first 10 years after surgery were obtained from national registers.
- The number of visits for self-harm and substance use disorder and the number of filled prescriptions for any psychiatric drug, antidepressants, and anxiolytics were other outcomes of interest.
TAKEAWAY:
- At 5 years before surgery, the prevalence of psychiatric healthcare visits (prevalence difference [Δ], 3.7%) and of psychiatric drug use (Δ, 6.2%) was higher in the surgery vs reference group.
- The preoperative trajectories continued and grew post-surgery, with the differences in psychiatric healthcare visits (Δ, ~12%) and psychiatric drug use (Δ, 20.4%) between the groups peaking at 9 and 10 years post surgery, respectively.
- A low prevalence of healthcare visits for substance use disorder in both groups grew to about 5% of adolescents in the surgery group after 10 years, driven primarily by alcohol use, compared with about 1% of adolescents in the reference group (Δ, 4.3%).
- Surgery is an obesity treatment, leading to sustainable weight loss, cardiometabolic health, and physical quality of life, but mental health improvements cannot be expected at the group level.
IN PRACTICE:
“Adolescent patients should be informed of the increased risk for alcohol use disorder and that they might continue needing mental health treatment,” the authors wrote.
SOURCE:
Gustaf Bruze, PhD, from the Department of Medicine, Clinical Epidemiology Division, Karolinska Institutet, Solna, and Kajsa Jarvholm, PhD, from the Department of Psychology, Lund University, Lund, Sweden, led this study, which was published online in The Lancet Child & Adolescent Health.
LIMITATIONS:
The findings may have limited generalizability to other settings, as the study was performed in Sweden with a predominantly White population undergoing Roux-en-Y gastric bypass in a universally accessible healthcare system. Moreover, there was a shortage of nonsurgically treated adolescents with severe obesity for comparison. Patients undergoing surgery may have easier access to healthcare than the general population, which could account for an increase in healthcare visits.
DISCLOSURES:
This study was supported by the Swedish Research Council and the Swedish Research Council for Health, Working Life, and Welfare. Two authors were the current or previous director of the Scandinavian Obesity Surgery Registry. Several authors declared receiving personal fees, participating in advisory boards and educational activities, and having other ties with Ethicon Johnson & Johnson, and Novo Nordisk.
A version of this article appeared on Medscape.com.
TOPLINE:
Adolescents with severe obesity who undergo bariatric surgery may have a continuing need for mental health treatment and an increased risk for alcohol use disorder after the procedure.
METHODOLOGY:
- Researchers evaluated the long-term effects of bariatric surgery on the mental health of 1554 adolescents (75% women) with severe obesity who underwent bariatric surgery in Sweden between 2007 and 2017.
- At the time of surgery, the mean age was 19.0 years, and the mean body mass index was 43.7.
- A general population reference group of 15,540 adolescents was created by matching 10 comparators each to adolescents in the surgery group by age, sex, and country of residence.
- Information on psychiatric healthcare use and filled psychiatric drug prescriptions for 5 years before surgery and the first 10 years after surgery were obtained from national registers.
- The number of visits for self-harm and substance use disorder and the number of filled prescriptions for any psychiatric drug, antidepressants, and anxiolytics were other outcomes of interest.
TAKEAWAY:
- At 5 years before surgery, the prevalence of psychiatric healthcare visits (prevalence difference [Δ], 3.7%) and of psychiatric drug use (Δ, 6.2%) was higher in the surgery vs reference group.
- The preoperative trajectories continued and grew post-surgery, with the differences in psychiatric healthcare visits (Δ, ~12%) and psychiatric drug use (Δ, 20.4%) between the groups peaking at 9 and 10 years post surgery, respectively.
- A low prevalence of healthcare visits for substance use disorder in both groups grew to about 5% of adolescents in the surgery group after 10 years, driven primarily by alcohol use, compared with about 1% of adolescents in the reference group (Δ, 4.3%).
- Surgery is an obesity treatment, leading to sustainable weight loss, cardiometabolic health, and physical quality of life, but mental health improvements cannot be expected at the group level.
IN PRACTICE:
“Adolescent patients should be informed of the increased risk for alcohol use disorder and that they might continue needing mental health treatment,” the authors wrote.
SOURCE:
Gustaf Bruze, PhD, from the Department of Medicine, Clinical Epidemiology Division, Karolinska Institutet, Solna, and Kajsa Jarvholm, PhD, from the Department of Psychology, Lund University, Lund, Sweden, led this study, which was published online in The Lancet Child & Adolescent Health.
LIMITATIONS:
The findings may have limited generalizability to other settings, as the study was performed in Sweden with a predominantly White population undergoing Roux-en-Y gastric bypass in a universally accessible healthcare system. Moreover, there was a shortage of nonsurgically treated adolescents with severe obesity for comparison. Patients undergoing surgery may have easier access to healthcare than the general population, which could account for an increase in healthcare visits.
DISCLOSURES:
This study was supported by the Swedish Research Council and the Swedish Research Council for Health, Working Life, and Welfare. Two authors were the current or previous director of the Scandinavian Obesity Surgery Registry. Several authors declared receiving personal fees, participating in advisory boards and educational activities, and having other ties with Ethicon Johnson & Johnson, and Novo Nordisk.
A version of this article appeared on Medscape.com.
Even Moderate Exposure to Radon Tied to Increased Stroke Risk
Exposure to even moderate concentrations of radon is associated with a significant increase in stroke risk, new research suggests.
Even moderate concentrations of radon were associated with a 6% higher stroke risk.
Radon is the second leading cause of lung cancer, but little was known about how exposure to the gas might affect stroke risk in women.
“Our research found an increased risk of stroke among participants exposed to radon above — and as many as 2 picocuries per liter (pCi/L) below — concentrations that usually trigger Environmental Protection Agency recommendations to install a home radon mitigation system,” senior author Eric A. Whitsel, MD, MPH, professor of epidemiology and medicine, University of North Carolina, Chapel Hill, said in a news release.
The study was published online on January 31, 2024, in Neurology.
Women Particularly Affected
Radon is a naturally occurring odorless radioactive gas produced when uranium or radium break down in rocks and soil. Its presence is increasing as a result of climate change, and it is increasingly being found in people’s homes. When inhaled, this air pollutant releases ionizing radiation in the lungs and is seen as second only to smoking as an established cause of lung cancer.
The National Radon Action Plan of the US Environmental Protection Agency (EPA) lays out testing and mitigation guidelines based on the known role of radon in lung carcinogenesis. But radon testing and mitigation are less common than recommended, and the EPA’s action plan doesn’t cover diseases other than lung cancer.
Compared with men, women have a higher rate of stroke and, in the US, typically spend about 11% more hours per day indoors at home, which investigators note highlights a “potential role of the residential environment among other risk factors specific to women.”
Researchers examined longitudinal associations between home radon exposure and incident stroke in 158,910 women at baseline (mean age 63.2 years; 83% White) over a mean follow-up of 13.4 years. During this time, participants experienced a total of 6979 strokes.
Participants’ home addresses were linked to radon concentration data drawn from the US Geological Survey and the EPA, which recommends that average indoor radon concentrations not exceed 4 pCi/L.
The highest radon exposure group resided in areas where average radon concentrations were < 4 pCi/L; the middle exposure group lived in regions with average concentrations of 2-4 pCi/L; and the lowest exposure group lived in areas with average concentrations < 2 pCi/L.
The researchers adjusted for demographic, social, behavioral, and clinical characteristics.
Public Health Implications
The incidence rates of stroke per 100,000 women in the lowest, middle, and highest radon concentration areas were 333, 343, and 349, respectively.
Stroke risk was 6% higher among those in the middle exposure group (adjusted hazard ratio [aHR], 1.06; 95% CI, 0.99-1.13) and 14% higher in the highest exposure group (aHR, 1.14; 95% CI, 1.05-1.22) compared with the lowest exposure group.
Notably, stroke risk was significant even at concentrations ranging from 2 to 4 pCi/L (P = .0004) vs < 2 pCi/L, which is below the EPA›s Radon Action Level for mitigation.
The findings remained robust in sensitivity analyses, although the associations were slightly stronger for ischemic stroke (especially cardioembolic, small-vessel occlusive, and very large artery atherosclerotic) compared with hemorrhagic stroke.
“Radon is an indoor air pollutant that can only be detected through testing that measures concentrations of the gas in homes,” Dr. Whitsel said in the release. “More studies are needed to confirm our findings. Confirmation would present an opportunity to improve public health by addressing an emerging risk factor for stroke.”
The study lacked gender and racial/ethnic diversity, so the findings may not be generalizable to other populations.
“Replication studies of individual-level radon exposures are needed to confirm this positive radon-stroke association,” the authors write. “Confirmation would present a potential opportunity to affect public health by addressing a pervasive environmental risk factor for stroke and thereby merit reconsideration of extant radon policy.”
The study was funded by the National Institute of Environmental Health Sciences and National Heart, Lung, and Blood Institute. Dr. Whitsel and coauthors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Exposure to even moderate concentrations of radon is associated with a significant increase in stroke risk, new research suggests.
Even moderate concentrations of radon were associated with a 6% higher stroke risk.
Radon is the second leading cause of lung cancer, but little was known about how exposure to the gas might affect stroke risk in women.
“Our research found an increased risk of stroke among participants exposed to radon above — and as many as 2 picocuries per liter (pCi/L) below — concentrations that usually trigger Environmental Protection Agency recommendations to install a home radon mitigation system,” senior author Eric A. Whitsel, MD, MPH, professor of epidemiology and medicine, University of North Carolina, Chapel Hill, said in a news release.
The study was published online on January 31, 2024, in Neurology.
Women Particularly Affected
Radon is a naturally occurring odorless radioactive gas produced when uranium or radium break down in rocks and soil. Its presence is increasing as a result of climate change, and it is increasingly being found in people’s homes. When inhaled, this air pollutant releases ionizing radiation in the lungs and is seen as second only to smoking as an established cause of lung cancer.
The National Radon Action Plan of the US Environmental Protection Agency (EPA) lays out testing and mitigation guidelines based on the known role of radon in lung carcinogenesis. But radon testing and mitigation are less common than recommended, and the EPA’s action plan doesn’t cover diseases other than lung cancer.
Compared with men, women have a higher rate of stroke and, in the US, typically spend about 11% more hours per day indoors at home, which investigators note highlights a “potential role of the residential environment among other risk factors specific to women.”
Researchers examined longitudinal associations between home radon exposure and incident stroke in 158,910 women at baseline (mean age 63.2 years; 83% White) over a mean follow-up of 13.4 years. During this time, participants experienced a total of 6979 strokes.
Participants’ home addresses were linked to radon concentration data drawn from the US Geological Survey and the EPA, which recommends that average indoor radon concentrations not exceed 4 pCi/L.
The highest radon exposure group resided in areas where average radon concentrations were < 4 pCi/L; the middle exposure group lived in regions with average concentrations of 2-4 pCi/L; and the lowest exposure group lived in areas with average concentrations < 2 pCi/L.
The researchers adjusted for demographic, social, behavioral, and clinical characteristics.
Public Health Implications
The incidence rates of stroke per 100,000 women in the lowest, middle, and highest radon concentration areas were 333, 343, and 349, respectively.
Stroke risk was 6% higher among those in the middle exposure group (adjusted hazard ratio [aHR], 1.06; 95% CI, 0.99-1.13) and 14% higher in the highest exposure group (aHR, 1.14; 95% CI, 1.05-1.22) compared with the lowest exposure group.
Notably, stroke risk was significant even at concentrations ranging from 2 to 4 pCi/L (P = .0004) vs < 2 pCi/L, which is below the EPA›s Radon Action Level for mitigation.
The findings remained robust in sensitivity analyses, although the associations were slightly stronger for ischemic stroke (especially cardioembolic, small-vessel occlusive, and very large artery atherosclerotic) compared with hemorrhagic stroke.
“Radon is an indoor air pollutant that can only be detected through testing that measures concentrations of the gas in homes,” Dr. Whitsel said in the release. “More studies are needed to confirm our findings. Confirmation would present an opportunity to improve public health by addressing an emerging risk factor for stroke.”
The study lacked gender and racial/ethnic diversity, so the findings may not be generalizable to other populations.
“Replication studies of individual-level radon exposures are needed to confirm this positive radon-stroke association,” the authors write. “Confirmation would present a potential opportunity to affect public health by addressing a pervasive environmental risk factor for stroke and thereby merit reconsideration of extant radon policy.”
The study was funded by the National Institute of Environmental Health Sciences and National Heart, Lung, and Blood Institute. Dr. Whitsel and coauthors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Exposure to even moderate concentrations of radon is associated with a significant increase in stroke risk, new research suggests.
Even moderate concentrations of radon were associated with a 6% higher stroke risk.
Radon is the second leading cause of lung cancer, but little was known about how exposure to the gas might affect stroke risk in women.
“Our research found an increased risk of stroke among participants exposed to radon above — and as many as 2 picocuries per liter (pCi/L) below — concentrations that usually trigger Environmental Protection Agency recommendations to install a home radon mitigation system,” senior author Eric A. Whitsel, MD, MPH, professor of epidemiology and medicine, University of North Carolina, Chapel Hill, said in a news release.
The study was published online on January 31, 2024, in Neurology.
Women Particularly Affected
Radon is a naturally occurring odorless radioactive gas produced when uranium or radium break down in rocks and soil. Its presence is increasing as a result of climate change, and it is increasingly being found in people’s homes. When inhaled, this air pollutant releases ionizing radiation in the lungs and is seen as second only to smoking as an established cause of lung cancer.
The National Radon Action Plan of the US Environmental Protection Agency (EPA) lays out testing and mitigation guidelines based on the known role of radon in lung carcinogenesis. But radon testing and mitigation are less common than recommended, and the EPA’s action plan doesn’t cover diseases other than lung cancer.
Compared with men, women have a higher rate of stroke and, in the US, typically spend about 11% more hours per day indoors at home, which investigators note highlights a “potential role of the residential environment among other risk factors specific to women.”
Researchers examined longitudinal associations between home radon exposure and incident stroke in 158,910 women at baseline (mean age 63.2 years; 83% White) over a mean follow-up of 13.4 years. During this time, participants experienced a total of 6979 strokes.
Participants’ home addresses were linked to radon concentration data drawn from the US Geological Survey and the EPA, which recommends that average indoor radon concentrations not exceed 4 pCi/L.
The highest radon exposure group resided in areas where average radon concentrations were < 4 pCi/L; the middle exposure group lived in regions with average concentrations of 2-4 pCi/L; and the lowest exposure group lived in areas with average concentrations < 2 pCi/L.
The researchers adjusted for demographic, social, behavioral, and clinical characteristics.
Public Health Implications
The incidence rates of stroke per 100,000 women in the lowest, middle, and highest radon concentration areas were 333, 343, and 349, respectively.
Stroke risk was 6% higher among those in the middle exposure group (adjusted hazard ratio [aHR], 1.06; 95% CI, 0.99-1.13) and 14% higher in the highest exposure group (aHR, 1.14; 95% CI, 1.05-1.22) compared with the lowest exposure group.
Notably, stroke risk was significant even at concentrations ranging from 2 to 4 pCi/L (P = .0004) vs < 2 pCi/L, which is below the EPA›s Radon Action Level for mitigation.
The findings remained robust in sensitivity analyses, although the associations were slightly stronger for ischemic stroke (especially cardioembolic, small-vessel occlusive, and very large artery atherosclerotic) compared with hemorrhagic stroke.
“Radon is an indoor air pollutant that can only be detected through testing that measures concentrations of the gas in homes,” Dr. Whitsel said in the release. “More studies are needed to confirm our findings. Confirmation would present an opportunity to improve public health by addressing an emerging risk factor for stroke.”
The study lacked gender and racial/ethnic diversity, so the findings may not be generalizable to other populations.
“Replication studies of individual-level radon exposures are needed to confirm this positive radon-stroke association,” the authors write. “Confirmation would present a potential opportunity to affect public health by addressing a pervasive environmental risk factor for stroke and thereby merit reconsideration of extant radon policy.”
The study was funded by the National Institute of Environmental Health Sciences and National Heart, Lung, and Blood Institute. Dr. Whitsel and coauthors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM NEUROLOGY
More Data Show Erectile Dysfunction Meds May Affect Alzheimer’s Risk
Men prescribed drugs to treat newly diagnosed erectile dysfunction (ED) are 18% less likely to develop Alzheimer’s disease (AD) during a 5-year follow-up period, new research shows.
The study is the second in recent years to suggest an association between the use of phosphodiesterase type 5 inhibitors (PDE5Is) such as sildenafil (Viagra) or tadalafil (Cialis) and AD risk. The findings contradict those in a third study, reported by this news organization, that showed no link between the two.
Although the research is interesting, outside experts noted that there is no evidence that the drugs can treat AD and urge caution when interpreting the findings.
Investigators agree but believe that the results offer a direction for future studies and underscore the importance of investigating whether existing approved therapies can be repurposed to treat AD.
“The positive findings from our large study in over 250,000 men is promising and can be used to enhance research capacity and knowledge, with a potential future impact on clinical use and public health policy,” senior author Ruth Brauer, PhD, of the University College London, told this news organization.
“However, before recommending PDE5I are used to reduce the risk of AD, more work is required to validate the findings of our work, particularly in a more generalizable population that includes women and men without erectile dysfunction,” she continued.
The findings were published online February 7 in Neurology.
Strong Association
The study drew on primary healthcare data from the United Kingdom and included 269,725 men (average age, 59 years) with newly diagnosed ED, 55% of whom had received prescriptions for PDE5Is.
Investigators accounted for a range of potential AD risk factors, including smoking status, alcohol use, body mass index, hypertension, diabetes, depression, anxiety, and concomitant medication use.
During the study period, 749 in the PDE5I group were diagnosed with AD, corresponding to a rate of 8.1 cases per 10,000 person-years. Among those who did not take the drugs, 370 developed AD, corresponding to a rate of 9.7 cases per 10,000 person-years.
Overall, initiation of a PDE5I was associated with an 18% lower risk for AD (adjusted hazard ration [aHR], 0.82; 95% CI, 0.72-0.93) compared with those with no prescriptions.
The association was stronger in people aged 70 years or older and those with a history of hypertension or diabetes. The greatest risk reduction was found in people with the most prescriptions during the study period. Those with 21-50 prescriptions had a 44% lower risk for AD (aHR, 0.56; 95% CI, 0.43-0.73) and those with more than 50 were 35% less likely to be diagnosed with AD (aHR, 0.65; 95% CI, 0.49-0.87).
There was no association with AD risk in individuals who received fewer than 20 prescriptions.
Investigators also analyzed associations after introducing a 1- and 3-year lag period after cohort entry to address the latent period between AD onset and diagnosis. The primary findings held with a 1-year lag period but lost significance with the inclusion of a 3-year lag period.
In subgroup analyses, investigators found evidence of reduced AD risk in those who received prescriptions for sildenafil (aHR, 0.81; 95% CI, 0.71-0.93), but there was no evidence for reduced risk compared with nonusers in those who received tadalafil and vardenafil.
Lower AD risk was found in patients with hypertension, diabetes, and in men aged 70 years or older, but there was no association in younger men or those with no history of hypertension or diabetes.
Although investigators controlled for a wide range of potential risk factors, Dr. Brauer noted that unmeasured confounders such as physical and sexual activity, which were not tracked and may predict PDE5I exposure, may have affected the results.
Interpret With Caution
Commenting on the findings, Ozama Ismail, PhD, Alzheimer’s Association director of scientific programs, noted that in addition to the limitations cited by the study authors, AD diagnoses were not made with the “gold standard” testing that typically includes imaging biomarkers and postmortem assessments.
“While this study is interesting and adds to a potential association, there is no evidence that these drugs are able to treat Alzheimer’s disease,” said Dr. Brauer, who was not part of the current study.
“People should not use over-the-counter phosphodiesterase type 5 inhibitors for prevention of Alzheimer’s or other dementias based on this very preliminary finding. Always consult with your physician before starting or changing your medications,” he cautioned.
However, Dr. Ismael added that the study does highlight a potential new avenue for drug repurposing.
“Repurposing of existing, already-approved treatments can be a valuable part of drug development because, through already-completed testing, we know much about their safety and side effects,” which can decrease cost and time needed for studies, he said.
“When considering repurposing an existing drug to an Alzheimer’s treatment, however, it is often important to conduct new studies over longer periods of time and in older people that reflect the diversity of individuals living with Alzheimer’s disease,” Dr. Ismael said.
Randomized Trials Needed
Dr. Brauer agreed, offering that such a trial should also include people with mild cognitive impairment and measure the effects of PDE5Is given in predefined doses plus an acetylcholinesterase inhibitor or placebo plus an acetylcholinesterase inhibitor.
“The primary outcome would be the change in baseline cognitive function,” she said. “This approach would provide a comprehensive understanding of the potential therapeutic benefits of PDE5I and AD.”
Studies are also needed to better understand the mechanisms by which these drugs might influence AD risk, Sevil Yasar, MD, PhD, and Lolita Nidadavolu, MD, PhD, from the Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, noted in an accompanying editorial.
The strong association between PDE5I use and AD risk in people with a history of hypertension or diabetes suggests “a potential neuroprotective effect through a vascular pathway,” they wrote.
In vitro studies on the role of inflammation and clearance of beta-amyloid could strengthen findings from studies like this one, and in vivo studies could help explain the mechanisms behind PDE5I use and lower AD risk, Dr. Yasar and Dr. Nidadavolu noted.
“In the end, however, further observational studies exploring mechanisms will not prove a causal association,” they wrote. “A well-designed randomized controlled trial is needed before PDE5I drugs can be prescribed for AD prevention.”
The study was unfunded. The study and editorial authors and Dr. Ismail report no relevant financial conflicts.
A version of this article appeared on Medscape.com.
Men prescribed drugs to treat newly diagnosed erectile dysfunction (ED) are 18% less likely to develop Alzheimer’s disease (AD) during a 5-year follow-up period, new research shows.
The study is the second in recent years to suggest an association between the use of phosphodiesterase type 5 inhibitors (PDE5Is) such as sildenafil (Viagra) or tadalafil (Cialis) and AD risk. The findings contradict those in a third study, reported by this news organization, that showed no link between the two.
Although the research is interesting, outside experts noted that there is no evidence that the drugs can treat AD and urge caution when interpreting the findings.
Investigators agree but believe that the results offer a direction for future studies and underscore the importance of investigating whether existing approved therapies can be repurposed to treat AD.
“The positive findings from our large study in over 250,000 men is promising and can be used to enhance research capacity and knowledge, with a potential future impact on clinical use and public health policy,” senior author Ruth Brauer, PhD, of the University College London, told this news organization.
“However, before recommending PDE5I are used to reduce the risk of AD, more work is required to validate the findings of our work, particularly in a more generalizable population that includes women and men without erectile dysfunction,” she continued.
The findings were published online February 7 in Neurology.
Strong Association
The study drew on primary healthcare data from the United Kingdom and included 269,725 men (average age, 59 years) with newly diagnosed ED, 55% of whom had received prescriptions for PDE5Is.
Investigators accounted for a range of potential AD risk factors, including smoking status, alcohol use, body mass index, hypertension, diabetes, depression, anxiety, and concomitant medication use.
During the study period, 749 in the PDE5I group were diagnosed with AD, corresponding to a rate of 8.1 cases per 10,000 person-years. Among those who did not take the drugs, 370 developed AD, corresponding to a rate of 9.7 cases per 10,000 person-years.
Overall, initiation of a PDE5I was associated with an 18% lower risk for AD (adjusted hazard ration [aHR], 0.82; 95% CI, 0.72-0.93) compared with those with no prescriptions.
The association was stronger in people aged 70 years or older and those with a history of hypertension or diabetes. The greatest risk reduction was found in people with the most prescriptions during the study period. Those with 21-50 prescriptions had a 44% lower risk for AD (aHR, 0.56; 95% CI, 0.43-0.73) and those with more than 50 were 35% less likely to be diagnosed with AD (aHR, 0.65; 95% CI, 0.49-0.87).
There was no association with AD risk in individuals who received fewer than 20 prescriptions.
Investigators also analyzed associations after introducing a 1- and 3-year lag period after cohort entry to address the latent period between AD onset and diagnosis. The primary findings held with a 1-year lag period but lost significance with the inclusion of a 3-year lag period.
In subgroup analyses, investigators found evidence of reduced AD risk in those who received prescriptions for sildenafil (aHR, 0.81; 95% CI, 0.71-0.93), but there was no evidence for reduced risk compared with nonusers in those who received tadalafil and vardenafil.
Lower AD risk was found in patients with hypertension, diabetes, and in men aged 70 years or older, but there was no association in younger men or those with no history of hypertension or diabetes.
Although investigators controlled for a wide range of potential risk factors, Dr. Brauer noted that unmeasured confounders such as physical and sexual activity, which were not tracked and may predict PDE5I exposure, may have affected the results.
Interpret With Caution
Commenting on the findings, Ozama Ismail, PhD, Alzheimer’s Association director of scientific programs, noted that in addition to the limitations cited by the study authors, AD diagnoses were not made with the “gold standard” testing that typically includes imaging biomarkers and postmortem assessments.
“While this study is interesting and adds to a potential association, there is no evidence that these drugs are able to treat Alzheimer’s disease,” said Dr. Brauer, who was not part of the current study.
“People should not use over-the-counter phosphodiesterase type 5 inhibitors for prevention of Alzheimer’s or other dementias based on this very preliminary finding. Always consult with your physician before starting or changing your medications,” he cautioned.
However, Dr. Ismael added that the study does highlight a potential new avenue for drug repurposing.
“Repurposing of existing, already-approved treatments can be a valuable part of drug development because, through already-completed testing, we know much about their safety and side effects,” which can decrease cost and time needed for studies, he said.
“When considering repurposing an existing drug to an Alzheimer’s treatment, however, it is often important to conduct new studies over longer periods of time and in older people that reflect the diversity of individuals living with Alzheimer’s disease,” Dr. Ismael said.
Randomized Trials Needed
Dr. Brauer agreed, offering that such a trial should also include people with mild cognitive impairment and measure the effects of PDE5Is given in predefined doses plus an acetylcholinesterase inhibitor or placebo plus an acetylcholinesterase inhibitor.
“The primary outcome would be the change in baseline cognitive function,” she said. “This approach would provide a comprehensive understanding of the potential therapeutic benefits of PDE5I and AD.”
Studies are also needed to better understand the mechanisms by which these drugs might influence AD risk, Sevil Yasar, MD, PhD, and Lolita Nidadavolu, MD, PhD, from the Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, noted in an accompanying editorial.
The strong association between PDE5I use and AD risk in people with a history of hypertension or diabetes suggests “a potential neuroprotective effect through a vascular pathway,” they wrote.
In vitro studies on the role of inflammation and clearance of beta-amyloid could strengthen findings from studies like this one, and in vivo studies could help explain the mechanisms behind PDE5I use and lower AD risk, Dr. Yasar and Dr. Nidadavolu noted.
“In the end, however, further observational studies exploring mechanisms will not prove a causal association,” they wrote. “A well-designed randomized controlled trial is needed before PDE5I drugs can be prescribed for AD prevention.”
The study was unfunded. The study and editorial authors and Dr. Ismail report no relevant financial conflicts.
A version of this article appeared on Medscape.com.
Men prescribed drugs to treat newly diagnosed erectile dysfunction (ED) are 18% less likely to develop Alzheimer’s disease (AD) during a 5-year follow-up period, new research shows.
The study is the second in recent years to suggest an association between the use of phosphodiesterase type 5 inhibitors (PDE5Is) such as sildenafil (Viagra) or tadalafil (Cialis) and AD risk. The findings contradict those in a third study, reported by this news organization, that showed no link between the two.
Although the research is interesting, outside experts noted that there is no evidence that the drugs can treat AD and urge caution when interpreting the findings.
Investigators agree but believe that the results offer a direction for future studies and underscore the importance of investigating whether existing approved therapies can be repurposed to treat AD.
“The positive findings from our large study in over 250,000 men is promising and can be used to enhance research capacity and knowledge, with a potential future impact on clinical use and public health policy,” senior author Ruth Brauer, PhD, of the University College London, told this news organization.
“However, before recommending PDE5I are used to reduce the risk of AD, more work is required to validate the findings of our work, particularly in a more generalizable population that includes women and men without erectile dysfunction,” she continued.
The findings were published online February 7 in Neurology.
Strong Association
The study drew on primary healthcare data from the United Kingdom and included 269,725 men (average age, 59 years) with newly diagnosed ED, 55% of whom had received prescriptions for PDE5Is.
Investigators accounted for a range of potential AD risk factors, including smoking status, alcohol use, body mass index, hypertension, diabetes, depression, anxiety, and concomitant medication use.
During the study period, 749 in the PDE5I group were diagnosed with AD, corresponding to a rate of 8.1 cases per 10,000 person-years. Among those who did not take the drugs, 370 developed AD, corresponding to a rate of 9.7 cases per 10,000 person-years.
Overall, initiation of a PDE5I was associated with an 18% lower risk for AD (adjusted hazard ration [aHR], 0.82; 95% CI, 0.72-0.93) compared with those with no prescriptions.
The association was stronger in people aged 70 years or older and those with a history of hypertension or diabetes. The greatest risk reduction was found in people with the most prescriptions during the study period. Those with 21-50 prescriptions had a 44% lower risk for AD (aHR, 0.56; 95% CI, 0.43-0.73) and those with more than 50 were 35% less likely to be diagnosed with AD (aHR, 0.65; 95% CI, 0.49-0.87).
There was no association with AD risk in individuals who received fewer than 20 prescriptions.
Investigators also analyzed associations after introducing a 1- and 3-year lag period after cohort entry to address the latent period between AD onset and diagnosis. The primary findings held with a 1-year lag period but lost significance with the inclusion of a 3-year lag period.
In subgroup analyses, investigators found evidence of reduced AD risk in those who received prescriptions for sildenafil (aHR, 0.81; 95% CI, 0.71-0.93), but there was no evidence for reduced risk compared with nonusers in those who received tadalafil and vardenafil.
Lower AD risk was found in patients with hypertension, diabetes, and in men aged 70 years or older, but there was no association in younger men or those with no history of hypertension or diabetes.
Although investigators controlled for a wide range of potential risk factors, Dr. Brauer noted that unmeasured confounders such as physical and sexual activity, which were not tracked and may predict PDE5I exposure, may have affected the results.
Interpret With Caution
Commenting on the findings, Ozama Ismail, PhD, Alzheimer’s Association director of scientific programs, noted that in addition to the limitations cited by the study authors, AD diagnoses were not made with the “gold standard” testing that typically includes imaging biomarkers and postmortem assessments.
“While this study is interesting and adds to a potential association, there is no evidence that these drugs are able to treat Alzheimer’s disease,” said Dr. Brauer, who was not part of the current study.
“People should not use over-the-counter phosphodiesterase type 5 inhibitors for prevention of Alzheimer’s or other dementias based on this very preliminary finding. Always consult with your physician before starting or changing your medications,” he cautioned.
However, Dr. Ismael added that the study does highlight a potential new avenue for drug repurposing.
“Repurposing of existing, already-approved treatments can be a valuable part of drug development because, through already-completed testing, we know much about their safety and side effects,” which can decrease cost and time needed for studies, he said.
“When considering repurposing an existing drug to an Alzheimer’s treatment, however, it is often important to conduct new studies over longer periods of time and in older people that reflect the diversity of individuals living with Alzheimer’s disease,” Dr. Ismael said.
Randomized Trials Needed
Dr. Brauer agreed, offering that such a trial should also include people with mild cognitive impairment and measure the effects of PDE5Is given in predefined doses plus an acetylcholinesterase inhibitor or placebo plus an acetylcholinesterase inhibitor.
“The primary outcome would be the change in baseline cognitive function,” she said. “This approach would provide a comprehensive understanding of the potential therapeutic benefits of PDE5I and AD.”
Studies are also needed to better understand the mechanisms by which these drugs might influence AD risk, Sevil Yasar, MD, PhD, and Lolita Nidadavolu, MD, PhD, from the Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, noted in an accompanying editorial.
The strong association between PDE5I use and AD risk in people with a history of hypertension or diabetes suggests “a potential neuroprotective effect through a vascular pathway,” they wrote.
In vitro studies on the role of inflammation and clearance of beta-amyloid could strengthen findings from studies like this one, and in vivo studies could help explain the mechanisms behind PDE5I use and lower AD risk, Dr. Yasar and Dr. Nidadavolu noted.
“In the end, however, further observational studies exploring mechanisms will not prove a causal association,” they wrote. “A well-designed randomized controlled trial is needed before PDE5I drugs can be prescribed for AD prevention.”
The study was unfunded. The study and editorial authors and Dr. Ismail report no relevant financial conflicts.
A version of this article appeared on Medscape.com.
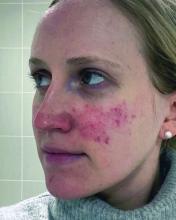
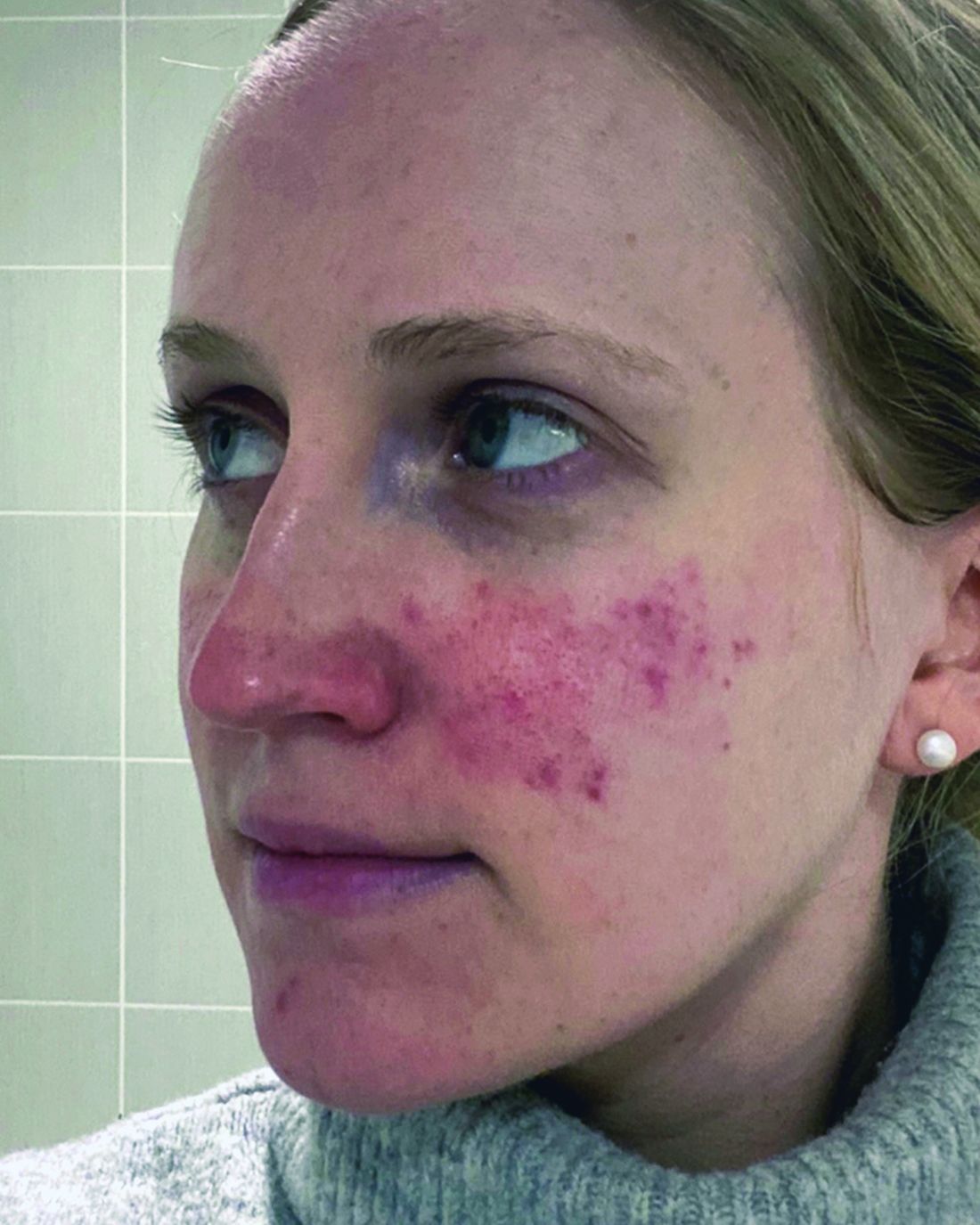
Despite An AI Assist, Imaging Study Shows Disparities in Diagnosing Different Skin Tones
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
When clinicians in a large-scale study viewed a series of digital images that showed skin diseases across skin tones and were asked to make a diagnosis, the accuracy was 38% among dermatologists and 19% among primary care physicians (PCPs). But when decision support from a deep learning system (DLS) was introduced, diagnostic accuracy increased by 33% among dermatologists and 69% among PCPs, results from a multicenter study showed.
However, the researchers found that across all images, diseases in dark skin (Fitzpatrick skin types 5 and 6) were diagnosed less accurately than diseases in light skin (Fitzpatrick skin types 1-4).
“,” researchers led by Matthew Groh, PhD, of Northwestern University’s Kellogg School of Management, wrote in their study, published online in Nature Medicine.
For the study, 389 board-certified dermatologists and 450 PCPs in 39 countries were presented with 364 images to view spanning 46 skin diseases and asked to submit up to four differential diagnoses. Nearly 80% of the images were of 8 diseases: atopic dermatitis, cutaneous T-cell lymphoma (CTCL), dermatomyositis, lichen planus, Lyme disease, pityriasis rosea, pityriasis rubra pilaris, and secondary syphilis.
Dermatologists and PCPs achieved a diagnostic accuracy of 38% and 19%, respectively, but both groups of clinicians were 4 percentage points less accurate for diagnosis of images of dark skin as compared with light skin. With assistance from DLS decision support, diagnostic accuracy increased by 33% among dermatologists and 69% among primary care physicians. Among dermatologists, DLS support generally increased diagnostic accuracy evenly across skin tones. However, among PCPs, DLS support increased their diagnostic accuracy more in light skin tones than in dark ones.
In the survey component of the study, when the participants were asked, “Do you feel you received sufficient training for diagnosing skin diseases in patients with skin of color (non-white patients)?” 67% of all PCPs and 33% of all dermatologists responded no. “Furthermore, we have found differences in how often BCDs [board-certified dermatologists] and PCPs refer patients with light and dark skin for biopsy,” the authors wrote. “Specifically, for CTCL (a life-threatening disease), we found that both BCDs and PCPs report that they would refer patients for biopsy significantly more often in light skin than dark skin. Moreover, for the common skin diseases atopic dermatitis and pityriasis rosea, we found that BCDs report they would refer patients for biopsy more often in dark skin than light skin, which creates an unnecessary overburden on patients with dark skin.”
In a press release about the study, Dr. Groh emphasized that he and other scientists who investigate human-computer interaction “have to find a way to incorporate underrepresented demographics in our research. That way we will be ready to accurately implement these models in the real world and build AI systems that serve as tools that are designed to avoid the kind of systematic errors we know humans and machines are prone to. Then you can update curricula, you can change norms in different fields and hopefully everyone gets better.”
Ronald Moy, MD, a dermatologist who practices in Beverly Hills, Calif., who was asked to comment on the work, said that the study contributes insights into physician-AI interaction and highlights the need for further training on diagnosing skin diseases in people with darker skin tones. “The strengths of this study include its large sample size of dermatologists and primary care physicians, use of quality-controlled images across skin tones, and thorough evaluation of diagnostic accuracy with and without AI assistance,” said Dr. Moy, who is a past president of the American Academy of Dermatology, the American Society for Dermatologic Surgery, and the American Board of Facial Cosmetic Surgery.
“The study is limited to diagnosis and skin tone estimation based purely on a single image, which does not fully represent a clinical evaluation,” he added. However, “it does provide important benchmark data on diagnostic accuracy disparities across skin tones, but also demonstrates that while AI assistance can improve overall diagnostic accuracy, it may exacerbate disparities for non-specialists.”
Funding for the study was provided by MIT Media Lab consortium members and the Harold Horowitz Student Research Fund. One of the study authors, P. Murali Doraiswamy, MBBS, disclosed that he has received grants, advisory fees, and/or stock from several biotechnology companies outside the scope of this work and that he is a co-inventor on several patents through Duke University. The remaining authors reported having no disclosures. Dr. Moy reported having no disclosures.
FROM NATURE MEDICINE
Preventing Gout Flares and Hospitalizations Means Targeting These Serum Urate Levels
Clinical efforts to get patients with a history of gout to reach specific target serum urate (SU) levels less than either 5 or 6 mg/dL could prevent the great majority of gout flares and hospitalizations for them, according to a new study that tracked patients for a mean of 8.3 years.
The findings, which appeared February 6 in JAMA, “support the value of target serum urate levels in gout flare prevention in primary care, where most gout patients are treated,” rheumatologist and study coauthor Hyon K. Choi, MD, DrPH, of Harvard Medical School and Massachusetts General Hospital, Boston, told this news organization. However, Dr. Choi noted that “the value of relying on target urate levels is not accepted in primary care practice,” and the author of an accompanying commentary said that the jury is still out about the best strategy to prevent flares.
Gout is caused by monosodium urate crystallization within the joints, which occurs when SU levels exceed the saturation point for uric acid crystallization in the body: approximately 6.8 mg/dL. “Studies have found strongly graded associations between serum urate levels above the saturation point and the risk of developing new cases of gout among individuals without gout at baseline,” Dr. Choi said. “However, associations between serum urate levels and the risk of recurrent flares among preexisting gout patients, which is relevant to clinical gout care practice, has not been established.”
Dr. Choi added that “despite the emphasis in US and European rheumatology guidelines on the use of urate-lowering therapy to treat-to-target serum urate level — eg, under 6 or 5 mg/dL — the proportions of flares associated with such target urate levels remained unknown.”
Study Shows Relationship Between SU Levels and Recurrent Flares
For the study, researchers tracked 3613 patients aged 40-69 with gout in the UK Biobank database from 2006-2010 to 2017 or 2020. The patients, 86% of whom were men, had a mean age of 60 years and about 96% were White.
Among the patients, 1773 new episodes of acute gout occurred in 27% of the patients (16% had one episode, 6% had two episodes, and 5% had at least three episodes). These were treated in primary care or required hospitalizations. The other 73% of patients had no new acute gout episodes.
Overall, 95% of flares occurred in those with baseline SU levels ≥ 6 mg/dL, and 98% occurred in those with levels ≥ 5 mg/dL.
Patients with baseline SU levels < 6.0 mg/dL had an acute gout flare rate of 10.6 per 1000 person-years. In comparison, relative risks for acute gout flares per 1000 person-years were 3.16 at baseline SU levels of 6.0-6.9 mg/dL, 6.20 for 7.0-7.9 mg/dL, 7.70 for 8.0-8.9 mg/dL, 9.80 for 9.0-9.9 mg/dL, and 11.26 for > 10 mg/dL after adjustment for various possible confounders (P < .001).
The researchers identified 64 hospitalizations with gout as the main discharge diagnosis, and 97% occurred in patients with baseline SU levels ≥ 6 mg/dL. All were in patients with baseline SU levels ≥ 5 mg/dL.
“An important feature of this study was that serum urate measurements were obtained from all gout patients at the study baseline, irrespective of clinical needs or flare status,” Dr. Choi said. “Prior studies failed to reveal the truly compelling nature of relations between serum urate levels and recurrent flares among preexisting gout patients.”
As for the cost of SU tests, Dr. Choi said they can run as low as $2. “Portable tests similar to home glucose measurement for diabetes patients are also being adopted by certain gout care practices,” he said.
The findings matter, Dr. Choi said, because SU is not tracked in the “vast majority of gout patients” in primary care. Instead, primary care doctors — as per the guidelines of the American College of Physicians — often adopt an approach that treats symptoms as needed instead of tracking and lowering SU levels, he said. In fact, “95% and 98% of gout flares can be potentially preventable at the population level if serum urate levels < 6 and < 5 mg/dL can be met, respectively, and 100% of hospitalizations for gout could be preventable with serum urate < 5 mg/dL,” he said.
As for limitations, the authors noted that participants in the UK Biobank “typically have a better socioeconomic status and are healthier than the UK general population,” and they added that “these data may underestimate the number of acute gout flares in the cohort.” Also, 55% of the total 502,490 patients in the UK Biobank were excluded owing to lack of primary care data.
Study ‘Offers the Kind of Evidence That We Need’
In an accompanying commentary, University of Alabama at Birmingham rheumatologist Angelo L. Gaffo, MD, MSPH, also noted that the study population was overwhelmingly White, had a low mean SU level (6.9 mg/dL), and had a low level of comorbidities, making the sample “poorly representative of the most commonly described gout populations.”
However, he also noted that there is “growing evidence linking serum urate levels with clinical outcomes,” with a pair of studies — one from 2021 and the other from 2022 — linking reductions in SU to < 6 md/dL to lower flare rates.
Dr. Gaffo told this news organization that although rheumatology guidelines support a treat-to-target strategy, “we haven›t generated a whole lot of important evidence to support it.”
The new study “offers the kind of evidence that we need,” he said, “but this is not going to be the ultimate answer.” That will only come from randomized clinical trials in the works that will pit the treat-to-target approach vs the primary care–favored strategy of titrating treatment until flares are controlled, he said.
Even though evidence is sparse, Dr. Gaffo said he still believes in the treat-to-target strategy: “I believe it is the best way to treat gout.”
What’s next? Researchers hope to understand how to better reach target SU goals in clinical practice, Dr. Choi said. “Involving nurses, pharmacists, or interactive online or app systems — as in other chronic treat-to-target care such as anticoagulation care, blood pressure, or lipid care — is actively being researched.”
He added that “we are trying to find the effective and safe medications and nonpharmacologic measures to reduce the urate burden, which can also simultaneously take care of gout’s frequent cardiovascular-kidney comorbidities.”
The US National Institutes of Health supported the study. Dr. Choi reports receiving grants from Horizon and serving on a board or committee for LG Chem, Shanton, and ANI Pharmaceuticals. Some other authors report an employment and stockholder relationship with Regeneron and support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, and Rheumatology Research Foundation. Dr. Gaffo reports personal fees from PK MED, SOBI/Selecta, Atom, and UpToDate.
A version of this article first appeared on Medscape.com.
Clinical efforts to get patients with a history of gout to reach specific target serum urate (SU) levels less than either 5 or 6 mg/dL could prevent the great majority of gout flares and hospitalizations for them, according to a new study that tracked patients for a mean of 8.3 years.
The findings, which appeared February 6 in JAMA, “support the value of target serum urate levels in gout flare prevention in primary care, where most gout patients are treated,” rheumatologist and study coauthor Hyon K. Choi, MD, DrPH, of Harvard Medical School and Massachusetts General Hospital, Boston, told this news organization. However, Dr. Choi noted that “the value of relying on target urate levels is not accepted in primary care practice,” and the author of an accompanying commentary said that the jury is still out about the best strategy to prevent flares.
Gout is caused by monosodium urate crystallization within the joints, which occurs when SU levels exceed the saturation point for uric acid crystallization in the body: approximately 6.8 mg/dL. “Studies have found strongly graded associations between serum urate levels above the saturation point and the risk of developing new cases of gout among individuals without gout at baseline,” Dr. Choi said. “However, associations between serum urate levels and the risk of recurrent flares among preexisting gout patients, which is relevant to clinical gout care practice, has not been established.”
Dr. Choi added that “despite the emphasis in US and European rheumatology guidelines on the use of urate-lowering therapy to treat-to-target serum urate level — eg, under 6 or 5 mg/dL — the proportions of flares associated with such target urate levels remained unknown.”
Study Shows Relationship Between SU Levels and Recurrent Flares
For the study, researchers tracked 3613 patients aged 40-69 with gout in the UK Biobank database from 2006-2010 to 2017 or 2020. The patients, 86% of whom were men, had a mean age of 60 years and about 96% were White.
Among the patients, 1773 new episodes of acute gout occurred in 27% of the patients (16% had one episode, 6% had two episodes, and 5% had at least three episodes). These were treated in primary care or required hospitalizations. The other 73% of patients had no new acute gout episodes.
Overall, 95% of flares occurred in those with baseline SU levels ≥ 6 mg/dL, and 98% occurred in those with levels ≥ 5 mg/dL.
Patients with baseline SU levels < 6.0 mg/dL had an acute gout flare rate of 10.6 per 1000 person-years. In comparison, relative risks for acute gout flares per 1000 person-years were 3.16 at baseline SU levels of 6.0-6.9 mg/dL, 6.20 for 7.0-7.9 mg/dL, 7.70 for 8.0-8.9 mg/dL, 9.80 for 9.0-9.9 mg/dL, and 11.26 for > 10 mg/dL after adjustment for various possible confounders (P < .001).
The researchers identified 64 hospitalizations with gout as the main discharge diagnosis, and 97% occurred in patients with baseline SU levels ≥ 6 mg/dL. All were in patients with baseline SU levels ≥ 5 mg/dL.
“An important feature of this study was that serum urate measurements were obtained from all gout patients at the study baseline, irrespective of clinical needs or flare status,” Dr. Choi said. “Prior studies failed to reveal the truly compelling nature of relations between serum urate levels and recurrent flares among preexisting gout patients.”
As for the cost of SU tests, Dr. Choi said they can run as low as $2. “Portable tests similar to home glucose measurement for diabetes patients are also being adopted by certain gout care practices,” he said.
The findings matter, Dr. Choi said, because SU is not tracked in the “vast majority of gout patients” in primary care. Instead, primary care doctors — as per the guidelines of the American College of Physicians — often adopt an approach that treats symptoms as needed instead of tracking and lowering SU levels, he said. In fact, “95% and 98% of gout flares can be potentially preventable at the population level if serum urate levels < 6 and < 5 mg/dL can be met, respectively, and 100% of hospitalizations for gout could be preventable with serum urate < 5 mg/dL,” he said.
As for limitations, the authors noted that participants in the UK Biobank “typically have a better socioeconomic status and are healthier than the UK general population,” and they added that “these data may underestimate the number of acute gout flares in the cohort.” Also, 55% of the total 502,490 patients in the UK Biobank were excluded owing to lack of primary care data.
Study ‘Offers the Kind of Evidence That We Need’
In an accompanying commentary, University of Alabama at Birmingham rheumatologist Angelo L. Gaffo, MD, MSPH, also noted that the study population was overwhelmingly White, had a low mean SU level (6.9 mg/dL), and had a low level of comorbidities, making the sample “poorly representative of the most commonly described gout populations.”
However, he also noted that there is “growing evidence linking serum urate levels with clinical outcomes,” with a pair of studies — one from 2021 and the other from 2022 — linking reductions in SU to < 6 md/dL to lower flare rates.
Dr. Gaffo told this news organization that although rheumatology guidelines support a treat-to-target strategy, “we haven›t generated a whole lot of important evidence to support it.”
The new study “offers the kind of evidence that we need,” he said, “but this is not going to be the ultimate answer.” That will only come from randomized clinical trials in the works that will pit the treat-to-target approach vs the primary care–favored strategy of titrating treatment until flares are controlled, he said.
Even though evidence is sparse, Dr. Gaffo said he still believes in the treat-to-target strategy: “I believe it is the best way to treat gout.”
What’s next? Researchers hope to understand how to better reach target SU goals in clinical practice, Dr. Choi said. “Involving nurses, pharmacists, or interactive online or app systems — as in other chronic treat-to-target care such as anticoagulation care, blood pressure, or lipid care — is actively being researched.”
He added that “we are trying to find the effective and safe medications and nonpharmacologic measures to reduce the urate burden, which can also simultaneously take care of gout’s frequent cardiovascular-kidney comorbidities.”
The US National Institutes of Health supported the study. Dr. Choi reports receiving grants from Horizon and serving on a board or committee for LG Chem, Shanton, and ANI Pharmaceuticals. Some other authors report an employment and stockholder relationship with Regeneron and support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, and Rheumatology Research Foundation. Dr. Gaffo reports personal fees from PK MED, SOBI/Selecta, Atom, and UpToDate.
A version of this article first appeared on Medscape.com.
Clinical efforts to get patients with a history of gout to reach specific target serum urate (SU) levels less than either 5 or 6 mg/dL could prevent the great majority of gout flares and hospitalizations for them, according to a new study that tracked patients for a mean of 8.3 years.
The findings, which appeared February 6 in JAMA, “support the value of target serum urate levels in gout flare prevention in primary care, where most gout patients are treated,” rheumatologist and study coauthor Hyon K. Choi, MD, DrPH, of Harvard Medical School and Massachusetts General Hospital, Boston, told this news organization. However, Dr. Choi noted that “the value of relying on target urate levels is not accepted in primary care practice,” and the author of an accompanying commentary said that the jury is still out about the best strategy to prevent flares.
Gout is caused by monosodium urate crystallization within the joints, which occurs when SU levels exceed the saturation point for uric acid crystallization in the body: approximately 6.8 mg/dL. “Studies have found strongly graded associations between serum urate levels above the saturation point and the risk of developing new cases of gout among individuals without gout at baseline,” Dr. Choi said. “However, associations between serum urate levels and the risk of recurrent flares among preexisting gout patients, which is relevant to clinical gout care practice, has not been established.”
Dr. Choi added that “despite the emphasis in US and European rheumatology guidelines on the use of urate-lowering therapy to treat-to-target serum urate level — eg, under 6 or 5 mg/dL — the proportions of flares associated with such target urate levels remained unknown.”
Study Shows Relationship Between SU Levels and Recurrent Flares
For the study, researchers tracked 3613 patients aged 40-69 with gout in the UK Biobank database from 2006-2010 to 2017 or 2020. The patients, 86% of whom were men, had a mean age of 60 years and about 96% were White.
Among the patients, 1773 new episodes of acute gout occurred in 27% of the patients (16% had one episode, 6% had two episodes, and 5% had at least three episodes). These were treated in primary care or required hospitalizations. The other 73% of patients had no new acute gout episodes.
Overall, 95% of flares occurred in those with baseline SU levels ≥ 6 mg/dL, and 98% occurred in those with levels ≥ 5 mg/dL.
Patients with baseline SU levels < 6.0 mg/dL had an acute gout flare rate of 10.6 per 1000 person-years. In comparison, relative risks for acute gout flares per 1000 person-years were 3.16 at baseline SU levels of 6.0-6.9 mg/dL, 6.20 for 7.0-7.9 mg/dL, 7.70 for 8.0-8.9 mg/dL, 9.80 for 9.0-9.9 mg/dL, and 11.26 for > 10 mg/dL after adjustment for various possible confounders (P < .001).
The researchers identified 64 hospitalizations with gout as the main discharge diagnosis, and 97% occurred in patients with baseline SU levels ≥ 6 mg/dL. All were in patients with baseline SU levels ≥ 5 mg/dL.
“An important feature of this study was that serum urate measurements were obtained from all gout patients at the study baseline, irrespective of clinical needs or flare status,” Dr. Choi said. “Prior studies failed to reveal the truly compelling nature of relations between serum urate levels and recurrent flares among preexisting gout patients.”
As for the cost of SU tests, Dr. Choi said they can run as low as $2. “Portable tests similar to home glucose measurement for diabetes patients are also being adopted by certain gout care practices,” he said.
The findings matter, Dr. Choi said, because SU is not tracked in the “vast majority of gout patients” in primary care. Instead, primary care doctors — as per the guidelines of the American College of Physicians — often adopt an approach that treats symptoms as needed instead of tracking and lowering SU levels, he said. In fact, “95% and 98% of gout flares can be potentially preventable at the population level if serum urate levels < 6 and < 5 mg/dL can be met, respectively, and 100% of hospitalizations for gout could be preventable with serum urate < 5 mg/dL,” he said.
As for limitations, the authors noted that participants in the UK Biobank “typically have a better socioeconomic status and are healthier than the UK general population,” and they added that “these data may underestimate the number of acute gout flares in the cohort.” Also, 55% of the total 502,490 patients in the UK Biobank were excluded owing to lack of primary care data.
Study ‘Offers the Kind of Evidence That We Need’
In an accompanying commentary, University of Alabama at Birmingham rheumatologist Angelo L. Gaffo, MD, MSPH, also noted that the study population was overwhelmingly White, had a low mean SU level (6.9 mg/dL), and had a low level of comorbidities, making the sample “poorly representative of the most commonly described gout populations.”
However, he also noted that there is “growing evidence linking serum urate levels with clinical outcomes,” with a pair of studies — one from 2021 and the other from 2022 — linking reductions in SU to < 6 md/dL to lower flare rates.
Dr. Gaffo told this news organization that although rheumatology guidelines support a treat-to-target strategy, “we haven›t generated a whole lot of important evidence to support it.”
The new study “offers the kind of evidence that we need,” he said, “but this is not going to be the ultimate answer.” That will only come from randomized clinical trials in the works that will pit the treat-to-target approach vs the primary care–favored strategy of titrating treatment until flares are controlled, he said.
Even though evidence is sparse, Dr. Gaffo said he still believes in the treat-to-target strategy: “I believe it is the best way to treat gout.”
What’s next? Researchers hope to understand how to better reach target SU goals in clinical practice, Dr. Choi said. “Involving nurses, pharmacists, or interactive online or app systems — as in other chronic treat-to-target care such as anticoagulation care, blood pressure, or lipid care — is actively being researched.”
He added that “we are trying to find the effective and safe medications and nonpharmacologic measures to reduce the urate burden, which can also simultaneously take care of gout’s frequent cardiovascular-kidney comorbidities.”
The US National Institutes of Health supported the study. Dr. Choi reports receiving grants from Horizon and serving on a board or committee for LG Chem, Shanton, and ANI Pharmaceuticals. Some other authors report an employment and stockholder relationship with Regeneron and support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, and Rheumatology Research Foundation. Dr. Gaffo reports personal fees from PK MED, SOBI/Selecta, Atom, and UpToDate.
A version of this article first appeared on Medscape.com.
FROM JAMA
Higher HDL Tied to Prediabetes Reversion — Up to a Point
TOPLINE:
Higher high-density lipoprotein cholesterol (HDL-C) levels show a positive association with prediabetes reversal to normoglycemia in Chinese adults, but only up to a certain threshold.
METHODOLOGY:
- Researchers examined the correlation between HDL-C levels and the reversion of people with prediabetes to normoglycemia in a secondary analysis of data from a population-based cohort study.
- The analysis included 15,420 Chinese patients with prediabetes who underwent health screening between 2010 and 2016 (mean age, 51 ± 13 years; 5414 (35%) women).
- The outcome measure, reversion to normoglycemia, was determined by no self-reported diabetic event and fasting plasma glucose < 5.6 mmol/L at follow-up.
- They categorized the adults into four groups on the basis of HDL-C quartiles.
- They used multiple statistical models to investigate the association between HDL-C levels and reversion from prediabetes, assess the linearity of the association, and account for independent variables and confounding factors.
TAKEAWAY:
- After a median follow-up of nearly 3 years, 6627 (43%) of patients with prediabetes had a reversion to normoglycemia.
- The groups with higher HDL-C levels had a higher likelihood of prediabetes reversal to normoglycemia (adjusted hazard ratio [HR], 1.90; P < .001).
- They found a nonlinear association and threshold effect: The probability of reversal from prediabetes to normoglycemia stabilized rather than continued to increase at an inflection point (1.54 mmol/L in men, 1.62 mmol/L in women).
- A significant positive correlation with reversal to normoglycemia was observed below the HDL-C threshold (men: HR, 2.78; 95% CI, 2.37-3.26; women: HR, 2.22; 95% CI, 1.80-2.73).
IN PRACTICE:
“Keeping HDL-C levels near the inflection point in patients with prediabetes may greatly increase the likelihood of reversion from prediabetes to normoglycemia,” the authors wrote.
SOURCE:
The study, with lead author Zihe Mo, Department of Physical Examination, Dongguan Tungwah Hospital, Dongguan, China, was published online in Scientific Reports.
LIMITATIONS:
The study included individuals of Chinese descent, necessitating more studies into the HDL-C and normoglycemia relationship across diverse genetic backgrounds. The study relied solely on fasting plasma glucose measurements and was unable to capture the entirety of prediabetes complexity. As a secondary analysis of previously published data, the study faces limitations in managing unmeasured variables not initially included in the dataset. The observational study cannot determine a causal relationship between HDL-C and reversion from prediabetes to normoglycemia.
DISCLOSURES:
The study was supported by the Natural Science Funding of China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher high-density lipoprotein cholesterol (HDL-C) levels show a positive association with prediabetes reversal to normoglycemia in Chinese adults, but only up to a certain threshold.
METHODOLOGY:
- Researchers examined the correlation between HDL-C levels and the reversion of people with prediabetes to normoglycemia in a secondary analysis of data from a population-based cohort study.
- The analysis included 15,420 Chinese patients with prediabetes who underwent health screening between 2010 and 2016 (mean age, 51 ± 13 years; 5414 (35%) women).
- The outcome measure, reversion to normoglycemia, was determined by no self-reported diabetic event and fasting plasma glucose < 5.6 mmol/L at follow-up.
- They categorized the adults into four groups on the basis of HDL-C quartiles.
- They used multiple statistical models to investigate the association between HDL-C levels and reversion from prediabetes, assess the linearity of the association, and account for independent variables and confounding factors.
TAKEAWAY:
- After a median follow-up of nearly 3 years, 6627 (43%) of patients with prediabetes had a reversion to normoglycemia.
- The groups with higher HDL-C levels had a higher likelihood of prediabetes reversal to normoglycemia (adjusted hazard ratio [HR], 1.90; P < .001).
- They found a nonlinear association and threshold effect: The probability of reversal from prediabetes to normoglycemia stabilized rather than continued to increase at an inflection point (1.54 mmol/L in men, 1.62 mmol/L in women).
- A significant positive correlation with reversal to normoglycemia was observed below the HDL-C threshold (men: HR, 2.78; 95% CI, 2.37-3.26; women: HR, 2.22; 95% CI, 1.80-2.73).
IN PRACTICE:
“Keeping HDL-C levels near the inflection point in patients with prediabetes may greatly increase the likelihood of reversion from prediabetes to normoglycemia,” the authors wrote.
SOURCE:
The study, with lead author Zihe Mo, Department of Physical Examination, Dongguan Tungwah Hospital, Dongguan, China, was published online in Scientific Reports.
LIMITATIONS:
The study included individuals of Chinese descent, necessitating more studies into the HDL-C and normoglycemia relationship across diverse genetic backgrounds. The study relied solely on fasting plasma glucose measurements and was unable to capture the entirety of prediabetes complexity. As a secondary analysis of previously published data, the study faces limitations in managing unmeasured variables not initially included in the dataset. The observational study cannot determine a causal relationship between HDL-C and reversion from prediabetes to normoglycemia.
DISCLOSURES:
The study was supported by the Natural Science Funding of China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher high-density lipoprotein cholesterol (HDL-C) levels show a positive association with prediabetes reversal to normoglycemia in Chinese adults, but only up to a certain threshold.
METHODOLOGY:
- Researchers examined the correlation between HDL-C levels and the reversion of people with prediabetes to normoglycemia in a secondary analysis of data from a population-based cohort study.
- The analysis included 15,420 Chinese patients with prediabetes who underwent health screening between 2010 and 2016 (mean age, 51 ± 13 years; 5414 (35%) women).
- The outcome measure, reversion to normoglycemia, was determined by no self-reported diabetic event and fasting plasma glucose < 5.6 mmol/L at follow-up.
- They categorized the adults into four groups on the basis of HDL-C quartiles.
- They used multiple statistical models to investigate the association between HDL-C levels and reversion from prediabetes, assess the linearity of the association, and account for independent variables and confounding factors.
TAKEAWAY:
- After a median follow-up of nearly 3 years, 6627 (43%) of patients with prediabetes had a reversion to normoglycemia.
- The groups with higher HDL-C levels had a higher likelihood of prediabetes reversal to normoglycemia (adjusted hazard ratio [HR], 1.90; P < .001).
- They found a nonlinear association and threshold effect: The probability of reversal from prediabetes to normoglycemia stabilized rather than continued to increase at an inflection point (1.54 mmol/L in men, 1.62 mmol/L in women).
- A significant positive correlation with reversal to normoglycemia was observed below the HDL-C threshold (men: HR, 2.78; 95% CI, 2.37-3.26; women: HR, 2.22; 95% CI, 1.80-2.73).
IN PRACTICE:
“Keeping HDL-C levels near the inflection point in patients with prediabetes may greatly increase the likelihood of reversion from prediabetes to normoglycemia,” the authors wrote.
SOURCE:
The study, with lead author Zihe Mo, Department of Physical Examination, Dongguan Tungwah Hospital, Dongguan, China, was published online in Scientific Reports.
LIMITATIONS:
The study included individuals of Chinese descent, necessitating more studies into the HDL-C and normoglycemia relationship across diverse genetic backgrounds. The study relied solely on fasting plasma glucose measurements and was unable to capture the entirety of prediabetes complexity. As a secondary analysis of previously published data, the study faces limitations in managing unmeasured variables not initially included in the dataset. The observational study cannot determine a causal relationship between HDL-C and reversion from prediabetes to normoglycemia.
DISCLOSURES:
The study was supported by the Natural Science Funding of China. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
10 Reasons to Refer Your Patient to an Endocrinologist
The blockbuster drugs of the century have arrived: glucagon-like peptide 1 receptor agonists (GLP-1 RAs). These drugs were developed to control blood sugar but have gained immense popularity for weight loss. Patients are clamoring for the drugs, and physicians are inundated with patient inquiries.
As doctors in primary care and other specialties are discovering, the GLP-1 RA drugs add another layer of complexity to the long-term management of a chronic disease. Managing diabetes and obesity requires a multidisciplinary team and a multispecialty treatment approach.
That’s why it’s more important than ever to know when and why to refer patients to an endocrinologist, who can offer unparalleled expertise as part of a multidisciplinary treatment approach.
Here are 10 reasons to refer your patients with diabetes to an endocrinologist.
1. To help make an optimal medication choice. Endocrinologists navigate diabetes management by considering individualized glycemic, cardiorenal, and weight goals as per guidelines, incorporating knowledge of medication side effects, simplifying regimens for adherence, and addressing practical factors like access and cost. Optimal medication selection is crucial, as a recent study found that nearly two thirds of patients altered their treatment by discontinuing their medication, switching their medication, or changing the dose of their medication within 12 months. Whether diabetes is controlled or uncontrolled, patients should consult an endocrinologist due to the potential complexity of cases, including late autoimmune onset of diabetes; medication-induced diabetes; and factors such as age, fragility, and chronic illnesses.
2. To facilitate medication approvals, alternatives, and authorizations. Attaining medication approval for patients entails a nuanced understanding and resources. Through experience and careful consideration, endocrinologists develop insights into potential barriers, especially in cases where approval for specific medications necessitates prior failures with multiple GLP-1 RAs or antihyperglycemic agents. This expertise positions them to advocate effectively for alternative options, often involving the meticulous process of prior authorizations. Certain endocrinology practices may augment this endeavor by offering dedicated resources, such as a specialized prior authorization team.
3. To deal with diabetes complications. Endocrinologists can help address emerging issues in GLP-1 RA drugs such as retinopathy, gastroparesis, and mental health effects. They can also help manage coexisting conditions, such as addressing thyroid nodules before considering the use of GLP-1 RAs. Recognizing the interconnected nature of diabetes and its influence on diverse body systems, endocrinologists ensure a thorough and integrated management strategy for their patients.
4. To titrate other glucose-lowering agents. Patients with diabetes are often on combination therapy. Endocrinologists adeptly adjust and titrate these treatments to optimize glucose control while minimizing side effects like hypoglycemia. Beyond insulin, their expertise encompasses various glucose-lowering agents. Notably, patients who use GLP-1 RAs in combination with medications such as insulin secretagogues (eg, sulfonylurea) and insulin face an elevated risk for hypoglycemia, including severe cases, necessitating careful titration to mitigate these effects.
5. To integrate advances in diabetes technology. Endocrinologists stay abreast of technological advancements in diabetes care, incorporating innovations in monitoring and treatment strategies such as continuous glucose monitors and insulin pumps. This ensures that patients benefit from the latest technologies for more precise management of their condition.
6. To ensure a comprehensive care team. Endocrinologists engage in collaborative efforts with a multidisciplinary team composed of professionals like nurses, diabetes educators, and nutritionists. These experts may be situated within endocrinology offices or accessible through a well-established referral network. Together, the team delivers thorough counseling on medication use and effectively addresses essential lifestyle factors, ensuring a comprehensive approach to diabetes management.
7. To counsel on side effects and management. Ensuring adherence and persistence with medication therapy poses considerable challenges. One study noted discontinuation rates for non-insulin diabetes medications of about 38%, with a higher 50% rate for GLP-1 RA drugs. The study didn›t provide specific reasons for discontinuation, but discontinuation was lower when medications were prescribed by an endocrinologist. Endocrinologists can provide valuable guidance on potential medication side effects and their management. This proactive approach not only fosters patient understanding but also empowers individuals to promptly address side effects, significantly enhancing treatment adherence and overall effectiveness.
8. To work around drug shortages. Given their frequent involvement in prescribing and obtaining medications for patients, endocrinologists adeptly utilize community relationships to navigate medication shortages. Their awareness of drug availability provides patients with a strategic advantage in overcoming supply challenges.
9. To determine dosing equivalents. In situations where supply-chain shortages persist, a thorough understanding of alternative options and dosing equivalents becomes paramount for ensuring uninterrupted care.
To provide follow-up. Endocrinologists prioritize regular follow-ups, providing patients with dedicated time slots for 10. ongoing monitoring and adjustments to their treatment plans. This commitment to follow-up care contributes to sustained, optimal outcomes in diabetes management.
Navigating the intricate healthcare landscape requires a delicate balance between primary care proficiency and specialist expertise, with endocrinologists playing a pivotal role in diabetes management. Our collaborative strength lies in acknowledging challenges and resource limitations, especially a physician’s familiarity with the latest diabetes medications.
Dr. Jaisinghani has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from Novo Nordisk.
A version of this article appeared on Medscape.com.
The blockbuster drugs of the century have arrived: glucagon-like peptide 1 receptor agonists (GLP-1 RAs). These drugs were developed to control blood sugar but have gained immense popularity for weight loss. Patients are clamoring for the drugs, and physicians are inundated with patient inquiries.
As doctors in primary care and other specialties are discovering, the GLP-1 RA drugs add another layer of complexity to the long-term management of a chronic disease. Managing diabetes and obesity requires a multidisciplinary team and a multispecialty treatment approach.
That’s why it’s more important than ever to know when and why to refer patients to an endocrinologist, who can offer unparalleled expertise as part of a multidisciplinary treatment approach.
Here are 10 reasons to refer your patients with diabetes to an endocrinologist.
1. To help make an optimal medication choice. Endocrinologists navigate diabetes management by considering individualized glycemic, cardiorenal, and weight goals as per guidelines, incorporating knowledge of medication side effects, simplifying regimens for adherence, and addressing practical factors like access and cost. Optimal medication selection is crucial, as a recent study found that nearly two thirds of patients altered their treatment by discontinuing their medication, switching their medication, or changing the dose of their medication within 12 months. Whether diabetes is controlled or uncontrolled, patients should consult an endocrinologist due to the potential complexity of cases, including late autoimmune onset of diabetes; medication-induced diabetes; and factors such as age, fragility, and chronic illnesses.
2. To facilitate medication approvals, alternatives, and authorizations. Attaining medication approval for patients entails a nuanced understanding and resources. Through experience and careful consideration, endocrinologists develop insights into potential barriers, especially in cases where approval for specific medications necessitates prior failures with multiple GLP-1 RAs or antihyperglycemic agents. This expertise positions them to advocate effectively for alternative options, often involving the meticulous process of prior authorizations. Certain endocrinology practices may augment this endeavor by offering dedicated resources, such as a specialized prior authorization team.
3. To deal with diabetes complications. Endocrinologists can help address emerging issues in GLP-1 RA drugs such as retinopathy, gastroparesis, and mental health effects. They can also help manage coexisting conditions, such as addressing thyroid nodules before considering the use of GLP-1 RAs. Recognizing the interconnected nature of diabetes and its influence on diverse body systems, endocrinologists ensure a thorough and integrated management strategy for their patients.
4. To titrate other glucose-lowering agents. Patients with diabetes are often on combination therapy. Endocrinologists adeptly adjust and titrate these treatments to optimize glucose control while minimizing side effects like hypoglycemia. Beyond insulin, their expertise encompasses various glucose-lowering agents. Notably, patients who use GLP-1 RAs in combination with medications such as insulin secretagogues (eg, sulfonylurea) and insulin face an elevated risk for hypoglycemia, including severe cases, necessitating careful titration to mitigate these effects.
5. To integrate advances in diabetes technology. Endocrinologists stay abreast of technological advancements in diabetes care, incorporating innovations in monitoring and treatment strategies such as continuous glucose monitors and insulin pumps. This ensures that patients benefit from the latest technologies for more precise management of their condition.
6. To ensure a comprehensive care team. Endocrinologists engage in collaborative efforts with a multidisciplinary team composed of professionals like nurses, diabetes educators, and nutritionists. These experts may be situated within endocrinology offices or accessible through a well-established referral network. Together, the team delivers thorough counseling on medication use and effectively addresses essential lifestyle factors, ensuring a comprehensive approach to diabetes management.
7. To counsel on side effects and management. Ensuring adherence and persistence with medication therapy poses considerable challenges. One study noted discontinuation rates for non-insulin diabetes medications of about 38%, with a higher 50% rate for GLP-1 RA drugs. The study didn›t provide specific reasons for discontinuation, but discontinuation was lower when medications were prescribed by an endocrinologist. Endocrinologists can provide valuable guidance on potential medication side effects and their management. This proactive approach not only fosters patient understanding but also empowers individuals to promptly address side effects, significantly enhancing treatment adherence and overall effectiveness.
8. To work around drug shortages. Given their frequent involvement in prescribing and obtaining medications for patients, endocrinologists adeptly utilize community relationships to navigate medication shortages. Their awareness of drug availability provides patients with a strategic advantage in overcoming supply challenges.
9. To determine dosing equivalents. In situations where supply-chain shortages persist, a thorough understanding of alternative options and dosing equivalents becomes paramount for ensuring uninterrupted care.
To provide follow-up. Endocrinologists prioritize regular follow-ups, providing patients with dedicated time slots for 10. ongoing monitoring and adjustments to their treatment plans. This commitment to follow-up care contributes to sustained, optimal outcomes in diabetes management.
Navigating the intricate healthcare landscape requires a delicate balance between primary care proficiency and specialist expertise, with endocrinologists playing a pivotal role in diabetes management. Our collaborative strength lies in acknowledging challenges and resource limitations, especially a physician’s familiarity with the latest diabetes medications.
Dr. Jaisinghani has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from Novo Nordisk.
A version of this article appeared on Medscape.com.
The blockbuster drugs of the century have arrived: glucagon-like peptide 1 receptor agonists (GLP-1 RAs). These drugs were developed to control blood sugar but have gained immense popularity for weight loss. Patients are clamoring for the drugs, and physicians are inundated with patient inquiries.
As doctors in primary care and other specialties are discovering, the GLP-1 RA drugs add another layer of complexity to the long-term management of a chronic disease. Managing diabetes and obesity requires a multidisciplinary team and a multispecialty treatment approach.
That’s why it’s more important than ever to know when and why to refer patients to an endocrinologist, who can offer unparalleled expertise as part of a multidisciplinary treatment approach.
Here are 10 reasons to refer your patients with diabetes to an endocrinologist.
1. To help make an optimal medication choice. Endocrinologists navigate diabetes management by considering individualized glycemic, cardiorenal, and weight goals as per guidelines, incorporating knowledge of medication side effects, simplifying regimens for adherence, and addressing practical factors like access and cost. Optimal medication selection is crucial, as a recent study found that nearly two thirds of patients altered their treatment by discontinuing their medication, switching their medication, or changing the dose of their medication within 12 months. Whether diabetes is controlled or uncontrolled, patients should consult an endocrinologist due to the potential complexity of cases, including late autoimmune onset of diabetes; medication-induced diabetes; and factors such as age, fragility, and chronic illnesses.
2. To facilitate medication approvals, alternatives, and authorizations. Attaining medication approval for patients entails a nuanced understanding and resources. Through experience and careful consideration, endocrinologists develop insights into potential barriers, especially in cases where approval for specific medications necessitates prior failures with multiple GLP-1 RAs or antihyperglycemic agents. This expertise positions them to advocate effectively for alternative options, often involving the meticulous process of prior authorizations. Certain endocrinology practices may augment this endeavor by offering dedicated resources, such as a specialized prior authorization team.
3. To deal with diabetes complications. Endocrinologists can help address emerging issues in GLP-1 RA drugs such as retinopathy, gastroparesis, and mental health effects. They can also help manage coexisting conditions, such as addressing thyroid nodules before considering the use of GLP-1 RAs. Recognizing the interconnected nature of diabetes and its influence on diverse body systems, endocrinologists ensure a thorough and integrated management strategy for their patients.
4. To titrate other glucose-lowering agents. Patients with diabetes are often on combination therapy. Endocrinologists adeptly adjust and titrate these treatments to optimize glucose control while minimizing side effects like hypoglycemia. Beyond insulin, their expertise encompasses various glucose-lowering agents. Notably, patients who use GLP-1 RAs in combination with medications such as insulin secretagogues (eg, sulfonylurea) and insulin face an elevated risk for hypoglycemia, including severe cases, necessitating careful titration to mitigate these effects.
5. To integrate advances in diabetes technology. Endocrinologists stay abreast of technological advancements in diabetes care, incorporating innovations in monitoring and treatment strategies such as continuous glucose monitors and insulin pumps. This ensures that patients benefit from the latest technologies for more precise management of their condition.
6. To ensure a comprehensive care team. Endocrinologists engage in collaborative efforts with a multidisciplinary team composed of professionals like nurses, diabetes educators, and nutritionists. These experts may be situated within endocrinology offices or accessible through a well-established referral network. Together, the team delivers thorough counseling on medication use and effectively addresses essential lifestyle factors, ensuring a comprehensive approach to diabetes management.
7. To counsel on side effects and management. Ensuring adherence and persistence with medication therapy poses considerable challenges. One study noted discontinuation rates for non-insulin diabetes medications of about 38%, with a higher 50% rate for GLP-1 RA drugs. The study didn›t provide specific reasons for discontinuation, but discontinuation was lower when medications were prescribed by an endocrinologist. Endocrinologists can provide valuable guidance on potential medication side effects and their management. This proactive approach not only fosters patient understanding but also empowers individuals to promptly address side effects, significantly enhancing treatment adherence and overall effectiveness.
8. To work around drug shortages. Given their frequent involvement in prescribing and obtaining medications for patients, endocrinologists adeptly utilize community relationships to navigate medication shortages. Their awareness of drug availability provides patients with a strategic advantage in overcoming supply challenges.
9. To determine dosing equivalents. In situations where supply-chain shortages persist, a thorough understanding of alternative options and dosing equivalents becomes paramount for ensuring uninterrupted care.
To provide follow-up. Endocrinologists prioritize regular follow-ups, providing patients with dedicated time slots for 10. ongoing monitoring and adjustments to their treatment plans. This commitment to follow-up care contributes to sustained, optimal outcomes in diabetes management.
Navigating the intricate healthcare landscape requires a delicate balance between primary care proficiency and specialist expertise, with endocrinologists playing a pivotal role in diabetes management. Our collaborative strength lies in acknowledging challenges and resource limitations, especially a physician’s familiarity with the latest diabetes medications.
Dr. Jaisinghani has disclosed the following relevant financial relationships: Received income in an amount equal to or greater than $250 from Novo Nordisk.
A version of this article appeared on Medscape.com.
A Neurotoxin, an Antidepressant, and More Emerging Options for Treating Rosacea
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
FROM ODAC 2024
RNA Vaccines: Risk for Heavy Menstrual Bleeding Clarified
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Cases of menstrual disorders, particularly unusually heavy menstrual bleeding, have been reported following RNA vaccination against COVID-19.
In France, this safety signal has been confirmed and added to the product characteristics summaries and vaccine leaflets for mRNA vaccines in October 2022. However, few studies have accurately measured this risk to date.
To address this gap in research, the French scientific interest group in the epidemiology of health products, ANSM-Cnam EPI-PHARE, conducted a study to assess the risk for heavy menstrual bleeding requiring hospitalization after COVID-19 vaccination in France.
“This study provides new evidence supporting the existence of an increased risk for heavy menstrual bleeding following COVID-19 vaccination with mRNA vaccines,” wrote the authors.
Study Details
The study included all women aged 15-50 years who were diagnosed with heavy menstrual bleeding in the hospital between May 12, 2021, and August 31, 2022. Participants were identified in the National Health Data System, and the study population totaled 4610 women.
Each participant was randomly matched with as many as 30 women who had not been hospitalized for abnormal genital bleeding and had similar characteristics in terms of age, department of residence, social deprivation index of the commune of residence, and contraceptive method.
Women who had a recent pregnancy, hysterectomy, or coagulation disorder within the specified time frames were excluded.
At the time of the study, 71% of cases and 70% of controls had received at least one dose of the COVID-19 vaccine. Among vaccinated participants, 68% and 66%, respectively, received a vaccination dose (first or second dose). An mRNA vaccine (Comirnaty or Spikevax) was the last vaccine for 99.8% of the population.
Increased Risk
Compared with control women, those hospitalized for heavy menstrual bleeding were more likely to have received their last dose of mRNA vaccine (Comirnaty or Spikevax) in the previous 1-3 months. This association was observed for vaccination doses (odds ratio [OR], 1.20), indicating a 20% increased risk, but it was not found for booster doses (OR, 1.07).
This association was particularly notable for women residing in socially disadvantaged communities (OR, 1.28) and women not using hormonal contraception (OR, 1.28).
The risk did not appear to be increased beyond 3 months after vaccination. Researchers noted that the increased risk may have occurred earlier, considering the likely interval between initial symptoms and hospitalization.
Assuming a causal relationship, the estimated number of cases attributable to vaccination was 8 cases per million vaccinated women, totaling 103 cases among all women aged 15-50 years who were vaccinated in France between May 12, 2021, and August 31, 2022.
As of the study date and in the 3 years before the study, none of the authors had any conflicts of interest with pharmaceutical companies.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Disparities Seen in Weight Loss Drug Prescriptions, Fills
Socioeconomic factors and insurance type greatly influence the odds of a person with obesity receiving a prescription for a weight loss medication and subsequently filling it, new research finds.
The results come from a retrospective study of Florida and Ohio electronic health records of more than 50,000 adults with a body mass index (BMI) of ≥ 30 kg/m2 who sought care for obesity from 2015 through June 2023.
The fill rate increased to 26% in 2022-2023 after the newer glucagon-like peptide 1 (GLP-1) agonists became available, but the identified disparities persisted throughout, study author Hamlet Gasoyan, PhD, told this news organization. “Things are changing, but this study provides a very good picture of who’s getting prescriptions and the implications for policy decisions.”
Dr. Gasoyan, of the Center for Value-Based Care Research at the Cleveland Clinic, Cleveland, Ohio, noted that Medicare doesn’t currently cover antiobesity medications nor do most Medicaid programs (neither Florida’s nor Ohio’s do), but there is now at least one bill in Congress to change that. “Medicare and other government payers are currently facing important policy decisions about antiobesity medication coverage. I think they should consider how their policies could impact existing inequalities in obesity care.”
Another noteworthy finding, Dr. Gasoyan said, is that “despite all the recent hype, the real data shows these medications are underutilized and probably will remain so.”
Asked to comment, David B. Sarwer, PhD, Director of the Center for Obesity Research and Education at Temple University, Philadelphia, Pennsylvania, told this news organization, “there’s a tremendous amount of enthusiasm in the obesity treatment community that these newer medications have the potential to be game-changers. I think what this study shows us, as does other work from this group and others, is that we still have some significant issues around access to care and long-term engagement with these medications that we need to address for them to realize their full potential.”
Dr. Sarwer acknowledged, as did Dr. Gasoyan, that the study timing is a limitation and more data will need to be collected prospectively with the new incretin drugs. As of now, though, “These medications are very expensive. While there are some insurance plans that are offering payment for them, many are not. Until we wrestle that to the ground there are always going to be questions about whether these medications are getting to the people who need them the most. I think one of the highlights of this paper is it reminds us that obesity is a disease that differentially impacts persons from underserved groups.”
Moreover, Dr. Sarwer noted, “In this day and age, many physicians don’t have a lot of time to spend with individual patients. Conversations around weight can be challenging and often very emotional for patients. I’m not sure we’ve trained physicians how to have productive, targeted conversations that lead to effective use of a weight-loss intervention. Maybe in some ways that’s what we’re seeing here.”
Disparities Seen in Both Prescriptions and Fills
The 50,678 study subjects all not only met BMI criteria (≥ 30 kg/m2) but also attended at least one weight management program (n = 48,711) and/or received a weight-loss medication prescription (n = 4047). “We know BMI isn’t a perfect measure of obesity, so we specifically looked at where the patient or provider had identified excess weight as an issue and wanted to do something about it…You would expect that in this group the use of antiobesity medications would be high, but it wasn’t, unfortunately,” Dr. Gasoyan commented.
Participants had a mean BMI of 38 kg/m2 and mean age 50 years. Slightly more than half (54%) were women, 66% were White individuals, 24% Black individuals, and 5.3% Hispanic individuals. A majority (56%) had private insurance, and 41% had diabetes. Mean follow-up time was 4.7 years.
The main measures were prescriptions for naltrexone-bupropion, orlistat, phentermine-topiramate, 3.0 mg liraglutide, 2.4 mg semaglutide, and a fill for one of those during the study follow-up.
Overall, 8.0% had a new anti-obesity medication prescription, and of those, 55% had at least one documented fill of the prescription. Among the fills, 39% were for naltrexone-buproprion, 29% for phentermine-topiramate, 19% for semaglutide, 11% for liraglutide, and 1.2% for orlistat.
In the multivariable model, receipt of an antiobesity medication prescription was significantly less likely among Black patients (adjusted odds ratio, 0.68), Hispanic individuals (0.72), and those from other racial or ethnic backgrounds (0.70) than among White patients. Men had lower odds than women (0.38).
Compared with privately insured patients, significantly lower odds of receiving prescriptions were seen in those with Medicaid (0.44), traditional Medicare (0.35), Medicare Advantage (0.36), and self-paying (0.65) and other insurance types (0.53). Those in the highest quartile of economic disadvantage also had lower antiobesity medication prescription odds (0.81).
Also associated with lower prescription odds were younger age, higher age-adjusted Charlson comorbidity score, presence of diabetes diagnosis, and a history of myocardial infarction or heart failure.
Factors associated with lower odds of filling antiobesity medication prescriptions included Hispanic ethnicity vs White ethnicity (0.51) but not Black race. Compared with private insurance, lower odds of filling the prescriptions were seen among those with Medicaid (0.41), traditional Medicare (0.38), and Medicare Advantage (0.37).
Over the study period, compared with naltrexone-buproprion, phentermine-topiramate had higher odds of being filled (1.27), while liraglutide (0.61) and orlistat (0.11) had lower odds, and semaglutide didn’t differ significantly (0.90).
Older age, female sex, and the presence of diabetes diagnosis were associated with higher odds of prescription fills, while deprivation quartile, history of myocardial infarction, history of heart failure, and age-adjusted Charlson comorbidity score were not significantly associated with medication fill.
Dr. Gasoyan told this news organization, “This study is unique in that we were able to look at patterns of use and barriers at several stages…We just recently published another study where we found patients weren’t often taking these medications long-term. So, patients are facing challenges on receiving obesity pharmacotherapy at several stages. …Hopefully these data will highlight the issues and inform future decisions. We see clear areas where we could obviously do better.”
Dr. Gasoyan had no disclosures. Dr. Sarwer received grant funding from the National Institutes of Health and declared having consulting relationships with NovoNordisk and Twenty30 Health.
A version of this article appeared on Medscape.com.
Socioeconomic factors and insurance type greatly influence the odds of a person with obesity receiving a prescription for a weight loss medication and subsequently filling it, new research finds.
The results come from a retrospective study of Florida and Ohio electronic health records of more than 50,000 adults with a body mass index (BMI) of ≥ 30 kg/m2 who sought care for obesity from 2015 through June 2023.
The fill rate increased to 26% in 2022-2023 after the newer glucagon-like peptide 1 (GLP-1) agonists became available, but the identified disparities persisted throughout, study author Hamlet Gasoyan, PhD, told this news organization. “Things are changing, but this study provides a very good picture of who’s getting prescriptions and the implications for policy decisions.”
Dr. Gasoyan, of the Center for Value-Based Care Research at the Cleveland Clinic, Cleveland, Ohio, noted that Medicare doesn’t currently cover antiobesity medications nor do most Medicaid programs (neither Florida’s nor Ohio’s do), but there is now at least one bill in Congress to change that. “Medicare and other government payers are currently facing important policy decisions about antiobesity medication coverage. I think they should consider how their policies could impact existing inequalities in obesity care.”
Another noteworthy finding, Dr. Gasoyan said, is that “despite all the recent hype, the real data shows these medications are underutilized and probably will remain so.”
Asked to comment, David B. Sarwer, PhD, Director of the Center for Obesity Research and Education at Temple University, Philadelphia, Pennsylvania, told this news organization, “there’s a tremendous amount of enthusiasm in the obesity treatment community that these newer medications have the potential to be game-changers. I think what this study shows us, as does other work from this group and others, is that we still have some significant issues around access to care and long-term engagement with these medications that we need to address for them to realize their full potential.”
Dr. Sarwer acknowledged, as did Dr. Gasoyan, that the study timing is a limitation and more data will need to be collected prospectively with the new incretin drugs. As of now, though, “These medications are very expensive. While there are some insurance plans that are offering payment for them, many are not. Until we wrestle that to the ground there are always going to be questions about whether these medications are getting to the people who need them the most. I think one of the highlights of this paper is it reminds us that obesity is a disease that differentially impacts persons from underserved groups.”
Moreover, Dr. Sarwer noted, “In this day and age, many physicians don’t have a lot of time to spend with individual patients. Conversations around weight can be challenging and often very emotional for patients. I’m not sure we’ve trained physicians how to have productive, targeted conversations that lead to effective use of a weight-loss intervention. Maybe in some ways that’s what we’re seeing here.”
Disparities Seen in Both Prescriptions and Fills
The 50,678 study subjects all not only met BMI criteria (≥ 30 kg/m2) but also attended at least one weight management program (n = 48,711) and/or received a weight-loss medication prescription (n = 4047). “We know BMI isn’t a perfect measure of obesity, so we specifically looked at where the patient or provider had identified excess weight as an issue and wanted to do something about it…You would expect that in this group the use of antiobesity medications would be high, but it wasn’t, unfortunately,” Dr. Gasoyan commented.
Participants had a mean BMI of 38 kg/m2 and mean age 50 years. Slightly more than half (54%) were women, 66% were White individuals, 24% Black individuals, and 5.3% Hispanic individuals. A majority (56%) had private insurance, and 41% had diabetes. Mean follow-up time was 4.7 years.
The main measures were prescriptions for naltrexone-bupropion, orlistat, phentermine-topiramate, 3.0 mg liraglutide, 2.4 mg semaglutide, and a fill for one of those during the study follow-up.
Overall, 8.0% had a new anti-obesity medication prescription, and of those, 55% had at least one documented fill of the prescription. Among the fills, 39% were for naltrexone-buproprion, 29% for phentermine-topiramate, 19% for semaglutide, 11% for liraglutide, and 1.2% for orlistat.
In the multivariable model, receipt of an antiobesity medication prescription was significantly less likely among Black patients (adjusted odds ratio, 0.68), Hispanic individuals (0.72), and those from other racial or ethnic backgrounds (0.70) than among White patients. Men had lower odds than women (0.38).
Compared with privately insured patients, significantly lower odds of receiving prescriptions were seen in those with Medicaid (0.44), traditional Medicare (0.35), Medicare Advantage (0.36), and self-paying (0.65) and other insurance types (0.53). Those in the highest quartile of economic disadvantage also had lower antiobesity medication prescription odds (0.81).
Also associated with lower prescription odds were younger age, higher age-adjusted Charlson comorbidity score, presence of diabetes diagnosis, and a history of myocardial infarction or heart failure.
Factors associated with lower odds of filling antiobesity medication prescriptions included Hispanic ethnicity vs White ethnicity (0.51) but not Black race. Compared with private insurance, lower odds of filling the prescriptions were seen among those with Medicaid (0.41), traditional Medicare (0.38), and Medicare Advantage (0.37).
Over the study period, compared with naltrexone-buproprion, phentermine-topiramate had higher odds of being filled (1.27), while liraglutide (0.61) and orlistat (0.11) had lower odds, and semaglutide didn’t differ significantly (0.90).
Older age, female sex, and the presence of diabetes diagnosis were associated with higher odds of prescription fills, while deprivation quartile, history of myocardial infarction, history of heart failure, and age-adjusted Charlson comorbidity score were not significantly associated with medication fill.
Dr. Gasoyan told this news organization, “This study is unique in that we were able to look at patterns of use and barriers at several stages…We just recently published another study where we found patients weren’t often taking these medications long-term. So, patients are facing challenges on receiving obesity pharmacotherapy at several stages. …Hopefully these data will highlight the issues and inform future decisions. We see clear areas where we could obviously do better.”
Dr. Gasoyan had no disclosures. Dr. Sarwer received grant funding from the National Institutes of Health and declared having consulting relationships with NovoNordisk and Twenty30 Health.
A version of this article appeared on Medscape.com.
Socioeconomic factors and insurance type greatly influence the odds of a person with obesity receiving a prescription for a weight loss medication and subsequently filling it, new research finds.
The results come from a retrospective study of Florida and Ohio electronic health records of more than 50,000 adults with a body mass index (BMI) of ≥ 30 kg/m2 who sought care for obesity from 2015 through June 2023.
The fill rate increased to 26% in 2022-2023 after the newer glucagon-like peptide 1 (GLP-1) agonists became available, but the identified disparities persisted throughout, study author Hamlet Gasoyan, PhD, told this news organization. “Things are changing, but this study provides a very good picture of who’s getting prescriptions and the implications for policy decisions.”
Dr. Gasoyan, of the Center for Value-Based Care Research at the Cleveland Clinic, Cleveland, Ohio, noted that Medicare doesn’t currently cover antiobesity medications nor do most Medicaid programs (neither Florida’s nor Ohio’s do), but there is now at least one bill in Congress to change that. “Medicare and other government payers are currently facing important policy decisions about antiobesity medication coverage. I think they should consider how their policies could impact existing inequalities in obesity care.”
Another noteworthy finding, Dr. Gasoyan said, is that “despite all the recent hype, the real data shows these medications are underutilized and probably will remain so.”
Asked to comment, David B. Sarwer, PhD, Director of the Center for Obesity Research and Education at Temple University, Philadelphia, Pennsylvania, told this news organization, “there’s a tremendous amount of enthusiasm in the obesity treatment community that these newer medications have the potential to be game-changers. I think what this study shows us, as does other work from this group and others, is that we still have some significant issues around access to care and long-term engagement with these medications that we need to address for them to realize their full potential.”
Dr. Sarwer acknowledged, as did Dr. Gasoyan, that the study timing is a limitation and more data will need to be collected prospectively with the new incretin drugs. As of now, though, “These medications are very expensive. While there are some insurance plans that are offering payment for them, many are not. Until we wrestle that to the ground there are always going to be questions about whether these medications are getting to the people who need them the most. I think one of the highlights of this paper is it reminds us that obesity is a disease that differentially impacts persons from underserved groups.”
Moreover, Dr. Sarwer noted, “In this day and age, many physicians don’t have a lot of time to spend with individual patients. Conversations around weight can be challenging and often very emotional for patients. I’m not sure we’ve trained physicians how to have productive, targeted conversations that lead to effective use of a weight-loss intervention. Maybe in some ways that’s what we’re seeing here.”
Disparities Seen in Both Prescriptions and Fills
The 50,678 study subjects all not only met BMI criteria (≥ 30 kg/m2) but also attended at least one weight management program (n = 48,711) and/or received a weight-loss medication prescription (n = 4047). “We know BMI isn’t a perfect measure of obesity, so we specifically looked at where the patient or provider had identified excess weight as an issue and wanted to do something about it…You would expect that in this group the use of antiobesity medications would be high, but it wasn’t, unfortunately,” Dr. Gasoyan commented.
Participants had a mean BMI of 38 kg/m2 and mean age 50 years. Slightly more than half (54%) were women, 66% were White individuals, 24% Black individuals, and 5.3% Hispanic individuals. A majority (56%) had private insurance, and 41% had diabetes. Mean follow-up time was 4.7 years.
The main measures were prescriptions for naltrexone-bupropion, orlistat, phentermine-topiramate, 3.0 mg liraglutide, 2.4 mg semaglutide, and a fill for one of those during the study follow-up.
Overall, 8.0% had a new anti-obesity medication prescription, and of those, 55% had at least one documented fill of the prescription. Among the fills, 39% were for naltrexone-buproprion, 29% for phentermine-topiramate, 19% for semaglutide, 11% for liraglutide, and 1.2% for orlistat.
In the multivariable model, receipt of an antiobesity medication prescription was significantly less likely among Black patients (adjusted odds ratio, 0.68), Hispanic individuals (0.72), and those from other racial or ethnic backgrounds (0.70) than among White patients. Men had lower odds than women (0.38).
Compared with privately insured patients, significantly lower odds of receiving prescriptions were seen in those with Medicaid (0.44), traditional Medicare (0.35), Medicare Advantage (0.36), and self-paying (0.65) and other insurance types (0.53). Those in the highest quartile of economic disadvantage also had lower antiobesity medication prescription odds (0.81).
Also associated with lower prescription odds were younger age, higher age-adjusted Charlson comorbidity score, presence of diabetes diagnosis, and a history of myocardial infarction or heart failure.
Factors associated with lower odds of filling antiobesity medication prescriptions included Hispanic ethnicity vs White ethnicity (0.51) but not Black race. Compared with private insurance, lower odds of filling the prescriptions were seen among those with Medicaid (0.41), traditional Medicare (0.38), and Medicare Advantage (0.37).
Over the study period, compared with naltrexone-buproprion, phentermine-topiramate had higher odds of being filled (1.27), while liraglutide (0.61) and orlistat (0.11) had lower odds, and semaglutide didn’t differ significantly (0.90).
Older age, female sex, and the presence of diabetes diagnosis were associated with higher odds of prescription fills, while deprivation quartile, history of myocardial infarction, history of heart failure, and age-adjusted Charlson comorbidity score were not significantly associated with medication fill.
Dr. Gasoyan told this news organization, “This study is unique in that we were able to look at patterns of use and barriers at several stages…We just recently published another study where we found patients weren’t often taking these medications long-term. So, patients are facing challenges on receiving obesity pharmacotherapy at several stages. …Hopefully these data will highlight the issues and inform future decisions. We see clear areas where we could obviously do better.”
Dr. Gasoyan had no disclosures. Dr. Sarwer received grant funding from the National Institutes of Health and declared having consulting relationships with NovoNordisk and Twenty30 Health.
A version of this article appeared on Medscape.com.