User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
First validated classification criteria for discoid lupus erythematosus unveiled
The first validated classification criteria for discoid lupus erythematosus has a sensitivity that ranges between 73.9% and 84.1% and a specificity that ranges between 75.9% and 92.9%.
“Discoid lupus erythematosus [DLE] is the most common type of chronic cutaneous lupus,” lead study author Scott A. Elman, MD, said during the virtual annual meeting of the American Academy of Dermatology. “It’s one of the most potentially disfiguring forms of cutaneous lupus erythematosus [CLE], which can lead to scarring, hair loss, and dyspigmentation if not treated early or promptly. It has a significant impact on patient quality of life and there are currently no classification criteria for DLE, which has led to problematic heterogeneity in observational and interventional research efforts. As there is increasing interest in drug development programs for CLE and DLE, there is a need to develop classification criteria.”
Dr. Elman, of the Harvard combined medicine-dermatology training program at Brigham and Women’s Hospital, Boston, pointed out that classification criteria are the standard definitions that are primarily intended to enroll uniform cohorts for research. “These emphasize high specificity, whereas diagnostic criteria reflect a more broad and variable set of features of a given disease, and therefore require a higher sensitivity,” he explained. “While classification criteria are not synonymous with diagnostic criteria, they typically mirror the list of criteria that are used for diagnosis.”
In 2017, Dr. Elman and colleagues generated an item list of 12 potential classification criteria using an international Delphi consensus process: 5 criteria represented disease morphology, 2 represented discoid lupus location, and 5 represented histopathology (J Am Acad Dermatol. 2017 Aug 1;77[2]:261-7). The purpose of the current study, which was presented as a late-breaking abstract, was to validate the proposed classification criteria in a multicenter, international trial. “The point is to be able to differentiate between discoid lupus and its disease mimickers, which could be confused in enrollment in clinical trials,” he said.
At nine participating sites, patients were identified at clinical visits as having either DLE or a DLE mimicker. After each visit, dermatologists determined if morphological features were present. One dermatopathologist at each site reviewed pathology, if available, to see if the histopathologic features were present. Diagnosis by clinical features and dermatopathology were tabulated and presented as counts and percentages. Clinical features among those with and without DLE were calculated and compared with chi-square or Fisher’s exact tests. The researchers used best subsets logistic regression analysis to identify candidate models.
A total of 215 patients were enrolled: 94 that were consistent with DLE and 121 that were consistent with a DLE mimicker. Most cases (83%) were from North America, 11% were from Asia, and 6% were from Europe. Only 86 cases (40%) had biopsies for dermatopathology review.
The following clinical features were found to be more commonly associated with DLE, compared with DLE mimickers: atrophic scarring (83% vs. 24%; P < .001), dyspigmentation (84% vs. 55%; P < .001), follicular hyperkeratosis/plugging (43% vs. 11%; P < .001), scarring alopecia (61% vs. 21%; P < .001), location in the conchal bowl (49% vs. 10%; P < .001), preference for the head and neck (87% vs. 49%; P < .001), and erythematous to violaceous in color (93% vs. 85%, a nonsignificant difference; P = .09).
When histopathological items were assessed, the following features were found to be more commonly associated with DLE, compared with DLE mimickers: interface/vacuolar dermatitis (83% vs. 53%; P = .004), perivascular and/or periappendageal lymphohistiocytic infiltrate (95% vs. 84%, a nonsignificant difference; P = .18), follicular keratin plugs (57% vs. 20%; P < .001), mucin deposition (73% vs. 39%; P = .002), and basement membrane thickening (57% vs. 14%; P < .001).
“There was good agreement between the diagnoses made by dermatologists and dermatopathologists, with a Cohen’s kappa statistic of 0.83,” Dr. Elman added. “Similarly, in many of the cases, the dermatopathologists and the dermatologists felt confident in their diagnosis.”
For the final model, the researchers excluded patients who had any missing data as well as those who had a diagnosis that was uncertain. This left 200 cases in the final model. Clinical variables associated with DLE were: atrophic scarring (odds ratio, 8.70; P < .001), location in the conchal bowl (OR, 6.80; P < .001), preference for head and neck (OR, 9.41; P < .001), dyspigmentation (OR, 3.23; P = .020), follicular hyperkeratosis/plugging (OR, 2.94; P = .054), and erythematous to violaceous in color (OR, 3.44; P = .056). The area under the curve for the model was 0.91.
According to Dr. Elman, the final model is a points-based model with 3 points assigned to atrophic scarring, 2 points assigned to location in the conchal bowl, 2 points assigned to preference for head and neck, 1 point assigned to dyspigmentation, 1 point assigned to follicular hyperkeratosis/plugging, and 1 point assigned to erythematous to violaceous in color. A score of 5 or greater yields a classification as DLE with 84.1% sensitivity and 75.9% specificity, while a score of 7 or greater yields a 73.9% sensitivity and 92.9% specificity.
Dr. Elman acknowledged certain limitations of the study, including the fact that information related to histopathology was not included in the final model. “This was a result of having only 40% of cases with relevant dermatopathology,” he said. “This limited our ability to meaningfully incorporate these items into a classification criteria set. However, with the data we’ve collected, efforts are under way to make a DLE-specific histopathology classification criteria.”
Another limitation is that the researchers relied on expert diagnosis as the preferred option. “Similarly, many of the cases came from large referral centers, and no demographic data were obtained, so this limits the generalizability of our study,” he said.
Dr. Elman reported having no financial disclosures.
The first validated classification criteria for discoid lupus erythematosus has a sensitivity that ranges between 73.9% and 84.1% and a specificity that ranges between 75.9% and 92.9%.
“Discoid lupus erythematosus [DLE] is the most common type of chronic cutaneous lupus,” lead study author Scott A. Elman, MD, said during the virtual annual meeting of the American Academy of Dermatology. “It’s one of the most potentially disfiguring forms of cutaneous lupus erythematosus [CLE], which can lead to scarring, hair loss, and dyspigmentation if not treated early or promptly. It has a significant impact on patient quality of life and there are currently no classification criteria for DLE, which has led to problematic heterogeneity in observational and interventional research efforts. As there is increasing interest in drug development programs for CLE and DLE, there is a need to develop classification criteria.”
Dr. Elman, of the Harvard combined medicine-dermatology training program at Brigham and Women’s Hospital, Boston, pointed out that classification criteria are the standard definitions that are primarily intended to enroll uniform cohorts for research. “These emphasize high specificity, whereas diagnostic criteria reflect a more broad and variable set of features of a given disease, and therefore require a higher sensitivity,” he explained. “While classification criteria are not synonymous with diagnostic criteria, they typically mirror the list of criteria that are used for diagnosis.”
In 2017, Dr. Elman and colleagues generated an item list of 12 potential classification criteria using an international Delphi consensus process: 5 criteria represented disease morphology, 2 represented discoid lupus location, and 5 represented histopathology (J Am Acad Dermatol. 2017 Aug 1;77[2]:261-7). The purpose of the current study, which was presented as a late-breaking abstract, was to validate the proposed classification criteria in a multicenter, international trial. “The point is to be able to differentiate between discoid lupus and its disease mimickers, which could be confused in enrollment in clinical trials,” he said.
At nine participating sites, patients were identified at clinical visits as having either DLE or a DLE mimicker. After each visit, dermatologists determined if morphological features were present. One dermatopathologist at each site reviewed pathology, if available, to see if the histopathologic features were present. Diagnosis by clinical features and dermatopathology were tabulated and presented as counts and percentages. Clinical features among those with and without DLE were calculated and compared with chi-square or Fisher’s exact tests. The researchers used best subsets logistic regression analysis to identify candidate models.
A total of 215 patients were enrolled: 94 that were consistent with DLE and 121 that were consistent with a DLE mimicker. Most cases (83%) were from North America, 11% were from Asia, and 6% were from Europe. Only 86 cases (40%) had biopsies for dermatopathology review.
The following clinical features were found to be more commonly associated with DLE, compared with DLE mimickers: atrophic scarring (83% vs. 24%; P < .001), dyspigmentation (84% vs. 55%; P < .001), follicular hyperkeratosis/plugging (43% vs. 11%; P < .001), scarring alopecia (61% vs. 21%; P < .001), location in the conchal bowl (49% vs. 10%; P < .001), preference for the head and neck (87% vs. 49%; P < .001), and erythematous to violaceous in color (93% vs. 85%, a nonsignificant difference; P = .09).
When histopathological items were assessed, the following features were found to be more commonly associated with DLE, compared with DLE mimickers: interface/vacuolar dermatitis (83% vs. 53%; P = .004), perivascular and/or periappendageal lymphohistiocytic infiltrate (95% vs. 84%, a nonsignificant difference; P = .18), follicular keratin plugs (57% vs. 20%; P < .001), mucin deposition (73% vs. 39%; P = .002), and basement membrane thickening (57% vs. 14%; P < .001).
“There was good agreement between the diagnoses made by dermatologists and dermatopathologists, with a Cohen’s kappa statistic of 0.83,” Dr. Elman added. “Similarly, in many of the cases, the dermatopathologists and the dermatologists felt confident in their diagnosis.”
For the final model, the researchers excluded patients who had any missing data as well as those who had a diagnosis that was uncertain. This left 200 cases in the final model. Clinical variables associated with DLE were: atrophic scarring (odds ratio, 8.70; P < .001), location in the conchal bowl (OR, 6.80; P < .001), preference for head and neck (OR, 9.41; P < .001), dyspigmentation (OR, 3.23; P = .020), follicular hyperkeratosis/plugging (OR, 2.94; P = .054), and erythematous to violaceous in color (OR, 3.44; P = .056). The area under the curve for the model was 0.91.
According to Dr. Elman, the final model is a points-based model with 3 points assigned to atrophic scarring, 2 points assigned to location in the conchal bowl, 2 points assigned to preference for head and neck, 1 point assigned to dyspigmentation, 1 point assigned to follicular hyperkeratosis/plugging, and 1 point assigned to erythematous to violaceous in color. A score of 5 or greater yields a classification as DLE with 84.1% sensitivity and 75.9% specificity, while a score of 7 or greater yields a 73.9% sensitivity and 92.9% specificity.
Dr. Elman acknowledged certain limitations of the study, including the fact that information related to histopathology was not included in the final model. “This was a result of having only 40% of cases with relevant dermatopathology,” he said. “This limited our ability to meaningfully incorporate these items into a classification criteria set. However, with the data we’ve collected, efforts are under way to make a DLE-specific histopathology classification criteria.”
Another limitation is that the researchers relied on expert diagnosis as the preferred option. “Similarly, many of the cases came from large referral centers, and no demographic data were obtained, so this limits the generalizability of our study,” he said.
Dr. Elman reported having no financial disclosures.
The first validated classification criteria for discoid lupus erythematosus has a sensitivity that ranges between 73.9% and 84.1% and a specificity that ranges between 75.9% and 92.9%.
“Discoid lupus erythematosus [DLE] is the most common type of chronic cutaneous lupus,” lead study author Scott A. Elman, MD, said during the virtual annual meeting of the American Academy of Dermatology. “It’s one of the most potentially disfiguring forms of cutaneous lupus erythematosus [CLE], which can lead to scarring, hair loss, and dyspigmentation if not treated early or promptly. It has a significant impact on patient quality of life and there are currently no classification criteria for DLE, which has led to problematic heterogeneity in observational and interventional research efforts. As there is increasing interest in drug development programs for CLE and DLE, there is a need to develop classification criteria.”
Dr. Elman, of the Harvard combined medicine-dermatology training program at Brigham and Women’s Hospital, Boston, pointed out that classification criteria are the standard definitions that are primarily intended to enroll uniform cohorts for research. “These emphasize high specificity, whereas diagnostic criteria reflect a more broad and variable set of features of a given disease, and therefore require a higher sensitivity,” he explained. “While classification criteria are not synonymous with diagnostic criteria, they typically mirror the list of criteria that are used for diagnosis.”
In 2017, Dr. Elman and colleagues generated an item list of 12 potential classification criteria using an international Delphi consensus process: 5 criteria represented disease morphology, 2 represented discoid lupus location, and 5 represented histopathology (J Am Acad Dermatol. 2017 Aug 1;77[2]:261-7). The purpose of the current study, which was presented as a late-breaking abstract, was to validate the proposed classification criteria in a multicenter, international trial. “The point is to be able to differentiate between discoid lupus and its disease mimickers, which could be confused in enrollment in clinical trials,” he said.
At nine participating sites, patients were identified at clinical visits as having either DLE or a DLE mimicker. After each visit, dermatologists determined if morphological features were present. One dermatopathologist at each site reviewed pathology, if available, to see if the histopathologic features were present. Diagnosis by clinical features and dermatopathology were tabulated and presented as counts and percentages. Clinical features among those with and without DLE were calculated and compared with chi-square or Fisher’s exact tests. The researchers used best subsets logistic regression analysis to identify candidate models.
A total of 215 patients were enrolled: 94 that were consistent with DLE and 121 that were consistent with a DLE mimicker. Most cases (83%) were from North America, 11% were from Asia, and 6% were from Europe. Only 86 cases (40%) had biopsies for dermatopathology review.
The following clinical features were found to be more commonly associated with DLE, compared with DLE mimickers: atrophic scarring (83% vs. 24%; P < .001), dyspigmentation (84% vs. 55%; P < .001), follicular hyperkeratosis/plugging (43% vs. 11%; P < .001), scarring alopecia (61% vs. 21%; P < .001), location in the conchal bowl (49% vs. 10%; P < .001), preference for the head and neck (87% vs. 49%; P < .001), and erythematous to violaceous in color (93% vs. 85%, a nonsignificant difference; P = .09).
When histopathological items were assessed, the following features were found to be more commonly associated with DLE, compared with DLE mimickers: interface/vacuolar dermatitis (83% vs. 53%; P = .004), perivascular and/or periappendageal lymphohistiocytic infiltrate (95% vs. 84%, a nonsignificant difference; P = .18), follicular keratin plugs (57% vs. 20%; P < .001), mucin deposition (73% vs. 39%; P = .002), and basement membrane thickening (57% vs. 14%; P < .001).
“There was good agreement between the diagnoses made by dermatologists and dermatopathologists, with a Cohen’s kappa statistic of 0.83,” Dr. Elman added. “Similarly, in many of the cases, the dermatopathologists and the dermatologists felt confident in their diagnosis.”
For the final model, the researchers excluded patients who had any missing data as well as those who had a diagnosis that was uncertain. This left 200 cases in the final model. Clinical variables associated with DLE were: atrophic scarring (odds ratio, 8.70; P < .001), location in the conchal bowl (OR, 6.80; P < .001), preference for head and neck (OR, 9.41; P < .001), dyspigmentation (OR, 3.23; P = .020), follicular hyperkeratosis/plugging (OR, 2.94; P = .054), and erythematous to violaceous in color (OR, 3.44; P = .056). The area under the curve for the model was 0.91.
According to Dr. Elman, the final model is a points-based model with 3 points assigned to atrophic scarring, 2 points assigned to location in the conchal bowl, 2 points assigned to preference for head and neck, 1 point assigned to dyspigmentation, 1 point assigned to follicular hyperkeratosis/plugging, and 1 point assigned to erythematous to violaceous in color. A score of 5 or greater yields a classification as DLE with 84.1% sensitivity and 75.9% specificity, while a score of 7 or greater yields a 73.9% sensitivity and 92.9% specificity.
Dr. Elman acknowledged certain limitations of the study, including the fact that information related to histopathology was not included in the final model. “This was a result of having only 40% of cases with relevant dermatopathology,” he said. “This limited our ability to meaningfully incorporate these items into a classification criteria set. However, with the data we’ve collected, efforts are under way to make a DLE-specific histopathology classification criteria.”
Another limitation is that the researchers relied on expert diagnosis as the preferred option. “Similarly, many of the cases came from large referral centers, and no demographic data were obtained, so this limits the generalizability of our study,” he said.
Dr. Elman reported having no financial disclosures.
FROM AAD 20
Daily Recap: Higher risk of severe COVID-19 seen in pregnancy, primary care practices at risk
Here are the stories our MDedge editors across specialties think you need to know about today:
Pregnant women at higher risk for severe COVID-19
Pregnant women may be at increased risk for severe COVID-19 illness, according to a report published online June 26 in Morbidity and Mortality Weekly Report.
Among reproductive-aged women (15-44 years) infected with SARS-CoV-2, pregnancy was associated with a greater likelihood of hospitalization, admission to the intensive care unit (ICU), and mechanical ventilation, but not death. Pregnant women were 5.4 times more likely to be hospitalized, 1.5 times more likely to be admitted to the ICU, and 1.7 times more likely to need mechanical ventilation, after adjustment for age, underlying conditions, and race/ethnicity.
CDC researchers said that preventing COVID-19 infection in pregnant women should be a priority and any potential barriers to compliance with preventive measures need to be removed.
“During pregnancy, women experience immunologic and physiologic changes that could increase their risk for more severe illness from respiratory infections,” they wrote. Read more.
Going out of business: Primary care practices at risk
In a recently published editorial, Tom Frieden, MD, MPH, former head of the Centers for Disease Control and Prevention, argued that primary care is in deep trouble, its long-standing financial problems exacerbated by the fallout from the COVID-19 pandemic. In an interview with Kenny Lin, MD, MPH, a family physician, Dr. Frieden discussed the future of primary care.
Here is a sample of Dr. Frieden’s observations:
“When I’ve looked around the United States, I’ve been extremely concerned about both the risk that primary care practitioners are subjected to in their everyday practice and the economic risk that we could lose many of our primary care practices around the country. It’s really striking to see that the number of visits has plummeted. Because of our payment structure, that means incomes have plummeted. We’re hearing about doctors’ offices getting boarded up and shuttering. As I write in the piece, it’s one thing for a theater or a restaurant or another important community entity to shut because of economic downturn, and these are real losses, but to lose their only primary care practice or one of the few in an area really is a matter of life and death for many communities.” Read more.
Surge in out-of-hospital cardiac arrests
The COVID-19 pandemic in New York City led to a surge in out-of-hospital cardiac arrests that placed a huge burden on first responders, according to a new analysis.
During the height of the pandemic in New York, there was a “dramatic increase in cardiopulmonary arrests, nearly all presented in non-shockable cardiac rhythms (> 90% fatality rate) and vulnerable patient populations were most affected,” David J. Prezant, MD, chief medical officer, Fire Department of New York (FDNY), said in an interview.
In a news release, Dr. Prezant noted that “relatively few, if any, patients were tested to confirm the presence of COVID-19,” making it impossible to distinguish between cardiac arrests as a result of COVID-19 and those that may have resulted from other health conditions.
“We also can’t rule out the possibility that some people may have died from delays in seeking or receiving treatment for non–COVID-19-related conditions. However, the dramatic increase in cardiac arrests compared to the same period in 2019 strongly indicates that the pandemic was directly or indirectly responsible for that surge in cardiac arrests and deaths,” said Dr. Prezant.
The study was published online June 19 in JAMA Cardiology.
Read more.
Fenfluramine approved for Dravet syndrome
The U.S. Food and Drug Administration has approved fenfluramine (Fintepla, Zogenix) oral solution, a Schedule IV controlled substance, for the treatment of seizures associated with Dravet syndrome in children age 2 years and older.
Dravet syndrome is a rare childhood-onset epilepsy characterized by frequent, drug-resistant convulsive seizures that may contribute to intellectual disability and impairments in motor control, behavior, and cognition, as well as an increased risk of sudden unexpected death in epilepsy.
Dravet syndrome takes a “tremendous toll on both patients and their families. Fintepla offers an additional effective treatment option for the treatment of seizures associated with Dravet syndrome,” Billy Dunn, MD, director, Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, said in a news release. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Pregnant women at higher risk for severe COVID-19
Pregnant women may be at increased risk for severe COVID-19 illness, according to a report published online June 26 in Morbidity and Mortality Weekly Report.
Among reproductive-aged women (15-44 years) infected with SARS-CoV-2, pregnancy was associated with a greater likelihood of hospitalization, admission to the intensive care unit (ICU), and mechanical ventilation, but not death. Pregnant women were 5.4 times more likely to be hospitalized, 1.5 times more likely to be admitted to the ICU, and 1.7 times more likely to need mechanical ventilation, after adjustment for age, underlying conditions, and race/ethnicity.
CDC researchers said that preventing COVID-19 infection in pregnant women should be a priority and any potential barriers to compliance with preventive measures need to be removed.
“During pregnancy, women experience immunologic and physiologic changes that could increase their risk for more severe illness from respiratory infections,” they wrote. Read more.
Going out of business: Primary care practices at risk
In a recently published editorial, Tom Frieden, MD, MPH, former head of the Centers for Disease Control and Prevention, argued that primary care is in deep trouble, its long-standing financial problems exacerbated by the fallout from the COVID-19 pandemic. In an interview with Kenny Lin, MD, MPH, a family physician, Dr. Frieden discussed the future of primary care.
Here is a sample of Dr. Frieden’s observations:
“When I’ve looked around the United States, I’ve been extremely concerned about both the risk that primary care practitioners are subjected to in their everyday practice and the economic risk that we could lose many of our primary care practices around the country. It’s really striking to see that the number of visits has plummeted. Because of our payment structure, that means incomes have plummeted. We’re hearing about doctors’ offices getting boarded up and shuttering. As I write in the piece, it’s one thing for a theater or a restaurant or another important community entity to shut because of economic downturn, and these are real losses, but to lose their only primary care practice or one of the few in an area really is a matter of life and death for many communities.” Read more.
Surge in out-of-hospital cardiac arrests
The COVID-19 pandemic in New York City led to a surge in out-of-hospital cardiac arrests that placed a huge burden on first responders, according to a new analysis.
During the height of the pandemic in New York, there was a “dramatic increase in cardiopulmonary arrests, nearly all presented in non-shockable cardiac rhythms (> 90% fatality rate) and vulnerable patient populations were most affected,” David J. Prezant, MD, chief medical officer, Fire Department of New York (FDNY), said in an interview.
In a news release, Dr. Prezant noted that “relatively few, if any, patients were tested to confirm the presence of COVID-19,” making it impossible to distinguish between cardiac arrests as a result of COVID-19 and those that may have resulted from other health conditions.
“We also can’t rule out the possibility that some people may have died from delays in seeking or receiving treatment for non–COVID-19-related conditions. However, the dramatic increase in cardiac arrests compared to the same period in 2019 strongly indicates that the pandemic was directly or indirectly responsible for that surge in cardiac arrests and deaths,” said Dr. Prezant.
The study was published online June 19 in JAMA Cardiology.
Read more.
Fenfluramine approved for Dravet syndrome
The U.S. Food and Drug Administration has approved fenfluramine (Fintepla, Zogenix) oral solution, a Schedule IV controlled substance, for the treatment of seizures associated with Dravet syndrome in children age 2 years and older.
Dravet syndrome is a rare childhood-onset epilepsy characterized by frequent, drug-resistant convulsive seizures that may contribute to intellectual disability and impairments in motor control, behavior, and cognition, as well as an increased risk of sudden unexpected death in epilepsy.
Dravet syndrome takes a “tremendous toll on both patients and their families. Fintepla offers an additional effective treatment option for the treatment of seizures associated with Dravet syndrome,” Billy Dunn, MD, director, Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, said in a news release. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Pregnant women at higher risk for severe COVID-19
Pregnant women may be at increased risk for severe COVID-19 illness, according to a report published online June 26 in Morbidity and Mortality Weekly Report.
Among reproductive-aged women (15-44 years) infected with SARS-CoV-2, pregnancy was associated with a greater likelihood of hospitalization, admission to the intensive care unit (ICU), and mechanical ventilation, but not death. Pregnant women were 5.4 times more likely to be hospitalized, 1.5 times more likely to be admitted to the ICU, and 1.7 times more likely to need mechanical ventilation, after adjustment for age, underlying conditions, and race/ethnicity.
CDC researchers said that preventing COVID-19 infection in pregnant women should be a priority and any potential barriers to compliance with preventive measures need to be removed.
“During pregnancy, women experience immunologic and physiologic changes that could increase their risk for more severe illness from respiratory infections,” they wrote. Read more.
Going out of business: Primary care practices at risk
In a recently published editorial, Tom Frieden, MD, MPH, former head of the Centers for Disease Control and Prevention, argued that primary care is in deep trouble, its long-standing financial problems exacerbated by the fallout from the COVID-19 pandemic. In an interview with Kenny Lin, MD, MPH, a family physician, Dr. Frieden discussed the future of primary care.
Here is a sample of Dr. Frieden’s observations:
“When I’ve looked around the United States, I’ve been extremely concerned about both the risk that primary care practitioners are subjected to in their everyday practice and the economic risk that we could lose many of our primary care practices around the country. It’s really striking to see that the number of visits has plummeted. Because of our payment structure, that means incomes have plummeted. We’re hearing about doctors’ offices getting boarded up and shuttering. As I write in the piece, it’s one thing for a theater or a restaurant or another important community entity to shut because of economic downturn, and these are real losses, but to lose their only primary care practice or one of the few in an area really is a matter of life and death for many communities.” Read more.
Surge in out-of-hospital cardiac arrests
The COVID-19 pandemic in New York City led to a surge in out-of-hospital cardiac arrests that placed a huge burden on first responders, according to a new analysis.
During the height of the pandemic in New York, there was a “dramatic increase in cardiopulmonary arrests, nearly all presented in non-shockable cardiac rhythms (> 90% fatality rate) and vulnerable patient populations were most affected,” David J. Prezant, MD, chief medical officer, Fire Department of New York (FDNY), said in an interview.
In a news release, Dr. Prezant noted that “relatively few, if any, patients were tested to confirm the presence of COVID-19,” making it impossible to distinguish between cardiac arrests as a result of COVID-19 and those that may have resulted from other health conditions.
“We also can’t rule out the possibility that some people may have died from delays in seeking or receiving treatment for non–COVID-19-related conditions. However, the dramatic increase in cardiac arrests compared to the same period in 2019 strongly indicates that the pandemic was directly or indirectly responsible for that surge in cardiac arrests and deaths,” said Dr. Prezant.
The study was published online June 19 in JAMA Cardiology.
Read more.
Fenfluramine approved for Dravet syndrome
The U.S. Food and Drug Administration has approved fenfluramine (Fintepla, Zogenix) oral solution, a Schedule IV controlled substance, for the treatment of seizures associated with Dravet syndrome in children age 2 years and older.
Dravet syndrome is a rare childhood-onset epilepsy characterized by frequent, drug-resistant convulsive seizures that may contribute to intellectual disability and impairments in motor control, behavior, and cognition, as well as an increased risk of sudden unexpected death in epilepsy.
Dravet syndrome takes a “tremendous toll on both patients and their families. Fintepla offers an additional effective treatment option for the treatment of seizures associated with Dravet syndrome,” Billy Dunn, MD, director, Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research, said in a news release. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
In scleroderma, GERD questionnaires are essential tools
Every rheumatologist ought to be comfortable in using a validated gastrointestinal symptom scale for evaluation of gastroesophageal reflux disease in patients with scleroderma, Tracy M. Frech, MD, declared at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
About 90% of scleroderma patients will develop GI tract involvement during the course of their connective tissue disease. And while any portion of the GI tract from esophagus to anus can be involved, the most common GI manifestation is gastroesophageal reflux disease (GERD), affecting up to 90% of scleroderma patients, observed Dr. Frech, a rheumatologist and director of the systemic sclerosis clinic at the University of Utah and the George E. Wahlen Department of Veterans Affairs Medical Center, both in Salt Lake City.
“It is essential to ask scleroderma patients questions in order to understand their gastrointestinal tract symptoms. The questionnaires are really critical for us to grade the severity and then properly order tests,” she explained. “The goal is symptom identification, ideally with minimal time burden and at no cost, to guide decisions that move our patients’ care forward.”
Three of the most useful validated instruments for assessment of GERD symptoms in scleroderma patients in routine clinical practice are the GerdQ, the University of California, Los Angeles, Scleroderma Clinical Trial Consortium GI Tract Questionnaire (UCLA GIT) 2.0 reflux scale, and the Patient-Reported Outcomes Measurement Information System (PROMIS) reflux scale.
The GerdQ is a six-item, self-administered questionnaire in which patients specify how many days in the past week they have experienced heartburn, regurgitation, nausea, sleep interference, upper abdominal pain, and need for medication. A free online tool is available for calculating the likelihood of having GERD based upon GerdQ score. A score of 8 or more points out of a possible 18 has the highest sensitivity and specificity for diagnosis of GERD.
The UCLA GIT 2.0 – the most commonly used instrument for GI symptom assessment in scleroderma patients – includes 34 items. It takes 6-8 minutes to complete the whole thing, but patients being assessed for GERD only need answer the eight GERD-specific questions. Six of these eight questions are the same as in the GerdQ. One of the two extra questions asks about difficulty in swallowing solid food, which if answered affirmatively warrants early referral to a gastroenterologist. The other question inquires about any food triggers for the reflux, providing an opportunity for a rheumatologist to educate the patient about the importance of avoiding acidic foods, such as tomatoes, and other food and drink generally considered healthy but which actually exacerbate GERD.
The National Institutes of Health PROMIS scale, the newest of the three instruments, is a 60-item questionnaire; however, only 20 questions relate to reflux and dysphagia and are thus germane to a focused GERD assessment in scleroderma.
When a clinical diagnosis of GERD is made in a scleroderma patient based upon symptoms elicited by questionnaire, guidelines recommend a trial of empiric proton pump inhibitor therapy and behavioral interventions, such as raising the head of the bed, in order to confirm the diagnosis. If the patient reports feeling better after these basic interventions, the diagnosis is confirmed. If not, it’s time to make a referral to a gastroenterologist for specialized care, Dr. Frech said.
Dr. Frech was a coinvestigator in an international, prospective, longitudinal study of patient-reported outcomes measures in 116 patients with scleroderma and GERD. All study participants had to complete the UCLA GIT 2.0, the PROMIS reflux scale, and a third patient-reported GERD measure both before and after the therapeutic intervention. The UCLA GIT 2.0 and PROMIS instruments demonstrated similarly robust sensitivity for identifying changes in GERD symptoms after therapeutic intervention.
“It doesn’t really matter what questionnaire we’re using,” according to the rheumatologist. “But I will point out that there is significant overlap in symptoms among GERD, gastroparesis, functional dyspepsia, and eosinophilic esophagitis, all of which cause symptoms of heartburn and regurgitation. So we don’t want to ask these questions just once, we want to make an intervention and then reask the questions to ensure that we’re continuously moving forward with the gastrointestinal tract management plan.”
Dr. Frech reported having no financial conflicts regarding her presentation.
Every rheumatologist ought to be comfortable in using a validated gastrointestinal symptom scale for evaluation of gastroesophageal reflux disease in patients with scleroderma, Tracy M. Frech, MD, declared at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
About 90% of scleroderma patients will develop GI tract involvement during the course of their connective tissue disease. And while any portion of the GI tract from esophagus to anus can be involved, the most common GI manifestation is gastroesophageal reflux disease (GERD), affecting up to 90% of scleroderma patients, observed Dr. Frech, a rheumatologist and director of the systemic sclerosis clinic at the University of Utah and the George E. Wahlen Department of Veterans Affairs Medical Center, both in Salt Lake City.
“It is essential to ask scleroderma patients questions in order to understand their gastrointestinal tract symptoms. The questionnaires are really critical for us to grade the severity and then properly order tests,” she explained. “The goal is symptom identification, ideally with minimal time burden and at no cost, to guide decisions that move our patients’ care forward.”
Three of the most useful validated instruments for assessment of GERD symptoms in scleroderma patients in routine clinical practice are the GerdQ, the University of California, Los Angeles, Scleroderma Clinical Trial Consortium GI Tract Questionnaire (UCLA GIT) 2.0 reflux scale, and the Patient-Reported Outcomes Measurement Information System (PROMIS) reflux scale.
The GerdQ is a six-item, self-administered questionnaire in which patients specify how many days in the past week they have experienced heartburn, regurgitation, nausea, sleep interference, upper abdominal pain, and need for medication. A free online tool is available for calculating the likelihood of having GERD based upon GerdQ score. A score of 8 or more points out of a possible 18 has the highest sensitivity and specificity for diagnosis of GERD.
The UCLA GIT 2.0 – the most commonly used instrument for GI symptom assessment in scleroderma patients – includes 34 items. It takes 6-8 minutes to complete the whole thing, but patients being assessed for GERD only need answer the eight GERD-specific questions. Six of these eight questions are the same as in the GerdQ. One of the two extra questions asks about difficulty in swallowing solid food, which if answered affirmatively warrants early referral to a gastroenterologist. The other question inquires about any food triggers for the reflux, providing an opportunity for a rheumatologist to educate the patient about the importance of avoiding acidic foods, such as tomatoes, and other food and drink generally considered healthy but which actually exacerbate GERD.
The National Institutes of Health PROMIS scale, the newest of the three instruments, is a 60-item questionnaire; however, only 20 questions relate to reflux and dysphagia and are thus germane to a focused GERD assessment in scleroderma.
When a clinical diagnosis of GERD is made in a scleroderma patient based upon symptoms elicited by questionnaire, guidelines recommend a trial of empiric proton pump inhibitor therapy and behavioral interventions, such as raising the head of the bed, in order to confirm the diagnosis. If the patient reports feeling better after these basic interventions, the diagnosis is confirmed. If not, it’s time to make a referral to a gastroenterologist for specialized care, Dr. Frech said.
Dr. Frech was a coinvestigator in an international, prospective, longitudinal study of patient-reported outcomes measures in 116 patients with scleroderma and GERD. All study participants had to complete the UCLA GIT 2.0, the PROMIS reflux scale, and a third patient-reported GERD measure both before and after the therapeutic intervention. The UCLA GIT 2.0 and PROMIS instruments demonstrated similarly robust sensitivity for identifying changes in GERD symptoms after therapeutic intervention.
“It doesn’t really matter what questionnaire we’re using,” according to the rheumatologist. “But I will point out that there is significant overlap in symptoms among GERD, gastroparesis, functional dyspepsia, and eosinophilic esophagitis, all of which cause symptoms of heartburn and regurgitation. So we don’t want to ask these questions just once, we want to make an intervention and then reask the questions to ensure that we’re continuously moving forward with the gastrointestinal tract management plan.”
Dr. Frech reported having no financial conflicts regarding her presentation.
Every rheumatologist ought to be comfortable in using a validated gastrointestinal symptom scale for evaluation of gastroesophageal reflux disease in patients with scleroderma, Tracy M. Frech, MD, declared at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
About 90% of scleroderma patients will develop GI tract involvement during the course of their connective tissue disease. And while any portion of the GI tract from esophagus to anus can be involved, the most common GI manifestation is gastroesophageal reflux disease (GERD), affecting up to 90% of scleroderma patients, observed Dr. Frech, a rheumatologist and director of the systemic sclerosis clinic at the University of Utah and the George E. Wahlen Department of Veterans Affairs Medical Center, both in Salt Lake City.
“It is essential to ask scleroderma patients questions in order to understand their gastrointestinal tract symptoms. The questionnaires are really critical for us to grade the severity and then properly order tests,” she explained. “The goal is symptom identification, ideally with minimal time burden and at no cost, to guide decisions that move our patients’ care forward.”
Three of the most useful validated instruments for assessment of GERD symptoms in scleroderma patients in routine clinical practice are the GerdQ, the University of California, Los Angeles, Scleroderma Clinical Trial Consortium GI Tract Questionnaire (UCLA GIT) 2.0 reflux scale, and the Patient-Reported Outcomes Measurement Information System (PROMIS) reflux scale.
The GerdQ is a six-item, self-administered questionnaire in which patients specify how many days in the past week they have experienced heartburn, regurgitation, nausea, sleep interference, upper abdominal pain, and need for medication. A free online tool is available for calculating the likelihood of having GERD based upon GerdQ score. A score of 8 or more points out of a possible 18 has the highest sensitivity and specificity for diagnosis of GERD.
The UCLA GIT 2.0 – the most commonly used instrument for GI symptom assessment in scleroderma patients – includes 34 items. It takes 6-8 minutes to complete the whole thing, but patients being assessed for GERD only need answer the eight GERD-specific questions. Six of these eight questions are the same as in the GerdQ. One of the two extra questions asks about difficulty in swallowing solid food, which if answered affirmatively warrants early referral to a gastroenterologist. The other question inquires about any food triggers for the reflux, providing an opportunity for a rheumatologist to educate the patient about the importance of avoiding acidic foods, such as tomatoes, and other food and drink generally considered healthy but which actually exacerbate GERD.
The National Institutes of Health PROMIS scale, the newest of the three instruments, is a 60-item questionnaire; however, only 20 questions relate to reflux and dysphagia and are thus germane to a focused GERD assessment in scleroderma.
When a clinical diagnosis of GERD is made in a scleroderma patient based upon symptoms elicited by questionnaire, guidelines recommend a trial of empiric proton pump inhibitor therapy and behavioral interventions, such as raising the head of the bed, in order to confirm the diagnosis. If the patient reports feeling better after these basic interventions, the diagnosis is confirmed. If not, it’s time to make a referral to a gastroenterologist for specialized care, Dr. Frech said.
Dr. Frech was a coinvestigator in an international, prospective, longitudinal study of patient-reported outcomes measures in 116 patients with scleroderma and GERD. All study participants had to complete the UCLA GIT 2.0, the PROMIS reflux scale, and a third patient-reported GERD measure both before and after the therapeutic intervention. The UCLA GIT 2.0 and PROMIS instruments demonstrated similarly robust sensitivity for identifying changes in GERD symptoms after therapeutic intervention.
“It doesn’t really matter what questionnaire we’re using,” according to the rheumatologist. “But I will point out that there is significant overlap in symptoms among GERD, gastroparesis, functional dyspepsia, and eosinophilic esophagitis, all of which cause symptoms of heartburn and regurgitation. So we don’t want to ask these questions just once, we want to make an intervention and then reask the questions to ensure that we’re continuously moving forward with the gastrointestinal tract management plan.”
Dr. Frech reported having no financial conflicts regarding her presentation.
FROM SOTA 2020
Eczema may increase lymphoma risk, cohort studies suggest
according to two matched longitudinal cohort studies from England and Denmark.
“In this study, no evidence was found that people with atopic eczema are at increased risk of most cancers. An exception is the observed association between atopic eczema and lymphoma, particularly NHL, [which] increased with eczema severity,” Kathryn E. Mansfield, PhD, wrote in JAMA Dermatology. Adjusted hazard ratios for NHL in the English cohort were 1.06 (99% CI, 0.90-1.25) for mild atopic eczema, 1.24 (99% CI, 1.04-1.48) for moderate eczema, and 2.08 (99% CI, 1.42-3.04) for severe eczema, reported Dr. Mansfield of the London School of Hygiene and Tropical Medicine and associates.
Past studies of a possible link between atopic eczema and cancer have produced conflicting evidence, which might reflect “two competing theories” – that cancer risk falls with greater immune surveillance, and that cancer risk rises with immune stimulation, the researchers wrote. Immunosuppressive treatment and an impaired skin barrier might also increase the risk of cancer, but the evidence is conflicting.
For the study, they analyzed electronic health records linked with hospital admissions and death records in England and national health registry data from Denmark. The English cohort included 471,970 adults with atopic eczema and 2,239,775 adults without atopic eczema. The Danish cohort was composed of individuals of any age, including 44,945 who had eczema and with 445,673 who did not. Participants were matched based on factors such as age, sex, and primary care practice. The researchers excluded individuals with a history of cancer, apart from nonmelanoma skin cancer or keratinocyte cancer. (For analyses of skin cancer risk, they also excluded individuals with a history of nonmelanoma skin cancer.)
Overall, there was “little evidence” for a link between atopic eczema and cancer (adjusted hazard ratio in England, 1.04; 99% CI, 1.02-1.06; aHR in Denmark, 1.05; 99% CI, 0.95-1.16) or for most specific types of cancer, the investigators wrote.
In England, however, eczema was associated with a significantly increased risk for noncutaneous lymphoma, with an adjusted HRs of 1.19 (99% CI, 1.07-1.34) for NHL, and 1.48 (99% CI, 1.07-2.04) for Hodgkin lymphoma. Lymphoma risk was highest among adults with severe eczema, defined as those who had been prescribed a systemic treatment for their disease, who had received phototherapy, or who had been referred to a specialist or admitted to a hospital for atopic eczema. Point estimates in the Danish cohort also revealed a higher risk for lymphoma among individuals with moderate to severe atopic eczema, compared with those with eczema, but the 99% CIs crossed 1.0.
The findings highlight the need to be aware of, screen for, and study the pathogenesis of heightened lymphoma risk among patients with atopic eczema, said Shawn Demehri, MD, PhD, of the department of dermatology and cancer center, at Massachusetts General Hospital, Boston, who was not involved in the study.
“Prospectively collected data from large cohorts of eczema patients is a strength of this study,” he said in an interview. “However, the age range included in the study is suboptimal for assessing cancer as an outcome. The lower incidence of cancer in younger individuals hinders the ability to detect differences in cancer risk between the two groups.” (Approximately 57% of individuals in the English cohort were aged 18-44 years, while approximately 70% of those in the Danish cohort were less than 18 years.)
Understanding how eczema affects the risk of non-Hodgkin lymphoma is an important future direction of research, Dr. Demehri emphasized. “The landscape of atopic eczema therapeutics has dramatically changed in the recent years. It will be very interesting to determine how new biologics impact cancer risk in eczema patients.”
Partial support for the work was provided by the Wellcome Trust, the Royal Society, the Dagmar Marshalls Fund, and the Aase and Ejnar Danielsens Fund. Dr. Mansfield disclosed support from a Wellcome Trust grant. Her coinvestigators disclosed ties to TARGET-DERM, Pfizer, and GlaxoSmithKline, and from the Wellcome Trust, Medical Research Council, the National Institute for Health Research, the British Heart Foundation, Diabetes UK, and IMI Horizon 2020 funding BIOMAP. Dr. Demehri reported having no relevant conflicts of interest.
SOURCE: Mansfield KE et al. JAMA Dermatol. 2020 Jun 24. doi: 10.1001/jamadermatol.2020.1948.
according to two matched longitudinal cohort studies from England and Denmark.
“In this study, no evidence was found that people with atopic eczema are at increased risk of most cancers. An exception is the observed association between atopic eczema and lymphoma, particularly NHL, [which] increased with eczema severity,” Kathryn E. Mansfield, PhD, wrote in JAMA Dermatology. Adjusted hazard ratios for NHL in the English cohort were 1.06 (99% CI, 0.90-1.25) for mild atopic eczema, 1.24 (99% CI, 1.04-1.48) for moderate eczema, and 2.08 (99% CI, 1.42-3.04) for severe eczema, reported Dr. Mansfield of the London School of Hygiene and Tropical Medicine and associates.
Past studies of a possible link between atopic eczema and cancer have produced conflicting evidence, which might reflect “two competing theories” – that cancer risk falls with greater immune surveillance, and that cancer risk rises with immune stimulation, the researchers wrote. Immunosuppressive treatment and an impaired skin barrier might also increase the risk of cancer, but the evidence is conflicting.
For the study, they analyzed electronic health records linked with hospital admissions and death records in England and national health registry data from Denmark. The English cohort included 471,970 adults with atopic eczema and 2,239,775 adults without atopic eczema. The Danish cohort was composed of individuals of any age, including 44,945 who had eczema and with 445,673 who did not. Participants were matched based on factors such as age, sex, and primary care practice. The researchers excluded individuals with a history of cancer, apart from nonmelanoma skin cancer or keratinocyte cancer. (For analyses of skin cancer risk, they also excluded individuals with a history of nonmelanoma skin cancer.)
Overall, there was “little evidence” for a link between atopic eczema and cancer (adjusted hazard ratio in England, 1.04; 99% CI, 1.02-1.06; aHR in Denmark, 1.05; 99% CI, 0.95-1.16) or for most specific types of cancer, the investigators wrote.
In England, however, eczema was associated with a significantly increased risk for noncutaneous lymphoma, with an adjusted HRs of 1.19 (99% CI, 1.07-1.34) for NHL, and 1.48 (99% CI, 1.07-2.04) for Hodgkin lymphoma. Lymphoma risk was highest among adults with severe eczema, defined as those who had been prescribed a systemic treatment for their disease, who had received phototherapy, or who had been referred to a specialist or admitted to a hospital for atopic eczema. Point estimates in the Danish cohort also revealed a higher risk for lymphoma among individuals with moderate to severe atopic eczema, compared with those with eczema, but the 99% CIs crossed 1.0.
The findings highlight the need to be aware of, screen for, and study the pathogenesis of heightened lymphoma risk among patients with atopic eczema, said Shawn Demehri, MD, PhD, of the department of dermatology and cancer center, at Massachusetts General Hospital, Boston, who was not involved in the study.
“Prospectively collected data from large cohorts of eczema patients is a strength of this study,” he said in an interview. “However, the age range included in the study is suboptimal for assessing cancer as an outcome. The lower incidence of cancer in younger individuals hinders the ability to detect differences in cancer risk between the two groups.” (Approximately 57% of individuals in the English cohort were aged 18-44 years, while approximately 70% of those in the Danish cohort were less than 18 years.)
Understanding how eczema affects the risk of non-Hodgkin lymphoma is an important future direction of research, Dr. Demehri emphasized. “The landscape of atopic eczema therapeutics has dramatically changed in the recent years. It will be very interesting to determine how new biologics impact cancer risk in eczema patients.”
Partial support for the work was provided by the Wellcome Trust, the Royal Society, the Dagmar Marshalls Fund, and the Aase and Ejnar Danielsens Fund. Dr. Mansfield disclosed support from a Wellcome Trust grant. Her coinvestigators disclosed ties to TARGET-DERM, Pfizer, and GlaxoSmithKline, and from the Wellcome Trust, Medical Research Council, the National Institute for Health Research, the British Heart Foundation, Diabetes UK, and IMI Horizon 2020 funding BIOMAP. Dr. Demehri reported having no relevant conflicts of interest.
SOURCE: Mansfield KE et al. JAMA Dermatol. 2020 Jun 24. doi: 10.1001/jamadermatol.2020.1948.
according to two matched longitudinal cohort studies from England and Denmark.
“In this study, no evidence was found that people with atopic eczema are at increased risk of most cancers. An exception is the observed association between atopic eczema and lymphoma, particularly NHL, [which] increased with eczema severity,” Kathryn E. Mansfield, PhD, wrote in JAMA Dermatology. Adjusted hazard ratios for NHL in the English cohort were 1.06 (99% CI, 0.90-1.25) for mild atopic eczema, 1.24 (99% CI, 1.04-1.48) for moderate eczema, and 2.08 (99% CI, 1.42-3.04) for severe eczema, reported Dr. Mansfield of the London School of Hygiene and Tropical Medicine and associates.
Past studies of a possible link between atopic eczema and cancer have produced conflicting evidence, which might reflect “two competing theories” – that cancer risk falls with greater immune surveillance, and that cancer risk rises with immune stimulation, the researchers wrote. Immunosuppressive treatment and an impaired skin barrier might also increase the risk of cancer, but the evidence is conflicting.
For the study, they analyzed electronic health records linked with hospital admissions and death records in England and national health registry data from Denmark. The English cohort included 471,970 adults with atopic eczema and 2,239,775 adults without atopic eczema. The Danish cohort was composed of individuals of any age, including 44,945 who had eczema and with 445,673 who did not. Participants were matched based on factors such as age, sex, and primary care practice. The researchers excluded individuals with a history of cancer, apart from nonmelanoma skin cancer or keratinocyte cancer. (For analyses of skin cancer risk, they also excluded individuals with a history of nonmelanoma skin cancer.)
Overall, there was “little evidence” for a link between atopic eczema and cancer (adjusted hazard ratio in England, 1.04; 99% CI, 1.02-1.06; aHR in Denmark, 1.05; 99% CI, 0.95-1.16) or for most specific types of cancer, the investigators wrote.
In England, however, eczema was associated with a significantly increased risk for noncutaneous lymphoma, with an adjusted HRs of 1.19 (99% CI, 1.07-1.34) for NHL, and 1.48 (99% CI, 1.07-2.04) for Hodgkin lymphoma. Lymphoma risk was highest among adults with severe eczema, defined as those who had been prescribed a systemic treatment for their disease, who had received phototherapy, or who had been referred to a specialist or admitted to a hospital for atopic eczema. Point estimates in the Danish cohort also revealed a higher risk for lymphoma among individuals with moderate to severe atopic eczema, compared with those with eczema, but the 99% CIs crossed 1.0.
The findings highlight the need to be aware of, screen for, and study the pathogenesis of heightened lymphoma risk among patients with atopic eczema, said Shawn Demehri, MD, PhD, of the department of dermatology and cancer center, at Massachusetts General Hospital, Boston, who was not involved in the study.
“Prospectively collected data from large cohorts of eczema patients is a strength of this study,” he said in an interview. “However, the age range included in the study is suboptimal for assessing cancer as an outcome. The lower incidence of cancer in younger individuals hinders the ability to detect differences in cancer risk between the two groups.” (Approximately 57% of individuals in the English cohort were aged 18-44 years, while approximately 70% of those in the Danish cohort were less than 18 years.)
Understanding how eczema affects the risk of non-Hodgkin lymphoma is an important future direction of research, Dr. Demehri emphasized. “The landscape of atopic eczema therapeutics has dramatically changed in the recent years. It will be very interesting to determine how new biologics impact cancer risk in eczema patients.”
Partial support for the work was provided by the Wellcome Trust, the Royal Society, the Dagmar Marshalls Fund, and the Aase and Ejnar Danielsens Fund. Dr. Mansfield disclosed support from a Wellcome Trust grant. Her coinvestigators disclosed ties to TARGET-DERM, Pfizer, and GlaxoSmithKline, and from the Wellcome Trust, Medical Research Council, the National Institute for Health Research, the British Heart Foundation, Diabetes UK, and IMI Horizon 2020 funding BIOMAP. Dr. Demehri reported having no relevant conflicts of interest.
SOURCE: Mansfield KE et al. JAMA Dermatol. 2020 Jun 24. doi: 10.1001/jamadermatol.2020.1948.
FROM JAMA DERMATOLOGY
What COVID-19 has taught us about senior care
Across the globe, there are marked differences in how countries responded to the COVID-19 outbreak, with varying degrees of success in limiting the spread of the virus. Some countries learned important lessons from previous outbreaks, including SARS and MERS, and put policies in place that contributed to lower infection and death rates from COVID-19 in these countries. Others struggled to respond appropriately to the outbreak.
The United States and most of the world was not affected significantly by SARS and MERS. Hence there is a need for different perspectives and observations on lessons that can be learned from this outbreak to help develop effective strategies and policies for the future. It also makes sense to focus intently on the demographic most affected by COVID-19 – the elderly.
Medical care, for the most part, is governed by protocols that clearly detail processes to be followed for the prevention and treatment of disease. Caring for older patients requires going above and beyond the protocols. That is one of the lessons learned from the COVID-19 pandemic – a wake-up call for a more proactive approach for at-risk patients, in this case everyone over the age of 60 years.
In this context, it is important for medical outreach to continue with the senior population long after the pandemic has run its course. Many seniors, particularly those susceptible to other illnesses or exhibiting ongoing issues, would benefit from a consistent and preplanned pattern of contacts by medical professionals and agencies that work with the aging population. These proactive follow-ups can facilitate prevention and treatment and, at the same time, reduce costs that would otherwise increase when health care is reactive.
Lessons in infectious disease containment
As COVID-19 spread globally, there were contrasting responses from individual countries in their efforts to contain the disease. Unfortunately, Italy suffered from its decision to lock down only specific regions of the country initially. The leadership in Italy may have ignored the advice of medical experts and been caught off guard by the intensity of the spread of COVID-19. In fact, they might not have taken strict actions right away because they did not want their responses to be viewed as an overreaction to the disease.
The government decided to shut down areas where the infection rates were high (“red zones”) rather than implement restrictions nationally. This may have inadvertently increased the spread as Italians vacated those “red zones” for other areas of the country not yet affected by COVID-19. Italy’s decentralized health care system also played a part in the effects of the disease, with some regions demonstrating more success in slowing the reach of the disease. According to an article in the Harvard Business Review, the neighboring regions of Lombardy and Veneto applied similar approaches to social distancing and retail closures. Veneto was more proactive, and its response to the outbreak was multipronged, including putting a “strong emphasis on home diagnosis and care” and “specific efforts to monitor and protect health care and other essential workers.” These measures most likely contributed to a slowdown of the spread of the disease in Veneto’s health care facilities, which lessened the load on medical providers.1
Conversely, Taiwan implemented proactive measures swiftly after learning about COVID-19. Taiwan was impacted adversely by the SARS outbreak in 2003 and, afterward, revised their medical policies and procedures to respond quickly to future infectious disease crises. In the beginning, little was known about COVID-19 or how it spread. However, Taiwan’s swift public health response to COVID-19 included early travel restrictions, patient screening, and quarantining of symptomatic patients. The government emphasized education and created real-time digital updates and alerts sent to their citizens, as well as partnering with media to broadcast crucial proactive health information and quickly disproving false information related to COVID-19. They coordinated with organizations throughout the country to increase supplies of personal protective equipment (PPE).2
Although countries and even cities within a country differ in terms of population demographics, health resources, government policies, and cultural practices, initial success stories have some similarities, including the following:
- Early travel restrictions from countries with positive cases, with some circumstances requiring compulsory quarantine periods and testing before entry.
- Extensive testing and proactive tracing of symptomatic cases early. Contacts of people testing positive were also tested, irrespective of being symptomatic or asymptomatic. If testing kits were unavailable, the contacts were self-quarantined.
- Emphasis on avoiding overburdening hospitals by having the public health infrastructure to divert people exhibiting symptoms, including using public health hotlines to send patients to dedicated testing sites and drive-through testing, rather than have patients presenting to emergency rooms and hospitals. This approach protected medical staff from exposure and allowed the focus to remain on treating severe symptomatic patients.
The vastly different response to the COVID-19 outbreak in these two countries illuminates the need for better preparation in the United States. At the onset of this outbreak, emergency room medical professionals, hospitalists, and outpatient primary care providers did not know how to screen for or treat this virus. Additionally, there was limited information on the most effective contact protocols for medical professionals, patients, and visitors. Finally, the lack of PPE and COVID-19 test kits hindered the U.S. response. Once the country is on the road to recovery from COVID-19, it is imperative to set the groundwork to prepare for future outbreaks and create mechanisms to quickly identify vulnerable populations when outbreaks occur.
Senior care in future infectious disease outbreaks
How can medical providers translate lessons learned from this outbreak into improving the quality of care for seniors? The National Institute on Aging (NIA) maintains a website with information about healthy aging. Seniors and their caregivers can use this website to learn more about chronic diseases, lifestyle modifications, disease prevention, and mental health.
In times of a pandemic, this website provides consistent and accurate information and education. One recommendation for reaching the elderly population during future outbreaks is for NIA to develop and implement strategies to increase the use of the website, including adding more audio and visual interfaces and developing a mobile app. Other recommendations for improving the quality of care for seniors include the following:
1. Identify which populations may be most affected when future outbreaks occur.
2. Consider nontraditional platforms, including social media, for communicating with the general population and for medical providers worldwide to learn from each other about new diseases, including the signs, symptoms, and treatment plans. Some medical professionals created specific WhatsApp groups to communicate, and the World Health Organization sent updated information about COVID-19 to anyone who texted them via WhatsApp.3
3. Create a checklist of signs and symptoms related to current infectious diseases and assess every vulnerable patient.
4. Share these guidelines with medical facilities that treat these populations, such as senior care, assisted living and rehabilitation facilities, hospitals, and outpatient treatment centers. Teach the staff at these medical facilities how to screen patients for signs and symptoms of the disease.
5. Implement social isolation strategies, travel and visitor restrictions, and testing and screening as soon as possible at these medical facilities.
6. Recognize that these strategies may affect the psychological and emotional well-being of seniors, increasing their risk for depression and anxiety and negatively affecting their immunity and mental health. Additionally, the use of PPE, either by the medical providers or the patient, may cause anxiety in seniors and those with mild cognitive impairment.
7. Encourage these medical facilities to improve coping strategies with older patients, such as incorporating communication technology that helps seniors stay connected with their families, and participating in physical and mental exercise, as well as religious activities.
8. Ask these medical facilities to create isolation or quarantine rooms for infected seniors.
9. Work with family members to proactively report to medical professionals any symptoms noticed in their senior relatives. Educate seniors to report symptoms earlier.
10. Offer incentives for medical professionals to conduct on-site testing in primary care offices or senior care facilities instead of sending patients to hospital emergency rooms for evaluation. This will only be effective if there are enough test kits available.
11. Urge insurance companies and Medicare to allow additional medical visits for screening vulnerable populations. Encourage the use of telemedicine in place of in-office visits (preferably billed at the same rate as an in-office visit) where appropriate, especially with nonambulatory patients or those with transportation issues. Many insurance companies, including Medicare, approved COVID-19–related coverage of telemedicine in place of office visits to limit the spread of the disease.
12. Provide community health care and integration and better coordination of local, state, and national health care.
13. Hold regular epidemic and pandemic preparedness exercises in every hospital, nursing home, and assisted living facility.
Proactive health care outreach
It is easier to identify the signs and symptoms of already identified infectious diseases as opposed to a novel one like COVID-19. The United States faced a steep learning curve with COVID-19. Hospitalists and other medical professionals were not able to learn about COVID-19 in a journal. At first, they did not know how to screen patients coming into the ER, how to protect staff, or what the treatment plan was for this new disease. As a result, the medical system experienced disorder and confusion. Investing in community health care and better coordination of local, state, and national health care resources is a priority.
The senior citizen population appears to be most vulnerable to this virus and may be just as vulnerable in future outbreaks. Yet the insights gained from this pandemic can lead to a more comprehensive outreach to senior patients and increased screenings for comorbidities and future contagious diseases. An emphasis on proactive health care and outreach for seniors, with a focus on identifying and treating comorbid conditions, improves the medical care system overall and may prevent or slow future community outbreaks.
Dr. Kasarla is a hospitalist with APOGEE Physicians at Wise Surgical at Parkway in Fort Worth, Tex. He did his internal medicine residency at Mercy Hospital & Medical Center, Chicago. Readers can contact him at [email protected]. Dr. Devireddy is a family physician at Positive Health Medical Center, Kingston, Jamaica. Contact him at [email protected].
References
1. Pisano GP et al. Lessons from Italy’s response to coronavirus. Harvard Business Review. 2020 Mar 27. https://hbr.org/2020/03/lessons-from-italys-response-to-coronavirus.
2. Tu C. Lessons from Taiwan’s experience with COVID-19. New Atlanticist. 2020 Apr 7. https://atlanticcouncil.org/blogs/new-atlanticist/lessons-from-taiwans-experience-with-covid-19/.
3. Newman LH. WhatsApp is at the center of coronavirus response. WIRED. 2020 Mar 20. https://www.wired.com/story/whatsapp-coronavirus-who-information-app/.
Across the globe, there are marked differences in how countries responded to the COVID-19 outbreak, with varying degrees of success in limiting the spread of the virus. Some countries learned important lessons from previous outbreaks, including SARS and MERS, and put policies in place that contributed to lower infection and death rates from COVID-19 in these countries. Others struggled to respond appropriately to the outbreak.
The United States and most of the world was not affected significantly by SARS and MERS. Hence there is a need for different perspectives and observations on lessons that can be learned from this outbreak to help develop effective strategies and policies for the future. It also makes sense to focus intently on the demographic most affected by COVID-19 – the elderly.
Medical care, for the most part, is governed by protocols that clearly detail processes to be followed for the prevention and treatment of disease. Caring for older patients requires going above and beyond the protocols. That is one of the lessons learned from the COVID-19 pandemic – a wake-up call for a more proactive approach for at-risk patients, in this case everyone over the age of 60 years.
In this context, it is important for medical outreach to continue with the senior population long after the pandemic has run its course. Many seniors, particularly those susceptible to other illnesses or exhibiting ongoing issues, would benefit from a consistent and preplanned pattern of contacts by medical professionals and agencies that work with the aging population. These proactive follow-ups can facilitate prevention and treatment and, at the same time, reduce costs that would otherwise increase when health care is reactive.
Lessons in infectious disease containment
As COVID-19 spread globally, there were contrasting responses from individual countries in their efforts to contain the disease. Unfortunately, Italy suffered from its decision to lock down only specific regions of the country initially. The leadership in Italy may have ignored the advice of medical experts and been caught off guard by the intensity of the spread of COVID-19. In fact, they might not have taken strict actions right away because they did not want their responses to be viewed as an overreaction to the disease.
The government decided to shut down areas where the infection rates were high (“red zones”) rather than implement restrictions nationally. This may have inadvertently increased the spread as Italians vacated those “red zones” for other areas of the country not yet affected by COVID-19. Italy’s decentralized health care system also played a part in the effects of the disease, with some regions demonstrating more success in slowing the reach of the disease. According to an article in the Harvard Business Review, the neighboring regions of Lombardy and Veneto applied similar approaches to social distancing and retail closures. Veneto was more proactive, and its response to the outbreak was multipronged, including putting a “strong emphasis on home diagnosis and care” and “specific efforts to monitor and protect health care and other essential workers.” These measures most likely contributed to a slowdown of the spread of the disease in Veneto’s health care facilities, which lessened the load on medical providers.1
Conversely, Taiwan implemented proactive measures swiftly after learning about COVID-19. Taiwan was impacted adversely by the SARS outbreak in 2003 and, afterward, revised their medical policies and procedures to respond quickly to future infectious disease crises. In the beginning, little was known about COVID-19 or how it spread. However, Taiwan’s swift public health response to COVID-19 included early travel restrictions, patient screening, and quarantining of symptomatic patients. The government emphasized education and created real-time digital updates and alerts sent to their citizens, as well as partnering with media to broadcast crucial proactive health information and quickly disproving false information related to COVID-19. They coordinated with organizations throughout the country to increase supplies of personal protective equipment (PPE).2
Although countries and even cities within a country differ in terms of population demographics, health resources, government policies, and cultural practices, initial success stories have some similarities, including the following:
- Early travel restrictions from countries with positive cases, with some circumstances requiring compulsory quarantine periods and testing before entry.
- Extensive testing and proactive tracing of symptomatic cases early. Contacts of people testing positive were also tested, irrespective of being symptomatic or asymptomatic. If testing kits were unavailable, the contacts were self-quarantined.
- Emphasis on avoiding overburdening hospitals by having the public health infrastructure to divert people exhibiting symptoms, including using public health hotlines to send patients to dedicated testing sites and drive-through testing, rather than have patients presenting to emergency rooms and hospitals. This approach protected medical staff from exposure and allowed the focus to remain on treating severe symptomatic patients.
The vastly different response to the COVID-19 outbreak in these two countries illuminates the need for better preparation in the United States. At the onset of this outbreak, emergency room medical professionals, hospitalists, and outpatient primary care providers did not know how to screen for or treat this virus. Additionally, there was limited information on the most effective contact protocols for medical professionals, patients, and visitors. Finally, the lack of PPE and COVID-19 test kits hindered the U.S. response. Once the country is on the road to recovery from COVID-19, it is imperative to set the groundwork to prepare for future outbreaks and create mechanisms to quickly identify vulnerable populations when outbreaks occur.
Senior care in future infectious disease outbreaks
How can medical providers translate lessons learned from this outbreak into improving the quality of care for seniors? The National Institute on Aging (NIA) maintains a website with information about healthy aging. Seniors and their caregivers can use this website to learn more about chronic diseases, lifestyle modifications, disease prevention, and mental health.
In times of a pandemic, this website provides consistent and accurate information and education. One recommendation for reaching the elderly population during future outbreaks is for NIA to develop and implement strategies to increase the use of the website, including adding more audio and visual interfaces and developing a mobile app. Other recommendations for improving the quality of care for seniors include the following:
1. Identify which populations may be most affected when future outbreaks occur.
2. Consider nontraditional platforms, including social media, for communicating with the general population and for medical providers worldwide to learn from each other about new diseases, including the signs, symptoms, and treatment plans. Some medical professionals created specific WhatsApp groups to communicate, and the World Health Organization sent updated information about COVID-19 to anyone who texted them via WhatsApp.3
3. Create a checklist of signs and symptoms related to current infectious diseases and assess every vulnerable patient.
4. Share these guidelines with medical facilities that treat these populations, such as senior care, assisted living and rehabilitation facilities, hospitals, and outpatient treatment centers. Teach the staff at these medical facilities how to screen patients for signs and symptoms of the disease.
5. Implement social isolation strategies, travel and visitor restrictions, and testing and screening as soon as possible at these medical facilities.
6. Recognize that these strategies may affect the psychological and emotional well-being of seniors, increasing their risk for depression and anxiety and negatively affecting their immunity and mental health. Additionally, the use of PPE, either by the medical providers or the patient, may cause anxiety in seniors and those with mild cognitive impairment.
7. Encourage these medical facilities to improve coping strategies with older patients, such as incorporating communication technology that helps seniors stay connected with their families, and participating in physical and mental exercise, as well as religious activities.
8. Ask these medical facilities to create isolation or quarantine rooms for infected seniors.
9. Work with family members to proactively report to medical professionals any symptoms noticed in their senior relatives. Educate seniors to report symptoms earlier.
10. Offer incentives for medical professionals to conduct on-site testing in primary care offices or senior care facilities instead of sending patients to hospital emergency rooms for evaluation. This will only be effective if there are enough test kits available.
11. Urge insurance companies and Medicare to allow additional medical visits for screening vulnerable populations. Encourage the use of telemedicine in place of in-office visits (preferably billed at the same rate as an in-office visit) where appropriate, especially with nonambulatory patients or those with transportation issues. Many insurance companies, including Medicare, approved COVID-19–related coverage of telemedicine in place of office visits to limit the spread of the disease.
12. Provide community health care and integration and better coordination of local, state, and national health care.
13. Hold regular epidemic and pandemic preparedness exercises in every hospital, nursing home, and assisted living facility.
Proactive health care outreach
It is easier to identify the signs and symptoms of already identified infectious diseases as opposed to a novel one like COVID-19. The United States faced a steep learning curve with COVID-19. Hospitalists and other medical professionals were not able to learn about COVID-19 in a journal. At first, they did not know how to screen patients coming into the ER, how to protect staff, or what the treatment plan was for this new disease. As a result, the medical system experienced disorder and confusion. Investing in community health care and better coordination of local, state, and national health care resources is a priority.
The senior citizen population appears to be most vulnerable to this virus and may be just as vulnerable in future outbreaks. Yet the insights gained from this pandemic can lead to a more comprehensive outreach to senior patients and increased screenings for comorbidities and future contagious diseases. An emphasis on proactive health care and outreach for seniors, with a focus on identifying and treating comorbid conditions, improves the medical care system overall and may prevent or slow future community outbreaks.
Dr. Kasarla is a hospitalist with APOGEE Physicians at Wise Surgical at Parkway in Fort Worth, Tex. He did his internal medicine residency at Mercy Hospital & Medical Center, Chicago. Readers can contact him at [email protected]. Dr. Devireddy is a family physician at Positive Health Medical Center, Kingston, Jamaica. Contact him at [email protected].
References
1. Pisano GP et al. Lessons from Italy’s response to coronavirus. Harvard Business Review. 2020 Mar 27. https://hbr.org/2020/03/lessons-from-italys-response-to-coronavirus.
2. Tu C. Lessons from Taiwan’s experience with COVID-19. New Atlanticist. 2020 Apr 7. https://atlanticcouncil.org/blogs/new-atlanticist/lessons-from-taiwans-experience-with-covid-19/.
3. Newman LH. WhatsApp is at the center of coronavirus response. WIRED. 2020 Mar 20. https://www.wired.com/story/whatsapp-coronavirus-who-information-app/.
Across the globe, there are marked differences in how countries responded to the COVID-19 outbreak, with varying degrees of success in limiting the spread of the virus. Some countries learned important lessons from previous outbreaks, including SARS and MERS, and put policies in place that contributed to lower infection and death rates from COVID-19 in these countries. Others struggled to respond appropriately to the outbreak.
The United States and most of the world was not affected significantly by SARS and MERS. Hence there is a need for different perspectives and observations on lessons that can be learned from this outbreak to help develop effective strategies and policies for the future. It also makes sense to focus intently on the demographic most affected by COVID-19 – the elderly.
Medical care, for the most part, is governed by protocols that clearly detail processes to be followed for the prevention and treatment of disease. Caring for older patients requires going above and beyond the protocols. That is one of the lessons learned from the COVID-19 pandemic – a wake-up call for a more proactive approach for at-risk patients, in this case everyone over the age of 60 years.
In this context, it is important for medical outreach to continue with the senior population long after the pandemic has run its course. Many seniors, particularly those susceptible to other illnesses or exhibiting ongoing issues, would benefit from a consistent and preplanned pattern of contacts by medical professionals and agencies that work with the aging population. These proactive follow-ups can facilitate prevention and treatment and, at the same time, reduce costs that would otherwise increase when health care is reactive.
Lessons in infectious disease containment
As COVID-19 spread globally, there were contrasting responses from individual countries in their efforts to contain the disease. Unfortunately, Italy suffered from its decision to lock down only specific regions of the country initially. The leadership in Italy may have ignored the advice of medical experts and been caught off guard by the intensity of the spread of COVID-19. In fact, they might not have taken strict actions right away because they did not want their responses to be viewed as an overreaction to the disease.
The government decided to shut down areas where the infection rates were high (“red zones”) rather than implement restrictions nationally. This may have inadvertently increased the spread as Italians vacated those “red zones” for other areas of the country not yet affected by COVID-19. Italy’s decentralized health care system also played a part in the effects of the disease, with some regions demonstrating more success in slowing the reach of the disease. According to an article in the Harvard Business Review, the neighboring regions of Lombardy and Veneto applied similar approaches to social distancing and retail closures. Veneto was more proactive, and its response to the outbreak was multipronged, including putting a “strong emphasis on home diagnosis and care” and “specific efforts to monitor and protect health care and other essential workers.” These measures most likely contributed to a slowdown of the spread of the disease in Veneto’s health care facilities, which lessened the load on medical providers.1
Conversely, Taiwan implemented proactive measures swiftly after learning about COVID-19. Taiwan was impacted adversely by the SARS outbreak in 2003 and, afterward, revised their medical policies and procedures to respond quickly to future infectious disease crises. In the beginning, little was known about COVID-19 or how it spread. However, Taiwan’s swift public health response to COVID-19 included early travel restrictions, patient screening, and quarantining of symptomatic patients. The government emphasized education and created real-time digital updates and alerts sent to their citizens, as well as partnering with media to broadcast crucial proactive health information and quickly disproving false information related to COVID-19. They coordinated with organizations throughout the country to increase supplies of personal protective equipment (PPE).2
Although countries and even cities within a country differ in terms of population demographics, health resources, government policies, and cultural practices, initial success stories have some similarities, including the following:
- Early travel restrictions from countries with positive cases, with some circumstances requiring compulsory quarantine periods and testing before entry.
- Extensive testing and proactive tracing of symptomatic cases early. Contacts of people testing positive were also tested, irrespective of being symptomatic or asymptomatic. If testing kits were unavailable, the contacts were self-quarantined.
- Emphasis on avoiding overburdening hospitals by having the public health infrastructure to divert people exhibiting symptoms, including using public health hotlines to send patients to dedicated testing sites and drive-through testing, rather than have patients presenting to emergency rooms and hospitals. This approach protected medical staff from exposure and allowed the focus to remain on treating severe symptomatic patients.
The vastly different response to the COVID-19 outbreak in these two countries illuminates the need for better preparation in the United States. At the onset of this outbreak, emergency room medical professionals, hospitalists, and outpatient primary care providers did not know how to screen for or treat this virus. Additionally, there was limited information on the most effective contact protocols for medical professionals, patients, and visitors. Finally, the lack of PPE and COVID-19 test kits hindered the U.S. response. Once the country is on the road to recovery from COVID-19, it is imperative to set the groundwork to prepare for future outbreaks and create mechanisms to quickly identify vulnerable populations when outbreaks occur.
Senior care in future infectious disease outbreaks
How can medical providers translate lessons learned from this outbreak into improving the quality of care for seniors? The National Institute on Aging (NIA) maintains a website with information about healthy aging. Seniors and their caregivers can use this website to learn more about chronic diseases, lifestyle modifications, disease prevention, and mental health.
In times of a pandemic, this website provides consistent and accurate information and education. One recommendation for reaching the elderly population during future outbreaks is for NIA to develop and implement strategies to increase the use of the website, including adding more audio and visual interfaces and developing a mobile app. Other recommendations for improving the quality of care for seniors include the following:
1. Identify which populations may be most affected when future outbreaks occur.
2. Consider nontraditional platforms, including social media, for communicating with the general population and for medical providers worldwide to learn from each other about new diseases, including the signs, symptoms, and treatment plans. Some medical professionals created specific WhatsApp groups to communicate, and the World Health Organization sent updated information about COVID-19 to anyone who texted them via WhatsApp.3
3. Create a checklist of signs and symptoms related to current infectious diseases and assess every vulnerable patient.
4. Share these guidelines with medical facilities that treat these populations, such as senior care, assisted living and rehabilitation facilities, hospitals, and outpatient treatment centers. Teach the staff at these medical facilities how to screen patients for signs and symptoms of the disease.
5. Implement social isolation strategies, travel and visitor restrictions, and testing and screening as soon as possible at these medical facilities.
6. Recognize that these strategies may affect the psychological and emotional well-being of seniors, increasing their risk for depression and anxiety and negatively affecting their immunity and mental health. Additionally, the use of PPE, either by the medical providers or the patient, may cause anxiety in seniors and those with mild cognitive impairment.
7. Encourage these medical facilities to improve coping strategies with older patients, such as incorporating communication technology that helps seniors stay connected with their families, and participating in physical and mental exercise, as well as religious activities.
8. Ask these medical facilities to create isolation or quarantine rooms for infected seniors.
9. Work with family members to proactively report to medical professionals any symptoms noticed in their senior relatives. Educate seniors to report symptoms earlier.
10. Offer incentives for medical professionals to conduct on-site testing in primary care offices or senior care facilities instead of sending patients to hospital emergency rooms for evaluation. This will only be effective if there are enough test kits available.
11. Urge insurance companies and Medicare to allow additional medical visits for screening vulnerable populations. Encourage the use of telemedicine in place of in-office visits (preferably billed at the same rate as an in-office visit) where appropriate, especially with nonambulatory patients or those with transportation issues. Many insurance companies, including Medicare, approved COVID-19–related coverage of telemedicine in place of office visits to limit the spread of the disease.
12. Provide community health care and integration and better coordination of local, state, and national health care.
13. Hold regular epidemic and pandemic preparedness exercises in every hospital, nursing home, and assisted living facility.
Proactive health care outreach
It is easier to identify the signs and symptoms of already identified infectious diseases as opposed to a novel one like COVID-19. The United States faced a steep learning curve with COVID-19. Hospitalists and other medical professionals were not able to learn about COVID-19 in a journal. At first, they did not know how to screen patients coming into the ER, how to protect staff, or what the treatment plan was for this new disease. As a result, the medical system experienced disorder and confusion. Investing in community health care and better coordination of local, state, and national health care resources is a priority.
The senior citizen population appears to be most vulnerable to this virus and may be just as vulnerable in future outbreaks. Yet the insights gained from this pandemic can lead to a more comprehensive outreach to senior patients and increased screenings for comorbidities and future contagious diseases. An emphasis on proactive health care and outreach for seniors, with a focus on identifying and treating comorbid conditions, improves the medical care system overall and may prevent or slow future community outbreaks.
Dr. Kasarla is a hospitalist with APOGEE Physicians at Wise Surgical at Parkway in Fort Worth, Tex. He did his internal medicine residency at Mercy Hospital & Medical Center, Chicago. Readers can contact him at [email protected]. Dr. Devireddy is a family physician at Positive Health Medical Center, Kingston, Jamaica. Contact him at [email protected].
References
1. Pisano GP et al. Lessons from Italy’s response to coronavirus. Harvard Business Review. 2020 Mar 27. https://hbr.org/2020/03/lessons-from-italys-response-to-coronavirus.
2. Tu C. Lessons from Taiwan’s experience with COVID-19. New Atlanticist. 2020 Apr 7. https://atlanticcouncil.org/blogs/new-atlanticist/lessons-from-taiwans-experience-with-covid-19/.
3. Newman LH. WhatsApp is at the center of coronavirus response. WIRED. 2020 Mar 20. https://www.wired.com/story/whatsapp-coronavirus-who-information-app/.
Microneedling plus 10% TCA peels bests CO2 laser alone for infraorbital dark circles
In a study of patients with mild to moderate infraorbital dark circles, treatment with carbon dioxide laser resurfacing did not produce a significant improvement in infraorbital hyperpigmentation. However, the combination of microneedling and 10% trichloroacetic acid peels did.
The finding comes from what is believed to be the first head-to-head comparison of the two procedures for infraorbital dark circles, which are a common cosmetic concern with increased age.
During a late-breaking abstract session at the virtual annual meeting of the American Academy of Dermatology, lead study author Banu Farabi, MD, said that dark circles seen in the periorbital area are defined as bilateral, round homogeneous pigmented macules whose etiology is thought to be multifactorial. Available treatments include bleaching creams, topical retinoids, chemical peels, lasers, autologous fat transplantation, injectable fillers, and blepharoplasty.
“Microneedling has been recently suggested as an effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi said. “This technique is based on creating microchannels that can stimulate the production of subcutaneous collagen and elastin. It also enhances the revascularization and fibroblast activity, which increases the skin thickness and gives a shiny appearance to the skin. The fractional CO2 has also been introduced as an effective procedure to remove infraorbital dark circles. However, there are some potential complications with that therapy.”
For the current study, Dr. Farabi, of the department of dermatology at Ankara (Turkey) University, and Mohamad Goldust, MD, of University Hospital Basel (Switzerland), They used the handheld Automatic Microneedle Therapy System-Handhold from MCure. After creating microchannels, the investigators topically applied 10% trichloroacetic acid peels to each infraorbital area and waited for 5 minutes.
In the carbon dioxide laser group, a Lutronic CO2 laser was used with a pulse energy of 10 J/cm2, a 100-microsecond pulse rate, 30 W of power, and a pulse width of 4 mm. The treatment outcome was assessed with the patient’s satisfaction and the physician’s judgment, which were no response, partial response, and complete response. Patients in both study groups were followed up for blinded-investigator assessment of infraorbital hyperpigmentation, adverse events, and improvement, compared with baseline.
The mean age of patients was 40 years, with a range between 27 and 58 years. About one-third of patients in each group had Fitzpatrick skin types II, III, and IV, respectively. In the blinded investigator assessment, the laser-resurfacing procedure did not demonstrate a significant improvement in infraorbital hyperpigmentation at day 90 (P = .24). However, the combination of microneedling and 10% trichloroacetic acid peels significantly improved infraorbital hyperpigmentation by day 90, with improvement maintained through day 180 (P = .012 and .002, respectively).
Adverse events were mild and temporary in both groups. In the laser-resurfacing group, 7 of the patients (22.5%) developed transient infraorbital hyperpigmentation postoperatively that lasted 4 weeks. In the combination treatment group, 18 patients (58%) developed transient erythema that lasted for up to 1 week.
“We suggest using microneedling plus 10% [trichloroacetic acid] as a cost-effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi concluded.
The researchers reported having no financial disclosures.
In a study of patients with mild to moderate infraorbital dark circles, treatment with carbon dioxide laser resurfacing did not produce a significant improvement in infraorbital hyperpigmentation. However, the combination of microneedling and 10% trichloroacetic acid peels did.
The finding comes from what is believed to be the first head-to-head comparison of the two procedures for infraorbital dark circles, which are a common cosmetic concern with increased age.
During a late-breaking abstract session at the virtual annual meeting of the American Academy of Dermatology, lead study author Banu Farabi, MD, said that dark circles seen in the periorbital area are defined as bilateral, round homogeneous pigmented macules whose etiology is thought to be multifactorial. Available treatments include bleaching creams, topical retinoids, chemical peels, lasers, autologous fat transplantation, injectable fillers, and blepharoplasty.
“Microneedling has been recently suggested as an effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi said. “This technique is based on creating microchannels that can stimulate the production of subcutaneous collagen and elastin. It also enhances the revascularization and fibroblast activity, which increases the skin thickness and gives a shiny appearance to the skin. The fractional CO2 has also been introduced as an effective procedure to remove infraorbital dark circles. However, there are some potential complications with that therapy.”
For the current study, Dr. Farabi, of the department of dermatology at Ankara (Turkey) University, and Mohamad Goldust, MD, of University Hospital Basel (Switzerland), They used the handheld Automatic Microneedle Therapy System-Handhold from MCure. After creating microchannels, the investigators topically applied 10% trichloroacetic acid peels to each infraorbital area and waited for 5 minutes.
In the carbon dioxide laser group, a Lutronic CO2 laser was used with a pulse energy of 10 J/cm2, a 100-microsecond pulse rate, 30 W of power, and a pulse width of 4 mm. The treatment outcome was assessed with the patient’s satisfaction and the physician’s judgment, which were no response, partial response, and complete response. Patients in both study groups were followed up for blinded-investigator assessment of infraorbital hyperpigmentation, adverse events, and improvement, compared with baseline.
The mean age of patients was 40 years, with a range between 27 and 58 years. About one-third of patients in each group had Fitzpatrick skin types II, III, and IV, respectively. In the blinded investigator assessment, the laser-resurfacing procedure did not demonstrate a significant improvement in infraorbital hyperpigmentation at day 90 (P = .24). However, the combination of microneedling and 10% trichloroacetic acid peels significantly improved infraorbital hyperpigmentation by day 90, with improvement maintained through day 180 (P = .012 and .002, respectively).
Adverse events were mild and temporary in both groups. In the laser-resurfacing group, 7 of the patients (22.5%) developed transient infraorbital hyperpigmentation postoperatively that lasted 4 weeks. In the combination treatment group, 18 patients (58%) developed transient erythema that lasted for up to 1 week.
“We suggest using microneedling plus 10% [trichloroacetic acid] as a cost-effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi concluded.
The researchers reported having no financial disclosures.
In a study of patients with mild to moderate infraorbital dark circles, treatment with carbon dioxide laser resurfacing did not produce a significant improvement in infraorbital hyperpigmentation. However, the combination of microneedling and 10% trichloroacetic acid peels did.
The finding comes from what is believed to be the first head-to-head comparison of the two procedures for infraorbital dark circles, which are a common cosmetic concern with increased age.
During a late-breaking abstract session at the virtual annual meeting of the American Academy of Dermatology, lead study author Banu Farabi, MD, said that dark circles seen in the periorbital area are defined as bilateral, round homogeneous pigmented macules whose etiology is thought to be multifactorial. Available treatments include bleaching creams, topical retinoids, chemical peels, lasers, autologous fat transplantation, injectable fillers, and blepharoplasty.
“Microneedling has been recently suggested as an effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi said. “This technique is based on creating microchannels that can stimulate the production of subcutaneous collagen and elastin. It also enhances the revascularization and fibroblast activity, which increases the skin thickness and gives a shiny appearance to the skin. The fractional CO2 has also been introduced as an effective procedure to remove infraorbital dark circles. However, there are some potential complications with that therapy.”
For the current study, Dr. Farabi, of the department of dermatology at Ankara (Turkey) University, and Mohamad Goldust, MD, of University Hospital Basel (Switzerland), They used the handheld Automatic Microneedle Therapy System-Handhold from MCure. After creating microchannels, the investigators topically applied 10% trichloroacetic acid peels to each infraorbital area and waited for 5 minutes.
In the carbon dioxide laser group, a Lutronic CO2 laser was used with a pulse energy of 10 J/cm2, a 100-microsecond pulse rate, 30 W of power, and a pulse width of 4 mm. The treatment outcome was assessed with the patient’s satisfaction and the physician’s judgment, which were no response, partial response, and complete response. Patients in both study groups were followed up for blinded-investigator assessment of infraorbital hyperpigmentation, adverse events, and improvement, compared with baseline.
The mean age of patients was 40 years, with a range between 27 and 58 years. About one-third of patients in each group had Fitzpatrick skin types II, III, and IV, respectively. In the blinded investigator assessment, the laser-resurfacing procedure did not demonstrate a significant improvement in infraorbital hyperpigmentation at day 90 (P = .24). However, the combination of microneedling and 10% trichloroacetic acid peels significantly improved infraorbital hyperpigmentation by day 90, with improvement maintained through day 180 (P = .012 and .002, respectively).
Adverse events were mild and temporary in both groups. In the laser-resurfacing group, 7 of the patients (22.5%) developed transient infraorbital hyperpigmentation postoperatively that lasted 4 weeks. In the combination treatment group, 18 patients (58%) developed transient erythema that lasted for up to 1 week.
“We suggest using microneedling plus 10% [trichloroacetic acid] as a cost-effective and efficient method for reducing infraorbital dark circles,” Dr. Farabi concluded.
The researchers reported having no financial disclosures.
FROM AAD 20
Telehealth and medical liability
The COVID-19 pandemic has led to the rapid uptake of telehealth nationwide in primary care and specialty practices. Over the last few months many practices have actually performed more telehealth visits than traditional in-person visits. The use of telehealth, which had been increasing slowly for the last few years, accelerated rapidly during the pandemic. Long term, telehealth has the potential to increase access to primary care and specialists, and make follow-up easier for many patients, changing how health care is delivered to millions of patients throughout the world.
Since telehealth will be a regular part of our practices from now on, it is important for clinicians to recognize how telehealth visits are viewed in a legal arena.
As is often the case with technological advances, the law needs time to adapt. Will a health care provider treating a patient using telemedicine be held to the same standard of care applicable to an in-person encounter? Stated differently, will consideration be given to the obvious limitations imposed by a telemedicine exam?
Standard of care in medical malpractice cases
The central question in most medical malpractice cases is whether the provider complied with the generally accepted standard of care when evaluating, diagnosing, or treating a patient. This standard typically takes into consideration the provider’s particular specialty as well as all the circumstances surrounding the encounter.1 Medical providers, not state legislators, usually define the standard of care for medical professionals. In malpractice cases, medical experts explain the applicable standard of care to the jury and guide its determination of whether, in the particular case, the standard of care was met. In this way, the law has long recognized that the medical profession itself is best suited to establish the appropriate standards of care under any particular set of circumstances. This standard of care is often referred to as the “reasonable professional under the circumstances” standard of care.
Telemedicine standard of care
Despite the fact that the complex and often nebulous concept of standard of care has been traditionally left to the medical experts to define, state legislators and regulators throughout the nation have chosen to weigh in on this issue in the context of telemedicine. Most states with telemedicine regulations have followed the model policy adopted by the Federation of State Medical Boards in April 2014 which states that “[t]reatment and consultation recommendations made in an online setting … will be held to the same standards of appropriate practice as those in traditional (in-person) settings.”2 States that have adopted this model policy have effectively created a “legal fiction” requiring a jury to ignore the fact that the care was provided virtually by telemedicine technologies and instead assume that the physician treated the patient in person, i.e, applying an “in-person” standard of care. Hawaii appears to be the lone notable exception. Its telemedicine law recognizes that an in-person standard of care should not be applied if there was not a face-to-face visit.3
Proponents of the in-person telemedicine standard claim that it is necessary to ensure patient safety, thus justifying the “legal fiction.” Holding the provider to the in-person standard, it is argued, forces the physician to err on the side of caution and require an actual in-person encounter to ensure the advantages of sight, touch, and sense of things are fully available.4 This discourages the use of telemedicine and deprives the population of its many benefits.
Telemedicine can overcome geographical barriers, increase clinical support, improve health outcomes, reduce health care costs, encourage patient input, reduce travel, and foster continuity of care. The pandemic, which has significantly limited the ability of providers to see patients in person, only underscores the benefits of telemedicine.
The legislatively imposed in-person telemedicine standard of care should be replaced with the “reasonable professional under the circumstances” standard in order to fairly judge physicians’ care and promote overall population health. The “reasonable professional under the circumstances” standard has applied to physicians and other health care professionals outside of telemedicine for decades, and it has served the medical community and public well. It is unfortunate that legislators felt the need to weigh in and define a distinctly different standard of care for telemedicine than for the rest of medicine, as this may present unforeseen obstacles to the use of telemedicine.
The in-person telemedicine standard of care remains a significant barrier for long-term telemedicine. Eliminating this legal fiction has the potential to further expand physicians’ use of telemedicine and fulfill its promise of improving access to care and improving population health.
Mr. Horner (partner), Mr. Milewski (partner), and Mr. Gajer (associate) are attorneys with White and Williams. They specialize in defending health care providers in medical malpractice lawsuits and other health care–related matters. Dr. Skolnik is professor of family and community Medicine at the Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Follow Dr. Skolnik, and feel free to submit questions to him on Twitter: @neilskolnik. The authors have no financial conflicts related to the content of this piece.
References
1. Cowan v. Doering, 111 N.J. 451-62,.1988.
2. Model Policy For The Appropriate Use Of Telemedicine Technologies In The Practice Of Medicine. State Medical Boards Appropriate Regulation of Telemedicine. April 2014..
3. Haw. Rev. Stat. Ann. § 453-1.3(c).
4. Kaspar BJ. Iowa Law Review. 2014 Jan;99:839-59.
The COVID-19 pandemic has led to the rapid uptake of telehealth nationwide in primary care and specialty practices. Over the last few months many practices have actually performed more telehealth visits than traditional in-person visits. The use of telehealth, which had been increasing slowly for the last few years, accelerated rapidly during the pandemic. Long term, telehealth has the potential to increase access to primary care and specialists, and make follow-up easier for many patients, changing how health care is delivered to millions of patients throughout the world.
Since telehealth will be a regular part of our practices from now on, it is important for clinicians to recognize how telehealth visits are viewed in a legal arena.
As is often the case with technological advances, the law needs time to adapt. Will a health care provider treating a patient using telemedicine be held to the same standard of care applicable to an in-person encounter? Stated differently, will consideration be given to the obvious limitations imposed by a telemedicine exam?
Standard of care in medical malpractice cases
The central question in most medical malpractice cases is whether the provider complied with the generally accepted standard of care when evaluating, diagnosing, or treating a patient. This standard typically takes into consideration the provider’s particular specialty as well as all the circumstances surrounding the encounter.1 Medical providers, not state legislators, usually define the standard of care for medical professionals. In malpractice cases, medical experts explain the applicable standard of care to the jury and guide its determination of whether, in the particular case, the standard of care was met. In this way, the law has long recognized that the medical profession itself is best suited to establish the appropriate standards of care under any particular set of circumstances. This standard of care is often referred to as the “reasonable professional under the circumstances” standard of care.
Telemedicine standard of care
Despite the fact that the complex and often nebulous concept of standard of care has been traditionally left to the medical experts to define, state legislators and regulators throughout the nation have chosen to weigh in on this issue in the context of telemedicine. Most states with telemedicine regulations have followed the model policy adopted by the Federation of State Medical Boards in April 2014 which states that “[t]reatment and consultation recommendations made in an online setting … will be held to the same standards of appropriate practice as those in traditional (in-person) settings.”2 States that have adopted this model policy have effectively created a “legal fiction” requiring a jury to ignore the fact that the care was provided virtually by telemedicine technologies and instead assume that the physician treated the patient in person, i.e, applying an “in-person” standard of care. Hawaii appears to be the lone notable exception. Its telemedicine law recognizes that an in-person standard of care should not be applied if there was not a face-to-face visit.3
Proponents of the in-person telemedicine standard claim that it is necessary to ensure patient safety, thus justifying the “legal fiction.” Holding the provider to the in-person standard, it is argued, forces the physician to err on the side of caution and require an actual in-person encounter to ensure the advantages of sight, touch, and sense of things are fully available.4 This discourages the use of telemedicine and deprives the population of its many benefits.
Telemedicine can overcome geographical barriers, increase clinical support, improve health outcomes, reduce health care costs, encourage patient input, reduce travel, and foster continuity of care. The pandemic, which has significantly limited the ability of providers to see patients in person, only underscores the benefits of telemedicine.
The legislatively imposed in-person telemedicine standard of care should be replaced with the “reasonable professional under the circumstances” standard in order to fairly judge physicians’ care and promote overall population health. The “reasonable professional under the circumstances” standard has applied to physicians and other health care professionals outside of telemedicine for decades, and it has served the medical community and public well. It is unfortunate that legislators felt the need to weigh in and define a distinctly different standard of care for telemedicine than for the rest of medicine, as this may present unforeseen obstacles to the use of telemedicine.
The in-person telemedicine standard of care remains a significant barrier for long-term telemedicine. Eliminating this legal fiction has the potential to further expand physicians’ use of telemedicine and fulfill its promise of improving access to care and improving population health.
Mr. Horner (partner), Mr. Milewski (partner), and Mr. Gajer (associate) are attorneys with White and Williams. They specialize in defending health care providers in medical malpractice lawsuits and other health care–related matters. Dr. Skolnik is professor of family and community Medicine at the Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Follow Dr. Skolnik, and feel free to submit questions to him on Twitter: @neilskolnik. The authors have no financial conflicts related to the content of this piece.
References
1. Cowan v. Doering, 111 N.J. 451-62,.1988.
2. Model Policy For The Appropriate Use Of Telemedicine Technologies In The Practice Of Medicine. State Medical Boards Appropriate Regulation of Telemedicine. April 2014..
3. Haw. Rev. Stat. Ann. § 453-1.3(c).
4. Kaspar BJ. Iowa Law Review. 2014 Jan;99:839-59.
The COVID-19 pandemic has led to the rapid uptake of telehealth nationwide in primary care and specialty practices. Over the last few months many practices have actually performed more telehealth visits than traditional in-person visits. The use of telehealth, which had been increasing slowly for the last few years, accelerated rapidly during the pandemic. Long term, telehealth has the potential to increase access to primary care and specialists, and make follow-up easier for many patients, changing how health care is delivered to millions of patients throughout the world.
Since telehealth will be a regular part of our practices from now on, it is important for clinicians to recognize how telehealth visits are viewed in a legal arena.
As is often the case with technological advances, the law needs time to adapt. Will a health care provider treating a patient using telemedicine be held to the same standard of care applicable to an in-person encounter? Stated differently, will consideration be given to the obvious limitations imposed by a telemedicine exam?
Standard of care in medical malpractice cases
The central question in most medical malpractice cases is whether the provider complied with the generally accepted standard of care when evaluating, diagnosing, or treating a patient. This standard typically takes into consideration the provider’s particular specialty as well as all the circumstances surrounding the encounter.1 Medical providers, not state legislators, usually define the standard of care for medical professionals. In malpractice cases, medical experts explain the applicable standard of care to the jury and guide its determination of whether, in the particular case, the standard of care was met. In this way, the law has long recognized that the medical profession itself is best suited to establish the appropriate standards of care under any particular set of circumstances. This standard of care is often referred to as the “reasonable professional under the circumstances” standard of care.
Telemedicine standard of care
Despite the fact that the complex and often nebulous concept of standard of care has been traditionally left to the medical experts to define, state legislators and regulators throughout the nation have chosen to weigh in on this issue in the context of telemedicine. Most states with telemedicine regulations have followed the model policy adopted by the Federation of State Medical Boards in April 2014 which states that “[t]reatment and consultation recommendations made in an online setting … will be held to the same standards of appropriate practice as those in traditional (in-person) settings.”2 States that have adopted this model policy have effectively created a “legal fiction” requiring a jury to ignore the fact that the care was provided virtually by telemedicine technologies and instead assume that the physician treated the patient in person, i.e, applying an “in-person” standard of care. Hawaii appears to be the lone notable exception. Its telemedicine law recognizes that an in-person standard of care should not be applied if there was not a face-to-face visit.3
Proponents of the in-person telemedicine standard claim that it is necessary to ensure patient safety, thus justifying the “legal fiction.” Holding the provider to the in-person standard, it is argued, forces the physician to err on the side of caution and require an actual in-person encounter to ensure the advantages of sight, touch, and sense of things are fully available.4 This discourages the use of telemedicine and deprives the population of its many benefits.
Telemedicine can overcome geographical barriers, increase clinical support, improve health outcomes, reduce health care costs, encourage patient input, reduce travel, and foster continuity of care. The pandemic, which has significantly limited the ability of providers to see patients in person, only underscores the benefits of telemedicine.
The legislatively imposed in-person telemedicine standard of care should be replaced with the “reasonable professional under the circumstances” standard in order to fairly judge physicians’ care and promote overall population health. The “reasonable professional under the circumstances” standard has applied to physicians and other health care professionals outside of telemedicine for decades, and it has served the medical community and public well. It is unfortunate that legislators felt the need to weigh in and define a distinctly different standard of care for telemedicine than for the rest of medicine, as this may present unforeseen obstacles to the use of telemedicine.
The in-person telemedicine standard of care remains a significant barrier for long-term telemedicine. Eliminating this legal fiction has the potential to further expand physicians’ use of telemedicine and fulfill its promise of improving access to care and improving population health.
Mr. Horner (partner), Mr. Milewski (partner), and Mr. Gajer (associate) are attorneys with White and Williams. They specialize in defending health care providers in medical malpractice lawsuits and other health care–related matters. Dr. Skolnik is professor of family and community Medicine at the Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Follow Dr. Skolnik, and feel free to submit questions to him on Twitter: @neilskolnik. The authors have no financial conflicts related to the content of this piece.
References
1. Cowan v. Doering, 111 N.J. 451-62,.1988.
2. Model Policy For The Appropriate Use Of Telemedicine Technologies In The Practice Of Medicine. State Medical Boards Appropriate Regulation of Telemedicine. April 2014..
3. Haw. Rev. Stat. Ann. § 453-1.3(c).
4. Kaspar BJ. Iowa Law Review. 2014 Jan;99:839-59.
COVID-19: Medicare data show long hospital stays, disparities
according to a new analysis by the Centers for Medicare & Medicaid Services.
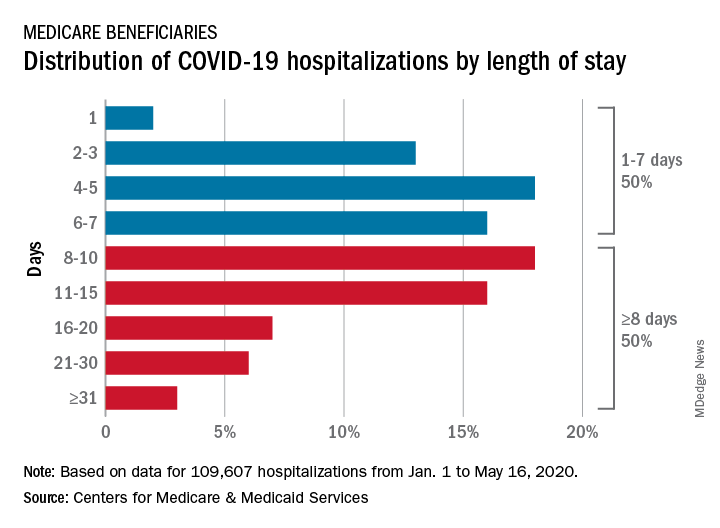
CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
according to a new analysis by the Centers for Medicare & Medicaid Services.

CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
according to a new analysis by the Centers for Medicare & Medicaid Services.

CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
Inside Mercy’s mission to care for non-COVID patients in Los Angeles
When the hospital ship USNS Mercy departed San Diego’s Naval Station North Island on March 23, 2020, to support the Department of Defense efforts in Los Angeles during the coronavirus outbreak, Commander Erin Blevins remembers the crew’s excitement was palpable.
“We normally do partnerships abroad and respond to tsunamis and earthquakes,” said Cdr. Blevins, MD, a pediatric hematologist-oncologist who served as director of medical services for the mission. “This was a slight change in situation, but still disaster relief in the form of a pandemic. We switched our mindset to putting together the best experts for an infectious disease pandemic versus an earthquake disaster relief.”
A new mission
The 1,000-bed Mercy ship – a converted San Clemente–class oil tanker that was delivered in 1986 – spent nearly 50 days pier side in Los Angeles as a referral hospital for non–COVID-19 patients, so that clinicians at Los Angeles area hospitals could care for an anticipated surge of COVID-19 patients. “We went into it with expectations of, ‘We’ll treat as many patients as you need us to take,” Cdr. Blevins recalled. “I don’t even think Los Angeles [health officials] knew exactly where they were going to peak and what the need was going to be.”
Between March 29 and May 15, about 1,071 medical personnel aboard the Mercy cared for 77 patients with an average age of 53 years who were referred from 11 Los Angeles area hospitals. The physicians, nurses, and other medical support personnel were drawn from military treatment facilities across the country. “We had additional people join us as we scoped the mission to be more medically heavy and surgically light,” said Captain John Rotruck, MD, an anesthesiologist who is commanding officer of Mercy’s medical treatment facility. “We did adjust to make sure that we had the right staffing mix to meet the parameters that we were assigned. That was the crux of the change: a change in flavors of staffing to ensure that we focused on ICU and ward medical care as opposed to very heavy surgical care in support of a combat operation.”
About 10% of the team consisted of reservists who volunteered for the mission. “There’s no way you could have walked around the ship and known who was active duty and who was reservist,” said Capt. Rotruck, who was formerly chief of staff at Walter Reed National Military Medical Center, Bethesda, Md. “They worked together so well, and I think that marriage of active duty who are used to working in a military medical treatment facility – in our case, a Navy medical treatment facility – together with our reservist physician colleagues who work in civilian facilities around the country, was beneficial. It was a synergistic relationship. I think both sides walked away learning quite a bit from each other.”
Start with screening
All crew members underwent a temperature check and completed a health screening questionnaire: once before departing their home of record and again before boarding Mercy. Based on those results, crew members and medical staff were screened for COVID-19 and tested as needed in order to minimize the risk of an outbreak aboard the ship.
Fewer than 1% of crew members developed COVID-19 or tested positive for the virus during the mission, according to Capt. Rotruck. Affected individuals were isolated and quarantined. “All staff have recovered and are doing well,” he said.
Mercy personnel worked with local health officials to ensure that all patients transferred to the ship tested negative for COVID-19. Physicians aboard the Mercy then worked directly with the patients’ civilian physician to ensure a safe and thorough turnover process before the patients were transferred.
From basic medical to trauma care
Care aboard the ship, which consists of open-bay medical wards, ranged from basic medical and surgical care to critical care and trauma. The most common procedures were cholecystectomies and orthopedic procedures, and the average length of stay was 4-5 days, according to Cdr. Blevins. Over the course of the mission, the medical professionals conducted 36 surgeries, 77 x-ray exams, 26 CT scans, and administered hundreds of ancillary studies ranging from routine labs to high-end x-rays and blood transfusion support.
“Within our ICU, we did have some end-of-life patients who ended up dying on our ship in comfort care,” Cdr. Blevins said. “Fortunately, we had a wonderful ICU team who had a great deal of experience with end-of-life care and were able to take care of these patients very comfortably and ensure good communication with family and loved ones during that time. In most instances we tried to make sure that people got to FaceTime or video chat with their loved one before they passed away.”
The Mercy, which includes 12 operating rooms, four x-ray units, and one CAT-scan unit, was not equipped to deliver pediatric or obstetrical care. Other unavailable services included psychiatry, oncology, cardiac and thoracic surgery, nuclear medicine, MRI, mammography, electrophysiology, cardiac catheterization, negative-pressure isolation, speech therapy, and occupational therapy.
Not your typical hospital experience
But for patients who did receive medical care aboard the Mercy – which made three 150-day deployments in recent years for the military-led humanitarian response known as Pacific Partnership in 2015, 2016, and 2018 – it was an experience that they are unlikely to forget.
“Every time a patient left the ship, our team on the ground surveyed them to see how their experience was and see what we could do to improve,” Cdr. Blevins said. “Across the board, they were all very appreciative of the medical care. We had a couple of veterans on board. They got [USNS Mercy] hats on their way out and seemed to very much enjoy a slightly different experience than they would get at a regular hospital.”
Capt. Rotruck added that the enthusiasm crew members had for supporting fellow Americans “really energized our team and really saturated that caring aspect of the people who interacted directly with patients,” he said. “It wasn’t just the physicians and nurses, but it was the staff delivering the food and coming to take blood samples and every other interaction that the patients had with our team. I think they really felt that enthusiasm for being there and supporting our neighbors in LA [Los Angeles].”
Crew life aboard the Mercy
Just as with any hospital on shore, personnel aboard the Mercy practiced preventive hygiene measures recommended by the Centers for Disease Control and Prevention to help prevent the spread of COVID-19, such as wearing cloth face masks, spacing out tables in the dining hall, closing indoor gyms, and devising creative ways to stay physically fit. Popular options included jogging around the perimeter of the ship and practicing yoga and calisthenics on the deck, “making sure you were physically distanced appropriately, and when you were done, putting your mask back on,” Cdr. Blevins said. Others supplemented their workouts with a pull-up bar on the deck. “In addition, we have a series of ramps that run on the starboard side of the ship that we can use for patient movement with litters on wheels or patient beds,” Capt. Rotruck said. “The uphill portion of those ramps represents a good workout opportunity as well.”
Downtime in an era of physical distancing also afforded crew members the opportunity to call or FaceTime with loved ones, watch streamed TV shows and movies, and work on their own professional development. Some continued with coursework for online degree programs offered by colleges and universities they were enrolled in, while some enlisted personnel used the time to complete the Navy Enlisted Warfare Qualification Programs Instruction, which issues the basic overarching requirements for the qualification and designation of all enlisted warfare programs.
“As you can imagine, people spend a lot of time learning how the ship works and how it integrates into larger naval forces and so forth,” Capt. Rotruck said. “Not just our ship but also other ships: their weapons systems and defense mechanisms and navigation systems. We had people spending a significant amount of time working on that. We had people complete their Enlisted Surface Warfare qualification while we were on the mission.”
End of the mission
Mercy returned to its home base in San Diego on May 15, but about 60 medical personnel stayed behind in Los Angeles to support Federal Emergency Management Agency (FEMA), state, and local health care professionals. Some worked at a site where clinicians provided care for COVID-19–positive patients who had been transferred from area skilled nursing facilities.
In addition, a team consisting of one nurse and five corpsmen “would go out to individual skilled nursing facilities and mainly conduct assessments and training, such as training in donning proper PPE [personal protective equipment] and determining what needs they had,” Capt. Rotruck said. “They met those needs if possible or [communicated with California officials] and let them know what the requirements were and what the needs were in that facility.” The assignment for those who stayed behind ended on May 31.
On the opposite coast, Mercy’s sister ship, USNS Comfort, arrived in New York Harbor from Norfolk, Va., on March 30 and spent 3½ weeks assisting area hospitals in the COVID-19 pandemic fight. A few days into the mission, Comfort’s internal spaces were reconfigured to create separate COVID-negative and COVID-positive sections. Medical teams aboard the ship cared for a total of 182 patients during the assignment.
Looking back on Mercy’s mission, Cdr. Blevins marveled at the sense of teamwork that unfolded. “We have quarterly training exercises with a core set of personnel, [and] we train getting ready for activation in 5 days,” she said. “All of that training kicks in and it comes to fruition in a mission like this. It was terrific to see a group of very disparate subject matter experts from all over the country come together with one purpose: which was to serve our own country during the pandemic.”
Capt. Rotruck pointed out that the experience enabled enlisted and nonenlisted physicians to maintain their skill sets during a time when military and civilian hospitals had stopped doing elective procedures and routine appointments. “The fact that those people were able to come on board the ship and continue to conduct their medical practice and maintain their skills and competencies in an environment that they weren’t quite used to is great,” he said. “Otherwise, some of those medical personnel would have been sitting idle, wherever they were from. This is the power of Navy medicine on behalf of our country.”
When the hospital ship USNS Mercy departed San Diego’s Naval Station North Island on March 23, 2020, to support the Department of Defense efforts in Los Angeles during the coronavirus outbreak, Commander Erin Blevins remembers the crew’s excitement was palpable.
“We normally do partnerships abroad and respond to tsunamis and earthquakes,” said Cdr. Blevins, MD, a pediatric hematologist-oncologist who served as director of medical services for the mission. “This was a slight change in situation, but still disaster relief in the form of a pandemic. We switched our mindset to putting together the best experts for an infectious disease pandemic versus an earthquake disaster relief.”
A new mission

The 1,000-bed Mercy ship – a converted San Clemente–class oil tanker that was delivered in 1986 – spent nearly 50 days pier side in Los Angeles as a referral hospital for non–COVID-19 patients, so that clinicians at Los Angeles area hospitals could care for an anticipated surge of COVID-19 patients. “We went into it with expectations of, ‘We’ll treat as many patients as you need us to take,” Cdr. Blevins recalled. “I don’t even think Los Angeles [health officials] knew exactly where they were going to peak and what the need was going to be.”
Between March 29 and May 15, about 1,071 medical personnel aboard the Mercy cared for 77 patients with an average age of 53 years who were referred from 11 Los Angeles area hospitals. The physicians, nurses, and other medical support personnel were drawn from military treatment facilities across the country. “We had additional people join us as we scoped the mission to be more medically heavy and surgically light,” said Captain John Rotruck, MD, an anesthesiologist who is commanding officer of Mercy’s medical treatment facility. “We did adjust to make sure that we had the right staffing mix to meet the parameters that we were assigned. That was the crux of the change: a change in flavors of staffing to ensure that we focused on ICU and ward medical care as opposed to very heavy surgical care in support of a combat operation.”
About 10% of the team consisted of reservists who volunteered for the mission. “There’s no way you could have walked around the ship and known who was active duty and who was reservist,” said Capt. Rotruck, who was formerly chief of staff at Walter Reed National Military Medical Center, Bethesda, Md. “They worked together so well, and I think that marriage of active duty who are used to working in a military medical treatment facility – in our case, a Navy medical treatment facility – together with our reservist physician colleagues who work in civilian facilities around the country, was beneficial. It was a synergistic relationship. I think both sides walked away learning quite a bit from each other.”
Start with screening
All crew members underwent a temperature check and completed a health screening questionnaire: once before departing their home of record and again before boarding Mercy. Based on those results, crew members and medical staff were screened for COVID-19 and tested as needed in order to minimize the risk of an outbreak aboard the ship.
Fewer than 1% of crew members developed COVID-19 or tested positive for the virus during the mission, according to Capt. Rotruck. Affected individuals were isolated and quarantined. “All staff have recovered and are doing well,” he said.
Mercy personnel worked with local health officials to ensure that all patients transferred to the ship tested negative for COVID-19. Physicians aboard the Mercy then worked directly with the patients’ civilian physician to ensure a safe and thorough turnover process before the patients were transferred.
From basic medical to trauma care

Care aboard the ship, which consists of open-bay medical wards, ranged from basic medical and surgical care to critical care and trauma. The most common procedures were cholecystectomies and orthopedic procedures, and the average length of stay was 4-5 days, according to Cdr. Blevins. Over the course of the mission, the medical professionals conducted 36 surgeries, 77 x-ray exams, 26 CT scans, and administered hundreds of ancillary studies ranging from routine labs to high-end x-rays and blood transfusion support.
“Within our ICU, we did have some end-of-life patients who ended up dying on our ship in comfort care,” Cdr. Blevins said. “Fortunately, we had a wonderful ICU team who had a great deal of experience with end-of-life care and were able to take care of these patients very comfortably and ensure good communication with family and loved ones during that time. In most instances we tried to make sure that people got to FaceTime or video chat with their loved one before they passed away.”

The Mercy, which includes 12 operating rooms, four x-ray units, and one CAT-scan unit, was not equipped to deliver pediatric or obstetrical care. Other unavailable services included psychiatry, oncology, cardiac and thoracic surgery, nuclear medicine, MRI, mammography, electrophysiology, cardiac catheterization, negative-pressure isolation, speech therapy, and occupational therapy.
Not your typical hospital experience
But for patients who did receive medical care aboard the Mercy – which made three 150-day deployments in recent years for the military-led humanitarian response known as Pacific Partnership in 2015, 2016, and 2018 – it was an experience that they are unlikely to forget.
“Every time a patient left the ship, our team on the ground surveyed them to see how their experience was and see what we could do to improve,” Cdr. Blevins said. “Across the board, they were all very appreciative of the medical care. We had a couple of veterans on board. They got [USNS Mercy] hats on their way out and seemed to very much enjoy a slightly different experience than they would get at a regular hospital.”
Capt. Rotruck added that the enthusiasm crew members had for supporting fellow Americans “really energized our team and really saturated that caring aspect of the people who interacted directly with patients,” he said. “It wasn’t just the physicians and nurses, but it was the staff delivering the food and coming to take blood samples and every other interaction that the patients had with our team. I think they really felt that enthusiasm for being there and supporting our neighbors in LA [Los Angeles].”
Crew life aboard the Mercy
Just as with any hospital on shore, personnel aboard the Mercy practiced preventive hygiene measures recommended by the Centers for Disease Control and Prevention to help prevent the spread of COVID-19, such as wearing cloth face masks, spacing out tables in the dining hall, closing indoor gyms, and devising creative ways to stay physically fit. Popular options included jogging around the perimeter of the ship and practicing yoga and calisthenics on the deck, “making sure you were physically distanced appropriately, and when you were done, putting your mask back on,” Cdr. Blevins said. Others supplemented their workouts with a pull-up bar on the deck. “In addition, we have a series of ramps that run on the starboard side of the ship that we can use for patient movement with litters on wheels or patient beds,” Capt. Rotruck said. “The uphill portion of those ramps represents a good workout opportunity as well.”
Downtime in an era of physical distancing also afforded crew members the opportunity to call or FaceTime with loved ones, watch streamed TV shows and movies, and work on their own professional development. Some continued with coursework for online degree programs offered by colleges and universities they were enrolled in, while some enlisted personnel used the time to complete the Navy Enlisted Warfare Qualification Programs Instruction, which issues the basic overarching requirements for the qualification and designation of all enlisted warfare programs.
“As you can imagine, people spend a lot of time learning how the ship works and how it integrates into larger naval forces and so forth,” Capt. Rotruck said. “Not just our ship but also other ships: their weapons systems and defense mechanisms and navigation systems. We had people spending a significant amount of time working on that. We had people complete their Enlisted Surface Warfare qualification while we were on the mission.”
End of the mission
Mercy returned to its home base in San Diego on May 15, but about 60 medical personnel stayed behind in Los Angeles to support Federal Emergency Management Agency (FEMA), state, and local health care professionals. Some worked at a site where clinicians provided care for COVID-19–positive patients who had been transferred from area skilled nursing facilities.
In addition, a team consisting of one nurse and five corpsmen “would go out to individual skilled nursing facilities and mainly conduct assessments and training, such as training in donning proper PPE [personal protective equipment] and determining what needs they had,” Capt. Rotruck said. “They met those needs if possible or [communicated with California officials] and let them know what the requirements were and what the needs were in that facility.” The assignment for those who stayed behind ended on May 31.
On the opposite coast, Mercy’s sister ship, USNS Comfort, arrived in New York Harbor from Norfolk, Va., on March 30 and spent 3½ weeks assisting area hospitals in the COVID-19 pandemic fight. A few days into the mission, Comfort’s internal spaces were reconfigured to create separate COVID-negative and COVID-positive sections. Medical teams aboard the ship cared for a total of 182 patients during the assignment.
Looking back on Mercy’s mission, Cdr. Blevins marveled at the sense of teamwork that unfolded. “We have quarterly training exercises with a core set of personnel, [and] we train getting ready for activation in 5 days,” she said. “All of that training kicks in and it comes to fruition in a mission like this. It was terrific to see a group of very disparate subject matter experts from all over the country come together with one purpose: which was to serve our own country during the pandemic.”
Capt. Rotruck pointed out that the experience enabled enlisted and nonenlisted physicians to maintain their skill sets during a time when military and civilian hospitals had stopped doing elective procedures and routine appointments. “The fact that those people were able to come on board the ship and continue to conduct their medical practice and maintain their skills and competencies in an environment that they weren’t quite used to is great,” he said. “Otherwise, some of those medical personnel would have been sitting idle, wherever they were from. This is the power of Navy medicine on behalf of our country.”
When the hospital ship USNS Mercy departed San Diego’s Naval Station North Island on March 23, 2020, to support the Department of Defense efforts in Los Angeles during the coronavirus outbreak, Commander Erin Blevins remembers the crew’s excitement was palpable.
“We normally do partnerships abroad and respond to tsunamis and earthquakes,” said Cdr. Blevins, MD, a pediatric hematologist-oncologist who served as director of medical services for the mission. “This was a slight change in situation, but still disaster relief in the form of a pandemic. We switched our mindset to putting together the best experts for an infectious disease pandemic versus an earthquake disaster relief.”
A new mission

The 1,000-bed Mercy ship – a converted San Clemente–class oil tanker that was delivered in 1986 – spent nearly 50 days pier side in Los Angeles as a referral hospital for non–COVID-19 patients, so that clinicians at Los Angeles area hospitals could care for an anticipated surge of COVID-19 patients. “We went into it with expectations of, ‘We’ll treat as many patients as you need us to take,” Cdr. Blevins recalled. “I don’t even think Los Angeles [health officials] knew exactly where they were going to peak and what the need was going to be.”
Between March 29 and May 15, about 1,071 medical personnel aboard the Mercy cared for 77 patients with an average age of 53 years who were referred from 11 Los Angeles area hospitals. The physicians, nurses, and other medical support personnel were drawn from military treatment facilities across the country. “We had additional people join us as we scoped the mission to be more medically heavy and surgically light,” said Captain John Rotruck, MD, an anesthesiologist who is commanding officer of Mercy’s medical treatment facility. “We did adjust to make sure that we had the right staffing mix to meet the parameters that we were assigned. That was the crux of the change: a change in flavors of staffing to ensure that we focused on ICU and ward medical care as opposed to very heavy surgical care in support of a combat operation.”
About 10% of the team consisted of reservists who volunteered for the mission. “There’s no way you could have walked around the ship and known who was active duty and who was reservist,” said Capt. Rotruck, who was formerly chief of staff at Walter Reed National Military Medical Center, Bethesda, Md. “They worked together so well, and I think that marriage of active duty who are used to working in a military medical treatment facility – in our case, a Navy medical treatment facility – together with our reservist physician colleagues who work in civilian facilities around the country, was beneficial. It was a synergistic relationship. I think both sides walked away learning quite a bit from each other.”
Start with screening
All crew members underwent a temperature check and completed a health screening questionnaire: once before departing their home of record and again before boarding Mercy. Based on those results, crew members and medical staff were screened for COVID-19 and tested as needed in order to minimize the risk of an outbreak aboard the ship.
Fewer than 1% of crew members developed COVID-19 or tested positive for the virus during the mission, according to Capt. Rotruck. Affected individuals were isolated and quarantined. “All staff have recovered and are doing well,” he said.
Mercy personnel worked with local health officials to ensure that all patients transferred to the ship tested negative for COVID-19. Physicians aboard the Mercy then worked directly with the patients’ civilian physician to ensure a safe and thorough turnover process before the patients were transferred.
From basic medical to trauma care

Care aboard the ship, which consists of open-bay medical wards, ranged from basic medical and surgical care to critical care and trauma. The most common procedures were cholecystectomies and orthopedic procedures, and the average length of stay was 4-5 days, according to Cdr. Blevins. Over the course of the mission, the medical professionals conducted 36 surgeries, 77 x-ray exams, 26 CT scans, and administered hundreds of ancillary studies ranging from routine labs to high-end x-rays and blood transfusion support.
“Within our ICU, we did have some end-of-life patients who ended up dying on our ship in comfort care,” Cdr. Blevins said. “Fortunately, we had a wonderful ICU team who had a great deal of experience with end-of-life care and were able to take care of these patients very comfortably and ensure good communication with family and loved ones during that time. In most instances we tried to make sure that people got to FaceTime or video chat with their loved one before they passed away.”

The Mercy, which includes 12 operating rooms, four x-ray units, and one CAT-scan unit, was not equipped to deliver pediatric or obstetrical care. Other unavailable services included psychiatry, oncology, cardiac and thoracic surgery, nuclear medicine, MRI, mammography, electrophysiology, cardiac catheterization, negative-pressure isolation, speech therapy, and occupational therapy.
Not your typical hospital experience
But for patients who did receive medical care aboard the Mercy – which made three 150-day deployments in recent years for the military-led humanitarian response known as Pacific Partnership in 2015, 2016, and 2018 – it was an experience that they are unlikely to forget.
“Every time a patient left the ship, our team on the ground surveyed them to see how their experience was and see what we could do to improve,” Cdr. Blevins said. “Across the board, they were all very appreciative of the medical care. We had a couple of veterans on board. They got [USNS Mercy] hats on their way out and seemed to very much enjoy a slightly different experience than they would get at a regular hospital.”
Capt. Rotruck added that the enthusiasm crew members had for supporting fellow Americans “really energized our team and really saturated that caring aspect of the people who interacted directly with patients,” he said. “It wasn’t just the physicians and nurses, but it was the staff delivering the food and coming to take blood samples and every other interaction that the patients had with our team. I think they really felt that enthusiasm for being there and supporting our neighbors in LA [Los Angeles].”
Crew life aboard the Mercy
Just as with any hospital on shore, personnel aboard the Mercy practiced preventive hygiene measures recommended by the Centers for Disease Control and Prevention to help prevent the spread of COVID-19, such as wearing cloth face masks, spacing out tables in the dining hall, closing indoor gyms, and devising creative ways to stay physically fit. Popular options included jogging around the perimeter of the ship and practicing yoga and calisthenics on the deck, “making sure you were physically distanced appropriately, and when you were done, putting your mask back on,” Cdr. Blevins said. Others supplemented their workouts with a pull-up bar on the deck. “In addition, we have a series of ramps that run on the starboard side of the ship that we can use for patient movement with litters on wheels or patient beds,” Capt. Rotruck said. “The uphill portion of those ramps represents a good workout opportunity as well.”
Downtime in an era of physical distancing also afforded crew members the opportunity to call or FaceTime with loved ones, watch streamed TV shows and movies, and work on their own professional development. Some continued with coursework for online degree programs offered by colleges and universities they were enrolled in, while some enlisted personnel used the time to complete the Navy Enlisted Warfare Qualification Programs Instruction, which issues the basic overarching requirements for the qualification and designation of all enlisted warfare programs.
“As you can imagine, people spend a lot of time learning how the ship works and how it integrates into larger naval forces and so forth,” Capt. Rotruck said. “Not just our ship but also other ships: their weapons systems and defense mechanisms and navigation systems. We had people spending a significant amount of time working on that. We had people complete their Enlisted Surface Warfare qualification while we were on the mission.”
End of the mission
Mercy returned to its home base in San Diego on May 15, but about 60 medical personnel stayed behind in Los Angeles to support Federal Emergency Management Agency (FEMA), state, and local health care professionals. Some worked at a site where clinicians provided care for COVID-19–positive patients who had been transferred from area skilled nursing facilities.
In addition, a team consisting of one nurse and five corpsmen “would go out to individual skilled nursing facilities and mainly conduct assessments and training, such as training in donning proper PPE [personal protective equipment] and determining what needs they had,” Capt. Rotruck said. “They met those needs if possible or [communicated with California officials] and let them know what the requirements were and what the needs were in that facility.” The assignment for those who stayed behind ended on May 31.
On the opposite coast, Mercy’s sister ship, USNS Comfort, arrived in New York Harbor from Norfolk, Va., on March 30 and spent 3½ weeks assisting area hospitals in the COVID-19 pandemic fight. A few days into the mission, Comfort’s internal spaces were reconfigured to create separate COVID-negative and COVID-positive sections. Medical teams aboard the ship cared for a total of 182 patients during the assignment.
Looking back on Mercy’s mission, Cdr. Blevins marveled at the sense of teamwork that unfolded. “We have quarterly training exercises with a core set of personnel, [and] we train getting ready for activation in 5 days,” she said. “All of that training kicks in and it comes to fruition in a mission like this. It was terrific to see a group of very disparate subject matter experts from all over the country come together with one purpose: which was to serve our own country during the pandemic.”
Capt. Rotruck pointed out that the experience enabled enlisted and nonenlisted physicians to maintain their skill sets during a time when military and civilian hospitals had stopped doing elective procedures and routine appointments. “The fact that those people were able to come on board the ship and continue to conduct their medical practice and maintain their skills and competencies in an environment that they weren’t quite used to is great,” he said. “Otherwise, some of those medical personnel would have been sitting idle, wherever they were from. This is the power of Navy medicine on behalf of our country.”
Guidance on infection prevention for health care personnel
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.


















