User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Sleepless nights, hair loss, and cracked teeth: Pandemic stress takes its toll
In late March, shortly after New York state closed nonessential businesses and asked people to stay home, Ashley Laderer began waking each morning with a throbbing headache.
“The pressure was so intense it felt like my head was going to explode,” recalled the 27-year-old freelance writer from Long Island.
She tried spending less time on the computer and taking over-the-counter pain medication, but the pounding kept breaking through – a constant drumbeat to accompany her equally incessant worries about COVID-19.
“Every day I lived in fear that I was going to get it and I was going to infect my whole family,” she said.
After a month and a half, Ms. Laderer decided to visit a neurologist, who ordered an MRI. But the doctor found no physical cause. The scan was clear.
Then he asked: “Are you under a lot of stress?”
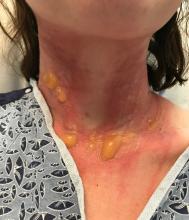
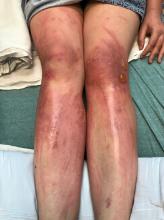
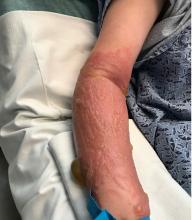
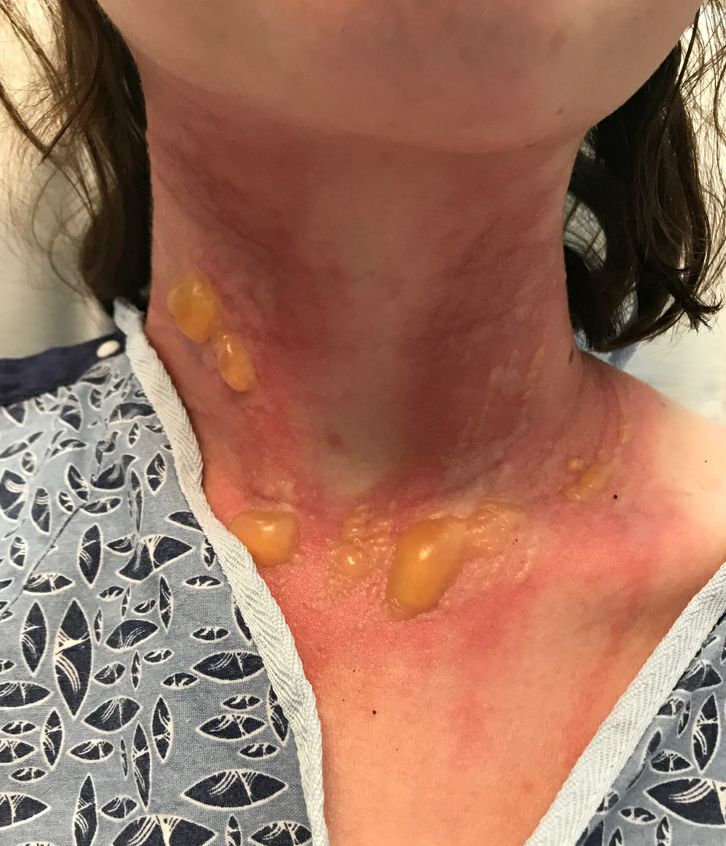
excruciating headaches, episodes of hair loss, upset stomach for weeks on end, sudden outbreaks of shingles, and flare-ups of autoimmune disorders. The disparate symptoms, often in otherwise-healthy individuals, have puzzled doctors and patients alike, sometimes resulting in a series of visits to specialists with few answers. But it turns out there’s a common thread among many of these conditions, one that has been months in the making: chronic stress.
Although people often underestimate the influence of the mind on the body, a growing catalog of research shows that high levels of stress over an extended time can drastically alter physical function and affect nearly every organ system.
Now, at least 8 months into the pandemic, alongside a divisive election cycle and racial unrest, those effects are showing up in a variety of symptoms.
“The mental health component of COVID is starting to come like a tsunami,” said Jennifer Love, MD, a California-based psychiatrist and coauthor of an upcoming book on how to heal from chronic stress.
Nationwide, surveys have found increasing rates of depression, anxiety and suicidal thoughts during the pandemic. But many medical experts said it’s too soon to measure the related physical symptoms, since they generally appear months after the stress begins.
Still, some early research, such as a small Chinese study and an online survey of more than 500 people in Turkey, points to an uptick.
In the United States, data from FAIR Health, a nonprofit database that provides cost information to the health industry and consumers, showed slight to moderate increases in the percentage of medical claims related to conditions triggered or exacerbated by stress, like multiple sclerosis and shingles. The portion of claims for the autoimmune disease lupus, for example, showed one of the biggest increases – 12% this year – compared with the same period last year (January to August).
Express Scripts, a major pharmacy benefit manager, reported that prescriptions for anti-insomnia medications increased 15% early in the pandemic.
Perhaps the strongest indicator comes from doctors reporting a growing number of patients with physical symptoms for which they can’t determine a cause.
Shilpi Khetarpal, MD, a dermatologist at the Cleveland Clinic, used to see about five patients a week with stress-related hair loss. Since mid-June, that number has jumped to 20 or 25. Mostly women, ages 20-80, are reporting hair coming out in fistfuls, Dr. Khetarpal said.
In Houston, at least a dozen patients have told fertility specialist Rashmi Kudesia, MD, they’re having irregular menstrual cycles, changes in cervical discharge and breast tenderness, despite normal hormone levels.
Stress is also the culprit dentists are pointing to for the rapid increase in patients with teeth grinding, teeth fractures, and temporomandibular joint dysfunction.
“We, as humans, like to have the idea that we are in control of our minds and that stress isn’t a big deal,” Dr. Love said. “But it’s simply not true.”
How mental stress becomes physical
Stress causes physical changes in the body that can affect nearly every organ system.
Although symptoms of chronic stress are often dismissed as being in one’s head, the pain is very real, said Kate Harkness, PhD, a professor of psychology and psychiatry at Queen’s University, Kingston, Ont.
When the body feels unsafe – whether it’s a physical threat of attack or a psychological fear of losing a job or catching a disease – the brain signals adrenal glands to pump stress hormones. Adrenaline and cortisol flood the body, activating the fight-or-flight response. They also disrupt bodily functions that aren’t necessary for immediate survival, like digestion and reproduction.
When the danger is over, the hormones return to normal levels. But during times of chronic stress, like a pandemic, the body keeps pumping out stress hormones until it tires itself out. This leads to increased inflammation throughout the body and brain, and a poorly functioning immune system.
Studies link chronic stress to heart disease, muscle tension, gastrointestinal issues and even physical shrinking of the hippocampus, an area of the brain associated with memory and learning. As the immune system acts up, some people can even develop new allergic reactions, Dr. Harkness said.
The good news is that many of these symptoms are reversible. But it’s important to recognize them early, especially when it comes to the brain, said Barbara Sahakian, FBA, FMedSci, a professor of clinical neuropsychology at the University of Cambridge (England).
“The brain is plastic, so we can to some extent modify it,” Dr. Sahakian said. “But we don’t know if there’s a cliff beyond which you can’t reverse a change. So the sooner you catch something, the better.”
The day-to-day impact
In some ways, mental health awareness has increased during the pandemic. TV shows are flush with ads for therapy and meditation apps, like Talkspace and Calm, and companies are announcing mental health days off for staff. But those spurts of attention fail to reveal the full impact of poor mental health on people’s daily lives.
For Alex Kostka, pandemic-related stress has brought on mood swings, nightmares, and jaw pain.
He’d been working at a Whole Foods coffee bar in New York City for only about a month before the pandemic hit, suddenly anointing him an essential worker. As deaths in the city soared, Mr. Kostka continued riding the subway to work, interacting with coworkers in the store and working longer hours for just a $2-per-hour wage increase. (Months later, he’d get a $500 bonus.) It left the 28-year-old feeling constantly unsafe and helpless.
“It was hard not to break down on the subway the minute I got on it,” Mr. Kostka said.
Soon he began waking in the middle of the night with pain from clenching his jaw so tightly. Often his teeth grinding and chomping were loud enough to wake his girlfriend.
Mr. Kostka tried Talkspace, but found texting about his troubles felt impersonal. By the end of the summer, he decided to start using the seven free counseling sessions offered by his employer. That’s helped, he said. But as the sessions run out, he worries the symptoms might return if he’s unable to find a new therapist covered by his insurance.
“Eventually, I will be able to leave this behind me, but it will take time,” Mr. Kostka said. “I’m still very much a work in progress.”
How to mitigate chronic stress
When it comes to chronic stress, seeing a doctor for stomach pain, headaches, or skin rashes may address those physical symptoms. But the root cause is mental, medical experts said.
That means the solution will often involve stress-management techniques. And there’s plenty we can do to feel better:
- Exercise. Even low- to moderate-intensity physical activity can help counteract stress-induced inflammation in the body. It can also increase neuronal connections in the brain.
- Meditation and mindfulness. Research shows this can lead to positive, structural, and functional changes in the brain.
- Fostering social connections. Talking to family and friends, even virtually, or staring into a pet’s eyes can release a hormone that may counteract inflammation.
- Learning something new. Whether it’s a formal class or taking up a casual hobby, learning supports brain plasticity, the ability to change and adapt as a result of experience, which can be protective against depression and other mental illness.
“We shouldn’t think of this stressful situation as a negative sentence for the brain,” said Dr. Harkness. “Because stress changes the brain, that means positive stuff can change the brain, too. And there is plenty we can do to help ourselves feel better in the face of adversity.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In late March, shortly after New York state closed nonessential businesses and asked people to stay home, Ashley Laderer began waking each morning with a throbbing headache.
“The pressure was so intense it felt like my head was going to explode,” recalled the 27-year-old freelance writer from Long Island.
She tried spending less time on the computer and taking over-the-counter pain medication, but the pounding kept breaking through – a constant drumbeat to accompany her equally incessant worries about COVID-19.
“Every day I lived in fear that I was going to get it and I was going to infect my whole family,” she said.
After a month and a half, Ms. Laderer decided to visit a neurologist, who ordered an MRI. But the doctor found no physical cause. The scan was clear.
Then he asked: “Are you under a lot of stress?”
excruciating headaches, episodes of hair loss, upset stomach for weeks on end, sudden outbreaks of shingles, and flare-ups of autoimmune disorders. The disparate symptoms, often in otherwise-healthy individuals, have puzzled doctors and patients alike, sometimes resulting in a series of visits to specialists with few answers. But it turns out there’s a common thread among many of these conditions, one that has been months in the making: chronic stress.
Although people often underestimate the influence of the mind on the body, a growing catalog of research shows that high levels of stress over an extended time can drastically alter physical function and affect nearly every organ system.
Now, at least 8 months into the pandemic, alongside a divisive election cycle and racial unrest, those effects are showing up in a variety of symptoms.
“The mental health component of COVID is starting to come like a tsunami,” said Jennifer Love, MD, a California-based psychiatrist and coauthor of an upcoming book on how to heal from chronic stress.
Nationwide, surveys have found increasing rates of depression, anxiety and suicidal thoughts during the pandemic. But many medical experts said it’s too soon to measure the related physical symptoms, since they generally appear months after the stress begins.
Still, some early research, such as a small Chinese study and an online survey of more than 500 people in Turkey, points to an uptick.
In the United States, data from FAIR Health, a nonprofit database that provides cost information to the health industry and consumers, showed slight to moderate increases in the percentage of medical claims related to conditions triggered or exacerbated by stress, like multiple sclerosis and shingles. The portion of claims for the autoimmune disease lupus, for example, showed one of the biggest increases – 12% this year – compared with the same period last year (January to August).
Express Scripts, a major pharmacy benefit manager, reported that prescriptions for anti-insomnia medications increased 15% early in the pandemic.
Perhaps the strongest indicator comes from doctors reporting a growing number of patients with physical symptoms for which they can’t determine a cause.
Shilpi Khetarpal, MD, a dermatologist at the Cleveland Clinic, used to see about five patients a week with stress-related hair loss. Since mid-June, that number has jumped to 20 or 25. Mostly women, ages 20-80, are reporting hair coming out in fistfuls, Dr. Khetarpal said.
In Houston, at least a dozen patients have told fertility specialist Rashmi Kudesia, MD, they’re having irregular menstrual cycles, changes in cervical discharge and breast tenderness, despite normal hormone levels.
Stress is also the culprit dentists are pointing to for the rapid increase in patients with teeth grinding, teeth fractures, and temporomandibular joint dysfunction.
“We, as humans, like to have the idea that we are in control of our minds and that stress isn’t a big deal,” Dr. Love said. “But it’s simply not true.”
How mental stress becomes physical
Stress causes physical changes in the body that can affect nearly every organ system.
Although symptoms of chronic stress are often dismissed as being in one’s head, the pain is very real, said Kate Harkness, PhD, a professor of psychology and psychiatry at Queen’s University, Kingston, Ont.
When the body feels unsafe – whether it’s a physical threat of attack or a psychological fear of losing a job or catching a disease – the brain signals adrenal glands to pump stress hormones. Adrenaline and cortisol flood the body, activating the fight-or-flight response. They also disrupt bodily functions that aren’t necessary for immediate survival, like digestion and reproduction.
When the danger is over, the hormones return to normal levels. But during times of chronic stress, like a pandemic, the body keeps pumping out stress hormones until it tires itself out. This leads to increased inflammation throughout the body and brain, and a poorly functioning immune system.
Studies link chronic stress to heart disease, muscle tension, gastrointestinal issues and even physical shrinking of the hippocampus, an area of the brain associated with memory and learning. As the immune system acts up, some people can even develop new allergic reactions, Dr. Harkness said.
The good news is that many of these symptoms are reversible. But it’s important to recognize them early, especially when it comes to the brain, said Barbara Sahakian, FBA, FMedSci, a professor of clinical neuropsychology at the University of Cambridge (England).
“The brain is plastic, so we can to some extent modify it,” Dr. Sahakian said. “But we don’t know if there’s a cliff beyond which you can’t reverse a change. So the sooner you catch something, the better.”
The day-to-day impact
In some ways, mental health awareness has increased during the pandemic. TV shows are flush with ads for therapy and meditation apps, like Talkspace and Calm, and companies are announcing mental health days off for staff. But those spurts of attention fail to reveal the full impact of poor mental health on people’s daily lives.
For Alex Kostka, pandemic-related stress has brought on mood swings, nightmares, and jaw pain.
He’d been working at a Whole Foods coffee bar in New York City for only about a month before the pandemic hit, suddenly anointing him an essential worker. As deaths in the city soared, Mr. Kostka continued riding the subway to work, interacting with coworkers in the store and working longer hours for just a $2-per-hour wage increase. (Months later, he’d get a $500 bonus.) It left the 28-year-old feeling constantly unsafe and helpless.
“It was hard not to break down on the subway the minute I got on it,” Mr. Kostka said.
Soon he began waking in the middle of the night with pain from clenching his jaw so tightly. Often his teeth grinding and chomping were loud enough to wake his girlfriend.
Mr. Kostka tried Talkspace, but found texting about his troubles felt impersonal. By the end of the summer, he decided to start using the seven free counseling sessions offered by his employer. That’s helped, he said. But as the sessions run out, he worries the symptoms might return if he’s unable to find a new therapist covered by his insurance.
“Eventually, I will be able to leave this behind me, but it will take time,” Mr. Kostka said. “I’m still very much a work in progress.”
How to mitigate chronic stress
When it comes to chronic stress, seeing a doctor for stomach pain, headaches, or skin rashes may address those physical symptoms. But the root cause is mental, medical experts said.
That means the solution will often involve stress-management techniques. And there’s plenty we can do to feel better:
- Exercise. Even low- to moderate-intensity physical activity can help counteract stress-induced inflammation in the body. It can also increase neuronal connections in the brain.
- Meditation and mindfulness. Research shows this can lead to positive, structural, and functional changes in the brain.
- Fostering social connections. Talking to family and friends, even virtually, or staring into a pet’s eyes can release a hormone that may counteract inflammation.
- Learning something new. Whether it’s a formal class or taking up a casual hobby, learning supports brain plasticity, the ability to change and adapt as a result of experience, which can be protective against depression and other mental illness.
“We shouldn’t think of this stressful situation as a negative sentence for the brain,” said Dr. Harkness. “Because stress changes the brain, that means positive stuff can change the brain, too. And there is plenty we can do to help ourselves feel better in the face of adversity.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
In late March, shortly after New York state closed nonessential businesses and asked people to stay home, Ashley Laderer began waking each morning with a throbbing headache.
“The pressure was so intense it felt like my head was going to explode,” recalled the 27-year-old freelance writer from Long Island.
She tried spending less time on the computer and taking over-the-counter pain medication, but the pounding kept breaking through – a constant drumbeat to accompany her equally incessant worries about COVID-19.
“Every day I lived in fear that I was going to get it and I was going to infect my whole family,” she said.
After a month and a half, Ms. Laderer decided to visit a neurologist, who ordered an MRI. But the doctor found no physical cause. The scan was clear.
Then he asked: “Are you under a lot of stress?”
excruciating headaches, episodes of hair loss, upset stomach for weeks on end, sudden outbreaks of shingles, and flare-ups of autoimmune disorders. The disparate symptoms, often in otherwise-healthy individuals, have puzzled doctors and patients alike, sometimes resulting in a series of visits to specialists with few answers. But it turns out there’s a common thread among many of these conditions, one that has been months in the making: chronic stress.
Although people often underestimate the influence of the mind on the body, a growing catalog of research shows that high levels of stress over an extended time can drastically alter physical function and affect nearly every organ system.
Now, at least 8 months into the pandemic, alongside a divisive election cycle and racial unrest, those effects are showing up in a variety of symptoms.
“The mental health component of COVID is starting to come like a tsunami,” said Jennifer Love, MD, a California-based psychiatrist and coauthor of an upcoming book on how to heal from chronic stress.
Nationwide, surveys have found increasing rates of depression, anxiety and suicidal thoughts during the pandemic. But many medical experts said it’s too soon to measure the related physical symptoms, since they generally appear months after the stress begins.
Still, some early research, such as a small Chinese study and an online survey of more than 500 people in Turkey, points to an uptick.
In the United States, data from FAIR Health, a nonprofit database that provides cost information to the health industry and consumers, showed slight to moderate increases in the percentage of medical claims related to conditions triggered or exacerbated by stress, like multiple sclerosis and shingles. The portion of claims for the autoimmune disease lupus, for example, showed one of the biggest increases – 12% this year – compared with the same period last year (January to August).
Express Scripts, a major pharmacy benefit manager, reported that prescriptions for anti-insomnia medications increased 15% early in the pandemic.
Perhaps the strongest indicator comes from doctors reporting a growing number of patients with physical symptoms for which they can’t determine a cause.
Shilpi Khetarpal, MD, a dermatologist at the Cleveland Clinic, used to see about five patients a week with stress-related hair loss. Since mid-June, that number has jumped to 20 or 25. Mostly women, ages 20-80, are reporting hair coming out in fistfuls, Dr. Khetarpal said.
In Houston, at least a dozen patients have told fertility specialist Rashmi Kudesia, MD, they’re having irregular menstrual cycles, changes in cervical discharge and breast tenderness, despite normal hormone levels.
Stress is also the culprit dentists are pointing to for the rapid increase in patients with teeth grinding, teeth fractures, and temporomandibular joint dysfunction.
“We, as humans, like to have the idea that we are in control of our minds and that stress isn’t a big deal,” Dr. Love said. “But it’s simply not true.”
How mental stress becomes physical
Stress causes physical changes in the body that can affect nearly every organ system.
Although symptoms of chronic stress are often dismissed as being in one’s head, the pain is very real, said Kate Harkness, PhD, a professor of psychology and psychiatry at Queen’s University, Kingston, Ont.
When the body feels unsafe – whether it’s a physical threat of attack or a psychological fear of losing a job or catching a disease – the brain signals adrenal glands to pump stress hormones. Adrenaline and cortisol flood the body, activating the fight-or-flight response. They also disrupt bodily functions that aren’t necessary for immediate survival, like digestion and reproduction.
When the danger is over, the hormones return to normal levels. But during times of chronic stress, like a pandemic, the body keeps pumping out stress hormones until it tires itself out. This leads to increased inflammation throughout the body and brain, and a poorly functioning immune system.
Studies link chronic stress to heart disease, muscle tension, gastrointestinal issues and even physical shrinking of the hippocampus, an area of the brain associated with memory and learning. As the immune system acts up, some people can even develop new allergic reactions, Dr. Harkness said.
The good news is that many of these symptoms are reversible. But it’s important to recognize them early, especially when it comes to the brain, said Barbara Sahakian, FBA, FMedSci, a professor of clinical neuropsychology at the University of Cambridge (England).
“The brain is plastic, so we can to some extent modify it,” Dr. Sahakian said. “But we don’t know if there’s a cliff beyond which you can’t reverse a change. So the sooner you catch something, the better.”
The day-to-day impact
In some ways, mental health awareness has increased during the pandemic. TV shows are flush with ads for therapy and meditation apps, like Talkspace and Calm, and companies are announcing mental health days off for staff. But those spurts of attention fail to reveal the full impact of poor mental health on people’s daily lives.
For Alex Kostka, pandemic-related stress has brought on mood swings, nightmares, and jaw pain.
He’d been working at a Whole Foods coffee bar in New York City for only about a month before the pandemic hit, suddenly anointing him an essential worker. As deaths in the city soared, Mr. Kostka continued riding the subway to work, interacting with coworkers in the store and working longer hours for just a $2-per-hour wage increase. (Months later, he’d get a $500 bonus.) It left the 28-year-old feeling constantly unsafe and helpless.
“It was hard not to break down on the subway the minute I got on it,” Mr. Kostka said.
Soon he began waking in the middle of the night with pain from clenching his jaw so tightly. Often his teeth grinding and chomping were loud enough to wake his girlfriend.
Mr. Kostka tried Talkspace, but found texting about his troubles felt impersonal. By the end of the summer, he decided to start using the seven free counseling sessions offered by his employer. That’s helped, he said. But as the sessions run out, he worries the symptoms might return if he’s unable to find a new therapist covered by his insurance.
“Eventually, I will be able to leave this behind me, but it will take time,” Mr. Kostka said. “I’m still very much a work in progress.”
How to mitigate chronic stress
When it comes to chronic stress, seeing a doctor for stomach pain, headaches, or skin rashes may address those physical symptoms. But the root cause is mental, medical experts said.
That means the solution will often involve stress-management techniques. And there’s plenty we can do to feel better:
- Exercise. Even low- to moderate-intensity physical activity can help counteract stress-induced inflammation in the body. It can also increase neuronal connections in the brain.
- Meditation and mindfulness. Research shows this can lead to positive, structural, and functional changes in the brain.
- Fostering social connections. Talking to family and friends, even virtually, or staring into a pet’s eyes can release a hormone that may counteract inflammation.
- Learning something new. Whether it’s a formal class or taking up a casual hobby, learning supports brain plasticity, the ability to change and adapt as a result of experience, which can be protective against depression and other mental illness.
“We shouldn’t think of this stressful situation as a negative sentence for the brain,” said Dr. Harkness. “Because stress changes the brain, that means positive stuff can change the brain, too. And there is plenty we can do to help ourselves feel better in the face of adversity.”
KHN (Kaiser Health News) is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Survey: Doctors lonely, burned out in COVID-19
Patrick Ross, MD, a critical care physician at Children’s Hospital of Los Angeles, was plagued with increasing worry about his health and that of his family, patients, and colleagues. While distancing from his wife and daughter, he became terrified of falling ill and dying alone.
As he grew more anxious, Ross withdrew from family, colleagues, and friends, although his clinical and academic responsibilities were unaffected. He barely ate; his weight plummeted, and he began to have suicidal thoughts.
Rebecca Margolis, DO, a pediatric anesthesiologist whom Ross was mentoring, noticed something was amiss and suggested that he go to a therapist. That suggestion may have saved him.
“Once I started therapy, I no longer had suicidal ideations, but I still remained anxious on a day-to-day basis,” said Ross, who is an associate professor of clinical anesthesiology and pediatrics at the University of Southern California, Los Angeles. “As soon as I learned to manage or mitigate the anxiety, I was no longer consumed to the degree I had been by the sense of day-to-day threat.”
Ross openly shares his story because “many other physicians may be going through versions of what I experienced, and I want to encourage them to get help if they’re feeling stressed, anxious, lonely, depressed, or burned out, and to recognize that they are not alone.”
Physicians feel a sense of betrayal
Ross’ experience, although extreme, is not unique. According to a Medscape survey of almost 7,500 physicians, about two-thirds (64%) of U.S. physicians reported experiencing more intense burnout, and close to half (46%) reported feeling more lonely and isolated during the pandemic.
“We know that stress, which was already significant in physicians, has increased dramatically for many physicians during the pandemic. That’s understandable, given the circumstances they’ve been working under,” said Christine A. Sinsky, MD, vice president of professional satisfaction at the American Medical Association.
Physicians are stressed about potentially contracting the virus or infecting family members; being overworked and fatigued; witnessing wrenching scenes of patients dying alone; grieving the loss of patients, colleagues, or family members; and sometimes lacking adequate personal protective equipment (PPE), she said.
Lack of PPE has been identified as one of the most significant contributors to burnout and stress among physicians and other health care professionals. In all eight countries surveyed by Medscape, a significant number of respondents reported lacking appropriate PPE “sometimes,” “often,” or “always” when treating COVID-19 patients. Only 54% of U.S. respondents said they were always adequately protected.
The PPE shortage not only jeopardizes physical health but also has a negative effect on mental health and morale. A U.S.-based rheumatologist said, “The fact that we were sent to take care of infectious patients without proper PPE makes me feel we were betrayed in this fight.”
Not what they signed up for
Many physicians expressed fear regarding their personal safety, but that was often superseded by concern for family – especially elderly relatives or young children. (Medscape’s survey found that 9% of US respondents had immediate family members who had been diagnosed with COVID-19.)
Larissa Thomas, MD, MPH, University of California, San Francisco, said her greatest fear was bringing the virus home to her new baby and other vulnerable family members. Thomas is associate clinical professor of medicine and is a faculty hospitalist at Zuckerberg San Francisco General Hospital.
“Although physicians assume risk in our work, we didn’t sign up to care for patients without adequate protection, and our families certainly didn’t sign up for that risk, so the concern was acutely stressful,” said Thomas, who is also associate program director for the UCSF Internal Medicine Residency Program and is director of well-being for UCSF Graduate Medical Education.
The impact of stay-at-home restrictions on family members’ mental health also affected many physicians.
David Marcus, MD, residency director of the Combined Program in Emergency/Internal/Critical Care Medicine and chair of the GME Physician Wellbeing Committee at Northwell Health, Long Island, New York, said that a large stressor during the pandemic was having an elderly father with multiple comorbidities who lived alone and was unable to go out because of stay-at-home restrictions.
“I was worried not only for his physical health but also that his cognition might slip due to lack of socialization,” said Marcus.
Marcus was also worried about his preschool-age daughter, who seemed to be regressing and becoming desocialized from no longer being at school. “Fortunately, school has reopened, but it was a constant weight on my wife and me to see the impact of the lockdown on her development,” he said.
New situations create more anxiety
Being redeployed to new clinical roles in settings such as the emergency department or intensive care, which were not in their area of specialty, created much stress for physicians, Thomas said.
Physicians in private practice also had to adjust to new ways of practicing. In Medscape’s survey, 39% of U.S. physicians reported that their medical practice never closed during the pandemic. Keeping a practice open often meant learning to see patients virtually or becoming extremely vigilant about reducing the risk for contagion when seeing patients in person.
Relationships became more challenging
Social distancing during the pandemic had a negative effect on personal relationships for 44% of respondents, both in the United States and abroad.
One physician described her relationship with her partner as “more stressful” and argumentative. A rheumatologist reported experiencing frustration at having college-aged children living at home. Another respondent said that being with young children 24/7 left her “short-tempered,” and an emergency medicine physician respondent said she and her family were “driving each other crazy.”
Social distancing was not the only challenge to relationships. An orthopedist identified long, taxing work hours as contributing to a “decline in spousal harmony.”
On the other hand, some physicians said their relationships improved by developing shared insight. An emergency medicine physician wrote that he and his wife were “having more quarrels” but were “trying very hard and succeeding at understanding that much of this is due to the changes in our living situation.”
As a volunteer with New York City’s Medical Reserve Corps, Wilfrid Noel Raby, PhD, MD, adjunct clinical professor of psychiatry, Albert Einstein College of Medicine, New York City, chose to keep his Teaneck, New Jersey–based office open and was taking overnight shifts at Lincoln Hospital in New York City during the acute physician shortage. “After my regular hospital job treating psychiatric patients and seeing patients in my private practice, I sometimes pulled 12-hour nights caring for very ill patients. It was grueling, and I came home drained and exhausted,” he recalled.
Raby’s wife, a surgical nurse, had been redeployed to care for COVID-19 patients in the ICU – a situation she found grueling as well. Adding to the stress were the “rigorous distancing and sanitation precautions we needed to practice at home.” Fear of contagion, together with exhaustion, resulted in “occasional moments of friction,” Raby acknowledged.
Still, some physicians managed to find a bit of a silver lining. “We tried to relax, get as much sleep as possible, and keep things simple, not taking on extra tasks that could be postponed,” Raby said. “It helped that we both recognized how difficult it was to reassure each other when we were stressed and scared, so we faced the crisis together, and I think it ultimately brought us closer.”
Thomas said that the pandemic has helped her to recognize what she can and cannot control and how to take things one day at a time.
“When my husband and I can both work from home, we are grateful to have that ability and grateful for the things that we do have. These small moments of gratitude have sustained us day to day,” Thomas said.
Socializing outside the box
Several physicians expressed a sense of loneliness because stay-at-home guidelines and social distancing prevented them from socializing with friends. In all countries, physician respondents to the Medscape survey reported feeling “more lonely” than prior to the pandemic. Over half (51%) of Portuguese physicians reported feeling lonelier; 48% of physicians in Brazil felt that way. The United States came in third, at 46%.
Many physicians feel cut off, even from other physicians, and are reluctant to share feelings of distress.
“Talking to colleagues about distress is an important human connection,” Margolis emphasized. “We need to rely on each other to commiserate and receive validation and comfort.”
Some institutions have formalized this process by instituting a “battle buddy” model – a term borrowed from the military – which involves pairing clinicians of similar specialty, career stage, and life circumstances to provide mutual peer support, Margolis said. A partner who notices concerning signs in the other partner can refer the person to resources for help.
Sinsky said that an organization called PeerRxMed offers physicians a chance to sign up for a “buddy,” even outside their own institution.
The importance of ‘fixing’ the workplace
Close to half (43%) of U.S. respondents to Medscape’s survey reported that their workplace offers activities to help physicians deal with grief and stress, but 39% said that their workplace does not offer this type of support, and 18% were not sure whether these services were offered.
At times of crisis, organizations need to offer “stress first aid,” Sinsky said. This includes providing for basic needs, such as child care, transportation, and healthy food, and having “open, transparent, and honest communication” from leadership regarding what is known and not known about the pandemic, clinician responsibilities, and stress reduction measures.
Marcus notes that, at his institution, psychiatric residents and other members of the psychiatry department have “stepped up and crafted process groups and peer support contexts to debrief, engage, explore productive outlets for feelings, and facilitate communication.” In particular, residents have found cognitive-behavioral therapy to be useful.
Despite the difficult situation, seeking help can be challenging for some physicians. One reason, Marcus says, is that doctors tend to think of themselves as being at the giving rather than the receiving end of help – especially during a crisis. “We do what we need to do, and we often don’t see the toll it takes on us,” he noted. Moreover, the pressure to be at the “giving” end can lead to stigma in acknowledging vulnerability.
Ross said he hopes his story will help to destigmatize reaching out for help. “It is possible that a silver lining of this terrible crisis is to normalize physicians receiving help for mental health issues.”
Marcus likewise openly shares his own experiences about struggles with burnout and depressive symptoms. “As a physician educator, I think it’s important for me to be public about these things, which validates help-seeking for residents and colleagues.”
For physicians seeking help not offered in their workplace, the Physician Support Line is a useful resource, added Margolis. She noted that its services are free and confidential.
This article first appeared on Medscape.com.
Patrick Ross, MD, a critical care physician at Children’s Hospital of Los Angeles, was plagued with increasing worry about his health and that of his family, patients, and colleagues. While distancing from his wife and daughter, he became terrified of falling ill and dying alone.
As he grew more anxious, Ross withdrew from family, colleagues, and friends, although his clinical and academic responsibilities were unaffected. He barely ate; his weight plummeted, and he began to have suicidal thoughts.
Rebecca Margolis, DO, a pediatric anesthesiologist whom Ross was mentoring, noticed something was amiss and suggested that he go to a therapist. That suggestion may have saved him.
“Once I started therapy, I no longer had suicidal ideations, but I still remained anxious on a day-to-day basis,” said Ross, who is an associate professor of clinical anesthesiology and pediatrics at the University of Southern California, Los Angeles. “As soon as I learned to manage or mitigate the anxiety, I was no longer consumed to the degree I had been by the sense of day-to-day threat.”
Ross openly shares his story because “many other physicians may be going through versions of what I experienced, and I want to encourage them to get help if they’re feeling stressed, anxious, lonely, depressed, or burned out, and to recognize that they are not alone.”
Physicians feel a sense of betrayal
Ross’ experience, although extreme, is not unique. According to a Medscape survey of almost 7,500 physicians, about two-thirds (64%) of U.S. physicians reported experiencing more intense burnout, and close to half (46%) reported feeling more lonely and isolated during the pandemic.
“We know that stress, which was already significant in physicians, has increased dramatically for many physicians during the pandemic. That’s understandable, given the circumstances they’ve been working under,” said Christine A. Sinsky, MD, vice president of professional satisfaction at the American Medical Association.
Physicians are stressed about potentially contracting the virus or infecting family members; being overworked and fatigued; witnessing wrenching scenes of patients dying alone; grieving the loss of patients, colleagues, or family members; and sometimes lacking adequate personal protective equipment (PPE), she said.
Lack of PPE has been identified as one of the most significant contributors to burnout and stress among physicians and other health care professionals. In all eight countries surveyed by Medscape, a significant number of respondents reported lacking appropriate PPE “sometimes,” “often,” or “always” when treating COVID-19 patients. Only 54% of U.S. respondents said they were always adequately protected.
The PPE shortage not only jeopardizes physical health but also has a negative effect on mental health and morale. A U.S.-based rheumatologist said, “The fact that we were sent to take care of infectious patients without proper PPE makes me feel we were betrayed in this fight.”
Not what they signed up for
Many physicians expressed fear regarding their personal safety, but that was often superseded by concern for family – especially elderly relatives or young children. (Medscape’s survey found that 9% of US respondents had immediate family members who had been diagnosed with COVID-19.)
Larissa Thomas, MD, MPH, University of California, San Francisco, said her greatest fear was bringing the virus home to her new baby and other vulnerable family members. Thomas is associate clinical professor of medicine and is a faculty hospitalist at Zuckerberg San Francisco General Hospital.
“Although physicians assume risk in our work, we didn’t sign up to care for patients without adequate protection, and our families certainly didn’t sign up for that risk, so the concern was acutely stressful,” said Thomas, who is also associate program director for the UCSF Internal Medicine Residency Program and is director of well-being for UCSF Graduate Medical Education.
The impact of stay-at-home restrictions on family members’ mental health also affected many physicians.
David Marcus, MD, residency director of the Combined Program in Emergency/Internal/Critical Care Medicine and chair of the GME Physician Wellbeing Committee at Northwell Health, Long Island, New York, said that a large stressor during the pandemic was having an elderly father with multiple comorbidities who lived alone and was unable to go out because of stay-at-home restrictions.
“I was worried not only for his physical health but also that his cognition might slip due to lack of socialization,” said Marcus.
Marcus was also worried about his preschool-age daughter, who seemed to be regressing and becoming desocialized from no longer being at school. “Fortunately, school has reopened, but it was a constant weight on my wife and me to see the impact of the lockdown on her development,” he said.
New situations create more anxiety
Being redeployed to new clinical roles in settings such as the emergency department or intensive care, which were not in their area of specialty, created much stress for physicians, Thomas said.
Physicians in private practice also had to adjust to new ways of practicing. In Medscape’s survey, 39% of U.S. physicians reported that their medical practice never closed during the pandemic. Keeping a practice open often meant learning to see patients virtually or becoming extremely vigilant about reducing the risk for contagion when seeing patients in person.
Relationships became more challenging
Social distancing during the pandemic had a negative effect on personal relationships for 44% of respondents, both in the United States and abroad.
One physician described her relationship with her partner as “more stressful” and argumentative. A rheumatologist reported experiencing frustration at having college-aged children living at home. Another respondent said that being with young children 24/7 left her “short-tempered,” and an emergency medicine physician respondent said she and her family were “driving each other crazy.”
Social distancing was not the only challenge to relationships. An orthopedist identified long, taxing work hours as contributing to a “decline in spousal harmony.”
On the other hand, some physicians said their relationships improved by developing shared insight. An emergency medicine physician wrote that he and his wife were “having more quarrels” but were “trying very hard and succeeding at understanding that much of this is due to the changes in our living situation.”
As a volunteer with New York City’s Medical Reserve Corps, Wilfrid Noel Raby, PhD, MD, adjunct clinical professor of psychiatry, Albert Einstein College of Medicine, New York City, chose to keep his Teaneck, New Jersey–based office open and was taking overnight shifts at Lincoln Hospital in New York City during the acute physician shortage. “After my regular hospital job treating psychiatric patients and seeing patients in my private practice, I sometimes pulled 12-hour nights caring for very ill patients. It was grueling, and I came home drained and exhausted,” he recalled.
Raby’s wife, a surgical nurse, had been redeployed to care for COVID-19 patients in the ICU – a situation she found grueling as well. Adding to the stress were the “rigorous distancing and sanitation precautions we needed to practice at home.” Fear of contagion, together with exhaustion, resulted in “occasional moments of friction,” Raby acknowledged.
Still, some physicians managed to find a bit of a silver lining. “We tried to relax, get as much sleep as possible, and keep things simple, not taking on extra tasks that could be postponed,” Raby said. “It helped that we both recognized how difficult it was to reassure each other when we were stressed and scared, so we faced the crisis together, and I think it ultimately brought us closer.”
Thomas said that the pandemic has helped her to recognize what she can and cannot control and how to take things one day at a time.
“When my husband and I can both work from home, we are grateful to have that ability and grateful for the things that we do have. These small moments of gratitude have sustained us day to day,” Thomas said.
Socializing outside the box
Several physicians expressed a sense of loneliness because stay-at-home guidelines and social distancing prevented them from socializing with friends. In all countries, physician respondents to the Medscape survey reported feeling “more lonely” than prior to the pandemic. Over half (51%) of Portuguese physicians reported feeling lonelier; 48% of physicians in Brazil felt that way. The United States came in third, at 46%.
Many physicians feel cut off, even from other physicians, and are reluctant to share feelings of distress.
“Talking to colleagues about distress is an important human connection,” Margolis emphasized. “We need to rely on each other to commiserate and receive validation and comfort.”
Some institutions have formalized this process by instituting a “battle buddy” model – a term borrowed from the military – which involves pairing clinicians of similar specialty, career stage, and life circumstances to provide mutual peer support, Margolis said. A partner who notices concerning signs in the other partner can refer the person to resources for help.
Sinsky said that an organization called PeerRxMed offers physicians a chance to sign up for a “buddy,” even outside their own institution.
The importance of ‘fixing’ the workplace
Close to half (43%) of U.S. respondents to Medscape’s survey reported that their workplace offers activities to help physicians deal with grief and stress, but 39% said that their workplace does not offer this type of support, and 18% were not sure whether these services were offered.
At times of crisis, organizations need to offer “stress first aid,” Sinsky said. This includes providing for basic needs, such as child care, transportation, and healthy food, and having “open, transparent, and honest communication” from leadership regarding what is known and not known about the pandemic, clinician responsibilities, and stress reduction measures.
Marcus notes that, at his institution, psychiatric residents and other members of the psychiatry department have “stepped up and crafted process groups and peer support contexts to debrief, engage, explore productive outlets for feelings, and facilitate communication.” In particular, residents have found cognitive-behavioral therapy to be useful.
Despite the difficult situation, seeking help can be challenging for some physicians. One reason, Marcus says, is that doctors tend to think of themselves as being at the giving rather than the receiving end of help – especially during a crisis. “We do what we need to do, and we often don’t see the toll it takes on us,” he noted. Moreover, the pressure to be at the “giving” end can lead to stigma in acknowledging vulnerability.
Ross said he hopes his story will help to destigmatize reaching out for help. “It is possible that a silver lining of this terrible crisis is to normalize physicians receiving help for mental health issues.”
Marcus likewise openly shares his own experiences about struggles with burnout and depressive symptoms. “As a physician educator, I think it’s important for me to be public about these things, which validates help-seeking for residents and colleagues.”
For physicians seeking help not offered in their workplace, the Physician Support Line is a useful resource, added Margolis. She noted that its services are free and confidential.
This article first appeared on Medscape.com.
Patrick Ross, MD, a critical care physician at Children’s Hospital of Los Angeles, was plagued with increasing worry about his health and that of his family, patients, and colleagues. While distancing from his wife and daughter, he became terrified of falling ill and dying alone.
As he grew more anxious, Ross withdrew from family, colleagues, and friends, although his clinical and academic responsibilities were unaffected. He barely ate; his weight plummeted, and he began to have suicidal thoughts.
Rebecca Margolis, DO, a pediatric anesthesiologist whom Ross was mentoring, noticed something was amiss and suggested that he go to a therapist. That suggestion may have saved him.
“Once I started therapy, I no longer had suicidal ideations, but I still remained anxious on a day-to-day basis,” said Ross, who is an associate professor of clinical anesthesiology and pediatrics at the University of Southern California, Los Angeles. “As soon as I learned to manage or mitigate the anxiety, I was no longer consumed to the degree I had been by the sense of day-to-day threat.”
Ross openly shares his story because “many other physicians may be going through versions of what I experienced, and I want to encourage them to get help if they’re feeling stressed, anxious, lonely, depressed, or burned out, and to recognize that they are not alone.”
Physicians feel a sense of betrayal
Ross’ experience, although extreme, is not unique. According to a Medscape survey of almost 7,500 physicians, about two-thirds (64%) of U.S. physicians reported experiencing more intense burnout, and close to half (46%) reported feeling more lonely and isolated during the pandemic.
“We know that stress, which was already significant in physicians, has increased dramatically for many physicians during the pandemic. That’s understandable, given the circumstances they’ve been working under,” said Christine A. Sinsky, MD, vice president of professional satisfaction at the American Medical Association.
Physicians are stressed about potentially contracting the virus or infecting family members; being overworked and fatigued; witnessing wrenching scenes of patients dying alone; grieving the loss of patients, colleagues, or family members; and sometimes lacking adequate personal protective equipment (PPE), she said.
Lack of PPE has been identified as one of the most significant contributors to burnout and stress among physicians and other health care professionals. In all eight countries surveyed by Medscape, a significant number of respondents reported lacking appropriate PPE “sometimes,” “often,” or “always” when treating COVID-19 patients. Only 54% of U.S. respondents said they were always adequately protected.
The PPE shortage not only jeopardizes physical health but also has a negative effect on mental health and morale. A U.S.-based rheumatologist said, “The fact that we were sent to take care of infectious patients without proper PPE makes me feel we were betrayed in this fight.”
Not what they signed up for
Many physicians expressed fear regarding their personal safety, but that was often superseded by concern for family – especially elderly relatives or young children. (Medscape’s survey found that 9% of US respondents had immediate family members who had been diagnosed with COVID-19.)
Larissa Thomas, MD, MPH, University of California, San Francisco, said her greatest fear was bringing the virus home to her new baby and other vulnerable family members. Thomas is associate clinical professor of medicine and is a faculty hospitalist at Zuckerberg San Francisco General Hospital.
“Although physicians assume risk in our work, we didn’t sign up to care for patients without adequate protection, and our families certainly didn’t sign up for that risk, so the concern was acutely stressful,” said Thomas, who is also associate program director for the UCSF Internal Medicine Residency Program and is director of well-being for UCSF Graduate Medical Education.
The impact of stay-at-home restrictions on family members’ mental health also affected many physicians.
David Marcus, MD, residency director of the Combined Program in Emergency/Internal/Critical Care Medicine and chair of the GME Physician Wellbeing Committee at Northwell Health, Long Island, New York, said that a large stressor during the pandemic was having an elderly father with multiple comorbidities who lived alone and was unable to go out because of stay-at-home restrictions.
“I was worried not only for his physical health but also that his cognition might slip due to lack of socialization,” said Marcus.
Marcus was also worried about his preschool-age daughter, who seemed to be regressing and becoming desocialized from no longer being at school. “Fortunately, school has reopened, but it was a constant weight on my wife and me to see the impact of the lockdown on her development,” he said.
New situations create more anxiety
Being redeployed to new clinical roles in settings such as the emergency department or intensive care, which were not in their area of specialty, created much stress for physicians, Thomas said.
Physicians in private practice also had to adjust to new ways of practicing. In Medscape’s survey, 39% of U.S. physicians reported that their medical practice never closed during the pandemic. Keeping a practice open often meant learning to see patients virtually or becoming extremely vigilant about reducing the risk for contagion when seeing patients in person.
Relationships became more challenging
Social distancing during the pandemic had a negative effect on personal relationships for 44% of respondents, both in the United States and abroad.
One physician described her relationship with her partner as “more stressful” and argumentative. A rheumatologist reported experiencing frustration at having college-aged children living at home. Another respondent said that being with young children 24/7 left her “short-tempered,” and an emergency medicine physician respondent said she and her family were “driving each other crazy.”
Social distancing was not the only challenge to relationships. An orthopedist identified long, taxing work hours as contributing to a “decline in spousal harmony.”
On the other hand, some physicians said their relationships improved by developing shared insight. An emergency medicine physician wrote that he and his wife were “having more quarrels” but were “trying very hard and succeeding at understanding that much of this is due to the changes in our living situation.”
As a volunteer with New York City’s Medical Reserve Corps, Wilfrid Noel Raby, PhD, MD, adjunct clinical professor of psychiatry, Albert Einstein College of Medicine, New York City, chose to keep his Teaneck, New Jersey–based office open and was taking overnight shifts at Lincoln Hospital in New York City during the acute physician shortage. “After my regular hospital job treating psychiatric patients and seeing patients in my private practice, I sometimes pulled 12-hour nights caring for very ill patients. It was grueling, and I came home drained and exhausted,” he recalled.
Raby’s wife, a surgical nurse, had been redeployed to care for COVID-19 patients in the ICU – a situation she found grueling as well. Adding to the stress were the “rigorous distancing and sanitation precautions we needed to practice at home.” Fear of contagion, together with exhaustion, resulted in “occasional moments of friction,” Raby acknowledged.
Still, some physicians managed to find a bit of a silver lining. “We tried to relax, get as much sleep as possible, and keep things simple, not taking on extra tasks that could be postponed,” Raby said. “It helped that we both recognized how difficult it was to reassure each other when we were stressed and scared, so we faced the crisis together, and I think it ultimately brought us closer.”
Thomas said that the pandemic has helped her to recognize what she can and cannot control and how to take things one day at a time.
“When my husband and I can both work from home, we are grateful to have that ability and grateful for the things that we do have. These small moments of gratitude have sustained us day to day,” Thomas said.
Socializing outside the box
Several physicians expressed a sense of loneliness because stay-at-home guidelines and social distancing prevented them from socializing with friends. In all countries, physician respondents to the Medscape survey reported feeling “more lonely” than prior to the pandemic. Over half (51%) of Portuguese physicians reported feeling lonelier; 48% of physicians in Brazil felt that way. The United States came in third, at 46%.
Many physicians feel cut off, even from other physicians, and are reluctant to share feelings of distress.
“Talking to colleagues about distress is an important human connection,” Margolis emphasized. “We need to rely on each other to commiserate and receive validation and comfort.”
Some institutions have formalized this process by instituting a “battle buddy” model – a term borrowed from the military – which involves pairing clinicians of similar specialty, career stage, and life circumstances to provide mutual peer support, Margolis said. A partner who notices concerning signs in the other partner can refer the person to resources for help.
Sinsky said that an organization called PeerRxMed offers physicians a chance to sign up for a “buddy,” even outside their own institution.
The importance of ‘fixing’ the workplace
Close to half (43%) of U.S. respondents to Medscape’s survey reported that their workplace offers activities to help physicians deal with grief and stress, but 39% said that their workplace does not offer this type of support, and 18% were not sure whether these services were offered.
At times of crisis, organizations need to offer “stress first aid,” Sinsky said. This includes providing for basic needs, such as child care, transportation, and healthy food, and having “open, transparent, and honest communication” from leadership regarding what is known and not known about the pandemic, clinician responsibilities, and stress reduction measures.
Marcus notes that, at his institution, psychiatric residents and other members of the psychiatry department have “stepped up and crafted process groups and peer support contexts to debrief, engage, explore productive outlets for feelings, and facilitate communication.” In particular, residents have found cognitive-behavioral therapy to be useful.
Despite the difficult situation, seeking help can be challenging for some physicians. One reason, Marcus says, is that doctors tend to think of themselves as being at the giving rather than the receiving end of help – especially during a crisis. “We do what we need to do, and we often don’t see the toll it takes on us,” he noted. Moreover, the pressure to be at the “giving” end can lead to stigma in acknowledging vulnerability.
Ross said he hopes his story will help to destigmatize reaching out for help. “It is possible that a silver lining of this terrible crisis is to normalize physicians receiving help for mental health issues.”
Marcus likewise openly shares his own experiences about struggles with burnout and depressive symptoms. “As a physician educator, I think it’s important for me to be public about these things, which validates help-seeking for residents and colleagues.”
For physicians seeking help not offered in their workplace, the Physician Support Line is a useful resource, added Margolis. She noted that its services are free and confidential.
This article first appeared on Medscape.com.
Low IgA levels associated with increased infection risk in SLE patients
A new study of immunoglobulin levels in adult patients with systemic lupus erythematosus (SLE) has found that acquired low levels of IgA are associated with a higher risk of infection.
To the knowledge of first author Ibrahim Almaghlouth, MD, of the division of rheumatology at the University of Toronto, and colleagues, “this is the first dedicated study to examine the relationship between acquired low immunoglobulins and infection risk in adult patients with SLE.” But as to whether there may be a “protective role for immunoglobulins and the potential effect of immunoglobulin replacement in a setting of recurrent or severe infection among SLE patients requires further study.”
To determine if the risk of infection was tied to acquired low immunoglobulin levels, the researchers launched a retrospective analysis of data from a prospective cohort study of adult SLE patients from a Toronto lupus cohort that was established in 1970. The study was published in Rheumatology.
A total of 448 patients with at least two low immunoglobulin tests were matched with 656 SLE patients with no low immunoglobulins according to enrollment decade. The average age of the low-immunoglobulin group was 41.8 years, compared with 39.3 years in the control group. Average disease duration was 11.2 years in the low-immunoglobulin group and 7.6 years in the control group.
Of the patients in the low-immunoglobulin group, 221 had consecutive low tests and 227 had nonconsecutive low tests. Overall, 98 of those patients had low IgG, 251 patients had low IgM, and 51 patients had low IgA. Only 48 patients had overlapping low levels, including 5 with all three.
Average levels among the low-immunoglobulin group at baseline were 11.5 (standard deviation, 6.1) g/L of IgG, 0.8 (1.1) g/L of IgM, and 2.4 (1.6) g/L of IgA, while average levels among the control group were 16.3 (6.4) g/L of IgG, 1.8 (1.2) g/L of IgM, and 3.2 (1.5) g/L of IgA. In the primary analysis, after adjustment using propensity scoring, there were 97 infections: 47 in the low-immunoglobulin group and 50 in the control group. The most common types were respiratory and urinary tract infections, and the rate of infection was higher in patients with low IgA. The IgA level associated with risk of infection was less than 0.75 g/L.
After Cox regression analysis, the only variable that significantly increased infection risk was a low IgA level (hazard ratio, 3.19; 95% confidence interval, 1.17-8.71), not a low IgG level (HR, 1.87; 95% CI, 0.77-4.54) or low IgM level (HR, 0.63; 95% CI, 0.34-1.17). In regard to recovery among the low-immunoglobulin group, 11 patients (2.5%) recovered from low immunoglobulins within in the first year, followed by 36 (8.2%) in the second year, 44 (10.1%) in the third year, and 80 (18.4%) in the fourth year. All told, 60% (263) of patients with acquired hypogammaglobulinemia recovered over a 4-year period.
Is there clinical relevance to low IgA?
“I don’t see us using this clinically immediately,” Karen Costenbader, MD, a rheumatologist at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said in an interview. “We do test immunoglobulins often, especially in patients who’ve had biologic therapy. Will we start thinking about their IgA levels? It’s not clear, and the researchers leave it up in the air as to what this means, beyond them being at high risk.”
That said, she added, “IgA levels are interesting, especially in a time of COVID, because they’re associated with mucosal immunity. Is this subset of patients going to be at particularly high risk for the coronavirus?”
She also noted that, though immunoglobulin replacement has been helpful in her patients, it’s an expensive therapy to recommend for low IgA levels without knowing exactly what is causing these deficiencies. “My question is, would it be useful to follow these levels in lupus patients, even we don’t know what to do about them?” she asked. “We know there are a lot of risk factors for infections, so is the IgA going to be useful above and beyond that, and then what can we do about it?”
The authors acknowledged their study’s potential limitations, including low infection rates and yearly measurements of immunoglobulin levels, which could’ve led to misclassifying a lab error as true low immunoglobulin. They also highlighted its strengths, including using various methods to reduce selection and confounding bias while also reporting consistent results after examining multiple definitions of low immunoglobulins and outcomes.
The study received no specific funding, and the authors reported no potential conflicts of interest.
SOURCE: Almaghlouth I et al. Rheumatology. 2020 Oct 2. doi: 10.1093/rheumatology/keaa641.
A new study of immunoglobulin levels in adult patients with systemic lupus erythematosus (SLE) has found that acquired low levels of IgA are associated with a higher risk of infection.
To the knowledge of first author Ibrahim Almaghlouth, MD, of the division of rheumatology at the University of Toronto, and colleagues, “this is the first dedicated study to examine the relationship between acquired low immunoglobulins and infection risk in adult patients with SLE.” But as to whether there may be a “protective role for immunoglobulins and the potential effect of immunoglobulin replacement in a setting of recurrent or severe infection among SLE patients requires further study.”
To determine if the risk of infection was tied to acquired low immunoglobulin levels, the researchers launched a retrospective analysis of data from a prospective cohort study of adult SLE patients from a Toronto lupus cohort that was established in 1970. The study was published in Rheumatology.
A total of 448 patients with at least two low immunoglobulin tests were matched with 656 SLE patients with no low immunoglobulins according to enrollment decade. The average age of the low-immunoglobulin group was 41.8 years, compared with 39.3 years in the control group. Average disease duration was 11.2 years in the low-immunoglobulin group and 7.6 years in the control group.
Of the patients in the low-immunoglobulin group, 221 had consecutive low tests and 227 had nonconsecutive low tests. Overall, 98 of those patients had low IgG, 251 patients had low IgM, and 51 patients had low IgA. Only 48 patients had overlapping low levels, including 5 with all three.
Average levels among the low-immunoglobulin group at baseline were 11.5 (standard deviation, 6.1) g/L of IgG, 0.8 (1.1) g/L of IgM, and 2.4 (1.6) g/L of IgA, while average levels among the control group were 16.3 (6.4) g/L of IgG, 1.8 (1.2) g/L of IgM, and 3.2 (1.5) g/L of IgA. In the primary analysis, after adjustment using propensity scoring, there were 97 infections: 47 in the low-immunoglobulin group and 50 in the control group. The most common types were respiratory and urinary tract infections, and the rate of infection was higher in patients with low IgA. The IgA level associated with risk of infection was less than 0.75 g/L.
After Cox regression analysis, the only variable that significantly increased infection risk was a low IgA level (hazard ratio, 3.19; 95% confidence interval, 1.17-8.71), not a low IgG level (HR, 1.87; 95% CI, 0.77-4.54) or low IgM level (HR, 0.63; 95% CI, 0.34-1.17). In regard to recovery among the low-immunoglobulin group, 11 patients (2.5%) recovered from low immunoglobulins within in the first year, followed by 36 (8.2%) in the second year, 44 (10.1%) in the third year, and 80 (18.4%) in the fourth year. All told, 60% (263) of patients with acquired hypogammaglobulinemia recovered over a 4-year period.
Is there clinical relevance to low IgA?
“I don’t see us using this clinically immediately,” Karen Costenbader, MD, a rheumatologist at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said in an interview. “We do test immunoglobulins often, especially in patients who’ve had biologic therapy. Will we start thinking about their IgA levels? It’s not clear, and the researchers leave it up in the air as to what this means, beyond them being at high risk.”
That said, she added, “IgA levels are interesting, especially in a time of COVID, because they’re associated with mucosal immunity. Is this subset of patients going to be at particularly high risk for the coronavirus?”
She also noted that, though immunoglobulin replacement has been helpful in her patients, it’s an expensive therapy to recommend for low IgA levels without knowing exactly what is causing these deficiencies. “My question is, would it be useful to follow these levels in lupus patients, even we don’t know what to do about them?” she asked. “We know there are a lot of risk factors for infections, so is the IgA going to be useful above and beyond that, and then what can we do about it?”
The authors acknowledged their study’s potential limitations, including low infection rates and yearly measurements of immunoglobulin levels, which could’ve led to misclassifying a lab error as true low immunoglobulin. They also highlighted its strengths, including using various methods to reduce selection and confounding bias while also reporting consistent results after examining multiple definitions of low immunoglobulins and outcomes.
The study received no specific funding, and the authors reported no potential conflicts of interest.
SOURCE: Almaghlouth I et al. Rheumatology. 2020 Oct 2. doi: 10.1093/rheumatology/keaa641.
A new study of immunoglobulin levels in adult patients with systemic lupus erythematosus (SLE) has found that acquired low levels of IgA are associated with a higher risk of infection.
To the knowledge of first author Ibrahim Almaghlouth, MD, of the division of rheumatology at the University of Toronto, and colleagues, “this is the first dedicated study to examine the relationship between acquired low immunoglobulins and infection risk in adult patients with SLE.” But as to whether there may be a “protective role for immunoglobulins and the potential effect of immunoglobulin replacement in a setting of recurrent or severe infection among SLE patients requires further study.”
To determine if the risk of infection was tied to acquired low immunoglobulin levels, the researchers launched a retrospective analysis of data from a prospective cohort study of adult SLE patients from a Toronto lupus cohort that was established in 1970. The study was published in Rheumatology.
A total of 448 patients with at least two low immunoglobulin tests were matched with 656 SLE patients with no low immunoglobulins according to enrollment decade. The average age of the low-immunoglobulin group was 41.8 years, compared with 39.3 years in the control group. Average disease duration was 11.2 years in the low-immunoglobulin group and 7.6 years in the control group.
Of the patients in the low-immunoglobulin group, 221 had consecutive low tests and 227 had nonconsecutive low tests. Overall, 98 of those patients had low IgG, 251 patients had low IgM, and 51 patients had low IgA. Only 48 patients had overlapping low levels, including 5 with all three.
Average levels among the low-immunoglobulin group at baseline were 11.5 (standard deviation, 6.1) g/L of IgG, 0.8 (1.1) g/L of IgM, and 2.4 (1.6) g/L of IgA, while average levels among the control group were 16.3 (6.4) g/L of IgG, 1.8 (1.2) g/L of IgM, and 3.2 (1.5) g/L of IgA. In the primary analysis, after adjustment using propensity scoring, there were 97 infections: 47 in the low-immunoglobulin group and 50 in the control group. The most common types were respiratory and urinary tract infections, and the rate of infection was higher in patients with low IgA. The IgA level associated with risk of infection was less than 0.75 g/L.
After Cox regression analysis, the only variable that significantly increased infection risk was a low IgA level (hazard ratio, 3.19; 95% confidence interval, 1.17-8.71), not a low IgG level (HR, 1.87; 95% CI, 0.77-4.54) or low IgM level (HR, 0.63; 95% CI, 0.34-1.17). In regard to recovery among the low-immunoglobulin group, 11 patients (2.5%) recovered from low immunoglobulins within in the first year, followed by 36 (8.2%) in the second year, 44 (10.1%) in the third year, and 80 (18.4%) in the fourth year. All told, 60% (263) of patients with acquired hypogammaglobulinemia recovered over a 4-year period.
Is there clinical relevance to low IgA?
“I don’t see us using this clinically immediately,” Karen Costenbader, MD, a rheumatologist at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston, said in an interview. “We do test immunoglobulins often, especially in patients who’ve had biologic therapy. Will we start thinking about their IgA levels? It’s not clear, and the researchers leave it up in the air as to what this means, beyond them being at high risk.”
That said, she added, “IgA levels are interesting, especially in a time of COVID, because they’re associated with mucosal immunity. Is this subset of patients going to be at particularly high risk for the coronavirus?”
She also noted that, though immunoglobulin replacement has been helpful in her patients, it’s an expensive therapy to recommend for low IgA levels without knowing exactly what is causing these deficiencies. “My question is, would it be useful to follow these levels in lupus patients, even we don’t know what to do about them?” she asked. “We know there are a lot of risk factors for infections, so is the IgA going to be useful above and beyond that, and then what can we do about it?”
The authors acknowledged their study’s potential limitations, including low infection rates and yearly measurements of immunoglobulin levels, which could’ve led to misclassifying a lab error as true low immunoglobulin. They also highlighted its strengths, including using various methods to reduce selection and confounding bias while also reporting consistent results after examining multiple definitions of low immunoglobulins and outcomes.
The study received no specific funding, and the authors reported no potential conflicts of interest.
SOURCE: Almaghlouth I et al. Rheumatology. 2020 Oct 2. doi: 10.1093/rheumatology/keaa641.
FROM RHEUMATOLOGY
Fauci: Cautious optimism for COVID-19 vaccine by end of 2020
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
with distribution of first doses possible before the end of the year, according to Anthony S. Fauci, MD, director, National Institute of Allergy and Infectious Diseases, Bethesda, Md.
“Given the rate of infection that’s going on in this country, and the distribution of the clinical trial sites involving tens of thousands of volunteers, we project that we will have an answer as to whether or not we have a safe and effective vaccine by November or December,” Dr. Fauci said today in his virtual keynote address during the annual meeting of the American College of Chest Physicians.
“It may come earlier -- this month, in October,” he added in his remarks. “That is unlikely – it is more likely that we’ll have an answer in November and December.”
If that timing does come to pass, Dr. Fauci said, it’s possible that distribution of doses could start at the end of the year, continuing throughout the beginning and middle of 2021.
Although there are no guarantees, Dr. Fauci said he is “cautiously optimistic” regarding the timeline.
He said that his optimism is based in part on animal studies and phase 1 data that demonstrate robust neutralizing antibody responses to a vaccine that are equivalent to, if not greater than, natural infection with the SARS-CoV-2 virus that causes COVID-19.
Rapid development gives reason for hope
Ryan C. Maves, MD, FCCP, a critical care and infectious disease specialist at Naval Medical Center San Diego, said there is reason to be hopeful that a vaccine will be available by the end of the calendar year. He cautioned, however, that this timing is based on the assumption that one of the vaccines will be proven safe and effective very soon.
“We’re lucky to have multiple phase 3 trials using multiple vaccine technologies in different platforms,” Dr. Maves said in a panel discussion following Dr. Fauci’s remarks. “I think the odds are very high that one of them will be effective.”
“I’m hoping that multiple vaccines will be effective,” Dr. Maves added. “Then we’ll be in a good position of determining which is the best of several good options, as a society and as a world.”
COVID-19 vaccine development over the past year has been remarkably fast, especially given the previous record set by the mumps vaccine, which took about four years to go from initial steps to rollout, Dr. Maves noted.
Dr. Fauci said the federal government has taken a “strategic approach” to the COVID-19 vaccine that includes direct involvement in the research and development of six different vaccine candidates, five of which are now in phase 3 trials.
As part of that strategic approach, the study protocols are harmonized to have a common data and safety monitoring board, common primary and secondary endpoints, and an independent statistical group to determine correlates of protection, Dr. Fauci said.
Prioritizing COVID-19 vaccine distribution
Who gets COVID-19 vaccine first will be a challenge for governmental organizations as well as bioethicists, who have proposed different strategies for fairly prioritizing different groups for access.
Reaching communities of color will be an important consideration for prioritization, according to Dr. Maves, given the disproportionate burden of disease on Black and Hispanic individuals, among other such populations.
COVID-19–related hospitalization rates have been substantially higher in communities of color, Dr. Fauci said in his keynote address. Age-adjusted hospitalization rates for Hispanic/Latinx and Black populations are 375 to 368 per 100,000, respectively, compared with just 82 per 100,000 for White non-Hispanics, according to data from the Centers for Disease Control and Prevention.
Outreach to those communities should include building trust in those populations that they will benefit from a safe and effective vaccine, and making sure that the vaccine is available to those communities as quickly as possible, Dr. Maves said.
Dr. Fauci and Dr. Maves provided no disclosures related to their presentations.
FROM CHEST 2020
Survey of Mohs surgeons highlights its use in invasive melanoma
of members of the American College of Mohs Surgery.
Of 513 survey participants, 40.9% reported using MMS to treat any subtype of melanoma. Most of these surgeons reported treating both lentigo maligna (97.5%) and other melanoma in situ (MIS) subtypes (91.4%). A slight majority – 58.6% – reported treating invasive T1 melanoma, and 20.5% reported treating invasive T2 and/or higher-stage melanoma with MMS.
The analysis, published in Dermatologic Surgery, was done by Spyros M. Siscos, MD, and a team of residents and faculty in the division of dermatology at the University of Kansas Medical Center, Kansas City.
It comes on the heels of an analysis of claims data for Mohs surgery, published last year in JAMA Dermatology, which showed a more than threefold increase in the use of Mohs surgery for melanoma from 2.6% of all surgical cases in 2001 to 7.9% in 2016.
With the increased use of MMS for treatment of melanoma, “Mohs surgeons who previously treated MIS with MMS may be increasingly doing so and/or expanding their scope of treatment to include invasive melanoma,” the University of Kansas investigators wrote.
That a slight majority now report treating invasive melanoma with MMS “may be due, in part, to upstaging during the MMS procedure and the increasing evidence demonstrating improved survival of early-invasive melanoma treated with MMS compared with [wide local excision],” as well as the advent of melanocytic immunohistochemical (IHC) stains, particularly melanoma antigen recognized by T-cells 1 (MART-1), they said. However, 29% of surveyed Mohs surgeons treating melanoma with MMS do not use IHC stains “despite growing evidence supporting” their use, the authors wrote.
The advent of IHC stains, particularly MART-1, has improved the accuracy of interpreting frozen sections of melanoma, they reported, noting that MMS without IHC has been associated with a recurrence rate as high as 33%. Of the 71% who reported using IHC stains, MART-1 was the primary IHC stain for virtually all of them (97.3%).
There was also variation in the number of surgeons who reported debulking MIS. Eighty-two percent take this approach, excising the clinically visible tumor before excising the initial Mohs stage – almost all with a scalpel. More than half of these surgeons – 58.5% – submit the entire debulked MIS specimen for permanent vertical sectioning (breadloafing) to evaluate for deeper tumor invasion.
The others reported submitting the entire debulked specimen for frozen vertical sectioning, or portions of the specimen for both permanent and frozen vertical sectioning. “It is unclear why a minority of surveyed Mohs surgeons reported not debulking MIS,” wrote Dr. Siscos and his colleagues.
The average margin size of the first Mohs stage for MIS was 4.96 ± 1.74 mm, which is at the lower end of the 0.5-1.0 cm range for wide local excision (WLE) recommended by the National Comprehensive Cancer Network (NCCN) and the American Academy of Dermatology (AAD), according to a clinical practice guideline. (The survey did not investigate initial margins for invasive melanoma treated with MMS.)
Jeremy R. Etzhorn, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and an author of a 2019 claims data analysis of excisional surgery practices for melanoma, said that the new survey findings – like the prior analysis – highlight the variability in approaches to using MMS for melanoma.
“Mohs for melanoma [seems] like a one-liner ... but really, there are [a lot] of different techniques that fall under that umbrella, if you parse out all the variations,” he said in an interview.
Per the 2016 claims analysis, he noted, IHC was used in less than 40% of Mohs surgery cases for melanoma, and there were wide geographic variations. “The biggest critique of Mohs surgery for melanoma over the last two decades has been that it’s hard to see the tumor,” he said. “But with the advent of IHC, that challenge was overcome.”
Surgical excision practices are evolving without the development of best practice guidelines, said Dr. Etzkorn, who is director of clinical research for the University of Pennsylvania dermatologic oncology center. Multisociety guidelines published in 2012 on appropriate use criteria for Mohs surgery do not offer specific recommendations on the use of MMS for invasive melanoma. Nor do guidelines from the AAD and the NCCN, he said.
“What this [new] study highlights and what’s being discussed amongst Moh’s surgeons” is that Mohs for melanoma “has be to be standardized” to some extent and then clinical trials conducted comparing Mohs to conventional excision. The studies that have been published in recent years comparing MMS with WLE for MIS and invasive melanoma are “not gold standard studies,” he said.
Practice guidelines then can be informed by high-quality evidence on its safety and efficacy, he said.
The 513 participants in the newly published survey represent a 31.5% response rate. Invasive T2 and/or higher stage melanoma was more likely to be treated with MMS in academic hospitals, compared with other practice settings (30.2% v. 18.1%), Dr. Siscos and his coauthors reported.
Participants who reported treating melanoma with MMS were more likely to report fellowship exposure and more likely to have received fellowship training on melanocytic IHC stains. The study “highlights the importance of fellowship exposure to MMS and IHC staining for melanoma,” the authors wrote, adding that postfellowship training opportunities in MMS and IHC staining for melanoma may help broaden its use among Mohs surgeons who received inadequate fellowship exposure.
Dr. Siscos and his colleagues reported no significant interest with commercial supporters. Dr. Etzkorn had no disclosures.
SOURCE: Siscos S et al. Dermatol Surg. 2020 Oct;46(10):1267-71.
of members of the American College of Mohs Surgery.
Of 513 survey participants, 40.9% reported using MMS to treat any subtype of melanoma. Most of these surgeons reported treating both lentigo maligna (97.5%) and other melanoma in situ (MIS) subtypes (91.4%). A slight majority – 58.6% – reported treating invasive T1 melanoma, and 20.5% reported treating invasive T2 and/or higher-stage melanoma with MMS.
The analysis, published in Dermatologic Surgery, was done by Spyros M. Siscos, MD, and a team of residents and faculty in the division of dermatology at the University of Kansas Medical Center, Kansas City.
It comes on the heels of an analysis of claims data for Mohs surgery, published last year in JAMA Dermatology, which showed a more than threefold increase in the use of Mohs surgery for melanoma from 2.6% of all surgical cases in 2001 to 7.9% in 2016.
With the increased use of MMS for treatment of melanoma, “Mohs surgeons who previously treated MIS with MMS may be increasingly doing so and/or expanding their scope of treatment to include invasive melanoma,” the University of Kansas investigators wrote.
That a slight majority now report treating invasive melanoma with MMS “may be due, in part, to upstaging during the MMS procedure and the increasing evidence demonstrating improved survival of early-invasive melanoma treated with MMS compared with [wide local excision],” as well as the advent of melanocytic immunohistochemical (IHC) stains, particularly melanoma antigen recognized by T-cells 1 (MART-1), they said. However, 29% of surveyed Mohs surgeons treating melanoma with MMS do not use IHC stains “despite growing evidence supporting” their use, the authors wrote.
The advent of IHC stains, particularly MART-1, has improved the accuracy of interpreting frozen sections of melanoma, they reported, noting that MMS without IHC has been associated with a recurrence rate as high as 33%. Of the 71% who reported using IHC stains, MART-1 was the primary IHC stain for virtually all of them (97.3%).
There was also variation in the number of surgeons who reported debulking MIS. Eighty-two percent take this approach, excising the clinically visible tumor before excising the initial Mohs stage – almost all with a scalpel. More than half of these surgeons – 58.5% – submit the entire debulked MIS specimen for permanent vertical sectioning (breadloafing) to evaluate for deeper tumor invasion.
The others reported submitting the entire debulked specimen for frozen vertical sectioning, or portions of the specimen for both permanent and frozen vertical sectioning. “It is unclear why a minority of surveyed Mohs surgeons reported not debulking MIS,” wrote Dr. Siscos and his colleagues.
The average margin size of the first Mohs stage for MIS was 4.96 ± 1.74 mm, which is at the lower end of the 0.5-1.0 cm range for wide local excision (WLE) recommended by the National Comprehensive Cancer Network (NCCN) and the American Academy of Dermatology (AAD), according to a clinical practice guideline. (The survey did not investigate initial margins for invasive melanoma treated with MMS.)
Jeremy R. Etzhorn, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and an author of a 2019 claims data analysis of excisional surgery practices for melanoma, said that the new survey findings – like the prior analysis – highlight the variability in approaches to using MMS for melanoma.
“Mohs for melanoma [seems] like a one-liner ... but really, there are [a lot] of different techniques that fall under that umbrella, if you parse out all the variations,” he said in an interview.
Per the 2016 claims analysis, he noted, IHC was used in less than 40% of Mohs surgery cases for melanoma, and there were wide geographic variations. “The biggest critique of Mohs surgery for melanoma over the last two decades has been that it’s hard to see the tumor,” he said. “But with the advent of IHC, that challenge was overcome.”
Surgical excision practices are evolving without the development of best practice guidelines, said Dr. Etzkorn, who is director of clinical research for the University of Pennsylvania dermatologic oncology center. Multisociety guidelines published in 2012 on appropriate use criteria for Mohs surgery do not offer specific recommendations on the use of MMS for invasive melanoma. Nor do guidelines from the AAD and the NCCN, he said.
“What this [new] study highlights and what’s being discussed amongst Moh’s surgeons” is that Mohs for melanoma “has be to be standardized” to some extent and then clinical trials conducted comparing Mohs to conventional excision. The studies that have been published in recent years comparing MMS with WLE for MIS and invasive melanoma are “not gold standard studies,” he said.
Practice guidelines then can be informed by high-quality evidence on its safety and efficacy, he said.
The 513 participants in the newly published survey represent a 31.5% response rate. Invasive T2 and/or higher stage melanoma was more likely to be treated with MMS in academic hospitals, compared with other practice settings (30.2% v. 18.1%), Dr. Siscos and his coauthors reported.
Participants who reported treating melanoma with MMS were more likely to report fellowship exposure and more likely to have received fellowship training on melanocytic IHC stains. The study “highlights the importance of fellowship exposure to MMS and IHC staining for melanoma,” the authors wrote, adding that postfellowship training opportunities in MMS and IHC staining for melanoma may help broaden its use among Mohs surgeons who received inadequate fellowship exposure.
Dr. Siscos and his colleagues reported no significant interest with commercial supporters. Dr. Etzkorn had no disclosures.
SOURCE: Siscos S et al. Dermatol Surg. 2020 Oct;46(10):1267-71.
of members of the American College of Mohs Surgery.
Of 513 survey participants, 40.9% reported using MMS to treat any subtype of melanoma. Most of these surgeons reported treating both lentigo maligna (97.5%) and other melanoma in situ (MIS) subtypes (91.4%). A slight majority – 58.6% – reported treating invasive T1 melanoma, and 20.5% reported treating invasive T2 and/or higher-stage melanoma with MMS.
The analysis, published in Dermatologic Surgery, was done by Spyros M. Siscos, MD, and a team of residents and faculty in the division of dermatology at the University of Kansas Medical Center, Kansas City.
It comes on the heels of an analysis of claims data for Mohs surgery, published last year in JAMA Dermatology, which showed a more than threefold increase in the use of Mohs surgery for melanoma from 2.6% of all surgical cases in 2001 to 7.9% in 2016.
With the increased use of MMS for treatment of melanoma, “Mohs surgeons who previously treated MIS with MMS may be increasingly doing so and/or expanding their scope of treatment to include invasive melanoma,” the University of Kansas investigators wrote.
That a slight majority now report treating invasive melanoma with MMS “may be due, in part, to upstaging during the MMS procedure and the increasing evidence demonstrating improved survival of early-invasive melanoma treated with MMS compared with [wide local excision],” as well as the advent of melanocytic immunohistochemical (IHC) stains, particularly melanoma antigen recognized by T-cells 1 (MART-1), they said. However, 29% of surveyed Mohs surgeons treating melanoma with MMS do not use IHC stains “despite growing evidence supporting” their use, the authors wrote.
The advent of IHC stains, particularly MART-1, has improved the accuracy of interpreting frozen sections of melanoma, they reported, noting that MMS without IHC has been associated with a recurrence rate as high as 33%. Of the 71% who reported using IHC stains, MART-1 was the primary IHC stain for virtually all of them (97.3%).
There was also variation in the number of surgeons who reported debulking MIS. Eighty-two percent take this approach, excising the clinically visible tumor before excising the initial Mohs stage – almost all with a scalpel. More than half of these surgeons – 58.5% – submit the entire debulked MIS specimen for permanent vertical sectioning (breadloafing) to evaluate for deeper tumor invasion.
The others reported submitting the entire debulked specimen for frozen vertical sectioning, or portions of the specimen for both permanent and frozen vertical sectioning. “It is unclear why a minority of surveyed Mohs surgeons reported not debulking MIS,” wrote Dr. Siscos and his colleagues.
The average margin size of the first Mohs stage for MIS was 4.96 ± 1.74 mm, which is at the lower end of the 0.5-1.0 cm range for wide local excision (WLE) recommended by the National Comprehensive Cancer Network (NCCN) and the American Academy of Dermatology (AAD), according to a clinical practice guideline. (The survey did not investigate initial margins for invasive melanoma treated with MMS.)
Jeremy R. Etzhorn, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, and an author of a 2019 claims data analysis of excisional surgery practices for melanoma, said that the new survey findings – like the prior analysis – highlight the variability in approaches to using MMS for melanoma.
“Mohs for melanoma [seems] like a one-liner ... but really, there are [a lot] of different techniques that fall under that umbrella, if you parse out all the variations,” he said in an interview.
Per the 2016 claims analysis, he noted, IHC was used in less than 40% of Mohs surgery cases for melanoma, and there were wide geographic variations. “The biggest critique of Mohs surgery for melanoma over the last two decades has been that it’s hard to see the tumor,” he said. “But with the advent of IHC, that challenge was overcome.”
Surgical excision practices are evolving without the development of best practice guidelines, said Dr. Etzkorn, who is director of clinical research for the University of Pennsylvania dermatologic oncology center. Multisociety guidelines published in 2012 on appropriate use criteria for Mohs surgery do not offer specific recommendations on the use of MMS for invasive melanoma. Nor do guidelines from the AAD and the NCCN, he said.
“What this [new] study highlights and what’s being discussed amongst Moh’s surgeons” is that Mohs for melanoma “has be to be standardized” to some extent and then clinical trials conducted comparing Mohs to conventional excision. The studies that have been published in recent years comparing MMS with WLE for MIS and invasive melanoma are “not gold standard studies,” he said.
Practice guidelines then can be informed by high-quality evidence on its safety and efficacy, he said.
The 513 participants in the newly published survey represent a 31.5% response rate. Invasive T2 and/or higher stage melanoma was more likely to be treated with MMS in academic hospitals, compared with other practice settings (30.2% v. 18.1%), Dr. Siscos and his coauthors reported.
Participants who reported treating melanoma with MMS were more likely to report fellowship exposure and more likely to have received fellowship training on melanocytic IHC stains. The study “highlights the importance of fellowship exposure to MMS and IHC staining for melanoma,” the authors wrote, adding that postfellowship training opportunities in MMS and IHC staining for melanoma may help broaden its use among Mohs surgeons who received inadequate fellowship exposure.
Dr. Siscos and his colleagues reported no significant interest with commercial supporters. Dr. Etzkorn had no disclosures.
SOURCE: Siscos S et al. Dermatol Surg. 2020 Oct;46(10):1267-71.
FROM DERMATOLOGIC SURGERY
A teen presents with a severe, tender rash on the extremities
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
“There’s rue for you, and here’s some for me; we may call it herb of grace o’ Sundays. O, you must wear your rue with a difference.”
— Ophelia in Hamlet by William Shakespeare
The patient was admitted to the hospital for IV fluids, pain control, and observation. The following day she admitted using the leaves of a plant on the trail as a bug repellent, as one time was taught by her grandfather. She rubbed some of the leaves on the brother as well. The grandfather shared some pictures of the bushes, and the plant was identified as Ruta graveolens.
The blisters were deroofed, cleaned with saline, and wrapped with triamcinolone ointment and petrolatum. The patient was also started on a prednisone taper and received analgesics for the severe pain.
Ruta graveolens also known as common rue or herb of grace, is an ornamental plant from the Rutaceae family. This plant is also used as a medicinal herb, condiment, and as an insect repellent. If ingested in large doses, it can cause severe abdominal pain and vomiting. It also can be hepatotoxic.
The herb contains furocumarines, such as 8-methoxypsoralen and 5-methoxypsoralen and furoquinoline alkaloids. These chemicals when exposed to UVA radiation cause cell injury and inflammation of the skin. This is considered a phototoxic reaction of the skin, compared with allergic reactions, such as poison ivy dermatitis, which need a prior sensitization to the allergen for the T cells to be activated and cause injury in the skin. Other common plants and fruits that can cause phytophotodermatitis include citrus fruits, figs, carrots, celery, parsnips, parsley, and other wildflowers like hogweed.
Depending on the degree of injury, the patients can be treated with topical corticosteroids, petrolatum wraps, and pain control. In severe cases like our patient, systemic prednisone may help stop the progression of the lesions and help with the inflammation. Skin hyperpigmentation after the initial injury may take months to clear, and some patient can develop scars.
The differential diagnosis should include severe bullous contact dermatitis like exposure to urushiol in poison ivy; second- and third-degree burns; severe medications reactions such Stevens-Johnson syndrome or toxic epidermal necrolysis, and inmunobullous diseases such as bullous lupus erythematosus, pemphigus vulgaris, or bullous pemphigoid. If there is no history of exposure or there are any other systemic symptoms, consider performing a skin biopsy of one of the lesions.
In this patient’s case, the history of exposure and skin findings helped the dermatologist on call make the right diagnosis.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
J Burn Care Res. 2018 Oct 23;39(6):1064-6.
Dermatitis. 2007 Mar;18(1):52-5.
BMJ Case Rep. 2015 Dec 23;2015:bcr2015213388.
She started taking lithium for depression and anxiety 3 weeks prior to her developing the rash. She denies taking any other medications, supplements, or recreational drugs.
She denied any prior history of photosensitivity, no history of mouth ulcers, joint pain, muscle weakness, hair loss, or any other symptoms.
Besides her brother, there are no other affected family members, and no history of immune bullous disorders or other skin conditions.
On physical exam, the girl appears in a lot of pain and is uncomfortable. The skin is red and hot, and there are tense bullae on the neck, arms, and legs. There are no ocular or mucosal lesions.
The unsteady state
As the COVID-19 pandemic continues to chug along, some communities feel it slowing to a pace at which they might feel comfortable about a return to, if not quite “business as usual,” at least “business as sort of normal-ish.” They are ready to accept a level of disease that signals they have reached a steady state. However, in other communities, the virus has picked up speed and is threatening to overwhelm the medical infrastructure. If you are in one of those fortunate and skillfully managed states in which folks are beginning to talk seriously, but with little evidence, that it is time to return to normal, it is probably far too early.
Eons ago in pandemic terms, the World Health Organization in Thailand published a list of criteria to aid in determining when a community could consider lifting the limits that seemed to have been effective in halting transmission of the virus (“Transitioning to and maintaining a steady state of low-level or no transmission,” WHO, Thailand, 2020 Apr 18). While much more has been learned about the behavior of the virus since the spring of 2020, the criteria from the WHO in Thailand are worth considering.
Here is my summary of their criteria for returning to normalcy. First, virus transmission is controlled to the point that only sporadic cases and small clusters exist, and that all of these are traceable in origin. Second, health care and public health systems are in place with sufficient capacities to manage a shift from detection to treatment should the case load increase dramatically; this capacity should include detection, testing, isolation, and quarantine. Third, outbreaks in high-risk populations such as nursing homes have been minimized. Fourth, workplace prevention strategies are in place and have been demonstrated to be effective. Fifth, risk of imported cases is at manageable levels. Finally, communities are engaged.
It is hard to argue with the rationale behind each of these criteria. However, the United States is not Thailand, and just thinking about how this country would go about meeting those criteria provides a window into some of the reasons why we have done so poorly and will continue to be challenged in dealing with the pandemic.
First, notice that the criteria make no mention of a vaccine. One gets the sense that from the top down our country is banking too heavily on the effectiveness and widespread delivery of a vaccine. Even if and when a vaccine is developed and delivered, all of these criteria still must be met and kept in mind for a future pandemic.
Second, the criteria call for an effective health care system, but it is abundantly clear that the United States does not have a cohesive health care system and probably won’t for the foreseeable future. The best we can hope for is individual states cobbling together their own systems, which may in turn serve as examples for those states who haven’t had the foresight. We have had a public health system of sorts, but its credibility and effectiveness has been neutered to the point that again we must rely on each state’s ability to see through the haze and create it’s own systems for detection, testing, tracking, isolating, and quarantining – often with little help in materiel support from the federal government. The sliver of good news is that, after a bit of a stumbling start, detecting and limiting the importation of cases from abroad is being addressed.
We continue to hear and see evidence that there are segments of the population who are not engaged in the activities that we have learned are necessary to stabilize the pandemic. My sense is that those people represent a very small minority. But, it is probably large enough to make the route to a steady state on a national level long and painful. This unfortunately is to be expected in a country that was built on a framework of personal freedoms. The best you can hope for in achieving a steady state is to live in one of the states that seems to be achieving the fine balance between personal freedoms and the common good.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the COVID-19 pandemic continues to chug along, some communities feel it slowing to a pace at which they might feel comfortable about a return to, if not quite “business as usual,” at least “business as sort of normal-ish.” They are ready to accept a level of disease that signals they have reached a steady state. However, in other communities, the virus has picked up speed and is threatening to overwhelm the medical infrastructure. If you are in one of those fortunate and skillfully managed states in which folks are beginning to talk seriously, but with little evidence, that it is time to return to normal, it is probably far too early.
Eons ago in pandemic terms, the World Health Organization in Thailand published a list of criteria to aid in determining when a community could consider lifting the limits that seemed to have been effective in halting transmission of the virus (“Transitioning to and maintaining a steady state of low-level or no transmission,” WHO, Thailand, 2020 Apr 18). While much more has been learned about the behavior of the virus since the spring of 2020, the criteria from the WHO in Thailand are worth considering.
Here is my summary of their criteria for returning to normalcy. First, virus transmission is controlled to the point that only sporadic cases and small clusters exist, and that all of these are traceable in origin. Second, health care and public health systems are in place with sufficient capacities to manage a shift from detection to treatment should the case load increase dramatically; this capacity should include detection, testing, isolation, and quarantine. Third, outbreaks in high-risk populations such as nursing homes have been minimized. Fourth, workplace prevention strategies are in place and have been demonstrated to be effective. Fifth, risk of imported cases is at manageable levels. Finally, communities are engaged.
It is hard to argue with the rationale behind each of these criteria. However, the United States is not Thailand, and just thinking about how this country would go about meeting those criteria provides a window into some of the reasons why we have done so poorly and will continue to be challenged in dealing with the pandemic.
First, notice that the criteria make no mention of a vaccine. One gets the sense that from the top down our country is banking too heavily on the effectiveness and widespread delivery of a vaccine. Even if and when a vaccine is developed and delivered, all of these criteria still must be met and kept in mind for a future pandemic.
Second, the criteria call for an effective health care system, but it is abundantly clear that the United States does not have a cohesive health care system and probably won’t for the foreseeable future. The best we can hope for is individual states cobbling together their own systems, which may in turn serve as examples for those states who haven’t had the foresight. We have had a public health system of sorts, but its credibility and effectiveness has been neutered to the point that again we must rely on each state’s ability to see through the haze and create it’s own systems for detection, testing, tracking, isolating, and quarantining – often with little help in materiel support from the federal government. The sliver of good news is that, after a bit of a stumbling start, detecting and limiting the importation of cases from abroad is being addressed.
We continue to hear and see evidence that there are segments of the population who are not engaged in the activities that we have learned are necessary to stabilize the pandemic. My sense is that those people represent a very small minority. But, it is probably large enough to make the route to a steady state on a national level long and painful. This unfortunately is to be expected in a country that was built on a framework of personal freedoms. The best you can hope for in achieving a steady state is to live in one of the states that seems to be achieving the fine balance between personal freedoms and the common good.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
As the COVID-19 pandemic continues to chug along, some communities feel it slowing to a pace at which they might feel comfortable about a return to, if not quite “business as usual,” at least “business as sort of normal-ish.” They are ready to accept a level of disease that signals they have reached a steady state. However, in other communities, the virus has picked up speed and is threatening to overwhelm the medical infrastructure. If you are in one of those fortunate and skillfully managed states in which folks are beginning to talk seriously, but with little evidence, that it is time to return to normal, it is probably far too early.
Eons ago in pandemic terms, the World Health Organization in Thailand published a list of criteria to aid in determining when a community could consider lifting the limits that seemed to have been effective in halting transmission of the virus (“Transitioning to and maintaining a steady state of low-level or no transmission,” WHO, Thailand, 2020 Apr 18). While much more has been learned about the behavior of the virus since the spring of 2020, the criteria from the WHO in Thailand are worth considering.
Here is my summary of their criteria for returning to normalcy. First, virus transmission is controlled to the point that only sporadic cases and small clusters exist, and that all of these are traceable in origin. Second, health care and public health systems are in place with sufficient capacities to manage a shift from detection to treatment should the case load increase dramatically; this capacity should include detection, testing, isolation, and quarantine. Third, outbreaks in high-risk populations such as nursing homes have been minimized. Fourth, workplace prevention strategies are in place and have been demonstrated to be effective. Fifth, risk of imported cases is at manageable levels. Finally, communities are engaged.
It is hard to argue with the rationale behind each of these criteria. However, the United States is not Thailand, and just thinking about how this country would go about meeting those criteria provides a window into some of the reasons why we have done so poorly and will continue to be challenged in dealing with the pandemic.
First, notice that the criteria make no mention of a vaccine. One gets the sense that from the top down our country is banking too heavily on the effectiveness and widespread delivery of a vaccine. Even if and when a vaccine is developed and delivered, all of these criteria still must be met and kept in mind for a future pandemic.
Second, the criteria call for an effective health care system, but it is abundantly clear that the United States does not have a cohesive health care system and probably won’t for the foreseeable future. The best we can hope for is individual states cobbling together their own systems, which may in turn serve as examples for those states who haven’t had the foresight. We have had a public health system of sorts, but its credibility and effectiveness has been neutered to the point that again we must rely on each state’s ability to see through the haze and create it’s own systems for detection, testing, tracking, isolating, and quarantining – often with little help in materiel support from the federal government. The sliver of good news is that, after a bit of a stumbling start, detecting and limiting the importation of cases from abroad is being addressed.
We continue to hear and see evidence that there are segments of the population who are not engaged in the activities that we have learned are necessary to stabilize the pandemic. My sense is that those people represent a very small minority. But, it is probably large enough to make the route to a steady state on a national level long and painful. This unfortunately is to be expected in a country that was built on a framework of personal freedoms. The best you can hope for in achieving a steady state is to live in one of the states that seems to be achieving the fine balance between personal freedoms and the common good.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Mastering mask communicating
. For those specialties not accustomed to wearing a mask all day, it’s frustrating: How many times have you had to repeat yourself today? Or ask your patient to say something again? (Ain’t no one got time to repeat a third time how to do that prednisone taper). Worse, we’re losing important nonverbal cues that help us connect with our patients. How can we be understood when our faces are covered and 6 feet away?
Masks muffle both verbal and nonverbal communication. For soft-spoken or high-pitched speakers, the verbal effect is significant. In particular, masks make hearing consonants more difficult. They can make the “sh,” “th,” “f,” and “s” sounds difficult to distinguish. Typically, we’d use context and lip reading to boost the signal, but this fix is blocked (and the clear mouth-window masks are kinda creepy).
Masks also prevent us from seeing facial microexpressions, critical information when you are trying to connect with someone or to build trust. A randomized controlled trial published in 2013 indeed showed that doctors wearing a mask were perceived as less empathetic and had diminished relational continuity with patients as compared to doctors not wearing a mask. There are a few things we can do to help.
Speak more loudly is obvious advice. Loud talking has limitations though, as it can feel rude, and it blunts inflections, which add richness and emotion. (Shouting “THIS WILL ONLY HURT A LITTLE” seems a mixed message). More important than the volume is your choice of words. Try to use simple terms and short sentences. Pause between points. Hit your consonants harder.
It’s also important that you have their full attention and are giving yours. As much as possible, try to align squared up with patients. Facing your computer exacerbates the problem. Look them in their eyes and be sure they are connected with you before any complex or difficult conversations. Hearing-impaired patients are now sometimes leaving out their aids because it’s too uncomfortable to wear them with their mask. You might ask them to put them back in. Check in with patients and repeat back what you heard them say. This can help with clarity and with connecting. Use your face more: if you’ve ever acted on stage, this would be your on-stage face. Exaggerate your expressions so it’s a little easier for them to read you.
Lastly, there are apps such as Ava or Google Live Translator, which can transcribe your speech real time. You could then share your screen with the patient so they can read exactly what you’ve said.
Some of us are natural communicators. Even if you are not, you can mitigate some of our current challenges. I’ll admit, it’s been a bit easier for me than for others. Between my prominent eyebrows and Italian-American upbringing, I can express my way through pretty much any face covering. If you’d like to learn how to use your hands better, then just watch this little girl: https://youtu.be/Z5wAWyqDrnc.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
. For those specialties not accustomed to wearing a mask all day, it’s frustrating: How many times have you had to repeat yourself today? Or ask your patient to say something again? (Ain’t no one got time to repeat a third time how to do that prednisone taper). Worse, we’re losing important nonverbal cues that help us connect with our patients. How can we be understood when our faces are covered and 6 feet away?
Masks muffle both verbal and nonverbal communication. For soft-spoken or high-pitched speakers, the verbal effect is significant. In particular, masks make hearing consonants more difficult. They can make the “sh,” “th,” “f,” and “s” sounds difficult to distinguish. Typically, we’d use context and lip reading to boost the signal, but this fix is blocked (and the clear mouth-window masks are kinda creepy).
Masks also prevent us from seeing facial microexpressions, critical information when you are trying to connect with someone or to build trust. A randomized controlled trial published in 2013 indeed showed that doctors wearing a mask were perceived as less empathetic and had diminished relational continuity with patients as compared to doctors not wearing a mask. There are a few things we can do to help.
Speak more loudly is obvious advice. Loud talking has limitations though, as it can feel rude, and it blunts inflections, which add richness and emotion. (Shouting “THIS WILL ONLY HURT A LITTLE” seems a mixed message). More important than the volume is your choice of words. Try to use simple terms and short sentences. Pause between points. Hit your consonants harder.
It’s also important that you have their full attention and are giving yours. As much as possible, try to align squared up with patients. Facing your computer exacerbates the problem. Look them in their eyes and be sure they are connected with you before any complex or difficult conversations. Hearing-impaired patients are now sometimes leaving out their aids because it’s too uncomfortable to wear them with their mask. You might ask them to put them back in. Check in with patients and repeat back what you heard them say. This can help with clarity and with connecting. Use your face more: if you’ve ever acted on stage, this would be your on-stage face. Exaggerate your expressions so it’s a little easier for them to read you.
Lastly, there are apps such as Ava or Google Live Translator, which can transcribe your speech real time. You could then share your screen with the patient so they can read exactly what you’ve said.
Some of us are natural communicators. Even if you are not, you can mitigate some of our current challenges. I’ll admit, it’s been a bit easier for me than for others. Between my prominent eyebrows and Italian-American upbringing, I can express my way through pretty much any face covering. If you’d like to learn how to use your hands better, then just watch this little girl: https://youtu.be/Z5wAWyqDrnc.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
. For those specialties not accustomed to wearing a mask all day, it’s frustrating: How many times have you had to repeat yourself today? Or ask your patient to say something again? (Ain’t no one got time to repeat a third time how to do that prednisone taper). Worse, we’re losing important nonverbal cues that help us connect with our patients. How can we be understood when our faces are covered and 6 feet away?
Masks muffle both verbal and nonverbal communication. For soft-spoken or high-pitched speakers, the verbal effect is significant. In particular, masks make hearing consonants more difficult. They can make the “sh,” “th,” “f,” and “s” sounds difficult to distinguish. Typically, we’d use context and lip reading to boost the signal, but this fix is blocked (and the clear mouth-window masks are kinda creepy).
Masks also prevent us from seeing facial microexpressions, critical information when you are trying to connect with someone or to build trust. A randomized controlled trial published in 2013 indeed showed that doctors wearing a mask were perceived as less empathetic and had diminished relational continuity with patients as compared to doctors not wearing a mask. There are a few things we can do to help.
Speak more loudly is obvious advice. Loud talking has limitations though, as it can feel rude, and it blunts inflections, which add richness and emotion. (Shouting “THIS WILL ONLY HURT A LITTLE” seems a mixed message). More important than the volume is your choice of words. Try to use simple terms and short sentences. Pause between points. Hit your consonants harder.
It’s also important that you have their full attention and are giving yours. As much as possible, try to align squared up with patients. Facing your computer exacerbates the problem. Look them in their eyes and be sure they are connected with you before any complex or difficult conversations. Hearing-impaired patients are now sometimes leaving out their aids because it’s too uncomfortable to wear them with their mask. You might ask them to put them back in. Check in with patients and repeat back what you heard them say. This can help with clarity and with connecting. Use your face more: if you’ve ever acted on stage, this would be your on-stage face. Exaggerate your expressions so it’s a little easier for them to read you.
Lastly, there are apps such as Ava or Google Live Translator, which can transcribe your speech real time. You could then share your screen with the patient so they can read exactly what you’ve said.
Some of us are natural communicators. Even if you are not, you can mitigate some of our current challenges. I’ll admit, it’s been a bit easier for me than for others. Between my prominent eyebrows and Italian-American upbringing, I can express my way through pretty much any face covering. If you’d like to learn how to use your hands better, then just watch this little girl: https://youtu.be/Z5wAWyqDrnc.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Guselkumab improvements for psoriatic arthritis persist through 1 year
Adults with active psoriatic arthritis (PsA) treated with guselkumab (Tremfya) showed significant improvement in American College of Rheumatology response criteria and disease activity after 1 year, based on data from the phase 3 DISCOVER-2 trial.
The findings, published in Arthritis & Rheumatology, extend the previously published 24-week, primary endpoint results of the trial, which tested guselkumab for adults with PsA who had not previously taken a biologic drug. Guselkumab was approved in July 2020 in the United States.
Iain B. McInnes, MD, PhD, of the University of Glasgow and his colleagues described guselkumab as “a fully-human monoclonal antibody specific to interleukin (IL)-23’s p19-subunit” that offers a potential alternative for PsA patients who discontinue their index tumor necrosis factor inhibitor because of insufficient efficacy.
The study enrolled 739 PsA patients at 118 sites worldwide. Participants were randomized to receive subcutaneous injections of 100 mg guselkumab every 4 weeks; 100 mg guselkumab at week 0 and 4, then every 8 weeks; or a placebo; 238 placebo-treated patients crossed over at 24 weeks to receive 100 mg guselkumab every 4 weeks. Patients on nonbiologic disease-modifying antirheumatic drugs at baseline were allowed to continue stable doses. Overall, about 93% of patients originally randomized to the three groups remained on guselkumab at 52 weeks.
Overall, 71% and 75% of 4-week and 8-week guselkumab patients, respectively, showed an improvement of at least 20% from baseline in ACR response criteria components at 52 weeks, which was up from 64% of patients seen at 24 weeks in both groups.
The study participants had an average disease duration of more than 5 years with no biologic treatment, and an average of 12-13 swollen joints and 20-22 tender joints at baseline. Approximately half were male, half had psoriasis or dactylitis, and two-thirds had enthesitis. Skin disease severity was assessed using the Investigator’s Global Assessment and Psoriasis Area Severity Index (PASI).
At 52 weeks, 75% and 58% of patients in the guselkumab groups had resolution of dactylitis and enthesitis, respectively. In addition, 86% of patients in both guselkumab groups achieved PASI 75 at 52 weeks, and 58% and 53% of the 4-week and 8-week groups, respectively, achieved PASI 100.
In addition, patients treated with guselkumab showed low levels of radiographic progression and significant improvements from baseline in measures of physical function and quality of life.
The most frequently reported adverse events in guselkumab patients were upper respiratory tract infections, nasopharyngitis, bronchitis, and investigator-reported laboratory values of increased alanine aminotransferase and aspartate aminotransferase; these rates were similar to those seen in the previously published 24-week data. Approximately 2% of guselkumab and placebo patients discontinued their treatments because of adverse events.
No patient developed an opportunistic infection or died during the study period.
The study findings were limited by several factors including the relatively short 1-year duration, the shorter duration of placebo, compared with guselkumab, and by potential confounding from missing data on patients who discontinued, the researchers noted. However, the results support the effectiveness of guselkumab for improving a range of manifestations of active PsA, and the overall treatment and safety profiles seen at 24 weeks were maintained, they said.
“Data obtained during the second year of DISCOVER-2 will augment current knowledge of the guselkumab benefit-risk profile and further our understanding of longer-term radiographic outcomes with both guselkumab dosing regimens,” they concluded.
The study was supported by Janssen. Many authors reported financial relationships with Janssen and other pharmaceutical companies. Nine of the 15 authors are employees of Janssen (a subsidiary of Johnson & Johnson) and own Johnson & Johnson stock or stock options.
SOURCE: McInnes IB et al. Arthritis Rheumatol. 2020 Oct 11. doi: 10.1002/art.41553.
Adults with active psoriatic arthritis (PsA) treated with guselkumab (Tremfya) showed significant improvement in American College of Rheumatology response criteria and disease activity after 1 year, based on data from the phase 3 DISCOVER-2 trial.
The findings, published in Arthritis & Rheumatology, extend the previously published 24-week, primary endpoint results of the trial, which tested guselkumab for adults with PsA who had not previously taken a biologic drug. Guselkumab was approved in July 2020 in the United States.
Iain B. McInnes, MD, PhD, of the University of Glasgow and his colleagues described guselkumab as “a fully-human monoclonal antibody specific to interleukin (IL)-23’s p19-subunit” that offers a potential alternative for PsA patients who discontinue their index tumor necrosis factor inhibitor because of insufficient efficacy.
The study enrolled 739 PsA patients at 118 sites worldwide. Participants were randomized to receive subcutaneous injections of 100 mg guselkumab every 4 weeks; 100 mg guselkumab at week 0 and 4, then every 8 weeks; or a placebo; 238 placebo-treated patients crossed over at 24 weeks to receive 100 mg guselkumab every 4 weeks. Patients on nonbiologic disease-modifying antirheumatic drugs at baseline were allowed to continue stable doses. Overall, about 93% of patients originally randomized to the three groups remained on guselkumab at 52 weeks.
Overall, 71% and 75% of 4-week and 8-week guselkumab patients, respectively, showed an improvement of at least 20% from baseline in ACR response criteria components at 52 weeks, which was up from 64% of patients seen at 24 weeks in both groups.
The study participants had an average disease duration of more than 5 years with no biologic treatment, and an average of 12-13 swollen joints and 20-22 tender joints at baseline. Approximately half were male, half had psoriasis or dactylitis, and two-thirds had enthesitis. Skin disease severity was assessed using the Investigator’s Global Assessment and Psoriasis Area Severity Index (PASI).
At 52 weeks, 75% and 58% of patients in the guselkumab groups had resolution of dactylitis and enthesitis, respectively. In addition, 86% of patients in both guselkumab groups achieved PASI 75 at 52 weeks, and 58% and 53% of the 4-week and 8-week groups, respectively, achieved PASI 100.
In addition, patients treated with guselkumab showed low levels of radiographic progression and significant improvements from baseline in measures of physical function and quality of life.
The most frequently reported adverse events in guselkumab patients were upper respiratory tract infections, nasopharyngitis, bronchitis, and investigator-reported laboratory values of increased alanine aminotransferase and aspartate aminotransferase; these rates were similar to those seen in the previously published 24-week data. Approximately 2% of guselkumab and placebo patients discontinued their treatments because of adverse events.
No patient developed an opportunistic infection or died during the study period.
The study findings were limited by several factors including the relatively short 1-year duration, the shorter duration of placebo, compared with guselkumab, and by potential confounding from missing data on patients who discontinued, the researchers noted. However, the results support the effectiveness of guselkumab for improving a range of manifestations of active PsA, and the overall treatment and safety profiles seen at 24 weeks were maintained, they said.
“Data obtained during the second year of DISCOVER-2 will augment current knowledge of the guselkumab benefit-risk profile and further our understanding of longer-term radiographic outcomes with both guselkumab dosing regimens,” they concluded.
The study was supported by Janssen. Many authors reported financial relationships with Janssen and other pharmaceutical companies. Nine of the 15 authors are employees of Janssen (a subsidiary of Johnson & Johnson) and own Johnson & Johnson stock or stock options.
SOURCE: McInnes IB et al. Arthritis Rheumatol. 2020 Oct 11. doi: 10.1002/art.41553.
Adults with active psoriatic arthritis (PsA) treated with guselkumab (Tremfya) showed significant improvement in American College of Rheumatology response criteria and disease activity after 1 year, based on data from the phase 3 DISCOVER-2 trial.
The findings, published in Arthritis & Rheumatology, extend the previously published 24-week, primary endpoint results of the trial, which tested guselkumab for adults with PsA who had not previously taken a biologic drug. Guselkumab was approved in July 2020 in the United States.
Iain B. McInnes, MD, PhD, of the University of Glasgow and his colleagues described guselkumab as “a fully-human monoclonal antibody specific to interleukin (IL)-23’s p19-subunit” that offers a potential alternative for PsA patients who discontinue their index tumor necrosis factor inhibitor because of insufficient efficacy.
The study enrolled 739 PsA patients at 118 sites worldwide. Participants were randomized to receive subcutaneous injections of 100 mg guselkumab every 4 weeks; 100 mg guselkumab at week 0 and 4, then every 8 weeks; or a placebo; 238 placebo-treated patients crossed over at 24 weeks to receive 100 mg guselkumab every 4 weeks. Patients on nonbiologic disease-modifying antirheumatic drugs at baseline were allowed to continue stable doses. Overall, about 93% of patients originally randomized to the three groups remained on guselkumab at 52 weeks.
Overall, 71% and 75% of 4-week and 8-week guselkumab patients, respectively, showed an improvement of at least 20% from baseline in ACR response criteria components at 52 weeks, which was up from 64% of patients seen at 24 weeks in both groups.
The study participants had an average disease duration of more than 5 years with no biologic treatment, and an average of 12-13 swollen joints and 20-22 tender joints at baseline. Approximately half were male, half had psoriasis or dactylitis, and two-thirds had enthesitis. Skin disease severity was assessed using the Investigator’s Global Assessment and Psoriasis Area Severity Index (PASI).
At 52 weeks, 75% and 58% of patients in the guselkumab groups had resolution of dactylitis and enthesitis, respectively. In addition, 86% of patients in both guselkumab groups achieved PASI 75 at 52 weeks, and 58% and 53% of the 4-week and 8-week groups, respectively, achieved PASI 100.
In addition, patients treated with guselkumab showed low levels of radiographic progression and significant improvements from baseline in measures of physical function and quality of life.
The most frequently reported adverse events in guselkumab patients were upper respiratory tract infections, nasopharyngitis, bronchitis, and investigator-reported laboratory values of increased alanine aminotransferase and aspartate aminotransferase; these rates were similar to those seen in the previously published 24-week data. Approximately 2% of guselkumab and placebo patients discontinued their treatments because of adverse events.
No patient developed an opportunistic infection or died during the study period.
The study findings were limited by several factors including the relatively short 1-year duration, the shorter duration of placebo, compared with guselkumab, and by potential confounding from missing data on patients who discontinued, the researchers noted. However, the results support the effectiveness of guselkumab for improving a range of manifestations of active PsA, and the overall treatment and safety profiles seen at 24 weeks were maintained, they said.
“Data obtained during the second year of DISCOVER-2 will augment current knowledge of the guselkumab benefit-risk profile and further our understanding of longer-term radiographic outcomes with both guselkumab dosing regimens,” they concluded.
The study was supported by Janssen. Many authors reported financial relationships with Janssen and other pharmaceutical companies. Nine of the 15 authors are employees of Janssen (a subsidiary of Johnson & Johnson) and own Johnson & Johnson stock or stock options.
SOURCE: McInnes IB et al. Arthritis Rheumatol. 2020 Oct 11. doi: 10.1002/art.41553.
FROM ARTHRITIS & RHEUMATOLOGY
Blood group O linked to decreased risk of SARS-CoV-2 infection
Blood group O was associated with a decreased risk for contracting SARS-CoV-2 infection, according to the results of large retrospective analysis of the Danish population.
Researchers Mike Bogetofte Barnkob, MD, of the Department of Clinical Immunology, Odense (Denmark) University Hospital, and colleagues performed a retrospective cohort analysis of all Danish individuals with a known ABO blood group who were tested for SARS-CoV-2 between Feb. 27, 2020, and July 30, 2020.
Of the 841,327 people tested, ABO and RhD blood groups could be identified for 473,654 individuals. ABO and RhD data from 2,204,742 (38% of the entire Danish population) were used as a reference, according to the online report in Blood Advances.
The primary outcome was status of ABO and RhD blood groups and test results for SARS-CoV-2. The secondary outcomes followed were hospitalization and death from COVID-19.
Reduced prevalence
The study found that ABO blood groups varied significantly between patients and the reference group, with only 38.41% (95% confidence interval, 37.30%-39.50%) of the patients belonging to blood group O, compared with 41.70% (95% CI, 41.60%-41.80%) in the controls, corresponding to a relative risk of 0.87 (95% CI, 0.83-0.91) for acquiring COVID-19.
There was a slight, but statistically significant, difference in blood group distribution between the SARS-CoV-22 individuals and the reference population (P < .001), according to the authors.
Among the SARS-CoV-2 individuals, fewer group O individuals were found (P < .001); while more A, B, and AB individuals were seen (P < .001, P = .011, and P = .091, respectively). There was no significant difference seen among A, B, and AB blood groups (P = .30). The RR for contracting SARS-CoV-2 were 1.09 (95% CI, 1.04-1.14) for A group individuals; 1.06 (95% CI, 0.99-1.14) for B group; and 1.15 (95% CI, 1.03-1.27) for AB group, respectively.
There was no difference found in the RhD group between positive test cases and the reference population (P = .15). In addition, there was no statistical difference (all P > .40) between ABO blood groups and clinical severity of COVID-19 for nonhospitalized patients versus hospitalized patients or for deceased patients versus living patients, the researchers added.
Possible causes
The authors speculated on two possible causes of the lower prevalence of SARS-CoV-2 infection in the blood group O population. The first is that anti-A and anti-B antibodies may have an effect on neutralizing SARS-CoV viruses and that anti-A and anti-B are present on mucosal surfaces in some individuals lacking the corresponding ABO blood group. The second is that the association between ABO blood groups and levels of von Willebrand factor, which is higher in non-O individuals and is tied to an increased likelihood of arterial and venous thrombosis, could have an indirect or unknown impact on susceptibility to infection, according to the authors.
“Given the known increased risk of thrombosis in non-O individuals and the evolving central role for thrombosis in the pathogenesis of COVID-19, it is important to explore this aspect more closely in larger patient cohorts (e.g., by examining ABO blood type and viral load, the severity of symptoms, and the long-term effects following COVID-19),” the researchers concluded.
One author reported receiving fees from Bristol Myers Squibb, Novartis, and Roche. The remaining authors reported they had no competing financial interests.
SOURCE: Barnkob MB et al. Blood Adv. 2020 Oct 14. doi: 10.1182/bloodadvances.2020002657.
Blood group O was associated with a decreased risk for contracting SARS-CoV-2 infection, according to the results of large retrospective analysis of the Danish population.
Researchers Mike Bogetofte Barnkob, MD, of the Department of Clinical Immunology, Odense (Denmark) University Hospital, and colleagues performed a retrospective cohort analysis of all Danish individuals with a known ABO blood group who were tested for SARS-CoV-2 between Feb. 27, 2020, and July 30, 2020.
Of the 841,327 people tested, ABO and RhD blood groups could be identified for 473,654 individuals. ABO and RhD data from 2,204,742 (38% of the entire Danish population) were used as a reference, according to the online report in Blood Advances.
The primary outcome was status of ABO and RhD blood groups and test results for SARS-CoV-2. The secondary outcomes followed were hospitalization and death from COVID-19.
Reduced prevalence
The study found that ABO blood groups varied significantly between patients and the reference group, with only 38.41% (95% confidence interval, 37.30%-39.50%) of the patients belonging to blood group O, compared with 41.70% (95% CI, 41.60%-41.80%) in the controls, corresponding to a relative risk of 0.87 (95% CI, 0.83-0.91) for acquiring COVID-19.
There was a slight, but statistically significant, difference in blood group distribution between the SARS-CoV-22 individuals and the reference population (P < .001), according to the authors.
Among the SARS-CoV-2 individuals, fewer group O individuals were found (P < .001); while more A, B, and AB individuals were seen (P < .001, P = .011, and P = .091, respectively). There was no significant difference seen among A, B, and AB blood groups (P = .30). The RR for contracting SARS-CoV-2 were 1.09 (95% CI, 1.04-1.14) for A group individuals; 1.06 (95% CI, 0.99-1.14) for B group; and 1.15 (95% CI, 1.03-1.27) for AB group, respectively.
There was no difference found in the RhD group between positive test cases and the reference population (P = .15). In addition, there was no statistical difference (all P > .40) between ABO blood groups and clinical severity of COVID-19 for nonhospitalized patients versus hospitalized patients or for deceased patients versus living patients, the researchers added.
Possible causes
The authors speculated on two possible causes of the lower prevalence of SARS-CoV-2 infection in the blood group O population. The first is that anti-A and anti-B antibodies may have an effect on neutralizing SARS-CoV viruses and that anti-A and anti-B are present on mucosal surfaces in some individuals lacking the corresponding ABO blood group. The second is that the association between ABO blood groups and levels of von Willebrand factor, which is higher in non-O individuals and is tied to an increased likelihood of arterial and venous thrombosis, could have an indirect or unknown impact on susceptibility to infection, according to the authors.
“Given the known increased risk of thrombosis in non-O individuals and the evolving central role for thrombosis in the pathogenesis of COVID-19, it is important to explore this aspect more closely in larger patient cohorts (e.g., by examining ABO blood type and viral load, the severity of symptoms, and the long-term effects following COVID-19),” the researchers concluded.
One author reported receiving fees from Bristol Myers Squibb, Novartis, and Roche. The remaining authors reported they had no competing financial interests.
SOURCE: Barnkob MB et al. Blood Adv. 2020 Oct 14. doi: 10.1182/bloodadvances.2020002657.
Blood group O was associated with a decreased risk for contracting SARS-CoV-2 infection, according to the results of large retrospective analysis of the Danish population.
Researchers Mike Bogetofte Barnkob, MD, of the Department of Clinical Immunology, Odense (Denmark) University Hospital, and colleagues performed a retrospective cohort analysis of all Danish individuals with a known ABO blood group who were tested for SARS-CoV-2 between Feb. 27, 2020, and July 30, 2020.
Of the 841,327 people tested, ABO and RhD blood groups could be identified for 473,654 individuals. ABO and RhD data from 2,204,742 (38% of the entire Danish population) were used as a reference, according to the online report in Blood Advances.
The primary outcome was status of ABO and RhD blood groups and test results for SARS-CoV-2. The secondary outcomes followed were hospitalization and death from COVID-19.
Reduced prevalence
The study found that ABO blood groups varied significantly between patients and the reference group, with only 38.41% (95% confidence interval, 37.30%-39.50%) of the patients belonging to blood group O, compared with 41.70% (95% CI, 41.60%-41.80%) in the controls, corresponding to a relative risk of 0.87 (95% CI, 0.83-0.91) for acquiring COVID-19.
There was a slight, but statistically significant, difference in blood group distribution between the SARS-CoV-22 individuals and the reference population (P < .001), according to the authors.
Among the SARS-CoV-2 individuals, fewer group O individuals were found (P < .001); while more A, B, and AB individuals were seen (P < .001, P = .011, and P = .091, respectively). There was no significant difference seen among A, B, and AB blood groups (P = .30). The RR for contracting SARS-CoV-2 were 1.09 (95% CI, 1.04-1.14) for A group individuals; 1.06 (95% CI, 0.99-1.14) for B group; and 1.15 (95% CI, 1.03-1.27) for AB group, respectively.
There was no difference found in the RhD group between positive test cases and the reference population (P = .15). In addition, there was no statistical difference (all P > .40) between ABO blood groups and clinical severity of COVID-19 for nonhospitalized patients versus hospitalized patients or for deceased patients versus living patients, the researchers added.
Possible causes
The authors speculated on two possible causes of the lower prevalence of SARS-CoV-2 infection in the blood group O population. The first is that anti-A and anti-B antibodies may have an effect on neutralizing SARS-CoV viruses and that anti-A and anti-B are present on mucosal surfaces in some individuals lacking the corresponding ABO blood group. The second is that the association between ABO blood groups and levels of von Willebrand factor, which is higher in non-O individuals and is tied to an increased likelihood of arterial and venous thrombosis, could have an indirect or unknown impact on susceptibility to infection, according to the authors.
“Given the known increased risk of thrombosis in non-O individuals and the evolving central role for thrombosis in the pathogenesis of COVID-19, it is important to explore this aspect more closely in larger patient cohorts (e.g., by examining ABO blood type and viral load, the severity of symptoms, and the long-term effects following COVID-19),” the researchers concluded.
One author reported receiving fees from Bristol Myers Squibb, Novartis, and Roche. The remaining authors reported they had no competing financial interests.
SOURCE: Barnkob MB et al. Blood Adv. 2020 Oct 14. doi: 10.1182/bloodadvances.2020002657.
FROM BLOOD ADVANCES