User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Exercising to lose weight is not for every ‘body’
Exercising to lose weight is not for every ‘body’
This first item comes from the “You’ve got to be kidding” section of LOTME’s supersecret topics-of-interest file.
Investigators at the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences and the University of Roehampton noticed that some people who enrolled in exercise programs to lose weight did just the opposite: they gained weight.
Being scientists, they decided to look at the effects of energy expenditure and how those effects varied among individuals. The likely culprit in this case, they determined, is something called compensatory mechanisms. One such mechanism involves eating more food because exercise stimulates appetite, and another might reduce energy expenditure on other components like resting metabolism so that the exercise is, in effect, less costly.
A look at the numbers shows how compensatory mechanisms worked in the study population of 1,750 adults. Among individuals with the highest BMI, 51% of the calories burned during activity translated into calories burned at the end of the day. For those with normal BMI, however, 72% of calories burned during activity were reflected in total expenditure.
“People living with obesity cut back their resting metabolism when they are more active. The result is that for every calorie they spend on exercise they save about half a calorie on resting,” the investigators explained.
In other words, some bodies will, unconsciously, work against the conscious effort of exercising to lose weight. Thank you very much, compensatory mechanisms, for the boundarylessness exhibited in exceeding your job description.
When it comes to the mix, walnuts go nuts
When it comes to mixed nuts, walnuts get no love. But we may be able to give you a reason to not pick them out: Your arteries.
Participants in a recent study who ate about a half-cup of walnuts every day for 2 years saw a drop in their low-density lipoprotein (LDL) cholesterol. The number and quality of LDL particles in healthy older adults also improved. How? Good ol’ omega-3 fatty acids.
Omega-3 is found in many foods linked to lower risks of heart disease, lower cholesterol levels, and lower blood sugar levels, but the one thing that makes the walnut a front runner for Miss Super Food 2021 is their ability to improve the quality of LDL particles.
“LDL particles come in various sizes [and] research has shown that small, dense LDL particles are more often associated with atherosclerosis, the plaque or fatty deposits that build up in the arteries,” Emilio Ros, MD, PhD, of the Hospital Clínic of Barcelona and the study’s senior investigator, said in a written statement.
The 708 participants, aged 63-79 years and mostly women, were divided into two groups: One received the walnut diet and the other did not. After 2 years, the walnut group had lower LDL levels by an average of 4.3 mg/dL. Total cholesterol was reduced by an average of 8.5 mg/dL. Also, their total LDL particle count was 4.3% lower and small LDL particles were down by 6.1%.
So instead of picking the walnuts out of the mix, try to find it in your heart to appreciate them. Your body already does.
Begun, the clone war has
Well, not quite yet, Master Yoda, but perhaps one day soon, if a study from Japan into the uncanny valley of the usage of cloned humanlike faces in robotics and artificial intelligence, published in PLOS One, is to be believed.
The study consisted of a number of six smaller experiments in which participants judged a series of images based on subjective eeriness, emotional valence, and realism. The images included people with the same cloned face; people with different faces; dogs; identical twins, triplets, quadruplets, etc.; and cloned animated characters. In the sixth experiment, the photos were the same as in the second (six cloned faces, six different faces, and a single face) but participants also answered the Disgust Scale–Revised to accurately analyze disgust sensitivity.
The results of all these experiments were quite clear: People found the cloned faces far creepier than the varied or single face, an effect the researchers called clone devaluation. Notably, this effect only applied to realistic human faces; most people didn’t find the cloned dogs or cloned animated characters creepy. However, those who did were more likely to find the human clones eerie on the Disgust Scale.
The authors noted that future robotics technology needs to be carefully considered to avoid the uncanny valley and this clone devaluation effect, which is a very good point. The last thing we need is a few million robots with identical faces getting angry at us and pulling a Terminator/Order 66 combo. We’re already in a viral apocalypse; we don’t need a robot one on top of that.
Congratulations to our new favorite reader
The winner of last week’s inaugural Pandemic Pandemonium comes to us from Tiffanie Roe. By getting her entry in first, just ahead of the flood of responses we received – and by flood we mean a very slow and very quickly repaired drip – Ms. Roe puts the gold medal for COVID-related insanity around the necks of Australian magpies, who may start attacking people wearing face masks during “swooping season” because the birds don’t recognize them.
Exercising to lose weight is not for every ‘body’
This first item comes from the “You’ve got to be kidding” section of LOTME’s supersecret topics-of-interest file.
Investigators at the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences and the University of Roehampton noticed that some people who enrolled in exercise programs to lose weight did just the opposite: they gained weight.
Being scientists, they decided to look at the effects of energy expenditure and how those effects varied among individuals. The likely culprit in this case, they determined, is something called compensatory mechanisms. One such mechanism involves eating more food because exercise stimulates appetite, and another might reduce energy expenditure on other components like resting metabolism so that the exercise is, in effect, less costly.
A look at the numbers shows how compensatory mechanisms worked in the study population of 1,750 adults. Among individuals with the highest BMI, 51% of the calories burned during activity translated into calories burned at the end of the day. For those with normal BMI, however, 72% of calories burned during activity were reflected in total expenditure.
“People living with obesity cut back their resting metabolism when they are more active. The result is that for every calorie they spend on exercise they save about half a calorie on resting,” the investigators explained.
In other words, some bodies will, unconsciously, work against the conscious effort of exercising to lose weight. Thank you very much, compensatory mechanisms, for the boundarylessness exhibited in exceeding your job description.
When it comes to the mix, walnuts go nuts
When it comes to mixed nuts, walnuts get no love. But we may be able to give you a reason to not pick them out: Your arteries.
Participants in a recent study who ate about a half-cup of walnuts every day for 2 years saw a drop in their low-density lipoprotein (LDL) cholesterol. The number and quality of LDL particles in healthy older adults also improved. How? Good ol’ omega-3 fatty acids.
Omega-3 is found in many foods linked to lower risks of heart disease, lower cholesterol levels, and lower blood sugar levels, but the one thing that makes the walnut a front runner for Miss Super Food 2021 is their ability to improve the quality of LDL particles.
“LDL particles come in various sizes [and] research has shown that small, dense LDL particles are more often associated with atherosclerosis, the plaque or fatty deposits that build up in the arteries,” Emilio Ros, MD, PhD, of the Hospital Clínic of Barcelona and the study’s senior investigator, said in a written statement.
The 708 participants, aged 63-79 years and mostly women, were divided into two groups: One received the walnut diet and the other did not. After 2 years, the walnut group had lower LDL levels by an average of 4.3 mg/dL. Total cholesterol was reduced by an average of 8.5 mg/dL. Also, their total LDL particle count was 4.3% lower and small LDL particles were down by 6.1%.
So instead of picking the walnuts out of the mix, try to find it in your heart to appreciate them. Your body already does.
Begun, the clone war has
Well, not quite yet, Master Yoda, but perhaps one day soon, if a study from Japan into the uncanny valley of the usage of cloned humanlike faces in robotics and artificial intelligence, published in PLOS One, is to be believed.
The study consisted of a number of six smaller experiments in which participants judged a series of images based on subjective eeriness, emotional valence, and realism. The images included people with the same cloned face; people with different faces; dogs; identical twins, triplets, quadruplets, etc.; and cloned animated characters. In the sixth experiment, the photos were the same as in the second (six cloned faces, six different faces, and a single face) but participants also answered the Disgust Scale–Revised to accurately analyze disgust sensitivity.
The results of all these experiments were quite clear: People found the cloned faces far creepier than the varied or single face, an effect the researchers called clone devaluation. Notably, this effect only applied to realistic human faces; most people didn’t find the cloned dogs or cloned animated characters creepy. However, those who did were more likely to find the human clones eerie on the Disgust Scale.
The authors noted that future robotics technology needs to be carefully considered to avoid the uncanny valley and this clone devaluation effect, which is a very good point. The last thing we need is a few million robots with identical faces getting angry at us and pulling a Terminator/Order 66 combo. We’re already in a viral apocalypse; we don’t need a robot one on top of that.
Congratulations to our new favorite reader
The winner of last week’s inaugural Pandemic Pandemonium comes to us from Tiffanie Roe. By getting her entry in first, just ahead of the flood of responses we received – and by flood we mean a very slow and very quickly repaired drip – Ms. Roe puts the gold medal for COVID-related insanity around the necks of Australian magpies, who may start attacking people wearing face masks during “swooping season” because the birds don’t recognize them.
Exercising to lose weight is not for every ‘body’
This first item comes from the “You’ve got to be kidding” section of LOTME’s supersecret topics-of-interest file.
Investigators at the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences and the University of Roehampton noticed that some people who enrolled in exercise programs to lose weight did just the opposite: they gained weight.
Being scientists, they decided to look at the effects of energy expenditure and how those effects varied among individuals. The likely culprit in this case, they determined, is something called compensatory mechanisms. One such mechanism involves eating more food because exercise stimulates appetite, and another might reduce energy expenditure on other components like resting metabolism so that the exercise is, in effect, less costly.
A look at the numbers shows how compensatory mechanisms worked in the study population of 1,750 adults. Among individuals with the highest BMI, 51% of the calories burned during activity translated into calories burned at the end of the day. For those with normal BMI, however, 72% of calories burned during activity were reflected in total expenditure.
“People living with obesity cut back their resting metabolism when they are more active. The result is that for every calorie they spend on exercise they save about half a calorie on resting,” the investigators explained.
In other words, some bodies will, unconsciously, work against the conscious effort of exercising to lose weight. Thank you very much, compensatory mechanisms, for the boundarylessness exhibited in exceeding your job description.
When it comes to the mix, walnuts go nuts
When it comes to mixed nuts, walnuts get no love. But we may be able to give you a reason to not pick them out: Your arteries.
Participants in a recent study who ate about a half-cup of walnuts every day for 2 years saw a drop in their low-density lipoprotein (LDL) cholesterol. The number and quality of LDL particles in healthy older adults also improved. How? Good ol’ omega-3 fatty acids.
Omega-3 is found in many foods linked to lower risks of heart disease, lower cholesterol levels, and lower blood sugar levels, but the one thing that makes the walnut a front runner for Miss Super Food 2021 is their ability to improve the quality of LDL particles.
“LDL particles come in various sizes [and] research has shown that small, dense LDL particles are more often associated with atherosclerosis, the plaque or fatty deposits that build up in the arteries,” Emilio Ros, MD, PhD, of the Hospital Clínic of Barcelona and the study’s senior investigator, said in a written statement.
The 708 participants, aged 63-79 years and mostly women, were divided into two groups: One received the walnut diet and the other did not. After 2 years, the walnut group had lower LDL levels by an average of 4.3 mg/dL. Total cholesterol was reduced by an average of 8.5 mg/dL. Also, their total LDL particle count was 4.3% lower and small LDL particles were down by 6.1%.
So instead of picking the walnuts out of the mix, try to find it in your heart to appreciate them. Your body already does.
Begun, the clone war has
Well, not quite yet, Master Yoda, but perhaps one day soon, if a study from Japan into the uncanny valley of the usage of cloned humanlike faces in robotics and artificial intelligence, published in PLOS One, is to be believed.
The study consisted of a number of six smaller experiments in which participants judged a series of images based on subjective eeriness, emotional valence, and realism. The images included people with the same cloned face; people with different faces; dogs; identical twins, triplets, quadruplets, etc.; and cloned animated characters. In the sixth experiment, the photos were the same as in the second (six cloned faces, six different faces, and a single face) but participants also answered the Disgust Scale–Revised to accurately analyze disgust sensitivity.
The results of all these experiments were quite clear: People found the cloned faces far creepier than the varied or single face, an effect the researchers called clone devaluation. Notably, this effect only applied to realistic human faces; most people didn’t find the cloned dogs or cloned animated characters creepy. However, those who did were more likely to find the human clones eerie on the Disgust Scale.
The authors noted that future robotics technology needs to be carefully considered to avoid the uncanny valley and this clone devaluation effect, which is a very good point. The last thing we need is a few million robots with identical faces getting angry at us and pulling a Terminator/Order 66 combo. We’re already in a viral apocalypse; we don’t need a robot one on top of that.
Congratulations to our new favorite reader
The winner of last week’s inaugural Pandemic Pandemonium comes to us from Tiffanie Roe. By getting her entry in first, just ahead of the flood of responses we received – and by flood we mean a very slow and very quickly repaired drip – Ms. Roe puts the gold medal for COVID-related insanity around the necks of Australian magpies, who may start attacking people wearing face masks during “swooping season” because the birds don’t recognize them.
Even highly allergic adults unlikely to react to COVID-19 vaccine
published Aug. 31, 2021, in JAMA Network Open. Symptoms resolved in a few hours with medication, and no patients required hospitalization.
Risk for allergic reaction has been one of several obstacles in global vaccination efforts, the authors, led by Nancy Agmon-Levin, MD, of the Sheba Medical Center, Ramat Gan, Israel, wrote. Clinical trials for the Moderna and Pfizer-BioNTech COVID-19 vaccines excluded individuals with allergies to any component of the vaccine or with previous allergies to other vaccines. Early reports of anaphylaxis in reaction to the vaccines caused concern among patients and practitioners. Soon after, the Centers for Disease Control and Prevention and other authorities released guidance on preparing for allergic reactions. “Despite these recommendations, uncertainty remains, particularly among patients with a history of anaphylaxis and/or multiple allergies,” the authors added.
In response to early concerns, the Sheba Medical Center opened a COVID-19 referral center to address safety questions and to conduct assessments of allergy risk for the Pfizer-BioNTech vaccine, the first COVID-19 vaccine approved in Israel. From Dec. 27, 2020, to Feb. 22, 2021, the referral center assessed 8,102 patients with allergies. Those who were not clearly at low risk filled out a questionnaire about prior allergic or anaphylactic reactions to drugs or vaccines, other allergies, and other relevant medical history. Patients were considered to be at high risk for allergic reactions if they met at least one of the following criteria: previous anaphylactic reaction to any drug or vaccine, multiple drug allergies, multiple other allergies, and mast cell disorders. Individuals were also classified as high risk if their health care practitioner deferred vaccination because of allergy concerns.
Nearly 95% of the cohort (7,668 individuals) were classified as low risk and received both Pfizer vaccine doses at standard immunization sites and underwent 30 minutes of observation after immunization. Although the study did not follow these lower-risk patients, “no serious allergic reactions were reported back to our referral center by patients or their general practitioner after immunization in the regular settings,” the authors wrote.
Five patients were considered ineligible for immunization because of known sensitivity to polyethylene glycol or multiple anaphylactic reactions to different injectable drugs, following recommendations from the Ministry of Health of Israel at the time. The remaining 429 individuals were deemed high risk and underwent observation for 2 hours from a dedicated allergy team after immunization. For these high-risk patients, both vaccine doses were administered in the same setting. Patients also reported any adverse reactions in the 21 days between the first and second dose.
Women made up most of the high-risk cohort (70.9%). The average age of participants was 52 years. Of the high-risk individuals, 63.2% reported prior anaphylaxis, 32.9% had multiple drug allergies, and 30.3% had multiple other allergies.
During the first 2 hours following immunization, nine individuals (2.1%), all women, experienced allergic reactions. Six individuals (1.4%) experienced minor reactions, including skin flushing, tongue or uvula swelling, or a cough that resolved with antihistamine treatment during the observation period. Three patients (0.7%) had anaphylactic reactions that occurred 10 to 20 minutes after injection. All three patients experienced significant bronchospasm, skin eruption, itching, and shortness of breath. Two patients experienced angioedema, and one patient had gastrointestinal symptoms. They were treated with adrenaline, antihistamines, and an inhaled bronchodilator. All symptoms resolved within 2-6 hours, and no patient required hospitalization.
In the days following vaccination, patients commonly reported pain at the injection site, fatigue, muscle pain, and headache; 14.7% of patients reported skin eruption, itching, or urticaria.
As of Feb. 22, 2021, 218 patients from this highly allergic cohort received their second dose of the vaccine. Four patients (1.8%) had mild allergic reactions. All four developed flushing, and one patient also developed a cough that resolved with antihistamine treatment. Three of these patients had experienced mild allergic reactions to the first dose and were premedicated for the second dose. One patient only reacted to the second dose.
The findings should be “very reassuring” to individuals hesitant to receive the vaccine, Elizabeth Phillips, MD, the director of the Center for Drug Safety and Immunology at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview. She was not involved with the research and wrote an invited commentary on the study. “The rates of anaphylaxis and allergic reactions are truly quite low,” she said. Although about 2% of the high-risk group developed allergic reactions to immunization, the overall percentage for the entire cohort would be much lower.
The study did not investigate specific risk factors for and mechanisms of allergic reactions to COVID-19 vaccines, Dr. Phillips said, which is a study limitation that the authors also acknowledge. The National Institute for Allergy and Infectious Diseases is currently trying to answer some of these questions with a multisite, randomized, double-blinded study. The study is intended to help understand why people have these allergic reactions, Dr. Phillips added. Vanderbilt is one of the sites for the study.
While researchers continue to hunt for answers, the algorithm developed by the authors provides “a great strategy to get people that are at higher risk vaccinated in a monitored setting,” she said. The results show that “people should not be avoiding vaccination because of a history of anaphylaxis.”
Dr. Phillips has received institutional grants from the National Institutes of Health and the National Health and Medical Research Council; royalties from UpToDate and Lexicomp; and consulting fees from Janssen, Vertex, Biocryst, and Regeneron.
A version of this article first appeared on Medscape.com.
published Aug. 31, 2021, in JAMA Network Open. Symptoms resolved in a few hours with medication, and no patients required hospitalization.
Risk for allergic reaction has been one of several obstacles in global vaccination efforts, the authors, led by Nancy Agmon-Levin, MD, of the Sheba Medical Center, Ramat Gan, Israel, wrote. Clinical trials for the Moderna and Pfizer-BioNTech COVID-19 vaccines excluded individuals with allergies to any component of the vaccine or with previous allergies to other vaccines. Early reports of anaphylaxis in reaction to the vaccines caused concern among patients and practitioners. Soon after, the Centers for Disease Control and Prevention and other authorities released guidance on preparing for allergic reactions. “Despite these recommendations, uncertainty remains, particularly among patients with a history of anaphylaxis and/or multiple allergies,” the authors added.
In response to early concerns, the Sheba Medical Center opened a COVID-19 referral center to address safety questions and to conduct assessments of allergy risk for the Pfizer-BioNTech vaccine, the first COVID-19 vaccine approved in Israel. From Dec. 27, 2020, to Feb. 22, 2021, the referral center assessed 8,102 patients with allergies. Those who were not clearly at low risk filled out a questionnaire about prior allergic or anaphylactic reactions to drugs or vaccines, other allergies, and other relevant medical history. Patients were considered to be at high risk for allergic reactions if they met at least one of the following criteria: previous anaphylactic reaction to any drug or vaccine, multiple drug allergies, multiple other allergies, and mast cell disorders. Individuals were also classified as high risk if their health care practitioner deferred vaccination because of allergy concerns.
Nearly 95% of the cohort (7,668 individuals) were classified as low risk and received both Pfizer vaccine doses at standard immunization sites and underwent 30 minutes of observation after immunization. Although the study did not follow these lower-risk patients, “no serious allergic reactions were reported back to our referral center by patients or their general practitioner after immunization in the regular settings,” the authors wrote.
Five patients were considered ineligible for immunization because of known sensitivity to polyethylene glycol or multiple anaphylactic reactions to different injectable drugs, following recommendations from the Ministry of Health of Israel at the time. The remaining 429 individuals were deemed high risk and underwent observation for 2 hours from a dedicated allergy team after immunization. For these high-risk patients, both vaccine doses were administered in the same setting. Patients also reported any adverse reactions in the 21 days between the first and second dose.
Women made up most of the high-risk cohort (70.9%). The average age of participants was 52 years. Of the high-risk individuals, 63.2% reported prior anaphylaxis, 32.9% had multiple drug allergies, and 30.3% had multiple other allergies.
During the first 2 hours following immunization, nine individuals (2.1%), all women, experienced allergic reactions. Six individuals (1.4%) experienced minor reactions, including skin flushing, tongue or uvula swelling, or a cough that resolved with antihistamine treatment during the observation period. Three patients (0.7%) had anaphylactic reactions that occurred 10 to 20 minutes after injection. All three patients experienced significant bronchospasm, skin eruption, itching, and shortness of breath. Two patients experienced angioedema, and one patient had gastrointestinal symptoms. They were treated with adrenaline, antihistamines, and an inhaled bronchodilator. All symptoms resolved within 2-6 hours, and no patient required hospitalization.
In the days following vaccination, patients commonly reported pain at the injection site, fatigue, muscle pain, and headache; 14.7% of patients reported skin eruption, itching, or urticaria.
As of Feb. 22, 2021, 218 patients from this highly allergic cohort received their second dose of the vaccine. Four patients (1.8%) had mild allergic reactions. All four developed flushing, and one patient also developed a cough that resolved with antihistamine treatment. Three of these patients had experienced mild allergic reactions to the first dose and were premedicated for the second dose. One patient only reacted to the second dose.
The findings should be “very reassuring” to individuals hesitant to receive the vaccine, Elizabeth Phillips, MD, the director of the Center for Drug Safety and Immunology at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview. She was not involved with the research and wrote an invited commentary on the study. “The rates of anaphylaxis and allergic reactions are truly quite low,” she said. Although about 2% of the high-risk group developed allergic reactions to immunization, the overall percentage for the entire cohort would be much lower.
The study did not investigate specific risk factors for and mechanisms of allergic reactions to COVID-19 vaccines, Dr. Phillips said, which is a study limitation that the authors also acknowledge. The National Institute for Allergy and Infectious Diseases is currently trying to answer some of these questions with a multisite, randomized, double-blinded study. The study is intended to help understand why people have these allergic reactions, Dr. Phillips added. Vanderbilt is one of the sites for the study.
While researchers continue to hunt for answers, the algorithm developed by the authors provides “a great strategy to get people that are at higher risk vaccinated in a monitored setting,” she said. The results show that “people should not be avoiding vaccination because of a history of anaphylaxis.”
Dr. Phillips has received institutional grants from the National Institutes of Health and the National Health and Medical Research Council; royalties from UpToDate and Lexicomp; and consulting fees from Janssen, Vertex, Biocryst, and Regeneron.
A version of this article first appeared on Medscape.com.
published Aug. 31, 2021, in JAMA Network Open. Symptoms resolved in a few hours with medication, and no patients required hospitalization.
Risk for allergic reaction has been one of several obstacles in global vaccination efforts, the authors, led by Nancy Agmon-Levin, MD, of the Sheba Medical Center, Ramat Gan, Israel, wrote. Clinical trials for the Moderna and Pfizer-BioNTech COVID-19 vaccines excluded individuals with allergies to any component of the vaccine or with previous allergies to other vaccines. Early reports of anaphylaxis in reaction to the vaccines caused concern among patients and practitioners. Soon after, the Centers for Disease Control and Prevention and other authorities released guidance on preparing for allergic reactions. “Despite these recommendations, uncertainty remains, particularly among patients with a history of anaphylaxis and/or multiple allergies,” the authors added.
In response to early concerns, the Sheba Medical Center opened a COVID-19 referral center to address safety questions and to conduct assessments of allergy risk for the Pfizer-BioNTech vaccine, the first COVID-19 vaccine approved in Israel. From Dec. 27, 2020, to Feb. 22, 2021, the referral center assessed 8,102 patients with allergies. Those who were not clearly at low risk filled out a questionnaire about prior allergic or anaphylactic reactions to drugs or vaccines, other allergies, and other relevant medical history. Patients were considered to be at high risk for allergic reactions if they met at least one of the following criteria: previous anaphylactic reaction to any drug or vaccine, multiple drug allergies, multiple other allergies, and mast cell disorders. Individuals were also classified as high risk if their health care practitioner deferred vaccination because of allergy concerns.
Nearly 95% of the cohort (7,668 individuals) were classified as low risk and received both Pfizer vaccine doses at standard immunization sites and underwent 30 minutes of observation after immunization. Although the study did not follow these lower-risk patients, “no serious allergic reactions were reported back to our referral center by patients or their general practitioner after immunization in the regular settings,” the authors wrote.
Five patients were considered ineligible for immunization because of known sensitivity to polyethylene glycol or multiple anaphylactic reactions to different injectable drugs, following recommendations from the Ministry of Health of Israel at the time. The remaining 429 individuals were deemed high risk and underwent observation for 2 hours from a dedicated allergy team after immunization. For these high-risk patients, both vaccine doses were administered in the same setting. Patients also reported any adverse reactions in the 21 days between the first and second dose.
Women made up most of the high-risk cohort (70.9%). The average age of participants was 52 years. Of the high-risk individuals, 63.2% reported prior anaphylaxis, 32.9% had multiple drug allergies, and 30.3% had multiple other allergies.
During the first 2 hours following immunization, nine individuals (2.1%), all women, experienced allergic reactions. Six individuals (1.4%) experienced minor reactions, including skin flushing, tongue or uvula swelling, or a cough that resolved with antihistamine treatment during the observation period. Three patients (0.7%) had anaphylactic reactions that occurred 10 to 20 minutes after injection. All three patients experienced significant bronchospasm, skin eruption, itching, and shortness of breath. Two patients experienced angioedema, and one patient had gastrointestinal symptoms. They were treated with adrenaline, antihistamines, and an inhaled bronchodilator. All symptoms resolved within 2-6 hours, and no patient required hospitalization.
In the days following vaccination, patients commonly reported pain at the injection site, fatigue, muscle pain, and headache; 14.7% of patients reported skin eruption, itching, or urticaria.
As of Feb. 22, 2021, 218 patients from this highly allergic cohort received their second dose of the vaccine. Four patients (1.8%) had mild allergic reactions. All four developed flushing, and one patient also developed a cough that resolved with antihistamine treatment. Three of these patients had experienced mild allergic reactions to the first dose and were premedicated for the second dose. One patient only reacted to the second dose.
The findings should be “very reassuring” to individuals hesitant to receive the vaccine, Elizabeth Phillips, MD, the director of the Center for Drug Safety and Immunology at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview. She was not involved with the research and wrote an invited commentary on the study. “The rates of anaphylaxis and allergic reactions are truly quite low,” she said. Although about 2% of the high-risk group developed allergic reactions to immunization, the overall percentage for the entire cohort would be much lower.
The study did not investigate specific risk factors for and mechanisms of allergic reactions to COVID-19 vaccines, Dr. Phillips said, which is a study limitation that the authors also acknowledge. The National Institute for Allergy and Infectious Diseases is currently trying to answer some of these questions with a multisite, randomized, double-blinded study. The study is intended to help understand why people have these allergic reactions, Dr. Phillips added. Vanderbilt is one of the sites for the study.
While researchers continue to hunt for answers, the algorithm developed by the authors provides “a great strategy to get people that are at higher risk vaccinated in a monitored setting,” she said. The results show that “people should not be avoiding vaccination because of a history of anaphylaxis.”
Dr. Phillips has received institutional grants from the National Institutes of Health and the National Health and Medical Research Council; royalties from UpToDate and Lexicomp; and consulting fees from Janssen, Vertex, Biocryst, and Regeneron.
A version of this article first appeared on Medscape.com.
COVID-clogged ICUs ‘terrify’ those with chronic or emergency illness
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A Severe Presentation of Plasma Cell Cheilitis
Plasma cell cheilitis (PCC), also known as plasmocytosis circumorificialis and plasmocytosis mucosae,1 is a poorly understood, uncommon inflammatory condition characterized by dense infiltration of mature plasma cells in the mucosal dermis of the lip.2-5 The etiology of PCC is unknown but is thought to be a reactive immune process triggered by infection, mechanical friction, trauma, or solar damage.1,5,6
The most common presentation of PCC is a slowly evolving, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 Secondary changes with disease progression can include erosion, ulceration, fissures, edema, bleeding, or crusting.5 The diagnosis of PCC is challenging because it can mimic neoplastic, infectious, and inflammatory conditions.8,9
Treatment strategies for PCC described in the literature vary, as does therapeutic response. Resolution of PCC has been documented after systemic steroids, intralesional steroids, systemic griseofulvin, and topical calcineurin inhibitors, among other agents.6,7,10-16
We present the case of a patient with a lip lesion who ultimately was diagnosed with PCC after it progressed to an advanced necrotic stage.
Case Report
An 80-year-old male veteran of the Armed Services initially presented to our institution via teledermatology with redness and crusting of the lower lip (Figure 1). He had a history of myelodysplastic syndrome and anemia requiring iron transfusion. The process appeared to be consistent with actinic cheilitis vs squamous cell carcinoma. In-person dermatology consultation was recommended; however, the patient did not follow through with that appointment.
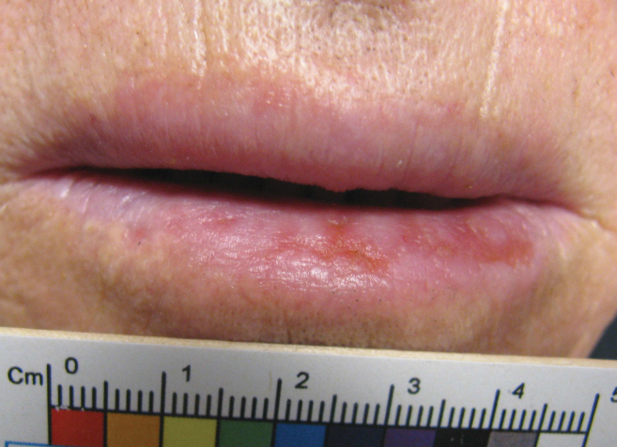
Five months later, additional photographs of the lesion were taken by the patient's primary care physician and sent through teledermatology, revealing progression to an erythematous, yellow-crusted erosion (Figure 2). The medical record indicated that a punch biopsy performed by the patient’s primary care physician showed hyperkeratosis and fungal organisms on periodic acid–Schiff staining. He subsequently applied ketoconazole and terbinafine cream to the lower lip without improvement. Prompt in-person evaluation by dermatology was again recommended.
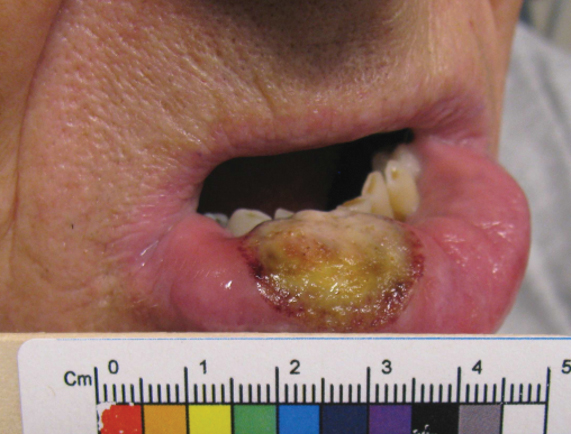
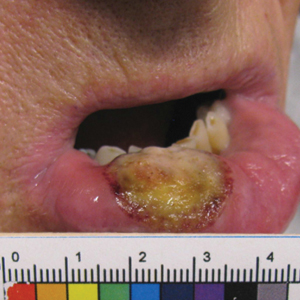
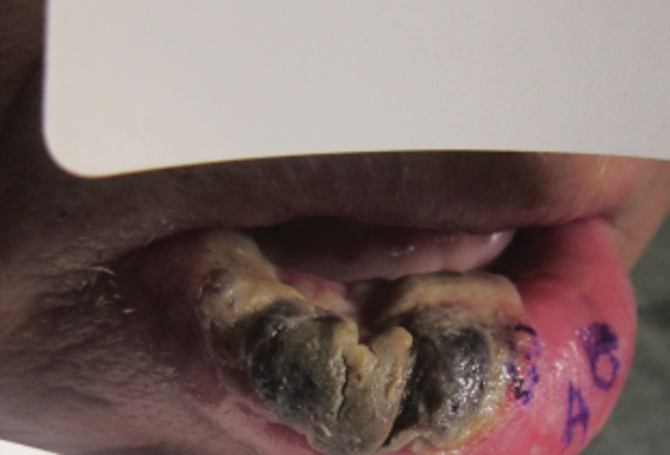
Ten days later, the patient was seen in our dermatology clinic, at which point his condition had rapidly progressed. The lower lip displayed a 3.0×2.5-cm, yellow and black, crusted, ulcerated plaque (Figure 3). He reported severe burning and pain of the lip as well as spontaneous bleeding. He had lost approximately 10 pounds over the last month due to poor oral intake. A second punch biopsy showed benign mucosa with extensive ulceration and formation of full-thickness granulation tissue. No fungi or bacteria were identified.
Consultation and Histologic Analysis
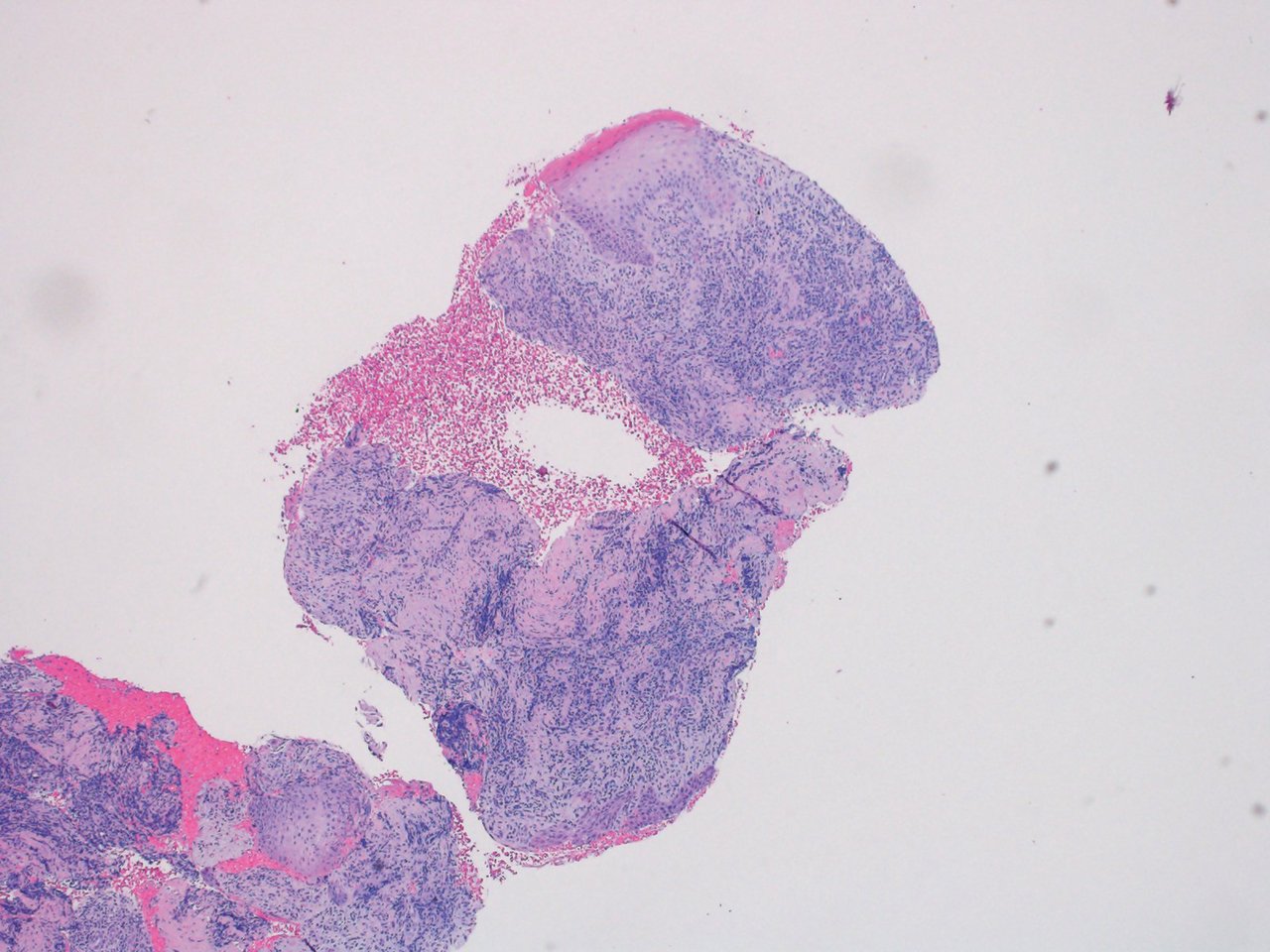
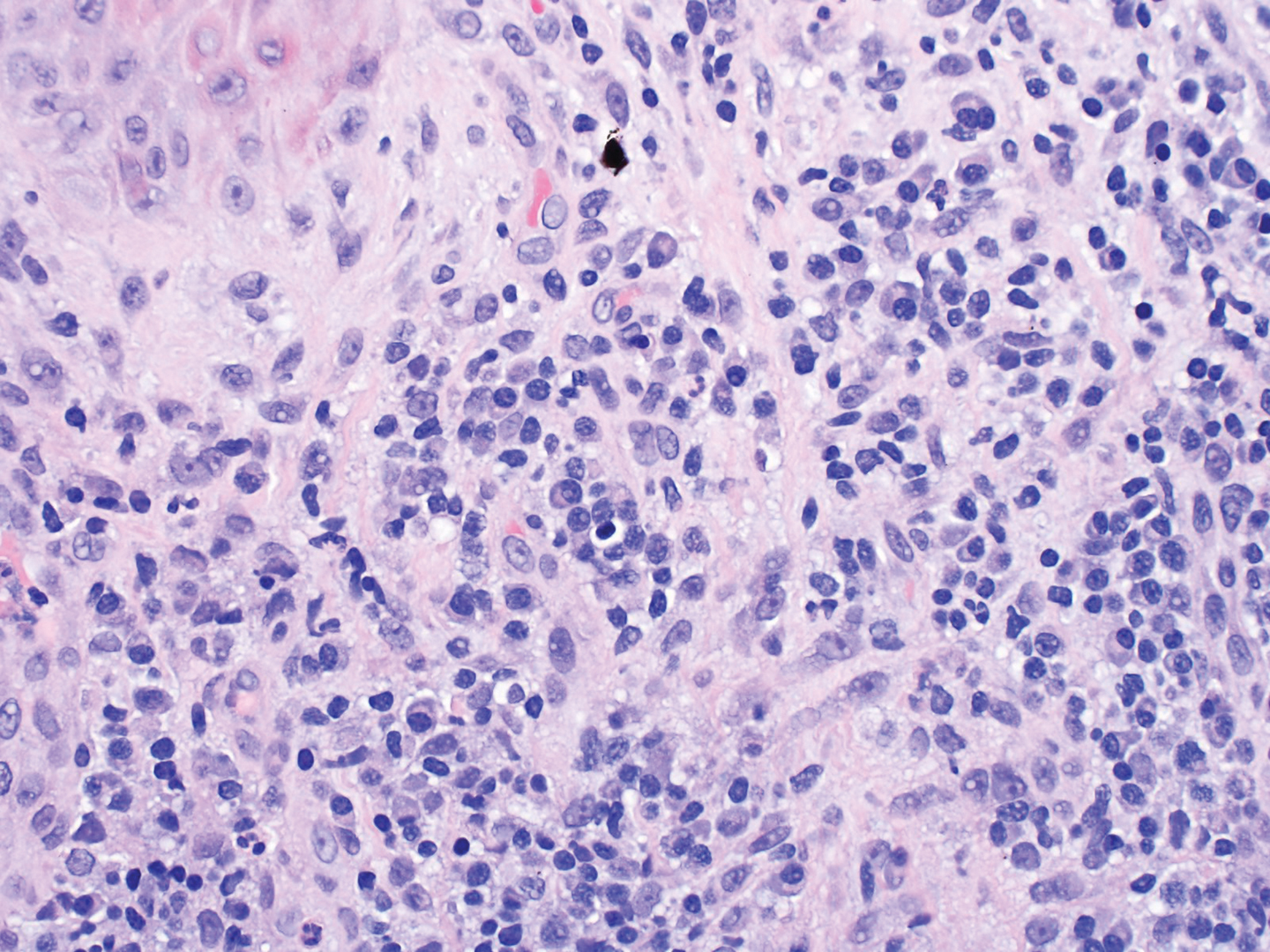
Dermatopathology was consulted and recommended a third punch biopsy for additional testing. A repeat biopsy demonstrated ulceration with lateral elements of retained epidermis and a dense submucosal chronic inflammatory infiltrate comprising plasma cells and lymphocytes (Figures 4 and 5). Immunohistochemical staining demonstrated a mixed inflammatory infiltrate with CD3+ T cells and CD20+ B cells. In situ hybridization studies demonstrated numerous lambda-positive and kappa-positive plasma cells without chain restriction. Periodic acid–Schiff with diastase and Grocott-Gomori methenamine-silver staining demonstrated no fungi. Findings were interpreted to be most consistent with a diagnosis of PCC.
Treatment and Follow-up
The patient was treated with clobetasol ointment 0.05% twice daily for 6 weeks and topical lidocaine as needed for pain. At 6-week follow-up, he displayed substantial improvement, with normal-appearing lips and complete resolution of symptoms.
Comment
The diagnosis and management of PCC is difficult because the condition is uncommon (though its true incidence is unknown) and the presentation is nonspecific, invoking a wide differential diagnosis. In the literature, PCC presents as a slowly progressive, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 The lesion can progress to become eroded, ulcerated, fissured, or edematous.5
Differential Diagnosis
The clinical differential diagnosis of PCC is broad and includes inflammatory, infectious, and neoplastic causes, such as actinic cheilitis, allergic contact cheilitis, exfoliative cheilitis, granulomatous cheilitis, lichen planus, candidiasis, syphilis, and squamous cell carcinoma of the lip.7,9 The histologic differential diagnosis includes allergic contact cheilitis, secondary syphilis, actinic cheilitis, squamous cell carcinoma, cheilitis granulomatosa, and plasmacytoma.17-19
Histopathology
On biopsy, PCC usually is characterized by plasma cells in a bandlike pattern in the upper submucosa or even more diffusely throughout the submucosa.20 In earlier studies, polyclonality of plasma cells with kappa and lambda light chains has been demonstrated5; in this case, such polyclonality militated against a plasma cell dyscrasia. There have been reports of a various number of eosinophils in PCC,5,20 but eosinophils were not a prominent feature in our case.
Treatment
As reported in the literature, treatment of PCC has been attempted using a broad range of strategies; however, the optimal regimen has yet to be elucidated.15 Numerous therapies, including excision, radiation, electrocauterization, cryotherapy, steroids, systemic griseofulvin, topical fusidic acid, and topical calcineurin inhibitors, have yielded variable success.6,7,10-16
The success of topical corticosteroids, as demonstrated in our case, has been unpredictable; the reported response has ranged from complete resolution to failure.9 This variability is thought to be related to epithelial width and the degree of acanthosis, with ulcerative lesions demonstrating a superior response to topical corticosteroids.9
Conclusion
Our case highlights the challenges of diagnosing and managing PCC, especially through teledermatology. Initial photographs of the lesion (Figure 1) that were submitted demonstrated a nonspecific erosion, which was concerning for any of several infectious, inflammatory, and malignant causes. Prompt in-person evaluation was warranted; regrettably, the patient’s condition worsened rapidly in the 10 days it took for him to be seen in-person by dermatology.
Furthermore, this case necessitated 3 separate biopsies because the pathology on the first 2 biopsies initially was equivocal, demonstrating ulceration and granulation tissue. The diagnosis was finally made after a third biopsy was recommended by a dermatopathologist, who eventually identified a bandlike distribution of polyclonal plasma cells in the upper submucosa, consistent with a diagnosis of PCC. Our patient’s final disease presentation (Figure 3) was exuberant and may represent the end point of untreated PCC.
- Senol M, Ozcan A, Aydin NE, et al. Intertriginous plasmacytosis with plasmoacanthoma: report of a typical case and review of the literature. Int J Dermatol. 2008;47:265-268. doi:10.1111/j.1365-4632.2008.03385.x
- Rocha N, Mota F, Horta M, et al. Plasma cell cheilitis. J Eur Acad Dermatol Venereol. 2004;18:96-98. doi:10.1111/j.1468-3083.2004.00791.x
- Farrier JN, Perkins CS. Plasma cell cheilitis. Br J Oral Maxillofac Surg. 2008;46:679-680. doi:10.1016/j.bjoms.2008.03.009
- Baughman RD, Berger P, Pringle WM. Plasma cell cheilitis. Arch Dermatol. 1974;110:725-726.
- Lee JY, Kim KH, Hahm JE, et al. Plasma cell cheilitis: a clinicopathological and immunohistochemical study of 13 cases. Ann Dermatol. 2017;29:536-542. doi:10.5021/ad.2017.29.5.536
- da Cunha Filho RR, Tochetto LB, Tochetto BB, et al. “Angular” plasma cell cheilitis. Dermatol Online J. 2014;20:doj_21759.
- Yang JH, Lee UH, Jang SJ, et al. Plasma cell cheilitis treated with intralesional injection of corticosteroids. J Dermatol. 2005;32:987-990. doi:10.1111/j.1346-8138.2005.tb00887.x
- Solomon LW, Wein RO, Rosenwald I, et al. Plasma cell mucositis of the oral cavity: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:853-860. doi:10.1016/j.tripleo.2008.08.016
- Dos Santos HT, Cunha JLS, Santana LAM, et al. Plasma cell cheilitis: the diagnosis of a disorder mimicking lip cancer. Autops Case Rep. 2019;9:e2018075. doi:10.4322/acr.2018.075
- Fujimura T, Furudate S, Ishibashi M, et al. Successful treatment of plasmacytosis circumorificialis with topical tacrolimus: two case reports and an immunohistochemical study. Case Rep Dermatol. 2013;5:79-83. doi:10.1159/000350184
- Tamaki K, Osada A, Tsukamoto K, et al. Treatment of plasma cell cheilitis with griseofulvin. J Am Acad Dermatol. 1994;30:789-790. doi:10.1016/s0190-9622(08)81515-0
- Choi JW, Choi M, Cho KH. Successful treatment of plasma cell cheilitis with topical calcineurin inhibitors. J Dermatol. 2009;36:669-671. doi:10.1111/j.1346-8138.2009.00733.x
- Hanami Y, Motoki Y, Yamamoto T. Successful treatment of plasma cell cheilitis with topical tacrolimus: report of two cases. Dermatol Online J. 2011;17:6.
- Jin SP, Cho KH, Huh CH. Plasma cell cheilitis, successfully treated with topical 0.03% tacrolimus ointment. J Dermatolog Treat. 2010;21:130-132. doi:10.1080/09546630903200620
- Tseng JT-P, Cheng C-J, Lee W-R, et al. Plasma-cell cheilitis: successful treatment with intralesional injections of corticosteroids. Clin Exp Dermatol. 2009;34:174-177. doi:10.1111/j.1365-2230.2008.02765.x
- Yoshimura K, Nakano S, Tsuruta D, et al. Successful treatment with 308-nm monochromatic excimer light and subsequent tacrolimus 0.03% ointment in refractory plasma cell cheilitis. J Dermatol. 2013;40:471-474. doi:10.1111/1346-8138.12152
- Fujimura Y, Natsuga K, Abe R, et al. Plasma cell cheilitis extending beyond vermillion border. J Dermatol. 2015;42:935-936. doi:10.1111/1346-8138.12985
- White JW Jr, Olsen KD, Banks PM. Plasma cell orificial mucositis. report of a case and review of the literature. Arch Dermatol. 1986;122:1321-1324. doi:10.1001/archderm.122.11.1321
- Román CC, Yuste CM, Gonzalez MA, et al. Plasma cell gingivitis. Cutis. 2002;69:41-45.
- Choe HC, Park HJ, Oh ST, et al. Clinicopathologic study of 8 patients with plasma cell cheilitis. Korean J Dermatol. 2003;41:174-178.
Plasma cell cheilitis (PCC), also known as plasmocytosis circumorificialis and plasmocytosis mucosae,1 is a poorly understood, uncommon inflammatory condition characterized by dense infiltration of mature plasma cells in the mucosal dermis of the lip.2-5 The etiology of PCC is unknown but is thought to be a reactive immune process triggered by infection, mechanical friction, trauma, or solar damage.1,5,6
The most common presentation of PCC is a slowly evolving, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 Secondary changes with disease progression can include erosion, ulceration, fissures, edema, bleeding, or crusting.5 The diagnosis of PCC is challenging because it can mimic neoplastic, infectious, and inflammatory conditions.8,9
Treatment strategies for PCC described in the literature vary, as does therapeutic response. Resolution of PCC has been documented after systemic steroids, intralesional steroids, systemic griseofulvin, and topical calcineurin inhibitors, among other agents.6,7,10-16
We present the case of a patient with a lip lesion who ultimately was diagnosed with PCC after it progressed to an advanced necrotic stage.
Case Report
An 80-year-old male veteran of the Armed Services initially presented to our institution via teledermatology with redness and crusting of the lower lip (Figure 1). He had a history of myelodysplastic syndrome and anemia requiring iron transfusion. The process appeared to be consistent with actinic cheilitis vs squamous cell carcinoma. In-person dermatology consultation was recommended; however, the patient did not follow through with that appointment.

Five months later, additional photographs of the lesion were taken by the patient's primary care physician and sent through teledermatology, revealing progression to an erythematous, yellow-crusted erosion (Figure 2). The medical record indicated that a punch biopsy performed by the patient’s primary care physician showed hyperkeratosis and fungal organisms on periodic acid–Schiff staining. He subsequently applied ketoconazole and terbinafine cream to the lower lip without improvement. Prompt in-person evaluation by dermatology was again recommended.

Ten days later, the patient was seen in our dermatology clinic, at which point his condition had rapidly progressed. The lower lip displayed a 3.0×2.5-cm, yellow and black, crusted, ulcerated plaque (Figure 3). He reported severe burning and pain of the lip as well as spontaneous bleeding. He had lost approximately 10 pounds over the last month due to poor oral intake. A second punch biopsy showed benign mucosa with extensive ulceration and formation of full-thickness granulation tissue. No fungi or bacteria were identified.

Consultation and Histologic Analysis
Dermatopathology was consulted and recommended a third punch biopsy for additional testing. A repeat biopsy demonstrated ulceration with lateral elements of retained epidermis and a dense submucosal chronic inflammatory infiltrate comprising plasma cells and lymphocytes (Figures 4 and 5). Immunohistochemical staining demonstrated a mixed inflammatory infiltrate with CD3+ T cells and CD20+ B cells. In situ hybridization studies demonstrated numerous lambda-positive and kappa-positive plasma cells without chain restriction. Periodic acid–Schiff with diastase and Grocott-Gomori methenamine-silver staining demonstrated no fungi. Findings were interpreted to be most consistent with a diagnosis of PCC.


Treatment and Follow-up
The patient was treated with clobetasol ointment 0.05% twice daily for 6 weeks and topical lidocaine as needed for pain. At 6-week follow-up, he displayed substantial improvement, with normal-appearing lips and complete resolution of symptoms.
Comment
The diagnosis and management of PCC is difficult because the condition is uncommon (though its true incidence is unknown) and the presentation is nonspecific, invoking a wide differential diagnosis. In the literature, PCC presents as a slowly progressive, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 The lesion can progress to become eroded, ulcerated, fissured, or edematous.5
Differential Diagnosis
The clinical differential diagnosis of PCC is broad and includes inflammatory, infectious, and neoplastic causes, such as actinic cheilitis, allergic contact cheilitis, exfoliative cheilitis, granulomatous cheilitis, lichen planus, candidiasis, syphilis, and squamous cell carcinoma of the lip.7,9 The histologic differential diagnosis includes allergic contact cheilitis, secondary syphilis, actinic cheilitis, squamous cell carcinoma, cheilitis granulomatosa, and plasmacytoma.17-19
Histopathology
On biopsy, PCC usually is characterized by plasma cells in a bandlike pattern in the upper submucosa or even more diffusely throughout the submucosa.20 In earlier studies, polyclonality of plasma cells with kappa and lambda light chains has been demonstrated5; in this case, such polyclonality militated against a plasma cell dyscrasia. There have been reports of a various number of eosinophils in PCC,5,20 but eosinophils were not a prominent feature in our case.
Treatment
As reported in the literature, treatment of PCC has been attempted using a broad range of strategies; however, the optimal regimen has yet to be elucidated.15 Numerous therapies, including excision, radiation, electrocauterization, cryotherapy, steroids, systemic griseofulvin, topical fusidic acid, and topical calcineurin inhibitors, have yielded variable success.6,7,10-16
The success of topical corticosteroids, as demonstrated in our case, has been unpredictable; the reported response has ranged from complete resolution to failure.9 This variability is thought to be related to epithelial width and the degree of acanthosis, with ulcerative lesions demonstrating a superior response to topical corticosteroids.9
Conclusion
Our case highlights the challenges of diagnosing and managing PCC, especially through teledermatology. Initial photographs of the lesion (Figure 1) that were submitted demonstrated a nonspecific erosion, which was concerning for any of several infectious, inflammatory, and malignant causes. Prompt in-person evaluation was warranted; regrettably, the patient’s condition worsened rapidly in the 10 days it took for him to be seen in-person by dermatology.
Furthermore, this case necessitated 3 separate biopsies because the pathology on the first 2 biopsies initially was equivocal, demonstrating ulceration and granulation tissue. The diagnosis was finally made after a third biopsy was recommended by a dermatopathologist, who eventually identified a bandlike distribution of polyclonal plasma cells in the upper submucosa, consistent with a diagnosis of PCC. Our patient’s final disease presentation (Figure 3) was exuberant and may represent the end point of untreated PCC.
Plasma cell cheilitis (PCC), also known as plasmocytosis circumorificialis and plasmocytosis mucosae,1 is a poorly understood, uncommon inflammatory condition characterized by dense infiltration of mature plasma cells in the mucosal dermis of the lip.2-5 The etiology of PCC is unknown but is thought to be a reactive immune process triggered by infection, mechanical friction, trauma, or solar damage.1,5,6
The most common presentation of PCC is a slowly evolving, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 Secondary changes with disease progression can include erosion, ulceration, fissures, edema, bleeding, or crusting.5 The diagnosis of PCC is challenging because it can mimic neoplastic, infectious, and inflammatory conditions.8,9
Treatment strategies for PCC described in the literature vary, as does therapeutic response. Resolution of PCC has been documented after systemic steroids, intralesional steroids, systemic griseofulvin, and topical calcineurin inhibitors, among other agents.6,7,10-16
We present the case of a patient with a lip lesion who ultimately was diagnosed with PCC after it progressed to an advanced necrotic stage.
Case Report
An 80-year-old male veteran of the Armed Services initially presented to our institution via teledermatology with redness and crusting of the lower lip (Figure 1). He had a history of myelodysplastic syndrome and anemia requiring iron transfusion. The process appeared to be consistent with actinic cheilitis vs squamous cell carcinoma. In-person dermatology consultation was recommended; however, the patient did not follow through with that appointment.

Five months later, additional photographs of the lesion were taken by the patient's primary care physician and sent through teledermatology, revealing progression to an erythematous, yellow-crusted erosion (Figure 2). The medical record indicated that a punch biopsy performed by the patient’s primary care physician showed hyperkeratosis and fungal organisms on periodic acid–Schiff staining. He subsequently applied ketoconazole and terbinafine cream to the lower lip without improvement. Prompt in-person evaluation by dermatology was again recommended.

Ten days later, the patient was seen in our dermatology clinic, at which point his condition had rapidly progressed. The lower lip displayed a 3.0×2.5-cm, yellow and black, crusted, ulcerated plaque (Figure 3). He reported severe burning and pain of the lip as well as spontaneous bleeding. He had lost approximately 10 pounds over the last month due to poor oral intake. A second punch biopsy showed benign mucosa with extensive ulceration and formation of full-thickness granulation tissue. No fungi or bacteria were identified.

Consultation and Histologic Analysis
Dermatopathology was consulted and recommended a third punch biopsy for additional testing. A repeat biopsy demonstrated ulceration with lateral elements of retained epidermis and a dense submucosal chronic inflammatory infiltrate comprising plasma cells and lymphocytes (Figures 4 and 5). Immunohistochemical staining demonstrated a mixed inflammatory infiltrate with CD3+ T cells and CD20+ B cells. In situ hybridization studies demonstrated numerous lambda-positive and kappa-positive plasma cells without chain restriction. Periodic acid–Schiff with diastase and Grocott-Gomori methenamine-silver staining demonstrated no fungi. Findings were interpreted to be most consistent with a diagnosis of PCC.


Treatment and Follow-up
The patient was treated with clobetasol ointment 0.05% twice daily for 6 weeks and topical lidocaine as needed for pain. At 6-week follow-up, he displayed substantial improvement, with normal-appearing lips and complete resolution of symptoms.
Comment
The diagnosis and management of PCC is difficult because the condition is uncommon (though its true incidence is unknown) and the presentation is nonspecific, invoking a wide differential diagnosis. In the literature, PCC presents as a slowly progressive, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 The lesion can progress to become eroded, ulcerated, fissured, or edematous.5
Differential Diagnosis
The clinical differential diagnosis of PCC is broad and includes inflammatory, infectious, and neoplastic causes, such as actinic cheilitis, allergic contact cheilitis, exfoliative cheilitis, granulomatous cheilitis, lichen planus, candidiasis, syphilis, and squamous cell carcinoma of the lip.7,9 The histologic differential diagnosis includes allergic contact cheilitis, secondary syphilis, actinic cheilitis, squamous cell carcinoma, cheilitis granulomatosa, and plasmacytoma.17-19
Histopathology
On biopsy, PCC usually is characterized by plasma cells in a bandlike pattern in the upper submucosa or even more diffusely throughout the submucosa.20 In earlier studies, polyclonality of plasma cells with kappa and lambda light chains has been demonstrated5; in this case, such polyclonality militated against a plasma cell dyscrasia. There have been reports of a various number of eosinophils in PCC,5,20 but eosinophils were not a prominent feature in our case.
Treatment
As reported in the literature, treatment of PCC has been attempted using a broad range of strategies; however, the optimal regimen has yet to be elucidated.15 Numerous therapies, including excision, radiation, electrocauterization, cryotherapy, steroids, systemic griseofulvin, topical fusidic acid, and topical calcineurin inhibitors, have yielded variable success.6,7,10-16
The success of topical corticosteroids, as demonstrated in our case, has been unpredictable; the reported response has ranged from complete resolution to failure.9 This variability is thought to be related to epithelial width and the degree of acanthosis, with ulcerative lesions demonstrating a superior response to topical corticosteroids.9
Conclusion
Our case highlights the challenges of diagnosing and managing PCC, especially through teledermatology. Initial photographs of the lesion (Figure 1) that were submitted demonstrated a nonspecific erosion, which was concerning for any of several infectious, inflammatory, and malignant causes. Prompt in-person evaluation was warranted; regrettably, the patient’s condition worsened rapidly in the 10 days it took for him to be seen in-person by dermatology.
Furthermore, this case necessitated 3 separate biopsies because the pathology on the first 2 biopsies initially was equivocal, demonstrating ulceration and granulation tissue. The diagnosis was finally made after a third biopsy was recommended by a dermatopathologist, who eventually identified a bandlike distribution of polyclonal plasma cells in the upper submucosa, consistent with a diagnosis of PCC. Our patient’s final disease presentation (Figure 3) was exuberant and may represent the end point of untreated PCC.
- Senol M, Ozcan A, Aydin NE, et al. Intertriginous plasmacytosis with plasmoacanthoma: report of a typical case and review of the literature. Int J Dermatol. 2008;47:265-268. doi:10.1111/j.1365-4632.2008.03385.x
- Rocha N, Mota F, Horta M, et al. Plasma cell cheilitis. J Eur Acad Dermatol Venereol. 2004;18:96-98. doi:10.1111/j.1468-3083.2004.00791.x
- Farrier JN, Perkins CS. Plasma cell cheilitis. Br J Oral Maxillofac Surg. 2008;46:679-680. doi:10.1016/j.bjoms.2008.03.009
- Baughman RD, Berger P, Pringle WM. Plasma cell cheilitis. Arch Dermatol. 1974;110:725-726.
- Lee JY, Kim KH, Hahm JE, et al. Plasma cell cheilitis: a clinicopathological and immunohistochemical study of 13 cases. Ann Dermatol. 2017;29:536-542. doi:10.5021/ad.2017.29.5.536
- da Cunha Filho RR, Tochetto LB, Tochetto BB, et al. “Angular” plasma cell cheilitis. Dermatol Online J. 2014;20:doj_21759.
- Yang JH, Lee UH, Jang SJ, et al. Plasma cell cheilitis treated with intralesional injection of corticosteroids. J Dermatol. 2005;32:987-990. doi:10.1111/j.1346-8138.2005.tb00887.x
- Solomon LW, Wein RO, Rosenwald I, et al. Plasma cell mucositis of the oral cavity: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:853-860. doi:10.1016/j.tripleo.2008.08.016
- Dos Santos HT, Cunha JLS, Santana LAM, et al. Plasma cell cheilitis: the diagnosis of a disorder mimicking lip cancer. Autops Case Rep. 2019;9:e2018075. doi:10.4322/acr.2018.075
- Fujimura T, Furudate S, Ishibashi M, et al. Successful treatment of plasmacytosis circumorificialis with topical tacrolimus: two case reports and an immunohistochemical study. Case Rep Dermatol. 2013;5:79-83. doi:10.1159/000350184
- Tamaki K, Osada A, Tsukamoto K, et al. Treatment of plasma cell cheilitis with griseofulvin. J Am Acad Dermatol. 1994;30:789-790. doi:10.1016/s0190-9622(08)81515-0
- Choi JW, Choi M, Cho KH. Successful treatment of plasma cell cheilitis with topical calcineurin inhibitors. J Dermatol. 2009;36:669-671. doi:10.1111/j.1346-8138.2009.00733.x
- Hanami Y, Motoki Y, Yamamoto T. Successful treatment of plasma cell cheilitis with topical tacrolimus: report of two cases. Dermatol Online J. 2011;17:6.
- Jin SP, Cho KH, Huh CH. Plasma cell cheilitis, successfully treated with topical 0.03% tacrolimus ointment. J Dermatolog Treat. 2010;21:130-132. doi:10.1080/09546630903200620
- Tseng JT-P, Cheng C-J, Lee W-R, et al. Plasma-cell cheilitis: successful treatment with intralesional injections of corticosteroids. Clin Exp Dermatol. 2009;34:174-177. doi:10.1111/j.1365-2230.2008.02765.x
- Yoshimura K, Nakano S, Tsuruta D, et al. Successful treatment with 308-nm monochromatic excimer light and subsequent tacrolimus 0.03% ointment in refractory plasma cell cheilitis. J Dermatol. 2013;40:471-474. doi:10.1111/1346-8138.12152
- Fujimura Y, Natsuga K, Abe R, et al. Plasma cell cheilitis extending beyond vermillion border. J Dermatol. 2015;42:935-936. doi:10.1111/1346-8138.12985
- White JW Jr, Olsen KD, Banks PM. Plasma cell orificial mucositis. report of a case and review of the literature. Arch Dermatol. 1986;122:1321-1324. doi:10.1001/archderm.122.11.1321
- Román CC, Yuste CM, Gonzalez MA, et al. Plasma cell gingivitis. Cutis. 2002;69:41-45.
- Choe HC, Park HJ, Oh ST, et al. Clinicopathologic study of 8 patients with plasma cell cheilitis. Korean J Dermatol. 2003;41:174-178.
- Senol M, Ozcan A, Aydin NE, et al. Intertriginous plasmacytosis with plasmoacanthoma: report of a typical case and review of the literature. Int J Dermatol. 2008;47:265-268. doi:10.1111/j.1365-4632.2008.03385.x
- Rocha N, Mota F, Horta M, et al. Plasma cell cheilitis. J Eur Acad Dermatol Venereol. 2004;18:96-98. doi:10.1111/j.1468-3083.2004.00791.x
- Farrier JN, Perkins CS. Plasma cell cheilitis. Br J Oral Maxillofac Surg. 2008;46:679-680. doi:10.1016/j.bjoms.2008.03.009
- Baughman RD, Berger P, Pringle WM. Plasma cell cheilitis. Arch Dermatol. 1974;110:725-726.
- Lee JY, Kim KH, Hahm JE, et al. Plasma cell cheilitis: a clinicopathological and immunohistochemical study of 13 cases. Ann Dermatol. 2017;29:536-542. doi:10.5021/ad.2017.29.5.536
- da Cunha Filho RR, Tochetto LB, Tochetto BB, et al. “Angular” plasma cell cheilitis. Dermatol Online J. 2014;20:doj_21759.
- Yang JH, Lee UH, Jang SJ, et al. Plasma cell cheilitis treated with intralesional injection of corticosteroids. J Dermatol. 2005;32:987-990. doi:10.1111/j.1346-8138.2005.tb00887.x
- Solomon LW, Wein RO, Rosenwald I, et al. Plasma cell mucositis of the oral cavity: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:853-860. doi:10.1016/j.tripleo.2008.08.016
- Dos Santos HT, Cunha JLS, Santana LAM, et al. Plasma cell cheilitis: the diagnosis of a disorder mimicking lip cancer. Autops Case Rep. 2019;9:e2018075. doi:10.4322/acr.2018.075
- Fujimura T, Furudate S, Ishibashi M, et al. Successful treatment of plasmacytosis circumorificialis with topical tacrolimus: two case reports and an immunohistochemical study. Case Rep Dermatol. 2013;5:79-83. doi:10.1159/000350184
- Tamaki K, Osada A, Tsukamoto K, et al. Treatment of plasma cell cheilitis with griseofulvin. J Am Acad Dermatol. 1994;30:789-790. doi:10.1016/s0190-9622(08)81515-0
- Choi JW, Choi M, Cho KH. Successful treatment of plasma cell cheilitis with topical calcineurin inhibitors. J Dermatol. 2009;36:669-671. doi:10.1111/j.1346-8138.2009.00733.x
- Hanami Y, Motoki Y, Yamamoto T. Successful treatment of plasma cell cheilitis with topical tacrolimus: report of two cases. Dermatol Online J. 2011;17:6.
- Jin SP, Cho KH, Huh CH. Plasma cell cheilitis, successfully treated with topical 0.03% tacrolimus ointment. J Dermatolog Treat. 2010;21:130-132. doi:10.1080/09546630903200620
- Tseng JT-P, Cheng C-J, Lee W-R, et al. Plasma-cell cheilitis: successful treatment with intralesional injections of corticosteroids. Clin Exp Dermatol. 2009;34:174-177. doi:10.1111/j.1365-2230.2008.02765.x
- Yoshimura K, Nakano S, Tsuruta D, et al. Successful treatment with 308-nm monochromatic excimer light and subsequent tacrolimus 0.03% ointment in refractory plasma cell cheilitis. J Dermatol. 2013;40:471-474. doi:10.1111/1346-8138.12152
- Fujimura Y, Natsuga K, Abe R, et al. Plasma cell cheilitis extending beyond vermillion border. J Dermatol. 2015;42:935-936. doi:10.1111/1346-8138.12985
- White JW Jr, Olsen KD, Banks PM. Plasma cell orificial mucositis. report of a case and review of the literature. Arch Dermatol. 1986;122:1321-1324. doi:10.1001/archderm.122.11.1321
- Román CC, Yuste CM, Gonzalez MA, et al. Plasma cell gingivitis. Cutis. 2002;69:41-45.
- Choe HC, Park HJ, Oh ST, et al. Clinicopathologic study of 8 patients with plasma cell cheilitis. Korean J Dermatol. 2003;41:174-178.
PRACTICE POINTS
- Plasma cell cheilitis (PCC) is a benign condition that affects the lower lip in older individuals, presenting as a nonspecific, red-brown patch or plaque that can progress slowly to erosions and edema.
- Our patient with PCC experienced full resolution of symptoms with application of a class I topical corticosteroid.
Verruca Vulgaris Arising Within the Red Portion of a Multicolored Tattoo
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.
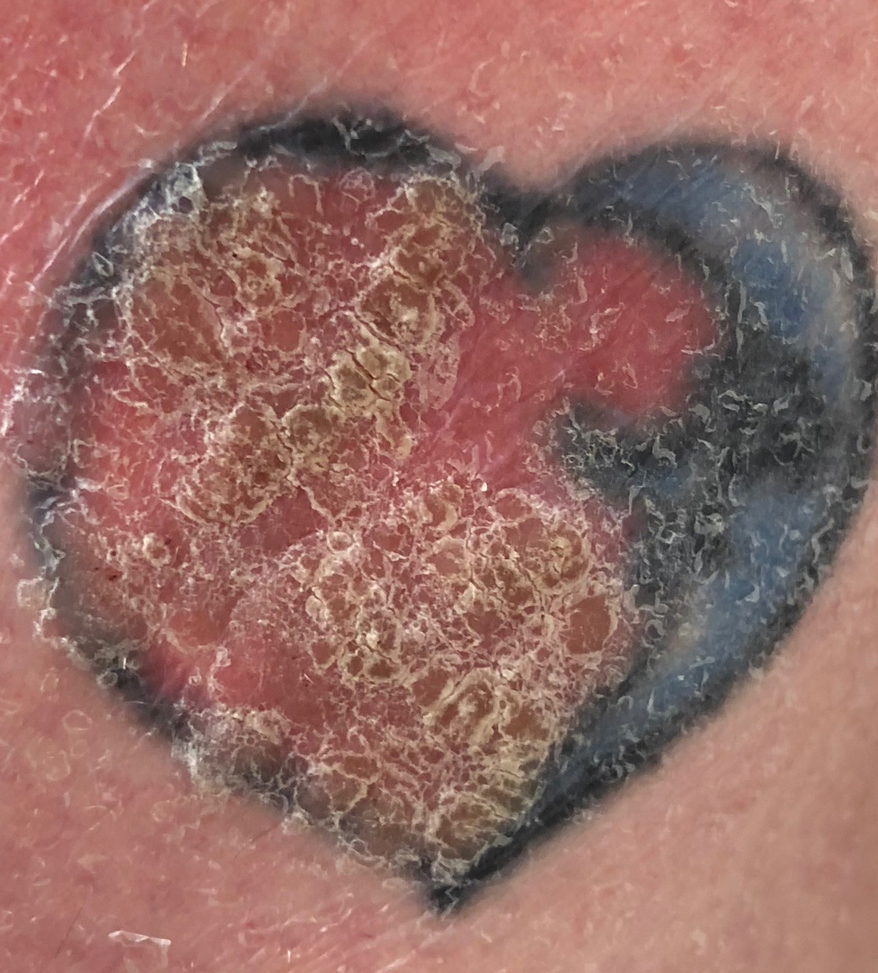
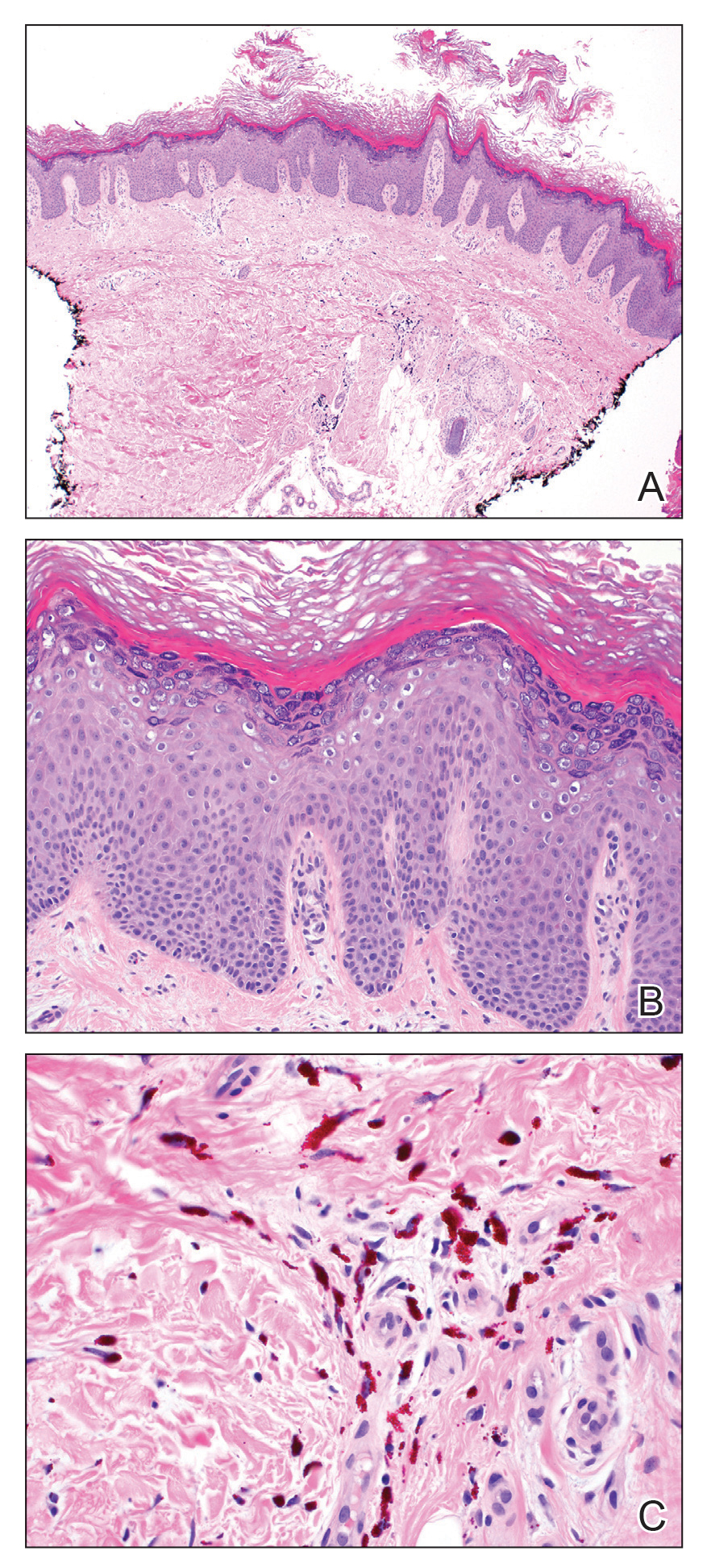
The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.


The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.


The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
Practice Points
- Various adverse reactions and infectious agents may involve tattoos.
- Verruca vulgaris may affect tattoos in a color-restricted manner and demonstrate latency of many years after tattoo placement.
- Timely diagnosis of the tattoo-involving process, confirmed by biopsy, allows for appropriate management.
Pediatric-Onset Refractory Lupus Erythematosus Panniculitis Treated With Rituximab
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.
Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.
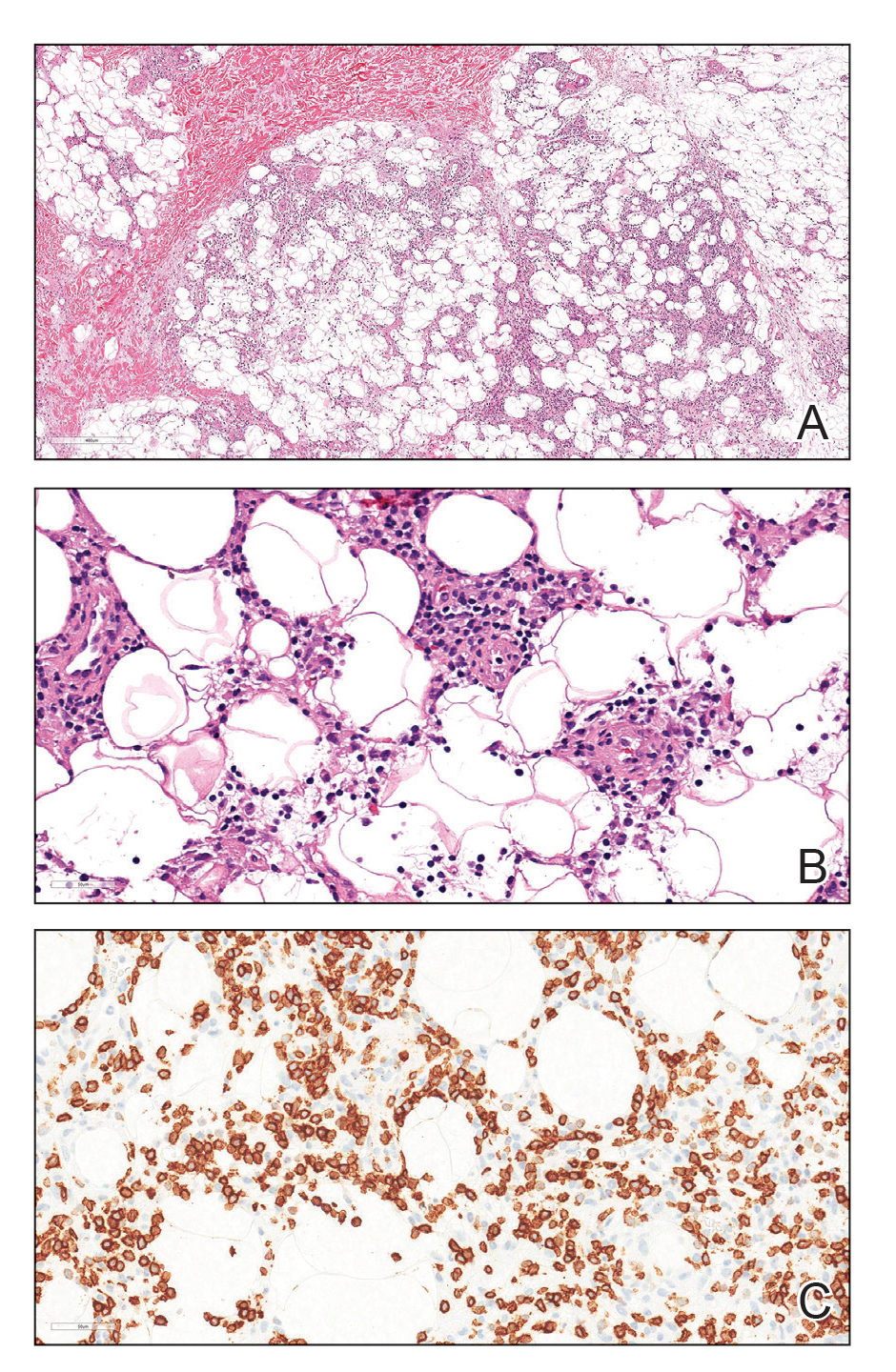

Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.

Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.


Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.

Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.


Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
Practice Points
- Lupus erythematosus panniculitis (LEP) is rare in the pediatric population and often is difficult to treat.
- Rituximab can be an effective treatment option for refractory LEP.
- Before the initiation of rituximab, a biopsy is warranted to rule out subcutaneous T-cell lymphoma, which can mimic LEP and hemophagocytic lymphohistiocytosis–associated panniculitis.
Volunteer Opportunities Within Dermatology: More than Skin Deep
The adage “so much to do, so little time” aptly describes the daily challenges facing dermatologists and dermatology residents. The time and attention required by direct patient care, writing notes, navigating electronic health records, and engaging in education and research as well as family commitments can drain even the most tireless clinician. In addition, dermatologists are expected to play a critical role in clinic and practice management to successfully curate an online presence and adapt their skills to successfully manage a teledermatology practice. Coupled with the time spent socializing with friends or colleagues and time for personal hobbies or exercise, it’s easy to see how sleep deprivation is common in many of our colleagues.
What’s being left out of these jam-packed schedules? Increasingly, it is the time and expertise dedicated to volunteering in our local communities. Two recent research letters highlighted how a dramatic increase in the number of research projects and publications is not mirrored by a similar increase in volunteer experiences as dermatology residency selection becomes more competitive.1,2
Although the rate of volunteerism among practicing dermatologists has yet to be studied, a brief review suggests a component of unmet dermatology need within our communities. It’s estimated that approximately 5% to 10% of all emergency department visits are for dermatologic concerns.3-5 In many cases, the reason for the visit is nonurgent and instead reflects a lack of other options for care. However, the need for dermatologists extends beyond the emergency department setting. A review of the prevalence of patients presenting for care to a group of regional free clinics found that 8% (N=5553) of all visitors sought care for dermatologic concerns.6 The benefit is not just for those seated on the examination table; research has shown that while many of the underlying factors resulting in physician burnout stem from systemic issues, participating in volunteer opportunities helps combat burnout in ourselves and our colleagues.7-9 Herein, opportunities that exist for dermatologists to reconnect with their communities, advocate for causes distinctive to the specialty, and care for neighbors most in need are highlighted.
Camp Wonder
Every year, children from across the United States living with chronic and debilitating skin conditions get the opportunity to join fellow campers and spend a week just being kids without the constant focus on being a patient. Camp Wonder’s founder and director, Francesca Tenconi, describes the camp as a place where kids “can form a community and can feel free to be themselves, without judgment, without stares. They get the chance to forget about their skin disease and be themselves” (oral communication, June 18, 2021). Tenconi and the camp’s cofounders and medical directors, Drs. Jenny Kim and Stefani Takahashi, envisioned the camp as a place for all campers regardless of their skin condition to feel safe and welcome. This overall mission guides camp leadership and staff every year over the course of the camp week where campers participate in a mix of traditional and nontraditional summer activities that are safe and accessible for all, from spending time in the pool to arts and crafts and a ropes course.
Camp Wonder is in its 21st year of hosting children and adolescents from across North America at its camp in Livermore, California. This year, Tenconi expects about 100 campers during the last week in July. Camp Wonder relies on medical staff volunteers to make the camp setting safe, inclusive, and fun. “Our dermatology residents and dermatology volunteers are a huge part of why we’re able to have camp,” said Tenconi. “A lot of our kids require very specific medical care throughout the week. We are able to provide this camp experience for them because we have this medical support system available, this specialized dermatology knowledge.” She also noted the benefit to the volunteers themselves, saying,“The feedback we get a lot from residents and dermatologists is that camp gave them a chance to understand the true-life impact of some of the skin diseases these kids and families are living with. Kids will open up to them and tell them how their disease has impacted them personally” (oral communication, June 18, 2021).
Volunteer medical providers help manage the medical needs of the campers beginning at check-in and work shifts in the infirmary as well as help with dispensing and administering medications, changing dressings, and applying ointments or other topical medications. When not assisting with medical care, medical staff can get to know the campers; help out with arts and crafts, games, sports, and other camp activities; and put on skits and plays for campers at nightly camp hangouts (Figure 1).

How to Get Involved
Visit the website (https://www.csdf.org/camp-wonder) for information on becoming a medical volunteer for 2022. Donations to help keep the camp running also are greatly appreciated, as attendance, including travel costs, is free for families through the Children’s Skin Disease Foundation. Finally, dermatologists can help by keeping their young patients with skin disease in mind as future campers. The camp welcomes kids from across the United States and Canada and invites questions from dermatologists and families on how to become a camper and what the experience is like.
Native American Health Services Rotation
Located in the southwestern United States, the Navajo Nation is North America’s largest Native American tribe by enrollment and resides on the largest reservation in the United States.10 Comprised of 27,000 square miles within portions of Arizona, New Mexico, and Utah, the reservation’s total area is greater than that of Massachusetts, Vermont, and New Hampshire combined.11 The reservation is home to an estimated 180,000 Navajo people, a population roughly the size of Salt Lake City, Utah. Yet, many homes on the reservation are without electricity, running water, telephones, or broadband access, and many roads on the reservation remain unpaved. Prior to the COVID-19 pandemic, 4 dermatology residents were selected each year to travel to this unique and remote location to work with the staff of the Chinle Comprehensive Health Care Facility (Chinle, Arizona), an Indian Health Service facility, as part of the American Academy of Dermatology (AAD)–sponsored Native American Health Services Resident Rotation (NAHSRR).
Dr. Lucinda Kohn, Assistant Professor of Dermatology at the University of Colorado and the director of the NAHSRR program discovered the value of this rotation firsthand as a dermatology resident. In 2017, she traveled to the area to spend 2 weeks serving within the community. “I went because of a personal connection. My husband is Native American, although not Navajo. I wanted to experience what it was like to provide dermatologic care for Native Americans. I found the Navajo people to be so friendly and so grateful for our care. The clinicians we worked with at Chinle were excited to have us share our expertise and to pass on their knowledge to us,” said Dr. Kohn (personal communication, June 24, 2021).
Rotating residents provide dermatologic care for the Navajo people and share their unique medical skill set to local primary care clinicians serving as preceptors. They also may have an opportunity to learn from Native healers about traditional Navajo beliefs and ceremonies used as part of a holistic approach to healing.
The program, similar to volunteer programs across the country, was put on hold during the height of the COVID-19 pandemic. “The Navajo nation witnessed a really tragic surge of COVID cases that required that limited medical resources be diverted to help cope with the pandemic,” says Dr. Kohn. “It really wasn’t safe for residents to travel to the reservation either, so the rotation had to be put on hold.” However, in April 2021, the health care staff of the Chinle Comprehensive Care Facility reached out to revive the program, which is now pending the green light from the AAD. It is unclear if or when AAD leadership will allow this rotation to restart. Dr. Kohn hopes to be able to start accepting new applications soon. “This rotation provides a wealth of benefits to all those involved, from the residents who get the chance to work with a unique population in need to the clinicians who gain a diverse understanding of dermatology treatment techniques. And of course, for the patients, who are so appreciative of the care they receive from our volunteers” (personal communication, June 25, 2021).
How to Get Involved
Dr. Kohn is happy to field questions regarding the rotation and requests for more information via email ([email protected]). Residents interested in this program also may reach out to the AAD’s Education and Volunteers Abroad Committee to express interest in the NAHSRR program’s reinstatement.
Destination Healthy Skin
Since 2017, the Skin Cancer Foundation’s Destination Healthy Skin (DHS) RV has been the setting for more than 3800 free skin cancer screenings provided by volunteers within underserved populations across the United States (Figure 2). After a year hiatus due to the pandemic, DHS hit the road again, starting in New York City on August 1 to 3, 2021. From there, the DHS RV will traverse the country in one large loop, starting with visits to large and small cities in the Midwest and the West Coast. Following a visit to San Diego, California, in early October, the RV will turn east, with stops in Arizona, Texas, and several southern states before ending in Philadelphia, Pennsylvania. Dr. Elizabeth Hale, Senior Vice President of the Skin Cancer Foundation, feels that increasing awareness of the importance of regular skin cancer screening for those at risk is more important than ever. “We know that many people in the past year put routine cancer screening on the back burner, but we’re beginning to appreciate that this has led to significant delays in skin cancer diagnosis and potentially more significant disease when cases are diagnosed.” Dr. Hale noted that as the country continues to return to a degree of normalcy, the backlog of patients now seeking their routine screening has led to longer wait times. She expects DHS may offer some relief. “There are no appointments necessary. If the RV is close to their hometown, patients have an advantage in being able to be seen first come, first served, without having to wait for an appointment or make sure their insurance is accepted. It’s a free screening that can increase access to dermatologists” (personal communication, June 21, 2021).

The program’s organizers acknowledge that DHS is not a long-term solution for improving dermatology access in the United States and recognize that more needs to be done to raise awareness, both of the value that screenings can provide and the importance of sun-protective behavior. “This is an important first step,” says Dr. Hale. “It’s important that we disseminate that no one is immune to skin cancer. It’s about education, and this is a tool to educate patients that everyone should have a skin check once a year, regardless of where you live or what your skin type is” (personal communication, June 21, 2021).
Volunteer dermatologists are needed to assist with screenings when the DHS RV arrives in their community. Providers complete a screening form identifying any concerning lesions and can document specific lesions using the patient’s cell phone. Following the screenings, participating dermatologists are welcome to invite participants to make appointments at their practices or suggest local clinics for follow-up care.
How to Get Involved
The schedule for this year’s screening events can be found online (https://www.skincancer.org/early-detection/destination-healthy-skin/). Consider volunteering (https://www.skincancer.org/early-detection/destination-healthy-skin/physician-volunteers/) or helping to raise awareness by reaching out to local dermatology societies or free clinics in your area. Residents and physician’s assistants are welcome to volunteer as well, as long as they are under the on-site supervision of a board-certified dermatologist.
Final Thoughts
As medical professionals, we all recognize there are valuable contributions we can make to groups and organizations that need our help. The stresses and pressure of work and everyday life can make finding the time to offer that help seem impossible. Although it may seem counterintuitive, volunteering our time to help others can help us better navigate the professional burnout that many medical professionals experience today.
- Ezekor M, Pona A, Cline A, et al. An increasing trend in the number of publications and research projects among dermatology residency applicants. J Am Acad Dermatol. 2020;83:214-216.
- Atluri S, Seivright JR, Shi VY, et al. Volunteer and work experiences among dermatology residency applicants. J Am Acad Dermatol. 2021;84:E97-E98.
- Abokwidir M, Davis SA, Fleischer AB, et al. Use of the emergency department for dermatologic care in the United States by ethnic group. J Dermatolog Treat. 2015;26:392-394.
- Uscher-Pines L, Pines J, Kellermann A, et al. Emergency department visits for nonurgent conditions: systematic literature review. Am J Manag Care. 2013;19:47-59.
- Jack AR, Spence AA, Nichols BJ, et al. Cutaneous conditions leading to dermatology consultations in the emergency department. West J Emerg Med. 2011;12:551-555.
- Ayoubi N, Mirza A-S, Swanson J, et al. Dermatologic care of uninsured patients managed at free clinics. J Am Acad Dermatol. 2019;81:433-437.
- Wright AA, Katz IT. Beyond burnout—redesigning care to restore meaning and sanity for physicians. N Engl J Med. 2018;378:309-311.
- Bull C, Aucoin JB. Voluntary association participation and life satisfaction: a replication note. J Gerontol. 1975;30:73-76.
- Iserson KV. Burnout syndrome: global medicine volunteering as a possible treatment strategy. J Emerg Med. 2018;54:516-521.
- Romero S. Navajo Nation becomes largest tribe in U.S. after pandemic enrollment surge. New York Times. May 21, 2021. Accessed August 19, 2021. https://www.nytimes.com/2021/05/21/us/navajo-cherokee-population.html
- Moore GR, Benally J, Tuttle S. The Navajo Nation: quick facts. University of Arizona website. Accessed August 19, 2021. https://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1471.pdf
The adage “so much to do, so little time” aptly describes the daily challenges facing dermatologists and dermatology residents. The time and attention required by direct patient care, writing notes, navigating electronic health records, and engaging in education and research as well as family commitments can drain even the most tireless clinician. In addition, dermatologists are expected to play a critical role in clinic and practice management to successfully curate an online presence and adapt their skills to successfully manage a teledermatology practice. Coupled with the time spent socializing with friends or colleagues and time for personal hobbies or exercise, it’s easy to see how sleep deprivation is common in many of our colleagues.
What’s being left out of these jam-packed schedules? Increasingly, it is the time and expertise dedicated to volunteering in our local communities. Two recent research letters highlighted how a dramatic increase in the number of research projects and publications is not mirrored by a similar increase in volunteer experiences as dermatology residency selection becomes more competitive.1,2
Although the rate of volunteerism among practicing dermatologists has yet to be studied, a brief review suggests a component of unmet dermatology need within our communities. It’s estimated that approximately 5% to 10% of all emergency department visits are for dermatologic concerns.3-5 In many cases, the reason for the visit is nonurgent and instead reflects a lack of other options for care. However, the need for dermatologists extends beyond the emergency department setting. A review of the prevalence of patients presenting for care to a group of regional free clinics found that 8% (N=5553) of all visitors sought care for dermatologic concerns.6 The benefit is not just for those seated on the examination table; research has shown that while many of the underlying factors resulting in physician burnout stem from systemic issues, participating in volunteer opportunities helps combat burnout in ourselves and our colleagues.7-9 Herein, opportunities that exist for dermatologists to reconnect with their communities, advocate for causes distinctive to the specialty, and care for neighbors most in need are highlighted.
Camp Wonder
Every year, children from across the United States living with chronic and debilitating skin conditions get the opportunity to join fellow campers and spend a week just being kids without the constant focus on being a patient. Camp Wonder’s founder and director, Francesca Tenconi, describes the camp as a place where kids “can form a community and can feel free to be themselves, without judgment, without stares. They get the chance to forget about their skin disease and be themselves” (oral communication, June 18, 2021). Tenconi and the camp’s cofounders and medical directors, Drs. Jenny Kim and Stefani Takahashi, envisioned the camp as a place for all campers regardless of their skin condition to feel safe and welcome. This overall mission guides camp leadership and staff every year over the course of the camp week where campers participate in a mix of traditional and nontraditional summer activities that are safe and accessible for all, from spending time in the pool to arts and crafts and a ropes course.
Camp Wonder is in its 21st year of hosting children and adolescents from across North America at its camp in Livermore, California. This year, Tenconi expects about 100 campers during the last week in July. Camp Wonder relies on medical staff volunteers to make the camp setting safe, inclusive, and fun. “Our dermatology residents and dermatology volunteers are a huge part of why we’re able to have camp,” said Tenconi. “A lot of our kids require very specific medical care throughout the week. We are able to provide this camp experience for them because we have this medical support system available, this specialized dermatology knowledge.” She also noted the benefit to the volunteers themselves, saying,“The feedback we get a lot from residents and dermatologists is that camp gave them a chance to understand the true-life impact of some of the skin diseases these kids and families are living with. Kids will open up to them and tell them how their disease has impacted them personally” (oral communication, June 18, 2021).
Volunteer medical providers help manage the medical needs of the campers beginning at check-in and work shifts in the infirmary as well as help with dispensing and administering medications, changing dressings, and applying ointments or other topical medications. When not assisting with medical care, medical staff can get to know the campers; help out with arts and crafts, games, sports, and other camp activities; and put on skits and plays for campers at nightly camp hangouts (Figure 1).

How to Get Involved
Visit the website (https://www.csdf.org/camp-wonder) for information on becoming a medical volunteer for 2022. Donations to help keep the camp running also are greatly appreciated, as attendance, including travel costs, is free for families through the Children’s Skin Disease Foundation. Finally, dermatologists can help by keeping their young patients with skin disease in mind as future campers. The camp welcomes kids from across the United States and Canada and invites questions from dermatologists and families on how to become a camper and what the experience is like.
Native American Health Services Rotation
Located in the southwestern United States, the Navajo Nation is North America’s largest Native American tribe by enrollment and resides on the largest reservation in the United States.10 Comprised of 27,000 square miles within portions of Arizona, New Mexico, and Utah, the reservation’s total area is greater than that of Massachusetts, Vermont, and New Hampshire combined.11 The reservation is home to an estimated 180,000 Navajo people, a population roughly the size of Salt Lake City, Utah. Yet, many homes on the reservation are without electricity, running water, telephones, or broadband access, and many roads on the reservation remain unpaved. Prior to the COVID-19 pandemic, 4 dermatology residents were selected each year to travel to this unique and remote location to work with the staff of the Chinle Comprehensive Health Care Facility (Chinle, Arizona), an Indian Health Service facility, as part of the American Academy of Dermatology (AAD)–sponsored Native American Health Services Resident Rotation (NAHSRR).
Dr. Lucinda Kohn, Assistant Professor of Dermatology at the University of Colorado and the director of the NAHSRR program discovered the value of this rotation firsthand as a dermatology resident. In 2017, she traveled to the area to spend 2 weeks serving within the community. “I went because of a personal connection. My husband is Native American, although not Navajo. I wanted to experience what it was like to provide dermatologic care for Native Americans. I found the Navajo people to be so friendly and so grateful for our care. The clinicians we worked with at Chinle were excited to have us share our expertise and to pass on their knowledge to us,” said Dr. Kohn (personal communication, June 24, 2021).
Rotating residents provide dermatologic care for the Navajo people and share their unique medical skill set to local primary care clinicians serving as preceptors. They also may have an opportunity to learn from Native healers about traditional Navajo beliefs and ceremonies used as part of a holistic approach to healing.
The program, similar to volunteer programs across the country, was put on hold during the height of the COVID-19 pandemic. “The Navajo nation witnessed a really tragic surge of COVID cases that required that limited medical resources be diverted to help cope with the pandemic,” says Dr. Kohn. “It really wasn’t safe for residents to travel to the reservation either, so the rotation had to be put on hold.” However, in April 2021, the health care staff of the Chinle Comprehensive Care Facility reached out to revive the program, which is now pending the green light from the AAD. It is unclear if or when AAD leadership will allow this rotation to restart. Dr. Kohn hopes to be able to start accepting new applications soon. “This rotation provides a wealth of benefits to all those involved, from the residents who get the chance to work with a unique population in need to the clinicians who gain a diverse understanding of dermatology treatment techniques. And of course, for the patients, who are so appreciative of the care they receive from our volunteers” (personal communication, June 25, 2021).
How to Get Involved
Dr. Kohn is happy to field questions regarding the rotation and requests for more information via email ([email protected]). Residents interested in this program also may reach out to the AAD’s Education and Volunteers Abroad Committee to express interest in the NAHSRR program’s reinstatement.
Destination Healthy Skin
Since 2017, the Skin Cancer Foundation’s Destination Healthy Skin (DHS) RV has been the setting for more than 3800 free skin cancer screenings provided by volunteers within underserved populations across the United States (Figure 2). After a year hiatus due to the pandemic, DHS hit the road again, starting in New York City on August 1 to 3, 2021. From there, the DHS RV will traverse the country in one large loop, starting with visits to large and small cities in the Midwest and the West Coast. Following a visit to San Diego, California, in early October, the RV will turn east, with stops in Arizona, Texas, and several southern states before ending in Philadelphia, Pennsylvania. Dr. Elizabeth Hale, Senior Vice President of the Skin Cancer Foundation, feels that increasing awareness of the importance of regular skin cancer screening for those at risk is more important than ever. “We know that many people in the past year put routine cancer screening on the back burner, but we’re beginning to appreciate that this has led to significant delays in skin cancer diagnosis and potentially more significant disease when cases are diagnosed.” Dr. Hale noted that as the country continues to return to a degree of normalcy, the backlog of patients now seeking their routine screening has led to longer wait times. She expects DHS may offer some relief. “There are no appointments necessary. If the RV is close to their hometown, patients have an advantage in being able to be seen first come, first served, without having to wait for an appointment or make sure their insurance is accepted. It’s a free screening that can increase access to dermatologists” (personal communication, June 21, 2021).

The program’s organizers acknowledge that DHS is not a long-term solution for improving dermatology access in the United States and recognize that more needs to be done to raise awareness, both of the value that screenings can provide and the importance of sun-protective behavior. “This is an important first step,” says Dr. Hale. “It’s important that we disseminate that no one is immune to skin cancer. It’s about education, and this is a tool to educate patients that everyone should have a skin check once a year, regardless of where you live or what your skin type is” (personal communication, June 21, 2021).
Volunteer dermatologists are needed to assist with screenings when the DHS RV arrives in their community. Providers complete a screening form identifying any concerning lesions and can document specific lesions using the patient’s cell phone. Following the screenings, participating dermatologists are welcome to invite participants to make appointments at their practices or suggest local clinics for follow-up care.
How to Get Involved
The schedule for this year’s screening events can be found online (https://www.skincancer.org/early-detection/destination-healthy-skin/). Consider volunteering (https://www.skincancer.org/early-detection/destination-healthy-skin/physician-volunteers/) or helping to raise awareness by reaching out to local dermatology societies or free clinics in your area. Residents and physician’s assistants are welcome to volunteer as well, as long as they are under the on-site supervision of a board-certified dermatologist.
Final Thoughts
As medical professionals, we all recognize there are valuable contributions we can make to groups and organizations that need our help. The stresses and pressure of work and everyday life can make finding the time to offer that help seem impossible. Although it may seem counterintuitive, volunteering our time to help others can help us better navigate the professional burnout that many medical professionals experience today.
The adage “so much to do, so little time” aptly describes the daily challenges facing dermatologists and dermatology residents. The time and attention required by direct patient care, writing notes, navigating electronic health records, and engaging in education and research as well as family commitments can drain even the most tireless clinician. In addition, dermatologists are expected to play a critical role in clinic and practice management to successfully curate an online presence and adapt their skills to successfully manage a teledermatology practice. Coupled with the time spent socializing with friends or colleagues and time for personal hobbies or exercise, it’s easy to see how sleep deprivation is common in many of our colleagues.
What’s being left out of these jam-packed schedules? Increasingly, it is the time and expertise dedicated to volunteering in our local communities. Two recent research letters highlighted how a dramatic increase in the number of research projects and publications is not mirrored by a similar increase in volunteer experiences as dermatology residency selection becomes more competitive.1,2
Although the rate of volunteerism among practicing dermatologists has yet to be studied, a brief review suggests a component of unmet dermatology need within our communities. It’s estimated that approximately 5% to 10% of all emergency department visits are for dermatologic concerns.3-5 In many cases, the reason for the visit is nonurgent and instead reflects a lack of other options for care. However, the need for dermatologists extends beyond the emergency department setting. A review of the prevalence of patients presenting for care to a group of regional free clinics found that 8% (N=5553) of all visitors sought care for dermatologic concerns.6 The benefit is not just for those seated on the examination table; research has shown that while many of the underlying factors resulting in physician burnout stem from systemic issues, participating in volunteer opportunities helps combat burnout in ourselves and our colleagues.7-9 Herein, opportunities that exist for dermatologists to reconnect with their communities, advocate for causes distinctive to the specialty, and care for neighbors most in need are highlighted.
Camp Wonder
Every year, children from across the United States living with chronic and debilitating skin conditions get the opportunity to join fellow campers and spend a week just being kids without the constant focus on being a patient. Camp Wonder’s founder and director, Francesca Tenconi, describes the camp as a place where kids “can form a community and can feel free to be themselves, without judgment, without stares. They get the chance to forget about their skin disease and be themselves” (oral communication, June 18, 2021). Tenconi and the camp’s cofounders and medical directors, Drs. Jenny Kim and Stefani Takahashi, envisioned the camp as a place for all campers regardless of their skin condition to feel safe and welcome. This overall mission guides camp leadership and staff every year over the course of the camp week where campers participate in a mix of traditional and nontraditional summer activities that are safe and accessible for all, from spending time in the pool to arts and crafts and a ropes course.
Camp Wonder is in its 21st year of hosting children and adolescents from across North America at its camp in Livermore, California. This year, Tenconi expects about 100 campers during the last week in July. Camp Wonder relies on medical staff volunteers to make the camp setting safe, inclusive, and fun. “Our dermatology residents and dermatology volunteers are a huge part of why we’re able to have camp,” said Tenconi. “A lot of our kids require very specific medical care throughout the week. We are able to provide this camp experience for them because we have this medical support system available, this specialized dermatology knowledge.” She also noted the benefit to the volunteers themselves, saying,“The feedback we get a lot from residents and dermatologists is that camp gave them a chance to understand the true-life impact of some of the skin diseases these kids and families are living with. Kids will open up to them and tell them how their disease has impacted them personally” (oral communication, June 18, 2021).
Volunteer medical providers help manage the medical needs of the campers beginning at check-in and work shifts in the infirmary as well as help with dispensing and administering medications, changing dressings, and applying ointments or other topical medications. When not assisting with medical care, medical staff can get to know the campers; help out with arts and crafts, games, sports, and other camp activities; and put on skits and plays for campers at nightly camp hangouts (Figure 1).

How to Get Involved
Visit the website (https://www.csdf.org/camp-wonder) for information on becoming a medical volunteer for 2022. Donations to help keep the camp running also are greatly appreciated, as attendance, including travel costs, is free for families through the Children’s Skin Disease Foundation. Finally, dermatologists can help by keeping their young patients with skin disease in mind as future campers. The camp welcomes kids from across the United States and Canada and invites questions from dermatologists and families on how to become a camper and what the experience is like.
Native American Health Services Rotation
Located in the southwestern United States, the Navajo Nation is North America’s largest Native American tribe by enrollment and resides on the largest reservation in the United States.10 Comprised of 27,000 square miles within portions of Arizona, New Mexico, and Utah, the reservation’s total area is greater than that of Massachusetts, Vermont, and New Hampshire combined.11 The reservation is home to an estimated 180,000 Navajo people, a population roughly the size of Salt Lake City, Utah. Yet, many homes on the reservation are without electricity, running water, telephones, or broadband access, and many roads on the reservation remain unpaved. Prior to the COVID-19 pandemic, 4 dermatology residents were selected each year to travel to this unique and remote location to work with the staff of the Chinle Comprehensive Health Care Facility (Chinle, Arizona), an Indian Health Service facility, as part of the American Academy of Dermatology (AAD)–sponsored Native American Health Services Resident Rotation (NAHSRR).
Dr. Lucinda Kohn, Assistant Professor of Dermatology at the University of Colorado and the director of the NAHSRR program discovered the value of this rotation firsthand as a dermatology resident. In 2017, she traveled to the area to spend 2 weeks serving within the community. “I went because of a personal connection. My husband is Native American, although not Navajo. I wanted to experience what it was like to provide dermatologic care for Native Americans. I found the Navajo people to be so friendly and so grateful for our care. The clinicians we worked with at Chinle were excited to have us share our expertise and to pass on their knowledge to us,” said Dr. Kohn (personal communication, June 24, 2021).
Rotating residents provide dermatologic care for the Navajo people and share their unique medical skill set to local primary care clinicians serving as preceptors. They also may have an opportunity to learn from Native healers about traditional Navajo beliefs and ceremonies used as part of a holistic approach to healing.
The program, similar to volunteer programs across the country, was put on hold during the height of the COVID-19 pandemic. “The Navajo nation witnessed a really tragic surge of COVID cases that required that limited medical resources be diverted to help cope with the pandemic,” says Dr. Kohn. “It really wasn’t safe for residents to travel to the reservation either, so the rotation had to be put on hold.” However, in April 2021, the health care staff of the Chinle Comprehensive Care Facility reached out to revive the program, which is now pending the green light from the AAD. It is unclear if or when AAD leadership will allow this rotation to restart. Dr. Kohn hopes to be able to start accepting new applications soon. “This rotation provides a wealth of benefits to all those involved, from the residents who get the chance to work with a unique population in need to the clinicians who gain a diverse understanding of dermatology treatment techniques. And of course, for the patients, who are so appreciative of the care they receive from our volunteers” (personal communication, June 25, 2021).
How to Get Involved
Dr. Kohn is happy to field questions regarding the rotation and requests for more information via email ([email protected]). Residents interested in this program also may reach out to the AAD’s Education and Volunteers Abroad Committee to express interest in the NAHSRR program’s reinstatement.
Destination Healthy Skin
Since 2017, the Skin Cancer Foundation’s Destination Healthy Skin (DHS) RV has been the setting for more than 3800 free skin cancer screenings provided by volunteers within underserved populations across the United States (Figure 2). After a year hiatus due to the pandemic, DHS hit the road again, starting in New York City on August 1 to 3, 2021. From there, the DHS RV will traverse the country in one large loop, starting with visits to large and small cities in the Midwest and the West Coast. Following a visit to San Diego, California, in early October, the RV will turn east, with stops in Arizona, Texas, and several southern states before ending in Philadelphia, Pennsylvania. Dr. Elizabeth Hale, Senior Vice President of the Skin Cancer Foundation, feels that increasing awareness of the importance of regular skin cancer screening for those at risk is more important than ever. “We know that many people in the past year put routine cancer screening on the back burner, but we’re beginning to appreciate that this has led to significant delays in skin cancer diagnosis and potentially more significant disease when cases are diagnosed.” Dr. Hale noted that as the country continues to return to a degree of normalcy, the backlog of patients now seeking their routine screening has led to longer wait times. She expects DHS may offer some relief. “There are no appointments necessary. If the RV is close to their hometown, patients have an advantage in being able to be seen first come, first served, without having to wait for an appointment or make sure their insurance is accepted. It’s a free screening that can increase access to dermatologists” (personal communication, June 21, 2021).

The program’s organizers acknowledge that DHS is not a long-term solution for improving dermatology access in the United States and recognize that more needs to be done to raise awareness, both of the value that screenings can provide and the importance of sun-protective behavior. “This is an important first step,” says Dr. Hale. “It’s important that we disseminate that no one is immune to skin cancer. It’s about education, and this is a tool to educate patients that everyone should have a skin check once a year, regardless of where you live or what your skin type is” (personal communication, June 21, 2021).
Volunteer dermatologists are needed to assist with screenings when the DHS RV arrives in their community. Providers complete a screening form identifying any concerning lesions and can document specific lesions using the patient’s cell phone. Following the screenings, participating dermatologists are welcome to invite participants to make appointments at their practices or suggest local clinics for follow-up care.
How to Get Involved
The schedule for this year’s screening events can be found online (https://www.skincancer.org/early-detection/destination-healthy-skin/). Consider volunteering (https://www.skincancer.org/early-detection/destination-healthy-skin/physician-volunteers/) or helping to raise awareness by reaching out to local dermatology societies or free clinics in your area. Residents and physician’s assistants are welcome to volunteer as well, as long as they are under the on-site supervision of a board-certified dermatologist.
Final Thoughts
As medical professionals, we all recognize there are valuable contributions we can make to groups and organizations that need our help. The stresses and pressure of work and everyday life can make finding the time to offer that help seem impossible. Although it may seem counterintuitive, volunteering our time to help others can help us better navigate the professional burnout that many medical professionals experience today.
- Ezekor M, Pona A, Cline A, et al. An increasing trend in the number of publications and research projects among dermatology residency applicants. J Am Acad Dermatol. 2020;83:214-216.
- Atluri S, Seivright JR, Shi VY, et al. Volunteer and work experiences among dermatology residency applicants. J Am Acad Dermatol. 2021;84:E97-E98.
- Abokwidir M, Davis SA, Fleischer AB, et al. Use of the emergency department for dermatologic care in the United States by ethnic group. J Dermatolog Treat. 2015;26:392-394.
- Uscher-Pines L, Pines J, Kellermann A, et al. Emergency department visits for nonurgent conditions: systematic literature review. Am J Manag Care. 2013;19:47-59.
- Jack AR, Spence AA, Nichols BJ, et al. Cutaneous conditions leading to dermatology consultations in the emergency department. West J Emerg Med. 2011;12:551-555.
- Ayoubi N, Mirza A-S, Swanson J, et al. Dermatologic care of uninsured patients managed at free clinics. J Am Acad Dermatol. 2019;81:433-437.
- Wright AA, Katz IT. Beyond burnout—redesigning care to restore meaning and sanity for physicians. N Engl J Med. 2018;378:309-311.
- Bull C, Aucoin JB. Voluntary association participation and life satisfaction: a replication note. J Gerontol. 1975;30:73-76.
- Iserson KV. Burnout syndrome: global medicine volunteering as a possible treatment strategy. J Emerg Med. 2018;54:516-521.
- Romero S. Navajo Nation becomes largest tribe in U.S. after pandemic enrollment surge. New York Times. May 21, 2021. Accessed August 19, 2021. https://www.nytimes.com/2021/05/21/us/navajo-cherokee-population.html
- Moore GR, Benally J, Tuttle S. The Navajo Nation: quick facts. University of Arizona website. Accessed August 19, 2021. https://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1471.pdf
- Ezekor M, Pona A, Cline A, et al. An increasing trend in the number of publications and research projects among dermatology residency applicants. J Am Acad Dermatol. 2020;83:214-216.
- Atluri S, Seivright JR, Shi VY, et al. Volunteer and work experiences among dermatology residency applicants. J Am Acad Dermatol. 2021;84:E97-E98.
- Abokwidir M, Davis SA, Fleischer AB, et al. Use of the emergency department for dermatologic care in the United States by ethnic group. J Dermatolog Treat. 2015;26:392-394.
- Uscher-Pines L, Pines J, Kellermann A, et al. Emergency department visits for nonurgent conditions: systematic literature review. Am J Manag Care. 2013;19:47-59.
- Jack AR, Spence AA, Nichols BJ, et al. Cutaneous conditions leading to dermatology consultations in the emergency department. West J Emerg Med. 2011;12:551-555.
- Ayoubi N, Mirza A-S, Swanson J, et al. Dermatologic care of uninsured patients managed at free clinics. J Am Acad Dermatol. 2019;81:433-437.
- Wright AA, Katz IT. Beyond burnout—redesigning care to restore meaning and sanity for physicians. N Engl J Med. 2018;378:309-311.
- Bull C, Aucoin JB. Voluntary association participation and life satisfaction: a replication note. J Gerontol. 1975;30:73-76.
- Iserson KV. Burnout syndrome: global medicine volunteering as a possible treatment strategy. J Emerg Med. 2018;54:516-521.
- Romero S. Navajo Nation becomes largest tribe in U.S. after pandemic enrollment surge. New York Times. May 21, 2021. Accessed August 19, 2021. https://www.nytimes.com/2021/05/21/us/navajo-cherokee-population.html
- Moore GR, Benally J, Tuttle S. The Navajo Nation: quick facts. University of Arizona website. Accessed August 19, 2021. https://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1471.pdf
Resident Pearl
- Volunteerism rates among dermatology residents seem to be decreasing. We should work to combat this trend by finding ways to give back to our communities and spur our colleagues to do the same.
Clinical Edge Journal Scan Commentary: Psoriasis September 2021
Several recent studies have evaluated that association between psoriasis and known comorbidities including cardiovascular disease, non-alcoholic fatty liver disease (NAFLD), and malignancy. Several recent studies have added to our understanding of the relationship between these conditions.
Using the largest database of hospitalized patients in the United States, Edgen et al found that hospitalization rates for patients with psoriasis are increasing. While the proportion of patients with psoriasis hospitalized with psoriasis as a primary diagnosis decreased about four-fold over a 20-year period (1999-2018), incidence of hospitalizations with any diagnosis of psoriasis has increased. Hospitalized psoriasis patients are increasingly more likely to have other comorbid conditions as during the study period the proportion of hospitalized psoriasis patients with a Charlson Comorbidity Index score of 3 or higher increased from 13.9% to 30.9%. Psoriasis severity, medication use, and reasons for hospitalization were not reported. The authors suggest that screening and management of comorbidities in the outpatient setting may help reduce preventable psoriasis hospitalizations.
Both NAFLD and cardiovascular disease are well-known psoriasis comorbidities, Gonzalez-Cantaro et al studied two cohorts of patients to better define the relationship between these two conditions. In a European cohort of 76 psoriasis patients and 76 control patients, psoriasis patients with NAFLD had a higher prevalence of subclinical atherosclerosis than both psoriasis patients without NAFLD (61% vs 23%) and age, sex, and BMI-matched controls with NAFLD (61% vs 32%). Psoriasis patients were also more likely that control patients to have insulin resistance, higher weight circumference, and dysplipidemia. Among 162 psoriasis patients who underwent PET and coronary CT angiography, higher hepatic FDG uptake (indicating NAFLD) was associated higher atherosclerotic disease burden. Importantly, both the NAFLD and CAD were subclinical in these patients. While the cross-sectional study design precludes any conclusions about causality, physicians should be aware that these two comorbidities are related. Lower waist circumference and greater physical activity were both associated with lower rates of NAFLD among patients with psoriasis, providing some guidance for counseling patients.
Several recent studies have found that cancer rates among patients with psoriasis are higher than what is observed in the general population. The association of psoriasis with lymphohematologic malignancies (LHM) has been controversial. A systematic review and meta-analysis of 25 observational studies including over 2.5 million subjects (Bellinato et al.) found a 1.55-fold increased risk of LHM in patients with moderate to severe psoriasis. Strikingly, the risk of cutaneous T cell lymphoma (CTCL) was increased 6.22-fold, with more severe psoriasis being associated with the highest risk of CTCL. A causal relationship cannot be established from this type of studies, but the authors hypothesize that drugs used to treat psoriasis or the chronic T cell activation caused by active disease may contribute to the risk of LMH. Additionally, psoriasis and CTCL can share clinical features and some cases may be due to misdiagnosis. Interestingly, two psoriasis comorbities, diabetes and obesity, are also associated with an increased risk of LHM.
Early identification and management of comorbidities can help in reducing morbidity and mortality. With so many psoriasis treatments available, understanding how different therapies may impact comorbid conditions is important in helping dermatologists to choose the best therapy for each individual patient.
Several recent studies have evaluated that association between psoriasis and known comorbidities including cardiovascular disease, non-alcoholic fatty liver disease (NAFLD), and malignancy. Several recent studies have added to our understanding of the relationship between these conditions.
Using the largest database of hospitalized patients in the United States, Edgen et al found that hospitalization rates for patients with psoriasis are increasing. While the proportion of patients with psoriasis hospitalized with psoriasis as a primary diagnosis decreased about four-fold over a 20-year period (1999-2018), incidence of hospitalizations with any diagnosis of psoriasis has increased. Hospitalized psoriasis patients are increasingly more likely to have other comorbid conditions as during the study period the proportion of hospitalized psoriasis patients with a Charlson Comorbidity Index score of 3 or higher increased from 13.9% to 30.9%. Psoriasis severity, medication use, and reasons for hospitalization were not reported. The authors suggest that screening and management of comorbidities in the outpatient setting may help reduce preventable psoriasis hospitalizations.
Both NAFLD and cardiovascular disease are well-known psoriasis comorbidities, Gonzalez-Cantaro et al studied two cohorts of patients to better define the relationship between these two conditions. In a European cohort of 76 psoriasis patients and 76 control patients, psoriasis patients with NAFLD had a higher prevalence of subclinical atherosclerosis than both psoriasis patients without NAFLD (61% vs 23%) and age, sex, and BMI-matched controls with NAFLD (61% vs 32%). Psoriasis patients were also more likely that control patients to have insulin resistance, higher weight circumference, and dysplipidemia. Among 162 psoriasis patients who underwent PET and coronary CT angiography, higher hepatic FDG uptake (indicating NAFLD) was associated higher atherosclerotic disease burden. Importantly, both the NAFLD and CAD were subclinical in these patients. While the cross-sectional study design precludes any conclusions about causality, physicians should be aware that these two comorbidities are related. Lower waist circumference and greater physical activity were both associated with lower rates of NAFLD among patients with psoriasis, providing some guidance for counseling patients.
Several recent studies have found that cancer rates among patients with psoriasis are higher than what is observed in the general population. The association of psoriasis with lymphohematologic malignancies (LHM) has been controversial. A systematic review and meta-analysis of 25 observational studies including over 2.5 million subjects (Bellinato et al.) found a 1.55-fold increased risk of LHM in patients with moderate to severe psoriasis. Strikingly, the risk of cutaneous T cell lymphoma (CTCL) was increased 6.22-fold, with more severe psoriasis being associated with the highest risk of CTCL. A causal relationship cannot be established from this type of studies, but the authors hypothesize that drugs used to treat psoriasis or the chronic T cell activation caused by active disease may contribute to the risk of LMH. Additionally, psoriasis and CTCL can share clinical features and some cases may be due to misdiagnosis. Interestingly, two psoriasis comorbities, diabetes and obesity, are also associated with an increased risk of LHM.
Early identification and management of comorbidities can help in reducing morbidity and mortality. With so many psoriasis treatments available, understanding how different therapies may impact comorbid conditions is important in helping dermatologists to choose the best therapy for each individual patient.
Several recent studies have evaluated that association between psoriasis and known comorbidities including cardiovascular disease, non-alcoholic fatty liver disease (NAFLD), and malignancy. Several recent studies have added to our understanding of the relationship between these conditions.
Using the largest database of hospitalized patients in the United States, Edgen et al found that hospitalization rates for patients with psoriasis are increasing. While the proportion of patients with psoriasis hospitalized with psoriasis as a primary diagnosis decreased about four-fold over a 20-year period (1999-2018), incidence of hospitalizations with any diagnosis of psoriasis has increased. Hospitalized psoriasis patients are increasingly more likely to have other comorbid conditions as during the study period the proportion of hospitalized psoriasis patients with a Charlson Comorbidity Index score of 3 or higher increased from 13.9% to 30.9%. Psoriasis severity, medication use, and reasons for hospitalization were not reported. The authors suggest that screening and management of comorbidities in the outpatient setting may help reduce preventable psoriasis hospitalizations.
Both NAFLD and cardiovascular disease are well-known psoriasis comorbidities, Gonzalez-Cantaro et al studied two cohorts of patients to better define the relationship between these two conditions. In a European cohort of 76 psoriasis patients and 76 control patients, psoriasis patients with NAFLD had a higher prevalence of subclinical atherosclerosis than both psoriasis patients without NAFLD (61% vs 23%) and age, sex, and BMI-matched controls with NAFLD (61% vs 32%). Psoriasis patients were also more likely that control patients to have insulin resistance, higher weight circumference, and dysplipidemia. Among 162 psoriasis patients who underwent PET and coronary CT angiography, higher hepatic FDG uptake (indicating NAFLD) was associated higher atherosclerotic disease burden. Importantly, both the NAFLD and CAD were subclinical in these patients. While the cross-sectional study design precludes any conclusions about causality, physicians should be aware that these two comorbidities are related. Lower waist circumference and greater physical activity were both associated with lower rates of NAFLD among patients with psoriasis, providing some guidance for counseling patients.
Several recent studies have found that cancer rates among patients with psoriasis are higher than what is observed in the general population. The association of psoriasis with lymphohematologic malignancies (LHM) has been controversial. A systematic review and meta-analysis of 25 observational studies including over 2.5 million subjects (Bellinato et al.) found a 1.55-fold increased risk of LHM in patients with moderate to severe psoriasis. Strikingly, the risk of cutaneous T cell lymphoma (CTCL) was increased 6.22-fold, with more severe psoriasis being associated with the highest risk of CTCL. A causal relationship cannot be established from this type of studies, but the authors hypothesize that drugs used to treat psoriasis or the chronic T cell activation caused by active disease may contribute to the risk of LMH. Additionally, psoriasis and CTCL can share clinical features and some cases may be due to misdiagnosis. Interestingly, two psoriasis comorbities, diabetes and obesity, are also associated with an increased risk of LHM.
Early identification and management of comorbidities can help in reducing morbidity and mortality. With so many psoriasis treatments available, understanding how different therapies may impact comorbid conditions is important in helping dermatologists to choose the best therapy for each individual patient.
Report urges complete residency overhaul
from the Graduate Medical Education Review Committee (UGRC) of the Coalition for Physician Accountability.
The 275-page report presents preliminary findings that were released in April 2021 and a long list of stakeholder comments. According to the report, the coalition will meet soon to discuss the final recommendations and consider next steps toward implementation.
The UGRC includes representatives of national medical organizations, medical schools, and residency programs. Among the organizations that participated in the report’s creation are the American Medical Association, the National Board of Medical Examiners, the American Osteopathic Association, the National Board of Osteopathic Medical Examiners, the Educational Commission for Foreign Medical Graduates, and the Association of American Medical Colleges.
The report identifies a list of challenges that affect the transition of medical students into residency programs and beyond. They include:
- Too much focus on finding and filling residency positions instead of “assuring learner competence and readiness for residency training”
- Inattention to assuring congruence between applicant goals and program missions
- Overreliance on licensure exam scores rather than “valid, trustworthy measures of students’ competence and clinical abilities”
- Increasing financial costs to students
- Individual and systemic biases in the UME-GME transition, as well as inequities related to international medical graduates
Seeking a common framework for competence
Overall, the report calls for increased standardization of how students are evaluated in medical school and how residency programs evaluate students. Less reliance should be placed on the numerical scores of the U.S. Medical Licensing Examination (USMLE), the report says, and more attention should be paid to the direct observation of student performance in clinical situations. In addition, the various organizations involved in the UME-GME transition process are asked to work better together.
To develop better methods of evaluating medical students and residents, UME and GME educators should jointly define and implement a common framework and set of competencies to apply to learners across the UME-GME transition, the report suggests.
While emphasizing the need for a broader student assessment framework, the report says, USMLE scores should also continue to be used in judging residency applicants. “Assessment information should be shared in residency applications and a postmatch learner handover. Licensing examinations should be used for their intended purpose to ensure requisite competence.”
Among the committee’s three dozen recommendations are the following:
- The Centers for Medicare & Medicaid Services should change the GME funding structure so that the initial residency period is calculated starting with the second year of postgraduate training. This change would allow residents to reconsider their career choices. Currently, if a resident decides to switch to another program or specialty after beginning training, the hospital may not receive full GME funding, so may be less likely to approve the change.
- Residency programs should improve recruitment practices to increase specialty-specific diversity of residents. Medical educators should also receive additional training regarding antiracism, avoiding bias, and ensuring equity.
- The self-reported demographic information of applicants to residency programs should be measured and shared with stakeholders, including the programs and medical schools, to promote equity. “A residency program that finds bias in its selection process could go back in real time to find qualified applicants who may have been missed, potentially improving outcomes,” the report notes.
- An interactive database of GME program and specialty track information should be created and made available to all applicants, medical schools, and residency programs at no cost to applicants. “Applicants and their advisors should be able to sort the information according to demographic and educational features that may significantly impact the likelihood of matching at a program.”
Less than half of applicants get in-depth reviews
The 2020 National Resident Matching Program Program Director Survey found that only 49% of applications received in-depth review. In light of this, the report suggests that the application system be updated to use modern information technology, including discrete fields for key data to expedite application reviews.
Many applications have been discarded because of various filters used to block consideration of certain applications. The report suggests that new filters be designed to ensure that each detects meaningful differences among applicants and promotes review based on mission alignment and likelihood of success in a program. Filters should be improved to decrease the likelihood of random exclusions of qualified applicants.
Specialty-specific, just-in-time training for all incoming first-year residents is also suggested to support the transition from the role of student to a physician ready to assume increased responsibility for patient care. In addition, the report urges adequate time be allowed between medical school graduation and residency to enable new residents to relocate and find homes.
The report also calls for a standardized process in the United States for initial licensing of doctors at entrance to residency in order to streamline the process of credentialing for both residency training and continuing practice.
Osteopathic students’ dilemma
To promote equitable treatment of applicants regardless of licensure examination requirements, comparable exams with different scales (COMLEX-USA and USMLE) should be reported within the electronic application system in a single field, the report said.
Osteopathic students, who make up 25% of U.S. medical students, must take the COMLEX-USA exam, but residency programs may filter them out if they don’t also take the USMLE exam. Thus, many osteopathic students take both exams, incurring extra time, cost, and stress.
The UGRC recommends creating a combined field in the electronic residency application service that normalizes the scores between the two exams. Residency programs could then filter applications based only on the single normalized score.
This approach makes sense from the viewpoint that it would reduce the pressure on osteopathic students to take the USMLE, Bryan Carmody, MD, an outspoken critic of various current training policies, said in an interview. But it could also have serious disadvantages.
For one thing, only osteopathic students can take the COMLEX-USA exam, he noted. If they don’t like their score, they can then take the USMLE test to get a higher score – an option that allopathic students don’t have. It’s not clear that they’d be prevented from doing this under the UGRC recommendation.
Second, he said, osteopathic students, on average, don’t do as well as allopathic students on the UMSLE exam. If they only take the COMLEX-USA test, they’re competing against other students who don’t do as well on tests as allopathic students do. If their scores were normalized with those of the USMLE test takers, they’d gain an unfair advantage against students who can only take the USMLE, including international medical graduates.
Although Dr. Carmody admitted that osteopathic students face a harder challenge than allopathic students in matching to residency programs, he said that the UGRC approach to the licensing exams might actually penalize them further. As a result of the scores of the two exams being averaged, residency program directors might discount the scores of all osteopathic students.
A version of this article first appeared on Medscape.com.
from the Graduate Medical Education Review Committee (UGRC) of the Coalition for Physician Accountability.
The 275-page report presents preliminary findings that were released in April 2021 and a long list of stakeholder comments. According to the report, the coalition will meet soon to discuss the final recommendations and consider next steps toward implementation.
The UGRC includes representatives of national medical organizations, medical schools, and residency programs. Among the organizations that participated in the report’s creation are the American Medical Association, the National Board of Medical Examiners, the American Osteopathic Association, the National Board of Osteopathic Medical Examiners, the Educational Commission for Foreign Medical Graduates, and the Association of American Medical Colleges.
The report identifies a list of challenges that affect the transition of medical students into residency programs and beyond. They include:
- Too much focus on finding and filling residency positions instead of “assuring learner competence and readiness for residency training”
- Inattention to assuring congruence between applicant goals and program missions
- Overreliance on licensure exam scores rather than “valid, trustworthy measures of students’ competence and clinical abilities”
- Increasing financial costs to students
- Individual and systemic biases in the UME-GME transition, as well as inequities related to international medical graduates
Seeking a common framework for competence
Overall, the report calls for increased standardization of how students are evaluated in medical school and how residency programs evaluate students. Less reliance should be placed on the numerical scores of the U.S. Medical Licensing Examination (USMLE), the report says, and more attention should be paid to the direct observation of student performance in clinical situations. In addition, the various organizations involved in the UME-GME transition process are asked to work better together.
To develop better methods of evaluating medical students and residents, UME and GME educators should jointly define and implement a common framework and set of competencies to apply to learners across the UME-GME transition, the report suggests.
While emphasizing the need for a broader student assessment framework, the report says, USMLE scores should also continue to be used in judging residency applicants. “Assessment information should be shared in residency applications and a postmatch learner handover. Licensing examinations should be used for their intended purpose to ensure requisite competence.”
Among the committee’s three dozen recommendations are the following:
- The Centers for Medicare & Medicaid Services should change the GME funding structure so that the initial residency period is calculated starting with the second year of postgraduate training. This change would allow residents to reconsider their career choices. Currently, if a resident decides to switch to another program or specialty after beginning training, the hospital may not receive full GME funding, so may be less likely to approve the change.
- Residency programs should improve recruitment practices to increase specialty-specific diversity of residents. Medical educators should also receive additional training regarding antiracism, avoiding bias, and ensuring equity.
- The self-reported demographic information of applicants to residency programs should be measured and shared with stakeholders, including the programs and medical schools, to promote equity. “A residency program that finds bias in its selection process could go back in real time to find qualified applicants who may have been missed, potentially improving outcomes,” the report notes.
- An interactive database of GME program and specialty track information should be created and made available to all applicants, medical schools, and residency programs at no cost to applicants. “Applicants and their advisors should be able to sort the information according to demographic and educational features that may significantly impact the likelihood of matching at a program.”
Less than half of applicants get in-depth reviews
The 2020 National Resident Matching Program Program Director Survey found that only 49% of applications received in-depth review. In light of this, the report suggests that the application system be updated to use modern information technology, including discrete fields for key data to expedite application reviews.
Many applications have been discarded because of various filters used to block consideration of certain applications. The report suggests that new filters be designed to ensure that each detects meaningful differences among applicants and promotes review based on mission alignment and likelihood of success in a program. Filters should be improved to decrease the likelihood of random exclusions of qualified applicants.
Specialty-specific, just-in-time training for all incoming first-year residents is also suggested to support the transition from the role of student to a physician ready to assume increased responsibility for patient care. In addition, the report urges adequate time be allowed between medical school graduation and residency to enable new residents to relocate and find homes.
The report also calls for a standardized process in the United States for initial licensing of doctors at entrance to residency in order to streamline the process of credentialing for both residency training and continuing practice.
Osteopathic students’ dilemma
To promote equitable treatment of applicants regardless of licensure examination requirements, comparable exams with different scales (COMLEX-USA and USMLE) should be reported within the electronic application system in a single field, the report said.
Osteopathic students, who make up 25% of U.S. medical students, must take the COMLEX-USA exam, but residency programs may filter them out if they don’t also take the USMLE exam. Thus, many osteopathic students take both exams, incurring extra time, cost, and stress.
The UGRC recommends creating a combined field in the electronic residency application service that normalizes the scores between the two exams. Residency programs could then filter applications based only on the single normalized score.
This approach makes sense from the viewpoint that it would reduce the pressure on osteopathic students to take the USMLE, Bryan Carmody, MD, an outspoken critic of various current training policies, said in an interview. But it could also have serious disadvantages.
For one thing, only osteopathic students can take the COMLEX-USA exam, he noted. If they don’t like their score, they can then take the USMLE test to get a higher score – an option that allopathic students don’t have. It’s not clear that they’d be prevented from doing this under the UGRC recommendation.
Second, he said, osteopathic students, on average, don’t do as well as allopathic students on the UMSLE exam. If they only take the COMLEX-USA test, they’re competing against other students who don’t do as well on tests as allopathic students do. If their scores were normalized with those of the USMLE test takers, they’d gain an unfair advantage against students who can only take the USMLE, including international medical graduates.
Although Dr. Carmody admitted that osteopathic students face a harder challenge than allopathic students in matching to residency programs, he said that the UGRC approach to the licensing exams might actually penalize them further. As a result of the scores of the two exams being averaged, residency program directors might discount the scores of all osteopathic students.
A version of this article first appeared on Medscape.com.
from the Graduate Medical Education Review Committee (UGRC) of the Coalition for Physician Accountability.
The 275-page report presents preliminary findings that were released in April 2021 and a long list of stakeholder comments. According to the report, the coalition will meet soon to discuss the final recommendations and consider next steps toward implementation.
The UGRC includes representatives of national medical organizations, medical schools, and residency programs. Among the organizations that participated in the report’s creation are the American Medical Association, the National Board of Medical Examiners, the American Osteopathic Association, the National Board of Osteopathic Medical Examiners, the Educational Commission for Foreign Medical Graduates, and the Association of American Medical Colleges.
The report identifies a list of challenges that affect the transition of medical students into residency programs and beyond. They include:
- Too much focus on finding and filling residency positions instead of “assuring learner competence and readiness for residency training”
- Inattention to assuring congruence between applicant goals and program missions
- Overreliance on licensure exam scores rather than “valid, trustworthy measures of students’ competence and clinical abilities”
- Increasing financial costs to students
- Individual and systemic biases in the UME-GME transition, as well as inequities related to international medical graduates
Seeking a common framework for competence
Overall, the report calls for increased standardization of how students are evaluated in medical school and how residency programs evaluate students. Less reliance should be placed on the numerical scores of the U.S. Medical Licensing Examination (USMLE), the report says, and more attention should be paid to the direct observation of student performance in clinical situations. In addition, the various organizations involved in the UME-GME transition process are asked to work better together.
To develop better methods of evaluating medical students and residents, UME and GME educators should jointly define and implement a common framework and set of competencies to apply to learners across the UME-GME transition, the report suggests.
While emphasizing the need for a broader student assessment framework, the report says, USMLE scores should also continue to be used in judging residency applicants. “Assessment information should be shared in residency applications and a postmatch learner handover. Licensing examinations should be used for their intended purpose to ensure requisite competence.”
Among the committee’s three dozen recommendations are the following:
- The Centers for Medicare & Medicaid Services should change the GME funding structure so that the initial residency period is calculated starting with the second year of postgraduate training. This change would allow residents to reconsider their career choices. Currently, if a resident decides to switch to another program or specialty after beginning training, the hospital may not receive full GME funding, so may be less likely to approve the change.
- Residency programs should improve recruitment practices to increase specialty-specific diversity of residents. Medical educators should also receive additional training regarding antiracism, avoiding bias, and ensuring equity.
- The self-reported demographic information of applicants to residency programs should be measured and shared with stakeholders, including the programs and medical schools, to promote equity. “A residency program that finds bias in its selection process could go back in real time to find qualified applicants who may have been missed, potentially improving outcomes,” the report notes.
- An interactive database of GME program and specialty track information should be created and made available to all applicants, medical schools, and residency programs at no cost to applicants. “Applicants and their advisors should be able to sort the information according to demographic and educational features that may significantly impact the likelihood of matching at a program.”
Less than half of applicants get in-depth reviews
The 2020 National Resident Matching Program Program Director Survey found that only 49% of applications received in-depth review. In light of this, the report suggests that the application system be updated to use modern information technology, including discrete fields for key data to expedite application reviews.
Many applications have been discarded because of various filters used to block consideration of certain applications. The report suggests that new filters be designed to ensure that each detects meaningful differences among applicants and promotes review based on mission alignment and likelihood of success in a program. Filters should be improved to decrease the likelihood of random exclusions of qualified applicants.
Specialty-specific, just-in-time training for all incoming first-year residents is also suggested to support the transition from the role of student to a physician ready to assume increased responsibility for patient care. In addition, the report urges adequate time be allowed between medical school graduation and residency to enable new residents to relocate and find homes.
The report also calls for a standardized process in the United States for initial licensing of doctors at entrance to residency in order to streamline the process of credentialing for both residency training and continuing practice.
Osteopathic students’ dilemma
To promote equitable treatment of applicants regardless of licensure examination requirements, comparable exams with different scales (COMLEX-USA and USMLE) should be reported within the electronic application system in a single field, the report said.
Osteopathic students, who make up 25% of U.S. medical students, must take the COMLEX-USA exam, but residency programs may filter them out if they don’t also take the USMLE exam. Thus, many osteopathic students take both exams, incurring extra time, cost, and stress.
The UGRC recommends creating a combined field in the electronic residency application service that normalizes the scores between the two exams. Residency programs could then filter applications based only on the single normalized score.
This approach makes sense from the viewpoint that it would reduce the pressure on osteopathic students to take the USMLE, Bryan Carmody, MD, an outspoken critic of various current training policies, said in an interview. But it could also have serious disadvantages.
For one thing, only osteopathic students can take the COMLEX-USA exam, he noted. If they don’t like their score, they can then take the USMLE test to get a higher score – an option that allopathic students don’t have. It’s not clear that they’d be prevented from doing this under the UGRC recommendation.
Second, he said, osteopathic students, on average, don’t do as well as allopathic students on the UMSLE exam. If they only take the COMLEX-USA test, they’re competing against other students who don’t do as well on tests as allopathic students do. If their scores were normalized with those of the USMLE test takers, they’d gain an unfair advantage against students who can only take the USMLE, including international medical graduates.
Although Dr. Carmody admitted that osteopathic students face a harder challenge than allopathic students in matching to residency programs, he said that the UGRC approach to the licensing exams might actually penalize them further. As a result of the scores of the two exams being averaged, residency program directors might discount the scores of all osteopathic students.
A version of this article first appeared on Medscape.com.
Verrucous Scalp Plaque and Widespread Eruption
The Diagnosis: Pemphigus Foliaceous
Laboratory workup including a complete blood cell count with differential, comprehensive metabolic panel, antinuclear antibodies, Sjögren syndrome A and B antibodies, hepatitis profile, rapid plasma reagin, HIV screen, aldolase, anti–Jo-1, T-Spot TB test (Quest Diagnostics), and tissue cultures was unremarkable. Two 4-mm punch biopsies were obtained from the left cheek and upper back, both of which demonstrated intragranular acantholysis suggestive of pemphigus foliaceous (Figure 1A). A subsequent punch biopsy from the right lower abdomen sent for direct immunofluorescence demonstrated netlike positivity of IgG and C3 in the upper epidermis (Figure 1B), and serum sent for indirect immunofluorescence demonstrated intercellular IgG antibodies to desmoglein (Dsg) 1 on monkey esophagus and positive Dsg-1 antibodies on enzyme-linked immunosorbent assay, confirming the diagnosis.
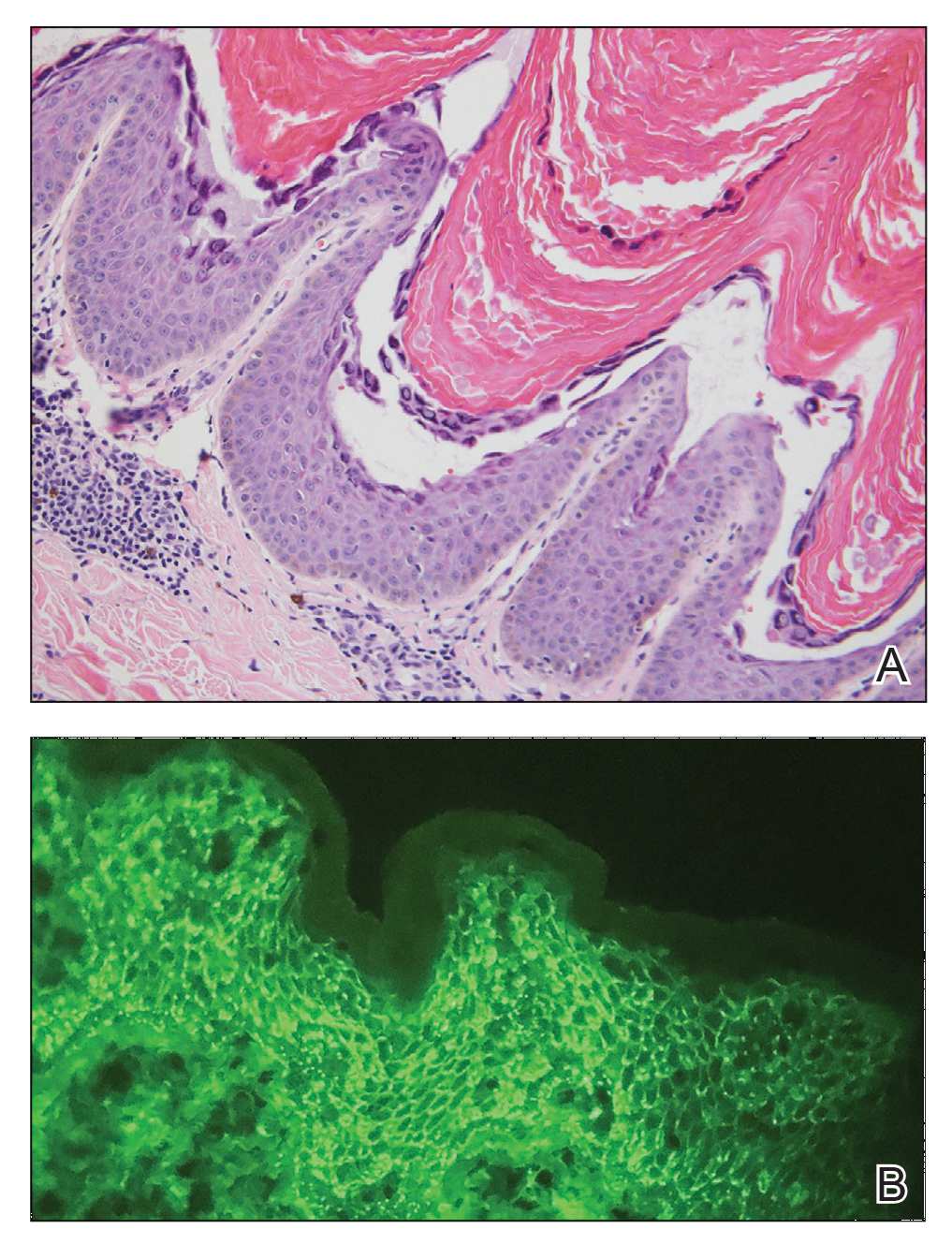
The patient was started on a 60-mg prednisone taper as well as dapsone 50 mg daily; the dapsone was titrated up to 100 mg daily. After tapering down to 10 mg daily of prednisone over 2 months and continuing dapsone with minimal improvement, he was given 2 infusions of rituximab 1000 mg 2 weeks apart. The scalp plaque was dramatically improved at 3-month follow-up (Figure 2), with partial improvement of the cheek plaques (Figure 3). Dapsone was increased to 150 mg daily, and he was encouraged to use triamcinolone acetonide ointment 0.1% twice daily, which led to further improvement.
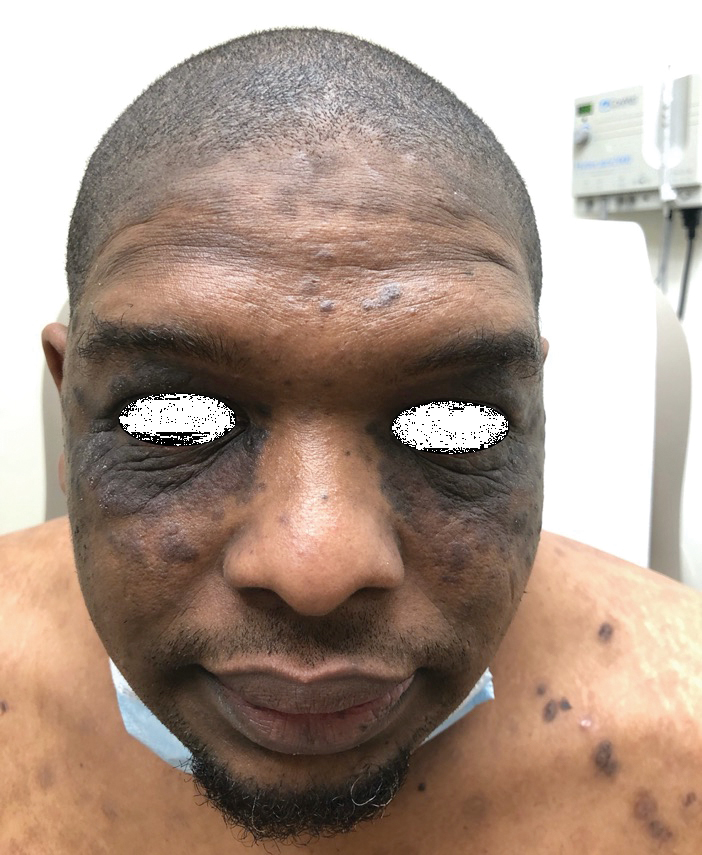
Pemphigus foliaceus is an autoimmune blistering disease that most commonly occurs in middle-aged adults. It generally is less common than pemphigus vulgaris, except in Finland, Tunisia, and Brazil, where there is an endemic condition with an identical clinical and histological presentation known as fogo selvagem.1
The pathogenesis of pemphigus foliaceous is characterized by IgG autoantibodies against Dsg-1, a transmembrane glycoprotein involved in the cellular adhesion of keratinocytes, which is preferentially expressed in the superficial epidermis.2-7 Dysfunction of Dsg-1 results in the separation of superficial epidermal cells, resulting in intraepidermal blisters.2,7 In contrast to pemphigus vulgaris, there typically is a lack of oral mucosal involvement due to compensation by Dsg-3 in the mucosa.4 Potential triggers for pemphigus foliaceous include exposure to UV radiation; radiotherapy; pregnancy; physiologic stress; and drugs, most commonly captopril, penicillamine, and thiols.8
Pemphigus foliaceous lesions clinically appear as eroded and crusted lesions on an erythematous base, commonly in a seborrheic distribution on the face, scalp, and trunk with sparing of the oral mucosa,2,6 but lesions can progress to a widespread and more severe exfoliative dermatitis.7 Lesions also can appear as psoriasiform plaques and often are initially misdiagnosed as psoriasis, particularly in patients with skin of color.9,10
Diagnosis of pemphigus foliaceous typically is made using a combination of histology as well as both direct and indirect immunofluorescence. Histologically, pemphigus foliaceus presents with subcorneal acantholysis, which is most prominent in the granular layer and occasionally the presence of neutrophils and eosinophils in the blister cavity.7 Direct immunofluorescence demonstrates netlike intercellular IgG and C3 in the upper portion of the epidermis.11 Indirect immunofluorescence can help detect circulating IgG antibodies to Dsg-1, with guinea pig esophagus being the ideal substrate.11,12
First-line treatment of pemphigus foliaceus consists of systemic glucocorticoid therapy, often administered with azathioprine, methotrexate, or mycophenolate mofetil.2,6,13 Although first-line treatment is effective in 60% to 80% of patients,2 relapsing cases can be treated with cyclophosphamide, intravenous immunoglobulin, immunoadsorption, plasmapheresis, or rituximab.2
Rituximab is a chimeric monoclonal antibody targeting CD20+ B cells, leading to decreased antibody production, which has been shown to be effective in treating severe and refractory cases of pemphigus foliaceus.6,13Rituximab with short-course prednisone has been found to be more effective in achieving complete remission at 24 months than prednisone alone.14 In patients with contraindications to systemic glucocorticoid therapy, rituximab has been shown as an effective first-line therapy.15 One-quarter of patients treated with rituximab relapsed within 2 years of treatment6 (average time to relapse, 6–26 months).16 High-dose rituximab regimens, along with a higher number of rituximab treatment cycles, have been shown to prolong time to relapse.6 Further, higher baseline levels of Dsg-1 antibody have been correlated to earlier relapse and can be used following rituximab therapy to monitor disease progression.6,16
The differential diagnosis for pemphigus foliaceous includes disseminated blastomycosis, hypertrophic lupus erythematosus, sebopsoriasis, and secondary syphilis. Disseminated blastomycosis presents with cutaneous manifestations such as nodules, papules, or pustules evolving over weeks to months into ulcers with subsequent scarring.17 Hypertrophic lupus erythematosus presents with papules and nodules with associated keratotic scaling on the face, palms, and extensor surfaces of the limbs.18 Sebopsoriasis is characterized by well-defined lesions with an overlying scale distributed on the scalp, face, and chest.19 Secondary syphilis presents as early hyperpigmented macules transitioning to acral papulosquamous lesions involving the palms and soles.20
- Hans-Filho G, Aoki V, Hans Bittner NR, et al. Fogo selvagem: endemic pemphigus foliaceus. An Bras Dermatol. 2018;93:638-650.
- Jenson KK, Burr DM, Edwards BC. Case report: reatment of refractory pemphigus foliaceus with rituximab. Practical Dermatology. February 2016:33-36. Accessed August 27, 2021. https://practicaldermatology.com/articles/2016-feb/case-report -treatment-of-refractory-pemphigus-foliaceus-with-rituximab -financial-matters-aad-asds-resources
- Amagai M, Hashimoto T, Green KJ, et al. Antigen-specific immunoadsorption of pathogenic autoantibodies in pemphigus foliaceus. J Invest Dermatol. 1995;104:895-901.
- Mahoney MG, Wang Z, Rothenberger K, et al. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103:461-468.
- Oktarina DAM, Sokol E, Kramer D, et al. Endocytosis of IgG, desmoglein 1, and plakoglobin in pemphigus foliaceus patient skin. Front Immunol. 2019;10:1-12.
- Kraft M, Worm M. Pemphigus foliaceus-repeated treatment with rituximab 7 years after initial response: a case report. Front Med. 2018;5:315.
- Hale EK. Pemphigus foliaceous. Dermatol Online J. 2002;8:9.
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106.
- A boobaker J, Morar N, Ramdial PK, et al. Pemphigus in South Africa. Int J Dermatol. 2001;40:115-119.
- Austin E, Millsop JW, Ely H, et al. Psoriasiform pemphigus foliaceus in an African American female: an important clinical manifestation. J Drugs Dermatol. 2018;17:471.
- Arbache ST, Nogueira TG, Delgado L, et al. Immunofluorescence testing in the diagnosis of autoimmune blistering diseases: overview of 10-year experience. An Bras Dermatol. 2014;89:885-889.
- Sabolinski ML, Beutner EH, Krasny S, et al. Substrate specificity of antiepithelial antibodies of pemphigus vulgaris and pemphigus foliaceus sera in immunofluorescence tests on monkey and guinea pig esophagus sections. J Invest Dermatol. 1987;88:545-549.
- Palacios-Álvarez I, Riquelme-McLoughlin C, Curto-Barredo L, et al. Rituximab treatment of pemphigus foliaceus: a retrospective study of 12 patients. J Am Acad Dermatol. 2021;85:484-486.
- Murrell DF, Sprecher E. Rituximab and short-course prednisone as the new gold standard for new-onset pemphigus vulgaris and pemphigus foliaceus. Br J Dermatol. 2017;177:1143-1144.
- Gregoriou S, Efthymiou O, Stefanaki C, et al. Management of pemphigus vulgaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2015;8:521-527.
- Saleh MA. A prospective study comparing patients with early and late relapsing pemphigus treated with rituximab. J Am Acad Dermatol. 2018;79:97-103.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264.
- Herzum A, Gasparini G, Emanuele C, et al. Atypical and rare forms of cutaneous lupus erythematosus: the importance of the diagnosis for the best management of patients. Dermatology. 2013;1-10.
- Tull TJ, Noy M, Bunker CB, et al. Sebopsoriasis in patients with HIV: a case series of 20 patients. Br J Dermatol. 2016; 173:813-815.
- Balagula Y, Mattei P, Wisco OJ, et al. The great imitator revised: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
The Diagnosis: Pemphigus Foliaceous
Laboratory workup including a complete blood cell count with differential, comprehensive metabolic panel, antinuclear antibodies, Sjögren syndrome A and B antibodies, hepatitis profile, rapid plasma reagin, HIV screen, aldolase, anti–Jo-1, T-Spot TB test (Quest Diagnostics), and tissue cultures was unremarkable. Two 4-mm punch biopsies were obtained from the left cheek and upper back, both of which demonstrated intragranular acantholysis suggestive of pemphigus foliaceous (Figure 1A). A subsequent punch biopsy from the right lower abdomen sent for direct immunofluorescence demonstrated netlike positivity of IgG and C3 in the upper epidermis (Figure 1B), and serum sent for indirect immunofluorescence demonstrated intercellular IgG antibodies to desmoglein (Dsg) 1 on monkey esophagus and positive Dsg-1 antibodies on enzyme-linked immunosorbent assay, confirming the diagnosis.

The patient was started on a 60-mg prednisone taper as well as dapsone 50 mg daily; the dapsone was titrated up to 100 mg daily. After tapering down to 10 mg daily of prednisone over 2 months and continuing dapsone with minimal improvement, he was given 2 infusions of rituximab 1000 mg 2 weeks apart. The scalp plaque was dramatically improved at 3-month follow-up (Figure 2), with partial improvement of the cheek plaques (Figure 3). Dapsone was increased to 150 mg daily, and he was encouraged to use triamcinolone acetonide ointment 0.1% twice daily, which led to further improvement.


Pemphigus foliaceus is an autoimmune blistering disease that most commonly occurs in middle-aged adults. It generally is less common than pemphigus vulgaris, except in Finland, Tunisia, and Brazil, where there is an endemic condition with an identical clinical and histological presentation known as fogo selvagem.1
The pathogenesis of pemphigus foliaceous is characterized by IgG autoantibodies against Dsg-1, a transmembrane glycoprotein involved in the cellular adhesion of keratinocytes, which is preferentially expressed in the superficial epidermis.2-7 Dysfunction of Dsg-1 results in the separation of superficial epidermal cells, resulting in intraepidermal blisters.2,7 In contrast to pemphigus vulgaris, there typically is a lack of oral mucosal involvement due to compensation by Dsg-3 in the mucosa.4 Potential triggers for pemphigus foliaceous include exposure to UV radiation; radiotherapy; pregnancy; physiologic stress; and drugs, most commonly captopril, penicillamine, and thiols.8
Pemphigus foliaceous lesions clinically appear as eroded and crusted lesions on an erythematous base, commonly in a seborrheic distribution on the face, scalp, and trunk with sparing of the oral mucosa,2,6 but lesions can progress to a widespread and more severe exfoliative dermatitis.7 Lesions also can appear as psoriasiform plaques and often are initially misdiagnosed as psoriasis, particularly in patients with skin of color.9,10
Diagnosis of pemphigus foliaceous typically is made using a combination of histology as well as both direct and indirect immunofluorescence. Histologically, pemphigus foliaceus presents with subcorneal acantholysis, which is most prominent in the granular layer and occasionally the presence of neutrophils and eosinophils in the blister cavity.7 Direct immunofluorescence demonstrates netlike intercellular IgG and C3 in the upper portion of the epidermis.11 Indirect immunofluorescence can help detect circulating IgG antibodies to Dsg-1, with guinea pig esophagus being the ideal substrate.11,12
First-line treatment of pemphigus foliaceus consists of systemic glucocorticoid therapy, often administered with azathioprine, methotrexate, or mycophenolate mofetil.2,6,13 Although first-line treatment is effective in 60% to 80% of patients,2 relapsing cases can be treated with cyclophosphamide, intravenous immunoglobulin, immunoadsorption, plasmapheresis, or rituximab.2
Rituximab is a chimeric monoclonal antibody targeting CD20+ B cells, leading to decreased antibody production, which has been shown to be effective in treating severe and refractory cases of pemphigus foliaceus.6,13Rituximab with short-course prednisone has been found to be more effective in achieving complete remission at 24 months than prednisone alone.14 In patients with contraindications to systemic glucocorticoid therapy, rituximab has been shown as an effective first-line therapy.15 One-quarter of patients treated with rituximab relapsed within 2 years of treatment6 (average time to relapse, 6–26 months).16 High-dose rituximab regimens, along with a higher number of rituximab treatment cycles, have been shown to prolong time to relapse.6 Further, higher baseline levels of Dsg-1 antibody have been correlated to earlier relapse and can be used following rituximab therapy to monitor disease progression.6,16
The differential diagnosis for pemphigus foliaceous includes disseminated blastomycosis, hypertrophic lupus erythematosus, sebopsoriasis, and secondary syphilis. Disseminated blastomycosis presents with cutaneous manifestations such as nodules, papules, or pustules evolving over weeks to months into ulcers with subsequent scarring.17 Hypertrophic lupus erythematosus presents with papules and nodules with associated keratotic scaling on the face, palms, and extensor surfaces of the limbs.18 Sebopsoriasis is characterized by well-defined lesions with an overlying scale distributed on the scalp, face, and chest.19 Secondary syphilis presents as early hyperpigmented macules transitioning to acral papulosquamous lesions involving the palms and soles.20
The Diagnosis: Pemphigus Foliaceous
Laboratory workup including a complete blood cell count with differential, comprehensive metabolic panel, antinuclear antibodies, Sjögren syndrome A and B antibodies, hepatitis profile, rapid plasma reagin, HIV screen, aldolase, anti–Jo-1, T-Spot TB test (Quest Diagnostics), and tissue cultures was unremarkable. Two 4-mm punch biopsies were obtained from the left cheek and upper back, both of which demonstrated intragranular acantholysis suggestive of pemphigus foliaceous (Figure 1A). A subsequent punch biopsy from the right lower abdomen sent for direct immunofluorescence demonstrated netlike positivity of IgG and C3 in the upper epidermis (Figure 1B), and serum sent for indirect immunofluorescence demonstrated intercellular IgG antibodies to desmoglein (Dsg) 1 on monkey esophagus and positive Dsg-1 antibodies on enzyme-linked immunosorbent assay, confirming the diagnosis.

The patient was started on a 60-mg prednisone taper as well as dapsone 50 mg daily; the dapsone was titrated up to 100 mg daily. After tapering down to 10 mg daily of prednisone over 2 months and continuing dapsone with minimal improvement, he was given 2 infusions of rituximab 1000 mg 2 weeks apart. The scalp plaque was dramatically improved at 3-month follow-up (Figure 2), with partial improvement of the cheek plaques (Figure 3). Dapsone was increased to 150 mg daily, and he was encouraged to use triamcinolone acetonide ointment 0.1% twice daily, which led to further improvement.


Pemphigus foliaceus is an autoimmune blistering disease that most commonly occurs in middle-aged adults. It generally is less common than pemphigus vulgaris, except in Finland, Tunisia, and Brazil, where there is an endemic condition with an identical clinical and histological presentation known as fogo selvagem.1
The pathogenesis of pemphigus foliaceous is characterized by IgG autoantibodies against Dsg-1, a transmembrane glycoprotein involved in the cellular adhesion of keratinocytes, which is preferentially expressed in the superficial epidermis.2-7 Dysfunction of Dsg-1 results in the separation of superficial epidermal cells, resulting in intraepidermal blisters.2,7 In contrast to pemphigus vulgaris, there typically is a lack of oral mucosal involvement due to compensation by Dsg-3 in the mucosa.4 Potential triggers for pemphigus foliaceous include exposure to UV radiation; radiotherapy; pregnancy; physiologic stress; and drugs, most commonly captopril, penicillamine, and thiols.8
Pemphigus foliaceous lesions clinically appear as eroded and crusted lesions on an erythematous base, commonly in a seborrheic distribution on the face, scalp, and trunk with sparing of the oral mucosa,2,6 but lesions can progress to a widespread and more severe exfoliative dermatitis.7 Lesions also can appear as psoriasiform plaques and often are initially misdiagnosed as psoriasis, particularly in patients with skin of color.9,10
Diagnosis of pemphigus foliaceous typically is made using a combination of histology as well as both direct and indirect immunofluorescence. Histologically, pemphigus foliaceus presents with subcorneal acantholysis, which is most prominent in the granular layer and occasionally the presence of neutrophils and eosinophils in the blister cavity.7 Direct immunofluorescence demonstrates netlike intercellular IgG and C3 in the upper portion of the epidermis.11 Indirect immunofluorescence can help detect circulating IgG antibodies to Dsg-1, with guinea pig esophagus being the ideal substrate.11,12
First-line treatment of pemphigus foliaceus consists of systemic glucocorticoid therapy, often administered with azathioprine, methotrexate, or mycophenolate mofetil.2,6,13 Although first-line treatment is effective in 60% to 80% of patients,2 relapsing cases can be treated with cyclophosphamide, intravenous immunoglobulin, immunoadsorption, plasmapheresis, or rituximab.2
Rituximab is a chimeric monoclonal antibody targeting CD20+ B cells, leading to decreased antibody production, which has been shown to be effective in treating severe and refractory cases of pemphigus foliaceus.6,13Rituximab with short-course prednisone has been found to be more effective in achieving complete remission at 24 months than prednisone alone.14 In patients with contraindications to systemic glucocorticoid therapy, rituximab has been shown as an effective first-line therapy.15 One-quarter of patients treated with rituximab relapsed within 2 years of treatment6 (average time to relapse, 6–26 months).16 High-dose rituximab regimens, along with a higher number of rituximab treatment cycles, have been shown to prolong time to relapse.6 Further, higher baseline levels of Dsg-1 antibody have been correlated to earlier relapse and can be used following rituximab therapy to monitor disease progression.6,16
The differential diagnosis for pemphigus foliaceous includes disseminated blastomycosis, hypertrophic lupus erythematosus, sebopsoriasis, and secondary syphilis. Disseminated blastomycosis presents with cutaneous manifestations such as nodules, papules, or pustules evolving over weeks to months into ulcers with subsequent scarring.17 Hypertrophic lupus erythematosus presents with papules and nodules with associated keratotic scaling on the face, palms, and extensor surfaces of the limbs.18 Sebopsoriasis is characterized by well-defined lesions with an overlying scale distributed on the scalp, face, and chest.19 Secondary syphilis presents as early hyperpigmented macules transitioning to acral papulosquamous lesions involving the palms and soles.20
- Hans-Filho G, Aoki V, Hans Bittner NR, et al. Fogo selvagem: endemic pemphigus foliaceus. An Bras Dermatol. 2018;93:638-650.
- Jenson KK, Burr DM, Edwards BC. Case report: reatment of refractory pemphigus foliaceus with rituximab. Practical Dermatology. February 2016:33-36. Accessed August 27, 2021. https://practicaldermatology.com/articles/2016-feb/case-report -treatment-of-refractory-pemphigus-foliaceus-with-rituximab -financial-matters-aad-asds-resources
- Amagai M, Hashimoto T, Green KJ, et al. Antigen-specific immunoadsorption of pathogenic autoantibodies in pemphigus foliaceus. J Invest Dermatol. 1995;104:895-901.
- Mahoney MG, Wang Z, Rothenberger K, et al. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103:461-468.
- Oktarina DAM, Sokol E, Kramer D, et al. Endocytosis of IgG, desmoglein 1, and plakoglobin in pemphigus foliaceus patient skin. Front Immunol. 2019;10:1-12.
- Kraft M, Worm M. Pemphigus foliaceus-repeated treatment with rituximab 7 years after initial response: a case report. Front Med. 2018;5:315.
- Hale EK. Pemphigus foliaceous. Dermatol Online J. 2002;8:9.
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106.
- A boobaker J, Morar N, Ramdial PK, et al. Pemphigus in South Africa. Int J Dermatol. 2001;40:115-119.
- Austin E, Millsop JW, Ely H, et al. Psoriasiform pemphigus foliaceus in an African American female: an important clinical manifestation. J Drugs Dermatol. 2018;17:471.
- Arbache ST, Nogueira TG, Delgado L, et al. Immunofluorescence testing in the diagnosis of autoimmune blistering diseases: overview of 10-year experience. An Bras Dermatol. 2014;89:885-889.
- Sabolinski ML, Beutner EH, Krasny S, et al. Substrate specificity of antiepithelial antibodies of pemphigus vulgaris and pemphigus foliaceus sera in immunofluorescence tests on monkey and guinea pig esophagus sections. J Invest Dermatol. 1987;88:545-549.
- Palacios-Álvarez I, Riquelme-McLoughlin C, Curto-Barredo L, et al. Rituximab treatment of pemphigus foliaceus: a retrospective study of 12 patients. J Am Acad Dermatol. 2021;85:484-486.
- Murrell DF, Sprecher E. Rituximab and short-course prednisone as the new gold standard for new-onset pemphigus vulgaris and pemphigus foliaceus. Br J Dermatol. 2017;177:1143-1144.
- Gregoriou S, Efthymiou O, Stefanaki C, et al. Management of pemphigus vulgaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2015;8:521-527.
- Saleh MA. A prospective study comparing patients with early and late relapsing pemphigus treated with rituximab. J Am Acad Dermatol. 2018;79:97-103.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264.
- Herzum A, Gasparini G, Emanuele C, et al. Atypical and rare forms of cutaneous lupus erythematosus: the importance of the diagnosis for the best management of patients. Dermatology. 2013;1-10.
- Tull TJ, Noy M, Bunker CB, et al. Sebopsoriasis in patients with HIV: a case series of 20 patients. Br J Dermatol. 2016; 173:813-815.
- Balagula Y, Mattei P, Wisco OJ, et al. The great imitator revised: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hans-Filho G, Aoki V, Hans Bittner NR, et al. Fogo selvagem: endemic pemphigus foliaceus. An Bras Dermatol. 2018;93:638-650.
- Jenson KK, Burr DM, Edwards BC. Case report: reatment of refractory pemphigus foliaceus with rituximab. Practical Dermatology. February 2016:33-36. Accessed August 27, 2021. https://practicaldermatology.com/articles/2016-feb/case-report -treatment-of-refractory-pemphigus-foliaceus-with-rituximab -financial-matters-aad-asds-resources
- Amagai M, Hashimoto T, Green KJ, et al. Antigen-specific immunoadsorption of pathogenic autoantibodies in pemphigus foliaceus. J Invest Dermatol. 1995;104:895-901.
- Mahoney MG, Wang Z, Rothenberger K, et al. Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103:461-468.
- Oktarina DAM, Sokol E, Kramer D, et al. Endocytosis of IgG, desmoglein 1, and plakoglobin in pemphigus foliaceus patient skin. Front Immunol. 2019;10:1-12.
- Kraft M, Worm M. Pemphigus foliaceus-repeated treatment with rituximab 7 years after initial response: a case report. Front Med. 2018;5:315.
- Hale EK. Pemphigus foliaceous. Dermatol Online J. 2002;8:9.
- Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol Res. 2018;310:95-106.
- A boobaker J, Morar N, Ramdial PK, et al. Pemphigus in South Africa. Int J Dermatol. 2001;40:115-119.
- Austin E, Millsop JW, Ely H, et al. Psoriasiform pemphigus foliaceus in an African American female: an important clinical manifestation. J Drugs Dermatol. 2018;17:471.
- Arbache ST, Nogueira TG, Delgado L, et al. Immunofluorescence testing in the diagnosis of autoimmune blistering diseases: overview of 10-year experience. An Bras Dermatol. 2014;89:885-889.
- Sabolinski ML, Beutner EH, Krasny S, et al. Substrate specificity of antiepithelial antibodies of pemphigus vulgaris and pemphigus foliaceus sera in immunofluorescence tests on monkey and guinea pig esophagus sections. J Invest Dermatol. 1987;88:545-549.
- Palacios-Álvarez I, Riquelme-McLoughlin C, Curto-Barredo L, et al. Rituximab treatment of pemphigus foliaceus: a retrospective study of 12 patients. J Am Acad Dermatol. 2021;85:484-486.
- Murrell DF, Sprecher E. Rituximab and short-course prednisone as the new gold standard for new-onset pemphigus vulgaris and pemphigus foliaceus. Br J Dermatol. 2017;177:1143-1144.
- Gregoriou S, Efthymiou O, Stefanaki C, et al. Management of pemphigus vulgaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2015;8:521-527.
- Saleh MA. A prospective study comparing patients with early and late relapsing pemphigus treated with rituximab. J Am Acad Dermatol. 2018;79:97-103.
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264.
- Herzum A, Gasparini G, Emanuele C, et al. Atypical and rare forms of cutaneous lupus erythematosus: the importance of the diagnosis for the best management of patients. Dermatology. 2013;1-10.
- Tull TJ, Noy M, Bunker CB, et al. Sebopsoriasis in patients with HIV: a case series of 20 patients. Br J Dermatol. 2016; 173:813-815.
- Balagula Y, Mattei P, Wisco OJ, et al. The great imitator revised: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
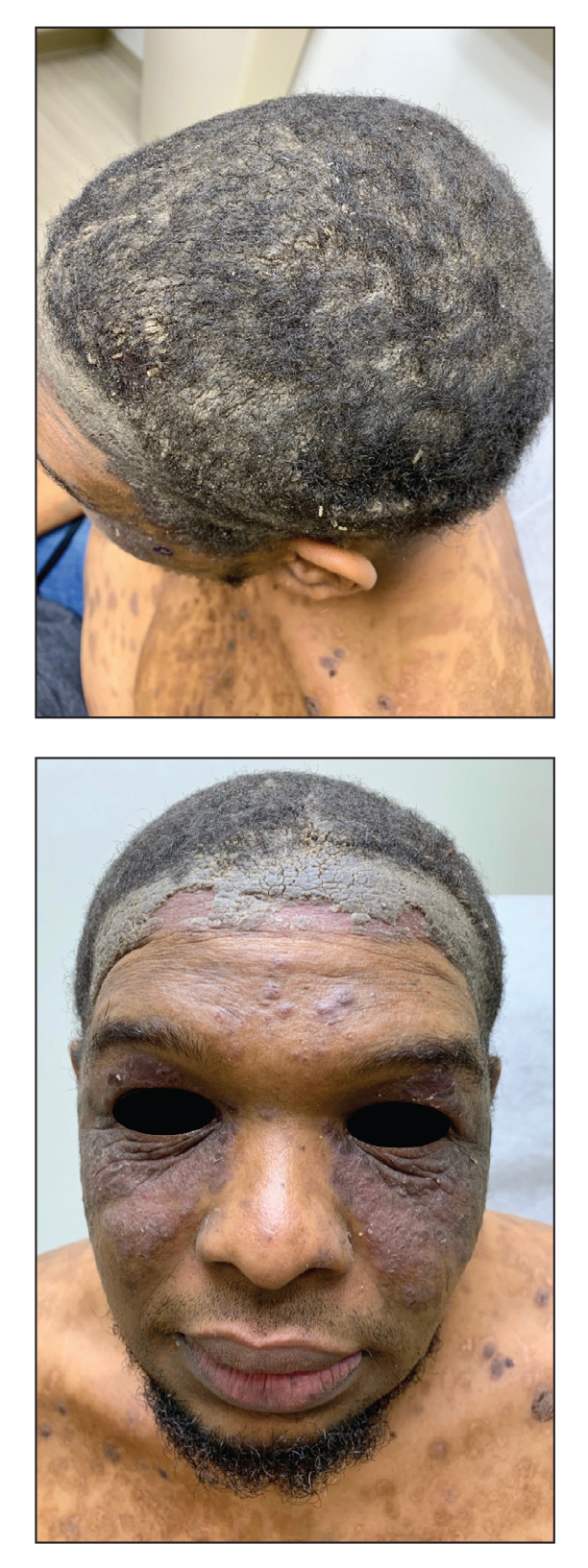
A 40-year-old Black man presented for evaluation of a thick plaque throughout the scalp (top), scaly plaques on the cheeks (bottom), and a spreading rash on the trunk that had progressed over the last few months. He had no relevant medical history, took no medications, and was in a monogamous relationship with a female partner. He previously saw an outside dermatologist who gave him triamcinolone cream, which was mildly helpful. Physical examination revealed a thick verrucous plaque throughout the scalp extending onto the forehead; thick plaques on the cheeks; and numerous, thinly eroded lesions on the trunk. Biopsies and a laboratory workup were performed.