User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Detection of COVID-19 in children in early January 2020 in Wuhan, China
Clinical question: What were the clinical characteristics of children in Wuhan, China hospitalized with SARS-CoV-2?
Background: The coronavirus disease 2019 (COVID-19) was recently described by researchers in Wuhan, China.1 However, there has been limited discussion on how the disease has affected children. Based on the Chinese Center for Disease Control and Prevention report, Wu et al. found that 1% of the affected population was less than 10 years, and another 1% of the affected population was 10-19 years.2 However, little information regarding hospitalizations of children with viral infections was previously reported.
Study design: A retrospective analysis of hospitalized children.
Setting: Three sites of a multisite urban teaching hospital in central Wuhan, China.
Synopsis: Over an 8-day period, hospitalized pediatric patients were retrospectively enrolled into this study. The authors defined pediatric patients as those aged 16 years or younger. The patients had one throat swab specimen collected on admission. Throat swab specimens were tested for viral etiologies. In response to the COVID-19 outbreak, the throat samples were retrospectively tested for SARS-CoV-2. If two independent experiments and a clinically verified diagnostic test confirmed the SARS-CoV-2, the cases were confirmed as COVID-19 cases. During the 8-day period, 366 hospitalized pediatric patients were included in the study. Of the 366 patients, 6 tested positive for SARS-CoV-2, while 23 tested positive for influenza A and 20 tested positive for influenza B. The median age of the six patients was 3 years (range, 1-7 years), and all were previously healthy. All six pediatric patients with COVID-19 had high fevers (greater than 39°C), cough, and lymphopenia. Four of the six affected patients had vomiting and leukopenia, while three of the six patients had neutropenia. Four of the six affected patients had pneumonia, as diagnosed on CT scans. Of the six patients, one patient was admitted to the ICU and received intravenous immunoglobulin. The patient admitted to ICU underwent a CT scan which showed “patchy ground-glass opacities in both lungs,” while three of the five children requiring non-ICU hospitalization had chest radiographs showing “patchy shadows in both lungs.” The median length of stay in the hospital was 7.5 days (range, 5-13 days).
Bottom line: COVID-19 causes moderate to severe respiratory illness in pediatric patients with SARS-CoV-2, possibly leading to critical illness. During this time period of the Wuhan COVID-19 outbreak, pediatric patients were more likely to be hospitalized with influenza A or B, than they were with SARS-CoV-2.
Citation: Liu W et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Dr. Kumar is clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of the Hospitalist.
References
1. Zhu N et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 (Epub ahead of print).
From the Hospitalist editors: The pediatrics “In the Literature” series generally focuses on original articles. However, given the urgency to learn more about SARS-CoV-2/COVID-19 pandemic and the limited literature about hospitalized pediatric patients with the disease, the editors of the Hospitalist thought it was appropriate to share an article reviewing this letter that was recently published in the New England Journal of Medicine.
Clinical question: What were the clinical characteristics of children in Wuhan, China hospitalized with SARS-CoV-2?
Background: The coronavirus disease 2019 (COVID-19) was recently described by researchers in Wuhan, China.1 However, there has been limited discussion on how the disease has affected children. Based on the Chinese Center for Disease Control and Prevention report, Wu et al. found that 1% of the affected population was less than 10 years, and another 1% of the affected population was 10-19 years.2 However, little information regarding hospitalizations of children with viral infections was previously reported.
Study design: A retrospective analysis of hospitalized children.
Setting: Three sites of a multisite urban teaching hospital in central Wuhan, China.
Synopsis: Over an 8-day period, hospitalized pediatric patients were retrospectively enrolled into this study. The authors defined pediatric patients as those aged 16 years or younger. The patients had one throat swab specimen collected on admission. Throat swab specimens were tested for viral etiologies. In response to the COVID-19 outbreak, the throat samples were retrospectively tested for SARS-CoV-2. If two independent experiments and a clinically verified diagnostic test confirmed the SARS-CoV-2, the cases were confirmed as COVID-19 cases. During the 8-day period, 366 hospitalized pediatric patients were included in the study. Of the 366 patients, 6 tested positive for SARS-CoV-2, while 23 tested positive for influenza A and 20 tested positive for influenza B. The median age of the six patients was 3 years (range, 1-7 years), and all were previously healthy. All six pediatric patients with COVID-19 had high fevers (greater than 39°C), cough, and lymphopenia. Four of the six affected patients had vomiting and leukopenia, while three of the six patients had neutropenia. Four of the six affected patients had pneumonia, as diagnosed on CT scans. Of the six patients, one patient was admitted to the ICU and received intravenous immunoglobulin. The patient admitted to ICU underwent a CT scan which showed “patchy ground-glass opacities in both lungs,” while three of the five children requiring non-ICU hospitalization had chest radiographs showing “patchy shadows in both lungs.” The median length of stay in the hospital was 7.5 days (range, 5-13 days).
Bottom line: COVID-19 causes moderate to severe respiratory illness in pediatric patients with SARS-CoV-2, possibly leading to critical illness. During this time period of the Wuhan COVID-19 outbreak, pediatric patients were more likely to be hospitalized with influenza A or B, than they were with SARS-CoV-2.
Citation: Liu W et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Dr. Kumar is clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of the Hospitalist.
References
1. Zhu N et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 (Epub ahead of print).
From the Hospitalist editors: The pediatrics “In the Literature” series generally focuses on original articles. However, given the urgency to learn more about SARS-CoV-2/COVID-19 pandemic and the limited literature about hospitalized pediatric patients with the disease, the editors of the Hospitalist thought it was appropriate to share an article reviewing this letter that was recently published in the New England Journal of Medicine.
Clinical question: What were the clinical characteristics of children in Wuhan, China hospitalized with SARS-CoV-2?
Background: The coronavirus disease 2019 (COVID-19) was recently described by researchers in Wuhan, China.1 However, there has been limited discussion on how the disease has affected children. Based on the Chinese Center for Disease Control and Prevention report, Wu et al. found that 1% of the affected population was less than 10 years, and another 1% of the affected population was 10-19 years.2 However, little information regarding hospitalizations of children with viral infections was previously reported.
Study design: A retrospective analysis of hospitalized children.
Setting: Three sites of a multisite urban teaching hospital in central Wuhan, China.
Synopsis: Over an 8-day period, hospitalized pediatric patients were retrospectively enrolled into this study. The authors defined pediatric patients as those aged 16 years or younger. The patients had one throat swab specimen collected on admission. Throat swab specimens were tested for viral etiologies. In response to the COVID-19 outbreak, the throat samples were retrospectively tested for SARS-CoV-2. If two independent experiments and a clinically verified diagnostic test confirmed the SARS-CoV-2, the cases were confirmed as COVID-19 cases. During the 8-day period, 366 hospitalized pediatric patients were included in the study. Of the 366 patients, 6 tested positive for SARS-CoV-2, while 23 tested positive for influenza A and 20 tested positive for influenza B. The median age of the six patients was 3 years (range, 1-7 years), and all were previously healthy. All six pediatric patients with COVID-19 had high fevers (greater than 39°C), cough, and lymphopenia. Four of the six affected patients had vomiting and leukopenia, while three of the six patients had neutropenia. Four of the six affected patients had pneumonia, as diagnosed on CT scans. Of the six patients, one patient was admitted to the ICU and received intravenous immunoglobulin. The patient admitted to ICU underwent a CT scan which showed “patchy ground-glass opacities in both lungs,” while three of the five children requiring non-ICU hospitalization had chest radiographs showing “patchy shadows in both lungs.” The median length of stay in the hospital was 7.5 days (range, 5-13 days).
Bottom line: COVID-19 causes moderate to severe respiratory illness in pediatric patients with SARS-CoV-2, possibly leading to critical illness. During this time period of the Wuhan COVID-19 outbreak, pediatric patients were more likely to be hospitalized with influenza A or B, than they were with SARS-CoV-2.
Citation: Liu W et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Dr. Kumar is clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of the Hospitalist.
References
1. Zhu N et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 (Epub ahead of print).
From the Hospitalist editors: The pediatrics “In the Literature” series generally focuses on original articles. However, given the urgency to learn more about SARS-CoV-2/COVID-19 pandemic and the limited literature about hospitalized pediatric patients with the disease, the editors of the Hospitalist thought it was appropriate to share an article reviewing this letter that was recently published in the New England Journal of Medicine.
Flattening the curve: Viral graphic shows COVID-19 containment needs
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
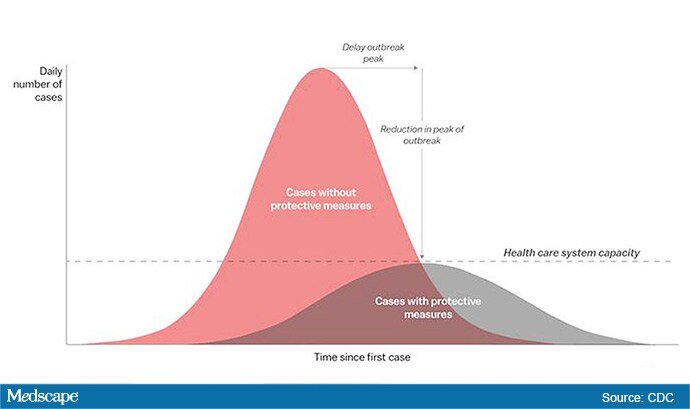
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.
Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.

Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The “Flattening the Curve” graphic, which has, to not use the term lightly, gone viral on social media, visually explains the best currently available strategy to stop the COVID-19 spread, experts told Medscape Medical News.
The height of the curve is the number of potential cases in the United States; along the horizontal X axis, or the breadth, is the amount of time. The line across the middle represents the point at which too many cases in too short a time overwhelm the healthcare system.

Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at the University of Alabama at Birmingham’s School of Medicine explained.
“Not only are you spreading out the new cases but the rate at which people recover,” she told Medscape Medical News. “You have time to get people out of the hospital so you can get new people in and clear out those beds.”
The strategy, with its own Twitter hashtag, #Flattenthecurve, “is about all we have,” without a vaccine, Marrazzo said.
Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said avoiding spikes in cases could mean fewer deaths.
“If you look at the curves of outbreaks, you know, they go big peaks, and then they come down. What we need to do is flatten that down,” Fauci said March 10 in a White House briefing. “You do that by trying to interfere with the natural flow of the outbreak.”
Wuhan, China, at the epicenter of the pandemic, “had an explosive curve” and quickly got overwhelmed without early containment measures, Marrazzo noted. “If you look at Italy right now, it’s clearly in the same situation.”
The Race Is On to Interrupt the Spread
The race is on in the US to interrupt the transmission of the virus and slow the spread, meaning containment measures have increasingly higher and wider stakes.
Closing down Broadway shows and some theme parks and massive sporting events; the escalating numbers of people working from home; and businesses cutting hours or closing all demonstrate the level of US confidence that “social distancing” will work, Marrazzo said.
“We’re clearly ready to disrupt the economy and social infrastructure,” she said.
That appears to have made a difference in Wuhan, Marrazzo said, as the new infections are coming down.
The question, she said, is “we’re not China – so are Americans really going to take to this? Americans greatly value their liberty and there’s some skepticism about public health and its directives. People have never seen a pandemic like this before.”
Dena Grayson, MD, PhD, a Florida-based expert in Ebola and other pandemic threats, told Medscape Medical News that EvergreenHealth in Kirkland, Washington, is a good example of what it means when a virus overwhelms healthcare operations.
The New York Times reported that supplies were so strained at the facility that staff were using sanitary napkins to pad protective helmets.
As of March 11, 65 people who had come into the hospital have tested positive for the virus, and 15 of them had died.
Grayson points out that the COVID-19 cases come on top of a severe flu season and the usual cases hospitals see, so the bar on the graphic is even lower than it usually would be.
“We have a relatively limited capacity with ICU beds to begin with,” she said.
So far, closures, postponements, and cancellations are woefully inadequate, Grayson said.
“We can’t stop this virus. We can hope to contain it and slow down the rate of infection,” she said.
“We need to right now shut down all the schools, preschools, and universities,” Grayson said. “We need to look at shutting down public transportation. We need people to stay home – and not for a day but for a couple of weeks.”
The graphic was developed by visual-data journalist Rosamund Pearce, based on a graphic that had appeared in a Centers for Disease Control and Prevention (CDC) article titled “Community Mitigation Guidelines to Prevent Pandemic Influenza,” the Times reports.
Marrazzo and Grayson have disclosed no relevant financial relationships.
This story first appeared on Medscape.com .
So you have a COVID-19 patient: How do you treat them?
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
Clinicians are working out how to manage patients with or suspected of having COVID-19.
“Over the past couple of weeks, we’ve been preparing for the oncoming onslaught of patients,” said Lillian Wu, MD, of the HealthPoint network in the Seattle area of greater King County and president elect of the Washington Academy of Family Physicians.
Step One: Triage
The first step, Wu says, is careful triage.
When patients call one of the 17 clinics in the HealthPoint system, nurses gauge how sick they are. High fever? Shortness of breath? Do they have a chronic illness, such as diabetes, cardiovascular disease, or a lung condition, that increases risk for infection and complications?
“If a patient has mild symptoms, we ask them to stay home or to check back in 24 hours, or we’ll reach out to them. For moderate symptoms, we ask them to come in, and [we] clearly mark on the schedule that it is a respiratory patient, who will be sent to a separate area. If the patient is severe, we don’t even see them and send them directly to the hospital to the ER,” Wu told Medscape Medical News.
These categories parallel the World Health Organization’s designations of uncomplicated illness, mild pneumonia, severe pneumonia, acute respiratory distress syndrome, sepsis, and septic shock. The Centers for Disease Control and Prevention (CDC) advises case by case regarding decisions as to outpatient or inpatient assignment.
“Patients who pass the initial phone triage are given masks, separated, and sent to different parts of the clinic or are required to wait in their cars until it’s time to be seen,” Wu said.
Step 2: Hospital Arrival
Once at the hospital, the CDC’s interim guidance kicks in.
“Any patient with fever, cough, and shortness of breath presenting with a history of travel to countries with high ongoing transmission or a credible history of exposure should be promptly evaluated for COVID-19,” said Raghavendra Tirupathi, MD, medical director, Keystone Infectious Diseases/HIV; chair in infection prevention, Summit Health; and clinical assistant professor of medicine, Penn State School of Medicine, Hershey, Pennsylvania.
“We recommend obtaining baseline CBC with differential, basic metabolic panel, liver function tests, and procalcitonin. Clues for COVID-19 include leukopenia, seen in 30% to 45% of patients, and lymphocytopenia, seen in 85% of the patients in the case series from China,” Tirupathi said. He uses a respiratory virus polymerase chain reaction panel to rule out other pathogens.
Wu concurs. “This is the one time we are grateful when someone tests positive for the flu! If flu is negative and other common respiratory infections are negative, then we do a COVID-19 test,” she said.
But test results may be delayed. “At the University of Washington, it takes 8 hours, but commercial labs take up to 4 days,” Wu said. All patients with respiratory symptoms are treated as persons under investigation, for whom isolation precautions are required. In addition, for these patients, use of personal protective equipment by caregivers is required.
For suspected pneumonia, the American College of Radiography recommends chest CT to identify peripheral basal ground-glass opacities characteristic of COVID-19.
However, diagnosis should be based on detection of SARS-CoV-2, because chest images for COVID-19 are nonspecific – associated signs can also be seen in H1N1 influenza, SARS, and MERS.
Step 3: Supportive Care
Once a patient is admitted, supportive care entails “maintaining fluid status and nutrition and supporting physiological functions until we heal. It’s treating complications and organ support, whether that means providing supplementary oxygen all the way to ventilator support, and just waiting it out. If a patient progresses to acute respiratory distress syndrome, it becomes tougher,” said David Liebers, MD, chief medical officer and an infectious disease specialist at Ellis Medicine in Schenectady, New York.
Efforts are ramping up to develop therapeutics. Remdesivir, an investigational antiviral drug developed to treat Ebola and Marburg hemorrhagic fevers, shows activity against SARS-CoV-2 in vitro.
Remdesivir has been used in a few patients on a compassionate-use basis outside of a clinical trial setting. “It’s a nucleotide analogue, and like other drugs of that class, it disrupts nucleic acid production. Some data suggest that it might have some efficacy,” Liebers said.
Antibiotics are reserved for patients suspected of having concomitant bacterial or fungal infections. Liebers said clinicians should be alerted to “the big three” signs of secondary infection – fever, elevated white blood cell count, and lactic acidosis. Immunosuppressed patients are at elevated risk for secondary infection.
Step 4: Managing Complications
Patients do die of COVID-19, mostly through an inability to ventilate, even when supported with oxygen, Liebers told Medscape Medical News. (According to Tirupathi, “The studies from China indicate that from 6%-10% of patients needed ventilators.”)
Liebers continued, “Others may develop sepsis or a syndrome of multisystem organ failure with renal and endothelial collapse, making it difficult to maintain blood pressure. Like with so many pathologies, it is a vicious circle in which everything gets overworked. Off-and-on treatments can sometimes break the cycle: supplementary oxygen, giving red blood cells, dialysis. We support those functions while waiting for healing to occur.”
A facility’s airborne-infection isolation rooms may become filled to capacity, but that isn’t critical, Liebers said. “Airborne precautions are standard to contain measles, tuberculosis, chickenpox, and herpes zoster, in which very small particles spread in the air,” he said.
Consensus is growing that SARS-CoV-2 spreads in large droplets, he added. Private rooms and closed doors may suffice.
Step 5: Discharge
Liebers said that as of now, the million-dollar question regards criteria for discharge.
Patients who clinically improve are sent home with instructions to remain in isolation. They may be tested again for virus before or after discharge.
Liebers and Wu pointed to the experience at EvergreenHealth Medical Center, in Kirkland, Washington, as guidance from the trenches. “They’re the ones who are learning firsthand and passing the experience along to everyone else,” Wu said.
“The situation is unprecedented,” said Liebers, who, like many others, has barely slept these past weeks. “We’re swimming in murky water right now.”
The epidemic in the United States is still months from peaking, Wu emphasized. “There is no vaccine, and many cases are subclinical. COVID-19 has to spread through the country before it infects a critical mass of people who will develop immunity. It’s too late to contain.”
Added Liebers, “It’s a constantly changing situation, and we are still being surprised – not that this wasn’t predicted.”
This article first appeared on Medscape.com.
Society of Hospital Medicine cancels 2020 Annual Conference
The Society of Hospital Medicine (SHM) has canceled its annual conference, scheduled for mid-April, joining a growing list of events shuttered by coronavirus (COVID-19) concerns.
In a March 13 announcement, SHM said it would be impossible for the society to host the Hospital Medicine 2020 conference amid the escalating health concerns regarding the global COVID-19 outbreak. For more information about the cancellation and the society’s refund policies, see the SHM website for a list of frequently answered questions.
The Society of Hospital Medicine (SHM) has canceled its annual conference, scheduled for mid-April, joining a growing list of events shuttered by coronavirus (COVID-19) concerns.
In a March 13 announcement, SHM said it would be impossible for the society to host the Hospital Medicine 2020 conference amid the escalating health concerns regarding the global COVID-19 outbreak. For more information about the cancellation and the society’s refund policies, see the SHM website for a list of frequently answered questions.
The Society of Hospital Medicine (SHM) has canceled its annual conference, scheduled for mid-April, joining a growing list of events shuttered by coronavirus (COVID-19) concerns.
In a March 13 announcement, SHM said it would be impossible for the society to host the Hospital Medicine 2020 conference amid the escalating health concerns regarding the global COVID-19 outbreak. For more information about the cancellation and the society’s refund policies, see the SHM website for a list of frequently answered questions.
President declares national emergency for COVID-19, ramps up testing capability
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
After weeks of decline, influenza activity increases slightly
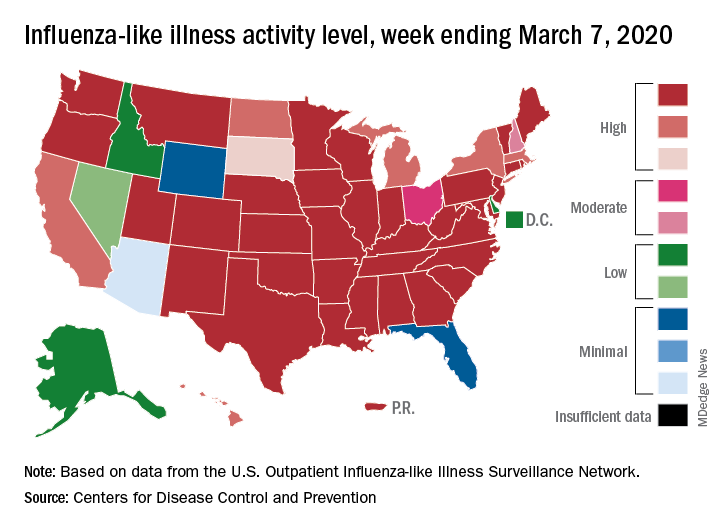
The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.

The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.

The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.
Hospital medicine physician leaders
The right skills and time to develop them
“When you get someone who knows what quality looks like and pair that with curiosity about new ways to think about leading, you end up with the people who are able to produce dramatic innovations in the field.”1
In medicine, a physician is trained to take charge in emergent situations and make potentially lifesaving efforts. However, when it comes to leading teams of individuals, not only must successful leaders have the right skills, they also need time to dedicate to the work of leadership.
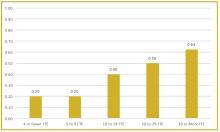
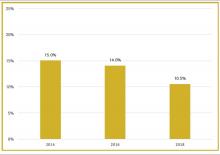
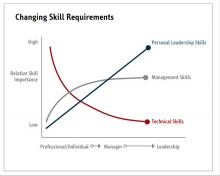
To better understand current approaches to dedicated hospital medicine group (HMG) leadership time, let’s examine the 2018 State of Hospital Medicine (SoHM) Report. The survey, upon which the Report was based, examined two aspects of leadership: 1) how much dedicated time a leader receives to manage the group; and 2) how the leader’s time is compensated. Looking closely at the data displayed in graphs from the SoHM Report (Figures 1, 2, and 3), we can see that dedicated administrative time is directly proportional to the size of the group.
In my current role as a regional medical director in the Dallas-Fort Worth market, I oversee some programs where the size is greater than 30 full-time equivalents (FTEs), and requires a full-time administrative physician leader to manage the group. Their daily administrative duties include, but are not limited to, addressing physician performance and behaviors, managing team performance metrics, dealing with consultants’ expectations, attending and leading various committee meetings at the hospital or the system level, attending and presenting performance reviews, leading and preparing for team meetings, as well as addressing and being innovative in leading new initiatives from the hospital partner system.
Although physician leaders are paid more for their work, the 2018 SoHM Report reveals a decline in the premium year over year. One of the reasons for the payment decline that I have encountered in various groups is that their incentives for leading the group are based on performance, as opposed to receiving a fixed stipend. Another reason is the presence of dedicated administrative support or the inclusion of a performance improvement staffer, such as an additional nurse or advanced practice provider, in the group.
Evidence suggests that organizations and patients benefit when physicians take on leadership roles. Physician leaders play critical roles in providing high-quality patient care. How can the Society of Hospital Medicine help? Management degrees and leadership workshops have become a common pathway for many physicians, including myself. SHM provides one of the most thorough and relevant experiences through the SHM Leadership Academy. The focus of the Leadership Academy is on developing a broad set of additional leadership competencies across a spectrum of experience.5 As hospitalist physicians are often expected to fulfill a broader leadership void, we must pay attention to developing the leadership skills depicted in Figure 3. Hospital medicine is an ideal “proving ground” for future physician executives and leaders, as they often share the same characteristics required for success.
The leadership paths available in my organization, Sound Physicians, were recently highlighted in a New York Times article.3 Sound Physicians employs more than 3,000 physicians across the country, and has a pipeline for doctors to advance through structured rungs of leadership – emphasizing a different mix of clinical, strategic, and business skills at each stage, from individual practitioner to the C-suite. The training includes in-person and online courses, as well as an annual conference, to help doctors develop management and leadership competencies, and learn how to apply these skills within their organizations. Since introducing its leadership development program, the company reports less turnover, higher morale, and better growth. I personally have gone through the leadership training provided by Sound Physicians, and reflecting back, it has been a transformational experience for me. Leadership is a journey, not a destination, and as physicians we should strive to learn more from the health care leaders around us.
The administrative workload for hospital-based physician leaders will increase with the arrival of value-based programs and alternative payment models promoted by the Centers for Medicare and Medicaid Services. Lead hospitalist duties are not limited to daily operations, but can extend to leading the strategic vision of the hospital or health system. The 2020 SoHM Report will reflect these changes, as well as provide further information about how to manage and set expectations for physician leaders, based on group size and employment model.
Dr. Patel is a regional medical director with Sound Physicians. He manages more than 100 FTE hospitalists and advanced-practice providers (APPs) within multiple health systems and hospitals in the Texas market. He also serves as a member of the SHM Practice Analysis Committee and as a vice president of SHM North Texas Chapter.
References
1. Angood P and Birk S. The Value of Physician Leadership. Physician Exec. 2014 May-Jun;40(3):6-20.
2. Rice JA. Expanding the Need for Physician Leaders. Executive Insight, Advance Healthcare Network, Nov 16, 2011. Available at: http://healthcare-executive-insight.advanceweb.com/Features/Articles/Expanding-the-Need-for-Physician-Leaders.aspx.
3. Khullar D. Good leaders make good doctors. New York Times. 2019 Nov 21.
4. Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
5. Harte B. Hospitalists can meet the demand for physician executives. Hospitalist. 2018 Nov 29.
The right skills and time to develop them
The right skills and time to develop them
“When you get someone who knows what quality looks like and pair that with curiosity about new ways to think about leading, you end up with the people who are able to produce dramatic innovations in the field.”1
In medicine, a physician is trained to take charge in emergent situations and make potentially lifesaving efforts. However, when it comes to leading teams of individuals, not only must successful leaders have the right skills, they also need time to dedicate to the work of leadership.
To better understand current approaches to dedicated hospital medicine group (HMG) leadership time, let’s examine the 2018 State of Hospital Medicine (SoHM) Report. The survey, upon which the Report was based, examined two aspects of leadership: 1) how much dedicated time a leader receives to manage the group; and 2) how the leader’s time is compensated. Looking closely at the data displayed in graphs from the SoHM Report (Figures 1, 2, and 3), we can see that dedicated administrative time is directly proportional to the size of the group.
In my current role as a regional medical director in the Dallas-Fort Worth market, I oversee some programs where the size is greater than 30 full-time equivalents (FTEs), and requires a full-time administrative physician leader to manage the group. Their daily administrative duties include, but are not limited to, addressing physician performance and behaviors, managing team performance metrics, dealing with consultants’ expectations, attending and leading various committee meetings at the hospital or the system level, attending and presenting performance reviews, leading and preparing for team meetings, as well as addressing and being innovative in leading new initiatives from the hospital partner system.
Although physician leaders are paid more for their work, the 2018 SoHM Report reveals a decline in the premium year over year. One of the reasons for the payment decline that I have encountered in various groups is that their incentives for leading the group are based on performance, as opposed to receiving a fixed stipend. Another reason is the presence of dedicated administrative support or the inclusion of a performance improvement staffer, such as an additional nurse or advanced practice provider, in the group.
Evidence suggests that organizations and patients benefit when physicians take on leadership roles. Physician leaders play critical roles in providing high-quality patient care. How can the Society of Hospital Medicine help? Management degrees and leadership workshops have become a common pathway for many physicians, including myself. SHM provides one of the most thorough and relevant experiences through the SHM Leadership Academy. The focus of the Leadership Academy is on developing a broad set of additional leadership competencies across a spectrum of experience.5 As hospitalist physicians are often expected to fulfill a broader leadership void, we must pay attention to developing the leadership skills depicted in Figure 3. Hospital medicine is an ideal “proving ground” for future physician executives and leaders, as they often share the same characteristics required for success.
The leadership paths available in my organization, Sound Physicians, were recently highlighted in a New York Times article.3 Sound Physicians employs more than 3,000 physicians across the country, and has a pipeline for doctors to advance through structured rungs of leadership – emphasizing a different mix of clinical, strategic, and business skills at each stage, from individual practitioner to the C-suite. The training includes in-person and online courses, as well as an annual conference, to help doctors develop management and leadership competencies, and learn how to apply these skills within their organizations. Since introducing its leadership development program, the company reports less turnover, higher morale, and better growth. I personally have gone through the leadership training provided by Sound Physicians, and reflecting back, it has been a transformational experience for me. Leadership is a journey, not a destination, and as physicians we should strive to learn more from the health care leaders around us.
The administrative workload for hospital-based physician leaders will increase with the arrival of value-based programs and alternative payment models promoted by the Centers for Medicare and Medicaid Services. Lead hospitalist duties are not limited to daily operations, but can extend to leading the strategic vision of the hospital or health system. The 2020 SoHM Report will reflect these changes, as well as provide further information about how to manage and set expectations for physician leaders, based on group size and employment model.
Dr. Patel is a regional medical director with Sound Physicians. He manages more than 100 FTE hospitalists and advanced-practice providers (APPs) within multiple health systems and hospitals in the Texas market. He also serves as a member of the SHM Practice Analysis Committee and as a vice president of SHM North Texas Chapter.
References
1. Angood P and Birk S. The Value of Physician Leadership. Physician Exec. 2014 May-Jun;40(3):6-20.
2. Rice JA. Expanding the Need for Physician Leaders. Executive Insight, Advance Healthcare Network, Nov 16, 2011. Available at: http://healthcare-executive-insight.advanceweb.com/Features/Articles/Expanding-the-Need-for-Physician-Leaders.aspx.
3. Khullar D. Good leaders make good doctors. New York Times. 2019 Nov 21.
4. Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
5. Harte B. Hospitalists can meet the demand for physician executives. Hospitalist. 2018 Nov 29.
“When you get someone who knows what quality looks like and pair that with curiosity about new ways to think about leading, you end up with the people who are able to produce dramatic innovations in the field.”1
In medicine, a physician is trained to take charge in emergent situations and make potentially lifesaving efforts. However, when it comes to leading teams of individuals, not only must successful leaders have the right skills, they also need time to dedicate to the work of leadership.
To better understand current approaches to dedicated hospital medicine group (HMG) leadership time, let’s examine the 2018 State of Hospital Medicine (SoHM) Report. The survey, upon which the Report was based, examined two aspects of leadership: 1) how much dedicated time a leader receives to manage the group; and 2) how the leader’s time is compensated. Looking closely at the data displayed in graphs from the SoHM Report (Figures 1, 2, and 3), we can see that dedicated administrative time is directly proportional to the size of the group.
In my current role as a regional medical director in the Dallas-Fort Worth market, I oversee some programs where the size is greater than 30 full-time equivalents (FTEs), and requires a full-time administrative physician leader to manage the group. Their daily administrative duties include, but are not limited to, addressing physician performance and behaviors, managing team performance metrics, dealing with consultants’ expectations, attending and leading various committee meetings at the hospital or the system level, attending and presenting performance reviews, leading and preparing for team meetings, as well as addressing and being innovative in leading new initiatives from the hospital partner system.
Although physician leaders are paid more for their work, the 2018 SoHM Report reveals a decline in the premium year over year. One of the reasons for the payment decline that I have encountered in various groups is that their incentives for leading the group are based on performance, as opposed to receiving a fixed stipend. Another reason is the presence of dedicated administrative support or the inclusion of a performance improvement staffer, such as an additional nurse or advanced practice provider, in the group.
Evidence suggests that organizations and patients benefit when physicians take on leadership roles. Physician leaders play critical roles in providing high-quality patient care. How can the Society of Hospital Medicine help? Management degrees and leadership workshops have become a common pathway for many physicians, including myself. SHM provides one of the most thorough and relevant experiences through the SHM Leadership Academy. The focus of the Leadership Academy is on developing a broad set of additional leadership competencies across a spectrum of experience.5 As hospitalist physicians are often expected to fulfill a broader leadership void, we must pay attention to developing the leadership skills depicted in Figure 3. Hospital medicine is an ideal “proving ground” for future physician executives and leaders, as they often share the same characteristics required for success.
The leadership paths available in my organization, Sound Physicians, were recently highlighted in a New York Times article.3 Sound Physicians employs more than 3,000 physicians across the country, and has a pipeline for doctors to advance through structured rungs of leadership – emphasizing a different mix of clinical, strategic, and business skills at each stage, from individual practitioner to the C-suite. The training includes in-person and online courses, as well as an annual conference, to help doctors develop management and leadership competencies, and learn how to apply these skills within their organizations. Since introducing its leadership development program, the company reports less turnover, higher morale, and better growth. I personally have gone through the leadership training provided by Sound Physicians, and reflecting back, it has been a transformational experience for me. Leadership is a journey, not a destination, and as physicians we should strive to learn more from the health care leaders around us.
The administrative workload for hospital-based physician leaders will increase with the arrival of value-based programs and alternative payment models promoted by the Centers for Medicare and Medicaid Services. Lead hospitalist duties are not limited to daily operations, but can extend to leading the strategic vision of the hospital or health system. The 2020 SoHM Report will reflect these changes, as well as provide further information about how to manage and set expectations for physician leaders, based on group size and employment model.
Dr. Patel is a regional medical director with Sound Physicians. He manages more than 100 FTE hospitalists and advanced-practice providers (APPs) within multiple health systems and hospitals in the Texas market. He also serves as a member of the SHM Practice Analysis Committee and as a vice president of SHM North Texas Chapter.
References
1. Angood P and Birk S. The Value of Physician Leadership. Physician Exec. 2014 May-Jun;40(3):6-20.
2. Rice JA. Expanding the Need for Physician Leaders. Executive Insight, Advance Healthcare Network, Nov 16, 2011. Available at: http://healthcare-executive-insight.advanceweb.com/Features/Articles/Expanding-the-Need-for-Physician-Leaders.aspx.
3. Khullar D. Good leaders make good doctors. New York Times. 2019 Nov 21.
4. Beresford L. The State of Hospital Medicine in 2018. Hospitalist. 2019;23(1):1-11.
5. Harte B. Hospitalists can meet the demand for physician executives. Hospitalist. 2018 Nov 29.
Lombardy ICU capacity stressed to breaking point by COVID-19 outbreak
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.
Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
REPORTING FROM JAMA LIVE STREAM
FDA warns of serious infection risk after FMT
The Food and Drug Administration has issued a Safety Alert warning of the potential risk of serious, life-threatening infection in patients who receive fecal microbiota transplant for Clostridioides difficile infection.
The FDA has received six reports of infection associated with fecal microbiota transplant from a stool bank company based in the United States: Two patients had enteropathogenic Escherichia coli (EPEC) infection, and four had shiga toxin–producing E. coli (STEC). The two EPEC infections came from two separate donors, but the four STEC infections came from a single donor, according to the FDA.
In addition, two patients died after receiving fecal microbiota transplant from the donor associated with the STEC infections. These patients died before any of the STEC infections were reported to the FDA; as their stool was not tested for STEC, it is unclear whether it contributed to their deaths.
The use of fecal microbiota transplant is still investigational, and as such, patients should be made aware by health care providers of the risks, which include the potential for transmission of pathogenic bacteria and the resultant adverse events, the FDA said in the press release.
The Food and Drug Administration has issued a Safety Alert warning of the potential risk of serious, life-threatening infection in patients who receive fecal microbiota transplant for Clostridioides difficile infection.
The FDA has received six reports of infection associated with fecal microbiota transplant from a stool bank company based in the United States: Two patients had enteropathogenic Escherichia coli (EPEC) infection, and four had shiga toxin–producing E. coli (STEC). The two EPEC infections came from two separate donors, but the four STEC infections came from a single donor, according to the FDA.
In addition, two patients died after receiving fecal microbiota transplant from the donor associated with the STEC infections. These patients died before any of the STEC infections were reported to the FDA; as their stool was not tested for STEC, it is unclear whether it contributed to their deaths.
The use of fecal microbiota transplant is still investigational, and as such, patients should be made aware by health care providers of the risks, which include the potential for transmission of pathogenic bacteria and the resultant adverse events, the FDA said in the press release.
The Food and Drug Administration has issued a Safety Alert warning of the potential risk of serious, life-threatening infection in patients who receive fecal microbiota transplant for Clostridioides difficile infection.
The FDA has received six reports of infection associated with fecal microbiota transplant from a stool bank company based in the United States: Two patients had enteropathogenic Escherichia coli (EPEC) infection, and four had shiga toxin–producing E. coli (STEC). The two EPEC infections came from two separate donors, but the four STEC infections came from a single donor, according to the FDA.
In addition, two patients died after receiving fecal microbiota transplant from the donor associated with the STEC infections. These patients died before any of the STEC infections were reported to the FDA; as their stool was not tested for STEC, it is unclear whether it contributed to their deaths.
The use of fecal microbiota transplant is still investigational, and as such, patients should be made aware by health care providers of the risks, which include the potential for transmission of pathogenic bacteria and the resultant adverse events, the FDA said in the press release.
Wuhan case review: COVID-19 characteristics differ in children vs. adults
Pediatric cases of COVID-19 infection are typically mild, but underlying coinfection may be more common in children than in adults, according to an analysis of clinical, laboratory, and chest CT features of pediatric inpatients in Wuhan, China.

The findings point toward a need for early chest CT with corresponding pathogen detection in children with suspected COVID-19 infection, Wei Xia, MD, of Huazhong University of Science and Technology, Wuhan, China, and colleagues reported in Pediatric Pulmonology.
The most common symptoms in 20 pediatric patients hospitalized between Jan. 23 and Feb. 8, 2020, with COVID-19 infection confirmed by the pharyngeal swab COVID-19 nucleic acid test were fever and cough, which occurred in 60% and 65% of patients, respectively. Coinfection was detected in eight patients (40%), they noted.
Clinical manifestations were similar to those seen in adults, but overall symptoms were relatively mild and overall prognosis was good. Of particular note, 7 of the 20 (35%) patients had a previously diagnosed congenital or acquired diseases, suggesting that children with underlying conditions may be more susceptible, Dr. Xia and colleagues wrote.
Laboratory findings also were notable in that 80% of the children had procalcitonin (PCT) elevations not typically seen in adults with COVID-19. PCT is a marker for bacterial infection and “[this finding] may suggest that routine antibacterial treatment should be considered in pediatric patients,” the investigators wrote.
As for imaging results, chest CT findings in children were similar to those in adults.“The typical manifestations were unilateral or bilateral subpleural ground-glass opacities, and consolidations with surrounding halo signs,” Dr. Xia and associates wrote, adding that consolidations with surrounding halo sign accounted for about half the pediatric cases and should be considered as “typical signs in pediatric patients.”
Pediatric cases were “rather rare” in the early days of the COVID-19 outbreak in Wuhan, where the first cases of infection were reported.
“As a pediatric group is usually susceptible to upper respiratory tract infection, because of their developing immune system, the delayed presence of pediatric patients is confusing,” the investigators wrote, noting that a low detection rate of pharyngeal swab COVID-19 nucleic acid test, distinguishing the virus from other common respiratory tract infectious pathogens in pediatric patients, “is still a problem.”
To better characterize the clinical and imaging features in children versus adults with COVID-19, Dr. Xia and associates reviewed these 20 pediatric cases, including 13 boys and 7 girls with ages ranging from less than 1 month to 14 years, 7 months (median 2 years, 1.5 months). Thirteen had an identified close contact with a COVID-19–diagnosed family member, and all were treated in an isolation ward. A total of 18 children were cured and discharged after an average stay of 13 days, and 2 neonates remained under observation because of positive swab results with negative CT findings. The investigators speculated that the different findings in neonates were perhaps caused by the influence of delivery on sampling or the specific CT manifestations for neonates, adding that more samples are needed for further clarification.
Based on these findings, “the CT imaging of COVID-19 infection should be differentiated with other virus pneumonias such as influenza virus, parainfluenza virus, respiratory syncytial virus, and adenovirus,” they concluded. It also should “be differentiated from bacterial pneumonia, mycoplasma pneumonia, and chlamydia pneumonia ... the density of pneumonia lesions caused by the latter pathogens is relatively higher.”
However, Dr. Xia and colleagues noted that chest CT manifestations of pneumonia caused by different pathogens overlap, and COVID-19 pneumonia “can be superimposed with serious and complex imaging manifestations, so epidemiological and etiological examinations should be combined.”
The investigators concluded that COVID-19 virus pneumonia in children is generally mild, and that the characteristic changes of subpleural ground-glass opacities and consolidations with surrounding halo on chest CT provide an “effective means for follow-up and evaluating the changes of lung lesions.”
“In the case that the positive rate of COVID-19 nucleic acid test from pharyngeal swab samples is not high, the early detection of lesions by CT is conducive to reasonable management and early treatment for pediatric patients. However, the diagnosis of COVID-19 pneumonia by CT imaging alone is not sufficient enough, especially in the case of coinfection with other pathogens,” Dr. Xia and associates wrote. “Therefore, early chest CT screening and timely follow-up, combined with corresponding pathogen detection, is a feasible clinical protocol in children.”
An early study
In a separate retrospective analysis described in a letter to the editor of the New England Journal of Medicine, Weiyong Liu, PhD, of Tongji Hospital of Huazhong University of Science and Technology and colleagues found that the most frequently detected pathogens in 366 children under the age of 16 years hospitalized with respiratory infections in Wuhan during Jan. 7-15, 2020, were influenza A virus (6.3% of cases) and influenza B virus (5.5% of cases), whereas COVID-19 was detected in 1.6% of cases.
The median age of the COVID-19 patients in that series was 3 years (range 1-7 years), and in contrast to the findings of Xia et al., all previously had been “completely healthy.” Common characteristics were high fever and cough in all six patients, and vomiting in four patients. Five had pneumonia as assessed by X-ray, and CTs showed typical viral pneumonia patterns.
One patient was admitted to a pediatric ICU. All patients received antiviral agents, antibiotic agents, and supportive therapies; all recovered after a median hospital stay of 7.5 days (median range, 5-13 days).
In contrast with the findings of Xia et al., the findings of Liu et al. showed COVID-19 caused moderate to severe respiratory illness in children, and that infections in children were occurring early in the epidemic.
Some perspective
In an interview regarding the findings by Xia et al., Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University, and director of pediatric infectious diseases at Boston Medical Center, noted the absence of fever in 40% of cases.
“This is important, as the criteria for testing by public health departments has been high fever, cough, and shortness of breath,” he said. “The absence of fever is not inconsistent with COVID-19 disease.”
Another important point regarding the findings by Xia et al. is that the highest attack rates appear to be in children under 1 year of age, he said, further noting that the finding of concurrent influenza A, influenza B, or respiratory syncytial virus underscores that “concurrent infection can occur, and the presence of another virus in diagnostic tests does not mean that COVID-19 is not causal.”
As for whether the finding of elevated procalcitonin levels in 80% of cases reflects COVID-19 disease or coinfection with bacteria, the answer is unclear. But none of the children in the study were proven to have bacterial disease, he said, adding that “this marker will need to be interpreted with caution in the setting of COVID-19 disease.”
Dr. Xia and colleagues reported having no disclosures. Dr. Liu and associates also reported having no disclosures. The study by Liu et al. was supported by the Ministry of Science and Technology of China, the National Mega Project on Major Infectious Disease Prevention, and the National Key Research and Development Program of China.
SOURCES: Xia W et al. Ped Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718; Liu W et al. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Pediatric cases of COVID-19 infection are typically mild, but underlying coinfection may be more common in children than in adults, according to an analysis of clinical, laboratory, and chest CT features of pediatric inpatients in Wuhan, China.

The findings point toward a need for early chest CT with corresponding pathogen detection in children with suspected COVID-19 infection, Wei Xia, MD, of Huazhong University of Science and Technology, Wuhan, China, and colleagues reported in Pediatric Pulmonology.
The most common symptoms in 20 pediatric patients hospitalized between Jan. 23 and Feb. 8, 2020, with COVID-19 infection confirmed by the pharyngeal swab COVID-19 nucleic acid test were fever and cough, which occurred in 60% and 65% of patients, respectively. Coinfection was detected in eight patients (40%), they noted.
Clinical manifestations were similar to those seen in adults, but overall symptoms were relatively mild and overall prognosis was good. Of particular note, 7 of the 20 (35%) patients had a previously diagnosed congenital or acquired diseases, suggesting that children with underlying conditions may be more susceptible, Dr. Xia and colleagues wrote.
Laboratory findings also were notable in that 80% of the children had procalcitonin (PCT) elevations not typically seen in adults with COVID-19. PCT is a marker for bacterial infection and “[this finding] may suggest that routine antibacterial treatment should be considered in pediatric patients,” the investigators wrote.
As for imaging results, chest CT findings in children were similar to those in adults.“The typical manifestations were unilateral or bilateral subpleural ground-glass opacities, and consolidations with surrounding halo signs,” Dr. Xia and associates wrote, adding that consolidations with surrounding halo sign accounted for about half the pediatric cases and should be considered as “typical signs in pediatric patients.”
Pediatric cases were “rather rare” in the early days of the COVID-19 outbreak in Wuhan, where the first cases of infection were reported.
“As a pediatric group is usually susceptible to upper respiratory tract infection, because of their developing immune system, the delayed presence of pediatric patients is confusing,” the investigators wrote, noting that a low detection rate of pharyngeal swab COVID-19 nucleic acid test, distinguishing the virus from other common respiratory tract infectious pathogens in pediatric patients, “is still a problem.”
To better characterize the clinical and imaging features in children versus adults with COVID-19, Dr. Xia and associates reviewed these 20 pediatric cases, including 13 boys and 7 girls with ages ranging from less than 1 month to 14 years, 7 months (median 2 years, 1.5 months). Thirteen had an identified close contact with a COVID-19–diagnosed family member, and all were treated in an isolation ward. A total of 18 children were cured and discharged after an average stay of 13 days, and 2 neonates remained under observation because of positive swab results with negative CT findings. The investigators speculated that the different findings in neonates were perhaps caused by the influence of delivery on sampling or the specific CT manifestations for neonates, adding that more samples are needed for further clarification.
Based on these findings, “the CT imaging of COVID-19 infection should be differentiated with other virus pneumonias such as influenza virus, parainfluenza virus, respiratory syncytial virus, and adenovirus,” they concluded. It also should “be differentiated from bacterial pneumonia, mycoplasma pneumonia, and chlamydia pneumonia ... the density of pneumonia lesions caused by the latter pathogens is relatively higher.”
However, Dr. Xia and colleagues noted that chest CT manifestations of pneumonia caused by different pathogens overlap, and COVID-19 pneumonia “can be superimposed with serious and complex imaging manifestations, so epidemiological and etiological examinations should be combined.”
The investigators concluded that COVID-19 virus pneumonia in children is generally mild, and that the characteristic changes of subpleural ground-glass opacities and consolidations with surrounding halo on chest CT provide an “effective means for follow-up and evaluating the changes of lung lesions.”
“In the case that the positive rate of COVID-19 nucleic acid test from pharyngeal swab samples is not high, the early detection of lesions by CT is conducive to reasonable management and early treatment for pediatric patients. However, the diagnosis of COVID-19 pneumonia by CT imaging alone is not sufficient enough, especially in the case of coinfection with other pathogens,” Dr. Xia and associates wrote. “Therefore, early chest CT screening and timely follow-up, combined with corresponding pathogen detection, is a feasible clinical protocol in children.”
An early study
In a separate retrospective analysis described in a letter to the editor of the New England Journal of Medicine, Weiyong Liu, PhD, of Tongji Hospital of Huazhong University of Science and Technology and colleagues found that the most frequently detected pathogens in 366 children under the age of 16 years hospitalized with respiratory infections in Wuhan during Jan. 7-15, 2020, were influenza A virus (6.3% of cases) and influenza B virus (5.5% of cases), whereas COVID-19 was detected in 1.6% of cases.
The median age of the COVID-19 patients in that series was 3 years (range 1-7 years), and in contrast to the findings of Xia et al., all previously had been “completely healthy.” Common characteristics were high fever and cough in all six patients, and vomiting in four patients. Five had pneumonia as assessed by X-ray, and CTs showed typical viral pneumonia patterns.
One patient was admitted to a pediatric ICU. All patients received antiviral agents, antibiotic agents, and supportive therapies; all recovered after a median hospital stay of 7.5 days (median range, 5-13 days).
In contrast with the findings of Xia et al., the findings of Liu et al. showed COVID-19 caused moderate to severe respiratory illness in children, and that infections in children were occurring early in the epidemic.
Some perspective
In an interview regarding the findings by Xia et al., Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University, and director of pediatric infectious diseases at Boston Medical Center, noted the absence of fever in 40% of cases.
“This is important, as the criteria for testing by public health departments has been high fever, cough, and shortness of breath,” he said. “The absence of fever is not inconsistent with COVID-19 disease.”
Another important point regarding the findings by Xia et al. is that the highest attack rates appear to be in children under 1 year of age, he said, further noting that the finding of concurrent influenza A, influenza B, or respiratory syncytial virus underscores that “concurrent infection can occur, and the presence of another virus in diagnostic tests does not mean that COVID-19 is not causal.”
As for whether the finding of elevated procalcitonin levels in 80% of cases reflects COVID-19 disease or coinfection with bacteria, the answer is unclear. But none of the children in the study were proven to have bacterial disease, he said, adding that “this marker will need to be interpreted with caution in the setting of COVID-19 disease.”
Dr. Xia and colleagues reported having no disclosures. Dr. Liu and associates also reported having no disclosures. The study by Liu et al. was supported by the Ministry of Science and Technology of China, the National Mega Project on Major Infectious Disease Prevention, and the National Key Research and Development Program of China.
SOURCES: Xia W et al. Ped Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718; Liu W et al. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Pediatric cases of COVID-19 infection are typically mild, but underlying coinfection may be more common in children than in adults, according to an analysis of clinical, laboratory, and chest CT features of pediatric inpatients in Wuhan, China.

The findings point toward a need for early chest CT with corresponding pathogen detection in children with suspected COVID-19 infection, Wei Xia, MD, of Huazhong University of Science and Technology, Wuhan, China, and colleagues reported in Pediatric Pulmonology.
The most common symptoms in 20 pediatric patients hospitalized between Jan. 23 and Feb. 8, 2020, with COVID-19 infection confirmed by the pharyngeal swab COVID-19 nucleic acid test were fever and cough, which occurred in 60% and 65% of patients, respectively. Coinfection was detected in eight patients (40%), they noted.
Clinical manifestations were similar to those seen in adults, but overall symptoms were relatively mild and overall prognosis was good. Of particular note, 7 of the 20 (35%) patients had a previously diagnosed congenital or acquired diseases, suggesting that children with underlying conditions may be more susceptible, Dr. Xia and colleagues wrote.
Laboratory findings also were notable in that 80% of the children had procalcitonin (PCT) elevations not typically seen in adults with COVID-19. PCT is a marker for bacterial infection and “[this finding] may suggest that routine antibacterial treatment should be considered in pediatric patients,” the investigators wrote.
As for imaging results, chest CT findings in children were similar to those in adults.“The typical manifestations were unilateral or bilateral subpleural ground-glass opacities, and consolidations with surrounding halo signs,” Dr. Xia and associates wrote, adding that consolidations with surrounding halo sign accounted for about half the pediatric cases and should be considered as “typical signs in pediatric patients.”
Pediatric cases were “rather rare” in the early days of the COVID-19 outbreak in Wuhan, where the first cases of infection were reported.
“As a pediatric group is usually susceptible to upper respiratory tract infection, because of their developing immune system, the delayed presence of pediatric patients is confusing,” the investigators wrote, noting that a low detection rate of pharyngeal swab COVID-19 nucleic acid test, distinguishing the virus from other common respiratory tract infectious pathogens in pediatric patients, “is still a problem.”
To better characterize the clinical and imaging features in children versus adults with COVID-19, Dr. Xia and associates reviewed these 20 pediatric cases, including 13 boys and 7 girls with ages ranging from less than 1 month to 14 years, 7 months (median 2 years, 1.5 months). Thirteen had an identified close contact with a COVID-19–diagnosed family member, and all were treated in an isolation ward. A total of 18 children were cured and discharged after an average stay of 13 days, and 2 neonates remained under observation because of positive swab results with negative CT findings. The investigators speculated that the different findings in neonates were perhaps caused by the influence of delivery on sampling or the specific CT manifestations for neonates, adding that more samples are needed for further clarification.
Based on these findings, “the CT imaging of COVID-19 infection should be differentiated with other virus pneumonias such as influenza virus, parainfluenza virus, respiratory syncytial virus, and adenovirus,” they concluded. It also should “be differentiated from bacterial pneumonia, mycoplasma pneumonia, and chlamydia pneumonia ... the density of pneumonia lesions caused by the latter pathogens is relatively higher.”
However, Dr. Xia and colleagues noted that chest CT manifestations of pneumonia caused by different pathogens overlap, and COVID-19 pneumonia “can be superimposed with serious and complex imaging manifestations, so epidemiological and etiological examinations should be combined.”
The investigators concluded that COVID-19 virus pneumonia in children is generally mild, and that the characteristic changes of subpleural ground-glass opacities and consolidations with surrounding halo on chest CT provide an “effective means for follow-up and evaluating the changes of lung lesions.”
“In the case that the positive rate of COVID-19 nucleic acid test from pharyngeal swab samples is not high, the early detection of lesions by CT is conducive to reasonable management and early treatment for pediatric patients. However, the diagnosis of COVID-19 pneumonia by CT imaging alone is not sufficient enough, especially in the case of coinfection with other pathogens,” Dr. Xia and associates wrote. “Therefore, early chest CT screening and timely follow-up, combined with corresponding pathogen detection, is a feasible clinical protocol in children.”
An early study
In a separate retrospective analysis described in a letter to the editor of the New England Journal of Medicine, Weiyong Liu, PhD, of Tongji Hospital of Huazhong University of Science and Technology and colleagues found that the most frequently detected pathogens in 366 children under the age of 16 years hospitalized with respiratory infections in Wuhan during Jan. 7-15, 2020, were influenza A virus (6.3% of cases) and influenza B virus (5.5% of cases), whereas COVID-19 was detected in 1.6% of cases.
The median age of the COVID-19 patients in that series was 3 years (range 1-7 years), and in contrast to the findings of Xia et al., all previously had been “completely healthy.” Common characteristics were high fever and cough in all six patients, and vomiting in four patients. Five had pneumonia as assessed by X-ray, and CTs showed typical viral pneumonia patterns.
One patient was admitted to a pediatric ICU. All patients received antiviral agents, antibiotic agents, and supportive therapies; all recovered after a median hospital stay of 7.5 days (median range, 5-13 days).
In contrast with the findings of Xia et al., the findings of Liu et al. showed COVID-19 caused moderate to severe respiratory illness in children, and that infections in children were occurring early in the epidemic.
Some perspective
In an interview regarding the findings by Xia et al., Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University, and director of pediatric infectious diseases at Boston Medical Center, noted the absence of fever in 40% of cases.
“This is important, as the criteria for testing by public health departments has been high fever, cough, and shortness of breath,” he said. “The absence of fever is not inconsistent with COVID-19 disease.”
Another important point regarding the findings by Xia et al. is that the highest attack rates appear to be in children under 1 year of age, he said, further noting that the finding of concurrent influenza A, influenza B, or respiratory syncytial virus underscores that “concurrent infection can occur, and the presence of another virus in diagnostic tests does not mean that COVID-19 is not causal.”
As for whether the finding of elevated procalcitonin levels in 80% of cases reflects COVID-19 disease or coinfection with bacteria, the answer is unclear. But none of the children in the study were proven to have bacterial disease, he said, adding that “this marker will need to be interpreted with caution in the setting of COVID-19 disease.”
Dr. Xia and colleagues reported having no disclosures. Dr. Liu and associates also reported having no disclosures. The study by Liu et al. was supported by the Ministry of Science and Technology of China, the National Mega Project on Major Infectious Disease Prevention, and the National Key Research and Development Program of China.
SOURCES: Xia W et al. Ped Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718; Liu W et al. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
FROM PEDIATRIC PULMONOLOGY