User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Certain opioids hold promise for treating itch
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
Certain opioids are proving to be effective in treating a variety of itch conditions, according to Brian S. Kim, MD.
“We know that opioids or opiates do cause itch in a significant number of patients,” Dr. Kim, a dermatologist who is codirector of the Center for the Study of Itch & Sensory Disorders at Washington University, St. Louis, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It’s thought to do this by way of acting as a pruritogen at times and stimulating sensory neurons [that] then activate the itch cascade. But it’s also been well known that endogenous kappa opioids can activate sensory neurons that can then suppress itch and gate out signals from these opiates, but perhaps other pruritogens as well.”
Multiple drugs differentially target kappa-opioid receptor (KOR) and mu-opioid receptor (MOR) pathways, he continued. For example, oral naltrexone is a MOR antagonist, oral nalfurafine and intravenous difelikefalin are KOR agonists, while intranasal butorphanol and oral nalbuphine have a dual mechanism.
Difelikefalin is the first Food and Drug Administration–approved treatment for uremic pruritus associated with dialysis, approved in August 2021 for moderate-to-severe pruritus associated with chronic kidney disease in adults undergoing hemodialysis; it is administered intravenously. During the 2021 annual congress of the European Academy of Dermatology and Venereology, Dr. Kim and colleagues presented findings from a phase 2 trial of 401 people with atopic dermatitis (AD) and moderate to severe pruritus, who were randomized to receive oral difelikefalin at a dose of 0.25 mg, 0.5 mg, or 1.0 mg, or placebo over a 12-week treatment period. The primary endpoint, change from baseline in Itch Numerical Rating Scale score, was not met in any of the difelikefalin dose groups in the overall study population, but patients with a body surface area of less than 10% experienced a significant improvement in itch at week 12 in the combined difelikefalin dose group in (P = .039). A significant reduction in itch with difelikefalin was seen in this group of patients with itch-dominant AD, as early as the second day of treatment.
In another trial, 373 hemodialysis patients with moderate or severe uremic pruritus were randomized in a 1: 1:1 ratio to nalbuphine extended-release tablets 120 mg, 60 mg, or placebo and treated for 8 weeks. The researchers found that nalbuphine 120 mg significantly reduced the itching intensity. Specifically, from a baseline numerical rate scale (NRS) of 6.9, the mean NRS declined by 3.5 and by 2.8 in the nalbuphine 120-mg and the placebo groups, respectively (P = .017).
In a separate, unpublished multicenter, randomized, phase 2/3 trial, researchers evaluated the safety and antipruritic efficacy of nalbuphine extended-release tablets dosed twice daily at 90 mg and 180 mg in 62 patients in the United States and Europe. The proportion of patients in the nalbuphine 180-mg arm who met 50% responder criteria at week 10 or last observed visit approached statistical significance (P = .083), and this arm met statistical significance for patients who completed treatment (P = .028).
Dr. Kim disclosed that he has served as a consultant for AbbVie, AstraZeneca, Cara Therapeutics, Galderma, GlaxoSmithKline, LEO Pharma, Lilly, Pfizer, Regeneron, Sanofi, Trevi Therapeutics. He also has conducted contracted research for Cara Therapeutics and LEO Pharma.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Fueling an ‘already raging fire’: Fifth COVID surge approaches
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
Validity of commercial serologic tests for dermatomyositis still questionable
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
, according to Jeffrey P. Callen, MD.
That’s because the validity and reproducibility of testing in commercial laboratories remain questionable, Dr. Callen, professor of medicine and chief of the division of dermatology at the University of Louisville, Ky., said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “The testing in research laboratories is not widely available and the results are often delayed by weeks to months,” he said.
In addition, while the associations between antibody results and risks of malignancy or pulmonary disease are “statistically valid,” he said, “there are patients with disease in whom antibodies are not present and those without associated disease in whom the testing was positive.” For example, there are patients positive for anti–transition initiation factor (TIF)-1gamma but don’t have a malignancy, “and the ones with anti-MDA-5 tend to have pulmonary disease, but there are patients with anti-MDA-5 who don’t have pulmonary disease.”
Compared with patients with systemic lupus erythematosus, patients with dermatomyositis tend to have more itching and they tend of have fewer serologic abnormalities, such as anti-Ro/SS-A antibody, “but there is overlap,” Dr. Callen said. “The reason to differentiate cutaneous lupus erythematosus from dermatomyositis is because we think that patients who have amyopathic dermatomyositis still have an increased risk of having or developing an internal malignancy,” he added. Another differentiating point that is substantive is the presence of Gottron papules.
In a recent development related to antibody testing, researchers demonstrated that the IgG2 isotype of anti-TIF-1gamma antibodies is a biomarker of cancer and mortality in adult dermatomyositis.
According to population-based studies, about 20%-25% of dermatomyositis patients have had, have, or will develop a cancer (Lancet 2001;357: 96-100). Amyopathic dermatomyositis patients may also have cancer. Polymyositis patients generally have lower rates and their risk of subsequent malignancy is much closer to that of the general population, suggesting that the presence of the association is due to a “diagnostic suspicion bias,” Dr. Callen said.
A large-scale multicenter cohort study that set out to identify the risk factors and prognosis of patients with cancer-associated myositis found that ovarian cancer seems to be overrepresented. The only serologic abnormality that was statistically significant was anti-TIF-1gamma antibody (P less than .001). Patients with cancer-associated myositis also have less overall survival compared with those with non–cancer-associated myositis (P = .004), with malignancy being the primary cause of death (P less than .001).
In what is believed to be the largest study of its kind, Dr. Callen and colleagues retrospectively examined the prevalence of malignancy and screening practices in 400 dermatomyositis patients. Of the 400 patients, 48 (12%) had malignancies, and 21 cancers (40%) were diagnosed within 1 year of the dermatomyositis diagnosis. Both classic dermatomyositis and amyopathic dermatomyositis were associated with cancer, and 27 patients (6.8%) had a cancer at the time of diagnosis. Of those, 59% were asymptomatic; their cancers were discovered with CT scans, suggesting that “blind” screening is effective in identifying cancers in DM patients.
Dr. Callen’s malignancy evaluation includes chest x-ray, CT of the chest and abdomen, stool Hematest in all dermatomyositis patients; a mammogram, pelvic ultrasound and/or CT of the pelvis in women; and age, race or ethnicity-related testing. “I generally reevaluate patients annually for 3 years, because data from epidemiologic studies suggest that after 3 years [from the initial diagnosis], the rates of malignancy return toward normal,” he said. “I also evaluate any new symptom that might be suggestive of malignancy. The remaining issue is how to handle a patient in remission for several years, but who develops a relapse. What I do is perform another malignancy assessment.”
According to results from a meta-analysis of risk factors and systematic review of screening approaches, factors that increase malignancy risk include dermatomyositis subtype (risk ratio, 2.21), older age (weighted mean difference 11.19), male gender (RR, 1.53), dysphagia (RR, 2.09), cutaneous necrosis (RR, 2.73), and positive anti-TIF-1gamma (RR, 4.41).
Factors associated with a decreased risk of malignancy include polymyositis (RR, 0.49), clinically amyopathic dermatomyositis subtypes (RR, 0.44), Raynaud’s phenomenon (RR, 0.61), interstitial lung disease (RR, 0.49), very high serum creatine kinase (WMD –1189.96) or lactate dehydrogenase levels (WMD –336.53), and anti-Jo1 (RR, 0.45) or anti-EJ (RR, 0.17) positivity.
The analysis also found that CT scanning of the thorax, abdomen and pelvis appeared to yield a high proportion of underlying asymptomatic cancers. Limited evidence relating to the utility of tumor markers and 18F-FDG PET/CT was available.
As for treatment, the use of tofacitinib for cutaneous lesions of dermatomyositis has been suggested in various studies. In a recent open-label study of 10 patients with dermatomyositis who took extended release the JAK inhibitor tofacitinib 11 mg daily for 12 weeks, half experienced moderate improvement in disease activity, and the other half experienced minimal improvement. JAK inhibitors have been used in patients with juvenile dermatomyositis.
Dr. Callen’s treatment approach with dermatomyositis patients includes recommendations for sunscreens and protective clothing, plus assessment of vitamin D levels. “I will use topical emollients, corticosteroids, and calcineurin inhibitors,” he said. “Antimalarials might be used. I generally reach for methotrexate or mycophenolate mofetil relatively early. IVIG has also been studied.” Off-label therapies that have been used include dapsone, thalidomide, leflunomide, sirolimus, chlorambucil, etanercept, infliximab, rituximab, apremilast, tofacitinib, lenabasum, and low-dose naltrexone.
Dr. Callen disclosed that he is a consultant to Genentech and is a member of the safety monitoring committee for Principia Biopharma. He holds equity in Celgene, Pfizer, 3M, Johnson & Johnson, Merck, Abbott Laboratories, AbbVie, Procter & Gamble, Gilead, Allergen, and Amgen.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Prevalence of undiagnosed vitiligo is ‘remarkably high’
A new

“The remarkably high number of participants with undiagnosed vitiligo” indicates a need for “the development and validation of teledermatology apps that allow for potential diagnosis,” Kavita Gandhi, MS, of the patient and health impact group at Pfizer in Collegeville, Pa., and associates said in JAMA Dermatology.
The estimated range of 0.76%-1.11% prevalence represents 1.9 million to 2.8 million adults with vitiligo in the general population, based on responses from 40,888 participants surveyed between Dec. 30, 2019, and March 11, 2020, and further physician evaluation of photos uploaded by 113 respondents, they explained. The investigators used a representative sample of the U.S. population, of people ages 18-85 years.
A prior vitiligo diagnosis was reported by 314 participants, and another 249 screened positive through the survey, for a self-reported overall prevalence of 1.38% in the adult population and a previously undiagnosed prevalence of 0.61%. The physician adjudication brought the overall prevalence down to 0.76% and the undiagnosed prevalence to 0.29%. “These findings suggest that up to 40% of adults with vitiligo in the U.S. may be undiagnosed,” the investigators wrote.
Survey questions covering the laterality of lesions broke the 1.38% overall prevalence down to 0.77% nonsegmental vitiligo (self-reported as bilateral) and 0.61% segmental (unilateral). The 0.76% overall prevalence provided by the three dermatologist reviewers worked out to 0.58% classified as nonsegmental and 0.18% as segmental, Ms. Gandhi and associates said.
“The distinction between segmental and nonsegmental vitiligo is of prime importance [since] patients are usually concerned by the spreading of the disease and its unpredictable course, which is the hallmark of nonsegmental vitiligo,” the researchers noted.
The analysis was the first, to the authors’ knowledge, to identify several trends among the undiagnosed population. The proportion of nonwhite adults was higher in the undiagnosed group (40.2%) than among those with a diagnosis (31.5%), as was Hispanic, Latino, or Spanish origin (21.3% vs. 15.3%). Unilateral presentation was seen in 54.2% of the undiagnosed adults and 37.3% of those with diagnosed vitiligo, they reported.
The study was sponsored by Pfizer, which employs several of the investigators. Two of the investigators disclosed multiple conflicts of interest involving other companies.
A new

“The remarkably high number of participants with undiagnosed vitiligo” indicates a need for “the development and validation of teledermatology apps that allow for potential diagnosis,” Kavita Gandhi, MS, of the patient and health impact group at Pfizer in Collegeville, Pa., and associates said in JAMA Dermatology.
The estimated range of 0.76%-1.11% prevalence represents 1.9 million to 2.8 million adults with vitiligo in the general population, based on responses from 40,888 participants surveyed between Dec. 30, 2019, and March 11, 2020, and further physician evaluation of photos uploaded by 113 respondents, they explained. The investigators used a representative sample of the U.S. population, of people ages 18-85 years.
A prior vitiligo diagnosis was reported by 314 participants, and another 249 screened positive through the survey, for a self-reported overall prevalence of 1.38% in the adult population and a previously undiagnosed prevalence of 0.61%. The physician adjudication brought the overall prevalence down to 0.76% and the undiagnosed prevalence to 0.29%. “These findings suggest that up to 40% of adults with vitiligo in the U.S. may be undiagnosed,” the investigators wrote.
Survey questions covering the laterality of lesions broke the 1.38% overall prevalence down to 0.77% nonsegmental vitiligo (self-reported as bilateral) and 0.61% segmental (unilateral). The 0.76% overall prevalence provided by the three dermatologist reviewers worked out to 0.58% classified as nonsegmental and 0.18% as segmental, Ms. Gandhi and associates said.
“The distinction between segmental and nonsegmental vitiligo is of prime importance [since] patients are usually concerned by the spreading of the disease and its unpredictable course, which is the hallmark of nonsegmental vitiligo,” the researchers noted.
The analysis was the first, to the authors’ knowledge, to identify several trends among the undiagnosed population. The proportion of nonwhite adults was higher in the undiagnosed group (40.2%) than among those with a diagnosis (31.5%), as was Hispanic, Latino, or Spanish origin (21.3% vs. 15.3%). Unilateral presentation was seen in 54.2% of the undiagnosed adults and 37.3% of those with diagnosed vitiligo, they reported.
The study was sponsored by Pfizer, which employs several of the investigators. Two of the investigators disclosed multiple conflicts of interest involving other companies.
A new

“The remarkably high number of participants with undiagnosed vitiligo” indicates a need for “the development and validation of teledermatology apps that allow for potential diagnosis,” Kavita Gandhi, MS, of the patient and health impact group at Pfizer in Collegeville, Pa., and associates said in JAMA Dermatology.
The estimated range of 0.76%-1.11% prevalence represents 1.9 million to 2.8 million adults with vitiligo in the general population, based on responses from 40,888 participants surveyed between Dec. 30, 2019, and March 11, 2020, and further physician evaluation of photos uploaded by 113 respondents, they explained. The investigators used a representative sample of the U.S. population, of people ages 18-85 years.
A prior vitiligo diagnosis was reported by 314 participants, and another 249 screened positive through the survey, for a self-reported overall prevalence of 1.38% in the adult population and a previously undiagnosed prevalence of 0.61%. The physician adjudication brought the overall prevalence down to 0.76% and the undiagnosed prevalence to 0.29%. “These findings suggest that up to 40% of adults with vitiligo in the U.S. may be undiagnosed,” the investigators wrote.
Survey questions covering the laterality of lesions broke the 1.38% overall prevalence down to 0.77% nonsegmental vitiligo (self-reported as bilateral) and 0.61% segmental (unilateral). The 0.76% overall prevalence provided by the three dermatologist reviewers worked out to 0.58% classified as nonsegmental and 0.18% as segmental, Ms. Gandhi and associates said.
“The distinction between segmental and nonsegmental vitiligo is of prime importance [since] patients are usually concerned by the spreading of the disease and its unpredictable course, which is the hallmark of nonsegmental vitiligo,” the researchers noted.
The analysis was the first, to the authors’ knowledge, to identify several trends among the undiagnosed population. The proportion of nonwhite adults was higher in the undiagnosed group (40.2%) than among those with a diagnosis (31.5%), as was Hispanic, Latino, or Spanish origin (21.3% vs. 15.3%). Unilateral presentation was seen in 54.2% of the undiagnosed adults and 37.3% of those with diagnosed vitiligo, they reported.
The study was sponsored by Pfizer, which employs several of the investigators. Two of the investigators disclosed multiple conflicts of interest involving other companies.
FROM JAMA DERMATOLOGY
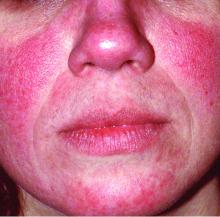
Rosacea is in the eye of the beholder, expert says
In the clinical experience of Emmy Graber, MD, MBA, rosacea is in the eye of the beholder.
“It’s not really up to us as the providers as to what’s important to the patient or how bad their rosacea is,” she said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It really is up to the patient,” added Dr. Graber, president of The Dermatology Institute of Boston, who recommends asking patients about how severe they consider their rosacea to be, and what about rosacea bothers them most. Their responses may be surprising.
A study published in 2017 showed that complete resolution of even mild rosacea prolongs remission of rosacea, and most importantly, improves the quality of life for patients. “So, don’t discount what you consider to be mild rosacea in patients,” she said.
Skin care recommendations
“And don’t forget about basic skin care,” she advised. A recently published Chinese study of 999 rosacea patients and 1,010 controls with healthy skin found that a high frequency of cleansing and expansive use of cleansers were positively correlated with rosacea occurrence, suggesting that overcleansing can be a risk factor for rosacea. “Ask your patient, ‘how often are you cleaning your face?’ ” Dr. Graber suggested. “You might find that they’re overdoing it by washing three or four times a day. Several studies have shown that basic skin care alone improves rosacea.”
Skin care recommendations for patients with rosacea include avoiding chemical or physical exfoliants and alcohol-based topical products, and moisturizing and washing their faces with mild, synthetic detergent-based products rather than traditional soaps, which may further alkalinize and irritate the skin. “Patients should also be counseled to use physical-based sunscreens rather than chemical-based sunscreens,” she said.
Treating erythema
For treating erythema with topicals, a systematic review published in 2019 found the most evidence for brimonidine 0.33% gel, an alpha2-adrenergic agonist, and oxymetazoline 1% cream, an alpha1-adrenergic agonist. “Both of these products functionally constrict facial blood vessels,” and are Food and Drug Administration approved for treating persistent erythema, Dr. Graber said. “These products improve erythema within 3 hours of and up to 12 hours after application and overall, they are well tolerated.”
Based on clinical trial results, about 15% of patients on brimonidine report adverse reactions such as dermatitis, burning, pruritus, and erythema, compared with 8% of patients on oxymetazoline. At the same time, up to 20% of individuals on brimonidine report rebound erythema, compared with fewer than 1% of those using oxymetazoline. Laser and light therapies such as pulse-dye lasers, potassium-titanyl-phosphate lasers, and intense-pulse light devices are also effective in treating persistent erythema but are less effective for transient flushing.
Treatment of papules and pustules
For treating papules and pustules, the 2019 systemic review also found high-certainty evidence for using azelaic acid and topical ivermectin, and moderate-certainty evidence for using topical metronidazole and topical minocycline. “Topical ivermectin was demonstrated to be the most effective topical treatment for papulopustular rosacea and to provide the greatest psychological benefit to these patients,” Dr. Graber said.
In a double-blind, multicenter 15-week trial comparing azelaic acid 15% gel with metronidazole 0.75% gel in patients with papulopustular rosacea, both agents were found to be effective. But those treated with azelaic acid 15% gel had a greater reduction in lesion counts and erythema, and improvement in global assessments, compared with metronidazole 0.75% gel. However, the azelaic acid 15% gel was associated with more stinging compared with metronidazole 0.75% gel, although it was usually transient.
Another study, a double-blind, single-center, 15-week trial, compared the efficacy of azelaic acid 20% cream with metronidazole 0.75% cream. Both agents were found to be effective and had similar levels of reductions in papules and pustules. However, patients in the azelaic acid 20% cream arm had significantly higher physician ratings of global improvement, as well as overall higher patient satisfaction.
More recently, a phase 3 study of 962 patients found that ivermectin 1% cream once daily improved quality of life slightly more than metronidazole 0.75% cream twice daily. No difference in adverse events were noted between the two agents.
Other options for treating papules and pustules include topical minocycline 1.5% foam, which is FDA approved for rosacea, as well as second-line agents topical sodium sulfacetamide with sulfur cleanser (cream or lotion), and permethrin, Dr. Graber said.
As for treating papules and pustules with oral agents, the strongest evidence favors oral tetracyclines and isotretinoin, she noted.
Doxycycline, minocycline, tetracycline, and sarecycline can be used as monotherapy or coadministered with topical agents. “The addition of topical agents may also help to shorten the duration of antibiotic use, which is very important,” Dr. Graber said.
She noted that oral beta-blockers might be useful to treat persistent erythema and flushing because they antagonize the effects of sympathetic nerve stimulation and circulating catecholamines at b-adrenoceptors. Carvedilol and propranolol have been the most studied. The most common potential side effects are hypotension and bradycardia.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
In the clinical experience of Emmy Graber, MD, MBA, rosacea is in the eye of the beholder.
“It’s not really up to us as the providers as to what’s important to the patient or how bad their rosacea is,” she said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It really is up to the patient,” added Dr. Graber, president of The Dermatology Institute of Boston, who recommends asking patients about how severe they consider their rosacea to be, and what about rosacea bothers them most. Their responses may be surprising.
A study published in 2017 showed that complete resolution of even mild rosacea prolongs remission of rosacea, and most importantly, improves the quality of life for patients. “So, don’t discount what you consider to be mild rosacea in patients,” she said.
Skin care recommendations
“And don’t forget about basic skin care,” she advised. A recently published Chinese study of 999 rosacea patients and 1,010 controls with healthy skin found that a high frequency of cleansing and expansive use of cleansers were positively correlated with rosacea occurrence, suggesting that overcleansing can be a risk factor for rosacea. “Ask your patient, ‘how often are you cleaning your face?’ ” Dr. Graber suggested. “You might find that they’re overdoing it by washing three or four times a day. Several studies have shown that basic skin care alone improves rosacea.”
Skin care recommendations for patients with rosacea include avoiding chemical or physical exfoliants and alcohol-based topical products, and moisturizing and washing their faces with mild, synthetic detergent-based products rather than traditional soaps, which may further alkalinize and irritate the skin. “Patients should also be counseled to use physical-based sunscreens rather than chemical-based sunscreens,” she said.
Treating erythema
For treating erythema with topicals, a systematic review published in 2019 found the most evidence for brimonidine 0.33% gel, an alpha2-adrenergic agonist, and oxymetazoline 1% cream, an alpha1-adrenergic agonist. “Both of these products functionally constrict facial blood vessels,” and are Food and Drug Administration approved for treating persistent erythema, Dr. Graber said. “These products improve erythema within 3 hours of and up to 12 hours after application and overall, they are well tolerated.”
Based on clinical trial results, about 15% of patients on brimonidine report adverse reactions such as dermatitis, burning, pruritus, and erythema, compared with 8% of patients on oxymetazoline. At the same time, up to 20% of individuals on brimonidine report rebound erythema, compared with fewer than 1% of those using oxymetazoline. Laser and light therapies such as pulse-dye lasers, potassium-titanyl-phosphate lasers, and intense-pulse light devices are also effective in treating persistent erythema but are less effective for transient flushing.
Treatment of papules and pustules
For treating papules and pustules, the 2019 systemic review also found high-certainty evidence for using azelaic acid and topical ivermectin, and moderate-certainty evidence for using topical metronidazole and topical minocycline. “Topical ivermectin was demonstrated to be the most effective topical treatment for papulopustular rosacea and to provide the greatest psychological benefit to these patients,” Dr. Graber said.
In a double-blind, multicenter 15-week trial comparing azelaic acid 15% gel with metronidazole 0.75% gel in patients with papulopustular rosacea, both agents were found to be effective. But those treated with azelaic acid 15% gel had a greater reduction in lesion counts and erythema, and improvement in global assessments, compared with metronidazole 0.75% gel. However, the azelaic acid 15% gel was associated with more stinging compared with metronidazole 0.75% gel, although it was usually transient.
Another study, a double-blind, single-center, 15-week trial, compared the efficacy of azelaic acid 20% cream with metronidazole 0.75% cream. Both agents were found to be effective and had similar levels of reductions in papules and pustules. However, patients in the azelaic acid 20% cream arm had significantly higher physician ratings of global improvement, as well as overall higher patient satisfaction.
More recently, a phase 3 study of 962 patients found that ivermectin 1% cream once daily improved quality of life slightly more than metronidazole 0.75% cream twice daily. No difference in adverse events were noted between the two agents.
Other options for treating papules and pustules include topical minocycline 1.5% foam, which is FDA approved for rosacea, as well as second-line agents topical sodium sulfacetamide with sulfur cleanser (cream or lotion), and permethrin, Dr. Graber said.
As for treating papules and pustules with oral agents, the strongest evidence favors oral tetracyclines and isotretinoin, she noted.
Doxycycline, minocycline, tetracycline, and sarecycline can be used as monotherapy or coadministered with topical agents. “The addition of topical agents may also help to shorten the duration of antibiotic use, which is very important,” Dr. Graber said.
She noted that oral beta-blockers might be useful to treat persistent erythema and flushing because they antagonize the effects of sympathetic nerve stimulation and circulating catecholamines at b-adrenoceptors. Carvedilol and propranolol have been the most studied. The most common potential side effects are hypotension and bradycardia.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
In the clinical experience of Emmy Graber, MD, MBA, rosacea is in the eye of the beholder.
“It’s not really up to us as the providers as to what’s important to the patient or how bad their rosacea is,” she said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “It really is up to the patient,” added Dr. Graber, president of The Dermatology Institute of Boston, who recommends asking patients about how severe they consider their rosacea to be, and what about rosacea bothers them most. Their responses may be surprising.
A study published in 2017 showed that complete resolution of even mild rosacea prolongs remission of rosacea, and most importantly, improves the quality of life for patients. “So, don’t discount what you consider to be mild rosacea in patients,” she said.
Skin care recommendations
“And don’t forget about basic skin care,” she advised. A recently published Chinese study of 999 rosacea patients and 1,010 controls with healthy skin found that a high frequency of cleansing and expansive use of cleansers were positively correlated with rosacea occurrence, suggesting that overcleansing can be a risk factor for rosacea. “Ask your patient, ‘how often are you cleaning your face?’ ” Dr. Graber suggested. “You might find that they’re overdoing it by washing three or four times a day. Several studies have shown that basic skin care alone improves rosacea.”
Skin care recommendations for patients with rosacea include avoiding chemical or physical exfoliants and alcohol-based topical products, and moisturizing and washing their faces with mild, synthetic detergent-based products rather than traditional soaps, which may further alkalinize and irritate the skin. “Patients should also be counseled to use physical-based sunscreens rather than chemical-based sunscreens,” she said.
Treating erythema
For treating erythema with topicals, a systematic review published in 2019 found the most evidence for brimonidine 0.33% gel, an alpha2-adrenergic agonist, and oxymetazoline 1% cream, an alpha1-adrenergic agonist. “Both of these products functionally constrict facial blood vessels,” and are Food and Drug Administration approved for treating persistent erythema, Dr. Graber said. “These products improve erythema within 3 hours of and up to 12 hours after application and overall, they are well tolerated.”
Based on clinical trial results, about 15% of patients on brimonidine report adverse reactions such as dermatitis, burning, pruritus, and erythema, compared with 8% of patients on oxymetazoline. At the same time, up to 20% of individuals on brimonidine report rebound erythema, compared with fewer than 1% of those using oxymetazoline. Laser and light therapies such as pulse-dye lasers, potassium-titanyl-phosphate lasers, and intense-pulse light devices are also effective in treating persistent erythema but are less effective for transient flushing.
Treatment of papules and pustules
For treating papules and pustules, the 2019 systemic review also found high-certainty evidence for using azelaic acid and topical ivermectin, and moderate-certainty evidence for using topical metronidazole and topical minocycline. “Topical ivermectin was demonstrated to be the most effective topical treatment for papulopustular rosacea and to provide the greatest psychological benefit to these patients,” Dr. Graber said.
In a double-blind, multicenter 15-week trial comparing azelaic acid 15% gel with metronidazole 0.75% gel in patients with papulopustular rosacea, both agents were found to be effective. But those treated with azelaic acid 15% gel had a greater reduction in lesion counts and erythema, and improvement in global assessments, compared with metronidazole 0.75% gel. However, the azelaic acid 15% gel was associated with more stinging compared with metronidazole 0.75% gel, although it was usually transient.
Another study, a double-blind, single-center, 15-week trial, compared the efficacy of azelaic acid 20% cream with metronidazole 0.75% cream. Both agents were found to be effective and had similar levels of reductions in papules and pustules. However, patients in the azelaic acid 20% cream arm had significantly higher physician ratings of global improvement, as well as overall higher patient satisfaction.
More recently, a phase 3 study of 962 patients found that ivermectin 1% cream once daily improved quality of life slightly more than metronidazole 0.75% cream twice daily. No difference in adverse events were noted between the two agents.
Other options for treating papules and pustules include topical minocycline 1.5% foam, which is FDA approved for rosacea, as well as second-line agents topical sodium sulfacetamide with sulfur cleanser (cream or lotion), and permethrin, Dr. Graber said.
As for treating papules and pustules with oral agents, the strongest evidence favors oral tetracyclines and isotretinoin, she noted.
Doxycycline, minocycline, tetracycline, and sarecycline can be used as monotherapy or coadministered with topical agents. “The addition of topical agents may also help to shorten the duration of antibiotic use, which is very important,” Dr. Graber said.
She noted that oral beta-blockers might be useful to treat persistent erythema and flushing because they antagonize the effects of sympathetic nerve stimulation and circulating catecholamines at b-adrenoceptors. Carvedilol and propranolol have been the most studied. The most common potential side effects are hypotension and bradycardia.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
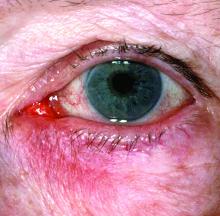
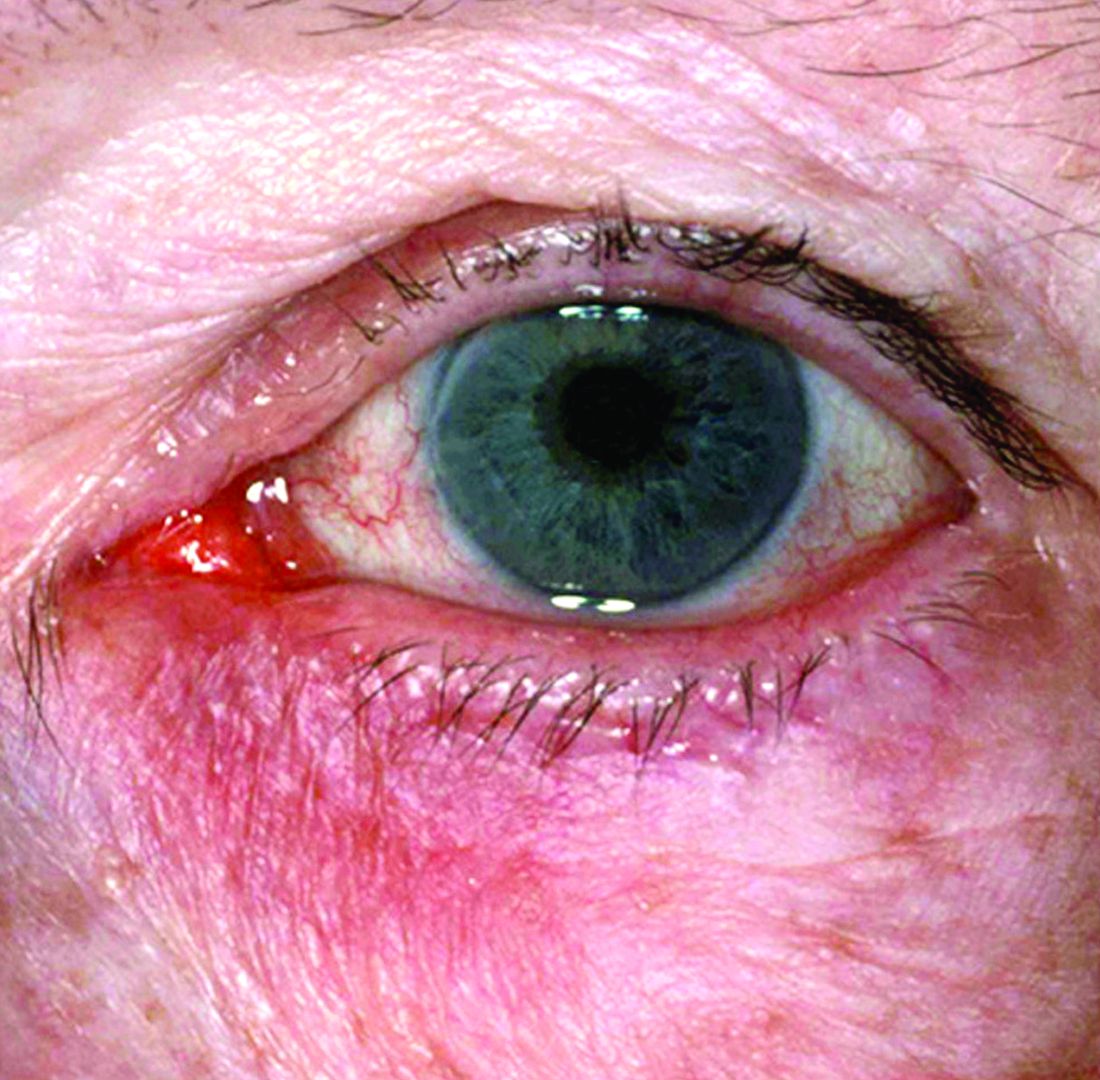
Microbiome studies among those awarded National Rosacea Society grants
A study on this year, as part of the organization’s research grants program.
The NRS research grants program was created to increase knowledge and understanding of not only the potential causes of rosacea, but other aspects of the disease that may inform prevention, treatment, or a potential cure, according to the press release announcing the recipients.
New research grant recipient Sezen Karakus, MD, of the Johns Hopkins Wilmer Eye Institute, Baltimore, received $15,000 for a study on the contribution of the ocular surface microbiome to the development of rosacea. Ocular rosacea can result in corneal complications severe enough to affect vision, and identifying the microorganisms on the ocular surface may lead to new treatment strategies, Dr. Karakus said in the release. He will collaborate on this research with dermatologist Noori Kim, MD, of Johns Hopkins University, Baltimore.
A second new research grant went to Emmanuel Contassot, MD, project leader in the dermatology department at of the University Hospital of Basel, Switzerland, who received $5,000 to investigate whether certain elevated intracellular signals in rosacea lesions may promote the skin inflammation that may be a root cause of the condition.
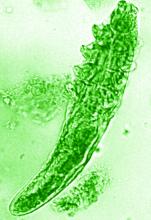
The NRS also renewed its support of a pair of ongoing studies. Michelle Trautwein, MD, of the Institute for Biodiversity Science and Sustainability at the California Academy of Sciences, continues her work on the first study to sequence the genome of Demodex mites; the study also identifies associated bacteria that may play a role in rosacea.
A second ongoing study by Tissa Hata, MD, of the University of California, San Diego, focuses on the normalization of the microbiome in people with rosacea. Dr. Hata’s work identifies types of bacteria associated with rosacea, as well as bacteria that may be associated with healthy skin after successful treatment of rosacea, including Cutibacterium acnes and Staphylococcus epidermidis.
The deadline to submit research proposals for next year’s grants is June 17, 2022. Researchers can find forms and instructions at the research grants section of the NRS website or by contacting the National Rosacea Society at 111 Lions Dr., Suite 216, Barrington, Ill., 60010, by telephone at 1-888-662-5874, or by email at [email protected].
A study on this year, as part of the organization’s research grants program.
The NRS research grants program was created to increase knowledge and understanding of not only the potential causes of rosacea, but other aspects of the disease that may inform prevention, treatment, or a potential cure, according to the press release announcing the recipients.
New research grant recipient Sezen Karakus, MD, of the Johns Hopkins Wilmer Eye Institute, Baltimore, received $15,000 for a study on the contribution of the ocular surface microbiome to the development of rosacea. Ocular rosacea can result in corneal complications severe enough to affect vision, and identifying the microorganisms on the ocular surface may lead to new treatment strategies, Dr. Karakus said in the release. He will collaborate on this research with dermatologist Noori Kim, MD, of Johns Hopkins University, Baltimore.
A second new research grant went to Emmanuel Contassot, MD, project leader in the dermatology department at of the University Hospital of Basel, Switzerland, who received $5,000 to investigate whether certain elevated intracellular signals in rosacea lesions may promote the skin inflammation that may be a root cause of the condition.
The NRS also renewed its support of a pair of ongoing studies. Michelle Trautwein, MD, of the Institute for Biodiversity Science and Sustainability at the California Academy of Sciences, continues her work on the first study to sequence the genome of Demodex mites; the study also identifies associated bacteria that may play a role in rosacea.
A second ongoing study by Tissa Hata, MD, of the University of California, San Diego, focuses on the normalization of the microbiome in people with rosacea. Dr. Hata’s work identifies types of bacteria associated with rosacea, as well as bacteria that may be associated with healthy skin after successful treatment of rosacea, including Cutibacterium acnes and Staphylococcus epidermidis.
The deadline to submit research proposals for next year’s grants is June 17, 2022. Researchers can find forms and instructions at the research grants section of the NRS website or by contacting the National Rosacea Society at 111 Lions Dr., Suite 216, Barrington, Ill., 60010, by telephone at 1-888-662-5874, or by email at [email protected].
A study on this year, as part of the organization’s research grants program.
The NRS research grants program was created to increase knowledge and understanding of not only the potential causes of rosacea, but other aspects of the disease that may inform prevention, treatment, or a potential cure, according to the press release announcing the recipients.
New research grant recipient Sezen Karakus, MD, of the Johns Hopkins Wilmer Eye Institute, Baltimore, received $15,000 for a study on the contribution of the ocular surface microbiome to the development of rosacea. Ocular rosacea can result in corneal complications severe enough to affect vision, and identifying the microorganisms on the ocular surface may lead to new treatment strategies, Dr. Karakus said in the release. He will collaborate on this research with dermatologist Noori Kim, MD, of Johns Hopkins University, Baltimore.
A second new research grant went to Emmanuel Contassot, MD, project leader in the dermatology department at of the University Hospital of Basel, Switzerland, who received $5,000 to investigate whether certain elevated intracellular signals in rosacea lesions may promote the skin inflammation that may be a root cause of the condition.
The NRS also renewed its support of a pair of ongoing studies. Michelle Trautwein, MD, of the Institute for Biodiversity Science and Sustainability at the California Academy of Sciences, continues her work on the first study to sequence the genome of Demodex mites; the study also identifies associated bacteria that may play a role in rosacea.
A second ongoing study by Tissa Hata, MD, of the University of California, San Diego, focuses on the normalization of the microbiome in people with rosacea. Dr. Hata’s work identifies types of bacteria associated with rosacea, as well as bacteria that may be associated with healthy skin after successful treatment of rosacea, including Cutibacterium acnes and Staphylococcus epidermidis.
The deadline to submit research proposals for next year’s grants is June 17, 2022. Researchers can find forms and instructions at the research grants section of the NRS website or by contacting the National Rosacea Society at 111 Lions Dr., Suite 216, Barrington, Ill., 60010, by telephone at 1-888-662-5874, or by email at [email protected].
COVID surge in Europe: A preview of what’s ahead for the U.S.?
Health experts are warning the United States could be headed for another COVID-19 surge just as we enter the holiday season, following a massive new wave of infections in Europe – a troubling pattern seen throughout the pandemic.
Eighteen months into the global health crisis that has killed 5.1 million people worldwide including more than 767,000 Americans, Europe has become the epicenter of the global health crisis once again.
And some infectious disease specialists say the United States may be next.
“It’s déjà vu, yet again,” says Eric Topol, M.D., founder and director of the Scripps Research Translational Institute. In a new analysis published in The Guardian, the professor of molecular medicine argues that it’s “wishful thinking” for U.S. authorities to believe the nation is “immune” to what’s happening in Europe.
Dr. Topol is also editor-in-chief of Medscape, MDedge’s sister site for medical professionals.
Three times over the past 18 months coronavirus surges in the United States followed similar spikes in Europe, where COVID-19 deaths grew by 10% this month.
Dr. Topol argues another wave may be in store for the states, as European countries implement new lockdowns. COVID-19 spikes are hitting some regions of the continent hard, including areas with high vaccination rates and strict control measures.
Eastern Europe and Russia, where vaccination rates are low, have experienced the worst of it. But even western countries, such as Germany, Austria and the United Kingdom, are reporting some of the highest daily infection figures in the world today.
Countries are responding in increasingly drastic ways.
In Russia, President Vladimir Putin ordered tens of thousands of workers to stay home earlier this month.
In the Dutch city of Utrecht, traditional Christmas celebrations have been canceled as the country is headed for a partial lockdown.
Austria announced a 20-day lockdown beginning Nov. 22 and on Nov. 19 leaders there announced that all 9 million residents will be required to be vaccinated by February. Leaders there are telling unvaccinated individuals to stay at home and out of restaurants, cafes, and other shops in hard-hit regions of the country.
And in Germany, where daily new-infection rates now stand at 50,000, officials have introduced stricter mask mandates and made proof of vaccination or past infection mandatory for entry to many venues. Berlin is also eyeing proposals to shut down the city’s traditional Christmas markets while authorities in Cologne have already called off holiday celebrations, after the ceremonial head of festivities tested positive for COVID-19. Bavaria canceled its popular Christmas markets and will order lockdowns in particularly vulnerable districts, while unvaccinated people will face serious restrictions on where they can go.
Former FDA Commissioner Scott Gottlieb, MD, says what’s happening across the European continent is troubling.
But he also believes it’s possible the United States may be better prepared to head off a similar surge this time around, with increased testing, vaccination and new therapies such as monoclonal antibodies, and antiviral therapeutics.
“Germany’s challenges are [a] caution to [the] world, the COVID pandemic isn’t over globally, won’t be for long time,” he says. “But [the] U.S. is further along than many other countries, in part because we already suffered more spread, in part because we’re making progress on vaccines, therapeutics, testing.”
Other experts agree the United States may not be as vulnerable to another wave of COVID-19 in coming weeks but have stopped short of suggesting we’re out of the woods.
“I don’t think that what we’re seeing in Europe necessarily means that we’re in for a huge surge of serious illness and death the way that we saw last year here in the states,” says David Dowdy, MD, PhD, an associate professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health and a general internist with Baltimore Medical Services.
“But I think anyone who says that they can predict the course of the pandemic for the next few months or few years has been proven wrong in the past and will probably be proven wrong in the future,” Dr. Dowdy says. “None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness.”
Looking back, and forward
What’s happening in Europe today mirrors past COVID-19 spikes that presaged big upticks in cases, hospitalizations, and deaths in the United States.
When the pandemic first hit Europe in March 2020, then-President Donald Trump downplayed the threat of the virus despite the warnings of his own advisors and independent public health experts who said COVID-19 could have dire impacts without an aggressive federal action plan.
By late spring the United States had become the epicenter of the pandemic, when case totals eclipsed those of other countries and New York City became a hot zone, according to data compiled by the Johns Hopkins Coronavirus Resource Center. Over the summer, spread of the disease slowed in New York, after tough control measures were instituted, but steadily increased in other states.
Then, later in the year, the Alpha variant of the virus took hold in the United Kingdom and the United States was again unprepared. By winter, the number of cases accelerated in every state in a major second surge that kept millions of Americans from traveling and gathering for the winter holidays.
With the rollout of COVID vaccines last December, cases in the United States – and in many parts of the world – began to fall. Some experts even suggested we’d turned a corner on the pandemic.
But then, last spring and summer, the Delta variant popped up in India and spread to the United Kingdom in a third major wave of COVID. Once again, the United States was unprepared, with 4 in 10 Americans refusing the vaccine and even some vaccinated individuals succumbing to breakthrough Delta infections.
The resulting Delta surge swept the country, preventing many businesses and schools from fully reopening and stressing hospitals in some areas of the country – particularly southern states – with new influxes of COVID-19 patients.
Now, Europe is facing another rise in COVID, with about 350 cases per 100,000 people and many countries hitting new record highs.
What’s driving the European resurgence?
So, what’s behind the new COVID-19 wave in Europe and what might it mean for the United States?
Shaun Truelove, PhD, an infectious disease epidemiologist and faculty member of the Johns Hopkins School of Public Health, says experts are examining several likely factors:
Waning immunity from the vaccines. Data from Johns Hopkins shows infections rising in nations with lower vaccination rates.
The impact of the Delta variant, which is three times more transmissible than the original virus and can even sicken some vaccinated individuals.
The spread of COVID-19 among teens and children; the easing of precautions (such as masking and social distancing); differences in the types of vaccines used in European nations and the United States.
“These are all possibilities,” says Dr. Truelove. “There are so many factors and so it’s difficult to pinpoint exactly what’s driving it and what effect each of those things might be having.”
As a result, it’s difficult to predict and prepare for what might lie ahead for the United States, he says.
“There’s a ton of uncertainty and we’re trying to understand what’s going to happen here over the next 6 months,” he says.
Even so, Dr. Truelove adds that what’s happening overseas might not be “super predictive” of a new wave of COVID in the United States.
For one thing, he says, the Pfizer and Moderna vaccines, the two mRNA vaccines used predominantly in the United States, are far more effective – 94-95% – than the Oxford/AstraZeneca COVID shot (63%) widely administered across Europe.
Secondly, European countries have imposed much stronger and stricter control measures throughout the pandemic than the United States. That might actually be driving the new surges because fewer unvaccinated people have been exposed to the virus, which means they have lower “natural immunity” from prior COVID infection.
Dr. Truelove explains: “Stronger and stricter control measures … have the consequence of leaving a lot more susceptible individuals in the population, [because] the stronger the controls, the fewer people get infected. And so, you have more individuals remaining in the population who are more susceptible and at risk of getting infected in the future.”
By contrast, he notes, a “large chunk” of the United States has not put strict lockdowns in place.
“So, what we’ve seen over the past couple months with the Delta wave is that in a lot of those states with lower vaccination coverage and lower controls this virus has really burned through a lot of the susceptible population. As a result, we’re seeing the curves coming down and what really looks like a lot of the built-up immunity in these states, especially southern states.”
But whether these differences will be enough for the United States to dodge another COVID-19 bullet this winter is uncertain.
“I don’t want to say that the [Europe] surge is NOT a predictor of what might come in the U.S., because I think that it very well could be,” Dr. Truelove says. “And so, people need to be aware of that, and be cautious and be sure get their vaccines and everything else.
“But I’m hopeful that because of some of the differences that maybe we’ll have a little bit of a different situation.”
The takeaway: How best to prepare?
Dr. Dowdy agrees that Europe’s current troubles might not necessarily mean a major new winter surge in the United States.
But he also points out that cases are beginning to head up again in New England, the Midwest, and other regions of the country that are just experiencing the first chill of winter.
“After reaching a low point about 3 weeks ago, cases due to COVID-19 have started to rise again in the United States,” he says. “Cases were falling consistently until mid-October, but over the last 3 weeks, cases have started to rise again in most states.
“Cases in Eastern and Central Europe have more than doubled during that time, meaning that the possibility of a winter surge here is very real.”
Even so, Dr. Dowdy believes the rising rates of vaccination could limit the number of Americans who will be hospitalized with severe disease or die this winter.
Still, he warns against being too optimistic, as Americans travel and get together for the winter holidays.
None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness, Dr. Dowdy says.”
The upshot?
“People need to realize that it’s not quite over,” Dr. Truelove says. “We still have a substantial amount of infection in our country. We’re still above 200 cases per million [and] 500,000 incident cases per week or so. That’s a lot of death and a lot of hospitalizations. So, we still have to be concerned and do our best to reduce transmission … by wearing masks, getting vaccinated, getting a booster shot, and getting your children vaccinated.”
Johns Hopkins social and behavioral scientist Rupali Limaye, PhD, MPH, adds that while COVID vaccines have been a “game changer” in the pandemic, more than a third of Americans have yet to receive one.
“That’s really what we need to be messaging around -- that people can still get COVID, there can still be breakthrough infections,” says Dr. Limaye, a health communications scholar. “But the great news is if you have been vaccinated, you are very much less likely, I think it’s 12 times, to be hospitalized or have severe COVID compared to those that are un-vaccinated.”
Dr. Topol agrees, adding: “Now is the time for the U.S. to heed the European signal for the first time, to pull out all the stops. Promote primary vaccination and boosters like there’s no tomorrow. Aggressively counter the pervasive misinformation and disinformation. Accelerate and expand the vaccine mandates ...
“Instead of succumbing to yet another major rise in cases and their sequelae, this is a chance for America to finally rise to the occasion, showing an ability to lead and execute.”
A version of this article first appeared on WebMD.com.
Health experts are warning the United States could be headed for another COVID-19 surge just as we enter the holiday season, following a massive new wave of infections in Europe – a troubling pattern seen throughout the pandemic.
Eighteen months into the global health crisis that has killed 5.1 million people worldwide including more than 767,000 Americans, Europe has become the epicenter of the global health crisis once again.
And some infectious disease specialists say the United States may be next.
“It’s déjà vu, yet again,” says Eric Topol, M.D., founder and director of the Scripps Research Translational Institute. In a new analysis published in The Guardian, the professor of molecular medicine argues that it’s “wishful thinking” for U.S. authorities to believe the nation is “immune” to what’s happening in Europe.
Dr. Topol is also editor-in-chief of Medscape, MDedge’s sister site for medical professionals.
Three times over the past 18 months coronavirus surges in the United States followed similar spikes in Europe, where COVID-19 deaths grew by 10% this month.
Dr. Topol argues another wave may be in store for the states, as European countries implement new lockdowns. COVID-19 spikes are hitting some regions of the continent hard, including areas with high vaccination rates and strict control measures.
Eastern Europe and Russia, where vaccination rates are low, have experienced the worst of it. But even western countries, such as Germany, Austria and the United Kingdom, are reporting some of the highest daily infection figures in the world today.
Countries are responding in increasingly drastic ways.
In Russia, President Vladimir Putin ordered tens of thousands of workers to stay home earlier this month.
In the Dutch city of Utrecht, traditional Christmas celebrations have been canceled as the country is headed for a partial lockdown.
Austria announced a 20-day lockdown beginning Nov. 22 and on Nov. 19 leaders there announced that all 9 million residents will be required to be vaccinated by February. Leaders there are telling unvaccinated individuals to stay at home and out of restaurants, cafes, and other shops in hard-hit regions of the country.
And in Germany, where daily new-infection rates now stand at 50,000, officials have introduced stricter mask mandates and made proof of vaccination or past infection mandatory for entry to many venues. Berlin is also eyeing proposals to shut down the city’s traditional Christmas markets while authorities in Cologne have already called off holiday celebrations, after the ceremonial head of festivities tested positive for COVID-19. Bavaria canceled its popular Christmas markets and will order lockdowns in particularly vulnerable districts, while unvaccinated people will face serious restrictions on where they can go.
Former FDA Commissioner Scott Gottlieb, MD, says what’s happening across the European continent is troubling.
But he also believes it’s possible the United States may be better prepared to head off a similar surge this time around, with increased testing, vaccination and new therapies such as monoclonal antibodies, and antiviral therapeutics.
“Germany’s challenges are [a] caution to [the] world, the COVID pandemic isn’t over globally, won’t be for long time,” he says. “But [the] U.S. is further along than many other countries, in part because we already suffered more spread, in part because we’re making progress on vaccines, therapeutics, testing.”
Other experts agree the United States may not be as vulnerable to another wave of COVID-19 in coming weeks but have stopped short of suggesting we’re out of the woods.
“I don’t think that what we’re seeing in Europe necessarily means that we’re in for a huge surge of serious illness and death the way that we saw last year here in the states,” says David Dowdy, MD, PhD, an associate professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health and a general internist with Baltimore Medical Services.
“But I think anyone who says that they can predict the course of the pandemic for the next few months or few years has been proven wrong in the past and will probably be proven wrong in the future,” Dr. Dowdy says. “None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness.”
Looking back, and forward
What’s happening in Europe today mirrors past COVID-19 spikes that presaged big upticks in cases, hospitalizations, and deaths in the United States.
When the pandemic first hit Europe in March 2020, then-President Donald Trump downplayed the threat of the virus despite the warnings of his own advisors and independent public health experts who said COVID-19 could have dire impacts without an aggressive federal action plan.
By late spring the United States had become the epicenter of the pandemic, when case totals eclipsed those of other countries and New York City became a hot zone, according to data compiled by the Johns Hopkins Coronavirus Resource Center. Over the summer, spread of the disease slowed in New York, after tough control measures were instituted, but steadily increased in other states.
Then, later in the year, the Alpha variant of the virus took hold in the United Kingdom and the United States was again unprepared. By winter, the number of cases accelerated in every state in a major second surge that kept millions of Americans from traveling and gathering for the winter holidays.
With the rollout of COVID vaccines last December, cases in the United States – and in many parts of the world – began to fall. Some experts even suggested we’d turned a corner on the pandemic.
But then, last spring and summer, the Delta variant popped up in India and spread to the United Kingdom in a third major wave of COVID. Once again, the United States was unprepared, with 4 in 10 Americans refusing the vaccine and even some vaccinated individuals succumbing to breakthrough Delta infections.
The resulting Delta surge swept the country, preventing many businesses and schools from fully reopening and stressing hospitals in some areas of the country – particularly southern states – with new influxes of COVID-19 patients.
Now, Europe is facing another rise in COVID, with about 350 cases per 100,000 people and many countries hitting new record highs.
What’s driving the European resurgence?
So, what’s behind the new COVID-19 wave in Europe and what might it mean for the United States?
Shaun Truelove, PhD, an infectious disease epidemiologist and faculty member of the Johns Hopkins School of Public Health, says experts are examining several likely factors:
Waning immunity from the vaccines. Data from Johns Hopkins shows infections rising in nations with lower vaccination rates.
The impact of the Delta variant, which is three times more transmissible than the original virus and can even sicken some vaccinated individuals.
The spread of COVID-19 among teens and children; the easing of precautions (such as masking and social distancing); differences in the types of vaccines used in European nations and the United States.
“These are all possibilities,” says Dr. Truelove. “There are so many factors and so it’s difficult to pinpoint exactly what’s driving it and what effect each of those things might be having.”
As a result, it’s difficult to predict and prepare for what might lie ahead for the United States, he says.
“There’s a ton of uncertainty and we’re trying to understand what’s going to happen here over the next 6 months,” he says.
Even so, Dr. Truelove adds that what’s happening overseas might not be “super predictive” of a new wave of COVID in the United States.
For one thing, he says, the Pfizer and Moderna vaccines, the two mRNA vaccines used predominantly in the United States, are far more effective – 94-95% – than the Oxford/AstraZeneca COVID shot (63%) widely administered across Europe.
Secondly, European countries have imposed much stronger and stricter control measures throughout the pandemic than the United States. That might actually be driving the new surges because fewer unvaccinated people have been exposed to the virus, which means they have lower “natural immunity” from prior COVID infection.
Dr. Truelove explains: “Stronger and stricter control measures … have the consequence of leaving a lot more susceptible individuals in the population, [because] the stronger the controls, the fewer people get infected. And so, you have more individuals remaining in the population who are more susceptible and at risk of getting infected in the future.”
By contrast, he notes, a “large chunk” of the United States has not put strict lockdowns in place.
“So, what we’ve seen over the past couple months with the Delta wave is that in a lot of those states with lower vaccination coverage and lower controls this virus has really burned through a lot of the susceptible population. As a result, we’re seeing the curves coming down and what really looks like a lot of the built-up immunity in these states, especially southern states.”
But whether these differences will be enough for the United States to dodge another COVID-19 bullet this winter is uncertain.
“I don’t want to say that the [Europe] surge is NOT a predictor of what might come in the U.S., because I think that it very well could be,” Dr. Truelove says. “And so, people need to be aware of that, and be cautious and be sure get their vaccines and everything else.
“But I’m hopeful that because of some of the differences that maybe we’ll have a little bit of a different situation.”
The takeaway: How best to prepare?
Dr. Dowdy agrees that Europe’s current troubles might not necessarily mean a major new winter surge in the United States.
But he also points out that cases are beginning to head up again in New England, the Midwest, and other regions of the country that are just experiencing the first chill of winter.
“After reaching a low point about 3 weeks ago, cases due to COVID-19 have started to rise again in the United States,” he says. “Cases were falling consistently until mid-October, but over the last 3 weeks, cases have started to rise again in most states.
“Cases in Eastern and Central Europe have more than doubled during that time, meaning that the possibility of a winter surge here is very real.”
Even so, Dr. Dowdy believes the rising rates of vaccination could limit the number of Americans who will be hospitalized with severe disease or die this winter.
Still, he warns against being too optimistic, as Americans travel and get together for the winter holidays.
None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness, Dr. Dowdy says.”
The upshot?
“People need to realize that it’s not quite over,” Dr. Truelove says. “We still have a substantial amount of infection in our country. We’re still above 200 cases per million [and] 500,000 incident cases per week or so. That’s a lot of death and a lot of hospitalizations. So, we still have to be concerned and do our best to reduce transmission … by wearing masks, getting vaccinated, getting a booster shot, and getting your children vaccinated.”
Johns Hopkins social and behavioral scientist Rupali Limaye, PhD, MPH, adds that while COVID vaccines have been a “game changer” in the pandemic, more than a third of Americans have yet to receive one.
“That’s really what we need to be messaging around -- that people can still get COVID, there can still be breakthrough infections,” says Dr. Limaye, a health communications scholar. “But the great news is if you have been vaccinated, you are very much less likely, I think it’s 12 times, to be hospitalized or have severe COVID compared to those that are un-vaccinated.”
Dr. Topol agrees, adding: “Now is the time for the U.S. to heed the European signal for the first time, to pull out all the stops. Promote primary vaccination and boosters like there’s no tomorrow. Aggressively counter the pervasive misinformation and disinformation. Accelerate and expand the vaccine mandates ...
“Instead of succumbing to yet another major rise in cases and their sequelae, this is a chance for America to finally rise to the occasion, showing an ability to lead and execute.”
A version of this article first appeared on WebMD.com.
Health experts are warning the United States could be headed for another COVID-19 surge just as we enter the holiday season, following a massive new wave of infections in Europe – a troubling pattern seen throughout the pandemic.
Eighteen months into the global health crisis that has killed 5.1 million people worldwide including more than 767,000 Americans, Europe has become the epicenter of the global health crisis once again.
And some infectious disease specialists say the United States may be next.
“It’s déjà vu, yet again,” says Eric Topol, M.D., founder and director of the Scripps Research Translational Institute. In a new analysis published in The Guardian, the professor of molecular medicine argues that it’s “wishful thinking” for U.S. authorities to believe the nation is “immune” to what’s happening in Europe.
Dr. Topol is also editor-in-chief of Medscape, MDedge’s sister site for medical professionals.
Three times over the past 18 months coronavirus surges in the United States followed similar spikes in Europe, where COVID-19 deaths grew by 10% this month.
Dr. Topol argues another wave may be in store for the states, as European countries implement new lockdowns. COVID-19 spikes are hitting some regions of the continent hard, including areas with high vaccination rates and strict control measures.
Eastern Europe and Russia, where vaccination rates are low, have experienced the worst of it. But even western countries, such as Germany, Austria and the United Kingdom, are reporting some of the highest daily infection figures in the world today.
Countries are responding in increasingly drastic ways.
In Russia, President Vladimir Putin ordered tens of thousands of workers to stay home earlier this month.
In the Dutch city of Utrecht, traditional Christmas celebrations have been canceled as the country is headed for a partial lockdown.
Austria announced a 20-day lockdown beginning Nov. 22 and on Nov. 19 leaders there announced that all 9 million residents will be required to be vaccinated by February. Leaders there are telling unvaccinated individuals to stay at home and out of restaurants, cafes, and other shops in hard-hit regions of the country.
And in Germany, where daily new-infection rates now stand at 50,000, officials have introduced stricter mask mandates and made proof of vaccination or past infection mandatory for entry to many venues. Berlin is also eyeing proposals to shut down the city’s traditional Christmas markets while authorities in Cologne have already called off holiday celebrations, after the ceremonial head of festivities tested positive for COVID-19. Bavaria canceled its popular Christmas markets and will order lockdowns in particularly vulnerable districts, while unvaccinated people will face serious restrictions on where they can go.
Former FDA Commissioner Scott Gottlieb, MD, says what’s happening across the European continent is troubling.
But he also believes it’s possible the United States may be better prepared to head off a similar surge this time around, with increased testing, vaccination and new therapies such as monoclonal antibodies, and antiviral therapeutics.
“Germany’s challenges are [a] caution to [the] world, the COVID pandemic isn’t over globally, won’t be for long time,” he says. “But [the] U.S. is further along than many other countries, in part because we already suffered more spread, in part because we’re making progress on vaccines, therapeutics, testing.”
Other experts agree the United States may not be as vulnerable to another wave of COVID-19 in coming weeks but have stopped short of suggesting we’re out of the woods.
“I don’t think that what we’re seeing in Europe necessarily means that we’re in for a huge surge of serious illness and death the way that we saw last year here in the states,” says David Dowdy, MD, PhD, an associate professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health and a general internist with Baltimore Medical Services.
“But I think anyone who says that they can predict the course of the pandemic for the next few months or few years has been proven wrong in the past and will probably be proven wrong in the future,” Dr. Dowdy says. “None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness.”
Looking back, and forward
What’s happening in Europe today mirrors past COVID-19 spikes that presaged big upticks in cases, hospitalizations, and deaths in the United States.
When the pandemic first hit Europe in March 2020, then-President Donald Trump downplayed the threat of the virus despite the warnings of his own advisors and independent public health experts who said COVID-19 could have dire impacts without an aggressive federal action plan.
By late spring the United States had become the epicenter of the pandemic, when case totals eclipsed those of other countries and New York City became a hot zone, according to data compiled by the Johns Hopkins Coronavirus Resource Center. Over the summer, spread of the disease slowed in New York, after tough control measures were instituted, but steadily increased in other states.
Then, later in the year, the Alpha variant of the virus took hold in the United Kingdom and the United States was again unprepared. By winter, the number of cases accelerated in every state in a major second surge that kept millions of Americans from traveling and gathering for the winter holidays.
With the rollout of COVID vaccines last December, cases in the United States – and in many parts of the world – began to fall. Some experts even suggested we’d turned a corner on the pandemic.
But then, last spring and summer, the Delta variant popped up in India and spread to the United Kingdom in a third major wave of COVID. Once again, the United States was unprepared, with 4 in 10 Americans refusing the vaccine and even some vaccinated individuals succumbing to breakthrough Delta infections.
The resulting Delta surge swept the country, preventing many businesses and schools from fully reopening and stressing hospitals in some areas of the country – particularly southern states – with new influxes of COVID-19 patients.
Now, Europe is facing another rise in COVID, with about 350 cases per 100,000 people and many countries hitting new record highs.
What’s driving the European resurgence?
So, what’s behind the new COVID-19 wave in Europe and what might it mean for the United States?
Shaun Truelove, PhD, an infectious disease epidemiologist and faculty member of the Johns Hopkins School of Public Health, says experts are examining several likely factors:
Waning immunity from the vaccines. Data from Johns Hopkins shows infections rising in nations with lower vaccination rates.
The impact of the Delta variant, which is three times more transmissible than the original virus and can even sicken some vaccinated individuals.
The spread of COVID-19 among teens and children; the easing of precautions (such as masking and social distancing); differences in the types of vaccines used in European nations and the United States.
“These are all possibilities,” says Dr. Truelove. “There are so many factors and so it’s difficult to pinpoint exactly what’s driving it and what effect each of those things might be having.”
As a result, it’s difficult to predict and prepare for what might lie ahead for the United States, he says.
“There’s a ton of uncertainty and we’re trying to understand what’s going to happen here over the next 6 months,” he says.
Even so, Dr. Truelove adds that what’s happening overseas might not be “super predictive” of a new wave of COVID in the United States.
For one thing, he says, the Pfizer and Moderna vaccines, the two mRNA vaccines used predominantly in the United States, are far more effective – 94-95% – than the Oxford/AstraZeneca COVID shot (63%) widely administered across Europe.
Secondly, European countries have imposed much stronger and stricter control measures throughout the pandemic than the United States. That might actually be driving the new surges because fewer unvaccinated people have been exposed to the virus, which means they have lower “natural immunity” from prior COVID infection.
Dr. Truelove explains: “Stronger and stricter control measures … have the consequence of leaving a lot more susceptible individuals in the population, [because] the stronger the controls, the fewer people get infected. And so, you have more individuals remaining in the population who are more susceptible and at risk of getting infected in the future.”
By contrast, he notes, a “large chunk” of the United States has not put strict lockdowns in place.
“So, what we’ve seen over the past couple months with the Delta wave is that in a lot of those states with lower vaccination coverage and lower controls this virus has really burned through a lot of the susceptible population. As a result, we’re seeing the curves coming down and what really looks like a lot of the built-up immunity in these states, especially southern states.”
But whether these differences will be enough for the United States to dodge another COVID-19 bullet this winter is uncertain.
“I don’t want to say that the [Europe] surge is NOT a predictor of what might come in the U.S., because I think that it very well could be,” Dr. Truelove says. “And so, people need to be aware of that, and be cautious and be sure get their vaccines and everything else.
“But I’m hopeful that because of some of the differences that maybe we’ll have a little bit of a different situation.”
The takeaway: How best to prepare?
Dr. Dowdy agrees that Europe’s current troubles might not necessarily mean a major new winter surge in the United States.
But he also points out that cases are beginning to head up again in New England, the Midwest, and other regions of the country that are just experiencing the first chill of winter.
“After reaching a low point about 3 weeks ago, cases due to COVID-19 have started to rise again in the United States,” he says. “Cases were falling consistently until mid-October, but over the last 3 weeks, cases have started to rise again in most states.
“Cases in Eastern and Central Europe have more than doubled during that time, meaning that the possibility of a winter surge here is very real.”
Even so, Dr. Dowdy believes the rising rates of vaccination could limit the number of Americans who will be hospitalized with severe disease or die this winter.
Still, he warns against being too optimistic, as Americans travel and get together for the winter holidays.
None of us knows the future of this pandemic, but I do think that we are in for an increase of cases, not necessarily of deaths and serious illness, Dr. Dowdy says.”
The upshot?
“People need to realize that it’s not quite over,” Dr. Truelove says. “We still have a substantial amount of infection in our country. We’re still above 200 cases per million [and] 500,000 incident cases per week or so. That’s a lot of death and a lot of hospitalizations. So, we still have to be concerned and do our best to reduce transmission … by wearing masks, getting vaccinated, getting a booster shot, and getting your children vaccinated.”
Johns Hopkins social and behavioral scientist Rupali Limaye, PhD, MPH, adds that while COVID vaccines have been a “game changer” in the pandemic, more than a third of Americans have yet to receive one.
“That’s really what we need to be messaging around -- that people can still get COVID, there can still be breakthrough infections,” says Dr. Limaye, a health communications scholar. “But the great news is if you have been vaccinated, you are very much less likely, I think it’s 12 times, to be hospitalized or have severe COVID compared to those that are un-vaccinated.”
Dr. Topol agrees, adding: “Now is the time for the U.S. to heed the European signal for the first time, to pull out all the stops. Promote primary vaccination and boosters like there’s no tomorrow. Aggressively counter the pervasive misinformation and disinformation. Accelerate and expand the vaccine mandates ...
“Instead of succumbing to yet another major rise in cases and their sequelae, this is a chance for America to finally rise to the occasion, showing an ability to lead and execute.”
A version of this article first appeared on WebMD.com.
Topical options for acne patients continue to expand
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
yet they are likely underused in today’s clinical practice.
A study of prescribing practices from 2012 to 2014 indicated that dermatologists prescribed retinoids for just 58.8% of acne cases, while nondermatologists prescribed them for only 32.4% of cases. “If the guidelines are telling us that we should use topical retinoids for almost all of our acne patients, why are we using them for half of the patients?” Emmy Graber, MD, MBA, asked during MedscapeLive’s annual Las Vegas Dermatology Seminar. “We have a lot of options today for topical retinoids,” she added, noting that, in the past few years, trifarotene cream 0.005% and new formulations of tazarotene lotion (0.045%) and tretinoin lotion (0.05%) have become available.
According to Dr. Graber, president of The Dermatology Institute of Boston, tazarotene has been considered the most efficacious topical retinoid but is generally the least well tolerated, while adapalene has often been considered to be one of the better-tolerated topical retinoids. “This is a broad generalization,” she said. “One should also take into account the concentration and formulation of the retinoid. Cutaneous adverse events increase in severity as the concentration increases regardless of the vehicle.” There are no studies comparing trifarotene with other topical retinoids, she added.
In two phase 2, double-blind, vehicle-controlled studies (PERFECT 1 and PERFECT 2), researchers randomized more than 2,400 patients with moderate facial or truncal acne to receive trifarotene cream or a vehicle for 12 weeks. The mean percent change from baseline in facial inflammatory lesions in the trifarotene-treated group was –54.4% and –66.2% in PERFECT 1, and PERFECT 2, respectively, while the mean percent change from baseline in facial noninflammatory lesions was –49.7% and –57.7%, respectively.
In addition, the mean percent change from baseline in truncal inflammatory lesions in the trifarotene-treated groups was –57.4% and –65.4%, respectively, while the mean percent change from baseline in truncal noninflammatory lesions was –49.1% and –55.2%, respectively.
The choice of vehicle may affect absorption of topical retinoids, and some formulations may increase skin hydration and decrease transepidermal water loss, “which is a good thing,” Dr. Graber said. “Also, vehicles aim to slow drug delivery over time while also making sure that the drug penetrates into the pilosebaceous unit.”
One recent advance is the honeycomb-like polymeric emulsion technology found in tretinoin 0.05% lotion and tazarotene 0.045% lotion. These formulations contain droplets of the tretinoin and tazarotene embedded in a honeycomb matrix with hydrating agents. “I think this is exciting and could enhance our patient compliance and tolerability,” she said. Another unique feature about these two products, especially the tretinoin product, is the very small particle size with this new formulation. “It’s small enough that it can penetrate down into the pilosebaceous unit,” which is different than with older formulations, in which the tretinoin “largely just sat on the surface of the skin and didn’t penetrate into the pilosebaceous unit.” In addition, she said, “there’s only 9% degradation of the tretinoin in UV light, compared to 72% degradation of standard tretinoin 0.025% gel, and with the new tretinoin formulation, there’s no degradation when used with benzoyl peroxide.”
Another new topical retinoid to consider is a fixed-dose combination of encapsulated benzoyl peroxide 3% and encapsulated tretinoin 0.1% cream (Twyneo), which was approved by the Food and Drug Administration in July 2021 for the treatment of acne in adults and children aged 9 years and older. “Typically, benzoyl peroxide and tretinoin cannot be mixed in the same tube to stability issues,” she said. “Here, each product is individually encapsulated in a silica shell so that they can be applied together.”
The approval was supported by positive results from two phase 3, randomized, double-blind, vehicle-controlled, multicenter studies (NCT03761784 and NCT03761810), in which Twyneo demonstrated efficacy and a favorable tolerability profile in patients aged 9 years and older with facial acne.
Another topical treatment option, dapsone, is now FDA approved for ages 9 and up, expanded from its initial indication for ages 12 and up. The new indication is based on a phase 4, multicenter, open-label study in which acne patients aged 9-11 years applied dapsone 7.5% gel once daily to the face and acne-affected areas on the upper chest, upper back, and shoulders for 12 weeks. After 12 weeks, facial acne was clear or almost clear in about 47% of patients. “Inflammatory, noninflammatory, and total lesions decreased from baseline, but there was a greater reduction in noninflammatory lesions, so if you have a very young patient with acne, now you can consider dapsone gel,” Dr. Graber said.
In August 2020, clascoterone cream became the first topical androgen receptor inhibitor approved for the treatment of acne in patients 12 years of age and older. It is a drug believed to address sebum and inflammation directly in the sebaceous gland and is structurally similar to dihydrotestosterone and spironolactone.
“This is a completely new drug category in acne,” she said. “Unlike all oral antiandrogen therapies, clascoterone cream can be used in both males and females with acne. It’s the first acne drug to have a new mechanism of action in almost 40 years, since isotretinoin was approved in 1982.”
In vitro, she continued, clascoterone competes with dihydrotestosterone for binding to the androgen receptor, inhibiting downstream signaling and leading to inhibited sebum production, reduced secretion of inflammatory cytokines, and inhibition of inflammatory pathways. Two phase 3 studies that led to its approval involved 1,440 patients with moderate to severe facial acne aged 9-58 years. The cream was applied twice a day for 12 weeks and treatment adherence was approximately 90%. The researchers found that clascoterone cream was significantly more effective than vehicle cream at achieving Investigator’s Global Assessment scores of 0 (clear) or 1 (almost clear), the definition of treatment success in the study, and reducing noninflammatory lesion and inflammatory lesion counts at week 12. “There were no safety issues noted during these studies, and clascoterone cream was well tolerated,” Dr. Graber said.
Dr. Graber disclosed that she is a consultant/adviser for Digital Diagnostics, Almirall, Hovione, Keratin Biosciences, La Roche Posay, Ortho Dermatologics, Sebacia, Sol-Gel, Verrica, and WebMD. She is also a research investigator for Hovione, Ortho Dermatologics, Sebacia, and she receives royalties from Wolters Kluwer Health.
MedscapeLive and this news organization are owned by the same parent company.
Commentary by Lawrence W. Eichenfield, MD
Acne vulgaris remains an issue of tremendous importance to preteens, teens, and young adults, with approximately 85% of individuals aged 12-24 being affected. Expanding options for topical treatments may help bring effective disease control. Dr. Graber pointed out that historically, pediatricians and other primary care practitioners utilize topical retinoids less often for acne care as compared with dermatologists or guidelines recommendations (either the AAP’s or AAD’s). There are now expanded options, including over-the-counter retinoids (adapalene 0.1% gel), generic and trade brand topical tretinoin products, prescription adapalene medications, older and recently approved tazarotene products, and a newer type of topical retinoid, trifarotene. Novel formulations and emulsion technology, as well as retinoid developed in combination products, give more options in patients down to 9 years of age. A novel topical anti-androgen, clascoterone, is in its own category, as the first topical “hormonal agent,” allowing hormonal therapy to be used for males as well as females (aged 12 years and up). A recent review in JAMA (2021 Nov 23;326[20]:2055-67) incorporates many of these newer medications into management suggestions, emphasizing that first-line therapies are topical retinoids, benzoyl peroxide, azelaic acid, or combinations of topicals, whereas in more severe disease, oral antibiotics such as doxycycline or minocycline, hormonal therapies such as combination oral conceptive agents or spironolactone, or isotretinoin are most effective.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Patient whips out smartphone and starts recording: Trouble ahead?
Joe Lindsey, a 48-year old Colorado-based journalist, has dealt with complex hearing loss for about 15 years. which has led to countless doctor’s visits, treatments, and even surgery in hopes of finding improvement. As time went on and Mr. Lindsey’s hearing deteriorated, he began recording his appointments in order to retain important information.
Mr. Lindsey had positive intentions, but not every patient does.
With smartphones everywhere, recording medical appointments can be fraught with downsides too. While there are clear-cut reasons for recording doctor visits, patients’ goals and how they carry out the taping are key. Audio only? Or also video? With the physician’s knowledge and permission, or without?
These are the legal and ethical weeds doctors find themselves in today, so it’s important to understand all sides of the issue.
The medical world is divided on its sentiments about patients recording their visits. The American Medical Association, in fact, failed to make progress on a recent policy (resolution 007) proposal to encourage that any “audio or video recording made during a medical encounter should require both physician and patient notification and consent.” Rather than voting on the resolution, the AMA house of delegates tabled it and chose to gather more information on the issue.
In most cases, patients are recording their visits in good faith, says Jeffrey Segal, MD, JD, the CEO and founder of Medical Justice, a risk mitigation and reputation management firm for healthcare clinicians. “When it comes to ‘Team, let’s record this,’ I’m a fan,” he says. “The most common reason patients record visits is that there’s a lot of information transferred from the doctor to the patient, and there’s just not enough time to absorb it all.”
While the option is there for patients to take notes, in the give-and-take nature of conversation, this can get difficult. “If they record the visit, they can then digest it all down the road,” says Dr. Segal. “A compliant patient is one who understands what’s expected. That’s the charitable explanation for recording, and I support it.”
It’s that question of good intent, however, that concerns some physicians in today’s highly litigious society. “The worry is that there’s a small subset of patients with an ulterior motive,” says Dr. Segal.
“Some patients do record in case of an event down the road,” he adds. “They want the recording to potentially talk to a lawyer, or to file a board complaint.”
Laws in the United States surrounding recordings are confusing, with variations from state to state. Currently, 39 U.S. states allow for one-party consent — meaning a patient can record a visit without consenting with the physician.
Monica Verduzco-Gutierrez, MD, professor and chair of rehabilitation medicine at University of Texas Health, San Antonio, resides in Texas, which is one of the 39 one-consent states. “Physicians must be aware of this fact and consider how it might be used against them,” she says. “A good practice is to set expectations with the patient from the start. Also, know your hospital’s policy — some may have boundaries surrounding recordings.”
The first step is to know what type of state you practice in. Regardless of whether you are in a one- or two-party consent state — but especially a one-party state — it’s a smart move to add a sign at your office saying that you support the recording of visits, provided the patient is open and transparent about it. “Let the patient know that if they plan to record, they should ask your permission,” says Dr. Segal. “Let them know it’s not appropriate if they haven’t received your permission.”
There are, of course, the occasional horror stories involving surreptitious recordings. “I remember a case where a patient left a phone actively recording in his bag of clothing, which went into the OR with him,” he says. “The background conversation was not flattering to the patient, who happened to be an employee of the hospital. When he came to and listened to the recording, he sued, winning his case.”
The age of video and telehealth
What about the rare situation when a patient pulls out a phone and begins to videotape a conversation? It can be a big slippery slope. “Patients can abuse a video recording with editing, and the recording becomes one-dimensional, which is unfair to the physician,” adds Dr. Segal.
Patients sometimes have other motives as well. “I’m aware of occasions where a doctor/patient visit got heated and the patient took out the phone to video record, sharing it to social media,” says Dr. Segal. “Once someone uses a phone to take video, just stop the conversation. Tell the patient, ‘We’re having a disagreement,’ and that it’s time to put an end to it.”
He adds that from the physician side, a video can be a protagonist in a conversation. “Frankly, a camera on your face changes the nature of things,” Dr. Segal says. “It’s much easier to have the phone sitting in a corner, quietly recording.”
Other scenarios might involve a patient’s family member accompanying the patient and bringing out their phone to record. “Doctors should consider how this might be used against them — it can blow up,” says Dr. Verduzco-Gutierrez. “Draw boundaries on this behavior, using your hospital’s policy if it has one.”
In today’s pandemic landscape, this is particularly important, she adds. “There’s generally more mistrust in the medical system right now,” says Dr. Verduzco-Gutierrez. “People are getting misinformation from sources that aren’t credible, and then want to record their visits because they aren’t receiving the treatment they want, for instance.”
COVID has also added the tricky element of telehealth, which has exploded since 2020. “You don’t know what a patient is doing on the other side of the screen,” Dr. Verduzco-Gutierrez explains. “Face-to-face, you might see them with their phones out, but anything goes with telehealth. You have to be open and communicative with your patients about your policies from the start to avoid any negative connotations.”
How taping can help patients
Mr. Lindsey, the Colorado journalist, is far from alone in his desire to use visit recordings in order to retain valuable information — and with good reason. According to the Dartmouth Institute for Health Policy and Clinical Practice’s Open Recordings Project, at least 1 in 10 patients records their doctor’s visits.
“I realized I was missing things and in a medical setting, that matters,” Mr. Lindsey says. “Last year, once COVID hit and we all began wearing masks, I lost my ability to read lips, one of my coping mechanisms. It became even more important that I had a backup recording to ensure I understood everything.”
Even if a patient doesn’t have hearing loss like Mr. Lindsey, having an audio record of a visit can be useful. According to a 2018 study on patient recall of key information 1 week out from their visits, 49% of decisions and recommendations were recalled accurately without prompting; 36% recalled with a prompt; and 15% recalled erroneously or not at all.
This squares with the personal experiences of Dr. Verduzco-Gutierrez. “I even see this with my mom, who doesn’t remember many details of her doctor’s visits when I ask her,” she says. “This can definitely impact treatment.”
For better or worse
Dr. Verduzco-Gutierrez says that often it comes down to how a patient learns best. “I teach my residents to keep this in mind and to ask the patient in advance what works best for them,” she says. “If a patient is a visual learner, they might want to take notes or have access to the appointment notes after the visit. If they will learn and retain the information best with an audio recording, then offer that option.”
Mr. Lindsey makes it a habit to inform his physicians that he will be making an audio recording of his visits. “I always let them know that I’m recording for accuracy and not to catch them in some sort of falsehood,” he says. “I can get the doctor’s notes, but those are often short and to the point; I can get more information by going back over the recording.”
To date, Mr. Lindsey hasn’t experienced any pushback from his physicians. “No one has balked at the idea or acted surprised that I want to do it,” he explains. “I think most doctors appreciate that we have a tool we can make use of for better care.”
In past coverage of the topic, some healthcare providers weighed in with support for recordings, usually citing personal reasons. “I am so very grateful for the physicians that allowed me to record the medical appointments that I attended with my parents,” said one. “As their adult daughter, I was painfully aware that my parents struggled to process and understand all of the new information coming their way.”
Another expressed support as well, stating that as a patient, he prefers recordings to notes, because the latter “bears little resemblance to the content of the meeting and discussion with the physician. If the patient straightforwardly asks for permission to record, then why not honor the good intent expressed thereby?”
More often than not, patients have good intentions when they decide to hit the record button in a medical visit. A little preparation goes a long way, however, says Dr. Segal: “Assume you’re being recorded, and act accordingly.”
A version of this article first appeared on Medscape.com.
Joe Lindsey, a 48-year old Colorado-based journalist, has dealt with complex hearing loss for about 15 years. which has led to countless doctor’s visits, treatments, and even surgery in hopes of finding improvement. As time went on and Mr. Lindsey’s hearing deteriorated, he began recording his appointments in order to retain important information.
Mr. Lindsey had positive intentions, but not every patient does.
With smartphones everywhere, recording medical appointments can be fraught with downsides too. While there are clear-cut reasons for recording doctor visits, patients’ goals and how they carry out the taping are key. Audio only? Or also video? With the physician’s knowledge and permission, or without?
These are the legal and ethical weeds doctors find themselves in today, so it’s important to understand all sides of the issue.
The medical world is divided on its sentiments about patients recording their visits. The American Medical Association, in fact, failed to make progress on a recent policy (resolution 007) proposal to encourage that any “audio or video recording made during a medical encounter should require both physician and patient notification and consent.” Rather than voting on the resolution, the AMA house of delegates tabled it and chose to gather more information on the issue.
In most cases, patients are recording their visits in good faith, says Jeffrey Segal, MD, JD, the CEO and founder of Medical Justice, a risk mitigation and reputation management firm for healthcare clinicians. “When it comes to ‘Team, let’s record this,’ I’m a fan,” he says. “The most common reason patients record visits is that there’s a lot of information transferred from the doctor to the patient, and there’s just not enough time to absorb it all.”
While the option is there for patients to take notes, in the give-and-take nature of conversation, this can get difficult. “If they record the visit, they can then digest it all down the road,” says Dr. Segal. “A compliant patient is one who understands what’s expected. That’s the charitable explanation for recording, and I support it.”
It’s that question of good intent, however, that concerns some physicians in today’s highly litigious society. “The worry is that there’s a small subset of patients with an ulterior motive,” says Dr. Segal.
“Some patients do record in case of an event down the road,” he adds. “They want the recording to potentially talk to a lawyer, or to file a board complaint.”
Laws in the United States surrounding recordings are confusing, with variations from state to state. Currently, 39 U.S. states allow for one-party consent — meaning a patient can record a visit without consenting with the physician.
Monica Verduzco-Gutierrez, MD, professor and chair of rehabilitation medicine at University of Texas Health, San Antonio, resides in Texas, which is one of the 39 one-consent states. “Physicians must be aware of this fact and consider how it might be used against them,” she says. “A good practice is to set expectations with the patient from the start. Also, know your hospital’s policy — some may have boundaries surrounding recordings.”
The first step is to know what type of state you practice in. Regardless of whether you are in a one- or two-party consent state — but especially a one-party state — it’s a smart move to add a sign at your office saying that you support the recording of visits, provided the patient is open and transparent about it. “Let the patient know that if they plan to record, they should ask your permission,” says Dr. Segal. “Let them know it’s not appropriate if they haven’t received your permission.”
There are, of course, the occasional horror stories involving surreptitious recordings. “I remember a case where a patient left a phone actively recording in his bag of clothing, which went into the OR with him,” he says. “The background conversation was not flattering to the patient, who happened to be an employee of the hospital. When he came to and listened to the recording, he sued, winning his case.”
The age of video and telehealth
What about the rare situation when a patient pulls out a phone and begins to videotape a conversation? It can be a big slippery slope. “Patients can abuse a video recording with editing, and the recording becomes one-dimensional, which is unfair to the physician,” adds Dr. Segal.
Patients sometimes have other motives as well. “I’m aware of occasions where a doctor/patient visit got heated and the patient took out the phone to video record, sharing it to social media,” says Dr. Segal. “Once someone uses a phone to take video, just stop the conversation. Tell the patient, ‘We’re having a disagreement,’ and that it’s time to put an end to it.”
He adds that from the physician side, a video can be a protagonist in a conversation. “Frankly, a camera on your face changes the nature of things,” Dr. Segal says. “It’s much easier to have the phone sitting in a corner, quietly recording.”
Other scenarios might involve a patient’s family member accompanying the patient and bringing out their phone to record. “Doctors should consider how this might be used against them — it can blow up,” says Dr. Verduzco-Gutierrez. “Draw boundaries on this behavior, using your hospital’s policy if it has one.”
In today’s pandemic landscape, this is particularly important, she adds. “There’s generally more mistrust in the medical system right now,” says Dr. Verduzco-Gutierrez. “People are getting misinformation from sources that aren’t credible, and then want to record their visits because they aren’t receiving the treatment they want, for instance.”
COVID has also added the tricky element of telehealth, which has exploded since 2020. “You don’t know what a patient is doing on the other side of the screen,” Dr. Verduzco-Gutierrez explains. “Face-to-face, you might see them with their phones out, but anything goes with telehealth. You have to be open and communicative with your patients about your policies from the start to avoid any negative connotations.”
How taping can help patients
Mr. Lindsey, the Colorado journalist, is far from alone in his desire to use visit recordings in order to retain valuable information — and with good reason. According to the Dartmouth Institute for Health Policy and Clinical Practice’s Open Recordings Project, at least 1 in 10 patients records their doctor’s visits.
“I realized I was missing things and in a medical setting, that matters,” Mr. Lindsey says. “Last year, once COVID hit and we all began wearing masks, I lost my ability to read lips, one of my coping mechanisms. It became even more important that I had a backup recording to ensure I understood everything.”
Even if a patient doesn’t have hearing loss like Mr. Lindsey, having an audio record of a visit can be useful. According to a 2018 study on patient recall of key information 1 week out from their visits, 49% of decisions and recommendations were recalled accurately without prompting; 36% recalled with a prompt; and 15% recalled erroneously or not at all.
This squares with the personal experiences of Dr. Verduzco-Gutierrez. “I even see this with my mom, who doesn’t remember many details of her doctor’s visits when I ask her,” she says. “This can definitely impact treatment.”
For better or worse
Dr. Verduzco-Gutierrez says that often it comes down to how a patient learns best. “I teach my residents to keep this in mind and to ask the patient in advance what works best for them,” she says. “If a patient is a visual learner, they might want to take notes or have access to the appointment notes after the visit. If they will learn and retain the information best with an audio recording, then offer that option.”
Mr. Lindsey makes it a habit to inform his physicians that he will be making an audio recording of his visits. “I always let them know that I’m recording for accuracy and not to catch them in some sort of falsehood,” he says. “I can get the doctor’s notes, but those are often short and to the point; I can get more information by going back over the recording.”
To date, Mr. Lindsey hasn’t experienced any pushback from his physicians. “No one has balked at the idea or acted surprised that I want to do it,” he explains. “I think most doctors appreciate that we have a tool we can make use of for better care.”
In past coverage of the topic, some healthcare providers weighed in with support for recordings, usually citing personal reasons. “I am so very grateful for the physicians that allowed me to record the medical appointments that I attended with my parents,” said one. “As their adult daughter, I was painfully aware that my parents struggled to process and understand all of the new information coming their way.”
Another expressed support as well, stating that as a patient, he prefers recordings to notes, because the latter “bears little resemblance to the content of the meeting and discussion with the physician. If the patient straightforwardly asks for permission to record, then why not honor the good intent expressed thereby?”
More often than not, patients have good intentions when they decide to hit the record button in a medical visit. A little preparation goes a long way, however, says Dr. Segal: “Assume you’re being recorded, and act accordingly.”
A version of this article first appeared on Medscape.com.
Joe Lindsey, a 48-year old Colorado-based journalist, has dealt with complex hearing loss for about 15 years. which has led to countless doctor’s visits, treatments, and even surgery in hopes of finding improvement. As time went on and Mr. Lindsey’s hearing deteriorated, he began recording his appointments in order to retain important information.
Mr. Lindsey had positive intentions, but not every patient does.
With smartphones everywhere, recording medical appointments can be fraught with downsides too. While there are clear-cut reasons for recording doctor visits, patients’ goals and how they carry out the taping are key. Audio only? Or also video? With the physician’s knowledge and permission, or without?
These are the legal and ethical weeds doctors find themselves in today, so it’s important to understand all sides of the issue.
The medical world is divided on its sentiments about patients recording their visits. The American Medical Association, in fact, failed to make progress on a recent policy (resolution 007) proposal to encourage that any “audio or video recording made during a medical encounter should require both physician and patient notification and consent.” Rather than voting on the resolution, the AMA house of delegates tabled it and chose to gather more information on the issue.
In most cases, patients are recording their visits in good faith, says Jeffrey Segal, MD, JD, the CEO and founder of Medical Justice, a risk mitigation and reputation management firm for healthcare clinicians. “When it comes to ‘Team, let’s record this,’ I’m a fan,” he says. “The most common reason patients record visits is that there’s a lot of information transferred from the doctor to the patient, and there’s just not enough time to absorb it all.”
While the option is there for patients to take notes, in the give-and-take nature of conversation, this can get difficult. “If they record the visit, they can then digest it all down the road,” says Dr. Segal. “A compliant patient is one who understands what’s expected. That’s the charitable explanation for recording, and I support it.”
It’s that question of good intent, however, that concerns some physicians in today’s highly litigious society. “The worry is that there’s a small subset of patients with an ulterior motive,” says Dr. Segal.
“Some patients do record in case of an event down the road,” he adds. “They want the recording to potentially talk to a lawyer, or to file a board complaint.”
Laws in the United States surrounding recordings are confusing, with variations from state to state. Currently, 39 U.S. states allow for one-party consent — meaning a patient can record a visit without consenting with the physician.
Monica Verduzco-Gutierrez, MD, professor and chair of rehabilitation medicine at University of Texas Health, San Antonio, resides in Texas, which is one of the 39 one-consent states. “Physicians must be aware of this fact and consider how it might be used against them,” she says. “A good practice is to set expectations with the patient from the start. Also, know your hospital’s policy — some may have boundaries surrounding recordings.”
The first step is to know what type of state you practice in. Regardless of whether you are in a one- or two-party consent state — but especially a one-party state — it’s a smart move to add a sign at your office saying that you support the recording of visits, provided the patient is open and transparent about it. “Let the patient know that if they plan to record, they should ask your permission,” says Dr. Segal. “Let them know it’s not appropriate if they haven’t received your permission.”
There are, of course, the occasional horror stories involving surreptitious recordings. “I remember a case where a patient left a phone actively recording in his bag of clothing, which went into the OR with him,” he says. “The background conversation was not flattering to the patient, who happened to be an employee of the hospital. When he came to and listened to the recording, he sued, winning his case.”
The age of video and telehealth
What about the rare situation when a patient pulls out a phone and begins to videotape a conversation? It can be a big slippery slope. “Patients can abuse a video recording with editing, and the recording becomes one-dimensional, which is unfair to the physician,” adds Dr. Segal.
Patients sometimes have other motives as well. “I’m aware of occasions where a doctor/patient visit got heated and the patient took out the phone to video record, sharing it to social media,” says Dr. Segal. “Once someone uses a phone to take video, just stop the conversation. Tell the patient, ‘We’re having a disagreement,’ and that it’s time to put an end to it.”
He adds that from the physician side, a video can be a protagonist in a conversation. “Frankly, a camera on your face changes the nature of things,” Dr. Segal says. “It’s much easier to have the phone sitting in a corner, quietly recording.”
Other scenarios might involve a patient’s family member accompanying the patient and bringing out their phone to record. “Doctors should consider how this might be used against them — it can blow up,” says Dr. Verduzco-Gutierrez. “Draw boundaries on this behavior, using your hospital’s policy if it has one.”
In today’s pandemic landscape, this is particularly important, she adds. “There’s generally more mistrust in the medical system right now,” says Dr. Verduzco-Gutierrez. “People are getting misinformation from sources that aren’t credible, and then want to record their visits because they aren’t receiving the treatment they want, for instance.”
COVID has also added the tricky element of telehealth, which has exploded since 2020. “You don’t know what a patient is doing on the other side of the screen,” Dr. Verduzco-Gutierrez explains. “Face-to-face, you might see them with their phones out, but anything goes with telehealth. You have to be open and communicative with your patients about your policies from the start to avoid any negative connotations.”
How taping can help patients
Mr. Lindsey, the Colorado journalist, is far from alone in his desire to use visit recordings in order to retain valuable information — and with good reason. According to the Dartmouth Institute for Health Policy and Clinical Practice’s Open Recordings Project, at least 1 in 10 patients records their doctor’s visits.
“I realized I was missing things and in a medical setting, that matters,” Mr. Lindsey says. “Last year, once COVID hit and we all began wearing masks, I lost my ability to read lips, one of my coping mechanisms. It became even more important that I had a backup recording to ensure I understood everything.”
Even if a patient doesn’t have hearing loss like Mr. Lindsey, having an audio record of a visit can be useful. According to a 2018 study on patient recall of key information 1 week out from their visits, 49% of decisions and recommendations were recalled accurately without prompting; 36% recalled with a prompt; and 15% recalled erroneously or not at all.
This squares with the personal experiences of Dr. Verduzco-Gutierrez. “I even see this with my mom, who doesn’t remember many details of her doctor’s visits when I ask her,” she says. “This can definitely impact treatment.”
For better or worse
Dr. Verduzco-Gutierrez says that often it comes down to how a patient learns best. “I teach my residents to keep this in mind and to ask the patient in advance what works best for them,” she says. “If a patient is a visual learner, they might want to take notes or have access to the appointment notes after the visit. If they will learn and retain the information best with an audio recording, then offer that option.”
Mr. Lindsey makes it a habit to inform his physicians that he will be making an audio recording of his visits. “I always let them know that I’m recording for accuracy and not to catch them in some sort of falsehood,” he says. “I can get the doctor’s notes, but those are often short and to the point; I can get more information by going back over the recording.”
To date, Mr. Lindsey hasn’t experienced any pushback from his physicians. “No one has balked at the idea or acted surprised that I want to do it,” he explains. “I think most doctors appreciate that we have a tool we can make use of for better care.”
In past coverage of the topic, some healthcare providers weighed in with support for recordings, usually citing personal reasons. “I am so very grateful for the physicians that allowed me to record the medical appointments that I attended with my parents,” said one. “As their adult daughter, I was painfully aware that my parents struggled to process and understand all of the new information coming their way.”
Another expressed support as well, stating that as a patient, he prefers recordings to notes, because the latter “bears little resemblance to the content of the meeting and discussion with the physician. If the patient straightforwardly asks for permission to record, then why not honor the good intent expressed thereby?”
More often than not, patients have good intentions when they decide to hit the record button in a medical visit. A little preparation goes a long way, however, says Dr. Segal: “Assume you’re being recorded, and act accordingly.”
A version of this article first appeared on Medscape.com.
CDC unveils mental health protection plan for health care workers
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.
Federal health officials have outlined a five-part plan to improve and protect the mental health and well-being of America’s health care workers (HCWs) and create sustainable change for the next generation of HCWs.
“It’s long past time for us to care for the people who care for all of us and address burnout in our health care workers,” U.S. Surgeon General Vivek H. Murthy, MD, MBA, said during a webinar hosted by the National Institute for Occupational Safety and Health, part of the U.S. Centers for Disease Control and Prevention.
“My hope is that, going forward, we will be able to embark on this journey together to create a health care system, a health care environment, a country where we can not only provide extraordinary care to all those who need it, but where we can take good care of those who have sacrificed so much and make sure that they are well,” Dr. Murthy said.
Burnout is not selective
There are 20 million HCWs in the United States, and no one is immune from burnout, said NIOSH Director John Howard, MD.
He noted that from June through Sept. of 2020 – the height of the COVID-19 pandemic – 93% of HCWs experienced some degree of stress, with 22% reporting moderate depression and post-traumatic stress disorder.
Looking at subsets of HCWs, a recent survey showed that one in five nurses contemplated leaving the profession because of insufficient staffing, intensity of workload, emotional and physical toll of the job, and lack of support, Dr. Howard noted.
Physician burnout was a significant issue even before the pandemic, with about 79% of physicians reporting burnout. , Dr. Howard said.
Women in health care jobs are especially vulnerable to burnout; 76% of health care jobs are held by women and 64% of physicians that feel burned-out are women, according to federal data.
“We have significant work to do in shoring up the safety and health of women in health care,” Dr. Howard said.
Mental health is also suffering among local and state public health workers. In a recent CDC survey of 26,000 of these workers, 53% reported symptoms of at least one mental health condition in the past 2 weeks.
“That is really an alarming proportion of public health workers who are as vital and essential as nurses and doctors are in our health care system,” Dr. Howard said.
Primary prevention approach
To tackle the burnout crisis, NIOSH plans to:
- Take a deep dive into understanding the personal, social, and economic burdens HCWs face on a daily basis.
- Assimilate the evidence and create a repository of best practices, resources, and interventions.
- Partner with key stakeholders, including the American Hospital Association, the American Nurses Association, National Nurses United, the Joint Commission.
- Identify and adapt tools for the health care workplace that emphasize stress reduction.
NIOSH also plans to “generate awareness through a national, multidimensional social marketing campaign to get the word out about stress so health care workers don’t feel so alone,” Dr. Howard said.
This five-part plan takes a primary prevention approach to identifying and eliminating risk factors for burnout and stress, he added.
Secondary prevention, “when damage has already been done and you’re trying to save a health care worker who is suffering from a mental health issue, that’s a lot harder than taking a good look at what you can do to organizational practices that lead to health care workers’ stress and burnout,” Dr. Howard said.
A version of this article first appeared on Medscape.com.