User login
News and Views that Matter to Physicians
Why is the mental health burden in EDs rising?
The mounting impact of mental illness on patients and the American health care system has been of growing concern, especially in recent years. As such, now more than ever, it is important to understand the mental health burden and investigate the factors contributing to the elevated use of emergency departments to treat patients with psychiatric illness.
In recent years, the overall prevalence of mental illness has not changed drastically. According to the 2014 National Survey of Drug Use and Health, 18.1% of adults indicated having “any mental illness,” a prevalence that had not changed much since 2008.1 It is possible, however, that despite the relative stability in the prevalence of mental illness, the acuity of mental illness may be on the rise. For instance, 4.1% of adults indicated having a “serious mental illness” (SMI) in 2014, a prevalence that was 0.4% higher than that of 2008 and 2009.1 Also, of note, the prevalence of SMI among the 18-to-25-year-old population in 2014 had increased in previous years.1 Meanwhile, 6.6% of adults indicated having experienced a major depressive episode at least once in the preceding 12 months. That prevalence has held relatively steady over recent years.1
Despite the rising need for mental health services, the number of inpatient psychiatric beds has declined. During the 32 years between 1970 and 2002, the United States experienced a staggering nearly 60% decline in the number of inpatient psychiatric beds.4 Moreover, the number of psychiatric beds within the national public sector fell from 50,509 in 2005 to 43,318 in 2010, which is about a 14% decline.5 This decrease translated to a decrease from 17.1 beds/100,000 people in 2005 to 14.1 beds/100,000 in 2010 – both of which fall drastically below the “minimum number of public psychiatric beds deemed necessary for adequate psychiatric services (50/100,000).”5 Similarly, psychiatric practice has been unable to keep up with the increasing population size – the population-adjusted median number of psychiatrists declined 10.2% between 2003 and 2013.6
While inpatient psychiatric beds and psychiatrist availability have declined, the frequency of ED use for mental health reasons has increased. Mental health or substance abuse diagnoses directly accounted for 4.3% of ED visits in 2007 and were associated with 12.5% of ED visits.7 Specifically, there was a 19.3% increase in the rate of nonmaternal treat-and-release ED visits for mental health reasons between 2008-2012.8 Moreover, in a study assessing frequent treat-and-release ED visits among Medicaid patients, investigators found that while most ED visits were for non–mental health purposes, the odds of frequent ED use were higher among patients with either a psychiatric disorder or substance use problem across all levels of overall health complexity.9
What factors have been driving adults to increasingly rely on ED visits for their mental health care? Given the immense complexity of the U.S. mental health delivery system, it is evident that there is no clear-cut explanation. However, several specific factors may have contributed and must be investigated to better our understanding of this public health conundrum. The opioid epidemic, transition out of the correctional system, and coverage changes under the Affordable Care Act are hypotheses that will be examined further in the context of this pressing issue.
References
1. “Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health.”
2. “Increase in Suicide in the United States, 1999-2014.” NCHS Data Brief No. 241, April 2016.
3. Web-Based Injury Statistics Query and Reporting System (WISQARS), Centers for Disease Control and Prevention.
4. National Health Policy Forum Issue Brief (2007 Aug 1;[823]:1-21).
5. “No Room at the Inn: Trends and Consequences of Closing Public Psychiatric Hospitals, 2005-2010,” Arlington, Va.: Treatment Advocacy Center, July 19, 2012.
6. “Population of U.S. Practicing Psychiatrists Declined, 2003-13, Which May Help Explain Poor Access to Mental Health Care,” Health Aff (Millwood). 2016 Jul 1;35[7]:1271-7.
7. “Mental Health and Substance Abuse-Related Emergency Department Visits Among Adults, 2007: Statistical Brief #92,” in Healthcare Cost and Utilization Project Statistical Briefs, (Rockville, Md.: Agency for Healthcare Research and Quality, 2010).
8. “Trends in Potentially Preventable Inpatient Hospital Admissions and Emergency Department Visits, 2015: Statistical Brief #195,” in Healthcare Cost and Utilization Project Statistical Briefs, (Rockville, Md.: Agency for Healthcare Research and Quality).
9. Nurs Res. 2015 Jan-Feb;64[1]3-12.
10. Ann Emerg Med. 2016 Apr;67[4]:525-30.
Ms. Kablanian is a 2nd-year medical student at the George Washington University, Washington, where she is enrolled in the Community and Urban Health Scholarly Concentration Program. Before attending medical school, she earned a master of public health degree in epidemiology from Columbia University, New York. She also holds a bachelor’s degree in biology and French from Scripps College, Claremont, Calif. Her interests include advocating for the urban underserved, contributing to medical curriculum development, and investigating population-level contributors to adverse health outcomes. Dr. Norris is assistant professor in the department of psychiatry & behavioral sciences, and assistant dean of student affairs at the George Washington University. He also is medical director of psychiatric & behavioral sciences at George Washington University Hospital. As part of his commitment to providing mental health care to patients with severe medical illness, Dr. Norris has been a leading voice within the psychiatric community on the value of palliative psychotherapy.
The mounting impact of mental illness on patients and the American health care system has been of growing concern, especially in recent years. As such, now more than ever, it is important to understand the mental health burden and investigate the factors contributing to the elevated use of emergency departments to treat patients with psychiatric illness.
In recent years, the overall prevalence of mental illness has not changed drastically. According to the 2014 National Survey of Drug Use and Health, 18.1% of adults indicated having “any mental illness,” a prevalence that had not changed much since 2008.1 It is possible, however, that despite the relative stability in the prevalence of mental illness, the acuity of mental illness may be on the rise. For instance, 4.1% of adults indicated having a “serious mental illness” (SMI) in 2014, a prevalence that was 0.4% higher than that of 2008 and 2009.1 Also, of note, the prevalence of SMI among the 18-to-25-year-old population in 2014 had increased in previous years.1 Meanwhile, 6.6% of adults indicated having experienced a major depressive episode at least once in the preceding 12 months. That prevalence has held relatively steady over recent years.1
Despite the rising need for mental health services, the number of inpatient psychiatric beds has declined. During the 32 years between 1970 and 2002, the United States experienced a staggering nearly 60% decline in the number of inpatient psychiatric beds.4 Moreover, the number of psychiatric beds within the national public sector fell from 50,509 in 2005 to 43,318 in 2010, which is about a 14% decline.5 This decrease translated to a decrease from 17.1 beds/100,000 people in 2005 to 14.1 beds/100,000 in 2010 – both of which fall drastically below the “minimum number of public psychiatric beds deemed necessary for adequate psychiatric services (50/100,000).”5 Similarly, psychiatric practice has been unable to keep up with the increasing population size – the population-adjusted median number of psychiatrists declined 10.2% between 2003 and 2013.6
While inpatient psychiatric beds and psychiatrist availability have declined, the frequency of ED use for mental health reasons has increased. Mental health or substance abuse diagnoses directly accounted for 4.3% of ED visits in 2007 and were associated with 12.5% of ED visits.7 Specifically, there was a 19.3% increase in the rate of nonmaternal treat-and-release ED visits for mental health reasons between 2008-2012.8 Moreover, in a study assessing frequent treat-and-release ED visits among Medicaid patients, investigators found that while most ED visits were for non–mental health purposes, the odds of frequent ED use were higher among patients with either a psychiatric disorder or substance use problem across all levels of overall health complexity.9
What factors have been driving adults to increasingly rely on ED visits for their mental health care? Given the immense complexity of the U.S. mental health delivery system, it is evident that there is no clear-cut explanation. However, several specific factors may have contributed and must be investigated to better our understanding of this public health conundrum. The opioid epidemic, transition out of the correctional system, and coverage changes under the Affordable Care Act are hypotheses that will be examined further in the context of this pressing issue.
References
1. “Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health.”
2. “Increase in Suicide in the United States, 1999-2014.” NCHS Data Brief No. 241, April 2016.
3. Web-Based Injury Statistics Query and Reporting System (WISQARS), Centers for Disease Control and Prevention.
4. National Health Policy Forum Issue Brief (2007 Aug 1;[823]:1-21).
5. “No Room at the Inn: Trends and Consequences of Closing Public Psychiatric Hospitals, 2005-2010,” Arlington, Va.: Treatment Advocacy Center, July 19, 2012.
6. “Population of U.S. Practicing Psychiatrists Declined, 2003-13, Which May Help Explain Poor Access to Mental Health Care,” Health Aff (Millwood). 2016 Jul 1;35[7]:1271-7.
7. “Mental Health and Substance Abuse-Related Emergency Department Visits Among Adults, 2007: Statistical Brief #92,” in Healthcare Cost and Utilization Project Statistical Briefs, (Rockville, Md.: Agency for Healthcare Research and Quality, 2010).
8. “Trends in Potentially Preventable Inpatient Hospital Admissions and Emergency Department Visits, 2015: Statistical Brief #195,” in Healthcare Cost and Utilization Project Statistical Briefs, (Rockville, Md.: Agency for Healthcare Research and Quality).
9. Nurs Res. 2015 Jan-Feb;64[1]3-12.
10. Ann Emerg Med. 2016 Apr;67[4]:525-30.
Ms. Kablanian is a 2nd-year medical student at the George Washington University, Washington, where she is enrolled in the Community and Urban Health Scholarly Concentration Program. Before attending medical school, she earned a master of public health degree in epidemiology from Columbia University, New York. She also holds a bachelor’s degree in biology and French from Scripps College, Claremont, Calif. Her interests include advocating for the urban underserved, contributing to medical curriculum development, and investigating population-level contributors to adverse health outcomes. Dr. Norris is assistant professor in the department of psychiatry & behavioral sciences, and assistant dean of student affairs at the George Washington University. He also is medical director of psychiatric & behavioral sciences at George Washington University Hospital. As part of his commitment to providing mental health care to patients with severe medical illness, Dr. Norris has been a leading voice within the psychiatric community on the value of palliative psychotherapy.
The mounting impact of mental illness on patients and the American health care system has been of growing concern, especially in recent years. As such, now more than ever, it is important to understand the mental health burden and investigate the factors contributing to the elevated use of emergency departments to treat patients with psychiatric illness.
In recent years, the overall prevalence of mental illness has not changed drastically. According to the 2014 National Survey of Drug Use and Health, 18.1% of adults indicated having “any mental illness,” a prevalence that had not changed much since 2008.1 It is possible, however, that despite the relative stability in the prevalence of mental illness, the acuity of mental illness may be on the rise. For instance, 4.1% of adults indicated having a “serious mental illness” (SMI) in 2014, a prevalence that was 0.4% higher than that of 2008 and 2009.1 Also, of note, the prevalence of SMI among the 18-to-25-year-old population in 2014 had increased in previous years.1 Meanwhile, 6.6% of adults indicated having experienced a major depressive episode at least once in the preceding 12 months. That prevalence has held relatively steady over recent years.1
Despite the rising need for mental health services, the number of inpatient psychiatric beds has declined. During the 32 years between 1970 and 2002, the United States experienced a staggering nearly 60% decline in the number of inpatient psychiatric beds.4 Moreover, the number of psychiatric beds within the national public sector fell from 50,509 in 2005 to 43,318 in 2010, which is about a 14% decline.5 This decrease translated to a decrease from 17.1 beds/100,000 people in 2005 to 14.1 beds/100,000 in 2010 – both of which fall drastically below the “minimum number of public psychiatric beds deemed necessary for adequate psychiatric services (50/100,000).”5 Similarly, psychiatric practice has been unable to keep up with the increasing population size – the population-adjusted median number of psychiatrists declined 10.2% between 2003 and 2013.6
While inpatient psychiatric beds and psychiatrist availability have declined, the frequency of ED use for mental health reasons has increased. Mental health or substance abuse diagnoses directly accounted for 4.3% of ED visits in 2007 and were associated with 12.5% of ED visits.7 Specifically, there was a 19.3% increase in the rate of nonmaternal treat-and-release ED visits for mental health reasons between 2008-2012.8 Moreover, in a study assessing frequent treat-and-release ED visits among Medicaid patients, investigators found that while most ED visits were for non–mental health purposes, the odds of frequent ED use were higher among patients with either a psychiatric disorder or substance use problem across all levels of overall health complexity.9
What factors have been driving adults to increasingly rely on ED visits for their mental health care? Given the immense complexity of the U.S. mental health delivery system, it is evident that there is no clear-cut explanation. However, several specific factors may have contributed and must be investigated to better our understanding of this public health conundrum. The opioid epidemic, transition out of the correctional system, and coverage changes under the Affordable Care Act are hypotheses that will be examined further in the context of this pressing issue.
References
1. “Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health.”
2. “Increase in Suicide in the United States, 1999-2014.” NCHS Data Brief No. 241, April 2016.
3. Web-Based Injury Statistics Query and Reporting System (WISQARS), Centers for Disease Control and Prevention.
4. National Health Policy Forum Issue Brief (2007 Aug 1;[823]:1-21).
5. “No Room at the Inn: Trends and Consequences of Closing Public Psychiatric Hospitals, 2005-2010,” Arlington, Va.: Treatment Advocacy Center, July 19, 2012.
6. “Population of U.S. Practicing Psychiatrists Declined, 2003-13, Which May Help Explain Poor Access to Mental Health Care,” Health Aff (Millwood). 2016 Jul 1;35[7]:1271-7.
7. “Mental Health and Substance Abuse-Related Emergency Department Visits Among Adults, 2007: Statistical Brief #92,” in Healthcare Cost and Utilization Project Statistical Briefs, (Rockville, Md.: Agency for Healthcare Research and Quality, 2010).
8. “Trends in Potentially Preventable Inpatient Hospital Admissions and Emergency Department Visits, 2015: Statistical Brief #195,” in Healthcare Cost and Utilization Project Statistical Briefs, (Rockville, Md.: Agency for Healthcare Research and Quality).
9. Nurs Res. 2015 Jan-Feb;64[1]3-12.
10. Ann Emerg Med. 2016 Apr;67[4]:525-30.
Ms. Kablanian is a 2nd-year medical student at the George Washington University, Washington, where she is enrolled in the Community and Urban Health Scholarly Concentration Program. Before attending medical school, she earned a master of public health degree in epidemiology from Columbia University, New York. She also holds a bachelor’s degree in biology and French from Scripps College, Claremont, Calif. Her interests include advocating for the urban underserved, contributing to medical curriculum development, and investigating population-level contributors to adverse health outcomes. Dr. Norris is assistant professor in the department of psychiatry & behavioral sciences, and assistant dean of student affairs at the George Washington University. He also is medical director of psychiatric & behavioral sciences at George Washington University Hospital. As part of his commitment to providing mental health care to patients with severe medical illness, Dr. Norris has been a leading voice within the psychiatric community on the value of palliative psychotherapy.
Home oxygen upped survival in PAH with severely impaired DLCO
LOS ANGELES – Pulmonary arterial hypertension (PAH) patients with severely impaired diffusing capacity of the lung for carbon monoxide (DLCO) were much more likely to survive when they received home oxygen therapy, according to a disease registry analysis.
“We all know that supplemental oxygen is widely used with PAH,” said Harrison W. Farber, MD, director of the pulmonary hypertension center at Boston University. But there are practically no data showing that it is successful, and there are even fewer data for patients with PAH who have very low diffusion capacity, he added.
That knowledge gap prompted Dr. Farber and his colleagues to analyze data from the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL), the largest disease registry in the world of patients with PAH.
“Patients in that group – the severe DLCO group – who got oxygen had poorer prognostic features but improved overall survival relative to those who didn’t,” Dr. Farber explained during a presentation at the annual meeting of the American College of Chest Physicians. “Based on this, it makes us think that home oxygen, supplemental oxygen treatment, is associated with improved survival in patients, especially those with severe DLCO and PAH.”
The 3,046 patients analyzed by Dr. Farber and his colleagues had World Health Organization Group 1 PAH with right heart catheterization hemodynamic criteria: a mean pulmonary artery pressure greater than 25 mm Hg, a pulmonary capillary wedge pressure less than or equal to 15 mm Hg, and a pulmonary vascular resistance of at least 3 Wood units (WU). Patients were at least 18 years of age and grouped by oxygen use, which was defined as any use at any time from study enrollment to the end of follow-up, and by DLCO group.
A total of 57% of the patients (1,734) received oxygen, and the remaining 43% of the patients (1,312) did not receive oxygen. Among the patients who received oxygen, 71% (1,227) received the therapy continuously, and 24% (408) received oxygen at night only.
The 424 patients with a DLCO of less than 40% were considered to have a severe DLCO impairment. The other two groups comprised 505 patients with a moderate DLCO impairment (at least 40%, but less than 60%) and 844 patients with a mild to normal DLCO (at least 60%). The DLCOs of 1,273 patients analyzed were unknown.
Among those patients with severe DLCO impairment, the risk of death was significantly lower in those who received oxygen, compared with those who did not receive oxygen (hazard ratio, 0.56; P = .0033). Oxygen use was associated with significant improvements in overall survival in both the newly diagnosed (HR, 0.47; P = .029) and previously diagnosed (HR, 0.59; P = .026) severe DLCO cohorts, Dr. Farber said.
Patients receiving oxygen were more likely to be treated with PAH-specific medications, regardless of their DLCO group.
Among the analysis’s limitations was that the lengths of time patients had been undergoing oxygen treatment were unknown. That prevented adjustments for duration of oxygen treatment, according to Dr. Farber.
Dr. Farber disclosed serving on the steering committees or advisory boards for Actelion, Bayer, Bellerophon, Gilead, and United Therapeutics. He has received research support from Actelion, Gilead, and United Therapeutics, and has been a speaker for Actelion, Bayer, and Gilead.
LOS ANGELES – Pulmonary arterial hypertension (PAH) patients with severely impaired diffusing capacity of the lung for carbon monoxide (DLCO) were much more likely to survive when they received home oxygen therapy, according to a disease registry analysis.
“We all know that supplemental oxygen is widely used with PAH,” said Harrison W. Farber, MD, director of the pulmonary hypertension center at Boston University. But there are practically no data showing that it is successful, and there are even fewer data for patients with PAH who have very low diffusion capacity, he added.
That knowledge gap prompted Dr. Farber and his colleagues to analyze data from the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL), the largest disease registry in the world of patients with PAH.
“Patients in that group – the severe DLCO group – who got oxygen had poorer prognostic features but improved overall survival relative to those who didn’t,” Dr. Farber explained during a presentation at the annual meeting of the American College of Chest Physicians. “Based on this, it makes us think that home oxygen, supplemental oxygen treatment, is associated with improved survival in patients, especially those with severe DLCO and PAH.”
The 3,046 patients analyzed by Dr. Farber and his colleagues had World Health Organization Group 1 PAH with right heart catheterization hemodynamic criteria: a mean pulmonary artery pressure greater than 25 mm Hg, a pulmonary capillary wedge pressure less than or equal to 15 mm Hg, and a pulmonary vascular resistance of at least 3 Wood units (WU). Patients were at least 18 years of age and grouped by oxygen use, which was defined as any use at any time from study enrollment to the end of follow-up, and by DLCO group.
A total of 57% of the patients (1,734) received oxygen, and the remaining 43% of the patients (1,312) did not receive oxygen. Among the patients who received oxygen, 71% (1,227) received the therapy continuously, and 24% (408) received oxygen at night only.
The 424 patients with a DLCO of less than 40% were considered to have a severe DLCO impairment. The other two groups comprised 505 patients with a moderate DLCO impairment (at least 40%, but less than 60%) and 844 patients with a mild to normal DLCO (at least 60%). The DLCOs of 1,273 patients analyzed were unknown.
Among those patients with severe DLCO impairment, the risk of death was significantly lower in those who received oxygen, compared with those who did not receive oxygen (hazard ratio, 0.56; P = .0033). Oxygen use was associated with significant improvements in overall survival in both the newly diagnosed (HR, 0.47; P = .029) and previously diagnosed (HR, 0.59; P = .026) severe DLCO cohorts, Dr. Farber said.
Patients receiving oxygen were more likely to be treated with PAH-specific medications, regardless of their DLCO group.
Among the analysis’s limitations was that the lengths of time patients had been undergoing oxygen treatment were unknown. That prevented adjustments for duration of oxygen treatment, according to Dr. Farber.
Dr. Farber disclosed serving on the steering committees or advisory boards for Actelion, Bayer, Bellerophon, Gilead, and United Therapeutics. He has received research support from Actelion, Gilead, and United Therapeutics, and has been a speaker for Actelion, Bayer, and Gilead.
LOS ANGELES – Pulmonary arterial hypertension (PAH) patients with severely impaired diffusing capacity of the lung for carbon monoxide (DLCO) were much more likely to survive when they received home oxygen therapy, according to a disease registry analysis.
“We all know that supplemental oxygen is widely used with PAH,” said Harrison W. Farber, MD, director of the pulmonary hypertension center at Boston University. But there are practically no data showing that it is successful, and there are even fewer data for patients with PAH who have very low diffusion capacity, he added.
That knowledge gap prompted Dr. Farber and his colleagues to analyze data from the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL), the largest disease registry in the world of patients with PAH.
“Patients in that group – the severe DLCO group – who got oxygen had poorer prognostic features but improved overall survival relative to those who didn’t,” Dr. Farber explained during a presentation at the annual meeting of the American College of Chest Physicians. “Based on this, it makes us think that home oxygen, supplemental oxygen treatment, is associated with improved survival in patients, especially those with severe DLCO and PAH.”
The 3,046 patients analyzed by Dr. Farber and his colleagues had World Health Organization Group 1 PAH with right heart catheterization hemodynamic criteria: a mean pulmonary artery pressure greater than 25 mm Hg, a pulmonary capillary wedge pressure less than or equal to 15 mm Hg, and a pulmonary vascular resistance of at least 3 Wood units (WU). Patients were at least 18 years of age and grouped by oxygen use, which was defined as any use at any time from study enrollment to the end of follow-up, and by DLCO group.
A total of 57% of the patients (1,734) received oxygen, and the remaining 43% of the patients (1,312) did not receive oxygen. Among the patients who received oxygen, 71% (1,227) received the therapy continuously, and 24% (408) received oxygen at night only.
The 424 patients with a DLCO of less than 40% were considered to have a severe DLCO impairment. The other two groups comprised 505 patients with a moderate DLCO impairment (at least 40%, but less than 60%) and 844 patients with a mild to normal DLCO (at least 60%). The DLCOs of 1,273 patients analyzed were unknown.
Among those patients with severe DLCO impairment, the risk of death was significantly lower in those who received oxygen, compared with those who did not receive oxygen (hazard ratio, 0.56; P = .0033). Oxygen use was associated with significant improvements in overall survival in both the newly diagnosed (HR, 0.47; P = .029) and previously diagnosed (HR, 0.59; P = .026) severe DLCO cohorts, Dr. Farber said.
Patients receiving oxygen were more likely to be treated with PAH-specific medications, regardless of their DLCO group.
Among the analysis’s limitations was that the lengths of time patients had been undergoing oxygen treatment were unknown. That prevented adjustments for duration of oxygen treatment, according to Dr. Farber.
Dr. Farber disclosed serving on the steering committees or advisory boards for Actelion, Bayer, Bellerophon, Gilead, and United Therapeutics. He has received research support from Actelion, Gilead, and United Therapeutics, and has been a speaker for Actelion, Bayer, and Gilead.
Key clinical point:
Major finding: PAH patients with severe DLCO impairment who received oxygen had a significantly higher probability of survival than those who didn’t receive oxygen (HR, 0.56; P = .0033).
Data source: An analysis of 3,046 patients in the U.S. multicenter, observational REVEAL disease registry.
Disclosures: Dr. Farber disclosed serving on the steering committees or advisory boards for Actelion, Bayer, Bellerophon, Gilead, and United Therapeutics. He has received research support from Actelion, Gilead, and United Therapeutics, and has been a speaker for Actelion, Bayer, and Gilead.
Novel antibiotic hits skin and soft tissue infections with one-two punch
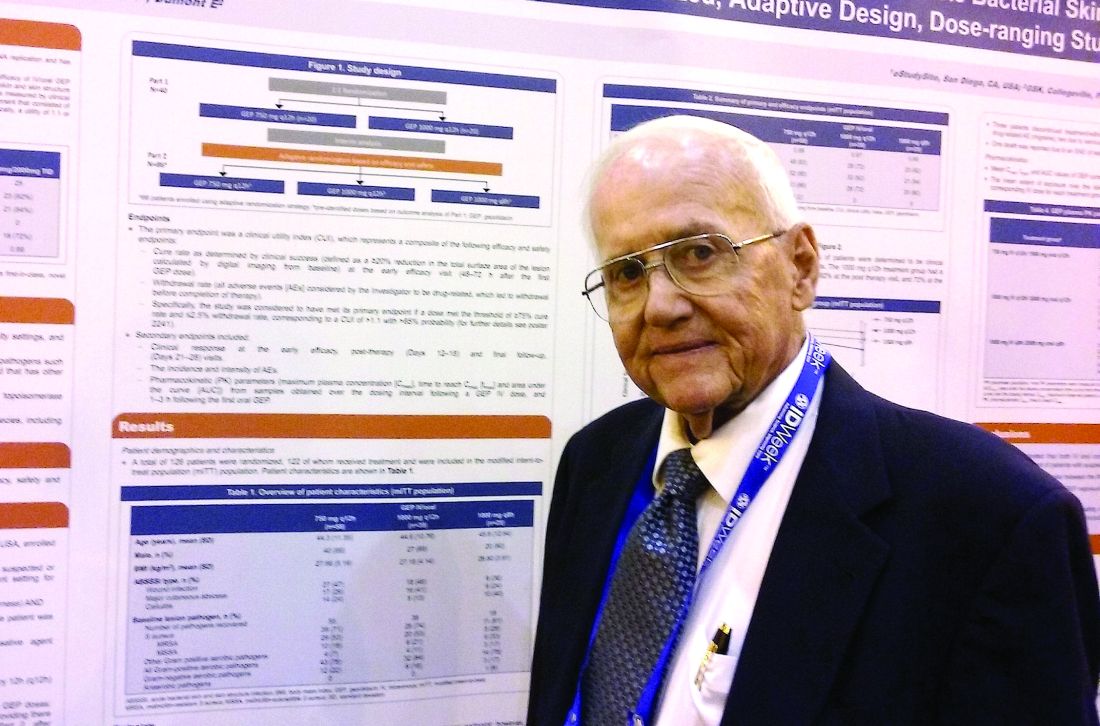
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
AT IDWEEK 2016
Key clinical point: A dual-mechanism-of-action antibiotic in development shows good efficacy and a low adverse event rate in a phase II study.
Major finding: A total 71 of 122 adult patients achieved clinical success within 48 to 72 hours with gepotidacin treatment.
Data source: 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis.
Disclosures: Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
What will the Trump administration mean for medicine?
The Affordable Care Act is in the crosshairs as the transition to the Trump administration begins Nov. 9.
The primary tenet of Donald J. Trump’s health care platform calls for Congress to repeal the ACA.
In fact, Mr. Trump has called for ACA repeal efforts to begin on his administration’s first day.
The Trump administration is likely to find plentiful allies in Congress as both the House and the Senate were projected at press time to have Republican majorities, albeit slim ones. Since the ACA’s passage in 2010, House Republicans have put forward repeal legislation scores of times.
While many medical specialty societies have supported the ACA and other major health care reforms enacted over the last 8 years – Meaningful Use from the HITECH ACT and value-based payment from MACRA among them – large numbers of physicians have chafed under the myriad reporting requirements and administrative hassles.
A recent survey commissioned by the Physicians Foundation and conducted by Merritt Hawkins found that nearly half (48%) of physicians are considering a change of practice – including leaving medicine – in the next 1-3 years. Reasons cited by survey respondents included the MACRA (Medicare Access and CHIP Reauthorization Act of 2015) transition to value-based care, the increased coding required by ICD-10, the growth of physician employment, the continued sale of private practices to hospitals and health systems, the increased number of patients in the system because of the ACA coupled with a shortage of physicians, and the “businessification” of heath care.
“If any of these [changes] occurred in a period of time, it would be impactful,” Walker Ray, MD, president of the Physicians Foundation, said in an interview. “But to have all occur simultaneously, we say now that to be a physician is to feel the ground shaking under your feet. This is the landscape in which the survey was taken.”
Mr. Trump supports several free market reforms to replace repealed provisions of the ACA, as well as address other issues in the health care system. The proposals include the following:
• Foster interstate insurance sales.
• Reinstate the tax deductibility of health insurance premiums.
• Promote the more widespread use of health savings accounts.
• Require price transparency so that patients can shop for medical procedures, exams, and tests.
• Block grant Medicaid to the states.
• Allow patients to import drugs from outside of the United States.
The Trump platform also promises to reduce fraud and waste, as well as save approximately $11 billion annually by not providing health care to illegal immigrants.
Speculation has also begun regarding who might lead health care agencies and policy for the Trump administration. Among the names that have been floated for secretary of Health and Human Services are Ben Carson, MD, the former presidential candidate and retired neurosurgeon; former House Speaker Newt Gingrich (who also has been suggested as a potential secretary of State); as well as Florida Gov. Rick Scott, former chief executive of Columbia/HCA, according to Politico.com.
[email protected]
On Twitter @denisefulton
Gregory Twachtman contributed to this story.
The Affordable Care Act is in the crosshairs as the transition to the Trump administration begins Nov. 9.
The primary tenet of Donald J. Trump’s health care platform calls for Congress to repeal the ACA.
In fact, Mr. Trump has called for ACA repeal efforts to begin on his administration’s first day.
The Trump administration is likely to find plentiful allies in Congress as both the House and the Senate were projected at press time to have Republican majorities, albeit slim ones. Since the ACA’s passage in 2010, House Republicans have put forward repeal legislation scores of times.
While many medical specialty societies have supported the ACA and other major health care reforms enacted over the last 8 years – Meaningful Use from the HITECH ACT and value-based payment from MACRA among them – large numbers of physicians have chafed under the myriad reporting requirements and administrative hassles.
A recent survey commissioned by the Physicians Foundation and conducted by Merritt Hawkins found that nearly half (48%) of physicians are considering a change of practice – including leaving medicine – in the next 1-3 years. Reasons cited by survey respondents included the MACRA (Medicare Access and CHIP Reauthorization Act of 2015) transition to value-based care, the increased coding required by ICD-10, the growth of physician employment, the continued sale of private practices to hospitals and health systems, the increased number of patients in the system because of the ACA coupled with a shortage of physicians, and the “businessification” of heath care.
“If any of these [changes] occurred in a period of time, it would be impactful,” Walker Ray, MD, president of the Physicians Foundation, said in an interview. “But to have all occur simultaneously, we say now that to be a physician is to feel the ground shaking under your feet. This is the landscape in which the survey was taken.”
Mr. Trump supports several free market reforms to replace repealed provisions of the ACA, as well as address other issues in the health care system. The proposals include the following:
• Foster interstate insurance sales.
• Reinstate the tax deductibility of health insurance premiums.
• Promote the more widespread use of health savings accounts.
• Require price transparency so that patients can shop for medical procedures, exams, and tests.
• Block grant Medicaid to the states.
• Allow patients to import drugs from outside of the United States.
The Trump platform also promises to reduce fraud and waste, as well as save approximately $11 billion annually by not providing health care to illegal immigrants.
Speculation has also begun regarding who might lead health care agencies and policy for the Trump administration. Among the names that have been floated for secretary of Health and Human Services are Ben Carson, MD, the former presidential candidate and retired neurosurgeon; former House Speaker Newt Gingrich (who also has been suggested as a potential secretary of State); as well as Florida Gov. Rick Scott, former chief executive of Columbia/HCA, according to Politico.com.
[email protected]
On Twitter @denisefulton
Gregory Twachtman contributed to this story.
The Affordable Care Act is in the crosshairs as the transition to the Trump administration begins Nov. 9.
The primary tenet of Donald J. Trump’s health care platform calls for Congress to repeal the ACA.
In fact, Mr. Trump has called for ACA repeal efforts to begin on his administration’s first day.
The Trump administration is likely to find plentiful allies in Congress as both the House and the Senate were projected at press time to have Republican majorities, albeit slim ones. Since the ACA’s passage in 2010, House Republicans have put forward repeal legislation scores of times.
While many medical specialty societies have supported the ACA and other major health care reforms enacted over the last 8 years – Meaningful Use from the HITECH ACT and value-based payment from MACRA among them – large numbers of physicians have chafed under the myriad reporting requirements and administrative hassles.
A recent survey commissioned by the Physicians Foundation and conducted by Merritt Hawkins found that nearly half (48%) of physicians are considering a change of practice – including leaving medicine – in the next 1-3 years. Reasons cited by survey respondents included the MACRA (Medicare Access and CHIP Reauthorization Act of 2015) transition to value-based care, the increased coding required by ICD-10, the growth of physician employment, the continued sale of private practices to hospitals and health systems, the increased number of patients in the system because of the ACA coupled with a shortage of physicians, and the “businessification” of heath care.
“If any of these [changes] occurred in a period of time, it would be impactful,” Walker Ray, MD, president of the Physicians Foundation, said in an interview. “But to have all occur simultaneously, we say now that to be a physician is to feel the ground shaking under your feet. This is the landscape in which the survey was taken.”
Mr. Trump supports several free market reforms to replace repealed provisions of the ACA, as well as address other issues in the health care system. The proposals include the following:
• Foster interstate insurance sales.
• Reinstate the tax deductibility of health insurance premiums.
• Promote the more widespread use of health savings accounts.
• Require price transparency so that patients can shop for medical procedures, exams, and tests.
• Block grant Medicaid to the states.
• Allow patients to import drugs from outside of the United States.
The Trump platform also promises to reduce fraud and waste, as well as save approximately $11 billion annually by not providing health care to illegal immigrants.
Speculation has also begun regarding who might lead health care agencies and policy for the Trump administration. Among the names that have been floated for secretary of Health and Human Services are Ben Carson, MD, the former presidential candidate and retired neurosurgeon; former House Speaker Newt Gingrich (who also has been suggested as a potential secretary of State); as well as Florida Gov. Rick Scott, former chief executive of Columbia/HCA, according to Politico.com.
[email protected]
On Twitter @denisefulton
Gregory Twachtman contributed to this story.
Diabetes drugs with cardiovascular benefits broaden cardiology’s turf
The dramatic reduction in cardiovascular death and heart failure hospitalization seen during treatment with empagliflozin (Jardiance) in the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial, for example, has prompted some cardiologists in the year since the first EMPA-REG report to become active prescribers of the drug to their patients who have type 2 diabetes and cardiovascular disease. The same evidence has driven other cardiologists who may not feel fully comfortable prescribing an antidiabetic drug on their own to enter into active partnerships with endocrinologists to work as a team to put diabetes patients with cardiovascular disease on empagliflozin.
In Dr. Fitchett’s practice, “if a patient with type 2 diabetes has an endocrinologist, then I will send a letter to that physician saying I think the patient should be on one of these drugs,” empagliflozin or liraglutide, he said. “If the patient is being treated by a primary care physician, then I will prescribe empagliflozin myself because most primary care physicians are not willing to prescribe it. I think more and more cardiologists are doing this. The great thing about empagliflozin and liraglutide is that they do not cause hypoglycemia and the adverse effect profiles are relatively good. As long as drug cost is not an issue, then as cardiologists we need to adjust glycemia control with cardiovascular benefit as we did years ago with statin treatment,” explained Dr. Fitchett, a cardiologist at St. Michael’s Hospital in Toronto and a senior collaborator and coauthor on the EMPA-REG study.
When results from the 4S [Scandinavian Simvastatin Survival Study] came out in 1994, proving that long-term statin treatment was both safe and increased survival in patients with coronary heart disease, “cardiologists took over lipid management from endocrinologists,” he recalled. “We now have a safe and simple treatment for glucose lowering that also cuts cardiovascular disease events, so cardiologists have to also be involved, at least to some extent. Their degree of involvement depends on their practice and who provides a patient’s primary diabetes care,” he said.
Cardiologists vary on empagliflozin
Other cardiologists are mixed in their take on personally prescribing antidiabetic drugs to high-risk patients with type 2 diabetes. Greg C. Fonarow, MD, has also aggressively taken to empagliflozin over the past year, especially for his patients with heart failure or at high risk for developing heart failure. The EMPA-REG results showed that empagliflozin’s potent impact on reducing cardiovascular death in patients linked closely with a reduction in heart failure hospitalizations. In his recent experience, endocrinologists as well as other physicians who care for patients with type 2 diabetes “are often reluctant to make any changes [in a patient’s hypoglycemic regimen], and in general they have not gravitated toward the treatments that have been shown to improve cardiovascular outcomes and instead focus solely on a patient’s hemoglobin A1c,” Dr. Fonarow said in an interview at the recent annual meeting of the Heart Failure Society of America.
He said he prescribes empagliflozin to patients with type 2 diabetes if they are hospitalized for heart failure or as outpatients, and he targets it to patients diagnosed with heart failure – including heart failure with preserved ejection fraction – as well as to patients with other forms of cardiovascular disease, closely following the EMPA-REG enrollment criteria. It’s too early in the experience with empagliflozin to use it preferentially in diabetes patients without cardiovascular disease or patients who in any other way fall outside the enrollment criteria for EMPA-REG, he said.
“I am happy to consult with their endocrinologist, or I tell patients to discuss this treatment with their endocrinologist. If the endocrinologist prescribes empagliflozin, great; if not, I feel an obligation to provide the best care I can to my patients. This is not a hard medication to use. The safety profile is good. Treatment with empagliflozin obviously has renal-function considerations, but that’s true for many drugs. The biggest challenge is what is covered by the patient’s insurance. We often need preauthorization.
“So far I have seen excellent responses in patients for both metabolic control and clinical responses in patients with heart failure. Their symptoms seem to improve,” said Dr. Fonarow, professor of medicine and co-chief of cardiology at the University of Southern California , Los Angeles.
While Dr. Fonarow cautioned that he also would not start empagliflozin in a patient with a HbA1c below 7%, he would seriously consider swapping out a patient’s drug for empagliflozin if it were a sulfonylurea or a dipeptidyl peptidase-4 inhibitor. He stopped short of suggesting a substitution of empagliflozin for metformin. In Dr. Fonarow’s opinion, the evidence for empagliflozin is also “more robust” than it has been for liraglutide or semaglutide. With what’s now known about the clinical impact of these drugs, he foresees a time when a combination between a SGLT-2 inhibitor, with its effect on heart failure, and a GLP-1 analogue, with its effect on atherosclerotic disease, may seem an ideal initial drug pairing for patients with type 2 diabetes and significant cardiovascular disease risk, with metformin relegated to a second-line role.
Other cardiologists endorsed a more collaborative approach to prescribing empagliflozin and liraglutide.
Another team-approach advocate is Robert O. Bonow, MD, cardiologist and professor of medicine at Northwestern University in Chicago. “Cardiologists are comfortable prescribing metformin and telling patients about lifestyle, but when it comes to newer antidiabetic drugs, that’s a new field, and a team approach may be best,” he said in an interview. “If possible, a cardiologist should have a friendly partnership with a diabetologist or endocrinologist who is expert in treating diabetes.” Many cardiologists now work in and for hospitals, and easy access to an endocrinologist is probably available, he noted.
But new analyses of the EMPA-REG data reported by Dr. Fitchett at the ESC congress showed that empagliflozin treatment exerted a similar benefit of reduced cardiovascular death regardless of whether patients had prevalent heart failure at entry into the study, incident heart failure during follow-up, or no heart failure of any sort.
Impact of heart failure in EMPA-REG
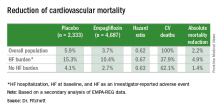
Roughly 10% of the 7,020 patients enrolled in EMPA-REG had heart failure at the time they entered the trial. During a median follow-up of just over 3 years, the incidence of new-onset heart failure – tallied as either a new heart failure hospitalization or a clinical episode deemed to be heart failure by an investigator – occurred in 4.6% of patients on empagliflozin and in 6.5% of patients in the placebo arm, a 1.9-percentage-point difference and a 30% relative risk reduction linked with empagliflozin use, Dr. Fitchett reported.
The main EMPA-REG outcome was a composite of cardiovascular death, nonfatal MI, and nonfatal stroke. This positive outcome in favor of empagliflozin treatment was primarily driven by a difference in the rate of cardiovascular death. In the new analysis, the relative reduction in cardiovascular deaths with empagliflozin compared with placebo was 29% among patients with prevalent heart failure at baseline, 35% among those who had an incident heart failure hospitalization during follow-up, 27% among patients with an incident heart failure episode diagnosed by an investigator during follow-up, 33% among the combined group of trial patients with any form of heart failure at trial entry or during the trial (those with prevalent heart failure at baseline plus those with an incident event), and 37% among the large number of patients in the trial who remained free from any indication of heart failure during follow-up.
In short, treatment with empagliflozin “reduced cardiovascular mortality by the same relative amount” regardless of whether patients did or did not have heart failure during the trial,” Dr. Fitchett concluded.
Additional secondary analyses from EMPA-REG reported at the ESC congress in August also documented that the benefit from empagliflozin treatment was roughly the same regardless of the age of patients enrolled in the trial and regardless of patients’ blood level of LDL cholesterol at entry into the study. These findings provide “confidence in the consistency of the effect” by empagliflozin, Dr. Fitchett said.
The endocrinologists’ view
“Most cardiologists are not thoroughly familiar with the full palette of medications for hyperglycemia. Selection of medication should not be made solely on the basis of results from a cardiovascular outcomes trial,” said Helena W. Rodbard, MD, a clinical endocrinologist in Rockville, Md.
“The EMPA-REG OUTCOMES and LEADER results are very exciting and encouraging. When all other factors are equal, the cardiovascular results could sway the decision about which medication to use. But an endocrinologist is in the best position to balance the many factors when choosing combination therapy and to set a target level for HbA1c, fasting blood glucose, and postprandial glucose, and to adjust therapy to minimize the risk of hypoglycemia,” Dr. Rodbard said in an interview.
He called empagliflozin a drug with “interesting promise,” especially for patients with incipient heart failure. The extra cardiovascular benefit from the GLP-1 analogues is “less settled,” although the liraglutide and semaglutide trial results are important and mean these drugs need more consideration and study. The EMPA-REG results were more clearly positive, he said.
“Metformin is still the initial drug” for most patients with type 2 diabetes, echoed Dr. Levy. Drugs like empagliflozin and liraglutide are usually used in combination with metformin.
“Like many endocrinologists, I have for some time used the oral SGLT-2 inhibitors and GLP-1 analogues in combination with metformin. It made sense before the recent cardiovascular data appeared, and it makes even more sense now,” said Dr. Jellinger, professor of clinical medicine and an endocrinologist at the University of Miami.
“Endocrinologists and diabetologists are aware that cardiologists have been taking a larger role in the care of patients with diabetes,” noted Dr. Rodbard. “I favor cardiologists and endocrinologists working in concert to improve the care of patients with diabetes.”
“Over the next few years, we will need to decide whether to treat patients with type 2 diabetes with an agent with proven benefits,” said Dr. Fitchett. “Until the results from EMPA-REG and the LEADER trial came out, there was no specific glucose-lowering agent that also reduced cardiovascular events. Some cardiologists might ask when they should get involved in managing patients with type 2 diabetes. What I would do for patients with a history of cardiovascular disease who develop new type 2 diabetes is start empagliflozin as their first drug,” Dr. Fitchett said, though he admitted that no evidence yet exists to back that approach.
The EMPA-REG trial was sponsored by Boehringer Ingelheim and by Eli Lilly, the companies that market empagliflozin. The LEADER trial was sponsored in part by Novo Nordisk, the company that markets liraglutide. Dr. Fitchett and Dr. Mentz were both researchers for EMPA-REG. Dr. Fitchett has been a consultant to AstraZeneca, Merck, and Amgen. Dr. Mentz has been an adviser to Boehringer Ingelheim. Dr. Fonarow has been an adviser to Amgen, Janssen, Novartis, and ZS Pharma. Dr. Bozkurt had no disclosures. Dr. Bonow has been a consultant to Gilead. Dr. Jellinger has been a speaker on behalf of Boehringer-Ingelheim, Novo Nordisk, Merck, and Janssen. Dr. Rodbard has been a consultant to or speaker for several drug companies including Boehringer-Ingelheim, Eli Lilly, and Novo Nordisk. Dr. Levy has been a speaker on behalf of Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and AstraZeneca. Dr. Hellman had no disclosures.
[email protected]
On Twitter @mitchelzoler
The dramatic reduction in cardiovascular death and heart failure hospitalization seen during treatment with empagliflozin (Jardiance) in the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial, for example, has prompted some cardiologists in the year since the first EMPA-REG report to become active prescribers of the drug to their patients who have type 2 diabetes and cardiovascular disease. The same evidence has driven other cardiologists who may not feel fully comfortable prescribing an antidiabetic drug on their own to enter into active partnerships with endocrinologists to work as a team to put diabetes patients with cardiovascular disease on empagliflozin.
In Dr. Fitchett’s practice, “if a patient with type 2 diabetes has an endocrinologist, then I will send a letter to that physician saying I think the patient should be on one of these drugs,” empagliflozin or liraglutide, he said. “If the patient is being treated by a primary care physician, then I will prescribe empagliflozin myself because most primary care physicians are not willing to prescribe it. I think more and more cardiologists are doing this. The great thing about empagliflozin and liraglutide is that they do not cause hypoglycemia and the adverse effect profiles are relatively good. As long as drug cost is not an issue, then as cardiologists we need to adjust glycemia control with cardiovascular benefit as we did years ago with statin treatment,” explained Dr. Fitchett, a cardiologist at St. Michael’s Hospital in Toronto and a senior collaborator and coauthor on the EMPA-REG study.
When results from the 4S [Scandinavian Simvastatin Survival Study] came out in 1994, proving that long-term statin treatment was both safe and increased survival in patients with coronary heart disease, “cardiologists took over lipid management from endocrinologists,” he recalled. “We now have a safe and simple treatment for glucose lowering that also cuts cardiovascular disease events, so cardiologists have to also be involved, at least to some extent. Their degree of involvement depends on their practice and who provides a patient’s primary diabetes care,” he said.
Cardiologists vary on empagliflozin
Other cardiologists are mixed in their take on personally prescribing antidiabetic drugs to high-risk patients with type 2 diabetes. Greg C. Fonarow, MD, has also aggressively taken to empagliflozin over the past year, especially for his patients with heart failure or at high risk for developing heart failure. The EMPA-REG results showed that empagliflozin’s potent impact on reducing cardiovascular death in patients linked closely with a reduction in heart failure hospitalizations. In his recent experience, endocrinologists as well as other physicians who care for patients with type 2 diabetes “are often reluctant to make any changes [in a patient’s hypoglycemic regimen], and in general they have not gravitated toward the treatments that have been shown to improve cardiovascular outcomes and instead focus solely on a patient’s hemoglobin A1c,” Dr. Fonarow said in an interview at the recent annual meeting of the Heart Failure Society of America.
He said he prescribes empagliflozin to patients with type 2 diabetes if they are hospitalized for heart failure or as outpatients, and he targets it to patients diagnosed with heart failure – including heart failure with preserved ejection fraction – as well as to patients with other forms of cardiovascular disease, closely following the EMPA-REG enrollment criteria. It’s too early in the experience with empagliflozin to use it preferentially in diabetes patients without cardiovascular disease or patients who in any other way fall outside the enrollment criteria for EMPA-REG, he said.
“I am happy to consult with their endocrinologist, or I tell patients to discuss this treatment with their endocrinologist. If the endocrinologist prescribes empagliflozin, great; if not, I feel an obligation to provide the best care I can to my patients. This is not a hard medication to use. The safety profile is good. Treatment with empagliflozin obviously has renal-function considerations, but that’s true for many drugs. The biggest challenge is what is covered by the patient’s insurance. We often need preauthorization.
“So far I have seen excellent responses in patients for both metabolic control and clinical responses in patients with heart failure. Their symptoms seem to improve,” said Dr. Fonarow, professor of medicine and co-chief of cardiology at the University of Southern California , Los Angeles.
While Dr. Fonarow cautioned that he also would not start empagliflozin in a patient with a HbA1c below 7%, he would seriously consider swapping out a patient’s drug for empagliflozin if it were a sulfonylurea or a dipeptidyl peptidase-4 inhibitor. He stopped short of suggesting a substitution of empagliflozin for metformin. In Dr. Fonarow’s opinion, the evidence for empagliflozin is also “more robust” than it has been for liraglutide or semaglutide. With what’s now known about the clinical impact of these drugs, he foresees a time when a combination between a SGLT-2 inhibitor, with its effect on heart failure, and a GLP-1 analogue, with its effect on atherosclerotic disease, may seem an ideal initial drug pairing for patients with type 2 diabetes and significant cardiovascular disease risk, with metformin relegated to a second-line role.
Other cardiologists endorsed a more collaborative approach to prescribing empagliflozin and liraglutide.
Another team-approach advocate is Robert O. Bonow, MD, cardiologist and professor of medicine at Northwestern University in Chicago. “Cardiologists are comfortable prescribing metformin and telling patients about lifestyle, but when it comes to newer antidiabetic drugs, that’s a new field, and a team approach may be best,” he said in an interview. “If possible, a cardiologist should have a friendly partnership with a diabetologist or endocrinologist who is expert in treating diabetes.” Many cardiologists now work in and for hospitals, and easy access to an endocrinologist is probably available, he noted.
But new analyses of the EMPA-REG data reported by Dr. Fitchett at the ESC congress showed that empagliflozin treatment exerted a similar benefit of reduced cardiovascular death regardless of whether patients had prevalent heart failure at entry into the study, incident heart failure during follow-up, or no heart failure of any sort.
Impact of heart failure in EMPA-REG
Roughly 10% of the 7,020 patients enrolled in EMPA-REG had heart failure at the time they entered the trial. During a median follow-up of just over 3 years, the incidence of new-onset heart failure – tallied as either a new heart failure hospitalization or a clinical episode deemed to be heart failure by an investigator – occurred in 4.6% of patients on empagliflozin and in 6.5% of patients in the placebo arm, a 1.9-percentage-point difference and a 30% relative risk reduction linked with empagliflozin use, Dr. Fitchett reported.
The main EMPA-REG outcome was a composite of cardiovascular death, nonfatal MI, and nonfatal stroke. This positive outcome in favor of empagliflozin treatment was primarily driven by a difference in the rate of cardiovascular death. In the new analysis, the relative reduction in cardiovascular deaths with empagliflozin compared with placebo was 29% among patients with prevalent heart failure at baseline, 35% among those who had an incident heart failure hospitalization during follow-up, 27% among patients with an incident heart failure episode diagnosed by an investigator during follow-up, 33% among the combined group of trial patients with any form of heart failure at trial entry or during the trial (those with prevalent heart failure at baseline plus those with an incident event), and 37% among the large number of patients in the trial who remained free from any indication of heart failure during follow-up.
In short, treatment with empagliflozin “reduced cardiovascular mortality by the same relative amount” regardless of whether patients did or did not have heart failure during the trial,” Dr. Fitchett concluded.
Additional secondary analyses from EMPA-REG reported at the ESC congress in August also documented that the benefit from empagliflozin treatment was roughly the same regardless of the age of patients enrolled in the trial and regardless of patients’ blood level of LDL cholesterol at entry into the study. These findings provide “confidence in the consistency of the effect” by empagliflozin, Dr. Fitchett said.
The endocrinologists’ view
“Most cardiologists are not thoroughly familiar with the full palette of medications for hyperglycemia. Selection of medication should not be made solely on the basis of results from a cardiovascular outcomes trial,” said Helena W. Rodbard, MD, a clinical endocrinologist in Rockville, Md.
“The EMPA-REG OUTCOMES and LEADER results are very exciting and encouraging. When all other factors are equal, the cardiovascular results could sway the decision about which medication to use. But an endocrinologist is in the best position to balance the many factors when choosing combination therapy and to set a target level for HbA1c, fasting blood glucose, and postprandial glucose, and to adjust therapy to minimize the risk of hypoglycemia,” Dr. Rodbard said in an interview.
He called empagliflozin a drug with “interesting promise,” especially for patients with incipient heart failure. The extra cardiovascular benefit from the GLP-1 analogues is “less settled,” although the liraglutide and semaglutide trial results are important and mean these drugs need more consideration and study. The EMPA-REG results were more clearly positive, he said.
“Metformin is still the initial drug” for most patients with type 2 diabetes, echoed Dr. Levy. Drugs like empagliflozin and liraglutide are usually used in combination with metformin.
“Like many endocrinologists, I have for some time used the oral SGLT-2 inhibitors and GLP-1 analogues in combination with metformin. It made sense before the recent cardiovascular data appeared, and it makes even more sense now,” said Dr. Jellinger, professor of clinical medicine and an endocrinologist at the University of Miami.
“Endocrinologists and diabetologists are aware that cardiologists have been taking a larger role in the care of patients with diabetes,” noted Dr. Rodbard. “I favor cardiologists and endocrinologists working in concert to improve the care of patients with diabetes.”
“Over the next few years, we will need to decide whether to treat patients with type 2 diabetes with an agent with proven benefits,” said Dr. Fitchett. “Until the results from EMPA-REG and the LEADER trial came out, there was no specific glucose-lowering agent that also reduced cardiovascular events. Some cardiologists might ask when they should get involved in managing patients with type 2 diabetes. What I would do for patients with a history of cardiovascular disease who develop new type 2 diabetes is start empagliflozin as their first drug,” Dr. Fitchett said, though he admitted that no evidence yet exists to back that approach.
The EMPA-REG trial was sponsored by Boehringer Ingelheim and by Eli Lilly, the companies that market empagliflozin. The LEADER trial was sponsored in part by Novo Nordisk, the company that markets liraglutide. Dr. Fitchett and Dr. Mentz were both researchers for EMPA-REG. Dr. Fitchett has been a consultant to AstraZeneca, Merck, and Amgen. Dr. Mentz has been an adviser to Boehringer Ingelheim. Dr. Fonarow has been an adviser to Amgen, Janssen, Novartis, and ZS Pharma. Dr. Bozkurt had no disclosures. Dr. Bonow has been a consultant to Gilead. Dr. Jellinger has been a speaker on behalf of Boehringer-Ingelheim, Novo Nordisk, Merck, and Janssen. Dr. Rodbard has been a consultant to or speaker for several drug companies including Boehringer-Ingelheim, Eli Lilly, and Novo Nordisk. Dr. Levy has been a speaker on behalf of Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and AstraZeneca. Dr. Hellman had no disclosures.
[email protected]
On Twitter @mitchelzoler
The dramatic reduction in cardiovascular death and heart failure hospitalization seen during treatment with empagliflozin (Jardiance) in the EMPA-REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial, for example, has prompted some cardiologists in the year since the first EMPA-REG report to become active prescribers of the drug to their patients who have type 2 diabetes and cardiovascular disease. The same evidence has driven other cardiologists who may not feel fully comfortable prescribing an antidiabetic drug on their own to enter into active partnerships with endocrinologists to work as a team to put diabetes patients with cardiovascular disease on empagliflozin.
In Dr. Fitchett’s practice, “if a patient with type 2 diabetes has an endocrinologist, then I will send a letter to that physician saying I think the patient should be on one of these drugs,” empagliflozin or liraglutide, he said. “If the patient is being treated by a primary care physician, then I will prescribe empagliflozin myself because most primary care physicians are not willing to prescribe it. I think more and more cardiologists are doing this. The great thing about empagliflozin and liraglutide is that they do not cause hypoglycemia and the adverse effect profiles are relatively good. As long as drug cost is not an issue, then as cardiologists we need to adjust glycemia control with cardiovascular benefit as we did years ago with statin treatment,” explained Dr. Fitchett, a cardiologist at St. Michael’s Hospital in Toronto and a senior collaborator and coauthor on the EMPA-REG study.
When results from the 4S [Scandinavian Simvastatin Survival Study] came out in 1994, proving that long-term statin treatment was both safe and increased survival in patients with coronary heart disease, “cardiologists took over lipid management from endocrinologists,” he recalled. “We now have a safe and simple treatment for glucose lowering that also cuts cardiovascular disease events, so cardiologists have to also be involved, at least to some extent. Their degree of involvement depends on their practice and who provides a patient’s primary diabetes care,” he said.
Cardiologists vary on empagliflozin
Other cardiologists are mixed in their take on personally prescribing antidiabetic drugs to high-risk patients with type 2 diabetes. Greg C. Fonarow, MD, has also aggressively taken to empagliflozin over the past year, especially for his patients with heart failure or at high risk for developing heart failure. The EMPA-REG results showed that empagliflozin’s potent impact on reducing cardiovascular death in patients linked closely with a reduction in heart failure hospitalizations. In his recent experience, endocrinologists as well as other physicians who care for patients with type 2 diabetes “are often reluctant to make any changes [in a patient’s hypoglycemic regimen], and in general they have not gravitated toward the treatments that have been shown to improve cardiovascular outcomes and instead focus solely on a patient’s hemoglobin A1c,” Dr. Fonarow said in an interview at the recent annual meeting of the Heart Failure Society of America.
He said he prescribes empagliflozin to patients with type 2 diabetes if they are hospitalized for heart failure or as outpatients, and he targets it to patients diagnosed with heart failure – including heart failure with preserved ejection fraction – as well as to patients with other forms of cardiovascular disease, closely following the EMPA-REG enrollment criteria. It’s too early in the experience with empagliflozin to use it preferentially in diabetes patients without cardiovascular disease or patients who in any other way fall outside the enrollment criteria for EMPA-REG, he said.
“I am happy to consult with their endocrinologist, or I tell patients to discuss this treatment with their endocrinologist. If the endocrinologist prescribes empagliflozin, great; if not, I feel an obligation to provide the best care I can to my patients. This is not a hard medication to use. The safety profile is good. Treatment with empagliflozin obviously has renal-function considerations, but that’s true for many drugs. The biggest challenge is what is covered by the patient’s insurance. We often need preauthorization.
“So far I have seen excellent responses in patients for both metabolic control and clinical responses in patients with heart failure. Their symptoms seem to improve,” said Dr. Fonarow, professor of medicine and co-chief of cardiology at the University of Southern California , Los Angeles.
While Dr. Fonarow cautioned that he also would not start empagliflozin in a patient with a HbA1c below 7%, he would seriously consider swapping out a patient’s drug for empagliflozin if it were a sulfonylurea or a dipeptidyl peptidase-4 inhibitor. He stopped short of suggesting a substitution of empagliflozin for metformin. In Dr. Fonarow’s opinion, the evidence for empagliflozin is also “more robust” than it has been for liraglutide or semaglutide. With what’s now known about the clinical impact of these drugs, he foresees a time when a combination between a SGLT-2 inhibitor, with its effect on heart failure, and a GLP-1 analogue, with its effect on atherosclerotic disease, may seem an ideal initial drug pairing for patients with type 2 diabetes and significant cardiovascular disease risk, with metformin relegated to a second-line role.
Other cardiologists endorsed a more collaborative approach to prescribing empagliflozin and liraglutide.
Another team-approach advocate is Robert O. Bonow, MD, cardiologist and professor of medicine at Northwestern University in Chicago. “Cardiologists are comfortable prescribing metformin and telling patients about lifestyle, but when it comes to newer antidiabetic drugs, that’s a new field, and a team approach may be best,” he said in an interview. “If possible, a cardiologist should have a friendly partnership with a diabetologist or endocrinologist who is expert in treating diabetes.” Many cardiologists now work in and for hospitals, and easy access to an endocrinologist is probably available, he noted.
But new analyses of the EMPA-REG data reported by Dr. Fitchett at the ESC congress showed that empagliflozin treatment exerted a similar benefit of reduced cardiovascular death regardless of whether patients had prevalent heart failure at entry into the study, incident heart failure during follow-up, or no heart failure of any sort.
Impact of heart failure in EMPA-REG
Roughly 10% of the 7,020 patients enrolled in EMPA-REG had heart failure at the time they entered the trial. During a median follow-up of just over 3 years, the incidence of new-onset heart failure – tallied as either a new heart failure hospitalization or a clinical episode deemed to be heart failure by an investigator – occurred in 4.6% of patients on empagliflozin and in 6.5% of patients in the placebo arm, a 1.9-percentage-point difference and a 30% relative risk reduction linked with empagliflozin use, Dr. Fitchett reported.
The main EMPA-REG outcome was a composite of cardiovascular death, nonfatal MI, and nonfatal stroke. This positive outcome in favor of empagliflozin treatment was primarily driven by a difference in the rate of cardiovascular death. In the new analysis, the relative reduction in cardiovascular deaths with empagliflozin compared with placebo was 29% among patients with prevalent heart failure at baseline, 35% among those who had an incident heart failure hospitalization during follow-up, 27% among patients with an incident heart failure episode diagnosed by an investigator during follow-up, 33% among the combined group of trial patients with any form of heart failure at trial entry or during the trial (those with prevalent heart failure at baseline plus those with an incident event), and 37% among the large number of patients in the trial who remained free from any indication of heart failure during follow-up.
In short, treatment with empagliflozin “reduced cardiovascular mortality by the same relative amount” regardless of whether patients did or did not have heart failure during the trial,” Dr. Fitchett concluded.
Additional secondary analyses from EMPA-REG reported at the ESC congress in August also documented that the benefit from empagliflozin treatment was roughly the same regardless of the age of patients enrolled in the trial and regardless of patients’ blood level of LDL cholesterol at entry into the study. These findings provide “confidence in the consistency of the effect” by empagliflozin, Dr. Fitchett said.
The endocrinologists’ view
“Most cardiologists are not thoroughly familiar with the full palette of medications for hyperglycemia. Selection of medication should not be made solely on the basis of results from a cardiovascular outcomes trial,” said Helena W. Rodbard, MD, a clinical endocrinologist in Rockville, Md.
“The EMPA-REG OUTCOMES and LEADER results are very exciting and encouraging. When all other factors are equal, the cardiovascular results could sway the decision about which medication to use. But an endocrinologist is in the best position to balance the many factors when choosing combination therapy and to set a target level for HbA1c, fasting blood glucose, and postprandial glucose, and to adjust therapy to minimize the risk of hypoglycemia,” Dr. Rodbard said in an interview.
He called empagliflozin a drug with “interesting promise,” especially for patients with incipient heart failure. The extra cardiovascular benefit from the GLP-1 analogues is “less settled,” although the liraglutide and semaglutide trial results are important and mean these drugs need more consideration and study. The EMPA-REG results were more clearly positive, he said.
“Metformin is still the initial drug” for most patients with type 2 diabetes, echoed Dr. Levy. Drugs like empagliflozin and liraglutide are usually used in combination with metformin.
“Like many endocrinologists, I have for some time used the oral SGLT-2 inhibitors and GLP-1 analogues in combination with metformin. It made sense before the recent cardiovascular data appeared, and it makes even more sense now,” said Dr. Jellinger, professor of clinical medicine and an endocrinologist at the University of Miami.
“Endocrinologists and diabetologists are aware that cardiologists have been taking a larger role in the care of patients with diabetes,” noted Dr. Rodbard. “I favor cardiologists and endocrinologists working in concert to improve the care of patients with diabetes.”
“Over the next few years, we will need to decide whether to treat patients with type 2 diabetes with an agent with proven benefits,” said Dr. Fitchett. “Until the results from EMPA-REG and the LEADER trial came out, there was no specific glucose-lowering agent that also reduced cardiovascular events. Some cardiologists might ask when they should get involved in managing patients with type 2 diabetes. What I would do for patients with a history of cardiovascular disease who develop new type 2 diabetes is start empagliflozin as their first drug,” Dr. Fitchett said, though he admitted that no evidence yet exists to back that approach.
The EMPA-REG trial was sponsored by Boehringer Ingelheim and by Eli Lilly, the companies that market empagliflozin. The LEADER trial was sponsored in part by Novo Nordisk, the company that markets liraglutide. Dr. Fitchett and Dr. Mentz were both researchers for EMPA-REG. Dr. Fitchett has been a consultant to AstraZeneca, Merck, and Amgen. Dr. Mentz has been an adviser to Boehringer Ingelheim. Dr. Fonarow has been an adviser to Amgen, Janssen, Novartis, and ZS Pharma. Dr. Bozkurt had no disclosures. Dr. Bonow has been a consultant to Gilead. Dr. Jellinger has been a speaker on behalf of Boehringer-Ingelheim, Novo Nordisk, Merck, and Janssen. Dr. Rodbard has been a consultant to or speaker for several drug companies including Boehringer-Ingelheim, Eli Lilly, and Novo Nordisk. Dr. Levy has been a speaker on behalf of Boehringer-Ingelheim, Eli Lilly, Novo Nordisk, and AstraZeneca. Dr. Hellman had no disclosures.
[email protected]
On Twitter @mitchelzoler
In era of infliximab, ulcerative colitis surgical outcomes worsen
WASHINGTON – The era of powerful biologics has led to unforeseen surgical outcomes in patients with ulcerative colitis.
Patients undergoing surgery for ulcerative colitis now are 38% more likely to die in the hospital than they were 15 years ago, before infliximab and other biologics were adopted as medical therapy for the disease. A database review covering 18 years found that other surgical outcomes are worse, too, Jonathan Abelson, MD, said at the annual clinical congress of the American College of Surgeons.
“These very powerful agents could be completely eliminating the need for surgery in patients with mild disease, leaving surgery for those who have very advanced disease and didn’t respond well to medical therapy,” said Dr. Abelson, a clinical research fellow at New York–Presbyterian Hospital, N.Y. “We are operating now only on patients with very severe disease, not the wider range of patients we had 15 years ago, when there weren’t as effective medical options.”
He and his colleagues used the New York Statewide Planning and Research Cooperative System (SPARCS) database to identify 7,070 patients who had undergone bowel resection for ulcerative colitis during two epochs: prebiologics (1995-2005) and postbiologics (2006-2013). The cohorts were about evenly split in numbers.
There were some statistically significant differences in baseline characteristics. Patients in epoch 2 were about a year older (51 vs. 50 years). Significantly more of them had at least two major comorbidities (28% vs. 18%). Minimally invasive surgery was significantly more common in epoch 2 (28% vs. 3%).
Significantly more surgeries in epoch 2 were staged into three or more procedures (14% vs. 9%). This finding probably reflects the level of disease severity in those presenting for surgery or the fact that they underwent surgery after recently receiving biologics, Dr. Abelson said.
“One of the limits of this study is that we don’t know exactly the reasons for these one-, two-, or three-stage surgeries. The theory is that patients who were more ill at presentation are more likely to have a multistaged surgery. Another reason could be that if they are on these powerful immunosuppressive regimens, the surgeon might be concerned about not healing well from a definitive one- or two-stage surgery.”
He then conducted a multivariate analysis that controlled for baseline factors, including a variety of individual comorbid conditions. In this analysis, patients in epoch 2 were 38% more likely to die in the hospital and 51% more likely to experience a major postoperative event, like shock, pulmonary embolism, stroke, or heart attack. The chance of a surgical complication was increased by 39%, and these patients were 25% more likely to need a transfusion during surgery than those from epoch 1.
The poorer outcomes held for an at least an entire year after surgery, Dr. Abelson said. At 1 year, patients in epoch 2 were 36% more likely to have a readmission than those in epoch 1. Major events and procedural complications were both 46% more likely. Patients were also 36% more likely to require an additional procedure.
“These are not the outcomes we want to see, especially in this era when our surgical techniques have improved so much,” Dr. Abelson said. “If what this represents, though, is that we are now operating on a higher-risk population, we can’t just say, ‘Well, that’s how it’s going to be.’ We need to figure out how to minimize morbidity and mortality in this high-risk patient population.”
One goal, he suggested, would be to assess response to a biologic regimen earlier in the hopes of determining who will respond well, and moving ahead with surgery in those who don’t.
This is a tough sell for patients, he said.
“There is a big fear of this surgery. It usually requires a temporary ileostomy and a stoma bag, and patients are terrified of that. There have been a few studies demonstrating that earlier referral to surgery improves quality of life; living with advanced ulcerative colitis can be extremely difficult and patients often feel a lot better after we remove their diseased colon. But getting there is a challenge.”
Dr. Abelson had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
WASHINGTON – The era of powerful biologics has led to unforeseen surgical outcomes in patients with ulcerative colitis.
Patients undergoing surgery for ulcerative colitis now are 38% more likely to die in the hospital than they were 15 years ago, before infliximab and other biologics were adopted as medical therapy for the disease. A database review covering 18 years found that other surgical outcomes are worse, too, Jonathan Abelson, MD, said at the annual clinical congress of the American College of Surgeons.
“These very powerful agents could be completely eliminating the need for surgery in patients with mild disease, leaving surgery for those who have very advanced disease and didn’t respond well to medical therapy,” said Dr. Abelson, a clinical research fellow at New York–Presbyterian Hospital, N.Y. “We are operating now only on patients with very severe disease, not the wider range of patients we had 15 years ago, when there weren’t as effective medical options.”
He and his colleagues used the New York Statewide Planning and Research Cooperative System (SPARCS) database to identify 7,070 patients who had undergone bowel resection for ulcerative colitis during two epochs: prebiologics (1995-2005) and postbiologics (2006-2013). The cohorts were about evenly split in numbers.
There were some statistically significant differences in baseline characteristics. Patients in epoch 2 were about a year older (51 vs. 50 years). Significantly more of them had at least two major comorbidities (28% vs. 18%). Minimally invasive surgery was significantly more common in epoch 2 (28% vs. 3%).
Significantly more surgeries in epoch 2 were staged into three or more procedures (14% vs. 9%). This finding probably reflects the level of disease severity in those presenting for surgery or the fact that they underwent surgery after recently receiving biologics, Dr. Abelson said.
“One of the limits of this study is that we don’t know exactly the reasons for these one-, two-, or three-stage surgeries. The theory is that patients who were more ill at presentation are more likely to have a multistaged surgery. Another reason could be that if they are on these powerful immunosuppressive regimens, the surgeon might be concerned about not healing well from a definitive one- or two-stage surgery.”
He then conducted a multivariate analysis that controlled for baseline factors, including a variety of individual comorbid conditions. In this analysis, patients in epoch 2 were 38% more likely to die in the hospital and 51% more likely to experience a major postoperative event, like shock, pulmonary embolism, stroke, or heart attack. The chance of a surgical complication was increased by 39%, and these patients were 25% more likely to need a transfusion during surgery than those from epoch 1.
The poorer outcomes held for an at least an entire year after surgery, Dr. Abelson said. At 1 year, patients in epoch 2 were 36% more likely to have a readmission than those in epoch 1. Major events and procedural complications were both 46% more likely. Patients were also 36% more likely to require an additional procedure.
“These are not the outcomes we want to see, especially in this era when our surgical techniques have improved so much,” Dr. Abelson said. “If what this represents, though, is that we are now operating on a higher-risk population, we can’t just say, ‘Well, that’s how it’s going to be.’ We need to figure out how to minimize morbidity and mortality in this high-risk patient population.”
One goal, he suggested, would be to assess response to a biologic regimen earlier in the hopes of determining who will respond well, and moving ahead with surgery in those who don’t.
This is a tough sell for patients, he said.
“There is a big fear of this surgery. It usually requires a temporary ileostomy and a stoma bag, and patients are terrified of that. There have been a few studies demonstrating that earlier referral to surgery improves quality of life; living with advanced ulcerative colitis can be extremely difficult and patients often feel a lot better after we remove their diseased colon. But getting there is a challenge.”
Dr. Abelson had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
WASHINGTON – The era of powerful biologics has led to unforeseen surgical outcomes in patients with ulcerative colitis.
Patients undergoing surgery for ulcerative colitis now are 38% more likely to die in the hospital than they were 15 years ago, before infliximab and other biologics were adopted as medical therapy for the disease. A database review covering 18 years found that other surgical outcomes are worse, too, Jonathan Abelson, MD, said at the annual clinical congress of the American College of Surgeons.
“These very powerful agents could be completely eliminating the need for surgery in patients with mild disease, leaving surgery for those who have very advanced disease and didn’t respond well to medical therapy,” said Dr. Abelson, a clinical research fellow at New York–Presbyterian Hospital, N.Y. “We are operating now only on patients with very severe disease, not the wider range of patients we had 15 years ago, when there weren’t as effective medical options.”
He and his colleagues used the New York Statewide Planning and Research Cooperative System (SPARCS) database to identify 7,070 patients who had undergone bowel resection for ulcerative colitis during two epochs: prebiologics (1995-2005) and postbiologics (2006-2013). The cohorts were about evenly split in numbers.
There were some statistically significant differences in baseline characteristics. Patients in epoch 2 were about a year older (51 vs. 50 years). Significantly more of them had at least two major comorbidities (28% vs. 18%). Minimally invasive surgery was significantly more common in epoch 2 (28% vs. 3%).
Significantly more surgeries in epoch 2 were staged into three or more procedures (14% vs. 9%). This finding probably reflects the level of disease severity in those presenting for surgery or the fact that they underwent surgery after recently receiving biologics, Dr. Abelson said.
“One of the limits of this study is that we don’t know exactly the reasons for these one-, two-, or three-stage surgeries. The theory is that patients who were more ill at presentation are more likely to have a multistaged surgery. Another reason could be that if they are on these powerful immunosuppressive regimens, the surgeon might be concerned about not healing well from a definitive one- or two-stage surgery.”
He then conducted a multivariate analysis that controlled for baseline factors, including a variety of individual comorbid conditions. In this analysis, patients in epoch 2 were 38% more likely to die in the hospital and 51% more likely to experience a major postoperative event, like shock, pulmonary embolism, stroke, or heart attack. The chance of a surgical complication was increased by 39%, and these patients were 25% more likely to need a transfusion during surgery than those from epoch 1.
The poorer outcomes held for an at least an entire year after surgery, Dr. Abelson said. At 1 year, patients in epoch 2 were 36% more likely to have a readmission than those in epoch 1. Major events and procedural complications were both 46% more likely. Patients were also 36% more likely to require an additional procedure.
“These are not the outcomes we want to see, especially in this era when our surgical techniques have improved so much,” Dr. Abelson said. “If what this represents, though, is that we are now operating on a higher-risk population, we can’t just say, ‘Well, that’s how it’s going to be.’ We need to figure out how to minimize morbidity and mortality in this high-risk patient population.”
One goal, he suggested, would be to assess response to a biologic regimen earlier in the hopes of determining who will respond well, and moving ahead with surgery in those who don’t.
This is a tough sell for patients, he said.
“There is a big fear of this surgery. It usually requires a temporary ileostomy and a stoma bag, and patients are terrified of that. There have been a few studies demonstrating that earlier referral to surgery improves quality of life; living with advanced ulcerative colitis can be extremely difficult and patients often feel a lot better after we remove their diseased colon. But getting there is a challenge.”
Dr. Abelson had no financial disclosures.
[email protected]
On Twitter @Alz_Gal
AT THE ACS CLINICAL CONGRESS
Key clinical point:
Major finding: Patients are 38% more likely to die in the hospital than they were 15 years ago.
Data source: The 18-year database review comprised more than 7,000 surgeries.
Disclosures: Dr. Abelson had no financial disclosures.
Scoring formula consolidates stroke, bleeding risk in atrial fib patients
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2016
Key clinical point:
Major finding: Among high-risk patients, edoxaban cut adverse events by 21 percentage points, compared with warfarin.
Data source: ENGAGE AF-TIMI 48, a multicenter trial with 21,105 patients.
Disclosures: ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban (Savaysa). Dr. Fanola had no relevant financial disclosures.
Is regional anesthesia safer in CEA?
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: General anesthesia for carotid endarterectomy carries a higher risk of complications and readmissions than regional anesthesia.
Major finding: Any morbidity after CEA with general anesthesia was 8.7% vs. 4.2% for regional anesthesia, and readmissions rates were 9.2% vs. 6.1%.
Data source: Retrospective analysis of 4,558 patients who had CEA between 2012 and 2014 at hospitals participating in the Michigan Surgical Quality Collaborative database.
Disclosures: Dr. Hussain reported having no financial disclosures.
Delayed bleeding possible with EBUS-TBNA on antiplatelets
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
LOS ANGELES – There might be a slight increase in delayed bleeding when patients have endobronchial ultrasound with transbronchial needle aspiration within 5 days of taking oral antiplatelets, according to a review of 404 patients at Riverside Methodist Hospital in Columbus, Ohio.
This study is unusual in that it looked at the 48 hour mark. Previous studies have tended to focus on immediate bleeding events that require the procedure to be stopped; only some of that research has found an increased bleeding risk with antiplatelet therapy.
In the study at Riverside Methodist, none of the 20 patients on dual antiplatelet therapy – clopidogrel (Plavix) plus aspirin – bled during the procedure, but one (5%) had a hemoglobin drop of more than 2 g within 48 hours and another was readmitted to the hospital within 48 hours for procedure-related hemoptysis. Overall, the delayed bleeding event rate for patients using the dual antiplatelet therapy was 10%. Additionally, one of the 13 patients (7.7%) on clopidogrel alone experienced a greater than 2 g drop in hemoglobin.
Among the 270 patients not exposed to antiplatelets, the overall bleeding event rate was 2.6%, and the event rate for delayed bleeding was 1.1%. Four patients (1.5%) bled during the procedure, two (0.7%) had hemoglobin drops greater than 2 g within 48 hours, and one (0.4%) was readmitted for hemoptysis.
There were no bleeding events in the 101 patients who only took aspirin.
“There was a trend toward delayed bleeding events in patients” on clopidogrel or dual antiplatelets. “It’s worth considering a thoughtful pause in decision making. Maybe with the bleeding events we’re seeing, it would be worthwhile, if possible, to defer” endobronchial ultrasound with transbronchial needle aspiration “until after the antiplatelet therapy,” said Kevin Swiatek, DO, a medicine resident at Riverside.
Patients were excluded from the study if they had histories of bleeding or clotting disorders; low platelet counts; or if they were on anticoagulation. Subjects on antiplatelets were about 10 years older, on average, than those who were not (about 68 versus 59 years old), and more likely to have had a heart attack or stroke, and to be hypertensive.
There was no industry funding for the work, and the investigators had no disclosures.
AT CHEST 2016
Key clinical point:
Major finding: Ten percent of patients on dual antiplatelet therapy bled within 48 hours, versus 1.1% of those not on antiplatelet therapy.
Data source: Single-center review of 404 patients.
Disclosures: There was no industry funding for the work, and the investigators had no disclosures.
CEA risk models fit for an app
COLUMBUS, OHIO – Carotid endarterectomy is an effective treatment for people with asymptomatic carotid artery disease when stroke rates are low and they survive long enough to benefit from the treatment. But determining who those patients are can be a challenge for vascular surgeons. A team of vascular specialists from around the country have developed risk prediction models to help surgeons better select asymptomatic patients for the procedure, Randall DeMartino, MD, said at the annual meeting of the Midwestern Vascular Surgical Society.
“These models will be used for mobile apps and web-based applications for point of care patient risk assessment,” said Dr. DeMartino of the Mayo Clinic in Rochester, Minn. He is the lead researcher for the study, which uses data from the Vascular Quality Initiative (VQI).
In developing the models, the researchers sampled asymptomatic patients in the VQI who had first-time elective CEA. There were 31,939 patients in the stroke analysis who had CEA from 2010-2015, and 24,086 patients in the mortality analysis who had procedures from 2010-2014. Dr. DeMartino and his colleagues evaluated all preoperative patient and surgeon characteristics, then used an algorithm to optimize the variables that were selected for the final logistic model.
The researchers also evaluated 30-day stroke rates and 1-year mortality at participating centers and found wide variability: an average of 0.9% for stroke, with a range of 0-8.3%; and 3.2% for mortality, with a range of 0-20%. “Actually, 22% of centers had a 1-year mortality rate that exceeded 5%,” Dr. DeMartino said.
The model for 1-year mortality identified the following variables associated with the highest risk of death 1 year after CEA: age greater than or equal to 80 years; a preoperative hemoglobin less than 10 mg/dL; oxygen-dependent chronic obstructive pulmonary disease; mild to severe congestive heart failure; American Society of Anesthesiologists classification of IV or V; stage 4 or 5 chronic kidney disease; and a contralateral occlusion.
“Conversely, a normal stress test, when performed, and preoperative statin use were associated with reduced risk of death over a year,” Dr. DeMartino said.
“These data have been used to provide Center Opportunity for Improvement reports through VQI where centers can identify if they are selecting patients with risk factors for stroke or mortality more often compared to other centers,” Dr. DeMartino said. “This allows centers to see where opportunities for improvement exist.”
Also, physicians can see the proportion of patients they select with a predicted mortality risk over 5% at one year – “a group of patients who may gain little benefit from prophylactic CEA,” he said. “Physicians can compare their patient selection to those in their region or nationally.”
Dr. DeMartino had no relationships to disclose.
COLUMBUS, OHIO – Carotid endarterectomy is an effective treatment for people with asymptomatic carotid artery disease when stroke rates are low and they survive long enough to benefit from the treatment. But determining who those patients are can be a challenge for vascular surgeons. A team of vascular specialists from around the country have developed risk prediction models to help surgeons better select asymptomatic patients for the procedure, Randall DeMartino, MD, said at the annual meeting of the Midwestern Vascular Surgical Society.
“These models will be used for mobile apps and web-based applications for point of care patient risk assessment,” said Dr. DeMartino of the Mayo Clinic in Rochester, Minn. He is the lead researcher for the study, which uses data from the Vascular Quality Initiative (VQI).
In developing the models, the researchers sampled asymptomatic patients in the VQI who had first-time elective CEA. There were 31,939 patients in the stroke analysis who had CEA from 2010-2015, and 24,086 patients in the mortality analysis who had procedures from 2010-2014. Dr. DeMartino and his colleagues evaluated all preoperative patient and surgeon characteristics, then used an algorithm to optimize the variables that were selected for the final logistic model.
The researchers also evaluated 30-day stroke rates and 1-year mortality at participating centers and found wide variability: an average of 0.9% for stroke, with a range of 0-8.3%; and 3.2% for mortality, with a range of 0-20%. “Actually, 22% of centers had a 1-year mortality rate that exceeded 5%,” Dr. DeMartino said.
The model for 1-year mortality identified the following variables associated with the highest risk of death 1 year after CEA: age greater than or equal to 80 years; a preoperative hemoglobin less than 10 mg/dL; oxygen-dependent chronic obstructive pulmonary disease; mild to severe congestive heart failure; American Society of Anesthesiologists classification of IV or V; stage 4 or 5 chronic kidney disease; and a contralateral occlusion.
“Conversely, a normal stress test, when performed, and preoperative statin use were associated with reduced risk of death over a year,” Dr. DeMartino said.
“These data have been used to provide Center Opportunity for Improvement reports through VQI where centers can identify if they are selecting patients with risk factors for stroke or mortality more often compared to other centers,” Dr. DeMartino said. “This allows centers to see where opportunities for improvement exist.”
Also, physicians can see the proportion of patients they select with a predicted mortality risk over 5% at one year – “a group of patients who may gain little benefit from prophylactic CEA,” he said. “Physicians can compare their patient selection to those in their region or nationally.”
Dr. DeMartino had no relationships to disclose.
COLUMBUS, OHIO – Carotid endarterectomy is an effective treatment for people with asymptomatic carotid artery disease when stroke rates are low and they survive long enough to benefit from the treatment. But determining who those patients are can be a challenge for vascular surgeons. A team of vascular specialists from around the country have developed risk prediction models to help surgeons better select asymptomatic patients for the procedure, Randall DeMartino, MD, said at the annual meeting of the Midwestern Vascular Surgical Society.
“These models will be used for mobile apps and web-based applications for point of care patient risk assessment,” said Dr. DeMartino of the Mayo Clinic in Rochester, Minn. He is the lead researcher for the study, which uses data from the Vascular Quality Initiative (VQI).
In developing the models, the researchers sampled asymptomatic patients in the VQI who had first-time elective CEA. There were 31,939 patients in the stroke analysis who had CEA from 2010-2015, and 24,086 patients in the mortality analysis who had procedures from 2010-2014. Dr. DeMartino and his colleagues evaluated all preoperative patient and surgeon characteristics, then used an algorithm to optimize the variables that were selected for the final logistic model.
The researchers also evaluated 30-day stroke rates and 1-year mortality at participating centers and found wide variability: an average of 0.9% for stroke, with a range of 0-8.3%; and 3.2% for mortality, with a range of 0-20%. “Actually, 22% of centers had a 1-year mortality rate that exceeded 5%,” Dr. DeMartino said.
The model for 1-year mortality identified the following variables associated with the highest risk of death 1 year after CEA: age greater than or equal to 80 years; a preoperative hemoglobin less than 10 mg/dL; oxygen-dependent chronic obstructive pulmonary disease; mild to severe congestive heart failure; American Society of Anesthesiologists classification of IV or V; stage 4 or 5 chronic kidney disease; and a contralateral occlusion.
“Conversely, a normal stress test, when performed, and preoperative statin use were associated with reduced risk of death over a year,” Dr. DeMartino said.
“These data have been used to provide Center Opportunity for Improvement reports through VQI where centers can identify if they are selecting patients with risk factors for stroke or mortality more often compared to other centers,” Dr. DeMartino said. “This allows centers to see where opportunities for improvement exist.”
Also, physicians can see the proportion of patients they select with a predicted mortality risk over 5% at one year – “a group of patients who may gain little benefit from prophylactic CEA,” he said. “Physicians can compare their patient selection to those in their region or nationally.”
Dr. DeMartino had no relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: Risk-prediction models may identify patients at greatest risk of stroke and 1-year death after carotid endarterectomy (CEA).
Major finding: Contralateral occlusion has odds ratios of 2.5 for 30-day stroke after CEA and 1.7 for death at 1 year.
Data source: Sampling of patients from the Vascular Quality Initiative who had first-time CEA: 31,939 in the stroke analysis and 24,086 in the mortality analysis.
Disclosures: Dr. DeMartino reported having no financial disclosures.