User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Physician couples draft wills, face tough questions amid COVID-19
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”
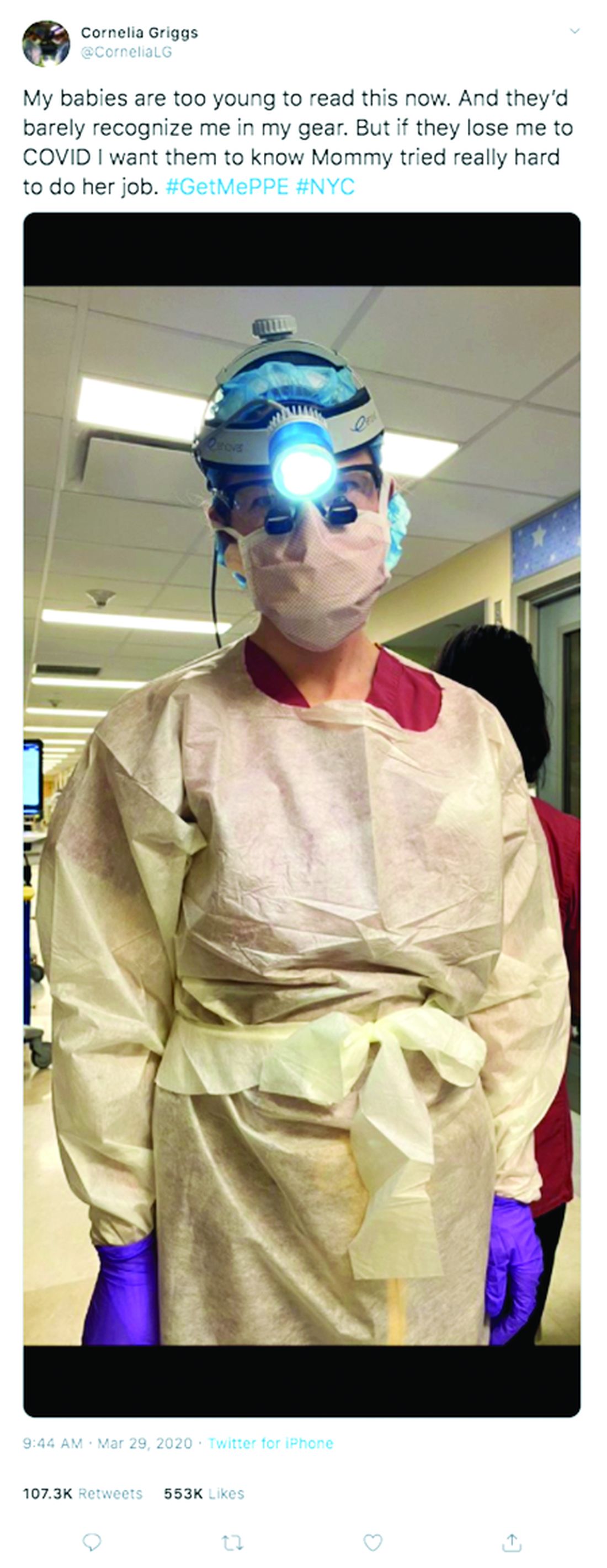
“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”

“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”

“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Dapagliflozin trial in CKD halted because of high efficacy
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.
AstraZeneca has announced that the phase 3 DAPA-CKD trial for dapagliflozin (Farxiga) in patients with chronic kidney disease has been halted early because of overwhelming efficacy of the drug, at the recommendation of an independent data monitoring committee.
DAPA-CKD is an international, multicenter, randomized, double-blinded trial in 4,245 patients with stage 2-4 chronic kidney disease. Patients received either 10 mg of the dapagliflozin once-daily or a placebo. The primary composite endpoint is worsening of renal function, defined as a composite of an estimated glomerular filtration rate decline of at least 50%, onset of end-stage kidney disease, and death from cardiovascular or renal cause.
The decision to stop the trial came after a routine assessment of efficacy and safety that showed dapagliflozin’s benefits significantly earlier than expected. AstraZeneca will initiate closure of the study, and results will be published and submitted for presentation at a forthcoming medical meeting.
Dapagliflozin is a sodium-glucose transporter 2 inhibitor currently indicated for the treatment type 2 diabetes patients with inadequately controlled type 2 diabetes and for reduction of the risk of hospitalization for heart failure. In August 2019, the drug was granted Fast Track status by the Food and Drug Administration for the treatment of chronic kidney disease. In January 2020, the agency also granted Fast Track status for the reduction of risk of cardiovascular death or worsening of heart failure in adult patients, regardless of diabetes status, with heart failure with reduced ejection fraction.
“Chronic kidney disease patients have limited treatment options, particularly those without type-2 diabetes. We are very pleased the data monitoring committee concluded that patients experienced overwhelming benefit. Farxiga has the potential to change the management of chronic kidney disease for patients around the world,” Mene Pangalos, executive vice president of BioPharmaceuticals R&D, said in the press release.
Flu activity measures continue COVID-19–related divergence
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
Critical care and COVID-19: Dr. Matt Aldrich
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
Larger absolute rivaroxaban benefit in diabetes: COMPASS
In the COMPASS trial of patients with stable coronary or peripheral artery disease (PAD), the combination of aspirin plus rivaroxaban, 2.5 mg twice daily, provided a larger absolute benefit on cardiovascular endpoints — including a threefold greater reduction in all-cause mortality — in patients with diabetes compared with the overall population.
The results of the diabetes subset of the COMPASS trial were presented by Deepak Bhatt, MD, Brigham and Women’s Hospital Heart & Vascular Center, Boston, Massachusetts, on March 28 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). They were also simultaneously published online in Circulation.
“Use of dual pathway inhibition with low-dose rivaroxaban plus aspirin is particularly attractive in high-risk patients such as those with diabetes,” Bhatt concluded.
The COMPASS trial was first reported in 2017 and showed a new low dose of rivaroxaban (2.5-mg twice-daily; Xarelto, Bayer/Janssen Pharmaceuticals) plus aspirin, 100 mg once daily, was associated with a reduction in ischemic events and mortality and a superior net clinical benefit, balancing ischemic benefit with severe bleeding, compared with aspirin alone for secondary prevention in patients with stable atherosclerotic vascular disease.
But clinicians have been slow to prescribe rivaroxaban in this new and very large population.
“It’s been more than 2 years now since main COMPASS results, and there isn’t a sense that this therapy has really caught on,” chair of the current ACC session at which the diabetes subgroup results were presented, Hadley Wilson, MD, Sanger Heart and Vascular Institute, Charlotte, North Carolina, commented:
He asked Bhatt whether the diabetes subgroup may be “the tipping point that will make people aware of rivaroxaban and then that may trickle down to other patients.”
Bhatt said that he hoped that would be the case. “We as a steering committee of this trial could say the results were positive so rivaroxaban should now be used in everyone with stable coronary or peripheral arterial disease, but that is impractical and as you out point out it hasn’t happened,” he replied.
“In PAD/vascular medicine we have embraced this new therapy. In the broader cardiology world there are a lot of patients with stable coronary arterial disease at high ischemic risk who could take rivaroxaban, but its use is bound to be limited by it being a branded drug and the fact that there is a bleeding risk,” Bhatt explained.
“I think we need to identify patients with the highest ischemic risk and focus drugs such as these with a financial cost and a bleeding risk on those who most likely will derive the greatest absolute reduction in risk,” he said. “The PAD subgroup is one group where this is the case, and now we have shown the diabetes subgroup is another. And there is no incremental bleeding risk in this group over the whole population, so they get a much greater benefit without a greater risk. I hope this helps get rivaroxaban at the new lower dose used much more often.”
A total of 18,278 patients were randomly assigned to the combination of rivaroxaban and aspirin or aspirin alone in the COMPASS trial. Of these, 6922 had diabetes mellitus at baseline and 11,356 did not have diabetes.
Results from the current analysis show a consistent and similar relative risk reduction for benefit of rivaroxaban plus aspirin vs placebo plus aspirin in patients both with and without diabetes for the primary efficacy endpoint, a composite of cardiovascular death, myocardial infarction (MI), or stroke, with a hazard ratio of 0.74 for patients with diabetes and 0.77 for those without diabetes, the researchers report.
Because of the higher baseline risk in the diabetes subgroup, these patients had numerically larger absolute risk reductions with rivaroxaban than those without diabetes for the primary efficacy endpoint at 3 years (2.3% vs 1.4%) and for all-cause mortality (1.9% vs 0.6%).
These results translate into a number needed to treat (NNT) with rivaroxaban for 3 years to prevent one CV death, MI, or stroke of 44 for the diabetes group vs 73 for the nondiabetes group; the NNT to prevent one all-cause death was 54 for the diabetes group vs 167 for the nondiabetes group, the authors write.
Because the bleeding hazards were similar among patients with and without diabetes, the absolute net clinical benefit (MI, stroke, cardiovascular death, or bleeding leading to death or symptomatic bleeding into a critical organ) for rivaroxaban was “particularly favorable” in the diabetes group (2.7% fewer events in the diabetes group vs 1.0% fewer events in the nondiabetes group), they add.
Panelist at the ACC Featured Clinical Research session at which these results were presented, Jennifer Robinson, MD, University of Iowa College of Public Health, Iowa City, asked Bhatt how clinicians were supposed to decide which of the many new agents now becoming available for patients with stable coronary artery disease to prescribe first.
“We often forget about rivaroxaban when we’re thinking about what to add next for our secondary prevention patients,” she said. “You also led the REDUCE-IT trial showing benefit of icosapent ethyl, icosapent ethyl icosapent ethyl icosapent ethyl and there is also ezetimibe, PCSK9 inhibitors and SGLT2 inhibitors. For your patients with coronary disease who are already on a high dose statin which one of these would you add next?”
“That is what physicians need to ponder all the time,” Bhatt replied. “And when a patient has several risk factors that are not well controlled, I guess it’s all important. I go through a checklist with my patients and try and figure what they’re not on that could further reduce their risk.”
“In the COMPASS trial there was an overall positive result with rivaroxaban in the whole population. And now we have shown that patients with diabetes had an even greater absolute risk reduction. That pattern has also been seen with other classes of agents including the statins, PCSK9 inhibitors, and icosapent ethyl,” Bhatt noted.
“In patients with diabetes, I will usually target whatever is standing out most at that time. If their glycemic control is completely out of whack, then that is what I would focus on first, and these days often with a SGLT2 inhibitor or GLP-1 agonist. If the LDL was out of control, I would add ezetimibe or a PCSK9 inhibitor. If the triglycerides were high, I would add icosapent ethyl. If multiple things were out of control, I would usually focus on the number most out of kilter first and try not to forget about everything else.”
But Bhatt noted that the challenge with rivaroxaban is that there is no test of thrombosis risk that would prompt the physician to take action. “Basically, the doctor just has to remember to do it. In that regard I would consider whether patients are at low bleeding risk and are they still at high ischemic risk despite controlling other risk factors and, if so, then I would add this low dose of rivaroxaban.”
Another panel member, Sekar Kathiresan, MD, asked Bhatt whether he recommended using available scores to assess the bleeding/thrombosis risk/benefits of adding an antithrombotic.
Bhatt replied: “That’s a terrific question. I guess the right answer is that we should be doing that, but in reality I have to concede that I don’t use these scores. They have shown appropriate C statistics in populations, but they are not fantastic in individual patients.”
“I have to confess that I use the eyeball test. There is nothing as good at predicting future bleeding as past bleeding. So if a patient has had bleeding problems on aspirin alone I wouldn’t add rivaroxaban. But if a patient hasn’t bled before, especially if they had some experience of dual antiplatelet therapy, then they would be good candidates for a low vascular dose of rivaroxaban,” he said.
“It is not as easy as with other drugs as there is always a bleeding trade-off with an antithrombotic. There is no such thing as a free lunch. So patients need careful assessment when considering prescribing rivaroxaban and regular reassessment over time to check if they have had any bleeding,” he added.
The COMPASS study was funded by Bayer. Bhatt reports honoraria from Bayer via the Population Health Research Institute for his role on the COMPASS trial and other research funding from Bayer to the Brigham & Women’s Hospital.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20544-ACC. Presented March 28, 2020.
Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
In the COMPASS trial of patients with stable coronary or peripheral artery disease (PAD), the combination of aspirin plus rivaroxaban, 2.5 mg twice daily, provided a larger absolute benefit on cardiovascular endpoints — including a threefold greater reduction in all-cause mortality — in patients with diabetes compared with the overall population.
The results of the diabetes subset of the COMPASS trial were presented by Deepak Bhatt, MD, Brigham and Women’s Hospital Heart & Vascular Center, Boston, Massachusetts, on March 28 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). They were also simultaneously published online in Circulation.
“Use of dual pathway inhibition with low-dose rivaroxaban plus aspirin is particularly attractive in high-risk patients such as those with diabetes,” Bhatt concluded.
The COMPASS trial was first reported in 2017 and showed a new low dose of rivaroxaban (2.5-mg twice-daily; Xarelto, Bayer/Janssen Pharmaceuticals) plus aspirin, 100 mg once daily, was associated with a reduction in ischemic events and mortality and a superior net clinical benefit, balancing ischemic benefit with severe bleeding, compared with aspirin alone for secondary prevention in patients with stable atherosclerotic vascular disease.
But clinicians have been slow to prescribe rivaroxaban in this new and very large population.
“It’s been more than 2 years now since main COMPASS results, and there isn’t a sense that this therapy has really caught on,” chair of the current ACC session at which the diabetes subgroup results were presented, Hadley Wilson, MD, Sanger Heart and Vascular Institute, Charlotte, North Carolina, commented:
He asked Bhatt whether the diabetes subgroup may be “the tipping point that will make people aware of rivaroxaban and then that may trickle down to other patients.”
Bhatt said that he hoped that would be the case. “We as a steering committee of this trial could say the results were positive so rivaroxaban should now be used in everyone with stable coronary or peripheral arterial disease, but that is impractical and as you out point out it hasn’t happened,” he replied.
“In PAD/vascular medicine we have embraced this new therapy. In the broader cardiology world there are a lot of patients with stable coronary arterial disease at high ischemic risk who could take rivaroxaban, but its use is bound to be limited by it being a branded drug and the fact that there is a bleeding risk,” Bhatt explained.
“I think we need to identify patients with the highest ischemic risk and focus drugs such as these with a financial cost and a bleeding risk on those who most likely will derive the greatest absolute reduction in risk,” he said. “The PAD subgroup is one group where this is the case, and now we have shown the diabetes subgroup is another. And there is no incremental bleeding risk in this group over the whole population, so they get a much greater benefit without a greater risk. I hope this helps get rivaroxaban at the new lower dose used much more often.”
A total of 18,278 patients were randomly assigned to the combination of rivaroxaban and aspirin or aspirin alone in the COMPASS trial. Of these, 6922 had diabetes mellitus at baseline and 11,356 did not have diabetes.
Results from the current analysis show a consistent and similar relative risk reduction for benefit of rivaroxaban plus aspirin vs placebo plus aspirin in patients both with and without diabetes for the primary efficacy endpoint, a composite of cardiovascular death, myocardial infarction (MI), or stroke, with a hazard ratio of 0.74 for patients with diabetes and 0.77 for those without diabetes, the researchers report.
Because of the higher baseline risk in the diabetes subgroup, these patients had numerically larger absolute risk reductions with rivaroxaban than those without diabetes for the primary efficacy endpoint at 3 years (2.3% vs 1.4%) and for all-cause mortality (1.9% vs 0.6%).
These results translate into a number needed to treat (NNT) with rivaroxaban for 3 years to prevent one CV death, MI, or stroke of 44 for the diabetes group vs 73 for the nondiabetes group; the NNT to prevent one all-cause death was 54 for the diabetes group vs 167 for the nondiabetes group, the authors write.
Because the bleeding hazards were similar among patients with and without diabetes, the absolute net clinical benefit (MI, stroke, cardiovascular death, or bleeding leading to death or symptomatic bleeding into a critical organ) for rivaroxaban was “particularly favorable” in the diabetes group (2.7% fewer events in the diabetes group vs 1.0% fewer events in the nondiabetes group), they add.
Panelist at the ACC Featured Clinical Research session at which these results were presented, Jennifer Robinson, MD, University of Iowa College of Public Health, Iowa City, asked Bhatt how clinicians were supposed to decide which of the many new agents now becoming available for patients with stable coronary artery disease to prescribe first.
“We often forget about rivaroxaban when we’re thinking about what to add next for our secondary prevention patients,” she said. “You also led the REDUCE-IT trial showing benefit of icosapent ethyl, icosapent ethyl icosapent ethyl icosapent ethyl and there is also ezetimibe, PCSK9 inhibitors and SGLT2 inhibitors. For your patients with coronary disease who are already on a high dose statin which one of these would you add next?”
“That is what physicians need to ponder all the time,” Bhatt replied. “And when a patient has several risk factors that are not well controlled, I guess it’s all important. I go through a checklist with my patients and try and figure what they’re not on that could further reduce their risk.”
“In the COMPASS trial there was an overall positive result with rivaroxaban in the whole population. And now we have shown that patients with diabetes had an even greater absolute risk reduction. That pattern has also been seen with other classes of agents including the statins, PCSK9 inhibitors, and icosapent ethyl,” Bhatt noted.
“In patients with diabetes, I will usually target whatever is standing out most at that time. If their glycemic control is completely out of whack, then that is what I would focus on first, and these days often with a SGLT2 inhibitor or GLP-1 agonist. If the LDL was out of control, I would add ezetimibe or a PCSK9 inhibitor. If the triglycerides were high, I would add icosapent ethyl. If multiple things were out of control, I would usually focus on the number most out of kilter first and try not to forget about everything else.”
But Bhatt noted that the challenge with rivaroxaban is that there is no test of thrombosis risk that would prompt the physician to take action. “Basically, the doctor just has to remember to do it. In that regard I would consider whether patients are at low bleeding risk and are they still at high ischemic risk despite controlling other risk factors and, if so, then I would add this low dose of rivaroxaban.”
Another panel member, Sekar Kathiresan, MD, asked Bhatt whether he recommended using available scores to assess the bleeding/thrombosis risk/benefits of adding an antithrombotic.
Bhatt replied: “That’s a terrific question. I guess the right answer is that we should be doing that, but in reality I have to concede that I don’t use these scores. They have shown appropriate C statistics in populations, but they are not fantastic in individual patients.”
“I have to confess that I use the eyeball test. There is nothing as good at predicting future bleeding as past bleeding. So if a patient has had bleeding problems on aspirin alone I wouldn’t add rivaroxaban. But if a patient hasn’t bled before, especially if they had some experience of dual antiplatelet therapy, then they would be good candidates for a low vascular dose of rivaroxaban,” he said.
“It is not as easy as with other drugs as there is always a bleeding trade-off with an antithrombotic. There is no such thing as a free lunch. So patients need careful assessment when considering prescribing rivaroxaban and regular reassessment over time to check if they have had any bleeding,” he added.
The COMPASS study was funded by Bayer. Bhatt reports honoraria from Bayer via the Population Health Research Institute for his role on the COMPASS trial and other research funding from Bayer to the Brigham & Women’s Hospital.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20544-ACC. Presented March 28, 2020.
Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
In the COMPASS trial of patients with stable coronary or peripheral artery disease (PAD), the combination of aspirin plus rivaroxaban, 2.5 mg twice daily, provided a larger absolute benefit on cardiovascular endpoints — including a threefold greater reduction in all-cause mortality — in patients with diabetes compared with the overall population.
The results of the diabetes subset of the COMPASS trial were presented by Deepak Bhatt, MD, Brigham and Women’s Hospital Heart & Vascular Center, Boston, Massachusetts, on March 28 at the “virtual” American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). They were also simultaneously published online in Circulation.
“Use of dual pathway inhibition with low-dose rivaroxaban plus aspirin is particularly attractive in high-risk patients such as those with diabetes,” Bhatt concluded.
The COMPASS trial was first reported in 2017 and showed a new low dose of rivaroxaban (2.5-mg twice-daily; Xarelto, Bayer/Janssen Pharmaceuticals) plus aspirin, 100 mg once daily, was associated with a reduction in ischemic events and mortality and a superior net clinical benefit, balancing ischemic benefit with severe bleeding, compared with aspirin alone for secondary prevention in patients with stable atherosclerotic vascular disease.
But clinicians have been slow to prescribe rivaroxaban in this new and very large population.
“It’s been more than 2 years now since main COMPASS results, and there isn’t a sense that this therapy has really caught on,” chair of the current ACC session at which the diabetes subgroup results were presented, Hadley Wilson, MD, Sanger Heart and Vascular Institute, Charlotte, North Carolina, commented:
He asked Bhatt whether the diabetes subgroup may be “the tipping point that will make people aware of rivaroxaban and then that may trickle down to other patients.”
Bhatt said that he hoped that would be the case. “We as a steering committee of this trial could say the results were positive so rivaroxaban should now be used in everyone with stable coronary or peripheral arterial disease, but that is impractical and as you out point out it hasn’t happened,” he replied.
“In PAD/vascular medicine we have embraced this new therapy. In the broader cardiology world there are a lot of patients with stable coronary arterial disease at high ischemic risk who could take rivaroxaban, but its use is bound to be limited by it being a branded drug and the fact that there is a bleeding risk,” Bhatt explained.
“I think we need to identify patients with the highest ischemic risk and focus drugs such as these with a financial cost and a bleeding risk on those who most likely will derive the greatest absolute reduction in risk,” he said. “The PAD subgroup is one group where this is the case, and now we have shown the diabetes subgroup is another. And there is no incremental bleeding risk in this group over the whole population, so they get a much greater benefit without a greater risk. I hope this helps get rivaroxaban at the new lower dose used much more often.”
A total of 18,278 patients were randomly assigned to the combination of rivaroxaban and aspirin or aspirin alone in the COMPASS trial. Of these, 6922 had diabetes mellitus at baseline and 11,356 did not have diabetes.
Results from the current analysis show a consistent and similar relative risk reduction for benefit of rivaroxaban plus aspirin vs placebo plus aspirin in patients both with and without diabetes for the primary efficacy endpoint, a composite of cardiovascular death, myocardial infarction (MI), or stroke, with a hazard ratio of 0.74 for patients with diabetes and 0.77 for those without diabetes, the researchers report.
Because of the higher baseline risk in the diabetes subgroup, these patients had numerically larger absolute risk reductions with rivaroxaban than those without diabetes for the primary efficacy endpoint at 3 years (2.3% vs 1.4%) and for all-cause mortality (1.9% vs 0.6%).
These results translate into a number needed to treat (NNT) with rivaroxaban for 3 years to prevent one CV death, MI, or stroke of 44 for the diabetes group vs 73 for the nondiabetes group; the NNT to prevent one all-cause death was 54 for the diabetes group vs 167 for the nondiabetes group, the authors write.
Because the bleeding hazards were similar among patients with and without diabetes, the absolute net clinical benefit (MI, stroke, cardiovascular death, or bleeding leading to death or symptomatic bleeding into a critical organ) for rivaroxaban was “particularly favorable” in the diabetes group (2.7% fewer events in the diabetes group vs 1.0% fewer events in the nondiabetes group), they add.
Panelist at the ACC Featured Clinical Research session at which these results were presented, Jennifer Robinson, MD, University of Iowa College of Public Health, Iowa City, asked Bhatt how clinicians were supposed to decide which of the many new agents now becoming available for patients with stable coronary artery disease to prescribe first.
“We often forget about rivaroxaban when we’re thinking about what to add next for our secondary prevention patients,” she said. “You also led the REDUCE-IT trial showing benefit of icosapent ethyl, icosapent ethyl icosapent ethyl icosapent ethyl and there is also ezetimibe, PCSK9 inhibitors and SGLT2 inhibitors. For your patients with coronary disease who are already on a high dose statin which one of these would you add next?”
“That is what physicians need to ponder all the time,” Bhatt replied. “And when a patient has several risk factors that are not well controlled, I guess it’s all important. I go through a checklist with my patients and try and figure what they’re not on that could further reduce their risk.”
“In the COMPASS trial there was an overall positive result with rivaroxaban in the whole population. And now we have shown that patients with diabetes had an even greater absolute risk reduction. That pattern has also been seen with other classes of agents including the statins, PCSK9 inhibitors, and icosapent ethyl,” Bhatt noted.
“In patients with diabetes, I will usually target whatever is standing out most at that time. If their glycemic control is completely out of whack, then that is what I would focus on first, and these days often with a SGLT2 inhibitor or GLP-1 agonist. If the LDL was out of control, I would add ezetimibe or a PCSK9 inhibitor. If the triglycerides were high, I would add icosapent ethyl. If multiple things were out of control, I would usually focus on the number most out of kilter first and try not to forget about everything else.”
But Bhatt noted that the challenge with rivaroxaban is that there is no test of thrombosis risk that would prompt the physician to take action. “Basically, the doctor just has to remember to do it. In that regard I would consider whether patients are at low bleeding risk and are they still at high ischemic risk despite controlling other risk factors and, if so, then I would add this low dose of rivaroxaban.”
Another panel member, Sekar Kathiresan, MD, asked Bhatt whether he recommended using available scores to assess the bleeding/thrombosis risk/benefits of adding an antithrombotic.
Bhatt replied: “That’s a terrific question. I guess the right answer is that we should be doing that, but in reality I have to concede that I don’t use these scores. They have shown appropriate C statistics in populations, but they are not fantastic in individual patients.”
“I have to confess that I use the eyeball test. There is nothing as good at predicting future bleeding as past bleeding. So if a patient has had bleeding problems on aspirin alone I wouldn’t add rivaroxaban. But if a patient hasn’t bled before, especially if they had some experience of dual antiplatelet therapy, then they would be good candidates for a low vascular dose of rivaroxaban,” he said.
“It is not as easy as with other drugs as there is always a bleeding trade-off with an antithrombotic. There is no such thing as a free lunch. So patients need careful assessment when considering prescribing rivaroxaban and regular reassessment over time to check if they have had any bleeding,” he added.
The COMPASS study was funded by Bayer. Bhatt reports honoraria from Bayer via the Population Health Research Institute for his role on the COMPASS trial and other research funding from Bayer to the Brigham & Women’s Hospital.
American College of Cardiology 2020 Scientific Session (ACC.20)/World Congress of Cardiology (WCC). Abstract 20-LB-20544-ACC. Presented March 28, 2020.
Circulation. Published online March 28, 2020. Full text.
This article first appeared on Medscape.com.
Before the COVID-19 surge hits your facility, take steps to boost capacity
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
REPORTING FROM AN ALLIANCE FOR HEALTH POLICY WEBINAR
FDA okays emergency use of convalescent plasma for seriously ill COVID-19 patients
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
Wilkie and the VA vs COVID-19: Who’s Winning?
US Department of Veterans Affairs (VA) Secretary Robert Wilkie is finding out what it means to be on wartime footing against a virus. He is overseeing the VA’s internal response to COVID-19 while deciding how to fulfil the VA’s fourth mission: providing reinforcement for the nation’s healthcare system in a national emergency. Meanwhile, he’s facing hostilities on a third front: criticism of his efforts so far.
In late February, when lawmakers asked whether the VA needed more resources to fight COVID-19, Wilkie said no. He told NPR on March 19 that “we are poised for the onslaught.” But on March 13, 2020, the VA was being attacked for not releasing a comprehensive emergency response to the incipient pandemic. Wilkie countered, “Before there was a single confirmed case in the US,” he wrote in a recent op-ed piece for Military Times, “the VA was already conducting emergency preparedness exercises.”
In the NPR interview, Wilkie said the VA had undertaken “a very aggressive public health response at an early stage.” Now, the VA has added other measures. The VA, he said, was the first health system to stop people from entering its facilities without being questioned or tested, and the first to adopt the “hard decision” of a no-visitor rule for veterans in nursing homes. Every veteran who comes to a VA facility with flu-like symptoms is screened. Further, via tweets and blog posts, Wilkie is “inviting” retired medical personnel back to work to help deal with the pandemic.
The VA is also the “buttress force,” Wilkie says, for the Federal Emergency Management Agency and the US Department of Health and Human Services if they need medical professionals for crises. “We plan for that every day,” he says. “We are gaming out emergency preparedness scenarios and we stand ready when the President needs us to expand our mission.” But in The American Prospect, Suzanne Gordon and Jasper Craven, both fellows at the Veterans Healthcare Policy Institute, write that “one quiet action is ominous”—the VA website has deleted any mention of the department’s credo of caring for civilians in times of crisis.
According to Gordon and Craven, on Wednesday Wilkie “came out of the woodwork” to express the department’s readiness to help in the crisis. The VA has established 19 emergency operations centers across the country, Wilkie says, and has stopped elective surgeries to free up thousands of beds. He touts the agency’s flexibility, saying it’s prepared to move resources around the country as needed. “Some veterans hospitals have not been impacted [by the virus],” Wilkie said. “So, I’m not going to keep 500 respirators in the middle of a state that has one veteran with the infection, when I can use that in Seattle or New Orleans, or New York City.”
Wilkie says the VA has stockpiled equipment and its supply chain is stable. However, in the NPR interview, Mary Louise Kelly said the NPR VA correspondent had been hearing complaints about lack of gear, such as masks. When pressed on his claim that the VA had adequate protective supplies, Wilkie said those complaints “have not reached us.” In fact, he said, “I can tell you that the arrangements that we have made on both the masks side and also on the testing side—we’re in a very good place.”
Nonetheless, on March 16, the employee unions representing nearly 350,000 VA healthcare workers issued a joint statement that called on VHA management to “work with us to ensure the nation’s VA health facilities can safely handle COVID-19.” It’s time, said Everett Kelley, National President of the American Federation of Government Employees, “for the VA to invite our members to the table, instead of kicking them off the property, so we can finally work together on a solution….”
“Instead of relaxing standards and efforts,” the unions said, “like we have seen the CDC do [in allowing healthcare workers to reuse facemasks and rely on simple surgical facemasks], “we need to be stepping it up.”
It all takes money. After weeks of debate, the US Senate has just released details of the $2 trillion coronavirus aid package. The US Department of Defense (DoD) seems about to get $10.5 billion in emergency funding and the VA another $19.6 billion. The money includes funding for National Guard deployments to help state governments respond to emerging health needs, the expansion of military hospitals and mobile medical centers if needed, and help with production of medical supplies. Nearly $16 billion will be used for direct care specifically in response to veterans’ health needs, covering treatment for COVID-19 in VA hospitals, community urgent care clinics and emergency departments; overtime for clinical staff; and purchase of protective equipment, tests, and other supplies.
Despite having one of the best telehealth systems in the US, the VA has also come under fire for its telehealth preparations to meet the current pandemic-related demand. Former VA Under Secretary of Health Kenneth Kizer wrote in an op-ed for Military Times, “Regrettably, so far, there is no coordinated strategy for ramping up and optimizing the use of telehealth to combat the growing epidemic in the US.” The relief package proposes $3 billion for new telemedicine efforts, including staffing and equipping mobile treatment sites.
In mid-March, the VA had 3,000 coronavirus test kits but still had not used roughly 90%, an article in Mother Jones charged. At a White house press conference around that time, Wilkie was asked how many veterans of those who needed to be tested had been. “We believe we’ve caught most of them,” he replied.
But that was in the early days of the crisis.
With results from the 322 tests administered by Mar. 18, the VA had confirmed five positive cases, was tracking 33 presumptive cases, and acknowledged the first veteran death linked to COVID-19. As of Mar. 26, the VA had administered roughly 7,500 COVID-19 tests nationwide.
Secretary Wilkie has promised that the department’s first focus will always be caring for veterans. In an interview with Military Times, he said, “We don’t release any beds if veterans are needing them. The veterans still are primary. We are a [health] bridge for the larger community, but that’s only after veterans are taken care of.”
US Department of Veterans Affairs (VA) Secretary Robert Wilkie is finding out what it means to be on wartime footing against a virus. He is overseeing the VA’s internal response to COVID-19 while deciding how to fulfil the VA’s fourth mission: providing reinforcement for the nation’s healthcare system in a national emergency. Meanwhile, he’s facing hostilities on a third front: criticism of his efforts so far.
In late February, when lawmakers asked whether the VA needed more resources to fight COVID-19, Wilkie said no. He told NPR on March 19 that “we are poised for the onslaught.” But on March 13, 2020, the VA was being attacked for not releasing a comprehensive emergency response to the incipient pandemic. Wilkie countered, “Before there was a single confirmed case in the US,” he wrote in a recent op-ed piece for Military Times, “the VA was already conducting emergency preparedness exercises.”
In the NPR interview, Wilkie said the VA had undertaken “a very aggressive public health response at an early stage.” Now, the VA has added other measures. The VA, he said, was the first health system to stop people from entering its facilities without being questioned or tested, and the first to adopt the “hard decision” of a no-visitor rule for veterans in nursing homes. Every veteran who comes to a VA facility with flu-like symptoms is screened. Further, via tweets and blog posts, Wilkie is “inviting” retired medical personnel back to work to help deal with the pandemic.
The VA is also the “buttress force,” Wilkie says, for the Federal Emergency Management Agency and the US Department of Health and Human Services if they need medical professionals for crises. “We plan for that every day,” he says. “We are gaming out emergency preparedness scenarios and we stand ready when the President needs us to expand our mission.” But in The American Prospect, Suzanne Gordon and Jasper Craven, both fellows at the Veterans Healthcare Policy Institute, write that “one quiet action is ominous”—the VA website has deleted any mention of the department’s credo of caring for civilians in times of crisis.
According to Gordon and Craven, on Wednesday Wilkie “came out of the woodwork” to express the department’s readiness to help in the crisis. The VA has established 19 emergency operations centers across the country, Wilkie says, and has stopped elective surgeries to free up thousands of beds. He touts the agency’s flexibility, saying it’s prepared to move resources around the country as needed. “Some veterans hospitals have not been impacted [by the virus],” Wilkie said. “So, I’m not going to keep 500 respirators in the middle of a state that has one veteran with the infection, when I can use that in Seattle or New Orleans, or New York City.”
Wilkie says the VA has stockpiled equipment and its supply chain is stable. However, in the NPR interview, Mary Louise Kelly said the NPR VA correspondent had been hearing complaints about lack of gear, such as masks. When pressed on his claim that the VA had adequate protective supplies, Wilkie said those complaints “have not reached us.” In fact, he said, “I can tell you that the arrangements that we have made on both the masks side and also on the testing side—we’re in a very good place.”
Nonetheless, on March 16, the employee unions representing nearly 350,000 VA healthcare workers issued a joint statement that called on VHA management to “work with us to ensure the nation’s VA health facilities can safely handle COVID-19.” It’s time, said Everett Kelley, National President of the American Federation of Government Employees, “for the VA to invite our members to the table, instead of kicking them off the property, so we can finally work together on a solution….”
“Instead of relaxing standards and efforts,” the unions said, “like we have seen the CDC do [in allowing healthcare workers to reuse facemasks and rely on simple surgical facemasks], “we need to be stepping it up.”
It all takes money. After weeks of debate, the US Senate has just released details of the $2 trillion coronavirus aid package. The US Department of Defense (DoD) seems about to get $10.5 billion in emergency funding and the VA another $19.6 billion. The money includes funding for National Guard deployments to help state governments respond to emerging health needs, the expansion of military hospitals and mobile medical centers if needed, and help with production of medical supplies. Nearly $16 billion will be used for direct care specifically in response to veterans’ health needs, covering treatment for COVID-19 in VA hospitals, community urgent care clinics and emergency departments; overtime for clinical staff; and purchase of protective equipment, tests, and other supplies.
Despite having one of the best telehealth systems in the US, the VA has also come under fire for its telehealth preparations to meet the current pandemic-related demand. Former VA Under Secretary of Health Kenneth Kizer wrote in an op-ed for Military Times, “Regrettably, so far, there is no coordinated strategy for ramping up and optimizing the use of telehealth to combat the growing epidemic in the US.” The relief package proposes $3 billion for new telemedicine efforts, including staffing and equipping mobile treatment sites.
In mid-March, the VA had 3,000 coronavirus test kits but still had not used roughly 90%, an article in Mother Jones charged. At a White house press conference around that time, Wilkie was asked how many veterans of those who needed to be tested had been. “We believe we’ve caught most of them,” he replied.
But that was in the early days of the crisis.
With results from the 322 tests administered by Mar. 18, the VA had confirmed five positive cases, was tracking 33 presumptive cases, and acknowledged the first veteran death linked to COVID-19. As of Mar. 26, the VA had administered roughly 7,500 COVID-19 tests nationwide.
Secretary Wilkie has promised that the department’s first focus will always be caring for veterans. In an interview with Military Times, he said, “We don’t release any beds if veterans are needing them. The veterans still are primary. We are a [health] bridge for the larger community, but that’s only after veterans are taken care of.”
US Department of Veterans Affairs (VA) Secretary Robert Wilkie is finding out what it means to be on wartime footing against a virus. He is overseeing the VA’s internal response to COVID-19 while deciding how to fulfil the VA’s fourth mission: providing reinforcement for the nation’s healthcare system in a national emergency. Meanwhile, he’s facing hostilities on a third front: criticism of his efforts so far.
In late February, when lawmakers asked whether the VA needed more resources to fight COVID-19, Wilkie said no. He told NPR on March 19 that “we are poised for the onslaught.” But on March 13, 2020, the VA was being attacked for not releasing a comprehensive emergency response to the incipient pandemic. Wilkie countered, “Before there was a single confirmed case in the US,” he wrote in a recent op-ed piece for Military Times, “the VA was already conducting emergency preparedness exercises.”
In the NPR interview, Wilkie said the VA had undertaken “a very aggressive public health response at an early stage.” Now, the VA has added other measures. The VA, he said, was the first health system to stop people from entering its facilities without being questioned or tested, and the first to adopt the “hard decision” of a no-visitor rule for veterans in nursing homes. Every veteran who comes to a VA facility with flu-like symptoms is screened. Further, via tweets and blog posts, Wilkie is “inviting” retired medical personnel back to work to help deal with the pandemic.
The VA is also the “buttress force,” Wilkie says, for the Federal Emergency Management Agency and the US Department of Health and Human Services if they need medical professionals for crises. “We plan for that every day,” he says. “We are gaming out emergency preparedness scenarios and we stand ready when the President needs us to expand our mission.” But in The American Prospect, Suzanne Gordon and Jasper Craven, both fellows at the Veterans Healthcare Policy Institute, write that “one quiet action is ominous”—the VA website has deleted any mention of the department’s credo of caring for civilians in times of crisis.
According to Gordon and Craven, on Wednesday Wilkie “came out of the woodwork” to express the department’s readiness to help in the crisis. The VA has established 19 emergency operations centers across the country, Wilkie says, and has stopped elective surgeries to free up thousands of beds. He touts the agency’s flexibility, saying it’s prepared to move resources around the country as needed. “Some veterans hospitals have not been impacted [by the virus],” Wilkie said. “So, I’m not going to keep 500 respirators in the middle of a state that has one veteran with the infection, when I can use that in Seattle or New Orleans, or New York City.”
Wilkie says the VA has stockpiled equipment and its supply chain is stable. However, in the NPR interview, Mary Louise Kelly said the NPR VA correspondent had been hearing complaints about lack of gear, such as masks. When pressed on his claim that the VA had adequate protective supplies, Wilkie said those complaints “have not reached us.” In fact, he said, “I can tell you that the arrangements that we have made on both the masks side and also on the testing side—we’re in a very good place.”
Nonetheless, on March 16, the employee unions representing nearly 350,000 VA healthcare workers issued a joint statement that called on VHA management to “work with us to ensure the nation’s VA health facilities can safely handle COVID-19.” It’s time, said Everett Kelley, National President of the American Federation of Government Employees, “for the VA to invite our members to the table, instead of kicking them off the property, so we can finally work together on a solution….”
“Instead of relaxing standards and efforts,” the unions said, “like we have seen the CDC do [in allowing healthcare workers to reuse facemasks and rely on simple surgical facemasks], “we need to be stepping it up.”
It all takes money. After weeks of debate, the US Senate has just released details of the $2 trillion coronavirus aid package. The US Department of Defense (DoD) seems about to get $10.5 billion in emergency funding and the VA another $19.6 billion. The money includes funding for National Guard deployments to help state governments respond to emerging health needs, the expansion of military hospitals and mobile medical centers if needed, and help with production of medical supplies. Nearly $16 billion will be used for direct care specifically in response to veterans’ health needs, covering treatment for COVID-19 in VA hospitals, community urgent care clinics and emergency departments; overtime for clinical staff; and purchase of protective equipment, tests, and other supplies.
Despite having one of the best telehealth systems in the US, the VA has also come under fire for its telehealth preparations to meet the current pandemic-related demand. Former VA Under Secretary of Health Kenneth Kizer wrote in an op-ed for Military Times, “Regrettably, so far, there is no coordinated strategy for ramping up and optimizing the use of telehealth to combat the growing epidemic in the US.” The relief package proposes $3 billion for new telemedicine efforts, including staffing and equipping mobile treatment sites.
In mid-March, the VA had 3,000 coronavirus test kits but still had not used roughly 90%, an article in Mother Jones charged. At a White house press conference around that time, Wilkie was asked how many veterans of those who needed to be tested had been. “We believe we’ve caught most of them,” he replied.
But that was in the early days of the crisis.
With results from the 322 tests administered by Mar. 18, the VA had confirmed five positive cases, was tracking 33 presumptive cases, and acknowledged the first veteran death linked to COVID-19. As of Mar. 26, the VA had administered roughly 7,500 COVID-19 tests nationwide.
Secretary Wilkie has promised that the department’s first focus will always be caring for veterans. In an interview with Military Times, he said, “We don’t release any beds if veterans are needing them. The veterans still are primary. We are a [health] bridge for the larger community, but that’s only after veterans are taken care of.”
Reports suggest possible in utero transmission of novel coronavirus 2019
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
FROM JAMA
Two decades of leadership
In recognition of Dr. Larry Wellikson’s contributions to SHM
It’s already been a few years since I exited the Society of Hospital Medicine’s Board of Directors (2 years, or maybe 3 – I’ve already lost count), and sitting in my proverbial rocking chair in the Old Hospitalists’ Home, I heard, as many of you did, that Larry Wellikson, MD, MHM, the first and only CEO in the Society’s history, is stepping down soon.
With all the idle time that I find myself with these days, I have had the opportunity to ruminate on what Larry has brought to SHM in his 2 decades of leadership. And among the many answers, two stand out for me.
The first is Larry’s deep appreciation of the value of relationships that he has developed and nurtured, an attribute which he has imprinted on many of us who have worked with him over the years. Although Larry speaks of the camaraderie of the first years of SHM and the bonds that he, Bob Wachter, Win Whitcomb, and John Nelson established, he also has kept in touch with a vast network of hospitalists over the last 20-plus years.
Go to lunch with Larry, and be amazed at how much he knows about the goings-on of many of our colleagues. The fondness that Larry has for the people in his life is without parallel. These aren’t just professional colleagues who have impacted him in some way – for Larry, every one of these is a true lifetime friendship, and he continues to establish new ones every year. He makes each of his friends feel truly special to him.
The second is the critical value of and need for change and disruption. The specialty of hospital medicine was, from its beginning, disruptive, and from his career as a physician executive, Larry understood and has brought to SHM an understanding of the necessity of disruption to encourage growth and fresh thinking. If one steps back and looks at, for example, the composition of the Board over the years, or the Journal of Hospital Medicine’s editorial staff, or of our committees, one sees a pattern – a commitment to continuously bringing on young leaders who are still on the early and ascending part of their career paths.
Other organizations identify Board candidates at the peak of their careers, but at SHM, many of us were elected when we had just enough experience to contribute but then continued to grow in our careers after finishing our terms. I joined the Board in 2012 (I think) and while I would probably be a more seasoned and stately Board member if I joined at this point in my life, I would also have less new and novel to offer – and therefore be less effective for what the Society needs. While SHM respects its past leaders, it does not revere them. Our past is important, but our present and future are more important. Larry brought that mentality to SHM.
Ironically, the one position within SHM which has not, until this year, been subject to that same kind of transition is the CEO position itself. And this year, that domino will fall as well. While transitions are hard, change is good – and I am confident that our Society’s commitment to seeking out new, talented leaders, and making transitions at all levels – Board, committees, chapters, speakers – with the intent of bringing new perspectives and creativity, is firmly entrenched in our culture. And Larry can join me in the rocking chair as we relive our common SHM experiences together – and create new memories as well.
Congratulations Larry, and thank you.
Dr. Harte is a past president of SHM, and president of Cleveland Clinic Akron (Ohio) General and the Southern Region. He formerly served as president of Cleveland Clinic Hillcrest Hospital and Cleveland Clinic South Pointe Hospital.
In recognition of Dr. Larry Wellikson’s contributions to SHM
In recognition of Dr. Larry Wellikson’s contributions to SHM
It’s already been a few years since I exited the Society of Hospital Medicine’s Board of Directors (2 years, or maybe 3 – I’ve already lost count), and sitting in my proverbial rocking chair in the Old Hospitalists’ Home, I heard, as many of you did, that Larry Wellikson, MD, MHM, the first and only CEO in the Society’s history, is stepping down soon.
With all the idle time that I find myself with these days, I have had the opportunity to ruminate on what Larry has brought to SHM in his 2 decades of leadership. And among the many answers, two stand out for me.
The first is Larry’s deep appreciation of the value of relationships that he has developed and nurtured, an attribute which he has imprinted on many of us who have worked with him over the years. Although Larry speaks of the camaraderie of the first years of SHM and the bonds that he, Bob Wachter, Win Whitcomb, and John Nelson established, he also has kept in touch with a vast network of hospitalists over the last 20-plus years.
Go to lunch with Larry, and be amazed at how much he knows about the goings-on of many of our colleagues. The fondness that Larry has for the people in his life is without parallel. These aren’t just professional colleagues who have impacted him in some way – for Larry, every one of these is a true lifetime friendship, and he continues to establish new ones every year. He makes each of his friends feel truly special to him.
The second is the critical value of and need for change and disruption. The specialty of hospital medicine was, from its beginning, disruptive, and from his career as a physician executive, Larry understood and has brought to SHM an understanding of the necessity of disruption to encourage growth and fresh thinking. If one steps back and looks at, for example, the composition of the Board over the years, or the Journal of Hospital Medicine’s editorial staff, or of our committees, one sees a pattern – a commitment to continuously bringing on young leaders who are still on the early and ascending part of their career paths.
Other organizations identify Board candidates at the peak of their careers, but at SHM, many of us were elected when we had just enough experience to contribute but then continued to grow in our careers after finishing our terms. I joined the Board in 2012 (I think) and while I would probably be a more seasoned and stately Board member if I joined at this point in my life, I would also have less new and novel to offer – and therefore be less effective for what the Society needs. While SHM respects its past leaders, it does not revere them. Our past is important, but our present and future are more important. Larry brought that mentality to SHM.
Ironically, the one position within SHM which has not, until this year, been subject to that same kind of transition is the CEO position itself. And this year, that domino will fall as well. While transitions are hard, change is good – and I am confident that our Society’s commitment to seeking out new, talented leaders, and making transitions at all levels – Board, committees, chapters, speakers – with the intent of bringing new perspectives and creativity, is firmly entrenched in our culture. And Larry can join me in the rocking chair as we relive our common SHM experiences together – and create new memories as well.
Congratulations Larry, and thank you.
Dr. Harte is a past president of SHM, and president of Cleveland Clinic Akron (Ohio) General and the Southern Region. He formerly served as president of Cleveland Clinic Hillcrest Hospital and Cleveland Clinic South Pointe Hospital.
It’s already been a few years since I exited the Society of Hospital Medicine’s Board of Directors (2 years, or maybe 3 – I’ve already lost count), and sitting in my proverbial rocking chair in the Old Hospitalists’ Home, I heard, as many of you did, that Larry Wellikson, MD, MHM, the first and only CEO in the Society’s history, is stepping down soon.
With all the idle time that I find myself with these days, I have had the opportunity to ruminate on what Larry has brought to SHM in his 2 decades of leadership. And among the many answers, two stand out for me.
The first is Larry’s deep appreciation of the value of relationships that he has developed and nurtured, an attribute which he has imprinted on many of us who have worked with him over the years. Although Larry speaks of the camaraderie of the first years of SHM and the bonds that he, Bob Wachter, Win Whitcomb, and John Nelson established, he also has kept in touch with a vast network of hospitalists over the last 20-plus years.
Go to lunch with Larry, and be amazed at how much he knows about the goings-on of many of our colleagues. The fondness that Larry has for the people in his life is without parallel. These aren’t just professional colleagues who have impacted him in some way – for Larry, every one of these is a true lifetime friendship, and he continues to establish new ones every year. He makes each of his friends feel truly special to him.
The second is the critical value of and need for change and disruption. The specialty of hospital medicine was, from its beginning, disruptive, and from his career as a physician executive, Larry understood and has brought to SHM an understanding of the necessity of disruption to encourage growth and fresh thinking. If one steps back and looks at, for example, the composition of the Board over the years, or the Journal of Hospital Medicine’s editorial staff, or of our committees, one sees a pattern – a commitment to continuously bringing on young leaders who are still on the early and ascending part of their career paths.
Other organizations identify Board candidates at the peak of their careers, but at SHM, many of us were elected when we had just enough experience to contribute but then continued to grow in our careers after finishing our terms. I joined the Board in 2012 (I think) and while I would probably be a more seasoned and stately Board member if I joined at this point in my life, I would also have less new and novel to offer – and therefore be less effective for what the Society needs. While SHM respects its past leaders, it does not revere them. Our past is important, but our present and future are more important. Larry brought that mentality to SHM.
Ironically, the one position within SHM which has not, until this year, been subject to that same kind of transition is the CEO position itself. And this year, that domino will fall as well. While transitions are hard, change is good – and I am confident that our Society’s commitment to seeking out new, talented leaders, and making transitions at all levels – Board, committees, chapters, speakers – with the intent of bringing new perspectives and creativity, is firmly entrenched in our culture. And Larry can join me in the rocking chair as we relive our common SHM experiences together – and create new memories as well.
Congratulations Larry, and thank you.
Dr. Harte is a past president of SHM, and president of Cleveland Clinic Akron (Ohio) General and the Southern Region. He formerly served as president of Cleveland Clinic Hillcrest Hospital and Cleveland Clinic South Pointe Hospital.













