User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
FDA approves point-of-care COVID-19 antigen test
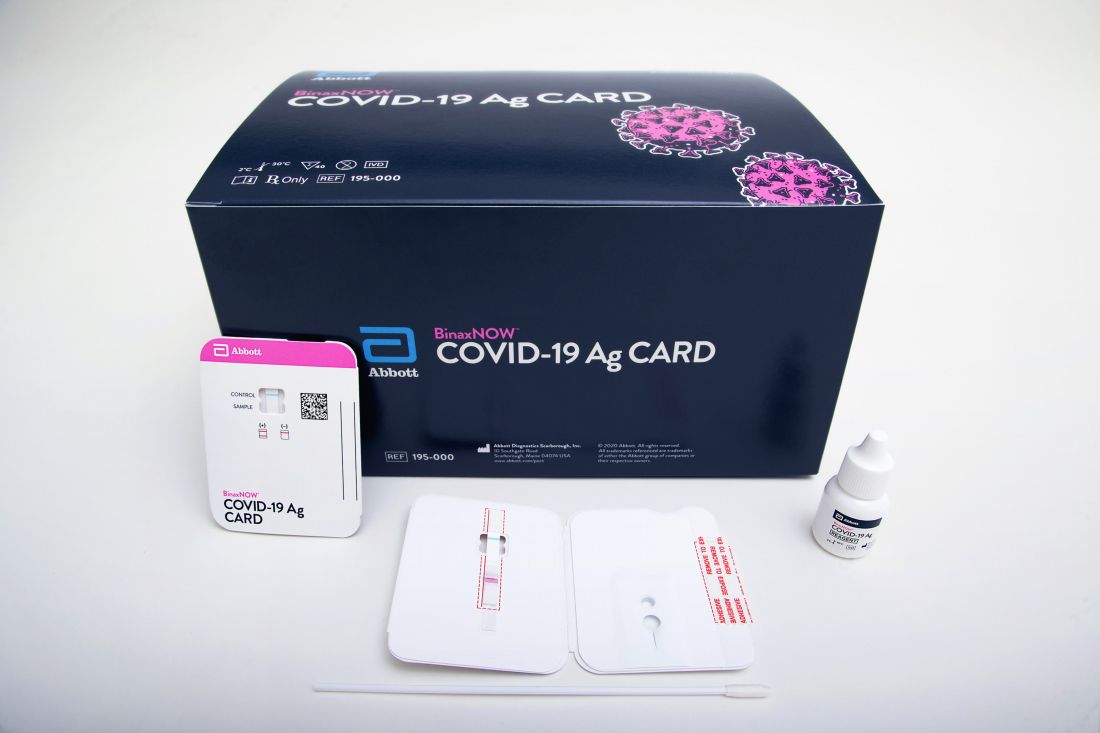
The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.

The BinaxNOW COVID-19 Ag Card (Abbott) is similar in some ways to a home pregnancy test. Clinicians read results on a card – one line for a negative result, two lines for positive.
A health care provider swabs a symptomatic patient’s nose, twirls the sample on a test card with a reagent, and waits approximately 15 minutes for results. No additional equipment is required.
Abbott expects the test to cost about $5.00, the company announced.
Office-based physicians, ED physicians, and school nurses could potentially use the product as a point-of-care test. The FDA granted the test emergency use authorization. It is approved for people suspected of having COVID-19 who are within 7 days of symptom onset.
“This new COVID-19 antigen test is an important addition to available tests because the results can be read in minutes, right off the testing card,” Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health, wrote in a news release. “This means people will know if they have the virus in almost real time.”
“This fits into the testing landscape as a simple, inexpensive test that does not require additional equipment,” Marcus Lynch, PhD, assistant manager of the Health Care Horizon Scanning program at ECRI, told Medscape Medical News when asked to comment. ECRI is an independent, nonprofit organization that reviews and analyses COVID-19 therapeutics and diagnostics.
The test could help with early triage of patients who test positive, perhaps alerting physicians to the need to start COVID-19 therapy, added Lynch, who specializes in immunology and vaccine development. The test also could be useful in low-resource settings.
The FDA included a caveat: antigen tests are generally less sensitive than molecular assays. “Due to the potential for decreased sensitivity compared to molecular assays, negative results from an antigen test may need to be confirmed with a molecular test prior to making treatment decisions,” the agency noted.
Lynch agreed and said that when a patient tests negative, physicians still need to use their clinical judgment on the basis of symptoms and other factors. The test is not designed for population-based screening of asymptomatic people, he added.
Abbott announced plans to make up to 50 million tests available per month in the United States starting in October. The product comes with a free smartphone app that people can use to share results with an employer or with others as needed.
This article first appeared on Medscape.com.
COVID-19 vaccine supply will be limited at first, ACIP says
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) yesterday held its third meeting this summer to discuss the vaccines and plan how initial vaccines will be allocated, inasmuch as supplies will likely be limited at first. Vaccines are expected to be more available as production ramps up and as more than one vaccine become available, but vaccine allocation initially will need to take place in phases.
Considerations include first getting the vaccine to individuals who need it the most, such as healthcare personnel and essential workers, as well as those at higher risk for severe illness or death, including the elderly, those with underlying conditions, and certain racial and ethnic minorities. Other factors include storage requirements that might be difficult to meet in certain settings and the fact that both vaccines must be given in two doses.
Vaccine allocation models
The group presented two possible models for allocating initial vaccine supplies.
The first population model considers risk status within each age group on the basis of underlying health conditions and occupational group, with priority given to healthcare personnel (paid or unpaid) and essential workers. The model considers partial reopening and social distancing, expected vaccine efficacy, prevaccination immunity, mortality, and the direct and indirect benefits of vaccination.
In this model, COVID-19 infections and deaths were reduced when healthcare personnel, essential workers, or adults with underlying conditions were vaccinated. There were smaller differences between the groups with respect to the impact of vaccination. Declines in infections were “more modest” and declines in deaths were greater when adults aged 65 years and older were vaccinated in comparison with other age groups.
The second model focused on vaccination of nursing home healthcare personnel and residents. Vaccinating nursing home healthcare personnel reduced infections and deaths more than vaccinating nursing home residents.
In settings such as long-term care facilities and correction facilities, where people gather in groups, cases increase first among staff. The vaccine working group suggests that vaccinating staff may also benefit individuals living in those facilities.
The working group expects that from 15 to 45 million doses of vaccine will be available by the end of December, depending on which vaccine is approved by then or whether both are approved.
Supplies won’t be nearly enough to vaccinate everyone: There are approximately 17 to 20 million healthcare workers in the United States and 60 to 80 million essential workers who do not work in healthcare. More than 100 million adults have underlying medical conditions that put them at higher risk for hospitalization and death, such as obesity, cardiovascular disease, diabetes, and chronic obstructive pulmonary disease. And approximately 53 million adults are aged 65 years or older.
The group reviewed promising early data for two vaccines under development.
The mRNA-1273 vaccine, made by Moderna with support from two federal agencies, is moving into phase 3 clinical trials – enrollment into the COVID-19 Efficacy and Safety (COVE) study is ongoing, according to Jacqueline M. Miller, MD, senior vice president and therapeutic area head of infectious diseases. The study’s primary objective will be to determine whether two doses can prevent symptomatic COVID-19, according to an NIH news release.
A second mRNA vaccine, BNT 162b2, made by Pfizer and BioNTech, is entering phase 2/3 trials. Nearly 20% of people enrolled are Black or Hispanic persons, and 4% are Asian persons. The team is also trying to recruit Native American participants, Nicholas Kitchin, MD, senior director in Pfizer’s vaccine clinical research and development group, said in a presentation to the advisory committee.
‘Ultra-cold’ temperatures required for storage
Both vaccines require storage at lower temperatures than is usually needed for vaccines. One vaccine must be distributed and stored at -20° C, and the other must be stored, distributed, and handled at -70° C.
This issue stands out most to ACIP Chair Jose Romero, MD. He says the “ultra-cold” temperatures required for storage and transportation of the vaccines will be a “significant problem” for those in rural areas.
High-risk populations such as meat processors and agricultural workers “may have to wait until we have a more stable vaccine that can be transported and delivered more or less at room temperature,” Romero explained. He is the chief medical officer at the Arkansas Department of Health and is a professor of pediatrics and pediatric infectious diseases at the University of Arkansas for Medical Sciences, both in Little Rock.
The advisory committee will meet again on September 22. At that time, they’ll vote on an interim plan for prioritization of the first COVID-19 vaccine.
This article first appeared on Medscape.com.
FDA approves topical antiandrogen for acne
Clascoterone is a topical androgen receptor inhibitor indicated for treatment of acne vulgaris in patients aged 12 years and older, according to the labeling from manufacturer Cassiopea. Clascoterone, which will be marketed as Winlevi, targets the androgen hormones that contribute to acne by inhibiting serum production and inflammation, according to a company press release.
“Although clascoterone’s exact mechanism of action is unknown, laboratory studies suggest clascoterone competes with androgens, specifically dihydrotestosterone, for binding to the androgen receptors within the sebaceous gland and hair follicles,” according to the release.
Approval was based in part on a pair of phase 3, double-blind, vehicle-controlled, 12-week, randomized trials including 1,440 patients aged 9 years and older with moderate to severe facial acne. The findings were published in April, in JAMA Dermatology .
Participants were randomized to twice-daily application of clascoterone or a control vehicle; treatment success was defined as having an Investigator’s Global Assessment score of 0 (clear) or 1 (almost clear), as well as at least a 2-grade improvement from baseline, and absolute change in noninflammatory and inflammatory lesion counts at week 12.
At 12 weeks, treatment success rates were 18.4% and 20.3% among those on clascoterone, compared with 9% and 6.5%, respectively, among controls. There were also significant reductions in noninflammatory and inflammatory lesions from baseline at 12 weeks, compared with controls.
In the studies, treatment was well tolerated, with a safety profile similar to safety in controls. Adverse events thought to be related to clascoterone in the studies (a total of 13) included application-site pain; erythema; oropharyngeal pain; hypersensitivity, dryness, or hypertrichosis at the application site; eye irritation; headache; and hair color changes. “Clascoterone targets androgen receptors at the site of application and is quickly metabolized to an inactive form, thus limiting systemic activity,” the authors of the study wrote.
Clascoterone is expected to be available in the United States in early 2021, according to the manufacturer.
Clascoterone is a topical androgen receptor inhibitor indicated for treatment of acne vulgaris in patients aged 12 years and older, according to the labeling from manufacturer Cassiopea. Clascoterone, which will be marketed as Winlevi, targets the androgen hormones that contribute to acne by inhibiting serum production and inflammation, according to a company press release.
“Although clascoterone’s exact mechanism of action is unknown, laboratory studies suggest clascoterone competes with androgens, specifically dihydrotestosterone, for binding to the androgen receptors within the sebaceous gland and hair follicles,” according to the release.
Approval was based in part on a pair of phase 3, double-blind, vehicle-controlled, 12-week, randomized trials including 1,440 patients aged 9 years and older with moderate to severe facial acne. The findings were published in April, in JAMA Dermatology .
Participants were randomized to twice-daily application of clascoterone or a control vehicle; treatment success was defined as having an Investigator’s Global Assessment score of 0 (clear) or 1 (almost clear), as well as at least a 2-grade improvement from baseline, and absolute change in noninflammatory and inflammatory lesion counts at week 12.
At 12 weeks, treatment success rates were 18.4% and 20.3% among those on clascoterone, compared with 9% and 6.5%, respectively, among controls. There were also significant reductions in noninflammatory and inflammatory lesions from baseline at 12 weeks, compared with controls.
In the studies, treatment was well tolerated, with a safety profile similar to safety in controls. Adverse events thought to be related to clascoterone in the studies (a total of 13) included application-site pain; erythema; oropharyngeal pain; hypersensitivity, dryness, or hypertrichosis at the application site; eye irritation; headache; and hair color changes. “Clascoterone targets androgen receptors at the site of application and is quickly metabolized to an inactive form, thus limiting systemic activity,” the authors of the study wrote.
Clascoterone is expected to be available in the United States in early 2021, according to the manufacturer.
Clascoterone is a topical androgen receptor inhibitor indicated for treatment of acne vulgaris in patients aged 12 years and older, according to the labeling from manufacturer Cassiopea. Clascoterone, which will be marketed as Winlevi, targets the androgen hormones that contribute to acne by inhibiting serum production and inflammation, according to a company press release.
“Although clascoterone’s exact mechanism of action is unknown, laboratory studies suggest clascoterone competes with androgens, specifically dihydrotestosterone, for binding to the androgen receptors within the sebaceous gland and hair follicles,” according to the release.
Approval was based in part on a pair of phase 3, double-blind, vehicle-controlled, 12-week, randomized trials including 1,440 patients aged 9 years and older with moderate to severe facial acne. The findings were published in April, in JAMA Dermatology .
Participants were randomized to twice-daily application of clascoterone or a control vehicle; treatment success was defined as having an Investigator’s Global Assessment score of 0 (clear) or 1 (almost clear), as well as at least a 2-grade improvement from baseline, and absolute change in noninflammatory and inflammatory lesion counts at week 12.
At 12 weeks, treatment success rates were 18.4% and 20.3% among those on clascoterone, compared with 9% and 6.5%, respectively, among controls. There were also significant reductions in noninflammatory and inflammatory lesions from baseline at 12 weeks, compared with controls.
In the studies, treatment was well tolerated, with a safety profile similar to safety in controls. Adverse events thought to be related to clascoterone in the studies (a total of 13) included application-site pain; erythema; oropharyngeal pain; hypersensitivity, dryness, or hypertrichosis at the application site; eye irritation; headache; and hair color changes. “Clascoterone targets androgen receptors at the site of application and is quickly metabolized to an inactive form, thus limiting systemic activity,” the authors of the study wrote.
Clascoterone is expected to be available in the United States in early 2021, according to the manufacturer.
Immunotherapy should not be withheld because of sex, age, or PS
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
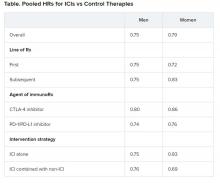
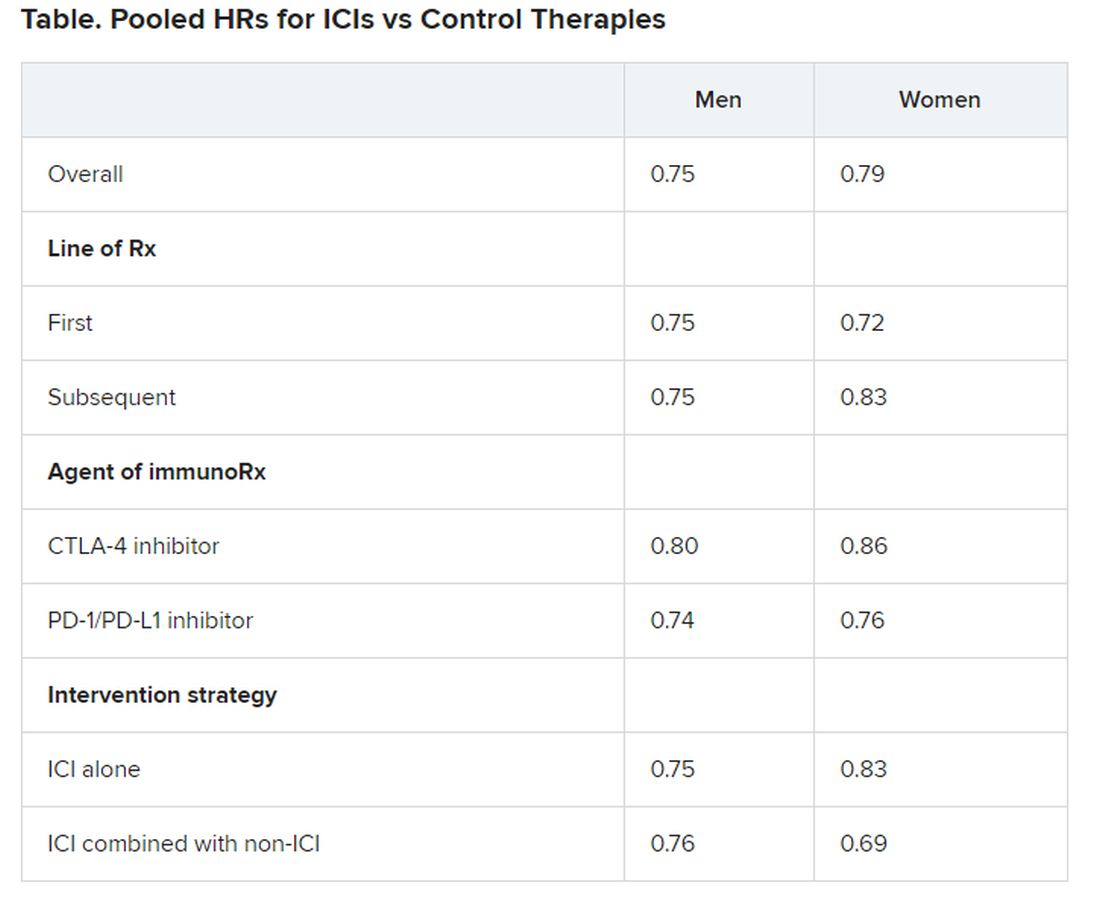
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
Asymptomatic SARS-CoV-2 infections in kids tied to local rates
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
As communities wrestle with the decision to send children back to school or opt for distance learning, a key question is how many children are likely to have asymptomatic SARS-CoV-2 infections.
“The strong association between prevalence of SARS-CoV-2 in children who are asymptomatic and contemporaneous weekly incidence of COVID-19 in the general population ... provides a simple means for institutions to estimate local pediatric asymptomatic prevalence from the publicly available Johns Hopkins University database,” researchers say in an article published online August 25 in JAMA Pediatrics.
Ana Marija Sola, BS, a researcher at the University of California, San Francisco, and colleagues examined the prevalence of SARS-CoV-2 infection among 33,041 children who underwent routine testing in April and May when hospitals resumed elective medical and surgical care. The hospitals performed reverse transcription–polymerase chain reaction tests for SARS-CoV-2 RNA before surgery, clinic visits, or hospital admissions. Pediatric otolaryngologists reported the prevalence data through May 29 as part of a quality improvement project.
In all, 250 patients tested positive for the virus, for an overall prevalence of 0.65%. Across 25 geographic areas, the prevalence ranged from 0% to 2.2%. By region, prevalence was highest in the Northeast, at 0.90%, and the Midwest, at 0.87%; prevalence was lower in the West, at 0.59%, and the South, at 0.52%.
To get a sense of how those rates compared with overall rates in the same geographic areas, the researchers used the Johns Hopkins University confirmed cases database to calculate the average weekly incidence of COVID-19 for the entire population for each geographic area.
“Asymptomatic pediatric prevalence was significantly associated with weekly incidence of COVID-19 in the general population during the 6-week period over which most testing of individuals without symptoms occurred,” Ms. Sola and colleagues reported. An analysis using additional data from 11 geographic areas demonstrated that this association persisted at a later time point.
The study provides “another window on the question of how likely is it that an asymptomatic child will be carrying coronavirus,” said Susan E. Coffin, MD, MPH, an attending physician for the division of infectious diseases at Children’s Hospital of Philadelphia. However, important related questions remain, said Dr. Coffin, who was not involved with the study.
For one, it is unclear how many children remain asymptomatic in comparison with those who were in a presymptomatic phase at the time of testing. And importantly, “what proportion of these children are infectious?” said Dr. Coffin. “There is some data to suggest that children with asymptomatic infection may be less infectious than children with symptomatic infection.”
It also could be that patients seen at children’s hospitals differ from the general pediatric population. “What does this look like if you do the exact same study in a group of randomly selected children, not children who are queueing up to have a procedure? ... And what do these numbers look like now that stay-at-home orders have been lifted?” Dr. Coffin asked.
Further studies are needed to establish that detection of COVID-19 in the general population is predictive of the prevalence of SARS-CoV-2 infection in asymptomatic children, Dr. Coffin said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
The interesting history of dermatologist-developed skin care
Those of you who have visited my dermatology practice in Miami know that the art in my office is dedicated to the history of the skin care industry. I collect , and I have written this historical column in honor of the 50th anniversary of Dermatology News.
The first doctor to market his own cosmetic product, Erasmus Wilson, MD, faced scrutiny from his colleagues. Although he had contributed much to the field of dermatology, he was criticized by other dermatologists when he promoted a hair wash. The next doctor in my story, William Pusey, MD, was criticized for helping the company that manufactured Camay soap because he allowed his name to be used in Camay advertisements. The scrutiny that these two well-respected dermatologists endured from their colleagues deterred dermatologists from entering the skin care business for decades. The professional jealousy from dermatologic colleagues left the skin care field wide open for imposters, charlatans, and nondermatologists who had no concern for efficacy and patient outcomes to flourish. This is the story of a group of brilliant entrepreneurial dermatologists and one chiropractor who misrepresented himself as a dermatologist and how they influenced skin care as we know it.
Erasmus Wilson, MD1 (1809-1884): In 1840, Erasmus Wilson2 was a physician in London who chose to specialize in dermatology at a time when that specialization was frowned upon. He was a subeditor for The Lancet and wrote several books on dermatology including “Diseases of the Skin – A Practical and Theoretical Treatise,” “Portraits of the Diseases of the Skin,” and “Student’s Book on Diseases of the Skin.” He was the first professor of dermatology in the College of Surgeons and by 1869, was the leading English-speaking dermatologist in the world. He contributed much to dermatology, including his pioneering characterizations of Demodex mites, lichen planus, exfoliative dermatitis, neurotic excoriations, and roseola. Dr. Wilson was knighted in 1881 for his good works and notable generosity. (He was known for giving his poor patients money for food, endowing chairs in dermatology, and donating a famous obelisk in London).
In 1854, Dr. Wilson wrote a book for laypeople called “Healthy Skin: A Popular Treatise on the Skin and Hair, Their Preservation and Management,” in which he advocated cleanliness and bathing, which led to the popularity of Turkish baths and bathing resorts in Europe. Despite his undeniable contributions to dermatology, he was widely criticized by his colleagues for promoting a “Hair Wash” and a turtle oil soap. I cannot find any information about whether or not he developed the hair wash and turtle soap himself, but it seems that he earned income from sales of these two products, even though he was said to have donated it all to charities.
William A. Pusey MD (1865-1940): Dr. Pusey was the first chairman of dermatology at the University of Illinois College of Medicine, Chicago. He published several books, including “Care of the Skin and Hair,” “Syphilis as a Modern Problem,” “The Principles and Practices of Dermatology,” and “History of Dermatology” among others. He is best known for his work in developing the use of x-rays (roentgen rays) and phototherapy in dermatology, and in 1907, he was the first dermatologist to describe the use of solid carbon dioxide to treat skin lesions. He was president of the American Dermatological Association in 1910, president of the Chicago Medical Society in 1918, editor of the Archives of Dermatology in 1920, and president of the American Medical Association in 1924.
In the early 1920s, skin care companies were beginning to advertise their products using endorsements from celebrities and socialites, and were making misleading claims. Dr. Pusey wanted to work with these companies to help them perform evidence-based trials so they could make scientifically correct claims. Proctor & Gamble asked Dr. Pusey to advise them on how they could advertise honestly about their new soap, “Camay.” In Dr. Pusey’s words,3 “they (Proctor & Gamble) wanted to give the public authoritative advice about the use of soap and water. They suggested that I get a group of dermatologists of my selection to examine the soap and prepare instructions for bathing and the use of soap, and, if they found this soap was of high quality, to certify to that effect.” The research was performed as he suggested, and he allowed his name to be used in the Camay soap ads from 1926 to 1929. He said that he allowed them to use his name hoping to promote the need for evidence-based research, in contrast to the skin care products endorsed by socialites and celebrities that were flooding the market around that time.
Herbert Rattner, MD, at Northwestern University, Chicago, was his friend and one of the many dermatologists who criticized Dr. Pusey for allowing his name to be used in the Camay ads. Dr. Pusey’s reply to the criticism (according to Dr. Rattner) was that Proctor & Gamble was “proposing to do what the medical profession always is criticizing commercial concerns for not doing, namely, coming to physicians for information on medical matters. Could the profession hope to have any influence with business concerns if it was always eager to criticize bad commercial practices but never willing to support good ones?”3
While Dr. Pusey felt his reasons for adding his name to the Camay ads and research were justified, many of his friends stated that in hindsight, he regretted the action because of the negative response of his colleagues. It was years before dermatologists began providing input again into the skin care industry. During that time, radio, television and print ads were rampant with misleading claims – which led the way for a dermatologic imposter to make a fortune on skin care.
John Woodbury (1851-1909): John Woodbury, a chiropractor, never went to medical school, but that did not stop him from claiming he was a dermatologist and cosmetic surgeon. In 1889, he opened the John H. Woodbury Dermatological Institute in New York City, and over the next few years, opened Woodbury Dermatological Institutes in at least 5 states and employed 25 “physicians” who were not licensed to practice medicine. He came out with face soaps, tonics, and cold creams and spent a fortune on advertising these products and his institutes. In 1901, he sold his “Woodbury Soap” to the Andrew Jergens Company for $212,500 and 10% in royalties.
Multiple lawsuits occurred from 1898 to 1907 because he continued using the Woodbury name on his own products, despite having sold the “Woodbury” trademark to Jergens. He was sued for practicing medicine without a medical license and claiming to be a dermatologist when he was not. He lost most of these lawsuits, including one in 1907 in which the court ruled that corporations may not employ unlicensed professionals to practice medicine. In 1909, John Woodbury committed suicide. The Woodbury Soap company flourished in the 1930s and 1940s, as part of Jergens, until the brand was discontinued in 1970 when Jergens was acquired by American Brands.
The next dermatologists to come along did not make the same mistakes as those of their predecessors. They all made scientific discoveries through their basic science research in the laboratory, filed patents, formed skin care companies, perfected the formulations, and conducted research trials of the final product. Their marketing focused on science and efficacy and only rarely used their names and images in advertising, allowing them to maintain their reputations in the dermatology field.
Eugene Van Scott, MD (1922-present): Dermatologist Dr. Van Scott and dermatopharmacologist Ruey Yu, PhD, filed a method patent in the early 1970s on the effectiveness of alpha hydroxy acids to treat ichthyosis. They invented the abbreviation “AHA” and have continued their work on organic acids to this day. They now have more than 125 patents, which they have licensed to 60 companies in the cosmetics and pharmaceutical industries.
In 1988, 14 years after their initial publication, they founded the company they named Polystrata, which grew into today’s NeoStrata.4 Over the years, they had to defend their patents because many personal care companies used their technologies without licensing them. In 2007, they won a $41 million settlement in a patent infringement suit against Mary Kay filed in March 2005. They have both been very philanthropic in the dermatology world5 and are highly respected in the field. Among many other honors, Dr. Van Scott was named a Master Dermatologist by the American Academy of Dermatology in 1998 and received the Dermatology Foundation’s Distinguished Service Medallion in 2004.
Sheldon Pinnell, MD (1937-2013): After Dr. Pinnell completed his dermatology residency at Harvard Medical School, he spent 2 years studying collagen chemistry at the Max Planck Institute in Munich, Germany. In 1973, he returned to Duke University where he had earned his undergraduate degree before attending Yale University. He remained at Duke for the duration of his career and was professor and chief of dermatology there for many years. Early in his career, he focused on the role of vitamin C in collagen biosynthesis and discovered some of the mechanisms by which sun exposure causes photoaging. He described the use of the first (and most popular) topically applied L-ascorbic acid (vitamin C) to prevent and treat skin aging.
Dr. Pinnell’s many discoveries include showing that the addition of ascorbic acid to fibroblast cultures increases collagen production and that topically applied L-ascorbic acid penetrates into the skin best at a pH of 2-2.5. Dr. Pinnell changed the way the world uses topical antioxidants today; he was widely respected and was a member of the American Dermatological Association and an honorary member of the Society of Investigative Dermatology. He published more than 200 scientific articles and held 10 patents. He started the skin care company Skinceuticals, based on his antioxidant technologies. It was acquired by L’Oreal in 2005.
Richard Fitzpatrick, MD (1944-2014): The dermatologist affectionately known as “Fitz” is credited with being the first to use lasers for skin resurfacing. He went to medical school at Emory University and did his dermatology residency at the University of California, Los Angeles. He authored more than 130 publications and was one of the first doctors to specialize in cosmetic dermatology. He realized that fibroblast cell cultures used to produce the collagen filler CosmoPlast (no longer on the market) generated many growth factors that could rejuvenate the skin, and in 1999, he launched the skin care brand SkinMedica. In 2000, he received a patent for fibroblast-derived growth factors used topically for antiaging – a formula he called Tissue Nutrient Solution. In 2001, the popular product TNS Recovery Complex was launched based on the patented growth factor technology. It is still the most popular growth factor technology on the market.
What can we learn from these pioneers? I have had several interesting discussions about this topic with Leonard Hoenig, MD, section editor for Reflections on Dermatology: Past, Present, and Future, in Clinics in Dermatology. (Dr. Hoenig told me the interesting story that Listerine mouthwash was named in honor of Joseph Lister but accounts vary as to whether he gave permission to do so. This makes Dr. Lister the most famous physician to endorse a personal care product.) When Dr. Hoenig and I discussed the ethics of dermatologists creating a skin care line or retailing skin care in their medical practice, he stated my sentiments perfectly: “We should rely on professional, ethical, and legal guidelines to help us do what is right. Most importantly, we should have the best interests of our patients at heart when recommending any treatments.”
Dermatologists have unique knowledge, experience, and perspective on treating the skin with topical agents and have the true desire to improve skin health. If we do not discover, research, patent, and develop efficacious skin care products, someone else will do it – and I do not think they will do it as well as a dermatologist can.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Everett MA. Int J Dermatol. 1978 May;17(4):345-52.
2. Moxon RK. N Engl J Med. 1976 Apr 1;294(14):762-4.
3. Rattner H. Arch Derm Syphilol. 1937;35(1):25-66.
4. Neostrata: More than Hope, by Elaine Strauss, U.S. 1 Newspaper, Feb. 24, 1999.
5. Two legends in the field of dermatology provide $1 million gift to Temple University school of medicine’s department of dermatology, Temple University, June 5, 2015.
Those of you who have visited my dermatology practice in Miami know that the art in my office is dedicated to the history of the skin care industry. I collect , and I have written this historical column in honor of the 50th anniversary of Dermatology News.
The first doctor to market his own cosmetic product, Erasmus Wilson, MD, faced scrutiny from his colleagues. Although he had contributed much to the field of dermatology, he was criticized by other dermatologists when he promoted a hair wash. The next doctor in my story, William Pusey, MD, was criticized for helping the company that manufactured Camay soap because he allowed his name to be used in Camay advertisements. The scrutiny that these two well-respected dermatologists endured from their colleagues deterred dermatologists from entering the skin care business for decades. The professional jealousy from dermatologic colleagues left the skin care field wide open for imposters, charlatans, and nondermatologists who had no concern for efficacy and patient outcomes to flourish. This is the story of a group of brilliant entrepreneurial dermatologists and one chiropractor who misrepresented himself as a dermatologist and how they influenced skin care as we know it.
Erasmus Wilson, MD1 (1809-1884): In 1840, Erasmus Wilson2 was a physician in London who chose to specialize in dermatology at a time when that specialization was frowned upon. He was a subeditor for The Lancet and wrote several books on dermatology including “Diseases of the Skin – A Practical and Theoretical Treatise,” “Portraits of the Diseases of the Skin,” and “Student’s Book on Diseases of the Skin.” He was the first professor of dermatology in the College of Surgeons and by 1869, was the leading English-speaking dermatologist in the world. He contributed much to dermatology, including his pioneering characterizations of Demodex mites, lichen planus, exfoliative dermatitis, neurotic excoriations, and roseola. Dr. Wilson was knighted in 1881 for his good works and notable generosity. (He was known for giving his poor patients money for food, endowing chairs in dermatology, and donating a famous obelisk in London).
In 1854, Dr. Wilson wrote a book for laypeople called “Healthy Skin: A Popular Treatise on the Skin and Hair, Their Preservation and Management,” in which he advocated cleanliness and bathing, which led to the popularity of Turkish baths and bathing resorts in Europe. Despite his undeniable contributions to dermatology, he was widely criticized by his colleagues for promoting a “Hair Wash” and a turtle oil soap. I cannot find any information about whether or not he developed the hair wash and turtle soap himself, but it seems that he earned income from sales of these two products, even though he was said to have donated it all to charities.
William A. Pusey MD (1865-1940): Dr. Pusey was the first chairman of dermatology at the University of Illinois College of Medicine, Chicago. He published several books, including “Care of the Skin and Hair,” “Syphilis as a Modern Problem,” “The Principles and Practices of Dermatology,” and “History of Dermatology” among others. He is best known for his work in developing the use of x-rays (roentgen rays) and phototherapy in dermatology, and in 1907, he was the first dermatologist to describe the use of solid carbon dioxide to treat skin lesions. He was president of the American Dermatological Association in 1910, president of the Chicago Medical Society in 1918, editor of the Archives of Dermatology in 1920, and president of the American Medical Association in 1924.
In the early 1920s, skin care companies were beginning to advertise their products using endorsements from celebrities and socialites, and were making misleading claims. Dr. Pusey wanted to work with these companies to help them perform evidence-based trials so they could make scientifically correct claims. Proctor & Gamble asked Dr. Pusey to advise them on how they could advertise honestly about their new soap, “Camay.” In Dr. Pusey’s words,3 “they (Proctor & Gamble) wanted to give the public authoritative advice about the use of soap and water. They suggested that I get a group of dermatologists of my selection to examine the soap and prepare instructions for bathing and the use of soap, and, if they found this soap was of high quality, to certify to that effect.” The research was performed as he suggested, and he allowed his name to be used in the Camay soap ads from 1926 to 1929. He said that he allowed them to use his name hoping to promote the need for evidence-based research, in contrast to the skin care products endorsed by socialites and celebrities that were flooding the market around that time.
Herbert Rattner, MD, at Northwestern University, Chicago, was his friend and one of the many dermatologists who criticized Dr. Pusey for allowing his name to be used in the Camay ads. Dr. Pusey’s reply to the criticism (according to Dr. Rattner) was that Proctor & Gamble was “proposing to do what the medical profession always is criticizing commercial concerns for not doing, namely, coming to physicians for information on medical matters. Could the profession hope to have any influence with business concerns if it was always eager to criticize bad commercial practices but never willing to support good ones?”3
While Dr. Pusey felt his reasons for adding his name to the Camay ads and research were justified, many of his friends stated that in hindsight, he regretted the action because of the negative response of his colleagues. It was years before dermatologists began providing input again into the skin care industry. During that time, radio, television and print ads were rampant with misleading claims – which led the way for a dermatologic imposter to make a fortune on skin care.
John Woodbury (1851-1909): John Woodbury, a chiropractor, never went to medical school, but that did not stop him from claiming he was a dermatologist and cosmetic surgeon. In 1889, he opened the John H. Woodbury Dermatological Institute in New York City, and over the next few years, opened Woodbury Dermatological Institutes in at least 5 states and employed 25 “physicians” who were not licensed to practice medicine. He came out with face soaps, tonics, and cold creams and spent a fortune on advertising these products and his institutes. In 1901, he sold his “Woodbury Soap” to the Andrew Jergens Company for $212,500 and 10% in royalties.
Multiple lawsuits occurred from 1898 to 1907 because he continued using the Woodbury name on his own products, despite having sold the “Woodbury” trademark to Jergens. He was sued for practicing medicine without a medical license and claiming to be a dermatologist when he was not. He lost most of these lawsuits, including one in 1907 in which the court ruled that corporations may not employ unlicensed professionals to practice medicine. In 1909, John Woodbury committed suicide. The Woodbury Soap company flourished in the 1930s and 1940s, as part of Jergens, until the brand was discontinued in 1970 when Jergens was acquired by American Brands.
The next dermatologists to come along did not make the same mistakes as those of their predecessors. They all made scientific discoveries through their basic science research in the laboratory, filed patents, formed skin care companies, perfected the formulations, and conducted research trials of the final product. Their marketing focused on science and efficacy and only rarely used their names and images in advertising, allowing them to maintain their reputations in the dermatology field.
Eugene Van Scott, MD (1922-present): Dermatologist Dr. Van Scott and dermatopharmacologist Ruey Yu, PhD, filed a method patent in the early 1970s on the effectiveness of alpha hydroxy acids to treat ichthyosis. They invented the abbreviation “AHA” and have continued their work on organic acids to this day. They now have more than 125 patents, which they have licensed to 60 companies in the cosmetics and pharmaceutical industries.
In 1988, 14 years after their initial publication, they founded the company they named Polystrata, which grew into today’s NeoStrata.4 Over the years, they had to defend their patents because many personal care companies used their technologies without licensing them. In 2007, they won a $41 million settlement in a patent infringement suit against Mary Kay filed in March 2005. They have both been very philanthropic in the dermatology world5 and are highly respected in the field. Among many other honors, Dr. Van Scott was named a Master Dermatologist by the American Academy of Dermatology in 1998 and received the Dermatology Foundation’s Distinguished Service Medallion in 2004.
Sheldon Pinnell, MD (1937-2013): After Dr. Pinnell completed his dermatology residency at Harvard Medical School, he spent 2 years studying collagen chemistry at the Max Planck Institute in Munich, Germany. In 1973, he returned to Duke University where he had earned his undergraduate degree before attending Yale University. He remained at Duke for the duration of his career and was professor and chief of dermatology there for many years. Early in his career, he focused on the role of vitamin C in collagen biosynthesis and discovered some of the mechanisms by which sun exposure causes photoaging. He described the use of the first (and most popular) topically applied L-ascorbic acid (vitamin C) to prevent and treat skin aging.
Dr. Pinnell’s many discoveries include showing that the addition of ascorbic acid to fibroblast cultures increases collagen production and that topically applied L-ascorbic acid penetrates into the skin best at a pH of 2-2.5. Dr. Pinnell changed the way the world uses topical antioxidants today; he was widely respected and was a member of the American Dermatological Association and an honorary member of the Society of Investigative Dermatology. He published more than 200 scientific articles and held 10 patents. He started the skin care company Skinceuticals, based on his antioxidant technologies. It was acquired by L’Oreal in 2005.
Richard Fitzpatrick, MD (1944-2014): The dermatologist affectionately known as “Fitz” is credited with being the first to use lasers for skin resurfacing. He went to medical school at Emory University and did his dermatology residency at the University of California, Los Angeles. He authored more than 130 publications and was one of the first doctors to specialize in cosmetic dermatology. He realized that fibroblast cell cultures used to produce the collagen filler CosmoPlast (no longer on the market) generated many growth factors that could rejuvenate the skin, and in 1999, he launched the skin care brand SkinMedica. In 2000, he received a patent for fibroblast-derived growth factors used topically for antiaging – a formula he called Tissue Nutrient Solution. In 2001, the popular product TNS Recovery Complex was launched based on the patented growth factor technology. It is still the most popular growth factor technology on the market.
What can we learn from these pioneers? I have had several interesting discussions about this topic with Leonard Hoenig, MD, section editor for Reflections on Dermatology: Past, Present, and Future, in Clinics in Dermatology. (Dr. Hoenig told me the interesting story that Listerine mouthwash was named in honor of Joseph Lister but accounts vary as to whether he gave permission to do so. This makes Dr. Lister the most famous physician to endorse a personal care product.) When Dr. Hoenig and I discussed the ethics of dermatologists creating a skin care line or retailing skin care in their medical practice, he stated my sentiments perfectly: “We should rely on professional, ethical, and legal guidelines to help us do what is right. Most importantly, we should have the best interests of our patients at heart when recommending any treatments.”
Dermatologists have unique knowledge, experience, and perspective on treating the skin with topical agents and have the true desire to improve skin health. If we do not discover, research, patent, and develop efficacious skin care products, someone else will do it – and I do not think they will do it as well as a dermatologist can.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Everett MA. Int J Dermatol. 1978 May;17(4):345-52.
2. Moxon RK. N Engl J Med. 1976 Apr 1;294(14):762-4.
3. Rattner H. Arch Derm Syphilol. 1937;35(1):25-66.
4. Neostrata: More than Hope, by Elaine Strauss, U.S. 1 Newspaper, Feb. 24, 1999.
5. Two legends in the field of dermatology provide $1 million gift to Temple University school of medicine’s department of dermatology, Temple University, June 5, 2015.
Those of you who have visited my dermatology practice in Miami know that the art in my office is dedicated to the history of the skin care industry. I collect , and I have written this historical column in honor of the 50th anniversary of Dermatology News.
The first doctor to market his own cosmetic product, Erasmus Wilson, MD, faced scrutiny from his colleagues. Although he had contributed much to the field of dermatology, he was criticized by other dermatologists when he promoted a hair wash. The next doctor in my story, William Pusey, MD, was criticized for helping the company that manufactured Camay soap because he allowed his name to be used in Camay advertisements. The scrutiny that these two well-respected dermatologists endured from their colleagues deterred dermatologists from entering the skin care business for decades. The professional jealousy from dermatologic colleagues left the skin care field wide open for imposters, charlatans, and nondermatologists who had no concern for efficacy and patient outcomes to flourish. This is the story of a group of brilliant entrepreneurial dermatologists and one chiropractor who misrepresented himself as a dermatologist and how they influenced skin care as we know it.
Erasmus Wilson, MD1 (1809-1884): In 1840, Erasmus Wilson2 was a physician in London who chose to specialize in dermatology at a time when that specialization was frowned upon. He was a subeditor for The Lancet and wrote several books on dermatology including “Diseases of the Skin – A Practical and Theoretical Treatise,” “Portraits of the Diseases of the Skin,” and “Student’s Book on Diseases of the Skin.” He was the first professor of dermatology in the College of Surgeons and by 1869, was the leading English-speaking dermatologist in the world. He contributed much to dermatology, including his pioneering characterizations of Demodex mites, lichen planus, exfoliative dermatitis, neurotic excoriations, and roseola. Dr. Wilson was knighted in 1881 for his good works and notable generosity. (He was known for giving his poor patients money for food, endowing chairs in dermatology, and donating a famous obelisk in London).
In 1854, Dr. Wilson wrote a book for laypeople called “Healthy Skin: A Popular Treatise on the Skin and Hair, Their Preservation and Management,” in which he advocated cleanliness and bathing, which led to the popularity of Turkish baths and bathing resorts in Europe. Despite his undeniable contributions to dermatology, he was widely criticized by his colleagues for promoting a “Hair Wash” and a turtle oil soap. I cannot find any information about whether or not he developed the hair wash and turtle soap himself, but it seems that he earned income from sales of these two products, even though he was said to have donated it all to charities.
William A. Pusey MD (1865-1940): Dr. Pusey was the first chairman of dermatology at the University of Illinois College of Medicine, Chicago. He published several books, including “Care of the Skin and Hair,” “Syphilis as a Modern Problem,” “The Principles and Practices of Dermatology,” and “History of Dermatology” among others. He is best known for his work in developing the use of x-rays (roentgen rays) and phototherapy in dermatology, and in 1907, he was the first dermatologist to describe the use of solid carbon dioxide to treat skin lesions. He was president of the American Dermatological Association in 1910, president of the Chicago Medical Society in 1918, editor of the Archives of Dermatology in 1920, and president of the American Medical Association in 1924.
In the early 1920s, skin care companies were beginning to advertise their products using endorsements from celebrities and socialites, and were making misleading claims. Dr. Pusey wanted to work with these companies to help them perform evidence-based trials so they could make scientifically correct claims. Proctor & Gamble asked Dr. Pusey to advise them on how they could advertise honestly about their new soap, “Camay.” In Dr. Pusey’s words,3 “they (Proctor & Gamble) wanted to give the public authoritative advice about the use of soap and water. They suggested that I get a group of dermatologists of my selection to examine the soap and prepare instructions for bathing and the use of soap, and, if they found this soap was of high quality, to certify to that effect.” The research was performed as he suggested, and he allowed his name to be used in the Camay soap ads from 1926 to 1929. He said that he allowed them to use his name hoping to promote the need for evidence-based research, in contrast to the skin care products endorsed by socialites and celebrities that were flooding the market around that time.
Herbert Rattner, MD, at Northwestern University, Chicago, was his friend and one of the many dermatologists who criticized Dr. Pusey for allowing his name to be used in the Camay ads. Dr. Pusey’s reply to the criticism (according to Dr. Rattner) was that Proctor & Gamble was “proposing to do what the medical profession always is criticizing commercial concerns for not doing, namely, coming to physicians for information on medical matters. Could the profession hope to have any influence with business concerns if it was always eager to criticize bad commercial practices but never willing to support good ones?”3
While Dr. Pusey felt his reasons for adding his name to the Camay ads and research were justified, many of his friends stated that in hindsight, he regretted the action because of the negative response of his colleagues. It was years before dermatologists began providing input again into the skin care industry. During that time, radio, television and print ads were rampant with misleading claims – which led the way for a dermatologic imposter to make a fortune on skin care.
John Woodbury (1851-1909): John Woodbury, a chiropractor, never went to medical school, but that did not stop him from claiming he was a dermatologist and cosmetic surgeon. In 1889, he opened the John H. Woodbury Dermatological Institute in New York City, and over the next few years, opened Woodbury Dermatological Institutes in at least 5 states and employed 25 “physicians” who were not licensed to practice medicine. He came out with face soaps, tonics, and cold creams and spent a fortune on advertising these products and his institutes. In 1901, he sold his “Woodbury Soap” to the Andrew Jergens Company for $212,500 and 10% in royalties.
Multiple lawsuits occurred from 1898 to 1907 because he continued using the Woodbury name on his own products, despite having sold the “Woodbury” trademark to Jergens. He was sued for practicing medicine without a medical license and claiming to be a dermatologist when he was not. He lost most of these lawsuits, including one in 1907 in which the court ruled that corporations may not employ unlicensed professionals to practice medicine. In 1909, John Woodbury committed suicide. The Woodbury Soap company flourished in the 1930s and 1940s, as part of Jergens, until the brand was discontinued in 1970 when Jergens was acquired by American Brands.
The next dermatologists to come along did not make the same mistakes as those of their predecessors. They all made scientific discoveries through their basic science research in the laboratory, filed patents, formed skin care companies, perfected the formulations, and conducted research trials of the final product. Their marketing focused on science and efficacy and only rarely used their names and images in advertising, allowing them to maintain their reputations in the dermatology field.
Eugene Van Scott, MD (1922-present): Dermatologist Dr. Van Scott and dermatopharmacologist Ruey Yu, PhD, filed a method patent in the early 1970s on the effectiveness of alpha hydroxy acids to treat ichthyosis. They invented the abbreviation “AHA” and have continued their work on organic acids to this day. They now have more than 125 patents, which they have licensed to 60 companies in the cosmetics and pharmaceutical industries.
In 1988, 14 years after their initial publication, they founded the company they named Polystrata, which grew into today’s NeoStrata.4 Over the years, they had to defend their patents because many personal care companies used their technologies without licensing them. In 2007, they won a $41 million settlement in a patent infringement suit against Mary Kay filed in March 2005. They have both been very philanthropic in the dermatology world5 and are highly respected in the field. Among many other honors, Dr. Van Scott was named a Master Dermatologist by the American Academy of Dermatology in 1998 and received the Dermatology Foundation’s Distinguished Service Medallion in 2004.
Sheldon Pinnell, MD (1937-2013): After Dr. Pinnell completed his dermatology residency at Harvard Medical School, he spent 2 years studying collagen chemistry at the Max Planck Institute in Munich, Germany. In 1973, he returned to Duke University where he had earned his undergraduate degree before attending Yale University. He remained at Duke for the duration of his career and was professor and chief of dermatology there for many years. Early in his career, he focused on the role of vitamin C in collagen biosynthesis and discovered some of the mechanisms by which sun exposure causes photoaging. He described the use of the first (and most popular) topically applied L-ascorbic acid (vitamin C) to prevent and treat skin aging.
Dr. Pinnell’s many discoveries include showing that the addition of ascorbic acid to fibroblast cultures increases collagen production and that topically applied L-ascorbic acid penetrates into the skin best at a pH of 2-2.5. Dr. Pinnell changed the way the world uses topical antioxidants today; he was widely respected and was a member of the American Dermatological Association and an honorary member of the Society of Investigative Dermatology. He published more than 200 scientific articles and held 10 patents. He started the skin care company Skinceuticals, based on his antioxidant technologies. It was acquired by L’Oreal in 2005.
Richard Fitzpatrick, MD (1944-2014): The dermatologist affectionately known as “Fitz” is credited with being the first to use lasers for skin resurfacing. He went to medical school at Emory University and did his dermatology residency at the University of California, Los Angeles. He authored more than 130 publications and was one of the first doctors to specialize in cosmetic dermatology. He realized that fibroblast cell cultures used to produce the collagen filler CosmoPlast (no longer on the market) generated many growth factors that could rejuvenate the skin, and in 1999, he launched the skin care brand SkinMedica. In 2000, he received a patent for fibroblast-derived growth factors used topically for antiaging – a formula he called Tissue Nutrient Solution. In 2001, the popular product TNS Recovery Complex was launched based on the patented growth factor technology. It is still the most popular growth factor technology on the market.
What can we learn from these pioneers? I have had several interesting discussions about this topic with Leonard Hoenig, MD, section editor for Reflections on Dermatology: Past, Present, and Future, in Clinics in Dermatology. (Dr. Hoenig told me the interesting story that Listerine mouthwash was named in honor of Joseph Lister but accounts vary as to whether he gave permission to do so. This makes Dr. Lister the most famous physician to endorse a personal care product.) When Dr. Hoenig and I discussed the ethics of dermatologists creating a skin care line or retailing skin care in their medical practice, he stated my sentiments perfectly: “We should rely on professional, ethical, and legal guidelines to help us do what is right. Most importantly, we should have the best interests of our patients at heart when recommending any treatments.”
Dermatologists have unique knowledge, experience, and perspective on treating the skin with topical agents and have the true desire to improve skin health. If we do not discover, research, patent, and develop efficacious skin care products, someone else will do it – and I do not think they will do it as well as a dermatologist can.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Everett MA. Int J Dermatol. 1978 May;17(4):345-52.
2. Moxon RK. N Engl J Med. 1976 Apr 1;294(14):762-4.
3. Rattner H. Arch Derm Syphilol. 1937;35(1):25-66.
4. Neostrata: More than Hope, by Elaine Strauss, U.S. 1 Newspaper, Feb. 24, 1999.
5. Two legends in the field of dermatology provide $1 million gift to Temple University school of medicine’s department of dermatology, Temple University, June 5, 2015.
Convalescent plasma actions spark trial recruitment concerns
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
The agency’s move took many investigators by surprise. The EUA was announced at the White House the day after President Donald J. Trump accused the FDA of delaying approval of therapeutics to hurt his re-election chances.
In a memo describing the decision, the FDA cited data from some controlled and uncontrolled studies and, primarily, data from an open-label expanded-access protocol overseen by the Mayo Clinic.
At the White House, FDA Commissioner Stephen Hahn, MD, said that plasma had been found to save the lives of 35 out of every 100 who were treated. That figure was later found to have been erroneous, and many experts pointed out that Hahn had conflated an absolute risk reduction with a relative reduction. After a firestorm of criticism, Hahn issued an apology.
“The criticism is entirely justified,” he tweeted. “What I should have said better is that the data show a relative risk reduction not an absolute risk reduction.”
About 15 randomized controlled trials – out of 54 total studies involving convalescent plasma – are underway in the United States, according to ClinicalTrials.gov. The FDA’s Aug. 23 emergency authorization gave clinicians wide leeway to employ convalescent plasma in patients hospitalized with COVID-19.
The agency noted, however, that “adequate and well-controlled randomized trials remain necessary for a definitive demonstration of COVID-19 convalescent plasma efficacy and to determine the optimal product attributes and appropriate patient populations for its use.”
But it’s not clear that people with COVID-19, especially those who are severely ill and hospitalized, will choose to enlist in a clinical trial – where they could receive a placebo – when they instead could get plasma.
“I’ve been asked repeatedly whether the EUA will affect our ability to recruit people into our hospitalized patient trial,” said Liise-anne Pirofski, MD, FIDSA, chief of the department of medicine, infectious diseases division at Albert Einstein College of Medicine and Montefiore Medical Center in the Bronx, New York. “I do not know,” she said, on a call with reporters organized by the Infectious Diseases Society of America.
“But,” she said, “I do know that the trial will continue and that we will discuss the evidence that we have with our patients and give them all that we can to help them weigh the evidence and make up their minds.”
Pirofski said the study being conducted at Montefiore and four other sites has since late April enrolled 190 patients out of a hoped-for 300.
When the study – which compares convalescent plasma to saline in hospitalized patients – was first designed, “there was not any funding for our trial and honestly not a whole lot of interest,” Pirofski told reporters. Individual donors helped support the initial rollout in late April and the trial quickly enrolled 150 patients as the pandemic peaked in the New York City area.
The National Institutes of Health has since given funding, which allowed the study to expand to New York University, Yale University, the University of Miami, and the University of Texas at Houston.
Hopeful, but a long way to go
Shmuel Shoham, MD, FIDSA, associate director of the transplant and oncology infectious diseases center at Johns Hopkins University School of Medicine in Baltimore, said that he’s hopeful that people will continue to enroll in his trial, which is seeking to determine if plasma can prevent COVID-19 in those who’ve been recently exposed.
“Volunteers joining the study is the only way that we’re going to get to know whether this stuff works for prevention and treatment,” Shoham said on the call. He urged physicians and other healthcare workers to talk with patients about considering trial participation.
Shoham’s study is being conducted at 30 US sites and one at the Navajo Nation. It has enrolled 25 out of a hoped-for 500 participants. “We have a long way to go,” said Shoham.
Another Hopkins study to determine whether plasma is helpful in shortening illness in nonhospitalized patients, which is being conducted at the same 31 sites, has enrolled 50 out of 600.
Shoham said recruiting patients with COVID for any study had proven to be difficult. “The vast majority of people that have coronavirus do not come to centers that do clinical trials or interventional trials,” he said, adding that, in addition, most of those “who have coronavirus don’t want to be in a trial. They just want to have coronavirus and get it over with.”
But it’s important to understand how to conduct trials in a pandemic – in part to get answers quickly, he said. Researchers have been looking at convalescent plasma for months, said Shoham. “Why don’t we have the randomized clinical trial data that we want?”
Pirofski noted that trials have also been hobbled in part by “the shifting areas of the pandemic.” Fewer cases make for fewer potential plasma donors.
Both Shoham and Pirofski also said that more needed to be done to encourage plasma donors to participate.
The US Department of Health & Human Services clarified in August that hospitals, physicians, health plans, and other health care workers could contact individuals who had recovered from COVID-19 without violating the HIPAA privacy rule.
Pirofski said she believes that trial investigators know it is legal to reach out to patients. But, she said, “it probably could be better known.”
This article first appeared on Medscape.com.
Prognosis for rural hospitals worsens with pandemic
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Jerome Antone said he is one of the lucky ones.
After becoming ill with COVID-19, Mr. Antone was hospitalized only 65 miles away from his small Alabama town. He is the mayor of Georgiana – population 1,700.
“It hit our rural community so rabid,” Mr. Antone said. The town’s hospital closed last year. If hospitals in nearby communities don’t have beds available, “you may have to go 4 or 5 hours away.”
Eighteen rural hospitals closed last year and the first 3 months of 2020 were “really big months,” said Mark Holmes, PhD, director of the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill. Many of the losses are in Southern states like Florida and Texas. More than 170 rural hospitals have closed nationwide since 2005, according to data collected by the Sheps Center.
It’s a dangerous scenario. “We know that a closure leads to higher mortality pretty quickly” among the populations served, said Dr. Holmes, who is also a professor at UNC Gillings School of Global Public Health. “That’s pretty clear.”
One 2019 study found that death rates in the surrounding communities increase nearly 6% after a rural hospital closes – and that’s when there’s not a pandemic.
Add to that what is known about the coronavirus: People who are obese or live with diabetes, hypertension, asthma, and other underlying health issues are more susceptible to COVID-19. Rural areas tend to have higher rates of these conditions. And rural residents are more likely to be older, sicker and poorer than those in urban areas. All this leaves rural communities particularly vulnerable to the coronavirus.
Congress approved billions in federal relief funds for health care providers. Initially, federal officials based what a hospital would get on its Medicare payments, but by late April they heeded criticism and carved out funds for rural hospitals and COVID-19 hot spots. Rural hospitals leapt at the chance to shore up already-negative budgets and prepare for the pandemic.
The funds “helped rural hospitals with the immediate storm,” said Don Williamson, MD, president of the Alabama Hospital Association. Nearly 80% of Alabama’s rural hospitals began the year with negative balance sheets and about 8 days’ worth of cash on hand.
Before the pandemic hit this year, hundreds of rural hospitals “were just trying to keep their doors open,” said Maggie Elehwany, vice president of government affairs with the National Rural Health Association. Then an estimated 70% of their income stopped as patients avoided the emergency room, doctor’s appointments, and elective surgeries.
“It was devastating,” Ms. Elehwany said.
Paul Taylor, chief executive of a 25-bed critical-access hospital and outpatient clinics in northwestern Arkansas, accepted millions in grants and loan money Congress approved this spring, largely through the CARES (Coronavirus Aid, Relief, and Economic Security) Act.
“For us, this was survival money and we spent it already,” Mr. Taylor said. With those funds, Ozarks Community Hospital increased surge capacity, expanding from 25 beds to 50 beds, adding negative pressure rooms and buying six ventilators. Taylor also ramped up COVID-19 testing at his hospital and clinics, located near some meat-processing plants.
Throughout June and July, Ozarks tested 1,000 patients a day and reported a 20% positive rate. The rate dropped to 16.9% in late July. But patients continue to avoid routine care.
Mr. Taylor said revenue is still constrained and he does not know how he will pay back $8 million that he borrowed from Medicare. The program allowed hospitals to borrow against future payments from the federal government, but stipulated that repayment would begin within 120 days.
For Mr. Taylor, this seems impossible. Medicare makes up 40% of Ozarks’ income. And he has to pay the loan back before he gets any more payments from Medicare. He’s hoping to refinance the hospital’s mortgage.
“If I get no relief and they take the money ... we won’t still be open,” Mr. Taylor said. Ozarks provides 625 jobs and serves an area with a population of about 75,000.
There are 1,300 small critical-access hospitals like Ozarks in rural America, and of those, 859 took advantage of the Medicare loans, sending about $3.1 billion into the local communities. But many rural communities have not yet experienced a surge in coronavirus cases – national leaders fear it will come as part of a new phase.
“There are pockets of rural America who say, ‘We haven’t seen a single COVID patient yet and we do not believe it’s real,’ ” Mr. Taylor said. “They will get hit sooner or later.”
Across the country, the reduced patient numbers and increased spending required to fight and prepare for the coronavirus was “like a knife cutting into a hospital’s blood supply,” said Ge Bai, PhD, associate professor of health policy and management at the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Dr. Bai said the way the federal government reimbursed small rural hospitals through federal programs like Medicare before the pandemic was faulty and inefficient. “They are too weak to survive,” she said.
In rural Texas, about 2 hours from Dallas, Titus Regional Medical Center chief executive officer Terry Scoggin cut staff and furloughed workers even as his rural hospital faced down the pandemic. Titus Regional lost about $4 million last fiscal year and broke even each of the three years before that.
Mr. Scoggin said he did not cut from his clinical staff, though. Titus is now facing its second surge of the virus in the community. “The last 7 days, we’ve been testing 30% positive,” he said, including the case of his father, who contracted it at a nursing home and survived.
“It’s personal and this is real,” Mr. Scoggin said. “You know the people who are infected. You know the people who are passing away.”
Of his roughly 700 employees, 48 have tested positive for the virus and 1 has died. They are short on testing kits, medication, and supplies.
“Right now the staff is strained,” Mr. Scoggin said. “I’ve been blown away by their selflessness and unbelievable spirit. We’re resilient, we’re nimble, and we will make it. We don’t have a choice.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Mapping melasma management
Melasma has such a high recurrence rate that, once the facial hyperpigmentation has been cleared, it’s best that treatment never entirely stops, Amit G. Pandya, MD, said at the virtual annual meeting of the American Academy of Dermatology.
He recommended alternating between a less-intensive maintenance therapy regimen in the winter months and an acute care regimen in the sunnier summer months. But . And that is largely a matter of location.
Location, location, location
Melasma has a distinctive symmetric bilateral distribution: “Melasma likes the central area of the forehead, whereas the lateral areas of the forehead are more involved in lichen planus pigmentosus. Melanoma likes the area above the eyebrow or under the eyebrow. However, it does not go below the superior orbital rim or above the inferior orbital rim,”said Dr. Pandya, a dermatologist at the Palo Alto Medical Foundation in Sunnyvale, Calif., who is also on the faculty at the University of Texas Southwestern Medical Center, Dallas.
Melasma is common on the bridge of the nose, but usually not along the nasolabial fold, where hyperpigmentation is much more likely to be due to seborrheic dermatitis or drug-induced hyperpigmentation. Melasma doesn’t affect the tip of the nose; that’s more likely a sign of sarcoidosis or drug-induced hyperpigmentation. Melasma is common on the zygomatic prominence, while acanthosis nigricans favors the concave area below the zygomatic prominence. And melasma stays above the mandible; pigmentation below the mandible is more suggestive of poikiloderma of Civatte. Lentigines are scattered broadly across sun-exposed areas of the face. They also tend to be less symmetrical than melasma, the dermatologist continued.
Acute treatment
Dr. Pandya’s acute treatment algorithm begins with topical 4% hydroquinone in patients who’ve never been on it before. A response to the drug, which blocks the tyrosine-to-melanin pathway, takes 4-6 weeks, with maximum effect not seen until 3-6 months or longer. Bluish-grey ochronosis is a rare side effect at the 4% concentration but becomes more common at higher concentrations or when the drug is used in combination therapy.
“Hydroquinone is a workhorse, the oldest and most effective depigmenting agent,” he said.
If the patient hasn’t responded positively by 3 months, Dr. Pandya moves on to daily use of the triple-drug combination of fluocinolone acetonide 0.01%/hydroquinone 4%/tretinoin 0.05% known as Tri-Luma, a kinder, gentler descendant of the 45-year-old Kligman-Willis compounded formula comprised of 0.1% dexamethasone, 5% hydroquinone, and 0.1% tretinoin.
If Tri-Luma also proves ineffective, Dr. Pandya turns to oral tranexamic acid. This is off-label therapy for the drug, a plasmin inhibitor, which is approved for the treatment of menorrhagia. But oral tranexamic acid is widely used for treatment of melasma in East Asia, and Dr. Pandya and others have evaluated it in placebo-controlled clinical trials. His conclusion is that oral tranexamic acid appears to be safe and effective for treatment of melasma.
“The drug is not approved for melasma, it’s approved for menorrhagia, so every doctor has to decide how much risk they want to take. The evidence suggests 500 mg per day is a good dose,” he said.
The collective clinical trials experience with oral tranexamic acid for melasma shows a side effect profile consisting of mild GI upset, headache, and myalgia. While increased thromboembolic risk is a theoretic concern, it hasn’t been an issue in the published studies, which typically exclude patients with a history of thromboembolic disease from enrollment. Patient satisfaction with the oral agent is high, according to Dr. Pandya.
In one randomized, open-label, 40-patient study, oral tranexamic acid plus a triple-combination cream featuring fluocinolone 0.01%, hydroquinone 2%, and tretinoin 0.05%, applied once a day, was significantly more effective and faster-acting than the topical therapy alone. At 8 weeks, the dual-therapy group averaged an 88% improvement in the Melasma Activity and Severity Index (MASI) scores, compared with 55% with the topical therapy alone (Indian J Dermatol. Sep-Oct 2015;60[5]:520).
Cysteamine 5% cream, which is available over the counter as Cyspera but is pricey, showed promising efficacy in a 40-patient, randomized, double-blind trial (J Dermatolog Treat. 2018 Mar;29[2]:182-9). Dr. Pandya said he’s looking forward to seeing further studies.
Chemical peels can be used, but multiple treatment sessions using a superficial peeling agent are required, and even then “the efficacy is usually not profound,” according to Dr. Pandya. Together with two colleagues he recently published a comprehensive systematic review of 113 published studies of all treatments for melasma in nearly 7,000 patients (Am J Clin Dermatol. 2020 Apr;21(2):173-225).
Newer lasers with various pulse lengths, fluences, wave lengths, and treatment frequency show “some promise,” but there have also been published reports of hypopigmentation and rebound hyperpigmentation. The optimal laser regimen remains elusive, he said.
Maintenance therapy
Dr. Pandya usually switches from hydroquinone to a different topical tyrosinase inhibitor for maintenance therapy, such as kojic acid, arbutin, or azelaic acid, all available OTC in many formulations. Alternatively, he might drop down to 2% hydroquinone for the winter months. Another option is triple-combination cream applied two or three times per week. A topical formulation of tranexamic acid is available, but studies of this agent in patients with melasma have yielded mixed results.
“I don’t think topical tranexamic acid is going to harm the patient, but I don’t think the efficacy is as good as with oral tranexamic acid,” he said.
Slap that melasma in irons
A comprehensive melasma management plan requires year-round frequent daily application of a broad spectrum sunscreen. And since it’s now evident that visible-wavelength light can worsen melasma through mechanisms similar to UVA and UVB, which are long recognized as the major drivers of the hyperpigmentation disorder, serious consideration should be given to the use of a tinted broad-spectrum sunscreen or makeup containing more than 3% iron oxide, which blocks visible light. In contrast, zinc oxide does not, Dr. Pandya noted.
In one influential study, aminolevulinic acid was applied on the arms of 20 patients; two sunscreens were applied on areas where the ALA was applied, and on one area, no sunscreen was applied. The minimal phototoxic dose of visible blue light was doubled with application of a broad-spectrum sunscreen containing titanium dioxide, zinc oxide, and 0.2% iron oxide, compared with no sunscreen, but increased 21-fold using a sunscreen containing titanium dioxide, zinc oxide, and 3.2% iron oxide (Dermatol Surg. 2008 Nov;34[11]:1469-76).
Moreover, in a double-blind, randomized trial including 61 patients with melasma, all on background 4% hydroquinone, those assigned to a broad-spectrum sunscreen containing iron oxide had a 78% improvement in MASI scores at 8 weeks, compared with a 62% improvement with a broad-spectrum UV-only sunscreen. Both sunscreens had a sun protection factor of at least 50 (Photodermatol Photoimmunol Photomed. 2014 Feb;30[1]:35-42).
Numerous sunscreen and makeup products containing more than 3% iron oxide are available OTC in various tints. It’s a matter of finding a color that matches the patient’s skin.
Concern has been raised that exposure to the visible blue light emitted by computer screens and cell phones could worsen melasma. Dr. Pandya noted that reassurance on that score was recently provided by French investigators. They measured the intensity of visible light at the wavelengths emitted by computer screens and laptops and determined that it was 100- to 1,000-fold less than sunlight in the same spectrum. They also conducted a prospective, randomized, split-face trial in 12 melasma patients. One side of the face was exposed to the visible blue light at the same wavelengths emitted by device screens, but at far greater intensity. Blinded evaluators found no split-face difference in modified MASI scores.
“These results suggest that at a 20-cm distance, a maximized use of a high-intensity computer screen for 8 hours per day during a 5-day period does not worsen melasma lesions. Although it is very unlikely that similar exposure during a longer period would start to affect melasma lesions, such a possibility cannot be ruled out,” according to the investigators (J Am Acad Dermatol. 2019 Dec 27;S0190-9622(19)33324-9. doi: 10.1016/j.jaad.2019.12.047).
Dr. Pandya reported serving as a consultant to Incyte, Pfizer, Viela Bio, and Villaris.
Melasma has such a high recurrence rate that, once the facial hyperpigmentation has been cleared, it’s best that treatment never entirely stops, Amit G. Pandya, MD, said at the virtual annual meeting of the American Academy of Dermatology.
He recommended alternating between a less-intensive maintenance therapy regimen in the winter months and an acute care regimen in the sunnier summer months. But . And that is largely a matter of location.
Location, location, location
Melasma has a distinctive symmetric bilateral distribution: “Melasma likes the central area of the forehead, whereas the lateral areas of the forehead are more involved in lichen planus pigmentosus. Melanoma likes the area above the eyebrow or under the eyebrow. However, it does not go below the superior orbital rim or above the inferior orbital rim,”said Dr. Pandya, a dermatologist at the Palo Alto Medical Foundation in Sunnyvale, Calif., who is also on the faculty at the University of Texas Southwestern Medical Center, Dallas.
Melasma is common on the bridge of the nose, but usually not along the nasolabial fold, where hyperpigmentation is much more likely to be due to seborrheic dermatitis or drug-induced hyperpigmentation. Melasma doesn’t affect the tip of the nose; that’s more likely a sign of sarcoidosis or drug-induced hyperpigmentation. Melasma is common on the zygomatic prominence, while acanthosis nigricans favors the concave area below the zygomatic prominence. And melasma stays above the mandible; pigmentation below the mandible is more suggestive of poikiloderma of Civatte. Lentigines are scattered broadly across sun-exposed areas of the face. They also tend to be less symmetrical than melasma, the dermatologist continued.
Acute treatment
Dr. Pandya’s acute treatment algorithm begins with topical 4% hydroquinone in patients who’ve never been on it before. A response to the drug, which blocks the tyrosine-to-melanin pathway, takes 4-6 weeks, with maximum effect not seen until 3-6 months or longer. Bluish-grey ochronosis is a rare side effect at the 4% concentration but becomes more common at higher concentrations or when the drug is used in combination therapy.
“Hydroquinone is a workhorse, the oldest and most effective depigmenting agent,” he said.
If the patient hasn’t responded positively by 3 months, Dr. Pandya moves on to daily use of the triple-drug combination of fluocinolone acetonide 0.01%/hydroquinone 4%/tretinoin 0.05% known as Tri-Luma, a kinder, gentler descendant of the 45-year-old Kligman-Willis compounded formula comprised of 0.1% dexamethasone, 5% hydroquinone, and 0.1% tretinoin.
If Tri-Luma also proves ineffective, Dr. Pandya turns to oral tranexamic acid. This is off-label therapy for the drug, a plasmin inhibitor, which is approved for the treatment of menorrhagia. But oral tranexamic acid is widely used for treatment of melasma in East Asia, and Dr. Pandya and others have evaluated it in placebo-controlled clinical trials. His conclusion is that oral tranexamic acid appears to be safe and effective for treatment of melasma.
“The drug is not approved for melasma, it’s approved for menorrhagia, so every doctor has to decide how much risk they want to take. The evidence suggests 500 mg per day is a good dose,” he said.
The collective clinical trials experience with oral tranexamic acid for melasma shows a side effect profile consisting of mild GI upset, headache, and myalgia. While increased thromboembolic risk is a theoretic concern, it hasn’t been an issue in the published studies, which typically exclude patients with a history of thromboembolic disease from enrollment. Patient satisfaction with the oral agent is high, according to Dr. Pandya.
In one randomized, open-label, 40-patient study, oral tranexamic acid plus a triple-combination cream featuring fluocinolone 0.01%, hydroquinone 2%, and tretinoin 0.05%, applied once a day, was significantly more effective and faster-acting than the topical therapy alone. At 8 weeks, the dual-therapy group averaged an 88% improvement in the Melasma Activity and Severity Index (MASI) scores, compared with 55% with the topical therapy alone (Indian J Dermatol. Sep-Oct 2015;60[5]:520).
Cysteamine 5% cream, which is available over the counter as Cyspera but is pricey, showed promising efficacy in a 40-patient, randomized, double-blind trial (J Dermatolog Treat. 2018 Mar;29[2]:182-9). Dr. Pandya said he’s looking forward to seeing further studies.
Chemical peels can be used, but multiple treatment sessions using a superficial peeling agent are required, and even then “the efficacy is usually not profound,” according to Dr. Pandya. Together with two colleagues he recently published a comprehensive systematic review of 113 published studies of all treatments for melasma in nearly 7,000 patients (Am J Clin Dermatol. 2020 Apr;21(2):173-225).
Newer lasers with various pulse lengths, fluences, wave lengths, and treatment frequency show “some promise,” but there have also been published reports of hypopigmentation and rebound hyperpigmentation. The optimal laser regimen remains elusive, he said.
Maintenance therapy
Dr. Pandya usually switches from hydroquinone to a different topical tyrosinase inhibitor for maintenance therapy, such as kojic acid, arbutin, or azelaic acid, all available OTC in many formulations. Alternatively, he might drop down to 2% hydroquinone for the winter months. Another option is triple-combination cream applied two or three times per week. A topical formulation of tranexamic acid is available, but studies of this agent in patients with melasma have yielded mixed results.
“I don’t think topical tranexamic acid is going to harm the patient, but I don’t think the efficacy is as good as with oral tranexamic acid,” he said.
Slap that melasma in irons
A comprehensive melasma management plan requires year-round frequent daily application of a broad spectrum sunscreen. And since it’s now evident that visible-wavelength light can worsen melasma through mechanisms similar to UVA and UVB, which are long recognized as the major drivers of the hyperpigmentation disorder, serious consideration should be given to the use of a tinted broad-spectrum sunscreen or makeup containing more than 3% iron oxide, which blocks visible light. In contrast, zinc oxide does not, Dr. Pandya noted.
In one influential study, aminolevulinic acid was applied on the arms of 20 patients; two sunscreens were applied on areas where the ALA was applied, and on one area, no sunscreen was applied. The minimal phototoxic dose of visible blue light was doubled with application of a broad-spectrum sunscreen containing titanium dioxide, zinc oxide, and 0.2% iron oxide, compared with no sunscreen, but increased 21-fold using a sunscreen containing titanium dioxide, zinc oxide, and 3.2% iron oxide (Dermatol Surg. 2008 Nov;34[11]:1469-76).
Moreover, in a double-blind, randomized trial including 61 patients with melasma, all on background 4% hydroquinone, those assigned to a broad-spectrum sunscreen containing iron oxide had a 78% improvement in MASI scores at 8 weeks, compared with a 62% improvement with a broad-spectrum UV-only sunscreen. Both sunscreens had a sun protection factor of at least 50 (Photodermatol Photoimmunol Photomed. 2014 Feb;30[1]:35-42).
Numerous sunscreen and makeup products containing more than 3% iron oxide are available OTC in various tints. It’s a matter of finding a color that matches the patient’s skin.
Concern has been raised that exposure to the visible blue light emitted by computer screens and cell phones could worsen melasma. Dr. Pandya noted that reassurance on that score was recently provided by French investigators. They measured the intensity of visible light at the wavelengths emitted by computer screens and laptops and determined that it was 100- to 1,000-fold less than sunlight in the same spectrum. They also conducted a prospective, randomized, split-face trial in 12 melasma patients. One side of the face was exposed to the visible blue light at the same wavelengths emitted by device screens, but at far greater intensity. Blinded evaluators found no split-face difference in modified MASI scores.
“These results suggest that at a 20-cm distance, a maximized use of a high-intensity computer screen for 8 hours per day during a 5-day period does not worsen melasma lesions. Although it is very unlikely that similar exposure during a longer period would start to affect melasma lesions, such a possibility cannot be ruled out,” according to the investigators (J Am Acad Dermatol. 2019 Dec 27;S0190-9622(19)33324-9. doi: 10.1016/j.jaad.2019.12.047).
Dr. Pandya reported serving as a consultant to Incyte, Pfizer, Viela Bio, and Villaris.
Melasma has such a high recurrence rate that, once the facial hyperpigmentation has been cleared, it’s best that treatment never entirely stops, Amit G. Pandya, MD, said at the virtual annual meeting of the American Academy of Dermatology.
He recommended alternating between a less-intensive maintenance therapy regimen in the winter months and an acute care regimen in the sunnier summer months. But . And that is largely a matter of location.
Location, location, location
Melasma has a distinctive symmetric bilateral distribution: “Melasma likes the central area of the forehead, whereas the lateral areas of the forehead are more involved in lichen planus pigmentosus. Melanoma likes the area above the eyebrow or under the eyebrow. However, it does not go below the superior orbital rim or above the inferior orbital rim,”said Dr. Pandya, a dermatologist at the Palo Alto Medical Foundation in Sunnyvale, Calif., who is also on the faculty at the University of Texas Southwestern Medical Center, Dallas.
Melasma is common on the bridge of the nose, but usually not along the nasolabial fold, where hyperpigmentation is much more likely to be due to seborrheic dermatitis or drug-induced hyperpigmentation. Melasma doesn’t affect the tip of the nose; that’s more likely a sign of sarcoidosis or drug-induced hyperpigmentation. Melasma is common on the zygomatic prominence, while acanthosis nigricans favors the concave area below the zygomatic prominence. And melasma stays above the mandible; pigmentation below the mandible is more suggestive of poikiloderma of Civatte. Lentigines are scattered broadly across sun-exposed areas of the face. They also tend to be less symmetrical than melasma, the dermatologist continued.
Acute treatment
Dr. Pandya’s acute treatment algorithm begins with topical 4% hydroquinone in patients who’ve never been on it before. A response to the drug, which blocks the tyrosine-to-melanin pathway, takes 4-6 weeks, with maximum effect not seen until 3-6 months or longer. Bluish-grey ochronosis is a rare side effect at the 4% concentration but becomes more common at higher concentrations or when the drug is used in combination therapy.
“Hydroquinone is a workhorse, the oldest and most effective depigmenting agent,” he said.
If the patient hasn’t responded positively by 3 months, Dr. Pandya moves on to daily use of the triple-drug combination of fluocinolone acetonide 0.01%/hydroquinone 4%/tretinoin 0.05% known as Tri-Luma, a kinder, gentler descendant of the 45-year-old Kligman-Willis compounded formula comprised of 0.1% dexamethasone, 5% hydroquinone, and 0.1% tretinoin.
If Tri-Luma also proves ineffective, Dr. Pandya turns to oral tranexamic acid. This is off-label therapy for the drug, a plasmin inhibitor, which is approved for the treatment of menorrhagia. But oral tranexamic acid is widely used for treatment of melasma in East Asia, and Dr. Pandya and others have evaluated it in placebo-controlled clinical trials. His conclusion is that oral tranexamic acid appears to be safe and effective for treatment of melasma.
“The drug is not approved for melasma, it’s approved for menorrhagia, so every doctor has to decide how much risk they want to take. The evidence suggests 500 mg per day is a good dose,” he said.
The collective clinical trials experience with oral tranexamic acid for melasma shows a side effect profile consisting of mild GI upset, headache, and myalgia. While increased thromboembolic risk is a theoretic concern, it hasn’t been an issue in the published studies, which typically exclude patients with a history of thromboembolic disease from enrollment. Patient satisfaction with the oral agent is high, according to Dr. Pandya.
In one randomized, open-label, 40-patient study, oral tranexamic acid plus a triple-combination cream featuring fluocinolone 0.01%, hydroquinone 2%, and tretinoin 0.05%, applied once a day, was significantly more effective and faster-acting than the topical therapy alone. At 8 weeks, the dual-therapy group averaged an 88% improvement in the Melasma Activity and Severity Index (MASI) scores, compared with 55% with the topical therapy alone (Indian J Dermatol. Sep-Oct 2015;60[5]:520).
Cysteamine 5% cream, which is available over the counter as Cyspera but is pricey, showed promising efficacy in a 40-patient, randomized, double-blind trial (J Dermatolog Treat. 2018 Mar;29[2]:182-9). Dr. Pandya said he’s looking forward to seeing further studies.
Chemical peels can be used, but multiple treatment sessions using a superficial peeling agent are required, and even then “the efficacy is usually not profound,” according to Dr. Pandya. Together with two colleagues he recently published a comprehensive systematic review of 113 published studies of all treatments for melasma in nearly 7,000 patients (Am J Clin Dermatol. 2020 Apr;21(2):173-225).
Newer lasers with various pulse lengths, fluences, wave lengths, and treatment frequency show “some promise,” but there have also been published reports of hypopigmentation and rebound hyperpigmentation. The optimal laser regimen remains elusive, he said.
Maintenance therapy
Dr. Pandya usually switches from hydroquinone to a different topical tyrosinase inhibitor for maintenance therapy, such as kojic acid, arbutin, or azelaic acid, all available OTC in many formulations. Alternatively, he might drop down to 2% hydroquinone for the winter months. Another option is triple-combination cream applied two or three times per week. A topical formulation of tranexamic acid is available, but studies of this agent in patients with melasma have yielded mixed results.
“I don’t think topical tranexamic acid is going to harm the patient, but I don’t think the efficacy is as good as with oral tranexamic acid,” he said.
Slap that melasma in irons
A comprehensive melasma management plan requires year-round frequent daily application of a broad spectrum sunscreen. And since it’s now evident that visible-wavelength light can worsen melasma through mechanisms similar to UVA and UVB, which are long recognized as the major drivers of the hyperpigmentation disorder, serious consideration should be given to the use of a tinted broad-spectrum sunscreen or makeup containing more than 3% iron oxide, which blocks visible light. In contrast, zinc oxide does not, Dr. Pandya noted.
In one influential study, aminolevulinic acid was applied on the arms of 20 patients; two sunscreens were applied on areas where the ALA was applied, and on one area, no sunscreen was applied. The minimal phototoxic dose of visible blue light was doubled with application of a broad-spectrum sunscreen containing titanium dioxide, zinc oxide, and 0.2% iron oxide, compared with no sunscreen, but increased 21-fold using a sunscreen containing titanium dioxide, zinc oxide, and 3.2% iron oxide (Dermatol Surg. 2008 Nov;34[11]:1469-76).
Moreover, in a double-blind, randomized trial including 61 patients with melasma, all on background 4% hydroquinone, those assigned to a broad-spectrum sunscreen containing iron oxide had a 78% improvement in MASI scores at 8 weeks, compared with a 62% improvement with a broad-spectrum UV-only sunscreen. Both sunscreens had a sun protection factor of at least 50 (Photodermatol Photoimmunol Photomed. 2014 Feb;30[1]:35-42).
Numerous sunscreen and makeup products containing more than 3% iron oxide are available OTC in various tints. It’s a matter of finding a color that matches the patient’s skin.
Concern has been raised that exposure to the visible blue light emitted by computer screens and cell phones could worsen melasma. Dr. Pandya noted that reassurance on that score was recently provided by French investigators. They measured the intensity of visible light at the wavelengths emitted by computer screens and laptops and determined that it was 100- to 1,000-fold less than sunlight in the same spectrum. They also conducted a prospective, randomized, split-face trial in 12 melasma patients. One side of the face was exposed to the visible blue light at the same wavelengths emitted by device screens, but at far greater intensity. Blinded evaluators found no split-face difference in modified MASI scores.
“These results suggest that at a 20-cm distance, a maximized use of a high-intensity computer screen for 8 hours per day during a 5-day period does not worsen melasma lesions. Although it is very unlikely that similar exposure during a longer period would start to affect melasma lesions, such a possibility cannot be ruled out,” according to the investigators (J Am Acad Dermatol. 2019 Dec 27;S0190-9622(19)33324-9. doi: 10.1016/j.jaad.2019.12.047).
Dr. Pandya reported serving as a consultant to Incyte, Pfizer, Viela Bio, and Villaris.
FROM AAD 20
Large study finds no link between TCI use, skin cancer in patients with AD
The results also suggest dose, frequency, and exposure duration to the topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are not associated with an increased risk of keratinocyte carcinomas (KCs), basal cell carcinomas (BCCs), and squamous cell carcinomas (SCCs) in patients with atopic dermatitis (AD), according to Maryam M. Asgari, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues. In 2006, the Food and Drug Administration announced the addition of the boxed warning to the labeling of TCIs regarding a possible risk of cancer associated with use of pimecrolimus (Elidel) and with tacrolimus (Protopic), because of an increased risk of KCs associated with oral calcineurin inhibitors and reports of skin cancer in patients on TCIs.
“Controversy has surrounded the association between TCI exposure and KC risk since the black-box warning was issued by the FDA. A hypothesized mechanism of action for TCIs increasing KC risk includes a direct effect of calcineurin inhibition on DNA repair and apoptosis, which could influence keratinocyte carcinogenesis,” the authors of the study wrote in JAMA Dermatology. But, they added, there have been “conflicting results” in research exploring this association.
In the retrospective cohort study, Dr. Asgari and coauthors evaluated 93,746 adult patients with AD at Kaiser Permanente Northern California, diagnosed between January 2002 and December 2013, comparing skin cancer risk among 7,033 patients exposed to TCIs, 73,674 patients taking topical corticosteroids, and 46,141 patients who had not been exposed to TCIs or topical corticosteroids. Results were adjusted in a multivariate Cox regression analysis for age, gender, race/ethnicity, calendar year, number of dermatology visits per year, history of KCs, immunosuppression, prior systemic AD treatment, autoimmune disease, treatment with ultraviolet therapy, chemotherapy, and radiotherapy.
The researchers also examined how TCI dose, frequency and exposure duration impacted skin cancer risk. Patients were grouped by high-dose (0.1%) and low-dose (0.03%) formulations of tacrolimus; and the 1% formulation of pimecrolimus. Frequency of use was defined as low (once daily or less) or high (twice daily or more), and exposure duration was based on short- (less than 2 years), moderate- (2-4 years), and long-term (4 years or more) use. Patients were at least 40 years old (mean age, 58.5 years), 58.7% were women, 50.5% were White, 20.6% were Asian, 12.2% were Hispanic, and 7.9% were Black. They were followed for a mean of 7.70 years.
Compared with patients who were exposed to topical corticosteroids, there was no association between risk of KCs and exposure to TCIs in patients with AD (adjusted hazard ratio, 1.02; 95% confidence interval, 0.93-1.13). There were also no significant differences in risk of BCCs and TCI exposure (aHR, 1.01; 95% CI, 0.90-1.14) and risk of SCCs and TCI exposure (aHR, 0.94; 95% CI, 0.82-1.08), compared with patients exposed to topical corticosteroids.
Results were similar for risk of KCs (aHR, 1.03; 95% CI, 0.92-1.14), BCCs (aHR, 1.04; 95% CI, 0.91-1.19), and SCCs (aHR, 0.91; 95% CI, 0.78-1.06) when patients exposed to TCIs were compared with those with AD who were unexposed to any medication. In secondary analyses, Dr. Asgari and coauthors found no association with overall risk of KCs, or risk of BCCs or SCCs, and the dose, frequency, or exposure duration to TCIs.
“Our findings appear to support those of smaller postmarketing surveillance studies of TCI and KC risk and may provide some reassurance about the safety profile of this class of topical agents in the treatment of AD,” they concluded.
In an interview, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said initial concerns surrounding TCIs were based on high doses potentially increasing the risk of malignancy, and off-label use of TCIs for inflammatory skin diseases other than AD.
“However, the FDA’s concerns may not have been justified,” he said. The manufacturers of pimecrolimus and tacrolimus have published results of 10-year observational registries that assess cancer risk, which “found no evidence of any associations between TCIs and malignancy,” noted Dr. Silverberg, who is also director of clinical research and contact dermatitis at George Washington University.
Elizabeth Hughes, MD, a dermatologist in private practice in San Antonio, said in an interview that initial enthusiasm was “huge” for use of TCIs like tacrolimus in patients with AD when they first became available, especially in the pediatric population, for whom clinicians are hesitant to use long-term strong topical steroids. However, parents of children taking the medication soon became concerned about potential side effects.
“The TCIs can be absorbed to a small extent through body surface area, so it was not a big leap to become concerned that infants and small children could absorb enough ... into the bloodstream to give a similar side effect profile as oral tacrolimus,” she said.
The addition of the boxed warning in 2006 was frustrating for dermatologists “because a medication we needed very much for a young population now was ‘labeled’ and parents were scared to use it,” Dr. Hughes explained.
Dr. Silverberg noted that, while the results of the new study are unlikely to change clinical practice, they are reassuring, and provide real-world data and “further confirmation of previous studies showing no associations between AD and malignancy.”
“Since AD and skin cancer are both commonly managed by dermatologists, there is potential for increased surveillance and detection of skin cancers in AD patients. So, the greatest chance of seeing a false-positive signal for malignancy would likely occur with skin cancers,” he pointed out. “Yet, even in the case of skin cancers, there were no demonstrable signals.”
Based on the results, “I think it is definitely reasonable to reconsider” the TCI boxed warning, but there isn’t much precedent for boxed warnings to be removed from labeling, Dr. Silverberg commented. “Unfortunately, the black-box warning may persist despite a lot of reassuring data.”
In a related editorial, Aaron M. Drucker, MD, ScM, and Mina Tadrous, PharmD, PhD, of the University of Toronto, said the boxed warning “had the intent of helping patients and clinicians understand possible risks,” but also carried the “potential for harm” if patients discontinued or did not adhere to treatment. “Safety warnings on topical medications could lead to undertreatment of atopic dermatitis, reduced quality of life and, potentially, increased use of more toxic systemic medications.”
Long-term studies of medications and cancer risk are challenging to perform, having to account for dose-response relationships, confounding by indication, and time bias, among other factors, and this study “recognizes and attempts to address many of these challenges,” Dr. Drucker and Dr. Tadrous wrote.
These results are similar to previous studies that have “consistently reported no or minimal association between TCI use and skin cancer,” they noted, adding that, “if an association exists, it is likely very small, meaning that skin cancer attributable to TCI use is rare. Clinicians can use this evidence to counsel and reassure patients for whom the benefits of ongoing treatment with TCIs may outweigh the harms.”
This study was funded by a grant from Valeant Pharmaceuticals. Dr. Asgari reported receiving grants from Valeant during the study, and from Pfizer not related to the study. The other authors reported no relevant conflicts of interest. Dr. Drucker reported relationships with the Canadian Agency for Drugs and Technology in Health, CME Outfitters, Eczema Society of Canada, Sanofi, Regeneron, and RTI Health Solutions in the form of paid fees, consultancies, honoraria, educational grants, and other compensation paid to him and/or his institution. Dr. Tadrous reported no relevant disclosures. Dr. Silverberg reported receiving honoraria for advisory board, speaker, and consultant services from numerous pharmaceutical manufacturers, and research grants for investigator services from GlaxoSmithKline and Galderma. Dr. Hughes Tichy reported no relevant financial disclosures. Dr. Silverberg is a member of the Dermatology News editorial advisory board.
SOURCE: Asgari MM et al. JAMA Dermatol. 2020 Aug 12. doi: 10.1001/jamadermatol.2020.2240.
The results also suggest dose, frequency, and exposure duration to the topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are not associated with an increased risk of keratinocyte carcinomas (KCs), basal cell carcinomas (BCCs), and squamous cell carcinomas (SCCs) in patients with atopic dermatitis (AD), according to Maryam M. Asgari, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues. In 2006, the Food and Drug Administration announced the addition of the boxed warning to the labeling of TCIs regarding a possible risk of cancer associated with use of pimecrolimus (Elidel) and with tacrolimus (Protopic), because of an increased risk of KCs associated with oral calcineurin inhibitors and reports of skin cancer in patients on TCIs.
“Controversy has surrounded the association between TCI exposure and KC risk since the black-box warning was issued by the FDA. A hypothesized mechanism of action for TCIs increasing KC risk includes a direct effect of calcineurin inhibition on DNA repair and apoptosis, which could influence keratinocyte carcinogenesis,” the authors of the study wrote in JAMA Dermatology. But, they added, there have been “conflicting results” in research exploring this association.
In the retrospective cohort study, Dr. Asgari and coauthors evaluated 93,746 adult patients with AD at Kaiser Permanente Northern California, diagnosed between January 2002 and December 2013, comparing skin cancer risk among 7,033 patients exposed to TCIs, 73,674 patients taking topical corticosteroids, and 46,141 patients who had not been exposed to TCIs or topical corticosteroids. Results were adjusted in a multivariate Cox regression analysis for age, gender, race/ethnicity, calendar year, number of dermatology visits per year, history of KCs, immunosuppression, prior systemic AD treatment, autoimmune disease, treatment with ultraviolet therapy, chemotherapy, and radiotherapy.
The researchers also examined how TCI dose, frequency and exposure duration impacted skin cancer risk. Patients were grouped by high-dose (0.1%) and low-dose (0.03%) formulations of tacrolimus; and the 1% formulation of pimecrolimus. Frequency of use was defined as low (once daily or less) or high (twice daily or more), and exposure duration was based on short- (less than 2 years), moderate- (2-4 years), and long-term (4 years or more) use. Patients were at least 40 years old (mean age, 58.5 years), 58.7% were women, 50.5% were White, 20.6% were Asian, 12.2% were Hispanic, and 7.9% were Black. They were followed for a mean of 7.70 years.
Compared with patients who were exposed to topical corticosteroids, there was no association between risk of KCs and exposure to TCIs in patients with AD (adjusted hazard ratio, 1.02; 95% confidence interval, 0.93-1.13). There were also no significant differences in risk of BCCs and TCI exposure (aHR, 1.01; 95% CI, 0.90-1.14) and risk of SCCs and TCI exposure (aHR, 0.94; 95% CI, 0.82-1.08), compared with patients exposed to topical corticosteroids.
Results were similar for risk of KCs (aHR, 1.03; 95% CI, 0.92-1.14), BCCs (aHR, 1.04; 95% CI, 0.91-1.19), and SCCs (aHR, 0.91; 95% CI, 0.78-1.06) when patients exposed to TCIs were compared with those with AD who were unexposed to any medication. In secondary analyses, Dr. Asgari and coauthors found no association with overall risk of KCs, or risk of BCCs or SCCs, and the dose, frequency, or exposure duration to TCIs.
“Our findings appear to support those of smaller postmarketing surveillance studies of TCI and KC risk and may provide some reassurance about the safety profile of this class of topical agents in the treatment of AD,” they concluded.
In an interview, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said initial concerns surrounding TCIs were based on high doses potentially increasing the risk of malignancy, and off-label use of TCIs for inflammatory skin diseases other than AD.
“However, the FDA’s concerns may not have been justified,” he said. The manufacturers of pimecrolimus and tacrolimus have published results of 10-year observational registries that assess cancer risk, which “found no evidence of any associations between TCIs and malignancy,” noted Dr. Silverberg, who is also director of clinical research and contact dermatitis at George Washington University.
Elizabeth Hughes, MD, a dermatologist in private practice in San Antonio, said in an interview that initial enthusiasm was “huge” for use of TCIs like tacrolimus in patients with AD when they first became available, especially in the pediatric population, for whom clinicians are hesitant to use long-term strong topical steroids. However, parents of children taking the medication soon became concerned about potential side effects.
“The TCIs can be absorbed to a small extent through body surface area, so it was not a big leap to become concerned that infants and small children could absorb enough ... into the bloodstream to give a similar side effect profile as oral tacrolimus,” she said.
The addition of the boxed warning in 2006 was frustrating for dermatologists “because a medication we needed very much for a young population now was ‘labeled’ and parents were scared to use it,” Dr. Hughes explained.
Dr. Silverberg noted that, while the results of the new study are unlikely to change clinical practice, they are reassuring, and provide real-world data and “further confirmation of previous studies showing no associations between AD and malignancy.”
“Since AD and skin cancer are both commonly managed by dermatologists, there is potential for increased surveillance and detection of skin cancers in AD patients. So, the greatest chance of seeing a false-positive signal for malignancy would likely occur with skin cancers,” he pointed out. “Yet, even in the case of skin cancers, there were no demonstrable signals.”
Based on the results, “I think it is definitely reasonable to reconsider” the TCI boxed warning, but there isn’t much precedent for boxed warnings to be removed from labeling, Dr. Silverberg commented. “Unfortunately, the black-box warning may persist despite a lot of reassuring data.”
In a related editorial, Aaron M. Drucker, MD, ScM, and Mina Tadrous, PharmD, PhD, of the University of Toronto, said the boxed warning “had the intent of helping patients and clinicians understand possible risks,” but also carried the “potential for harm” if patients discontinued or did not adhere to treatment. “Safety warnings on topical medications could lead to undertreatment of atopic dermatitis, reduced quality of life and, potentially, increased use of more toxic systemic medications.”
Long-term studies of medications and cancer risk are challenging to perform, having to account for dose-response relationships, confounding by indication, and time bias, among other factors, and this study “recognizes and attempts to address many of these challenges,” Dr. Drucker and Dr. Tadrous wrote.
These results are similar to previous studies that have “consistently reported no or minimal association between TCI use and skin cancer,” they noted, adding that, “if an association exists, it is likely very small, meaning that skin cancer attributable to TCI use is rare. Clinicians can use this evidence to counsel and reassure patients for whom the benefits of ongoing treatment with TCIs may outweigh the harms.”
This study was funded by a grant from Valeant Pharmaceuticals. Dr. Asgari reported receiving grants from Valeant during the study, and from Pfizer not related to the study. The other authors reported no relevant conflicts of interest. Dr. Drucker reported relationships with the Canadian Agency for Drugs and Technology in Health, CME Outfitters, Eczema Society of Canada, Sanofi, Regeneron, and RTI Health Solutions in the form of paid fees, consultancies, honoraria, educational grants, and other compensation paid to him and/or his institution. Dr. Tadrous reported no relevant disclosures. Dr. Silverberg reported receiving honoraria for advisory board, speaker, and consultant services from numerous pharmaceutical manufacturers, and research grants for investigator services from GlaxoSmithKline and Galderma. Dr. Hughes Tichy reported no relevant financial disclosures. Dr. Silverberg is a member of the Dermatology News editorial advisory board.
SOURCE: Asgari MM et al. JAMA Dermatol. 2020 Aug 12. doi: 10.1001/jamadermatol.2020.2240.
The results also suggest dose, frequency, and exposure duration to the topical calcineurin inhibitors (TCIs) tacrolimus and pimecrolimus are not associated with an increased risk of keratinocyte carcinomas (KCs), basal cell carcinomas (BCCs), and squamous cell carcinomas (SCCs) in patients with atopic dermatitis (AD), according to Maryam M. Asgari, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues. In 2006, the Food and Drug Administration announced the addition of the boxed warning to the labeling of TCIs regarding a possible risk of cancer associated with use of pimecrolimus (Elidel) and with tacrolimus (Protopic), because of an increased risk of KCs associated with oral calcineurin inhibitors and reports of skin cancer in patients on TCIs.
“Controversy has surrounded the association between TCI exposure and KC risk since the black-box warning was issued by the FDA. A hypothesized mechanism of action for TCIs increasing KC risk includes a direct effect of calcineurin inhibition on DNA repair and apoptosis, which could influence keratinocyte carcinogenesis,” the authors of the study wrote in JAMA Dermatology. But, they added, there have been “conflicting results” in research exploring this association.
In the retrospective cohort study, Dr. Asgari and coauthors evaluated 93,746 adult patients with AD at Kaiser Permanente Northern California, diagnosed between January 2002 and December 2013, comparing skin cancer risk among 7,033 patients exposed to TCIs, 73,674 patients taking topical corticosteroids, and 46,141 patients who had not been exposed to TCIs or topical corticosteroids. Results were adjusted in a multivariate Cox regression analysis for age, gender, race/ethnicity, calendar year, number of dermatology visits per year, history of KCs, immunosuppression, prior systemic AD treatment, autoimmune disease, treatment with ultraviolet therapy, chemotherapy, and radiotherapy.
The researchers also examined how TCI dose, frequency and exposure duration impacted skin cancer risk. Patients were grouped by high-dose (0.1%) and low-dose (0.03%) formulations of tacrolimus; and the 1% formulation of pimecrolimus. Frequency of use was defined as low (once daily or less) or high (twice daily or more), and exposure duration was based on short- (less than 2 years), moderate- (2-4 years), and long-term (4 years or more) use. Patients were at least 40 years old (mean age, 58.5 years), 58.7% were women, 50.5% were White, 20.6% were Asian, 12.2% were Hispanic, and 7.9% were Black. They were followed for a mean of 7.70 years.
Compared with patients who were exposed to topical corticosteroids, there was no association between risk of KCs and exposure to TCIs in patients with AD (adjusted hazard ratio, 1.02; 95% confidence interval, 0.93-1.13). There were also no significant differences in risk of BCCs and TCI exposure (aHR, 1.01; 95% CI, 0.90-1.14) and risk of SCCs and TCI exposure (aHR, 0.94; 95% CI, 0.82-1.08), compared with patients exposed to topical corticosteroids.
Results were similar for risk of KCs (aHR, 1.03; 95% CI, 0.92-1.14), BCCs (aHR, 1.04; 95% CI, 0.91-1.19), and SCCs (aHR, 0.91; 95% CI, 0.78-1.06) when patients exposed to TCIs were compared with those with AD who were unexposed to any medication. In secondary analyses, Dr. Asgari and coauthors found no association with overall risk of KCs, or risk of BCCs or SCCs, and the dose, frequency, or exposure duration to TCIs.
“Our findings appear to support those of smaller postmarketing surveillance studies of TCI and KC risk and may provide some reassurance about the safety profile of this class of topical agents in the treatment of AD,” they concluded.
In an interview, Jonathan Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, said initial concerns surrounding TCIs were based on high doses potentially increasing the risk of malignancy, and off-label use of TCIs for inflammatory skin diseases other than AD.
“However, the FDA’s concerns may not have been justified,” he said. The manufacturers of pimecrolimus and tacrolimus have published results of 10-year observational registries that assess cancer risk, which “found no evidence of any associations between TCIs and malignancy,” noted Dr. Silverberg, who is also director of clinical research and contact dermatitis at George Washington University.
Elizabeth Hughes, MD, a dermatologist in private practice in San Antonio, said in an interview that initial enthusiasm was “huge” for use of TCIs like tacrolimus in patients with AD when they first became available, especially in the pediatric population, for whom clinicians are hesitant to use long-term strong topical steroids. However, parents of children taking the medication soon became concerned about potential side effects.
“The TCIs can be absorbed to a small extent through body surface area, so it was not a big leap to become concerned that infants and small children could absorb enough ... into the bloodstream to give a similar side effect profile as oral tacrolimus,” she said.
The addition of the boxed warning in 2006 was frustrating for dermatologists “because a medication we needed very much for a young population now was ‘labeled’ and parents were scared to use it,” Dr. Hughes explained.
Dr. Silverberg noted that, while the results of the new study are unlikely to change clinical practice, they are reassuring, and provide real-world data and “further confirmation of previous studies showing no associations between AD and malignancy.”
“Since AD and skin cancer are both commonly managed by dermatologists, there is potential for increased surveillance and detection of skin cancers in AD patients. So, the greatest chance of seeing a false-positive signal for malignancy would likely occur with skin cancers,” he pointed out. “Yet, even in the case of skin cancers, there were no demonstrable signals.”
Based on the results, “I think it is definitely reasonable to reconsider” the TCI boxed warning, but there isn’t much precedent for boxed warnings to be removed from labeling, Dr. Silverberg commented. “Unfortunately, the black-box warning may persist despite a lot of reassuring data.”
In a related editorial, Aaron M. Drucker, MD, ScM, and Mina Tadrous, PharmD, PhD, of the University of Toronto, said the boxed warning “had the intent of helping patients and clinicians understand possible risks,” but also carried the “potential for harm” if patients discontinued or did not adhere to treatment. “Safety warnings on topical medications could lead to undertreatment of atopic dermatitis, reduced quality of life and, potentially, increased use of more toxic systemic medications.”
Long-term studies of medications and cancer risk are challenging to perform, having to account for dose-response relationships, confounding by indication, and time bias, among other factors, and this study “recognizes and attempts to address many of these challenges,” Dr. Drucker and Dr. Tadrous wrote.
These results are similar to previous studies that have “consistently reported no or minimal association between TCI use and skin cancer,” they noted, adding that, “if an association exists, it is likely very small, meaning that skin cancer attributable to TCI use is rare. Clinicians can use this evidence to counsel and reassure patients for whom the benefits of ongoing treatment with TCIs may outweigh the harms.”
This study was funded by a grant from Valeant Pharmaceuticals. Dr. Asgari reported receiving grants from Valeant during the study, and from Pfizer not related to the study. The other authors reported no relevant conflicts of interest. Dr. Drucker reported relationships with the Canadian Agency for Drugs and Technology in Health, CME Outfitters, Eczema Society of Canada, Sanofi, Regeneron, and RTI Health Solutions in the form of paid fees, consultancies, honoraria, educational grants, and other compensation paid to him and/or his institution. Dr. Tadrous reported no relevant disclosures. Dr. Silverberg reported receiving honoraria for advisory board, speaker, and consultant services from numerous pharmaceutical manufacturers, and research grants for investigator services from GlaxoSmithKline and Galderma. Dr. Hughes Tichy reported no relevant financial disclosures. Dr. Silverberg is a member of the Dermatology News editorial advisory board.
SOURCE: Asgari MM et al. JAMA Dermatol. 2020 Aug 12. doi: 10.1001/jamadermatol.2020.2240.
FROM JAMA DERMATOLOGY